User login
Pseudo-Pedicle Heterotopic Ossification From Use of Recombinant Human Bone Morphogenetic Protein 2 (rhBMP-2) in Transforaminal Lumbar Interbody Fusion Cages
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
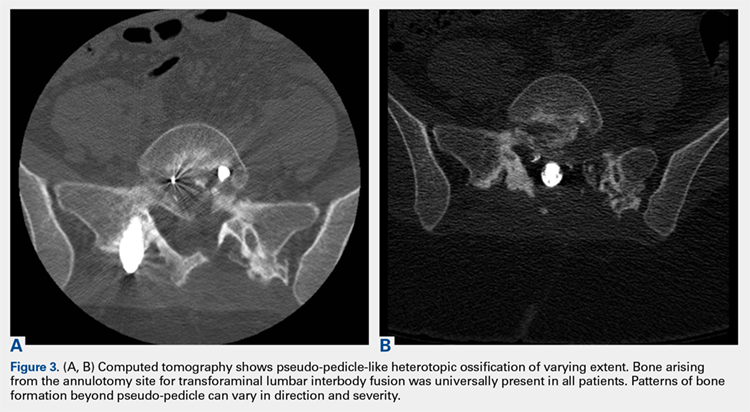
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.
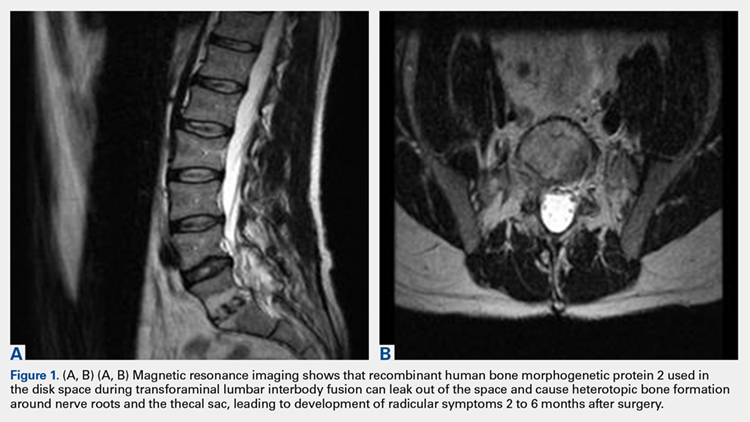
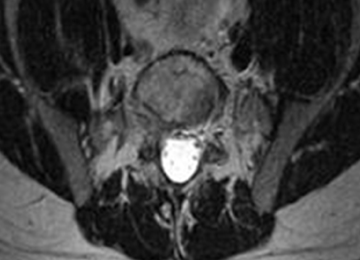
2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.
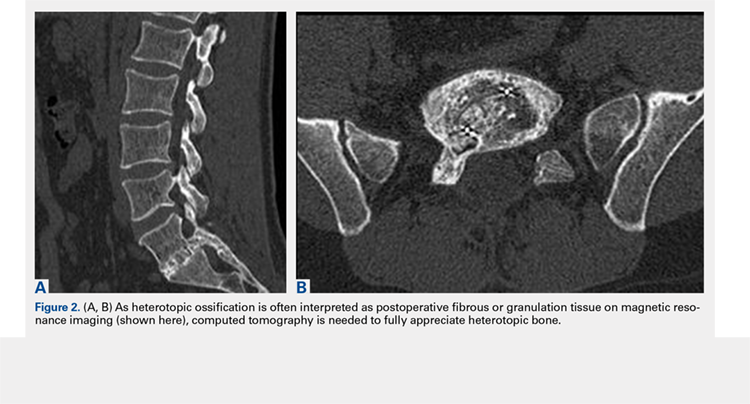
3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.
4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.

2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.

3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.

4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
ABSTRACT
We conducted a study to determine the common characteristics of patients who developed radiculopathy symptoms and corresponding heterotopic ossification (HO) from transforaminal lumbar interbody fusions (TLIF) using recombinant human bone morphogenetic protein 2 (rhBMP-2). HO can arise from a disk space with rhBMP-2 use in TLIF. Formation of bone around nerve roots or the thecal sac can cause a radiculopathy with a consistent pattern of symptoms.
We identified 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years who developed radiculopathy symptoms and corresponding HO from TLIF with rhBMP-2 in the disk space between 2002 and 2015. To document this complication and improve its recognition, we recorded common patterns of symptom development and radiologic findings: specifically, time from implantation of rhBMP-2 to symptom development, consistency with side of TLIF placement, and radiologic findings.
Radicular pain generally developed a mean (SD) of 3.8 (1.0) months after TLIF with rhBMP-2. Development of radiculopathy symptoms corresponded to consistent “pseudo-pedicle”-like HO. In all 38 patients, HO arising from the annulotomy site showed a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. In addition, development of radiculopathy symptoms and corresponding HO appear to be independent of amount of rhBMP-2. HO resulting from TLIF with rhBMP-2 in the disk space is a pain generator and a recognizable complication that can be diagnosed by assessment of symptoms and computed tomography characteristics.
Continue to: Bone morphogenetic proteins...
Bone morphogenetic proteins (BMPs), first isolated by Urist in 19641, are a family of growth factors that stimulate the cascade of bone formation. Recombinant human BMP (rhBMP), specifically rhBMP-2 and rhBMP-7 (also known as osteogenic protein 1 [OP-1]), was developed in the 1990s after the advent of gene splicing. Then, in 2002, the US Food and Drug Administration (FDA) approved use of rhBMP to stimulate fusion in the human spine. Specifically, rhBMP-2 (Medtronic) was approved for use in combination with a specific brand of interbody cage in 1-level anterior lumbar interbody fusion.2 Over the past decade, off-label use of rhBMP-2 to achieve osseous union has increased dramatically, particularly in spinal surgery: transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion, and posterolateral lumbar fusion.3-9 However, this widespread off-label use for posterior spinal fusion began despite FDA data indicating that specific complications were underreported in the peer-reviewed literature.10,11 Although rhBMP-2 is very effective in increasing osteoblast formation and improving osteogenesis and subsequent bone healing in spinal surgery,12,13 its use in TLIF resulted in significant adverse side effects, including radiculopathy with and without neuroforaminal heterotopic ossification (HO); 14-24 complications in the FDA studies; 14,22,25-27 and osteolysis causing intervertebral cage subsidence, inflammatory radiculitis, genitourinary complications, infections, possible systemic effects, and significant HO complications.10,28-30 Of these, HO complications involved rhBMP leakage through the annulotomy to the disk space that led to HO. Specifically, rhBMP leaked directly out of the disk space and formed a pillar of bone that encased the nerve roots and dura, which led to occlusion of the foramen and symptoms of radiculopathy.10,28-30
Despite this frequent finding of HO in the intervertebral space outside the target fusion area, use of rhBMP-2 with intervertebral cages increased so rapidly that rhBMP-2 was used more often than autologous bone.5,11,17,31 In this study, we reviewed the common characteristics of patients who developed HO and subsequent radiculopathy from TLIF with rhBMP.
METHODS
After this study received Institutional Review Board approval, we retrospectively reviewed cases of radiculopathy symptoms that developed after TLIF with rhBMP between January 2002 and January 2015. During this period, 38 patients (26 males, 12 females) with a mean (SD) age of 50.8 (7.5) years and radiculopathy symptoms arising from TLIF with rhBMP-2 were identified to determine commonalities and defining characteristics that will help facilitate diagnosis.
Inclusion criteria were computed tomography (CT)–documented HO arising from the TLIF annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell with contouring around the thecal sac or nerve roots, as well as recurrence or initial occurrence of radiculopathy with signs and symptoms corresponding to the CT site of aberrant bone growth in terms of laterality and particular nerve root(s) involved. Exclusion criteria were malplacement of interbody cage or pedicle screws, disk herniation, systemic neuropathic disease, and new or unresolved radiculopathy immediately after index surgery.
To improve recognition of this complication, we also documented the amount of BMP used, common patterns of radiculopathy symptom development, and radiologic findings. Type and timing of radiculopathy symptom onset and consistency with side of TLIF placement were documented as well. Radiculopathy symptoms included shooting pain in the legs, incontinence, sexual dysfunction, and severe paralysis. Radiologic findings were specific to bone formation from the disk space (detected with CT).
