User login
Auditory Auras May Signal Poor Surgical Outcomes
Patients with temporal lobe epilepsy are more likely to fare poorly after surgery if they experience auditory auras. When Ali A. Asadi-Pooya and associates performed a retrospective analysis of 1186 drug-resistant patients who had had a temporal resection, they found that those with auditory auras were more likely to relapse after surgery, when compared to those who did not experience the auras (P=.03). The side of the head in which the procedure was performed did not affect postoperative prognosis.
Asadi-Pooya AA, Wyeth D, Nei M, et al. Postsurgical outcome in patients with auditory auras and drug-resistant epilepsy. Epilepsy Behav. 2017;66:49-52.
Patients with temporal lobe epilepsy are more likely to fare poorly after surgery if they experience auditory auras. When Ali A. Asadi-Pooya and associates performed a retrospective analysis of 1186 drug-resistant patients who had had a temporal resection, they found that those with auditory auras were more likely to relapse after surgery, when compared to those who did not experience the auras (P=.03). The side of the head in which the procedure was performed did not affect postoperative prognosis.
Asadi-Pooya AA, Wyeth D, Nei M, et al. Postsurgical outcome in patients with auditory auras and drug-resistant epilepsy. Epilepsy Behav. 2017;66:49-52.
Patients with temporal lobe epilepsy are more likely to fare poorly after surgery if they experience auditory auras. When Ali A. Asadi-Pooya and associates performed a retrospective analysis of 1186 drug-resistant patients who had had a temporal resection, they found that those with auditory auras were more likely to relapse after surgery, when compared to those who did not experience the auras (P=.03). The side of the head in which the procedure was performed did not affect postoperative prognosis.
Asadi-Pooya AA, Wyeth D, Nei M, et al. Postsurgical outcome in patients with auditory auras and drug-resistant epilepsy. Epilepsy Behav. 2017;66:49-52.
Older, Poorer Patients With Epilepsy Less Likely to Take their Medication
Approximately one-third of older adults with epilepsy do not adhere very well to their antiepileptic drug regimen, with older minority patients even less compliant. That’s the conclusion reached by researchers who analyzed Medicare claims from 2008 to 2010, using a 5% random sample of beneficiaries and augmenting it with minority patients. Piper et al looked at 36,912 cases of epilepsy and found 31.8% were nonadherent; that included 24.1% of whites and 34.3% of African Americans. They also found that Medicare beneficiaries who lived in high poverty areas were more likely to be noncompliant.
Piper K, Richman J, Faught E, at al. Adherence to antiepileptic drugs among diverse older Americans on Part D Medicare. Epilepsy Behav. 2017;66:68-73.
Approximately one-third of older adults with epilepsy do not adhere very well to their antiepileptic drug regimen, with older minority patients even less compliant. That’s the conclusion reached by researchers who analyzed Medicare claims from 2008 to 2010, using a 5% random sample of beneficiaries and augmenting it with minority patients. Piper et al looked at 36,912 cases of epilepsy and found 31.8% were nonadherent; that included 24.1% of whites and 34.3% of African Americans. They also found that Medicare beneficiaries who lived in high poverty areas were more likely to be noncompliant.
Piper K, Richman J, Faught E, at al. Adherence to antiepileptic drugs among diverse older Americans on Part D Medicare. Epilepsy Behav. 2017;66:68-73.
Approximately one-third of older adults with epilepsy do not adhere very well to their antiepileptic drug regimen, with older minority patients even less compliant. That’s the conclusion reached by researchers who analyzed Medicare claims from 2008 to 2010, using a 5% random sample of beneficiaries and augmenting it with minority patients. Piper et al looked at 36,912 cases of epilepsy and found 31.8% were nonadherent; that included 24.1% of whites and 34.3% of African Americans. They also found that Medicare beneficiaries who lived in high poverty areas were more likely to be noncompliant.
Piper K, Richman J, Faught E, at al. Adherence to antiepileptic drugs among diverse older Americans on Part D Medicare. Epilepsy Behav. 2017;66:68-73.
Senate takes first step toward repealing ACA
With a Jan. 12 early morning procedural passed on party lines, the Senate has set the stage for the repeal of the revenue aspects of the Affordable Care Act.
Republican Senators will be using the budget reconciliation process, which will allow them to move forward with repealing certain provisions of the health care reform law without any Democratic support, although passage of any replacement will require some bipartisan support as Republicans do not have the required 60 votes to guarantee passage.
The budget resolution contains no details about what could be repealed or whether there will be a replacement, but it does direct the key committees to write draft legislation by Jan. 27.
Senate Republicans “plan to rescue those trapped in a failing system, to replace that system with a functional market, or markets, and then repeal Obamacare for good,” he said.
Sen. Alexander said the process will come in three parts. The first will protect the 11 million people who have purchased health insurance through the exchanges so that they don’t lose coverage.
“Second, we will build better systems providing Americans with more choices that cost less,” he said. “Note I say systems, not one system. If anyone is expecting [Senate Majority Leader Mitch] McConnell [R-Ky.] to roll a wheelbarrow on the Senate floor with a comprehensive Republican health care plan, they’re going to be waiting a long time because we don’t believe in that. We don’t want to replace a failed Obamacare federal system with another failed federal system.”
The last part will be to repeal what remains of the law after the new plan is in place.
Sen. Alexander reiterated that any future bill will keep the ban on coverage denials for preexisting conditions and the allowance of coverage of children up to the age of 26 who are on their parents’ plans.
He stated that this reform effort will not address Medicare reform, which will be the subject of separate legislative action.
The AGA opposes repealing the ACA unless a viable, equitable replacement is in place. Patients who have received coverage through the ACA should be able to maintain coverage without interruption, and any replacement package must ensure patient access and coverage of specialty care, provide for preventive screenings without cost-sharing, not discriminate on the basis of a pre-existing condition or gender, cover children until they are age 26, and ban annual and lifetime caps on coverage.
With a Jan. 12 early morning procedural passed on party lines, the Senate has set the stage for the repeal of the revenue aspects of the Affordable Care Act.
Republican Senators will be using the budget reconciliation process, which will allow them to move forward with repealing certain provisions of the health care reform law without any Democratic support, although passage of any replacement will require some bipartisan support as Republicans do not have the required 60 votes to guarantee passage.
The budget resolution contains no details about what could be repealed or whether there will be a replacement, but it does direct the key committees to write draft legislation by Jan. 27.
Senate Republicans “plan to rescue those trapped in a failing system, to replace that system with a functional market, or markets, and then repeal Obamacare for good,” he said.
Sen. Alexander said the process will come in three parts. The first will protect the 11 million people who have purchased health insurance through the exchanges so that they don’t lose coverage.
“Second, we will build better systems providing Americans with more choices that cost less,” he said. “Note I say systems, not one system. If anyone is expecting [Senate Majority Leader Mitch] McConnell [R-Ky.] to roll a wheelbarrow on the Senate floor with a comprehensive Republican health care plan, they’re going to be waiting a long time because we don’t believe in that. We don’t want to replace a failed Obamacare federal system with another failed federal system.”
