User login
Unique microbiota mix found in guts of T1D patients
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
potentially offering early insight into possible links between the disease and gut germs.
The findings by an Italian team don’t confirm any connection between bacteria in the digestive system and diabetes. Still, “this study is probably the best example to date in the literature of inflammatory events happening in the gut that are correlated with type 1 diabetes,” said Aleksandar Kostic, PhD, of the department of microbiology and immunobiology at Harvard Medical School, Boston, who conducts similar research.
At issue: What role, if any, does the gut play in the development of type 1 diabetes (T1D)? Scientists already believe that the gut microbiome directly affects metabolism and the development of type 2 diabetes, according to Dr. Kostic. But T1D is an autoimmune disease, not a metabolic one, he said, “and the mechanisms are very different. For type 1, we don’t know a whole lot. We’re in the very early days.”
Still, “there’s a theory that inflammatory stimulus in the gut that is somehow partially responsible for causing T1D. The idea is that the microbiome is less diverse, which means that it loses its integrity in some way and loses the ability to crowd out inflammatory organisms,” he said in an interview.
For the new study, researchers led by scientists at Milan’s IRCCS San Raffaele Scientific Institute measured inflammation and the microbiome in the duodenal mucosa of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls. They reported their findings online Jan. 19 (J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3222).
The researchers found a unique inflammation profile through an analysis of gene expression in the patients with T1D. They called it a “peculiar signature” that’s notable for increased numbers of infiltration from the monocyte/macrophage lineage.
“In T1D patients, we didn’t observe any correlation between gene expression and [hemoglobin A1c] level, duration of diabetes, presence of secondary complications or the reason that led to endoscopy, indicating that gene expression was not influenced by these variables,” the researchers write.
They also found a “specific microbiota composition” featuring a reduction in the role of Proteobacteria and an increase in Firmicutes; this was unique to the T1D patients. Bacteroidetes “showed a trend to reduction” in both T1D and celiac patients compared to the controls.
“The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in duodenum,” the researchers added.
Elena Barengolts, MD, of the division of endocrinology, diabetes, and metabolism at the University of Illinois, Chicago, who’s familiar with the study, said it appears to be valid. However, the methods used have limited powers to define specific types of bacteria, making it difficult to know if the germs in question are “bad” or “good,” she said in an interview.
For his part, Dr. Kostic said the findings are “really neat” and consistent with previous findings regarding the role of the gut microbiome and T1D. He pointed to a study he led that found less-diverse microbiomes in the guts of Finnish infants with T1D (Cell Host Microbe. 2015 Feb 11;17[2]:260-73).
As a result, the gut microbiome is “functionally capable of doing fewer things, and the community gets overrun by certain pathogens,” he said. “We saw that a lot of organisms were capable of promoting inflammation in the gut.”
Dr. Kostic, Dr. Barengolts, and the study authors report no relevant disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Key clinical point: Patients with type 1 diabetes (T1D) show signs of unique inflammation and microbiota in the duodenal mucosa, compared with controls and celiac patients.
Major finding: T1D patients had a “peculiar” inflammation signature and a unique microbiota composition, and there’s a sign of a link between inflammation and bacteria levels.
Data source: An analysis of 19 patients with T1D, 19 with celiac disease, and 16 healthy controls.
Disclosures: The study was supported by institutional funds, and the authors report no relevant disclosures.
Experts say don’t SPRINT to adopt low blood pressure target
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.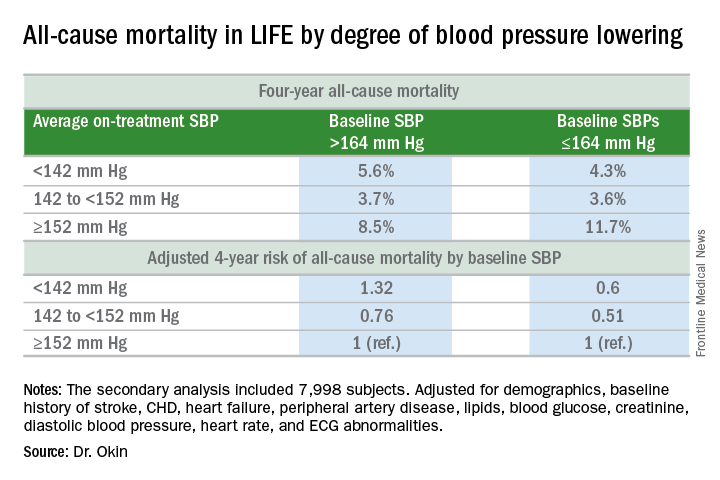
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Deferring RT for brain mets in EGFR-mutated NSCLC shortens survival
Deferring radiotherapy to administer epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Upfront therapy using EGFR TKIs such as erlotinib has been proposed as a way to avoid radiotherapy altogether in this patient population, or to at least defer it and any related toxicities until intracranial disease progresses, said William J. Magnuson, MD, of Yale University, New Haven, Conn., and his associates.
To assess the advantages and disadvantages of upfront EGFR TKIs vs. initial radiotherapy, the researchers pooled survival data for 351 patients treated at six academic medical centers during 2008-2015. A total of 131 (37%) received upfront EGFR TKIs followed by stereotactic radiosurgery or whole-brain radiotherapy when the brain metastases progressed, 120 (34%) received whole-brain radiotherapy followed by EGFR TKIs, and 100 (29%) received stereotactic radiosurgery followed by EGFR TKIs. These patients were followed for a median of 22 months.
Median overall survival was 25 months for upfront EGFR TKIs, compared with 30 months for initial whole-brain radiotherapy and 46 months for initial stereotactic radiosurgery. At 2 years, overall survival rates for the three study groups were 51%, 62%, and 78%, respectively. Both forms of initial radiotherapy were associated with improved overall survival relative to EGFR TKIs, with a hazard ratio of 0.39 for stereotactic radiosurgery and a hazard ratio of 0.70 for whole-brain irradiation.
This survival advantage was even more pronounced in the subgroup of patients who had more favorable prognostic features at baseline. These patients had a median overall survival of 64 months if they received radiotherapy followed by EGFR TKIs, compared with only 32 months if EGFR TKIs were taken before radiotherapy, the investigators said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.69.7144).
These findings have the potential to change clinical practice, but prospective randomized data to confirm the results are urgently needed. “Until such a study is conducted and published, the standard-of-care treatment of newly diagnosed brain metastases should remain stereotactic radiosurgery followed by systemic therapy,” Dr. Magnuson and his associates said.
No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
The findings of Magnuson et al. suggest that initial brain radiotherapy, especially stereotactic radiosurgery, is critical for patients who have EGFR-mutated NSCLC with brain metastases.
However, prospective studies are needed to confirm these results, and outcomes other than survival – including quality of life and neurocognitive function – must be addressed. The authors were unable to assess these outcomes in their pooled retrospective analysis.
In addition, potentially synergetic cognitive toxicities caused by combined or sequential therapies are still unclear, and are especially important for patients who do achieve long-term survival.
Lin Zhou, MD, and associates are at West China Hospital and Sichuan University, Chengdu, China. Dr. Zhou reported having no relevant financial disclosures; one of Dr. Zhou’s associates reported having ties to AstraZeneca. Hoffman-La Roche, Eli Lilly, Pfizer, Elekta, and Varian Medical Systems. Dr. Zhou and associates made these remarks in an editorial accompanying Dr. Magnuson’s report (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.5706).
The findings of Magnuson et al. suggest that initial brain radiotherapy, especially stereotactic radiosurgery, is critical for patients who have EGFR-mutated NSCLC with brain metastases.
However, prospective studies are needed to confirm these results, and outcomes other than survival – including quality of life and neurocognitive function – must be addressed. The authors were unable to assess these outcomes in their pooled retrospective analysis.
In addition, potentially synergetic cognitive toxicities caused by combined or sequential therapies are still unclear, and are especially important for patients who do achieve long-term survival.
Lin Zhou, MD, and associates are at West China Hospital and Sichuan University, Chengdu, China. Dr. Zhou reported having no relevant financial disclosures; one of Dr. Zhou’s associates reported having ties to AstraZeneca. Hoffman-La Roche, Eli Lilly, Pfizer, Elekta, and Varian Medical Systems. Dr. Zhou and associates made these remarks in an editorial accompanying Dr. Magnuson’s report (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.5706).
The findings of Magnuson et al. suggest that initial brain radiotherapy, especially stereotactic radiosurgery, is critical for patients who have EGFR-mutated NSCLC with brain metastases.
However, prospective studies are needed to confirm these results, and outcomes other than survival – including quality of life and neurocognitive function – must be addressed. The authors were unable to assess these outcomes in their pooled retrospective analysis.
In addition, potentially synergetic cognitive toxicities caused by combined or sequential therapies are still unclear, and are especially important for patients who do achieve long-term survival.
Lin Zhou, MD, and associates are at West China Hospital and Sichuan University, Chengdu, China. Dr. Zhou reported having no relevant financial disclosures; one of Dr. Zhou’s associates reported having ties to AstraZeneca. Hoffman-La Roche, Eli Lilly, Pfizer, Elekta, and Varian Medical Systems. Dr. Zhou and associates made these remarks in an editorial accompanying Dr. Magnuson’s report (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.71.5706).
Deferring radiotherapy to administer epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Upfront therapy using EGFR TKIs such as erlotinib has been proposed as a way to avoid radiotherapy altogether in this patient population, or to at least defer it and any related toxicities until intracranial disease progresses, said William J. Magnuson, MD, of Yale University, New Haven, Conn., and his associates.
To assess the advantages and disadvantages of upfront EGFR TKIs vs. initial radiotherapy, the researchers pooled survival data for 351 patients treated at six academic medical centers during 2008-2015. A total of 131 (37%) received upfront EGFR TKIs followed by stereotactic radiosurgery or whole-brain radiotherapy when the brain metastases progressed, 120 (34%) received whole-brain radiotherapy followed by EGFR TKIs, and 100 (29%) received stereotactic radiosurgery followed by EGFR TKIs. These patients were followed for a median of 22 months.
Median overall survival was 25 months for upfront EGFR TKIs, compared with 30 months for initial whole-brain radiotherapy and 46 months for initial stereotactic radiosurgery. At 2 years, overall survival rates for the three study groups were 51%, 62%, and 78%, respectively. Both forms of initial radiotherapy were associated with improved overall survival relative to EGFR TKIs, with a hazard ratio of 0.39 for stereotactic radiosurgery and a hazard ratio of 0.70 for whole-brain irradiation.
This survival advantage was even more pronounced in the subgroup of patients who had more favorable prognostic features at baseline. These patients had a median overall survival of 64 months if they received radiotherapy followed by EGFR TKIs, compared with only 32 months if EGFR TKIs were taken before radiotherapy, the investigators said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.69.7144).
These findings have the potential to change clinical practice, but prospective randomized data to confirm the results are urgently needed. “Until such a study is conducted and published, the standard-of-care treatment of newly diagnosed brain metastases should remain stereotactic radiosurgery followed by systemic therapy,” Dr. Magnuson and his associates said.
No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
Deferring radiotherapy to administer epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Upfront therapy using EGFR TKIs such as erlotinib has been proposed as a way to avoid radiotherapy altogether in this patient population, or to at least defer it and any related toxicities until intracranial disease progresses, said William J. Magnuson, MD, of Yale University, New Haven, Conn., and his associates.
To assess the advantages and disadvantages of upfront EGFR TKIs vs. initial radiotherapy, the researchers pooled survival data for 351 patients treated at six academic medical centers during 2008-2015. A total of 131 (37%) received upfront EGFR TKIs followed by stereotactic radiosurgery or whole-brain radiotherapy when the brain metastases progressed, 120 (34%) received whole-brain radiotherapy followed by EGFR TKIs, and 100 (29%) received stereotactic radiosurgery followed by EGFR TKIs. These patients were followed for a median of 22 months.
