User login
Which Cognitive Domains Predict Progression From MCI to Dementia in Parkinson’s Disease?
MIAMI—Among patients with Parkinson’s disease–associated mild cognitive impairment (MCI), Montreal Cognitive Assessment (MoCA) subscores in visuospatial function, attention, language, and orientation are the most useful in predicting conversion to dementia, according to research presented at the First Pan American Parkinson’s Disease and Movement Disorders Congress. Melissa Mackenzie, of the Division of Neurology at the University of British Columbia in Vancouver, Canada, and colleagues conducted a study to evaluate which subscores on the cognitive assessment predict conversion to dementia in patients with Parkinson’s disease–associated MCI.
The investigators searched the Pacific Parkinson’s Research Centre Database to identify patients with a diagnosis of idiopathic Parkinson’s disease who completed an itemized MoCA in the MCI range (ie, they had a corrected total score between 21 and 27) and who completed at least one other MoCA at least one year later. Patients taking potentially cognitive enhancing medications were excluded.
The researchers included in their study 529 assessments from 164 patients. They separated patients into three groups based on their last MoCA score—those who developed dementia (33 patients), those who returned to normal cognition (48 patients), and those who maintained MoCA scores in the MCI range (83 patients). In a model that predicted future MoCA score categories with 78% accuracy, the most important subscores were visuospatial, attention, language, and orientation, “but, interestingly, not delayed recall,” Dr. Mackenzie and colleagues said.
“A prevailing theory of cognitive decline in Parkinson’s disease postulates that visuospatial ‘posterior-cortical’ impairments are due to Lewy body deposition, whereas frontal executive dysfunction reflects ‘on–off’ state,” the researchers said. “Interestingly, language scores and memory function in delayed recall were the items that improved the most” in patients who reverted from MCI to normal cognition. “Whether the best approach to assess risk of conversion to dementia is to focus exclusively on these MoCA sections, or alternatively, employing multiple tests that target these cognitive domains, remains to be seen,” the researchers concluded. Patients with Parkinson’s disease–associated MCI at any time “should likely be followed more closely for cognitive decline, as they seem to be at increased risk for developing dementia, even if there is interval maintenance of MCI or return to normal cognition.”
—Jake Remaly
MIAMI—Among patients with Parkinson’s disease–associated mild cognitive impairment (MCI), Montreal Cognitive Assessment (MoCA) subscores in visuospatial function, attention, language, and orientation are the most useful in predicting conversion to dementia, according to research presented at the First Pan American Parkinson’s Disease and Movement Disorders Congress. Melissa Mackenzie, of the Division of Neurology at the University of British Columbia in Vancouver, Canada, and colleagues conducted a study to evaluate which subscores on the cognitive assessment predict conversion to dementia in patients with Parkinson’s disease–associated MCI.
The investigators searched the Pacific Parkinson’s Research Centre Database to identify patients with a diagnosis of idiopathic Parkinson’s disease who completed an itemized MoCA in the MCI range (ie, they had a corrected total score between 21 and 27) and who completed at least one other MoCA at least one year later. Patients taking potentially cognitive enhancing medications were excluded.
The researchers included in their study 529 assessments from 164 patients. They separated patients into three groups based on their last MoCA score—those who developed dementia (33 patients), those who returned to normal cognition (48 patients), and those who maintained MoCA scores in the MCI range (83 patients). In a model that predicted future MoCA score categories with 78% accuracy, the most important subscores were visuospatial, attention, language, and orientation, “but, interestingly, not delayed recall,” Dr. Mackenzie and colleagues said.
“A prevailing theory of cognitive decline in Parkinson’s disease postulates that visuospatial ‘posterior-cortical’ impairments are due to Lewy body deposition, whereas frontal executive dysfunction reflects ‘on–off’ state,” the researchers said. “Interestingly, language scores and memory function in delayed recall were the items that improved the most” in patients who reverted from MCI to normal cognition. “Whether the best approach to assess risk of conversion to dementia is to focus exclusively on these MoCA sections, or alternatively, employing multiple tests that target these cognitive domains, remains to be seen,” the researchers concluded. Patients with Parkinson’s disease–associated MCI at any time “should likely be followed more closely for cognitive decline, as they seem to be at increased risk for developing dementia, even if there is interval maintenance of MCI or return to normal cognition.”
—Jake Remaly
MIAMI—Among patients with Parkinson’s disease–associated mild cognitive impairment (MCI), Montreal Cognitive Assessment (MoCA) subscores in visuospatial function, attention, language, and orientation are the most useful in predicting conversion to dementia, according to research presented at the First Pan American Parkinson’s Disease and Movement Disorders Congress. Melissa Mackenzie, of the Division of Neurology at the University of British Columbia in Vancouver, Canada, and colleagues conducted a study to evaluate which subscores on the cognitive assessment predict conversion to dementia in patients with Parkinson’s disease–associated MCI.
The investigators searched the Pacific Parkinson’s Research Centre Database to identify patients with a diagnosis of idiopathic Parkinson’s disease who completed an itemized MoCA in the MCI range (ie, they had a corrected total score between 21 and 27) and who completed at least one other MoCA at least one year later. Patients taking potentially cognitive enhancing medications were excluded.
The researchers included in their study 529 assessments from 164 patients. They separated patients into three groups based on their last MoCA score—those who developed dementia (33 patients), those who returned to normal cognition (48 patients), and those who maintained MoCA scores in the MCI range (83 patients). In a model that predicted future MoCA score categories with 78% accuracy, the most important subscores were visuospatial, attention, language, and orientation, “but, interestingly, not delayed recall,” Dr. Mackenzie and colleagues said.
“A prevailing theory of cognitive decline in Parkinson’s disease postulates that visuospatial ‘posterior-cortical’ impairments are due to Lewy body deposition, whereas frontal executive dysfunction reflects ‘on–off’ state,” the researchers said. “Interestingly, language scores and memory function in delayed recall were the items that improved the most” in patients who reverted from MCI to normal cognition. “Whether the best approach to assess risk of conversion to dementia is to focus exclusively on these MoCA sections, or alternatively, employing multiple tests that target these cognitive domains, remains to be seen,” the researchers concluded. Patients with Parkinson’s disease–associated MCI at any time “should likely be followed more closely for cognitive decline, as they seem to be at increased risk for developing dementia, even if there is interval maintenance of MCI or return to normal cognition.”
—Jake Remaly
House leaders ‘came up short’ in effort to kill Obamacare
Despite days of intense negotiations and last-minute concessions to win over wavering GOP conservatives and moderates, House Republican leaders Friday failed to secure enough support to pass their plan to repeal and replace the Affordable Care Act.
House Speaker Paul Ryan pulled the bill from consideration after he rushed to the White House to tell President Donald Trump that there weren’t the 216 votes necessary for passage.
“We came really close today, but we came up short,” he told reporters at a hastily called news conference.
When pressed about what happens to the federal health law, he added, “Obamacare is the law of the land. … We’re going to be living with Obamacare for the foreseeable future.”
President Trump laid the blame at the feet of Democrats, complaining that not one was willing to help Republicans on the measure, and he warned again that the Obamacare insurance markets are in serious danger. “Bad things are going to happen to Obamacare,” he told reporters at the White House. “There’s not much you can do to help it. I’ve been saying that for a year and a half. I said, look, eventually, it’s not sustainable. The insurance companies are leaving.”
But he said the collapse of the bill might allow Republicans and Democrats to work on a replacement. “I honestly believe the Democrats will come to us and say, ‘Look, let’s get together and get a great health care bill or plan that’s really great for the people of our country,’” he said.
Mr. Ryan originally had hoped to hold a floor vote on the measure Thursday – timed to coincide with the 7th anniversary of the ACA – but decided to delay that effort because GOP leaders didn’t have enough “yes” votes. The House was in session Friday, before his announcement, while members debated the bill.
House Democratic leader Nancy Pelosi (Calif.) said the speaker’s decision to pull the bill “is pretty exciting for us … a victory for the Affordable Care Act, more importantly for the American people.”
The legislation was damaged by a variety of issues raised by competing factions of the party. Many members were nervous about reports by the Congressional Budget Office showing that the bill would lead eventually to 24 million people losing insurance, while some moderate Republicans worried that ending the ACA’s Medicaid expansion would hurt low-income Americans.
At the same time, conservatives, especially the hard-right House Freedom Caucus that often has needled party leaders, complained that the bill kept too much of the ACA structure in place. They wanted a straight repeal of Obamacare, but party leaders said that couldn’t pass the Senate, where Republicans don’t have enough votes to stop a filibuster. They were hoping to use a complicated legislative strategy called budget reconciliation that would allow them to repeal parts of the ACA that only affect federal spending.
The decision came after a chaotic week of negotiations, as party leaders sought to woo more conservatives. The president lobbied 120 members through personal meetings or phone calls, according to a count provided Friday by his spokesman, Sean Spicer. “The president and the team here have left everything on the field,” Mr. Spicer said.
On Thursday evening, Mr. Trump dispatched Office of Management and Budget Director Mick Mulvaney to tell his former House GOP colleagues that the president wanted a vote on Friday. It was time to move on to other priorities, including tax reform, he told House Republicans.
“He said the president needs this, the president has said he wants a vote tomorrow, up or down. If for any reason it goes down, we’re just going to move forward with additional parts of his agenda. This is our moment in time,” Rep. Chris Collins (R-N.Y.), a loyal Trump ally, told reporters late Thursday. “If it doesn’t pass, we’re moving beyond health care. … We are done negotiating.”
Trump’s edict clearly irked some lawmakers, including the Freedom Caucus chairman, Rep. Mark Meadows (R-N.C), whose group of more than two dozen members represented the strongest bloc against the measure.
“Anytime you don’t have 216 votes, negotiations are not totally over,” he told reporters who had surrounded him in a Capitol basement hallway as he headed in to the party’s caucus meeting.
President Trump, Speaker Ryan, and other GOP lawmakers tweaked their initial package in a variety of ways to win over both conservatives and moderates. But every time one change was made to win votes in one camp, it repelled support in another.
The White House on Thursday accepted conservatives’ demands that the legislation strip federal guarantees of essential health benefits from insurance policies. But that was another problem for moderates, and Democrats suggested the provision would not survive in the Senate.
Republican moderates in the House – as well as the Senate – objected to the bill’s provisions that would shift Medicaid from an open-ended entitlement to a set amount of funding for states that also would give governors and state lawmakers more flexibility over the program. Moderates also were concerned that the package’s tax credits would not be generous enough to help older Americans – who could be charged five times more for coverage than would their younger counterparts – afford coverage.
The House package also lost the support of key GOP allies, including the Club for Growth and Heritage Action. Physician, patient and hospital groups also opposed it.
But Mr. Ryan’s comments made clear how difficult this decision was. “This is a disappointing day for us,” he said. “Doing big things is hard. All of us. All of us – myself included – we will need time to reflect on how we got to this moment, what we could have done to do it better.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Despite days of intense negotiations and last-minute concessions to win over wavering GOP conservatives and moderates, House Republican leaders Friday failed to secure enough support to pass their plan to repeal and replace the Affordable Care Act.
House Speaker Paul Ryan pulled the bill from consideration after he rushed to the White House to tell President Donald Trump that there weren’t the 216 votes necessary for passage.
“We came really close today, but we came up short,” he told reporters at a hastily called news conference.
When pressed about what happens to the federal health law, he added, “Obamacare is the law of the land. … We’re going to be living with Obamacare for the foreseeable future.”
President Trump laid the blame at the feet of Democrats, complaining that not one was willing to help Republicans on the measure, and he warned again that the Obamacare insurance markets are in serious danger. “Bad things are going to happen to Obamacare,” he told reporters at the White House. “There’s not much you can do to help it. I’ve been saying that for a year and a half. I said, look, eventually, it’s not sustainable. The insurance companies are leaving.”
But he said the collapse of the bill might allow Republicans and Democrats to work on a replacement. “I honestly believe the Democrats will come to us and say, ‘Look, let’s get together and get a great health care bill or plan that’s really great for the people of our country,’” he said.
Mr. Ryan originally had hoped to hold a floor vote on the measure Thursday – timed to coincide with the 7th anniversary of the ACA – but decided to delay that effort because GOP leaders didn’t have enough “yes” votes. The House was in session Friday, before his announcement, while members debated the bill.
House Democratic leader Nancy Pelosi (Calif.) said the speaker’s decision to pull the bill “is pretty exciting for us … a victory for the Affordable Care Act, more importantly for the American people.”
The legislation was damaged by a variety of issues raised by competing factions of the party. Many members were nervous about reports by the Congressional Budget Office showing that the bill would lead eventually to 24 million people losing insurance, while some moderate Republicans worried that ending the ACA’s Medicaid expansion would hurt low-income Americans.
At the same time, conservatives, especially the hard-right House Freedom Caucus that often has needled party leaders, complained that the bill kept too much of the ACA structure in place. They wanted a straight repeal of Obamacare, but party leaders said that couldn’t pass the Senate, where Republicans don’t have enough votes to stop a filibuster. They were hoping to use a complicated legislative strategy called budget reconciliation that would allow them to repeal parts of the ACA that only affect federal spending.
The decision came after a chaotic week of negotiations, as party leaders sought to woo more conservatives. The president lobbied 120 members through personal meetings or phone calls, according to a count provided Friday by his spokesman, Sean Spicer. “The president and the team here have left everything on the field,” Mr. Spicer said.
On Thursday evening, Mr. Trump dispatched Office of Management and Budget Director Mick Mulvaney to tell his former House GOP colleagues that the president wanted a vote on Friday. It was time to move on to other priorities, including tax reform, he told House Republicans.
“He said the president needs this, the president has said he wants a vote tomorrow, up or down. If for any reason it goes down, we’re just going to move forward with additional parts of his agenda. This is our moment in time,” Rep. Chris Collins (R-N.Y.), a loyal Trump ally, told reporters late Thursday. “If it doesn’t pass, we’re moving beyond health care. … We are done negotiating.”
Trump’s edict clearly irked some lawmakers, including the Freedom Caucus chairman, Rep. Mark Meadows (R-N.C), whose group of more than two dozen members represented the strongest bloc against the measure.
“Anytime you don’t have 216 votes, negotiations are not totally over,” he told reporters who had surrounded him in a Capitol basement hallway as he headed in to the party’s caucus meeting.
President Trump, Speaker Ryan, and other GOP lawmakers tweaked their initial package in a variety of ways to win over both conservatives and moderates. But every time one change was made to win votes in one camp, it repelled support in another.
