User login
57% drop in central venous catheter–related infections after QI interventions
Quality improvement (QI) interventions related to the use of central venous catheters (CVCs) were, on average, associated with 57% fewer infections and $1.85 million in net savings to hospitals within 1-3 years of implementation, based on the results of a meta-analysis of data from 113 hospitals.
“Hospitals that have already attained very low infection rates (through the use of quality improvement checklists) would likely see smaller clinical benefits and savings than in the studies we have reviewed,” said Dr. Teryl Nuckols of Cedars-Sinai Medical Center, Los Angeles. “Nonetheless, we found that QI interventions can be associated with declines in CLABSI (central line-associated bloodstream infection) and/or CRBSI (catheter-related bloodstream infection) and net savings when checklists are already in use, and when hospitals have CLABSI rates as low as 1.7-3.7 per 1,000 CVC-days.”
Studies were eligible for the analysis if they reported or estimated the quality improvement intervention’s clinical effectiveness, measured or modeled its costs, compared alternatives to the intervention, and reported both program and infection-related costs.
Insertion checklists were examined in 12 studies, physician education in 11 studies, ultrasound-guided placement of catheters in 3 studies, all-inclusive catheter kits in 5 studies, sterile dressings in 5 studies, chlorhexidine gluconate sponge or antimicrobial dressing in 2 studies, and antimicrobial catheters in 2 studies.
Overall, the weighted mean incidence rate ratio was 0.43 (95% confidence interval, 0.35-0.51) and incremental net savings were $1.85 million (95% CI, $1.30 million to $2.40 million) per hospital over 3 years (2015 U.S. dollars). Each $100,000 increase in program cost was associated with $315,000 greater savings (95% CI, $166 000-$464 000; P less than .001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7-3.7 per 1,000 catheter-days. (doi: 10.1001/jamainternmed.2016.6610)
Dr. Nuckols acknowledged that the price tag for achieving these savings “may be burdensome for hospitals with limited financial resources … wages and benefits account for two-thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years. We found that, for CLABSI- and CRBSI-prevention interventions, median program costs were about $270,000 per hospital over 3 years – but reached $500,000 to $750,000 in some studies.”
The researchers recommended that “future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.”
Quality improvement (QI) interventions related to the use of central venous catheters (CVCs) were, on average, associated with 57% fewer infections and $1.85 million in net savings to hospitals within 1-3 years of implementation, based on the results of a meta-analysis of data from 113 hospitals.
“Hospitals that have already attained very low infection rates (through the use of quality improvement checklists) would likely see smaller clinical benefits and savings than in the studies we have reviewed,” said Dr. Teryl Nuckols of Cedars-Sinai Medical Center, Los Angeles. “Nonetheless, we found that QI interventions can be associated with declines in CLABSI (central line-associated bloodstream infection) and/or CRBSI (catheter-related bloodstream infection) and net savings when checklists are already in use, and when hospitals have CLABSI rates as low as 1.7-3.7 per 1,000 CVC-days.”
Studies were eligible for the analysis if they reported or estimated the quality improvement intervention’s clinical effectiveness, measured or modeled its costs, compared alternatives to the intervention, and reported both program and infection-related costs.
Insertion checklists were examined in 12 studies, physician education in 11 studies, ultrasound-guided placement of catheters in 3 studies, all-inclusive catheter kits in 5 studies, sterile dressings in 5 studies, chlorhexidine gluconate sponge or antimicrobial dressing in 2 studies, and antimicrobial catheters in 2 studies.
Overall, the weighted mean incidence rate ratio was 0.43 (95% confidence interval, 0.35-0.51) and incremental net savings were $1.85 million (95% CI, $1.30 million to $2.40 million) per hospital over 3 years (2015 U.S. dollars). Each $100,000 increase in program cost was associated with $315,000 greater savings (95% CI, $166 000-$464 000; P less than .001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7-3.7 per 1,000 catheter-days. (doi: 10.1001/jamainternmed.2016.6610)
Dr. Nuckols acknowledged that the price tag for achieving these savings “may be burdensome for hospitals with limited financial resources … wages and benefits account for two-thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years. We found that, for CLABSI- and CRBSI-prevention interventions, median program costs were about $270,000 per hospital over 3 years – but reached $500,000 to $750,000 in some studies.”
The researchers recommended that “future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.”
Quality improvement (QI) interventions related to the use of central venous catheters (CVCs) were, on average, associated with 57% fewer infections and $1.85 million in net savings to hospitals within 1-3 years of implementation, based on the results of a meta-analysis of data from 113 hospitals.
“Hospitals that have already attained very low infection rates (through the use of quality improvement checklists) would likely see smaller clinical benefits and savings than in the studies we have reviewed,” said Dr. Teryl Nuckols of Cedars-Sinai Medical Center, Los Angeles. “Nonetheless, we found that QI interventions can be associated with declines in CLABSI (central line-associated bloodstream infection) and/or CRBSI (catheter-related bloodstream infection) and net savings when checklists are already in use, and when hospitals have CLABSI rates as low as 1.7-3.7 per 1,000 CVC-days.”
Studies were eligible for the analysis if they reported or estimated the quality improvement intervention’s clinical effectiveness, measured or modeled its costs, compared alternatives to the intervention, and reported both program and infection-related costs.
Insertion checklists were examined in 12 studies, physician education in 11 studies, ultrasound-guided placement of catheters in 3 studies, all-inclusive catheter kits in 5 studies, sterile dressings in 5 studies, chlorhexidine gluconate sponge or antimicrobial dressing in 2 studies, and antimicrobial catheters in 2 studies.
Overall, the weighted mean incidence rate ratio was 0.43 (95% confidence interval, 0.35-0.51) and incremental net savings were $1.85 million (95% CI, $1.30 million to $2.40 million) per hospital over 3 years (2015 U.S. dollars). Each $100,000 increase in program cost was associated with $315,000 greater savings (95% CI, $166 000-$464 000; P less than .001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7-3.7 per 1,000 catheter-days. (doi: 10.1001/jamainternmed.2016.6610)
Dr. Nuckols acknowledged that the price tag for achieving these savings “may be burdensome for hospitals with limited financial resources … wages and benefits account for two-thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years. We found that, for CLABSI- and CRBSI-prevention interventions, median program costs were about $270,000 per hospital over 3 years – but reached $500,000 to $750,000 in some studies.”
The researchers recommended that “future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.”
PET-directed induction therapy improves esophageal cancer outcomes
SAN FRANCISCO – The use of positron emission tomography to assess esophageal tumor response to induction chemotherapy and to guide a regimen change in those who failed to respond was associated with an improved pathologic complete response (pCR) rate in patients in a phase II randomized trial.
Of 257 patients with esophageal and gastroesophageal junction (GEJ) adenocarcinomas who were randomized after a baseline PET scan to receive induction chemotherapy with either modified FOLFOX-6 (oxaliplatin, leucovorin, and 5-fluorouracil) on days 1, 15, and 29, or carboplatin/paclitaxel (CP) on days 1, 8, 22, and 29, 39 patients and 49 patients, respectively, were found on a repeat PET scan performed between days 36 and 42 after initiation of therapy to be nonresponders. Those patients were switched to the alternative regimen during preoperative chemoradiation therapy (CRT). Eligible subjects underwent surgical resection 6 weeks after CRT. The pCR rate after surgery among those who were switched because of initial nonresponse was 18%, compared with an expected rate of 5% based on data from prior studies in which chemotherapy was not changed in nonresponders. The rate was 26% in PET responders, Karyn A. Goodman, MD, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology..
