User login
FDA approves ibrutinib for refractory MZL
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
[email protected]
On Twitter @HematologyNews1
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
[email protected]
On Twitter @HematologyNews1
The Food and Drug Administration has approved ibrutinib for the treatment of patients with relapsed or refractory marginal zone lymphoma (MZL), the drug’s manufacturers report.
The approval marks the fifth indication for ibrutinib (Imbruvica) in just over 4 years, and ibrutinib is the first agent specifically approved for relapsed/refractory MZL, according to press releases issued by Janssen Biotech and Pharmacyclics, the two manufacturers that jointly developed and marketed the Bruton tyrosine kinase inhibitor.
After receiving various fast-track, breakthrough therapy, priority review, and accelerated approval designations from the FDA, ibrutinib was previously approved to treat mantle cell lymphoma; refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); CLL/SLL with 17p deletion; and Waldenstrom’s macroglobulinemia, another rare form of non-Hodgkin lymphoma. The MCL and MZL approvals are based on overall response rates, and full approval is likely to require additional confirmatory data.
The new indication is based on data from a phase II, open-label, single-arm manufacturer-sponsored study that showed a 46% overall response rate (95% confidence interval, 33.4-59.1) in a cohort of 63 MZL patients who had failed one or more prior therapies. Of these, 3.2% had a complete response and 42.9% had a partial response. The median duration of response was not reached (NR) (range, 16.7 months–NR), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (2.3-16.4 months).
All three MZL subtypes were represented in the cohort, and ibrutinib appeared to be effective across subtypes. Thrombocytopenia, fatigue, anemia, diarrhea, bruising, and musculoskeletal pain were commonly reported adverse events.
[email protected]
On Twitter @HematologyNews1
Left ventricle dose predicts heart events after BC radiation
The risk of acute coronary events following radiotherapy for breast cancer is better predicted by the volume of the left ventricle that received 5 Gy than by the mean dose of radiation to the heart, according to a Dutch investigation of 910 women who underwent radiation treatment following breast-conserving surgery.
The finding follows up a 2013 report that found that the risk of acute coronary events (ACE) after breast cancer (BC) radiation could be predicted by the mean radiation heart dose (MHD), the presence of cardiac risk factors, and age (N Engl J Med. 2013 Mar 14;368[11]:987-98. doi: 0.1056/NEJMoa1209825).
The new study validated those findings, but also found that risk prediction was better when mean heart dose (MHD) was replaced by the volume of the left ventricle receiving 5 Gy (LV-V5); the substitution improved the c-statistic to 0.80 (95% confidence interval, 0.72-0.88). Using a weighted ACE risk score based on baseline diabetes, hypertension, and ischemic event history – instead of the risk factor yes-or-no approach from 2013 – further improved predictive power, with a c-statistic of 0.83 (95% CI, 0.75-0.91). Anything over a c-statistic of 0.8 is considered strong; 0.5 is chance, 1.0 is perfect prediction.
For instance, a 70-year-old woman with an LV-V5 of 50% and no cardiac risk factors had an excess ACE risk in the new system of 2.52% within 9 years of radiotherapy (RT). If she had a history of ischemic heart disease, the excess risk increased to 8.42%, the investigators said (J Clin Oncol. 2017 Jan 17. doi: 10.1200/JCO.2016.69.8480).
“Model performance was significantly improved by replacing MHD with LV-V5 and using the weighted ACE risk score.” However, “because we were not able to externally validate the LV-V5 model, this model” requires validation “before it can be used in routine clinical practice,” said investigators, led by Veerle van den Bogaard, MD, of the University of Groningen, the Netherlands.
The women were a median of 59 years old, and they were followed for a median of 7.6 years, with a range of 0.1-10.1 years. Radiation dose information was derived from CT planning scans. The median MHD was 2.37 Gy.
Thirty patients (3.3%) had an ACE, defined as myocardial infarction, coronary revascularization, or death due to ischemic heart disease; 17 had events in the first 5 years. The 5- and 9-year cumulative ACE incidences were 1.9% and 3.9%. Ten of the 30 women died from their cardiac complication.
The model predicted a cumulative ACE incidence at 9 years of 3.5%, which was in line with the observed rate of 3.9%. The excess cumulative risk related to RT was 1.13%. Overall, about 10 patients had an ACE that could be attributed to RT. The cumulative incidence of ACE increased by 16.5% per Gy (95% CI, 0.6-35.0; P = .042). The findings were consistent with the 2013 study.
ACE incidence was not significantly associated with the maximum dose of radiation to the heart.
LV-V5 was the most important prognostic dose-volume parameter associated with the cumulative incidence of ACE, with a hazard ratio of 1.016 (95% CI, 1.002-1.030; P = .016). “Because of this strong association, we chose to include LV-V5 in the model,” the investigators said.
There was no external funding. The lead investigator had no disclosures, but two authors reported institutional research funding from Philips, Roche, and other companies. One was an advisor and speaker for IBA.
The limitations of this study are substantial. As the authors note, their work was based on only a small number of events, and their length of follow-up prevented them from being able to examine risk in the period of 10 years and beyond.
Also, the likelihood is high that the risk of radiation-induced heart disease can be reduced below that seen in the study. Cardiac exposure in many recent studies has been lower. Techniques such as deep inspiration breath holding or treatment in the prone position often can further decrease this exposure. Several randomized trials comparing partial-breast with whole-breast irradiation have found equal local control rates with both approaches, strongly suggesting that judicious individualization of field borders and cardiac blocks are unlikely to compromise outcome for selected patients treated with partial mastectomy.
Nonetheless, at some point, compromising coverage of the breast, chest wall, or nodal target volumes because an arbitrary threshold of a cardiac dose-volume parameter has been exceeded may be dangerous. Computer programs can likely be created to perform calculations of an individual’s excess risk of cardiac events or death in relation to dose-volume and clinical parameters, although I suspect it will be some years before they provide sufficiently validated, narrow estimates of risk to be clinically useful. Perhaps by then we will also have better ways to explain such sobering decisions to patients.
Abram Recht, MD , is a professor of radiation oncology at Harvard Medical School, Boston. He is also an advisor for CareCore and US Oncology, and receives institutional research funding form Genomic Health. He made his comments in an editorial (J Clin Oncol. 2017 Jan 17. doi: 0.1200/JCO.2016.71.4113 ).
The limitations of this study are substantial. As the authors note, their work was based on only a small number of events, and their length of follow-up prevented them from being able to examine risk in the period of 10 years and beyond.
Also, the likelihood is high that the risk of radiation-induced heart disease can be reduced below that seen in the study. Cardiac exposure in many recent studies has been lower. Techniques such as deep inspiration breath holding or treatment in the prone position often can further decrease this exposure. Several randomized trials comparing partial-breast with whole-breast irradiation have found equal local control rates with both approaches, strongly suggesting that judicious individualization of field borders and cardiac blocks are unlikely to compromise outcome for selected patients treated with partial mastectomy.
Nonetheless, at some point, compromising coverage of the breast, chest wall, or nodal target volumes because an arbitrary threshold of a cardiac dose-volume parameter has been exceeded may be dangerous. Computer programs can likely be created to perform calculations of an individual’s excess risk of cardiac events or death in relation to dose-volume and clinical parameters, although I suspect it will be some years before they provide sufficiently validated, narrow estimates of risk to be clinically useful. Perhaps by then we will also have better ways to explain such sobering decisions to patients.
Abram Recht, MD , is a professor of radiation oncology at Harvard Medical School, Boston. He is also an advisor for CareCore and US Oncology, and receives institutional research funding form Genomic Health. He made his comments in an editorial (J Clin Oncol. 2017 Jan 17. doi: 0.1200/JCO.2016.71.4113 ).
The limitations of this study are substantial. As the authors note, their work was based on only a small number of events, and their length of follow-up prevented them from being able to examine risk in the period of 10 years and beyond.
Also, the likelihood is high that the risk of radiation-induced heart disease can be reduced below that seen in the study. Cardiac exposure in many recent studies has been lower. Techniques such as deep inspiration breath holding or treatment in the prone position often can further decrease this exposure. Several randomized trials comparing partial-breast with whole-breast irradiation have found equal local control rates with both approaches, strongly suggesting that judicious individualization of field borders and cardiac blocks are unlikely to compromise outcome for selected patients treated with partial mastectomy.
Nonetheless, at some point, compromising coverage of the breast, chest wall, or nodal target volumes because an arbitrary threshold of a cardiac dose-volume parameter has been exceeded may be dangerous. Computer programs can likely be created to perform calculations of an individual’s excess risk of cardiac events or death in relation to dose-volume and clinical parameters, although I suspect it will be some years before they provide sufficiently validated, narrow estimates of risk to be clinically useful. Perhaps by then we will also have better ways to explain such sobering decisions to patients.
Abram Recht, MD , is a professor of radiation oncology at Harvard Medical School, Boston. He is also an advisor for CareCore and US Oncology, and receives institutional research funding form Genomic Health. He made his comments in an editorial (J Clin Oncol. 2017 Jan 17. doi: 0.1200/JCO.2016.71.4113 ).
The risk of acute coronary events following radiotherapy for breast cancer is better predicted by the volume of the left ventricle that received 5 Gy than by the mean dose of radiation to the heart, according to a Dutch investigation of 910 women who underwent radiation treatment following breast-conserving surgery.
The finding follows up a 2013 report that found that the risk of acute coronary events (ACE) after breast cancer (BC) radiation could be predicted by the mean radiation heart dose (MHD), the presence of cardiac risk factors, and age (N Engl J Med. 2013 Mar 14;368[11]:987-98. doi: 0.1056/NEJMoa1209825).
The new study validated those findings, but also found that risk prediction was better when mean heart dose (MHD) was replaced by the volume of the left ventricle receiving 5 Gy (LV-V5); the substitution improved the c-statistic to 0.80 (95% confidence interval, 0.72-0.88). Using a weighted ACE risk score based on baseline diabetes, hypertension, and ischemic event history – instead of the risk factor yes-or-no approach from 2013 – further improved predictive power, with a c-statistic of 0.83 (95% CI, 0.75-0.91). Anything over a c-statistic of 0.8 is considered strong; 0.5 is chance, 1.0 is perfect prediction.
