User login
Rehabilitation Program Improves Symptoms of Functional Movement Disorders
RIVIERA BEACH, FL—A comprehensive, interdisciplinary rehabilitation program for functional movement disorders (FMD) is providing high rates of sustained improvement in patients with this challenging clinical problem, according to an initial analysis. In this program, which is administered during a one-week in-hospital stay, the emphasis is on relearning normal movement through physical therapy, but attention is also paid to the psychological component of the disorder.
What Is FMD?
FMD includes movement abnormalities such as tremor, gait disturbances, or dystonia that are not explained by organic lesions or diseases. FMD is common and occurs in 3% to 5% of patients presenting at movement disorder clinics, according to Dr. LaFaver. Although FMD has an important psychogenic component, Dr. LaFaver said that the symptoms can be as persistent and debilitating as those associated with organic disorders. As with organic diseases, the consequences of FMD include chronic disability.
This last point was emphasized in the demographics of a series of 32 patients with FMD presented by Dr. LaFaver. The mean duration of symptoms was seven years, and 56% of patients were on disability at the time of enrollment into the MoRe program. Consistent with other series of patients with FMD, the population was predominantly female (75%), and a substantial proportion reported posttraumatic stress disorder (53%), sexual abuse (48%), and physical abuse (41%). Mean scores on the Beck Depression Inventory (16.59) and the State-Trait Anxiety Index (40.79) indicated that mood disorders were common. This result also has been previously reported in patients with FMD.
The MoRe Program
Over the course of the inpatient MoRe program, patients begin with simple, repetitive, and structured exercises relevant to their FMD, progressing to more complex motor tasks as they improve. Positive gains with physical therapy, which is provided for five consecutive days, are reinforced with structured cognitive behavioral therapy (CBT). The motor reprogramming provided is analogous to that offered for various neurologic symptoms associated with organic diseases, such as paraplegia or hemiparesis. Importantly, participants in the MoRe program are encouraged to think of their disorder as definable and treatable, even if the psychogenic component is not concealed.
“We do set the expectation that they will be normal by the end of the week,” said Dr. LaFaver, who explains to patients that neurologic abnormalities are likely to be involved, even if they cannot be objectively demonstrated.
MoRe is run as an inpatient program to permit an adequate intensity of physical therapy and to allow patients to develop trust in their physical and psychological therapists, Dr. LaFaver said. She also suggested that the emphasis on physical therapy in the MoRe program allows patients to frame the goals of treatment in a useful way. The psychological support is essentially adjunctive.
“It can be helpful to use analogies, such as describing the movement disorder as a software [problem] rather than a hardware problem,” Dr. LaFaver explained. The emphasis is on engaging patients to participate in treatment that will reverse adverse changes in the neurologic circuitry that is driving the symptoms. Citing recent functional MRI (fMRI) studies that have shown changes in right temporoparietal junction connectivity in patients with FMD, Dr. LaFaver suggested that the premise of a change in brain function with FMD has evidential support.
Rehabilitation Yields Improvements
In the series of 32 patients treated during a two-year period starting in 2014, the predominant symptoms were abnormal gait in 31%, dystonia in 31%, tremor in 13%, chorea in 13%, myoclonus in 6%, and weakness in 6%. On video rating performed by a movement disorder specialist to compare symptom severity at baseline with that following treatment, movement symptoms improved by 59% on average from day 1 to day 5 of treatment.
The improvement in video ratings was supported by patient self-assessment. On a descending scale of 7 to 1, with 1 signifying the greatest symptom control, the mean patient-assessment score was 2.07 immediately after completing the MoRe program and 2.78 at the six-month follow-up, according to Dr. LaFaver.
Patient satisfaction with the program was high. On an ascending scale of 0 to 10, with 10 providing the best rating, patients gave physical therapy an average rating of 9.23 and psychological skills training an average rating of 8.87. For mental practice training, another aspect of the MoRe program used to reinforce motor reprogramming, the average patient rating was 8.62. Ninety-six percent of patients reported that they would participate in the program again.
Taking a Systematic Approach
Patient selection is important, according to Dr. LaFaver. Although she does not believe it is necessary to rule out all organic diseases with an exhaustive series of diagnostic studies, she did suggest that a movement disorder specialist capable of performing a detailed differential diagnosis should be engaged to confirm FMD. She also suggested that patients are more likely to respond to a program like MoRe after they have accepted a diagnosis of FMD over other potential etiologies, such as Lyme disease.
So far, patients accepted into the MoRe program have typically had significant disability, which has facilitated the justification for inpatient treatment, according to Dr. LaFaver. As a result, third-party reimbursement is usually obtained. For patients with lower symptom burden, such as isolated tremor, similar principles have been employed in an outpatient basis with encouraging rates of response, said Dr. LaFaver. These responses emphasize the value of a systematic approach to a condition that deserves greater public awareness, as well as further clinical research, she added.
Even if FMD is primarily a psychogenic disorder, “patients treated with psychotherapy alone often do not get better,” Dr. LaFaver observed. “It is our job as neurologists to try to make a difference for these patients,” she added. She believes that the principles employed in the MoRe program, many of which were borrowed from an outpatient program at the Mayo Clinic in Rochester, Minnesota (where Dr. LaFaver trained), are broadly applicable in FMD.
Dr. LaFaver reported participation in studies of Parkinson’s disease and Huntington’s disease that had been sponsored by industry and by the NIH, but had no disclosures relevant to FMD.
—Theodore Bosworth
RIVIERA BEACH, FL—A comprehensive, interdisciplinary rehabilitation program for functional movement disorders (FMD) is providing high rates of sustained improvement in patients with this challenging clinical problem, according to an initial analysis. In this program, which is administered during a one-week in-hospital stay, the emphasis is on relearning normal movement through physical therapy, but attention is also paid to the psychological component of the disorder.
What Is FMD?
FMD includes movement abnormalities such as tremor, gait disturbances, or dystonia that are not explained by organic lesions or diseases. FMD is common and occurs in 3% to 5% of patients presenting at movement disorder clinics, according to Dr. LaFaver. Although FMD has an important psychogenic component, Dr. LaFaver said that the symptoms can be as persistent and debilitating as those associated with organic disorders. As with organic diseases, the consequences of FMD include chronic disability.
This last point was emphasized in the demographics of a series of 32 patients with FMD presented by Dr. LaFaver. The mean duration of symptoms was seven years, and 56% of patients were on disability at the time of enrollment into the MoRe program. Consistent with other series of patients with FMD, the population was predominantly female (75%), and a substantial proportion reported posttraumatic stress disorder (53%), sexual abuse (48%), and physical abuse (41%). Mean scores on the Beck Depression Inventory (16.59) and the State-Trait Anxiety Index (40.79) indicated that mood disorders were common. This result also has been previously reported in patients with FMD.
The MoRe Program
Over the course of the inpatient MoRe program, patients begin with simple, repetitive, and structured exercises relevant to their FMD, progressing to more complex motor tasks as they improve. Positive gains with physical therapy, which is provided for five consecutive days, are reinforced with structured cognitive behavioral therapy (CBT). The motor reprogramming provided is analogous to that offered for various neurologic symptoms associated with organic diseases, such as paraplegia or hemiparesis. Importantly, participants in the MoRe program are encouraged to think of their disorder as definable and treatable, even if the psychogenic component is not concealed.
“We do set the expectation that they will be normal by the end of the week,” said Dr. LaFaver, who explains to patients that neurologic abnormalities are likely to be involved, even if they cannot be objectively demonstrated.
MoRe is run as an inpatient program to permit an adequate intensity of physical therapy and to allow patients to develop trust in their physical and psychological therapists, Dr. LaFaver said. She also suggested that the emphasis on physical therapy in the MoRe program allows patients to frame the goals of treatment in a useful way. The psychological support is essentially adjunctive.
“It can be helpful to use analogies, such as describing the movement disorder as a software [problem] rather than a hardware problem,” Dr. LaFaver explained. The emphasis is on engaging patients to participate in treatment that will reverse adverse changes in the neurologic circuitry that is driving the symptoms. Citing recent functional MRI (fMRI) studies that have shown changes in right temporoparietal junction connectivity in patients with FMD, Dr. LaFaver suggested that the premise of a change in brain function with FMD has evidential support.
Rehabilitation Yields Improvements
In the series of 32 patients treated during a two-year period starting in 2014, the predominant symptoms were abnormal gait in 31%, dystonia in 31%, tremor in 13%, chorea in 13%, myoclonus in 6%, and weakness in 6%. On video rating performed by a movement disorder specialist to compare symptom severity at baseline with that following treatment, movement symptoms improved by 59% on average from day 1 to day 5 of treatment.
The improvement in video ratings was supported by patient self-assessment. On a descending scale of 7 to 1, with 1 signifying the greatest symptom control, the mean patient-assessment score was 2.07 immediately after completing the MoRe program and 2.78 at the six-month follow-up, according to Dr. LaFaver.
Patient satisfaction with the program was high. On an ascending scale of 0 to 10, with 10 providing the best rating, patients gave physical therapy an average rating of 9.23 and psychological skills training an average rating of 8.87. For mental practice training, another aspect of the MoRe program used to reinforce motor reprogramming, the average patient rating was 8.62. Ninety-six percent of patients reported that they would participate in the program again.
Taking a Systematic Approach
Patient selection is important, according to Dr. LaFaver. Although she does not believe it is necessary to rule out all organic diseases with an exhaustive series of diagnostic studies, she did suggest that a movement disorder specialist capable of performing a detailed differential diagnosis should be engaged to confirm FMD. She also suggested that patients are more likely to respond to a program like MoRe after they have accepted a diagnosis of FMD over other potential etiologies, such as Lyme disease.
So far, patients accepted into the MoRe program have typically had significant disability, which has facilitated the justification for inpatient treatment, according to Dr. LaFaver. As a result, third-party reimbursement is usually obtained. For patients with lower symptom burden, such as isolated tremor, similar principles have been employed in an outpatient basis with encouraging rates of response, said Dr. LaFaver. These responses emphasize the value of a systematic approach to a condition that deserves greater public awareness, as well as further clinical research, she added.
Even if FMD is primarily a psychogenic disorder, “patients treated with psychotherapy alone often do not get better,” Dr. LaFaver observed. “It is our job as neurologists to try to make a difference for these patients,” she added. She believes that the principles employed in the MoRe program, many of which were borrowed from an outpatient program at the Mayo Clinic in Rochester, Minnesota (where Dr. LaFaver trained), are broadly applicable in FMD.
Dr. LaFaver reported participation in studies of Parkinson’s disease and Huntington’s disease that had been sponsored by industry and by the NIH, but had no disclosures relevant to FMD.
—Theodore Bosworth
RIVIERA BEACH, FL—A comprehensive, interdisciplinary rehabilitation program for functional movement disorders (FMD) is providing high rates of sustained improvement in patients with this challenging clinical problem, according to an initial analysis. In this program, which is administered during a one-week in-hospital stay, the emphasis is on relearning normal movement through physical therapy, but attention is also paid to the psychological component of the disorder.
What Is FMD?
FMD includes movement abnormalities such as tremor, gait disturbances, or dystonia that are not explained by organic lesions or diseases. FMD is common and occurs in 3% to 5% of patients presenting at movement disorder clinics, according to Dr. LaFaver. Although FMD has an important psychogenic component, Dr. LaFaver said that the symptoms can be as persistent and debilitating as those associated with organic disorders. As with organic diseases, the consequences of FMD include chronic disability.
This last point was emphasized in the demographics of a series of 32 patients with FMD presented by Dr. LaFaver. The mean duration of symptoms was seven years, and 56% of patients were on disability at the time of enrollment into the MoRe program. Consistent with other series of patients with FMD, the population was predominantly female (75%), and a substantial proportion reported posttraumatic stress disorder (53%), sexual abuse (48%), and physical abuse (41%). Mean scores on the Beck Depression Inventory (16.59) and the State-Trait Anxiety Index (40.79) indicated that mood disorders were common. This result also has been previously reported in patients with FMD.
The MoRe Program
Over the course of the inpatient MoRe program, patients begin with simple, repetitive, and structured exercises relevant to their FMD, progressing to more complex motor tasks as they improve. Positive gains with physical therapy, which is provided for five consecutive days, are reinforced with structured cognitive behavioral therapy (CBT). The motor reprogramming provided is analogous to that offered for various neurologic symptoms associated with organic diseases, such as paraplegia or hemiparesis. Importantly, participants in the MoRe program are encouraged to think of their disorder as definable and treatable, even if the psychogenic component is not concealed.
“We do set the expectation that they will be normal by the end of the week,” said Dr. LaFaver, who explains to patients that neurologic abnormalities are likely to be involved, even if they cannot be objectively demonstrated.
MoRe is run as an inpatient program to permit an adequate intensity of physical therapy and to allow patients to develop trust in their physical and psychological therapists, Dr. LaFaver said. She also suggested that the emphasis on physical therapy in the MoRe program allows patients to frame the goals of treatment in a useful way. The psychological support is essentially adjunctive.
“It can be helpful to use analogies, such as describing the movement disorder as a software [problem] rather than a hardware problem,” Dr. LaFaver explained. The emphasis is on engaging patients to participate in treatment that will reverse adverse changes in the neurologic circuitry that is driving the symptoms. Citing recent functional MRI (fMRI) studies that have shown changes in right temporoparietal junction connectivity in patients with FMD, Dr. LaFaver suggested that the premise of a change in brain function with FMD has evidential support.
Rehabilitation Yields Improvements
In the series of 32 patients treated during a two-year period starting in 2014, the predominant symptoms were abnormal gait in 31%, dystonia in 31%, tremor in 13%, chorea in 13%, myoclonus in 6%, and weakness in 6%. On video rating performed by a movement disorder specialist to compare symptom severity at baseline with that following treatment, movement symptoms improved by 59% on average from day 1 to day 5 of treatment.
The improvement in video ratings was supported by patient self-assessment. On a descending scale of 7 to 1, with 1 signifying the greatest symptom control, the mean patient-assessment score was 2.07 immediately after completing the MoRe program and 2.78 at the six-month follow-up, according to Dr. LaFaver.
Patient satisfaction with the program was high. On an ascending scale of 0 to 10, with 10 providing the best rating, patients gave physical therapy an average rating of 9.23 and psychological skills training an average rating of 8.87. For mental practice training, another aspect of the MoRe program used to reinforce motor reprogramming, the average patient rating was 8.62. Ninety-six percent of patients reported that they would participate in the program again.
Taking a Systematic Approach
Patient selection is important, according to Dr. LaFaver. Although she does not believe it is necessary to rule out all organic diseases with an exhaustive series of diagnostic studies, she did suggest that a movement disorder specialist capable of performing a detailed differential diagnosis should be engaged to confirm FMD. She also suggested that patients are more likely to respond to a program like MoRe after they have accepted a diagnosis of FMD over other potential etiologies, such as Lyme disease.
So far, patients accepted into the MoRe program have typically had significant disability, which has facilitated the justification for inpatient treatment, according to Dr. LaFaver. As a result, third-party reimbursement is usually obtained. For patients with lower symptom burden, such as isolated tremor, similar principles have been employed in an outpatient basis with encouraging rates of response, said Dr. LaFaver. These responses emphasize the value of a systematic approach to a condition that deserves greater public awareness, as well as further clinical research, she added.
Even if FMD is primarily a psychogenic disorder, “patients treated with psychotherapy alone often do not get better,” Dr. LaFaver observed. “It is our job as neurologists to try to make a difference for these patients,” she added. She believes that the principles employed in the MoRe program, many of which were borrowed from an outpatient program at the Mayo Clinic in Rochester, Minnesota (where Dr. LaFaver trained), are broadly applicable in FMD.
Dr. LaFaver reported participation in studies of Parkinson’s disease and Huntington’s disease that had been sponsored by industry and by the NIH, but had no disclosures relevant to FMD.
—Theodore Bosworth
Collagenous and Elastotic Marginal Plaques of the Hands
To the Editor:
Collagenous and elastotic marginal plaques of the hands (CEMPHs) has several names including degenerative collagenous plaques of the hands, keratoelastoidosis marginalis, and digital papular calcific elastosis. This rare disorder is an acquired, slowly progressive, asymptomatic, dermal connective tissue abnormality that is underrecognized and underdiagnosed. Clinical presentation includes hyperkeratotic translucent papules arranged linearly on the radial aspect of the hands.
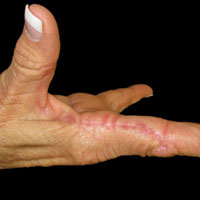
A 74-year-old woman described having "rough hands" of more than 20 years' duration. She presented with 4-cm wide longitudinal, erythematous, firm, depressed plaques along the lateral edge of the second finger and extending to the medial thumb in both hands (Figure 1). She had attempted multiple treatments by her primary care physician, including topical and oral medications unknown to the patient and light therapy, all without benefit over a period of several years. We have attempted salicylic acid 40%, clobetasol cream 0.05%, and emollient creams containing α-hydroxy acid. At best the condition fluctuated between a subtle raised scale at the edge to smooth and occasionally more red-pink, seemingly unrelated to any treatments.
