User login
Hospital infections top WHO’s list of priority pathogens
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
A practical framework for understanding and reducing medical overuse: Conceptualizing overuse through the patient-clinician interaction
Medical services overuse is the provision of healthcare services for which there is no medical basis or for which harms equal or exceed benefits.1 This overuse drives poor-quality care and unnecessary cost.2,3 The high prevalence of overuse is recognized by patients,4 clinicians,5 and policymakers.6 Initiatives to reduce overuse have targeted physicians,7 the public,8 and medical educators9,10 but have had limited impact.11,12 Few studies have addressed methods for reducing overuse, and de-implementation of nonbeneficial practices has proved challenging.1,13,14 Models for reducing overuse are only theoretical15 or are focused on administrative decisions.16,17 We think a practical framework is needed. We used an iterative process, informed by expert opinion and discussion, to design such a framework.
METHODS
The authors, who have expertise in overuse, value, medical education, evidence-based medicine, and implementation science, reviewed related conceptual frameworks18 and evidence regarding drivers of overuse. We organized these drivers into domains to create a draft framework, which we presented at Preventing Overdiagnosis 2015, a meeting of clinicians, patients, and policymakers interested in overuse. We incorporated feedback from meeting attendees to modify framework domains, and we performed structured searches (using key words in Pubmed) to explore, and estimate the strength of, evidence supporting items within each domain. We rated supporting evidence as strong (studies found a clear correlation between a factor and overuse), moderate (evidence suggests such a correlation or demonstrates a correlation between a particular factor and utilization but not overuse per se), weak (only indirect evidence exists), or absent (no studies identified evaluating a particular factor). All authors reached consensus on ratings.
Framework Principles and Evidence
Patient-centered definition of overuse. During framework development, defining clinical appropriateness emerged as the primary challenge to identifying and reducing overuse. Although some care generally is appropriate based on strong evidence of benefit, and some is inappropriate given a clear lack of benefit or harm, much care is of unclear or variable benefit. Practice guidelines can help identify overuse, but their utility may be limited by lack of evidence in specific clinical situations,19 and their recommendations may apply poorly to an individual patient. This presents challenges to using guidelines to identify and reduce overuse.
Despite limitations, the scope of overuse has been estimated by applying broad, often guideline-based, criteria for care appropriateness to administrative data.20 Unfortunately, these estimates provide little direction to clinicians and patients partnering to make usage decisions. During framework development, we identified the importance of a patient-level, patient-specific definition of overuse. This approach reinforces the importance of meeting patient needs while standardizing treatments to reduce overuse. A patient-centered approach may also assist professional societies and advocacy groups in developing actionable campaigns and may uncover evidence gaps.
Centrality of patient-clinician interaction. During framework development, the patient–clinician interaction emerged as the nexus through which drivers of overuse exert influence. The centrality of this interaction has been demonstrated in studies of the relationship between care continuity and overuse21 or utilization,22,23 by evidence that communication and patient–clinician relationships affect utilization,24 and by the observation that clinician training in shared decision-making reduces overuse.25 A patient-centered framework assumes that, at least in the weighing of clinically reasonable options, a patient-centered approach optimizes outcomes for that patient.
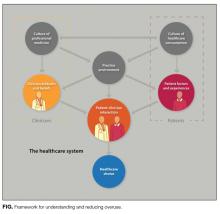
Incorporating drivers of overuse. We incorporated drivers of overuse into domains and related them to the patient–clinician interaction.26 Domains included the culture of healthcare consumption, patient factors and experiences, the practice environment, the culture of professional medicine, and clinician attitudes and beliefs.
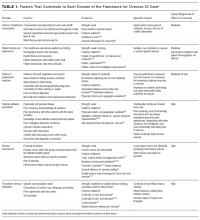
We characterized the evidence illustrating how drivers within each domain influence healthcare use. The evidence for each domain is listed in Table 1.
RESULTS
The final framework is shown in the Figure. Within the healthcare system, patients are influenced by the culture of healthcare consumption, which varies within and among countries.27 Clinicians are influenced by the culture of medical care, which varies by practice setting,28 and by their training environment.29 Both clinicians and patients are influenced by the practice environment and by personal experiences. Ultimately, clinical decisions occur within the specific patient–clinician interaction.24 Table 1 lists each domain’s components, likely impact on overuse, and estimated strength of supporting evidence. Interventions can be conceptualized within appropriate domains or through the interaction between patient and clinician.
DISCUSSION
We developed a novel and practical conceptual framework for characterizing drivers of overuse and potential intervention points. To our knowledge, this is the first framework incorporating a patient-specific approach to overuse and emphasizing the patient–clinician interaction. Key strengths of framework development are inclusion of a range of perspectives and characterization of the evidence within each domain. Limitations include lack of a formal systematic review and broad, qualitative assessments of evidence strength. However, we believe this framework provides an important conceptual foundation for the study of overuse and interventions to reduce overuse.
Framework Applications
This framework, which highlights the many drivers of overuse, can facilitate understanding of overuse and help conceptualize change, prioritize research goals, and inform specific interventions. For policymakers, the framework can inform efforts to reduce overuse by emphasizing the need for complex interventions and by clarifying the likely impact of interventions targeting specific domains. Similarly, for clinicians and quality improvement professionals, the framework can ground root cause analyses of overuse-related problems and inform allocation of limited resources. Finally, the relatively weak evidence on the role of most acknowledged drivers of overuse suggests an important research agenda. Specifically, several pressing needs have been identified: defining relevant physician and patient cultural factors, investigating interventions to impact culture, defining practice environment features that optimize care appropriateness, and describing specific patient–clinician interaction practices that minimize overuse while providing needed care.
Targeting Interventions
Domains within the framework are influenced by different types of interventions, and different stakeholders may target different domains. For example:
- The culture of healthcare consumption may be influenced through public education (eg, Choosing Wisely® patient resources)30-32 and public health campaigns.
- The practice environment may be influenced by initiatives to align clinician incentives,33 team care,34 electronic health record interventions,35 and improved access.36
- Clinician attitudes and beliefs may be influenced by audit and feedback,37-40 reflection,41 role modeling,42 and education.43-45
- Patient attitudes and beliefs may be influenced by education, access to price and quality information, and increased engagement in care.46,47
- For clinicians, the patient–clinician interaction can be improved through training in communication and shared decision-making,25 through access to information (eg, costs) that can be easily shared with patients,48,49 and through novel visit structures (eg, scribes).50
- On the patient side, this interaction can be optimized with improved access (eg, through telemedicine)51,52 or with patient empowerment during hospitalization.
- The culture of medicine is difficult to influence. Change likely will occur through:
○ Regulatory interventions (eg, Transforming Clinical Practice Initiative of Center for Medicare & Medicaid Innovation).
○ Educational initiatives (eg, high-value care curricula of Alliance for Academic Internal Medicine/American College of Physicians53).
○ Medical journal features (eg, “Less Is More” in JAMA Internal Medicine54 and “Things We Do for No Reason” in Journal of Hospital Medicine).
○ Professional organizations (eg, Choosing Wisely®).
As organizations implement quality improvement initiatives to reduce overuse of services, the framework can be used to target interventions to relevant domains. For example, a hospital leader who wants to reduce opioid prescribing may use the framework to identify the factors that encourage prescribing in each domain—poor understanding of pain treatment (a clinician factor), desire for early discharge encouraging overly aggressive pain management (an environmental factor), patient demand for opioids combined with poor understanding of harms (patient factors), and poor communication regarding pain (a patient–clinician interaction factor). Although not all relevant factors can be addressed, their classification by domain facilitates intervention, in this case perhaps leading to a focus on clinician and patient education on opioids and development of a practical communication tool that targets 3 domains. Table 2 lists ways in which the framework informs approaches to this and other overused services in the hospital setting. Note that some drivers can be acknowledged without identifying targeted interventions.
Moving Forward
Through a multi-stakeholder iterative process, we developed a practical framework for understanding medical overuse and interventions to reduce it. Centered on the patient–clinician interaction, this framework explains overuse as the product of medical and patient culture, the practice environment and incentives, and other clinician and patient factors. Ultimately, care is implemented during the patient–clinician interaction, though few interventions to reduce overuse have focused on that domain.
Conceptualizing overuse through the patient–clinician interaction maintains focus on patients while promoting population health that is both better and lower in cost. This framework can guide interventions to reduce overuse in important parts of the healthcare system while ensuring the final goal of high-quality individualized patient care.
Acknowledgments
The authors thank Valerie Pocus for helping with the artistic design of Framework. An early version of Framework was presented at the 2015 Preventing Overdiagnosis meeting in Bethesda, Maryland.
Disclosures
Dr. Morgan received research support from the VA Health Services Research (CRE 12-307), Agency for Healthcare Research and Quality (AHRQ) (K08- HS18111). Dr. Leppin’s work was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Dr. Korenstein’s work on this paper was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). Dr. Morgan provided a self-developed lecture in a 3M-sponsored series on hospital epidemiology and has received honoraria for serving as a book and journal editor for Springer Publishing. Dr. Smith is employed by the American College of Physicians and owns stock in Merck, where her husband is employed. The other authors report no potential conflicts of interest.
1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. PubMed
2. Hood VL, Weinberger SE. High value, cost-conscious care: an international imperative. Eur J Intern Med. 2012;23(6):495-498. PubMed
3. Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171-178. PubMed
4. How SKH, Shih A, Lau J, Schoen C. Public Views on U.S. Health System Organization: A Call for New Directions. http://www.commonwealthfund.org/publications/data-briefs/2008/aug/public-views-on-u-s--health-system-organization--a-call-for-new-directions. Published August 1, 2008. Accessed December 11, 2015.
5. Sirovich BE, Woloshin S, Schwartz LM. Too little? Too much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171(17):1582-1585. PubMed
6. Joint Commission, American Medical Association–Convened Physician Consortium for Performance Improvement. Proceedings From the National Summit on Overuse. https://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf. Published September 24, 2012. Accessed July 8, 2016.
7. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. PubMed
8. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the Choosing Wisely campaign. Acad Med. 2014;89(7):990-995. PubMed
9. Smith CD, Levinson WS. A commitment to high-value care education from the internal medicine community. Ann Int Med. 2015;162(9):639-640. PubMed
10. Korenstein D, Kale M, Levinson W. Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671-1672. PubMed
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. PubMed
12. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
13. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. PubMed
14. Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff (Millwood). 2015;34(2):239-244. PubMed
15. Powell AA, Bloomfield HE, Burgess DJ, Wilt TJ, Partin MR. A conceptual framework for understanding and reducing overuse by primary care providers. Med Care Res Rev. 2013;70(5):451-472. PubMed
16. Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1-6. PubMed
17. Segal JB, Nassery N, Chang HY, Chang E, Chan K, Bridges JF. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. PubMed
18. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555-S561. PubMed
19. Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529-535. PubMed
20. Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1(2):185-194. PubMed
21. Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148-1154. PubMed
22. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med. 2015;13(2):123-129. PubMed
23. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient-centered medical home reduces emergency department visits. PloS One. 2014;9(5):e96356. PubMed
24. Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555-563. PubMed
25. Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012;184(13):E726-E734. PubMed
26. PerryUndum Research/Communication; for ABIM Foundation. Unnecessary Tests and Procedures in the Health Care System: What Physicians Say About the Problem, the Causes, and the Solutions: Results From a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2015/04/Final-Choosing-Wisely-Survey-Report.pdf. Published May 1, 2014. Accessed July 8, 2016.
27. Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5-14. PubMed
28. Cutler D, Skinner JS, Stern AD, Wennberg DE. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. NBER Working Paper No. 19320. Cambridge, MA: National Bureau of Economic Research; 2013. http://www.nber.org/papers/w19320. Published August 2013. Accessed July 8, 2016.
29. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. PubMed
30. Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17-31. PubMed
31. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103-3109. PubMed
32. Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009;6(6):e1000084. PubMed
33. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. PubMed
34. Yoon J, Rose DE, Canelo I, et al. Medical home features of VHA primary care clinics and avoidable hospitalizations. J Gen Intern Med. 2013;28(9):1188-1194. PubMed
35. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. PubMed
36. Davis MM, Balasubramanian BA, Cifuentes M, et al. Clinician staffing, scheduling, and engagement strategies among primary care practices delivering integrated care. J Am Board Fam Med. 2015;28(suppl 1):S32-S40. PubMed
37. Dine CJ, Miller J, Fuld A, Bellini LM, Iwashyna TJ. Educating physicians-in-training about resource utilization and their own outcomes of care in the inpatient setting. J Grad Med Educ. 2010;2(2):175-180. PubMed
38. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361. PubMed
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345-2352. PubMed
40. Taggart LR, Leung E, Muller MP, Matukas LM, Daneman N. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480. PubMed
41. Hughes DR, Sunshine JH, Bhargavan M, Forman H. Physician self-referral for imaging and the cost of chronic care for Medicare beneficiaries. Med Care. 2011;49(9):857-864. PubMed
42. Ryskina KL, Pesko MF, Gossey JT, Caesar EP, Bishop TF. Brand name statin prescribing in a resident ambulatory practice: implications for teaching cost-conscious medicine. J Grad Med Educ. 2014;6(3):484-488. PubMed
43. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. PubMed
44. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72. PubMed
45. Wilson I, Cowin LS, Johnson M, Young H. Professional identity in medical students: pedagogical challenges to medical education. Teach Learn Med. 2013;25(4):369-373. PubMed
46. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
47. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annu Symp Proc. 2014;2014:486-495. PubMed
48. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. PubMed
49. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. PubMed
50. Bank AJ, Gage RM. Annual impact of scribes on physician productivity and revenue in a cardiology clinic. Clinicoecon Outcomes Res. 2015;7:489-495. PubMed
51. Lyles CR, Sarkar U, Schillinger D, et al. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28-e33. PubMed
52. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res. 2015;17(2):e44. PubMed
53. Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians curriculum. Ann Intern Med. 2012;157(4):284-286. PubMed
54. Redberg RF. Less is more. Arch Intern Med. 2010;170(7):584. PubMed
65. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. PubMed
66. Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557-564. PubMed
67. Tubbs EP, Elrod JA, Flum DR. Risk taking and tolerance of uncertainty: implications for surgeons. J Surg Res. 2006;131(1):1-6. PubMed
68. Zaat JO, van Eijk JT. General practitioners’ uncertainty, risk preference, and use of laboratory tests. Med Care. 1992;30(9):846-854. PubMed
69. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
70. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362. PubMed
71. Yasaitis LC, Bynum JP, Skinner JS. Association between physician supply, local practice norms, and outpatient visit rates. Med Care. 2013;51(6):524-531. PubMed
72. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. PubMed
73. Ryskina KL, Smith CD, Weissman A, et al. U.S. internal medicine residents’ knowledge and practice of high-value care: a national survey. Acad Med. 2015;90(10):1373-1379. PubMed
74. Khullar D, Chokshi DA, Kocher R, et al. Behavioral economics and physician compensation—promise and challenges. N Engl J Med. 2015;372(24):2281-2283. PubMed
75. Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39(8):889-905. PubMed
76. Fanari Z, Abraham N, Kolm P, et al. Aggressive measures to decrease “door to balloon” time and incidence of unnecessary cardiac catheterization: potential risks and role of quality improvement. Mayo Clin Proc. 2015;90(12):1614-1622. PubMed
77. Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP. Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med. 2012;172(12):938-945. PubMed
78. Verhofstede R, Smets T, Cohen J, Costantini M, Van Den Noortgate N, Deliens L. Implementing the care programme for the last days of life in an acute geriatric hospital ward: a phase 2 mixed method study. BMC Palliat Care. 2016;15:27. PubMed
Medical services overuse is the provision of healthcare services for which there is no medical basis or for which harms equal or exceed benefits.1 This overuse drives poor-quality care and unnecessary cost.2,3 The high prevalence of overuse is recognized by patients,4 clinicians,5 and policymakers.6 Initiatives to reduce overuse have targeted physicians,7 the public,8 and medical educators9,10 but have had limited impact.11,12 Few studies have addressed methods for reducing overuse, and de-implementation of nonbeneficial practices has proved challenging.1,13,14 Models for reducing overuse are only theoretical15 or are focused on administrative decisions.16,17 We think a practical framework is needed. We used an iterative process, informed by expert opinion and discussion, to design such a framework.
METHODS
The authors, who have expertise in overuse, value, medical education, evidence-based medicine, and implementation science, reviewed related conceptual frameworks18 and evidence regarding drivers of overuse. We organized these drivers into domains to create a draft framework, which we presented at Preventing Overdiagnosis 2015, a meeting of clinicians, patients, and policymakers interested in overuse. We incorporated feedback from meeting attendees to modify framework domains, and we performed structured searches (using key words in Pubmed) to explore, and estimate the strength of, evidence supporting items within each domain. We rated supporting evidence as strong (studies found a clear correlation between a factor and overuse), moderate (evidence suggests such a correlation or demonstrates a correlation between a particular factor and utilization but not overuse per se), weak (only indirect evidence exists), or absent (no studies identified evaluating a particular factor). All authors reached consensus on ratings.
Framework Principles and Evidence
Patient-centered definition of overuse. During framework development, defining clinical appropriateness emerged as the primary challenge to identifying and reducing overuse. Although some care generally is appropriate based on strong evidence of benefit, and some is inappropriate given a clear lack of benefit or harm, much care is of unclear or variable benefit. Practice guidelines can help identify overuse, but their utility may be limited by lack of evidence in specific clinical situations,19 and their recommendations may apply poorly to an individual patient. This presents challenges to using guidelines to identify and reduce overuse.
Despite limitations, the scope of overuse has been estimated by applying broad, often guideline-based, criteria for care appropriateness to administrative data.20 Unfortunately, these estimates provide little direction to clinicians and patients partnering to make usage decisions. During framework development, we identified the importance of a patient-level, patient-specific definition of overuse. This approach reinforces the importance of meeting patient needs while standardizing treatments to reduce overuse. A patient-centered approach may also assist professional societies and advocacy groups in developing actionable campaigns and may uncover evidence gaps.
