User login
Six Steps to Reduce Taxes on Investments: Minimizing What You Pay in a Tough Environment
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Orthopedic physicians in the highest income tax brackets may have been presented with an unpleasant surprise in recent years when they learned of their investment tax liability. A prolonged period of strong domestic stock performance from 2009 to 2016, combined with the implementation of The American Taxpayer Relief Act of 2012, may have resulted in significantly higher taxes for many of you.
The top ordinary income tax rates increased by 24% when including the Net Investment Income surtax, while the top capital gains rate was increased by more than 58%. Writing a large check to the Internal Revenue Service serves as a harsh reminder that tax planning requires attention throughout the year, and is not a technique you can properly manage a few weeks before an April 15 deadline.
Proper tax planning became more critical as we moved into an era of higher taxes. A multi-year bull market for domestic stocks has caused many traditional investment vehicles to hold large amounts of unrealized gains, which can become realized gains if you are not careful. Most major equity indices took a breath in 2015 and finished the year in the red, which created a planning opportunity for astute investors and their advisors. Stocks in the US and emerging market countries quickly bounced back in 2016; however, European stocks struggled and continue to trade well below peak levels reached nearly a decade ago. Investors who missed the opportunity to offset gains of the prior 2 years may have an opportunity to reduce their tax bill in 2017.
In this article, we will provide you with 6 suggestions that could save you thousands of dollars in investment taxes over the next several years.
1. Account Registration Matters: A common mistake investors make is the failure to implement a tax diversification strategy. Brokerage accounts, Roth IRAs, and qualified plans are subject to various forms of taxation. It is important to utilize the tax advantages of these tools to ensure they work for you in the most productive manner possible. A properly integrated approach is critical during your accumulation phase. Further, it is just as important when you enter the distribution period of your investment life cycle (ie, retirement).
Master Limited Partnerships offer a potentially advantageous income stream for a brokerage account, while it is generally preferable for qualified accounts to own high yield bonds and corporate debt, as they are taxed at ordinary income rates. There are countless additional examples we could discuss, but the lesson is simple: it is important to review the pieces of your plan with an advisor who will consider both tax diversification and security diversification as they relate to your specific circumstances.
2. Consider Owning Municipal Bonds in Taxable Accounts: Most municipal bonds are exempt from federal taxation. Certain issues may also be exempt from state and local taxes. If you are in the highest federal tax bracket, you may be paying tax on investment income at a rate of 43.4%. Under these circumstances, a municipal bond yielding 3% will provide a superior after tax return in comparison to a corporate bond yielding 5% in an individual or joint registration, a pass-through LLC, or in many trust accounts. Therefore, it is important in many circumstances to make certain your long-term plan utilizes the advantages of owning certain municipal bonds in taxable accounts.
3. Be Cognizant of Holding Periods: Long-term capital gains rates are much more favorable than short-term rates. Holding a security for a period of 12 months presents an opportunity to save nearly 20% on the taxation of your appreciated position. For example, an initial investment of $50,000 which grows to $100,000 represents a $50,000 unrealized gain. If an investor in the highest tax bracket simply delays liquidation of the position (assuming the security price does not change) the tax savings in this scenario would be $9,800. Although an awareness of the holding period of a security would appear to be a basic principal of investing, many mutual funds and managed accounts are not designed for tax sensitivity. High income investors should be aware that the average client of most advisors is not in the highest federal tax bracket. Therefore, it is generally advantageous to seek the advice of a financial professional with experience executing an appropriate exit strategy that is aware of holding periods.
4. Proactively Realize Losses to Offset Gains: As mentioned in the opening paragraphs of the article, 2015 presented investors with an opportunity to realize losses in domestic stocks for the first time in 4 years. Clients with a diversified portfolio may still have an opportunity to offset gains in domestic stocks by selling foreign equities. One benefit of diversifying across asset classes is that if the portfolio is structured properly, the securities typically will not move in tandem. This divergence of returns among asset classes not only reduces portfolio volatility, but it creates a tax planning opportunity. Domestic equities experienced tremendous appreciation over a 5-year period through 2014; however, international stocks, commodities, and multiple fixed income investments experienced down years. Astute advisors were presented with the opportunity to save clients thousands of dollars in taxes by performing strategic tax swaps prior to year-end. It is important to understand the rules relating to wash sales when executing such tactics. The laws are confusing, and if a mistake is made your loss could be disallowed. Make certain your advisor is well-versed in utilizing tax offsets.
5. Think Twice About Gifting Cash: This is not to discourage your charitable intentions. Quite the opposite is true. However, a successful investor can occasionally find themselves in a precarious position. You may have allocated 5% of your portfolio to a growth stock with significant upside. Several years have passed, the security has experienced explosive growth, and it now represents 15% of your investable assets. Suddenly your portfolio has a concentrated position with significant gains, and the level of risk is no longer consistent with your long-term objectives. The sound practice of rebalancing your portfolio then becomes very costly, because liquidation of the stock could create a taxable event that may negatively impact your net return.
By planning ahead of time, you may be able to gift a portion of the appreciated security to a charitable organization able to accept this type of donation. The value of your gift can be replaced with the cash you originally intended to donate to the charitable organization and, in this scenario, your cash will create a new cost basis. The charity can liquidate the stock without paying tax, and you have removed a future tax liability from your portfolio. Implementing the aforementioned gifting strategy offers the potential to save thousands of dollars in taxes over the life of your portfolio.
6. Understand your Mutual Fund’s Tax Cost Ratio: The technical detail behind a mutual fund’s tax cost ratio is beyond the scope of this article. Our intent is to simply bring this topic to your attention. Tax cost ratio represents the percentage of an investor’s assets that are lost to taxes. Mutual funds avoid double taxation, provided they pay at least 90% of net investment income and realized capital gains to shareholders at the end of the calendar year. But all mutual funds are not created equally, and proper research will allow you to identify funds that are tax efficient.
A well-managed mutual fund will add diversification to a portfolio while creating the opportunity to outperform asset classes with inefficient markets. You do need to be aware of funds with excessive turnover. An understanding of when a fund pays its capital gains distributions is a critical component of successful investing. A poorly timed fund purchase can result in acquiring another investor’s tax liability. It is not unusual for an investor to experience a negative return in a calendar year, yet find himself on the receiving end of a capital gains distribution. Understanding the tax cost ratios of the funds that make up portions of your investment plan will enable you to take advantage of the many benefits of owning mutual funds.
The above steps are by no means the only tax strategies experienced advisors can execute on behalf of their clients. This article highlights several strategies you should discuss with your advisor to determine if implementation is appropriate for your unique portfolio and overall financial situation. Successful investing requires discipline that extends beyond proper security selection. While gross returns are important and should not be ignored, the percentage return you see on your statements does not tell the full story.
In today’s tax environment, successful investors must choose an advisor who will help them look beyond portfolio earnings and focus on strategic after-tax asset growth.
To receive a free hardcopy of Wealth Protection Planning for Orthopaedic Surgeons, please call 877-656-4362. Visit www.ojmbookstore.com and enter promotional code AJO30 for a free ebook download of Wealth Protection Planning or one of our other ebooks for your Kindle or iPad.
Bone marrow transplantation for epidermolysis bullosa continues to evolve
CHICAGO – Bone marrow transplantation is evolving as a promising treatment for patients with the most severe forms of epidermolysis bullosa.
“Is this a cure? It’s not,” Dr. Jakub Tolar, MD, PhD, said at the World Congress of Pediatric Dermatology. “It is, however, a path toward understanding how we can treat this grave disorder in a systemic way.”
The University of Minnesota BMT Team has also observed a correlation between the engraftment in the blood and engraftment in the skin. “We have skin engraftment as high as 50%, which is good,” Dr. Tolar said. “The more donor cells engrafted in the skin, the more types of collagen you express.”
The clinicians have also been able to reduce the amount of chemotherapy and radiation patients require prior to transplant, for the BMT to work and skin to heal. “We were able to make it so that the last 11 patients are surviving and having benefit from the transplant, with the exception of one,” Dr. Tolar said. “How does this work? We still don’t entirely know. This is not a shot in the dark, however, this is the continuation of a very long process where we were first able to show that bone marrow transplant is an efficient stem cell therapy for leukemia, and about 20 years ago for the lysosomal enzyme deficiencies.” Their hunt for the cell that travels from the bone marrow to skin and produces type 7 collagen is continuing. “What haunts me is that BMT, which works in recessive dystrophic EB, works only in some types of junctional EB, those with alpha-3 chain deficiency of laminin 322.” he continued. “There has been no benefit to bone marrow transplantation for children with mutations of beta-3 chain of laminin 322, so we have closed enrollment for this one form of junctional EB. Survival in this group was 40%. Other types of junctional EB continue to be eligible for the study.”
Dr. Tolar recommended keratinocyte-driven or thymic epithelium cell type–driven therapy for patients with mutations of beta-3. “The deficiency of thymic function seems to be key in the inability to benefit from BMT in this form of junctional EB,” he said. “We have seen children who have engrafted, their skin got better, and then they died of infection many months after transplant. When we look at the immune profile and the thymic epithelial cells, they are both deficient – very abnormal.”
Despite current challenges, Dr. Tolar expressed optimism about the future of BMT in EB patients. “We have the same approach that we have in cancer care: deep empathy for all patients, radical international collaboration, and rapid laboratory and clinical prototyping,” he said. “It’s time to move from two-dimensional science to three-dimensional science; we need to study all aspects of EB simultaneously, from gene to cell to tissue to individual to patient population, and to understand the properties of the whole EB pathobiology that emerge at each level of biological complexity. By connecting information from these layers of disease network, we can better understand EB and create comb
Dr. Tolar reported having no financial disclosures.
CHICAGO – Bone marrow transplantation is evolving as a promising treatment for patients with the most severe forms of epidermolysis bullosa.
“Is this a cure? It’s not,” Dr. Jakub Tolar, MD, PhD, said at the World Congress of Pediatric Dermatology. “It is, however, a path toward understanding how we can treat this grave disorder in a systemic way.”
The University of Minnesota BMT Team has also observed a correlation between the engraftment in the blood and engraftment in the skin. “We have skin engraftment as high as 50%, which is good,” Dr. Tolar said. “The more donor cells engrafted in the skin, the more types of collagen you express.”
The clinicians have also been able to reduce the amount of chemotherapy and radiation patients require prior to transplant, for the BMT to work and skin to heal. “We were able to make it so that the last 11 patients are surviving and having benefit from the transplant, with the exception of one,” Dr. Tolar said. “How does this work? We still don’t entirely know. This is not a shot in the dark, however, this is the continuation of a very long process where we were first able to show that bone marrow transplant is an efficient stem cell therapy for leukemia, and about 20 years ago for the lysosomal enzyme deficiencies.” Their hunt for the cell that travels from the bone marrow to skin and produces type 7 collagen is continuing. “What haunts me is that BMT, which works in recessive dystrophic EB, works only in some types of junctional EB, those with alpha-3 chain deficiency of laminin 322.” he continued. “There has been no benefit to bone marrow transplantation for children with mutations of beta-3 chain of laminin 322, so we have closed enrollment for this one form of junctional EB. Survival in this group was 40%. Other types of junctional EB continue to be eligible for the study.”
Dr. Tolar recommended keratinocyte-driven or thymic epithelium cell type–driven therapy for patients with mutations of beta-3. “The deficiency of thymic function seems to be key in the inability to benefit from BMT in this form of junctional EB,” he said. “We have seen children who have engrafted, their skin got better, and then they died of infection many months after transplant. When we look at the immune profile and the thymic epithelial cells, they are both deficient – very abnormal.”