“Preoperative chemoradiotherapy is an accepted standard of care for patients with operable esophageal or gastroesophageal junction adenocarcinoma, but even with this aggressive approach, 5-year overall survival rates are on the order of 40%-50%, and most patients die of systemic disease. Optimal systemic therapy for esophageal and gastroesophageal junction cancers remains undefined, so better methods to identify effective therapies are needed,” she said.
Based on findings from the German, phase II, multicenter MUNICON trial, which showed that early PET responders to chemotherapy had significantly better event-free survival than did PET nonresponders (30 vs. 14 months), and that immediate surgical resection among those identified as nonresponders was also associated with improved outcomes, Dr. Goodman and her colleagues “set out to do CALGB 80803 [the current trial] to evaluate the use of early assessment of chemotherapy responsiveness by PET imaging to direct further therapy … with the goal of improving treatment responses,” she said.
Among secondary CALGB 80803 endpoints was the PET response in the treatment arms.
The rate of response to induction chemotherapy was 57% in the FOLFOX group, and 50% in the CP group. The nonresponders were switched to the alternate regimen, and 84% of patients went on to surgery.
The overall pCR rate after surgery was 19% for those who switched from FOLFOX to CP, and 17% for those who switched from CP to FOLFOX.
“The efficacy criteria for changing therapy was met for both induction arms,” she said.
“Of note, patients who were PET responders who had induction and concurrent FOLFOX had a pCR rate of almost 38%. The pCR rate for patients who started on the induction FOLFOX arm was 31%, and for those who started on the induction CP arm it was 14%, and for all patients enrolled on the study, the pCR rate was 22.7%” she said.
PET scans are routinely used to guide therapy decisions in patients with lymphoma, but are only beginning to be explored for this purpose in solid tumors. CALGB 80803 is among the first to show the benefit of PET imaging in directing presurgery treatment decisions for esophageal cancer.
“We’ve shown that a short course of induction chemotherapy followed by early response assessment using PET imaging to determine whether to switch from ineffective therapy to alternative chemotherapy during preoperative chemoradiation for PET nonresponders is feasible for esophageal and GEJ cancers,” she said, adding that the findings demonstrate a benefit with “a new paradigm of using metabolic imaging … to individualize multimodality therapy and improve outcomes in this poor-prognosis population.”
Further, while the study was not powered for a head-to-head comparison of FOLFOX and CP, the “very promising” 38% pCR rate with FOLFOX induction and concurrent therapy is hypothesis generating, she said.
“How these improvements in pCR translate into survival benefit will be determined with longer follow-up,” she added, noting in response to a question about whether these findings will change practice that “this is really the first step in the process. If we can improve outcomes by changing treatment early on, this should be standard of care, but we really do need to get survival outcome information.”
PET at baseline is already standard; adding another scan adds additional cost, but this potentially may be offset by the prevention of costly toxicities and other problems that have to be addressed, she said, concluding that “going forward, early-response assessment using PET imaging can be incorporated into studies to identify more effective new regimens for esophageal and GEJ cancers.”
During a preconference press cast on the findings, press cast moderator Nancy Baxter, MD, of ASCO said that PET scans may prove to be a valuable tool for fine-tuning the use of chemotherapy for esophageal cancer, and for maximizing the benefit of chemotherapy for each patient.
“This is heartening evidence for a new approach to treating a disease where innovation is sorely needed,” she said.
CALGB 80803 was funded by grants from the National Cancer Institute. Dr. Goodman and Dr. Baxter reported having no disclosures.
SAN FRANCISCO – The use of positron emission tomography to assess esophageal tumor response to induction chemotherapy and to guide a regimen change in those who failed to respond was associated with an improved pathologic complete response (pCR) rate in patients in a phase II randomized trial.
Of 257 patients with esophageal and gastroesophageal junction (GEJ) adenocarcinomas who were randomized after a baseline PET scan to receive induction chemotherapy with either modified FOLFOX-6 (oxaliplatin, leucovorin, and 5-fluorouracil) on days 1, 15, and 29, or carboplatin/paclitaxel (CP) on days 1, 8, 22, and 29, 39 patients and 49 patients, respectively, were found on a repeat PET scan performed between days 36 and 42 after initiation of therapy to be nonresponders. Those patients were switched to the alternative regimen during preoperative chemoradiation therapy (CRT). Eligible subjects underwent surgical resection 6 weeks after CRT. The pCR rate after surgery among those who were switched because of initial nonresponse was 18%, compared with an expected rate of 5% based on data from prior studies in which chemotherapy was not changed in nonresponders. The rate was 26% in PET responders, Karyn A. Goodman, MD, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology..
“Preoperative chemoradiotherapy is an accepted standard of care for patients with operable esophageal or gastroesophageal junction adenocarcinoma, but even with this aggressive approach, 5-year overall survival rates are on the order of 40%-50%, and most patients die of systemic disease. Optimal systemic therapy for esophageal and gastroesophageal junction cancers remains undefined, so better methods to identify effective therapies are needed,” she said.
Based on findings from the German, phase II, multicenter MUNICON trial, which showed that early PET responders to chemotherapy had significantly better event-free survival than did PET nonresponders (30 vs. 14 months), and that immediate surgical resection among those identified as nonresponders was also associated with improved outcomes, Dr. Goodman and her colleagues “set out to do CALGB 80803 [the current trial] to evaluate the use of early assessment of chemotherapy responsiveness by PET imaging to direct further therapy … with the goal of improving treatment responses,” she said.
Among secondary CALGB 80803 endpoints was the PET response in the treatment arms.
The rate of response to induction chemotherapy was 57% in the FOLFOX group, and 50% in the CP group. The nonresponders were switched to the alternate regimen, and 84% of patients went on to surgery.
The overall pCR rate after surgery was 19% for those who switched from FOLFOX to CP, and 17% for those who switched from CP to FOLFOX.
“The efficacy criteria for changing therapy was met for both induction arms,” she said.
“Of note, patients who were PET responders who had induction and concurrent FOLFOX had a pCR rate of almost 38%. The pCR rate for patients who started on the induction FOLFOX arm was 31%, and for those who started on the induction CP arm it was 14%, and for all patients enrolled on the study, the pCR rate was 22.7%” she said.
PET scans are routinely used to guide therapy decisions in patients with lymphoma, but are only beginning to be explored for this purpose in solid tumors. CALGB 80803 is among the first to show the benefit of PET imaging in directing presurgery treatment decisions for esophageal cancer.
“We’ve shown that a short course of induction chemotherapy followed by early response assessment using PET imaging to determine whether to switch from ineffective therapy to alternative chemotherapy during preoperative chemoradiation for PET nonresponders is feasible for esophageal and GEJ cancers,” she said, adding that the findings demonstrate a benefit with “a new paradigm of using metabolic imaging … to individualize multimodality therapy and improve outcomes in this poor-prognosis population.”
Further, while the study was not powered for a head-to-head comparison of FOLFOX and CP, the “very promising” 38% pCR rate with FOLFOX induction and concurrent therapy is hypothesis generating, she said.
“How these improvements in pCR translate into survival benefit will be determined with longer follow-up,” she added, noting in response to a question about whether these findings will change practice that “this is really the first step in the process. If we can improve outcomes by changing treatment early on, this should be standard of care, but we really do need to get survival outcome information.”
