User login
Chronic Cough in Children: Is it Asthma?
CAM in MS: What Works?
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
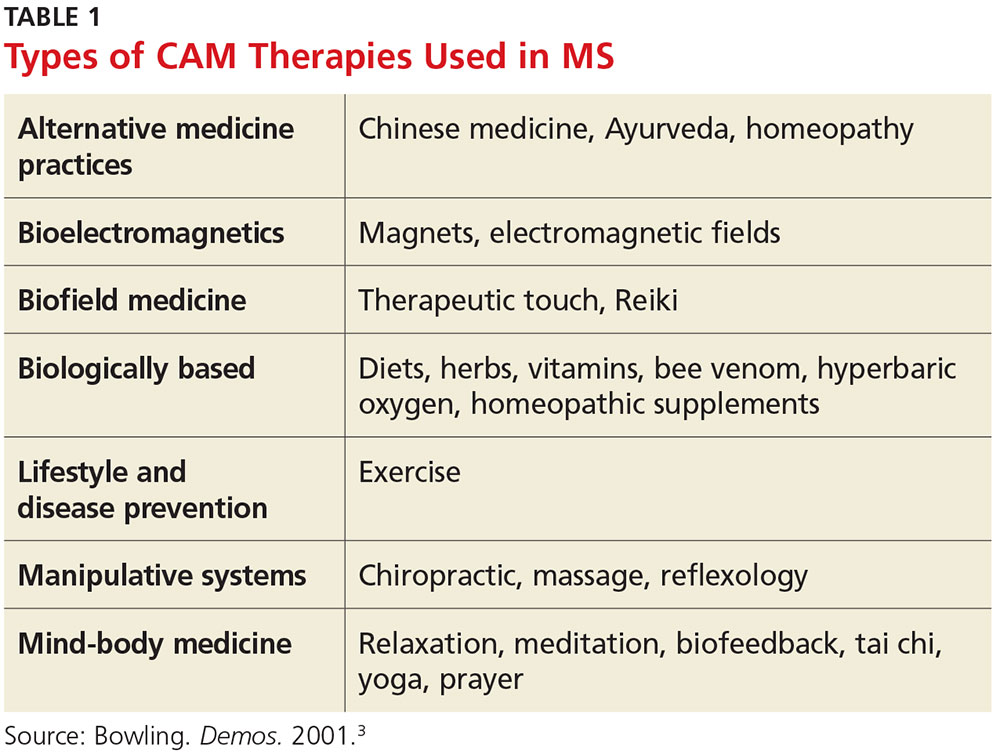
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.
Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.

Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.

Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.

Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
Q)How is complementary and alternative medicine used in multiple sclerosis, and how can I safely recommend it to my patients?
Complementary and alternative medicine (CAM) is a non-mainstream practice used in conjunction with conventional medicine.1 Its use is prevalent among people with and without chronic illnesses, including those living with multiple sclerosis (MS). Up to 70% of Americans with MS have used some type of CAM therapy, compared with 36% of the general population.1,2 CAM use is higher in women than in men and is highest among persons ages 35 to 49—two demographics also associated with MS.3
CAM practices include a myriad of therapies from different disciplines (see Table 1).3 Because most people who use CAM do not discuss it with their health care providers, it is important that providers inquire about patient use and are armed with basic safety and efficacy information.

Office visits for MS should include safety and efficacy discussions about all therapeutic treatments (disease modifying, relapse, and symptom management). Some issues—such as adverse effects—are obvious, while others, such as cost, are less so. A patient with MS may pursue an extremely expensive CAM therapy that lacks substantial evidence for the condition. Providers should therefore consider cost as part of the safety equation and be aware that while some CAM therapies have been studied in MS, most have not (or the research has been of poor quality).4
For many commonly used therapies, there is insufficient scientific evidence to support their usefulness in MS. These include acupuncture, biofeedback, Chinese medicine, chiropractic care, replacing amalgam dental fillings, equine therapy, hyperbaric oxygen treatment, low-dose naltrexone, massage therapy, tai chi, and yoga. While many of these practices are relatively safe and inexpensive, others may cause financial harm. Conversely, something considered safe and inexpensive (eg, a low-fat diet with omega-3 supplementation) may be found to be ineffective. Although recommending this type of diet for a person with MS is safe, realistic expectations must be discussed regarding its effect (or lack thereof) on the condition.4
The effectiveness of medical marijuana for MS is another popular deliberation. While data suggest that several administration methods of oral cannabinoids may be effective for spasticity and pain reduction, there is inadequate evidence to support the use of smoked cannabis. The deleterious effects of cannabis on cognition also need to be considered.5
Dietary supplements (eg, vitamins, minerals, botanicals, dietary substances) are often regarded as safe by patients because they are “natural.” As clinicians, we must be direct in asking patients about everything they are taking—many dietary supplements have drug interactions and/or toxic effects and may adversely stimulate the immune system. Vitamin D, for example, is one supplement that has been heavily studied in MS; lower levels of vitamin D have been shown to increase the risk for MS, and higher levels may be associated with lower relapse and disability rates. Therefore, standard of practice is to monitor vitamin D levels and supplement accordingly.
CAM can be safely and effectively recommended to people living with MS with due diligence. A question guide to aid recommendations is listed in Table 2.

Currently, no CAM therapies have been shown to modify MS, and CAM should not be recommended in place of disease-modifying treatment. However, if the proper questions are addressed, many CAM therapies can be safely recommended for common MS symptoms. Insurance coverage varies significantly among policies, but some treatments (eg, acupuncture and chiropractic care) are gaining coverage.
Finally, it is safe and a good standard of care to recommend a healthy anti-inflammatory diet, such as the Mediterranean diet, to people living with MS in order to improve general health. —MW
Megan Weigel, DNP, ARNP-C, MSCN
President of IOMSN
Baptist Neurology, Jacksonville Beach, Florida
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
1. CDC. Complementary and alternative medicine use among adults: United States, 2002. http://nccih.nih.gov/sites/nccam.nih.gov/files/news/camstats/2002/report.pdf. Accessed October 28, 2016.
2. Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. 2010;6(3):381-395.
3. Bowling AC. Alternative Medicine and Multiple Sclerosis. New York: Demos; 2001.
4. Bowling AC. Complementary and alternative medicine in MS. www.nationalmssociety.org/nationalmssociety/media/msnationalfiles/brochures/clinical_bulletin_complementary-and-alternative-medicine-in-ms.pdf. Accessed November 2, 2016.
5. Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76(13):1153-1160.
Fiber may play role in lessening knee pain, OA development
Consumption of dietary fiber at the recommended average intake of 25 g per day was associated with lower risks of developing symptomatic knee osteoarthritis and moderate or severe knee pain over 4-8 years in two separate analyses of Osteoarthritis Initiative participants conducted by investigators at Boston University.
The studies are the first to describe an association between total dietary fiber and lower risk of symptomatic OA and pain worsening in the knee, as well as a lower risk of moderate and severe pain patterns. The lowered risks were partially mediated by body mass index (BMI) but persisted even after adjustment for the variable.
In both studies, the investigators estimated dietary fiber intake by using a validated food frequency questionnaire at baseline that summed the fibers from grains, fruits and vegetables, and nuts and legumes.
Fiber and symptomatic knee OA
At the end of 4 years of follow-up, Dr. Dai and her colleagues identified 152 knees with incident radiographic OA (defined as a knee newly developing a Kellgren and Lawrence grade of 2 or higher), 869 knees with incident symptomatic OA (defined as new onset of both radiographic OA and a painful knee on most days in past month), and 1,964 knees with pain worsening as defined by an increase of at least 14% of the baseline Western Ontario and McMaster Universities (WOMAC) Index pain subscale score at each annual exam. This analysis excluded 540 people who were lost to follow-up and 205 who had invalid caloric intake recordings, leaving 4,051 in the study. The outcomes also excluded people with prevalent radiographic or symptomatic knee OA or knee pain worsening at baseline.
There was a significant trend for lower risk of both symptomatic OA (P less than .002) and pain worsening (P = .005) across four quartiles of daily total dietary fiber intake (mean of 9.1 g, 13 g, 16 g, and 21.9 g in quartiles 1-4). Quartile 4 daily intake was associated with a statistically significant 30% reduction (95% confidence interval, 6%-48%) in the odds of symptomatic knee OA and a 19% reduction (95% CI, 6%-29%) in the odds of pain worsening. Both comparisons were adjusted for age, sex, race, total energy intake, education, smoking status, physical activity, intake of other dietary factors (including polyunsaturated fat and other fats, vitamin C, vitamin D, vitamin E, vitamin K, dairy products, sweets, and soda), and nonsteroidal anti-inflammatory drug use (for the pain-worsening comparison).
Even though approximately 34% of the association between total fiber intake and symptomatic OA and 22% of the association between total fiber intake and pain worsening were mediated through reduced BMI, further adjustment of the comparisons for baseline BMI yielded similarly significant results.
The investigators found no associations between total dietary fiber intake and radiographic knee OA or for other fiber intake with either symptomatic or radiographic knee OA.
“The strongest protection was suggested at the highest quartile, which is in line with the current dietary guidelines for daily fiber intake. For older people [aged 51 years and older], for women it’s 22 g per day and for men it’s 28 g per day,” Dr. Dai said at the meeting.
Fiber and knee pain trajectories
Dr. Dai and her associates identified distinct, relatively homogeneous clusters of WOMAC pain trajectories over the 8-year study course in patients with and without radiographic knee OA at baseline in the Arthritis Care & Research study, and then examined their relationship to participants’ total dietary fiber intake, divided into quartiles (Arthritis Res Care. 2016; Nov 29. doi: 10.1002/acr.23158). The investigators found four pain trajectory patterns, including no pain (34.5%), mild pain (38.1%), moderate pain (21.2%), and severe pain (6.2%).
Individuals who consumed the most total fiber also had the highest representation in the no pain pattern (38.1%) and the lowest representation in the severe pain pattern (4.3%). A high total fiber intake was associated with lower risk of having a moderate or severe pain pattern when compared with those in the no pain trajectory (both P for trend less than .01). Intake of fiber in the highest quartile was associated with a 24% lower likelihood (95% CI, 7%-39%) of belonging to the moderate pain pattern and a 44% lower likelihood (95% CI, 2%-59%) of being in the severe pain pattern, compared with individuals in the lowest intake quartile.
The same four pain trajectory patterns existed in individuals with radiographic knee OA at baseline, but the proportions were shifted slightly lower for no pain (26.1%) and higher for severe pain (7.9%). There was an even greater effect magnitude for the association between dietary total fiber and moderate or severe pain pattern among individuals with radiographic knee OA at baseline. Similar results were found for participants without radiographic knee OA at baseline. The relationships between total dietary fiber intake and pain patterns were somewhat attenuated after adjustment for depression scores and BMI at baseline but still remained statistically significant.