Despite current challenges, Dr. Tolar expressed optimism about the future of BMT in EB patients. “We have the same approach that we have in cancer care: deep empathy for all patients, radical international collaboration, and rapid laboratory and clinical prototyping,” he said. “It’s time to move from two-dimensional science to three-dimensional science; we need to study all aspects of EB simultaneously, from gene to cell to tissue to individual to patient population, and to understand the properties of the whole EB pathobiology that emerge at each level of biological complexity. By connecting information from these layers of disease network, we can better understand EB and create comb
Dr. Tolar reported having no financial disclosures.
CHICAGO – Bone marrow transplantation is evolving as a promising treatment for patients with the most severe forms of epidermolysis bullosa.
“Is this a cure? It’s not,” Dr. Jakub Tolar, MD, PhD, said at the World Congress of Pediatric Dermatology. “It is, however, a path toward understanding how we can treat this grave disorder in a systemic way.”
The University of Minnesota BMT Team has also observed a correlation between the engraftment in the blood and engraftment in the skin. “We have skin engraftment as high as 50%, which is good,” Dr. Tolar said. “The more donor cells engrafted in the skin, the more types of collagen you express.”
The clinicians have also been able to reduce the amount of chemotherapy and radiation patients require prior to transplant, for the BMT to work and skin to heal. “We were able to make it so that the last 11 patients are surviving and having benefit from the transplant, with the exception of one,” Dr. Tolar said. “How does this work? We still don’t entirely know. This is not a shot in the dark, however, this is the continuation of a very long process where we were first able to show that bone marrow transplant is an efficient stem cell therapy for leukemia, and about 20 years ago for the lysosomal enzyme deficiencies.” Their hunt for the cell that travels from the bone marrow to skin and produces type 7 collagen is continuing. “What haunts me is that BMT, which works in recessive dystrophic EB, works only in some types of junctional EB, those with alpha-3 chain deficiency of laminin 322.” he continued. “There has been no benefit to bone marrow transplantation for children with mutations of beta-3 chain of laminin 322, so we have closed enrollment for this one form of junctional EB. Survival in this group was 40%. Other types of junctional EB continue to be eligible for the study.”
Dr. Tolar recommended keratinocyte-driven or thymic epithelium cell type–driven therapy for patients with mutations of beta-3. “The deficiency of thymic function seems to be key in the inability to benefit from BMT in this form of junctional EB,” he said. “We have seen children who have engrafted, their skin got better, and then they died of infection many months after transplant. When we look at the immune profile and the thymic epithelial cells, they are both deficient – very abnormal.”
Despite current challenges, Dr. Tolar expressed optimism about the future of BMT in EB patients. “We have the same approach that we have in cancer care: deep empathy for all patients, radical international collaboration, and rapid laboratory and clinical prototyping,” he said. “It’s time to move from two-dimensional science to three-dimensional science; we need to study all aspects of EB simultaneously, from gene to cell to tissue to individual to patient population, and to understand the properties of the whole EB pathobiology that emerge at each level of biological complexity. By connecting information from these layers of disease network, we can better understand EB and create comb
Dr. Tolar reported having no financial disclosures.
AT WCPD 2017
Is Simultaneous Bilateral Total Knee Arthroplasty (BTKA) as Safe as Staged BTKA?
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
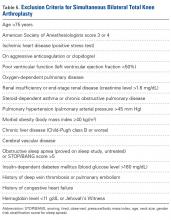
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
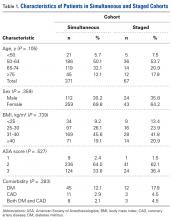
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
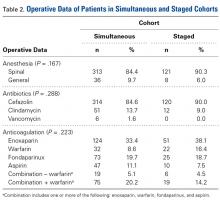
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
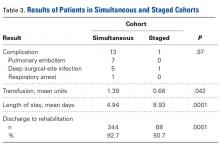
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
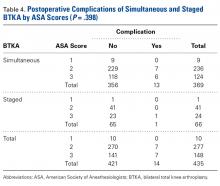
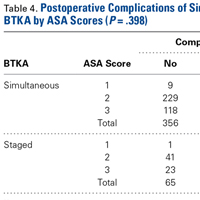
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Complication rates did not statistically significantly differ between simultaneous and staged TKA.
- Length of stay of 2 TKA admissions was greater than 1 BTKA admission.
- Transfusion requirements were greater in BTKA.
- Avoid bilateral procedures in ASA 3 patients.
- Develop institutional protocols for BTKA with multidisciplinary input.
In the United States, osteoarthritis is the most common cause of knee pain and one of the leading causes of disability.1 Total knee arthroplasty (TKA) is an effective treatment for end-stage osteoarthritis of the knee.2 Whether patients with severe, debilitating bilateral disease should undergo simultaneous bilateral TKA (BTKA) or staged BTKA (2 separate procedures during separate hospital admissions) continues to be debated. The relative risks and benefits of simultaneous BTKA relative to staged BTKA or unilateral TKA are controversial.3-6 Proponents of simultaneous BTKA have argued that this surgery results in shorter hospital length of stay (LOS) and higher patient satisfaction without increased risk of perioperative complications,7-9 and opponents have argued that it leads to increased perioperative mortality and complications and should not be performed routinely.10,11
The safety of simultaneous BTKA cannot necessarily be extrapolated from data on unilateral TKA. Authors have argued that the complication rate for simultaneous BTKA is not comparable to the rate for unilateral TKA but instead is double the rate.12 Although a doubled rate may more closely approximate the true risk of simultaneous BTKA, it still does not account for the increased surgical impact of 2 procedures (vs 1 procedure) on a patient. In this regard, comparing simultaneous and staged BTKA provides a more accurate assessment of risk, as long as the interval between surgeries is not excessive. The major stress experienced during TKA affects the cardiovascular, pulmonary, and musculoskeletal systems, and full recovery may take up to 6 months.13-15 Outcome studies have found significant improvement in validated measures of function and pain up to but not past 6 months.13,15 Furthermore, a large study comparing American Society of Anesthesiologists (ASA) scores with morbidity and mortality rates recorded in the New Zealand Total Joint Database established 6 months as a best approximation of postoperative mortality and morbidity risk.14 Given these data, we propose that the most accurate analysis of postoperative morbidity and mortality would be a comparison of simultaneous BTKA with BTKA staged <6 months apart. The staged procedures fall within the crucial postoperative period when increased morbidity and mortality would more likely be present. A between-surgeries interval >6 months would effectively separate the 2 procedures, rendering their risks not truly representative.
We retrospectively analyzed all simultaneous BTKA and staged BTKA (<6 months apart) surgeries performed at our orthopedic specialty hospital between 2005 and 2009. We hypothesized there would be no significant difference in perioperative morbidity or mortality between the groups.
Methods and Materials
Our institution’s Institutional Review Board approved this study. All patients who underwent either simultaneous BTKA or staged BTKA (<6 months apart) at a single orthopedic specialty hospital between 2005 and 2009 were retrospectively identified. Twenty-five surgeons performed the procedures. Which procedure to perform (simultaneous or staged) was decided by the attending surgeon in consultation with an anesthesiologist. Preoperative medical diagnostic testing was determined by the internist, who provided medical clearance, and was subject to review by the anesthesiologist. A patient was excluded from simultaneous BTKA only if the medical or anesthesiology consultant deemed the patient too high risk for bilateral procedures. Revision TKAs were excluded from the study.
Implant, approach, tourniquet use, and TKA technique were selected by the individual surgeons. Strategies for the simultaneous procedures were (1) single surgeon, single team, sequential, start second knee after closure of first, and (2) single surgeon, single team, sequential, start second knee after implantation of first but before closure. The decision to proceed with the second knee was confirmed in consultation with the anesthesiologist after implantation and deflation of the tourniquet on the first knee.
Individual electronic patient charts were reviewed for information on demographics, comorbidities, anesthesia type, antibiotics, and postoperative venous thromboembolism prophylaxis. Demographic variables included age, sex, height, weight, and body mass index (BMI). Comorbidities recorded were diabetes mellitus, coronary artery disease, prior myocardial infarction, stroke, and endocrinopathies. In addition, available ASA scores were recorded. The primary outcome was perioperative complications, defined as any complications that occurred within 6 months after surgery. These included death, pulmonary embolism (PE), and deep surgical-site infections (SSIs). Secondary outcome measures were LOS, discharge location (rehabilitation or home), and blood transfusion requirements.
The 2 groups (simultaneous BTKA, staged BTKA) were compared using Student t test for continuous variables and χ2 test for categorical variables. Subgroup analysis was performed to compare healthier patients (ASA score 1 or 2) with patients who had more severe comorbidities (ASA score 3). Statistical significance was set at P < .05.
Results
Between 2005 and 2009, 371 patients had simultaneous BTKA, and 67 had staged BTKA (134 procedures) <6 months apart (Table 1).
Most surgeries (84.4% simultaneous, 90.3% staged) were performed with the patient under spinal anesthesia, and there was a trend (P = .167) toward more frequent use of general anesthesia in the simultaneous group relative to the staged group (Table 2).
The 2 cohorts’ perioperative complication rates were not statistically significantly different (P = .97) (Table 3).
There was no statistically significant difference (P = .398) in occurrence of postoperative complications between the 2 cohorts compared on ASA scores, and the difference between patients with ASA score 1 or 2 and those with ASA score 3 was not statistically significant (P = .200) (Table 4).
Discussion
Although there was no significant difference in postoperative complication rates within 6 months after surgery between the simultaneous and staged BTKA groups, the incidence of complications in the simultaneous group was notable. The disproportionate size of the 2 comparison groups limited the power of our study to analyze individual perioperative complications. This study may be underpowered to detect differences in complications occurring relatively infrequently, which may explain why the difference in number of complications (13 in simultaneous group, 1 in staged group) did not achieve statistical significance (β = 0.89). Post hoc power analysis showed 956 patients would be needed in each group to adequately power for such small complication rates. However, our results are consistent with those of other studies.13-15 The 1.9% PE rate in our simultaneous BTKA group does not vary from the average PE rate for TKA in the literature and is actually lower than the PE rate in a previous study at our institution.16 Fat embolism traditionally is considered more of a concern in bilateral cases than in unilateral cases. Although fat embolism surely is inherent to the physiologic alterations caused by TKA, we did not find clinically significant fat embolism in either cohort.
Similarly, the 1.08% rate of deep SSIs is within the range for postoperative TKA infections at our institution and others.17 Our staged BTKA group’s complication rate, 0.75% (1 SSI), was slightly lower than expected. However, 0.75% is in keeping with institutional norms (typical rate, ~1%). We would have expected a nonzero rate for venous thromboembolism, and perhaps such a rate would have come with an inclusion period longer than 6 months. Last, the death in the simultaneous BTKA group was not an outlier, given the published rate of mortality after elective total joint surgery.18The characteristics of our simultaneous and staged BTKA groups were very similar (Table 1), though the larger number of staged-group patients with diabetes mellitus and coronary artery disease may represent selection bias. Nevertheless, the proportions of patients with each of 3 ASA scores were similar. It is also important to note that, in this context, a high percentage of patients in each group (33.6% simultaneous, 37.5% staged) received ASA score 3 from the anesthesiologist (P > .05). This may be an important factor in explaining the larger though not statistically significant number of complications in the simultaneous group (13) relative to the staged group (1).
