User login
Update on the VA Precision Oncology Program
Purpose: To inform VA stakeholders of the availability of precision oncology (PO) services for Veterans with advanced cancer.
Background: PO offers the promise of effective, low-toxicity targeted therapies tailored to individual tumor genomics but is unequally available within VHA. A systemwide PO program (POP), including patients in rural areas, launched in July 2016.
Methods: Patients tested with multigene next generation sequencing (NGS) tumor testing through 2 contracted vendors were identified from POP records and cancer characteristics were extracted from POP and medical records. Drug use data were obtained from the VA Corporate Data Warehouse. NGS testing results, and annotations were extracted from POP records.
Results: 1,442 tumor samples were sent for NGS testing as of 5/21/17 from 61 facilities. Rural patient testing (35%) was similar to VHA rurality (33%) and more than twice the US rate (14%). Most common diagnoses: lung (688: adeno 482, squamous 134), unknown (114), colorectal (103), skin (96), prostate (76), and H&N (66). Sample test requests increased rapidly after national implementation in July 2016 (23 samples/month prior to implementation to mean 126 samples/month 3 months later) as did the number of participating facilities (10/quarter to 39/month). Sequencing success rate increased from 68% to 71% over the same interval, while mean turn around time remained similar at 19.7 and 19.1 days, respectively. To date, 26 patients received a recommended drug outside a clinical trial, some more than 9 months after NGS. 5 additional patients had received an NGS-recommended drug prior to testing. NGS results are available for a cohort of 344 patients including: lung 200 (adeno 138, squamous 51), skin 28, LN 20, liver 19, GI 16. 979 variants were found most commonly in TP53, KRAS, STK11, APC, PIK3CA, and CDKN2A. 228 patients (66%) had actionable results (on-label drug 24, off-label drug 165, clinical trial 213). A PO consultation service (available by IFC) and a liquid biopsy are now available nationally.
Conclusions: Implementation of tumor NGS testing in VHA has been successful. Further program expansion, addition of hematological malignancies, deployment of informatics tools and efforts to expand access to appropriate drugs are ongoing.
Purpose: To inform VA stakeholders of the availability of precision oncology (PO) services for Veterans with advanced cancer.
Background: PO offers the promise of effective, low-toxicity targeted therapies tailored to individual tumor genomics but is unequally available within VHA. A systemwide PO program (POP), including patients in rural areas, launched in July 2016.
Methods: Patients tested with multigene next generation sequencing (NGS) tumor testing through 2 contracted vendors were identified from POP records and cancer characteristics were extracted from POP and medical records. Drug use data were obtained from the VA Corporate Data Warehouse. NGS testing results, and annotations were extracted from POP records.
Results: 1,442 tumor samples were sent for NGS testing as of 5/21/17 from 61 facilities. Rural patient testing (35%) was similar to VHA rurality (33%) and more than twice the US rate (14%). Most common diagnoses: lung (688: adeno 482, squamous 134), unknown (114), colorectal (103), skin (96), prostate (76), and H&N (66). Sample test requests increased rapidly after national implementation in July 2016 (23 samples/month prior to implementation to mean 126 samples/month 3 months later) as did the number of participating facilities (10/quarter to 39/month). Sequencing success rate increased from 68% to 71% over the same interval, while mean turn around time remained similar at 19.7 and 19.1 days, respectively. To date, 26 patients received a recommended drug outside a clinical trial, some more than 9 months after NGS. 5 additional patients had received an NGS-recommended drug prior to testing. NGS results are available for a cohort of 344 patients including: lung 200 (adeno 138, squamous 51), skin 28, LN 20, liver 19, GI 16. 979 variants were found most commonly in TP53, KRAS, STK11, APC, PIK3CA, and CDKN2A. 228 patients (66%) had actionable results (on-label drug 24, off-label drug 165, clinical trial 213). A PO consultation service (available by IFC) and a liquid biopsy are now available nationally.
Conclusions: Implementation of tumor NGS testing in VHA has been successful. Further program expansion, addition of hematological malignancies, deployment of informatics tools and efforts to expand access to appropriate drugs are ongoing.
Purpose: To inform VA stakeholders of the availability of precision oncology (PO) services for Veterans with advanced cancer.
Background: PO offers the promise of effective, low-toxicity targeted therapies tailored to individual tumor genomics but is unequally available within VHA. A systemwide PO program (POP), including patients in rural areas, launched in July 2016.
Methods: Patients tested with multigene next generation sequencing (NGS) tumor testing through 2 contracted vendors were identified from POP records and cancer characteristics were extracted from POP and medical records. Drug use data were obtained from the VA Corporate Data Warehouse. NGS testing results, and annotations were extracted from POP records.
Results: 1,442 tumor samples were sent for NGS testing as of 5/21/17 from 61 facilities. Rural patient testing (35%) was similar to VHA rurality (33%) and more than twice the US rate (14%). Most common diagnoses: lung (688: adeno 482, squamous 134), unknown (114), colorectal (103), skin (96), prostate (76), and H&N (66). Sample test requests increased rapidly after national implementation in July 2016 (23 samples/month prior to implementation to mean 126 samples/month 3 months later) as did the number of participating facilities (10/quarter to 39/month). Sequencing success rate increased from 68% to 71% over the same interval, while mean turn around time remained similar at 19.7 and 19.1 days, respectively. To date, 26 patients received a recommended drug outside a clinical trial, some more than 9 months after NGS. 5 additional patients had received an NGS-recommended drug prior to testing. NGS results are available for a cohort of 344 patients including: lung 200 (adeno 138, squamous 51), skin 28, LN 20, liver 19, GI 16. 979 variants were found most commonly in TP53, KRAS, STK11, APC, PIK3CA, and CDKN2A. 228 patients (66%) had actionable results (on-label drug 24, off-label drug 165, clinical trial 213). A PO consultation service (available by IFC) and a liquid biopsy are now available nationally.
Conclusions: Implementation of tumor NGS testing in VHA has been successful. Further program expansion, addition of hematological malignancies, deployment of informatics tools and efforts to expand access to appropriate drugs are ongoing.
DDSEP® 8 Quick quiz - August 2017 Question 2
Q2: Answer: D
The history of weight loss, intermittent diarrhea, and bloating are suspicious for celiac disease. While lactose intolerance can explain the pain, diarrhea, and bloating, there does not appear to be any correlation with the ingestion of particular foods, nor should there be any weight loss. While inflammatory bowel disease is certainly a possible explanation for his symptoms, it would be premature to jump to upper and lower endoscopy as initial evaluations.
Tissue transglutaminase antibodies are a sensitive and specific screening test for celiac disease, with published sensitivities and specificities greater than 95%. Obtaining a total serum IgA level at the time of screening is recommended to exclude IgA deficiency, which may result in a false-negative test.
Reference
1. Husby S., Koletzko S., Korponay-Szabo I.R., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
2. Olen O., Gudjonsdottir A., Browaldh L., et al. Antibodies against deamindated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:695-700.
Q2: Answer: D
The history of weight loss, intermittent diarrhea, and bloating are suspicious for celiac disease. While lactose intolerance can explain the pain, diarrhea, and bloating, there does not appear to be any correlation with the ingestion of particular foods, nor should there be any weight loss. While inflammatory bowel disease is certainly a possible explanation for his symptoms, it would be premature to jump to upper and lower endoscopy as initial evaluations.
Tissue transglutaminase antibodies are a sensitive and specific screening test for celiac disease, with published sensitivities and specificities greater than 95%. Obtaining a total serum IgA level at the time of screening is recommended to exclude IgA deficiency, which may result in a false-negative test.
Reference
1. Husby S., Koletzko S., Korponay-Szabo I.R., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
2. Olen O., Gudjonsdottir A., Browaldh L., et al. Antibodies against deamindated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:695-700.
Q2: Answer: D
The history of weight loss, intermittent diarrhea, and bloating are suspicious for celiac disease. While lactose intolerance can explain the pain, diarrhea, and bloating, there does not appear to be any correlation with the ingestion of particular foods, nor should there be any weight loss. While inflammatory bowel disease is certainly a possible explanation for his symptoms, it would be premature to jump to upper and lower endoscopy as initial evaluations.
Tissue transglutaminase antibodies are a sensitive and specific screening test for celiac disease, with published sensitivities and specificities greater than 95%. Obtaining a total serum IgA level at the time of screening is recommended to exclude IgA deficiency, which may result in a false-negative test.
Reference
1. Husby S., Koletzko S., Korponay-Szabo I.R., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-60.
2. Olen O., Gudjonsdottir A., Browaldh L., et al. Antibodies against deamindated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:695-700.
A 10-year-old boy is referred after he was noted to have lost weight over the past year during a routine physical exam. He denies trying to lose weight. He has occasional abdominal pain and intermittent watery nonbloody diarrhea, which do not seem associated with particular foods. He also complains of feeling bloated and his mother reports that “his belly always looks swollen.” He has had no other symptoms of illness. On physical exam, he is slender and has a mildly distended and tympanitic abdomen.
Opioid abuse and overdose: Keep your patients safe
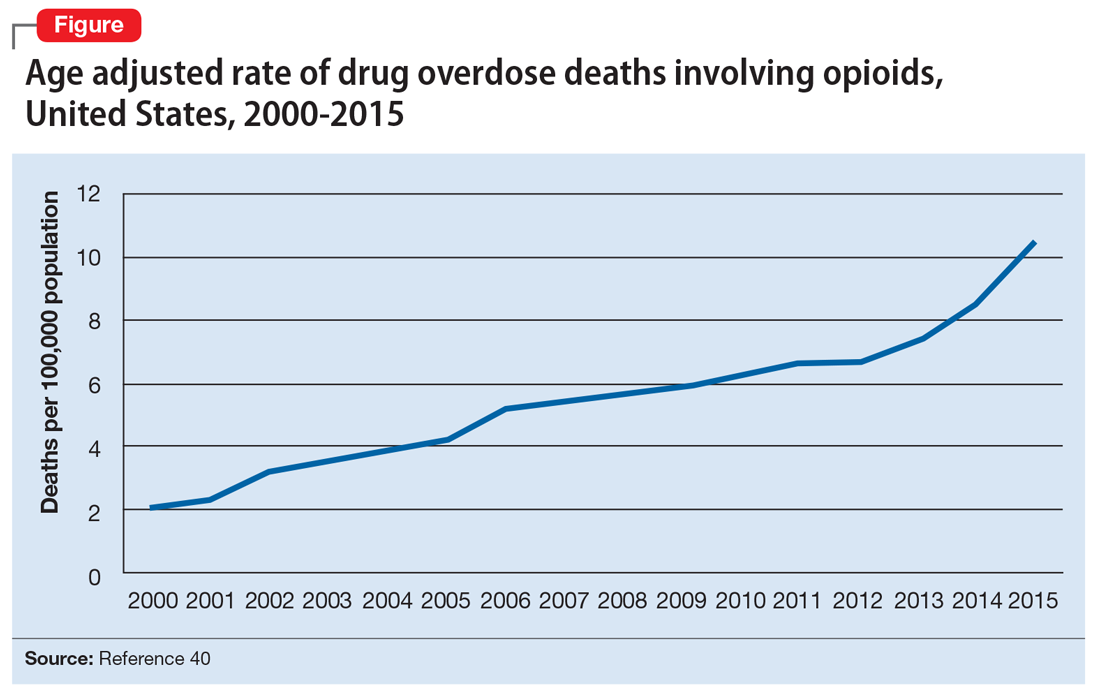
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13

Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”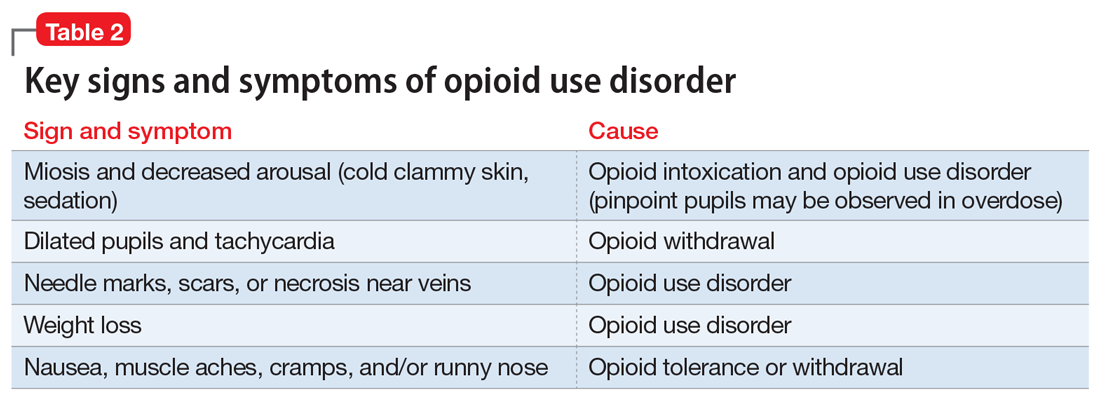
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28

MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.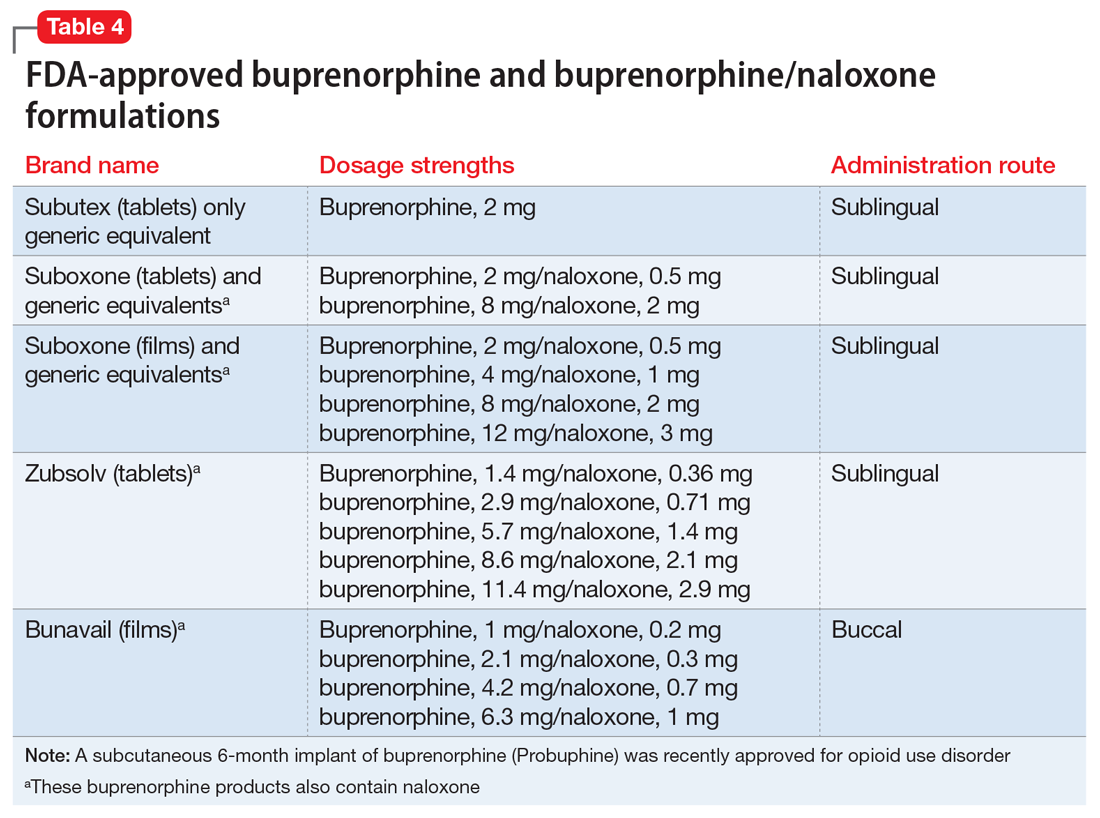
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5

Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13

Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28

MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5

Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13

Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28

MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
Evaluating suicidality
For first-episode psychosis, psychiatrists should behave like cardiologists
Myocardial infarction (MI) is the leading cause of death in the United States, and schizophrenia is the leading cause of disability. But while cardiologists manage the first heart attack very aggressively to prevent a second MI, we psychiatrists generally do not manage first-episode psychosis (FEP) as aggressively to prevent the more malignant second psychotic episode. Yet abundant evidence indicates that psychiatrists must behave like cardiologists at the onset of schizophrenia and other serious psychosis.
Individuals who survive the first heart attack, which permanently destroys part of the myocardium, are at high risk for a second MI, which may lead to death or weaken the heart so much that heart transplantation becomes necessary. Only implementation of aggressive medical intervention will prevent the likelihood of death due to a second MI in a person who has already suffered a first MI.
Similarly, the FEP of schizophrenia destroys brain tissue, about 10 to 12 cc containing millions of glial cells and billions of synapses.2 This neurotoxicity of psychosis is mediated by neuroinflammation and oxidative stress.3 In most FEP patients, the risk of a second psychotic episode is high, and the tissue destruction of the brain’s gray and white matter infrastructure is even more extensive, leading to clinical deterioration, treatment resistance, and functional disability. That is the grim turning point in the trajectory of schizophrenia.
Although most FEP patients respond well to antipsychotic medications and often return to their baseline social and vocational functioning, after a second episode, they are much more likely to become disabled. Unlike physical death, the mental, cognitive, social, and vocational death of chronic schizophrenia goes on for decades with much suffering, misery, and inability to have love and work, which is what life is all about (according to Freud).
But what is the most common psychiatric practice for a patient who suffers a FEP after he (she) is admitted to an acute inpatient ward? The patient is started on an oral antipsychotic but a long-acting injectable (LAI) antipsychotic, which is the best protection against future episodes, is never considered, let alone recommended. The patient is given a prescription for an oral antipsychotic at discharge and the family is told to find a private psychiatrist or a community mental health center for follow-up. This practice pattern will likely guarantee a relapse into a second psychotic episode for the following reasons:
- patients’ lack of insight (anosognosia) and refusal to believe they are sick or need medications
- adverse effects, especially extrapyramidal symptoms, to which FEP patients are particularly vulnerable unless they are started on small doses
- apathy and lack of motivation to take medication due to negative symptoms, which impair ability to initiate actions (avolition)
- severe memory impairment that leads to forgetting medications
- substance use, such as marijuana, stimulants, and hallucinogens, as well as alcohol, interferes with adherence.
Most patients and families are ignorant about FEP of schizophrenia and its recurrence and devastating effects.
Thus, because of the almost ubiquitous inability to adhere fully to antipsychotic medications after discharge, FEP patients are essentially destined (ie, doomed) to experience a destructive second psychotic episode, whose neurotoxicity starts the patient on a downhill journey of lifetime disability.4 LAI antipsychotics are the optimal solution to this serious problem, yet 99.99% of psychiatrists never start LAI during a FEP. This is inexplicable considering the body of evidence that supports early use of LAI to prevent relapse. Of the multipronged strategy that should be used for FEP patients to circumvent a second episode and avoid disability, starting LAI in FEP is the most important interventional tactic.5 Consider the following studies that support initiating LAIs during the FEP:
In South Africa, Emsley et al6 conducted the first study of LAI in FEP. In a 2-year follow-up, 64% of patients had complete remission and returned to their baseline functioning with restoration of insight and good quality of life. When the study ended and patients were returned to their referring psychiatrists after 2 years, all patients were switched to oral antipsychotics, because it was the standard practice among psychiatrists there. All patients relapsed within a few months due to poor adherence to oral medications. When they were placed back on the LAI they had received, a sobering (even shocking) clinical finding emerged: 16% of those who had responded so well to LAI for 2 years no longer responded!7 This rapid emergence of treatment resistance after only a second psychotic episode demonstrates how the brain changes drastically after a second episode and validates the recent adoption of “stages” in schizophrenia, similar to cancer stages.8 Many more patients will develop treatment resistance after subsequent episodes.
Subotnik et al9 compared LAI vs oral risperidone in 86 FEP patients. At the end of 1 year, they reported a 650% higher relapse rate in the oral medication group compared with the LAI group (33% vs 5%).9 This well-done study is a wake-up call for psychiatrists to help FEP patients avoid a brain-damaging second episode by using LAI as a first-line option in FEP.
In a separate study, Subotnik et al10 reported that when the blood level of a patient receiving an antipsychotic is measured at the time of discharge from FEP and every month for a year, all it took for a relapse was a drop of 25%.10 Thus, skipping an antipsychotic just 1 day out of 4 (partial nonadherence) is enough to cause a psychotic relapse.
So what should psychiatrists and nurse practitioners do to protect FEP patients from losing their lives to the permanent disability that begins with a second psychotic episode? They must simply change their attitude and their old-fashioned (antiquated?) prescribing habits that keep failing, and start administering LAI during the initial hospitalization right after a few (usually 3 or 4) days of receiving oral antipsychotics (with nursing-assured swallowing of pills) (Table 2). By starting the patient with oral antipsychotics, the presence of an allergic reaction is ruled out, and efficacy onset begins within 2 to 3 days.11 LAI can then be administered several days before discharge and continued in the outpatient setting.

However, various essential psychosocial interventions should be provided along with LAI to ensure progress toward remission and functional recovery after an FEP. The recently published National Institute of Mental Health-sponsored RAISE study12 is a prime example of the synergy between a multimodal and multidisciplinary team-based approach and antipsychotic medication to improve outcome and quality of life after emerging from FEP.
As psychiatric practitioners, we must be clinically aggressive during the “FEP window of opportunity” to avoid a second episode, thereby bending the curve of the downhill trajectory that occurs after second episodes. We must behave like cardiologists, and relentlessly protect patients who suffer a first “brain attack” from experiencing a relapse. No doubt, any psychiatrists who have a family member with FEP would channel their inner cardiologist and implement the evidence-based recommendations described above. But then, shouldn’t we apply the same standard of care to every FEP patient we see?
1. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
2. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
3. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog
4. Alvarez-Jiménoz M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33,38-45,e3.
6. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
7. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
8. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
9. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
10. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
11. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
12. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372.
Myocardial infarction (MI) is the leading cause of death in the United States, and schizophrenia is the leading cause of disability. But while cardiologists manage the first heart attack very aggressively to prevent a second MI, we psychiatrists generally do not manage first-episode psychosis (FEP) as aggressively to prevent the more malignant second psychotic episode. Yet abundant evidence indicates that psychiatrists must behave like cardiologists at the onset of schizophrenia and other serious psychosis.
Individuals who survive the first heart attack, which permanently destroys part of the myocardium, are at high risk for a second MI, which may lead to death or weaken the heart so much that heart transplantation becomes necessary. Only implementation of aggressive medical intervention will prevent the likelihood of death due to a second MI in a person who has already suffered a first MI.
Similarly, the FEP of schizophrenia destroys brain tissue, about 10 to 12 cc containing millions of glial cells and billions of synapses.2 This neurotoxicity of psychosis is mediated by neuroinflammation and oxidative stress.3 In most FEP patients, the risk of a second psychotic episode is high, and the tissue destruction of the brain’s gray and white matter infrastructure is even more extensive, leading to clinical deterioration, treatment resistance, and functional disability. That is the grim turning point in the trajectory of schizophrenia.
Although most FEP patients respond well to antipsychotic medications and often return to their baseline social and vocational functioning, after a second episode, they are much more likely to become disabled. Unlike physical death, the mental, cognitive, social, and vocational death of chronic schizophrenia goes on for decades with much suffering, misery, and inability to have love and work, which is what life is all about (according to Freud).
But what is the most common psychiatric practice for a patient who suffers a FEP after he (she) is admitted to an acute inpatient ward? The patient is started on an oral antipsychotic but a long-acting injectable (LAI) antipsychotic, which is the best protection against future episodes, is never considered, let alone recommended. The patient is given a prescription for an oral antipsychotic at discharge and the family is told to find a private psychiatrist or a community mental health center for follow-up. This practice pattern will likely guarantee a relapse into a second psychotic episode for the following reasons:
- patients’ lack of insight (anosognosia) and refusal to believe they are sick or need medications
- adverse effects, especially extrapyramidal symptoms, to which FEP patients are particularly vulnerable unless they are started on small doses
- apathy and lack of motivation to take medication due to negative symptoms, which impair ability to initiate actions (avolition)
- severe memory impairment that leads to forgetting medications
- substance use, such as marijuana, stimulants, and hallucinogens, as well as alcohol, interferes with adherence.
Most patients and families are ignorant about FEP of schizophrenia and its recurrence and devastating effects.
Thus, because of the almost ubiquitous inability to adhere fully to antipsychotic medications after discharge, FEP patients are essentially destined (ie, doomed) to experience a destructive second psychotic episode, whose neurotoxicity starts the patient on a downhill journey of lifetime disability.4 LAI antipsychotics are the optimal solution to this serious problem, yet 99.99% of psychiatrists never start LAI during a FEP. This is inexplicable considering the body of evidence that supports early use of LAI to prevent relapse. Of the multipronged strategy that should be used for FEP patients to circumvent a second episode and avoid disability, starting LAI in FEP is the most important interventional tactic.5 Consider the following studies that support initiating LAIs during the FEP:
In South Africa, Emsley et al6 conducted the first study of LAI in FEP. In a 2-year follow-up, 64% of patients had complete remission and returned to their baseline functioning with restoration of insight and good quality of life. When the study ended and patients were returned to their referring psychiatrists after 2 years, all patients were switched to oral antipsychotics, because it was the standard practice among psychiatrists there. All patients relapsed within a few months due to poor adherence to oral medications. When they were placed back on the LAI they had received, a sobering (even shocking) clinical finding emerged: 16% of those who had responded so well to LAI for 2 years no longer responded!7 This rapid emergence of treatment resistance after only a second psychotic episode demonstrates how the brain changes drastically after a second episode and validates the recent adoption of “stages” in schizophrenia, similar to cancer stages.8 Many more patients will develop treatment resistance after subsequent episodes.
Subotnik et al9 compared LAI vs oral risperidone in 86 FEP patients. At the end of 1 year, they reported a 650% higher relapse rate in the oral medication group compared with the LAI group (33% vs 5%).9 This well-done study is a wake-up call for psychiatrists to help FEP patients avoid a brain-damaging second episode by using LAI as a first-line option in FEP.
In a separate study, Subotnik et al10 reported that when the blood level of a patient receiving an antipsychotic is measured at the time of discharge from FEP and every month for a year, all it took for a relapse was a drop of 25%.10 Thus, skipping an antipsychotic just 1 day out of 4 (partial nonadherence) is enough to cause a psychotic relapse.
So what should psychiatrists and nurse practitioners do to protect FEP patients from losing their lives to the permanent disability that begins with a second psychotic episode? They must simply change their attitude and their old-fashioned (antiquated?) prescribing habits that keep failing, and start administering LAI during the initial hospitalization right after a few (usually 3 or 4) days of receiving oral antipsychotics (with nursing-assured swallowing of pills) (Table 2). By starting the patient with oral antipsychotics, the presence of an allergic reaction is ruled out, and efficacy onset begins within 2 to 3 days.11 LAI can then be administered several days before discharge and continued in the outpatient setting.

However, various essential psychosocial interventions should be provided along with LAI to ensure progress toward remission and functional recovery after an FEP. The recently published National Institute of Mental Health-sponsored RAISE study12 is a prime example of the synergy between a multimodal and multidisciplinary team-based approach and antipsychotic medication to improve outcome and quality of life after emerging from FEP.
As psychiatric practitioners, we must be clinically aggressive during the “FEP window of opportunity” to avoid a second episode, thereby bending the curve of the downhill trajectory that occurs after second episodes. We must behave like cardiologists, and relentlessly protect patients who suffer a first “brain attack” from experiencing a relapse. No doubt, any psychiatrists who have a family member with FEP would channel their inner cardiologist and implement the evidence-based recommendations described above. But then, shouldn’t we apply the same standard of care to every FEP patient we see?
Myocardial infarction (MI) is the leading cause of death in the United States, and schizophrenia is the leading cause of disability. But while cardiologists manage the first heart attack very aggressively to prevent a second MI, we psychiatrists generally do not manage first-episode psychosis (FEP) as aggressively to prevent the more malignant second psychotic episode. Yet abundant evidence indicates that psychiatrists must behave like cardiologists at the onset of schizophrenia and other serious psychosis.
Individuals who survive the first heart attack, which permanently destroys part of the myocardium, are at high risk for a second MI, which may lead to death or weaken the heart so much that heart transplantation becomes necessary. Only implementation of aggressive medical intervention will prevent the likelihood of death due to a second MI in a person who has already suffered a first MI.
Similarly, the FEP of schizophrenia destroys brain tissue, about 10 to 12 cc containing millions of glial cells and billions of synapses.2 This neurotoxicity of psychosis is mediated by neuroinflammation and oxidative stress.3 In most FEP patients, the risk of a second psychotic episode is high, and the tissue destruction of the brain’s gray and white matter infrastructure is even more extensive, leading to clinical deterioration, treatment resistance, and functional disability. That is the grim turning point in the trajectory of schizophrenia.
Although most FEP patients respond well to antipsychotic medications and often return to their baseline social and vocational functioning, after a second episode, they are much more likely to become disabled. Unlike physical death, the mental, cognitive, social, and vocational death of chronic schizophrenia goes on for decades with much suffering, misery, and inability to have love and work, which is what life is all about (according to Freud).
But what is the most common psychiatric practice for a patient who suffers a FEP after he (she) is admitted to an acute inpatient ward? The patient is started on an oral antipsychotic but a long-acting injectable (LAI) antipsychotic, which is the best protection against future episodes, is never considered, let alone recommended. The patient is given a prescription for an oral antipsychotic at discharge and the family is told to find a private psychiatrist or a community mental health center for follow-up. This practice pattern will likely guarantee a relapse into a second psychotic episode for the following reasons:
- patients’ lack of insight (anosognosia) and refusal to believe they are sick or need medications
- adverse effects, especially extrapyramidal symptoms, to which FEP patients are particularly vulnerable unless they are started on small doses
- apathy and lack of motivation to take medication due to negative symptoms, which impair ability to initiate actions (avolition)
- severe memory impairment that leads to forgetting medications
- substance use, such as marijuana, stimulants, and hallucinogens, as well as alcohol, interferes with adherence.
Most patients and families are ignorant about FEP of schizophrenia and its recurrence and devastating effects.
Thus, because of the almost ubiquitous inability to adhere fully to antipsychotic medications after discharge, FEP patients are essentially destined (ie, doomed) to experience a destructive second psychotic episode, whose neurotoxicity starts the patient on a downhill journey of lifetime disability.4 LAI antipsychotics are the optimal solution to this serious problem, yet 99.99% of psychiatrists never start LAI during a FEP. This is inexplicable considering the body of evidence that supports early use of LAI to prevent relapse. Of the multipronged strategy that should be used for FEP patients to circumvent a second episode and avoid disability, starting LAI in FEP is the most important interventional tactic.5 Consider the following studies that support initiating LAIs during the FEP:
In South Africa, Emsley et al6 conducted the first study of LAI in FEP. In a 2-year follow-up, 64% of patients had complete remission and returned to their baseline functioning with restoration of insight and good quality of life. When the study ended and patients were returned to their referring psychiatrists after 2 years, all patients were switched to oral antipsychotics, because it was the standard practice among psychiatrists there. All patients relapsed within a few months due to poor adherence to oral medications. When they were placed back on the LAI they had received, a sobering (even shocking) clinical finding emerged: 16% of those who had responded so well to LAI for 2 years no longer responded!7 This rapid emergence of treatment resistance after only a second psychotic episode demonstrates how the brain changes drastically after a second episode and validates the recent adoption of “stages” in schizophrenia, similar to cancer stages.8 Many more patients will develop treatment resistance after subsequent episodes.
Subotnik et al9 compared LAI vs oral risperidone in 86 FEP patients. At the end of 1 year, they reported a 650% higher relapse rate in the oral medication group compared with the LAI group (33% vs 5%).9 This well-done study is a wake-up call for psychiatrists to help FEP patients avoid a brain-damaging second episode by using LAI as a first-line option in FEP.
In a separate study, Subotnik et al10 reported that when the blood level of a patient receiving an antipsychotic is measured at the time of discharge from FEP and every month for a year, all it took for a relapse was a drop of 25%.10 Thus, skipping an antipsychotic just 1 day out of 4 (partial nonadherence) is enough to cause a psychotic relapse.
So what should psychiatrists and nurse practitioners do to protect FEP patients from losing their lives to the permanent disability that begins with a second psychotic episode? They must simply change their attitude and their old-fashioned (antiquated?) prescribing habits that keep failing, and start administering LAI during the initial hospitalization right after a few (usually 3 or 4) days of receiving oral antipsychotics (with nursing-assured swallowing of pills) (Table 2). By starting the patient with oral antipsychotics, the presence of an allergic reaction is ruled out, and efficacy onset begins within 2 to 3 days.11 LAI can then be administered several days before discharge and continued in the outpatient setting.