The last part will be to repeal what remains of the law after the new plan is in place.
Sen. Alexander reiterated that any future bill will keep the ban on coverage denials for preexisting conditions and the allowance of coverage of children up to the age of 26 who are on their parents’ plans.
He stated that this reform effort will not address Medicare reform, which will be the subject of separate legislative action.
The AGA opposes repealing the ACA unless a viable, equitable replacement is in place. Patients who have received coverage through the ACA should be able to maintain coverage without interruption, and any replacement package must ensure patient access and coverage of specialty care, provide for preventive screenings without cost-sharing, not discriminate on the basis of a pre-existing condition or gender, cover children until they are age 26, and ban annual and lifetime caps on coverage.
With a Jan. 12 early morning procedural passed on party lines, the Senate has set the stage for the repeal of the revenue aspects of the Affordable Care Act.
Republican Senators will be using the budget reconciliation process, which will allow them to move forward with repealing certain provisions of the health care reform law without any Democratic support, although passage of any replacement will require some bipartisan support as Republicans do not have the required 60 votes to guarantee passage.
The budget resolution contains no details about what could be repealed or whether there will be a replacement, but it does direct the key committees to write draft legislation by Jan. 27.
Senate Republicans “plan to rescue those trapped in a failing system, to replace that system with a functional market, or markets, and then repeal Obamacare for good,” he said.
Sen. Alexander said the process will come in three parts. The first will protect the 11 million people who have purchased health insurance through the exchanges so that they don’t lose coverage.
“Second, we will build better systems providing Americans with more choices that cost less,” he said. “Note I say systems, not one system. If anyone is expecting [Senate Majority Leader Mitch] McConnell [R-Ky.] to roll a wheelbarrow on the Senate floor with a comprehensive Republican health care plan, they’re going to be waiting a long time because we don’t believe in that. We don’t want to replace a failed Obamacare federal system with another failed federal system.”
The last part will be to repeal what remains of the law after the new plan is in place.
Sen. Alexander reiterated that any future bill will keep the ban on coverage denials for preexisting conditions and the allowance of coverage of children up to the age of 26 who are on their parents’ plans.
He stated that this reform effort will not address Medicare reform, which will be the subject of separate legislative action.
The AGA opposes repealing the ACA unless a viable, equitable replacement is in place. Patients who have received coverage through the ACA should be able to maintain coverage without interruption, and any replacement package must ensure patient access and coverage of specialty care, provide for preventive screenings without cost-sharing, not discriminate on the basis of a pre-existing condition or gender, cover children until they are age 26, and ban annual and lifetime caps on coverage.
CMS nixes Part B drug payment demonstration
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services. The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
The AGA expressed concern that many of the drugs that gastroenterologists administer would be included in this proposed new payment model and that the model would affect the patients treated for the most complex conditions, such as Crohn’s disease and ulcerative colitis. Ultimately, this payment model would limit patient access to specialist care. The AGA urged CMS to include all stakeholders in the development of approaches to control Part B costs.
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services. The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
The AGA expressed concern that many of the drugs that gastroenterologists administer would be included in this proposed new payment model and that the model would affect the patients treated for the most complex conditions, such as Crohn’s disease and ulcerative colitis. Ultimately, this payment model would limit patient access to specialist care. The AGA urged CMS to include all stakeholders in the development of approaches to control Part B costs.
A controversial demonstration project that would have tested new methods to pay for the drugs administered in medical offices has been canceled by the Centers for Medicare & Medicaid Services. The agency received considerable backlash from physicians, Congress, and others when the demonstration project was announced in March 2016.
The agency said it received “a great deal of support from some” for the proposed demonstration. However, “a number of stakeholders expressed strong concerns about the model. While CMS was working to address these concerns, the complexity of the issues and the limited time available led to the decision not to finalize the rule at this time.”
The demonstration project was designed to test new methods to “improve how Medicare Part B pays for prescription drugs and supports physicians and other clinicians in delivering high quality care,” according to a fact sheet published in March.
Under the project, medical practices would have been divided into two groups. A control group would continue to be paid for Part B drugs at the current rate of 106% of average sales price (ASP), while the other would have been paid at 102.5% of ASP plus a flat fee of $16.80 per drug payment. Starting in January 2017, each group would have been further subdivided with a portion of each being subjected to value-based purchasing tools.
One key criticism of the demonstration project centered on the proposed randomization of practices, which was based on primary care service areas (clusters of zip codes with similar Part B medical care patterns). That randomization scheme could have caused different payment levels – and patient out-of-pocket spending – for geographically close areas. Further, participation in the demonstration project would have been mandatory, with no mechanism to opt out.
“This is a model for how Washington should, but often doesn’t, work,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “We are grateful that CMS came to the right decision after listening to stakeholders.”
An analysis of the proposed demonstration project by Avalere found that specialists would likely see a decrease in their drug payments under the proposal, while primary care doctors would likely see an increase, and that 7 of the 10 drugs most affected by this proposal were drugs used to treat cancer.
The AGA expressed concern that many of the drugs that gastroenterologists administer would be included in this proposed new payment model and that the model would affect the patients treated for the most complex conditions, such as Crohn’s disease and ulcerative colitis. Ultimately, this payment model would limit patient access to specialist care. The AGA urged CMS to include all stakeholders in the development of approaches to control Part B costs.
Continuous glucose monitoring benefits patients with type 1 diabetes
, according to two separate randomized trials reported online Jan. 24 in JAMA.
Compared with usual care, continuous glucose monitoring decreased mean HbA1c levels by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden. Both research groups noted that lengthier trials are needed to assess longer-term effectiveness of continuous glucose monitoring in this patient population, the possible adverse effects of long-term use, and whether the reduction in HbA1c levels translates into improved clinical outcomes.
The first trial, which was conducted at 24 U.S. endocrinology practices, involved patients aged 25 and older (mean age, 48 years) who had had type 1 diabetes for a median of 19 years and whose baseline HbA1c levels ranged from 7.5% to 10%. A total of 105 of these participants were randomly assigned to use continuous glucose monitoring (CGM group) and 53 to receive usual care (control group) for 24 weeks, said Roy W. Beck, MD, PhD, of the Jaeb Center for Health Research, Tampa, and his associates.
The CGM group was instructed to wear the device on at least 85% of days and to calibrate it at least twice per day, and they were to verify their glucose level by doing blood glucose meter testing at least three times daily before injecting insulin. The control group was instructed to do blood glucose meter testing at least four times per day.
The primary outcome, reduction in HbA1c level, was 1.1% at 12 weeks and 1% at 24 weeks with CGM, compared with 0.5% at 12 weeks and 0.4% at 24 weeks with usual care. At the end of the treatment period, the mean difference between the two study groups in HbA1c reduction was 0.6%.
Secondary outcomes also favored CGM, including the time spent with glucose levels within the target range of 70-180 mg/dL, duration of hypoglycemia, duration of hyperglycemia, and glycemic variability. In addition, patients reported a high level of satisfaction with CGM, Dr. Beck and his associates said (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19975).
