User login
Team devises new way to manufacture RBCs
Researchers say they have generated the first human immortalized adult erythroid line that provides a sustainable supply of erythroid cells.
The team says this cell line, known as Bristol Erythroid Line Adult (BEL-A), is the first erythroid line to fully recapitulate normal erythropoiesis.
It produced mature reticulocytes that proved functionally and molecularly similar to adult reticulocytes cultured in vitro.
In addition, in vivo survival rates of BEL-A reticulocytes were similar to the survival rates of red blood cells (RBCs) from adult donors.
The researchers described their generation and testing of the BEL-A line in Nature Communications.
“Previous approaches to producing red blood cells have relied on various sources of stem cells, which can only presently produce very limited quantities,” said study author Jan Frayne, PhD, of the University of Bristol in the UK.
“By taking an alternative approach, we have generated the first human immortalized adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.”
Dr Frayne and her colleagues began with CD34+ cells from adult bone marrow. The researchers transduced these cells with an HPV16-E6/E7 construct and maintained them in the primary medium of the team’s erythroid culture system for 4 days.
On day 5, the researchers transferred the cells to an expansion medium containing doxycycline. After 190 days of continuous proliferation, the cells were frozen for storage.
Some cells were frozen throughout this time period as well, and all of the samples re-established efficiently in culture after thawing.
The researchers said these immortalized BEL-A cells were pro- to early basophilic erythroblasts, and there was no change in morphology over time.
After 100 days in continuous culture, the researchers transferred BEL-A cells to the primary erythroid culture medium containing doxycycline. The cells remained there for 6 days and were then moved to a medium without doxycycline to induce differentiation to mature erythroblasts and reticulocytes.
The researchers said the BEL-A cells exhibited an “unchanged ability to differentiate,” and were consistently able to expand.
The team also noted that BEL-A reticulocytes were similar to normal adult reticulocytes. The cells had similar diameters, comparable deformability indexes, and they bound and released oxygen in the same way.
The researchers tested the in vivo survival of BEL-A reticulocytes as well. They transfused BEL-A reticulocytes and donor RBCs into macrophage-depleted NSG mice. There was no difference in survival between the 2 cell types.
Dr Frayne and her colleagues said their results suggest immortalized erythroid lines can be used for the manufacture of RBCs for clinical use and for the study of erythropoiesis. ![]()
Researchers say they have generated the first human immortalized adult erythroid line that provides a sustainable supply of erythroid cells.
The team says this cell line, known as Bristol Erythroid Line Adult (BEL-A), is the first erythroid line to fully recapitulate normal erythropoiesis.
It produced mature reticulocytes that proved functionally and molecularly similar to adult reticulocytes cultured in vitro.
In addition, in vivo survival rates of BEL-A reticulocytes were similar to the survival rates of red blood cells (RBCs) from adult donors.
The researchers described their generation and testing of the BEL-A line in Nature Communications.
“Previous approaches to producing red blood cells have relied on various sources of stem cells, which can only presently produce very limited quantities,” said study author Jan Frayne, PhD, of the University of Bristol in the UK.
“By taking an alternative approach, we have generated the first human immortalized adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.”
Dr Frayne and her colleagues began with CD34+ cells from adult bone marrow. The researchers transduced these cells with an HPV16-E6/E7 construct and maintained them in the primary medium of the team’s erythroid culture system for 4 days.
On day 5, the researchers transferred the cells to an expansion medium containing doxycycline. After 190 days of continuous proliferation, the cells were frozen for storage.
Some cells were frozen throughout this time period as well, and all of the samples re-established efficiently in culture after thawing.
The researchers said these immortalized BEL-A cells were pro- to early basophilic erythroblasts, and there was no change in morphology over time.
After 100 days in continuous culture, the researchers transferred BEL-A cells to the primary erythroid culture medium containing doxycycline. The cells remained there for 6 days and were then moved to a medium without doxycycline to induce differentiation to mature erythroblasts and reticulocytes.
The researchers said the BEL-A cells exhibited an “unchanged ability to differentiate,” and were consistently able to expand.
The team also noted that BEL-A reticulocytes were similar to normal adult reticulocytes. The cells had similar diameters, comparable deformability indexes, and they bound and released oxygen in the same way.
The researchers tested the in vivo survival of BEL-A reticulocytes as well. They transfused BEL-A reticulocytes and donor RBCs into macrophage-depleted NSG mice. There was no difference in survival between the 2 cell types.
Dr Frayne and her colleagues said their results suggest immortalized erythroid lines can be used for the manufacture of RBCs for clinical use and for the study of erythropoiesis. ![]()
Researchers say they have generated the first human immortalized adult erythroid line that provides a sustainable supply of erythroid cells.
The team says this cell line, known as Bristol Erythroid Line Adult (BEL-A), is the first erythroid line to fully recapitulate normal erythropoiesis.
It produced mature reticulocytes that proved functionally and molecularly similar to adult reticulocytes cultured in vitro.
In addition, in vivo survival rates of BEL-A reticulocytes were similar to the survival rates of red blood cells (RBCs) from adult donors.
The researchers described their generation and testing of the BEL-A line in Nature Communications.
“Previous approaches to producing red blood cells have relied on various sources of stem cells, which can only presently produce very limited quantities,” said study author Jan Frayne, PhD, of the University of Bristol in the UK.
“By taking an alternative approach, we have generated the first human immortalized adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.”
Dr Frayne and her colleagues began with CD34+ cells from adult bone marrow. The researchers transduced these cells with an HPV16-E6/E7 construct and maintained them in the primary medium of the team’s erythroid culture system for 4 days.
On day 5, the researchers transferred the cells to an expansion medium containing doxycycline. After 190 days of continuous proliferation, the cells were frozen for storage.
Some cells were frozen throughout this time period as well, and all of the samples re-established efficiently in culture after thawing.
The researchers said these immortalized BEL-A cells were pro- to early basophilic erythroblasts, and there was no change in morphology over time.
After 100 days in continuous culture, the researchers transferred BEL-A cells to the primary erythroid culture medium containing doxycycline. The cells remained there for 6 days and were then moved to a medium without doxycycline to induce differentiation to mature erythroblasts and reticulocytes.
The researchers said the BEL-A cells exhibited an “unchanged ability to differentiate,” and were consistently able to expand.
The team also noted that BEL-A reticulocytes were similar to normal adult reticulocytes. The cells had similar diameters, comparable deformability indexes, and they bound and released oxygen in the same way.
The researchers tested the in vivo survival of BEL-A reticulocytes as well. They transfused BEL-A reticulocytes and donor RBCs into macrophage-depleted NSG mice. There was no difference in survival between the 2 cell types.
Dr Frayne and her colleagues said their results suggest immortalized erythroid lines can be used for the manufacture of RBCs for clinical use and for the study of erythropoiesis. ![]()
Protein may prevent transformation from MDS to AML
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
Model could advance fight against malaria
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
PARP inhibitor approved as maintenance for recurrent ovarian cancer
The Food and Drug Administration has approved niraparib, a poly ADP-ribose polymerase (PARP) inhibitor, for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy.
The indication does not require a suspected or confirmed deleterious BRCA mutation.
Median PFS in women with germline BRCA mutations who received niraparib was 21 months, compared with 5.5 months for those on placebo (hazard ratio, 0.26; 95% confidence interval: 0.17-0.41; P less than .0001), according to a study presented at the 2016 European Society for Medical Oncology Congress and published simultaneously in the New England Journal of Medicine.
Among patients who did not have a germline BRCA mutation, median PFS for patients on niraparib was 9.3 months, compared with 3.9 months for those on placebo (HR, 0.45; 95% CI, 0.34-0.61; P less than .0001).
Patients enrolled in NOVA were randomized (2:1) within 8 weeks of the last therapy to either niraparib (300 mg orally daily) or matched placebo. Patients were assigned to one of two cohorts based on the BRACAnalysis CDx. Patients with deleterious or suspected deleterious germline BRCA mutations were assigned to the germline BRCA-mutated cohort (n = 203), and those without germline BRCA mutations were assigned to the nonmutated cohort (n = 350).