However, various essential psychosocial interventions should be provided along with LAI to ensure progress toward remission and functional recovery after an FEP. The recently published National Institute of Mental Health-sponsored RAISE study12 is a prime example of the synergy between a multimodal and multidisciplinary team-based approach and antipsychotic medication to improve outcome and quality of life after emerging from FEP.
As psychiatric practitioners, we must be clinically aggressive during the “FEP window of opportunity” to avoid a second episode, thereby bending the curve of the downhill trajectory that occurs after second episodes. We must behave like cardiologists, and relentlessly protect patients who suffer a first “brain attack” from experiencing a relapse. No doubt, any psychiatrists who have a family member with FEP would channel their inner cardiologist and implement the evidence-based recommendations described above. But then, shouldn’t we apply the same standard of care to every FEP patient we see?
1. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
2. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
3. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog
4. Alvarez-Jiménoz M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33,38-45,e3.
6. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
7. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
8. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
9. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
10. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
11. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
12. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372.
1. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
2. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
3. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog
4. Alvarez-Jiménoz M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33,38-45,e3.
6. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
7. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
8. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
9. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
10. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
11. Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60(12):1228-1235.
12. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am J Psychiatry. 2016;173(4):362-372.
DDSEP® 8 Quick quiz - August 2017 Question 1
Q2: Answer: B
This patient has chronic hepatitis E infection, as demonstrated by the positive hepatitis IgG antibody. It is recommended that HEV RNA be identified in serum or stool for diagnosis of hepatitis E. However, HEV RNA PCR is not readily available outside of research settings and therefore the Centers for Disease Control and Prevention states that the diagnosis can be confirmed only by testing for the presence of antibody against HEV or HEV RNA. Providers must be aware of the possibility of false positives and negatives for HEV serologies.
In immunocompetent individuals, hepatitis E is generally a self-limited condition, but in solid-organ transplant recipients, chronic infection can ensue. Hepatitis E infection in solid-organ transplant recipients has been linked to consumption of game meat, pork, and mussels. The infection is largely asymptomatic, but occasionally presents with jaundice. The liver test elevations are mild, with ALT levels up to 300 U/L. Approximately 60% of transplant recipients who are infected with hepatitis E develop chronic infections.
The best treatment for chronic hepatitis E in solid-organ transplant recipients is ribavirin. In one study, the sustained virologic response rate was 78% after a course of approximately 3 months of ribavirin. Pegylated interferon has been used for treatment of hepatitis E, but has less evidence to support its use and has a less favorable side effect profile. Sofosbuvir is a treatment for hepatitis C and therefore is not correct, though there are recent data suggesting that sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin.
Observation is not a correct answer, as about 10% of patients with chronic hepatitis E may develop cirrhosis. Although not one of the provided answers, lowering the overall immunosuppression would be part of the treatment approach in a solid-organ transplant recipient with chronic hepatitis E.
Reference
1. Kamar N., Izopet J., Tripon S., et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014 Mar 20;370(12):1111-20.
2. Dao Thi V.L., Debing Y., Wu X. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016 Jan;150(1):82-5.
Q2: Answer: B
This patient has chronic hepatitis E infection, as demonstrated by the positive hepatitis IgG antibody. It is recommended that HEV RNA be identified in serum or stool for diagnosis of hepatitis E. However, HEV RNA PCR is not readily available outside of research settings and therefore the Centers for Disease Control and Prevention states that the diagnosis can be confirmed only by testing for the presence of antibody against HEV or HEV RNA. Providers must be aware of the possibility of false positives and negatives for HEV serologies.
In immunocompetent individuals, hepatitis E is generally a self-limited condition, but in solid-organ transplant recipients, chronic infection can ensue. Hepatitis E infection in solid-organ transplant recipients has been linked to consumption of game meat, pork, and mussels. The infection is largely asymptomatic, but occasionally presents with jaundice. The liver test elevations are mild, with ALT levels up to 300 U/L. Approximately 60% of transplant recipients who are infected with hepatitis E develop chronic infections.
The best treatment for chronic hepatitis E in solid-organ transplant recipients is ribavirin. In one study, the sustained virologic response rate was 78% after a course of approximately 3 months of ribavirin. Pegylated interferon has been used for treatment of hepatitis E, but has less evidence to support its use and has a less favorable side effect profile. Sofosbuvir is a treatment for hepatitis C and therefore is not correct, though there are recent data suggesting that sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin.
Observation is not a correct answer, as about 10% of patients with chronic hepatitis E may develop cirrhosis. Although not one of the provided answers, lowering the overall immunosuppression would be part of the treatment approach in a solid-organ transplant recipient with chronic hepatitis E.
Reference
1. Kamar N., Izopet J., Tripon S., et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014 Mar 20;370(12):1111-20.
2. Dao Thi V.L., Debing Y., Wu X. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016 Jan;150(1):82-5.
Q2: Answer: B
This patient has chronic hepatitis E infection, as demonstrated by the positive hepatitis IgG antibody. It is recommended that HEV RNA be identified in serum or stool for diagnosis of hepatitis E. However, HEV RNA PCR is not readily available outside of research settings and therefore the Centers for Disease Control and Prevention states that the diagnosis can be confirmed only by testing for the presence of antibody against HEV or HEV RNA. Providers must be aware of the possibility of false positives and negatives for HEV serologies.
In immunocompetent individuals, hepatitis E is generally a self-limited condition, but in solid-organ transplant recipients, chronic infection can ensue. Hepatitis E infection in solid-organ transplant recipients has been linked to consumption of game meat, pork, and mussels. The infection is largely asymptomatic, but occasionally presents with jaundice. The liver test elevations are mild, with ALT levels up to 300 U/L. Approximately 60% of transplant recipients who are infected with hepatitis E develop chronic infections.
The best treatment for chronic hepatitis E in solid-organ transplant recipients is ribavirin. In one study, the sustained virologic response rate was 78% after a course of approximately 3 months of ribavirin. Pegylated interferon has been used for treatment of hepatitis E, but has less evidence to support its use and has a less favorable side effect profile. Sofosbuvir is a treatment for hepatitis C and therefore is not correct, though there are recent data suggesting that sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin.
Observation is not a correct answer, as about 10% of patients with chronic hepatitis E may develop cirrhosis. Although not one of the provided answers, lowering the overall immunosuppression would be part of the treatment approach in a solid-organ transplant recipient with chronic hepatitis E.
Reference
1. Kamar N., Izopet J., Tripon S., et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014 Mar 20;370(12):1111-20.
2. Dao Thi V.L., Debing Y., Wu X. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016 Jan;150(1):82-5.
A 64-year-old man arrives at the transplant clinic for his annual posttransplant assessment. He received a deceased-donor liver transplant 4 years ago for nonalcoholic steatohepatitis (NASH)–related cirrhosis. His immediate postoperative course was unremarkable, but he does have posttransplant hypertension, diabetes mellitus (diet controlled), and obesity. His alanine aminotransferase and aspartate aminotransferase levels have been modestly elevated at 1-3 times the upper limit of normal for 2.5 years.
Multiple liver biopsies have shown only nonspecific inflammation, with no features of cellular- or antibody-mediated rejection, or recurrent NASH. Medications include tacrolimus, mycophenolate mofetil, amlodipine, and low-dose aspirin. Tacrolimus trough levels have ranged from 8 to 10 ng/mL intentionally as it was thought that the liver test abnormalities may be an immunologically driven phenomenon despite the lack of objective liver biopsy–based evidence. As a new provider for this patient, you decide to recheck several laboratory values to rule out alternative reasons for the elevated aminotransferases. The lab results are as follows: hepatitis B DNA negative, hepatitis C RNA negative, smooth muscle antibody negative, anti-nuclear antibody negative, pANCA negative, hepatitis E IgG positive.
Triple-bead mixed amphetamine salt for ADHD
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3
Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3
Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3

In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3

Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3

Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3

In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
Stimulants are first-line psychopharmacologic interventions for attention-deficit/hyperactivity disorder (ADHD), and their efficacy is supported by clinical trials and meta-analyses in children and adolescents1 as well as adults.2 Despite decades of tolerability and efficacy data supporting their use, a major drawback of stimulants is that their salutary therapeutic effects wane once the medication is cleared or metabolized. Both mixed amphetamine- and methylphenidate-based preparations have short half-lives, necessitating multiple doses per day (eg, 3 or 4 times a day) when short-acting preparations are used. Over the past 15 years, nearly a dozen formulations were developed that extend the duration of action through delayed release, delayed absorption, or utilizing prodrugs.
The encapsulated preparation contains 3 MAS beads: an immediate-release amphetamine salt bead, a pulsed-delayed release bead, and an extended-release bead (Figure 1), which give rise to a unique pharmacokinetic profile (Figure 2).3

Mechanism of action
Like all MAS, this formulation blocks the reuptake of norepinephrine and dopamine, increasing synaptic concentrations of these monoamine neurotransmitters. Additionally, amphetamine salts may inhibit the activity of monoamine oxidase (MAO), further increasing synaptic levels of monoamines.4 Enhancing noradrenergic, dopaminergic neurotransmission, particularly within the prefrontal cortex, increases attention, working memory, and processing speed in patients with ADHD.4
Pharmacokinetics
Cmax occurs approximately 7 to 10 hours and 8 hours following administration in adolescent and adult patients, respectively (Figure 2).3 In adolescents who were administered a single dose of long-acting, triple-bead MAS, Cmax and area under the curve (AUC) for d- and l-amphetamine were both 21% to 31% higher compared with adults3 and did not appear to be affected by sex or race.3

Half-life is 10 to 11 hours for d-amphetamine and 10 to 13 hours for l-amphetamine and does not statistically differ between pediatric and adult studies.3
Metabolism and elimination. Amphetamines are partially metabolized through cytochrome 450 (CYP) 2D6-dependent mechanisms, and thus in CYP2D6 poor metabolizers medication exposure may be increased, while decreased exposure may occur in ultra-rapid metabolizers; however, there are no guidelines from the Clinical Pharmacogenetics Implementation Consortium regarding alternate dosing strategies for patients based on CYP2D6 genotype or activity phenotype.5 Because amphetamines are renally excreted, dosages should be adjusted in patients with renal impairment.
Drug interactions. Medications that affect gastrointestinal and urinary pH may affect serum concentrations of amphetamine. Specifically, agents that increase gastric pH (eg, proton pump inhibitors) as well as urinary alkalinizing agents (eg, acetazolamide, some thiazide diuretics) increase serum amphetamine concentrations.3 Because amphetamine is a weak MAOI, there is a theoretical risk of serotonin syndrome when amphetamine-based preparations are used concurrently with SSRIs, TCAs, and MAOIs. However, the concurrent use of MAS and SSRIs generally is considered safe and common practice in patients with ADHD and co-occurring anxiety6,7 or depressive disorders.
Dosing
Long-acting, triple-bead MAS is available in 12.5-, 25-, 37.5-, and 50-mg capsules. The capsule may be opened and sprinkled in food for patients who cannot swallow capsules. Opening of the capsule results in similar absorption relative to oral administration of the intact capsule.3
In adults with ADHD, long-acting, triple-bead MAS should be initiated at 12.5 mg in the morning (Table 2). However, in some individuals, long-acting, triple-bead MAS may be initiated at 25 mg each morning. Titration should occur in 12.5-mg weekly increments to a maximum dosage of 50 mg/d.3

