User login
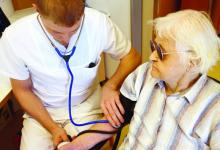
Health Canada expands indication for lenalidomide

Photo courtesy of Celgene
Health Canada has expanded the approved indication for lenalidomide (Revlimid®) to include the treatment of patients with multiple myeloma (MM).
Lenalidomide is now approved for use in combination with dexamethasone to treat patients newly diagnosed with MM who are not eligible for stem cell transplant.
Lenalidomide was previously approved in Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities.
Lenalidomide is a product of Celgene Corporation.
“The expanded indication of Revlimid® provides [MM] patients with a treatment much earlier in their disease and offers this patient population an all-oral, melphalan-free option for a disease that continues to be difficult to treat,” said Donna Reece, MD, of Princess Margaret Hospital in Toronto, Ontario, Canada.
The expanded approval of lenalidomide is based on safety and efficacy results from the phase 3 FIRST trial. Updated results from this study were published in the Journal of Clinical Oncology last November.
The trial included 1623 patients with newly diagnosed MM who were not eligible for stem cell transplant.
Patients were randomized to receive:
- Lenalidomide and low-dose dexamethasone (Rd) in 28-day cycles until disease progression (n=535)
- 18 cycles of Rd (Rd18) for 72 weeks (n=541)
- Melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
In the intent-to-treat population, the overall response rate was 81% for the continuous Rd group, 79% for the Rd18 group, and 67% in the MPT group. The complete response rates were 21%, 20%, and 12%, respectively.
The median progression-free survival (PFS) was 26.0 months in the continuous Rd group, 21.0 months in the Rd18 group, and 21.9 months in the MPT group. At 4 years, the PFS rates were 33%, 14%, and 13%, respectively.
The median overall survival (OS) was 58.9 months in the continuous Rd group, 56.7 months in the Rd18 group, and 48.5 months in the MPT group. At 4 years, the OS rates were 60%, 57%, and 51%, respectively.
The most frequent grade 3/4 hematologic treatment-emergent adverse events were neutropenia and anemia. The rate of grade 3/4 neutropenia was higher in the MPT group than the continuous Rd or Rd18 groups.
Infections were the most common grade 3/4 non-hematologic treatment-emergent adverse events. The rate of grade 3/4 infections was higher in the Rd groups than the MPT group.
“With this new clinical evidence, we know that keeping newly diagnosed multiple myeloma patients on Revlimid® may help delay disease progression and reduce the risk of death,” Dr Reece said. “As such, we are looking forward to having Revlimid® as a key option in the first-line setting for the appropriate patients.” ![]()

Photo courtesy of Celgene
Health Canada has expanded the approved indication for lenalidomide (Revlimid®) to include the treatment of patients with multiple myeloma (MM).
Lenalidomide is now approved for use in combination with dexamethasone to treat patients newly diagnosed with MM who are not eligible for stem cell transplant.
Lenalidomide was previously approved in Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities.
Lenalidomide is a product of Celgene Corporation.
“The expanded indication of Revlimid® provides [MM] patients with a treatment much earlier in their disease and offers this patient population an all-oral, melphalan-free option for a disease that continues to be difficult to treat,” said Donna Reece, MD, of Princess Margaret Hospital in Toronto, Ontario, Canada.
The expanded approval of lenalidomide is based on safety and efficacy results from the phase 3 FIRST trial. Updated results from this study were published in the Journal of Clinical Oncology last November.
The trial included 1623 patients with newly diagnosed MM who were not eligible for stem cell transplant.
Patients were randomized to receive:
- Lenalidomide and low-dose dexamethasone (Rd) in 28-day cycles until disease progression (n=535)
- 18 cycles of Rd (Rd18) for 72 weeks (n=541)
- Melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
In the intent-to-treat population, the overall response rate was 81% for the continuous Rd group, 79% for the Rd18 group, and 67% in the MPT group. The complete response rates were 21%, 20%, and 12%, respectively.
The median progression-free survival (PFS) was 26.0 months in the continuous Rd group, 21.0 months in the Rd18 group, and 21.9 months in the MPT group. At 4 years, the PFS rates were 33%, 14%, and 13%, respectively.
The median overall survival (OS) was 58.9 months in the continuous Rd group, 56.7 months in the Rd18 group, and 48.5 months in the MPT group. At 4 years, the OS rates were 60%, 57%, and 51%, respectively.
The most frequent grade 3/4 hematologic treatment-emergent adverse events were neutropenia and anemia. The rate of grade 3/4 neutropenia was higher in the MPT group than the continuous Rd or Rd18 groups.
Infections were the most common grade 3/4 non-hematologic treatment-emergent adverse events. The rate of grade 3/4 infections was higher in the Rd groups than the MPT group.
“With this new clinical evidence, we know that keeping newly diagnosed multiple myeloma patients on Revlimid® may help delay disease progression and reduce the risk of death,” Dr Reece said. “As such, we are looking forward to having Revlimid® as a key option in the first-line setting for the appropriate patients.” ![]()

Photo courtesy of Celgene
Health Canada has expanded the approved indication for lenalidomide (Revlimid®) to include the treatment of patients with multiple myeloma (MM).
Lenalidomide is now approved for use in combination with dexamethasone to treat patients newly diagnosed with MM who are not eligible for stem cell transplant.
Lenalidomide was previously approved in Canada for the treatment of patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality, with or without additional cytogenetic abnormalities.
Lenalidomide is a product of Celgene Corporation.
“The expanded indication of Revlimid® provides [MM] patients with a treatment much earlier in their disease and offers this patient population an all-oral, melphalan-free option for a disease that continues to be difficult to treat,” said Donna Reece, MD, of Princess Margaret Hospital in Toronto, Ontario, Canada.
The expanded approval of lenalidomide is based on safety and efficacy results from the phase 3 FIRST trial. Updated results from this study were published in the Journal of Clinical Oncology last November.
The trial included 1623 patients with newly diagnosed MM who were not eligible for stem cell transplant.
Patients were randomized to receive:
- Lenalidomide and low-dose dexamethasone (Rd) in 28-day cycles until disease progression (n=535)
- 18 cycles of Rd (Rd18) for 72 weeks (n=541)
- Melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
In the intent-to-treat population, the overall response rate was 81% for the continuous Rd group, 79% for the Rd18 group, and 67% in the MPT group. The complete response rates were 21%, 20%, and 12%, respectively.
The median progression-free survival (PFS) was 26.0 months in the continuous Rd group, 21.0 months in the Rd18 group, and 21.9 months in the MPT group. At 4 years, the PFS rates were 33%, 14%, and 13%, respectively.
The median overall survival (OS) was 58.9 months in the continuous Rd group, 56.7 months in the Rd18 group, and 48.5 months in the MPT group. At 4 years, the OS rates were 60%, 57%, and 51%, respectively.
The most frequent grade 3/4 hematologic treatment-emergent adverse events were neutropenia and anemia. The rate of grade 3/4 neutropenia was higher in the MPT group than the continuous Rd or Rd18 groups.
Infections were the most common grade 3/4 non-hematologic treatment-emergent adverse events. The rate of grade 3/4 infections was higher in the Rd groups than the MPT group.
“With this new clinical evidence, we know that keeping newly diagnosed multiple myeloma patients on Revlimid® may help delay disease progression and reduce the risk of death,” Dr Reece said. “As such, we are looking forward to having Revlimid® as a key option in the first-line setting for the appropriate patients.” ![]()
Do not use steroids in patients with severe sepsis without shock
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
HHS Secretary-nominee avoids specifics on Medicaid funding during second hearing
WASHINGTON – Rep. Tom Price, MD (R-Ga.), dodged specifics on Medicaid reform and the issue of block grants for funding Medicaid during a hearing Jan. 24 before the Senate Financing Committee.
The committee will be voting to move forward to the full Senate his nomination as secretary of the Department of Health & Human Services.
Sen. Robert Casey (D-Penn.) queried Rep. Price about guarantees as to whether people with disabilities covered by Medicaid would continue to be covered under a block grant program. Rep. Price responded that the “metrics that we will use ... [are] the quality of care and whether or not they are receiving that care.”
Rep. Price added that he is committed “to make it so they have that [current level of existing] coverage or greater.” Sen. Casey questioned whether that goal could be achieved, considering the amount of funding that could potentially be lost to a block grant program.
When further pressed on the 2017 budget he prepared as House Budget Committee chairman that included block grants for Medicaid, Rep. Price would not state clearly his promotion of the concept. Instead, he said he was committed to creating a system that is affordable, accessible, of high quality, and responsive to patient needs, as well as one that incentivizes innovation and provides choice.
Rep. Price was also pressed on his advocacy of high-risk pools, particularly for those who have high-cost, preexisting conditions and might not be able to get coverage in other areas of the reformed market. He voiced his support for such pools as well as for pools that would allow people without a common economic link, such as an employer, to band together for insurance coverage.
Sen. Debbie Stabenow (D-Mich.) noted that the history of high-risk pools has been less than stellar, with insurance rates typically 150%-200% higher than the rates of other plans and, typically, lifetime caps on coverage.
Rep. Price additionally called for a “better” system that puts patients at the center of health care decisions. In response to discussion with Sen. Chuck Grassley (R-Iowa), Rep. Price said transparency, specifically in relation to the Physician Payments Sunshine Act, was “vital,” and expanded the notion of transparency to include outcomes and pricing so that patients could have the best information to make decisions about their own health care.
It is “virtually impossible” for patients to know their true health care costs, Rep. Price said. To be informed, patients need better outcome measures, which would be “a priority” if he is confirmed as secretary.
Rep. Price also agreed that the Children’s Health Insurance Plan should be extended, and when asked about extending the program for 5 years, he responded that “8 years would be better.”
In the area of mental health, he suggested treatment models similar to those used to address physical health.
