User login
EHR price alert doesn’t reduce lab orders
Displaying Medicare allowable fees in the electronic health record at the time of order entry did not significantly reduce the number of inpatient lab tests at three Philadelphia hospitals.
In a study involving 98,529 patients and 142,921 admissions, Medicare payment information popped up randomly in the EHR when standard tests including complete blood cell counts, metabolic panels, and liver function tests were ordered. The costs of the labs varied depending on their extent. The message mentioned that “the dollar amount represents Medicare reimbursement for the test. Actual costs to the consumer may vary by patient insurance status.” Just over a third of the patients were actually on Medicare; most had private insurance.
The idea of the study was to see if cost information would curb unnecessary testing, and save money. “There is growing interest in using price transparency to influence medical decision-making toward higher value care,” Mina Sedrak, MD, and her colleagues said in a paper presented at the annual meeting of the Society of General Internal Medicine.
It didn’t work out that way. Four tests ordered per patient day when the messages appeared, and 2.34 when they did not. With messaging, the mean lab fee per patient day was $38.85, versus $27.59 without it. In an adjusted analyses comparing the intervention to the control group, there were no significant changes in overall test ordering (0.05 tests ordered per patient day, P = .06) or associated fees when pricing information was displayed ($0.24 per patient day; P = .47).
In a subset analysis, the investigators did find a small decrease orders for the most expensive labs and a small but significant increase in orders for the least expensive ones when physicians aware of cost (top quartile of tests based on fee value: -0.01; P = .04; bottom quartile: 0.03, P = .04).
Despite the overall negative results, there’s still a likely role for cost information in value improvement programs; what the study shows is that there’s a better way to use it, according to Dr. Sedrak, currently of the City of Hope Comprehensive Cancer Center in Duarte, Calif., and colleagues.
The investigators made several suggestions when reviewing their work.
“First, the price transparency intervention in this study was always displayed regardless of the clinical scenario. The presence of this information for appropriate tests may have diminished its impact when tests were inappropriate. Future efforts may consider more selective targeting of price transparency.” It might also be a good idea to price out different testing options for providers, and use actual charges and other more on-point forms of cost estimates, they said, instead of Medicare fees that have little to do with what many patients are actually charged. Targeting only the most expensive tests might also help (JAMA Intern Med. 2017 April 21. doi: 10.1001/jamainternmed.2017.1144).
The investigators also noticed a problem when labs are ordered to repeat automatically; clinicians did not see the price information every day, and so missed cost information “when it would be most salient.”
The mean age in the study was 54.7 years; 52% of the patients were white, 39% black, and 57% women. The mean length of stay was about 6 days, and over 80% of the patients were discharged home.
The authors evaluated what happens when one randomizes which tests have price information; other studies examine what happens when one randomizes which physicians have price information. All contemporary studies conclude the same – no effect of price information on physician ordering behavior.
One possible conclusion is that making health care prices available at the point of care is not an effective strategy to decrease wasteful spending, yet we believe this is not the case.
The disconnect suggests that current price transparency initiatives are not enough to infuse clinical care with price information and encourage consumers and physicians to consider the value of health care decisions. This does not mean we should give up on increased price transparency in health care. Rather, a more thoughtful approach to the design, point of delivery, and context for health care price information is needed to achieve the promise of price transparency.
Little has been done to deliver [out-of-pocket cost] information to patients at the time when patients are making health care decisions. Also, if both patients and physicians could see prices for episodes or bundles of care, then it could allow them to assess value together. Future interventions need to deliver price and quality information together.
Anna Sinaiko, PhD, is a research scientist at the Harvard School of Public Health, Boston. Alyna Chien, MD, is an assistant professor of pediatrics at the Harvard Medical School and a healthcare quality researcher. They made their comments in an editorial, and had no relevant disclosures (JAMA Intern Med. 2017 April 21. doi: 10.1001/jamainternmed.2017.1676 ).
The authors evaluated what happens when one randomizes which tests have price information; other studies examine what happens when one randomizes which physicians have price information. All contemporary studies conclude the same – no effect of price information on physician ordering behavior.
One possible conclusion is that making health care prices available at the point of care is not an effective strategy to decrease wasteful spending, yet we believe this is not the case.
The disconnect suggests that current price transparency initiatives are not enough to infuse clinical care with price information and encourage consumers and physicians to consider the value of health care decisions. This does not mean we should give up on increased price transparency in health care. Rather, a more thoughtful approach to the design, point of delivery, and context for health care price information is needed to achieve the promise of price transparency.
Little has been done to deliver [out-of-pocket cost] information to patients at the time when patients are making health care decisions. Also, if both patients and physicians could see prices for episodes or bundles of care, then it could allow them to assess value together. Future interventions need to deliver price and quality information together.
Anna Sinaiko, PhD, is a research scientist at the Harvard School of Public Health, Boston. Alyna Chien, MD, is an assistant professor of pediatrics at the Harvard Medical School and a healthcare quality researcher. They made their comments in an editorial, and had no relevant disclosures (JAMA Intern Med. 2017 April 21. doi: 10.1001/jamainternmed.2017.1676 ).
The authors evaluated what happens when one randomizes which tests have price information; other studies examine what happens when one randomizes which physicians have price information. All contemporary studies conclude the same – no effect of price information on physician ordering behavior.
One possible conclusion is that making health care prices available at the point of care is not an effective strategy to decrease wasteful spending, yet we believe this is not the case.
The disconnect suggests that current price transparency initiatives are not enough to infuse clinical care with price information and encourage consumers and physicians to consider the value of health care decisions. This does not mean we should give up on increased price transparency in health care. Rather, a more thoughtful approach to the design, point of delivery, and context for health care price information is needed to achieve the promise of price transparency.
Little has been done to deliver [out-of-pocket cost] information to patients at the time when patients are making health care decisions. Also, if both patients and physicians could see prices for episodes or bundles of care, then it could allow them to assess value together. Future interventions need to deliver price and quality information together.
Anna Sinaiko, PhD, is a research scientist at the Harvard School of Public Health, Boston. Alyna Chien, MD, is an assistant professor of pediatrics at the Harvard Medical School and a healthcare quality researcher. They made their comments in an editorial, and had no relevant disclosures (JAMA Intern Med. 2017 April 21. doi: 10.1001/jamainternmed.2017.1676 ).
Displaying Medicare allowable fees in the electronic health record at the time of order entry did not significantly reduce the number of inpatient lab tests at three Philadelphia hospitals.
In a study involving 98,529 patients and 142,921 admissions, Medicare payment information popped up randomly in the EHR when standard tests including complete blood cell counts, metabolic panels, and liver function tests were ordered. The costs of the labs varied depending on their extent. The message mentioned that “the dollar amount represents Medicare reimbursement for the test. Actual costs to the consumer may vary by patient insurance status.” Just over a third of the patients were actually on Medicare; most had private insurance.
The idea of the study was to see if cost information would curb unnecessary testing, and save money. “There is growing interest in using price transparency to influence medical decision-making toward higher value care,” Mina Sedrak, MD, and her colleagues said in a paper presented at the annual meeting of the Society of General Internal Medicine.
It didn’t work out that way. Four tests ordered per patient day when the messages appeared, and 2.34 when they did not. With messaging, the mean lab fee per patient day was $38.85, versus $27.59 without it. In an adjusted analyses comparing the intervention to the control group, there were no significant changes in overall test ordering (0.05 tests ordered per patient day, P = .06) or associated fees when pricing information was displayed ($0.24 per patient day; P = .47).
In a subset analysis, the investigators did find a small decrease orders for the most expensive labs and a small but significant increase in orders for the least expensive ones when physicians aware of cost (top quartile of tests based on fee value: -0.01; P = .04; bottom quartile: 0.03, P = .04).
Despite the overall negative results, there’s still a likely role for cost information in value improvement programs; what the study shows is that there’s a better way to use it, according to Dr. Sedrak, currently of the City of Hope Comprehensive Cancer Center in Duarte, Calif., and colleagues.
The investigators made several suggestions when reviewing their work.
“First, the price transparency intervention in this study was always displayed regardless of the clinical scenario. The presence of this information for appropriate tests may have diminished its impact when tests were inappropriate. Future efforts may consider more selective targeting of price transparency.” It might also be a good idea to price out different testing options for providers, and use actual charges and other more on-point forms of cost estimates, they said, instead of Medicare fees that have little to do with what many patients are actually charged. Targeting only the most expensive tests might also help (JAMA Intern Med. 2017 April 21. doi: 10.1001/jamainternmed.2017.1144).
The investigators also noticed a problem when labs are ordered to repeat automatically; clinicians did not see the price information every day, and so missed cost information “when it would be most salient.”