In each of the comparisons and sensitivity analyses, the highest quartile of cereal grain fiber intake was also significant on its own in lowering risk for being in the moderate or severe pain trajectory patterns. However, no significant results were found for fiber from fruits and vegetables or from nuts and legumes.
The studies were supported by grants from the National Institutes of Health. None of the authors had conflicts of interest to disclose.
Consumption of dietary fiber at the recommended average intake of 25 g per day was associated with lower risks of developing symptomatic knee osteoarthritis and moderate or severe knee pain over 4-8 years in two separate analyses of Osteoarthritis Initiative participants conducted by investigators at Boston University.
The studies are the first to describe an association between total dietary fiber and lower risk of symptomatic OA and pain worsening in the knee, as well as a lower risk of moderate and severe pain patterns. The lowered risks were partially mediated by body mass index (BMI) but persisted even after adjustment for the variable.
In both studies, the investigators estimated dietary fiber intake by using a validated food frequency questionnaire at baseline that summed the fibers from grains, fruits and vegetables, and nuts and legumes.
Fiber and symptomatic knee OA
At the end of 4 years of follow-up, Dr. Dai and her colleagues identified 152 knees with incident radiographic OA (defined as a knee newly developing a Kellgren and Lawrence grade of 2 or higher), 869 knees with incident symptomatic OA (defined as new onset of both radiographic OA and a painful knee on most days in past month), and 1,964 knees with pain worsening as defined by an increase of at least 14% of the baseline Western Ontario and McMaster Universities (WOMAC) Index pain subscale score at each annual exam. This analysis excluded 540 people who were lost to follow-up and 205 who had invalid caloric intake recordings, leaving 4,051 in the study. The outcomes also excluded people with prevalent radiographic or symptomatic knee OA or knee pain worsening at baseline.
There was a significant trend for lower risk of both symptomatic OA (P less than .002) and pain worsening (P = .005) across four quartiles of daily total dietary fiber intake (mean of 9.1 g, 13 g, 16 g, and 21.9 g in quartiles 1-4). Quartile 4 daily intake was associated with a statistically significant 30% reduction (95% confidence interval, 6%-48%) in the odds of symptomatic knee OA and a 19% reduction (95% CI, 6%-29%) in the odds of pain worsening. Both comparisons were adjusted for age, sex, race, total energy intake, education, smoking status, physical activity, intake of other dietary factors (including polyunsaturated fat and other fats, vitamin C, vitamin D, vitamin E, vitamin K, dairy products, sweets, and soda), and nonsteroidal anti-inflammatory drug use (for the pain-worsening comparison).
Even though approximately 34% of the association between total fiber intake and symptomatic OA and 22% of the association between total fiber intake and pain worsening were mediated through reduced BMI, further adjustment of the comparisons for baseline BMI yielded similarly significant results.
The investigators found no associations between total dietary fiber intake and radiographic knee OA or for other fiber intake with either symptomatic or radiographic knee OA.
“The strongest protection was suggested at the highest quartile, which is in line with the current dietary guidelines for daily fiber intake. For older people [aged 51 years and older], for women it’s 22 g per day and for men it’s 28 g per day,” Dr. Dai said at the meeting.
Fiber and knee pain trajectories
Dr. Dai and her associates identified distinct, relatively homogeneous clusters of WOMAC pain trajectories over the 8-year study course in patients with and without radiographic knee OA at baseline in the Arthritis Care & Research study, and then examined their relationship to participants’ total dietary fiber intake, divided into quartiles (Arthritis Res Care. 2016; Nov 29. doi: 10.1002/acr.23158). The investigators found four pain trajectory patterns, including no pain (34.5%), mild pain (38.1%), moderate pain (21.2%), and severe pain (6.2%).
Individuals who consumed the most total fiber also had the highest representation in the no pain pattern (38.1%) and the lowest representation in the severe pain pattern (4.3%). A high total fiber intake was associated with lower risk of having a moderate or severe pain pattern when compared with those in the no pain trajectory (both P for trend less than .01). Intake of fiber in the highest quartile was associated with a 24% lower likelihood (95% CI, 7%-39%) of belonging to the moderate pain pattern and a 44% lower likelihood (95% CI, 2%-59%) of being in the severe pain pattern, compared with individuals in the lowest intake quartile.
The same four pain trajectory patterns existed in individuals with radiographic knee OA at baseline, but the proportions were shifted slightly lower for no pain (26.1%) and higher for severe pain (7.9%). There was an even greater effect magnitude for the association between dietary total fiber and moderate or severe pain pattern among individuals with radiographic knee OA at baseline. Similar results were found for participants without radiographic knee OA at baseline. The relationships between total dietary fiber intake and pain patterns were somewhat attenuated after adjustment for depression scores and BMI at baseline but still remained statistically significant.
In each of the comparisons and sensitivity analyses, the highest quartile of cereal grain fiber intake was also significant on its own in lowering risk for being in the moderate or severe pain trajectory patterns. However, no significant results were found for fiber from fruits and vegetables or from nuts and legumes.
The studies were supported by grants from the National Institutes of Health. None of the authors had conflicts of interest to disclose.
Consumption of dietary fiber at the recommended average intake of 25 g per day was associated with lower risks of developing symptomatic knee osteoarthritis and moderate or severe knee pain over 4-8 years in two separate analyses of Osteoarthritis Initiative participants conducted by investigators at Boston University.
The studies are the first to describe an association between total dietary fiber and lower risk of symptomatic OA and pain worsening in the knee, as well as a lower risk of moderate and severe pain patterns. The lowered risks were partially mediated by body mass index (BMI) but persisted even after adjustment for the variable.
In both studies, the investigators estimated dietary fiber intake by using a validated food frequency questionnaire at baseline that summed the fibers from grains, fruits and vegetables, and nuts and legumes.
Fiber and symptomatic knee OA
At the end of 4 years of follow-up, Dr. Dai and her colleagues identified 152 knees with incident radiographic OA (defined as a knee newly developing a Kellgren and Lawrence grade of 2 or higher), 869 knees with incident symptomatic OA (defined as new onset of both radiographic OA and a painful knee on most days in past month), and 1,964 knees with pain worsening as defined by an increase of at least 14% of the baseline Western Ontario and McMaster Universities (WOMAC) Index pain subscale score at each annual exam. This analysis excluded 540 people who were lost to follow-up and 205 who had invalid caloric intake recordings, leaving 4,051 in the study. The outcomes also excluded people with prevalent radiographic or symptomatic knee OA or knee pain worsening at baseline.
There was a significant trend for lower risk of both symptomatic OA (P less than .002) and pain worsening (P = .005) across four quartiles of daily total dietary fiber intake (mean of 9.1 g, 13 g, 16 g, and 21.9 g in quartiles 1-4). Quartile 4 daily intake was associated with a statistically significant 30% reduction (95% confidence interval, 6%-48%) in the odds of symptomatic knee OA and a 19% reduction (95% CI, 6%-29%) in the odds of pain worsening. Both comparisons were adjusted for age, sex, race, total energy intake, education, smoking status, physical activity, intake of other dietary factors (including polyunsaturated fat and other fats, vitamin C, vitamin D, vitamin E, vitamin K, dairy products, sweets, and soda), and nonsteroidal anti-inflammatory drug use (for the pain-worsening comparison).
Even though approximately 34% of the association between total fiber intake and symptomatic OA and 22% of the association between total fiber intake and pain worsening were mediated through reduced BMI, further adjustment of the comparisons for baseline BMI yielded similarly significant results.
The investigators found no associations between total dietary fiber intake and radiographic knee OA or for other fiber intake with either symptomatic or radiographic knee OA.
“The strongest protection was suggested at the highest quartile, which is in line with the current dietary guidelines for daily fiber intake. For older people [aged 51 years and older], for women it’s 22 g per day and for men it’s 28 g per day,” Dr. Dai said at the meeting.
Fiber and knee pain trajectories
Dr. Dai and her associates identified distinct, relatively homogeneous clusters of WOMAC pain trajectories over the 8-year study course in patients with and without radiographic knee OA at baseline in the Arthritis Care & Research study, and then examined their relationship to participants’ total dietary fiber intake, divided into quartiles (Arthritis Res Care. 2016; Nov 29. doi: 10.1002/acr.23158). The investigators found four pain trajectory patterns, including no pain (34.5%), mild pain (38.1%), moderate pain (21.2%), and severe pain (6.2%).
Individuals who consumed the most total fiber also had the highest representation in the no pain pattern (38.1%) and the lowest representation in the severe pain pattern (4.3%). A high total fiber intake was associated with lower risk of having a moderate or severe pain pattern when compared with those in the no pain trajectory (both P for trend less than .01). Intake of fiber in the highest quartile was associated with a 24% lower likelihood (95% CI, 7%-39%) of belonging to the moderate pain pattern and a 44% lower likelihood (95% CI, 2%-59%) of being in the severe pain pattern, compared with individuals in the lowest intake quartile.
The same four pain trajectory patterns existed in individuals with radiographic knee OA at baseline, but the proportions were shifted slightly lower for no pain (26.1%) and higher for severe pain (7.9%). There was an even greater effect magnitude for the association between dietary total fiber and moderate or severe pain pattern among individuals with radiographic knee OA at baseline. Similar results were found for participants without radiographic knee OA at baseline. The relationships between total dietary fiber intake and pain patterns were somewhat attenuated after adjustment for depression scores and BMI at baseline but still remained statistically significant.
In each of the comparisons and sensitivity analyses, the highest quartile of cereal grain fiber intake was also significant on its own in lowering risk for being in the moderate or severe pain trajectory patterns. However, no significant results were found for fiber from fruits and vegetables or from nuts and legumes.
The studies were supported by grants from the National Institutes of Health. None of the authors had conflicts of interest to disclose.
FROM ARTHRITIS CARE & RESEARCH AND THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The highest quartile of daily dietary fiber intake was associated with a statistically significant 30% reduction (95% CI, 6%-48%) in the odds of symptomatic knee OA and a 19% reduction (95% CI, 6%-29%) in the odds of pain worsening.
Data source: Two analyses of the prospective, multicenter Osteoarthritis Initiative cohort of 4,796 men and women aged 45-79 years with or at risk for knee osteoarthritis.
Disclosures: The studies were supported by grants from the National Institutes of Health. None of the authors had conflicts of interest to disclose.
Legislators commit to bipartisan support of Alzheimer’s funding
WASHINGTON – The nation’s political sea-change won’t wash Alzheimer’s disease research funding offtrack, two legislators vowed at a Washington briefing.