Median overall survival was 25 months for upfront EGFR TKIs, compared with 30 months for initial whole-brain radiotherapy and 46 months for initial stereotactic radiosurgery. At 2 years, overall survival rates for the three study groups were 51%, 62%, and 78%, respectively. Both forms of initial radiotherapy were associated with improved overall survival relative to EGFR TKIs, with a hazard ratio of 0.39 for stereotactic radiosurgery and a hazard ratio of 0.70 for whole-brain irradiation.
This survival advantage was even more pronounced in the subgroup of patients who had more favorable prognostic features at baseline. These patients had a median overall survival of 64 months if they received radiotherapy followed by EGFR TKIs, compared with only 32 months if EGFR TKIs were taken before radiotherapy, the investigators said (J Clin Oncol. 2017 Jan 23. doi: 10.1200/JCO.2016.69.7144).
These findings have the potential to change clinical practice, but prospective randomized data to confirm the results are urgently needed. “Until such a study is conducted and published, the standard-of-care treatment of newly diagnosed brain metastases should remain stereotactic radiosurgery followed by systemic therapy,” Dr. Magnuson and his associates said.
No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Deferring radiotherapy to administer EGFR tyrosine kinase inhibitors first doesn’t prolong overall survival, it shortens survival in patients who have brain metastases of EGFR-mutated non–small-cell lung cancer.
Major finding: At 2 years, overall survival rates were 51% with upfront EGFR TKIs, 62% with initial whole-brain radiotherapy, and 78% with stereotactic radiosurgery.
Data source: A retrospective multicenter pooled analysis of survival data for 351 patients with NSCLC brain metastases followed for a median of 22 months.
Disclosures: No funding source was cited for this study. Dr. Magnuson reported having no relevant financial disclosures; his associates reported having ties to numerous industry sources.
Cardiac events after NSCLC radiotherapy occur early
Cardiac events are “relatively common,” affecting 23% of patients, and occur earlier than previously thought following radiotherapy for non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Radiation-associated cardiac toxicity has long been recognized in patients treated for other thoracic cancers, but the conventional wisdom has been that it isn’t a consideration in patients with stage III NSCLC because “there are few long-term survivors to experience toxicity, given the typically long latency of radiotherapy-associated heart injury and the poor prognosis” of this cancer. However, the findings “challenge the perception that minimizing heart dose is not important in the treatment of patients with stage III NSCLC,” said Kyle Wang, MD, of University of North Carolina Hospitals, Chapel Hill, and his associates.
“Our data support minimization of heart radiation exposure whenever possible to doses lower than commonly recommended in patients with stage III NSCLC, to reduce risks of [cardiac] toxicity,” they noted.
Dr. Wang and his associates performed a retrospective post hoc analysis of data pooled from six prospective phase I and II trials that the University of North Carolina was involved in between 1996 and 2009. The studies assessed both dose-escalated radiotherapy and various chemotherapeutic regimens in 112 patients who were followed for a median of 8.8 years (range, 2.3-17.3 years). All the patients received induction chemotherapy, 90% received concurrent chemotherapy, and 25% received consolidation chemotherapy.
A total of 26 patients (23%) had at least one symptomatic cardiac event following radiotherapy: pericardial effusion (7 patients), MI (5 patients), unstable angina (3 patients), pericarditis (2 patients), significant arrhythmia (12 patients), and heart failure (1 patient). After the data were adjusted to account for competing risks of death, the 2-year rate of symptomatic cardiac toxicity was 10% and the 4-year rate was 18%. The first adverse cardiac event occurred at a median of 26 months (range, 1-84 months).
The risk of cardiac toxicities rose with increasing radiation exposure: At 2 years, the rate of cardiac events was 4% for those exposed to less than 10 Gy, 7% for those exposed to 10-20 Gy, and 21% for those exposed to greater than 20 Gy. At 4 years, those rates were 4%, 13%, and 41%, respectively. Patients whose hearts were exposed to greater than 20 Gy had a significantly higher rate of cardiac events than did those exposed to less than 10 Gy (HR, 5.47) or to 10-20 Gy (HR, 2.76).
Even though the prognosis may be poor in patients with stage III NSCLC, “they generally receive higher heart doses and may also have more comorbidities and smoking history, thus increasing risk and perhaps shortening the latency between radiotherapy and resultant heart disease,” Dr. Wang and his associates said (J Clin Oncol. 2017 Jan 23 [doi: 10.1200/JCO.2016.70.0229]).
“In our opinion, tumor coverage should rarely be compromised to meet a heart dose constraint. However, it would be reasonable to try to limit heart mean dose to less than 20 Gy (lower if possible) on the basis of the high event rate we observed in patients exceeding this dose (21% at 2 years and 41% at 4 years). Sophisticated radiation treatment planning techniques (e.g., [intensity-modulated radiation therapy]) and charged-particle therapy with protons or carbon ions may provide increased flexibility to generate more conformal treatment plans and reduce heart dose, which could potentially improve the clinical outcomes in patients with stage III NSCLC,” they added.
This is the first report to clearly associate radiation doses with clinically significant cardiac events in patients with locally advanced NSCLC treated with modern radiotherapy techniques, and it suggests that these events happen much earlier than conventionally believed.
Perhaps cardiac risk didn’t matter so much when, historically, the life expectancy of this patient population was only 2 years. But given the improvements in survival over the last 2 decades, together with the findings of Wang et al., the era of indiscriminate irradiation to the heart should end.
Charles B. Simone II, MD, is at the University of Maryland Medical Center, Baltimore. He reported having no relevant financial disclosures. Dr. Simone made these remarks in an editorial accompanying Dr. Wang’s report (J Clin Oncol. 2017 January 23 [doi: 10.1200/JCO.2016.71.5581]).
This is the first report to clearly associate radiation doses with clinically significant cardiac events in patients with locally advanced NSCLC treated with modern radiotherapy techniques, and it suggests that these events happen much earlier than conventionally believed.
Perhaps cardiac risk didn’t matter so much when, historically, the life expectancy of this patient population was only 2 years. But given the improvements in survival over the last 2 decades, together with the findings of Wang et al., the era of indiscriminate irradiation to the heart should end.
Charles B. Simone II, MD, is at the University of Maryland Medical Center, Baltimore. He reported having no relevant financial disclosures. Dr. Simone made these remarks in an editorial accompanying Dr. Wang’s report (J Clin Oncol. 2017 January 23 [doi: 10.1200/JCO.2016.71.5581]).
This is the first report to clearly associate radiation doses with clinically significant cardiac events in patients with locally advanced NSCLC treated with modern radiotherapy techniques, and it suggests that these events happen much earlier than conventionally believed.
Perhaps cardiac risk didn’t matter so much when, historically, the life expectancy of this patient population was only 2 years. But given the improvements in survival over the last 2 decades, together with the findings of Wang et al., the era of indiscriminate irradiation to the heart should end.
Charles B. Simone II, MD, is at the University of Maryland Medical Center, Baltimore. He reported having no relevant financial disclosures. Dr. Simone made these remarks in an editorial accompanying Dr. Wang’s report (J Clin Oncol. 2017 January 23 [doi: 10.1200/JCO.2016.71.5581]).
Cardiac events are “relatively common,” affecting 23% of patients, and occur earlier than previously thought following radiotherapy for non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Radiation-associated cardiac toxicity has long been recognized in patients treated for other thoracic cancers, but the conventional wisdom has been that it isn’t a consideration in patients with stage III NSCLC because “there are few long-term survivors to experience toxicity, given the typically long latency of radiotherapy-associated heart injury and the poor prognosis” of this cancer. However, the findings “challenge the perception that minimizing heart dose is not important in the treatment of patients with stage III NSCLC,” said Kyle Wang, MD, of University of North Carolina Hospitals, Chapel Hill, and his associates.
“Our data support minimization of heart radiation exposure whenever possible to doses lower than commonly recommended in patients with stage III NSCLC, to reduce risks of [cardiac] toxicity,” they noted.
Dr. Wang and his associates performed a retrospective post hoc analysis of data pooled from six prospective phase I and II trials that the University of North Carolina was involved in between 1996 and 2009. The studies assessed both dose-escalated radiotherapy and various chemotherapeutic regimens in 112 patients who were followed for a median of 8.8 years (range, 2.3-17.3 years). All the patients received induction chemotherapy, 90% received concurrent chemotherapy, and 25% received consolidation chemotherapy.
A total of 26 patients (23%) had at least one symptomatic cardiac event following radiotherapy: pericardial effusion (7 patients), MI (5 patients), unstable angina (3 patients), pericarditis (2 patients), significant arrhythmia (12 patients), and heart failure (1 patient). After the data were adjusted to account for competing risks of death, the 2-year rate of symptomatic cardiac toxicity was 10% and the 4-year rate was 18%. The first adverse cardiac event occurred at a median of 26 months (range, 1-84 months).
The risk of cardiac toxicities rose with increasing radiation exposure: At 2 years, the rate of cardiac events was 4% for those exposed to less than 10 Gy, 7% for those exposed to 10-20 Gy, and 21% for those exposed to greater than 20 Gy. At 4 years, those rates were 4%, 13%, and 41%, respectively. Patients whose hearts were exposed to greater than 20 Gy had a significantly higher rate of cardiac events than did those exposed to less than 10 Gy (HR, 5.47) or to 10-20 Gy (HR, 2.76).
Even though the prognosis may be poor in patients with stage III NSCLC, “they generally receive higher heart doses and may also have more comorbidities and smoking history, thus increasing risk and perhaps shortening the latency between radiotherapy and resultant heart disease,” Dr. Wang and his associates said (J Clin Oncol. 2017 Jan 23 [doi: 10.1200/JCO.2016.70.0229]).
“In our opinion, tumor coverage should rarely be compromised to meet a heart dose constraint. However, it would be reasonable to try to limit heart mean dose to less than 20 Gy (lower if possible) on the basis of the high event rate we observed in patients exceeding this dose (21% at 2 years and 41% at 4 years). Sophisticated radiation treatment planning techniques (e.g., [intensity-modulated radiation therapy]) and charged-particle therapy with protons or carbon ions may provide increased flexibility to generate more conformal treatment plans and reduce heart dose, which could potentially improve the clinical outcomes in patients with stage III NSCLC,” they added.
Cardiac events are “relatively common,” affecting 23% of patients, and occur earlier than previously thought following radiotherapy for non–small-cell lung cancer (NSCLC), according to a report in the Journal of Clinical Oncology.
Radiation-associated cardiac toxicity has long been recognized in patients treated for other thoracic cancers, but the conventional wisdom has been that it isn’t a consideration in patients with stage III NSCLC because “there are few long-term survivors to experience toxicity, given the typically long latency of radiotherapy-associated heart injury and the poor prognosis” of this cancer. However, the findings “challenge the perception that minimizing heart dose is not important in the treatment of patients with stage III NSCLC,” said Kyle Wang, MD, of University of North Carolina Hospitals, Chapel Hill, and his associates.
“Our data support minimization of heart radiation exposure whenever possible to doses lower than commonly recommended in patients with stage III NSCLC, to reduce risks of [cardiac] toxicity,” they noted.
Dr. Wang and his associates performed a retrospective post hoc analysis of data pooled from six prospective phase I and II trials that the University of North Carolina was involved in between 1996 and 2009. The studies assessed both dose-escalated radiotherapy and various chemotherapeutic regimens in 112 patients who were followed for a median of 8.8 years (range, 2.3-17.3 years). All the patients received induction chemotherapy, 90% received concurrent chemotherapy, and 25% received consolidation chemotherapy.