The mean age in the study was 54.7 years; 52% of the patients were white, 39% black, and 57% women. The mean length of stay was about 6 days, and over 80% of the patients were discharged home.
Displaying Medicare allowable fees in the electronic health record at the time of order entry did not significantly reduce the number of inpatient lab tests at three Philadelphia hospitals.
In a study involving 98,529 patients and 142,921 admissions, Medicare payment information popped up randomly in the EHR when standard tests including complete blood cell counts, metabolic panels, and liver function tests were ordered. The costs of the labs varied depending on their extent. The message mentioned that “the dollar amount represents Medicare reimbursement for the test. Actual costs to the consumer may vary by patient insurance status.” Just over a third of the patients were actually on Medicare; most had private insurance.
The idea of the study was to see if cost information would curb unnecessary testing, and save money. “There is growing interest in using price transparency to influence medical decision-making toward higher value care,” Mina Sedrak, MD, and her colleagues said in a paper presented at the annual meeting of the Society of General Internal Medicine.
It didn’t work out that way. Four tests ordered per patient day when the messages appeared, and 2.34 when they did not. With messaging, the mean lab fee per patient day was $38.85, versus $27.59 without it. In an adjusted analyses comparing the intervention to the control group, there were no significant changes in overall test ordering (0.05 tests ordered per patient day, P = .06) or associated fees when pricing information was displayed ($0.24 per patient day; P = .47).
In a subset analysis, the investigators did find a small decrease orders for the most expensive labs and a small but significant increase in orders for the least expensive ones when physicians aware of cost (top quartile of tests based on fee value: -0.01; P = .04; bottom quartile: 0.03, P = .04).
Despite the overall negative results, there’s still a likely role for cost information in value improvement programs; what the study shows is that there’s a better way to use it, according to Dr. Sedrak, currently of the City of Hope Comprehensive Cancer Center in Duarte, Calif., and colleagues.
The investigators made several suggestions when reviewing their work.
“First, the price transparency intervention in this study was always displayed regardless of the clinical scenario. The presence of this information for appropriate tests may have diminished its impact when tests were inappropriate. Future efforts may consider more selective targeting of price transparency.” It might also be a good idea to price out different testing options for providers, and use actual charges and other more on-point forms of cost estimates, they said, instead of Medicare fees that have little to do with what many patients are actually charged. Targeting only the most expensive tests might also help (JAMA Intern Med. 2017 April 21. doi: 10.1001/jamainternmed.2017.1144).
The investigators also noticed a problem when labs are ordered to repeat automatically; clinicians did not see the price information every day, and so missed cost information “when it would be most salient.”
The mean age in the study was 54.7 years; 52% of the patients were white, 39% black, and 57% women. The mean length of stay was about 6 days, and over 80% of the patients were discharged home.
Key clinical point:
Major finding: There were no significant changes in overall test ordering (0.05 tests ordered per patient day, P = .06) or associated fees when pricing information was displayed ($0.24 per patient day; P = .47).
Data source: Analysis involving 98,529 patients and 142,921 admissions of the effect of Medicare reimbursement information on lab test ordering
Disclosures: This study was funded by the University of Pennsylvania Health System. The senior investigator Mitesh Patel, MD, an assistant professor of medicine at the University of Pennsylvania, Philadelphia, is a principal at Catalyst Health, a technology and behavioral change consulting firm. The authors had no other disclosures.
HIV demographics shifting in U.S.
The number of people living with diagnosed HIV in the United States will increase to 1,232,054 by 2045, up from 917,294 in 2013, according to projections based on the National HIV Surveillance System and U.S. census data.
The number of people with HIV over age 55 will more than double from 232,113 to 470,221, though the annual growth rate will slow from 1.8% to 0.8%. By 2025, older people will comprise about 38% of the entire HIV population, said investigators led by epidemiologist Julia Hood, of the University of Washington Department of Epidemiology, Seattle.
The number of Hispanic and black people living with HIV is projected to grow consistently, shifting the demographics of people living with HIV from 32% to 23% white; 42% to 38% black; and 20% to 32% Hispanic by 2045 (AIDS Care. 2017 Apr 10:1-8).
The growing number of black patients “reflects disparities in HIV incidence; while among Hispanics/ Latinos, the growing number … is largely driven by immigration. These results underscore the importance of expanding coverage of HIV care, treatment, and prevention programs to ethnic/racial minorities, ensuring such programs are culturally and linguistically appropriate,” they said.
Overall, “these changing population dynamics should be considered in current decisions about resource allocation, human resource needs, HIV-prevention programs, and design of research studies.” However, “major technological advancements in HIV prevention (e.g., vaccines) and cure could occur in future decades, potentially nullifying the applicability of our projections,” the investigators said.
The authors had no conflicts of interest.
The number of people living with diagnosed HIV in the United States will increase to 1,232,054 by 2045, up from 917,294 in 2013, according to projections based on the National HIV Surveillance System and U.S. census data.
The number of people with HIV over age 55 will more than double from 232,113 to 470,221, though the annual growth rate will slow from 1.8% to 0.8%. By 2025, older people will comprise about 38% of the entire HIV population, said investigators led by epidemiologist Julia Hood, of the University of Washington Department of Epidemiology, Seattle.
The number of Hispanic and black people living with HIV is projected to grow consistently, shifting the demographics of people living with HIV from 32% to 23% white; 42% to 38% black; and 20% to 32% Hispanic by 2045 (AIDS Care. 2017 Apr 10:1-8).
The growing number of black patients “reflects disparities in HIV incidence; while among Hispanics/ Latinos, the growing number … is largely driven by immigration. These results underscore the importance of expanding coverage of HIV care, treatment, and prevention programs to ethnic/racial minorities, ensuring such programs are culturally and linguistically appropriate,” they said.
Overall, “these changing population dynamics should be considered in current decisions about resource allocation, human resource needs, HIV-prevention programs, and design of research studies.” However, “major technological advancements in HIV prevention (e.g., vaccines) and cure could occur in future decades, potentially nullifying the applicability of our projections,” the investigators said.
The authors had no conflicts of interest.
The number of people living with diagnosed HIV in the United States will increase to 1,232,054 by 2045, up from 917,294 in 2013, according to projections based on the National HIV Surveillance System and U.S. census data.
The number of people with HIV over age 55 will more than double from 232,113 to 470,221, though the annual growth rate will slow from 1.8% to 0.8%. By 2025, older people will comprise about 38% of the entire HIV population, said investigators led by epidemiologist Julia Hood, of the University of Washington Department of Epidemiology, Seattle.
The number of Hispanic and black people living with HIV is projected to grow consistently, shifting the demographics of people living with HIV from 32% to 23% white; 42% to 38% black; and 20% to 32% Hispanic by 2045 (AIDS Care. 2017 Apr 10:1-8).
The growing number of black patients “reflects disparities in HIV incidence; while among Hispanics/ Latinos, the growing number … is largely driven by immigration. These results underscore the importance of expanding coverage of HIV care, treatment, and prevention programs to ethnic/racial minorities, ensuring such programs are culturally and linguistically appropriate,” they said.
Overall, “these changing population dynamics should be considered in current decisions about resource allocation, human resource needs, HIV-prevention programs, and design of research studies.” However, “major technological advancements in HIV prevention (e.g., vaccines) and cure could occur in future decades, potentially nullifying the applicability of our projections,” the investigators said.
The authors had no conflicts of interest.
ALL, HL guidelines added to radiation therapy resource
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
Risks are reduced when angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers are held before noncardiac surgery
Clinical question: Is withholding angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) prior to noncardiac surgery associated with a lower risk of a 30-day composite outcome of all-cause death, myocardial injury after noncardiac surgery, and stroke when compared with continuing them on the day of surgery?
Background: The current American College of Cardiology/American Heart Association guidelines recommend continuing ACEI and ARBs for noncardiac surgery. However, many clinicians, including anesthesiologists, withhold these medications to prevent intraoperative hypotension. Because of the lack of strong evidence regarding clinical outcomes, the decision to withhold ACEI and ARBs prior to noncardiac surgery is currently dictated by physician preference and local policy.
Study Design: Prospective cohort study.
Setting: Analysis sample from the VISION study (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), which included 12 centers in eight countries.
Synopsis: A sample analysis was performed on 14,687 patients from the VISION study, who were at least 45 years old and undergoing noncardiac surgery and who required an overnight hospital admission. A total of 4,802 patients were taking ACEI/ARBs at baseline, and, for 1,245 (25.9%) of those patients, ACEI/ARBs were withheld at least 24 hours before surgery. Using multivariable regression models, the authors found that patients for whom ACEI/ARBs were withheld were less likely to suffer from the primary composite outcome of 30-day all-cause death, myocardial injury after noncardiac surgery, and stroke (12% vs 12.9%; adjusted relative risk, 0.82; 95% confidence interval, 0.7-0.96; P = .01). Withholding ACEI/ARBs prior to surgery was also associated with less risk of clinically important intraoperative hypotension, while the risk of postoperative hypotension was similar between the two groups.
