User login
Preoperative VTEs occurred in 10% of cancer patients
SEATTLE – Venous thromboembolism (VTE) is common in cancer, but 10% of asymptomatic patients undergoing major oncologic surgery have a preoperative VTE, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
The incidence of preoperative VTE was associated with increasing age, a history of previous VTE, and a diagnosis of sepsis 1 month prior to undergoing oncologic surgery.
Surprisingly, noted study author Dr. Melanie Gainsbury of Cedar’s Sinai Medical Center, Los Angeles, it was not associated with oncologic factors such as locally recurrent disease, metastatic disease, or the receipt of neoadjuvant therapy.
“One may argue that patients undergoing oncologic surgery should receive preoperative lower-extremity duplex screening,” especially those who appear to be at high risk, she said.
About one in five cases of VTE is cancer related, and postoperative VTE is a leading cause of morbidity in cancer patients. However, Dr. Gainsbury noted, the incidence of preoperative VTE has not been well established or studied.
In this study, she and her colleagues evaluated the prevalence and risk factors associated with preoperative VTE in asymptomatic patients who were undergoing major oncologic surgery at an academic medical center.
In their retrospective analysis, the investigators identified 412 patients from the hospital’s database who underwent open abdominopelvic oncologic surgery between 2009 to 2016. All patients in the cohort had received a preoperative lower-extremity venous duplex scan (VDS).
The authors found that the overall incidence of preoperative VTE detected on VDS in this asymptomatic population was 10.1%. Of this group, 48.6% of the VTEs were acute, 42.9% were chronic, and a small subset (8.5%) was classified as subacute.
The majority of VTEs (62.9%) were located below the knee, and all of those patients with above-the-knee VTEs (37.1%) received inferior vena cava filters prior to surgery.
None of the patients in this cohort experienced a postoperative pulmonary embolism.
The investigators also looked at various risk factors that could predispose patients to a higher risk of developing a VTE. They did not find any statistically significant differences between those with a preoperative VTE and those without one when looking at gender, body mass index, or cancer type.
There was, however, a statistically significant difference in age, with older age being significantly associated with preoperative VTE. Further analysis showed that patients were 1.3 times more likely to have a preoperative DVT for every 5-year increase in age (odds ratio, 1.3; 95% confidence interval, 1.1-1.6).
In addition, patients with preoperative VTEs were significantly more likely to experience postoperative complications, with an almost twofold increased incidence (25.7% vs. 13.2%, P = .046).
“Patients with preoperative VTE were 1.95 times more likely to develop a postoperative complication than patients without a preoperative VTE,” Dr. Gainsbury said.
In terms of comorbidities, there was no statistically significant difference in regards to history of a known lung disease, varicose veins, a known coagulation mutation, congestive heart failure, and inflammatory bowel disease.
There were also no statistical differences between hormone use or anticoagulants in patients with and without VTEs.
Of note, a recent history of sepsis appeared to be an important factor that put patients at risk for a subsequent VTE. “The preoperative VTE group had a higher rate of diagnosed sepsis during the month prior to surgery,” she said. “We believe that the preoperative diagnosis of sepsis represents a prior hospitalization and perhaps a sicker population at risk for VTEs.”
There was no funding source disclosed in the abstract. Dr. Gainsbury and her coauthors had no disclosures.
SEATTLE – Venous thromboembolism (VTE) is common in cancer, but 10% of asymptomatic patients undergoing major oncologic surgery have a preoperative VTE, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
The incidence of preoperative VTE was associated with increasing age, a history of previous VTE, and a diagnosis of sepsis 1 month prior to undergoing oncologic surgery.
Surprisingly, noted study author Dr. Melanie Gainsbury of Cedar’s Sinai Medical Center, Los Angeles, it was not associated with oncologic factors such as locally recurrent disease, metastatic disease, or the receipt of neoadjuvant therapy.
“One may argue that patients undergoing oncologic surgery should receive preoperative lower-extremity duplex screening,” especially those who appear to be at high risk, she said.
About one in five cases of VTE is cancer related, and postoperative VTE is a leading cause of morbidity in cancer patients. However, Dr. Gainsbury noted, the incidence of preoperative VTE has not been well established or studied.
In this study, she and her colleagues evaluated the prevalence and risk factors associated with preoperative VTE in asymptomatic patients who were undergoing major oncologic surgery at an academic medical center.
In their retrospective analysis, the investigators identified 412 patients from the hospital’s database who underwent open abdominopelvic oncologic surgery between 2009 to 2016. All patients in the cohort had received a preoperative lower-extremity venous duplex scan (VDS).
The authors found that the overall incidence of preoperative VTE detected on VDS in this asymptomatic population was 10.1%. Of this group, 48.6% of the VTEs were acute, 42.9% were chronic, and a small subset (8.5%) was classified as subacute.
The majority of VTEs (62.9%) were located below the knee, and all of those patients with above-the-knee VTEs (37.1%) received inferior vena cava filters prior to surgery.
None of the patients in this cohort experienced a postoperative pulmonary embolism.
The investigators also looked at various risk factors that could predispose patients to a higher risk of developing a VTE. They did not find any statistically significant differences between those with a preoperative VTE and those without one when looking at gender, body mass index, or cancer type.
There was, however, a statistically significant difference in age, with older age being significantly associated with preoperative VTE. Further analysis showed that patients were 1.3 times more likely to have a preoperative DVT for every 5-year increase in age (odds ratio, 1.3; 95% confidence interval, 1.1-1.6).
In addition, patients with preoperative VTEs were significantly more likely to experience postoperative complications, with an almost twofold increased incidence (25.7% vs. 13.2%, P = .046).
“Patients with preoperative VTE were 1.95 times more likely to develop a postoperative complication than patients without a preoperative VTE,” Dr. Gainsbury said.
In terms of comorbidities, there was no statistically significant difference in regards to history of a known lung disease, varicose veins, a known coagulation mutation, congestive heart failure, and inflammatory bowel disease.
There were also no statistical differences between hormone use or anticoagulants in patients with and without VTEs.
Of note, a recent history of sepsis appeared to be an important factor that put patients at risk for a subsequent VTE. “The preoperative VTE group had a higher rate of diagnosed sepsis during the month prior to surgery,” she said. “We believe that the preoperative diagnosis of sepsis represents a prior hospitalization and perhaps a sicker population at risk for VTEs.”
There was no funding source disclosed in the abstract. Dr. Gainsbury and her coauthors had no disclosures.
SEATTLE – Venous thromboembolism (VTE) is common in cancer, but 10% of asymptomatic patients undergoing major oncologic surgery have a preoperative VTE, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
The incidence of preoperative VTE was associated with increasing age, a history of previous VTE, and a diagnosis of sepsis 1 month prior to undergoing oncologic surgery.
Surprisingly, noted study author Dr. Melanie Gainsbury of Cedar’s Sinai Medical Center, Los Angeles, it was not associated with oncologic factors such as locally recurrent disease, metastatic disease, or the receipt of neoadjuvant therapy.
“One may argue that patients undergoing oncologic surgery should receive preoperative lower-extremity duplex screening,” especially those who appear to be at high risk, she said.
About one in five cases of VTE is cancer related, and postoperative VTE is a leading cause of morbidity in cancer patients. However, Dr. Gainsbury noted, the incidence of preoperative VTE has not been well established or studied.
In this study, she and her colleagues evaluated the prevalence and risk factors associated with preoperative VTE in asymptomatic patients who were undergoing major oncologic surgery at an academic medical center.
In their retrospective analysis, the investigators identified 412 patients from the hospital’s database who underwent open abdominopelvic oncologic surgery between 2009 to 2016. All patients in the cohort had received a preoperative lower-extremity venous duplex scan (VDS).
The authors found that the overall incidence of preoperative VTE detected on VDS in this asymptomatic population was 10.1%. Of this group, 48.6% of the VTEs were acute, 42.9% were chronic, and a small subset (8.5%) was classified as subacute.
The majority of VTEs (62.9%) were located below the knee, and all of those patients with above-the-knee VTEs (37.1%) received inferior vena cava filters prior to surgery.
None of the patients in this cohort experienced a postoperative pulmonary embolism.
The investigators also looked at various risk factors that could predispose patients to a higher risk of developing a VTE. They did not find any statistically significant differences between those with a preoperative VTE and those without one when looking at gender, body mass index, or cancer type.
There was, however, a statistically significant difference in age, with older age being significantly associated with preoperative VTE. Further analysis showed that patients were 1.3 times more likely to have a preoperative DVT for every 5-year increase in age (odds ratio, 1.3; 95% confidence interval, 1.1-1.6).
In addition, patients with preoperative VTEs were significantly more likely to experience postoperative complications, with an almost twofold increased incidence (25.7% vs. 13.2%, P = .046).
“Patients with preoperative VTE were 1.95 times more likely to develop a postoperative complication than patients without a preoperative VTE,” Dr. Gainsbury said.
In terms of comorbidities, there was no statistically significant difference in regards to history of a known lung disease, varicose veins, a known coagulation mutation, congestive heart failure, and inflammatory bowel disease.
There were also no statistical differences between hormone use or anticoagulants in patients with and without VTEs.
