User login
Retroperitoneal lymphadenectomy did not impact OS and DFS for high risk, nonmetastatic renal cell carcinoma
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Lymphadenectomy did not improve overall survival or disease-free survival in patients with high-risk, nonmetastatic renal cell carcinoma who received either adjuvant therapy or placebo.
Major finding: There was no overall survival benefit for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20).
Study details: Patients enrolled prospectively in the ASSURE trial.
Disclosures: The study was funded by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Although one author did report conflicts of interest with Pfizer, Novartis, and Argos, the rest reported no conflicts of interest.
Source: Ristau BT et al. J Urol. Jan 2018. doi: 10.1016/j.juro.2017.07.042.
‘Smoker’s paradox’ found in study of IBD patients
Las Vegas – Smoking is more prevalent in Crohn’s disease (CD) patients than in patients with ulcerative colitis (UC), results from a retrospective analysis of national data showed. In addition, , a so-called “smoker’s paradox.”
“This paradox seems to be real, because we know that it has been shown in some heart diseases, that the patients who were smokers had better outcomes,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. In fact, a recent analysis of a nationwide cohort of patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction found that smokers had significantly lower risk‐adjusted in‐hospital mortality, compared with nonsmokers (J Am Heart Assoc. 2016 Apr 22;5:e003370. doi: 10.1161/JAHA.116.003370).
Between 2002 and 2014, a higher proportion of CD patients than UC patients were smokers (25.1% vs. 17.2%; P less than .001), while CD patients who smoked were more likely to be younger than age 50 years, compared with UC patients who smoked (53.9% vs. 36.9%; P less than .001). The researchers also found that African Americans with CD were more likely than were those with UC to smoke (10% vs. 7.8%, respectively; P less than .001). On the other hand, both Hispanics and Asians with UC were more likely to be smokers than were their counterparts with CD (5% vs. 2.9% and 3.4% vs. 2.5%, respectively). From a geographical standpoint, UC patients in the Northeast and Western United States were more likely to be smokers, compared with CD patients in those regions (20.7% vs. 18.3% and 21.4% vs. 15%, respectively). Meanwhile, CD patients in the Midwest and South were more likely to be smokers, compared with UC patients in those regions (29.3% vs 26% and 37.2% vs. 31.9%, respectively).
Dr. Khan and his associates also found that a higher proportion of female CD patients were smokers, compared with female UC patients (57% vs. 47.3%; P less than .001), and that mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
“I would certainly not encourage IBD patients to smoke, but maybe we need to so some more prospective studies to better understand this smoker’s paradox,” Dr. Khan said. He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan et al. Crohn’s & Colitis Congress, Poster 213.
Las Vegas – Smoking is more prevalent in Crohn’s disease (CD) patients than in patients with ulcerative colitis (UC), results from a retrospective analysis of national data showed. In addition, , a so-called “smoker’s paradox.”
“This paradox seems to be real, because we know that it has been shown in some heart diseases, that the patients who were smokers had better outcomes,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. In fact, a recent analysis of a nationwide cohort of patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction found that smokers had significantly lower risk‐adjusted in‐hospital mortality, compared with nonsmokers (J Am Heart Assoc. 2016 Apr 22;5:e003370. doi: 10.1161/JAHA.116.003370).
Between 2002 and 2014, a higher proportion of CD patients than UC patients were smokers (25.1% vs. 17.2%; P less than .001), while CD patients who smoked were more likely to be younger than age 50 years, compared with UC patients who smoked (53.9% vs. 36.9%; P less than .001). The researchers also found that African Americans with CD were more likely than were those with UC to smoke (10% vs. 7.8%, respectively; P less than .001). On the other hand, both Hispanics and Asians with UC were more likely to be smokers than were their counterparts with CD (5% vs. 2.9% and 3.4% vs. 2.5%, respectively). From a geographical standpoint, UC patients in the Northeast and Western United States were more likely to be smokers, compared with CD patients in those regions (20.7% vs. 18.3% and 21.4% vs. 15%, respectively). Meanwhile, CD patients in the Midwest and South were more likely to be smokers, compared with UC patients in those regions (29.3% vs 26% and 37.2% vs. 31.9%, respectively).
Dr. Khan and his associates also found that a higher proportion of female CD patients were smokers, compared with female UC patients (57% vs. 47.3%; P less than .001), and that mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
“I would certainly not encourage IBD patients to smoke, but maybe we need to so some more prospective studies to better understand this smoker’s paradox,” Dr. Khan said. He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan et al. Crohn’s & Colitis Congress, Poster 213.
Las Vegas – Smoking is more prevalent in Crohn’s disease (CD) patients than in patients with ulcerative colitis (UC), results from a retrospective analysis of national data showed. In addition, , a so-called “smoker’s paradox.”
“This paradox seems to be real, because we know that it has been shown in some heart diseases, that the patients who were smokers had better outcomes,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. In fact, a recent analysis of a nationwide cohort of patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction found that smokers had significantly lower risk‐adjusted in‐hospital mortality, compared with nonsmokers (J Am Heart Assoc. 2016 Apr 22;5:e003370. doi: 10.1161/JAHA.116.003370).
Between 2002 and 2014, a higher proportion of CD patients than UC patients were smokers (25.1% vs. 17.2%; P less than .001), while CD patients who smoked were more likely to be younger than age 50 years, compared with UC patients who smoked (53.9% vs. 36.9%; P less than .001). The researchers also found that African Americans with CD were more likely than were those with UC to smoke (10% vs. 7.8%, respectively; P less than .001). On the other hand, both Hispanics and Asians with UC were more likely to be smokers than were their counterparts with CD (5% vs. 2.9% and 3.4% vs. 2.5%, respectively). From a geographical standpoint, UC patients in the Northeast and Western United States were more likely to be smokers, compared with CD patients in those regions (20.7% vs. 18.3% and 21.4% vs. 15%, respectively). Meanwhile, CD patients in the Midwest and South were more likely to be smokers, compared with UC patients in those regions (29.3% vs 26% and 37.2% vs. 31.9%, respectively).
Dr. Khan and his associates also found that a higher proportion of female CD patients were smokers, compared with female UC patients (57% vs. 47.3%; P less than .001), and that mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
“I would certainly not encourage IBD patients to smoke, but maybe we need to so some more prospective studies to better understand this smoker’s paradox,” Dr. Khan said. He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan et al. Crohn’s & Colitis Congress, Poster 213.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: In IBD patients, smoker status was paradoxically associated with mortality and other outcomes.
Major finding: Mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
Study details: An analysis of 22,620 patients with a primary or secondary discharge diagnosis of IBD during 2002-2014.
Disclosures: Dr. Khan reported having no financial disclosures.
Source: Khan et al. Crohn’s & Colitis Congress, Poster 213.
States judged on smoking cessation services
Minnesota and South Carolina are at the top of the class for access to shows that the treatment coverage in most states earned barely passable or failing grades.
In fact, 31 states received either a D (11 states) or an F (20 states) on the grading system. There were also 11 C’s and 7 B’s to go along with the two A’s, the ALA said in “State of Tobacco Control 2018.”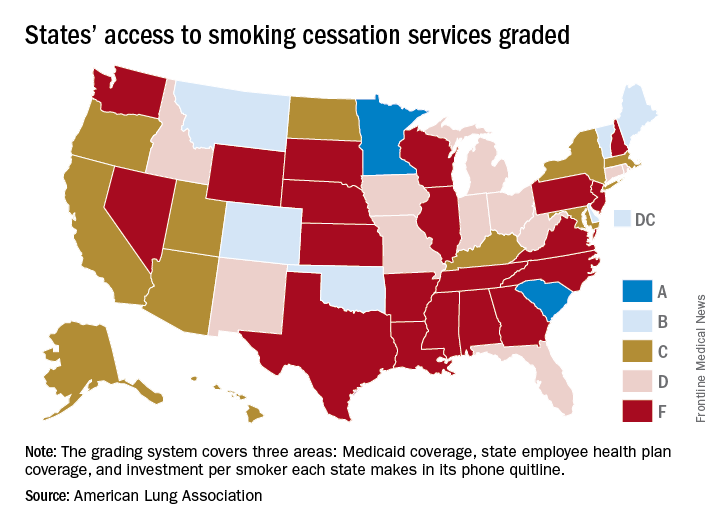
Minnesota received 66 points and South Carolina earned 63 after a 5-point deduction for not expanding Medicaid up to Affordable Care Act standards. The highest-finishing states with B’s were Vermont with 62 points and Maine with 61, and the lowest total score was the 23 points earned by Virginia and Washington, although Washington’s grade did not include the state employee category since the state did not provide data on its plan, the ALA noted.
The Department of Health & Human Services recommends that tobacco cessation coverage include the use of five nicotine-replacement therapies (gum, patch, lozenge, nasal spray, inhaler), bupropion and varenicline (nonnicotine medications), and three types of counseling (individual, group, and phone), the report said.
“It’s imperative that all state Medicaid programs cover a comprehensive tobacco cessation benefit, with no barriers, to help smokers quit, including all seven [Food and Drug Administration]–approved medications and three forms of counseling for Medicaid enrollees. In 2017, only Kentucky, Missouri, and South Carolina provided this coverage,” wrote Harold P. Wimmer, national president and CEO of the ALA.
Minnesota and South Carolina are at the top of the class for access to shows that the treatment coverage in most states earned barely passable or failing grades.
In fact, 31 states received either a D (11 states) or an F (20 states) on the grading system. There were also 11 C’s and 7 B’s to go along with the two A’s, the ALA said in “State of Tobacco Control 2018.”
Minnesota received 66 points and South Carolina earned 63 after a 5-point deduction for not expanding Medicaid up to Affordable Care Act standards. The highest-finishing states with B’s were Vermont with 62 points and Maine with 61, and the lowest total score was the 23 points earned by Virginia and Washington, although Washington’s grade did not include the state employee category since the state did not provide data on its plan, the ALA noted.
The Department of Health & Human Services recommends that tobacco cessation coverage include the use of five nicotine-replacement therapies (gum, patch, lozenge, nasal spray, inhaler), bupropion and varenicline (nonnicotine medications), and three types of counseling (individual, group, and phone), the report said.
“It’s imperative that all state Medicaid programs cover a comprehensive tobacco cessation benefit, with no barriers, to help smokers quit, including all seven [Food and Drug Administration]–approved medications and three forms of counseling for Medicaid enrollees. In 2017, only Kentucky, Missouri, and South Carolina provided this coverage,” wrote Harold P. Wimmer, national president and CEO of the ALA.
Minnesota and South Carolina are at the top of the class for access to shows that the treatment coverage in most states earned barely passable or failing grades.
In fact, 31 states received either a D (11 states) or an F (20 states) on the grading system. There were also 11 C’s and 7 B’s to go along with the two A’s, the ALA said in “State of Tobacco Control 2018.”
