User login
Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis
Toxic epidermal necrolysis (TEN) is a rare, life-threatening adverse drug reaction with an estimated incidence of 0.4 to 1.9 cases per million persons per year worldwide and an estimated mortality rate of 25% to 35%.1,2 This dermatologic emergency is characterized by extensive detachment of the epidermis and erosions of the mucous membranes secondary to massive keratinocyte cell death via apoptosis, evolving quickly into full-thickness epidermal necrosis.
Primary treatment of TEN includes (1) prompt discontinuation of the suspected medication; (2) rapid transfer to an intensive care unit, burn center, or other specialty unit; and (3) supportive care, including wound care, fluid and electrolyte maintenance, and treatment of infections. Aside from the primary treatment, controversy remains over the most effective adjunctive therapy for TEN, as none has proven consistent superiority over well-conducted primary treatment alone. Therefore, established therapeutic guidelines do not exist.1-3
The use of adjunctive systemic therapy in TEN (eg, corticosteroids, intravenous immunoglobulin [IVIG], cyclosporine, plasmapheresis, granulocyte-colony stimulating factor) is based primarily on theories of pathogenesis, which unfortunately remain unclear. Activated CD8+ T cells are thought to increase the expression and production of granulysin, granzyme B, and perforins, leading to keratinocyte apoptosis. Fas ligand and tumor necrosis factor α (TNF-α) also are implicated as secondary mediators of cell death via the inducible nitric oxide synthase pathway.1,4-6
Since TNF-α was found to be elevated in serum and blister fluid in patients with TEN,7,8 medications aimed at decreasing the TNF-α concentration, such as pentoxifylline (PTX) and thalidomide, have been attempted for treatment.9,10 Biologic inhibitors of TNF-α, such as infliximab and etanercept, are novel therapeutic options in the treatment of TEN, as numerous reports document their successful use in the treatment of this disease.11-24 The purpose of this study is to systematically review the current literature on the use of TNF-α antagonists in the treatment of TEN.
METHODS
A PubMed search of all available articles indexed for MEDLINE using the terms toxic epidermal necrolysis and TNF-alpha and pentoxifylline or thalidomide or infliximab or etanercept or adalimumab was conducted.
RESULTS