Of note, a recent history of sepsis appeared to be an important factor that put patients at risk for a subsequent VTE. “The preoperative VTE group had a higher rate of diagnosed sepsis during the month prior to surgery,” she said. “We believe that the preoperative diagnosis of sepsis represents a prior hospitalization and perhaps a sicker population at risk for VTEs.”
There was no funding source disclosed in the abstract. Dr. Gainsbury and her coauthors had no disclosures.
AT SSO 2017
Key clinical point: 10% of asymptomatic cancer patients undergoing major cancer surgery have preoperative venous thromboembolism.
Major finding: Patients with preoperative VTEs were significantly more likely to experience postoperative complications, with an almost twofold increased incidence (25.7% vs. 13.2%, P = .046).
Data source: Retrospective analysis that included 412 who underwent open abdominopelvic oncologic surgery at a single academic center.
Disclosures: There was no funding source disclosed in the abstract. Dr. Gainsbury and her coauthors had no disclosures.
Flu Activity Falling Across the U.S.
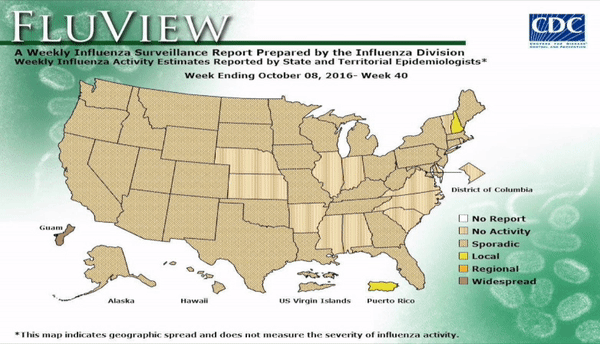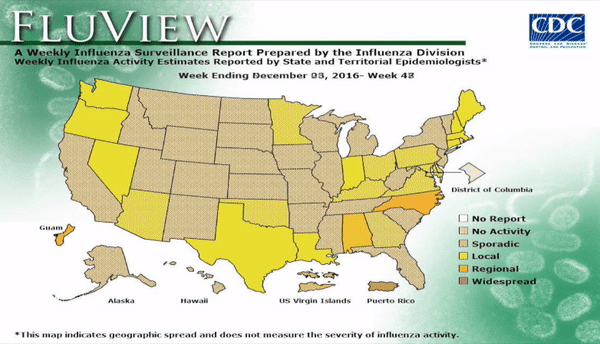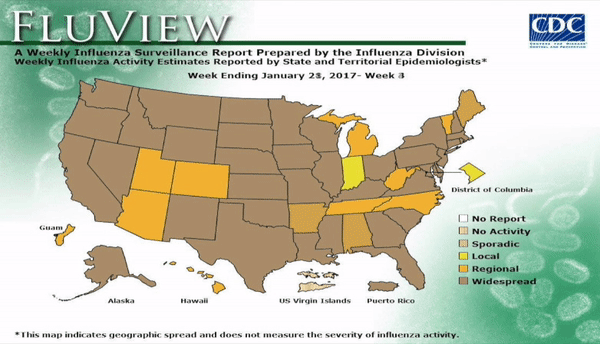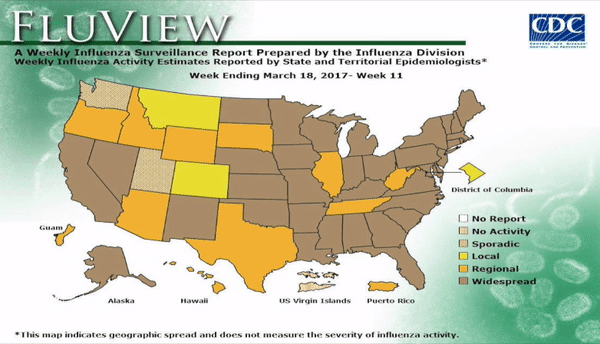
Flu activity remained elevated for the entire U.S. for the week ending March 18, 2017, according the most recent report from the Centers for Disease Control and Prevention (CDC). The percentage of respiratory specimens testing positive for influenza in clinical laboratories also is falling, suggesting that flu season is coming to a close. The most frequently identified influenza continues to be virus subtype was influenza A (H3).
According to the CDC, 31,673 influenza positive specimens have been collected and reported by public health laboratories in the U.S. during the 2016-2017 season. The CDC genetically characterized 1,510 influenza viruses and the HA gene segment of all influenza A (H1N1)pdm09 viruses analyzed belonged to genetic group 6B.1. Influenza A (H3N2) virus HA gene segments analyzed belonged to genetic groups 3C.2a or 3C.3a. Genetic group 3C.2a includes a newly emerging subgroup known as 3C.2a1. The HA of influenza B/Victoria-lineage viruses all belonged to genetic group V1A. The HA of influenza B/Yamagata-lineage viruses analyzed all belonged to genetic group Y3.
Outpatient visits for influenza-like illness (ILI) dropped to 3.2%, still well above the national baseline (2.2%). Seven of 10 regions continued to experience high ILI activity. Alabama, Georgia, Indiana, Kansas, Kentucky, Louisiana, Maryland, Minnesota, Mississippi, Oklahoma, South Carolina, and Virginia all experienced high ILI activity. New York City, Puerto Rico, Colorado, Connecticut, Delaware, Florida, Hawaii, Idaho, Iowa, Maine, Massachusetts, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New York, North Dakota, Ohio, Oregon, Utah, Vermont, Washington, West Virginia, and Wyoming all experienced minimal ILI activity.
Two more influenza-associated pediatric deaths were reported to CDC during the first week of March 2017. A total of 55 influenza-associated pediatric deaths have been reported for the 2016-2017 season..

Flu activity remained elevated for the entire U.S. for the week ending March 18, 2017, according the most recent report from the Centers for Disease Control and Prevention (CDC). The percentage of respiratory specimens testing positive for influenza in clinical laboratories also is falling, suggesting that flu season is coming to a close. The most frequently identified influenza continues to be virus subtype was influenza A (H3).
According to the CDC, 31,673 influenza positive specimens have been collected and reported by public health laboratories in the U.S. during the 2016-2017 season. The CDC genetically characterized 1,510 influenza viruses and the HA gene segment of all influenza A (H1N1)pdm09 viruses analyzed belonged to genetic group 6B.1. Influenza A (H3N2) virus HA gene segments analyzed belonged to genetic groups 3C.2a or 3C.3a. Genetic group 3C.2a includes a newly emerging subgroup known as 3C.2a1. The HA of influenza B/Victoria-lineage viruses all belonged to genetic group V1A. The HA of influenza B/Yamagata-lineage viruses analyzed all belonged to genetic group Y3.
Outpatient visits for influenza-like illness (ILI) dropped to 3.2%, still well above the national baseline (2.2%). Seven of 10 regions continued to experience high ILI activity. Alabama, Georgia, Indiana, Kansas, Kentucky, Louisiana, Maryland, Minnesota, Mississippi, Oklahoma, South Carolina, and Virginia all experienced high ILI activity. New York City, Puerto Rico, Colorado, Connecticut, Delaware, Florida, Hawaii, Idaho, Iowa, Maine, Massachusetts, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New York, North Dakota, Ohio, Oregon, Utah, Vermont, Washington, West Virginia, and Wyoming all experienced minimal ILI activity.
Two more influenza-associated pediatric deaths were reported to CDC during the first week of March 2017. A total of 55 influenza-associated pediatric deaths have been reported for the 2016-2017 season..

Flu activity remained elevated for the entire U.S. for the week ending March 18, 2017, according the most recent report from the Centers for Disease Control and Prevention (CDC). The percentage of respiratory specimens testing positive for influenza in clinical laboratories also is falling, suggesting that flu season is coming to a close. The most frequently identified influenza continues to be virus subtype was influenza A (H3).
According to the CDC, 31,673 influenza positive specimens have been collected and reported by public health laboratories in the U.S. during the 2016-2017 season. The CDC genetically characterized 1,510 influenza viruses and the HA gene segment of all influenza A (H1N1)pdm09 viruses analyzed belonged to genetic group 6B.1. Influenza A (H3N2) virus HA gene segments analyzed belonged to genetic groups 3C.2a or 3C.3a. Genetic group 3C.2a includes a newly emerging subgroup known as 3C.2a1. The HA of influenza B/Victoria-lineage viruses all belonged to genetic group V1A. The HA of influenza B/Yamagata-lineage viruses analyzed all belonged to genetic group Y3.
Outpatient visits for influenza-like illness (ILI) dropped to 3.2%, still well above the national baseline (2.2%). Seven of 10 regions continued to experience high ILI activity. Alabama, Georgia, Indiana, Kansas, Kentucky, Louisiana, Maryland, Minnesota, Mississippi, Oklahoma, South Carolina, and Virginia all experienced high ILI activity. New York City, Puerto Rico, Colorado, Connecticut, Delaware, Florida, Hawaii, Idaho, Iowa, Maine, Massachusetts, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New York, North Dakota, Ohio, Oregon, Utah, Vermont, Washington, West Virginia, and Wyoming all experienced minimal ILI activity.