Continue to: RESULTS
RESULTS
All 38 selected patients had radiculopathy symptoms from HO out of the intervertebral space. The Table lists the patients’ overall characteristics. The left side had the most radiculopathy symptoms (31/38 patients), followed by the right side (5/38) and both sides (2/38). Radiculopathy symptoms began a mean (SD) of 3.8 (1.0) months (range, 2-6 months) after index surgery. The 38 patients had 4 characteristics in common:
Table. Transforaminal Lumbar Interbody Fusion With Recombinant Human Bone Morphogenetic Protein 2: Onset Time for Radiculopathy Symptoms, Surgery Level, Side of Pseudo-Pedicle Bone Formation, and Subsequent Complications
| Pt | Sympton Onset, mo | Surgery Level(s) | Side(s) | Complication(s) |
| 1 | 3 | L3-L5 (2) | Both | Radiculopathy, pseudo-pedicle, urine |
| 2 | 3 | L4-L5 (2) | R | Radiculopathy, pseudo-pedicle |
| 3 | 4 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 4 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 5 | 4 | L4-S1 (2) | L | Radiculopathy, pseudo-pedicle, subsidence |
| 6 | 5 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 7 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 8 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 9 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 10 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 11 | 2 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, subsidence, neurologic |
| 12 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 13 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, neurologic |
| 14 | 2 | L2-L3 (1) | R | Radiculopathy, pseudo-pedicle |
| 15 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 16 | 3 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 17 | 3 | L2-L3, L4-L5 (2) | L | Radiculopathy, pseudo-pedicle |
| 18 | 3 | L4-L5, L2-L3 (1) | L | Radiculopathy, pseudo-pedicle, nonunion |
| 19 | 4 | L4-L5 (1) | R | Radiculopathy, pseudo-pedicle |
| 20 | 5 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 21 | 5 | L5-S1 (1) | R | Radiculopathy, pseudo-pedicle |
| 22 | 3 | L3-L4, L5-S1 (2) | Both | Radiculopathy, pseudo-pedicle |
| 23 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 24 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 25 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 26 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle, urine, bowel |
| 27 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 28 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 29 | 6 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 30 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 31 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 32 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 33 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 34 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 35 | 4 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 36 | 3 | L5-S1 (1) | L | Radiculopathy, pseudo-pedicle |
| 37 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
| 38 | 4 | L4-L5 (1) | L | Radiculopathy, pseudo-pedicle |
1. Bone growing out of the annulotomy site for TLIF cage placement was present and in continuity with the disk space in 33 (87%) of the 38 cases. In the other 5 cases (13%), HO was present around the neural tissue, but not necessarily in continuity with the disk space. This bone appeared ectopic and not osteophytic and facet-related, as it formed a shell around either the nerve root or the thecal sac, contouring to the structure.

2. The common, novel finding on CT was a “pseudo-pedicle” (Figures 1A, 1B), which appeared as ectopic growth from the disk space—a solid piece of bone in the same direction as the anatomical pedicle. Confusing similarity to the anatomical pedicle is present on axial cuts and during surgery. The pseudo-pedicle varied in thickness and extent out of the disk space, but was always presented as a bar of bone arising from the annulotomy site. After arising from the disk space, the HO could disperse in any direction, further calcifying neural structures or the facet joints above or below. There was no apparent distinguishable repeating pattern, given the variable nature of arthritic facet changes, scoliotic deformities, size of annulotomies, amount of rhBMP used, and placement in cage and disk space or only in cage.

3. In 36 (95%) of the 38 cases, the initial interpretation of HO on magnetic resonance imaging (MRI) was of tissue other than bone, such as fibrous tissue, granulation tissue, recurrent disk herniation, or postoperative changes. However, this tissue was later determined to be bone from HO complications, which we confirmed with CT in all 38 cases. It is important to note that HO on MRI (Figures 2A, 2B) was initially interpreted by a radiologist as fibrous tissue, but same-level CT of the same case (Figures 3A, 3B) showed clear HO.

4. The radiculopathy symptoms caused by HO were independent of the amount of rhBMP-2 used in TLIF. Of the 38 patients, 19 had 1 rhBMP-2 sponge placed in the cage, 12 had a small kit sponge (1.05 mg), 5 had 1 sponge placed in the cage and 1 sponge placed directly in the disk space before cage placement (no notation of precise size or amount of rhBMP-2), and 2 had 1 sponge placed in the cage (no notation of rhBMP-2 amount). The data showed that HO can occur with even a small amount of rhBMP-2.
Continue to: Bone formation with rhBMP-2...
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
DISCUSSION
We identified 38 patients with a recognizable and consistent pattern of complications of off-label use of rhBMP-2 in TLIF performed at our institution between 2002 and 2015. This pattern included consistent radiculopathy symptoms with corresponding HO at the annulotomy site in continuity with bone in the disk space or ectopic bone forming a distinctive shell around the thecal sac or nerve roots, as well as showing a distinct pseudo-pedicle pattern encompassing nerve roots and the thecal sac. Our finding differs from other findings of similar complication characteristics, but with much larger variations without consistency within the patient population.19,20,22,24 Specifically, previous studies found an association between off-label rhBMP-2 use in the posterior spine and radiculopathy with and without neuroforaminal HO. However, our study found consistent radiculopathy symptoms with pseudo-pedicle-like HO complications in all its 38 patients a mean (SD) of 3.8 (1.0) months after surgery.
In this study, consistent radiculopathy symptoms with pseudo-pedicle-like HO complications were independent of the amount of rhBMP-2 used, as some complications occurred with use of small pack rhBMP-2 with TLIF. It is well understood that high doses of rhBMP-2 may be required to improve fusion rates, but to our knowledge an optimal dosing strategy for TLIF has not been reported, particularly with respect to potential complications.8,20,31-33 For anterior lumbar interbody fusion surgery, the FDA-approved use of rhBMP-2 appears to have a significantly decreased risk of neuroforaminal HO complications. This may be attributable to the protective presence of the intact posterior annulus and longitudinal ligament for this procedure.20,33 For TLIF, it has been suggested that rhBMP-2 should be placed only along the anterior annulus with a posterior strut and morselized bone allograft barricade,33 and that fibrin glue should be used to limit BMP diffusion through the annulotomy site31 to prevent this complication.
Our study results suggest that radiculopathy symptoms with pseudo-pedicle-like HO complications appear to be caused by leakage of rhBMP-2 from the disk space through the annulotomy site. This was often initially interpreted incorrectly on MRI in the first year after surgery as being fibrous or granulation tissue, or even postoperative changes that the heterotopic tissue was bone was obvious only on CT. Even then the tissue may be incorrectly identified, as the encasing nerve roots in bone are similar to the scar tissue having no compressive effect. HO may compress, but it also has an inflammatory component that the scars lack. Additionally, the HO from the disk space, caused by leakage of the BMP placed in or around the fusion cage, can create a pseudo-pedicle of varying size and extent. This was present in all 38 of our cases.
This retrospective case series had its limitations. Its clinical and radiographic findings were not blinded. Confounding variables cannot be isolated for causal relationships, if any, to the complication in a case series such as this.
Bone formation with rhBMP-2 is robust and beneficial, but HO-related complications are significant, and identifiable on assessment of radiculopathy symptoms and CT characteristics.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
1. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
2. Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181-1189.
3. Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo award in clinical studies. Spine. 2002;27(23):2662-2673.
4. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25(3):376-381.
5. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538.
6. Meisel HJ, Schnöring M, Hohaus C, et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17(12):1735-1744.
7. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
8. Shimer AL, Oner FC, Vaccaro AR. Spinal reconstruction and bone morphogenetic proteins: open questions. Injury. 2009;40(suppl 3):S32-S38.
9. Slosar PJ, Josey R, Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7(3):301-307.
10. Knox JB, Dai JM 3rd, Orchowski J. Osteolysis in transforaminal lumbar interbody fusion with bone morphogenetic protein-2. Spine. 2011;36(8):672-676.
11. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2011;20(4):612-617.
12. Mindea SA, Shih P, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480-1484.
13. Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29(23):2603-2611.
14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.
16. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12(1):40-46.
17. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885-2890.
18. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech. 2006;19(7):483-486.
19. Mroz TE, Wang JC, Hashimoto R, Norvell DC. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010;35(9 suppl):S86-S104.
20. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1-e6.
21. Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794-1800.
22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623-629.
23. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557-562.
24. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8(6):1011-1018.
25. Delawi D, Dhert WJ, Rillardon L, et al. A prospective, randomized, controlled, multicenter study of osteogenic protein-1 in instrumented posterolateral fusions: report on safety and feasibility. Spine. 2010;35(12):1185-1191.
26. Vaccaro AR, Patel T, Fischgrund J, et al. A pilot study evaluating the safety and efficacy of OP-1 putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine. 2004;29(17):1885-1892.
27. Vaidya R, Weir R, Sethi A, Meisterling S, Hakeos W, Wybo CD. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342-345.
28. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849-1854.
29. Helgeson MD, Lehman RA Jr, Patzkowski JC, Dmitriev AE, Rosner MK, Mack AW. Adjacent vertebral body osteolysis with bone morphogenetic protein use in transforaminal lumbar interbody fusion. Spine J. 2011;11(6):507-510.
30. Hoffmann MF, Jones CB, Sietsema DL. Adjuncts in posterior lumbar spine fusion: comparison of complications and efficacy. Arch Orthop Trauma Surg. 2012;132(8):1105-1110.
31. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine. 2005;3(6):436-443.
32. Patel VV, Zhao L, Wong P, et al. Controlling bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth using fibrin glue. Spine. 2006;31(11):1201-1206.
33. Zhang H, Sucato DJ, Welch RD. Recombinant human bone morphogenic protein-2-enhanced anterior spine fusion without bone encroachment into the spinal canal: a histomorphometric study in a thoracoscopically instrumented porcine model. Spine. 2005;30(5):512-518.
TAKE-HOME POINTS
- Use of rhBMP-2 in TLIF cages can result in HO out of the cage into the spinal canal.
- HO from rhBMP-2 in TLIF cages can result in a radiculopathy from compression or inflammatory reaction.
- HO out of the cage into the spinal canal resulting from use of rhBMP-2 in TLIF cages can be adequately diagnosed only with CT.
- HO can appear as a pedicle or pseudo-pedicle.
- Consider potential HO when using rhBMP-2 in TLIF cages.
Tough patient cases from 2017
AGA’s member-only online networking platform, the AGA Community, was the hub for clinical case scenarios in 2017. About 100 deidentified patient cases were submitted to the forum, generating over 475 private and public responses from your peers.
Here is a summary of the three cases that sparked the most discussion among AGA members. You can view all discussions in the forum at community.gastro.org/discussions.
#3 “Esophageal hyperkeratosis” (February 2017)
Patient scenario: Patient was having dysphagia. EGD showed circumferential thickening of esophageal lining in the lower half of the esophagus causing partial obstruction; lumen diameter was 7 mm (scope was able to pass with mild resistance). Human papillomavirus (HPV) stain was negative. Multiple biopsies were negative for malignancy, so the practice did not recommend esophagectomy and believed the symptoms were consistent with hyperkeratosis of esophagus. Endoscopic cryotherapy was being considered.
Question: Has anyone come across a case like this?
#2 Thickened stomach (May 2017)
Patient scenario: A 74-year-old male presented early satiety, anemia, and dyspepsia. EGD showed diffuse moderate erythema of the stomach sparing the antrum, and two small superficial duodenal ulcers. Biopsies showed mild chronic inflammation, duodenitis, and negative for H. pylori. The patient was started on a proton pump inhibitor (PPI).
One month later, patient reported early satiety, a 40-pound weight loss over last few months, nausea and vomiting, with minimal improvement while using the PPI. A CT scan of the abdomen and pelvis showed diffuse thickening of the stomach, but was otherwise unremarkable.
One month after that, a repeated EGD showed moderate erythema with enlarged gastric folds, cobblestone of mucosa, again all sparing the antrum. The colonoscopy results were unremarkable. Gastric biopsies showed mild chronic inflammation. Endoscopic ultrasound showed a thickened gastric wall to 14 mm (normal 5 mm) and fine needle aspiration showed normal gastric foveolar epithelium. The patient received a PEG-J tube to maintain nutrition, and then had a laparoscopic assisted full thickness gastric biopsy, which showed benign hypertrophic gastric smooth muscle tissue.
Serum protein electrophoresis and urine protein electrophoresis test results were normal, with total IgG and IgA normal, total IgM low at 31 (normal 60-265), albumin low, other proteins normal, and immunofixation negative. Prealbumin was low at 5 (normal 15-45). Albumin initially normal and over a couple of days low at 2.6 (normal 3.4-5.0). Total protein initially normal and over a couple of days was low at 6.3 (normal 6.8-8.8). Gastrin level was insignificant on the PPI, in the 400s. Zollinger Ellison gastrin not impressive, and the patient is HIV negative.
Question: With a negative biopsy and other test results, Menetrier’s, malignancy, sarcoidosis, eosinophilic gastroenteritis, and amyloidosis can be ruled out. What could the diagnosis be?
#1 IBD and prior hep B (July 2017)
Patient scenario: A 53-year-old male diagnosed with ulcerative colitis (UC) at outside hospital after presenting with abdominal pain, perforation of sigmoid colon. He underwent total colectomy with ileostomy, which showed he has remnant rectum, and the path of colon showed UC with sigmoid stricture. There is no malignancy or dysplasia, and the terminal ileum included in the resection was normal. He had complicated post-op course with enterocutaneous fistula.
He underwent takedown of ileostomy, small bowel resection and ileostomy revision. Path showed segmental small bowel showing viable mucosa with acute serositis and serial adhesions. Ileal mucosa was normal. Rectum has inflammation, and he has symptoms of mucus, urgency, and blood. He had rectal burning and did not tolerate CANASA® suppository. He did not seem to improve with hydrocortisone suppository either.
In trying to decipher next treatment step, hepatitis panel was done, which showed positive hepatitis B core antibody (IgM). Hepatitis B viral load was undetectable. Hepatitis B surface antibody test (HBsAb) quantitative was 6 (not quite the range for immunity of greater than 10). Hepatitis B “e” antigen (HBeAg) negative and hepatitis B “e” antibody (HBeAb) positive. This patient’s hep B core total was positive and hep B surface antigen was negative.
Question: How would you treat this patient? Would you use Imuran?
Share your difficult patient case for the GI community to help you solve at community.gastro.org/quickpost.
AGA’s member-only online networking platform, the AGA Community, was the hub for clinical case scenarios in 2017. About 100 deidentified patient cases were submitted to the forum, generating over 475 private and public responses from your peers.
Here is a summary of the three cases that sparked the most discussion among AGA members. You can view all discussions in the forum at community.gastro.org/discussions.
#3 “Esophageal hyperkeratosis” (February 2017)
Patient scenario: Patient was having dysphagia. EGD showed circumferential thickening of esophageal lining in the lower half of the esophagus causing partial obstruction; lumen diameter was 7 mm (scope was able to pass with mild resistance). Human papillomavirus (HPV) stain was negative. Multiple biopsies were negative for malignancy, so the practice did not recommend esophagectomy and believed the symptoms were consistent with hyperkeratosis of esophagus. Endoscopic cryotherapy was being considered.
Question: Has anyone come across a case like this?
#2 Thickened stomach (May 2017)
Patient scenario: A 74-year-old male presented early satiety, anemia, and dyspepsia. EGD showed diffuse moderate erythema of the stomach sparing the antrum, and two small superficial duodenal ulcers. Biopsies showed mild chronic inflammation, duodenitis, and negative for H. pylori. The patient was started on a proton pump inhibitor (PPI).
One month later, patient reported early satiety, a 40-pound weight loss over last few months, nausea and vomiting, with minimal improvement while using the PPI. A CT scan of the abdomen and pelvis showed diffuse thickening of the stomach, but was otherwise unremarkable.