PET at baseline is already standard; adding another scan adds additional cost, but this potentially may be offset by the prevention of costly toxicities and other problems that have to be addressed, she said, concluding that “going forward, early-response assessment using PET imaging can be incorporated into studies to identify more effective new regimens for esophageal and GEJ cancers.”
During a preconference press cast on the findings, press cast moderator Nancy Baxter, MD, of ASCO said that PET scans may prove to be a valuable tool for fine-tuning the use of chemotherapy for esophageal cancer, and for maximizing the benefit of chemotherapy for each patient.
“This is heartening evidence for a new approach to treating a disease where innovation is sorely needed,” she said.
CALGB 80803 was funded by grants from the National Cancer Institute. Dr. Goodman and Dr. Baxter reported having no disclosures.
SAN FRANCISCO – The use of positron emission tomography to assess esophageal tumor response to induction chemotherapy and to guide a regimen change in those who failed to respond was associated with an improved pathologic complete response (pCR) rate in patients in a phase II randomized trial.
Of 257 patients with esophageal and gastroesophageal junction (GEJ) adenocarcinomas who were randomized after a baseline PET scan to receive induction chemotherapy with either modified FOLFOX-6 (oxaliplatin, leucovorin, and 5-fluorouracil) on days 1, 15, and 29, or carboplatin/paclitaxel (CP) on days 1, 8, 22, and 29, 39 patients and 49 patients, respectively, were found on a repeat PET scan performed between days 36 and 42 after initiation of therapy to be nonresponders. Those patients were switched to the alternative regimen during preoperative chemoradiation therapy (CRT). Eligible subjects underwent surgical resection 6 weeks after CRT. The pCR rate after surgery among those who were switched because of initial nonresponse was 18%, compared with an expected rate of 5% based on data from prior studies in which chemotherapy was not changed in nonresponders. The rate was 26% in PET responders, Karyn A. Goodman, MD, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology..
“Preoperative chemoradiotherapy is an accepted standard of care for patients with operable esophageal or gastroesophageal junction adenocarcinoma, but even with this aggressive approach, 5-year overall survival rates are on the order of 40%-50%, and most patients die of systemic disease. Optimal systemic therapy for esophageal and gastroesophageal junction cancers remains undefined, so better methods to identify effective therapies are needed,” she said.
Based on findings from the German, phase II, multicenter MUNICON trial, which showed that early PET responders to chemotherapy had significantly better event-free survival than did PET nonresponders (30 vs. 14 months), and that immediate surgical resection among those identified as nonresponders was also associated with improved outcomes, Dr. Goodman and her colleagues “set out to do CALGB 80803 [the current trial] to evaluate the use of early assessment of chemotherapy responsiveness by PET imaging to direct further therapy … with the goal of improving treatment responses,” she said.
Among secondary CALGB 80803 endpoints was the PET response in the treatment arms.
The rate of response to induction chemotherapy was 57% in the FOLFOX group, and 50% in the CP group. The nonresponders were switched to the alternate regimen, and 84% of patients went on to surgery.
The overall pCR rate after surgery was 19% for those who switched from FOLFOX to CP, and 17% for those who switched from CP to FOLFOX.
“The efficacy criteria for changing therapy was met for both induction arms,” she said.
“Of note, patients who were PET responders who had induction and concurrent FOLFOX had a pCR rate of almost 38%. The pCR rate for patients who started on the induction FOLFOX arm was 31%, and for those who started on the induction CP arm it was 14%, and for all patients enrolled on the study, the pCR rate was 22.7%” she said.
PET scans are routinely used to guide therapy decisions in patients with lymphoma, but are only beginning to be explored for this purpose in solid tumors. CALGB 80803 is among the first to show the benefit of PET imaging in directing presurgery treatment decisions for esophageal cancer.
“We’ve shown that a short course of induction chemotherapy followed by early response assessment using PET imaging to determine whether to switch from ineffective therapy to alternative chemotherapy during preoperative chemoradiation for PET nonresponders is feasible for esophageal and GEJ cancers,” she said, adding that the findings demonstrate a benefit with “a new paradigm of using metabolic imaging … to individualize multimodality therapy and improve outcomes in this poor-prognosis population.”
Further, while the study was not powered for a head-to-head comparison of FOLFOX and CP, the “very promising” 38% pCR rate with FOLFOX induction and concurrent therapy is hypothesis generating, she said.
“How these improvements in pCR translate into survival benefit will be determined with longer follow-up,” she added, noting in response to a question about whether these findings will change practice that “this is really the first step in the process. If we can improve outcomes by changing treatment early on, this should be standard of care, but we really do need to get survival outcome information.”
PET at baseline is already standard; adding another scan adds additional cost, but this potentially may be offset by the prevention of costly toxicities and other problems that have to be addressed, she said, concluding that “going forward, early-response assessment using PET imaging can be incorporated into studies to identify more effective new regimens for esophageal and GEJ cancers.”
During a preconference press cast on the findings, press cast moderator Nancy Baxter, MD, of ASCO said that PET scans may prove to be a valuable tool for fine-tuning the use of chemotherapy for esophageal cancer, and for maximizing the benefit of chemotherapy for each patient.
“This is heartening evidence for a new approach to treating a disease where innovation is sorely needed,” she said.
CALGB 80803 was funded by grants from the National Cancer Institute. Dr. Goodman and Dr. Baxter reported having no disclosures.
AT THE 2017 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point:
Major finding: The pathologic completer response rate among PET nonresponders who switched therapies was 18% vs. an expected rate of 5%.
Data source: The phase II randomized CALGB 80803 of 257 esophageal and GEJ cancer patients.
Disclosures: CALGB 80803 was funded by grants from the National Cancer Institute. Dr. Goodman and Dr. Baxter reported having no disclosures.
Meta-analysis supports statins as VTE prophylaxis

Photo courtesy of the CDC
Results of a meta-analysis support the idea that statins can reduce the risk of venous thromboembolism (VTE).
Researchers analyzed data from 36 studies involving more than 3.2 million people and found an “extensive body of evidence on the clinical benefit of statin[s] in the occurrence of VTE.”
The team believes these findings could potentially lead to new guidelines and an expansion of the use of statins.
“These findings underscore a potential beneficial role of statin therapy on VTE in addition to its established role in cardiovascular disease prevention,” said Kamlesh Khunti, MD, PhD, of the University of Leicester in the UK.
Dr Khunti and his colleagues reported these findings in The Lancet Haematology.
The researchers analyzed data from 36 studies. This included 13 cohort studies with a total of 3,148,259 subjects and 23 randomized, controlled trials (RCTs) of 118,464 subjects in which statins were compared to placebo or no treatment.
In the observational studies, statin use was associated with a significant reduction in the risk of VTE, when compared to no statin use. The pooled relative risk (RR) was 0.75 (P<0.0001).
Statin use was associated with a significant reduction in the risk of deep vein thrombosis (DVT), with an RR of 0.77 (P<0.0001). (There were only 2 observational studies looking specifically at DVT outcomes.)
However, there was no significant reduction in the risk of pulmonary embolism (PE) with statin use. The RR was 1.02 (P=0.90). (There was only 1 observational study reporting specifically on PE outcomes.)
In the RCTs, statin use was associated with a significant reduction in the risk of VTE, with an RR of 0.85 (P=0.038).