A total of 26 patients (23%) had at least one symptomatic cardiac event following radiotherapy: pericardial effusion (7 patients), MI (5 patients), unstable angina (3 patients), pericarditis (2 patients), significant arrhythmia (12 patients), and heart failure (1 patient). After the data were adjusted to account for competing risks of death, the 2-year rate of symptomatic cardiac toxicity was 10% and the 4-year rate was 18%. The first adverse cardiac event occurred at a median of 26 months (range, 1-84 months).
The risk of cardiac toxicities rose with increasing radiation exposure: At 2 years, the rate of cardiac events was 4% for those exposed to less than 10 Gy, 7% for those exposed to 10-20 Gy, and 21% for those exposed to greater than 20 Gy. At 4 years, those rates were 4%, 13%, and 41%, respectively. Patients whose hearts were exposed to greater than 20 Gy had a significantly higher rate of cardiac events than did those exposed to less than 10 Gy (HR, 5.47) or to 10-20 Gy (HR, 2.76).
Even though the prognosis may be poor in patients with stage III NSCLC, “they generally receive higher heart doses and may also have more comorbidities and smoking history, thus increasing risk and perhaps shortening the latency between radiotherapy and resultant heart disease,” Dr. Wang and his associates said (J Clin Oncol. 2017 Jan 23 [doi: 10.1200/JCO.2016.70.0229]).
“In our opinion, tumor coverage should rarely be compromised to meet a heart dose constraint. However, it would be reasonable to try to limit heart mean dose to less than 20 Gy (lower if possible) on the basis of the high event rate we observed in patients exceeding this dose (21% at 2 years and 41% at 4 years). Sophisticated radiation treatment planning techniques (e.g., [intensity-modulated radiation therapy]) and charged-particle therapy with protons or carbon ions may provide increased flexibility to generate more conformal treatment plans and reduce heart dose, which could potentially improve the clinical outcomes in patients with stage III NSCLC,” they added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Cardiac events are “relatively common” and occur earlier than previously thought following radiotherapy for NSCLC.
Major finding: Following radiotherapy, 26 of 112 patients (23%) had at least one symptomatic cardiac event: pericardial effusion (7 patients), MI (5 patients), unstable angina (3 patients), pericarditis (2 patients), significant arrhythmia (12 patients), and heart failure (1 patient).
Data source: A retrospective post hoc analysis of data from six phase I and II trials involving 112 patients who received radiotherapy for stage-III NSCLC during 1996-2009.
Disclosures: This study was supported in part by the National Institutes of Health. Dr. Wang reported having no relevant financial disclosures; his associates disclosed ties to La Jolla Pharmaceutical, Vision RT, Medtronic, Novartis, Elekta, Morphomics, Accuray, and Varian Medical Systems.
Judge blocks Aetna-Humana merger
A federal judge has blocked a megamerger between health insurance giants Aetna and Humana, ruling that the consolidation would violate antitrust laws and reduce competition.
In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. The court was unpersuaded that the efficiencies generated by the merger would be “sufficient to mitigate the anticompetitive effects for consumers in the challenged markets,” Judge Bates said in his opinion.
The U.S. Department of Justice, which challenged the merger, called the ruling a victory for patients, particularly seniors and working families.
“Competition spurs health insurers to offer higher quality and more affordable health insurance to seniors who choose Medicare Advantage plans and to low-income families and individuals who purchase insurance from public exchanges,” Brent Snyder, deputy assistant attorney general, said in a statement. “Aetna attempted to buy a formidable rival, Humana, instead of competing independently to win customers. Millions of consumers have benefited from competition between Aetna and Humana, and will continue to benefit because of today’s decision to block this merger.”
The Department of Justice, eight states, and the District of Columbia sued Aetna and Humana after an investigation into their proposed July 2015 merger. The government argued that the companies compete head-to-head in the Medicare Advantage and public exchange markets, and that such competition would be lost following the merger to the significant detriment of patients. Aetna and Humana argued their proposed merger would not substantially lessen competition because of the government’s regulatory authority over Medicare Advantage, the threat of entry by new competitors, and the defendants’ proposed divestiture of a portion of their Medicare Advantage business to insurer Molina Healthcare. The insurers also asserted that no current competition between the two companies exists in the 17 complaint counties because Aetna has decided not to compete in those counties in 2017. Judge Bates disagreed.
“The merger would likely substantially lessen competition in the market for individual Medicare Advantage in all 364 complaint counties,” Judge Bates said in his opinion. “This conclusion is based on identification of the proper product market, the overwhelming market concentration figures generated by the merger, and the considerable evidence of valuable head-to-head competition between Aetna and Humana, which the merger would eliminate. The companies’ rebuttal arguments are unpersuasive.”
The American Medical Association praised the ruling.
“Elderly patients were the big winners today as a federal court imposed an injunction on Aetna’s $37 billion acquisition of Humana,” AMA president Andrew W. Gurman, MD, said in a statement. “Aetna’s strategy to eliminate head-to-head competition with rival Humana posed a clear and present threat to the quality, accessibility, and affordability of health care for millions of seniors. The AMA applauds the extraordinarily well-documented, comprehensive, fact-based ruling of U.S. District Judge John D. Bates, which acknowledged that meaningful action was needed to preserve competition and protect high-quality medical care from unprecedented market power that Aetna would acquire from the merger deal.”
Another ruling is expected soon in the $48 billion planned merger between Anthem and Cigna, which is also being challenged by the Justice department. A trial in that case wrapped up in late December in front of U.S. District Court for the District of Columbia Judge Amy Berman.
[email protected]
On Twitter @legal_med
A federal judge has blocked a megamerger between health insurance giants Aetna and Humana, ruling that the consolidation would violate antitrust laws and reduce competition.
In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. The court was unpersuaded that the efficiencies generated by the merger would be “sufficient to mitigate the anticompetitive effects for consumers in the challenged markets,” Judge Bates said in his opinion.
The U.S. Department of Justice, which challenged the merger, called the ruling a victory for patients, particularly seniors and working families.
“Competition spurs health insurers to offer higher quality and more affordable health insurance to seniors who choose Medicare Advantage plans and to low-income families and individuals who purchase insurance from public exchanges,” Brent Snyder, deputy assistant attorney general, said in a statement. “Aetna attempted to buy a formidable rival, Humana, instead of competing independently to win customers. Millions of consumers have benefited from competition between Aetna and Humana, and will continue to benefit because of today’s decision to block this merger.”
The Department of Justice, eight states, and the District of Columbia sued Aetna and Humana after an investigation into their proposed July 2015 merger. The government argued that the companies compete head-to-head in the Medicare Advantage and public exchange markets, and that such competition would be lost following the merger to the significant detriment of patients. Aetna and Humana argued their proposed merger would not substantially lessen competition because of the government’s regulatory authority over Medicare Advantage, the threat of entry by new competitors, and the defendants’ proposed divestiture of a portion of their Medicare Advantage business to insurer Molina Healthcare. The insurers also asserted that no current competition between the two companies exists in the 17 complaint counties because Aetna has decided not to compete in those counties in 2017. Judge Bates disagreed.
“The merger would likely substantially lessen competition in the market for individual Medicare Advantage in all 364 complaint counties,” Judge Bates said in his opinion. “This conclusion is based on identification of the proper product market, the overwhelming market concentration figures generated by the merger, and the considerable evidence of valuable head-to-head competition between Aetna and Humana, which the merger would eliminate. The companies’ rebuttal arguments are unpersuasive.”
The American Medical Association praised the ruling.
“Elderly patients were the big winners today as a federal court imposed an injunction on Aetna’s $37 billion acquisition of Humana,” AMA president Andrew W. Gurman, MD, said in a statement. “Aetna’s strategy to eliminate head-to-head competition with rival Humana posed a clear and present threat to the quality, accessibility, and affordability of health care for millions of seniors. The AMA applauds the extraordinarily well-documented, comprehensive, fact-based ruling of U.S. District Judge John D. Bates, which acknowledged that meaningful action was needed to preserve competition and protect high-quality medical care from unprecedented market power that Aetna would acquire from the merger deal.”
Another ruling is expected soon in the $48 billion planned merger between Anthem and Cigna, which is also being challenged by the Justice department. A trial in that case wrapped up in late December in front of U.S. District Court for the District of Columbia Judge Amy Berman.
[email protected]
On Twitter @legal_med
A federal judge has blocked a megamerger between health insurance giants Aetna and Humana, ruling that the consolidation would violate antitrust laws and reduce competition.
In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. The court was unpersuaded that the efficiencies generated by the merger would be “sufficient to mitigate the anticompetitive effects for consumers in the challenged markets,” Judge Bates said in his opinion.
The U.S. Department of Justice, which challenged the merger, called the ruling a victory for patients, particularly seniors and working families.
“Competition spurs health insurers to offer higher quality and more affordable health insurance to seniors who choose Medicare Advantage plans and to low-income families and individuals who purchase insurance from public exchanges,” Brent Snyder, deputy assistant attorney general, said in a statement. “Aetna attempted to buy a formidable rival, Humana, instead of competing independently to win customers. Millions of consumers have benefited from competition between Aetna and Humana, and will continue to benefit because of today’s decision to block this merger.”
The Department of Justice, eight states, and the District of Columbia sued Aetna and Humana after an investigation into their proposed July 2015 merger. The government argued that the companies compete head-to-head in the Medicare Advantage and public exchange markets, and that such competition would be lost following the merger to the significant detriment of patients. Aetna and Humana argued their proposed merger would not substantially lessen competition because of the government’s regulatory authority over Medicare Advantage, the threat of entry by new competitors, and the defendants’ proposed divestiture of a portion of their Medicare Advantage business to insurer Molina Healthcare. The insurers also asserted that no current competition between the two companies exists in the 17 complaint counties because Aetna has decided not to compete in those counties in 2017. Judge Bates disagreed.
“The merger would likely substantially lessen competition in the market for individual Medicare Advantage in all 364 complaint counties,” Judge Bates said in his opinion. “This conclusion is based on identification of the proper product market, the overwhelming market concentration figures generated by the merger, and the considerable evidence of valuable head-to-head competition between Aetna and Humana, which the merger would eliminate. The companies’ rebuttal arguments are unpersuasive.”
The American Medical Association praised the ruling.
“Elderly patients were the big winners today as a federal court imposed an injunction on Aetna’s $37 billion acquisition of Humana,” AMA president Andrew W. Gurman, MD, said in a statement. “Aetna’s strategy to eliminate head-to-head competition with rival Humana posed a clear and present threat to the quality, accessibility, and affordability of health care for millions of seniors. The AMA applauds the extraordinarily well-documented, comprehensive, fact-based ruling of U.S. District Judge John D. Bates, which acknowledged that meaningful action was needed to preserve competition and protect high-quality medical care from unprecedented market power that Aetna would acquire from the merger deal.”
Another ruling is expected soon in the $48 billion planned merger between Anthem and Cigna, which is also being challenged by the Justice department. A trial in that case wrapped up in late December in front of U.S. District Court for the District of Columbia Judge Amy Berman.
[email protected]
On Twitter @legal_med
AAP: Teen access to abortion care is a right
.
“Genuine concern for the best interests of minors argues strongly against mandatory parental consent and notification laws,” the statement authors wrote in the updated policy statement, “The Adolescent’s Right to Confidential Care When Considering Abortion,” published online in Pediatrics.
The AAP Committee on Adolescence, which wrote the policy statement, encourages adolescents to voluntarily involve their parents – or other adults they trust – in decisions surrounding an unintended pregnancy, stating that teens who do will “likely benefit from adult experience, wisdom, emotional support, and financial support.” However, the policy statement also stresses that legally emphasizing parental involvement over a teen’s autonomy can result in barriers to care when timely access is most crucial, especially if a teen is reluctant to tell her parents of the situation (Pediatrics. 2017. doi: 10.1542/peds.2016-3861).
