User login
Study supports NCCN recommendations on risk-reducing salpingo-oophorectomy
NATIONAL HARBOR, MD – A large hereditary cancer study supports National Comprehensive Cancer Network guidance to consider risk-reducing salpingo-oophorectomy (RRSO) between ages 45 and 50 years for women with BRIP1, RAD51C, or RAD51D mutations, Lydia Usha, MD, said at the annual meeting of the Society of Gynecologic Oncology.
The average ages for an ovarian cancer diagnosis were 56 years for women with RAD51D mutations, 61 years for RAD51C mutations, and 64 years for BRIP1 mutations, said Dr. Usha of Rush Medical College, Chicago. When appropriate, delaying RRSO “avoids the psychosocial and medical complications of premature menopause,” she said.
Among all women, mutation prevalence was 0.3% for BRIP1, 0.1% for RAD51C and RAD51D, 1.2% for BRCA1, and 1.3% for BRCA2 mutations, Dr. Usha reported. Among 18,719 women who had a personal history of ovarian cancer, the most common mutation was BRCA1 (3.5%), followed by BRCA2 (2.7%). In contrast, the combined prevalence of BRIP1, RAD51C, and RAD51D mutations among cancer patients was only 1.6%.
Cancer prevalence was highest among women who had mutations of RAD51C (22%), followed by RAD51D (19%), BRCA1 and BRIP1 (16% in each case), and BRCA2 (11%). Thus, while BRIP1 and RAD51D mutations were uncommon, their presence signified an ovarian cancer risk that was similar to that with BRCA1 mutations, and a greater risk than with BRCA2, Dr. Usha said.
The average ages for ovarian cancer diagnosis were 64 years for BRIP1, 61 years for RAD51C, 60 years for BRCA2, 56 years for RAD51C, and 54 years for BRCA1. “More than 80% of women with ovarian cancer who had a mutation in BRIP1, RAD51C, or BRCA2 were diagnosed after age 50,” Dr. Usha noted. These findings support considering RRSO closer to age 45 years for RAD51D mutation carriers and closer to age 50 years for women with pathogenic variants of BRIP1, added discussant Kari Ring, MD, of the University of Virginia, Charlottesville.
Mutation type did not significantly correlate with ethnicity or type of ovarian cancer, Dr. Usha noted. “Collectively, these findings may aid clinical decisions about the medical management of women with mutations in these genes,” she said. “Our data may also assist with reproductive decisions, such as age of childbearing.”
Dr. Usha did not report external funding sources, but disclosed travel expenses from Myriad Genetics.
NATIONAL HARBOR, MD – A large hereditary cancer study supports National Comprehensive Cancer Network guidance to consider risk-reducing salpingo-oophorectomy (RRSO) between ages 45 and 50 years for women with BRIP1, RAD51C, or RAD51D mutations, Lydia Usha, MD, said at the annual meeting of the Society of Gynecologic Oncology.
The average ages for an ovarian cancer diagnosis were 56 years for women with RAD51D mutations, 61 years for RAD51C mutations, and 64 years for BRIP1 mutations, said Dr. Usha of Rush Medical College, Chicago. When appropriate, delaying RRSO “avoids the psychosocial and medical complications of premature menopause,” she said.
Among all women, mutation prevalence was 0.3% for BRIP1, 0.1% for RAD51C and RAD51D, 1.2% for BRCA1, and 1.3% for BRCA2 mutations, Dr. Usha reported. Among 18,719 women who had a personal history of ovarian cancer, the most common mutation was BRCA1 (3.5%), followed by BRCA2 (2.7%). In contrast, the combined prevalence of BRIP1, RAD51C, and RAD51D mutations among cancer patients was only 1.6%.
Cancer prevalence was highest among women who had mutations of RAD51C (22%), followed by RAD51D (19%), BRCA1 and BRIP1 (16% in each case), and BRCA2 (11%). Thus, while BRIP1 and RAD51D mutations were uncommon, their presence signified an ovarian cancer risk that was similar to that with BRCA1 mutations, and a greater risk than with BRCA2, Dr. Usha said.
The average ages for ovarian cancer diagnosis were 64 years for BRIP1, 61 years for RAD51C, 60 years for BRCA2, 56 years for RAD51C, and 54 years for BRCA1. “More than 80% of women with ovarian cancer who had a mutation in BRIP1, RAD51C, or BRCA2 were diagnosed after age 50,” Dr. Usha noted. These findings support considering RRSO closer to age 45 years for RAD51D mutation carriers and closer to age 50 years for women with pathogenic variants of BRIP1, added discussant Kari Ring, MD, of the University of Virginia, Charlottesville.
Mutation type did not significantly correlate with ethnicity or type of ovarian cancer, Dr. Usha noted. “Collectively, these findings may aid clinical decisions about the medical management of women with mutations in these genes,” she said. “Our data may also assist with reproductive decisions, such as age of childbearing.”
Dr. Usha did not report external funding sources, but disclosed travel expenses from Myriad Genetics.
NATIONAL HARBOR, MD – A large hereditary cancer study supports National Comprehensive Cancer Network guidance to consider risk-reducing salpingo-oophorectomy (RRSO) between ages 45 and 50 years for women with BRIP1, RAD51C, or RAD51D mutations, Lydia Usha, MD, said at the annual meeting of the Society of Gynecologic Oncology.
The average ages for an ovarian cancer diagnosis were 56 years for women with RAD51D mutations, 61 years for RAD51C mutations, and 64 years for BRIP1 mutations, said Dr. Usha of Rush Medical College, Chicago. When appropriate, delaying RRSO “avoids the psychosocial and medical complications of premature menopause,” she said.
Among all women, mutation prevalence was 0.3% for BRIP1, 0.1% for RAD51C and RAD51D, 1.2% for BRCA1, and 1.3% for BRCA2 mutations, Dr. Usha reported. Among 18,719 women who had a personal history of ovarian cancer, the most common mutation was BRCA1 (3.5%), followed by BRCA2 (2.7%). In contrast, the combined prevalence of BRIP1, RAD51C, and RAD51D mutations among cancer patients was only 1.6%.
Cancer prevalence was highest among women who had mutations of RAD51C (22%), followed by RAD51D (19%), BRCA1 and BRIP1 (16% in each case), and BRCA2 (11%). Thus, while BRIP1 and RAD51D mutations were uncommon, their presence signified an ovarian cancer risk that was similar to that with BRCA1 mutations, and a greater risk than with BRCA2, Dr. Usha said.
The average ages for ovarian cancer diagnosis were 64 years for BRIP1, 61 years for RAD51C, 60 years for BRCA2, 56 years for RAD51C, and 54 years for BRCA1. “More than 80% of women with ovarian cancer who had a mutation in BRIP1, RAD51C, or BRCA2 were diagnosed after age 50,” Dr. Usha noted. These findings support considering RRSO closer to age 45 years for RAD51D mutation carriers and closer to age 50 years for women with pathogenic variants of BRIP1, added discussant Kari Ring, MD, of the University of Virginia, Charlottesville.
Mutation type did not significantly correlate with ethnicity or type of ovarian cancer, Dr. Usha noted. “Collectively, these findings may aid clinical decisions about the medical management of women with mutations in these genes,” she said. “Our data may also assist with reproductive decisions, such as age of childbearing.”
Dr. Usha did not report external funding sources, but disclosed travel expenses from Myriad Genetics.
AT THE ANNUAL MEETING ON WOMEN'S CANCER
Key clinical point: A large hereditary cancer study supports National Comprehensive Cancer Network guidance to consider risk-reducing salpingo-oophorectomy between age 45 and 50 years for women with BRIP1, RAD51C, or RAD51D mutations.
Major finding: Average ages for an ovarian cancer diagnosis were 56 years for women with RAD51D mutations, 61 years for RAD51C mutations, and 64 years for BRIP1 mutations.
Data source: Analyses of a 25-gene hereditary panel performed in 345,667 women.
Disclosures: Dr. Usha did not report external funding sources, but disclosed travel expenses from Myriad Genetics.
Dupilumab: FDA approves first biologic for atopic dermatitis
Dupilumab, a monoclonal antibody that targets both interleukin-4 and interleukin-13, has been approved for the treatment of moderate to severe atopic dermatitis in adults not adequately controlled with topical prescription therapies or for whom topicals are not appropriate.
The approval marks the first biologic approved for treating AD, according to a March 28 announcement from the Food and Drug Administration.
Dupilumab “inhibits signaling of IL-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in the pathogenesis of the disease,” according to Regeneron, which will market dupilumab.
Approval was based on three phase III pivotal studies of adults with moderate to severe AD whose disease was not adequately controlled with topical prescription treatments: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
The 16-week data from the SOLO-1 and -2 studies were presented at the 2016 annual congress of the European Academy for Dermatology and Venereology.
In two phase III trials of identical design involving patients with atopic dermatitis, dupilumab, administered weekly or every 2 weeks, improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (P less than .001 for all comparisons).
Dupilumab also was associated with improvement in other clinical endpoints, including reductions in pruritus and symptoms of anxiety or depression, and an improvement in quality of life. Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups. The results were published in the New England Journal of Medicine (2016;375:2335-48).
More recently, 52-week data from the CHRONOS study were reported at the annual meeting of the American Academy of Dermatology in March. In that study of 740 adults with moderate to severe AD that was not controlled with topical medications – including corticosteroids with or without calcineurin inhibitors – those randomized to 300 mg of dupilumab once a week, plus topical corticosteroids, showed significantly greater improvements in measures of overall disease severity at 16 weeks and at 52 weeks, compared with those treated with steroids alone. Measures used included Eczema Area and Severity Index and the Pruritus Numerical Rating Scale, Patient Oriented Eczema Measure, Dermatology Life Quality Index.
Adverse events experienced with dupilumab included injection site reactions, eye and eyelid inflammation, and cold sores on the mouth or lips, according to a Regeneron statement.
