User login
The Cardiovascular Safety of Nonsteroidal Anti-Inflammatory Drugs: Putting the Evidence in Perspective
Topics Include:
- Key Prospective Clinical Trials
- Danish Registry Study
- Meta-Analyses
- FDA Actions
- PRECISION Trial
Faculty/Faculty Disclosure:
Martin Quan, MD
Professor of Clinical Family Medicine
David Geffen School of Medicine at UCLA
Vice Chair for Academic Affairs
UCLA Department of Family Medicine
Los Angeles, CA
Dr. Quan discloses that he has no real or apparent conflicts to report.
Topics Include:
- Key Prospective Clinical Trials
- Danish Registry Study
- Meta-Analyses
- FDA Actions
- PRECISION Trial
Faculty/Faculty Disclosure:
Martin Quan, MD
Professor of Clinical Family Medicine
David Geffen School of Medicine at UCLA
Vice Chair for Academic Affairs
UCLA Department of Family Medicine
Los Angeles, CA
Dr. Quan discloses that he has no real or apparent conflicts to report.
Topics Include:
- Key Prospective Clinical Trials
- Danish Registry Study
- Meta-Analyses
- FDA Actions
- PRECISION Trial
Faculty/Faculty Disclosure:
Martin Quan, MD
Professor of Clinical Family Medicine
David Geffen School of Medicine at UCLA
Vice Chair for Academic Affairs
UCLA Department of Family Medicine
Los Angeles, CA
Dr. Quan discloses that he has no real or apparent conflicts to report.
Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record
A patient’s prognosis can strongly influence their medical care. Decisions about diagnostic modalities, treatment options, and the use of preventive therapies can all be affected by the likelihood of a patient’s death in the near future. For example, patients with severely limited survival might forego prophylactic therapy, avoid interventions for asymptomatic issues, and cease screening interventions. Knowing survival probability would also be very helpful as a controlling variable in research analyses whenever death risk might be a possible confounder.
Sixteen indices that aim to predict patient death risk have been described by Yourman et al.1 They were all created from secondary analyses of clinical and administrative datasets, were applicable to patients in a variety of settings (including the community, nursing home, or hospital), and predicted survival probabilities in time horizons ranging from 6 months to 5 years. Prognostic factors that were most commonly included in these indices were comorbidity and functional status. In validation populations, the discrimination of these indices for 1-year survival in hospitalized patients was moderate (with C statistics that ranged from 0.64 to 0.79) with good calibration for broad prognostic ranges.
In 2014, we published the Hospitalized-patient One-year Mortality Risk (HOMR) score.2 This study used health administrative data for all adult Ontarians admitted in 2011 to hospital under nonpsychiatric services (n = 640,022) to estimate the probability of dying within 1 year of admission to hospital (which happened in 11.7% of people). The HOMR score included 12 patient and hospitalization factors (Table 1). It was highly discriminative (C statistic, 0.923; [0.922-0.924]) and well calibrated (the mean relative difference between observed and expected death risk was 2.0% [range, 0.0% to 7.0%]). It was externally validated in more than 3 million adults from Ontario, Alberta, and Boston in whom the C statistic ranged from 0.89 to 0.92 and calibration was excellent.3 We concluded from these studies that the HOMR score is excellent for prognosticating a diverse group of patients using health administrative data.
However, we do not know whether the HOMR score can be applied to patients using primary data (ie, those taken directly from the chart). This question is important for 2 reasons. First, if HOMR accurately predicts death risk using data abstracted from the medical record, it could be used in the clinical setting to assist in clinical decision-making. Second, HOMR uses multiple administrative datasets that are difficult to access and use by most clinical researchers; it is, therefore, important to determine if HOMR is accurate for clinical research based on primary medical record review. The primary objective of this study was to determine the accuracy of the HOMR score when calculated using data abstracted from clinical notes that were available when patients were admitted to hospital. Secondary objectives included determining whether functional measures abstracted were significantly associated with death risk beyond the HOMR score and whether HOMR scores calculated from chart review deviated from those calculated from administrative data.
METHODS
Study Cohort
The study, which was approved by our local research ethics board, took place at the Ottawa Hospital, a 1000-bed teaching hospital that is the primary referral center in our region. We used the hospital admission registry to identify all people 18 years or older who were admitted to a nonpsychiatric service at our hospital between January 1, 2011 and December 31, 2011 (this time frame corresponds with the year used to derive the HOMR score). We excluded overnight patients in the same-day surgery or the bone-marrow transplant units (since they would not have been included in the original study) and those without a valid health card number (which was required to link to provincial data to identify outcomes). From this list, we randomly selected 5000 patients.
Primary Data Collection
For each patient, we retrieved all data required to calculate the HOMR score from the medical record (Table 1). Patient registration information in our electronic medical record was used to identify patient age, sex, admitting service, number of emergency department (ED) visits in the previous year, number of admissions in the previous year (the nursing triage note was reviewed for each admission to determine if it was by ambulance), and whether or not the patient had been discharged from hospital in the previous 30 days. The admitting service consult note was used to determine the admitting diagnosis and whether or not the patient was admitted directly to the intensive care unit. If they were present, the emergency nursing triage note, the ED record of treatment, the admission consult note, the pre-operative consult note, and consult notes were all used to determine the patient’s comorbidities, living status, and home oxygen status. Admission urgency was determined using information from the patient registration information and the ED nursing triage note. All data were abstracted from information that had been registered prior to when the patient was physically transferred to their hospital bed. This ensured that we used only data available at the start of the admission.
Patient functional status has been shown to be strongly associated with survival4 but HOMR only indirectly captures functional information (through the patient’s living status). We, therefore, collected more detailed functional information from the medical record by determining if the patient was dependent for any activities of daily living (ADL) from the emergency nursing triage note, the ED record of treatment, the admission consult note, and the pre-operative consultation. We also collected information that might indicate frailty, which we defined per Clegg et al.5 as “a state of increased vulnerability to poor resolution of homeostasis following a stress.” This information included: delirium or more than 1 fall recorded on the emergency nursing triage note, the ED record of treatment, or the admission consultation note; or whether a geriatric nursing specialist assessment occurred in the ED in the previous 6 months. Finally, we recorded possible indicators of limited social support (no fixed address [from patient registration and nursing triage note], primary contact is not a family member [from the emergency notes, consult, and patient registration], and no religion noted in system [from patient registration]). Patients for whom religion status was missing were classified as having “no religion.”
Analysis
These data were encrypted and linked anonymously to population-based databases to determine whether patients died within 1 year of admission to hospital. We calculated the chart-HOMR score using information from the chart review and determined its association with the outcome using bivariate logistic regression. We compared observed and expected risk of death within 1 year of admission to hospital for each chart-HOMR score value, with expected risks determined from the external validation study.3 We regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups to ensure adequate numbers in each group); and we gauged overall deviations from expected risk and the relationship between the observed and expected death risk (based on the chart-HOMR score) using the line’s intercept and slope, respectively.6 Next, we replicated methods from our studies2,3 to calculate the administrative-HOMR score in our study cohort using administrative databases. We compared these chart-HOMR and administrative-HOMR scores (and scores for each of its components). Finally, we determined which of the socio-functional factors were associated with 1-year death risk independent of the chart-HOMR score. We used the likelihood ratio test to determine whether these additional socio-functional factors significantly improved the model beyond the chart-HOMR score.7 This test subtracted the -2 logL value of the full model from that containing the chart-HOMR score alone, comparing its value to the χ2 distribution (with degrees of freedom equivalent to the number of additional parameters in the nested model) to determine statistical significance. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
There were 43,883 overnight hospitalizations at our hospital in 2011, and 38,886 hospitalizations were excluded: 1883 hospitalizations were in the same-day surgery or the bone-marrow transplant unit; 2485 did not have a valid health card number; 34,515 were not randomly selected; the records of 3 randomly selected patients had been blocked by our hospital’s privacy department; and 1 patient could not be linked with the population-based administrative datasets.
The 4996 study patients were middle-aged and predominantly female (Table 2). The extensive majority of patients was admitted from the community, was independent for ADL, had a family member as the principal contact, and had no admissions by ambulance in the previous year. Most people had no significant comorbidities or ED visits in the year prior to their admission. The mean chart-HOMR score was 22 (standard deviation [SD], 12), which is associated with a 1.2% expected risk of death within 1 year of hospital admission (Appendix 1).3
A total of 563 patients (11.3%) died within 1 year of admission to hospital (Table 2). In the study cohort, each chart-HOMR component was associated with death status. People who died were older, more likely to be male, had a greater number of important comorbidities, had more ED visits and admissions by ambulance in the previous year, and were more likely to have been discharged in the previous 30 days, and were admitted urgently, directly to the intensive care unit, or with complicated diagnoses. The mean chart-HOMR score differed extensively by survival status (37.4 [SD, 7.5] in those who died vs. 19.9 [SD, 12.2] in those who survived). Three of the socio-functional variables (delirium and falls noted on admission documents, and dependent for any ADL) also varied with death status.
The chart-HOMR score was strongly associated with the likelihood of death within 1 year of admission. When included in a logistic regression model having 1-year death as the outcome, a 1-point increase in the chart-HOMR score was associated with a 19% increase in the odds of death (P < 0.0001). This model (with only the chart-HOMR score) was highly discriminative (C statistic, 0.888) and well calibrated (Hosmer-Lemeshow test, 12.9 [8 df, P = 0.11]).
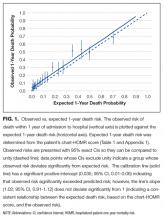
Observed and expected death risks by chart-HOMR score were similar (Figure 1). The observed total number of deaths (n = 563; 11.3%) exceeded the expected number of deaths (n = 437, 8.7%). When we regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups), the Hosmer-Lemeshow test was significant, indicating that differences between observed and expected risks were beyond that expected by chance (Hosmer-Lemeshow test, 141.9, 21 df, P < 0.0001). The intercept of this model (0.035; 95% CI, 0.01-0.06) was statistically significant (P = 0.01), indicating that the observed number of cases significantly exceeded the expected; however, its calibration slope (1.02; 95% CI, 0.89-1.16) did not deviate significantly from unity, indicating that the relationship between the observed and expected death risk (based on the chart-HOMR score) remained intact (Figure 1).
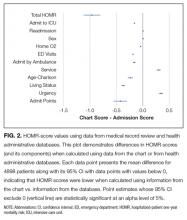
The deviations between observed and expected death risks reflected deviations between the c chart-HOMR score and the administrative-HOMR score, with the former being significantly lower than the latter (Figure 2). Overall, the chart-HOMR score was 0.96 points lower (95% CI, 0.81-1.12) than the administrative-HOMR score. The HOMR score components that were notably underestimated using chart data included those for the age-Charlson Comorbidity Index interaction, living status, and admit points. Points for only 2 components (admitting service and admission urgency) were higher when calculated using chart data.
Four additional socio-functional variables collected from medical record review were significantly associated with 1-year death risk independent of the chart-HOMR score (Table 3). Admission documentation noting either delirium or falls were both associated with a significantly increased death risk (adjusted odds ratio [OR], 1.92 [95% CI, 1.24-2.96] and OR 1.96 [95% CI, 1.29-2.99], respectively). An independently increased death risk was also noted in patients who were dependent for any ADL (adjusted OR, 1.99 [95% CI, 1.24-3.19]). The presence of an ED geriatrics consultation within the previous 6 months was associated with a significantly decreased death risk of 60% (adjusted OR, 0.40 [95% CI, 0.20-0.81]). Adding these covariates to the logistic model with the chart-HOMR score significantly improved predictions (likelihood ratio statistic = 33.569, 4df, P < 0.00001).
DISCUSSION
In a large random sample of patients from our hospital, we found that the HOMR score using data abstracted from the medical record was significantly associated with 1-year death risk. The expected death risk based on the chart-HOMR score underestimated observed death risk but the relationship between the chart-HOMR score and death risk was similar to that in studies using administrative data. The HOMR score calculated using data from the chart was lower than that calculated using data from population-based administrative datasets; additional variables indicating patient frailty were significantly associated with 1-year death risk independent of the chart-HOMR score. Since the HOMR score was derived and initially validated using health administrative data, this study using data abstracted from the health record shows that the HOMR score has methodological generalizability.8
We think that our study has several notable findings. First, we found that data abstracted from the medical record can be used to calculate the HOMR score to accurately predict individual death risk. The chart-HOMR score discriminated very well between patients who did and did not die (C statistic, 0.88), which extensively exceeds the discrimination of published death risk indices (whose C statistics range between 0.69 and 0.82). It is also possible that chart abstraction for the HOMR score—without functional status—is simpler than other indices since its components are primarily very objective. (Other indices for hospital-based patients required factors that could be difficult to abstract reliably from the medical record including meeting more than 1 guideline for noncancer hospice care9; ambulation difficulties10; scales such as the Exton-Smith Scale or the Short Portable Mental Status Questionnaire11; weight loss12; functional status4; and pressure sore risk.13) Although expected risks for the chart-HOMR consistently underestimated observed risks (Figure 1), the mean deviation was small (with an absolute difference of 3.5% that can be used as a correction factor when determining expected risks with HOMR scores calculated from chart review), but it was an association between the chart-HOMR score and death risk that remained consistent through the cohort. Second, we found a small but significant decrease in the chart-HOMR score vs. the administrative-HOMR score (Figure 2). Some of these underestimates such as those for the number of ED visits or admissions by ambulance were expected since population-based health administrative databases would best capture such data. However, we were surprised that the comorbidity score was less when calculated using chart vs. database data (Figure 2). This finding is distinct from studies finding that particular comorbidities are documented in the chart are sometimes not coded.14,15 However, we identified comorbidities in the administrative databases using a 1-year ‘look-back’ period so that diagnostic codes from multiple hospitalizations (and from multiple hospitals) could be used to calculate the Charlson Comorbidity Index for a particular patient; this has been shown to increase the capture of comorbidities.16 Third, we found that variables from the chart review indicating frailty were predictive of 1-year death risk independent of the chart-HOMR score (Table 2). This illustrates that mortality risk prediction can be improved for particular patient groups by adding new covariates to the HOMR. Further work is required to determine how to incorporate these (and possibly other) covariates into the HOMR to create a unique chart-HOMR score. Finally, we found that a geriatrics assessment in the ED was associated with a significant (and notable) decrease in death risk. With these data, we are unable to indicate whether this association is causative. However, these findings indicate that the influence of emergency geriatric assessments on patient survival needs to be explored in more detail.
Several issues about our study should be considered when interpreting its results. First, this was a single-center study and the generalizability of our results to other centers is unknown. However, our study had the largest sample size of all primary data prognostic index validation studies1 ensuring that our results are, at the very least, internally reliable. In addition, our simple random sample ensured that we studied a broad assortment of patients to be certain that our results are representative of our institution. Second, we used a single abstractor for the study, which could limit the generalizability of our results. However, almost all the data points that were abstracted for our study were very objective.
In summary, our study shows that the HOMR score can be used to accurately predict 1-year death risk using data abstracted from the patient record. These findings will aid in individual patient prognostication for clinicians and researchers.
Disclosure
The authors report no financial conflicts of interest.
1. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. PubMed
2. van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicts long term death risk in hospitalized patients. J Clin Epidemiol. 2014;67(9):1025-1034. PubMed
3. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ. 2015;187(10):725-733. PubMed
4. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
5. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. The Lancet 2002;381:752-762. PubMed
6. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692-1706. PubMed
7. Harrell FE Jr. Overview of Maximum Likelihood Estimation. Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001: 179-212.
8. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515-524. PubMed
9. Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285-292. PubMed
10. Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83. PubMed
11. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151-161. PubMed
12. Teno JM, Harrell FE Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 suppl):S16-S24. PubMed
13. Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783-791. PubMed
14. Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52(2):137-142. PubMed
15. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. PubMed
16. Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson cComorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128-1139. PubMed
A patient’s prognosis can strongly influence their medical care. Decisions about diagnostic modalities, treatment options, and the use of preventive therapies can all be affected by the likelihood of a patient’s death in the near future. For example, patients with severely limited survival might forego prophylactic therapy, avoid interventions for asymptomatic issues, and cease screening interventions. Knowing survival probability would also be very helpful as a controlling variable in research analyses whenever death risk might be a possible confounder.
Sixteen indices that aim to predict patient death risk have been described by Yourman et al.1 They were all created from secondary analyses of clinical and administrative datasets, were applicable to patients in a variety of settings (including the community, nursing home, or hospital), and predicted survival probabilities in time horizons ranging from 6 months to 5 years. Prognostic factors that were most commonly included in these indices were comorbidity and functional status. In validation populations, the discrimination of these indices for 1-year survival in hospitalized patients was moderate (with C statistics that ranged from 0.64 to 0.79) with good calibration for broad prognostic ranges.
In 2014, we published the Hospitalized-patient One-year Mortality Risk (HOMR) score.2 This study used health administrative data for all adult Ontarians admitted in 2011 to hospital under nonpsychiatric services (n = 640,022) to estimate the probability of dying within 1 year of admission to hospital (which happened in 11.7% of people). The HOMR score included 12 patient and hospitalization factors (Table 1). It was highly discriminative (C statistic, 0.923; [0.922-0.924]) and well calibrated (the mean relative difference between observed and expected death risk was 2.0% [range, 0.0% to 7.0%]). It was externally validated in more than 3 million adults from Ontario, Alberta, and Boston in whom the C statistic ranged from 0.89 to 0.92 and calibration was excellent.3 We concluded from these studies that the HOMR score is excellent for prognosticating a diverse group of patients using health administrative data.
However, we do not know whether the HOMR score can be applied to patients using primary data (ie, those taken directly from the chart). This question is important for 2 reasons. First, if HOMR accurately predicts death risk using data abstracted from the medical record, it could be used in the clinical setting to assist in clinical decision-making. Second, HOMR uses multiple administrative datasets that are difficult to access and use by most clinical researchers; it is, therefore, important to determine if HOMR is accurate for clinical research based on primary medical record review. The primary objective of this study was to determine the accuracy of the HOMR score when calculated using data abstracted from clinical notes that were available when patients were admitted to hospital. Secondary objectives included determining whether functional measures abstracted were significantly associated with death risk beyond the HOMR score and whether HOMR scores calculated from chart review deviated from those calculated from administrative data.
METHODS
Study Cohort
The study, which was approved by our local research ethics board, took place at the Ottawa Hospital, a 1000-bed teaching hospital that is the primary referral center in our region. We used the hospital admission registry to identify all people 18 years or older who were admitted to a nonpsychiatric service at our hospital between January 1, 2011 and December 31, 2011 (this time frame corresponds with the year used to derive the HOMR score). We excluded overnight patients in the same-day surgery or the bone-marrow transplant units (since they would not have been included in the original study) and those without a valid health card number (which was required to link to provincial data to identify outcomes). From this list, we randomly selected 5000 patients.
Primary Data Collection
For each patient, we retrieved all data required to calculate the HOMR score from the medical record (Table 1). Patient registration information in our electronic medical record was used to identify patient age, sex, admitting service, number of emergency department (ED) visits in the previous year, number of admissions in the previous year (the nursing triage note was reviewed for each admission to determine if it was by ambulance), and whether or not the patient had been discharged from hospital in the previous 30 days. The admitting service consult note was used to determine the admitting diagnosis and whether or not the patient was admitted directly to the intensive care unit. If they were present, the emergency nursing triage note, the ED record of treatment, the admission consult note, the pre-operative consult note, and consult notes were all used to determine the patient’s comorbidities, living status, and home oxygen status. Admission urgency was determined using information from the patient registration information and the ED nursing triage note. All data were abstracted from information that had been registered prior to when the patient was physically transferred to their hospital bed. This ensured that we used only data available at the start of the admission.
Patient functional status has been shown to be strongly associated with survival4 but HOMR only indirectly captures functional information (through the patient’s living status). We, therefore, collected more detailed functional information from the medical record by determining if the patient was dependent for any activities of daily living (ADL) from the emergency nursing triage note, the ED record of treatment, the admission consult note, and the pre-operative consultation. We also collected information that might indicate frailty, which we defined per Clegg et al.5 as “a state of increased vulnerability to poor resolution of homeostasis following a stress.” This information included: delirium or more than 1 fall recorded on the emergency nursing triage note, the ED record of treatment, or the admission consultation note; or whether a geriatric nursing specialist assessment occurred in the ED in the previous 6 months. Finally, we recorded possible indicators of limited social support (no fixed address [from patient registration and nursing triage note], primary contact is not a family member [from the emergency notes, consult, and patient registration], and no religion noted in system [from patient registration]). Patients for whom religion status was missing were classified as having “no religion.”
Analysis
These data were encrypted and linked anonymously to population-based databases to determine whether patients died within 1 year of admission to hospital. We calculated the chart-HOMR score using information from the chart review and determined its association with the outcome using bivariate logistic regression. We compared observed and expected risk of death within 1 year of admission to hospital for each chart-HOMR score value, with expected risks determined from the external validation study.3 We regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups to ensure adequate numbers in each group); and we gauged overall deviations from expected risk and the relationship between the observed and expected death risk (based on the chart-HOMR score) using the line’s intercept and slope, respectively.6 Next, we replicated methods from our studies2,3 to calculate the administrative-HOMR score in our study cohort using administrative databases. We compared these chart-HOMR and administrative-HOMR scores (and scores for each of its components). Finally, we determined which of the socio-functional factors were associated with 1-year death risk independent of the chart-HOMR score. We used the likelihood ratio test to determine whether these additional socio-functional factors significantly improved the model beyond the chart-HOMR score.7 This test subtracted the -2 logL value of the full model from that containing the chart-HOMR score alone, comparing its value to the χ2 distribution (with degrees of freedom equivalent to the number of additional parameters in the nested model) to determine statistical significance. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
There were 43,883 overnight hospitalizations at our hospital in 2011, and 38,886 hospitalizations were excluded: 1883 hospitalizations were in the same-day surgery or the bone-marrow transplant unit; 2485 did not have a valid health card number; 34,515 were not randomly selected; the records of 3 randomly selected patients had been blocked by our hospital’s privacy department; and 1 patient could not be linked with the population-based administrative datasets.
The 4996 study patients were middle-aged and predominantly female (Table 2). The extensive majority of patients was admitted from the community, was independent for ADL, had a family member as the principal contact, and had no admissions by ambulance in the previous year. Most people had no significant comorbidities or ED visits in the year prior to their admission. The mean chart-HOMR score was 22 (standard deviation [SD], 12), which is associated with a 1.2% expected risk of death within 1 year of hospital admission (Appendix 1).3
A total of 563 patients (11.3%) died within 1 year of admission to hospital (Table 2). In the study cohort, each chart-HOMR component was associated with death status. People who died were older, more likely to be male, had a greater number of important comorbidities, had more ED visits and admissions by ambulance in the previous year, and were more likely to have been discharged in the previous 30 days, and were admitted urgently, directly to the intensive care unit, or with complicated diagnoses. The mean chart-HOMR score differed extensively by survival status (37.4 [SD, 7.5] in those who died vs. 19.9 [SD, 12.2] in those who survived). Three of the socio-functional variables (delirium and falls noted on admission documents, and dependent for any ADL) also varied with death status.
The chart-HOMR score was strongly associated with the likelihood of death within 1 year of admission. When included in a logistic regression model having 1-year death as the outcome, a 1-point increase in the chart-HOMR score was associated with a 19% increase in the odds of death (P < 0.0001). This model (with only the chart-HOMR score) was highly discriminative (C statistic, 0.888) and well calibrated (Hosmer-Lemeshow test, 12.9 [8 df, P = 0.11]).
Observed and expected death risks by chart-HOMR score were similar (Figure 1). The observed total number of deaths (n = 563; 11.3%) exceeded the expected number of deaths (n = 437, 8.7%). When we regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups), the Hosmer-Lemeshow test was significant, indicating that differences between observed and expected risks were beyond that expected by chance (Hosmer-Lemeshow test, 141.9, 21 df, P < 0.0001). The intercept of this model (0.035; 95% CI, 0.01-0.06) was statistically significant (P = 0.01), indicating that the observed number of cases significantly exceeded the expected; however, its calibration slope (1.02; 95% CI, 0.89-1.16) did not deviate significantly from unity, indicating that the relationship between the observed and expected death risk (based on the chart-HOMR score) remained intact (Figure 1).