Other authors have studied the safety of simultaneous vs staged BTKA and drawn conflicting conclusions.11,19-21 Walmsley and colleagues21 found no differences in 90-day mortality between 3 groups: patients with simultaneous BTKA, patients with BTKA staged within 5 years, and patients with unilateral TKA. Stefánsdóttir and colleagues11 found that, compared with simultaneous BTKA, BTKA staged within 1 year had a lower 30-day mortality rate. Meehan and colleagues20 compared simultaneous BTKA with BTKA staged within 1 year and found a lower risk of infection and device malfunction and a higher risk of adverse cardiovascular outcomes in the simultaneous group. A recent meta-analysis found that, compared with staged BTKA, simultaneous BTKA had a higher risk of perioperative complications.19 A systematic review of retrospective studies found simultaneous BTKA had higher rates of mortality, PE, and transfusion and lower rates of deep SSI and revision.22 A survey of Medicare data found higher 90-day mortality and myocardial infarction rates for simultaneous BTKA but no difference in infection and revision rates.23 Clearly, there is no consensus as to whether simultaneous BTKA carries higher risks relative to staged BTKA.
The amount of blood transfused in our simultaneous BTKA group was more than double that in the 2 staged TKAs combined. It is intuitive that the blood loss in 2 concurrent TKAs is always more than in 1 TKA, but the clinical relevance of this fact is unknown. Transfusions have potential complications, and this risk needs to be addressed in the preoperative discussion.
LOS for simultaneous BTKA was on average 4 days shorter than the combined LOS (2 hospitalizations) for staged BTKA. This shorter LOS has been shown to provide the healthcare system with a cost savings.8 However, not considered in the equation is the difference in cost of rehabilitations, 2 vs 1. In the present study, 92.7% of simultaneous BTKA patients and only 50.7% of staged BTKA patients were discharged to an inpatient acute rehabilitation unit. Interestingly, the majority of the staged patients who went to inpatient rehabilitation did so after the second surgery. At our institution at the time of this study, simultaneous BTKA patients, and staged BTKA patients with the second surgery completed, were more likely than unilateral TKA patients to qualify for inpatient acute rehabilitation. Staged BTKA patients’ higher cost for 2 rehabilitations, rather than 1, adds to the cost savings realized with simultaneous BTKA. In the context of an episode-based payment system, the cost of posthospital rehabilitation enters the overall cost equation and may lead to an increase in the number of simultaneous BTKAs being performed.
Conclusion
In this study, the incidence of postoperative complications was higher for simultaneous BTKA than for staged BTKA performed <6 months apart, but the difference was not significantly different. There were significant differences in LOS and blood transfusion rates between the groups, as expected. At present, only patients with ASA score 1 or 2 are considered for simultaneous BTKA at our institution. Patients with ASA score 3 or higher are not eligible.
Am J Orthop. 2017;46(4):E224-E229. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
1. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
2. Kolettis GT, Wixson RL, Peruzzi WT, Blake MJ, Wardell S, Stulberg SD. Safety of 1-stage bilateral total knee arthroplasty. Clin Orthop Relat Res. 1994;(309):102-109.
3. Kim YH, Choi YW, Kim JS. Simultaneous bilateral sequential total knee replacement is as safe as unilateral total knee replacement. J Bone Joint Surg Br. 2009;91(1):64-68.
4. Luscombe JC, Theivendran K, Abudu A, Carter SR. The relative safety of one-stage bilateral total knee arthroplasty. Int Orthop. 2009;33(1):101-104.
5. Memtsoudis SG, Ma Y, González Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111(6):1206-1216.
6. Zeni JA Jr, Snyder-Mackler L. Clinical outcomes after simultaneous bilateral total knee arthroplasty: comparison to unilateral total knee arthroplasty and healthy controls. J Arthroplasty. 2010;25(4):541-546.
7. March LM, Cross M, Tribe KL, et al; Arthritis C.O.S.T. Study Project Group. Two knees or not two knees? Patient costs and outcomes following bilateral and unilateral total knee joint replacement surgery for OA. Osteoarthritis Cartilage. 2004;12(5):400-408.
8. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
9. Ritter MA, Harty LD. Debate: simultaneous bilateral knee replacements: the outcomes justify its use. Clin Orthop Relat Res. 2004;(428):84-86.
10. Restrepo C, Parvizi J, Dietrich T, Einhorn TA. Safety of simultaneous bilateral total knee arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2007;89(6):1220-1226.
11. Stefánsdóttir A, Lidgren L, Robertsson O. Higher early mortality with simultaneous rather than staged bilateral TKAs: results from the Swedish Knee Arthroplasty Register. Clin Orthop Relat Res. 2008;466(12):3066-3070.
12. Noble J, Goodall J, Noble D. Simultaneous bilateral total knee replacement: a persistent controversy. Knee. 2009;16(6):420-426.
13. Fortin PR, Penrod JR, Clarke AE, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327-3330.
14. Hooper GJ, Rothwell AG, Hooper NM, Frampton C. The relationship between the American Society of Anesthesiologists physical rating and outcome following total hip and knee arthroplasty: an analysis of the New Zealand Joint Registry. J Bone Joint Surg Am. 2012;94(12):1065-1070.
15. MacWilliam CH, Yood MU, Verner JJ, McCarthy BD, Ward RE. Patient-related risk factors that predict poor outcome after total hip replacement. Health Serv Res. 1996;31(5):623-638.
16. Hadley SR, Lee M, Reid M, Dweck E, Steiger D. Predictors of pulmonary embolism in orthopaedic patient population. Abstract presented at: 43rd Annual Meeting of the Eastern Orthopaedic Association; June 20-23, 2012; Bolton Landing, NY.
17. Hadley S, Immerman I, Hutzler L, Slover J, Bosco J. Staphylococcus aureus decolonization protocol decreases surgical site infections for total joint replacement. Arthritis. 2010;2010:924518.
18. Singh JA, Lewallen DG. Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty. 2012;27(8):1417-1422.e1.
19. Hu J, Liu Y, Lv Z, Li X, Qin X, Fan W. Mortality and morbidity associated with simultaneous bilateral or staged bilateral total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2011;131(9):1291-1298.
20. Meehan JP, Danielsen B, Tancredi DJ, Kim S, Jamali AA, White RH. A population-based comparison of the incidence of adverse outcomes after simultaneous-bilateral and staged-bilateral total knee arthroplasty. J Bone Joint Surg Am. 2011;93(23):2203-2213.
21. Walmsley P, Murray A, Brenkel IJ. The practice of bilateral, simultaneous total knee replacement in Scotland over the last decade. Data from the Scottish Arthroplasty Project. Knee. 2006;13(2):102-105.
22. Fu D, Li G, Chen K, Zeng H, Zhang X, Cai Z. Comparison of clinical outcome between simultaneous-bilateral and staged-bilateral total knee arthroplasty: a systematic review of retrospective studies. J Arthroplasty. 2013;28(7):1141-1147.
23. Bolognesi MP, Watters TS, Attarian DE, Wellman SS, Setoguchi S. Simultaneous vs staged bilateral total knee arthroplasty among Medicare beneficiaries, 2000–2009. J Arthroplasty. 2013;28(8 suppl):87-91.
Severity of Depression May Affect Epilepsy Risk and Outcomes
Severity of depression may be associated with epilepsy risk and outcomes, according to a study published in the May issue of JAMA Neurology. “Treated depression is associated with worse epilepsy outcomes, suggesting that this [factor] may be a surrogate for more severe depression and that severity of depression is associated with severity of epilepsy,” said Colin B. Josephson, MD, Assistant Professor of Neurology at the University of Calgary in Alberta, and colleagues.
“If one assumes that depression treatment is a surrogate marker for depression severity, then there … appears to be a biological gradient,” the investigators said. “This assertion is corroborated by the incremental hazard of epilepsy among those receiving counseling alone (lowest hazard), antidepressants alone (intermediate hazard), and a combination of counseling and antidepressants (highest hazard).”
Prior research has found that the incidence and prevalence of depression are significantly elevated before and after an epilepsy diagnosis. Studies have not assessed, however, the risk of epilepsy after an incident diagnosis of depression or whether depression severity affects the level of risk or seizure outcomes, the authors said.
Two Data Sources
Dr. Josephson and colleagues examined two independent sources of data to explore the relationship between depression and epilepsy further. First, they conducted an observational study using The Health Improvement Network (THIN) database, a population-based primary care cohort. The THIN database contains prospective data that are broadly representative of the general population of the United Kingdom. All patients included in the study were free of prevalent depression and epilepsy and between the ages of 18 and 90. Separately, they analyzed prospective data from the Calgary Comprehensive Epilepsy Programme, a registry of adult outpatient encounters.
Of the more than 10.5 million eligible patients in the THIN database, 229,164 (2.2%) developed depression and 97,177 (0.9%) developed epilepsy. The median age was 44 among patients with depression and 56 among patients with epilepsy. Sixty-three percent of the patients with depression and 56% of the patients with epilepsy were women.
Incident epilepsy was associated with an increased hazard of developing depression (hazard ratio [HR], 2.04), and incident depression was associated with an increased hazard of developing epilepsy (HR, 2.55). “The association with incident epilepsy was even stronger for those with treated depression (HR, 3.42), compared with a combination of those who did not develop depression and those who developed depression not requiring therapy.”
In a sensitivity analysis controlling for age, sex, socioeconomic status, and Charlson Comorbidity Index, “there was an incremental hazard according to depression treatment type, with lowest risk for those receiving counselling alone (HR, 1.84), an intermediate risk for those receiving antidepressants alone (HR, 3.43), and the highest risk for those receiving both (HR, 9.85).”
Likelihood of Seizure Freedom
To assess the effect of depression on seizure outcomes, the researchers analyzed data from 2,573 patients in the Calgary Comprehensive Epilepsy Programme cohort. Data were derived from patients’ first-visit evaluation forms. A total of 504 (20%) of the patients had achieved one-year seizure freedom during the year prior to their first clinic visit. Patients with past or current depression had higher odds of failing to achieve seizure freedom during the previous year, compared with patients without depression (odds ratio, 1.41). When considering only patients with current or past depression (n = 738), current depression treatment (ie, antidepressants, counseling, or both) was associated with significantly higher odds of failing to achieve one-year seizure freedom (odds ratio, 1.75) after controlling for age at onset of epilepsy, sex, lesional epilepsy detected by MRI, history of generalized tonic-clonic seizures, and current antiepileptic drug use.
Overall, the results “indicate a shared relationship between depression and epilepsy, with each appearing to act as a risk factor for the other, and, therefore, should have a direct effect on counseling and management,” Dr. Josephson and colleagues said.
Limitations
The researchers noted that they were unable to control for etiology, epilepsy subtype, or conditions that could cause epilepsy and depression, and they did not have information about the duration, dose, or frequency of depression treatments. Although depression severity may not necessarily determine treatment type, primary care guidelines recommend counseling as first-line treatment for mild depression and combination therapy for severe depression, the researchers said.
Other possible interpretations of the results, such as the idea that antidepressant treatment augments the risk of epilepsy or that patients are more severely depressed because they have not achieved one-year seizure freedom, are less likely. “Counseling interacted with antidepressant exposure and resulted in a three
—Jake Remaly
Suggested Reading
Josephson CB, Lowerison M, Vallerand I, et al. Association of depression and treated depression with epilepsy and seizure outcomes: A multicohort analysis. JAMA Neurol. 2017;74(5):533-539.
Kanner AM. Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: A review of the evidence. Epilepsy Behav. 2016;61:282-286.