Centrality of patient-clinician interaction. During framework development, the patient–clinician interaction emerged as the nexus through which drivers of overuse exert influence. The centrality of this interaction has been demonstrated in studies of the relationship between care continuity and overuse21 or utilization,22,23 by evidence that communication and patient–clinician relationships affect utilization,24 and by the observation that clinician training in shared decision-making reduces overuse.25 A patient-centered framework assumes that, at least in the weighing of clinically reasonable options, a patient-centered approach optimizes outcomes for that patient.
Incorporating drivers of overuse. We incorporated drivers of overuse into domains and related them to the patient–clinician interaction.26 Domains included the culture of healthcare consumption, patient factors and experiences, the practice environment, the culture of professional medicine, and clinician attitudes and beliefs.
We characterized the evidence illustrating how drivers within each domain influence healthcare use. The evidence for each domain is listed in Table 1.
RESULTS
The final framework is shown in the Figure. Within the healthcare system, patients are influenced by the culture of healthcare consumption, which varies within and among countries.27 Clinicians are influenced by the culture of medical care, which varies by practice setting,28 and by their training environment.29 Both clinicians and patients are influenced by the practice environment and by personal experiences. Ultimately, clinical decisions occur within the specific patient–clinician interaction.24 Table 1 lists each domain’s components, likely impact on overuse, and estimated strength of supporting evidence. Interventions can be conceptualized within appropriate domains or through the interaction between patient and clinician.
DISCUSSION
We developed a novel and practical conceptual framework for characterizing drivers of overuse and potential intervention points. To our knowledge, this is the first framework incorporating a patient-specific approach to overuse and emphasizing the patient–clinician interaction. Key strengths of framework development are inclusion of a range of perspectives and characterization of the evidence within each domain. Limitations include lack of a formal systematic review and broad, qualitative assessments of evidence strength. However, we believe this framework provides an important conceptual foundation for the study of overuse and interventions to reduce overuse.
Framework Applications
This framework, which highlights the many drivers of overuse, can facilitate understanding of overuse and help conceptualize change, prioritize research goals, and inform specific interventions. For policymakers, the framework can inform efforts to reduce overuse by emphasizing the need for complex interventions and by clarifying the likely impact of interventions targeting specific domains. Similarly, for clinicians and quality improvement professionals, the framework can ground root cause analyses of overuse-related problems and inform allocation of limited resources. Finally, the relatively weak evidence on the role of most acknowledged drivers of overuse suggests an important research agenda. Specifically, several pressing needs have been identified: defining relevant physician and patient cultural factors, investigating interventions to impact culture, defining practice environment features that optimize care appropriateness, and describing specific patient–clinician interaction practices that minimize overuse while providing needed care.
Targeting Interventions
Domains within the framework are influenced by different types of interventions, and different stakeholders may target different domains. For example:
- The culture of healthcare consumption may be influenced through public education (eg, Choosing Wisely® patient resources)30-32 and public health campaigns.
- The practice environment may be influenced by initiatives to align clinician incentives,33 team care,34 electronic health record interventions,35 and improved access.36
- Clinician attitudes and beliefs may be influenced by audit and feedback,37-40 reflection,41 role modeling,42 and education.43-45
- Patient attitudes and beliefs may be influenced by education, access to price and quality information, and increased engagement in care.46,47
- For clinicians, the patient–clinician interaction can be improved through training in communication and shared decision-making,25 through access to information (eg, costs) that can be easily shared with patients,48,49 and through novel visit structures (eg, scribes).50
- On the patient side, this interaction can be optimized with improved access (eg, through telemedicine)51,52 or with patient empowerment during hospitalization.
- The culture of medicine is difficult to influence. Change likely will occur through:
○ Regulatory interventions (eg, Transforming Clinical Practice Initiative of Center for Medicare & Medicaid Innovation).
○ Educational initiatives (eg, high-value care curricula of Alliance for Academic Internal Medicine/American College of Physicians53).
○ Medical journal features (eg, “Less Is More” in JAMA Internal Medicine54 and “Things We Do for No Reason” in Journal of Hospital Medicine).
○ Professional organizations (eg, Choosing Wisely®).
As organizations implement quality improvement initiatives to reduce overuse of services, the framework can be used to target interventions to relevant domains. For example, a hospital leader who wants to reduce opioid prescribing may use the framework to identify the factors that encourage prescribing in each domain—poor understanding of pain treatment (a clinician factor), desire for early discharge encouraging overly aggressive pain management (an environmental factor), patient demand for opioids combined with poor understanding of harms (patient factors), and poor communication regarding pain (a patient–clinician interaction factor). Although not all relevant factors can be addressed, their classification by domain facilitates intervention, in this case perhaps leading to a focus on clinician and patient education on opioids and development of a practical communication tool that targets 3 domains. Table 2 lists ways in which the framework informs approaches to this and other overused services in the hospital setting. Note that some drivers can be acknowledged without identifying targeted interventions.
Moving Forward
Through a multi-stakeholder iterative process, we developed a practical framework for understanding medical overuse and interventions to reduce it. Centered on the patient–clinician interaction, this framework explains overuse as the product of medical and patient culture, the practice environment and incentives, and other clinician and patient factors. Ultimately, care is implemented during the patient–clinician interaction, though few interventions to reduce overuse have focused on that domain.
Conceptualizing overuse through the patient–clinician interaction maintains focus on patients while promoting population health that is both better and lower in cost. This framework can guide interventions to reduce overuse in important parts of the healthcare system while ensuring the final goal of high-quality individualized patient care.
Acknowledgments
The authors thank Valerie Pocus for helping with the artistic design of Framework. An early version of Framework was presented at the 2015 Preventing Overdiagnosis meeting in Bethesda, Maryland.
Disclosures
Dr. Morgan received research support from the VA Health Services Research (CRE 12-307), Agency for Healthcare Research and Quality (AHRQ) (K08- HS18111). Dr. Leppin’s work was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Dr. Korenstein’s work on this paper was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). Dr. Morgan provided a self-developed lecture in a 3M-sponsored series on hospital epidemiology and has received honoraria for serving as a book and journal editor for Springer Publishing. Dr. Smith is employed by the American College of Physicians and owns stock in Merck, where her husband is employed. The other authors report no potential conflicts of interest.
Medical services overuse is the provision of healthcare services for which there is no medical basis or for which harms equal or exceed benefits.1 This overuse drives poor-quality care and unnecessary cost.2,3 The high prevalence of overuse is recognized by patients,4 clinicians,5 and policymakers.6 Initiatives to reduce overuse have targeted physicians,7 the public,8 and medical educators9,10 but have had limited impact.11,12 Few studies have addressed methods for reducing overuse, and de-implementation of nonbeneficial practices has proved challenging.1,13,14 Models for reducing overuse are only theoretical15 or are focused on administrative decisions.16,17 We think a practical framework is needed. We used an iterative process, informed by expert opinion and discussion, to design such a framework.
METHODS
The authors, who have expertise in overuse, value, medical education, evidence-based medicine, and implementation science, reviewed related conceptual frameworks18 and evidence regarding drivers of overuse. We organized these drivers into domains to create a draft framework, which we presented at Preventing Overdiagnosis 2015, a meeting of clinicians, patients, and policymakers interested in overuse. We incorporated feedback from meeting attendees to modify framework domains, and we performed structured searches (using key words in Pubmed) to explore, and estimate the strength of, evidence supporting items within each domain. We rated supporting evidence as strong (studies found a clear correlation between a factor and overuse), moderate (evidence suggests such a correlation or demonstrates a correlation between a particular factor and utilization but not overuse per se), weak (only indirect evidence exists), or absent (no studies identified evaluating a particular factor). All authors reached consensus on ratings.
Framework Principles and Evidence
Patient-centered definition of overuse. During framework development, defining clinical appropriateness emerged as the primary challenge to identifying and reducing overuse. Although some care generally is appropriate based on strong evidence of benefit, and some is inappropriate given a clear lack of benefit or harm, much care is of unclear or variable benefit. Practice guidelines can help identify overuse, but their utility may be limited by lack of evidence in specific clinical situations,19 and their recommendations may apply poorly to an individual patient. This presents challenges to using guidelines to identify and reduce overuse.
Despite limitations, the scope of overuse has been estimated by applying broad, often guideline-based, criteria for care appropriateness to administrative data.20 Unfortunately, these estimates provide little direction to clinicians and patients partnering to make usage decisions. During framework development, we identified the importance of a patient-level, patient-specific definition of overuse. This approach reinforces the importance of meeting patient needs while standardizing treatments to reduce overuse. A patient-centered approach may also assist professional societies and advocacy groups in developing actionable campaigns and may uncover evidence gaps.
Centrality of patient-clinician interaction. During framework development, the patient–clinician interaction emerged as the nexus through which drivers of overuse exert influence. The centrality of this interaction has been demonstrated in studies of the relationship between care continuity and overuse21 or utilization,22,23 by evidence that communication and patient–clinician relationships affect utilization,24 and by the observation that clinician training in shared decision-making reduces overuse.25 A patient-centered framework assumes that, at least in the weighing of clinically reasonable options, a patient-centered approach optimizes outcomes for that patient.
Incorporating drivers of overuse. We incorporated drivers of overuse into domains and related them to the patient–clinician interaction.26 Domains included the culture of healthcare consumption, patient factors and experiences, the practice environment, the culture of professional medicine, and clinician attitudes and beliefs.
We characterized the evidence illustrating how drivers within each domain influence healthcare use. The evidence for each domain is listed in Table 1.
RESULTS
The final framework is shown in the Figure. Within the healthcare system, patients are influenced by the culture of healthcare consumption, which varies within and among countries.27 Clinicians are influenced by the culture of medical care, which varies by practice setting,28 and by their training environment.29 Both clinicians and patients are influenced by the practice environment and by personal experiences. Ultimately, clinical decisions occur within the specific patient–clinician interaction.24 Table 1 lists each domain’s components, likely impact on overuse, and estimated strength of supporting evidence. Interventions can be conceptualized within appropriate domains or through the interaction between patient and clinician.
DISCUSSION
We developed a novel and practical conceptual framework for characterizing drivers of overuse and potential intervention points. To our knowledge, this is the first framework incorporating a patient-specific approach to overuse and emphasizing the patient–clinician interaction. Key strengths of framework development are inclusion of a range of perspectives and characterization of the evidence within each domain. Limitations include lack of a formal systematic review and broad, qualitative assessments of evidence strength. However, we believe this framework provides an important conceptual foundation for the study of overuse and interventions to reduce overuse.
Framework Applications
This framework, which highlights the many drivers of overuse, can facilitate understanding of overuse and help conceptualize change, prioritize research goals, and inform specific interventions. For policymakers, the framework can inform efforts to reduce overuse by emphasizing the need for complex interventions and by clarifying the likely impact of interventions targeting specific domains. Similarly, for clinicians and quality improvement professionals, the framework can ground root cause analyses of overuse-related problems and inform allocation of limited resources. Finally, the relatively weak evidence on the role of most acknowledged drivers of overuse suggests an important research agenda. Specifically, several pressing needs have been identified: defining relevant physician and patient cultural factors, investigating interventions to impact culture, defining practice environment features that optimize care appropriateness, and describing specific patient–clinician interaction practices that minimize overuse while providing needed care.
Targeting Interventions
Domains within the framework are influenced by different types of interventions, and different stakeholders may target different domains. For example:
- The culture of healthcare consumption may be influenced through public education (eg, Choosing Wisely® patient resources)30-32 and public health campaigns.
- The practice environment may be influenced by initiatives to align clinician incentives,33 team care,34 electronic health record interventions,35 and improved access.36
- Clinician attitudes and beliefs may be influenced by audit and feedback,37-40 reflection,41 role modeling,42 and education.43-45
- Patient attitudes and beliefs may be influenced by education, access to price and quality information, and increased engagement in care.46,47
- For clinicians, the patient–clinician interaction can be improved through training in communication and shared decision-making,25 through access to information (eg, costs) that can be easily shared with patients,48,49 and through novel visit structures (eg, scribes).50
- On the patient side, this interaction can be optimized with improved access (eg, through telemedicine)51,52 or with patient empowerment during hospitalization.
- The culture of medicine is difficult to influence. Change likely will occur through:
○ Regulatory interventions (eg, Transforming Clinical Practice Initiative of Center for Medicare & Medicaid Innovation).
○ Educational initiatives (eg, high-value care curricula of Alliance for Academic Internal Medicine/American College of Physicians53).
○ Medical journal features (eg, “Less Is More” in JAMA Internal Medicine54 and “Things We Do for No Reason” in Journal of Hospital Medicine).
○ Professional organizations (eg, Choosing Wisely®).
As organizations implement quality improvement initiatives to reduce overuse of services, the framework can be used to target interventions to relevant domains. For example, a hospital leader who wants to reduce opioid prescribing may use the framework to identify the factors that encourage prescribing in each domain—poor understanding of pain treatment (a clinician factor), desire for early discharge encouraging overly aggressive pain management (an environmental factor), patient demand for opioids combined with poor understanding of harms (patient factors), and poor communication regarding pain (a patient–clinician interaction factor). Although not all relevant factors can be addressed, their classification by domain facilitates intervention, in this case perhaps leading to a focus on clinician and patient education on opioids and development of a practical communication tool that targets 3 domains. Table 2 lists ways in which the framework informs approaches to this and other overused services in the hospital setting. Note that some drivers can be acknowledged without identifying targeted interventions.
Moving Forward
Through a multi-stakeholder iterative process, we developed a practical framework for understanding medical overuse and interventions to reduce it. Centered on the patient–clinician interaction, this framework explains overuse as the product of medical and patient culture, the practice environment and incentives, and other clinician and patient factors. Ultimately, care is implemented during the patient–clinician interaction, though few interventions to reduce overuse have focused on that domain.
Conceptualizing overuse through the patient–clinician interaction maintains focus on patients while promoting population health that is both better and lower in cost. This framework can guide interventions to reduce overuse in important parts of the healthcare system while ensuring the final goal of high-quality individualized patient care.
Acknowledgments
The authors thank Valerie Pocus for helping with the artistic design of Framework. An early version of Framework was presented at the 2015 Preventing Overdiagnosis meeting in Bethesda, Maryland.
Disclosures
Dr. Morgan received research support from the VA Health Services Research (CRE 12-307), Agency for Healthcare Research and Quality (AHRQ) (K08- HS18111). Dr. Leppin’s work was supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Dr. Korenstein’s work on this paper was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). Dr. Morgan provided a self-developed lecture in a 3M-sponsored series on hospital epidemiology and has received honoraria for serving as a book and journal editor for Springer Publishing. Dr. Smith is employed by the American College of Physicians and owns stock in Merck, where her husband is employed. The other authors report no potential conflicts of interest.
1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. PubMed
2. Hood VL, Weinberger SE. High value, cost-conscious care: an international imperative. Eur J Intern Med. 2012;23(6):495-498. PubMed
3. Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171-178. PubMed
4. How SKH, Shih A, Lau J, Schoen C. Public Views on U.S. Health System Organization: A Call for New Directions. http://www.commonwealthfund.org/publications/data-briefs/2008/aug/public-views-on-u-s--health-system-organization--a-call-for-new-directions. Published August 1, 2008. Accessed December 11, 2015.
5. Sirovich BE, Woloshin S, Schwartz LM. Too little? Too much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171(17):1582-1585. PubMed
6. Joint Commission, American Medical Association–Convened Physician Consortium for Performance Improvement. Proceedings From the National Summit on Overuse. https://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf. Published September 24, 2012. Accessed July 8, 2016.
7. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. PubMed
8. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the Choosing Wisely campaign. Acad Med. 2014;89(7):990-995. PubMed
9. Smith CD, Levinson WS. A commitment to high-value care education from the internal medicine community. Ann Int Med. 2015;162(9):639-640. PubMed
10. Korenstein D, Kale M, Levinson W. Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671-1672. PubMed
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. PubMed
12. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
13. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. PubMed
14. Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff (Millwood). 2015;34(2):239-244. PubMed
15. Powell AA, Bloomfield HE, Burgess DJ, Wilt TJ, Partin MR. A conceptual framework for understanding and reducing overuse by primary care providers. Med Care Res Rev. 2013;70(5):451-472. PubMed
16. Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1-6. PubMed
17. Segal JB, Nassery N, Chang HY, Chang E, Chan K, Bridges JF. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. PubMed
18. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555-S561. PubMed
19. Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529-535. PubMed
20. Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1(2):185-194. PubMed
21. Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148-1154. PubMed
22. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med. 2015;13(2):123-129. PubMed
23. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient-centered medical home reduces emergency department visits. PloS One. 2014;9(5):e96356. PubMed
24. Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555-563. PubMed
25. Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012;184(13):E726-E734. PubMed
26. PerryUndum Research/Communication; for ABIM Foundation. Unnecessary Tests and Procedures in the Health Care System: What Physicians Say About the Problem, the Causes, and the Solutions: Results From a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2015/04/Final-Choosing-Wisely-Survey-Report.pdf. Published May 1, 2014. Accessed July 8, 2016.
27. Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5-14. PubMed
28. Cutler D, Skinner JS, Stern AD, Wennberg DE. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. NBER Working Paper No. 19320. Cambridge, MA: National Bureau of Economic Research; 2013. http://www.nber.org/papers/w19320. Published August 2013. Accessed July 8, 2016.
29. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. PubMed
30. Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17-31. PubMed
31. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103-3109. PubMed
32. Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009;6(6):e1000084. PubMed
33. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. PubMed
34. Yoon J, Rose DE, Canelo I, et al. Medical home features of VHA primary care clinics and avoidable hospitalizations. J Gen Intern Med. 2013;28(9):1188-1194. PubMed
35. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. PubMed
36. Davis MM, Balasubramanian BA, Cifuentes M, et al. Clinician staffing, scheduling, and engagement strategies among primary care practices delivering integrated care. J Am Board Fam Med. 2015;28(suppl 1):S32-S40. PubMed
37. Dine CJ, Miller J, Fuld A, Bellini LM, Iwashyna TJ. Educating physicians-in-training about resource utilization and their own outcomes of care in the inpatient setting. J Grad Med Educ. 2010;2(2):175-180. PubMed
38. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361. PubMed
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345-2352. PubMed
40. Taggart LR, Leung E, Muller MP, Matukas LM, Daneman N. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480. PubMed
41. Hughes DR, Sunshine JH, Bhargavan M, Forman H. Physician self-referral for imaging and the cost of chronic care for Medicare beneficiaries. Med Care. 2011;49(9):857-864. PubMed
42. Ryskina KL, Pesko MF, Gossey JT, Caesar EP, Bishop TF. Brand name statin prescribing in a resident ambulatory practice: implications for teaching cost-conscious medicine. J Grad Med Educ. 2014;6(3):484-488. PubMed
43. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. PubMed
44. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72. PubMed
45. Wilson I, Cowin LS, Johnson M, Young H. Professional identity in medical students: pedagogical challenges to medical education. Teach Learn Med. 2013;25(4):369-373. PubMed
46. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
47. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annu Symp Proc. 2014;2014:486-495. PubMed
48. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. PubMed
49. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. PubMed
50. Bank AJ, Gage RM. Annual impact of scribes on physician productivity and revenue in a cardiology clinic. Clinicoecon Outcomes Res. 2015;7:489-495. PubMed
51. Lyles CR, Sarkar U, Schillinger D, et al. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28-e33. PubMed
52. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res. 2015;17(2):e44. PubMed
53. Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians curriculum. Ann Intern Med. 2012;157(4):284-286. PubMed
54. Redberg RF. Less is more. Arch Intern Med. 2010;170(7):584. PubMed
65. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. PubMed
66. Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557-564. PubMed
67. Tubbs EP, Elrod JA, Flum DR. Risk taking and tolerance of uncertainty: implications for surgeons. J Surg Res. 2006;131(1):1-6. PubMed
68. Zaat JO, van Eijk JT. General practitioners’ uncertainty, risk preference, and use of laboratory tests. Med Care. 1992;30(9):846-854. PubMed
69. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
70. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362. PubMed
71. Yasaitis LC, Bynum JP, Skinner JS. Association between physician supply, local practice norms, and outpatient visit rates. Med Care. 2013;51(6):524-531. PubMed
72. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. PubMed
73. Ryskina KL, Smith CD, Weissman A, et al. U.S. internal medicine residents’ knowledge and practice of high-value care: a national survey. Acad Med. 2015;90(10):1373-1379. PubMed
74. Khullar D, Chokshi DA, Kocher R, et al. Behavioral economics and physician compensation—promise and challenges. N Engl J Med. 2015;372(24):2281-2283. PubMed
75. Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39(8):889-905. PubMed
76. Fanari Z, Abraham N, Kolm P, et al. Aggressive measures to decrease “door to balloon” time and incidence of unnecessary cardiac catheterization: potential risks and role of quality improvement. Mayo Clin Proc. 2015;90(12):1614-1622. PubMed
77. Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP. Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med. 2012;172(12):938-945. PubMed
78. Verhofstede R, Smets T, Cohen J, Costantini M, Van Den Noortgate N, Deliens L. Implementing the care programme for the last days of life in an acute geriatric hospital ward: a phase 2 mixed method study. BMC Palliat Care. 2016;15:27. PubMed
1. Morgan DJ, Brownlee S, Leppin AL, et al. Setting a research agenda for medical overuse. BMJ. 2015;351:h4534. PubMed
2. Hood VL, Weinberger SE. High value, cost-conscious care: an international imperative. Eur J Intern Med. 2012;23(6):495-498. PubMed
3. Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172(2):171-178. PubMed
4. How SKH, Shih A, Lau J, Schoen C. Public Views on U.S. Health System Organization: A Call for New Directions. http://www.commonwealthfund.org/publications/data-briefs/2008/aug/public-views-on-u-s--health-system-organization--a-call-for-new-directions. Published August 1, 2008. Accessed December 11, 2015.
5. Sirovich BE, Woloshin S, Schwartz LM. Too little? Too much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171(17):1582-1585. PubMed
6. Joint Commission, American Medical Association–Convened Physician Consortium for Performance Improvement. Proceedings From the National Summit on Overuse. https://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf. Published September 24, 2012. Accessed July 8, 2016.
7. Cassel CK, Guest JA. Choosing Wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801-1802. PubMed
8. Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the Choosing Wisely campaign. Acad Med. 2014;89(7):990-995. PubMed
9. Smith CD, Levinson WS. A commitment to high-value care education from the internal medicine community. Ann Int Med. 2015;162(9):639-640. PubMed
10. Korenstein D, Kale M, Levinson W. Teaching value in academic environments: shifting the ivory tower. JAMA. 2013;310(16):1671-1672. PubMed
11. Kale MS, Bishop TF, Federman AD, Keyhani S. Trends in the overuse of ambulatory health care services in the United States. JAMA Intern Med. 2013;173(2):142-148. PubMed
12. Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. PubMed
13. Prasad V, Ioannidis JP. Evidence-based de-implementation for contradicted, unproven, and aspiring healthcare practices. Implement Sci. 2014;9:1. PubMed
14. Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff (Millwood). 2015;34(2):239-244. PubMed
15. Powell AA, Bloomfield HE, Burgess DJ, Wilt TJ, Partin MR. A conceptual framework for understanding and reducing overuse by primary care providers. Med Care Res Rev. 2013;70(5):451-472. PubMed
16. Nassery N, Segal JB, Chang E, Bridges JF. Systematic overuse of healthcare services: a conceptual model. Appl Health Econ Health Policy. 2015;13(1):1-6. PubMed
17. Segal JB, Nassery N, Chang HY, Chang E, Chan K, Bridges JF. An index for measuring overuse of health care resources with Medicare claims. Med Care. 2015;53(3):230-236. PubMed
18. Reschovsky JD, Rich EC, Lake TK. Factors contributing to variations in physicians’ use of evidence at the point of care: a conceptual model. J Gen Intern Med. 2015;30(suppl 3):S555-S561. PubMed
19. Feinstein AR, Horwitz RI. Problems in the “evidence” of “evidence-based medicine.” Am J Med. 1997;103(6):529-535. PubMed
20. Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1(2):185-194. PubMed
21. Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148-1154. PubMed
22. Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Effect of continuity of care on hospital utilization for seniors with multiple medical conditions in an integrated health care system. Ann Fam Med. 2015;13(2):123-129. PubMed
23. Chaiyachati KH, Gordon K, Long T, et al. Continuity in a VA patient-centered medical home reduces emergency department visits. PloS One. 2014;9(5):e96356. PubMed
24. Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39(5):555-563. PubMed
25. Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ. 2012;184(13):E726-E734. PubMed
26. PerryUndum Research/Communication; for ABIM Foundation. Unnecessary Tests and Procedures in the Health Care System: What Physicians Say About the Problem, the Causes, and the Solutions: Results From a National Survey of Physicians. http://www.choosingwisely.org/wp-content/uploads/2015/04/Final-Choosing-Wisely-Survey-Report.pdf. Published May 1, 2014. Accessed July 8, 2016.
27. Corallo AN, Croxford R, Goodman DC, Bryan EL, Srivastava D, Stukel TA. A systematic review of medical practice variation in OECD countries. Health Policy. 2014;114(1):5-14. PubMed
28. Cutler D, Skinner JS, Stern AD, Wennberg DE. Physician Beliefs and Patient Preferences: A New Look at Regional Variation in Health Care Spending. NBER Working Paper No. 19320. Cambridge, MA: National Bureau of Economic Research; 2013. http://www.nber.org/papers/w19320. Published August 2013. Accessed July 8, 2016.
29. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. PubMed
30. Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17-31. PubMed
31. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103-3109. PubMed
32. Sabuncu E, David J, Bernede-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002-2007. PLoS Med. 2009;6(6):e1000084. PubMed
33. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. PubMed
34. Yoon J, Rose DE, Canelo I, et al. Medical home features of VHA primary care clinics and avoidable hospitalizations. J Gen Intern Med. 2013;28(9):1188-1194. PubMed
35. Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267-273. PubMed
36. Davis MM, Balasubramanian BA, Cifuentes M, et al. Clinician staffing, scheduling, and engagement strategies among primary care practices delivering integrated care. J Am Board Fam Med. 2015;28(suppl 1):S32-S40. PubMed
37. Dine CJ, Miller J, Fuld A, Bellini LM, Iwashyna TJ. Educating physicians-in-training about resource utilization and their own outcomes of care in the inpatient setting. J Grad Med Educ. 2010;2(2):175-180. PubMed
38. Elligsen M, Walker SA, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol. 2012;33(4):354-361. PubMed
39. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345-2352. PubMed
40. Taggart LR, Leung E, Muller MP, Matukas LM, Daneman N. Differential outcome of an antimicrobial stewardship audit and feedback program in two intensive care units: a controlled interrupted time series study. BMC Infect Dis. 2015;15:480. PubMed
41. Hughes DR, Sunshine JH, Bhargavan M, Forman H. Physician self-referral for imaging and the cost of chronic care for Medicare beneficiaries. Med Care. 2011;49(9):857-864. PubMed
42. Ryskina KL, Pesko MF, Gossey JT, Caesar EP, Bishop TF. Brand name statin prescribing in a resident ambulatory practice: implications for teaching cost-conscious medicine. J Grad Med Educ. 2014;6(3):484-488. PubMed
43. Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545-555. PubMed
44. Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii-iv, 1-72. PubMed
45. Wilson I, Cowin LS, Johnson M, Young H. Professional identity in medical students: pedagogical challenges to medical education. Teach Learn Med. 2013;25(4):369-373. PubMed
46. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. PubMed
47. Dykes PC, Stade D, Chang F, et al. Participatory design and development of a patient-centered toolkit to engage hospitalized patients and care partners in their plan of care. AMIA Annu Symp Proc. 2014;2014:486-495. PubMed
48. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev. 2015;(11):CD010907. PubMed
49. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431. PubMed
50. Bank AJ, Gage RM. Annual impact of scribes on physician productivity and revenue in a cardiology clinic. Clinicoecon Outcomes Res. 2015;7:489-495. PubMed
51. Lyles CR, Sarkar U, Schillinger D, et al. Refilling medications through an online patient portal: consistent improvements in adherence across racial/ethnic groups. J Am Med Inform Assoc. 2016;23(e1):e28-e33. PubMed
52. Kruse CS, Bolton K, Freriks G. The effect of patient portals on quality outcomes and its implications to meaningful use: a systematic review. J Med Internet Res. 2015;17(2):e44. PubMed
53. Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians curriculum. Ann Intern Med. 2012;157(4):284-286. PubMed
54. Redberg RF. Less is more. Arch Intern Med. 2010;170(7):584. PubMed
65. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. PubMed
66. Pearson SD, Goldman L, Orav EJ, et al. Triage decisions for emergency department patients with chest pain: do physicians’ risk attitudes make the difference? J Gen Intern Med. 1995;10(10):557-564. PubMed
67. Tubbs EP, Elrod JA, Flum DR. Risk taking and tolerance of uncertainty: implications for surgeons. J Surg Res. 2006;131(1):1-6. PubMed
68. Zaat JO, van Eijk JT. General practitioners’ uncertainty, risk preference, and use of laboratory tests. Med Care. 1992;30(9):846-854. PubMed
69. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
70. Fisher ES, Wennberg JE, Stukel TA, et al. Associations among hospital capacity, utilization, and mortality of US Medicare beneficiaries, controlling for sociodemographic factors. Health Serv Res. 2000;34(6):1351-1362. PubMed
71. Yasaitis LC, Bynum JP, Skinner JS. Association between physician supply, local practice norms, and outpatient visit rates. Med Care. 2013;51(6):524-531. PubMed
72. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. PubMed
73. Ryskina KL, Smith CD, Weissman A, et al. U.S. internal medicine residents’ knowledge and practice of high-value care: a national survey. Acad Med. 2015;90(10):1373-1379. PubMed
74. Khullar D, Chokshi DA, Kocher R, et al. Behavioral economics and physician compensation—promise and challenges. N Engl J Med. 2015;372(24):2281-2283. PubMed
75. Landon BE, Reschovsky J, Reed M, Blumenthal D. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39(8):889-905. PubMed
76. Fanari Z, Abraham N, Kolm P, et al. Aggressive measures to decrease “door to balloon” time and incidence of unnecessary cardiac catheterization: potential risks and role of quality improvement. Mayo Clin Proc. 2015;90(12):1614-1622. PubMed
77. Kerr EA, Lucatorto MA, Holleman R, Hogan MM, Klamerus ML, Hofer TP. Monitoring performance for blood pressure management among patients with diabetes mellitus: too much of a good thing? Arch Intern Med. 2012;172(12):938-945. PubMed
78. Verhofstede R, Smets T, Cohen J, Costantini M, Van Den Noortgate N, Deliens L. Implementing the care programme for the last days of life in an acute geriatric hospital ward: a phase 2 mixed method study. BMC Palliat Care. 2016;15:27. PubMed
© 2017 Society of Hospital Medicine
Battling biases with the 5 Rs of cultural humility
How do we, as hospitalists, win the hearts and minds of patients, families, and care team members whom we do not know? What are the obstacles that we face when encountering patients and gaining the trust needed to improve patient care and patient experience?
With these questions in mind, the Cultural Humility Work Group, part of SHM’s Practice Management Committee, set out to develop a simple, universal framework to provide a foundation for strengthening communication skills and raising awareness of the basic tenets of cultural humility. According to Tervalon and Murray-Garcia, cultural humility is defined as a “process that requires humility as individuals continually engage in self-reflection and self-critique as lifelong learners and reflective practitioners. It requires humility in how physicians bring into check the power imbalances that exist in the dynamics of physician-patient communication by using patient-focused interviewing and care, and it is a process that requires humility to develop and maintain mutually respectful and dynamic partnerships with communities” (Tervalon, M. & Murray-García, J. “Cultural Humility Versus Cultural Competence: A Critical Distinction in Defining Physician Training Outcomes in Multicultural Education.” J Health Care Poor Underserved. 1998;9[2]:117-25).
How do we win this battle? The first step is to simply be aware that everyone is a victim of unconscious biases. Once we come to this (often uncomfortable) realization, we must make a conscious effort to change our mindset and make conscious decisions to not allow these biases to manifest.
Practicing cultural humility is extremely important in this process. It puts everyone on the same platform because there is no “minority,” “majority,” or “ethnicity” associated with it. It takes away the need to know everything about a certain culture and encourages us to approach every patient encounter acknowledging that we will humble ourselves, learn what is important to the patient, and leave having learned something from the interaction.
The work group developed “The 5 Rs of Cultural Humility” as a simple tool for hospitalists to incorporate into their practice. The first four Rs (Reflection, Respect, Regard and Relevance) are extrinsically focused, while the 5th R (Resiliency) is intrinsic. Our theory posits that, if you attain the first 4 Rs in every interaction, these will serve to build on and develop your own personal resiliency. Here are the 5 Rs:
- Reflection – Hospitalists will approach every encounter with humility and understanding that there is always something to learn from everyone.
- Respect – Hospitalists will treat every person with the utmost respect and strive to preserve dignity at all times.
- Regard – Hospitalists will hold every person in their highest regard while being aware of and not allowing unconscious biases to interfere in any interactions.
- Relevance – Hospitalists will expect cultural humility to be relevant to the patient and apply this practice to every encounter.
- Resiliency – Hospitalists will embody the practice of cultural humility to enhance personal resilience and globally focused compassion.
The content will be available as a downloadable pocket card that can be easily referenced on rounds and shared with colleagues. Our hope is to achieve heightened awareness of effective interaction. In addition to the definitions of each of the Rs, the card will feature questions to ask yourself before, during, and after every interaction to aid in attaining cultural humility.
The card will be printed and disseminated at Hospital Medicine 2017, and the 5 Rs will be discussed in a few sessions: “Making ‘Everything We Say and Do’ a Positive Patient Experience” in the Practice Management track on Thursday, May 4, and during a 20-minute “MEDtalk” in Product Theater 1 on May 3, at 10:15 a.m.
Keep on the lookout for future blog posts, where you’ll read about the 5 R’s in action through vignettes and a deeper dive into each aspect.
For more information and the downloadable pocket card, visit www.hospitalmedicine.org/5Rs.
Dr. Ansari is associate professor and associate division director of hospital medicine at Loyola University Medical Center, Maywood, Ill., and serves on SHM’s Cultural Humility Work Group.
How do we, as hospitalists, win the hearts and minds of patients, families, and care team members whom we do not know? What are the obstacles that we face when encountering patients and gaining the trust needed to improve patient care and patient experience?
With these questions in mind, the Cultural Humility Work Group, part of SHM’s Practice Management Committee, set out to develop a simple, universal framework to provide a foundation for strengthening communication skills and raising awareness of the basic tenets of cultural humility. According to Tervalon and Murray-Garcia, cultural humility is defined as a “process that requires humility as individuals continually engage in self-reflection and self-critique as lifelong learners and reflective practitioners. It requires humility in how physicians bring into check the power imbalances that exist in the dynamics of physician-patient communication by using patient-focused interviewing and care, and it is a process that requires humility to develop and maintain mutually respectful and dynamic partnerships with communities” (Tervalon, M. & Murray-García, J. “Cultural Humility Versus Cultural Competence: A Critical Distinction in Defining Physician Training Outcomes in Multicultural Education.” J Health Care Poor Underserved. 1998;9[2]:117-25).
How do we win this battle? The first step is to simply be aware that everyone is a victim of unconscious biases. Once we come to this (often uncomfortable) realization, we must make a conscious effort to change our mindset and make conscious decisions to not allow these biases to manifest.
Practicing cultural humility is extremely important in this process. It puts everyone on the same platform because there is no “minority,” “majority,” or “ethnicity” associated with it. It takes away the need to know everything about a certain culture and encourages us to approach every patient encounter acknowledging that we will humble ourselves, learn what is important to the patient, and leave having learned something from the interaction.