Rather than descend into partisan budget-bickering under the new administration, lawmakers should reach across the aisle and pass funding bills to vigorously propel the nation toward its goal of having an effective disease-modifying Alzheimer’s disease therapy by 2025.
“If we make this a high-enough priority, we can meet that goal,” Rep. Paul Tonko (D-NY) said at a briefing sponsored by The Hill newspaper, with support by Eli Lilly. “We should prioritize the work we need to do to achieve it in both the House and Senate, and move forward both aggressively and progressively.”
“I am a strong proponent of investing in research. We simply must continue to put research dollars into Alzheimer’s. If we don’t invest, try to find a cure or treatment, Alzheimer’s will become the single largest driver of health care costs on both a federal and state level. We have to recognize this: We could, potentially, not even be able to provide care for all patients we will have, unless we find a treatment or a cure.”
After a decade of struggle, federal dollars for Alzheimer’s research have begun to creep up. Last year, Congress passed a historic $350 million increase in Alzheimer’s research funding at the National Institutes of Health, raising the total spending to $991 million for fiscal year 2016. Then, in June, the Senate Appropriations Committee approved a landmark $400 million funding increase. In July, the House Appropriations Committee approved its own $350 million bump.
In August, the NIH recommended a $414 million increase for fiscal year 2018. If both the fiscal year 2017 increase and fiscal year 2018 request are ultimately passed, funding levels would be very close to the $2 billion/year federal commitment that researchers and Alzheimer’s policy mavens say is necessary to achieve the 2025 goal, set forth in the National Plan to Address Alzheimer’s Disease.
“There’s no such thing as too much research funding,” Sen. Tillis said during the briefing. But, he added, that federal generosity must be wisely husbanded.
“There must also be a sense of discipline along with the funding. Money like that should be targeted in terms of which diseases we go after – they should be areas that have the broadest impact. In a world of scarce resources, we can’t afford to simply throw money around.”
Alzheimer’s research is a perfect example of this careful resource management, he said. The NIH has prepared its second “bypass budget,” a funding proposal that passes the normal legislative channels and goes directly to the President.
Based on the consensus of scientists involved in search for a disease-modifying therapy, this budget proposal estimates the additional money needed to meet the 2025 goal, above the NIH’s baseline Alzheimer’s funding.
Only two other areas of medicine have such a budget: cancer and HIV-AIDS, said Robert J. Egge, chief public policy officer of the Alzheimer’s Association.
“This budget goes right from the scientists to Congress with no filter by the Office of Management and Budget, and lands directly on the President’s desk,” he said in an interview. “It tells legislators what scientists need in order to accomplish that 2025 goal.”
The $2 billion/year figure, Mr. Egge noted, is a ground-floor suggestion. “That’s what we need right now to stay on track. We think the best way to figure out where we need to be in the long-term is to let the scientists at NIH tell us what they need, and we need Congress and the Administration to follow this year to year.”
Like Sen. Tillis and Rep. Tonko, Mr. Egge was upbeat in anticipating steady funding progress.
“The champions of Alzheimer’s funding who have made such a difference for us are all back and in their same positions,” with unstinting commitment to the cause.
Those legislators include:
• Rep. Tom Cole (R-OK), chairman of the House Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, which funds the NIH. In 2015, Rep. Cole shepherded through a $300 million appropriation for Alzheimer’s research, and the pending $350 million appropriation for fiscal year 2017.
• Sen. Roy Blunt (R-MO), who, as chairman of the Senate Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, secured June’s $400 million and a $350 million bump in 2015.
• Ranking member Sen. Patty Murray (D-WA), who cosponsored the Alzheimer’s Breakthrough Act of 2009, and who worked with Sen. Blunt to secure the recent Alzheimer’s funding increases.
Mr. Egge is not overly troubled about the antiscience rhetoric bandied about by some potential members of President-elect Trump’s administration. He predicted those rumblings will settle down and not negatively affect Alzheimer’s funding.
“I think all the talk of not believing in the science of biomedical research was premature,” he said. “I think it’s completely appropriate to say ‘We are going to watch closely and form opinions,’ but I have never been overly alarmed by this. As an Alzheimer’s advocate for strong science, I am waiting to see what the next steps are. President-elect Trump has said on the campaign trail that Alzheimer’s would be a top priority. Our champions from both parties are fully committed to this fight and recognize that science is the fundamental path to do so. I share this optimism. It’s not complacent optimism – it’s vigilant optimism that we will continue with the momentum we need to finally, squarely and effectively, address this disease.”
[email protected]
On Twitter @alz_gal
WASHINGTON – The nation’s political sea-change won’t wash Alzheimer’s disease research funding offtrack, two legislators vowed at a Washington briefing.
Rather than descend into partisan budget-bickering under the new administration, lawmakers should reach across the aisle and pass funding bills to vigorously propel the nation toward its goal of having an effective disease-modifying Alzheimer’s disease therapy by 2025.
“If we make this a high-enough priority, we can meet that goal,” Rep. Paul Tonko (D-NY) said at a briefing sponsored by The Hill newspaper, with support by Eli Lilly. “We should prioritize the work we need to do to achieve it in both the House and Senate, and move forward both aggressively and progressively.”
“I am a strong proponent of investing in research. We simply must continue to put research dollars into Alzheimer’s. If we don’t invest, try to find a cure or treatment, Alzheimer’s will become the single largest driver of health care costs on both a federal and state level. We have to recognize this: We could, potentially, not even be able to provide care for all patients we will have, unless we find a treatment or a cure.”
After a decade of struggle, federal dollars for Alzheimer’s research have begun to creep up. Last year, Congress passed a historic $350 million increase in Alzheimer’s research funding at the National Institutes of Health, raising the total spending to $991 million for fiscal year 2016. Then, in June, the Senate Appropriations Committee approved a landmark $400 million funding increase. In July, the House Appropriations Committee approved its own $350 million bump.
In August, the NIH recommended a $414 million increase for fiscal year 2018. If both the fiscal year 2017 increase and fiscal year 2018 request are ultimately passed, funding levels would be very close to the $2 billion/year federal commitment that researchers and Alzheimer’s policy mavens say is necessary to achieve the 2025 goal, set forth in the National Plan to Address Alzheimer’s Disease.
“There’s no such thing as too much research funding,” Sen. Tillis said during the briefing. But, he added, that federal generosity must be wisely husbanded.
“There must also be a sense of discipline along with the funding. Money like that should be targeted in terms of which diseases we go after – they should be areas that have the broadest impact. In a world of scarce resources, we can’t afford to simply throw money around.”
Alzheimer’s research is a perfect example of this careful resource management, he said. The NIH has prepared its second “bypass budget,” a funding proposal that passes the normal legislative channels and goes directly to the President.
Based on the consensus of scientists involved in search for a disease-modifying therapy, this budget proposal estimates the additional money needed to meet the 2025 goal, above the NIH’s baseline Alzheimer’s funding.
Only two other areas of medicine have such a budget: cancer and HIV-AIDS, said Robert J. Egge, chief public policy officer of the Alzheimer’s Association.
“This budget goes right from the scientists to Congress with no filter by the Office of Management and Budget, and lands directly on the President’s desk,” he said in an interview. “It tells legislators what scientists need in order to accomplish that 2025 goal.”
The $2 billion/year figure, Mr. Egge noted, is a ground-floor suggestion. “That’s what we need right now to stay on track. We think the best way to figure out where we need to be in the long-term is to let the scientists at NIH tell us what they need, and we need Congress and the Administration to follow this year to year.”
Like Sen. Tillis and Rep. Tonko, Mr. Egge was upbeat in anticipating steady funding progress.
“The champions of Alzheimer’s funding who have made such a difference for us are all back and in their same positions,” with unstinting commitment to the cause.
Those legislators include:
• Rep. Tom Cole (R-OK), chairman of the House Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, which funds the NIH. In 2015, Rep. Cole shepherded through a $300 million appropriation for Alzheimer’s research, and the pending $350 million appropriation for fiscal year 2017.
• Sen. Roy Blunt (R-MO), who, as chairman of the Senate Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, secured June’s $400 million and a $350 million bump in 2015.
• Ranking member Sen. Patty Murray (D-WA), who cosponsored the Alzheimer’s Breakthrough Act of 2009, and who worked with Sen. Blunt to secure the recent Alzheimer’s funding increases.
Mr. Egge is not overly troubled about the antiscience rhetoric bandied about by some potential members of President-elect Trump’s administration. He predicted those rumblings will settle down and not negatively affect Alzheimer’s funding.
“I think all the talk of not believing in the science of biomedical research was premature,” he said. “I think it’s completely appropriate to say ‘We are going to watch closely and form opinions,’ but I have never been overly alarmed by this. As an Alzheimer’s advocate for strong science, I am waiting to see what the next steps are. President-elect Trump has said on the campaign trail that Alzheimer’s would be a top priority. Our champions from both parties are fully committed to this fight and recognize that science is the fundamental path to do so. I share this optimism. It’s not complacent optimism – it’s vigilant optimism that we will continue with the momentum we need to finally, squarely and effectively, address this disease.”
[email protected]
On Twitter @alz_gal
WASHINGTON – The nation’s political sea-change won’t wash Alzheimer’s disease research funding offtrack, two legislators vowed at a Washington briefing.
Rather than descend into partisan budget-bickering under the new administration, lawmakers should reach across the aisle and pass funding bills to vigorously propel the nation toward its goal of having an effective disease-modifying Alzheimer’s disease therapy by 2025.
“If we make this a high-enough priority, we can meet that goal,” Rep. Paul Tonko (D-NY) said at a briefing sponsored by The Hill newspaper, with support by Eli Lilly. “We should prioritize the work we need to do to achieve it in both the House and Senate, and move forward both aggressively and progressively.”
“I am a strong proponent of investing in research. We simply must continue to put research dollars into Alzheimer’s. If we don’t invest, try to find a cure or treatment, Alzheimer’s will become the single largest driver of health care costs on both a federal and state level. We have to recognize this: We could, potentially, not even be able to provide care for all patients we will have, unless we find a treatment or a cure.”
After a decade of struggle, federal dollars for Alzheimer’s research have begun to creep up. Last year, Congress passed a historic $350 million increase in Alzheimer’s research funding at the National Institutes of Health, raising the total spending to $991 million for fiscal year 2016. Then, in June, the Senate Appropriations Committee approved a landmark $400 million funding increase. In July, the House Appropriations Committee approved its own $350 million bump.
In August, the NIH recommended a $414 million increase for fiscal year 2018. If both the fiscal year 2017 increase and fiscal year 2018 request are ultimately passed, funding levels would be very close to the $2 billion/year federal commitment that researchers and Alzheimer’s policy mavens say is necessary to achieve the 2025 goal, set forth in the National Plan to Address Alzheimer’s Disease.