The second trial was conducted at 15 medical centers in Sweden and involved 161 adults aged 18 and older (mean age, 44 years) whose baseline HbA1c levels were 7.5% or higher. The participants served as their own controls, randomly assigned to use either CGM or usual care for 26 weeks and then to crossover to the other group for 26 weeks, said Marcus Lind, MD, PhD, of the diabetes outpatient clinic, Uddevalla (Sweden) Hospital, and his associates.
The primary outcome, reduction in HbA1c level, was lower by 0.4% with CGM than with usual care. In addition, secondary outcomes also favored CGM, including treatment satisfaction, patient concern about having a hypoglycemic episode, overall well-being, and mean glucose levels. However, in this study, patients measured their blood glucose levels less often with CGM (2.7 measurements per day) than with usual care (3.7 measurements per day).
One patient developed an allergic reaction to the device’s internal sensor and had it removed, according to Dr. Lind and his associates (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19976).
Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
Both of these studies show a clear benefit with continuous glucose monitoring in patients with type 1 diabetes, but there are a few concerns.
First, CGM is expensive, and insurers may be reluctant to pay for this device in light of the relatively modest benefits reported by Beck et al. and Lind et al. Second, CGM is invasive and still requires patients to monitor their blood glucose with needle sticks several times per day – two factors that may limit its acceptability to many patients.
Third, the clinicians in these trials were experienced with using CGM and instructing patients in its use. Most clinicians who are not endocrinologists, and even some endocrinologists, would not have the time to manage the volume of data generated by the device and to guide patients’ lifestyle and insulin dosage changes accordingly, given the current time constraints in managing diabetes care.
Mayer B. Davidson, MD, is at Charles R. Drew University of Medicine and Science, Los Angeles. He reported having no relevant financial disclosures. Dr. Davidson made these remarks in an editorial accompanying the two reports (JAMA. 2017;317:363-4).
Both of these studies show a clear benefit with continuous glucose monitoring in patients with type 1 diabetes, but there are a few concerns.
First, CGM is expensive, and insurers may be reluctant to pay for this device in light of the relatively modest benefits reported by Beck et al. and Lind et al. Second, CGM is invasive and still requires patients to monitor their blood glucose with needle sticks several times per day – two factors that may limit its acceptability to many patients.
Third, the clinicians in these trials were experienced with using CGM and instructing patients in its use. Most clinicians who are not endocrinologists, and even some endocrinologists, would not have the time to manage the volume of data generated by the device and to guide patients’ lifestyle and insulin dosage changes accordingly, given the current time constraints in managing diabetes care.
Mayer B. Davidson, MD, is at Charles R. Drew University of Medicine and Science, Los Angeles. He reported having no relevant financial disclosures. Dr. Davidson made these remarks in an editorial accompanying the two reports (JAMA. 2017;317:363-4).
Both of these studies show a clear benefit with continuous glucose monitoring in patients with type 1 diabetes, but there are a few concerns.
First, CGM is expensive, and insurers may be reluctant to pay for this device in light of the relatively modest benefits reported by Beck et al. and Lind et al. Second, CGM is invasive and still requires patients to monitor their blood glucose with needle sticks several times per day – two factors that may limit its acceptability to many patients.
Third, the clinicians in these trials were experienced with using CGM and instructing patients in its use. Most clinicians who are not endocrinologists, and even some endocrinologists, would not have the time to manage the volume of data generated by the device and to guide patients’ lifestyle and insulin dosage changes accordingly, given the current time constraints in managing diabetes care.
Mayer B. Davidson, MD, is at Charles R. Drew University of Medicine and Science, Los Angeles. He reported having no relevant financial disclosures. Dr. Davidson made these remarks in an editorial accompanying the two reports (JAMA. 2017;317:363-4).
, according to two separate randomized trials reported online Jan. 24 in JAMA.
Compared with usual care, continuous glucose monitoring decreased mean HbA1c levels by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden. Both research groups noted that lengthier trials are needed to assess longer-term effectiveness of continuous glucose monitoring in this patient population, the possible adverse effects of long-term use, and whether the reduction in HbA1c levels translates into improved clinical outcomes.
The first trial, which was conducted at 24 U.S. endocrinology practices, involved patients aged 25 and older (mean age, 48 years) who had had type 1 diabetes for a median of 19 years and whose baseline HbA1c levels ranged from 7.5% to 10%. A total of 105 of these participants were randomly assigned to use continuous glucose monitoring (CGM group) and 53 to receive usual care (control group) for 24 weeks, said Roy W. Beck, MD, PhD, of the Jaeb Center for Health Research, Tampa, and his associates.
The CGM group was instructed to wear the device on at least 85% of days and to calibrate it at least twice per day, and they were to verify their glucose level by doing blood glucose meter testing at least three times daily before injecting insulin. The control group was instructed to do blood glucose meter testing at least four times per day.
The primary outcome, reduction in HbA1c level, was 1.1% at 12 weeks and 1% at 24 weeks with CGM, compared with 0.5% at 12 weeks and 0.4% at 24 weeks with usual care. At the end of the treatment period, the mean difference between the two study groups in HbA1c reduction was 0.6%.
Secondary outcomes also favored CGM, including the time spent with glucose levels within the target range of 70-180 mg/dL, duration of hypoglycemia, duration of hyperglycemia, and glycemic variability. In addition, patients reported a high level of satisfaction with CGM, Dr. Beck and his associates said (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19975).
The second trial was conducted at 15 medical centers in Sweden and involved 161 adults aged 18 and older (mean age, 44 years) whose baseline HbA1c levels were 7.5% or higher. The participants served as their own controls, randomly assigned to use either CGM or usual care for 26 weeks and then to crossover to the other group for 26 weeks, said Marcus Lind, MD, PhD, of the diabetes outpatient clinic, Uddevalla (Sweden) Hospital, and his associates.
The primary outcome, reduction in HbA1c level, was lower by 0.4% with CGM than with usual care. In addition, secondary outcomes also favored CGM, including treatment satisfaction, patient concern about having a hypoglycemic episode, overall well-being, and mean glucose levels. However, in this study, patients measured their blood glucose levels less often with CGM (2.7 measurements per day) than with usual care (3.7 measurements per day).
One patient developed an allergic reaction to the device’s internal sensor and had it removed, according to Dr. Lind and his associates (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19976).
Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
, according to two separate randomized trials reported online Jan. 24 in JAMA.
Compared with usual care, continuous glucose monitoring decreased mean HbA1c levels by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden. Both research groups noted that lengthier trials are needed to assess longer-term effectiveness of continuous glucose monitoring in this patient population, the possible adverse effects of long-term use, and whether the reduction in HbA1c levels translates into improved clinical outcomes.
The first trial, which was conducted at 24 U.S. endocrinology practices, involved patients aged 25 and older (mean age, 48 years) who had had type 1 diabetes for a median of 19 years and whose baseline HbA1c levels ranged from 7.5% to 10%. A total of 105 of these participants were randomly assigned to use continuous glucose monitoring (CGM group) and 53 to receive usual care (control group) for 24 weeks, said Roy W. Beck, MD, PhD, of the Jaeb Center for Health Research, Tampa, and his associates.
