User login
Apomorphine Reduces Off Time for People with Advanced Parkinson’s Disease
BOSTON—Apomorphine may provide relief from off time for people with advanced Parkinson’s disease, according to a study presented at the 69th Annual Meeting of the American Academy of Neurology.
“If a person with Parkinson’s disease can reduce their off times, that can have a great impact on their everyday life,” said study author Regina Katzenschlager, MD, of Danube Hospital, which is affiliated with the Medical University of Vienna. “In some patients in the trial, the insecurity of unpredictable periods of incapacity was completely alleviated.”
Open-Label Data Suggest Efficacy
Levodopa has long been the gold standard in the treatment of Parkinson’s disease, but the effects of the medication can partially wear off more quickly as the disease progresses. Patients consequently experience off time that includes symptoms such as slowness and muscle rigidity.
The drug apomorphine, which first was produced in 1865, began to be used to treat advanced Parkinson’s disease in the United States in 1950. Its use grew in the 1990s when European doctors started administering subcutaneous infusions of the drug to treat fluctuations in mobility that could not be controlled by levodopa.
Extensive data from open-label studies of apomorphine have demonstrated its efficacy in reducing off time, dyskinesias, and oral levodopa dose in patients with severe motor fluctuations that are poorly controlled by conventional therapy. Evidence from randomized, blinded studies of the drug has been lacking, however.
Comparing Apomorphine With Placebo
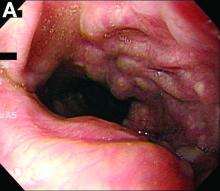
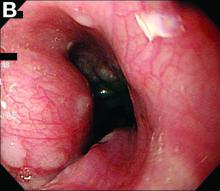
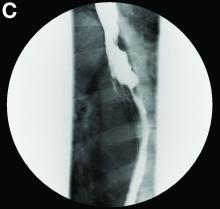
In a double-blind phase III study, researchers recruited 107 people with advanced Parkinson’s disease from 23 centers in seven countries.
Participants were randomized to either apomorphine subcutaneous infusion (≤ 8 mg/h) or a placebo saline infusion. The infusion was administered over a period of 14 to 18 hours each day through a small portable pump similar to the type used in the treatment of type 1 diabetes. The hourly flow rate of the infusion and dose of concomitant antiparkinsonian medication were adjusted during the first four weeks, based on efficacy and tolerability. The study’s primary end point was the absolute change in off time from baseline to Week 12 based on patient diaries.
Patients who received apomorphine had a significantly greater reduction of off time than those who received the placebo infusion. Active patients had, on average, 2.5 hours less off time per day, while participants who received the placebo infusion had an average of 35 minutes less off time per day. This improvement was apparent within the first week of treatment and was sustained over 12 weeks. At the same time, patients who received apomorphine had a significantly greater increase of on time without the dyskinesias that often are observed with levodopa, compared with placebo.
Participants were also asked to evaluate how well they thought the treatment worked. Those who received apomorphine gave their treatment higher scores at week 12 than those who received the placebo infusion. In the apomorphine group, 71% of patients felt improved, compared with 18% of patients who received placebo. Furthermore, 19% of patients worsened on apomorphine, compared with 45% on placebo. Apomorphine was generally well tolerated, and the researchers observed no serious side effects.
“It is our hope that these findings confirming the efficacy of apomorphine infusion will encourage doctors in the United States to offer this treatment to their patients and assess its efficacy in their own clinical practice,” Dr. Katzenschlager
concluded.
The study was supported by Britannia Pharmaceuticals, the maker of apomorphine.
BOSTON—Apomorphine may provide relief from off time for people with advanced Parkinson’s disease, according to a study presented at the 69th Annual Meeting of the American Academy of Neurology.
“If a person with Parkinson’s disease can reduce their off times, that can have a great impact on their everyday life,” said study author Regina Katzenschlager, MD, of Danube Hospital, which is affiliated with the Medical University of Vienna. “In some patients in the trial, the insecurity of unpredictable periods of incapacity was completely alleviated.”
Open-Label Data Suggest Efficacy
Levodopa has long been the gold standard in the treatment of Parkinson’s disease, but the effects of the medication can partially wear off more quickly as the disease progresses. Patients consequently experience off time that includes symptoms such as slowness and muscle rigidity.
The drug apomorphine, which first was produced in 1865, began to be used to treat advanced Parkinson’s disease in the United States in 1950. Its use grew in the 1990s when European doctors started administering subcutaneous infusions of the drug to treat fluctuations in mobility that could not be controlled by levodopa.
Extensive data from open-label studies of apomorphine have demonstrated its efficacy in reducing off time, dyskinesias, and oral levodopa dose in patients with severe motor fluctuations that are poorly controlled by conventional therapy. Evidence from randomized, blinded studies of the drug has been lacking, however.
Comparing Apomorphine With Placebo
In a double-blind phase III study, researchers recruited 107 people with advanced Parkinson’s disease from 23 centers in seven countries.
Participants were randomized to either apomorphine subcutaneous infusion (≤ 8 mg/h) or a placebo saline infusion. The infusion was administered over a period of 14 to 18 hours each day through a small portable pump similar to the type used in the treatment of type 1 diabetes. The hourly flow rate of the infusion and dose of concomitant antiparkinsonian medication were adjusted during the first four weeks, based on efficacy and tolerability. The study’s primary end point was the absolute change in off time from baseline to Week 12 based on patient diaries.
Patients who received apomorphine had a significantly greater reduction of off time than those who received the placebo infusion. Active patients had, on average, 2.5 hours less off time per day, while participants who received the placebo infusion had an average of 35 minutes less off time per day. This improvement was apparent within the first week of treatment and was sustained over 12 weeks. At the same time, patients who received apomorphine had a significantly greater increase of on time without the dyskinesias that often are observed with levodopa, compared with placebo.
Participants were also asked to evaluate how well they thought the treatment worked. Those who received apomorphine gave their treatment higher scores at week 12 than those who received the placebo infusion. In the apomorphine group, 71% of patients felt improved, compared with 18% of patients who received placebo. Furthermore, 19% of patients worsened on apomorphine, compared with 45% on placebo. Apomorphine was generally well tolerated, and the researchers observed no serious side effects.
“It is our hope that these findings confirming the efficacy of apomorphine infusion will encourage doctors in the United States to offer this treatment to their patients and assess its efficacy in their own clinical practice,” Dr. Katzenschlager
concluded.
The study was supported by Britannia Pharmaceuticals, the maker of apomorphine.
BOSTON—Apomorphine may provide relief from off time for people with advanced Parkinson’s disease, according to a study presented at the 69th Annual Meeting of the American Academy of Neurology.
“If a person with Parkinson’s disease can reduce their off times, that can have a great impact on their everyday life,” said study author Regina Katzenschlager, MD, of Danube Hospital, which is affiliated with the Medical University of Vienna. “In some patients in the trial, the insecurity of unpredictable periods of incapacity was completely alleviated.”
Open-Label Data Suggest Efficacy
Levodopa has long been the gold standard in the treatment of Parkinson’s disease, but the effects of the medication can partially wear off more quickly as the disease progresses. Patients consequently experience off time that includes symptoms such as slowness and muscle rigidity.
The drug apomorphine, which first was produced in 1865, began to be used to treat advanced Parkinson’s disease in the United States in 1950. Its use grew in the 1990s when European doctors started administering subcutaneous infusions of the drug to treat fluctuations in mobility that could not be controlled by levodopa.
Extensive data from open-label studies of apomorphine have demonstrated its efficacy in reducing off time, dyskinesias, and oral levodopa dose in patients with severe motor fluctuations that are poorly controlled by conventional therapy. Evidence from randomized, blinded studies of the drug has been lacking, however.
Comparing Apomorphine With Placebo
In a double-blind phase III study, researchers recruited 107 people with advanced Parkinson’s disease from 23 centers in seven countries.
Participants were randomized to either apomorphine subcutaneous infusion (≤ 8 mg/h) or a placebo saline infusion. The infusion was administered over a period of 14 to 18 hours each day through a small portable pump similar to the type used in the treatment of type 1 diabetes. The hourly flow rate of the infusion and dose of concomitant antiparkinsonian medication were adjusted during the first four weeks, based on efficacy and tolerability. The study’s primary end point was the absolute change in off time from baseline to Week 12 based on patient diaries.
Patients who received apomorphine had a significantly greater reduction of off time than those who received the placebo infusion. Active patients had, on average, 2.5 hours less off time per day, while participants who received the placebo infusion had an average of 35 minutes less off time per day. This improvement was apparent within the first week of treatment and was sustained over 12 weeks. At the same time, patients who received apomorphine had a significantly greater increase of on time without the dyskinesias that often are observed with levodopa, compared with placebo.
Participants were also asked to evaluate how well they thought the treatment worked. Those who received apomorphine gave their treatment higher scores at week 12 than those who received the placebo infusion. In the apomorphine group, 71% of patients felt improved, compared with 18% of patients who received placebo. Furthermore, 19% of patients worsened on apomorphine, compared with 45% on placebo. Apomorphine was generally well tolerated, and the researchers observed no serious side effects.
“It is our hope that these findings confirming the efficacy of apomorphine infusion will encourage doctors in the United States to offer this treatment to their patients and assess its efficacy in their own clinical practice,” Dr. Katzenschlager
concluded.
The study was supported by Britannia Pharmaceuticals, the maker of apomorphine.
May 2017 Quiz 2
Q2: Answer: B
Objective: Diagnose HELLP syndrome
Rationale: This patient’s presentation and laboratory findings are consistent with HELLP syndrome – the syndrome of hemolysis, elevated liver enzymes, and low platelets. HELLP is on the preeclampsia spectrum, which encompasses preeclampsia, eclampsia, and HELLP. Patients with HELLP will have hypertension (BP above 140/90), thrombocytopenia to less than 100,000/mm3 and aminotransferase levels above 70 U/L.
The diagnosis can be confirmed with an LDH (lactate dehydrogenase) greater than 600 U/L and microangiopathic hemolytic anemia on peripheral blood smear. On liver biopsy, HELLP is characterized by periportal or focal parenchyma necrosis with hyaline deposition of fibrin material in the sinusoids. However, liver biopsies are rarely performed in this setting as it likely will not change management (delivery of the fetus) and it exposes the mother and fetus to additional risks.
There is significant overlap between HELLP and acute fatty liver of pregnancy, although elevated prothrombin and partial thromboplastin time, severe hypoglycemia, and elevated creatinine are more common in acute fatty liver of pregnancy. Hypertension is more common in HELLP, and therefore this patient’s presentation is more consistent with HELLP.