Minnesota received 66 points and South Carolina earned 63 after a 5-point deduction for not expanding Medicaid up to Affordable Care Act standards. The highest-finishing states with B’s were Vermont with 62 points and Maine with 61, and the lowest total score was the 23 points earned by Virginia and Washington, although Washington’s grade did not include the state employee category since the state did not provide data on its plan, the ALA noted.
The Department of Health & Human Services recommends that tobacco cessation coverage include the use of five nicotine-replacement therapies (gum, patch, lozenge, nasal spray, inhaler), bupropion and varenicline (nonnicotine medications), and three types of counseling (individual, group, and phone), the report said.
“It’s imperative that all state Medicaid programs cover a comprehensive tobacco cessation benefit, with no barriers, to help smokers quit, including all seven [Food and Drug Administration]–approved medications and three forms of counseling for Medicaid enrollees. In 2017, only Kentucky, Missouri, and South Carolina provided this coverage,” wrote Harold P. Wimmer, national president and CEO of the ALA.
Postcolonoscopy cancer rates persist despite quality protocols
The number of colorectal cancers diagnosed after a colonoscopy remained consistent at approximately 8% over a 15-year period despite the introduction of quality improvement measures, according to data from a population-based cohort study of more than 1 million individuals in Canada.
“It is believed that the majority of PCCRCs [postcolonoscopy colorectal cancers] arise due to cancers or near cancers that were either missed or incompletely treated during colonoscopy,” wrote Sanjay K. Murthy, MD, of the University of Ottawa, and colleagues.
Established quality improvement measures included adenoma detection rate, cecal intubation rate, colonoscopy withdrawal time, and endoscopy training standards, but how well the measures have been implemented remains uncertain, the researchers said. In a study published in Gastrointestinal Endoscopy (2018 Jan 6. doi: 10.1016/j.gie.2017.12.027), the researchers assessed data from 1,093,658 eligible adults aged 50-74 years over a 15-year period. The time period was divided into three sections: July 1, 1996, to June 30, 2001; July 1, 2001, to June 30, 2006; and July 1, 2006, to Dec. 31, 2010.
Overall, the number of colonoscopy procedures increased during the study period, from 305 per 10,000 people in 1996-1997 to 870 per 10,000 people in 2010-2011, and the percentage of individuals who underwent complete colonoscopies increased from 67% in the 1996-2001 period to 88% in the 2006-2010 period. “There was a considerable increase in the proportion of colonoscopies performed in younger age groups and community clinics in successive study periods,” the researchers noted.
Comparing the 2006-2010 and 1996-2001 time periods yielded adjusted odds of PCCRC, distal PCCRC, and proximal PCCRC of 1.14, 1.11, and 1.14, respectively; the trends were not affected by endoscopist specialty or institutional setting.
“Our findings are concerning for lack of improvement in colonoscopy practice quality in Ontario, particularly in the wake of greater emphasis having been placed on colonoscopy quality metrics during the study period,” the researchers said. The findings contrast with the decline in PCCRC rates in the United Kingdom reported in a previous study of a similar time period, they noted.
The study findings were limited by several factors, including possible patient and outcome misclassification, an unvalidated definition for PCCRC, and unmeasured confounders such as changes in practice or changes in the definition of PCCRC. Although more research is needed in other jurisdictions to confirm, the results “call for increased population-based practice audit as well as endoscopy educational programs and certification requirements.”
The study was supported by a research grant to Dr. Murthy from the University of Ottawa. The researchers had no financial conflicts to disclose.
SOURCE: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027
Postcolonoscopy colorectal cancers (PCCRCs) are those cancers that occur between 6 and 36 months after a complete colonoscopy. For cancers diagnosed less than 6 months from exam, it is presumed that the exam itself was diagnostic. Most of these cancers grow from cancers or near cancers missed or incompletely resected during the baseline colonoscopy. Clinical researchers have published extensively about reasons for missed lesions and we know that age, female sex, and proximal location of cancers increase rates of PCCRC. GI societies worldwide have developed training initiatives, performance metrics (adenoma detection rate or ADR, withdrawal time, and prep quality documentation), and postcolonoscopy guidelines, all intended to mitigate risk of PCCRCs. It would be nice to know whether such efforts have made a difference.
Murthy and colleagues studied PCCRC rates in Ottawa, Canada during three different time periods to determine whether quality and educational efforts impacted PCCRC rates. More than 99% of this population has health care covered under a single public payer system where all encounters are carefully tracked. Using population-level health data derived from over 1 million people (screen eligible people, 50-74 years of age with low to moderate CRC risk) they identified cancers diagnosed within 36 months of a colonoscopy and compared three 5-year periods (1996-2001, 2001-2006, and 2006-2010).
Their method of calculating PCCRC rates essentially says, “If I am destined to develop CRC in the next 3 years, what is my chance of a false-negative colonoscopy?” There are five published methods of calculating PCCRC rates (summarized in Gut 2015;64:1248-56) and each method uses different inclusion criteria and denominators. The question posed above yields “rates” that would terrify patients (4%-10%) without a detailed explanation (it took me about an hour of focused attention to finally understand this methodology). In essence, if we could, a priori, identify and examine only patients who have a prevalent cancer or near cancer, how close can we come to 100% accuracy with a colonoscopy? Turns out, that rate is somewhere between 90% and 96% and really hasn’t changed over time. Thus, these studies speak to the impact of our efforts around colonoscopy quality.
John I. Allen MD, MBA, AGAF, professor of medicine, department of gastroenterology and hepatology, University of Michigan, Ann Arbor, and Editor in Chief of GI & Hepatology News.
Postcolonoscopy colorectal cancers (PCCRCs) are those cancers that occur between 6 and 36 months after a complete colonoscopy. For cancers diagnosed less than 6 months from exam, it is presumed that the exam itself was diagnostic. Most of these cancers grow from cancers or near cancers missed or incompletely resected during the baseline colonoscopy. Clinical researchers have published extensively about reasons for missed lesions and we know that age, female sex, and proximal location of cancers increase rates of PCCRC. GI societies worldwide have developed training initiatives, performance metrics (adenoma detection rate or ADR, withdrawal time, and prep quality documentation), and postcolonoscopy guidelines, all intended to mitigate risk of PCCRCs. It would be nice to know whether such efforts have made a difference.
Murthy and colleagues studied PCCRC rates in Ottawa, Canada during three different time periods to determine whether quality and educational efforts impacted PCCRC rates. More than 99% of this population has health care covered under a single public payer system where all encounters are carefully tracked. Using population-level health data derived from over 1 million people (screen eligible people, 50-74 years of age with low to moderate CRC risk) they identified cancers diagnosed within 36 months of a colonoscopy and compared three 5-year periods (1996-2001, 2001-2006, and 2006-2010).
Their method of calculating PCCRC rates essentially says, “If I am destined to develop CRC in the next 3 years, what is my chance of a false-negative colonoscopy?” There are five published methods of calculating PCCRC rates (summarized in Gut 2015;64:1248-56) and each method uses different inclusion criteria and denominators. The question posed above yields “rates” that would terrify patients (4%-10%) without a detailed explanation (it took me about an hour of focused attention to finally understand this methodology). In essence, if we could, a priori, identify and examine only patients who have a prevalent cancer or near cancer, how close can we come to 100% accuracy with a colonoscopy? Turns out, that rate is somewhere between 90% and 96% and really hasn’t changed over time. Thus, these studies speak to the impact of our efforts around colonoscopy quality.
John I. Allen MD, MBA, AGAF, professor of medicine, department of gastroenterology and hepatology, University of Michigan, Ann Arbor, and Editor in Chief of GI & Hepatology News.
Postcolonoscopy colorectal cancers (PCCRCs) are those cancers that occur between 6 and 36 months after a complete colonoscopy. For cancers diagnosed less than 6 months from exam, it is presumed that the exam itself was diagnostic. Most of these cancers grow from cancers or near cancers missed or incompletely resected during the baseline colonoscopy. Clinical researchers have published extensively about reasons for missed lesions and we know that age, female sex, and proximal location of cancers increase rates of PCCRC. GI societies worldwide have developed training initiatives, performance metrics (adenoma detection rate or ADR, withdrawal time, and prep quality documentation), and postcolonoscopy guidelines, all intended to mitigate risk of PCCRCs. It would be nice to know whether such efforts have made a difference.
Murthy and colleagues studied PCCRC rates in Ottawa, Canada during three different time periods to determine whether quality and educational efforts impacted PCCRC rates. More than 99% of this population has health care covered under a single public payer system where all encounters are carefully tracked. Using population-level health data derived from over 1 million people (screen eligible people, 50-74 years of age with low to moderate CRC risk) they identified cancers diagnosed within 36 months of a colonoscopy and compared three 5-year periods (1996-2001, 2001-2006, and 2006-2010).
Their method of calculating PCCRC rates essentially says, “If I am destined to develop CRC in the next 3 years, what is my chance of a false-negative colonoscopy?” There are five published methods of calculating PCCRC rates (summarized in Gut 2015;64:1248-56) and each method uses different inclusion criteria and denominators. The question posed above yields “rates” that would terrify patients (4%-10%) without a detailed explanation (it took me about an hour of focused attention to finally understand this methodology). In essence, if we could, a priori, identify and examine only patients who have a prevalent cancer or near cancer, how close can we come to 100% accuracy with a colonoscopy? Turns out, that rate is somewhere between 90% and 96% and really hasn’t changed over time. Thus, these studies speak to the impact of our efforts around colonoscopy quality.
John I. Allen MD, MBA, AGAF, professor of medicine, department of gastroenterology and hepatology, University of Michigan, Ann Arbor, and Editor in Chief of GI & Hepatology News.
The number of colorectal cancers diagnosed after a colonoscopy remained consistent at approximately 8% over a 15-year period despite the introduction of quality improvement measures, according to data from a population-based cohort study of more than 1 million individuals in Canada.
“It is believed that the majority of PCCRCs [postcolonoscopy colorectal cancers] arise due to cancers or near cancers that were either missed or incompletely treated during colonoscopy,” wrote Sanjay K. Murthy, MD, of the University of Ottawa, and colleagues.
Established quality improvement measures included adenoma detection rate, cecal intubation rate, colonoscopy withdrawal time, and endoscopy training standards, but how well the measures have been implemented remains uncertain, the researchers said. In a study published in Gastrointestinal Endoscopy (2018 Jan 6. doi: 10.1016/j.gie.2017.12.027), the researchers assessed data from 1,093,658 eligible adults aged 50-74 years over a 15-year period. The time period was divided into three sections: July 1, 1996, to June 30, 2001; July 1, 2001, to June 30, 2006; and July 1, 2006, to Dec. 31, 2010.
Overall, the number of colonoscopy procedures increased during the study period, from 305 per 10,000 people in 1996-1997 to 870 per 10,000 people in 2010-2011, and the percentage of individuals who underwent complete colonoscopies increased from 67% in the 1996-2001 period to 88% in the 2006-2010 period. “There was a considerable increase in the proportion of colonoscopies performed in younger age groups and community clinics in successive study periods,” the researchers noted.