For instance, a 70-year-old woman with an LV-V5 of 50% and no cardiac risk factors had an excess ACE risk in the new system of 2.52% within 9 years of radiotherapy (RT). If she had a history of ischemic heart disease, the excess risk increased to 8.42%, the investigators said (J Clin Oncol. 2017 Jan 17. doi: 10.1200/JCO.2016.69.8480).
“Model performance was significantly improved by replacing MHD with LV-V5 and using the weighted ACE risk score.” However, “because we were not able to externally validate the LV-V5 model, this model” requires validation “before it can be used in routine clinical practice,” said investigators, led by Veerle van den Bogaard, MD, of the University of Groningen, the Netherlands.
The women were a median of 59 years old, and they were followed for a median of 7.6 years, with a range of 0.1-10.1 years. Radiation dose information was derived from CT planning scans. The median MHD was 2.37 Gy.
Thirty patients (3.3%) had an ACE, defined as myocardial infarction, coronary revascularization, or death due to ischemic heart disease; 17 had events in the first 5 years. The 5- and 9-year cumulative ACE incidences were 1.9% and 3.9%. Ten of the 30 women died from their cardiac complication.
The model predicted a cumulative ACE incidence at 9 years of 3.5%, which was in line with the observed rate of 3.9%. The excess cumulative risk related to RT was 1.13%. Overall, about 10 patients had an ACE that could be attributed to RT. The cumulative incidence of ACE increased by 16.5% per Gy (95% CI, 0.6-35.0; P = .042). The findings were consistent with the 2013 study.
ACE incidence was not significantly associated with the maximum dose of radiation to the heart.
LV-V5 was the most important prognostic dose-volume parameter associated with the cumulative incidence of ACE, with a hazard ratio of 1.016 (95% CI, 1.002-1.030; P = .016). “Because of this strong association, we chose to include LV-V5 in the model,” the investigators said.
There was no external funding. The lead investigator had no disclosures, but two authors reported institutional research funding from Philips, Roche, and other companies. One was an advisor and speaker for IBA.
The risk of acute coronary events following radiotherapy for breast cancer is better predicted by the volume of the left ventricle that received 5 Gy than by the mean dose of radiation to the heart, according to a Dutch investigation of 910 women who underwent radiation treatment following breast-conserving surgery.
The finding follows up a 2013 report that found that the risk of acute coronary events (ACE) after breast cancer (BC) radiation could be predicted by the mean radiation heart dose (MHD), the presence of cardiac risk factors, and age (N Engl J Med. 2013 Mar 14;368[11]:987-98. doi: 0.1056/NEJMoa1209825).
The new study validated those findings, but also found that risk prediction was better when mean heart dose (MHD) was replaced by the volume of the left ventricle receiving 5 Gy (LV-V5); the substitution improved the c-statistic to 0.80 (95% confidence interval, 0.72-0.88). Using a weighted ACE risk score based on baseline diabetes, hypertension, and ischemic event history – instead of the risk factor yes-or-no approach from 2013 – further improved predictive power, with a c-statistic of 0.83 (95% CI, 0.75-0.91). Anything over a c-statistic of 0.8 is considered strong; 0.5 is chance, 1.0 is perfect prediction.
For instance, a 70-year-old woman with an LV-V5 of 50% and no cardiac risk factors had an excess ACE risk in the new system of 2.52% within 9 years of radiotherapy (RT). If she had a history of ischemic heart disease, the excess risk increased to 8.42%, the investigators said (J Clin Oncol. 2017 Jan 17. doi: 10.1200/JCO.2016.69.8480).
“Model performance was significantly improved by replacing MHD with LV-V5 and using the weighted ACE risk score.” However, “because we were not able to externally validate the LV-V5 model, this model” requires validation “before it can be used in routine clinical practice,” said investigators, led by Veerle van den Bogaard, MD, of the University of Groningen, the Netherlands.
The women were a median of 59 years old, and they were followed for a median of 7.6 years, with a range of 0.1-10.1 years. Radiation dose information was derived from CT planning scans. The median MHD was 2.37 Gy.
Thirty patients (3.3%) had an ACE, defined as myocardial infarction, coronary revascularization, or death due to ischemic heart disease; 17 had events in the first 5 years. The 5- and 9-year cumulative ACE incidences were 1.9% and 3.9%. Ten of the 30 women died from their cardiac complication.
The model predicted a cumulative ACE incidence at 9 years of 3.5%, which was in line with the observed rate of 3.9%. The excess cumulative risk related to RT was 1.13%. Overall, about 10 patients had an ACE that could be attributed to RT. The cumulative incidence of ACE increased by 16.5% per Gy (95% CI, 0.6-35.0; P = .042). The findings were consistent with the 2013 study.
ACE incidence was not significantly associated with the maximum dose of radiation to the heart.
LV-V5 was the most important prognostic dose-volume parameter associated with the cumulative incidence of ACE, with a hazard ratio of 1.016 (95% CI, 1.002-1.030; P = .016). “Because of this strong association, we chose to include LV-V5 in the model,” the investigators said.
There was no external funding. The lead investigator had no disclosures, but two authors reported institutional research funding from Philips, Roche, and other companies. One was an advisor and speaker for IBA.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Replacing the mean heart dose with the volume of the left ventricle receiving 5 Gy improves the predictive c-statistic to 0.80.
Data source: Investigation of 910 women who underwent radiation treatment following breast-conserving surgery.
Disclosures: There was no external funding. The lead investigator had no disclosures, but two authors reported institutional research funding from Philips, Roche, and other companies. One was an advisor and speaker for IBA.
ASCO offers practice guidance on small renal masses
All patients with small renal masses detected on imaging should be considered for renal tumor biopsy when there is a likelihood that the results may affect management of the patient, says a new clinical oncology practice guideline from the American Society of Clinical Oncology.
The guideline defines small renal masses as incidentally image-detected, contrast-enhancing renal tumors 4 cm in diameter or less that are usually consistent with stage T1a renal cell carcinoma (RCC). Approximately one-fourth of all small renal masses turn out to be benign lesions such as oncocytoma or metanephric adenoma, and another 25% may be indolent tumors that can be managed more conservatively, the guidelines note.
Not too long ago, nearly all patients with small renal masses would have undergone radical nephrectomy for lesions of any size. Today, however, partial nephrectomy and percutaneous thermal ablation are safe and less debilitating surgical options for many patients, the authors point out. The purpose of the guideline, therefore, is to help clinicians manage patients with clinically localized small renal masses with evidence-based clinical recommendations.
Recommendations summarized
The guideline, developed with consensus from a multidisciplinary panel, includes six evidence-based recommendations, all based on intermediate quality sources, with recommendation strengths running from moderate to strong. In summary, the guideline recommends:
- All patients with a small renal mass should be considered for renal tumor biopsy “when the results may alter management.”
- For patients with significant comorbidities and a limited life expectancy, active surveillance should be one of the initial management options. Absolute indications for active surveillance include if the patient is at high risk for anesthesia and intervention or has a life expectancy of less than 5 years. Active surveillance is a relative indication for those patients with significant risk of end-stage renal disease if treated, small renal masses less than 1 cm, or a life expectancy of less than 10 years.
- For all patients for whom an intervention is indicated and who have a tumor amenable to limited resection, partial nephrectomy should be the standard treatment offered.
- Percutaneous thermal ablation can be considered as an option for patients whose tumors can be completely ablated. A biopsy should be performed either prior to or at the time of ablation.
- Radical nephrectomy for small renal masses should be reserved only for patients whose tumors are significantly complex to allow for successful partial nephrectomy or for whom or where partial nephrectomy “may result in unacceptable morbidity even when performed at centers with expertise. Referral to a surgeon and a center with experience in partial nephrectomy should be considered.”
- If the patient has chronic kidney disease (CKD), defined as an estimated glomerular filtration rate less than 45 mL/min per 1.73 m2, or develops progressive CKD after treatment, he or she should be considered for referral to a nephrologist, especially if the CKD is associated with proteinuria.
The guideline also offers advice for clinicians on communicating with patients and coordinating all aspects of care in a complex care environment.
“To begin, remember that today’s empowered patient will expect a greater role in his or her care. This means taking steps to ensure the patient is well educated and informed. Clinicians should take the time to orient the patient to his or her care but also make available recommended sources for information, including both print materials and online information,” the guideline authors advise.
They also recommend that clinicians share the details of pathology reports and test results with patients, families, and caregivers using terminology they can understand, including a thorough explanation of cancer staging, tumor types, and clinical options. Patients should also be informed, if appropriate, about the availability of clinical trials.
The guideline is sponsored by ASCO, Dr. Finelli and multiple coauthors disclosed relationships with various drug and/or device companies.
All patients with small renal masses detected on imaging should be considered for renal tumor biopsy when there is a likelihood that the results may affect management of the patient, says a new clinical oncology practice guideline from the American Society of Clinical Oncology.
The guideline defines small renal masses as incidentally image-detected, contrast-enhancing renal tumors 4 cm in diameter or less that are usually consistent with stage T1a renal cell carcinoma (RCC). Approximately one-fourth of all small renal masses turn out to be benign lesions such as oncocytoma or metanephric adenoma, and another 25% may be indolent tumors that can be managed more conservatively, the guidelines note.
Not too long ago, nearly all patients with small renal masses would have undergone radical nephrectomy for lesions of any size. Today, however, partial nephrectomy and percutaneous thermal ablation are safe and less debilitating surgical options for many patients, the authors point out. The purpose of the guideline, therefore, is to help clinicians manage patients with clinically localized small renal masses with evidence-based clinical recommendations.
Recommendations summarized
The guideline, developed with consensus from a multidisciplinary panel, includes six evidence-based recommendations, all based on intermediate quality sources, with recommendation strengths running from moderate to strong. In summary, the guideline recommends:
- All patients with a small renal mass should be considered for renal tumor biopsy “when the results may alter management.”