The deviations between observed and expected death risks reflected deviations between the c chart-HOMR score and the administrative-HOMR score, with the former being significantly lower than the latter (Figure 2). Overall, the chart-HOMR score was 0.96 points lower (95% CI, 0.81-1.12) than the administrative-HOMR score. The HOMR score components that were notably underestimated using chart data included those for the age-Charlson Comorbidity Index interaction, living status, and admit points. Points for only 2 components (admitting service and admission urgency) were higher when calculated using chart data.
Four additional socio-functional variables collected from medical record review were significantly associated with 1-year death risk independent of the chart-HOMR score (Table 3). Admission documentation noting either delirium or falls were both associated with a significantly increased death risk (adjusted odds ratio [OR], 1.92 [95% CI, 1.24-2.96] and OR 1.96 [95% CI, 1.29-2.99], respectively). An independently increased death risk was also noted in patients who were dependent for any ADL (adjusted OR, 1.99 [95% CI, 1.24-3.19]). The presence of an ED geriatrics consultation within the previous 6 months was associated with a significantly decreased death risk of 60% (adjusted OR, 0.40 [95% CI, 0.20-0.81]). Adding these covariates to the logistic model with the chart-HOMR score significantly improved predictions (likelihood ratio statistic = 33.569, 4df, P < 0.00001).
DISCUSSION
In a large random sample of patients from our hospital, we found that the HOMR score using data abstracted from the medical record was significantly associated with 1-year death risk. The expected death risk based on the chart-HOMR score underestimated observed death risk but the relationship between the chart-HOMR score and death risk was similar to that in studies using administrative data. The HOMR score calculated using data from the chart was lower than that calculated using data from population-based administrative datasets; additional variables indicating patient frailty were significantly associated with 1-year death risk independent of the chart-HOMR score. Since the HOMR score was derived and initially validated using health administrative data, this study using data abstracted from the health record shows that the HOMR score has methodological generalizability.8
We think that our study has several notable findings. First, we found that data abstracted from the medical record can be used to calculate the HOMR score to accurately predict individual death risk. The chart-HOMR score discriminated very well between patients who did and did not die (C statistic, 0.88), which extensively exceeds the discrimination of published death risk indices (whose C statistics range between 0.69 and 0.82). It is also possible that chart abstraction for the HOMR score—without functional status—is simpler than other indices since its components are primarily very objective. (Other indices for hospital-based patients required factors that could be difficult to abstract reliably from the medical record including meeting more than 1 guideline for noncancer hospice care9; ambulation difficulties10; scales such as the Exton-Smith Scale or the Short Portable Mental Status Questionnaire11; weight loss12; functional status4; and pressure sore risk.13) Although expected risks for the chart-HOMR consistently underestimated observed risks (Figure 1), the mean deviation was small (with an absolute difference of 3.5% that can be used as a correction factor when determining expected risks with HOMR scores calculated from chart review), but it was an association between the chart-HOMR score and death risk that remained consistent through the cohort. Second, we found a small but significant decrease in the chart-HOMR score vs. the administrative-HOMR score (Figure 2). Some of these underestimates such as those for the number of ED visits or admissions by ambulance were expected since population-based health administrative databases would best capture such data. However, we were surprised that the comorbidity score was less when calculated using chart vs. database data (Figure 2). This finding is distinct from studies finding that particular comorbidities are documented in the chart are sometimes not coded.14,15 However, we identified comorbidities in the administrative databases using a 1-year ‘look-back’ period so that diagnostic codes from multiple hospitalizations (and from multiple hospitals) could be used to calculate the Charlson Comorbidity Index for a particular patient; this has been shown to increase the capture of comorbidities.16 Third, we found that variables from the chart review indicating frailty were predictive of 1-year death risk independent of the chart-HOMR score (Table 2). This illustrates that mortality risk prediction can be improved for particular patient groups by adding new covariates to the HOMR. Further work is required to determine how to incorporate these (and possibly other) covariates into the HOMR to create a unique chart-HOMR score. Finally, we found that a geriatrics assessment in the ED was associated with a significant (and notable) decrease in death risk. With these data, we are unable to indicate whether this association is causative. However, these findings indicate that the influence of emergency geriatric assessments on patient survival needs to be explored in more detail.
Several issues about our study should be considered when interpreting its results. First, this was a single-center study and the generalizability of our results to other centers is unknown. However, our study had the largest sample size of all primary data prognostic index validation studies1 ensuring that our results are, at the very least, internally reliable. In addition, our simple random sample ensured that we studied a broad assortment of patients to be certain that our results are representative of our institution. Second, we used a single abstractor for the study, which could limit the generalizability of our results. However, almost all the data points that were abstracted for our study were very objective.
In summary, our study shows that the HOMR score can be used to accurately predict 1-year death risk using data abstracted from the patient record. These findings will aid in individual patient prognostication for clinicians and researchers.
Disclosure
The authors report no financial conflicts of interest.
A patient’s prognosis can strongly influence their medical care. Decisions about diagnostic modalities, treatment options, and the use of preventive therapies can all be affected by the likelihood of a patient’s death in the near future. For example, patients with severely limited survival might forego prophylactic therapy, avoid interventions for asymptomatic issues, and cease screening interventions. Knowing survival probability would also be very helpful as a controlling variable in research analyses whenever death risk might be a possible confounder.
Sixteen indices that aim to predict patient death risk have been described by Yourman et al.1 They were all created from secondary analyses of clinical and administrative datasets, were applicable to patients in a variety of settings (including the community, nursing home, or hospital), and predicted survival probabilities in time horizons ranging from 6 months to 5 years. Prognostic factors that were most commonly included in these indices were comorbidity and functional status. In validation populations, the discrimination of these indices for 1-year survival in hospitalized patients was moderate (with C statistics that ranged from 0.64 to 0.79) with good calibration for broad prognostic ranges.
In 2014, we published the Hospitalized-patient One-year Mortality Risk (HOMR) score.2 This study used health administrative data for all adult Ontarians admitted in 2011 to hospital under nonpsychiatric services (n = 640,022) to estimate the probability of dying within 1 year of admission to hospital (which happened in 11.7% of people). The HOMR score included 12 patient and hospitalization factors (Table 1). It was highly discriminative (C statistic, 0.923; [0.922-0.924]) and well calibrated (the mean relative difference between observed and expected death risk was 2.0% [range, 0.0% to 7.0%]). It was externally validated in more than 3 million adults from Ontario, Alberta, and Boston in whom the C statistic ranged from 0.89 to 0.92 and calibration was excellent.3 We concluded from these studies that the HOMR score is excellent for prognosticating a diverse group of patients using health administrative data.
However, we do not know whether the HOMR score can be applied to patients using primary data (ie, those taken directly from the chart). This question is important for 2 reasons. First, if HOMR accurately predicts death risk using data abstracted from the medical record, it could be used in the clinical setting to assist in clinical decision-making. Second, HOMR uses multiple administrative datasets that are difficult to access and use by most clinical researchers; it is, therefore, important to determine if HOMR is accurate for clinical research based on primary medical record review. The primary objective of this study was to determine the accuracy of the HOMR score when calculated using data abstracted from clinical notes that were available when patients were admitted to hospital. Secondary objectives included determining whether functional measures abstracted were significantly associated with death risk beyond the HOMR score and whether HOMR scores calculated from chart review deviated from those calculated from administrative data.
METHODS
Study Cohort
The study, which was approved by our local research ethics board, took place at the Ottawa Hospital, a 1000-bed teaching hospital that is the primary referral center in our region. We used the hospital admission registry to identify all people 18 years or older who were admitted to a nonpsychiatric service at our hospital between January 1, 2011 and December 31, 2011 (this time frame corresponds with the year used to derive the HOMR score). We excluded overnight patients in the same-day surgery or the bone-marrow transplant units (since they would not have been included in the original study) and those without a valid health card number (which was required to link to provincial data to identify outcomes). From this list, we randomly selected 5000 patients.
Primary Data Collection
For each patient, we retrieved all data required to calculate the HOMR score from the medical record (Table 1). Patient registration information in our electronic medical record was used to identify patient age, sex, admitting service, number of emergency department (ED) visits in the previous year, number of admissions in the previous year (the nursing triage note was reviewed for each admission to determine if it was by ambulance), and whether or not the patient had been discharged from hospital in the previous 30 days. The admitting service consult note was used to determine the admitting diagnosis and whether or not the patient was admitted directly to the intensive care unit. If they were present, the emergency nursing triage note, the ED record of treatment, the admission consult note, the pre-operative consult note, and consult notes were all used to determine the patient’s comorbidities, living status, and home oxygen status. Admission urgency was determined using information from the patient registration information and the ED nursing triage note. All data were abstracted from information that had been registered prior to when the patient was physically transferred to their hospital bed. This ensured that we used only data available at the start of the admission.
Patient functional status has been shown to be strongly associated with survival4 but HOMR only indirectly captures functional information (through the patient’s living status). We, therefore, collected more detailed functional information from the medical record by determining if the patient was dependent for any activities of daily living (ADL) from the emergency nursing triage note, the ED record of treatment, the admission consult note, and the pre-operative consultation. We also collected information that might indicate frailty, which we defined per Clegg et al.5 as “a state of increased vulnerability to poor resolution of homeostasis following a stress.” This information included: delirium or more than 1 fall recorded on the emergency nursing triage note, the ED record of treatment, or the admission consultation note; or whether a geriatric nursing specialist assessment occurred in the ED in the previous 6 months. Finally, we recorded possible indicators of limited social support (no fixed address [from patient registration and nursing triage note], primary contact is not a family member [from the emergency notes, consult, and patient registration], and no religion noted in system [from patient registration]). Patients for whom religion status was missing were classified as having “no religion.”
Analysis
These data were encrypted and linked anonymously to population-based databases to determine whether patients died within 1 year of admission to hospital. We calculated the chart-HOMR score using information from the chart review and determined its association with the outcome using bivariate logistic regression. We compared observed and expected risk of death within 1 year of admission to hospital for each chart-HOMR score value, with expected risks determined from the external validation study.3 We regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups to ensure adequate numbers in each group); and we gauged overall deviations from expected risk and the relationship between the observed and expected death risk (based on the chart-HOMR score) using the line’s intercept and slope, respectively.6 Next, we replicated methods from our studies2,3 to calculate the administrative-HOMR score in our study cohort using administrative databases. We compared these chart-HOMR and administrative-HOMR scores (and scores for each of its components). Finally, we determined which of the socio-functional factors were associated with 1-year death risk independent of the chart-HOMR score. We used the likelihood ratio test to determine whether these additional socio-functional factors significantly improved the model beyond the chart-HOMR score.7 This test subtracted the -2 logL value of the full model from that containing the chart-HOMR score alone, comparing its value to the χ2 distribution (with degrees of freedom equivalent to the number of additional parameters in the nested model) to determine statistical significance. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
There were 43,883 overnight hospitalizations at our hospital in 2011, and 38,886 hospitalizations were excluded: 1883 hospitalizations were in the same-day surgery or the bone-marrow transplant unit; 2485 did not have a valid health card number; 34,515 were not randomly selected; the records of 3 randomly selected patients had been blocked by our hospital’s privacy department; and 1 patient could not be linked with the population-based administrative datasets.
The 4996 study patients were middle-aged and predominantly female (Table 2). The extensive majority of patients was admitted from the community, was independent for ADL, had a family member as the principal contact, and had no admissions by ambulance in the previous year. Most people had no significant comorbidities or ED visits in the year prior to their admission. The mean chart-HOMR score was 22 (standard deviation [SD], 12), which is associated with a 1.2% expected risk of death within 1 year of hospital admission (Appendix 1).3
A total of 563 patients (11.3%) died within 1 year of admission to hospital (Table 2). In the study cohort, each chart-HOMR component was associated with death status. People who died were older, more likely to be male, had a greater number of important comorbidities, had more ED visits and admissions by ambulance in the previous year, and were more likely to have been discharged in the previous 30 days, and were admitted urgently, directly to the intensive care unit, or with complicated diagnoses. The mean chart-HOMR score differed extensively by survival status (37.4 [SD, 7.5] in those who died vs. 19.9 [SD, 12.2] in those who survived). Three of the socio-functional variables (delirium and falls noted on admission documents, and dependent for any ADL) also varied with death status.
The chart-HOMR score was strongly associated with the likelihood of death within 1 year of admission. When included in a logistic regression model having 1-year death as the outcome, a 1-point increase in the chart-HOMR score was associated with a 19% increase in the odds of death (P < 0.0001). This model (with only the chart-HOMR score) was highly discriminative (C statistic, 0.888) and well calibrated (Hosmer-Lemeshow test, 12.9 [8 df, P = 0.11]).
Observed and expected death risks by chart-HOMR score were similar (Figure 1). The observed total number of deaths (n = 563; 11.3%) exceeded the expected number of deaths (n = 437, 8.7%). When we regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups), the Hosmer-Lemeshow test was significant, indicating that differences between observed and expected risks were beyond that expected by chance (Hosmer-Lemeshow test, 141.9, 21 df, P < 0.0001). The intercept of this model (0.035; 95% CI, 0.01-0.06) was statistically significant (P = 0.01), indicating that the observed number of cases significantly exceeded the expected; however, its calibration slope (1.02; 95% CI, 0.89-1.16) did not deviate significantly from unity, indicating that the relationship between the observed and expected death risk (based on the chart-HOMR score) remained intact (Figure 1).
The deviations between observed and expected death risks reflected deviations between the c chart-HOMR score and the administrative-HOMR score, with the former being significantly lower than the latter (Figure 2). Overall, the chart-HOMR score was 0.96 points lower (95% CI, 0.81-1.12) than the administrative-HOMR score. The HOMR score components that were notably underestimated using chart data included those for the age-Charlson Comorbidity Index interaction, living status, and admit points. Points for only 2 components (admitting service and admission urgency) were higher when calculated using chart data.
Four additional socio-functional variables collected from medical record review were significantly associated with 1-year death risk independent of the chart-HOMR score (Table 3). Admission documentation noting either delirium or falls were both associated with a significantly increased death risk (adjusted odds ratio [OR], 1.92 [95% CI, 1.24-2.96] and OR 1.96 [95% CI, 1.29-2.99], respectively). An independently increased death risk was also noted in patients who were dependent for any ADL (adjusted OR, 1.99 [95% CI, 1.24-3.19]). The presence of an ED geriatrics consultation within the previous 6 months was associated with a significantly decreased death risk of 60% (adjusted OR, 0.40 [95% CI, 0.20-0.81]). Adding these covariates to the logistic model with the chart-HOMR score significantly improved predictions (likelihood ratio statistic = 33.569, 4df, P < 0.00001).
DISCUSSION
In a large random sample of patients from our hospital, we found that the HOMR score using data abstracted from the medical record was significantly associated with 1-year death risk. The expected death risk based on the chart-HOMR score underestimated observed death risk but the relationship between the chart-HOMR score and death risk was similar to that in studies using administrative data. The HOMR score calculated using data from the chart was lower than that calculated using data from population-based administrative datasets; additional variables indicating patient frailty were significantly associated with 1-year death risk independent of the chart-HOMR score. Since the HOMR score was derived and initially validated using health administrative data, this study using data abstracted from the health record shows that the HOMR score has methodological generalizability.8
We think that our study has several notable findings. First, we found that data abstracted from the medical record can be used to calculate the HOMR score to accurately predict individual death risk. The chart-HOMR score discriminated very well between patients who did and did not die (C statistic, 0.88), which extensively exceeds the discrimination of published death risk indices (whose C statistics range between 0.69 and 0.82). It is also possible that chart abstraction for the HOMR score—without functional status—is simpler than other indices since its components are primarily very objective. (Other indices for hospital-based patients required factors that could be difficult to abstract reliably from the medical record including meeting more than 1 guideline for noncancer hospice care9; ambulation difficulties10; scales such as the Exton-Smith Scale or the Short Portable Mental Status Questionnaire11; weight loss12; functional status4; and pressure sore risk.13) Although expected risks for the chart-HOMR consistently underestimated observed risks (Figure 1), the mean deviation was small (with an absolute difference of 3.5% that can be used as a correction factor when determining expected risks with HOMR scores calculated from chart review), but it was an association between the chart-HOMR score and death risk that remained consistent through the cohort. Second, we found a small but significant decrease in the chart-HOMR score vs. the administrative-HOMR score (Figure 2). Some of these underestimates such as those for the number of ED visits or admissions by ambulance were expected since population-based health administrative databases would best capture such data. However, we were surprised that the comorbidity score was less when calculated using chart vs. database data (Figure 2). This finding is distinct from studies finding that particular comorbidities are documented in the chart are sometimes not coded.14,15 However, we identified comorbidities in the administrative databases using a 1-year ‘look-back’ period so that diagnostic codes from multiple hospitalizations (and from multiple hospitals) could be used to calculate the Charlson Comorbidity Index for a particular patient; this has been shown to increase the capture of comorbidities.16 Third, we found that variables from the chart review indicating frailty were predictive of 1-year death risk independent of the chart-HOMR score (Table 2). This illustrates that mortality risk prediction can be improved for particular patient groups by adding new covariates to the HOMR. Further work is required to determine how to incorporate these (and possibly other) covariates into the HOMR to create a unique chart-HOMR score. Finally, we found that a geriatrics assessment in the ED was associated with a significant (and notable) decrease in death risk. With these data, we are unable to indicate whether this association is causative. However, these findings indicate that the influence of emergency geriatric assessments on patient survival needs to be explored in more detail.
Several issues about our study should be considered when interpreting its results. First, this was a single-center study and the generalizability of our results to other centers is unknown. However, our study had the largest sample size of all primary data prognostic index validation studies1 ensuring that our results are, at the very least, internally reliable. In addition, our simple random sample ensured that we studied a broad assortment of patients to be certain that our results are representative of our institution. Second, we used a single abstractor for the study, which could limit the generalizability of our results. However, almost all the data points that were abstracted for our study were very objective.
In summary, our study shows that the HOMR score can be used to accurately predict 1-year death risk using data abstracted from the patient record. These findings will aid in individual patient prognostication for clinicians and researchers.
Disclosure
The authors report no financial conflicts of interest.
1. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. PubMed
2. van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicts long term death risk in hospitalized patients. J Clin Epidemiol. 2014;67(9):1025-1034. PubMed
3. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ. 2015;187(10):725-733. PubMed
4. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
5. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. The Lancet 2002;381:752-762. PubMed
6. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692-1706. PubMed
7. Harrell FE Jr. Overview of Maximum Likelihood Estimation. Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001: 179-212.
8. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515-524. PubMed
9. Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285-292. PubMed
10. Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83. PubMed
11. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151-161. PubMed
12. Teno JM, Harrell FE Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 suppl):S16-S24. PubMed
13. Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783-791. PubMed
14. Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52(2):137-142. PubMed
15. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. PubMed
16. Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson cComorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128-1139. PubMed
1. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. PubMed
2. van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicts long term death risk in hospitalized patients. J Clin Epidemiol. 2014;67(9):1025-1034. PubMed
3. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ. 2015;187(10):725-733. PubMed
4. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
5. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. The Lancet 2002;381:752-762. PubMed
6. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692-1706. PubMed
7. Harrell FE Jr. Overview of Maximum Likelihood Estimation. Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001: 179-212.
8. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515-524. PubMed
9. Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285-292. PubMed
10. Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83. PubMed
11. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151-161. PubMed
12. Teno JM, Harrell FE Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 suppl):S16-S24. PubMed
13. Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783-791. PubMed
14. Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52(2):137-142. PubMed
15. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. PubMed
16. Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson cComorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128-1139. PubMed
© 2017 Society of Hospital Medicine
Predicting 30-day pneumonia readmissions using electronic health record data
Pneumonia is a leading cause of hospitalizations in the U.S., accounting for more than 1.1 million discharges annually.1 Pneumonia is frequently complicated by hospital readmission, which is costly and potentially avoidable.2,3 Due to financial penalties imposed on hospitals for higher than expected 30-day readmission rates, there is increasing attention to implementing interventions to reduce readmissions in this population.4,5 However, because these programs are resource-intensive, interventions are thought to be most cost-effective if they are targeted to high-risk individuals who are most likely to benefit.6-8
Current pneumonia-specific readmission risk-prediction models that could enable identification of high-risk patients suffer from poor predictive ability, greatly limiting their use, and most were validated among older adults or by using data from single academic medical centers, limiting their generalizability.9-14 A potential reason for poor predictive accuracy is the omission of known robust clinical predictors of pneumonia-related outcomes, including pneumonia severity of illness and stability on discharge.15-17 Approaches using electronic health record (EHR) data, which include this clinically granular data, could enable hospitals to more accurately and pragmatically identify high-risk patients during the index hospitalization and enable interventions to be initiated prior to discharge.
An alternative strategy to identifying high-risk patients for readmission is to use a multi-condition risk-prediction model. Developing and implementing models for every condition may be time-consuming and costly. We have derived and validated 2 multi-condition risk-prediction models using EHR data—1 using data from the first day of hospital admission (‘first-day’ model), and the second incorporating data from the entire hospitalization (‘full-stay’ model) to reflect in-hospital complications and clinical stability at discharge.18,19 However, it is unknown if a multi-condition model for pneumonia would perform as well as a disease-specific model.
This study aimed to develop 2 EHR-based pneumonia-specific readmission risk-prediction models using data routinely collected in clinical practice—a ‘first-day’ and a ‘full-stay’ model—and compare the performance of each model to: 1) one another; 2) the corresponding multi-condition EHR model; and 3) to other potentially useful models in predicting pneumonia readmissions (the Centers for Medicare and Medicaid Services [CMS] pneumonia model, and 2 commonly used pneumonia severity of illness scores validated for predicting mortality). We hypothesized that the pneumonia-specific EHR models would outperform other models; and the full-stay pneumonia-specific model would outperform the first-day pneumonia-specific model.
METHODS
Study Design, Population, and Data Sources
We conducted an observational study using EHR data collected from 6 hospitals (including safety net, community, teaching, and nonteaching hospitals) in north Texas between November 2009 and October 2010, All hospitals used the Epic EHR (Epic Systems Corporation, Verona, WI). Details of this cohort have been published.18,19
We included consecutive hospitalizations among adults 18 years and older discharged from any medicine service with principal discharge diagnoses of pneumonia (ICD-9-CM codes 480-483, 485, 486-487), sepsis (ICD-9-CM codes 038, 995.91, 995.92, 785.52), or respiratory failure (ICD-9-CM codes 518.81, 518.82, 518.84, 799.1) when the latter 2 were also accompanied by a secondary diagnosis of pneumonia.20 For individuals with multiple hospitalizations during the study period, we included only the first hospitalization. We excluded individuals who died during the index hospitalization or within 30 days of discharge, were transferred to another acute care facility, or left against medical advice.
Outcomes
The primary outcome was all-cause 30-day readmission, defined as a nonelective hospitalization within 30 days of discharge to any of 75 acute care hospitals within a 100-mile radius of Dallas, ascertained from an all-payer regional hospitalization database.
Predictor Variables for the Pneumonia-Specific Readmission Models
The selection of candidate predictors was informed by our validated multi-condition risk-prediction models using EHR data available within 24 hours of admission (‘first-day’ multi-condition EHR model) or during the entire hospitalization (‘full-stay’ multi-condition EHR model).18,19 For the pneumonia-specific models, we included all variables in our published multi-condition models as candidate predictors, including sociodemographics, prior utilization, Charlson Comorbidity Index, select laboratory and vital sign abnormalities, length of stay, hospital complications (eg, venous thromboembolism), vital sign instabilities, and disposition status (see Supplemental Table 1 for complete list of variables). We also assessed additional variables specific to pneumonia for inclusion that were: (1) available in the EHR of all participating hospitals; (2) routinely collected or available at the time of admission or discharge; and (3) plausible predictors of adverse outcomes based on literature and clinical expertise. These included select comorbidities (eg, psychiatric conditions, chronic lung disease, history of pneumonia),10,11,21,22 the pneumonia severity index (PSI),16,23,24 intensive care unit stay, and receipt of invasive or noninvasive ventilation. We used a modified PSI score because certain data elements were missing. The modified PSI (henceforth referred to as PSI) did not include nursing home residence and included diagnostic codes as proxies for the presence of pleural effusion (ICD-9-CM codes 510, 511.1, and 511.9) and altered mental status (ICD-9-CM codes 780.0X, 780.97, 293.0, 293.1, and 348.3X).