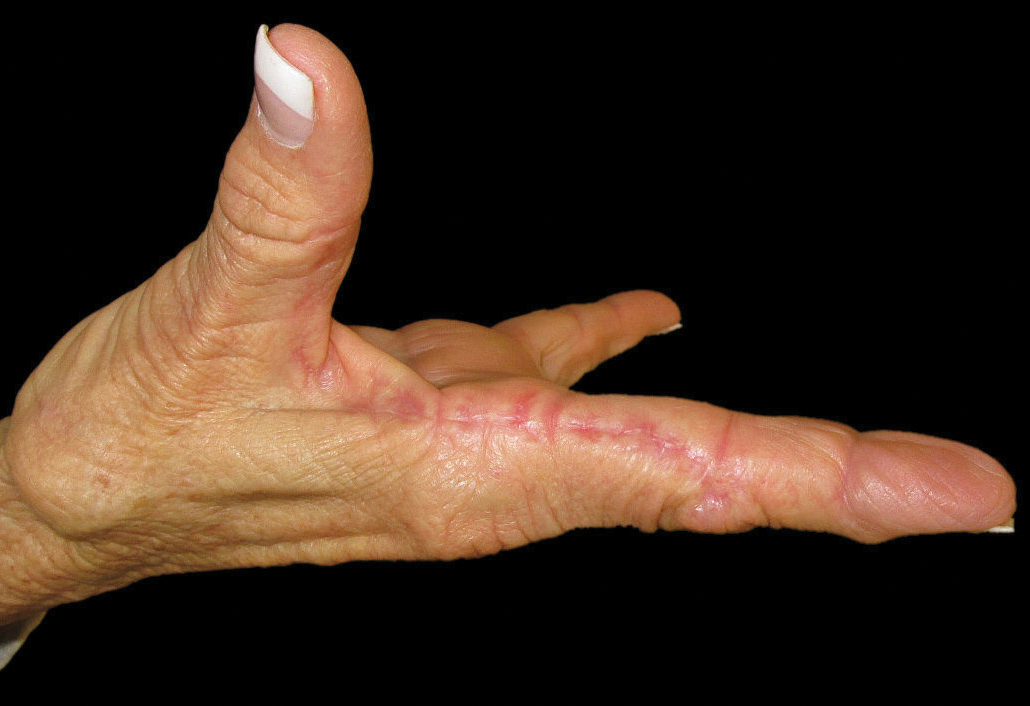
The patient did not have plaques elsewhere on the body, and notably, the feet were clear. She did not have a history of repeated trauma to the hands and did not engage in manual labor. She denied excessive sun exposure, though she had Fitzpatrick skin type III and a history of multiple precancers and nonmelanoma skin cancers 7 years prior to presentation.
Histology of CEMPH reveals a hyperkeratotic epidermis with an avascular and acellular replacement of the superficial reticular dermis by haphazardly arranged, thickened collagen fibers (Figure 2A-2C). Collagen fibers were oriented perpendicularly to the epidermal surface. Intervening amorphous basophilic elastotic masses were present in the upper dermis with occasional calcification and degenerative elastic fibers (Figure 2D).

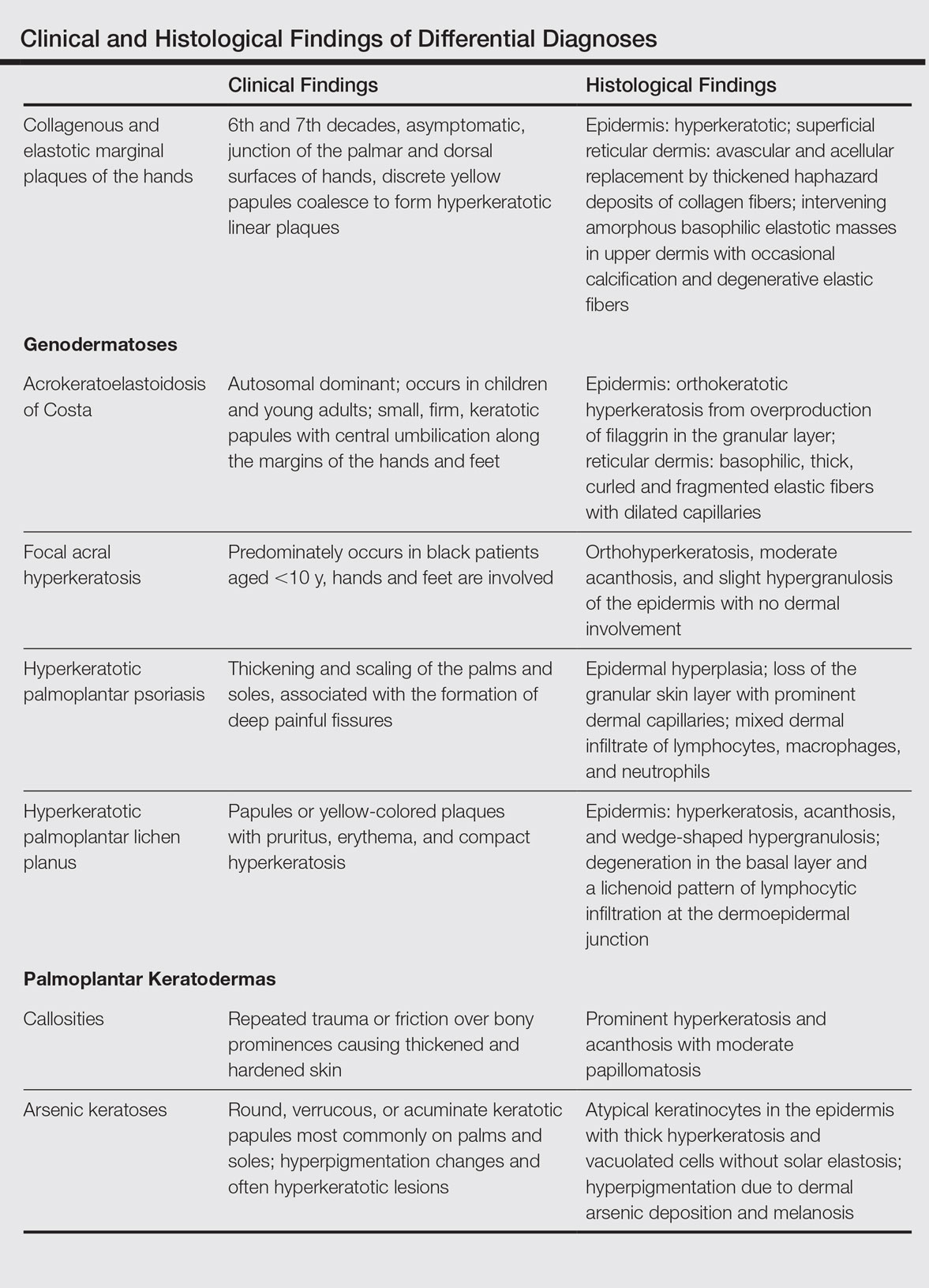
Collagenous and elastotic marginal plaques of the hands is a chronic, asymptomatic, sclerotic skin disorder described in a 1960 case series of 5 patients reported by Burks et al.1 Although it has many names, the most common is CEMPH. Collagenous and elastotic marginal plaques of the hands most often presents in white men aged 50 to 60 years.2 Patients typically are asymptomatic with plaques limited to the junction of the palmar and dorsal surfaces of the hands with only minimal intermittent stiffness around the flexor creases. Lesions begin as discrete yellow papules that coalesce to form hyperkeratotic linear plaques with occasional telangiectasia.3
The etiology of CEMPH is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.4,5 The 3 stages of degeneration include an initial linear padded stage, an intermediate padded plaque stage, and an advanced padded hyperkeratotic plaque stage.4 Vascular compromise is seen from the enlarged and fused thickened collagen and elastic fibers that in turn lead to ischemic changes, hyperkeratosis with epidermal atrophy, and papillary dermis telangiectasia. Absence or weak expression of keratins 14 and 10 and strong expression of keratin 16 have been reported in the epidermis of CEMPH patients.4
Collagenous and elastotic marginal plaques of the hands do not have a specific treatment, as it is a benign, slowly progressive condition. Several treatments such as laser therapy, high-potency topical corticosteroids, topical tazarotene and tretinoin, oral isotretinoin, and cryotherapy have been tried with little long-term success.4 Moisturizing may help reduce fissuring, and patients are advised to avoid the sun and repeated trauma to the hands.
The differential diagnosis of CEMPH is summarized in the Table. Two genodermatoses—acrokeratoelastoidosis of Costa and focal acral hyperkeratosis—clinically resemble CEMPH. Acrokeratoelastoidosis of Costa is an autosomal-dominant condition that occurs without trauma in children and young adults. Histopathology shows orthokeratotic hyperkeratosis due to an overproduction of filaggrin in the granular layer of the epidermis. The reticular dermis shows basophilic, thick, curled and fragmented elastic fibers with dilated capillaries that can be seen with Weigert elastic, Verhoeff-van Gieson, or orcein stains. Focal acral hyperkeratosis occurs on the hands and feet, predominantly in black patients. On histology, the epidermis shows a characteristic orthohyperkeratosis, moderate acanthosis, and slight hypergranulosis with no dermal involvment.6
Chronic hyperkeratotic eczematous dermatitis is another common entity in the differential characterized by hyperkeratotic plaques that scale and fissure. Biopsy demonstrates a spongiotic acanthotic epidermis.7,8
Psoriasis of the hands, specifically hyperkeratotic palmoplantar psoriasis, is associated with manual labor, similar to CEMPH. Histology shows epidermal hyperplasia; regular acanthosis; loss of the granular skin layer with prominent dermal capillaries; and a mixed dermal infiltrate of lymphocytes, macrophages, and neutrophils.9 Hyperkeratotic palmoplantar lichen planus presents with pruritic papules in the third and fifth decades of life. Histologically, hyperkeratosis, acanthosis, and wedge-shaped hypergranulosis with a lichenoid lymphocytic infiltration at the dermoepidermal junction is seen.10
Palmoplantar keratodermas due to inflammatory reactive dermatoses include callosities that develop in response to repeated trauma or friction on the skin. On histology, there is prominent hyperkeratosis and acanthosis with moderate papillomatosis.11 Drug-related palmoplantar keratodermas such as those from arsenic exposure can lead to multiple, irregular, verrucous, keratotic, and pigmented lesions on the palms and soles. Histologically, atypical keratinocytes are seen in the epidermis with thick hyperkeratosis and vacuolated cells without solar elastosis.12
In conclusion, CEMPH is an underdiagnosed and underrecognized condition characterized by asymptomatic hyperkeratotic linear plaques along the medial aspect of the thumb and radial aspect of the index finger. It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It also is imperative to separate it from other diseases and avoid misdiagnosing this degenerative collagenous and elastotic disease as a malignant lesion.
- Burks JW, Wise LJ, Clark WH. Degenerative collagenous plaques of the hands. Arch Dermatol. 1960;82:362-366.
- Jordaan HF, Rossouw DJ. Digital papular calcific elastosis: a histopathological, histochemical and ultrastructural study of 20 patients. J Cutan Pathol. 1990;17:358-370.
- Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42:211-213.
- Tieu KD, Satter EK. Thickened plaques on the hands. Collagenous and elastotic marginal plaques of the hands (CEMPH). Arch Dermatol. 2011;147:499-504.
- Todd D, Al-Aboosi M, Hameed O, et al. The role of UV light in the pathogenesis of digital papular calcific elastosis. Arch Dermatol. 2001;137:379-381.
- Mengesha YM, Kayal JD, Swerlick RA. Keratoelastoidosis marginalis. J Cutan Med Surg. 2002;6:23-25.
- MacKee MG, Lewis MG. Keratolysis exfoliativa and the mosaic fungus. Arch Dermatol. 1931;23:445-447.
- Walling HW, Swick BL, Storrs FJ, et al. Frictional hyperkeratotic hand dermatitis responding to Grenz ray therapy. Contact Dermatitis. 2008;58:49-51.
- Farley E, Masrour S, McKey J, et al. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-1031.
- Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
- Unal VS, Sevin A, Dayican A. Palmar callus formation as a result of mechanical trauma during sailing. Plast Reconstr Surg. 2005;115:2161-2162.
- Cöl M, Cöl C, Soran A, et al. Arsenic-related Bowen's disease, palmar keratosis, and skin cancer. Environ Health Perspect. 1999;107:687-689.
To the Editor:
Collagenous and elastotic marginal plaques of the hands (CEMPHs) has several names including degenerative collagenous plaques of the hands, keratoelastoidosis marginalis, and digital papular calcific elastosis. This rare disorder is an acquired, slowly progressive, asymptomatic, dermal connective tissue abnormality that is underrecognized and underdiagnosed. Clinical presentation includes hyperkeratotic translucent papules arranged linearly on the radial aspect of the hands.
A 74-year-old woman described having "rough hands" of more than 20 years' duration. She presented with 4-cm wide longitudinal, erythematous, firm, depressed plaques along the lateral edge of the second finger and extending to the medial thumb in both hands (Figure 1). She had attempted multiple treatments by her primary care physician, including topical and oral medications unknown to the patient and light therapy, all without benefit over a period of several years. We have attempted salicylic acid 40%, clobetasol cream 0.05%, and emollient creams containing α-hydroxy acid. At best the condition fluctuated between a subtle raised scale at the edge to smooth and occasionally more red-pink, seemingly unrelated to any treatments.

The patient did not have plaques elsewhere on the body, and notably, the feet were clear. She did not have a history of repeated trauma to the hands and did not engage in manual labor. She denied excessive sun exposure, though she had Fitzpatrick skin type III and a history of multiple precancers and nonmelanoma skin cancers 7 years prior to presentation.
Histology of CEMPH reveals a hyperkeratotic epidermis with an avascular and acellular replacement of the superficial reticular dermis by haphazardly arranged, thickened collagen fibers (Figure 2A-2C). Collagen fibers were oriented perpendicularly to the epidermal surface. Intervening amorphous basophilic elastotic masses were present in the upper dermis with occasional calcification and degenerative elastic fibers (Figure 2D).

Collagenous and elastotic marginal plaques of the hands is a chronic, asymptomatic, sclerotic skin disorder described in a 1960 case series of 5 patients reported by Burks et al.1 Although it has many names, the most common is CEMPH. Collagenous and elastotic marginal plaques of the hands most often presents in white men aged 50 to 60 years.2 Patients typically are asymptomatic with plaques limited to the junction of the palmar and dorsal surfaces of the hands with only minimal intermittent stiffness around the flexor creases. Lesions begin as discrete yellow papules that coalesce to form hyperkeratotic linear plaques with occasional telangiectasia.3
The etiology of CEMPH is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.4,5 The 3 stages of degeneration include an initial linear padded stage, an intermediate padded plaque stage, and an advanced padded hyperkeratotic plaque stage.4 Vascular compromise is seen from the enlarged and fused thickened collagen and elastic fibers that in turn lead to ischemic changes, hyperkeratosis with epidermal atrophy, and papillary dermis telangiectasia. Absence or weak expression of keratins 14 and 10 and strong expression of keratin 16 have been reported in the epidermis of CEMPH patients.4
Collagenous and elastotic marginal plaques of the hands do not have a specific treatment, as it is a benign, slowly progressive condition. Several treatments such as laser therapy, high-potency topical corticosteroids, topical tazarotene and tretinoin, oral isotretinoin, and cryotherapy have been tried with little long-term success.4 Moisturizing may help reduce fissuring, and patients are advised to avoid the sun and repeated trauma to the hands.
The differential diagnosis of CEMPH is summarized in the Table. Two genodermatoses—acrokeratoelastoidosis of Costa and focal acral hyperkeratosis—clinically resemble CEMPH. Acrokeratoelastoidosis of Costa is an autosomal-dominant condition that occurs without trauma in children and young adults. Histopathology shows orthokeratotic hyperkeratosis due to an overproduction of filaggrin in the granular layer of the epidermis. The reticular dermis shows basophilic, thick, curled and fragmented elastic fibers with dilated capillaries that can be seen with Weigert elastic, Verhoeff-van Gieson, or orcein stains. Focal acral hyperkeratosis occurs on the hands and feet, predominantly in black patients. On histology, the epidermis shows a characteristic orthohyperkeratosis, moderate acanthosis, and slight hypergranulosis with no dermal involvment.6

Chronic hyperkeratotic eczematous dermatitis is another common entity in the differential characterized by hyperkeratotic plaques that scale and fissure. Biopsy demonstrates a spongiotic acanthotic epidermis.7,8
Psoriasis of the hands, specifically hyperkeratotic palmoplantar psoriasis, is associated with manual labor, similar to CEMPH. Histology shows epidermal hyperplasia; regular acanthosis; loss of the granular skin layer with prominent dermal capillaries; and a mixed dermal infiltrate of lymphocytes, macrophages, and neutrophils.9 Hyperkeratotic palmoplantar lichen planus presents with pruritic papules in the third and fifth decades of life. Histologically, hyperkeratosis, acanthosis, and wedge-shaped hypergranulosis with a lichenoid lymphocytic infiltration at the dermoepidermal junction is seen.10
Palmoplantar keratodermas due to inflammatory reactive dermatoses include callosities that develop in response to repeated trauma or friction on the skin. On histology, there is prominent hyperkeratosis and acanthosis with moderate papillomatosis.11 Drug-related palmoplantar keratodermas such as those from arsenic exposure can lead to multiple, irregular, verrucous, keratotic, and pigmented lesions on the palms and soles. Histologically, atypical keratinocytes are seen in the epidermis with thick hyperkeratosis and vacuolated cells without solar elastosis.12
In conclusion, CEMPH is an underdiagnosed and underrecognized condition characterized by asymptomatic hyperkeratotic linear plaques along the medial aspect of the thumb and radial aspect of the index finger. It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It also is imperative to separate it from other diseases and avoid misdiagnosing this degenerative collagenous and elastotic disease as a malignant lesion.