“There’s no such thing as too much research funding,” Sen. Tillis said during the briefing. But, he added, that federal generosity must be wisely husbanded.
“There must also be a sense of discipline along with the funding. Money like that should be targeted in terms of which diseases we go after – they should be areas that have the broadest impact. In a world of scarce resources, we can’t afford to simply throw money around.”
Alzheimer’s research is a perfect example of this careful resource management, he said. The NIH has prepared its second “bypass budget,” a funding proposal that passes the normal legislative channels and goes directly to the President.
Based on the consensus of scientists involved in search for a disease-modifying therapy, this budget proposal estimates the additional money needed to meet the 2025 goal, above the NIH’s baseline Alzheimer’s funding.
Only two other areas of medicine have such a budget: cancer and HIV-AIDS, said Robert J. Egge, chief public policy officer of the Alzheimer’s Association.
“This budget goes right from the scientists to Congress with no filter by the Office of Management and Budget, and lands directly on the President’s desk,” he said in an interview. “It tells legislators what scientists need in order to accomplish that 2025 goal.”
The $2 billion/year figure, Mr. Egge noted, is a ground-floor suggestion. “That’s what we need right now to stay on track. We think the best way to figure out where we need to be in the long-term is to let the scientists at NIH tell us what they need, and we need Congress and the Administration to follow this year to year.”
Like Sen. Tillis and Rep. Tonko, Mr. Egge was upbeat in anticipating steady funding progress.
“The champions of Alzheimer’s funding who have made such a difference for us are all back and in their same positions,” with unstinting commitment to the cause.
Those legislators include:
• Rep. Tom Cole (R-OK), chairman of the House Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, which funds the NIH. In 2015, Rep. Cole shepherded through a $300 million appropriation for Alzheimer’s research, and the pending $350 million appropriation for fiscal year 2017.
• Sen. Roy Blunt (R-MO), who, as chairman of the Senate Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, secured June’s $400 million and a $350 million bump in 2015.
• Ranking member Sen. Patty Murray (D-WA), who cosponsored the Alzheimer’s Breakthrough Act of 2009, and who worked with Sen. Blunt to secure the recent Alzheimer’s funding increases.
Mr. Egge is not overly troubled about the antiscience rhetoric bandied about by some potential members of President-elect Trump’s administration. He predicted those rumblings will settle down and not negatively affect Alzheimer’s funding.
“I think all the talk of not believing in the science of biomedical research was premature,” he said. “I think it’s completely appropriate to say ‘We are going to watch closely and form opinions,’ but I have never been overly alarmed by this. As an Alzheimer’s advocate for strong science, I am waiting to see what the next steps are. President-elect Trump has said on the campaign trail that Alzheimer’s would be a top priority. Our champions from both parties are fully committed to this fight and recognize that science is the fundamental path to do so. I share this optimism. It’s not complacent optimism – it’s vigilant optimism that we will continue with the momentum we need to finally, squarely and effectively, address this disease.”
[email protected]
On Twitter @alz_gal
Cultural approach to vaccine hesitancy essential for ethnic communities
ATLANTA – Research into vaccine hesitancy in the United States tends to focus on overall trends among native-born Americans or immigrants who have mostly assimilated into American culture. But the nation is dotted with tight-knit ethnic communities which have immigrated to the United States, including refugee communities that retain much of the culture and practices of their home country.
Developing interventions to address vaccine hesitancy in these communities may require a significantly different approach than it would in fully assimilated groups, with a need to start by learning about the culture, fears, values and priorities of that particular community.
A 2000 study had shown Somali parents were generally supportive of immunization, but that perception had changed by summer of 2008, explained co-presenter Lynn Bahta, RN, PHN, an immunization clinical consultant at the Minnesota Department of Health Immunization Program. A local TV station ran a story about Somali parents’ concern that a disproportionately higher number of Somali children were in early childhood special education programs for autism.
“In the middle of the report, a parent stated, ‘It’s the vaccines,’ ” Ms. Bahta said. Because they did not have a word for autism in Somali, parents’ online searches led them to groups promoting the misconception that the MMR vaccine and autism were linked. Clinicians in Minnesota began to report Somali parents’ refusal to get their children’s 12-month vaccines. Then a 2011 measles outbreak led the Minnesota Department of Health to look at MMR vaccination rates among local Somalis.
Somalis had a higher rate of MMR coverage in 24-month-old children than did non-Somalis in 2004 – 90%, compared with 84% – according to the Minnesota Immunization Information Connection. But MMR rates among Somali 24-month-olds began dropping in 2005, reaching 82% in 2007 and 63% in 2009.
“The data we got instilled a bit of panic in the immunization team,” Ms. Bahta said. “Parents were still supporting immunizations, but they weren’t getting that MMR.”
Traditional strategies to increase vaccination – distributing travel immunization information, promoting YouTube videos about immunization and autism, using diverse media for information campaigns – failed.
So they joined with the community and family health department, where co-presenter Asli Ashkir, RN, MPH, is a senior nurse consultant in the Children & Youth with Special Health Needs program. They also hired Somali staff and began to improve their cultural knowledge and competence.
With Somalis, social life revolves around family ties, the community, and faith, explained Ms. Ashkir, a Somali woman herself. Somali culture is based on oral tradition, one that shares information among themselves and provides unsolicited advice to one another, and they persuade each other easily. But issues of health, life, and death are in the hands of Allah only, she said.
“There is a time you will die, whether you are vaccinated or not,” Ms. Ashkir explained. “That doesn’t mean we don’t practice preventive service or health promotion – we do – but at the back of our head, when our time is over, you’re going to go. These are the people we are working with.”
Two other potential obstacles involve Somali beliefs about sin and mental illness.
“We believe if someone is ill, their sins will be cleansed,” she said, explaining why Somalis with minor health problems don’t seek health care. “Parents with kids who have autism keep kids in their apartment until they are 8 years old because mental illness has a negative stigma.”
The Minnesota Department of Health conducted a study on the experience of having a child with autism in the Somali community and discovered four key themes. First, the parents greatly feared autism: Every Somali interviewed said they did not get the MMR because they wanted to avoid autism. Second, parents lacked information about normal child development, autism, and the diseases that vaccines prevent.
“We were expecting parents to identify developmental delays, but parents look not at the development but the growth, at the physical size of the child,” Ms. Ashkir said. And when they learned that the MMR prevented measles – the No. 3 killer of children in Somalia – parents often wanted the shot immediately.
The other two discoveries were that it was impossible to talk about immunization issues in isolation – they were too intricately entwined with discussions about autism – and that Somalis wanted to hear information from respected community sources.
These findings were applied in a pilot program that aimed to improve parents’ knowledge about child growth and development, autism, and vaccine-preventable diseases. Six mothers attended the training program, and tracking their contacts revealed that the information had traveled to 82 other family, friends, and neighbors within the first 3 months. All the women found the program “very helpful” with no negative responses.
The success of this program led to a more comprehensive approach that included training and outreach, engaging the community, disease mitigation and control, and creating and expanding partnerships with organizations such as the state American Academy of Pediatrics chapter, the Somali American Parent Association, the Minnesota Medical Association, and Parents in Community Action.
Training included all-Somali speakers with messages from spiritual leaders and parents of children with autism. Community outreach involved one-on-one conversations among Somalis at information tables in places such as malls, mosques, community centers, and libraries.
“Among this group, there are four parents who have children with autism,” Ms. Ashkir said. “Two of these parents are very, very vocal and talk about their children who have autism, and that they did not give them the MMR. They tell people ‘You have wrong information.’ ”
As of March 2016, the decline in MMR vaccination rates among Somalis had started to flatten. The annual drop of 5%-7% a year in MMR rates became 0.89% last year, which the Minnesota Department of Health finds encouraging.
“Our initial efforts, which included a typical repertoire of public health interventions, were ineffective, so we had to go back and dig deep to understand the core concerns,” Ms. Bahta said. “Our information had to address the core concerns of the community, not what we assumed to be the issue.”
Credibility came from the cultural relevancy of the message, and the fact that those providing the message were parents who had vaccinated their children, she said.
“Each cultural group needs unique approaches, and this is certainly true in this situation – to understand the unique perspective of the community and develop an effective approach required bringing in culturally competent staff and engaging the community,” Ms. Bahta said.
ATLANTA – Research into vaccine hesitancy in the United States tends to focus on overall trends among native-born Americans or immigrants who have mostly assimilated into American culture. But the nation is dotted with tight-knit ethnic communities which have immigrated to the United States, including refugee communities that retain much of the culture and practices of their home country.
Developing interventions to address vaccine hesitancy in these communities may require a significantly different approach than it would in fully assimilated groups, with a need to start by learning about the culture, fears, values and priorities of that particular community.
A 2000 study had shown Somali parents were generally supportive of immunization, but that perception had changed by summer of 2008, explained co-presenter Lynn Bahta, RN, PHN, an immunization clinical consultant at the Minnesota Department of Health Immunization Program. A local TV station ran a story about Somali parents’ concern that a disproportionately higher number of Somali children were in early childhood special education programs for autism.
“In the middle of the report, a parent stated, ‘It’s the vaccines,’ ” Ms. Bahta said. Because they did not have a word for autism in Somali, parents’ online searches led them to groups promoting the misconception that the MMR vaccine and autism were linked. Clinicians in Minnesota began to report Somali parents’ refusal to get their children’s 12-month vaccines. Then a 2011 measles outbreak led the Minnesota Department of Health to look at MMR vaccination rates among local Somalis.
Somalis had a higher rate of MMR coverage in 24-month-old children than did non-Somalis in 2004 – 90%, compared with 84% – according to the Minnesota Immunization Information Connection. But MMR rates among Somali 24-month-olds began dropping in 2005, reaching 82% in 2007 and 63% in 2009.
“The data we got instilled a bit of panic in the immunization team,” Ms. Bahta said. “Parents were still supporting immunizations, but they weren’t getting that MMR.”
Traditional strategies to increase vaccination – distributing travel immunization information, promoting YouTube videos about immunization and autism, using diverse media for information campaigns – failed.
So they joined with the community and family health department, where co-presenter Asli Ashkir, RN, MPH, is a senior nurse consultant in the Children & Youth with Special Health Needs program. They also hired Somali staff and began to improve their cultural knowledge and competence.
With Somalis, social life revolves around family ties, the community, and faith, explained Ms. Ashkir, a Somali woman herself. Somali culture is based on oral tradition, one that shares information among themselves and provides unsolicited advice to one another, and they persuade each other easily. But issues of health, life, and death are in the hands of Allah only, she said.
“There is a time you will die, whether you are vaccinated or not,” Ms. Ashkir explained. “That doesn’t mean we don’t practice preventive service or health promotion – we do – but at the back of our head, when our time is over, you’re going to go. These are the people we are working with.”