Comparing the 2006-2010 and 1996-2001 time periods yielded adjusted odds of PCCRC, distal PCCRC, and proximal PCCRC of 1.14, 1.11, and 1.14, respectively; the trends were not affected by endoscopist specialty or institutional setting.
“Our findings are concerning for lack of improvement in colonoscopy practice quality in Ontario, particularly in the wake of greater emphasis having been placed on colonoscopy quality metrics during the study period,” the researchers said. The findings contrast with the decline in PCCRC rates in the United Kingdom reported in a previous study of a similar time period, they noted.
The study findings were limited by several factors, including possible patient and outcome misclassification, an unvalidated definition for PCCRC, and unmeasured confounders such as changes in practice or changes in the definition of PCCRC. Although more research is needed in other jurisdictions to confirm, the results “call for increased population-based practice audit as well as endoscopy educational programs and certification requirements.”
The study was supported by a research grant to Dr. Murthy from the University of Ottawa. The researchers had no financial conflicts to disclose.
SOURCE: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027
The number of colorectal cancers diagnosed after a colonoscopy remained consistent at approximately 8% over a 15-year period despite the introduction of quality improvement measures, according to data from a population-based cohort study of more than 1 million individuals in Canada.
“It is believed that the majority of PCCRCs [postcolonoscopy colorectal cancers] arise due to cancers or near cancers that were either missed or incompletely treated during colonoscopy,” wrote Sanjay K. Murthy, MD, of the University of Ottawa, and colleagues.
Established quality improvement measures included adenoma detection rate, cecal intubation rate, colonoscopy withdrawal time, and endoscopy training standards, but how well the measures have been implemented remains uncertain, the researchers said. In a study published in Gastrointestinal Endoscopy (2018 Jan 6. doi: 10.1016/j.gie.2017.12.027), the researchers assessed data from 1,093,658 eligible adults aged 50-74 years over a 15-year period. The time period was divided into three sections: July 1, 1996, to June 30, 2001; July 1, 2001, to June 30, 2006; and July 1, 2006, to Dec. 31, 2010.
Overall, the number of colonoscopy procedures increased during the study period, from 305 per 10,000 people in 1996-1997 to 870 per 10,000 people in 2010-2011, and the percentage of individuals who underwent complete colonoscopies increased from 67% in the 1996-2001 period to 88% in the 2006-2010 period. “There was a considerable increase in the proportion of colonoscopies performed in younger age groups and community clinics in successive study periods,” the researchers noted.
Comparing the 2006-2010 and 1996-2001 time periods yielded adjusted odds of PCCRC, distal PCCRC, and proximal PCCRC of 1.14, 1.11, and 1.14, respectively; the trends were not affected by endoscopist specialty or institutional setting.
“Our findings are concerning for lack of improvement in colonoscopy practice quality in Ontario, particularly in the wake of greater emphasis having been placed on colonoscopy quality metrics during the study period,” the researchers said. The findings contrast with the decline in PCCRC rates in the United Kingdom reported in a previous study of a similar time period, they noted.
The study findings were limited by several factors, including possible patient and outcome misclassification, an unvalidated definition for PCCRC, and unmeasured confounders such as changes in practice or changes in the definition of PCCRC. Although more research is needed in other jurisdictions to confirm, the results “call for increased population-based practice audit as well as endoscopy educational programs and certification requirements.”
The study was supported by a research grant to Dr. Murthy from the University of Ottawa. The researchers had no financial conflicts to disclose.
SOURCE: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Rates of postcolonoscopy colorectal cancer have not declined despite the introduction of quality improvement measures.
Major finding: The rate of colorectal cancers diagnosed after a colonoscopy has remained at approximately 8% over the past 15 years.
Study details: A population-based retrospective cohort study of Canadian adults aged 50-74 years without risk factors for CRC.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027.
Beta-blocker therapy post AMI
reported that the treatment of AMI in patients with propranolol, the beta-blocker of the day, reduced mortality by over 25% over 3 years (JAMA. 1981 Nov 6;246[18]:2073-4), and a European trial of timolol confirmed those findings.
New medications have been developed, including ACE inhibitors, statins, and a variety of drugs that modify the thrombotic process that occurs with the event. In addition, endovascular procedures have modified the obstructive coronary vascular anatomy that precipitated the event. At the same time, the definition of an AMI has changed dramatically, now depending in many instances on transitory elevation of the highly sensitive troponins. The BHAT definition depended largely on electrocardiographic changes associated with the event, which were ST-segment elevations in 79% of the occurrences, or significant ST-segment changes associated often with elevation of the insensitive enzyme, serum glutamic transaminase, and significant symptoms. The characteristics of the BHAT patient only faintly resemble the patients who we now classify with AMIs, and its definition expanded well beyond the BHAT patients. And yet beta-blocker therapy is still part of the class I or II recommendations for the treatment of an AMI.
Reasonable questions have been raised as to the wisdom of throwing the full bag of therapy at this population rather than a more selective choice of drugs. In order to gain further insight into the benefits of beta-blocker therapy in the total spectrum of post MI therapy, large populations studies using a variety of statistical gymnastics have tried to identify the unique benefit that can be attributed to beta-blocker therapy relative to the other drugs and surgical interventions used in the post-AMI populations. The result has been a mixed message without any clear guidance. One can make a good case for carrying out clinical trials in specific subsets of the currently defined post-AMI population. Such an endeavor is unlikely. Pharma is clearly not interested in old drug research and the National Heart, Lung, and Blood Institute doesn’t have the funds. So it comes down to clinician’s decisions.
As an avowed beta-blocker enthusiast, I use it in most of my post-AMI patients, particularly if they have left ventricular dysfunction, and in patients with concomitant hypertension. I do not use it in patents with transitory troponin elevations without convincing evidence of clinical symptoms. I will continue beta-blockade as long as I am able to write the prescription or until someone has answered the question.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
reported that the treatment of AMI in patients with propranolol, the beta-blocker of the day, reduced mortality by over 25% over 3 years (JAMA. 1981 Nov 6;246[18]:2073-4), and a European trial of timolol confirmed those findings.
New medications have been developed, including ACE inhibitors, statins, and a variety of drugs that modify the thrombotic process that occurs with the event. In addition, endovascular procedures have modified the obstructive coronary vascular anatomy that precipitated the event. At the same time, the definition of an AMI has changed dramatically, now depending in many instances on transitory elevation of the highly sensitive troponins. The BHAT definition depended largely on electrocardiographic changes associated with the event, which were ST-segment elevations in 79% of the occurrences, or significant ST-segment changes associated often with elevation of the insensitive enzyme, serum glutamic transaminase, and significant symptoms. The characteristics of the BHAT patient only faintly resemble the patients who we now classify with AMIs, and its definition expanded well beyond the BHAT patients. And yet beta-blocker therapy is still part of the class I or II recommendations for the treatment of an AMI.
Reasonable questions have been raised as to the wisdom of throwing the full bag of therapy at this population rather than a more selective choice of drugs. In order to gain further insight into the benefits of beta-blocker therapy in the total spectrum of post MI therapy, large populations studies using a variety of statistical gymnastics have tried to identify the unique benefit that can be attributed to beta-blocker therapy relative to the other drugs and surgical interventions used in the post-AMI populations. The result has been a mixed message without any clear guidance. One can make a good case for carrying out clinical trials in specific subsets of the currently defined post-AMI population. Such an endeavor is unlikely. Pharma is clearly not interested in old drug research and the National Heart, Lung, and Blood Institute doesn’t have the funds. So it comes down to clinician’s decisions.
As an avowed beta-blocker enthusiast, I use it in most of my post-AMI patients, particularly if they have left ventricular dysfunction, and in patients with concomitant hypertension. I do not use it in patents with transitory troponin elevations without convincing evidence of clinical symptoms. I will continue beta-blockade as long as I am able to write the prescription or until someone has answered the question.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
reported that the treatment of AMI in patients with propranolol, the beta-blocker of the day, reduced mortality by over 25% over 3 years (JAMA. 1981 Nov 6;246[18]:2073-4), and a European trial of timolol confirmed those findings.
New medications have been developed, including ACE inhibitors, statins, and a variety of drugs that modify the thrombotic process that occurs with the event. In addition, endovascular procedures have modified the obstructive coronary vascular anatomy that precipitated the event. At the same time, the definition of an AMI has changed dramatically, now depending in many instances on transitory elevation of the highly sensitive troponins. The BHAT definition depended largely on electrocardiographic changes associated with the event, which were ST-segment elevations in 79% of the occurrences, or significant ST-segment changes associated often with elevation of the insensitive enzyme, serum glutamic transaminase, and significant symptoms. The characteristics of the BHAT patient only faintly resemble the patients who we now classify with AMIs, and its definition expanded well beyond the BHAT patients. And yet beta-blocker therapy is still part of the class I or II recommendations for the treatment of an AMI.
Reasonable questions have been raised as to the wisdom of throwing the full bag of therapy at this population rather than a more selective choice of drugs. In order to gain further insight into the benefits of beta-blocker therapy in the total spectrum of post MI therapy, large populations studies using a variety of statistical gymnastics have tried to identify the unique benefit that can be attributed to beta-blocker therapy relative to the other drugs and surgical interventions used in the post-AMI populations. The result has been a mixed message without any clear guidance. One can make a good case for carrying out clinical trials in specific subsets of the currently defined post-AMI population. Such an endeavor is unlikely. Pharma is clearly not interested in old drug research and the National Heart, Lung, and Blood Institute doesn’t have the funds. So it comes down to clinician’s decisions.
As an avowed beta-blocker enthusiast, I use it in most of my post-AMI patients, particularly if they have left ventricular dysfunction, and in patients with concomitant hypertension. I do not use it in patents with transitory troponin elevations without convincing evidence of clinical symptoms. I will continue beta-blockade as long as I am able to write the prescription or until someone has answered the question.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
First-line ramucirumab nets small PFS benefit in gastric cancer
SAN FRANCISCO – Adding ramucirumab to first-line chemotherapy for gastric and gastroesophageal junction cancer prolongs progression-free survival, but not by much, according to results of the phase 3 international RAINFALL trial.
A total of 645 patients were randomized to receive chemotherapy (cisplatin with capecitabine or 5FU) plus either placebo or ramucirumab (Cyramza), an antibody that targets human vascular endothelial growth factor receptor 2. The antiangiogenic antibody has been found to prolong overall survival in the second-line setting, as shown in the REGARD monotherapy and RAINBOW combination therapy trials.
“In patients with treatment-naive gastric and gastroesophageal junction adenocarcinoma, the addition of ramucirumab to first-line chemotherapy conferred a significant improvement in the primary endpoint of investigator-assessed progression-free survival, but did not confer any improvement in overall survival,” said lead investigator Charles S. Fuchs, MD, director of the Yale Cancer Center and physician-in-chief of the Smilow Cancer Hospital, New Haven, Connecticut. “Ramucirumab in combination with first-line chemotherapy did appear to be well tolerated.”
A session attendee asked, “Given the high cost and limited benefit in progression-free survival, what do you think of the statistical significance versus the clinical significance of the findings of this study?”