- For patients with significant comorbidities and a limited life expectancy, active surveillance should be one of the initial management options. Absolute indications for active surveillance include if the patient is at high risk for anesthesia and intervention or has a life expectancy of less than 5 years. Active surveillance is a relative indication for those patients with significant risk of end-stage renal disease if treated, small renal masses less than 1 cm, or a life expectancy of less than 10 years.
- For all patients for whom an intervention is indicated and who have a tumor amenable to limited resection, partial nephrectomy should be the standard treatment offered.
- Percutaneous thermal ablation can be considered as an option for patients whose tumors can be completely ablated. A biopsy should be performed either prior to or at the time of ablation.
- Radical nephrectomy for small renal masses should be reserved only for patients whose tumors are significantly complex to allow for successful partial nephrectomy or for whom or where partial nephrectomy “may result in unacceptable morbidity even when performed at centers with expertise. Referral to a surgeon and a center with experience in partial nephrectomy should be considered.”
- If the patient has chronic kidney disease (CKD), defined as an estimated glomerular filtration rate less than 45 mL/min per 1.73 m2, or develops progressive CKD after treatment, he or she should be considered for referral to a nephrologist, especially if the CKD is associated with proteinuria.
The guideline also offers advice for clinicians on communicating with patients and coordinating all aspects of care in a complex care environment.
“To begin, remember that today’s empowered patient will expect a greater role in his or her care. This means taking steps to ensure the patient is well educated and informed. Clinicians should take the time to orient the patient to his or her care but also make available recommended sources for information, including both print materials and online information,” the guideline authors advise.
They also recommend that clinicians share the details of pathology reports and test results with patients, families, and caregivers using terminology they can understand, including a thorough explanation of cancer staging, tumor types, and clinical options. Patients should also be informed, if appropriate, about the availability of clinical trials.
The guideline is sponsored by ASCO, Dr. Finelli and multiple coauthors disclosed relationships with various drug and/or device companies.
All patients with small renal masses detected on imaging should be considered for renal tumor biopsy when there is a likelihood that the results may affect management of the patient, says a new clinical oncology practice guideline from the American Society of Clinical Oncology.
The guideline defines small renal masses as incidentally image-detected, contrast-enhancing renal tumors 4 cm in diameter or less that are usually consistent with stage T1a renal cell carcinoma (RCC). Approximately one-fourth of all small renal masses turn out to be benign lesions such as oncocytoma or metanephric adenoma, and another 25% may be indolent tumors that can be managed more conservatively, the guidelines note.
Not too long ago, nearly all patients with small renal masses would have undergone radical nephrectomy for lesions of any size. Today, however, partial nephrectomy and percutaneous thermal ablation are safe and less debilitating surgical options for many patients, the authors point out. The purpose of the guideline, therefore, is to help clinicians manage patients with clinically localized small renal masses with evidence-based clinical recommendations.
Recommendations summarized
The guideline, developed with consensus from a multidisciplinary panel, includes six evidence-based recommendations, all based on intermediate quality sources, with recommendation strengths running from moderate to strong. In summary, the guideline recommends:
- All patients with a small renal mass should be considered for renal tumor biopsy “when the results may alter management.”
- For patients with significant comorbidities and a limited life expectancy, active surveillance should be one of the initial management options. Absolute indications for active surveillance include if the patient is at high risk for anesthesia and intervention or has a life expectancy of less than 5 years. Active surveillance is a relative indication for those patients with significant risk of end-stage renal disease if treated, small renal masses less than 1 cm, or a life expectancy of less than 10 years.
- For all patients for whom an intervention is indicated and who have a tumor amenable to limited resection, partial nephrectomy should be the standard treatment offered.
- Percutaneous thermal ablation can be considered as an option for patients whose tumors can be completely ablated. A biopsy should be performed either prior to or at the time of ablation.
- Radical nephrectomy for small renal masses should be reserved only for patients whose tumors are significantly complex to allow for successful partial nephrectomy or for whom or where partial nephrectomy “may result in unacceptable morbidity even when performed at centers with expertise. Referral to a surgeon and a center with experience in partial nephrectomy should be considered.”
- If the patient has chronic kidney disease (CKD), defined as an estimated glomerular filtration rate less than 45 mL/min per 1.73 m2, or develops progressive CKD after treatment, he or she should be considered for referral to a nephrologist, especially if the CKD is associated with proteinuria.
The guideline also offers advice for clinicians on communicating with patients and coordinating all aspects of care in a complex care environment.
“To begin, remember that today’s empowered patient will expect a greater role in his or her care. This means taking steps to ensure the patient is well educated and informed. Clinicians should take the time to orient the patient to his or her care but also make available recommended sources for information, including both print materials and online information,” the guideline authors advise.
They also recommend that clinicians share the details of pathology reports and test results with patients, families, and caregivers using terminology they can understand, including a thorough explanation of cancer staging, tumor types, and clinical options. Patients should also be informed, if appropriate, about the availability of clinical trials.
The guideline is sponsored by ASCO, Dr. Finelli and multiple coauthors disclosed relationships with various drug and/or device companies.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The guideline recommends renal tumor biopsy for most patients with incidentally detected renal masses 4 cm or smaller.
Major finding: Approximately 25% of patients with incidental small renal masses will have benign lesions.
Data source: Evidence-based clinical guideline developed by a multidisciplinary panel.
Disclosures: The guideline is sponsored by ASCO, Dr. Finelli and multiple coauthors disclosed relationships with various drug and/or device companies.
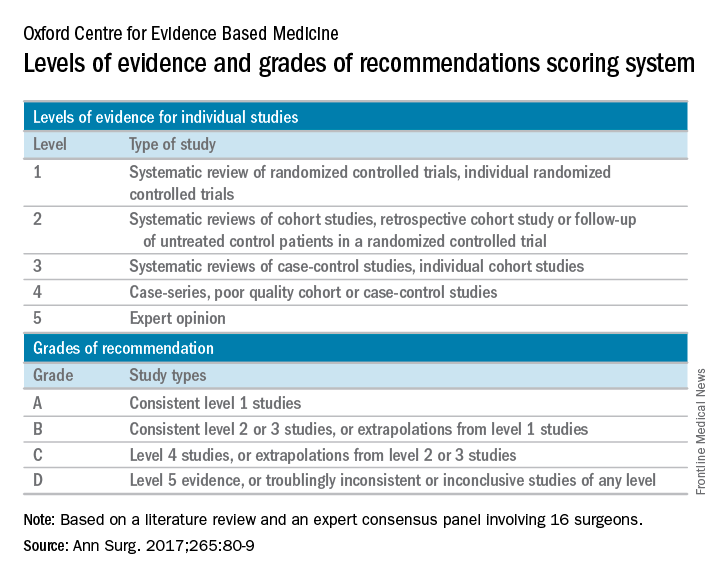
Expert panel reaches consensus on hernia management recommendations
Those are key conclusions from a consensus statement based on a systematic review of existing evidence in the medical literature about ventral hernia management that were published in the January 2017 issue of the Annals of Surgery.
“Despite ventral hernias (VH) being one of the most common pathologies seen by clinicians, significant variability in management exists,” wrote the researchers, led by Mike K. Liang, MD, of the University of Texas Health Science Center at Houston. “Surveys of clinicians and review of nationwide databases of patients undergoing elective ventral hernia repair (VHR) demonstrate substantial heterogeneity in patient selection and clinical practice.”
The panelists agreed that complications with VHR increase in obese patients (grade A evidence), current smokers (grade A), and in patients with glycosylated hemoglobin A1c (HbA1c) of 6.5% or greater (grade B). They did not recommend elective VHR in patients with a body mass index of 50 kg/m2 or greater (grade C), in current smokers (grade A), or patients with an HbA1c of 8.0% or greater (grade B). They also agreed that patients with a BMI of 30-50 kg/m2 or an HbA1c level of 6.5%-8.0% require individualized interventions to reduce surgical risk (grade C, grade B, respectively). The panelists considered nonoperative management to have a low risk of short-term morbidity (grade C) and they recommended mesh reinforcement for repair of hernias 2 cm or greater in size (grade A).
The panelists failed to reach agreement on several areas where high-quality data were limited, including mesh type. “Categories include ultra-light weight, light-weight, mid-weight, heavy-weight, and super-heavy weight, though precise definitions for each category are variable,” authors of the consensus statement wrote. “Randomized controlled trials are needed to compare synthetic, biological, and bioabsorbable meshes in all VH types and clinical settings.”
The authors of the consensus statement also called for further high-quality studies to better assess the management of VH in complex patients, which they defined as those presenting acutely, patients with cirrhosis, patients with inflammatory bowel disease, and patients who are pregnant.
The authors acknowledged certain limitations of the statement, including the fact that not all VH experts were included on the consensus panel. “However, the panel consisted of a large group of national experts with a primary practice focus of VHR,” they wrote. “The panelists have diverse views and unique areas of knowledge in the realm of hernia repair. The differing backgrounds among panelists was intended to make the guidelines that were developed more generalizable, as there is a wide variety of experience and skill level in the surgical community. In addition, there are no objective criteria to define an ‘expert’ in VH management.”
This work was supported by the Center for Clinical and Translational Sciences. The authors reported having no financial disclosures.
[email protected]
Those are key conclusions from a consensus statement based on a systematic review of existing evidence in the medical literature about ventral hernia management that were published in the January 2017 issue of the Annals of Surgery.
“Despite ventral hernias (VH) being one of the most common pathologies seen by clinicians, significant variability in management exists,” wrote the researchers, led by Mike K. Liang, MD, of the University of Texas Health Science Center at Houston. “Surveys of clinicians and review of nationwide databases of patients undergoing elective ventral hernia repair (VHR) demonstrate substantial heterogeneity in patient selection and clinical practice.”
The panelists agreed that complications with VHR increase in obese patients (grade A evidence), current smokers (grade A), and in patients with glycosylated hemoglobin A1c (HbA1c) of 6.5% or greater (grade B). They did not recommend elective VHR in patients with a body mass index of 50 kg/m2 or greater (grade C), in current smokers (grade A), or patients with an HbA1c of 8.0% or greater (grade B). They also agreed that patients with a BMI of 30-50 kg/m2 or an HbA1c level of 6.5%-8.0% require individualized interventions to reduce surgical risk (grade C, grade B, respectively). The panelists considered nonoperative management to have a low risk of short-term morbidity (grade C) and they recommended mesh reinforcement for repair of hernias 2 cm or greater in size (grade A).