The CGM group was instructed to wear the device on at least 85% of days and to calibrate it at least twice per day, and they were to verify their glucose level by doing blood glucose meter testing at least three times daily before injecting insulin. The control group was instructed to do blood glucose meter testing at least four times per day.
The primary outcome, reduction in HbA1c level, was 1.1% at 12 weeks and 1% at 24 weeks with CGM, compared with 0.5% at 12 weeks and 0.4% at 24 weeks with usual care. At the end of the treatment period, the mean difference between the two study groups in HbA1c reduction was 0.6%.
Secondary outcomes also favored CGM, including the time spent with glucose levels within the target range of 70-180 mg/dL, duration of hypoglycemia, duration of hyperglycemia, and glycemic variability. In addition, patients reported a high level of satisfaction with CGM, Dr. Beck and his associates said (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19975).
The second trial was conducted at 15 medical centers in Sweden and involved 161 adults aged 18 and older (mean age, 44 years) whose baseline HbA1c levels were 7.5% or higher. The participants served as their own controls, randomly assigned to use either CGM or usual care for 26 weeks and then to crossover to the other group for 26 weeks, said Marcus Lind, MD, PhD, of the diabetes outpatient clinic, Uddevalla (Sweden) Hospital, and his associates.
The primary outcome, reduction in HbA1c level, was lower by 0.4% with CGM than with usual care. In addition, secondary outcomes also favored CGM, including treatment satisfaction, patient concern about having a hypoglycemic episode, overall well-being, and mean glucose levels. However, in this study, patients measured their blood glucose levels less often with CGM (2.7 measurements per day) than with usual care (3.7 measurements per day).
One patient developed an allergic reaction to the device’s internal sensor and had it removed, according to Dr. Lind and his associates (JAMA. 2017 Jan 24. doi: 10.1001/jama.2016.19976).
Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: A 6-month course of continuous glucose monitoring modestly reduced HbA1c levels in patients with type 1 diabetes who used multiple daily insulin injections.
Major finding: Compared with usual care, continuous glucose monitoring decreased mean HbA1c by 0.6% in a multicenter open-label U.S. study involving 158 participants and by 0.4% in a multicenter open-label crossover trial in Sweden.
Data source: Two separate short-term randomized trials comparing the effect of continuous glucose monitoring against usual care in 319 adults with type 1 diabetes.
Disclosures: Dr. Beck’s study was sponsored by Dexcom, maker of the CGM device, which also participated in designing the study, writing the protocol, reviewing and approving the manuscripts, and interpreting the data. Dr. Beck reported financial relationships with Dexcom and Abbott Diabetes Care, and his associates reported ties to numerous industry sources. Dr. Lind’s study was sponsored by the NU Hospital Group and Dexcom. Dr. Lind reported financial relationships with AstraZeneca, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, and Rubin Medical, and his associates reported ties to numerous industry sources.
USPSTF punts on sleep apnea screening
because the current evidence is inadequate to assess the benefits and harms of doing so, according to a Recommendation Statement published online Jan. 23 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the Recommendation Statement addresses adults who don’t snore excessively; gasp or choke while sleeping; or report the daytime sleepiness, impaired cognition, or mood changes typically associated with obstructive sleep apnea, said Kirsten Bobbins-Domingo, PhD, MD, chair of the organization and lead author of the Recommendation Statement, and her associates (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.20325).
The USPSTF commissioned a comprehensive review of the literature to examine whether screening such patients by primary caregivers would effectively identify those who have obstructive sleep apnea and lead to treatment that would prevent the elevated rates of death, cognitive impairment, motor vehicle crashes, cardiovascular events, and cerebrovascular events related to the disorder. Daniel E. Jonas, MD, of the University of North Carolina at Chapel Hill and his associates reviewed 110 relevant studies involving 46,188 participants.
They found that the accuracy and clinical utility of numerous OSA screening tools was uncertain. In particular, the Epworth Sleepiness Scale, the STOP (Snoring, Tiredness, Observed Apnea, and High Blood Pressure) questionnaire, the STOP-BANG (STOP plus BMI, Age, Neck Circumference, and Gender) questionnaire, the Berlin Questionnaire, the Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction (MVAP) tool have not been adequately validated in primary care settings.
Moreover, no studies directly assessed whether screening had an impact on actual health outcomes. Several treatments, notably CPAP and mandibular advancement devices, did improve intermediate outcomes such as scores on the apnea-hypopnea index, scores on the Epworth Sleepiness Scale, and blood pressure levels, but the evidence did not show that this in turn improved mortality, cardiovascular events, or the other “hard” outcomes of interest, Dr. Jonas and his associates said in their Evidence Report (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.19635).
Dr. Bobbins-Domingo and her associates on the task force noted that this Recommendation Statement is consistent with that of the American Academy of Family Physicians, which also concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening asymptomatic adults for obstructive sleep apnea.
The American College of Physicians offers a “weak” recommendation based on low-quality evidence that patients with unexplained daytime sleepiness and patients suspected of having apnea undergo a sleep study, said Dr. Bobbins-Domingo, professor of medicine at the University of California, San Francisco, and her associates.
In contrast, the American Academy of Sleep Medicine recommends that routine health visits should include questions about OSA and evaluation of risk factors such as obesity, retrognathia, and treatment-refractory hypertension. If there are positive findings, a comprehensive sleep evaluation should follow, according to the AASM.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality as mandated by the U.S. Congress. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
This recommendation must not be misinterpreted. If clinicians are discouraged from directly questioning patients about apnea signs and symptoms or from using short screening questionnaires to identify those at high risk for the disorder, it would negatively influence public health.
Susan Redline, MD, is at the Sleep Health Institute and in the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital and Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston. She reported ties to Jazz Pharmaceuticals, RosMed Inc., and the Beckman Company, as well as serving on the American Academy of Sleep Medicine’s board of directors. Dr. Redline made these remarks in an editorial accompanying the USPSTF reports (JAMA 2017;317:368-70).
This recommendation must not be misinterpreted. If clinicians are discouraged from directly questioning patients about apnea signs and symptoms or from using short screening questionnaires to identify those at high risk for the disorder, it would negatively influence public health.
Susan Redline, MD, is at the Sleep Health Institute and in the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital and Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston. She reported ties to Jazz Pharmaceuticals, RosMed Inc., and the Beckman Company, as well as serving on the American Academy of Sleep Medicine’s board of directors. Dr. Redline made these remarks in an editorial accompanying the USPSTF reports (JAMA 2017;317:368-70).
This recommendation must not be misinterpreted. If clinicians are discouraged from directly questioning patients about apnea signs and symptoms or from using short screening questionnaires to identify those at high risk for the disorder, it would negatively influence public health.