Rep. Price was not grilled on his investments at the Finance Committee hearing as he was at the Health, Education, Labor and Pensions Committee hearing, where he maintained he did nothing unethical or against the rules of the Senate.
Separate from the hearing, eight Democratic senators, led by Ranking Member Patty Murray of Washington, sent a Jan. 23 letter to the U.S. Securities and Exchange Commission to investigate whether Rep. Price potentially engaged in insider trading or other violations in relation to his specific purchase of stock in Innate Immunotherapeutics.
WASHINGTON – Rep. Tom Price, MD (R-Ga.), dodged specifics on Medicaid reform and the issue of block grants for funding Medicaid during a hearing Jan. 24 before the Senate Financing Committee.
The committee will be voting to move forward to the full Senate his nomination as secretary of the Department of Health & Human Services.
Sen. Robert Casey (D-Penn.) queried Rep. Price about guarantees as to whether people with disabilities covered by Medicaid would continue to be covered under a block grant program. Rep. Price responded that the “metrics that we will use ... [are] the quality of care and whether or not they are receiving that care.”
Rep. Price added that he is committed “to make it so they have that [current level of existing] coverage or greater.” Sen. Casey questioned whether that goal could be achieved, considering the amount of funding that could potentially be lost to a block grant program.
When further pressed on the 2017 budget he prepared as House Budget Committee chairman that included block grants for Medicaid, Rep. Price would not state clearly his promotion of the concept. Instead, he said he was committed to creating a system that is affordable, accessible, of high quality, and responsive to patient needs, as well as one that incentivizes innovation and provides choice.
Rep. Price was also pressed on his advocacy of high-risk pools, particularly for those who have high-cost, preexisting conditions and might not be able to get coverage in other areas of the reformed market. He voiced his support for such pools as well as for pools that would allow people without a common economic link, such as an employer, to band together for insurance coverage.
Sen. Debbie Stabenow (D-Mich.) noted that the history of high-risk pools has been less than stellar, with insurance rates typically 150%-200% higher than the rates of other plans and, typically, lifetime caps on coverage.
Rep. Price additionally called for a “better” system that puts patients at the center of health care decisions. In response to discussion with Sen. Chuck Grassley (R-Iowa), Rep. Price said transparency, specifically in relation to the Physician Payments Sunshine Act, was “vital,” and expanded the notion of transparency to include outcomes and pricing so that patients could have the best information to make decisions about their own health care.
It is “virtually impossible” for patients to know their true health care costs, Rep. Price said. To be informed, patients need better outcome measures, which would be “a priority” if he is confirmed as secretary.
Rep. Price also agreed that the Children’s Health Insurance Plan should be extended, and when asked about extending the program for 5 years, he responded that “8 years would be better.”
In the area of mental health, he suggested treatment models similar to those used to address physical health.
Rep. Price was not grilled on his investments at the Finance Committee hearing as he was at the Health, Education, Labor and Pensions Committee hearing, where he maintained he did nothing unethical or against the rules of the Senate.
Separate from the hearing, eight Democratic senators, led by Ranking Member Patty Murray of Washington, sent a Jan. 23 letter to the U.S. Securities and Exchange Commission to investigate whether Rep. Price potentially engaged in insider trading or other violations in relation to his specific purchase of stock in Innate Immunotherapeutics.
WASHINGTON – Rep. Tom Price, MD (R-Ga.), dodged specifics on Medicaid reform and the issue of block grants for funding Medicaid during a hearing Jan. 24 before the Senate Financing Committee.
The committee will be voting to move forward to the full Senate his nomination as secretary of the Department of Health & Human Services.
Sen. Robert Casey (D-Penn.) queried Rep. Price about guarantees as to whether people with disabilities covered by Medicaid would continue to be covered under a block grant program. Rep. Price responded that the “metrics that we will use ... [are] the quality of care and whether or not they are receiving that care.”
Rep. Price added that he is committed “to make it so they have that [current level of existing] coverage or greater.” Sen. Casey questioned whether that goal could be achieved, considering the amount of funding that could potentially be lost to a block grant program.
When further pressed on the 2017 budget he prepared as House Budget Committee chairman that included block grants for Medicaid, Rep. Price would not state clearly his promotion of the concept. Instead, he said he was committed to creating a system that is affordable, accessible, of high quality, and responsive to patient needs, as well as one that incentivizes innovation and provides choice.
Rep. Price was also pressed on his advocacy of high-risk pools, particularly for those who have high-cost, preexisting conditions and might not be able to get coverage in other areas of the reformed market. He voiced his support for such pools as well as for pools that would allow people without a common economic link, such as an employer, to band together for insurance coverage.
Sen. Debbie Stabenow (D-Mich.) noted that the history of high-risk pools has been less than stellar, with insurance rates typically 150%-200% higher than the rates of other plans and, typically, lifetime caps on coverage.
Rep. Price additionally called for a “better” system that puts patients at the center of health care decisions. In response to discussion with Sen. Chuck Grassley (R-Iowa), Rep. Price said transparency, specifically in relation to the Physician Payments Sunshine Act, was “vital,” and expanded the notion of transparency to include outcomes and pricing so that patients could have the best information to make decisions about their own health care.
It is “virtually impossible” for patients to know their true health care costs, Rep. Price said. To be informed, patients need better outcome measures, which would be “a priority” if he is confirmed as secretary.
Rep. Price also agreed that the Children’s Health Insurance Plan should be extended, and when asked about extending the program for 5 years, he responded that “8 years would be better.”
In the area of mental health, he suggested treatment models similar to those used to address physical health.
Rep. Price was not grilled on his investments at the Finance Committee hearing as he was at the Health, Education, Labor and Pensions Committee hearing, where he maintained he did nothing unethical or against the rules of the Senate.
Separate from the hearing, eight Democratic senators, led by Ranking Member Patty Murray of Washington, sent a Jan. 23 letter to the U.S. Securities and Exchange Commission to investigate whether Rep. Price potentially engaged in insider trading or other violations in relation to his specific purchase of stock in Innate Immunotherapeutics.
Intracardiac echo safely guides LAA occluder placement
ORLANDO – Intracardiac echocardiography (ICE) can be safely substituted for transesophageal echocardiography (TEE) as a less invasive option in the placement of an investigational percutaneous device for left atrial appendage (LAA) occlusion, according to data presented at the annual International AF Symposium.
The relative safety and efficacy of ICE and TEE for placement of the device, called the Amplatzer Amulet, was evaluated as a subanalysis of a large, nonrandomized, observational study, according to Boris Schmidt, MD, of Cardiovascular Center Bethanien, Frankfurt, Germany.
The Amplatzer Amulet device, which is designed to prevent LAA-associated thromboembolism in patients with nonvalvular atrial fibrillation (AF), has been available in Europe for several years. It functions much like Boston Scientific’s Watchman implant. A registration trial in the United States was initiated in 2016.
In the study that provided the basis for this analysis, 1,088 AF patients were implanted. The average age was 75 years, and the population was relatively high risk for both stroke and bleeding. The CHA2DS2-VASc score was at least 4 in 65% of patients, with an average score of 4.2. Prior stroke (28%) and transient ischemic attack (11%) had occurred in more than one-third. Reflecting the fact that 72% had a history of a major bleed, the average HAS-BLED score was 3.3. More than 80% were hypertensive.
The decision to place the device with TEE, which Dr. Schmidt characterized as the standard, or ICE was left up to the discretion of the implanter. Ultimately, 958 (88.4%) of the devices were placed with TEE and 126 (11.6%) with ICE.
There were no significant differences in implant success or safety when the two methods for guiding implantation were compared. Specifically, success was achieved in 99.2% of the ICE group with 90.5% of cases requiring only one device. In the TEE group, the device was successfully implanted in 98.4%, and 94.4% needed only one device. The first device to be selected was ultimately implanted in 96.5% of the ICE group and 94.4% of the TEE group. The LAA closure rate, defined as gap of less than 3 mm, was 100% at implant and 3 months after transplant in the ICE group, when evaluated by an independent core laboratory. The closure rates in the TEE group at the time of implant and 3 months later were 99.8% and 98.6%, respectively. No difference between imaging methods approached statistical significance.
Adverse events, which were also adjudicated by independent investigators, occurred at low rates and also did not differ by imaging strategy. In the ICE and TEE groups, respectively, these included vascular complications in 0.9% and 1.6%, pericardial effusion in 1.0% and 0.8%, stroke in 0.3% and 0%, and embolization in 0.1% and 0%. With a median follow-up of 6.6 months, there have been four deaths. Two were considered to be device related by the adjudicators. One involved a perforation and another a cardiac arrest. Both were observed in the TEE group.
The Amplatzer Amulet device consists of a lobe that is designed to fit inside the LAA neck and a disk that provides a complete seal at the orifice. It was first introduced in Europe in 2008 but has undergone several modifications. In the United States, the Watchman, which opens like an umbrella in order to block passage of thromboemboli when deployed in the LAA, received FDA approval in 2015. Other LAA closure strategies are in development. There are no large randomized trials in which LAA closure devices have been compared.
Dr. Schmidt has financial relationships with Boston Scientific and St. Jude Medical.
ORLANDO – Intracardiac echocardiography (ICE) can be safely substituted for transesophageal echocardiography (TEE) as a less invasive option in the placement of an investigational percutaneous device for left atrial appendage (LAA) occlusion, according to data presented at the annual International AF Symposium.
The relative safety and efficacy of ICE and TEE for placement of the device, called the Amplatzer Amulet, was evaluated as a subanalysis of a large, nonrandomized, observational study, according to Boris Schmidt, MD, of Cardiovascular Center Bethanien, Frankfurt, Germany.