Reference
1. Kia L, Rinella ME. Interpretation and management of hepatic abnormalities in pregnancy. Clin Gastroenterol Hepatol. 2013;11(11):1392-8.
Q2: Answer: B
Objective: Diagnose HELLP syndrome
Rationale: This patient’s presentation and laboratory findings are consistent with HELLP syndrome – the syndrome of hemolysis, elevated liver enzymes, and low platelets. HELLP is on the preeclampsia spectrum, which encompasses preeclampsia, eclampsia, and HELLP. Patients with HELLP will have hypertension (BP above 140/90), thrombocytopenia to less than 100,000/mm3 and aminotransferase levels above 70 U/L.
The diagnosis can be confirmed with an LDH (lactate dehydrogenase) greater than 600 U/L and microangiopathic hemolytic anemia on peripheral blood smear. On liver biopsy, HELLP is characterized by periportal or focal parenchyma necrosis with hyaline deposition of fibrin material in the sinusoids. However, liver biopsies are rarely performed in this setting as it likely will not change management (delivery of the fetus) and it exposes the mother and fetus to additional risks.
There is significant overlap between HELLP and acute fatty liver of pregnancy, although elevated prothrombin and partial thromboplastin time, severe hypoglycemia, and elevated creatinine are more common in acute fatty liver of pregnancy. Hypertension is more common in HELLP, and therefore this patient’s presentation is more consistent with HELLP.
Reference
1. Kia L, Rinella ME. Interpretation and management of hepatic abnormalities in pregnancy. Clin Gastroenterol Hepatol. 2013;11(11):1392-8.
Q2: Answer: B
Objective: Diagnose HELLP syndrome
Rationale: This patient’s presentation and laboratory findings are consistent with HELLP syndrome – the syndrome of hemolysis, elevated liver enzymes, and low platelets. HELLP is on the preeclampsia spectrum, which encompasses preeclampsia, eclampsia, and HELLP. Patients with HELLP will have hypertension (BP above 140/90), thrombocytopenia to less than 100,000/mm3 and aminotransferase levels above 70 U/L.
The diagnosis can be confirmed with an LDH (lactate dehydrogenase) greater than 600 U/L and microangiopathic hemolytic anemia on peripheral blood smear. On liver biopsy, HELLP is characterized by periportal or focal parenchyma necrosis with hyaline deposition of fibrin material in the sinusoids. However, liver biopsies are rarely performed in this setting as it likely will not change management (delivery of the fetus) and it exposes the mother and fetus to additional risks.
There is significant overlap between HELLP and acute fatty liver of pregnancy, although elevated prothrombin and partial thromboplastin time, severe hypoglycemia, and elevated creatinine are more common in acute fatty liver of pregnancy. Hypertension is more common in HELLP, and therefore this patient’s presentation is more consistent with HELLP.
Reference
1. Kia L, Rinella ME. Interpretation and management of hepatic abnormalities in pregnancy. Clin Gastroenterol Hepatol. 2013;11(11):1392-8.
Q2. A consult is requested for a 32-year-old woman who is 29 weeks pregnant and has presented to the emergency department with nausea, vomiting, and right upper quadrant abdominal pain. She is afebrile, pulse 89, BP 160/105. On exam, she has mild to moderate epigastric and right upper quadrant tenderness. Her labs are notable for WBCs 13.0 x 109/L, Hgb 8.9 g/dL, platelets 55,000 x 109/L, AST 145 U/L, total bilirubin 2.1 mg/dL; PT and PTT are normal, blood glucose is 110 mg/dL.
After the epidemic, Ebola’s destructive power still haunts survivors
VIENNA – The Ebola crisis may be over in Sierra Leone, but the suffering is not.
The last patient from the epidemic was discharged in February 2016, but 78% of survivors now appear to have one or more sequelae of the infection. Some problems are mild, but some are so debilitating that life may never be the same.
Janet Scott, MD, of the University of Liverpool (England), heads a task force studying Ebola’s lingering aftereffects. These fall into four categories, Dr. Scott said at the European Society of Clinical Microbiology and Infectious Diseases annual congress: musculoskeletal pain, headache, eye problems, and psychological disorders.
They add up to an enormous risk of disability – survivors are more than 200 times more likely than controls to express at least moderate disability.
Dr. Scott and her team of researchers are partnering with clinicians and data managers in the Ebola treatment unit in the 34th Regimental Military Hospital (MH34) in Freetown, Sierra Leone. In Sierra Leone alone, she said, there were nearly 9,000 cases and 3,500 deaths from the virus; about 5,000 patients survived. So far, Dr. Scott and her team have collected data on about 500 patients for whom they also provide free health care.
The project has six arms, each headed by an expert: clinical care, data collection, disability, neurology, ophthalmology, and psychiatry.
The team sees patients in a large tent sectioned by a plywood wall*. Wireless Internet access, which she said is “enormously expensive” in Sierra Leone, has been donated by Omline Business Communications*. It’s the team’s lifeline, allowing them to transmit data between Freetown and participating units around the world. Members also Skype regularly, talking with patients and with each other.
All patients who come into the clinic have an initial visit that includes collection of demographics, their Ebola and clinical history (including an exploration of comorbidities), a maternal health screening for women, vital signs and symptom assessment, medication dispensing, and a treatment plan.
Then they visit the specialists, either onsite or through local referral*. These specialist modules include joints, eyes, headache, ears, neurology, cardiac, respiratory, gastrointestinal, renal and urologic, reproductive health for both genders, and psychiatry.
Last year, Dr. Scott published initial data on 44 patients. At ECCMID 2017, she expanded that report to include 203 survivors. They spanned all ages, but about 67% were in their most productive adult years, aged 20-39 years.
Her findings are striking: About 78% report musculoskeletal pain, with many saying they have trouble walking even short distances, climbing stairs, or picking up their children.
Headache was the next most common problem, reported by nearly 40%. About 15% report ocular problems, which include anterior uveitis, cataracts – even in very young children – and retinal lesions. Abdominal and chest pain affect about 10% of the survivors.
Although she didn’t present specific numbers, Dr. Scott also said that many of the survivors experience psychological sequelae, including insomnia, anxiety, and depression. Whether this is related to viral pathology isn’t clear; it could be a not-unexpected response to the trauma of living through the epidemic.
“Many of these people have lost their entire family, and those that are left now shun them,” she said in a live video interview on Facebook. “It’s almost like a post-traumatic stress reaction.”
The other symptoms probably are related to the disease pathology, she observed. “Unfortunately, we don’t have all the clinical details of the acute phase for everyone, but for those for whom we do have details, we are seeing correlation between some of the problems with viral loads at admission, and even episodes of becoming unconscious during the acute illness.”
Patrick Howlett, MD, of the King’s Sierra Leone Partnership, Freetown, leads the neurology study. So far, the researchers have collected data on 19 patients with severe neurological consequences. Of those, 12 (63%) experienced a period of unconsciousness during their acute Ebola episode. In a comparator group of 21 with nonsevere neurologic sequelae, 33% had experienced unconsciousness.
Headache was present in nine (47%) of the patients. Migraine was the most common diagnosis. “We don’t have money for migraine medications, but fortunately, most of our migraine patients seem to be doing well on beta blockers,” Dr. Scott said.
CT scans were performed on 17 patients: three showed cerebral or cerebellar atrophy and two had confirmed stroke.
The brain injuries were severe in two, including a 42-year-old with extensive gliosis in the left middle cerebral artery region and a dilated left ventricle secondary to loss of volume in that hemisphere. A 12-year-old girl showed extensive parietal and temporal lobe atrophy. She is now so disabled that her family can’t care for her at home.
Other neurological problems include peripheral neuropathy, brachial plexus neuropathy, and asymmetric lower limb muscular atrophy.
Paul Steptoe, MD, an ophthalmic registrar from St. Paul’s Eye Unit at the Royal Liverpool Hospital, heads the eye study. He has observed dense cataracts, even in children, and anterior uveitis that has blinded some patients. There is concern about live virus persisting in vitreal fluid, but two eye taps have been negative, Dr. Scott said.
The most exciting recent finding, however, was made possible by the donation of a digital retinal camera, which “enabled us to get dozens of amazing images,” Dr. Scott said. With it, Dr. Steptoe conducted a case-control study of 81 Ebola survivors and 106 community controls. The findings of this study are potentially very, very important, Dr. Scott said.
“The first thing we found out is that retinal scarring is pervasive in our control patients,” she said. “There is just a lot of it out here in the community. But more interesting is that Dr. Steptoe seems to have identified a characteristic retinal lesion seen only in our survivors. It could be evidence of neurotropic aspects of the Ebola virus.”
The lesions occurred in 12 (15%) of the survivors and none of the controls. They are of a striking and consistent shape: straight-edged and sharply angulated. The lesions are only on the surface of the retina and do not penetrate into deeper levels. Nor do they interfere with vision. Dr. Steptoe has proposed that they take their angular shape from the retina’s underlying structures. His paper documenting this finding has been accepted and will be published shortly in the journal Emerging Infectious Diseases.
All of the post-Ebola sequelae add up to general disability for survivors, Dr. Scott said. Soushieta Jagadesh, of the* Liverpool School of Tropical Medicine, is conducting a disability survey. The comparison between 27 survivors and 54 community controls employed the Washington Group extended disability questionnaire. “We noted major limitations 1 year after discharge in mobility, vision, cognition, and affect,” Dr. Scott said.
The hazard ratios for these issues are enormous: Overall, compared with controls, survivors were 23 times more likely to have some level of disability. They were 94 times more likely to have walking limitations and 65 times more likely to have problems with stairs. Survivors were over 200 times more likely to have moderate disability than were their unaffected neighbors.
If funding for the project is renewed – and Dr. Scott admitted this is an “if,” not a “when” – caring for and studying these survivors will continue. Just in this one city, she said, the need is huge.
According to data from the U.S. Centers for Disease Control and Prevention, more than 17,000 patients in Sierra Leone, Liberia, and Guinea survived the 2014 Ebola outbreak.
If the assessments of Freetown survivors hold true across this population, thousands of survivors face life-limiting sequelae of the disease.
“We still have patients walk in every day with musculoskeletal pain, headaches, and ocular issues,” Dr. Scott said. “At the beginning of the epidemic, we were just focusing on containing it and reducing transmission. Now, we are faced with the long-term consequences.”
The Wellcome Trust supported the study. The authors have been awarded a grant from the Enhancing Research Activity in Epidemic Situations (ERAES) program, funded by the Wellcome Trust to support further research into the sequelae of Ebola virus disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @Alz_gal
*This story was updated May 2, 2017.
VIENNA – The Ebola crisis may be over in Sierra Leone, but the suffering is not.
The last patient from the epidemic was discharged in February 2016, but 78% of survivors now appear to have one or more sequelae of the infection. Some problems are mild, but some are so debilitating that life may never be the same.