Two other potential obstacles involve Somali beliefs about sin and mental illness.
“We believe if someone is ill, their sins will be cleansed,” she said, explaining why Somalis with minor health problems don’t seek health care. “Parents with kids who have autism keep kids in their apartment until they are 8 years old because mental illness has a negative stigma.”
The Minnesota Department of Health conducted a study on the experience of having a child with autism in the Somali community and discovered four key themes. First, the parents greatly feared autism: Every Somali interviewed said they did not get the MMR because they wanted to avoid autism. Second, parents lacked information about normal child development, autism, and the diseases that vaccines prevent.
“We were expecting parents to identify developmental delays, but parents look not at the development but the growth, at the physical size of the child,” Ms. Ashkir said. And when they learned that the MMR prevented measles – the No. 3 killer of children in Somalia – parents often wanted the shot immediately.
The other two discoveries were that it was impossible to talk about immunization issues in isolation – they were too intricately entwined with discussions about autism – and that Somalis wanted to hear information from respected community sources.
These findings were applied in a pilot program that aimed to improve parents’ knowledge about child growth and development, autism, and vaccine-preventable diseases. Six mothers attended the training program, and tracking their contacts revealed that the information had traveled to 82 other family, friends, and neighbors within the first 3 months. All the women found the program “very helpful” with no negative responses.
The success of this program led to a more comprehensive approach that included training and outreach, engaging the community, disease mitigation and control, and creating and expanding partnerships with organizations such as the state American Academy of Pediatrics chapter, the Somali American Parent Association, the Minnesota Medical Association, and Parents in Community Action.
Training included all-Somali speakers with messages from spiritual leaders and parents of children with autism. Community outreach involved one-on-one conversations among Somalis at information tables in places such as malls, mosques, community centers, and libraries.
“Among this group, there are four parents who have children with autism,” Ms. Ashkir said. “Two of these parents are very, very vocal and talk about their children who have autism, and that they did not give them the MMR. They tell people ‘You have wrong information.’ ”
As of March 2016, the decline in MMR vaccination rates among Somalis had started to flatten. The annual drop of 5%-7% a year in MMR rates became 0.89% last year, which the Minnesota Department of Health finds encouraging.
“Our initial efforts, which included a typical repertoire of public health interventions, were ineffective, so we had to go back and dig deep to understand the core concerns,” Ms. Bahta said. “Our information had to address the core concerns of the community, not what we assumed to be the issue.”
Credibility came from the cultural relevancy of the message, and the fact that those providing the message were parents who had vaccinated their children, she said.
“Each cultural group needs unique approaches, and this is certainly true in this situation – to understand the unique perspective of the community and develop an effective approach required bringing in culturally competent staff and engaging the community,” Ms. Bahta said.
ATLANTA – Research into vaccine hesitancy in the United States tends to focus on overall trends among native-born Americans or immigrants who have mostly assimilated into American culture. But the nation is dotted with tight-knit ethnic communities which have immigrated to the United States, including refugee communities that retain much of the culture and practices of their home country.
Developing interventions to address vaccine hesitancy in these communities may require a significantly different approach than it would in fully assimilated groups, with a need to start by learning about the culture, fears, values and priorities of that particular community.
A 2000 study had shown Somali parents were generally supportive of immunization, but that perception had changed by summer of 2008, explained co-presenter Lynn Bahta, RN, PHN, an immunization clinical consultant at the Minnesota Department of Health Immunization Program. A local TV station ran a story about Somali parents’ concern that a disproportionately higher number of Somali children were in early childhood special education programs for autism.
“In the middle of the report, a parent stated, ‘It’s the vaccines,’ ” Ms. Bahta said. Because they did not have a word for autism in Somali, parents’ online searches led them to groups promoting the misconception that the MMR vaccine and autism were linked. Clinicians in Minnesota began to report Somali parents’ refusal to get their children’s 12-month vaccines. Then a 2011 measles outbreak led the Minnesota Department of Health to look at MMR vaccination rates among local Somalis.
Somalis had a higher rate of MMR coverage in 24-month-old children than did non-Somalis in 2004 – 90%, compared with 84% – according to the Minnesota Immunization Information Connection. But MMR rates among Somali 24-month-olds began dropping in 2005, reaching 82% in 2007 and 63% in 2009.
“The data we got instilled a bit of panic in the immunization team,” Ms. Bahta said. “Parents were still supporting immunizations, but they weren’t getting that MMR.”
Traditional strategies to increase vaccination – distributing travel immunization information, promoting YouTube videos about immunization and autism, using diverse media for information campaigns – failed.
So they joined with the community and family health department, where co-presenter Asli Ashkir, RN, MPH, is a senior nurse consultant in the Children & Youth with Special Health Needs program. They also hired Somali staff and began to improve their cultural knowledge and competence.
With Somalis, social life revolves around family ties, the community, and faith, explained Ms. Ashkir, a Somali woman herself. Somali culture is based on oral tradition, one that shares information among themselves and provides unsolicited advice to one another, and they persuade each other easily. But issues of health, life, and death are in the hands of Allah only, she said.
“There is a time you will die, whether you are vaccinated or not,” Ms. Ashkir explained. “That doesn’t mean we don’t practice preventive service or health promotion – we do – but at the back of our head, when our time is over, you’re going to go. These are the people we are working with.”
Two other potential obstacles involve Somali beliefs about sin and mental illness.
“We believe if someone is ill, their sins will be cleansed,” she said, explaining why Somalis with minor health problems don’t seek health care. “Parents with kids who have autism keep kids in their apartment until they are 8 years old because mental illness has a negative stigma.”
The Minnesota Department of Health conducted a study on the experience of having a child with autism in the Somali community and discovered four key themes. First, the parents greatly feared autism: Every Somali interviewed said they did not get the MMR because they wanted to avoid autism. Second, parents lacked information about normal child development, autism, and the diseases that vaccines prevent.
“We were expecting parents to identify developmental delays, but parents look not at the development but the growth, at the physical size of the child,” Ms. Ashkir said. And when they learned that the MMR prevented measles – the No. 3 killer of children in Somalia – parents often wanted the shot immediately.
The other two discoveries were that it was impossible to talk about immunization issues in isolation – they were too intricately entwined with discussions about autism – and that Somalis wanted to hear information from respected community sources.
These findings were applied in a pilot program that aimed to improve parents’ knowledge about child growth and development, autism, and vaccine-preventable diseases. Six mothers attended the training program, and tracking their contacts revealed that the information had traveled to 82 other family, friends, and neighbors within the first 3 months. All the women found the program “very helpful” with no negative responses.
The success of this program led to a more comprehensive approach that included training and outreach, engaging the community, disease mitigation and control, and creating and expanding partnerships with organizations such as the state American Academy of Pediatrics chapter, the Somali American Parent Association, the Minnesota Medical Association, and Parents in Community Action.
Training included all-Somali speakers with messages from spiritual leaders and parents of children with autism. Community outreach involved one-on-one conversations among Somalis at information tables in places such as malls, mosques, community centers, and libraries.
“Among this group, there are four parents who have children with autism,” Ms. Ashkir said. “Two of these parents are very, very vocal and talk about their children who have autism, and that they did not give them the MMR. They tell people ‘You have wrong information.’ ”
As of March 2016, the decline in MMR vaccination rates among Somalis had started to flatten. The annual drop of 5%-7% a year in MMR rates became 0.89% last year, which the Minnesota Department of Health finds encouraging.
“Our initial efforts, which included a typical repertoire of public health interventions, were ineffective, so we had to go back and dig deep to understand the core concerns,” Ms. Bahta said. “Our information had to address the core concerns of the community, not what we assumed to be the issue.”
Credibility came from the cultural relevancy of the message, and the fact that those providing the message were parents who had vaccinated their children, she said.
“Each cultural group needs unique approaches, and this is certainly true in this situation – to understand the unique perspective of the community and develop an effective approach required bringing in culturally competent staff and engaging the community,” Ms. Bahta said.
AT THE NATIONAL IMMUNIZATION CONFERENCE
Key clinical point:
Major finding: The decline in MMR vaccination among Somali children in Minnesota went from a 5%-7% annual drop to a 0.89% drop in 2015.
Data source: The findings are based on a comprehensive training and outreach program developed at the Minnesota Department of Health.
Disclosures: The initiative was funded by the Minnesota Department of Health. Ms. Ashkir and Ms. Bahta reported they had no conflicts to disclose.
HPV vaccination rates tripled with practice’s comprehensive intervention
ATLANTA – A multifaceted comprehensive intervention significantly improved human papillomavirus (HPV) vaccination rates in a Florida pediatric health care group practice.
Alix G. Casler, MD, chief of pediatrics at Orlando Health Physician Associates, described how her practice put into place practices to improve the overall HPV vaccination rate of their clients.
She described the critical components of a vaccination quality improvement project: set specific goals, know your practice’s actual rates, identify areas of weakness and/or opportunity, and then implement effective and sustainable processes for improvement. Their initial goal was to show any improvement at all in the first year and then to meet the highest national rates 2 years later.
“We started by agreeing we would become transparent to one another,” Dr. Casler explained. “This is called peer influence. What we didn’t want to be was the one who deviated from standard practice.”
As they got further along into their initiative, this transparency led physicians to ask others with better rates for help. “It’s not just a motivator in terms of not wanting to be the worse; it’s also a motivator in knowing how to get help,” said Dr. Casler, also at Florida State College of Medicine in Tallahassee and the University of Central Florida in Orlando.
Individual physicians’ rates were first shared privately with that physician, then shared with the department, and then published monthly and eventually only quarterly.
Then they developed the interventions to improve rates: verification and clean-up of their data, physician and staff education, physician incentives, previsit planning, electronic follow-up orders for the second and third doses, reminder calls, manufacturer tools, and clinical summaries.
The physician education program involved first making HPV vaccination a priority even when multiple competing priorities exist at each well visit.
“Our doctors felt, as all doctors feel, that we have 75 things to do and it’s not possible to do them all,” Dr. Casler said. “If we don’t have a fast and dirty way of doing something, it won’t get done.”
Part of prioritizing the vaccine was making physicians aware of how common HPV and HPV diseases were, which many did not realize. Then the training addressed providers’ discomfort about discussing the vaccine. They provided a script that included a clear recommendation for the HPV vaccine – sandwiched between the recommendations for the meningitis and Tdap vaccines – without adding unnecessary extra information unless the parent requested it.
During staff training, her practice found similar obstacles as with the doctors. “They had different competing priorities, they didn’t really know what HPV was, and they didn’t want to talk about sex,” Dr. Casler said.