Given that this was an observational study, analysis is limited because of the inability to account for every potential confounding factor.
Bottom Line: The study suggests a lower risk of postoperative death, stroke, and myocardial injury in patients for whom ACEI/ARBs were withheld prior to noncardiac surgery. A large randomized trial is needed to confirm the findings suggested by this analysis.
Citation: Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery.” Anesthesiology. 2017 Jan;126(1):16-27.
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Clinical question: Is withholding angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) prior to noncardiac surgery associated with a lower risk of a 30-day composite outcome of all-cause death, myocardial injury after noncardiac surgery, and stroke when compared with continuing them on the day of surgery?
Background: The current American College of Cardiology/American Heart Association guidelines recommend continuing ACEI and ARBs for noncardiac surgery. However, many clinicians, including anesthesiologists, withhold these medications to prevent intraoperative hypotension. Because of the lack of strong evidence regarding clinical outcomes, the decision to withhold ACEI and ARBs prior to noncardiac surgery is currently dictated by physician preference and local policy.
Study Design: Prospective cohort study.
Setting: Analysis sample from the VISION study (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), which included 12 centers in eight countries.
Synopsis: A sample analysis was performed on 14,687 patients from the VISION study, who were at least 45 years old and undergoing noncardiac surgery and who required an overnight hospital admission. A total of 4,802 patients were taking ACEI/ARBs at baseline, and, for 1,245 (25.9%) of those patients, ACEI/ARBs were withheld at least 24 hours before surgery. Using multivariable regression models, the authors found that patients for whom ACEI/ARBs were withheld were less likely to suffer from the primary composite outcome of 30-day all-cause death, myocardial injury after noncardiac surgery, and stroke (12% vs 12.9%; adjusted relative risk, 0.82; 95% confidence interval, 0.7-0.96; P = .01). Withholding ACEI/ARBs prior to surgery was also associated with less risk of clinically important intraoperative hypotension, while the risk of postoperative hypotension was similar between the two groups.
Given that this was an observational study, analysis is limited because of the inability to account for every potential confounding factor.
Bottom Line: The study suggests a lower risk of postoperative death, stroke, and myocardial injury in patients for whom ACEI/ARBs were withheld prior to noncardiac surgery. A large randomized trial is needed to confirm the findings suggested by this analysis.
Citation: Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery.” Anesthesiology. 2017 Jan;126(1):16-27.
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Clinical question: Is withholding angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARBs) prior to noncardiac surgery associated with a lower risk of a 30-day composite outcome of all-cause death, myocardial injury after noncardiac surgery, and stroke when compared with continuing them on the day of surgery?
Background: The current American College of Cardiology/American Heart Association guidelines recommend continuing ACEI and ARBs for noncardiac surgery. However, many clinicians, including anesthesiologists, withhold these medications to prevent intraoperative hypotension. Because of the lack of strong evidence regarding clinical outcomes, the decision to withhold ACEI and ARBs prior to noncardiac surgery is currently dictated by physician preference and local policy.
Study Design: Prospective cohort study.
Setting: Analysis sample from the VISION study (Vascular Events in Noncardiac Surgery Patients Cohort Evaluation), which included 12 centers in eight countries.
Synopsis: A sample analysis was performed on 14,687 patients from the VISION study, who were at least 45 years old and undergoing noncardiac surgery and who required an overnight hospital admission. A total of 4,802 patients were taking ACEI/ARBs at baseline, and, for 1,245 (25.9%) of those patients, ACEI/ARBs were withheld at least 24 hours before surgery. Using multivariable regression models, the authors found that patients for whom ACEI/ARBs were withheld were less likely to suffer from the primary composite outcome of 30-day all-cause death, myocardial injury after noncardiac surgery, and stroke (12% vs 12.9%; adjusted relative risk, 0.82; 95% confidence interval, 0.7-0.96; P = .01). Withholding ACEI/ARBs prior to surgery was also associated with less risk of clinically important intraoperative hypotension, while the risk of postoperative hypotension was similar between the two groups.
Given that this was an observational study, analysis is limited because of the inability to account for every potential confounding factor.
Bottom Line: The study suggests a lower risk of postoperative death, stroke, and myocardial injury in patients for whom ACEI/ARBs were withheld prior to noncardiac surgery. A large randomized trial is needed to confirm the findings suggested by this analysis.
Citation: Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery.” Anesthesiology. 2017 Jan;126(1):16-27.
Dr. Libot is assistant professor in the division of hospital medicine, Loyola University Chicago, Maywood, Ill.
Ebola research update: March 2017
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Malaria parasite coinfections were common in patients presenting to Ebola treatment units in Sierra Leone and conferred an increased mortality risk in patients infected with Ebola virus, according to a study in the Lancet Infectious Diseases.
New oral vaccine technologies hold great promise as a tool for protecting endangered tropical wildlife from Ebola virus disease, according to a study on captive chimpanzees published in Scientific Reports.
A European study in JAMA found that immunity after heterologous primary and booster vaccination with Ebola virus vaccines Ad26.ZEBOV and MVA-BN-Filo persisted at 1 year. The researchers said a strategy of preemptive use of an Ad26.ZEBOV, followed by MVA-BN-Filo immunization schedule in at-risk populations may offer advantages over reactive use of single-dose vaccine regimens.
An analysis in Cell Host & Microbe concluded that the Ebola virus glycoprotein (GP) acquired an A82V change during the West Africa epidemic and that this change altered the capacity of GP to be activated by host factors, enhancing infection of human cells.
The overall decrease in bushmeat consumption in West Africa associated with the Ebola crisis may have had a short-term positive effect on vulnerable wildlife populations, according to a recent study.
A study in the Journal of Virology found that limiting the excessive TLR4-mediated proinflammatory response in Ebola virus infection should be considered as a potential supportive treatment option for Ebola virus disease.
The Clinical Data Interchange Standards Consortium and the Infectious Diseases Data Observatory have announced the availability of a new standard to assist in the collection, aggregation, and analysis of Ebola virus disease research data.
A small study published in PLOS One found that treatment with interferon beta-1a of people with Ebola virus disease may be associated with clearance of virus from blood, better clinical features, and potentially, improved survival.
Finally, medical evacuation of patients with suspected potentially fatal, infectious diseases such as Ebola virus is feasible using a light isolator containment pod for patients without critical dysfunctions, according to a study in BMC Emergency Medicine.
[email protected]
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Malaria parasite coinfections were common in patients presenting to Ebola treatment units in Sierra Leone and conferred an increased mortality risk in patients infected with Ebola virus, according to a study in the Lancet Infectious Diseases.
New oral vaccine technologies hold great promise as a tool for protecting endangered tropical wildlife from Ebola virus disease, according to a study on captive chimpanzees published in Scientific Reports.
A European study in JAMA found that immunity after heterologous primary and booster vaccination with Ebola virus vaccines Ad26.ZEBOV and MVA-BN-Filo persisted at 1 year. The researchers said a strategy of preemptive use of an Ad26.ZEBOV, followed by MVA-BN-Filo immunization schedule in at-risk populations may offer advantages over reactive use of single-dose vaccine regimens.
An analysis in Cell Host & Microbe concluded that the Ebola virus glycoprotein (GP) acquired an A82V change during the West Africa epidemic and that this change altered the capacity of GP to be activated by host factors, enhancing infection of human cells.
The overall decrease in bushmeat consumption in West Africa associated with the Ebola crisis may have had a short-term positive effect on vulnerable wildlife populations, according to a recent study.
A study in the Journal of Virology found that limiting the excessive TLR4-mediated proinflammatory response in Ebola virus infection should be considered as a potential supportive treatment option for Ebola virus disease.
The Clinical Data Interchange Standards Consortium and the Infectious Diseases Data Observatory have announced the availability of a new standard to assist in the collection, aggregation, and analysis of Ebola virus disease research data.
A small study published in PLOS One found that treatment with interferon beta-1a of people with Ebola virus disease may be associated with clearance of virus from blood, better clinical features, and potentially, improved survival.
Finally, medical evacuation of patients with suspected potentially fatal, infectious diseases such as Ebola virus is feasible using a light isolator containment pod for patients without critical dysfunctions, according to a study in BMC Emergency Medicine.
[email protected]
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Malaria parasite coinfections were common in patients presenting to Ebola treatment units in Sierra Leone and conferred an increased mortality risk in patients infected with Ebola virus, according to a study in the Lancet Infectious Diseases.
New oral vaccine technologies hold great promise as a tool for protecting endangered tropical wildlife from Ebola virus disease, according to a study on captive chimpanzees published in Scientific Reports.