“We did have a statistically significant benefit in progression-free survival but did not see a survival benefit by any measure, so I wouldn’t purport that ramucirumab should be a first-line therapy,” Dr. Fuchs replied. “Should it supplant existing therapy in the first line? I don’t think the data support that.”
Clinical implications
“RAINFALL did meet its primary endpoint of progression-free survival. However, it was disappointing not to see some survival benefit,” commented invited discussant Stephen Leong, MD, of the University of Colorado Comprehensive Cancer Center, Denver. “Ramucirumab’s role in the first-line setting is debatable.”
The gain in median progression-free survival of 0.3 months, or 9 days, comes at an approximate drug cost of $67,112, he noted. And that does not include port or infusion costs.
The trial still leaves some important questions to be answered, he said. In particular, the secondary endpoint of quality of life and biomarker analyses have not yet been reported.
“Will ramucirumab get FDA approval for a first-line indication? No. The answer is quite simple. [The manufacturer] has already indicated that they are not going to pursue a first-line indication,” Dr. Leong said.
“Will this replace our current standard of care? Right now the standard of care is the doublet or triplet with a 5FU or platinum backbone. This is not going to replace that,” he said. Finally, “will the NCCN add ramucirumab to the first-line setting? This will be debated shortly.”
Study details
In RAINFALL, median investigator-assessed progression-free survival in the final intention-to-treat analysis was 5.85 months with ramucirumab and 5.55 months with placebo (hazard ratio, 0.75; P = .0024), Dr. Fuchs reported at the symposium, which is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Median overall survival was 11.17 months with ramucirumab and 10.74 months with placebo, a nonsignificant difference. Findings for both endpoints were consistent across subgroups stratified by patient, disease, and treatment characteristics.
The overall response rate was 41% and 36%, respectively (P = .17), and the disease control rate was 82% and 77%, respectively (P = .10).
Treatment-emergent adverse events of grade 3 or worse were generally similar for the two groups, Dr. Fuchs reported. Patients in the ramucirumab group had higher rates of certain grade 3 or worse adverse events known to be related to the antibody’s mechanism of action (26% vs. 15%), such as hypertension (9.9% vs 1.5%) and gastrointestinal perforation (4.0% vs. 0.3%).
After the study, about half of patients in each group went on to receive additional (second-line) systemic therapy, mainly paclitaxel and (more) ramucirumab.
In an exploratory analysis, patients who received poststudy ramucirumab versus some other systemic therapy tended to have better overall survival, whether originally in the ramucirumab group (16.2 vs. 13.2 months) or the placebo group (14.9 vs. 13.0 months). Findings were similar when overall survival was assessed from initiation of the poststudy therapy.
Dr. Fuchs disclosed that he is a consultant to Agios, Bayer, Eli Lilly, Entrinsic Health, Five Prime Therapeutics, Genentech, Merck, Sanofi, and Taiho Pharmaceutical, and that he is on the board of directors of CytomX. The trial was sponsored by Eli Lilly.
SOURCE: Fuchs CS et al. GI CANCERS SYMPOSIUM Abstract 5
SAN FRANCISCO – Adding ramucirumab to first-line chemotherapy for gastric and gastroesophageal junction cancer prolongs progression-free survival, but not by much, according to results of the phase 3 international RAINFALL trial.
A total of 645 patients were randomized to receive chemotherapy (cisplatin with capecitabine or 5FU) plus either placebo or ramucirumab (Cyramza), an antibody that targets human vascular endothelial growth factor receptor 2. The antiangiogenic antibody has been found to prolong overall survival in the second-line setting, as shown in the REGARD monotherapy and RAINBOW combination therapy trials.
“In patients with treatment-naive gastric and gastroesophageal junction adenocarcinoma, the addition of ramucirumab to first-line chemotherapy conferred a significant improvement in the primary endpoint of investigator-assessed progression-free survival, but did not confer any improvement in overall survival,” said lead investigator Charles S. Fuchs, MD, director of the Yale Cancer Center and physician-in-chief of the Smilow Cancer Hospital, New Haven, Connecticut. “Ramucirumab in combination with first-line chemotherapy did appear to be well tolerated.”
A session attendee asked, “Given the high cost and limited benefit in progression-free survival, what do you think of the statistical significance versus the clinical significance of the findings of this study?”
“We did have a statistically significant benefit in progression-free survival but did not see a survival benefit by any measure, so I wouldn’t purport that ramucirumab should be a first-line therapy,” Dr. Fuchs replied. “Should it supplant existing therapy in the first line? I don’t think the data support that.”
Clinical implications
“RAINFALL did meet its primary endpoint of progression-free survival. However, it was disappointing not to see some survival benefit,” commented invited discussant Stephen Leong, MD, of the University of Colorado Comprehensive Cancer Center, Denver. “Ramucirumab’s role in the first-line setting is debatable.”
The gain in median progression-free survival of 0.3 months, or 9 days, comes at an approximate drug cost of $67,112, he noted. And that does not include port or infusion costs.
The trial still leaves some important questions to be answered, he said. In particular, the secondary endpoint of quality of life and biomarker analyses have not yet been reported.
“Will ramucirumab get FDA approval for a first-line indication? No. The answer is quite simple. [The manufacturer] has already indicated that they are not going to pursue a first-line indication,” Dr. Leong said.
“Will this replace our current standard of care? Right now the standard of care is the doublet or triplet with a 5FU or platinum backbone. This is not going to replace that,” he said. Finally, “will the NCCN add ramucirumab to the first-line setting? This will be debated shortly.”
Study details
In RAINFALL, median investigator-assessed progression-free survival in the final intention-to-treat analysis was 5.85 months with ramucirumab and 5.55 months with placebo (hazard ratio, 0.75; P = .0024), Dr. Fuchs reported at the symposium, which is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Median overall survival was 11.17 months with ramucirumab and 10.74 months with placebo, a nonsignificant difference. Findings for both endpoints were consistent across subgroups stratified by patient, disease, and treatment characteristics.
The overall response rate was 41% and 36%, respectively (P = .17), and the disease control rate was 82% and 77%, respectively (P = .10).
Treatment-emergent adverse events of grade 3 or worse were generally similar for the two groups, Dr. Fuchs reported. Patients in the ramucirumab group had higher rates of certain grade 3 or worse adverse events known to be related to the antibody’s mechanism of action (26% vs. 15%), such as hypertension (9.9% vs 1.5%) and gastrointestinal perforation (4.0% vs. 0.3%).
After the study, about half of patients in each group went on to receive additional (second-line) systemic therapy, mainly paclitaxel and (more) ramucirumab.
In an exploratory analysis, patients who received poststudy ramucirumab versus some other systemic therapy tended to have better overall survival, whether originally in the ramucirumab group (16.2 vs. 13.2 months) or the placebo group (14.9 vs. 13.0 months). Findings were similar when overall survival was assessed from initiation of the poststudy therapy.
Dr. Fuchs disclosed that he is a consultant to Agios, Bayer, Eli Lilly, Entrinsic Health, Five Prime Therapeutics, Genentech, Merck, Sanofi, and Taiho Pharmaceutical, and that he is on the board of directors of CytomX. The trial was sponsored by Eli Lilly.
SOURCE: Fuchs CS et al. GI CANCERS SYMPOSIUM Abstract 5
SAN FRANCISCO – Adding ramucirumab to first-line chemotherapy for gastric and gastroesophageal junction cancer prolongs progression-free survival, but not by much, according to results of the phase 3 international RAINFALL trial.
A total of 645 patients were randomized to receive chemotherapy (cisplatin with capecitabine or 5FU) plus either placebo or ramucirumab (Cyramza), an antibody that targets human vascular endothelial growth factor receptor 2. The antiangiogenic antibody has been found to prolong overall survival in the second-line setting, as shown in the REGARD monotherapy and RAINBOW combination therapy trials.
“In patients with treatment-naive gastric and gastroesophageal junction adenocarcinoma, the addition of ramucirumab to first-line chemotherapy conferred a significant improvement in the primary endpoint of investigator-assessed progression-free survival, but did not confer any improvement in overall survival,” said lead investigator Charles S. Fuchs, MD, director of the Yale Cancer Center and physician-in-chief of the Smilow Cancer Hospital, New Haven, Connecticut. “Ramucirumab in combination with first-line chemotherapy did appear to be well tolerated.”
A session attendee asked, “Given the high cost and limited benefit in progression-free survival, what do you think of the statistical significance versus the clinical significance of the findings of this study?”
“We did have a statistically significant benefit in progression-free survival but did not see a survival benefit by any measure, so I wouldn’t purport that ramucirumab should be a first-line therapy,” Dr. Fuchs replied. “Should it supplant existing therapy in the first line? I don’t think the data support that.”
Clinical implications
“RAINFALL did meet its primary endpoint of progression-free survival. However, it was disappointing not to see some survival benefit,” commented invited discussant Stephen Leong, MD, of the University of Colorado Comprehensive Cancer Center, Denver. “Ramucirumab’s role in the first-line setting is debatable.”
The gain in median progression-free survival of 0.3 months, or 9 days, comes at an approximate drug cost of $67,112, he noted. And that does not include port or infusion costs.
The trial still leaves some important questions to be answered, he said. In particular, the secondary endpoint of quality of life and biomarker analyses have not yet been reported.
“Will ramucirumab get FDA approval for a first-line indication? No. The answer is quite simple. [The manufacturer] has already indicated that they are not going to pursue a first-line indication,” Dr. Leong said.
“Will this replace our current standard of care? Right now the standard of care is the doublet or triplet with a 5FU or platinum backbone. This is not going to replace that,” he said. Finally, “will the NCCN add ramucirumab to the first-line setting? This will be debated shortly.”
Study details
In RAINFALL, median investigator-assessed progression-free survival in the final intention-to-treat analysis was 5.85 months with ramucirumab and 5.55 months with placebo (hazard ratio, 0.75; P = .0024), Dr. Fuchs reported at the symposium, which is sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Median overall survival was 11.17 months with ramucirumab and 10.74 months with placebo, a nonsignificant difference. Findings for both endpoints were consistent across subgroups stratified by patient, disease, and treatment characteristics.
The overall response rate was 41% and 36%, respectively (P = .17), and the disease control rate was 82% and 77%, respectively (P = .10).
Treatment-emergent adverse events of grade 3 or worse were generally similar for the two groups, Dr. Fuchs reported. Patients in the ramucirumab group had higher rates of certain grade 3 or worse adverse events known to be related to the antibody’s mechanism of action (26% vs. 15%), such as hypertension (9.9% vs 1.5%) and gastrointestinal perforation (4.0% vs. 0.3%).
After the study, about half of patients in each group went on to receive additional (second-line) systemic therapy, mainly paclitaxel and (more) ramucirumab.
In an exploratory analysis, patients who received poststudy ramucirumab versus some other systemic therapy tended to have better overall survival, whether originally in the ramucirumab group (16.2 vs. 13.2 months) or the placebo group (14.9 vs. 13.0 months). Findings were similar when overall survival was assessed from initiation of the poststudy therapy.
