User login
An alternative device for ESRD patients with central venous obstruction
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
Key clinical point: Combined graft-catheter device may preserve femoral access for hemodialysis for patients with central venous obstruction.
Major finding: One-year primary potency rate was 22% and secondary patency rate 60% for device recipients.
Data source: Literature review, including pooled results from eight studies involving 408 subjects.
Disclosures: Dr. Wong reported having no financial disclosures.
Rates of Deep Vein Thrombosis Occurring After Osteotomy About the Knee
Take-Home Points
- DVT and PE are uncommon complications following osteotomies about the knee.
- Use of oral contraceptives can increase the risk of a patient sustaining a postoperative DVT and PE following osteotomies about the knee.
- In the absence of significant risk factors, postoperative chemical DVT prophylaxis may be unnecessary in patients undergoing osteotomies about the knee.
High tibial osteotomy (HTO), distal femoral osteotomy (DFO), and tibial tubercle osteotomy (TTO) are viable treatment options for deformities about the knee and patella maltracking.1-4 Although TTO can be performed in many ways (eg, anteriorization, anteromedialization, medialization), the basic idea is to move the tibial tubercle to improve patellar tracking or to offload a patellar facet that has sustained trauma or degenerated.2 DFO is a surgical option for treating a valgus knee deformity (the lateral tibiofemoral compartment is offloaded) or for protecting a knee compartment after cartilage or meniscal restoration (medial closing wedge or lateral opening wedge).1 Similarly, HTO is an option for treating a varus knee deformity or isolated medial compartment arthritis; the diseased compartment is offloaded, and any malalignment is corrected. Akin to DFO, HTO is often performed to protect a knee compartment, typically the medial tibiofemoral compartment, after cartilage or meniscal restoration.2-4
Compared to most arthroscopic knee surgeries, these osteotomies are much more involved, have longer operative times, and restrict postoperative weight-bearing and range of motion.2-4 The rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) after these osteotomies are not well documented. In addition, there is no documentation of the risks in patients who smoke, are obese, or are using oral contraceptives (OCs) at time of surgery, despite the increased DVT and PE risks posed by smoking, obesity, and OC use in other surgical procedures.5-7 Although the American Academy of Orthopaedic Surgeons (AAOS) issued clinical practice guidelines for DVT/PE prophylaxis after hip and knee arthroplasty, there is no standard prophylaxis guidelines for DVT/PE prevention after HTO, DFO, or TTO.8,9 Last, rates of DVT after total knee arthroplasty (TKA) are well defined; they range from 2% to 12%.10,11 These rates may be surrogates for osteotomies about the knee, but this is only conjecture.
We conducted a study to determine the rates of symptomatic DVT and PE after HTO, DFO, or TTO in patients who did not receive postoperative DVT/PE prophylaxis. We also wanted to determine if age, body mass index (BMI), and smoking status have associations with the risk of developing either DVT or PE after HTO, DFO, or TTO. We hypothesized that the DVT and PE rates would both be <1%.
Methods
After this study was approved by our university’s Institutional Review Board, we searched the surgical database of Dr. Cole, a sports medicine fellowship–trained surgeon, to identify all patients who had HTO, DFO, or TTO performed between September 1, 2009 and September 30, 2014. Current Procedural Terminology (CPT) codes were used for the search. The code for HTO was 27457: osteotomy, proximal tibia, including fibular excision or osteotomy (includes correction of genu varus [bowleg] or genu valgus [knock-knee]); after epiphyseal closure). The code for DFO was 27450: osteotomy, femur, shaft or supracondylar; with fixation. Last, the code for TTO was 27418: anterior tibial tubercleplasty (eg, Maquet-type procedure). The 141 patients identified in the search were treated by Dr. Cole at a single institution and were included in the study. Study inclusion did not require a minimum follow-up. Follow-up duration was defined as the time between surgery and the final clinic note in the patient chart. No patient was excluded for lack of follow-up clinic visits, and none was lost to follow-up.
Age, BMI, smoking status, and OC use were recorded for all patients. For each procedure, the surgeon’s technique remained the same throughout the study period: HTO, medial opening-wedge osteotomy with plate-and-screw fixation; DFO, lateral opening-wedge osteotomy with plate-and-screw fixation; and TTO, mostly anteromedialization with screw fixation (though this was dictated by patellar contact pressures). A tourniquet was used in all cases. Each patient’s hospital electronic medical record and outpatient office notes were reviewed to determine if symptomatic DVT or PE developed after surgery. The diagnosis of symptomatic DVT was based on clinical symptoms and confirmatory ultrasound, and the PE diagnosis was based on computed tomography. Doppler ultrasound was performed only in symptomatic patients (ie, it was not routinely performed).
Per surgeon protocol, postoperative DVT prophylaxis was not administered. Patients were encouraged to begin dorsiflexion and plantar flexion of the ankle (ankle pumps) immediately and to mobilize as soon as comfortable. Each patient received a cold therapy machine with compression sleeve. Patients were allowed toe-touch weight-bearing for 6 weeks, and then progressed 25% per week for 4 weeks to full weight-bearing by 10 weeks. After surgery, each patient was placed in a brace, which was kept locked in extension for 10 days; when the brace was unlocked, the patient was allowed to range the knee.
Continuous variable data are reported as weighted means and weighted standard deviations. Categorical variable data are reported as frequencies and percentages.
Results
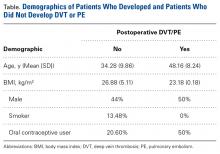
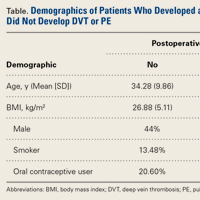
Our database search identified 141 patients (44% male, 56% female) who underwent HTO (47 patients, 33.3%), DFO (13 patients, 9.2%), or TTO (81 patients, 57.5%). Mean (SD) age was 34.28 (9.86) years, mean (SD) BMI was 26.88 (5.11) kg/m2, and mean (SD) follow-up was 17.1 (4.1) months. Of the female patients, 36.7% were using OCs at time of surgery. Of all patients, 13.48% were smokers.
Two patients (1.42%) had clinical symptoms consistent with DVT. In each case, the diagnosis was confirmed with Doppler ultrasound. The below-knee DVT was unilateral in 1 case and bilateral in the other.
The unilateral DVT occurred in a patient who underwent anteromedialization of the tibial tubercle and osteochondral allograft transfer to the lateral femoral condyle for patellar maltracking and a focal trochlear defect. The DVT was diagnosed 8 days after surgery and was treated with warfarin. Low-molecular-weight heparin (LMWH) was used as a bridge until the warfarin level was therapeutic (4 days). This male patient had no significant medical history.
The bilateral DVT with PE occurred in a patient who underwent a medial opening-wedge HTO for a varus deformity with right medial compartment osteoarthritis and a meniscal tear. The DVT and PE were diagnosed 48 hours after surgery, when the patient complained of lightheadedness and lost consciousness. She had no medical problems but was using OCs at time of surgery. The patient died 3 days after surgery and subsequently was found to have a maternal-side family history of DVT (the patient and her family physician had been unaware of this history).
Discussion
As the rates of DVT and PE after osteotomies about the knee have not been well studied, we wanted to determine these rates after HTO, DFO, and TTO in patients who did not receive postoperative DVT prophylaxis. We hypothesized that DVT and PE rates would both be <1%, and this hypothesis was partly confirmed: The rate of PE after HTO, DFO, and TTO was <1%, and the rate of symptomatic DVT was >1%. Similarly, the patients who developed these complications were nonsmokers and had a BMI no higher than that of the patients who did not develop DVT or PE. In addition, only 1 patient developed DVT and PE, and she was using OCs and had a family history of DVT. Last, the patients who developed these complications were on average 14 years older than the patients who did not develop DVT or PE.
Although there is a plethora of reports on the incidence of DVT and PE after TKA, there is little on the incidence after osteotomies about the knee.8,12 The rate of DVT after TKA varies, but many studies place it between 2% and 12%, and routinely find a PE rate of <0.5%.10,11,13,14 Although the AAOS issued a clinical practice guideline for postoperative DVT prophylaxis after TKA, and evaluated the best available evidence, it could not reach consensus on a specific type of DVT prophylaxis, though the workgroup did recommend that patients be administered postoperative DVT prophylaxis of some kind.8,9 Similarly, the American College of Chest Physicians (ACCP) issued clinical practice guidelines for preventing DVT and PE after elective TKA and total hip arthroplasty.15 According to the ACCP guidelines, patients should receive prophylaxis—LMWH, fondaparinux, apixaban, dabigatran, rivaroxaban, low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin, or an intermittent pneumatic compression device—for a minimum of 14 days. Unfortunately, though there are similarities between TKAs and peri-knee osteotomies, these procedures are markedly different, and it is difficult to extrapolate and adapt recommendations and produce a consensus statement for knee arthroplasties. In addition, guidelines exist for hospitalized patients who are being treated for medical conditions or have undergone surgery, but all the patients in the present study had their osteotomies performed on an outpatient basis.
Martin and colleagues16 reviewed 323 cases of medial opening-wedge HTO and found a DVT rate of 1.4% in the absence of routine DVT prophylaxis, except in patients with a history of DVT. Their rate is almost identical to ours, but we also included other osteotomies in our study. Miller and colleagues17 reviewed 46 cases of medial opening-wedge HTO and found a 4.3% DVT rate, despite routine prophylaxis with once-daily 325-mg aspirin and ankle pumps. This finding contrasts with our 1.42% DVT rate in the absence of postoperative chemical DVT prophylaxis. Motycka and colleagues18 reviewed 65 HTO cases in which DVT prophylaxis (oral anticoagulant) was given for 6 weeks, and they found a DVT rate of 9.7%. Turner and colleagues19 performed venous ultrasound on 81 consecutive patients who underwent HTO and received DVT prophylaxis (twice-daily subcutaneous heparin), and they found a DVT rate of 41% and a PE rate of 1.2%, though only 8.6% of the DVT cases were symptomatic. Of note, whereas the lowest postoperative DVT rate was for patients who did not receive postoperative DVT prophylaxis, the rate of symptomatic DVT after these osteotomies ranged from 1.4% to 8.6% in patients who received prophylaxis.16,19 Given this evidence and our study results, it appears routine chemical DVT prophylaxis after osteotomies about the knee may not be necessary, though higher level evidence is needed in order to make definitive recommendations.
In the present study, the 2 patients who developed symptomatic DVT (1 subsequently developed PE) were nonsmokers in good health. The female patient (DVT plus PE) was using OCs at time of surgery. Studies have shown that patients who smoke and who use OCs are at increased risk for developing DVT or PE after surgery.5,6,12 Given that only 2 of our patients developed DVT/PE, and neither was a smoker, smoking was not associated with increased DVT or PE risk in this study population, in which 13.48% of patients were smokers at time of surgery. In addition, given that the 1 female patient who developed DVT/PE was using OCs and that 36.7% of all female patients in the study were using OCs, it is difficult to conclude whether OC use increased the female patient’s risk for DVT or PE. Furthermore, neither the literature nor the AAOS consensus statement supports discontinuing OCs for this surgical procedure.
Patients in this study did not receive chemical or mechanical DVT prophylaxis after surgery. Regarding various post-TKA DVT prophylaxis regimens, aspirin is as effective as LMWH in preventing DVT, and the risk for postoperative blood loss and wound complications is lower with aspirin than with rivaroxaban.20,21 Given that the present study’s postoperative rates of DVT (1.42%) and PE (0.71%) are equal to or less than rates already reported in the literature, routine DVT prophylaxis after osteotomies about the knee may be unnecessary in the absence of other significant risk factors.16,19 However, our study considered only symptomatic DVT and PE, so it is possible that the number of asymptomatic DVT cases is higher in this patient population. Definitively answering our study’s clinical question will require a multicenter registry study (prospective cohort study).
Study Limitations
The strengths of this study include the large number of patients treated by a single surgeon using the same postoperative protocol. Limitations of this study include the lack of a control group. Although we found a DVT rate of 1.42% and a PE rate of 0.71%, the literature on the accepted risks for DVT and PE after HTO, DFO, and TTO is unclear. With our results stratified by procedure, the DVT rate was 2% in the HTO group, 0% in the DFO group, and 1% in the TTO group. However, we were unable to reliably stratify these results by each specific procedure, as the number of patients in each group would be too low. This study involved reviewing charts; as patients were not contacted, it is possible a patient developed DVT or PE, was treated at an outside facility, and then never followed up with the treating surgeon. Patients were identified by CPT codes, so, if a patient underwent HTO, DFO, or TTO that was recorded under a different CPT code, it is possible the patient was missed by our search. All patients were seen after surgery, and we reviewed the outpatient office notes that were taken, so unless the DVT or PE occurred after a patient’s final postoperative visit, it would have been recorded. Similarly, the DVT and PE rates reported here cannot be extrapolated to overall risks for DVT and PE after osteotomies about the knee in all patients—only in patients who did not receive DVT prophylaxis after surgery.
Conclusion
The rates of DVT and PE after HTO, DFO, and TTO in patients who did not receive chemical prophylaxis are low: 1.42% and 0.71%, respectively. After these osteotomies, DVT/PE prophylaxis in the absence of known risk factors may not be warranted.
Am J Orthop. 2017;46(1):E23-E27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19(10):590-599.
2. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
3. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279-289.
4. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015.
5. Ng WM, Chan KY, Lim AB, Gan EC. The incidence of deep venous thrombosis following arthroscopic knee surgery. Med J Malaysia. 2005;60(suppl C):14-16.
6. Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755-760.
7. Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22(7):918-927.
8. Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801-1811.
9. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):777-778.
10. Kim YH, Kulkarni SS, Park JW, Kim JS. Prevalence of deep vein thrombosis and pulmonary embolism treated with mechanical compression device after total knee arthroplasty in Asian patients. J Arthroplasty. 2015;30(9):1633-1637.
11. Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22(7):974-980.
12. Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482-488.
13. Won MH, Lee GW, Lee TJ, Moon KH. Prevalence and risk factors of thromboembolism after joint arthroplasty without chemical thromboprophylaxis in an Asian population. J Arthroplasty. 2011;26(7):1106-1111.
14. Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, Auerbach AD. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053-1060.
15. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
16. Martin R, Birmingham TB, Willits K, Litchfield R, Lebel ME, Giffin JR. Adverse event rates and classifications in medial opening wedge high tibial osteotomy. Am J Sports Med. 2014;42(5):1118-1126.
17. Miller BS, Downie B, McDonough EB, Wojtys EM. Complications after medial opening wedge high tibial osteotomy. Arthroscopy. 2009;25(6):639-646.
18. Motycka T, Eggerth G, Landsiedl F. The incidence of thrombosis in high tibial osteotomies with and without the use of a tourniquet. Arch Orthop Trauma Surg. 2000;120(3-4):157-159.
19. Turner RS, Griffiths H, Heatley FW. The incidence of deep-vein thrombosis after upper tibial osteotomy. A venographic study. J Bone Joint Surg Br. 1993;75(6):942-944.
20. Jiang Y, Du H, Liu J, Zhou Y. Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl). 2014;127(12):2201-2205.
21. Zou Y, Tian S, Wang Y, Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis. 2014;25(7):660-664.
Take-Home Points
- DVT and PE are uncommon complications following osteotomies about the knee.
- Use of oral contraceptives can increase the risk of a patient sustaining a postoperative DVT and PE following osteotomies about the knee.
- In the absence of significant risk factors, postoperative chemical DVT prophylaxis may be unnecessary in patients undergoing osteotomies about the knee.