Niraparib’s safety was evaluated in 367 patients with platinum-sensitive recurrent ovarian, fallopian tube, and primary peritoneal cancer in the NOVA trial, according to a statement from the FDA.
The most common adverse reactions were thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, constipation, vomiting, abdominal pain/distention, mucositis/stomatitis, diarrhea, dyspepsia, dry mouth, fatigue, decreased appetite, urinary tract infection, AST/ALT elevation, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, and hypertension.
Myelodysplastic syndrome and/or acute myeloid leukemia occurred in 5 of 367 (1.4%) patients receiving niraparib and in 2 of 179 (1.1%) patients assigned to placebo. Grade 3-4 hypertension occurred in 9% of niraparib-treated patients, compared with 2% of patients assigned to placebo, according to the FDA.
Niraparib is the third PARP inhibitor to receive approval for ovarian cancer, following approval of olaparib (Lynparza) for patients with advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy and whose tumors have a deleterious BRCA mutation as detected by an FDA-approved test and accelerated approval of rucaparib (Rubraca) for women with advanced ovarian cancer who have been treated with two or more chemotherapies and whose tumors have a deleterious BRCA mutation as identified by an FDA-approved companion diagnostic test.
The recommended dose and schedule of niraparib is 300 mg taken once daily with or without food.
Full prescribing information is available here.
Niraparib is being marketed as Zejula by Tesaro. Following FDA announcement of the approval, Tesaro announced an expanded development program for niraparib focused on the treatment of front-line metastatic ovarian and lung cancers and metastatic breast cancer.
[email protected]
On Twitter @NikolaidesLaura
The Food and Drug Administration has approved niraparib, a poly ADP-ribose polymerase (PARP) inhibitor, for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy.
The indication does not require a suspected or confirmed deleterious BRCA mutation.
Median PFS in women with germline BRCA mutations who received niraparib was 21 months, compared with 5.5 months for those on placebo (hazard ratio, 0.26; 95% confidence interval: 0.17-0.41; P less than .0001), according to a study presented at the 2016 European Society for Medical Oncology Congress and published simultaneously in the New England Journal of Medicine.
Among patients who did not have a germline BRCA mutation, median PFS for patients on niraparib was 9.3 months, compared with 3.9 months for those on placebo (HR, 0.45; 95% CI, 0.34-0.61; P less than .0001).
Patients enrolled in NOVA were randomized (2:1) within 8 weeks of the last therapy to either niraparib (300 mg orally daily) or matched placebo. Patients were assigned to one of two cohorts based on the BRACAnalysis CDx. Patients with deleterious or suspected deleterious germline BRCA mutations were assigned to the germline BRCA-mutated cohort (n = 203), and those without germline BRCA mutations were assigned to the nonmutated cohort (n = 350).
Niraparib’s safety was evaluated in 367 patients with platinum-sensitive recurrent ovarian, fallopian tube, and primary peritoneal cancer in the NOVA trial, according to a statement from the FDA.
The most common adverse reactions were thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, constipation, vomiting, abdominal pain/distention, mucositis/stomatitis, diarrhea, dyspepsia, dry mouth, fatigue, decreased appetite, urinary tract infection, AST/ALT elevation, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, and hypertension.
Myelodysplastic syndrome and/or acute myeloid leukemia occurred in 5 of 367 (1.4%) patients receiving niraparib and in 2 of 179 (1.1%) patients assigned to placebo. Grade 3-4 hypertension occurred in 9% of niraparib-treated patients, compared with 2% of patients assigned to placebo, according to the FDA.
Niraparib is the third PARP inhibitor to receive approval for ovarian cancer, following approval of olaparib (Lynparza) for patients with advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy and whose tumors have a deleterious BRCA mutation as detected by an FDA-approved test and accelerated approval of rucaparib (Rubraca) for women with advanced ovarian cancer who have been treated with two or more chemotherapies and whose tumors have a deleterious BRCA mutation as identified by an FDA-approved companion diagnostic test.
The recommended dose and schedule of niraparib is 300 mg taken once daily with or without food.
Full prescribing information is available here.
Niraparib is being marketed as Zejula by Tesaro. Following FDA announcement of the approval, Tesaro announced an expanded development program for niraparib focused on the treatment of front-line metastatic ovarian and lung cancers and metastatic breast cancer.
[email protected]
On Twitter @NikolaidesLaura
The Food and Drug Administration has approved niraparib, a poly ADP-ribose polymerase (PARP) inhibitor, for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy.
The indication does not require a suspected or confirmed deleterious BRCA mutation.
Median PFS in women with germline BRCA mutations who received niraparib was 21 months, compared with 5.5 months for those on placebo (hazard ratio, 0.26; 95% confidence interval: 0.17-0.41; P less than .0001), according to a study presented at the 2016 European Society for Medical Oncology Congress and published simultaneously in the New England Journal of Medicine.
Among patients who did not have a germline BRCA mutation, median PFS for patients on niraparib was 9.3 months, compared with 3.9 months for those on placebo (HR, 0.45; 95% CI, 0.34-0.61; P less than .0001).
Patients enrolled in NOVA were randomized (2:1) within 8 weeks of the last therapy to either niraparib (300 mg orally daily) or matched placebo. Patients were assigned to one of two cohorts based on the BRACAnalysis CDx. Patients with deleterious or suspected deleterious germline BRCA mutations were assigned to the germline BRCA-mutated cohort (n = 203), and those without germline BRCA mutations were assigned to the nonmutated cohort (n = 350).
Niraparib’s safety was evaluated in 367 patients with platinum-sensitive recurrent ovarian, fallopian tube, and primary peritoneal cancer in the NOVA trial, according to a statement from the FDA.
The most common adverse reactions were thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, constipation, vomiting, abdominal pain/distention, mucositis/stomatitis, diarrhea, dyspepsia, dry mouth, fatigue, decreased appetite, urinary tract infection, AST/ALT elevation, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, and hypertension.
Myelodysplastic syndrome and/or acute myeloid leukemia occurred in 5 of 367 (1.4%) patients receiving niraparib and in 2 of 179 (1.1%) patients assigned to placebo. Grade 3-4 hypertension occurred in 9% of niraparib-treated patients, compared with 2% of patients assigned to placebo, according to the FDA.
Niraparib is the third PARP inhibitor to receive approval for ovarian cancer, following approval of olaparib (Lynparza) for patients with advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy and whose tumors have a deleterious BRCA mutation as detected by an FDA-approved test and accelerated approval of rucaparib (Rubraca) for women with advanced ovarian cancer who have been treated with two or more chemotherapies and whose tumors have a deleterious BRCA mutation as identified by an FDA-approved companion diagnostic test.
The recommended dose and schedule of niraparib is 300 mg taken once daily with or without food.
Full prescribing information is available here.
Niraparib is being marketed as Zejula by Tesaro. Following FDA announcement of the approval, Tesaro announced an expanded development program for niraparib focused on the treatment of front-line metastatic ovarian and lung cancers and metastatic breast cancer.
[email protected]
On Twitter @NikolaidesLaura
Testosterone Affects Outcomes in Men With MS
ORLANDO—Although multiple sclerosis (MS) is more common in women, when men develop MS, it tends to be worse. Studies indicate that MS in men is associated with a faster accrual of disability and worse relapse recovery. In addition, research suggests that male gender is a predictor for more severe forms of MS. Also, there appears to be a higher male-to-female ratio in primary progressive MS (PPMS), compared with relapsing forms of MS.
Role of Sex Chromosomes and Hormones
Studies suggest that women are predisposed to higher rates of MS relapses than men. Kalincik et al found that there was approximately a 1:1 male-to-female ratio in PPMS onset, compared with a 1:3 male-to-female ratio in relapsing-onset MS. “One of the interesting paradigms they looked at was the proportion of relapses or the relapse count over the course of 10 years and the female-to-male ratio and how it has increased to become predominately female with a higher relapse count. This certainly suggests that hormones and sex may be driving a relapsing phenotype,” said Dr. Chitnis.