Severity of depression may be associated with epilepsy risk and outcomes, according to a study published in the May issue of JAMA Neurology. “Treated depression is associated with worse epilepsy outcomes, suggesting that this [factor] may be a surrogate for more severe depression and that severity of depression is associated with severity of epilepsy,” said Colin B. Josephson, MD, Assistant Professor of Neurology at the University of Calgary in Alberta, and colleagues.
“If one assumes that depression treatment is a surrogate marker for depression severity, then there … appears to be a biological gradient,” the investigators said. “This assertion is corroborated by the incremental hazard of epilepsy among those receiving counseling alone (lowest hazard), antidepressants alone (intermediate hazard), and a combination of counseling and antidepressants (highest hazard).”
Prior research has found that the incidence and prevalence of depression are significantly elevated before and after an epilepsy diagnosis. Studies have not assessed, however, the risk of epilepsy after an incident diagnosis of depression or whether depression severity affects the level of risk or seizure outcomes, the authors said.
Two Data Sources
Dr. Josephson and colleagues examined two independent sources of data to explore the relationship between depression and epilepsy further. First, they conducted an observational study using The Health Improvement Network (THIN) database, a population-based primary care cohort. The THIN database contains prospective data that are broadly representative of the general population of the United Kingdom. All patients included in the study were free of prevalent depression and epilepsy and between the ages of 18 and 90. Separately, they analyzed prospective data from the Calgary Comprehensive Epilepsy Programme, a registry of adult outpatient encounters.
Of the more than 10.5 million eligible patients in the THIN database, 229,164 (2.2%) developed depression and 97,177 (0.9%) developed epilepsy. The median age was 44 among patients with depression and 56 among patients with epilepsy. Sixty-three percent of the patients with depression and 56% of the patients with epilepsy were women.
Incident epilepsy was associated with an increased hazard of developing depression (hazard ratio [HR], 2.04), and incident depression was associated with an increased hazard of developing epilepsy (HR, 2.55). “The association with incident epilepsy was even stronger for those with treated depression (HR, 3.42), compared with a combination of those who did not develop depression and those who developed depression not requiring therapy.”
In a sensitivity analysis controlling for age, sex, socioeconomic status, and Charlson Comorbidity Index, “there was an incremental hazard according to depression treatment type, with lowest risk for those receiving counselling alone (HR, 1.84), an intermediate risk for those receiving antidepressants alone (HR, 3.43), and the highest risk for those receiving both (HR, 9.85).”
Likelihood of Seizure Freedom
To assess the effect of depression on seizure outcomes, the researchers analyzed data from 2,573 patients in the Calgary Comprehensive Epilepsy Programme cohort. Data were derived from patients’ first-visit evaluation forms. A total of 504 (20%) of the patients had achieved one-year seizure freedom during the year prior to their first clinic visit. Patients with past or current depression had higher odds of failing to achieve seizure freedom during the previous year, compared with patients without depression (odds ratio, 1.41). When considering only patients with current or past depression (n = 738), current depression treatment (ie, antidepressants, counseling, or both) was associated with significantly higher odds of failing to achieve one-year seizure freedom (odds ratio, 1.75) after controlling for age at onset of epilepsy, sex, lesional epilepsy detected by MRI, history of generalized tonic-clonic seizures, and current antiepileptic drug use.
Overall, the results “indicate a shared relationship between depression and epilepsy, with each appearing to act as a risk factor for the other, and, therefore, should have a direct effect on counseling and management,” Dr. Josephson and colleagues said.
Limitations
The researchers noted that they were unable to control for etiology, epilepsy subtype, or conditions that could cause epilepsy and depression, and they did not have information about the duration, dose, or frequency of depression treatments. Although depression severity may not necessarily determine treatment type, primary care guidelines recommend counseling as first-line treatment for mild depression and combination therapy for severe depression, the researchers said.
Other possible interpretations of the results, such as the idea that antidepressant treatment augments the risk of epilepsy or that patients are more severely depressed because they have not achieved one-year seizure freedom, are less likely. “Counseling interacted with antidepressant exposure and resulted in a three
—Jake Remaly
Suggested Reading
Josephson CB, Lowerison M, Vallerand I, et al. Association of depression and treated depression with epilepsy and seizure outcomes: A multicohort analysis. JAMA Neurol. 2017;74(5):533-539.
Kanner AM. Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: A review of the evidence. Epilepsy Behav. 2016;61:282-286.
Severity of depression may be associated with epilepsy risk and outcomes, according to a study published in the May issue of JAMA Neurology. “Treated depression is associated with worse epilepsy outcomes, suggesting that this [factor] may be a surrogate for more severe depression and that severity of depression is associated with severity of epilepsy,” said Colin B. Josephson, MD, Assistant Professor of Neurology at the University of Calgary in Alberta, and colleagues.
“If one assumes that depression treatment is a surrogate marker for depression severity, then there … appears to be a biological gradient,” the investigators said. “This assertion is corroborated by the incremental hazard of epilepsy among those receiving counseling alone (lowest hazard), antidepressants alone (intermediate hazard), and a combination of counseling and antidepressants (highest hazard).”
Prior research has found that the incidence and prevalence of depression are significantly elevated before and after an epilepsy diagnosis. Studies have not assessed, however, the risk of epilepsy after an incident diagnosis of depression or whether depression severity affects the level of risk or seizure outcomes, the authors said.
Two Data Sources
Dr. Josephson and colleagues examined two independent sources of data to explore the relationship between depression and epilepsy further. First, they conducted an observational study using The Health Improvement Network (THIN) database, a population-based primary care cohort. The THIN database contains prospective data that are broadly representative of the general population of the United Kingdom. All patients included in the study were free of prevalent depression and epilepsy and between the ages of 18 and 90. Separately, they analyzed prospective data from the Calgary Comprehensive Epilepsy Programme, a registry of adult outpatient encounters.
Of the more than 10.5 million eligible patients in the THIN database, 229,164 (2.2%) developed depression and 97,177 (0.9%) developed epilepsy. The median age was 44 among patients with depression and 56 among patients with epilepsy. Sixty-three percent of the patients with depression and 56% of the patients with epilepsy were women.
Incident epilepsy was associated with an increased hazard of developing depression (hazard ratio [HR], 2.04), and incident depression was associated with an increased hazard of developing epilepsy (HR, 2.55). “The association with incident epilepsy was even stronger for those with treated depression (HR, 3.42), compared with a combination of those who did not develop depression and those who developed depression not requiring therapy.”
In a sensitivity analysis controlling for age, sex, socioeconomic status, and Charlson Comorbidity Index, “there was an incremental hazard according to depression treatment type, with lowest risk for those receiving counselling alone (HR, 1.84), an intermediate risk for those receiving antidepressants alone (HR, 3.43), and the highest risk for those receiving both (HR, 9.85).”
Likelihood of Seizure Freedom
To assess the effect of depression on seizure outcomes, the researchers analyzed data from 2,573 patients in the Calgary Comprehensive Epilepsy Programme cohort. Data were derived from patients’ first-visit evaluation forms. A total of 504 (20%) of the patients had achieved one-year seizure freedom during the year prior to their first clinic visit. Patients with past or current depression had higher odds of failing to achieve seizure freedom during the previous year, compared with patients without depression (odds ratio, 1.41). When considering only patients with current or past depression (n = 738), current depression treatment (ie, antidepressants, counseling, or both) was associated with significantly higher odds of failing to achieve one-year seizure freedom (odds ratio, 1.75) after controlling for age at onset of epilepsy, sex, lesional epilepsy detected by MRI, history of generalized tonic-clonic seizures, and current antiepileptic drug use.
Overall, the results “indicate a shared relationship between depression and epilepsy, with each appearing to act as a risk factor for the other, and, therefore, should have a direct effect on counseling and management,” Dr. Josephson and colleagues said.
Limitations
The researchers noted that they were unable to control for etiology, epilepsy subtype, or conditions that could cause epilepsy and depression, and they did not have information about the duration, dose, or frequency of depression treatments. Although depression severity may not necessarily determine treatment type, primary care guidelines recommend counseling as first-line treatment for mild depression and combination therapy for severe depression, the researchers said.
Other possible interpretations of the results, such as the idea that antidepressant treatment augments the risk of epilepsy or that patients are more severely depressed because they have not achieved one-year seizure freedom, are less likely. “Counseling interacted with antidepressant exposure and resulted in a three
—Jake Remaly
Suggested Reading
Josephson CB, Lowerison M, Vallerand I, et al. Association of depression and treated depression with epilepsy and seizure outcomes: A multicohort analysis. JAMA Neurol. 2017;74(5):533-539.
Kanner AM. Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: A review of the evidence. Epilepsy Behav. 2016;61:282-286.
The team and I
As a physician or a patient, you probably have noticed that the quality of health care is better when there is a continuous relationship between the physician and the patient. Discontinuity can make doctor-patient communication less fluid, but familiarity can breed comfort and confidence. Patients often complain when they see a different physician at every visit. And physicians know they are less efficient when they are seeing a patient they have never seen before.
They discovered that less continuity was associated with more ambulatory sick visits and more ambulatory sensitive hospitalizations, particularly for children with chronic conditions. Interestingly, they could find no association between continuity measured at well visits and patients’ health outcomes.
With only a gut level and personal relationship with the subject, I wondered how the researchers measured something as nebulous as continuity. It turns out there are several ways to measure continuity, of which the investigators focused on two. The Usual Provider of Care is calculated by dividing the number of visits with the most common provider by the total number of primary care visits. The Bice and Boxerman Continuity of Care Index is more difficult to calculate because, rather than using a single provider, it lumps a small core of providers together (such as a team) as the most the common provider.
As a curmudgeonly, old school, egotistical kind of guy, I was surprised and disappointed to learn from this paper’s references of another study that found, in at least one scenario, the individual-based (Usual Provider of Care) and team-based (Bice and Boxerman Continuity of Care Index) methods of defining continuity yielded comparable results (Med Care. 2016 May;54[5]:e30-4). I always have assumed that, regardless of how well it had been crafted, that I could provide better continuity than a team of providers.
I know what you are thinking: This guy hasn’t bought into the maxim that “There is no I in team.” No, no, I do believe in it, but in the context of continuity of care, it seemed to me that sometimes the more links there are in the chain, the more chances there are for miscommunication. And we all know that primary care is 90% communication.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
As a physician or a patient, you probably have noticed that the quality of health care is better when there is a continuous relationship between the physician and the patient. Discontinuity can make doctor-patient communication less fluid, but familiarity can breed comfort and confidence. Patients often complain when they see a different physician at every visit. And physicians know they are less efficient when they are seeing a patient they have never seen before.
They discovered that less continuity was associated with more ambulatory sick visits and more ambulatory sensitive hospitalizations, particularly for children with chronic conditions. Interestingly, they could find no association between continuity measured at well visits and patients’ health outcomes.
With only a gut level and personal relationship with the subject, I wondered how the researchers measured something as nebulous as continuity. It turns out there are several ways to measure continuity, of which the investigators focused on two. The Usual Provider of Care is calculated by dividing the number of visits with the most common provider by the total number of primary care visits. The Bice and Boxerman Continuity of Care Index is more difficult to calculate because, rather than using a single provider, it lumps a small core of providers together (such as a team) as the most the common provider.
As a curmudgeonly, old school, egotistical kind of guy, I was surprised and disappointed to learn from this paper’s references of another study that found, in at least one scenario, the individual-based (Usual Provider of Care) and team-based (Bice and Boxerman Continuity of Care Index) methods of defining continuity yielded comparable results (Med Care. 2016 May;54[5]:e30-4). I always have assumed that, regardless of how well it had been crafted, that I could provide better continuity than a team of providers.