The panelists failed to reach agreement on several areas where high-quality data were limited, including mesh type. “Categories include ultra-light weight, light-weight, mid-weight, heavy-weight, and super-heavy weight, though precise definitions for each category are variable,” authors of the consensus statement wrote. “Randomized controlled trials are needed to compare synthetic, biological, and bioabsorbable meshes in all VH types and clinical settings.”

The authors of the consensus statement also called for further high-quality studies to better assess the management of VH in complex patients, which they defined as those presenting acutely, patients with cirrhosis, patients with inflammatory bowel disease, and patients who are pregnant.
The authors acknowledged certain limitations of the statement, including the fact that not all VH experts were included on the consensus panel. “However, the panel consisted of a large group of national experts with a primary practice focus of VHR,” they wrote. “The panelists have diverse views and unique areas of knowledge in the realm of hernia repair. The differing backgrounds among panelists was intended to make the guidelines that were developed more generalizable, as there is a wide variety of experience and skill level in the surgical community. In addition, there are no objective criteria to define an ‘expert’ in VH management.”
This work was supported by the Center for Clinical and Translational Sciences. The authors reported having no financial disclosures.
[email protected]
Those are key conclusions from a consensus statement based on a systematic review of existing evidence in the medical literature about ventral hernia management that were published in the January 2017 issue of the Annals of Surgery.
“Despite ventral hernias (VH) being one of the most common pathologies seen by clinicians, significant variability in management exists,” wrote the researchers, led by Mike K. Liang, MD, of the University of Texas Health Science Center at Houston. “Surveys of clinicians and review of nationwide databases of patients undergoing elective ventral hernia repair (VHR) demonstrate substantial heterogeneity in patient selection and clinical practice.”
The panelists agreed that complications with VHR increase in obese patients (grade A evidence), current smokers (grade A), and in patients with glycosylated hemoglobin A1c (HbA1c) of 6.5% or greater (grade B). They did not recommend elective VHR in patients with a body mass index of 50 kg/m2 or greater (grade C), in current smokers (grade A), or patients with an HbA1c of 8.0% or greater (grade B). They also agreed that patients with a BMI of 30-50 kg/m2 or an HbA1c level of 6.5%-8.0% require individualized interventions to reduce surgical risk (grade C, grade B, respectively). The panelists considered nonoperative management to have a low risk of short-term morbidity (grade C) and they recommended mesh reinforcement for repair of hernias 2 cm or greater in size (grade A).
The panelists failed to reach agreement on several areas where high-quality data were limited, including mesh type. “Categories include ultra-light weight, light-weight, mid-weight, heavy-weight, and super-heavy weight, though precise definitions for each category are variable,” authors of the consensus statement wrote. “Randomized controlled trials are needed to compare synthetic, biological, and bioabsorbable meshes in all VH types and clinical settings.”

The authors of the consensus statement also called for further high-quality studies to better assess the management of VH in complex patients, which they defined as those presenting acutely, patients with cirrhosis, patients with inflammatory bowel disease, and patients who are pregnant.
The authors acknowledged certain limitations of the statement, including the fact that not all VH experts were included on the consensus panel. “However, the panel consisted of a large group of national experts with a primary practice focus of VHR,” they wrote. “The panelists have diverse views and unique areas of knowledge in the realm of hernia repair. The differing backgrounds among panelists was intended to make the guidelines that were developed more generalizable, as there is a wide variety of experience and skill level in the surgical community. In addition, there are no objective criteria to define an ‘expert’ in VH management.”
This work was supported by the Center for Clinical and Translational Sciences. The authors reported having no financial disclosures.
[email protected]
FROM ANNALS OF SURGERY
Esophageal cancers: Apples and oranges wrongly lumped together
Genomic analysis suggests that esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are two separate diseases that should not be combined in clinical trials and may benefit from different treatments, according to the results of a molecular study of 559 esophageal and gastric carcinoma tumors obtained from around the world.
The comprehensive molecular analysis comprised 164 esophageal tumors, 359 gastric adenocarcinomas, and 36 additional adenocarcinomas spanning the gastroesophageal junction.
The results of their analysis “call into question the premise of envisioning esophageal carcinoma as a single entity” and “argue against approaches that combine EAC and ESCC for clinical trials of neoadjuvant, adjuvant, or systemic therapies,” wrote the members of The Cancer Genome Atlas Research Network under the coordination of the National Cancer Institute and the National Human Genome Research Institute project.
The researchers evaluated the 164 esophageal carcinomas using integrated clustering of somatic copy number aberrations, DNA methylation, mRNA, and microRNA expression.
Gene expression analysis showed EACs had increased E-cadherin (CDH1) signaling and upregulation of ARF6 and FOXA pathways, which regulate E-cadherin. In contrast, ESCCs showed upregulation of Wnt, syndecan; p63 pathways, which are essential for squamous epithelial cell differentiation, were also upregulated. “These data suggest the presence of lineage-specific alterations that drive progression in EACs and ESCCs,” according to the researchers.
Somatic genome alterations showed that many of the same genetic pathways were altered in both EAC and ESCC, but the specific genes affected were dissimilar, suggesting distinct pathophysiologies between the two types of cancer. This could signal the need for different treatment approaches and led the researchers to caution against lumping EAC and ESCC in the same clinical trials.
Molecular subtype analysis of the ESCC cancers showed three molecular subtypes: ESCC1 (50 tumors), ESCC2 (36) and ESCC3 (4), distinguished by their mutation types. ESCC1, for example, was characterized by alterations in the NRF2 pathway, mutations in which are associated with poor prognosis and resistance to chemotherapy.
The three subtypes also showed trends for geographic associations, with Vietnamese patients (the only Asian population studied) showing a predominance of ESCC1 (27/41), and all 4 ESCC3 tumors being derived from United States patients.
The researchers also evaluated the molecular association between ESCC and human papillomavirus (HPV), which has been shown to have a pathogenic role in cervical SCC and head and neck (HN)SCC. They found that ESCC mRNA sequencing showed that ESCC-HPV transcript levels were similar to HPV-negative HNSCC tumors, diminishing the likelihood of an etiological role for HPV in ESCC.
In evaluating EACs in comparison to chromosomal instability (CIN) gastric cancers, the researchers found “clear similarity between chromosomal aberrations” in the two cancer types, with a stronger similarity between EAC and CIN gastric cancers than between EAC and ESCC, further differentiating the two esophageal cancers.
“The notable molecular similarity between EACs and CIN gastric cancers provides indirect support for gastric origin of Barrett’s esophagus and EAC and indicates that we may view GEA [gastroesophageal adenocarcinoma] as a singular entity, analogous to colorectal adenocarcinoma,” the authors added.
A notable anatomic gradient showed up in the progression of DNA methylation as seen from proximal to distal GEA-CIN tumors, with the most frequent hypermethylation seen in EACs, compared with gastric CIN cancers, a significant difference.
“These molecular data show that EAC and ESCC are distinct in their molecular characteristics across all platforms tested. ESCC emerges as a disease more reminiscent of other SCCs than of EAC, which itself bears striking resemblance to CIN gastric cancer,” the researchers concluded.
The authors reported that they had no competing financial interests.
This article published in Nature summarizes an integrated genomic analysis of esophageal cancer with careful comparisons to other cancers in the neighborhood (head and neck, lung, and gastric cancer). While clinically apparent to physicians taking care of esophageal cancer throughout the world, these analyses confirm that esophageal squamous cell cancer and esophageal adenocarcinoma are essentially two different diseases with distinct genomic characteristics. This has an important implication in clinical trial design: These pathologies should not be analyzed together, but instead should be studied distinctly.
In addition to the above major conclusion, several other features deserve to be noted. First, esophageal cancer does not seem to be associated with HPV as the HPV transcript levels in these tumors resemble those in HPV-negative head and neck cancers. Second, there are significant differences in the genomic characteristics of esophageal squamous cell cancer depending on geographic location. Third, esophageal adenocarcinoma is most like one particular molecular variant of gastric cancer (chromosomal instability type) and as one moves from the gastric antrum to the esophagus, there is an enrichment of this type of cancer. Such a gradient is found in methylation patterns as well, suggesting a similar cell of origin between gastric and esophageal cancers.
This study brings into focus the overarching theme that cancers may soon be treated based on molecular characteristics rather than anatomic location and clinical trials may have to be grouped based on genetic changes rather than organ systems.
Sai Yendamuri, MD, FACS, is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y.
This article published in Nature summarizes an integrated genomic analysis of esophageal cancer with careful comparisons to other cancers in the neighborhood (head and neck, lung, and gastric cancer). While clinically apparent to physicians taking care of esophageal cancer throughout the world, these analyses confirm that esophageal squamous cell cancer and esophageal adenocarcinoma are essentially two different diseases with distinct genomic characteristics. This has an important implication in clinical trial design: These pathologies should not be analyzed together, but instead should be studied distinctly.
In addition to the above major conclusion, several other features deserve to be noted. First, esophageal cancer does not seem to be associated with HPV as the HPV transcript levels in these tumors resemble those in HPV-negative head and neck cancers. Second, there are significant differences in the genomic characteristics of esophageal squamous cell cancer depending on geographic location. Third, esophageal adenocarcinoma is most like one particular molecular variant of gastric cancer (chromosomal instability type) and as one moves from the gastric antrum to the esophagus, there is an enrichment of this type of cancer. Such a gradient is found in methylation patterns as well, suggesting a similar cell of origin between gastric and esophageal cancers.
This study brings into focus the overarching theme that cancers may soon be treated based on molecular characteristics rather than anatomic location and clinical trials may have to be grouped based on genetic changes rather than organ systems.
Sai Yendamuri, MD, FACS, is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y.