Statin use was also associated with a significant reduction in DVT risk, with an RR of 0.45, but not PE risk, with an RR of 0.77 (no P values available). (There was only 1 RCT that reported specifically on DVT and PE outcomes.)
The RCTs also showed significant differences in VTE risk according to type of statin used. Patients receiving rosuvastatin had the lowest risk of VTE when compared to those receiving other statins, with an RR of 0.57 (P=0.015).
“Currently, statins are only approved for lipid lowering in the primary and secondary prevention of cardiovascular disease,” said study author Setor Kunutsor, MBChB, PhD, of the University of Bristol in the UK.
“But they have shown great promise beyond their established lipid-lowering effects, and these include potential beneficial impact on multiple disease conditions. These results provide an extensive body of evidence on the clinical benefit of statin[s] in the occurrence of VTE and may support a true protective effect.” ![]()

Photo courtesy of the CDC
Results of a meta-analysis support the idea that statins can reduce the risk of venous thromboembolism (VTE).
Researchers analyzed data from 36 studies involving more than 3.2 million people and found an “extensive body of evidence on the clinical benefit of statin[s] in the occurrence of VTE.”
The team believes these findings could potentially lead to new guidelines and an expansion of the use of statins.
“These findings underscore a potential beneficial role of statin therapy on VTE in addition to its established role in cardiovascular disease prevention,” said Kamlesh Khunti, MD, PhD, of the University of Leicester in the UK.
Dr Khunti and his colleagues reported these findings in The Lancet Haematology.
The researchers analyzed data from 36 studies. This included 13 cohort studies with a total of 3,148,259 subjects and 23 randomized, controlled trials (RCTs) of 118,464 subjects in which statins were compared to placebo or no treatment.
In the observational studies, statin use was associated with a significant reduction in the risk of VTE, when compared to no statin use. The pooled relative risk (RR) was 0.75 (P<0.0001).
Statin use was associated with a significant reduction in the risk of deep vein thrombosis (DVT), with an RR of 0.77 (P<0.0001). (There were only 2 observational studies looking specifically at DVT outcomes.)
However, there was no significant reduction in the risk of pulmonary embolism (PE) with statin use. The RR was 1.02 (P=0.90). (There was only 1 observational study reporting specifically on PE outcomes.)
In the RCTs, statin use was associated with a significant reduction in the risk of VTE, with an RR of 0.85 (P=0.038).
Statin use was also associated with a significant reduction in DVT risk, with an RR of 0.45, but not PE risk, with an RR of 0.77 (no P values available). (There was only 1 RCT that reported specifically on DVT and PE outcomes.)
The RCTs also showed significant differences in VTE risk according to type of statin used. Patients receiving rosuvastatin had the lowest risk of VTE when compared to those receiving other statins, with an RR of 0.57 (P=0.015).
“Currently, statins are only approved for lipid lowering in the primary and secondary prevention of cardiovascular disease,” said study author Setor Kunutsor, MBChB, PhD, of the University of Bristol in the UK.
“But they have shown great promise beyond their established lipid-lowering effects, and these include potential beneficial impact on multiple disease conditions. These results provide an extensive body of evidence on the clinical benefit of statin[s] in the occurrence of VTE and may support a true protective effect.” ![]()

Photo courtesy of the CDC
Results of a meta-analysis support the idea that statins can reduce the risk of venous thromboembolism (VTE).
Researchers analyzed data from 36 studies involving more than 3.2 million people and found an “extensive body of evidence on the clinical benefit of statin[s] in the occurrence of VTE.”
The team believes these findings could potentially lead to new guidelines and an expansion of the use of statins.
“These findings underscore a potential beneficial role of statin therapy on VTE in addition to its established role in cardiovascular disease prevention,” said Kamlesh Khunti, MD, PhD, of the University of Leicester in the UK.
Dr Khunti and his colleagues reported these findings in The Lancet Haematology.
The researchers analyzed data from 36 studies. This included 13 cohort studies with a total of 3,148,259 subjects and 23 randomized, controlled trials (RCTs) of 118,464 subjects in which statins were compared to placebo or no treatment.
In the observational studies, statin use was associated with a significant reduction in the risk of VTE, when compared to no statin use. The pooled relative risk (RR) was 0.75 (P<0.0001).
Statin use was associated with a significant reduction in the risk of deep vein thrombosis (DVT), with an RR of 0.77 (P<0.0001). (There were only 2 observational studies looking specifically at DVT outcomes.)
However, there was no significant reduction in the risk of pulmonary embolism (PE) with statin use. The RR was 1.02 (P=0.90). (There was only 1 observational study reporting specifically on PE outcomes.)
In the RCTs, statin use was associated with a significant reduction in the risk of VTE, with an RR of 0.85 (P=0.038).
Statin use was also associated with a significant reduction in DVT risk, with an RR of 0.45, but not PE risk, with an RR of 0.77 (no P values available). (There was only 1 RCT that reported specifically on DVT and PE outcomes.)
The RCTs also showed significant differences in VTE risk according to type of statin used. Patients receiving rosuvastatin had the lowest risk of VTE when compared to those receiving other statins, with an RR of 0.57 (P=0.015).
“Currently, statins are only approved for lipid lowering in the primary and secondary prevention of cardiovascular disease,” said study author Setor Kunutsor, MBChB, PhD, of the University of Bristol in the UK.
“But they have shown great promise beyond their established lipid-lowering effects, and these include potential beneficial impact on multiple disease conditions. These results provide an extensive body of evidence on the clinical benefit of statin[s] in the occurrence of VTE and may support a true protective effect.” ![]()
New guidelines for patients requiring red blood cell transfusion
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Eight things hospitalists need to know about post-acute care
Whether you’re a hospitalist who works only in a hospital, a hospitalist who works only in a post-acute care (PAC) setting, or a hospitalist who works in both types of facilities, knowing about current trends at PAC facilities and what the future may hold can help you excel in your current capacity and, ultimately, improve patient care.
The Hospitalist tapped experts in the post-acute space to tell us what they thought HM should know about working in PAC – which, in many ways, is quite different from the hospital setting. Here’s a compilation of their top eight must-knows.
1. PAC settings rely more on mid-level medical staff than hospitals do.
PAC facilities employ more mid-level providers, such as nurse practitioners and physician assistants, because they can support the level of medical complexity and decision making 95% of the time, says James D. Tollman, MD, FHM, president of Essex Inpatient Physicians in Boxford, Mass. Further, they are more heavily staffed by licensed practical nurses than are acute-care settings.
Usually, there is no physician or nurse practitioner presence at night. Clinicians rely on nursing staff’s assessment to make decisions regarding changes in patient status during off-hours, says Virginia Cummings, MD, director of long-term care, gerontology division, at Boston-based Beth Israel Deaconess Medical Center.
2. Testing takes longer, and options are limited.
Access to some acute urgent resources such as laboratory testing, imaging tests, and pharmacy products is more challenging at PAC facilities because most of these resources are not on-site. Consequently, there is a time lag between ordering tests and new medications and implementing these orders.
“If a patient needs something performed diagnostically immediately, they usually have to be transported to the emergency room or a facility with the necessary testing equipment,” Dr. Tollman says.