Two more influenza-associated pediatric deaths were reported to CDC during the first week of March 2017. A total of 55 influenza-associated pediatric deaths have been reported for the 2016-2017 season..
Lower risk of heart attack with VKAs than with DOACs, aspirin
A large, retrospective study suggests patients with atrial fibrillation may have a lower risk of acute myocardial infarction (AMI) if they receive vitamin K antagonists (VKAs) rather than other anticoagulants.
Investigators found that patients taking direct oral anticoagulants (DOACs, rivaroxaban or dabigatran) had more than twice the AMI risk of patients taking VKAs.
And the AMI risk for patients taking low-dose aspirin was nearly double that of those taking VKAs.
Leo M. Stolk, PharmD, PhD, of Maastricht University Medical Centre in the Netherlands, and his colleagues reported these results in the British Journal of Clinical Pharmacology.
The investigators analyzed data on 30,146 adults with atrial fibrillation who were new users of DOACs (n=1266), VKAs (n=13,098), low-dose aspirin (n=15,400), or mixed anticoagulants (n=382). Most DOAC users were taking rivaroxaban (71.6%), but some were taking dabigatran (28.4%).
The mean follow-up was 0.95 years for DOAC users, 2.72 years for VKA users, 2.86 years for low-dose aspirin users, and 2.99 years for mixed medication users.
The investigators estimated the hazard ratio (HR) of AMI for users of DOACs, aspirin, or mixed medications versus VKAs. The team adjusted their analysis for age, sex, lifestyle, risk factors, comorbidities, and use of other medications.
Compared to VKA users, the risk of AMI was significantly higher for DOAC users (HR=2.11; 95% CI 1.08 – 4.12, P<0.05) and aspirin users (HR=1.91; 95% CI 1.45-2.51, P<0.05). The risk was not significantly higher for mixed medication users (HR=1.69; 95% CI 0.69, 4.16).
When patients were stratified by gender, there was a significantly increased risk of AMI for aspirin users who were male (HR=1.60; 95% CI 1.10, 2.33, P<0.05) or female (HR=2.33; 95% CI 1.55, 3.50, P<0.05), when compared to VKA users. Neither DOACs nor mixed medications were associated with a significantly increased risk of AMI in this analysis.
The investigators also stratified patients according to their CHA2DS2-VASc score at the index date.
Among patients with a high score (≥4), there was a significantly increased risk of AMI for aspirin users compared to VKA users (HR=2.21; 95% CI 1.37,3.55, P<0.05).
Among patients with a medium score (>1 and <4), the risk of AMI was significantly higher for DOAC users (HR=2.67; 95% CI 1.11, 6.40, P<0.05) and aspirin users (HR=1.82; 95% CI 1.23, 2.68, P<0.05) compared to VKA users.
The investigators said this study suggests VKAs probably have greater beneficial effects on AMI than DOACs, and ongoing research is needed as the use of DOACs increases. ![]()
A large, retrospective study suggests patients with atrial fibrillation may have a lower risk of acute myocardial infarction (AMI) if they receive vitamin K antagonists (VKAs) rather than other anticoagulants.
Investigators found that patients taking direct oral anticoagulants (DOACs, rivaroxaban or dabigatran) had more than twice the AMI risk of patients taking VKAs.
And the AMI risk for patients taking low-dose aspirin was nearly double that of those taking VKAs.
Leo M. Stolk, PharmD, PhD, of Maastricht University Medical Centre in the Netherlands, and his colleagues reported these results in the British Journal of Clinical Pharmacology.
The investigators analyzed data on 30,146 adults with atrial fibrillation who were new users of DOACs (n=1266), VKAs (n=13,098), low-dose aspirin (n=15,400), or mixed anticoagulants (n=382). Most DOAC users were taking rivaroxaban (71.6%), but some were taking dabigatran (28.4%).
The mean follow-up was 0.95 years for DOAC users, 2.72 years for VKA users, 2.86 years for low-dose aspirin users, and 2.99 years for mixed medication users.
The investigators estimated the hazard ratio (HR) of AMI for users of DOACs, aspirin, or mixed medications versus VKAs. The team adjusted their analysis for age, sex, lifestyle, risk factors, comorbidities, and use of other medications.
Compared to VKA users, the risk of AMI was significantly higher for DOAC users (HR=2.11; 95% CI 1.08 – 4.12, P<0.05) and aspirin users (HR=1.91; 95% CI 1.45-2.51, P<0.05). The risk was not significantly higher for mixed medication users (HR=1.69; 95% CI 0.69, 4.16).
When patients were stratified by gender, there was a significantly increased risk of AMI for aspirin users who were male (HR=1.60; 95% CI 1.10, 2.33, P<0.05) or female (HR=2.33; 95% CI 1.55, 3.50, P<0.05), when compared to VKA users. Neither DOACs nor mixed medications were associated with a significantly increased risk of AMI in this analysis.
The investigators also stratified patients according to their CHA2DS2-VASc score at the index date.
Among patients with a high score (≥4), there was a significantly increased risk of AMI for aspirin users compared to VKA users (HR=2.21; 95% CI 1.37,3.55, P<0.05).
Among patients with a medium score (>1 and <4), the risk of AMI was significantly higher for DOAC users (HR=2.67; 95% CI 1.11, 6.40, P<0.05) and aspirin users (HR=1.82; 95% CI 1.23, 2.68, P<0.05) compared to VKA users.
The investigators said this study suggests VKAs probably have greater beneficial effects on AMI than DOACs, and ongoing research is needed as the use of DOACs increases. ![]()
A large, retrospective study suggests patients with atrial fibrillation may have a lower risk of acute myocardial infarction (AMI) if they receive vitamin K antagonists (VKAs) rather than other anticoagulants.
Investigators found that patients taking direct oral anticoagulants (DOACs, rivaroxaban or dabigatran) had more than twice the AMI risk of patients taking VKAs.
And the AMI risk for patients taking low-dose aspirin was nearly double that of those taking VKAs.
Leo M. Stolk, PharmD, PhD, of Maastricht University Medical Centre in the Netherlands, and his colleagues reported these results in the British Journal of Clinical Pharmacology.
The investigators analyzed data on 30,146 adults with atrial fibrillation who were new users of DOACs (n=1266), VKAs (n=13,098), low-dose aspirin (n=15,400), or mixed anticoagulants (n=382). Most DOAC users were taking rivaroxaban (71.6%), but some were taking dabigatran (28.4%).
The mean follow-up was 0.95 years for DOAC users, 2.72 years for VKA users, 2.86 years for low-dose aspirin users, and 2.99 years for mixed medication users.
The investigators estimated the hazard ratio (HR) of AMI for users of DOACs, aspirin, or mixed medications versus VKAs. The team adjusted their analysis for age, sex, lifestyle, risk factors, comorbidities, and use of other medications.
Compared to VKA users, the risk of AMI was significantly higher for DOAC users (HR=2.11; 95% CI 1.08 – 4.12, P<0.05) and aspirin users (HR=1.91; 95% CI 1.45-2.51, P<0.05). The risk was not significantly higher for mixed medication users (HR=1.69; 95% CI 0.69, 4.16).
When patients were stratified by gender, there was a significantly increased risk of AMI for aspirin users who were male (HR=1.60; 95% CI 1.10, 2.33, P<0.05) or female (HR=2.33; 95% CI 1.55, 3.50, P<0.05), when compared to VKA users. Neither DOACs nor mixed medications were associated with a significantly increased risk of AMI in this analysis.
The investigators also stratified patients according to their CHA2DS2-VASc score at the index date.
Among patients with a high score (≥4), there was a significantly increased risk of AMI for aspirin users compared to VKA users (HR=2.21; 95% CI 1.37,3.55, P<0.05).
Among patients with a medium score (>1 and <4), the risk of AMI was significantly higher for DOAC users (HR=2.67; 95% CI 1.11, 6.40, P<0.05) and aspirin users (HR=1.82; 95% CI 1.23, 2.68, P<0.05) compared to VKA users.
The investigators said this study suggests VKAs probably have greater beneficial effects on AMI than DOACs, and ongoing research is needed as the use of DOACs increases. ![]()
Company again withdraws application for pacritinib
CTI BioPharma has withdrawn its application for marketing authorization of pacritinib (Enpaxiq) in the European Union, according to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).
The company was seeking approval for pacritinib, a JAK2/FLT3 inhibitor, to treat splenomegaly or symptoms of myelofibrosis (MF) in adults with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
When the application was withdrawn, the CHMP was of the provisional opinion that pacritinib could not have been approved for this indication.
Last year, CTI BioPharma withdrew its application for approval of pacritinib in the US.
Issues preventing approval
The CHMP said it had a number of concerns related to the PERSIST-1 trial, which was used to support the application for approval in the European Union. In this trial, researchers compared pacritinib to best available therapy, excluding JAK inhibitors, in patients with MF.
The CHMP said the reduction in spleen size, which was the main efficacy outcome in the study, appeared to be lower with pacritinib than with another medicine of its class, with no improvement in symptom scores.
In addition, the incidence of thrombocytopenia was higher in patients treated with pacritinib.
And more deaths occurred in patients taking pacritinib than in those receiving best available therapy, including deaths due to bleeding and adverse effects on the heart.