The Amplatzer Amulet device, which is designed to prevent LAA-associated thromboembolism in patients with nonvalvular atrial fibrillation (AF), has been available in Europe for several years. It functions much like Boston Scientific’s Watchman implant. A registration trial in the United States was initiated in 2016.
In the study that provided the basis for this analysis, 1,088 AF patients were implanted. The average age was 75 years, and the population was relatively high risk for both stroke and bleeding. The CHA2DS2-VASc score was at least 4 in 65% of patients, with an average score of 4.2. Prior stroke (28%) and transient ischemic attack (11%) had occurred in more than one-third. Reflecting the fact that 72% had a history of a major bleed, the average HAS-BLED score was 3.3. More than 80% were hypertensive.
The decision to place the device with TEE, which Dr. Schmidt characterized as the standard, or ICE was left up to the discretion of the implanter. Ultimately, 958 (88.4%) of the devices were placed with TEE and 126 (11.6%) with ICE.
There were no significant differences in implant success or safety when the two methods for guiding implantation were compared. Specifically, success was achieved in 99.2% of the ICE group with 90.5% of cases requiring only one device. In the TEE group, the device was successfully implanted in 98.4%, and 94.4% needed only one device. The first device to be selected was ultimately implanted in 96.5% of the ICE group and 94.4% of the TEE group. The LAA closure rate, defined as gap of less than 3 mm, was 100% at implant and 3 months after transplant in the ICE group, when evaluated by an independent core laboratory. The closure rates in the TEE group at the time of implant and 3 months later were 99.8% and 98.6%, respectively. No difference between imaging methods approached statistical significance.
Adverse events, which were also adjudicated by independent investigators, occurred at low rates and also did not differ by imaging strategy. In the ICE and TEE groups, respectively, these included vascular complications in 0.9% and 1.6%, pericardial effusion in 1.0% and 0.8%, stroke in 0.3% and 0%, and embolization in 0.1% and 0%. With a median follow-up of 6.6 months, there have been four deaths. Two were considered to be device related by the adjudicators. One involved a perforation and another a cardiac arrest. Both were observed in the TEE group.
The Amplatzer Amulet device consists of a lobe that is designed to fit inside the LAA neck and a disk that provides a complete seal at the orifice. It was first introduced in Europe in 2008 but has undergone several modifications. In the United States, the Watchman, which opens like an umbrella in order to block passage of thromboemboli when deployed in the LAA, received FDA approval in 2015. Other LAA closure strategies are in development. There are no large randomized trials in which LAA closure devices have been compared.
Dr. Schmidt has financial relationships with Boston Scientific and St. Jude Medical.
ORLANDO – Intracardiac echocardiography (ICE) can be safely substituted for transesophageal echocardiography (TEE) as a less invasive option in the placement of an investigational percutaneous device for left atrial appendage (LAA) occlusion, according to data presented at the annual International AF Symposium.
The relative safety and efficacy of ICE and TEE for placement of the device, called the Amplatzer Amulet, was evaluated as a subanalysis of a large, nonrandomized, observational study, according to Boris Schmidt, MD, of Cardiovascular Center Bethanien, Frankfurt, Germany.
The Amplatzer Amulet device, which is designed to prevent LAA-associated thromboembolism in patients with nonvalvular atrial fibrillation (AF), has been available in Europe for several years. It functions much like Boston Scientific’s Watchman implant. A registration trial in the United States was initiated in 2016.
In the study that provided the basis for this analysis, 1,088 AF patients were implanted. The average age was 75 years, and the population was relatively high risk for both stroke and bleeding. The CHA2DS2-VASc score was at least 4 in 65% of patients, with an average score of 4.2. Prior stroke (28%) and transient ischemic attack (11%) had occurred in more than one-third. Reflecting the fact that 72% had a history of a major bleed, the average HAS-BLED score was 3.3. More than 80% were hypertensive.
The decision to place the device with TEE, which Dr. Schmidt characterized as the standard, or ICE was left up to the discretion of the implanter. Ultimately, 958 (88.4%) of the devices were placed with TEE and 126 (11.6%) with ICE.
There were no significant differences in implant success or safety when the two methods for guiding implantation were compared. Specifically, success was achieved in 99.2% of the ICE group with 90.5% of cases requiring only one device. In the TEE group, the device was successfully implanted in 98.4%, and 94.4% needed only one device. The first device to be selected was ultimately implanted in 96.5% of the ICE group and 94.4% of the TEE group. The LAA closure rate, defined as gap of less than 3 mm, was 100% at implant and 3 months after transplant in the ICE group, when evaluated by an independent core laboratory. The closure rates in the TEE group at the time of implant and 3 months later were 99.8% and 98.6%, respectively. No difference between imaging methods approached statistical significance.
Adverse events, which were also adjudicated by independent investigators, occurred at low rates and also did not differ by imaging strategy. In the ICE and TEE groups, respectively, these included vascular complications in 0.9% and 1.6%, pericardial effusion in 1.0% and 0.8%, stroke in 0.3% and 0%, and embolization in 0.1% and 0%. With a median follow-up of 6.6 months, there have been four deaths. Two were considered to be device related by the adjudicators. One involved a perforation and another a cardiac arrest. Both were observed in the TEE group.
The Amplatzer Amulet device consists of a lobe that is designed to fit inside the LAA neck and a disk that provides a complete seal at the orifice. It was first introduced in Europe in 2008 but has undergone several modifications. In the United States, the Watchman, which opens like an umbrella in order to block passage of thromboemboli when deployed in the LAA, received FDA approval in 2015. Other LAA closure strategies are in development. There are no large randomized trials in which LAA closure devices have been compared.
Dr. Schmidt has financial relationships with Boston Scientific and St. Jude Medical.
Key clinical point: Intracardiac echocardiography is a viable option for guiding placement of an experimental left atrial appendage thromboembolism blocker.
Major finding: LAA closure rates at 3 months were 100% with ICE and 98.6% with transesophageal echocardiograph.
Data source: Prospective, multicenter, observational study.
Disclosures: Dr. Schmidt has financial relationships with Boston Scientific and St. Jude Medical.
Hypothermia confers no benefits in children with cardiac arrest
Comatose children who survived cardiac arrest in the hospital do not benefit more from treatment with therapeutic hypothermia than from keeping their body temperatures normal, according to results from a randomized trial conducted in 37 hospitals in three countries.
The findings were presented in Honolulu at the Critical Care Congress, sponsored by the Society for Critical Care Medicine, and published online Jan. 24 in the New England Journal of Medicine (2017 Jan 24. doi: 10.1056/NEJMoa1610493). They add to a growing consensus from adult studies that the use of induced hypothermia to prevent fevers and neurologic injury after cardiac arrest does not confer additional survival or functional benefit over normothermia. Less was known about children, particularly those whose cardiac arrest occurred in a hospital setting.
Frank W. Moler, MD, of the University of Michigan, Ann Arbor, led the study, which randomized 329 comatose children, from newborns to age 18 years, to either 120 hours of normothermia (target temperature, 36.8° C) or 48 hours of hypothermia (33°) followed by normal temperature maintenance to 120 days following an in-hospital cardiac arrest.
Fever prevention in both groups was achieved through active intervention, with hypothermia-treated patients also having been pharmacologically paralyzed and sedated. The investigators used the Vineland Adaptive Behavioral Scales to measure neurobehavioral function, with a score of 70 or higher deemed indicative of good function.
The study’s primary outcome was survival at 12 months after cardiac arrest and a favorable neurobehavioral outcome. In the 257 children with scores of 70 or higher before cardiac arrest, no significant differences were seen between the two different groups, with 36% of the hypothermia-treated patients (48/133) and 39% of normothermia-treated patients (48/124) surviving with a favorable neurobehavioral outcome (relative risk, 0.92; 95% confidence interval, 0.67-1.27; P = .63). In 317 children who could be evaluated for changes in neurobehavioral function, the changes from baseline between groups did not reach statistical significance (P = .70), and 1-year survival also did not differ significantly (49% for hypothermia-treated vs. 46% for normothermia; RR, 1.07; 95% CI, 0.85-1.34, P = .56). Adverse events did not differ significantly between groups.
The trial was stopped early for futility, leaving fewer than the hoped-for number of patients available for analysis, and wider confidence intervals. However, the investigators said their hypothesized 15 percentage point benefit for hypothermia treatment could be ruled out. Dr. Moler and his colleagues wrote in their analysis that unanswered questions remain regarding the role of body temperature interventions in this population, noting that different duration of treatment, different temperatures, and combination of temperature management with neuroprotective agents are worth considering for future studies. Dr. Moler and his colleagues’ study was funded by the National Heart, Lung, and Blood Institute. Four of its 49 coauthors disclosed commercial conflicts of interest.
Comatose children who survived cardiac arrest in the hospital do not benefit more from treatment with therapeutic hypothermia than from keeping their body temperatures normal, according to results from a randomized trial conducted in 37 hospitals in three countries.
The findings were presented in Honolulu at the Critical Care Congress, sponsored by the Society for Critical Care Medicine, and published online Jan. 24 in the New England Journal of Medicine (2017 Jan 24. doi: 10.1056/NEJMoa1610493). They add to a growing consensus from adult studies that the use of induced hypothermia to prevent fevers and neurologic injury after cardiac arrest does not confer additional survival or functional benefit over normothermia. Less was known about children, particularly those whose cardiac arrest occurred in a hospital setting.
Frank W. Moler, MD, of the University of Michigan, Ann Arbor, led the study, which randomized 329 comatose children, from newborns to age 18 years, to either 120 hours of normothermia (target temperature, 36.8° C) or 48 hours of hypothermia (33°) followed by normal temperature maintenance to 120 days following an in-hospital cardiac arrest.
Fever prevention in both groups was achieved through active intervention, with hypothermia-treated patients also having been pharmacologically paralyzed and sedated. The investigators used the Vineland Adaptive Behavioral Scales to measure neurobehavioral function, with a score of 70 or higher deemed indicative of good function.