Sixteen articles published between 1994 and 2014 were retrieved from PubMed and reviewed.9-24 Fourteen articles were case reports and case series involving the use of TNF-α inhibitors as either monotherapy, second-line agents, or in combination with other medications in the treatment of TEN, providing a total of 28 patients.9,11-23 Two articles were prospective trials, one evaluating the efficacy of thalidomide10 and the other infliximab24 in treating TEN. All studies implemented primary treatment (ie, prompt discontinuation of the suspected medication and aggressive supportive care) in addition to TNF-α inhibition.
Pentoxifylline
The first case report describing the use of an anti–TNF-α inhibitor for TEN was with PTX in 1994.9 Pentoxifylline, a vasoactive drug with immunomodulatory properties including the downregulation of TNF-α synthesis, was used to treat a 26-year-old woman with TEN on phenylhydantoin 15 days following resection of a grade II astrocytoma. The patient initially received intravenous N-acetylcysteine (NAC) (9 g once daily) and S-adenosyl-L-methionine (100 mg once daily) for antioxidant effects. On the second day of treatment, intravenous PTX (900 mg once daily) was added for TNF-α inhibition. Following PTX administration, the investigators reported quick stabilization of the eruption and achievement of reepithelialization after 7 days of therapy. Upon cessation of PTX therapy, a recurrence of generalized erythema occurred, suggesting a relapse of TEN; therefore, PTX was reinitiated for an additional 3 days, and the patient’s skin remained clear.9
Thalidomide
The earliest prospective trial we reviewed using anti–TNF-α therapy in TEN occurred in 1998 with thalidomide, a moderate inhibitor of TNF-α.10 In this randomized controlled trial, 22 TEN patients received either a 5-day course of thalidomide (400 mg once daily) or placebo. There was increased mortality in the thalidomide group (10/12 [83.3%]) versus the placebo group (3/10 [30.0%]). Additionally, the plasma TNF-α concentrations in the thalidomide group were higher than the control group. This study was stopped prematurely due to the excess mortality in the thalidomide group.10
Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
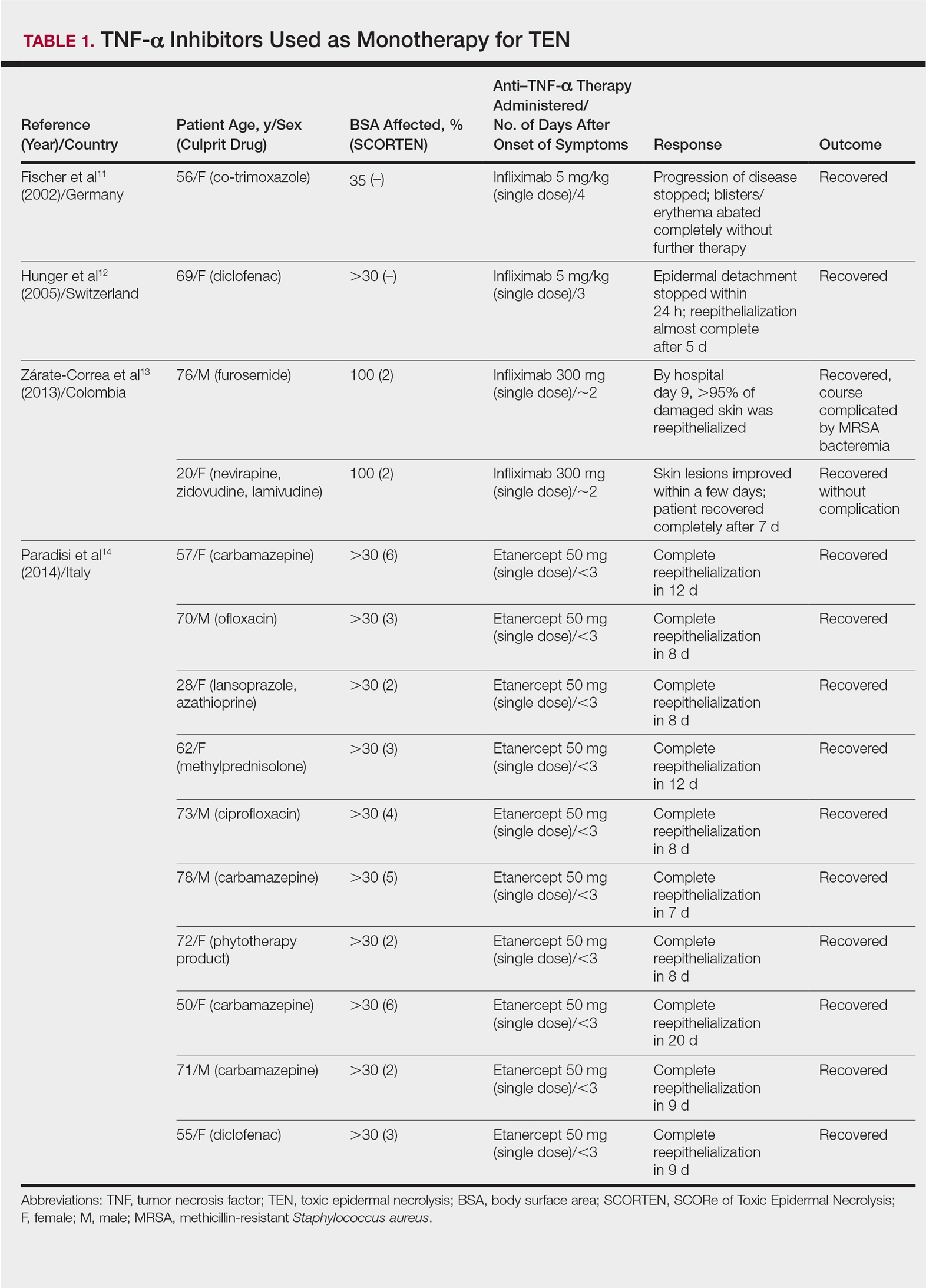
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14
TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
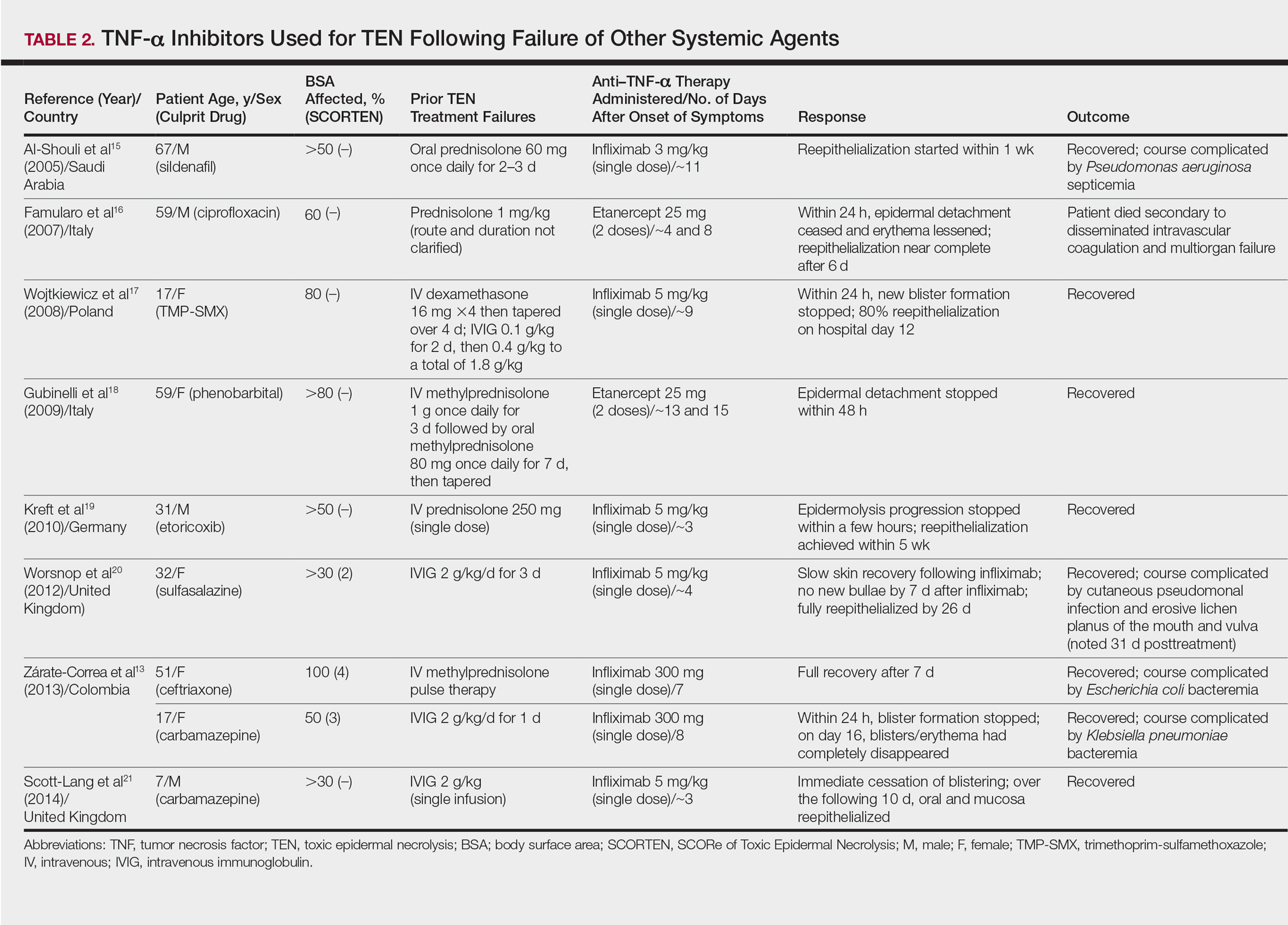
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.
Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
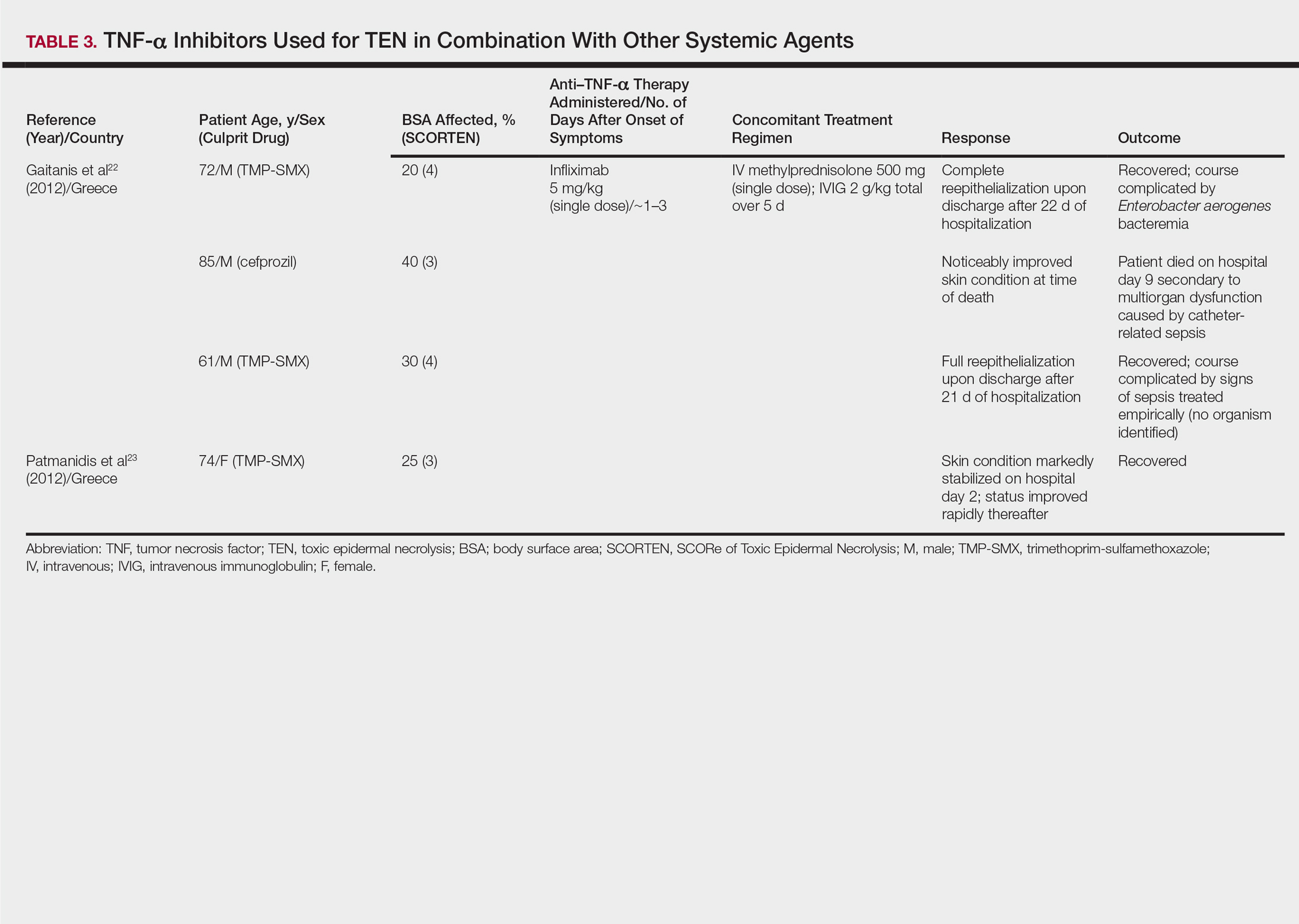
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.