Currently, 37 states require some level of parental involvement in an adolescent’s decision to pursue an abortion. Most of these states will allow a minor to terminate a pregnancy without parental consent in the case of a medical emergency; about half waive the parental involvement requirement when there is evidence of incest, abuse, or neglect. All states with parental involvement laws also have a so-called judicial bypass, allowing a minor to obtain an abortion with a court’s approval; however, because the process can take as long as several weeks, access to medical treatment can be delayed, upping the risk of complications from later-term abortions. Data cited in the statement indicate that following the enactment of parental involvement laws in three states, second-trimester abortion rates increased by as much as 21% (N Engl J Med. 2006;354[10]:1031-8; Fam Plann Perspect. 1995;27[3]:120-2; Women Health. 1995;22[3]:47-58.
Even when a judicial bypass is obtained, a study of 12,000 such petitions obtained in Minnesota and Massachusetts showed that only 21 of them were denied, and half of those were overturned, meaning the outcome was the same, but the potential risks of delaying care were higher (J Adolesc Health. 1991;12[2]:143-7). The AAP statement suggests physicians learn their state requirements for judicial bypass, if any.
The updated statement also refutes the notion that parental involvement laws improve communication within families and lead to better health outcomes for teens facing an unintended pregnancy. Instead, the statement says that on average, minors who discuss pregnancy termination with their parents do so at the same rate as in states with and without such laws, and that a teen is more likely to involve her parents than not, a likelihood that increases the younger a teen is. When she doesn’t choose to discuss it with parents, fear of some sort of danger such as an escalation in any ongoing family tensions, coercion into a decision, or abuse of some kind is often why (Contraception. 2010 Oct;82[4]:310-3; Fam Plann Perspect. 1992 Jul-Aug;24[4]:148-54, 173).
“In a perfect world of butterflies and unicorns, parents and kids have a perfect relationship, but we know that’s not always true. There can be significant discord in families, and parental notification could result in harm to the adolescent, or risk to the family’s tapestry with significant consequences for the family and the adolescent,” Dr. Breuner said.
Dr. Beers argued that while she believed most parents would rather be the one their teen turns to for advice in such a situation, it’s not possible to legislate trust within families, each of which has its own history and unique family culture and style of communication. “This is the type of conversation that doesn’t begin with the acute event. This is the kind of conversation that should begin [at] a very early age between a parent and a child,” she said.
The physician’s role, according to Dr. Beers, is to ensure parents and their children have “a shared understanding of the facts to [rely on] when they talk about what is important to their family and what their family values are.”
The updated statement, originally issued in 1993, roughly coincides with the release of a new Guttmacher Institute report indicating that abortion rates in the United States are at their lowest since passage of Roe v. Wade in 1973. The report credits lower unintended pregnancy rates and better access to contraception, not restrictive abortion legislation, for the drop. The AAP’s updated statement also comes within days of the inauguration of President Donald Trump, whose run-up to election featured rhetoric challenging the status quo of federal abortion laws.
Dr. Breuner said the timing was a coincidence, and that plans to reissue the statement began over 3 years ago as she and her colleagues drew up plans to recommit their membership to protecting the reproductive rights of their patients. “With all of the regulations, restrictions, and decreased access to [abortion] care that are occurring, the academy wanted members to know just how much more difficult it is to obtain an abortion,” she said, noting that access and confidentiality are not the same. “If you can’t get an abortion, what difference does confidentiality make?”
Last year, the Supreme Court reversed a law that would have greatly limited access to abortion in Texas by closing all but nine clinics statewide, burdening them with care for tens of thousands of women annually. Also in 2016, citing a Constitutional violation of a woman’s right to privacy, a federal judge blocked an Indiana law signed by then governor and now vice president Mike Pence, that restricted the reasons a woman could cite to seek an abortion.
Dr. Breuner views this as an erosion of the rights of all women to comprehensive reproductive care, including those of adolescents. “You can’t ‘Google Map’ how to figure this out anymore. The roads are closed and all the ways people say you can get there don’t really lead you there,” she said, noting that the AAP’s statement is in line with several other professional medical societies, including the American College of Obstetricians and Gynecologists and the American Medical Association.
Regardless of timing, the statement should be viewed not through a political – or even partisan – lens, but as a reaffirmation of “core values,” according to Dr. Beers. “We do live in divided times. I think it’s important to circle back to our core values as pediatricians. That’s the agenda – making sure adolescents are healthy, safe, and supported.”
[email protected]
On Twitter @whitneymcknight
.
“Genuine concern for the best interests of minors argues strongly against mandatory parental consent and notification laws,” the statement authors wrote in the updated policy statement, “The Adolescent’s Right to Confidential Care When Considering Abortion,” published online in Pediatrics.
The AAP Committee on Adolescence, which wrote the policy statement, encourages adolescents to voluntarily involve their parents – or other adults they trust – in decisions surrounding an unintended pregnancy, stating that teens who do will “likely benefit from adult experience, wisdom, emotional support, and financial support.” However, the policy statement also stresses that legally emphasizing parental involvement over a teen’s autonomy can result in barriers to care when timely access is most crucial, especially if a teen is reluctant to tell her parents of the situation (Pediatrics. 2017. doi: 10.1542/peds.2016-3861).
Currently, 37 states require some level of parental involvement in an adolescent’s decision to pursue an abortion. Most of these states will allow a minor to terminate a pregnancy without parental consent in the case of a medical emergency; about half waive the parental involvement requirement when there is evidence of incest, abuse, or neglect. All states with parental involvement laws also have a so-called judicial bypass, allowing a minor to obtain an abortion with a court’s approval; however, because the process can take as long as several weeks, access to medical treatment can be delayed, upping the risk of complications from later-term abortions. Data cited in the statement indicate that following the enactment of parental involvement laws in three states, second-trimester abortion rates increased by as much as 21% (N Engl J Med. 2006;354[10]:1031-8; Fam Plann Perspect. 1995;27[3]:120-2; Women Health. 1995;22[3]:47-58.
Even when a judicial bypass is obtained, a study of 12,000 such petitions obtained in Minnesota and Massachusetts showed that only 21 of them were denied, and half of those were overturned, meaning the outcome was the same, but the potential risks of delaying care were higher (J Adolesc Health. 1991;12[2]:143-7). The AAP statement suggests physicians learn their state requirements for judicial bypass, if any.
The updated statement also refutes the notion that parental involvement laws improve communication within families and lead to better health outcomes for teens facing an unintended pregnancy. Instead, the statement says that on average, minors who discuss pregnancy termination with their parents do so at the same rate as in states with and without such laws, and that a teen is more likely to involve her parents than not, a likelihood that increases the younger a teen is. When she doesn’t choose to discuss it with parents, fear of some sort of danger such as an escalation in any ongoing family tensions, coercion into a decision, or abuse of some kind is often why (Contraception. 2010 Oct;82[4]:310-3; Fam Plann Perspect. 1992 Jul-Aug;24[4]:148-54, 173).
“In a perfect world of butterflies and unicorns, parents and kids have a perfect relationship, but we know that’s not always true. There can be significant discord in families, and parental notification could result in harm to the adolescent, or risk to the family’s tapestry with significant consequences for the family and the adolescent,” Dr. Breuner said.
Dr. Beers argued that while she believed most parents would rather be the one their teen turns to for advice in such a situation, it’s not possible to legislate trust within families, each of which has its own history and unique family culture and style of communication. “This is the type of conversation that doesn’t begin with the acute event. This is the kind of conversation that should begin [at] a very early age between a parent and a child,” she said.
The physician’s role, according to Dr. Beers, is to ensure parents and their children have “a shared understanding of the facts to [rely on] when they talk about what is important to their family and what their family values are.”
The updated statement, originally issued in 1993, roughly coincides with the release of a new Guttmacher Institute report indicating that abortion rates in the United States are at their lowest since passage of Roe v. Wade in 1973. The report credits lower unintended pregnancy rates and better access to contraception, not restrictive abortion legislation, for the drop. The AAP’s updated statement also comes within days of the inauguration of President Donald Trump, whose run-up to election featured rhetoric challenging the status quo of federal abortion laws.
Dr. Breuner said the timing was a coincidence, and that plans to reissue the statement began over 3 years ago as she and her colleagues drew up plans to recommit their membership to protecting the reproductive rights of their patients. “With all of the regulations, restrictions, and decreased access to [abortion] care that are occurring, the academy wanted members to know just how much more difficult it is to obtain an abortion,” she said, noting that access and confidentiality are not the same. “If you can’t get an abortion, what difference does confidentiality make?”
Last year, the Supreme Court reversed a law that would have greatly limited access to abortion in Texas by closing all but nine clinics statewide, burdening them with care for tens of thousands of women annually. Also in 2016, citing a Constitutional violation of a woman’s right to privacy, a federal judge blocked an Indiana law signed by then governor and now vice president Mike Pence, that restricted the reasons a woman could cite to seek an abortion.
Dr. Breuner views this as an erosion of the rights of all women to comprehensive reproductive care, including those of adolescents. “You can’t ‘Google Map’ how to figure this out anymore. The roads are closed and all the ways people say you can get there don’t really lead you there,” she said, noting that the AAP’s statement is in line with several other professional medical societies, including the American College of Obstetricians and Gynecologists and the American Medical Association.
Regardless of timing, the statement should be viewed not through a political – or even partisan – lens, but as a reaffirmation of “core values,” according to Dr. Beers. “We do live in divided times. I think it’s important to circle back to our core values as pediatricians. That’s the agenda – making sure adolescents are healthy, safe, and supported.”
[email protected]
On Twitter @whitneymcknight
.
“Genuine concern for the best interests of minors argues strongly against mandatory parental consent and notification laws,” the statement authors wrote in the updated policy statement, “The Adolescent’s Right to Confidential Care When Considering Abortion,” published online in Pediatrics.
The AAP Committee on Adolescence, which wrote the policy statement, encourages adolescents to voluntarily involve their parents – or other adults they trust – in decisions surrounding an unintended pregnancy, stating that teens who do will “likely benefit from adult experience, wisdom, emotional support, and financial support.” However, the policy statement also stresses that legally emphasizing parental involvement over a teen’s autonomy can result in barriers to care when timely access is most crucial, especially if a teen is reluctant to tell her parents of the situation (Pediatrics. 2017. doi: 10.1542/peds.2016-3861).
Currently, 37 states require some level of parental involvement in an adolescent’s decision to pursue an abortion. Most of these states will allow a minor to terminate a pregnancy without parental consent in the case of a medical emergency; about half waive the parental involvement requirement when there is evidence of incest, abuse, or neglect. All states with parental involvement laws also have a so-called judicial bypass, allowing a minor to obtain an abortion with a court’s approval; however, because the process can take as long as several weeks, access to medical treatment can be delayed, upping the risk of complications from later-term abortions. Data cited in the statement indicate that following the enactment of parental involvement laws in three states, second-trimester abortion rates increased by as much as 21% (N Engl J Med. 2006;354[10]:1031-8; Fam Plann Perspect. 1995;27[3]:120-2; Women Health. 1995;22[3]:47-58.
Even when a judicial bypass is obtained, a study of 12,000 such petitions obtained in Minnesota and Massachusetts showed that only 21 of them were denied, and half of those were overturned, meaning the outcome was the same, but the potential risks of delaying care were higher (J Adolesc Health. 1991;12[2]:143-7). The AAP statement suggests physicians learn their state requirements for judicial bypass, if any.
The updated statement also refutes the notion that parental involvement laws improve communication within families and lead to better health outcomes for teens facing an unintended pregnancy. Instead, the statement says that on average, minors who discuss pregnancy termination with their parents do so at the same rate as in states with and without such laws, and that a teen is more likely to involve her parents than not, a likelihood that increases the younger a teen is. When she doesn’t choose to discuss it with parents, fear of some sort of danger such as an escalation in any ongoing family tensions, coercion into a decision, or abuse of some kind is often why (Contraception. 2010 Oct;82[4]:310-3; Fam Plann Perspect. 1992 Jul-Aug;24[4]:148-54, 173).