Raghavan et al found that men appeared to have higher Expanded Disability Status Scale scores over disease duration in relapsing forms of MS compared with PPMS. In addition, when researchers observed optic neuritis as a model of relapse recovery, they found that male gender was associated with worse recovery from optic neuritis despite adjusting for disease severity as well as vitamin D levels.
Is Testosterone Neuroprotective?
Previous studies in MS suggest that low testosterone is associated with worse disability. Studies have also associated low testosterone levels with more cognitive decline in MS. Following up on these clinical observations, Dr. Chitnis and colleagues conducted the CLIMB study, a long-term study of how MS changes over time, to observe testosterone levels in men with MS. Data from the study, which involved nearly 100 men with MS, revealed that there were significantly reduced testosterone levels in a majority of the males.
Although lower levels of testosterone may be a risk factor for rapid disability accrual, testosterone supplementation appears to be neuroprotective. “There is a vast amount of literature now demonstrating in animal models particularly that testosterone does appear to be neuroprotective and anti-inflammatory,” said Dr. Chitnis.
According to Ziehn et al, testosterone seemed to be protective against synaptic preservation in animal models. In addition, research has elucidated that testosterone is able to cross the blood-brain barrier in its free form and directly influence neuronal cells. Other studies suggest that testosterone protects spinal cord neurons from glutamate toxicity, and protects from oxidative stress in neuronal cell lines.
Sex-Stratified MS Risk, Prenatal Exposure, and Puberty
To understand whether testosterone levels predispose men to MS, researchers studied a measure called the second-digit-fourth-digit ratio (2D:4D) and found that it was associated with MS in men. Researchers have also discovered that there may be prenatal risk factors that may increase susceptibility in men. While studies suggest that breast-feeding is associated with a reduced risk of MS in boys, maternal illness and pesticide exposure in pregnancy and during the prenatal stage were associated with an increased risk for pediatric-onset MS.
Puberty also may be a turning point for the initiation of MS in boys, said Dr. Chitnis. “Younger males with MS show evidence of earlier puberty than regional controls. It is unclear if they have lower testosterone, however, research does suggest a disturbance in sex hormones early on,” she said.
Testosterone as an MS Therapy?
Several benefits have been associated with testosterone as a potential therapy, such as improved libido, increased muscle strength, improved bone density, and potentially improved memory and other cognitive measures. However, there are serious risks associated with testosterone therapy such as an increased prostate specific antigen, increased susceptibility to cancer, emotional lability, hypertension, increased hemoglobin, and increased cardiovascular events.
There may be benefits with regard to slowing disease progression and cognitive decline that warrant further study, said Dr. Chitnis. She added that future studies should focus on “mechanisms and interactions of sex hormones and testosterone in boys with MS, as well as safety and efficacy of testosterone in men with MS.”
—Erica Tricarico
Suggested Reading
Bove R, Malik MT, Diaz-Cruz C, et al. The 2D:4D ratio, a proxy for prenatal androgen levels, differs in men with and without MS. Neurology. 2015;85(14):1209-1213.
Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609-3617.
Raghavan K, Healy BC, Carruthers RL, Chitnis T. Progression rates and sample size estimates for PPMS based on the CLIMB study population. Mult Scler. 2015;21(2):180-188.
Ziehn MO, Avedisian AA, Dervin SM, et al. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci. 2012; 32(36):12312-12324.
ORLANDO—Although multiple sclerosis (MS) is more common in women, when men develop MS, it tends to be worse. Studies indicate that MS in men is associated with a faster accrual of disability and worse relapse recovery. In addition, research suggests that male gender is a predictor for more severe forms of MS. Also, there appears to be a higher male-to-female ratio in primary progressive MS (PPMS), compared with relapsing forms of MS.
Role of Sex Chromosomes and Hormones
Studies suggest that women are predisposed to higher rates of MS relapses than men. Kalincik et al found that there was approximately a 1:1 male-to-female ratio in PPMS onset, compared with a 1:3 male-to-female ratio in relapsing-onset MS. “One of the interesting paradigms they looked at was the proportion of relapses or the relapse count over the course of 10 years and the female-to-male ratio and how it has increased to become predominately female with a higher relapse count. This certainly suggests that hormones and sex may be driving a relapsing phenotype,” said Dr. Chitnis.
Raghavan et al found that men appeared to have higher Expanded Disability Status Scale scores over disease duration in relapsing forms of MS compared with PPMS. In addition, when researchers observed optic neuritis as a model of relapse recovery, they found that male gender was associated with worse recovery from optic neuritis despite adjusting for disease severity as well as vitamin D levels.
Is Testosterone Neuroprotective?
Previous studies in MS suggest that low testosterone is associated with worse disability. Studies have also associated low testosterone levels with more cognitive decline in MS. Following up on these clinical observations, Dr. Chitnis and colleagues conducted the CLIMB study, a long-term study of how MS changes over time, to observe testosterone levels in men with MS. Data from the study, which involved nearly 100 men with MS, revealed that there were significantly reduced testosterone levels in a majority of the males.
Although lower levels of testosterone may be a risk factor for rapid disability accrual, testosterone supplementation appears to be neuroprotective. “There is a vast amount of literature now demonstrating in animal models particularly that testosterone does appear to be neuroprotective and anti-inflammatory,” said Dr. Chitnis.
According to Ziehn et al, testosterone seemed to be protective against synaptic preservation in animal models. In addition, research has elucidated that testosterone is able to cross the blood-brain barrier in its free form and directly influence neuronal cells. Other studies suggest that testosterone protects spinal cord neurons from glutamate toxicity, and protects from oxidative stress in neuronal cell lines.
Sex-Stratified MS Risk, Prenatal Exposure, and Puberty
To understand whether testosterone levels predispose men to MS, researchers studied a measure called the second-digit-fourth-digit ratio (2D:4D) and found that it was associated with MS in men. Researchers have also discovered that there may be prenatal risk factors that may increase susceptibility in men. While studies suggest that breast-feeding is associated with a reduced risk of MS in boys, maternal illness and pesticide exposure in pregnancy and during the prenatal stage were associated with an increased risk for pediatric-onset MS.
Puberty also may be a turning point for the initiation of MS in boys, said Dr. Chitnis. “Younger males with MS show evidence of earlier puberty than regional controls. It is unclear if they have lower testosterone, however, research does suggest a disturbance in sex hormones early on,” she said.
Testosterone as an MS Therapy?
Several benefits have been associated with testosterone as a potential therapy, such as improved libido, increased muscle strength, improved bone density, and potentially improved memory and other cognitive measures. However, there are serious risks associated with testosterone therapy such as an increased prostate specific antigen, increased susceptibility to cancer, emotional lability, hypertension, increased hemoglobin, and increased cardiovascular events.
There may be benefits with regard to slowing disease progression and cognitive decline that warrant further study, said Dr. Chitnis. She added that future studies should focus on “mechanisms and interactions of sex hormones and testosterone in boys with MS, as well as safety and efficacy of testosterone in men with MS.”
—Erica Tricarico
Suggested Reading
Bove R, Malik MT, Diaz-Cruz C, et al. The 2D:4D ratio, a proxy for prenatal androgen levels, differs in men with and without MS. Neurology. 2015;85(14):1209-1213.
Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609-3617.
Raghavan K, Healy BC, Carruthers RL, Chitnis T. Progression rates and sample size estimates for PPMS based on the CLIMB study population. Mult Scler. 2015;21(2):180-188.
Ziehn MO, Avedisian AA, Dervin SM, et al. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci. 2012; 32(36):12312-12324.
ORLANDO—Although multiple sclerosis (MS) is more common in women, when men develop MS, it tends to be worse. Studies indicate that MS in men is associated with a faster accrual of disability and worse relapse recovery. In addition, research suggests that male gender is a predictor for more severe forms of MS. Also, there appears to be a higher male-to-female ratio in primary progressive MS (PPMS), compared with relapsing forms of MS.