The study’s primary outcome was survival at 12 months after cardiac arrest and a favorable neurobehavioral outcome. In the 257 children with scores of 70 or higher before cardiac arrest, no significant differences were seen between the two different groups, with 36% of the hypothermia-treated patients (48/133) and 39% of normothermia-treated patients (48/124) surviving with a favorable neurobehavioral outcome (relative risk, 0.92; 95% confidence interval, 0.67-1.27; P = .63). In 317 children who could be evaluated for changes in neurobehavioral function, the changes from baseline between groups did not reach statistical significance (P = .70), and 1-year survival also did not differ significantly (49% for hypothermia-treated vs. 46% for normothermia; RR, 1.07; 95% CI, 0.85-1.34, P = .56). Adverse events did not differ significantly between groups.
The trial was stopped early for futility, leaving fewer than the hoped-for number of patients available for analysis, and wider confidence intervals. However, the investigators said their hypothesized 15 percentage point benefit for hypothermia treatment could be ruled out. Dr. Moler and his colleagues wrote in their analysis that unanswered questions remain regarding the role of body temperature interventions in this population, noting that different duration of treatment, different temperatures, and combination of temperature management with neuroprotective agents are worth considering for future studies. Dr. Moler and his colleagues’ study was funded by the National Heart, Lung, and Blood Institute. Four of its 49 coauthors disclosed commercial conflicts of interest.
Comatose children who survived cardiac arrest in the hospital do not benefit more from treatment with therapeutic hypothermia than from keeping their body temperatures normal, according to results from a randomized trial conducted in 37 hospitals in three countries.
The findings were presented in Honolulu at the Critical Care Congress, sponsored by the Society for Critical Care Medicine, and published online Jan. 24 in the New England Journal of Medicine (2017 Jan 24. doi: 10.1056/NEJMoa1610493). They add to a growing consensus from adult studies that the use of induced hypothermia to prevent fevers and neurologic injury after cardiac arrest does not confer additional survival or functional benefit over normothermia. Less was known about children, particularly those whose cardiac arrest occurred in a hospital setting.
Frank W. Moler, MD, of the University of Michigan, Ann Arbor, led the study, which randomized 329 comatose children, from newborns to age 18 years, to either 120 hours of normothermia (target temperature, 36.8° C) or 48 hours of hypothermia (33°) followed by normal temperature maintenance to 120 days following an in-hospital cardiac arrest.
Fever prevention in both groups was achieved through active intervention, with hypothermia-treated patients also having been pharmacologically paralyzed and sedated. The investigators used the Vineland Adaptive Behavioral Scales to measure neurobehavioral function, with a score of 70 or higher deemed indicative of good function.
The study’s primary outcome was survival at 12 months after cardiac arrest and a favorable neurobehavioral outcome. In the 257 children with scores of 70 or higher before cardiac arrest, no significant differences were seen between the two different groups, with 36% of the hypothermia-treated patients (48/133) and 39% of normothermia-treated patients (48/124) surviving with a favorable neurobehavioral outcome (relative risk, 0.92; 95% confidence interval, 0.67-1.27; P = .63). In 317 children who could be evaluated for changes in neurobehavioral function, the changes from baseline between groups did not reach statistical significance (P = .70), and 1-year survival also did not differ significantly (49% for hypothermia-treated vs. 46% for normothermia; RR, 1.07; 95% CI, 0.85-1.34, P = .56). Adverse events did not differ significantly between groups.
The trial was stopped early for futility, leaving fewer than the hoped-for number of patients available for analysis, and wider confidence intervals. However, the investigators said their hypothesized 15 percentage point benefit for hypothermia treatment could be ruled out. Dr. Moler and his colleagues wrote in their analysis that unanswered questions remain regarding the role of body temperature interventions in this population, noting that different duration of treatment, different temperatures, and combination of temperature management with neuroprotective agents are worth considering for future studies. Dr. Moler and his colleagues’ study was funded by the National Heart, Lung, and Blood Institute. Four of its 49 coauthors disclosed commercial conflicts of interest.
FROM THE CRITICAL CARE CONGRESS
Key clinical point: Treating comatose children with hypothermia following cardiac arrest did not produce better neurobehavioral or survival outcomes at 1 year, compared with children whose body temperatures were held to normal.
Major finding: 36% of hypothermia-treated patients and 39% of normothermia-treated patients survived with a favorable neurobehavioral outcome (RR, 0.92; 95% CI, 0.67-1.27; P = .63).
Data source: A multisite, international trial randomizing 329 infants and children comatose after cardiac arrest while in hospital to hypothermia or normothermia.
Disclosures: The National Heart, Lung, and Blood Institute sponsored the study. Several investigators disclosed National Institutes of Health or university funding while four disclosed commercial conflicts.
Immune-suppressing drugs in IBD linked to higher skin cancer rates
In another sign that immune-suppressing drugs may cause skin cancer, a new Irish study links immunomodulator use in younger patients with inflammatory bowel disease (IBD) to higher rates of nonmelanoma skin cancer (NMSC).
The 19-year study lacks information about medication doses or duration, and it doesn’t confirm a cause-and-effect link. Still, researchers recommend that all patients with IBD be urged to comply with skin cancer prevention guidelines.
As the study notes, previous research has linked immunosuppression – such as that in transplant patients and those with AIDS and lymphoma – to higher rates of NMSC.
Studies have also linked IBD to higher rates of NMSC even before the age of 50, possibly as the result of immune system dysfunction and exposure to immunomodulators, especially thiopurines. The risk of tumor necrosis factor–alpha (TNF-alpha) inhibitors, the study says, is less clear.
To better understand the risk of immunomodulators, researchers led by Julianne Clowry, MBBCh, of St Vincent’s University Hospital in Dublin tracked 2,053 IBD patients at a tertiary adult hospital from 1994 to 2013.
The median age at IBD diagnosis was 31 with a median of 19.6 years of illness, and the patients had both Crohn’s disease (41%) and ulcerative colitis (59%). Fifty-seven percent of patients had taken immunomodulating medication, although the database used didn’t disclose details about dose or duration, and 43% had not.
The study findings appeared Jan. 3 in the Journal of the European Academy of Dermatology and Venereology (doi: 10.1111/jdv.14105).
NMSC was diagnosed in 1.7% of the entire cohort, 1.4% of patients who’d taken immunosuppressants, and 1.9% of those who had not.
Older ages may explain the higher rate in those who didn’t take the medications. The researchers found that the standardized incidence ratio for the patients who took immunomodulators overall was 1.76 [confidence interval, 1.0-2.7], compared with a matched general population cohort, while the ratio was not considered significant among the nonimmunosuppressed [1.07; CI, 0.6-1.6].
The study links use of thiopurines alone and use of both thiopurines and TNF-alpha inhibitors to higher rates of NMSC [odds ratio, 5.26; 95% CI, 2.15-12.93; P less than .001, and OR: 6.45; 95% CI, 2.69-15.95; P less than .001, respectively].
The researchers note that 82% of those who had taken a TNF-alpha inhibitor also took a thiopurine at some point.
The study says the “relatively high” standardized incident ratios are worrisome amid more use of dual immunomodulators and higher IBD rates in kids and younger adults. But the medications are “vital,” the study says, and the researchers suggest “targeted dermatology referrals for IBD patients, particularly those exposed to dual immunomodulatory therapy from an early age.”
The study authors disclose no source of funding and report no relevant disclosures.
In another sign that immune-suppressing drugs may cause skin cancer, a new Irish study links immunomodulator use in younger patients with inflammatory bowel disease (IBD) to higher rates of nonmelanoma skin cancer (NMSC).
The 19-year study lacks information about medication doses or duration, and it doesn’t confirm a cause-and-effect link. Still, researchers recommend that all patients with IBD be urged to comply with skin cancer prevention guidelines.
As the study notes, previous research has linked immunosuppression – such as that in transplant patients and those with AIDS and lymphoma – to higher rates of NMSC.
Studies have also linked IBD to higher rates of NMSC even before the age of 50, possibly as the result of immune system dysfunction and exposure to immunomodulators, especially thiopurines. The risk of tumor necrosis factor–alpha (TNF-alpha) inhibitors, the study says, is less clear.
To better understand the risk of immunomodulators, researchers led by Julianne Clowry, MBBCh, of St Vincent’s University Hospital in Dublin tracked 2,053 IBD patients at a tertiary adult hospital from 1994 to 2013.
The median age at IBD diagnosis was 31 with a median of 19.6 years of illness, and the patients had both Crohn’s disease (41%) and ulcerative colitis (59%). Fifty-seven percent of patients had taken immunomodulating medication, although the database used didn’t disclose details about dose or duration, and 43% had not.
The study findings appeared Jan. 3 in the Journal of the European Academy of Dermatology and Venereology (doi: 10.1111/jdv.14105).
NMSC was diagnosed in 1.7% of the entire cohort, 1.4% of patients who’d taken immunosuppressants, and 1.9% of those who had not.
Older ages may explain the higher rate in those who didn’t take the medications. The researchers found that the standardized incidence ratio for the patients who took immunomodulators overall was 1.76 [confidence interval, 1.0-2.7], compared with a matched general population cohort, while the ratio was not considered significant among the nonimmunosuppressed [1.07; CI, 0.6-1.6].
The study links use of thiopurines alone and use of both thiopurines and TNF-alpha inhibitors to higher rates of NMSC [odds ratio, 5.26; 95% CI, 2.15-12.93; P less than .001, and OR: 6.45; 95% CI, 2.69-15.95; P less than .001, respectively].
The researchers note that 82% of those who had taken a TNF-alpha inhibitor also took a thiopurine at some point.