“In a perfect world of butterflies and unicorns, parents and kids have a perfect relationship, but we know that’s not always true. There can be significant discord in families, and parental notification could result in harm to the adolescent, or risk to the family’s tapestry with significant consequences for the family and the adolescent,” Dr. Breuner said.
Dr. Beers argued that while she believed most parents would rather be the one their teen turns to for advice in such a situation, it’s not possible to legislate trust within families, each of which has its own history and unique family culture and style of communication. “This is the type of conversation that doesn’t begin with the acute event. This is the kind of conversation that should begin [at] a very early age between a parent and a child,” she said.
The physician’s role, according to Dr. Beers, is to ensure parents and their children have “a shared understanding of the facts to [rely on] when they talk about what is important to their family and what their family values are.”
The updated statement, originally issued in 1993, roughly coincides with the release of a new Guttmacher Institute report indicating that abortion rates in the United States are at their lowest since passage of Roe v. Wade in 1973. The report credits lower unintended pregnancy rates and better access to contraception, not restrictive abortion legislation, for the drop. The AAP’s updated statement also comes within days of the inauguration of President Donald Trump, whose run-up to election featured rhetoric challenging the status quo of federal abortion laws.
Dr. Breuner said the timing was a coincidence, and that plans to reissue the statement began over 3 years ago as she and her colleagues drew up plans to recommit their membership to protecting the reproductive rights of their patients. “With all of the regulations, restrictions, and decreased access to [abortion] care that are occurring, the academy wanted members to know just how much more difficult it is to obtain an abortion,” she said, noting that access and confidentiality are not the same. “If you can’t get an abortion, what difference does confidentiality make?”
Last year, the Supreme Court reversed a law that would have greatly limited access to abortion in Texas by closing all but nine clinics statewide, burdening them with care for tens of thousands of women annually. Also in 2016, citing a Constitutional violation of a woman’s right to privacy, a federal judge blocked an Indiana law signed by then governor and now vice president Mike Pence, that restricted the reasons a woman could cite to seek an abortion.
Dr. Breuner views this as an erosion of the rights of all women to comprehensive reproductive care, including those of adolescents. “You can’t ‘Google Map’ how to figure this out anymore. The roads are closed and all the ways people say you can get there don’t really lead you there,” she said, noting that the AAP’s statement is in line with several other professional medical societies, including the American College of Obstetricians and Gynecologists and the American Medical Association.
Regardless of timing, the statement should be viewed not through a political – or even partisan – lens, but as a reaffirmation of “core values,” according to Dr. Beers. “We do live in divided times. I think it’s important to circle back to our core values as pediatricians. That’s the agenda – making sure adolescents are healthy, safe, and supported.”
[email protected]
On Twitter @whitneymcknight
Symptoms don’t worsen during pregnancy in most PsA patients
Psoriatic arthritis disease activity tended to improve or stabilize during pregnancy and the first year after giving birth, even outpacing controls, in a small retrospective cohort study.
“The outcome of pregnancy in PsA [psoriatic arthritis] patients is excellent,” investigators from the University of Toronto Psoriatic Arthritis Program reported. They found that “arthritis activity has a favorable course during pregnancy in almost 60% of the pregnancies, while the skin disease shows a favorable course in close to 90% of the pregnancies.”
According to the researchers, led by Ari Polachek, MD, of the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital, previous research has found that rheumatoid arthritis improves in most pregnant women with the condition but then worsens in the year after birth, while most patients with ankylosing spondylitis don’t get better or worse. Limited research suggests psoriasis tends to improve during pregnancy and then flares afterward.
For the new study, researchers tracked 29 pregnant PsA patients with 42 total pregnancies who had a mean age of about 34 years at the beginning of pregnancy and matched them with 67 control patients with PsA who were not pregnant and had an average age of about 35 years. They had all visited the University of Toronto Psoriatic Arthritis Clinic during 1990-2015 (Semin Arthritis Rheum. 2017 Jan 16. doi: 10.1016/j.semarthrit.2017.01.002).
Among the 41 pregnancies in women who began follow-up prior to pregnancy, 13 (32%) had an unfavorable course of disease activity marked by worsening during 8 (20%) of the pregnancies or stable high disease activity during 5 (12%). The course of disease activity was more favorable for 24 (59%) pregnancies, in which it improved in 11 (27%) and stayed stable at a low disease level in 13 (32%). Four pregnancies (10%) had a mixed pattern of improvement followed by worsening.
The 1-year postpartum period during 40 pregnancies showed that stable or worsening symptoms were most common: 8 (20%) had improvement and 13 (32.5%) had stable low disease activity, while another 16 (4%) worsened and 3 (8%) had a mixed course of improvement followed by worsening.
After they controlled the results to account for various factors, the researchers found that pregnancy appeared to be especially beneficial for skin-related PsA symptoms. The likelihood of improved skin activity during pregnancy rose significantly (odds ratio, 7.7; 95% confidence interval, 1.8-23.5; P = .004) when compared with a matched period among controls, but this was no longer the case during the year after birth (OR, 0.4; 95% CI, 0.2-1.4; P = .60).
The likelihood of improved joint symptoms during pregnancy also rose, but not to a significant extent (OR, 2.1; 95% CI, 0.8-5.1; P = .10), and the effect on joints was not significant in the year after pregnancy (OR, 1.4; 95% CI, 0.5-3.4; P = .50).
There was a declining use of medications among the patients during pregnancy, particularly in the second and third trimesters, but two-thirds of the patients were treated with medications for PsA during this time, including NSAIDs (41%), disease-modifying antirheumatic drugs (35%), and biologic agents (26%).
The researchers speculated that hormonal changes during pregnancy may explain the improvement in skin activity. However, they noted that women with PsA who have multiple pregnancies don’t tend to have the same experiences each time: “Most of the women with more than one pregnancy had different joint disease course during their own different pregnancies and postpartum periods. Accordingly, this suggests that each woman needs specific evaluation and treatment adjustment during each pregnancy.”
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. Dr. Polachek is supported by an educational grant from Janssen Canada.
Psoriatic arthritis disease activity tended to improve or stabilize during pregnancy and the first year after giving birth, even outpacing controls, in a small retrospective cohort study.
“The outcome of pregnancy in PsA [psoriatic arthritis] patients is excellent,” investigators from the University of Toronto Psoriatic Arthritis Program reported. They found that “arthritis activity has a favorable course during pregnancy in almost 60% of the pregnancies, while the skin disease shows a favorable course in close to 90% of the pregnancies.”
According to the researchers, led by Ari Polachek, MD, of the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital, previous research has found that rheumatoid arthritis improves in most pregnant women with the condition but then worsens in the year after birth, while most patients with ankylosing spondylitis don’t get better or worse. Limited research suggests psoriasis tends to improve during pregnancy and then flares afterward.
For the new study, researchers tracked 29 pregnant PsA patients with 42 total pregnancies who had a mean age of about 34 years at the beginning of pregnancy and matched them with 67 control patients with PsA who were not pregnant and had an average age of about 35 years. They had all visited the University of Toronto Psoriatic Arthritis Clinic during 1990-2015 (Semin Arthritis Rheum. 2017 Jan 16. doi: 10.1016/j.semarthrit.2017.01.002).
Among the 41 pregnancies in women who began follow-up prior to pregnancy, 13 (32%) had an unfavorable course of disease activity marked by worsening during 8 (20%) of the pregnancies or stable high disease activity during 5 (12%). The course of disease activity was more favorable for 24 (59%) pregnancies, in which it improved in 11 (27%) and stayed stable at a low disease level in 13 (32%). Four pregnancies (10%) had a mixed pattern of improvement followed by worsening.
The 1-year postpartum period during 40 pregnancies showed that stable or worsening symptoms were most common: 8 (20%) had improvement and 13 (32.5%) had stable low disease activity, while another 16 (4%) worsened and 3 (8%) had a mixed course of improvement followed by worsening.
After they controlled the results to account for various factors, the researchers found that pregnancy appeared to be especially beneficial for skin-related PsA symptoms. The likelihood of improved skin activity during pregnancy rose significantly (odds ratio, 7.7; 95% confidence interval, 1.8-23.5; P = .004) when compared with a matched period among controls, but this was no longer the case during the year after birth (OR, 0.4; 95% CI, 0.2-1.4; P = .60).
The likelihood of improved joint symptoms during pregnancy also rose, but not to a significant extent (OR, 2.1; 95% CI, 0.8-5.1; P = .10), and the effect on joints was not significant in the year after pregnancy (OR, 1.4; 95% CI, 0.5-3.4; P = .50).
There was a declining use of medications among the patients during pregnancy, particularly in the second and third trimesters, but two-thirds of the patients were treated with medications for PsA during this time, including NSAIDs (41%), disease-modifying antirheumatic drugs (35%), and biologic agents (26%).
The researchers speculated that hormonal changes during pregnancy may explain the improvement in skin activity. However, they noted that women with PsA who have multiple pregnancies don’t tend to have the same experiences each time: “Most of the women with more than one pregnancy had different joint disease course during their own different pregnancies and postpartum periods. Accordingly, this suggests that each woman needs specific evaluation and treatment adjustment during each pregnancy.”
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. Dr. Polachek is supported by an educational grant from Janssen Canada.
Psoriatic arthritis disease activity tended to improve or stabilize during pregnancy and the first year after giving birth, even outpacing controls, in a small retrospective cohort study.
“The outcome of pregnancy in PsA [psoriatic arthritis] patients is excellent,” investigators from the University of Toronto Psoriatic Arthritis Program reported. They found that “arthritis activity has a favorable course during pregnancy in almost 60% of the pregnancies, while the skin disease shows a favorable course in close to 90% of the pregnancies.”
According to the researchers, led by Ari Polachek, MD, of the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital, previous research has found that rheumatoid arthritis improves in most pregnant women with the condition but then worsens in the year after birth, while most patients with ankylosing spondylitis don’t get better or worse. Limited research suggests psoriasis tends to improve during pregnancy and then flares afterward.
For the new study, researchers tracked 29 pregnant PsA patients with 42 total pregnancies who had a mean age of about 34 years at the beginning of pregnancy and matched them with 67 control patients with PsA who were not pregnant and had an average age of about 35 years. They had all visited the University of Toronto Psoriatic Arthritis Clinic during 1990-2015 (Semin Arthritis Rheum. 2017 Jan 16. doi: 10.1016/j.semarthrit.2017.01.002).
Among the 41 pregnancies in women who began follow-up prior to pregnancy, 13 (32%) had an unfavorable course of disease activity marked by worsening during 8 (20%) of the pregnancies or stable high disease activity during 5 (12%). The course of disease activity was more favorable for 24 (59%) pregnancies, in which it improved in 11 (27%) and stayed stable at a low disease level in 13 (32%). Four pregnancies (10%) had a mixed pattern of improvement followed by worsening.
The 1-year postpartum period during 40 pregnancies showed that stable or worsening symptoms were most common: 8 (20%) had improvement and 13 (32.5%) had stable low disease activity, while another 16 (4%) worsened and 3 (8%) had a mixed course of improvement followed by worsening.
After they controlled the results to account for various factors, the researchers found that pregnancy appeared to be especially beneficial for skin-related PsA symptoms. The likelihood of improved skin activity during pregnancy rose significantly (odds ratio, 7.7; 95% confidence interval, 1.8-23.5; P = .004) when compared with a matched period among controls, but this was no longer the case during the year after birth (OR, 0.4; 95% CI, 0.2-1.4; P = .60).