I know what you are thinking: This guy hasn’t bought into the maxim that “There is no I in team.” No, no, I do believe in it, but in the context of continuity of care, it seemed to me that sometimes the more links there are in the chain, the more chances there are for miscommunication. And we all know that primary care is 90% communication.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
As a physician or a patient, you probably have noticed that the quality of health care is better when there is a continuous relationship between the physician and the patient. Discontinuity can make doctor-patient communication less fluid, but familiarity can breed comfort and confidence. Patients often complain when they see a different physician at every visit. And physicians know they are less efficient when they are seeing a patient they have never seen before.
They discovered that less continuity was associated with more ambulatory sick visits and more ambulatory sensitive hospitalizations, particularly for children with chronic conditions. Interestingly, they could find no association between continuity measured at well visits and patients’ health outcomes.
With only a gut level and personal relationship with the subject, I wondered how the researchers measured something as nebulous as continuity. It turns out there are several ways to measure continuity, of which the investigators focused on two. The Usual Provider of Care is calculated by dividing the number of visits with the most common provider by the total number of primary care visits. The Bice and Boxerman Continuity of Care Index is more difficult to calculate because, rather than using a single provider, it lumps a small core of providers together (such as a team) as the most the common provider.
As a curmudgeonly, old school, egotistical kind of guy, I was surprised and disappointed to learn from this paper’s references of another study that found, in at least one scenario, the individual-based (Usual Provider of Care) and team-based (Bice and Boxerman Continuity of Care Index) methods of defining continuity yielded comparable results (Med Care. 2016 May;54[5]:e30-4). I always have assumed that, regardless of how well it had been crafted, that I could provide better continuity than a team of providers.
I know what you are thinking: This guy hasn’t bought into the maxim that “There is no I in team.” No, no, I do believe in it, but in the context of continuity of care, it seemed to me that sometimes the more links there are in the chain, the more chances there are for miscommunication. And we all know that primary care is 90% communication.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Successful teams
As physicians, we know, and have been taught, that success comes from being smart and answering questions correctly. By doing so, we excelled in school, scored high on standardized tests, and confidently raised our hands in class. As a result, we were chosen for plum assignments like class officer, yearbook editor, and team captain. Success bred success and we were initiated into honor societies, accepted into prestigious internships, and allowed to work on important research projects.
These achievements guaranteed further success in medical school where the tradition of competition continued as we positioned for the attention of those who would someday write our letters of recommendation. This model of individual success served us very well until we joined a hematology group to begin our practice.
When we join a group of physicians, we join a team. The team might organize around diagnoses, laboratories, or geographic location, but it is a team nonetheless. The team, of course, includes more than just physicians. As a team member, we are expected to fulfill a role that advances the mission of the team and the larger organization. That mission may include some combination of academic, educational, and clinical productivity.
As physicians trained to compete with other individuals, teamwork does not come naturally. We might think that so long as the team consists of more and mor
As Margaret Heffernan brilliantly points out in “Margaret Heffernan: Why it’s time to forget the pecking order at work,” a TED talk video posted on YouTube, teams consisting of those skilled in interpersonal relationships are much more effective, efficient, and productive than are teams consisting of those skilled in problem-solving with superior IQs. The teams that function most successfully are those that create a culture of trust and helpfulness no matter the individual talents of those on the team.
As a department chair, I try to see past the thickness of the CV to ensure that I see into the person’s ability to relate with other people. If the CV is thick and the emotional intelligence high, then that is the person I want on my team, but I would rather have the latter than the former, and so would teammates and patients.
Research has shown repeatedly that no individuals can hope to achieve on their own what a good, functional team can achieve as a group. Yet, the academic hierarchy rewards individuals, not teams. Hopefully, the rewarded individual will recognize the support and effort of the team, but that is not always the case and not always done adequately.
How can academic departments develop social capital in their teams and reward them, in addition to individuals, for their successes? Social capital grows when the relationships between people become stronger. Those bonds develop though informal interaction outside the work environment. The more the team knows each other, the more they trust each other, the more they are willing to help each other, and the more they are likely to be civil to one another. Teams of friends are much more likely to solve problems quickly for less cost than teams of strangers.
Reward and recognition for a team is not as easy as it sounds. Everyone wants individual recognition for their work, not just as an integral member of a team. The world loves All-Stars and MVPs as much as they love their teams. We do not need to change the way we reward individuals, but we do need to also include teams for reward and recognition. How can a leader determine which teams are most deserving of the highest reward? Many institutions measure employee engagement through surveys. High-functioning teams are likely to be highly engaged and high scores on surveys should be recognized and rewarded. Teams with low employee turnover help reduce training costs and should be appreciated.
Successful implementation of continuous improvement projects are an often overlooked opportunity for an expression of gratitude. I am sure there are other ways to acknowledge a team’s efforts and I hope readers will write to us with their ideas for all to share. Perhaps by doing so, we can accelerate the cultural transformation of academic medicine from one of personal achievement to one of team success.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
As physicians, we know, and have been taught, that success comes from being smart and answering questions correctly. By doing so, we excelled in school, scored high on standardized tests, and confidently raised our hands in class. As a result, we were chosen for plum assignments like class officer, yearbook editor, and team captain. Success bred success and we were initiated into honor societies, accepted into prestigious internships, and allowed to work on important research projects.
These achievements guaranteed further success in medical school where the tradition of competition continued as we positioned for the attention of those who would someday write our letters of recommendation. This model of individual success served us very well until we joined a hematology group to begin our practice.
When we join a group of physicians, we join a team. The team might organize around diagnoses, laboratories, or geographic location, but it is a team nonetheless. The team, of course, includes more than just physicians. As a team member, we are expected to fulfill a role that advances the mission of the team and the larger organization. That mission may include some combination of academic, educational, and clinical productivity.
As physicians trained to compete with other individuals, teamwork does not come naturally. We might think that so long as the team consists of more and mor
As Margaret Heffernan brilliantly points out in “Margaret Heffernan: Why it’s time to forget the pecking order at work,” a TED talk video posted on YouTube, teams consisting of those skilled in interpersonal relationships are much more effective, efficient, and productive than are teams consisting of those skilled in problem-solving with superior IQs. The teams that function most successfully are those that create a culture of trust and helpfulness no matter the individual talents of those on the team.
As a department chair, I try to see past the thickness of the CV to ensure that I see into the person’s ability to relate with other people. If the CV is thick and the emotional intelligence high, then that is the person I want on my team, but I would rather have the latter than the former, and so would teammates and patients.
Research has shown repeatedly that no individuals can hope to achieve on their own what a good, functional team can achieve as a group. Yet, the academic hierarchy rewards individuals, not teams. Hopefully, the rewarded individual will recognize the support and effort of the team, but that is not always the case and not always done adequately.
How can academic departments develop social capital in their teams and reward them, in addition to individuals, for their successes? Social capital grows when the relationships between people become stronger. Those bonds develop though informal interaction outside the work environment. The more the team knows each other, the more they trust each other, the more they are willing to help each other, and the more they are likely to be civil to one another. Teams of friends are much more likely to solve problems quickly for less cost than teams of strangers.
Reward and recognition for a team is not as easy as it sounds. Everyone wants individual recognition for their work, not just as an integral member of a team. The world loves All-Stars and MVPs as much as they love their teams. We do not need to change the way we reward individuals, but we do need to also include teams for reward and recognition. How can a leader determine which teams are most deserving of the highest reward? Many institutions measure employee engagement through surveys. High-functioning teams are likely to be highly engaged and high scores on surveys should be recognized and rewarded. Teams with low employee turnover help reduce training costs and should be appreciated.
Successful implementation of continuous improvement projects are an often overlooked opportunity for an expression of gratitude. I am sure there are other ways to acknowledge a team’s efforts and I hope readers will write to us with their ideas for all to share. Perhaps by doing so, we can accelerate the cultural transformation of academic medicine from one of personal achievement to one of team success.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
As physicians, we know, and have been taught, that success comes from being smart and answering questions correctly. By doing so, we excelled in school, scored high on standardized tests, and confidently raised our hands in class. As a result, we were chosen for plum assignments like class officer, yearbook editor, and team captain. Success bred success and we were initiated into honor societies, accepted into prestigious internships, and allowed to work on important research projects.
These achievements guaranteed further success in medical school where the tradition of competition continued as we positioned for the attention of those who would someday write our letters of recommendation. This model of individual success served us very well until we joined a hematology group to begin our practice.
When we join a group of physicians, we join a team. The team might organize around diagnoses, laboratories, or geographic location, but it is a team nonetheless. The team, of course, includes more than just physicians. As a team member, we are expected to fulfill a role that advances the mission of the team and the larger organization. That mission may include some combination of academic, educational, and clinical productivity.
As physicians trained to compete with other individuals, teamwork does not come naturally. We might think that so long as the team consists of more and mor
As Margaret Heffernan brilliantly points out in “Margaret Heffernan: Why it’s time to forget the pecking order at work,” a TED talk video posted on YouTube, teams consisting of those skilled in interpersonal relationships are much more effective, efficient, and productive than are teams consisting of those skilled in problem-solving with superior IQs. The teams that function most successfully are those that create a culture of trust and helpfulness no matter the individual talents of those on the team.
As a department chair, I try to see past the thickness of the CV to ensure that I see into the person’s ability to relate with other people. If the CV is thick and the emotional intelligence high, then that is the person I want on my team, but I would rather have the latter than the former, and so would teammates and patients.
Research has shown repeatedly that no individuals can hope to achieve on their own what a good, functional team can achieve as a group. Yet, the academic hierarchy rewards individuals, not teams. Hopefully, the rewarded individual will recognize the support and effort of the team, but that is not always the case and not always done adequately.
How can academic departments develop social capital in their teams and reward them, in addition to individuals, for their successes? Social capital grows when the relationships between people become stronger. Those bonds develop though informal interaction outside the work environment. The more the team knows each other, the more they trust each other, the more they are willing to help each other, and the more they are likely to be civil to one another. Teams of friends are much more likely to solve problems quickly for less cost than teams of strangers.
Reward and recognition for a team is not as easy as it sounds. Everyone wants individual recognition for their work, not just as an integral member of a team. The world loves All-Stars and MVPs as much as they love their teams. We do not need to change the way we reward individuals, but we do need to also include teams for reward and recognition. How can a leader determine which teams are most deserving of the highest reward? Many institutions measure employee engagement through surveys. High-functioning teams are likely to be highly engaged and high scores on surveys should be recognized and rewarded. Teams with low employee turnover help reduce training costs and should be appreciated.
Successful implementation of continuous improvement projects are an often overlooked opportunity for an expression of gratitude. I am sure there are other ways to acknowledge a team’s efforts and I hope readers will write to us with their ideas for all to share. Perhaps by doing so, we can accelerate the cultural transformation of academic medicine from one of personal achievement to one of team success.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
Spinal Cord Stimulation May Improve Gait in Patients With Advanced Parkinson’s Disease
VANCOUVER—Spinal cord stimulation improves gait in patients with advanced Parkinson’s disease, according to a pilot study described at the 21st International Congress of Parkinson’s Disease and Movement Disorders. The technique allows neurologists to determine which stimulation parameters provide the greatest benefit for each patient, said Mandar Jog, MD, Professor of Neurology and Engineering and Director of the Movement Disorders Center at Western University in London, Ontario.
“Each participant demonstrated unique gait c
Treatment-Resistant Symptoms
Gait dysfunction and freezing of gait are common in advanced Parkinson’s disease, and gait symptoms are largely resistant to dopamine replacement therapy. Studies have suggested that epidural spinal cord stimulation, which is an approved therapy for pain, may help treat levodopa-resistant motor symptoms in Parkinson’s disease.