The work group developed “The 5 Rs of Cultural Humility” as a simple tool for hospitalists to incorporate into their practice. The first four Rs (Reflection, Respect, Regard and Relevance) are extrinsically focused, while the 5th R (Resiliency) is intrinsic. Our theory posits that, if you attain the first 4 Rs in every interaction, these will serve to build on and develop your own personal resiliency. Here are the 5 Rs:
- Reflection – Hospitalists will approach every encounter with humility and understanding that there is always something to learn from everyone.
- Respect – Hospitalists will treat every person with the utmost respect and strive to preserve dignity at all times.
- Regard – Hospitalists will hold every person in their highest regard while being aware of and not allowing unconscious biases to interfere in any interactions.
- Relevance – Hospitalists will expect cultural humility to be relevant to the patient and apply this practice to every encounter.
- Resiliency – Hospitalists will embody the practice of cultural humility to enhance personal resilience and globally focused compassion.
The content will be available as a downloadable pocket card that can be easily referenced on rounds and shared with colleagues. Our hope is to achieve heightened awareness of effective interaction. In addition to the definitions of each of the Rs, the card will feature questions to ask yourself before, during, and after every interaction to aid in attaining cultural humility.
The card will be printed and disseminated at Hospital Medicine 2017, and the 5 Rs will be discussed in a few sessions: “Making ‘Everything We Say and Do’ a Positive Patient Experience” in the Practice Management track on Thursday, May 4, and during a 20-minute “MEDtalk” in Product Theater 1 on May 3, at 10:15 a.m.
Keep on the lookout for future blog posts, where you’ll read about the 5 R’s in action through vignettes and a deeper dive into each aspect.
For more information and the downloadable pocket card, visit www.hospitalmedicine.org/5Rs.
Dr. Ansari is associate professor and associate division director of hospital medicine at Loyola University Medical Center, Maywood, Ill., and serves on SHM’s Cultural Humility Work Group.
How do we, as hospitalists, win the hearts and minds of patients, families, and care team members whom we do not know? What are the obstacles that we face when encountering patients and gaining the trust needed to improve patient care and patient experience?
With these questions in mind, the Cultural Humility Work Group, part of SHM’s Practice Management Committee, set out to develop a simple, universal framework to provide a foundation for strengthening communication skills and raising awareness of the basic tenets of cultural humility. According to Tervalon and Murray-Garcia, cultural humility is defined as a “process that requires humility as individuals continually engage in self-reflection and self-critique as lifelong learners and reflective practitioners. It requires humility in how physicians bring into check the power imbalances that exist in the dynamics of physician-patient communication by using patient-focused interviewing and care, and it is a process that requires humility to develop and maintain mutually respectful and dynamic partnerships with communities” (Tervalon, M. & Murray-García, J. “Cultural Humility Versus Cultural Competence: A Critical Distinction in Defining Physician Training Outcomes in Multicultural Education.” J Health Care Poor Underserved. 1998;9[2]:117-25).
How do we win this battle? The first step is to simply be aware that everyone is a victim of unconscious biases. Once we come to this (often uncomfortable) realization, we must make a conscious effort to change our mindset and make conscious decisions to not allow these biases to manifest.
Practicing cultural humility is extremely important in this process. It puts everyone on the same platform because there is no “minority,” “majority,” or “ethnicity” associated with it. It takes away the need to know everything about a certain culture and encourages us to approach every patient encounter acknowledging that we will humble ourselves, learn what is important to the patient, and leave having learned something from the interaction.
The work group developed “The 5 Rs of Cultural Humility” as a simple tool for hospitalists to incorporate into their practice. The first four Rs (Reflection, Respect, Regard and Relevance) are extrinsically focused, while the 5th R (Resiliency) is intrinsic. Our theory posits that, if you attain the first 4 Rs in every interaction, these will serve to build on and develop your own personal resiliency. Here are the 5 Rs:
- Reflection – Hospitalists will approach every encounter with humility and understanding that there is always something to learn from everyone.
- Respect – Hospitalists will treat every person with the utmost respect and strive to preserve dignity at all times.
- Regard – Hospitalists will hold every person in their highest regard while being aware of and not allowing unconscious biases to interfere in any interactions.
- Relevance – Hospitalists will expect cultural humility to be relevant to the patient and apply this practice to every encounter.
- Resiliency – Hospitalists will embody the practice of cultural humility to enhance personal resilience and globally focused compassion.
The content will be available as a downloadable pocket card that can be easily referenced on rounds and shared with colleagues. Our hope is to achieve heightened awareness of effective interaction. In addition to the definitions of each of the Rs, the card will feature questions to ask yourself before, during, and after every interaction to aid in attaining cultural humility.
The card will be printed and disseminated at Hospital Medicine 2017, and the 5 Rs will be discussed in a few sessions: “Making ‘Everything We Say and Do’ a Positive Patient Experience” in the Practice Management track on Thursday, May 4, and during a 20-minute “MEDtalk” in Product Theater 1 on May 3, at 10:15 a.m.
Keep on the lookout for future blog posts, where you’ll read about the 5 R’s in action through vignettes and a deeper dive into each aspect.
For more information and the downloadable pocket card, visit www.hospitalmedicine.org/5Rs.
Dr. Ansari is associate professor and associate division director of hospital medicine at Loyola University Medical Center, Maywood, Ill., and serves on SHM’s Cultural Humility Work Group.
How to reduce the open hysterectomy rate
SAN ANTONIO – About a decade ago, the Northern California Permanente Medical Group realized it had a problem: There were too many open hysterectomies being performed.
“We had a large number of low-volume surgeons doing a lot of open hysterectomies,” said Andrew Walter, MD, a gynecologic surgeon at the Permanente Medical Group (TPMG) campus in Roseville, Calif., part of Kaiser Permanente. Across 15 hospitals in the system, “our open rate was 64% in 2007.”
To address the problem, “our leadership basically set targets; we then provided surgical education and training in minimally invasive hysterectomy,” Dr. Walter said at the annual scientific meeting of the Society of Gynecologic Surgeons.
Kaiser Permanente is a capitated system, but even so, its experience in reducing open hysterectomy rates may be useful for other systems dealing with the same problem.
At first, Dr. Walter and his colleagues didn’t know how much of an improvement could be made. “In 2008, we said, ‘Okay, 60% seems doable’” as a minimally invasive target rate, Dr. Walter said. TPMG hit that target, and “the chiefs looked at the numbers and said we can do a little bit better.” With some more effort, the rate of minimally invasive hysterectomies hit 80%, and then 90%, about where it stands today, with a corresponding drop in open rates.
“It will be interesting to see if someone says, ‘Let’s try 95%,’ ” Dr. Walter said.
However, the process hasn’t been easy, and it hasn’t been entirely evidence based, he said. “We are working with hundreds of gynecologists, and very few of them have had advanced training. But ultimately, it worked.”
Surgeons trained with TPMG peers experienced in minimally invasive hysterectomy, and both surgeons were kept on full salary during the learning process. It took about 5-15 cases before learner surgeons were considered proficient. “Funded proctoring is the most important aspect of the program,” he said.
TPMG also reduced the number of physicians doing hysterectomies from 416 to 228 in 2015. “This was the hard part, deciding who is a surgeon and who is not,” Dr. Walter said. “I would like to tell you that these were Kumbaya moments, but there was consternation, and there remains consternation about the process.” A number of ob.gyns. voluntarily gave up their surgical privileges, saying, ‘Thank God I don’t have to operate anymore,’ ” he said.
For low-volume surgeons – those who performed 10 or fewer hysterectomies a year – who wanted stay in the operating room, “we either had to push them into this training or encourage them to give up their surgical practices.” The more than 3,600 hysterectomies performed annually at TPMG facilities are now mostly done by surgeons doing at least 11 of these procedures a year, and often more than 20.
TPMG also paid for training courses at outside institutions, and department chiefs were held accountable for performance.
“Obviously, there are unique processes within Kaiser Permanente that facilitated this, but some of them are not unique. Physician support by enhanced training – that’s something that can be done. The barriers are reimbursement, and deciding who is a surgeon,” he said.
The next target is vaginal hysterectomy. Rates have been stable lately at about 30%, but “we have found that many patients, when reviewed, are vaginal hysterectomy candidates. We’ve set a target of 40%.” The procedure needs to be incentivized, Dr. Walter said, but it’s unclear how to do that at this point.
Dr. Walter reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – About a decade ago, the Northern California Permanente Medical Group realized it had a problem: There were too many open hysterectomies being performed.
“We had a large number of low-volume surgeons doing a lot of open hysterectomies,” said Andrew Walter, MD, a gynecologic surgeon at the Permanente Medical Group (TPMG) campus in Roseville, Calif., part of Kaiser Permanente. Across 15 hospitals in the system, “our open rate was 64% in 2007.”
To address the problem, “our leadership basically set targets; we then provided surgical education and training in minimally invasive hysterectomy,” Dr. Walter said at the annual scientific meeting of the Society of Gynecologic Surgeons.
Kaiser Permanente is a capitated system, but even so, its experience in reducing open hysterectomy rates may be useful for other systems dealing with the same problem.
At first, Dr. Walter and his colleagues didn’t know how much of an improvement could be made. “In 2008, we said, ‘Okay, 60% seems doable’” as a minimally invasive target rate, Dr. Walter said. TPMG hit that target, and “the chiefs looked at the numbers and said we can do a little bit better.” With some more effort, the rate of minimally invasive hysterectomies hit 80%, and then 90%, about where it stands today, with a corresponding drop in open rates.
“It will be interesting to see if someone says, ‘Let’s try 95%,’ ” Dr. Walter said.
However, the process hasn’t been easy, and it hasn’t been entirely evidence based, he said. “We are working with hundreds of gynecologists, and very few of them have had advanced training. But ultimately, it worked.”
Surgeons trained with TPMG peers experienced in minimally invasive hysterectomy, and both surgeons were kept on full salary during the learning process. It took about 5-15 cases before learner surgeons were considered proficient. “Funded proctoring is the most important aspect of the program,” he said.
TPMG also reduced the number of physicians doing hysterectomies from 416 to 228 in 2015. “This was the hard part, deciding who is a surgeon and who is not,” Dr. Walter said. “I would like to tell you that these were Kumbaya moments, but there was consternation, and there remains consternation about the process.” A number of ob.gyns. voluntarily gave up their surgical privileges, saying, ‘Thank God I don’t have to operate anymore,’ ” he said.
For low-volume surgeons – those who performed 10 or fewer hysterectomies a year – who wanted stay in the operating room, “we either had to push them into this training or encourage them to give up their surgical practices.” The more than 3,600 hysterectomies performed annually at TPMG facilities are now mostly done by surgeons doing at least 11 of these procedures a year, and often more than 20.
TPMG also paid for training courses at outside institutions, and department chiefs were held accountable for performance.
“Obviously, there are unique processes within Kaiser Permanente that facilitated this, but some of them are not unique. Physician support by enhanced training – that’s something that can be done. The barriers are reimbursement, and deciding who is a surgeon,” he said.
The next target is vaginal hysterectomy. Rates have been stable lately at about 30%, but “we have found that many patients, when reviewed, are vaginal hysterectomy candidates. We’ve set a target of 40%.” The procedure needs to be incentivized, Dr. Walter said, but it’s unclear how to do that at this point.
Dr. Walter reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – About a decade ago, the Northern California Permanente Medical Group realized it had a problem: There were too many open hysterectomies being performed.
“We had a large number of low-volume surgeons doing a lot of open hysterectomies,” said Andrew Walter, MD, a gynecologic surgeon at the Permanente Medical Group (TPMG) campus in Roseville, Calif., part of Kaiser Permanente. Across 15 hospitals in the system, “our open rate was 64% in 2007.”
To address the problem, “our leadership basically set targets; we then provided surgical education and training in minimally invasive hysterectomy,” Dr. Walter said at the annual scientific meeting of the Society of Gynecologic Surgeons.
Kaiser Permanente is a capitated system, but even so, its experience in reducing open hysterectomy rates may be useful for other systems dealing with the same problem.
At first, Dr. Walter and his colleagues didn’t know how much of an improvement could be made. “In 2008, we said, ‘Okay, 60% seems doable’” as a minimally invasive target rate, Dr. Walter said. TPMG hit that target, and “the chiefs looked at the numbers and said we can do a little bit better.” With some more effort, the rate of minimally invasive hysterectomies hit 80%, and then 90%, about where it stands today, with a corresponding drop in open rates.
“It will be interesting to see if someone says, ‘Let’s try 95%,’ ” Dr. Walter said.
However, the process hasn’t been easy, and it hasn’t been entirely evidence based, he said. “We are working with hundreds of gynecologists, and very few of them have had advanced training. But ultimately, it worked.”
Surgeons trained with TPMG peers experienced in minimally invasive hysterectomy, and both surgeons were kept on full salary during the learning process. It took about 5-15 cases before learner surgeons were considered proficient. “Funded proctoring is the most important aspect of the program,” he said.
TPMG also reduced the number of physicians doing hysterectomies from 416 to 228 in 2015. “This was the hard part, deciding who is a surgeon and who is not,” Dr. Walter said. “I would like to tell you that these were Kumbaya moments, but there was consternation, and there remains consternation about the process.” A number of ob.gyns. voluntarily gave up their surgical privileges, saying, ‘Thank God I don’t have to operate anymore,’ ” he said.
For low-volume surgeons – those who performed 10 or fewer hysterectomies a year – who wanted stay in the operating room, “we either had to push them into this training or encourage them to give up their surgical practices.” The more than 3,600 hysterectomies performed annually at TPMG facilities are now mostly done by surgeons doing at least 11 of these procedures a year, and often more than 20.
TPMG also paid for training courses at outside institutions, and department chiefs were held accountable for performance.
“Obviously, there are unique processes within Kaiser Permanente that facilitated this, but some of them are not unique. Physician support by enhanced training – that’s something that can be done. The barriers are reimbursement, and deciding who is a surgeon,” he said.
The next target is vaginal hysterectomy. Rates have been stable lately at about 30%, but “we have found that many patients, when reviewed, are vaginal hysterectomy candidates. We’ve set a target of 40%.” The procedure needs to be incentivized, Dr. Walter said, but it’s unclear how to do that at this point.
Dr. Walter reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
EXPERT ANALYSIS FROM SGS 2017
WHO’s malaria pilot vaccine: No silver bullet, but a potential strike at malaria’s heart
EXPERT ANALYSIS FROM ECCMID 2017
VIENNA – The first malaria vaccine to enter a national pilot project is not a silver bullet against the disease that kills half a million every year, but it still might be powerful enough to significantly reduce global disease burden, and even impact transmission, according to infectious disease specialist Nick Beeching, MD.
The vaccine, RTS,S (Mosquirix; GlaxoSmithKline), will be tested in three African countries beginning next year, the World Health Organization announced on April 25. The pilot programs will target 720,000 children aged 5-17 months in high-risk areas of the three countries.
Even though it’s the first malaria vaccine to pass its pivotal phase III trial, RTS,S isn’t terribly effective by any standards, said Dr. Beeching of the Royal Liverpool (England) University.
April 25, 2017, is World Malaria Day, and Anthony S. Fauci, MD, and B. Fenton Hall, MD, PhD, of the U.S. National Institute of Allergy and Infectious Diseases, said in a statement, “Safe and effective vaccines are critical tools for future efforts to control, eliminate, and, ultimately, eradicate malaria. NIAID is supporting the development of numerous malaria vaccine candidates, 10 of which are in clinical trials. In 2015, an estimated 212 million new malaria cases and 429,000 deaths occurred. Nearly 90% of these cases were among children under the age of 5 years in Africa, where malaria claims the life of a child every 2 minutes.”
GSK has been working on this vaccine since 1985, according to the company’s RTS,S literature. It is a recombinant protein that targets the circumsporozoite protein of the Plasmodium falciparum parasite at an early stage, before it enters the liver and begins to embed in erythrocytes. The aim, Dr. Beeching said, was to develop an antigen that would mobilize the immune system from the moment a mosquito injected the sporozoites through a bite, “well before they have a chance to hide in the liver.”
The 2- and 3-year follow-up results of the phase III trial, conducted in 15,500 children, were published in the Lancet in 2015. RTS,S was administered as a three-dose series, plus a booster dose, beginning at 5 months of age. The primary immunizations were given with a minimum 4-week interval between doses, with the booster administered 18 months after the last dose.
The primary series reduced clinical cases by 26%. With the booster dose, cases were reduced by 39% overall. The vaccine averted 1,774 episodes of clinical malaria per 1,000 vaccinated children, and 983 cases per 1,000 vaccinated infants. But vaccine efficacy waned over time, disappearing completely in children who got only the three-dose series. The booster dose improved response stability somewhat; during the 12 months after the fourth dose, vaccine efficacy was about 25%.
Based on these results, GSK received approval from the European Medicines Agency in 2015, and the WHO recommended a large-scale implementation of the vaccine be carried out last year. GSK will provide the vaccine at no cost, and each country’s government will decide which regions to include in the pilot study.
This real-world use will put RTS,S to the ultimate test, Dr. Beeching said: “There is always the practical problem of how do you get four doses of vaccine into people. It’s easy to do in a clinical trial, but the operations and the logistics of getting it right on the ground are what really matter. We don’t know how good less than four doses would be, and we still don’t know how long the protective effect of the full series plus booster will last. I think there’s concern that it might wane with time.”
Still, he said, even a 39% reduction in disease burden is worth aggressively pursuing, not only because of the thousands of children’s lives that could be saved, but because unvaccinated children and adults could potentially be protected as well: “We could see a knock-on effect. By reducing the burden of malaria in children, it may also reduce transmission to other people who haven’t been vaccinated.”
The vaccine certainly won’t eradicate malaria, Dr. Beeching said. It needs to be viewed as an addition to WHO’s core vector control strategy, which includes insecticide-impregnated bed nets and mosquito eradication programs.
Cost is an unresolved issue. According to the Malaria Vaccine Initiative, which is partnering with GSK to launch RTS,S, the company won’t charge for the vaccine in the pilot project, and is committed to making sure the children who need it get it.
“In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms,” the company said in a press release. “The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access.
“GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around 5%, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.”