Janet Scott, MD, of the University of Liverpool (England), heads a task force studying Ebola’s lingering aftereffects. These fall into four categories, Dr. Scott said at the European Society of Clinical Microbiology and Infectious Diseases annual congress: musculoskeletal pain, headache, eye problems, and psychological disorders.
They add up to an enormous risk of disability – survivors are more than 200 times more likely than controls to express at least moderate disability.
Dr. Scott and her team of researchers are partnering with clinicians and data managers in the Ebola treatment unit in the 34th Regimental Military Hospital (MH34) in Freetown, Sierra Leone. In Sierra Leone alone, she said, there were nearly 9,000 cases and 3,500 deaths from the virus; about 5,000 patients survived. So far, Dr. Scott and her team have collected data on about 500 patients for whom they also provide free health care.
The project has six arms, each headed by an expert: clinical care, data collection, disability, neurology, ophthalmology, and psychiatry.
The team sees patients in a large tent sectioned by a plywood wall*. Wireless Internet access, which she said is “enormously expensive” in Sierra Leone, has been donated by Omline Business Communications*. It’s the team’s lifeline, allowing them to transmit data between Freetown and participating units around the world. Members also Skype regularly, talking with patients and with each other.
All patients who come into the clinic have an initial visit that includes collection of demographics, their Ebola and clinical history (including an exploration of comorbidities), a maternal health screening for women, vital signs and symptom assessment, medication dispensing, and a treatment plan.
Then they visit the specialists, either onsite or through local referral*. These specialist modules include joints, eyes, headache, ears, neurology, cardiac, respiratory, gastrointestinal, renal and urologic, reproductive health for both genders, and psychiatry.
Last year, Dr. Scott published initial data on 44 patients. At ECCMID 2017, she expanded that report to include 203 survivors. They spanned all ages, but about 67% were in their most productive adult years, aged 20-39 years.
Her findings are striking: About 78% report musculoskeletal pain, with many saying they have trouble walking even short distances, climbing stairs, or picking up their children.
Headache was the next most common problem, reported by nearly 40%. About 15% report ocular problems, which include anterior uveitis, cataracts – even in very young children – and retinal lesions. Abdominal and chest pain affect about 10% of the survivors.
Although she didn’t present specific numbers, Dr. Scott also said that many of the survivors experience psychological sequelae, including insomnia, anxiety, and depression. Whether this is related to viral pathology isn’t clear; it could be a not-unexpected response to the trauma of living through the epidemic.
“Many of these people have lost their entire family, and those that are left now shun them,” she said in a live video interview on Facebook. “It’s almost like a post-traumatic stress reaction.”
The other symptoms probably are related to the disease pathology, she observed. “Unfortunately, we don’t have all the clinical details of the acute phase for everyone, but for those for whom we do have details, we are seeing correlation between some of the problems with viral loads at admission, and even episodes of becoming unconscious during the acute illness.”
Patrick Howlett, MD, of the King’s Sierra Leone Partnership, Freetown, leads the neurology study. So far, the researchers have collected data on 19 patients with severe neurological consequences. Of those, 12 (63%) experienced a period of unconsciousness during their acute Ebola episode. In a comparator group of 21 with nonsevere neurologic sequelae, 33% had experienced unconsciousness.
Headache was present in nine (47%) of the patients. Migraine was the most common diagnosis. “We don’t have money for migraine medications, but fortunately, most of our migraine patients seem to be doing well on beta blockers,” Dr. Scott said.
CT scans were performed on 17 patients: three showed cerebral or cerebellar atrophy and two had confirmed stroke.
The brain injuries were severe in two, including a 42-year-old with extensive gliosis in the left middle cerebral artery region and a dilated left ventricle secondary to loss of volume in that hemisphere. A 12-year-old girl showed extensive parietal and temporal lobe atrophy. She is now so disabled that her family can’t care for her at home.
Other neurological problems include peripheral neuropathy, brachial plexus neuropathy, and asymmetric lower limb muscular atrophy.
Paul Steptoe, MD, an ophthalmic registrar from St. Paul’s Eye Unit at the Royal Liverpool Hospital, heads the eye study. He has observed dense cataracts, even in children, and anterior uveitis that has blinded some patients. There is concern about live virus persisting in vitreal fluid, but two eye taps have been negative, Dr. Scott said.
The most exciting recent finding, however, was made possible by the donation of a digital retinal camera, which “enabled us to get dozens of amazing images,” Dr. Scott said. With it, Dr. Steptoe conducted a case-control study of 81 Ebola survivors and 106 community controls. The findings of this study are potentially very, very important, Dr. Scott said.
“The first thing we found out is that retinal scarring is pervasive in our control patients,” she said. “There is just a lot of it out here in the community. But more interesting is that Dr. Steptoe seems to have identified a characteristic retinal lesion seen only in our survivors. It could be evidence of neurotropic aspects of the Ebola virus.”
The lesions occurred in 12 (15%) of the survivors and none of the controls. They are of a striking and consistent shape: straight-edged and sharply angulated. The lesions are only on the surface of the retina and do not penetrate into deeper levels. Nor do they interfere with vision. Dr. Steptoe has proposed that they take their angular shape from the retina’s underlying structures. His paper documenting this finding has been accepted and will be published shortly in the journal Emerging Infectious Diseases.
All of the post-Ebola sequelae add up to general disability for survivors, Dr. Scott said. Soushieta Jagadesh, of the* Liverpool School of Tropical Medicine, is conducting a disability survey. The comparison between 27 survivors and 54 community controls employed the Washington Group extended disability questionnaire. “We noted major limitations 1 year after discharge in mobility, vision, cognition, and affect,” Dr. Scott said.
The hazard ratios for these issues are enormous: Overall, compared with controls, survivors were 23 times more likely to have some level of disability. They were 94 times more likely to have walking limitations and 65 times more likely to have problems with stairs. Survivors were over 200 times more likely to have moderate disability than were their unaffected neighbors.
If funding for the project is renewed – and Dr. Scott admitted this is an “if,” not a “when” – caring for and studying these survivors will continue. Just in this one city, she said, the need is huge.
According to data from the U.S. Centers for Disease Control and Prevention, more than 17,000 patients in Sierra Leone, Liberia, and Guinea survived the 2014 Ebola outbreak.
If the assessments of Freetown survivors hold true across this population, thousands of survivors face life-limiting sequelae of the disease.
“We still have patients walk in every day with musculoskeletal pain, headaches, and ocular issues,” Dr. Scott said. “At the beginning of the epidemic, we were just focusing on containing it and reducing transmission. Now, we are faced with the long-term consequences.”
The Wellcome Trust supported the study. The authors have been awarded a grant from the Enhancing Research Activity in Epidemic Situations (ERAES) program, funded by the Wellcome Trust to support further research into the sequelae of Ebola virus disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @Alz_gal
*This story was updated May 2, 2017.
VIENNA – The Ebola crisis may be over in Sierra Leone, but the suffering is not.
The last patient from the epidemic was discharged in February 2016, but 78% of survivors now appear to have one or more sequelae of the infection. Some problems are mild, but some are so debilitating that life may never be the same.
Janet Scott, MD, of the University of Liverpool (England), heads a task force studying Ebola’s lingering aftereffects. These fall into four categories, Dr. Scott said at the European Society of Clinical Microbiology and Infectious Diseases annual congress: musculoskeletal pain, headache, eye problems, and psychological disorders.
They add up to an enormous risk of disability – survivors are more than 200 times more likely than controls to express at least moderate disability.
Dr. Scott and her team of researchers are partnering with clinicians and data managers in the Ebola treatment unit in the 34th Regimental Military Hospital (MH34) in Freetown, Sierra Leone. In Sierra Leone alone, she said, there were nearly 9,000 cases and 3,500 deaths from the virus; about 5,000 patients survived. So far, Dr. Scott and her team have collected data on about 500 patients for whom they also provide free health care.
The project has six arms, each headed by an expert: clinical care, data collection, disability, neurology, ophthalmology, and psychiatry.
The team sees patients in a large tent sectioned by a plywood wall*. Wireless Internet access, which she said is “enormously expensive” in Sierra Leone, has been donated by Omline Business Communications*. It’s the team’s lifeline, allowing them to transmit data between Freetown and participating units around the world. Members also Skype regularly, talking with patients and with each other.
All patients who come into the clinic have an initial visit that includes collection of demographics, their Ebola and clinical history (including an exploration of comorbidities), a maternal health screening for women, vital signs and symptom assessment, medication dispensing, and a treatment plan.
Then they visit the specialists, either onsite or through local referral*. These specialist modules include joints, eyes, headache, ears, neurology, cardiac, respiratory, gastrointestinal, renal and urologic, reproductive health for both genders, and psychiatry.
Last year, Dr. Scott published initial data on 44 patients. At ECCMID 2017, she expanded that report to include 203 survivors. They spanned all ages, but about 67% were in their most productive adult years, aged 20-39 years.
Her findings are striking: About 78% report musculoskeletal pain, with many saying they have trouble walking even short distances, climbing stairs, or picking up their children.
Headache was the next most common problem, reported by nearly 40%. About 15% report ocular problems, which include anterior uveitis, cataracts – even in very young children – and retinal lesions. Abdominal and chest pain affect about 10% of the survivors.
Although she didn’t present specific numbers, Dr. Scott also said that many of the survivors experience psychological sequelae, including insomnia, anxiety, and depression. Whether this is related to viral pathology isn’t clear; it could be a not-unexpected response to the trauma of living through the epidemic.
“Many of these people have lost their entire family, and those that are left now shun them,” she said in a live video interview on Facebook. “It’s almost like a post-traumatic stress reaction.”
The other symptoms probably are related to the disease pathology, she observed. “Unfortunately, we don’t have all the clinical details of the acute phase for everyone, but for those for whom we do have details, we are seeing correlation between some of the problems with viral loads at admission, and even episodes of becoming unconscious during the acute illness.”
Patrick Howlett, MD, of the King’s Sierra Leone Partnership, Freetown, leads the neurology study. So far, the researchers have collected data on 19 patients with severe neurological consequences. Of those, 12 (63%) experienced a period of unconsciousness during their acute Ebola episode. In a comparator group of 21 with nonsevere neurologic sequelae, 33% had experienced unconsciousness.
Headache was present in nine (47%) of the patients. Migraine was the most common diagnosis. “We don’t have money for migraine medications, but fortunately, most of our migraine patients seem to be doing well on beta blockers,” Dr. Scott said.
CT scans were performed on 17 patients: three showed cerebral or cerebellar atrophy and two had confirmed stroke.