This article published in Nature summarizes an integrated genomic analysis of esophageal cancer with careful comparisons to other cancers in the neighborhood (head and neck, lung, and gastric cancer). While clinically apparent to physicians taking care of esophageal cancer throughout the world, these analyses confirm that esophageal squamous cell cancer and esophageal adenocarcinoma are essentially two different diseases with distinct genomic characteristics. This has an important implication in clinical trial design: These pathologies should not be analyzed together, but instead should be studied distinctly.
In addition to the above major conclusion, several other features deserve to be noted. First, esophageal cancer does not seem to be associated with HPV as the HPV transcript levels in these tumors resemble those in HPV-negative head and neck cancers. Second, there are significant differences in the genomic characteristics of esophageal squamous cell cancer depending on geographic location. Third, esophageal adenocarcinoma is most like one particular molecular variant of gastric cancer (chromosomal instability type) and as one moves from the gastric antrum to the esophagus, there is an enrichment of this type of cancer. Such a gradient is found in methylation patterns as well, suggesting a similar cell of origin between gastric and esophageal cancers.
This study brings into focus the overarching theme that cancers may soon be treated based on molecular characteristics rather than anatomic location and clinical trials may have to be grouped based on genetic changes rather than organ systems.
Sai Yendamuri, MD, FACS, is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y.
Genomic analysis suggests that esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are two separate diseases that should not be combined in clinical trials and may benefit from different treatments, according to the results of a molecular study of 559 esophageal and gastric carcinoma tumors obtained from around the world.
The comprehensive molecular analysis comprised 164 esophageal tumors, 359 gastric adenocarcinomas, and 36 additional adenocarcinomas spanning the gastroesophageal junction.
The results of their analysis “call into question the premise of envisioning esophageal carcinoma as a single entity” and “argue against approaches that combine EAC and ESCC for clinical trials of neoadjuvant, adjuvant, or systemic therapies,” wrote the members of The Cancer Genome Atlas Research Network under the coordination of the National Cancer Institute and the National Human Genome Research Institute project.
The researchers evaluated the 164 esophageal carcinomas using integrated clustering of somatic copy number aberrations, DNA methylation, mRNA, and microRNA expression.
Gene expression analysis showed EACs had increased E-cadherin (CDH1) signaling and upregulation of ARF6 and FOXA pathways, which regulate E-cadherin. In contrast, ESCCs showed upregulation of Wnt, syndecan; p63 pathways, which are essential for squamous epithelial cell differentiation, were also upregulated. “These data suggest the presence of lineage-specific alterations that drive progression in EACs and ESCCs,” according to the researchers.
Somatic genome alterations showed that many of the same genetic pathways were altered in both EAC and ESCC, but the specific genes affected were dissimilar, suggesting distinct pathophysiologies between the two types of cancer. This could signal the need for different treatment approaches and led the researchers to caution against lumping EAC and ESCC in the same clinical trials.
Molecular subtype analysis of the ESCC cancers showed three molecular subtypes: ESCC1 (50 tumors), ESCC2 (36) and ESCC3 (4), distinguished by their mutation types. ESCC1, for example, was characterized by alterations in the NRF2 pathway, mutations in which are associated with poor prognosis and resistance to chemotherapy.
The three subtypes also showed trends for geographic associations, with Vietnamese patients (the only Asian population studied) showing a predominance of ESCC1 (27/41), and all 4 ESCC3 tumors being derived from United States patients.
The researchers also evaluated the molecular association between ESCC and human papillomavirus (HPV), which has been shown to have a pathogenic role in cervical SCC and head and neck (HN)SCC. They found that ESCC mRNA sequencing showed that ESCC-HPV transcript levels were similar to HPV-negative HNSCC tumors, diminishing the likelihood of an etiological role for HPV in ESCC.
In evaluating EACs in comparison to chromosomal instability (CIN) gastric cancers, the researchers found “clear similarity between chromosomal aberrations” in the two cancer types, with a stronger similarity between EAC and CIN gastric cancers than between EAC and ESCC, further differentiating the two esophageal cancers.
“The notable molecular similarity between EACs and CIN gastric cancers provides indirect support for gastric origin of Barrett’s esophagus and EAC and indicates that we may view GEA [gastroesophageal adenocarcinoma] as a singular entity, analogous to colorectal adenocarcinoma,” the authors added.
A notable anatomic gradient showed up in the progression of DNA methylation as seen from proximal to distal GEA-CIN tumors, with the most frequent hypermethylation seen in EACs, compared with gastric CIN cancers, a significant difference.
“These molecular data show that EAC and ESCC are distinct in their molecular characteristics across all platforms tested. ESCC emerges as a disease more reminiscent of other SCCs than of EAC, which itself bears striking resemblance to CIN gastric cancer,” the researchers concluded.
The authors reported that they had no competing financial interests.
Genomic analysis suggests that esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are two separate diseases that should not be combined in clinical trials and may benefit from different treatments, according to the results of a molecular study of 559 esophageal and gastric carcinoma tumors obtained from around the world.
The comprehensive molecular analysis comprised 164 esophageal tumors, 359 gastric adenocarcinomas, and 36 additional adenocarcinomas spanning the gastroesophageal junction.
The results of their analysis “call into question the premise of envisioning esophageal carcinoma as a single entity” and “argue against approaches that combine EAC and ESCC for clinical trials of neoadjuvant, adjuvant, or systemic therapies,” wrote the members of The Cancer Genome Atlas Research Network under the coordination of the National Cancer Institute and the National Human Genome Research Institute project.
The researchers evaluated the 164 esophageal carcinomas using integrated clustering of somatic copy number aberrations, DNA methylation, mRNA, and microRNA expression.
Gene expression analysis showed EACs had increased E-cadherin (CDH1) signaling and upregulation of ARF6 and FOXA pathways, which regulate E-cadherin. In contrast, ESCCs showed upregulation of Wnt, syndecan; p63 pathways, which are essential for squamous epithelial cell differentiation, were also upregulated. “These data suggest the presence of lineage-specific alterations that drive progression in EACs and ESCCs,” according to the researchers.
Somatic genome alterations showed that many of the same genetic pathways were altered in both EAC and ESCC, but the specific genes affected were dissimilar, suggesting distinct pathophysiologies between the two types of cancer. This could signal the need for different treatment approaches and led the researchers to caution against lumping EAC and ESCC in the same clinical trials.
Molecular subtype analysis of the ESCC cancers showed three molecular subtypes: ESCC1 (50 tumors), ESCC2 (36) and ESCC3 (4), distinguished by their mutation types. ESCC1, for example, was characterized by alterations in the NRF2 pathway, mutations in which are associated with poor prognosis and resistance to chemotherapy.
The three subtypes also showed trends for geographic associations, with Vietnamese patients (the only Asian population studied) showing a predominance of ESCC1 (27/41), and all 4 ESCC3 tumors being derived from United States patients.
The researchers also evaluated the molecular association between ESCC and human papillomavirus (HPV), which has been shown to have a pathogenic role in cervical SCC and head and neck (HN)SCC. They found that ESCC mRNA sequencing showed that ESCC-HPV transcript levels were similar to HPV-negative HNSCC tumors, diminishing the likelihood of an etiological role for HPV in ESCC.
In evaluating EACs in comparison to chromosomal instability (CIN) gastric cancers, the researchers found “clear similarity between chromosomal aberrations” in the two cancer types, with a stronger similarity between EAC and CIN gastric cancers than between EAC and ESCC, further differentiating the two esophageal cancers.
“The notable molecular similarity between EACs and CIN gastric cancers provides indirect support for gastric origin of Barrett’s esophagus and EAC and indicates that we may view GEA [gastroesophageal adenocarcinoma] as a singular entity, analogous to colorectal adenocarcinoma,” the authors added.
A notable anatomic gradient showed up in the progression of DNA methylation as seen from proximal to distal GEA-CIN tumors, with the most frequent hypermethylation seen in EACs, compared with gastric CIN cancers, a significant difference.
“These molecular data show that EAC and ESCC are distinct in their molecular characteristics across all platforms tested. ESCC emerges as a disease more reminiscent of other SCCs than of EAC, which itself bears striking resemblance to CIN gastric cancer,” the researchers concluded.
The authors reported that they had no competing financial interests.
FROM NATURE
Key clinical point:
Major finding: Molecular analysis showed esophageal squamous cell carcinoma is more like other squamous cell carcinomas than esophageal adenocarcinoma, which itself resembles chromosomal-instability gastric cancer.
Data source: A molecular study of 559 esophageal and gastric carcinoma tumors obtained from around the world.
Disclosures: The authors reported that they had no competing financial interests.
Scleroligation procedure proposed for gastroesophageal varices
In the treatment of gastroesophageal varices, scleroligation – a hybrid procedure that combines sclerotherapy and band ligation – performed as well as did band ligation, but required fewer sessions and had a shorter overall treatment duration. Sclerotherapy involves the injection of sclerosant to prompt occlusion of the varices, while ligation involves banding the varices to cut off blood flow.
The new approach combines them. The researchers ligated the varix 3-5 cm from the gastroesophageal junction and injected the sclerosant into the varix, below the ligated section. They reasoned that ligation should increase the contact time between the sclerosant and endothelial cells, and thus improve efficacy.
He noted that in the United States, band ligation is the standard of therapy, and the new study won’t change that. “These preliminary results from a single center are quite promising, and if they can be confirmed in a larger, multicenter trial, then this is something that can be added to our therapeutic armamentarium,” said Dr. Kwo.
Band ligation replaced sclerotherapy as the preferred treatment for gastroesophageal varices because it has equal efficacy but fewer severe side effects. However, it also suffers from a higher rate of recurrence because the bands cannot destroy deeper varices and perforating veins.
The combination technique, scleroligation, has been demonstrated in the treatment of esophageal varices, which prompted the authors’ investigation into gastroesophageal varices.
At a single center, the researchers recruited 120 patients with cirrhosis and acute gastroesophageal variceal bleeding above the gastroesophageal junction. They were randomized 1:1 to undergo endoscopic band ligation (EBL) or scleroligation (SL).