A European study in JAMA found that immunity after heterologous primary and booster vaccination with Ebola virus vaccines Ad26.ZEBOV and MVA-BN-Filo persisted at 1 year. The researchers said a strategy of preemptive use of an Ad26.ZEBOV, followed by MVA-BN-Filo immunization schedule in at-risk populations may offer advantages over reactive use of single-dose vaccine regimens.
An analysis in Cell Host & Microbe concluded that the Ebola virus glycoprotein (GP) acquired an A82V change during the West Africa epidemic and that this change altered the capacity of GP to be activated by host factors, enhancing infection of human cells.
The overall decrease in bushmeat consumption in West Africa associated with the Ebola crisis may have had a short-term positive effect on vulnerable wildlife populations, according to a recent study.
A study in the Journal of Virology found that limiting the excessive TLR4-mediated proinflammatory response in Ebola virus infection should be considered as a potential supportive treatment option for Ebola virus disease.
The Clinical Data Interchange Standards Consortium and the Infectious Diseases Data Observatory have announced the availability of a new standard to assist in the collection, aggregation, and analysis of Ebola virus disease research data.
A small study published in PLOS One found that treatment with interferon beta-1a of people with Ebola virus disease may be associated with clearance of virus from blood, better clinical features, and potentially, improved survival.
Finally, medical evacuation of patients with suspected potentially fatal, infectious diseases such as Ebola virus is feasible using a light isolator containment pod for patients without critical dysfunctions, according to a study in BMC Emergency Medicine.
[email protected]
On Twitter @richpizzi
ACA brought down veterans’ uninsured rate
The percentage of uninsured veterans dropped by almost 40% in the first 2 years after the Affordable Care Act’s major coverage provisions were implemented, according to a report from the Robert Wood Johnson Foundation and the Urban Institute.
In 2015, 5.9% of the almost 9.4 million veterans aged 19-64 years were uninsured, down from 9.6% in 2013 – the statistically significant difference of 3.8 percentage points representing a relative decline of 38.5%. The difference was even greater for veterans living in the states that had expanded Medicaid by mid-2015, who saw their uninsured rate fall from 9% to 4.8%, compared with those in states that had not, whose uninsured rate declined from 10.3% to 7.1%, the report’s authors said.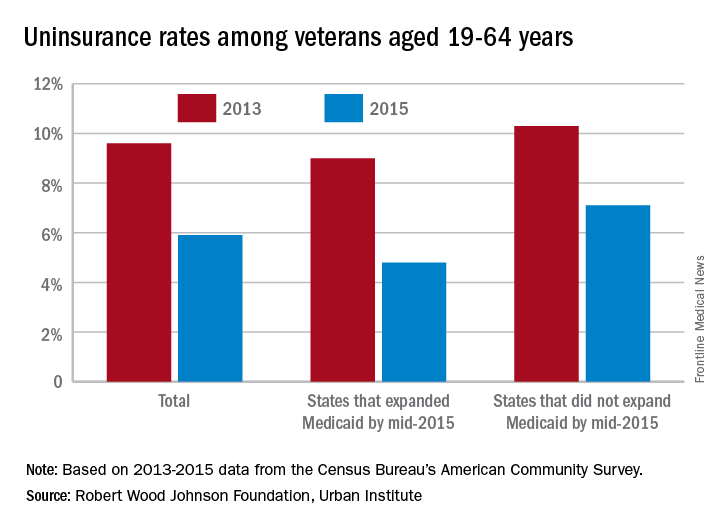
“These findings suggest that repeal of the ACA or particular components of the ACA (such as the Medicaid expansion) could reverse these coverage gains, increasing the number of veterans without health insurance coverage,” they wrote.
Of those currently uninsured, the report notes that “one-quarter are eligible for Medicaid but not enrolled, and … a number of uninsured veterans may qualify for VA care.” One option from the Department of Veterans Affairs, the Veterans Choice Program, was just extended beyond its expiration in August, ensuring that veterans will “continue to have access to care through local community providers [and] will not have to wait weeks or months, or drive long distances, to get the care they need,” Patrice A. Harris, MD, chair of the American Medical Association’s Board of Trustees, said in a statement.
The study was conducted by the Urban Institute with funding by the Robert Wood Johnson Foundation. Data for the analysis came from the Census Bureau’s American Community Survey and from the Centers for Disease Control and Prevention’s National Health Interview Survey.
The percentage of uninsured veterans dropped by almost 40% in the first 2 years after the Affordable Care Act’s major coverage provisions were implemented, according to a report from the Robert Wood Johnson Foundation and the Urban Institute.
In 2015, 5.9% of the almost 9.4 million veterans aged 19-64 years were uninsured, down from 9.6% in 2013 – the statistically significant difference of 3.8 percentage points representing a relative decline of 38.5%. The difference was even greater for veterans living in the states that had expanded Medicaid by mid-2015, who saw their uninsured rate fall from 9% to 4.8%, compared with those in states that had not, whose uninsured rate declined from 10.3% to 7.1%, the report’s authors said.
“These findings suggest that repeal of the ACA or particular components of the ACA (such as the Medicaid expansion) could reverse these coverage gains, increasing the number of veterans without health insurance coverage,” they wrote.
Of those currently uninsured, the report notes that “one-quarter are eligible for Medicaid but not enrolled, and … a number of uninsured veterans may qualify for VA care.” One option from the Department of Veterans Affairs, the Veterans Choice Program, was just extended beyond its expiration in August, ensuring that veterans will “continue to have access to care through local community providers [and] will not have to wait weeks or months, or drive long distances, to get the care they need,” Patrice A. Harris, MD, chair of the American Medical Association’s Board of Trustees, said in a statement.
The study was conducted by the Urban Institute with funding by the Robert Wood Johnson Foundation. Data for the analysis came from the Census Bureau’s American Community Survey and from the Centers for Disease Control and Prevention’s National Health Interview Survey.
The percentage of uninsured veterans dropped by almost 40% in the first 2 years after the Affordable Care Act’s major coverage provisions were implemented, according to a report from the Robert Wood Johnson Foundation and the Urban Institute.
In 2015, 5.9% of the almost 9.4 million veterans aged 19-64 years were uninsured, down from 9.6% in 2013 – the statistically significant difference of 3.8 percentage points representing a relative decline of 38.5%. The difference was even greater for veterans living in the states that had expanded Medicaid by mid-2015, who saw their uninsured rate fall from 9% to 4.8%, compared with those in states that had not, whose uninsured rate declined from 10.3% to 7.1%, the report’s authors said.
“These findings suggest that repeal of the ACA or particular components of the ACA (such as the Medicaid expansion) could reverse these coverage gains, increasing the number of veterans without health insurance coverage,” they wrote.
Of those currently uninsured, the report notes that “one-quarter are eligible for Medicaid but not enrolled, and … a number of uninsured veterans may qualify for VA care.” One option from the Department of Veterans Affairs, the Veterans Choice Program, was just extended beyond its expiration in August, ensuring that veterans will “continue to have access to care through local community providers [and] will not have to wait weeks or months, or drive long distances, to get the care they need,” Patrice A. Harris, MD, chair of the American Medical Association’s Board of Trustees, said in a statement.
The study was conducted by the Urban Institute with funding by the Robert Wood Johnson Foundation. Data for the analysis came from the Census Bureau’s American Community Survey and from the Centers for Disease Control and Prevention’s National Health Interview Survey.
VIDEO: Surgery succeeds with select hidradenitis suppurativa patients
WASHINGTON – Medication has its limits for some patients with more severe hidradenitis suppurativa, and these patients can often benefit from surgical treatment, Chris Sayed, MD, said at an educational session held by George Washington University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Especially if patients have relatively limited areas of sinus, being able to do some local procedures [is] what will get the patient a lot better,” Dr. Sayed of the department of dermatology at the University of North Carolina, Chapel Hill, said in a video interview. “Whereas the medicines would never have made that sinus go away.”
The meeting was supported by AbbVie. Dr. Sayed disclosed financial relationships with the company.
WASHINGTON – Medication has its limits for some patients with more severe hidradenitis suppurativa, and these patients can often benefit from surgical treatment, Chris Sayed, MD, said at an educational session held by George Washington University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Especially if patients have relatively limited areas of sinus, being able to do some local procedures [is] what will get the patient a lot better,” Dr. Sayed of the department of dermatology at the University of North Carolina, Chapel Hill, said in a video interview. “Whereas the medicines would never have made that sinus go away.”
The meeting was supported by AbbVie. Dr. Sayed disclosed financial relationships with the company.