The likelihood of improved joint symptoms during pregnancy also rose, but not to a significant extent (OR, 2.1; 95% CI, 0.8-5.1; P = .10), and the effect on joints was not significant in the year after pregnancy (OR, 1.4; 95% CI, 0.5-3.4; P = .50).
There was a declining use of medications among the patients during pregnancy, particularly in the second and third trimesters, but two-thirds of the patients were treated with medications for PsA during this time, including NSAIDs (41%), disease-modifying antirheumatic drugs (35%), and biologic agents (26%).
The researchers speculated that hormonal changes during pregnancy may explain the improvement in skin activity. However, they noted that women with PsA who have multiple pregnancies don’t tend to have the same experiences each time: “Most of the women with more than one pregnancy had different joint disease course during their own different pregnancies and postpartum periods. Accordingly, this suggests that each woman needs specific evaluation and treatment adjustment during each pregnancy.”
The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. Dr. Polachek is supported by an educational grant from Janssen Canada.
Key clinical point:
Major finding: PsA symptoms improved in 27% of pregnancies, stabilized at a low level in 32%, and worsened in 20%.
Data source: 29 pregnant PsA patients with 42 total pregnancies and 67 non-pregnant PsA patients.
Disclosures: The University of Toronto Psoriatic Arthritis Program is supported by a grant from the Krembil Foundation. Dr. Polachek is supported by an educational grant from Janssen Canada.
Lyme arthritis changes its immune response profile if it persists after antibiotics
The persistence of Lyme arthritis after treatment with antibiotics comes with a shift from an immune response expected for bacterial infection to one characterized by chronic inflammation, synovial proliferation, and breakdown of wound repair processes, according to an analysis of microRNA expression in patients before, during, and after infection.
Based on the findings, investigators led by Robert B. Lochhead, PhD, of Massachusetts General Hospital, Boston, said that they “suspect that, in humans, genetic variables determine whether the response to B. burgdorferi infection elicits an appropriate wound repair response … or a maladaptive inflammatory cellular response and arrest of wound repair processes.”
The investigators studied synovial fluid or tissue from 32 patients with LA who were representative of the spectrum of disease severity and treatment responses seen in this disease. In 18 patients for whom synovial fluid samples were available, 5 illustrated the differences in miRNA expression that occur from the time before any antibiotic treatment to the time after antibiotic treatment that successfully resolves arthritis symptoms, and 13 showed how an incomplete response to antibiotic treatment can affect miRNA expression. The expression profile of miRNA in synovial tissue samples from another 14 patients who underwent arthroscopic synovectomies 4-48 months after oral and intravenous antibiotics demonstrated the change in immune response seen in postinfectious LA.
The group of five patients with synovial fluid samples who were referred prior to antibiotic therapy when they had active B. burgdorferi infection (group 1) had a history of mild to severe knee swelling and pain for a median duration of 1 month prior to evaluation and the start of antibiotic treatment. A 1-month course of oral doxycycline resolved arthritis in three of the patients, and the other two continued to have marked knee swelling that later resolved after 1 month of IV ceftriaxone. Of six different miRNAs that the investigators measured, five were at low levels in these patients. The lone exception was a hematopoietic-specific miRNA, miR-223, which is abundantly expressed in polymorphonuclear lymphocytes (PMNs) and is associated with downregulation of acute inflammation and tissue remodeling.
In the group of 13 who still had joint swelling despite oral or IV antibiotics (group 2), only 2 had resolution of their swelling after IV antibiotics. Most of the remaining 11 had successful treatment with methotrexate. The synovial fluid samples for seven patients were collected after oral antibiotics but before IV antibiotics, and in the other six the samples were collected after both therapies. These 13 patients had significantly lower white blood cell counts in synovial fluid, fewer PMNs, and greater percentages of lymphocytes and monocytes than did group 1 patients. The five miRNAs that were found at low levels among the patients in group 1 were higher in these patients, which “suggested that the nature of the arthritis had changed after spirochetal killing.” The higher miR-223 levels seen in group 1 also occurred in group 2.
A separate analysis of synovial fluid samples from four patients with osteoarthritis and six patients with RA showed that levels of the six miRNAs from RA patients were similar to those of patients from group 2, and the low levels that were observed for most of the miRNAs in group 1 were similar to those seen in OA patients.
The investigators found that the five miRNAs with elevated levels in group 2 patients, but not miR-223, were positively correlated with arthritis duration after start of oral antibiotic therapy. B. burgdorferi IgG antibody titers negatively correlated with some of the elevated miRNAs in group 2.
The investigators analyzed a larger set of miRNAs in the synovial tissue samples obtained from 14 patients who underwent synovectomies for treatment of persistent synovitis a median of 15.5 months after they had undergone 2-3 months of antibiotic therapy (group 3). Some of the miRNAs overexpressed in these postinfectious LA samples, relative to samples from five OA patients, were associated with proliferative, tumor-associated responses and regulation of inflammatory processes. However, miRNAs overexpressed in tissues from OA patients relative to postinfectious LA patients were thought to affect genes that control tissue remodeling and cell proliferation, but not inflammation. The “distinct oncogenic miRNA profile” exhibited by postinfectious synovial tissue, according to the investigators, showed that “in these patients, the transition to the postinfectious phase was blocked by chronic inflammation, which stalled the wound repair process.”
The investigators concluded that “miRNAs hold promise as potential biomarkers to identify LA patients who are developing maladaptive immune responses during the period of infection. In such patients, it will be important to learn whether simultaneous treatment with antibiotics and DMARDs, rather than sequential treatment with these medications, will reduce the period of therapy and improve outcome, creating a new paradigm in treatment of this form of chronic inflammatory arthritis.”
The study was supported by grants from the National Institutes of Health, the Lucius N. Littauer Foundation, the Roland Foundation, the Lyme Disease and Arthritis Research Fund at Massachusetts General Hospital, the Rheumatology Research Foundation, and the English, Bonter, Mitchell Foundation.
The persistence of Lyme arthritis after treatment with antibiotics comes with a shift from an immune response expected for bacterial infection to one characterized by chronic inflammation, synovial proliferation, and breakdown of wound repair processes, according to an analysis of microRNA expression in patients before, during, and after infection.
Based on the findings, investigators led by Robert B. Lochhead, PhD, of Massachusetts General Hospital, Boston, said that they “suspect that, in humans, genetic variables determine whether the response to B. burgdorferi infection elicits an appropriate wound repair response … or a maladaptive inflammatory cellular response and arrest of wound repair processes.”
The investigators studied synovial fluid or tissue from 32 patients with LA who were representative of the spectrum of disease severity and treatment responses seen in this disease. In 18 patients for whom synovial fluid samples were available, 5 illustrated the differences in miRNA expression that occur from the time before any antibiotic treatment to the time after antibiotic treatment that successfully resolves arthritis symptoms, and 13 showed how an incomplete response to antibiotic treatment can affect miRNA expression. The expression profile of miRNA in synovial tissue samples from another 14 patients who underwent arthroscopic synovectomies 4-48 months after oral and intravenous antibiotics demonstrated the change in immune response seen in postinfectious LA.
The group of five patients with synovial fluid samples who were referred prior to antibiotic therapy when they had active B. burgdorferi infection (group 1) had a history of mild to severe knee swelling and pain for a median duration of 1 month prior to evaluation and the start of antibiotic treatment. A 1-month course of oral doxycycline resolved arthritis in three of the patients, and the other two continued to have marked knee swelling that later resolved after 1 month of IV ceftriaxone. Of six different miRNAs that the investigators measured, five were at low levels in these patients. The lone exception was a hematopoietic-specific miRNA, miR-223, which is abundantly expressed in polymorphonuclear lymphocytes (PMNs) and is associated with downregulation of acute inflammation and tissue remodeling.
In the group of 13 who still had joint swelling despite oral or IV antibiotics (group 2), only 2 had resolution of their swelling after IV antibiotics. Most of the remaining 11 had successful treatment with methotrexate. The synovial fluid samples for seven patients were collected after oral antibiotics but before IV antibiotics, and in the other six the samples were collected after both therapies. These 13 patients had significantly lower white blood cell counts in synovial fluid, fewer PMNs, and greater percentages of lymphocytes and monocytes than did group 1 patients. The five miRNAs that were found at low levels among the patients in group 1 were higher in these patients, which “suggested that the nature of the arthritis had changed after spirochetal killing.” The higher miR-223 levels seen in group 1 also occurred in group 2.
A separate analysis of synovial fluid samples from four patients with osteoarthritis and six patients with RA showed that levels of the six miRNAs from RA patients were similar to those of patients from group 2, and the low levels that were observed for most of the miRNAs in group 1 were similar to those seen in OA patients.
The investigators found that the five miRNAs with elevated levels in group 2 patients, but not miR-223, were positively correlated with arthritis duration after start of oral antibiotic therapy. B. burgdorferi IgG antibody titers negatively correlated with some of the elevated miRNAs in group 2.
The investigators analyzed a larger set of miRNAs in the synovial tissue samples obtained from 14 patients who underwent synovectomies for treatment of persistent synovitis a median of 15.5 months after they had undergone 2-3 months of antibiotic therapy (group 3). Some of the miRNAs overexpressed in these postinfectious LA samples, relative to samples from five OA patients, were associated with proliferative, tumor-associated responses and regulation of inflammatory processes. However, miRNAs overexpressed in tissues from OA patients relative to postinfectious LA patients were thought to affect genes that control tissue remodeling and cell proliferation, but not inflammation. The “distinct oncogenic miRNA profile” exhibited by postinfectious synovial tissue, according to the investigators, showed that “in these patients, the transition to the postinfectious phase was blocked by chronic inflammation, which stalled the wound repair process.”
The investigators concluded that “miRNAs hold promise as potential biomarkers to identify LA patients who are developing maladaptive immune responses during the period of infection. In such patients, it will be important to learn whether simultaneous treatment with antibiotics and DMARDs, rather than sequential treatment with these medications, will reduce the period of therapy and improve outcome, creating a new paradigm in treatment of this form of chronic inflammatory arthritis.”
The study was supported by grants from the National Institutes of Health, the Lucius N. Littauer Foundation, the Roland Foundation, the Lyme Disease and Arthritis Research Fund at Massachusetts General Hospital, the Rheumatology Research Foundation, and the English, Bonter, Mitchell Foundation.
The persistence of Lyme arthritis after treatment with antibiotics comes with a shift from an immune response expected for bacterial infection to one characterized by chronic inflammation, synovial proliferation, and breakdown of wound repair processes, according to an analysis of microRNA expression in patients before, during, and after infection.
Based on the findings, investigators led by Robert B. Lochhead, PhD, of Massachusetts General Hospital, Boston, said that they “suspect that, in humans, genetic variables determine whether the response to B. burgdorferi infection elicits an appropriate wound repair response … or a maladaptive inflammatory cellular response and arrest of wound repair processes.”
The investigators studied synovial fluid or tissue from 32 patients with LA who were representative of the spectrum of disease severity and treatment responses seen in this disease. In 18 patients for whom synovial fluid samples were available, 5 illustrated the differences in miRNA expression that occur from the time before any antibiotic treatment to the time after antibiotic treatment that successfully resolves arthritis symptoms, and 13 showed how an incomplete response to antibiotic treatment can affect miRNA expression. The expression profile of miRNA in synovial tissue samples from another 14 patients who underwent arthroscopic synovectomies 4-48 months after oral and intravenous antibiotics demonstrated the change in immune response seen in postinfectious LA.
The group of five patients with synovial fluid samples who were referred prior to antibiotic therapy when they had active B. burgdorferi infection (group 1) had a history of mild to severe knee swelling and pain for a median duration of 1 month prior to evaluation and the start of antibiotic treatment. A 1-month course of oral doxycycline resolved arthritis in three of the patients, and the other two continued to have marked knee swelling that later resolved after 1 month of IV ceftriaxone. Of six different miRNAs that the investigators measured, five were at low levels in these patients. The lone exception was a hematopoietic-specific miRNA, miR-223, which is abundantly expressed in polymorphonuclear lymphocytes (PMNs) and is associated with downregulation of acute inflammation and tissue remodeling.