Role of Sex Chromosomes and Hormones
Studies suggest that women are predisposed to higher rates of MS relapses than men. Kalincik et al found that there was approximately a 1:1 male-to-female ratio in PPMS onset, compared with a 1:3 male-to-female ratio in relapsing-onset MS. “One of the interesting paradigms they looked at was the proportion of relapses or the relapse count over the course of 10 years and the female-to-male ratio and how it has increased to become predominately female with a higher relapse count. This certainly suggests that hormones and sex may be driving a relapsing phenotype,” said Dr. Chitnis.
Raghavan et al found that men appeared to have higher Expanded Disability Status Scale scores over disease duration in relapsing forms of MS compared with PPMS. In addition, when researchers observed optic neuritis as a model of relapse recovery, they found that male gender was associated with worse recovery from optic neuritis despite adjusting for disease severity as well as vitamin D levels.
Is Testosterone Neuroprotective?
Previous studies in MS suggest that low testosterone is associated with worse disability. Studies have also associated low testosterone levels with more cognitive decline in MS. Following up on these clinical observations, Dr. Chitnis and colleagues conducted the CLIMB study, a long-term study of how MS changes over time, to observe testosterone levels in men with MS. Data from the study, which involved nearly 100 men with MS, revealed that there were significantly reduced testosterone levels in a majority of the males.
Although lower levels of testosterone may be a risk factor for rapid disability accrual, testosterone supplementation appears to be neuroprotective. “There is a vast amount of literature now demonstrating in animal models particularly that testosterone does appear to be neuroprotective and anti-inflammatory,” said Dr. Chitnis.
According to Ziehn et al, testosterone seemed to be protective against synaptic preservation in animal models. In addition, research has elucidated that testosterone is able to cross the blood-brain barrier in its free form and directly influence neuronal cells. Other studies suggest that testosterone protects spinal cord neurons from glutamate toxicity, and protects from oxidative stress in neuronal cell lines.
Sex-Stratified MS Risk, Prenatal Exposure, and Puberty
To understand whether testosterone levels predispose men to MS, researchers studied a measure called the second-digit-fourth-digit ratio (2D:4D) and found that it was associated with MS in men. Researchers have also discovered that there may be prenatal risk factors that may increase susceptibility in men. While studies suggest that breast-feeding is associated with a reduced risk of MS in boys, maternal illness and pesticide exposure in pregnancy and during the prenatal stage were associated with an increased risk for pediatric-onset MS.
Puberty also may be a turning point for the initiation of MS in boys, said Dr. Chitnis. “Younger males with MS show evidence of earlier puberty than regional controls. It is unclear if they have lower testosterone, however, research does suggest a disturbance in sex hormones early on,” she said.
Testosterone as an MS Therapy?
Several benefits have been associated with testosterone as a potential therapy, such as improved libido, increased muscle strength, improved bone density, and potentially improved memory and other cognitive measures. However, there are serious risks associated with testosterone therapy such as an increased prostate specific antigen, increased susceptibility to cancer, emotional lability, hypertension, increased hemoglobin, and increased cardiovascular events.
There may be benefits with regard to slowing disease progression and cognitive decline that warrant further study, said Dr. Chitnis. She added that future studies should focus on “mechanisms and interactions of sex hormones and testosterone in boys with MS, as well as safety and efficacy of testosterone in men with MS.”
—Erica Tricarico
Suggested Reading
Bove R, Malik MT, Diaz-Cruz C, et al. The 2D:4D ratio, a proxy for prenatal androgen levels, differs in men with and without MS. Neurology. 2015;85(14):1209-1213.
Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609-3617.
Raghavan K, Healy BC, Carruthers RL, Chitnis T. Progression rates and sample size estimates for PPMS based on the CLIMB study population. Mult Scler. 2015;21(2):180-188.
Ziehn MO, Avedisian AA, Dervin SM, et al. Therapeutic testosterone administration preserves excitatory synaptic transmission in the hippocampus during autoimmune demyelinating disease. J Neurosci. 2012; 32(36):12312-12324.
ACP: Substance use disorder is a chronic medical condition
Substance use disorder should be approached as a chronic medical condition, with treatment programs in place of incarceration and improved training programs with which physicians can treat patients with substance abuse.
Such programs are among the eight major recommendations the American College of Physicians has made to improve the nation’s approach to preventing and treating substance abuse in a position paper released March 27 (Ann Intern Med. 2017. doi: 10.7326/M16-2953).
The ACP’s recommendations address the growing rates of opioid addiction, as well as the financial burden of substance abuse on the medical system.
“In 2014, 22.5 million people in the United States needed treatment for an illicit drug or alcohol use problem, but only 18% received any treatment,” according to the paper’s authors. “The medical complications of untreated substance use disorder also drive up health care system costs. Hospitalizations for opioid use disorder rose from nearly 302,000 to more than 520,000 from 2002 to 2012, and costs for such care quadrupled to $15 billion in 2012.”
The complete list of the ACP’s recommendations are:
1) Substance abuse should be approached as a chronic medical disorder. Substance abuse can be treated through evidence-based health programs, according to the ACP. Effort should be put into developing research initiatives, as well as combating the social stigmas associated with substance use disorders.
2) The ACP encourages the establishment of substance abuse programs as a replacement for incarceration. Treatment for substance abuse is a time-sensitive matter, which should be given to patients as soon as possible, including those found guilty of the sale or possession of illegal substances.
3) Policy makers should consider reducing the punishments for drug-related crimes committed by nonviolent offenders. Officials should consider decriminalization, legalization, or treatment alternatives for crimes regarding certain drugs based on the potential risk associated with that drug, the accessibility of treatment in criminal facilities, any disproportionate affects on different sections of the population, and the potential decrease in rates of abuses.
4) There should be multiple stakeholders involved in the creation of programs to eliminate substance abuse. Physicians, policymakers, advocacy groups, and health care professionals are encouraged to work together to create strategies to combat and prevent substance abuse, including programs that expand naloxone access for opioid users or the establishment of a national prescription drug monitoring program. Extensive education programs on proper pain reduction methods should also be made available to physicians to help prevent future dependencies.
5) Coverage of substance use and mental disorder treatments should be mandatory for health insurance companies. Evidence-based treatments for mental health conditions and substance abuse, including counseling, medications, legal services, and education, should be covered by patients’ health insurance. The ACP asserts strict oversight would be essential; however, it is also essential that patients receive nonpharmacologic treatments, which are usually not covered by insurance.
6) There should be an increase in professionals trained to treat substance abuse. There are 4,500 health care professionals in the United States who have mental health and/or substance abuse training, according to the Health Resources and Services Administration. This number shows a high demand for those qualified to treat mental health conditions and substance abuse. Efforts should also be focused on creating a more ethnically diverse group among trained professionals to further increase access to these services.
7) Substance abuse treatment methods should be added to professionals’ continuing medical education. In 2000, 17% of primary care physicians felt very prepared to identify illegal drug use, and 30% could identify drug misuse, according to a study conducted by the National Center on Addiction and Substance Abuse. In response, education for physicians “should be rigorously evaluated to ensure effectiveness and continued access to care and should be designed to prevent onerous burdens on patients and physicians,” according to the paper’s authors.
8) Further study should be conducted on effectiveness of substance abuse programs. Current substance abuse intervention methods should be evaluated to see how effective they are. Among those, safe injection sites should especially be encouraged, as these initiatives have proven effective in reducing unsafe needle sharing in Canada, Australia, and Denmark.
The researchers had no relevant financial disclosures.
[email protected] On Twitter @EAZTweets
Substance use disorder should be approached as a chronic medical condition, with treatment programs in place of incarceration and improved training programs with which physicians can treat patients with substance abuse.
Such programs are among the eight major recommendations the American College of Physicians has made to improve the nation’s approach to preventing and treating substance abuse in a position paper released March 27 (Ann Intern Med. 2017. doi: 10.7326/M16-2953).