One month after that, a repeated EGD showed moderate erythema with enlarged gastric folds, cobblestone of mucosa, again all sparing the antrum. The colonoscopy results were unremarkable. Gastric biopsies showed mild chronic inflammation. Endoscopic ultrasound showed a thickened gastric wall to 14 mm (normal 5 mm) and fine needle aspiration showed normal gastric foveolar epithelium. The patient received a PEG-J tube to maintain nutrition, and then had a laparoscopic assisted full thickness gastric biopsy, which showed benign hypertrophic gastric smooth muscle tissue.
Serum protein electrophoresis and urine protein electrophoresis test results were normal, with total IgG and IgA normal, total IgM low at 31 (normal 60-265), albumin low, other proteins normal, and immunofixation negative. Prealbumin was low at 5 (normal 15-45). Albumin initially normal and over a couple of days low at 2.6 (normal 3.4-5.0). Total protein initially normal and over a couple of days was low at 6.3 (normal 6.8-8.8). Gastrin level was insignificant on the PPI, in the 400s. Zollinger Ellison gastrin not impressive, and the patient is HIV negative.
Question: With a negative biopsy and other test results, Menetrier’s, malignancy, sarcoidosis, eosinophilic gastroenteritis, and amyloidosis can be ruled out. What could the diagnosis be?
#1 IBD and prior hep B (July 2017)
Patient scenario: A 53-year-old male diagnosed with ulcerative colitis (UC) at outside hospital after presenting with abdominal pain, perforation of sigmoid colon. He underwent total colectomy with ileostomy, which showed he has remnant rectum, and the path of colon showed UC with sigmoid stricture. There is no malignancy or dysplasia, and the terminal ileum included in the resection was normal. He had complicated post-op course with enterocutaneous fistula.
He underwent takedown of ileostomy, small bowel resection and ileostomy revision. Path showed segmental small bowel showing viable mucosa with acute serositis and serial adhesions. Ileal mucosa was normal. Rectum has inflammation, and he has symptoms of mucus, urgency, and blood. He had rectal burning and did not tolerate CANASA® suppository. He did not seem to improve with hydrocortisone suppository either.
In trying to decipher next treatment step, hepatitis panel was done, which showed positive hepatitis B core antibody (IgM). Hepatitis B viral load was undetectable. Hepatitis B surface antibody test (HBsAb) quantitative was 6 (not quite the range for immunity of greater than 10). Hepatitis B “e” antigen (HBeAg) negative and hepatitis B “e” antibody (HBeAb) positive. This patient’s hep B core total was positive and hep B surface antigen was negative.
Question: How would you treat this patient? Would you use Imuran?
Share your difficult patient case for the GI community to help you solve at community.gastro.org/quickpost.
AGA’s member-only online networking platform, the AGA Community, was the hub for clinical case scenarios in 2017. About 100 deidentified patient cases were submitted to the forum, generating over 475 private and public responses from your peers.
Here is a summary of the three cases that sparked the most discussion among AGA members. You can view all discussions in the forum at community.gastro.org/discussions.
#3 “Esophageal hyperkeratosis” (February 2017)
Patient scenario: Patient was having dysphagia. EGD showed circumferential thickening of esophageal lining in the lower half of the esophagus causing partial obstruction; lumen diameter was 7 mm (scope was able to pass with mild resistance). Human papillomavirus (HPV) stain was negative. Multiple biopsies were negative for malignancy, so the practice did not recommend esophagectomy and believed the symptoms were consistent with hyperkeratosis of esophagus. Endoscopic cryotherapy was being considered.
Question: Has anyone come across a case like this?
#2 Thickened stomach (May 2017)
Patient scenario: A 74-year-old male presented early satiety, anemia, and dyspepsia. EGD showed diffuse moderate erythema of the stomach sparing the antrum, and two small superficial duodenal ulcers. Biopsies showed mild chronic inflammation, duodenitis, and negative for H. pylori. The patient was started on a proton pump inhibitor (PPI).
One month later, patient reported early satiety, a 40-pound weight loss over last few months, nausea and vomiting, with minimal improvement while using the PPI. A CT scan of the abdomen and pelvis showed diffuse thickening of the stomach, but was otherwise unremarkable.
One month after that, a repeated EGD showed moderate erythema with enlarged gastric folds, cobblestone of mucosa, again all sparing the antrum. The colonoscopy results were unremarkable. Gastric biopsies showed mild chronic inflammation. Endoscopic ultrasound showed a thickened gastric wall to 14 mm (normal 5 mm) and fine needle aspiration showed normal gastric foveolar epithelium. The patient received a PEG-J tube to maintain nutrition, and then had a laparoscopic assisted full thickness gastric biopsy, which showed benign hypertrophic gastric smooth muscle tissue.
Serum protein electrophoresis and urine protein electrophoresis test results were normal, with total IgG and IgA normal, total IgM low at 31 (normal 60-265), albumin low, other proteins normal, and immunofixation negative. Prealbumin was low at 5 (normal 15-45). Albumin initially normal and over a couple of days low at 2.6 (normal 3.4-5.0). Total protein initially normal and over a couple of days was low at 6.3 (normal 6.8-8.8). Gastrin level was insignificant on the PPI, in the 400s. Zollinger Ellison gastrin not impressive, and the patient is HIV negative.
Question: With a negative biopsy and other test results, Menetrier’s, malignancy, sarcoidosis, eosinophilic gastroenteritis, and amyloidosis can be ruled out. What could the diagnosis be?
#1 IBD and prior hep B (July 2017)
Patient scenario: A 53-year-old male diagnosed with ulcerative colitis (UC) at outside hospital after presenting with abdominal pain, perforation of sigmoid colon. He underwent total colectomy with ileostomy, which showed he has remnant rectum, and the path of colon showed UC with sigmoid stricture. There is no malignancy or dysplasia, and the terminal ileum included in the resection was normal. He had complicated post-op course with enterocutaneous fistula.
He underwent takedown of ileostomy, small bowel resection and ileostomy revision. Path showed segmental small bowel showing viable mucosa with acute serositis and serial adhesions. Ileal mucosa was normal. Rectum has inflammation, and he has symptoms of mucus, urgency, and blood. He had rectal burning and did not tolerate CANASA® suppository. He did not seem to improve with hydrocortisone suppository either.
In trying to decipher next treatment step, hepatitis panel was done, which showed positive hepatitis B core antibody (IgM). Hepatitis B viral load was undetectable. Hepatitis B surface antibody test (HBsAb) quantitative was 6 (not quite the range for immunity of greater than 10). Hepatitis B “e” antigen (HBeAg) negative and hepatitis B “e” antibody (HBeAb) positive. This patient’s hep B core total was positive and hep B surface antigen was negative.
Question: How would you treat this patient? Would you use Imuran?
Share your difficult patient case for the GI community to help you solve at community.gastro.org/quickpost.
Insurance barriers should not hinder step therapy treatment for IBD
As part of Crohn’s and Colitis Awareness Week 2017 (Dec. 1-7), AGA participated in a congressional briefing sponsored by Takeda and the California Life Sciences Association highlighting advances in inflammatory bowel disease (IBD) therapies, as well as the barriers that patients face in receiving proper treatment for managing their disease.
Physician perspective
Michael Weinstein, MD, representing AGA and the Digestive Health Physicians Association, discussed how treatment options have changed considerably since he began practicing in the 1980s when the only treatment options were immunosuppressive drugs or high-dose steroids that led to dangerous side effects. He highlighted the burden that physicians face with prior authorization practices, especially step therapy in which a patient is required to fail several therapies before being granted coverage to the preferred, physician-prescribed therapy. These insurance protocols can have dire effects on patient care and can be very disruptive to patients who may be so ill that they cannot work or go to school. Dr. Weinstein stated that the burden step therapy places on his practice requires him to have a full-time employee just to navigate the various insurance policies. Many small practices do not have the resources to handle these burdens. Read more from Dr. Weinstein in his op-ed from The Hill.
Patient perspective
Members of Congress and congressional staff heard compelling testimony from Kate Detwiler, an IBD patient who spoke of her family history of IBD, her experience with the disease, and how disruptive it has been to find the best provider and treatments to manage her disease. She and Dr. Weinstein both stressed the financial burdens that the disease puts on families and how limiting it can be to patients who are starting out in their careers or school.