On average, 15.6 weeks were required to obliterate the varices in the EBL group versus 8.64 weeks in the SL group (P less than .001). The EBL group required an average of 3.43 sessions to reach that endpoint, compared with 2.22 sessions in the SL group (P less than .001). The EBL group required an average of 13.72 bands per patient, compared with 8.88 bands in the SL group (P less than .001). The EBL group also had a higher average number of units of blood transfused (2.30 vs 1.53; P less than .001).
No patients in either group experienced perforation, chest empyema, pericardial effusion, or strictures.
The good safety outcomes may be related to the small volume of sclerosant used, just 2 mL. “It’s probably because of their meticulous approach that they were able to reduce the complications that we have historically seen with sclerotherapy alone,” said Dr. Kwo.
There was no difference in the number of rebleeds or recurrences at follow-up, total cost, mortality due to rebleeding, or 12-month survival.
No funding source was disclosed. The authors reported no financial disclosures.
In the treatment of gastroesophageal varices, scleroligation – a hybrid procedure that combines sclerotherapy and band ligation – performed as well as did band ligation, but required fewer sessions and had a shorter overall treatment duration. Sclerotherapy involves the injection of sclerosant to prompt occlusion of the varices, while ligation involves banding the varices to cut off blood flow.
The new approach combines them. The researchers ligated the varix 3-5 cm from the gastroesophageal junction and injected the sclerosant into the varix, below the ligated section. They reasoned that ligation should increase the contact time between the sclerosant and endothelial cells, and thus improve efficacy.
He noted that in the United States, band ligation is the standard of therapy, and the new study won’t change that. “These preliminary results from a single center are quite promising, and if they can be confirmed in a larger, multicenter trial, then this is something that can be added to our therapeutic armamentarium,” said Dr. Kwo.
Band ligation replaced sclerotherapy as the preferred treatment for gastroesophageal varices because it has equal efficacy but fewer severe side effects. However, it also suffers from a higher rate of recurrence because the bands cannot destroy deeper varices and perforating veins.
The combination technique, scleroligation, has been demonstrated in the treatment of esophageal varices, which prompted the authors’ investigation into gastroesophageal varices.
At a single center, the researchers recruited 120 patients with cirrhosis and acute gastroesophageal variceal bleeding above the gastroesophageal junction. They were randomized 1:1 to undergo endoscopic band ligation (EBL) or scleroligation (SL).
On average, 15.6 weeks were required to obliterate the varices in the EBL group versus 8.64 weeks in the SL group (P less than .001). The EBL group required an average of 3.43 sessions to reach that endpoint, compared with 2.22 sessions in the SL group (P less than .001). The EBL group required an average of 13.72 bands per patient, compared with 8.88 bands in the SL group (P less than .001). The EBL group also had a higher average number of units of blood transfused (2.30 vs 1.53; P less than .001).
No patients in either group experienced perforation, chest empyema, pericardial effusion, or strictures.
The good safety outcomes may be related to the small volume of sclerosant used, just 2 mL. “It’s probably because of their meticulous approach that they were able to reduce the complications that we have historically seen with sclerotherapy alone,” said Dr. Kwo.
There was no difference in the number of rebleeds or recurrences at follow-up, total cost, mortality due to rebleeding, or 12-month survival.
No funding source was disclosed. The authors reported no financial disclosures.
In the treatment of gastroesophageal varices, scleroligation – a hybrid procedure that combines sclerotherapy and band ligation – performed as well as did band ligation, but required fewer sessions and had a shorter overall treatment duration. Sclerotherapy involves the injection of sclerosant to prompt occlusion of the varices, while ligation involves banding the varices to cut off blood flow.
The new approach combines them. The researchers ligated the varix 3-5 cm from the gastroesophageal junction and injected the sclerosant into the varix, below the ligated section. They reasoned that ligation should increase the contact time between the sclerosant and endothelial cells, and thus improve efficacy.
He noted that in the United States, band ligation is the standard of therapy, and the new study won’t change that. “These preliminary results from a single center are quite promising, and if they can be confirmed in a larger, multicenter trial, then this is something that can be added to our therapeutic armamentarium,” said Dr. Kwo.
Band ligation replaced sclerotherapy as the preferred treatment for gastroesophageal varices because it has equal efficacy but fewer severe side effects. However, it also suffers from a higher rate of recurrence because the bands cannot destroy deeper varices and perforating veins.
The combination technique, scleroligation, has been demonstrated in the treatment of esophageal varices, which prompted the authors’ investigation into gastroesophageal varices.
At a single center, the researchers recruited 120 patients with cirrhosis and acute gastroesophageal variceal bleeding above the gastroesophageal junction. They were randomized 1:1 to undergo endoscopic band ligation (EBL) or scleroligation (SL).
On average, 15.6 weeks were required to obliterate the varices in the EBL group versus 8.64 weeks in the SL group (P less than .001). The EBL group required an average of 3.43 sessions to reach that endpoint, compared with 2.22 sessions in the SL group (P less than .001). The EBL group required an average of 13.72 bands per patient, compared with 8.88 bands in the SL group (P less than .001). The EBL group also had a higher average number of units of blood transfused (2.30 vs 1.53; P less than .001).
No patients in either group experienced perforation, chest empyema, pericardial effusion, or strictures.
The good safety outcomes may be related to the small volume of sclerosant used, just 2 mL. “It’s probably because of their meticulous approach that they were able to reduce the complications that we have historically seen with sclerotherapy alone,” said Dr. Kwo.
There was no difference in the number of rebleeds or recurrences at follow-up, total cost, mortality due to rebleeding, or 12-month survival.
No funding source was disclosed. The authors reported no financial disclosures.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point:
Major finding: Scleroligation required 2.22 sessions on average, compared with 3.43 for endoscopic band ligation.
Data source: Single-center randomized trial of 120 patients.
Disclosures: No funding source was disclosed. The authors reported no financial disclosures.
Double-dose influenza vaccine effective against type B strains
A double-dose inactivated quadrivalent influenza vaccine (IIV4) could be administered to all children aged 6-35 months, as it not only offers the best protection against influenza type B, but it also allows for simplifying the current vaccination schedule considerably.
“The introduction of IIV4 provides an opportunity to review long-accepted practices in administration of influenza vaccines,” explained Varsha K. Jain, MD, formerly employed by GlaxoSmithKline Vaccines, King of Prussia, Pa., and associates.
Giving a lower dose to young children was planned to reduce reactogenicity and febrile convulsions observed with the whole virus vaccines that were in use in the 1970s. But young children have a variable immune response to lower doses, especially against vaccine B strains, they noted (J Ped Infect Dis. 2017 Jan 6. doi: 10.1093/jpids/piw068).
Dr. Jain and coauthors enrolled 2,430 children aged 6-35 months during the 2014-2015 influenza season in the United States and Mexico in this phase III study. Children were randomized into one of two cohorts: one cohort received a standard-doze IIV4 vaccination, while the other received a double-dose. Data on age (6-17 months, 18-35 months), health care center, and influenza primer status also were taken into consideration.
The standard-dose vaccine contained 7.5 mcg of A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), B/Brisbane/60/2008 (B/Victoria), and B/Massachusetts/2/2012 (B/Yamagata), while the double-dose vaccine contained 15 mcg, or twice the amount each, of the same strains. The former was developed by Sanofi Pasteur and the latter by GSK Vaccines.
Primed children who completed the study numbered 1,173; 586 received the standard-dose and 587 received the double-dose. On the unprimed side, 868 completed the study: 442 standard-dose and 426 double-dose. Each dose’s immunogenic noninferiority was quantified by calculating the geometric mean titer (GMT) ratio.
“Immunogenicity was higher in the double-dose group compared with the standard-dose group, particularly against vaccine B strains in children 6-17 months of age and unprimed children,” Dr. Vain and associates said. Both vaccines performed well against the influenza B strain, with the double-dose yielding a GMT of 1.89 against the B/Yamagata strain and 2.13 against the B/Victoria in children aged 6-17 months. Across the entire age spectrum of the study population, unprimed children registered a GMT of 1.85 and 2.04 against the same strains, respectively. For comparison, none of the A strains in any cohort based on age or primed/unprimed registered a GMT above 1.5.
“Increased protection against influenza B [would] be a beneficial clinical outcome [and] use of the same vaccine dose for all eligible ages would also simplify the annual influenza vaccine campaign and reduce cost and logistic complexity,” the authors concluded. “This study provides evidence to support a change in clinical practice to use [double-dose IIV4] in all children 6 months of age and older, once that dosing for a vaccine product has been approved.”
Dr. Jain now is employed by the Bill and Melinda Gates Foundation.
Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
A double-dose inactivated quadrivalent influenza vaccine (IIV4) could be administered to all children aged 6-35 months, as it not only offers the best protection against influenza type B, but it also allows for simplifying the current vaccination schedule considerably.
“The introduction of IIV4 provides an opportunity to review long-accepted practices in administration of influenza vaccines,” explained Varsha K. Jain, MD, formerly employed by GlaxoSmithKline Vaccines, King of Prussia, Pa., and associates.
Giving a lower dose to young children was planned to reduce reactogenicity and febrile convulsions observed with the whole virus vaccines that were in use in the 1970s. But young children have a variable immune response to lower doses, especially against vaccine B strains, they noted (J Ped Infect Dis. 2017 Jan 6. doi: 10.1093/jpids/piw068).
Dr. Jain and coauthors enrolled 2,430 children aged 6-35 months during the 2014-2015 influenza season in the United States and Mexico in this phase III study. Children were randomized into one of two cohorts: one cohort received a standard-doze IIV4 vaccination, while the other received a double-dose. Data on age (6-17 months, 18-35 months), health care center, and influenza primer status also were taken into consideration.
The standard-dose vaccine contained 7.5 mcg of A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), B/Brisbane/60/2008 (B/Victoria), and B/Massachusetts/2/2012 (B/Yamagata), while the double-dose vaccine contained 15 mcg, or twice the amount each, of the same strains. The former was developed by Sanofi Pasteur and the latter by GSK Vaccines.