The brain injuries were severe in two, including a 42-year-old with extensive gliosis in the left middle cerebral artery region and a dilated left ventricle secondary to loss of volume in that hemisphere. A 12-year-old girl showed extensive parietal and temporal lobe atrophy. She is now so disabled that her family can’t care for her at home.
Other neurological problems include peripheral neuropathy, brachial plexus neuropathy, and asymmetric lower limb muscular atrophy.
Paul Steptoe, MD, an ophthalmic registrar from St. Paul’s Eye Unit at the Royal Liverpool Hospital, heads the eye study. He has observed dense cataracts, even in children, and anterior uveitis that has blinded some patients. There is concern about live virus persisting in vitreal fluid, but two eye taps have been negative, Dr. Scott said.
The most exciting recent finding, however, was made possible by the donation of a digital retinal camera, which “enabled us to get dozens of amazing images,” Dr. Scott said. With it, Dr. Steptoe conducted a case-control study of 81 Ebola survivors and 106 community controls. The findings of this study are potentially very, very important, Dr. Scott said.
“The first thing we found out is that retinal scarring is pervasive in our control patients,” she said. “There is just a lot of it out here in the community. But more interesting is that Dr. Steptoe seems to have identified a characteristic retinal lesion seen only in our survivors. It could be evidence of neurotropic aspects of the Ebola virus.”
The lesions occurred in 12 (15%) of the survivors and none of the controls. They are of a striking and consistent shape: straight-edged and sharply angulated. The lesions are only on the surface of the retina and do not penetrate into deeper levels. Nor do they interfere with vision. Dr. Steptoe has proposed that they take their angular shape from the retina’s underlying structures. His paper documenting this finding has been accepted and will be published shortly in the journal Emerging Infectious Diseases.
All of the post-Ebola sequelae add up to general disability for survivors, Dr. Scott said. Soushieta Jagadesh, of the* Liverpool School of Tropical Medicine, is conducting a disability survey. The comparison between 27 survivors and 54 community controls employed the Washington Group extended disability questionnaire. “We noted major limitations 1 year after discharge in mobility, vision, cognition, and affect,” Dr. Scott said.
The hazard ratios for these issues are enormous: Overall, compared with controls, survivors were 23 times more likely to have some level of disability. They were 94 times more likely to have walking limitations and 65 times more likely to have problems with stairs. Survivors were over 200 times more likely to have moderate disability than were their unaffected neighbors.
If funding for the project is renewed – and Dr. Scott admitted this is an “if,” not a “when” – caring for and studying these survivors will continue. Just in this one city, she said, the need is huge.
According to data from the U.S. Centers for Disease Control and Prevention, more than 17,000 patients in Sierra Leone, Liberia, and Guinea survived the 2014 Ebola outbreak.
If the assessments of Freetown survivors hold true across this population, thousands of survivors face life-limiting sequelae of the disease.
“We still have patients walk in every day with musculoskeletal pain, headaches, and ocular issues,” Dr. Scott said. “At the beginning of the epidemic, we were just focusing on containing it and reducing transmission. Now, we are faced with the long-term consequences.”
The Wellcome Trust supported the study. The authors have been awarded a grant from the Enhancing Research Activity in Epidemic Situations (ERAES) program, funded by the Wellcome Trust to support further research into the sequelae of Ebola virus disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @Alz_gal
*This story was updated May 2, 2017.
AT ECCMID 2017
May 2017 Quiz 1
Q1: Answer: A
The Food and Drug Administration issued a postmarketing warning about potential for interaction between sofosbuvir and amiodarone. Nine patients taking sofosbuvir (with other antiviral agents) and amiodarone developed significant bradycardia. Seven patients were on concomitant beta-blockade. One patient died of cardiac arrest while three others required pacemaker placement. Two-thirds of the events occurred within 24 hours of coadministration while the other third occurred within 12 days. Three patients had recurrence of bradycardia with rechallenge of sofosbuvir treatment while on amiodarone. The mechanism of bradycardia is not fully understood. Amiodarone is considered an absolute contraindication to the use of a sofosbuvir-containing regimen. The sofosbuvir-containing regimens listed are endorsed by the AASLD/IDSA joint guidelines for treatment of genotype 1a hepatitis C, as long as the patient is not on amiodarone, although the combination of sofosbuvir and daclatasvir is not FDA approved for genotype 1.
Reference
1. Fontaine H, Lazarus A, Pol S, et al.; Cochin Hepatology and Cardiology Group. N Engl J Med. 2015 Nov 5;373(19):1886-8.
Q1: Answer: A
The Food and Drug Administration issued a postmarketing warning about potential for interaction between sofosbuvir and amiodarone. Nine patients taking sofosbuvir (with other antiviral agents) and amiodarone developed significant bradycardia. Seven patients were on concomitant beta-blockade. One patient died of cardiac arrest while three others required pacemaker placement. Two-thirds of the events occurred within 24 hours of coadministration while the other third occurred within 12 days. Three patients had recurrence of bradycardia with rechallenge of sofosbuvir treatment while on amiodarone. The mechanism of bradycardia is not fully understood. Amiodarone is considered an absolute contraindication to the use of a sofosbuvir-containing regimen. The sofosbuvir-containing regimens listed are endorsed by the AASLD/IDSA joint guidelines for treatment of genotype 1a hepatitis C, as long as the patient is not on amiodarone, although the combination of sofosbuvir and daclatasvir is not FDA approved for genotype 1.
Reference
1. Fontaine H, Lazarus A, Pol S, et al.; Cochin Hepatology and Cardiology Group. N Engl J Med. 2015 Nov 5;373(19):1886-8.
Q1: Answer: A
The Food and Drug Administration issued a postmarketing warning about potential for interaction between sofosbuvir and amiodarone. Nine patients taking sofosbuvir (with other antiviral agents) and amiodarone developed significant bradycardia. Seven patients were on concomitant beta-blockade. One patient died of cardiac arrest while three others required pacemaker placement. Two-thirds of the events occurred within 24 hours of coadministration while the other third occurred within 12 days. Three patients had recurrence of bradycardia with rechallenge of sofosbuvir treatment while on amiodarone. The mechanism of bradycardia is not fully understood. Amiodarone is considered an absolute contraindication to the use of a sofosbuvir-containing regimen. The sofosbuvir-containing regimens listed are endorsed by the AASLD/IDSA joint guidelines for treatment of genotype 1a hepatitis C, as long as the patient is not on amiodarone, although the combination of sofosbuvir and daclatasvir is not FDA approved for genotype 1.
Reference
1. Fontaine H, Lazarus A, Pol S, et al.; Cochin Hepatology and Cardiology Group. N Engl J Med. 2015 Nov 5;373(19):1886-8.
Q1. A 58-year-old woman with genotype 1a HCV presents for reevaluation. She is treatment naive and a recent transient elastography reveals stage 3 fibrosis. Her past medical history is notable for atrial fibrillation, hypertension, and dyslipidemia. Medications include amiodarone, lisinopril, and atorvastatin.
High readmits after peripheral arterial procedures
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
WASHINGTON – More than one in six patients who undergo a lower extremity arterial endovascular or surgical procedure are readmitted within 30 days, according to a large national study.
The annual total cost of these early readmissions is high, in excess of $360 million. But because there turned out to be surprisingly little difference in readmission rates between hospitals, 30-day readmissions may not be a rational quality measure on which to base institutional reimbursement or withholding of payment for peripheral arterial interventions, Eric A. Secemsky, MD, said at the annual meeting of the American College of Cardiology.
Forty-seven percent of patients had an endovascular procedure, 42% had surgery, and the remainder had hybrid procedures in which both endovascular and surgical interventions took place during the same admission. Patients with hybrid procedures contributed data to both treatment groups.
In-hospital mortality occurred in 2.5% of patients.
Of the patients who survived to discharge, 21,589, or 17.4%, were readmitted within 30 days. The early readmission rate was higher following endovascular procedures, at 18.7%, than the 16.1% rate in the surgical group. The average cost of a readmission was $15,876. Death during readmission occurred in 4.2% of patients.
The median rate ratio – a measure of the amount of variance in readmission rates between hospitals – was 1.12. That’s a low figure.
“If the median rate ratio is lower, like here, it says there’s not a lot of interhospital variability across the country. So overall this burden seems to be pretty uniform across the institutions included in our analysis,” Dr. Secemsky explained.
This observation drew the attention of session comoderator Naomi M. Hamburg, MD.
“It’s interesting that you didn’t see a lot of heterogeneity across hospitals, because we often think of readmissions as a potentially modifiable quality metric. Do you think it’s modifiable, or is this just the nature of the disease?” asked Dr. Hamburg of Boston Medical Center.
It’s the disease process, Dr. Secemsky replied.
“We were surprised by the lack of hospital variation,” he added. None of the institutional characteristics examined, including teaching hospital status, bed size, and procedural volume, had a significant impact on readmission rates.
But that doesn’t mean there aren’t opportunities to whittle down those readmissions, according to Dr. Secemsky.
He noted that the high readmission rates were driven by procedural complications such as graft or stent failure. Indeed, procedural complications accounted for 29% of all early readmissions. The procedural complication rate was about 20% following endovascular procedures and 39% after surgery. It’s likely that identification and implementation of best practices could trim those high rates. Unfortunately, however, the nationwide database relies upon ICD-9 codes, which don’t provide the granular level of detail required to home in on specific best practices. That will require further studies, according to Dr. Secemsky.
A distant second on the list of causes of early readmission was peripheral atherosclerosis, meaning persistent claudication or rest pain. This accounted for 8.8% of readmissions. Rounding out the top five causes of readmission were sepsis, which was the reason for 6.7% of readmissions; diabetes with complications, at 4.7%; and heart failure, at 4.6%.
The strongest predictors of readmission included having renal disease at baseline, Medicare rather than private insurance, and discharge to a subacute nursing facility or home with home care.
Dr. Hamburg commented that a focus on reducing readmissions for sepsis as well as for skin and soft tissue infections, which accounted for 2.1% of 30-day hospitalizations, could be fruitful.
Dr. Secemsky reported having no financial conflicts regarding his study.
AT ACC 2017
Key clinical point:
Major finding: Readmission within 30 days after a peripheral arterial procedure occurred nationally in 17.4% of patients, with little between-hospital variation in rates.
Data source: A retrospective analysis of nearly 124,000 hospital admissions for lower extremity arterial endovascular or surgical procedures.
Disclosures: The study presenter reported having no financial conflicts of interest.
Drugmakers Dramatically Boosted Lobbying Spending In Trump’s First Quarter
Eight pharmaceutical companies more than doubled their lobbying spending in the first three months of 2017, when the Affordable Care Act was on the chopping block and high drug prices were clearly in the crosshairs of Congress and President Donald Trump.