Legislator perspective
Rep. Brad Weinstrup, R-OH, and Rep. Raul Ruiz, D-CA, addressed the briefing as the lead sponsors of HR 2077, the Restoring Patient’s Voice Act, which would provide patients and providers with a clear, equitable, and transparent appeals process when subject to step therapy protocols. Both Rep. Wenstrup and Rep. Ruiz are physicians and have seen the real-life consequences of these policies and their impact on patient care. Both representatives stressed that this is a bipartisan, commonsense solution to ensuring that patients have access to the care that they need when they need it.
AGA continues to advocate for support and passage of HR 2077 and thanks those members who have contacted their members of Congress to request their support. If you haven’t already, please call on your legislator to support this legislation. We will continue to work to garner additional support for the bill in this Congress.
As part of Crohn’s and Colitis Awareness Week 2017 (Dec. 1-7), AGA participated in a congressional briefing sponsored by Takeda and the California Life Sciences Association highlighting advances in inflammatory bowel disease (IBD) therapies, as well as the barriers that patients face in receiving proper treatment for managing their disease.
Physician perspective
Michael Weinstein, MD, representing AGA and the Digestive Health Physicians Association, discussed how treatment options have changed considerably since he began practicing in the 1980s when the only treatment options were immunosuppressive drugs or high-dose steroids that led to dangerous side effects. He highlighted the burden that physicians face with prior authorization practices, especially step therapy in which a patient is required to fail several therapies before being granted coverage to the preferred, physician-prescribed therapy. These insurance protocols can have dire effects on patient care and can be very disruptive to patients who may be so ill that they cannot work or go to school. Dr. Weinstein stated that the burden step therapy places on his practice requires him to have a full-time employee just to navigate the various insurance policies. Many small practices do not have the resources to handle these burdens. Read more from Dr. Weinstein in his op-ed from The Hill.
Patient perspective
Members of Congress and congressional staff heard compelling testimony from Kate Detwiler, an IBD patient who spoke of her family history of IBD, her experience with the disease, and how disruptive it has been to find the best provider and treatments to manage her disease. She and Dr. Weinstein both stressed the financial burdens that the disease puts on families and how limiting it can be to patients who are starting out in their careers or school.
Legislator perspective
Rep. Brad Weinstrup, R-OH, and Rep. Raul Ruiz, D-CA, addressed the briefing as the lead sponsors of HR 2077, the Restoring Patient’s Voice Act, which would provide patients and providers with a clear, equitable, and transparent appeals process when subject to step therapy protocols. Both Rep. Wenstrup and Rep. Ruiz are physicians and have seen the real-life consequences of these policies and their impact on patient care. Both representatives stressed that this is a bipartisan, commonsense solution to ensuring that patients have access to the care that they need when they need it.
AGA continues to advocate for support and passage of HR 2077 and thanks those members who have contacted their members of Congress to request their support. If you haven’t already, please call on your legislator to support this legislation. We will continue to work to garner additional support for the bill in this Congress.
As part of Crohn’s and Colitis Awareness Week 2017 (Dec. 1-7), AGA participated in a congressional briefing sponsored by Takeda and the California Life Sciences Association highlighting advances in inflammatory bowel disease (IBD) therapies, as well as the barriers that patients face in receiving proper treatment for managing their disease.
Physician perspective
Michael Weinstein, MD, representing AGA and the Digestive Health Physicians Association, discussed how treatment options have changed considerably since he began practicing in the 1980s when the only treatment options were immunosuppressive drugs or high-dose steroids that led to dangerous side effects. He highlighted the burden that physicians face with prior authorization practices, especially step therapy in which a patient is required to fail several therapies before being granted coverage to the preferred, physician-prescribed therapy. These insurance protocols can have dire effects on patient care and can be very disruptive to patients who may be so ill that they cannot work or go to school. Dr. Weinstein stated that the burden step therapy places on his practice requires him to have a full-time employee just to navigate the various insurance policies. Many small practices do not have the resources to handle these burdens. Read more from Dr. Weinstein in his op-ed from The Hill.
Patient perspective
Members of Congress and congressional staff heard compelling testimony from Kate Detwiler, an IBD patient who spoke of her family history of IBD, her experience with the disease, and how disruptive it has been to find the best provider and treatments to manage her disease. She and Dr. Weinstein both stressed the financial burdens that the disease puts on families and how limiting it can be to patients who are starting out in their careers or school.
Legislator perspective
Rep. Brad Weinstrup, R-OH, and Rep. Raul Ruiz, D-CA, addressed the briefing as the lead sponsors of HR 2077, the Restoring Patient’s Voice Act, which would provide patients and providers with a clear, equitable, and transparent appeals process when subject to step therapy protocols. Both Rep. Wenstrup and Rep. Ruiz are physicians and have seen the real-life consequences of these policies and their impact on patient care. Both representatives stressed that this is a bipartisan, commonsense solution to ensuring that patients have access to the care that they need when they need it.
AGA continues to advocate for support and passage of HR 2077 and thanks those members who have contacted their members of Congress to request their support. If you haven’t already, please call on your legislator to support this legislation. We will continue to work to garner additional support for the bill in this Congress.
Registration open for DDW® 2018 and 2018 AGA Postgraduate Course
Digestive Disease Week® (DDW), taking place in Washington, D.C., June 2-5, is the gold standard digestive disease meeting. Attend to learn monumental developments in science and medicine, meet leaders in the field, network with colleagues, and attain practical takeaways that you can implement in your career. Visit www.ddw.org to register, for general meeting information, details about the host city, and much more.
On the DDW website, you can also register for the AGA Postgraduate Course taking place during DDW, June 2-3. The 2018 AGA Postgraduate Course will provide a comprehensive look at the latest medical, surgical, and technological advances over the past 12 months that aim to keep you up to date in a field that is rapidly changing. Each presenter will turn abstract ideas into concrete action items that you can immediately implement in your practice. At the end of the course, the take-home points will be compiled and distributed with the eSyllabus.
Digestive Disease Week® (DDW), taking place in Washington, D.C., June 2-5, is the gold standard digestive disease meeting. Attend to learn monumental developments in science and medicine, meet leaders in the field, network with colleagues, and attain practical takeaways that you can implement in your career. Visit www.ddw.org to register, for general meeting information, details about the host city, and much more.
On the DDW website, you can also register for the AGA Postgraduate Course taking place during DDW, June 2-3. The 2018 AGA Postgraduate Course will provide a comprehensive look at the latest medical, surgical, and technological advances over the past 12 months that aim to keep you up to date in a field that is rapidly changing. Each presenter will turn abstract ideas into concrete action items that you can immediately implement in your practice. At the end of the course, the take-home points will be compiled and distributed with the eSyllabus.
Digestive Disease Week® (DDW), taking place in Washington, D.C., June 2-5, is the gold standard digestive disease meeting. Attend to learn monumental developments in science and medicine, meet leaders in the field, network with colleagues, and attain practical takeaways that you can implement in your career. Visit www.ddw.org to register, for general meeting information, details about the host city, and much more.
On the DDW website, you can also register for the AGA Postgraduate Course taking place during DDW, June 2-3. The 2018 AGA Postgraduate Course will provide a comprehensive look at the latest medical, surgical, and technological advances over the past 12 months that aim to keep you up to date in a field that is rapidly changing. Each presenter will turn abstract ideas into concrete action items that you can immediately implement in your practice. At the end of the course, the take-home points will be compiled and distributed with the eSyllabus.
Career Choices: Consultation-liaison psychiatry
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Peter Ganpat, MD, a consultation-liaison (C-L) psychiatrist at Florida Hospital, where he provides guidance to various medical specialties on managing acute and chronic mental illness and substance use disorders. In addition, he also is the medical director for the repetitive transcranial magnetic stimulation service and staffs the inpatient unit.
Dr. Stanciu: What made you choose to become a C-L psychiatrist?
Dr. Ganpat: In my opinion, C-L is the most challenging area of psychiatry because not only are you thinking along the realms of a psychiatrist, but you’re also considering the viewpoint of the other subspecialties at the same time. For me, it brings together my medical background with my passion for psychiatry, and the patients I see daily allow for this incorporation.
Dr. Stanciu: How did your career path prepare you to become a C-L psychiatrist?