Dupilumab will be available “later this week,” according to the statement, which noted the wholesale acquisition cost of the medication is expected to be $37,000 annually.
The FDA approval announcement noted that the safety and efficacy of dupilumab had not been established in patients with asthma.
Dupilumab currently is being studied for children with AD in phase II studies and is being studied for other indications: eosinophilic esophagitis in phase II studies, and asthma and nasal polyps in phase III studies.
Dupilumab will be marketed as Dupixent by Regeneron.
Dupilumab, a monoclonal antibody that targets both interleukin-4 and interleukin-13, has been approved for the treatment of moderate to severe atopic dermatitis in adults not adequately controlled with topical prescription therapies or for whom topicals are not appropriate.
The approval marks the first biologic approved for treating AD, according to a March 28 announcement from the Food and Drug Administration.
Dupilumab “inhibits signaling of IL-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in the pathogenesis of the disease,” according to Regeneron, which will market dupilumab.
Approval was based on three phase III pivotal studies of adults with moderate to severe AD whose disease was not adequately controlled with topical prescription treatments: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
The 16-week data from the SOLO-1 and -2 studies were presented at the 2016 annual congress of the European Academy for Dermatology and Venereology.
In two phase III trials of identical design involving patients with atopic dermatitis, dupilumab, administered weekly or every 2 weeks, improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (P less than .001 for all comparisons).
Dupilumab also was associated with improvement in other clinical endpoints, including reductions in pruritus and symptoms of anxiety or depression, and an improvement in quality of life. Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups. The results were published in the New England Journal of Medicine (2016;375:2335-48).
More recently, 52-week data from the CHRONOS study were reported at the annual meeting of the American Academy of Dermatology in March. In that study of 740 adults with moderate to severe AD that was not controlled with topical medications – including corticosteroids with or without calcineurin inhibitors – those randomized to 300 mg of dupilumab once a week, plus topical corticosteroids, showed significantly greater improvements in measures of overall disease severity at 16 weeks and at 52 weeks, compared with those treated with steroids alone. Measures used included Eczema Area and Severity Index and the Pruritus Numerical Rating Scale, Patient Oriented Eczema Measure, Dermatology Life Quality Index.
Adverse events experienced with dupilumab included injection site reactions, eye and eyelid inflammation, and cold sores on the mouth or lips, according to a Regeneron statement.
Dupilumab will be available “later this week,” according to the statement, which noted the wholesale acquisition cost of the medication is expected to be $37,000 annually.
The FDA approval announcement noted that the safety and efficacy of dupilumab had not been established in patients with asthma.
Dupilumab currently is being studied for children with AD in phase II studies and is being studied for other indications: eosinophilic esophagitis in phase II studies, and asthma and nasal polyps in phase III studies.
Dupilumab will be marketed as Dupixent by Regeneron.
Dupilumab, a monoclonal antibody that targets both interleukin-4 and interleukin-13, has been approved for the treatment of moderate to severe atopic dermatitis in adults not adequately controlled with topical prescription therapies or for whom topicals are not appropriate.
The approval marks the first biologic approved for treating AD, according to a March 28 announcement from the Food and Drug Administration.
Dupilumab “inhibits signaling of IL-4 and IL-13, two key cytokines required for the type 2 (including Th2) immune response, which is believed to be a major driver in the pathogenesis of the disease,” according to Regeneron, which will market dupilumab.
Approval was based on three phase III pivotal studies of adults with moderate to severe AD whose disease was not adequately controlled with topical prescription treatments: SOLO-1 and SOLO-2, which evaluated dupilumab as monotherapy, and the CHRONOS study, which compared dupilumab with topical corticosteroids to treatment with topical corticosteroids alone.
The 16-week data from the SOLO-1 and -2 studies were presented at the 2016 annual congress of the European Academy for Dermatology and Venereology.
In two phase III trials of identical design involving patients with atopic dermatitis, dupilumab, administered weekly or every 2 weeks, improved the signs and symptoms of atopic dermatitis, including pruritus, symptoms of anxiety and depression, and quality of life, as compared with placebo. Eczema Area and Severity Index was reported in significantly more patients who received each regimen of dupilumab than in patients who received placebo (P less than .001 for all comparisons).
Dupilumab also was associated with improvement in other clinical endpoints, including reductions in pruritus and symptoms of anxiety or depression, and an improvement in quality of life. Injection-site reactions and conjunctivitis were more frequent in the dupilumab groups than in the placebo groups. The results were published in the New England Journal of Medicine (2016;375:2335-48).
More recently, 52-week data from the CHRONOS study were reported at the annual meeting of the American Academy of Dermatology in March. In that study of 740 adults with moderate to severe AD that was not controlled with topical medications – including corticosteroids with or without calcineurin inhibitors – those randomized to 300 mg of dupilumab once a week, plus topical corticosteroids, showed significantly greater improvements in measures of overall disease severity at 16 weeks and at 52 weeks, compared with those treated with steroids alone. Measures used included Eczema Area and Severity Index and the Pruritus Numerical Rating Scale, Patient Oriented Eczema Measure, Dermatology Life Quality Index.
Adverse events experienced with dupilumab included injection site reactions, eye and eyelid inflammation, and cold sores on the mouth or lips, according to a Regeneron statement.
Dupilumab will be available “later this week,” according to the statement, which noted the wholesale acquisition cost of the medication is expected to be $37,000 annually.
The FDA approval announcement noted that the safety and efficacy of dupilumab had not been established in patients with asthma.
Dupilumab currently is being studied for children with AD in phase II studies and is being studied for other indications: eosinophilic esophagitis in phase II studies, and asthma and nasal polyps in phase III studies.
Dupilumab will be marketed as Dupixent by Regeneron.
Liver disease likely to become increasing indication for bariatric surgery
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
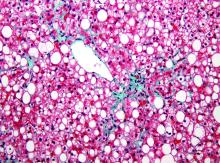
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
PHILADELPHIA – There is a long list of benefits from bariatric surgery in the morbidly obese, but prevention of end-stage liver disease and the need for a first or second liver transplant is likely to grow as an indication, according to an overview of weight loss surgery at Digestive Diseases: New Advances, held by Rutgers, the State University of New Jersey, and Global Academy for Medical Education.
“Bariatric surgery is associated with significant improvement not just in diabetes, dyslipidemia, hypertension, and other complications of metabolic disorders but for me more interestingly, it is effective for treating fatty liver disease where you can see a 90% improvement in steatosis,” reported Subhashini Ayloo, MD, chief of minimally invasive robotic hepato-pancreato-biliary surgery and liver transplantation at New Jersey Medical School, Newark.
Trained in both bariatric surgery and liver transplant, Dr. Ayloo predicts that these fields will become increasingly connected because of the obesity epidemic and the related rise in nonalcoholic fatty liver disease (NAFLD). Dr. Ayloo reported that bariatric surgery is already being used in her center to avoid a second liver transplant in obese patients who are unable to lose sufficient weight to prevent progressive NAFLD after a first transplant.
The emphasis Dr. Ayloo placed on the role of bariatric surgery in preventing progression of NAFLD to nonalcoholic steatohepatitis and the inflammatory process that leads to fibrosis, cirrhosis, and liver decompensation, was drawn from her interest in these two fields. However, she did not ignore the potential of protection from obesity control for other diseases.
“Obesity adversely affects every organ in the body,” Dr. Ayloo pointed out. As a result of weight loss achieved with bariatric surgery, there is now a large body of evidence supporting broad benefits, not just those related to fat deposited in hepatocytes.
“We have a couple of decades of experience that has been published [with bariatric surgery], and this has shown that it maintains weight loss long term, it improves all the obesity-associated comorbidities, and it is cost effective,” Dr. Ayloo said. Now with long-term follow-up, “all of the studies are showing that bariatric surgery improves survival.”
Although most of the survival data have been generated by retrospective cohort studies, Dr. Ayloo cited nine sets of data showing odds ratios associating bariatric surgery with up to a 90% reduction in death over periods of up to 10 years of follow-up. In a summary slide presented by Dr. Ayloo, the estimated mortality benefit over 5 years was listed as 85%. The same summary slide listed large improvements in relevant measures of morbidity for more than 10 organ systems, such as improvement or resolution of dyslipidemia and hypertension in the circulatory system, improvement or resolution of asthma and other diseases affecting the respiratory system, and resolution or improvement of gastroesophageal reflux disease and other diseases affecting the gastrointestinal system.
Specific to the liver, these benefits included a nearly 40% reduction in liver inflammation and 20% reduction in fibrosis. According to Dr. Ayloo, who noted that NAFLD is expected to overtake hepatitis C virus as the No. 1 cause of liver transplant within the next 5 years, these data are important for drawing attention to bariatric surgery as a strategy to control liver disease. She suggested that there is a need to create a tighter link between efforts to treat morbid obesity and advanced liver disease.
“There is an established literature showing that if somebody is morbidly obese, the rate of liver transplant is lower than when compared to patients with normal weight,” Dr. Ayloo said. “There is a call out in the transplant community that we need to address this and we cannot just be throwing this under the table.”
Because of the strong relationship between obesity and NAFLD, a systematic approach is needed to consider liver disease in obese patients and obesity in patients with liver disease, she said. The close relationship is relevant when planning interventions for either. Liver disease should be assessed prior to bariatric surgery regardless of the indication and then monitored closely as part of postoperative care, she said.
Dr. Ayloo identified weight control as an essential part of posttransplant care to prevent hepatic fat deposition that threatens transplant-free survival.
Global Academy and this news organization are owned by the same company. Dr. Ayloo reports no relevant financial relationships.
What Is the Impact of a High-Salt Diet in Patients With MS?
ORLANDO—A high-salt diet might be a key environmental risk factor for multiple sclerosis (MS), according to an overview presented at the ACTRIMS 2017 Forum. Since most research has been performed in vitro and in animal models, it remains unclear how a high-salt diet affects patients with MS. Researchers have found in experimental models, however, that salt induces inflammation by several mechanisms: it increases frequency of inflammatory TH17 cells, decreases function of suppressor cells, and increases inflammation of antigen-presenting cells.
