User login
Getting it right at the end of life
Although the concept of the living will was first proposed in 1969
In contrast, an informal search of PubMed reveals that at least 38 articles on advance directives and end-of-life care have been published during the first 7 months of 2017. And a feature article in this month’s issue of JFP makes one more. Why is there such strong interest now in an issue that seldom arose when I began practice in 1978?
More complex, less personalized medicine. As medical care has become more sophisticated, there is a great deal more we can do to keep people alive as they approach the end of life, and a great many more decisions to be made.
Additionally, people are much less likely today to be cared for in their dying days by a family physician who knows them, their wishes, and their family well. In my early years in small-town practice, I was present when my patients were dying, and I usually knew their family members. Family meetings were easy to arrange, and we quickly came to a consensus about what to do and what not to do. If I was not available, one of my practice partners was. We cared for our patients in the office, nursing home, and hospital. Now, most dying hospitalized patients are cared for by hospitalists who may be meeting the patient for the first time.
Getting more people to participate. Consequently, it is important to understand patients’ wishes for end-of-life care and to document those wishes in writing, using things like a POLST (Physician Orders for Life-Sustaining Treatment) form. Although randomized trials support the value of advance care planning, especially in primary care settings,3,4 two-thirds of Americans have not completed an advance directive.5 Rolnick suggests we “delegalize” the process to remove barriers and make it easier for people to execute such documents and integrate them into health care systems.6
Make it part of your office routine. A 70-year-old patient of mine with advanced COPD arrived at his office visit last month with advance directive and POLST forms in hand. We had an excellent, frank conversation, spiced with humor that he supplied, about his wishes for end-of-life care. Just like so many other tasks that we must squeeze into our busy schedules, this is one that we should hard-wire into our office systems and routines.
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. Schneiderman LJ, Arras JD. Counseling patients to counsel physicians on future care in the event of patient incompetence. Ann Intern Med. 1985;102:693-698.
3. Weathers E, O’Caoimh R, Cornally N, et al. Advance care planning: a systematic review of randomised controlled trials conducted with older adults. Maturitas. 2016;91:101-109.
4. Tierney WM, Dexter PR, Gramelspacher GP, et al. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16:32-40.
5. Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood). 2017;36:1244-1251.
6. Rolnick JA, Asch DA, Halpern SD. Delegalizing advance directives—facilitating advance care planning. N Engl J Med. 2017;376:2105-2107.
Although the concept of the living will was first proposed in 1969
In contrast, an informal search of PubMed reveals that at least 38 articles on advance directives and end-of-life care have been published during the first 7 months of 2017. And a feature article in this month’s issue of JFP makes one more. Why is there such strong interest now in an issue that seldom arose when I began practice in 1978?
More complex, less personalized medicine. As medical care has become more sophisticated, there is a great deal more we can do to keep people alive as they approach the end of life, and a great many more decisions to be made.
Additionally, people are much less likely today to be cared for in their dying days by a family physician who knows them, their wishes, and their family well. In my early years in small-town practice, I was present when my patients were dying, and I usually knew their family members. Family meetings were easy to arrange, and we quickly came to a consensus about what to do and what not to do. If I was not available, one of my practice partners was. We cared for our patients in the office, nursing home, and hospital. Now, most dying hospitalized patients are cared for by hospitalists who may be meeting the patient for the first time.
Getting more people to participate. Consequently, it is important to understand patients’ wishes for end-of-life care and to document those wishes in writing, using things like a POLST (Physician Orders for Life-Sustaining Treatment) form. Although randomized trials support the value of advance care planning, especially in primary care settings,3,4 two-thirds of Americans have not completed an advance directive.5 Rolnick suggests we “delegalize” the process to remove barriers and make it easier for people to execute such documents and integrate them into health care systems.6
Make it part of your office routine. A 70-year-old patient of mine with advanced COPD arrived at his office visit last month with advance directive and POLST forms in hand. We had an excellent, frank conversation, spiced with humor that he supplied, about his wishes for end-of-life care. Just like so many other tasks that we must squeeze into our busy schedules, this is one that we should hard-wire into our office systems and routines.
Although the concept of the living will was first proposed in 1969
In contrast, an informal search of PubMed reveals that at least 38 articles on advance directives and end-of-life care have been published during the first 7 months of 2017. And a feature article in this month’s issue of JFP makes one more. Why is there such strong interest now in an issue that seldom arose when I began practice in 1978?
More complex, less personalized medicine. As medical care has become more sophisticated, there is a great deal more we can do to keep people alive as they approach the end of life, and a great many more decisions to be made.
Additionally, people are much less likely today to be cared for in their dying days by a family physician who knows them, their wishes, and their family well. In my early years in small-town practice, I was present when my patients were dying, and I usually knew their family members. Family meetings were easy to arrange, and we quickly came to a consensus about what to do and what not to do. If I was not available, one of my practice partners was. We cared for our patients in the office, nursing home, and hospital. Now, most dying hospitalized patients are cared for by hospitalists who may be meeting the patient for the first time.
Getting more people to participate. Consequently, it is important to understand patients’ wishes for end-of-life care and to document those wishes in writing, using things like a POLST (Physician Orders for Life-Sustaining Treatment) form. Although randomized trials support the value of advance care planning, especially in primary care settings,3,4 two-thirds of Americans have not completed an advance directive.5 Rolnick suggests we “delegalize” the process to remove barriers and make it easier for people to execute such documents and integrate them into health care systems.6
Make it part of your office routine. A 70-year-old patient of mine with advanced COPD arrived at his office visit last month with advance directive and POLST forms in hand. We had an excellent, frank conversation, spiced with humor that he supplied, about his wishes for end-of-life care. Just like so many other tasks that we must squeeze into our busy schedules, this is one that we should hard-wire into our office systems and routines.
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. Schneiderman LJ, Arras JD. Counseling patients to counsel physicians on future care in the event of patient incompetence. Ann Intern Med. 1985;102:693-698.
3. Weathers E, O’Caoimh R, Cornally N, et al. Advance care planning: a systematic review of randomised controlled trials conducted with older adults. Maturitas. 2016;91:101-109.
4. Tierney WM, Dexter PR, Gramelspacher GP, et al. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16:32-40.
5. Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood). 2017;36:1244-1251.
6. Rolnick JA, Asch DA, Halpern SD. Delegalizing advance directives—facilitating advance care planning. N Engl J Med. 2017;376:2105-2107.
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. Schneiderman LJ, Arras JD. Counseling patients to counsel physicians on future care in the event of patient incompetence. Ann Intern Med. 1985;102:693-698.
3. Weathers E, O’Caoimh R, Cornally N, et al. Advance care planning: a systematic review of randomised controlled trials conducted with older adults. Maturitas. 2016;91:101-109.
4. Tierney WM, Dexter PR, Gramelspacher GP, et al. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16:32-40.
5. Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood). 2017;36:1244-1251.
6. Rolnick JA, Asch DA, Halpern SD. Delegalizing advance directives—facilitating advance care planning. N Engl J Med. 2017;376:2105-2107.
Low-grade fever, erythematous rash in pregnant woman • Dx?
THE CASE
A 31-year-old woman presented to her obstetrician’s office at 16 weeks’ gestation with a 2-day history of low-grade fever and an erythematous rash measuring 1 x 4 cm on her right groin. She had a medical history of a penicillin allergy (urticaria) and her outdoor activities included gardening and picnicking.
We suspected that she was experiencing an allergic reaction and recommended an antihistamine (diphenhydramine). The patient returned 4 days later with new symptoms including headache, photophobia, neck pain, unilateral large joint pain, and periorbital cellulitis, as well as expansion of her rash. She was afebrile and an examination revealed that the 1 x 4 cm rash on her groin had grown; it was now a demarcated erythematous rash with faint central clearing measuring 5 x 8 cm. Right periorbital erythema and nuchal rigidity were also noted.
Because of her expanding rash and nuchal rigidity, we suspected Lyme meningitis and we referred her to the emergency department. Within 24 hours, the rash had spread to her abdomen, thigh, and wrist, and was consistent with erythema migrans.
Laboratory evaluation revealed an increased number of white bloods cells (13.5 million cells/mcL; normal range 4.5-11.0 million cells/mcL), and an increased number of neutrophils (10.8 million cells/mcL; normal range 1.8-8 million cells/mcL), indicating leukocytosis with a left shift. Lab tests also revealed a low hemoglobin level (10.6 g/dL; normal range 12-16 g/dL) and a mean corpuscular volume of 85.6 fL/red cell (normal range 80-100 fL/red cell), indicating microcytic anemia. A lumbar puncture was negative for disseminated Lyme disease by Gram stain, culture, and polymerase chain reaction.
THE DIAGNOSIS
A diagnosis of Lyme disease was confirmed with a positive Lyme titer serology via an enzyme-linked immunosorbent assay. The rash and other symptoms responded promptly to intravenous ceftriaxone 2 g, and the patient was discharged home on oral cefuroxime 500 mg bid for 14 days.
DISCUSSION
Lyme disease is the most common vector-borne illness in the United States, concentrated heavily in the Northeast and upper Midwest.1 The most recent information released by the Centers for Disease Control and Prevention (CDC) lists Vermont, Maine, Pennsylvania, Rhode Island, Connecticut, New Jersey, Massachusetts, Delaware, New Hampshire, and Minnesota as the states with the highest incidence of Lyme disease.2
The number of reported cases in the United States has increased over the past 2 decades, from approximately 11,000 in 1995 to about 28,000 in 2015.3 Over the past year, we have seen several cases of Lyme disease in the obstetric population of our own practice.
Prompt treatment is crucial. Pregnant women who are acutely infected with Borrelia burgdorferi (the primary cause of Lyme disease) and do not receive treatment have experienced multiple adverse pregnancy outcomes, including preterm delivery, infants born with rash, and stillbirth.4 Additional concern exists for fetal cardiac anomalies, with data showing that there are twice as many cardiac defects in children born to mothers residing in endemic regions.5
What animal studies have taught us about Lyme disease
The potential causal relationship between Lyme disease and fetal demise was first studied in 2007. This case report involved the stillbirth of a full-term baby from an acutely infected woman who did not receive treatment. She experienced erythema multiforme 6 weeks prior to delivery.6
The vast majority of research on Lyme disease in pregnancy comes from work with mice and dogs. These studies confirmed that acute infection with Lyme disease is associated with an increased risk of adverse fetal outcomes, specifically fetal death.7
Silver et al further researched the association using murine models in the 1980s. They found that fetal death occurred in 12% of acutely infected mice, compared with none of the mice that were chronically infected.7
In 2010, Lakos and Solymosi examined the effects of Lyme disease on pregnancy outcomes in acutely infected women. Seven out of 95 pregnant women acutely infected with B burgdorferi experienced fetal demise, further supporting the association seen in animal experiments.8
Treating pregnant patients
Doxycycline and tetracycline, which are routinely used to treat Lyme disease, are not appropriate in the obstetric population. The CDC recommends up to a 3-week course of antibiotics; the standard regimen is amoxicillin 500 mg by mouth tid. For women who are allergic to penicillin, as was the case with our patient, cefuroxime 500 mg by mouth bid is the treatment of choice.9
Our patient underwent a detailed ultrasound at 21 weeks, which revealed normal fetal anatomy and no evidence of cardiac malformations. The remainder of her pregnancy was uncomplicated, and she gave birth vaginally at 41 weeks to a baby boy weighing 3700 g.
THE TAKEAWAY
There is a need to increase awareness of Lyme disease in pregnancy on a national level. It is the responsibility of every practitioner caring for obstetric patients in endemic regions to address new-onset rash promptly. There have been cases of women who experienced erythema migrans and arthralgias after exposure to a tick bite, later delivering infants with cardiac anomalies such as atrial and ventricular septal defects.10 In obstetric patients acutely infected during the first trimester, a fetal echocardiogram is reasonable, given the demonstrated high potential for fetal cardiac anomalies.
Preventing adverse fetal outcomes requires early treatment with antibiotics. The CDC maintains that there have been no life-threatening adverse fetal effects from Lyme disease seen in women who are appropriately treated, as well as no transmission of Lyme disease in the breast milk of lactating mothers.9
1. Centers for Disease Control and Prevention. Lyme disease. Data and statistics. Available at: https://www.cdc.gov/lyme/stats/. Accessed July 5, 2017.
2. Centers for Disease Control and Prevention. Lyme disease data tables. Reported cases of Lyme disease by state or locality, 2005-2015. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html. Accessed July 5, 2017.
3. Centers for Disease Control and Prevention. Lyme disease graphs. Reported cases of Lyme disease by year, United States, 1995-2015. Available at: https://www.cdc.gov/lyme/stats/graphs.html. Accessed July 5, 2017.
4. Maraspin V, Cimperman J, Lotric-Furlan S, et al. Erythema migrans in pregnancy. Wien Klin Wochenschr. 1999;111:933-940.
5. Strobino BA, Williams CL, Abid S, et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367-374.
6. Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27-2007. A 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918-925.
7. Silver RM, Yang L, Daynes RA, et al. Fetal outcome in murine Lyme disease. Infect Immun. 1995;63:66-72.
8. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcomes. Int J Infect Dis. 2010;14:e494-e498.
9. Centers for Disease Control and Prevention. Ticks and Lyme disease. Pregnancy and Lyme disease. Available at: https://www.cdc.gov/lyme/resources/toolkit/factsheets/10_508_Lyme%20disease_PregnantWoman_FACTSheet.pdf. Accessed July 5, 2017.
10. O’Brien JM, Martens MG. Lyme disease in pregnancy: a New Jersey medical advisory. MD Advis. 2014;7:24-27.
THE CASE
A 31-year-old woman presented to her obstetrician’s office at 16 weeks’ gestation with a 2-day history of low-grade fever and an erythematous rash measuring 1 x 4 cm on her right groin. She had a medical history of a penicillin allergy (urticaria) and her outdoor activities included gardening and picnicking.
We suspected that she was experiencing an allergic reaction and recommended an antihistamine (diphenhydramine). The patient returned 4 days later with new symptoms including headache, photophobia, neck pain, unilateral large joint pain, and periorbital cellulitis, as well as expansion of her rash. She was afebrile and an examination revealed that the 1 x 4 cm rash on her groin had grown; it was now a demarcated erythematous rash with faint central clearing measuring 5 x 8 cm. Right periorbital erythema and nuchal rigidity were also noted.
Because of her expanding rash and nuchal rigidity, we suspected Lyme meningitis and we referred her to the emergency department. Within 24 hours, the rash had spread to her abdomen, thigh, and wrist, and was consistent with erythema migrans.
Laboratory evaluation revealed an increased number of white bloods cells (13.5 million cells/mcL; normal range 4.5-11.0 million cells/mcL), and an increased number of neutrophils (10.8 million cells/mcL; normal range 1.8-8 million cells/mcL), indicating leukocytosis with a left shift. Lab tests also revealed a low hemoglobin level (10.6 g/dL; normal range 12-16 g/dL) and a mean corpuscular volume of 85.6 fL/red cell (normal range 80-100 fL/red cell), indicating microcytic anemia. A lumbar puncture was negative for disseminated Lyme disease by Gram stain, culture, and polymerase chain reaction.
THE DIAGNOSIS
A diagnosis of Lyme disease was confirmed with a positive Lyme titer serology via an enzyme-linked immunosorbent assay. The rash and other symptoms responded promptly to intravenous ceftriaxone 2 g, and the patient was discharged home on oral cefuroxime 500 mg bid for 14 days.
DISCUSSION
Lyme disease is the most common vector-borne illness in the United States, concentrated heavily in the Northeast and upper Midwest.1 The most recent information released by the Centers for Disease Control and Prevention (CDC) lists Vermont, Maine, Pennsylvania, Rhode Island, Connecticut, New Jersey, Massachusetts, Delaware, New Hampshire, and Minnesota as the states with the highest incidence of Lyme disease.2
The number of reported cases in the United States has increased over the past 2 decades, from approximately 11,000 in 1995 to about 28,000 in 2015.3 Over the past year, we have seen several cases of Lyme disease in the obstetric population of our own practice.
Prompt treatment is crucial. Pregnant women who are acutely infected with Borrelia burgdorferi (the primary cause of Lyme disease) and do not receive treatment have experienced multiple adverse pregnancy outcomes, including preterm delivery, infants born with rash, and stillbirth.4 Additional concern exists for fetal cardiac anomalies, with data showing that there are twice as many cardiac defects in children born to mothers residing in endemic regions.5
What animal studies have taught us about Lyme disease
The potential causal relationship between Lyme disease and fetal demise was first studied in 2007. This case report involved the stillbirth of a full-term baby from an acutely infected woman who did not receive treatment. She experienced erythema multiforme 6 weeks prior to delivery.6
The vast majority of research on Lyme disease in pregnancy comes from work with mice and dogs. These studies confirmed that acute infection with Lyme disease is associated with an increased risk of adverse fetal outcomes, specifically fetal death.7
Silver et al further researched the association using murine models in the 1980s. They found that fetal death occurred in 12% of acutely infected mice, compared with none of the mice that were chronically infected.7
In 2010, Lakos and Solymosi examined the effects of Lyme disease on pregnancy outcomes in acutely infected women. Seven out of 95 pregnant women acutely infected with B burgdorferi experienced fetal demise, further supporting the association seen in animal experiments.8
Treating pregnant patients
Doxycycline and tetracycline, which are routinely used to treat Lyme disease, are not appropriate in the obstetric population. The CDC recommends up to a 3-week course of antibiotics; the standard regimen is amoxicillin 500 mg by mouth tid. For women who are allergic to penicillin, as was the case with our patient, cefuroxime 500 mg by mouth bid is the treatment of choice.9
Our patient underwent a detailed ultrasound at 21 weeks, which revealed normal fetal anatomy and no evidence of cardiac malformations. The remainder of her pregnancy was uncomplicated, and she gave birth vaginally at 41 weeks to a baby boy weighing 3700 g.
THE TAKEAWAY
There is a need to increase awareness of Lyme disease in pregnancy on a national level. It is the responsibility of every practitioner caring for obstetric patients in endemic regions to address new-onset rash promptly. There have been cases of women who experienced erythema migrans and arthralgias after exposure to a tick bite, later delivering infants with cardiac anomalies such as atrial and ventricular septal defects.10 In obstetric patients acutely infected during the first trimester, a fetal echocardiogram is reasonable, given the demonstrated high potential for fetal cardiac anomalies.
Preventing adverse fetal outcomes requires early treatment with antibiotics. The CDC maintains that there have been no life-threatening adverse fetal effects from Lyme disease seen in women who are appropriately treated, as well as no transmission of Lyme disease in the breast milk of lactating mothers.9
THE CASE
A 31-year-old woman presented to her obstetrician’s office at 16 weeks’ gestation with a 2-day history of low-grade fever and an erythematous rash measuring 1 x 4 cm on her right groin. She had a medical history of a penicillin allergy (urticaria) and her outdoor activities included gardening and picnicking.
We suspected that she was experiencing an allergic reaction and recommended an antihistamine (diphenhydramine). The patient returned 4 days later with new symptoms including headache, photophobia, neck pain, unilateral large joint pain, and periorbital cellulitis, as well as expansion of her rash. She was afebrile and an examination revealed that the 1 x 4 cm rash on her groin had grown; it was now a demarcated erythematous rash with faint central clearing measuring 5 x 8 cm. Right periorbital erythema and nuchal rigidity were also noted.
Because of her expanding rash and nuchal rigidity, we suspected Lyme meningitis and we referred her to the emergency department. Within 24 hours, the rash had spread to her abdomen, thigh, and wrist, and was consistent with erythema migrans.
Laboratory evaluation revealed an increased number of white bloods cells (13.5 million cells/mcL; normal range 4.5-11.0 million cells/mcL), and an increased number of neutrophils (10.8 million cells/mcL; normal range 1.8-8 million cells/mcL), indicating leukocytosis with a left shift. Lab tests also revealed a low hemoglobin level (10.6 g/dL; normal range 12-16 g/dL) and a mean corpuscular volume of 85.6 fL/red cell (normal range 80-100 fL/red cell), indicating microcytic anemia. A lumbar puncture was negative for disseminated Lyme disease by Gram stain, culture, and polymerase chain reaction.