To the Editor:
Collagenous and elastotic marginal plaques of the hands (CEMPHs) has several names including degenerative collagenous plaques of the hands, keratoelastoidosis marginalis, and digital papular calcific elastosis. This rare disorder is an acquired, slowly progressive, asymptomatic, dermal connective tissue abnormality that is underrecognized and underdiagnosed. Clinical presentation includes hyperkeratotic translucent papules arranged linearly on the radial aspect of the hands.
A 74-year-old woman described having "rough hands" of more than 20 years' duration. She presented with 4-cm wide longitudinal, erythematous, firm, depressed plaques along the lateral edge of the second finger and extending to the medial thumb in both hands (Figure 1). She had attempted multiple treatments by her primary care physician, including topical and oral medications unknown to the patient and light therapy, all without benefit over a period of several years. We have attempted salicylic acid 40%, clobetasol cream 0.05%, and emollient creams containing α-hydroxy acid. At best the condition fluctuated between a subtle raised scale at the edge to smooth and occasionally more red-pink, seemingly unrelated to any treatments.

The patient did not have plaques elsewhere on the body, and notably, the feet were clear. She did not have a history of repeated trauma to the hands and did not engage in manual labor. She denied excessive sun exposure, though she had Fitzpatrick skin type III and a history of multiple precancers and nonmelanoma skin cancers 7 years prior to presentation.
Histology of CEMPH reveals a hyperkeratotic epidermis with an avascular and acellular replacement of the superficial reticular dermis by haphazardly arranged, thickened collagen fibers (Figure 2A-2C). Collagen fibers were oriented perpendicularly to the epidermal surface. Intervening amorphous basophilic elastotic masses were present in the upper dermis with occasional calcification and degenerative elastic fibers (Figure 2D).

Collagenous and elastotic marginal plaques of the hands is a chronic, asymptomatic, sclerotic skin disorder described in a 1960 case series of 5 patients reported by Burks et al.1 Although it has many names, the most common is CEMPH. Collagenous and elastotic marginal plaques of the hands most often presents in white men aged 50 to 60 years.2 Patients typically are asymptomatic with plaques limited to the junction of the palmar and dorsal surfaces of the hands with only minimal intermittent stiffness around the flexor creases. Lesions begin as discrete yellow papules that coalesce to form hyperkeratotic linear plaques with occasional telangiectasia.3
The etiology of CEMPH is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.4,5 The 3 stages of degeneration include an initial linear padded stage, an intermediate padded plaque stage, and an advanced padded hyperkeratotic plaque stage.4 Vascular compromise is seen from the enlarged and fused thickened collagen and elastic fibers that in turn lead to ischemic changes, hyperkeratosis with epidermal atrophy, and papillary dermis telangiectasia. Absence or weak expression of keratins 14 and 10 and strong expression of keratin 16 have been reported in the epidermis of CEMPH patients.4
Collagenous and elastotic marginal plaques of the hands do not have a specific treatment, as it is a benign, slowly progressive condition. Several treatments such as laser therapy, high-potency topical corticosteroids, topical tazarotene and tretinoin, oral isotretinoin, and cryotherapy have been tried with little long-term success.4 Moisturizing may help reduce fissuring, and patients are advised to avoid the sun and repeated trauma to the hands.
The differential diagnosis of CEMPH is summarized in the Table. Two genodermatoses—acrokeratoelastoidosis of Costa and focal acral hyperkeratosis—clinically resemble CEMPH. Acrokeratoelastoidosis of Costa is an autosomal-dominant condition that occurs without trauma in children and young adults. Histopathology shows orthokeratotic hyperkeratosis due to an overproduction of filaggrin in the granular layer of the epidermis. The reticular dermis shows basophilic, thick, curled and fragmented elastic fibers with dilated capillaries that can be seen with Weigert elastic, Verhoeff-van Gieson, or orcein stains. Focal acral hyperkeratosis occurs on the hands and feet, predominantly in black patients. On histology, the epidermis shows a characteristic orthohyperkeratosis, moderate acanthosis, and slight hypergranulosis with no dermal involvment.6

Chronic hyperkeratotic eczematous dermatitis is another common entity in the differential characterized by hyperkeratotic plaques that scale and fissure. Biopsy demonstrates a spongiotic acanthotic epidermis.7,8
Psoriasis of the hands, specifically hyperkeratotic palmoplantar psoriasis, is associated with manual labor, similar to CEMPH. Histology shows epidermal hyperplasia; regular acanthosis; loss of the granular skin layer with prominent dermal capillaries; and a mixed dermal infiltrate of lymphocytes, macrophages, and neutrophils.9 Hyperkeratotic palmoplantar lichen planus presents with pruritic papules in the third and fifth decades of life. Histologically, hyperkeratosis, acanthosis, and wedge-shaped hypergranulosis with a lichenoid lymphocytic infiltration at the dermoepidermal junction is seen.10
Palmoplantar keratodermas due to inflammatory reactive dermatoses include callosities that develop in response to repeated trauma or friction on the skin. On histology, there is prominent hyperkeratosis and acanthosis with moderate papillomatosis.11 Drug-related palmoplantar keratodermas such as those from arsenic exposure can lead to multiple, irregular, verrucous, keratotic, and pigmented lesions on the palms and soles. Histologically, atypical keratinocytes are seen in the epidermis with thick hyperkeratosis and vacuolated cells without solar elastosis.12
In conclusion, CEMPH is an underdiagnosed and underrecognized condition characterized by asymptomatic hyperkeratotic linear plaques along the medial aspect of the thumb and radial aspect of the index finger. It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It also is imperative to separate it from other diseases and avoid misdiagnosing this degenerative collagenous and elastotic disease as a malignant lesion.
- Burks JW, Wise LJ, Clark WH. Degenerative collagenous plaques of the hands. Arch Dermatol. 1960;82:362-366.
- Jordaan HF, Rossouw DJ. Digital papular calcific elastosis: a histopathological, histochemical and ultrastructural study of 20 patients. J Cutan Pathol. 1990;17:358-370.
- Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42:211-213.
- Tieu KD, Satter EK. Thickened plaques on the hands. Collagenous and elastotic marginal plaques of the hands (CEMPH). Arch Dermatol. 2011;147:499-504.
- Todd D, Al-Aboosi M, Hameed O, et al. The role of UV light in the pathogenesis of digital papular calcific elastosis. Arch Dermatol. 2001;137:379-381.
- Mengesha YM, Kayal JD, Swerlick RA. Keratoelastoidosis marginalis. J Cutan Med Surg. 2002;6:23-25.
- MacKee MG, Lewis MG. Keratolysis exfoliativa and the mosaic fungus. Arch Dermatol. 1931;23:445-447.
- Walling HW, Swick BL, Storrs FJ, et al. Frictional hyperkeratotic hand dermatitis responding to Grenz ray therapy. Contact Dermatitis. 2008;58:49-51.
- Farley E, Masrour S, McKey J, et al. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-1031.
- Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
- Unal VS, Sevin A, Dayican A. Palmar callus formation as a result of mechanical trauma during sailing. Plast Reconstr Surg. 2005;115:2161-2162.
- Cöl M, Cöl C, Soran A, et al. Arsenic-related Bowen's disease, palmar keratosis, and skin cancer. Environ Health Perspect. 1999;107:687-689.
- Burks JW, Wise LJ, Clark WH. Degenerative collagenous plaques of the hands. Arch Dermatol. 1960;82:362-366.
- Jordaan HF, Rossouw DJ. Digital papular calcific elastosis: a histopathological, histochemical and ultrastructural study of 20 patients. J Cutan Pathol. 1990;17:358-370.
- Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42:211-213.
- Tieu KD, Satter EK. Thickened plaques on the hands. Collagenous and elastotic marginal plaques of the hands (CEMPH). Arch Dermatol. 2011;147:499-504.
- Todd D, Al-Aboosi M, Hameed O, et al. The role of UV light in the pathogenesis of digital papular calcific elastosis. Arch Dermatol. 2001;137:379-381.
- Mengesha YM, Kayal JD, Swerlick RA. Keratoelastoidosis marginalis. J Cutan Med Surg. 2002;6:23-25.
- MacKee MG, Lewis MG. Keratolysis exfoliativa and the mosaic fungus. Arch Dermatol. 1931;23:445-447.
- Walling HW, Swick BL, Storrs FJ, et al. Frictional hyperkeratotic hand dermatitis responding to Grenz ray therapy. Contact Dermatitis. 2008;58:49-51.
- Farley E, Masrour S, McKey J, et al. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-1031.
- Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
- Unal VS, Sevin A, Dayican A. Palmar callus formation as a result of mechanical trauma during sailing. Plast Reconstr Surg. 2005;115:2161-2162.
- Cöl M, Cöl C, Soran A, et al. Arsenic-related Bowen's disease, palmar keratosis, and skin cancer. Environ Health Perspect. 1999;107:687-689.
Practice Points
- The etiology of collagenous and elastotic marginal plaques of the hands (CEMPHs) is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.
- It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It should be separated from other diseases and avoid being misdiagnosed as a malignant lesion.
FDA approves first treatment for metastatic Merkel cell carcinoma
The Food and Drug Administration has granted accelerated approval to avelumab for the treatment of metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients aged 12 years and older.
Avelumab, a programmed death-ligand 1 (PD-L1)–blocking human IgG1 lambda monoclonal antibody, is the first FDA-approved treatment for metastatic MCC.
Approval was based on a 33% overall response rate in a single arm trial (JAVELIN Merkel 200 trial) of 88 patients with metastatic MCC who had been previously treated with at least one prior chemotherapy regimen, the FDA said in a written statement.
The response duration among that 33% ranged from 2.8 to 23.3+ months, and 86% of responses were durable for 6 months or more. “Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel cell polyomavirus,” the FDA said.
There were safety data in 1,738 patients, who received 10 mg/kg of avelumab every 2 weeks. Immune-mediated adverse reactions (pneumonitis, colitis, hepatitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions were the most common, serious adverse events associated with avelumab. Of the 88 patients in the JAVELIN Merkel 200 trial, the most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. Serious adverse reactions that occurred in more than one patient in the trial were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis, the FDA said.
The recommended dose of avelumab is 10 mg/kg administered in an intravenous infusion over 60 minutes every 2 weeks. Labeling includes the recommendation that all patients should be premedicated with an antihistamine and acetaminophen before each of the first four infusions.
“As a condition of accelerated approval, an additional study is required to confirm the clinical benefit of avelumab for this indication,” according to the FDA.
The drug is being marketed as Bavencio by EMD Serono.
The Food and Drug Administration has granted accelerated approval to avelumab for the treatment of metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients aged 12 years and older.
Avelumab, a programmed death-ligand 1 (PD-L1)–blocking human IgG1 lambda monoclonal antibody, is the first FDA-approved treatment for metastatic MCC.
Approval was based on a 33% overall response rate in a single arm trial (JAVELIN Merkel 200 trial) of 88 patients with metastatic MCC who had been previously treated with at least one prior chemotherapy regimen, the FDA said in a written statement.
The response duration among that 33% ranged from 2.8 to 23.3+ months, and 86% of responses were durable for 6 months or more. “Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel cell polyomavirus,” the FDA said.
There were safety data in 1,738 patients, who received 10 mg/kg of avelumab every 2 weeks. Immune-mediated adverse reactions (pneumonitis, colitis, hepatitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions were the most common, serious adverse events associated with avelumab. Of the 88 patients in the JAVELIN Merkel 200 trial, the most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. Serious adverse reactions that occurred in more than one patient in the trial were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis, the FDA said.
The recommended dose of avelumab is 10 mg/kg administered in an intravenous infusion over 60 minutes every 2 weeks. Labeling includes the recommendation that all patients should be premedicated with an antihistamine and acetaminophen before each of the first four infusions.
“As a condition of accelerated approval, an additional study is required to confirm the clinical benefit of avelumab for this indication,” according to the FDA.
The drug is being marketed as Bavencio by EMD Serono.
The Food and Drug Administration has granted accelerated approval to avelumab for the treatment of metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients aged 12 years and older.
Avelumab, a programmed death-ligand 1 (PD-L1)–blocking human IgG1 lambda monoclonal antibody, is the first FDA-approved treatment for metastatic MCC.
Approval was based on a 33% overall response rate in a single arm trial (JAVELIN Merkel 200 trial) of 88 patients with metastatic MCC who had been previously treated with at least one prior chemotherapy regimen, the FDA said in a written statement.
The response duration among that 33% ranged from 2.8 to 23.3+ months, and 86% of responses were durable for 6 months or more. “Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel cell polyomavirus,” the FDA said.
There were safety data in 1,738 patients, who received 10 mg/kg of avelumab every 2 weeks. Immune-mediated adverse reactions (pneumonitis, colitis, hepatitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions were the most common, serious adverse events associated with avelumab. Of the 88 patients in the JAVELIN Merkel 200 trial, the most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. Serious adverse reactions that occurred in more than one patient in the trial were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis, the FDA said.
The recommended dose of avelumab is 10 mg/kg administered in an intravenous infusion over 60 minutes every 2 weeks. Labeling includes the recommendation that all patients should be premedicated with an antihistamine and acetaminophen before each of the first four infusions.
“As a condition of accelerated approval, an additional study is required to confirm the clinical benefit of avelumab for this indication,” according to the FDA.
The drug is being marketed as Bavencio by EMD Serono.
Grandparents aid in early diagnosis of autism
Family members other than parents can play a key role in initial recognition of problems prior to diagnosis of autism, which may lead to earlier diagnosis and potentially better outcomes, a study found.
Of 477 parents of children with autism who were surveyed online, 25% of parents reported that other individuals indicated their child might have a serious condition before they themselves suspected it. “The two most common categories of individuals are maternal grandmothers (27%) and teachers (24%),” Nachum Sicherman, PhD, of Columbia Business School, New York, and associates said. “If one adds maternal and paternal grandmothers and grandfathers together, then 59% of respondents who reported that anyone had raised concerns before they were aware that their child had a problem identified grandparents as having done so” (Autism. 2017. doi: 10.1177/1362361316679632)”
Children with no siblings were diagnosed 6-8 months earlier than children with siblings (P less than .01).Children with an older sibling were diagnosed approximately 10 months earlier, relative to those with only younger siblings. (P less than .01).
Thus, the presence of grandparents and siblings significantly affects age of diagnosis in autistic children, Dr. Sicherman and associates said.
*This article was update 3/27/2018
Family members other than parents can play a key role in initial recognition of problems prior to diagnosis of autism, which may lead to earlier diagnosis and potentially better outcomes, a study found.
Of 477 parents of children with autism who were surveyed online, 25% of parents reported that other individuals indicated their child might have a serious condition before they themselves suspected it. “The two most common categories of individuals are maternal grandmothers (27%) and teachers (24%),” Nachum Sicherman, PhD, of Columbia Business School, New York, and associates said. “If one adds maternal and paternal grandmothers and grandfathers together, then 59% of respondents who reported that anyone had raised concerns before they were aware that their child had a problem identified grandparents as having done so” (Autism. 2017. doi: 10.1177/1362361316679632)”
Children with no siblings were diagnosed 6-8 months earlier than children with siblings (P less than .01).Children with an older sibling were diagnosed approximately 10 months earlier, relative to those with only younger siblings. (P less than .01).
Thus, the presence of grandparents and siblings significantly affects age of diagnosis in autistic children, Dr. Sicherman and associates said.
*This article was update 3/27/2018
Family members other than parents can play a key role in initial recognition of problems prior to diagnosis of autism, which may lead to earlier diagnosis and potentially better outcomes, a study found.
Of 477 parents of children with autism who were surveyed online, 25% of parents reported that other individuals indicated their child might have a serious condition before they themselves suspected it. “The two most common categories of individuals are maternal grandmothers (27%) and teachers (24%),” Nachum Sicherman, PhD, of Columbia Business School, New York, and associates said. “If one adds maternal and paternal grandmothers and grandfathers together, then 59% of respondents who reported that anyone had raised concerns before they were aware that their child had a problem identified grandparents as having done so” (Autism. 2017. doi: 10.1177/1362361316679632)”
Children with no siblings were diagnosed 6-8 months earlier than children with siblings (P less than .01).Children with an older sibling were diagnosed approximately 10 months earlier, relative to those with only younger siblings. (P less than .01).
Thus, the presence of grandparents and siblings significantly affects age of diagnosis in autistic children, Dr. Sicherman and associates said.
*This article was update 3/27/2018
How to stop penicillin allergy relabeling
ATLANTA – About a third to half of patients who test negative for penicillin allergies and have the label removed from their charts will, somewhere down the line, be relabeled as penicillin allergic.