Statistical Analysis
Model Derivation. Candidate predictor variables were classified as available in the EHR within 24 hours of admission and/or at the time of discharge. For example, socioeconomic factors could be ascertained within the first day of hospitalization, whereas length of stay would not be available until the day of discharge. Predictors with missing values were assumed to be normal (less than 1% missing for each variable). Univariate relationships between readmission and each candidate predictor were assessed in the overall cohort using a pre-specified significance threshold of P ≤ 0.10. Significant variables were entered in the respective first-day and full-stay pneumonia-specific multivariable logistic regression models using stepwise-backward selection with a pre-specified significance threshold of P ≤ 0.05. In sensitivity analyses, we alternately derived our models using stepwise-forward selection, as well as stepwise-backward selection minimizing the Bayesian information criterion and Akaike information criterion separately. These alternate modeling strategies yielded identical predictors to our final models.
Model Validation. Model validation was performed using 5-fold cross-validation, with the overall cohort randomly divided into 5 equal-size subsets.25 For each cycle, 4 subsets were used for training to estimate model coefficients, and the fifth subset was used for validation. This cycle was repeated 5 times with each randomly-divided subset used once as the validation set. We repeated this entire process 50 times and averaged the C statistic estimates to derive an optimism-corrected C statistic. Model calibration was assessed qualitatively by comparing predicted to observed probabilities of readmission by quintiles of predicted risk, and with the Hosmer-Lemeshow goodness-of-fit test.
Comparison to Other Models. The main comparisons of the first-day and full-stay pneumonia-specific EHR model performance were to each other and the corresponding multi-condition EHR model.18,19 The multi-condition EHR models were separately derived and validated within the larger parent cohort from which this study cohort was derived, and outperformed the CMS all-cause model, the HOSPITAL model, and the LACE index.19 To further triangulate our findings, given the lack of other rigorously validated pneumonia-specific risk-prediction models for readmission,14 we compared the pneumonia-specific EHR models to the CMS pneumonia model derived from administrative claims data,10 and 2 commonly used risk-prediction scores for short-term mortality among patients with community-acquired pneumonia, the PSI and CURB-65 scores.16 Although derived and validated using patient-level data, the CMS model was developed to benchmark hospitals according to hospital-level readmission rates.10 The CURB-65 score in this study was also modified to include the same altered mental status diagnostic codes according to the modified PSI as a proxy for “confusion.” Both the PSI and CURB-65 scores were calculated using the most abnormal values within the first 24 hours of admission. The ‘updated’ PSI and the ‘updated’ CURB-65 were calculated using the most abnormal values within 24 hours prior to discharge, or the last known observation prior to discharge if no results were recorded within this time period. A complete list of variables for each of the comparison models are shown in Supplemental Table 1.
We assessed model performance by calculating the C statistic, integrated discrimination index, and net reclassification index (NRI) compared to our pneumonia-specific models. The integrated discrimination index is the difference in the mean predicted probability of readmission between patients who were and were not actually readmitted between 2 models, where more positive values suggest improvement in model performance compared to a reference model.26 The NRI is defined as the sum of the net proportions of correctly reclassified persons with and without the event of interest.27 Here, we calculated a category-based NRI to evaluate the performance of pneumonia-specific models in correctly classifying individuals with and without readmissions into the 2 highest readmission risk quintiles vs the lowest 3 risk quintiles compared to other models.27 This pre-specified cutoff is relevant for hospitals interested in identifying the highest risk individuals for targeted intervention.7 Finally, we assessed calibration of comparator models in our cohort by comparing predicted probability to observed probability of readmission by quintiles of risk for each model. We conducted all analyses using Stata 12.1 (StataCorp, College Station, Texas). This study was approved by the University of Texas Southwestern Medical Center Institutional Review Board.
RESULTS
Of 1463 index hospitalizations (Supplemental Figure 1), the 30-day all-cause readmission rate was 13.6%. Individuals with a 30-day readmission had markedly different sociodemographic and clinical characteristics compared to those not readmitted (Table 1; see Supplemental Table 2 for additional clinical characteristics).
Derivation, Validation, and Performance of the Pneumonia-Specific Readmission Risk-Prediction Models
The final first-day pneumonia-specific EHR model included 7 variables, including sociodemographic characteristics; prior hospitalizations; thrombocytosis, and PSI (Table 2). The first-day pneumonia-specific model had adequate discrimination (C statistic, 0.695; optimism-corrected C statistic 0.675, 95% confidence interval [CI], 0.667-0.685; Table 3). It also effectively stratified individuals across a broad range of risk (average predicted decile of risk ranged from 4% to 33%; Table 3) and was well calibrated (Supplemental Table 3).
The final full-stay pneumonia-specific EHR readmission model included 8 predictors, including 3 variables from the first-day model (median income, thrombocytosis, and prior hospitalizations; Table 2). The full-stay pneumonia-specific EHR model also included vital sign instabilities on discharge, updated PSI, and disposition status (ie, being discharged with home health or to a post-acute care facility was associated with greater odds of readmission, and hospice with lower odds). The full-stay pneumonia-specific EHR model had good discrimination (C statistic, 0.731; optimism-corrected C statistic, 0.714; 95% CI, 0.706-0.720), and stratified individuals across a broad range of risk (average predicted decile of risk ranged from 3% to 37%; Table 3), and was also well calibrated (Supplemental Table 3).
First-Day Pneumonia-Specific EHR Model vs First-Day Multi-Condition EHR Model
The first-day pneumonia-specific EHR model outperformed the first-day multi-condition EHR model with better discrimination (P = 0.029) and more correctly classified individuals in the top 2 highest risk quintiles vs the bottom 3 risk quintiles (Table 3, Supplemental Table 4, and Supplemental Figure 2A). With respect to calibration, the first-day multi-condition EHR model overestimated risk among the highest quintile risk group compared to the first-day pneumonia-specific EHR model (Figure 1A, 1B).
Full-Stay Pneumonia-Specific EHR Model vs Other Models
The full-stay pneumonia-specific EHR model comparatively outperformed the corresponding full-stay multi-condition EHR model, as well as the first-day pneumonia-specific EHR model, the CMS pneumonia model, the updated PSI, and the updated CURB-65 (Table 3, Supplemental Table 5, Supplemental Table 6, and Supplemental Figures 2B and 2C). Compared to the full-stay multi-condition and first-day pneumonia-specific EHR models, the full-stay pneumonia-specific EHR model had better discrimination, better reclassification (NRI, 0.09 and 0.08, respectively), and was able to stratify individuals across a broader range of readmission risk (Table 3). It also had better calibration in the highest quintile risk group compared to the full-stay multi-condition EHR model (Figure 1C and 1D).
Updated vs First-Day Modified PSI and CURB-65 Scores
The updated PSI was more strongly predictive of readmission than the PSI calculated on the day of admission (Wald test, 9.83; P = 0.002). Each 10-point increase in the updated PSI was associated with a 22% increased odds of readmission vs an 11% increase for the PSI calculated upon admission (Table 2). The improved predictive ability of the updated PSI and CURB-65 scores was also reflected in the superior discrimination and calibration vs the respective first-day pneumonia severity of illness scores (Table 3).
DISCUSSION
Using routinely available EHR data from 6 diverse hospitals, we developed 2 pneumonia-specific readmission risk-prediction models that aimed to allow hospitals to identify patients hospitalized with pneumonia at high risk for readmission. Overall, we found that a pneumonia-specific model using EHR data from the entire hospitalization outperformed all other models—including the first-day pneumonia-specific model using data present only on admission, our own multi-condition EHR models, and the CMS pneumonia model based on administrative claims data—in all aspects of model performance (discrimination, calibration, and reclassification). We found that socioeconomic status, prior hospitalizations, thrombocytosis, and measures of clinical severity and stability were important predictors of 30-day all-cause readmissions among patients hospitalized with pneumonia. Additionally, an updated discharge PSI score was a stronger independent predictor of readmissions compared to the PSI score calculated upon admission; and inclusion of the updated PSI in our full-stay pneumonia model led to improved prediction of 30-day readmissions.
The marked improvement in performance of the full-stay pneumonia-specific EHR model compared to the first-day pneumonia-specific model suggests that clinical stability and trajectory during hospitalization (as modeled through disposition status, updated PSI, and vital sign instabilities at discharge) are important predictors of 30-day readmission among patients hospitalized for pneumonia, which was not the case for our EHR-based multi-condition models.19 With the inclusion of these measures, the full-stay pneumonia-specific model correctly reclassified an additional 8% of patients according to their true risk compared to the first-day pneumonia-specific model. One implication of these findings is that hospitals interested in targeting their highest risk individuals with pneumonia for transitional care interventions could do so using the first-day pneumonia-specific EHR model and could refine their targeted strategy at the time of discharge by using the full-stay pneumonia model. This staged risk-prediction strategy would enable hospitals to initiate transitional care interventions for high-risk individuals in the inpatient setting (ie, patient education).7 Then, hospitals could enroll both persistent and newly identified high-risk individuals for outpatient interventions (ie, follow-up telephone call) in the immediate post-discharge period, an interval characterized by heightened vulnerability for adverse events,28 based on patients’ illness severity and stability at discharge. This approach can be implemented by hospitals by building these risk-prediction models directly into the EHR, or by extracting EHR data in near real time as our group has done successfully for heart failure.7
Another key implication of our study is that, for pneumonia, a disease-specific modeling approach has better predictive ability than using a multi-condition model. Compared to multi-condition models, the first-day and full-stay pneumonia-specific EHR models correctly reclassified an additional 6% and 9% of patients, respectively. Thus, hospitals interested in identifying the highest risk patients with pneumonia for targeted interventions should do so using the disease-specific models, if the costs and resources of doing so are within reach of the healthcare system.
An additional novel finding of our study is the added value of an updated PSI for predicting adverse events. Studies of pneumonia severity of illness scores have calculated the PSI and CURB-65 scores using data present only on admission.16,24 While our study also confirms that the PSI calculated upon admission is a significant predictor of readmission,23,29 this study extends this work by showing that an updated PSI score calculated at the time of discharge is an even stronger predictor for readmission, and its inclusion in the model significantly improves risk stratification and prognostication.
Our study was noteworthy for several strengths. First, we used data from a common EHR system, thus potentially allowing for the implementation of the pneumonia-specific models in real time across a number of hospitals. The use of routinely collected data for risk-prediction modeling makes this approach scalable and sustainable, because it obviates the need for burdensome data collection and entry. Second, to our knowledge, this is the first study to measure the additive influence of illness severity and stability at discharge on the readmission risk among patients hospitalized with pneumonia. Third, our study population was derived from 6 hospitals diverse in payer status, age, race/ethnicity, and socioeconomic status. Fourth, our models are less likely to be overfit to the idiosyncrasies of our data given that several predictors included in our final pneumonia-specific models have been associated with readmission in this population, including marital status,13,30 income,11,31 prior hospitalizations,11,13 thrombocytosis,32-34 and vital sign instabilities on discharge.17 Lastly, the discrimination of the CMS pneumonia model in our cohort (C statistic, 0.64) closely matched the discrimination observed in 4 independent cohorts (C statistic, 0.63), suggesting adequate generalizability of our study setting and population.10,12
Our results should be interpreted in the context of several limitations. First, generalizability to other regions beyond north Texas is unknown. Second, although we included a diverse cohort of safety net, community, teaching, and nonteaching hospitals, the pneumonia-specific models were not externally validated in a separate cohort, which may lead to more optimistic estimates of model performance. Third, PSI and CURB-65 scores were modified to use diagnostic codes for altered mental status and pleural effusion, and omitted nursing home residence. Thus, the independent associations for the PSI and CURB-65 scores and their predictive ability are likely attenuated. Fourth, we were unable to include data on medications (antibiotics and steroid use) and outpatient visits, which may influence readmission risk.2,9,13,35-40 Fifth, we included only the first pneumonia hospitalization per patient in this study. Had we included multiple hospitalizations per patient, we anticipate better model performance for the 2 pneumonia-specific EHR models since prior hospitalization was a robust predictor of readmission.
In conclusion, the full-stay pneumonia-specific EHR readmission risk-prediction model outperformed the first-day pneumonia-specific model, multi-condition EHR models, and the CMS pneumonia model. This suggests that: measures of clinical severity and stability at the time of discharge are important predictors for identifying patients at highest risk for readmission; and that EHR data routinely collected for clinical practice can be used to accurately predict risk of readmission among patients hospitalized for pneumonia.
Acknowledgments
The authors would like to acknowledge Ruben Amarasingham, MD, MBA, president and chief executive officer of Parkland Center for Clinical Innovation, and Ferdinand Velasco, MD, chief health information officer at Texas Health Resources for their assistance in assembling the 6-hospital cohort used in this study.
Disclosures
This work was supported by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24 HS022418-01); the Commonwealth Foundation (#20100323); the UT Southwestern KL2 Scholars Program supported by the National Institutes of Health (KL2 TR001103 to ANM and OKN); and the National Center for Advancing Translational Sciences at the National Institute of Health (U54 RFA-TR-12-006 to E.A.H.). The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no financial conflicts of interest to disclose
1. Centers for Disease Control and Prevention. Pneumonia. http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed January 26, 2016.
33. Prina E, Ferrer M, Ranzani OT, et al. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest. 2013;143(3):767-775. PubMed
34. Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. J Thromb Haemost. 2014;12(9):1391-1400. PubMed
35. Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441-1447. PubMed
36. Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post-discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565-571. PubMed
37. Spatz ES, Sheth SD, Gosch KL, et al. Usual source of care and outcomes following acute myocardial infarction. J Gen Intern Med. 2014;29(6):862-869. PubMed
38. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828. PubMed
39. Adamuz J, Viasus D, Campreciós-Rodriguez P, et al. A prospective cohort study of healthcare visits and rehospitalizations after discharge of patients with community-acquired pneumonia. Respirology. 2011;16(7):1119-1126. PubMed
40. Shorr AF, Zilberberg MD, Reichley R, et al. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin Infect Dis. 2013;57(3):362-367. PubMed
Pneumonia is a leading cause of hospitalizations in the U.S., accounting for more than 1.1 million discharges annually.1 Pneumonia is frequently complicated by hospital readmission, which is costly and potentially avoidable.2,3 Due to financial penalties imposed on hospitals for higher than expected 30-day readmission rates, there is increasing attention to implementing interventions to reduce readmissions in this population.4,5 However, because these programs are resource-intensive, interventions are thought to be most cost-effective if they are targeted to high-risk individuals who are most likely to benefit.6-8
Current pneumonia-specific readmission risk-prediction models that could enable identification of high-risk patients suffer from poor predictive ability, greatly limiting their use, and most were validated among older adults or by using data from single academic medical centers, limiting their generalizability.9-14 A potential reason for poor predictive accuracy is the omission of known robust clinical predictors of pneumonia-related outcomes, including pneumonia severity of illness and stability on discharge.15-17 Approaches using electronic health record (EHR) data, which include this clinically granular data, could enable hospitals to more accurately and pragmatically identify high-risk patients during the index hospitalization and enable interventions to be initiated prior to discharge.
An alternative strategy to identifying high-risk patients for readmission is to use a multi-condition risk-prediction model. Developing and implementing models for every condition may be time-consuming and costly. We have derived and validated 2 multi-condition risk-prediction models using EHR data—1 using data from the first day of hospital admission (‘first-day’ model), and the second incorporating data from the entire hospitalization (‘full-stay’ model) to reflect in-hospital complications and clinical stability at discharge.18,19 However, it is unknown if a multi-condition model for pneumonia would perform as well as a disease-specific model.
This study aimed to develop 2 EHR-based pneumonia-specific readmission risk-prediction models using data routinely collected in clinical practice—a ‘first-day’ and a ‘full-stay’ model—and compare the performance of each model to: 1) one another; 2) the corresponding multi-condition EHR model; and 3) to other potentially useful models in predicting pneumonia readmissions (the Centers for Medicare and Medicaid Services [CMS] pneumonia model, and 2 commonly used pneumonia severity of illness scores validated for predicting mortality). We hypothesized that the pneumonia-specific EHR models would outperform other models; and the full-stay pneumonia-specific model would outperform the first-day pneumonia-specific model.
METHODS
Study Design, Population, and Data Sources
We conducted an observational study using EHR data collected from 6 hospitals (including safety net, community, teaching, and nonteaching hospitals) in north Texas between November 2009 and October 2010, All hospitals used the Epic EHR (Epic Systems Corporation, Verona, WI). Details of this cohort have been published.18,19
We included consecutive hospitalizations among adults 18 years and older discharged from any medicine service with principal discharge diagnoses of pneumonia (ICD-9-CM codes 480-483, 485, 486-487), sepsis (ICD-9-CM codes 038, 995.91, 995.92, 785.52), or respiratory failure (ICD-9-CM codes 518.81, 518.82, 518.84, 799.1) when the latter 2 were also accompanied by a secondary diagnosis of pneumonia.20 For individuals with multiple hospitalizations during the study period, we included only the first hospitalization. We excluded individuals who died during the index hospitalization or within 30 days of discharge, were transferred to another acute care facility, or left against medical advice.
Outcomes
The primary outcome was all-cause 30-day readmission, defined as a nonelective hospitalization within 30 days of discharge to any of 75 acute care hospitals within a 100-mile radius of Dallas, ascertained from an all-payer regional hospitalization database.
Predictor Variables for the Pneumonia-Specific Readmission Models
The selection of candidate predictors was informed by our validated multi-condition risk-prediction models using EHR data available within 24 hours of admission (‘first-day’ multi-condition EHR model) or during the entire hospitalization (‘full-stay’ multi-condition EHR model).18,19 For the pneumonia-specific models, we included all variables in our published multi-condition models as candidate predictors, including sociodemographics, prior utilization, Charlson Comorbidity Index, select laboratory and vital sign abnormalities, length of stay, hospital complications (eg, venous thromboembolism), vital sign instabilities, and disposition status (see Supplemental Table 1 for complete list of variables). We also assessed additional variables specific to pneumonia for inclusion that were: (1) available in the EHR of all participating hospitals; (2) routinely collected or available at the time of admission or discharge; and (3) plausible predictors of adverse outcomes based on literature and clinical expertise. These included select comorbidities (eg, psychiatric conditions, chronic lung disease, history of pneumonia),10,11,21,22 the pneumonia severity index (PSI),16,23,24 intensive care unit stay, and receipt of invasive or noninvasive ventilation. We used a modified PSI score because certain data elements were missing. The modified PSI (henceforth referred to as PSI) did not include nursing home residence and included diagnostic codes as proxies for the presence of pleural effusion (ICD-9-CM codes 510, 511.1, and 511.9) and altered mental status (ICD-9-CM codes 780.0X, 780.97, 293.0, 293.1, and 348.3X).
Statistical Analysis
Model Derivation. Candidate predictor variables were classified as available in the EHR within 24 hours of admission and/or at the time of discharge. For example, socioeconomic factors could be ascertained within the first day of hospitalization, whereas length of stay would not be available until the day of discharge. Predictors with missing values were assumed to be normal (less than 1% missing for each variable). Univariate relationships between readmission and each candidate predictor were assessed in the overall cohort using a pre-specified significance threshold of P ≤ 0.10. Significant variables were entered in the respective first-day and full-stay pneumonia-specific multivariable logistic regression models using stepwise-backward selection with a pre-specified significance threshold of P ≤ 0.05. In sensitivity analyses, we alternately derived our models using stepwise-forward selection, as well as stepwise-backward selection minimizing the Bayesian information criterion and Akaike information criterion separately. These alternate modeling strategies yielded identical predictors to our final models.
Model Validation. Model validation was performed using 5-fold cross-validation, with the overall cohort randomly divided into 5 equal-size subsets.25 For each cycle, 4 subsets were used for training to estimate model coefficients, and the fifth subset was used for validation. This cycle was repeated 5 times with each randomly-divided subset used once as the validation set. We repeated this entire process 50 times and averaged the C statistic estimates to derive an optimism-corrected C statistic. Model calibration was assessed qualitatively by comparing predicted to observed probabilities of readmission by quintiles of predicted risk, and with the Hosmer-Lemeshow goodness-of-fit test.
Comparison to Other Models. The main comparisons of the first-day and full-stay pneumonia-specific EHR model performance were to each other and the corresponding multi-condition EHR model.18,19 The multi-condition EHR models were separately derived and validated within the larger parent cohort from which this study cohort was derived, and outperformed the CMS all-cause model, the HOSPITAL model, and the LACE index.19 To further triangulate our findings, given the lack of other rigorously validated pneumonia-specific risk-prediction models for readmission,14 we compared the pneumonia-specific EHR models to the CMS pneumonia model derived from administrative claims data,10 and 2 commonly used risk-prediction scores for short-term mortality among patients with community-acquired pneumonia, the PSI and CURB-65 scores.16 Although derived and validated using patient-level data, the CMS model was developed to benchmark hospitals according to hospital-level readmission rates.10 The CURB-65 score in this study was also modified to include the same altered mental status diagnostic codes according to the modified PSI as a proxy for “confusion.” Both the PSI and CURB-65 scores were calculated using the most abnormal values within the first 24 hours of admission. The ‘updated’ PSI and the ‘updated’ CURB-65 were calculated using the most abnormal values within 24 hours prior to discharge, or the last known observation prior to discharge if no results were recorded within this time period. A complete list of variables for each of the comparison models are shown in Supplemental Table 1.
We assessed model performance by calculating the C statistic, integrated discrimination index, and net reclassification index (NRI) compared to our pneumonia-specific models. The integrated discrimination index is the difference in the mean predicted probability of readmission between patients who were and were not actually readmitted between 2 models, where more positive values suggest improvement in model performance compared to a reference model.26 The NRI is defined as the sum of the net proportions of correctly reclassified persons with and without the event of interest.27 Here, we calculated a category-based NRI to evaluate the performance of pneumonia-specific models in correctly classifying individuals with and without readmissions into the 2 highest readmission risk quintiles vs the lowest 3 risk quintiles compared to other models.27 This pre-specified cutoff is relevant for hospitals interested in identifying the highest risk individuals for targeted intervention.7 Finally, we assessed calibration of comparator models in our cohort by comparing predicted probability to observed probability of readmission by quintiles of risk for each model. We conducted all analyses using Stata 12.1 (StataCorp, College Station, Texas). This study was approved by the University of Texas Southwestern Medical Center Institutional Review Board.
RESULTS
Of 1463 index hospitalizations (Supplemental Figure 1), the 30-day all-cause readmission rate was 13.6%. Individuals with a 30-day readmission had markedly different sociodemographic and clinical characteristics compared to those not readmitted (Table 1; see Supplemental Table 2 for additional clinical characteristics).
Derivation, Validation, and Performance of the Pneumonia-Specific Readmission Risk-Prediction Models
The final first-day pneumonia-specific EHR model included 7 variables, including sociodemographic characteristics; prior hospitalizations; thrombocytosis, and PSI (Table 2). The first-day pneumonia-specific model had adequate discrimination (C statistic, 0.695; optimism-corrected C statistic 0.675, 95% confidence interval [CI], 0.667-0.685; Table 3). It also effectively stratified individuals across a broad range of risk (average predicted decile of risk ranged from 4% to 33%; Table 3) and was well calibrated (Supplemental Table 3).