High tibial osteotomy (HTO), distal femoral osteotomy (DFO), and tibial tubercle osteotomy (TTO) are viable treatment options for deformities about the knee and patella maltracking.1-4 Although TTO can be performed in many ways (eg, anteriorization, anteromedialization, medialization), the basic idea is to move the tibial tubercle to improve patellar tracking or to offload a patellar facet that has sustained trauma or degenerated.2 DFO is a surgical option for treating a valgus knee deformity (the lateral tibiofemoral compartment is offloaded) or for protecting a knee compartment after cartilage or meniscal restoration (medial closing wedge or lateral opening wedge).1 Similarly, HTO is an option for treating a varus knee deformity or isolated medial compartment arthritis; the diseased compartment is offloaded, and any malalignment is corrected. Akin to DFO, HTO is often performed to protect a knee compartment, typically the medial tibiofemoral compartment, after cartilage or meniscal restoration.2-4
Compared to most arthroscopic knee surgeries, these osteotomies are much more involved, have longer operative times, and restrict postoperative weight-bearing and range of motion.2-4 The rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) after these osteotomies are not well documented. In addition, there is no documentation of the risks in patients who smoke, are obese, or are using oral contraceptives (OCs) at time of surgery, despite the increased DVT and PE risks posed by smoking, obesity, and OC use in other surgical procedures.5-7 Although the American Academy of Orthopaedic Surgeons (AAOS) issued clinical practice guidelines for DVT/PE prophylaxis after hip and knee arthroplasty, there is no standard prophylaxis guidelines for DVT/PE prevention after HTO, DFO, or TTO.8,9 Last, rates of DVT after total knee arthroplasty (TKA) are well defined; they range from 2% to 12%.10,11 These rates may be surrogates for osteotomies about the knee, but this is only conjecture.
We conducted a study to determine the rates of symptomatic DVT and PE after HTO, DFO, or TTO in patients who did not receive postoperative DVT/PE prophylaxis. We also wanted to determine if age, body mass index (BMI), and smoking status have associations with the risk of developing either DVT or PE after HTO, DFO, or TTO. We hypothesized that the DVT and PE rates would both be <1%.
Methods
After this study was approved by our university’s Institutional Review Board, we searched the surgical database of Dr. Cole, a sports medicine fellowship–trained surgeon, to identify all patients who had HTO, DFO, or TTO performed between September 1, 2009 and September 30, 2014. Current Procedural Terminology (CPT) codes were used for the search. The code for HTO was 27457: osteotomy, proximal tibia, including fibular excision or osteotomy (includes correction of genu varus [bowleg] or genu valgus [knock-knee]); after epiphyseal closure). The code for DFO was 27450: osteotomy, femur, shaft or supracondylar; with fixation. Last, the code for TTO was 27418: anterior tibial tubercleplasty (eg, Maquet-type procedure). The 141 patients identified in the search were treated by Dr. Cole at a single institution and were included in the study. Study inclusion did not require a minimum follow-up. Follow-up duration was defined as the time between surgery and the final clinic note in the patient chart. No patient was excluded for lack of follow-up clinic visits, and none was lost to follow-up.
Age, BMI, smoking status, and OC use were recorded for all patients. For each procedure, the surgeon’s technique remained the same throughout the study period: HTO, medial opening-wedge osteotomy with plate-and-screw fixation; DFO, lateral opening-wedge osteotomy with plate-and-screw fixation; and TTO, mostly anteromedialization with screw fixation (though this was dictated by patellar contact pressures). A tourniquet was used in all cases. Each patient’s hospital electronic medical record and outpatient office notes were reviewed to determine if symptomatic DVT or PE developed after surgery. The diagnosis of symptomatic DVT was based on clinical symptoms and confirmatory ultrasound, and the PE diagnosis was based on computed tomography. Doppler ultrasound was performed only in symptomatic patients (ie, it was not routinely performed).
Per surgeon protocol, postoperative DVT prophylaxis was not administered. Patients were encouraged to begin dorsiflexion and plantar flexion of the ankle (ankle pumps) immediately and to mobilize as soon as comfortable. Each patient received a cold therapy machine with compression sleeve. Patients were allowed toe-touch weight-bearing for 6 weeks, and then progressed 25% per week for 4 weeks to full weight-bearing by 10 weeks. After surgery, each patient was placed in a brace, which was kept locked in extension for 10 days; when the brace was unlocked, the patient was allowed to range the knee.
Continuous variable data are reported as weighted means and weighted standard deviations. Categorical variable data are reported as frequencies and percentages.
Results
Our database search identified 141 patients (44% male, 56% female) who underwent HTO (47 patients, 33.3%), DFO (13 patients, 9.2%), or TTO (81 patients, 57.5%). Mean (SD) age was 34.28 (9.86) years, mean (SD) BMI was 26.88 (5.11) kg/m2, and mean (SD) follow-up was 17.1 (4.1) months. Of the female patients, 36.7% were using OCs at time of surgery. Of all patients, 13.48% were smokers.
Two patients (1.42%) had clinical symptoms consistent with DVT. In each case, the diagnosis was confirmed with Doppler ultrasound. The below-knee DVT was unilateral in 1 case and bilateral in the other.
The unilateral DVT occurred in a patient who underwent anteromedialization of the tibial tubercle and osteochondral allograft transfer to the lateral femoral condyle for patellar maltracking and a focal trochlear defect. The DVT was diagnosed 8 days after surgery and was treated with warfarin. Low-molecular-weight heparin (LMWH) was used as a bridge until the warfarin level was therapeutic (4 days). This male patient had no significant medical history.
The bilateral DVT with PE occurred in a patient who underwent a medial opening-wedge HTO for a varus deformity with right medial compartment osteoarthritis and a meniscal tear. The DVT and PE were diagnosed 48 hours after surgery, when the patient complained of lightheadedness and lost consciousness. She had no medical problems but was using OCs at time of surgery. The patient died 3 days after surgery and subsequently was found to have a maternal-side family history of DVT (the patient and her family physician had been unaware of this history).
Discussion
As the rates of DVT and PE after osteotomies about the knee have not been well studied, we wanted to determine these rates after HTO, DFO, and TTO in patients who did not receive postoperative DVT prophylaxis. We hypothesized that DVT and PE rates would both be <1%, and this hypothesis was partly confirmed: The rate of PE after HTO, DFO, and TTO was <1%, and the rate of symptomatic DVT was >1%. Similarly, the patients who developed these complications were nonsmokers and had a BMI no higher than that of the patients who did not develop DVT or PE. In addition, only 1 patient developed DVT and PE, and she was using OCs and had a family history of DVT. Last, the patients who developed these complications were on average 14 years older than the patients who did not develop DVT or PE.
Although there is a plethora of reports on the incidence of DVT and PE after TKA, there is little on the incidence after osteotomies about the knee.8,12 The rate of DVT after TKA varies, but many studies place it between 2% and 12%, and routinely find a PE rate of <0.5%.10,11,13,14 Although the AAOS issued a clinical practice guideline for postoperative DVT prophylaxis after TKA, and evaluated the best available evidence, it could not reach consensus on a specific type of DVT prophylaxis, though the workgroup did recommend that patients be administered postoperative DVT prophylaxis of some kind.8,9 Similarly, the American College of Chest Physicians (ACCP) issued clinical practice guidelines for preventing DVT and PE after elective TKA and total hip arthroplasty.15 According to the ACCP guidelines, patients should receive prophylaxis—LMWH, fondaparinux, apixaban, dabigatran, rivaroxaban, low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin, or an intermittent pneumatic compression device—for a minimum of 14 days. Unfortunately, though there are similarities between TKAs and peri-knee osteotomies, these procedures are markedly different, and it is difficult to extrapolate and adapt recommendations and produce a consensus statement for knee arthroplasties. In addition, guidelines exist for hospitalized patients who are being treated for medical conditions or have undergone surgery, but all the patients in the present study had their osteotomies performed on an outpatient basis.
Martin and colleagues16 reviewed 323 cases of medial opening-wedge HTO and found a DVT rate of 1.4% in the absence of routine DVT prophylaxis, except in patients with a history of DVT. Their rate is almost identical to ours, but we also included other osteotomies in our study. Miller and colleagues17 reviewed 46 cases of medial opening-wedge HTO and found a 4.3% DVT rate, despite routine prophylaxis with once-daily 325-mg aspirin and ankle pumps. This finding contrasts with our 1.42% DVT rate in the absence of postoperative chemical DVT prophylaxis. Motycka and colleagues18 reviewed 65 HTO cases in which DVT prophylaxis (oral anticoagulant) was given for 6 weeks, and they found a DVT rate of 9.7%. Turner and colleagues19 performed venous ultrasound on 81 consecutive patients who underwent HTO and received DVT prophylaxis (twice-daily subcutaneous heparin), and they found a DVT rate of 41% and a PE rate of 1.2%, though only 8.6% of the DVT cases were symptomatic. Of note, whereas the lowest postoperative DVT rate was for patients who did not receive postoperative DVT prophylaxis, the rate of symptomatic DVT after these osteotomies ranged from 1.4% to 8.6% in patients who received prophylaxis.16,19 Given this evidence and our study results, it appears routine chemical DVT prophylaxis after osteotomies about the knee may not be necessary, though higher level evidence is needed in order to make definitive recommendations.
In the present study, the 2 patients who developed symptomatic DVT (1 subsequently developed PE) were nonsmokers in good health. The female patient (DVT plus PE) was using OCs at time of surgery. Studies have shown that patients who smoke and who use OCs are at increased risk for developing DVT or PE after surgery.5,6,12 Given that only 2 of our patients developed DVT/PE, and neither was a smoker, smoking was not associated with increased DVT or PE risk in this study population, in which 13.48% of patients were smokers at time of surgery. In addition, given that the 1 female patient who developed DVT/PE was using OCs and that 36.7% of all female patients in the study were using OCs, it is difficult to conclude whether OC use increased the female patient’s risk for DVT or PE. Furthermore, neither the literature nor the AAOS consensus statement supports discontinuing OCs for this surgical procedure.
Patients in this study did not receive chemical or mechanical DVT prophylaxis after surgery. Regarding various post-TKA DVT prophylaxis regimens, aspirin is as effective as LMWH in preventing DVT, and the risk for postoperative blood loss and wound complications is lower with aspirin than with rivaroxaban.20,21 Given that the present study’s postoperative rates of DVT (1.42%) and PE (0.71%) are equal to or less than rates already reported in the literature, routine DVT prophylaxis after osteotomies about the knee may be unnecessary in the absence of other significant risk factors.16,19 However, our study considered only symptomatic DVT and PE, so it is possible that the number of asymptomatic DVT cases is higher in this patient population. Definitively answering our study’s clinical question will require a multicenter registry study (prospective cohort study).
Study Limitations
The strengths of this study include the large number of patients treated by a single surgeon using the same postoperative protocol. Limitations of this study include the lack of a control group. Although we found a DVT rate of 1.42% and a PE rate of 0.71%, the literature on the accepted risks for DVT and PE after HTO, DFO, and TTO is unclear. With our results stratified by procedure, the DVT rate was 2% in the HTO group, 0% in the DFO group, and 1% in the TTO group. However, we were unable to reliably stratify these results by each specific procedure, as the number of patients in each group would be too low. This study involved reviewing charts; as patients were not contacted, it is possible a patient developed DVT or PE, was treated at an outside facility, and then never followed up with the treating surgeon. Patients were identified by CPT codes, so, if a patient underwent HTO, DFO, or TTO that was recorded under a different CPT code, it is possible the patient was missed by our search. All patients were seen after surgery, and we reviewed the outpatient office notes that were taken, so unless the DVT or PE occurred after a patient’s final postoperative visit, it would have been recorded. Similarly, the DVT and PE rates reported here cannot be extrapolated to overall risks for DVT and PE after osteotomies about the knee in all patients—only in patients who did not receive DVT prophylaxis after surgery.
Conclusion
The rates of DVT and PE after HTO, DFO, and TTO in patients who did not receive chemical prophylaxis are low: 1.42% and 0.71%, respectively. After these osteotomies, DVT/PE prophylaxis in the absence of known risk factors may not be warranted.
Am J Orthop. 2017;46(1):E23-E27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- DVT and PE are uncommon complications following osteotomies about the knee.
- Use of oral contraceptives can increase the risk of a patient sustaining a postoperative DVT and PE following osteotomies about the knee.
- In the absence of significant risk factors, postoperative chemical DVT prophylaxis may be unnecessary in patients undergoing osteotomies about the knee.
High tibial osteotomy (HTO), distal femoral osteotomy (DFO), and tibial tubercle osteotomy (TTO) are viable treatment options for deformities about the knee and patella maltracking.1-4 Although TTO can be performed in many ways (eg, anteriorization, anteromedialization, medialization), the basic idea is to move the tibial tubercle to improve patellar tracking or to offload a patellar facet that has sustained trauma or degenerated.2 DFO is a surgical option for treating a valgus knee deformity (the lateral tibiofemoral compartment is offloaded) or for protecting a knee compartment after cartilage or meniscal restoration (medial closing wedge or lateral opening wedge).1 Similarly, HTO is an option for treating a varus knee deformity or isolated medial compartment arthritis; the diseased compartment is offloaded, and any malalignment is corrected. Akin to DFO, HTO is often performed to protect a knee compartment, typically the medial tibiofemoral compartment, after cartilage or meniscal restoration.2-4
Compared to most arthroscopic knee surgeries, these osteotomies are much more involved, have longer operative times, and restrict postoperative weight-bearing and range of motion.2-4 The rates of deep vein thrombosis (DVT) and pulmonary embolism (PE) after these osteotomies are not well documented. In addition, there is no documentation of the risks in patients who smoke, are obese, or are using oral contraceptives (OCs) at time of surgery, despite the increased DVT and PE risks posed by smoking, obesity, and OC use in other surgical procedures.5-7 Although the American Academy of Orthopaedic Surgeons (AAOS) issued clinical practice guidelines for DVT/PE prophylaxis after hip and knee arthroplasty, there is no standard prophylaxis guidelines for DVT/PE prevention after HTO, DFO, or TTO.8,9 Last, rates of DVT after total knee arthroplasty (TKA) are well defined; they range from 2% to 12%.10,11 These rates may be surrogates for osteotomies about the knee, but this is only conjecture.
We conducted a study to determine the rates of symptomatic DVT and PE after HTO, DFO, or TTO in patients who did not receive postoperative DVT/PE prophylaxis. We also wanted to determine if age, body mass index (BMI), and smoking status have associations with the risk of developing either DVT or PE after HTO, DFO, or TTO. We hypothesized that the DVT and PE rates would both be <1%.
Methods
After this study was approved by our university’s Institutional Review Board, we searched the surgical database of Dr. Cole, a sports medicine fellowship–trained surgeon, to identify all patients who had HTO, DFO, or TTO performed between September 1, 2009 and September 30, 2014. Current Procedural Terminology (CPT) codes were used for the search. The code for HTO was 27457: osteotomy, proximal tibia, including fibular excision or osteotomy (includes correction of genu varus [bowleg] or genu valgus [knock-knee]); after epiphyseal closure). The code for DFO was 27450: osteotomy, femur, shaft or supracondylar; with fixation. Last, the code for TTO was 27418: anterior tibial tubercleplasty (eg, Maquet-type procedure). The 141 patients identified in the search were treated by Dr. Cole at a single institution and were included in the study. Study inclusion did not require a minimum follow-up. Follow-up duration was defined as the time between surgery and the final clinic note in the patient chart. No patient was excluded for lack of follow-up clinic visits, and none was lost to follow-up.