Dr. Ganpat: My career path was unique in that I completed a family medicine residency, and then immediately pursued training in psychiatry. Some may consider this as “overkill” for C-L, but as I’ve come to learn, this background grants me a level of understanding and confidence to step in when dealing with a complex case and lend a hand to the consulting physician beyond psychiatry. I do not feel a fellowship is required to practice C-L psychiatry. However, a psychosomatic fellowship will definitely provide the experience needed for this career path, and also will enable one to get a second American Board of Psychiatry and Neurology board certification.
Dr. Stanciu: What types of clinical conditions are you asked to provide input on managing, and how do you find working alongside other specialties?
Dr. Ganpat: I have been managing the full breadth of psychiatry, and in some cases I also provide medical management. Practicing in a metropolitan area with a high influx of tourists also brings in unique cultural cases. The level of respect that the other specialties give is impressive, because they have now seen what a C-L psychiatrist can do. Their performance scores also have improved as a result of my involvement. They greatly appreciate my efforts to shed light on cases or assist with the ever-challenging patient whose psychiatric complexity impedes care.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Ganpat: The perfect candidate for this role should be capable of abstract as well as objective thinking. Having a good understanding of the other medical specialties and being able to solve problems is essential, because often it isn’t a clear-cut picture. It is imperative for the C-L psychiatrist to have sound teaching abilities and to be able to educate and communicate his (her) reasoning to the consulting team. It also is important to be well-versed in the psychiatric manifestations of various medical disorders and the psychiatric iatrogenesis of widely used prescription medications.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Ganpat: I think the largest challenge that I have encountered is the lack of resources. Substance abuse is a major problem here, especially opioids, and there are limited community resources for these patients, so they wind up in the hospital.
Dr. Stanciu: What are the disadvantages of C-L compared with other branches of psychiatry?
Dr. Ganpat: There isn’t much continuity of care with C-L psychiatry over the long run, but you do get to see patients improve during the duration of their hospitalization, which is very rewarding.
Dr. Stanciu: What is the typical reimbursement model for a C-L psychiatrist, and have you run into difficulties with insurance providers in this setting?
Dr. Ganpat: The reimbursement model varies from one system to the next. The common model is to bill just as any other hospital service would, based on the time or level of complexity. Obviously, the more consults you have, the more billing is generated. Most insurance carriers recognize this and so I haven’t had much of an issue with reimbursement, although some unexpected problems may arise.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a C-L career?
Dr. Ganpat: If you enjoy working in the hospital and interfacing with other specialties, then consider C-L psychiatry. It is challenging but intellectually stimulating. Make sure you request a C-L rotation during your training, because the Accreditation Council for Graduate Medical Education requires it during a psychiatric residency.
Dr. Stanciu: What is the future outlook of C-L?
Dr. Ganpat: There is a shortage of C-L psychiatrists because >50% of practicing psychiatrists are in private practice in an outpatient setting. Because access to psychiatric care outside of a hospital setting is an issue, and much care is being driven to hospitals, there will be an increasing need for C-L psychiatrists.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Peter Ganpat, MD, a consultation-liaison (C-L) psychiatrist at Florida Hospital, where he provides guidance to various medical specialties on managing acute and chronic mental illness and substance use disorders. In addition, he also is the medical director for the repetitive transcranial magnetic stimulation service and staffs the inpatient unit.
Dr. Stanciu: What made you choose to become a C-L psychiatrist?
Dr. Ganpat: In my opinion, C-L is the most challenging area of psychiatry because not only are you thinking along the realms of a psychiatrist, but you’re also considering the viewpoint of the other subspecialties at the same time. For me, it brings together my medical background with my passion for psychiatry, and the patients I see daily allow for this incorporation.
Dr. Stanciu: How did your career path prepare you to become a C-L psychiatrist?
Dr. Ganpat: My career path was unique in that I completed a family medicine residency, and then immediately pursued training in psychiatry. Some may consider this as “overkill” for C-L, but as I’ve come to learn, this background grants me a level of understanding and confidence to step in when dealing with a complex case and lend a hand to the consulting physician beyond psychiatry. I do not feel a fellowship is required to practice C-L psychiatry. However, a psychosomatic fellowship will definitely provide the experience needed for this career path, and also will enable one to get a second American Board of Psychiatry and Neurology board certification.
Dr. Stanciu: What types of clinical conditions are you asked to provide input on managing, and how do you find working alongside other specialties?
Dr. Ganpat: I have been managing the full breadth of psychiatry, and in some cases I also provide medical management. Practicing in a metropolitan area with a high influx of tourists also brings in unique cultural cases. The level of respect that the other specialties give is impressive, because they have now seen what a C-L psychiatrist can do. Their performance scores also have improved as a result of my involvement. They greatly appreciate my efforts to shed light on cases or assist with the ever-challenging patient whose psychiatric complexity impedes care.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Ganpat: The perfect candidate for this role should be capable of abstract as well as objective thinking. Having a good understanding of the other medical specialties and being able to solve problems is essential, because often it isn’t a clear-cut picture. It is imperative for the C-L psychiatrist to have sound teaching abilities and to be able to educate and communicate his (her) reasoning to the consulting team. It also is important to be well-versed in the psychiatric manifestations of various medical disorders and the psychiatric iatrogenesis of widely used prescription medications.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Ganpat: I think the largest challenge that I have encountered is the lack of resources. Substance abuse is a major problem here, especially opioids, and there are limited community resources for these patients, so they wind up in the hospital.
Dr. Stanciu: What are the disadvantages of C-L compared with other branches of psychiatry?
Dr. Ganpat: There isn’t much continuity of care with C-L psychiatry over the long run, but you do get to see patients improve during the duration of their hospitalization, which is very rewarding.
Dr. Stanciu: What is the typical reimbursement model for a C-L psychiatrist, and have you run into difficulties with insurance providers in this setting?
Dr. Ganpat: The reimbursement model varies from one system to the next. The common model is to bill just as any other hospital service would, based on the time or level of complexity. Obviously, the more consults you have, the more billing is generated. Most insurance carriers recognize this and so I haven’t had much of an issue with reimbursement, although some unexpected problems may arise.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a C-L career?
Dr. Ganpat: If you enjoy working in the hospital and interfacing with other specialties, then consider C-L psychiatry. It is challenging but intellectually stimulating. Make sure you request a C-L rotation during your training, because the Accreditation Council for Graduate Medical Education requires it during a psychiatric residency.
Dr. Stanciu: What is the future outlook of C-L?
Dr. Ganpat: There is a shortage of C-L psychiatrists because >50% of practicing psychiatrists are in private practice in an outpatient setting. Because access to psychiatric care outside of a hospital setting is an issue, and much care is being driven to hospitals, there will be an increasing need for C-L psychiatrists.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he (she) has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Cornel Stanciu, MD, talked with Peter Ganpat, MD, a consultation-liaison (C-L) psychiatrist at Florida Hospital, where he provides guidance to various medical specialties on managing acute and chronic mental illness and substance use disorders. In addition, he also is the medical director for the repetitive transcranial magnetic stimulation service and staffs the inpatient unit.
Dr. Stanciu: What made you choose to become a C-L psychiatrist?
Dr. Ganpat: In my opinion, C-L is the most challenging area of psychiatry because not only are you thinking along the realms of a psychiatrist, but you’re also considering the viewpoint of the other subspecialties at the same time. For me, it brings together my medical background with my passion for psychiatry, and the patients I see daily allow for this incorporation.
Dr. Stanciu: How did your career path prepare you to become a C-L psychiatrist?
Dr. Ganpat: My career path was unique in that I completed a family medicine residency, and then immediately pursued training in psychiatry. Some may consider this as “overkill” for C-L, but as I’ve come to learn, this background grants me a level of understanding and confidence to step in when dealing with a complex case and lend a hand to the consulting physician beyond psychiatry. I do not feel a fellowship is required to practice C-L psychiatry. However, a psychosomatic fellowship will definitely provide the experience needed for this career path, and also will enable one to get a second American Board of Psychiatry and Neurology board certification.
Dr. Stanciu: What types of clinical conditions are you asked to provide input on managing, and how do you find working alongside other specialties?