N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24
COMMENT
Tumor necrosis factor α inhibition in the treatment of TEN was first utilized in the 1990s with PTX and thalidomide.9,10 In 1994, PTX in addition to antioxidant therapy was found to successfully treat a 26-year-old woman with TEN attributed to anticonvulsant therapy.9 Other reports of PTX in the treatment of TEN were not found; however, there is a case series describing the successful treatment of 2 pediatric patients with Stevens-Johnson syndrome (SJS) and SJS-TEN overlap with PTX.25 Thalidomide, however, proved detrimental to patients with TEN as evidenced by an increased mortality in the 1998 trial.10 Paradoxically, the treatment group was found to have increased rather than decreased TNF-α concentrations, which was hypothesized to be the cause of increased mortality. This finding furthered the theory that TNF-α is an important mediator in TEN pathogenesis and a potential novel target in disease management.10
Since the PTX case report and the thalidomide trial, many physicians have reported the beneficial effects of biologic TNF-α inhibitors in the course of TEN; however, most of the literature is composed of case reports and case series describing a small number of patients. Therefore, the beneficial effects of anti–TNF-α therapy in TEN cannot be conclusively derived. Furthermore, cases using TNF-α inhibitors in combination with or after other systemic agents complicate the effects of TNF-α inhibitors themselves. Most of these case reports and case series describe the beneficial effects of TNF-α inhibitors in TEN; however, it is important to remember that cases in which these agents were ineffective are less likely to be published. The strongest evidence for TNF-α inhibitor use in the treatment TEN comes from the Paradisi et al14 case series, which showed a decrease in expected mortality with etanercept monotherapy in a relatively large cohort of patients. However, when evaluated prospectively by Paquet et al,24 there was no benefit seen by adding infliximab to NAC therapy and possibly an increased mortality in the group treated with both agents.
In the cases reviewed, a total of 32 patients were treated with infliximab or etanercept, and of these patients there were 4 deaths (12.5%).16,22,24 Three deaths were attributed to infection and 1 was attributed to disseminated intravascular coagulation. Furthermore, infection complicated the hospital course of 9 (28.1%) patients.13,15,22,24 The bacteria cultured from these patients included methicillin-resistant S aureus, P aeruginosa, E coli, Enterobacter aerogenes, and K pneumoniae. Patients who received TNF-α antagonists in combination with or after other systemic immunosuppressants appeared to have a higher incidence of infections. All patients treated with TNF-α antagonists in TEN should undergo careful evaluation and monitoring for infections due to the immunosuppressant effect of these drugs.
In our review, a total of 3 pediatric/adolescent patients received a TNF-α inhibitor for the treatment of TEN.13,17,21 Two patients received infliximab as a second-line medication after failure of IVIG to arrest progression of disease13,17 and one patient received infliximab as a second-line medication after dexamethasone.21 Each of these patients recovered without any reported infections or long-term complications.
Although excluded from this review, both infliximab and etanercept have been reported to show benefit in acute generalized exanthematous pustulosis/TEN overlap.26,27 Interestingly, in postmarketing surveillance, rare reports have implicated both infliximab and etanercept in causing both SJS and TEN.28 Also, there have been case reports of adalimumab causing SJS, but no cases of it causing TEN were identified.29,30
CONCLUSION
Rapid discontinuation of the culprit drug and aggressive supportive care remain the primary treatment of TEN. Tumor necrosis factor α inhibitors as monotherapy or as second-line agents show promise in the treatment of this complex disease state in both the adult and pediatric populations. The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections. Additional studies are needed to further characterize the role of TNF-α inhibition in the treatment of TEN.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173-186.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187-203.
- Fernando S. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;55:165-171.
- Paquet P, Paquet F, Saleh W, et al. Immunoregulatory effector cells in drug-induced toxic epidermal necrolysis. Am J Dermatopathol. 2000;22:413-417.
- Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850-855.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and INF-γ are potential inducers of Fas-mediated keratinocyte apoptosis thought activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Paquet P, Pierard G. Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med. 1998;1:459-462.
- Correia O, Delgado L, Barbosa I, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- Redondo P, Rutz de Erenchun F, Iglesias M, et al. Toxic epidermal necrolysis. treatment with pentoxifylline. Br J Dermatol. 1994;130:688-689.
- Wolkenstein P, Latarjet J, Roujeau J, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch W, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-708.
- Hunger R, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Al-Shouli S, Bogusz M, Al Tufail M, et al. Toxic epidermal necrosis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-553.
- Famularo G, Di Dona B, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Worsnop F, Wee J, Moosa Y, et al. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37:879-881.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulins. Dermatology. 2012;224:134-139.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Paquet P, Jennes S, Rousseua A, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;1:1-6.
- Sanclemente G, De le Rouche C, Escobar C, et al. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 1998;38:878-879.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumor necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South Med J. 2009;102:1133-1140.
- Muna S, Lawrance I. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Mounach A, Rezgi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
Toxic epidermal necrolysis (TEN) is a rare, life-threatening adverse drug reaction with an estimated incidence of 0.4 to 1.9 cases per million persons per year worldwide and an estimated mortality rate of 25% to 35%.1,2 This dermatologic emergency is characterized by extensive detachment of the epidermis and erosions of the mucous membranes secondary to massive keratinocyte cell death via apoptosis, evolving quickly into full-thickness epidermal necrosis.
Primary treatment of TEN includes (1) prompt discontinuation of the suspected medication; (2) rapid transfer to an intensive care unit, burn center, or other specialty unit; and (3) supportive care, including wound care, fluid and electrolyte maintenance, and treatment of infections. Aside from the primary treatment, controversy remains over the most effective adjunctive therapy for TEN, as none has proven consistent superiority over well-conducted primary treatment alone. Therefore, established therapeutic guidelines do not exist.1-3
The use of adjunctive systemic therapy in TEN (eg, corticosteroids, intravenous immunoglobulin [IVIG], cyclosporine, plasmapheresis, granulocyte-colony stimulating factor) is based primarily on theories of pathogenesis, which unfortunately remain unclear. Activated CD8+ T cells are thought to increase the expression and production of granulysin, granzyme B, and perforins, leading to keratinocyte apoptosis. Fas ligand and tumor necrosis factor α (TNF-α) also are implicated as secondary mediators of cell death via the inducible nitric oxide synthase pathway.1,4-6
Since TNF-α was found to be elevated in serum and blister fluid in patients with TEN,7,8 medications aimed at decreasing the TNF-α concentration, such as pentoxifylline (PTX) and thalidomide, have been attempted for treatment.9,10 Biologic inhibitors of TNF-α, such as infliximab and etanercept, are novel therapeutic options in the treatment of TEN, as numerous reports document their successful use in the treatment of this disease.11-24 The purpose of this study is to systematically review the current literature on the use of TNF-α antagonists in the treatment of TEN.