Congressional records show that those eight, including Celgene and Mylan, kicked in an extra $4.42 million versus that quarter last year. Industry giant Teva Pharmaceutical Industries spent $2.67 million, up 115% from a year ago as several companies embroiled in controversies raised their outlays significantly.
“It’s certainly a rare event” when lobbying dollars double, noted Timothy LaPira, PhD, an associate professor of political science at James Madison University. “These spikes are usually timed when Congress in particular is going to be really hammering home on a particular issue. Right now, that’s health care and taxes.”
Trump has come down hard on drugmakers, stating in a press conference before his inauguration that the industry is “getting away with murder.” He has promised to lower drug prices and increase competition with faster approvals and fewer regulations. Sen. Bernie Sanders (I-Vt.), Sen. John McCain (R-Ariz.), and Rep. Elijah E. Cummings (D-Md.) have introduced bills to allow lower-cost drug imports from Canada or other countries.
Lobbyists weren’t expecting much by way of big policy changes during the comparatively sleepy end of the Obama administration this time last year, but, with a surprise Trump administration and a Republican-controlled House and Senate, trade groups and companies are probably “going all in,” Dr. LaPira said.
Thirty-eight major drugmakers and trade groups spent a total of $50.9 million, up $10.1 million from the first quarter of last year, according to a Kaiser Health News analysis. They deployed 600 lobbyists in all.
PhRMA, the drug industry’s largest trade group, spent $7.98 million during the quarter – more than in any single quarter in almost a decade, congressional records show, topping even its quarterly lobbying ahead of the Affordable Care Act’s passage in 2010.
In their congressional disclosures, companies listed Medicare price negotiation, the American Health Care Act, drug importation, and the orphan drug program as issues they were lobbying for or against. They do not have to disclose on which side of an issue they lobbied.
When Medicare prices are on the table, it should come as no surprise that pharmaceutical companies are interested in influencing congress.
“It’s quite literally hitting their bottom line,” LaPira said.
Drugmakers, under fire, more than doubled their lobbying dollars. Mylan spent $1.45 million during the quarter, up from $610,000 last year. The company’s CEO faced a congressional hearing in the fall when it raised the price of EpiPen to over $600.
Marathon Pharmaceuticals spent $230,000, which was $120,000 more than last year. Marathon was criticized in February after setting the price of Emflaza, a steroid to treat Duchenne muscular dystrophy, at $89,000 a year. That angered advocates, Congress, and patients who had been importing the same drug for as little as $1,000 a year. Marathon has since sold the drug to another company, and the price may come down.
Teva and Shire also more than doubled their spending. Teva was accused, as part of an alleged generic price-fixing scheme in December, and the Federal Trade Commission sued Shire because one of its recently acquired companies allegedly filed “sham” petitions with the Food and Drug Administration to stave off generics.
Companies that make drugs for rare diseases also more than doubled lobbying dollars as congressional leaders and the Government Accountability Office work to determine whether the Orphan Drug Act is being abused. Those firms include BioMarin, Celgene, and Vertex Pharmaceuticals. Celgene, which makes a rare cancer drug, more than tripled its first quarter lobbying to more than $1 million.
Despite efforts to make good on campaign promises to repeal the Affordable Care Act, House Republicans canceled a floor vote on the American Health Care Act in March after multiple studies estimated that millions of people would lose coverage if it passed, and neither Democrats nor ultraconservatives lined up in opposition to the bill’s provisions. Drug prices weren’t a key part of the package.
KHN’s coverage of prescription drug development, costs, and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Eight pharmaceutical companies more than doubled their lobbying spending in the first three months of 2017, when the Affordable Care Act was on the chopping block and high drug prices were clearly in the crosshairs of Congress and President Donald Trump.
Congressional records show that those eight, including Celgene and Mylan, kicked in an extra $4.42 million versus that quarter last year. Industry giant Teva Pharmaceutical Industries spent $2.67 million, up 115% from a year ago as several companies embroiled in controversies raised their outlays significantly.
“It’s certainly a rare event” when lobbying dollars double, noted Timothy LaPira, PhD, an associate professor of political science at James Madison University. “These spikes are usually timed when Congress in particular is going to be really hammering home on a particular issue. Right now, that’s health care and taxes.”
Trump has come down hard on drugmakers, stating in a press conference before his inauguration that the industry is “getting away with murder.” He has promised to lower drug prices and increase competition with faster approvals and fewer regulations. Sen. Bernie Sanders (I-Vt.), Sen. John McCain (R-Ariz.), and Rep. Elijah E. Cummings (D-Md.) have introduced bills to allow lower-cost drug imports from Canada or other countries.
Lobbyists weren’t expecting much by way of big policy changes during the comparatively sleepy end of the Obama administration this time last year, but, with a surprise Trump administration and a Republican-controlled House and Senate, trade groups and companies are probably “going all in,” Dr. LaPira said.
Thirty-eight major drugmakers and trade groups spent a total of $50.9 million, up $10.1 million from the first quarter of last year, according to a Kaiser Health News analysis. They deployed 600 lobbyists in all.
PhRMA, the drug industry’s largest trade group, spent $7.98 million during the quarter – more than in any single quarter in almost a decade, congressional records show, topping even its quarterly lobbying ahead of the Affordable Care Act’s passage in 2010.
In their congressional disclosures, companies listed Medicare price negotiation, the American Health Care Act, drug importation, and the orphan drug program as issues they were lobbying for or against. They do not have to disclose on which side of an issue they lobbied.
When Medicare prices are on the table, it should come as no surprise that pharmaceutical companies are interested in influencing congress.
“It’s quite literally hitting their bottom line,” LaPira said.
Drugmakers, under fire, more than doubled their lobbying dollars. Mylan spent $1.45 million during the quarter, up from $610,000 last year. The company’s CEO faced a congressional hearing in the fall when it raised the price of EpiPen to over $600.
Marathon Pharmaceuticals spent $230,000, which was $120,000 more than last year. Marathon was criticized in February after setting the price of Emflaza, a steroid to treat Duchenne muscular dystrophy, at $89,000 a year. That angered advocates, Congress, and patients who had been importing the same drug for as little as $1,000 a year. Marathon has since sold the drug to another company, and the price may come down.
Teva and Shire also more than doubled their spending. Teva was accused, as part of an alleged generic price-fixing scheme in December, and the Federal Trade Commission sued Shire because one of its recently acquired companies allegedly filed “sham” petitions with the Food and Drug Administration to stave off generics.
Companies that make drugs for rare diseases also more than doubled lobbying dollars as congressional leaders and the Government Accountability Office work to determine whether the Orphan Drug Act is being abused. Those firms include BioMarin, Celgene, and Vertex Pharmaceuticals. Celgene, which makes a rare cancer drug, more than tripled its first quarter lobbying to more than $1 million.
Despite efforts to make good on campaign promises to repeal the Affordable Care Act, House Republicans canceled a floor vote on the American Health Care Act in March after multiple studies estimated that millions of people would lose coverage if it passed, and neither Democrats nor ultraconservatives lined up in opposition to the bill’s provisions. Drug prices weren’t a key part of the package.
KHN’s coverage of prescription drug development, costs, and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Eight pharmaceutical companies more than doubled their lobbying spending in the first three months of 2017, when the Affordable Care Act was on the chopping block and high drug prices were clearly in the crosshairs of Congress and President Donald Trump.
Congressional records show that those eight, including Celgene and Mylan, kicked in an extra $4.42 million versus that quarter last year. Industry giant Teva Pharmaceutical Industries spent $2.67 million, up 115% from a year ago as several companies embroiled in controversies raised their outlays significantly.
“It’s certainly a rare event” when lobbying dollars double, noted Timothy LaPira, PhD, an associate professor of political science at James Madison University. “These spikes are usually timed when Congress in particular is going to be really hammering home on a particular issue. Right now, that’s health care and taxes.”
Trump has come down hard on drugmakers, stating in a press conference before his inauguration that the industry is “getting away with murder.” He has promised to lower drug prices and increase competition with faster approvals and fewer regulations. Sen. Bernie Sanders (I-Vt.), Sen. John McCain (R-Ariz.), and Rep. Elijah E. Cummings (D-Md.) have introduced bills to allow lower-cost drug imports from Canada or other countries.
Lobbyists weren’t expecting much by way of big policy changes during the comparatively sleepy end of the Obama administration this time last year, but, with a surprise Trump administration and a Republican-controlled House and Senate, trade groups and companies are probably “going all in,” Dr. LaPira said.
Thirty-eight major drugmakers and trade groups spent a total of $50.9 million, up $10.1 million from the first quarter of last year, according to a Kaiser Health News analysis. They deployed 600 lobbyists in all.
PhRMA, the drug industry’s largest trade group, spent $7.98 million during the quarter – more than in any single quarter in almost a decade, congressional records show, topping even its quarterly lobbying ahead of the Affordable Care Act’s passage in 2010.
In their congressional disclosures, companies listed Medicare price negotiation, the American Health Care Act, drug importation, and the orphan drug program as issues they were lobbying for or against. They do not have to disclose on which side of an issue they lobbied.
When Medicare prices are on the table, it should come as no surprise that pharmaceutical companies are interested in influencing congress.
“It’s quite literally hitting their bottom line,” LaPira said.
Drugmakers, under fire, more than doubled their lobbying dollars. Mylan spent $1.45 million during the quarter, up from $610,000 last year. The company’s CEO faced a congressional hearing in the fall when it raised the price of EpiPen to over $600.
Marathon Pharmaceuticals spent $230,000, which was $120,000 more than last year. Marathon was criticized in February after setting the price of Emflaza, a steroid to treat Duchenne muscular dystrophy, at $89,000 a year. That angered advocates, Congress, and patients who had been importing the same drug for as little as $1,000 a year. Marathon has since sold the drug to another company, and the price may come down.
Teva and Shire also more than doubled their spending. Teva was accused, as part of an alleged generic price-fixing scheme in December, and the Federal Trade Commission sued Shire because one of its recently acquired companies allegedly filed “sham” petitions with the Food and Drug Administration to stave off generics.
Companies that make drugs for rare diseases also more than doubled lobbying dollars as congressional leaders and the Government Accountability Office work to determine whether the Orphan Drug Act is being abused. Those firms include BioMarin, Celgene, and Vertex Pharmaceuticals. Celgene, which makes a rare cancer drug, more than tripled its first quarter lobbying to more than $1 million.
Despite efforts to make good on campaign promises to repeal the Affordable Care Act, House Republicans canceled a floor vote on the American Health Care Act in March after multiple studies estimated that millions of people would lose coverage if it passed, and neither Democrats nor ultraconservatives lined up in opposition to the bill’s provisions. Drug prices weren’t a key part of the package.