Dr. Jog and colleagues analyzed objective gait measurements to understand which spinal cord stimulation settings most improved gait. They tested combinations of pulse widths and frequencies that induced paresthesia in patients’ lower limbs.
The six-month study included five men with Parkinson’s disease who had significant gait disturbances on levodopa therapy. The patients were not eligible for deep brain stimulation. Mean age was 71, and mean disease duration was 14 years. The patients underwent mid-thoracic spinal cord stimulation surgery, and a dorsal spinal cord stimulator was implanted in the epidural space (T7–10). The researchers evaluated patients before the operation and at weeks 2, 4, 6, 8, 10, 12, 16, and 24 after surgery.
The investigators conducted randomized testing of different spinal cord stimulation settings at weeks 2, 4, 6, 8, 10, 12, and 16. At week 24, researchers evaluated patients when they had been off stimulation for 48 hours and then with stimulation.
Patients completed walking tasks on a 20-foot walkway with sensors that relayed each footfall to a computer with gait analysis software. The researchers grouped gait variables according to whether they were expected to increase with improvement (eg, step length and stride velocity) or decrease with improvement (eg, stride width and step time).
Determining Optimal Settings
For two of the patients, the most effective spinal cord stimulation setting was a pulse width of 400 µs and a frequency of 60 Hz. The optimal settings for the remaining three patients were 300 µs and 30 Hz, 300 µs and 130 Hz, and 300 or 400 µs and 130 Hz.
Key gait variables improved with spinal cord stimulation. For instance, stride velocity significantly improved by 54% with the optimal settings, compared with baseline. Mean number of freezing of gait episodes significantly decreased. Unified Parkinson’s Disease Rating Scale motor scores and patients’ confidence in performing activities of daily living also significantly improved, compared with baseline.
Patients achieved a greater degree of independence after receiving spinal cord stimulation, Dr. Jog said. For example, one patient no longer had to rely on a wheelchair or scooter to get around, and one patient no longer needed help getting out of a chair. One patient was able to resume working in fields, and one patient was able to walk on a beach. “Everybody improved to different levels. The improvement occurred immediately in the laboratory,” he said.
No adverse events were reported. The researchers plan to conduct further studies. Because spinal cord stimulation already is available for the treatment of pain, it may be feasible to pair a spinal cord stimulation device with a sensor system that allows physicians to optimize the stimulator’s settings for the treatment of gait dysfunction in Parkinson’s disease, Dr. Jog said.
—Jake Remaly
Suggested Reading
de Andrade EM, Ghilardi MG, Cury RG, et al. Spinal cord stimulation for Parkinson’s disease: a systematic review. Neurosurg Rev. 2016;39(1):27-35.
Pinto de Souza C, Hamani C, Oliveira Souza C, et al. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov Disord. 2017;32(2):278-282.
VANCOUVER—Spinal cord stimulation improves gait in patients with advanced Parkinson’s disease, according to a pilot study described at the 21st International Congress of Parkinson’s Disease and Movement Disorders. The technique allows neurologists to determine which stimulation parameters provide the greatest benefit for each patient, said Mandar Jog, MD, Professor of Neurology and Engineering and Director of the Movement Disorders Center at Western University in London, Ontario.
“Each participant demonstrated unique gait c
Treatment-Resistant Symptoms
Gait dysfunction and freezing of gait are common in advanced Parkinson’s disease, and gait symptoms are largely resistant to dopamine replacement therapy. Studies have suggested that epidural spinal cord stimulation, which is an approved therapy for pain, may help treat levodopa-resistant motor symptoms in Parkinson’s disease.
Dr. Jog and colleagues analyzed objective gait measurements to understand which spinal cord stimulation settings most improved gait. They tested combinations of pulse widths and frequencies that induced paresthesia in patients’ lower limbs.
The six-month study included five men with Parkinson’s disease who had significant gait disturbances on levodopa therapy. The patients were not eligible for deep brain stimulation. Mean age was 71, and mean disease duration was 14 years. The patients underwent mid-thoracic spinal cord stimulation surgery, and a dorsal spinal cord stimulator was implanted in the epidural space (T7–10). The researchers evaluated patients before the operation and at weeks 2, 4, 6, 8, 10, 12, 16, and 24 after surgery.
The investigators conducted randomized testing of different spinal cord stimulation settings at weeks 2, 4, 6, 8, 10, 12, and 16. At week 24, researchers evaluated patients when they had been off stimulation for 48 hours and then with stimulation.
Patients completed walking tasks on a 20-foot walkway with sensors that relayed each footfall to a computer with gait analysis software. The researchers grouped gait variables according to whether they were expected to increase with improvement (eg, step length and stride velocity) or decrease with improvement (eg, stride width and step time).
Determining Optimal Settings
For two of the patients, the most effective spinal cord stimulation setting was a pulse width of 400 µs and a frequency of 60 Hz. The optimal settings for the remaining three patients were 300 µs and 30 Hz, 300 µs and 130 Hz, and 300 or 400 µs and 130 Hz.
Key gait variables improved with spinal cord stimulation. For instance, stride velocity significantly improved by 54% with the optimal settings, compared with baseline. Mean number of freezing of gait episodes significantly decreased. Unified Parkinson’s Disease Rating Scale motor scores and patients’ confidence in performing activities of daily living also significantly improved, compared with baseline.
Patients achieved a greater degree of independence after receiving spinal cord stimulation, Dr. Jog said. For example, one patient no longer had to rely on a wheelchair or scooter to get around, and one patient no longer needed help getting out of a chair. One patient was able to resume working in fields, and one patient was able to walk on a beach. “Everybody improved to different levels. The improvement occurred immediately in the laboratory,” he said.
No adverse events were reported. The researchers plan to conduct further studies. Because spinal cord stimulation already is available for the treatment of pain, it may be feasible to pair a spinal cord stimulation device with a sensor system that allows physicians to optimize the stimulator’s settings for the treatment of gait dysfunction in Parkinson’s disease, Dr. Jog said.
—Jake Remaly
Suggested Reading
de Andrade EM, Ghilardi MG, Cury RG, et al. Spinal cord stimulation for Parkinson’s disease: a systematic review. Neurosurg Rev. 2016;39(1):27-35.
Pinto de Souza C, Hamani C, Oliveira Souza C, et al. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov Disord. 2017;32(2):278-282.
VANCOUVER—Spinal cord stimulation improves gait in patients with advanced Parkinson’s disease, according to a pilot study described at the 21st International Congress of Parkinson’s Disease and Movement Disorders. The technique allows neurologists to determine which stimulation parameters provide the greatest benefit for each patient, said Mandar Jog, MD, Professor of Neurology and Engineering and Director of the Movement Disorders Center at Western University in London, Ontario.
“Each participant demonstrated unique gait c
Treatment-Resistant Symptoms
Gait dysfunction and freezing of gait are common in advanced Parkinson’s disease, and gait symptoms are largely resistant to dopamine replacement therapy. Studies have suggested that epidural spinal cord stimulation, which is an approved therapy for pain, may help treat levodopa-resistant motor symptoms in Parkinson’s disease.
Dr. Jog and colleagues analyzed objective gait measurements to understand which spinal cord stimulation settings most improved gait. They tested combinations of pulse widths and frequencies that induced paresthesia in patients’ lower limbs.
The six-month study included five men with Parkinson’s disease who had significant gait disturbances on levodopa therapy. The patients were not eligible for deep brain stimulation. Mean age was 71, and mean disease duration was 14 years. The patients underwent mid-thoracic spinal cord stimulation surgery, and a dorsal spinal cord stimulator was implanted in the epidural space (T7–10). The researchers evaluated patients before the operation and at weeks 2, 4, 6, 8, 10, 12, 16, and 24 after surgery.
The investigators conducted randomized testing of different spinal cord stimulation settings at weeks 2, 4, 6, 8, 10, 12, and 16. At week 24, researchers evaluated patients when they had been off stimulation for 48 hours and then with stimulation.
Patients completed walking tasks on a 20-foot walkway with sensors that relayed each footfall to a computer with gait analysis software. The researchers grouped gait variables according to whether they were expected to increase with improvement (eg, step length and stride velocity) or decrease with improvement (eg, stride width and step time).
Determining Optimal Settings
For two of the patients, the most effective spinal cord stimulation setting was a pulse width of 400 µs and a frequency of 60 Hz. The optimal settings for the remaining three patients were 300 µs and 30 Hz, 300 µs and 130 Hz, and 300 or 400 µs and 130 Hz.
Key gait variables improved with spinal cord stimulation. For instance, stride velocity significantly improved by 54% with the optimal settings, compared with baseline. Mean number of freezing of gait episodes significantly decreased. Unified Parkinson’s Disease Rating Scale motor scores and patients’ confidence in performing activities of daily living also significantly improved, compared with baseline.
Patients achieved a greater degree of independence after receiving spinal cord stimulation, Dr. Jog said. For example, one patient no longer had to rely on a wheelchair or scooter to get around, and one patient no longer needed help getting out of a chair. One patient was able to resume working in fields, and one patient was able to walk on a beach. “Everybody improved to different levels. The improvement occurred immediately in the laboratory,” he said.
No adverse events were reported. The researchers plan to conduct further studies. Because spinal cord stimulation already is available for the treatment of pain, it may be feasible to pair a spinal cord stimulation device with a sensor system that allows physicians to optimize the stimulator’s settings for the treatment of gait dysfunction in Parkinson’s disease, Dr. Jog said.
—Jake Remaly
Suggested Reading
de Andrade EM, Ghilardi MG, Cury RG, et al. Spinal cord stimulation for Parkinson’s disease: a systematic review. Neurosurg Rev. 2016;39(1):27-35.
Pinto de Souza C, Hamani C, Oliveira Souza C, et al. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov Disord. 2017;32(2):278-282.
The impact of Election 2016
Because of the health care policy work I have done over the years, I often get asked about what to expect from Capitol Hill and from federal policy makers in D.C. Since the surprise election results in November, the most common questions revolve around what impact the Trump administration is likely to have on the delivery system reform work done since the passage of the Affordable Care Act (ACA).
While much uncertainty remains, events since the election have given us some clues to answer these and other questions.
Let’s address the ACA. It’s important to recognize that the ACA cannot be repealed completely for at least two reasons. First, it does not even exist as it was passed, having undergone several changes, including adjustments and exemptions. Second, parts of the bill would require 60 votes in the Senate to repeal, and those votes are not available to the party seeking repeal.
Yes, parts of the bill could be changed significantly with only Republican votes. However, the reality is that many changes would have occurred even if Hillary Clinton had won the election; there are elements of the current law that are not working and that both sides acknowledge need to be fixed, such as state individual insurance exchanges.
There also are parts of the ACA that neither party would like to see rescinded, which are unlikely to be removed in a new law – for example, losing insurance for preexisting conditions.
From the standpoint of providers, the most notable aspect of the current discussion is that proposed changes have largely been limited to addressing areas of insurance reform. This has potential impact on who is covered under a revised plan. In the meantime, the important work of delivery system reform – the elements of the ACA that providers care the most about (and that will have the most impact on their careers) – have been left untouched. There are strong signs that this will remain the case and that this important work will continue.