WASHINGTON – Medication has its limits for some patients with more severe hidradenitis suppurativa, and these patients can often benefit from surgical treatment, Chris Sayed, MD, said at an educational session held by George Washington University.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Especially if patients have relatively limited areas of sinus, being able to do some local procedures [is] what will get the patient a lot better,” Dr. Sayed of the department of dermatology at the University of North Carolina, Chapel Hill, said in a video interview. “Whereas the medicines would never have made that sinus go away.”
The meeting was supported by AbbVie. Dr. Sayed disclosed financial relationships with the company.
Renflexis approved as second infliximab biosimilar
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
[email protected]
On Twitter @denisefulton
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
[email protected]
On Twitter @denisefulton
Infliximab-abda is the second infliximab biosimilar approved by the Food and Drug Administration, the agency announced April 21.
Infliximab-abda, to be marketed as Renflexis, is approved for all indications as the reference product, including Crohn’s diseases in adults and children, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, according to the product label.
Like Remicade, Renflexis will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections, lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Renflexis will be marketed by Merck Sharp & Dohme and is manufactured by Samsung Bioepis.
[email protected]
On Twitter @denisefulton
New and Noteworthy Information—May 2017
The Biomarkers for Infant Brain Injury Score can identify infants with acute intracranial hemorrhage, according to a study published online ahead of print April 10 in JAMA Pediatrics. Binary logistic regression was used to develop a multivariable model incorporating three serum biomarkers and one clinical variable (total hemoglobin). The Biomarkers for Infant Brain Injury Score was applied to 599 infants (mean age, 4.7 months) at increased risk for abusive head trauma. Fifty-two percent were boys, 78% were white, and 8% were Hispanic. At a cutoff of 0.182, the model was 89.3% sensitive and 48.0% specific for acute intracranial hemorrhage. Positive and negative predictive values were 21.3% and 95.6%, respectively. The model was neither sensitive nor specific for atraumatic brain abnormalities, isolated skull fractures, or chronic intracranial hemorrhage.
The FDA has approved Ocrevus (ocrelizumab), an IV infusion, to treat adults with relapsing forms of multiple sclerosis (MS) and primary progressive MS. This drug is the first to be approved by the FDA for primary progressive MS. The efficacy of Ocrevus for the treatment of relapsing-remitting MS was shown in two clinical trials including 1,656 participants treated for 96 weeks. In both studies, patients receiving Ocrevus had reduced relapse rates and reduced worsening of disability, compared with patients receiving interferon beta-1a. In a study of 732 participants with primary progressive MS treated for at least 120 weeks, participants receiving Ocrevus had a longer time to the worsening of disability, compared with participants receiving placebo. Common side effects include infusion-related reactions and upper respiratory tract infection. Genentech markets Ocrevus.
Unemployed men and women and reemployed men have an increased risk of hemorrhagic and ischemic stroke and mortality, according to a study published online ahead of print April 6 in Stroke. This prospective study included 21,902 men and 19,826 women, ages 40 to 59, from nine public health centers across Japan. Participants were followed up from 1990–1993 to the end of 2009–2014. During the follow-up period, 973 incident strokes and 275 deaths from stroke occurred in men, as well as 460 strokes and 131 deaths from stroke in women. Compared with continuously employed subjects, the multivariable hazard ratio (HR) for total stroke incidence was 1.58 in unemployed men and 1.51 in unemployed women. HR for total stroke mortality was 2.22 in men and 2.48 in women.
In people with uncomplicated childhood-onset epilepsy and five-year terminal remission, young adult social outcomes are comparable to those of sibling controls, according to a study published online ahead of print April 4 in Epilepsia. Long-term social outcomes were assessed at the 15-year follow-up of the Connecticut Study of Epilepsy, which included 361 individuals with epilepsy and 173 controls. Social outcomes for cases with uncomplicated epilepsy with five or more years’ terminal remission were comparable to those of controls. Cases with uncomplicated epilepsy and less than five years of seizure freedom were more likely to be less productive and not to have a driver’s license. Complicated cases with epilepsy and less than five years of seizure freedom had worse outcomes across multiple domains, including not graduating high school.
Deep brain stimulation of the ventralis oralis internus and centromedian-parafascicular thalamus is an effective and relatively safe treatment for severe, refractory Tourette syndrome, according to a study published online ahead of print April 7 in the Journal of Neurosurgery. Researchers retrospectively reviewed outcomes in 13 patients with refractory Tourette syndrome who underwent medial thalamic deep brain stimulation for seven years. Patients were evaluated by a multidisciplinary team, and preoperative objective assessments were performed using the Yale Global Tic Severity Scale and Yale-Brown Obsessive Compulsive Scale. Patients showed an average decrease of 37% in total tic severity at their first postoperative visit. During their most recent visit, patient scores decreased from preoperative scores by an average of 50%, which was statistically significant. Device-related complications occurred in two patients, necessitating additional surgeries.
The FDA has approved Ingrezza (valbenazine) capsules for the treatment of adults with tardive dyskinesia. Ingrezza is a selective vesicular monoamine transporter 2 inhibitor. The approval of Ingrezza was based on data from the Kinect 3 study, a phase III, randomized, double-blind, placebo-controlled, parallel-group, fixed-dose study comparing once-daily Ingrezza (80 mg and 40 mg) to placebo over six weeks in patients with schizophrenia, schizoaffective disorder, or mood disorder. The mean change from baseline to week six in the AIMS dyskinesia total score was –3.2 for patients receiving 80 mg/day of Ingrezza, compared with –0.1 for controls. Ingrezza also was generally well tolerated, with somnolence as the only adverse event occurring at a rate of 5% or greater and twice the rate associated with placebo. Neurocrine Biosciences markets Ingrezza.
Benzodiazepine use is associated with an increased risk of pneumonia among patients with Alzheimer’s disease, according to a study published April 10 in CMAJ. Researchers obtained data on all community-dwelling adults with a recent diagnosis of Alzheimer disease in Finland between 2005 and 2011 from the Medication use and Alzheimer’s disease cohort. Incident users of benzodiazepines and nonbenzodiazepines were identified using a one-year washout period and matched with nonusers through propensity scores. Among 49,484 eligible participants with Alzheimer’s disease, 5,232 taking benzodiazepines and 3,269 taking nonbenzodiazepines were matched 1:1 with people not taking these drugs. Benzodiazepine and nonbenzodiazepine use was associated with a 22% increased risk of pneumonia. When analyzed separately, benzodiazepine use was significantly associated with a 28% increased risk of pneumonia, but nonbenzodiazepine use was not.
Hospitalization rates for acute ischemic stroke in young adults are increasing, along with the prevalence of traditional stroke risk factors, according to a study published online ahead of print April 10 in JAMA Neurology. Hospitalization data from the National Inpatient Sample from 1995 through 2012 were used to analyze acute stroke hospitalization rates among people ages 18 to 64. The 2003–2004 set included 362,339 hospitalizations, and the 2011–2012 set included 421,815 hospitalizations. Acute ischemic stroke hospitalization rates increased significantly for men and women, and for certain racial and ethnic groups, among younger adults ages 18 to 54. The prevalence of stroke risk factors among people hospitalized for acute ischemic stroke continued to increase from 2003–2004 through 2011–2012 for men and women ages 18 to 64.
A polygenic hazard score may help quantify individual differences in age-specific genetic risk for Alzheimer’s disease, according to a study published March 21 in PLOS Medicine. The investigators reviewed single-nucleotide polymorphisms associated with Alzheimer’s disease risk. Using a Cox proportional hazard model, they calculated polygenic hazard scores for participants in the Alzheimer’s Disease Genetics Consortium and tested them in two independent cohorts. People in the top polygenic hazard score quartile developed Alzheimer’s disease at a considerably lower age and had the highest yearly Alzheimer’s disease incidence rate. Among people who did not have the APOE ε3 allele, polygenic hazard score modified the age of expected onset by more than 10 years between the lowest and highest deciles. In independent cohorts, the polygenic hazard score strongly predicted age of Alzheimer’s disease onset.
Generalized anxiety disorder (GAD) is associated with migraine, according to a study published in the March issue of Headache. Researchers performed a secondary data analysis of the 2012 Canadian Community Health Survey-Mental Health. The first subsample included people with and without migraine. The second subsample was restricted to people with migraine. Six percent of people with migraine had had GAD in the previous year, compared with 2.1% of people without migraine. The adjusted odds of previous year GAD were 2.5 times higher among people with migraine than among people without. In the sample containing only migraineurs, the factors associated with higher odds of 12-month GAD included having a university degree, having low income, being without a confidant, and being male.