Susan Redline, MD, is at the Sleep Health Institute and in the Division of Sleep and Circadian Disorders at Brigham and Women’s Hospital and Harvard Medical School and Beth Israel Deaconess Medical Center, all in Boston. She reported ties to Jazz Pharmaceuticals, RosMed Inc., and the Beckman Company, as well as serving on the American Academy of Sleep Medicine’s board of directors. Dr. Redline made these remarks in an editorial accompanying the USPSTF reports (JAMA 2017;317:368-70).
because the current evidence is inadequate to assess the benefits and harms of doing so, according to a Recommendation Statement published online Jan. 23 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the Recommendation Statement addresses adults who don’t snore excessively; gasp or choke while sleeping; or report the daytime sleepiness, impaired cognition, or mood changes typically associated with obstructive sleep apnea, said Kirsten Bobbins-Domingo, PhD, MD, chair of the organization and lead author of the Recommendation Statement, and her associates (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.20325).
The USPSTF commissioned a comprehensive review of the literature to examine whether screening such patients by primary caregivers would effectively identify those who have obstructive sleep apnea and lead to treatment that would prevent the elevated rates of death, cognitive impairment, motor vehicle crashes, cardiovascular events, and cerebrovascular events related to the disorder. Daniel E. Jonas, MD, of the University of North Carolina at Chapel Hill and his associates reviewed 110 relevant studies involving 46,188 participants.
They found that the accuracy and clinical utility of numerous OSA screening tools was uncertain. In particular, the Epworth Sleepiness Scale, the STOP (Snoring, Tiredness, Observed Apnea, and High Blood Pressure) questionnaire, the STOP-BANG (STOP plus BMI, Age, Neck Circumference, and Gender) questionnaire, the Berlin Questionnaire, the Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction (MVAP) tool have not been adequately validated in primary care settings.
Moreover, no studies directly assessed whether screening had an impact on actual health outcomes. Several treatments, notably CPAP and mandibular advancement devices, did improve intermediate outcomes such as scores on the apnea-hypopnea index, scores on the Epworth Sleepiness Scale, and blood pressure levels, but the evidence did not show that this in turn improved mortality, cardiovascular events, or the other “hard” outcomes of interest, Dr. Jonas and his associates said in their Evidence Report (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.19635).
Dr. Bobbins-Domingo and her associates on the task force noted that this Recommendation Statement is consistent with that of the American Academy of Family Physicians, which also concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening asymptomatic adults for obstructive sleep apnea.
The American College of Physicians offers a “weak” recommendation based on low-quality evidence that patients with unexplained daytime sleepiness and patients suspected of having apnea undergo a sleep study, said Dr. Bobbins-Domingo, professor of medicine at the University of California, San Francisco, and her associates.
In contrast, the American Academy of Sleep Medicine recommends that routine health visits should include questions about OSA and evaluation of risk factors such as obesity, retrognathia, and treatment-refractory hypertension. If there are positive findings, a comprehensive sleep evaluation should follow, according to the AASM.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality as mandated by the U.S. Congress. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
because the current evidence is inadequate to assess the benefits and harms of doing so, according to a Recommendation Statement published online Jan. 23 in JAMA.
The USPSTF makes recommendations about the effectiveness of specific health care services for patients who don’t have related signs or symptoms. In this case, the Recommendation Statement addresses adults who don’t snore excessively; gasp or choke while sleeping; or report the daytime sleepiness, impaired cognition, or mood changes typically associated with obstructive sleep apnea, said Kirsten Bobbins-Domingo, PhD, MD, chair of the organization and lead author of the Recommendation Statement, and her associates (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.20325).
The USPSTF commissioned a comprehensive review of the literature to examine whether screening such patients by primary caregivers would effectively identify those who have obstructive sleep apnea and lead to treatment that would prevent the elevated rates of death, cognitive impairment, motor vehicle crashes, cardiovascular events, and cerebrovascular events related to the disorder. Daniel E. Jonas, MD, of the University of North Carolina at Chapel Hill and his associates reviewed 110 relevant studies involving 46,188 participants.
They found that the accuracy and clinical utility of numerous OSA screening tools was uncertain. In particular, the Epworth Sleepiness Scale, the STOP (Snoring, Tiredness, Observed Apnea, and High Blood Pressure) questionnaire, the STOP-BANG (STOP plus BMI, Age, Neck Circumference, and Gender) questionnaire, the Berlin Questionnaire, the Wisconsin Sleep Questionnaire, and the Multivariable Apnea Prediction (MVAP) tool have not been adequately validated in primary care settings.
Moreover, no studies directly assessed whether screening had an impact on actual health outcomes. Several treatments, notably CPAP and mandibular advancement devices, did improve intermediate outcomes such as scores on the apnea-hypopnea index, scores on the Epworth Sleepiness Scale, and blood pressure levels, but the evidence did not show that this in turn improved mortality, cardiovascular events, or the other “hard” outcomes of interest, Dr. Jonas and his associates said in their Evidence Report (JAMA 2017 Jan 23. doi: 10.1001/jama.2016.19635).
Dr. Bobbins-Domingo and her associates on the task force noted that this Recommendation Statement is consistent with that of the American Academy of Family Physicians, which also concluded that the current evidence is insufficient to assess the balance of benefits and harms of screening asymptomatic adults for obstructive sleep apnea.
The American College of Physicians offers a “weak” recommendation based on low-quality evidence that patients with unexplained daytime sleepiness and patients suspected of having apnea undergo a sleep study, said Dr. Bobbins-Domingo, professor of medicine at the University of California, San Francisco, and her associates.
In contrast, the American Academy of Sleep Medicine recommends that routine health visits should include questions about OSA and evaluation of risk factors such as obesity, retrognathia, and treatment-refractory hypertension. If there are positive findings, a comprehensive sleep evaluation should follow, according to the AASM.
The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality as mandated by the U.S. Congress. The authors’ conflict of interest disclosures are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
FDA approves topical oxymetazoline for rosacea
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
Plesiomonas shigelloides Periprosthetic Knee Infection After Consumption of Raw Oysters
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
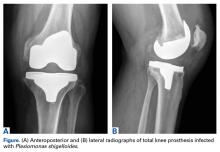
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Topical crisaborole new option for AD
There’s a new addition to the armamentarium for atopic dermatitis: a topical phosphodiesterase 4 (PDE-4) inhibitor.
“It’s a real boon to patients, now that we have crisaborole as a first-line treatment for mild to moderate atopic dermatitis,” Joseph F. Fowler Jr., MD, of the University of Louisville (Ky.), said in an interview. “In the trials leading up to its approval, crisaborole was impressive in that it was well tolerated, and the results held up across all age groups over time.” Dr. Fowler spoke at the meeting provided by Global Academy for Medical Education.
Topical ointment crisaborole 2% (Eucrisa) was approved by the Food and Drug Administration in December 2016 for mild to moderate atopic dermatitis in patients aged 2 years and older. Approval was based on two placebo-controlled trials with more than 1,500 participants between the ages of 2 and 79 years with mild to moderate atopic dermatitis.
“That it’s not a steroid is important since there are so few options for this pesky condition. We have topical steroids, but a lot of patients get nervous about the overuse of those, just as they do about the box warning for cancer risk on topical calcineurin inhibitors,” Dr. Fowler said. The most common treatment-related adverse event recorded during the trials were burning and stinging at the site of application.
“While topical corticosteroids are certainly effective, I am concerned about the risk of atrophy, especially in children and on sensitive areas such as the face and intertriginous areas. This new treatment does not have that potential adverse effect,” he said.