The final full-stay pneumonia-specific EHR readmission model included 8 predictors, including 3 variables from the first-day model (median income, thrombocytosis, and prior hospitalizations; Table 2). The full-stay pneumonia-specific EHR model also included vital sign instabilities on discharge, updated PSI, and disposition status (ie, being discharged with home health or to a post-acute care facility was associated with greater odds of readmission, and hospice with lower odds). The full-stay pneumonia-specific EHR model had good discrimination (C statistic, 0.731; optimism-corrected C statistic, 0.714; 95% CI, 0.706-0.720), and stratified individuals across a broad range of risk (average predicted decile of risk ranged from 3% to 37%; Table 3), and was also well calibrated (Supplemental Table 3).
First-Day Pneumonia-Specific EHR Model vs First-Day Multi-Condition EHR Model
The first-day pneumonia-specific EHR model outperformed the first-day multi-condition EHR model with better discrimination (P = 0.029) and more correctly classified individuals in the top 2 highest risk quintiles vs the bottom 3 risk quintiles (Table 3, Supplemental Table 4, and Supplemental Figure 2A). With respect to calibration, the first-day multi-condition EHR model overestimated risk among the highest quintile risk group compared to the first-day pneumonia-specific EHR model (Figure 1A, 1B).
Full-Stay Pneumonia-Specific EHR Model vs Other Models
The full-stay pneumonia-specific EHR model comparatively outperformed the corresponding full-stay multi-condition EHR model, as well as the first-day pneumonia-specific EHR model, the CMS pneumonia model, the updated PSI, and the updated CURB-65 (Table 3, Supplemental Table 5, Supplemental Table 6, and Supplemental Figures 2B and 2C). Compared to the full-stay multi-condition and first-day pneumonia-specific EHR models, the full-stay pneumonia-specific EHR model had better discrimination, better reclassification (NRI, 0.09 and 0.08, respectively), and was able to stratify individuals across a broader range of readmission risk (Table 3). It also had better calibration in the highest quintile risk group compared to the full-stay multi-condition EHR model (Figure 1C and 1D).
Updated vs First-Day Modified PSI and CURB-65 Scores
The updated PSI was more strongly predictive of readmission than the PSI calculated on the day of admission (Wald test, 9.83; P = 0.002). Each 10-point increase in the updated PSI was associated with a 22% increased odds of readmission vs an 11% increase for the PSI calculated upon admission (Table 2). The improved predictive ability of the updated PSI and CURB-65 scores was also reflected in the superior discrimination and calibration vs the respective first-day pneumonia severity of illness scores (Table 3).
DISCUSSION
Using routinely available EHR data from 6 diverse hospitals, we developed 2 pneumonia-specific readmission risk-prediction models that aimed to allow hospitals to identify patients hospitalized with pneumonia at high risk for readmission. Overall, we found that a pneumonia-specific model using EHR data from the entire hospitalization outperformed all other models—including the first-day pneumonia-specific model using data present only on admission, our own multi-condition EHR models, and the CMS pneumonia model based on administrative claims data—in all aspects of model performance (discrimination, calibration, and reclassification). We found that socioeconomic status, prior hospitalizations, thrombocytosis, and measures of clinical severity and stability were important predictors of 30-day all-cause readmissions among patients hospitalized with pneumonia. Additionally, an updated discharge PSI score was a stronger independent predictor of readmissions compared to the PSI score calculated upon admission; and inclusion of the updated PSI in our full-stay pneumonia model led to improved prediction of 30-day readmissions.
The marked improvement in performance of the full-stay pneumonia-specific EHR model compared to the first-day pneumonia-specific model suggests that clinical stability and trajectory during hospitalization (as modeled through disposition status, updated PSI, and vital sign instabilities at discharge) are important predictors of 30-day readmission among patients hospitalized for pneumonia, which was not the case for our EHR-based multi-condition models.19 With the inclusion of these measures, the full-stay pneumonia-specific model correctly reclassified an additional 8% of patients according to their true risk compared to the first-day pneumonia-specific model. One implication of these findings is that hospitals interested in targeting their highest risk individuals with pneumonia for transitional care interventions could do so using the first-day pneumonia-specific EHR model and could refine their targeted strategy at the time of discharge by using the full-stay pneumonia model. This staged risk-prediction strategy would enable hospitals to initiate transitional care interventions for high-risk individuals in the inpatient setting (ie, patient education).7 Then, hospitals could enroll both persistent and newly identified high-risk individuals for outpatient interventions (ie, follow-up telephone call) in the immediate post-discharge period, an interval characterized by heightened vulnerability for adverse events,28 based on patients’ illness severity and stability at discharge. This approach can be implemented by hospitals by building these risk-prediction models directly into the EHR, or by extracting EHR data in near real time as our group has done successfully for heart failure.7
Another key implication of our study is that, for pneumonia, a disease-specific modeling approach has better predictive ability than using a multi-condition model. Compared to multi-condition models, the first-day and full-stay pneumonia-specific EHR models correctly reclassified an additional 6% and 9% of patients, respectively. Thus, hospitals interested in identifying the highest risk patients with pneumonia for targeted interventions should do so using the disease-specific models, if the costs and resources of doing so are within reach of the healthcare system.
An additional novel finding of our study is the added value of an updated PSI for predicting adverse events. Studies of pneumonia severity of illness scores have calculated the PSI and CURB-65 scores using data present only on admission.16,24 While our study also confirms that the PSI calculated upon admission is a significant predictor of readmission,23,29 this study extends this work by showing that an updated PSI score calculated at the time of discharge is an even stronger predictor for readmission, and its inclusion in the model significantly improves risk stratification and prognostication.
Our study was noteworthy for several strengths. First, we used data from a common EHR system, thus potentially allowing for the implementation of the pneumonia-specific models in real time across a number of hospitals. The use of routinely collected data for risk-prediction modeling makes this approach scalable and sustainable, because it obviates the need for burdensome data collection and entry. Second, to our knowledge, this is the first study to measure the additive influence of illness severity and stability at discharge on the readmission risk among patients hospitalized with pneumonia. Third, our study population was derived from 6 hospitals diverse in payer status, age, race/ethnicity, and socioeconomic status. Fourth, our models are less likely to be overfit to the idiosyncrasies of our data given that several predictors included in our final pneumonia-specific models have been associated with readmission in this population, including marital status,13,30 income,11,31 prior hospitalizations,11,13 thrombocytosis,32-34 and vital sign instabilities on discharge.17 Lastly, the discrimination of the CMS pneumonia model in our cohort (C statistic, 0.64) closely matched the discrimination observed in 4 independent cohorts (C statistic, 0.63), suggesting adequate generalizability of our study setting and population.10,12
Our results should be interpreted in the context of several limitations. First, generalizability to other regions beyond north Texas is unknown. Second, although we included a diverse cohort of safety net, community, teaching, and nonteaching hospitals, the pneumonia-specific models were not externally validated in a separate cohort, which may lead to more optimistic estimates of model performance. Third, PSI and CURB-65 scores were modified to use diagnostic codes for altered mental status and pleural effusion, and omitted nursing home residence. Thus, the independent associations for the PSI and CURB-65 scores and their predictive ability are likely attenuated. Fourth, we were unable to include data on medications (antibiotics and steroid use) and outpatient visits, which may influence readmission risk.2,9,13,35-40 Fifth, we included only the first pneumonia hospitalization per patient in this study. Had we included multiple hospitalizations per patient, we anticipate better model performance for the 2 pneumonia-specific EHR models since prior hospitalization was a robust predictor of readmission.
In conclusion, the full-stay pneumonia-specific EHR readmission risk-prediction model outperformed the first-day pneumonia-specific model, multi-condition EHR models, and the CMS pneumonia model. This suggests that: measures of clinical severity and stability at the time of discharge are important predictors for identifying patients at highest risk for readmission; and that EHR data routinely collected for clinical practice can be used to accurately predict risk of readmission among patients hospitalized for pneumonia.
Acknowledgments
The authors would like to acknowledge Ruben Amarasingham, MD, MBA, president and chief executive officer of Parkland Center for Clinical Innovation, and Ferdinand Velasco, MD, chief health information officer at Texas Health Resources for their assistance in assembling the 6-hospital cohort used in this study.
Disclosures
This work was supported by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24 HS022418-01); the Commonwealth Foundation (#20100323); the UT Southwestern KL2 Scholars Program supported by the National Institutes of Health (KL2 TR001103 to ANM and OKN); and the National Center for Advancing Translational Sciences at the National Institute of Health (U54 RFA-TR-12-006 to E.A.H.). The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no financial conflicts of interest to disclose
Pneumonia is a leading cause of hospitalizations in the U.S., accounting for more than 1.1 million discharges annually.1 Pneumonia is frequently complicated by hospital readmission, which is costly and potentially avoidable.2,3 Due to financial penalties imposed on hospitals for higher than expected 30-day readmission rates, there is increasing attention to implementing interventions to reduce readmissions in this population.4,5 However, because these programs are resource-intensive, interventions are thought to be most cost-effective if they are targeted to high-risk individuals who are most likely to benefit.6-8
Current pneumonia-specific readmission risk-prediction models that could enable identification of high-risk patients suffer from poor predictive ability, greatly limiting their use, and most were validated among older adults or by using data from single academic medical centers, limiting their generalizability.9-14 A potential reason for poor predictive accuracy is the omission of known robust clinical predictors of pneumonia-related outcomes, including pneumonia severity of illness and stability on discharge.15-17 Approaches using electronic health record (EHR) data, which include this clinically granular data, could enable hospitals to more accurately and pragmatically identify high-risk patients during the index hospitalization and enable interventions to be initiated prior to discharge.
An alternative strategy to identifying high-risk patients for readmission is to use a multi-condition risk-prediction model. Developing and implementing models for every condition may be time-consuming and costly. We have derived and validated 2 multi-condition risk-prediction models using EHR data—1 using data from the first day of hospital admission (‘first-day’ model), and the second incorporating data from the entire hospitalization (‘full-stay’ model) to reflect in-hospital complications and clinical stability at discharge.18,19 However, it is unknown if a multi-condition model for pneumonia would perform as well as a disease-specific model.
This study aimed to develop 2 EHR-based pneumonia-specific readmission risk-prediction models using data routinely collected in clinical practice—a ‘first-day’ and a ‘full-stay’ model—and compare the performance of each model to: 1) one another; 2) the corresponding multi-condition EHR model; and 3) to other potentially useful models in predicting pneumonia readmissions (the Centers for Medicare and Medicaid Services [CMS] pneumonia model, and 2 commonly used pneumonia severity of illness scores validated for predicting mortality). We hypothesized that the pneumonia-specific EHR models would outperform other models; and the full-stay pneumonia-specific model would outperform the first-day pneumonia-specific model.
METHODS
Study Design, Population, and Data Sources
We conducted an observational study using EHR data collected from 6 hospitals (including safety net, community, teaching, and nonteaching hospitals) in north Texas between November 2009 and October 2010, All hospitals used the Epic EHR (Epic Systems Corporation, Verona, WI). Details of this cohort have been published.18,19
We included consecutive hospitalizations among adults 18 years and older discharged from any medicine service with principal discharge diagnoses of pneumonia (ICD-9-CM codes 480-483, 485, 486-487), sepsis (ICD-9-CM codes 038, 995.91, 995.92, 785.52), or respiratory failure (ICD-9-CM codes 518.81, 518.82, 518.84, 799.1) when the latter 2 were also accompanied by a secondary diagnosis of pneumonia.20 For individuals with multiple hospitalizations during the study period, we included only the first hospitalization. We excluded individuals who died during the index hospitalization or within 30 days of discharge, were transferred to another acute care facility, or left against medical advice.
Outcomes
The primary outcome was all-cause 30-day readmission, defined as a nonelective hospitalization within 30 days of discharge to any of 75 acute care hospitals within a 100-mile radius of Dallas, ascertained from an all-payer regional hospitalization database.
Predictor Variables for the Pneumonia-Specific Readmission Models
The selection of candidate predictors was informed by our validated multi-condition risk-prediction models using EHR data available within 24 hours of admission (‘first-day’ multi-condition EHR model) or during the entire hospitalization (‘full-stay’ multi-condition EHR model).18,19 For the pneumonia-specific models, we included all variables in our published multi-condition models as candidate predictors, including sociodemographics, prior utilization, Charlson Comorbidity Index, select laboratory and vital sign abnormalities, length of stay, hospital complications (eg, venous thromboembolism), vital sign instabilities, and disposition status (see Supplemental Table 1 for complete list of variables). We also assessed additional variables specific to pneumonia for inclusion that were: (1) available in the EHR of all participating hospitals; (2) routinely collected or available at the time of admission or discharge; and (3) plausible predictors of adverse outcomes based on literature and clinical expertise. These included select comorbidities (eg, psychiatric conditions, chronic lung disease, history of pneumonia),10,11,21,22 the pneumonia severity index (PSI),16,23,24 intensive care unit stay, and receipt of invasive or noninvasive ventilation. We used a modified PSI score because certain data elements were missing. The modified PSI (henceforth referred to as PSI) did not include nursing home residence and included diagnostic codes as proxies for the presence of pleural effusion (ICD-9-CM codes 510, 511.1, and 511.9) and altered mental status (ICD-9-CM codes 780.0X, 780.97, 293.0, 293.1, and 348.3X).
Statistical Analysis
Model Derivation. Candidate predictor variables were classified as available in the EHR within 24 hours of admission and/or at the time of discharge. For example, socioeconomic factors could be ascertained within the first day of hospitalization, whereas length of stay would not be available until the day of discharge. Predictors with missing values were assumed to be normal (less than 1% missing for each variable). Univariate relationships between readmission and each candidate predictor were assessed in the overall cohort using a pre-specified significance threshold of P ≤ 0.10. Significant variables were entered in the respective first-day and full-stay pneumonia-specific multivariable logistic regression models using stepwise-backward selection with a pre-specified significance threshold of P ≤ 0.05. In sensitivity analyses, we alternately derived our models using stepwise-forward selection, as well as stepwise-backward selection minimizing the Bayesian information criterion and Akaike information criterion separately. These alternate modeling strategies yielded identical predictors to our final models.
Model Validation. Model validation was performed using 5-fold cross-validation, with the overall cohort randomly divided into 5 equal-size subsets.25 For each cycle, 4 subsets were used for training to estimate model coefficients, and the fifth subset was used for validation. This cycle was repeated 5 times with each randomly-divided subset used once as the validation set. We repeated this entire process 50 times and averaged the C statistic estimates to derive an optimism-corrected C statistic. Model calibration was assessed qualitatively by comparing predicted to observed probabilities of readmission by quintiles of predicted risk, and with the Hosmer-Lemeshow goodness-of-fit test.
Comparison to Other Models. The main comparisons of the first-day and full-stay pneumonia-specific EHR model performance were to each other and the corresponding multi-condition EHR model.18,19 The multi-condition EHR models were separately derived and validated within the larger parent cohort from which this study cohort was derived, and outperformed the CMS all-cause model, the HOSPITAL model, and the LACE index.19 To further triangulate our findings, given the lack of other rigorously validated pneumonia-specific risk-prediction models for readmission,14 we compared the pneumonia-specific EHR models to the CMS pneumonia model derived from administrative claims data,10 and 2 commonly used risk-prediction scores for short-term mortality among patients with community-acquired pneumonia, the PSI and CURB-65 scores.16 Although derived and validated using patient-level data, the CMS model was developed to benchmark hospitals according to hospital-level readmission rates.10 The CURB-65 score in this study was also modified to include the same altered mental status diagnostic codes according to the modified PSI as a proxy for “confusion.” Both the PSI and CURB-65 scores were calculated using the most abnormal values within the first 24 hours of admission. The ‘updated’ PSI and the ‘updated’ CURB-65 were calculated using the most abnormal values within 24 hours prior to discharge, or the last known observation prior to discharge if no results were recorded within this time period. A complete list of variables for each of the comparison models are shown in Supplemental Table 1.
We assessed model performance by calculating the C statistic, integrated discrimination index, and net reclassification index (NRI) compared to our pneumonia-specific models. The integrated discrimination index is the difference in the mean predicted probability of readmission between patients who were and were not actually readmitted between 2 models, where more positive values suggest improvement in model performance compared to a reference model.26 The NRI is defined as the sum of the net proportions of correctly reclassified persons with and without the event of interest.27 Here, we calculated a category-based NRI to evaluate the performance of pneumonia-specific models in correctly classifying individuals with and without readmissions into the 2 highest readmission risk quintiles vs the lowest 3 risk quintiles compared to other models.27 This pre-specified cutoff is relevant for hospitals interested in identifying the highest risk individuals for targeted intervention.7 Finally, we assessed calibration of comparator models in our cohort by comparing predicted probability to observed probability of readmission by quintiles of risk for each model. We conducted all analyses using Stata 12.1 (StataCorp, College Station, Texas). This study was approved by the University of Texas Southwestern Medical Center Institutional Review Board.
RESULTS
Of 1463 index hospitalizations (Supplemental Figure 1), the 30-day all-cause readmission rate was 13.6%. Individuals with a 30-day readmission had markedly different sociodemographic and clinical characteristics compared to those not readmitted (Table 1; see Supplemental Table 2 for additional clinical characteristics).
Derivation, Validation, and Performance of the Pneumonia-Specific Readmission Risk-Prediction Models
The final first-day pneumonia-specific EHR model included 7 variables, including sociodemographic characteristics; prior hospitalizations; thrombocytosis, and PSI (Table 2). The first-day pneumonia-specific model had adequate discrimination (C statistic, 0.695; optimism-corrected C statistic 0.675, 95% confidence interval [CI], 0.667-0.685; Table 3). It also effectively stratified individuals across a broad range of risk (average predicted decile of risk ranged from 4% to 33%; Table 3) and was well calibrated (Supplemental Table 3).
The final full-stay pneumonia-specific EHR readmission model included 8 predictors, including 3 variables from the first-day model (median income, thrombocytosis, and prior hospitalizations; Table 2). The full-stay pneumonia-specific EHR model also included vital sign instabilities on discharge, updated PSI, and disposition status (ie, being discharged with home health or to a post-acute care facility was associated with greater odds of readmission, and hospice with lower odds). The full-stay pneumonia-specific EHR model had good discrimination (C statistic, 0.731; optimism-corrected C statistic, 0.714; 95% CI, 0.706-0.720), and stratified individuals across a broad range of risk (average predicted decile of risk ranged from 3% to 37%; Table 3), and was also well calibrated (Supplemental Table 3).
First-Day Pneumonia-Specific EHR Model vs First-Day Multi-Condition EHR Model
The first-day pneumonia-specific EHR model outperformed the first-day multi-condition EHR model with better discrimination (P = 0.029) and more correctly classified individuals in the top 2 highest risk quintiles vs the bottom 3 risk quintiles (Table 3, Supplemental Table 4, and Supplemental Figure 2A). With respect to calibration, the first-day multi-condition EHR model overestimated risk among the highest quintile risk group compared to the first-day pneumonia-specific EHR model (Figure 1A, 1B).
Full-Stay Pneumonia-Specific EHR Model vs Other Models
The full-stay pneumonia-specific EHR model comparatively outperformed the corresponding full-stay multi-condition EHR model, as well as the first-day pneumonia-specific EHR model, the CMS pneumonia model, the updated PSI, and the updated CURB-65 (Table 3, Supplemental Table 5, Supplemental Table 6, and Supplemental Figures 2B and 2C). Compared to the full-stay multi-condition and first-day pneumonia-specific EHR models, the full-stay pneumonia-specific EHR model had better discrimination, better reclassification (NRI, 0.09 and 0.08, respectively), and was able to stratify individuals across a broader range of readmission risk (Table 3). It also had better calibration in the highest quintile risk group compared to the full-stay multi-condition EHR model (Figure 1C and 1D).
Updated vs First-Day Modified PSI and CURB-65 Scores
The updated PSI was more strongly predictive of readmission than the PSI calculated on the day of admission (Wald test, 9.83; P = 0.002). Each 10-point increase in the updated PSI was associated with a 22% increased odds of readmission vs an 11% increase for the PSI calculated upon admission (Table 2). The improved predictive ability of the updated PSI and CURB-65 scores was also reflected in the superior discrimination and calibration vs the respective first-day pneumonia severity of illness scores (Table 3).
DISCUSSION
Using routinely available EHR data from 6 diverse hospitals, we developed 2 pneumonia-specific readmission risk-prediction models that aimed to allow hospitals to identify patients hospitalized with pneumonia at high risk for readmission. Overall, we found that a pneumonia-specific model using EHR data from the entire hospitalization outperformed all other models—including the first-day pneumonia-specific model using data present only on admission, our own multi-condition EHR models, and the CMS pneumonia model based on administrative claims data—in all aspects of model performance (discrimination, calibration, and reclassification). We found that socioeconomic status, prior hospitalizations, thrombocytosis, and measures of clinical severity and stability were important predictors of 30-day all-cause readmissions among patients hospitalized with pneumonia. Additionally, an updated discharge PSI score was a stronger independent predictor of readmissions compared to the PSI score calculated upon admission; and inclusion of the updated PSI in our full-stay pneumonia model led to improved prediction of 30-day readmissions.
The marked improvement in performance of the full-stay pneumonia-specific EHR model compared to the first-day pneumonia-specific model suggests that clinical stability and trajectory during hospitalization (as modeled through disposition status, updated PSI, and vital sign instabilities at discharge) are important predictors of 30-day readmission among patients hospitalized for pneumonia, which was not the case for our EHR-based multi-condition models.19 With the inclusion of these measures, the full-stay pneumonia-specific model correctly reclassified an additional 8% of patients according to their true risk compared to the first-day pneumonia-specific model. One implication of these findings is that hospitals interested in targeting their highest risk individuals with pneumonia for transitional care interventions could do so using the first-day pneumonia-specific EHR model and could refine their targeted strategy at the time of discharge by using the full-stay pneumonia model. This staged risk-prediction strategy would enable hospitals to initiate transitional care interventions for high-risk individuals in the inpatient setting (ie, patient education).7 Then, hospitals could enroll both persistent and newly identified high-risk individuals for outpatient interventions (ie, follow-up telephone call) in the immediate post-discharge period, an interval characterized by heightened vulnerability for adverse events,28 based on patients’ illness severity and stability at discharge. This approach can be implemented by hospitals by building these risk-prediction models directly into the EHR, or by extracting EHR data in near real time as our group has done successfully for heart failure.7
Another key implication of our study is that, for pneumonia, a disease-specific modeling approach has better predictive ability than using a multi-condition model. Compared to multi-condition models, the first-day and full-stay pneumonia-specific EHR models correctly reclassified an additional 6% and 9% of patients, respectively. Thus, hospitals interested in identifying the highest risk patients with pneumonia for targeted interventions should do so using the disease-specific models, if the costs and resources of doing so are within reach of the healthcare system.
An additional novel finding of our study is the added value of an updated PSI for predicting adverse events. Studies of pneumonia severity of illness scores have calculated the PSI and CURB-65 scores using data present only on admission.16,24 While our study also confirms that the PSI calculated upon admission is a significant predictor of readmission,23,29 this study extends this work by showing that an updated PSI score calculated at the time of discharge is an even stronger predictor for readmission, and its inclusion in the model significantly improves risk stratification and prognostication.
Our study was noteworthy for several strengths. First, we used data from a common EHR system, thus potentially allowing for the implementation of the pneumonia-specific models in real time across a number of hospitals. The use of routinely collected data for risk-prediction modeling makes this approach scalable and sustainable, because it obviates the need for burdensome data collection and entry. Second, to our knowledge, this is the first study to measure the additive influence of illness severity and stability at discharge on the readmission risk among patients hospitalized with pneumonia. Third, our study population was derived from 6 hospitals diverse in payer status, age, race/ethnicity, and socioeconomic status. Fourth, our models are less likely to be overfit to the idiosyncrasies of our data given that several predictors included in our final pneumonia-specific models have been associated with readmission in this population, including marital status,13,30 income,11,31 prior hospitalizations,11,13 thrombocytosis,32-34 and vital sign instabilities on discharge.17 Lastly, the discrimination of the CMS pneumonia model in our cohort (C statistic, 0.64) closely matched the discrimination observed in 4 independent cohorts (C statistic, 0.63), suggesting adequate generalizability of our study setting and population.10,12
Our results should be interpreted in the context of several limitations. First, generalizability to other regions beyond north Texas is unknown. Second, although we included a diverse cohort of safety net, community, teaching, and nonteaching hospitals, the pneumonia-specific models were not externally validated in a separate cohort, which may lead to more optimistic estimates of model performance. Third, PSI and CURB-65 scores were modified to use diagnostic codes for altered mental status and pleural effusion, and omitted nursing home residence. Thus, the independent associations for the PSI and CURB-65 scores and their predictive ability are likely attenuated. Fourth, we were unable to include data on medications (antibiotics and steroid use) and outpatient visits, which may influence readmission risk.2,9,13,35-40 Fifth, we included only the first pneumonia hospitalization per patient in this study. Had we included multiple hospitalizations per patient, we anticipate better model performance for the 2 pneumonia-specific EHR models since prior hospitalization was a robust predictor of readmission.