METHODS
A PubMed search of all available articles indexed for MEDLINE using the terms toxic epidermal necrolysis and TNF-alpha and pentoxifylline or thalidomide or infliximab or etanercept or adalimumab was conducted.
RESULTS
Sixteen articles published between 1994 and 2014 were retrieved from PubMed and reviewed.9-24 Fourteen articles were case reports and case series involving the use of TNF-α inhibitors as either monotherapy, second-line agents, or in combination with other medications in the treatment of TEN, providing a total of 28 patients.9,11-23 Two articles were prospective trials, one evaluating the efficacy of thalidomide10 and the other infliximab24 in treating TEN. All studies implemented primary treatment (ie, prompt discontinuation of the suspected medication and aggressive supportive care) in addition to TNF-α inhibition.
Pentoxifylline
The first case report describing the use of an anti–TNF-α inhibitor for TEN was with PTX in 1994.9 Pentoxifylline, a vasoactive drug with immunomodulatory properties including the downregulation of TNF-α synthesis, was used to treat a 26-year-old woman with TEN on phenylhydantoin 15 days following resection of a grade II astrocytoma. The patient initially received intravenous N-acetylcysteine (NAC) (9 g once daily) and S-adenosyl-L-methionine (100 mg once daily) for antioxidant effects. On the second day of treatment, intravenous PTX (900 mg once daily) was added for TNF-α inhibition. Following PTX administration, the investigators reported quick stabilization of the eruption and achievement of reepithelialization after 7 days of therapy. Upon cessation of PTX therapy, a recurrence of generalized erythema occurred, suggesting a relapse of TEN; therefore, PTX was reinitiated for an additional 3 days, and the patient’s skin remained clear.9
Thalidomide
The earliest prospective trial we reviewed using anti–TNF-α therapy in TEN occurred in 1998 with thalidomide, a moderate inhibitor of TNF-α.10 In this randomized controlled trial, 22 TEN patients received either a 5-day course of thalidomide (400 mg once daily) or placebo. There was increased mortality in the thalidomide group (10/12 [83.3%]) versus the placebo group (3/10 [30.0%]). Additionally, the plasma TNF-α concentrations in the thalidomide group were higher than the control group. This study was stopped prematurely due to the excess mortality in the thalidomide group.10
Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14

TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.

Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.

N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24
COMMENT
Tumor necrosis factor α inhibition in the treatment of TEN was first utilized in the 1990s with PTX and thalidomide.9,10 In 1994, PTX in addition to antioxidant therapy was found to successfully treat a 26-year-old woman with TEN attributed to anticonvulsant therapy.9 Other reports of PTX in the treatment of TEN were not found; however, there is a case series describing the successful treatment of 2 pediatric patients with Stevens-Johnson syndrome (SJS) and SJS-TEN overlap with PTX.25 Thalidomide, however, proved detrimental to patients with TEN as evidenced by an increased mortality in the 1998 trial.10 Paradoxically, the treatment group was found to have increased rather than decreased TNF-α concentrations, which was hypothesized to be the cause of increased mortality. This finding furthered the theory that TNF-α is an important mediator in TEN pathogenesis and a potential novel target in disease management.10
Since the PTX case report and the thalidomide trial, many physicians have reported the beneficial effects of biologic TNF-α inhibitors in the course of TEN; however, most of the literature is composed of case reports and case series describing a small number of patients. Therefore, the beneficial effects of anti–TNF-α therapy in TEN cannot be conclusively derived. Furthermore, cases using TNF-α inhibitors in combination with or after other systemic agents complicate the effects of TNF-α inhibitors themselves. Most of these case reports and case series describe the beneficial effects of TNF-α inhibitors in TEN; however, it is important to remember that cases in which these agents were ineffective are less likely to be published. The strongest evidence for TNF-α inhibitor use in the treatment TEN comes from the Paradisi et al14 case series, which showed a decrease in expected mortality with etanercept monotherapy in a relatively large cohort of patients. However, when evaluated prospectively by Paquet et al,24 there was no benefit seen by adding infliximab to NAC therapy and possibly an increased mortality in the group treated with both agents.
In the cases reviewed, a total of 32 patients were treated with infliximab or etanercept, and of these patients there were 4 deaths (12.5%).16,22,24 Three deaths were attributed to infection and 1 was attributed to disseminated intravascular coagulation. Furthermore, infection complicated the hospital course of 9 (28.1%) patients.13,15,22,24 The bacteria cultured from these patients included methicillin-resistant S aureus, P aeruginosa, E coli, Enterobacter aerogenes, and K pneumoniae. Patients who received TNF-α antagonists in combination with or after other systemic immunosuppressants appeared to have a higher incidence of infections. All patients treated with TNF-α antagonists in TEN should undergo careful evaluation and monitoring for infections due to the immunosuppressant effect of these drugs.
In our review, a total of 3 pediatric/adolescent patients received a TNF-α inhibitor for the treatment of TEN.13,17,21 Two patients received infliximab as a second-line medication after failure of IVIG to arrest progression of disease13,17 and one patient received infliximab as a second-line medication after dexamethasone.21 Each of these patients recovered without any reported infections or long-term complications.
Although excluded from this review, both infliximab and etanercept have been reported to show benefit in acute generalized exanthematous pustulosis/TEN overlap.26,27 Interestingly, in postmarketing surveillance, rare reports have implicated both infliximab and etanercept in causing both SJS and TEN.28 Also, there have been case reports of adalimumab causing SJS, but no cases of it causing TEN were identified.29,30
CONCLUSION
Rapid discontinuation of the culprit drug and aggressive supportive care remain the primary treatment of TEN. Tumor necrosis factor α inhibitors as monotherapy or as second-line agents show promise in the treatment of this complex disease state in both the adult and pediatric populations. The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections. Additional studies are needed to further characterize the role of TNF-α inhibition in the treatment of TEN.
Toxic epidermal necrolysis (TEN) is a rare, life-threatening adverse drug reaction with an estimated incidence of 0.4 to 1.9 cases per million persons per year worldwide and an estimated mortality rate of 25% to 35%.1,2 This dermatologic emergency is characterized by extensive detachment of the epidermis and erosions of the mucous membranes secondary to massive keratinocyte cell death via apoptosis, evolving quickly into full-thickness epidermal necrosis.