Dr. Ganpat: I have been managing the full breadth of psychiatry, and in some cases I also provide medical management. Practicing in a metropolitan area with a high influx of tourists also brings in unique cultural cases. The level of respect that the other specialties give is impressive, because they have now seen what a C-L psychiatrist can do. Their performance scores also have improved as a result of my involvement. They greatly appreciate my efforts to shed light on cases or assist with the ever-challenging patient whose psychiatric complexity impedes care.
Dr. Stanciu: How would you describe a physician who is well-suited for such a setting?
Dr. Ganpat: The perfect candidate for this role should be capable of abstract as well as objective thinking. Having a good understanding of the other medical specialties and being able to solve problems is essential, because often it isn’t a clear-cut picture. It is imperative for the C-L psychiatrist to have sound teaching abilities and to be able to educate and communicate his (her) reasoning to the consulting team. It also is important to be well-versed in the psychiatric manifestations of various medical disorders and the psychiatric iatrogenesis of widely used prescription medications.
Dr. Stanciu: What challenges and surprises did you encounter when you first began to practice in this setting?
Dr. Ganpat: I think the largest challenge that I have encountered is the lack of resources. Substance abuse is a major problem here, especially opioids, and there are limited community resources for these patients, so they wind up in the hospital.
Dr. Stanciu: What are the disadvantages of C-L compared with other branches of psychiatry?
Dr. Ganpat: There isn’t much continuity of care with C-L psychiatry over the long run, but you do get to see patients improve during the duration of their hospitalization, which is very rewarding.
Dr. Stanciu: What is the typical reimbursement model for a C-L psychiatrist, and have you run into difficulties with insurance providers in this setting?
Dr. Ganpat: The reimbursement model varies from one system to the next. The common model is to bill just as any other hospital service would, based on the time or level of complexity. Obviously, the more consults you have, the more billing is generated. Most insurance carriers recognize this and so I haven’t had much of an issue with reimbursement, although some unexpected problems may arise.
Dr. Stanciu: What advice do you have for early career psychiatrists and trainees who are contemplating a C-L career?
Dr. Ganpat: If you enjoy working in the hospital and interfacing with other specialties, then consider C-L psychiatry. It is challenging but intellectually stimulating. Make sure you request a C-L rotation during your training, because the Accreditation Council for Graduate Medical Education requires it during a psychiatric residency.
Dr. Stanciu: What is the future outlook of C-L?
Dr. Ganpat: There is a shortage of C-L psychiatrists because >50% of practicing psychiatrists are in private practice in an outpatient setting. Because access to psychiatric care outside of a hospital setting is an issue, and much care is being driven to hospitals, there will be an increasing need for C-L psychiatrists.
Short takes
Pacing in syncope for select patients only
The 2017 ACC/AHA/HRD guideline for syncope evaluation and management concludes that the evidence does not yet support the use of pacing for reflex-mediated except among those with both recurrent vasovagal syncope and asystole documented by implantable loop recorder.
Citation: Varosy P et al. Pacing as a treatment for reflex-mediated (vasovagal, situational, or carotid sinus hypersensitivity) syncope: A systematic review for the 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;20(5):664-79.
Postoperative opioids often underutilized
Between 67% and 92% of patients report postoperative opioid oversupply, defined as filled but unused opioid prescriptions or unfilled opioid prescriptions. Half of the filled prescriptions were unused, with the majority reporting that the narcotics were not stored in locked containers.
Citation: Bicket MC et al. Prescription opioid analgesics commonly unused after surgery: A systematic review. JAMA Surg. 2017 Aug 2. doi: 10.1001/jamasurg.2017.0831
5-hour protocol for contrast allergy safe and effective
Observational study showing a 5-hour IV steroid protocol was noninferior to a traditional 13-hour oral premedication regimen in patients at high risk for IV contrast reactions.
Citation: Mervak BM et al. Intravenous corticosteroid premedication administered 5 hours before CT, compared with a traditional 13-hour oral regimen. Radiology. 2017 Nov;285(2):425-33.
Poor food intake and chills predict true bacteremia in hospitalized patients
Observational study showing that poor food consumption had a sensitivity of 93.7% and shaking chills a specificity of 95.1% in diagnosing true bacteremia based on blood culture results.
Citation: Komatsu T et al. A simple algorithm for predicting bacteremia using food consumption and shaking chills: a prospective observational study. J Hosp Med. 2017 Jul;12(7),510-5.
Lower readmission rates do not lead to increased postdischarge mortality at 30 days
Post–Affordable Care Act reductions in 30-day hospital risk-adjusted readmission rates for heart failure, acute MI, and pneumonia among Medicare beneficiaries did not increase but were weakly associated with decreased 30-day post–hospital discharge risk-adjusted mortality.
Citation: Dharmarajan K et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017Jul 18;318(3):270-8.
Pacing in syncope for select patients only
The 2017 ACC/AHA/HRD guideline for syncope evaluation and management concludes that the evidence does not yet support the use of pacing for reflex-mediated except among those with both recurrent vasovagal syncope and asystole documented by implantable loop recorder.
Citation: Varosy P et al. Pacing as a treatment for reflex-mediated (vasovagal, situational, or carotid sinus hypersensitivity) syncope: A systematic review for the 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;20(5):664-79.
Postoperative opioids often underutilized
Between 67% and 92% of patients report postoperative opioid oversupply, defined as filled but unused opioid prescriptions or unfilled opioid prescriptions. Half of the filled prescriptions were unused, with the majority reporting that the narcotics were not stored in locked containers.
Citation: Bicket MC et al. Prescription opioid analgesics commonly unused after surgery: A systematic review. JAMA Surg. 2017 Aug 2. doi: 10.1001/jamasurg.2017.0831
5-hour protocol for contrast allergy safe and effective
Observational study showing a 5-hour IV steroid protocol was noninferior to a traditional 13-hour oral premedication regimen in patients at high risk for IV contrast reactions.
Citation: Mervak BM et al. Intravenous corticosteroid premedication administered 5 hours before CT, compared with a traditional 13-hour oral regimen. Radiology. 2017 Nov;285(2):425-33.
Poor food intake and chills predict true bacteremia in hospitalized patients
Observational study showing that poor food consumption had a sensitivity of 93.7% and shaking chills a specificity of 95.1% in diagnosing true bacteremia based on blood culture results.
Citation: Komatsu T et al. A simple algorithm for predicting bacteremia using food consumption and shaking chills: a prospective observational study. J Hosp Med. 2017 Jul;12(7),510-5.
Lower readmission rates do not lead to increased postdischarge mortality at 30 days
Post–Affordable Care Act reductions in 30-day hospital risk-adjusted readmission rates for heart failure, acute MI, and pneumonia among Medicare beneficiaries did not increase but were weakly associated with decreased 30-day post–hospital discharge risk-adjusted mortality.
Citation: Dharmarajan K et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017Jul 18;318(3):270-8.
Pacing in syncope for select patients only
The 2017 ACC/AHA/HRD guideline for syncope evaluation and management concludes that the evidence does not yet support the use of pacing for reflex-mediated except among those with both recurrent vasovagal syncope and asystole documented by implantable loop recorder.
Citation: Varosy P et al. Pacing as a treatment for reflex-mediated (vasovagal, situational, or carotid sinus hypersensitivity) syncope: A systematic review for the 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol. 2017 Aug 1;20(5):664-79.
Postoperative opioids often underutilized
Between 67% and 92% of patients report postoperative opioid oversupply, defined as filled but unused opioid prescriptions or unfilled opioid prescriptions. Half of the filled prescriptions were unused, with the majority reporting that the narcotics were not stored in locked containers.
Citation: Bicket MC et al. Prescription opioid analgesics commonly unused after surgery: A systematic review. JAMA Surg. 2017 Aug 2. doi: 10.1001/jamasurg.2017.0831
5-hour protocol for contrast allergy safe and effective
Observational study showing a 5-hour IV steroid protocol was noninferior to a traditional 13-hour oral premedication regimen in patients at high risk for IV contrast reactions.
Citation: Mervak BM et al. Intravenous corticosteroid premedication administered 5 hours before CT, compared with a traditional 13-hour oral regimen. Radiology. 2017 Nov;285(2):425-33.
Poor food intake and chills predict true bacteremia in hospitalized patients
Observational study showing that poor food consumption had a sensitivity of 93.7% and shaking chills a specificity of 95.1% in diagnosing true bacteremia based on blood culture results.