In conclusion, the full-stay pneumonia-specific EHR readmission risk-prediction model outperformed the first-day pneumonia-specific model, multi-condition EHR models, and the CMS pneumonia model. This suggests that: measures of clinical severity and stability at the time of discharge are important predictors for identifying patients at highest risk for readmission; and that EHR data routinely collected for clinical practice can be used to accurately predict risk of readmission among patients hospitalized for pneumonia.
Acknowledgments
The authors would like to acknowledge Ruben Amarasingham, MD, MBA, president and chief executive officer of Parkland Center for Clinical Innovation, and Ferdinand Velasco, MD, chief health information officer at Texas Health Resources for their assistance in assembling the 6-hospital cohort used in this study.
Disclosures
This work was supported by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24 HS022418-01); the Commonwealth Foundation (#20100323); the UT Southwestern KL2 Scholars Program supported by the National Institutes of Health (KL2 TR001103 to ANM and OKN); and the National Center for Advancing Translational Sciences at the National Institute of Health (U54 RFA-TR-12-006 to E.A.H.). The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no financial conflicts of interest to disclose
1. Centers for Disease Control and Prevention. Pneumonia. http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed January 26, 2016.
33. Prina E, Ferrer M, Ranzani OT, et al. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest. 2013;143(3):767-775. PubMed
34. Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. J Thromb Haemost. 2014;12(9):1391-1400. PubMed
35. Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441-1447. PubMed
36. Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post-discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565-571. PubMed
37. Spatz ES, Sheth SD, Gosch KL, et al. Usual source of care and outcomes following acute myocardial infarction. J Gen Intern Med. 2014;29(6):862-869. PubMed
38. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828. PubMed
39. Adamuz J, Viasus D, Campreciós-Rodriguez P, et al. A prospective cohort study of healthcare visits and rehospitalizations after discharge of patients with community-acquired pneumonia. Respirology. 2011;16(7):1119-1126. PubMed
40. Shorr AF, Zilberberg MD, Reichley R, et al. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin Infect Dis. 2013;57(3):362-367. PubMed
1. Centers for Disease Control and Prevention. Pneumonia. http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed January 26, 2016.
33. Prina E, Ferrer M, Ranzani OT, et al. Thrombocytosis is a marker of poor outcome in community-acquired pneumonia. Chest. 2013;143(3):767-775. PubMed
34. Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. J Thromb Haemost. 2014;12(9):1391-1400. PubMed
35. Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441-1447. PubMed
36. Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post-discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565-571. PubMed
37. Spatz ES, Sheth SD, Gosch KL, et al. Usual source of care and outcomes following acute myocardial infarction. J Gen Intern Med. 2014;29(6):862-869. PubMed
38. Brooke BS, Stone DH, Cronenwett JL, et al. Early primary care provider follow-up and readmission after high-risk surgery. JAMA Surg. 2014;149(8):821-828. PubMed
39. Adamuz J, Viasus D, Campreciós-Rodriguez P, et al. A prospective cohort study of healthcare visits and rehospitalizations after discharge of patients with community-acquired pneumonia. Respirology. 2011;16(7):1119-1126. PubMed
40. Shorr AF, Zilberberg MD, Reichley R, et al. Readmission following hospitalization for pneumonia: the impact of pneumonia type and its implication for hospitals. Clin Infect Dis. 2013;57(3):362-367. PubMed
© 2017 Society of Hospital Medicine
Anxiety in children during a new administration; Why medical psychiatry is vital for my patients; And more
Anxiety in children during a new administration
Since the current administration took office, many children continue to grapple with the initial shock of the election results and the uncertainty of what the next 4 years will bring. In the days after the election, several patients sat in my office and spoke of intense feelings of sadness, anger, and worry. Their stress levels were elevated, and they searched desperately for refuge from the unknown. On the other side of the hospital, patients expressing suicidal ideation filed into the emergency room. A similar scene played out nationally when suicide prevention hotlines experienced a sharp increase in calls.
During this emotional time, it is critical to support our children. Some will be more affected than others. Children from immigrant backgrounds might be particularly fearful of what this means for them and their families. In the days after the election, a video surfaced from a middle school in Michigan featuring kids at lunch chanting, “Build the wall!”
Bullying also is a concern. Despite being a third-generation American, an 8-year-old boy woke up the day after the election confused and scared. One mother told me that a student confronted her 11-year-old son at school, yelling that the election outcome was a “good thing” and he should “go back to his country.” Like his mother, the 11-year-old was born in the United States.
Kids get their cues from the adults in their lives. Parents and teachers play an important role in modeling behavior and providing comfort. Adults need to support children and to do that properly they need make sure they have processed their own feelings. They do not need to be unrealistic or overly positive, but should offer hope and trust in our democratic system. With discussion, children should have ample opportunity to express how they feel. Psychiatrists can evaluate a child’s symptoms and presentation. Are current medications helping enough with the recent changes? Does a child need a medication adjustment or to be seen more often? Does he (she) need to be admitted to the hospital for evaluation of suicidal ideation? As a psychiatrist, do you need to revisit the list of resources in the community and give children a crisis hotline number? Also consider referring a child to a psychotherapist if needed. Some schools offered counseling after the election. It is worthwhile to contact school officials if a student is struggling or could benefit from additional support.
Although many unknowns remain, 1 thing is certain: children will have more questions and we must be ready to answer.
Balkozar S. Adam, MD
Associate Professor of Clinical Psychiatry Child and Adolescent Psychiatry
University of Missouri
Columbia, Missouri
Co-Editor
Missouri Psychiatry Newsletter
Jefferson City, Missouri
Two additional adjunctive therapies for mental health
I was excited to read Dr. Nasrallah’s editorial about adjunctive therapies for mental health disorders (Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,
The article missed 2 important vitamins that play a crucial role in positive mental health treatment outcomes: folic acid and vitamin B12. In my practice, I have found up to 50% of my patients with depression have a vitamin B12 deficiency. After supplementation, these patients’ symptoms improve to the point that we often can reduce or eliminate medication. Folic acid deficiency has been found among individuals with depression and linked to poor response to treatment.1 Higher serum levels of homocysteine—a consequence of low folic acid levels—are linked to increased risk of developing depression later in life, as well as higher risk of cardiovascular disease.2,3 Folate also can be used for enhancing treatment response to antidepressants by increasing production of neurotransmitters.2
Another factor to consider is methylenetetrahydrofolate reductase (MTHFR) variants. Approximately 20% of the population cannot methylate B vitamins because of a variation on the MTHFR gene.4,5 These patients are at increased risk for depression because they are unable to use B vitamins, which are essential in the synthesis of serotonin and dopamine. These patients do not respond to B12 and folate supplements. For these individuals, I recommend methylated products, which can be purchased online.
I have found these practices, as well as many of those listed in the editorial, are effective in treating depression and anxiety.
Lara Kain, PA-C, MPAS
Psychiatric Physician Assistant
Tidewater Psychotherapy Services
Virginia Beach, Virginia
References
1. Kaner G, Soylu M, Yüksel N, et al. Evaluation of nutritional status of patients with depression. Biomed Res Int. 2015;2015:521481. doi: 10.1155/2015/521481.
2. Seppälä J, Koponen H, Kautiainen H, et al. Association between vitamin B12 and melancholic depressive symptoms: a Finnish population-based study. BMC Psychiatry. 2013;13:145. doi: 10.1186/1471-244X-13-145.
3. Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systemic review and meta-analysis. Aging Ment Health. 2016;20(9):965-973.
4. Lynch B. MTHFR mutations and the conditions they cause. MTHFR.Net. http://mthfr.net/mthfr-mutations-and-the-conditions-they-cause/2011/09/07. Accessed February 16, 2017.
5. Eszlari N, Kovacs D, Petschner P, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: a potential risk mechanism for depression. Transl Psychiatry. 2016;6(3):e745. doi: 10.1038/tp.2016.19.
An honest perspective on Cannabis in therapy
I enjoyed Dr. Nasrallah’s editorial “Maddening therapies: How hallucinogens morphed into novel treatments” (From the
As a psychiatrist specializing in bipolar and psychotic disorders—as well as the founder and Board President of Doctors for Cannabis Regulation—I appreciate his reservations about the potential of Cannabis to trigger psychosis in vulnerable individuals. My reading of the literature is there is good evidence for marijuana as a trigger—not as a cause—of the disease. However, what is the evidence for hallucinogens?
Cannabis can have adverse effects on brain development, but it is not clear whether those effects are worse than those caused by alcohol. In the absence of any head-to-head studies, how can we proceed?
David L. Nathan, MD, DFAPA
Clinical Associate Professor
Rutgers Robert Wood Johnson Medical School
Director of Continuing Medical Education
Princeton HealthCare System
Princeton, New Jersey
Dr. Nasrallah responds
LSD can cause psychosis, paranoid delusions, and altered thinking in addition to vivid visual hallucinations in some individuals but not all, because vulnerability occurs on a spectrum. I postulate that the recently discovered inverse agonist of the serotonin 5-HT2A receptor, pimavanserin (FDA-approved for visual hallucinations and delusions of Parkinson’s disease psychosis), might be effective for LSD psychosis because this hallucinogen has a strong binding affinity to the serotonin 5-HT2A receptors.
Studies show that marijuana can induce apoptosis, which would adversely affect brain development. Patients with schizophrenia who abuse marijuana have a lower gray matter volume than those who do not abuse the drug, and both groups have lower gray matter volume than matched healthy controls. I strongly advise a pregnant woman against smoking marijuana because it could impair the fetus’s brain development.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Self-administering LSD: Solution or abuse
Dr. Nasrallah’s editorial (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
I am not aware of any systematic data about self-prescribed use of microdoses of LSD to reduce anxiety and depression. Among persons with anxiety and depression who have not had access to psychiatric care, self-medicating with agents such as alcohol, stimulants, ketamine, or LSD is regarded as substance abuse. It also is questionable whether people can determine which microdose of LSD to use. Finally, most drugs of abuse are not “pure,” and many are laced with potentially harmful contaminants.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Why medical psychiatry is vital for my patients
Dr. Paul Summergrad’s guest editorial “Medical psychiatry: The skill of integrating medical and psychiatric care” (
For me, the most inspiring sentence in Dr. Summergrad’s editorial was, “It is incumbent on us to pursue the medical differential of patients when we think it is needed, even if other physicians disagree.” I believe that this describes our job as physicians who specialize in psychiatry. To have a clinician of Dr. Summergrad’s stature write this was inspiring because it goes to the core of what more of us should do.
Ruth Myers, MD
Psychiatrist
The Community Circle PLLC
Burnsville, Minnesota
Anxiety in children during a new administration
Since the current administration took office, many children continue to grapple with the initial shock of the election results and the uncertainty of what the next 4 years will bring. In the days after the election, several patients sat in my office and spoke of intense feelings of sadness, anger, and worry. Their stress levels were elevated, and they searched desperately for refuge from the unknown. On the other side of the hospital, patients expressing suicidal ideation filed into the emergency room. A similar scene played out nationally when suicide prevention hotlines experienced a sharp increase in calls.
During this emotional time, it is critical to support our children. Some will be more affected than others. Children from immigrant backgrounds might be particularly fearful of what this means for them and their families. In the days after the election, a video surfaced from a middle school in Michigan featuring kids at lunch chanting, “Build the wall!”
Bullying also is a concern. Despite being a third-generation American, an 8-year-old boy woke up the day after the election confused and scared. One mother told me that a student confronted her 11-year-old son at school, yelling that the election outcome was a “good thing” and he should “go back to his country.” Like his mother, the 11-year-old was born in the United States.
Kids get their cues from the adults in their lives. Parents and teachers play an important role in modeling behavior and providing comfort. Adults need to support children and to do that properly they need make sure they have processed their own feelings. They do not need to be unrealistic or overly positive, but should offer hope and trust in our democratic system. With discussion, children should have ample opportunity to express how they feel. Psychiatrists can evaluate a child’s symptoms and presentation. Are current medications helping enough with the recent changes? Does a child need a medication adjustment or to be seen more often? Does he (she) need to be admitted to the hospital for evaluation of suicidal ideation? As a psychiatrist, do you need to revisit the list of resources in the community and give children a crisis hotline number? Also consider referring a child to a psychotherapist if needed. Some schools offered counseling after the election. It is worthwhile to contact school officials if a student is struggling or could benefit from additional support.
Although many unknowns remain, 1 thing is certain: children will have more questions and we must be ready to answer.
Balkozar S. Adam, MD
Associate Professor of Clinical Psychiatry Child and Adolescent Psychiatry
University of Missouri
Columbia, Missouri
Co-Editor
Missouri Psychiatry Newsletter
Jefferson City, Missouri
Two additional adjunctive therapies for mental health
I was excited to read Dr. Nasrallah’s editorial about adjunctive therapies for mental health disorders (Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,
The article missed 2 important vitamins that play a crucial role in positive mental health treatment outcomes: folic acid and vitamin B12. In my practice, I have found up to 50% of my patients with depression have a vitamin B12 deficiency. After supplementation, these patients’ symptoms improve to the point that we often can reduce or eliminate medication. Folic acid deficiency has been found among individuals with depression and linked to poor response to treatment.1 Higher serum levels of homocysteine—a consequence of low folic acid levels—are linked to increased risk of developing depression later in life, as well as higher risk of cardiovascular disease.2,3 Folate also can be used for enhancing treatment response to antidepressants by increasing production of neurotransmitters.2
Another factor to consider is methylenetetrahydrofolate reductase (MTHFR) variants. Approximately 20% of the population cannot methylate B vitamins because of a variation on the MTHFR gene.4,5 These patients are at increased risk for depression because they are unable to use B vitamins, which are essential in the synthesis of serotonin and dopamine. These patients do not respond to B12 and folate supplements. For these individuals, I recommend methylated products, which can be purchased online.
I have found these practices, as well as many of those listed in the editorial, are effective in treating depression and anxiety.
Lara Kain, PA-C, MPAS
Psychiatric Physician Assistant
Tidewater Psychotherapy Services
Virginia Beach, Virginia
References
1. Kaner G, Soylu M, Yüksel N, et al. Evaluation of nutritional status of patients with depression. Biomed Res Int. 2015;2015:521481. doi: 10.1155/2015/521481.
2. Seppälä J, Koponen H, Kautiainen H, et al. Association between vitamin B12 and melancholic depressive symptoms: a Finnish population-based study. BMC Psychiatry. 2013;13:145. doi: 10.1186/1471-244X-13-145.
3. Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systemic review and meta-analysis. Aging Ment Health. 2016;20(9):965-973.
4. Lynch B. MTHFR mutations and the conditions they cause. MTHFR.Net. http://mthfr.net/mthfr-mutations-and-the-conditions-they-cause/2011/09/07. Accessed February 16, 2017.
5. Eszlari N, Kovacs D, Petschner P, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: a potential risk mechanism for depression. Transl Psychiatry. 2016;6(3):e745. doi: 10.1038/tp.2016.19.
An honest perspective on Cannabis in therapy
I enjoyed Dr. Nasrallah’s editorial “Maddening therapies: How hallucinogens morphed into novel treatments” (From the
As a psychiatrist specializing in bipolar and psychotic disorders—as well as the founder and Board President of Doctors for Cannabis Regulation—I appreciate his reservations about the potential of Cannabis to trigger psychosis in vulnerable individuals. My reading of the literature is there is good evidence for marijuana as a trigger—not as a cause—of the disease. However, what is the evidence for hallucinogens?
Cannabis can have adverse effects on brain development, but it is not clear whether those effects are worse than those caused by alcohol. In the absence of any head-to-head studies, how can we proceed?
David L. Nathan, MD, DFAPA
Clinical Associate Professor
Rutgers Robert Wood Johnson Medical School
Director of Continuing Medical Education
Princeton HealthCare System
Princeton, New Jersey
Dr. Nasrallah responds
LSD can cause psychosis, paranoid delusions, and altered thinking in addition to vivid visual hallucinations in some individuals but not all, because vulnerability occurs on a spectrum. I postulate that the recently discovered inverse agonist of the serotonin 5-HT2A receptor, pimavanserin (FDA-approved for visual hallucinations and delusions of Parkinson’s disease psychosis), might be effective for LSD psychosis because this hallucinogen has a strong binding affinity to the serotonin 5-HT2A receptors.
Studies show that marijuana can induce apoptosis, which would adversely affect brain development. Patients with schizophrenia who abuse marijuana have a lower gray matter volume than those who do not abuse the drug, and both groups have lower gray matter volume than matched healthy controls. I strongly advise a pregnant woman against smoking marijuana because it could impair the fetus’s brain development.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Self-administering LSD: Solution or abuse
Dr. Nasrallah’s editorial (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
I am not aware of any systematic data about self-prescribed use of microdoses of LSD to reduce anxiety and depression. Among persons with anxiety and depression who have not had access to psychiatric care, self-medicating with agents such as alcohol, stimulants, ketamine, or LSD is regarded as substance abuse. It also is questionable whether people can determine which microdose of LSD to use. Finally, most drugs of abuse are not “pure,” and many are laced with potentially harmful contaminants.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Why medical psychiatry is vital for my patients
Dr. Paul Summergrad’s guest editorial “Medical psychiatry: The skill of integrating medical and psychiatric care” (
For me, the most inspiring sentence in Dr. Summergrad’s editorial was, “It is incumbent on us to pursue the medical differential of patients when we think it is needed, even if other physicians disagree.” I believe that this describes our job as physicians who specialize in psychiatry. To have a clinician of Dr. Summergrad’s stature write this was inspiring because it goes to the core of what more of us should do.
Ruth Myers, MD
Psychiatrist
The Community Circle PLLC
Burnsville, Minnesota
Anxiety in children during a new administration
Since the current administration took office, many children continue to grapple with the initial shock of the election results and the uncertainty of what the next 4 years will bring. In the days after the election, several patients sat in my office and spoke of intense feelings of sadness, anger, and worry. Their stress levels were elevated, and they searched desperately for refuge from the unknown. On the other side of the hospital, patients expressing suicidal ideation filed into the emergency room. A similar scene played out nationally when suicide prevention hotlines experienced a sharp increase in calls.
During this emotional time, it is critical to support our children. Some will be more affected than others. Children from immigrant backgrounds might be particularly fearful of what this means for them and their families. In the days after the election, a video surfaced from a middle school in Michigan featuring kids at lunch chanting, “Build the wall!”
Bullying also is a concern. Despite being a third-generation American, an 8-year-old boy woke up the day after the election confused and scared. One mother told me that a student confronted her 11-year-old son at school, yelling that the election outcome was a “good thing” and he should “go back to his country.” Like his mother, the 11-year-old was born in the United States.
Kids get their cues from the adults in their lives. Parents and teachers play an important role in modeling behavior and providing comfort. Adults need to support children and to do that properly they need make sure they have processed their own feelings. They do not need to be unrealistic or overly positive, but should offer hope and trust in our democratic system. With discussion, children should have ample opportunity to express how they feel. Psychiatrists can evaluate a child’s symptoms and presentation. Are current medications helping enough with the recent changes? Does a child need a medication adjustment or to be seen more often? Does he (she) need to be admitted to the hospital for evaluation of suicidal ideation? As a psychiatrist, do you need to revisit the list of resources in the community and give children a crisis hotline number? Also consider referring a child to a psychotherapist if needed. Some schools offered counseling after the election. It is worthwhile to contact school officials if a student is struggling or could benefit from additional support.
Although many unknowns remain, 1 thing is certain: children will have more questions and we must be ready to answer.
Balkozar S. Adam, MD
Associate Professor of Clinical Psychiatry Child and Adolescent Psychiatry
University of Missouri
Columbia, Missouri
Co-Editor
Missouri Psychiatry Newsletter
Jefferson City, Missouri
Two additional adjunctive therapies for mental health
I was excited to read Dr. Nasrallah’s editorial about adjunctive therapies for mental health disorders (Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,
The article missed 2 important vitamins that play a crucial role in positive mental health treatment outcomes: folic acid and vitamin B12. In my practice, I have found up to 50% of my patients with depression have a vitamin B12 deficiency. After supplementation, these patients’ symptoms improve to the point that we often can reduce or eliminate medication. Folic acid deficiency has been found among individuals with depression and linked to poor response to treatment.1 Higher serum levels of homocysteine—a consequence of low folic acid levels—are linked to increased risk of developing depression later in life, as well as higher risk of cardiovascular disease.2,3 Folate also can be used for enhancing treatment response to antidepressants by increasing production of neurotransmitters.2
Another factor to consider is methylenetetrahydrofolate reductase (MTHFR) variants. Approximately 20% of the population cannot methylate B vitamins because of a variation on the MTHFR gene.4,5 These patients are at increased risk for depression because they are unable to use B vitamins, which are essential in the synthesis of serotonin and dopamine. These patients do not respond to B12 and folate supplements. For these individuals, I recommend methylated products, which can be purchased online.
I have found these practices, as well as many of those listed in the editorial, are effective in treating depression and anxiety.
Lara Kain, PA-C, MPAS
Psychiatric Physician Assistant
Tidewater Psychotherapy Services
Virginia Beach, Virginia
References
1. Kaner G, Soylu M, Yüksel N, et al. Evaluation of nutritional status of patients with depression. Biomed Res Int. 2015;2015:521481. doi: 10.1155/2015/521481.
2. Seppälä J, Koponen H, Kautiainen H, et al. Association between vitamin B12 and melancholic depressive symptoms: a Finnish population-based study. BMC Psychiatry. 2013;13:145. doi: 10.1186/1471-244X-13-145.
3. Petridou ET, Kousoulis AA, Michelakos T, et al. Folate and B12 serum levels in association with depression in the aged: a systemic review and meta-analysis. Aging Ment Health. 2016;20(9):965-973.
4. Lynch B. MTHFR mutations and the conditions they cause. MTHFR.Net. http://mthfr.net/mthfr-mutations-and-the-conditions-they-cause/2011/09/07. Accessed February 16, 2017.
5. Eszlari N, Kovacs D, Petschner P, et al. Distinct effects of folate pathway genes MTHFR and MTHFD1L on ruminative response style: a potential risk mechanism for depression. Transl Psychiatry. 2016;6(3):e745. doi: 10.1038/tp.2016.19.
An honest perspective on Cannabis in therapy
I enjoyed Dr. Nasrallah’s editorial “Maddening therapies: How hallucinogens morphed into novel treatments” (From the
As a psychiatrist specializing in bipolar and psychotic disorders—as well as the founder and Board President of Doctors for Cannabis Regulation—I appreciate his reservations about the potential of Cannabis to trigger psychosis in vulnerable individuals. My reading of the literature is there is good evidence for marijuana as a trigger—not as a cause—of the disease. However, what is the evidence for hallucinogens?
Cannabis can have adverse effects on brain development, but it is not clear whether those effects are worse than those caused by alcohol. In the absence of any head-to-head studies, how can we proceed?
David L. Nathan, MD, DFAPA
Clinical Associate Professor
Rutgers Robert Wood Johnson Medical School
Director of Continuing Medical Education
Princeton HealthCare System
Princeton, New Jersey
Dr. Nasrallah responds
LSD can cause psychosis, paranoid delusions, and altered thinking in addition to vivid visual hallucinations in some individuals but not all, because vulnerability occurs on a spectrum. I postulate that the recently discovered inverse agonist of the serotonin 5-HT2A receptor, pimavanserin (FDA-approved for visual hallucinations and delusions of Parkinson’s disease psychosis), might be effective for LSD psychosis because this hallucinogen has a strong binding affinity to the serotonin 5-HT2A receptors.