Primed children who completed the study numbered 1,173; 586 received the standard-dose and 587 received the double-dose. On the unprimed side, 868 completed the study: 442 standard-dose and 426 double-dose. Each dose’s immunogenic noninferiority was quantified by calculating the geometric mean titer (GMT) ratio.
“Immunogenicity was higher in the double-dose group compared with the standard-dose group, particularly against vaccine B strains in children 6-17 months of age and unprimed children,” Dr. Vain and associates said. Both vaccines performed well against the influenza B strain, with the double-dose yielding a GMT of 1.89 against the B/Yamagata strain and 2.13 against the B/Victoria in children aged 6-17 months. Across the entire age spectrum of the study population, unprimed children registered a GMT of 1.85 and 2.04 against the same strains, respectively. For comparison, none of the A strains in any cohort based on age or primed/unprimed registered a GMT above 1.5.
“Increased protection against influenza B [would] be a beneficial clinical outcome [and] use of the same vaccine dose for all eligible ages would also simplify the annual influenza vaccine campaign and reduce cost and logistic complexity,” the authors concluded. “This study provides evidence to support a change in clinical practice to use [double-dose IIV4] in all children 6 months of age and older, once that dosing for a vaccine product has been approved.”
Dr. Jain now is employed by the Bill and Melinda Gates Foundation.
Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
A double-dose inactivated quadrivalent influenza vaccine (IIV4) could be administered to all children aged 6-35 months, as it not only offers the best protection against influenza type B, but it also allows for simplifying the current vaccination schedule considerably.
“The introduction of IIV4 provides an opportunity to review long-accepted practices in administration of influenza vaccines,” explained Varsha K. Jain, MD, formerly employed by GlaxoSmithKline Vaccines, King of Prussia, Pa., and associates.
Giving a lower dose to young children was planned to reduce reactogenicity and febrile convulsions observed with the whole virus vaccines that were in use in the 1970s. But young children have a variable immune response to lower doses, especially against vaccine B strains, they noted (J Ped Infect Dis. 2017 Jan 6. doi: 10.1093/jpids/piw068).
Dr. Jain and coauthors enrolled 2,430 children aged 6-35 months during the 2014-2015 influenza season in the United States and Mexico in this phase III study. Children were randomized into one of two cohorts: one cohort received a standard-doze IIV4 vaccination, while the other received a double-dose. Data on age (6-17 months, 18-35 months), health care center, and influenza primer status also were taken into consideration.
The standard-dose vaccine contained 7.5 mcg of A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), B/Brisbane/60/2008 (B/Victoria), and B/Massachusetts/2/2012 (B/Yamagata), while the double-dose vaccine contained 15 mcg, or twice the amount each, of the same strains. The former was developed by Sanofi Pasteur and the latter by GSK Vaccines.
Primed children who completed the study numbered 1,173; 586 received the standard-dose and 587 received the double-dose. On the unprimed side, 868 completed the study: 442 standard-dose and 426 double-dose. Each dose’s immunogenic noninferiority was quantified by calculating the geometric mean titer (GMT) ratio.
“Immunogenicity was higher in the double-dose group compared with the standard-dose group, particularly against vaccine B strains in children 6-17 months of age and unprimed children,” Dr. Vain and associates said. Both vaccines performed well against the influenza B strain, with the double-dose yielding a GMT of 1.89 against the B/Yamagata strain and 2.13 against the B/Victoria in children aged 6-17 months. Across the entire age spectrum of the study population, unprimed children registered a GMT of 1.85 and 2.04 against the same strains, respectively. For comparison, none of the A strains in any cohort based on age or primed/unprimed registered a GMT above 1.5.
“Increased protection against influenza B [would] be a beneficial clinical outcome [and] use of the same vaccine dose for all eligible ages would also simplify the annual influenza vaccine campaign and reduce cost and logistic complexity,” the authors concluded. “This study provides evidence to support a change in clinical practice to use [double-dose IIV4] in all children 6 months of age and older, once that dosing for a vaccine product has been approved.”
Dr. Jain now is employed by the Bill and Melinda Gates Foundation.
Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
FROM THE JOURNAL OF THE PEDIATRIC INFECTIOUS DISEASES SOCIETY
Key clinical point:
Major finding: Geometric mean titer (GMT) ratios showed that the double-dose IIV4 vaccine was the most effective against influenza type B in children aged 6-17 months (GMT = 1.89) and in unprimed children aged 6-35 months (GMT = 1.85).
Data source: Phase III, randomized trial of 2,041 children aged 6-35 months.
Disclosures: Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
Switch from fingolimod to alemtuzumab might trigger MS relapse
Nine relapsing multiple sclerosis patients had significant and unexpected disease activity within 12 months of switching from fingolimod to alemtuzumab, according to a report from six European neuroscience centers.
The centers treated 174 patients with alemtuzumab (Lemtrada); 36 had been on fingolimod (Gilenya) beforehand. “Therefore, these nine patients ... represent 25% of the fingolimod-alemtuzumab cohort,” said investigators led by Mark Willis, MBBCh, a clinical research fellow at Cardiff University, Wales (Neurol Neuroimmunol Neuroinflamm. 2017 Jan 10. doi: 10.1212/NXI.0000000000000320).
“Careful consideration needs to be given to mode of action of individual therapies and sequential treatment effects,” the team concluded.
The nine patients were on fingolimod for 5-33 months, but it wasn’t working well so they were started on alemtuzumab following a median fingolimod washout period of 6 weeks. Eight patients had at least one clinical relapse within 12 months of the first alemtuzumab infusion cycle; the median time to relapse following alemtuzumab induction was 4.5 months. All nine patients had radiologic evidence of new disease activity.
Five patients had lymphocyte counts below normal when started on alemtuzumab.
Fingolimod has a half-life of 6-9 days, so “lymphocytes would be expected to normalize 2-4 weeks after discontinuation. However, there are case reports of prolonged lymphopenia following prolonged drug exposure, up to 37 months after discontinuation.” It has “been suggested that patients continue on an alternative [disease-modifying treatment] after fingolimod discontinuation, preferably until peripheral lymphocyte counts have normalized.” However, “there is currently no consensus as to which subsequent therapeutic agent is optimal,” the investigators said.
Despite the presence of disease activity in the first 12 months, all nine patients went on to the second planned infusion of alemtuzumab; eight were relapse free during a mean follow-up of 6 months after the second treatment cycle. Four of seven were radiologically stable, but three had new T2 lesions and one with a single new gadolinium-enhancing lesion.
Even so, the findings offers “some support to the hypothesis that, after a period, sequestrated lymphocytes eventually become available for depletion by alemtuzumab,” the researchers noted.
The mean age of the patients when diagnosed with MS was 21 years. The median disease duration to alemtuzumab treatment was 94 months.
There was no external funding. Dr. Willis had no disclosures. Other researchers reported ties to a number of companies, including Genzyme, maker of alemtuzumab, and Novartis, fingolimod’s maker.
Nine relapsing multiple sclerosis patients had significant and unexpected disease activity within 12 months of switching from fingolimod to alemtuzumab, according to a report from six European neuroscience centers.
The centers treated 174 patients with alemtuzumab (Lemtrada); 36 had been on fingolimod (Gilenya) beforehand. “Therefore, these nine patients ... represent 25% of the fingolimod-alemtuzumab cohort,” said investigators led by Mark Willis, MBBCh, a clinical research fellow at Cardiff University, Wales (Neurol Neuroimmunol Neuroinflamm. 2017 Jan 10. doi: 10.1212/NXI.0000000000000320).
“Careful consideration needs to be given to mode of action of individual therapies and sequential treatment effects,” the team concluded.
The nine patients were on fingolimod for 5-33 months, but it wasn’t working well so they were started on alemtuzumab following a median fingolimod washout period of 6 weeks. Eight patients had at least one clinical relapse within 12 months of the first alemtuzumab infusion cycle; the median time to relapse following alemtuzumab induction was 4.5 months. All nine patients had radiologic evidence of new disease activity.
Five patients had lymphocyte counts below normal when started on alemtuzumab.
Fingolimod has a half-life of 6-9 days, so “lymphocytes would be expected to normalize 2-4 weeks after discontinuation. However, there are case reports of prolonged lymphopenia following prolonged drug exposure, up to 37 months after discontinuation.” It has “been suggested that patients continue on an alternative [disease-modifying treatment] after fingolimod discontinuation, preferably until peripheral lymphocyte counts have normalized.” However, “there is currently no consensus as to which subsequent therapeutic agent is optimal,” the investigators said.
Despite the presence of disease activity in the first 12 months, all nine patients went on to the second planned infusion of alemtuzumab; eight were relapse free during a mean follow-up of 6 months after the second treatment cycle. Four of seven were radiologically stable, but three had new T2 lesions and one with a single new gadolinium-enhancing lesion.
Even so, the findings offers “some support to the hypothesis that, after a period, sequestrated lymphocytes eventually become available for depletion by alemtuzumab,” the researchers noted.
The mean age of the patients when diagnosed with MS was 21 years. The median disease duration to alemtuzumab treatment was 94 months.
There was no external funding. Dr. Willis had no disclosures. Other researchers reported ties to a number of companies, including Genzyme, maker of alemtuzumab, and Novartis, fingolimod’s maker.
Nine relapsing multiple sclerosis patients had significant and unexpected disease activity within 12 months of switching from fingolimod to alemtuzumab, according to a report from six European neuroscience centers.
The centers treated 174 patients with alemtuzumab (Lemtrada); 36 had been on fingolimod (Gilenya) beforehand. “Therefore, these nine patients ... represent 25% of the fingolimod-alemtuzumab cohort,” said investigators led by Mark Willis, MBBCh, a clinical research fellow at Cardiff University, Wales (Neurol Neuroimmunol Neuroinflamm. 2017 Jan 10. doi: 10.1212/NXI.0000000000000320).
“Careful consideration needs to be given to mode of action of individual therapies and sequential treatment effects,” the team concluded.
The nine patients were on fingolimod for 5-33 months, but it wasn’t working well so they were started on alemtuzumab following a median fingolimod washout period of 6 weeks. Eight patients had at least one clinical relapse within 12 months of the first alemtuzumab infusion cycle; the median time to relapse following alemtuzumab induction was 4.5 months. All nine patients had radiologic evidence of new disease activity.