The rates of childhood epilepsy increase with maternal overweight or obesity in a dose-response manner, according to a study published online ahead of print April 3 in JAMA Neurology. Researchers conducted a population-based cohort study of 1,441,623 live single births at 22 or more gestational weeks in Sweden from January 1, 1997, to December 31, 2011. The risk of childhood epilepsy increased by maternal BMI from 6.30 per 10,000 child-years among normal-weight women to 12.4 per 10,000 child-years among women with grade III obesity. Risk of epilepsy increased by 11% in children of overweight mothers, compared with children of normal-weight mothers. Grade I obesity was associated with a 20% increased risk, grade II obesity was associated with a 30% increased risk, and grade III obesity was associated with an 82% increased risk.
The FDA has approved a label expansion for Trokendi XR (topiramate) to include migraine prophylaxis in adults and adolescents age 12 and older. Trokendi XR is a once-daily extended release formulation. The drug previously was approved as initial monotherapy and adjunctive therapy in patients age 6 and older with partial onset or primary generalized tonic-clonic seizures and as adjunctive therapy in patients age 6 and older with seizures associated with Lennox-Gastaut syndrome. Trokendi XR is available in 25-mg, 50-mg, 100-mg, and 200-mg capsules. The drug may cause sudden decrease in vision, secondary angle closure glaucoma, or decreased sweating. Approximately one in 500 people who take Trokendi XR may have suicidal thoughts. Supernus Pharmaceuticals markets Trokendi XR.
The FDA has approved Austedo (deutetrabenazine) tablets for the treatment of chorea associated with Huntington’s disease. Austedo was previously referred to by the name SD-809 and was granted Orphan Drug Designation by the FDA. Austedo is the second product approved for Huntington’s disease. The approval was based on phase III results in a randomized, double-blind, placebo-controlled, multicenter trial conducted in 90 ambulatory patients with manifest chorea associated with Huntington’s disease. Total Maximal Chorea Scores for patients receiving Austedo improved by approximately 4.4 units from baseline to the maintenance period, compared with approximately 1.9 units in the placebo group. At the week 13 follow-up visit (one week after discontinuation of the study drug), the Total Maximal Chorea Scores of patients who had received Austedo returned to baseline levels. Teva Pharmaceutical Industries markets Austedo.
—Kimberly Williams
The Biomarkers for Infant Brain Injury Score can identify infants with acute intracranial hemorrhage, according to a study published online ahead of print April 10 in JAMA Pediatrics. Binary logistic regression was used to develop a multivariable model incorporating three serum biomarkers and one clinical variable (total hemoglobin). The Biomarkers for Infant Brain Injury Score was applied to 599 infants (mean age, 4.7 months) at increased risk for abusive head trauma. Fifty-two percent were boys, 78% were white, and 8% were Hispanic. At a cutoff of 0.182, the model was 89.3% sensitive and 48.0% specific for acute intracranial hemorrhage. Positive and negative predictive values were 21.3% and 95.6%, respectively. The model was neither sensitive nor specific for atraumatic brain abnormalities, isolated skull fractures, or chronic intracranial hemorrhage.
The FDA has approved Ocrevus (ocrelizumab), an IV infusion, to treat adults with relapsing forms of multiple sclerosis (MS) and primary progressive MS. This drug is the first to be approved by the FDA for primary progressive MS. The efficacy of Ocrevus for the treatment of relapsing-remitting MS was shown in two clinical trials including 1,656 participants treated for 96 weeks. In both studies, patients receiving Ocrevus had reduced relapse rates and reduced worsening of disability, compared with patients receiving interferon beta-1a. In a study of 732 participants with primary progressive MS treated for at least 120 weeks, participants receiving Ocrevus had a longer time to the worsening of disability, compared with participants receiving placebo. Common side effects include infusion-related reactions and upper respiratory tract infection. Genentech markets Ocrevus.
Unemployed men and women and reemployed men have an increased risk of hemorrhagic and ischemic stroke and mortality, according to a study published online ahead of print April 6 in Stroke. This prospective study included 21,902 men and 19,826 women, ages 40 to 59, from nine public health centers across Japan. Participants were followed up from 1990–1993 to the end of 2009–2014. During the follow-up period, 973 incident strokes and 275 deaths from stroke occurred in men, as well as 460 strokes and 131 deaths from stroke in women. Compared with continuously employed subjects, the multivariable hazard ratio (HR) for total stroke incidence was 1.58 in unemployed men and 1.51 in unemployed women. HR for total stroke mortality was 2.22 in men and 2.48 in women.
In people with uncomplicated childhood-onset epilepsy and five-year terminal remission, young adult social outcomes are comparable to those of sibling controls, according to a study published online ahead of print April 4 in Epilepsia. Long-term social outcomes were assessed at the 15-year follow-up of the Connecticut Study of Epilepsy, which included 361 individuals with epilepsy and 173 controls. Social outcomes for cases with uncomplicated epilepsy with five or more years’ terminal remission were comparable to those of controls. Cases with uncomplicated epilepsy and less than five years of seizure freedom were more likely to be less productive and not to have a driver’s license. Complicated cases with epilepsy and less than five years of seizure freedom had worse outcomes across multiple domains, including not graduating high school.
Deep brain stimulation of the ventralis oralis internus and centromedian-parafascicular thalamus is an effective and relatively safe treatment for severe, refractory Tourette syndrome, according to a study published online ahead of print April 7 in the Journal of Neurosurgery. Researchers retrospectively reviewed outcomes in 13 patients with refractory Tourette syndrome who underwent medial thalamic deep brain stimulation for seven years. Patients were evaluated by a multidisciplinary team, and preoperative objective assessments were performed using the Yale Global Tic Severity Scale and Yale-Brown Obsessive Compulsive Scale. Patients showed an average decrease of 37% in total tic severity at their first postoperative visit. During their most recent visit, patient scores decreased from preoperative scores by an average of 50%, which was statistically significant. Device-related complications occurred in two patients, necessitating additional surgeries.
The FDA has approved Ingrezza (valbenazine) capsules for the treatment of adults with tardive dyskinesia. Ingrezza is a selective vesicular monoamine transporter 2 inhibitor. The approval of Ingrezza was based on data from the Kinect 3 study, a phase III, randomized, double-blind, placebo-controlled, parallel-group, fixed-dose study comparing once-daily Ingrezza (80 mg and 40 mg) to placebo over six weeks in patients with schizophrenia, schizoaffective disorder, or mood disorder. The mean change from baseline to week six in the AIMS dyskinesia total score was –3.2 for patients receiving 80 mg/day of Ingrezza, compared with –0.1 for controls. Ingrezza also was generally well tolerated, with somnolence as the only adverse event occurring at a rate of 5% or greater and twice the rate associated with placebo. Neurocrine Biosciences markets Ingrezza.
Benzodiazepine use is associated with an increased risk of pneumonia among patients with Alzheimer’s disease, according to a study published April 10 in CMAJ. Researchers obtained data on all community-dwelling adults with a recent diagnosis of Alzheimer disease in Finland between 2005 and 2011 from the Medication use and Alzheimer’s disease cohort. Incident users of benzodiazepines and nonbenzodiazepines were identified using a one-year washout period and matched with nonusers through propensity scores. Among 49,484 eligible participants with Alzheimer’s disease, 5,232 taking benzodiazepines and 3,269 taking nonbenzodiazepines were matched 1:1 with people not taking these drugs. Benzodiazepine and nonbenzodiazepine use was associated with a 22% increased risk of pneumonia. When analyzed separately, benzodiazepine use was significantly associated with a 28% increased risk of pneumonia, but nonbenzodiazepine use was not.
Hospitalization rates for acute ischemic stroke in young adults are increasing, along with the prevalence of traditional stroke risk factors, according to a study published online ahead of print April 10 in JAMA Neurology. Hospitalization data from the National Inpatient Sample from 1995 through 2012 were used to analyze acute stroke hospitalization rates among people ages 18 to 64. The 2003–2004 set included 362,339 hospitalizations, and the 2011–2012 set included 421,815 hospitalizations. Acute ischemic stroke hospitalization rates increased significantly for men and women, and for certain racial and ethnic groups, among younger adults ages 18 to 54. The prevalence of stroke risk factors among people hospitalized for acute ischemic stroke continued to increase from 2003–2004 through 2011–2012 for men and women ages 18 to 64.
A polygenic hazard score may help quantify individual differences in age-specific genetic risk for Alzheimer’s disease, according to a study published March 21 in PLOS Medicine. The investigators reviewed single-nucleotide polymorphisms associated with Alzheimer’s disease risk. Using a Cox proportional hazard model, they calculated polygenic hazard scores for participants in the Alzheimer’s Disease Genetics Consortium and tested them in two independent cohorts. People in the top polygenic hazard score quartile developed Alzheimer’s disease at a considerably lower age and had the highest yearly Alzheimer’s disease incidence rate. Among people who did not have the APOE ε3 allele, polygenic hazard score modified the age of expected onset by more than 10 years between the lowest and highest deciles. In independent cohorts, the polygenic hazard score strongly predicted age of Alzheimer’s disease onset.