The study says the “relatively high” standardized incident ratios are worrisome amid more use of dual immunomodulators and higher IBD rates in kids and younger adults. But the medications are “vital,” the study says, and the researchers suggest “targeted dermatology referrals for IBD patients, particularly those exposed to dual immunomodulatory therapy from an early age.”
The study authors disclose no source of funding and report no relevant disclosures.
In another sign that immune-suppressing drugs may cause skin cancer, a new Irish study links immunomodulator use in younger patients with inflammatory bowel disease (IBD) to higher rates of nonmelanoma skin cancer (NMSC).
The 19-year study lacks information about medication doses or duration, and it doesn’t confirm a cause-and-effect link. Still, researchers recommend that all patients with IBD be urged to comply with skin cancer prevention guidelines.
As the study notes, previous research has linked immunosuppression – such as that in transplant patients and those with AIDS and lymphoma – to higher rates of NMSC.
Studies have also linked IBD to higher rates of NMSC even before the age of 50, possibly as the result of immune system dysfunction and exposure to immunomodulators, especially thiopurines. The risk of tumor necrosis factor–alpha (TNF-alpha) inhibitors, the study says, is less clear.
To better understand the risk of immunomodulators, researchers led by Julianne Clowry, MBBCh, of St Vincent’s University Hospital in Dublin tracked 2,053 IBD patients at a tertiary adult hospital from 1994 to 2013.
The median age at IBD diagnosis was 31 with a median of 19.6 years of illness, and the patients had both Crohn’s disease (41%) and ulcerative colitis (59%). Fifty-seven percent of patients had taken immunomodulating medication, although the database used didn’t disclose details about dose or duration, and 43% had not.
The study findings appeared Jan. 3 in the Journal of the European Academy of Dermatology and Venereology (doi: 10.1111/jdv.14105).
NMSC was diagnosed in 1.7% of the entire cohort, 1.4% of patients who’d taken immunosuppressants, and 1.9% of those who had not.
Older ages may explain the higher rate in those who didn’t take the medications. The researchers found that the standardized incidence ratio for the patients who took immunomodulators overall was 1.76 [confidence interval, 1.0-2.7], compared with a matched general population cohort, while the ratio was not considered significant among the nonimmunosuppressed [1.07; CI, 0.6-1.6].
The study links use of thiopurines alone and use of both thiopurines and TNF-alpha inhibitors to higher rates of NMSC [odds ratio, 5.26; 95% CI, 2.15-12.93; P less than .001, and OR: 6.45; 95% CI, 2.69-15.95; P less than .001, respectively].
The researchers note that 82% of those who had taken a TNF-alpha inhibitor also took a thiopurine at some point.
The study says the “relatively high” standardized incident ratios are worrisome amid more use of dual immunomodulators and higher IBD rates in kids and younger adults. But the medications are “vital,” the study says, and the researchers suggest “targeted dermatology referrals for IBD patients, particularly those exposed to dual immunomodulatory therapy from an early age.”
The study authors disclose no source of funding and report no relevant disclosures.
Key clinical point: Younger inflammatory bowel disease (IBD) patients who’ve taken immunomodulating drugs have higher rates of nonmelanoma skin cancer (NMSC).
Major finding: IBD patients who took thiopurines alone and both thiopurines and TNF-alpha inhibitors had higher rates of NMSC [OR, 5.26; 95% CI, 2.15-12.93; P less than .001, and OR, 6.45; 95% CI, 2.69-15.95; P less than .001, respectively], compared with an age-matched general population cohort.
Data source: Retrospective single-center cohort study over 19 years of 2,053 IBD patients with Crohn’s disease (41%) and ulcerative colitis (59%); 57% had taken immunomodulating medications.
Disclosures: The study authors disclose no source of funding and report no relevant disclosures.
SDEF experts tackle atopic dermatitis
Atopic dermatitis (AD) is among the nuts and bolts of any dermatology practice, and as such, gets its time in the spotlight at SDEF’s Annual Hawaii Dermatology Seminar.
This year, SDEF celebrates 41 years of educating dermatologists with the latest in dermatology, featuring presentations from experts on topics ranging from AD treatments and skin cancer chemoprevention to botulinum toxin injections and fillers.
Also last year, Joseph F. Fowler Jr., MD, meeting codirector and clinical professor of dermatology at the University of Louisville (Ky.), shared in a video interview his perspective on addressing parents’ safety concerns about the boxed warning for topical calcineurin inhibitors.
Watch for more coverage and comments from experts at this year’s meeting.
SDEF and this news organization are owned by the same parent company.
Atopic dermatitis (AD) is among the nuts and bolts of any dermatology practice, and as such, gets its time in the spotlight at SDEF’s Annual Hawaii Dermatology Seminar.
This year, SDEF celebrates 41 years of educating dermatologists with the latest in dermatology, featuring presentations from experts on topics ranging from AD treatments and skin cancer chemoprevention to botulinum toxin injections and fillers.
Also last year, Joseph F. Fowler Jr., MD, meeting codirector and clinical professor of dermatology at the University of Louisville (Ky.), shared in a video interview his perspective on addressing parents’ safety concerns about the boxed warning for topical calcineurin inhibitors.
Watch for more coverage and comments from experts at this year’s meeting.
SDEF and this news organization are owned by the same parent company.
Atopic dermatitis (AD) is among the nuts and bolts of any dermatology practice, and as such, gets its time in the spotlight at SDEF’s Annual Hawaii Dermatology Seminar.
This year, SDEF celebrates 41 years of educating dermatologists with the latest in dermatology, featuring presentations from experts on topics ranging from AD treatments and skin cancer chemoprevention to botulinum toxin injections and fillers.
Also last year, Joseph F. Fowler Jr., MD, meeting codirector and clinical professor of dermatology at the University of Louisville (Ky.), shared in a video interview his perspective on addressing parents’ safety concerns about the boxed warning for topical calcineurin inhibitors.
Watch for more coverage and comments from experts at this year’s meeting.
SDEF and this news organization are owned by the same parent company.
An insider’s guide to aesthetic dermatology
When aesthetic dermatology takes the stage at SDEF’s Annual Hawaii Dermatology Seminar, experts will address the latest tips for successful outcomes with filler and toxins, offer guidance on choosing devices, and explain how to make the most of cosmeceuticals in a dermatology practice.
Christopher B. Zachary, MD, meeting codirector and professor and chair of the department of dermatology at the University of California, Irvine, cochairs a pair of aesthetic and procedural dermatology sessions with Michael S. Kaminer, MD, of Yale University, New Haven, Conn., and SkinCare Physicians, Chestnut Hill, Mass.
Last year, Dr. Zachary and a panel of expert aesthetic dermatologists addressed the enduring value of topical retinoids for facial rejuvenation. Read their comments here.
Stay tuned for the latest tips and techniques in aesthetic dermatology from the 2017 SDEF Hawaii Dermatology Seminar.
When aesthetic dermatology takes the stage at SDEF’s Annual Hawaii Dermatology Seminar, experts will address the latest tips for successful outcomes with filler and toxins, offer guidance on choosing devices, and explain how to make the most of cosmeceuticals in a dermatology practice.
Christopher B. Zachary, MD, meeting codirector and professor and chair of the department of dermatology at the University of California, Irvine, cochairs a pair of aesthetic and procedural dermatology sessions with Michael S. Kaminer, MD, of Yale University, New Haven, Conn., and SkinCare Physicians, Chestnut Hill, Mass.
Last year, Dr. Zachary and a panel of expert aesthetic dermatologists addressed the enduring value of topical retinoids for facial rejuvenation. Read their comments here.
Stay tuned for the latest tips and techniques in aesthetic dermatology from the 2017 SDEF Hawaii Dermatology Seminar.
When aesthetic dermatology takes the stage at SDEF’s Annual Hawaii Dermatology Seminar, experts will address the latest tips for successful outcomes with filler and toxins, offer guidance on choosing devices, and explain how to make the most of cosmeceuticals in a dermatology practice.
Christopher B. Zachary, MD, meeting codirector and professor and chair of the department of dermatology at the University of California, Irvine, cochairs a pair of aesthetic and procedural dermatology sessions with Michael S. Kaminer, MD, of Yale University, New Haven, Conn., and SkinCare Physicians, Chestnut Hill, Mass.
Last year, Dr. Zachary and a panel of expert aesthetic dermatologists addressed the enduring value of topical retinoids for facial rejuvenation. Read their comments here.
Stay tuned for the latest tips and techniques in aesthetic dermatology from the 2017 SDEF Hawaii Dermatology Seminar.
Guideline: Keep blood pressure below 150 mm Hg in healthy elderly
Treat to lower a persistent systolic blood pressure of 150 mm Hg or more in patients aged 60 years or older who are otherwise healthy, the American College of Physicians and the American Academy of Family Physicians recommended in a new guideline for managing blood pressure in older patients.
The recommendation was “strong,” based on high-quality evidence from the 24 studies reviewed. The groups also made a weak recommendation based on lower-quality evidence to keep systolic blood pressure below 140 mm Hg in patients aged 60 years and older who have a history of stroke, transient ischemic attack, or high cardiovascular risks.
For those patients who are otherwise well, “most patients aged 60 years or older with a SPB [systolic blood pressure] of 150 mm Hg or greater who receive antihypertensive medications will have benefit with acceptable harms and costs from treatment to a BP target of less than 150/90 mm Hg,” according to the guideline’s authors.
“Although some benefit is achieved by aiming for lower BP targets, most benefit occurs with acceptable harms and costs in the pharmacologic treatment of patients who have an SBP of 150 mm Hg or greater,” said the authors, led by Amir Qaseem, MD, PhD, ACP’s vice president of clinical policy (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M16-1754).
Meanwhile, treating hypertension to an SBP of 130-140 mm Hg in older adults with previous transient ischemic attacks or strokes reduces stroke recurrence, according to the guideline. In addition, an SBP of less than 140 mm Hg “is a reasonable goal for some patients with increased cardiovascular risk,” including those with vascular disease, diabetes, chronic kidney disease, or metabolic syndrome.