In adults with severe renal impairment (glomerular filtrate rate, 15 to 30 mL/min/1.73 m2), the recommended starting dose is 12.5 mg/d, with a maximum dosage of 25 mg/d.3
The efficacy of long-acting, triple-bead MAS in adults with ADHD was demonstrated in 3 studies involving adults ages 18 to 55, and the effectiveness of the medication, with regard to duration of action, was assessed using the Time-Sensitive ADHD Symptom Scale—a self-report scale that consists of items indexed by the ADHD Rating Scale-IV (ADHD-RS-IV) which assesses ADHD symptom severity. Doses up to 75 mg/d were studied; however, there were no significant effects. It should be noted that this maximum daily dose was not determined by any safety parameter.
Study 1 (dose-optimization, triple-bead MAS, n = 137; placebo, n = 135, dosing: 12.5 to 75 mg) and Study 2 (forced dose-titration study, triple-bead MAS, n = 308; placebo, n = 104, dosing: 25 mg, 50 mg, 75 mg) demonstrated efficacy of triple-bead MAS for treating ADHD in adults. Despite differences in study designs, statistically significant and similar clinically relevant improvement was observed with triple-bead MAS (vs placebo) on ADHD-RS-IV total scores in both Study 1 and Study 2.8 An additional study in adults ages 18 to 55 (N = 275) with ADHD (DSM-5 criteria) involved randomization to either 12.5 mg (fixed dose) or forced titration (12.5 to 37.5 mg) or placebo and, as with the first 2 studies, improvement in ADHD symptoms was observed in triple-bead MAS-treated patients relative to those who had received placebo. (See Reference 3 for a summary of the clinical trials of triple-bead MAS in adults with ADHD.)
The tolerability of this medication was evaluated in a 12-month open-label study of adults with ADHD (DSM-IV-TR criteria) in which discontinuation was higher at doses >25 mg/d.7 Treatment-related increases in blood pressure and heart rate were consistent with the known hemodynamic adverse effect profile of stimulants.9
In adolescents with ADHD ages 13 to 17, long-acting, triple-bead MAS should be initiated at 12.5 mg/d and may be increased to 25 mg/d (Table 2). Importantly, in younger patients, including those younger than age 12, triple-bead MAS was associated with an increased risk of adverse events including insomnia and anorexia, and this was thought to be related to increased drug exposure (ie, AUC).
The efficacy of long-acting, triple-bead MAS was evaluated in 2 studies of adolescents ages 13 to 17, including 1 fixed-dose trial (25 mg/d) and 1 flexibly-dosed trial (12.5 to 25 mg/d). These unpublished studies utilized the ADHD-RS-IV score and the Average Permanent Product Measure of Performance, an age-adjusted math test and measure of sustained attention, and revealed statistically significant differences between medication and placebo in the primary outcomes.3
Adverse effects
Long-acting, triple-bead MAS was developed to treat ADHD symptoms throughout the day, and serum concentrations of the medication may be higher with this formulation compared with other long-acting preparations. Therefore, adverse effects that are directly related to plasma exposure (eg, insomnia and appetite suppression) may occur at higher rates with this preparation compared with alternatives. For example, in some of the registration trials, insomnia occurred in more than one-third of patients receiving the active medication (38%).9 Although insomnia was the most frequently reported adverse event in adults with ADHD, most reports of insomnia occurred early in the course of treatment. Of these insomnia-related adverse events, 94% were mild to moderate and resulted in discontinuation of the medication in approximately 2% of patients. Further, 73.9% of treatment-emergent, insomnia–related adverse events resolved during the course of the study. It is also important to note that the Pittsburgh Sleep Quality Index did not differ from placebo in studies of triple-bead MAS in adults with ADHD.10 Similarly, rates of stimulant-induced appetite suppression may be higher with this preparation compared with other long-acting preparations.9
Adverse effects observed in adults with ADHD that occurred in ≥2% of patients receiving triple-bead MAS and at least twice the incidence in patients randomized to placebo included:
- anxiety (7% vs 3%)
- feeling jittery (2% vs 1%)
- agitation (2% vs 0%)
- insomnia (31% vs 8%)
- depression (3% vs 0%)
- decreased appetite (30% vs 4%)
- weight loss (9% vs 0%)
- xerostomia (23% vs 4%)
- diarrhea (3% vs 0%)
- increased heart rate (9% vs 0%)
- palpitations (4% vs 2%)
- dysmenorrhea (4% vs 2%)
- erectile dysfunction (2% vs 1%).
In adolescents receiving triple-bea
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
1. Punja S, Shamseer L, Hartling L, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2016;2016(2):CD009996.
2. Castells X, Ramos-Quiroga J, Bosch R, et al. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2011;(6):CD007813.
3. Mydayis [package insert]. Lexington, MA: Shire; 2017.
4. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
5. Hoffman JM, Dunnenberger HM, Kevin Hicks J, et al. Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc. 2016;23(4):766-801.
6. Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766.
7. Connolly SD, Bernstein GA; Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283.
8. Goodman DW, Spencer TJ, Adler LA, et al. Clinical evaluation of triple-bead mixed amphetamine salts in adult ADHD. Presented at: 54th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 25, 2007; Boston, MA.
9. Adler LA, Frick G, Yan B. A Long-term, open-label, safety study of triple-bead mixed amphetamine salts (SHP465) in adults with ADHD [published online April 1, 2017]. J Atten Disord. doi: 10.1177/1087054717696770.
10. Backhaus J, Junghanns K, Broocks A, et al. test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740.
Treating comorbid posttraumatic stress disorder and cardiovascular disease
Mr. S, 64, has a history of posttraumatic stress disorder (PTSD), which has been well controlled for the past 15 years with cognitive-processing therapy and fluoxetine, 40 mg/d. However, over the past 6 weeks, Mr. S has experienced increased hypervigilance, nightmares, and flashbacks. He states that his primary care provider recommended an adjustment in pharmacotherapy to address this exacerbation of symptoms. Previous medication trials include sertraline, 200 mg/d, discontinued due to lack of perceived efficacy, and venlafaxine, 150 mg/d, discontinued due to increased blood pressure.
Mr. S’s medical history includes hypertension, dyslipidemia, and myocardial infarction (MI) 5 years ago. His family history includes sudden cardiac death (mother and father) and major depressive disorder (sister). His blood pressure is currently uncontrolled on lisinopril, 5 mg/d, and metoprolol succinate, 50 mg/d. Today, serial blood pressure readings measured approximately 180/90 mm Hg, with a pulse 50-60 beats per minute.
What is the next step in treating Mr. S’s hypertension and PTSD symptoms? Is there any evidence to support concomitant therapy?
PTSD is characterized by emotional and behavioral symptoms following exposure to a traumatic event. Its 12-month prevalence in the United States is estimated at 3.5%. Diagnostic criteria necessitate the presence of intrusive symptoms, persistent effortful avoidance of distressing trauma-related stimuli, negative cognitions or mood, and alterations in arousal and reactivity. PTSD negatively impacts social and occupational functioning.1
Studies have revealed a correlation between the presence of psychosocial factors, such as depression and anxiety, and the occurrence of cardiovascular events. The mechanism appears to consist of a behavioral component (eg, poor diet, tobacco use) and a direct pathophysiologic component (eg, excessive sympathetic nervous system activation) (Table 13).4 Management of concomitant PTSD and CVD presents a challenge to clinicians.
This article summarizes the evidence for the use of CVD medications in treating PTSD (Table 2) and how to apply these principles in patient care (Table 35-14).
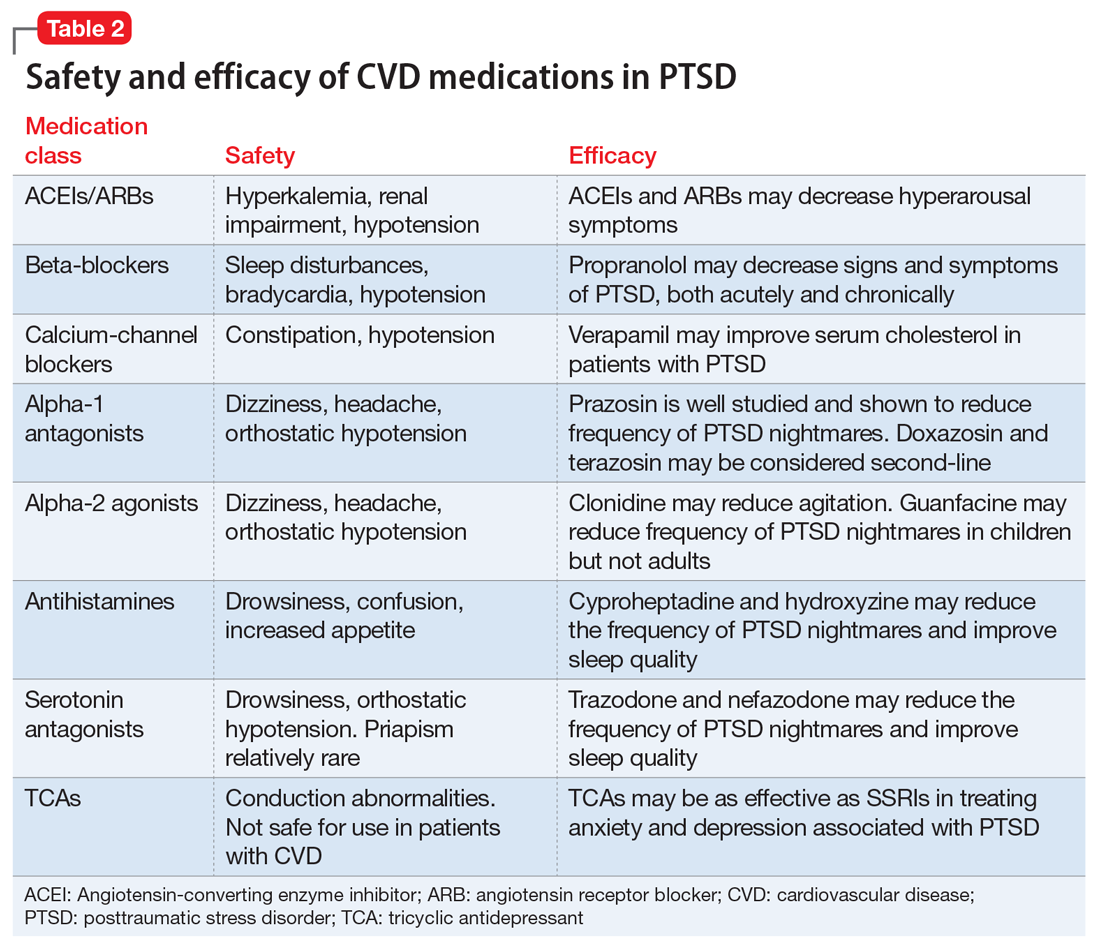
ACEIs, ARBs, beta blockers, and calcium channel blockers
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) inhibit the renin-angiotensin system: ACEIs prevent formation of angiotensin II, a potent vasoconstrictor, and ARBs prevent interaction between angiotensin II and its receptor. In one study, patients were recruited from a large public hospital serving primarily a highly traumatized, low-income population. Patients taking an ACEI or ARB who had experienced at least 1 traumatic event exhibited significantly decreased hyperarousal symptoms and decreased intrusive thoughts on the PTSD Symptom Scale and Clinician Administered PTSD Scale.5 Other studies have reported that blockade of angiotensin II AT1 receptors may result in decreased stress, anxiety, and inflammation.15
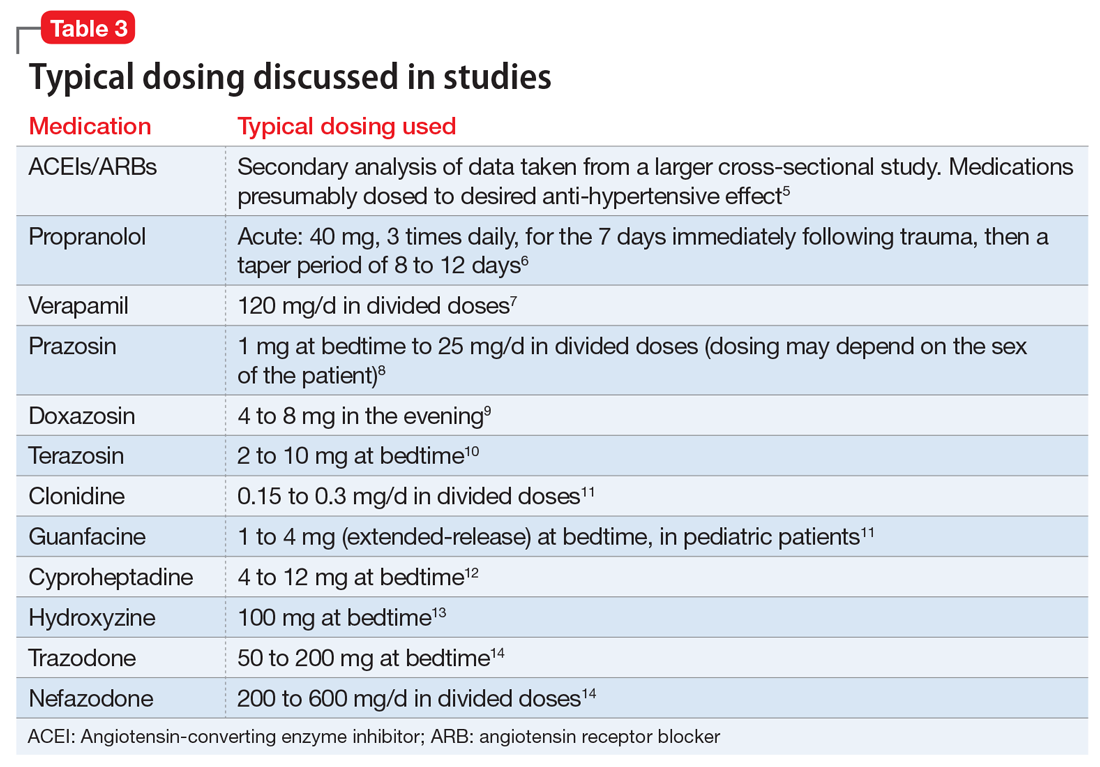
Evidence supports the use of the centrally acting, beta-adrenergic antagonist propranolol for decreasing the physiologic reactivity to acute trauma. Emotional arousal enhances the consolidation of emotional experiences into long-term memories via the adrenal stress hormones epinephrine and corticosterone. The amygdala mediates these stress hormones and releases norepinephrine, which subsequently activates noradrenergic receptors essential for memory enhancement. Several studies have reported that patients who received propranolol within several hours of a traumatic event experienced fewer physiologic signs of PTSD at follow-up 1 month later.16 Moreover, researchers have hypothesized that chronic treatment with propranolol may be effective in decreasing hyperarousal symptoms in patients with chronic PTSD by reducing tonically elevated norepinephrine signaling.6
Chronic elevation of noradrenergic activity may induce lipoprotein lipase and suppress low-density lipoprotein (LDL) receptor activity, which in turn elevates serum cholesterol levels. The results of one study suggested that verapamil, a non-dihydropyridine calcium channel blocker, significantly improves serum cholesterol levels in patients with PTSD by increasing LDL receptor activity and decreasing norepinephrine release.7
Alpha-1 and alpha-2 antagonists
Alpha-1 antagonists relax vascular smooth muscle by blocking norepinephrine stimulation at postsynaptic α-1-adrenergic receptors. They frequently are prescribed for hypertension and benign prostatic hypertrophy. One α-1 antagonist in particular, prazosin, appears especially useful in treating sleep disturbances, which occur in up to 90% of patients with PTSD.17 Because of its relatively greater lipophilicity, prazosin crosses the blood–brain barrier and acts centrally to reduce the fight-or-flight and hyperarousal reactions related to nightmares caused by PTSD.18 Common adverse effects include dizziness and orthostatic hypotension. These usually can be mitigated with titration to effective dose. In a study of active-duty soldiers who returned from Iraq and Afghanistan, Raskind et al8 found that prazosin doses up to 25 mg/d in men and 12 mg/d in women were tolerated with weekly adjustments and blood pressure monitoring.
Other α-1 antagonists have shown efficacy in a limited number of trials and may be considered second-line treatment of PTSD hyperarousal symptoms. Doxazosin has a longer half-life compared with prazosin (22 hours vs 3 hours) and may be useful in treating daytime hyperarousal with once-daily dosing. However, its hydrophilicity prevents it from crossing the blood–brain barrier to the same degree as prazosin.19 Terazosin also has a longer half-life (12 hours) and reaches peak plasma concentration in 1 hour. It undergoes minimal first-pass metabolism, leaving almost the entire circulating dose in the parent form, but clinical data are limited to only a small case report.10
Alpha-2 agonists inhibit sympathetic outflow in the CNS, which ultimately relaxes vascular smooth muscle like α-1 antagonists. Clonidine exhibits sedative properties, which derive from its nonspecific binding to α-2a-, -2b-, and -2c-adrenergic receptors. Several case studies have described a reduction in agitation in PTSD patients with the use of clonidine, likely through the induction of sleep and relaxation. Guanfacine, on the other hand, selectively binds to the α-2a-adrenergic receptor and therefore lacks the sedative properties of clonidine. Several placebo-controlled trials showed no alleviation of PTSD symptoms in adults with the use of guanfacine.11 However, case reports and open-label trials have suggested that guanfacine may reduce trauma-induced nightmares in pediatric patients. Further investigation is needed to clarify the potential use of guanfacine in pediatric PTSD.19
Antihistamines and antidepressants
Several second-line pharmacologic agents may be useful in patients with PTSD who are already taking cardiovascular medication. A limited number of studies have demonstrated reduced frequency of PTSD nightmares with the histamine-1 antagonists cyproheptadine and hydroxyzine, both of which exhibit minor anti-serotonergic properties.12,13 Likewise, the serotonin antagonists nefazodone and trazodone have been shown to reduce the frequency of PTSD nightmares, as well as improve overall sleep quality.14 Nefazodone should be considered an option only after treatment failure of multiple other medications, because it is associated with a small, but significant, risk of life-threatening hepatotoxicity.20
Tricyclic antidepressants (TCAs) may reduce anxiety and depression associated with PTSD to the same degree as SSRIs.21 However, their effect on PTSD-associated sleep disturbances is much less pronounced than other available medications.14 TCAs should be avoided in patients with CVD because they may exacerbate cardiac conduction abnormalities. This is especially true for those recovering from acute MI.22
CASE CONTINUED
Mr. S is started on prazosin, 1 mg at bedtime, titrated weekly to 6 mg at bedtime with regular blood pressure monitoring because of the risk of orthostatic hypotension. Although the frequency of his nightmares decreases to 1 or 2 per month, he still experiences flashbacks at the same frequency and intensity as before. Prazosin, 1 mg every morning, is added, titrated weekly to 4 mg every morning. This combination of morning and bedtime dosing leads to resolution of both nightmares and flashbacks along with a significant reduction in hyperarousal. Lisinopril is increased from 5 to 10 mg/d to address Mr. S’s uncontrolled hypertension; this change also could have contributed to the reduction in hyperarousal. CPT and fluoxetine are continued.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl 25):S1-S49.
3. Cohen BE, Marmar C, Ren L, et al. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302(5):489-492.
4. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192-2217.
5. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849-855.
6. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26-33.
7. Ansari MA, Ahmed S. Calcium channel blocker verapamil: a new intervention for high cholesterol levels in patients with PTSD. Turk Jem. 2007;11:93-97.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. De Jong J, Wauben P, Huijbrechts I, et al. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30(1):84-85.
10. Nirmalani-Gandhy A, Sanchez D, Catalano G. Terazosin for the treatment of trauma-related nightmares: a report of four cases. Clin Neuropharmacol. 2015;38(3):109-111.
11. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
12. Gupta S, Popli A, Bathurst E, et al. Efficacy of cyproheptadine for nightmares associated with posttraumatic stress disorder. Compr Psychiatry. 1998;39(3):160-164.
13. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
14. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
15. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1-18.
16. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402-10407.
17. Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: a critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24-33.
18. Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87(9):890-900.
19. Arnsten AF, Raskind MA, Taylor FB, et al. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89-99.
20. Serzone [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529. doi: 10.1371/journal. pone.0126529.
22. Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annu Rev Med. 1984;35:503-511.
Mr. S, 64, has a history of posttraumatic stress disorder (PTSD), which has been well controlled for the past 15 years with cognitive-processing therapy and fluoxetine, 40 mg/d. However, over the past 6 weeks, Mr. S has experienced increased hypervigilance, nightmares, and flashbacks. He states that his primary care provider recommended an adjustment in pharmacotherapy to address this exacerbation of symptoms. Previous medication trials include sertraline, 200 mg/d, discontinued due to lack of perceived efficacy, and venlafaxine, 150 mg/d, discontinued due to increased blood pressure.
Mr. S’s medical history includes hypertension, dyslipidemia, and myocardial infarction (MI) 5 years ago. His family history includes sudden cardiac death (mother and father) and major depressive disorder (sister). His blood pressure is currently uncontrolled on lisinopril, 5 mg/d, and metoprolol succinate, 50 mg/d. Today, serial blood pressure readings measured approximately 180/90 mm Hg, with a pulse 50-60 beats per minute.
What is the next step in treating Mr. S’s hypertension and PTSD symptoms? Is there any evidence to support concomitant therapy?
PTSD is characterized by emotional and behavioral symptoms following exposure to a traumatic event. Its 12-month prevalence in the United States is estimated at 3.5%. Diagnostic criteria necessitate the presence of intrusive symptoms, persistent effortful avoidance of distressing trauma-related stimuli, negative cognitions or mood, and alterations in arousal and reactivity. PTSD negatively impacts social and occupational functioning.1
Studies have revealed a correlation between the presence of psychosocial factors, such as depression and anxiety, and the occurrence of cardiovascular events. The mechanism appears to consist of a behavioral component (eg, poor diet, tobacco use) and a direct pathophysiologic component (eg, excessive sympathetic nervous system activation) (Table 13).4 Management of concomitant PTSD and CVD presents a challenge to clinicians.
This article summarizes the evidence for the use of CVD medications in treating PTSD (Table 2) and how to apply these principles in patient care (Table 35-14).