The White House on Thursday accepted conservatives’ demands that the legislation strip federal guarantees of essential health benefits from insurance policies. But that was another problem for moderates, and Democrats suggested the provision would not survive in the Senate.
Republican moderates in the House – as well as the Senate – objected to the bill’s provisions that would shift Medicaid from an open-ended entitlement to a set amount of funding for states that also would give governors and state lawmakers more flexibility over the program. Moderates also were concerned that the package’s tax credits would not be generous enough to help older Americans – who could be charged five times more for coverage than would their younger counterparts – afford coverage.
The House package also lost the support of key GOP allies, including the Club for Growth and Heritage Action. Physician, patient and hospital groups also opposed it.
But Mr. Ryan’s comments made clear how difficult this decision was. “This is a disappointing day for us,” he said. “Doing big things is hard. All of us. All of us – myself included – we will need time to reflect on how we got to this moment, what we could have done to do it better.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Despite days of intense negotiations and last-minute concessions to win over wavering GOP conservatives and moderates, House Republican leaders Friday failed to secure enough support to pass their plan to repeal and replace the Affordable Care Act.
House Speaker Paul Ryan pulled the bill from consideration after he rushed to the White House to tell President Donald Trump that there weren’t the 216 votes necessary for passage.
“We came really close today, but we came up short,” he told reporters at a hastily called news conference.
When pressed about what happens to the federal health law, he added, “Obamacare is the law of the land. … We’re going to be living with Obamacare for the foreseeable future.”
President Trump laid the blame at the feet of Democrats, complaining that not one was willing to help Republicans on the measure, and he warned again that the Obamacare insurance markets are in serious danger. “Bad things are going to happen to Obamacare,” he told reporters at the White House. “There’s not much you can do to help it. I’ve been saying that for a year and a half. I said, look, eventually, it’s not sustainable. The insurance companies are leaving.”
But he said the collapse of the bill might allow Republicans and Democrats to work on a replacement. “I honestly believe the Democrats will come to us and say, ‘Look, let’s get together and get a great health care bill or plan that’s really great for the people of our country,’” he said.
Mr. Ryan originally had hoped to hold a floor vote on the measure Thursday – timed to coincide with the 7th anniversary of the ACA – but decided to delay that effort because GOP leaders didn’t have enough “yes” votes. The House was in session Friday, before his announcement, while members debated the bill.
House Democratic leader Nancy Pelosi (Calif.) said the speaker’s decision to pull the bill “is pretty exciting for us … a victory for the Affordable Care Act, more importantly for the American people.”
The legislation was damaged by a variety of issues raised by competing factions of the party. Many members were nervous about reports by the Congressional Budget Office showing that the bill would lead eventually to 24 million people losing insurance, while some moderate Republicans worried that ending the ACA’s Medicaid expansion would hurt low-income Americans.
At the same time, conservatives, especially the hard-right House Freedom Caucus that often has needled party leaders, complained that the bill kept too much of the ACA structure in place. They wanted a straight repeal of Obamacare, but party leaders said that couldn’t pass the Senate, where Republicans don’t have enough votes to stop a filibuster. They were hoping to use a complicated legislative strategy called budget reconciliation that would allow them to repeal parts of the ACA that only affect federal spending.
The decision came after a chaotic week of negotiations, as party leaders sought to woo more conservatives. The president lobbied 120 members through personal meetings or phone calls, according to a count provided Friday by his spokesman, Sean Spicer. “The president and the team here have left everything on the field,” Mr. Spicer said.
On Thursday evening, Mr. Trump dispatched Office of Management and Budget Director Mick Mulvaney to tell his former House GOP colleagues that the president wanted a vote on Friday. It was time to move on to other priorities, including tax reform, he told House Republicans.
“He said the president needs this, the president has said he wants a vote tomorrow, up or down. If for any reason it goes down, we’re just going to move forward with additional parts of his agenda. This is our moment in time,” Rep. Chris Collins (R-N.Y.), a loyal Trump ally, told reporters late Thursday. “If it doesn’t pass, we’re moving beyond health care. … We are done negotiating.”
Trump’s edict clearly irked some lawmakers, including the Freedom Caucus chairman, Rep. Mark Meadows (R-N.C), whose group of more than two dozen members represented the strongest bloc against the measure.
“Anytime you don’t have 216 votes, negotiations are not totally over,” he told reporters who had surrounded him in a Capitol basement hallway as he headed in to the party’s caucus meeting.
President Trump, Speaker Ryan, and other GOP lawmakers tweaked their initial package in a variety of ways to win over both conservatives and moderates. But every time one change was made to win votes in one camp, it repelled support in another.
The White House on Thursday accepted conservatives’ demands that the legislation strip federal guarantees of essential health benefits from insurance policies. But that was another problem for moderates, and Democrats suggested the provision would not survive in the Senate.
Republican moderates in the House – as well as the Senate – objected to the bill’s provisions that would shift Medicaid from an open-ended entitlement to a set amount of funding for states that also would give governors and state lawmakers more flexibility over the program. Moderates also were concerned that the package’s tax credits would not be generous enough to help older Americans – who could be charged five times more for coverage than would their younger counterparts – afford coverage.
The House package also lost the support of key GOP allies, including the Club for Growth and Heritage Action. Physician, patient and hospital groups also opposed it.
But Mr. Ryan’s comments made clear how difficult this decision was. “This is a disappointing day for us,” he said. “Doing big things is hard. All of us. All of us – myself included – we will need time to reflect on how we got to this moment, what we could have done to do it better.”
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
2016-2017 flu season continues to wind down
Influenza activity took another healthy step down as outpatient visits continued to drop, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was down to 3.2% for the week ending March 18, 2017, the CDC reported, compared with 3.6% the week before. (The figure of 3.7% previously reported for last week has been adjusted this week, so the halt in the decline in outpatient visits was actually more of a slowdown.) The national baseline for outpatient ILI visits is 2.2%.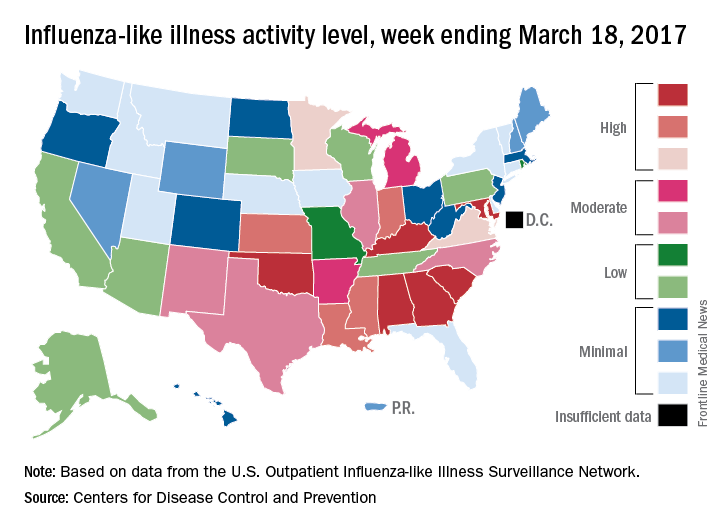
Two flu-related pediatric deaths were reported during the week of March 18, but both occurred earlier: one during the week ending Feb. 18 and the other in the week ending Feb. 25, the CDC reported. The total number of pediatric flu deaths reported is now 55 for the 2016-2017 season.
Influenza activity took another healthy step down as outpatient visits continued to drop, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was down to 3.2% for the week ending March 18, 2017, the CDC reported, compared with 3.6% the week before. (The figure of 3.7% previously reported for last week has been adjusted this week, so the halt in the decline in outpatient visits was actually more of a slowdown.) The national baseline for outpatient ILI visits is 2.2%.
Two flu-related pediatric deaths were reported during the week of March 18, but both occurred earlier: one during the week ending Feb. 18 and the other in the week ending Feb. 25, the CDC reported. The total number of pediatric flu deaths reported is now 55 for the 2016-2017 season.
Influenza activity took another healthy step down as outpatient visits continued to drop, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was down to 3.2% for the week ending March 18, 2017, the CDC reported, compared with 3.6% the week before. (The figure of 3.7% previously reported for last week has been adjusted this week, so the halt in the decline in outpatient visits was actually more of a slowdown.) The national baseline for outpatient ILI visits is 2.2%.
Two flu-related pediatric deaths were reported during the week of March 18, but both occurred earlier: one during the week ending Feb. 18 and the other in the week ending Feb. 25, the CDC reported. The total number of pediatric flu deaths reported is now 55 for the 2016-2017 season.
SPECT reveals perfusion problems in antiphospholipid syndrome
MELBOURNE – SPECT imaging can identify abnormalities in brain perfusion in patients with multiple antiphospholipid antibodies and neuropsychiatric symptoms, but without a history of thrombosis, according to a study presented at an international congress on systemic lupus erythematosus.
The retrospective study by researchers from National Taiwan University Hospital addresses the challenge posed by patients who have antiphospholipid antibodies and neuropsychiatric symptoms but do not meet the full criteria for antiphospholipid syndrome because of a lack of a history of thromboembolism. Current antiphospholipid syndrome classification criteria are based on thrombosis or pregnancy loss, with a third category of noncriteria manifestations that include a range of neuropsychiatric symptoms, said presenter Ting-Syuan Lin, MD, of the Yun-Lin Branch of National Taiwan University Hospital.
“Some physicians may not give the patient early treatment because they do not fill the criteria,” Dr. Lin said in an interview. “But if we have SPECT image to document the abnormality, then the physician can have more confidence to give them early treatment.”
Dr. Lin and his colleagues looked at the brain SPECT images of 54 patients with a history of positive antiphospholipid antibodies and neuropsychiatric symptoms, but who had no history of thromboembolism or other lupus-related antibodies such as antibodies to double-stranded DNA. When the researchers looked simply at mean brain perfusion according to the number of antiphospholipid antibodies each patient had, they found no significant differences between the groups, including a control group of six patients without antiphospholipid antibodies.
But when they examined heterogeneity of brain perfusion, they saw significantly greater heterogeneity (P = .01) in patients with four antiphospholipid antibodies compared with patients who had no antibodies.
The patients enrolled in the study presented with a range of neuropsychiatric symptoms. The most common was headache (56.7%), followed by dizziness (41.7%), depression (28.3%), psychosis (15%), vertigo (8.3%), and seizures (6.7%). The mean age of the patients was 38 years, and 52 of the patients were women.
One of the patients – a 39-year-old woman with more than four antiphospholipid antibodies – had a normal CT scan but showed significant heterogeneity in brain perfusion on the SPECT imaging. She experienced a stroke 1 year after the study.
Commenting on the presentation, session cochair Timothy Godfrey, MBBS, of St. Vincent’s Hospital in Melbourne, said neuropsychiatric lupus was particularly problematic, especially when patients had normal imaging.
“You’re wondering is the patient just depressed or is there some other explanation, or do they truly have a manifestation of lupus which may require immunosuppression or anticoagulation?” he said in an interview.
This study “is highlighting the fact that maybe we do need to do these tests when the MRI is normal, particularly in people that have documented abnormalities in their blood test.”
Dr. Lin said the next phase of the study would look at whether treatment was associated with changes in brain perfusion on SPECT, and whether the abnormality of the SPECT imaging correlated with clinical outcomes.
No conflicts of interest were declared.
MELBOURNE – SPECT imaging can identify abnormalities in brain perfusion in patients with multiple antiphospholipid antibodies and neuropsychiatric symptoms, but without a history of thrombosis, according to a study presented at an international congress on systemic lupus erythematosus.
The retrospective study by researchers from National Taiwan University Hospital addresses the challenge posed by patients who have antiphospholipid antibodies and neuropsychiatric symptoms but do not meet the full criteria for antiphospholipid syndrome because of a lack of a history of thromboembolism. Current antiphospholipid syndrome classification criteria are based on thrombosis or pregnancy loss, with a third category of noncriteria manifestations that include a range of neuropsychiatric symptoms, said presenter Ting-Syuan Lin, MD, of the Yun-Lin Branch of National Taiwan University Hospital.
“Some physicians may not give the patient early treatment because they do not fill the criteria,” Dr. Lin said in an interview. “But if we have SPECT image to document the abnormality, then the physician can have more confidence to give them early treatment.”
Dr. Lin and his colleagues looked at the brain SPECT images of 54 patients with a history of positive antiphospholipid antibodies and neuropsychiatric symptoms, but who had no history of thromboembolism or other lupus-related antibodies such as antibodies to double-stranded DNA. When the researchers looked simply at mean brain perfusion according to the number of antiphospholipid antibodies each patient had, they found no significant differences between the groups, including a control group of six patients without antiphospholipid antibodies.
But when they examined heterogeneity of brain perfusion, they saw significantly greater heterogeneity (P = .01) in patients with four antiphospholipid antibodies compared with patients who had no antibodies.
The patients enrolled in the study presented with a range of neuropsychiatric symptoms. The most common was headache (56.7%), followed by dizziness (41.7%), depression (28.3%), psychosis (15%), vertigo (8.3%), and seizures (6.7%). The mean age of the patients was 38 years, and 52 of the patients were women.
One of the patients – a 39-year-old woman with more than four antiphospholipid antibodies – had a normal CT scan but showed significant heterogeneity in brain perfusion on the SPECT imaging. She experienced a stroke 1 year after the study.
Commenting on the presentation, session cochair Timothy Godfrey, MBBS, of St. Vincent’s Hospital in Melbourne, said neuropsychiatric lupus was particularly problematic, especially when patients had normal imaging.
“You’re wondering is the patient just depressed or is there some other explanation, or do they truly have a manifestation of lupus which may require immunosuppression or anticoagulation?” he said in an interview.
This study “is highlighting the fact that maybe we do need to do these tests when the MRI is normal, particularly in people that have documented abnormalities in their blood test.”
Dr. Lin said the next phase of the study would look at whether treatment was associated with changes in brain perfusion on SPECT, and whether the abnormality of the SPECT imaging correlated with clinical outcomes.
No conflicts of interest were declared.
MELBOURNE – SPECT imaging can identify abnormalities in brain perfusion in patients with multiple antiphospholipid antibodies and neuropsychiatric symptoms, but without a history of thrombosis, according to a study presented at an international congress on systemic lupus erythematosus.
The retrospective study by researchers from National Taiwan University Hospital addresses the challenge posed by patients who have antiphospholipid antibodies and neuropsychiatric symptoms but do not meet the full criteria for antiphospholipid syndrome because of a lack of a history of thromboembolism. Current antiphospholipid syndrome classification criteria are based on thrombosis or pregnancy loss, with a third category of noncriteria manifestations that include a range of neuropsychiatric symptoms, said presenter Ting-Syuan Lin, MD, of the Yun-Lin Branch of National Taiwan University Hospital.