Primary treatment of TEN includes (1) prompt discontinuation of the suspected medication; (2) rapid transfer to an intensive care unit, burn center, or other specialty unit; and (3) supportive care, including wound care, fluid and electrolyte maintenance, and treatment of infections. Aside from the primary treatment, controversy remains over the most effective adjunctive therapy for TEN, as none has proven consistent superiority over well-conducted primary treatment alone. Therefore, established therapeutic guidelines do not exist.1-3
The use of adjunctive systemic therapy in TEN (eg, corticosteroids, intravenous immunoglobulin [IVIG], cyclosporine, plasmapheresis, granulocyte-colony stimulating factor) is based primarily on theories of pathogenesis, which unfortunately remain unclear. Activated CD8+ T cells are thought to increase the expression and production of granulysin, granzyme B, and perforins, leading to keratinocyte apoptosis. Fas ligand and tumor necrosis factor α (TNF-α) also are implicated as secondary mediators of cell death via the inducible nitric oxide synthase pathway.1,4-6
Since TNF-α was found to be elevated in serum and blister fluid in patients with TEN,7,8 medications aimed at decreasing the TNF-α concentration, such as pentoxifylline (PTX) and thalidomide, have been attempted for treatment.9,10 Biologic inhibitors of TNF-α, such as infliximab and etanercept, are novel therapeutic options in the treatment of TEN, as numerous reports document their successful use in the treatment of this disease.11-24 The purpose of this study is to systematically review the current literature on the use of TNF-α antagonists in the treatment of TEN.
METHODS
A PubMed search of all available articles indexed for MEDLINE using the terms toxic epidermal necrolysis and TNF-alpha and pentoxifylline or thalidomide or infliximab or etanercept or adalimumab was conducted.
RESULTS
Sixteen articles published between 1994 and 2014 were retrieved from PubMed and reviewed.9-24 Fourteen articles were case reports and case series involving the use of TNF-α inhibitors as either monotherapy, second-line agents, or in combination with other medications in the treatment of TEN, providing a total of 28 patients.9,11-23 Two articles were prospective trials, one evaluating the efficacy of thalidomide10 and the other infliximab24 in treating TEN. All studies implemented primary treatment (ie, prompt discontinuation of the suspected medication and aggressive supportive care) in addition to TNF-α inhibition.
Pentoxifylline
The first case report describing the use of an anti–TNF-α inhibitor for TEN was with PTX in 1994.9 Pentoxifylline, a vasoactive drug with immunomodulatory properties including the downregulation of TNF-α synthesis, was used to treat a 26-year-old woman with TEN on phenylhydantoin 15 days following resection of a grade II astrocytoma. The patient initially received intravenous N-acetylcysteine (NAC) (9 g once daily) and S-adenosyl-L-methionine (100 mg once daily) for antioxidant effects. On the second day of treatment, intravenous PTX (900 mg once daily) was added for TNF-α inhibition. Following PTX administration, the investigators reported quick stabilization of the eruption and achievement of reepithelialization after 7 days of therapy. Upon cessation of PTX therapy, a recurrence of generalized erythema occurred, suggesting a relapse of TEN; therefore, PTX was reinitiated for an additional 3 days, and the patient’s skin remained clear.9
Thalidomide
The earliest prospective trial we reviewed using anti–TNF-α therapy in TEN occurred in 1998 with thalidomide, a moderate inhibitor of TNF-α.10 In this randomized controlled trial, 22 TEN patients received either a 5-day course of thalidomide (400 mg once daily) or placebo. There was increased mortality in the thalidomide group (10/12 [83.3%]) versus the placebo group (3/10 [30.0%]). Additionally, the plasma TNF-α concentrations in the thalidomide group were higher than the control group. This study was stopped prematurely due to the excess mortality in the thalidomide group.10
Biologic TNF-α Antagonists
Following the PTX case report and the thalidomide trial, there was increased interest in using newer-generation TNF-α inhibitors, such as the monoclonal antibody infliximab or the fusion protein etanercept, in the treatment of TEN. To date, there are 10 known published case reports,11,12,15-21,23 3 case series,13,14,22 and 1 trial24 describing the use of these agents; however, treatment protocols vary. Categories of treatment protocols include the use of TNF-α inhibitors as monotherapy, following failure of other systemic agents, and in combination with other systemic therapies.
TNF-α Inhibitors as Monotherapy
Review of the literature yielded 2 case reports using infliximab monotherapy11,12 and 2 case series using infliximab or etanercept monotherapy13,14 with a total of 14 patients (Table 1). Fischer et al11 was the first of these reports to describe a patient successfully treated with supportive care and a single dose of infliximab 5 mg/kg. The dose was given 4 days after the onset of symptoms, and the rapid progression of the disease was stopped, with complete recovery in less than 4 weeks.11 Hunger et al12 also described the successful treatment of a patient using a similar protocol: a single dose of infliximab 5 mg/kg given 3 days after symptom onset. Epidermal detachment was abated within 24 hours and the patient had almost complete reepithelialization within 5 days.12 In a case series published by Zárate-Correa et al,13 2 patients with near 100% body surface area involvement were successfully treated with a single dose of infliximab 300 mg. Although both of these patients experienced fairly rapid recoveries, one patient’s course was complicated by methicillin-resistant Staphylococcus aureus bacteremia.13 Paradisi et al14 described 10 consecutive patients treated with a single dose of etanercept 50 mg given within 6 hours of hospital admission and within 72 hours of symptom onset. The SCORTEN (SCORe of Toxic Epidermal Necrolysis) scale—a severity-of-illness assessment for TEN based on body surface area involvement, comorbidities, and metabolic abnormalities—was used to predict mortality in these patients. The investigators reported an expected mortality of 46.9%; however, the observed mortality was 0%, and there were no reported infections.14

TNF-α Inhibitors Following Failure of Other Systemic Agents in TEN
Seven case reports and 1 case series using anti–TNF-α therapy following failure of other systemic agents were reviewed for a total of 9 patients (3 pediatric/adolescent patients, 6 adult patients)(Table 2).13,15-21 Seven patients were treated with infliximab,13,15,17,19-21 and the remaining 2 patients were treated with etanercept.16,18 All patients were treated initially with corticosteroids and/or IVIG. In each case, anti–TNF-α therapy was introduced when prior treatment failed to halt the progression of TEN. Most reports claimed a rapid and beneficial response to anti–TNF-α therapy. Eight of 9 (88.9%) patients recovered.13,15,17-21 Famularo et al16 described 1 patient who was treated with 2 doses of etanercept following prednisolone but died on the tenth day of hospitalization secondary to disseminated intravascular coagulation and multiorgan failure; however, the patient reportedly had near-complete reepithelialization of the skin on the sixth day of the hospital course.16 Of the 8 surviving patients, 3 (37.5%) experienced hospital courses complicated by nosocomial gram-negative bacteremia, including Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.13,15 Interestingly, a patient described by Worsnop et al20 developed erosive lichen planus of the mouth and vulva 31 days after infliximab infusion.

Combination of TNF-α Inhibitor With Other Systemic Agents in TEN
One case series22 and 1 case report23 using infliximab in combination with other systemic therapies were reviewed with a total of 4 patients (Table 3). Both reports utilized the same treatment protocol, which consisted of a single bolus of intravenous methylprednisolone 500 mg followed by a single dose of infliximab 5 mg/kg and then IVIG 2 g/kg over 5 days. Three of 4 (75%) patients recovered.22,23 Gaitanis et al22 reported a patient who died on the ninth day of hospitalization secondary to multiorgan dysfunction caused by a catheter-related bacteremia. Similar to the patient described by Famularo et al,16 this patient also was noted to have remarkably improved skin prior to death. Two of the other 3 patients that survived had their hospital course complicated by infection, requiring antibiotics.22 In the Gaitanis et al22 series, the average predicted mortality according to a SCORTEN assessment was 50.8%; however, mortality was observed in 33.3% (1/3) of patients in the case series.