ACEIs, ARBs, beta blockers, and calcium channel blockers
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) inhibit the renin-angiotensin system: ACEIs prevent formation of angiotensin II, a potent vasoconstrictor, and ARBs prevent interaction between angiotensin II and its receptor. In one study, patients were recruited from a large public hospital serving primarily a highly traumatized, low-income population. Patients taking an ACEI or ARB who had experienced at least 1 traumatic event exhibited significantly decreased hyperarousal symptoms and decreased intrusive thoughts on the PTSD Symptom Scale and Clinician Administered PTSD Scale.5 Other studies have reported that blockade of angiotensin II AT1 receptors may result in decreased stress, anxiety, and inflammation.15

Evidence supports the use of the centrally acting, beta-adrenergic antagonist propranolol for decreasing the physiologic reactivity to acute trauma. Emotional arousal enhances the consolidation of emotional experiences into long-term memories via the adrenal stress hormones epinephrine and corticosterone. The amygdala mediates these stress hormones and releases norepinephrine, which subsequently activates noradrenergic receptors essential for memory enhancement. Several studies have reported that patients who received propranolol within several hours of a traumatic event experienced fewer physiologic signs of PTSD at follow-up 1 month later.16 Moreover, researchers have hypothesized that chronic treatment with propranolol may be effective in decreasing hyperarousal symptoms in patients with chronic PTSD by reducing tonically elevated norepinephrine signaling.6
Chronic elevation of noradrenergic activity may induce lipoprotein lipase and suppress low-density lipoprotein (LDL) receptor activity, which in turn elevates serum cholesterol levels. The results of one study suggested that verapamil, a non-dihydropyridine calcium channel blocker, significantly improves serum cholesterol levels in patients with PTSD by increasing LDL receptor activity and decreasing norepinephrine release.7
Alpha-1 and alpha-2 antagonists
Alpha-1 antagonists relax vascular smooth muscle by blocking norepinephrine stimulation at postsynaptic α-1-adrenergic receptors. They frequently are prescribed for hypertension and benign prostatic hypertrophy. One α-1 antagonist in particular, prazosin, appears especially useful in treating sleep disturbances, which occur in up to 90% of patients with PTSD.17 Because of its relatively greater lipophilicity, prazosin crosses the blood–brain barrier and acts centrally to reduce the fight-or-flight and hyperarousal reactions related to nightmares caused by PTSD.18 Common adverse effects include dizziness and orthostatic hypotension. These usually can be mitigated with titration to effective dose. In a study of active-duty soldiers who returned from Iraq and Afghanistan, Raskind et al8 found that prazosin doses up to 25 mg/d in men and 12 mg/d in women were tolerated with weekly adjustments and blood pressure monitoring.
Other α-1 antagonists have shown efficacy in a limited number of trials and may be considered second-line treatment of PTSD hyperarousal symptoms. Doxazosin has a longer half-life compared with prazosin (22 hours vs 3 hours) and may be useful in treating daytime hyperarousal with once-daily dosing. However, its hydrophilicity prevents it from crossing the blood–brain barrier to the same degree as prazosin.19 Terazosin also has a longer half-life (12 hours) and reaches peak plasma concentration in 1 hour. It undergoes minimal first-pass metabolism, leaving almost the entire circulating dose in the parent form, but clinical data are limited to only a small case report.10
Alpha-2 agonists inhibit sympathetic outflow in the CNS, which ultimately relaxes vascular smooth muscle like α-1 antagonists. Clonidine exhibits sedative properties, which derive from its nonspecific binding to α-2a-, -2b-, and -2c-adrenergic receptors. Several case studies have described a reduction in agitation in PTSD patients with the use of clonidine, likely through the induction of sleep and relaxation. Guanfacine, on the other hand, selectively binds to the α-2a-adrenergic receptor and therefore lacks the sedative properties of clonidine. Several placebo-controlled trials showed no alleviation of PTSD symptoms in adults with the use of guanfacine.11 However, case reports and open-label trials have suggested that guanfacine may reduce trauma-induced nightmares in pediatric patients. Further investigation is needed to clarify the potential use of guanfacine in pediatric PTSD.19
Antihistamines and antidepressants
Several second-line pharmacologic agents may be useful in patients with PTSD who are already taking cardiovascular medication. A limited number of studies have demonstrated reduced frequency of PTSD nightmares with the histamine-1 antagonists cyproheptadine and hydroxyzine, both of which exhibit minor anti-serotonergic properties.12,13 Likewise, the serotonin antagonists nefazodone and trazodone have been shown to reduce the frequency of PTSD nightmares, as well as improve overall sleep quality.14 Nefazodone should be considered an option only after treatment failure of multiple other medications, because it is associated with a small, but significant, risk of life-threatening hepatotoxicity.20
Tricyclic antidepressants (TCAs) may reduce anxiety and depression associated with PTSD to the same degree as SSRIs.21 However, their effect on PTSD-associated sleep disturbances is much less pronounced than other available medications.14 TCAs should be avoided in patients with CVD because they may exacerbate cardiac conduction abnormalities. This is especially true for those recovering from acute MI.22
CASE CONTINUED
Mr. S is started on prazosin, 1 mg at bedtime, titrated weekly to 6 mg at bedtime with regular blood pressure monitoring because of the risk of orthostatic hypotension. Although the frequency of his nightmares decreases to 1 or 2 per month, he still experiences flashbacks at the same frequency and intensity as before. Prazosin, 1 mg every morning, is added, titrated weekly to 4 mg every morning. This combination of morning and bedtime dosing leads to resolution of both nightmares and flashbacks along with a significant reduction in hyperarousal. Lisinopril is increased from 5 to 10 mg/d to address Mr. S’s uncontrolled hypertension; this change also could have contributed to the reduction in hyperarousal. CPT and fluoxetine are continued.
Mr. S, 64, has a history of posttraumatic stress disorder (PTSD), which has been well controlled for the past 15 years with cognitive-processing therapy and fluoxetine, 40 mg/d. However, over the past 6 weeks, Mr. S has experienced increased hypervigilance, nightmares, and flashbacks. He states that his primary care provider recommended an adjustment in pharmacotherapy to address this exacerbation of symptoms. Previous medication trials include sertraline, 200 mg/d, discontinued due to lack of perceived efficacy, and venlafaxine, 150 mg/d, discontinued due to increased blood pressure.
Mr. S’s medical history includes hypertension, dyslipidemia, and myocardial infarction (MI) 5 years ago. His family history includes sudden cardiac death (mother and father) and major depressive disorder (sister). His blood pressure is currently uncontrolled on lisinopril, 5 mg/d, and metoprolol succinate, 50 mg/d. Today, serial blood pressure readings measured approximately 180/90 mm Hg, with a pulse 50-60 beats per minute.
What is the next step in treating Mr. S’s hypertension and PTSD symptoms? Is there any evidence to support concomitant therapy?
PTSD is characterized by emotional and behavioral symptoms following exposure to a traumatic event. Its 12-month prevalence in the United States is estimated at 3.5%. Diagnostic criteria necessitate the presence of intrusive symptoms, persistent effortful avoidance of distressing trauma-related stimuli, negative cognitions or mood, and alterations in arousal and reactivity. PTSD negatively impacts social and occupational functioning.1
Studies have revealed a correlation between the presence of psychosocial factors, such as depression and anxiety, and the occurrence of cardiovascular events. The mechanism appears to consist of a behavioral component (eg, poor diet, tobacco use) and a direct pathophysiologic component (eg, excessive sympathetic nervous system activation) (Table 13).4 Management of concomitant PTSD and CVD presents a challenge to clinicians.
This article summarizes the evidence for the use of CVD medications in treating PTSD (Table 2) and how to apply these principles in patient care (Table 35-14).

ACEIs, ARBs, beta blockers, and calcium channel blockers
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) inhibit the renin-angiotensin system: ACEIs prevent formation of angiotensin II, a potent vasoconstrictor, and ARBs prevent interaction between angiotensin II and its receptor. In one study, patients were recruited from a large public hospital serving primarily a highly traumatized, low-income population. Patients taking an ACEI or ARB who had experienced at least 1 traumatic event exhibited significantly decreased hyperarousal symptoms and decreased intrusive thoughts on the PTSD Symptom Scale and Clinician Administered PTSD Scale.5 Other studies have reported that blockade of angiotensin II AT1 receptors may result in decreased stress, anxiety, and inflammation.15