Generalized anxiety disorder (GAD) is associated with migraine, according to a study published in the March issue of Headache. Researchers performed a secondary data analysis of the 2012 Canadian Community Health Survey-Mental Health. The first subsample included people with and without migraine. The second subsample was restricted to people with migraine. Six percent of people with migraine had had GAD in the previous year, compared with 2.1% of people without migraine. The adjusted odds of previous year GAD were 2.5 times higher among people with migraine than among people without. In the sample containing only migraineurs, the factors associated with higher odds of 12-month GAD included having a university degree, having low income, being without a confidant, and being male.
The rates of childhood epilepsy increase with maternal overweight or obesity in a dose-response manner, according to a study published online ahead of print April 3 in JAMA Neurology. Researchers conducted a population-based cohort study of 1,441,623 live single births at 22 or more gestational weeks in Sweden from January 1, 1997, to December 31, 2011. The risk of childhood epilepsy increased by maternal BMI from 6.30 per 10,000 child-years among normal-weight women to 12.4 per 10,000 child-years among women with grade III obesity. Risk of epilepsy increased by 11% in children of overweight mothers, compared with children of normal-weight mothers. Grade I obesity was associated with a 20% increased risk, grade II obesity was associated with a 30% increased risk, and grade III obesity was associated with an 82% increased risk.
The FDA has approved a label expansion for Trokendi XR (topiramate) to include migraine prophylaxis in adults and adolescents age 12 and older. Trokendi XR is a once-daily extended release formulation. The drug previously was approved as initial monotherapy and adjunctive therapy in patients age 6 and older with partial onset or primary generalized tonic-clonic seizures and as adjunctive therapy in patients age 6 and older with seizures associated with Lennox-Gastaut syndrome. Trokendi XR is available in 25-mg, 50-mg, 100-mg, and 200-mg capsules. The drug may cause sudden decrease in vision, secondary angle closure glaucoma, or decreased sweating. Approximately one in 500 people who take Trokendi XR may have suicidal thoughts. Supernus Pharmaceuticals markets Trokendi XR.
The FDA has approved Austedo (deutetrabenazine) tablets for the treatment of chorea associated with Huntington’s disease. Austedo was previously referred to by the name SD-809 and was granted Orphan Drug Designation by the FDA. Austedo is the second product approved for Huntington’s disease. The approval was based on phase III results in a randomized, double-blind, placebo-controlled, multicenter trial conducted in 90 ambulatory patients with manifest chorea associated with Huntington’s disease. Total Maximal Chorea Scores for patients receiving Austedo improved by approximately 4.4 units from baseline to the maintenance period, compared with approximately 1.9 units in the placebo group. At the week 13 follow-up visit (one week after discontinuation of the study drug), the Total Maximal Chorea Scores of patients who had received Austedo returned to baseline levels. Teva Pharmaceutical Industries markets Austedo.
—Kimberly Williams
The Biomarkers for Infant Brain Injury Score can identify infants with acute intracranial hemorrhage, according to a study published online ahead of print April 10 in JAMA Pediatrics. Binary logistic regression was used to develop a multivariable model incorporating three serum biomarkers and one clinical variable (total hemoglobin). The Biomarkers for Infant Brain Injury Score was applied to 599 infants (mean age, 4.7 months) at increased risk for abusive head trauma. Fifty-two percent were boys, 78% were white, and 8% were Hispanic. At a cutoff of 0.182, the model was 89.3% sensitive and 48.0% specific for acute intracranial hemorrhage. Positive and negative predictive values were 21.3% and 95.6%, respectively. The model was neither sensitive nor specific for atraumatic brain abnormalities, isolated skull fractures, or chronic intracranial hemorrhage.
The FDA has approved Ocrevus (ocrelizumab), an IV infusion, to treat adults with relapsing forms of multiple sclerosis (MS) and primary progressive MS. This drug is the first to be approved by the FDA for primary progressive MS. The efficacy of Ocrevus for the treatment of relapsing-remitting MS was shown in two clinical trials including 1,656 participants treated for 96 weeks. In both studies, patients receiving Ocrevus had reduced relapse rates and reduced worsening of disability, compared with patients receiving interferon beta-1a. In a study of 732 participants with primary progressive MS treated for at least 120 weeks, participants receiving Ocrevus had a longer time to the worsening of disability, compared with participants receiving placebo. Common side effects include infusion-related reactions and upper respiratory tract infection. Genentech markets Ocrevus.
Unemployed men and women and reemployed men have an increased risk of hemorrhagic and ischemic stroke and mortality, according to a study published online ahead of print April 6 in Stroke. This prospective study included 21,902 men and 19,826 women, ages 40 to 59, from nine public health centers across Japan. Participants were followed up from 1990–1993 to the end of 2009–2014. During the follow-up period, 973 incident strokes and 275 deaths from stroke occurred in men, as well as 460 strokes and 131 deaths from stroke in women. Compared with continuously employed subjects, the multivariable hazard ratio (HR) for total stroke incidence was 1.58 in unemployed men and 1.51 in unemployed women. HR for total stroke mortality was 2.22 in men and 2.48 in women.
In people with uncomplicated childhood-onset epilepsy and five-year terminal remission, young adult social outcomes are comparable to those of sibling controls, according to a study published online ahead of print April 4 in Epilepsia. Long-term social outcomes were assessed at the 15-year follow-up of the Connecticut Study of Epilepsy, which included 361 individuals with epilepsy and 173 controls. Social outcomes for cases with uncomplicated epilepsy with five or more years’ terminal remission were comparable to those of controls. Cases with uncomplicated epilepsy and less than five years of seizure freedom were more likely to be less productive and not to have a driver’s license. Complicated cases with epilepsy and less than five years of seizure freedom had worse outcomes across multiple domains, including not graduating high school.
Deep brain stimulation of the ventralis oralis internus and centromedian-parafascicular thalamus is an effective and relatively safe treatment for severe, refractory Tourette syndrome, according to a study published online ahead of print April 7 in the Journal of Neurosurgery. Researchers retrospectively reviewed outcomes in 13 patients with refractory Tourette syndrome who underwent medial thalamic deep brain stimulation for seven years. Patients were evaluated by a multidisciplinary team, and preoperative objective assessments were performed using the Yale Global Tic Severity Scale and Yale-Brown Obsessive Compulsive Scale. Patients showed an average decrease of 37% in total tic severity at their first postoperative visit. During their most recent visit, patient scores decreased from preoperative scores by an average of 50%, which was statistically significant. Device-related complications occurred in two patients, necessitating additional surgeries.
The FDA has approved Ingrezza (valbenazine) capsules for the treatment of adults with tardive dyskinesia. Ingrezza is a selective vesicular monoamine transporter 2 inhibitor. The approval of Ingrezza was based on data from the Kinect 3 study, a phase III, randomized, double-blind, placebo-controlled, parallel-group, fixed-dose study comparing once-daily Ingrezza (80 mg and 40 mg) to placebo over six weeks in patients with schizophrenia, schizoaffective disorder, or mood disorder. The mean change from baseline to week six in the AIMS dyskinesia total score was –3.2 for patients receiving 80 mg/day of Ingrezza, compared with –0.1 for controls. Ingrezza also was generally well tolerated, with somnolence as the only adverse event occurring at a rate of 5% or greater and twice the rate associated with placebo. Neurocrine Biosciences markets Ingrezza.
Benzodiazepine use is associated with an increased risk of pneumonia among patients with Alzheimer’s disease, according to a study published April 10 in CMAJ. Researchers obtained data on all community-dwelling adults with a recent diagnosis of Alzheimer disease in Finland between 2005 and 2011 from the Medication use and Alzheimer’s disease cohort. Incident users of benzodiazepines and nonbenzodiazepines were identified using a one-year washout period and matched with nonusers through propensity scores. Among 49,484 eligible participants with Alzheimer’s disease, 5,232 taking benzodiazepines and 3,269 taking nonbenzodiazepines were matched 1:1 with people not taking these drugs. Benzodiazepine and nonbenzodiazepine use was associated with a 22% increased risk of pneumonia. When analyzed separately, benzodiazepine use was significantly associated with a 28% increased risk of pneumonia, but nonbenzodiazepine use was not.
Hospitalization rates for acute ischemic stroke in young adults are increasing, along with the prevalence of traditional stroke risk factors, according to a study published online ahead of print April 10 in JAMA Neurology. Hospitalization data from the National Inpatient Sample from 1995 through 2012 were used to analyze acute stroke hospitalization rates among people ages 18 to 64. The 2003–2004 set included 362,339 hospitalizations, and the 2011–2012 set included 421,815 hospitalizations. Acute ischemic stroke hospitalization rates increased significantly for men and women, and for certain racial and ethnic groups, among younger adults ages 18 to 54. The prevalence of stroke risk factors among people hospitalized for acute ischemic stroke continued to increase from 2003–2004 through 2011–2012 for men and women ages 18 to 64.