KHN’s coverage of prescription drug development, costs, and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Targeted drugs transform CLL management
NEW YORK – New, targeted treatments, especially ibrutinib (Imbruvica), have sharply shifted prognosis for patients with chronic lymphocytic leukemia (CLL) and raised new issues for managing these patients now that they survive years longer.
“Ibrutinib has produced a profound change in survival” of patients with CLL, Timothy G. Call, MD, a hematologist/oncologist at the Mayo Clinic in Rochester, Minn., said at a conference held by Imedex. It “has changed the playing field.” No other new agent so far “has produced the same level of progression-free survival in CLL.”
An analysis published in early 2017 projected a greater than 50% jump in U.S. patients living with CLL from 2011, before the advent of targeted oral drugs, to 2025, when the study predicted that there will be nearly 200,000 U.S. patients living with CLL (J Clin Oncol. 2017 January;35[2]:166-74). With targeted drugs like ibrutinib and idelalisib (Zydelig) costing about $130,000 per patient each year, the projected cost for managing the U.S. CLL population is on track to rise to more than $5 billion by 2025, a nearly sixfold increase, compared with CLL patient expenditures in 2011, according to this analysis.
The impact of the higher cost of treatment is already being felt more acutely by many patients because of recent cuts in assistance from the Patient Access Network, which helps patients with copays but recently had to put a lid on CLL assistance availability when its funding availability hit a wall, Dr. Call said.
On the clinical side, there are new considerations triggered by greater patient longevity. “As we make patients live longer with CLL, we need to double down on the diagnosis and treatment of its complications,” such as watching for development of secondary cancers, Dr. Call said in an interview. This stems from the reduced immunosurveillance in patients with CLL and their resulting increased susceptibility to developing environmentally-triggered malignancies like lung and skin cancers. Other long-term implications of impaired immunosurveillance include increased infection susceptibility, an ongoing risk for Richter’s or Hodgkin lymphoma transformation, and a risk for autoimmune complications, such as red blood cell aplasia and transfusion-associated graft versus host disease.
Patients with CLL can also be susceptible to complications from long-term use of the targeted drug they’re on. The new targeted agents can trigger bruising and bleeding, diarrhea, rash, fatigue, muscle and joint aches, and arrhythmia, he noted.
Potential adverse effects, specifically from ibrutinib, include a 3% risk for a major bleed, a 10% rate of new-onset atrial fibrillation, and a 20% risk for new hypertension, Dr. Call said. Before starting ibrutinib, patients should undergo screening for hepatitis B virus infection and receive prophylaxis against herpes zoster activation with acyclovir or valacyclovir. If the patient starts with a CD4 cell count below 200 cells/mm3, it might be prudent to prophylax the patient against Pneumocystis jirovecii pneumonia.
However, even if a toxicity develops on ibrutinib, Dr. Call recommended reducing the dosage rather than discontinuing the drug. “I rarely see a loss in response from a reduced dosage of ibrutinib,” he said.
Because ibrutinib is primarily metabolized via the liver enzyme cytochrome P450 3A (CYP3A), other drugs that enhance or reduce the activity of this enzyme produce significant changes in ibrutinib levels. The Food and Drug Administration considers ibrutinib a “sensitive substrate” for fluctuations in CYP3A activity. Strong CYP3A inhibitors include clarithromycin, ketoconazole, and various anti-HIV medications; moderate CYP3A4 inhibitors include ciprofloxacin, and verapamil; and inducers of CYP3A include phenytoin and rifampin. A more complete list of the drugs that inhibit or induce CYP3A activity can be found at the FDA website: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#table2-2.
Dr. Call presented the combined experience from several U.S. Mayo Clinic centers for 118 patients treated with ibrutinib after the drug received FDA marketing approval in November 2013. The clinicians identified 75 patients (64%) who were on a concurrent medication that could potentially increase the risk for ibrutinib toxicity and 4 patients (3%) who were on a concurrent drug with the potential to reduce ibrutinib efficacy (Leukemia Lymphoma, 2017;58[6]:1376-83).
“We don’t change the dosage of ibrutinib when the patient is on a CYP3A inducer, but, if the patient is getting a CYP3A inhibitor, we change that to another drug or reduce the ibrutinib dosage,” Dr. Call said.
Dr. Call had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW YORK – New, targeted treatments, especially ibrutinib (Imbruvica), have sharply shifted prognosis for patients with chronic lymphocytic leukemia (CLL) and raised new issues for managing these patients now that they survive years longer.
“Ibrutinib has produced a profound change in survival” of patients with CLL, Timothy G. Call, MD, a hematologist/oncologist at the Mayo Clinic in Rochester, Minn., said at a conference held by Imedex. It “has changed the playing field.” No other new agent so far “has produced the same level of progression-free survival in CLL.”
An analysis published in early 2017 projected a greater than 50% jump in U.S. patients living with CLL from 2011, before the advent of targeted oral drugs, to 2025, when the study predicted that there will be nearly 200,000 U.S. patients living with CLL (J Clin Oncol. 2017 January;35[2]:166-74). With targeted drugs like ibrutinib and idelalisib (Zydelig) costing about $130,000 per patient each year, the projected cost for managing the U.S. CLL population is on track to rise to more than $5 billion by 2025, a nearly sixfold increase, compared with CLL patient expenditures in 2011, according to this analysis.
The impact of the higher cost of treatment is already being felt more acutely by many patients because of recent cuts in assistance from the Patient Access Network, which helps patients with copays but recently had to put a lid on CLL assistance availability when its funding availability hit a wall, Dr. Call said.
On the clinical side, there are new considerations triggered by greater patient longevity. “As we make patients live longer with CLL, we need to double down on the diagnosis and treatment of its complications,” such as watching for development of secondary cancers, Dr. Call said in an interview. This stems from the reduced immunosurveillance in patients with CLL and their resulting increased susceptibility to developing environmentally-triggered malignancies like lung and skin cancers. Other long-term implications of impaired immunosurveillance include increased infection susceptibility, an ongoing risk for Richter’s or Hodgkin lymphoma transformation, and a risk for autoimmune complications, such as red blood cell aplasia and transfusion-associated graft versus host disease.
Patients with CLL can also be susceptible to complications from long-term use of the targeted drug they’re on. The new targeted agents can trigger bruising and bleeding, diarrhea, rash, fatigue, muscle and joint aches, and arrhythmia, he noted.
Potential adverse effects, specifically from ibrutinib, include a 3% risk for a major bleed, a 10% rate of new-onset atrial fibrillation, and a 20% risk for new hypertension, Dr. Call said. Before starting ibrutinib, patients should undergo screening for hepatitis B virus infection and receive prophylaxis against herpes zoster activation with acyclovir or valacyclovir. If the patient starts with a CD4 cell count below 200 cells/mm3, it might be prudent to prophylax the patient against Pneumocystis jirovecii pneumonia.
However, even if a toxicity develops on ibrutinib, Dr. Call recommended reducing the dosage rather than discontinuing the drug. “I rarely see a loss in response from a reduced dosage of ibrutinib,” he said.
Because ibrutinib is primarily metabolized via the liver enzyme cytochrome P450 3A (CYP3A), other drugs that enhance or reduce the activity of this enzyme produce significant changes in ibrutinib levels. The Food and Drug Administration considers ibrutinib a “sensitive substrate” for fluctuations in CYP3A activity. Strong CYP3A inhibitors include clarithromycin, ketoconazole, and various anti-HIV medications; moderate CYP3A4 inhibitors include ciprofloxacin, and verapamil; and inducers of CYP3A include phenytoin and rifampin. A more complete list of the drugs that inhibit or induce CYP3A activity can be found at the FDA website: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#table2-2.
Dr. Call presented the combined experience from several U.S. Mayo Clinic centers for 118 patients treated with ibrutinib after the drug received FDA marketing approval in November 2013. The clinicians identified 75 patients (64%) who were on a concurrent medication that could potentially increase the risk for ibrutinib toxicity and 4 patients (3%) who were on a concurrent drug with the potential to reduce ibrutinib efficacy (Leukemia Lymphoma, 2017;58[6]:1376-83).
“We don’t change the dosage of ibrutinib when the patient is on a CYP3A inducer, but, if the patient is getting a CYP3A inhibitor, we change that to another drug or reduce the ibrutinib dosage,” Dr. Call said.
Dr. Call had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW YORK – New, targeted treatments, especially ibrutinib (Imbruvica), have sharply shifted prognosis for patients with chronic lymphocytic leukemia (CLL) and raised new issues for managing these patients now that they survive years longer.
“Ibrutinib has produced a profound change in survival” of patients with CLL, Timothy G. Call, MD, a hematologist/oncologist at the Mayo Clinic in Rochester, Minn., said at a conference held by Imedex. It “has changed the playing field.” No other new agent so far “has produced the same level of progression-free survival in CLL.”
An analysis published in early 2017 projected a greater than 50% jump in U.S. patients living with CLL from 2011, before the advent of targeted oral drugs, to 2025, when the study predicted that there will be nearly 200,000 U.S. patients living with CLL (J Clin Oncol. 2017 January;35[2]:166-74). With targeted drugs like ibrutinib and idelalisib (Zydelig) costing about $130,000 per patient each year, the projected cost for managing the U.S. CLL population is on track to rise to more than $5 billion by 2025, a nearly sixfold increase, compared with CLL patient expenditures in 2011, according to this analysis.
The impact of the higher cost of treatment is already being felt more acutely by many patients because of recent cuts in assistance from the Patient Access Network, which helps patients with copays but recently had to put a lid on CLL assistance availability when its funding availability hit a wall, Dr. Call said.
On the clinical side, there are new considerations triggered by greater patient longevity. “As we make patients live longer with CLL, we need to double down on the diagnosis and treatment of its complications,” such as watching for development of secondary cancers, Dr. Call said in an interview. This stems from the reduced immunosurveillance in patients with CLL and their resulting increased susceptibility to developing environmentally-triggered malignancies like lung and skin cancers. Other long-term implications of impaired immunosurveillance include increased infection susceptibility, an ongoing risk for Richter’s or Hodgkin lymphoma transformation, and a risk for autoimmune complications, such as red blood cell aplasia and transfusion-associated graft versus host disease.
Patients with CLL can also be susceptible to complications from long-term use of the targeted drug they’re on. The new targeted agents can trigger bruising and bleeding, diarrhea, rash, fatigue, muscle and joint aches, and arrhythmia, he noted.
Potential adverse effects, specifically from ibrutinib, include a 3% risk for a major bleed, a 10% rate of new-onset atrial fibrillation, and a 20% risk for new hypertension, Dr. Call said. Before starting ibrutinib, patients should undergo screening for hepatitis B virus infection and receive prophylaxis against herpes zoster activation with acyclovir or valacyclovir. If the patient starts with a CD4 cell count below 200 cells/mm3, it might be prudent to prophylax the patient against Pneumocystis jirovecii pneumonia.