Studies show that marijuana can induce apoptosis, which would adversely affect brain development. Patients with schizophrenia who abuse marijuana have a lower gray matter volume than those who do not abuse the drug, and both groups have lower gray matter volume than matched healthy controls. I strongly advise a pregnant woman against smoking marijuana because it could impair the fetus’s brain development.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Self-administering LSD: Solution or abuse
Dr. Nasrallah’s editorial (From the Editor,
H. Steven Moffic, MD
Retired Tenured Professor of Psychiatry
Medical College of Wisconsin
Milwaukee, Wisconsin
Dr. Nasrallah responds
I am not aware of any systematic data about self-prescribed use of microdoses of LSD to reduce anxiety and depression. Among persons with anxiety and depression who have not had access to psychiatric care, self-medicating with agents such as alcohol, stimulants, ketamine, or LSD is regarded as substance abuse. It also is questionable whether people can determine which microdose of LSD to use. Finally, most drugs of abuse are not “pure,” and many are laced with potentially harmful contaminants.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
Why medical psychiatry is vital for my patients
Dr. Paul Summergrad’s guest editorial “Medical psychiatry: The skill of integrating medical and psychiatric care” (
For me, the most inspiring sentence in Dr. Summergrad’s editorial was, “It is incumbent on us to pursue the medical differential of patients when we think it is needed, even if other physicians disagree.” I believe that this describes our job as physicians who specialize in psychiatry. To have a clinician of Dr. Summergrad’s stature write this was inspiring because it goes to the core of what more of us should do.
Ruth Myers, MD
Psychiatrist
The Community Circle PLLC
Burnsville, Minnesota
Evaluating automated rules for rapid response system alarm triggers in medical and surgical patients
Patients typically show signs and symptoms of deterioration hours to days prior to cardiorespiratory arrest.1,2 The rate of inhospital cardiorespiratory arrest (CRA) requiring cardiopulmonary resuscitation is estimated to be 0.174 per bed per year in the United States.3 After CRA, survival to discharge is estimated to be as low as 18%.3,4 Efforts to predict and prevent arrest could prove beneficial.1,2
Rapid response systems (RRS) have been proposed as a means of identifying clinical deterioration and facilitating a timely response. These systems were designed to bring clinicians with critical care expertise to the bedside to prevent unnecessary deaths. They typically include an afferent limb (detects deteriorating patients), an efferent limb (responds to calls and acts to avoid further deterioration), and administrative and data analysis limbs.5,6 Automatic provision of recommendations and computer-based systems are desirable components of the afferent limb of the detection system.6 Both are independent predictors of improved clinical practices for clinical decision support systems.7 However, the existing early warning scores (EWS) may not be ready for automation due to low positive predictive values (PPV) and sensitivities.8
It is possible that the low discriminatory accuracy of the published EWS may be secondary to the use of aggregate patient populations for derivation of scores. We hypothesized that these EWS perform differently in medical and in surgical subpopulations. Also, the EWS need to be tested in a time-dependent manner to serve as a realistic clinical support tool for hospitalized patients.
STUDY AIM
The aim of this study was to evaluate the differential performance of widely used EWS in medical vs surgical patients.
METHODS
Site
The study was conducted in an academic center with 2 hospitals in Southeastern Minnesota totaling approximately 1500 general care nonintensive care unit (ICU) beds. The Mayo Clinic Institutional Review Board approved the research proposal.
Subjects
Our retrospective cohort was comprised of all adult inpatients discharged from 2 academic hospitals between January 1, 2011 and December 31, 2011 who spent any time in a general care (non-ICU) unit. We excluded patients younger than 18 years, psychiatric or rehabilitation inpatients, those without research authorization, and patients admitted for research purposes.
Study patients were divided into medical and surgical cohorts. Hospitalizations were considered surgical if patients had surgery at any time during their hospital stay according to billing data. A trigger was an instance in which a patient met the conditions of a specific rule (score/vital sign exceeded the published/defined threshold).
A resuscitation call was defined as a call for cardiopulmonary resuscitation when a patient has a CRA.
An event was an occurrence of 1 of the following in a general care setting: unplanned transfer to the ICU, resuscitation call, or RRS activation.
The RRS activation criteria consisted of an “acute and persistent change” in any 1 or more of the following: oxygen saturations less than 90%, heart rate less than 40 or greater than 130 beats/minute, systolic blood pressure less than 90 mm Hg, or respiratory rate less than 10 or greater than 28 breaths/minute. The RRS activation requires health provider action; they are not electronically generated. Nurses and physicians may also activate the RRS if they are concerned about a patient, even if calling criteria are not met. This is in contrast to the EWS analyzed, which are aggregate composites of multiple parameters. However, whether or not a derangement in vital signs is considered an “acute and persistent change” still involves clinical judgment. Any movement from a general care bed to an ICU bed, or from a general care bed to a procedure area, and from there to an ICU, was considered unplanned. Transfers to the ICU directly from the emergency department or operating room (OR) were not considered as an unplanned transfer and were not included in the analyses.
Coverage time was the period observed for events after a rule was triggered. In this analysis, a coverage time of 24 hours was considered, with a 1-hour look-back. A trigger was counted as a true positive if an event occurred during the following 24 hours. The 1-hour look-back was included to take into account the nursing clinical process of prioritizing a call to the RRS followed by documentation of the altered vital signs that prompted the call.
An episode was the continuous time on the general care floor within a hospitalization, excluding times when a patient was in the OR or ICU. For example, if a patient was admitted to a general bed on a surgery floor, subsequently went to the OR, and then returned to the surgery floor, the 2 episodes were considered separate: the time on the floor before surgery, and the time on the floor after surgery.
Assessment of implementation of RRS in our hospitals showed a significant drop in the failure-to-rescue rate (issues considered related to delay or failure to identify or intervene appropriately when a patient was deteriorating, as identified through mortality review) and a decrease in non-ICU mortality.9,10 This suggests that our current process captures many of the relevant episodes of acute deterioration when a rapid response team is needed and supports using RRS activation as outcomes.
Data Sources
We developed a time-stamped longitudinal database of patient data from the electronic health record, including vital signs, laboratory test results, demographics (age, sex), administrative data (including length of stay), comorbidities, resuscitation code status, location in hospital, and at the minute level throughout each patient’s hospital stay. Physiologically impossible values (eg, blood pressures of 1200 mm Hg) were considered entered in error and eliminated from the database. Time spent in the OR or ICU was excluded because RRS activation would not be applied in these already highly monitored areas. SAS Statistical software (SAS Institute Inc. Cary, North Carolina) was used for database creation.
We applied the current RRS calling criteria in our institution and calculated the Kirkland score,11 along with some of the most widely used early warning scores:12 Modified Early Warning System (MEWS),13 Standardized Early Warning Scoring System (SEWS),14 Global Modified Early Warning Score (GMEWS),15 Worthing physiologic scoring system,16 National Early Warning Score (NEWS),17 and VitaPAC Early Warning Score (ViEWS).18 Published thresholds for these scores were used to create rule triggers in the data. Once a trigger was created to calculate the number of false positives and true positives, all subsequent triggers were ignored until the end of the episode or until 24 hours elapsed. We calculated triggers in a rolling fashion throughout the episodes of care. The EWS score was updated every time a new parameter was entered into the analytical electronic health record, and the most recent value for each was used to calculate the score. SAS statistical software was used for calculation of scores and identification of outcomes.
For our analysis, events were treated as dependent variables, and triggers were independent variables. We calculated the score for each EWS to the minute level throughout our retrospective database. If the score for a specific EWS was higher than the published/recommended threshold for that EWS, an alert was considered to have been issued, and the patient was followed for 24 hours. If the patient had an event in the subsequent 24 hours, or 1 hour before (1-hour look-back), the alert was considered a true positive; if not, a false positive. Events that were not preceded by an alert were false negatives, and 24-hour intervals without either an alert or an event were considered true negatives. This simulation exercise was performed for each EWS in both subcohorts (medical and surgical). Clusters of RRS calls followed by transfers to the ICU within 3 hours were considered as a single adverse event (RRS calls, as it was the first event to occur) to avoid double counting. We have described how well this simulation methodology,8 correlates with results from prospective studies.19
Statistical Analysis
To calculate whether results were statistically significant for subgroups, a jackknife method of calculating variance20 was used. The jackknife method calculates variance by repeating the calculations of the statistic leaving out 1 sample at a time. In our case, we repeated the calculation of sensitivity and PPV leaving out 1 patient at a time. Once the simulation method had been run and the false/true positives/negatives had been assigned, calculation of each metric (PPV and sensitivity) was repeated for n subsamples, each leaving out 1 patient. The variance was calculated and 2 Student t tests were performed for each EWS: 1 for PPV and another for sensitivity. SAS statistical software v 9.3 was used for the simulation analysis; R statistical software v 3.0.2 (The R Foundation, Vienna, Austria) was used for the calculation of the statistical significance of results. A univariable analysis was also performed to assess the sensitivity and PPVs for the published thresholds of the most common variables in each EWS: respiratory rate, systolic blood pressure, heart rate, temperature, and mental status as measured by the modified Richmond Agitation Sedation Score.21
RESULTS
The initial cohort included 60,020 hospitalizations, of which the following were excluded: 2751 because of a lack of appropriate research authorization; 6433 because the patients were younger than 18 years; 2129 as psychiatric admissions; 284 as rehabilitation admissions; 872 as research purposes-only admissions; and 1185 because the patient was never in a general care bed (eg, they were either admitted directly to the ICU, or they were admitted for an outpatient surgical procedure and spent time in the postanesthesia care unit).
Table 1 summarizes patient and trigger characteristics, overall and by subgroup. The final cohort included 75,240 total episodes in 46,366 hospitalizations, from 34,898 unique patients, of which 48.7% were male. There were 23,831 medical and 22,535 surgical hospitalizations. Median length of episode was 2 days both for medical and surgical patients. Median length of stay was 3 days, both for medical and for surgical patients.
There were 3332 events in total, of which 1709 were RRS calls, 185 were resuscitation calls, and 1438 were unscheduled transfers to the ICU. The rate of events was 4.67 events per 100 episodes in the aggregate adult population. There were 3.93 events per 100 episodes for surgical hospitalizations, and 5.86 events per 100 episodes for medical hospitalizations (P < .001). The number of CRAs in our cohort was 0.27 per 100 episodes, 0.128 per hospital bed per year, or 4.37 per 1000 hospital admissions, similar to other reported numbers in the literature.3, 22,23
The total number of EWS triggers varied greatly between EWS rules, with the volume ranging during the study year from 1363 triggers with the GMEWS rule to 77,711 triggers with the ViEWS score.
All scores had PPVs less than 25%. As seen in Table 2 and shown graphically in the Figure, all scores performed better on medical patients (blue) than on surgical patients (yellow). The P value was < .0001 for both PPV and sensitivity. The Worthing score had the highest sensitivity (0.78 for medical and 0.68 for surgical) but a very low PPV (0.04 for medical and 0.03 for surgical), while GMEWS was the opposite: low sensitivity (0.10 and 0.07) but the highest PPV (0.22 and 0.18).
The results of the univariable analysis can be seen in Table 3. Most of the criteria performed better (higher sensitivity and PPV) as predictors in the medical hospitalizations than in the surgical hospitalizations.
DISCUSSION
We hypothesized that EWS may perform differently when applied to medical rather than surgical patients. Studies had not analyzed this in a time-dependent manner,24-26 which limited the applicability of the results.8
All analyzed scores performed better in medical patients than in surgical patients (Figure). This could reflect a behavioral difference by the teams on surgical and medical floors in the decision to activate the RRS, or a bias of the clinicians who designed the scores (mostly nonsurgeons). The difference could also mean that physiological deteriorations are intrinsically different in patients who have undergone anesthesia and surgery. For example, in surgical patients, a bleeding episode is more likely to be the cause of their physiological deterioration, or the lingering effects of anesthesia could mask underlying deterioration. Such patients would benefit from scores where variables such as heart rate, blood pressure, or hemoglobin had more influence.
When comparing the different scores, it was much easier for a patient to meet the alerting score with the Worthing score than with GMEWS. In the Worthing score, a respiratory rate greater than 22 breaths per minute, or a systolic blood pressure less than 100 mm Hg, already meet alerting criteria. Similar vital signs result in 0 and 1 points (respectively) in GMEWS, far from its alerting score of 5. This reflects the intrinsic tradeoff of EWS: as the threshold for considering a patient “at risk” drops, not only does the number of true positives (and the sensitivity) increase, but also the number of false positives, thus lowering the PPV.
However, none of the scores analyzed were considered to perform well based on their PPV and sensitivity, particularly in the surgical subpopulation. Focusing on another metric, the area under the receiver operator curve can give misleadingly optimistic results.24,27 However, the extremely low prevalence of acute physiological deterioration can produce low PPVs even when specificity seems acceptable, which is why it is important to evaluate PPV directly.28
To use EWS effectively to activate RRS, they need to be combined with clinical judgment to avoid high levels of false alerts, particularly in surgical patients. It has been reported that RRS is activated only 30% of the time a patient meets RRS calling criteria.29 While there may be cultural characteristics inhibiting the decision to call,30 our study hints at another explanation: if RRS was activated every time a patient met calling criteria based on the scores analyzed, the number of RRS calls would be very high and difficult to manage. So health providers may be doing the right thing when “filtering” RRS calls and not applying the criteria strictly, but in conjunction with clinical judgment.
A limitation of any study like this is how to define “acute physiological deterioration.” We defined an event as recognized episodes of acute physiological deterioration that are signaled by escalations of care (eg, RRS, resuscitation calls, or transfers to an ICU) or unexpected death. By definition, our calculated PPV is affected by clinicians’ recognition of clinical deteriorations. This definition, common in the literature, has the limitation of potentially underestimating EWS’ performance by missing some events that are resolved by the primary care team without an escalation of care. However, we believe our interpretation is not unreasonable since the purpose of EWS is to trigger escalations of care in a timely fashion. Prospective studies could define an event in a way that is less affected by the clinicians’ judgment.
Regarding patient demographics, age was similar between the 2 groups (average, 58.2 years for medical vs 58.9 years for surgical), and there was only a small difference in gender ratios (45.1% male in the medical vs 51.4% in the surgical group). These differences are unlikely to have affected the results significantly, but unknown differences in demographics or other patient characteristics between groups may account for differences in score performance between surgical and medical patients.
Several of the EWS analyzed had overlapping trigger criteria with our own RRS activation criteria (although as single-parameter triggers and not as aggregate). To test how these potential biases could affect our results, we performed a post hoc sensitivity analysis eliminating calls to the RRS as an outcome (so using the alternative outcome of unexpected transfers to the ICU and resuscitation calls). The results are similar to those of our main analysis, with all analyzed scores having lower sensitivity and PPV in surgical hospitalizations when compared to medical hospitalizations.
Our study suggests that, to optimize detection of physiological deterioration events, EWS should try to take into account different patient types, with the most basic distinction being surgical vs medical. This tailoring will make EWS more complex, and less suited for paper-based calculation, but new electronic health records are increasingly able to incorporate decision support, and some EWS have been developed for electronic calculation only. Of particular interest in this regard is the score developed by Escobar et al,31 which groups patients into categories according to the reason for admission, and calculates a different subscore based on that category. While the score by Escobar et al. does not split patients based on medical or surgical status, a more general interpretation of our results suggests that a score may be more accurate if it classifies patients into subgroups with different subscores. This seems to be confirmed by the fact that the score by Escobar et al performs better than MEWS.28 Unfortunately, the paper describing it does not provide enough detail to use it in our database.
A recent systematic review showed increasing evidence that RRS may be effective in reducing CRAs occurring in a non-ICU setting and, more important, overall inhospital mortality.32 While differing implementation strategies (eg, different length of the educational effort, changes in the frequency of vital signs monitoring) can impact the success of such an initiative, it has been speculated that the afferent limb (which often includes an EWS) might be the most critical part of the system.33 Our results show that the most widely used EWS perform significantly worse on surgical patients, and suggest that a way to improve the accuracy of EWS would be to tailor the risk calculation to different patient subgroups (eg, medical and surgical patients). Plausible next steps would be to demonstrate that tailoring risk calculation to medical and surgical patients separately can improve risk predictions and accuracy of EWS.
Disclosure
The authors report no financial conflicts of interest.
1. Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999; 171(1):22-25. PubMed
2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. PubMed
3. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003; 58(3):297-308. PubMed
4. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57. PubMed
5. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463-2478. PubMed
6. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. PubMed
7. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
8. Romero-Brufau S, Huddleston JM, Naessens JM, Johnson MG, Hickman J, Morlan BW, et al. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549-552. PubMed
9. Huddleston JM, Diedrich DA, Kinsey GC, Enzler MJ, Manning DM. Learning from every death. J Patient Saf. 2014;10(1):6-12. PubMed
10. Moriarty JP, Schiebel NE, Johnson MG, Jensen JB, Caples SM, Morlan BW, et al. Evaluating implementation of a rapid response team: considering alternative outcome measures. Int J Qual Health Care. 2014;26(1):49-57. PubMed
11. Kirkland LL, Malinchoc M, O’Byrne M, Benson JT, Kashiwagi DT, Burton MC, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142. PubMed
12. Griffiths JR, Kidney EM. Current use of early warning scores in UK emergency departments. Emerg Med J. 2012;29(1):65-66. PubMed
13. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-526. PubMed
14. Paterson R, MacLeod DC, Thetford D, Beattie A, Graham C, Lam S, et al.. Prediction of in-hospital mortality and length of stay using an early warning scoring system: clinical audit. Clin Med (Lond). 2006;6(3):281-284. PubMed
15. Harrison GA, Jacques T, McLaws ML, Kilborn G. Combinations of early signs of critical illness predict in-hospital death–the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71(3):327-334. PubMed
16. Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007; 98(6):769-774. PubMed
17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-470. PubMed
18. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932-937. PubMed
19. Romero-Brufau S, Huddleston JM. Reply to letter: widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(10):e159. PubMed
20. Efron B, Stein C. The jackknife estimate of variance. Annals of Statistics. 1981;586-596.
21. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. PubMed
22. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. PubMed
23. Goncales PD, Polessi JA, Bass LM, Santos Gde P, Yokota PK, Laselva CR, et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442-448. PubMed
24. Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. PubMed
25. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88(6):571-575. PubMed
26. Stenhouse C, Coates S, Tivey M, Allsop P, Parker T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. British Journal of Anaesthesia. 2000;84(5):663-663.
27. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation. 2008;77(2):170-179. PubMed
28. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015; 19:285. PubMed
29. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. PubMed
30. Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569-575. PubMed
31. Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. PubMed
32. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417-425. PubMed
33. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-146. PubMed
Patients typically show signs and symptoms of deterioration hours to days prior to cardiorespiratory arrest.1,2 The rate of inhospital cardiorespiratory arrest (CRA) requiring cardiopulmonary resuscitation is estimated to be 0.174 per bed per year in the United States.3 After CRA, survival to discharge is estimated to be as low as 18%.3,4 Efforts to predict and prevent arrest could prove beneficial.1,2
Rapid response systems (RRS) have been proposed as a means of identifying clinical deterioration and facilitating a timely response. These systems were designed to bring clinicians with critical care expertise to the bedside to prevent unnecessary deaths. They typically include an afferent limb (detects deteriorating patients), an efferent limb (responds to calls and acts to avoid further deterioration), and administrative and data analysis limbs.5,6 Automatic provision of recommendations and computer-based systems are desirable components of the afferent limb of the detection system.6 Both are independent predictors of improved clinical practices for clinical decision support systems.7 However, the existing early warning scores (EWS) may not be ready for automation due to low positive predictive values (PPV) and sensitivities.8
It is possible that the low discriminatory accuracy of the published EWS may be secondary to the use of aggregate patient populations for derivation of scores. We hypothesized that these EWS perform differently in medical and in surgical subpopulations. Also, the EWS need to be tested in a time-dependent manner to serve as a realistic clinical support tool for hospitalized patients.
STUDY AIM
The aim of this study was to evaluate the differential performance of widely used EWS in medical vs surgical patients.
METHODS
Site
The study was conducted in an academic center with 2 hospitals in Southeastern Minnesota totaling approximately 1500 general care nonintensive care unit (ICU) beds. The Mayo Clinic Institutional Review Board approved the research proposal.
Subjects
Our retrospective cohort was comprised of all adult inpatients discharged from 2 academic hospitals between January 1, 2011 and December 31, 2011 who spent any time in a general care (non-ICU) unit. We excluded patients younger than 18 years, psychiatric or rehabilitation inpatients, those without research authorization, and patients admitted for research purposes.
Study patients were divided into medical and surgical cohorts. Hospitalizations were considered surgical if patients had surgery at any time during their hospital stay according to billing data. A trigger was an instance in which a patient met the conditions of a specific rule (score/vital sign exceeded the published/defined threshold).
A resuscitation call was defined as a call for cardiopulmonary resuscitation when a patient has a CRA.
An event was an occurrence of 1 of the following in a general care setting: unplanned transfer to the ICU, resuscitation call, or RRS activation.
The RRS activation criteria consisted of an “acute and persistent change” in any 1 or more of the following: oxygen saturations less than 90%, heart rate less than 40 or greater than 130 beats/minute, systolic blood pressure less than 90 mm Hg, or respiratory rate less than 10 or greater than 28 breaths/minute. The RRS activation requires health provider action; they are not electronically generated. Nurses and physicians may also activate the RRS if they are concerned about a patient, even if calling criteria are not met. This is in contrast to the EWS analyzed, which are aggregate composites of multiple parameters. However, whether or not a derangement in vital signs is considered an “acute and persistent change” still involves clinical judgment. Any movement from a general care bed to an ICU bed, or from a general care bed to a procedure area, and from there to an ICU, was considered unplanned. Transfers to the ICU directly from the emergency department or operating room (OR) were not considered as an unplanned transfer and were not included in the analyses.
Coverage time was the period observed for events after a rule was triggered. In this analysis, a coverage time of 24 hours was considered, with a 1-hour look-back. A trigger was counted as a true positive if an event occurred during the following 24 hours. The 1-hour look-back was included to take into account the nursing clinical process of prioritizing a call to the RRS followed by documentation of the altered vital signs that prompted the call.
An episode was the continuous time on the general care floor within a hospitalization, excluding times when a patient was in the OR or ICU. For example, if a patient was admitted to a general bed on a surgery floor, subsequently went to the OR, and then returned to the surgery floor, the 2 episodes were considered separate: the time on the floor before surgery, and the time on the floor after surgery.
Assessment of implementation of RRS in our hospitals showed a significant drop in the failure-to-rescue rate (issues considered related to delay or failure to identify or intervene appropriately when a patient was deteriorating, as identified through mortality review) and a decrease in non-ICU mortality.9,10 This suggests that our current process captures many of the relevant episodes of acute deterioration when a rapid response team is needed and supports using RRS activation as outcomes.
Data Sources
We developed a time-stamped longitudinal database of patient data from the electronic health record, including vital signs, laboratory test results, demographics (age, sex), administrative data (including length of stay), comorbidities, resuscitation code status, location in hospital, and at the minute level throughout each patient’s hospital stay. Physiologically impossible values (eg, blood pressures of 1200 mm Hg) were considered entered in error and eliminated from the database. Time spent in the OR or ICU was excluded because RRS activation would not be applied in these already highly monitored areas. SAS Statistical software (SAS Institute Inc. Cary, North Carolina) was used for database creation.