It’s a vexing problem for the increasing number of patients who undergo confirmatory skin testing for penicillin sensitivity while in hospital. It’s become clear in recent years that at least 90% of people who say they have a penicillin allergy don’t really have one. Without confirmatory testing, they end up on expensive, second-line antibiotics, and don’t do well.
It’s unclear why people are relabeled after negative tests. Maybe patients don’t trust the results. Maybe doctors don’t hear about them or err on the side of caution despite negative testing. “I suspect it’s a combination of patient and provider factors,” said Sheenal Patel, MD, an allergy and immunology fellow at the University of Texas Southwestern Medical Center, Dallas.
Whatever the reason, UT Southwestern has taken steps over the past few years to make sure negative test results stick in patients’ records, he said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
First, the time and place of negative tests were documented in the EHR, primary care providers were notified of the results, and pharmacists counseled patients at the time of negative testing to make sure they understood the results.
Next, pharmacists began to counsel patients after discharge to reaffirm the message, either face to face or over the phone. The center added an alert to the EHR that pops up if someone tries to relabel a patient and it notifies the pharmacists running the penicillin allergy testing program that an attempt was made. They call the patient’s primary care provider to find out what’s going on.
As of late, patients go home with a wallet card that documents their negative test results, to show providers who aren’t on the UT Southwestern EHR system, and family members.
It’s all made a difference. Only 31 of 225 (13.8%) were relabeled in a review presented by Dr. Patel. All 225 patients had at least 90 days of postdischarge follow-up in the UT Southwestern EHR system.
Rates of relabeling varied according to the specific intervention. Five of 27 (18.5%) who had only pharmacist counseling at the time of negative testing, documentation of negative results in the EHR, and the alert added to their electronic record were relabeled, versus just 1 of 15 patients (6.7%) who received all of the interventions, including pre- and postdischarge counseling and the wallet card. The relabel rate was 14.3% (14) among the 98 patients counseled by a pharmacist when they tested negative, with the results documented in their electronic record – the largest patient subset in the study.
Given the small numbers, it’s hard to know which intervention gave the most bang for the buck, but “pharmacist counseling and EHR documentation had clear benefit.” Postdischarge counseling, EHR alerts, and the wallet cards probably helped, too, Dr. Patel said.
Older patients were more likely to be relabeled, but the trend didn’t reach significance (P = .07). The risk of relabeling was unrelated to race, gender, infection risk factors, number of drug allergies, allergy symptoms, or how long ago the alleged penicillin reaction occurred; most patients reported it was more than 20 years ago. About half of the relabels were on the outpatient side, almost a third in the ED, and the rest in a hospital.
The study didn’t address why they occurred. “Some patients who have had this label for 20 or 30 years will just say they are penicillin allergic despite all our counseling efforts, and whoever is reviewing their allergy list has to decide what to do with that. [Often,] they put it back in the chart. There’s a fear of penicillin allergy histories not only among patients, but also among many providers, and that contributes a lot to this,” Dr. Patel said.
There was no external funding for the work. Dr. Patel had no disclosures.
ATLANTA – About a third to half of patients who test negative for penicillin allergies and have the label removed from their charts will, somewhere down the line, be relabeled as penicillin allergic.
It’s a vexing problem for the increasing number of patients who undergo confirmatory skin testing for penicillin sensitivity while in hospital. It’s become clear in recent years that at least 90% of people who say they have a penicillin allergy don’t really have one. Without confirmatory testing, they end up on expensive, second-line antibiotics, and don’t do well.
It’s unclear why people are relabeled after negative tests. Maybe patients don’t trust the results. Maybe doctors don’t hear about them or err on the side of caution despite negative testing. “I suspect it’s a combination of patient and provider factors,” said Sheenal Patel, MD, an allergy and immunology fellow at the University of Texas Southwestern Medical Center, Dallas.
Whatever the reason, UT Southwestern has taken steps over the past few years to make sure negative test results stick in patients’ records, he said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
First, the time and place of negative tests were documented in the EHR, primary care providers were notified of the results, and pharmacists counseled patients at the time of negative testing to make sure they understood the results.
Next, pharmacists began to counsel patients after discharge to reaffirm the message, either face to face or over the phone. The center added an alert to the EHR that pops up if someone tries to relabel a patient and it notifies the pharmacists running the penicillin allergy testing program that an attempt was made. They call the patient’s primary care provider to find out what’s going on.
As of late, patients go home with a wallet card that documents their negative test results, to show providers who aren’t on the UT Southwestern EHR system, and family members.
It’s all made a difference. Only 31 of 225 (13.8%) were relabeled in a review presented by Dr. Patel. All 225 patients had at least 90 days of postdischarge follow-up in the UT Southwestern EHR system.
Rates of relabeling varied according to the specific intervention. Five of 27 (18.5%) who had only pharmacist counseling at the time of negative testing, documentation of negative results in the EHR, and the alert added to their electronic record were relabeled, versus just 1 of 15 patients (6.7%) who received all of the interventions, including pre- and postdischarge counseling and the wallet card. The relabel rate was 14.3% (14) among the 98 patients counseled by a pharmacist when they tested negative, with the results documented in their electronic record – the largest patient subset in the study.
Given the small numbers, it’s hard to know which intervention gave the most bang for the buck, but “pharmacist counseling and EHR documentation had clear benefit.” Postdischarge counseling, EHR alerts, and the wallet cards probably helped, too, Dr. Patel said.
Older patients were more likely to be relabeled, but the trend didn’t reach significance (P = .07). The risk of relabeling was unrelated to race, gender, infection risk factors, number of drug allergies, allergy symptoms, or how long ago the alleged penicillin reaction occurred; most patients reported it was more than 20 years ago. About half of the relabels were on the outpatient side, almost a third in the ED, and the rest in a hospital.
The study didn’t address why they occurred. “Some patients who have had this label for 20 or 30 years will just say they are penicillin allergic despite all our counseling efforts, and whoever is reviewing their allergy list has to decide what to do with that. [Often,] they put it back in the chart. There’s a fear of penicillin allergy histories not only among patients, but also among many providers, and that contributes a lot to this,” Dr. Patel said.
There was no external funding for the work. Dr. Patel had no disclosures.
ATLANTA – About a third to half of patients who test negative for penicillin allergies and have the label removed from their charts will, somewhere down the line, be relabeled as penicillin allergic.
It’s a vexing problem for the increasing number of patients who undergo confirmatory skin testing for penicillin sensitivity while in hospital. It’s become clear in recent years that at least 90% of people who say they have a penicillin allergy don’t really have one. Without confirmatory testing, they end up on expensive, second-line antibiotics, and don’t do well.
It’s unclear why people are relabeled after negative tests. Maybe patients don’t trust the results. Maybe doctors don’t hear about them or err on the side of caution despite negative testing. “I suspect it’s a combination of patient and provider factors,” said Sheenal Patel, MD, an allergy and immunology fellow at the University of Texas Southwestern Medical Center, Dallas.
Whatever the reason, UT Southwestern has taken steps over the past few years to make sure negative test results stick in patients’ records, he said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
First, the time and place of negative tests were documented in the EHR, primary care providers were notified of the results, and pharmacists counseled patients at the time of negative testing to make sure they understood the results.
Next, pharmacists began to counsel patients after discharge to reaffirm the message, either face to face or over the phone. The center added an alert to the EHR that pops up if someone tries to relabel a patient and it notifies the pharmacists running the penicillin allergy testing program that an attempt was made. They call the patient’s primary care provider to find out what’s going on.
As of late, patients go home with a wallet card that documents their negative test results, to show providers who aren’t on the UT Southwestern EHR system, and family members.
It’s all made a difference. Only 31 of 225 (13.8%) were relabeled in a review presented by Dr. Patel. All 225 patients had at least 90 days of postdischarge follow-up in the UT Southwestern EHR system.
Rates of relabeling varied according to the specific intervention. Five of 27 (18.5%) who had only pharmacist counseling at the time of negative testing, documentation of negative results in the EHR, and the alert added to their electronic record were relabeled, versus just 1 of 15 patients (6.7%) who received all of the interventions, including pre- and postdischarge counseling and the wallet card. The relabel rate was 14.3% (14) among the 98 patients counseled by a pharmacist when they tested negative, with the results documented in their electronic record – the largest patient subset in the study.
Given the small numbers, it’s hard to know which intervention gave the most bang for the buck, but “pharmacist counseling and EHR documentation had clear benefit.” Postdischarge counseling, EHR alerts, and the wallet cards probably helped, too, Dr. Patel said.
Older patients were more likely to be relabeled, but the trend didn’t reach significance (P = .07). The risk of relabeling was unrelated to race, gender, infection risk factors, number of drug allergies, allergy symptoms, or how long ago the alleged penicillin reaction occurred; most patients reported it was more than 20 years ago. About half of the relabels were on the outpatient side, almost a third in the ED, and the rest in a hospital.
The study didn’t address why they occurred. “Some patients who have had this label for 20 or 30 years will just say they are penicillin allergic despite all our counseling efforts, and whoever is reviewing their allergy list has to decide what to do with that. [Often,] they put it back in the chart. There’s a fear of penicillin allergy histories not only among patients, but also among many providers, and that contributes a lot to this,” Dr. Patel said.
There was no external funding for the work. Dr. Patel had no disclosures.
Key clinical point:
Major finding: Only 31 of 225 patients (13.8%) with negative results were relabeled as penicillin allergic after pharmacist counseling, a far lower rate than in previous reports.
Data source: Follow up of inpatients who tested negative for penicillin allergies.
Disclosures: There was no external funding for the work. The lead investigator had no disclosures.
Digoxin definitively dissed for AF
WASHINGTON – In what could prove to be the final word in the clinical controversy over the safety of prescribing digoxin in patients with atrial fibrillation, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial has come down emphatically on the side of avoiding the venerable drug.
“The clinical implications of our analysis are that in the absence of randomized trial data showing its safety and efficacy, digoxin should generally not be prescribed for patients with atrial fibrillation, particularly if symptoms can be alleviated with other treatments. And in patients with atrial fibrillation already taking digoxin, monitoring its serum concentration may be important, targeting blood levels below 1.2 ng/mL,” Renato D. Lopes, MD, PhD, said at the annual meeting of the American College of Cardiology.
A randomized clinical trial of digoxin in AF is extremely unlikely, added Dr. Lopes, professor of medicine at Duke University in Durham, N.C.
ARISTOTLE was a randomized trial of apixaban (Eliquis) versus warfarin for stroke prevention in AF. The results of this landmark study, previously reported (N Engl J Med. 2011 Sep 15;365[11]:981-92), demonstrated that apixaban was the superior oral anticoagulant in preventing stroke or systemic embolism, caused less bleeding, and resulted in lower mortality.
ARISTOTLE had some unique features that rendered the study database an exceptional resource for use in a large observational study of digoxin’s safety in patients with AF. It included a detailed serial assessment of concomitant medications as well as measurements of serum digoxin levels, left ventricular ejection fraction, creatinine clearance, and biomarkers including vasoactive intestinal peptide, troponins T and I, N-terminal pro–brain-type natriuretic peptide, and growth differentiation factor 15. These were among the 48 clinical variables included in multivariate adjusted analyses of mortality risk.
One-third of ARISTOTLE participants were on digoxin at study entry, a prevalence typical of what’s seen in clinical practice. Among the 5,824 subjects with AF already on digoxin at the start of the trial, the risk of death during follow-up proved independently related to baseline serum digoxin concentration. Patients with a level from 0.9 ng/mL to less than 1.2 ng/mL had a 16% increased risk of death during study follow-up, compared with digoxin nonusers, a trend that didn’t reach statistical significance. However, the 11% of AF patients with a serum concentration of 1.2 ng/mL or above were at a significant 56% increased risk for death.
When serum digoxin concentration is looked at as a continuous, rather than dichotomous variable, for each 0.5-ng/mL increase in drug concentration, the adjusted risk of all-cause mortality at 1 year of study follow-up climbed by 19%.
Moreover, among 781 AF patients who initiated digoxin during the study, the risk of death was increased by 78%, compared with that of 2,343 extensively matched controls. The most common cause of this excess mortality was sudden death, and in a closer look at that endpoint, the investigators found that the risk of sudden death was increased fourfold in new users of digoxin. This increased risk occurred early: Most sudden deaths occurred within the first 6 months after going on the drug, suggesting a causal relationship, although not providing definitive proof, Dr. Lopes noted.
Forty-three percent of ARISTOTLE participants had heart failure at enrollment. Interestingly, the increased risk of death associated with on-study initiation of digoxin was of similar magnitude, regardless of whether comorbid heart failure was present. The mortality risk was 58% greater in new users with heart failure, compared with matched nonusers with heart failure, and twofold greater in new users without heart failure than in their matched controls.
The benefits of apixaban over warfarin were consistent regardless of whether or not patients were on digoxin.
Discussant Kristen K. Patton, MD, was effusive in her response to the new ARISTOTLE findings.
“This was a really, truly, beautiful observational analysis,” declared Dr. Patton, an electrophysiologist at the University of Washington, Seattle.
“I think in cardiology, where our hearts have been broken before due to flawed observational studies, it’s really important for people to understand that observational data, when analyzed well, with appropriate propensity matching, with new-user analysis and close attention to clinical variables that are important, can really change practice in a good way. I think that’s what we see here,” she said.
A beaming Dr. Lopes responded that it’s likely that some of the past conflicting studies were marred by survival bias – that is, an inability to account for the fact that patients already on digoxin at the outset of a study have already declared themselves to be more tolerant of the drug. Past studies also didn’t adjust for biomarker levels.
“We could adjust for things we know today are associated with death in atrial fibrillation,” he observed.
Dr. Patton added that the most surprising study finding to her involved the new users of digoxin. She suspects that the reported figure of a 78% increased risk of all-cause mortality during study follow-up actually markedly underestimates the true size of that risk during the initial months on the drug. Dr. Lopes agreed.
She also said she found worrisome and disappointing the increased mortality risk reported with initiation of digoxin in AF patients with heart failure. That hasn’t been seen in other studies.
Dr. Lopes said the investigators utilized multiple means of identifying patients with heart failure and are certain they captured the full population of affected patients.
“We feel very confident that, when you have atrial fibrillation together with heart failure, it might be a different story than without atrial fibrillation,” the cardiologist said.
Discussant Jagmeet P. Singh, MD, associate chief of cardiology at Massachusetts General Hospital and professor of medicine at Harvard Medical School, Boston, said the ARISTOTLE analysis carries an eye-opening take-home message: “If you have to initiate digoxin, you have to follow the serum levels more closely than we ever have before. How frequently, I don’t know – maybe monthly instead of at the 6-monthly intervals that we often do. And I think maybe arrhythmia monitoring in the initial stages of putting patients on digoxin will be key to see if there are any additional proarrhythmic effects.”
The original ARISTOTLE trial was sponsored by Bristol-Myers Squibb and Pfizer. However, the ARISTOTLE digoxin analysis was sponsored by the Duke Clinical Research Institute. Dr. Lopes reported serving as a consultant to and/or receiving research grants from Bristol-Myers Squibb, Pfizer, Bayer, Boehringer Ingleheim, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, and Portola.
WASHINGTON – In what could prove to be the final word in the clinical controversy over the safety of prescribing digoxin in patients with atrial fibrillation, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial has come down emphatically on the side of avoiding the venerable drug.
“The clinical implications of our analysis are that in the absence of randomized trial data showing its safety and efficacy, digoxin should generally not be prescribed for patients with atrial fibrillation, particularly if symptoms can be alleviated with other treatments. And in patients with atrial fibrillation already taking digoxin, monitoring its serum concentration may be important, targeting blood levels below 1.2 ng/mL,” Renato D. Lopes, MD, PhD, said at the annual meeting of the American College of Cardiology.
A randomized clinical trial of digoxin in AF is extremely unlikely, added Dr. Lopes, professor of medicine at Duke University in Durham, N.C.
ARISTOTLE was a randomized trial of apixaban (Eliquis) versus warfarin for stroke prevention in AF. The results of this landmark study, previously reported (N Engl J Med. 2011 Sep 15;365[11]:981-92), demonstrated that apixaban was the superior oral anticoagulant in preventing stroke or systemic embolism, caused less bleeding, and resulted in lower mortality.
ARISTOTLE had some unique features that rendered the study database an exceptional resource for use in a large observational study of digoxin’s safety in patients with AF. It included a detailed serial assessment of concomitant medications as well as measurements of serum digoxin levels, left ventricular ejection fraction, creatinine clearance, and biomarkers including vasoactive intestinal peptide, troponins T and I, N-terminal pro–brain-type natriuretic peptide, and growth differentiation factor 15. These were among the 48 clinical variables included in multivariate adjusted analyses of mortality risk.
One-third of ARISTOTLE participants were on digoxin at study entry, a prevalence typical of what’s seen in clinical practice. Among the 5,824 subjects with AF already on digoxin at the start of the trial, the risk of death during follow-up proved independently related to baseline serum digoxin concentration. Patients with a level from 0.9 ng/mL to less than 1.2 ng/mL had a 16% increased risk of death during study follow-up, compared with digoxin nonusers, a trend that didn’t reach statistical significance. However, the 11% of AF patients with a serum concentration of 1.2 ng/mL or above were at a significant 56% increased risk for death.
When serum digoxin concentration is looked at as a continuous, rather than dichotomous variable, for each 0.5-ng/mL increase in drug concentration, the adjusted risk of all-cause mortality at 1 year of study follow-up climbed by 19%.