Global Academy and this news organization are owned by the same parent company.
Dr. Fowler disclosed that he has financial relationships with Anacor Pharmaceuticals – the manufacturer of crisaborole – and Pfizer, among other companies.
[email protected]
On Twitter @whitneymcknight
There’s a new addition to the armamentarium for atopic dermatitis: a topical phosphodiesterase 4 (PDE-4) inhibitor.
“It’s a real boon to patients, now that we have crisaborole as a first-line treatment for mild to moderate atopic dermatitis,” Joseph F. Fowler Jr., MD, of the University of Louisville (Ky.), said in an interview. “In the trials leading up to its approval, crisaborole was impressive in that it was well tolerated, and the results held up across all age groups over time.” Dr. Fowler spoke at the meeting provided by Global Academy for Medical Education.
Topical ointment crisaborole 2% (Eucrisa) was approved by the Food and Drug Administration in December 2016 for mild to moderate atopic dermatitis in patients aged 2 years and older. Approval was based on two placebo-controlled trials with more than 1,500 participants between the ages of 2 and 79 years with mild to moderate atopic dermatitis.
“That it’s not a steroid is important since there are so few options for this pesky condition. We have topical steroids, but a lot of patients get nervous about the overuse of those, just as they do about the box warning for cancer risk on topical calcineurin inhibitors,” Dr. Fowler said. The most common treatment-related adverse event recorded during the trials were burning and stinging at the site of application.
“While topical corticosteroids are certainly effective, I am concerned about the risk of atrophy, especially in children and on sensitive areas such as the face and intertriginous areas. This new treatment does not have that potential adverse effect,” he said.
Global Academy and this news organization are owned by the same parent company.
Dr. Fowler disclosed that he has financial relationships with Anacor Pharmaceuticals – the manufacturer of crisaborole – and Pfizer, among other companies.
[email protected]
On Twitter @whitneymcknight
There’s a new addition to the armamentarium for atopic dermatitis: a topical phosphodiesterase 4 (PDE-4) inhibitor.
“It’s a real boon to patients, now that we have crisaborole as a first-line treatment for mild to moderate atopic dermatitis,” Joseph F. Fowler Jr., MD, of the University of Louisville (Ky.), said in an interview. “In the trials leading up to its approval, crisaborole was impressive in that it was well tolerated, and the results held up across all age groups over time.” Dr. Fowler spoke at the meeting provided by Global Academy for Medical Education.
Topical ointment crisaborole 2% (Eucrisa) was approved by the Food and Drug Administration in December 2016 for mild to moderate atopic dermatitis in patients aged 2 years and older. Approval was based on two placebo-controlled trials with more than 1,500 participants between the ages of 2 and 79 years with mild to moderate atopic dermatitis.
“That it’s not a steroid is important since there are so few options for this pesky condition. We have topical steroids, but a lot of patients get nervous about the overuse of those, just as they do about the box warning for cancer risk on topical calcineurin inhibitors,” Dr. Fowler said. The most common treatment-related adverse event recorded during the trials were burning and stinging at the site of application.
“While topical corticosteroids are certainly effective, I am concerned about the risk of atrophy, especially in children and on sensitive areas such as the face and intertriginous areas. This new treatment does not have that potential adverse effect,” he said.
Global Academy and this news organization are owned by the same parent company.
Dr. Fowler disclosed that he has financial relationships with Anacor Pharmaceuticals – the manufacturer of crisaborole – and Pfizer, among other companies.
[email protected]
On Twitter @whitneymcknight
FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Reports of new-onset joint pain differ after starting vedolizumab
Two recent reports that provide opposing evidence about the potential for inflammatory bowel disease patients to develop articular manifestations after starting vedolizumab raise questions for future studies to answer in regard to a plausible mechanism for the adverse event and its relative importance.
The two reports, one a case series of 5 patients with inflammatory bowel disease (IBD) who developed articular manifestations after beginning vedolizumab (Entyvio) and the other a prospective cohort study of 53 patients with IBD who started vedolizumab without any subsequent cases of induction or flare of arthritis and/or sacroiliitis, came to somewhat different conclusions about the beneficial or paradoxical effects of vedolizumab’s blockade of the alpha4beta7 receptor on articular manifestations of IBD.
The five-patient cases series reported by Gaëlle Varkas, MD, a doctoral student at the University of Ghent, Belgium, and her colleagues consisted of five IBD patients, aged 26-50 years, who developed either new onset or an exacerbation of sacroiliitis or arthritis soon after starting vedolizumab. All but one of the patients was female (Ann Rheum Dis. 2016 Nov 29. doi: 10.1136/annrheumdis-2016-210233). In these patients, the investigators said, vedolizumab did not “seem to show any efficacy in and might even induce arthritis and/or sacroiliitis.”
The first was a 50-year-old woman who had progressive back pain with MRI-confirmed bilateral sacroiliitis about 2 months after beginning vedolizumab. The second patient, a 28-year-old woman with no previous history of spondyloarthropathy, had lower limb pain, a painful left shoulder, and arthritis of one wrist. Ultrasound examination confirmed intercarpal effusions and synovial hyperproliferation.
The third patient was 30 years old and male. He had both ankylosing spondylitis and Crohn’s disease, and experienced arthralgias, elevated C-reactive protein, and MRI-confirmed axial skeletal inflammation 4 weeks after starting vedolizumab.
The fourth patient was a 47-year-old woman with no previous history of spondyloarthropathy who developed MRI-confirmed sacroiliitis after beginning vedolizumab. The fifth patient, a 26-year-old woman, developed polyarticular joint pain after starting vedolizumab. Examination of this patient showed synovitis and enthesitis of multiple joints of the appendicular skeleton.
In discussion, Dr. Varkas and her colleagues noted that “one of the many hypotheses is that integrins and adhesion molecules play a role in the interception of recirculating activated lymphocytes between the gut and the synovial membrane due to the inhibition of the alpha4beta7 integrin homing at the level of the gut.” However, the investigators also acknowledged that other hypotheses may also account for their findings. “Alternatively, in the presence of vedolizumab, cellular recruitment may be mediated by yet to be determined adhesion molecules. This recirculation theory might explain the short mean interval of 64 days between vedolizumab initiation and the expression of symptoms.”
Another group, publishing its prospective cohort study in a letter, had different findings (Ann Rheum Dis. 2017 Jan 17. doi: 10.1136/annrheumdis-2016-211011).
“Although the hypotheses proposed by the authors to explain such events sounds reasonable,” wrote Ambrogio Orlando, MD, and his coauthors, their experience of the effect of vedolizumab on spondyloarthritis differed.
In the report on 53 patients who began treatment with vedolizumab at Villa Sofia-Cervello Hospital, Palermo, Italy, where Dr. Orlando and his associates work, almost all (96%) had been steroid dependent and 81% had been treated with at least one tumor necrosis factor inhibitor. About two-thirds had completed the induction phase of vedolizumab treatment during follow-up, which lasted a mean of 2.6 months. Of the 14 patients (26%) who had active IBD-associated spondyloarthropathy when starting vedolizumab, 6 (46.2%) saw “a sharp clinical benefit after the initiation of vedolizumab,” wrote Dr. Orlando and his colleagues. Five of these six patients experienced clinical remission of gut symptoms by 12 weeks of therapy.