We applied the current RRS calling criteria in our institution and calculated the Kirkland score,11 along with some of the most widely used early warning scores:12 Modified Early Warning System (MEWS),13 Standardized Early Warning Scoring System (SEWS),14 Global Modified Early Warning Score (GMEWS),15 Worthing physiologic scoring system,16 National Early Warning Score (NEWS),17 and VitaPAC Early Warning Score (ViEWS).18 Published thresholds for these scores were used to create rule triggers in the data. Once a trigger was created to calculate the number of false positives and true positives, all subsequent triggers were ignored until the end of the episode or until 24 hours elapsed. We calculated triggers in a rolling fashion throughout the episodes of care. The EWS score was updated every time a new parameter was entered into the analytical electronic health record, and the most recent value for each was used to calculate the score. SAS statistical software was used for calculation of scores and identification of outcomes.
For our analysis, events were treated as dependent variables, and triggers were independent variables. We calculated the score for each EWS to the minute level throughout our retrospective database. If the score for a specific EWS was higher than the published/recommended threshold for that EWS, an alert was considered to have been issued, and the patient was followed for 24 hours. If the patient had an event in the subsequent 24 hours, or 1 hour before (1-hour look-back), the alert was considered a true positive; if not, a false positive. Events that were not preceded by an alert were false negatives, and 24-hour intervals without either an alert or an event were considered true negatives. This simulation exercise was performed for each EWS in both subcohorts (medical and surgical). Clusters of RRS calls followed by transfers to the ICU within 3 hours were considered as a single adverse event (RRS calls, as it was the first event to occur) to avoid double counting. We have described how well this simulation methodology,8 correlates with results from prospective studies.19
Statistical Analysis
To calculate whether results were statistically significant for subgroups, a jackknife method of calculating variance20 was used. The jackknife method calculates variance by repeating the calculations of the statistic leaving out 1 sample at a time. In our case, we repeated the calculation of sensitivity and PPV leaving out 1 patient at a time. Once the simulation method had been run and the false/true positives/negatives had been assigned, calculation of each metric (PPV and sensitivity) was repeated for n subsamples, each leaving out 1 patient. The variance was calculated and 2 Student t tests were performed for each EWS: 1 for PPV and another for sensitivity. SAS statistical software v 9.3 was used for the simulation analysis; R statistical software v 3.0.2 (The R Foundation, Vienna, Austria) was used for the calculation of the statistical significance of results. A univariable analysis was also performed to assess the sensitivity and PPVs for the published thresholds of the most common variables in each EWS: respiratory rate, systolic blood pressure, heart rate, temperature, and mental status as measured by the modified Richmond Agitation Sedation Score.21
RESULTS
The initial cohort included 60,020 hospitalizations, of which the following were excluded: 2751 because of a lack of appropriate research authorization; 6433 because the patients were younger than 18 years; 2129 as psychiatric admissions; 284 as rehabilitation admissions; 872 as research purposes-only admissions; and 1185 because the patient was never in a general care bed (eg, they were either admitted directly to the ICU, or they were admitted for an outpatient surgical procedure and spent time in the postanesthesia care unit).
Table 1 summarizes patient and trigger characteristics, overall and by subgroup. The final cohort included 75,240 total episodes in 46,366 hospitalizations, from 34,898 unique patients, of which 48.7% were male. There were 23,831 medical and 22,535 surgical hospitalizations. Median length of episode was 2 days both for medical and surgical patients. Median length of stay was 3 days, both for medical and for surgical patients.
There were 3332 events in total, of which 1709 were RRS calls, 185 were resuscitation calls, and 1438 were unscheduled transfers to the ICU. The rate of events was 4.67 events per 100 episodes in the aggregate adult population. There were 3.93 events per 100 episodes for surgical hospitalizations, and 5.86 events per 100 episodes for medical hospitalizations (P < .001). The number of CRAs in our cohort was 0.27 per 100 episodes, 0.128 per hospital bed per year, or 4.37 per 1000 hospital admissions, similar to other reported numbers in the literature.3, 22,23
The total number of EWS triggers varied greatly between EWS rules, with the volume ranging during the study year from 1363 triggers with the GMEWS rule to 77,711 triggers with the ViEWS score.
All scores had PPVs less than 25%. As seen in Table 2 and shown graphically in the Figure, all scores performed better on medical patients (blue) than on surgical patients (yellow). The P value was < .0001 for both PPV and sensitivity. The Worthing score had the highest sensitivity (0.78 for medical and 0.68 for surgical) but a very low PPV (0.04 for medical and 0.03 for surgical), while GMEWS was the opposite: low sensitivity (0.10 and 0.07) but the highest PPV (0.22 and 0.18).
The results of the univariable analysis can be seen in Table 3. Most of the criteria performed better (higher sensitivity and PPV) as predictors in the medical hospitalizations than in the surgical hospitalizations.
DISCUSSION
We hypothesized that EWS may perform differently when applied to medical rather than surgical patients. Studies had not analyzed this in a time-dependent manner,24-26 which limited the applicability of the results.8
All analyzed scores performed better in medical patients than in surgical patients (Figure). This could reflect a behavioral difference by the teams on surgical and medical floors in the decision to activate the RRS, or a bias of the clinicians who designed the scores (mostly nonsurgeons). The difference could also mean that physiological deteriorations are intrinsically different in patients who have undergone anesthesia and surgery. For example, in surgical patients, a bleeding episode is more likely to be the cause of their physiological deterioration, or the lingering effects of anesthesia could mask underlying deterioration. Such patients would benefit from scores where variables such as heart rate, blood pressure, or hemoglobin had more influence.
When comparing the different scores, it was much easier for a patient to meet the alerting score with the Worthing score than with GMEWS. In the Worthing score, a respiratory rate greater than 22 breaths per minute, or a systolic blood pressure less than 100 mm Hg, already meet alerting criteria. Similar vital signs result in 0 and 1 points (respectively) in GMEWS, far from its alerting score of 5. This reflects the intrinsic tradeoff of EWS: as the threshold for considering a patient “at risk” drops, not only does the number of true positives (and the sensitivity) increase, but also the number of false positives, thus lowering the PPV.
However, none of the scores analyzed were considered to perform well based on their PPV and sensitivity, particularly in the surgical subpopulation. Focusing on another metric, the area under the receiver operator curve can give misleadingly optimistic results.24,27 However, the extremely low prevalence of acute physiological deterioration can produce low PPVs even when specificity seems acceptable, which is why it is important to evaluate PPV directly.28
To use EWS effectively to activate RRS, they need to be combined with clinical judgment to avoid high levels of false alerts, particularly in surgical patients. It has been reported that RRS is activated only 30% of the time a patient meets RRS calling criteria.29 While there may be cultural characteristics inhibiting the decision to call,30 our study hints at another explanation: if RRS was activated every time a patient met calling criteria based on the scores analyzed, the number of RRS calls would be very high and difficult to manage. So health providers may be doing the right thing when “filtering” RRS calls and not applying the criteria strictly, but in conjunction with clinical judgment.
A limitation of any study like this is how to define “acute physiological deterioration.” We defined an event as recognized episodes of acute physiological deterioration that are signaled by escalations of care (eg, RRS, resuscitation calls, or transfers to an ICU) or unexpected death. By definition, our calculated PPV is affected by clinicians’ recognition of clinical deteriorations. This definition, common in the literature, has the limitation of potentially underestimating EWS’ performance by missing some events that are resolved by the primary care team without an escalation of care. However, we believe our interpretation is not unreasonable since the purpose of EWS is to trigger escalations of care in a timely fashion. Prospective studies could define an event in a way that is less affected by the clinicians’ judgment.
Regarding patient demographics, age was similar between the 2 groups (average, 58.2 years for medical vs 58.9 years for surgical), and there was only a small difference in gender ratios (45.1% male in the medical vs 51.4% in the surgical group). These differences are unlikely to have affected the results significantly, but unknown differences in demographics or other patient characteristics between groups may account for differences in score performance between surgical and medical patients.
Several of the EWS analyzed had overlapping trigger criteria with our own RRS activation criteria (although as single-parameter triggers and not as aggregate). To test how these potential biases could affect our results, we performed a post hoc sensitivity analysis eliminating calls to the RRS as an outcome (so using the alternative outcome of unexpected transfers to the ICU and resuscitation calls). The results are similar to those of our main analysis, with all analyzed scores having lower sensitivity and PPV in surgical hospitalizations when compared to medical hospitalizations.
Our study suggests that, to optimize detection of physiological deterioration events, EWS should try to take into account different patient types, with the most basic distinction being surgical vs medical. This tailoring will make EWS more complex, and less suited for paper-based calculation, but new electronic health records are increasingly able to incorporate decision support, and some EWS have been developed for electronic calculation only. Of particular interest in this regard is the score developed by Escobar et al,31 which groups patients into categories according to the reason for admission, and calculates a different subscore based on that category. While the score by Escobar et al. does not split patients based on medical or surgical status, a more general interpretation of our results suggests that a score may be more accurate if it classifies patients into subgroups with different subscores. This seems to be confirmed by the fact that the score by Escobar et al performs better than MEWS.28 Unfortunately, the paper describing it does not provide enough detail to use it in our database.
A recent systematic review showed increasing evidence that RRS may be effective in reducing CRAs occurring in a non-ICU setting and, more important, overall inhospital mortality.32 While differing implementation strategies (eg, different length of the educational effort, changes in the frequency of vital signs monitoring) can impact the success of such an initiative, it has been speculated that the afferent limb (which often includes an EWS) might be the most critical part of the system.33 Our results show that the most widely used EWS perform significantly worse on surgical patients, and suggest that a way to improve the accuracy of EWS would be to tailor the risk calculation to different patient subgroups (eg, medical and surgical patients). Plausible next steps would be to demonstrate that tailoring risk calculation to medical and surgical patients separately can improve risk predictions and accuracy of EWS.
Disclosure
The authors report no financial conflicts of interest.
Patients typically show signs and symptoms of deterioration hours to days prior to cardiorespiratory arrest.1,2 The rate of inhospital cardiorespiratory arrest (CRA) requiring cardiopulmonary resuscitation is estimated to be 0.174 per bed per year in the United States.3 After CRA, survival to discharge is estimated to be as low as 18%.3,4 Efforts to predict and prevent arrest could prove beneficial.1,2
Rapid response systems (RRS) have been proposed as a means of identifying clinical deterioration and facilitating a timely response. These systems were designed to bring clinicians with critical care expertise to the bedside to prevent unnecessary deaths. They typically include an afferent limb (detects deteriorating patients), an efferent limb (responds to calls and acts to avoid further deterioration), and administrative and data analysis limbs.5,6 Automatic provision of recommendations and computer-based systems are desirable components of the afferent limb of the detection system.6 Both are independent predictors of improved clinical practices for clinical decision support systems.7 However, the existing early warning scores (EWS) may not be ready for automation due to low positive predictive values (PPV) and sensitivities.8
It is possible that the low discriminatory accuracy of the published EWS may be secondary to the use of aggregate patient populations for derivation of scores. We hypothesized that these EWS perform differently in medical and in surgical subpopulations. Also, the EWS need to be tested in a time-dependent manner to serve as a realistic clinical support tool for hospitalized patients.
STUDY AIM
The aim of this study was to evaluate the differential performance of widely used EWS in medical vs surgical patients.
METHODS
Site
The study was conducted in an academic center with 2 hospitals in Southeastern Minnesota totaling approximately 1500 general care nonintensive care unit (ICU) beds. The Mayo Clinic Institutional Review Board approved the research proposal.
Subjects
Our retrospective cohort was comprised of all adult inpatients discharged from 2 academic hospitals between January 1, 2011 and December 31, 2011 who spent any time in a general care (non-ICU) unit. We excluded patients younger than 18 years, psychiatric or rehabilitation inpatients, those without research authorization, and patients admitted for research purposes.
Study patients were divided into medical and surgical cohorts. Hospitalizations were considered surgical if patients had surgery at any time during their hospital stay according to billing data. A trigger was an instance in which a patient met the conditions of a specific rule (score/vital sign exceeded the published/defined threshold).
A resuscitation call was defined as a call for cardiopulmonary resuscitation when a patient has a CRA.
An event was an occurrence of 1 of the following in a general care setting: unplanned transfer to the ICU, resuscitation call, or RRS activation.
The RRS activation criteria consisted of an “acute and persistent change” in any 1 or more of the following: oxygen saturations less than 90%, heart rate less than 40 or greater than 130 beats/minute, systolic blood pressure less than 90 mm Hg, or respiratory rate less than 10 or greater than 28 breaths/minute. The RRS activation requires health provider action; they are not electronically generated. Nurses and physicians may also activate the RRS if they are concerned about a patient, even if calling criteria are not met. This is in contrast to the EWS analyzed, which are aggregate composites of multiple parameters. However, whether or not a derangement in vital signs is considered an “acute and persistent change” still involves clinical judgment. Any movement from a general care bed to an ICU bed, or from a general care bed to a procedure area, and from there to an ICU, was considered unplanned. Transfers to the ICU directly from the emergency department or operating room (OR) were not considered as an unplanned transfer and were not included in the analyses.
Coverage time was the period observed for events after a rule was triggered. In this analysis, a coverage time of 24 hours was considered, with a 1-hour look-back. A trigger was counted as a true positive if an event occurred during the following 24 hours. The 1-hour look-back was included to take into account the nursing clinical process of prioritizing a call to the RRS followed by documentation of the altered vital signs that prompted the call.
An episode was the continuous time on the general care floor within a hospitalization, excluding times when a patient was in the OR or ICU. For example, if a patient was admitted to a general bed on a surgery floor, subsequently went to the OR, and then returned to the surgery floor, the 2 episodes were considered separate: the time on the floor before surgery, and the time on the floor after surgery.
Assessment of implementation of RRS in our hospitals showed a significant drop in the failure-to-rescue rate (issues considered related to delay or failure to identify or intervene appropriately when a patient was deteriorating, as identified through mortality review) and a decrease in non-ICU mortality.9,10 This suggests that our current process captures many of the relevant episodes of acute deterioration when a rapid response team is needed and supports using RRS activation as outcomes.
Data Sources
We developed a time-stamped longitudinal database of patient data from the electronic health record, including vital signs, laboratory test results, demographics (age, sex), administrative data (including length of stay), comorbidities, resuscitation code status, location in hospital, and at the minute level throughout each patient’s hospital stay. Physiologically impossible values (eg, blood pressures of 1200 mm Hg) were considered entered in error and eliminated from the database. Time spent in the OR or ICU was excluded because RRS activation would not be applied in these already highly monitored areas. SAS Statistical software (SAS Institute Inc. Cary, North Carolina) was used for database creation.
We applied the current RRS calling criteria in our institution and calculated the Kirkland score,11 along with some of the most widely used early warning scores:12 Modified Early Warning System (MEWS),13 Standardized Early Warning Scoring System (SEWS),14 Global Modified Early Warning Score (GMEWS),15 Worthing physiologic scoring system,16 National Early Warning Score (NEWS),17 and VitaPAC Early Warning Score (ViEWS).18 Published thresholds for these scores were used to create rule triggers in the data. Once a trigger was created to calculate the number of false positives and true positives, all subsequent triggers were ignored until the end of the episode or until 24 hours elapsed. We calculated triggers in a rolling fashion throughout the episodes of care. The EWS score was updated every time a new parameter was entered into the analytical electronic health record, and the most recent value for each was used to calculate the score. SAS statistical software was used for calculation of scores and identification of outcomes.
For our analysis, events were treated as dependent variables, and triggers were independent variables. We calculated the score for each EWS to the minute level throughout our retrospective database. If the score for a specific EWS was higher than the published/recommended threshold for that EWS, an alert was considered to have been issued, and the patient was followed for 24 hours. If the patient had an event in the subsequent 24 hours, or 1 hour before (1-hour look-back), the alert was considered a true positive; if not, a false positive. Events that were not preceded by an alert were false negatives, and 24-hour intervals without either an alert or an event were considered true negatives. This simulation exercise was performed for each EWS in both subcohorts (medical and surgical). Clusters of RRS calls followed by transfers to the ICU within 3 hours were considered as a single adverse event (RRS calls, as it was the first event to occur) to avoid double counting. We have described how well this simulation methodology,8 correlates with results from prospective studies.19
Statistical Analysis
To calculate whether results were statistically significant for subgroups, a jackknife method of calculating variance20 was used. The jackknife method calculates variance by repeating the calculations of the statistic leaving out 1 sample at a time. In our case, we repeated the calculation of sensitivity and PPV leaving out 1 patient at a time. Once the simulation method had been run and the false/true positives/negatives had been assigned, calculation of each metric (PPV and sensitivity) was repeated for n subsamples, each leaving out 1 patient. The variance was calculated and 2 Student t tests were performed for each EWS: 1 for PPV and another for sensitivity. SAS statistical software v 9.3 was used for the simulation analysis; R statistical software v 3.0.2 (The R Foundation, Vienna, Austria) was used for the calculation of the statistical significance of results. A univariable analysis was also performed to assess the sensitivity and PPVs for the published thresholds of the most common variables in each EWS: respiratory rate, systolic blood pressure, heart rate, temperature, and mental status as measured by the modified Richmond Agitation Sedation Score.21
RESULTS
The initial cohort included 60,020 hospitalizations, of which the following were excluded: 2751 because of a lack of appropriate research authorization; 6433 because the patients were younger than 18 years; 2129 as psychiatric admissions; 284 as rehabilitation admissions; 872 as research purposes-only admissions; and 1185 because the patient was never in a general care bed (eg, they were either admitted directly to the ICU, or they were admitted for an outpatient surgical procedure and spent time in the postanesthesia care unit).
Table 1 summarizes patient and trigger characteristics, overall and by subgroup. The final cohort included 75,240 total episodes in 46,366 hospitalizations, from 34,898 unique patients, of which 48.7% were male. There were 23,831 medical and 22,535 surgical hospitalizations. Median length of episode was 2 days both for medical and surgical patients. Median length of stay was 3 days, both for medical and for surgical patients.
There were 3332 events in total, of which 1709 were RRS calls, 185 were resuscitation calls, and 1438 were unscheduled transfers to the ICU. The rate of events was 4.67 events per 100 episodes in the aggregate adult population. There were 3.93 events per 100 episodes for surgical hospitalizations, and 5.86 events per 100 episodes for medical hospitalizations (P < .001). The number of CRAs in our cohort was 0.27 per 100 episodes, 0.128 per hospital bed per year, or 4.37 per 1000 hospital admissions, similar to other reported numbers in the literature.3, 22,23
The total number of EWS triggers varied greatly between EWS rules, with the volume ranging during the study year from 1363 triggers with the GMEWS rule to 77,711 triggers with the ViEWS score.
All scores had PPVs less than 25%. As seen in Table 2 and shown graphically in the Figure, all scores performed better on medical patients (blue) than on surgical patients (yellow). The P value was < .0001 for both PPV and sensitivity. The Worthing score had the highest sensitivity (0.78 for medical and 0.68 for surgical) but a very low PPV (0.04 for medical and 0.03 for surgical), while GMEWS was the opposite: low sensitivity (0.10 and 0.07) but the highest PPV (0.22 and 0.18).
The results of the univariable analysis can be seen in Table 3. Most of the criteria performed better (higher sensitivity and PPV) as predictors in the medical hospitalizations than in the surgical hospitalizations.
DISCUSSION
We hypothesized that EWS may perform differently when applied to medical rather than surgical patients. Studies had not analyzed this in a time-dependent manner,24-26 which limited the applicability of the results.8
All analyzed scores performed better in medical patients than in surgical patients (Figure). This could reflect a behavioral difference by the teams on surgical and medical floors in the decision to activate the RRS, or a bias of the clinicians who designed the scores (mostly nonsurgeons). The difference could also mean that physiological deteriorations are intrinsically different in patients who have undergone anesthesia and surgery. For example, in surgical patients, a bleeding episode is more likely to be the cause of their physiological deterioration, or the lingering effects of anesthesia could mask underlying deterioration. Such patients would benefit from scores where variables such as heart rate, blood pressure, or hemoglobin had more influence.
When comparing the different scores, it was much easier for a patient to meet the alerting score with the Worthing score than with GMEWS. In the Worthing score, a respiratory rate greater than 22 breaths per minute, or a systolic blood pressure less than 100 mm Hg, already meet alerting criteria. Similar vital signs result in 0 and 1 points (respectively) in GMEWS, far from its alerting score of 5. This reflects the intrinsic tradeoff of EWS: as the threshold for considering a patient “at risk” drops, not only does the number of true positives (and the sensitivity) increase, but also the number of false positives, thus lowering the PPV.
However, none of the scores analyzed were considered to perform well based on their PPV and sensitivity, particularly in the surgical subpopulation. Focusing on another metric, the area under the receiver operator curve can give misleadingly optimistic results.24,27 However, the extremely low prevalence of acute physiological deterioration can produce low PPVs even when specificity seems acceptable, which is why it is important to evaluate PPV directly.28
To use EWS effectively to activate RRS, they need to be combined with clinical judgment to avoid high levels of false alerts, particularly in surgical patients. It has been reported that RRS is activated only 30% of the time a patient meets RRS calling criteria.29 While there may be cultural characteristics inhibiting the decision to call,30 our study hints at another explanation: if RRS was activated every time a patient met calling criteria based on the scores analyzed, the number of RRS calls would be very high and difficult to manage. So health providers may be doing the right thing when “filtering” RRS calls and not applying the criteria strictly, but in conjunction with clinical judgment.
A limitation of any study like this is how to define “acute physiological deterioration.” We defined an event as recognized episodes of acute physiological deterioration that are signaled by escalations of care (eg, RRS, resuscitation calls, or transfers to an ICU) or unexpected death. By definition, our calculated PPV is affected by clinicians’ recognition of clinical deteriorations. This definition, common in the literature, has the limitation of potentially underestimating EWS’ performance by missing some events that are resolved by the primary care team without an escalation of care. However, we believe our interpretation is not unreasonable since the purpose of EWS is to trigger escalations of care in a timely fashion. Prospective studies could define an event in a way that is less affected by the clinicians’ judgment.
Regarding patient demographics, age was similar between the 2 groups (average, 58.2 years for medical vs 58.9 years for surgical), and there was only a small difference in gender ratios (45.1% male in the medical vs 51.4% in the surgical group). These differences are unlikely to have affected the results significantly, but unknown differences in demographics or other patient characteristics between groups may account for differences in score performance between surgical and medical patients.
Several of the EWS analyzed had overlapping trigger criteria with our own RRS activation criteria (although as single-parameter triggers and not as aggregate). To test how these potential biases could affect our results, we performed a post hoc sensitivity analysis eliminating calls to the RRS as an outcome (so using the alternative outcome of unexpected transfers to the ICU and resuscitation calls). The results are similar to those of our main analysis, with all analyzed scores having lower sensitivity and PPV in surgical hospitalizations when compared to medical hospitalizations.
Our study suggests that, to optimize detection of physiological deterioration events, EWS should try to take into account different patient types, with the most basic distinction being surgical vs medical. This tailoring will make EWS more complex, and less suited for paper-based calculation, but new electronic health records are increasingly able to incorporate decision support, and some EWS have been developed for electronic calculation only. Of particular interest in this regard is the score developed by Escobar et al,31 which groups patients into categories according to the reason for admission, and calculates a different subscore based on that category. While the score by Escobar et al. does not split patients based on medical or surgical status, a more general interpretation of our results suggests that a score may be more accurate if it classifies patients into subgroups with different subscores. This seems to be confirmed by the fact that the score by Escobar et al performs better than MEWS.28 Unfortunately, the paper describing it does not provide enough detail to use it in our database.
A recent systematic review showed increasing evidence that RRS may be effective in reducing CRAs occurring in a non-ICU setting and, more important, overall inhospital mortality.32 While differing implementation strategies (eg, different length of the educational effort, changes in the frequency of vital signs monitoring) can impact the success of such an initiative, it has been speculated that the afferent limb (which often includes an EWS) might be the most critical part of the system.33 Our results show that the most widely used EWS perform significantly worse on surgical patients, and suggest that a way to improve the accuracy of EWS would be to tailor the risk calculation to different patient subgroups (eg, medical and surgical patients). Plausible next steps would be to demonstrate that tailoring risk calculation to medical and surgical patients separately can improve risk predictions and accuracy of EWS.
Disclosure
The authors report no financial conflicts of interest.
1. Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999; 171(1):22-25. PubMed
2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. PubMed
3. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003; 58(3):297-308. PubMed
4. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57. PubMed
5. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463-2478. PubMed
6. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. PubMed
7. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
8. Romero-Brufau S, Huddleston JM, Naessens JM, Johnson MG, Hickman J, Morlan BW, et al. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549-552. PubMed
9. Huddleston JM, Diedrich DA, Kinsey GC, Enzler MJ, Manning DM. Learning from every death. J Patient Saf. 2014;10(1):6-12. PubMed
10. Moriarty JP, Schiebel NE, Johnson MG, Jensen JB, Caples SM, Morlan BW, et al. Evaluating implementation of a rapid response team: considering alternative outcome measures. Int J Qual Health Care. 2014;26(1):49-57. PubMed
11. Kirkland LL, Malinchoc M, O’Byrne M, Benson JT, Kashiwagi DT, Burton MC, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142. PubMed
12. Griffiths JR, Kidney EM. Current use of early warning scores in UK emergency departments. Emerg Med J. 2012;29(1):65-66. PubMed
13. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-526. PubMed
14. Paterson R, MacLeod DC, Thetford D, Beattie A, Graham C, Lam S, et al.. Prediction of in-hospital mortality and length of stay using an early warning scoring system: clinical audit. Clin Med (Lond). 2006;6(3):281-284. PubMed
15. Harrison GA, Jacques T, McLaws ML, Kilborn G. Combinations of early signs of critical illness predict in-hospital death–the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71(3):327-334. PubMed
16. Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007; 98(6):769-774. PubMed
17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-470. PubMed
18. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932-937. PubMed
19. Romero-Brufau S, Huddleston JM. Reply to letter: widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(10):e159. PubMed
20. Efron B, Stein C. The jackknife estimate of variance. Annals of Statistics. 1981;586-596.
21. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. PubMed
22. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. PubMed
23. Goncales PD, Polessi JA, Bass LM, Santos Gde P, Yokota PK, Laselva CR, et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442-448. PubMed
24. Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. PubMed
25. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88(6):571-575. PubMed
26. Stenhouse C, Coates S, Tivey M, Allsop P, Parker T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. British Journal of Anaesthesia. 2000;84(5):663-663.
27. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation. 2008;77(2):170-179. PubMed
28. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015; 19:285. PubMed
29. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. PubMed
30. Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569-575. PubMed
31. Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. PubMed
32. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417-425. PubMed
33. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-146. PubMed
1. Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999; 171(1):22-25. PubMed
2. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98(6):1388-1392. PubMed
3. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003; 58(3):297-308. PubMed
4. Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50-57. PubMed
5. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34(9):2463-2478. PubMed
6. DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”--a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. PubMed
7. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. PubMed
8. Romero-Brufau S, Huddleston JM, Naessens JM, Johnson MG, Hickman J, Morlan BW, et al. Widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(4):549-552. PubMed
9. Huddleston JM, Diedrich DA, Kinsey GC, Enzler MJ, Manning DM. Learning from every death. J Patient Saf. 2014;10(1):6-12. PubMed
10. Moriarty JP, Schiebel NE, Johnson MG, Jensen JB, Caples SM, Morlan BW, et al. Evaluating implementation of a rapid response team: considering alternative outcome measures. Int J Qual Health Care. 2014;26(1):49-57. PubMed
11. Kirkland LL, Malinchoc M, O’Byrne M, Benson JT, Kashiwagi DT, Burton MC, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142. PubMed
12. Griffiths JR, Kidney EM. Current use of early warning scores in UK emergency departments. Emerg Med J. 2012;29(1):65-66. PubMed
13. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521-526. PubMed
14. Paterson R, MacLeod DC, Thetford D, Beattie A, Graham C, Lam S, et al.. Prediction of in-hospital mortality and length of stay using an early warning scoring system: clinical audit. Clin Med (Lond). 2006;6(3):281-284. PubMed
15. Harrison GA, Jacques T, McLaws ML, Kilborn G. Combinations of early signs of critical illness predict in-hospital death–the SOCCER study (signs of critical conditions and emergency responses). Resuscitation. 2006;71(3):327-334. PubMed
16. Duckitt RW, Buxton-Thomas R, Walker J, Cheek E, Bewick V, Venn R, et al. Worthing physiological scoring system: derivation and validation of a physiological early-warning system for medical admissions. An observational, population-based single-centre study. Br J Anaesth. 2007; 98(6):769-774. PubMed
17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465-470. PubMed
18. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932-937. PubMed
19. Romero-Brufau S, Huddleston JM. Reply to letter: widely used track and trigger scores: are they ready for automation in practice? Resuscitation. 2014;85(10):e159. PubMed
20. Efron B, Stein C. The jackknife estimate of variance. Annals of Statistics. 1981;586-596.
21. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. PubMed
22. DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Medical Emergency Response Improvement Team (MERIT). Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13(4):251-254. PubMed
23. Goncales PD, Polessi JA, Bass LM, Santos Gde P, Yokota PK, Laselva CR, et al. Reduced frequency of cardiopulmonary arrests by rapid response teams. Einstein (Sao Paulo). 2012;10(4):442-448. PubMed
24. Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. PubMed
25. Gardner-Thorpe J, Love N, Wrightson J, Walsh S, Keeling N. The value of Modified Early Warning Score (MEWS) in surgical in-patients: a prospective observational study. Ann R Coll Surg Engl. 2006;88(6):571-575. PubMed
26. Stenhouse C, Coates S, Tivey M, Allsop P, Parker T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. British Journal of Anaesthesia. 2000;84(5):663-663.
27. Smith GB, Prytherch DR, Schmidt PE, Featherstone PI. Review and performance evaluation of aggregate weighted ‘track and trigger’ systems. Resuscitation. 2008;77(2):170-179. PubMed
28. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015; 19:285. PubMed
29. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005;365(9477):2091-2097. PubMed
30. Shearer B, Marshall S, Buist MD, Finnigan M, Kitto S, Hore T, et al. What stops hospital clinical staff from following protocols? An analysis of the incidence and factors behind the failure of bedside clinical staff to activate the rapid response system in a multi-campus Australian metropolitan healthcare service. BMJ Qual Saf. 2012;21(7):569-575. PubMed
31. Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. PubMed
32. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):417-425. PubMed
33. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011;365(2):139-146. PubMed
© 2017 Society of Hospital Medicine
Nonoperative management of pediatric appendicitis appears feasible
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
This is the first data synthesis on the effectiveness of nonoperative management compared with appendectomy in children, and it shows that the evidence at this time is simply insufficient to warrant a change in clinical practice. Appendectomy remains the standard of care for this disease.
Despite the high early “success rate” for nonoperative treatment, patients in this group were nearly nine times more likely to have “treatment failure” than those who underwent immediate appendectomy.
The nonoperative approach remains an experimental proposition and should be offered only under protocol in a clinical trial setting. It clearly merits ongoing consideration, but much more data from high-quality clinical trials are needed.
Monica E. Lopez, MD, and David E. Wesson, MD, are both with the division of pediatric surgery at Baylor College of Medicine and the department of surgery at Texas Children’s Hospital, both in Houston. They reported having no relevant financial disclosures. Dr. Lopez and Dr. Wesson made these remarks in an editorial accompanying Dr. Huang’s report (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0056).
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
Nonoperative management of uncomplicated acute appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach, investigators reported March 27 in JAMA Pediatrics.
Nonoperative management, based on antibiotic treatment and close monitoring of the patient, is accepted as safe and effective in adults but has not been well studied in children and adolescents. “Owing to specific anatomical and pathophysiologic features of children, the clinical scenario of acute appendicitis in pediatric patients is different from that in adults, and treatment decisions for children are more difficult,” said Libin Huang, MD, of West China Hospital and Sichuan University, Chengdu, and his associates.
The few clinical trials that have been performed in children have had small sample sizes, so the investigators performed a meta-analysis to pool the results for 404 patients aged 5-18 years. They analyzed data from four single-center prospective but nonrandomized controlled trials and one single-center randomized controlled trial to compare outcomes between 168 patients initially treated with antibiotics and 236 who underwent immediate appendectomy.
Sixteen patients in the nonoperative group (9.5%) had treatment failure, defined as appendectomy within 48 hours (11 patients) or within 1 month of follow-up (5 patients). Three of these patients developed a complication (perforated appendicitis). In comparison, none of the surgery group had treatment failure, and one developed a complication requiring reoperation. Thus, the rate of success in the nonoperative group was 152 of 168 patients, or 90.5%, and the rate of complications was not significantly different between the two study groups, Dr. Huang and his associates said (JAMA Ped. 2017 Mar 27. doi: 10.1001/jamapediatrics.2017.0057).
During the following year, 27 patients in the nonoperative group had a histopathologically confirmed recurrence of appendicitis and underwent appendectomy; another 8 had the surgery because of parents’ requests. Nonoperative management was significantly more likely to fail in patients who had an appendicolith, so this approach should be considered inappropriate for this subgroup of patients, the investigators said.
Larger clinical trials with a randomized design, standardized criteria for antibiotic therapy, and longer follow-up are needed to confirm these preliminary findings, they added.
No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Nonoperative management of uncomplicated appendicitis in the pediatric population appeared feasible and didn’t raise the risk of complications in the first metaanalysis to examine this approach.
Major finding: The rate of treatment success in the nonoperative group was 90.5% (152 of 168 patients).
Data source: A metaanalysis of five single-center clinical trials involving 404 patients aged 5-18 years.
Disclosures: No sponsor was cited for this study. Dr. Huang and his associates reported having no relevant financial disclosures.
Mood and Memory Problems Associated With Deep Brain Stimulation
Bilateral deep brain stimulation of the anterior nucleus of the thalamus can help control seizures, but there are reports that suggest it also causes memory problems and depression. When Tröster et al analyzed data from a randomized trial (SANTE), they did find subjective evidence of both adverse events but were unable to confirm the presence of these problems with objective neurobehavioral measures. Nonetheless, they recommend that patients undergoing deep brain stimulation be monitored and undergo neuropsychological assessment for depression and memory problems.
Tröster AI, Meador KJ, Irwin CP, Fisher RS. Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure. 2017;45:133-141.
Bilateral deep brain stimulation of the anterior nucleus of the thalamus can help control seizures, but there are reports that suggest it also causes memory problems and depression. When Tröster et al analyzed data from a randomized trial (SANTE), they did find subjective evidence of both adverse events but were unable to confirm the presence of these problems with objective neurobehavioral measures. Nonetheless, they recommend that patients undergoing deep brain stimulation be monitored and undergo neuropsychological assessment for depression and memory problems.
Tröster AI, Meador KJ, Irwin CP, Fisher RS. Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure. 2017;45:133-141.
Bilateral deep brain stimulation of the anterior nucleus of the thalamus can help control seizures, but there are reports that suggest it also causes memory problems and depression. When Tröster et al analyzed data from a randomized trial (SANTE), they did find subjective evidence of both adverse events but were unable to confirm the presence of these problems with objective neurobehavioral measures. Nonetheless, they recommend that patients undergoing deep brain stimulation be monitored and undergo neuropsychological assessment for depression and memory problems.
Tröster AI, Meador KJ, Irwin CP, Fisher RS. Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure. 2017;45:133-141.
Why Do Patients Vary in Their Response to Cortical Electric Stimulation?
Patients vary widely in their response to cortical electric stimulation (CES). A retrospective analysis of 92 patients with medically intractable epilepsy who underwent CES was unable to detect any clinical or demographic factors that would explain the varied response to the procedure. Corley et al also found striking variability and a wide range of motor, sensory, and speech response thresholds between patients and within the different regions of the brain in the same patient.
Corley JA, Nazari P, Rossi VJ, et al. Cortical stimulation parameters for functional mapping. Seizure. 2017;45:36-71.
Patients vary widely in their response to cortical electric stimulation (CES). A retrospective analysis of 92 patients with medically intractable epilepsy who underwent CES was unable to detect any clinical or demographic factors that would explain the varied response to the procedure. Corley et al also found striking variability and a wide range of motor, sensory, and speech response thresholds between patients and within the different regions of the brain in the same patient.
Corley JA, Nazari P, Rossi VJ, et al. Cortical stimulation parameters for functional mapping. Seizure. 2017;45:36-71.
Patients vary widely in their response to cortical electric stimulation (CES). A retrospective analysis of 92 patients with medically intractable epilepsy who underwent CES was unable to detect any clinical or demographic factors that would explain the varied response to the procedure. Corley et al also found striking variability and a wide range of motor, sensory, and speech response thresholds between patients and within the different regions of the brain in the same patient.
Corley JA, Nazari P, Rossi VJ, et al. Cortical stimulation parameters for functional mapping. Seizure. 2017;45:36-71.
Deciphering the Significance of Generalized Periodic Discharges
Dementia, poor mental status during electroencephalogram (EEG), chronic focal abnormalities on neuroimaging, cardiac arrest, and chronic obstructive pulmonary disease (COPD) were independently associated with increased in-hospital mortality in patients with generalized periodic discharges (GPDs), according to an analysis of 113 patients at 3 hospitals. To determine the prognostic significance of GPDs observed during EEGs, Jadeja et al reviewed EEG tracings of the patients, of whom there were 60 inpatient deaths (53.1%).
Jadeja N, Zarnegar R, Legatt AD. Clinical outcomes in patients with generalized periodic discharges. Seizure. 2017; 45:114-118.
Dementia, poor mental status during electroencephalogram (EEG), chronic focal abnormalities on neuroimaging, cardiac arrest, and chronic obstructive pulmonary disease (COPD) were independently associated with increased in-hospital mortality in patients with generalized periodic discharges (GPDs), according to an analysis of 113 patients at 3 hospitals. To determine the prognostic significance of GPDs observed during EEGs, Jadeja et al reviewed EEG tracings of the patients, of whom there were 60 inpatient deaths (53.1%).
Jadeja N, Zarnegar R, Legatt AD. Clinical outcomes in patients with generalized periodic discharges. Seizure. 2017; 45:114-118.
Dementia, poor mental status during electroencephalogram (EEG), chronic focal abnormalities on neuroimaging, cardiac arrest, and chronic obstructive pulmonary disease (COPD) were independently associated with increased in-hospital mortality in patients with generalized periodic discharges (GPDs), according to an analysis of 113 patients at 3 hospitals. To determine the prognostic significance of GPDs observed during EEGs, Jadeja et al reviewed EEG tracings of the patients, of whom there were 60 inpatient deaths (53.1%).
Jadeja N, Zarnegar R, Legatt AD. Clinical outcomes in patients with generalized periodic discharges. Seizure. 2017; 45:114-118.
ACGME finalizes return of trainees’ 24-hour max shift
First-year residents will once again be permitted to work up to 24 consecutive hours following a reversal of a rule implemented in 2011 that restricted them to 16 hours, the Accreditation Council for Graduate Medical Education (ACGME) announced.
According to a memo issued by the ACGME March 10, 2017, the change reverting back to the 24-hour ceiling was evidence based.
“The preponderance of the evidence from a number of studies conducted after the current 16-hour cap was implemented in 2011 suggests that it may not have had an incremental benefit in patient safety, and that there might be significant negative impacts to the quality of physician education and professional development,” the memo states. The work week is still capped at 80 hours worked per week, with 1 day free from clinical experience or education in 7, and in-house call no more frequent than every third night.
An ACGME task force determined “that the hypothesized benefits associated with the changes made to first-year resident scheduled hours in 2011 have not been realized, and the disruption of team-based care and supervisory systems has had a significant negative impact on the professional education of the first-year resident, and the effectiveness of care delivery of the team as a whole.”
Sharmila Dissanaike, MD, FACS, chair and professor of surgery at Texas Tech University, Lubbock, said in an interview that the change back to a 24-hour ceiling provides “an increased flexibility for all residents in order to allow completion of immediate patient care responsibilities, such as finishing an operation, and ensure smooth handoffs. Both of these should improve work flow for both trainees and supervisors.”
Mark A. Malangoni, MD, FACS, associate executive director of the American Board of Surgery, said he believes the change back to 24 hours is a positive thing.
“The 16-hour requirement posed a lot of scheduling problems,” he said in an interview. “In essence, what it meant was you had residents that either couldn’t take call or they did take call, it was very limited in what they could do.”
Complicating the issue of time is the nature of what needs to be taught to residents.
“What has definitely changed is the breadth of knowledge and repertoire of technical skills that must be learned by today’s residents,” Dr. Dissanaike said. “As scientific knowledge and technical capabilities expand, there is ever more to learn and increasingly less time in which to learn it.”
Dr. Malangoni added that the time restraints didn’t allow for residents to see the natural progression of the patient’s condition. “You don’t have the chance to continue to assess that over a longer period of time,” he said. “That is really important in learning when to operate on someone, but also when not to operate on someone because they will get better without an operation.”
Compounding that is a greater need for reporting to meet regulatory requirements.
“Concurrently, we have increased requirements for documentation and clerical tasks, and reduced time available to do it,” Dr. Dissanaike continued. “All of this has led to a severe ‘work-compression’ for the modern resident, and I suspect the high rates of burnout and depression that are being reported in many specialties are at least partly a result of this phenomenon.”
That being said, Dr. Dissanaike was quick to add that this latest change should not be considered a “final solution” and that there are “many ongoing issues around resident fatigue, as well as adequacy of educational experience that still need to be addressed.”
Dr. Malangoni added that residents need to be more mindful and take more responsibility for their own health and well-being.
“I think what residents need to understand is they are really in charge of their own well-being. Making that point is really a key,” he said. “So it’s not only what you do while you are in the hospital, but you are also responsible for what you do when you are not in the hospital. ACGME cannot regulate what people do in their free time. If you work a 24-hour shift and you decide you are not going to sleep the next day for whatever reason, your well-being is likely not going to be what you want it to be and what, I think, your patients want it to be.”
First-year residents will once again be permitted to work up to 24 consecutive hours following a reversal of a rule implemented in 2011 that restricted them to 16 hours, the Accreditation Council for Graduate Medical Education (ACGME) announced.
According to a memo issued by the ACGME March 10, 2017, the change reverting back to the 24-hour ceiling was evidence based.
“The preponderance of the evidence from a number of studies conducted after the current 16-hour cap was implemented in 2011 suggests that it may not have had an incremental benefit in patient safety, and that there might be significant negative impacts to the quality of physician education and professional development,” the memo states. The work week is still capped at 80 hours worked per week, with 1 day free from clinical experience or education in 7, and in-house call no more frequent than every third night.
An ACGME task force determined “that the hypothesized benefits associated with the changes made to first-year resident scheduled hours in 2011 have not been realized, and the disruption of team-based care and supervisory systems has had a significant negative impact on the professional education of the first-year resident, and the effectiveness of care delivery of the team as a whole.”
Sharmila Dissanaike, MD, FACS, chair and professor of surgery at Texas Tech University, Lubbock, said in an interview that the change back to a 24-hour ceiling provides “an increased flexibility for all residents in order to allow completion of immediate patient care responsibilities, such as finishing an operation, and ensure smooth handoffs. Both of these should improve work flow for both trainees and supervisors.”
Mark A. Malangoni, MD, FACS, associate executive director of the American Board of Surgery, said he believes the change back to 24 hours is a positive thing.
“The 16-hour requirement posed a lot of scheduling problems,” he said in an interview. “In essence, what it meant was you had residents that either couldn’t take call or they did take call, it was very limited in what they could do.”
Complicating the issue of time is the nature of what needs to be taught to residents.
“What has definitely changed is the breadth of knowledge and repertoire of technical skills that must be learned by today’s residents,” Dr. Dissanaike said. “As scientific knowledge and technical capabilities expand, there is ever more to learn and increasingly less time in which to learn it.”
Dr. Malangoni added that the time restraints didn’t allow for residents to see the natural progression of the patient’s condition. “You don’t have the chance to continue to assess that over a longer period of time,” he said. “That is really important in learning when to operate on someone, but also when not to operate on someone because they will get better without an operation.”
Compounding that is a greater need for reporting to meet regulatory requirements.
“Concurrently, we have increased requirements for documentation and clerical tasks, and reduced time available to do it,” Dr. Dissanaike continued. “All of this has led to a severe ‘work-compression’ for the modern resident, and I suspect the high rates of burnout and depression that are being reported in many specialties are at least partly a result of this phenomenon.”
That being said, Dr. Dissanaike was quick to add that this latest change should not be considered a “final solution” and that there are “many ongoing issues around resident fatigue, as well as adequacy of educational experience that still need to be addressed.”
Dr. Malangoni added that residents need to be more mindful and take more responsibility for their own health and well-being.
“I think what residents need to understand is they are really in charge of their own well-being. Making that point is really a key,” he said. “So it’s not only what you do while you are in the hospital, but you are also responsible for what you do when you are not in the hospital. ACGME cannot regulate what people do in their free time. If you work a 24-hour shift and you decide you are not going to sleep the next day for whatever reason, your well-being is likely not going to be what you want it to be and what, I think, your patients want it to be.”
First-year residents will once again be permitted to work up to 24 consecutive hours following a reversal of a rule implemented in 2011 that restricted them to 16 hours, the Accreditation Council for Graduate Medical Education (ACGME) announced.
According to a memo issued by the ACGME March 10, 2017, the change reverting back to the 24-hour ceiling was evidence based.
“The preponderance of the evidence from a number of studies conducted after the current 16-hour cap was implemented in 2011 suggests that it may not have had an incremental benefit in patient safety, and that there might be significant negative impacts to the quality of physician education and professional development,” the memo states. The work week is still capped at 80 hours worked per week, with 1 day free from clinical experience or education in 7, and in-house call no more frequent than every third night.
An ACGME task force determined “that the hypothesized benefits associated with the changes made to first-year resident scheduled hours in 2011 have not been realized, and the disruption of team-based care and supervisory systems has had a significant negative impact on the professional education of the first-year resident, and the effectiveness of care delivery of the team as a whole.”
Sharmila Dissanaike, MD, FACS, chair and professor of surgery at Texas Tech University, Lubbock, said in an interview that the change back to a 24-hour ceiling provides “an increased flexibility for all residents in order to allow completion of immediate patient care responsibilities, such as finishing an operation, and ensure smooth handoffs. Both of these should improve work flow for both trainees and supervisors.”
Mark A. Malangoni, MD, FACS, associate executive director of the American Board of Surgery, said he believes the change back to 24 hours is a positive thing.
“The 16-hour requirement posed a lot of scheduling problems,” he said in an interview. “In essence, what it meant was you had residents that either couldn’t take call or they did take call, it was very limited in what they could do.”
Complicating the issue of time is the nature of what needs to be taught to residents.
“What has definitely changed is the breadth of knowledge and repertoire of technical skills that must be learned by today’s residents,” Dr. Dissanaike said. “As scientific knowledge and technical capabilities expand, there is ever more to learn and increasingly less time in which to learn it.”
Dr. Malangoni added that the time restraints didn’t allow for residents to see the natural progression of the patient’s condition. “You don’t have the chance to continue to assess that over a longer period of time,” he said. “That is really important in learning when to operate on someone, but also when not to operate on someone because they will get better without an operation.”
Compounding that is a greater need for reporting to meet regulatory requirements.
“Concurrently, we have increased requirements for documentation and clerical tasks, and reduced time available to do it,” Dr. Dissanaike continued. “All of this has led to a severe ‘work-compression’ for the modern resident, and I suspect the high rates of burnout and depression that are being reported in many specialties are at least partly a result of this phenomenon.”
That being said, Dr. Dissanaike was quick to add that this latest change should not be considered a “final solution” and that there are “many ongoing issues around resident fatigue, as well as adequacy of educational experience that still need to be addressed.”
Dr. Malangoni added that residents need to be more mindful and take more responsibility for their own health and well-being.
“I think what residents need to understand is they are really in charge of their own well-being. Making that point is really a key,” he said. “So it’s not only what you do while you are in the hospital, but you are also responsible for what you do when you are not in the hospital. ACGME cannot regulate what people do in their free time. If you work a 24-hour shift and you decide you are not going to sleep the next day for whatever reason, your well-being is likely not going to be what you want it to be and what, I think, your patients want it to be.”