Dr. Fuchs disclosed that he is a consultant to Agios, Bayer, Eli Lilly, Entrinsic Health, Five Prime Therapeutics, Genentech, Merck, Sanofi, and Taiho Pharmaceutical, and that he is on the board of directors of CytomX. The trial was sponsored by Eli Lilly.
SOURCE: Fuchs CS et al. GI CANCERS SYMPOSIUM Abstract 5
REPORTING FROM THE 2018 GI CANCERS SYMPOSIUM
Key clinical point:
Major finding: Median progression-free survival was 5.85 months with ramucirumab and 5.55 months with placebo (hazard ratio, 0.75; P = .0024).
Data source: A phase 3 randomized, controlled trial among 645 patients with metastatic gastric or gastroesophageal junction adenocarcinoma given first-line chemotherapy plus ramucirumab or placebo (RAINFALL trial).
Disclosures: Dr. Fuchs disclosed that he is a consultant to Agios, Bayer, Eli Lilly, Entrinsic Health, Five Prime Therapeutics, Genentech, Merck, Sanofi, and Taiho Pharmaceutical, and that he is on the board of directors of CytomX. Eli Lilly sponsored the trial.
Source: Fuchs CS et al. GI CANCERS SYMPOSIUM Abstract 5.
Expert: Eat walnuts!
ANAHEIM, CALIF. – Eating an ounce and a half of walnuts daily led to favorable changes in gut microbiome composition and diversity in a prospective randomized controlled trial, Klaus G. Parhofer, MD, reported at the American Heart Association scientific session.
These changes in intestinal flora may account for the reductions in LDL cholesterol, triglycerides, apolipoprotein B, and non-HDL cholesterol previously documented with walnut consumption in the same trial, according to Dr. Parhofer, professor of endocrinology and metabolism at the University of Munich.
The gut microbiome composition during the walnut and control phases differed by about 5%. The proportion of the microbiome composed of probiotic and butyric acid–producing organisms in the phylum Bacteroidetes – especially Ruminococcacaeae and Bifidobacteria – increased during the walnut-eating phase, while the Clostridium cluster XIVa microorganisms in the genera Blautia and Anaerostipes decreased significantly, compared with the control diet.
“It is unclear whether these changes are preserved during longer walnut consumption and how these changes can be associated with the observed changes in lipid metabolism,” the endocrinologist noted.
An earlier report focused on the fasting lipid reductions seen in response to walnut consumption in the full study population. LDL cholesterol fell by an average of 7.4 mg/dL after 8 weeks of daily walnut consumption, compared with a 1.7-mg/dL reduction on the nut-free diet; triglycerides fell by 5.0 mg/dL, while increasing by 3.7 mg/dL on the control diet; non-HDL cholesterol declined by 10.3 mg/dL on the walnut diet and by a far more modest 1.4 mg/dL during the control phase; and apolipoprotein B dropped by 6.7 mg/dL compared with a 0.5 mg/dL reduction on the nut-free diet. All of these differences were statistically significant. However, levels of HDL and lipoprotein (a) weren’t affected.
The California Walnut Commission provided Dr. Parhofer with a research grant to conduct the trial.
ANAHEIM, CALIF. – Eating an ounce and a half of walnuts daily led to favorable changes in gut microbiome composition and diversity in a prospective randomized controlled trial, Klaus G. Parhofer, MD, reported at the American Heart Association scientific session.
These changes in intestinal flora may account for the reductions in LDL cholesterol, triglycerides, apolipoprotein B, and non-HDL cholesterol previously documented with walnut consumption in the same trial, according to Dr. Parhofer, professor of endocrinology and metabolism at the University of Munich.
The gut microbiome composition during the walnut and control phases differed by about 5%. The proportion of the microbiome composed of probiotic and butyric acid–producing organisms in the phylum Bacteroidetes – especially Ruminococcacaeae and Bifidobacteria – increased during the walnut-eating phase, while the Clostridium cluster XIVa microorganisms in the genera Blautia and Anaerostipes decreased significantly, compared with the control diet.
“It is unclear whether these changes are preserved during longer walnut consumption and how these changes can be associated with the observed changes in lipid metabolism,” the endocrinologist noted.
An earlier report focused on the fasting lipid reductions seen in response to walnut consumption in the full study population. LDL cholesterol fell by an average of 7.4 mg/dL after 8 weeks of daily walnut consumption, compared with a 1.7-mg/dL reduction on the nut-free diet; triglycerides fell by 5.0 mg/dL, while increasing by 3.7 mg/dL on the control diet; non-HDL cholesterol declined by 10.3 mg/dL on the walnut diet and by a far more modest 1.4 mg/dL during the control phase; and apolipoprotein B dropped by 6.7 mg/dL compared with a 0.5 mg/dL reduction on the nut-free diet. All of these differences were statistically significant. However, levels of HDL and lipoprotein (a) weren’t affected.
The California Walnut Commission provided Dr. Parhofer with a research grant to conduct the trial.
ANAHEIM, CALIF. – Eating an ounce and a half of walnuts daily led to favorable changes in gut microbiome composition and diversity in a prospective randomized controlled trial, Klaus G. Parhofer, MD, reported at the American Heart Association scientific session.
These changes in intestinal flora may account for the reductions in LDL cholesterol, triglycerides, apolipoprotein B, and non-HDL cholesterol previously documented with walnut consumption in the same trial, according to Dr. Parhofer, professor of endocrinology and metabolism at the University of Munich.
The gut microbiome composition during the walnut and control phases differed by about 5%. The proportion of the microbiome composed of probiotic and butyric acid–producing organisms in the phylum Bacteroidetes – especially Ruminococcacaeae and Bifidobacteria – increased during the walnut-eating phase, while the Clostridium cluster XIVa microorganisms in the genera Blautia and Anaerostipes decreased significantly, compared with the control diet.
“It is unclear whether these changes are preserved during longer walnut consumption and how these changes can be associated with the observed changes in lipid metabolism,” the endocrinologist noted.
An earlier report focused on the fasting lipid reductions seen in response to walnut consumption in the full study population. LDL cholesterol fell by an average of 7.4 mg/dL after 8 weeks of daily walnut consumption, compared with a 1.7-mg/dL reduction on the nut-free diet; triglycerides fell by 5.0 mg/dL, while increasing by 3.7 mg/dL on the control diet; non-HDL cholesterol declined by 10.3 mg/dL on the walnut diet and by a far more modest 1.4 mg/dL during the control phase; and apolipoprotein B dropped by 6.7 mg/dL compared with a 0.5 mg/dL reduction on the nut-free diet. All of these differences were statistically significant. However, levels of HDL and lipoprotein (a) weren’t affected.
The California Walnut Commission provided Dr. Parhofer with a research grant to conduct the trial.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: , which may be linked to the nut’s lipid-lowering effects.
Major finding: The proportion of the gut microbiome comprising probiotic and butyric acid–producing species increased, while Clostridium species decreased in response to daily walnut consumption.
Study details: This prospective randomized controlled trial included 204 healthy white men and women who were assigned to 8 weeks of a diet that included 43 g of shelled walnuts per day or to an isocaloric nut-free control diet, then crossed over to the other study arm after a washout period.
Disclosures: The study was sponsored by the California Walnut Commission.
Neoadjuvant dabrafenib and trametinib improves event-free survival in resectable melanoma
For patients with surgically resectable, BRAF-mutated melanoma, neoadjuvant and adjuvant treatment with the combination of dabrafenib and trametinib resulted in significantly longer event-free survival compared with standard care, according to results of a randomized study.
The trial was closed early because of the “large difference” in event-free survival favoring the neoadjuvant approach, the authors wrote (Lancet Oncol. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
While early closure limits interpretation of results, they do provide important proof-of-concept and data for future studies, wrote the authors, led by Rodabe N Amaria, MD, of the department of medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“The clinical and translational results strongly support the rationale for further assessment of neoadjuvant therapy in patients with high-risk, surgically resectable melanoma,” Dr. Amaria and colleagues said in the report on the randomized trial, believed to be the first to evaluate the role of neoadjuvant therapy versus standard care in BRAF-mutated melanoma.
Dabrafenib and trametinib combination therapy is approved as a treatment for patients with unresectable or metastatic stage IV melanoma and a BRAFV600 mutation, which is found in about half of cutaneous melanomas, authors said.
To evaluate the combination in earlier stage disease, Dr. Amaria and coinvestigators conducted a single-center, open-label, randomized, phase 2 trial of 21 patients with surgically resectable clinical stage III or oligometastatic stage IV melanoma with BRAFV600E or BRAFV600K mutations.
Patients were randomized 2:1 to receive the neoadjuvant/adjuvant treatment or to standard of care, which consisted of standard surgery plus consideration for adjuvant therapy, the authors said. Those patients assigned to the targeted therapy arm received 8 weeks of neoadjuvant dabrafenib and trametinib followed by surgery, then adjuvant dabrafenib and trametinib for up to 44 weeks.
Event-free survival, the primary endpoint of the trial, was a median of 19.7 months for neoadjuvant plus adjuvant dabrafenib and trametinib, versus 2.9 months for standard care (P less than .0001), the investigators reported.
Dabrafenib and trametinib combination therapy was well tolerated as neoadjuvant and adjuvant therapy, with no grade 4 adverse events or treatment related deaths, according to the investigators. The most common grade 3 adverse event seen with the combination was diarrhea, occurring in 2 patients (15%).
The trial is continuing as a single-arm study of neoadjuvant plus adjuvant dabrafenib and trametinib.
Dr. Amaria and colleagues reported individual disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others, including Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study.
SOURCE: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9
Although results of the study by Amaria et al. are “promising,” the role of neoadjuvant therapy in treatment of stage III–IV oligometastatic melanoma in clinical practice “is unclear for now,” melanoma specialists Paolo A. Ascierto, MD, and Alexander M. M. Eggermont, MD, PhD, wrote in an editorial.
Amaria et al. have presented results of the first randomized trial to evaluate neoadjuvant therapy versus standard care in patients with high-risk resectable BRAF-mutated melanoma.
Patients who received both neoadjuvant and adjuvant treatment with the dabrafenib/trametinib combination had superior event-free survival versus standard surgery and consideration for adjuvant therapy, published results show.
However, previous studies have already shown good results on this, that adjuvant dabrafenib plus trametinib (as well as nivolumab monotherapy) in this setting, “raising the question of whether a neoadjuvant approach is really needed, especially given a possible reduction of the role of surgery in the future,” Dr. Ascierto and Dr. Eggermont wrote.
Alternatively, adjuvant therapy with newer, more effective agents may be a “better way forward,” they said, noting that three patients in the trial by Amaria et al. who progressed after neoadjuvant/adjuvant dabrafenib and trametinib relapsed at first with brain metastases, raising the question of whether the treatment “might induce a resistant phenotype predisposed to the development of CNS metastases.”
That said, effectively combining neoadjuvant with adjuvant therapy could reduce the extent of surgery, make radiotherapy redundant, or increase distant metastasis-free survival and overall survival, among other benefits.