“Some physicians may not give the patient early treatment because they do not fill the criteria,” Dr. Lin said in an interview. “But if we have SPECT image to document the abnormality, then the physician can have more confidence to give them early treatment.”
Dr. Lin and his colleagues looked at the brain SPECT images of 54 patients with a history of positive antiphospholipid antibodies and neuropsychiatric symptoms, but who had no history of thromboembolism or other lupus-related antibodies such as antibodies to double-stranded DNA. When the researchers looked simply at mean brain perfusion according to the number of antiphospholipid antibodies each patient had, they found no significant differences between the groups, including a control group of six patients without antiphospholipid antibodies.
But when they examined heterogeneity of brain perfusion, they saw significantly greater heterogeneity (P = .01) in patients with four antiphospholipid antibodies compared with patients who had no antibodies.
The patients enrolled in the study presented with a range of neuropsychiatric symptoms. The most common was headache (56.7%), followed by dizziness (41.7%), depression (28.3%), psychosis (15%), vertigo (8.3%), and seizures (6.7%). The mean age of the patients was 38 years, and 52 of the patients were women.
One of the patients – a 39-year-old woman with more than four antiphospholipid antibodies – had a normal CT scan but showed significant heterogeneity in brain perfusion on the SPECT imaging. She experienced a stroke 1 year after the study.
Commenting on the presentation, session cochair Timothy Godfrey, MBBS, of St. Vincent’s Hospital in Melbourne, said neuropsychiatric lupus was particularly problematic, especially when patients had normal imaging.
“You’re wondering is the patient just depressed or is there some other explanation, or do they truly have a manifestation of lupus which may require immunosuppression or anticoagulation?” he said in an interview.
This study “is highlighting the fact that maybe we do need to do these tests when the MRI is normal, particularly in people that have documented abnormalities in their blood test.”
Dr. Lin said the next phase of the study would look at whether treatment was associated with changes in brain perfusion on SPECT, and whether the abnormality of the SPECT imaging correlated with clinical outcomes.
No conflicts of interest were declared.
AT LUPUS 2017
Key clinical point: SPECT may be worthwhile in patients who don’t meet antiphospholipid syndrome criteria but have aPL antibodies, neuropsychiatric symptoms, and no thrombosis history.
Major finding: Patients with four antiphospholipid antibodies have significantly greater heterogeneity in brain perfusion on SPECT imaging than do patients with no antiphospholipid antibodies.
Data source: A retrospective cohort study in 54 patients with antiphospholipid syndrome.
Disclosures: No conflicts of interest were declared.
Oral agent found promising for subset of chronic rhinosinusitis patients
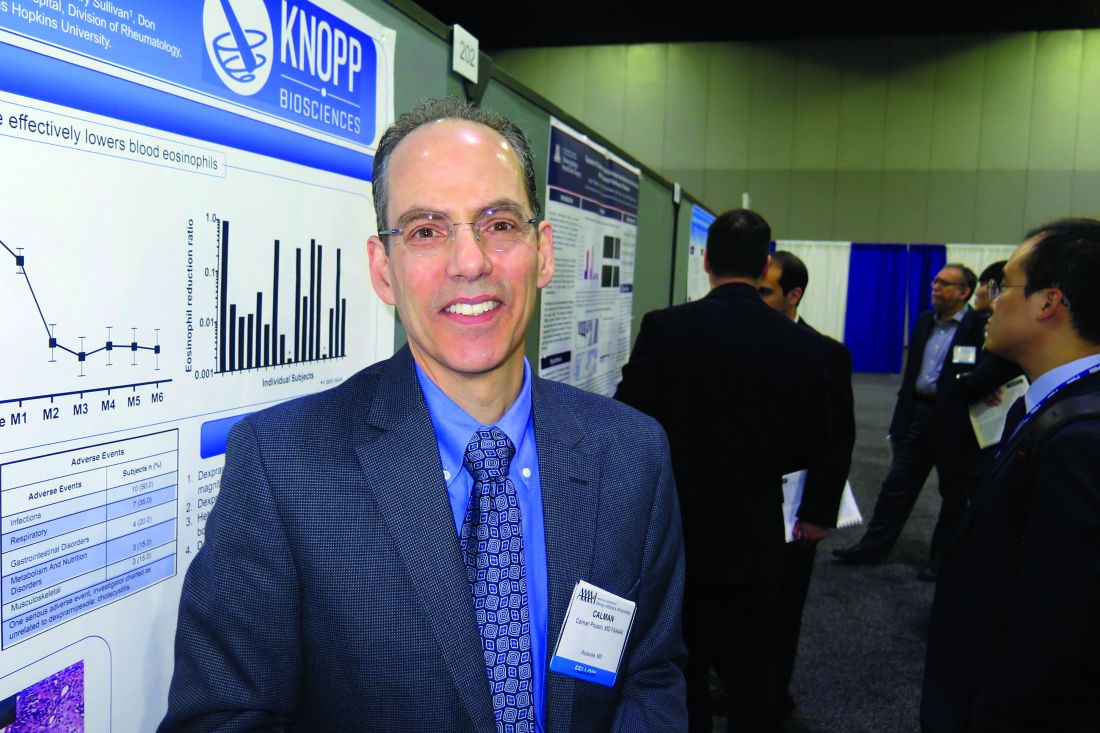
ATLANTA – The use of dexpramipexole by patients with chronic rhinosinusitis was well tolerated and showed robust and tissue eosinophil–lowering activity, according to results from a small study.
Dexpramipexole is an investigational oral agent that has been studied in previous clinical trials for patients with amyotrophic lateral sclerosis, Calman Prussin, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The drug did not meet the clinical endpoint for ALS patients, but its investigators noted that it lowered eosinophil counts by about 50%. “It was a serendipitous finding,” said Dr. Prussin, senior director of clinical and translational medicine for Pittsburgh-based Knopp Biosciences. “We do not have a mechanism of action, but we think it’s working on progenitor cells in the bone marrow.”
In all, 16 of the 20 patients completed the trial. Dr. Prussin and his associates found that the baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than.001). “I don’t think any of us expected to see this,” he said, noting that the drug’s maximal eosinophil-lowering effect was maximal after 2 months. No reduction in total polyp score was observed.
Biopsies conducted in 12 of the patients revealed that polyp tissue eosinophilia was reduced from a mean of 233 to 5 eosinophils/high-powered field, a drop of 97% (P = .001). No serious drug-related adverse effects occurred. The most common adverse event was infection (50%), followed by respiratory symptoms (35%) and gastrointestinal disorders (20%).
Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
ATLANTA – The use of dexpramipexole by patients with chronic rhinosinusitis was well tolerated and showed robust and tissue eosinophil–lowering activity, according to results from a small study.
Dexpramipexole is an investigational oral agent that has been studied in previous clinical trials for patients with amyotrophic lateral sclerosis, Calman Prussin, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The drug did not meet the clinical endpoint for ALS patients, but its investigators noted that it lowered eosinophil counts by about 50%. “It was a serendipitous finding,” said Dr. Prussin, senior director of clinical and translational medicine for Pittsburgh-based Knopp Biosciences. “We do not have a mechanism of action, but we think it’s working on progenitor cells in the bone marrow.”
In all, 16 of the 20 patients completed the trial. Dr. Prussin and his associates found that the baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than.001). “I don’t think any of us expected to see this,” he said, noting that the drug’s maximal eosinophil-lowering effect was maximal after 2 months. No reduction in total polyp score was observed.
Biopsies conducted in 12 of the patients revealed that polyp tissue eosinophilia was reduced from a mean of 233 to 5 eosinophils/high-powered field, a drop of 97% (P = .001). No serious drug-related adverse effects occurred. The most common adverse event was infection (50%), followed by respiratory symptoms (35%) and gastrointestinal disorders (20%).
Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
ATLANTA – The use of dexpramipexole by patients with chronic rhinosinusitis was well tolerated and showed robust and tissue eosinophil–lowering activity, according to results from a small study.
Dexpramipexole is an investigational oral agent that has been studied in previous clinical trials for patients with amyotrophic lateral sclerosis, Calman Prussin, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. The drug did not meet the clinical endpoint for ALS patients, but its investigators noted that it lowered eosinophil counts by about 50%. “It was a serendipitous finding,” said Dr. Prussin, senior director of clinical and translational medicine for Pittsburgh-based Knopp Biosciences. “We do not have a mechanism of action, but we think it’s working on progenitor cells in the bone marrow.”
In all, 16 of the 20 patients completed the trial. Dr. Prussin and his associates found that the baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than.001). “I don’t think any of us expected to see this,” he said, noting that the drug’s maximal eosinophil-lowering effect was maximal after 2 months. No reduction in total polyp score was observed.
Biopsies conducted in 12 of the patients revealed that polyp tissue eosinophilia was reduced from a mean of 233 to 5 eosinophils/high-powered field, a drop of 97% (P = .001). No serious drug-related adverse effects occurred. The most common adverse event was infection (50%), followed by respiratory symptoms (35%) and gastrointestinal disorders (20%).
Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: The baseline eosinophil count fell from 0.525 x 109/L to 0.031 x 109/L at 6 months, a reduction of 94% (P less than .001).
Data source: Results from a open-label trial in 16 chronic rhinosinusitis patients who received dexpramipexole 150 mg b.i.d. for 6 months.
Disclosures: Knopp Biosciences funded the study. Dr. Prussin is an employee of the company.
Freezing of Gait May Be Associated With Anxiety and Depression in Parkinson’s Disease
MIAMI—Freezing of gait in Parkinson's disease may be associated with higher levels of anxiety and depressive symptoms, as well as recurrent falls and lower quality of life, according to research presented at the First Pan American Parkinson's Disease and Movement Disorders Congress.
"Our data suggest that people with Parkinson's disease and freezing of gait have advanced disease, functional limitations, lower balance confidence, and a higher level of anxiety and depressive symptoms, which may negatively impact their quality of life," said Milla Pimenta, a medical student at the Bahiana School of Medicine and Public Health in Brazil, and colleagues. "Future prospective studies should elucidate whether the treatment of anxiety can contribute to reduce the frequency or severity of freezing of gait episodes."
To identify the association between freezing of gait and symptoms of anxiety and depression, the researchers recruited consecutive patients with idiopathic Parkinson's disease and independent walking ability from the Movement Disorders Clinic at the State of Bahia Health Attention Center for the Elderly in Brazil. They excluded patients with other neurologic conditions or comorbidities that affect balance.
The investigators assessed patients' demographics, Parkinson's disease severity and symptoms, medication, disability, freezing, anxiety, depression, self-efficacy, and quality of life.
A total of 78 people with Parkinson's disease (mean age, 70.5; mean Unified Parkinson's Disease Rating Scale motor score, 32; Hoehn and Yahr stages between 1.5 and 4) were included in the study.
Twenty-seven participants (35%) were identified as having freezing of gait (ie, they scored at least 1 point on item 3 of the Freezing of Gait Questionnaire).
Patients with freezing of gait had higher Hospital Anxiety and Depression Scale scores and lower Activities-Specific Balance Confidence Scale scores, compared with patients without freezing.
Patients with freezing of gait were more likely to have had recurrent falls in the previous year. In addition, patients with freezing had longer median disease duration (nine years versus four years) and received a higher median levodopa equivalent dose (800 mg/day vs 532 mg/day) than patients without freezing. Quality of life, as assessed by the eight-item Parkinson's Disease Questionnaire, was worse in patients with freezing of gait (40.6 vs 25).
—Jake Remaly
MIAMI—Freezing of gait in Parkinson's disease may be associated with higher levels of anxiety and depressive symptoms, as well as recurrent falls and lower quality of life, according to research presented at the First Pan American Parkinson's Disease and Movement Disorders Congress.
"Our data suggest that people with Parkinson's disease and freezing of gait have advanced disease, functional limitations, lower balance confidence, and a higher level of anxiety and depressive symptoms, which may negatively impact their quality of life," said Milla Pimenta, a medical student at the Bahiana School of Medicine and Public Health in Brazil, and colleagues. "Future prospective studies should elucidate whether the treatment of anxiety can contribute to reduce the frequency or severity of freezing of gait episodes."
To identify the association between freezing of gait and symptoms of anxiety and depression, the researchers recruited consecutive patients with idiopathic Parkinson's disease and independent walking ability from the Movement Disorders Clinic at the State of Bahia Health Attention Center for the Elderly in Brazil. They excluded patients with other neurologic conditions or comorbidities that affect balance.
The investigators assessed patients' demographics, Parkinson's disease severity and symptoms, medication, disability, freezing, anxiety, depression, self-efficacy, and quality of life.
A total of 78 people with Parkinson's disease (mean age, 70.5; mean Unified Parkinson's Disease Rating Scale motor score, 32; Hoehn and Yahr stages between 1.5 and 4) were included in the study.
Twenty-seven participants (35%) were identified as having freezing of gait (ie, they scored at least 1 point on item 3 of the Freezing of Gait Questionnaire).
Patients with freezing of gait had higher Hospital Anxiety and Depression Scale scores and lower Activities-Specific Balance Confidence Scale scores, compared with patients without freezing.
Patients with freezing of gait were more likely to have had recurrent falls in the previous year. In addition, patients with freezing had longer median disease duration (nine years versus four years) and received a higher median levodopa equivalent dose (800 mg/day vs 532 mg/day) than patients without freezing. Quality of life, as assessed by the eight-item Parkinson's Disease Questionnaire, was worse in patients with freezing of gait (40.6 vs 25).
—Jake Remaly
MIAMI—Freezing of gait in Parkinson's disease may be associated with higher levels of anxiety and depressive symptoms, as well as recurrent falls and lower quality of life, according to research presented at the First Pan American Parkinson's Disease and Movement Disorders Congress.
"Our data suggest that people with Parkinson's disease and freezing of gait have advanced disease, functional limitations, lower balance confidence, and a higher level of anxiety and depressive symptoms, which may negatively impact their quality of life," said Milla Pimenta, a medical student at the Bahiana School of Medicine and Public Health in Brazil, and colleagues. "Future prospective studies should elucidate whether the treatment of anxiety can contribute to reduce the frequency or severity of freezing of gait episodes."
To identify the association between freezing of gait and symptoms of anxiety and depression, the researchers recruited consecutive patients with idiopathic Parkinson's disease and independent walking ability from the Movement Disorders Clinic at the State of Bahia Health Attention Center for the Elderly in Brazil. They excluded patients with other neurologic conditions or comorbidities that affect balance.