“There’s been an epidemic of human autoimmune disease over the past 70 years,” said Dr. Hafler. Researchers are working to discover why there has been such a significant rise in cases. “Genetics cannot allow this to happen. There must be something in the environment,” said Dr. Hafler. He and his colleagues sought to understand how a high-salt diet affects MS.
“What we found is if you added sodium chloride to cultures of T cells, you have autoimmune increases in the frequency of TH17 cells,” he said. In addition, salt decreases function of suppressor cells, the Tregs, and increases inflammation of antigen-presenting cells of the immune system, said Dr. Hafler.
Previous research found that mice fed a high-salt diet were more prone to severe experimental autoimmune encephalomyelitis. When the mice were given a high-salt, high-fat diet that mimicked fast food and probiotics, researchers observed a decrease in inflammation.
“It is really surprising to me how diet can really influence the degree of inflammation in these animals,” said Dr. Hafler. “We do not know if this will work in humans, but probiotics may decrease inflammation. We have no information [on] whether a diet with a probiotic would help prevent or treat MS.”
According to Farez et al, a higher sodium intake is associated with increased clinical and radiological disease activity in patients with MS. Other studies have found that environmental factors such as smoking and low vitamin D levels are associated with MS risk, and the risk is genetically mediated. What may be an environmental risk for one individual may not be a risk for another individual, said Dr. Hafler. “It is unlikely that any of these factors by themselves—smoking, vitamin D, fat, and salt—would be critically important, but together, they might have a strong effect,” he said.
Since there is limited research on a high-salt diet in humans, Dr. Hafler does not recommend his patients with MS go on a strict low-salt diet. However, he does advise patients to stay away from processed foods and fast food. He also encourages patients to get most of their calories from fruits and vegetables.
“We are now doing studies in which we put patients and control subjects on a high-salt diet and low[-salt] diet to observe the direct effect in those individuals,” said Dr. Hafler.
—Erica Tricarico
Suggested Reading
Farez MF, Fiol MP, Gaitán MI, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry.
Jörg S, Kissel J, Manzel A, et al. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol. 2016;279:212-222.
Kleinewietfeld M, Manzel A, Tize J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518-522.
Paling D, Solanky BS, Riemer F, et al. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain. 2013;136(Pt 7):2305-2317.
ORLANDO—A high-salt diet might be a key environmental risk factor for multiple sclerosis (MS), according to an overview presented at the ACTRIMS 2017 Forum. Since most research has been performed in vitro and in animal models, it remains unclear how a high-salt diet affects patients with MS. Researchers have found in experimental models, however, that salt induces inflammation by several mechanisms: it increases frequency of inflammatory TH17 cells, decreases function of suppressor cells, and increases inflammation of antigen-presenting cells.
“There’s been an epidemic of human autoimmune disease over the past 70 years,” said Dr. Hafler. Researchers are working to discover why there has been such a significant rise in cases. “Genetics cannot allow this to happen. There must be something in the environment,” said Dr. Hafler. He and his colleagues sought to understand how a high-salt diet affects MS.
“What we found is if you added sodium chloride to cultures of T cells, you have autoimmune increases in the frequency of TH17 cells,” he said. In addition, salt decreases function of suppressor cells, the Tregs, and increases inflammation of antigen-presenting cells of the immune system, said Dr. Hafler.
Previous research found that mice fed a high-salt diet were more prone to severe experimental autoimmune encephalomyelitis. When the mice were given a high-salt, high-fat diet that mimicked fast food and probiotics, researchers observed a decrease in inflammation.
“It is really surprising to me how diet can really influence the degree of inflammation in these animals,” said Dr. Hafler. “We do not know if this will work in humans, but probiotics may decrease inflammation. We have no information [on] whether a diet with a probiotic would help prevent or treat MS.”
According to Farez et al, a higher sodium intake is associated with increased clinical and radiological disease activity in patients with MS. Other studies have found that environmental factors such as smoking and low vitamin D levels are associated with MS risk, and the risk is genetically mediated. What may be an environmental risk for one individual may not be a risk for another individual, said Dr. Hafler. “It is unlikely that any of these factors by themselves—smoking, vitamin D, fat, and salt—would be critically important, but together, they might have a strong effect,” he said.
Since there is limited research on a high-salt diet in humans, Dr. Hafler does not recommend his patients with MS go on a strict low-salt diet. However, he does advise patients to stay away from processed foods and fast food. He also encourages patients to get most of their calories from fruits and vegetables.
“We are now doing studies in which we put patients and control subjects on a high-salt diet and low[-salt] diet to observe the direct effect in those individuals,” said Dr. Hafler.
—Erica Tricarico
Suggested Reading
Farez MF, Fiol MP, Gaitán MI, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry.
Jörg S, Kissel J, Manzel A, et al. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol. 2016;279:212-222.
Kleinewietfeld M, Manzel A, Tize J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518-522.
Paling D, Solanky BS, Riemer F, et al. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain. 2013;136(Pt 7):2305-2317.
ORLANDO—A high-salt diet might be a key environmental risk factor for multiple sclerosis (MS), according to an overview presented at the ACTRIMS 2017 Forum. Since most research has been performed in vitro and in animal models, it remains unclear how a high-salt diet affects patients with MS. Researchers have found in experimental models, however, that salt induces inflammation by several mechanisms: it increases frequency of inflammatory TH17 cells, decreases function of suppressor cells, and increases inflammation of antigen-presenting cells.
“There’s been an epidemic of human autoimmune disease over the past 70 years,” said Dr. Hafler. Researchers are working to discover why there has been such a significant rise in cases. “Genetics cannot allow this to happen. There must be something in the environment,” said Dr. Hafler. He and his colleagues sought to understand how a high-salt diet affects MS.
“What we found is if you added sodium chloride to cultures of T cells, you have autoimmune increases in the frequency of TH17 cells,” he said. In addition, salt decreases function of suppressor cells, the Tregs, and increases inflammation of antigen-presenting cells of the immune system, said Dr. Hafler.
Previous research found that mice fed a high-salt diet were more prone to severe experimental autoimmune encephalomyelitis. When the mice were given a high-salt, high-fat diet that mimicked fast food and probiotics, researchers observed a decrease in inflammation.
“It is really surprising to me how diet can really influence the degree of inflammation in these animals,” said Dr. Hafler. “We do not know if this will work in humans, but probiotics may decrease inflammation. We have no information [on] whether a diet with a probiotic would help prevent or treat MS.”
According to Farez et al, a higher sodium intake is associated with increased clinical and radiological disease activity in patients with MS. Other studies have found that environmental factors such as smoking and low vitamin D levels are associated with MS risk, and the risk is genetically mediated. What may be an environmental risk for one individual may not be a risk for another individual, said Dr. Hafler. “It is unlikely that any of these factors by themselves—smoking, vitamin D, fat, and salt—would be critically important, but together, they might have a strong effect,” he said.
Since there is limited research on a high-salt diet in humans, Dr. Hafler does not recommend his patients with MS go on a strict low-salt diet. However, he does advise patients to stay away from processed foods and fast food. He also encourages patients to get most of their calories from fruits and vegetables.
“We are now doing studies in which we put patients and control subjects on a high-salt diet and low[-salt] diet to observe the direct effect in those individuals,” said Dr. Hafler.
—Erica Tricarico
Suggested Reading
Farez MF, Fiol MP, Gaitán MI, et al. Sodium intake is associated with increased disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry.
Jörg S, Kissel J, Manzel A, et al. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol. 2016;279:212-222.
Kleinewietfeld M, Manzel A, Tize J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518-522.
Paling D, Solanky BS, Riemer F, et al. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain. 2013;136(Pt 7):2305-2317.
Hot Threads in ACS Communities
Your colleagues have a lot to say! Here are the top discussion threads in ACS Communities in March (all of these threads are from the General Surgery community):
1. Sully
3. Time has changed
4. Who fires your EEA staplers?
5. Neurosurgeon Sentenced to Life in Prison
6. Close the current VA health system as it is …
7. Surgery resident hours
8. Diagnostic laparotomy/laparoscopy
9. High jejunal resection in critically ill
10. Consults: Phone call or Text
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Your colleagues have a lot to say! Here are the top discussion threads in ACS Communities in March (all of these threads are from the General Surgery community):
1. Sully
3. Time has changed
4. Who fires your EEA staplers?
5. Neurosurgeon Sentenced to Life in Prison
6. Close the current VA health system as it is …
7. Surgery resident hours
8. Diagnostic laparotomy/laparoscopy
9. High jejunal resection in critically ill
10. Consults: Phone call or Text
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Your colleagues have a lot to say! Here are the top discussion threads in ACS Communities in March (all of these threads are from the General Surgery community):
1. Sully
3. Time has changed
4. Who fires your EEA staplers?
5. Neurosurgeon Sentenced to Life in Prison
6. Close the current VA health system as it is …
7. Surgery resident hours
8. Diagnostic laparotomy/laparoscopy
9. High jejunal resection in critically ill
10. Consults: Phone call or Text
To join communities, log in to ACS Communities at http://acscommunities.facs.org/home, go to “Browse All Communities” near the top of any page, and click the blue “Join” button next to the community you’d like to join. If you have any questions, please send them to [email protected].
Hemorrhagic Stroke Increases Risk of Depression and Subsequent Dementia
HOUSTON—Hemorrhagic stroke sharply increases the risk of new-onset depression which, in turn, is associated with a 30% increased risk of dementia within five years, according to research presented at the International Stroke Conference 2017.
“This is of great importance from a research and clinical standpoint, as it may represent a marker of ongoing cognitive deterioration,” said Dr. Biffi.