“The next generation of adjuvant trials should aim to address these outstanding questions,” they concluded.
Dr. Ascierto is with Istituto Nazionale Tumori Fondazione “G Pascale,” Napoli, Italy, and Dr. Eggermont is with Cancer Institute Gustave Roussy, University Paris-Sud, France. This commentary is based on their editorial appearing in The Lancet Oncology (2018 Jan 17. doi: 10.1016/S1470-2045[18]30016-0). The authors reported disclosures related to Novartis, Merck Serono, Bristol-Myers Squibb, Amgen, and others.
Although results of the study by Amaria et al. are “promising,” the role of neoadjuvant therapy in treatment of stage III–IV oligometastatic melanoma in clinical practice “is unclear for now,” melanoma specialists Paolo A. Ascierto, MD, and Alexander M. M. Eggermont, MD, PhD, wrote in an editorial.
Amaria et al. have presented results of the first randomized trial to evaluate neoadjuvant therapy versus standard care in patients with high-risk resectable BRAF-mutated melanoma.
Patients who received both neoadjuvant and adjuvant treatment with the dabrafenib/trametinib combination had superior event-free survival versus standard surgery and consideration for adjuvant therapy, published results show.
However, previous studies have already shown good results on this, that adjuvant dabrafenib plus trametinib (as well as nivolumab monotherapy) in this setting, “raising the question of whether a neoadjuvant approach is really needed, especially given a possible reduction of the role of surgery in the future,” Dr. Ascierto and Dr. Eggermont wrote.
Alternatively, adjuvant therapy with newer, more effective agents may be a “better way forward,” they said, noting that three patients in the trial by Amaria et al. who progressed after neoadjuvant/adjuvant dabrafenib and trametinib relapsed at first with brain metastases, raising the question of whether the treatment “might induce a resistant phenotype predisposed to the development of CNS metastases.”
That said, effectively combining neoadjuvant with adjuvant therapy could reduce the extent of surgery, make radiotherapy redundant, or increase distant metastasis-free survival and overall survival, among other benefits.
“The next generation of adjuvant trials should aim to address these outstanding questions,” they concluded.
Dr. Ascierto is with Istituto Nazionale Tumori Fondazione “G Pascale,” Napoli, Italy, and Dr. Eggermont is with Cancer Institute Gustave Roussy, University Paris-Sud, France. This commentary is based on their editorial appearing in The Lancet Oncology (2018 Jan 17. doi: 10.1016/S1470-2045[18]30016-0). The authors reported disclosures related to Novartis, Merck Serono, Bristol-Myers Squibb, Amgen, and others.
Although results of the study by Amaria et al. are “promising,” the role of neoadjuvant therapy in treatment of stage III–IV oligometastatic melanoma in clinical practice “is unclear for now,” melanoma specialists Paolo A. Ascierto, MD, and Alexander M. M. Eggermont, MD, PhD, wrote in an editorial.
Amaria et al. have presented results of the first randomized trial to evaluate neoadjuvant therapy versus standard care in patients with high-risk resectable BRAF-mutated melanoma.
Patients who received both neoadjuvant and adjuvant treatment with the dabrafenib/trametinib combination had superior event-free survival versus standard surgery and consideration for adjuvant therapy, published results show.
However, previous studies have already shown good results on this, that adjuvant dabrafenib plus trametinib (as well as nivolumab monotherapy) in this setting, “raising the question of whether a neoadjuvant approach is really needed, especially given a possible reduction of the role of surgery in the future,” Dr. Ascierto and Dr. Eggermont wrote.
Alternatively, adjuvant therapy with newer, more effective agents may be a “better way forward,” they said, noting that three patients in the trial by Amaria et al. who progressed after neoadjuvant/adjuvant dabrafenib and trametinib relapsed at first with brain metastases, raising the question of whether the treatment “might induce a resistant phenotype predisposed to the development of CNS metastases.”
That said, effectively combining neoadjuvant with adjuvant therapy could reduce the extent of surgery, make radiotherapy redundant, or increase distant metastasis-free survival and overall survival, among other benefits.
“The next generation of adjuvant trials should aim to address these outstanding questions,” they concluded.
Dr. Ascierto is with Istituto Nazionale Tumori Fondazione “G Pascale,” Napoli, Italy, and Dr. Eggermont is with Cancer Institute Gustave Roussy, University Paris-Sud, France. This commentary is based on their editorial appearing in The Lancet Oncology (2018 Jan 17. doi: 10.1016/S1470-2045[18]30016-0). The authors reported disclosures related to Novartis, Merck Serono, Bristol-Myers Squibb, Amgen, and others.
For patients with surgically resectable, BRAF-mutated melanoma, neoadjuvant and adjuvant treatment with the combination of dabrafenib and trametinib resulted in significantly longer event-free survival compared with standard care, according to results of a randomized study.
The trial was closed early because of the “large difference” in event-free survival favoring the neoadjuvant approach, the authors wrote (Lancet Oncol. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
While early closure limits interpretation of results, they do provide important proof-of-concept and data for future studies, wrote the authors, led by Rodabe N Amaria, MD, of the department of medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“The clinical and translational results strongly support the rationale for further assessment of neoadjuvant therapy in patients with high-risk, surgically resectable melanoma,” Dr. Amaria and colleagues said in the report on the randomized trial, believed to be the first to evaluate the role of neoadjuvant therapy versus standard care in BRAF-mutated melanoma.
Dabrafenib and trametinib combination therapy is approved as a treatment for patients with unresectable or metastatic stage IV melanoma and a BRAFV600 mutation, which is found in about half of cutaneous melanomas, authors said.
To evaluate the combination in earlier stage disease, Dr. Amaria and coinvestigators conducted a single-center, open-label, randomized, phase 2 trial of 21 patients with surgically resectable clinical stage III or oligometastatic stage IV melanoma with BRAFV600E or BRAFV600K mutations.
Patients were randomized 2:1 to receive the neoadjuvant/adjuvant treatment or to standard of care, which consisted of standard surgery plus consideration for adjuvant therapy, the authors said. Those patients assigned to the targeted therapy arm received 8 weeks of neoadjuvant dabrafenib and trametinib followed by surgery, then adjuvant dabrafenib and trametinib for up to 44 weeks.
Event-free survival, the primary endpoint of the trial, was a median of 19.7 months for neoadjuvant plus adjuvant dabrafenib and trametinib, versus 2.9 months for standard care (P less than .0001), the investigators reported.
Dabrafenib and trametinib combination therapy was well tolerated as neoadjuvant and adjuvant therapy, with no grade 4 adverse events or treatment related deaths, according to the investigators. The most common grade 3 adverse event seen with the combination was diarrhea, occurring in 2 patients (15%).
The trial is continuing as a single-arm study of neoadjuvant plus adjuvant dabrafenib and trametinib.
Dr. Amaria and colleagues reported individual disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others, including Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study.
SOURCE: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9
For patients with surgically resectable, BRAF-mutated melanoma, neoadjuvant and adjuvant treatment with the combination of dabrafenib and trametinib resulted in significantly longer event-free survival compared with standard care, according to results of a randomized study.
The trial was closed early because of the “large difference” in event-free survival favoring the neoadjuvant approach, the authors wrote (Lancet Oncol. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
While early closure limits interpretation of results, they do provide important proof-of-concept and data for future studies, wrote the authors, led by Rodabe N Amaria, MD, of the department of medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“The clinical and translational results strongly support the rationale for further assessment of neoadjuvant therapy in patients with high-risk, surgically resectable melanoma,” Dr. Amaria and colleagues said in the report on the randomized trial, believed to be the first to evaluate the role of neoadjuvant therapy versus standard care in BRAF-mutated melanoma.
Dabrafenib and trametinib combination therapy is approved as a treatment for patients with unresectable or metastatic stage IV melanoma and a BRAFV600 mutation, which is found in about half of cutaneous melanomas, authors said.
To evaluate the combination in earlier stage disease, Dr. Amaria and coinvestigators conducted a single-center, open-label, randomized, phase 2 trial of 21 patients with surgically resectable clinical stage III or oligometastatic stage IV melanoma with BRAFV600E or BRAFV600K mutations.
Patients were randomized 2:1 to receive the neoadjuvant/adjuvant treatment or to standard of care, which consisted of standard surgery plus consideration for adjuvant therapy, the authors said. Those patients assigned to the targeted therapy arm received 8 weeks of neoadjuvant dabrafenib and trametinib followed by surgery, then adjuvant dabrafenib and trametinib for up to 44 weeks.
Event-free survival, the primary endpoint of the trial, was a median of 19.7 months for neoadjuvant plus adjuvant dabrafenib and trametinib, versus 2.9 months for standard care (P less than .0001), the investigators reported.
Dabrafenib and trametinib combination therapy was well tolerated as neoadjuvant and adjuvant therapy, with no grade 4 adverse events or treatment related deaths, according to the investigators. The most common grade 3 adverse event seen with the combination was diarrhea, occurring in 2 patients (15%).
The trial is continuing as a single-arm study of neoadjuvant plus adjuvant dabrafenib and trametinib.
Dr. Amaria and colleagues reported individual disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others, including Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study.
SOURCE: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9
FROM THE LANCET ONCOLOGY
Key clinical point: In patients with high-risk, surgically resectable, clinical stage III-IV melanoma, dabrafenib and trametinib given in both the neoadjuvant and adjuvant setting improved event-free survival compared with standard care.
Major finding: Median event-free survival was 19.7 months for neoadjuvant/adjuvant dabrafenib and trametinib versus 2.9 months for standard upfront surgery including consideration for standard adjuvant therapy (P less than .0001).
Data source: A single-center, open-label, randomized, phase 2 trial including 21 patients with surgically resectable clinical stage III or oligometastatic stage IV BRAF-mutated melanoma.
Disclosures: Investigators reported ties to Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study, and disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others.
Source: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
Higher BMI linked to problems for IBD patients
LAS VEGAS – Higher body mass index among inflammatory bowel disease patients is independently associated with an increased risk of treatment failure and IBD-related surgery or hospitalization, a single-center, retrospective cohort study demonstrated.
“The problem of IBD and obesity is on the rise,” Soumya Kurnool said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “Today, 15%-40% of IBD patients are obese. This is significant because there is a decreased prevalence of remission and an increased risk of relapse in obese IBD patients. These patients also have a higher annual burden of hospitalization.”
She and her associates set out to evaluate the effect of obesity on response to biologic therapy in patients with ulcerative colitis (UC). They conducted a single-center, retrospective cohort study of biologic-treated adults with UC who started therapy during 2011-2016. The researchers excluded patients who had undergone a prior colectomy, as well as those who were underweight at the time of starting a biologic agent and those who had fewer than 6 months of follow-up data.
The primary outcome was time to treatment failure, defined as a composite of IBD-related surgery, hospitalization, and/or treatment modification. Secondary outcomes were time to IBD-related surgery and/or hospitalization and whether the patient achieved endoscopic remission within 1 year of starting biologic therapy. They conducted multivariate Cox proportional hazard analyses after adjusting for key confounders.