The investigators assessed patients' demographics, Parkinson's disease severity and symptoms, medication, disability, freezing, anxiety, depression, self-efficacy, and quality of life.
A total of 78 people with Parkinson's disease (mean age, 70.5; mean Unified Parkinson's Disease Rating Scale motor score, 32; Hoehn and Yahr stages between 1.5 and 4) were included in the study.
Twenty-seven participants (35%) were identified as having freezing of gait (ie, they scored at least 1 point on item 3 of the Freezing of Gait Questionnaire).
Patients with freezing of gait had higher Hospital Anxiety and Depression Scale scores and lower Activities-Specific Balance Confidence Scale scores, compared with patients without freezing.
Patients with freezing of gait were more likely to have had recurrent falls in the previous year. In addition, patients with freezing had longer median disease duration (nine years versus four years) and received a higher median levodopa equivalent dose (800 mg/day vs 532 mg/day) than patients without freezing. Quality of life, as assessed by the eight-item Parkinson's Disease Questionnaire, was worse in patients with freezing of gait (40.6 vs 25).
—Jake Remaly
Advanced CLL treatment approach depends on comorbidity burden
ORLANDO – The choice of first-line therapy in symptomatic chronic lymphocytic leukemia patients depends largely on comorbidity burden, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
“This is a disease of elderly patients. Frequently they have comorbidities,” he said. Categorizing these patients as having a low or high comorbidity burden can be done with the Cumulative Index Rating Scale score, which involves scoring of all organ systems on a 0-5 scale representing “not affected” to “extremely disabled.”
“We use this to determine first-line therapy,” said Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York. Dr. Zelenetz is chair of the NCCN Non-Hodgkin Lymphoma Guidelines panel.
Patients with a score of greater than 12 on the 0- to 56-point scale, are “no-go” patients with respect to therapy, and are typically treated only with palliative approaches. Those with a score of 7-12 (“slow-go” patients) have a significant comorbidity burden, but can undergo treatment, thought typically to be at reduced intensity. Those with a score of 0-6 are “go-go” patients with respect to treatment, as they are physically fit, have excellent renal function, and have no significant comorbidities, he said.
Treatment options for ‘go-go’ CLL patients
Among the treatment options for the latter is FCR–the combination of fludarabine, cyclophosphamide, and rituximab, which was shown in the phase III CLL10 trial of patients with advanced CLL to be associated with improved complete response rates compared with the popular regimen of bendamustine and rituximab (BR), both overall and in patients under age 65. In older patients, the advantage disappeared, Dr. Zelenetz said.
FCR was also associated with improved outcomes vs. BR in patients with del(11q).
The primary endpoint of the study was progression-free survival, which favored FCR (median of 55.2 vs. 41.7 months; hazard ratio, 1.643), he said, noting that no difference was seen between the two regimens in terms of overall survival.
In a recent publication, MD Anderson Cancer Center reported its experience with its first 300 CLL patients treated with FCR. With long-term follow-up of at least 9-10 years (median of 12.8 years), patients in this trial have done extremely well.
“But interestingly, when you stratify these patients by whether they have IGHV [immunoglobulin heavy chain variable] mutated or unmutated [disease], the IGHV mutated patients have something that looks a whole lot like a survival plateau, and that survival plateau is not trivial – it’s about 60%,” he said. “So there is a group of patients with CLL who are, in fact, curable with conventional chemoimmunotherapy.
“This is an appropriate treatment for a young, fit, ‘go-go’ patient, and it has a big implication,” he said. That is, patients who are young and fit require IGHV mutation testing, as “you will absolutely choose FCR chemotherapy for the fit, young patients who has IGHV mutated disease.
“In that setting IGHV testing is now mandatory,” he stressed, noting that the benefits in this population extend to overall survival as well as progression-free survival.
Dr. Zelenetz also emphasized the need for increasing the single dose of rituximab from 375 mg/m2 during cycle 1 to 500 mg/m2 during cycles 2-6 in those receiving FCR, as this is often forgotten.
The data demonstrating the efficacy of FCR were based on this approach, he said.
Fludarabine is to be given at a dose of 25 mg/m2, and cyclophosphamide at a dose of 250 mg/m2 – both for 2-4 days during cycle 1 and for 1-3 days during cycles 2-6.
Treatment options for ‘slow-go’ CLL patients
In “slow-go” patients, an interesting approach is to use new anti-CD20 antibodies such as ofatumumab and obinutuzumab, which have features that are distinct from rituximab.
Both have been studied in CLL. The CLL11 trial compared chlorambucil, rituximab+chlorambucil, and obinutuzumab+chlorambucil, and the latest analysis showed substantial improvement in progression-free survival with obinutuzumab+chlorambucil vs. the other two regimens (26.7 months vs. 11.1 and 16.3 months, respectively), Dr. Zelenetz said, noting that rituximab+chlorambucil was also superior to chlorambucil alone, but that only the obinutuzumab regimen had an overall survival advantage vs. chlorambucil alone.
An updated analysis to be reported soon will show emerging evidence of a survival advantage of obinutuzumab+chlorambucil vs. rituximab+chlorambucil, he said.
“This suggests that obinutuzumab is a far better antibody,” he added, noting that the reasons for that are under debate, “but the way it’s given, it works better in CLL, and that, I think is unequivocal.”
A similar study looking at chlorambucil with and without ofatumumab in “slow-go” patients also demonstrated an improvement in PFS with ofatumumab, but showed “no difference whatsoever in overall survival.”
“This is actually very similar to the rituximab result, and I actually call this the ‘death of ofatumumab’ study, because clearly obinutuzumab in CLL is, I think, a superior anti-CD20 antibody,” Dr. Zelenetz said.
Studies in which obinutuzumab is substituted for rituximab in the FCR combination are currently underway as are a number of other studies of obinutuzumab, he noted.
Another treatment option in the up-front setting is ibrutinib, which was shown to be effective in the RESONATE 2 trial .
“But notice, a very, very small [complete response rate]. CRs are very difficult to achieve with ibrutinib alone, so this drug is given continuously, lifelong,” Dr. Zelenetz said, noting that it was, however, associated with an overall survival advantage vs. chlorambucil.
“Should this be the standard of care? I think it is in patients who have del(17p) or mutation of TP53. Outside of that setting, I’m still concerned about the cost of long-term tolerability of the agent,” he said.
Future of first-line CLL treatment
Avoidance of long-term therapy and conventional chemotherapy in patients with CLL is a goal, he added, noting that new understanding from studies in patients in the relapsed/refractory CLL setting – such as recent findings from a phase Ib study of venetoclax plus rituximab, which demonstrated potentially durable responses after treatment discontinuation in minimal residual disease (MRD)–negative patients – are providing insights into achieving MRD negativity that could be applied in the front line treatment setting.
“We’re still trying to figure out how to best use this. We want to try to use some of this knowledge about achievement of MRD negativity in the up-front setting so we don’t have to give patients long-term therapy, and we would like to avoid conventional chemotherapy,” he said. “So I’m hoping we’re going to be able to replace chronic long-term therapy of CLL with a defined course of treatment with high levels of MRD negativity.”
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Acerta Pharma, Amgen Inc., BeiGene, Bristol-Myers Squibb, Celgene Corporation, Genentech, Gilead Sciences, Janssen Pharmaceutica Products, MEI Pharma, NanoString Technologies, Pharmacyclics, Portola Pharmaceuticals, Roche Laboratories, and Takeda Pharmaceuticals North America.
ORLANDO – The choice of first-line therapy in symptomatic chronic lymphocytic leukemia patients depends largely on comorbidity burden, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
“This is a disease of elderly patients. Frequently they have comorbidities,” he said. Categorizing these patients as having a low or high comorbidity burden can be done with the Cumulative Index Rating Scale score, which involves scoring of all organ systems on a 0-5 scale representing “not affected” to “extremely disabled.”
“We use this to determine first-line therapy,” said Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York. Dr. Zelenetz is chair of the NCCN Non-Hodgkin Lymphoma Guidelines panel.
Patients with a score of greater than 12 on the 0- to 56-point scale, are “no-go” patients with respect to therapy, and are typically treated only with palliative approaches. Those with a score of 7-12 (“slow-go” patients) have a significant comorbidity burden, but can undergo treatment, thought typically to be at reduced intensity. Those with a score of 0-6 are “go-go” patients with respect to treatment, as they are physically fit, have excellent renal function, and have no significant comorbidities, he said.
Treatment options for ‘go-go’ CLL patients
Among the treatment options for the latter is FCR–the combination of fludarabine, cyclophosphamide, and rituximab, which was shown in the phase III CLL10 trial of patients with advanced CLL to be associated with improved complete response rates compared with the popular regimen of bendamustine and rituximab (BR), both overall and in patients under age 65. In older patients, the advantage disappeared, Dr. Zelenetz said.
FCR was also associated with improved outcomes vs. BR in patients with del(11q).
The primary endpoint of the study was progression-free survival, which favored FCR (median of 55.2 vs. 41.7 months; hazard ratio, 1.643), he said, noting that no difference was seen between the two regimens in terms of overall survival.
In a recent publication, MD Anderson Cancer Center reported its experience with its first 300 CLL patients treated with FCR. With long-term follow-up of at least 9-10 years (median of 12.8 years), patients in this trial have done extremely well.
“But interestingly, when you stratify these patients by whether they have IGHV [immunoglobulin heavy chain variable] mutated or unmutated [disease], the IGHV mutated patients have something that looks a whole lot like a survival plateau, and that survival plateau is not trivial – it’s about 60%,” he said. “So there is a group of patients with CLL who are, in fact, curable with conventional chemoimmunotherapy.
“This is an appropriate treatment for a young, fit, ‘go-go’ patient, and it has a big implication,” he said. That is, patients who are young and fit require IGHV mutation testing, as “you will absolutely choose FCR chemotherapy for the fit, young patients who has IGHV mutated disease.
“In that setting IGHV testing is now mandatory,” he stressed, noting that the benefits in this population extend to overall survival as well as progression-free survival.
Dr. Zelenetz also emphasized the need for increasing the single dose of rituximab from 375 mg/m2 during cycle 1 to 500 mg/m2 during cycles 2-6 in those receiving FCR, as this is often forgotten.
The data demonstrating the efficacy of FCR were based on this approach, he said.
Fludarabine is to be given at a dose of 25 mg/m2, and cyclophosphamide at a dose of 250 mg/m2 – both for 2-4 days during cycle 1 and for 1-3 days during cycles 2-6.
Treatment options for ‘slow-go’ CLL patients
In “slow-go” patients, an interesting approach is to use new anti-CD20 antibodies such as ofatumumab and obinutuzumab, which have features that are distinct from rituximab.
Both have been studied in CLL. The CLL11 trial compared chlorambucil, rituximab+chlorambucil, and obinutuzumab+chlorambucil, and the latest analysis showed substantial improvement in progression-free survival with obinutuzumab+chlorambucil vs. the other two regimens (26.7 months vs. 11.1 and 16.3 months, respectively), Dr. Zelenetz said, noting that rituximab+chlorambucil was also superior to chlorambucil alone, but that only the obinutuzumab regimen had an overall survival advantage vs. chlorambucil alone.
An updated analysis to be reported soon will show emerging evidence of a survival advantage of obinutuzumab+chlorambucil vs. rituximab+chlorambucil, he said.
“This suggests that obinutuzumab is a far better antibody,” he added, noting that the reasons for that are under debate, “but the way it’s given, it works better in CLL, and that, I think is unequivocal.”
A similar study looking at chlorambucil with and without ofatumumab in “slow-go” patients also demonstrated an improvement in PFS with ofatumumab, but showed “no difference whatsoever in overall survival.”
“This is actually very similar to the rituximab result, and I actually call this the ‘death of ofatumumab’ study, because clearly obinutuzumab in CLL is, I think, a superior anti-CD20 antibody,” Dr. Zelenetz said.
Studies in which obinutuzumab is substituted for rituximab in the FCR combination are currently underway as are a number of other studies of obinutuzumab, he noted.
Another treatment option in the up-front setting is ibrutinib, which was shown to be effective in the RESONATE 2 trial .
“But notice, a very, very small [complete response rate]. CRs are very difficult to achieve with ibrutinib alone, so this drug is given continuously, lifelong,” Dr. Zelenetz said, noting that it was, however, associated with an overall survival advantage vs. chlorambucil.
“Should this be the standard of care? I think it is in patients who have del(17p) or mutation of TP53. Outside of that setting, I’m still concerned about the cost of long-term tolerability of the agent,” he said.
Future of first-line CLL treatment
Avoidance of long-term therapy and conventional chemotherapy in patients with CLL is a goal, he added, noting that new understanding from studies in patients in the relapsed/refractory CLL setting – such as recent findings from a phase Ib study of venetoclax plus rituximab, which demonstrated potentially durable responses after treatment discontinuation in minimal residual disease (MRD)–negative patients – are providing insights into achieving MRD negativity that could be applied in the front line treatment setting.
“We’re still trying to figure out how to best use this. We want to try to use some of this knowledge about achievement of MRD negativity in the up-front setting so we don’t have to give patients long-term therapy, and we would like to avoid conventional chemotherapy,” he said. “So I’m hoping we’re going to be able to replace chronic long-term therapy of CLL with a defined course of treatment with high levels of MRD negativity.”
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Acerta Pharma, Amgen Inc., BeiGene, Bristol-Myers Squibb, Celgene Corporation, Genentech, Gilead Sciences, Janssen Pharmaceutica Products, MEI Pharma, NanoString Technologies, Pharmacyclics, Portola Pharmaceuticals, Roche Laboratories, and Takeda Pharmaceuticals North America.
ORLANDO – The choice of first-line therapy in symptomatic chronic lymphocytic leukemia patients depends largely on comorbidity burden, Andrew D. Zelenetz, MD, PhD, said at the annual conference of the National Comprehensive Cancer Network.
“This is a disease of elderly patients. Frequently they have comorbidities,” he said. Categorizing these patients as having a low or high comorbidity burden can be done with the Cumulative Index Rating Scale score, which involves scoring of all organ systems on a 0-5 scale representing “not affected” to “extremely disabled.”
“We use this to determine first-line therapy,” said Dr. Zelenetz of Memorial Sloan Kettering Cancer Center, New York. Dr. Zelenetz is chair of the NCCN Non-Hodgkin Lymphoma Guidelines panel.
Patients with a score of greater than 12 on the 0- to 56-point scale, are “no-go” patients with respect to therapy, and are typically treated only with palliative approaches. Those with a score of 7-12 (“slow-go” patients) have a significant comorbidity burden, but can undergo treatment, thought typically to be at reduced intensity. Those with a score of 0-6 are “go-go” patients with respect to treatment, as they are physically fit, have excellent renal function, and have no significant comorbidities, he said.