Hemorrhagic Stroke and Mood Disorders
Previous studies have found that patients with ICH have a significantly increased risk of mood disorders and cognitive decline. “There is probably a link between mood disorders and cognition after ICH, as is the case for a number of other neurologic conditions,” said Dr. Biffi. “Cerebrovascular small-vessel disease is likely to be involved in the underlying pathogenesis for these disorders, as it is also a risk factor for late-life depression in the general population. Therefore, depression and dementia after ICH may share some etiologic connections.”
Dr. Biffi and his colleagues enrolled 695 patients with ICH into their study and followed them for a mean of five years. None of the subjects had ever been diagnosed with a mood disorder or cognitive decline. The researchers conducted telephone interviews with patients every six months.
At baseline, investigators collected CT and MRI imaging data, epidemiologic exposure data, and apolipoprotein E4 genotype. The outcomes were new-onset depression and incident dementia.
Subjects had a mean age of 74 at baseline. In addition, approximately 70% of patients had hypertension, and 15% had heart disease. Less than 1% of the cohort was positive for the APOE e4 gene. Imaging-confirmed white matter disease was present in 65% of participants. During the follow-up period, new-onset depression developed in 278 (40%) patients. The temporal incidence of this outcome was consistent at about 7% per year.
Factors That Influenced Risk of Dementia
Researchers discovered that having more than a single copy of the APOE e4 allele at baseline (hazard ratio [HR], 1.7) and the presence of white matter disease at baseline (HR, 1.82), were significantly associated with new-onset depression. Having had at least 10 years of school protected against depression (HR, 0.75), as did functional independence (HR, 0.52). By the end of the follow-up period, dementia had developed in 80% of individuals with depression (220). In 81% of cases, depression preceded dementia, with an average time lag of 1.5 years, said Dr. Biffi.
In a multivariate analysis, several factors were significantly associated with incident dementia. Higher education reduced the risk by 40% (HR, 0.60). Factors that increased the risk of dementia were black race (HR, 1.48), APOE e4 gene (HR, 2.12), white matter disease (HR, 1.7), and poststroke new-onset depression (HR, 1.29). The study shows only association, said Dr. Biffi. “No causal relationship can be inferred by this study. We also cannot capture the severity of the mood symptoms, and we are unable to examine the relationship between cognition and apathy, which is another highly relevant neuropsychiatric manifestation of small-vessel disease,” he said.
—Michelle G. Sullivan
HOUSTON—Hemorrhagic stroke sharply increases the risk of new-onset depression which, in turn, is associated with a 30% increased risk of dementia within five years, according to research presented at the International Stroke Conference 2017.
“This is of great importance from a research and clinical standpoint, as it may represent a marker of ongoing cognitive deterioration,” said Dr. Biffi.
Hemorrhagic Stroke and Mood Disorders
Previous studies have found that patients with ICH have a significantly increased risk of mood disorders and cognitive decline. “There is probably a link between mood disorders and cognition after ICH, as is the case for a number of other neurologic conditions,” said Dr. Biffi. “Cerebrovascular small-vessel disease is likely to be involved in the underlying pathogenesis for these disorders, as it is also a risk factor for late-life depression in the general population. Therefore, depression and dementia after ICH may share some etiologic connections.”
Dr. Biffi and his colleagues enrolled 695 patients with ICH into their study and followed them for a mean of five years. None of the subjects had ever been diagnosed with a mood disorder or cognitive decline. The researchers conducted telephone interviews with patients every six months.
At baseline, investigators collected CT and MRI imaging data, epidemiologic exposure data, and apolipoprotein E4 genotype. The outcomes were new-onset depression and incident dementia.
Subjects had a mean age of 74 at baseline. In addition, approximately 70% of patients had hypertension, and 15% had heart disease. Less than 1% of the cohort was positive for the APOE e4 gene. Imaging-confirmed white matter disease was present in 65% of participants. During the follow-up period, new-onset depression developed in 278 (40%) patients. The temporal incidence of this outcome was consistent at about 7% per year.
Factors That Influenced Risk of Dementia
Researchers discovered that having more than a single copy of the APOE e4 allele at baseline (hazard ratio [HR], 1.7) and the presence of white matter disease at baseline (HR, 1.82), were significantly associated with new-onset depression. Having had at least 10 years of school protected against depression (HR, 0.75), as did functional independence (HR, 0.52). By the end of the follow-up period, dementia had developed in 80% of individuals with depression (220). In 81% of cases, depression preceded dementia, with an average time lag of 1.5 years, said Dr. Biffi.
In a multivariate analysis, several factors were significantly associated with incident dementia. Higher education reduced the risk by 40% (HR, 0.60). Factors that increased the risk of dementia were black race (HR, 1.48), APOE e4 gene (HR, 2.12), white matter disease (HR, 1.7), and poststroke new-onset depression (HR, 1.29). The study shows only association, said Dr. Biffi. “No causal relationship can be inferred by this study. We also cannot capture the severity of the mood symptoms, and we are unable to examine the relationship between cognition and apathy, which is another highly relevant neuropsychiatric manifestation of small-vessel disease,” he said.
—Michelle G. Sullivan
HOUSTON—Hemorrhagic stroke sharply increases the risk of new-onset depression which, in turn, is associated with a 30% increased risk of dementia within five years, according to research presented at the International Stroke Conference 2017.
“This is of great importance from a research and clinical standpoint, as it may represent a marker of ongoing cognitive deterioration,” said Dr. Biffi.
Hemorrhagic Stroke and Mood Disorders
Previous studies have found that patients with ICH have a significantly increased risk of mood disorders and cognitive decline. “There is probably a link between mood disorders and cognition after ICH, as is the case for a number of other neurologic conditions,” said Dr. Biffi. “Cerebrovascular small-vessel disease is likely to be involved in the underlying pathogenesis for these disorders, as it is also a risk factor for late-life depression in the general population. Therefore, depression and dementia after ICH may share some etiologic connections.”
Dr. Biffi and his colleagues enrolled 695 patients with ICH into their study and followed them for a mean of five years. None of the subjects had ever been diagnosed with a mood disorder or cognitive decline. The researchers conducted telephone interviews with patients every six months.
At baseline, investigators collected CT and MRI imaging data, epidemiologic exposure data, and apolipoprotein E4 genotype. The outcomes were new-onset depression and incident dementia.
Subjects had a mean age of 74 at baseline. In addition, approximately 70% of patients had hypertension, and 15% had heart disease. Less than 1% of the cohort was positive for the APOE e4 gene. Imaging-confirmed white matter disease was present in 65% of participants. During the follow-up period, new-onset depression developed in 278 (40%) patients. The temporal incidence of this outcome was consistent at about 7% per year.
Factors That Influenced Risk of Dementia
Researchers discovered that having more than a single copy of the APOE e4 allele at baseline (hazard ratio [HR], 1.7) and the presence of white matter disease at baseline (HR, 1.82), were significantly associated with new-onset depression. Having had at least 10 years of school protected against depression (HR, 0.75), as did functional independence (HR, 0.52). By the end of the follow-up period, dementia had developed in 80% of individuals with depression (220). In 81% of cases, depression preceded dementia, with an average time lag of 1.5 years, said Dr. Biffi.
In a multivariate analysis, several factors were significantly associated with incident dementia. Higher education reduced the risk by 40% (HR, 0.60). Factors that increased the risk of dementia were black race (HR, 1.48), APOE e4 gene (HR, 2.12), white matter disease (HR, 1.7), and poststroke new-onset depression (HR, 1.29). The study shows only association, said Dr. Biffi. “No causal relationship can be inferred by this study. We also cannot capture the severity of the mood symptoms, and we are unable to examine the relationship between cognition and apathy, which is another highly relevant neuropsychiatric manifestation of small-vessel disease,” he said.
—Michelle G. Sullivan
Removal of the Distal Aspect of a Broken Tibial Nail
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
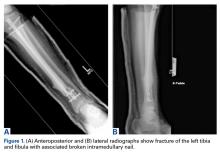
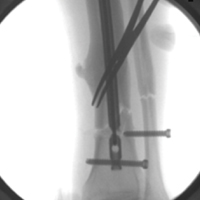
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
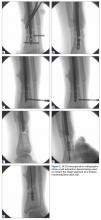
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
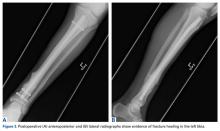
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Nail breakage is a known complication of intramedullary nail (IMN) fixation of tibial fractures.
- Several techniques have been described for broken IMN extraction.
Intramedullary nail (IMN) fixation is reliably used to manage tibial fractures and has become very popular for managing fractures of varying complexity.1-4 An occasional complication of intramedullary nailing is nail breakage,5-7 which can result from a fatigue fracture (from excessive fracture site instability caused by inadequate nail diameter, delayed fracture healing, or fracture nonunion) and direct traumatic impact.5-7 Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from tibias and femurs.4,8-16 In this article, we describe an efficient technique for extracting broken tibial IMNs—a technique that can be used before attempting more invasive extraction methods. The patient provided written informed consent for print and electronic publication of this case report.
Case Report and Surgical Technique
A 34-year-old male logger presented to our facility (Department of Orthopaedics, Warren Alpert School of Medicine, Brown University) with a new fracture of the left tibia and fibula with an associated broken IMN after a tree fell on his leg at work (Figures 1A, 1B).
The original IMN had been placed through a paramedian incision, with lateral to medial distal locking screws. The tibial shaft fracture and broken nail were displaced in the coronal plane (Figures 1A, 1B). For restoration of the central canal of the nail, closed reduction was performed in the operating room (Figure 2A). Once the fracture was reduced, the more proximal of the 2 distal interlocking screws was partially backed out so the extraction hook could be passed antegrade into the distal segment of the nail (Figure 2A).