Evidence supports the use of the centrally acting, beta-adrenergic antagonist propranolol for decreasing the physiologic reactivity to acute trauma. Emotional arousal enhances the consolidation of emotional experiences into long-term memories via the adrenal stress hormones epinephrine and corticosterone. The amygdala mediates these stress hormones and releases norepinephrine, which subsequently activates noradrenergic receptors essential for memory enhancement. Several studies have reported that patients who received propranolol within several hours of a traumatic event experienced fewer physiologic signs of PTSD at follow-up 1 month later.16 Moreover, researchers have hypothesized that chronic treatment with propranolol may be effective in decreasing hyperarousal symptoms in patients with chronic PTSD by reducing tonically elevated norepinephrine signaling.6
Chronic elevation of noradrenergic activity may induce lipoprotein lipase and suppress low-density lipoprotein (LDL) receptor activity, which in turn elevates serum cholesterol levels. The results of one study suggested that verapamil, a non-dihydropyridine calcium channel blocker, significantly improves serum cholesterol levels in patients with PTSD by increasing LDL receptor activity and decreasing norepinephrine release.7
Alpha-1 and alpha-2 antagonists
Alpha-1 antagonists relax vascular smooth muscle by blocking norepinephrine stimulation at postsynaptic α-1-adrenergic receptors. They frequently are prescribed for hypertension and benign prostatic hypertrophy. One α-1 antagonist in particular, prazosin, appears especially useful in treating sleep disturbances, which occur in up to 90% of patients with PTSD.17 Because of its relatively greater lipophilicity, prazosin crosses the blood–brain barrier and acts centrally to reduce the fight-or-flight and hyperarousal reactions related to nightmares caused by PTSD.18 Common adverse effects include dizziness and orthostatic hypotension. These usually can be mitigated with titration to effective dose. In a study of active-duty soldiers who returned from Iraq and Afghanistan, Raskind et al8 found that prazosin doses up to 25 mg/d in men and 12 mg/d in women were tolerated with weekly adjustments and blood pressure monitoring.
Other α-1 antagonists have shown efficacy in a limited number of trials and may be considered second-line treatment of PTSD hyperarousal symptoms. Doxazosin has a longer half-life compared with prazosin (22 hours vs 3 hours) and may be useful in treating daytime hyperarousal with once-daily dosing. However, its hydrophilicity prevents it from crossing the blood–brain barrier to the same degree as prazosin.19 Terazosin also has a longer half-life (12 hours) and reaches peak plasma concentration in 1 hour. It undergoes minimal first-pass metabolism, leaving almost the entire circulating dose in the parent form, but clinical data are limited to only a small case report.10
Alpha-2 agonists inhibit sympathetic outflow in the CNS, which ultimately relaxes vascular smooth muscle like α-1 antagonists. Clonidine exhibits sedative properties, which derive from its nonspecific binding to α-2a-, -2b-, and -2c-adrenergic receptors. Several case studies have described a reduction in agitation in PTSD patients with the use of clonidine, likely through the induction of sleep and relaxation. Guanfacine, on the other hand, selectively binds to the α-2a-adrenergic receptor and therefore lacks the sedative properties of clonidine. Several placebo-controlled trials showed no alleviation of PTSD symptoms in adults with the use of guanfacine.11 However, case reports and open-label trials have suggested that guanfacine may reduce trauma-induced nightmares in pediatric patients. Further investigation is needed to clarify the potential use of guanfacine in pediatric PTSD.19
Antihistamines and antidepressants
Several second-line pharmacologic agents may be useful in patients with PTSD who are already taking cardiovascular medication. A limited number of studies have demonstrated reduced frequency of PTSD nightmares with the histamine-1 antagonists cyproheptadine and hydroxyzine, both of which exhibit minor anti-serotonergic properties.12,13 Likewise, the serotonin antagonists nefazodone and trazodone have been shown to reduce the frequency of PTSD nightmares, as well as improve overall sleep quality.14 Nefazodone should be considered an option only after treatment failure of multiple other medications, because it is associated with a small, but significant, risk of life-threatening hepatotoxicity.20
Tricyclic antidepressants (TCAs) may reduce anxiety and depression associated with PTSD to the same degree as SSRIs.21 However, their effect on PTSD-associated sleep disturbances is much less pronounced than other available medications.14 TCAs should be avoided in patients with CVD because they may exacerbate cardiac conduction abnormalities. This is especially true for those recovering from acute MI.22
CASE CONTINUED
Mr. S is started on prazosin, 1 mg at bedtime, titrated weekly to 6 mg at bedtime with regular blood pressure monitoring because of the risk of orthostatic hypotension. Although the frequency of his nightmares decreases to 1 or 2 per month, he still experiences flashbacks at the same frequency and intensity as before. Prazosin, 1 mg every morning, is added, titrated weekly to 4 mg every morning. This combination of morning and bedtime dosing leads to resolution of both nightmares and flashbacks along with a significant reduction in hyperarousal. Lisinopril is increased from 5 to 10 mg/d to address Mr. S’s uncontrolled hypertension; this change also could have contributed to the reduction in hyperarousal. CPT and fluoxetine are continued.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl 25):S1-S49.
3. Cohen BE, Marmar C, Ren L, et al. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302(5):489-492.
4. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192-2217.
5. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849-855.
6. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26-33.
7. Ansari MA, Ahmed S. Calcium channel blocker verapamil: a new intervention for high cholesterol levels in patients with PTSD. Turk Jem. 2007;11:93-97.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. De Jong J, Wauben P, Huijbrechts I, et al. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30(1):84-85.
10. Nirmalani-Gandhy A, Sanchez D, Catalano G. Terazosin for the treatment of trauma-related nightmares: a report of four cases. Clin Neuropharmacol. 2015;38(3):109-111.
11. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
12. Gupta S, Popli A, Bathurst E, et al. Efficacy of cyproheptadine for nightmares associated with posttraumatic stress disorder. Compr Psychiatry. 1998;39(3):160-164.
13. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
14. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
15. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1-18.
16. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402-10407.
17. Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: a critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24-33.
18. Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87(9):890-900.
19. Arnsten AF, Raskind MA, Taylor FB, et al. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89-99.
20. Serzone [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529. doi: 10.1371/journal. pone.0126529.
22. Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annu Rev Med. 1984;35:503-511.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Laslett LJ, Alagona P Jr, Clark BA 3rd, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl 25):S1-S49.
3. Cohen BE, Marmar C, Ren L, et al. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302(5):489-492.
4. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99(16):2192-2217.
5. Khoury NM, Marvar PJ, Gillespie CF, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73(6):849-855.
6. Giustino TF, Fitzgerald PJ, Maren S. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem. 2016;130:26-33.
7. Ansari MA, Ahmed S. Calcium channel blocker verapamil: a new intervention for high cholesterol levels in patients with PTSD. Turk Jem. 2007;11:93-97.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. De Jong J, Wauben P, Huijbrechts I, et al. Doxazosin treatment for posttraumatic stress disorder. J Clin Psychopharmacol. 2010;30(1):84-85.
10. Nirmalani-Gandhy A, Sanchez D, Catalano G. Terazosin for the treatment of trauma-related nightmares: a report of four cases. Clin Neuropharmacol. 2015;38(3):109-111.
11. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
12. Gupta S, Popli A, Bathurst E, et al. Efficacy of cyproheptadine for nightmares associated with posttraumatic stress disorder. Compr Psychiatry. 1998;39(3):160-164.
13. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
14. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567-590.
15. Saavedra JM, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation, and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36(1):1-18.
16. McGaugh JL. Making lasting memories: remembering the significant. Proc Natl Acad Sci U S A. 2013;110(suppl 2):10402-10407.
17. Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: a critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24-33.
18. Kung S, Espinel Z, Lapid MI. Treatment of nightmares with prazosin: a systematic review. Mayo Clin Proc. 2012;87(9):890-900.
19. Arnsten AF, Raskind MA, Taylor FB, et al. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89-99.
20. Serzone [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2003.
21. Puetz TW, Youngstedt SD, Herring MP. Effects of pharmacotherapy on combat-related PTSD, anxiety, and depression: a systematic review and meta-regression analysis. PLoS One. 2015;10(5):e0126529. doi: 10.1371/journal. pone.0126529.
22. Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annu Rev Med. 1984;35:503-511.
Paranoia and suicidality after starting treatment for lupus
CASE Unusual behavior, thoughts
Mr. L, age 28, an immigrant from Burma, is brought to his primary care physician’s clinic by his wife for follow-up on a rash. During the evaluation, his wife reports that Mr. L recently has had suicidal ideation, depression, and increased anger. She says Mr. L had made statements about wanting to kill himself with a gun. Mr. L had driven his car to a soccer field with a knife in hand and was contemplating suicide. She is concerned about her own safety and their children’s safety because of Mr. L’s anger. The physician refers Mr. L to the emergency department, and he is admitted to the medical floor for a rheumatological flare-up and suicidal ideation.
Mr. L starts displaying inappropriate behaviors, including masturbating in front of the patient safety attendant, telling the attendant “You are going to die today,” and assaulting a female attendant by trying to grab her breasts. He is given IM haloperidol, 2 mg, which effectively alleviates these behaviors. Between episodes of unusual behavior and outbursts, Mr. L is docile, quiet, and cooperative, and denies any memory of these episodes.
One month earlier, Mr. L had been hospitalized for progressive weakness and inability to ambulate. He was diagnosed with necrotizing myositis and a rash consistent with subacute cutaneous lupus. He was started on IV methylprednisolone, 1 g, and transitioned to oral prednisolone, 40 mg/d, which he continued taking after discharge. He also started taking azathioprine, which was increased from 50 to 100 mg/d. His condition improved shortly after beginning this regimen.
[polldaddy:9796586]
The authors’ observations
DSM-5 defines brief psychotic disorder as positive symptoms or disorganized or catatonic behavior appearing suddenly and lasting between 1 day to 1 month.1 Mr. L had a sudden onset of his symptoms and marked stressors as a result of his worsening health. However, the possibility of his general medical conditions or medications causing his symptoms needed to be investigated and ruled out before this diagnosis could be assigned.
Another consideration is the culture-bound syndrome amok. Although DSM-5 does not use the term “culture-bound syndrome,” which was used in DSM-IV, it does recognize cultural conceptualizations of distress. Amok is described as a dissociative episode in which an individual has a period of brooding followed by outbursts that include violent, aggressive, and suicidal and/or homicidal ideation. The individual may exhibit persecutory and paranoid thinking, amnesia of the outbursts, and a return to typical behavior when the episode concludes.2 However, it remained unclear whether Mr. L’s violent behavior was a manifestation of psychiatric or organic disease.
Identifying the possibility of amok is important not only for alleviating the patient’s distress but also for preventing violent outbursts that can result in injury or death.3 Amok should be considered only in the context of possible psychiatric or organic brain disease, such as corticosteroid-induced psychosis (CIP) or systemic lupus erythematosus-induced psychosis (SLEIP).4
EVALUATION Informants, labs
Mr. L immigrated to the United States when he was 5 years old. He does not speak English, and interviews are conducted with interpreting services at the hospital. Mr. L answers most questions with or 1 to 2 words. His medical and psychiatric histories are notable for hypothyroidism, hepatitis, non-ischemic cardiomyopathy, necrotizing myositis, subacute cutaneous lupus, and depression. Mr. L denies a personal or family history of mental illness; however, records show he has a history of unspecified depressive disorder.
Mr. L reports his current mood is “okay,” but he has felt different in the past few weeks. He denies auditory or visual hallucinations, or suicidal or homicidal ideation, but exhibits paranoid thoughts. Mr. L believes everyone “lied” to him, and he repeats this frequently. Collateral information from friends reveals that he had threatened to burn down their houses. A family friend states that Mr. L has been depressed and angry over the past 5 days.
During his prior and current hospitalizations, many labs were completed. Thyroid, urine drug screen, C-reactive protein, urine analysis, ethanol, complete blood count, and comprehensive metabolic panel were negative. Erythrocyte sedimentation rate was 30. Lumbar puncture cell count was notable for mildly elevated lymphocytes at 84%. Antinuclear antibody (ANA) was positive. Lupus anticoagulant panel revealed a mildly prolonged partial thromboplastin time at 38.9 seconds. DNA double-stranded antibody (anti-dsDNA) was positive. Anti-Smith antibody was negative. Anti-Ro/SSA and anti-La/SSB antibodies were elevated. Albumin was low. A MRI of the brain showed dystrophic-appearing right parieto-occipital calcification and mild cerebral volume loss.
Based on Mr. L’s presentation and imaging, the rheumatology team suspects CNS lupus and that his prescribed steroids could be playing a role in his behavior.
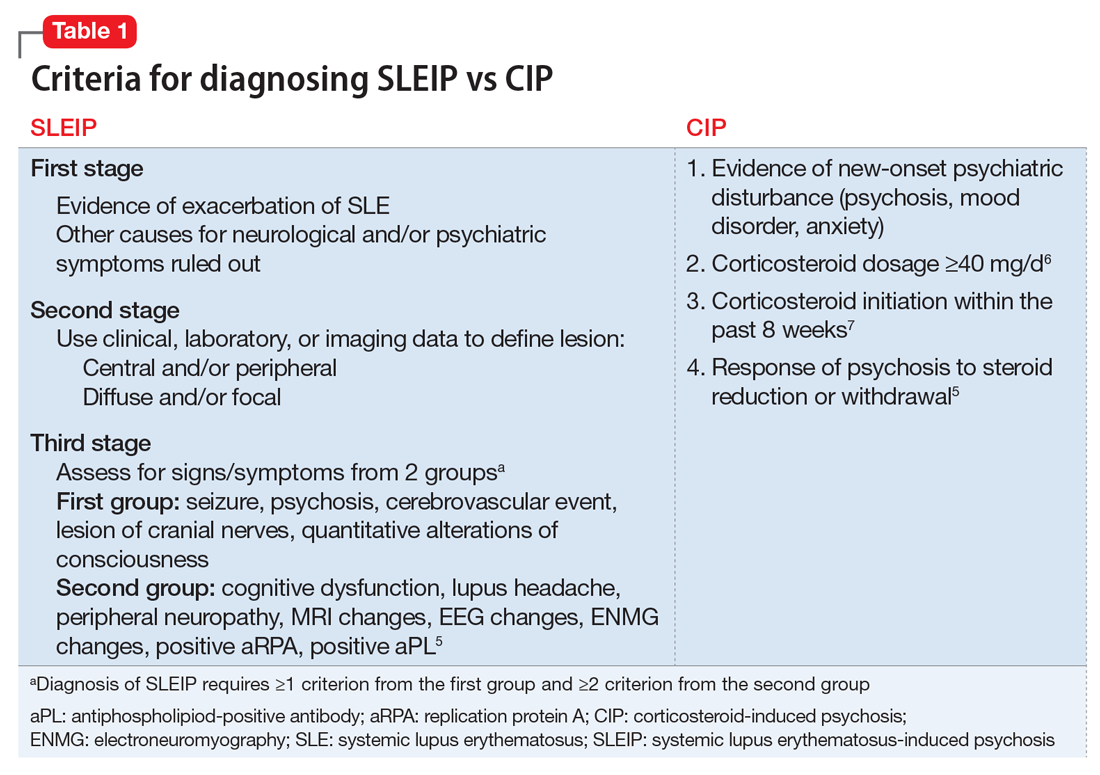
The authors’ observations
Differentiating CIP from SLEIP can be difficult. The clinical features and criteria for CIP and SLEIP are listed in Table 1.5-7 Several studies have highlighted the difficulties in separating the 2 diagnoses:
- Kampylafka et al8 found that CNS involvement, including stroke, myelopathy, seizures, optic neuritis, and meningitis, was present in 4.3% of their sample of patients with systematic lupus erythematosus (SLE), of whom 6.3% presented with SLEIP. Of patients with CNS involvement, 94% had positive ANA and 69% had positive anti-dsDNA antibodies. It remains difficult to definitively diagnose SLEIP rather than CIP, however, because 100% of patients in this study were taking corticosteroids, with 25% taking azathioprine, as was Mr. L.8
- Appenzeller et al9 found that acute psychosis was associated with SLE in 11.3% of their sample. Psychosis in patients with SLE was accompanied by other manifestations of CNS involvement. On follow-up these patients had mild increases in white blood cell count in their CSF, and MRI demonstrated hyperdense lesions and cerebral atrophy. Hypoalbuminemia, although often seen in SLEIP, also is observed in patients with CIP and cannot be used to differentiate these 2 conditions.9
- Monov and Monova5 recommended criteria for SLEIP that include 3 stages. The first stage is determining that there is evidence of an exacerbation of SLE, and ruling out other causes for neurologic and psychiatric symptoms. The second stage involves using clinical, laboratory, or imaging tests to define the lesion as central and/or peripheral and diffuse and/or focal. The third stage requires diagnosing SLEIP using criteria from 2 groups of signs and symptoms: the first group includes seizure, psychosis, cerebrovascular event, lesion of cranial nerves, and quantitative alterations of consciousness; the second group includes cognitive dysfunction, lupus headache, peripheral neuropathy, MRI changes, EEG changes, electroneuromyography changes, and a positive replication protein A or antiphospholipid-positive antibody. Diagnosing SLEIP requires ≥1 criterion from group 1 and ≥2 criteria from group 2.5
- Patten and Neutel6 found that patients taking prednisolone, Symbol Std<40 mg/d, had significantly higher rates of psychosis than those taking <40 mg/d.6
- Bhangle et Myriad Proal7 found that one of the major distinguishing factors between CIP and SLEIP is the timing of the onset of symptoms, with CIP occurring within 8 weeks of initiation of a corticosteroid, and SLEIP being more likely to occur when additional CNS symptoms are present.7
TREATMENT Decreased dosage
Mr. L starts quetiapine, 25 mg at bedtime, increased to 75 mg at bedtime. Prednisolone is decreased to 10 mg/d. Over the next few days Mr. L’s mood, psychosis, and aggression improve. He becomes calm and cooperative, and denies suicidal or homicidal ideation. Mr. L’s wife, who was initially scared to visit him, comes to see him and confirms that he has improved. After 3 consecutive days with no abnormal behaviors or psychiatric symptoms, Mr. L is discharged and continues taking quetiapine, 75 mg at bedtime, and prednisolone, 10 mg/d, with outpatient follow-up.
The authors’ observations
Table 210,11 describes approaches to treating CIP and SLEIP. Managing CIP typically consists of reducing the corticosteroid dosage. CIP treatment also includes adjunct therapy with psychotropics if the corticosteroid dose cannot be lowered enough to reduce psychiatric symptoms while suppressing symptoms of the disease for which the corticosteroid was prescribed.6
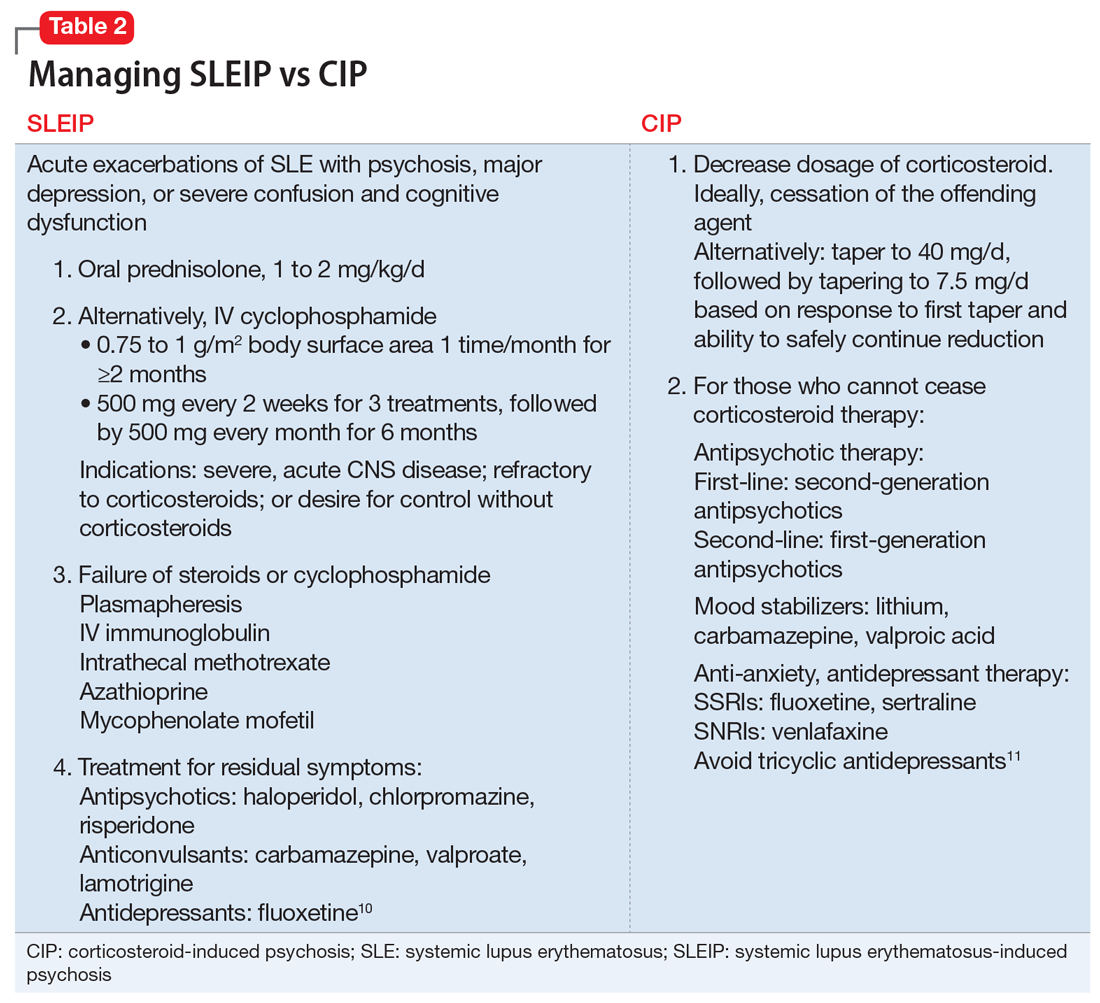
When treating SLEIP, the corticosteroid dosage often is increased. Corticosteroids often are used to treat SLEIP while suppressing symptoms of SLE.10 The main treatment of SLEIP is focused on the disease and using psychotropic medications to control symptoms that don’t respond after exacerbation of the disease has been controlled.10
The presence of Mr. L’s multiple SLE symptoms, as well as MRI findings, could indicate SLEIP. However, corticosteroids also were a possible cause of his psychotic symptoms. Mr. L’s psychosis began within 8 weeks of starting a corticosteroid (prednisolone, 40 mg/d), and his symptoms improved when the corticosteroid dosage was reduced. The difference between CIP and SLEIP may best be distinguished by reducing the corticosteroid dosage and seeing if psychotic symptoms improve. Because it is important to control SLE symptoms in those with CIP, prescribing psychotropics may be warranted, as well as alternative treatments for immunosuppression.
Because steroids are frequently prescribed for lupus, it is important for clinicians to be aware of their psychiatric effects as well as how to manage those effects. When distinguishing CIP from SLEIP, consider decreasing the corticosteroid dosage and see if psychotic symptoms improve. Use adjunct therapy as needed.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Saint Martin ML. Running amok: A modern perspective on a culture-bound syndrome. Prim Care Companion J Clin Psychiatry. 1999;1(3):66-70.
4. Flaskerud JH. Case studies in amok? Issues Ment Health Nurs. 2012;33(12):898-900.
5. Monov S, Monova D. Classification criteria for neuropsychiatric systemic lupus erythematosus: do they need a discussion? Hippokratia. 2008;12(2):103-107.
6. Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis and management. Drug Saf. 2000;22(2):111-122.
7. Bhangle SD, Kramer N, Rosenstein, ED. Corticosteroid-induced neuropsychiatric disorders: review and contrast with neuropsychiatric lupus. Rheumatol Int. 2013;33(8):1923-1932.
8. Kampylafka EI, Alexopoulos H, Kosmidis ML, et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3-year prospective study of 370 patients. PLoS One. 2013;8(2):e55843. d
9. Appenzeller S, Cendes F, Costallat LT. Acute psychosisin systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
10. Sanna G, Bertolaccini ML, Khamashta MA. Neuropsychiatric involvement in systemic lupus erythematosus: current therapeutic approach. Curr Pharm Des. 2008;14(13):1261-1269.
11. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
CASE Unusual behavior, thoughts
Mr. L, age 28, an immigrant from Burma, is brought to his primary care physician’s clinic by his wife for follow-up on a rash. During the evaluation, his wife reports that Mr. L recently has had suicidal ideation, depression, and increased anger. She says Mr. L had made statements about wanting to kill himself with a gun. Mr. L had driven his car to a soccer field with a knife in hand and was contemplating suicide. She is concerned about her own safety and their children’s safety because of Mr. L’s anger. The physician refers Mr. L to the emergency department, and he is admitted to the medical floor for a rheumatological flare-up and suicidal ideation.
Mr. L starts displaying inappropriate behaviors, including masturbating in front of the patient safety attendant, telling the attendant “You are going to die today,” and assaulting a female attendant by trying to grab her breasts. He is given IM haloperidol, 2 mg, which effectively alleviates these behaviors. Between episodes of unusual behavior and outbursts, Mr. L is docile, quiet, and cooperative, and denies any memory of these episodes.
One month earlier, Mr. L had been hospitalized for progressive weakness and inability to ambulate. He was diagnosed with necrotizing myositis and a rash consistent with subacute cutaneous lupus. He was started on IV methylprednisolone, 1 g, and transitioned to oral prednisolone, 40 mg/d, which he continued taking after discharge. He also started taking azathioprine, which was increased from 50 to 100 mg/d. His condition improved shortly after beginning this regimen.
[polldaddy:9796586]
The authors’ observations
DSM-5 defines brief psychotic disorder as positive symptoms or disorganized or catatonic behavior appearing suddenly and lasting between 1 day to 1 month.1 Mr. L had a sudden onset of his symptoms and marked stressors as a result of his worsening health. However, the possibility of his general medical conditions or medications causing his symptoms needed to be investigated and ruled out before this diagnosis could be assigned.
Another consideration is the culture-bound syndrome amok. Although DSM-5 does not use the term “culture-bound syndrome,” which was used in DSM-IV, it does recognize cultural conceptualizations of distress. Amok is described as a dissociative episode in which an individual has a period of brooding followed by outbursts that include violent, aggressive, and suicidal and/or homicidal ideation. The individual may exhibit persecutory and paranoid thinking, amnesia of the outbursts, and a return to typical behavior when the episode concludes.2 However, it remained unclear whether Mr. L’s violent behavior was a manifestation of psychiatric or organic disease.
Identifying the possibility of amok is important not only for alleviating the patient’s distress but also for preventing violent outbursts that can result in injury or death.3 Amok should be considered only in the context of possible psychiatric or organic brain disease, such as corticosteroid-induced psychosis (CIP) or systemic lupus erythematosus-induced psychosis (SLEIP).4
EVALUATION Informants, labs
Mr. L immigrated to the United States when he was 5 years old. He does not speak English, and interviews are conducted with interpreting services at the hospital. Mr. L answers most questions with or 1 to 2 words. His medical and psychiatric histories are notable for hypothyroidism, hepatitis, non-ischemic cardiomyopathy, necrotizing myositis, subacute cutaneous lupus, and depression. Mr. L denies a personal or family history of mental illness; however, records show he has a history of unspecified depressive disorder.
Mr. L reports his current mood is “okay,” but he has felt different in the past few weeks. He denies auditory or visual hallucinations, or suicidal or homicidal ideation, but exhibits paranoid thoughts. Mr. L believes everyone “lied” to him, and he repeats this frequently. Collateral information from friends reveals that he had threatened to burn down their houses. A family friend states that Mr. L has been depressed and angry over the past 5 days.
During his prior and current hospitalizations, many labs were completed. Thyroid, urine drug screen, C-reactive protein, urine analysis, ethanol, complete blood count, and comprehensive metabolic panel were negative. Erythrocyte sedimentation rate was 30. Lumbar puncture cell count was notable for mildly elevated lymphocytes at 84%. Antinuclear antibody (ANA) was positive. Lupus anticoagulant panel revealed a mildly prolonged partial thromboplastin time at 38.9 seconds. DNA double-stranded antibody (anti-dsDNA) was positive. Anti-Smith antibody was negative. Anti-Ro/SSA and anti-La/SSB antibodies were elevated. Albumin was low. A MRI of the brain showed dystrophic-appearing right parieto-occipital calcification and mild cerebral volume loss.
Based on Mr. L’s presentation and imaging, the rheumatology team suspects CNS lupus and that his prescribed steroids could be playing a role in his behavior.