The CHMP also said it needs more information about the starting materials used in the manufacture of pacritinib and how the drug acts on target proteins.
Given these concerns, the CHMP was of the opinion that pacritinib’s benefits had not been shown to outweigh its risks.
CTI BioPharma said it could address the CHMP’s concerns by providing data from a second study of pacritinib, PERSIST-2.
However, there was not enough time in the current application procedure to provide the data, so the company decided to withdraw the application.
CTI BioPharma said it intends to integrate data from PERSIST-2 into its current dossier before approaching the European Medicines Agency to discuss a new application.
The company also said the withdrawal of its application will not affect patients currently enrolled in clinical trials of pacritinib or compassionate use programs for the drug.
Previous withdrawal, clinical hold
CTI BioPharma withdrew its application for approval of pacritinib in the US after the US Food and Drug Administration (FDA) placed a full clinical hold on trials of the drug.
The FDA placed the hold on pacritinib trials in February 2016 after results from PERSIST-1 and PERSIST-2 showed excess mortality in patients who received pacritinib.
The FDA lifted the hold in January 2017 after CTI BioPharma agreed to conduct dose-exploration studies for pacritinib, submit final study reports and data sets for PERSIST-1 and PERSIST-2, and make modifications to protocols and study-related documents. ![]()
CTI BioPharma has withdrawn its application for marketing authorization of pacritinib (Enpaxiq) in the European Union, according to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).
The company was seeking approval for pacritinib, a JAK2/FLT3 inhibitor, to treat splenomegaly or symptoms of myelofibrosis (MF) in adults with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
When the application was withdrawn, the CHMP was of the provisional opinion that pacritinib could not have been approved for this indication.
Last year, CTI BioPharma withdrew its application for approval of pacritinib in the US.
Issues preventing approval
The CHMP said it had a number of concerns related to the PERSIST-1 trial, which was used to support the application for approval in the European Union. In this trial, researchers compared pacritinib to best available therapy, excluding JAK inhibitors, in patients with MF.
The CHMP said the reduction in spleen size, which was the main efficacy outcome in the study, appeared to be lower with pacritinib than with another medicine of its class, with no improvement in symptom scores.
In addition, the incidence of thrombocytopenia was higher in patients treated with pacritinib.
And more deaths occurred in patients taking pacritinib than in those receiving best available therapy, including deaths due to bleeding and adverse effects on the heart.
The CHMP also said it needs more information about the starting materials used in the manufacture of pacritinib and how the drug acts on target proteins.
Given these concerns, the CHMP was of the opinion that pacritinib’s benefits had not been shown to outweigh its risks.
CTI BioPharma said it could address the CHMP’s concerns by providing data from a second study of pacritinib, PERSIST-2.
However, there was not enough time in the current application procedure to provide the data, so the company decided to withdraw the application.
CTI BioPharma said it intends to integrate data from PERSIST-2 into its current dossier before approaching the European Medicines Agency to discuss a new application.
The company also said the withdrawal of its application will not affect patients currently enrolled in clinical trials of pacritinib or compassionate use programs for the drug.
Previous withdrawal, clinical hold
CTI BioPharma withdrew its application for approval of pacritinib in the US after the US Food and Drug Administration (FDA) placed a full clinical hold on trials of the drug.
The FDA placed the hold on pacritinib trials in February 2016 after results from PERSIST-1 and PERSIST-2 showed excess mortality in patients who received pacritinib.
The FDA lifted the hold in January 2017 after CTI BioPharma agreed to conduct dose-exploration studies for pacritinib, submit final study reports and data sets for PERSIST-1 and PERSIST-2, and make modifications to protocols and study-related documents. ![]()
CTI BioPharma has withdrawn its application for marketing authorization of pacritinib (Enpaxiq) in the European Union, according to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).
The company was seeking approval for pacritinib, a JAK2/FLT3 inhibitor, to treat splenomegaly or symptoms of myelofibrosis (MF) in adults with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
When the application was withdrawn, the CHMP was of the provisional opinion that pacritinib could not have been approved for this indication.
Last year, CTI BioPharma withdrew its application for approval of pacritinib in the US.
Issues preventing approval
The CHMP said it had a number of concerns related to the PERSIST-1 trial, which was used to support the application for approval in the European Union. In this trial, researchers compared pacritinib to best available therapy, excluding JAK inhibitors, in patients with MF.
The CHMP said the reduction in spleen size, which was the main efficacy outcome in the study, appeared to be lower with pacritinib than with another medicine of its class, with no improvement in symptom scores.
In addition, the incidence of thrombocytopenia was higher in patients treated with pacritinib.
And more deaths occurred in patients taking pacritinib than in those receiving best available therapy, including deaths due to bleeding and adverse effects on the heart.
The CHMP also said it needs more information about the starting materials used in the manufacture of pacritinib and how the drug acts on target proteins.
Given these concerns, the CHMP was of the opinion that pacritinib’s benefits had not been shown to outweigh its risks.
CTI BioPharma said it could address the CHMP’s concerns by providing data from a second study of pacritinib, PERSIST-2.
However, there was not enough time in the current application procedure to provide the data, so the company decided to withdraw the application.
CTI BioPharma said it intends to integrate data from PERSIST-2 into its current dossier before approaching the European Medicines Agency to discuss a new application.
The company also said the withdrawal of its application will not affect patients currently enrolled in clinical trials of pacritinib or compassionate use programs for the drug.
Previous withdrawal, clinical hold
CTI BioPharma withdrew its application for approval of pacritinib in the US after the US Food and Drug Administration (FDA) placed a full clinical hold on trials of the drug.
The FDA placed the hold on pacritinib trials in February 2016 after results from PERSIST-1 and PERSIST-2 showed excess mortality in patients who received pacritinib.
The FDA lifted the hold in January 2017 after CTI BioPharma agreed to conduct dose-exploration studies for pacritinib, submit final study reports and data sets for PERSIST-1 and PERSIST-2, and make modifications to protocols and study-related documents. ![]()
Alpha blockers may facilitate the expulsion of larger ureteric stones
Clinical Question: Are alpha blockers efficacious in patients with ureteric stones?
Background: A multicenter, randomized controlled trial by Pickard and colleagues demonstrated an alpha blocker to be no more efficacious than placebo as medical expulsive therapy. There are no systematic reviews that include this recent study.
Setting: Randomized controlled trials (RCTs); most conducted in Europe and Asia.
Synopsis: Fifty-five unique RCTs (5,990 subjects) examining alpha blockers as the main treatment of ureteric stones versus placebo or control were included regardless of language and publication status.
Treatment with alpha blockers resulted in a 49% greater likelihood of stone passage (RR, 1.49; CI, 1.39-1.61) with a number needed to treat of four. A priori subgroup analysis revealed treatment was only beneficial in patients with larger stones (5mm or greater) independent of stone location or type of alpha blocker.
Secondary outcomes included reduced time to stone passage, fewer episodes of pain, decreased risk of surgical intervention, and lower risk of hospital admission with alpha blocker treatment without an increase in serious adverse events.
The meta-analysis was limited by the overall lack of methodological rigor of and clinical heterogeneity between the pooled studies.
Bottom Line: Based on available evidence, it is reasonable to utilize an alpha blocker as medical expulsive therapy in patients with larger ureteric stones.
Citations: Hollingsworth JM, Canales BK, Rogers MA, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
Dr. Ecker is the assistant director of education, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: Are alpha blockers efficacious in patients with ureteric stones?
Background: A multicenter, randomized controlled trial by Pickard and colleagues demonstrated an alpha blocker to be no more efficacious than placebo as medical expulsive therapy. There are no systematic reviews that include this recent study.
Setting: Randomized controlled trials (RCTs); most conducted in Europe and Asia.
Synopsis: Fifty-five unique RCTs (5,990 subjects) examining alpha blockers as the main treatment of ureteric stones versus placebo or control were included regardless of language and publication status.
Treatment with alpha blockers resulted in a 49% greater likelihood of stone passage (RR, 1.49; CI, 1.39-1.61) with a number needed to treat of four. A priori subgroup analysis revealed treatment was only beneficial in patients with larger stones (5mm or greater) independent of stone location or type of alpha blocker.
Secondary outcomes included reduced time to stone passage, fewer episodes of pain, decreased risk of surgical intervention, and lower risk of hospital admission with alpha blocker treatment without an increase in serious adverse events.
The meta-analysis was limited by the overall lack of methodological rigor of and clinical heterogeneity between the pooled studies.
Bottom Line: Based on available evidence, it is reasonable to utilize an alpha blocker as medical expulsive therapy in patients with larger ureteric stones.
Citations: Hollingsworth JM, Canales BK, Rogers MA, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
Dr. Ecker is the assistant director of education, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: Are alpha blockers efficacious in patients with ureteric stones?
Background: A multicenter, randomized controlled trial by Pickard and colleagues demonstrated an alpha blocker to be no more efficacious than placebo as medical expulsive therapy. There are no systematic reviews that include this recent study.
Setting: Randomized controlled trials (RCTs); most conducted in Europe and Asia.
Synopsis: Fifty-five unique RCTs (5,990 subjects) examining alpha blockers as the main treatment of ureteric stones versus placebo or control were included regardless of language and publication status.