Following training, they distributed tools such as posters and fact sheets to physicians and developed incentives: competition among each other, a quality bonus structure, and wine. “It’s amazing what will motivate people,” Dr. Casler said with a smile. “Again, this is the real world.”
Daily previsit planning meant documenting on patient lists the priorities for each patient, including the HPV vaccine as well as needs such as flu shots; other vaccines; screening for asthma, depression, and STIs; smoking assessment; diet and exercise counseling; and risk factor assessments.
“That is one of the most valuable interventions and got a tremendous amount of feedback from the staff,” Dr. Casler said. “Any practice can do this for free. I look at every metric that needs to be covered with that patient during that visit.”
Patients then are required to schedule their second and third doses on their way out. “If someone no-shows or doesn’t reschedule, my secretary knows what HPV is and what it does,” Dr. Casler said. “She will call the parents and leave a message, ‘Call me tomorrow to reschedule your appointment... so that your child doesn’t get cancer.”
In evaluating the program, Dr. Casler said the most popular interventions were the physician and staff education programs, scheduling subsequent doses in real time, and using manufacturer-supplied tools such as magnets and cling posters. Staff involvement turned out to be a critical resource in the overall intervention as well.
As a result of the program begun in August 2013, the practice’s rates of girls and boys receiving one dose of the HPV vaccine increased to 65% and 57%, respectively, by the end of 2014. Further, 43% of girls and 30% of boys received all three doses. By June 2016, 75% of girls and 72% of boys were receiving their first dose of HPV vaccine, and 55% of girls and 47% of boys were receiving all three doses.
Dr. Casler reported previous consulting and speaking for Merck and Sanofi Pasteur. No external funding was reported.
ATLANTA – A multifaceted comprehensive intervention significantly improved human papillomavirus (HPV) vaccination rates in a Florida pediatric health care group practice.
Alix G. Casler, MD, chief of pediatrics at Orlando Health Physician Associates, described how her practice put into place practices to improve the overall HPV vaccination rate of their clients.
She described the critical components of a vaccination quality improvement project: set specific goals, know your practice’s actual rates, identify areas of weakness and/or opportunity, and then implement effective and sustainable processes for improvement. Their initial goal was to show any improvement at all in the first year and then to meet the highest national rates 2 years later.
“We started by agreeing we would become transparent to one another,” Dr. Casler explained. “This is called peer influence. What we didn’t want to be was the one who deviated from standard practice.”
As they got further along into their initiative, this transparency led physicians to ask others with better rates for help. “It’s not just a motivator in terms of not wanting to be the worse; it’s also a motivator in knowing how to get help,” said Dr. Casler, also at Florida State College of Medicine in Tallahassee and the University of Central Florida in Orlando.
Individual physicians’ rates were first shared privately with that physician, then shared with the department, and then published monthly and eventually only quarterly.
Then they developed the interventions to improve rates: verification and clean-up of their data, physician and staff education, physician incentives, previsit planning, electronic follow-up orders for the second and third doses, reminder calls, manufacturer tools, and clinical summaries.
The physician education program involved first making HPV vaccination a priority even when multiple competing priorities exist at each well visit.
“Our doctors felt, as all doctors feel, that we have 75 things to do and it’s not possible to do them all,” Dr. Casler said. “If we don’t have a fast and dirty way of doing something, it won’t get done.”
Part of prioritizing the vaccine was making physicians aware of how common HPV and HPV diseases were, which many did not realize. Then the training addressed providers’ discomfort about discussing the vaccine. They provided a script that included a clear recommendation for the HPV vaccine – sandwiched between the recommendations for the meningitis and Tdap vaccines – without adding unnecessary extra information unless the parent requested it.
During staff training, her practice found similar obstacles as with the doctors. “They had different competing priorities, they didn’t really know what HPV was, and they didn’t want to talk about sex,” Dr. Casler said.
Following training, they distributed tools such as posters and fact sheets to physicians and developed incentives: competition among each other, a quality bonus structure, and wine. “It’s amazing what will motivate people,” Dr. Casler said with a smile. “Again, this is the real world.”
Daily previsit planning meant documenting on patient lists the priorities for each patient, including the HPV vaccine as well as needs such as flu shots; other vaccines; screening for asthma, depression, and STIs; smoking assessment; diet and exercise counseling; and risk factor assessments.
“That is one of the most valuable interventions and got a tremendous amount of feedback from the staff,” Dr. Casler said. “Any practice can do this for free. I look at every metric that needs to be covered with that patient during that visit.”
Patients then are required to schedule their second and third doses on their way out. “If someone no-shows or doesn’t reschedule, my secretary knows what HPV is and what it does,” Dr. Casler said. “She will call the parents and leave a message, ‘Call me tomorrow to reschedule your appointment... so that your child doesn’t get cancer.”
In evaluating the program, Dr. Casler said the most popular interventions were the physician and staff education programs, scheduling subsequent doses in real time, and using manufacturer-supplied tools such as magnets and cling posters. Staff involvement turned out to be a critical resource in the overall intervention as well.
As a result of the program begun in August 2013, the practice’s rates of girls and boys receiving one dose of the HPV vaccine increased to 65% and 57%, respectively, by the end of 2014. Further, 43% of girls and 30% of boys received all three doses. By June 2016, 75% of girls and 72% of boys were receiving their first dose of HPV vaccine, and 55% of girls and 47% of boys were receiving all three doses.
Dr. Casler reported previous consulting and speaking for Merck and Sanofi Pasteur. No external funding was reported.
ATLANTA – A multifaceted comprehensive intervention significantly improved human papillomavirus (HPV) vaccination rates in a Florida pediatric health care group practice.
Alix G. Casler, MD, chief of pediatrics at Orlando Health Physician Associates, described how her practice put into place practices to improve the overall HPV vaccination rate of their clients.
She described the critical components of a vaccination quality improvement project: set specific goals, know your practice’s actual rates, identify areas of weakness and/or opportunity, and then implement effective and sustainable processes for improvement. Their initial goal was to show any improvement at all in the first year and then to meet the highest national rates 2 years later.
“We started by agreeing we would become transparent to one another,” Dr. Casler explained. “This is called peer influence. What we didn’t want to be was the one who deviated from standard practice.”
As they got further along into their initiative, this transparency led physicians to ask others with better rates for help. “It’s not just a motivator in terms of not wanting to be the worse; it’s also a motivator in knowing how to get help,” said Dr. Casler, also at Florida State College of Medicine in Tallahassee and the University of Central Florida in Orlando.
Individual physicians’ rates were first shared privately with that physician, then shared with the department, and then published monthly and eventually only quarterly.
Then they developed the interventions to improve rates: verification and clean-up of their data, physician and staff education, physician incentives, previsit planning, electronic follow-up orders for the second and third doses, reminder calls, manufacturer tools, and clinical summaries.
The physician education program involved first making HPV vaccination a priority even when multiple competing priorities exist at each well visit.
“Our doctors felt, as all doctors feel, that we have 75 things to do and it’s not possible to do them all,” Dr. Casler said. “If we don’t have a fast and dirty way of doing something, it won’t get done.”
Part of prioritizing the vaccine was making physicians aware of how common HPV and HPV diseases were, which many did not realize. Then the training addressed providers’ discomfort about discussing the vaccine. They provided a script that included a clear recommendation for the HPV vaccine – sandwiched between the recommendations for the meningitis and Tdap vaccines – without adding unnecessary extra information unless the parent requested it.
During staff training, her practice found similar obstacles as with the doctors. “They had different competing priorities, they didn’t really know what HPV was, and they didn’t want to talk about sex,” Dr. Casler said.
Following training, they distributed tools such as posters and fact sheets to physicians and developed incentives: competition among each other, a quality bonus structure, and wine. “It’s amazing what will motivate people,” Dr. Casler said with a smile. “Again, this is the real world.”
Daily previsit planning meant documenting on patient lists the priorities for each patient, including the HPV vaccine as well as needs such as flu shots; other vaccines; screening for asthma, depression, and STIs; smoking assessment; diet and exercise counseling; and risk factor assessments.
“That is one of the most valuable interventions and got a tremendous amount of feedback from the staff,” Dr. Casler said. “Any practice can do this for free. I look at every metric that needs to be covered with that patient during that visit.”
Patients then are required to schedule their second and third doses on their way out. “If someone no-shows or doesn’t reschedule, my secretary knows what HPV is and what it does,” Dr. Casler said. “She will call the parents and leave a message, ‘Call me tomorrow to reschedule your appointment... so that your child doesn’t get cancer.”
In evaluating the program, Dr. Casler said the most popular interventions were the physician and staff education programs, scheduling subsequent doses in real time, and using manufacturer-supplied tools such as magnets and cling posters. Staff involvement turned out to be a critical resource in the overall intervention as well.
As a result of the program begun in August 2013, the practice’s rates of girls and boys receiving one dose of the HPV vaccine increased to 65% and 57%, respectively, by the end of 2014. Further, 43% of girls and 30% of boys received all three doses. By June 2016, 75% of girls and 72% of boys were receiving their first dose of HPV vaccine, and 55% of girls and 47% of boys were receiving all three doses.
Dr. Casler reported previous consulting and speaking for Merck and Sanofi Pasteur. No external funding was reported.
AT THE NATIONAL IMMUNIZATION CONFERENCE
Key clinical point: A multifaceted comprehensive intervention significantly improved HPV vaccination rates in a pediatric health care group practice.
Major finding: Girls and boys receiving any HPV vaccine dose increased from 23% and 12% in 2013 to 75% and 72% in June 2016, respectively. Rates of three doses increased from 14% of girls and 3% of boys in 2013 to 55% of girls and 47% of boys in June 2016.
Data source: The findings are based on internal assessment of an intervention at a large multispecialty health care group with 22 pediatricians and with 23,000 patients at least 11 years old.
Disclosures: Dr. Casler reported previous consulting and speaking for Merck and Sanofi Pasteur. No external funding was reported.
GOLD: Base COPD treatment on symptoms, exacerbation risk
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
New guidelines provide standardized hypoglycemia values for clinical evaluation
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
New PAD guidelines expected to boost awareness and treatment
NEW ORLEANS – The latest revision of U.S. guidelines for diagnosing and managing lower-extremity peripheral artery disease is seen by several experts as primarily a renewed call to action by American physicians to more diligently identify at-risk people in their practices, diagnose the disease with ankle-brachial index measurement, and appropriately treat patients who have the disease.
The new lower-extremity peripheral artery disease (PAD) guidelines “give us a platform to get something done,” said Heather L. Gornik, MD, vice chair of the guidelines panel and medical director of the noninvasive vascular lab at the Cleveland Clinic. Improved PAD identification and care “starts with the fundamentals of recognizing who is at risk for PAD and then doing some clinical investigation to find it, because it’s there. You just need to ask patients if they have leg symptoms, have them take off their socks and examine their feet,” Dr. Gornik said in an interview following a program devoted to the revised guidelines at the American Heart Association scientific sessions.