However, Paul T. Liistro, managing partner, Arbors of Hop Brook Limited Partnership in Manchester, Conn., and Vernon Manor Health Care Center in Vernon, Conn., and administrator, Manchester Manor Health Care Center, notes that it’s possible for a laboratory service or mobile diagnostic unit to provide laboratory testing or certain imaging at a PAC facility. More-involved diagnostics, such as an MRI or a PET scan, typically require testing at a remote location.
3. Patient populations mainly include rehab and terminally ill patients.
Patients are typically sent to a PAC facility either to recover from an illness or injury or because they are chronically ill and have exhausted treatment options. Regarding the latter, “They are mostly there for palliation; we don’t perform daily tests or prescribe aggressive medications on these patients,” Dr. Nazir says.
Dr. Cummings explains that PAC clinicians go through “the dying process with the patient.”
“They may or may not have assistance from hospice organizations,” she says, “and when they don’t, [hospitalists] take on the role of palliative-care providers.”
Dr. Cummings has seen an increase in psychiatric patients entering PAC facilities.
“Many patients with chronic psychological problems are aging, and there are fewer inpatient psychiatric beds available to those with concurrent medical and psychiatric problems,” she says. Much of this work is now being done in PAC settings. 
4. You can build a relationship with your patient.
Because the pace of a PAC facility is slower and a patient typically stays in a PAC facility longer than at a hospital, there’s time for a hospitalist to have more in-depth conversations with patients and their families.
“Building a deeper relationship with a patient may give the hospitalist an opportunity to discover the cause of an acute problem,” Dr. Nazir says. “They can go in-depth into the psychosocial aspect of medicine and may be able to find out what led to the initial problem and the real root cause, which can help prevent future recurrences, such as repeat falls or forgetting to take a medication.”
5. Using EHRs can improve transitions.
Care transitions between a hospital and PAC facility can be compromised by a lack of information sharing, and they can affect the quality and safety of patient care, says Dori Cross, a doctoral candidate in health services organization and policy at the University of Michigan School of Public Health in Ann Arbor. Handoffs between providers require information continuity – information that is complete, timely, and in a usable format – to ensure appropriate medical decisions and to provide high-quality care during and after transition.
Electronic health records (EHRs) as well as health information exchanges (HIEs) allow providers to communicate and share patient information. For example, hospitals can send information electronically to PAC facilities (“push” exchange) or make information available online securely for PAC providers to log in and access (“pull” exchange). According to a 2014 survey data by the American Hospital Association, more than 50% of hospitals report sending structured summary-of-care records electronically to long-term care settings; a little less than half of those hospitals (23% of the total sample of hospitals) were also receiving information electronically from long-term care sites.1
“This bidirectional exchange, in particular, can make it easier to share information across provider organizations electronically and, in turn, improve care delivery,” says Ms. Cross, who authored an accepted paper on the subject in the Journal of Post-Acute and Long-Term Medicine.
6. Hospitalists can work with providers in PAC settings to improve transitions.
Despite improvements in the electronic transfer of medical information, gaps still exist and can cause problems. One chasm when discharging patients to a PAC facility, is when a hospital IT system is incapable of communicating with the PAC facility system. In this instance, Dr. Nazir says, the hospitalist “can help bridge the gap.”
“[We] can verbally relay relevant information to physicians at PAC facilities so they understand the patient’s status, needs, and expectations,” he says. “Furthermore, hospitalists and a PAC facility’s administration can brainstorm methods to improve the systems of care so the patient receives more effective and timely care.”
7. Hospitalists switching to the PAC setting should have formal training.
The two main obstacles for hospitalists who change from working in a hospital to a PAC facility are the lack of exposure to PAC work in training and the assumption that it requires the same skills sets of a typical hospitalist, according to Manoj K. Mathew, MD, SFHM, national medical director of Los Angeles–based Agilon Health. The PAC setting has quite a number of differences compared with a hospital setting. For example, some regulations apply specifically to PAC facilities. In addition to formal training, hospitalists can benefit from using SHM’s Post-Acute Care Transitions Toolkit, having a mentor, or using resources from other organizations that function in this space such as The Society for Post-Acute and Long-Term Care Medicine, Dr. Nazir says.
8. A variety of payors and payment models are in play.
Commercial insurers continue to be major payors for PAC, especially for individuals younger than 65 years. Medicare and Medicaid, administered by the Centers for Medicare & Medicaid Services, are the primary payors for patients aged 65 years and older.
“These scorecards are using a variety of criteria to rank providers, such as length of stay, cost, readmissions to hospitals, and quality.”
Because Medicare Part A covers many patients discharged to a PAC setting, any changes in payment incentives or benefit structures by the Medicare program will drive changes in PAC.
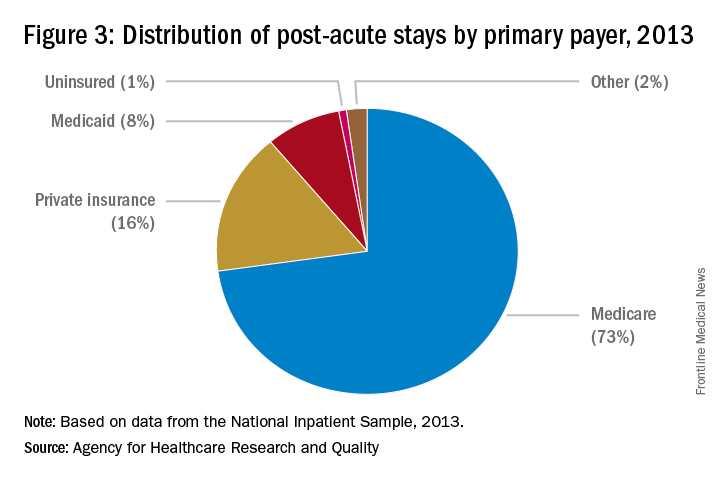
“For example, as Medicare implements payment adjustments for hospitals that have high rates of readmissions, hospitals have a new incentive to work closely with SNFs and other providers of PAC to ensure patients can avoid unnecessary readmissions,” says Tiffany A. Radcliff, PhD, a health economist and associate professor in the department of health policy and management at Texas A&M University School of Public Health in College Station.
Providers must follow the billing rules for each payor. The rules for Medicare payments are outlined on CMS’ website. Bundled payments for PAC under the Medicare Part A program are scheduled to be implemented by 2018.
Reference
Cross DA, Adler-Milstein J. Investing in post-acute care transitions: electronic information exchange between hospitals and long-term care facilities [published online ahead of print Sept. 14, 2016]. JAMDA. doi: http://dx.doi.org/10.1016/j.jamda.2016.07.024.
Whether you’re a hospitalist who works only in a hospital, a hospitalist who works only in a post-acute care (PAC) setting, or a hospitalist who works in both types of facilities, knowing about current trends at PAC facilities and what the future may hold can help you excel in your current capacity and, ultimately, improve patient care.
The Hospitalist tapped experts in the post-acute space to tell us what they thought HM should know about working in PAC – which, in many ways, is quite different from the hospital setting. Here’s a compilation of their top eight must-knows.
1. PAC settings rely more on mid-level medical staff than hospitals do.
PAC facilities employ more mid-level providers, such as nurse practitioners and physician assistants, because they can support the level of medical complexity and decision making 95% of the time, says James D. Tollman, MD, FHM, president of Essex Inpatient Physicians in Boxford, Mass. Further, they are more heavily staffed by licensed practical nurses than are acute-care settings.