Ms. Kurnool reported results from 160 patients with a median age of 36 years. Half were male, and the mean follow-up was 24 months. The median BMI of the cohort was 24.3 kg/m2; 26% were overweight and 18% were obese. More than half of patients (55%) were on infliximab with weight-based dosing and 45% were on other fixed-dosing regimens, including 19% on vedolizumab. In terms of outcomes, 68% of patients experienced treatment failure. All who failed treatment underwent treatment modifications; 15% had IBD-related surgery, and 19% had IBD-related hospitalization.
After adjusting for age, sex, disease duration, prior hospitalization, prior anti-TNF therapy, steroid use, and albumin level, Ms. Kurnool and her associates found that every 1-kg/m2 increase in BMI was associated with a 4% higher risk of treatment failure (adjusted hazard ratio, 1.04), an 8% higher risk of surgery or hospitalization (adjusted HR, 1.08), and a 6% lower risk of achieving endoscopic remission (adjusted HR, 0.94).
“This increase in the risk of treatment failure and IBD-related surgery or hospitalization was consistent across strata of patients treated with infliximab and fixed-dosing regimens,” she said. “Based on these findings, physicians should consider proactive monitoring in obese patients treated with biologic agents.”
Ms. Kurnool reported having received a National Institutes of Health Short Term Training Grant from the University of California, San Diego.
*This story was updated on 3/26.
SOURCE: Kurnool S et al. Crohn’s & Colitis Congress, Clinical Abstract 24.
LAS VEGAS – Higher body mass index among inflammatory bowel disease patients is independently associated with an increased risk of treatment failure and IBD-related surgery or hospitalization, a single-center, retrospective cohort study demonstrated.
“The problem of IBD and obesity is on the rise,” Soumya Kurnool said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “Today, 15%-40% of IBD patients are obese. This is significant because there is a decreased prevalence of remission and an increased risk of relapse in obese IBD patients. These patients also have a higher annual burden of hospitalization.”
She and her associates set out to evaluate the effect of obesity on response to biologic therapy in patients with ulcerative colitis (UC). They conducted a single-center, retrospective cohort study of biologic-treated adults with UC who started therapy during 2011-2016. The researchers excluded patients who had undergone a prior colectomy, as well as those who were underweight at the time of starting a biologic agent and those who had fewer than 6 months of follow-up data.
The primary outcome was time to treatment failure, defined as a composite of IBD-related surgery, hospitalization, and/or treatment modification. Secondary outcomes were time to IBD-related surgery and/or hospitalization and whether the patient achieved endoscopic remission within 1 year of starting biologic therapy. They conducted multivariate Cox proportional hazard analyses after adjusting for key confounders.
Ms. Kurnool reported results from 160 patients with a median age of 36 years. Half were male, and the mean follow-up was 24 months. The median BMI of the cohort was 24.3 kg/m2; 26% were overweight and 18% were obese. More than half of patients (55%) were on infliximab with weight-based dosing and 45% were on other fixed-dosing regimens, including 19% on vedolizumab. In terms of outcomes, 68% of patients experienced treatment failure. All who failed treatment underwent treatment modifications; 15% had IBD-related surgery, and 19% had IBD-related hospitalization.
After adjusting for age, sex, disease duration, prior hospitalization, prior anti-TNF therapy, steroid use, and albumin level, Ms. Kurnool and her associates found that every 1-kg/m2 increase in BMI was associated with a 4% higher risk of treatment failure (adjusted hazard ratio, 1.04), an 8% higher risk of surgery or hospitalization (adjusted HR, 1.08), and a 6% lower risk of achieving endoscopic remission (adjusted HR, 0.94).
“This increase in the risk of treatment failure and IBD-related surgery or hospitalization was consistent across strata of patients treated with infliximab and fixed-dosing regimens,” she said. “Based on these findings, physicians should consider proactive monitoring in obese patients treated with biologic agents.”
Ms. Kurnool reported having received a National Institutes of Health Short Term Training Grant from the University of California, San Diego.
*This story was updated on 3/26.
SOURCE: Kurnool S et al. Crohn’s & Colitis Congress, Clinical Abstract 24.
LAS VEGAS – Higher body mass index among inflammatory bowel disease patients is independently associated with an increased risk of treatment failure and IBD-related surgery or hospitalization, a single-center, retrospective cohort study demonstrated.
“The problem of IBD and obesity is on the rise,” Soumya Kurnool said at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “Today, 15%-40% of IBD patients are obese. This is significant because there is a decreased prevalence of remission and an increased risk of relapse in obese IBD patients. These patients also have a higher annual burden of hospitalization.”
She and her associates set out to evaluate the effect of obesity on response to biologic therapy in patients with ulcerative colitis (UC). They conducted a single-center, retrospective cohort study of biologic-treated adults with UC who started therapy during 2011-2016. The researchers excluded patients who had undergone a prior colectomy, as well as those who were underweight at the time of starting a biologic agent and those who had fewer than 6 months of follow-up data.
The primary outcome was time to treatment failure, defined as a composite of IBD-related surgery, hospitalization, and/or treatment modification. Secondary outcomes were time to IBD-related surgery and/or hospitalization and whether the patient achieved endoscopic remission within 1 year of starting biologic therapy. They conducted multivariate Cox proportional hazard analyses after adjusting for key confounders.
Ms. Kurnool reported results from 160 patients with a median age of 36 years. Half were male, and the mean follow-up was 24 months. The median BMI of the cohort was 24.3 kg/m2; 26% were overweight and 18% were obese. More than half of patients (55%) were on infliximab with weight-based dosing and 45% were on other fixed-dosing regimens, including 19% on vedolizumab. In terms of outcomes, 68% of patients experienced treatment failure. All who failed treatment underwent treatment modifications; 15% had IBD-related surgery, and 19% had IBD-related hospitalization.
After adjusting for age, sex, disease duration, prior hospitalization, prior anti-TNF therapy, steroid use, and albumin level, Ms. Kurnool and her associates found that every 1-kg/m2 increase in BMI was associated with a 4% higher risk of treatment failure (adjusted hazard ratio, 1.04), an 8% higher risk of surgery or hospitalization (adjusted HR, 1.08), and a 6% lower risk of achieving endoscopic remission (adjusted HR, 0.94).
“This increase in the risk of treatment failure and IBD-related surgery or hospitalization was consistent across strata of patients treated with infliximab and fixed-dosing regimens,” she said. “Based on these findings, physicians should consider proactive monitoring in obese patients treated with biologic agents.”
Ms. Kurnool reported having received a National Institutes of Health Short Term Training Grant from the University of California, San Diego.
*This story was updated on 3/26.
SOURCE: Kurnool S et al. Crohn’s & Colitis Congress, Clinical Abstract 24.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Consider proactive monitoring in obese patients treated with biologic agents.
Major finding: Every 1-kg/m2 increase in BMI was associated with a 4% higher risk of treatment failure (adjusted HR, 1.04).
Study details: A single-center retrospective analysis of 160 IBD patients.
Disclosures: Ms. Kurnool reported having received a National Institutes of Health Short-Term Training Grant from the University of California, San Diego.
Source: Kurnool S et al. Crohn’s & Colitis Congress, Clinical Abstract 24.
The care of ‘down there’
Let face it, it’s hard enough to get a teen girl to look up long enough to answer basic questions during an exam, let alone start a completely uncomfortable conversation about vaginal hygiene. Realistically, though, if we don’t have the conversation, who will? Sure, moms give some instructions on how to wipe properly and remind teens to change their pads frequently, but are they giving the correct advice? Are many women just suffering in silence, assuming it’s just something women deal with? Or are they continuing harmful practices that have been passed down through the generations?
Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial syndrome characterized by an imbalance of resident bacteria flora in the vagina.1 The normal flora is predominantly lactobacilli and produces hydrogen peroxide, which keeps the vaginal pH around 4.5. When normal flora is disrupted, other bacteria such as Gardnerella vaginalis, Mycoplasma hominis, and Prevotella bacteroides, to name just a few, can take over, resulting in an unpleasant odor, a watery discharge, and a lower pH. Although originally thought to be sexually transmitted, BV can occur at any age and without having intercourse.2
The incidence of BV varies among races, among socioeconomic classes, and with age. Cultural practices and resources play more of a role than physiologic differences.2 For example, African American women, particularly Caribbean blacks, have higher rates than white women, but douching also is more common among African American and Caribbean blacks than whites. Washing with harsh antiseptics or perfumed soaps also can increase risk, and BV can be sexually transmitted, so the number of partners a woman has can increase that risk.
The presence of BV also has significant social, interpersonal, and work effects and, for some women, is the source of extreme anxiety and distress, which is why many women turn to extreme measures such as douching to control it.3,5
Furthermore, BV is associated with preterm labor and low birth weight infants. Studies have shown that women who are culture positive in their second trimester are at greater risk for adverse outcomes.6
Douching
Douching began in the mid-1800s with the advent of the Eguisier irrigator, which was sold in French pharmacies and consisted of a plunger and a nozzle and was used to prevent pregnancy. Then, in the 1920’s, Lysol was used as the antiseptic, with claims that it acted as a spermicide. Rinsing out the vagina after coitus was believed to kill any sperm in the body and prevent pregnancy. It wasn’t until the 1980’s that the ill effects of Lysol on the vagina were acknowledged and the practice was discontinued.4
Although, generally, douching has fallen out of favor and most authorities advise against it,studies have shown that there can be a benefit when used for vaginosis or vaginitis in relieving symptoms.5,2 Those benefits do not outweigh the possible adverse effects. The process of douching allows for a pressurized solution to be injected into the vagina, thereby flushing bacteria throughout the vagina and into the uterus. In adolescence, the endothelial lining is more prone to adherence of the bacteria, so contracting a sexually transmitted infection is more likely and can increase the risk for ectopic pregnancy.2 The mucus lining of the vagina also tends to be thick; using harsh soaps thins the mucosa, again increasing the likelihood of infections. Furthermore, studies have confirmed that there also is a higher transmission rate of HIV and chlamydia when BV is present.2
Treating and preventing BV
The treatment of choice for BV is metronidazole taken orally or introduced vaginally. Studies have shown that recolonization of the lactobacilli can be slow, so the addition of lactic acid can be helpful. Clindamycin orally or vaginally also is a reasonable choice. Given that most of the bacteria causing BV have beta-lactamase, penicillin is not effective.7
Probiotics taken orally have a natural migration to the vaginal area and promote recolonization.7 Taking 250 mg of vitamin C 6 days/month for 6 months also has been shown to be helpful in recolonization and prevention of recurrence.8
A discussion of proper vaginal hygiene is important for adolescents and teens. Poor hygiene can significantly affect their social and interpersonal relationships, as well as their self-esteem. It puts them at greater risk for contracting sexually transmitted infections, and if they become pregnant, of having an adverse outcome.