The ACP’s recommendations address the growing rates of opioid addiction, as well as the financial burden of substance abuse on the medical system.
“In 2014, 22.5 million people in the United States needed treatment for an illicit drug or alcohol use problem, but only 18% received any treatment,” according to the paper’s authors. “The medical complications of untreated substance use disorder also drive up health care system costs. Hospitalizations for opioid use disorder rose from nearly 302,000 to more than 520,000 from 2002 to 2012, and costs for such care quadrupled to $15 billion in 2012.”
The complete list of the ACP’s recommendations are:
1) Substance abuse should be approached as a chronic medical disorder. Substance abuse can be treated through evidence-based health programs, according to the ACP. Effort should be put into developing research initiatives, as well as combating the social stigmas associated with substance use disorders.
2) The ACP encourages the establishment of substance abuse programs as a replacement for incarceration. Treatment for substance abuse is a time-sensitive matter, which should be given to patients as soon as possible, including those found guilty of the sale or possession of illegal substances.
3) Policy makers should consider reducing the punishments for drug-related crimes committed by nonviolent offenders. Officials should consider decriminalization, legalization, or treatment alternatives for crimes regarding certain drugs based on the potential risk associated with that drug, the accessibility of treatment in criminal facilities, any disproportionate affects on different sections of the population, and the potential decrease in rates of abuses.
4) There should be multiple stakeholders involved in the creation of programs to eliminate substance abuse. Physicians, policymakers, advocacy groups, and health care professionals are encouraged to work together to create strategies to combat and prevent substance abuse, including programs that expand naloxone access for opioid users or the establishment of a national prescription drug monitoring program. Extensive education programs on proper pain reduction methods should also be made available to physicians to help prevent future dependencies.
5) Coverage of substance use and mental disorder treatments should be mandatory for health insurance companies. Evidence-based treatments for mental health conditions and substance abuse, including counseling, medications, legal services, and education, should be covered by patients’ health insurance. The ACP asserts strict oversight would be essential; however, it is also essential that patients receive nonpharmacologic treatments, which are usually not covered by insurance.
6) There should be an increase in professionals trained to treat substance abuse. There are 4,500 health care professionals in the United States who have mental health and/or substance abuse training, according to the Health Resources and Services Administration. This number shows a high demand for those qualified to treat mental health conditions and substance abuse. Efforts should also be focused on creating a more ethnically diverse group among trained professionals to further increase access to these services.
7) Substance abuse treatment methods should be added to professionals’ continuing medical education. In 2000, 17% of primary care physicians felt very prepared to identify illegal drug use, and 30% could identify drug misuse, according to a study conducted by the National Center on Addiction and Substance Abuse. In response, education for physicians “should be rigorously evaluated to ensure effectiveness and continued access to care and should be designed to prevent onerous burdens on patients and physicians,” according to the paper’s authors.
8) Further study should be conducted on effectiveness of substance abuse programs. Current substance abuse intervention methods should be evaluated to see how effective they are. Among those, safe injection sites should especially be encouraged, as these initiatives have proven effective in reducing unsafe needle sharing in Canada, Australia, and Denmark.
The researchers had no relevant financial disclosures.
[email protected] On Twitter @EAZTweets
Substance use disorder should be approached as a chronic medical condition, with treatment programs in place of incarceration and improved training programs with which physicians can treat patients with substance abuse.
Such programs are among the eight major recommendations the American College of Physicians has made to improve the nation’s approach to preventing and treating substance abuse in a position paper released March 27 (Ann Intern Med. 2017. doi: 10.7326/M16-2953).
The ACP’s recommendations address the growing rates of opioid addiction, as well as the financial burden of substance abuse on the medical system.
“In 2014, 22.5 million people in the United States needed treatment for an illicit drug or alcohol use problem, but only 18% received any treatment,” according to the paper’s authors. “The medical complications of untreated substance use disorder also drive up health care system costs. Hospitalizations for opioid use disorder rose from nearly 302,000 to more than 520,000 from 2002 to 2012, and costs for such care quadrupled to $15 billion in 2012.”
The complete list of the ACP’s recommendations are:
1) Substance abuse should be approached as a chronic medical disorder. Substance abuse can be treated through evidence-based health programs, according to the ACP. Effort should be put into developing research initiatives, as well as combating the social stigmas associated with substance use disorders.
2) The ACP encourages the establishment of substance abuse programs as a replacement for incarceration. Treatment for substance abuse is a time-sensitive matter, which should be given to patients as soon as possible, including those found guilty of the sale or possession of illegal substances.
3) Policy makers should consider reducing the punishments for drug-related crimes committed by nonviolent offenders. Officials should consider decriminalization, legalization, or treatment alternatives for crimes regarding certain drugs based on the potential risk associated with that drug, the accessibility of treatment in criminal facilities, any disproportionate affects on different sections of the population, and the potential decrease in rates of abuses.
4) There should be multiple stakeholders involved in the creation of programs to eliminate substance abuse. Physicians, policymakers, advocacy groups, and health care professionals are encouraged to work together to create strategies to combat and prevent substance abuse, including programs that expand naloxone access for opioid users or the establishment of a national prescription drug monitoring program. Extensive education programs on proper pain reduction methods should also be made available to physicians to help prevent future dependencies.
5) Coverage of substance use and mental disorder treatments should be mandatory for health insurance companies. Evidence-based treatments for mental health conditions and substance abuse, including counseling, medications, legal services, and education, should be covered by patients’ health insurance. The ACP asserts strict oversight would be essential; however, it is also essential that patients receive nonpharmacologic treatments, which are usually not covered by insurance.
6) There should be an increase in professionals trained to treat substance abuse. There are 4,500 health care professionals in the United States who have mental health and/or substance abuse training, according to the Health Resources and Services Administration. This number shows a high demand for those qualified to treat mental health conditions and substance abuse. Efforts should also be focused on creating a more ethnically diverse group among trained professionals to further increase access to these services.
7) Substance abuse treatment methods should be added to professionals’ continuing medical education. In 2000, 17% of primary care physicians felt very prepared to identify illegal drug use, and 30% could identify drug misuse, according to a study conducted by the National Center on Addiction and Substance Abuse. In response, education for physicians “should be rigorously evaluated to ensure effectiveness and continued access to care and should be designed to prevent onerous burdens on patients and physicians,” according to the paper’s authors.
8) Further study should be conducted on effectiveness of substance abuse programs. Current substance abuse intervention methods should be evaluated to see how effective they are. Among those, safe injection sites should especially be encouraged, as these initiatives have proven effective in reducing unsafe needle sharing in Canada, Australia, and Denmark.
The researchers had no relevant financial disclosures.
[email protected] On Twitter @EAZTweets
South dominates rankings of most obese U.S. cities
Jackson, Miss., is the most obese city in the United States for 2017, according to the personal finance website WalletHub.
The city topped the ranking of the 100 heaviest metro areas in the country with a score of 84.9 out of a possible 100 points based on 17 key metrics in three broad categories: obese and overweight people (50 points), weight-related health problems (30 points), and health environment (20 points), according to WalletHub.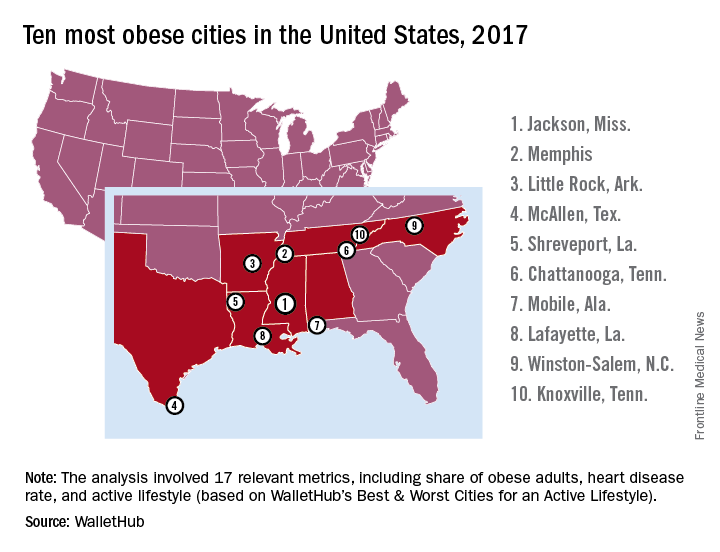
The second-most obese city for 2017 is Memphis, with Little Rock, Ark.; McAllen, Tex.; and Shreveport, La., occupying the rest of the top five. All of the cities in the top 10 – all of the cities in the top 20, actually – are located in the South, with the first non–Southern city (Indianapolis) making its appearance at number 21, the WalletHub analysis shows. At number 100 in the rankings is Seattle/Tacoma.