The authors’ observations
Differentiating CIP from SLEIP can be difficult. The clinical features and criteria for CIP and SLEIP are listed in Table 1.5-7 Several studies have highlighted the difficulties in separating the 2 diagnoses:
- Kampylafka et al8 found that CNS involvement, including stroke, myelopathy, seizures, optic neuritis, and meningitis, was present in 4.3% of their sample of patients with systematic lupus erythematosus (SLE), of whom 6.3% presented with SLEIP. Of patients with CNS involvement, 94% had positive ANA and 69% had positive anti-dsDNA antibodies. It remains difficult to definitively diagnose SLEIP rather than CIP, however, because 100% of patients in this study were taking corticosteroids, with 25% taking azathioprine, as was Mr. L.8
- Appenzeller et al9 found that acute psychosis was associated with SLE in 11.3% of their sample. Psychosis in patients with SLE was accompanied by other manifestations of CNS involvement. On follow-up these patients had mild increases in white blood cell count in their CSF, and MRI demonstrated hyperdense lesions and cerebral atrophy. Hypoalbuminemia, although often seen in SLEIP, also is observed in patients with CIP and cannot be used to differentiate these 2 conditions.9
- Monov and Monova5 recommended criteria for SLEIP that include 3 stages. The first stage is determining that there is evidence of an exacerbation of SLE, and ruling out other causes for neurologic and psychiatric symptoms. The second stage involves using clinical, laboratory, or imaging tests to define the lesion as central and/or peripheral and diffuse and/or focal. The third stage requires diagnosing SLEIP using criteria from 2 groups of signs and symptoms: the first group includes seizure, psychosis, cerebrovascular event, lesion of cranial nerves, and quantitative alterations of consciousness; the second group includes cognitive dysfunction, lupus headache, peripheral neuropathy, MRI changes, EEG changes, electroneuromyography changes, and a positive replication protein A or antiphospholipid-positive antibody. Diagnosing SLEIP requires ≥1 criterion from group 1 and ≥2 criteria from group 2.5
- Patten and Neutel6 found that patients taking prednisolone, Symbol Std<40 mg/d, had significantly higher rates of psychosis than those taking <40 mg/d.6
- Bhangle et Myriad Proal7 found that one of the major distinguishing factors between CIP and SLEIP is the timing of the onset of symptoms, with CIP occurring within 8 weeks of initiation of a corticosteroid, and SLEIP being more likely to occur when additional CNS symptoms are present.7
TREATMENT Decreased dosage
Mr. L starts quetiapine, 25 mg at bedtime, increased to 75 mg at bedtime. Prednisolone is decreased to 10 mg/d. Over the next few days Mr. L’s mood, psychosis, and aggression improve. He becomes calm and cooperative, and denies suicidal or homicidal ideation. Mr. L’s wife, who was initially scared to visit him, comes to see him and confirms that he has improved. After 3 consecutive days with no abnormal behaviors or psychiatric symptoms, Mr. L is discharged and continues taking quetiapine, 75 mg at bedtime, and prednisolone, 10 mg/d, with outpatient follow-up.
The authors’ observations
Table 210,11 describes approaches to treating CIP and SLEIP. Managing CIP typically consists of reducing the corticosteroid dosage. CIP treatment also includes adjunct therapy with psychotropics if the corticosteroid dose cannot be lowered enough to reduce psychiatric symptoms while suppressing symptoms of the disease for which the corticosteroid was prescribed.6

When treating SLEIP, the corticosteroid dosage often is increased. Corticosteroids often are used to treat SLEIP while suppressing symptoms of SLE.10 The main treatment of SLEIP is focused on the disease and using psychotropic medications to control symptoms that don’t respond after exacerbation of the disease has been controlled.10
The presence of Mr. L’s multiple SLE symptoms, as well as MRI findings, could indicate SLEIP. However, corticosteroids also were a possible cause of his psychotic symptoms. Mr. L’s psychosis began within 8 weeks of starting a corticosteroid (prednisolone, 40 mg/d), and his symptoms improved when the corticosteroid dosage was reduced. The difference between CIP and SLEIP may best be distinguished by reducing the corticosteroid dosage and seeing if psychotic symptoms improve. Because it is important to control SLE symptoms in those with CIP, prescribing psychotropics may be warranted, as well as alternative treatments for immunosuppression.
Because steroids are frequently prescribed for lupus, it is important for clinicians to be aware of their psychiatric effects as well as how to manage those effects. When distinguishing CIP from SLEIP, consider decreasing the corticosteroid dosage and see if psychotic symptoms improve. Use adjunct therapy as needed.
CASE Unusual behavior, thoughts
Mr. L, age 28, an immigrant from Burma, is brought to his primary care physician’s clinic by his wife for follow-up on a rash. During the evaluation, his wife reports that Mr. L recently has had suicidal ideation, depression, and increased anger. She says Mr. L had made statements about wanting to kill himself with a gun. Mr. L had driven his car to a soccer field with a knife in hand and was contemplating suicide. She is concerned about her own safety and their children’s safety because of Mr. L’s anger. The physician refers Mr. L to the emergency department, and he is admitted to the medical floor for a rheumatological flare-up and suicidal ideation.
Mr. L starts displaying inappropriate behaviors, including masturbating in front of the patient safety attendant, telling the attendant “You are going to die today,” and assaulting a female attendant by trying to grab her breasts. He is given IM haloperidol, 2 mg, which effectively alleviates these behaviors. Between episodes of unusual behavior and outbursts, Mr. L is docile, quiet, and cooperative, and denies any memory of these episodes.
One month earlier, Mr. L had been hospitalized for progressive weakness and inability to ambulate. He was diagnosed with necrotizing myositis and a rash consistent with subacute cutaneous lupus. He was started on IV methylprednisolone, 1 g, and transitioned to oral prednisolone, 40 mg/d, which he continued taking after discharge. He also started taking azathioprine, which was increased from 50 to 100 mg/d. His condition improved shortly after beginning this regimen.
[polldaddy:9796586]
The authors’ observations
DSM-5 defines brief psychotic disorder as positive symptoms or disorganized or catatonic behavior appearing suddenly and lasting between 1 day to 1 month.1 Mr. L had a sudden onset of his symptoms and marked stressors as a result of his worsening health. However, the possibility of his general medical conditions or medications causing his symptoms needed to be investigated and ruled out before this diagnosis could be assigned.
Another consideration is the culture-bound syndrome amok. Although DSM-5 does not use the term “culture-bound syndrome,” which was used in DSM-IV, it does recognize cultural conceptualizations of distress. Amok is described as a dissociative episode in which an individual has a period of brooding followed by outbursts that include violent, aggressive, and suicidal and/or homicidal ideation. The individual may exhibit persecutory and paranoid thinking, amnesia of the outbursts, and a return to typical behavior when the episode concludes.2 However, it remained unclear whether Mr. L’s violent behavior was a manifestation of psychiatric or organic disease.
Identifying the possibility of amok is important not only for alleviating the patient’s distress but also for preventing violent outbursts that can result in injury or death.3 Amok should be considered only in the context of possible psychiatric or organic brain disease, such as corticosteroid-induced psychosis (CIP) or systemic lupus erythematosus-induced psychosis (SLEIP).4
EVALUATION Informants, labs
Mr. L immigrated to the United States when he was 5 years old. He does not speak English, and interviews are conducted with interpreting services at the hospital. Mr. L answers most questions with or 1 to 2 words. His medical and psychiatric histories are notable for hypothyroidism, hepatitis, non-ischemic cardiomyopathy, necrotizing myositis, subacute cutaneous lupus, and depression. Mr. L denies a personal or family history of mental illness; however, records show he has a history of unspecified depressive disorder.
Mr. L reports his current mood is “okay,” but he has felt different in the past few weeks. He denies auditory or visual hallucinations, or suicidal or homicidal ideation, but exhibits paranoid thoughts. Mr. L believes everyone “lied” to him, and he repeats this frequently. Collateral information from friends reveals that he had threatened to burn down their houses. A family friend states that Mr. L has been depressed and angry over the past 5 days.
During his prior and current hospitalizations, many labs were completed. Thyroid, urine drug screen, C-reactive protein, urine analysis, ethanol, complete blood count, and comprehensive metabolic panel were negative. Erythrocyte sedimentation rate was 30. Lumbar puncture cell count was notable for mildly elevated lymphocytes at 84%. Antinuclear antibody (ANA) was positive. Lupus anticoagulant panel revealed a mildly prolonged partial thromboplastin time at 38.9 seconds. DNA double-stranded antibody (anti-dsDNA) was positive. Anti-Smith antibody was negative. Anti-Ro/SSA and anti-La/SSB antibodies were elevated. Albumin was low. A MRI of the brain showed dystrophic-appearing right parieto-occipital calcification and mild cerebral volume loss.
Based on Mr. L’s presentation and imaging, the rheumatology team suspects CNS lupus and that his prescribed steroids could be playing a role in his behavior.