N-Acetylcysteine and Infliximab
The combination of NAC and infliximab was studied in a randomized controlled trial using TNF-α inhibition in TEN.24 In this study, 10 patients were admitted to a burn unit and treated with either 3 doses of intravenous NAC (150 mg/kg per dose) plus 1 dose of infliximab 5 mg/kg or NAC alone. Unlike some of the previously described articles, Paquet et al24 utilized an illness auxiliary score (IAS), which predicts both disease duration and mortality. An IAS was taken at admission and again 48 hours after completion of NAC and/or infliximab administration. The mean clinical IAS score was reported to have remained unchanged at treatment completion in the NAC group and slightly worsened in the NAC-infliximab group. One patient died in the NAC group and 2 patients died in the NAC-infliximab group, each due to infection. These fatalities corresponded to a mean mortality of 20% in the NAC-treated group and 40% for the NAC-infliximab group. To compare, the predicted mortalities based on the IAS were 20.4% and 21.4%, respectively.24
COMMENT
Tumor necrosis factor α inhibition in the treatment of TEN was first utilized in the 1990s with PTX and thalidomide.9,10 In 1994, PTX in addition to antioxidant therapy was found to successfully treat a 26-year-old woman with TEN attributed to anticonvulsant therapy.9 Other reports of PTX in the treatment of TEN were not found; however, there is a case series describing the successful treatment of 2 pediatric patients with Stevens-Johnson syndrome (SJS) and SJS-TEN overlap with PTX.25 Thalidomide, however, proved detrimental to patients with TEN as evidenced by an increased mortality in the 1998 trial.10 Paradoxically, the treatment group was found to have increased rather than decreased TNF-α concentrations, which was hypothesized to be the cause of increased mortality. This finding furthered the theory that TNF-α is an important mediator in TEN pathogenesis and a potential novel target in disease management.10
Since the PTX case report and the thalidomide trial, many physicians have reported the beneficial effects of biologic TNF-α inhibitors in the course of TEN; however, most of the literature is composed of case reports and case series describing a small number of patients. Therefore, the beneficial effects of anti–TNF-α therapy in TEN cannot be conclusively derived. Furthermore, cases using TNF-α inhibitors in combination with or after other systemic agents complicate the effects of TNF-α inhibitors themselves. Most of these case reports and case series describe the beneficial effects of TNF-α inhibitors in TEN; however, it is important to remember that cases in which these agents were ineffective are less likely to be published. The strongest evidence for TNF-α inhibitor use in the treatment TEN comes from the Paradisi et al14 case series, which showed a decrease in expected mortality with etanercept monotherapy in a relatively large cohort of patients. However, when evaluated prospectively by Paquet et al,24 there was no benefit seen by adding infliximab to NAC therapy and possibly an increased mortality in the group treated with both agents.
In the cases reviewed, a total of 32 patients were treated with infliximab or etanercept, and of these patients there were 4 deaths (12.5%).16,22,24 Three deaths were attributed to infection and 1 was attributed to disseminated intravascular coagulation. Furthermore, infection complicated the hospital course of 9 (28.1%) patients.13,15,22,24 The bacteria cultured from these patients included methicillin-resistant S aureus, P aeruginosa, E coli, Enterobacter aerogenes, and K pneumoniae. Patients who received TNF-α antagonists in combination with or after other systemic immunosuppressants appeared to have a higher incidence of infections. All patients treated with TNF-α antagonists in TEN should undergo careful evaluation and monitoring for infections due to the immunosuppressant effect of these drugs.
In our review, a total of 3 pediatric/adolescent patients received a TNF-α inhibitor for the treatment of TEN.13,17,21 Two patients received infliximab as a second-line medication after failure of IVIG to arrest progression of disease13,17 and one patient received infliximab as a second-line medication after dexamethasone.21 Each of these patients recovered without any reported infections or long-term complications.
Although excluded from this review, both infliximab and etanercept have been reported to show benefit in acute generalized exanthematous pustulosis/TEN overlap.26,27 Interestingly, in postmarketing surveillance, rare reports have implicated both infliximab and etanercept in causing both SJS and TEN.28 Also, there have been case reports of adalimumab causing SJS, but no cases of it causing TEN were identified.29,30
CONCLUSION
Rapid discontinuation of the culprit drug and aggressive supportive care remain the primary treatment of TEN. Tumor necrosis factor α inhibitors as monotherapy or as second-line agents show promise in the treatment of this complex disease state in both the adult and pediatric populations. The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections. Additional studies are needed to further characterize the role of TNF-α inhibition in the treatment of TEN.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173-186.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187-203.
- Fernando S. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;55:165-171.
- Paquet P, Paquet F, Saleh W, et al. Immunoregulatory effector cells in drug-induced toxic epidermal necrolysis. Am J Dermatopathol. 2000;22:413-417.
- Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850-855.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and INF-γ are potential inducers of Fas-mediated keratinocyte apoptosis thought activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Paquet P, Pierard G. Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med. 1998;1:459-462.
- Correia O, Delgado L, Barbosa I, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- Redondo P, Rutz de Erenchun F, Iglesias M, et al. Toxic epidermal necrolysis. treatment with pentoxifylline. Br J Dermatol. 1994;130:688-689.
- Wolkenstein P, Latarjet J, Roujeau J, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch W, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-708.
- Hunger R, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Al-Shouli S, Bogusz M, Al Tufail M, et al. Toxic epidermal necrosis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-553.
- Famularo G, Di Dona B, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Worsnop F, Wee J, Moosa Y, et al. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37:879-881.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulins. Dermatology. 2012;224:134-139.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Paquet P, Jennes S, Rousseua A, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;1:1-6.
- Sanclemente G, De le Rouche C, Escobar C, et al. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 1998;38:878-879.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumor necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South Med J. 2009;102:1133-1140.
- Muna S, Lawrance I. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Mounach A, Rezgi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173-186.
- Schwartz R, McDonough P, Lee B. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187-203.
- Fernando S. The management of toxic epidermal necrolysis. Australas J Dermatol. 2012;55:165-171.
- Paquet P, Paquet F, Saleh W, et al. Immunoregulatory effector cells in drug-induced toxic epidermal necrolysis. Am J Dermatopathol. 2000;22:413-417.
- Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123:850-855.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and INF-γ are potential inducers of Fas-mediated keratinocyte apoptosis thought activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Paquet P, Pierard G. Soluble fractions of tumor necrosis factor-alpha, interleukin-6 and of their receptors in toxic epidermal necrolysis: a comparison with second-degree burns. Int J Mol Med. 1998;1:459-462.
- Correia O, Delgado L, Barbosa I, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- Redondo P, Rutz de Erenchun F, Iglesias M, et al. Toxic epidermal necrolysis. treatment with pentoxifylline. Br J Dermatol. 1994;130:688-689.
- Wolkenstein P, Latarjet J, Roujeau J, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch W, et al. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-708.
- Hunger R, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Al-Shouli S, Bogusz M, Al Tufail M, et al. Toxic epidermal necrosis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-553.