Data for the analysis came from the Centers for Disease Control and Prevention, County Health Rankings, the Department of Agriculture’s Economic Research Service, the Child and Adolescent Health Measurement Initiative, the Trust for America’s Health, and WalletHub’s own research, including its report Best & Worst Cities for an Active Lifestyle.
Jackson, Miss., is the most obese city in the United States for 2017, according to the personal finance website WalletHub.
The city topped the ranking of the 100 heaviest metro areas in the country with a score of 84.9 out of a possible 100 points based on 17 key metrics in three broad categories: obese and overweight people (50 points), weight-related health problems (30 points), and health environment (20 points), according to WalletHub.
The second-most obese city for 2017 is Memphis, with Little Rock, Ark.; McAllen, Tex.; and Shreveport, La., occupying the rest of the top five. All of the cities in the top 10 – all of the cities in the top 20, actually – are located in the South, with the first non–Southern city (Indianapolis) making its appearance at number 21, the WalletHub analysis shows. At number 100 in the rankings is Seattle/Tacoma.
Data for the analysis came from the Centers for Disease Control and Prevention, County Health Rankings, the Department of Agriculture’s Economic Research Service, the Child and Adolescent Health Measurement Initiative, the Trust for America’s Health, and WalletHub’s own research, including its report Best & Worst Cities for an Active Lifestyle.
Jackson, Miss., is the most obese city in the United States for 2017, according to the personal finance website WalletHub.
The city topped the ranking of the 100 heaviest metro areas in the country with a score of 84.9 out of a possible 100 points based on 17 key metrics in three broad categories: obese and overweight people (50 points), weight-related health problems (30 points), and health environment (20 points), according to WalletHub.
The second-most obese city for 2017 is Memphis, with Little Rock, Ark.; McAllen, Tex.; and Shreveport, La., occupying the rest of the top five. All of the cities in the top 10 – all of the cities in the top 20, actually – are located in the South, with the first non–Southern city (Indianapolis) making its appearance at number 21, the WalletHub analysis shows. At number 100 in the rankings is Seattle/Tacoma.
Data for the analysis came from the Centers for Disease Control and Prevention, County Health Rankings, the Department of Agriculture’s Economic Research Service, the Child and Adolescent Health Measurement Initiative, the Trust for America’s Health, and WalletHub’s own research, including its report Best & Worst Cities for an Active Lifestyle.
ACA: How would you improve it?
With the March 24 demise of the American Health Care Act, Republican leaders in Congress and in the White House have said that the Affordable Care Act will remain in place for the foreseeable future. While the ACA has brought about a number of health reforms that benefit physicians and their patients, other provisions have increased physician hassle and headache.
[polldaddy:9708248]
Check back soon to see if your colleagues agree on what part of the ACA should be changed, or email me your thoughts on the business of medicine.
On Twitter @denisefulton
With the March 24 demise of the American Health Care Act, Republican leaders in Congress and in the White House have said that the Affordable Care Act will remain in place for the foreseeable future. While the ACA has brought about a number of health reforms that benefit physicians and their patients, other provisions have increased physician hassle and headache.
[polldaddy:9708248]
Check back soon to see if your colleagues agree on what part of the ACA should be changed, or email me your thoughts on the business of medicine.
On Twitter @denisefulton
With the March 24 demise of the American Health Care Act, Republican leaders in Congress and in the White House have said that the Affordable Care Act will remain in place for the foreseeable future. While the ACA has brought about a number of health reforms that benefit physicians and their patients, other provisions have increased physician hassle and headache.
[polldaddy:9708248]
Check back soon to see if your colleagues agree on what part of the ACA should be changed, or email me your thoughts on the business of medicine.
On Twitter @denisefulton
Midlife Cardiovascular Risk Factors Increase Dementia Risk
HOUSTON—Cardiovascular risk factors in middle age may increase the risk of dementia in later years, according to a subanalysis of a 25-year atherosclerosis study.
Other conditions that significantly increased the likelihood of late-life dementia were hypertension and smoking, both of which augmented the risk by 40%.
Dr. Gottesman’s subanalysis of the biracial Atherosclerosis Risk in Communities-Neurocognitive Study (ARIC-NCS) also identified racial differences in dementia risk. Smoking was a risk factor for dementia in whites, but not in blacks. In addition, although the results were not significantly different by race, diabetes appeared to increase the risk more among blacks than whites, and hypertension increased the risk of dementia more among whites than blacks.
Analyzing Data From a Study of Atherosclerosis
The ARIC study, sponsored by the National Heart, Lung, and Blood Institute, is a prospective epidemiologic study conducted in four US communities. ARIC is designed to investigate the causes and clinical outcomes of atherosclerosis, and variation in cardiovascular risk factors, medical care, and disease by race, gender, location, and date. The ARIC project has led to the publication of more than 1,700 articles in peer-reviewed journals to date.
A total of 15,792 participants have received an extensive examination, including medical, social, and demographic data. The first screen occurred between 1987 and 1989, and participants were reexamined every three years through 1998, and then again 15 years later. Follow-up occurs yearly by telephone to maintain contact with participants and to assess the health status of the cohort.
The ARIC-NCS study includes about 10,000 of the ARIC participants. Of these patients, 6,471 completed the fifth visit, which occurred during 2011–2013. The participants have undergone cognitive and neurologic assessments to diagnose mild cognitive impairment or dementia and assign an etiology for any cognitive disorder; some also have undergone brain imaging. Last year, investigators published preliminary findings from the study, including that approximately 30% of participants had received a diagnosis of dementia or mild cognitive impairment.
Dr. Gottesman sought to determine the extent to which these subjects’ baseline cardiovascular risk factors influenced their risk of cognitive decline or dementia. She assessed risk for the entire cohort, and then assessed the risk for black and white subjects separately.
Genetic Status Had Largest Effect
In all, 1,516 participants (23%) developed dementia. In the total cohort, dementia was significantly associated with increasing age. Subjects between ages 50 and 54 when first examined had twice the risk of dementia during follow-up, compared with younger subjects, while those between ages 60 and 66 had eight times greater risk. Black race conferred an increased risk, compared with white race (hazard ratio [HR], 1.3). Education of less than a high school degree was associated with a 40% increased risk of dementia. Having at least one copy of the APOE4 allele doubled the risk of dementia.
Increasing BMI did not increase the risk of dementia, Dr. Gottesman noted. Smoking, however, increased the risk of dementia by 40%, as did prehypertension and hypertension. Dyslipidemia was not associated with any increased risk. Diabetes increased the risk of dementia by 80%.
Dr. Gottesman found significant differences in the way the factors affected risk in white and black subjects. Age exerted a greater influence on dementia risk in whites than it did in blacks. The risk was approximately doubled in both groups for people between ages 50 and 54 at baseline. But for patients between ages 55 and 59 at baseline, dementia risk was significantly higher in whites than in blacks (HR, 4.4 vs 3.5). The risk differential was even greater between whites and blacks who were between ages 60 and 66 (HR, 9.5 vs 6.2).
Blacks with low education had a greater risk of dementia than did whites (HR, 1.6 vs 1.3). APOE4 status (ie, having at least one allele) more than doubled the risk of dementia for whites (HR, 2.2), but had a weaker effect in blacks (HR, 1.6).