In the group of 13 who still had joint swelling despite oral or IV antibiotics (group 2), only 2 had resolution of their swelling after IV antibiotics. Most of the remaining 11 had successful treatment with methotrexate. The synovial fluid samples for seven patients were collected after oral antibiotics but before IV antibiotics, and in the other six the samples were collected after both therapies. These 13 patients had significantly lower white blood cell counts in synovial fluid, fewer PMNs, and greater percentages of lymphocytes and monocytes than did group 1 patients. The five miRNAs that were found at low levels among the patients in group 1 were higher in these patients, which “suggested that the nature of the arthritis had changed after spirochetal killing.” The higher miR-223 levels seen in group 1 also occurred in group 2.
A separate analysis of synovial fluid samples from four patients with osteoarthritis and six patients with RA showed that levels of the six miRNAs from RA patients were similar to those of patients from group 2, and the low levels that were observed for most of the miRNAs in group 1 were similar to those seen in OA patients.
The investigators found that the five miRNAs with elevated levels in group 2 patients, but not miR-223, were positively correlated with arthritis duration after start of oral antibiotic therapy. B. burgdorferi IgG antibody titers negatively correlated with some of the elevated miRNAs in group 2.
The investigators analyzed a larger set of miRNAs in the synovial tissue samples obtained from 14 patients who underwent synovectomies for treatment of persistent synovitis a median of 15.5 months after they had undergone 2-3 months of antibiotic therapy (group 3). Some of the miRNAs overexpressed in these postinfectious LA samples, relative to samples from five OA patients, were associated with proliferative, tumor-associated responses and regulation of inflammatory processes. However, miRNAs overexpressed in tissues from OA patients relative to postinfectious LA patients were thought to affect genes that control tissue remodeling and cell proliferation, but not inflammation. The “distinct oncogenic miRNA profile” exhibited by postinfectious synovial tissue, according to the investigators, showed that “in these patients, the transition to the postinfectious phase was blocked by chronic inflammation, which stalled the wound repair process.”
The investigators concluded that “miRNAs hold promise as potential biomarkers to identify LA patients who are developing maladaptive immune responses during the period of infection. In such patients, it will be important to learn whether simultaneous treatment with antibiotics and DMARDs, rather than sequential treatment with these medications, will reduce the period of therapy and improve outcome, creating a new paradigm in treatment of this form of chronic inflammatory arthritis.”
The study was supported by grants from the National Institutes of Health, the Lucius N. Littauer Foundation, the Roland Foundation, the Lyme Disease and Arthritis Research Fund at Massachusetts General Hospital, the Rheumatology Research Foundation, and the English, Bonter, Mitchell Foundation.
Key clinical point:
Major finding: miRNAs overexpressed in synovial tissue samples from 14 patients with postinfectious Lyme arthritis, relative to samples from five OA patients, were associated with proliferative, tumor-associated responses and regulation of inflammatory processes.
Data source: A retrospective study of synovial fluid or tissue samples from 32 patients with Lyme arthritis.
Disclosures: The study was supported by grants from the National Institutes of Health, the Lucius N. Littauer Foundation, the Roland Foundation, the Lyme Disease and Arthritis Research Fund at Massachusetts General Hospital, the Rheumatology Research Foundation, and the English, Bonter, Mitchell Foundation.
Ohio progestogen program reduced early preterm births
A statewide progestogen promotion program that aimed to reduce early premature births in Ohio by 10% is exceeding its goals, thanks to a joint effort of maternity hospitals and clinics, Ohio’s Medicaid program, Medicaid insurers, and service agencies.
The organizations joined forces via the Ohio Perinatal Quality Collaborative (PQC) beginning in 2014, and by February 2016 a sustained reduction in singleton births before 32 weeks of gestation was evident. The reduction was particularly pronounced among women with a prior preterm birth, African American women, and women on Medicaid, with reductions of 20.5%, 20.3%, and 17.1%, respectively, according to Jay D. Iams, MD, the obstetrics lead for the collaborative and emeritus professor at Ohio State University; Mary S. Applegate, MD, medical director for the Ohio Department of Medicaid; and their colleagues.
What was the key driver of their success? A collaborative effort among local- and system-level organizations and individuals to overcome the numerous barriers to providing the preventive, highly effective progestogen treatments to at-risk women, according to Dr. Iams and Dr. Applegate.
“Ohio has one of the worst rates of infant mortality and a high rate of premature birth – especially very early premature birth [before 32 weeks],” Dr. Iams said in an interview.
Those very early births account for more than half of infants who die before their first birthday, so while 13% may seem like a small number, it has the potential to have a very large effect on long-term health and infant mortality, he said.
The Ohio program was developed in the wake of practice guidelines from the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists, both issued in 2012, on the use of progestogens to reduce the incidence of preterm birth (Am J Obstet Gynecol 2012;206:376-86 and Obstet Gynecol 2012;120:964-73).
Large, high-quality, randomized placebo-controlled trials supported the use of 17-alpha hydroxyprogesterone caproate (17-p) injections or progesterone administered vaginally, and the Ohio Department of Medicaid and Ohio Department of Health asked the Ohio PQC – a volunteer network of stakeholders dedicated to improving perinatal health outcomes – to design a statewide quality improvement project to promote progestogen prescribing for eligible women.
To facilitate identification and management of women at risk for preterm birth, clinicians were asked to notify the Ohio Medicaid agency when a patient became pregnant so that a care manager could be assigned to help remove barriers to care.
“It turned out that piece of it was key,” she said. “It was the communication between these two, and then just dealing with whatever the issues were that actually made the difference.”
Care managers helped the patients navigate the system by notifying the county to make sure they didn’t “fall off Medicaid,” by addressing transportation issues to ensure patients could get to their weekly treatment visits, and by addressing cultural issues, for instance.
“It never really occurred to clinicians that Medicaid could actually be helpful in finding women and actually doing the right thing for them,” Dr. Applegate said, explaining that most only thought of Medicaid as “after the fact ... the doctors do what they need to and afterward we pay claims.”
“So this was kind of a new concept to them,” she added.
In fact, a quality improvement plan was structured on the managed care plan side, just like one was structured on the clinical side, she said. “In essence we have these parallel systems that were actually connecting, identifying barriers as we went,” Dr. Applegate said.
In the article in Obstetrics & Gynecology, the investigators describe the quality improvement processes, including training, evidence reviews, and strategy sharing. Steps toward efficient identification of eligible patients and prescription of progestogens were developed, and participating sites were encouraged to follow them.
At the system level, efforts focused on maintaining the patient’s Medicaid coverage, expanding eligibility for Medicaid, and streamlining forms and processes to improve efficiency and improve data collection.
The effects of these cumulative efforts emerged over time, with the drop in early premature births becoming apparent about 16-18 months after the project started. The investigators reviewed other possible causes for the decrease, such as an increase in the rate of cervical cerclage, but confirmed that the change was likely the result of the progestogen promotion program, Dr. Iams said.
In all, 2,562 women were eligible for progestogen at participating clinics between Jan. 1, 2014, and Nov. 30, 2015. Most (93%) were eligible because of a prior preterm birth, and the remainder had a short cervix on ultrasound. A progestogen was prescribed at or before 20 6/7 weeks of gestation in 64%, and at or before 24 6/7 weeks gestation in 72%. Injections were prescribed in 65%, and vaginal preparations in 30%; 5% were prescribed both or had no documentation of the formulation.
The progestogen program had no effect on the overall rate of births before 37 weeks. “So we can’t say that we changed the prematurity rate, but we changed the rate of births that are most likely to result in infant death and we were pretty excited about that,” Dr. Iams said.
Next steps for the program include expansion to rural areas and development of an electronic notification system to further streamline communication between the various players (pharmacies, insurance companies, Medicaid, etc.). Dr. Applegate said she also hopes to harness the lessons of this program for use in other high-risk scenarios, such as pregnancies complicated by substance abuse.
As for whether other states will follow Ohio’s lead, Dr. Iams said almost all the states are involved in quality improvement efforts, including several with programs similar to Ohio’s.
In fact, the Centers for Disease Control and Prevention is currently providing support to PQCs in California, Illinois, Massachusetts, North Carolina, and New York, in addition to the Ohio PQC, according to Zsakeba Henderson, MD, a medical officer on the CDC division of reproductive health in Atlanta.
“CDC has developed a resource guide to help develop and advance the work of state PQCs, and in collaboration with March of Dimes has spearheaded the development and launch of the National Network of Perinatal Quality Collaboratives,” she said. “This network is a consultative resource for state PQCs, with a mission is to support the development and enhance the ability of state perinatal quality collaboratives to make measurable improvements in statewide maternal and infant health care and health outcomes.”
Dr. Applegate stressed the importance of collaboration in achieving results.
“I think the message is you can’t do it in a silo. It really has taken this collaborative and fairly comprehensive approach to understand not only how complex the system is, but how complicated people’s lives are. You just have to hang in there over lots and lots of weeks to get the outcome you want,” she said, adding that the effort is well worth it.
“These are babies that are born weighing less than a pound and a half. When we’re helping them be born closer to term that changes the next 60 years of their life,” she said. “It totally changes not just that baby’s life, but the hardship that comes to that family as well, so the impact is actually huge.”
A statewide progestogen promotion program that aimed to reduce early premature births in Ohio by 10% is exceeding its goals, thanks to a joint effort of maternity hospitals and clinics, Ohio’s Medicaid program, Medicaid insurers, and service agencies.
The organizations joined forces via the Ohio Perinatal Quality Collaborative (PQC) beginning in 2014, and by February 2016 a sustained reduction in singleton births before 32 weeks of gestation was evident. The reduction was particularly pronounced among women with a prior preterm birth, African American women, and women on Medicaid, with reductions of 20.5%, 20.3%, and 17.1%, respectively, according to Jay D. Iams, MD, the obstetrics lead for the collaborative and emeritus professor at Ohio State University; Mary S. Applegate, MD, medical director for the Ohio Department of Medicaid; and their colleagues.
What was the key driver of their success? A collaborative effort among local- and system-level organizations and individuals to overcome the numerous barriers to providing the preventive, highly effective progestogen treatments to at-risk women, according to Dr. Iams and Dr. Applegate.
“Ohio has one of the worst rates of infant mortality and a high rate of premature birth – especially very early premature birth [before 32 weeks],” Dr. Iams said in an interview.
Those very early births account for more than half of infants who die before their first birthday, so while 13% may seem like a small number, it has the potential to have a very large effect on long-term health and infant mortality, he said.
The Ohio program was developed in the wake of practice guidelines from the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists, both issued in 2012, on the use of progestogens to reduce the incidence of preterm birth (Am J Obstet Gynecol 2012;206:376-86 and Obstet Gynecol 2012;120:964-73).
Large, high-quality, randomized placebo-controlled trials supported the use of 17-alpha hydroxyprogesterone caproate (17-p) injections or progesterone administered vaginally, and the Ohio Department of Medicaid and Ohio Department of Health asked the Ohio PQC – a volunteer network of stakeholders dedicated to improving perinatal health outcomes – to design a statewide quality improvement project to promote progestogen prescribing for eligible women.
To facilitate identification and management of women at risk for preterm birth, clinicians were asked to notify the Ohio Medicaid agency when a patient became pregnant so that a care manager could be assigned to help remove barriers to care.
“It turned out that piece of it was key,” she said. “It was the communication between these two, and then just dealing with whatever the issues were that actually made the difference.”