Age, BMI, smoking status, and OC use were recorded for all patients. For each procedure, the surgeon’s technique remained the same throughout the study period: HTO, medial opening-wedge osteotomy with plate-and-screw fixation; DFO, lateral opening-wedge osteotomy with plate-and-screw fixation; and TTO, mostly anteromedialization with screw fixation (though this was dictated by patellar contact pressures). A tourniquet was used in all cases. Each patient’s hospital electronic medical record and outpatient office notes were reviewed to determine if symptomatic DVT or PE developed after surgery. The diagnosis of symptomatic DVT was based on clinical symptoms and confirmatory ultrasound, and the PE diagnosis was based on computed tomography. Doppler ultrasound was performed only in symptomatic patients (ie, it was not routinely performed).
Per surgeon protocol, postoperative DVT prophylaxis was not administered. Patients were encouraged to begin dorsiflexion and plantar flexion of the ankle (ankle pumps) immediately and to mobilize as soon as comfortable. Each patient received a cold therapy machine with compression sleeve. Patients were allowed toe-touch weight-bearing for 6 weeks, and then progressed 25% per week for 4 weeks to full weight-bearing by 10 weeks. After surgery, each patient was placed in a brace, which was kept locked in extension for 10 days; when the brace was unlocked, the patient was allowed to range the knee.
Continuous variable data are reported as weighted means and weighted standard deviations. Categorical variable data are reported as frequencies and percentages.
Results
Our database search identified 141 patients (44% male, 56% female) who underwent HTO (47 patients, 33.3%), DFO (13 patients, 9.2%), or TTO (81 patients, 57.5%). Mean (SD) age was 34.28 (9.86) years, mean (SD) BMI was 26.88 (5.11) kg/m2, and mean (SD) follow-up was 17.1 (4.1) months. Of the female patients, 36.7% were using OCs at time of surgery. Of all patients, 13.48% were smokers.
Two patients (1.42%) had clinical symptoms consistent with DVT. In each case, the diagnosis was confirmed with Doppler ultrasound. The below-knee DVT was unilateral in 1 case and bilateral in the other.
The unilateral DVT occurred in a patient who underwent anteromedialization of the tibial tubercle and osteochondral allograft transfer to the lateral femoral condyle for patellar maltracking and a focal trochlear defect. The DVT was diagnosed 8 days after surgery and was treated with warfarin. Low-molecular-weight heparin (LMWH) was used as a bridge until the warfarin level was therapeutic (4 days). This male patient had no significant medical history.
The bilateral DVT with PE occurred in a patient who underwent a medial opening-wedge HTO for a varus deformity with right medial compartment osteoarthritis and a meniscal tear. The DVT and PE were diagnosed 48 hours after surgery, when the patient complained of lightheadedness and lost consciousness. She had no medical problems but was using OCs at time of surgery. The patient died 3 days after surgery and subsequently was found to have a maternal-side family history of DVT (the patient and her family physician had been unaware of this history).
Discussion
As the rates of DVT and PE after osteotomies about the knee have not been well studied, we wanted to determine these rates after HTO, DFO, and TTO in patients who did not receive postoperative DVT prophylaxis. We hypothesized that DVT and PE rates would both be <1%, and this hypothesis was partly confirmed: The rate of PE after HTO, DFO, and TTO was <1%, and the rate of symptomatic DVT was >1%. Similarly, the patients who developed these complications were nonsmokers and had a BMI no higher than that of the patients who did not develop DVT or PE. In addition, only 1 patient developed DVT and PE, and she was using OCs and had a family history of DVT. Last, the patients who developed these complications were on average 14 years older than the patients who did not develop DVT or PE.
Although there is a plethora of reports on the incidence of DVT and PE after TKA, there is little on the incidence after osteotomies about the knee.8,12 The rate of DVT after TKA varies, but many studies place it between 2% and 12%, and routinely find a PE rate of <0.5%.10,11,13,14 Although the AAOS issued a clinical practice guideline for postoperative DVT prophylaxis after TKA, and evaluated the best available evidence, it could not reach consensus on a specific type of DVT prophylaxis, though the workgroup did recommend that patients be administered postoperative DVT prophylaxis of some kind.8,9 Similarly, the American College of Chest Physicians (ACCP) issued clinical practice guidelines for preventing DVT and PE after elective TKA and total hip arthroplasty.15 According to the ACCP guidelines, patients should receive prophylaxis—LMWH, fondaparinux, apixaban, dabigatran, rivaroxaban, low-dose unfractionated heparin, adjusted-dose vitamin K antagonist, aspirin, or an intermittent pneumatic compression device—for a minimum of 14 days. Unfortunately, though there are similarities between TKAs and peri-knee osteotomies, these procedures are markedly different, and it is difficult to extrapolate and adapt recommendations and produce a consensus statement for knee arthroplasties. In addition, guidelines exist for hospitalized patients who are being treated for medical conditions or have undergone surgery, but all the patients in the present study had their osteotomies performed on an outpatient basis.
Martin and colleagues16 reviewed 323 cases of medial opening-wedge HTO and found a DVT rate of 1.4% in the absence of routine DVT prophylaxis, except in patients with a history of DVT. Their rate is almost identical to ours, but we also included other osteotomies in our study. Miller and colleagues17 reviewed 46 cases of medial opening-wedge HTO and found a 4.3% DVT rate, despite routine prophylaxis with once-daily 325-mg aspirin and ankle pumps. This finding contrasts with our 1.42% DVT rate in the absence of postoperative chemical DVT prophylaxis. Motycka and colleagues18 reviewed 65 HTO cases in which DVT prophylaxis (oral anticoagulant) was given for 6 weeks, and they found a DVT rate of 9.7%. Turner and colleagues19 performed venous ultrasound on 81 consecutive patients who underwent HTO and received DVT prophylaxis (twice-daily subcutaneous heparin), and they found a DVT rate of 41% and a PE rate of 1.2%, though only 8.6% of the DVT cases were symptomatic. Of note, whereas the lowest postoperative DVT rate was for patients who did not receive postoperative DVT prophylaxis, the rate of symptomatic DVT after these osteotomies ranged from 1.4% to 8.6% in patients who received prophylaxis.16,19 Given this evidence and our study results, it appears routine chemical DVT prophylaxis after osteotomies about the knee may not be necessary, though higher level evidence is needed in order to make definitive recommendations.
In the present study, the 2 patients who developed symptomatic DVT (1 subsequently developed PE) were nonsmokers in good health. The female patient (DVT plus PE) was using OCs at time of surgery. Studies have shown that patients who smoke and who use OCs are at increased risk for developing DVT or PE after surgery.5,6,12 Given that only 2 of our patients developed DVT/PE, and neither was a smoker, smoking was not associated with increased DVT or PE risk in this study population, in which 13.48% of patients were smokers at time of surgery. In addition, given that the 1 female patient who developed DVT/PE was using OCs and that 36.7% of all female patients in the study were using OCs, it is difficult to conclude whether OC use increased the female patient’s risk for DVT or PE. Furthermore, neither the literature nor the AAOS consensus statement supports discontinuing OCs for this surgical procedure.
Patients in this study did not receive chemical or mechanical DVT prophylaxis after surgery. Regarding various post-TKA DVT prophylaxis regimens, aspirin is as effective as LMWH in preventing DVT, and the risk for postoperative blood loss and wound complications is lower with aspirin than with rivaroxaban.20,21 Given that the present study’s postoperative rates of DVT (1.42%) and PE (0.71%) are equal to or less than rates already reported in the literature, routine DVT prophylaxis after osteotomies about the knee may be unnecessary in the absence of other significant risk factors.16,19 However, our study considered only symptomatic DVT and PE, so it is possible that the number of asymptomatic DVT cases is higher in this patient population. Definitively answering our study’s clinical question will require a multicenter registry study (prospective cohort study).
Study Limitations
The strengths of this study include the large number of patients treated by a single surgeon using the same postoperative protocol. Limitations of this study include the lack of a control group. Although we found a DVT rate of 1.42% and a PE rate of 0.71%, the literature on the accepted risks for DVT and PE after HTO, DFO, and TTO is unclear. With our results stratified by procedure, the DVT rate was 2% in the HTO group, 0% in the DFO group, and 1% in the TTO group. However, we were unable to reliably stratify these results by each specific procedure, as the number of patients in each group would be too low. This study involved reviewing charts; as patients were not contacted, it is possible a patient developed DVT or PE, was treated at an outside facility, and then never followed up with the treating surgeon. Patients were identified by CPT codes, so, if a patient underwent HTO, DFO, or TTO that was recorded under a different CPT code, it is possible the patient was missed by our search. All patients were seen after surgery, and we reviewed the outpatient office notes that were taken, so unless the DVT or PE occurred after a patient’s final postoperative visit, it would have been recorded. Similarly, the DVT and PE rates reported here cannot be extrapolated to overall risks for DVT and PE after osteotomies about the knee in all patients—only in patients who did not receive DVT prophylaxis after surgery.
Conclusion
The rates of DVT and PE after HTO, DFO, and TTO in patients who did not receive chemical prophylaxis are low: 1.42% and 0.71%, respectively. After these osteotomies, DVT/PE prophylaxis in the absence of known risk factors may not be warranted.
Am J Orthop. 2017;46(1):E23-E27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19(10):590-599.
2. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
3. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279-289.
4. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015.
5. Ng WM, Chan KY, Lim AB, Gan EC. The incidence of deep venous thrombosis following arthroscopic knee surgery. Med J Malaysia. 2005;60(suppl C):14-16.
6. Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755-760.
7. Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22(7):918-927.
8. Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801-1811.
9. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):777-778.
10. Kim YH, Kulkarni SS, Park JW, Kim JS. Prevalence of deep vein thrombosis and pulmonary embolism treated with mechanical compression device after total knee arthroplasty in Asian patients. J Arthroplasty. 2015;30(9):1633-1637.
11. Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22(7):974-980.
12. Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482-488.
13. Won MH, Lee GW, Lee TJ, Moon KH. Prevalence and risk factors of thromboembolism after joint arthroplasty without chemical thromboprophylaxis in an Asian population. J Arthroplasty. 2011;26(7):1106-1111.
14. Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, Auerbach AD. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053-1060.
15. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
16. Martin R, Birmingham TB, Willits K, Litchfield R, Lebel ME, Giffin JR. Adverse event rates and classifications in medial opening wedge high tibial osteotomy. Am J Sports Med. 2014;42(5):1118-1126.
17. Miller BS, Downie B, McDonough EB, Wojtys EM. Complications after medial opening wedge high tibial osteotomy. Arthroscopy. 2009;25(6):639-646.
18. Motycka T, Eggerth G, Landsiedl F. The incidence of thrombosis in high tibial osteotomies with and without the use of a tourniquet. Arch Orthop Trauma Surg. 2000;120(3-4):157-159.
19. Turner RS, Griffiths H, Heatley FW. The incidence of deep-vein thrombosis after upper tibial osteotomy. A venographic study. J Bone Joint Surg Br. 1993;75(6):942-944.
20. Jiang Y, Du H, Liu J, Zhou Y. Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl). 2014;127(12):2201-2205.
21. Zou Y, Tian S, Wang Y, Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis. 2014;25(7):660-664.
1. Rossi R, Bonasia DE, Amendola A. The role of high tibial osteotomy in the varus knee. J Am Acad Orthop Surg. 2011;19(10):590-599.
2. Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med. 2014;42(8):2006-2017.
3. Wright JM, Crockett HC, Slawski DP, Madsen MW, Windsor RE. High tibial osteotomy. J Am Acad Orthop Surg. 2005;13(4):279-289.
4. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015.
5. Ng WM, Chan KY, Lim AB, Gan EC. The incidence of deep venous thrombosis following arthroscopic knee surgery. Med J Malaysia. 2005;60(suppl C):14-16.
6. Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop. 2006;77(5):755-760.
7. Wallace G, Judge A, Prieto-Alhambra D, de Vries F, Arden NK, Cooper C. The effect of body mass index on the risk of post-operative complications during the 6 months following total hip replacement or total knee replacement surgery. Osteoarthritis Cartilage. 2014;22(7):918-927.
8. Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(19):1801-1811.
9. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg. 2011;19(12):777-778.
10. Kim YH, Kulkarni SS, Park JW, Kim JS. Prevalence of deep vein thrombosis and pulmonary embolism treated with mechanical compression device after total knee arthroplasty in Asian patients. J Arthroplasty. 2015;30(9):1633-1637.
11. Kim YH, Yoo JH, Kim JS. Factors leading to decreased rates of deep vein thrombosis and pulmonary embolism after total knee arthroplasty. J Arthroplasty. 2007;22(7):974-980.
12. Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472(2):482-488.
13. Won MH, Lee GW, Lee TJ, Moon KH. Prevalence and risk factors of thromboembolism after joint arthroplasty without chemical thromboprophylaxis in an Asian population. J Arthroplasty. 2011;26(7):1106-1111.
14. Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, Auerbach AD. Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty. 2010;25(7):1053-1060.
15. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
16. Martin R, Birmingham TB, Willits K, Litchfield R, Lebel ME, Giffin JR. Adverse event rates and classifications in medial opening wedge high tibial osteotomy. Am J Sports Med. 2014;42(5):1118-1126.
17. Miller BS, Downie B, McDonough EB, Wojtys EM. Complications after medial opening wedge high tibial osteotomy. Arthroscopy. 2009;25(6):639-646.
18. Motycka T, Eggerth G, Landsiedl F. The incidence of thrombosis in high tibial osteotomies with and without the use of a tourniquet. Arch Orthop Trauma Surg. 2000;120(3-4):157-159.
19. Turner RS, Griffiths H, Heatley FW. The incidence of deep-vein thrombosis after upper tibial osteotomy. A venographic study. J Bone Joint Surg Br. 1993;75(6):942-944.
20. Jiang Y, Du H, Liu J, Zhou Y. Aspirin combined with mechanical measures to prevent venous thromboembolism after total knee arthroplasty: a randomized controlled trial. Chin Med J (Engl). 2014;127(12):2201-2205.
21. Zou Y, Tian S, Wang Y, Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis. 2014;25(7):660-664.
Now is time to embrace emerging PAD interventions
CHICAGO – Bioresorbable scaffolds, new drugs, adjuvant interventions, and stem and progenitor cell therapy will change how vascular surgeons treat peripheral artery disease in the next 5 years, so they must embrace these emerging treatments or run the risk of being displaced by other specialists, according to a presentation at a symposium on vascular surgery sponsored by Northwestern University.
“Vascular surgeons must position their practices to be the nexus for the evaluation and treatment of the patient and proactively engage in the critical trials of these new technologies,” said Patrick J. Geraghty, MD, of Washington University, St. Louis. “If our specialty fails to adapt to new treatment options, we risk getting sidelined as critical limb ischemia (CLI) treatment moves into a multimodality model.”
Dr. Geraghty focused on several future directions for PAD treatment: improved drug-eluting stents (DES) for superficial femoral artery disease; drug-coated balloons and modified DES for infrapopliteal disease; biologic modifiers for claudication and CLI; and bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
“You’re not simply a plumber anymore; you’re a biological response modifier,” Dr. Geraghty said, explaining that biologic response modification technologies are the logical successor where standard surgical and endovascular techniques have either fallen short (as in early patency loss due to restenosis) or failed to offer effective alternatives (as in no-option advanced CLI patients). “And that takes many of us out of our comfort zone,” he said.