- Famularo G, Di Dona B, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Worsnop F, Wee J, Moosa Y, et al. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37:879-881.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulins. Dermatology. 2012;224:134-139.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Paquet P, Jennes S, Rousseua A, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;1:1-6.
- Sanclemente G, De le Rouche C, Escobar C, et al. Pentoxifylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int J Dermatol. 1998;38:878-879.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumor necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Borras-Blasco J, Navarro-Ruiz A, Borras C, et al. Adverse cutaneous reactions induced by TNF-α antagonist therapy. South Med J. 2009;102:1133-1140.
- Muna S, Lawrance I. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Mounach A, Rezgi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
Practice Points
- Controversy remains over the most effective adjunctive therapy for toxic epidermal necrolysis (TEN), as none have consistently displayed superiority over rapid discontinuation of the culprit drug and aggressive supportive care alone.
- Since tumor necrosis factor α (TNF-α) was implicated in the pathogenesis of TEN, TNF-α inhibition has been attempted in treatment of the disease. These medications have shown positive outcomes.
- The risks of these potent immunosuppressants must be weighed, and if administered, patients must be closely monitored for infections.
In Throwers With Posterior Instability, Rotator Cuff Tears Are Common but Do Not Affect Surgical Outcomes
ABSTRACT
In a previous study, compared with throwing athletes with superior labral anterior posterior (SLAP) tears, those with concomitant SLAP tears and rotator cuff tears (RCTs) had significantly poorer outcome scores and return to play. Posterior shoulder instability also occurs in throwing athletes, but no studies currently exist regarding outcomes of these patients with concomitant RCTs.
The authors hypothesized that throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent rotator cuff pathology would have poorer outcome scores and return to play.
Fifty-six consecutive throwing athletes with unidirectional posterior shoulder instability underwent arthroscopic capsulolabral repair. Preoperative and postoperative patient-centered outcomes of pain, stability, function, range of motion, strength, and American Shoulder and Elbow Surgeons Shoulder (ASES) scores, as well as return to play, were evaluated. Patients with and without rotator cuff pathology were compared.
Forty-three percent (24/56) of throwing athletes had rotator cuff pathology in addition to posterior capsulolabral pathology. All RCTs were débrided. At a mean of 3 years, there were no differences in preoperative and postoperative patient-centered outcomes between those with and without RCTs. Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport (P = .414) and 67% (16/24) returned to the same level (P = .430).
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike the previous study evaluating throwers outcomes after surgical treatment for concomitant SLAP tears and RCTs, the authors found no difference in patient-reported outcome measures or return to play for throwing athletes with concomitant posterior shoulder instability and RCTs. In throwing athletes with concomitant posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
Continue to: Posterior shoulder instability...
Posterior shoulder instability is an important and increasingly recognized pathology among throwers. Like the superior labrum, the posterior capsulolabral complex is also susceptible to injury during the throwing motion; the posterior labrum being most at risk during the late cocking and follow-through phases. Recent studies have found that arthroscopic capsulolabral reconstruction in posterior shoulder instability is successful in allowing athletes to return to their preinjury sports activities, with 2 studies detailing outcomes in throwing athletes.1-4 However, superior labral anterior posterior (SLAP) tears are common in throwing athletes and have been treated with varying and limited success. Further, in a study of outcomes of arthroscopic repair of SLAP lesions, Neri and colleagues5 found that, compared with throwing athletes with SLAP tears, throwing athletes with concomitant SLAP tears and partial-thickness rotator cuff tears (RCTs) had significantly poorer outcomes and return-to-play rates after surgical repair.
The purpose of this study was to determine outcome scores and return to play of throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent RCTs and to compare them with outcome scores as well as return to play of throwing athletes with isolated posterior shoulder instability. It was hypothesized that throwing athletes with a combination of posterior shoulder instability and RCT would have poorer outcomes and poorer return to play after surgery.5
METHODS
PATIENT SELECTION
After Institutional Review Board approval, informed consent was obtained, and consecutive throwing athletes who underwent arthroscopic posterior capsulolabral reconstruction for posterior shoulder instability were followed in the perioperative period. Inclusion criteria were throwing athletes participating in competitive sports at the high school, collegiate, or professional level, minimum 1-year follow-up, presence of unidirectional posterior instability, and absence of symptoms of instability in any direction other than posterior. Patients with inferior instability, SLAP pathology on examination and on magnetic resonance imaging, multidirectional instability, or habitual or psychogenic voluntary shoulder subluxations were excluded. Patients with diagnoses of both posterior shoulder instability and impingement treated with subacromial decompression and distal clavicle resection were also excluded.
After this cohort was identified, patient records were reviewed for pertinent operative data, such as procedure, complications, and evidence of RCT by operative report and arthroscopic photographs. A partial RCT was defined as a tear of 10% to 50%; those with rotator cuff fraying were determined not to be significant.
PATIENT EVALUATION
Surgeries were performed between January 1998 and December 2009 by the senior author (JPB). All patients were followed with clinical examinations, radiographs, and subjective grading scales. Recorded patient demographic data included age, sex, sport, position, competition level, and follow-up duration.
Continue to: All patients had...
All patients had symptomatic posterior shoulder instability, including posterior shoulder pain, clicking, a sensation of subluxation, or instability/apprehension with motion. Each athlete’s shoulder was palpated for tenderness and tested for impingement. Specific posterior glenohumeral instability tests, including the Kim test,6 the circumduction test, the jerk test,7 the posterior load-and-shift test,8 and the posterior stress test,9 were performed on all patients. Patients with multidirectional instability on the sulcus test, as well as provocative tests indicating SLAP pathology, such as the Crank test and the active compression test, were not included. Standard radiography and magnetic resonance arthrography (MRA) were performed to further narrow inclusion and exclusion criteria.
Both before surgery and at latest follow-up, patient outcomes were evaluated using the American Shoulder and Elbow Surgeons (ASES) score (range, 0-100) which combines a subjective functional scale measuring activities of daily living (0-3 for each of 10 tasks, with a total of 0-30) and a subjective pain scale (0-10, with 10 being worst pain). Values >80 were described as excellent, and failures were defined as scores <60 after surgery.10 A subjective stability scale (0-10, with 0 indicating completely stable and 10 completely unstable), strength scale (0-3, with 0 indicating none, 1 markedly decreased, 2 slightly decreased, and 3 normal), and ROM scale (0-3, with 0 indicating poor, 1 limited, 2 satisfactory, and 3 full) were evaluated both before surgery and at the latest follow-up. A stability score >5 after surgery was defined as a failure.1,2,11 Patients were also asked if, based on their current state, they would undergo surgery again. Intraoperative findings and specific surgical procedures performed were correlated with the aforementioned subjective and objective outcome scores.