THE DIAGNOSIS
A diagnosis of Lyme disease was confirmed with a positive Lyme titer serology via an enzyme-linked immunosorbent assay. The rash and other symptoms responded promptly to intravenous ceftriaxone 2 g, and the patient was discharged home on oral cefuroxime 500 mg bid for 14 days.
DISCUSSION
Lyme disease is the most common vector-borne illness in the United States, concentrated heavily in the Northeast and upper Midwest.1 The most recent information released by the Centers for Disease Control and Prevention (CDC) lists Vermont, Maine, Pennsylvania, Rhode Island, Connecticut, New Jersey, Massachusetts, Delaware, New Hampshire, and Minnesota as the states with the highest incidence of Lyme disease.2
The number of reported cases in the United States has increased over the past 2 decades, from approximately 11,000 in 1995 to about 28,000 in 2015.3 Over the past year, we have seen several cases of Lyme disease in the obstetric population of our own practice.
Prompt treatment is crucial. Pregnant women who are acutely infected with Borrelia burgdorferi (the primary cause of Lyme disease) and do not receive treatment have experienced multiple adverse pregnancy outcomes, including preterm delivery, infants born with rash, and stillbirth.4 Additional concern exists for fetal cardiac anomalies, with data showing that there are twice as many cardiac defects in children born to mothers residing in endemic regions.5
What animal studies have taught us about Lyme disease
The potential causal relationship between Lyme disease and fetal demise was first studied in 2007. This case report involved the stillbirth of a full-term baby from an acutely infected woman who did not receive treatment. She experienced erythema multiforme 6 weeks prior to delivery.6
The vast majority of research on Lyme disease in pregnancy comes from work with mice and dogs. These studies confirmed that acute infection with Lyme disease is associated with an increased risk of adverse fetal outcomes, specifically fetal death.7
Silver et al further researched the association using murine models in the 1980s. They found that fetal death occurred in 12% of acutely infected mice, compared with none of the mice that were chronically infected.7
In 2010, Lakos and Solymosi examined the effects of Lyme disease on pregnancy outcomes in acutely infected women. Seven out of 95 pregnant women acutely infected with B burgdorferi experienced fetal demise, further supporting the association seen in animal experiments.8
Treating pregnant patients
Doxycycline and tetracycline, which are routinely used to treat Lyme disease, are not appropriate in the obstetric population. The CDC recommends up to a 3-week course of antibiotics; the standard regimen is amoxicillin 500 mg by mouth tid. For women who are allergic to penicillin, as was the case with our patient, cefuroxime 500 mg by mouth bid is the treatment of choice.9
Our patient underwent a detailed ultrasound at 21 weeks, which revealed normal fetal anatomy and no evidence of cardiac malformations. The remainder of her pregnancy was uncomplicated, and she gave birth vaginally at 41 weeks to a baby boy weighing 3700 g.
THE TAKEAWAY
There is a need to increase awareness of Lyme disease in pregnancy on a national level. It is the responsibility of every practitioner caring for obstetric patients in endemic regions to address new-onset rash promptly. There have been cases of women who experienced erythema migrans and arthralgias after exposure to a tick bite, later delivering infants with cardiac anomalies such as atrial and ventricular septal defects.10 In obstetric patients acutely infected during the first trimester, a fetal echocardiogram is reasonable, given the demonstrated high potential for fetal cardiac anomalies.
Preventing adverse fetal outcomes requires early treatment with antibiotics. The CDC maintains that there have been no life-threatening adverse fetal effects from Lyme disease seen in women who are appropriately treated, as well as no transmission of Lyme disease in the breast milk of lactating mothers.9
1. Centers for Disease Control and Prevention. Lyme disease. Data and statistics. Available at: https://www.cdc.gov/lyme/stats/. Accessed July 5, 2017.
2. Centers for Disease Control and Prevention. Lyme disease data tables. Reported cases of Lyme disease by state or locality, 2005-2015. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html. Accessed July 5, 2017.
3. Centers for Disease Control and Prevention. Lyme disease graphs. Reported cases of Lyme disease by year, United States, 1995-2015. Available at: https://www.cdc.gov/lyme/stats/graphs.html. Accessed July 5, 2017.
4. Maraspin V, Cimperman J, Lotric-Furlan S, et al. Erythema migrans in pregnancy. Wien Klin Wochenschr. 1999;111:933-940.
5. Strobino BA, Williams CL, Abid S, et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367-374.
6. Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27-2007. A 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918-925.
7. Silver RM, Yang L, Daynes RA, et al. Fetal outcome in murine Lyme disease. Infect Immun. 1995;63:66-72.
8. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcomes. Int J Infect Dis. 2010;14:e494-e498.
9. Centers for Disease Control and Prevention. Ticks and Lyme disease. Pregnancy and Lyme disease. Available at: https://www.cdc.gov/lyme/resources/toolkit/factsheets/10_508_Lyme%20disease_PregnantWoman_FACTSheet.pdf. Accessed July 5, 2017.
10. O’Brien JM, Martens MG. Lyme disease in pregnancy: a New Jersey medical advisory. MD Advis. 2014;7:24-27.
1. Centers for Disease Control and Prevention. Lyme disease. Data and statistics. Available at: https://www.cdc.gov/lyme/stats/. Accessed July 5, 2017.
2. Centers for Disease Control and Prevention. Lyme disease data tables. Reported cases of Lyme disease by state or locality, 2005-2015. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html. Accessed July 5, 2017.
3. Centers for Disease Control and Prevention. Lyme disease graphs. Reported cases of Lyme disease by year, United States, 1995-2015. Available at: https://www.cdc.gov/lyme/stats/graphs.html. Accessed July 5, 2017.
4. Maraspin V, Cimperman J, Lotric-Furlan S, et al. Erythema migrans in pregnancy. Wien Klin Wochenschr. 1999;111:933-940.
5. Strobino BA, Williams CL, Abid S, et al. Lyme disease and pregnancy outcome: a prospective study of two thousand prenatal patients. Am J Obstet Gynecol. 1993;169:367-374.
6. Gibbs RS, Roberts DJ. Case records of the Massachusetts General Hospital. Case 27-2007. A 30-year-old pregnant woman with intrauterine fetal death. N Engl J Med. 2007;357:918-925.
7. Silver RM, Yang L, Daynes RA, et al. Fetal outcome in murine Lyme disease. Infect Immun. 1995;63:66-72.
8. Lakos A, Solymosi N. Maternal Lyme borreliosis and pregnancy outcomes. Int J Infect Dis. 2010;14:e494-e498.
9. Centers for Disease Control and Prevention. Ticks and Lyme disease. Pregnancy and Lyme disease. Available at: https://www.cdc.gov/lyme/resources/toolkit/factsheets/10_508_Lyme%20disease_PregnantWoman_FACTSheet.pdf. Accessed July 5, 2017.
10. O’Brien JM, Martens MG. Lyme disease in pregnancy: a New Jersey medical advisory. MD Advis. 2014;7:24-27.
Progressive hair loss
A 66-year-old white woman presented to her primary care clinic with concerns about hair loss, which began 2 years ago. Recently, she had noticed some “bumps” on her cheeks, as well.
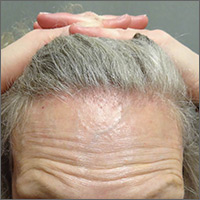
On physical examination, the physician noted hair loss in a symmetric 2-cm band-like distribution across her frontal and temporal scalp (FIGURES 1 and 2). In both areas, there was moderate perifollicular erythema, scale, and what appeared to be scarring.
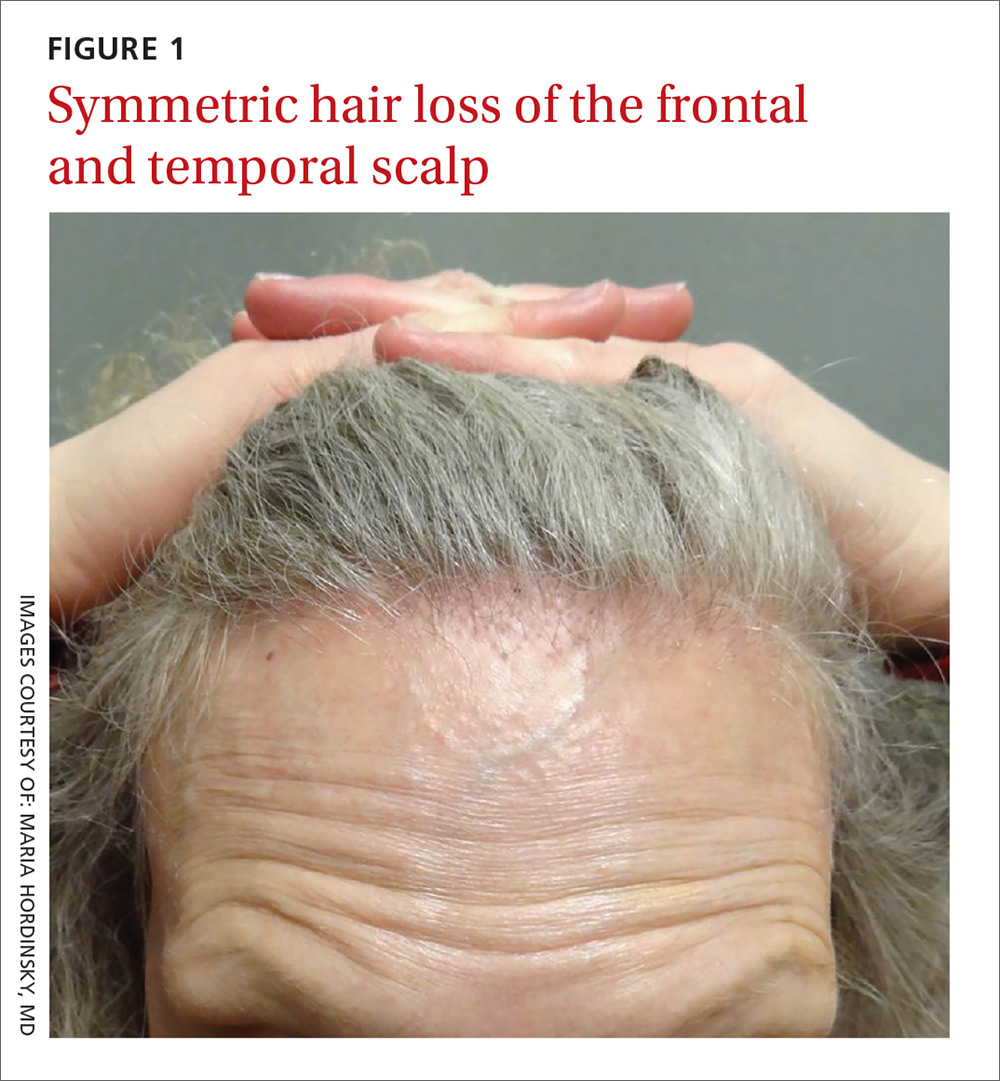
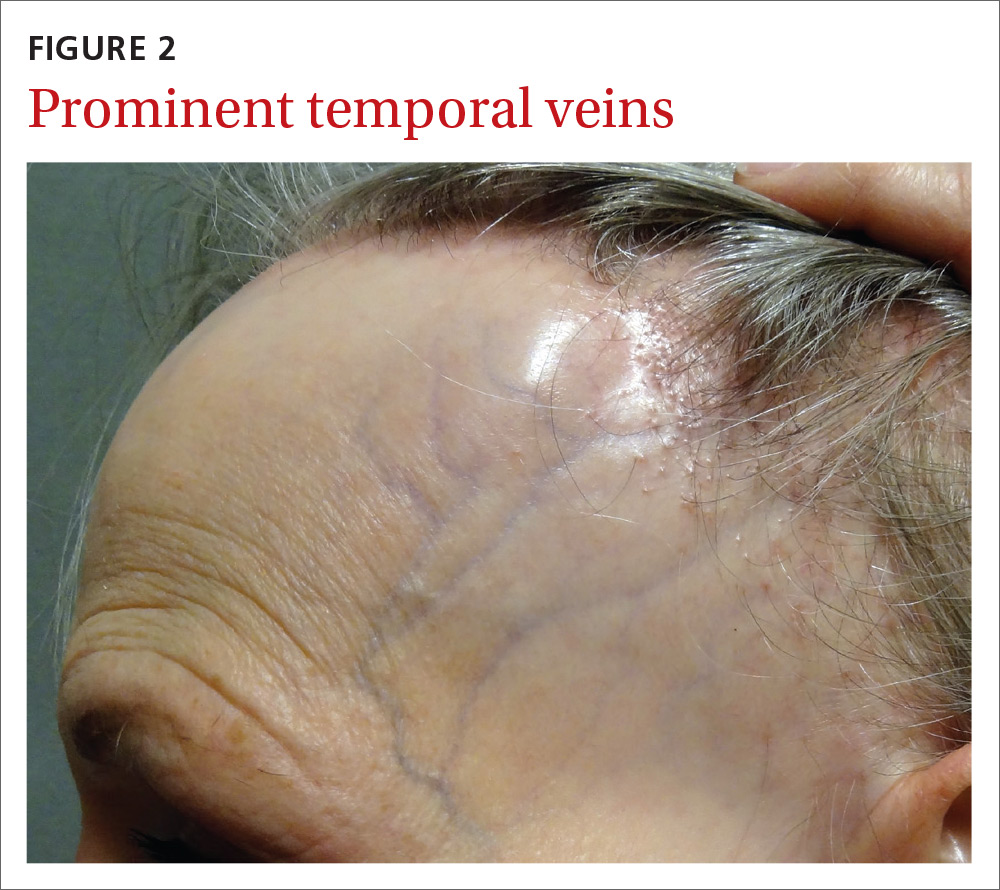
The patient had lost most of her eyebrow hairs, and had prominent temporal veins (FIGURE 2) and flesh-colored papules on her cheeks. She had no significant medical history, was emotionally stable, and recently had a satisfactory health care maintenance exam. The postmenopausal patient’s last menses was 15 years earlier, and she was not taking hormone replacement.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Frontal fibrosing alopecia
The patient was referred to our dermatology clinic, which specializes in hair loss. Based on the clinical findings, we suspected that this was a case of frontal fibrosing alopecia (FFA), a primary lymphocytic cicatricial (scarring) alopecia. A dermatopathologist confirmed the diagnosis via histologic review.
A condition on the rise. The incidence of FFA has been steadily increasing internationally since the condition was first described in 1994.1 Among patients referred to a specialty clinic for hair loss, diagnosis of FFA has increased from 1.6% in 2000 to 17% in 2011.2
FFA is characterized by symmetric band-like hair loss with evidence of scarring in the frontal and temporal regions of the scalp. (The extent of hair loss can be assessed by retracting the patient’s hair and having the patient raise his or her eyebrows and wrinkle the forehead in a surprised look.) FFA is accompanied by eyebrow loss in 73% to 95% of patients.2,3 Mild to severe perifollicular (and possibly more generalized) erythema and scale are usually present. In addition, erythematous or skin-colored papules may appear on the face,3 and marked exaggeration of the temporal veins is a common finding.
Most patients with FFA (83%) are postmenopausal women and nearly all (98.6%) have Fitzpatrick skin type 1 or 2 (white skin that burns easily and doesn’t readily tan).4 Other pertinent findings include the absence of oral lesions, nail changes, or other skin diseases.
A subtype of another condition? Because they are similar histologically, some consider FFA to be a subtype of lichen planopilaris. (See “Scarring alopecia in a woman with psoriasis,” J Fam Pract. 2015;64:E1-E3.)
A punch biopsy to confirm the diagnosis of FFA should be taken from the leading edge of the hair loss and, ideally, reviewed by a dermatopathologist. Histologic examination will reveal a lichenoid lymphocytic infiltrate (predominantly around the hair follicle where the follicular stem cells reside), resulting in fibrosis and scarring.5
Rule out other causes of hair loss
In addition to confirming the diagnosis with histologic examination, you’ll also need to have ruled out the following conditions in the differential.
Alopecia areata may mimic the ophiasis (band-like) pattern of hair loss seen with FFA, but it is a non-scarring disorder that typically lacks any signs of inflammation.
Female pattern hair loss is characterized by a decrease in hair density and thinning. The condition is non-scarring and usually involves the frontal and vertex (crown) regions of the scalp.
Discoid lupus erythematosus is characterized by circular scarring hair loss with a central patch of inflammation, as well as depigmentation.
Central centrifugal cicatricial alopecia predominantly affects black women and is characterized by circular hair loss of the vertex, with perifollicular inflammation and scarring.
Traction alopecia can occur in the same location as FFA, but is not usually associated with perifollicular inflammation. This condition can cause scarring if traction has been longstanding and persistent. There is usually a history of certain hairstyles (such as braiding) associated with chronic tension on hair fibers.
Numerous Tx strategies exist, but they are not well studied
Because there are no published randomized clinical trials on treatment for FFA, few evidence-based treatment strategies exist.6 In addition, the prognosis is variable. Experts have employed numerous treatment strategies, including topical and intralesional steroids, immunosuppressive medications, antibiotics, and anti-androgen therapy, with varying results.4,6 For most primary care physicians, it’s best to refer patients to a dermatologist to initiate treatment.
Intralesional steroids such as triamcinolone acetonide (5-10 mg/cc), as well as high-potency topical steroids, are generally helpful to stabilize the disease. There is also some evidence of benefit from oral dutasteride or finasteride at variable doses.6 Immunosuppressants such as hydroxychloroquine may also be used as second-line treatments, although the benefit-to-risk ratio needs to be taken into consideration.7
Early detection is key. In general, treatment should be initiated as soon as possible to prevent disease progression and reduce permanent scarring and hair loss. The Lichen Planopilaris Activity Index7 is a tool that clinicians can use to measure disease severity and track changes in disease activity through patient report of symptoms and measurements of scalp inflammation.
Our patient was started on a regimen of topical high-potency steroids (clobetasol foam, 0.05%), with targeted, intralesional injection of steroids (10 mg/cc of triamcinolone acetonide) to areas with the most inflammation. The patient was advised to use ketoconazole 2% shampoo while showering.
These interventions decreased our patient’s symptoms dramatically. Her scalp erythema and scale improved, but the hair did not regrow. One year later, her hairline was clinically stable with no evidence of disease progression. She continues to see a dermatologist annually.
CORRESPONDENCE
David V. Power, MB, MPH, Department of Family Medicine and Community Health, University of Minnesota, 516 Delaware St. SE, Minneapolis, MN 55455; [email protected].
1. Kossard S. Postmenopausal frontal fibrosing alopecia: Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
2. MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
3. Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
4. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
5. Poblet E, Jiménez F, Pascual A, et al. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45:375-380.
6. Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
7. Chiang C, Sah D, Cho BK, et al. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62:387-392.
A 66-year-old white woman presented to her primary care clinic with concerns about hair loss, which began 2 years ago. Recently, she had noticed some “bumps” on her cheeks, as well.
On physical examination, the physician noted hair loss in a symmetric 2-cm band-like distribution across her frontal and temporal scalp (FIGURES 1 and 2). In both areas, there was moderate perifollicular erythema, scale, and what appeared to be scarring.

The patient had lost most of her eyebrow hairs, and had prominent temporal veins (FIGURE 2) and flesh-colored papules on her cheeks. She had no significant medical history, was emotionally stable, and recently had a satisfactory health care maintenance exam. The postmenopausal patient’s last menses was 15 years earlier, and she was not taking hormone replacement.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Frontal fibrosing alopecia
The patient was referred to our dermatology clinic, which specializes in hair loss. Based on the clinical findings, we suspected that this was a case of frontal fibrosing alopecia (FFA), a primary lymphocytic cicatricial (scarring) alopecia. A dermatopathologist confirmed the diagnosis via histologic review.