Dr. Geraghty noted the VIBRANT trial (J Vasc Surg. 2013;58[2]:386-95) and similar studies of non–drug eluting constructs identified early restenosis as the primary culprit in endovascular patency loss. “If you could reduce those early patency losses, you’d have an admirable primary patency rate for these complex lesions,” he said. “We’re able to reconstruct a vessel lumen. The question is, how to best maintain it?”
To answer that, Dr. Geraghty noted that the SIROCCO II trial (J Vasc Interv Radiol. 2005;16[3]:331-8) failed to show an advantage for a sirolimus-eluting stent over bare nitinol stent for superficial femoral artery (SFA) disease, but the subsequent Zilver PTX trial showed the benefits of paclitaxel-eluting stents over 5 years (Circulation. 2016;133[15]:1472-83).
He noted that drug-coated balloons (DCBs) trials have yielded mixed results in infrapopliteal intervention. Most notably, the multicenter In.Pact DEEP trial (Circulation. 2015;131[5]:495-502) failed to show treatment efficacy, Dr. Geraghty said. “The In.Pact DEEP results sharply contrasted with the positive data from trials of similar DCBs in the SFA” (N Engl J Med. 2015;373[2]:145-53).
With regard to DES for infrapopliteal disease, Dr. Geraghty noted the promise of positive results of the ACHILLES (J Am Coll Cardiol. 2012;60[22]:2290-5) and DESTINY (J Vasc Surg. 2012;55[2]:390-9) trials, along with the modest structural changes needed to convert from coronary to proximal tibial applications.
Bioresorbable vascular scaffolds (BVS) for CLI have also made recent advances. “It has been a slow road, but I’m happy that industry has pursued this aggressively,” Dr. Geraghty said. He pointed out that the ESPRIT I trial of bioresorbable everolimus-eluting vascular scaffolds in PAD involving the external iliac artery and SFA reported restenosis rates of 12.1% and 16.1% at 1 and 2 years, respectively (JACC Cardiovasc Interv. 2016;9[11]:1178-87). A trial of the Absorb BVS (Abbott) for short infrapopliteal lesions showed primary patency rates of 96% and 85% at 1 and 2 years, he said (JACC Cardiovasc Interv. 2016;9[7]:715-24).
“Vascular surgeons should be tracking BVS technology closely,” Dr. Geraghty said. “It achieves multiple desirable goals: immediate scaffolding for luminal restoration; mitigation of the restenotic stimulus via stent resorption; drug delivery for inhibition of restenosis; and the prospect of simpler re-interventions.”
Stem/progenitor cell therapies may also provide new solutions for no-option vasculature. One trial that showed “promising trends,” Dr. Geraghty said, is the RESTORE-CLI study of bone marrow aspiration (Mol Ther. 2012;20[6]:1280-6). “This trial reported a trend toward improved time to failure and reduced amputation-free survival, but did not meet its primary endpoint,” he said. “Likewise, the recently presented Biomet MOBILE data failed to meet its primary endpoint, but showed favorable trends in some treatment subgroups” (J Vasc Surg. 2011;54[6]:1650-8).
Dr. Geraghty noted that trial design in this field may need to change directions. “Look at the Delphi consensus matrices for the WIfI (Wound, Ischemia, foot Infection) Threatened limb Classification System (J Vasc Surg. 2014;59[1]:220-34). These show that complex wounds bear a significant risk of amputation, perhaps unmitigated by successful revascularization.” In addition, he called amputation-free survival “a rather blunt instrument” for evaluating how therapies impact limb outcomes and said it can confound the analysis of their effectiveness.
“Instead of confining the progenitor-cell therapies to no-option CLI trials, I’m eager to also see them investigated for treatment of claudication,” Dr. Geraghty said. “Can cell-based therapies possibly displace endovascular interventions as the first-line, least-harmful option for claudication?”
Dr. Geraghty also touched on intra/extravascular adjuvant therapies: antithrombin nanoparticles; inhibitory nanoparticles and polymeric wraps; and adventitial drug delivery techniques, among others.
“It’s critically important for vascular surgeons to position themselves for continued success in CLI treatment,” he said. “That involves aggressive practice branding, active trial participation, critical analysis of new technologies, and adoption of new, even disruptive, treatment modalities that show patient benefit.”
Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
CHICAGO – Bioresorbable scaffolds, new drugs, adjuvant interventions, and stem and progenitor cell therapy will change how vascular surgeons treat peripheral artery disease in the next 5 years, so they must embrace these emerging treatments or run the risk of being displaced by other specialists, according to a presentation at a symposium on vascular surgery sponsored by Northwestern University.
“Vascular surgeons must position their practices to be the nexus for the evaluation and treatment of the patient and proactively engage in the critical trials of these new technologies,” said Patrick J. Geraghty, MD, of Washington University, St. Louis. “If our specialty fails to adapt to new treatment options, we risk getting sidelined as critical limb ischemia (CLI) treatment moves into a multimodality model.”
Dr. Geraghty focused on several future directions for PAD treatment: improved drug-eluting stents (DES) for superficial femoral artery disease; drug-coated balloons and modified DES for infrapopliteal disease; biologic modifiers for claudication and CLI; and bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
“You’re not simply a plumber anymore; you’re a biological response modifier,” Dr. Geraghty said, explaining that biologic response modification technologies are the logical successor where standard surgical and endovascular techniques have either fallen short (as in early patency loss due to restenosis) or failed to offer effective alternatives (as in no-option advanced CLI patients). “And that takes many of us out of our comfort zone,” he said.
Dr. Geraghty noted the VIBRANT trial (J Vasc Surg. 2013;58[2]:386-95) and similar studies of non–drug eluting constructs identified early restenosis as the primary culprit in endovascular patency loss. “If you could reduce those early patency losses, you’d have an admirable primary patency rate for these complex lesions,” he said. “We’re able to reconstruct a vessel lumen. The question is, how to best maintain it?”
To answer that, Dr. Geraghty noted that the SIROCCO II trial (J Vasc Interv Radiol. 2005;16[3]:331-8) failed to show an advantage for a sirolimus-eluting stent over bare nitinol stent for superficial femoral artery (SFA) disease, but the subsequent Zilver PTX trial showed the benefits of paclitaxel-eluting stents over 5 years (Circulation. 2016;133[15]:1472-83).
He noted that drug-coated balloons (DCBs) trials have yielded mixed results in infrapopliteal intervention. Most notably, the multicenter In.Pact DEEP trial (Circulation. 2015;131[5]:495-502) failed to show treatment efficacy, Dr. Geraghty said. “The In.Pact DEEP results sharply contrasted with the positive data from trials of similar DCBs in the SFA” (N Engl J Med. 2015;373[2]:145-53).
With regard to DES for infrapopliteal disease, Dr. Geraghty noted the promise of positive results of the ACHILLES (J Am Coll Cardiol. 2012;60[22]:2290-5) and DESTINY (J Vasc Surg. 2012;55[2]:390-9) trials, along with the modest structural changes needed to convert from coronary to proximal tibial applications.
Bioresorbable vascular scaffolds (BVS) for CLI have also made recent advances. “It has been a slow road, but I’m happy that industry has pursued this aggressively,” Dr. Geraghty said. He pointed out that the ESPRIT I trial of bioresorbable everolimus-eluting vascular scaffolds in PAD involving the external iliac artery and SFA reported restenosis rates of 12.1% and 16.1% at 1 and 2 years, respectively (JACC Cardiovasc Interv. 2016;9[11]:1178-87). A trial of the Absorb BVS (Abbott) for short infrapopliteal lesions showed primary patency rates of 96% and 85% at 1 and 2 years, he said (JACC Cardiovasc Interv. 2016;9[7]:715-24).
“Vascular surgeons should be tracking BVS technology closely,” Dr. Geraghty said. “It achieves multiple desirable goals: immediate scaffolding for luminal restoration; mitigation of the restenotic stimulus via stent resorption; drug delivery for inhibition of restenosis; and the prospect of simpler re-interventions.”
Stem/progenitor cell therapies may also provide new solutions for no-option vasculature. One trial that showed “promising trends,” Dr. Geraghty said, is the RESTORE-CLI study of bone marrow aspiration (Mol Ther. 2012;20[6]:1280-6). “This trial reported a trend toward improved time to failure and reduced amputation-free survival, but did not meet its primary endpoint,” he said. “Likewise, the recently presented Biomet MOBILE data failed to meet its primary endpoint, but showed favorable trends in some treatment subgroups” (J Vasc Surg. 2011;54[6]:1650-8).
Dr. Geraghty noted that trial design in this field may need to change directions. “Look at the Delphi consensus matrices for the WIfI (Wound, Ischemia, foot Infection) Threatened limb Classification System (J Vasc Surg. 2014;59[1]:220-34). These show that complex wounds bear a significant risk of amputation, perhaps unmitigated by successful revascularization.” In addition, he called amputation-free survival “a rather blunt instrument” for evaluating how therapies impact limb outcomes and said it can confound the analysis of their effectiveness.
“Instead of confining the progenitor-cell therapies to no-option CLI trials, I’m eager to also see them investigated for treatment of claudication,” Dr. Geraghty said. “Can cell-based therapies possibly displace endovascular interventions as the first-line, least-harmful option for claudication?”
Dr. Geraghty also touched on intra/extravascular adjuvant therapies: antithrombin nanoparticles; inhibitory nanoparticles and polymeric wraps; and adventitial drug delivery techniques, among others.
“It’s critically important for vascular surgeons to position themselves for continued success in CLI treatment,” he said. “That involves aggressive practice branding, active trial participation, critical analysis of new technologies, and adoption of new, even disruptive, treatment modalities that show patient benefit.”
Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
CHICAGO – Bioresorbable scaffolds, new drugs, adjuvant interventions, and stem and progenitor cell therapy will change how vascular surgeons treat peripheral artery disease in the next 5 years, so they must embrace these emerging treatments or run the risk of being displaced by other specialists, according to a presentation at a symposium on vascular surgery sponsored by Northwestern University.
“Vascular surgeons must position their practices to be the nexus for the evaluation and treatment of the patient and proactively engage in the critical trials of these new technologies,” said Patrick J. Geraghty, MD, of Washington University, St. Louis. “If our specialty fails to adapt to new treatment options, we risk getting sidelined as critical limb ischemia (CLI) treatment moves into a multimodality model.”
Dr. Geraghty focused on several future directions for PAD treatment: improved drug-eluting stents (DES) for superficial femoral artery disease; drug-coated balloons and modified DES for infrapopliteal disease; biologic modifiers for claudication and CLI; and bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
“You’re not simply a plumber anymore; you’re a biological response modifier,” Dr. Geraghty said, explaining that biologic response modification technologies are the logical successor where standard surgical and endovascular techniques have either fallen short (as in early patency loss due to restenosis) or failed to offer effective alternatives (as in no-option advanced CLI patients). “And that takes many of us out of our comfort zone,” he said.
Dr. Geraghty noted the VIBRANT trial (J Vasc Surg. 2013;58[2]:386-95) and similar studies of non–drug eluting constructs identified early restenosis as the primary culprit in endovascular patency loss. “If you could reduce those early patency losses, you’d have an admirable primary patency rate for these complex lesions,” he said. “We’re able to reconstruct a vessel lumen. The question is, how to best maintain it?”
To answer that, Dr. Geraghty noted that the SIROCCO II trial (J Vasc Interv Radiol. 2005;16[3]:331-8) failed to show an advantage for a sirolimus-eluting stent over bare nitinol stent for superficial femoral artery (SFA) disease, but the subsequent Zilver PTX trial showed the benefits of paclitaxel-eluting stents over 5 years (Circulation. 2016;133[15]:1472-83).
He noted that drug-coated balloons (DCBs) trials have yielded mixed results in infrapopliteal intervention. Most notably, the multicenter In.Pact DEEP trial (Circulation. 2015;131[5]:495-502) failed to show treatment efficacy, Dr. Geraghty said. “The In.Pact DEEP results sharply contrasted with the positive data from trials of similar DCBs in the SFA” (N Engl J Med. 2015;373[2]:145-53).
With regard to DES for infrapopliteal disease, Dr. Geraghty noted the promise of positive results of the ACHILLES (J Am Coll Cardiol. 2012;60[22]:2290-5) and DESTINY (J Vasc Surg. 2012;55[2]:390-9) trials, along with the modest structural changes needed to convert from coronary to proximal tibial applications.
Bioresorbable vascular scaffolds (BVS) for CLI have also made recent advances. “It has been a slow road, but I’m happy that industry has pursued this aggressively,” Dr. Geraghty said. He pointed out that the ESPRIT I trial of bioresorbable everolimus-eluting vascular scaffolds in PAD involving the external iliac artery and SFA reported restenosis rates of 12.1% and 16.1% at 1 and 2 years, respectively (JACC Cardiovasc Interv. 2016;9[11]:1178-87). A trial of the Absorb BVS (Abbott) for short infrapopliteal lesions showed primary patency rates of 96% and 85% at 1 and 2 years, he said (JACC Cardiovasc Interv. 2016;9[7]:715-24).
“Vascular surgeons should be tracking BVS technology closely,” Dr. Geraghty said. “It achieves multiple desirable goals: immediate scaffolding for luminal restoration; mitigation of the restenotic stimulus via stent resorption; drug delivery for inhibition of restenosis; and the prospect of simpler re-interventions.”
Stem/progenitor cell therapies may also provide new solutions for no-option vasculature. One trial that showed “promising trends,” Dr. Geraghty said, is the RESTORE-CLI study of bone marrow aspiration (Mol Ther. 2012;20[6]:1280-6). “This trial reported a trend toward improved time to failure and reduced amputation-free survival, but did not meet its primary endpoint,” he said. “Likewise, the recently presented Biomet MOBILE data failed to meet its primary endpoint, but showed favorable trends in some treatment subgroups” (J Vasc Surg. 2011;54[6]:1650-8).
Dr. Geraghty noted that trial design in this field may need to change directions. “Look at the Delphi consensus matrices for the WIfI (Wound, Ischemia, foot Infection) Threatened limb Classification System (J Vasc Surg. 2014;59[1]:220-34). These show that complex wounds bear a significant risk of amputation, perhaps unmitigated by successful revascularization.” In addition, he called amputation-free survival “a rather blunt instrument” for evaluating how therapies impact limb outcomes and said it can confound the analysis of their effectiveness.
“Instead of confining the progenitor-cell therapies to no-option CLI trials, I’m eager to also see them investigated for treatment of claudication,” Dr. Geraghty said. “Can cell-based therapies possibly displace endovascular interventions as the first-line, least-harmful option for claudication?”
Dr. Geraghty also touched on intra/extravascular adjuvant therapies: antithrombin nanoparticles; inhibitory nanoparticles and polymeric wraps; and adventitial drug delivery techniques, among others.
“It’s critically important for vascular surgeons to position themselves for continued success in CLI treatment,” he said. “That involves aggressive practice branding, active trial participation, critical analysis of new technologies, and adoption of new, even disruptive, treatment modalities that show patient benefit.”
Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: Emerging treatments for lower-extremity interventions range from improved drug-eluting stents for the superficial femoral artery and infrapopliteal disease to bioresorbable, drug-eluting scaffolds for infrainguinal interventions.
Major finding: The future of minimally invasive revascularization hinges on reliably reopening stenosed or occluded arteries, maintaining vessel patency and using therapies to stimulate arteriogenesis or angiogenesis without reintervention.
Data source: Review of literature.
Disclosures: Dr. Geraghty disclosed stock ownership in Pulse Therapeutics; consultant fees from Bard Peripheral Vascular, Boston Scientific, Intact Vascular, Bard/Lutonix and Spectranetics; and serving as principal investigator for trials by Cook Medical, Bard/Lutonix, and Intact Vascular, with fees going to Washington University Medical School.