However, even if a toxicity develops on ibrutinib, Dr. Call recommended reducing the dosage rather than discontinuing the drug. “I rarely see a loss in response from a reduced dosage of ibrutinib,” he said.
Because ibrutinib is primarily metabolized via the liver enzyme cytochrome P450 3A (CYP3A), other drugs that enhance or reduce the activity of this enzyme produce significant changes in ibrutinib levels. The Food and Drug Administration considers ibrutinib a “sensitive substrate” for fluctuations in CYP3A activity. Strong CYP3A inhibitors include clarithromycin, ketoconazole, and various anti-HIV medications; moderate CYP3A4 inhibitors include ciprofloxacin, and verapamil; and inducers of CYP3A include phenytoin and rifampin. A more complete list of the drugs that inhibit or induce CYP3A activity can be found at the FDA website: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#table2-2.
Dr. Call presented the combined experience from several U.S. Mayo Clinic centers for 118 patients treated with ibrutinib after the drug received FDA marketing approval in November 2013. The clinicians identified 75 patients (64%) who were on a concurrent medication that could potentially increase the risk for ibrutinib toxicity and 4 patients (3%) who were on a concurrent drug with the potential to reduce ibrutinib efficacy (Leukemia Lymphoma, 2017;58[6]:1376-83).
“We don’t change the dosage of ibrutinib when the patient is on a CYP3A inducer, but, if the patient is getting a CYP3A inhibitor, we change that to another drug or reduce the ibrutinib dosage,” Dr. Call said.
Dr. Call had no disclosures.
[email protected]
On Twitter @mitchelzoler
Clinical Challenges - May 2017 What's Your Diagnosis?
What's Your Diagnosis?
Answer to “What’s your diagnosis?” on page 2: Arteriovenous fistulas arising from the subclavian and coronary arteries
We performed aortography along with coronary angiography to find the feeding vessels for the vascular bundle (). There was an arteriovenous fistula that arose from the left subclavian artery, ran over the left mediastinum with the complex plexus, and emptied into the venous system of the left thorax. Multiple coronary artery fistulas originated in the left coronary artery, traversed the left and right mediastinum, and eventually emptied into the venous system of the mediastinum. The left anterior oblique view revealed a coronary artery fistula that arose from the distal right coronary artery and drained into the venous system of the thorax. In transthoracic echocardiography, the sizes of the left atrium and left ventricle were mildly dilated, but left ventricular systolic functions were preserved with an ejection fraction of 61%. We recommended surgery to the patient, but he refused invasive treatment. He will be followed with close observation.
A coronary artery fistula is usually of congenital origin, and connects a major coronary artery directly with the cardiac chamber, coronary sinus, superior vena cava, or pulmonary artery. However, its connection with a systemic venous system is extremely rare. Congenital subclavian arteriovenous fistulas are rare because they usually occur as a complication of previous trauma, percutaneous catheterization, or surgery.1 Complications include “steal” from the adjacent myocardium causing myocardial ischemia, thrombosis/embolism, cardiac failure, atrial fibrillation, rupture, endocarditis/endarteritis, and arrhythmia.2 Treatment options include close medical observation, surgical ligation, and catheter embolization.3
References
1. Brountzos, E.N., Kelekis, N.L., Danassi-Afentaki, D. et al. Congenital subclavian artery-to-subclavian vein fistula in an adult: treatment with transcatheter embolization. Cardiovasc Intervent Radiol. 2004;27:675-7.
2. Wilde, P., Watt, I. Congenital coronary artery fistulae: six new cases with a collective review. Clin Radiol. 1980;31:301-11.
3. Mangukia, C.V. Coronary artery fistula. Ann Thorac Surg. 2012;93:2084-92.
Answer to “What’s your diagnosis?” on page 2: Arteriovenous fistulas arising from the subclavian and coronary arteries
We performed aortography along with coronary angiography to find the feeding vessels for the vascular bundle (). There was an arteriovenous fistula that arose from the left subclavian artery, ran over the left mediastinum with the complex plexus, and emptied into the venous system of the left thorax. Multiple coronary artery fistulas originated in the left coronary artery, traversed the left and right mediastinum, and eventually emptied into the venous system of the mediastinum. The left anterior oblique view revealed a coronary artery fistula that arose from the distal right coronary artery and drained into the venous system of the thorax. In transthoracic echocardiography, the sizes of the left atrium and left ventricle were mildly dilated, but left ventricular systolic functions were preserved with an ejection fraction of 61%. We recommended surgery to the patient, but he refused invasive treatment. He will be followed with close observation.
A coronary artery fistula is usually of congenital origin, and connects a major coronary artery directly with the cardiac chamber, coronary sinus, superior vena cava, or pulmonary artery. However, its connection with a systemic venous system is extremely rare. Congenital subclavian arteriovenous fistulas are rare because they usually occur as a complication of previous trauma, percutaneous catheterization, or surgery.1 Complications include “steal” from the adjacent myocardium causing myocardial ischemia, thrombosis/embolism, cardiac failure, atrial fibrillation, rupture, endocarditis/endarteritis, and arrhythmia.2 Treatment options include close medical observation, surgical ligation, and catheter embolization.3
References
1. Brountzos, E.N., Kelekis, N.L., Danassi-Afentaki, D. et al. Congenital subclavian artery-to-subclavian vein fistula in an adult: treatment with transcatheter embolization. Cardiovasc Intervent Radiol. 2004;27:675-7.
2. Wilde, P., Watt, I. Congenital coronary artery fistulae: six new cases with a collective review. Clin Radiol. 1980;31:301-11.
3. Mangukia, C.V. Coronary artery fistula. Ann Thorac Surg. 2012;93:2084-92.
Answer to “What’s your diagnosis?” on page 2: Arteriovenous fistulas arising from the subclavian and coronary arteries
We performed aortography along with coronary angiography to find the feeding vessels for the vascular bundle (). There was an arteriovenous fistula that arose from the left subclavian artery, ran over the left mediastinum with the complex plexus, and emptied into the venous system of the left thorax. Multiple coronary artery fistulas originated in the left coronary artery, traversed the left and right mediastinum, and eventually emptied into the venous system of the mediastinum. The left anterior oblique view revealed a coronary artery fistula that arose from the distal right coronary artery and drained into the venous system of the thorax. In transthoracic echocardiography, the sizes of the left atrium and left ventricle were mildly dilated, but left ventricular systolic functions were preserved with an ejection fraction of 61%. We recommended surgery to the patient, but he refused invasive treatment. He will be followed with close observation.
A coronary artery fistula is usually of congenital origin, and connects a major coronary artery directly with the cardiac chamber, coronary sinus, superior vena cava, or pulmonary artery. However, its connection with a systemic venous system is extremely rare. Congenital subclavian arteriovenous fistulas are rare because they usually occur as a complication of previous trauma, percutaneous catheterization, or surgery.1 Complications include “steal” from the adjacent myocardium causing myocardial ischemia, thrombosis/embolism, cardiac failure, atrial fibrillation, rupture, endocarditis/endarteritis, and arrhythmia.2 Treatment options include close medical observation, surgical ligation, and catheter embolization.3
References
1. Brountzos, E.N., Kelekis, N.L., Danassi-Afentaki, D. et al. Congenital subclavian artery-to-subclavian vein fistula in an adult: treatment with transcatheter embolization. Cardiovasc Intervent Radiol. 2004;27:675-7.
2. Wilde, P., Watt, I. Congenital coronary artery fistulae: six new cases with a collective review. Clin Radiol. 1980;31:301-11.
3. Mangukia, C.V. Coronary artery fistula. Ann Thorac Surg. 2012;93:2084-92.
What's Your Diagnosis?
What's Your Diagnosis?
What’s your diagnosis?
By Ki-Hyun Ryu, MD, Tae-Hee Lee, MD, and Taek-Geun Kwon, MD. Published previously in Gastroenterology (2013;144;35, 253).
Reflectance confocal microscopy offers one-stop solution for BCC
For selected patients with basal cell carcinoma, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach of obtaining a punch biopsy to diagnose and subtype the lesion in one visit and performing surgical excision in a separate visit.
Those were the findings of an open-label, randomized, noninferiority trial in the Netherlands comparing the two approaches in 95 adults with suspected basal cell carcinoma (BCC), investigators reported.
In addition to reducing the number of visits and the total time required for treatment, this new approach uses noninvasive reflectance confocal microscopy in the place of punch biopsy, which patients will likely prefer, said Daniel J. Kadouch, MD, of the department of dermatology, Academic Medical Center, Amsterdam, and his associates (British J Derm. 2017 Apr 9. doi: 10.1111/bjd.15559).
The study excluded patients who had lesions in a high-risk location of the face, lesions larger than 20 mm, recurrent lesions, and macroscopic ulcerating lesions, as well as patients who had basal cell nevus syndrome. Another 22 patients who were found to have non-BCC lesions (1 melanoma, 2 squamous cell carcinomas, 5 cases of Bowen’s disease, and 11 nonmalignant lesions) also were excluded, leaving 40 patients with BCC in the one-stop shopping group and 33 in the standard of care (control) group.
The primary outcome – the proportion of patients with tumor-free margins on the final pathology report after surgical excision – was 100% (40 of 40) in the one-stop shopping group and 94% (31 of 33) in the control group, which demonstrates the noninferiority of the new, less invasive approach, Dr. Kadouch and his associates said.
The mean total treatment time was 2 hours and 23 minutes for the one-stop shopping group. The total treatment time could not be determined for the control group because their surgical times weren’t recorded.
Adverse events included four postoperative wound infections in the one-stop shopping group, all of which were successfully treated with oral antibiotics, and one case of excessive postoperative bleeding in the control group, which required 3 days of hospitalization.
This study was limited in that it excluded patients with large lesions and those with BCC on high-risk areas of the face, which reduces the generalizability of the findings. In addition, a follow-up time of at least 1 year would be needed to detect signs of BCC recurrence in the study participants, the investigators said.
For selected patients with basal cell carcinoma, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach of obtaining a punch biopsy to diagnose and subtype the lesion in one visit and performing surgical excision in a separate visit.
Those were the findings of an open-label, randomized, noninferiority trial in the Netherlands comparing the two approaches in 95 adults with suspected basal cell carcinoma (BCC), investigators reported.
In addition to reducing the number of visits and the total time required for treatment, this new approach uses noninvasive reflectance confocal microscopy in the place of punch biopsy, which patients will likely prefer, said Daniel J. Kadouch, MD, of the department of dermatology, Academic Medical Center, Amsterdam, and his associates (British J Derm. 2017 Apr 9. doi: 10.1111/bjd.15559).