Trials with lower BP targets had higher rates of hypotension, electrolyte abnormalities, abnormal renal function, cough, and withdrawals because of side effects.
Older “patients might theoretically benefit from more aggressive BP treatment because of higher cardiovascular risks,” the guideline authors noted. “However, they are more likely to be susceptible to serious harm[s] from higher rates of syncope and hypotension, which were seen in some trials. Moreover, the absolute benefits of more aggressive BP treatment in elderly persons, those with multimorbidity, or those who are frail are not well known, given limitations of the trials.”
The advice is based on 21 randomized, controlled trials of hypertension treatment intensity through September 2016, plus three observational studies of harms. Antihypertensive selection varied widely across the studies. The guideline notes the various lifestyle and pharmacy options, but did not recommend any specific treatment.
Nine trials provided high-strength evidence that BP control to less than 150/90 mm Hg reduces mortality (relative risk, 0.90; 95% confidence interval, 0.83-0.98), cardiac events (RR, 0.77; 95% CI, 0.68-0.89), and stroke (RR, 0.74; 95% CI, 0.65-0.84), according to the evidence review (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M16-1754).
Low- to moderate-strength evidence suggested targets at or below 140/85 mm Hg in older people, but there was only a modest decrease in cardiac events (RR, 0.82; 95% CI, 0.64-1.00) and stroke (RR, 0.79; 95% CI, 0.59-0.99), and a statistically insignificant trend toward fewer deaths (RR, 0.86; 95% CI, 0.69-1.06).
Evidence was insufficient for targeting treatment according to diastolic BP.
Patients have to be involved with decisions about BP targets. Ongoing discussions about the risks and benefits of target options need to be a part of routine care, the guideline authors said.
The work was funded by ACP and the U.S. Department of Veterans Affairs. The authors had no disclosures.
To prevent hypertension-related morbidity and mortality, providers should develop an office-based program with the following features:
• High-fidelity BP measurement support, including office BP measurement by well-trained staff, resources to train patients in home monitoring or making ambulatory BP monitoring available, and ongoing quality assurance efforts.
• Routine assessment of global CVD risk in all patients 40 years or older, as well as in younger patients with multiple risk factors or extreme elevations of a single risk factor.
• Provider training in shared decision making for hypertension treatment and CVD risk reduction.
• A registry to track patients who receive hypertension treatment.
• Nonvisit-based follow-up for patients with moderate to severe hypertension who have had treatment initiation or changes.
Such programs, when implemented, have been associated with large improvements in BP control.
Michael Pignone, MD , is the chair of internal medicine at the University of Texas, Austin. Anthony Viera, MD , is the director of the hypertension research program at the University of North Carolina at Chapel Hill. They had no commercial disclosures and made these recommendations in an editorial (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M17-0034 ).
To prevent hypertension-related morbidity and mortality, providers should develop an office-based program with the following features:
• High-fidelity BP measurement support, including office BP measurement by well-trained staff, resources to train patients in home monitoring or making ambulatory BP monitoring available, and ongoing quality assurance efforts.
• Routine assessment of global CVD risk in all patients 40 years or older, as well as in younger patients with multiple risk factors or extreme elevations of a single risk factor.
• Provider training in shared decision making for hypertension treatment and CVD risk reduction.
• A registry to track patients who receive hypertension treatment.
• Nonvisit-based follow-up for patients with moderate to severe hypertension who have had treatment initiation or changes.
Such programs, when implemented, have been associated with large improvements in BP control.
Michael Pignone, MD , is the chair of internal medicine at the University of Texas, Austin. Anthony Viera, MD , is the director of the hypertension research program at the University of North Carolina at Chapel Hill. They had no commercial disclosures and made these recommendations in an editorial (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M17-0034 ).
To prevent hypertension-related morbidity and mortality, providers should develop an office-based program with the following features:
• High-fidelity BP measurement support, including office BP measurement by well-trained staff, resources to train patients in home monitoring or making ambulatory BP monitoring available, and ongoing quality assurance efforts.
• Routine assessment of global CVD risk in all patients 40 years or older, as well as in younger patients with multiple risk factors or extreme elevations of a single risk factor.
• Provider training in shared decision making for hypertension treatment and CVD risk reduction.
• A registry to track patients who receive hypertension treatment.
• Nonvisit-based follow-up for patients with moderate to severe hypertension who have had treatment initiation or changes.
Such programs, when implemented, have been associated with large improvements in BP control.
Michael Pignone, MD , is the chair of internal medicine at the University of Texas, Austin. Anthony Viera, MD , is the director of the hypertension research program at the University of North Carolina at Chapel Hill. They had no commercial disclosures and made these recommendations in an editorial (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M17-0034 ).
Treat to lower a persistent systolic blood pressure of 150 mm Hg or more in patients aged 60 years or older who are otherwise healthy, the American College of Physicians and the American Academy of Family Physicians recommended in a new guideline for managing blood pressure in older patients.
The recommendation was “strong,” based on high-quality evidence from the 24 studies reviewed. The groups also made a weak recommendation based on lower-quality evidence to keep systolic blood pressure below 140 mm Hg in patients aged 60 years and older who have a history of stroke, transient ischemic attack, or high cardiovascular risks.
For those patients who are otherwise well, “most patients aged 60 years or older with a SPB [systolic blood pressure] of 150 mm Hg or greater who receive antihypertensive medications will have benefit with acceptable harms and costs from treatment to a BP target of less than 150/90 mm Hg,” according to the guideline’s authors.
“Although some benefit is achieved by aiming for lower BP targets, most benefit occurs with acceptable harms and costs in the pharmacologic treatment of patients who have an SBP of 150 mm Hg or greater,” said the authors, led by Amir Qaseem, MD, PhD, ACP’s vice president of clinical policy (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M16-1754).
Meanwhile, treating hypertension to an SBP of 130-140 mm Hg in older adults with previous transient ischemic attacks or strokes reduces stroke recurrence, according to the guideline. In addition, an SBP of less than 140 mm Hg “is a reasonable goal for some patients with increased cardiovascular risk,” including those with vascular disease, diabetes, chronic kidney disease, or metabolic syndrome.
Trials with lower BP targets had higher rates of hypotension, electrolyte abnormalities, abnormal renal function, cough, and withdrawals because of side effects.
Older “patients might theoretically benefit from more aggressive BP treatment because of higher cardiovascular risks,” the guideline authors noted. “However, they are more likely to be susceptible to serious harm[s] from higher rates of syncope and hypotension, which were seen in some trials. Moreover, the absolute benefits of more aggressive BP treatment in elderly persons, those with multimorbidity, or those who are frail are not well known, given limitations of the trials.”
The advice is based on 21 randomized, controlled trials of hypertension treatment intensity through September 2016, plus three observational studies of harms. Antihypertensive selection varied widely across the studies. The guideline notes the various lifestyle and pharmacy options, but did not recommend any specific treatment.
Nine trials provided high-strength evidence that BP control to less than 150/90 mm Hg reduces mortality (relative risk, 0.90; 95% confidence interval, 0.83-0.98), cardiac events (RR, 0.77; 95% CI, 0.68-0.89), and stroke (RR, 0.74; 95% CI, 0.65-0.84), according to the evidence review (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M16-1754).
Low- to moderate-strength evidence suggested targets at or below 140/85 mm Hg in older people, but there was only a modest decrease in cardiac events (RR, 0.82; 95% CI, 0.64-1.00) and stroke (RR, 0.79; 95% CI, 0.59-0.99), and a statistically insignificant trend toward fewer deaths (RR, 0.86; 95% CI, 0.69-1.06).
Evidence was insufficient for targeting treatment according to diastolic BP.
Patients have to be involved with decisions about BP targets. Ongoing discussions about the risks and benefits of target options need to be a part of routine care, the guideline authors said.
The work was funded by ACP and the U.S. Department of Veterans Affairs. The authors had no disclosures.
Treat to lower a persistent systolic blood pressure of 150 mm Hg or more in patients aged 60 years or older who are otherwise healthy, the American College of Physicians and the American Academy of Family Physicians recommended in a new guideline for managing blood pressure in older patients.
The recommendation was “strong,” based on high-quality evidence from the 24 studies reviewed. The groups also made a weak recommendation based on lower-quality evidence to keep systolic blood pressure below 140 mm Hg in patients aged 60 years and older who have a history of stroke, transient ischemic attack, or high cardiovascular risks.
For those patients who are otherwise well, “most patients aged 60 years or older with a SPB [systolic blood pressure] of 150 mm Hg or greater who receive antihypertensive medications will have benefit with acceptable harms and costs from treatment to a BP target of less than 150/90 mm Hg,” according to the guideline’s authors.
“Although some benefit is achieved by aiming for lower BP targets, most benefit occurs with acceptable harms and costs in the pharmacologic treatment of patients who have an SBP of 150 mm Hg or greater,” said the authors, led by Amir Qaseem, MD, PhD, ACP’s vice president of clinical policy (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M16-1754).
Meanwhile, treating hypertension to an SBP of 130-140 mm Hg in older adults with previous transient ischemic attacks or strokes reduces stroke recurrence, according to the guideline. In addition, an SBP of less than 140 mm Hg “is a reasonable goal for some patients with increased cardiovascular risk,” including those with vascular disease, diabetes, chronic kidney disease, or metabolic syndrome.
Trials with lower BP targets had higher rates of hypotension, electrolyte abnormalities, abnormal renal function, cough, and withdrawals because of side effects.
Older “patients might theoretically benefit from more aggressive BP treatment because of higher cardiovascular risks,” the guideline authors noted. “However, they are more likely to be susceptible to serious harm[s] from higher rates of syncope and hypotension, which were seen in some trials. Moreover, the absolute benefits of more aggressive BP treatment in elderly persons, those with multimorbidity, or those who are frail are not well known, given limitations of the trials.”