A condition on the rise. The incidence of FFA has been steadily increasing internationally since the condition was first described in 1994.1 Among patients referred to a specialty clinic for hair loss, diagnosis of FFA has increased from 1.6% in 2000 to 17% in 2011.2
FFA is characterized by symmetric band-like hair loss with evidence of scarring in the frontal and temporal regions of the scalp. (The extent of hair loss can be assessed by retracting the patient’s hair and having the patient raise his or her eyebrows and wrinkle the forehead in a surprised look.) FFA is accompanied by eyebrow loss in 73% to 95% of patients.2,3 Mild to severe perifollicular (and possibly more generalized) erythema and scale are usually present. In addition, erythematous or skin-colored papules may appear on the face,3 and marked exaggeration of the temporal veins is a common finding.
Most patients with FFA (83%) are postmenopausal women and nearly all (98.6%) have Fitzpatrick skin type 1 or 2 (white skin that burns easily and doesn’t readily tan).4 Other pertinent findings include the absence of oral lesions, nail changes, or other skin diseases.
A subtype of another condition? Because they are similar histologically, some consider FFA to be a subtype of lichen planopilaris. (See “Scarring alopecia in a woman with psoriasis,” J Fam Pract. 2015;64:E1-E3.)
A punch biopsy to confirm the diagnosis of FFA should be taken from the leading edge of the hair loss and, ideally, reviewed by a dermatopathologist. Histologic examination will reveal a lichenoid lymphocytic infiltrate (predominantly around the hair follicle where the follicular stem cells reside), resulting in fibrosis and scarring.5
Rule out other causes of hair loss
In addition to confirming the diagnosis with histologic examination, you’ll also need to have ruled out the following conditions in the differential.
Alopecia areata may mimic the ophiasis (band-like) pattern of hair loss seen with FFA, but it is a non-scarring disorder that typically lacks any signs of inflammation.
Female pattern hair loss is characterized by a decrease in hair density and thinning. The condition is non-scarring and usually involves the frontal and vertex (crown) regions of the scalp.
Discoid lupus erythematosus is characterized by circular scarring hair loss with a central patch of inflammation, as well as depigmentation.
Central centrifugal cicatricial alopecia predominantly affects black women and is characterized by circular hair loss of the vertex, with perifollicular inflammation and scarring.
Traction alopecia can occur in the same location as FFA, but is not usually associated with perifollicular inflammation. This condition can cause scarring if traction has been longstanding and persistent. There is usually a history of certain hairstyles (such as braiding) associated with chronic tension on hair fibers.
Numerous Tx strategies exist, but they are not well studied
Because there are no published randomized clinical trials on treatment for FFA, few evidence-based treatment strategies exist.6 In addition, the prognosis is variable. Experts have employed numerous treatment strategies, including topical and intralesional steroids, immunosuppressive medications, antibiotics, and anti-androgen therapy, with varying results.4,6 For most primary care physicians, it’s best to refer patients to a dermatologist to initiate treatment.
Intralesional steroids such as triamcinolone acetonide (5-10 mg/cc), as well as high-potency topical steroids, are generally helpful to stabilize the disease. There is also some evidence of benefit from oral dutasteride or finasteride at variable doses.6 Immunosuppressants such as hydroxychloroquine may also be used as second-line treatments, although the benefit-to-risk ratio needs to be taken into consideration.7
Early detection is key. In general, treatment should be initiated as soon as possible to prevent disease progression and reduce permanent scarring and hair loss. The Lichen Planopilaris Activity Index7 is a tool that clinicians can use to measure disease severity and track changes in disease activity through patient report of symptoms and measurements of scalp inflammation.
Our patient was started on a regimen of topical high-potency steroids (clobetasol foam, 0.05%), with targeted, intralesional injection of steroids (10 mg/cc of triamcinolone acetonide) to areas with the most inflammation. The patient was advised to use ketoconazole 2% shampoo while showering.
These interventions decreased our patient’s symptoms dramatically. Her scalp erythema and scale improved, but the hair did not regrow. One year later, her hairline was clinically stable with no evidence of disease progression. She continues to see a dermatologist annually.
CORRESPONDENCE
David V. Power, MB, MPH, Department of Family Medicine and Community Health, University of Minnesota, 516 Delaware St. SE, Minneapolis, MN 55455; [email protected].
A 66-year-old white woman presented to her primary care clinic with concerns about hair loss, which began 2 years ago. Recently, she had noticed some “bumps” on her cheeks, as well.
On physical examination, the physician noted hair loss in a symmetric 2-cm band-like distribution across her frontal and temporal scalp (FIGURES 1 and 2). In both areas, there was moderate perifollicular erythema, scale, and what appeared to be scarring.

The patient had lost most of her eyebrow hairs, and had prominent temporal veins (FIGURE 2) and flesh-colored papules on her cheeks. She had no significant medical history, was emotionally stable, and recently had a satisfactory health care maintenance exam. The postmenopausal patient’s last menses was 15 years earlier, and she was not taking hormone replacement.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Frontal fibrosing alopecia
The patient was referred to our dermatology clinic, which specializes in hair loss. Based on the clinical findings, we suspected that this was a case of frontal fibrosing alopecia (FFA), a primary lymphocytic cicatricial (scarring) alopecia. A dermatopathologist confirmed the diagnosis via histologic review.
A condition on the rise. The incidence of FFA has been steadily increasing internationally since the condition was first described in 1994.1 Among patients referred to a specialty clinic for hair loss, diagnosis of FFA has increased from 1.6% in 2000 to 17% in 2011.2
FFA is characterized by symmetric band-like hair loss with evidence of scarring in the frontal and temporal regions of the scalp. (The extent of hair loss can be assessed by retracting the patient’s hair and having the patient raise his or her eyebrows and wrinkle the forehead in a surprised look.) FFA is accompanied by eyebrow loss in 73% to 95% of patients.2,3 Mild to severe perifollicular (and possibly more generalized) erythema and scale are usually present. In addition, erythematous or skin-colored papules may appear on the face,3 and marked exaggeration of the temporal veins is a common finding.
Most patients with FFA (83%) are postmenopausal women and nearly all (98.6%) have Fitzpatrick skin type 1 or 2 (white skin that burns easily and doesn’t readily tan).4 Other pertinent findings include the absence of oral lesions, nail changes, or other skin diseases.
A subtype of another condition? Because they are similar histologically, some consider FFA to be a subtype of lichen planopilaris. (See “Scarring alopecia in a woman with psoriasis,” J Fam Pract. 2015;64:E1-E3.)
A punch biopsy to confirm the diagnosis of FFA should be taken from the leading edge of the hair loss and, ideally, reviewed by a dermatopathologist. Histologic examination will reveal a lichenoid lymphocytic infiltrate (predominantly around the hair follicle where the follicular stem cells reside), resulting in fibrosis and scarring.5
Rule out other causes of hair loss
In addition to confirming the diagnosis with histologic examination, you’ll also need to have ruled out the following conditions in the differential.
Alopecia areata may mimic the ophiasis (band-like) pattern of hair loss seen with FFA, but it is a non-scarring disorder that typically lacks any signs of inflammation.
Female pattern hair loss is characterized by a decrease in hair density and thinning. The condition is non-scarring and usually involves the frontal and vertex (crown) regions of the scalp.
Discoid lupus erythematosus is characterized by circular scarring hair loss with a central patch of inflammation, as well as depigmentation.
Central centrifugal cicatricial alopecia predominantly affects black women and is characterized by circular hair loss of the vertex, with perifollicular inflammation and scarring.
Traction alopecia can occur in the same location as FFA, but is not usually associated with perifollicular inflammation. This condition can cause scarring if traction has been longstanding and persistent. There is usually a history of certain hairstyles (such as braiding) associated with chronic tension on hair fibers.
Numerous Tx strategies exist, but they are not well studied
Because there are no published randomized clinical trials on treatment for FFA, few evidence-based treatment strategies exist.6 In addition, the prognosis is variable. Experts have employed numerous treatment strategies, including topical and intralesional steroids, immunosuppressive medications, antibiotics, and anti-androgen therapy, with varying results.4,6 For most primary care physicians, it’s best to refer patients to a dermatologist to initiate treatment.
Intralesional steroids such as triamcinolone acetonide (5-10 mg/cc), as well as high-potency topical steroids, are generally helpful to stabilize the disease. There is also some evidence of benefit from oral dutasteride or finasteride at variable doses.6 Immunosuppressants such as hydroxychloroquine may also be used as second-line treatments, although the benefit-to-risk ratio needs to be taken into consideration.7
Early detection is key. In general, treatment should be initiated as soon as possible to prevent disease progression and reduce permanent scarring and hair loss. The Lichen Planopilaris Activity Index7 is a tool that clinicians can use to measure disease severity and track changes in disease activity through patient report of symptoms and measurements of scalp inflammation.
Our patient was started on a regimen of topical high-potency steroids (clobetasol foam, 0.05%), with targeted, intralesional injection of steroids (10 mg/cc of triamcinolone acetonide) to areas with the most inflammation. The patient was advised to use ketoconazole 2% shampoo while showering.
These interventions decreased our patient’s symptoms dramatically. Her scalp erythema and scale improved, but the hair did not regrow. One year later, her hairline was clinically stable with no evidence of disease progression. She continues to see a dermatologist annually.
CORRESPONDENCE
David V. Power, MB, MPH, Department of Family Medicine and Community Health, University of Minnesota, 516 Delaware St. SE, Minneapolis, MN 55455; [email protected].
1. Kossard S. Postmenopausal frontal fibrosing alopecia: Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
2. MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
3. Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
4. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
5. Poblet E, Jiménez F, Pascual A, et al. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45:375-380.
6. Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
7. Chiang C, Sah D, Cho BK, et al. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62:387-392.
1. Kossard S. Postmenopausal frontal fibrosing alopecia: Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
2. MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
3. Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755.
4. Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
5. Poblet E, Jiménez F, Pascual A, et al. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45:375-380.
6. Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
7. Chiang C, Sah D, Cho BK, et al. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62:387-392.
Active 46-year-old man with right-sided visual loss and no family history of stroke • Dx?
THE CASE
A 46-year-old man presented to the emergency department (ED) with sudden-onset right-sided visual loss. He had a history of asthma, but no family history of hypercoagulability, deep vein thrombosis (DVT), or stroke. The patient had an active lifestyle that included scuba diving, mountain biking, and hockey (coaching and playing). The physical examination revealed a right homonymous upper quadrantanopia. The neurologic examination was within normal limits, except for the visual deficit and unequal pupil size. A computerized tomography scan of the patient’s head did not reveal any lesions.
Based on the patient’s clinical picture, the ED physician prescribed alteplase, a tissue plasminogen activator (tPA), and admitted him to the intensive care unit for monitoring.
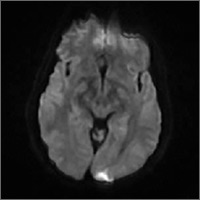
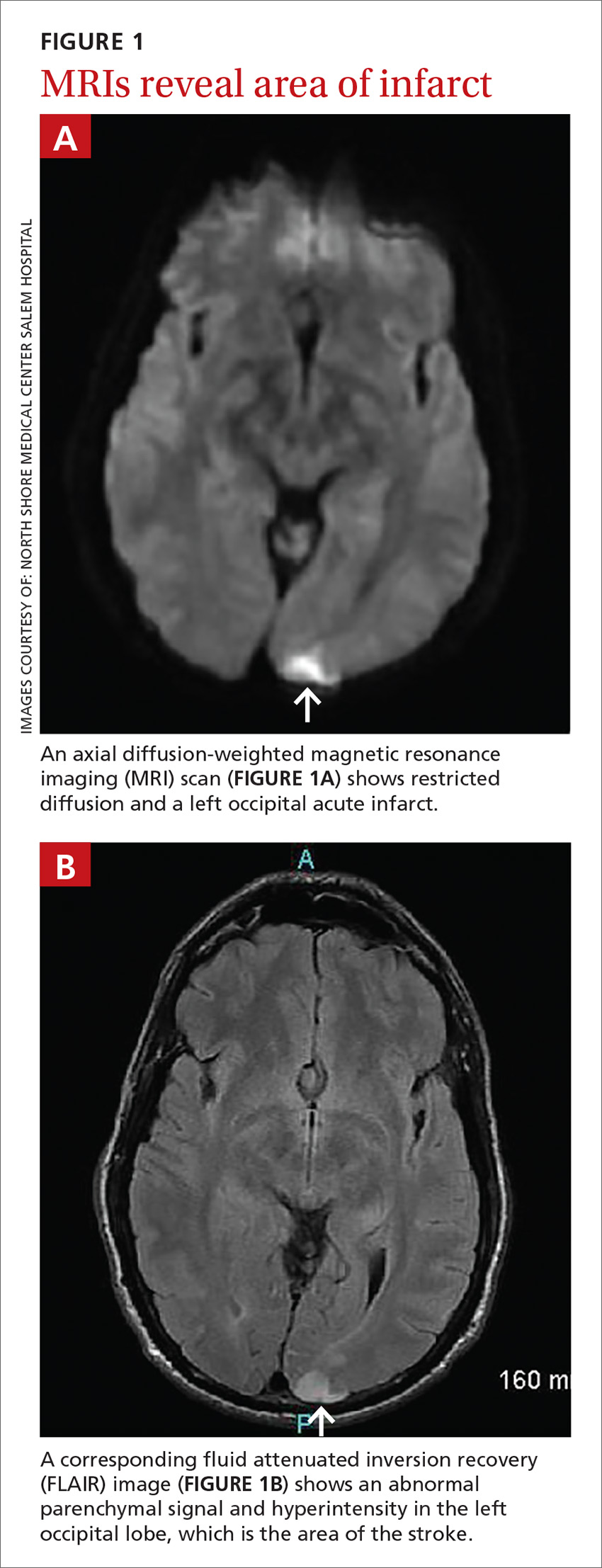
Subsequent magnetic resonance imaging (MRI) of the brain showed multiple small areas of acute infarct in the posterior circulation territory bilaterally, with involvement of small portions of the bilateral cerebellar hemispheres and parts of the left occipital lobe (FIGURE 1A and 1B).
An electrocardiogram showed no evidence of atrial fibrillation, and hypercoagulability studies were within normal limits. There was no evidence of May-Thurner anatomy, and an ultrasound of the lower extremities showed no DVT.
THE DIAGNOSIS
An echocardiogram with bubble study confirmed a diagnosis of patent foramen ovale (PFO) with bidirectional flow, a normal ejection fraction, and no evidence of left ventricular or left atrial thrombus. We started the patient on the anticoagulant enoxaparin 70 mg bid bridged with warfarin 5 mg/d.
Taking the patient’s active lifestyle into consideration, he was approved for PFO closure by the PFO committee and underwent closure. Following treatment, the patient was left with a residual 2-mm blind spot in the right visual field. At a 2-year follow-up visit, he showed no new focal deficits or recurrent symptoms.
DISCUSSION
Since 1988 when Lechat et al reported increased incidence of PFO in young stroke patients,1 many studies have supported the association between PFO and cryptogenic stroke (CS) in young adults.2 Because it remained controversial as to whether PFO is a risk factor for stroke or transient ischemic attack recurrence,3 researchers investigated PFO closure as a preventive measure to decrease stroke recurrence in patients with both CS and PFO.
A 2012 meta-analysis showed possible benefits of closure compared with medical management using antiplatelet or anticoagulation therapies.4 However, these results were not supported by results of other studies. These include the CLOSURE I trial,5 which compared device closure of PFO with medical therapy, and the RESPECT6 and PC trials,7 which did not show a significant difference in the primary end point of recurrent stroke between patients who received medical therapy and those who had PFO closure.
American Heart Association/American Stroke Association’s 2011 guidelines recommend only antiplatelet therapy for patients with CS and PFO.8 While there is consensus that surgical closure is not better than a medical approach to patients with CS and PFO, cases should be individualized, as a patient’s clinical or social factors may dictate otherwise.
Lifestyle may warrant PFO closure
No previous studies have considered occupation or hobbies as an indication for PFO closure in patients with CS. Our patient’s active lifestyle, particularly his scuba diving and participation in contact sports, made him a poor candidate for anticoagulation. Scuba diving is associated with decompression sickness and air emboli, which can be a mechanism of cerebral ischemia, especially in patients with a right-to-left shunt, such as with PFO.9
We did not observe a strong temporal relationship between diving and stroke in our patient. MRI findings suggested that he had multiple minor embolic events over time, which is consistent with a prior case report.9 This suggested air emboli as a possible source of stroke, in which case, our patient might not benefit from antiplatelet or anticoagulation therapy.
THE TAKEAWAY
This case illustrates the importance of a thorough social history and knowledge of the patient’s hobbies, occupation, and preferences in evaluating and treating individuals with CS associated with PFO. The current literature does not provide complete answers to the cause, diagnosis, and management of CS; additional research is needed.
The work-up involved in defining the etiology of stroke includes, but is not limited to, head and brain imaging, an echocardiogram, hypercoagulability tests, and vascular imaging. The work of Sanna et al showed that approximately 12% of patients with CS have atrial fibrillation when monitored over a one-year period, suggesting atrial fibrillation as a possible cause in some cases.10
As the case described here demonstrates, further research is warranted regarding how a patient’s occupation and lifestyle factor into decision-making for patients with PFO.
1. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152.
2. Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085-1096.
3. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol. 2010;257:1777-1787.
4. Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431.
5. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276.
9. Menkin M, Schwartzman RJ. Cerebral air embolism. Report of five cases and review of the literature. Arch Neurol. 1977;34:168-170.
10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
THE CASE
A 46-year-old man presented to the emergency department (ED) with sudden-onset right-sided visual loss. He had a history of asthma, but no family history of hypercoagulability, deep vein thrombosis (DVT), or stroke. The patient had an active lifestyle that included scuba diving, mountain biking, and hockey (coaching and playing). The physical examination revealed a right homonymous upper quadrantanopia. The neurologic examination was within normal limits, except for the visual deficit and unequal pupil size. A computerized tomography scan of the patient’s head did not reveal any lesions.
Based on the patient’s clinical picture, the ED physician prescribed alteplase, a tissue plasminogen activator (tPA), and admitted him to the intensive care unit for monitoring.
Subsequent magnetic resonance imaging (MRI) of the brain showed multiple small areas of acute infarct in the posterior circulation territory bilaterally, with involvement of small portions of the bilateral cerebellar hemispheres and parts of the left occipital lobe (FIGURE 1A and 1B).

An electrocardiogram showed no evidence of atrial fibrillation, and hypercoagulability studies were within normal limits. There was no evidence of May-Thurner anatomy, and an ultrasound of the lower extremities showed no DVT.
THE DIAGNOSIS
An echocardiogram with bubble study confirmed a diagnosis of patent foramen ovale (PFO) with bidirectional flow, a normal ejection fraction, and no evidence of left ventricular or left atrial thrombus. We started the patient on the anticoagulant enoxaparin 70 mg bid bridged with warfarin 5 mg/d.
Taking the patient’s active lifestyle into consideration, he was approved for PFO closure by the PFO committee and underwent closure. Following treatment, the patient was left with a residual 2-mm blind spot in the right visual field. At a 2-year follow-up visit, he showed no new focal deficits or recurrent symptoms.
DISCUSSION
Since 1988 when Lechat et al reported increased incidence of PFO in young stroke patients,1 many studies have supported the association between PFO and cryptogenic stroke (CS) in young adults.2 Because it remained controversial as to whether PFO is a risk factor for stroke or transient ischemic attack recurrence,3 researchers investigated PFO closure as a preventive measure to decrease stroke recurrence in patients with both CS and PFO.
A 2012 meta-analysis showed possible benefits of closure compared with medical management using antiplatelet or anticoagulation therapies.4 However, these results were not supported by results of other studies. These include the CLOSURE I trial,5 which compared device closure of PFO with medical therapy, and the RESPECT6 and PC trials,7 which did not show a significant difference in the primary end point of recurrent stroke between patients who received medical therapy and those who had PFO closure.
American Heart Association/American Stroke Association’s 2011 guidelines recommend only antiplatelet therapy for patients with CS and PFO.8 While there is consensus that surgical closure is not better than a medical approach to patients with CS and PFO, cases should be individualized, as a patient’s clinical or social factors may dictate otherwise.