Finally, Dr. Beeching said, there’s no way to know to know how long any malaria vaccine would retain its effectiveness.
“Making a malaria vaccine has been a dream for years, and a tough one. The antigens change according to the stage of the parasite, and there is always continuous genetic variation. So there is a possibility of escape from vaccine coverage. These are very clever parasites,” he said.
Dr. Beeching has no financial interest in the vaccine.
[email protected]
On Twitter @Alz_gal
EXPERT ANALYSIS FROM ECCMID 2017
VIENNA – The first malaria vaccine to enter a national pilot project is not a silver bullet against the disease that kills half a million every year, but it still might be powerful enough to significantly reduce global disease burden, and even impact transmission, according to infectious disease specialist Nick Beeching, MD.
The vaccine, RTS,S (Mosquirix; GlaxoSmithKline), will be tested in three African countries beginning next year, the World Health Organization announced on April 25. The pilot programs will target 720,000 children aged 5-17 months in high-risk areas of the three countries.
Even though it’s the first malaria vaccine to pass its pivotal phase III trial, RTS,S isn’t terribly effective by any standards, said Dr. Beeching of the Royal Liverpool (England) University.
April 25, 2017, is World Malaria Day, and Anthony S. Fauci, MD, and B. Fenton Hall, MD, PhD, of the U.S. National Institute of Allergy and Infectious Diseases, said in a statement, “Safe and effective vaccines are critical tools for future efforts to control, eliminate, and, ultimately, eradicate malaria. NIAID is supporting the development of numerous malaria vaccine candidates, 10 of which are in clinical trials. In 2015, an estimated 212 million new malaria cases and 429,000 deaths occurred. Nearly 90% of these cases were among children under the age of 5 years in Africa, where malaria claims the life of a child every 2 minutes.”
GSK has been working on this vaccine since 1985, according to the company’s RTS,S literature. It is a recombinant protein that targets the circumsporozoite protein of the Plasmodium falciparum parasite at an early stage, before it enters the liver and begins to embed in erythrocytes. The aim, Dr. Beeching said, was to develop an antigen that would mobilize the immune system from the moment a mosquito injected the sporozoites through a bite, “well before they have a chance to hide in the liver.”
The 2- and 3-year follow-up results of the phase III trial, conducted in 15,500 children, were published in the Lancet in 2015. RTS,S was administered as a three-dose series, plus a booster dose, beginning at 5 months of age. The primary immunizations were given with a minimum 4-week interval between doses, with the booster administered 18 months after the last dose.
The primary series reduced clinical cases by 26%. With the booster dose, cases were reduced by 39% overall. The vaccine averted 1,774 episodes of clinical malaria per 1,000 vaccinated children, and 983 cases per 1,000 vaccinated infants. But vaccine efficacy waned over time, disappearing completely in children who got only the three-dose series. The booster dose improved response stability somewhat; during the 12 months after the fourth dose, vaccine efficacy was about 25%.
Based on these results, GSK received approval from the European Medicines Agency in 2015, and the WHO recommended a large-scale implementation of the vaccine be carried out last year. GSK will provide the vaccine at no cost, and each country’s government will decide which regions to include in the pilot study.
This real-world use will put RTS,S to the ultimate test, Dr. Beeching said: “There is always the practical problem of how do you get four doses of vaccine into people. It’s easy to do in a clinical trial, but the operations and the logistics of getting it right on the ground are what really matter. We don’t know how good less than four doses would be, and we still don’t know how long the protective effect of the full series plus booster will last. I think there’s concern that it might wane with time.”
Still, he said, even a 39% reduction in disease burden is worth aggressively pursuing, not only because of the thousands of children’s lives that could be saved, but because unvaccinated children and adults could potentially be protected as well: “We could see a knock-on effect. By reducing the burden of malaria in children, it may also reduce transmission to other people who haven’t been vaccinated.”
The vaccine certainly won’t eradicate malaria, Dr. Beeching said. It needs to be viewed as an addition to WHO’s core vector control strategy, which includes insecticide-impregnated bed nets and mosquito eradication programs.
Cost is an unresolved issue. According to the Malaria Vaccine Initiative, which is partnering with GSK to launch RTS,S, the company won’t charge for the vaccine in the pilot project, and is committed to making sure the children who need it get it.
“In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms,” the company said in a press release. “The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access.
“GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around 5%, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.”
Finally, Dr. Beeching said, there’s no way to know to know how long any malaria vaccine would retain its effectiveness.
“Making a malaria vaccine has been a dream for years, and a tough one. The antigens change according to the stage of the parasite, and there is always continuous genetic variation. So there is a possibility of escape from vaccine coverage. These are very clever parasites,” he said.
Dr. Beeching has no financial interest in the vaccine.
[email protected]
On Twitter @Alz_gal
EXPERT ANALYSIS FROM ECCMID 2017
VIENNA – The first malaria vaccine to enter a national pilot project is not a silver bullet against the disease that kills half a million every year, but it still might be powerful enough to significantly reduce global disease burden, and even impact transmission, according to infectious disease specialist Nick Beeching, MD.
The vaccine, RTS,S (Mosquirix; GlaxoSmithKline), will be tested in three African countries beginning next year, the World Health Organization announced on April 25. The pilot programs will target 720,000 children aged 5-17 months in high-risk areas of the three countries.
Even though it’s the first malaria vaccine to pass its pivotal phase III trial, RTS,S isn’t terribly effective by any standards, said Dr. Beeching of the Royal Liverpool (England) University.
April 25, 2017, is World Malaria Day, and Anthony S. Fauci, MD, and B. Fenton Hall, MD, PhD, of the U.S. National Institute of Allergy and Infectious Diseases, said in a statement, “Safe and effective vaccines are critical tools for future efforts to control, eliminate, and, ultimately, eradicate malaria. NIAID is supporting the development of numerous malaria vaccine candidates, 10 of which are in clinical trials. In 2015, an estimated 212 million new malaria cases and 429,000 deaths occurred. Nearly 90% of these cases were among children under the age of 5 years in Africa, where malaria claims the life of a child every 2 minutes.”
GSK has been working on this vaccine since 1985, according to the company’s RTS,S literature. It is a recombinant protein that targets the circumsporozoite protein of the Plasmodium falciparum parasite at an early stage, before it enters the liver and begins to embed in erythrocytes. The aim, Dr. Beeching said, was to develop an antigen that would mobilize the immune system from the moment a mosquito injected the sporozoites through a bite, “well before they have a chance to hide in the liver.”
The 2- and 3-year follow-up results of the phase III trial, conducted in 15,500 children, were published in the Lancet in 2015. RTS,S was administered as a three-dose series, plus a booster dose, beginning at 5 months of age. The primary immunizations were given with a minimum 4-week interval between doses, with the booster administered 18 months after the last dose.
The primary series reduced clinical cases by 26%. With the booster dose, cases were reduced by 39% overall. The vaccine averted 1,774 episodes of clinical malaria per 1,000 vaccinated children, and 983 cases per 1,000 vaccinated infants. But vaccine efficacy waned over time, disappearing completely in children who got only the three-dose series. The booster dose improved response stability somewhat; during the 12 months after the fourth dose, vaccine efficacy was about 25%.
Based on these results, GSK received approval from the European Medicines Agency in 2015, and the WHO recommended a large-scale implementation of the vaccine be carried out last year. GSK will provide the vaccine at no cost, and each country’s government will decide which regions to include in the pilot study.
This real-world use will put RTS,S to the ultimate test, Dr. Beeching said: “There is always the practical problem of how do you get four doses of vaccine into people. It’s easy to do in a clinical trial, but the operations and the logistics of getting it right on the ground are what really matter. We don’t know how good less than four doses would be, and we still don’t know how long the protective effect of the full series plus booster will last. I think there’s concern that it might wane with time.”
Still, he said, even a 39% reduction in disease burden is worth aggressively pursuing, not only because of the thousands of children’s lives that could be saved, but because unvaccinated children and adults could potentially be protected as well: “We could see a knock-on effect. By reducing the burden of malaria in children, it may also reduce transmission to other people who haven’t been vaccinated.”
The vaccine certainly won’t eradicate malaria, Dr. Beeching said. It needs to be viewed as an addition to WHO’s core vector control strategy, which includes insecticide-impregnated bed nets and mosquito eradication programs.
Cost is an unresolved issue. According to the Malaria Vaccine Initiative, which is partnering with GSK to launch RTS,S, the company won’t charge for the vaccine in the pilot project, and is committed to making sure the children who need it get it.
“In many African countries, childhood vaccines are provided at no cost to children or their families, thanks to existing international and national financing mechanisms,” the company said in a press release. “The RTS,S partnership anticipates that similar mechanisms would be implemented for a malaria vaccine. A shared goal is to have the cost of a malaria vaccine not be a barrier to access.
“GSK has previously stated that the price of RTS,S will cover the cost of manufacturing the vaccine together with a small return of around 5%, which will be reinvested in research and development for next-generation malaria vaccines or vaccines against other neglected tropical diseases.”
Finally, Dr. Beeching said, there’s no way to know to know how long any malaria vaccine would retain its effectiveness.
“Making a malaria vaccine has been a dream for years, and a tough one. The antigens change according to the stage of the parasite, and there is always continuous genetic variation. So there is a possibility of escape from vaccine coverage. These are very clever parasites,” he said.
Dr. Beeching has no financial interest in the vaccine.
[email protected]
On Twitter @Alz_gal
Long-term albumin shows survival benefit in decompensated cirrhosis
AMSTERDAM – Long-term treatment with human albumin improved the overall survival of patients with decompensated liver cirrhosis, compared with standard medical care, in a randomized, controlled trial presented at the International Liver Congress.
The final results of the ANSWER study showed that a 38% reduction in the risk of death could be achieved at 18 months’ follow-up by giving patients human albumin, with an overall survival of 78% vs. 66% in the two groups, respectively (hazard ratio, 0.62; 95% confidence interval, 0.40-0.95; P = .028).
“Long-term albumin administration to patients with decompensated cirrhosis may be seen as a disease-modifying treatment,” said the presenting study author, Mauro Bernardi, MD, professor in the department of medical and surgical sciences at the University of Bologna (Italy).
Not only was the overall survival improved, but there was improvement in the management of ascites, a reduction in the incidence of severe complications (such as spontaneous bacterial peritonitis, renal dysfunction, and hepatic encephalopathy), a reduction in the number of hospitalizations and duration of in-hospital treatment, and a signal for improved quality of life, he said.
“We know that it is good to give albumin as an infusion in many, many circumstances,” said Frank Tacke, MD, PhD, who was not involved in the study, during a press briefing at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
“What we did not know before was if there was a role for giving albumin – which is unfortunately quite expensive – for a longer period of time in patients with liver cirrhosis,” said Dr. Tacke, who is the EASL vice-secretary and professor of medicine in the department of gastroenterology, metabolic diseases and intensive care medicine at University Hospital Aachen (Germany).
Although long-term treatment with human albumin is a relatively expensive treatment, particularly because it requires weekly infusion, Dr. Bernardi noted that a cost analysis was being performed, and potentially the cost of treatment could be offset by the reduction in paracentesis, duration of hospitalization, and reduced need for treating patients with complications, compared with standard medical care alone.
“The idea of supplying albumin to patients with advanced cirrhosis is quite an old one, and there is long debate. The point is that a reliable study that could resolve this was simply lacking up to now,” Dr. Bernard said at the briefing.
The results could mean that patients with decompensated cirrhosis now have a much-needed therapeutic option. These patients have “a very poor prognosis,” Dr. Bernardi said. The 1-year probability of survival is about 20%, and the only curative therapy at present is liver transplantation.
A total of 440 patients, mostly male with an average age of 61 years, with cirrhosis and uncomplicated ascites were randomized at 33 Italian centers to receive standard medical treatment alone (n = 213) or with human albumin (n = 218) given at an infused dose of 40 g twice a week for the first 2 weeks, then 40 g every week. The albumin used in the trial was provided by five different pharmaceutical companies and sent to a central location for generic relabeling and distribution out to the participating trial centers.
Significantly fewer patients who were given human albumin than those who were not (66% vs. 38%, P less than .001) needed at least one paracentesis. The incidence rate for the removal of peritoneal fluid in the standard medical treatment arm was 3.5/person per year. There was a 54% reduction in this rate by the addition of human albumin (HR = 0.46; 95% CI, 0.40-0.53; P less than .0001). There was also a significant 46% reduction in the incidence of refractory ascites (48% vs. 25%, P less than .0001).
Patients who received standard medical treatment plus albumin needed fewer hospitalizations and fewer days of in-hospital care per person per year than those in the standard care–only arm. The use of human albumin reduced the number of hospital stays by 35% (HR = 0.65; 95% CI, 0.55-0.77; P less than .0001) and the duration of days in hospital by 45% (HR = 0.55; 95% CI, 0.52-0.58; P less than .0001).
Although not statistically significant, a trend for greater improvements and fewer decreases in quality of life, as measured using the EQ-5D visual assessment scale, at 3, 6, and 12 months, was seen with the use of human albumin.
Four patients had adverse drug reactions: two were mild allergic reactions, and two were potentially life-threatening septic shock that needed intensive care treatment. One of the latter cases might have been caused by pneumonia, and the other required study interruption. But in all cases the patients recovered, Dr. Bernardi reported.
The study was funded by the Italian Drug Agency. Dr. Bernardi had acted as a speaker for and consultant to CLS Behring and Baxter Healthcare, and as a speaker to the Plasma Protein Therapeutics Association’s Europe division, Grifols, Gilead Sciences, and AbbVie Italia. Dr. Tacke had nothing to disclose.
AMSTERDAM – Long-term treatment with human albumin improved the overall survival of patients with decompensated liver cirrhosis, compared with standard medical care, in a randomized, controlled trial presented at the International Liver Congress.
The final results of the ANSWER study showed that a 38% reduction in the risk of death could be achieved at 18 months’ follow-up by giving patients human albumin, with an overall survival of 78% vs. 66% in the two groups, respectively (hazard ratio, 0.62; 95% confidence interval, 0.40-0.95; P = .028).
“Long-term albumin administration to patients with decompensated cirrhosis may be seen as a disease-modifying treatment,” said the presenting study author, Mauro Bernardi, MD, professor in the department of medical and surgical sciences at the University of Bologna (Italy).
Not only was the overall survival improved, but there was improvement in the management of ascites, a reduction in the incidence of severe complications (such as spontaneous bacterial peritonitis, renal dysfunction, and hepatic encephalopathy), a reduction in the number of hospitalizations and duration of in-hospital treatment, and a signal for improved quality of life, he said.
“We know that it is good to give albumin as an infusion in many, many circumstances,” said Frank Tacke, MD, PhD, who was not involved in the study, during a press briefing at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
“What we did not know before was if there was a role for giving albumin – which is unfortunately quite expensive – for a longer period of time in patients with liver cirrhosis,” said Dr. Tacke, who is the EASL vice-secretary and professor of medicine in the department of gastroenterology, metabolic diseases and intensive care medicine at University Hospital Aachen (Germany).
Although long-term treatment with human albumin is a relatively expensive treatment, particularly because it requires weekly infusion, Dr. Bernardi noted that a cost analysis was being performed, and potentially the cost of treatment could be offset by the reduction in paracentesis, duration of hospitalization, and reduced need for treating patients with complications, compared with standard medical care alone.
“The idea of supplying albumin to patients with advanced cirrhosis is quite an old one, and there is long debate. The point is that a reliable study that could resolve this was simply lacking up to now,” Dr. Bernard said at the briefing.
The results could mean that patients with decompensated cirrhosis now have a much-needed therapeutic option. These patients have “a very poor prognosis,” Dr. Bernardi said. The 1-year probability of survival is about 20%, and the only curative therapy at present is liver transplantation.
A total of 440 patients, mostly male with an average age of 61 years, with cirrhosis and uncomplicated ascites were randomized at 33 Italian centers to receive standard medical treatment alone (n = 213) or with human albumin (n = 218) given at an infused dose of 40 g twice a week for the first 2 weeks, then 40 g every week. The albumin used in the trial was provided by five different pharmaceutical companies and sent to a central location for generic relabeling and distribution out to the participating trial centers.
Significantly fewer patients who were given human albumin than those who were not (66% vs. 38%, P less than .001) needed at least one paracentesis. The incidence rate for the removal of peritoneal fluid in the standard medical treatment arm was 3.5/person per year. There was a 54% reduction in this rate by the addition of human albumin (HR = 0.46; 95% CI, 0.40-0.53; P less than .0001). There was also a significant 46% reduction in the incidence of refractory ascites (48% vs. 25%, P less than .0001).
Patients who received standard medical treatment plus albumin needed fewer hospitalizations and fewer days of in-hospital care per person per year than those in the standard care–only arm. The use of human albumin reduced the number of hospital stays by 35% (HR = 0.65; 95% CI, 0.55-0.77; P less than .0001) and the duration of days in hospital by 45% (HR = 0.55; 95% CI, 0.52-0.58; P less than .0001).
Although not statistically significant, a trend for greater improvements and fewer decreases in quality of life, as measured using the EQ-5D visual assessment scale, at 3, 6, and 12 months, was seen with the use of human albumin.
Four patients had adverse drug reactions: two were mild allergic reactions, and two were potentially life-threatening septic shock that needed intensive care treatment. One of the latter cases might have been caused by pneumonia, and the other required study interruption. But in all cases the patients recovered, Dr. Bernardi reported.
The study was funded by the Italian Drug Agency. Dr. Bernardi had acted as a speaker for and consultant to CLS Behring and Baxter Healthcare, and as a speaker to the Plasma Protein Therapeutics Association’s Europe division, Grifols, Gilead Sciences, and AbbVie Italia. Dr. Tacke had nothing to disclose.
AMSTERDAM – Long-term treatment with human albumin improved the overall survival of patients with decompensated liver cirrhosis, compared with standard medical care, in a randomized, controlled trial presented at the International Liver Congress.
The final results of the ANSWER study showed that a 38% reduction in the risk of death could be achieved at 18 months’ follow-up by giving patients human albumin, with an overall survival of 78% vs. 66% in the two groups, respectively (hazard ratio, 0.62; 95% confidence interval, 0.40-0.95; P = .028).