A ball-tipped guide wire was then passed down again, and reaming was carried out distally to 11.5 mm. A new tibial nail (10 mm × 315 mm) was placed down the intramedullary canal over the guide wire. The tibia was derotated to obtain better anatomical alignment using the fracture as an osteotomy, and 2 new distal interlocking screws were placed. The nail was then back-slapped to obtain impaction, and a single proximal dynamic interlocking screw was placed.
After surgery, the patient was allowed a gradual weight-bearing protocol.
Discussion
IMN fixation of tibial fractures is reliable.1-4 An occasional complication of intramedullary nailing is nail breakage. Several case reports have described unique methods used to facilitate removal of broken hollow and solid IMNs from knees and femurs.4,8-16
Our patient’s case involved a cannulated tibial IMN that broke secondary to an acute traumatic event. Several techniques have been used to remove the distal segment of broken cannulated tibial IMNs.8,9,14,17 Abdelgawad and Kanlic8 described a technique in which a small distractor hook was introduced past the distal end of the broken distal piece, and a small (~2 in) piece of flexible nail was introduced into the slot of the distal interlocking screw hole. The hook was pulled back and became incarcerated in the nail by the flexible nail piece, allowing the hook to extract the distal segment of the nail.
Charnley and Farrington9 used Petelin laparoscopic grasping forceps to extract the distal segment of a broken cannulated tibial IMN under fluoroscopic guidance. This tibial canal was initially reamed before inserting the instrument and removing the distal segment of the nail.
Levine and Georgiadis14 used a 4.5-mm bit to drill a hole in the distal aspect of the medial malleolus. A smooth Steinmann pin was used to engage the tip of the IMN. The nail was hammered several centimeters up the medullary canal of the tibia. A 3.0-mm ball-tipped guide wire was inserted in the hole in the medial malleolus and advanced through the distal aspect of the nail under fluoroscopic guidance. The guide wire was advanced through the extent of the nail proximally until it emerged through the knee incision. The distal segment of the broken nail was extracted with the guide wire; the end of the guide wire with the ball engaged the distal aspect of the nail.
Our technique allowed us to use a nail extraction device to extract the distal segment of a broken tibial IMN. This device is usually on hand for routine nail extraction. We used the more distal of the 2 distal interlocking screws to push the extraction hook over the distal lip of the nail, allowing for extraction without additional incisions or additional drill holes in bone. Our technique was efficient in this particular situation and avoided more time-consuming extraction methods. In cases in which the extraction hook does not engage the distal aspect of the nail secondary to bone ingrowth, our technique should be used before attempting other extraction methods.
Am J Orthop. 2017;46(2):E112-E115. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
1. Bone LB, Kassman S, Stegemann P, France J. Prospective study of union rate of open tibial fractures treated with locked, unreamed intramedullary nails. J Orthop Trauma. 1994;8(1):45-49.
2. Blachut PA, O’Brien PJ, Meek RN, Broekhuyse HM. Interlocking intramedullary nailing with and without reaming for the treatment of closed fractures of the tibial shaft. A prospective, randomized study. J Bone Joint Surg Am. 1997;79(5):640-646.
3. Bonnevialle P, Savorit L, Combes JM, Rongières M, Bellumore Y, Mansat M. Value of intramedullary locked nailing in distal fractures of the tibia [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1996;82(5):428-436.
4. Polat A, Kose O, Canbora K, Yanık S, Guler F. Intramedullary nailing versus minimally invasive plate osteosynthesis for distal extra-articular tibial fractures: a prospective randomized clinical trial. J Orthop Sci. 2015;20(4):695-701.
5. Bucholz RW, Ross SE, Lawrence KL. Fatigue fracture of the interlocking nail in the treatment of fractures of the distal part of the femoral shaft. J Bone Joint Surg Am. 1987;69(9):1391-1399.
6. Zimmerman KW, Klasen HJ. Mechanical failure of intramedullary nails after fracture union. J Bone Joint Surg Br. 1983;65(3):274-275.
7. Hahn D, Bradbury N, Hartley R, Radford PJ. Intramedullary nail breakage in distal fractures of the tibia. Injury. 1996;27(5):323-327.
8. Abdelgawad AA, Kanlic E. Removal of a broken cannulated intramedullary nail: review of the literature and a case report of a new technique. Case Rep Orthop. 2013;2013:461703.
9. Charnley GJ, Farrington WJ. Laparoscopic forceps removal of a broken tibial intramedullary nail. Injury. 1998;29(6):489-490.
10. Georgilas I, Mouzopoulos G, Neila C, Morakis E, Tzurbakis M. Removal of broken distal intramedullary nail with a simple method: a case report. Arch Orthop Trauma Surg. 2008;129(2):203-205.
11. Giannoudis PV, Matthews SJ, Smith RM. Removal of the retained fragment of broken solid nails by the intra-medullary route. Injury. 2001;32(5):407-410.
12. Gosling T, Allami M, Koenemann B, Hankemeier S, Krettek C. Minimally invasive exchange tibial nailing for a broken solid nail: case report and description of a new technique. J Orthop Trauma. 2005;19(10):744-747.
13. Hellemondt FJ, Haeff MJ. Removal of a broken solid intramedullary interlocking nail. A technical note. Acta Orthop Scand. 1996;67(5):512.
14. Levine JW, Georgiadis GM. Removal of a broken cannulated tibial nail: a simple intramedullary technique. J Orthop Trauma. 2004;18(4):247-249.
15. Schmidgen A, Naumann O, Wentzensen A. A simple and rapid method for removal of broken unreamed tibial nails [in German]. Unfallchirurg. 1999;102(12):975-978.
16. Steinberg EL, Luger E, Menahem A, Helfet DL. Removal of a broken distal closed section intramedullary nail: report of a case using a simple method. J Orthop Trauma. 2004;18(4):233-235.
17. Marwan M, Ibrahim M. Simple method for retrieval of distal segment of the broken interlocking intramedullary nail. Injury. 1999;30(5):333-335.
Laparoscopic and abdominal hysterectomy yield equivalent survival
Laparoscopic hysterectomy yields equivalent disease-free and overall survival at 4.5 years, compared with abdominal hysterectomy in stage I endometrial cancer, according to a report published online March 28 in JAMA.
Several short-term advantages with the laparoscopic approach have been well documented, including less pain, less morbidity, better quality of life, decreased risk of surgery-related adverse events, and cost savings. But until now, no large international trial has demonstrated that longer-term survival outcomes are at least as good with laparoscopic as with open abdominal hysterectomy in this patient population, reported Monika Janda, PhD, of Queensland University of Technology, Brisbane (Australia) and her colleagues.
They conducted the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial, a randomized equivalence study involving 760 women treated at 20 medical centers in Australia, New Zealand, and Hong Kong during 2005-2010. The women were followed for a median of 4.5 years.
All of the women had histologically confirmed stage I adenocarcinoma of the endometrium. A total of 407 patients were randomly assigned to undergo total laparoscopic hysterectomy and 353 patients to undergo total abdominal hysterectomy. Medical comorbidities were equally distributed between the two study groups, and there were no significant between-group differences in tumor type, histologic grade, number of involved lymph nodes, or adjuvant treatments.
Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy, meeting the criteria for equivalence. Overall survival at 4.5 years was 92.0% and 92.4%, respectively. Cancer recurred near the operative site in 3% of each group and at a regional or distant site in 2% or less of each group. Causes of death also were similar between the two study groups, with 56% of all deaths attributed to endometrial cancer (JAMA. 2017;317[12]:1224-33).
Of note, two patients who underwent laparoscopic surgery developed port-site metastases and two patients who underwent abdominal surgery developed metastases at the site of the abdominal wound.
The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and numerous others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
This study adds to a growing body of literature that suggests laparoscopic hysterectomy is not only safe, but also the preferred modality of hysterectomy for women with endometrial cancer.
Despite the clear benefits of laparoscopic hysterectomy, the findings from the LACE trial should be interpreted in the context of the study design. Importantly, patients randomized to the study represent a highly select group of women with endometrial cancer. The study entry criteria involved a low-risk population of women with stage I tumors of endometrioid histology with a uterine size of less than 10 weeks’ gestation. In practice, laparoscopic hysterectomy is now routinely used for women with nonendometrioid histologies and in those with more advanced disease.
The LACE trial reported by Janda et al. provides confirmation that laparoscopic hysterectomy is a safe and effective treatment modality for women with early-stage endometrial cancer. The favorable short-term outcomes along with equivalent oncological outcomes make laparoscopic hysterectomy the preferred surgical modality in this setting. Even though the road to defining the benefits of laparoscopic hysterectomy has been long, efforts to promote the procedure for women with endometrial cancer should now be a priority.
Jason D. Wright, MD, is at the Herbert Irving Comprehensive Cancer Center and the department of ob.gyn. at Columbia University, New York. He reported having no relevant financial disclosures. These comments are excerpted from an accompanying editorial (JAMA 2017;317[12]:1215-6).
This study adds to a growing body of literature that suggests laparoscopic hysterectomy is not only safe, but also the preferred modality of hysterectomy for women with endometrial cancer.
Despite the clear benefits of laparoscopic hysterectomy, the findings from the LACE trial should be interpreted in the context of the study design. Importantly, patients randomized to the study represent a highly select group of women with endometrial cancer. The study entry criteria involved a low-risk population of women with stage I tumors of endometrioid histology with a uterine size of less than 10 weeks’ gestation. In practice, laparoscopic hysterectomy is now routinely used for women with nonendometrioid histologies and in those with more advanced disease.
The LACE trial reported by Janda et al. provides confirmation that laparoscopic hysterectomy is a safe and effective treatment modality for women with early-stage endometrial cancer. The favorable short-term outcomes along with equivalent oncological outcomes make laparoscopic hysterectomy the preferred surgical modality in this setting. Even though the road to defining the benefits of laparoscopic hysterectomy has been long, efforts to promote the procedure for women with endometrial cancer should now be a priority.
Jason D. Wright, MD, is at the Herbert Irving Comprehensive Cancer Center and the department of ob.gyn. at Columbia University, New York. He reported having no relevant financial disclosures. These comments are excerpted from an accompanying editorial (JAMA 2017;317[12]:1215-6).