Questioning the Specificity and Sensitivity of ELISA for Bullous Pemphigoid Diagnosis
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.

Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Practice Points
- A low serum level of autoantibodies to BP180 should be interpreted with caution because it is more likely to represent a false-positive than a high serum level.
- Rely on the gold standard for diagnosis of bullous pemphigoid: clinical presentation along with direct immunofluorescence, which can be supported by histology, indirect immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) rather than ELISA alone.
Incompatible Type A plasma found safe for initial resuscitation of trauma patients
HOLLYWOOD, FLA. – Incompatible Type A plasma appears to be a safe and effective part of an initial resuscitation protocol for trauma patients who need a massive transfusion.
There were no increases in morbidity, mortality, or transfusion-related acute lung injury among 120 patients who received Type A plasma, compared with those who got compatible plasma, Bryan C. Morse, MD, said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Type AB blood products are preferred for initial transfusions for trauma patients with unknown blood type. While type AB blood products are universally acceptable to patients, they are also in short supply. In an attempt to mitigate this shortage, some trauma centers are relying on anecdotal data, much drawn from real-life combat experience dating from World War II to present times, suggesting that Type A plasma is safe for initial resuscitation protocols. But the body of data from well-constructed trials is small, said Dr. Morse of Emory University, Atlanta. Thus, EAST sponsored this retrospective registry study, which examined outcomes in 1,536 trauma patients who received plasma transfusions as part of a massive transfusion protocol from 2012 to 2016.
The primary endpoints were overall morbidity, and mortality at four time points: 6 and 24 hours, and 7 and 28 days. Eight trauma centers contributed data to the study.
The group was largely male (75%) with a mean age of 37 years. Patients were seriously injured, with a mean Injury Severity Score (ISS) of 25. About 60% suffered from blunt-force trauma. Among the entire group, 120 (8%) received incompatible type A plasma.
About 28% of patients (434) experienced an adverse event. These were numerically but not significantly more common among the incompatible A plasma group (35% vs. 28%; P = .14). Events included acute respiratory distress syndrome (6% vs. 7.6%), thromboembolism (9% vs. 7%), pneumonia (19% vs. 15%), and acute kidney injury (8% each).
There were two cases of transfusion-related acute lung injury, both of which occurred in the compatible type A group.
Mortality was similar at every time point: 6 hours (16% vs. 15%), 24 hours (25% vs. 22%), 7 days (35% vs. 32%), and 28 days (38% vs. 35%).
A multivariate regression model controlled for treatment center, ISS, units of packed red cells given by 4 hours, mechanism of injury, Type A plasma incompatibility, and age.
In the morbidity analysis, only ISS and units of red blood cells at 4 hours were associated with a significant increase in risk (odd ratio 1.02). Incompatible Type A plasma did not significantly increase the risk of morbidity.
In the mortality analysis, units of red cells, ISS, and age were significantly associated with increased risk. Again, incompatible Type A plasma did not significantly increase the risk of death.
Dr. Morse had no financial declaration.
[email protected]
On Twitter @Alz_Gal
HOLLYWOOD, FLA. – Incompatible Type A plasma appears to be a safe and effective part of an initial resuscitation protocol for trauma patients who need a massive transfusion.
There were no increases in morbidity, mortality, or transfusion-related acute lung injury among 120 patients who received Type A plasma, compared with those who got compatible plasma, Bryan C. Morse, MD, said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Type AB blood products are preferred for initial transfusions for trauma patients with unknown blood type. While type AB blood products are universally acceptable to patients, they are also in short supply. In an attempt to mitigate this shortage, some trauma centers are relying on anecdotal data, much drawn from real-life combat experience dating from World War II to present times, suggesting that Type A plasma is safe for initial resuscitation protocols. But the body of data from well-constructed trials is small, said Dr. Morse of Emory University, Atlanta. Thus, EAST sponsored this retrospective registry study, which examined outcomes in 1,536 trauma patients who received plasma transfusions as part of a massive transfusion protocol from 2012 to 2016.
The primary endpoints were overall morbidity, and mortality at four time points: 6 and 24 hours, and 7 and 28 days. Eight trauma centers contributed data to the study.
The group was largely male (75%) with a mean age of 37 years. Patients were seriously injured, with a mean Injury Severity Score (ISS) of 25. About 60% suffered from blunt-force trauma. Among the entire group, 120 (8%) received incompatible type A plasma.
About 28% of patients (434) experienced an adverse event. These were numerically but not significantly more common among the incompatible A plasma group (35% vs. 28%; P = .14). Events included acute respiratory distress syndrome (6% vs. 7.6%), thromboembolism (9% vs. 7%), pneumonia (19% vs. 15%), and acute kidney injury (8% each).
There were two cases of transfusion-related acute lung injury, both of which occurred in the compatible type A group.
Mortality was similar at every time point: 6 hours (16% vs. 15%), 24 hours (25% vs. 22%), 7 days (35% vs. 32%), and 28 days (38% vs. 35%).
A multivariate regression model controlled for treatment center, ISS, units of packed red cells given by 4 hours, mechanism of injury, Type A plasma incompatibility, and age.
In the morbidity analysis, only ISS and units of red blood cells at 4 hours were associated with a significant increase in risk (odd ratio 1.02). Incompatible Type A plasma did not significantly increase the risk of morbidity.
In the mortality analysis, units of red cells, ISS, and age were significantly associated with increased risk. Again, incompatible Type A plasma did not significantly increase the risk of death.
Dr. Morse had no financial declaration.
[email protected]
On Twitter @Alz_Gal
HOLLYWOOD, FLA. – Incompatible Type A plasma appears to be a safe and effective part of an initial resuscitation protocol for trauma patients who need a massive transfusion.
There were no increases in morbidity, mortality, or transfusion-related acute lung injury among 120 patients who received Type A plasma, compared with those who got compatible plasma, Bryan C. Morse, MD, said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Type AB blood products are preferred for initial transfusions for trauma patients with unknown blood type. While type AB blood products are universally acceptable to patients, they are also in short supply. In an attempt to mitigate this shortage, some trauma centers are relying on anecdotal data, much drawn from real-life combat experience dating from World War II to present times, suggesting that Type A plasma is safe for initial resuscitation protocols. But the body of data from well-constructed trials is small, said Dr. Morse of Emory University, Atlanta. Thus, EAST sponsored this retrospective registry study, which examined outcomes in 1,536 trauma patients who received plasma transfusions as part of a massive transfusion protocol from 2012 to 2016.
The primary endpoints were overall morbidity, and mortality at four time points: 6 and 24 hours, and 7 and 28 days. Eight trauma centers contributed data to the study.
The group was largely male (75%) with a mean age of 37 years. Patients were seriously injured, with a mean Injury Severity Score (ISS) of 25. About 60% suffered from blunt-force trauma. Among the entire group, 120 (8%) received incompatible type A plasma.
About 28% of patients (434) experienced an adverse event. These were numerically but not significantly more common among the incompatible A plasma group (35% vs. 28%; P = .14). Events included acute respiratory distress syndrome (6% vs. 7.6%), thromboembolism (9% vs. 7%), pneumonia (19% vs. 15%), and acute kidney injury (8% each).
There were two cases of transfusion-related acute lung injury, both of which occurred in the compatible type A group.
Mortality was similar at every time point: 6 hours (16% vs. 15%), 24 hours (25% vs. 22%), 7 days (35% vs. 32%), and 28 days (38% vs. 35%).
A multivariate regression model controlled for treatment center, ISS, units of packed red cells given by 4 hours, mechanism of injury, Type A plasma incompatibility, and age.
In the morbidity analysis, only ISS and units of red blood cells at 4 hours were associated with a significant increase in risk (odd ratio 1.02). Incompatible Type A plasma did not significantly increase the risk of morbidity.
In the mortality analysis, units of red cells, ISS, and age were significantly associated with increased risk. Again, incompatible Type A plasma did not significantly increase the risk of death.
Dr. Morse had no financial declaration.
[email protected]
On Twitter @Alz_Gal
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point:
Major finding: Adverse events were not significantly more common among the incompatible A plasma group (35% vs. 28%; P = .14).
Data source: The retrospective study comprised 1,536 patients.
Disclosures: Dr. Morse had no financial disclosures.
How to prepare to care for transgender adolescents
As pediatric and adolescent gynecologists, we are seeing an increasing number of adolescents with gender identity issues and have come to believe that all obstetrician-gynecologists need to have an understanding of varying gender identities, as well as their role in managing these patients’ care.
We had the honor to assist the American College of Obstetricians and Gynecologists’ (ACOG) Committee on Adolescent Health Care in the development of a new Committee Opinion to guide ob.gyns. in caring for transgender adolescents (Obstet Gynecol 2017;129:e11-6). As our culture grows more aware of the nuances and spectrum of gender identity, our health care practices must grow as well. Ob.gyns. are often uniquely positioned as being among the first people transgender adolescents present to – whether it’s signaling disassociation with their gender when answering routine medical questions or directly addressing gender with them as a trusted and private resource. Even when seeing a patient too young to consider hormone therapy, an ob.gyn. can offer vital early behavioral health support, educational and community resources, and specialist referrals.
Transgender adolescent patients have likely faced negative stereotypes and stigmas in social settings or through media that make them cautious and protective of their identity. They are also more likely to face social ostracism such as bullying and/or dissent and rejection from their parents, deepening the vulnerability of their situation. As a result, transgender adolescents can have increased instances of anxiety, depression, sexual harassment, homelessness, and risk-taking behavior. Medical practices can signal to transgender patients that they are safe and welcoming from the start by offering gender neutral forms, brochures, and information for LGBT patients in the waiting room, and having sensitive employees at every step – from the front desk onward.
As we’ve just outlined, transgender adolescent patients face unique challenges, including increased rates of social and mental health risks. In response, ob.gyns. must be prepared to have a comprehensive conversation about health and well-being beyond sexual and reproductive health. They must also be equipped to address the psychosocial issues associated with transgender adolescents. This includes knowledge of what to look for and offering patients resources, education, and referrals to guarantee their health and safety.
It is important that ob.gyns. are aware that transgender men have female reproductive organs and can present with common gynecological problems such as abnormal bleeding, ovarian cysts, and torsion, as well as pregnancy and pregnancy complications. Finally, ob.gyns. can serve a unique role in counseling about fertility and fertility preservation. Thus, not only do we provide essential health care, including ongoing primary care, but we can position ourselves as part of the support network for these adolescents and their families.
Most importantly, when addressing an adolescent transgender patient, we must understand there is no uniform transgender experience. Expressing gender, sexual identity, and behavior patterns will vary from patient to patient. There are a wide range of treatment options available for transgender patients, from hormone to surgical therapies. An ob.gyn.’s responsibility is to help each individual make an informed decision, and help that patient think ahead to the future.
While this all may seem like a lot, it’s important to remember the essential components of our role as health care providers do not change because an adolescent patient is transgender. Care should always include education about their bodies, safe sex, deliberate and thoughtful assessment of symptoms or concerns, and preventive care services such as STI screenings and contraception. We are simply adding more nuanced cultural and medical understanding to those practices.
Dr. Gomez-Lobo is director of pediatric and adolescent obstetrics and gynecology, Medstar Washington Hospital Center/Children’s National Health System, Washington, D.C. Dr. Sokkary is associate professor of ob.gyn. at Navicent Health Center/Mercer School of Medicine in Macon, Ga. They are members of the ACOG Committee on Adolescent Health Care. They reported having no relevant financial disclosures.
As pediatric and adolescent gynecologists, we are seeing an increasing number of adolescents with gender identity issues and have come to believe that all obstetrician-gynecologists need to have an understanding of varying gender identities, as well as their role in managing these patients’ care.
We had the honor to assist the American College of Obstetricians and Gynecologists’ (ACOG) Committee on Adolescent Health Care in the development of a new Committee Opinion to guide ob.gyns. in caring for transgender adolescents (Obstet Gynecol 2017;129:e11-6). As our culture grows more aware of the nuances and spectrum of gender identity, our health care practices must grow as well. Ob.gyns. are often uniquely positioned as being among the first people transgender adolescents present to – whether it’s signaling disassociation with their gender when answering routine medical questions or directly addressing gender with them as a trusted and private resource. Even when seeing a patient too young to consider hormone therapy, an ob.gyn. can offer vital early behavioral health support, educational and community resources, and specialist referrals.
Transgender adolescent patients have likely faced negative stereotypes and stigmas in social settings or through media that make them cautious and protective of their identity. They are also more likely to face social ostracism such as bullying and/or dissent and rejection from their parents, deepening the vulnerability of their situation. As a result, transgender adolescents can have increased instances of anxiety, depression, sexual harassment, homelessness, and risk-taking behavior. Medical practices can signal to transgender patients that they are safe and welcoming from the start by offering gender neutral forms, brochures, and information for LGBT patients in the waiting room, and having sensitive employees at every step – from the front desk onward.
As we’ve just outlined, transgender adolescent patients face unique challenges, including increased rates of social and mental health risks. In response, ob.gyns. must be prepared to have a comprehensive conversation about health and well-being beyond sexual and reproductive health. They must also be equipped to address the psychosocial issues associated with transgender adolescents. This includes knowledge of what to look for and offering patients resources, education, and referrals to guarantee their health and safety.
It is important that ob.gyns. are aware that transgender men have female reproductive organs and can present with common gynecological problems such as abnormal bleeding, ovarian cysts, and torsion, as well as pregnancy and pregnancy complications. Finally, ob.gyns. can serve a unique role in counseling about fertility and fertility preservation. Thus, not only do we provide essential health care, including ongoing primary care, but we can position ourselves as part of the support network for these adolescents and their families.
Most importantly, when addressing an adolescent transgender patient, we must understand there is no uniform transgender experience. Expressing gender, sexual identity, and behavior patterns will vary from patient to patient. There are a wide range of treatment options available for transgender patients, from hormone to surgical therapies. An ob.gyn.’s responsibility is to help each individual make an informed decision, and help that patient think ahead to the future.
While this all may seem like a lot, it’s important to remember the essential components of our role as health care providers do not change because an adolescent patient is transgender. Care should always include education about their bodies, safe sex, deliberate and thoughtful assessment of symptoms or concerns, and preventive care services such as STI screenings and contraception. We are simply adding more nuanced cultural and medical understanding to those practices.
Dr. Gomez-Lobo is director of pediatric and adolescent obstetrics and gynecology, Medstar Washington Hospital Center/Children’s National Health System, Washington, D.C. Dr. Sokkary is associate professor of ob.gyn. at Navicent Health Center/Mercer School of Medicine in Macon, Ga. They are members of the ACOG Committee on Adolescent Health Care. They reported having no relevant financial disclosures.
As pediatric and adolescent gynecologists, we are seeing an increasing number of adolescents with gender identity issues and have come to believe that all obstetrician-gynecologists need to have an understanding of varying gender identities, as well as their role in managing these patients’ care.
We had the honor to assist the American College of Obstetricians and Gynecologists’ (ACOG) Committee on Adolescent Health Care in the development of a new Committee Opinion to guide ob.gyns. in caring for transgender adolescents (Obstet Gynecol 2017;129:e11-6). As our culture grows more aware of the nuances and spectrum of gender identity, our health care practices must grow as well. Ob.gyns. are often uniquely positioned as being among the first people transgender adolescents present to – whether it’s signaling disassociation with their gender when answering routine medical questions or directly addressing gender with them as a trusted and private resource. Even when seeing a patient too young to consider hormone therapy, an ob.gyn. can offer vital early behavioral health support, educational and community resources, and specialist referrals.