Treatment options for ‘go-go’ CLL patients
Among the treatment options for the latter is FCR–the combination of fludarabine, cyclophosphamide, and rituximab, which was shown in the phase III CLL10 trial of patients with advanced CLL to be associated with improved complete response rates compared with the popular regimen of bendamustine and rituximab (BR), both overall and in patients under age 65. In older patients, the advantage disappeared, Dr. Zelenetz said.
FCR was also associated with improved outcomes vs. BR in patients with del(11q).
The primary endpoint of the study was progression-free survival, which favored FCR (median of 55.2 vs. 41.7 months; hazard ratio, 1.643), he said, noting that no difference was seen between the two regimens in terms of overall survival.
In a recent publication, MD Anderson Cancer Center reported its experience with its first 300 CLL patients treated with FCR. With long-term follow-up of at least 9-10 years (median of 12.8 years), patients in this trial have done extremely well.
“But interestingly, when you stratify these patients by whether they have IGHV [immunoglobulin heavy chain variable] mutated or unmutated [disease], the IGHV mutated patients have something that looks a whole lot like a survival plateau, and that survival plateau is not trivial – it’s about 60%,” he said. “So there is a group of patients with CLL who are, in fact, curable with conventional chemoimmunotherapy.
“This is an appropriate treatment for a young, fit, ‘go-go’ patient, and it has a big implication,” he said. That is, patients who are young and fit require IGHV mutation testing, as “you will absolutely choose FCR chemotherapy for the fit, young patients who has IGHV mutated disease.
“In that setting IGHV testing is now mandatory,” he stressed, noting that the benefits in this population extend to overall survival as well as progression-free survival.
Dr. Zelenetz also emphasized the need for increasing the single dose of rituximab from 375 mg/m2 during cycle 1 to 500 mg/m2 during cycles 2-6 in those receiving FCR, as this is often forgotten.
The data demonstrating the efficacy of FCR were based on this approach, he said.
Fludarabine is to be given at a dose of 25 mg/m2, and cyclophosphamide at a dose of 250 mg/m2 – both for 2-4 days during cycle 1 and for 1-3 days during cycles 2-6.
Treatment options for ‘slow-go’ CLL patients
In “slow-go” patients, an interesting approach is to use new anti-CD20 antibodies such as ofatumumab and obinutuzumab, which have features that are distinct from rituximab.
Both have been studied in CLL. The CLL11 trial compared chlorambucil, rituximab+chlorambucil, and obinutuzumab+chlorambucil, and the latest analysis showed substantial improvement in progression-free survival with obinutuzumab+chlorambucil vs. the other two regimens (26.7 months vs. 11.1 and 16.3 months, respectively), Dr. Zelenetz said, noting that rituximab+chlorambucil was also superior to chlorambucil alone, but that only the obinutuzumab regimen had an overall survival advantage vs. chlorambucil alone.
An updated analysis to be reported soon will show emerging evidence of a survival advantage of obinutuzumab+chlorambucil vs. rituximab+chlorambucil, he said.
“This suggests that obinutuzumab is a far better antibody,” he added, noting that the reasons for that are under debate, “but the way it’s given, it works better in CLL, and that, I think is unequivocal.”
A similar study looking at chlorambucil with and without ofatumumab in “slow-go” patients also demonstrated an improvement in PFS with ofatumumab, but showed “no difference whatsoever in overall survival.”
“This is actually very similar to the rituximab result, and I actually call this the ‘death of ofatumumab’ study, because clearly obinutuzumab in CLL is, I think, a superior anti-CD20 antibody,” Dr. Zelenetz said.
Studies in which obinutuzumab is substituted for rituximab in the FCR combination are currently underway as are a number of other studies of obinutuzumab, he noted.
Another treatment option in the up-front setting is ibrutinib, which was shown to be effective in the RESONATE 2 trial .
“But notice, a very, very small [complete response rate]. CRs are very difficult to achieve with ibrutinib alone, so this drug is given continuously, lifelong,” Dr. Zelenetz said, noting that it was, however, associated with an overall survival advantage vs. chlorambucil.
“Should this be the standard of care? I think it is in patients who have del(17p) or mutation of TP53. Outside of that setting, I’m still concerned about the cost of long-term tolerability of the agent,” he said.
Future of first-line CLL treatment
Avoidance of long-term therapy and conventional chemotherapy in patients with CLL is a goal, he added, noting that new understanding from studies in patients in the relapsed/refractory CLL setting – such as recent findings from a phase Ib study of venetoclax plus rituximab, which demonstrated potentially durable responses after treatment discontinuation in minimal residual disease (MRD)–negative patients – are providing insights into achieving MRD negativity that could be applied in the front line treatment setting.
“We’re still trying to figure out how to best use this. We want to try to use some of this knowledge about achievement of MRD negativity in the up-front setting so we don’t have to give patients long-term therapy, and we would like to avoid conventional chemotherapy,” he said. “So I’m hoping we’re going to be able to replace chronic long-term therapy of CLL with a defined course of treatment with high levels of MRD negativity.”
Dr. Zelenetz reported receiving consulting fees, honoraria, and/or grant/research support from Acerta Pharma, Amgen Inc., BeiGene, Bristol-Myers Squibb, Celgene Corporation, Genentech, Gilead Sciences, Janssen Pharmaceutica Products, MEI Pharma, NanoString Technologies, Pharmacyclics, Portola Pharmaceuticals, Roche Laboratories, and Takeda Pharmaceuticals North America.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONFERENCE
Is your patient’s valproic acid dosage too low or high? Adjust it with this equation
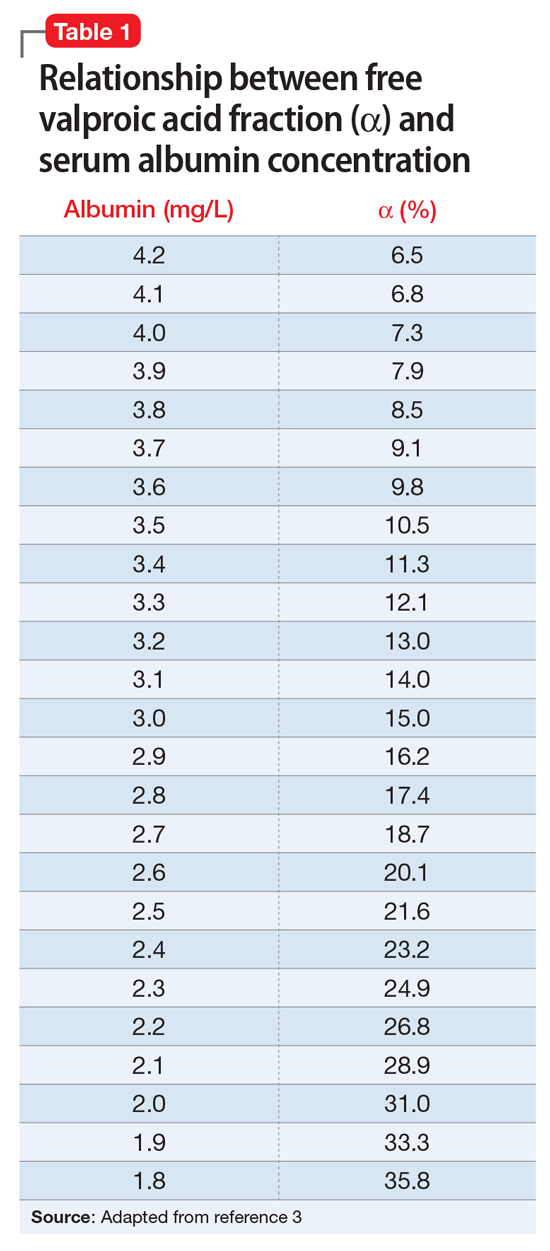
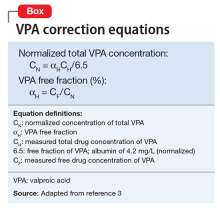
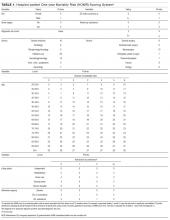
Valproic acid (VPA) often is used to treat mania in bipolar disorder, and it has a therapeutic range of 50 to 125 µg/mL of total serum concentration.1 VPA binds highly to albumin, resulting in free drug concentrations (5 to 15 mg/L) that are responsible for its therapeutic effect.2 Monitoring total VPA levels in patients with hypoalbuminemia could reveal seemingly subtherapeutic VPA levels, which could lead to unnecessary dosage adjustments or drug toxicity. Hermida et al3 devised a correction equation to normalize total VPA serum concentrations <75 µg/mL in patients with hypoalbuminemia (Table 1, Box).
We present a case employing this equation in a patient
Case
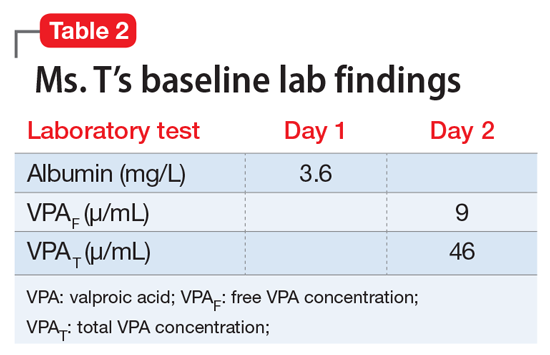
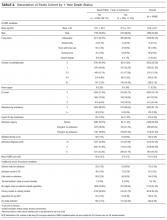
Ms. T, age 75, is admitted to the hospital with delusional, paranoid, assaultive, and combative behavior. By applying Ms. T’s baseline lab findings (Table 2) to the equation, a normalized total VPA level and estimated free VPA level of 70 µg/mL and 7 µg/mL, respectively, can be approximated. These estimates fall within the therapeutic range and are validated by the measured free VPA level of 9 µg/mL.
VPA serum levels should be assessed 2 to 4 days after initiation or dosage adjustments.1 Also, consider patient-specific goals and intended clinical effect when adjusting VPA dosage. In practice settings, where free VPA levels are not routinely monitored or are cost prohibitive, this equation can guide clinical decision-making.3
1. Depakote [divalproex sodium]. North Chicago, IL: AbbVie Inc; 2016.
2. DeVane CL. Pharmacokinetics, drug interactions, and tolerability of valproate. Psychopharmacol Bull. 2003;37(suppl 2):25-42.
3. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97(4):489-493.
Valproic acid (VPA) often is used to treat mania in bipolar disorder, and it has a therapeutic range of 50 to 125 µg/mL of total serum concentration.1 VPA binds highly to albumin, resulting in free drug concentrations (5 to 15 mg/L) that are responsible for its therapeutic effect.2 Monitoring total VPA levels in patients with hypoalbuminemia could reveal seemingly subtherapeutic VPA levels, which could lead to unnecessary dosage adjustments or drug toxicity. Hermida et al3 devised a correction equation to normalize total VPA serum concentrations <75 µg/mL in patients with hypoalbuminemia (Table 1, Box).
We present a case employing this equation in a patient
Case
Ms. T, age 75, is admitted to the hospital with delusional, paranoid, assaultive, and combative behavior. By applying Ms. T’s baseline lab findings (Table 2) to the equation, a normalized total VPA level and estimated free VPA level of 70 µg/mL and 7 µg/mL, respectively, can be approximated. These estimates fall within the therapeutic range and are validated by the measured free VPA level of 9 µg/mL.
VPA serum levels should be assessed 2 to 4 days after initiation or dosage adjustments.1 Also, consider patient-specific goals and intended clinical effect when adjusting VPA dosage. In practice settings, where free VPA levels are not routinely monitored or are cost prohibitive, this equation can guide clinical decision-making.3
Valproic acid (VPA) often is used to treat mania in bipolar disorder, and it has a therapeutic range of 50 to 125 µg/mL of total serum concentration.1 VPA binds highly to albumin, resulting in free drug concentrations (5 to 15 mg/L) that are responsible for its therapeutic effect.2 Monitoring total VPA levels in patients with hypoalbuminemia could reveal seemingly subtherapeutic VPA levels, which could lead to unnecessary dosage adjustments or drug toxicity. Hermida et al3 devised a correction equation to normalize total VPA serum concentrations <75 µg/mL in patients with hypoalbuminemia (Table 1, Box).
We present a case employing this equation in a patient
Case
Ms. T, age 75, is admitted to the hospital with delusional, paranoid, assaultive, and combative behavior. By applying Ms. T’s baseline lab findings (Table 2) to the equation, a normalized total VPA level and estimated free VPA level of 70 µg/mL and 7 µg/mL, respectively, can be approximated. These estimates fall within the therapeutic range and are validated by the measured free VPA level of 9 µg/mL.
VPA serum levels should be assessed 2 to 4 days after initiation or dosage adjustments.1 Also, consider patient-specific goals and intended clinical effect when adjusting VPA dosage. In practice settings, where free VPA levels are not routinely monitored or are cost prohibitive, this equation can guide clinical decision-making.3
1. Depakote [divalproex sodium]. North Chicago, IL: AbbVie Inc; 2016.
2. DeVane CL. Pharmacokinetics, drug interactions, and tolerability of valproate. Psychopharmacol Bull. 2003;37(suppl 2):25-42.
3. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97(4):489-493.
1. Depakote [divalproex sodium]. North Chicago, IL: AbbVie Inc; 2016.
2. DeVane CL. Pharmacokinetics, drug interactions, and tolerability of valproate. Psychopharmacol Bull. 2003;37(suppl 2):25-42.
3. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97(4):489-493.
The Cardiovascular Safety of Nonsteroidal Anti-Inflammatory Drugs: Putting the Evidence in Perspective
Topics Include:
- Key Prospective Clinical Trials
- Danish Registry Study
- Meta-Analyses
- FDA Actions
- PRECISION Trial
Faculty/Faculty Disclosure:
Martin Quan, MD
Professor of Clinical Family Medicine
David Geffen School of Medicine at UCLA
Vice Chair for Academic Affairs
UCLA Department of Family Medicine
Los Angeles, CA
Dr. Quan discloses that he has no real or apparent conflicts to report.
Topics Include:
- Key Prospective Clinical Trials
- Danish Registry Study
- Meta-Analyses
- FDA Actions
- PRECISION Trial
Faculty/Faculty Disclosure:
Martin Quan, MD
Professor of Clinical Family Medicine
David Geffen School of Medicine at UCLA
Vice Chair for Academic Affairs
UCLA Department of Family Medicine
Los Angeles, CA
Dr. Quan discloses that he has no real or apparent conflicts to report.