This study adds to a growing body of literature that suggests laparoscopic hysterectomy is not only safe, but also the preferred modality of hysterectomy for women with endometrial cancer.
Despite the clear benefits of laparoscopic hysterectomy, the findings from the LACE trial should be interpreted in the context of the study design. Importantly, patients randomized to the study represent a highly select group of women with endometrial cancer. The study entry criteria involved a low-risk population of women with stage I tumors of endometrioid histology with a uterine size of less than 10 weeks’ gestation. In practice, laparoscopic hysterectomy is now routinely used for women with nonendometrioid histologies and in those with more advanced disease.
The LACE trial reported by Janda et al. provides confirmation that laparoscopic hysterectomy is a safe and effective treatment modality for women with early-stage endometrial cancer. The favorable short-term outcomes along with equivalent oncological outcomes make laparoscopic hysterectomy the preferred surgical modality in this setting. Even though the road to defining the benefits of laparoscopic hysterectomy has been long, efforts to promote the procedure for women with endometrial cancer should now be a priority.
Jason D. Wright, MD, is at the Herbert Irving Comprehensive Cancer Center and the department of ob.gyn. at Columbia University, New York. He reported having no relevant financial disclosures. These comments are excerpted from an accompanying editorial (JAMA 2017;317[12]:1215-6).
Laparoscopic hysterectomy yields equivalent disease-free and overall survival at 4.5 years, compared with abdominal hysterectomy in stage I endometrial cancer, according to a report published online March 28 in JAMA.
Several short-term advantages with the laparoscopic approach have been well documented, including less pain, less morbidity, better quality of life, decreased risk of surgery-related adverse events, and cost savings. But until now, no large international trial has demonstrated that longer-term survival outcomes are at least as good with laparoscopic as with open abdominal hysterectomy in this patient population, reported Monika Janda, PhD, of Queensland University of Technology, Brisbane (Australia) and her colleagues.
They conducted the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial, a randomized equivalence study involving 760 women treated at 20 medical centers in Australia, New Zealand, and Hong Kong during 2005-2010. The women were followed for a median of 4.5 years.
All of the women had histologically confirmed stage I adenocarcinoma of the endometrium. A total of 407 patients were randomly assigned to undergo total laparoscopic hysterectomy and 353 patients to undergo total abdominal hysterectomy. Medical comorbidities were equally distributed between the two study groups, and there were no significant between-group differences in tumor type, histologic grade, number of involved lymph nodes, or adjuvant treatments.
Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy, meeting the criteria for equivalence. Overall survival at 4.5 years was 92.0% and 92.4%, respectively. Cancer recurred near the operative site in 3% of each group and at a regional or distant site in 2% or less of each group. Causes of death also were similar between the two study groups, with 56% of all deaths attributed to endometrial cancer (JAMA. 2017;317[12]:1224-33).
Of note, two patients who underwent laparoscopic surgery developed port-site metastases and two patients who underwent abdominal surgery developed metastases at the site of the abdominal wound.
The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and numerous others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
Laparoscopic hysterectomy yields equivalent disease-free and overall survival at 4.5 years, compared with abdominal hysterectomy in stage I endometrial cancer, according to a report published online March 28 in JAMA.
Several short-term advantages with the laparoscopic approach have been well documented, including less pain, less morbidity, better quality of life, decreased risk of surgery-related adverse events, and cost savings. But until now, no large international trial has demonstrated that longer-term survival outcomes are at least as good with laparoscopic as with open abdominal hysterectomy in this patient population, reported Monika Janda, PhD, of Queensland University of Technology, Brisbane (Australia) and her colleagues.
They conducted the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial, a randomized equivalence study involving 760 women treated at 20 medical centers in Australia, New Zealand, and Hong Kong during 2005-2010. The women were followed for a median of 4.5 years.
All of the women had histologically confirmed stage I adenocarcinoma of the endometrium. A total of 407 patients were randomly assigned to undergo total laparoscopic hysterectomy and 353 patients to undergo total abdominal hysterectomy. Medical comorbidities were equally distributed between the two study groups, and there were no significant between-group differences in tumor type, histologic grade, number of involved lymph nodes, or adjuvant treatments.
Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy, meeting the criteria for equivalence. Overall survival at 4.5 years was 92.0% and 92.4%, respectively. Cancer recurred near the operative site in 3% of each group and at a regional or distant site in 2% or less of each group. Causes of death also were similar between the two study groups, with 56% of all deaths attributed to endometrial cancer (JAMA. 2017;317[12]:1224-33).
Of note, two patients who underwent laparoscopic surgery developed port-site metastases and two patients who underwent abdominal surgery developed metastases at the site of the abdominal wound.
The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and numerous others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
FROM JAMA
Key clinical point:
Major finding: Disease-free survival at 4.5 years was 81.6% with laparoscopic hysterectomy and 81.3% with abdominal hysterectomy.
Data source: An international, randomized, phase III equivalence trial involving 760 women treated with total abdominal or total laparoscopic hysterectomy.
Disclosures: The study was funded by Cancer Councils in Australia, the National Health and Medical Research Council, Cancer Australia, QLD Health, and others. Dr. Janda reported having no relevant financial disclosures; one of her coauthors reported ties to the O.R. Company, SurgicalPerformance Pty, and Covidien.
One peanut daily might maintain childhood immunotherapy gains
ATLANTA – One year or more after peanut immunotherapy, 27 of 33 (82%) children were eating peanuts regularly, most without problems, in a survey from the Children’s Hospital of Philadelphia.
The finding speaks to the durability of peanut immunotherapy, something that’s been a concern for physicians and families. It suggests that peanut immunotherapy might give children long-term protection from accidental exposure, so long as they continue to eat a small amount of peanut almost every day after desensitization.
She and her team surveyed the families of 15 children who completed a trial of epicutaneous peanut immunotherapy (EPIT) and 9 who completed a trial of oral immunotherapy (OIT) about a year after the studies ended. They also surveyed families of nine children about 2 years after they completed a trial of OIT plus omalizumab (Xolair) for peanut allergy. The investigators and families chose maintenance doses based on results from the final peanut challenges, and children were warned against running around within 2 hours of their dose, to prevent exercised-induced reactions.
The point of using omalizumab in the one trial was to see if it helped children ramp up immunotherapy more quickly and tolerate higher final peanut doses. It did, and the nine omalizumab children were on the highest maintenance doses of peanut at 2-year follow-up, with almost all of them consuming an average of at least one peanut a day. Perhaps because of that, three of the four anaphylactic reactions were in the omalizumab group.
The fourth reaction was in a child who completed the OIT trial. There was no anaphylaxis in EPIT children. About 60% in both groups reported eating an average of at least a peanut a day at 1-year follow-up.
About three-quarters of the children ate peanut-containing candy to get their maintenance dose. Others ate peanuts or peanut butter or sprinkled peanut flour on their food. Just six children, all from the EPIT cohort, said they liked the taste of peanuts.
Meanwhile, 6 of the 33 children (18%) – 1 in the omalizumab group, 3 in the EPIT arm, and 2 in the OIT group – refused to eat peanuts after their immunotherapy trials.
Posttrial peanut dosing ranged from once a month to daily, and the majority of subjects ate peanut about five times per week. All of the anaphylaxis children recovered without incident and resumed peanut maintenance. A couple of the children in the EPIT group had an itch in their throat when they switched to eating peanuts, but it resolved on its own. One child in the omalizumab group and one in the OIT group reported gastrointestinal symptoms with maintenance dosing.
The original trials funded the follow-up. Ms. Ott Lewis had no relevant financial disclosures.
ATLANTA – One year or more after peanut immunotherapy, 27 of 33 (82%) children were eating peanuts regularly, most without problems, in a survey from the Children’s Hospital of Philadelphia.
The finding speaks to the durability of peanut immunotherapy, something that’s been a concern for physicians and families. It suggests that peanut immunotherapy might give children long-term protection from accidental exposure, so long as they continue to eat a small amount of peanut almost every day after desensitization.
She and her team surveyed the families of 15 children who completed a trial of epicutaneous peanut immunotherapy (EPIT) and 9 who completed a trial of oral immunotherapy (OIT) about a year after the studies ended. They also surveyed families of nine children about 2 years after they completed a trial of OIT plus omalizumab (Xolair) for peanut allergy. The investigators and families chose maintenance doses based on results from the final peanut challenges, and children were warned against running around within 2 hours of their dose, to prevent exercised-induced reactions.
The point of using omalizumab in the one trial was to see if it helped children ramp up immunotherapy more quickly and tolerate higher final peanut doses. It did, and the nine omalizumab children were on the highest maintenance doses of peanut at 2-year follow-up, with almost all of them consuming an average of at least one peanut a day. Perhaps because of that, three of the four anaphylactic reactions were in the omalizumab group.
The fourth reaction was in a child who completed the OIT trial. There was no anaphylaxis in EPIT children. About 60% in both groups reported eating an average of at least a peanut a day at 1-year follow-up.
About three-quarters of the children ate peanut-containing candy to get their maintenance dose. Others ate peanuts or peanut butter or sprinkled peanut flour on their food. Just six children, all from the EPIT cohort, said they liked the taste of peanuts.
Meanwhile, 6 of the 33 children (18%) – 1 in the omalizumab group, 3 in the EPIT arm, and 2 in the OIT group – refused to eat peanuts after their immunotherapy trials.
Posttrial peanut dosing ranged from once a month to daily, and the majority of subjects ate peanut about five times per week. All of the anaphylaxis children recovered without incident and resumed peanut maintenance. A couple of the children in the EPIT group had an itch in their throat when they switched to eating peanuts, but it resolved on its own. One child in the omalizumab group and one in the OIT group reported gastrointestinal symptoms with maintenance dosing.