The advice is based on 21 randomized, controlled trials of hypertension treatment intensity through September 2016, plus three observational studies of harms. Antihypertensive selection varied widely across the studies. The guideline notes the various lifestyle and pharmacy options, but did not recommend any specific treatment.
Nine trials provided high-strength evidence that BP control to less than 150/90 mm Hg reduces mortality (relative risk, 0.90; 95% confidence interval, 0.83-0.98), cardiac events (RR, 0.77; 95% CI, 0.68-0.89), and stroke (RR, 0.74; 95% CI, 0.65-0.84), according to the evidence review (Ann Intern Med. 2017 Jan 17. doi: 10.7326/M16-1754).
Low- to moderate-strength evidence suggested targets at or below 140/85 mm Hg in older people, but there was only a modest decrease in cardiac events (RR, 0.82; 95% CI, 0.64-1.00) and stroke (RR, 0.79; 95% CI, 0.59-0.99), and a statistically insignificant trend toward fewer deaths (RR, 0.86; 95% CI, 0.69-1.06).
Evidence was insufficient for targeting treatment according to diastolic BP.
Patients have to be involved with decisions about BP targets. Ongoing discussions about the risks and benefits of target options need to be a part of routine care, the guideline authors said.
The work was funded by ACP and the U.S. Department of Veterans Affairs. The authors had no disclosures.
Developing a personal scale for evaluating agitation on inpatient units
Agitation. As a mental health professional, you know it when you see it. While technically, it is the behavior that precedes aggression and violence, every clinical setting has its own flavor, from the voluntary psychiatric unit where I see my most acutely ill patients to the state hospital where I witnessed the type of violence that sent another patient to the emergency department.
Over the past 2 years of residency, I have developed a personal scale for measuring and responding to patient agitation. Through experiences that have provided the lower, upper (and even more upper) bounds to this scale, I have evolved from a nervous first-year intern to become a resident conscious of the need for a cool demeanor and continued engagement with the patient with escalating agitation.
The 2003 Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines1 provides an excellent visual scale to measure agitation. It begins with a patient’s refusal to cooperate and ascends stepwise toward motor restlessness, lability and loud speech, intimidation, aggression against property, and hostile verbal behavior, and it ends with directly threatening or assaultive behavior.2 Only when I witnessed signs of clinically significant agitation, hostile countenance, unpredictable anger, pacing, clenched fists, yelling, and threats, did I develop a personal understanding and approach for measuring agitation on the inpatient unit.
The upper bound for my scale initially was defined by my first incident of severe agitation and aggression while on call. I no longer remember if the patient was agitated and psychotic or just angry and agitated. Though schizophrenia and bipolar disorder often are the underlying causes of agitation, personality disorders and substance use complicate or contribute to agitation.3 The nurse called me, saying: “Can you come to the unit right now? Mr. X is agitated and has ripped the soap dispenser off the wall in his bathroom.” It was just after midnight, and already several nursing calls into this situation. Verbal de-escalation had failed with Mr. X, who moved from agitation to aggressive behavior. He already had received two doses of haloperidol and lorazepam since my evening shift began. I looked into his bedroom and saw him wrestling with the sink, which did not come off the wall as easily as the soap dispenser. The thought process of an inexperienced intern went like this: “How much haloperidol is too much haloperidol? Will this night never end?” I called my attending for help, and there was desperation in my voice as I explained: “The medications aren’t working.
These situations usually result in good clinical lessons: Medications take time to work, and in some individuals, the “standard cocktail” might not be the best option. Nonetheless, the lag time can be excruciating. As a more experienced resident, I now consider how certain medications may fail an individual, and there might be a better alternative to haloperidol and lorazepam. I’ve expanded my repertoire of pharmacological methods and opt often for second-generation antipsychotics, such as olanzapine or risperidone, without or without a benzodiazepine, when possible.2
Of course, not all agitation becomes a behavioral health emergency, and an integral part of my training as a resident has been watching an attending run an intervention smoothly. It requires coordination and experience, skills that I’m gaining. In these cases, the agitation is addressed before it escalates, nursing staff and the physicians collaborate to deliver treatment, and the patient responds to verbal redirection and, if offered, accepts oral medications. These types of patients help cement the lower bound for my agitation scale.
Nonetheless, the patients who challenge the positive archetype are the ones who cement lessons for physicians. I remember a man who with his history of serious mental illness had adverse reactions to haloperidol, aripiprazole, olanzapine, and fluphenazine. To address his agitation, the nurses prepared 2 mg of lorazepam and 50 mg of diphenhydramine. As the patient ramped up, I heard a nurse sigh, “Why can’t we add IM thorazine?” I commiserated with the nurse; the psychiatric unit is a dangerous place to work. Psychiatry and emergency department nurses, compared with their counterparts in other units, are the most likely to be assaulted at work.4,5 It is personal for me as well. Studies suggest that 30%-40% of psychiatric residents will be attacked during their 4-year training, and I am in that 70%-90% of residents who has been verbally threatened more than once.6
With time and training, my verbal de-escalation techniques have improved, as I’ve learned to avoid threatening and judgmental body language, avoiding a natural tendency to stand with my arms crossed over my chest or hands on my hips. I now more accurately and incisively inquire about a patient’s mental state. How can I address their frustration? In a nonaccusatory way, I let the patients know that they are behaving in a way that is frightening and that continued behavior may have consequences. Even when faced by the heat of agitation, I try to value the patients’ choice: This event will affect our therapeutic relationship in the longer term.
Whenever possible, I want the patients to choose their medication formulation or at least be able to ask them, “Would you be willing to take … ?” With time, I am earning that cool demeanor psychiatrists are known for. I can model calm behavior and effectively use my knowledge about mentalization to try to de-escalate the situation.
My scale of measuring of agitation and violence had its upper level increased significantly from just a soap dispenser being ripped off the wall. One patient really upped the ante by swiping a public telephone off the wall and then went tearing down the hallway to pull the fire extinguisher out of its supposed “safe” case and hurl it. So on a recent night shift, when I heard yelling through the door of the call room, it was with a sense of understanding rather than trepidation that my co-resident and I approached the patient, already being corralled to his room by nursing staff. He was an enormous man, angry, paranoid, pacing, and shouting about how the other patients wanted to attack him. Despite his size, his menacing posture, and that somewhere in his agitation he had ripped off his scrub shirt, I couldn’t help but think, “Well at least the phone and the fire extinguisher are still attached to the wall.”
References
1 J Psychiatr Pract. 2003 Jan;9(1):16-38. Treatment of behavioral emergencies: A summary of the expert consensus guidelines.
2 J Psychiatr Pract. 2005 Nov;11 Suppl 1:5-108; quiz 110-2. The expert consensus guideline series. Treatment of behavioral emergencies 2005.
3 Clin Pract Epidemiol Ment Health. 2016 Oct 27;12:75-86. State of acute agitation at psychiatric emergencies in Europe: The STAGE study.
4 J Emerg Nurs. 2014 May;40(3):218-28; quiz 295. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors.
5 Work. 2010;35(2):191-200. Physical assault among nursing staff employed in acute care.
6 Psychiatr Serv. 1999 Mar;50(3):381-3. Assaults by patients on psychiatric residents: a survey and training recommendations.
Dr. Posada is a second-year resident in the psychiatry & behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at the George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Agitation. As a mental health professional, you know it when you see it. While technically, it is the behavior that precedes aggression and violence, every clinical setting has its own flavor, from the voluntary psychiatric unit where I see my most acutely ill patients to the state hospital where I witnessed the type of violence that sent another patient to the emergency department.
Over the past 2 years of residency, I have developed a personal scale for measuring and responding to patient agitation. Through experiences that have provided the lower, upper (and even more upper) bounds to this scale, I have evolved from a nervous first-year intern to become a resident conscious of the need for a cool demeanor and continued engagement with the patient with escalating agitation.
The 2003 Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines1 provides an excellent visual scale to measure agitation. It begins with a patient’s refusal to cooperate and ascends stepwise toward motor restlessness, lability and loud speech, intimidation, aggression against property, and hostile verbal behavior, and it ends with directly threatening or assaultive behavior.2 Only when I witnessed signs of clinically significant agitation, hostile countenance, unpredictable anger, pacing, clenched fists, yelling, and threats, did I develop a personal understanding and approach for measuring agitation on the inpatient unit.
The upper bound for my scale initially was defined by my first incident of severe agitation and aggression while on call. I no longer remember if the patient was agitated and psychotic or just angry and agitated. Though schizophrenia and bipolar disorder often are the underlying causes of agitation, personality disorders and substance use complicate or contribute to agitation.3 The nurse called me, saying: “Can you come to the unit right now? Mr. X is agitated and has ripped the soap dispenser off the wall in his bathroom.” It was just after midnight, and already several nursing calls into this situation. Verbal de-escalation had failed with Mr. X, who moved from agitation to aggressive behavior. He already had received two doses of haloperidol and lorazepam since my evening shift began. I looked into his bedroom and saw him wrestling with the sink, which did not come off the wall as easily as the soap dispenser. The thought process of an inexperienced intern went like this: “How much haloperidol is too much haloperidol? Will this night never end?” I called my attending for help, and there was desperation in my voice as I explained: “The medications aren’t working.
These situations usually result in good clinical lessons: Medications take time to work, and in some individuals, the “standard cocktail” might not be the best option. Nonetheless, the lag time can be excruciating. As a more experienced resident, I now consider how certain medications may fail an individual, and there might be a better alternative to haloperidol and lorazepam. I’ve expanded my repertoire of pharmacological methods and opt often for second-generation antipsychotics, such as olanzapine or risperidone, without or without a benzodiazepine, when possible.2
Of course, not all agitation becomes a behavioral health emergency, and an integral part of my training as a resident has been watching an attending run an intervention smoothly. It requires coordination and experience, skills that I’m gaining. In these cases, the agitation is addressed before it escalates, nursing staff and the physicians collaborate to deliver treatment, and the patient responds to verbal redirection and, if offered, accepts oral medications. These types of patients help cement the lower bound for my agitation scale.