The new guidelines are “a clarion call to action,” commented Alan T. Hirsch, MD, professor of medicine, epidemiology, and community health and director of the vascular medicine program at the University of Minnesota in Minneapolis. PAD “is the single most morbid and fatal of all cardiovascular diseases, so why in 2016 are we challenged to have every cardiologist trained in basic PAD competency?” asked Dr. Hirsch, who chaired the 2005 guidelines panel.
The AHA wants to “elevate awareness of PAD among the public and health professionals, and we want to better understand how we can be a catalyst for change,” said Terri Wiggins, the organization’s vice president for vascular health programs. AHA publications now stress that clinicians need to have patients “take off their socks and look at the patient’s feet,” she said.
The problem with the way many U.S. physicians handle PAD goes beyond a failure to properly screen and diagnose the disease. Analysis of data collected by the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey during 2006-2013 showed that, among an average of 3.9 million patients seen each year with PAD, just 38% received treatment with an antiplatelet drug, 35% received a statin, and among those patients who smoked, 36% received counseling for smoking cessation, Jeffrey S. Berger, MD, reported in a separate talk at the meeting. These rates were roughly constant throughout the 8-year period he examined.
“These data are eye-opening. They highlight a clear opportunity to improve the care of patients with PAD with guideline-directed therapy. PAD is problematic because only 10%-15% of patients have typical symptoms, so many physicians have a false perception that these patients are not at high risk,” Dr. Berger said in an interview. “What the AHA is doing is great” for raising awareness, he added.
The revised 2016 PAD guidelines made several changes, compared with what had been on the books from the 2005 guidelines and 2011 update, including classifying vorapaxar (Zontivity) treatment a class IIb recommendation with “uncertain” incremental benefit when used as an add-on agent on top of standard antiplatelet therapy, endorsement of annual influenza vaccination as something every PAD patient should receive and a class I recommendation, and acknowledgment that selected patients with critical limb ischemia are candidates for an “endovascular first” approach to revascularization,
Perhaps just as important was inclusion for the first time in the new guidelines of three advocacy priorities for initiatives by various professional societies with an interest in PAD: easy availability of ankle-brachial index measurement as the initial test to establish a diagnosis of PAD in patients with physical examination findings suggestive of the disease; access to supervised exercise programs for patients diagnosed with PAD; and incorporation of patient-centered outcomes into the regulatory approval process of new medical therapies and revascularization technologies for treating PAD.
The new guidelines “ form the basis for the AHA establishing a Get With The Guidelines program for PAD so that clinicians can be held accountable for delivering these treatments,” said Naomi M. Hamburg, MD, chief of vascular biology at Boston University and a member of the guidelines panel.
Dr. Gornik has an ownership interest in Summit Doppler Systems and Zin Medical and has received research support from AstraZeneca and Theravasc. Dr. Hamburg has been a consultant to Acceleron and has received research support from Everest Genomics, Hershey’s, Unex, and Welch’s. Dr. Hirsch, Ms. Wiggins, and Dr. Berger had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – The latest revision of U.S. guidelines for diagnosing and managing lower-extremity peripheral artery disease is seen by several experts as primarily a renewed call to action by American physicians to more diligently identify at-risk people in their practices, diagnose the disease with ankle-brachial index measurement, and appropriately treat patients who have the disease.
The new lower-extremity peripheral artery disease (PAD) guidelines “give us a platform to get something done,” said Heather L. Gornik, MD, vice chair of the guidelines panel and medical director of the noninvasive vascular lab at the Cleveland Clinic. Improved PAD identification and care “starts with the fundamentals of recognizing who is at risk for PAD and then doing some clinical investigation to find it, because it’s there. You just need to ask patients if they have leg symptoms, have them take off their socks and examine their feet,” Dr. Gornik said in an interview following a program devoted to the revised guidelines at the American Heart Association scientific sessions.
The new guidelines are “a clarion call to action,” commented Alan T. Hirsch, MD, professor of medicine, epidemiology, and community health and director of the vascular medicine program at the University of Minnesota in Minneapolis. PAD “is the single most morbid and fatal of all cardiovascular diseases, so why in 2016 are we challenged to have every cardiologist trained in basic PAD competency?” asked Dr. Hirsch, who chaired the 2005 guidelines panel.
The AHA wants to “elevate awareness of PAD among the public and health professionals, and we want to better understand how we can be a catalyst for change,” said Terri Wiggins, the organization’s vice president for vascular health programs. AHA publications now stress that clinicians need to have patients “take off their socks and look at the patient’s feet,” she said.
The problem with the way many U.S. physicians handle PAD goes beyond a failure to properly screen and diagnose the disease. Analysis of data collected by the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey during 2006-2013 showed that, among an average of 3.9 million patients seen each year with PAD, just 38% received treatment with an antiplatelet drug, 35% received a statin, and among those patients who smoked, 36% received counseling for smoking cessation, Jeffrey S. Berger, MD, reported in a separate talk at the meeting. These rates were roughly constant throughout the 8-year period he examined.
“These data are eye-opening. They highlight a clear opportunity to improve the care of patients with PAD with guideline-directed therapy. PAD is problematic because only 10%-15% of patients have typical symptoms, so many physicians have a false perception that these patients are not at high risk,” Dr. Berger said in an interview. “What the AHA is doing is great” for raising awareness, he added.
The revised 2016 PAD guidelines made several changes, compared with what had been on the books from the 2005 guidelines and 2011 update, including classifying vorapaxar (Zontivity) treatment a class IIb recommendation with “uncertain” incremental benefit when used as an add-on agent on top of standard antiplatelet therapy, endorsement of annual influenza vaccination as something every PAD patient should receive and a class I recommendation, and acknowledgment that selected patients with critical limb ischemia are candidates for an “endovascular first” approach to revascularization,
Perhaps just as important was inclusion for the first time in the new guidelines of three advocacy priorities for initiatives by various professional societies with an interest in PAD: easy availability of ankle-brachial index measurement as the initial test to establish a diagnosis of PAD in patients with physical examination findings suggestive of the disease; access to supervised exercise programs for patients diagnosed with PAD; and incorporation of patient-centered outcomes into the regulatory approval process of new medical therapies and revascularization technologies for treating PAD.
The new guidelines “ form the basis for the AHA establishing a Get With The Guidelines program for PAD so that clinicians can be held accountable for delivering these treatments,” said Naomi M. Hamburg, MD, chief of vascular biology at Boston University and a member of the guidelines panel.
Dr. Gornik has an ownership interest in Summit Doppler Systems and Zin Medical and has received research support from AstraZeneca and Theravasc. Dr. Hamburg has been a consultant to Acceleron and has received research support from Everest Genomics, Hershey’s, Unex, and Welch’s. Dr. Hirsch, Ms. Wiggins, and Dr. Berger had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – The latest revision of U.S. guidelines for diagnosing and managing lower-extremity peripheral artery disease is seen by several experts as primarily a renewed call to action by American physicians to more diligently identify at-risk people in their practices, diagnose the disease with ankle-brachial index measurement, and appropriately treat patients who have the disease.
The new lower-extremity peripheral artery disease (PAD) guidelines “give us a platform to get something done,” said Heather L. Gornik, MD, vice chair of the guidelines panel and medical director of the noninvasive vascular lab at the Cleveland Clinic. Improved PAD identification and care “starts with the fundamentals of recognizing who is at risk for PAD and then doing some clinical investigation to find it, because it’s there. You just need to ask patients if they have leg symptoms, have them take off their socks and examine their feet,” Dr. Gornik said in an interview following a program devoted to the revised guidelines at the American Heart Association scientific sessions.
The new guidelines are “a clarion call to action,” commented Alan T. Hirsch, MD, professor of medicine, epidemiology, and community health and director of the vascular medicine program at the University of Minnesota in Minneapolis. PAD “is the single most morbid and fatal of all cardiovascular diseases, so why in 2016 are we challenged to have every cardiologist trained in basic PAD competency?” asked Dr. Hirsch, who chaired the 2005 guidelines panel.
The AHA wants to “elevate awareness of PAD among the public and health professionals, and we want to better understand how we can be a catalyst for change,” said Terri Wiggins, the organization’s vice president for vascular health programs. AHA publications now stress that clinicians need to have patients “take off their socks and look at the patient’s feet,” she said.
The problem with the way many U.S. physicians handle PAD goes beyond a failure to properly screen and diagnose the disease. Analysis of data collected by the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey during 2006-2013 showed that, among an average of 3.9 million patients seen each year with PAD, just 38% received treatment with an antiplatelet drug, 35% received a statin, and among those patients who smoked, 36% received counseling for smoking cessation, Jeffrey S. Berger, MD, reported in a separate talk at the meeting. These rates were roughly constant throughout the 8-year period he examined.
“These data are eye-opening. They highlight a clear opportunity to improve the care of patients with PAD with guideline-directed therapy. PAD is problematic because only 10%-15% of patients have typical symptoms, so many physicians have a false perception that these patients are not at high risk,” Dr. Berger said in an interview. “What the AHA is doing is great” for raising awareness, he added.
The revised 2016 PAD guidelines made several changes, compared with what had been on the books from the 2005 guidelines and 2011 update, including classifying vorapaxar (Zontivity) treatment a class IIb recommendation with “uncertain” incremental benefit when used as an add-on agent on top of standard antiplatelet therapy, endorsement of annual influenza vaccination as something every PAD patient should receive and a class I recommendation, and acknowledgment that selected patients with critical limb ischemia are candidates for an “endovascular first” approach to revascularization,
Perhaps just as important was inclusion for the first time in the new guidelines of three advocacy priorities for initiatives by various professional societies with an interest in PAD: easy availability of ankle-brachial index measurement as the initial test to establish a diagnosis of PAD in patients with physical examination findings suggestive of the disease; access to supervised exercise programs for patients diagnosed with PAD; and incorporation of patient-centered outcomes into the regulatory approval process of new medical therapies and revascularization technologies for treating PAD.
The new guidelines “ form the basis for the AHA establishing a Get With The Guidelines program for PAD so that clinicians can be held accountable for delivering these treatments,” said Naomi M. Hamburg, MD, chief of vascular biology at Boston University and a member of the guidelines panel.
Dr. Gornik has an ownership interest in Summit Doppler Systems and Zin Medical and has received research support from AstraZeneca and Theravasc. Dr. Hamburg has been a consultant to Acceleron and has received research support from Everest Genomics, Hershey’s, Unex, and Welch’s. Dr. Hirsch, Ms. Wiggins, and Dr. Berger had no disclosures.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Age correlated with occult cancer in VTE patients
A newly devised risk score may help identify which patients with acute venous thromboembolism (VTE) are most likely to have occult cancer and where they are likely to have it, according to a report published online in CHEST.