Lifestyle may warrant PFO closure
No previous studies have considered occupation or hobbies as an indication for PFO closure in patients with CS. Our patient’s active lifestyle, particularly his scuba diving and participation in contact sports, made him a poor candidate for anticoagulation. Scuba diving is associated with decompression sickness and air emboli, which can be a mechanism of cerebral ischemia, especially in patients with a right-to-left shunt, such as with PFO.9
We did not observe a strong temporal relationship between diving and stroke in our patient. MRI findings suggested that he had multiple minor embolic events over time, which is consistent with a prior case report.9 This suggested air emboli as a possible source of stroke, in which case, our patient might not benefit from antiplatelet or anticoagulation therapy.
THE TAKEAWAY
This case illustrates the importance of a thorough social history and knowledge of the patient’s hobbies, occupation, and preferences in evaluating and treating individuals with CS associated with PFO. The current literature does not provide complete answers to the cause, diagnosis, and management of CS; additional research is needed.
The work-up involved in defining the etiology of stroke includes, but is not limited to, head and brain imaging, an echocardiogram, hypercoagulability tests, and vascular imaging. The work of Sanna et al showed that approximately 12% of patients with CS have atrial fibrillation when monitored over a one-year period, suggesting atrial fibrillation as a possible cause in some cases.10
As the case described here demonstrates, further research is warranted regarding how a patient’s occupation and lifestyle factor into decision-making for patients with PFO.
THE CASE
A 46-year-old man presented to the emergency department (ED) with sudden-onset right-sided visual loss. He had a history of asthma, but no family history of hypercoagulability, deep vein thrombosis (DVT), or stroke. The patient had an active lifestyle that included scuba diving, mountain biking, and hockey (coaching and playing). The physical examination revealed a right homonymous upper quadrantanopia. The neurologic examination was within normal limits, except for the visual deficit and unequal pupil size. A computerized tomography scan of the patient’s head did not reveal any lesions.
Based on the patient’s clinical picture, the ED physician prescribed alteplase, a tissue plasminogen activator (tPA), and admitted him to the intensive care unit for monitoring.
Subsequent magnetic resonance imaging (MRI) of the brain showed multiple small areas of acute infarct in the posterior circulation territory bilaterally, with involvement of small portions of the bilateral cerebellar hemispheres and parts of the left occipital lobe (FIGURE 1A and 1B).

An electrocardiogram showed no evidence of atrial fibrillation, and hypercoagulability studies were within normal limits. There was no evidence of May-Thurner anatomy, and an ultrasound of the lower extremities showed no DVT.
THE DIAGNOSIS
An echocardiogram with bubble study confirmed a diagnosis of patent foramen ovale (PFO) with bidirectional flow, a normal ejection fraction, and no evidence of left ventricular or left atrial thrombus. We started the patient on the anticoagulant enoxaparin 70 mg bid bridged with warfarin 5 mg/d.
Taking the patient’s active lifestyle into consideration, he was approved for PFO closure by the PFO committee and underwent closure. Following treatment, the patient was left with a residual 2-mm blind spot in the right visual field. At a 2-year follow-up visit, he showed no new focal deficits or recurrent symptoms.
DISCUSSION
Since 1988 when Lechat et al reported increased incidence of PFO in young stroke patients,1 many studies have supported the association between PFO and cryptogenic stroke (CS) in young adults.2 Because it remained controversial as to whether PFO is a risk factor for stroke or transient ischemic attack recurrence,3 researchers investigated PFO closure as a preventive measure to decrease stroke recurrence in patients with both CS and PFO.
A 2012 meta-analysis showed possible benefits of closure compared with medical management using antiplatelet or anticoagulation therapies.4 However, these results were not supported by results of other studies. These include the CLOSURE I trial,5 which compared device closure of PFO with medical therapy, and the RESPECT6 and PC trials,7 which did not show a significant difference in the primary end point of recurrent stroke between patients who received medical therapy and those who had PFO closure.
American Heart Association/American Stroke Association’s 2011 guidelines recommend only antiplatelet therapy for patients with CS and PFO.8 While there is consensus that surgical closure is not better than a medical approach to patients with CS and PFO, cases should be individualized, as a patient’s clinical or social factors may dictate otherwise.
Lifestyle may warrant PFO closure
No previous studies have considered occupation or hobbies as an indication for PFO closure in patients with CS. Our patient’s active lifestyle, particularly his scuba diving and participation in contact sports, made him a poor candidate for anticoagulation. Scuba diving is associated with decompression sickness and air emboli, which can be a mechanism of cerebral ischemia, especially in patients with a right-to-left shunt, such as with PFO.9
We did not observe a strong temporal relationship between diving and stroke in our patient. MRI findings suggested that he had multiple minor embolic events over time, which is consistent with a prior case report.9 This suggested air emboli as a possible source of stroke, in which case, our patient might not benefit from antiplatelet or anticoagulation therapy.
THE TAKEAWAY
This case illustrates the importance of a thorough social history and knowledge of the patient’s hobbies, occupation, and preferences in evaluating and treating individuals with CS associated with PFO. The current literature does not provide complete answers to the cause, diagnosis, and management of CS; additional research is needed.
The work-up involved in defining the etiology of stroke includes, but is not limited to, head and brain imaging, an echocardiogram, hypercoagulability tests, and vascular imaging. The work of Sanna et al showed that approximately 12% of patients with CS have atrial fibrillation when monitored over a one-year period, suggesting atrial fibrillation as a possible cause in some cases.10
As the case described here demonstrates, further research is warranted regarding how a patient’s occupation and lifestyle factor into decision-making for patients with PFO.
1. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152.
2. Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085-1096.
3. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol. 2010;257:1777-1787.
4. Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431.
5. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276.
9. Menkin M, Schwartzman RJ. Cerebral air embolism. Report of five cases and review of the literature. Arch Neurol. 1977;34:168-170.
10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
1. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152.
2. Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085-1096.
3. Cotter PE, Belham M, Martin PJ. Stroke in younger patients: the heart of the matter. J Neurol. 2010;257:1777-1787.
4. Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422-431.
5. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999.
6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100.
7. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091.
8. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227-276.
9. Menkin M, Schwartzman RJ. Cerebral air embolism. Report of five cases and review of the literature. Arch Neurol. 1977;34:168-170.
10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478-2486.
Radiation therapy: Managing GI tract complications
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.
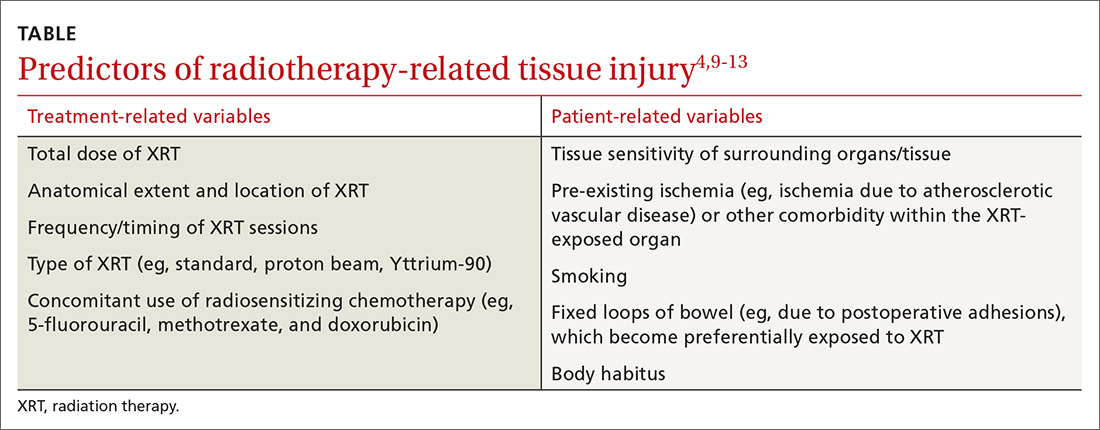
Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; [email protected].
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.

Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; [email protected].
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.

Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; [email protected].
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
PRACTICE RECOMMENDATIONS
› Correlate the patient’s symptoms with the radiation therapy history to determine if the onset, anatomical location, and nature of the symptoms suggest a (causal) relationship. B
› Refer patients for radiographic, endoscopic, or other diagnostic modalities according to the suspected pathology and treat (eg, pharmacologically, endoscopically, or surgically) when possible. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Direct oral anticoagulants or warfarin for A fib?
ILLUSTRATIVE CASE
A 66-year-old man with a history of hypertension and diabetes mellitus type 2 is hospitalized for palpitations and dizziness, and is given a diagnosis of atrial fibrillation (AF). His heart rate is successfully controlled with a beta-blocker. His CHA2DS2-VASc score is 3, meaning he is a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with AF results in stroke and death and can be decreased with appropriate use of antithrombotic therapy. Evidence-based guidelines recommend patients with AF at intermediate or high risk of stroke (CHADS2 score ≥ 2 or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends the use of the direct oral anticoagulant (DOAC) dabigatran over warfarin for those patients with nonvalvular AF with an estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) of individual DOACs (dabigatran [a direct thrombin inhibitor], rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk=1.25; 95% CI, 1.01 to 1.55) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, 3 separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications result in a significant reduction in embolic stroke and reduced the risk of major bleeds and hemorrhagic stroke when compared with warfarin.5-7
However, we know less about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any of these agents, are superior to others. Moreover, only about half of the patients in the United States with AF who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
One DOAC is better than warfarin at one thing; 2 others are better at another
This large cohort study examined the effectiveness of 3 DOACs compared with warfarin in 61,678 patients with AF by combining data from 3 Danish national databases. The patients had newly diagnosed AF (without valvular disease or venous thromboembolism) and were prescribed standard doses of DOACs (dabigatran 150 bid [N=12,701], rivaroxaban 20 mg/d [N=7192], apixaban 5 mg bid [N=6349]) or dose-adjusted warfarin to an INR goal of 2 to 3 (N=35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1702 ischemic strokes or systemic emboli. The incidence of ischemic stroke or systemic embolism was either the same or better for each of the 3 DOAC treatments than for warfarin (DOACs, 2.9-3.9 events per 100 person-years; warfarin, 3.3 events per 100 person-years; no P value provided). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group compared with warfarin at one year (hazard ratio [HR]=0.83; 95% confidence interval [CI], 0.69-0.99) and after 2.5 years (HR=0.80; 95% CI, 0.69-0.94). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at one year and 2.5 years.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR=0.63; 95% CI, 0.53-0.76) and dabigatran group (HR=0.61; 95% CI, 0.51-0.74) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk of death. Compared with warfarin, the risk of death after one year of treatment was lower in the apixaban (HR=0.65; 95% CI, 0.56-0.75) and dabigatran (HR=0.63; 95% CI, 0.48-0.82) groups, and there was no significant difference in the rivaroxaban group (HR=0.92; 95% CI, 0.82-1.03).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, and that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk of bleeding than warfarin.
CAVEATS
This non-randomized cohort trial lacked INR data
This study was a non-randomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin was not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. We feel that this study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
ILLUSTRATIVE CASE
A 66-year-old man with a history of hypertension and diabetes mellitus type 2 is hospitalized for palpitations and dizziness, and is given a diagnosis of atrial fibrillation (AF). His heart rate is successfully controlled with a beta-blocker. His CHA2DS2-VASc score is 3, meaning he is a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with AF results in stroke and death and can be decreased with appropriate use of antithrombotic therapy. Evidence-based guidelines recommend patients with AF at intermediate or high risk of stroke (CHADS2 score ≥ 2 or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends the use of the direct oral anticoagulant (DOAC) dabigatran over warfarin for those patients with nonvalvular AF with an estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) of individual DOACs (dabigatran [a direct thrombin inhibitor], rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk=1.25; 95% CI, 1.01 to 1.55) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, 3 separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications result in a significant reduction in embolic stroke and reduced the risk of major bleeds and hemorrhagic stroke when compared with warfarin.5-7
However, we know less about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any of these agents, are superior to others. Moreover, only about half of the patients in the United States with AF who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
One DOAC is better than warfarin at one thing; 2 others are better at another
This large cohort study examined the effectiveness of 3 DOACs compared with warfarin in 61,678 patients with AF by combining data from 3 Danish national databases. The patients had newly diagnosed AF (without valvular disease or venous thromboembolism) and were prescribed standard doses of DOACs (dabigatran 150 bid [N=12,701], rivaroxaban 20 mg/d [N=7192], apixaban 5 mg bid [N=6349]) or dose-adjusted warfarin to an INR goal of 2 to 3 (N=35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1702 ischemic strokes or systemic emboli. The incidence of ischemic stroke or systemic embolism was either the same or better for each of the 3 DOAC treatments than for warfarin (DOACs, 2.9-3.9 events per 100 person-years; warfarin, 3.3 events per 100 person-years; no P value provided). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group compared with warfarin at one year (hazard ratio [HR]=0.83; 95% confidence interval [CI], 0.69-0.99) and after 2.5 years (HR=0.80; 95% CI, 0.69-0.94). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at one year and 2.5 years.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR=0.63; 95% CI, 0.53-0.76) and dabigatran group (HR=0.61; 95% CI, 0.51-0.74) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk of death. Compared with warfarin, the risk of death after one year of treatment was lower in the apixaban (HR=0.65; 95% CI, 0.56-0.75) and dabigatran (HR=0.63; 95% CI, 0.48-0.82) groups, and there was no significant difference in the rivaroxaban group (HR=0.92; 95% CI, 0.82-1.03).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, and that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk of bleeding than warfarin.
CAVEATS
This non-randomized cohort trial lacked INR data
This study was a non-randomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin was not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. We feel that this study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 66-year-old man with a history of hypertension and diabetes mellitus type 2 is hospitalized for palpitations and dizziness, and is given a diagnosis of atrial fibrillation (AF). His heart rate is successfully controlled with a beta-blocker. His CHA2DS2-VASc score is 3, meaning he is a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with AF results in stroke and death and can be decreased with appropriate use of antithrombotic therapy. Evidence-based guidelines recommend patients with AF at intermediate or high risk of stroke (CHADS2 score ≥ 2 or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends the use of the direct oral anticoagulant (DOAC) dabigatran over warfarin for those patients with nonvalvular AF with an estimated glomerular filtration rate (eGFR) ≥15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) of individual DOACs (dabigatran [a direct thrombin inhibitor], rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk=1.25; 95% CI, 1.01 to 1.55) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, 3 separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications result in a significant reduction in embolic stroke and reduced the risk of major bleeds and hemorrhagic stroke when compared with warfarin.5-7
However, we know less about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any of these agents, are superior to others. Moreover, only about half of the patients in the United States with AF who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
One DOAC is better than warfarin at one thing; 2 others are better at another
This large cohort study examined the effectiveness of 3 DOACs compared with warfarin in 61,678 patients with AF by combining data from 3 Danish national databases. The patients had newly diagnosed AF (without valvular disease or venous thromboembolism) and were prescribed standard doses of DOACs (dabigatran 150 bid [N=12,701], rivaroxaban 20 mg/d [N=7192], apixaban 5 mg bid [N=6349]) or dose-adjusted warfarin to an INR goal of 2 to 3 (N=35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1702 ischemic strokes or systemic emboli. The incidence of ischemic stroke or systemic embolism was either the same or better for each of the 3 DOAC treatments than for warfarin (DOACs, 2.9-3.9 events per 100 person-years; warfarin, 3.3 events per 100 person-years; no P value provided). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group compared with warfarin at one year (hazard ratio [HR]=0.83; 95% confidence interval [CI], 0.69-0.99) and after 2.5 years (HR=0.80; 95% CI, 0.69-0.94). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at one year and 2.5 years.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR=0.63; 95% CI, 0.53-0.76) and dabigatran group (HR=0.61; 95% CI, 0.51-0.74) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk of death. Compared with warfarin, the risk of death after one year of treatment was lower in the apixaban (HR=0.65; 95% CI, 0.56-0.75) and dabigatran (HR=0.63; 95% CI, 0.48-0.82) groups, and there was no significant difference in the rivaroxaban group (HR=0.92; 95% CI, 0.82-1.03).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, and that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk of bleeding than warfarin.
CAVEATS
This non-randomized cohort trial lacked INR data
This study was a non-randomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin was not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. We feel that this study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Use direct oral anticoagulants instead of warfarin in patients with atrial fibrillation because they are just as effective at preventing ischemic stroke and systemic emboli as warfarin, and because apixaban and dabigatran have lower bleeding rates.
STRENGTH OF RECOMMENDATION
B: Based on a single, prospective, cohort study.
Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.1
Obsessive-compulsive disorder: Under-recognized and responsive to treatment
THE CASE
Ms. L is a 26-year-old woman in acute distress because of a recurrent thought. She worries: “What if I sexually molest my son?” She says she has no desire to act on this recurring thought and recognizes that it is unlikely to be true. The thought is so upsetting, however, that she has begun having panic attacks and avoids being left alone with her child.
She also reports past episodes of thoughts that she may be homosexual and thoughts that something catastrophic happened without her awareness (eg, that she unknowingly ran over someone while driving). She has attempted self-management, including trying to reason with herself, trying to stop thinking the thoughts, and seeking reassurance from her boyfriend and medical providers.
These compulsive behaviors lowered her distress temporarily, thereby reinforcing her need to check and seek reassurance.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with a 12-month prevalence of 1.2% in the United States and internationally.1 Like other psychiatric disorders, patients with OCD present more often to primary care than specialty settings.2 Despite high distress and impairment levels, individuals with OCD are often undiagnosed and do not receive evidence-based care.3,4 This can be particularly problematic in fast-paced primary care settings due to high medical utilization and increased costs associated with OCD.5
A time-consuming disorder associated with distress
OCD is characterized by obsessive thoughts and/or compulsions.1
Obsessions are repeated, unwanted, distressing thoughts or images (eg, of being contaminated by dirt/germs, fears of causing harm to others without wanting to). Individuals with OCD attempt to avoid these thoughts by suppressing or neutralizing them.
Compulsions are mental or behavioral rituals that the individual feels compelled to perform to reduce distress or prevent a feared consequence (eg, hand-washing, checking locks, counting). Compulsions are not reasonable safety efforts, but are instead out-of-proportion reactions to the situation.
Onset of OCD usually occurs by young adulthood, but may be present in children. Pregnancy and postpartum periods may be associated with increased risk for symptoms.6 The course is typically chronic if left untreated, although symptoms can occur episodically.1
Intrusive thoughts and compulsive behaviors are surprisingly common in the general population. One study found that most individuals in a non-clinical sample reported having occasional intrusive thoughts such as whether they may have accidentally left the stove on, running their car off the road, or engaging in a “disgusting” sex act.7 With OCD, however, obsessions and compulsions are time-consuming and associated with distress and/or impairment.1
For example, an individual with OCD may restrict their diet due to fears of handling foods that other people may have touched or may limit contact with people for fear they will lose control and act violently. This is partly the result of overestimating the significance of the thoughts.8 Individuals with OCD may believe that the thoughts mean something negative about them (eg, that they are immoral) or could lead to serious consequences (eg, thinking about a car accident makes it more likely to occur).
Distinguishing features of OCD
OCD is commonly misdiagnosed,9 which may contribute to the long duration of untreated illness (average 17 years).10 In one study, primary care providers were given vignettes describing OCD symptoms; half of these cases were misidentified.9 Certain types of obsessions (eg, aggression, fear of saying certain things, homosexuality, pedophilia) were misdiagnosed 70% to 85% of the time.9
Although OCD shares characteristics with other disorders, several features can help family physicians correctly identify OCD. Fears associated with OCD are usually not about everyday concerns or worries. For example, a patient with social anxiety disorder may report fear of embarrassing themselves in public, whereas a patient with OCD may report fear that they will lose control and do something outlandish such as start swearing loudly.
Additionally, obsessions and compulsions in OCD are not exclusively tied to a traumatic experience as in posttraumatic stress disorder. Someone with OCD who has harm-related or sexual obsessions (eg, homosexuality) will report that this is not consistent with their interests and desires. Furthermore, a small subset of people with OCD may have poor insight, meaning they have low self-awareness of the nature of their obsessions or compulsions, but they do not experience psychotic symptoms.