Dr. Orlando and his colleagues wrote that “our preliminary prospective data indicate a potential benefit of vedolizumab on IBD-associated spondyloarthropathy.”
Looking for mechanistic reasons for this apparent benefit, Dr. Orlando and his collaborators wrote that “the previous demonstration of alpha4beta7 in the joint and the recent evidence of the upregulation of mucosal vascular address in cell adhesion molecule (MadCAM-1) in the high endothelial venules of bone marrow in patients with active axial SpA seem to strengthen the hypothesis of a beneficial rather than a paradoxical effect of alpha4beta7 blockade on articular manifestations of IBD.”
Two authors of the case series reported relationships with multiple pharmaceutical companies, as did Dr. Orlando and two other authors of the letter describing the prospective study.
[email protected]
On Twitter @karioakes
Vedolizumab is gut-selective, and therefore a question that’s been raised is whether it would uncover extra-intestinal manifestations of inflammatory bowel disease (IBD).
When extra-intestinal manifestations of IBD occur with new treatments, we try to discern whether there is still active disease in the bowel. If the bowel is responding, we try to determine if the extra-intestinal symptoms are occurring in parallel to the bowel disease or if they represent a unique side effect of the medication.
In February 2017, at ECCO [the annual meeting of the European Crohn’s and Colitis Organisation], we will be presenting a post hoc analysis of the data from the vedolizumab pivotal clinical trial that examines whether joint pain was independently associated with administration of vedolizumab.
The individual case reports of joint pain with vedolizumab have not limited our using the drug for the patients who need it. It’s a matter of weighing risks and benefits, and the safety profile of this medication is overall so good that we don’t hesitate to use it. In our clinic, we have treated more than 400 IBD patients with vedolizumab, and I can only recall one patient who had to stop using it due to joint pain.
David Rubin, MD, is professor of medicine and chief of the gastroenterology, hepatology, and nutrition section of the University of Chicago. He reported that he is a consultant for and has received grant support from Takeda Pharmaceuticals. These remarks were drawn from an interview.
Vedolizumab is gut-selective, and therefore a question that’s been raised is whether it would uncover extra-intestinal manifestations of inflammatory bowel disease (IBD).
When extra-intestinal manifestations of IBD occur with new treatments, we try to discern whether there is still active disease in the bowel. If the bowel is responding, we try to determine if the extra-intestinal symptoms are occurring in parallel to the bowel disease or if they represent a unique side effect of the medication.
In February 2017, at ECCO [the annual meeting of the European Crohn’s and Colitis Organisation], we will be presenting a post hoc analysis of the data from the vedolizumab pivotal clinical trial that examines whether joint pain was independently associated with administration of vedolizumab.
The individual case reports of joint pain with vedolizumab have not limited our using the drug for the patients who need it. It’s a matter of weighing risks and benefits, and the safety profile of this medication is overall so good that we don’t hesitate to use it. In our clinic, we have treated more than 400 IBD patients with vedolizumab, and I can only recall one patient who had to stop using it due to joint pain.
David Rubin, MD, is professor of medicine and chief of the gastroenterology, hepatology, and nutrition section of the University of Chicago. He reported that he is a consultant for and has received grant support from Takeda Pharmaceuticals. These remarks were drawn from an interview.
Vedolizumab is gut-selective, and therefore a question that’s been raised is whether it would uncover extra-intestinal manifestations of inflammatory bowel disease (IBD).
When extra-intestinal manifestations of IBD occur with new treatments, we try to discern whether there is still active disease in the bowel. If the bowel is responding, we try to determine if the extra-intestinal symptoms are occurring in parallel to the bowel disease or if they represent a unique side effect of the medication.
In February 2017, at ECCO [the annual meeting of the European Crohn’s and Colitis Organisation], we will be presenting a post hoc analysis of the data from the vedolizumab pivotal clinical trial that examines whether joint pain was independently associated with administration of vedolizumab.
The individual case reports of joint pain with vedolizumab have not limited our using the drug for the patients who need it. It’s a matter of weighing risks and benefits, and the safety profile of this medication is overall so good that we don’t hesitate to use it. In our clinic, we have treated more than 400 IBD patients with vedolizumab, and I can only recall one patient who had to stop using it due to joint pain.
David Rubin, MD, is professor of medicine and chief of the gastroenterology, hepatology, and nutrition section of the University of Chicago. He reported that he is a consultant for and has received grant support from Takeda Pharmaceuticals. These remarks were drawn from an interview.
Two recent reports that provide opposing evidence about the potential for inflammatory bowel disease patients to develop articular manifestations after starting vedolizumab raise questions for future studies to answer in regard to a plausible mechanism for the adverse event and its relative importance.
The two reports, one a case series of 5 patients with inflammatory bowel disease (IBD) who developed articular manifestations after beginning vedolizumab (Entyvio) and the other a prospective cohort study of 53 patients with IBD who started vedolizumab without any subsequent cases of induction or flare of arthritis and/or sacroiliitis, came to somewhat different conclusions about the beneficial or paradoxical effects of vedolizumab’s blockade of the alpha4beta7 receptor on articular manifestations of IBD.
The five-patient cases series reported by Gaëlle Varkas, MD, a doctoral student at the University of Ghent, Belgium, and her colleagues consisted of five IBD patients, aged 26-50 years, who developed either new onset or an exacerbation of sacroiliitis or arthritis soon after starting vedolizumab. All but one of the patients was female (Ann Rheum Dis. 2016 Nov 29. doi: 10.1136/annrheumdis-2016-210233). In these patients, the investigators said, vedolizumab did not “seem to show any efficacy in and might even induce arthritis and/or sacroiliitis.”
The first was a 50-year-old woman who had progressive back pain with MRI-confirmed bilateral sacroiliitis about 2 months after beginning vedolizumab. The second patient, a 28-year-old woman with no previous history of spondyloarthropathy, had lower limb pain, a painful left shoulder, and arthritis of one wrist. Ultrasound examination confirmed intercarpal effusions and synovial hyperproliferation.
The third patient was 30 years old and male. He had both ankylosing spondylitis and Crohn’s disease, and experienced arthralgias, elevated C-reactive protein, and MRI-confirmed axial skeletal inflammation 4 weeks after starting vedolizumab.
The fourth patient was a 47-year-old woman with no previous history of spondyloarthropathy who developed MRI-confirmed sacroiliitis after beginning vedolizumab. The fifth patient, a 26-year-old woman, developed polyarticular joint pain after starting vedolizumab. Examination of this patient showed synovitis and enthesitis of multiple joints of the appendicular skeleton.
In discussion, Dr. Varkas and her colleagues noted that “one of the many hypotheses is that integrins and adhesion molecules play a role in the interception of recirculating activated lymphocytes between the gut and the synovial membrane due to the inhibition of the alpha4beta7 integrin homing at the level of the gut.” However, the investigators also acknowledged that other hypotheses may also account for their findings. “Alternatively, in the presence of vedolizumab, cellular recruitment may be mediated by yet to be determined adhesion molecules. This recirculation theory might explain the short mean interval of 64 days between vedolizumab initiation and the expression of symptoms.”