Usually, there is no physician or nurse practitioner presence at night. Clinicians rely on nursing staff’s assessment to make decisions regarding changes in patient status during off-hours, says Virginia Cummings, MD, director of long-term care, gerontology division, at Boston-based Beth Israel Deaconess Medical Center.
2. Testing takes longer, and options are limited.
Access to some acute urgent resources such as laboratory testing, imaging tests, and pharmacy products is more challenging at PAC facilities because most of these resources are not on-site. Consequently, there is a time lag between ordering tests and new medications and implementing these orders.
“If a patient needs something performed diagnostically immediately, they usually have to be transported to the emergency room or a facility with the necessary testing equipment,” Dr. Tollman says.
However, Paul T. Liistro, managing partner, Arbors of Hop Brook Limited Partnership in Manchester, Conn., and Vernon Manor Health Care Center in Vernon, Conn., and administrator, Manchester Manor Health Care Center, notes that it’s possible for a laboratory service or mobile diagnostic unit to provide laboratory testing or certain imaging at a PAC facility. More-involved diagnostics, such as an MRI or a PET scan, typically require testing at a remote location.
3. Patient populations mainly include rehab and terminally ill patients.
Patients are typically sent to a PAC facility either to recover from an illness or injury or because they are chronically ill and have exhausted treatment options. Regarding the latter, “They are mostly there for palliation; we don’t perform daily tests or prescribe aggressive medications on these patients,” Dr. Nazir says.
Dr. Cummings explains that PAC clinicians go through “the dying process with the patient.”
“They may or may not have assistance from hospice organizations,” she says, “and when they don’t, [hospitalists] take on the role of palliative-care providers.”
Dr. Cummings has seen an increase in psychiatric patients entering PAC facilities.
“Many patients with chronic psychological problems are aging, and there are fewer inpatient psychiatric beds available to those with concurrent medical and psychiatric problems,” she says. Much of this work is now being done in PAC settings. 
4. You can build a relationship with your patient.
Because the pace of a PAC facility is slower and a patient typically stays in a PAC facility longer than at a hospital, there’s time for a hospitalist to have more in-depth conversations with patients and their families.
“Building a deeper relationship with a patient may give the hospitalist an opportunity to discover the cause of an acute problem,” Dr. Nazir says. “They can go in-depth into the psychosocial aspect of medicine and may be able to find out what led to the initial problem and the real root cause, which can help prevent future recurrences, such as repeat falls or forgetting to take a medication.”
5. Using EHRs can improve transitions.
Care transitions between a hospital and PAC facility can be compromised by a lack of information sharing, and they can affect the quality and safety of patient care, says Dori Cross, a doctoral candidate in health services organization and policy at the University of Michigan School of Public Health in Ann Arbor. Handoffs between providers require information continuity – information that is complete, timely, and in a usable format – to ensure appropriate medical decisions and to provide high-quality care during and after transition.
Electronic health records (EHRs) as well as health information exchanges (HIEs) allow providers to communicate and share patient information. For example, hospitals can send information electronically to PAC facilities (“push” exchange) or make information available online securely for PAC providers to log in and access (“pull” exchange). According to a 2014 survey data by the American Hospital Association, more than 50% of hospitals report sending structured summary-of-care records electronically to long-term care settings; a little less than half of those hospitals (23% of the total sample of hospitals) were also receiving information electronically from long-term care sites.1
“This bidirectional exchange, in particular, can make it easier to share information across provider organizations electronically and, in turn, improve care delivery,” says Ms. Cross, who authored an accepted paper on the subject in the Journal of Post-Acute and Long-Term Medicine.
6. Hospitalists can work with providers in PAC settings to improve transitions.
Despite improvements in the electronic transfer of medical information, gaps still exist and can cause problems. One chasm when discharging patients to a PAC facility, is when a hospital IT system is incapable of communicating with the PAC facility system. In this instance, Dr. Nazir says, the hospitalist “can help bridge the gap.”
“[We] can verbally relay relevant information to physicians at PAC facilities so they understand the patient’s status, needs, and expectations,” he says. “Furthermore, hospitalists and a PAC facility’s administration can brainstorm methods to improve the systems of care so the patient receives more effective and timely care.”
7. Hospitalists switching to the PAC setting should have formal training.
The two main obstacles for hospitalists who change from working in a hospital to a PAC facility are the lack of exposure to PAC work in training and the assumption that it requires the same skills sets of a typical hospitalist, according to Manoj K. Mathew, MD, SFHM, national medical director of Los Angeles–based Agilon Health. The PAC setting has quite a number of differences compared with a hospital setting. For example, some regulations apply specifically to PAC facilities. In addition to formal training, hospitalists can benefit from using SHM’s Post-Acute Care Transitions Toolkit, having a mentor, or using resources from other organizations that function in this space such as The Society for Post-Acute and Long-Term Care Medicine, Dr. Nazir says.
8. A variety of payors and payment models are in play.
Commercial insurers continue to be major payors for PAC, especially for individuals younger than 65 years. Medicare and Medicaid, administered by the Centers for Medicare & Medicaid Services, are the primary payors for patients aged 65 years and older.
“These scorecards are using a variety of criteria to rank providers, such as length of stay, cost, readmissions to hospitals, and quality.”
Because Medicare Part A covers many patients discharged to a PAC setting, any changes in payment incentives or benefit structures by the Medicare program will drive changes in PAC.

“For example, as Medicare implements payment adjustments for hospitals that have high rates of readmissions, hospitals have a new incentive to work closely with SNFs and other providers of PAC to ensure patients can avoid unnecessary readmissions,” says Tiffany A. Radcliff, PhD, a health economist and associate professor in the department of health policy and management at Texas A&M University School of Public Health in College Station.
Providers must follow the billing rules for each payor. The rules for Medicare payments are outlined on CMS’ website. Bundled payments for PAC under the Medicare Part A program are scheduled to be implemented by 2018.
Reference
Cross DA, Adler-Milstein J. Investing in post-acute care transitions: electronic information exchange between hospitals and long-term care facilities [published online ahead of print Sept. 14, 2016]. JAMDA. doi: http://dx.doi.org/10.1016/j.jamda.2016.07.024.
Whether you’re a hospitalist who works only in a hospital, a hospitalist who works only in a post-acute care (PAC) setting, or a hospitalist who works in both types of facilities, knowing about current trends at PAC facilities and what the future may hold can help you excel in your current capacity and, ultimately, improve patient care.
The Hospitalist tapped experts in the post-acute space to tell us what they thought HM should know about working in PAC – which, in many ways, is quite different from the hospital setting. Here’s a compilation of their top eight must-knows.
1. PAC settings rely more on mid-level medical staff than hospitals do.
PAC facilities employ more mid-level providers, such as nurse practitioners and physician assistants, because they can support the level of medical complexity and decision making 95% of the time, says James D. Tollman, MD, FHM, president of Essex Inpatient Physicians in Boxford, Mass. Further, they are more heavily staffed by licensed practical nurses than are acute-care settings.
Usually, there is no physician or nurse practitioner presence at night. Clinicians rely on nursing staff’s assessment to make decisions regarding changes in patient status during off-hours, says Virginia Cummings, MD, director of long-term care, gerontology division, at Boston-based Beth Israel Deaconess Medical Center.
2. Testing takes longer, and options are limited.
Access to some acute urgent resources such as laboratory testing, imaging tests, and pharmacy products is more challenging at PAC facilities because most of these resources are not on-site. Consequently, there is a time lag between ordering tests and new medications and implementing these orders.