The authors’ observations
Differentiating CIP from SLEIP can be difficult. The clinical features and criteria for CIP and SLEIP are listed in Table 1.5-7 Several studies have highlighted the difficulties in separating the 2 diagnoses:
- Kampylafka et al8 found that CNS involvement, including stroke, myelopathy, seizures, optic neuritis, and meningitis, was present in 4.3% of their sample of patients with systematic lupus erythematosus (SLE), of whom 6.3% presented with SLEIP. Of patients with CNS involvement, 94% had positive ANA and 69% had positive anti-dsDNA antibodies. It remains difficult to definitively diagnose SLEIP rather than CIP, however, because 100% of patients in this study were taking corticosteroids, with 25% taking azathioprine, as was Mr. L.8
- Appenzeller et al9 found that acute psychosis was associated with SLE in 11.3% of their sample. Psychosis in patients with SLE was accompanied by other manifestations of CNS involvement. On follow-up these patients had mild increases in white blood cell count in their CSF, and MRI demonstrated hyperdense lesions and cerebral atrophy. Hypoalbuminemia, although often seen in SLEIP, also is observed in patients with CIP and cannot be used to differentiate these 2 conditions.9
- Monov and Monova5 recommended criteria for SLEIP that include 3 stages. The first stage is determining that there is evidence of an exacerbation of SLE, and ruling out other causes for neurologic and psychiatric symptoms. The second stage involves using clinical, laboratory, or imaging tests to define the lesion as central and/or peripheral and diffuse and/or focal. The third stage requires diagnosing SLEIP using criteria from 2 groups of signs and symptoms: the first group includes seizure, psychosis, cerebrovascular event, lesion of cranial nerves, and quantitative alterations of consciousness; the second group includes cognitive dysfunction, lupus headache, peripheral neuropathy, MRI changes, EEG changes, electroneuromyography changes, and a positive replication protein A or antiphospholipid-positive antibody. Diagnosing SLEIP requires ≥1 criterion from group 1 and ≥2 criteria from group 2.5
- Patten and Neutel6 found that patients taking prednisolone, Symbol Std<40 mg/d, had significantly higher rates of psychosis than those taking <40 mg/d.6
- Bhangle et Myriad Proal7 found that one of the major distinguishing factors between CIP and SLEIP is the timing of the onset of symptoms, with CIP occurring within 8 weeks of initiation of a corticosteroid, and SLEIP being more likely to occur when additional CNS symptoms are present.7
TREATMENT Decreased dosage
Mr. L starts quetiapine, 25 mg at bedtime, increased to 75 mg at bedtime. Prednisolone is decreased to 10 mg/d. Over the next few days Mr. L’s mood, psychosis, and aggression improve. He becomes calm and cooperative, and denies suicidal or homicidal ideation. Mr. L’s wife, who was initially scared to visit him, comes to see him and confirms that he has improved. After 3 consecutive days with no abnormal behaviors or psychiatric symptoms, Mr. L is discharged and continues taking quetiapine, 75 mg at bedtime, and prednisolone, 10 mg/d, with outpatient follow-up.
The authors’ observations
Table 210,11 describes approaches to treating CIP and SLEIP. Managing CIP typically consists of reducing the corticosteroid dosage. CIP treatment also includes adjunct therapy with psychotropics if the corticosteroid dose cannot be lowered enough to reduce psychiatric symptoms while suppressing symptoms of the disease for which the corticosteroid was prescribed.6

When treating SLEIP, the corticosteroid dosage often is increased. Corticosteroids often are used to treat SLEIP while suppressing symptoms of SLE.10 The main treatment of SLEIP is focused on the disease and using psychotropic medications to control symptoms that don’t respond after exacerbation of the disease has been controlled.10
The presence of Mr. L’s multiple SLE symptoms, as well as MRI findings, could indicate SLEIP. However, corticosteroids also were a possible cause of his psychotic symptoms. Mr. L’s psychosis began within 8 weeks of starting a corticosteroid (prednisolone, 40 mg/d), and his symptoms improved when the corticosteroid dosage was reduced. The difference between CIP and SLEIP may best be distinguished by reducing the corticosteroid dosage and seeing if psychotic symptoms improve. Because it is important to control SLE symptoms in those with CIP, prescribing psychotropics may be warranted, as well as alternative treatments for immunosuppression.
Because steroids are frequently prescribed for lupus, it is important for clinicians to be aware of their psychiatric effects as well as how to manage those effects. When distinguishing CIP from SLEIP, consider decreasing the corticosteroid dosage and see if psychotic symptoms improve. Use adjunct therapy as needed.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Saint Martin ML. Running amok: A modern perspective on a culture-bound syndrome. Prim Care Companion J Clin Psychiatry. 1999;1(3):66-70.
4. Flaskerud JH. Case studies in amok? Issues Ment Health Nurs. 2012;33(12):898-900.
5. Monov S, Monova D. Classification criteria for neuropsychiatric systemic lupus erythematosus: do they need a discussion? Hippokratia. 2008;12(2):103-107.
6. Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis and management. Drug Saf. 2000;22(2):111-122.
7. Bhangle SD, Kramer N, Rosenstein, ED. Corticosteroid-induced neuropsychiatric disorders: review and contrast with neuropsychiatric lupus. Rheumatol Int. 2013;33(8):1923-1932.
8. Kampylafka EI, Alexopoulos H, Kosmidis ML, et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3-year prospective study of 370 patients. PLoS One. 2013;8(2):e55843. d
9. Appenzeller S, Cendes F, Costallat LT. Acute psychosisin systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
10. Sanna G, Bertolaccini ML, Khamashta MA. Neuropsychiatric involvement in systemic lupus erythematosus: current therapeutic approach. Curr Pharm Des. 2008;14(13):1261-1269.
11. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
2. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Saint Martin ML. Running amok: A modern perspective on a culture-bound syndrome. Prim Care Companion J Clin Psychiatry. 1999;1(3):66-70.
4. Flaskerud JH. Case studies in amok? Issues Ment Health Nurs. 2012;33(12):898-900.
5. Monov S, Monova D. Classification criteria for neuropsychiatric systemic lupus erythematosus: do they need a discussion? Hippokratia. 2008;12(2):103-107.
6. Patten SB, Neutel CI. Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis and management. Drug Saf. 2000;22(2):111-122.
7. Bhangle SD, Kramer N, Rosenstein, ED. Corticosteroid-induced neuropsychiatric disorders: review and contrast with neuropsychiatric lupus. Rheumatol Int. 2013;33(8):1923-1932.
8. Kampylafka EI, Alexopoulos H, Kosmidis ML, et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3-year prospective study of 370 patients. PLoS One. 2013;8(2):e55843. d
9. Appenzeller S, Cendes F, Costallat LT. Acute psychosisin systemic lupus erythematosus. Rheumatol Int. 2008;28(3):237-243.
10. Sanna G, Bertolaccini ML, Khamashta MA. Neuropsychiatric involvement in systemic lupus erythematosus: current therapeutic approach. Curr Pharm Des. 2008;14(13):1261-1269.
11. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367.
Caring for medical marijuana patients who request controlled prescriptions
Twenty-eight states and Washington, DC, have legalized marijuana for treating certain medical conditions, but the United States Drug Enforcement Administration (DEA) still classifies marijuana as a Schedule I drug “with no currently accepted medical use and a high potential for abuse.”1 In certain states, clinicians can recommend, but not prescribe, medical marijuana. There is limited guidance in caring for patients who use medical marijuana and request or use DEA-controlled prescription medications, such as benzodiazepines, stimulants, and/or opiates. Physicians can take the following steps to ensure safe care for patients who use medical marijuana and request or take a DEA-controlled prescription medication:
1. Understand your patients’ point of view. Talk with patients who use medical marijuana about the history, frequency, and method of use, and reasons for using medical marijuana. Assess for psychiatric illnesses and any past or active treatment with DEA-controlled prescription medications.
2. Perform screens. Screen for risk factors, past psychiatric history, and prior or current substance use disorders. Treat any existing substance use disorders as appropriate.
3. Provide education. Discuss the risks of marijuana use and its potential adverse effects on the patient’s illness. Explain that marijuana is not currently an FDA-approved treatment and that there often are safer, efficacious alternatives.
4. Set clear boundaries. Be upfront about what is safe clinical practice or the usual standard of medical care and practice within the scope of state and federal laws. Document treatment agreements, utilize prescription drug monitoring programs, and use blood and/or urine toxicology screens as needed. Be aware that a routine drug screen can detect marijuana exposure but may vary in detecting the quantity or length of marijuana use.2
5. Try harm reduction. Any marijuana use, including use that falls short of a Cannabis use disorder, may adversely impact cognition, mood, and/or anxiety.3 Reducing use or abstaining from marijuana use for at least 4 weeks4,5 or reducing or discontinuing the DEA-controlled medication if a patient continues marijuana use are reasonable interventions to see if psychiatric symptoms improve or remit. Polypharmacy with marijuana may place a patient at risk for substance use disorders or additive adverse effects or can hinder the recovery process.
6. Consider alternatives. If a patient feels strongly about continuing medical marijuana use, and you feel that their marijuana use is not clinically harmful and that psychiatric symptoms require treatment, consider medications without a known potential for abuse (eg, antidepressants, buspirone, or hydroxyzine for anxiety; alpha-agonists or atomoxetine for attention-deficit/hyperactivity disorder, etc.). Start such medications at low dosages, titrate slowly, and monitor for benefits and adverse effects.
7. Continue the conversation. Maintain an open and nonjudgmental stance when discussing medical marijuana. Roll with resistance, and frame discussions toward a shared goal of improving the patient’s mental health as safely as possible while using the best medical evidence available.
8. Offer additional support. Refer patients any additional services as appropriate, which may include psychotherapy, a pain specialist, or a substance abuse specialist.
1. United States Drug Enforcement Administration. Drug scheduling. https://www.dea.gov/druginfo/ds.shtml. Accessed June 22, 2017.
2. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004:26(2);200-205.
3. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014:370(23);2219-2227.
4. Schuster RM, Fontaine M, Nip E, et al. Prolonged cannabis withdrawal in young adults with lifetime psychiatric illness [published online February 27, 2017]. Prev Med. pii: S0091-7435(17)30075-0.
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017:8:9-37.
Twenty-eight states and Washington, DC, have legalized marijuana for treating certain medical conditions, but the United States Drug Enforcement Administration (DEA) still classifies marijuana as a Schedule I drug “with no currently accepted medical use and a high potential for abuse.”1 In certain states, clinicians can recommend, but not prescribe, medical marijuana. There is limited guidance in caring for patients who use medical marijuana and request or use DEA-controlled prescription medications, such as benzodiazepines, stimulants, and/or opiates. Physicians can take the following steps to ensure safe care for patients who use medical marijuana and request or take a DEA-controlled prescription medication:
1. Understand your patients’ point of view. Talk with patients who use medical marijuana about the history, frequency, and method of use, and reasons for using medical marijuana. Assess for psychiatric illnesses and any past or active treatment with DEA-controlled prescription medications.
2. Perform screens. Screen for risk factors, past psychiatric history, and prior or current substance use disorders. Treat any existing substance use disorders as appropriate.
3. Provide education. Discuss the risks of marijuana use and its potential adverse effects on the patient’s illness. Explain that marijuana is not currently an FDA-approved treatment and that there often are safer, efficacious alternatives.
4. Set clear boundaries. Be upfront about what is safe clinical practice or the usual standard of medical care and practice within the scope of state and federal laws. Document treatment agreements, utilize prescription drug monitoring programs, and use blood and/or urine toxicology screens as needed. Be aware that a routine drug screen can detect marijuana exposure but may vary in detecting the quantity or length of marijuana use.2
5. Try harm reduction. Any marijuana use, including use that falls short of a Cannabis use disorder, may adversely impact cognition, mood, and/or anxiety.3 Reducing use or abstaining from marijuana use for at least 4 weeks4,5 or reducing or discontinuing the DEA-controlled medication if a patient continues marijuana use are reasonable interventions to see if psychiatric symptoms improve or remit. Polypharmacy with marijuana may place a patient at risk for substance use disorders or additive adverse effects or can hinder the recovery process.
6. Consider alternatives. If a patient feels strongly about continuing medical marijuana use, and you feel that their marijuana use is not clinically harmful and that psychiatric symptoms require treatment, consider medications without a known potential for abuse (eg, antidepressants, buspirone, or hydroxyzine for anxiety; alpha-agonists or atomoxetine for attention-deficit/hyperactivity disorder, etc.). Start such medications at low dosages, titrate slowly, and monitor for benefits and adverse effects.
7. Continue the conversation. Maintain an open and nonjudgmental stance when discussing medical marijuana. Roll with resistance, and frame discussions toward a shared goal of improving the patient’s mental health as safely as possible while using the best medical evidence available.
8. Offer additional support. Refer patients any additional services as appropriate, which may include psychotherapy, a pain specialist, or a substance abuse specialist.
Twenty-eight states and Washington, DC, have legalized marijuana for treating certain medical conditions, but the United States Drug Enforcement Administration (DEA) still classifies marijuana as a Schedule I drug “with no currently accepted medical use and a high potential for abuse.”1 In certain states, clinicians can recommend, but not prescribe, medical marijuana. There is limited guidance in caring for patients who use medical marijuana and request or use DEA-controlled prescription medications, such as benzodiazepines, stimulants, and/or opiates. Physicians can take the following steps to ensure safe care for patients who use medical marijuana and request or take a DEA-controlled prescription medication:
1. Understand your patients’ point of view. Talk with patients who use medical marijuana about the history, frequency, and method of use, and reasons for using medical marijuana. Assess for psychiatric illnesses and any past or active treatment with DEA-controlled prescription medications.
2. Perform screens. Screen for risk factors, past psychiatric history, and prior or current substance use disorders. Treat any existing substance use disorders as appropriate.
3. Provide education. Discuss the risks of marijuana use and its potential adverse effects on the patient’s illness. Explain that marijuana is not currently an FDA-approved treatment and that there often are safer, efficacious alternatives.
4. Set clear boundaries. Be upfront about what is safe clinical practice or the usual standard of medical care and practice within the scope of state and federal laws. Document treatment agreements, utilize prescription drug monitoring programs, and use blood and/or urine toxicology screens as needed. Be aware that a routine drug screen can detect marijuana exposure but may vary in detecting the quantity or length of marijuana use.2
5. Try harm reduction. Any marijuana use, including use that falls short of a Cannabis use disorder, may adversely impact cognition, mood, and/or anxiety.3 Reducing use or abstaining from marijuana use for at least 4 weeks4,5 or reducing or discontinuing the DEA-controlled medication if a patient continues marijuana use are reasonable interventions to see if psychiatric symptoms improve or remit. Polypharmacy with marijuana may place a patient at risk for substance use disorders or additive adverse effects or can hinder the recovery process.
6. Consider alternatives. If a patient feels strongly about continuing medical marijuana use, and you feel that their marijuana use is not clinically harmful and that psychiatric symptoms require treatment, consider medications without a known potential for abuse (eg, antidepressants, buspirone, or hydroxyzine for anxiety; alpha-agonists or atomoxetine for attention-deficit/hyperactivity disorder, etc.). Start such medications at low dosages, titrate slowly, and monitor for benefits and adverse effects.
7. Continue the conversation. Maintain an open and nonjudgmental stance when discussing medical marijuana. Roll with resistance, and frame discussions toward a shared goal of improving the patient’s mental health as safely as possible while using the best medical evidence available.
8. Offer additional support. Refer patients any additional services as appropriate, which may include psychotherapy, a pain specialist, or a substance abuse specialist.
1. United States Drug Enforcement Administration. Drug scheduling. https://www.dea.gov/druginfo/ds.shtml. Accessed June 22, 2017.
2. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004:26(2);200-205.
3. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014:370(23);2219-2227.
4. Schuster RM, Fontaine M, Nip E, et al. Prolonged cannabis withdrawal in young adults with lifetime psychiatric illness [published online February 27, 2017]. Prev Med. pii: S0091-7435(17)30075-0.
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017:8:9-37.
1. United States Drug Enforcement Administration. Drug scheduling. https://www.dea.gov/druginfo/ds.shtml. Accessed June 22, 2017.
2. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004:26(2);200-205.
3. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014:370(23);2219-2227.
4. Schuster RM, Fontaine M, Nip E, et al. Prolonged cannabis withdrawal in young adults with lifetime psychiatric illness [published online February 27, 2017]. Prev Med. pii: S0091-7435(17)30075-0.
5. Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017:8:9-37.