What are those signs? First of all, neither the “repeal” bill passed by the House nor any of the bills considered by the Senate made any mention of interrupting any of the important work being done by the Center for Medicare & Medicaid Innovation (CMMI), the part of the Centers for Medicare & Medicaid Services created by the ACA to develop and test alternative payment models (APMs), like accountable care organizations, bundled payments, etc. If successful, this work will improve quality while lowering the growth of health care costs and may save a health care system that, if unchecked, will create a crushing financial burden that threatens the Medicare Trust Fund. It also is a strong and clear sign that the CMMI continues its work today under the same effective leadership that first created excitement about its potential to improve the delivery system.
But probably the clearest sign that delivery system reform will continue was the strong bipartisan support shown in the passage of the Medicare Access and CHIP Reauthorization Act (MACRA) in April of 2015. This landmark piece of legislation creates a pathway that moves the entire health care system away from fee for service and toward payment models that will reward providers for innovations that will lower the cost of care, eliminate waste, improve safety, and achieve better outcomes. It puts in place a plan that will use APMs to offer providers the incentives to create care models that may be the salvation of our health care system. In the long run, isn’t this what matters the most?
Politicians in Washington can’t save our system. They can create or remove entitlements or support one segment of the population at the expense of another. But, in the end, they are only moving dollars around from one pocket to another, rearranging deck chairs on the Titanic of the American health care system.
The reality is that the only thing that can save our health care system is to lower the cost of care. And we all know that, as providers, only we can do that. SHM will be helping its members lead the way, providing educational content, training, advocacy, and policy leadership.
It will be up to the nation’s caregivers to reform the delivery system in a way that is sustainable for our generation and generations to come. We continue that work today, and I see no evidence that anyone on Capitol Hill wants us to stop.
Dr. Greeno is president of the Society of Hospital Medicine and senior adviser for medical affairs at TeamHealth.
Because of the health care policy work I have done over the years, I often get asked about what to expect from Capitol Hill and from federal policy makers in D.C. Since the surprise election results in November, the most common questions revolve around what impact the Trump administration is likely to have on the delivery system reform work done since the passage of the Affordable Care Act (ACA).
While much uncertainty remains, events since the election have given us some clues to answer these and other questions.
Let’s address the ACA. It’s important to recognize that the ACA cannot be repealed completely for at least two reasons. First, it does not even exist as it was passed, having undergone several changes, including adjustments and exemptions. Second, parts of the bill would require 60 votes in the Senate to repeal, and those votes are not available to the party seeking repeal.
Yes, parts of the bill could be changed significantly with only Republican votes. However, the reality is that many changes would have occurred even if Hillary Clinton had won the election; there are elements of the current law that are not working and that both sides acknowledge need to be fixed, such as state individual insurance exchanges.
There also are parts of the ACA that neither party would like to see rescinded, which are unlikely to be removed in a new law – for example, losing insurance for preexisting conditions.
From the standpoint of providers, the most notable aspect of the current discussion is that proposed changes have largely been limited to addressing areas of insurance reform. This has potential impact on who is covered under a revised plan. In the meantime, the important work of delivery system reform – the elements of the ACA that providers care the most about (and that will have the most impact on their careers) – have been left untouched. There are strong signs that this will remain the case and that this important work will continue.
What are those signs? First of all, neither the “repeal” bill passed by the House nor any of the bills considered by the Senate made any mention of interrupting any of the important work being done by the Center for Medicare & Medicaid Innovation (CMMI), the part of the Centers for Medicare & Medicaid Services created by the ACA to develop and test alternative payment models (APMs), like accountable care organizations, bundled payments, etc. If successful, this work will improve quality while lowering the growth of health care costs and may save a health care system that, if unchecked, will create a crushing financial burden that threatens the Medicare Trust Fund. It also is a strong and clear sign that the CMMI continues its work today under the same effective leadership that first created excitement about its potential to improve the delivery system.
But probably the clearest sign that delivery system reform will continue was the strong bipartisan support shown in the passage of the Medicare Access and CHIP Reauthorization Act (MACRA) in April of 2015. This landmark piece of legislation creates a pathway that moves the entire health care system away from fee for service and toward payment models that will reward providers for innovations that will lower the cost of care, eliminate waste, improve safety, and achieve better outcomes. It puts in place a plan that will use APMs to offer providers the incentives to create care models that may be the salvation of our health care system. In the long run, isn’t this what matters the most?
Politicians in Washington can’t save our system. They can create or remove entitlements or support one segment of the population at the expense of another. But, in the end, they are only moving dollars around from one pocket to another, rearranging deck chairs on the Titanic of the American health care system.
The reality is that the only thing that can save our health care system is to lower the cost of care. And we all know that, as providers, only we can do that. SHM will be helping its members lead the way, providing educational content, training, advocacy, and policy leadership.
It will be up to the nation’s caregivers to reform the delivery system in a way that is sustainable for our generation and generations to come. We continue that work today, and I see no evidence that anyone on Capitol Hill wants us to stop.
Dr. Greeno is president of the Society of Hospital Medicine and senior adviser for medical affairs at TeamHealth.
Because of the health care policy work I have done over the years, I often get asked about what to expect from Capitol Hill and from federal policy makers in D.C. Since the surprise election results in November, the most common questions revolve around what impact the Trump administration is likely to have on the delivery system reform work done since the passage of the Affordable Care Act (ACA).
While much uncertainty remains, events since the election have given us some clues to answer these and other questions.
Let’s address the ACA. It’s important to recognize that the ACA cannot be repealed completely for at least two reasons. First, it does not even exist as it was passed, having undergone several changes, including adjustments and exemptions. Second, parts of the bill would require 60 votes in the Senate to repeal, and those votes are not available to the party seeking repeal.
Yes, parts of the bill could be changed significantly with only Republican votes. However, the reality is that many changes would have occurred even if Hillary Clinton had won the election; there are elements of the current law that are not working and that both sides acknowledge need to be fixed, such as state individual insurance exchanges.
There also are parts of the ACA that neither party would like to see rescinded, which are unlikely to be removed in a new law – for example, losing insurance for preexisting conditions.
From the standpoint of providers, the most notable aspect of the current discussion is that proposed changes have largely been limited to addressing areas of insurance reform. This has potential impact on who is covered under a revised plan. In the meantime, the important work of delivery system reform – the elements of the ACA that providers care the most about (and that will have the most impact on their careers) – have been left untouched. There are strong signs that this will remain the case and that this important work will continue.
What are those signs? First of all, neither the “repeal” bill passed by the House nor any of the bills considered by the Senate made any mention of interrupting any of the important work being done by the Center for Medicare & Medicaid Innovation (CMMI), the part of the Centers for Medicare & Medicaid Services created by the ACA to develop and test alternative payment models (APMs), like accountable care organizations, bundled payments, etc. If successful, this work will improve quality while lowering the growth of health care costs and may save a health care system that, if unchecked, will create a crushing financial burden that threatens the Medicare Trust Fund. It also is a strong and clear sign that the CMMI continues its work today under the same effective leadership that first created excitement about its potential to improve the delivery system.
But probably the clearest sign that delivery system reform will continue was the strong bipartisan support shown in the passage of the Medicare Access and CHIP Reauthorization Act (MACRA) in April of 2015. This landmark piece of legislation creates a pathway that moves the entire health care system away from fee for service and toward payment models that will reward providers for innovations that will lower the cost of care, eliminate waste, improve safety, and achieve better outcomes. It puts in place a plan that will use APMs to offer providers the incentives to create care models that may be the salvation of our health care system. In the long run, isn’t this what matters the most?
Politicians in Washington can’t save our system. They can create or remove entitlements or support one segment of the population at the expense of another. But, in the end, they are only moving dollars around from one pocket to another, rearranging deck chairs on the Titanic of the American health care system.
The reality is that the only thing that can save our health care system is to lower the cost of care. And we all know that, as providers, only we can do that. SHM will be helping its members lead the way, providing educational content, training, advocacy, and policy leadership.
It will be up to the nation’s caregivers to reform the delivery system in a way that is sustainable for our generation and generations to come. We continue that work today, and I see no evidence that anyone on Capitol Hill wants us to stop.
Dr. Greeno is president of the Society of Hospital Medicine and senior adviser for medical affairs at TeamHealth.
VIDEO: How to discharge new pediatric diabetes cases in 2 days
NASHVILLE, TENN. – Pediatric endocrinologist Cassandra Brady, MD, caught the attention of her audience at Pediatric Hospital Medicine when she mentioned that children presenting with new-onset diabetes rarely spend more than 2 days at Vanderbilt University’s children’s hospital, even if they present in diabetic ketoacidosis.
In many places, children with new-onset diabetes spend quite a bit longer in the hospital – even if they are medically stable and feeling fine – for diabetes education.
That’s not the case at Vanderbilt, where Dr. Brady is an assistant professor. Once kids are stabilized, they and their parents undergo a 3-hour crash course – sometimes even in the PICU – on diabetes survival skills, and then they’re sent home with insulin. They learn the finer points about carbohydrate counting and tight glucose control at subsequent outpatient visits.
More and more payers are probably going to push for that model, Dr. Brady noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
For those interested in making the transition to outpatient eduction, she explained in an interview exactly how Vanderbilt’s been doing it safely for years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Pediatric endocrinologist Cassandra Brady, MD, caught the attention of her audience at Pediatric Hospital Medicine when she mentioned that children presenting with new-onset diabetes rarely spend more than 2 days at Vanderbilt University’s children’s hospital, even if they present in diabetic ketoacidosis.
In many places, children with new-onset diabetes spend quite a bit longer in the hospital – even if they are medically stable and feeling fine – for diabetes education.
That’s not the case at Vanderbilt, where Dr. Brady is an assistant professor. Once kids are stabilized, they and their parents undergo a 3-hour crash course – sometimes even in the PICU – on diabetes survival skills, and then they’re sent home with insulin. They learn the finer points about carbohydrate counting and tight glucose control at subsequent outpatient visits.
More and more payers are probably going to push for that model, Dr. Brady noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
For those interested in making the transition to outpatient eduction, she explained in an interview exactly how Vanderbilt’s been doing it safely for years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Pediatric endocrinologist Cassandra Brady, MD, caught the attention of her audience at Pediatric Hospital Medicine when she mentioned that children presenting with new-onset diabetes rarely spend more than 2 days at Vanderbilt University’s children’s hospital, even if they present in diabetic ketoacidosis.
In many places, children with new-onset diabetes spend quite a bit longer in the hospital – even if they are medically stable and feeling fine – for diabetes education.
That’s not the case at Vanderbilt, where Dr. Brady is an assistant professor. Once kids are stabilized, they and their parents undergo a 3-hour crash course – sometimes even in the PICU – on diabetes survival skills, and then they’re sent home with insulin. They learn the finer points about carbohydrate counting and tight glucose control at subsequent outpatient visits.
More and more payers are probably going to push for that model, Dr. Brady noted at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
For those interested in making the transition to outpatient eduction, she explained in an interview exactly how Vanderbilt’s been doing it safely for years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT PHM 2017
Treating RLS With Dopamine Agonists May Increase Risk for New-Onset Mental Disorders
BOSTON—Among patients with primary restless legs syndrome (RLS) and without a history of psychiatric disorders, patients who receive de novo dopamine agonist treatment are approximately twice as likely to subsequently develop a mental disorder as those who do not receive dopamine agonist treatment, according to a large-scale retrospective study presented at the 31st Annual Meeting of the Associated Professional Sleep Societies.
Previous research has demonstrated an increased risk of mental disorders among patients with Parkinson’s disease who are treated with dopamine agonists. Many patients with RLS are also treated with dopamine agonists, although at lower doses than patients with Parkinson’s disease. Given these lower doses, clinicians assumed that the risk of dopamine agonist-induced mental disorders in RLS would be small. Clinical case studies suggest a higher-than-anticipated risk, however.