Transgender adolescent patients have likely faced negative stereotypes and stigmas in social settings or through media that make them cautious and protective of their identity. They are also more likely to face social ostracism such as bullying and/or dissent and rejection from their parents, deepening the vulnerability of their situation. As a result, transgender adolescents can have increased instances of anxiety, depression, sexual harassment, homelessness, and risk-taking behavior. Medical practices can signal to transgender patients that they are safe and welcoming from the start by offering gender neutral forms, brochures, and information for LGBT patients in the waiting room, and having sensitive employees at every step – from the front desk onward.
As we’ve just outlined, transgender adolescent patients face unique challenges, including increased rates of social and mental health risks. In response, ob.gyns. must be prepared to have a comprehensive conversation about health and well-being beyond sexual and reproductive health. They must also be equipped to address the psychosocial issues associated with transgender adolescents. This includes knowledge of what to look for and offering patients resources, education, and referrals to guarantee their health and safety.
It is important that ob.gyns. are aware that transgender men have female reproductive organs and can present with common gynecological problems such as abnormal bleeding, ovarian cysts, and torsion, as well as pregnancy and pregnancy complications. Finally, ob.gyns. can serve a unique role in counseling about fertility and fertility preservation. Thus, not only do we provide essential health care, including ongoing primary care, but we can position ourselves as part of the support network for these adolescents and their families.
Most importantly, when addressing an adolescent transgender patient, we must understand there is no uniform transgender experience. Expressing gender, sexual identity, and behavior patterns will vary from patient to patient. There are a wide range of treatment options available for transgender patients, from hormone to surgical therapies. An ob.gyn.’s responsibility is to help each individual make an informed decision, and help that patient think ahead to the future.
While this all may seem like a lot, it’s important to remember the essential components of our role as health care providers do not change because an adolescent patient is transgender. Care should always include education about their bodies, safe sex, deliberate and thoughtful assessment of symptoms or concerns, and preventive care services such as STI screenings and contraception. We are simply adding more nuanced cultural and medical understanding to those practices.
Dr. Gomez-Lobo is director of pediatric and adolescent obstetrics and gynecology, Medstar Washington Hospital Center/Children’s National Health System, Washington, D.C. Dr. Sokkary is associate professor of ob.gyn. at Navicent Health Center/Mercer School of Medicine in Macon, Ga. They are members of the ACOG Committee on Adolescent Health Care. They reported having no relevant financial disclosures.
Patient Knowledge and Attitudes About Fecal Microbiota Therapy for Clostridium difficile Infection
Clostridium difficile (C difficile) infection (CDI) is a leading cause of infectious diarrhea among hospitalized patients and, increasingly, in ambulatory patients.1,2 The high prevalence of CDI and the high recurrence rates (15%-30%) led the CDC to categorize C difficile as an "urgent" threat (the highest category) in its 2013 Antimicrobial Resistance Threat Report.3-5 The Infectious Diseases Society of America guideline recommended treatment for CDI is vancomycin or metronidazole; more recent studies also support fidaxomicin use.4,6,7
Patients experiencing recurrent CDI are at risk for further recurrences, such that after the third CDI episode, the risk of subsequent recurrences exceeds 50%.8 This recurrence rate has stimulated research into other treatments, including fecal microbiota transplantation (FMT). A recent systematic review of FMT reports that 85% of patients have resolution of symptoms without recurrence after FMT, although this is based on data from case series and 2 small randomized clinical trials.9
A commonly cited barrier to FMT is patient acceptance. In response to this concern, a previous survey demonstrated that 81% of respondents would opt for FMT to treat a hypothetical case of recurrent CDI.10 However, the surveyed population did not have CDI, and the 48% response rate is concerning, since those with a favorable opinion of FMT might be more willing to complete a survey than would other patients. Accordingly, the authors systematically surveyed hospitalized veterans with active CDI to assess their knowledge, attitudes, and opinions about FMT as a treatment for CDI.
Methods
In-person patient interviews were conducted by one of the study authors at the Minneapolis VA Health Care System (MVAHCS), consisting of 13 to 18 questions. Questions addressed any prior CDI episodes and knowledge of the following: CDI, recurrence risk, and FMT; preferred route and location of FMT administration; concerns regarding FMT; likelihood of agreeing to undergo FMT (if available); and likelihood of enrollment in a hypothetical study comparing FMT to standard antibiotic treatment. The survey was developed internally and was not validated. Questions used the Likert-scale (Survey).
Patients with CDI were identified by monitoring for positive C difficile polymerase chain reaction (PCR) stool tests and then screened for inclusion by medical record review. Inclusion criteria were (1) MVAHCS hospitalization; and (2) written informed consent. Exclusion criteria were the inability to communicate or participate in an interview. Patient responses regarding their likelihood of agreeing to FMT for CDI treatment under different circumstances were compared using Wilcoxon rank sum test. These circumstances included FMT for their current episode of CDI, FMT for a subsequent episode, and FMT if recommended by their physician. Possible concerns regarding FMT also were solicited, including infection risk, effectiveness, and procedural aesthetics. The MVAHCS institutional review board approved the study.
Results
Stool PCR tests for CDI were monitored for 158 days from 2013 to 2014 (based on availability of study staff), yielding 106 positive results. Of those, 31 (29%) were from outpatients and not addressed further. Of the 75 positive CDI tests from 66 hospitalized patients (9 patients had duplicate tests), 18 of 66 (27%) were not able to provide consent and were excluded, leaving 48 eligible patients. Six (13%) were missed for logistic reasons (patient at a test or procedure, discharged before approached, etc), leaving 42 patients who were approached for participation. Among these, 34 (81%) consented to participate in the survey. Two subjects (6%) found the topic so unappealing that they terminated the interview.
The majority of enrolled subjects were men (32/34, 94%), with a mean age of 65.3 years (range, 31-89). Eleven subjects (32%) reported a prior CDI episode, with 10 reporting 1 such episode, and the other 2 episodes. Those with prior CDI reported the effect of CDI on their overall quality of life as 5.1 (1 = no limitation, 10 = severe limitation). Respondents were fairly accurate regarding the risk of recurrence after an initial episode of CDI, with the average expectedrecurrence rate estimated at 33%. In contrast, their estimation of the risk of recurrence after a second CDI episode was lower (28%), although the risk of recurrent episodes increases with each CDI recurrence.
Regarding FMT, 5 subjects indicated awareness of the procedure: 2 learning of it from a news source, 1 from family, 1 from a health care provider, and 1 was unsure of the source. After subjects received a description of FMT, their opinions regarding the procedure were elicited. When asked which route of delivery they would prefer if they were to undergo FMT, the 33 subjects who provided a response indicated a strong preference for either enema (15, 45%) or colonoscopy (10, 30%), compared with just 4 (12%) indicating no preference, 2 (6%) choosing nasogastric tube administration, and 2 (6%) indicating that they would not undergo FMT by any route (P < .001).
Regarding the location of FMT administration (hospital setting vs self-administered at home), 31 of 33 respondents (94%) indicated they would prefer FMT to occur in the hospital vs 2 (6%) preferring self-administration at home (P < .001). The preferred source of donor stool was more evenly distributed, with 14 of 32 respondents (44%) indicating a preference for an anonymous donor, 11 preferring a family member (34%), and 7 (21%) with no preference (P = .21).
Subjects were asked about concerns regarding FMT, and asked to rate each on a 5-point Likert scale (1 = not at all concerning; 5 = overwhelming concern). Concerns regarding risk of infection and effectiveness received an average score of 2.74 and 2.72, respectively, whereas concern regarding the aesthetics, or "yuck factor" was slightly lower (2.1: P = NS for all comparisons). Subjects also were asked to rate the likelihood of undergoing FMT, if it were available, for their current episode of CDI, a subsequent episode of CDI, or if their physician recommended undergoing FMT (10 point scale: 1 = not at all likely; 10 = certainly agree to FMT). The mean scores (SD) for agreeing to FMT for the current or a subsequent episode were 4.8 (SD 2.7) and 5.6 (SD 3.0); P = .12, but increased to 7.1 (SD 3.23) if FMT were recommended by their physician (P < .001 for FMT if physician recommended vs FMT for current episode; P = .001 for FMT if physician recommended vs FMT for a subsequent episode). Finally, subjects were asked about the likelihood of enrolling in a study comparing FMT to standard antimicrobial treatment, with answers ranging from 1 (almost certainly would not enroll) to 5 (almost certainly would enroll). Among the 32 respondents to this question, 17 (53%) answered either "probably would enroll" or "almost certainly would enroll," with a mean score of 3.2.
Discussion
Overall, VA patients with a current episode of CDI were not aware of FMT, with just 15% knowing about the procedure. However, after learning about FMT, patients expressed clear opinions regarding the route and setting of FMT administration, with enema or colonoscopy being the preferred routes, and a hospital the preferred setting. In contrast, subjects expressed ambivalence with regard to the source of donor stool, with no clear preference for stool from an anonymous donor vs from a family member.
When asked about concerns regarding FMT, none of the presented options (risk of infection, uncertain effectiveness, or procedural aesthetics) emerged as significantly more important than did others, although the oft-cited concern regarding FMT aesthetics engendered the lowest overall level of concern. In terms of FMT acceptance, 4 subjects (12%) were opposed to the procedure, indicating that they were not at all likely to agree to FMT for all scenarios (defined as a score of 1 or 2 on the 10-point Likert scale) or by terminating the survey because of the questions. However, 15 (44%) indicated that they would certainly agree to FMT (defined as a score of 9 or 10 on the 10-point Likert scale) if their physician recommended it. Physician recommendation for FMT resulted in the highest overall likelihood of agreeing to FMT, a finding in agreement with a previous survey of FMT for CDI.10 Most subjects indicated likely enrollment in a potential study comparing FMT with standard antimicrobial therapy.
Strengths/Limitations
Study strengths included surveying patients with current CDI, such that patients had personal experience with the disease in question. Use of in-person interviews also resulted in a robust response rate of 81% and allowed subjects to clarify any unclear questions with study personnel. Weaknesses included a relatively small sample size, underrepresentation of women, and lack of detail regarding respondent characteristics. Additionally, capsule delivery of FMT was not assessed since this method of delivery had not been published at the time of survey administration.
Conclusion
This survey of VA patients with CDI suggests that aesthetic concerns are not a critical deterrent for this population, and interest in FMT for the treatment of recurrent CDI exists. Physician recommendation to undergo FMT seems to be the most influential factor affecting the likelihood of agreeing to undergo FMT. These results support the feasibility of conducting clinical trials of FMT in the VA system.
1. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
2. Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201-1205.
3. Johnson S, Louie TJ, Gerding DN, et al; Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354.
4. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013. Updated July 17, 2014. Accessed November 16.2016.
6. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
7. Cornely OA, Crook DW, Esposito R, et al; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
8. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403-410.
9. Drekonja DM, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection--a systematic review. Ann Intern Med. 2015;162(9):630-638.
10. Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652-1658.
Clostridium difficile (C difficile) infection (CDI) is a leading cause of infectious diarrhea among hospitalized patients and, increasingly, in ambulatory patients.1,2 The high prevalence of CDI and the high recurrence rates (15%-30%) led the CDC to categorize C difficile as an "urgent" threat (the highest category) in its 2013 Antimicrobial Resistance Threat Report.3-5 The Infectious Diseases Society of America guideline recommended treatment for CDI is vancomycin or metronidazole; more recent studies also support fidaxomicin use.4,6,7
Patients experiencing recurrent CDI are at risk for further recurrences, such that after the third CDI episode, the risk of subsequent recurrences exceeds 50%.8 This recurrence rate has stimulated research into other treatments, including fecal microbiota transplantation (FMT). A recent systematic review of FMT reports that 85% of patients have resolution of symptoms without recurrence after FMT, although this is based on data from case series and 2 small randomized clinical trials.9
A commonly cited barrier to FMT is patient acceptance. In response to this concern, a previous survey demonstrated that 81% of respondents would opt for FMT to treat a hypothetical case of recurrent CDI.10 However, the surveyed population did not have CDI, and the 48% response rate is concerning, since those with a favorable opinion of FMT might be more willing to complete a survey than would other patients. Accordingly, the authors systematically surveyed hospitalized veterans with active CDI to assess their knowledge, attitudes, and opinions about FMT as a treatment for CDI.
Methods
In-person patient interviews were conducted by one of the study authors at the Minneapolis VA Health Care System (MVAHCS), consisting of 13 to 18 questions. Questions addressed any prior CDI episodes and knowledge of the following: CDI, recurrence risk, and FMT; preferred route and location of FMT administration; concerns regarding FMT; likelihood of agreeing to undergo FMT (if available); and likelihood of enrollment in a hypothetical study comparing FMT to standard antibiotic treatment. The survey was developed internally and was not validated. Questions used the Likert-scale (Survey).
Patients with CDI were identified by monitoring for positive C difficile polymerase chain reaction (PCR) stool tests and then screened for inclusion by medical record review. Inclusion criteria were (1) MVAHCS hospitalization; and (2) written informed consent. Exclusion criteria were the inability to communicate or participate in an interview. Patient responses regarding their likelihood of agreeing to FMT for CDI treatment under different circumstances were compared using Wilcoxon rank sum test. These circumstances included FMT for their current episode of CDI, FMT for a subsequent episode, and FMT if recommended by their physician. Possible concerns regarding FMT also were solicited, including infection risk, effectiveness, and procedural aesthetics. The MVAHCS institutional review board approved the study.
Results
Stool PCR tests for CDI were monitored for 158 days from 2013 to 2014 (based on availability of study staff), yielding 106 positive results. Of those, 31 (29%) were from outpatients and not addressed further. Of the 75 positive CDI tests from 66 hospitalized patients (9 patients had duplicate tests), 18 of 66 (27%) were not able to provide consent and were excluded, leaving 48 eligible patients. Six (13%) were missed for logistic reasons (patient at a test or procedure, discharged before approached, etc), leaving 42 patients who were approached for participation. Among these, 34 (81%) consented to participate in the survey. Two subjects (6%) found the topic so unappealing that they terminated the interview.
The majority of enrolled subjects were men (32/34, 94%), with a mean age of 65.3 years (range, 31-89). Eleven subjects (32%) reported a prior CDI episode, with 10 reporting 1 such episode, and the other 2 episodes. Those with prior CDI reported the effect of CDI on their overall quality of life as 5.1 (1 = no limitation, 10 = severe limitation). Respondents were fairly accurate regarding the risk of recurrence after an initial episode of CDI, with the average expectedrecurrence rate estimated at 33%. In contrast, their estimation of the risk of recurrence after a second CDI episode was lower (28%), although the risk of recurrent episodes increases with each CDI recurrence.
Regarding FMT, 5 subjects indicated awareness of the procedure: 2 learning of it from a news source, 1 from family, 1 from a health care provider, and 1 was unsure of the source. After subjects received a description of FMT, their opinions regarding the procedure were elicited. When asked which route of delivery they would prefer if they were to undergo FMT, the 33 subjects who provided a response indicated a strong preference for either enema (15, 45%) or colonoscopy (10, 30%), compared with just 4 (12%) indicating no preference, 2 (6%) choosing nasogastric tube administration, and 2 (6%) indicating that they would not undergo FMT by any route (P < .001).