Obesity increased the risk of dementia by 22% for whites, but it had no influence on risk among blacks. Current smoking increased the risk for whites by 62%, but was not a significant risk factor for blacks. Prehypertension also had a greater effect among whites, increasing the risk by 35%, compared with a nonsignificant 17% increase for blacks, but the difference was not statistically significant. Hypertension increased the risk of dementia similarly in both groups (37% and 36%, respectively). Diabetes increased the risk of dementia more in blacks than it did in whites (85% vs 69%), but the racial difference was not statistically significant.
“We do not have a clear explanation of these disparities in dementia risk with regard to race,” said Dr. Gottesman. “It could be, though, that even if a risk factor has the same relationship with dementia in both groups, if it is more prevalent in one group, that may somewhat account for this larger population-attributable risk.”
—Michelle G. Sullivan
Suggested Reading
Baumgart M, Snyder HM, Carrillo MC, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718-726.
Exalto LG, Quesenberry CP, Barnes D, et al. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10(5):562-570.
Hessler JB, Ander KH, Brönner M, et al. Predicting dementia in primary care patients with a cardiovascular health metric: a prospective population-based study. BMC Neurol. 2016;16:116.
Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11.
Virta JJ, Heikkilä K, Perola M, et al. Midlife cardiovascular risk factors and late cognitive impairment. Eur J Epidemiol. 2013;28(5):405-416.
HOUSTON—Cardiovascular risk factors in middle age may increase the risk of dementia in later years, according to a subanalysis of a 25-year atherosclerosis study.
Other conditions that significantly increased the likelihood of late-life dementia were hypertension and smoking, both of which augmented the risk by 40%.
Dr. Gottesman’s subanalysis of the biracial Atherosclerosis Risk in Communities-Neurocognitive Study (ARIC-NCS) also identified racial differences in dementia risk. Smoking was a risk factor for dementia in whites, but not in blacks. In addition, although the results were not significantly different by race, diabetes appeared to increase the risk more among blacks than whites, and hypertension increased the risk of dementia more among whites than blacks.
Analyzing Data From a Study of Atherosclerosis
The ARIC study, sponsored by the National Heart, Lung, and Blood Institute, is a prospective epidemiologic study conducted in four US communities. ARIC is designed to investigate the causes and clinical outcomes of atherosclerosis, and variation in cardiovascular risk factors, medical care, and disease by race, gender, location, and date. The ARIC project has led to the publication of more than 1,700 articles in peer-reviewed journals to date.
A total of 15,792 participants have received an extensive examination, including medical, social, and demographic data. The first screen occurred between 1987 and 1989, and participants were reexamined every three years through 1998, and then again 15 years later. Follow-up occurs yearly by telephone to maintain contact with participants and to assess the health status of the cohort.
The ARIC-NCS study includes about 10,000 of the ARIC participants. Of these patients, 6,471 completed the fifth visit, which occurred during 2011–2013. The participants have undergone cognitive and neurologic assessments to diagnose mild cognitive impairment or dementia and assign an etiology for any cognitive disorder; some also have undergone brain imaging. Last year, investigators published preliminary findings from the study, including that approximately 30% of participants had received a diagnosis of dementia or mild cognitive impairment.
Dr. Gottesman sought to determine the extent to which these subjects’ baseline cardiovascular risk factors influenced their risk of cognitive decline or dementia. She assessed risk for the entire cohort, and then assessed the risk for black and white subjects separately.
Genetic Status Had Largest Effect
In all, 1,516 participants (23%) developed dementia. In the total cohort, dementia was significantly associated with increasing age. Subjects between ages 50 and 54 when first examined had twice the risk of dementia during follow-up, compared with younger subjects, while those between ages 60 and 66 had eight times greater risk. Black race conferred an increased risk, compared with white race (hazard ratio [HR], 1.3). Education of less than a high school degree was associated with a 40% increased risk of dementia. Having at least one copy of the APOE4 allele doubled the risk of dementia.
Increasing BMI did not increase the risk of dementia, Dr. Gottesman noted. Smoking, however, increased the risk of dementia by 40%, as did prehypertension and hypertension. Dyslipidemia was not associated with any increased risk. Diabetes increased the risk of dementia by 80%.
Dr. Gottesman found significant differences in the way the factors affected risk in white and black subjects. Age exerted a greater influence on dementia risk in whites than it did in blacks. The risk was approximately doubled in both groups for people between ages 50 and 54 at baseline. But for patients between ages 55 and 59 at baseline, dementia risk was significantly higher in whites than in blacks (HR, 4.4 vs 3.5). The risk differential was even greater between whites and blacks who were between ages 60 and 66 (HR, 9.5 vs 6.2).
Blacks with low education had a greater risk of dementia than did whites (HR, 1.6 vs 1.3). APOE4 status (ie, having at least one allele) more than doubled the risk of dementia for whites (HR, 2.2), but had a weaker effect in blacks (HR, 1.6).
Obesity increased the risk of dementia by 22% for whites, but it had no influence on risk among blacks. Current smoking increased the risk for whites by 62%, but was not a significant risk factor for blacks. Prehypertension also had a greater effect among whites, increasing the risk by 35%, compared with a nonsignificant 17% increase for blacks, but the difference was not statistically significant. Hypertension increased the risk of dementia similarly in both groups (37% and 36%, respectively). Diabetes increased the risk of dementia more in blacks than it did in whites (85% vs 69%), but the racial difference was not statistically significant.
“We do not have a clear explanation of these disparities in dementia risk with regard to race,” said Dr. Gottesman. “It could be, though, that even if a risk factor has the same relationship with dementia in both groups, if it is more prevalent in one group, that may somewhat account for this larger population-attributable risk.”
—Michelle G. Sullivan
Suggested Reading
Baumgart M, Snyder HM, Carrillo MC, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718-726.
Exalto LG, Quesenberry CP, Barnes D, et al. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10(5):562-570.
Hessler JB, Ander KH, Brönner M, et al. Predicting dementia in primary care patients with a cardiovascular health metric: a prospective population-based study. BMC Neurol. 2016;16:116.
Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11.
Virta JJ, Heikkilä K, Perola M, et al. Midlife cardiovascular risk factors and late cognitive impairment. Eur J Epidemiol. 2013;28(5):405-416.
HOUSTON—Cardiovascular risk factors in middle age may increase the risk of dementia in later years, according to a subanalysis of a 25-year atherosclerosis study.
Other conditions that significantly increased the likelihood of late-life dementia were hypertension and smoking, both of which augmented the risk by 40%.
Dr. Gottesman’s subanalysis of the biracial Atherosclerosis Risk in Communities-Neurocognitive Study (ARIC-NCS) also identified racial differences in dementia risk. Smoking was a risk factor for dementia in whites, but not in blacks. In addition, although the results were not significantly different by race, diabetes appeared to increase the risk more among blacks than whites, and hypertension increased the risk of dementia more among whites than blacks.
Analyzing Data From a Study of Atherosclerosis
The ARIC study, sponsored by the National Heart, Lung, and Blood Institute, is a prospective epidemiologic study conducted in four US communities. ARIC is designed to investigate the causes and clinical outcomes of atherosclerosis, and variation in cardiovascular risk factors, medical care, and disease by race, gender, location, and date. The ARIC project has led to the publication of more than 1,700 articles in peer-reviewed journals to date.
A total of 15,792 participants have received an extensive examination, including medical, social, and demographic data. The first screen occurred between 1987 and 1989, and participants were reexamined every three years through 1998, and then again 15 years later. Follow-up occurs yearly by telephone to maintain contact with participants and to assess the health status of the cohort.
The ARIC-NCS study includes about 10,000 of the ARIC participants. Of these patients, 6,471 completed the fifth visit, which occurred during 2011–2013. The participants have undergone cognitive and neurologic assessments to diagnose mild cognitive impairment or dementia and assign an etiology for any cognitive disorder; some also have undergone brain imaging. Last year, investigators published preliminary findings from the study, including that approximately 30% of participants had received a diagnosis of dementia or mild cognitive impairment.