Another group, publishing its prospective cohort study in a letter, had different findings (Ann Rheum Dis. 2017 Jan 17. doi: 10.1136/annrheumdis-2016-211011).
“Although the hypotheses proposed by the authors to explain such events sounds reasonable,” wrote Ambrogio Orlando, MD, and his coauthors, their experience of the effect of vedolizumab on spondyloarthritis differed.
In the report on 53 patients who began treatment with vedolizumab at Villa Sofia-Cervello Hospital, Palermo, Italy, where Dr. Orlando and his associates work, almost all (96%) had been steroid dependent and 81% had been treated with at least one tumor necrosis factor inhibitor. About two-thirds had completed the induction phase of vedolizumab treatment during follow-up, which lasted a mean of 2.6 months. Of the 14 patients (26%) who had active IBD-associated spondyloarthropathy when starting vedolizumab, 6 (46.2%) saw “a sharp clinical benefit after the initiation of vedolizumab,” wrote Dr. Orlando and his colleagues. Five of these six patients experienced clinical remission of gut symptoms by 12 weeks of therapy.
Dr. Orlando and his colleagues wrote that “our preliminary prospective data indicate a potential benefit of vedolizumab on IBD-associated spondyloarthropathy.”
Looking for mechanistic reasons for this apparent benefit, Dr. Orlando and his collaborators wrote that “the previous demonstration of alpha4beta7 in the joint and the recent evidence of the upregulation of mucosal vascular address in cell adhesion molecule (MadCAM-1) in the high endothelial venules of bone marrow in patients with active axial SpA seem to strengthen the hypothesis of a beneficial rather than a paradoxical effect of alpha4beta7 blockade on articular manifestations of IBD.”
Two authors of the case series reported relationships with multiple pharmaceutical companies, as did Dr. Orlando and two other authors of the letter describing the prospective study.
[email protected]
On Twitter @karioakes
Two recent reports that provide opposing evidence about the potential for inflammatory bowel disease patients to develop articular manifestations after starting vedolizumab raise questions for future studies to answer in regard to a plausible mechanism for the adverse event and its relative importance.
The two reports, one a case series of 5 patients with inflammatory bowel disease (IBD) who developed articular manifestations after beginning vedolizumab (Entyvio) and the other a prospective cohort study of 53 patients with IBD who started vedolizumab without any subsequent cases of induction or flare of arthritis and/or sacroiliitis, came to somewhat different conclusions about the beneficial or paradoxical effects of vedolizumab’s blockade of the alpha4beta7 receptor on articular manifestations of IBD.
The five-patient cases series reported by Gaëlle Varkas, MD, a doctoral student at the University of Ghent, Belgium, and her colleagues consisted of five IBD patients, aged 26-50 years, who developed either new onset or an exacerbation of sacroiliitis or arthritis soon after starting vedolizumab. All but one of the patients was female (Ann Rheum Dis. 2016 Nov 29. doi: 10.1136/annrheumdis-2016-210233). In these patients, the investigators said, vedolizumab did not “seem to show any efficacy in and might even induce arthritis and/or sacroiliitis.”
The first was a 50-year-old woman who had progressive back pain with MRI-confirmed bilateral sacroiliitis about 2 months after beginning vedolizumab. The second patient, a 28-year-old woman with no previous history of spondyloarthropathy, had lower limb pain, a painful left shoulder, and arthritis of one wrist. Ultrasound examination confirmed intercarpal effusions and synovial hyperproliferation.
The third patient was 30 years old and male. He had both ankylosing spondylitis and Crohn’s disease, and experienced arthralgias, elevated C-reactive protein, and MRI-confirmed axial skeletal inflammation 4 weeks after starting vedolizumab.
The fourth patient was a 47-year-old woman with no previous history of spondyloarthropathy who developed MRI-confirmed sacroiliitis after beginning vedolizumab. The fifth patient, a 26-year-old woman, developed polyarticular joint pain after starting vedolizumab. Examination of this patient showed synovitis and enthesitis of multiple joints of the appendicular skeleton.
In discussion, Dr. Varkas and her colleagues noted that “one of the many hypotheses is that integrins and adhesion molecules play a role in the interception of recirculating activated lymphocytes between the gut and the synovial membrane due to the inhibition of the alpha4beta7 integrin homing at the level of the gut.” However, the investigators also acknowledged that other hypotheses may also account for their findings. “Alternatively, in the presence of vedolizumab, cellular recruitment may be mediated by yet to be determined adhesion molecules. This recirculation theory might explain the short mean interval of 64 days between vedolizumab initiation and the expression of symptoms.”
Another group, publishing its prospective cohort study in a letter, had different findings (Ann Rheum Dis. 2017 Jan 17. doi: 10.1136/annrheumdis-2016-211011).
“Although the hypotheses proposed by the authors to explain such events sounds reasonable,” wrote Ambrogio Orlando, MD, and his coauthors, their experience of the effect of vedolizumab on spondyloarthritis differed.
In the report on 53 patients who began treatment with vedolizumab at Villa Sofia-Cervello Hospital, Palermo, Italy, where Dr. Orlando and his associates work, almost all (96%) had been steroid dependent and 81% had been treated with at least one tumor necrosis factor inhibitor. About two-thirds had completed the induction phase of vedolizumab treatment during follow-up, which lasted a mean of 2.6 months. Of the 14 patients (26%) who had active IBD-associated spondyloarthropathy when starting vedolizumab, 6 (46.2%) saw “a sharp clinical benefit after the initiation of vedolizumab,” wrote Dr. Orlando and his colleagues. Five of these six patients experienced clinical remission of gut symptoms by 12 weeks of therapy.
Dr. Orlando and his colleagues wrote that “our preliminary prospective data indicate a potential benefit of vedolizumab on IBD-associated spondyloarthropathy.”
Looking for mechanistic reasons for this apparent benefit, Dr. Orlando and his collaborators wrote that “the previous demonstration of alpha4beta7 in the joint and the recent evidence of the upregulation of mucosal vascular address in cell adhesion molecule (MadCAM-1) in the high endothelial venules of bone marrow in patients with active axial SpA seem to strengthen the hypothesis of a beneficial rather than a paradoxical effect of alpha4beta7 blockade on articular manifestations of IBD.”
Two authors of the case series reported relationships with multiple pharmaceutical companies, as did Dr. Orlando and two other authors of the letter describing the prospective study.
[email protected]
On Twitter @karioakes
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Imaging-confirmed arthritis or sacroiliitis after starting vedolizumab was seen in a case series of 5 patients; a prospective study of 53 patients saw zero new-onset cases of joint pain.
Data source: Case series of 5 IBD patients starting vedolizumab, and prospective surveillance at another facility of 53 IBD patients receiving vedolizumab.
Disclosures: Two authors of the case series reported multiple relationships with pharmaceutical companies, as did three authors of a letter describing a prospective study.