Regarding the location of FMT administration (hospital setting vs self-administered at home), 31 of 33 respondents (94%) indicated they would prefer FMT to occur in the hospital vs 2 (6%) preferring self-administration at home (P < .001). The preferred source of donor stool was more evenly distributed, with 14 of 32 respondents (44%) indicating a preference for an anonymous donor, 11 preferring a family member (34%), and 7 (21%) with no preference (P = .21).
Subjects were asked about concerns regarding FMT, and asked to rate each on a 5-point Likert scale (1 = not at all concerning; 5 = overwhelming concern). Concerns regarding risk of infection and effectiveness received an average score of 2.74 and 2.72, respectively, whereas concern regarding the aesthetics, or "yuck factor" was slightly lower (2.1: P = NS for all comparisons). Subjects also were asked to rate the likelihood of undergoing FMT, if it were available, for their current episode of CDI, a subsequent episode of CDI, or if their physician recommended undergoing FMT (10 point scale: 1 = not at all likely; 10 = certainly agree to FMT). The mean scores (SD) for agreeing to FMT for the current or a subsequent episode were 4.8 (SD 2.7) and 5.6 (SD 3.0); P = .12, but increased to 7.1 (SD 3.23) if FMT were recommended by their physician (P < .001 for FMT if physician recommended vs FMT for current episode; P = .001 for FMT if physician recommended vs FMT for a subsequent episode). Finally, subjects were asked about the likelihood of enrolling in a study comparing FMT to standard antimicrobial treatment, with answers ranging from 1 (almost certainly would not enroll) to 5 (almost certainly would enroll). Among the 32 respondents to this question, 17 (53%) answered either "probably would enroll" or "almost certainly would enroll," with a mean score of 3.2.
Discussion
Overall, VA patients with a current episode of CDI were not aware of FMT, with just 15% knowing about the procedure. However, after learning about FMT, patients expressed clear opinions regarding the route and setting of FMT administration, with enema or colonoscopy being the preferred routes, and a hospital the preferred setting. In contrast, subjects expressed ambivalence with regard to the source of donor stool, with no clear preference for stool from an anonymous donor vs from a family member.
When asked about concerns regarding FMT, none of the presented options (risk of infection, uncertain effectiveness, or procedural aesthetics) emerged as significantly more important than did others, although the oft-cited concern regarding FMT aesthetics engendered the lowest overall level of concern. In terms of FMT acceptance, 4 subjects (12%) were opposed to the procedure, indicating that they were not at all likely to agree to FMT for all scenarios (defined as a score of 1 or 2 on the 10-point Likert scale) or by terminating the survey because of the questions. However, 15 (44%) indicated that they would certainly agree to FMT (defined as a score of 9 or 10 on the 10-point Likert scale) if their physician recommended it. Physician recommendation for FMT resulted in the highest overall likelihood of agreeing to FMT, a finding in agreement with a previous survey of FMT for CDI.10 Most subjects indicated likely enrollment in a potential study comparing FMT with standard antimicrobial therapy.
Strengths/Limitations
Study strengths included surveying patients with current CDI, such that patients had personal experience with the disease in question. Use of in-person interviews also resulted in a robust response rate of 81% and allowed subjects to clarify any unclear questions with study personnel. Weaknesses included a relatively small sample size, underrepresentation of women, and lack of detail regarding respondent characteristics. Additionally, capsule delivery of FMT was not assessed since this method of delivery had not been published at the time of survey administration.
Conclusion
This survey of VA patients with CDI suggests that aesthetic concerns are not a critical deterrent for this population, and interest in FMT for the treatment of recurrent CDI exists. Physician recommendation to undergo FMT seems to be the most influential factor affecting the likelihood of agreeing to undergo FMT. These results support the feasibility of conducting clinical trials of FMT in the VA system.
Clostridium difficile (C difficile) infection (CDI) is a leading cause of infectious diarrhea among hospitalized patients and, increasingly, in ambulatory patients.1,2 The high prevalence of CDI and the high recurrence rates (15%-30%) led the CDC to categorize C difficile as an "urgent" threat (the highest category) in its 2013 Antimicrobial Resistance Threat Report.3-5 The Infectious Diseases Society of America guideline recommended treatment for CDI is vancomycin or metronidazole; more recent studies also support fidaxomicin use.4,6,7
Patients experiencing recurrent CDI are at risk for further recurrences, such that after the third CDI episode, the risk of subsequent recurrences exceeds 50%.8 This recurrence rate has stimulated research into other treatments, including fecal microbiota transplantation (FMT). A recent systematic review of FMT reports that 85% of patients have resolution of symptoms without recurrence after FMT, although this is based on data from case series and 2 small randomized clinical trials.9
A commonly cited barrier to FMT is patient acceptance. In response to this concern, a previous survey demonstrated that 81% of respondents would opt for FMT to treat a hypothetical case of recurrent CDI.10 However, the surveyed population did not have CDI, and the 48% response rate is concerning, since those with a favorable opinion of FMT might be more willing to complete a survey than would other patients. Accordingly, the authors systematically surveyed hospitalized veterans with active CDI to assess their knowledge, attitudes, and opinions about FMT as a treatment for CDI.
Methods
In-person patient interviews were conducted by one of the study authors at the Minneapolis VA Health Care System (MVAHCS), consisting of 13 to 18 questions. Questions addressed any prior CDI episodes and knowledge of the following: CDI, recurrence risk, and FMT; preferred route and location of FMT administration; concerns regarding FMT; likelihood of agreeing to undergo FMT (if available); and likelihood of enrollment in a hypothetical study comparing FMT to standard antibiotic treatment. The survey was developed internally and was not validated. Questions used the Likert-scale (Survey).
Patients with CDI were identified by monitoring for positive C difficile polymerase chain reaction (PCR) stool tests and then screened for inclusion by medical record review. Inclusion criteria were (1) MVAHCS hospitalization; and (2) written informed consent. Exclusion criteria were the inability to communicate or participate in an interview. Patient responses regarding their likelihood of agreeing to FMT for CDI treatment under different circumstances were compared using Wilcoxon rank sum test. These circumstances included FMT for their current episode of CDI, FMT for a subsequent episode, and FMT if recommended by their physician. Possible concerns regarding FMT also were solicited, including infection risk, effectiveness, and procedural aesthetics. The MVAHCS institutional review board approved the study.
Results
Stool PCR tests for CDI were monitored for 158 days from 2013 to 2014 (based on availability of study staff), yielding 106 positive results. Of those, 31 (29%) were from outpatients and not addressed further. Of the 75 positive CDI tests from 66 hospitalized patients (9 patients had duplicate tests), 18 of 66 (27%) were not able to provide consent and were excluded, leaving 48 eligible patients. Six (13%) were missed for logistic reasons (patient at a test or procedure, discharged before approached, etc), leaving 42 patients who were approached for participation. Among these, 34 (81%) consented to participate in the survey. Two subjects (6%) found the topic so unappealing that they terminated the interview.
The majority of enrolled subjects were men (32/34, 94%), with a mean age of 65.3 years (range, 31-89). Eleven subjects (32%) reported a prior CDI episode, with 10 reporting 1 such episode, and the other 2 episodes. Those with prior CDI reported the effect of CDI on their overall quality of life as 5.1 (1 = no limitation, 10 = severe limitation). Respondents were fairly accurate regarding the risk of recurrence after an initial episode of CDI, with the average expectedrecurrence rate estimated at 33%. In contrast, their estimation of the risk of recurrence after a second CDI episode was lower (28%), although the risk of recurrent episodes increases with each CDI recurrence.
Regarding FMT, 5 subjects indicated awareness of the procedure: 2 learning of it from a news source, 1 from family, 1 from a health care provider, and 1 was unsure of the source. After subjects received a description of FMT, their opinions regarding the procedure were elicited. When asked which route of delivery they would prefer if they were to undergo FMT, the 33 subjects who provided a response indicated a strong preference for either enema (15, 45%) or colonoscopy (10, 30%), compared with just 4 (12%) indicating no preference, 2 (6%) choosing nasogastric tube administration, and 2 (6%) indicating that they would not undergo FMT by any route (P < .001).
Regarding the location of FMT administration (hospital setting vs self-administered at home), 31 of 33 respondents (94%) indicated they would prefer FMT to occur in the hospital vs 2 (6%) preferring self-administration at home (P < .001). The preferred source of donor stool was more evenly distributed, with 14 of 32 respondents (44%) indicating a preference for an anonymous donor, 11 preferring a family member (34%), and 7 (21%) with no preference (P = .21).
Subjects were asked about concerns regarding FMT, and asked to rate each on a 5-point Likert scale (1 = not at all concerning; 5 = overwhelming concern). Concerns regarding risk of infection and effectiveness received an average score of 2.74 and 2.72, respectively, whereas concern regarding the aesthetics, or "yuck factor" was slightly lower (2.1: P = NS for all comparisons). Subjects also were asked to rate the likelihood of undergoing FMT, if it were available, for their current episode of CDI, a subsequent episode of CDI, or if their physician recommended undergoing FMT (10 point scale: 1 = not at all likely; 10 = certainly agree to FMT). The mean scores (SD) for agreeing to FMT for the current or a subsequent episode were 4.8 (SD 2.7) and 5.6 (SD 3.0); P = .12, but increased to 7.1 (SD 3.23) if FMT were recommended by their physician (P < .001 for FMT if physician recommended vs FMT for current episode; P = .001 for FMT if physician recommended vs FMT for a subsequent episode). Finally, subjects were asked about the likelihood of enrolling in a study comparing FMT to standard antimicrobial treatment, with answers ranging from 1 (almost certainly would not enroll) to 5 (almost certainly would enroll). Among the 32 respondents to this question, 17 (53%) answered either "probably would enroll" or "almost certainly would enroll," with a mean score of 3.2.
Discussion
Overall, VA patients with a current episode of CDI were not aware of FMT, with just 15% knowing about the procedure. However, after learning about FMT, patients expressed clear opinions regarding the route and setting of FMT administration, with enema or colonoscopy being the preferred routes, and a hospital the preferred setting. In contrast, subjects expressed ambivalence with regard to the source of donor stool, with no clear preference for stool from an anonymous donor vs from a family member.
When asked about concerns regarding FMT, none of the presented options (risk of infection, uncertain effectiveness, or procedural aesthetics) emerged as significantly more important than did others, although the oft-cited concern regarding FMT aesthetics engendered the lowest overall level of concern. In terms of FMT acceptance, 4 subjects (12%) were opposed to the procedure, indicating that they were not at all likely to agree to FMT for all scenarios (defined as a score of 1 or 2 on the 10-point Likert scale) or by terminating the survey because of the questions. However, 15 (44%) indicated that they would certainly agree to FMT (defined as a score of 9 or 10 on the 10-point Likert scale) if their physician recommended it. Physician recommendation for FMT resulted in the highest overall likelihood of agreeing to FMT, a finding in agreement with a previous survey of FMT for CDI.10 Most subjects indicated likely enrollment in a potential study comparing FMT with standard antimicrobial therapy.
Strengths/Limitations
Study strengths included surveying patients with current CDI, such that patients had personal experience with the disease in question. Use of in-person interviews also resulted in a robust response rate of 81% and allowed subjects to clarify any unclear questions with study personnel. Weaknesses included a relatively small sample size, underrepresentation of women, and lack of detail regarding respondent characteristics. Additionally, capsule delivery of FMT was not assessed since this method of delivery had not been published at the time of survey administration.
Conclusion
This survey of VA patients with CDI suggests that aesthetic concerns are not a critical deterrent for this population, and interest in FMT for the treatment of recurrent CDI exists. Physician recommendation to undergo FMT seems to be the most influential factor affecting the likelihood of agreeing to undergo FMT. These results support the feasibility of conducting clinical trials of FMT in the VA system.
1. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
2. Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201-1205.
3. Johnson S, Louie TJ, Gerding DN, et al; Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354.
4. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013. Updated July 17, 2014. Accessed November 16.2016.
6. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
7. Cornely OA, Crook DW, Esposito R, et al; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
8. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403-410.
9. Drekonja DM, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection--a systematic review. Ann Intern Med. 2015;162(9):630-638.
10. Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652-1658.
1. Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
2. Centers for Disease Control and Prevention. Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201-1205.
3. Johnson S, Louie TJ, Gerding DN, et al; Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59(3):345-354.
4. Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-431.
5. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013. Updated July 17, 2014. Accessed November 16.2016.
6. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
7. Cornely OA, Crook DW, Esposito R, et al; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281-289.
8. Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403-410.
9. Drekonja DM, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection--a systematic review. Ann Intern Med. 2015;162(9):630-638.
10. Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652-1658.
Sexually Transmitted Disease Cases Hit a High
Cases of the most commonly reported STDs reached an “unprecedented” high in the US in 2015, with > 1.5 million chlamydia cases, nearly 400,000 gonorrhea cases, and nearly 24,000 cases of primary and secondary syphilis.
According to the CDC’s annual Sexually Transmitted Disease Surveillance Report, between 2014 and 2015, the number of syphilis cases rose by 19%, followed by gonorrhea (12.8%) and chlamydia (5.9%). Young people aged 15 to 24 accounted for nearly two thirds of chlamydia diagnoses and half of gonorrhea diagnoses. Men who have sex with men accounted for most new gonorrhea and syphilis cases. The report also notes that antibiotic-resistant gonorrhea may be higher in this group.
Syphilis diagnoses in women jumped by > 27% in 1 year, which presents a serious risk for infants. For example, reported congenital syphilis (transmitted from a pregnant woman to the baby) rose by 6%.
But all 3 of those STDs are not only treatable, they’re curable with antibiotics. Widespread access to screening and treatment would reduce the spread. Undiagnosed and untreated, these diseases pose severe and often irreversible health consequences, including infertility, chronic pain, and a greater risk of acquiring HIV. The CDC also estimates a ”substantial economic burden” of nearly $16 billion a year.
In recent years, > 50% of state and local STD programs have had their budgets cut, the report notes, and > 20 health department STD clinics closed their doors in 1 year alone. “STD prevention resources across the nation are stretched thin,” said Dr. Jonathan Mermin, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention.
The CDC says an effective national response to the epidemic requires engagement from many players. One suggestion: making screening a standard part of medical care, especially for pregnant women, and integrating STD prevention and treatment into prenatal care and other routine visits.
Cases of the most commonly reported STDs reached an “unprecedented” high in the US in 2015, with > 1.5 million chlamydia cases, nearly 400,000 gonorrhea cases, and nearly 24,000 cases of primary and secondary syphilis.
According to the CDC’s annual Sexually Transmitted Disease Surveillance Report, between 2014 and 2015, the number of syphilis cases rose by 19%, followed by gonorrhea (12.8%) and chlamydia (5.9%). Young people aged 15 to 24 accounted for nearly two thirds of chlamydia diagnoses and half of gonorrhea diagnoses. Men who have sex with men accounted for most new gonorrhea and syphilis cases. The report also notes that antibiotic-resistant gonorrhea may be higher in this group.
Syphilis diagnoses in women jumped by > 27% in 1 year, which presents a serious risk for infants. For example, reported congenital syphilis (transmitted from a pregnant woman to the baby) rose by 6%.
But all 3 of those STDs are not only treatable, they’re curable with antibiotics. Widespread access to screening and treatment would reduce the spread. Undiagnosed and untreated, these diseases pose severe and often irreversible health consequences, including infertility, chronic pain, and a greater risk of acquiring HIV. The CDC also estimates a ”substantial economic burden” of nearly $16 billion a year.
In recent years, > 50% of state and local STD programs have had their budgets cut, the report notes, and > 20 health department STD clinics closed their doors in 1 year alone. “STD prevention resources across the nation are stretched thin,” said Dr. Jonathan Mermin, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention.