Moreover, among 781 AF patients who initiated digoxin during the study, the risk of death was increased by 78%, compared with that of 2,343 extensively matched controls. The most common cause of this excess mortality was sudden death, and in a closer look at that endpoint, the investigators found that the risk of sudden death was increased fourfold in new users of digoxin. This increased risk occurred early: Most sudden deaths occurred within the first 6 months after going on the drug, suggesting a causal relationship, although not providing definitive proof, Dr. Lopes noted.
Forty-three percent of ARISTOTLE participants had heart failure at enrollment. Interestingly, the increased risk of death associated with on-study initiation of digoxin was of similar magnitude, regardless of whether comorbid heart failure was present. The mortality risk was 58% greater in new users with heart failure, compared with matched nonusers with heart failure, and twofold greater in new users without heart failure than in their matched controls.
The benefits of apixaban over warfarin were consistent regardless of whether or not patients were on digoxin.
Discussant Kristen K. Patton, MD, was effusive in her response to the new ARISTOTLE findings.
“This was a really, truly, beautiful observational analysis,” declared Dr. Patton, an electrophysiologist at the University of Washington, Seattle.
“I think in cardiology, where our hearts have been broken before due to flawed observational studies, it’s really important for people to understand that observational data, when analyzed well, with appropriate propensity matching, with new-user analysis and close attention to clinical variables that are important, can really change practice in a good way. I think that’s what we see here,” she said.
A beaming Dr. Lopes responded that it’s likely that some of the past conflicting studies were marred by survival bias – that is, an inability to account for the fact that patients already on digoxin at the outset of a study have already declared themselves to be more tolerant of the drug. Past studies also didn’t adjust for biomarker levels.
“We could adjust for things we know today are associated with death in atrial fibrillation,” he observed.
Dr. Patton added that the most surprising study finding to her involved the new users of digoxin. She suspects that the reported figure of a 78% increased risk of all-cause mortality during study follow-up actually markedly underestimates the true size of that risk during the initial months on the drug. Dr. Lopes agreed.
She also said she found worrisome and disappointing the increased mortality risk reported with initiation of digoxin in AF patients with heart failure. That hasn’t been seen in other studies.
Dr. Lopes said the investigators utilized multiple means of identifying patients with heart failure and are certain they captured the full population of affected patients.
“We feel very confident that, when you have atrial fibrillation together with heart failure, it might be a different story than without atrial fibrillation,” the cardiologist said.
Discussant Jagmeet P. Singh, MD, associate chief of cardiology at Massachusetts General Hospital and professor of medicine at Harvard Medical School, Boston, said the ARISTOTLE analysis carries an eye-opening take-home message: “If you have to initiate digoxin, you have to follow the serum levels more closely than we ever have before. How frequently, I don’t know – maybe monthly instead of at the 6-monthly intervals that we often do. And I think maybe arrhythmia monitoring in the initial stages of putting patients on digoxin will be key to see if there are any additional proarrhythmic effects.”
The original ARISTOTLE trial was sponsored by Bristol-Myers Squibb and Pfizer. However, the ARISTOTLE digoxin analysis was sponsored by the Duke Clinical Research Institute. Dr. Lopes reported serving as a consultant to and/or receiving research grants from Bristol-Myers Squibb, Pfizer, Bayer, Boehringer Ingleheim, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, and Portola.
WASHINGTON – In what could prove to be the final word in the clinical controversy over the safety of prescribing digoxin in patients with atrial fibrillation, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial has come down emphatically on the side of avoiding the venerable drug.
“The clinical implications of our analysis are that in the absence of randomized trial data showing its safety and efficacy, digoxin should generally not be prescribed for patients with atrial fibrillation, particularly if symptoms can be alleviated with other treatments. And in patients with atrial fibrillation already taking digoxin, monitoring its serum concentration may be important, targeting blood levels below 1.2 ng/mL,” Renato D. Lopes, MD, PhD, said at the annual meeting of the American College of Cardiology.
A randomized clinical trial of digoxin in AF is extremely unlikely, added Dr. Lopes, professor of medicine at Duke University in Durham, N.C.
ARISTOTLE was a randomized trial of apixaban (Eliquis) versus warfarin for stroke prevention in AF. The results of this landmark study, previously reported (N Engl J Med. 2011 Sep 15;365[11]:981-92), demonstrated that apixaban was the superior oral anticoagulant in preventing stroke or systemic embolism, caused less bleeding, and resulted in lower mortality.
ARISTOTLE had some unique features that rendered the study database an exceptional resource for use in a large observational study of digoxin’s safety in patients with AF. It included a detailed serial assessment of concomitant medications as well as measurements of serum digoxin levels, left ventricular ejection fraction, creatinine clearance, and biomarkers including vasoactive intestinal peptide, troponins T and I, N-terminal pro–brain-type natriuretic peptide, and growth differentiation factor 15. These were among the 48 clinical variables included in multivariate adjusted analyses of mortality risk.
One-third of ARISTOTLE participants were on digoxin at study entry, a prevalence typical of what’s seen in clinical practice. Among the 5,824 subjects with AF already on digoxin at the start of the trial, the risk of death during follow-up proved independently related to baseline serum digoxin concentration. Patients with a level from 0.9 ng/mL to less than 1.2 ng/mL had a 16% increased risk of death during study follow-up, compared with digoxin nonusers, a trend that didn’t reach statistical significance. However, the 11% of AF patients with a serum concentration of 1.2 ng/mL or above were at a significant 56% increased risk for death.
When serum digoxin concentration is looked at as a continuous, rather than dichotomous variable, for each 0.5-ng/mL increase in drug concentration, the adjusted risk of all-cause mortality at 1 year of study follow-up climbed by 19%.
Moreover, among 781 AF patients who initiated digoxin during the study, the risk of death was increased by 78%, compared with that of 2,343 extensively matched controls. The most common cause of this excess mortality was sudden death, and in a closer look at that endpoint, the investigators found that the risk of sudden death was increased fourfold in new users of digoxin. This increased risk occurred early: Most sudden deaths occurred within the first 6 months after going on the drug, suggesting a causal relationship, although not providing definitive proof, Dr. Lopes noted.
Forty-three percent of ARISTOTLE participants had heart failure at enrollment. Interestingly, the increased risk of death associated with on-study initiation of digoxin was of similar magnitude, regardless of whether comorbid heart failure was present. The mortality risk was 58% greater in new users with heart failure, compared with matched nonusers with heart failure, and twofold greater in new users without heart failure than in their matched controls.
The benefits of apixaban over warfarin were consistent regardless of whether or not patients were on digoxin.
Discussant Kristen K. Patton, MD, was effusive in her response to the new ARISTOTLE findings.
“This was a really, truly, beautiful observational analysis,” declared Dr. Patton, an electrophysiologist at the University of Washington, Seattle.
“I think in cardiology, where our hearts have been broken before due to flawed observational studies, it’s really important for people to understand that observational data, when analyzed well, with appropriate propensity matching, with new-user analysis and close attention to clinical variables that are important, can really change practice in a good way. I think that’s what we see here,” she said.
A beaming Dr. Lopes responded that it’s likely that some of the past conflicting studies were marred by survival bias – that is, an inability to account for the fact that patients already on digoxin at the outset of a study have already declared themselves to be more tolerant of the drug. Past studies also didn’t adjust for biomarker levels.
“We could adjust for things we know today are associated with death in atrial fibrillation,” he observed.
Dr. Patton added that the most surprising study finding to her involved the new users of digoxin. She suspects that the reported figure of a 78% increased risk of all-cause mortality during study follow-up actually markedly underestimates the true size of that risk during the initial months on the drug. Dr. Lopes agreed.
She also said she found worrisome and disappointing the increased mortality risk reported with initiation of digoxin in AF patients with heart failure. That hasn’t been seen in other studies.
Dr. Lopes said the investigators utilized multiple means of identifying patients with heart failure and are certain they captured the full population of affected patients.
“We feel very confident that, when you have atrial fibrillation together with heart failure, it might be a different story than without atrial fibrillation,” the cardiologist said.
Discussant Jagmeet P. Singh, MD, associate chief of cardiology at Massachusetts General Hospital and professor of medicine at Harvard Medical School, Boston, said the ARISTOTLE analysis carries an eye-opening take-home message: “If you have to initiate digoxin, you have to follow the serum levels more closely than we ever have before. How frequently, I don’t know – maybe monthly instead of at the 6-monthly intervals that we often do. And I think maybe arrhythmia monitoring in the initial stages of putting patients on digoxin will be key to see if there are any additional proarrhythmic effects.”
The original ARISTOTLE trial was sponsored by Bristol-Myers Squibb and Pfizer. However, the ARISTOTLE digoxin analysis was sponsored by the Duke Clinical Research Institute. Dr. Lopes reported serving as a consultant to and/or receiving research grants from Bristol-Myers Squibb, Pfizer, Bayer, Boehringer Ingleheim, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, and Portola.
AT ACC 17
Key clinical point:
Major finding: Patients with atrial fibrillation who initiated digoxin therapy had a fourfold increased risk of sudden death, compared with extensively matched controls.
Data source: This was a secondary observational analysis drawn from the roughly 18,000-patient, randomized, multicenter ARISTOTLE trial.
Disclosures: This ARISTOTLE digoxin analysis was sponsored by the Duke Clinical Research Institute. The study presenter reported serving as a consultant to and/or recipient of research grants from numerous companies, including Bristol-Myers Squibb and Pfizer, which cosponsored the original ARISTOTLE trial.
Successful Treatment of Ota Nevus With the 532-nm Solid-State Picosecond Laser
Ota nevus is a dermal melanocytosis that is typically characterized by blue, gray, or brown pigmented patches in the periorbital region.1 The condition has a prevalence of 0.04% in a Philadelphia study of 6915 patients and is most notable in patients with skin of color, affecting up to 0.6% of Asians,2 0.038% of white individuals, and 0.014% of black individuals.3,4 The appearance of an Ota nevus often imparts a negative psychosocial impact on the patient, prompting requests for treatment and/or removal.5 Laser treatment of Ota nevi must be carefully implemented, especially in Fitzpatrick skin types IV through VI. Although 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,5,6 typically only moderate improvement is seen; further treatment at higher fluences will only increase the risk for dyspigmentation and scarring.6
We report a case of successful treatment of an Ota nevus following 2 treatment sessions with the 532-nm solid-state picosecond laser, which is a novel application in patients with skin of color (Fitzpatrick skin types IV-VI). The Q-switched nanosecond laser has been shown to be moderately effective at treating Ota nevi.6
Case Report
An 18-year-old woman with Fitzpatrick skin type IV presented for cosmetic removal of an 8×5-cm dark brown-blue patch on the right temple and malar and buccal cheek present since birth that had failed to respond to an unknown laser treatment that was administered outside of the United States (Figure, A). To ascertain the diagnosis, a biopsy was performed, showing histology consistent with Ota nevus. Initially, the 755-nm Q-switched nanosecond laser was recommended for treatment. Over the course of 7 months (1 treatment session per month [Table]), the patient saw improvement but not to the desired extent. The patient then underwent 2 treatments at 4-week intervals with the 1064-nm solid-state picosecond and nanosecond lasers; however, no improvement was seen following these 2 sessions (Table).
The next month the patient received treatment with a novel 532-nm solid-state picosecond laser using the following parameters: fluence, 0.5 J/cm2; spot size, 6 mm; repetition rate, 1 Hz; pulse duration, 750 picoseconds; 339 pulses. The end point was whitening. A remarkable clinical response was demonstrated 6 weeks later (Figure, B). A second treatment with the 532-nm solid-state picosecond laser was then performed at 14 months. On a return visit 2 months after the second treatment, the patient showed dramatic improvement, almost to the degree of complete resolution (Figure, C).
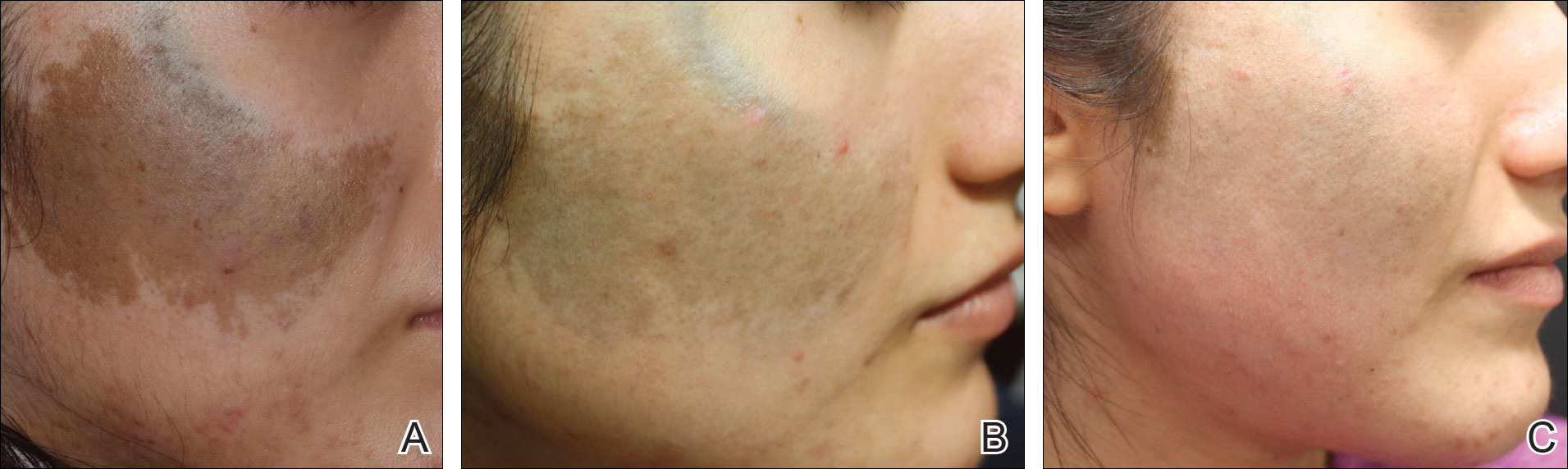
Comment
Pigmentation disorders are more common in patients with skin of color, and those affected may experience psychological effects secondary to these dermatoses, prompting requests for treatment and/or removal.7 Although the 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,3 the challenge remains for patients with skin of color, as these lasers work through photothermolysis, which generates heat and may cause thermal damage by targeting melanin. Because more melanin is present in skin of color patients, the threshold for too much heat is lower and these patients are at a higher risk for adverse events such as scarring and hyperpigmentation.6,8
By delivering energy in shorter pulses, the novel 532-nm solid-state picosecond laser shows greater fragmentation of melanosomes into melanin particles that are eventually phagocytosed.8 In our patient, dramatic improvement was noted after only 2 treatments, as evidenced by other picosecond treatments on Ota nevi,6,8 suggesting that fewer treatments are necessary when using the 532-nm solid-state picosecond laser for Ota nevi.
Although the 532-nm solid-state picosecond laser was cleared by the US Food and Drug Administration for tattoo removal, this laser shows potential use in other pigmentary disorders, particularly in patients with skin of color, as demonstrated in our case. With continued understanding through further studies, this picosecond laser with a shorter pulse duration may prove to be a safer and more effective alternative to the Q-switched nanosecond laser.
Conclusion
As shown in our case, the 532-nm solid-state picosecond laser appears to be a safe and effective modality for treating Ota nevi. This case demonstrates the potential utility of this laser in patients desiring more complete clearing, as it removes pigment more rapidly with lower risk for serious adverse effects. The 9th Cosmetic Surgery Forum will be held November 29-December 2, 2017, in Las Vegas, Nevada. Get more information at www.cosmeticsurgeryforum.com.
- Kim JY, Lee HG, Kim MJ, et al. The efficacy and safety of episcleral pigmentation removal from pig eyes: using a 532-nm quality-switched Nd: YAG laser. Cornea. 2012;31:1449-1454.
- Watanabe S, Takahashi H. Treatment of nevus of Ota with the Q-switched ruby laser. N Engl J Med. 1994;331:1745-1750.
- Yates B, Que SK, D'Souza L, et al. Laser treatment of periocular skin conditions. Clin Dermatol. 2015;33:197-206.
- Gonder JR, Ezell PC, Shields JA, et al. Ocular melanocytosis. a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950-952.
- Chesnut C, Diehl J, Lask G. Treatment of nevus of Ota with a picosecond 755-nm alexandrite laser. Dermatol Surg. 2015;41:508-510.
- Moreno-Arias GA, Camps-Fresneda A. Treatment of nevus of Ota with the Q-switched alexandrite laser. Lasers Surg Med. 2001;28:451-455.
- Manuskiatti W, Eimpunth S, Wanitphakdeedecha R. Effect of cold air cooling on the incidence of postinflammatory hyperpigmentation after Q-switched Nd:YAG laser treatment of acquired bilateral nevus of Ota like macules. Arch Dermatol. 2007;143:1139-1143.
- Levin MK, Ng E, Bae YS, et al. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: a retrospective photographic review. Lasers Surg Med. 2016;48:181-187.