Dr. Gottesman sought to determine the extent to which these subjects’ baseline cardiovascular risk factors influenced their risk of cognitive decline or dementia. She assessed risk for the entire cohort, and then assessed the risk for black and white subjects separately.
Genetic Status Had Largest Effect
In all, 1,516 participants (23%) developed dementia. In the total cohort, dementia was significantly associated with increasing age. Subjects between ages 50 and 54 when first examined had twice the risk of dementia during follow-up, compared with younger subjects, while those between ages 60 and 66 had eight times greater risk. Black race conferred an increased risk, compared with white race (hazard ratio [HR], 1.3). Education of less than a high school degree was associated with a 40% increased risk of dementia. Having at least one copy of the APOE4 allele doubled the risk of dementia.
Increasing BMI did not increase the risk of dementia, Dr. Gottesman noted. Smoking, however, increased the risk of dementia by 40%, as did prehypertension and hypertension. Dyslipidemia was not associated with any increased risk. Diabetes increased the risk of dementia by 80%.
Dr. Gottesman found significant differences in the way the factors affected risk in white and black subjects. Age exerted a greater influence on dementia risk in whites than it did in blacks. The risk was approximately doubled in both groups for people between ages 50 and 54 at baseline. But for patients between ages 55 and 59 at baseline, dementia risk was significantly higher in whites than in blacks (HR, 4.4 vs 3.5). The risk differential was even greater between whites and blacks who were between ages 60 and 66 (HR, 9.5 vs 6.2).
Blacks with low education had a greater risk of dementia than did whites (HR, 1.6 vs 1.3). APOE4 status (ie, having at least one allele) more than doubled the risk of dementia for whites (HR, 2.2), but had a weaker effect in blacks (HR, 1.6).
Obesity increased the risk of dementia by 22% for whites, but it had no influence on risk among blacks. Current smoking increased the risk for whites by 62%, but was not a significant risk factor for blacks. Prehypertension also had a greater effect among whites, increasing the risk by 35%, compared with a nonsignificant 17% increase for blacks, but the difference was not statistically significant. Hypertension increased the risk of dementia similarly in both groups (37% and 36%, respectively). Diabetes increased the risk of dementia more in blacks than it did in whites (85% vs 69%), but the racial difference was not statistically significant.
“We do not have a clear explanation of these disparities in dementia risk with regard to race,” said Dr. Gottesman. “It could be, though, that even if a risk factor has the same relationship with dementia in both groups, if it is more prevalent in one group, that may somewhat account for this larger population-attributable risk.”
—Michelle G. Sullivan
Suggested Reading
Baumgart M, Snyder HM, Carrillo MC, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718-726.
Exalto LG, Quesenberry CP, Barnes D, et al. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10(5):562-570.
Hessler JB, Ander KH, Brönner M, et al. Predicting dementia in primary care patients with a cardiovascular health metric: a prospective population-based study. BMC Neurol. 2016;16:116.
Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11.
Virta JJ, Heikkilä K, Perola M, et al. Midlife cardiovascular risk factors and late cognitive impairment. Eur J Epidemiol. 2013;28(5):405-416.
Guideline Provides Recommendations for Pharmacologic Treatment of Chronic Insomnia
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.
All patients with chronic insomnia should receive cognitive behavioral therapy as a primary intervention, and clinicians should use a shared decision-making approach when determining whether patients should start pharmacotherapy, according to a new guideline developed by the American Academy of Sleep Medicine (AASM). The guideline, which includes 14 recommendations, was published in the February 15 issue of the Journal of Clinical Sleep Medicine. It is the first guideline from the AASM to provide comprehensive evidence-based analyses of individual drugs commonly used to treat chronic insomnia disorder.
Chronic insomnia is associated with increased work absenteeism and impairment in functional status. Studies have also identified persistent insomnia as a significant risk factor for the development of psychiatric disorders, especially mood disorder. Pharmacologic treatment of insomnia is the most widely used approach to therapy, after treatment of comorbidities. No evidence-based clinical guideline had been published previously, however. The AASM commissioned a task force of four sleep medicine experts to develop the clinical guideline for treatment of chronic insomnia in adults.
The task force conducted a systematic review to identify randomized controlled trials. In addition, the group used the Grading of Recommendations Assessment, Development, and Evaluation process to assess the evidence. Recommendations were then made based on the quality of evidence, the balance of benefits and harms, and patient values and preferences.
Sleep Onset Insomnia Treatment Recommendations
The guideline recommends eszopiclone for the treatment of sleep onset insomnia, based on trials of 2-mg and 3-mg doses of the drug. Mean reduction of sleep latency was 14 minutes greater with eszopiclone than with placebo. Researchers also observed a moderate-to-large improvement in quality of sleep when they compared eszopiclone with placebo.
The experts also recommend ramelteon, based on trials of 8-mg doses of the drug. Mean reduction of sleep latency was nine minutes greater with ramelteon than with placebo. Ramelteon may not improve quality of sleep, however. Clinicians also are advised to use temazepam, based on trials of 15-mg doses. Temazepam reduced sleep latency by 37 minutes, compared with placebo, and provided a small improvement in quality of sleep.
The quality of evidence for the use of triazolam was high, and the drug reduced sleep latency by nine minutes, compared with placebo. The task force determined that the majority of patients would be more likely to use triazolam, compared with no treatment, but many would not use it. Zaleplon was recommended based on trials of 5-mg and 10-mg doses. It reduced sleep latency by 10 minutes, compared with placebo. Finally, zolpidem reduced sleep latency by five to 12 minutes, compared with placebo, and was associated with a moderate improvement in quality of sleep. The quality of evidence was very low, however.
Sleep Maintenance Insomnia Recommendations
The task force found four studies evaluating a 3-mg dose of doxepin and four studies investigating a 6-mg dose in sleep maintenance insomnia. Mean improvement in total sleep time with the drug was 26–32 min longer, compared with placebo. The mean reduction in wake after sleep onset (WASO) associated with doxepin was 22–23 min greater, compared with placebo.
The recommendation for the use of eszopiclone was based on trials of 2-mg and 3-mg doses of the drug. Mean improvement in total sleep time was 28–57 min longer with eszopiclone, compared with placebo. Mean reduction in WASO was 10–14 min greater with eszopiclone, compared with placebo.
Trials of 15-mg doses of temazepam were the basis for the task force’s recommendation of the drug. Mean improvement in total sleep time with temazepam was 99 min longer, compared with placebo. The drug’s effect on WASO was not reported. Compared with placebo, temazepam may provide a small improvement in quality of sleep.
Suvorexant was evaluated in trials of 10-mg, 15–20-mg, and 20-mg doses. Mean improvement in total sleep time was 10 min longer with suvorexant, compared with placebo. Mean reduction in WASO was 16–28 min greater with suvorexant, compared to placebo.
The guideline also recommends zolpidem, based on trials of 10-mg doses. Zolpidem provided a mean improvement in total sleep time that was 29 min longer, compared with placebo. The mean reduction in WASO was 25 min greater with zolpidem, compared with placebo.
Certain Drugs Are Not Recommended
Several drugs were not recommended for treating sleep onset or sleep maintenance insomnia. The task force suggests that clinicians not use diphenhydramine, because the evidence suggests that it is not effective for improving sleep onset or total sleep time. Similarly, evidence suggests that the effects of tiagabine on objective and subjective measures of sleep latency are clinically insignificant, and the drug’s harms may outweigh its benefits. Sleep outcome variables also did not improve with trazodone to a clinically significant degree. The task force also suggested that clinicians not use melatonin, valerian, or L-tryptophan because of the treatments’ lack of clinically significant effects.
All recommendations were classified as weak, due to the task force’s low degree of certainty in the appropriateness of the patient-care strategy. Further study is required to determine the drugs’ efficacy or lack thereof, said the task force. “Clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects,” the authors concluded.
—Erica Tricarico
Suggested Reading
Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349.