“Long-term albumin administration to patients with decompensated cirrhosis may be seen as a disease-modifying treatment,” said the presenting study author, Mauro Bernardi, MD, professor in the department of medical and surgical sciences at the University of Bologna (Italy).
Not only was the overall survival improved, but there was improvement in the management of ascites, a reduction in the incidence of severe complications (such as spontaneous bacterial peritonitis, renal dysfunction, and hepatic encephalopathy), a reduction in the number of hospitalizations and duration of in-hospital treatment, and a signal for improved quality of life, he said.
“We know that it is good to give albumin as an infusion in many, many circumstances,” said Frank Tacke, MD, PhD, who was not involved in the study, during a press briefing at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
“What we did not know before was if there was a role for giving albumin – which is unfortunately quite expensive – for a longer period of time in patients with liver cirrhosis,” said Dr. Tacke, who is the EASL vice-secretary and professor of medicine in the department of gastroenterology, metabolic diseases and intensive care medicine at University Hospital Aachen (Germany).
Although long-term treatment with human albumin is a relatively expensive treatment, particularly because it requires weekly infusion, Dr. Bernardi noted that a cost analysis was being performed, and potentially the cost of treatment could be offset by the reduction in paracentesis, duration of hospitalization, and reduced need for treating patients with complications, compared with standard medical care alone.
“The idea of supplying albumin to patients with advanced cirrhosis is quite an old one, and there is long debate. The point is that a reliable study that could resolve this was simply lacking up to now,” Dr. Bernard said at the briefing.
The results could mean that patients with decompensated cirrhosis now have a much-needed therapeutic option. These patients have “a very poor prognosis,” Dr. Bernardi said. The 1-year probability of survival is about 20%, and the only curative therapy at present is liver transplantation.
A total of 440 patients, mostly male with an average age of 61 years, with cirrhosis and uncomplicated ascites were randomized at 33 Italian centers to receive standard medical treatment alone (n = 213) or with human albumin (n = 218) given at an infused dose of 40 g twice a week for the first 2 weeks, then 40 g every week. The albumin used in the trial was provided by five different pharmaceutical companies and sent to a central location for generic relabeling and distribution out to the participating trial centers.
Significantly fewer patients who were given human albumin than those who were not (66% vs. 38%, P less than .001) needed at least one paracentesis. The incidence rate for the removal of peritoneal fluid in the standard medical treatment arm was 3.5/person per year. There was a 54% reduction in this rate by the addition of human albumin (HR = 0.46; 95% CI, 0.40-0.53; P less than .0001). There was also a significant 46% reduction in the incidence of refractory ascites (48% vs. 25%, P less than .0001).
Patients who received standard medical treatment plus albumin needed fewer hospitalizations and fewer days of in-hospital care per person per year than those in the standard care–only arm. The use of human albumin reduced the number of hospital stays by 35% (HR = 0.65; 95% CI, 0.55-0.77; P less than .0001) and the duration of days in hospital by 45% (HR = 0.55; 95% CI, 0.52-0.58; P less than .0001).
Although not statistically significant, a trend for greater improvements and fewer decreases in quality of life, as measured using the EQ-5D visual assessment scale, at 3, 6, and 12 months, was seen with the use of human albumin.
Four patients had adverse drug reactions: two were mild allergic reactions, and two were potentially life-threatening septic shock that needed intensive care treatment. One of the latter cases might have been caused by pneumonia, and the other required study interruption. But in all cases the patients recovered, Dr. Bernardi reported.
The study was funded by the Italian Drug Agency. Dr. Bernardi had acted as a speaker for and consultant to CLS Behring and Baxter Healthcare, and as a speaker to the Plasma Protein Therapeutics Association’s Europe division, Grifols, Gilead Sciences, and AbbVie Italia. Dr. Tacke had nothing to disclose.
AT ILC 2017
Key clinical point: A weekly infusion of human albumin has a beneficial effect in patients with decompensated cirrhosis.
Major finding: Overall survival was 78% vs. 66% for standard medical care with albumin vs. no albumin (HR, 0.62; 95% CI, 0.40-0.95; P = .028).
Data source: The ANSWER study, a multicenter, open-label, randomized clinical trial of 440 patients with decompensated cirrhosis.
Disclosures: The study was funded by the Italian Drug Agency. Dr. Bernardi had acted as a speaker for and consultant to CLS Behring and Baxter Healthcare, and as a speaker to the Plasma Protein Therapeutics Association’s Europe division, Grifols, Gilead Sciences, and AbbVie Italia. Dr. Tacke had nothing to disclose.
USPSTF says check BP at each visit to screen for preeclampsia
Blood pressure should be checked at every prenatal visit to screen for preeclampsia, according to a new recommendation from the U.S. Preventive Services Task Force.
The USPSTF commissioned a review of the current literature to update its initial recommendation regarding preeclampsia screening, which was issued in 1996. The update was considered important “given changes to screening, diagnosis, and management practices, as well as population health, over the past two decades.”
However, the evidence regarding different screening approaches is still quite limited, and the USPSTF did not change its recommendation except to indicate blood pressure checks at every visit rather than “periodically.” The current advice is a “B” recommendation, meaning that the USPSTF recommends the service.
Blood pressure assessment remains the most reliable, least harmful strategy for identifying preeclampsia as early as possible, said Kirsten Bobbins-Domingo, MD, PhD, chair of the USPSTF and lead author of the recommendation statement, and her associates.
“The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit,” the researchers wrote (JAMA. 2017;317[16]:1661-7).
The evidence report supporting this recommendation included 21 studies involving 13,982 pregnant women. But no studies directly compared the effectiveness of blood pressure screening between screened and unscreened populations, Jillian T. Henderson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates said in the report.
Fourteen studies assessed tests for protein in the urine to identify preeclampsia, but the accuracy of such tests was highly variable. Sensitivity ranged from 22% to 100% and specificity ranged from 36% to 100%, so the USPSTF did not recommend urine testing for proteinuria as a screen for preeclampsia.
In addition, Dr. Henderson and her associates reviewed four studies (involving 7,123 women) assessing 16 different risk-prediction models. Again, the findings did not support the routine use of such tools in clinical practice: their positive predictive value was just 4% in the largest validation cohort, the investigators reported (JAMA. 2017;317[16]:1668-83).
The American College of Obstetricians and Gynecologists recommends monitoring blood pressure at every prenatal visit to screen for preeclampsia, as well as obtaining a detailed medical history to assess risk factors for the disorder. In contrast, the Society of Obstetricians and Gynaecologists of Canada and the National Institute for Health and Care Excellence in the United Kingdom recommend urinalysis for proteinuria in addition to blood pressure screening.
The full recommendation statement and evidence report are available at www.uspreventiveservicestaskforce.org.
The use of blood pressure measurement at every prenatal visit is a simple and effective way to identify and treat at-risk women without adding any substantial risks. In contrast, the risks related to preeclampsia are very high. Although risk assessment tools for preeclampsia are available, their low positive predictive value currently limits their usefulness for screening. Given the incidence of preeclampsia and the lack of predictability, it is important that all pregnant women have routine blood pressure assessments at every visit during the prenatal period.
The emerging data relating preeclampsia to the risk of future cardiovascular disease make this pregnancy-related issue important not just for obstetricians for the acute risks but also for internists and cardiologists for the long-term risks.
Martha Gulati, MD, is in the division of cardiology at the University of Arizona, Phoenix. She reported having no relevant financial disclosures. These remarks are excerpted from an editorial (JAMA Cardiol. 2017 Apr 25. doi: 10.1001/jamacardio.2017.1276).
The use of blood pressure measurement at every prenatal visit is a simple and effective way to identify and treat at-risk women without adding any substantial risks. In contrast, the risks related to preeclampsia are very high. Although risk assessment tools for preeclampsia are available, their low positive predictive value currently limits their usefulness for screening. Given the incidence of preeclampsia and the lack of predictability, it is important that all pregnant women have routine blood pressure assessments at every visit during the prenatal period.
The emerging data relating preeclampsia to the risk of future cardiovascular disease make this pregnancy-related issue important not just for obstetricians for the acute risks but also for internists and cardiologists for the long-term risks.
Martha Gulati, MD, is in the division of cardiology at the University of Arizona, Phoenix. She reported having no relevant financial disclosures. These remarks are excerpted from an editorial (JAMA Cardiol. 2017 Apr 25. doi: 10.1001/jamacardio.2017.1276).
The use of blood pressure measurement at every prenatal visit is a simple and effective way to identify and treat at-risk women without adding any substantial risks. In contrast, the risks related to preeclampsia are very high. Although risk assessment tools for preeclampsia are available, their low positive predictive value currently limits their usefulness for screening. Given the incidence of preeclampsia and the lack of predictability, it is important that all pregnant women have routine blood pressure assessments at every visit during the prenatal period.
The emerging data relating preeclampsia to the risk of future cardiovascular disease make this pregnancy-related issue important not just for obstetricians for the acute risks but also for internists and cardiologists for the long-term risks.
Martha Gulati, MD, is in the division of cardiology at the University of Arizona, Phoenix. She reported having no relevant financial disclosures. These remarks are excerpted from an editorial (JAMA Cardiol. 2017 Apr 25. doi: 10.1001/jamacardio.2017.1276).
Blood pressure should be checked at every prenatal visit to screen for preeclampsia, according to a new recommendation from the U.S. Preventive Services Task Force.
The USPSTF commissioned a review of the current literature to update its initial recommendation regarding preeclampsia screening, which was issued in 1996. The update was considered important “given changes to screening, diagnosis, and management practices, as well as population health, over the past two decades.”
However, the evidence regarding different screening approaches is still quite limited, and the USPSTF did not change its recommendation except to indicate blood pressure checks at every visit rather than “periodically.” The current advice is a “B” recommendation, meaning that the USPSTF recommends the service.
Blood pressure assessment remains the most reliable, least harmful strategy for identifying preeclampsia as early as possible, said Kirsten Bobbins-Domingo, MD, PhD, chair of the USPSTF and lead author of the recommendation statement, and her associates.
“The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit,” the researchers wrote (JAMA. 2017;317[16]:1661-7).
The evidence report supporting this recommendation included 21 studies involving 13,982 pregnant women. But no studies directly compared the effectiveness of blood pressure screening between screened and unscreened populations, Jillian T. Henderson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates said in the report.
Fourteen studies assessed tests for protein in the urine to identify preeclampsia, but the accuracy of such tests was highly variable. Sensitivity ranged from 22% to 100% and specificity ranged from 36% to 100%, so the USPSTF did not recommend urine testing for proteinuria as a screen for preeclampsia.
In addition, Dr. Henderson and her associates reviewed four studies (involving 7,123 women) assessing 16 different risk-prediction models. Again, the findings did not support the routine use of such tools in clinical practice: their positive predictive value was just 4% in the largest validation cohort, the investigators reported (JAMA. 2017;317[16]:1668-83).
The American College of Obstetricians and Gynecologists recommends monitoring blood pressure at every prenatal visit to screen for preeclampsia, as well as obtaining a detailed medical history to assess risk factors for the disorder. In contrast, the Society of Obstetricians and Gynaecologists of Canada and the National Institute for Health and Care Excellence in the United Kingdom recommend urinalysis for proteinuria in addition to blood pressure screening.
The full recommendation statement and evidence report are available at www.uspreventiveservicestaskforce.org.
Blood pressure should be checked at every prenatal visit to screen for preeclampsia, according to a new recommendation from the U.S. Preventive Services Task Force.
The USPSTF commissioned a review of the current literature to update its initial recommendation regarding preeclampsia screening, which was issued in 1996. The update was considered important “given changes to screening, diagnosis, and management practices, as well as population health, over the past two decades.”
However, the evidence regarding different screening approaches is still quite limited, and the USPSTF did not change its recommendation except to indicate blood pressure checks at every visit rather than “periodically.” The current advice is a “B” recommendation, meaning that the USPSTF recommends the service.
Blood pressure assessment remains the most reliable, least harmful strategy for identifying preeclampsia as early as possible, said Kirsten Bobbins-Domingo, MD, PhD, chair of the USPSTF and lead author of the recommendation statement, and her associates.
“The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit,” the researchers wrote (JAMA. 2017;317[16]:1661-7).
The evidence report supporting this recommendation included 21 studies involving 13,982 pregnant women. But no studies directly compared the effectiveness of blood pressure screening between screened and unscreened populations, Jillian T. Henderson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates said in the report.
Fourteen studies assessed tests for protein in the urine to identify preeclampsia, but the accuracy of such tests was highly variable. Sensitivity ranged from 22% to 100% and specificity ranged from 36% to 100%, so the USPSTF did not recommend urine testing for proteinuria as a screen for preeclampsia.
In addition, Dr. Henderson and her associates reviewed four studies (involving 7,123 women) assessing 16 different risk-prediction models. Again, the findings did not support the routine use of such tools in clinical practice: their positive predictive value was just 4% in the largest validation cohort, the investigators reported (JAMA. 2017;317[16]:1668-83).
The American College of Obstetricians and Gynecologists recommends monitoring blood pressure at every prenatal visit to screen for preeclampsia, as well as obtaining a detailed medical history to assess risk factors for the disorder. In contrast, the Society of Obstetricians and Gynaecologists of Canada and the National Institute for Health and Care Excellence in the United Kingdom recommend urinalysis for proteinuria in addition to blood pressure screening.
The full recommendation statement and evidence report are available at www.uspreventiveservicestaskforce.org.
Key clinical point:
Major finding: The 14 studies about testing for proteinuria and the 4 studies about 16 different risk prediction tools did not yield evidence to support either approach as a screen for preeclampsia.
Data source: A review of 21 studies involving 13,982 pregnant women, performed since the initial USPSTF recommendation was issued in 1996.
Disclosures: The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality.
Does Gender Influence Pain Sensitivity?
STOWE, VT—Mounting evidence suggests that gender affects the modulation of pain to a greater extent than has been understood, according to an overview presented at the 27th Annual Headache Cooperative of New England Stowe Headache Symposium. Data indicate that the differences are biologic, and researchers are examining whether social and psychologic differences also may influence pain.
“If biomedicine ever comes up with a treatment, reproductive issues aside, that works and gets approval for one sex and not the other for treating the same underlying condition, it is going to happen in pain first,” said Jeffrey S. Mogil, PhD, Canada Research Chair in Genetics of Pain at McGill University in Montreal. “Maybe we are only 10 or 15 years away from seeing such a thing occur.”
A Clear Gender Difference
The first investigation of gender differences in pain was published in 1997, according to Dr. Mogil. It indicated that a greater number of chronic pain syndromes were more prevalent in women, compared with those that were more prevalent in men. Furthermore, the syndromes that were more prevalent in women were more common overall. The study also estimated that approximately 70% of patients with chronic pain are women. This result, however, could follow from reluctance among men to consult a physician, said Dr. Mogil.
When Dr. Mogil reviewed epidemiologic studies of pain, he found that women were between 5% and 10% more likely than men to endorse the symptoms of chronic pain. Although the studies used different definitions of chronic pain, each study used the same definition for men and women. A possible explanation for the result is that women are more susceptible to painful diseases.
But when he examined laboratory data from controlled experiments involving painful stimuli, Dr. Mogil found that “regardless of what type of pain you are looking at, and regardless of how it is measured, women are more sensitive to pain than men.” The difference in pain sensitivity is not great and depends on various factors, but it is clear and unmistakable, he added.
Biology Influences Pain Sensitivity
Biologic differences may explain gender differences in pain sensitivity. Research during the past 20 years has suggested that microglia play an important role in nociception. Newer data, however, indicate that microglial involvement in pain may be specific to males. Because the majority of animal research had been performed in male rodents, this observation had not been made previously, said Dr. Mogil.
He and his colleagues injured male and female mice to induce mechanical allodynia. The mice exhibited the same amount of mechanical allodynia, regardless of gender. The investigators next administered minocycline, a glial inhibitor, to the mice. The intervention reversed the allodynia in male mice, but not in female mice. Using fluorocitrate or propentofylline in place of minocycline produces the same result, said Dr. Mogil. Research into the biologic basis for pain modulation in females is ongoing.
Gender, Stress, and Analgesia
A patient’s sensitivity to pain may be influenced not only by his or her gender, but also by the gender of a person that the patient encounters. The results of certain mouse studies prompted researchers to hypothesize that the experimenters themselves may have produced analgesia, and Dr. Mogil decided to test this hypothesis.
When an investigator injected zymosan, an inflammatory agent, into a mouse’s ankle and left the room, the injection caused pain and grimacing in the mouse. When a male investigator administered zymosan to a mouse and remained in the room, the rate of grimacing decreased by approximately 40%. “In fact, experimenters are causing analgesia,” said Dr. Mogil. A female investigator did not produce the same effect, however.
Further research indicated that the effect has an olfactory origin. When injured mice are exposed to clothing previously worn by a male, or to bedding previously used by any male animal, they exhibit the same reduction in pain. “This is a stress phenomenon,” said Dr. Mogil. A mouse’s level of corticosterone, an equivalent to cortisol in humans, increases after exposure to male experimenters or their clothing.
The mice appear to be responding to axillary chemosignals (eg, androstadienone and androstenone) that males emit. The most important compound may be 3-Methyl-2-hexenoic acid, because it is “the only chemosignal that can produce this effect at reasonable concentrations in the nanomolar range,” said Dr. Mogil. He and his colleagues are studying whether these chemosignals produce the same responses in humans. “I would expect the effect to be much smaller and last for not quite as long,” he said. They also are studying the social modulation of pain in animals and humans.
—Erik Greb
Suggested Reading
Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371-380; discussion 435-513.
Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859-866.
Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081-1083.
Sorge RE, Martin LJ, Isbester KA, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629-632.
STOWE, VT—Mounting evidence suggests that gender affects the modulation of pain to a greater extent than has been understood, according to an overview presented at the 27th Annual Headache Cooperative of New England Stowe Headache Symposium. Data indicate that the differences are biologic, and researchers are examining whether social and psychologic differences also may influence pain.