Although VTE is known to occur before an occult cancer becomes symptomatic in a minority of patients, clinicians don’t agree on which patients with VTE should be screened for occult cancer or on how extensive that screening should be. Some favor a basic screening with only a clinical history, physical exam, simple lab tests, and a chest X-ray, while others advocate a more thorough work-up to improve the patient’s chance for a cure. “The potential benefits and harms of such screening are controversial, partly because there is little evidence” concerning which patients are at highest risk and which cancer types/sites should be assessed, said Luis Jara-Palomares, MD, PhD, of the medical surgical unit of respiratory diseases, Virgen del Rocio Hospital, Seville, Spain, and his associates.
Dr. Jara-Palomares and his associates devised a prognostic score by assigning points to the variables they found to be most significantly associated with occult cancer. For example, male sex was accorded 1 point, chronic lung disease or a high platelet count was accorded 1 point, age over 70 years or anemia was accorded 2 points, and postoperative or prior VTE was accorded negative 2 points. The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
The mean age of these study participants was 63 years, and approximately half were women. A total of 444 (7.6%) were diagnosed as having cancer at 1-24 months after presenting with VTE. Most of these cancers were discovered within 6 months of the VTE.
Patients who had occult cancer were significantly more likely than those who did not to be male and older than 70 years of age; to have chronic lung disease, an elevated platelet count, and/or anemia; and to have a history of recent surgery and/or prior VTE.
The percentage of VTE patients who had occult cancer increased with advancing age, from 2%-3% in the youngest age group (younger than 50 years) to 8%-12% in the oldest age group (older than 70 years). Among men with occult cancer, the most frequently affected sites were the lung (26%), prostate (17%), and colon/rectum (10%). Among women, the most frequent types of cancer were colorectal (19%), breast (12%), uterine (9.1%), hematologic (8.6%), pancreatic (7.6%), and stomach (6.6%).
Overall, more than half of the men who had occult cancer had cancer affecting the lung, prostate, or colon/rectum, while two-thirds of the women who had occult cancer had cancer affecting the colon/rectum, breast, or abdomen. “This is important because [cancer] screening is not necessary in all VTE patients, but any information suggesting [which] patients are at increased risk and [which] sites are more common may be of help to decide the most appropriate work-up for each patient,” the investigators noted.
To determine which patients are most likely to have occult cancer and which cancer types are most likely to develop, the investigators analyzed data from RIETE (the Computerized Registry of Patients With Venous Thromboembolism), an international registry of more than 50,000 consecutive patients with confirmed acute VTE. They focused on 5,863 patients who presented with pulmonary embolism (34%), deep vein thrombosis (48%), or both disorders (18%) and were followed for at least 2 years for the development of cancer.
The accuracy of this risk scoring system must be tested further in a large validation cohort before it can be widely adopted. If it proves to be accurate, then patients with low total risk scores could forgo the expense, discomfort, and psychological stress of extensive cancer screening, Dr. Jara-Palomares and his associates said.
Men whose total score is 3 points or more could benefit from a rectal exam and fecal occult blood test to rule out colorectal cancer, a rectal exam and prostate-specific antigen test to rule out prostate cancer, and a chest CT to rule out lung cancer. Women whose total score is 3 points or higher could benefit from a fecal occult blood test to rule out colorectal cancer, a mammogram to rule out breast cancer, and abdominopelvic CT to rule out uterine, pancreatic, and stomach cancer, they noted.
The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
A newly devised risk score may help identify which patients with acute venous thromboembolism (VTE) are most likely to have occult cancer and where they are likely to have it, according to a report published online in CHEST.
Although VTE is known to occur before an occult cancer becomes symptomatic in a minority of patients, clinicians don’t agree on which patients with VTE should be screened for occult cancer or on how extensive that screening should be. Some favor a basic screening with only a clinical history, physical exam, simple lab tests, and a chest X-ray, while others advocate a more thorough work-up to improve the patient’s chance for a cure. “The potential benefits and harms of such screening are controversial, partly because there is little evidence” concerning which patients are at highest risk and which cancer types/sites should be assessed, said Luis Jara-Palomares, MD, PhD, of the medical surgical unit of respiratory diseases, Virgen del Rocio Hospital, Seville, Spain, and his associates.
Dr. Jara-Palomares and his associates devised a prognostic score by assigning points to the variables they found to be most significantly associated with occult cancer. For example, male sex was accorded 1 point, chronic lung disease or a high platelet count was accorded 1 point, age over 70 years or anemia was accorded 2 points, and postoperative or prior VTE was accorded negative 2 points. The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
The mean age of these study participants was 63 years, and approximately half were women. A total of 444 (7.6%) were diagnosed as having cancer at 1-24 months after presenting with VTE. Most of these cancers were discovered within 6 months of the VTE.
Patients who had occult cancer were significantly more likely than those who did not to be male and older than 70 years of age; to have chronic lung disease, an elevated platelet count, and/or anemia; and to have a history of recent surgery and/or prior VTE.
The percentage of VTE patients who had occult cancer increased with advancing age, from 2%-3% in the youngest age group (younger than 50 years) to 8%-12% in the oldest age group (older than 70 years). Among men with occult cancer, the most frequently affected sites were the lung (26%), prostate (17%), and colon/rectum (10%). Among women, the most frequent types of cancer were colorectal (19%), breast (12%), uterine (9.1%), hematologic (8.6%), pancreatic (7.6%), and stomach (6.6%).
Overall, more than half of the men who had occult cancer had cancer affecting the lung, prostate, or colon/rectum, while two-thirds of the women who had occult cancer had cancer affecting the colon/rectum, breast, or abdomen. “This is important because [cancer] screening is not necessary in all VTE patients, but any information suggesting [which] patients are at increased risk and [which] sites are more common may be of help to decide the most appropriate work-up for each patient,” the investigators noted.
To determine which patients are most likely to have occult cancer and which cancer types are most likely to develop, the investigators analyzed data from RIETE (the Computerized Registry of Patients With Venous Thromboembolism), an international registry of more than 50,000 consecutive patients with confirmed acute VTE. They focused on 5,863 patients who presented with pulmonary embolism (34%), deep vein thrombosis (48%), or both disorders (18%) and were followed for at least 2 years for the development of cancer.
The accuracy of this risk scoring system must be tested further in a large validation cohort before it can be widely adopted. If it proves to be accurate, then patients with low total risk scores could forgo the expense, discomfort, and psychological stress of extensive cancer screening, Dr. Jara-Palomares and his associates said.
Men whose total score is 3 points or more could benefit from a rectal exam and fecal occult blood test to rule out colorectal cancer, a rectal exam and prostate-specific antigen test to rule out prostate cancer, and a chest CT to rule out lung cancer. Women whose total score is 3 points or higher could benefit from a fecal occult blood test to rule out colorectal cancer, a mammogram to rule out breast cancer, and abdominopelvic CT to rule out uterine, pancreatic, and stomach cancer, they noted.
The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
A newly devised risk score may help identify which patients with acute venous thromboembolism (VTE) are most likely to have occult cancer and where they are likely to have it, according to a report published online in CHEST.
Although VTE is known to occur before an occult cancer becomes symptomatic in a minority of patients, clinicians don’t agree on which patients with VTE should be screened for occult cancer or on how extensive that screening should be. Some favor a basic screening with only a clinical history, physical exam, simple lab tests, and a chest X-ray, while others advocate a more thorough work-up to improve the patient’s chance for a cure. “The potential benefits and harms of such screening are controversial, partly because there is little evidence” concerning which patients are at highest risk and which cancer types/sites should be assessed, said Luis Jara-Palomares, MD, PhD, of the medical surgical unit of respiratory diseases, Virgen del Rocio Hospital, Seville, Spain, and his associates.
Dr. Jara-Palomares and his associates devised a prognostic score by assigning points to the variables they found to be most significantly associated with occult cancer. For example, male sex was accorded 1 point, chronic lung disease or a high platelet count was accorded 1 point, age over 70 years or anemia was accorded 2 points, and postoperative or prior VTE was accorded negative 2 points. The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
The mean age of these study participants was 63 years, and approximately half were women. A total of 444 (7.6%) were diagnosed as having cancer at 1-24 months after presenting with VTE. Most of these cancers were discovered within 6 months of the VTE.
Patients who had occult cancer were significantly more likely than those who did not to be male and older than 70 years of age; to have chronic lung disease, an elevated platelet count, and/or anemia; and to have a history of recent surgery and/or prior VTE.
The percentage of VTE patients who had occult cancer increased with advancing age, from 2%-3% in the youngest age group (younger than 50 years) to 8%-12% in the oldest age group (older than 70 years). Among men with occult cancer, the most frequently affected sites were the lung (26%), prostate (17%), and colon/rectum (10%). Among women, the most frequent types of cancer were colorectal (19%), breast (12%), uterine (9.1%), hematologic (8.6%), pancreatic (7.6%), and stomach (6.6%).
Overall, more than half of the men who had occult cancer had cancer affecting the lung, prostate, or colon/rectum, while two-thirds of the women who had occult cancer had cancer affecting the colon/rectum, breast, or abdomen. “This is important because [cancer] screening is not necessary in all VTE patients, but any information suggesting [which] patients are at increased risk and [which] sites are more common may be of help to decide the most appropriate work-up for each patient,” the investigators noted.
To determine which patients are most likely to have occult cancer and which cancer types are most likely to develop, the investigators analyzed data from RIETE (the Computerized Registry of Patients With Venous Thromboembolism), an international registry of more than 50,000 consecutive patients with confirmed acute VTE. They focused on 5,863 patients who presented with pulmonary embolism (34%), deep vein thrombosis (48%), or both disorders (18%) and were followed for at least 2 years for the development of cancer.
The accuracy of this risk scoring system must be tested further in a large validation cohort before it can be widely adopted. If it proves to be accurate, then patients with low total risk scores could forgo the expense, discomfort, and psychological stress of extensive cancer screening, Dr. Jara-Palomares and his associates said.
Men whose total score is 3 points or more could benefit from a rectal exam and fecal occult blood test to rule out colorectal cancer, a rectal exam and prostate-specific antigen test to rule out prostate cancer, and a chest CT to rule out lung cancer. Women whose total score is 3 points or higher could benefit from a fecal occult blood test to rule out colorectal cancer, a mammogram to rule out breast cancer, and abdominopelvic CT to rule out uterine, pancreatic, and stomach cancer, they noted.
The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
FROM CHEST
Key clinical point:
Major finding: The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
Data source: A retrospective cohort study involving 5,863 consecutive VTE patients in an international observational registry.
Disclosures: The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.