Topics Include:
- Key Prospective Clinical Trials
- Danish Registry Study
- Meta-Analyses
- FDA Actions
- PRECISION Trial
Faculty/Faculty Disclosure:
Martin Quan, MD
Professor of Clinical Family Medicine
David Geffen School of Medicine at UCLA
Vice Chair for Academic Affairs
UCLA Department of Family Medicine
Los Angeles, CA
Dr. Quan discloses that he has no real or apparent conflicts to report.
Prognosticating with the hospitalized-patient one-year mortality risk score using information abstracted from the medical record
A patient’s prognosis can strongly influence their medical care. Decisions about diagnostic modalities, treatment options, and the use of preventive therapies can all be affected by the likelihood of a patient’s death in the near future. For example, patients with severely limited survival might forego prophylactic therapy, avoid interventions for asymptomatic issues, and cease screening interventions. Knowing survival probability would also be very helpful as a controlling variable in research analyses whenever death risk might be a possible confounder.
Sixteen indices that aim to predict patient death risk have been described by Yourman et al.1 They were all created from secondary analyses of clinical and administrative datasets, were applicable to patients in a variety of settings (including the community, nursing home, or hospital), and predicted survival probabilities in time horizons ranging from 6 months to 5 years. Prognostic factors that were most commonly included in these indices were comorbidity and functional status. In validation populations, the discrimination of these indices for 1-year survival in hospitalized patients was moderate (with C statistics that ranged from 0.64 to 0.79) with good calibration for broad prognostic ranges.
In 2014, we published the Hospitalized-patient One-year Mortality Risk (HOMR) score.2 This study used health administrative data for all adult Ontarians admitted in 2011 to hospital under nonpsychiatric services (n = 640,022) to estimate the probability of dying within 1 year of admission to hospital (which happened in 11.7% of people). The HOMR score included 12 patient and hospitalization factors (Table 1). It was highly discriminative (C statistic, 0.923; [0.922-0.924]) and well calibrated (the mean relative difference between observed and expected death risk was 2.0% [range, 0.0% to 7.0%]). It was externally validated in more than 3 million adults from Ontario, Alberta, and Boston in whom the C statistic ranged from 0.89 to 0.92 and calibration was excellent.3 We concluded from these studies that the HOMR score is excellent for prognosticating a diverse group of patients using health administrative data.
However, we do not know whether the HOMR score can be applied to patients using primary data (ie, those taken directly from the chart). This question is important for 2 reasons. First, if HOMR accurately predicts death risk using data abstracted from the medical record, it could be used in the clinical setting to assist in clinical decision-making. Second, HOMR uses multiple administrative datasets that are difficult to access and use by most clinical researchers; it is, therefore, important to determine if HOMR is accurate for clinical research based on primary medical record review. The primary objective of this study was to determine the accuracy of the HOMR score when calculated using data abstracted from clinical notes that were available when patients were admitted to hospital. Secondary objectives included determining whether functional measures abstracted were significantly associated with death risk beyond the HOMR score and whether HOMR scores calculated from chart review deviated from those calculated from administrative data.
METHODS
Study Cohort
The study, which was approved by our local research ethics board, took place at the Ottawa Hospital, a 1000-bed teaching hospital that is the primary referral center in our region. We used the hospital admission registry to identify all people 18 years or older who were admitted to a nonpsychiatric service at our hospital between January 1, 2011 and December 31, 2011 (this time frame corresponds with the year used to derive the HOMR score). We excluded overnight patients in the same-day surgery or the bone-marrow transplant units (since they would not have been included in the original study) and those without a valid health card number (which was required to link to provincial data to identify outcomes). From this list, we randomly selected 5000 patients.
Primary Data Collection
For each patient, we retrieved all data required to calculate the HOMR score from the medical record (Table 1). Patient registration information in our electronic medical record was used to identify patient age, sex, admitting service, number of emergency department (ED) visits in the previous year, number of admissions in the previous year (the nursing triage note was reviewed for each admission to determine if it was by ambulance), and whether or not the patient had been discharged from hospital in the previous 30 days. The admitting service consult note was used to determine the admitting diagnosis and whether or not the patient was admitted directly to the intensive care unit. If they were present, the emergency nursing triage note, the ED record of treatment, the admission consult note, the pre-operative consult note, and consult notes were all used to determine the patient’s comorbidities, living status, and home oxygen status. Admission urgency was determined using information from the patient registration information and the ED nursing triage note. All data were abstracted from information that had been registered prior to when the patient was physically transferred to their hospital bed. This ensured that we used only data available at the start of the admission.
Patient functional status has been shown to be strongly associated with survival4 but HOMR only indirectly captures functional information (through the patient’s living status). We, therefore, collected more detailed functional information from the medical record by determining if the patient was dependent for any activities of daily living (ADL) from the emergency nursing triage note, the ED record of treatment, the admission consult note, and the pre-operative consultation. We also collected information that might indicate frailty, which we defined per Clegg et al.5 as “a state of increased vulnerability to poor resolution of homeostasis following a stress.” This information included: delirium or more than 1 fall recorded on the emergency nursing triage note, the ED record of treatment, or the admission consultation note; or whether a geriatric nursing specialist assessment occurred in the ED in the previous 6 months. Finally, we recorded possible indicators of limited social support (no fixed address [from patient registration and nursing triage note], primary contact is not a family member [from the emergency notes, consult, and patient registration], and no religion noted in system [from patient registration]). Patients for whom religion status was missing were classified as having “no religion.”
Analysis
These data were encrypted and linked anonymously to population-based databases to determine whether patients died within 1 year of admission to hospital. We calculated the chart-HOMR score using information from the chart review and determined its association with the outcome using bivariate logistic regression. We compared observed and expected risk of death within 1 year of admission to hospital for each chart-HOMR score value, with expected risks determined from the external validation study.3 We regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups to ensure adequate numbers in each group); and we gauged overall deviations from expected risk and the relationship between the observed and expected death risk (based on the chart-HOMR score) using the line’s intercept and slope, respectively.6 Next, we replicated methods from our studies2,3 to calculate the administrative-HOMR score in our study cohort using administrative databases. We compared these chart-HOMR and administrative-HOMR scores (and scores for each of its components). Finally, we determined which of the socio-functional factors were associated with 1-year death risk independent of the chart-HOMR score. We used the likelihood ratio test to determine whether these additional socio-functional factors significantly improved the model beyond the chart-HOMR score.7 This test subtracted the -2 logL value of the full model from that containing the chart-HOMR score alone, comparing its value to the χ2 distribution (with degrees of freedom equivalent to the number of additional parameters in the nested model) to determine statistical significance. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
There were 43,883 overnight hospitalizations at our hospital in 2011, and 38,886 hospitalizations were excluded: 1883 hospitalizations were in the same-day surgery or the bone-marrow transplant unit; 2485 did not have a valid health card number; 34,515 were not randomly selected; the records of 3 randomly selected patients had been blocked by our hospital’s privacy department; and 1 patient could not be linked with the population-based administrative datasets.
The 4996 study patients were middle-aged and predominantly female (Table 2). The extensive majority of patients was admitted from the community, was independent for ADL, had a family member as the principal contact, and had no admissions by ambulance in the previous year. Most people had no significant comorbidities or ED visits in the year prior to their admission. The mean chart-HOMR score was 22 (standard deviation [SD], 12), which is associated with a 1.2% expected risk of death within 1 year of hospital admission (Appendix 1).3
A total of 563 patients (11.3%) died within 1 year of admission to hospital (Table 2). In the study cohort, each chart-HOMR component was associated with death status. People who died were older, more likely to be male, had a greater number of important comorbidities, had more ED visits and admissions by ambulance in the previous year, and were more likely to have been discharged in the previous 30 days, and were admitted urgently, directly to the intensive care unit, or with complicated diagnoses. The mean chart-HOMR score differed extensively by survival status (37.4 [SD, 7.5] in those who died vs. 19.9 [SD, 12.2] in those who survived). Three of the socio-functional variables (delirium and falls noted on admission documents, and dependent for any ADL) also varied with death status.
The chart-HOMR score was strongly associated with the likelihood of death within 1 year of admission. When included in a logistic regression model having 1-year death as the outcome, a 1-point increase in the chart-HOMR score was associated with a 19% increase in the odds of death (P < 0.0001). This model (with only the chart-HOMR score) was highly discriminative (C statistic, 0.888) and well calibrated (Hosmer-Lemeshow test, 12.9 [8 df, P = 0.11]).
Observed and expected death risks by chart-HOMR score were similar (Figure 1). The observed total number of deaths (n = 563; 11.3%) exceeded the expected number of deaths (n = 437, 8.7%). When we regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups), the Hosmer-Lemeshow test was significant, indicating that differences between observed and expected risks were beyond that expected by chance (Hosmer-Lemeshow test, 141.9, 21 df, P < 0.0001). The intercept of this model (0.035; 95% CI, 0.01-0.06) was statistically significant (P = 0.01), indicating that the observed number of cases significantly exceeded the expected; however, its calibration slope (1.02; 95% CI, 0.89-1.16) did not deviate significantly from unity, indicating that the relationship between the observed and expected death risk (based on the chart-HOMR score) remained intact (Figure 1).
The deviations between observed and expected death risks reflected deviations between the c chart-HOMR score and the administrative-HOMR score, with the former being significantly lower than the latter (Figure 2). Overall, the chart-HOMR score was 0.96 points lower (95% CI, 0.81-1.12) than the administrative-HOMR score. The HOMR score components that were notably underestimated using chart data included those for the age-Charlson Comorbidity Index interaction, living status, and admit points. Points for only 2 components (admitting service and admission urgency) were higher when calculated using chart data.
Four additional socio-functional variables collected from medical record review were significantly associated with 1-year death risk independent of the chart-HOMR score (Table 3). Admission documentation noting either delirium or falls were both associated with a significantly increased death risk (adjusted odds ratio [OR], 1.92 [95% CI, 1.24-2.96] and OR 1.96 [95% CI, 1.29-2.99], respectively). An independently increased death risk was also noted in patients who were dependent for any ADL (adjusted OR, 1.99 [95% CI, 1.24-3.19]). The presence of an ED geriatrics consultation within the previous 6 months was associated with a significantly decreased death risk of 60% (adjusted OR, 0.40 [95% CI, 0.20-0.81]). Adding these covariates to the logistic model with the chart-HOMR score significantly improved predictions (likelihood ratio statistic = 33.569, 4df, P < 0.00001).
DISCUSSION
In a large random sample of patients from our hospital, we found that the HOMR score using data abstracted from the medical record was significantly associated with 1-year death risk. The expected death risk based on the chart-HOMR score underestimated observed death risk but the relationship between the chart-HOMR score and death risk was similar to that in studies using administrative data. The HOMR score calculated using data from the chart was lower than that calculated using data from population-based administrative datasets; additional variables indicating patient frailty were significantly associated with 1-year death risk independent of the chart-HOMR score. Since the HOMR score was derived and initially validated using health administrative data, this study using data abstracted from the health record shows that the HOMR score has methodological generalizability.8
We think that our study has several notable findings. First, we found that data abstracted from the medical record can be used to calculate the HOMR score to accurately predict individual death risk. The chart-HOMR score discriminated very well between patients who did and did not die (C statistic, 0.88), which extensively exceeds the discrimination of published death risk indices (whose C statistics range between 0.69 and 0.82). It is also possible that chart abstraction for the HOMR score—without functional status—is simpler than other indices since its components are primarily very objective. (Other indices for hospital-based patients required factors that could be difficult to abstract reliably from the medical record including meeting more than 1 guideline for noncancer hospice care9; ambulation difficulties10; scales such as the Exton-Smith Scale or the Short Portable Mental Status Questionnaire11; weight loss12; functional status4; and pressure sore risk.13) Although expected risks for the chart-HOMR consistently underestimated observed risks (Figure 1), the mean deviation was small (with an absolute difference of 3.5% that can be used as a correction factor when determining expected risks with HOMR scores calculated from chart review), but it was an association between the chart-HOMR score and death risk that remained consistent through the cohort. Second, we found a small but significant decrease in the chart-HOMR score vs. the administrative-HOMR score (Figure 2). Some of these underestimates such as those for the number of ED visits or admissions by ambulance were expected since population-based health administrative databases would best capture such data. However, we were surprised that the comorbidity score was less when calculated using chart vs. database data (Figure 2). This finding is distinct from studies finding that particular comorbidities are documented in the chart are sometimes not coded.14,15 However, we identified comorbidities in the administrative databases using a 1-year ‘look-back’ period so that diagnostic codes from multiple hospitalizations (and from multiple hospitals) could be used to calculate the Charlson Comorbidity Index for a particular patient; this has been shown to increase the capture of comorbidities.16 Third, we found that variables from the chart review indicating frailty were predictive of 1-year death risk independent of the chart-HOMR score (Table 2). This illustrates that mortality risk prediction can be improved for particular patient groups by adding new covariates to the HOMR. Further work is required to determine how to incorporate these (and possibly other) covariates into the HOMR to create a unique chart-HOMR score. Finally, we found that a geriatrics assessment in the ED was associated with a significant (and notable) decrease in death risk. With these data, we are unable to indicate whether this association is causative. However, these findings indicate that the influence of emergency geriatric assessments on patient survival needs to be explored in more detail.
Several issues about our study should be considered when interpreting its results. First, this was a single-center study and the generalizability of our results to other centers is unknown. However, our study had the largest sample size of all primary data prognostic index validation studies1 ensuring that our results are, at the very least, internally reliable. In addition, our simple random sample ensured that we studied a broad assortment of patients to be certain that our results are representative of our institution. Second, we used a single abstractor for the study, which could limit the generalizability of our results. However, almost all the data points that were abstracted for our study were very objective.
In summary, our study shows that the HOMR score can be used to accurately predict 1-year death risk using data abstracted from the patient record. These findings will aid in individual patient prognostication for clinicians and researchers.
Disclosure
The authors report no financial conflicts of interest.
1. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. PubMed
2. van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicts long term death risk in hospitalized patients. J Clin Epidemiol. 2014;67(9):1025-1034. PubMed
3. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ. 2015;187(10):725-733. PubMed
4. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
5. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. The Lancet 2002;381:752-762. PubMed
6. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692-1706. PubMed
7. Harrell FE Jr. Overview of Maximum Likelihood Estimation. Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001: 179-212.
8. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515-524. PubMed
9. Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285-292. PubMed
10. Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83. PubMed
11. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151-161. PubMed
12. Teno JM, Harrell FE Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 suppl):S16-S24. PubMed
13. Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783-791. PubMed
14. Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52(2):137-142. PubMed
15. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. PubMed
16. Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson cComorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128-1139. PubMed
A patient’s prognosis can strongly influence their medical care. Decisions about diagnostic modalities, treatment options, and the use of preventive therapies can all be affected by the likelihood of a patient’s death in the near future. For example, patients with severely limited survival might forego prophylactic therapy, avoid interventions for asymptomatic issues, and cease screening interventions. Knowing survival probability would also be very helpful as a controlling variable in research analyses whenever death risk might be a possible confounder.
Sixteen indices that aim to predict patient death risk have been described by Yourman et al.1 They were all created from secondary analyses of clinical and administrative datasets, were applicable to patients in a variety of settings (including the community, nursing home, or hospital), and predicted survival probabilities in time horizons ranging from 6 months to 5 years. Prognostic factors that were most commonly included in these indices were comorbidity and functional status. In validation populations, the discrimination of these indices for 1-year survival in hospitalized patients was moderate (with C statistics that ranged from 0.64 to 0.79) with good calibration for broad prognostic ranges.
In 2014, we published the Hospitalized-patient One-year Mortality Risk (HOMR) score.2 This study used health administrative data for all adult Ontarians admitted in 2011 to hospital under nonpsychiatric services (n = 640,022) to estimate the probability of dying within 1 year of admission to hospital (which happened in 11.7% of people). The HOMR score included 12 patient and hospitalization factors (Table 1). It was highly discriminative (C statistic, 0.923; [0.922-0.924]) and well calibrated (the mean relative difference between observed and expected death risk was 2.0% [range, 0.0% to 7.0%]). It was externally validated in more than 3 million adults from Ontario, Alberta, and Boston in whom the C statistic ranged from 0.89 to 0.92 and calibration was excellent.3 We concluded from these studies that the HOMR score is excellent for prognosticating a diverse group of patients using health administrative data.
However, we do not know whether the HOMR score can be applied to patients using primary data (ie, those taken directly from the chart). This question is important for 2 reasons. First, if HOMR accurately predicts death risk using data abstracted from the medical record, it could be used in the clinical setting to assist in clinical decision-making. Second, HOMR uses multiple administrative datasets that are difficult to access and use by most clinical researchers; it is, therefore, important to determine if HOMR is accurate for clinical research based on primary medical record review. The primary objective of this study was to determine the accuracy of the HOMR score when calculated using data abstracted from clinical notes that were available when patients were admitted to hospital. Secondary objectives included determining whether functional measures abstracted were significantly associated with death risk beyond the HOMR score and whether HOMR scores calculated from chart review deviated from those calculated from administrative data.
METHODS
Study Cohort
The study, which was approved by our local research ethics board, took place at the Ottawa Hospital, a 1000-bed teaching hospital that is the primary referral center in our region. We used the hospital admission registry to identify all people 18 years or older who were admitted to a nonpsychiatric service at our hospital between January 1, 2011 and December 31, 2011 (this time frame corresponds with the year used to derive the HOMR score). We excluded overnight patients in the same-day surgery or the bone-marrow transplant units (since they would not have been included in the original study) and those without a valid health card number (which was required to link to provincial data to identify outcomes). From this list, we randomly selected 5000 patients.
Primary Data Collection
For each patient, we retrieved all data required to calculate the HOMR score from the medical record (Table 1). Patient registration information in our electronic medical record was used to identify patient age, sex, admitting service, number of emergency department (ED) visits in the previous year, number of admissions in the previous year (the nursing triage note was reviewed for each admission to determine if it was by ambulance), and whether or not the patient had been discharged from hospital in the previous 30 days. The admitting service consult note was used to determine the admitting diagnosis and whether or not the patient was admitted directly to the intensive care unit. If they were present, the emergency nursing triage note, the ED record of treatment, the admission consult note, the pre-operative consult note, and consult notes were all used to determine the patient’s comorbidities, living status, and home oxygen status. Admission urgency was determined using information from the patient registration information and the ED nursing triage note. All data were abstracted from information that had been registered prior to when the patient was physically transferred to their hospital bed. This ensured that we used only data available at the start of the admission.
Patient functional status has been shown to be strongly associated with survival4 but HOMR only indirectly captures functional information (through the patient’s living status). We, therefore, collected more detailed functional information from the medical record by determining if the patient was dependent for any activities of daily living (ADL) from the emergency nursing triage note, the ED record of treatment, the admission consult note, and the pre-operative consultation. We also collected information that might indicate frailty, which we defined per Clegg et al.5 as “a state of increased vulnerability to poor resolution of homeostasis following a stress.” This information included: delirium or more than 1 fall recorded on the emergency nursing triage note, the ED record of treatment, or the admission consultation note; or whether a geriatric nursing specialist assessment occurred in the ED in the previous 6 months. Finally, we recorded possible indicators of limited social support (no fixed address [from patient registration and nursing triage note], primary contact is not a family member [from the emergency notes, consult, and patient registration], and no religion noted in system [from patient registration]). Patients for whom religion status was missing were classified as having “no religion.”
Analysis
These data were encrypted and linked anonymously to population-based databases to determine whether patients died within 1 year of admission to hospital. We calculated the chart-HOMR score using information from the chart review and determined its association with the outcome using bivariate logistic regression. We compared observed and expected risk of death within 1 year of admission to hospital for each chart-HOMR score value, with expected risks determined from the external validation study.3 We regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups to ensure adequate numbers in each group); and we gauged overall deviations from expected risk and the relationship between the observed and expected death risk (based on the chart-HOMR score) using the line’s intercept and slope, respectively.6 Next, we replicated methods from our studies2,3 to calculate the administrative-HOMR score in our study cohort using administrative databases. We compared these chart-HOMR and administrative-HOMR scores (and scores for each of its components). Finally, we determined which of the socio-functional factors were associated with 1-year death risk independent of the chart-HOMR score. We used the likelihood ratio test to determine whether these additional socio-functional factors significantly improved the model beyond the chart-HOMR score.7 This test subtracted the -2 logL value of the full model from that containing the chart-HOMR score alone, comparing its value to the χ2 distribution (with degrees of freedom equivalent to the number of additional parameters in the nested model) to determine statistical significance. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
There were 43,883 overnight hospitalizations at our hospital in 2011, and 38,886 hospitalizations were excluded: 1883 hospitalizations were in the same-day surgery or the bone-marrow transplant unit; 2485 did not have a valid health card number; 34,515 were not randomly selected; the records of 3 randomly selected patients had been blocked by our hospital’s privacy department; and 1 patient could not be linked with the population-based administrative datasets.
The 4996 study patients were middle-aged and predominantly female (Table 2). The extensive majority of patients was admitted from the community, was independent for ADL, had a family member as the principal contact, and had no admissions by ambulance in the previous year. Most people had no significant comorbidities or ED visits in the year prior to their admission. The mean chart-HOMR score was 22 (standard deviation [SD], 12), which is associated with a 1.2% expected risk of death within 1 year of hospital admission (Appendix 1).3
A total of 563 patients (11.3%) died within 1 year of admission to hospital (Table 2). In the study cohort, each chart-HOMR component was associated with death status. People who died were older, more likely to be male, had a greater number of important comorbidities, had more ED visits and admissions by ambulance in the previous year, and were more likely to have been discharged in the previous 30 days, and were admitted urgently, directly to the intensive care unit, or with complicated diagnoses. The mean chart-HOMR score differed extensively by survival status (37.4 [SD, 7.5] in those who died vs. 19.9 [SD, 12.2] in those who survived). Three of the socio-functional variables (delirium and falls noted on admission documents, and dependent for any ADL) also varied with death status.
The chart-HOMR score was strongly associated with the likelihood of death within 1 year of admission. When included in a logistic regression model having 1-year death as the outcome, a 1-point increase in the chart-HOMR score was associated with a 19% increase in the odds of death (P < 0.0001). This model (with only the chart-HOMR score) was highly discriminative (C statistic, 0.888) and well calibrated (Hosmer-Lemeshow test, 12.9 [8 df, P = 0.11]).
Observed and expected death risks by chart-HOMR score were similar (Figure 1). The observed total number of deaths (n = 563; 11.3%) exceeded the expected number of deaths (n = 437, 8.7%). When we regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups), the Hosmer-Lemeshow test was significant, indicating that differences between observed and expected risks were beyond that expected by chance (Hosmer-Lemeshow test, 141.9, 21 df, P < 0.0001). The intercept of this model (0.035; 95% CI, 0.01-0.06) was statistically significant (P = 0.01), indicating that the observed number of cases significantly exceeded the expected; however, its calibration slope (1.02; 95% CI, 0.89-1.16) did not deviate significantly from unity, indicating that the relationship between the observed and expected death risk (based on the chart-HOMR score) remained intact (Figure 1).
The deviations between observed and expected death risks reflected deviations between the c chart-HOMR score and the administrative-HOMR score, with the former being significantly lower than the latter (Figure 2). Overall, the chart-HOMR score was 0.96 points lower (95% CI, 0.81-1.12) than the administrative-HOMR score. The HOMR score components that were notably underestimated using chart data included those for the age-Charlson Comorbidity Index interaction, living status, and admit points. Points for only 2 components (admitting service and admission urgency) were higher when calculated using chart data.
Four additional socio-functional variables collected from medical record review were significantly associated with 1-year death risk independent of the chart-HOMR score (Table 3). Admission documentation noting either delirium or falls were both associated with a significantly increased death risk (adjusted odds ratio [OR], 1.92 [95% CI, 1.24-2.96] and OR 1.96 [95% CI, 1.29-2.99], respectively). An independently increased death risk was also noted in patients who were dependent for any ADL (adjusted OR, 1.99 [95% CI, 1.24-3.19]). The presence of an ED geriatrics consultation within the previous 6 months was associated with a significantly decreased death risk of 60% (adjusted OR, 0.40 [95% CI, 0.20-0.81]). Adding these covariates to the logistic model with the chart-HOMR score significantly improved predictions (likelihood ratio statistic = 33.569, 4df, P < 0.00001).
DISCUSSION
In a large random sample of patients from our hospital, we found that the HOMR score using data abstracted from the medical record was significantly associated with 1-year death risk. The expected death risk based on the chart-HOMR score underestimated observed death risk but the relationship between the chart-HOMR score and death risk was similar to that in studies using administrative data. The HOMR score calculated using data from the chart was lower than that calculated using data from population-based administrative datasets; additional variables indicating patient frailty were significantly associated with 1-year death risk independent of the chart-HOMR score. Since the HOMR score was derived and initially validated using health administrative data, this study using data abstracted from the health record shows that the HOMR score has methodological generalizability.8
We think that our study has several notable findings. First, we found that data abstracted from the medical record can be used to calculate the HOMR score to accurately predict individual death risk. The chart-HOMR score discriminated very well between patients who did and did not die (C statistic, 0.88), which extensively exceeds the discrimination of published death risk indices (whose C statistics range between 0.69 and 0.82). It is also possible that chart abstraction for the HOMR score—without functional status—is simpler than other indices since its components are primarily very objective. (Other indices for hospital-based patients required factors that could be difficult to abstract reliably from the medical record including meeting more than 1 guideline for noncancer hospice care9; ambulation difficulties10; scales such as the Exton-Smith Scale or the Short Portable Mental Status Questionnaire11; weight loss12; functional status4; and pressure sore risk.13) Although expected risks for the chart-HOMR consistently underestimated observed risks (Figure 1), the mean deviation was small (with an absolute difference of 3.5% that can be used as a correction factor when determining expected risks with HOMR scores calculated from chart review), but it was an association between the chart-HOMR score and death risk that remained consistent through the cohort. Second, we found a small but significant decrease in the chart-HOMR score vs. the administrative-HOMR score (Figure 2). Some of these underestimates such as those for the number of ED visits or admissions by ambulance were expected since population-based health administrative databases would best capture such data. However, we were surprised that the comorbidity score was less when calculated using chart vs. database data (Figure 2). This finding is distinct from studies finding that particular comorbidities are documented in the chart are sometimes not coded.14,15 However, we identified comorbidities in the administrative databases using a 1-year ‘look-back’ period so that diagnostic codes from multiple hospitalizations (and from multiple hospitals) could be used to calculate the Charlson Comorbidity Index for a particular patient; this has been shown to increase the capture of comorbidities.16 Third, we found that variables from the chart review indicating frailty were predictive of 1-year death risk independent of the chart-HOMR score (Table 2). This illustrates that mortality risk prediction can be improved for particular patient groups by adding new covariates to the HOMR. Further work is required to determine how to incorporate these (and possibly other) covariates into the HOMR to create a unique chart-HOMR score. Finally, we found that a geriatrics assessment in the ED was associated with a significant (and notable) decrease in death risk. With these data, we are unable to indicate whether this association is causative. However, these findings indicate that the influence of emergency geriatric assessments on patient survival needs to be explored in more detail.
Several issues about our study should be considered when interpreting its results. First, this was a single-center study and the generalizability of our results to other centers is unknown. However, our study had the largest sample size of all primary data prognostic index validation studies1 ensuring that our results are, at the very least, internally reliable. In addition, our simple random sample ensured that we studied a broad assortment of patients to be certain that our results are representative of our institution. Second, we used a single abstractor for the study, which could limit the generalizability of our results. However, almost all the data points that were abstracted for our study were very objective.
In summary, our study shows that the HOMR score can be used to accurately predict 1-year death risk using data abstracted from the patient record. These findings will aid in individual patient prognostication for clinicians and researchers.
Disclosure
The authors report no financial conflicts of interest.
A patient’s prognosis can strongly influence their medical care. Decisions about diagnostic modalities, treatment options, and the use of preventive therapies can all be affected by the likelihood of a patient’s death in the near future. For example, patients with severely limited survival might forego prophylactic therapy, avoid interventions for asymptomatic issues, and cease screening interventions. Knowing survival probability would also be very helpful as a controlling variable in research analyses whenever death risk might be a possible confounder.
Sixteen indices that aim to predict patient death risk have been described by Yourman et al.1 They were all created from secondary analyses of clinical and administrative datasets, were applicable to patients in a variety of settings (including the community, nursing home, or hospital), and predicted survival probabilities in time horizons ranging from 6 months to 5 years. Prognostic factors that were most commonly included in these indices were comorbidity and functional status. In validation populations, the discrimination of these indices for 1-year survival in hospitalized patients was moderate (with C statistics that ranged from 0.64 to 0.79) with good calibration for broad prognostic ranges.