“If biomedicine ever comes up with a treatment, reproductive issues aside, that works and gets approval for one sex and not the other for treating the same underlying condition, it is going to happen in pain first,” said Jeffrey S. Mogil, PhD, Canada Research Chair in Genetics of Pain at McGill University in Montreal. “Maybe we are only 10 or 15 years away from seeing such a thing occur.”
A Clear Gender Difference
The first investigation of gender differences in pain was published in 1997, according to Dr. Mogil. It indicated that a greater number of chronic pain syndromes were more prevalent in women, compared with those that were more prevalent in men. Furthermore, the syndromes that were more prevalent in women were more common overall. The study also estimated that approximately 70% of patients with chronic pain are women. This result, however, could follow from reluctance among men to consult a physician, said Dr. Mogil.
When Dr. Mogil reviewed epidemiologic studies of pain, he found that women were between 5% and 10% more likely than men to endorse the symptoms of chronic pain. Although the studies used different definitions of chronic pain, each study used the same definition for men and women. A possible explanation for the result is that women are more susceptible to painful diseases.
But when he examined laboratory data from controlled experiments involving painful stimuli, Dr. Mogil found that “regardless of what type of pain you are looking at, and regardless of how it is measured, women are more sensitive to pain than men.” The difference in pain sensitivity is not great and depends on various factors, but it is clear and unmistakable, he added.
Biology Influences Pain Sensitivity
Biologic differences may explain gender differences in pain sensitivity. Research during the past 20 years has suggested that microglia play an important role in nociception. Newer data, however, indicate that microglial involvement in pain may be specific to males. Because the majority of animal research had been performed in male rodents, this observation had not been made previously, said Dr. Mogil.
He and his colleagues injured male and female mice to induce mechanical allodynia. The mice exhibited the same amount of mechanical allodynia, regardless of gender. The investigators next administered minocycline, a glial inhibitor, to the mice. The intervention reversed the allodynia in male mice, but not in female mice. Using fluorocitrate or propentofylline in place of minocycline produces the same result, said Dr. Mogil. Research into the biologic basis for pain modulation in females is ongoing.
Gender, Stress, and Analgesia
A patient’s sensitivity to pain may be influenced not only by his or her gender, but also by the gender of a person that the patient encounters. The results of certain mouse studies prompted researchers to hypothesize that the experimenters themselves may have produced analgesia, and Dr. Mogil decided to test this hypothesis.
When an investigator injected zymosan, an inflammatory agent, into a mouse’s ankle and left the room, the injection caused pain and grimacing in the mouse. When a male investigator administered zymosan to a mouse and remained in the room, the rate of grimacing decreased by approximately 40%. “In fact, experimenters are causing analgesia,” said Dr. Mogil. A female investigator did not produce the same effect, however.
Further research indicated that the effect has an olfactory origin. When injured mice are exposed to clothing previously worn by a male, or to bedding previously used by any male animal, they exhibit the same reduction in pain. “This is a stress phenomenon,” said Dr. Mogil. A mouse’s level of corticosterone, an equivalent to cortisol in humans, increases after exposure to male experimenters or their clothing.
The mice appear to be responding to axillary chemosignals (eg, androstadienone and androstenone) that males emit. The most important compound may be 3-Methyl-2-hexenoic acid, because it is “the only chemosignal that can produce this effect at reasonable concentrations in the nanomolar range,” said Dr. Mogil. He and his colleagues are studying whether these chemosignals produce the same responses in humans. “I would expect the effect to be much smaller and last for not quite as long,” he said. They also are studying the social modulation of pain in animals and humans.
—Erik Greb
Suggested Reading
Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371-380; discussion 435-513.
Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859-866.
Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081-1083.
Sorge RE, Martin LJ, Isbester KA, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629-632.
STOWE, VT—Mounting evidence suggests that gender affects the modulation of pain to a greater extent than has been understood, according to an overview presented at the 27th Annual Headache Cooperative of New England Stowe Headache Symposium. Data indicate that the differences are biologic, and researchers are examining whether social and psychologic differences also may influence pain.
“If biomedicine ever comes up with a treatment, reproductive issues aside, that works and gets approval for one sex and not the other for treating the same underlying condition, it is going to happen in pain first,” said Jeffrey S. Mogil, PhD, Canada Research Chair in Genetics of Pain at McGill University in Montreal. “Maybe we are only 10 or 15 years away from seeing such a thing occur.”
A Clear Gender Difference
The first investigation of gender differences in pain was published in 1997, according to Dr. Mogil. It indicated that a greater number of chronic pain syndromes were more prevalent in women, compared with those that were more prevalent in men. Furthermore, the syndromes that were more prevalent in women were more common overall. The study also estimated that approximately 70% of patients with chronic pain are women. This result, however, could follow from reluctance among men to consult a physician, said Dr. Mogil.
When Dr. Mogil reviewed epidemiologic studies of pain, he found that women were between 5% and 10% more likely than men to endorse the symptoms of chronic pain. Although the studies used different definitions of chronic pain, each study used the same definition for men and women. A possible explanation for the result is that women are more susceptible to painful diseases.
But when he examined laboratory data from controlled experiments involving painful stimuli, Dr. Mogil found that “regardless of what type of pain you are looking at, and regardless of how it is measured, women are more sensitive to pain than men.” The difference in pain sensitivity is not great and depends on various factors, but it is clear and unmistakable, he added.
Biology Influences Pain Sensitivity
Biologic differences may explain gender differences in pain sensitivity. Research during the past 20 years has suggested that microglia play an important role in nociception. Newer data, however, indicate that microglial involvement in pain may be specific to males. Because the majority of animal research had been performed in male rodents, this observation had not been made previously, said Dr. Mogil.
He and his colleagues injured male and female mice to induce mechanical allodynia. The mice exhibited the same amount of mechanical allodynia, regardless of gender. The investigators next administered minocycline, a glial inhibitor, to the mice. The intervention reversed the allodynia in male mice, but not in female mice. Using fluorocitrate or propentofylline in place of minocycline produces the same result, said Dr. Mogil. Research into the biologic basis for pain modulation in females is ongoing.
Gender, Stress, and Analgesia
A patient’s sensitivity to pain may be influenced not only by his or her gender, but also by the gender of a person that the patient encounters. The results of certain mouse studies prompted researchers to hypothesize that the experimenters themselves may have produced analgesia, and Dr. Mogil decided to test this hypothesis.
When an investigator injected zymosan, an inflammatory agent, into a mouse’s ankle and left the room, the injection caused pain and grimacing in the mouse. When a male investigator administered zymosan to a mouse and remained in the room, the rate of grimacing decreased by approximately 40%. “In fact, experimenters are causing analgesia,” said Dr. Mogil. A female investigator did not produce the same effect, however.
Further research indicated that the effect has an olfactory origin. When injured mice are exposed to clothing previously worn by a male, or to bedding previously used by any male animal, they exhibit the same reduction in pain. “This is a stress phenomenon,” said Dr. Mogil. A mouse’s level of corticosterone, an equivalent to cortisol in humans, increases after exposure to male experimenters or their clothing.
The mice appear to be responding to axillary chemosignals (eg, androstadienone and androstenone) that males emit. The most important compound may be 3-Methyl-2-hexenoic acid, because it is “the only chemosignal that can produce this effect at reasonable concentrations in the nanomolar range,” said Dr. Mogil. He and his colleagues are studying whether these chemosignals produce the same responses in humans. “I would expect the effect to be much smaller and last for not quite as long,” he said. They also are studying the social modulation of pain in animals and humans.
—Erik Greb
Suggested Reading
Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371-380; discussion 435-513.
Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859-866.
Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081-1083.
Sorge RE, Martin LJ, Isbester KA, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629-632.
VIDEO: Pilot stem cell trial for multiple system atrophy shows promising results
BOSTON – Intrathecal, autologous mesenchymal stem cell (MSC) treatment provided encouraging results for modifying the disease course of multiple system atrophy with relative safety and tolerability in a phase I/II trial.
The efficacy of MSCs on slowing multiple system atrophy (MSA) progression in a small trial of 24 patients appeared to be dependent on the dose, and, in the highest dose individuals, had a painful implantation response, trial investigator Wolfgang Singer, MD, reported at the annual meeting of the American Academy of Neurology.
Dr. Singer and his colleagues at the Mayo Clinic in Rochester, Minn., chose to investigate MSCs as a treatment because they are multipotent and capable of differentiating into different cell types, including glial cells, and they secrete neurotrophic factors, such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, which are thought to occur at pathologically low levels in individuals with MSA. The intrinsic immunomodulatory action of MSCs may also contribute to a potential benefit on neuroinflammatory aspects of MSA pathology.
MSCs have previously shown promising results on slowing disease progression in an open-label study of Korean patients with MSA, who received an intra-arterial infusion of MSCs into the internal carotids and dominant vertebral artery, followed by intravenous infusions (Clin Pharmacol Ther. 2008 May;83[5]:723-30). Those results were confirmed in a double-blind, placebo-controlled study (Ann Neurol. 2012 Jul;72[1]:32-40), but evidence of microemboli raised concerns with the Mayo Clinic team about stroke risk with the intra-arterial approach, Dr. Singer said.
The intrathecal route of administration also may be advantageous over an intra-arterial approach by reaching the targets of MSCs in the brain “in a more widespread fashion,” Dr. Singer said.
The relative safety and hint of efficacy with the different route of MSC administration in the Mayo Clinic study make it “a really groundbreaking direction to take,” session comoderator Christopher H. Gibbons, MD, said in an interview. “I think this a very good, small, but critical, step in demonstrating that ... you can do this, and maybe there’s a signal that it is, in fact, working at slowing down disease progression, which I think is incredibly important in this disease.”
In the current study, Dr. Singer and his associates intrathecally administered between 10 and 200 million autologous, fat-derived MSCs via lumbar puncture in predefined dose tiers and then followed patients over 1 year. Overall, 8 patients received a single dose of 10 million cells, 12 received a dose of 50 million cells followed by a second 50-million cell dose 4 weeks later, and 4 received a dose of 100 million cells followed by a second 100-million cell dose 4 weeks later.
The 24 patients in the study all met consensus criteria for probable MSA, had at least moderate laboratory evidence of autonomic failure, and were at an early stage of disease with a Unified MSA Rating Scale part 1 score of 18 or less.
In the primary outcome of safety, the investigators reported no treatment–attributable serious adverse events and said that the treatment was generally well-tolerated. All 16 patients who took either the high or medium doses had MRI abnormalities that showed thickening/enhancement of cauda equina nerve roots at the level of the puncture that were asymptomatic and did not lead to neurologic deficits.
The 12 medium-dose patients had variable elevation of cerebrospinal fluid protein and cell counts, and 5 had mild and transient low back pain. In the highest-dose tier, three of four patients developed low back pain, some of which was severe, and the same MRI findings, which signaled to the investigators that a dose-limiting toxicity had been reached.
Some patients also reported headaches after lumbar punctures, which were expected. Two patients also developed mild febrile reactions after administrations that were self-limited.
Treatment with MSCs led to a significantly lower rate of disease progression as measured by total score on the Unified MSA Rating Scale (0.43 vs. 1.22 points/month; P = .009), and this difference was even greater for the medium-dose tier than for the low-dose tier. The investigators used the placebo group from their recently completed rifampicin treatment trial in MSA to make the efficacy assessment.
There was no change between the treatment groups and the historical control group on the Composite Autonomic Symptom Scale or the Composite Autonomic Severity Score from baseline to 1 year.
Based on the promising findings, the Food and Drug Administration granted permission for a compassionate extension study for apparent responders to receive additional injections every 6 months, and, so far, 16 patients have been reinjected, according to Dr. Singer.
Dr. Singer and his associates have used these results as the basis for a multicenter, double-blind, placebo-controlled phase II/III study that is in the late planning stages. This kind of trial will be important for determining whether it’s possible to hold the quality of the MSC treatment steady and get the same sort of response across many centers, said Dr. Gibbons, a clinical neurophysiologist at Beth Israel Deaconess Medical Center, Boston, and past-president of the American Autonomic Society.
The trial was supported by grants from the National Institutes of Health, the Cure MSA Foundation, the Food and Drug Administration, the Autonomic Rare Disease Consortium, and Mayo Clinic funds. Dr. Singer and Dr. Gibbons reported having no relevant financial disclosures.
You can watch a video interview with Dr. Singer here.

BOSTON – Intrathecal, autologous mesenchymal stem cell (MSC) treatment provided encouraging results for modifying the disease course of multiple system atrophy with relative safety and tolerability in a phase I/II trial.
The efficacy of MSCs on slowing multiple system atrophy (MSA) progression in a small trial of 24 patients appeared to be dependent on the dose, and, in the highest dose individuals, had a painful implantation response, trial investigator Wolfgang Singer, MD, reported at the annual meeting of the American Academy of Neurology.
Dr. Singer and his colleagues at the Mayo Clinic in Rochester, Minn., chose to investigate MSCs as a treatment because they are multipotent and capable of differentiating into different cell types, including glial cells, and they secrete neurotrophic factors, such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, which are thought to occur at pathologically low levels in individuals with MSA. The intrinsic immunomodulatory action of MSCs may also contribute to a potential benefit on neuroinflammatory aspects of MSA pathology.
MSCs have previously shown promising results on slowing disease progression in an open-label study of Korean patients with MSA, who received an intra-arterial infusion of MSCs into the internal carotids and dominant vertebral artery, followed by intravenous infusions (Clin Pharmacol Ther. 2008 May;83[5]:723-30). Those results were confirmed in a double-blind, placebo-controlled study (Ann Neurol. 2012 Jul;72[1]:32-40), but evidence of microemboli raised concerns with the Mayo Clinic team about stroke risk with the intra-arterial approach, Dr. Singer said.
The intrathecal route of administration also may be advantageous over an intra-arterial approach by reaching the targets of MSCs in the brain “in a more widespread fashion,” Dr. Singer said.
The relative safety and hint of efficacy with the different route of MSC administration in the Mayo Clinic study make it “a really groundbreaking direction to take,” session comoderator Christopher H. Gibbons, MD, said in an interview. “I think this a very good, small, but critical, step in demonstrating that ... you can do this, and maybe there’s a signal that it is, in fact, working at slowing down disease progression, which I think is incredibly important in this disease.”
In the current study, Dr. Singer and his associates intrathecally administered between 10 and 200 million autologous, fat-derived MSCs via lumbar puncture in predefined dose tiers and then followed patients over 1 year. Overall, 8 patients received a single dose of 10 million cells, 12 received a dose of 50 million cells followed by a second 50-million cell dose 4 weeks later, and 4 received a dose of 100 million cells followed by a second 100-million cell dose 4 weeks later.
The 24 patients in the study all met consensus criteria for probable MSA, had at least moderate laboratory evidence of autonomic failure, and were at an early stage of disease with a Unified MSA Rating Scale part 1 score of 18 or less.
In the primary outcome of safety, the investigators reported no treatment–attributable serious adverse events and said that the treatment was generally well-tolerated. All 16 patients who took either the high or medium doses had MRI abnormalities that showed thickening/enhancement of cauda equina nerve roots at the level of the puncture that were asymptomatic and did not lead to neurologic deficits.
The 12 medium-dose patients had variable elevation of cerebrospinal fluid protein and cell counts, and 5 had mild and transient low back pain. In the highest-dose tier, three of four patients developed low back pain, some of which was severe, and the same MRI findings, which signaled to the investigators that a dose-limiting toxicity had been reached.
Some patients also reported headaches after lumbar punctures, which were expected. Two patients also developed mild febrile reactions after administrations that were self-limited.
Treatment with MSCs led to a significantly lower rate of disease progression as measured by total score on the Unified MSA Rating Scale (0.43 vs. 1.22 points/month; P = .009), and this difference was even greater for the medium-dose tier than for the low-dose tier. The investigators used the placebo group from their recently completed rifampicin treatment trial in MSA to make the efficacy assessment.
There was no change between the treatment groups and the historical control group on the Composite Autonomic Symptom Scale or the Composite Autonomic Severity Score from baseline to 1 year.
Based on the promising findings, the Food and Drug Administration granted permission for a compassionate extension study for apparent responders to receive additional injections every 6 months, and, so far, 16 patients have been reinjected, according to Dr. Singer.
Dr. Singer and his associates have used these results as the basis for a multicenter, double-blind, placebo-controlled phase II/III study that is in the late planning stages. This kind of trial will be important for determining whether it’s possible to hold the quality of the MSC treatment steady and get the same sort of response across many centers, said Dr. Gibbons, a clinical neurophysiologist at Beth Israel Deaconess Medical Center, Boston, and past-president of the American Autonomic Society.
The trial was supported by grants from the National Institutes of Health, the Cure MSA Foundation, the Food and Drug Administration, the Autonomic Rare Disease Consortium, and Mayo Clinic funds. Dr. Singer and Dr. Gibbons reported having no relevant financial disclosures.
You can watch a video interview with Dr. Singer here.

BOSTON – Intrathecal, autologous mesenchymal stem cell (MSC) treatment provided encouraging results for modifying the disease course of multiple system atrophy with relative safety and tolerability in a phase I/II trial.
The efficacy of MSCs on slowing multiple system atrophy (MSA) progression in a small trial of 24 patients appeared to be dependent on the dose, and, in the highest dose individuals, had a painful implantation response, trial investigator Wolfgang Singer, MD, reported at the annual meeting of the American Academy of Neurology.
Dr. Singer and his colleagues at the Mayo Clinic in Rochester, Minn., chose to investigate MSCs as a treatment because they are multipotent and capable of differentiating into different cell types, including glial cells, and they secrete neurotrophic factors, such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, which are thought to occur at pathologically low levels in individuals with MSA. The intrinsic immunomodulatory action of MSCs may also contribute to a potential benefit on neuroinflammatory aspects of MSA pathology.