How to make the diagnosis
A clinical interview is an essential component of assessing OCD in primary care. Ask patients who have mood or anxiety concerns about OCD symptoms, due to the high comorbidity rates of these entities.1 If a family history of OCD is known, assess the patient for this disorder, as higher rates exist among family members.10 Although primary care providers should indeed screen for OCD and provide provisional diagnoses as warranted, additional assessment by a behavioral health practitioner is recommended, given their specialty training in this area.
Patients with OCD are often reluctant to disclose intrusive thoughts due to perceiving them as shameful or unacceptable. Consider asking direct questions to facilitate the evaluation:
- “Do you ever feel bothered by unwanted or unusual thoughts that you cannot get out of your mind even though you try to?”
- “Do you feel that you have to do anything to get rid of these thoughts or prevent something bad from happening?”
- “Will you feel very uncomfortable if you don’t do something a specific way?”
Evidence-based self-report measures are also available. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS)11 has become the gold standard psychometric measure for OCD. The updated version (Y-BOCS-II)12 and child/adolescent version (CY-BOCS)11 are also available.
Treatment: A tandem approach is most effective
Patient characteristics (eg, comorbid depression, level of adherence to treatment) may also help guide prescribing choices.10 For patients not responding to pharmacologic treatment in 8 to 12 weeks, consider referral to a psychiatrist.14
For OCD, CBT provided by a trained specialist typically involves exposure with response prevention (ERP). This entails confronting difficult thoughts and feared situations through exposure therapy and learning to reduce compulsive and excessive safety behaviors (eg, thinking about being contaminated by germs and then refraining from washing hands). Research suggests that CBT with ERP produces outcomes equivalent or superior to those achieved with pharmacotherapy.13 In addition to finding large effect sizes, clinical trials have demonstrated a treatment response of 86% in those completing CBT with ERP, compared with 48% of those receiving clomipramine.15 And Y-BOCS symptom scores have been reduced by 50% to 60% with CBT and ERP.16
How best to navigate coordination-of-care issues
When selecting a psychotherapy treatment provider for a patient with OCD, ask whether they are trained in ERP. If a trained psychotherapist is not available in the local health care system, you may refer to the International OCD Foundation (iocdf.org), which maintains an online directory of psychotherapists specializing in OCD.
Primary care physicians can also work with psychiatrists or psychotherapy providers to develop shared treatment plans. Part of this plan may involve reducing excess medical utilization and checking/reassurance (eg, requesting repeat medical tests). When there is concern about safety issues (eg, intrusive homicidal or suicidal thoughts), a risk assessment is strongly recommended.
CASE
An on-site psychologist evaluated Ms. L and diagnosed OCD. Ms. L had talked to the doctor about the possibility of medication, but she preferred to try behavioral treatment first. Ms. L agreed to participate in CBT with ERP. Treatment included imaginal and situational exposure exercises to decrease emotional reactivity associated with the thoughts, and to challenge beliefs that the thoughts are meaningful (eg, that having the thought means that she may act on it or that she is an unfit mother).
For example, Ms. L practiced repeating the thoughts aloud and going into feared situations (eg, being alone with her child). This was paired with response prevention, meaning that Ms. L was instructed to avoid checking with, or seeking, reassurance from others. She engaged in 4 ERP sessions in the primary care setting, and treatment led to significant symptom improvement that was maintained at follow-up with her primary care provider 6 and 12 months later.
CORRESPONDENCE
Jared L. Skillings, PhD, ABPP, Division of Psychiatry and Behavioral Medicine, Spectrum Health System, 2750 East Beltline NE, Grand Rapids, MI 49525; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22:344-350.
3. Glazier K, Calizte RM, Rothschild R, et al. High rates of OCD symptom misidentification by mental health professionals. Ann Clin Psychiatry. 2013;25:201-209.
4. Schwartz C, Schlegl S, Kuelz AK, et al. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsessive Compuls Relat Disord. 2013;2:448-456.
5. Marciniak M, Lage MJ, Landbloom RP, et al. Medical and productivity costs of anxiety disorders: case control study. Depress Anxiety. 2004;19:112-120.
6. Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061-1068.
7. Purdon C, Clark DA. Obsessive intrusive thoughts in nonclinical subjects. Part I. Content and relation with depressive, anxious and obsessional symptoms. Behav Res Ther. 1993;31:713-720.
8. Clark DA. Cognitive-Behavioral Therapy for OCD. New York: The Guilford Press; 2002.
9. Glazier K, Swing M, McGinn LK. Half of obsessive-compulsive disorder cases misdiagnosed: vignette-based survey of primary care physicians. J Clin Psychiatry. 2015;26:e761-e767.
10. Fineberg NA, Reghunandanan S, Simpson HB, et al. Obsessive-compulsive disorder (OCD): practical strategies for pharmacological and somatic treatment in adults. Psych Res. 2015;227:114-125.
11. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006-1011.
12. Storch EA, Larson MJ, Goodman WK, et al. Development and psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale—second edition. Psychol Assess. 2010;22:223-232.
13. Katzman MA, Bleau P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder in primary care. Int J Psychiatry Clin. 2012;16:77-84.
15. Foa FB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
16. Abramowitz JS. The psychological treatment of obsessive compulsive disorder. Can J Psychiatry. 2006;51:407-416.
THE CASE
Ms. L is a 26-year-old woman in acute distress because of a recurrent thought. She worries: “What if I sexually molest my son?” She says she has no desire to act on this recurring thought and recognizes that it is unlikely to be true. The thought is so upsetting, however, that she has begun having panic attacks and avoids being left alone with her child.
She also reports past episodes of thoughts that she may be homosexual and thoughts that something catastrophic happened without her awareness (eg, that she unknowingly ran over someone while driving). She has attempted self-management, including trying to reason with herself, trying to stop thinking the thoughts, and seeking reassurance from her boyfriend and medical providers.
These compulsive behaviors lowered her distress temporarily, thereby reinforcing her need to check and seek reassurance.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with a 12-month prevalence of 1.2% in the United States and internationally.1 Like other psychiatric disorders, patients with OCD present more often to primary care than specialty settings.2 Despite high distress and impairment levels, individuals with OCD are often undiagnosed and do not receive evidence-based care.3,4 This can be particularly problematic in fast-paced primary care settings due to high medical utilization and increased costs associated with OCD.5
A time-consuming disorder associated with distress
OCD is characterized by obsessive thoughts and/or compulsions.1
Obsessions are repeated, unwanted, distressing thoughts or images (eg, of being contaminated by dirt/germs, fears of causing harm to others without wanting to). Individuals with OCD attempt to avoid these thoughts by suppressing or neutralizing them.
Compulsions are mental or behavioral rituals that the individual feels compelled to perform to reduce distress or prevent a feared consequence (eg, hand-washing, checking locks, counting). Compulsions are not reasonable safety efforts, but are instead out-of-proportion reactions to the situation.
Onset of OCD usually occurs by young adulthood, but may be present in children. Pregnancy and postpartum periods may be associated with increased risk for symptoms.6 The course is typically chronic if left untreated, although symptoms can occur episodically.1
Intrusive thoughts and compulsive behaviors are surprisingly common in the general population. One study found that most individuals in a non-clinical sample reported having occasional intrusive thoughts such as whether they may have accidentally left the stove on, running their car off the road, or engaging in a “disgusting” sex act.7 With OCD, however, obsessions and compulsions are time-consuming and associated with distress and/or impairment.1
For example, an individual with OCD may restrict their diet due to fears of handling foods that other people may have touched or may limit contact with people for fear they will lose control and act violently. This is partly the result of overestimating the significance of the thoughts.8 Individuals with OCD may believe that the thoughts mean something negative about them (eg, that they are immoral) or could lead to serious consequences (eg, thinking about a car accident makes it more likely to occur).
Distinguishing features of OCD
OCD is commonly misdiagnosed,9 which may contribute to the long duration of untreated illness (average 17 years).10 In one study, primary care providers were given vignettes describing OCD symptoms; half of these cases were misidentified.9 Certain types of obsessions (eg, aggression, fear of saying certain things, homosexuality, pedophilia) were misdiagnosed 70% to 85% of the time.9
Although OCD shares characteristics with other disorders, several features can help family physicians correctly identify OCD. Fears associated with OCD are usually not about everyday concerns or worries. For example, a patient with social anxiety disorder may report fear of embarrassing themselves in public, whereas a patient with OCD may report fear that they will lose control and do something outlandish such as start swearing loudly.
Additionally, obsessions and compulsions in OCD are not exclusively tied to a traumatic experience as in posttraumatic stress disorder. Someone with OCD who has harm-related or sexual obsessions (eg, homosexuality) will report that this is not consistent with their interests and desires. Furthermore, a small subset of people with OCD may have poor insight, meaning they have low self-awareness of the nature of their obsessions or compulsions, but they do not experience psychotic symptoms.
How to make the diagnosis
A clinical interview is an essential component of assessing OCD in primary care. Ask patients who have mood or anxiety concerns about OCD symptoms, due to the high comorbidity rates of these entities.1 If a family history of OCD is known, assess the patient for this disorder, as higher rates exist among family members.10 Although primary care providers should indeed screen for OCD and provide provisional diagnoses as warranted, additional assessment by a behavioral health practitioner is recommended, given their specialty training in this area.
Patients with OCD are often reluctant to disclose intrusive thoughts due to perceiving them as shameful or unacceptable. Consider asking direct questions to facilitate the evaluation:
- “Do you ever feel bothered by unwanted or unusual thoughts that you cannot get out of your mind even though you try to?”
- “Do you feel that you have to do anything to get rid of these thoughts or prevent something bad from happening?”
- “Will you feel very uncomfortable if you don’t do something a specific way?”
Evidence-based self-report measures are also available. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS)11 has become the gold standard psychometric measure for OCD. The updated version (Y-BOCS-II)12 and child/adolescent version (CY-BOCS)11 are also available.
Treatment: A tandem approach is most effective
Patient characteristics (eg, comorbid depression, level of adherence to treatment) may also help guide prescribing choices.10 For patients not responding to pharmacologic treatment in 8 to 12 weeks, consider referral to a psychiatrist.14
For OCD, CBT provided by a trained specialist typically involves exposure with response prevention (ERP). This entails confronting difficult thoughts and feared situations through exposure therapy and learning to reduce compulsive and excessive safety behaviors (eg, thinking about being contaminated by germs and then refraining from washing hands). Research suggests that CBT with ERP produces outcomes equivalent or superior to those achieved with pharmacotherapy.13 In addition to finding large effect sizes, clinical trials have demonstrated a treatment response of 86% in those completing CBT with ERP, compared with 48% of those receiving clomipramine.15 And Y-BOCS symptom scores have been reduced by 50% to 60% with CBT and ERP.16
How best to navigate coordination-of-care issues
When selecting a psychotherapy treatment provider for a patient with OCD, ask whether they are trained in ERP. If a trained psychotherapist is not available in the local health care system, you may refer to the International OCD Foundation (iocdf.org), which maintains an online directory of psychotherapists specializing in OCD.
Primary care physicians can also work with psychiatrists or psychotherapy providers to develop shared treatment plans. Part of this plan may involve reducing excess medical utilization and checking/reassurance (eg, requesting repeat medical tests). When there is concern about safety issues (eg, intrusive homicidal or suicidal thoughts), a risk assessment is strongly recommended.
CASE
An on-site psychologist evaluated Ms. L and diagnosed OCD. Ms. L had talked to the doctor about the possibility of medication, but she preferred to try behavioral treatment first. Ms. L agreed to participate in CBT with ERP. Treatment included imaginal and situational exposure exercises to decrease emotional reactivity associated with the thoughts, and to challenge beliefs that the thoughts are meaningful (eg, that having the thought means that she may act on it or that she is an unfit mother).
For example, Ms. L practiced repeating the thoughts aloud and going into feared situations (eg, being alone with her child). This was paired with response prevention, meaning that Ms. L was instructed to avoid checking with, or seeking, reassurance from others. She engaged in 4 ERP sessions in the primary care setting, and treatment led to significant symptom improvement that was maintained at follow-up with her primary care provider 6 and 12 months later.
CORRESPONDENCE
Jared L. Skillings, PhD, ABPP, Division of Psychiatry and Behavioral Medicine, Spectrum Health System, 2750 East Beltline NE, Grand Rapids, MI 49525; [email protected].
THE CASE
Ms. L is a 26-year-old woman in acute distress because of a recurrent thought. She worries: “What if I sexually molest my son?” She says she has no desire to act on this recurring thought and recognizes that it is unlikely to be true. The thought is so upsetting, however, that she has begun having panic attacks and avoids being left alone with her child.
She also reports past episodes of thoughts that she may be homosexual and thoughts that something catastrophic happened without her awareness (eg, that she unknowingly ran over someone while driving). She has attempted self-management, including trying to reason with herself, trying to stop thinking the thoughts, and seeking reassurance from her boyfriend and medical providers.
These compulsive behaviors lowered her distress temporarily, thereby reinforcing her need to check and seek reassurance.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with a 12-month prevalence of 1.2% in the United States and internationally.1 Like other psychiatric disorders, patients with OCD present more often to primary care than specialty settings.2 Despite high distress and impairment levels, individuals with OCD are often undiagnosed and do not receive evidence-based care.3,4 This can be particularly problematic in fast-paced primary care settings due to high medical utilization and increased costs associated with OCD.5
A time-consuming disorder associated with distress
OCD is characterized by obsessive thoughts and/or compulsions.1
Obsessions are repeated, unwanted, distressing thoughts or images (eg, of being contaminated by dirt/germs, fears of causing harm to others without wanting to). Individuals with OCD attempt to avoid these thoughts by suppressing or neutralizing them.
Compulsions are mental or behavioral rituals that the individual feels compelled to perform to reduce distress or prevent a feared consequence (eg, hand-washing, checking locks, counting). Compulsions are not reasonable safety efforts, but are instead out-of-proportion reactions to the situation.
Onset of OCD usually occurs by young adulthood, but may be present in children. Pregnancy and postpartum periods may be associated with increased risk for symptoms.6 The course is typically chronic if left untreated, although symptoms can occur episodically.1
Intrusive thoughts and compulsive behaviors are surprisingly common in the general population. One study found that most individuals in a non-clinical sample reported having occasional intrusive thoughts such as whether they may have accidentally left the stove on, running their car off the road, or engaging in a “disgusting” sex act.7 With OCD, however, obsessions and compulsions are time-consuming and associated with distress and/or impairment.1
For example, an individual with OCD may restrict their diet due to fears of handling foods that other people may have touched or may limit contact with people for fear they will lose control and act violently. This is partly the result of overestimating the significance of the thoughts.8 Individuals with OCD may believe that the thoughts mean something negative about them (eg, that they are immoral) or could lead to serious consequences (eg, thinking about a car accident makes it more likely to occur).
Distinguishing features of OCD
OCD is commonly misdiagnosed,9 which may contribute to the long duration of untreated illness (average 17 years).10 In one study, primary care providers were given vignettes describing OCD symptoms; half of these cases were misidentified.9 Certain types of obsessions (eg, aggression, fear of saying certain things, homosexuality, pedophilia) were misdiagnosed 70% to 85% of the time.9
Although OCD shares characteristics with other disorders, several features can help family physicians correctly identify OCD. Fears associated with OCD are usually not about everyday concerns or worries. For example, a patient with social anxiety disorder may report fear of embarrassing themselves in public, whereas a patient with OCD may report fear that they will lose control and do something outlandish such as start swearing loudly.
Additionally, obsessions and compulsions in OCD are not exclusively tied to a traumatic experience as in posttraumatic stress disorder. Someone with OCD who has harm-related or sexual obsessions (eg, homosexuality) will report that this is not consistent with their interests and desires. Furthermore, a small subset of people with OCD may have poor insight, meaning they have low self-awareness of the nature of their obsessions or compulsions, but they do not experience psychotic symptoms.
How to make the diagnosis
A clinical interview is an essential component of assessing OCD in primary care. Ask patients who have mood or anxiety concerns about OCD symptoms, due to the high comorbidity rates of these entities.1 If a family history of OCD is known, assess the patient for this disorder, as higher rates exist among family members.10 Although primary care providers should indeed screen for OCD and provide provisional diagnoses as warranted, additional assessment by a behavioral health practitioner is recommended, given their specialty training in this area.
Patients with OCD are often reluctant to disclose intrusive thoughts due to perceiving them as shameful or unacceptable. Consider asking direct questions to facilitate the evaluation:
- “Do you ever feel bothered by unwanted or unusual thoughts that you cannot get out of your mind even though you try to?”
- “Do you feel that you have to do anything to get rid of these thoughts or prevent something bad from happening?”
- “Will you feel very uncomfortable if you don’t do something a specific way?”
Evidence-based self-report measures are also available. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS)11 has become the gold standard psychometric measure for OCD. The updated version (Y-BOCS-II)12 and child/adolescent version (CY-BOCS)11 are also available.
Treatment: A tandem approach is most effective
Patient characteristics (eg, comorbid depression, level of adherence to treatment) may also help guide prescribing choices.10 For patients not responding to pharmacologic treatment in 8 to 12 weeks, consider referral to a psychiatrist.14
For OCD, CBT provided by a trained specialist typically involves exposure with response prevention (ERP). This entails confronting difficult thoughts and feared situations through exposure therapy and learning to reduce compulsive and excessive safety behaviors (eg, thinking about being contaminated by germs and then refraining from washing hands). Research suggests that CBT with ERP produces outcomes equivalent or superior to those achieved with pharmacotherapy.13 In addition to finding large effect sizes, clinical trials have demonstrated a treatment response of 86% in those completing CBT with ERP, compared with 48% of those receiving clomipramine.15 And Y-BOCS symptom scores have been reduced by 50% to 60% with CBT and ERP.16
How best to navigate coordination-of-care issues
When selecting a psychotherapy treatment provider for a patient with OCD, ask whether they are trained in ERP. If a trained psychotherapist is not available in the local health care system, you may refer to the International OCD Foundation (iocdf.org), which maintains an online directory of psychotherapists specializing in OCD.
Primary care physicians can also work with psychiatrists or psychotherapy providers to develop shared treatment plans. Part of this plan may involve reducing excess medical utilization and checking/reassurance (eg, requesting repeat medical tests). When there is concern about safety issues (eg, intrusive homicidal or suicidal thoughts), a risk assessment is strongly recommended.
CASE
An on-site psychologist evaluated Ms. L and diagnosed OCD. Ms. L had talked to the doctor about the possibility of medication, but she preferred to try behavioral treatment first. Ms. L agreed to participate in CBT with ERP. Treatment included imaginal and situational exposure exercises to decrease emotional reactivity associated with the thoughts, and to challenge beliefs that the thoughts are meaningful (eg, that having the thought means that she may act on it or that she is an unfit mother).
For example, Ms. L practiced repeating the thoughts aloud and going into feared situations (eg, being alone with her child). This was paired with response prevention, meaning that Ms. L was instructed to avoid checking with, or seeking, reassurance from others. She engaged in 4 ERP sessions in the primary care setting, and treatment led to significant symptom improvement that was maintained at follow-up with her primary care provider 6 and 12 months later.
CORRESPONDENCE
Jared L. Skillings, PhD, ABPP, Division of Psychiatry and Behavioral Medicine, Spectrum Health System, 2750 East Beltline NE, Grand Rapids, MI 49525; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22:344-350.
3. Glazier K, Calizte RM, Rothschild R, et al. High rates of OCD symptom misidentification by mental health professionals. Ann Clin Psychiatry. 2013;25:201-209.
4. Schwartz C, Schlegl S, Kuelz AK, et al. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsessive Compuls Relat Disord. 2013;2:448-456.
5. Marciniak M, Lage MJ, Landbloom RP, et al. Medical and productivity costs of anxiety disorders: case control study. Depress Anxiety. 2004;19:112-120.
6. Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061-1068.
7. Purdon C, Clark DA. Obsessive intrusive thoughts in nonclinical subjects. Part I. Content and relation with depressive, anxious and obsessional symptoms. Behav Res Ther. 1993;31:713-720.
8. Clark DA. Cognitive-Behavioral Therapy for OCD. New York: The Guilford Press; 2002.
9. Glazier K, Swing M, McGinn LK. Half of obsessive-compulsive disorder cases misdiagnosed: vignette-based survey of primary care physicians. J Clin Psychiatry. 2015;26:e761-e767.