Ota nevus is a dermal melanocytosis that is typically characterized by blue, gray, or brown pigmented patches in the periorbital region.1 The condition has a prevalence of 0.04% in a Philadelphia study of 6915 patients and is most notable in patients with skin of color, affecting up to 0.6% of Asians,2 0.038% of white individuals, and 0.014% of black individuals.3,4 The appearance of an Ota nevus often imparts a negative psychosocial impact on the patient, prompting requests for treatment and/or removal.5 Laser treatment of Ota nevi must be carefully implemented, especially in Fitzpatrick skin types IV through VI. Although 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,5,6 typically only moderate improvement is seen; further treatment at higher fluences will only increase the risk for dyspigmentation and scarring.6
We report a case of successful treatment of an Ota nevus following 2 treatment sessions with the 532-nm solid-state picosecond laser, which is a novel application in patients with skin of color (Fitzpatrick skin types IV-VI). The Q-switched nanosecond laser has been shown to be moderately effective at treating Ota nevi.6
Case Report
An 18-year-old woman with Fitzpatrick skin type IV presented for cosmetic removal of an 8×5-cm dark brown-blue patch on the right temple and malar and buccal cheek present since birth that had failed to respond to an unknown laser treatment that was administered outside of the United States (Figure, A). To ascertain the diagnosis, a biopsy was performed, showing histology consistent with Ota nevus. Initially, the 755-nm Q-switched nanosecond laser was recommended for treatment. Over the course of 7 months (1 treatment session per month [Table]), the patient saw improvement but not to the desired extent. The patient then underwent 2 treatments at 4-week intervals with the 1064-nm solid-state picosecond and nanosecond lasers; however, no improvement was seen following these 2 sessions (Table).

The next month the patient received treatment with a novel 532-nm solid-state picosecond laser using the following parameters: fluence, 0.5 J/cm2; spot size, 6 mm; repetition rate, 1 Hz; pulse duration, 750 picoseconds; 339 pulses. The end point was whitening. A remarkable clinical response was demonstrated 6 weeks later (Figure, B). A second treatment with the 532-nm solid-state picosecond laser was then performed at 14 months. On a return visit 2 months after the second treatment, the patient showed dramatic improvement, almost to the degree of complete resolution (Figure, C).

Comment
Pigmentation disorders are more common in patients with skin of color, and those affected may experience psychological effects secondary to these dermatoses, prompting requests for treatment and/or removal.7 Although the 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,3 the challenge remains for patients with skin of color, as these lasers work through photothermolysis, which generates heat and may cause thermal damage by targeting melanin. Because more melanin is present in skin of color patients, the threshold for too much heat is lower and these patients are at a higher risk for adverse events such as scarring and hyperpigmentation.6,8
By delivering energy in shorter pulses, the novel 532-nm solid-state picosecond laser shows greater fragmentation of melanosomes into melanin particles that are eventually phagocytosed.8 In our patient, dramatic improvement was noted after only 2 treatments, as evidenced by other picosecond treatments on Ota nevi,6,8 suggesting that fewer treatments are necessary when using the 532-nm solid-state picosecond laser for Ota nevi.
Although the 532-nm solid-state picosecond laser was cleared by the US Food and Drug Administration for tattoo removal, this laser shows potential use in other pigmentary disorders, particularly in patients with skin of color, as demonstrated in our case. With continued understanding through further studies, this picosecond laser with a shorter pulse duration may prove to be a safer and more effective alternative to the Q-switched nanosecond laser.
Conclusion
As shown in our case, the 532-nm solid-state picosecond laser appears to be a safe and effective modality for treating Ota nevi. This case demonstrates the potential utility of this laser in patients desiring more complete clearing, as it removes pigment more rapidly with lower risk for serious adverse effects. The 9th Cosmetic Surgery Forum will be held November 29-December 2, 2017, in Las Vegas, Nevada. Get more information at www.cosmeticsurgeryforum.com.
Ota nevus is a dermal melanocytosis that is typically characterized by blue, gray, or brown pigmented patches in the periorbital region.1 The condition has a prevalence of 0.04% in a Philadelphia study of 6915 patients and is most notable in patients with skin of color, affecting up to 0.6% of Asians,2 0.038% of white individuals, and 0.014% of black individuals.3,4 The appearance of an Ota nevus often imparts a negative psychosocial impact on the patient, prompting requests for treatment and/or removal.5 Laser treatment of Ota nevi must be carefully implemented, especially in Fitzpatrick skin types IV through VI. Although 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,5,6 typically only moderate improvement is seen; further treatment at higher fluences will only increase the risk for dyspigmentation and scarring.6
We report a case of successful treatment of an Ota nevus following 2 treatment sessions with the 532-nm solid-state picosecond laser, which is a novel application in patients with skin of color (Fitzpatrick skin types IV-VI). The Q-switched nanosecond laser has been shown to be moderately effective at treating Ota nevi.6
Case Report
An 18-year-old woman with Fitzpatrick skin type IV presented for cosmetic removal of an 8×5-cm dark brown-blue patch on the right temple and malar and buccal cheek present since birth that had failed to respond to an unknown laser treatment that was administered outside of the United States (Figure, A). To ascertain the diagnosis, a biopsy was performed, showing histology consistent with Ota nevus. Initially, the 755-nm Q-switched nanosecond laser was recommended for treatment. Over the course of 7 months (1 treatment session per month [Table]), the patient saw improvement but not to the desired extent. The patient then underwent 2 treatments at 4-week intervals with the 1064-nm solid-state picosecond and nanosecond lasers; however, no improvement was seen following these 2 sessions (Table).

The next month the patient received treatment with a novel 532-nm solid-state picosecond laser using the following parameters: fluence, 0.5 J/cm2; spot size, 6 mm; repetition rate, 1 Hz; pulse duration, 750 picoseconds; 339 pulses. The end point was whitening. A remarkable clinical response was demonstrated 6 weeks later (Figure, B). A second treatment with the 532-nm solid-state picosecond laser was then performed at 14 months. On a return visit 2 months after the second treatment, the patient showed dramatic improvement, almost to the degree of complete resolution (Figure, C).

Comment
Pigmentation disorders are more common in patients with skin of color, and those affected may experience psychological effects secondary to these dermatoses, prompting requests for treatment and/or removal.7 Although the 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,3 the challenge remains for patients with skin of color, as these lasers work through photothermolysis, which generates heat and may cause thermal damage by targeting melanin. Because more melanin is present in skin of color patients, the threshold for too much heat is lower and these patients are at a higher risk for adverse events such as scarring and hyperpigmentation.6,8
By delivering energy in shorter pulses, the novel 532-nm solid-state picosecond laser shows greater fragmentation of melanosomes into melanin particles that are eventually phagocytosed.8 In our patient, dramatic improvement was noted after only 2 treatments, as evidenced by other picosecond treatments on Ota nevi,6,8 suggesting that fewer treatments are necessary when using the 532-nm solid-state picosecond laser for Ota nevi.
Although the 532-nm solid-state picosecond laser was cleared by the US Food and Drug Administration for tattoo removal, this laser shows potential use in other pigmentary disorders, particularly in patients with skin of color, as demonstrated in our case. With continued understanding through further studies, this picosecond laser with a shorter pulse duration may prove to be a safer and more effective alternative to the Q-switched nanosecond laser.
Conclusion
As shown in our case, the 532-nm solid-state picosecond laser appears to be a safe and effective modality for treating Ota nevi. This case demonstrates the potential utility of this laser in patients desiring more complete clearing, as it removes pigment more rapidly with lower risk for serious adverse effects. The 9th Cosmetic Surgery Forum will be held November 29-December 2, 2017, in Las Vegas, Nevada. Get more information at www.cosmeticsurgeryforum.com.
- Kim JY, Lee HG, Kim MJ, et al. The efficacy and safety of episcleral pigmentation removal from pig eyes: using a 532-nm quality-switched Nd: YAG laser. Cornea. 2012;31:1449-1454.
- Watanabe S, Takahashi H. Treatment of nevus of Ota with the Q-switched ruby laser. N Engl J Med. 1994;331:1745-1750.
- Yates B, Que SK, D'Souza L, et al. Laser treatment of periocular skin conditions. Clin Dermatol. 2015;33:197-206.
- Gonder JR, Ezell PC, Shields JA, et al. Ocular melanocytosis. a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950-952.
- Chesnut C, Diehl J, Lask G. Treatment of nevus of Ota with a picosecond 755-nm alexandrite laser. Dermatol Surg. 2015;41:508-510.
- Moreno-Arias GA, Camps-Fresneda A. Treatment of nevus of Ota with the Q-switched alexandrite laser. Lasers Surg Med. 2001;28:451-455.
- Manuskiatti W, Eimpunth S, Wanitphakdeedecha R. Effect of cold air cooling on the incidence of postinflammatory hyperpigmentation after Q-switched Nd:YAG laser treatment of acquired bilateral nevus of Ota like macules. Arch Dermatol. 2007;143:1139-1143.
- Levin MK, Ng E, Bae YS, et al. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: a retrospective photographic review. Lasers Surg Med. 2016;48:181-187.
- Kim JY, Lee HG, Kim MJ, et al. The efficacy and safety of episcleral pigmentation removal from pig eyes: using a 532-nm quality-switched Nd: YAG laser. Cornea. 2012;31:1449-1454.
- Watanabe S, Takahashi H. Treatment of nevus of Ota with the Q-switched ruby laser. N Engl J Med. 1994;331:1745-1750.
- Yates B, Que SK, D'Souza L, et al. Laser treatment of periocular skin conditions. Clin Dermatol. 2015;33:197-206.
- Gonder JR, Ezell PC, Shields JA, et al. Ocular melanocytosis. a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950-952.
- Chesnut C, Diehl J, Lask G. Treatment of nevus of Ota with a picosecond 755-nm alexandrite laser. Dermatol Surg. 2015;41:508-510.
- Moreno-Arias GA, Camps-Fresneda A. Treatment of nevus of Ota with the Q-switched alexandrite laser. Lasers Surg Med. 2001;28:451-455.
- Manuskiatti W, Eimpunth S, Wanitphakdeedecha R. Effect of cold air cooling on the incidence of postinflammatory hyperpigmentation after Q-switched Nd:YAG laser treatment of acquired bilateral nevus of Ota like macules. Arch Dermatol. 2007;143:1139-1143.
- Levin MK, Ng E, Bae YS, et al. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: a retrospective photographic review. Lasers Surg Med. 2016;48:181-187.
Resident Pearl
The Q-switched 532-nm picosecond laser delivers energy in short pulses, creating fragmentation of melanosomes into melanin particles that eventually become phagocytosed. This process may be safer for patients with Fitzpatrick skin types IV to VI, as it decreases the risk for dyschromia and scarring.
CDC reports two new Zika-related pregnancy losses
The first pregnancy losses with Zika-related birth defects since last summer were reported March 14 by the Centers for Disease Control and Prevention.
Two new cases were reported to the U.S. Zika Pregnancy Registry between Feb. 21 and March 14 – the time period covered by the most recent release of data from the CDC. That brings the total number of Zika virus–related pregnancy losses to seven since the beginning of 2016 in the 50 states and the District of Columbia, along with 54 liveborn infants with Zika-related birth defects. The CDC stopped reporting adverse pregnancy outcomes in Puerto Rico and the other U.S. territories in early October 2016 because Puerto Rico’s Zika Active Pregnancy Surveillance System is not using the same inclusion criteria as its mainland counterpart.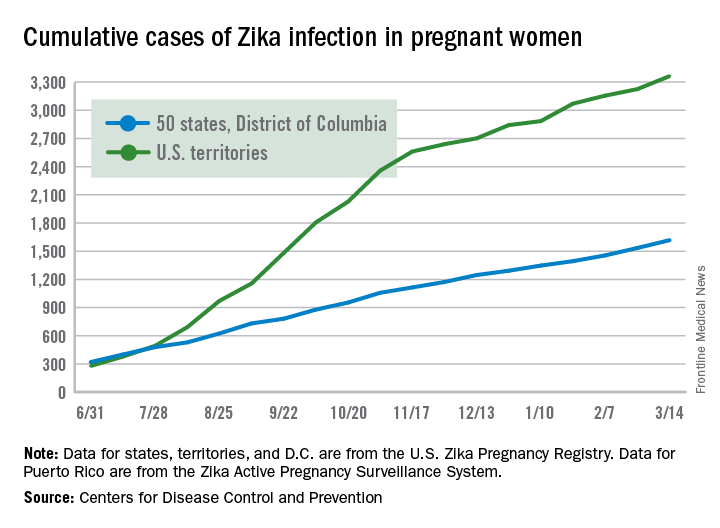
These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
The first pregnancy losses with Zika-related birth defects since last summer were reported March 14 by the Centers for Disease Control and Prevention.
Two new cases were reported to the U.S. Zika Pregnancy Registry between Feb. 21 and March 14 – the time period covered by the most recent release of data from the CDC. That brings the total number of Zika virus–related pregnancy losses to seven since the beginning of 2016 in the 50 states and the District of Columbia, along with 54 liveborn infants with Zika-related birth defects. The CDC stopped reporting adverse pregnancy outcomes in Puerto Rico and the other U.S. territories in early October 2016 because Puerto Rico’s Zika Active Pregnancy Surveillance System is not using the same inclusion criteria as its mainland counterpart.
These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
The first pregnancy losses with Zika-related birth defects since last summer were reported March 14 by the Centers for Disease Control and Prevention.
Two new cases were reported to the U.S. Zika Pregnancy Registry between Feb. 21 and March 14 – the time period covered by the most recent release of data from the CDC. That brings the total number of Zika virus–related pregnancy losses to seven since the beginning of 2016 in the 50 states and the District of Columbia, along with 54 liveborn infants with Zika-related birth defects. The CDC stopped reporting adverse pregnancy outcomes in Puerto Rico and the other U.S. territories in early October 2016 because Puerto Rico’s Zika Active Pregnancy Surveillance System is not using the same inclusion criteria as its mainland counterpart.
These are not real-time data and reflect only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, although it is not known if Zika virus was the cause of the poor outcomes. Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, or termination with evidence of birth defects.
Survivorship care models work, some better than others
ORLANDO – Accumulating experience is showing the benefits of various models of care for cancer survivors in terms of health care use and costs, while also suggesting that some provide higher-quality care than others, according to a pair of studies reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Initiative for breast cancer survivors
“In 2011, Cancer Care Ontario did a quick environmental scan of our 14 regional cancer centers and found that the transition of breast cancer survivors from oncologists to primary care was very variable, and that centers often didn’t transition patients very frequently,” said Nicole Mittmann, PhD, first author on one of the studies, chief research officer for Cancer Care Ontario, and an investigator at Sunnybrook Research Institute, Toronto.
The advisory organization therefore implemented the Well Follow-Up Care Initiative to facilitate appropriate transition of breast cancer survivors. Each regional center was given a $100,000 incentive to roll out a model of the initiative.
Dr. Mittmann and her coinvestigators used provincial administrative databases to compare health care use and associated costs between 2,324 breast cancer survivors who were transitioned with the initiative and 2,324 propensity-matched control survivors who were not. The survivors were about 5 years out from their breast cancer diagnosis at baseline and had median follow-up of 2 years.
Study results reported at the symposium showed that the mean annual total cost of care per patient paid for by the provincial health ministry was $6,575 for the transitioned group and $10,832 for the nontransitioned group, a difference of $4,257 (39%). The main drivers were reduced costs of long-term care and cancer clinic visits.
Findings were similar for median annual costs, which amounted to $2,261 for the transitioned group and $2,903 for the control group, a difference of $638.
Compared with the nontransitioned group, the transitioned group had significantly fewer annual visits to medical oncologists (0.39 vs. 1.29) and radiation oncologists (0.16 vs. 0.36), while visits to general or family practitioners were statistically indistinguishable (7.35 and 7.91), Dr. Mittmann reported. There was also a trend toward fewer emergency department visits.
The transitioned group had fewer bone scans, CT and MRI scans, and radiographs annually, but differences were not significant.
Reassuringly, Dr. Mittmann said, survivors who were transitioned did not fare worse than their nontransitioned counterparts in overall survival; if anything, they tended to live longer. “We think that because the individual cancer centers enrolled patients that they thought were very well that this is a very well and highly selected and maybe a biased group,” Dr. Mittmann acknowledged. “But we certainly see that they are not doing worse than the control group.”
“About $1.4 million was distributed to the cancer centers” for the initiative, she noted. “That generated a savings for the health system of $1.5 million, if you are looking at median costs, to $9.9 million, if you are looking at mean costs.
“The transition of appropriate breast cancer survivors to the community appears to be safe and effective outside of a clinical trial, at least based on this particular retrospective analysis using databases,” she said. “The overall costs are not increased, and they may actually be decreased based on our data, and certainly these results will inform policy.”
The investigators plan several next steps, such as encouraging senior leadership at Cancer Care Ontario and the Ministry of Health to endorse the findings, according to Dr. Mittmann. In addition, “[we plan to] engage with both oncology and primary care leadership and think about how we can potentially roll out a program like this, and develop tools, whether those are letters or information packages, and education, to … appropriately transition individuals.”
Considerations in interpreting the study’s findings include the quality of the matching of survivors, according to invited discussant Monika K. Krzyzanowska, MD, a medical oncologist at Princess Margaret Cancer Centre, an associate professor at the University of Toronto, and a clinical lead of Quality Care and Access, Systemic Treatment Program, at Cancer Care Ontario. “The quality of that match depends on what’s in the model, so there could be potential for residual confounding, and administrative data may not have all of the elements that you would need to get a perfect match.”