Treatment with alpha blockers resulted in a 49% greater likelihood of stone passage (RR, 1.49; CI, 1.39-1.61) with a number needed to treat of four. A priori subgroup analysis revealed treatment was only beneficial in patients with larger stones (5mm or greater) independent of stone location or type of alpha blocker.
Secondary outcomes included reduced time to stone passage, fewer episodes of pain, decreased risk of surgical intervention, and lower risk of hospital admission with alpha blocker treatment without an increase in serious adverse events.
The meta-analysis was limited by the overall lack of methodological rigor of and clinical heterogeneity between the pooled studies.
Bottom Line: Based on available evidence, it is reasonable to utilize an alpha blocker as medical expulsive therapy in patients with larger ureteric stones.
Citations: Hollingsworth JM, Canales BK, Rogers MA, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
Dr. Ecker is the assistant director of education, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Interventions, especially those that are organization-directed, reduce burnout in physicians
Clinical Question: How efficacious are interventions to reduce burnout in physicians?
Background: Burnout is characterized by emotional exhaustion, depersonalization, and a diminished sense of personal accomplishment. It is driven by workplace stressors and affects nearly half of physicians practicing in the U.S.
Setting: Randomized controlled trials and controlled before-after studies in primary, secondary, or intensive care settings; most conducted in North America and Europe.
Synopsis: Twenty independent comparisons from 19 studies (1,550 physicians of any specialty including trainees) were included. All reported burnout outcomes after either physician- or organization-directed interventions designed to relieve stress and/or improve physician performance. Most physician-directed interventions utilized mindfulness-based stress reduction techniques or other educational interventions. Most organizational-directed interventions introduced reductions in workload or schedule changes.
Interventions were associated with small, significant reductions in burnout (standardized mean difference, –0.29; CI –0.42 to –0.16). A pre-specified subgroup analysis revealed organization-directed interventions had significantly improved effects, compared with physician-directed ones.
The generalizability of this meta-analysis is limited as the included studies significantly differed in their methodologies.
Bottom Line: Burnout intervention programs for physicians are associated with small benefits, and the increased efficacy of organization-directed interventions suggest burnout is a problem of the health care system, rather than of individuals.
Citations: Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(2):195-205.
Dr. Ecker is the assistant director of education, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: How efficacious are interventions to reduce burnout in physicians?
Background: Burnout is characterized by emotional exhaustion, depersonalization, and a diminished sense of personal accomplishment. It is driven by workplace stressors and affects nearly half of physicians practicing in the U.S.
Setting: Randomized controlled trials and controlled before-after studies in primary, secondary, or intensive care settings; most conducted in North America and Europe.
Synopsis: Twenty independent comparisons from 19 studies (1,550 physicians of any specialty including trainees) were included. All reported burnout outcomes after either physician- or organization-directed interventions designed to relieve stress and/or improve physician performance. Most physician-directed interventions utilized mindfulness-based stress reduction techniques or other educational interventions. Most organizational-directed interventions introduced reductions in workload or schedule changes.
Interventions were associated with small, significant reductions in burnout (standardized mean difference, –0.29; CI –0.42 to –0.16). A pre-specified subgroup analysis revealed organization-directed interventions had significantly improved effects, compared with physician-directed ones.
The generalizability of this meta-analysis is limited as the included studies significantly differed in their methodologies.
Bottom Line: Burnout intervention programs for physicians are associated with small benefits, and the increased efficacy of organization-directed interventions suggest burnout is a problem of the health care system, rather than of individuals.
Citations: Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(2):195-205.
Dr. Ecker is the assistant director of education, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: How efficacious are interventions to reduce burnout in physicians?
Background: Burnout is characterized by emotional exhaustion, depersonalization, and a diminished sense of personal accomplishment. It is driven by workplace stressors and affects nearly half of physicians practicing in the U.S.
Setting: Randomized controlled trials and controlled before-after studies in primary, secondary, or intensive care settings; most conducted in North America and Europe.
Synopsis: Twenty independent comparisons from 19 studies (1,550 physicians of any specialty including trainees) were included. All reported burnout outcomes after either physician- or organization-directed interventions designed to relieve stress and/or improve physician performance. Most physician-directed interventions utilized mindfulness-based stress reduction techniques or other educational interventions. Most organizational-directed interventions introduced reductions in workload or schedule changes.
Interventions were associated with small, significant reductions in burnout (standardized mean difference, –0.29; CI –0.42 to –0.16). A pre-specified subgroup analysis revealed organization-directed interventions had significantly improved effects, compared with physician-directed ones.
The generalizability of this meta-analysis is limited as the included studies significantly differed in their methodologies.
Bottom Line: Burnout intervention programs for physicians are associated with small benefits, and the increased efficacy of organization-directed interventions suggest burnout is a problem of the health care system, rather than of individuals.
Citations: Panagioti M, Panagopoulou E, Bower P, et al. Controlled interventions to reduce burnout in physicians: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(2):195-205.
Dr. Ecker is the assistant director of education, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Disease site determines QOL, pain in recurrent rectal cancer
SEATTLE – Disease anatomy is the main determinant of subsequent quality of life (QOL) in patients with locally recurrent rectal cancer at both base line and in the long term, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
Posterior recurrences were associated with the worst QOL scores and the most severe pain.
Recurrent rectal cancer is a morbid disease state, leading to pain and disability. “Patients experience a multitude of symptoms, including disability as a result of tumor growth in a confined space in the pelvis and invasion of adjacent organs as well as complications from surgery and neoadjuvant treatment,” said lead author Dr. Tarik Sammour of the University of Texas MD Anderson Cancer Center in Houston.
He noted, however, that “it is not all doom and gloom.” Survival outcomes have been improving in this population, with the median 5-year survival now approaching 40%-50%.
“Decade by decade, the 5-year survival outcomes are increasing,” explained Dr. Sammour.
Increasing survival also begs the question: “Are we helping patients or affording them the option of living longer with pain and disability? In other words, we need to measure patient-centered outcomes,” he said.
Data for recurrent rectal cancer are very limited, particularly when it comes to measuring patient outcomes. Most studies have been retrospective in design, making it difficult to gauge symptoms and quality of life.
The majority of studies also have focused on surgery, have short follow-up times, and may be missing data.
“Very few measure baseline quality of life, so it makes it difficult to measure trajectories,” Dr. Sammour said. “So overall we don’t know very much about the quality of life of these patients, so we thought to remedy that with a prospective study.”
Dr. Sammour and his colleagues examined the longitudinal trajectory of cancer survivorship in recurrent rectal cancer over a 7-year period. A total of 104 patients diagnosed with recurrent rectal cancer were enrolled between 2008 and 2015, and they prospectively self reported QOL using the validated EORTC QLQ-C30 and EORTC QLQ-CR29. Pain was measured by the Brief Pain Inventory.
Symptoms were measured at baseline and then every 6 months for 5 years or until death.
Within this cohort, 73 (70.2%) patients were amenable to salvage surgery with curative intent. A variety of types of surgery were performed, and R0 resection was achieved in 75% of cases.
The 30-day complication rate was 49% (21% with grade 3/4), and 5-year disease-free survival was 40%. There was no immediate mortality from the surgery.
When looking at differences between patients who underwent surgery and those who didn’t, there was a significant difference in the location of the disease. The nonsurgical group was more likely to have posterior recurrences (26%) than was the surgical group (19%).
“This makes sense since these are more difficult to achieve a R0 result with, so it may be less likely that they were offered the procedure,” Dr. Sammour said.
There was also a significant difference in estimated 5-year survival. Overall survival was 7.7% in the nonsurgical group vs. 50.9% in the surgical group (P less than .0001), and cancer-specific survival was 11.5% vs. 59.6% (P less than .0001).
As for pain and QOL scores, there were no differences between groups at baseline.
“So when they arrived at clinic they had roughly equivalent quality of life,” he said.
At follow-up, male patients were more likely to experience severe pain, but “we felt it was due to the anatomical location. Men have a narrower pelvis and don’t have the luxury of a uterus to protect the genitourinary organs,” he explained. “We suspect this may have played a role in the severity of their pain.”
Patients with posterior recurrences also had worse pain, and this was true for both surgical and nonsurgical patients.
The only determinant for QOL in those who underwent surgery was a positive margin (global health score 70.4 for negative margin and 61.9 for positive margins [P = .024]), but otherwise there were no differences by type of surgery or postoperative complications.
At a median follow-up of 33 months, patients who underwent surgery showed gradual sustained improvement in QOL but not pain scores.
“We were encouraged to see improvement,” concluded Dr. Sammour. “Surgery does improve quality of life in resectable cases.”
No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
SEATTLE – Disease anatomy is the main determinant of subsequent quality of life (QOL) in patients with locally recurrent rectal cancer at both base line and in the long term, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
Posterior recurrences were associated with the worst QOL scores and the most severe pain.
Recurrent rectal cancer is a morbid disease state, leading to pain and disability. “Patients experience a multitude of symptoms, including disability as a result of tumor growth in a confined space in the pelvis and invasion of adjacent organs as well as complications from surgery and neoadjuvant treatment,” said lead author Dr. Tarik Sammour of the University of Texas MD Anderson Cancer Center in Houston.