An Examination of Claims Data
To investigate whether dopamine agonists increase the risk of developing mental disorders in patients with RLS, Cheryl Hankin, PhD, President and Chief Scientific Officer of BioMedEcon, a health economics and outcomes research firm in Moss Beach, California, and colleagues examined Truven MarketScan Commercial and Medicare Supplemental databases of claims filed between July 1, 2008, and December 31, 2014. From a pool of 539,399 patients with a diagnosis of RLS, investigators identified adults with two or more years of claims data preceding and following their index RLS diagnosis dates.
Patients were excluded from the analysis if, in the two or more years preceding RLS diagnosis, they received a diagnosis of mental disorder or filled a prescription for an antidepressant or antipsychotic. Also excluded were patients who filled a prescription for a dopamine agonist in the two or more years preceding RLS diagnosis. Patients who were ever diagnosed with Parkinson’s disease, kidney disease, iron deficiency, or pregnancy were assumed to have secondary RLS and were also excluded.
The investigators identified 5,419 eligible participants. Of this group, 1,649 patients received dopamine agonists after RLS diagnosis. Specifically, 571 participants received pramipexole, 915 received ropinirole, and 163 received both. Approximately 65% of patients were female. Patients residing in the Northeast were significantly less likely to receive dopamine agonists, compared with patients residing in the Midwest or the South. The investigators found no significant differences in treatment status by comorbid illness burden or by sex. The investigators also found no significant differences in demographic characteristics between patients receiving pramipexole and those receiving ropinirole.
Risk Was Significantly Greater in Patients Receiving Dopamine Agonist
Next, from this pool of eligible subjects, the researchers matched 1,080 patients treated with dopamine agonists with 1,080 dopamine agonist-naïve patients on sex, age at RLS diagnosis, region, employment, and illness burden. Dr. Hankin and colleagues found a significant increase in mental disorder diagnoses (eg, bipolar disorder, anxiety, depression, and substance abuse) among patients treated with dopamine agonists, compared with dopamine agonist-naïve patients. Among patients receiving dopamine agonists, the odds ratio for severe mental disorder (eg, psychoses and bipolar disorder) was 2.2, the odds ratio for moderate to severe mental disorder (eg, posttraumatic stress disorder and major depression) was 1.8, and the odds ratio for mild mental disorder (eg, anxiety disorders) was 1.9, compared with dopamine agonist-naïve patients.
“This is the first large-scale, real-world, claims-based study to examine the association between treatment of RLS with dopamine agonists and the development of psychiatric adverse events. Our findings are compelling, but need to be replicated in other patient populations,” said Dr. Hankin.
“Our retrospective analysis required careful consideration of matching,” said Daniel On-Fai Lee, MD, Clinical Professor of Neurology at the University of Kentucky College of Medicine in Lexington, who collaborated on the study. Although the investigators took care to match participants and to remove cases of secondary RLS from the analysis, they may have inadvertently overlooked one or more important matching variables that could affect the outcome, he added.
Arbor Pharmaceuticals provided funding for the study, but did not influence its methodology, analysis, results, or conclusion, said Dr. Lee.
—Erik Greb
Suggested Reading
Sierra M, Carnicella S, Strafella AP, et al. Apathy and impulse control disorders: yin & yang of dopamine dependent behaviors. J Parkinsons Dis. 2015;5(3):625-636.
Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173(7):496-505.
BOSTON—Among patients with primary restless legs syndrome (RLS) and without a history of psychiatric disorders, patients who receive de novo dopamine agonist treatment are approximately twice as likely to subsequently develop a mental disorder as those who do not receive dopamine agonist treatment, according to a large-scale retrospective study presented at the 31st Annual Meeting of the Associated Professional Sleep Societies.
Previous research has demonstrated an increased risk of mental disorders among patients with Parkinson’s disease who are treated with dopamine agonists. Many patients with RLS are also treated with dopamine agonists, although at lower doses than patients with Parkinson’s disease. Given these lower doses, clinicians assumed that the risk of dopamine agonist-induced mental disorders in RLS would be small. Clinical case studies suggest a higher-than-anticipated risk, however.
An Examination of Claims Data
To investigate whether dopamine agonists increase the risk of developing mental disorders in patients with RLS, Cheryl Hankin, PhD, President and Chief Scientific Officer of BioMedEcon, a health economics and outcomes research firm in Moss Beach, California, and colleagues examined Truven MarketScan Commercial and Medicare Supplemental databases of claims filed between July 1, 2008, and December 31, 2014. From a pool of 539,399 patients with a diagnosis of RLS, investigators identified adults with two or more years of claims data preceding and following their index RLS diagnosis dates.
Patients were excluded from the analysis if, in the two or more years preceding RLS diagnosis, they received a diagnosis of mental disorder or filled a prescription for an antidepressant or antipsychotic. Also excluded were patients who filled a prescription for a dopamine agonist in the two or more years preceding RLS diagnosis. Patients who were ever diagnosed with Parkinson’s disease, kidney disease, iron deficiency, or pregnancy were assumed to have secondary RLS and were also excluded.
The investigators identified 5,419 eligible participants. Of this group, 1,649 patients received dopamine agonists after RLS diagnosis. Specifically, 571 participants received pramipexole, 915 received ropinirole, and 163 received both. Approximately 65% of patients were female. Patients residing in the Northeast were significantly less likely to receive dopamine agonists, compared with patients residing in the Midwest or the South. The investigators found no significant differences in treatment status by comorbid illness burden or by sex. The investigators also found no significant differences in demographic characteristics between patients receiving pramipexole and those receiving ropinirole.
Risk Was Significantly Greater in Patients Receiving Dopamine Agonist
Next, from this pool of eligible subjects, the researchers matched 1,080 patients treated with dopamine agonists with 1,080 dopamine agonist-naïve patients on sex, age at RLS diagnosis, region, employment, and illness burden. Dr. Hankin and colleagues found a significant increase in mental disorder diagnoses (eg, bipolar disorder, anxiety, depression, and substance abuse) among patients treated with dopamine agonists, compared with dopamine agonist-naïve patients. Among patients receiving dopamine agonists, the odds ratio for severe mental disorder (eg, psychoses and bipolar disorder) was 2.2, the odds ratio for moderate to severe mental disorder (eg, posttraumatic stress disorder and major depression) was 1.8, and the odds ratio for mild mental disorder (eg, anxiety disorders) was 1.9, compared with dopamine agonist-naïve patients.
“This is the first large-scale, real-world, claims-based study to examine the association between treatment of RLS with dopamine agonists and the development of psychiatric adverse events. Our findings are compelling, but need to be replicated in other patient populations,” said Dr. Hankin.
“Our retrospective analysis required careful consideration of matching,” said Daniel On-Fai Lee, MD, Clinical Professor of Neurology at the University of Kentucky College of Medicine in Lexington, who collaborated on the study. Although the investigators took care to match participants and to remove cases of secondary RLS from the analysis, they may have inadvertently overlooked one or more important matching variables that could affect the outcome, he added.
Arbor Pharmaceuticals provided funding for the study, but did not influence its methodology, analysis, results, or conclusion, said Dr. Lee.
—Erik Greb
Suggested Reading
Sierra M, Carnicella S, Strafella AP, et al. Apathy and impulse control disorders: yin & yang of dopamine dependent behaviors. J Parkinsons Dis. 2015;5(3):625-636.
Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173(7):496-505.
BOSTON—Among patients with primary restless legs syndrome (RLS) and without a history of psychiatric disorders, patients who receive de novo dopamine agonist treatment are approximately twice as likely to subsequently develop a mental disorder as those who do not receive dopamine agonist treatment, according to a large-scale retrospective study presented at the 31st Annual Meeting of the Associated Professional Sleep Societies.
Previous research has demonstrated an increased risk of mental disorders among patients with Parkinson’s disease who are treated with dopamine agonists. Many patients with RLS are also treated with dopamine agonists, although at lower doses than patients with Parkinson’s disease. Given these lower doses, clinicians assumed that the risk of dopamine agonist-induced mental disorders in RLS would be small. Clinical case studies suggest a higher-than-anticipated risk, however.
An Examination of Claims Data
To investigate whether dopamine agonists increase the risk of developing mental disorders in patients with RLS, Cheryl Hankin, PhD, President and Chief Scientific Officer of BioMedEcon, a health economics and outcomes research firm in Moss Beach, California, and colleagues examined Truven MarketScan Commercial and Medicare Supplemental databases of claims filed between July 1, 2008, and December 31, 2014. From a pool of 539,399 patients with a diagnosis of RLS, investigators identified adults with two or more years of claims data preceding and following their index RLS diagnosis dates.
Patients were excluded from the analysis if, in the two or more years preceding RLS diagnosis, they received a diagnosis of mental disorder or filled a prescription for an antidepressant or antipsychotic. Also excluded were patients who filled a prescription for a dopamine agonist in the two or more years preceding RLS diagnosis. Patients who were ever diagnosed with Parkinson’s disease, kidney disease, iron deficiency, or pregnancy were assumed to have secondary RLS and were also excluded.
The investigators identified 5,419 eligible participants. Of this group, 1,649 patients received dopamine agonists after RLS diagnosis. Specifically, 571 participants received pramipexole, 915 received ropinirole, and 163 received both. Approximately 65% of patients were female. Patients residing in the Northeast were significantly less likely to receive dopamine agonists, compared with patients residing in the Midwest or the South. The investigators found no significant differences in treatment status by comorbid illness burden or by sex. The investigators also found no significant differences in demographic characteristics between patients receiving pramipexole and those receiving ropinirole.
Risk Was Significantly Greater in Patients Receiving Dopamine Agonist
Next, from this pool of eligible subjects, the researchers matched 1,080 patients treated with dopamine agonists with 1,080 dopamine agonist-naïve patients on sex, age at RLS diagnosis, region, employment, and illness burden. Dr. Hankin and colleagues found a significant increase in mental disorder diagnoses (eg, bipolar disorder, anxiety, depression, and substance abuse) among patients treated with dopamine agonists, compared with dopamine agonist-naïve patients. Among patients receiving dopamine agonists, the odds ratio for severe mental disorder (eg, psychoses and bipolar disorder) was 2.2, the odds ratio for moderate to severe mental disorder (eg, posttraumatic stress disorder and major depression) was 1.8, and the odds ratio for mild mental disorder (eg, anxiety disorders) was 1.9, compared with dopamine agonist-naïve patients.
“This is the first large-scale, real-world, claims-based study to examine the association between treatment of RLS with dopamine agonists and the development of psychiatric adverse events. Our findings are compelling, but need to be replicated in other patient populations,” said Dr. Hankin.
“Our retrospective analysis required careful consideration of matching,” said Daniel On-Fai Lee, MD, Clinical Professor of Neurology at the University of Kentucky College of Medicine in Lexington, who collaborated on the study. Although the investigators took care to match participants and to remove cases of secondary RLS from the analysis, they may have inadvertently overlooked one or more important matching variables that could affect the outcome, he added.
Arbor Pharmaceuticals provided funding for the study, but did not influence its methodology, analysis, results, or conclusion, said Dr. Lee.
—Erik Greb
Suggested Reading
Sierra M, Carnicella S, Strafella AP, et al. Apathy and impulse control disorders: yin & yang of dopamine dependent behaviors. J Parkinsons Dis. 2015;5(3):625-636.
Wilt TJ, MacDonald R, Ouellette J, et al. Pharmacologic therapy for primary restless legs syndrome: a systematic review and meta-analysis. JAMA Intern Med. 2013;173(7):496-505.