“If a patient needs something performed diagnostically immediately, they usually have to be transported to the emergency room or a facility with the necessary testing equipment,” Dr. Tollman says.
However, Paul T. Liistro, managing partner, Arbors of Hop Brook Limited Partnership in Manchester, Conn., and Vernon Manor Health Care Center in Vernon, Conn., and administrator, Manchester Manor Health Care Center, notes that it’s possible for a laboratory service or mobile diagnostic unit to provide laboratory testing or certain imaging at a PAC facility. More-involved diagnostics, such as an MRI or a PET scan, typically require testing at a remote location.
3. Patient populations mainly include rehab and terminally ill patients.
Patients are typically sent to a PAC facility either to recover from an illness or injury or because they are chronically ill and have exhausted treatment options. Regarding the latter, “They are mostly there for palliation; we don’t perform daily tests or prescribe aggressive medications on these patients,” Dr. Nazir says.
Dr. Cummings explains that PAC clinicians go through “the dying process with the patient.”
“They may or may not have assistance from hospice organizations,” she says, “and when they don’t, [hospitalists] take on the role of palliative-care providers.”
Dr. Cummings has seen an increase in psychiatric patients entering PAC facilities.
“Many patients with chronic psychological problems are aging, and there are fewer inpatient psychiatric beds available to those with concurrent medical and psychiatric problems,” she says. Much of this work is now being done in PAC settings. 
4. You can build a relationship with your patient.
Because the pace of a PAC facility is slower and a patient typically stays in a PAC facility longer than at a hospital, there’s time for a hospitalist to have more in-depth conversations with patients and their families.
“Building a deeper relationship with a patient may give the hospitalist an opportunity to discover the cause of an acute problem,” Dr. Nazir says. “They can go in-depth into the psychosocial aspect of medicine and may be able to find out what led to the initial problem and the real root cause, which can help prevent future recurrences, such as repeat falls or forgetting to take a medication.”
5. Using EHRs can improve transitions.
Care transitions between a hospital and PAC facility can be compromised by a lack of information sharing, and they can affect the quality and safety of patient care, says Dori Cross, a doctoral candidate in health services organization and policy at the University of Michigan School of Public Health in Ann Arbor. Handoffs between providers require information continuity – information that is complete, timely, and in a usable format – to ensure appropriate medical decisions and to provide high-quality care during and after transition.
Electronic health records (EHRs) as well as health information exchanges (HIEs) allow providers to communicate and share patient information. For example, hospitals can send information electronically to PAC facilities (“push” exchange) or make information available online securely for PAC providers to log in and access (“pull” exchange). According to a 2014 survey data by the American Hospital Association, more than 50% of hospitals report sending structured summary-of-care records electronically to long-term care settings; a little less than half of those hospitals (23% of the total sample of hospitals) were also receiving information electronically from long-term care sites.1
“This bidirectional exchange, in particular, can make it easier to share information across provider organizations electronically and, in turn, improve care delivery,” says Ms. Cross, who authored an accepted paper on the subject in the Journal of Post-Acute and Long-Term Medicine.
6. Hospitalists can work with providers in PAC settings to improve transitions.
Despite improvements in the electronic transfer of medical information, gaps still exist and can cause problems. One chasm when discharging patients to a PAC facility, is when a hospital IT system is incapable of communicating with the PAC facility system. In this instance, Dr. Nazir says, the hospitalist “can help bridge the gap.”
“[We] can verbally relay relevant information to physicians at PAC facilities so they understand the patient’s status, needs, and expectations,” he says. “Furthermore, hospitalists and a PAC facility’s administration can brainstorm methods to improve the systems of care so the patient receives more effective and timely care.”
7. Hospitalists switching to the PAC setting should have formal training.
The two main obstacles for hospitalists who change from working in a hospital to a PAC facility are the lack of exposure to PAC work in training and the assumption that it requires the same skills sets of a typical hospitalist, according to Manoj K. Mathew, MD, SFHM, national medical director of Los Angeles–based Agilon Health. The PAC setting has quite a number of differences compared with a hospital setting. For example, some regulations apply specifically to PAC facilities. In addition to formal training, hospitalists can benefit from using SHM’s Post-Acute Care Transitions Toolkit, having a mentor, or using resources from other organizations that function in this space such as The Society for Post-Acute and Long-Term Care Medicine, Dr. Nazir says.
8. A variety of payors and payment models are in play.
Commercial insurers continue to be major payors for PAC, especially for individuals younger than 65 years. Medicare and Medicaid, administered by the Centers for Medicare & Medicaid Services, are the primary payors for patients aged 65 years and older.
“These scorecards are using a variety of criteria to rank providers, such as length of stay, cost, readmissions to hospitals, and quality.”
Because Medicare Part A covers many patients discharged to a PAC setting, any changes in payment incentives or benefit structures by the Medicare program will drive changes in PAC.

“For example, as Medicare implements payment adjustments for hospitals that have high rates of readmissions, hospitals have a new incentive to work closely with SNFs and other providers of PAC to ensure patients can avoid unnecessary readmissions,” says Tiffany A. Radcliff, PhD, a health economist and associate professor in the department of health policy and management at Texas A&M University School of Public Health in College Station.
Providers must follow the billing rules for each payor. The rules for Medicare payments are outlined on CMS’ website. Bundled payments for PAC under the Medicare Part A program are scheduled to be implemented by 2018.
Reference
Cross DA, Adler-Milstein J. Investing in post-acute care transitions: electronic information exchange between hospitals and long-term care facilities [published online ahead of print Sept. 14, 2016]. JAMDA. doi: http://dx.doi.org/10.1016/j.jamda.2016.07.024.
VIDEO: Health law changes under new administration
WASHINGTON – A new president is taking office along with new staffers to lead the country’s top health care agencies.
In this video, Joyce Hall, chair of the American Bar Association Health Law Section, discusses what changes she foresees in health law issues under the new administration and what to expect from the leadership transition. Ms. Hall also speaks on potential alterations to the Medicare Access and CHIP Reauthorization Act of 2015 and whether the potential appointment of Rep. Tom Price (R-Ga.) as U.S. Department of Health and Human Services Secretary will be positive or negative for health care providers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
WASHINGTON – A new president is taking office along with new staffers to lead the country’s top health care agencies.
In this video, Joyce Hall, chair of the American Bar Association Health Law Section, discusses what changes she foresees in health law issues under the new administration and what to expect from the leadership transition. Ms. Hall also speaks on potential alterations to the Medicare Access and CHIP Reauthorization Act of 2015 and whether the potential appointment of Rep. Tom Price (R-Ga.) as U.S. Department of Health and Human Services Secretary will be positive or negative for health care providers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
WASHINGTON – A new president is taking office along with new staffers to lead the country’s top health care agencies.
In this video, Joyce Hall, chair of the American Bar Association Health Law Section, discusses what changes she foresees in health law issues under the new administration and what to expect from the leadership transition. Ms. Hall also speaks on potential alterations to the Medicare Access and CHIP Reauthorization Act of 2015 and whether the potential appointment of Rep. Tom Price (R-Ga.) as U.S. Department of Health and Human Services Secretary will be positive or negative for health care providers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
AT THE WASHINGTON HEALTH LAW SUMMIT
Echocardiography can benefit use of stented bovine graft for MVR in infants
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Preoperative echocardiography may help guide placement of modified stented jugular vein grafts in infants and small children with hypoplastic mitral and aortic valves.