The study excluded patients who had lesions in a high-risk location of the face, lesions larger than 20 mm, recurrent lesions, and macroscopic ulcerating lesions, as well as patients who had basal cell nevus syndrome. Another 22 patients who were found to have non-BCC lesions (1 melanoma, 2 squamous cell carcinomas, 5 cases of Bowen’s disease, and 11 nonmalignant lesions) also were excluded, leaving 40 patients with BCC in the one-stop shopping group and 33 in the standard of care (control) group.
The primary outcome – the proportion of patients with tumor-free margins on the final pathology report after surgical excision – was 100% (40 of 40) in the one-stop shopping group and 94% (31 of 33) in the control group, which demonstrates the noninferiority of the new, less invasive approach, Dr. Kadouch and his associates said.
The mean total treatment time was 2 hours and 23 minutes for the one-stop shopping group. The total treatment time could not be determined for the control group because their surgical times weren’t recorded.
Adverse events included four postoperative wound infections in the one-stop shopping group, all of which were successfully treated with oral antibiotics, and one case of excessive postoperative bleeding in the control group, which required 3 days of hospitalization.
This study was limited in that it excluded patients with large lesions and those with BCC on high-risk areas of the face, which reduces the generalizability of the findings. In addition, a follow-up time of at least 1 year would be needed to detect signs of BCC recurrence in the study participants, the investigators said.
For selected patients with basal cell carcinoma, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach of obtaining a punch biopsy to diagnose and subtype the lesion in one visit and performing surgical excision in a separate visit.
Those were the findings of an open-label, randomized, noninferiority trial in the Netherlands comparing the two approaches in 95 adults with suspected basal cell carcinoma (BCC), investigators reported.
In addition to reducing the number of visits and the total time required for treatment, this new approach uses noninvasive reflectance confocal microscopy in the place of punch biopsy, which patients will likely prefer, said Daniel J. Kadouch, MD, of the department of dermatology, Academic Medical Center, Amsterdam, and his associates (British J Derm. 2017 Apr 9. doi: 10.1111/bjd.15559).
The study excluded patients who had lesions in a high-risk location of the face, lesions larger than 20 mm, recurrent lesions, and macroscopic ulcerating lesions, as well as patients who had basal cell nevus syndrome. Another 22 patients who were found to have non-BCC lesions (1 melanoma, 2 squamous cell carcinomas, 5 cases of Bowen’s disease, and 11 nonmalignant lesions) also were excluded, leaving 40 patients with BCC in the one-stop shopping group and 33 in the standard of care (control) group.
The primary outcome – the proportion of patients with tumor-free margins on the final pathology report after surgical excision – was 100% (40 of 40) in the one-stop shopping group and 94% (31 of 33) in the control group, which demonstrates the noninferiority of the new, less invasive approach, Dr. Kadouch and his associates said.
The mean total treatment time was 2 hours and 23 minutes for the one-stop shopping group. The total treatment time could not be determined for the control group because their surgical times weren’t recorded.
Adverse events included four postoperative wound infections in the one-stop shopping group, all of which were successfully treated with oral antibiotics, and one case of excessive postoperative bleeding in the control group, which required 3 days of hospitalization.
This study was limited in that it excluded patients with large lesions and those with BCC on high-risk areas of the face, which reduces the generalizability of the findings. In addition, a follow-up time of at least 1 year would be needed to detect signs of BCC recurrence in the study participants, the investigators said.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: For selected patients with BCC, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach using punch biopsy.
Major finding: The percentage of patients with tumor-free margins after surgical excision was 100% (40 of 40) in the one-stop-shopping group and 94% (31 of 33) in the control group.
Data source: An open-label, randomized, controlled, noninferiority trial involving 95 adults.
Disclosures: The study received no outside funding. Dr. Kadouch and his associates reported having no relevant financial disclosures.
Blood donor age, sex do not affect recipient survival
The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation, according to a report published online April 24 in JAMA Internal Medicine.
A recent observational Canadian study suggested that blood from young donors and female donors increased the recipients’ risk of death – a finding which, if confirmed, would have immediate implications for medical practice.
A separate group of Scandinavian researchers attempted to replicate these findings by performing a retrospective cohort study using similar but more nuanced statistical methods. Gustaf Edgren, MD, PhD, of the department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, and his associates analyzed information collected on 968,264 patients over a 10-year period from a Swedish and Danish transfusion database.
In initial, unadjusted analyses, both extremes of age (young and old) and female sex in the donor were associated with reduced survival in the recipient. However, that association disappeared when the data were adjusted to account for the total number of transfusions a patient received, a marker of their severity of illness. The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99. This pattern also occurred in sensitivity analyses, the investigators noted (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0890).
“When studying associations between ... transfusions with a particular characteristic and the risk of death in the recipient, [the] underlying disease severity ... may still confound the association. However, with meticulous adjustment for total number of transfusions, it should be possible to block the confounding effect of patient disease severity entirely,” they noted.
“We believe that, rather than reflecting true biologic effects, the Canadian results can be explained by residual confounding (i.e., that the observations resulted from incomplete adjustment for the number of transfusions),” Dr. Edgren and his associates said.
“In addition, we believe these data reinforce the importance of extreme caution in assessing epidemiologic analyses in this field, given the tremendous clinical and logistical implications of false-positive findings,” they added.
The findings of Edgren et al. provide reassurance regarding the safety of current transfusion practice.
They present a convincing argument that differences in the statistical approach for controlling confounding likely explained the discrepant results of the Canadian study and their study.
This subtle confounding stems from the fact that increased transfusions expose the recipient to a greater total number of blood products, which in turn is associated with higher comorbidity, greater severity of illness, and higher mortality.
Nareg Roubinian, MD, is at the Blood Systems Research Institute, San Francisco, and in the division of research at Kaiser Permanente Northern California, Oakland. He and his associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Edgren’s report (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0914).
The findings of Edgren et al. provide reassurance regarding the safety of current transfusion practice.
They present a convincing argument that differences in the statistical approach for controlling confounding likely explained the discrepant results of the Canadian study and their study.
This subtle confounding stems from the fact that increased transfusions expose the recipient to a greater total number of blood products, which in turn is associated with higher comorbidity, greater severity of illness, and higher mortality.
Nareg Roubinian, MD, is at the Blood Systems Research Institute, San Francisco, and in the division of research at Kaiser Permanente Northern California, Oakland. He and his associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Edgren’s report (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0914).
The findings of Edgren et al. provide reassurance regarding the safety of current transfusion practice.
They present a convincing argument that differences in the statistical approach for controlling confounding likely explained the discrepant results of the Canadian study and their study.
This subtle confounding stems from the fact that increased transfusions expose the recipient to a greater total number of blood products, which in turn is associated with higher comorbidity, greater severity of illness, and higher mortality.
Nareg Roubinian, MD, is at the Blood Systems Research Institute, San Francisco, and in the division of research at Kaiser Permanente Northern California, Oakland. He and his associates reported having no relevant financial disclosures. They made these remarks in an invited commentary accompanying Dr. Edgren’s report (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0914).
The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation, according to a report published online April 24 in JAMA Internal Medicine.
A recent observational Canadian study suggested that blood from young donors and female donors increased the recipients’ risk of death – a finding which, if confirmed, would have immediate implications for medical practice.
A separate group of Scandinavian researchers attempted to replicate these findings by performing a retrospective cohort study using similar but more nuanced statistical methods. Gustaf Edgren, MD, PhD, of the department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, and his associates analyzed information collected on 968,264 patients over a 10-year period from a Swedish and Danish transfusion database.
In initial, unadjusted analyses, both extremes of age (young and old) and female sex in the donor were associated with reduced survival in the recipient. However, that association disappeared when the data were adjusted to account for the total number of transfusions a patient received, a marker of their severity of illness. The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99. This pattern also occurred in sensitivity analyses, the investigators noted (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0890).
“When studying associations between ... transfusions with a particular characteristic and the risk of death in the recipient, [the] underlying disease severity ... may still confound the association. However, with meticulous adjustment for total number of transfusions, it should be possible to block the confounding effect of patient disease severity entirely,” they noted.
“We believe that, rather than reflecting true biologic effects, the Canadian results can be explained by residual confounding (i.e., that the observations resulted from incomplete adjustment for the number of transfusions),” Dr. Edgren and his associates said.
“In addition, we believe these data reinforce the importance of extreme caution in assessing epidemiologic analyses in this field, given the tremendous clinical and logistical implications of false-positive findings,” they added.
The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation, according to a report published online April 24 in JAMA Internal Medicine.
A recent observational Canadian study suggested that blood from young donors and female donors increased the recipients’ risk of death – a finding which, if confirmed, would have immediate implications for medical practice.
A separate group of Scandinavian researchers attempted to replicate these findings by performing a retrospective cohort study using similar but more nuanced statistical methods. Gustaf Edgren, MD, PhD, of the department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, and his associates analyzed information collected on 968,264 patients over a 10-year period from a Swedish and Danish transfusion database.
In initial, unadjusted analyses, both extremes of age (young and old) and female sex in the donor were associated with reduced survival in the recipient. However, that association disappeared when the data were adjusted to account for the total number of transfusions a patient received, a marker of their severity of illness. The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99. This pattern also occurred in sensitivity analyses, the investigators noted (JAMA Intern. Med. 2017 April 24. doi: 10.1001/jamainternmed.2017.0890).
“When studying associations between ... transfusions with a particular characteristic and the risk of death in the recipient, [the] underlying disease severity ... may still confound the association. However, with meticulous adjustment for total number of transfusions, it should be possible to block the confounding effect of patient disease severity entirely,” they noted.
“We believe that, rather than reflecting true biologic effects, the Canadian results can be explained by residual confounding (i.e., that the observations resulted from incomplete adjustment for the number of transfusions),” Dr. Edgren and his associates said.
“In addition, we believe these data reinforce the importance of extreme caution in assessing epidemiologic analyses in this field, given the tremendous clinical and logistical implications of false-positive findings,” they added.
Key clinical point: The age and sex of blood donors do not affect the recipient’s survival and do not need to be considered in blood allocation.
Major finding: The hazard ratio per transfusion from a donor younger than age 20 was 0.98, and the hazard ratio per transfusion from a female donor was 0.99.
Data source: A retrospective cohort study involving 968,264 transfusion recipients in Sweden and Denmark during a 10-year period.
Disclosures: The Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, Karolinska Institutet’s Strategic Research Program, and the Danish Council for Independent Research supported the study. Dr. Edgren and his associates reported having no relevant financial disclosures.