The original trials funded the follow-up. Ms. Ott Lewis had no relevant financial disclosures.
ATLANTA – One year or more after peanut immunotherapy, 27 of 33 (82%) children were eating peanuts regularly, most without problems, in a survey from the Children’s Hospital of Philadelphia.
The finding speaks to the durability of peanut immunotherapy, something that’s been a concern for physicians and families. It suggests that peanut immunotherapy might give children long-term protection from accidental exposure, so long as they continue to eat a small amount of peanut almost every day after desensitization.
She and her team surveyed the families of 15 children who completed a trial of epicutaneous peanut immunotherapy (EPIT) and 9 who completed a trial of oral immunotherapy (OIT) about a year after the studies ended. They also surveyed families of nine children about 2 years after they completed a trial of OIT plus omalizumab (Xolair) for peanut allergy. The investigators and families chose maintenance doses based on results from the final peanut challenges, and children were warned against running around within 2 hours of their dose, to prevent exercised-induced reactions.
The point of using omalizumab in the one trial was to see if it helped children ramp up immunotherapy more quickly and tolerate higher final peanut doses. It did, and the nine omalizumab children were on the highest maintenance doses of peanut at 2-year follow-up, with almost all of them consuming an average of at least one peanut a day. Perhaps because of that, three of the four anaphylactic reactions were in the omalizumab group.
The fourth reaction was in a child who completed the OIT trial. There was no anaphylaxis in EPIT children. About 60% in both groups reported eating an average of at least a peanut a day at 1-year follow-up.
About three-quarters of the children ate peanut-containing candy to get their maintenance dose. Others ate peanuts or peanut butter or sprinkled peanut flour on their food. Just six children, all from the EPIT cohort, said they liked the taste of peanuts.
Meanwhile, 6 of the 33 children (18%) – 1 in the omalizumab group, 3 in the EPIT arm, and 2 in the OIT group – refused to eat peanuts after their immunotherapy trials.
Posttrial peanut dosing ranged from once a month to daily, and the majority of subjects ate peanut about five times per week. All of the anaphylaxis children recovered without incident and resumed peanut maintenance. A couple of the children in the EPIT group had an itch in their throat when they switched to eating peanuts, but it resolved on its own. One child in the omalizumab group and one in the OIT group reported gastrointestinal symptoms with maintenance dosing.
The original trials funded the follow-up. Ms. Ott Lewis had no relevant financial disclosures.
Key clinical point: Eating more than that might increase the risk of reaction.
Major finding: There were four anaphylactic reactions, three of which were among children on the highest maintenance doses.
Data source: Follow-up surveys a year or more after peanut desensitization trials in 33 children.
Disclosures: The original trials funded the follow-up. The lead investigator had no relevant financial disclosures.
How to Manage the Risks Associated With Epilepsy and Pregnancy
Taking Precautions Before Pregnancy
“In keeping with the CDC recommendations to help prevent spina bifida and other birth defects, we should encourage women with epilepsy to start taking folate at menarche and to keep it up through menopause or until they have a hysterectomy or tubal ligation,” Dr. Meador said. He pointed out that half of pregnancies in the US are not planned, whether the woman is married or not. “So, if you wait for a woman to say, ‘I want to get pregnant,’ before you address AED pregnancy risks and the need for folate supplementation, you are going to miss half of the children who may be adversely affected.”
Among women with epilepsy, folate may confer additional benefits. In the Neurodevelopmental Effects of AEDs (NEAD) study, Dr. Meador and colleagues found that mean IQ was 6 points higher at age 6 in children whose mothers had taken periconceptional folate than in those whose mothers had not. Data from NEAD, a prospective, observational, multicenter study in the US and UK, have been used to assess various outcomes in children born to women with epilepsy taking carbamazepine, lamotrigine, phenytoin, or valproate monotherapy during pregnancy who were enrolled between October 1999 and February 2004.
For women who do not want to become pregnant, it is important to note that carbamazepine, phenobarbital, phenytoin, topiramate at doses greater than 200 mg, oxcarbazepine at doses greater than 1,200 mg, and other AEDs may lessen the effect of hormonal contraceptives. Some drugs, such as clonazepam, levetiracetam, and lamotrigine, do not significantly affect blood levels of hormonal contraceptive agents, Dr. Meador said. Estradiol has been shown to lower blood levels of lamotrigine, and while valproate does not appear to interact significantly with contraceptive agents, it can interact with other drugs.
Hormonal contraception may increase seizure rates significantly across all AED categories, compared with nonhormonal contraception, according to the preliminary findings of the Epilepsy Birth Control Registry. Compared with combined pills, both hormonal patch and progestin-only pills had greater risk ratios for seizure increase.
“My favorite reversible contraception for women with epilepsy is the intrauterine device, because it does not have systemic effects, and AEDs do not interfere with it,” Dr. Meador said.
AED Clearance During Pregnancy
Pregnancy appears to affect blood levels of AEDs. In a retrospective analysis of 115 pregnancies in 95 women with epilepsy, significant changes in clearance during pregnancy were observed for lamotrigine and levetiracetam, with average peak clearance increases of 191% and 207%, respectively. The investigators recommended the monitoring of serum AED concentrations in pregnant women who have epilepsy, with dosage adjustments as needed. “My approach is to assess blood levels either before pregnancy or early on, to draw blood monthly, and to maintain the blood level at the preconception level if they were seizure-free prior to pregnancy,” Dr. Meador said. “AED dosages should be adjusted within seven to 10 days after the baby is delivered to avoid toxicity, because the metabolism goes back to normal.”
Congenital Malformations
Although the majority of children born to women with epilepsy are healthy, congenital malformations associated with fetal exposure to AEDs can include heart defects, orofacial clefts, and skeletal, urologic, and neural tube defects, Dr. Meador pointed out. The European and International Registry of AEDs in Pregnancy showed an increase in malformation rates with increasing dose at the time of conception of monotherapy with carbamazepine, lamotrigine, valproic acid, and phenobarbital. Lamotrigine at doses lower than 300 mg/day and carbamazepine at doses lower than 400 mg/day had significantly lower risks of malformations than did valproic acid and phenobarbital at all investigated doses, and low-dose lamotrigine had lower risks than carbamazepine had at doses of 400 mg/day or greater.
A review of studies using the European Surveillance of Congenital Anomalies database showed a 2.6 odds ratio for spina bifida with fetal exposure to carbamazepine during the first trimester, compared with no use of an AED. The odds ratio for spina bifida after valproate exposure was 12.7, compared with no use of an AED. Fetal exposure to valproate also was associated with significantly increased odds for atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis.
“We made many advances in the past 20 years in our knowledge of congenital malformations through these epilepsy registries around the world,” Dr. Meador said.
“I encourage physicians to urge women, especially those on new anticonvulsant drugs or who are on polytherapy, to join the North American AED Pregnancy Registry.”
Cognitive Defects
In the NEAD study, the mean IQ of 3-year-olds who had been exposed in utero to valproate was 9 points lower than that of children exposed in utero to lamotrigine, 7 points lower than that of children exposed in utero to phenytoin, and 6 points lower than that of children exposed in utero to carbamazepine. The association between valproate exposure and IQ was dose-dependent. “Verbal abilities were lower for valproate, compared with the other three drugs,” Dr. Meador said. “Children exposed to lamotrigine had better nonverbal abilities than those exposed to valproate, and the other two drugs had a trend in the same direction.”
At age 6, the same children who had been exposed to valproate did poorly on measures of verbal and memory abilities, compared with children exposed to the other AEDs, and on nonverbal and executive functions, compared with children exposed to lamotrigine (but not compared with children exposed to carbamazepine or phenytoin). High doses of valproate were negatively associated with IQ, verbal abilities, nonverbal abilities, memory, and executive function, but other AEDs were not. IQ at age 6 correlated with IQ at younger ages, and IQ improved with age for infants exposed to any AED.
Breastfeeding
Research has established that breastfeeding is beneficial for mother and child. “A concern has been raised that there is a risk that exposure to AEDs in breast milk might cause damage to the baby,” Dr. Meador remarked. To analyze that possibility, he and his colleagues examined data for the 42.9% of study children who were breastfed for a mean of 7.2 months. There were no differences in breastfeeding rates and duration between drugs. Although more study is needed to fully delineate the effects of all AEDs, the adjusted IQ was 4 points higher at age 6 for breastfed children than for children who were not breastfed, and verbal abilities were greater, as well.
—Adriene Marshall
Taking Precautions Before Pregnancy
“In keeping with the CDC recommendations to help prevent spina bifida and other birth defects, we should encourage women with epilepsy to start taking folate at menarche and to keep it up through menopause or until they have a hysterectomy or tubal ligation,” Dr. Meador said. He pointed out that half of pregnancies in the US are not planned, whether the woman is married or not. “So, if you wait for a woman to say, ‘I want to get pregnant,’ before you address AED pregnancy risks and the need for folate supplementation, you are going to miss half of the children who may be adversely affected.”
Among women with epilepsy, folate may confer additional benefits. In the Neurodevelopmental Effects of AEDs (NEAD) study, Dr. Meador and colleagues found that mean IQ was 6 points higher at age 6 in children whose mothers had taken periconceptional folate than in those whose mothers had not. Data from NEAD, a prospective, observational, multicenter study in the US and UK, have been used to assess various outcomes in children born to women with epilepsy taking carbamazepine, lamotrigine, phenytoin, or valproate monotherapy during pregnancy who were enrolled between October 1999 and February 2004.
For women who do not want to become pregnant, it is important to note that carbamazepine, phenobarbital, phenytoin, topiramate at doses greater than 200 mg, oxcarbazepine at doses greater than 1,200 mg, and other AEDs may lessen the effect of hormonal contraceptives. Some drugs, such as clonazepam, levetiracetam, and lamotrigine, do not significantly affect blood levels of hormonal contraceptive agents, Dr. Meador said. Estradiol has been shown to lower blood levels of lamotrigine, and while valproate does not appear to interact significantly with contraceptive agents, it can interact with other drugs.