He noted, however, that “it is not all doom and gloom.” Survival outcomes have been improving in this population, with the median 5-year survival now approaching 40%-50%.
“Decade by decade, the 5-year survival outcomes are increasing,” explained Dr. Sammour.
Increasing survival also begs the question: “Are we helping patients or affording them the option of living longer with pain and disability? In other words, we need to measure patient-centered outcomes,” he said.
Data for recurrent rectal cancer are very limited, particularly when it comes to measuring patient outcomes. Most studies have been retrospective in design, making it difficult to gauge symptoms and quality of life.
The majority of studies also have focused on surgery, have short follow-up times, and may be missing data.
“Very few measure baseline quality of life, so it makes it difficult to measure trajectories,” Dr. Sammour said. “So overall we don’t know very much about the quality of life of these patients, so we thought to remedy that with a prospective study.”
Dr. Sammour and his colleagues examined the longitudinal trajectory of cancer survivorship in recurrent rectal cancer over a 7-year period. A total of 104 patients diagnosed with recurrent rectal cancer were enrolled between 2008 and 2015, and they prospectively self reported QOL using the validated EORTC QLQ-C30 and EORTC QLQ-CR29. Pain was measured by the Brief Pain Inventory.
Symptoms were measured at baseline and then every 6 months for 5 years or until death.
Within this cohort, 73 (70.2%) patients were amenable to salvage surgery with curative intent. A variety of types of surgery were performed, and R0 resection was achieved in 75% of cases.
The 30-day complication rate was 49% (21% with grade 3/4), and 5-year disease-free survival was 40%. There was no immediate mortality from the surgery.
When looking at differences between patients who underwent surgery and those who didn’t, there was a significant difference in the location of the disease. The nonsurgical group was more likely to have posterior recurrences (26%) than was the surgical group (19%).
“This makes sense since these are more difficult to achieve a R0 result with, so it may be less likely that they were offered the procedure,” Dr. Sammour said.
There was also a significant difference in estimated 5-year survival. Overall survival was 7.7% in the nonsurgical group vs. 50.9% in the surgical group (P less than .0001), and cancer-specific survival was 11.5% vs. 59.6% (P less than .0001).
As for pain and QOL scores, there were no differences between groups at baseline.
“So when they arrived at clinic they had roughly equivalent quality of life,” he said.
At follow-up, male patients were more likely to experience severe pain, but “we felt it was due to the anatomical location. Men have a narrower pelvis and don’t have the luxury of a uterus to protect the genitourinary organs,” he explained. “We suspect this may have played a role in the severity of their pain.”
Patients with posterior recurrences also had worse pain, and this was true for both surgical and nonsurgical patients.
The only determinant for QOL in those who underwent surgery was a positive margin (global health score 70.4 for negative margin and 61.9 for positive margins [P = .024]), but otherwise there were no differences by type of surgery or postoperative complications.
At a median follow-up of 33 months, patients who underwent surgery showed gradual sustained improvement in QOL but not pain scores.
“We were encouraged to see improvement,” concluded Dr. Sammour. “Surgery does improve quality of life in resectable cases.”
No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
SEATTLE – Disease anatomy is the main determinant of subsequent quality of life (QOL) in patients with locally recurrent rectal cancer at both base line and in the long term, according to findings presented at the annual Society of Surgical Oncology Cancer Symposium.
Posterior recurrences were associated with the worst QOL scores and the most severe pain.
Recurrent rectal cancer is a morbid disease state, leading to pain and disability. “Patients experience a multitude of symptoms, including disability as a result of tumor growth in a confined space in the pelvis and invasion of adjacent organs as well as complications from surgery and neoadjuvant treatment,” said lead author Dr. Tarik Sammour of the University of Texas MD Anderson Cancer Center in Houston.
He noted, however, that “it is not all doom and gloom.” Survival outcomes have been improving in this population, with the median 5-year survival now approaching 40%-50%.
“Decade by decade, the 5-year survival outcomes are increasing,” explained Dr. Sammour.
Increasing survival also begs the question: “Are we helping patients or affording them the option of living longer with pain and disability? In other words, we need to measure patient-centered outcomes,” he said.
Data for recurrent rectal cancer are very limited, particularly when it comes to measuring patient outcomes. Most studies have been retrospective in design, making it difficult to gauge symptoms and quality of life.
The majority of studies also have focused on surgery, have short follow-up times, and may be missing data.
“Very few measure baseline quality of life, so it makes it difficult to measure trajectories,” Dr. Sammour said. “So overall we don’t know very much about the quality of life of these patients, so we thought to remedy that with a prospective study.”
Dr. Sammour and his colleagues examined the longitudinal trajectory of cancer survivorship in recurrent rectal cancer over a 7-year period. A total of 104 patients diagnosed with recurrent rectal cancer were enrolled between 2008 and 2015, and they prospectively self reported QOL using the validated EORTC QLQ-C30 and EORTC QLQ-CR29. Pain was measured by the Brief Pain Inventory.
Symptoms were measured at baseline and then every 6 months for 5 years or until death.
Within this cohort, 73 (70.2%) patients were amenable to salvage surgery with curative intent. A variety of types of surgery were performed, and R0 resection was achieved in 75% of cases.
The 30-day complication rate was 49% (21% with grade 3/4), and 5-year disease-free survival was 40%. There was no immediate mortality from the surgery.
When looking at differences between patients who underwent surgery and those who didn’t, there was a significant difference in the location of the disease. The nonsurgical group was more likely to have posterior recurrences (26%) than was the surgical group (19%).
“This makes sense since these are more difficult to achieve a R0 result with, so it may be less likely that they were offered the procedure,” Dr. Sammour said.
There was also a significant difference in estimated 5-year survival. Overall survival was 7.7% in the nonsurgical group vs. 50.9% in the surgical group (P less than .0001), and cancer-specific survival was 11.5% vs. 59.6% (P less than .0001).
As for pain and QOL scores, there were no differences between groups at baseline.
“So when they arrived at clinic they had roughly equivalent quality of life,” he said.
At follow-up, male patients were more likely to experience severe pain, but “we felt it was due to the anatomical location. Men have a narrower pelvis and don’t have the luxury of a uterus to protect the genitourinary organs,” he explained. “We suspect this may have played a role in the severity of their pain.”
Patients with posterior recurrences also had worse pain, and this was true for both surgical and nonsurgical patients.
The only determinant for QOL in those who underwent surgery was a positive margin (global health score 70.4 for negative margin and 61.9 for positive margins [P = .024]), but otherwise there were no differences by type of surgery or postoperative complications.
At a median follow-up of 33 months, patients who underwent surgery showed gradual sustained improvement in QOL but not pain scores.
“We were encouraged to see improvement,” concluded Dr. Sammour. “Surgery does improve quality of life in resectable cases.”
No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
AT SSO 2017
Key clinical point: Posterior recurrence in recurrent rectal cancer was associated with worst quality of life and pain.
Major finding: Surgery improved overall survival (7.7% in the nonsurgical group vs. 50.9% in the surgical group [P less than .0001]) and quality of life.
Data source: Prospective study involved 104 patients with recurrent rectal cancer.
Disclosures: No funding source was disclosed. Dr. Sammour and his coauthors had no disclosures.
NCCN: Myelofibrosis guideline is first in series on MPNs
ORLANDO – A guideline published late last year for the diagnostic work-up of myeloproliferative neoplasms and for the management of myelofibrosis in particular is just the first in a series of National Comprehensive Cancer Network guidelines on this “family of myeloid neoplasms,” according to the guideline panel chair, Ruben A. Mesa, MD.
The myeloproliferative neoplasm (MPN) guideline panel first worked to develop a framework based on existing understanding of the MPNs. Members consulted with two other panels working in the area of chronic myeloid diseases, including chronic myeloid leukemia and myelodysplastic syndrome.
“We were in agreement that these are different entities, and our treatments are different, our guidelines are different,” Dr. Mesa of the Mayo Clinic Cancer Center, Phoenix, said at the annual conference of the National Comprehensive Cancer Network.
That said, there are also some shared circumstances. For example, all three sets of diseases can progress to acute myeloid leukemia.
“Indeed, I view this very much as pieces in a jigsaw puzzle. … It is important that we recognize their interdependencies as well as those aspects that are disease specific,” he said.
In essence, however, the MPN guideline development is a from-scratch effort, as these are the first guidelines for these disorders, he noted.
The effort is timely, as the diagnosis and management of patients with MPNs have rapidly evolved since the identification of mutations that activate the JAK pathway, including JAK2, CALR, and MPL mutations. Further, the development of targeted therapies – such as the JAK1 and JAK2 inhibitor ruxolitinib, which was the first drug approved for the treatment of myelofibrosis – has resulted in significant improvements in disease-related symptoms and quality of life.
The panel is focusing first on the “core classic” Philadelphia chromosome–negative MPNs: myelofibrosis, polycythemia vera, and essential thrombocythemia. The first piece to be placed in the MPN puzzle was the guideline for the diagnostic work-up of these entities and for risk stratification, treatment, and supportive care strategies for the management of myelofibrosis, which the panel considered “the greatest unmet need and the most urgent in terms of guidance,” Dr. Mesa said.