Additional considerations include costs not covered by the payer, impact of the initiative on delivery of guideline-recommended care and patient and provider satisfaction, generalizability of the findings, and long-term outcomes.
“This is a proof of concept, certainly, that transition of low-risk cancer survivors to primary care is feasible and potentially economically attractive,” Dr. Krzyzanowska concluded. “It would be useful to have a formal evaluation of effectiveness that would inform a comprehensive value assessment. And we do have data from a randomized trial about the safety of this particular approach, but it would be nice to see that following implementation in real practices, those safety considerations played out the same way.”
Comparison of survivorship care models
Two-thirds of the large and growing population of cancer survivors are at least 5 years out from diagnosis, stimulating considerable discussion in the oncology community about how to best address their needs, according to Sarah Raskin, PhD, senior author on the second study and a research scientist at the Institute for Patient-Centered Initiatives and Health Equity at George Washington University Cancer Center, Washington.
“Yet, for a lack of cancer survivorship–specific guidelines from research or practice, cancer centers are increasingly developing survivorship care in a variety of ways, many of which are ad hoc or unproven as yet,” she said.
Dr. Raskin and her colleagues compared three emerging models of survivorship care: a specialized consultative model and a specialized longitudinal model – whereby patients have a single or multiple formalized survivorship visits, respectively, with care typically led by an oncology nurse-practitioner – and an oncology-embedded model – whereby survivorship is addressed as a part of ongoing oncology follow-up care, typically by the oncologist.
The investigators worked with survivors to develop the Patient-Prioritized Measure of High-Quality Survivorship Care, a 46-question scale assessing nine components of survivorship care that capture the health care priorities and needs that matter most to patients. Each component is rated on a scale from 0 (not at all met) to 1 (somewhat met) to 2 (definitely met).
Analyses were based on responses of 827 survivors of breast, colorectal, and prostate cancer who received care at 28 U.S. institutions using one of the above models and who were surveyed by telephone about the care received 1 week after their initial survivorship visit.
Results showed that survivors cared for under the three models differed significantly with respect to scores for seven of the nine components of quality of care, Dr. Raskin reported. The exceptions were practical life support, where the mean score was about 0.6-0.8 across the board, and having a medical home, where the mean score was about 1.8-1.9 across the board.
The specialized consult model of care had the highest scores for mental health and social support, information and resources, and supportive and prepared clinicians. The specialized longitudinal model of care had the highest scores for empowered and engaged patients, open patient-clinician communication, care coordination and transitions, and access to full spectrum of care. The oncology-embedded model had the lowest scores. Analysis of the tool’s 46 individual questions showed that patients cared for at institutions using the oncology-embedded model were significantly less likely than were counterparts cared for at institutions using the specialized models to report that the institution performed various activities such as offering a treatment summary, inquiring about the patient’s biggest worries or problems, and explaining the reasons why tests were needed (P less than .05 for each).
For some metrics, the overall proportion reporting that an activity was performed was low, regardless of the model being used. For example, only 48% of all patients reported being helped to set goals or make short-term plans to manage follow-up care and improve health, merely 24% reported being provided emotional and social support to deal with changes in relationships, and just 19% reported being referred to special providers for other medical problems.
“Overall, all three models are performing highly in terms of providing survivors with a medical home and communicating with patients. However, all three are performing quite low in terms of providing mental health and social support, as well as practical life support,” said Dr. Raskin.
“By model, we see that the embedded ongoing care model is significantly underperforming compared with both specialized models on seven of nine components, and we have some hypotheses from our early work with [Commission on Cancer]–accredited centers to explain this,” she added. “Embedded survivorship models have a lot of variability – many are high performers but others are low performers as compared with specialized programs. Embedded survivorship care models are typically led by the treating oncologist, who historically has focused on treating sick patients and less so on providing social supports for follow-up of well patients or ‘well-er’ patients. At the same time, specialized models focus predominantly on survivorship care and providing services and referrals for survivors, which may explain their high scores.
“We know that the higher quality of care measures presented here do not necessarily translate to better patient outcomes, and that’s actually going to be the next phase of our analysis,” she concluded.
The study sample may have had some selection bias, and it is unclear how well validated the tool was, according to Dr. Krzyzanowska, the discussant. Another issue was its assessment of quality of care at only a single time point.
Nonetheless, the findings show “that measuring quality of survivorship care from a patient perspective is feasible and valuable. We have already heard about [need for] survivorship plans in survivorship care, so certainly the work that was just presented is extremely important to help to fill some of these gaps,” she said.
“I’m not sure that we yet know what the optimal model of survivorship care is without the information of the other outcomes. Furthermore, there’s different survivor populations and different ways that health care is organized, so perhaps there isn’t really one optimal model, but the model has to fit with the context,” Dr. Krzyzanowska concluded. “That being said … the tool that they have created can be a great tool for existing survivorship care programs to assess and improve the quality of their care.”
Dr. Mittmann and Dr. Raskin had no disclosures to report.
ORLANDO – Accumulating experience is showing the benefits of various models of care for cancer survivors in terms of health care use and costs, while also suggesting that some provide higher-quality care than others, according to a pair of studies reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Initiative for breast cancer survivors
“In 2011, Cancer Care Ontario did a quick environmental scan of our 14 regional cancer centers and found that the transition of breast cancer survivors from oncologists to primary care was very variable, and that centers often didn’t transition patients very frequently,” said Nicole Mittmann, PhD, first author on one of the studies, chief research officer for Cancer Care Ontario, and an investigator at Sunnybrook Research Institute, Toronto.
The advisory organization therefore implemented the Well Follow-Up Care Initiative to facilitate appropriate transition of breast cancer survivors. Each regional center was given a $100,000 incentive to roll out a model of the initiative.
Dr. Mittmann and her coinvestigators used provincial administrative databases to compare health care use and associated costs between 2,324 breast cancer survivors who were transitioned with the initiative and 2,324 propensity-matched control survivors who were not. The survivors were about 5 years out from their breast cancer diagnosis at baseline and had median follow-up of 2 years.
Study results reported at the symposium showed that the mean annual total cost of care per patient paid for by the provincial health ministry was $6,575 for the transitioned group and $10,832 for the nontransitioned group, a difference of $4,257 (39%). The main drivers were reduced costs of long-term care and cancer clinic visits.
Findings were similar for median annual costs, which amounted to $2,261 for the transitioned group and $2,903 for the control group, a difference of $638.
Compared with the nontransitioned group, the transitioned group had significantly fewer annual visits to medical oncologists (0.39 vs. 1.29) and radiation oncologists (0.16 vs. 0.36), while visits to general or family practitioners were statistically indistinguishable (7.35 and 7.91), Dr. Mittmann reported. There was also a trend toward fewer emergency department visits.
The transitioned group had fewer bone scans, CT and MRI scans, and radiographs annually, but differences were not significant.
Reassuringly, Dr. Mittmann said, survivors who were transitioned did not fare worse than their nontransitioned counterparts in overall survival; if anything, they tended to live longer. “We think that because the individual cancer centers enrolled patients that they thought were very well that this is a very well and highly selected and maybe a biased group,” Dr. Mittmann acknowledged. “But we certainly see that they are not doing worse than the control group.”
“About $1.4 million was distributed to the cancer centers” for the initiative, she noted. “That generated a savings for the health system of $1.5 million, if you are looking at median costs, to $9.9 million, if you are looking at mean costs.
“The transition of appropriate breast cancer survivors to the community appears to be safe and effective outside of a clinical trial, at least based on this particular retrospective analysis using databases,” she said. “The overall costs are not increased, and they may actually be decreased based on our data, and certainly these results will inform policy.”
The investigators plan several next steps, such as encouraging senior leadership at Cancer Care Ontario and the Ministry of Health to endorse the findings, according to Dr. Mittmann. In addition, “[we plan to] engage with both oncology and primary care leadership and think about how we can potentially roll out a program like this, and develop tools, whether those are letters or information packages, and education, to … appropriately transition individuals.”
Considerations in interpreting the study’s findings include the quality of the matching of survivors, according to invited discussant Monika K. Krzyzanowska, MD, a medical oncologist at Princess Margaret Cancer Centre, an associate professor at the University of Toronto, and a clinical lead of Quality Care and Access, Systemic Treatment Program, at Cancer Care Ontario. “The quality of that match depends on what’s in the model, so there could be potential for residual confounding, and administrative data may not have all of the elements that you would need to get a perfect match.”
Additional considerations include costs not covered by the payer, impact of the initiative on delivery of guideline-recommended care and patient and provider satisfaction, generalizability of the findings, and long-term outcomes.
“This is a proof of concept, certainly, that transition of low-risk cancer survivors to primary care is feasible and potentially economically attractive,” Dr. Krzyzanowska concluded. “It would be useful to have a formal evaluation of effectiveness that would inform a comprehensive value assessment. And we do have data from a randomized trial about the safety of this particular approach, but it would be nice to see that following implementation in real practices, those safety considerations played out the same way.”
Comparison of survivorship care models
Two-thirds of the large and growing population of cancer survivors are at least 5 years out from diagnosis, stimulating considerable discussion in the oncology community about how to best address their needs, according to Sarah Raskin, PhD, senior author on the second study and a research scientist at the Institute for Patient-Centered Initiatives and Health Equity at George Washington University Cancer Center, Washington.
“Yet, for a lack of cancer survivorship–specific guidelines from research or practice, cancer centers are increasingly developing survivorship care in a variety of ways, many of which are ad hoc or unproven as yet,” she said.
Dr. Raskin and her colleagues compared three emerging models of survivorship care: a specialized consultative model and a specialized longitudinal model – whereby patients have a single or multiple formalized survivorship visits, respectively, with care typically led by an oncology nurse-practitioner – and an oncology-embedded model – whereby survivorship is addressed as a part of ongoing oncology follow-up care, typically by the oncologist.
The investigators worked with survivors to develop the Patient-Prioritized Measure of High-Quality Survivorship Care, a 46-question scale assessing nine components of survivorship care that capture the health care priorities and needs that matter most to patients. Each component is rated on a scale from 0 (not at all met) to 1 (somewhat met) to 2 (definitely met).
Analyses were based on responses of 827 survivors of breast, colorectal, and prostate cancer who received care at 28 U.S. institutions using one of the above models and who were surveyed by telephone about the care received 1 week after their initial survivorship visit.
Results showed that survivors cared for under the three models differed significantly with respect to scores for seven of the nine components of quality of care, Dr. Raskin reported. The exceptions were practical life support, where the mean score was about 0.6-0.8 across the board, and having a medical home, where the mean score was about 1.8-1.9 across the board.
The specialized consult model of care had the highest scores for mental health and social support, information and resources, and supportive and prepared clinicians. The specialized longitudinal model of care had the highest scores for empowered and engaged patients, open patient-clinician communication, care coordination and transitions, and access to full spectrum of care. The oncology-embedded model had the lowest scores. Analysis of the tool’s 46 individual questions showed that patients cared for at institutions using the oncology-embedded model were significantly less likely than were counterparts cared for at institutions using the specialized models to report that the institution performed various activities such as offering a treatment summary, inquiring about the patient’s biggest worries or problems, and explaining the reasons why tests were needed (P less than .05 for each).
For some metrics, the overall proportion reporting that an activity was performed was low, regardless of the model being used. For example, only 48% of all patients reported being helped to set goals or make short-term plans to manage follow-up care and improve health, merely 24% reported being provided emotional and social support to deal with changes in relationships, and just 19% reported being referred to special providers for other medical problems.
“Overall, all three models are performing highly in terms of providing survivors with a medical home and communicating with patients. However, all three are performing quite low in terms of providing mental health and social support, as well as practical life support,” said Dr. Raskin.
“By model, we see that the embedded ongoing care model is significantly underperforming compared with both specialized models on seven of nine components, and we have some hypotheses from our early work with [Commission on Cancer]–accredited centers to explain this,” she added. “Embedded survivorship models have a lot of variability – many are high performers but others are low performers as compared with specialized programs. Embedded survivorship care models are typically led by the treating oncologist, who historically has focused on treating sick patients and less so on providing social supports for follow-up of well patients or ‘well-er’ patients. At the same time, specialized models focus predominantly on survivorship care and providing services and referrals for survivors, which may explain their high scores.
“We know that the higher quality of care measures presented here do not necessarily translate to better patient outcomes, and that’s actually going to be the next phase of our analysis,” she concluded.
The study sample may have had some selection bias, and it is unclear how well validated the tool was, according to Dr. Krzyzanowska, the discussant. Another issue was its assessment of quality of care at only a single time point.
Nonetheless, the findings show “that measuring quality of survivorship care from a patient perspective is feasible and valuable. We have already heard about [need for] survivorship plans in survivorship care, so certainly the work that was just presented is extremely important to help to fill some of these gaps,” she said.
“I’m not sure that we yet know what the optimal model of survivorship care is without the information of the other outcomes. Furthermore, there’s different survivor populations and different ways that health care is organized, so perhaps there isn’t really one optimal model, but the model has to fit with the context,” Dr. Krzyzanowska concluded. “That being said … the tool that they have created can be a great tool for existing survivorship care programs to assess and improve the quality of their care.”
Dr. Mittmann and Dr. Raskin had no disclosures to report.
ORLANDO – Accumulating experience is showing the benefits of various models of care for cancer survivors in terms of health care use and costs, while also suggesting that some provide higher-quality care than others, according to a pair of studies reported at a symposium on quality care sponsored by the American Society of Clinical Oncology.
Initiative for breast cancer survivors
“In 2011, Cancer Care Ontario did a quick environmental scan of our 14 regional cancer centers and found that the transition of breast cancer survivors from oncologists to primary care was very variable, and that centers often didn’t transition patients very frequently,” said Nicole Mittmann, PhD, first author on one of the studies, chief research officer for Cancer Care Ontario, and an investigator at Sunnybrook Research Institute, Toronto.
The advisory organization therefore implemented the Well Follow-Up Care Initiative to facilitate appropriate transition of breast cancer survivors. Each regional center was given a $100,000 incentive to roll out a model of the initiative.
Dr. Mittmann and her coinvestigators used provincial administrative databases to compare health care use and associated costs between 2,324 breast cancer survivors who were transitioned with the initiative and 2,324 propensity-matched control survivors who were not. The survivors were about 5 years out from their breast cancer diagnosis at baseline and had median follow-up of 2 years.
Study results reported at the symposium showed that the mean annual total cost of care per patient paid for by the provincial health ministry was $6,575 for the transitioned group and $10,832 for the nontransitioned group, a difference of $4,257 (39%). The main drivers were reduced costs of long-term care and cancer clinic visits.
Findings were similar for median annual costs, which amounted to $2,261 for the transitioned group and $2,903 for the control group, a difference of $638.
Compared with the nontransitioned group, the transitioned group had significantly fewer annual visits to medical oncologists (0.39 vs. 1.29) and radiation oncologists (0.16 vs. 0.36), while visits to general or family practitioners were statistically indistinguishable (7.35 and 7.91), Dr. Mittmann reported. There was also a trend toward fewer emergency department visits.
The transitioned group had fewer bone scans, CT and MRI scans, and radiographs annually, but differences were not significant.
Reassuringly, Dr. Mittmann said, survivors who were transitioned did not fare worse than their nontransitioned counterparts in overall survival; if anything, they tended to live longer. “We think that because the individual cancer centers enrolled patients that they thought were very well that this is a very well and highly selected and maybe a biased group,” Dr. Mittmann acknowledged. “But we certainly see that they are not doing worse than the control group.”
“About $1.4 million was distributed to the cancer centers” for the initiative, she noted. “That generated a savings for the health system of $1.5 million, if you are looking at median costs, to $9.9 million, if you are looking at mean costs.
“The transition of appropriate breast cancer survivors to the community appears to be safe and effective outside of a clinical trial, at least based on this particular retrospective analysis using databases,” she said. “The overall costs are not increased, and they may actually be decreased based on our data, and certainly these results will inform policy.”
The investigators plan several next steps, such as encouraging senior leadership at Cancer Care Ontario and the Ministry of Health to endorse the findings, according to Dr. Mittmann. In addition, “[we plan to] engage with both oncology and primary care leadership and think about how we can potentially roll out a program like this, and develop tools, whether those are letters or information packages, and education, to … appropriately transition individuals.”
Considerations in interpreting the study’s findings include the quality of the matching of survivors, according to invited discussant Monika K. Krzyzanowska, MD, a medical oncologist at Princess Margaret Cancer Centre, an associate professor at the University of Toronto, and a clinical lead of Quality Care and Access, Systemic Treatment Program, at Cancer Care Ontario. “The quality of that match depends on what’s in the model, so there could be potential for residual confounding, and administrative data may not have all of the elements that you would need to get a perfect match.”
Additional considerations include costs not covered by the payer, impact of the initiative on delivery of guideline-recommended care and patient and provider satisfaction, generalizability of the findings, and long-term outcomes.
“This is a proof of concept, certainly, that transition of low-risk cancer survivors to primary care is feasible and potentially economically attractive,” Dr. Krzyzanowska concluded. “It would be useful to have a formal evaluation of effectiveness that would inform a comprehensive value assessment. And we do have data from a randomized trial about the safety of this particular approach, but it would be nice to see that following implementation in real practices, those safety considerations played out the same way.”