10. Fineberg NA, Reghunandanan S, Simpson HB, et al. Obsessive-compulsive disorder (OCD): practical strategies for pharmacological and somatic treatment in adults. Psych Res. 2015;227:114-125.
11. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006-1011.
12. Storch EA, Larson MJ, Goodman WK, et al. Development and psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale—second edition. Psychol Assess. 2010;22:223-232.
13. Katzman MA, Bleau P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder in primary care. Int J Psychiatry Clin. 2012;16:77-84.
15. Foa FB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
16. Abramowitz JS. The psychological treatment of obsessive compulsive disorder. Can J Psychiatry. 2006;51:407-416.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22:344-350.
3. Glazier K, Calizte RM, Rothschild R, et al. High rates of OCD symptom misidentification by mental health professionals. Ann Clin Psychiatry. 2013;25:201-209.
4. Schwartz C, Schlegl S, Kuelz AK, et al. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsessive Compuls Relat Disord. 2013;2:448-456.
5. Marciniak M, Lage MJ, Landbloom RP, et al. Medical and productivity costs of anxiety disorders: case control study. Depress Anxiety. 2004;19:112-120.
6. Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061-1068.
7. Purdon C, Clark DA. Obsessive intrusive thoughts in nonclinical subjects. Part I. Content and relation with depressive, anxious and obsessional symptoms. Behav Res Ther. 1993;31:713-720.
8. Clark DA. Cognitive-Behavioral Therapy for OCD. New York: The Guilford Press; 2002.
9. Glazier K, Swing M, McGinn LK. Half of obsessive-compulsive disorder cases misdiagnosed: vignette-based survey of primary care physicians. J Clin Psychiatry. 2015;26:e761-e767.
10. Fineberg NA, Reghunandanan S, Simpson HB, et al. Obsessive-compulsive disorder (OCD): practical strategies for pharmacological and somatic treatment in adults. Psych Res. 2015;227:114-125.
11. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006-1011.
12. Storch EA, Larson MJ, Goodman WK, et al. Development and psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale—second edition. Psychol Assess. 2010;22:223-232.
13. Katzman MA, Bleau P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder in primary care. Int J Psychiatry Clin. 2012;16:77-84.
15. Foa FB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
16. Abramowitz JS. The psychological treatment of obsessive compulsive disorder. Can J Psychiatry. 2006;51:407-416.
Advance care planning: Making it easier for patients (and you)
With the number of aging Americans projected to grow dramatically in the next several years, the need for primary palliative care and advance care planning (ACP) is more important than ever. Patients and their families want and expect palliative care when needed, but initial conversations about ACP can be difficult for them. Appropriate timing in raising this subject and clear communication can give patients the opportunity, while they are still independent, to set their goals for medical care.
For the past several decades, political decisions and judicial cases have shaped palliative care as we know it today. And its shape is still evolving. In support of ACP, advocacy groups at a national level are developing models that practitioners can use to engage patients in setting goals. And Medicare is now reimbursing primary care providers for this work that they have been doing for years (although many still may not be billing for the service).
Finally, the busy primary care office may have its own set of challenges in addressing ACP. Our aim in this review is to identify the barriers we face and the solutions we can implement to make a difference in our patients’ end-of-life care planning.
[polldaddy:9795119]
Landmark events have defined advance care planning today
In 1969, Luis Kutner, an Illinois attorney, proposed the idea of a “living will,” envisioned as a document specifying the types of treatment a person would be willing to receive were they unable at a later time to participate in making a decision.1 In 1976, California became the first state to give living wills the power of the law through the Natural Death Act.2
Throughout the 1970s and '80s, several high-profile court cases brought this idea into the national spotlight. In 1975, the New Jersey Supreme Court granted the parents of 21-year-old Karen Ann Quinlan the right to discontinue the treatment sustaining her in a persistent vegetative state. Ms. Quinlan was removed from the ventilator and lived 9 more months before dying in a nursing home.
A few years later, Nancy Cruzan, a 32-year-old woman involved in a 1983 motor vehicle accident, was also in a persistent vegetative state and remained so until 1988 when her parents asked that her feeding tube be removed. The hospital refused, indicating that it would lead to her death. The family sued and the case eventually went to the US Supreme Court in 1989.
In a 5-to-4 decision, the Supreme Court ruled that a state was legally able to require “clear and convincing evidence” of a patient’s wish for removal of life-sustaining therapies. Cruzan’s family was able to provide such evidence and her artificial nutrition was withheld. She died 12 days later.
The Cruzan case was instrumental in furthering ACP, leading to the passage of the Patient Self Determination Act (PSDA) by Congress in 1990. All federally funded health care facilities were now required to educate patients of their rights in determining their medical care and to ask about advance directives.3 The ACP movement gained additional momentum from the landmark SUPPORT study that documented shortcomings in communication between physicians and patients/families about treatment preferences and end-of-life care in US hospitals.4
In the Terri Schiavo case, the patient’s husband disagreed with the life-sustaining decisions of his wife’s parents given her persistent vegetative state and the fact that she had no chance of meaningful recovery. After a prolonged national debate, it was ultimately decided that the husband could elect to withhold artificial nutrition. (She died in 2005.) The Schiavo case, as well as the Institute of Medicine’s report on “Dying in America,”5 influenced Congress in 2016 to pass legislation funding ACP conversations.
The demonstrated benefits of advance care planning
When done comprehensively, ACP yields many benefits for patients and families and for the health care system. A systematic review demonstrated that, despite the few studies examining the economic cost of ACP, the process may lead to decreased health care costs in certain populations (nursing home residents, community dwelling adults with dementia, and those living in high health care spending regions) and at the very least does not increase health care costs.6 ACP has increased the number of do-not-resuscitate orders7 and has decreased hospitalizations,8 admissions to intensive care units,7,8 and rates of cardiopulmonary resuscitation,7,8 mechanical ventilation,7,8 and use of tube feeding.8
More noteworthy than the decrease in resource utilization and potential cost savings is the impact that ACP can have on a patient’s quality of life. Patients who receive aggressive care at the end of life tend to experience decreased quality of life compared with those receiving hospice care.7 Quality-of-life scores for patients in hospice improved with the length of enrollment in that care.7 When ACP discussions have taken place, the care patients receive at the end of life tends to conform more closely to their wishes and to increase family satisfaction.9-11
One reason that practitioners often give for not completing ACP is the fear of increasing patient or family anxiety or post-traumatic stress disorder (PTSD). However, studies have shown this concern to be unfounded.7,12 While ACP studies have not shown a decrease in rates of anxiety or PTSD, no study has shown an increase in these psychological morbidities.8
Caveats to keep in mind. Not all studies have shown unambiguous benefits related to ACP. Among the systematic reviews previously noted, there was significant variability in quality of data. Additionally, some experts argue that the traditional view of ACP (ie, completion of a single advance directive/living will) is outdated and should be replaced with a method that prepares patients and families to anticipate “in-the-moment decision making.”13 While we still believe that completion of an advance directive is useful, the experts’ point is well taken, especially since many patients change their preferences over time (and typically towards more aggressive care).14,15 While the advance directive serves a role, it is more important to help patients recognize their goals and preferences and to facilitate ongoing discussions between the patient and their families/surrogate decision maker and providers.
A snapshot of participation in advance care planning
Despite the ACP movement and the likely benefits associated with it, most individuals have not participated. Rates of completion do seem to be rising, but there is still room for improvement. Among all individuals older than 18 years, only 26.3% have an advance directive.16 In a cohort of older patients seen in an emergency department, only 40% had a living will, while nearly 54% had a designated health care power of attorney.17 Perhaps more alarming is the lack of ACP for those patients almost all physicians would agree need it—the long-term care population. The National Center for Health Statistics has reported that only 28% of home health care patients, 65% of nursing home residents, and 88% of hospice patients have an advance directive on file.18
Physician and patient barriers to advance care planning
If ACP can decrease resource utilization and improve caregiver compliance with a patient’s wishes for end of life, the obvious question is: Why isn’t it done more often? A longstanding barrier for physicians has been that these types of discussions are time intensive and have not been billable. However, since January 1, 2016, we are now able to bill for these discussions. (More on this in a bit.) Physicians do cite other barriers, though.
A recent systematic review showed that ACP is hindered by time constraints imposed by other clinical and administrative tasks that are heavily monitored.19 Barriers to engaging in ACP reported by patients include a reluctance to think about dying, a belief that family or physicians will know what to do, difficulty understanding ACP forms, and the absence of a person who can serve as a surrogate decision-maker.20,21
There are national models to help with implementation
The percentage of individuals with an advance directive in the United States has not increased significantly over the past decade.22 The lack of traction in completion and use of advance directives has lead several authors to question the utility of this older model of ACP.22 Several experts in the field believe that more robust, ongoing goals-of-care conversations between patients, families, and providers are equally, or even more, important than the completion of actual advance directive documents.23,24
National models such as the POLST (Physician Orders for Life-Sustaining Treatment) paradigm have become popular in several states (http://www.polst.org). Intended for those with estimated life expectancy of less than one year, POLST is not an advance directive but a physician order for these seriously ill patients. Emergency medical service workers are legally able to follow a POLST document but not a living will or advance directive—a significant reason for those with end-stage illness to consider completing a POLST document with their health care provider. Programs such as, “Respecting Choices,” have incorporated POLST documentation as part of ongoing goals-of-care conversations between patients and health care providers (http://www.gundersenhealth.org/respecting-choices).
Many groups have developed products to encourage patients and their families to initiate conversations at home. An example is the Conversation Project, a free online resource available in multiple languages that can help break the ice for patients and get them talking about their wishes for end-of-life care (http://www.theconversationproject.org). It poses simple stimulating questions such as: “What kind of role do you want to have in the decision-making process?” and “What are your concerns about treatment?”
How-to tips for advance care planning in the outpatient setting
When approaching the topic of ACP with patients, it’s important to do so over time, starting as soon as possible with older patients and those with chronic illness conferring a high risk of significant morbidity or mortality. Assess each patient’s understanding of ACP and readiness to discuss the topic. Many patients think of ACP in the context of a document (eg, living will), so asking about the existence of a living will may help to start the conversation. Alternatively, consider inquiring about whether the patient has had experience with family or friends at the end of life or during a difficult medical situation, and whether the patient has thought about making personal plans for such a situation.25
When a patient is ready to have this conversation, your goal should be three-fold: 26
- Help the patient articulate personal values, goals, and preferences.
- Ask the patient
to formally assign health care power of attorney (POA) to a trusted individual or to name a surrogate decision-maker. Document this decision in the medical record. - Help the patient translate expressed values into specific medical care plans, if applicable.
Because ACP conversations are often time consuming, it’s a good idea to schedule separate appointments to focus on this alone. If, however, a patient is unable to return for a dedicated ACP visit, a first step that can be completed in a reasonably short period would be choosing a surrogate decision-maker.
Helping a patient articulate personal values may be eased by asking such questions as: “Have you ever thought about what kind of care you would want if the time came when you could not make your own decisions?” or “What worries you the most about possibly not being able to make your own decisions?”27 If the patient is able to identify a surrogate decision maker before the ACP appointment, ask that this person attend. A family member or close friend may remember instances in which the patient expressed health care preferences, and their presence can help to minimize gaps in communication.
Once the patient’s preferences are clear, document them in the medical record. Some preferences may be suitable for translation into POLST orders or an advance directive, but this is less important than the overall discussion. ACP should be an ongoing conversation, since a patient’s goals may change over time. And encourage the patient to share any desired change in plans with their surrogate decision-
Be sure to bill for advance care planning services
To encourage office-based providers to conduct ACP, CMS implemented payment for CPT codes 99497 and 99498.
CPT code 99497 covers the first 30 minutes of face-to-face time with patients or their family members or medical decision-makers. This time can be used to discuss living wills or advance directives.
CPT code 99498 can be applied to each additional 30 minutes of ACP services. Typically, this billing code would be used as an add-on for a particular diagnosis such as heart failure, chronic obstructive pulmonary disease, or pancreatic cancer.
CPT Code 99497 equates to 2.40 relative-value units (RVU) with an estimated payment of $85.99, while CPT code 99498 equates to 2.09 RVU with an estimated payment of $74.88.28
According to CMS, there is no annual limit to the number of times the ACP codes can be billed for a particular patient. And there are no restrictions regarding location of service, meaning a provider could perform this in an outpatient setting, an inpatient setting, or a long-term care facility. Both physicians and non-physician practitioners are allowed to bill with this code. Also worth noting: You don’t need to complete any particular documentation for a visit to be billed as an ACP service. CMS provides a helpful Q & A at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf.
CORRESPONDENCE
John Liantonio, MD, Thomas Jefferson University Hospital, Department of Family and Community Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected]
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. California Law Revision Commission. 2000 Health Care Decisions Law and Revised Power of Attorney Law. Available at: http://www.clrc.ca.gov/pub/Printed-Reports/Pub208.pdf. Accessed May 15, 2017.
3. H.R. 5067 - 101st Congress. Patient Self Determination Act of 1990. Available at: https://www.govtrack.us/congress/bills/101/hr5067. Accessed November 16, 2016
4. The SUPPORT Principle Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591-1598.
5. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
6. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869-884.
7. Wright AA, Ray A, Mack JW, et al. Associations between end-of-life discussions, patient mental-health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
8. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000-1025.
9. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
10. Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc. 2005;53:290-294.
11. Schamp R, Tenkku L. Managed death in a PACE: pathways in present and advance directives. J Am Med Dir Assoc. 2006;7:339-344.
12. Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99:3-16.
13. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256-261.
14. Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors study. J Am Geriatr Soc. 2004;52:577-582.
15. Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890-895.
16. Rao JK, Anderson LA, Lin F, et al. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46:65-70.
17. Grudzen CR, Buonocore P, Steinberg J, et al; AAHPM Research Committee Writing Group. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647-651.
18. Jones AL, Moss AJ, Harris-Kojetin LD. Use of advance directives in long-term care populations. NCHS Data Brief. 2011;(54):1-8.
19. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10:e0116629.
20. Fried TR, Bullock K, Iannone L, et al. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547-1555.
21. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39.
22. Winter L, Parks SM, Diamond JJ. Ask a different question, get a different answer: why living wills are poor guides to care preferences at the end of life. J Pall Med. 2010;13:567-572.
23. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at: https://www.nap.edu/read/18748/chapter/1. Accessed May 15, 2017.
24. Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006-1013.
25. McMahan RD, Knight SJ, Fried TR, et al. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46:355-365.
26. Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391-403.
27. Lum HD, Sudore RL. Advance care planning and goals of care communication in older adults with cardiovascular disease and multi-morbidity. Clin Geriatr Med. 2016;32:247-260.
28. American College of Physicians. Advanced Care Planning: Implementation for practices. Available at: https://www.acponline.org/system/files/documents/practice-resources/business-resources/payment/advance_care_planning_toolkit.pdf. Accessed May 15, 2017.
With the number of aging Americans projected to grow dramatically in the next several years, the need for primary palliative care and advance care planning (ACP) is more important than ever. Patients and their families want and expect palliative care when needed, but initial conversations about ACP can be difficult for them. Appropriate timing in raising this subject and clear communication can give patients the opportunity, while they are still independent, to set their goals for medical care.
For the past several decades, political decisions and judicial cases have shaped palliative care as we know it today. And its shape is still evolving. In support of ACP, advocacy groups at a national level are developing models that practitioners can use to engage patients in setting goals. And Medicare is now reimbursing primary care providers for this work that they have been doing for years (although many still may not be billing for the service).
Finally, the busy primary care office may have its own set of challenges in addressing ACP. Our aim in this review is to identify the barriers we face and the solutions we can implement to make a difference in our patients’ end-of-life care planning.
[polldaddy:9795119]
Landmark events have defined advance care planning today
In 1969, Luis Kutner, an Illinois attorney, proposed the idea of a “living will,” envisioned as a document specifying the types of treatment a person would be willing to receive were they unable at a later time to participate in making a decision.1 In 1976, California became the first state to give living wills the power of the law through the Natural Death Act.2
Throughout the 1970s and '80s, several high-profile court cases brought this idea into the national spotlight. In 1975, the New Jersey Supreme Court granted the parents of 21-year-old Karen Ann Quinlan the right to discontinue the treatment sustaining her in a persistent vegetative state. Ms. Quinlan was removed from the ventilator and lived 9 more months before dying in a nursing home.
A few years later, Nancy Cruzan, a 32-year-old woman involved in a 1983 motor vehicle accident, was also in a persistent vegetative state and remained so until 1988 when her parents asked that her feeding tube be removed. The hospital refused, indicating that it would lead to her death. The family sued and the case eventually went to the US Supreme Court in 1989.
In a 5-to-4 decision, the Supreme Court ruled that a state was legally able to require “clear and convincing evidence” of a patient’s wish for removal of life-sustaining therapies. Cruzan’s family was able to provide such evidence and her artificial nutrition was withheld. She died 12 days later.
The Cruzan case was instrumental in furthering ACP, leading to the passage of the Patient Self Determination Act (PSDA) by Congress in 1990. All federally funded health care facilities were now required to educate patients of their rights in determining their medical care and to ask about advance directives.3 The ACP movement gained additional momentum from the landmark SUPPORT study that documented shortcomings in communication between physicians and patients/families about treatment preferences and end-of-life care in US hospitals.4
In the Terri Schiavo case, the patient’s husband disagreed with the life-sustaining decisions of his wife’s parents given her persistent vegetative state and the fact that she had no chance of meaningful recovery. After a prolonged national debate, it was ultimately decided that the husband could elect to withhold artificial nutrition. (She died in 2005.) The Schiavo case, as well as the Institute of Medicine’s report on “Dying in America,”5 influenced Congress in 2016 to pass legislation funding ACP conversations.
The demonstrated benefits of advance care planning
When done comprehensively, ACP yields many benefits for patients and families and for the health care system. A systematic review demonstrated that, despite the few studies examining the economic cost of ACP, the process may lead to decreased health care costs in certain populations (nursing home residents, community dwelling adults with dementia, and those living in high health care spending regions) and at the very least does not increase health care costs.6 ACP has increased the number of do-not-resuscitate orders7 and has decreased hospitalizations,8 admissions to intensive care units,7,8 and rates of cardiopulmonary resuscitation,7,8 mechanical ventilation,7,8 and use of tube feeding.8
More noteworthy than the decrease in resource utilization and potential cost savings is the impact that ACP can have on a patient’s quality of life. Patients who receive aggressive care at the end of life tend to experience decreased quality of life compared with those receiving hospice care.7 Quality-of-life scores for patients in hospice improved with the length of enrollment in that care.7 When ACP discussions have taken place, the care patients receive at the end of life tends to conform more closely to their wishes and to increase family satisfaction.9-11
One reason that practitioners often give for not completing ACP is the fear of increasing patient or family anxiety or post-traumatic stress disorder (PTSD). However, studies have shown this concern to be unfounded.7,12 While ACP studies have not shown a decrease in rates of anxiety or PTSD, no study has shown an increase in these psychological morbidities.8
Caveats to keep in mind. Not all studies have shown unambiguous benefits related to ACP. Among the systematic reviews previously noted, there was significant variability in quality of data. Additionally, some experts argue that the traditional view of ACP (ie, completion of a single advance directive/living will) is outdated and should be replaced with a method that prepares patients and families to anticipate “in-the-moment decision making.”13 While we still believe that completion of an advance directive is useful, the experts’ point is well taken, especially since many patients change their preferences over time (and typically towards more aggressive care).14,15 While the advance directive serves a role, it is more important to help patients recognize their goals and preferences and to facilitate ongoing discussions between the patient and their families/surrogate decision maker and providers.
A snapshot of participation in advance care planning
Despite the ACP movement and the likely benefits associated with it, most individuals have not participated. Rates of completion do seem to be rising, but there is still room for improvement. Among all individuals older than 18 years, only 26.3% have an advance directive.16 In a cohort of older patients seen in an emergency department, only 40% had a living will, while nearly 54% had a designated health care power of attorney.17 Perhaps more alarming is the lack of ACP for those patients almost all physicians would agree need it—the long-term care population. The National Center for Health Statistics has reported that only 28% of home health care patients, 65% of nursing home residents, and 88% of hospice patients have an advance directive on file.18
Physician and patient barriers to advance care planning
If ACP can decrease resource utilization and improve caregiver compliance with a patient’s wishes for end of life, the obvious question is: Why isn’t it done more often? A longstanding barrier for physicians has been that these types of discussions are time intensive and have not been billable. However, since January 1, 2016, we are now able to bill for these discussions. (More on this in a bit.) Physicians do cite other barriers, though.