Major finding: Echocardiography showed that a ratio of the narrowest subaortic region in systole to the actual mitral valve dimension of less than 0.5 was associated with postoperative left ventricular outflow tract obstruction.
Data source: Single-center, retrospective review of 24 patients who underwent mitral valve replacement with modified stented jugular vein grafts from March 2010 to March 2015.
Disclosures: Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
AGA comment on ABIM announcement
For more than a year, AGA has pushed the American Board of Internal Medicine (ABIM) to eliminate high-stakes testing and reform the maintenance of certification (MOC) system into one that’s personalized and reflective of the realities of practice.
ABIM’s listening tour is over. In December 2016, they announced the addition of an option for a 2-year “knowledge check-in.” Although ABIM can point to nominal progress by making the assessment available outside its testing centers, they have not addressed cost, personalization, or the impact on patient care of such assessments.
Despite AGA’s diligent efforts to co-create a new MOC process – which included creating G-APP, constant communication, and participation in numerous summits – ABIM deemed AGA’s approach to be inconsistent with its own philosophy. Nonetheless, we are still in the midst of an evolution. AGA will continue to work with our sister GI and internal medicine societies to bring about change that supports meaningful lifelong learning through the least intrusive means possible.
In the meantime, if your professional situation requires you to maintain certification, please visit ABIM’s blog for more information. AGA tools such as the Digestive Diseases Self-Education Program® can help you prepare.
Visit http://www.gastro.org/career-center/maintenance-of-certification for the latest updates and information on MOC.
For more than a year, AGA has pushed the American Board of Internal Medicine (ABIM) to eliminate high-stakes testing and reform the maintenance of certification (MOC) system into one that’s personalized and reflective of the realities of practice.
ABIM’s listening tour is over. In December 2016, they announced the addition of an option for a 2-year “knowledge check-in.” Although ABIM can point to nominal progress by making the assessment available outside its testing centers, they have not addressed cost, personalization, or the impact on patient care of such assessments.
Despite AGA’s diligent efforts to co-create a new MOC process – which included creating G-APP, constant communication, and participation in numerous summits – ABIM deemed AGA’s approach to be inconsistent with its own philosophy. Nonetheless, we are still in the midst of an evolution. AGA will continue to work with our sister GI and internal medicine societies to bring about change that supports meaningful lifelong learning through the least intrusive means possible.
In the meantime, if your professional situation requires you to maintain certification, please visit ABIM’s blog for more information. AGA tools such as the Digestive Diseases Self-Education Program® can help you prepare.
Visit http://www.gastro.org/career-center/maintenance-of-certification for the latest updates and information on MOC.
For more than a year, AGA has pushed the American Board of Internal Medicine (ABIM) to eliminate high-stakes testing and reform the maintenance of certification (MOC) system into one that’s personalized and reflective of the realities of practice.
ABIM’s listening tour is over. In December 2016, they announced the addition of an option for a 2-year “knowledge check-in.” Although ABIM can point to nominal progress by making the assessment available outside its testing centers, they have not addressed cost, personalization, or the impact on patient care of such assessments.
Despite AGA’s diligent efforts to co-create a new MOC process – which included creating G-APP, constant communication, and participation in numerous summits – ABIM deemed AGA’s approach to be inconsistent with its own philosophy. Nonetheless, we are still in the midst of an evolution. AGA will continue to work with our sister GI and internal medicine societies to bring about change that supports meaningful lifelong learning through the least intrusive means possible.
In the meantime, if your professional situation requires you to maintain certification, please visit ABIM’s blog for more information. AGA tools such as the Digestive Diseases Self-Education Program® can help you prepare.
Visit http://www.gastro.org/career-center/maintenance-of-certification for the latest updates and information on MOC.
Access our MACRA resource collection
Prepare for 2017 with AGA’s Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) resources, which are available in the AGA Community resource library. This includes webinars, a tip sheet, and discussion threads.
The webinars and discussions in the community are available to members only, and contain information on the following topics:
- Intro to MACRA.
- 2016 PQRS Quality Reporting through the AGA Digestive Health Recognition Program (DHRP).
- Preparing for MIPS.
The materials were collected from a series of webinars and eQ&As in December, when topic experts presented a series of webinars on relevant MACRA protocols to help clinicians prepare for Medicare changes starting this year.
Each webinar preceded an Ask the Expert session in the AGA Community forum. Members brought their wide range of questions to the forum, including discussions about MACRA basics, as well as meticulous situation-based recording scenarios.
This members-only library can be accessed at community.gastro.org/MACRA. For more information, including a timeline, downloadable guides, and the latest MACRA news, visit gastro.org/MACRA.
Prepare for 2017 with AGA’s Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) resources, which are available in the AGA Community resource library. This includes webinars, a tip sheet, and discussion threads.
The webinars and discussions in the community are available to members only, and contain information on the following topics:
- Intro to MACRA.
- 2016 PQRS Quality Reporting through the AGA Digestive Health Recognition Program (DHRP).
- Preparing for MIPS.
The materials were collected from a series of webinars and eQ&As in December, when topic experts presented a series of webinars on relevant MACRA protocols to help clinicians prepare for Medicare changes starting this year.
Each webinar preceded an Ask the Expert session in the AGA Community forum. Members brought their wide range of questions to the forum, including discussions about MACRA basics, as well as meticulous situation-based recording scenarios.
This members-only library can be accessed at community.gastro.org/MACRA. For more information, including a timeline, downloadable guides, and the latest MACRA news, visit gastro.org/MACRA.
Prepare for 2017 with AGA’s Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) resources, which are available in the AGA Community resource library. This includes webinars, a tip sheet, and discussion threads.
The webinars and discussions in the community are available to members only, and contain information on the following topics:
- Intro to MACRA.
- 2016 PQRS Quality Reporting through the AGA Digestive Health Recognition Program (DHRP).
- Preparing for MIPS.
The materials were collected from a series of webinars and eQ&As in December, when topic experts presented a series of webinars on relevant MACRA protocols to help clinicians prepare for Medicare changes starting this year.
Each webinar preceded an Ask the Expert session in the AGA Community forum. Members brought their wide range of questions to the forum, including discussions about MACRA basics, as well as meticulous situation-based recording scenarios.
This members-only library can be accessed at community.gastro.org/MACRA. For more information, including a timeline, downloadable guides, and the latest MACRA news, visit gastro.org/MACRA.
Register for DDW® before the early-bird deadline
Registration for AGA members opened Jan. 11, and general registration opened on Jan. 18. Register by March 22 to save at least $80; registration is complimentary up until this date for member trainees, students, and postdoctoral fellows.
Why attend DDW?
DDW is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
For more information regarding why you should attend, what’s included in registration and more, visit www.ddw.org.
Registration for AGA members opened Jan. 11, and general registration opened on Jan. 18. Register by March 22 to save at least $80; registration is complimentary up until this date for member trainees, students, and postdoctoral fellows.
Why attend DDW?
DDW is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
For more information regarding why you should attend, what’s included in registration and more, visit www.ddw.org.
Registration for AGA members opened Jan. 11, and general registration opened on Jan. 18. Register by March 22 to save at least $80; registration is complimentary up until this date for member trainees, students, and postdoctoral fellows.
Why attend DDW?
DDW is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
For more information regarding why you should attend, what’s included in registration and more, visit www.ddw.org.