In addition, inform them that douching with harsh or perfumed soaps changes the pH of the vagina, which can lead to bacterial overgrowth, so douching should be avoided. Advise them to change pads used during the menstrual cycle every 4-6 hours, and that cotton underwear and loose-fitting clothes also can reduce vaginal irritation. Lastly, advise teens to drink lots of fluids, eat yogurt, and take vitamin C and probiotics to reduce the risk of recurrence.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures.
References
1. Das P et al. PLoS One. 2015 Jun 30;10(6):e0130777.
2. Martino JL et al. Epidemiologic reviews. 2002;24(2):109-24.
3. Bilardi JE et al. PLoS ONE 2013;8(9):e74378.
4. www.Timeline.com/sexist-history-douching-bcc39f3d216c. 2016 Aug 14.
5. Fashemi B et al. Microb Ecol Health Dis. 2013 Feb 25. doi: 10.3402/mehd.v24i0.19703.
6. Hillier SL et al. N Engl J Med. 1995 Dec 28;333(26):1737-42.
7. Kumar N et al. J Pharm Bioallied Sci. 2011 Oct;3(4):496-503.
8. Krasnopolsky VN et al. J Clin Med Res. 2013 Aug;5(4):309-15..
Let face it, it’s hard enough to get a teen girl to look up long enough to answer basic questions during an exam, let alone start a completely uncomfortable conversation about vaginal hygiene. Realistically, though, if we don’t have the conversation, who will? Sure, moms give some instructions on how to wipe properly and remind teens to change their pads frequently, but are they giving the correct advice? Are many women just suffering in silence, assuming it’s just something women deal with? Or are they continuing harmful practices that have been passed down through the generations?
Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial syndrome characterized by an imbalance of resident bacteria flora in the vagina.1 The normal flora is predominantly lactobacilli and produces hydrogen peroxide, which keeps the vaginal pH around 4.5. When normal flora is disrupted, other bacteria such as Gardnerella vaginalis, Mycoplasma hominis, and Prevotella bacteroides, to name just a few, can take over, resulting in an unpleasant odor, a watery discharge, and a lower pH. Although originally thought to be sexually transmitted, BV can occur at any age and without having intercourse.2
The incidence of BV varies among races, among socioeconomic classes, and with age. Cultural practices and resources play more of a role than physiologic differences.2 For example, African American women, particularly Caribbean blacks, have higher rates than white women, but douching also is more common among African American and Caribbean blacks than whites. Washing with harsh antiseptics or perfumed soaps also can increase risk, and BV can be sexually transmitted, so the number of partners a woman has can increase that risk.
The presence of BV also has significant social, interpersonal, and work effects and, for some women, is the source of extreme anxiety and distress, which is why many women turn to extreme measures such as douching to control it.3,5
Furthermore, BV is associated with preterm labor and low birth weight infants. Studies have shown that women who are culture positive in their second trimester are at greater risk for adverse outcomes.6
Douching
Douching began in the mid-1800s with the advent of the Eguisier irrigator, which was sold in French pharmacies and consisted of a plunger and a nozzle and was used to prevent pregnancy. Then, in the 1920’s, Lysol was used as the antiseptic, with claims that it acted as a spermicide. Rinsing out the vagina after coitus was believed to kill any sperm in the body and prevent pregnancy. It wasn’t until the 1980’s that the ill effects of Lysol on the vagina were acknowledged and the practice was discontinued.4
Although, generally, douching has fallen out of favor and most authorities advise against it,studies have shown that there can be a benefit when used for vaginosis or vaginitis in relieving symptoms.5,2 Those benefits do not outweigh the possible adverse effects. The process of douching allows for a pressurized solution to be injected into the vagina, thereby flushing bacteria throughout the vagina and into the uterus. In adolescence, the endothelial lining is more prone to adherence of the bacteria, so contracting a sexually transmitted infection is more likely and can increase the risk for ectopic pregnancy.2 The mucus lining of the vagina also tends to be thick; using harsh soaps thins the mucosa, again increasing the likelihood of infections. Furthermore, studies have confirmed that there also is a higher transmission rate of HIV and chlamydia when BV is present.2
Treating and preventing BV
The treatment of choice for BV is metronidazole taken orally or introduced vaginally. Studies have shown that recolonization of the lactobacilli can be slow, so the addition of lactic acid can be helpful. Clindamycin orally or vaginally also is a reasonable choice. Given that most of the bacteria causing BV have beta-lactamase, penicillin is not effective.7
Probiotics taken orally have a natural migration to the vaginal area and promote recolonization.7 Taking 250 mg of vitamin C 6 days/month for 6 months also has been shown to be helpful in recolonization and prevention of recurrence.8
A discussion of proper vaginal hygiene is important for adolescents and teens. Poor hygiene can significantly affect their social and interpersonal relationships, as well as their self-esteem. It puts them at greater risk for contracting sexually transmitted infections, and if they become pregnant, of having an adverse outcome.
In addition, inform them that douching with harsh or perfumed soaps changes the pH of the vagina, which can lead to bacterial overgrowth, so douching should be avoided. Advise them to change pads used during the menstrual cycle every 4-6 hours, and that cotton underwear and loose-fitting clothes also can reduce vaginal irritation. Lastly, advise teens to drink lots of fluids, eat yogurt, and take vitamin C and probiotics to reduce the risk of recurrence.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures.
References
1. Das P et al. PLoS One. 2015 Jun 30;10(6):e0130777.
2. Martino JL et al. Epidemiologic reviews. 2002;24(2):109-24.
3. Bilardi JE et al. PLoS ONE 2013;8(9):e74378.
4. www.Timeline.com/sexist-history-douching-bcc39f3d216c. 2016 Aug 14.
5. Fashemi B et al. Microb Ecol Health Dis. 2013 Feb 25. doi: 10.3402/mehd.v24i0.19703.
6. Hillier SL et al. N Engl J Med. 1995 Dec 28;333(26):1737-42.
7. Kumar N et al. J Pharm Bioallied Sci. 2011 Oct;3(4):496-503.
8. Krasnopolsky VN et al. J Clin Med Res. 2013 Aug;5(4):309-15..
Let face it, it’s hard enough to get a teen girl to look up long enough to answer basic questions during an exam, let alone start a completely uncomfortable conversation about vaginal hygiene. Realistically, though, if we don’t have the conversation, who will? Sure, moms give some instructions on how to wipe properly and remind teens to change their pads frequently, but are they giving the correct advice? Are many women just suffering in silence, assuming it’s just something women deal with? Or are they continuing harmful practices that have been passed down through the generations?
Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial syndrome characterized by an imbalance of resident bacteria flora in the vagina.1 The normal flora is predominantly lactobacilli and produces hydrogen peroxide, which keeps the vaginal pH around 4.5. When normal flora is disrupted, other bacteria such as Gardnerella vaginalis, Mycoplasma hominis, and Prevotella bacteroides, to name just a few, can take over, resulting in an unpleasant odor, a watery discharge, and a lower pH. Although originally thought to be sexually transmitted, BV can occur at any age and without having intercourse.2
The incidence of BV varies among races, among socioeconomic classes, and with age. Cultural practices and resources play more of a role than physiologic differences.2 For example, African American women, particularly Caribbean blacks, have higher rates than white women, but douching also is more common among African American and Caribbean blacks than whites. Washing with harsh antiseptics or perfumed soaps also can increase risk, and BV can be sexually transmitted, so the number of partners a woman has can increase that risk.
The presence of BV also has significant social, interpersonal, and work effects and, for some women, is the source of extreme anxiety and distress, which is why many women turn to extreme measures such as douching to control it.3,5
Furthermore, BV is associated with preterm labor and low birth weight infants. Studies have shown that women who are culture positive in their second trimester are at greater risk for adverse outcomes.6
Douching
Douching began in the mid-1800s with the advent of the Eguisier irrigator, which was sold in French pharmacies and consisted of a plunger and a nozzle and was used to prevent pregnancy. Then, in the 1920’s, Lysol was used as the antiseptic, with claims that it acted as a spermicide. Rinsing out the vagina after coitus was believed to kill any sperm in the body and prevent pregnancy. It wasn’t until the 1980’s that the ill effects of Lysol on the vagina were acknowledged and the practice was discontinued.4
Although, generally, douching has fallen out of favor and most authorities advise against it,studies have shown that there can be a benefit when used for vaginosis or vaginitis in relieving symptoms.5,2 Those benefits do not outweigh the possible adverse effects. The process of douching allows for a pressurized solution to be injected into the vagina, thereby flushing bacteria throughout the vagina and into the uterus. In adolescence, the endothelial lining is more prone to adherence of the bacteria, so contracting a sexually transmitted infection is more likely and can increase the risk for ectopic pregnancy.2 The mucus lining of the vagina also tends to be thick; using harsh soaps thins the mucosa, again increasing the likelihood of infections. Furthermore, studies have confirmed that there also is a higher transmission rate of HIV and chlamydia when BV is present.2
Treating and preventing BV
The treatment of choice for BV is metronidazole taken orally or introduced vaginally. Studies have shown that recolonization of the lactobacilli can be slow, so the addition of lactic acid can be helpful. Clindamycin orally or vaginally also is a reasonable choice. Given that most of the bacteria causing BV have beta-lactamase, penicillin is not effective.7
Probiotics taken orally have a natural migration to the vaginal area and promote recolonization.7 Taking 250 mg of vitamin C 6 days/month for 6 months also has been shown to be helpful in recolonization and prevention of recurrence.8
A discussion of proper vaginal hygiene is important for adolescents and teens. Poor hygiene can significantly affect their social and interpersonal relationships, as well as their self-esteem. It puts them at greater risk for contracting sexually transmitted infections, and if they become pregnant, of having an adverse outcome.
In addition, inform them that douching with harsh or perfumed soaps changes the pH of the vagina, which can lead to bacterial overgrowth, so douching should be avoided. Advise them to change pads used during the menstrual cycle every 4-6 hours, and that cotton underwear and loose-fitting clothes also can reduce vaginal irritation. Lastly, advise teens to drink lots of fluids, eat yogurt, and take vitamin C and probiotics to reduce the risk of recurrence.
Dr. Pearce is a pediatrician in Frankfort, Ill. She said she had no relevant financial disclosures.
References
1. Das P et al. PLoS One. 2015 Jun 30;10(6):e0130777.
2. Martino JL et al. Epidemiologic reviews. 2002;24(2):109-24.
3. Bilardi JE et al. PLoS ONE 2013;8(9):e74378.
4. www.Timeline.com/sexist-history-douching-bcc39f3d216c. 2016 Aug 14.
5. Fashemi B et al. Microb Ecol Health Dis. 2013 Feb 25. doi: 10.3402/mehd.v24i0.19703.
6. Hillier SL et al. N Engl J Med. 1995 Dec 28;333(26):1737-42.
7. Kumar N et al. J Pharm Bioallied Sci. 2011 Oct;3(4):496-503.
8. Krasnopolsky VN et al. J Clin Med Res. 2013 Aug;5(4):309-15..