This initial MPN guideline was published in December (J Natl Compr Canc Netw. 2016;14:1572-611) and, because of the evolving understanding of MPNs, updates are already under consideration as additional MPN guidelines are being developed.
“We have been actively working … to develop the next set of treatment guidelines, which are the treatment guidelines for polycythemia vera and essential thrombocythemia. Finally, we will work to include the atypical MPNs,” he said, noting that the latter include hypereosinophilic disease, systemic mast cell disease, and other atypical illnesses.
These represent a small number of patients, but “their management is key, it’s distinct from the others, and there is no good guidance,” Dr. Mesa said.
“Once this is fleshed out, we will then have a fully developed set of guidelines that then will be maintained along the traditional process that NCCN follows, which is first an annual review, but second, a monitoring in real time of key developments that could impact the guidelines during the off-cycle,” he said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
ORLANDO – A guideline published late last year for the diagnostic work-up of myeloproliferative neoplasms and for the management of myelofibrosis in particular is just the first in a series of National Comprehensive Cancer Network guidelines on this “family of myeloid neoplasms,” according to the guideline panel chair, Ruben A. Mesa, MD.
The myeloproliferative neoplasm (MPN) guideline panel first worked to develop a framework based on existing understanding of the MPNs. Members consulted with two other panels working in the area of chronic myeloid diseases, including chronic myeloid leukemia and myelodysplastic syndrome.
“We were in agreement that these are different entities, and our treatments are different, our guidelines are different,” Dr. Mesa of the Mayo Clinic Cancer Center, Phoenix, said at the annual conference of the National Comprehensive Cancer Network.
That said, there are also some shared circumstances. For example, all three sets of diseases can progress to acute myeloid leukemia.
“Indeed, I view this very much as pieces in a jigsaw puzzle. … It is important that we recognize their interdependencies as well as those aspects that are disease specific,” he said.
In essence, however, the MPN guideline development is a from-scratch effort, as these are the first guidelines for these disorders, he noted.
The effort is timely, as the diagnosis and management of patients with MPNs have rapidly evolved since the identification of mutations that activate the JAK pathway, including JAK2, CALR, and MPL mutations. Further, the development of targeted therapies – such as the JAK1 and JAK2 inhibitor ruxolitinib, which was the first drug approved for the treatment of myelofibrosis – has resulted in significant improvements in disease-related symptoms and quality of life.
The panel is focusing first on the “core classic” Philadelphia chromosome–negative MPNs: myelofibrosis, polycythemia vera, and essential thrombocythemia. The first piece to be placed in the MPN puzzle was the guideline for the diagnostic work-up of these entities and for risk stratification, treatment, and supportive care strategies for the management of myelofibrosis, which the panel considered “the greatest unmet need and the most urgent in terms of guidance,” Dr. Mesa said.
This initial MPN guideline was published in December (J Natl Compr Canc Netw. 2016;14:1572-611) and, because of the evolving understanding of MPNs, updates are already under consideration as additional MPN guidelines are being developed.
“We have been actively working … to develop the next set of treatment guidelines, which are the treatment guidelines for polycythemia vera and essential thrombocythemia. Finally, we will work to include the atypical MPNs,” he said, noting that the latter include hypereosinophilic disease, systemic mast cell disease, and other atypical illnesses.
These represent a small number of patients, but “their management is key, it’s distinct from the others, and there is no good guidance,” Dr. Mesa said.
“Once this is fleshed out, we will then have a fully developed set of guidelines that then will be maintained along the traditional process that NCCN follows, which is first an annual review, but second, a monitoring in real time of key developments that could impact the guidelines during the off-cycle,” he said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
ORLANDO – A guideline published late last year for the diagnostic work-up of myeloproliferative neoplasms and for the management of myelofibrosis in particular is just the first in a series of National Comprehensive Cancer Network guidelines on this “family of myeloid neoplasms,” according to the guideline panel chair, Ruben A. Mesa, MD.
The myeloproliferative neoplasm (MPN) guideline panel first worked to develop a framework based on existing understanding of the MPNs. Members consulted with two other panels working in the area of chronic myeloid diseases, including chronic myeloid leukemia and myelodysplastic syndrome.
“We were in agreement that these are different entities, and our treatments are different, our guidelines are different,” Dr. Mesa of the Mayo Clinic Cancer Center, Phoenix, said at the annual conference of the National Comprehensive Cancer Network.
That said, there are also some shared circumstances. For example, all three sets of diseases can progress to acute myeloid leukemia.
“Indeed, I view this very much as pieces in a jigsaw puzzle. … It is important that we recognize their interdependencies as well as those aspects that are disease specific,” he said.
In essence, however, the MPN guideline development is a from-scratch effort, as these are the first guidelines for these disorders, he noted.
The effort is timely, as the diagnosis and management of patients with MPNs have rapidly evolved since the identification of mutations that activate the JAK pathway, including JAK2, CALR, and MPL mutations. Further, the development of targeted therapies – such as the JAK1 and JAK2 inhibitor ruxolitinib, which was the first drug approved for the treatment of myelofibrosis – has resulted in significant improvements in disease-related symptoms and quality of life.
The panel is focusing first on the “core classic” Philadelphia chromosome–negative MPNs: myelofibrosis, polycythemia vera, and essential thrombocythemia. The first piece to be placed in the MPN puzzle was the guideline for the diagnostic work-up of these entities and for risk stratification, treatment, and supportive care strategies for the management of myelofibrosis, which the panel considered “the greatest unmet need and the most urgent in terms of guidance,” Dr. Mesa said.
This initial MPN guideline was published in December (J Natl Compr Canc Netw. 2016;14:1572-611) and, because of the evolving understanding of MPNs, updates are already under consideration as additional MPN guidelines are being developed.
“We have been actively working … to develop the next set of treatment guidelines, which are the treatment guidelines for polycythemia vera and essential thrombocythemia. Finally, we will work to include the atypical MPNs,” he said, noting that the latter include hypereosinophilic disease, systemic mast cell disease, and other atypical illnesses.
These represent a small number of patients, but “their management is key, it’s distinct from the others, and there is no good guidance,” Dr. Mesa said.
“Once this is fleshed out, we will then have a fully developed set of guidelines that then will be maintained along the traditional process that NCCN follows, which is first an annual review, but second, a monitoring in real time of key developments that could impact the guidelines during the off-cycle,” he said.
Dr. Mesa disclosed that he has received consulting fees, honoraria, and/or grant/research support from ARIAD Pharmaceuticals, Celgene, CTI BioPharma, Galena Biopharma, Gilead Sciences, Incyte, Novartis Pharmaceuticals, and Promedior.
AT THE NCCN ANNUAL CONFERENCE
CHMP recommends product for hemophilia B
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for nonacog beta pegol (N9-GP, Refixia®).
N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
The CHMP has recommended N9-GP for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
The CHMP’s opinion has been forwarded to the European Commission, which will decide whether or not to grant marketing authorization for N9-GP. The product is being developed by Novo Nordisk.
Trial results
The CHMP’s recommendation for N9-GP is based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in a pair of phase 3 trials known as paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined.![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for nonacog beta pegol (N9-GP, Refixia®).
N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
The CHMP has recommended N9-GP for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
The CHMP’s opinion has been forwarded to the European Commission, which will decide whether or not to grant marketing authorization for N9-GP. The product is being developed by Novo Nordisk.
Trial results
The CHMP’s recommendation for N9-GP is based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in a pair of phase 3 trials known as paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined.![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for nonacog beta pegol (N9-GP, Refixia®).
N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
The CHMP has recommended N9-GP for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
The CHMP’s opinion has been forwarded to the European Commission, which will decide whether or not to grant marketing authorization for N9-GP. The product is being developed by Novo Nordisk.
Trial results
The CHMP’s recommendation for N9-GP is based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in a pair of phase 3 trials known as paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined.![]()
Complete resection tied to improved survival in low-grade serous ovarian cancer
NATIONAL HARBOR, MD. – Surgical resection to the point of no residual macroscopic disease significantly improved survival among patients with low-grade serous ovarian carcinoma, based on the findings of a large multicenter retrospective cohort study.
Adjuvant platinum-based therapy, however, did not appear to boost survival in the analysis, Tamayaa May, MD, MSc, said at the annual meeting of the Society of Gynecologic Oncology. “Genotyping and targeted sequencing of low-grade serous ovarian carcinoma often identifies actionable mutations, and treatment with MEK-based combination therapy might be a viable therapeutic strategy in patients with KRAS or NRAS mutations,” added Dr. May of Princess Margaret Cancer Center at the University of Toronto. She and her associates plan to examine more subgroups to determine if genomic alterations predict systemic response, she said.
Low-grade (Silverberg grade 1) serous ovarian tumors are slow growing but tend to resist chemotherapy, making optimal debulking a crucial part of treatment. In past studies, debulking that eliminated macroscopic evidence of disease was associated with a median survival time of about 115 months, compared with about 43 months if patients had residual disease, Dr. May noted.