The CDC says an effective national response to the epidemic requires engagement from many players. One suggestion: making screening a standard part of medical care, especially for pregnant women, and integrating STD prevention and treatment into prenatal care and other routine visits.
Cases of the most commonly reported STDs reached an “unprecedented” high in the US in 2015, with > 1.5 million chlamydia cases, nearly 400,000 gonorrhea cases, and nearly 24,000 cases of primary and secondary syphilis.
According to the CDC’s annual Sexually Transmitted Disease Surveillance Report, between 2014 and 2015, the number of syphilis cases rose by 19%, followed by gonorrhea (12.8%) and chlamydia (5.9%). Young people aged 15 to 24 accounted for nearly two thirds of chlamydia diagnoses and half of gonorrhea diagnoses. Men who have sex with men accounted for most new gonorrhea and syphilis cases. The report also notes that antibiotic-resistant gonorrhea may be higher in this group.
Syphilis diagnoses in women jumped by > 27% in 1 year, which presents a serious risk for infants. For example, reported congenital syphilis (transmitted from a pregnant woman to the baby) rose by 6%.
But all 3 of those STDs are not only treatable, they’re curable with antibiotics. Widespread access to screening and treatment would reduce the spread. Undiagnosed and untreated, these diseases pose severe and often irreversible health consequences, including infertility, chronic pain, and a greater risk of acquiring HIV. The CDC also estimates a ”substantial economic burden” of nearly $16 billion a year.
In recent years, > 50% of state and local STD programs have had their budgets cut, the report notes, and > 20 health department STD clinics closed their doors in 1 year alone. “STD prevention resources across the nation are stretched thin,” said Dr. Jonathan Mermin, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention.
The CDC says an effective national response to the epidemic requires engagement from many players. One suggestion: making screening a standard part of medical care, especially for pregnant women, and integrating STD prevention and treatment into prenatal care and other routine visits.
Anticoagulants often unnecessary after surgery, analysis suggests

Many surgical patients may be receiving anticoagulants they don’t need, according to research published in Annals of Surgery.
The study challenges standard of care guidelines, which recommend that all general surgery patients receive treatment to prevent venous thromboembolism (VTE).
The new findings suggest that anticoagulants may be unnecessary for most surgical patients and could even be harmful to some.
“A ‘one-size-fits-all approach’ doesn’t always make sense,” said study author Christopher Pannucci, MD, of the University of Utah in Salt Lake City.
“A healthy 35-year-old is very different from someone who is 85 and has a history of clots. Our research indicates that there could be a substantial number of people who are being over-treated.”
Dr Pannucci and his colleagues reviewed data from 13 studies to determine which surgical patients were most likely, and least likely, to benefit from anticoagulants. There was data on VTE events in 11 studies (n=14,776) and data on clinically relevant bleeding in 8 studies (n=7590).
In most of the studies, patients received mechanical VTE prophylaxis, which meant elastic compression and/or sequential compression devices.
Some studies compared mechanical prophylaxis to anticoagulants, including heparin, low-molecular-weight heparin, direct factor Xa inhibitors, direct thrombin inhibitors, warfarin, dextran, and aspirin.
The studies included a broad range of surgical patients, from individuals with few VTE risk factors to those with multiple risk factors, such as obesity, advanced age, and personal or family history of VTE.
The patients were divided into 1 of 5 categories indicating overall VTE risk. Assessment was based on the Caprini score.
VTE risk without anticoagulant treatment
There were 11 studies in which some patients did not receive anticoagulants (n=6085).
Among these patients, those who were classified as having the highest risk of VTE were 14 times more likely to develop VTE than patients in the low-risk category—10.7% vs 0.7%.
These findings were independent of surgery type.
“It was eye-opening to see that there is this huge variability in risk among the overall group of patients that walk into your office,” Dr Pannucci said. “Unless you consider a patient’s risk based on their individual factors, you would never know.”
VTE outcomes by risk score
When given, anticoagulants did significantly reduce the risk of VTE for the overall study population and for high-risk patients.
The odds ratios (ORs) were 0.66 (P=0.001) for the overall population, 0.60 (P=0.04) for patients with Caprini scores of 7 to 8, and 0.41 (P=0.0002) for patients with scores higher than 8.
Unfortunately, anticoagulants did not make a significant difference in VTE rates for mid- or low-risk patients.
The ORs were 0.45 (P=0.31) for patients with Caprini scores of 0 to 2, 1.31 (P=0.57) for patients with scores of 3 to 4, and 0.96 (P=0.85) for patients with scores of 5 to 6.
Risk of bleeding
Anticoagulants significantly increased clinically relevant bleeding for the overall population. The OR was 1.69 (P=0.006).
Patients who received anticoagulants were not significantly more likely to have clinically relevant bleeding if they had risk scores of 0 to 2 (OR=2.47, P=0.61), 3 to 4 (OR=1.05, P=0.87), 5 to 6 (OR=2.10, P=0.06), 7 to 8 (OR=3.15, P=0.16), or >8 (OR=2.31, P=0.16).
“For the first time, we have data that prophylaxis for the highest-risk groups is beneficial, and data that suggests that lower-risk patients may need no prophylaxis,” said study author Peter Henke, MD, of the University of Michigan in Ann Arbor.
He and his colleagues noted, however, that prospective studies are needed to confirm these findings. ![]()

Many surgical patients may be receiving anticoagulants they don’t need, according to research published in Annals of Surgery.
The study challenges standard of care guidelines, which recommend that all general surgery patients receive treatment to prevent venous thromboembolism (VTE).
The new findings suggest that anticoagulants may be unnecessary for most surgical patients and could even be harmful to some.
“A ‘one-size-fits-all approach’ doesn’t always make sense,” said study author Christopher Pannucci, MD, of the University of Utah in Salt Lake City.
“A healthy 35-year-old is very different from someone who is 85 and has a history of clots. Our research indicates that there could be a substantial number of people who are being over-treated.”
Dr Pannucci and his colleagues reviewed data from 13 studies to determine which surgical patients were most likely, and least likely, to benefit from anticoagulants. There was data on VTE events in 11 studies (n=14,776) and data on clinically relevant bleeding in 8 studies (n=7590).
In most of the studies, patients received mechanical VTE prophylaxis, which meant elastic compression and/or sequential compression devices.
Some studies compared mechanical prophylaxis to anticoagulants, including heparin, low-molecular-weight heparin, direct factor Xa inhibitors, direct thrombin inhibitors, warfarin, dextran, and aspirin.
The studies included a broad range of surgical patients, from individuals with few VTE risk factors to those with multiple risk factors, such as obesity, advanced age, and personal or family history of VTE.
The patients were divided into 1 of 5 categories indicating overall VTE risk. Assessment was based on the Caprini score.
VTE risk without anticoagulant treatment
There were 11 studies in which some patients did not receive anticoagulants (n=6085).
Among these patients, those who were classified as having the highest risk of VTE were 14 times more likely to develop VTE than patients in the low-risk category—10.7% vs 0.7%.
These findings were independent of surgery type.
“It was eye-opening to see that there is this huge variability in risk among the overall group of patients that walk into your office,” Dr Pannucci said. “Unless you consider a patient’s risk based on their individual factors, you would never know.”
VTE outcomes by risk score
When given, anticoagulants did significantly reduce the risk of VTE for the overall study population and for high-risk patients.
The odds ratios (ORs) were 0.66 (P=0.001) for the overall population, 0.60 (P=0.04) for patients with Caprini scores of 7 to 8, and 0.41 (P=0.0002) for patients with scores higher than 8.
Unfortunately, anticoagulants did not make a significant difference in VTE rates for mid- or low-risk patients.
The ORs were 0.45 (P=0.31) for patients with Caprini scores of 0 to 2, 1.31 (P=0.57) for patients with scores of 3 to 4, and 0.96 (P=0.85) for patients with scores of 5 to 6.
Risk of bleeding
Anticoagulants significantly increased clinically relevant bleeding for the overall population. The OR was 1.69 (P=0.006).
Patients who received anticoagulants were not significantly more likely to have clinically relevant bleeding if they had risk scores of 0 to 2 (OR=2.47, P=0.61), 3 to 4 (OR=1.05, P=0.87), 5 to 6 (OR=2.10, P=0.06), 7 to 8 (OR=3.15, P=0.16), or >8 (OR=2.31, P=0.16).
“For the first time, we have data that prophylaxis for the highest-risk groups is beneficial, and data that suggests that lower-risk patients may need no prophylaxis,” said study author Peter Henke, MD, of the University of Michigan in Ann Arbor.
He and his colleagues noted, however, that prospective studies are needed to confirm these findings. ![]()

Many surgical patients may be receiving anticoagulants they don’t need, according to research published in Annals of Surgery.
The study challenges standard of care guidelines, which recommend that all general surgery patients receive treatment to prevent venous thromboembolism (VTE).
The new findings suggest that anticoagulants may be unnecessary for most surgical patients and could even be harmful to some.
“A ‘one-size-fits-all approach’ doesn’t always make sense,” said study author Christopher Pannucci, MD, of the University of Utah in Salt Lake City.
“A healthy 35-year-old is very different from someone who is 85 and has a history of clots. Our research indicates that there could be a substantial number of people who are being over-treated.”
Dr Pannucci and his colleagues reviewed data from 13 studies to determine which surgical patients were most likely, and least likely, to benefit from anticoagulants. There was data on VTE events in 11 studies (n=14,776) and data on clinically relevant bleeding in 8 studies (n=7590).
In most of the studies, patients received mechanical VTE prophylaxis, which meant elastic compression and/or sequential compression devices.
Some studies compared mechanical prophylaxis to anticoagulants, including heparin, low-molecular-weight heparin, direct factor Xa inhibitors, direct thrombin inhibitors, warfarin, dextran, and aspirin.
The studies included a broad range of surgical patients, from individuals with few VTE risk factors to those with multiple risk factors, such as obesity, advanced age, and personal or family history of VTE.
The patients were divided into 1 of 5 categories indicating overall VTE risk. Assessment was based on the Caprini score.
VTE risk without anticoagulant treatment
There were 11 studies in which some patients did not receive anticoagulants (n=6085).
Among these patients, those who were classified as having the highest risk of VTE were 14 times more likely to develop VTE than patients in the low-risk category—10.7% vs 0.7%.
These findings were independent of surgery type.
“It was eye-opening to see that there is this huge variability in risk among the overall group of patients that walk into your office,” Dr Pannucci said. “Unless you consider a patient’s risk based on their individual factors, you would never know.”
VTE outcomes by risk score
When given, anticoagulants did significantly reduce the risk of VTE for the overall study population and for high-risk patients.
The odds ratios (ORs) were 0.66 (P=0.001) for the overall population, 0.60 (P=0.04) for patients with Caprini scores of 7 to 8, and 0.41 (P=0.0002) for patients with scores higher than 8.
Unfortunately, anticoagulants did not make a significant difference in VTE rates for mid- or low-risk patients.
The ORs were 0.45 (P=0.31) for patients with Caprini scores of 0 to 2, 1.31 (P=0.57) for patients with scores of 3 to 4, and 0.96 (P=0.85) for patients with scores of 5 to 6.
Risk of bleeding
Anticoagulants significantly increased clinically relevant bleeding for the overall population. The OR was 1.69 (P=0.006).
Patients who received anticoagulants were not significantly more likely to have clinically relevant bleeding if they had risk scores of 0 to 2 (OR=2.47, P=0.61), 3 to 4 (OR=1.05, P=0.87), 5 to 6 (OR=2.10, P=0.06), 7 to 8 (OR=3.15, P=0.16), or >8 (OR=2.31, P=0.16).
“For the first time, we have data that prophylaxis for the highest-risk groups is beneficial, and data that suggests that lower-risk patients may need no prophylaxis,” said study author Peter Henke, MD, of the University of Michigan in Ann Arbor.
He and his colleagues noted, however, that prospective studies are needed to confirm these findings. ![]()
Study quantifies 5-year survival rates for blood cancers

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()

chemotherapy
Photo by Rhoda Baer
A new study shows that 5-year survival rates for US patients with hematologic malignancies have increased greatly since the 1950s, but there is still room for improvement, particularly for patients with acute myeloid leukemia (AML).
Researchers found the absolute difference in improvement for 5-year survival from 1950-1954 to 2008-2013 ranged from 38.2% for non-Hodgkin lymphoma (NHL) to 56.6% for Hodgkin lymphoma.
And although the 5-year survival rate for Hodgkin lymphoma patients reached 86.6% for 2008-2013, the 5-year survival rate for patients with AML only reached 27.4%.
This study also revealed large disparities in overall cancer mortality rates between different counties across the country.
Ali H. Mokdad, PhD, of the Institute for Health Metrics and Evaluation in Seattle, Washington, and his colleagues reported these findings in JAMA.
Overall cancer deaths
The researchers found there were 19,511,910 cancer deaths recorded in the US between 1980 and 2014. Cancer mortality decreased by 20.1% between 1980 and 2014, from 240.2 deaths per 100,000 people to 192.0 deaths per 100,000 people.
In 1980, cancer mortality ranged from 130.6 per 100,000 in Summit County, Colorado, to 386.9 per 100,000 in North Slope Borough, Alaska.
In 2014, cancer mortality ranged from 70.7 per 100,000 in Summit County, Colorado, to 503.1 per 100,000 in Union County, Florida.
“Such significant disparities among US counties is unacceptable,” Dr Mokdad said. “Every person should have access to early screenings for cancer, as well as adequate treatment.”
Mortality rates for hematologic malignancies
In 2014, the mortality rates, per 100,000 people, for hematologic malignancies were:
- 0.4 for Hodgkin lymphoma (rank out of all cancers, 27)
- 8.3 for NHL (rank, 7)
- 3.9 for multiple myeloma (rank, 16)
- 9.0 for all leukemias (rank, 6)
- 0.7 for acute lymphoid leukemia (ALL)
- 2.6 for chronic lymphoid leukemia (CLL)
- 5.1 for AML
- 0.6 for chronic myeloid leukemia (CML).
The leukemia subtypes were not assigned a rank.
5-year survival rates for hematologic malignancies
Hodgkin lymphoma
- 30% for 1950-54
- 68.6% for 1973-77
- 72.1% for 1978-82
- 86.6% for 2008-2013
- Absolute difference (between the first and latest year of data), 56.6%.
NHL
- 33% for 1950-54
- 45.3% for 1973-77
- 48.7% for 1978-82
- 71.2% for 2008-2013
- Absolute difference, 38.2%.
Multiple myeloma
- 6% for 1950-54
- 23.4% for 1973-77
- 26.6% for 1978-82
- 49.8% for 2008-2013
- Absolute difference, 43.8%.
Leukemia
- 10% for 1950-54
- 34% for 1973-77
- 36.3% for 1978-82
- 60.1% for 2008-2013
- Absolute difference, 50.1%.
ALL
- 39.2% for 1973-77
- 50.5% for 1978-82
- 68.1% for 2008-2013
- Absolute difference, 28.9%.
CLL
- 67% for 1973-77
- 66.3% for 1978-82
- 82.5% for 2008-2013
- Absolute difference, 15.5%.
AML
- 6.2% for 1973-77
- 7.9% for 1978-82
- 27.4% for 2008-2013
- Absolute difference, 21.2%.
CML
- 21.1% for 1973-77
- 25.8% for 1978-82
- 66.4% for 2008-2013
- Absolute difference, 45.3%.
For the leukemia subtypes, there was no data for 1950 to 1954. ![]()