Five patients had lymphocyte counts below normal when started on alemtuzumab.
Fingolimod has a half-life of 6-9 days, so “lymphocytes would be expected to normalize 2-4 weeks after discontinuation. However, there are case reports of prolonged lymphopenia following prolonged drug exposure, up to 37 months after discontinuation.” It has “been suggested that patients continue on an alternative [disease-modifying treatment] after fingolimod discontinuation, preferably until peripheral lymphocyte counts have normalized.” However, “there is currently no consensus as to which subsequent therapeutic agent is optimal,” the investigators said.
Despite the presence of disease activity in the first 12 months, all nine patients went on to the second planned infusion of alemtuzumab; eight were relapse free during a mean follow-up of 6 months after the second treatment cycle. Four of seven were radiologically stable, but three had new T2 lesions and one with a single new gadolinium-enhancing lesion.
Even so, the findings offers “some support to the hypothesis that, after a period, sequestrated lymphocytes eventually become available for depletion by alemtuzumab,” the researchers noted.
The mean age of the patients when diagnosed with MS was 21 years. The median disease duration to alemtuzumab treatment was 94 months.
There was no external funding. Dr. Willis had no disclosures. Other researchers reported ties to a number of companies, including Genzyme, maker of alemtuzumab, and Novartis, fingolimod’s maker.
FROM NEUROLOGY: NEUROIMMUNOLOGY AND NEUROINFLAMMATION
Fixed combination topical shows promise for psoriasis
MIAMI – In an ongoing drive to identify an alternative to the mainstay treatment of psoriasis with topical corticosteroids, researchers evaluated a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion compared to its monads and vehicle in 212 people with moderate to severe psoriasis.
After 8 weeks of once-daily applications, combination therapy yielded significantly greater reductions in erythema, plaque elevation, and scaling at the target lesion, compared with the other groups in the phase II trial. The investigators also reported at safety profile consistent with halobetasol propionate or tazarotene monotherapy, meaning no new safety concerns emerged with the combination.
Participants were randomized to the combination, halobetasol propionate alone, tazarotene monotherapy, or vehicle in the multicenter, double blind study.
Efficacy evaluation
At 8 weeks, 53% of the combination group achieved treatment success, defined as at least a 2-point gain in Investigator’s Global Assessment of Disease Severity and a score of “clear” or “almost clear.” In contrast, 33% of the halobetasol propionate group, 19% in the tazarotene, and 10% of the vehicle only group achieved those endpoints in the intent-to-treat-analysis. The difference in efficacy between the combination group and vehicle was statistically significant (P less than .001).
On individual clinical efficacy measures, more than half of the patients in the combination treatment group achieved at least a 2-point improvement from baseline: 54% for erythema, 68% for plaque elevation, 65% for scaling. Each of these outcomes was significantly superior compared to the tazarotene or vehicle group (P less than or equal to .001).
“I was not surprised by the findings as the drugs work by complimentary mechanisms of action – so combination therapy should be effective,” Dr. Stein Gold said.
At baseline, IGA severity score of 3 was most common in each group and for each psoriasis sign at the target lesions. In each group, the mean age of participants ranged from 48 to 56 years; the proportion of men ranged from 59% to 68%; and 87% to 95% were white. The median target lesion sizes ranged from 25 to 32 cm2.
Safety outcomes
One patient in the vehicle-only group died. Three other participants experienced serious adverse events – one in each of the noncombination groups. None of these events were related to the study medications, the investigators noted. Application site reactions were the most common treatment-associated adverse events. Some skin atrophy was reported.
“These results show that the fixed combination of halobetasol propionate and tazarotene was effective and well tolerated,” said Dr. Stein Gold, who serves as division head of dermatology at Henry Ford Health System, West Bloomfield, Mich. “This would be a nice addition to our treatment armamentarium for patients with plaque psoriasis.”
Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
MIAMI – In an ongoing drive to identify an alternative to the mainstay treatment of psoriasis with topical corticosteroids, researchers evaluated a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion compared to its monads and vehicle in 212 people with moderate to severe psoriasis.
After 8 weeks of once-daily applications, combination therapy yielded significantly greater reductions in erythema, plaque elevation, and scaling at the target lesion, compared with the other groups in the phase II trial. The investigators also reported at safety profile consistent with halobetasol propionate or tazarotene monotherapy, meaning no new safety concerns emerged with the combination.
Participants were randomized to the combination, halobetasol propionate alone, tazarotene monotherapy, or vehicle in the multicenter, double blind study.
Efficacy evaluation
At 8 weeks, 53% of the combination group achieved treatment success, defined as at least a 2-point gain in Investigator’s Global Assessment of Disease Severity and a score of “clear” or “almost clear.” In contrast, 33% of the halobetasol propionate group, 19% in the tazarotene, and 10% of the vehicle only group achieved those endpoints in the intent-to-treat-analysis. The difference in efficacy between the combination group and vehicle was statistically significant (P less than .001).
On individual clinical efficacy measures, more than half of the patients in the combination treatment group achieved at least a 2-point improvement from baseline: 54% for erythema, 68% for plaque elevation, 65% for scaling. Each of these outcomes was significantly superior compared to the tazarotene or vehicle group (P less than or equal to .001).
“I was not surprised by the findings as the drugs work by complimentary mechanisms of action – so combination therapy should be effective,” Dr. Stein Gold said.
At baseline, IGA severity score of 3 was most common in each group and for each psoriasis sign at the target lesions. In each group, the mean age of participants ranged from 48 to 56 years; the proportion of men ranged from 59% to 68%; and 87% to 95% were white. The median target lesion sizes ranged from 25 to 32 cm2.
Safety outcomes
One patient in the vehicle-only group died. Three other participants experienced serious adverse events – one in each of the noncombination groups. None of these events were related to the study medications, the investigators noted. Application site reactions were the most common treatment-associated adverse events. Some skin atrophy was reported.
“These results show that the fixed combination of halobetasol propionate and tazarotene was effective and well tolerated,” said Dr. Stein Gold, who serves as division head of dermatology at Henry Ford Health System, West Bloomfield, Mich. “This would be a nice addition to our treatment armamentarium for patients with plaque psoriasis.”
Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
MIAMI – In an ongoing drive to identify an alternative to the mainstay treatment of psoriasis with topical corticosteroids, researchers evaluated a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion compared to its monads and vehicle in 212 people with moderate to severe psoriasis.
After 8 weeks of once-daily applications, combination therapy yielded significantly greater reductions in erythema, plaque elevation, and scaling at the target lesion, compared with the other groups in the phase II trial. The investigators also reported at safety profile consistent with halobetasol propionate or tazarotene monotherapy, meaning no new safety concerns emerged with the combination.
Participants were randomized to the combination, halobetasol propionate alone, tazarotene monotherapy, or vehicle in the multicenter, double blind study.
Efficacy evaluation
At 8 weeks, 53% of the combination group achieved treatment success, defined as at least a 2-point gain in Investigator’s Global Assessment of Disease Severity and a score of “clear” or “almost clear.” In contrast, 33% of the halobetasol propionate group, 19% in the tazarotene, and 10% of the vehicle only group achieved those endpoints in the intent-to-treat-analysis. The difference in efficacy between the combination group and vehicle was statistically significant (P less than .001).
On individual clinical efficacy measures, more than half of the patients in the combination treatment group achieved at least a 2-point improvement from baseline: 54% for erythema, 68% for plaque elevation, 65% for scaling. Each of these outcomes was significantly superior compared to the tazarotene or vehicle group (P less than or equal to .001).
“I was not surprised by the findings as the drugs work by complimentary mechanisms of action – so combination therapy should be effective,” Dr. Stein Gold said.
At baseline, IGA severity score of 3 was most common in each group and for each psoriasis sign at the target lesions. In each group, the mean age of participants ranged from 48 to 56 years; the proportion of men ranged from 59% to 68%; and 87% to 95% were white. The median target lesion sizes ranged from 25 to 32 cm2.
Safety outcomes
One patient in the vehicle-only group died. Three other participants experienced serious adverse events – one in each of the noncombination groups. None of these events were related to the study medications, the investigators noted. Application site reactions were the most common treatment-associated adverse events. Some skin atrophy was reported.
“These results show that the fixed combination of halobetasol propionate and tazarotene was effective and well tolerated,” said Dr. Stein Gold, who serves as division head of dermatology at Henry Ford Health System, West Bloomfield, Mich. “This would be a nice addition to our treatment armamentarium for patients with plaque psoriasis.”
Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
AT ODAC 2017
Key clinical point: A fixed dose combination of topical halobetasol propionate 0.01% and tazarotene 0.045% proved superior to either monotherapy or vehicle.
Major finding: Treatment success was achieved by 53% of a combination group compared with 33% of a halobetasol propionate group, 19% of a tazarotene group, and 10% of the vehicle-only group in an intent-to-treat-analysis.
Data source: A multicenter, randomized, double blind phase II study of 212 people with moderate to severe psoriasis.
Disclosures: Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
CKD death rate highest in Mexico
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
A global measure of chronic kidney disease dropped by 3.9% from 2005 to 2015, but CKD remains a top-10 burden in 63 countries, according to the Global Burden of Disease 2015 study.
The age-standardized rate of years of life lost (YLL) for CKD dropped by 3.9%, even though its global YLL rank rose from 21st to 17th and total CKD mortality was up by almost 32%, the Global Burden of Disease 2015 Mortality and Causes of Death Collaborators reported (Lancet. 2016 Oct 8;388[10053]:1459-544). The increase in the number of deaths comes largely “because of improved estimates within countries with large populations such as China, India, and Russia,” the collaborators pointed out.
“In 2015, Latin America had the highest chronic kidney disease death rates in the world. Within Mexico, the country with the highest chronic kidney disease death rate, more than half of patients with incident end-stage renal disease have an underlying diagnosis of diabetes mellitus,” the investigators wrote.
The study is funded by the Bill & Melinda Gates Foundation.
FROM THE LANCET