Care managers helped the patients navigate the system by notifying the county to make sure they didn’t “fall off Medicaid,” by addressing transportation issues to ensure patients could get to their weekly treatment visits, and by addressing cultural issues, for instance.
“It never really occurred to clinicians that Medicaid could actually be helpful in finding women and actually doing the right thing for them,” Dr. Applegate said, explaining that most only thought of Medicaid as “after the fact ... the doctors do what they need to and afterward we pay claims.”
“So this was kind of a new concept to them,” she added.
In fact, a quality improvement plan was structured on the managed care plan side, just like one was structured on the clinical side, she said. “In essence we have these parallel systems that were actually connecting, identifying barriers as we went,” Dr. Applegate said.
In the article in Obstetrics & Gynecology, the investigators describe the quality improvement processes, including training, evidence reviews, and strategy sharing. Steps toward efficient identification of eligible patients and prescription of progestogens were developed, and participating sites were encouraged to follow them.
At the system level, efforts focused on maintaining the patient’s Medicaid coverage, expanding eligibility for Medicaid, and streamlining forms and processes to improve efficiency and improve data collection.
The effects of these cumulative efforts emerged over time, with the drop in early premature births becoming apparent about 16-18 months after the project started. The investigators reviewed other possible causes for the decrease, such as an increase in the rate of cervical cerclage, but confirmed that the change was likely the result of the progestogen promotion program, Dr. Iams said.
In all, 2,562 women were eligible for progestogen at participating clinics between Jan. 1, 2014, and Nov. 30, 2015. Most (93%) were eligible because of a prior preterm birth, and the remainder had a short cervix on ultrasound. A progestogen was prescribed at or before 20 6/7 weeks of gestation in 64%, and at or before 24 6/7 weeks gestation in 72%. Injections were prescribed in 65%, and vaginal preparations in 30%; 5% were prescribed both or had no documentation of the formulation.
The progestogen program had no effect on the overall rate of births before 37 weeks. “So we can’t say that we changed the prematurity rate, but we changed the rate of births that are most likely to result in infant death and we were pretty excited about that,” Dr. Iams said.
Next steps for the program include expansion to rural areas and development of an electronic notification system to further streamline communication between the various players (pharmacies, insurance companies, Medicaid, etc.). Dr. Applegate said she also hopes to harness the lessons of this program for use in other high-risk scenarios, such as pregnancies complicated by substance abuse.
As for whether other states will follow Ohio’s lead, Dr. Iams said almost all the states are involved in quality improvement efforts, including several with programs similar to Ohio’s.
In fact, the Centers for Disease Control and Prevention is currently providing support to PQCs in California, Illinois, Massachusetts, North Carolina, and New York, in addition to the Ohio PQC, according to Zsakeba Henderson, MD, a medical officer on the CDC division of reproductive health in Atlanta.
“CDC has developed a resource guide to help develop and advance the work of state PQCs, and in collaboration with March of Dimes has spearheaded the development and launch of the National Network of Perinatal Quality Collaboratives,” she said. “This network is a consultative resource for state PQCs, with a mission is to support the development and enhance the ability of state perinatal quality collaboratives to make measurable improvements in statewide maternal and infant health care and health outcomes.”
Dr. Applegate stressed the importance of collaboration in achieving results.
“I think the message is you can’t do it in a silo. It really has taken this collaborative and fairly comprehensive approach to understand not only how complex the system is, but how complicated people’s lives are. You just have to hang in there over lots and lots of weeks to get the outcome you want,” she said, adding that the effort is well worth it.
“These are babies that are born weighing less than a pound and a half. When we’re helping them be born closer to term that changes the next 60 years of their life,” she said. “It totally changes not just that baby’s life, but the hardship that comes to that family as well, so the impact is actually huge.”
A statewide progestogen promotion program that aimed to reduce early premature births in Ohio by 10% is exceeding its goals, thanks to a joint effort of maternity hospitals and clinics, Ohio’s Medicaid program, Medicaid insurers, and service agencies.
The organizations joined forces via the Ohio Perinatal Quality Collaborative (PQC) beginning in 2014, and by February 2016 a sustained reduction in singleton births before 32 weeks of gestation was evident. The reduction was particularly pronounced among women with a prior preterm birth, African American women, and women on Medicaid, with reductions of 20.5%, 20.3%, and 17.1%, respectively, according to Jay D. Iams, MD, the obstetrics lead for the collaborative and emeritus professor at Ohio State University; Mary S. Applegate, MD, medical director for the Ohio Department of Medicaid; and their colleagues.
What was the key driver of their success? A collaborative effort among local- and system-level organizations and individuals to overcome the numerous barriers to providing the preventive, highly effective progestogen treatments to at-risk women, according to Dr. Iams and Dr. Applegate.
“Ohio has one of the worst rates of infant mortality and a high rate of premature birth – especially very early premature birth [before 32 weeks],” Dr. Iams said in an interview.
Those very early births account for more than half of infants who die before their first birthday, so while 13% may seem like a small number, it has the potential to have a very large effect on long-term health and infant mortality, he said.
The Ohio program was developed in the wake of practice guidelines from the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists, both issued in 2012, on the use of progestogens to reduce the incidence of preterm birth (Am J Obstet Gynecol 2012;206:376-86 and Obstet Gynecol 2012;120:964-73).
Large, high-quality, randomized placebo-controlled trials supported the use of 17-alpha hydroxyprogesterone caproate (17-p) injections or progesterone administered vaginally, and the Ohio Department of Medicaid and Ohio Department of Health asked the Ohio PQC – a volunteer network of stakeholders dedicated to improving perinatal health outcomes – to design a statewide quality improvement project to promote progestogen prescribing for eligible women.
To facilitate identification and management of women at risk for preterm birth, clinicians were asked to notify the Ohio Medicaid agency when a patient became pregnant so that a care manager could be assigned to help remove barriers to care.
“It turned out that piece of it was key,” she said. “It was the communication between these two, and then just dealing with whatever the issues were that actually made the difference.”
Care managers helped the patients navigate the system by notifying the county to make sure they didn’t “fall off Medicaid,” by addressing transportation issues to ensure patients could get to their weekly treatment visits, and by addressing cultural issues, for instance.
“It never really occurred to clinicians that Medicaid could actually be helpful in finding women and actually doing the right thing for them,” Dr. Applegate said, explaining that most only thought of Medicaid as “after the fact ... the doctors do what they need to and afterward we pay claims.”
“So this was kind of a new concept to them,” she added.
In fact, a quality improvement plan was structured on the managed care plan side, just like one was structured on the clinical side, she said. “In essence we have these parallel systems that were actually connecting, identifying barriers as we went,” Dr. Applegate said.
In the article in Obstetrics & Gynecology, the investigators describe the quality improvement processes, including training, evidence reviews, and strategy sharing. Steps toward efficient identification of eligible patients and prescription of progestogens were developed, and participating sites were encouraged to follow them.
At the system level, efforts focused on maintaining the patient’s Medicaid coverage, expanding eligibility for Medicaid, and streamlining forms and processes to improve efficiency and improve data collection.
The effects of these cumulative efforts emerged over time, with the drop in early premature births becoming apparent about 16-18 months after the project started. The investigators reviewed other possible causes for the decrease, such as an increase in the rate of cervical cerclage, but confirmed that the change was likely the result of the progestogen promotion program, Dr. Iams said.
In all, 2,562 women were eligible for progestogen at participating clinics between Jan. 1, 2014, and Nov. 30, 2015. Most (93%) were eligible because of a prior preterm birth, and the remainder had a short cervix on ultrasound. A progestogen was prescribed at or before 20 6/7 weeks of gestation in 64%, and at or before 24 6/7 weeks gestation in 72%. Injections were prescribed in 65%, and vaginal preparations in 30%; 5% were prescribed both or had no documentation of the formulation.
The progestogen program had no effect on the overall rate of births before 37 weeks. “So we can’t say that we changed the prematurity rate, but we changed the rate of births that are most likely to result in infant death and we were pretty excited about that,” Dr. Iams said.
Next steps for the program include expansion to rural areas and development of an electronic notification system to further streamline communication between the various players (pharmacies, insurance companies, Medicaid, etc.). Dr. Applegate said she also hopes to harness the lessons of this program for use in other high-risk scenarios, such as pregnancies complicated by substance abuse.
As for whether other states will follow Ohio’s lead, Dr. Iams said almost all the states are involved in quality improvement efforts, including several with programs similar to Ohio’s.
In fact, the Centers for Disease Control and Prevention is currently providing support to PQCs in California, Illinois, Massachusetts, North Carolina, and New York, in addition to the Ohio PQC, according to Zsakeba Henderson, MD, a medical officer on the CDC division of reproductive health in Atlanta.
“CDC has developed a resource guide to help develop and advance the work of state PQCs, and in collaboration with March of Dimes has spearheaded the development and launch of the National Network of Perinatal Quality Collaboratives,” she said. “This network is a consultative resource for state PQCs, with a mission is to support the development and enhance the ability of state perinatal quality collaboratives to make measurable improvements in statewide maternal and infant health care and health outcomes.”
Dr. Applegate stressed the importance of collaboration in achieving results.
“I think the message is you can’t do it in a silo. It really has taken this collaborative and fairly comprehensive approach to understand not only how complex the system is, but how complicated people’s lives are. You just have to hang in there over lots and lots of weeks to get the outcome you want,” she said, adding that the effort is well worth it.
“These are babies that are born weighing less than a pound and a half. When we’re helping them be born closer to term that changes the next 60 years of their life,” she said. “It totally changes not just that baby’s life, but the hardship that comes to that family as well, so the impact is actually huge.”
2-week left-sided pelvic pain
(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.
(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.
(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.
(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.

(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.

(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.

(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3

(A) Simple ovarian cyst INCORRECT
Here is an example of a well circumscribed round or oval anechoic, avascular simple ovarian cyst with posterior acoustic enhancement and thin smooth walls.1 No septations or solid components are identified.

(B) Hemorrhagic cyst CORRECT
This type of cyst is well circumscribed and hypoechoic, with posterior acoustic enhancement and demonstrates a lacy reticular pattern of internal echoes due to fibrin strands (long arrow). The internal echoes also may be solid appearing with concave margins (short arrow) due to retractile hemorrhagic clot.1 The absence of internal vascular flow on color Doppler helps differentiate it from the solid components seen in ovarian neoplasm.

(C) Endometrioma INCORRECT
This mass is a well-circumscribed hypoechoic cyst with homogeneous ground glass or low level echoes and increased through transmission.1 It is also avascular without solid components.

(D) Dermoid INCORRECT
This common benign ovarian tumor has varying appearances. The most common appearance is a cystic lesion with a focal echogenic nodule (long arrow) protruding into the cyst (Rokitansky nodule).2 The second most common appearance is a focal or diffuse hyperechoic mass with areas of acoustic shadowing (arrowhead) from the sebaceous material and hair (tip of the iceberg sign). A third common appearance is a cystic lesion with multiple thin echogenic bands (lines and dots), which are hair floating within the cyst (short arrow). There is no internal vascular flow identified.

(E) Cystic ovarian neoplasm INCORRECT
These are large complex masses with both cystic and solid components demonstrating internal vascular flow. They usually demonstrate a thick irregular wall, multiple septations, and nodular papillary projections.3

- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2010;256:(3):943−954.
- Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475−490.
- Wasnik AP, Menias CO, Platt JF, Lalchandani UR, Bedi DG, Elsayes KM. Multimodality imaging of ovarian cystic lesions: review with an imaging based algorithmic approach. World J Radiol. 2013;5(3):113−125.
A 37-year-old woman presents to the emergency department reporting left-sided pelvic pain for 2 weeks duration. She has a negative urine pregnancy test. Pelvic ultrasonography of the left adnexa is performed with gray scale (A) and color Doppler images (B). Figures shown above.