A polygenic hazard score may help quantify individual differences in age-specific genetic risk for Alzheimer’s disease, according to a study published March 21 in PLOS Medicine. The investigators reviewed single-nucleotide polymorphisms associated with Alzheimer’s disease risk. Using a Cox proportional hazard model, they calculated polygenic hazard scores for participants in the Alzheimer’s Disease Genetics Consortium and tested them in two independent cohorts. People in the top polygenic hazard score quartile developed Alzheimer’s disease at a considerably lower age and had the highest yearly Alzheimer’s disease incidence rate. Among people who did not have the APOE ε3 allele, polygenic hazard score modified the age of expected onset by more than 10 years between the lowest and highest deciles. In independent cohorts, the polygenic hazard score strongly predicted age of Alzheimer’s disease onset.
Generalized anxiety disorder (GAD) is associated with migraine, according to a study published in the March issue of Headache. Researchers performed a secondary data analysis of the 2012 Canadian Community Health Survey-Mental Health. The first subsample included people with and without migraine. The second subsample was restricted to people with migraine. Six percent of people with migraine had had GAD in the previous year, compared with 2.1% of people without migraine. The adjusted odds of previous year GAD were 2.5 times higher among people with migraine than among people without. In the sample containing only migraineurs, the factors associated with higher odds of 12-month GAD included having a university degree, having low income, being without a confidant, and being male.
The rates of childhood epilepsy increase with maternal overweight or obesity in a dose-response manner, according to a study published online ahead of print April 3 in JAMA Neurology. Researchers conducted a population-based cohort study of 1,441,623 live single births at 22 or more gestational weeks in Sweden from January 1, 1997, to December 31, 2011. The risk of childhood epilepsy increased by maternal BMI from 6.30 per 10,000 child-years among normal-weight women to 12.4 per 10,000 child-years among women with grade III obesity. Risk of epilepsy increased by 11% in children of overweight mothers, compared with children of normal-weight mothers. Grade I obesity was associated with a 20% increased risk, grade II obesity was associated with a 30% increased risk, and grade III obesity was associated with an 82% increased risk.
The FDA has approved a label expansion for Trokendi XR (topiramate) to include migraine prophylaxis in adults and adolescents age 12 and older. Trokendi XR is a once-daily extended release formulation. The drug previously was approved as initial monotherapy and adjunctive therapy in patients age 6 and older with partial onset or primary generalized tonic-clonic seizures and as adjunctive therapy in patients age 6 and older with seizures associated with Lennox-Gastaut syndrome. Trokendi XR is available in 25-mg, 50-mg, 100-mg, and 200-mg capsules. The drug may cause sudden decrease in vision, secondary angle closure glaucoma, or decreased sweating. Approximately one in 500 people who take Trokendi XR may have suicidal thoughts. Supernus Pharmaceuticals markets Trokendi XR.
The FDA has approved Austedo (deutetrabenazine) tablets for the treatment of chorea associated with Huntington’s disease. Austedo was previously referred to by the name SD-809 and was granted Orphan Drug Designation by the FDA. Austedo is the second product approved for Huntington’s disease. The approval was based on phase III results in a randomized, double-blind, placebo-controlled, multicenter trial conducted in 90 ambulatory patients with manifest chorea associated with Huntington’s disease. Total Maximal Chorea Scores for patients receiving Austedo improved by approximately 4.4 units from baseline to the maintenance period, compared with approximately 1.9 units in the placebo group. At the week 13 follow-up visit (one week after discontinuation of the study drug), the Total Maximal Chorea Scores of patients who had received Austedo returned to baseline levels. Teva Pharmaceutical Industries markets Austedo.
—Kimberly Williams
For doctors, a clampdown on visas could have an uneven effect in the U.S.
Limiting the number of foreign doctors who can get visas to practice in the United States could have a significant impact on certain hospitals and states that rely on them, according to a new study.
The research, published online in JAMA this week, found that more than 2,100 U.S. employers were certified to fill nearly 10,500 physician jobs nationwide, in 2016. That represents 1.4% of the physician workforce overall. There were wide variations by state and employer, however (JAMA. 2017 Apr 17. doi: 10.1001/jama.2017.4877).
Employers in New York, Michigan and Illinois accounted for the most H-1B visa applications for foreign physicians, nearly a third of the total. North Dakota, however, had the most applicants as a percentage of its physician workforce: 4.7%.
The top three employers that submitted applications for the most doctors through the visa program were William Beaumont Hospital in Royal Oak, Mich., with 470 physician applications, Bronx-Lebanon (N.Y.) Hospital Center, with 213, and Cleveland Clinic Foundation, with 180.
“People underestimate the fragility of certain hospitals and their reliance on certain physicians for their functioning,” said study coauthor Peter Kahn, who graduates from Albert Einstein College of Medicine, New York, this spring.
The H-1B visa program allows employers to hire highly skilled professionals from abroad to fill employment gaps in the United States, typically in high-tech, science, engineering, and math jobs. But hospitals use the program as well, often to recruit doctors to serve in rural or underserved urban areas. The number of visas is capped at 85,000 annually.
That could change. On Tuesday, President Donald Trump signed an executive order reiterating his administration’s priority to buy American goods and hire American workers. Among other things, it requires federal agencies to suggest reforms to the H-1B visa program to ensure the visas are awarded appropriately.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Limiting the number of foreign doctors who can get visas to practice in the United States could have a significant impact on certain hospitals and states that rely on them, according to a new study.
The research, published online in JAMA this week, found that more than 2,100 U.S. employers were certified to fill nearly 10,500 physician jobs nationwide, in 2016. That represents 1.4% of the physician workforce overall. There were wide variations by state and employer, however (JAMA. 2017 Apr 17. doi: 10.1001/jama.2017.4877).
Employers in New York, Michigan and Illinois accounted for the most H-1B visa applications for foreign physicians, nearly a third of the total. North Dakota, however, had the most applicants as a percentage of its physician workforce: 4.7%.
The top three employers that submitted applications for the most doctors through the visa program were William Beaumont Hospital in Royal Oak, Mich., with 470 physician applications, Bronx-Lebanon (N.Y.) Hospital Center, with 213, and Cleveland Clinic Foundation, with 180.
“People underestimate the fragility of certain hospitals and their reliance on certain physicians for their functioning,” said study coauthor Peter Kahn, who graduates from Albert Einstein College of Medicine, New York, this spring.
The H-1B visa program allows employers to hire highly skilled professionals from abroad to fill employment gaps in the United States, typically in high-tech, science, engineering, and math jobs. But hospitals use the program as well, often to recruit doctors to serve in rural or underserved urban areas. The number of visas is capped at 85,000 annually.
That could change. On Tuesday, President Donald Trump signed an executive order reiterating his administration’s priority to buy American goods and hire American workers. Among other things, it requires federal agencies to suggest reforms to the H-1B visa program to ensure the visas are awarded appropriately.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Limiting the number of foreign doctors who can get visas to practice in the United States could have a significant impact on certain hospitals and states that rely on them, according to a new study.
The research, published online in JAMA this week, found that more than 2,100 U.S. employers were certified to fill nearly 10,500 physician jobs nationwide, in 2016. That represents 1.4% of the physician workforce overall. There were wide variations by state and employer, however (JAMA. 2017 Apr 17. doi: 10.1001/jama.2017.4877).
Employers in New York, Michigan and Illinois accounted for the most H-1B visa applications for foreign physicians, nearly a third of the total. North Dakota, however, had the most applicants as a percentage of its physician workforce: 4.7%.
The top three employers that submitted applications for the most doctors through the visa program were William Beaumont Hospital in Royal Oak, Mich., with 470 physician applications, Bronx-Lebanon (N.Y.) Hospital Center, with 213, and Cleveland Clinic Foundation, with 180.
“People underestimate the fragility of certain hospitals and their reliance on certain physicians for their functioning,” said study coauthor Peter Kahn, who graduates from Albert Einstein College of Medicine, New York, this spring.
The H-1B visa program allows employers to hire highly skilled professionals from abroad to fill employment gaps in the United States, typically in high-tech, science, engineering, and math jobs. But hospitals use the program as well, often to recruit doctors to serve in rural or underserved urban areas. The number of visas is capped at 85,000 annually.
That could change. On Tuesday, President Donald Trump signed an executive order reiterating his administration’s priority to buy American goods and hire American workers. Among other things, it requires federal agencies to suggest reforms to the H-1B visa program to ensure the visas are awarded appropriately.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.