To further explore outcomes after surgical resection, and to help clarify the role of systemic platinum-based therapy in low-grade serous ovarian carcinoma, she and her associates analyzed clinical data from 714 patients with low-grade serous ovarian carcinomas, including 40 from her institution and 674 from the Ovarian Cancer Association Consortium Registry. Most (60%) patients had stage III disease at diagnosis.
Complete data on surgical outcomes were available for 382 patients, of whom 202 (53%) had residual macroscopic disease and 43% did not. Among 439 patients with complete treatment data, 170 (39%) received first-line platinum-based chemotherapy. For the 391 patients with complete data on progression-free survival (PFS), the median follow-up was 4.9 years and median PFS was 3.1 years (95% confidence interval, 2.6-4.5 years). Residual macroscopic disease correlated with shorter PFS (P less than .001), as did higher tumor stage and baseline CA125 (P less than .001), but platinum-based therapy did not (P = .1).
A multivariable analysis of 333 patients confirmed these findings, Dr. May said. Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; 95% CI, 1.68-3.37; P less than .001), older age (HR, 1.15; 95% CI, 1.02-1.30; P = .02), and stage III (HR, 3.28; 95% CI, 1.87-5.76; P less than .001) or stage IV disease (HR, 5.68; 95% CI, 2.73-11.83; P less than .001), compared with stage I disease. In contrast, platinum-based therapy did not correlate with survival (HR, 0.94; 95% CI, 0.69-1.28; P = .69).
The overall survival analysis also linked mortality with higher tumor stage, increased baseline CA125, and residual disease (P less than .001 for each association), but not with platinum-based therapy (P = .2). The multivariable analysis independently tied mortality to older age (HR, 1.25; P less than .001), stage III (HR, 2.31; P = .006) or IV disease (HR, 3.86; P less than .001), and residual disease (HR, 2.53; P less than .001), but not to platinum-based therapy (HR, 1.05; P = .77).
Data consistency and completeness were issues in this study: most notably, 45% of patients had no PFS data, Dr. May commented. Nonetheless, this type of large retrospective multicenter analysis is one of the best ways to study rare tumors, including low-grade serous ovarian carcinoma, she said.
The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Surgical resection to the point of no residual macroscopic disease significantly improved survival among patients with low-grade serous ovarian carcinoma, based on the findings of a large multicenter retrospective cohort study.
Adjuvant platinum-based therapy, however, did not appear to boost survival in the analysis, Tamayaa May, MD, MSc, said at the annual meeting of the Society of Gynecologic Oncology. “Genotyping and targeted sequencing of low-grade serous ovarian carcinoma often identifies actionable mutations, and treatment with MEK-based combination therapy might be a viable therapeutic strategy in patients with KRAS or NRAS mutations,” added Dr. May of Princess Margaret Cancer Center at the University of Toronto. She and her associates plan to examine more subgroups to determine if genomic alterations predict systemic response, she said.
Low-grade (Silverberg grade 1) serous ovarian tumors are slow growing but tend to resist chemotherapy, making optimal debulking a crucial part of treatment. In past studies, debulking that eliminated macroscopic evidence of disease was associated with a median survival time of about 115 months, compared with about 43 months if patients had residual disease, Dr. May noted.
To further explore outcomes after surgical resection, and to help clarify the role of systemic platinum-based therapy in low-grade serous ovarian carcinoma, she and her associates analyzed clinical data from 714 patients with low-grade serous ovarian carcinomas, including 40 from her institution and 674 from the Ovarian Cancer Association Consortium Registry. Most (60%) patients had stage III disease at diagnosis.
Complete data on surgical outcomes were available for 382 patients, of whom 202 (53%) had residual macroscopic disease and 43% did not. Among 439 patients with complete treatment data, 170 (39%) received first-line platinum-based chemotherapy. For the 391 patients with complete data on progression-free survival (PFS), the median follow-up was 4.9 years and median PFS was 3.1 years (95% confidence interval, 2.6-4.5 years). Residual macroscopic disease correlated with shorter PFS (P less than .001), as did higher tumor stage and baseline CA125 (P less than .001), but platinum-based therapy did not (P = .1).
A multivariable analysis of 333 patients confirmed these findings, Dr. May said. Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; 95% CI, 1.68-3.37; P less than .001), older age (HR, 1.15; 95% CI, 1.02-1.30; P = .02), and stage III (HR, 3.28; 95% CI, 1.87-5.76; P less than .001) or stage IV disease (HR, 5.68; 95% CI, 2.73-11.83; P less than .001), compared with stage I disease. In contrast, platinum-based therapy did not correlate with survival (HR, 0.94; 95% CI, 0.69-1.28; P = .69).
The overall survival analysis also linked mortality with higher tumor stage, increased baseline CA125, and residual disease (P less than .001 for each association), but not with platinum-based therapy (P = .2). The multivariable analysis independently tied mortality to older age (HR, 1.25; P less than .001), stage III (HR, 2.31; P = .006) or IV disease (HR, 3.86; P less than .001), and residual disease (HR, 2.53; P less than .001), but not to platinum-based therapy (HR, 1.05; P = .77).
Data consistency and completeness were issues in this study: most notably, 45% of patients had no PFS data, Dr. May commented. Nonetheless, this type of large retrospective multicenter analysis is one of the best ways to study rare tumors, including low-grade serous ovarian carcinoma, she said.
The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Surgical resection to the point of no residual macroscopic disease significantly improved survival among patients with low-grade serous ovarian carcinoma, based on the findings of a large multicenter retrospective cohort study.
Adjuvant platinum-based therapy, however, did not appear to boost survival in the analysis, Tamayaa May, MD, MSc, said at the annual meeting of the Society of Gynecologic Oncology. “Genotyping and targeted sequencing of low-grade serous ovarian carcinoma often identifies actionable mutations, and treatment with MEK-based combination therapy might be a viable therapeutic strategy in patients with KRAS or NRAS mutations,” added Dr. May of Princess Margaret Cancer Center at the University of Toronto. She and her associates plan to examine more subgroups to determine if genomic alterations predict systemic response, she said.
Low-grade (Silverberg grade 1) serous ovarian tumors are slow growing but tend to resist chemotherapy, making optimal debulking a crucial part of treatment. In past studies, debulking that eliminated macroscopic evidence of disease was associated with a median survival time of about 115 months, compared with about 43 months if patients had residual disease, Dr. May noted.
To further explore outcomes after surgical resection, and to help clarify the role of systemic platinum-based therapy in low-grade serous ovarian carcinoma, she and her associates analyzed clinical data from 714 patients with low-grade serous ovarian carcinomas, including 40 from her institution and 674 from the Ovarian Cancer Association Consortium Registry. Most (60%) patients had stage III disease at diagnosis.
Complete data on surgical outcomes were available for 382 patients, of whom 202 (53%) had residual macroscopic disease and 43% did not. Among 439 patients with complete treatment data, 170 (39%) received first-line platinum-based chemotherapy. For the 391 patients with complete data on progression-free survival (PFS), the median follow-up was 4.9 years and median PFS was 3.1 years (95% confidence interval, 2.6-4.5 years). Residual macroscopic disease correlated with shorter PFS (P less than .001), as did higher tumor stage and baseline CA125 (P less than .001), but platinum-based therapy did not (P = .1).
A multivariable analysis of 333 patients confirmed these findings, Dr. May said. Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; 95% CI, 1.68-3.37; P less than .001), older age (HR, 1.15; 95% CI, 1.02-1.30; P = .02), and stage III (HR, 3.28; 95% CI, 1.87-5.76; P less than .001) or stage IV disease (HR, 5.68; 95% CI, 2.73-11.83; P less than .001), compared with stage I disease. In contrast, platinum-based therapy did not correlate with survival (HR, 0.94; 95% CI, 0.69-1.28; P = .69).
The overall survival analysis also linked mortality with higher tumor stage, increased baseline CA125, and residual disease (P less than .001 for each association), but not with platinum-based therapy (P = .2). The multivariable analysis independently tied mortality to older age (HR, 1.25; P less than .001), stage III (HR, 2.31; P = .006) or IV disease (HR, 3.86; P less than .001), and residual disease (HR, 2.53; P less than .001), but not to platinum-based therapy (HR, 1.05; P = .77).
Data consistency and completeness were issues in this study: most notably, 45% of patients had no PFS data, Dr. May commented. Nonetheless, this type of large retrospective multicenter analysis is one of the best ways to study rare tumors, including low-grade serous ovarian carcinoma, she said.
The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Unlike platinum-based chemotherapy, resection to the point of no residual disease was associated with improved survival among patients with low-grade serous ovarian carcinoma.
Major finding: Independent correlates of death or disease progression included residual macroscopic disease (hazard ratio, 2.38; P less than .001), older age (HR, 1.15; P = .02), and stage III (HR, 3.28; P less than .001) or stage IV (HR, 5.68; P less than .001) disease, compared with stage I disease. Platinum-based therapy was not associated with improved survival (HR, 0.94; P = 69).
Data source: A retrospective cohort study of 714 patients with low-grade (Silverberg grade 1) serous tumors from the Ovarian Cancer Association Consortium Registry.
Disclosures: The Ovarian Cancer Research Fund provides financial support for OCAC. Dr. May reported having no conflicts of interest.