In 2014, we published the Hospitalized-patient One-year Mortality Risk (HOMR) score.2 This study used health administrative data for all adult Ontarians admitted in 2011 to hospital under nonpsychiatric services (n = 640,022) to estimate the probability of dying within 1 year of admission to hospital (which happened in 11.7% of people). The HOMR score included 12 patient and hospitalization factors (Table 1). It was highly discriminative (C statistic, 0.923; [0.922-0.924]) and well calibrated (the mean relative difference between observed and expected death risk was 2.0% [range, 0.0% to 7.0%]). It was externally validated in more than 3 million adults from Ontario, Alberta, and Boston in whom the C statistic ranged from 0.89 to 0.92 and calibration was excellent.3 We concluded from these studies that the HOMR score is excellent for prognosticating a diverse group of patients using health administrative data.
However, we do not know whether the HOMR score can be applied to patients using primary data (ie, those taken directly from the chart). This question is important for 2 reasons. First, if HOMR accurately predicts death risk using data abstracted from the medical record, it could be used in the clinical setting to assist in clinical decision-making. Second, HOMR uses multiple administrative datasets that are difficult to access and use by most clinical researchers; it is, therefore, important to determine if HOMR is accurate for clinical research based on primary medical record review. The primary objective of this study was to determine the accuracy of the HOMR score when calculated using data abstracted from clinical notes that were available when patients were admitted to hospital. Secondary objectives included determining whether functional measures abstracted were significantly associated with death risk beyond the HOMR score and whether HOMR scores calculated from chart review deviated from those calculated from administrative data.
METHODS
Study Cohort
The study, which was approved by our local research ethics board, took place at the Ottawa Hospital, a 1000-bed teaching hospital that is the primary referral center in our region. We used the hospital admission registry to identify all people 18 years or older who were admitted to a nonpsychiatric service at our hospital between January 1, 2011 and December 31, 2011 (this time frame corresponds with the year used to derive the HOMR score). We excluded overnight patients in the same-day surgery or the bone-marrow transplant units (since they would not have been included in the original study) and those without a valid health card number (which was required to link to provincial data to identify outcomes). From this list, we randomly selected 5000 patients.
Primary Data Collection
For each patient, we retrieved all data required to calculate the HOMR score from the medical record (Table 1). Patient registration information in our electronic medical record was used to identify patient age, sex, admitting service, number of emergency department (ED) visits in the previous year, number of admissions in the previous year (the nursing triage note was reviewed for each admission to determine if it was by ambulance), and whether or not the patient had been discharged from hospital in the previous 30 days. The admitting service consult note was used to determine the admitting diagnosis and whether or not the patient was admitted directly to the intensive care unit. If they were present, the emergency nursing triage note, the ED record of treatment, the admission consult note, the pre-operative consult note, and consult notes were all used to determine the patient’s comorbidities, living status, and home oxygen status. Admission urgency was determined using information from the patient registration information and the ED nursing triage note. All data were abstracted from information that had been registered prior to when the patient was physically transferred to their hospital bed. This ensured that we used only data available at the start of the admission.
Patient functional status has been shown to be strongly associated with survival4 but HOMR only indirectly captures functional information (through the patient’s living status). We, therefore, collected more detailed functional information from the medical record by determining if the patient was dependent for any activities of daily living (ADL) from the emergency nursing triage note, the ED record of treatment, the admission consult note, and the pre-operative consultation. We also collected information that might indicate frailty, which we defined per Clegg et al.5 as “a state of increased vulnerability to poor resolution of homeostasis following a stress.” This information included: delirium or more than 1 fall recorded on the emergency nursing triage note, the ED record of treatment, or the admission consultation note; or whether a geriatric nursing specialist assessment occurred in the ED in the previous 6 months. Finally, we recorded possible indicators of limited social support (no fixed address [from patient registration and nursing triage note], primary contact is not a family member [from the emergency notes, consult, and patient registration], and no religion noted in system [from patient registration]). Patients for whom religion status was missing were classified as having “no religion.”
Analysis
These data were encrypted and linked anonymously to population-based databases to determine whether patients died within 1 year of admission to hospital. We calculated the chart-HOMR score using information from the chart review and determined its association with the outcome using bivariate logistic regression. We compared observed and expected risk of death within 1 year of admission to hospital for each chart-HOMR score value, with expected risks determined from the external validation study.3 We regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups to ensure adequate numbers in each group); and we gauged overall deviations from expected risk and the relationship between the observed and expected death risk (based on the chart-HOMR score) using the line’s intercept and slope, respectively.6 Next, we replicated methods from our studies2,3 to calculate the administrative-HOMR score in our study cohort using administrative databases. We compared these chart-HOMR and administrative-HOMR scores (and scores for each of its components). Finally, we determined which of the socio-functional factors were associated with 1-year death risk independent of the chart-HOMR score. We used the likelihood ratio test to determine whether these additional socio-functional factors significantly improved the model beyond the chart-HOMR score.7 This test subtracted the -2 logL value of the full model from that containing the chart-HOMR score alone, comparing its value to the χ2 distribution (with degrees of freedom equivalent to the number of additional parameters in the nested model) to determine statistical significance. All analyses were completed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
There were 43,883 overnight hospitalizations at our hospital in 2011, and 38,886 hospitalizations were excluded: 1883 hospitalizations were in the same-day surgery or the bone-marrow transplant unit; 2485 did not have a valid health card number; 34,515 were not randomly selected; the records of 3 randomly selected patients had been blocked by our hospital’s privacy department; and 1 patient could not be linked with the population-based administrative datasets.
The 4996 study patients were middle-aged and predominantly female (Table 2). The extensive majority of patients was admitted from the community, was independent for ADL, had a family member as the principal contact, and had no admissions by ambulance in the previous year. Most people had no significant comorbidities or ED visits in the year prior to their admission. The mean chart-HOMR score was 22 (standard deviation [SD], 12), which is associated with a 1.2% expected risk of death within 1 year of hospital admission (Appendix 1).3
A total of 563 patients (11.3%) died within 1 year of admission to hospital (Table 2). In the study cohort, each chart-HOMR component was associated with death status. People who died were older, more likely to be male, had a greater number of important comorbidities, had more ED visits and admissions by ambulance in the previous year, and were more likely to have been discharged in the previous 30 days, and were admitted urgently, directly to the intensive care unit, or with complicated diagnoses. The mean chart-HOMR score differed extensively by survival status (37.4 [SD, 7.5] in those who died vs. 19.9 [SD, 12.2] in those who survived). Three of the socio-functional variables (delirium and falls noted on admission documents, and dependent for any ADL) also varied with death status.
The chart-HOMR score was strongly associated with the likelihood of death within 1 year of admission. When included in a logistic regression model having 1-year death as the outcome, a 1-point increase in the chart-HOMR score was associated with a 19% increase in the odds of death (P < 0.0001). This model (with only the chart-HOMR score) was highly discriminative (C statistic, 0.888) and well calibrated (Hosmer-Lemeshow test, 12.9 [8 df, P = 0.11]).
Observed and expected death risks by chart-HOMR score were similar (Figure 1). The observed total number of deaths (n = 563; 11.3%) exceeded the expected number of deaths (n = 437, 8.7%). When we regressed observed death risks on expected death risks for chart-HOMR scores (clustered into 22 groups), the Hosmer-Lemeshow test was significant, indicating that differences between observed and expected risks were beyond that expected by chance (Hosmer-Lemeshow test, 141.9, 21 df, P < 0.0001). The intercept of this model (0.035; 95% CI, 0.01-0.06) was statistically significant (P = 0.01), indicating that the observed number of cases significantly exceeded the expected; however, its calibration slope (1.02; 95% CI, 0.89-1.16) did not deviate significantly from unity, indicating that the relationship between the observed and expected death risk (based on the chart-HOMR score) remained intact (Figure 1).
The deviations between observed and expected death risks reflected deviations between the c chart-HOMR score and the administrative-HOMR score, with the former being significantly lower than the latter (Figure 2). Overall, the chart-HOMR score was 0.96 points lower (95% CI, 0.81-1.12) than the administrative-HOMR score. The HOMR score components that were notably underestimated using chart data included those for the age-Charlson Comorbidity Index interaction, living status, and admit points. Points for only 2 components (admitting service and admission urgency) were higher when calculated using chart data.
Four additional socio-functional variables collected from medical record review were significantly associated with 1-year death risk independent of the chart-HOMR score (Table 3). Admission documentation noting either delirium or falls were both associated with a significantly increased death risk (adjusted odds ratio [OR], 1.92 [95% CI, 1.24-2.96] and OR 1.96 [95% CI, 1.29-2.99], respectively). An independently increased death risk was also noted in patients who were dependent for any ADL (adjusted OR, 1.99 [95% CI, 1.24-3.19]). The presence of an ED geriatrics consultation within the previous 6 months was associated with a significantly decreased death risk of 60% (adjusted OR, 0.40 [95% CI, 0.20-0.81]). Adding these covariates to the logistic model with the chart-HOMR score significantly improved predictions (likelihood ratio statistic = 33.569, 4df, P < 0.00001).
DISCUSSION
In a large random sample of patients from our hospital, we found that the HOMR score using data abstracted from the medical record was significantly associated with 1-year death risk. The expected death risk based on the chart-HOMR score underestimated observed death risk but the relationship between the chart-HOMR score and death risk was similar to that in studies using administrative data. The HOMR score calculated using data from the chart was lower than that calculated using data from population-based administrative datasets; additional variables indicating patient frailty were significantly associated with 1-year death risk independent of the chart-HOMR score. Since the HOMR score was derived and initially validated using health administrative data, this study using data abstracted from the health record shows that the HOMR score has methodological generalizability.8
We think that our study has several notable findings. First, we found that data abstracted from the medical record can be used to calculate the HOMR score to accurately predict individual death risk. The chart-HOMR score discriminated very well between patients who did and did not die (C statistic, 0.88), which extensively exceeds the discrimination of published death risk indices (whose C statistics range between 0.69 and 0.82). It is also possible that chart abstraction for the HOMR score—without functional status—is simpler than other indices since its components are primarily very objective. (Other indices for hospital-based patients required factors that could be difficult to abstract reliably from the medical record including meeting more than 1 guideline for noncancer hospice care9; ambulation difficulties10; scales such as the Exton-Smith Scale or the Short Portable Mental Status Questionnaire11; weight loss12; functional status4; and pressure sore risk.13) Although expected risks for the chart-HOMR consistently underestimated observed risks (Figure 1), the mean deviation was small (with an absolute difference of 3.5% that can be used as a correction factor when determining expected risks with HOMR scores calculated from chart review), but it was an association between the chart-HOMR score and death risk that remained consistent through the cohort. Second, we found a small but significant decrease in the chart-HOMR score vs. the administrative-HOMR score (Figure 2). Some of these underestimates such as those for the number of ED visits or admissions by ambulance were expected since population-based health administrative databases would best capture such data. However, we were surprised that the comorbidity score was less when calculated using chart vs. database data (Figure 2). This finding is distinct from studies finding that particular comorbidities are documented in the chart are sometimes not coded.14,15 However, we identified comorbidities in the administrative databases using a 1-year ‘look-back’ period so that diagnostic codes from multiple hospitalizations (and from multiple hospitals) could be used to calculate the Charlson Comorbidity Index for a particular patient; this has been shown to increase the capture of comorbidities.16 Third, we found that variables from the chart review indicating frailty were predictive of 1-year death risk independent of the chart-HOMR score (Table 2). This illustrates that mortality risk prediction can be improved for particular patient groups by adding new covariates to the HOMR. Further work is required to determine how to incorporate these (and possibly other) covariates into the HOMR to create a unique chart-HOMR score. Finally, we found that a geriatrics assessment in the ED was associated with a significant (and notable) decrease in death risk. With these data, we are unable to indicate whether this association is causative. However, these findings indicate that the influence of emergency geriatric assessments on patient survival needs to be explored in more detail.
Several issues about our study should be considered when interpreting its results. First, this was a single-center study and the generalizability of our results to other centers is unknown. However, our study had the largest sample size of all primary data prognostic index validation studies1 ensuring that our results are, at the very least, internally reliable. In addition, our simple random sample ensured that we studied a broad assortment of patients to be certain that our results are representative of our institution. Second, we used a single abstractor for the study, which could limit the generalizability of our results. However, almost all the data points that were abstracted for our study were very objective.
In summary, our study shows that the HOMR score can be used to accurately predict 1-year death risk using data abstracted from the patient record. These findings will aid in individual patient prognostication for clinicians and researchers.
Disclosure
The authors report no financial conflicts of interest.
1. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. PubMed
2. van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicts long term death risk in hospitalized patients. J Clin Epidemiol. 2014;67(9):1025-1034. PubMed
3. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ. 2015;187(10):725-733. PubMed
4. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
5. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. The Lancet 2002;381:752-762. PubMed
6. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692-1706. PubMed
7. Harrell FE Jr. Overview of Maximum Likelihood Estimation. Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001: 179-212.
8. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515-524. PubMed
9. Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285-292. PubMed
10. Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83. PubMed
11. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151-161. PubMed
12. Teno JM, Harrell FE Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 suppl):S16-S24. PubMed
13. Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783-791. PubMed
14. Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52(2):137-142. PubMed
15. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. PubMed
16. Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson cComorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128-1139. PubMed
1. Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182-192. PubMed
2. van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicts long term death risk in hospitalized patients. J Clin Epidemiol. 2014;67(9):1025-1034. PubMed
3. van Walraven C, McAlister FA, Bakal JA, Hawken S, Donzé J. External validation of the Hospital-patient One-year Mortality Risk (HOMR) model for predicting death within 1 year after hospital admission. CMAJ. 2015;187(10):725-733. PubMed
4. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987-2994. PubMed
5. Clegg A, Young J, Iliffe S et al. Frailty in elderly people. The Lancet 2002;381:752-762. PubMed
6. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692-1706. PubMed
7. Harrell FE Jr. Overview of Maximum Likelihood Estimation. Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001: 179-212.
8. Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515-524. PubMed
9. Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285-292. PubMed
10. Inouye SK, Bogardus ST, Jr, Vitagliano G, et al. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70-83. PubMed
11. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151-161. PubMed
12. Teno JM, Harrell FE Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 suppl):S16-S24. PubMed
13. Dramé M, Novella JL, Lang PO, et al. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783-791. PubMed
14. Kieszak SM, Flanders WD, Kosinski AS, Shipp CC, Karp H. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52(2):137-142. PubMed
15. Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42(8):801-809. PubMed
16. Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson cComorbidity adjustment in Medicare claims. Med Care. 1999;37(11):1128-1139. PubMed
© 2017 Society of Hospital Medicine