Citation: Komatsu T et al. A simple algorithm for predicting bacteremia using food consumption and shaking chills: a prospective observational study. J Hosp Med. 2017 Jul;12(7),510-5.
Lower readmission rates do not lead to increased postdischarge mortality at 30 days
Post–Affordable Care Act reductions in 30-day hospital risk-adjusted readmission rates for heart failure, acute MI, and pneumonia among Medicare beneficiaries did not increase but were weakly associated with decreased 30-day post–hospital discharge risk-adjusted mortality.
Citation: Dharmarajan K et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017Jul 18;318(3):270-8.
MDedge Daily News: For Zika birth defects, it matters where you live
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Zika-linked birth defects are climbing, elderly trauma patients are at risk after leaving the hospital, junk food can damage the teenage brain, and the Food and Drug Administration has approved a starting dose of roflumilast for COPD patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Zika-linked birth defects are climbing, elderly trauma patients are at risk after leaving the hospital, junk food can damage the teenage brain, and the Food and Drug Administration has approved a starting dose of roflumilast for COPD patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Zika-linked birth defects are climbing, elderly trauma patients are at risk after leaving the hospital, junk food can damage the teenage brain, and the Food and Drug Administration has approved a starting dose of roflumilast for COPD patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
IMPDH inhibitors could treat ALL
A mutation that leads to relapse in patients with acute lymphoblastic leukemia (ALL) also causes a weakness that could be exploited to kill leukemia cells, according to research published in Nature.
Investigators found evidence to suggest that mutations in the NT5C2 gene make leukemic cells resistant to a common chemotherapy drug but vulnerable to a class of drugs called IMPDH inhibitors.
“Increased sensitivity to IMPDH inhibition shows proof of principle that the pathway for resistance provides a new therapeutic target,” said Adolfo Ferrando, MD, PhD, of Columbia University’s Herbert Irving Comprehensive Cancer Center in New York, New York.
Dr Ferrando’s lab had previously found that cancer cells from relapsed ALL patients frequently have mutations in NT5C2, which drives resistance to 6-mercaptopurine.
However, the investigators didn’t know how these mutations emerge as cancer recurs after remission.
In analyzing samples from ALL patients, the team found they could detect the NT5C2 mutation R367Q in cancer cells before patients were clinically diagnosed as relapsed. However, the mutation was not detectable in most cases at the time of diagnosis.
These findings suggest that cells with the R367Q mutation only multiply in response to treatment, and the mutation may help predict relapse.
“This seems to be a late mutation involved in disease progression,” Dr Ferrando said. “Our data support that it may not be present at diagnosis in many cases, and that, in cases where it may be present, it represents a very minor population.”
The investigators also found the R367Q mutation impaired leukemia cell growth and leukemia-initiating cell activity. This was “associated with excess export of purines to the extracellular space and depletion of the intracellular purine-nucleotide pool.”
These findings led the investigators to test mizoribine, an IMPDH inhibitor, in Nt5c2+/R367Q mutant and Nt5c2+/co-R367Q wild-type ALL lymphoblasts. The team found the mutant cells were significantly more sensitive to mizoribine.
In Nt5c2+/R367Q leukemia-bearing mice, treatment with mizoribine produced a “marked” anti-leukemic response and significantly prolonged survival.
In immunodeficient mice transplanted with an NT5C2(R367Q) xenograft, mizoribine decreased tumor burden and tumor infiltration.
“IMPDH inhibitors could eventually emerge as relevant antileukemic drugs, but this would require additional preclinical work before clinical testing,” Dr Ferrando said. ![]()
A mutation that leads to relapse in patients with acute lymphoblastic leukemia (ALL) also causes a weakness that could be exploited to kill leukemia cells, according to research published in Nature.
Investigators found evidence to suggest that mutations in the NT5C2 gene make leukemic cells resistant to a common chemotherapy drug but vulnerable to a class of drugs called IMPDH inhibitors.
“Increased sensitivity to IMPDH inhibition shows proof of principle that the pathway for resistance provides a new therapeutic target,” said Adolfo Ferrando, MD, PhD, of Columbia University’s Herbert Irving Comprehensive Cancer Center in New York, New York.
Dr Ferrando’s lab had previously found that cancer cells from relapsed ALL patients frequently have mutations in NT5C2, which drives resistance to 6-mercaptopurine.
However, the investigators didn’t know how these mutations emerge as cancer recurs after remission.
In analyzing samples from ALL patients, the team found they could detect the NT5C2 mutation R367Q in cancer cells before patients were clinically diagnosed as relapsed. However, the mutation was not detectable in most cases at the time of diagnosis.
These findings suggest that cells with the R367Q mutation only multiply in response to treatment, and the mutation may help predict relapse.
“This seems to be a late mutation involved in disease progression,” Dr Ferrando said. “Our data support that it may not be present at diagnosis in many cases, and that, in cases where it may be present, it represents a very minor population.”
The investigators also found the R367Q mutation impaired leukemia cell growth and leukemia-initiating cell activity. This was “associated with excess export of purines to the extracellular space and depletion of the intracellular purine-nucleotide pool.”
These findings led the investigators to test mizoribine, an IMPDH inhibitor, in Nt5c2+/R367Q mutant and Nt5c2+/co-R367Q wild-type ALL lymphoblasts. The team found the mutant cells were significantly more sensitive to mizoribine.
In Nt5c2+/R367Q leukemia-bearing mice, treatment with mizoribine produced a “marked” anti-leukemic response and significantly prolonged survival.
In immunodeficient mice transplanted with an NT5C2(R367Q) xenograft, mizoribine decreased tumor burden and tumor infiltration.
“IMPDH inhibitors could eventually emerge as relevant antileukemic drugs, but this would require additional preclinical work before clinical testing,” Dr Ferrando said. ![]()
A mutation that leads to relapse in patients with acute lymphoblastic leukemia (ALL) also causes a weakness that could be exploited to kill leukemia cells, according to research published in Nature.
Investigators found evidence to suggest that mutations in the NT5C2 gene make leukemic cells resistant to a common chemotherapy drug but vulnerable to a class of drugs called IMPDH inhibitors.
“Increased sensitivity to IMPDH inhibition shows proof of principle that the pathway for resistance provides a new therapeutic target,” said Adolfo Ferrando, MD, PhD, of Columbia University’s Herbert Irving Comprehensive Cancer Center in New York, New York.
Dr Ferrando’s lab had previously found that cancer cells from relapsed ALL patients frequently have mutations in NT5C2, which drives resistance to 6-mercaptopurine.
However, the investigators didn’t know how these mutations emerge as cancer recurs after remission.
In analyzing samples from ALL patients, the team found they could detect the NT5C2 mutation R367Q in cancer cells before patients were clinically diagnosed as relapsed. However, the mutation was not detectable in most cases at the time of diagnosis.
These findings suggest that cells with the R367Q mutation only multiply in response to treatment, and the mutation may help predict relapse.
“This seems to be a late mutation involved in disease progression,” Dr Ferrando said. “Our data support that it may not be present at diagnosis in many cases, and that, in cases where it may be present, it represents a very minor population.”
The investigators also found the R367Q mutation impaired leukemia cell growth and leukemia-initiating cell activity. This was “associated with excess export of purines to the extracellular space and depletion of the intracellular purine-nucleotide pool.”
These findings led the investigators to test mizoribine, an IMPDH inhibitor, in Nt5c2+/R367Q mutant and Nt5c2+/co-R367Q wild-type ALL lymphoblasts. The team found the mutant cells were significantly more sensitive to mizoribine.
In Nt5c2+/R367Q leukemia-bearing mice, treatment with mizoribine produced a “marked” anti-leukemic response and significantly prolonged survival.
In immunodeficient mice transplanted with an NT5C2(R367Q) xenograft, mizoribine decreased tumor burden and tumor infiltration.
“IMPDH inhibitors could eventually emerge as relevant antileukemic drugs, but this would require additional preclinical work before clinical testing,” Dr Ferrando said. ![]()
How to Investigate Thyroid Nodules Like A Pro
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
Chronic constipation: Practical approaches and novel therapies
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance
While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance

While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance

While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.