Hormonal contraception may increase seizure rates significantly across all AED categories, compared with nonhormonal contraception, according to the preliminary findings of the Epilepsy Birth Control Registry. Compared with combined pills, both hormonal patch and progestin-only pills had greater risk ratios for seizure increase.
“My favorite reversible contraception for women with epilepsy is the intrauterine device, because it does not have systemic effects, and AEDs do not interfere with it,” Dr. Meador said.
AED Clearance During Pregnancy
Pregnancy appears to affect blood levels of AEDs. In a retrospective analysis of 115 pregnancies in 95 women with epilepsy, significant changes in clearance during pregnancy were observed for lamotrigine and levetiracetam, with average peak clearance increases of 191% and 207%, respectively. The investigators recommended the monitoring of serum AED concentrations in pregnant women who have epilepsy, with dosage adjustments as needed. “My approach is to assess blood levels either before pregnancy or early on, to draw blood monthly, and to maintain the blood level at the preconception level if they were seizure-free prior to pregnancy,” Dr. Meador said. “AED dosages should be adjusted within seven to 10 days after the baby is delivered to avoid toxicity, because the metabolism goes back to normal.”
Congenital Malformations
Although the majority of children born to women with epilepsy are healthy, congenital malformations associated with fetal exposure to AEDs can include heart defects, orofacial clefts, and skeletal, urologic, and neural tube defects, Dr. Meador pointed out. The European and International Registry of AEDs in Pregnancy showed an increase in malformation rates with increasing dose at the time of conception of monotherapy with carbamazepine, lamotrigine, valproic acid, and phenobarbital. Lamotrigine at doses lower than 300 mg/day and carbamazepine at doses lower than 400 mg/day had significantly lower risks of malformations than did valproic acid and phenobarbital at all investigated doses, and low-dose lamotrigine had lower risks than carbamazepine had at doses of 400 mg/day or greater.
A review of studies using the European Surveillance of Congenital Anomalies database showed a 2.6 odds ratio for spina bifida with fetal exposure to carbamazepine during the first trimester, compared with no use of an AED. The odds ratio for spina bifida after valproate exposure was 12.7, compared with no use of an AED. Fetal exposure to valproate also was associated with significantly increased odds for atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis.
“We made many advances in the past 20 years in our knowledge of congenital malformations through these epilepsy registries around the world,” Dr. Meador said.
“I encourage physicians to urge women, especially those on new anticonvulsant drugs or who are on polytherapy, to join the North American AED Pregnancy Registry.”
Cognitive Defects
In the NEAD study, the mean IQ of 3-year-olds who had been exposed in utero to valproate was 9 points lower than that of children exposed in utero to lamotrigine, 7 points lower than that of children exposed in utero to phenytoin, and 6 points lower than that of children exposed in utero to carbamazepine. The association between valproate exposure and IQ was dose-dependent. “Verbal abilities were lower for valproate, compared with the other three drugs,” Dr. Meador said. “Children exposed to lamotrigine had better nonverbal abilities than those exposed to valproate, and the other two drugs had a trend in the same direction.”
At age 6, the same children who had been exposed to valproate did poorly on measures of verbal and memory abilities, compared with children exposed to the other AEDs, and on nonverbal and executive functions, compared with children exposed to lamotrigine (but not compared with children exposed to carbamazepine or phenytoin). High doses of valproate were negatively associated with IQ, verbal abilities, nonverbal abilities, memory, and executive function, but other AEDs were not. IQ at age 6 correlated with IQ at younger ages, and IQ improved with age for infants exposed to any AED.
Breastfeeding
Research has established that breastfeeding is beneficial for mother and child. “A concern has been raised that there is a risk that exposure to AEDs in breast milk might cause damage to the baby,” Dr. Meador remarked. To analyze that possibility, he and his colleagues examined data for the 42.9% of study children who were breastfed for a mean of 7.2 months. There were no differences in breastfeeding rates and duration between drugs. Although more study is needed to fully delineate the effects of all AEDs, the adjusted IQ was 4 points higher at age 6 for breastfed children than for children who were not breastfed, and verbal abilities were greater, as well.
—Adriene Marshall
Taking Precautions Before Pregnancy
“In keeping with the CDC recommendations to help prevent spina bifida and other birth defects, we should encourage women with epilepsy to start taking folate at menarche and to keep it up through menopause or until they have a hysterectomy or tubal ligation,” Dr. Meador said. He pointed out that half of pregnancies in the US are not planned, whether the woman is married or not. “So, if you wait for a woman to say, ‘I want to get pregnant,’ before you address AED pregnancy risks and the need for folate supplementation, you are going to miss half of the children who may be adversely affected.”
Among women with epilepsy, folate may confer additional benefits. In the Neurodevelopmental Effects of AEDs (NEAD) study, Dr. Meador and colleagues found that mean IQ was 6 points higher at age 6 in children whose mothers had taken periconceptional folate than in those whose mothers had not. Data from NEAD, a prospective, observational, multicenter study in the US and UK, have been used to assess various outcomes in children born to women with epilepsy taking carbamazepine, lamotrigine, phenytoin, or valproate monotherapy during pregnancy who were enrolled between October 1999 and February 2004.
For women who do not want to become pregnant, it is important to note that carbamazepine, phenobarbital, phenytoin, topiramate at doses greater than 200 mg, oxcarbazepine at doses greater than 1,200 mg, and other AEDs may lessen the effect of hormonal contraceptives. Some drugs, such as clonazepam, levetiracetam, and lamotrigine, do not significantly affect blood levels of hormonal contraceptive agents, Dr. Meador said. Estradiol has been shown to lower blood levels of lamotrigine, and while valproate does not appear to interact significantly with contraceptive agents, it can interact with other drugs.
Hormonal contraception may increase seizure rates significantly across all AED categories, compared with nonhormonal contraception, according to the preliminary findings of the Epilepsy Birth Control Registry. Compared with combined pills, both hormonal patch and progestin-only pills had greater risk ratios for seizure increase.
“My favorite reversible contraception for women with epilepsy is the intrauterine device, because it does not have systemic effects, and AEDs do not interfere with it,” Dr. Meador said.
AED Clearance During Pregnancy
Pregnancy appears to affect blood levels of AEDs. In a retrospective analysis of 115 pregnancies in 95 women with epilepsy, significant changes in clearance during pregnancy were observed for lamotrigine and levetiracetam, with average peak clearance increases of 191% and 207%, respectively. The investigators recommended the monitoring of serum AED concentrations in pregnant women who have epilepsy, with dosage adjustments as needed. “My approach is to assess blood levels either before pregnancy or early on, to draw blood monthly, and to maintain the blood level at the preconception level if they were seizure-free prior to pregnancy,” Dr. Meador said. “AED dosages should be adjusted within seven to 10 days after the baby is delivered to avoid toxicity, because the metabolism goes back to normal.”
Congenital Malformations
Although the majority of children born to women with epilepsy are healthy, congenital malformations associated with fetal exposure to AEDs can include heart defects, orofacial clefts, and skeletal, urologic, and neural tube defects, Dr. Meador pointed out. The European and International Registry of AEDs in Pregnancy showed an increase in malformation rates with increasing dose at the time of conception of monotherapy with carbamazepine, lamotrigine, valproic acid, and phenobarbital. Lamotrigine at doses lower than 300 mg/day and carbamazepine at doses lower than 400 mg/day had significantly lower risks of malformations than did valproic acid and phenobarbital at all investigated doses, and low-dose lamotrigine had lower risks than carbamazepine had at doses of 400 mg/day or greater.
A review of studies using the European Surveillance of Congenital Anomalies database showed a 2.6 odds ratio for spina bifida with fetal exposure to carbamazepine during the first trimester, compared with no use of an AED. The odds ratio for spina bifida after valproate exposure was 12.7, compared with no use of an AED. Fetal exposure to valproate also was associated with significantly increased odds for atrial septal defect, cleft palate, hypospadias, polydactyly, and craniosynostosis.
“We made many advances in the past 20 years in our knowledge of congenital malformations through these epilepsy registries around the world,” Dr. Meador said.
“I encourage physicians to urge women, especially those on new anticonvulsant drugs or who are on polytherapy, to join the North American AED Pregnancy Registry.”
Cognitive Defects
In the NEAD study, the mean IQ of 3-year-olds who had been exposed in utero to valproate was 9 points lower than that of children exposed in utero to lamotrigine, 7 points lower than that of children exposed in utero to phenytoin, and 6 points lower than that of children exposed in utero to carbamazepine. The association between valproate exposure and IQ was dose-dependent. “Verbal abilities were lower for valproate, compared with the other three drugs,” Dr. Meador said. “Children exposed to lamotrigine had better nonverbal abilities than those exposed to valproate, and the other two drugs had a trend in the same direction.”
At age 6, the same children who had been exposed to valproate did poorly on measures of verbal and memory abilities, compared with children exposed to the other AEDs, and on nonverbal and executive functions, compared with children exposed to lamotrigine (but not compared with children exposed to carbamazepine or phenytoin). High doses of valproate were negatively associated with IQ, verbal abilities, nonverbal abilities, memory, and executive function, but other AEDs were not. IQ at age 6 correlated with IQ at younger ages, and IQ improved with age for infants exposed to any AED.
Breastfeeding
Research has established that breastfeeding is beneficial for mother and child. “A concern has been raised that there is a risk that exposure to AEDs in breast milk might cause damage to the baby,” Dr. Meador remarked. To analyze that possibility, he and his colleagues examined data for the 42.9% of study children who were breastfed for a mean of 7.2 months. There were no differences in breastfeeding rates and duration between drugs. Although more study is needed to fully delineate the effects of all AEDs, the adjusted IQ was 4 points higher at age 6 for breastfed children than for children who were not breastfed, and verbal abilities were greater, as well.
—Adriene Marshall