A recent systematic review showed that ACP is hindered by time constraints imposed by other clinical and administrative tasks that are heavily monitored.19 Barriers to engaging in ACP reported by patients include a reluctance to think about dying, a belief that family or physicians will know what to do, difficulty understanding ACP forms, and the absence of a person who can serve as a surrogate decision-maker.20,21
There are national models to help with implementation
The percentage of individuals with an advance directive in the United States has not increased significantly over the past decade.22 The lack of traction in completion and use of advance directives has lead several authors to question the utility of this older model of ACP.22 Several experts in the field believe that more robust, ongoing goals-of-care conversations between patients, families, and providers are equally, or even more, important than the completion of actual advance directive documents.23,24
National models such as the POLST (Physician Orders for Life-Sustaining Treatment) paradigm have become popular in several states (http://www.polst.org). Intended for those with estimated life expectancy of less than one year, POLST is not an advance directive but a physician order for these seriously ill patients. Emergency medical service workers are legally able to follow a POLST document but not a living will or advance directive—a significant reason for those with end-stage illness to consider completing a POLST document with their health care provider. Programs such as, “Respecting Choices,” have incorporated POLST documentation as part of ongoing goals-of-care conversations between patients and health care providers (http://www.gundersenhealth.org/respecting-choices).
Many groups have developed products to encourage patients and their families to initiate conversations at home. An example is the Conversation Project, a free online resource available in multiple languages that can help break the ice for patients and get them talking about their wishes for end-of-life care (http://www.theconversationproject.org). It poses simple stimulating questions such as: “What kind of role do you want to have in the decision-making process?” and “What are your concerns about treatment?”
How-to tips for advance care planning in the outpatient setting
When approaching the topic of ACP with patients, it’s important to do so over time, starting as soon as possible with older patients and those with chronic illness conferring a high risk of significant morbidity or mortality. Assess each patient’s understanding of ACP and readiness to discuss the topic. Many patients think of ACP in the context of a document (eg, living will), so asking about the existence of a living will may help to start the conversation. Alternatively, consider inquiring about whether the patient has had experience with family or friends at the end of life or during a difficult medical situation, and whether the patient has thought about making personal plans for such a situation.25
When a patient is ready to have this conversation, your goal should be three-fold: 26
- Help the patient articulate personal values, goals, and preferences.
- Ask the patient
to formally assign health care power of attorney (POA) to a trusted individual or to name a surrogate decision-maker. Document this decision in the medical record. - Help the patient translate expressed values into specific medical care plans, if applicable.
Because ACP conversations are often time consuming, it’s a good idea to schedule separate appointments to focus on this alone. If, however, a patient is unable to return for a dedicated ACP visit, a first step that can be completed in a reasonably short period would be choosing a surrogate decision-maker.
Helping a patient articulate personal values may be eased by asking such questions as: “Have you ever thought about what kind of care you would want if the time came when you could not make your own decisions?” or “What worries you the most about possibly not being able to make your own decisions?”27 If the patient is able to identify a surrogate decision maker before the ACP appointment, ask that this person attend. A family member or close friend may remember instances in which the patient expressed health care preferences, and their presence can help to minimize gaps in communication.
Once the patient’s preferences are clear, document them in the medical record. Some preferences may be suitable for translation into POLST orders or an advance directive, but this is less important than the overall discussion. ACP should be an ongoing conversation, since a patient’s goals may change over time. And encourage the patient to share any desired change in plans with their surrogate decision-
Be sure to bill for advance care planning services
To encourage office-based providers to conduct ACP, CMS implemented payment for CPT codes 99497 and 99498.
CPT code 99497 covers the first 30 minutes of face-to-face time with patients or their family members or medical decision-makers. This time can be used to discuss living wills or advance directives.
CPT code 99498 can be applied to each additional 30 minutes of ACP services. Typically, this billing code would be used as an add-on for a particular diagnosis such as heart failure, chronic obstructive pulmonary disease, or pancreatic cancer.
CPT Code 99497 equates to 2.40 relative-value units (RVU) with an estimated payment of $85.99, while CPT code 99498 equates to 2.09 RVU with an estimated payment of $74.88.28
According to CMS, there is no annual limit to the number of times the ACP codes can be billed for a particular patient. And there are no restrictions regarding location of service, meaning a provider could perform this in an outpatient setting, an inpatient setting, or a long-term care facility. Both physicians and non-physician practitioners are allowed to bill with this code. Also worth noting: You don’t need to complete any particular documentation for a visit to be billed as an ACP service. CMS provides a helpful Q & A at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf.
CORRESPONDENCE
John Liantonio, MD, Thomas Jefferson University Hospital, Department of Family and Community Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected]
With the number of aging Americans projected to grow dramatically in the next several years, the need for primary palliative care and advance care planning (ACP) is more important than ever. Patients and their families want and expect palliative care when needed, but initial conversations about ACP can be difficult for them. Appropriate timing in raising this subject and clear communication can give patients the opportunity, while they are still independent, to set their goals for medical care.
For the past several decades, political decisions and judicial cases have shaped palliative care as we know it today. And its shape is still evolving. In support of ACP, advocacy groups at a national level are developing models that practitioners can use to engage patients in setting goals. And Medicare is now reimbursing primary care providers for this work that they have been doing for years (although many still may not be billing for the service).
Finally, the busy primary care office may have its own set of challenges in addressing ACP. Our aim in this review is to identify the barriers we face and the solutions we can implement to make a difference in our patients’ end-of-life care planning.
[polldaddy:9795119]
Landmark events have defined advance care planning today
In 1969, Luis Kutner, an Illinois attorney, proposed the idea of a “living will,” envisioned as a document specifying the types of treatment a person would be willing to receive were they unable at a later time to participate in making a decision.1 In 1976, California became the first state to give living wills the power of the law through the Natural Death Act.2
Throughout the 1970s and '80s, several high-profile court cases brought this idea into the national spotlight. In 1975, the New Jersey Supreme Court granted the parents of 21-year-old Karen Ann Quinlan the right to discontinue the treatment sustaining her in a persistent vegetative state. Ms. Quinlan was removed from the ventilator and lived 9 more months before dying in a nursing home.
A few years later, Nancy Cruzan, a 32-year-old woman involved in a 1983 motor vehicle accident, was also in a persistent vegetative state and remained so until 1988 when her parents asked that her feeding tube be removed. The hospital refused, indicating that it would lead to her death. The family sued and the case eventually went to the US Supreme Court in 1989.
In a 5-to-4 decision, the Supreme Court ruled that a state was legally able to require “clear and convincing evidence” of a patient’s wish for removal of life-sustaining therapies. Cruzan’s family was able to provide such evidence and her artificial nutrition was withheld. She died 12 days later.
The Cruzan case was instrumental in furthering ACP, leading to the passage of the Patient Self Determination Act (PSDA) by Congress in 1990. All federally funded health care facilities were now required to educate patients of their rights in determining their medical care and to ask about advance directives.3 The ACP movement gained additional momentum from the landmark SUPPORT study that documented shortcomings in communication between physicians and patients/families about treatment preferences and end-of-life care in US hospitals.4
In the Terri Schiavo case, the patient’s husband disagreed with the life-sustaining decisions of his wife’s parents given her persistent vegetative state and the fact that she had no chance of meaningful recovery. After a prolonged national debate, it was ultimately decided that the husband could elect to withhold artificial nutrition. (She died in 2005.) The Schiavo case, as well as the Institute of Medicine’s report on “Dying in America,”5 influenced Congress in 2016 to pass legislation funding ACP conversations.
The demonstrated benefits of advance care planning
When done comprehensively, ACP yields many benefits for patients and families and for the health care system. A systematic review demonstrated that, despite the few studies examining the economic cost of ACP, the process may lead to decreased health care costs in certain populations (nursing home residents, community dwelling adults with dementia, and those living in high health care spending regions) and at the very least does not increase health care costs.6 ACP has increased the number of do-not-resuscitate orders7 and has decreased hospitalizations,8 admissions to intensive care units,7,8 and rates of cardiopulmonary resuscitation,7,8 mechanical ventilation,7,8 and use of tube feeding.8
More noteworthy than the decrease in resource utilization and potential cost savings is the impact that ACP can have on a patient’s quality of life. Patients who receive aggressive care at the end of life tend to experience decreased quality of life compared with those receiving hospice care.7 Quality-of-life scores for patients in hospice improved with the length of enrollment in that care.7 When ACP discussions have taken place, the care patients receive at the end of life tends to conform more closely to their wishes and to increase family satisfaction.9-11
One reason that practitioners often give for not completing ACP is the fear of increasing patient or family anxiety or post-traumatic stress disorder (PTSD). However, studies have shown this concern to be unfounded.7,12 While ACP studies have not shown a decrease in rates of anxiety or PTSD, no study has shown an increase in these psychological morbidities.8
Caveats to keep in mind. Not all studies have shown unambiguous benefits related to ACP. Among the systematic reviews previously noted, there was significant variability in quality of data. Additionally, some experts argue that the traditional view of ACP (ie, completion of a single advance directive/living will) is outdated and should be replaced with a method that prepares patients and families to anticipate “in-the-moment decision making.”13 While we still believe that completion of an advance directive is useful, the experts’ point is well taken, especially since many patients change their preferences over time (and typically towards more aggressive care).14,15 While the advance directive serves a role, it is more important to help patients recognize their goals and preferences and to facilitate ongoing discussions between the patient and their families/surrogate decision maker and providers.
A snapshot of participation in advance care planning
Despite the ACP movement and the likely benefits associated with it, most individuals have not participated. Rates of completion do seem to be rising, but there is still room for improvement. Among all individuals older than 18 years, only 26.3% have an advance directive.16 In a cohort of older patients seen in an emergency department, only 40% had a living will, while nearly 54% had a designated health care power of attorney.17 Perhaps more alarming is the lack of ACP for those patients almost all physicians would agree need it—the long-term care population. The National Center for Health Statistics has reported that only 28% of home health care patients, 65% of nursing home residents, and 88% of hospice patients have an advance directive on file.18
Physician and patient barriers to advance care planning
If ACP can decrease resource utilization and improve caregiver compliance with a patient’s wishes for end of life, the obvious question is: Why isn’t it done more often? A longstanding barrier for physicians has been that these types of discussions are time intensive and have not been billable. However, since January 1, 2016, we are now able to bill for these discussions. (More on this in a bit.) Physicians do cite other barriers, though.
A recent systematic review showed that ACP is hindered by time constraints imposed by other clinical and administrative tasks that are heavily monitored.19 Barriers to engaging in ACP reported by patients include a reluctance to think about dying, a belief that family or physicians will know what to do, difficulty understanding ACP forms, and the absence of a person who can serve as a surrogate decision-maker.20,21
There are national models to help with implementation
The percentage of individuals with an advance directive in the United States has not increased significantly over the past decade.22 The lack of traction in completion and use of advance directives has lead several authors to question the utility of this older model of ACP.22 Several experts in the field believe that more robust, ongoing goals-of-care conversations between patients, families, and providers are equally, or even more, important than the completion of actual advance directive documents.23,24
National models such as the POLST (Physician Orders for Life-Sustaining Treatment) paradigm have become popular in several states (http://www.polst.org). Intended for those with estimated life expectancy of less than one year, POLST is not an advance directive but a physician order for these seriously ill patients. Emergency medical service workers are legally able to follow a POLST document but not a living will or advance directive—a significant reason for those with end-stage illness to consider completing a POLST document with their health care provider. Programs such as, “Respecting Choices,” have incorporated POLST documentation as part of ongoing goals-of-care conversations between patients and health care providers (http://www.gundersenhealth.org/respecting-choices).
Many groups have developed products to encourage patients and their families to initiate conversations at home. An example is the Conversation Project, a free online resource available in multiple languages that can help break the ice for patients and get them talking about their wishes for end-of-life care (http://www.theconversationproject.org). It poses simple stimulating questions such as: “What kind of role do you want to have in the decision-making process?” and “What are your concerns about treatment?”
How-to tips for advance care planning in the outpatient setting
When approaching the topic of ACP with patients, it’s important to do so over time, starting as soon as possible with older patients and those with chronic illness conferring a high risk of significant morbidity or mortality. Assess each patient’s understanding of ACP and readiness to discuss the topic. Many patients think of ACP in the context of a document (eg, living will), so asking about the existence of a living will may help to start the conversation. Alternatively, consider inquiring about whether the patient has had experience with family or friends at the end of life or during a difficult medical situation, and whether the patient has thought about making personal plans for such a situation.25
When a patient is ready to have this conversation, your goal should be three-fold: 26
- Help the patient articulate personal values, goals, and preferences.
- Ask the patient
to formally assign health care power of attorney (POA) to a trusted individual or to name a surrogate decision-maker. Document this decision in the medical record. - Help the patient translate expressed values into specific medical care plans, if applicable.
Because ACP conversations are often time consuming, it’s a good idea to schedule separate appointments to focus on this alone. If, however, a patient is unable to return for a dedicated ACP visit, a first step that can be completed in a reasonably short period would be choosing a surrogate decision-maker.
Helping a patient articulate personal values may be eased by asking such questions as: “Have you ever thought about what kind of care you would want if the time came when you could not make your own decisions?” or “What worries you the most about possibly not being able to make your own decisions?”27 If the patient is able to identify a surrogate decision maker before the ACP appointment, ask that this person attend. A family member or close friend may remember instances in which the patient expressed health care preferences, and their presence can help to minimize gaps in communication.
Once the patient’s preferences are clear, document them in the medical record. Some preferences may be suitable for translation into POLST orders or an advance directive, but this is less important than the overall discussion. ACP should be an ongoing conversation, since a patient’s goals may change over time. And encourage the patient to share any desired change in plans with their surrogate decision-
Be sure to bill for advance care planning services
To encourage office-based providers to conduct ACP, CMS implemented payment for CPT codes 99497 and 99498.
CPT code 99497 covers the first 30 minutes of face-to-face time with patients or their family members or medical decision-makers. This time can be used to discuss living wills or advance directives.
CPT code 99498 can be applied to each additional 30 minutes of ACP services. Typically, this billing code would be used as an add-on for a particular diagnosis such as heart failure, chronic obstructive pulmonary disease, or pancreatic cancer.
CPT Code 99497 equates to 2.40 relative-value units (RVU) with an estimated payment of $85.99, while CPT code 99498 equates to 2.09 RVU with an estimated payment of $74.88.28
According to CMS, there is no annual limit to the number of times the ACP codes can be billed for a particular patient. And there are no restrictions regarding location of service, meaning a provider could perform this in an outpatient setting, an inpatient setting, or a long-term care facility. Both physicians and non-physician practitioners are allowed to bill with this code. Also worth noting: You don’t need to complete any particular documentation for a visit to be billed as an ACP service. CMS provides a helpful Q & A at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-Advance-Care-Planning.pdf.
CORRESPONDENCE
John Liantonio, MD, Thomas Jefferson University Hospital, Department of Family and Community Medicine, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected]
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. California Law Revision Commission. 2000 Health Care Decisions Law and Revised Power of Attorney Law. Available at: http://www.clrc.ca.gov/pub/Printed-Reports/Pub208.pdf. Accessed May 15, 2017.
3. H.R. 5067 - 101st Congress. Patient Self Determination Act of 1990. Available at: https://www.govtrack.us/congress/bills/101/hr5067. Accessed November 16, 2016
4. The SUPPORT Principle Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591-1598.
5. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
6. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869-884.
7. Wright AA, Ray A, Mack JW, et al. Associations between end-of-life discussions, patient mental-health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
8. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000-1025.
9. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
10. Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc. 2005;53:290-294.
11. Schamp R, Tenkku L. Managed death in a PACE: pathways in present and advance directives. J Am Med Dir Assoc. 2006;7:339-344.
12. Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99:3-16.
13. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256-261.
14. Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors study. J Am Geriatr Soc. 2004;52:577-582.
15. Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890-895.
16. Rao JK, Anderson LA, Lin F, et al. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46:65-70.
17. Grudzen CR, Buonocore P, Steinberg J, et al; AAHPM Research Committee Writing Group. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647-651.
18. Jones AL, Moss AJ, Harris-Kojetin LD. Use of advance directives in long-term care populations. NCHS Data Brief. 2011;(54):1-8.
19. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10:e0116629.
20. Fried TR, Bullock K, Iannone L, et al. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547-1555.
21. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39.
22. Winter L, Parks SM, Diamond JJ. Ask a different question, get a different answer: why living wills are poor guides to care preferences at the end of life. J Pall Med. 2010;13:567-572.
23. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at: https://www.nap.edu/read/18748/chapter/1. Accessed May 15, 2017.
24. Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006-1013.
25. McMahan RD, Knight SJ, Fried TR, et al. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46:355-365.
26. Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391-403.
27. Lum HD, Sudore RL. Advance care planning and goals of care communication in older adults with cardiovascular disease and multi-morbidity. Clin Geriatr Med. 2016;32:247-260.
28. American College of Physicians. Advanced Care Planning: Implementation for practices. Available at: https://www.acponline.org/system/files/documents/practice-resources/business-resources/payment/advance_care_planning_toolkit.pdf. Accessed May 15, 2017.
1. Kutner L. Due process of euthanasia: the living will, a proposal. Indiana Law J. 1969;44:539-554.
2. California Law Revision Commission. 2000 Health Care Decisions Law and Revised Power of Attorney Law. Available at: http://www.clrc.ca.gov/pub/Printed-Reports/Pub208.pdf. Accessed May 15, 2017.
3. H.R. 5067 - 101st Congress. Patient Self Determination Act of 1990. Available at: https://www.govtrack.us/congress/bills/101/hr5067. Accessed November 16, 2016
4. The SUPPORT Principle Investigators. A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274:1591-1598.
5. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
6. Dixon J, Matosevic T, Knapp M. The economic evidence for advance care planning: systematic review of evidence. Palliat Med. 2015;29:869-884.
7. Wright AA, Ray A, Mack JW, et al. Associations between end-of-life discussions, patient mental-health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
8. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000-1025.
9. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345.
10. Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc. 2005;53:290-294.
11. Schamp R, Tenkku L. Managed death in a PACE: pathways in present and advance directives. J Am Med Dir Assoc. 2006;7:339-344.
12. Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns. 2016;99:3-16.
13. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153:256-261.
14. Straton JB, Wang NY, Meoni LA, et al. Physical functioning, depression, and preferences for treatment at the end of life: the Johns Hopkins Precursors study. J Am Geriatr Soc. 2004;52:577-582.
15. Fried TR, Byers AL, Gallo WT, et al. Prospective study of health status preferences and changes in preferences over time in older adults. Arch Intern Med. 2006;166:890-895.
16. Rao JK, Anderson LA, Lin F, et al. Completion of advance directives among U.S. consumers. Am J Prev Med. 2014;46:65-70.
17. Grudzen CR, Buonocore P, Steinberg J, et al; AAHPM Research Committee Writing Group. Concordance of advance care plans with inpatient directives in the electronic medical record for older patients admitted from the emergency department. J Pain Symptom Manage. 2016;51:647-651.
18. Jones AL, Moss AJ, Harris-Kojetin LD. Use of advance directives in long-term care populations. NCHS Data Brief. 2011;(54):1-8.
19. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10:e0116629.
20. Fried TR, Bullock K, Iannone L, et al. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547-1555.
21. Schickedanz AD, Schillinger D, Landefeld CS, et al. A clinical framework for improving the advance care planning process: start with patients’ self-identified barriers. J Am Geriatr Soc. 2009;57:31-39.
22. Winter L, Parks SM, Diamond JJ. Ask a different question, get a different answer: why living wills are poor guides to care preferences at the end of life. J Pall Med. 2010;13:567-572.
23. Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Available at: https://www.nap.edu/read/18748/chapter/1. Accessed May 15, 2017.
24. Sudore RL, Schickedanz AD, Landefeld CS, et al. Engagement in multiple steps of the advance care planning process: a descriptive study of diverse older adults. J Am Geriatr Soc. 2008;56:1006-1013.
25. McMahan RD, Knight SJ, Fried TR, et al. Advance care planning beyond advance directives: perspectives from patients and surrogates. J Pain Symptom Manage. 2013;46:355-365.
26. Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391-403.
27. Lum HD, Sudore RL. Advance care planning and goals of care communication in older adults with cardiovascular disease and multi-morbidity. Clin Geriatr Med. 2016;32:247-260.
28. American College of Physicians. Advanced Care Planning: Implementation for practices. Available at: https://www.acponline.org/system/files/documents/practice-resources/business-resources/payment/advance_care_planning_toolkit.pdf. Accessed May 15, 2017.
PRACTICE RECOMMENDATIONS
› Schedule visits dedicated to advance care planning (ACP) to remove time barriers and ensure that ACP is completed. C
› Give priority to identifying a health care representative. C
› Bill Centers for Medicare and Medicaid Services (CMS) for primary care ACP visits with CPT codes 99497 and 99498. Most private insurers are following CMS recommendations. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin care Q and A for your staff
in helping us be more efficient with our time. I asked my staff to put together a list of the most common patient skin care questions. This column can be used as a guide for your staff when answering questions about skin care.
Q: Should my sunscreen be applied first or last?
A: The best way to remember is that medications should be applied closest to the skin and sunscreen should be applied closest to the sun. I recommend that morning skin care be applied in the following order:
Step 1. Facial cleanser
Step 2. Eye products (protect the delicate eye area from the medication)
Step 3. Treatment product (medications, or the most important active ingredients)
Step 4. Moisturizer (if needed)
Step 5. Sunscreen
Q: When can I restart my normal skin care regimen after receiving dermal fillers?
A: The morning after receiving dermal fillers, you can resume your normal facial skin care regimen.
Q: How do I treat my skin after receiving cosmetic injections such as Botox?
A: After you are injected with dermal fillers, keep the Arnica gel, Arnica pads, or post procedure product on your face until bedtime. Prior to bed, wash your face with a gentle cleanser and lukewarm water. Do not use any scrubs, facial brushes, or hydroxy acids. Apply a soothing gel or soothing moisturizer. Do not use hot water on the face for 48 hours. Avoid hot showers, saunas, facial steaming, facial massage, and exercise for 48 hours. If you must exercise, try to keep the face cool with an ice filled bottle of water. Heat makes you more likely to bruise.
Q: Will retinol make my skin sun sensitive?
A: Retinol breaks down upon sun exposure so it should only be used at night. It is a myth that retinol makes your skin sun sensitive. Retinol is a form of vitamin A that helps protect your skin from sun damage by reducing levels of an enzyme called collagenase that is produced after sun exposure. For this and other reasons, retinol can help protect your skin from sun damage. However, if your skin gets irritated from the retinol and you go into the sun, you can develop postinflammatory hyperpigmentation – or dark patches on the skin. It is a good idea to always wear sunscreen in the morning every day whether you are using retinol or not.
Q: What do I do to treat the dark circles under my eyes?
A: Dark circles can be caused by sluggish blood flow around the eyes leading to deposition of a pigment found in blood called hemosiderin. Dark circles around the eyes also can be caused by an increased amount of the pigment melanin that occurs from sun exposure, heat, estrogen, and stress. Treatments include lifestyle habits that increase blood circulation, such as smoking cessation and exercise. Supplements such as horse chestnut may help improve circulation. Ingredients such as the tyrosinase inhibitors ascorbic acid, kojic acid, arbutin, and hydroquinone will help decrease melanin production, and may improve dark circles caused by increased melanin. Wearing a daily sunscreen around the eyes is a very important part of improving the appearance of dark circles under the eyes.
Q: Do I need an eye cream?
A: Most people need an eye product whether it is a serum, gel, or cream. Eye treatment products are usually one of the first skin care products that male patients purchase. The most common indications for eye treatment products are dark circles under the eyes, puffy eyes, dryness, fine lines, or redness. Each one of these indications requires very different ingredients; therefore, most people need more than one eye product depending on what eye issues they are experiencing. Eye treatment products can protect the delicate eye area from medications such as tretinoin that gets on the pillowcase and is transferred to the eye area at night. Eye products that have humectants, such as heparan sulfate or hyaluronic acid, should be used to treat fines lines and dryness, but these may make eyes puffy. For those with dry and puffy eyes, a second eye product with vasoconstrictive ingredients should be used in the daytime while the hydrating one is used at night. Antiaging eye products with retinol and hydroxy acids may irritate sensitive skin types so they can be paired with a soothing eye product. Consider using different eye products for the morning and evening depending on what eye issues need to be treated.
Q: Will moisturizer make my acne worse?
A: Noncomedogenic moisturizers can help improve acne and speed recovery. Using the proper cleanser and moisturizer will make acne medications more tolerable. Many patients cannot tolerate their acne medications every day. The bacteria that cause acne reproduce every 12 hours. For this reason, acne medications must be used consistently twice a day. Using the proper cleanser and moisturizer makes this possible in most patients. Avoid moisturizers with coconut oil, isopropyl myristate, and other ingredients known to cause acne.
Q: Why does my face get so red after washing it?
A: Rosacea-prone skin types get red from the friction of washing the face – even if just using water. If this occurs, use skin care products with anti-inflammatory ingredients such as argan oil, green tea, fever few, chamomile, caffeine, resveratrol, and niacinamide to sooth the skin. These can be paired with prescription rosacea medications such as Rhofade (oxymetazoline hydrochloride) to reduce facial redness. People with rosacea-prone skin types should avoid facial brushes, scrubs, and vigorous exfoliation.
Q: Why is my melasma not getting better on skin lighteners?
Educating our staff on the basics of skin care can make our office much more efficient. If you liked the format of this column or have questions that you hear often from patients, please email me at [email protected].
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, Aclaris, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
in helping us be more efficient with our time. I asked my staff to put together a list of the most common patient skin care questions. This column can be used as a guide for your staff when answering questions about skin care.
Q: Should my sunscreen be applied first or last?
A: The best way to remember is that medications should be applied closest to the skin and sunscreen should be applied closest to the sun. I recommend that morning skin care be applied in the following order:
Step 1. Facial cleanser
Step 2. Eye products (protect the delicate eye area from the medication)
Step 3. Treatment product (medications, or the most important active ingredients)
Step 4. Moisturizer (if needed)
Step 5. Sunscreen
Q: When can I restart my normal skin care regimen after receiving dermal fillers?
A: The morning after receiving dermal fillers, you can resume your normal facial skin care regimen.
Q: How do I treat my skin after receiving cosmetic injections such as Botox?
A: After you are injected with dermal fillers, keep the Arnica gel, Arnica pads, or post procedure product on your face until bedtime. Prior to bed, wash your face with a gentle cleanser and lukewarm water. Do not use any scrubs, facial brushes, or hydroxy acids. Apply a soothing gel or soothing moisturizer. Do not use hot water on the face for 48 hours. Avoid hot showers, saunas, facial steaming, facial massage, and exercise for 48 hours. If you must exercise, try to keep the face cool with an ice filled bottle of water. Heat makes you more likely to bruise.
Q: Will retinol make my skin sun sensitive?
A: Retinol breaks down upon sun exposure so it should only be used at night. It is a myth that retinol makes your skin sun sensitive. Retinol is a form of vitamin A that helps protect your skin from sun damage by reducing levels of an enzyme called collagenase that is produced after sun exposure. For this and other reasons, retinol can help protect your skin from sun damage. However, if your skin gets irritated from the retinol and you go into the sun, you can develop postinflammatory hyperpigmentation – or dark patches on the skin. It is a good idea to always wear sunscreen in the morning every day whether you are using retinol or not.
Q: What do I do to treat the dark circles under my eyes?
A: Dark circles can be caused by sluggish blood flow around the eyes leading to deposition of a pigment found in blood called hemosiderin. Dark circles around the eyes also can be caused by an increased amount of the pigment melanin that occurs from sun exposure, heat, estrogen, and stress. Treatments include lifestyle habits that increase blood circulation, such as smoking cessation and exercise. Supplements such as horse chestnut may help improve circulation. Ingredients such as the tyrosinase inhibitors ascorbic acid, kojic acid, arbutin, and hydroquinone will help decrease melanin production, and may improve dark circles caused by increased melanin. Wearing a daily sunscreen around the eyes is a very important part of improving the appearance of dark circles under the eyes.
Q: Do I need an eye cream?
A: Most people need an eye product whether it is a serum, gel, or cream. Eye treatment products are usually one of the first skin care products that male patients purchase. The most common indications for eye treatment products are dark circles under the eyes, puffy eyes, dryness, fine lines, or redness. Each one of these indications requires very different ingredients; therefore, most people need more than one eye product depending on what eye issues they are experiencing. Eye treatment products can protect the delicate eye area from medications such as tretinoin that gets on the pillowcase and is transferred to the eye area at night. Eye products that have humectants, such as heparan sulfate or hyaluronic acid, should be used to treat fines lines and dryness, but these may make eyes puffy. For those with dry and puffy eyes, a second eye product with vasoconstrictive ingredients should be used in the daytime while the hydrating one is used at night. Antiaging eye products with retinol and hydroxy acids may irritate sensitive skin types so they can be paired with a soothing eye product. Consider using different eye products for the morning and evening depending on what eye issues need to be treated.
Q: Will moisturizer make my acne worse?
A: Noncomedogenic moisturizers can help improve acne and speed recovery. Using the proper cleanser and moisturizer will make acne medications more tolerable. Many patients cannot tolerate their acne medications every day. The bacteria that cause acne reproduce every 12 hours. For this reason, acne medications must be used consistently twice a day. Using the proper cleanser and moisturizer makes this possible in most patients. Avoid moisturizers with coconut oil, isopropyl myristate, and other ingredients known to cause acne.
Q: Why does my face get so red after washing it?
A: Rosacea-prone skin types get red from the friction of washing the face – even if just using water. If this occurs, use skin care products with anti-inflammatory ingredients such as argan oil, green tea, fever few, chamomile, caffeine, resveratrol, and niacinamide to sooth the skin. These can be paired with prescription rosacea medications such as Rhofade (oxymetazoline hydrochloride) to reduce facial redness. People with rosacea-prone skin types should avoid facial brushes, scrubs, and vigorous exfoliation.
Q: Why is my melasma not getting better on skin lighteners?
Educating our staff on the basics of skin care can make our office much more efficient. If you liked the format of this column or have questions that you hear often from patients, please email me at [email protected].
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, Aclaris, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
in helping us be more efficient with our time. I asked my staff to put together a list of the most common patient skin care questions. This column can be used as a guide for your staff when answering questions about skin care.
Q: Should my sunscreen be applied first or last?
A: The best way to remember is that medications should be applied closest to the skin and sunscreen should be applied closest to the sun. I recommend that morning skin care be applied in the following order:
Step 1. Facial cleanser
Step 2. Eye products (protect the delicate eye area from the medication)
Step 3. Treatment product (medications, or the most important active ingredients)
Step 4. Moisturizer (if needed)
Step 5. Sunscreen
Q: When can I restart my normal skin care regimen after receiving dermal fillers?
A: The morning after receiving dermal fillers, you can resume your normal facial skin care regimen.
Q: How do I treat my skin after receiving cosmetic injections such as Botox?
A: After you are injected with dermal fillers, keep the Arnica gel, Arnica pads, or post procedure product on your face until bedtime. Prior to bed, wash your face with a gentle cleanser and lukewarm water. Do not use any scrubs, facial brushes, or hydroxy acids. Apply a soothing gel or soothing moisturizer. Do not use hot water on the face for 48 hours. Avoid hot showers, saunas, facial steaming, facial massage, and exercise for 48 hours. If you must exercise, try to keep the face cool with an ice filled bottle of water. Heat makes you more likely to bruise.
Q: Will retinol make my skin sun sensitive?
A: Retinol breaks down upon sun exposure so it should only be used at night. It is a myth that retinol makes your skin sun sensitive. Retinol is a form of vitamin A that helps protect your skin from sun damage by reducing levels of an enzyme called collagenase that is produced after sun exposure. For this and other reasons, retinol can help protect your skin from sun damage. However, if your skin gets irritated from the retinol and you go into the sun, you can develop postinflammatory hyperpigmentation – or dark patches on the skin. It is a good idea to always wear sunscreen in the morning every day whether you are using retinol or not.
Q: What do I do to treat the dark circles under my eyes?
A: Dark circles can be caused by sluggish blood flow around the eyes leading to deposition of a pigment found in blood called hemosiderin. Dark circles around the eyes also can be caused by an increased amount of the pigment melanin that occurs from sun exposure, heat, estrogen, and stress. Treatments include lifestyle habits that increase blood circulation, such as smoking cessation and exercise. Supplements such as horse chestnut may help improve circulation. Ingredients such as the tyrosinase inhibitors ascorbic acid, kojic acid, arbutin, and hydroquinone will help decrease melanin production, and may improve dark circles caused by increased melanin. Wearing a daily sunscreen around the eyes is a very important part of improving the appearance of dark circles under the eyes.
Q: Do I need an eye cream?
A: Most people need an eye product whether it is a serum, gel, or cream. Eye treatment products are usually one of the first skin care products that male patients purchase. The most common indications for eye treatment products are dark circles under the eyes, puffy eyes, dryness, fine lines, or redness. Each one of these indications requires very different ingredients; therefore, most people need more than one eye product depending on what eye issues they are experiencing. Eye treatment products can protect the delicate eye area from medications such as tretinoin that gets on the pillowcase and is transferred to the eye area at night. Eye products that have humectants, such as heparan sulfate or hyaluronic acid, should be used to treat fines lines and dryness, but these may make eyes puffy. For those with dry and puffy eyes, a second eye product with vasoconstrictive ingredients should be used in the daytime while the hydrating one is used at night. Antiaging eye products with retinol and hydroxy acids may irritate sensitive skin types so they can be paired with a soothing eye product. Consider using different eye products for the morning and evening depending on what eye issues need to be treated.
Q: Will moisturizer make my acne worse?
A: Noncomedogenic moisturizers can help improve acne and speed recovery. Using the proper cleanser and moisturizer will make acne medications more tolerable. Many patients cannot tolerate their acne medications every day. The bacteria that cause acne reproduce every 12 hours. For this reason, acne medications must be used consistently twice a day. Using the proper cleanser and moisturizer makes this possible in most patients. Avoid moisturizers with coconut oil, isopropyl myristate, and other ingredients known to cause acne.
Q: Why does my face get so red after washing it?
A: Rosacea-prone skin types get red from the friction of washing the face – even if just using water. If this occurs, use skin care products with anti-inflammatory ingredients such as argan oil, green tea, fever few, chamomile, caffeine, resveratrol, and niacinamide to sooth the skin. These can be paired with prescription rosacea medications such as Rhofade (oxymetazoline hydrochloride) to reduce facial redness. People with rosacea-prone skin types should avoid facial brushes, scrubs, and vigorous exfoliation.
Q: Why is my melasma not getting better on skin lighteners?
Educating our staff on the basics of skin care can make our office much more efficient. If you liked the format of this column or have questions that you hear often from patients, please email me at [email protected].
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, Aclaris, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
Letter from the Editor: Stay alert
This week (July 13, 2017) the US Senate released the next iteration of Repeal and Replace. Most involved in health care delivery oppose Medicaid cuts, relaxation of insurance coverage regulations, making the essential benefit set optional, and other parts of this legislation. Although most Americans oppose this legislation, if I were a hedge fund manager, I would be shorting the ACA.
The Editors have gathered an eclectic group of articles for your education and reading pleasure. On the cover, you read about threats from cybercrime, research questioning the safety of long-term use of proton pump inhibitors (I am going on my 20th year of a PPI) and a story about physician burnout.
Be sure and read about the new reversal agent for dabigatran (Pradaxa) – something we have been waiting for. It turns out that SUNSHINE (vitamin D) may be helpful in metastatic colon cancer. Relatives of NAFLD patients are at increased risk of NAFLD themselves and liver disease doubles the risk of colon cancer. EASL published new PBC guidelines.
From the Annals of Internal Medicine, we summarize a large retrospective study again linking interval colon cancers with polypectomy rates of physicians performing screening colonoscopy. The unique twist here is that African American patients tended to be examined by physicians with lower ADR’s compared to whites and they indeed had higher interval cancer rates. We must advocate for reduction in avoidable variations in health outcomes tied to diversity.
We are beginning to present summaries of AGA Presidential Plenary presentations, led off by my colleague from Michigan, Anna Lok (current President of the AASLD). I hope you enjoy this series.
We end this month’s issue with an article I wrote for CGH entitled, “From Obamacare to Trumpcare: Implications for gastroenterologists”. If you have not guessed, the implications are substantial.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This week (July 13, 2017) the US Senate released the next iteration of Repeal and Replace. Most involved in health care delivery oppose Medicaid cuts, relaxation of insurance coverage regulations, making the essential benefit set optional, and other parts of this legislation. Although most Americans oppose this legislation, if I were a hedge fund manager, I would be shorting the ACA.
The Editors have gathered an eclectic group of articles for your education and reading pleasure. On the cover, you read about threats from cybercrime, research questioning the safety of long-term use of proton pump inhibitors (I am going on my 20th year of a PPI) and a story about physician burnout.
Be sure and read about the new reversal agent for dabigatran (Pradaxa) – something we have been waiting for. It turns out that SUNSHINE (vitamin D) may be helpful in metastatic colon cancer. Relatives of NAFLD patients are at increased risk of NAFLD themselves and liver disease doubles the risk of colon cancer. EASL published new PBC guidelines.
From the Annals of Internal Medicine, we summarize a large retrospective study again linking interval colon cancers with polypectomy rates of physicians performing screening colonoscopy. The unique twist here is that African American patients tended to be examined by physicians with lower ADR’s compared to whites and they indeed had higher interval cancer rates. We must advocate for reduction in avoidable variations in health outcomes tied to diversity.
We are beginning to present summaries of AGA Presidential Plenary presentations, led off by my colleague from Michigan, Anna Lok (current President of the AASLD). I hope you enjoy this series.
We end this month’s issue with an article I wrote for CGH entitled, “From Obamacare to Trumpcare: Implications for gastroenterologists”. If you have not guessed, the implications are substantial.
John I. Allen, MD, MBA, AGAF
Editor in Chief
This week (July 13, 2017) the US Senate released the next iteration of Repeal and Replace. Most involved in health care delivery oppose Medicaid cuts, relaxation of insurance coverage regulations, making the essential benefit set optional, and other parts of this legislation. Although most Americans oppose this legislation, if I were a hedge fund manager, I would be shorting the ACA.
The Editors have gathered an eclectic group of articles for your education and reading pleasure. On the cover, you read about threats from cybercrime, research questioning the safety of long-term use of proton pump inhibitors (I am going on my 20th year of a PPI) and a story about physician burnout.
Be sure and read about the new reversal agent for dabigatran (Pradaxa) – something we have been waiting for. It turns out that SUNSHINE (vitamin D) may be helpful in metastatic colon cancer. Relatives of NAFLD patients are at increased risk of NAFLD themselves and liver disease doubles the risk of colon cancer. EASL published new PBC guidelines.
From the Annals of Internal Medicine, we summarize a large retrospective study again linking interval colon cancers with polypectomy rates of physicians performing screening colonoscopy. The unique twist here is that African American patients tended to be examined by physicians with lower ADR’s compared to whites and they indeed had higher interval cancer rates. We must advocate for reduction in avoidable variations in health outcomes tied to diversity.
We are beginning to present summaries of AGA Presidential Plenary presentations, led off by my colleague from Michigan, Anna Lok (current President of the AASLD). I hope you enjoy this series.
We end this month’s issue with an article I wrote for CGH entitled, “From Obamacare to Trumpcare: Implications for gastroenterologists”. If you have not guessed, the implications are substantial.
John I. Allen, MD, MBA, AGAF
Editor in Chief