MSCs have previously shown promising results on slowing disease progression in an open-label study of Korean patients with MSA, who received an intra-arterial infusion of MSCs into the internal carotids and dominant vertebral artery, followed by intravenous infusions (Clin Pharmacol Ther. 2008 May;83[5]:723-30). Those results were confirmed in a double-blind, placebo-controlled study (Ann Neurol. 2012 Jul;72[1]:32-40), but evidence of microemboli raised concerns with the Mayo Clinic team about stroke risk with the intra-arterial approach, Dr. Singer said.
The intrathecal route of administration also may be advantageous over an intra-arterial approach by reaching the targets of MSCs in the brain “in a more widespread fashion,” Dr. Singer said.
The relative safety and hint of efficacy with the different route of MSC administration in the Mayo Clinic study make it “a really groundbreaking direction to take,” session comoderator Christopher H. Gibbons, MD, said in an interview. “I think this a very good, small, but critical, step in demonstrating that ... you can do this, and maybe there’s a signal that it is, in fact, working at slowing down disease progression, which I think is incredibly important in this disease.”
In the current study, Dr. Singer and his associates intrathecally administered between 10 and 200 million autologous, fat-derived MSCs via lumbar puncture in predefined dose tiers and then followed patients over 1 year. Overall, 8 patients received a single dose of 10 million cells, 12 received a dose of 50 million cells followed by a second 50-million cell dose 4 weeks later, and 4 received a dose of 100 million cells followed by a second 100-million cell dose 4 weeks later.
The 24 patients in the study all met consensus criteria for probable MSA, had at least moderate laboratory evidence of autonomic failure, and were at an early stage of disease with a Unified MSA Rating Scale part 1 score of 18 or less.
In the primary outcome of safety, the investigators reported no treatment–attributable serious adverse events and said that the treatment was generally well-tolerated. All 16 patients who took either the high or medium doses had MRI abnormalities that showed thickening/enhancement of cauda equina nerve roots at the level of the puncture that were asymptomatic and did not lead to neurologic deficits.
The 12 medium-dose patients had variable elevation of cerebrospinal fluid protein and cell counts, and 5 had mild and transient low back pain. In the highest-dose tier, three of four patients developed low back pain, some of which was severe, and the same MRI findings, which signaled to the investigators that a dose-limiting toxicity had been reached.
Some patients also reported headaches after lumbar punctures, which were expected. Two patients also developed mild febrile reactions after administrations that were self-limited.
Treatment with MSCs led to a significantly lower rate of disease progression as measured by total score on the Unified MSA Rating Scale (0.43 vs. 1.22 points/month; P = .009), and this difference was even greater for the medium-dose tier than for the low-dose tier. The investigators used the placebo group from their recently completed rifampicin treatment trial in MSA to make the efficacy assessment.
There was no change between the treatment groups and the historical control group on the Composite Autonomic Symptom Scale or the Composite Autonomic Severity Score from baseline to 1 year.
Based on the promising findings, the Food and Drug Administration granted permission for a compassionate extension study for apparent responders to receive additional injections every 6 months, and, so far, 16 patients have been reinjected, according to Dr. Singer.
Dr. Singer and his associates have used these results as the basis for a multicenter, double-blind, placebo-controlled phase II/III study that is in the late planning stages. This kind of trial will be important for determining whether it’s possible to hold the quality of the MSC treatment steady and get the same sort of response across many centers, said Dr. Gibbons, a clinical neurophysiologist at Beth Israel Deaconess Medical Center, Boston, and past-president of the American Autonomic Society.
The trial was supported by grants from the National Institutes of Health, the Cure MSA Foundation, the Food and Drug Administration, the Autonomic Rare Disease Consortium, and Mayo Clinic funds. Dr. Singer and Dr. Gibbons reported having no relevant financial disclosures.
You can watch a video interview with Dr. Singer here.

AT AAN 2017
Key clinical point:
Major finding: Treatment with MSCs led to a significantly lower rate of disease progression as measured by total score on the Unified MSA Rating Scale (0.43 vs. 1.22 points/month in historical placebo group; P = .009).
Data source: A phase I/II trial of 24 patients with MSA treated intrathecally with autologous MSCs and compared against a historical control group.
Disclosures: The trial was supported by grants from the National Institutes of Health, the Cure MSA Foundation, the Food and Drug Administration, the Autonomic Rare Disease Consortium, and Mayo Clinic funds. Dr. Singer and Dr. Gibbons reported having no relevant financial disclosures.
Why state and school policies matter
Recently North Carolina proposed a bill (House Bill 780) that will ban same-sex marriage in the state, even though the U.S. Supreme Court ruled in 2015 that no states can ban same-sex marriage because doing so violates the 14th Amendment. Although many mainly will argue that H.B. 780 is unconstitutional, this bill also can be detrimental to the health of lesbian, gay, and bisexual (LGB) youth.
In March 2017, JAMA Pediatrics published a study on the association between same-sex marriage laws and the rates of suicide attempts.1 Using data from the Youth Risk Behavior Surveillance System, they analyzed the relationship between state policies that permitted same-sex marriage and self-report of suicide attempts within the last 12 months. Thirty percent of LGB youth (13% of the survey population) reported a suicide attempt in the past year prior to any state policies that permitted same-sex marriage, compared with about 9% of the general population. After states implemented pro–same-sex marriage policies, LGB youth suicide attempts dropped to about 26% – a 14% relative decline. This was not limited to LGB youth. The general youth suicide rates declined from 8.6% to 8% – a 7% relative decline. Although the change in suicide attempts was small, the authors determined that the likelihood it occurred by chance was very slim, and concluded that policies enabling same-sex marriage may be associated with an improvement in population health, especially for LGB youth.
This was not the first study examining the relationship between policies and the health of LGB youth. Mark Hatzenbuehler, PhD, of Columbia University, New York, and his associates have published multiple studies on this topic. Four years earlier, Hatzenbuehler et al. published a study on the relationship between antibullying policies and suicide among LGB youth. Using data from the Oregon Healthy Teen survey (2006-2008), he found that LGB youth who live in counties where there are fewer districts with antibullying policies are more than twice as likely to attempt suicide, compared with LGB youth who live in counties where more districts had antibullying policies. Furthermore, the type of antibullying policy mattered. If a district’s antibullying policy did not prohibit bullying based on sexual orientation, then it had no impact on the suicide attempt rates of LGB youth in that area.2 Similar results were found when the relationship between anti–homophobic bullying policies and LGB suicide attempts was examined in the larger Youth Risk Behavior Surveillance System.3
State and local policies also influence other health outcomes among LGB youth. Hatzenbuehler et al. did another study that examined community-level determinants of tobacco use among LGB youth. Again using the data from the Oregon Healthy Teen Survey, they found that LGB youth living in communities that were more supportive of LGB youth (i.e., communities with a high proportion of same-sex couples living in the area, a high proportion of gay-straight alliances at schools, and LGB-specific antibullying policies) were less likely to smoke cigarettes, compared with LGB youth living in communities that were less supportive.4 A similar study in Canada by Konishi et al. found that schools with gay-straight alliances and anti–homophobic bullying policies were less likely to have LGB youth engaging in risky alcohol or illicit drug use.5
Why do these policies matter? A common theme among these policies is that they can either cause or alleviate stress for LGB youth. States that restrict same-sex marriages or schools that do not have any gay-straight alliances may signal to LGB youth that they are not valued or welcomed, or at the very worse, are despised. This creates a hostile and stressful environment for LGB youth, which raises the risk for mental health problems, such as depression and anxiety, which in turn, raises the risk for substance use and suicide.6 Conversely, the presence of a gay-straight alliance at school or a state that allows same-sex marriage may indicate to LGB youth that they are welcomed, if not just tolerated, and may alleviate this risk. Furthermore, antibullying policies seem to reduce the stress associated with bullying among LGB youth because it may serve as a deterrent to bullying based on sexual orientation. Although passing pro-LGB policies will not solve all the health problems among LGB youth, these policies certainly have an impact.
There might be trepidation among some pediatricians about being vocal on a politically charged policy proposal such as H.B. 780. However, suicide and substance use are major concerns for all physicians – especially pediatricians. Policy makers considering passing laws that can affect their LGB constituents should read these studies to see what kind of influence these proposals can have on the health and well-being of LGB youth. Moreover, health care providers should use their expertise, influence, and standing in their community to support policies that encourage protection for LGB youth and oppose policies that can harm LGB youth. These leaders are responsible for ensuring the health of the people they serve.
Dr. Montano is clinical instructor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC.
References
1. JAMA Pediatr. 2017. doi: 10.1001/jamapediatrics.2016.4529.
2. J Adolesc Health. 2013:S21-6. doi: 10.1016/j.jadohealth.2012.08.010.
3. Am J Public Health. 2014. doi: 10.2105/AJPH.2013.301508.
4. Arch Pediatr Adolesc Med. 2011. doi: 10.1001/archpediatrics.2011.64.
5. Prev Med. 2013. doi: 10.1016/j.ypmed.2013.06.031.
6. Psychol Bull. 2003. doi: 10.1037/0033-2909.129.5.674.
Recently North Carolina proposed a bill (House Bill 780) that will ban same-sex marriage in the state, even though the U.S. Supreme Court ruled in 2015 that no states can ban same-sex marriage because doing so violates the 14th Amendment. Although many mainly will argue that H.B. 780 is unconstitutional, this bill also can be detrimental to the health of lesbian, gay, and bisexual (LGB) youth.
In March 2017, JAMA Pediatrics published a study on the association between same-sex marriage laws and the rates of suicide attempts.1 Using data from the Youth Risk Behavior Surveillance System, they analyzed the relationship between state policies that permitted same-sex marriage and self-report of suicide attempts within the last 12 months. Thirty percent of LGB youth (13% of the survey population) reported a suicide attempt in the past year prior to any state policies that permitted same-sex marriage, compared with about 9% of the general population. After states implemented pro–same-sex marriage policies, LGB youth suicide attempts dropped to about 26% – a 14% relative decline. This was not limited to LGB youth. The general youth suicide rates declined from 8.6% to 8% – a 7% relative decline. Although the change in suicide attempts was small, the authors determined that the likelihood it occurred by chance was very slim, and concluded that policies enabling same-sex marriage may be associated with an improvement in population health, especially for LGB youth.
This was not the first study examining the relationship between policies and the health of LGB youth. Mark Hatzenbuehler, PhD, of Columbia University, New York, and his associates have published multiple studies on this topic. Four years earlier, Hatzenbuehler et al. published a study on the relationship between antibullying policies and suicide among LGB youth. Using data from the Oregon Healthy Teen survey (2006-2008), he found that LGB youth who live in counties where there are fewer districts with antibullying policies are more than twice as likely to attempt suicide, compared with LGB youth who live in counties where more districts had antibullying policies. Furthermore, the type of antibullying policy mattered. If a district’s antibullying policy did not prohibit bullying based on sexual orientation, then it had no impact on the suicide attempt rates of LGB youth in that area.2 Similar results were found when the relationship between anti–homophobic bullying policies and LGB suicide attempts was examined in the larger Youth Risk Behavior Surveillance System.3
State and local policies also influence other health outcomes among LGB youth. Hatzenbuehler et al. did another study that examined community-level determinants of tobacco use among LGB youth. Again using the data from the Oregon Healthy Teen Survey, they found that LGB youth living in communities that were more supportive of LGB youth (i.e., communities with a high proportion of same-sex couples living in the area, a high proportion of gay-straight alliances at schools, and LGB-specific antibullying policies) were less likely to smoke cigarettes, compared with LGB youth living in communities that were less supportive.4 A similar study in Canada by Konishi et al. found that schools with gay-straight alliances and anti–homophobic bullying policies were less likely to have LGB youth engaging in risky alcohol or illicit drug use.5
Why do these policies matter? A common theme among these policies is that they can either cause or alleviate stress for LGB youth. States that restrict same-sex marriages or schools that do not have any gay-straight alliances may signal to LGB youth that they are not valued or welcomed, or at the very worse, are despised. This creates a hostile and stressful environment for LGB youth, which raises the risk for mental health problems, such as depression and anxiety, which in turn, raises the risk for substance use and suicide.6 Conversely, the presence of a gay-straight alliance at school or a state that allows same-sex marriage may indicate to LGB youth that they are welcomed, if not just tolerated, and may alleviate this risk. Furthermore, antibullying policies seem to reduce the stress associated with bullying among LGB youth because it may serve as a deterrent to bullying based on sexual orientation. Although passing pro-LGB policies will not solve all the health problems among LGB youth, these policies certainly have an impact.
There might be trepidation among some pediatricians about being vocal on a politically charged policy proposal such as H.B. 780. However, suicide and substance use are major concerns for all physicians – especially pediatricians. Policy makers considering passing laws that can affect their LGB constituents should read these studies to see what kind of influence these proposals can have on the health and well-being of LGB youth. Moreover, health care providers should use their expertise, influence, and standing in their community to support policies that encourage protection for LGB youth and oppose policies that can harm LGB youth. These leaders are responsible for ensuring the health of the people they serve.
Dr. Montano is clinical instructor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC.
References
1. JAMA Pediatr. 2017. doi: 10.1001/jamapediatrics.2016.4529.
2. J Adolesc Health. 2013:S21-6. doi: 10.1016/j.jadohealth.2012.08.010.
3. Am J Public Health. 2014. doi: 10.2105/AJPH.2013.301508.
4. Arch Pediatr Adolesc Med. 2011. doi: 10.1001/archpediatrics.2011.64.
5. Prev Med. 2013. doi: 10.1016/j.ypmed.2013.06.031.
6. Psychol Bull. 2003. doi: 10.1037/0033-2909.129.5.674.
Recently North Carolina proposed a bill (House Bill 780) that will ban same-sex marriage in the state, even though the U.S. Supreme Court ruled in 2015 that no states can ban same-sex marriage because doing so violates the 14th Amendment. Although many mainly will argue that H.B. 780 is unconstitutional, this bill also can be detrimental to the health of lesbian, gay, and bisexual (LGB) youth.
In March 2017, JAMA Pediatrics published a study on the association between same-sex marriage laws and the rates of suicide attempts.1 Using data from the Youth Risk Behavior Surveillance System, they analyzed the relationship between state policies that permitted same-sex marriage and self-report of suicide attempts within the last 12 months. Thirty percent of LGB youth (13% of the survey population) reported a suicide attempt in the past year prior to any state policies that permitted same-sex marriage, compared with about 9% of the general population. After states implemented pro–same-sex marriage policies, LGB youth suicide attempts dropped to about 26% – a 14% relative decline. This was not limited to LGB youth. The general youth suicide rates declined from 8.6% to 8% – a 7% relative decline. Although the change in suicide attempts was small, the authors determined that the likelihood it occurred by chance was very slim, and concluded that policies enabling same-sex marriage may be associated with an improvement in population health, especially for LGB youth.
This was not the first study examining the relationship between policies and the health of LGB youth. Mark Hatzenbuehler, PhD, of Columbia University, New York, and his associates have published multiple studies on this topic. Four years earlier, Hatzenbuehler et al. published a study on the relationship between antibullying policies and suicide among LGB youth. Using data from the Oregon Healthy Teen survey (2006-2008), he found that LGB youth who live in counties where there are fewer districts with antibullying policies are more than twice as likely to attempt suicide, compared with LGB youth who live in counties where more districts had antibullying policies. Furthermore, the type of antibullying policy mattered. If a district’s antibullying policy did not prohibit bullying based on sexual orientation, then it had no impact on the suicide attempt rates of LGB youth in that area.2 Similar results were found when the relationship between anti–homophobic bullying policies and LGB suicide attempts was examined in the larger Youth Risk Behavior Surveillance System.3
State and local policies also influence other health outcomes among LGB youth. Hatzenbuehler et al. did another study that examined community-level determinants of tobacco use among LGB youth. Again using the data from the Oregon Healthy Teen Survey, they found that LGB youth living in communities that were more supportive of LGB youth (i.e., communities with a high proportion of same-sex couples living in the area, a high proportion of gay-straight alliances at schools, and LGB-specific antibullying policies) were less likely to smoke cigarettes, compared with LGB youth living in communities that were less supportive.4 A similar study in Canada by Konishi et al. found that schools with gay-straight alliances and anti–homophobic bullying policies were less likely to have LGB youth engaging in risky alcohol or illicit drug use.5
Why do these policies matter? A common theme among these policies is that they can either cause or alleviate stress for LGB youth. States that restrict same-sex marriages or schools that do not have any gay-straight alliances may signal to LGB youth that they are not valued or welcomed, or at the very worse, are despised. This creates a hostile and stressful environment for LGB youth, which raises the risk for mental health problems, such as depression and anxiety, which in turn, raises the risk for substance use and suicide.6 Conversely, the presence of a gay-straight alliance at school or a state that allows same-sex marriage may indicate to LGB youth that they are welcomed, if not just tolerated, and may alleviate this risk. Furthermore, antibullying policies seem to reduce the stress associated with bullying among LGB youth because it may serve as a deterrent to bullying based on sexual orientation. Although passing pro-LGB policies will not solve all the health problems among LGB youth, these policies certainly have an impact.
There might be trepidation among some pediatricians about being vocal on a politically charged policy proposal such as H.B. 780. However, suicide and substance use are major concerns for all physicians – especially pediatricians. Policy makers considering passing laws that can affect their LGB constituents should read these studies to see what kind of influence these proposals can have on the health and well-being of LGB youth. Moreover, health care providers should use their expertise, influence, and standing in their community to support policies that encourage protection for LGB youth and oppose policies that can harm LGB youth. These leaders are responsible for ensuring the health of the people they serve.
Dr. Montano is clinical instructor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC.
References
1. JAMA Pediatr. 2017. doi: 10.1001/jamapediatrics.2016.4529.
2. J Adolesc Health. 2013:S21-6. doi: 10.1016/j.jadohealth.2012.08.010.
3. Am J Public Health. 2014. doi: 10.2105/AJPH.2013.301508.
4. Arch Pediatr Adolesc Med. 2011. doi: 10.1001/archpediatrics.2011.64.
5. Prev Med. 2013. doi: 10.1016/j.ypmed.2013.06.031.
6. Psychol Bull. 2003. doi: 10.1037/0033-2909.129.5.674.