Nonetheless, the patients who challenge the positive archetype are the ones who cement lessons for physicians. I remember a man who with his history of serious mental illness had adverse reactions to haloperidol, aripiprazole, olanzapine, and fluphenazine. To address his agitation, the nurses prepared 2 mg of lorazepam and 50 mg of diphenhydramine. As the patient ramped up, I heard a nurse sigh, “Why can’t we add IM thorazine?” I commiserated with the nurse; the psychiatric unit is a dangerous place to work. Psychiatry and emergency department nurses, compared with their counterparts in other units, are the most likely to be assaulted at work.4,5 It is personal for me as well. Studies suggest that 30%-40% of psychiatric residents will be attacked during their 4-year training, and I am in that 70%-90% of residents who has been verbally threatened more than once.6
With time and training, my verbal de-escalation techniques have improved, as I’ve learned to avoid threatening and judgmental body language, avoiding a natural tendency to stand with my arms crossed over my chest or hands on my hips. I now more accurately and incisively inquire about a patient’s mental state. How can I address their frustration? In a nonaccusatory way, I let the patients know that they are behaving in a way that is frightening and that continued behavior may have consequences. Even when faced by the heat of agitation, I try to value the patients’ choice: This event will affect our therapeutic relationship in the longer term.
Whenever possible, I want the patients to choose their medication formulation or at least be able to ask them, “Would you be willing to take … ?” With time, I am earning that cool demeanor psychiatrists are known for. I can model calm behavior and effectively use my knowledge about mentalization to try to de-escalate the situation.
My scale of measuring of agitation and violence had its upper level increased significantly from just a soap dispenser being ripped off the wall. One patient really upped the ante by swiping a public telephone off the wall and then went tearing down the hallway to pull the fire extinguisher out of its supposed “safe” case and hurl it. So on a recent night shift, when I heard yelling through the door of the call room, it was with a sense of understanding rather than trepidation that my co-resident and I approached the patient, already being corralled to his room by nursing staff. He was an enormous man, angry, paranoid, pacing, and shouting about how the other patients wanted to attack him. Despite his size, his menacing posture, and that somewhere in his agitation he had ripped off his scrub shirt, I couldn’t help but think, “Well at least the phone and the fire extinguisher are still attached to the wall.”
References
1 J Psychiatr Pract. 2003 Jan;9(1):16-38. Treatment of behavioral emergencies: A summary of the expert consensus guidelines.
2 J Psychiatr Pract. 2005 Nov;11 Suppl 1:5-108; quiz 110-2. The expert consensus guideline series. Treatment of behavioral emergencies 2005.
3 Clin Pract Epidemiol Ment Health. 2016 Oct 27;12:75-86. State of acute agitation at psychiatric emergencies in Europe: The STAGE study.
4 J Emerg Nurs. 2014 May;40(3):218-28; quiz 295. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors.
5 Work. 2010;35(2):191-200. Physical assault among nursing staff employed in acute care.
6 Psychiatr Serv. 1999 Mar;50(3):381-3. Assaults by patients on psychiatric residents: a survey and training recommendations.
Dr. Posada is a second-year resident in the psychiatry & behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at the George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.
Agitation. As a mental health professional, you know it when you see it. While technically, it is the behavior that precedes aggression and violence, every clinical setting has its own flavor, from the voluntary psychiatric unit where I see my most acutely ill patients to the state hospital where I witnessed the type of violence that sent another patient to the emergency department.
Over the past 2 years of residency, I have developed a personal scale for measuring and responding to patient agitation. Through experiences that have provided the lower, upper (and even more upper) bounds to this scale, I have evolved from a nervous first-year intern to become a resident conscious of the need for a cool demeanor and continued engagement with the patient with escalating agitation.
The 2003 Treatment of Behavioral Emergencies: A Summary of the Expert Consensus Guidelines1 provides an excellent visual scale to measure agitation. It begins with a patient’s refusal to cooperate and ascends stepwise toward motor restlessness, lability and loud speech, intimidation, aggression against property, and hostile verbal behavior, and it ends with directly threatening or assaultive behavior.2 Only when I witnessed signs of clinically significant agitation, hostile countenance, unpredictable anger, pacing, clenched fists, yelling, and threats, did I develop a personal understanding and approach for measuring agitation on the inpatient unit.
The upper bound for my scale initially was defined by my first incident of severe agitation and aggression while on call. I no longer remember if the patient was agitated and psychotic or just angry and agitated. Though schizophrenia and bipolar disorder often are the underlying causes of agitation, personality disorders and substance use complicate or contribute to agitation.3 The nurse called me, saying: “Can you come to the unit right now? Mr. X is agitated and has ripped the soap dispenser off the wall in his bathroom.” It was just after midnight, and already several nursing calls into this situation. Verbal de-escalation had failed with Mr. X, who moved from agitation to aggressive behavior. He already had received two doses of haloperidol and lorazepam since my evening shift began. I looked into his bedroom and saw him wrestling with the sink, which did not come off the wall as easily as the soap dispenser. The thought process of an inexperienced intern went like this: “How much haloperidol is too much haloperidol? Will this night never end?” I called my attending for help, and there was desperation in my voice as I explained: “The medications aren’t working.
These situations usually result in good clinical lessons: Medications take time to work, and in some individuals, the “standard cocktail” might not be the best option. Nonetheless, the lag time can be excruciating. As a more experienced resident, I now consider how certain medications may fail an individual, and there might be a better alternative to haloperidol and lorazepam. I’ve expanded my repertoire of pharmacological methods and opt often for second-generation antipsychotics, such as olanzapine or risperidone, without or without a benzodiazepine, when possible.2
Of course, not all agitation becomes a behavioral health emergency, and an integral part of my training as a resident has been watching an attending run an intervention smoothly. It requires coordination and experience, skills that I’m gaining. In these cases, the agitation is addressed before it escalates, nursing staff and the physicians collaborate to deliver treatment, and the patient responds to verbal redirection and, if offered, accepts oral medications. These types of patients help cement the lower bound for my agitation scale.
Nonetheless, the patients who challenge the positive archetype are the ones who cement lessons for physicians. I remember a man who with his history of serious mental illness had adverse reactions to haloperidol, aripiprazole, olanzapine, and fluphenazine. To address his agitation, the nurses prepared 2 mg of lorazepam and 50 mg of diphenhydramine. As the patient ramped up, I heard a nurse sigh, “Why can’t we add IM thorazine?” I commiserated with the nurse; the psychiatric unit is a dangerous place to work. Psychiatry and emergency department nurses, compared with their counterparts in other units, are the most likely to be assaulted at work.4,5 It is personal for me as well. Studies suggest that 30%-40% of psychiatric residents will be attacked during their 4-year training, and I am in that 70%-90% of residents who has been verbally threatened more than once.6
With time and training, my verbal de-escalation techniques have improved, as I’ve learned to avoid threatening and judgmental body language, avoiding a natural tendency to stand with my arms crossed over my chest or hands on my hips. I now more accurately and incisively inquire about a patient’s mental state. How can I address their frustration? In a nonaccusatory way, I let the patients know that they are behaving in a way that is frightening and that continued behavior may have consequences. Even when faced by the heat of agitation, I try to value the patients’ choice: This event will affect our therapeutic relationship in the longer term.
Whenever possible, I want the patients to choose their medication formulation or at least be able to ask them, “Would you be willing to take … ?” With time, I am earning that cool demeanor psychiatrists are known for. I can model calm behavior and effectively use my knowledge about mentalization to try to de-escalate the situation.
My scale of measuring of agitation and violence had its upper level increased significantly from just a soap dispenser being ripped off the wall. One patient really upped the ante by swiping a public telephone off the wall and then went tearing down the hallway to pull the fire extinguisher out of its supposed “safe” case and hurl it. So on a recent night shift, when I heard yelling through the door of the call room, it was with a sense of understanding rather than trepidation that my co-resident and I approached the patient, already being corralled to his room by nursing staff. He was an enormous man, angry, paranoid, pacing, and shouting about how the other patients wanted to attack him. Despite his size, his menacing posture, and that somewhere in his agitation he had ripped off his scrub shirt, I couldn’t help but think, “Well at least the phone and the fire extinguisher are still attached to the wall.”
References
1 J Psychiatr Pract. 2003 Jan;9(1):16-38. Treatment of behavioral emergencies: A summary of the expert consensus guidelines.
2 J Psychiatr Pract. 2005 Nov;11 Suppl 1:5-108; quiz 110-2. The expert consensus guideline series. Treatment of behavioral emergencies 2005.
3 Clin Pract Epidemiol Ment Health. 2016 Oct 27;12:75-86. State of acute agitation at psychiatric emergencies in Europe: The STAGE study.
4 J Emerg Nurs. 2014 May;40(3):218-28; quiz 295. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors.
5 Work. 2010;35(2):191-200. Physical assault among nursing staff employed in acute care.
6 Psychiatr Serv. 1999 Mar;50(3):381-3. Assaults by patients on psychiatric residents: a survey and training recommendations.
Dr. Posada is a second-year resident in the psychiatry & behavioral sciences department at George Washington University, Washington. She completed a bachelor’s degree at the George Washington University. For 2 years after her undergraduate education, she worked at the National Institutes of Allergy and Infectious Diseases studying HIV pathogenesis. Dr. Posada completed her medical degree at the University of Texas Medical Branch in Galveston. Her interests include public psychiatry, health care policy, health disparities, and psychosomatic medicine.