Comparison of survivorship care models
Two-thirds of the large and growing population of cancer survivors are at least 5 years out from diagnosis, stimulating considerable discussion in the oncology community about how to best address their needs, according to Sarah Raskin, PhD, senior author on the second study and a research scientist at the Institute for Patient-Centered Initiatives and Health Equity at George Washington University Cancer Center, Washington.
“Yet, for a lack of cancer survivorship–specific guidelines from research or practice, cancer centers are increasingly developing survivorship care in a variety of ways, many of which are ad hoc or unproven as yet,” she said.
Dr. Raskin and her colleagues compared three emerging models of survivorship care: a specialized consultative model and a specialized longitudinal model – whereby patients have a single or multiple formalized survivorship visits, respectively, with care typically led by an oncology nurse-practitioner – and an oncology-embedded model – whereby survivorship is addressed as a part of ongoing oncology follow-up care, typically by the oncologist.
The investigators worked with survivors to develop the Patient-Prioritized Measure of High-Quality Survivorship Care, a 46-question scale assessing nine components of survivorship care that capture the health care priorities and needs that matter most to patients. Each component is rated on a scale from 0 (not at all met) to 1 (somewhat met) to 2 (definitely met).
Analyses were based on responses of 827 survivors of breast, colorectal, and prostate cancer who received care at 28 U.S. institutions using one of the above models and who were surveyed by telephone about the care received 1 week after their initial survivorship visit.
Results showed that survivors cared for under the three models differed significantly with respect to scores for seven of the nine components of quality of care, Dr. Raskin reported. The exceptions were practical life support, where the mean score was about 0.6-0.8 across the board, and having a medical home, where the mean score was about 1.8-1.9 across the board.
The specialized consult model of care had the highest scores for mental health and social support, information and resources, and supportive and prepared clinicians. The specialized longitudinal model of care had the highest scores for empowered and engaged patients, open patient-clinician communication, care coordination and transitions, and access to full spectrum of care. The oncology-embedded model had the lowest scores. Analysis of the tool’s 46 individual questions showed that patients cared for at institutions using the oncology-embedded model were significantly less likely than were counterparts cared for at institutions using the specialized models to report that the institution performed various activities such as offering a treatment summary, inquiring about the patient’s biggest worries or problems, and explaining the reasons why tests were needed (P less than .05 for each).
For some metrics, the overall proportion reporting that an activity was performed was low, regardless of the model being used. For example, only 48% of all patients reported being helped to set goals or make short-term plans to manage follow-up care and improve health, merely 24% reported being provided emotional and social support to deal with changes in relationships, and just 19% reported being referred to special providers for other medical problems.
“Overall, all three models are performing highly in terms of providing survivors with a medical home and communicating with patients. However, all three are performing quite low in terms of providing mental health and social support, as well as practical life support,” said Dr. Raskin.
“By model, we see that the embedded ongoing care model is significantly underperforming compared with both specialized models on seven of nine components, and we have some hypotheses from our early work with [Commission on Cancer]–accredited centers to explain this,” she added. “Embedded survivorship models have a lot of variability – many are high performers but others are low performers as compared with specialized programs. Embedded survivorship care models are typically led by the treating oncologist, who historically has focused on treating sick patients and less so on providing social supports for follow-up of well patients or ‘well-er’ patients. At the same time, specialized models focus predominantly on survivorship care and providing services and referrals for survivors, which may explain their high scores.
“We know that the higher quality of care measures presented here do not necessarily translate to better patient outcomes, and that’s actually going to be the next phase of our analysis,” she concluded.
The study sample may have had some selection bias, and it is unclear how well validated the tool was, according to Dr. Krzyzanowska, the discussant. Another issue was its assessment of quality of care at only a single time point.
Nonetheless, the findings show “that measuring quality of survivorship care from a patient perspective is feasible and valuable. We have already heard about [need for] survivorship plans in survivorship care, so certainly the work that was just presented is extremely important to help to fill some of these gaps,” she said.
“I’m not sure that we yet know what the optimal model of survivorship care is without the information of the other outcomes. Furthermore, there’s different survivor populations and different ways that health care is organized, so perhaps there isn’t really one optimal model, but the model has to fit with the context,” Dr. Krzyzanowska concluded. “That being said … the tool that they have created can be a great tool for existing survivorship care programs to assess and improve the quality of their care.”
Dr. Mittmann and Dr. Raskin had no disclosures to report.
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: Mean annual health care costs were $4,257 (39%) lower for breast cancer survivors actively transitioned to primary care versus control peers. Specialized consult and specialized longitudinal models outperformed an oncology-embedded model on seven quality metrics.
Data source: A cohort study of 2,324 breast cancer survivors transitioned to primary care and 2,324 not transitioned. A cohort study of 827 survivors of breast, colorectal, and prostate cancer receiving care under three differing models.
Disclosures: Dr. Mittmann and Dr. Raskin had no disclosures to report.
Preoperative opioid use linked to worse outcomes following abdominal surgery
Surgeons need to do more to identify patients who are taking opioids preoperatively, because this is a population that appears to be at a higher risk of worse surgical outcomes, according to a large retrospective investigation.
“Opioid users represent a potentially high-risk surgical population and may require tailored perioperative care [and] the prevalence and clinical impact of this problem in the general surgery population remain unclear,” wrote the authors of a study, led by David C. Cron, a medical student of the University of Michigan, Ann Arbor. “Given the impact of pain control and gastrointestinal function on hospital stay, it is intuitive that opioid users may have increased hospital length of stay (LOS) and may incur more costs [and] also be at higher risk for surgical complications.”
The study was published in the Annals of Surgery (2017;465[4]696-701).
Mr. Cron and his coauthors made a study cohort retrospectively from abdominopelvic surgery patients from the Michigan Surgical Quality Collaborative (MSQC) database who had surgery between 2008 and 2014. All patients were treated at the University of Michigan, and were admitted within 2 days of undergoing their operation. Any patient with data indicating use of buprenorphine prior to surgery, or those were who opioid naive before admission but received opioids after being admitted, were excluded.
Investigators found a total of 3,107 patients in the MSQC database that underwent abdominopelvic surgery at the University of Michigan during the designated time frame; from that group, 2,413 were ultimately found to match the inclusion criteria for the study. The primary outcomes were 90-day total hospital costs, along with patient LOS, and 30-day rates of complications and readmissions. Data underwent covariate risk adjustment to account for age, race, gender, body mass index, insurance class, and other factors.
“Major complications are recorded by the MSQC and include: surgical site infection, deep venous thrombosis, acute renal failure, postoperative bleeding requiring transfusion, stroke, unplanned intubation, fascial dehiscence, prolonged mechanical ventilation longer than 48 hours, myocardial infarction, pneumonia, pulmonary embolism, sepsis, vascular graft loss, and renal insufficiency,” the authors noted.
Of the 2,413 subjects overall, 502 (20.8%) were found to use opioids before surgery. Differences between opioid users and nonusers were not significant in terms of age (P = .10), gender (P = .76), and race (P = .78). After adjustment, data indicated that preoperative opioid users had 9.2% higher hospital costs than nonusers (95% confidence interval, 2.8%-15.6%, P = .005) and 12.4% longer hospital stay (95% CI, 2.3%-23.5%; P = .015). Complications and readmission rates were quantified by odds ratios, which were also found to be significantly higher in subjects who were preoperative opioid users: OR = 1.36 (95% CI, 1.04-1.78; P = .023) and OR = 1.57 (95% CI, 1.08-2.29; P = .018), respectively.
The study had some limitations, including being conducted at a single center and potentially not generalizable to other types of health care facilities and population types. Additionally, information on opioid dosage and duration of use was lacking, making it that “possible that some opioid users in our study were using opioids over a shorter time period for pain related to their surgical disease,” according to the investigators.
“These results argue the potential cost-effectiveness of intervention in this unique patient population,” Mr. Cron and his colleagues concluded. “Opioid use is a potentially modifiable risk factor, and major surgery can provide powerful leverage to improve health behavior [and] our institution has implemented a preoperative program to optimize high-risk patients for surgery.”
Mr. Cron received funding from the 2015 AOA Carolyn L. Kuckein Student Research Fellowship and the Blue Cross Blue Shield of Michigan Foundation Student Research Award for this study. He and his coauthors reported no relevant financial disclosures.
The opioid epidemic is probably the biggest health care problem in the United States, and it’s getting worse. Opioids are absolutely affecting surgical outcomes on a large scale. Unfortunately, there’s no great solution in terms of how to better manage these patients – in a perfect world, we’d take the time to wean them off opioids before operating, but that isn’t very pragmatic. With such a large and expanding proportion of our patients presenting to the operating room on long-term opioids, we have developed a new cohort of high-risk surgical patients over the past several years.
Michael J. Englesbe, MD , is a professor of surgery at the University of Michigan, Ann Arbor, and a coauthor on this study.
The opioid epidemic is probably the biggest health care problem in the United States, and it’s getting worse. Opioids are absolutely affecting surgical outcomes on a large scale. Unfortunately, there’s no great solution in terms of how to better manage these patients – in a perfect world, we’d take the time to wean them off opioids before operating, but that isn’t very pragmatic. With such a large and expanding proportion of our patients presenting to the operating room on long-term opioids, we have developed a new cohort of high-risk surgical patients over the past several years.
Michael J. Englesbe, MD , is a professor of surgery at the University of Michigan, Ann Arbor, and a coauthor on this study.
The opioid epidemic is probably the biggest health care problem in the United States, and it’s getting worse. Opioids are absolutely affecting surgical outcomes on a large scale. Unfortunately, there’s no great solution in terms of how to better manage these patients – in a perfect world, we’d take the time to wean them off opioids before operating, but that isn’t very pragmatic. With such a large and expanding proportion of our patients presenting to the operating room on long-term opioids, we have developed a new cohort of high-risk surgical patients over the past several years.
Michael J. Englesbe, MD , is a professor of surgery at the University of Michigan, Ann Arbor, and a coauthor on this study.
Surgeons need to do more to identify patients who are taking opioids preoperatively, because this is a population that appears to be at a higher risk of worse surgical outcomes, according to a large retrospective investigation.
“Opioid users represent a potentially high-risk surgical population and may require tailored perioperative care [and] the prevalence and clinical impact of this problem in the general surgery population remain unclear,” wrote the authors of a study, led by David C. Cron, a medical student of the University of Michigan, Ann Arbor. “Given the impact of pain control and gastrointestinal function on hospital stay, it is intuitive that opioid users may have increased hospital length of stay (LOS) and may incur more costs [and] also be at higher risk for surgical complications.”
The study was published in the Annals of Surgery (2017;465[4]696-701).
Mr. Cron and his coauthors made a study cohort retrospectively from abdominopelvic surgery patients from the Michigan Surgical Quality Collaborative (MSQC) database who had surgery between 2008 and 2014. All patients were treated at the University of Michigan, and were admitted within 2 days of undergoing their operation. Any patient with data indicating use of buprenorphine prior to surgery, or those were who opioid naive before admission but received opioids after being admitted, were excluded.
Investigators found a total of 3,107 patients in the MSQC database that underwent abdominopelvic surgery at the University of Michigan during the designated time frame; from that group, 2,413 were ultimately found to match the inclusion criteria for the study. The primary outcomes were 90-day total hospital costs, along with patient LOS, and 30-day rates of complications and readmissions. Data underwent covariate risk adjustment to account for age, race, gender, body mass index, insurance class, and other factors.
“Major complications are recorded by the MSQC and include: surgical site infection, deep venous thrombosis, acute renal failure, postoperative bleeding requiring transfusion, stroke, unplanned intubation, fascial dehiscence, prolonged mechanical ventilation longer than 48 hours, myocardial infarction, pneumonia, pulmonary embolism, sepsis, vascular graft loss, and renal insufficiency,” the authors noted.
Of the 2,413 subjects overall, 502 (20.8%) were found to use opioids before surgery. Differences between opioid users and nonusers were not significant in terms of age (P = .10), gender (P = .76), and race (P = .78). After adjustment, data indicated that preoperative opioid users had 9.2% higher hospital costs than nonusers (95% confidence interval, 2.8%-15.6%, P = .005) and 12.4% longer hospital stay (95% CI, 2.3%-23.5%; P = .015). Complications and readmission rates were quantified by odds ratios, which were also found to be significantly higher in subjects who were preoperative opioid users: OR = 1.36 (95% CI, 1.04-1.78; P = .023) and OR = 1.57 (95% CI, 1.08-2.29; P = .018), respectively.
The study had some limitations, including being conducted at a single center and potentially not generalizable to other types of health care facilities and population types. Additionally, information on opioid dosage and duration of use was lacking, making it that “possible that some opioid users in our study were using opioids over a shorter time period for pain related to their surgical disease,” according to the investigators.
“These results argue the potential cost-effectiveness of intervention in this unique patient population,” Mr. Cron and his colleagues concluded. “Opioid use is a potentially modifiable risk factor, and major surgery can provide powerful leverage to improve health behavior [and] our institution has implemented a preoperative program to optimize high-risk patients for surgery.”
Mr. Cron received funding from the 2015 AOA Carolyn L. Kuckein Student Research Fellowship and the Blue Cross Blue Shield of Michigan Foundation Student Research Award for this study. He and his coauthors reported no relevant financial disclosures.
Surgeons need to do more to identify patients who are taking opioids preoperatively, because this is a population that appears to be at a higher risk of worse surgical outcomes, according to a large retrospective investigation.
“Opioid users represent a potentially high-risk surgical population and may require tailored perioperative care [and] the prevalence and clinical impact of this problem in the general surgery population remain unclear,” wrote the authors of a study, led by David C. Cron, a medical student of the University of Michigan, Ann Arbor. “Given the impact of pain control and gastrointestinal function on hospital stay, it is intuitive that opioid users may have increased hospital length of stay (LOS) and may incur more costs [and] also be at higher risk for surgical complications.”
The study was published in the Annals of Surgery (2017;465[4]696-701).
Mr. Cron and his coauthors made a study cohort retrospectively from abdominopelvic surgery patients from the Michigan Surgical Quality Collaborative (MSQC) database who had surgery between 2008 and 2014. All patients were treated at the University of Michigan, and were admitted within 2 days of undergoing their operation. Any patient with data indicating use of buprenorphine prior to surgery, or those were who opioid naive before admission but received opioids after being admitted, were excluded.
Investigators found a total of 3,107 patients in the MSQC database that underwent abdominopelvic surgery at the University of Michigan during the designated time frame; from that group, 2,413 were ultimately found to match the inclusion criteria for the study. The primary outcomes were 90-day total hospital costs, along with patient LOS, and 30-day rates of complications and readmissions. Data underwent covariate risk adjustment to account for age, race, gender, body mass index, insurance class, and other factors.
“Major complications are recorded by the MSQC and include: surgical site infection, deep venous thrombosis, acute renal failure, postoperative bleeding requiring transfusion, stroke, unplanned intubation, fascial dehiscence, prolonged mechanical ventilation longer than 48 hours, myocardial infarction, pneumonia, pulmonary embolism, sepsis, vascular graft loss, and renal insufficiency,” the authors noted.
Of the 2,413 subjects overall, 502 (20.8%) were found to use opioids before surgery. Differences between opioid users and nonusers were not significant in terms of age (P = .10), gender (P = .76), and race (P = .78). After adjustment, data indicated that preoperative opioid users had 9.2% higher hospital costs than nonusers (95% confidence interval, 2.8%-15.6%, P = .005) and 12.4% longer hospital stay (95% CI, 2.3%-23.5%; P = .015). Complications and readmission rates were quantified by odds ratios, which were also found to be significantly higher in subjects who were preoperative opioid users: OR = 1.36 (95% CI, 1.04-1.78; P = .023) and OR = 1.57 (95% CI, 1.08-2.29; P = .018), respectively.
The study had some limitations, including being conducted at a single center and potentially not generalizable to other types of health care facilities and population types. Additionally, information on opioid dosage and duration of use was lacking, making it that “possible that some opioid users in our study were using opioids over a shorter time period for pain related to their surgical disease,” according to the investigators.
“These results argue the potential cost-effectiveness of intervention in this unique patient population,” Mr. Cron and his colleagues concluded. “Opioid use is a potentially modifiable risk factor, and major surgery can provide powerful leverage to improve health behavior [and] our institution has implemented a preoperative program to optimize high-risk patients for surgery.”
Mr. Cron received funding from the 2015 AOA Carolyn L. Kuckein Student Research Fellowship and the Blue Cross Blue Shield of Michigan Foundation Student Research Award for this study. He and his coauthors reported no relevant financial disclosures.
FROM THE ANNALS OF SURGERY
Key clinical point:
Major finding: Preoperative opioid users had 9.2% higher costs and 12.4% longer LOS, with higher ORs for complications and readmissions, than patients who did not take opioids.
Data source: Retrospective analysis of data on 2,413 abdominopelvic surgery patients during 2008-2014.
Disclosures: Study funded by grants awarded to Mr. Cron from the 2015 AOA Carolyn L. Kuckein Student Research Fellowship and the Blue Cross Blue Shield of Michigan Foundation Student Research Award. Authors reported no relevant financial disclosures.