OPERATIVE TREATMENT
Throwing athletes who met inclusion criteria and failed nonoperative management underwent surgery by the senior author (JPB). Each patient was examined under anesthesia and, with the patient in the lateral decubitus position, a diagnostic arthroscopy was performed to identify posterior capsulolabral complex pathology, including a patulous capsule, capsular tears, labral fraying, and labral tears. A careful examination for rotator cuff pathology was also performed. Based on preoperative clinical examination, MRA, examination under anesthesia, pathologic findings at diagnostic arthroscopic surgery, and surgeon experience, capsulolabral plication was performed with or without suture anchors.2,5 After capsulolabral repair, the capsule was evaluated for residual laxity, and additional plication sutures were placed, as indicated, with care to avoid overconstraint in these throwing athletes.1 Posterior glenohumeral stability restoration was judged by removing traction and performing posterior load-and-shift and posterior stress tests. Any RCT with <50% thickness was débrided. Postoperative care and rehabilitation were carried out as previously described and were not altered by the presence or absence of a RCT.3
STATISTICAL ANALYSIS
Preoperative and latest follow-up ASES scores, stability scores, functional scores, and pain-level findings were compared using paired-samples Comparisons between groups, including throwing athletes with and without rotator cuff pathology, were done using the Student t test. Outcome comparisons between multiple groups, which included intraoperative findings and surgical fixation methods, were analyzed with c2 modeling for nonparametric data. Statistical significance was set at P < .05. A power analysis found that this study was able to detect a meaningful difference of 10 ASES points.
RESULTS
PATIENT DEMOGRAPHIC CHARACTERISTICS
Of the 56 throwing athletes who met the inclusion criteria, 24 were found to have rotator cuff pathology in addition to posterior capsulolabral pathology, while 32 were found to have capsulolabral pathology alone. Demographic data are listed in Table 1. Mean age was 20.1 years for patients with rotator cuff pathology and 17.8 years for patients without RCTs. All 24 athletes with rotator cuff pathology were treated with arthroscopic débridement. Mean follow-up was 38.6 months (range, 16.5-63.6 months) for patients with RCTs and 39.1 months (range, 12-98.8 months) for patients without RCTs. No significant difference was found in age, sports level, or follow-up between groups.
Table 1. Demographic Data for Athletes With Posterior Instability With and Without Rotator Cuff Tears (N = 56 Shoulders)a
Characteristic | Rotator Cuff Tears | |
Yes | No | |
| Total | 24 | 32 |
| Sex | ||
| Male | 16 | 27 |
| Female | 8 | 5 |
| Mean age, y | 20.1 | 17.8 |
| Mean follow up, mo | 38.6 | 39.1 |
| Participation level | ||
| Professional | 1 | 0 |
| College | 4 | 4 |
| High school | 17 | 26 |
| Recreational | 2 | 2 |
aThe majority of athletes were males in high school and their mean follow-up was 3 years.
Continue to: Outcomes
OUTCOMES
Table 2 lists the preoperative and postoperative scores for shoulder performance in throwing athletes with posterior shoulder instability, with and without RCTs.
Table 2. Preoperative and Postoperative Scores for Shoulder Performance in Throwing Athletes With Posterior Shoulder Instability With and Without Rotator Cuff Tearsa
| With Rotator Cuff Tears (n=24 shoulders) | Without Rotator Cuff Tears (n=32 shoulders) | |||||||||
| Preoperative | Latest Follow-Up | Preoperative | Latest Follow-Up | |||||||
| Outcome Measure | Mean Score | Range | Mean Score | Range | P | Mean Score | Range | Mean Score | Range | P |
ASES 0-100 0 = worst | 41.8 | 20-70 | 85.4 | 67-100 | <.05 | 49.7 | 20-85 | 83.1 | 25-100 | <.05 |
Stability 0-10 0 = most stable | 6.7 | 2-10 | 2.4 | 0-6 | <.05 | 7.8 | 0-10 | 2.4 | 0-8 | <.05 |
Pain 0-10 10 = worst | 7.6 | 5-10 | 1.9 | 0-5 | <.05 | 6.3 | 0-10 | 2.2 | 0-7 | <.05 |
Function 0-30 0 = worst | 18.5 | 6-27 | 27 | 16-30 | <.05 | 19.0 | 8-26 | 26.4 | 6-30 | <.05 |
aThere was no difference in ASES, stability, pain, or functional scores between athletes with posterior instability alone compared with patients with concomitant rotator cuff tears.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
ASES Scores. Mean preoperative ASES scores for patients with RCTs improved significantly (t = –13.8, P < .001), as did those for patients without rotator cuff pathology (t = –8.9, P < .001). No significant differences in ASES score were found between patients with and without rotator cuff pathology before or after surgery (t = 1.9, P = .07; t = .58, P = .06). In addition, 70.8% (17/24) of throwing athletes with rotator cuff pathology had an excellent postoperative outcome (ASES score >80), and 29.2% (7/24) had a satisfactory outcome (ASES score, 60-80). Thus, 100% of those with concomitant posterior shoulder instability and RCTs had a good or excellent outcome after surgical intervention. In those without rotator cuff pathology, 78.1% (25/32) had an excellent outcome, 12.5% (4/32) had a satisfactory outcome, and 9.4% (3/32) had a poor outcome. Thus, 91% of those without rotator cuff pathology had a good or excellent outcome after surgery.
Stability. Preoperative stability scores improved significantly after surgery in both groups (t = 7.2, P < .001; t = 10.5, P < .001). There were no statistical differences between preoperative or postoperative stability scores in those with or without rotator cuff pathology (t = 1.7, P = .095; t = .03, P = .975). Of throwing athletes with RCTs, 54.2% (13/24) had an excellent outcome, 33.3% (8/24) a good outcome, and 12.5% (3/24) a satisfactory outcome. Thus, 87.5% (21/24) of those with RCTs had a good or excellent outcome in terms of stability. In those without rotator cuff pathology, 46.9% (15/32) had excellent stability, 46.9% (15/32) had good stability, and 3.1% (1/32) had satisfactory stability after surgery. Thus, 93.8% (30/32) of throwing athletes without rotator cuff pathology had good or excellent stability after surgery.
Pain. Mean preoperative pain scores for those with and without rotator cuff pathology improved significantly (t = 13.4, P < .001; t = 7.1, P < .001). There was no statistical difference in preoperative or postoperative pain scores between those with and without rotator cuff pathology (t = 1.99, P = .051; t = .49, P = .627).
Function. Mean preoperative function scores for both groups improved significantly (t = 7.7, P < .001; t = 8.0, P < .001). There was no difference in improvement in functional scores between the two groups before or after surgery (t = .36, P = .721; t = .5, P = .622).
Continue to: ROM
ROM. Of those with rotator cuff pathology, 54% (13/24) had normal ROM, 42% (10/24) had satisfactory ROM, and 4% (1/24) had limited ROM. In throwing athletes without rotator cuff pathology, 34% (11/32) had normal ROM, 53.1% (17/32) had satisfactory ROM, and 9% (3/32) had limited ROM after surgery. There was no significant difference in ROM between the groups (c2 = 2.7, P = .260).
Strength. Of those with RCTs, 67% (16/24) reported normal strength, 29% (7/24) slightly decreased strength, and 4% (1/24) markedly decreased strength. Of those throwing athletes without rotator cuff pathology, 50% (16/32) had normal strength, 41% (13/32) had slightly decreased strength, and 9% (3/32) had markedly decreased strength. No statistical difference was noted between the two groups (c2 = 1.7, P = .429).
Return to Sport. Of those with RCTs, 92% (22/24) returned to sport while 84% (27/32) of throwing athletes without RCTs returned to sport. There was no difference between the two groups (c2 = .667, P = .414). Sixty-seven percent (16/24) of those with RCTs and 56% (18/32) of those without RCTs returned to the same level of sport. No statistical difference was found in return to play between throwing athletes with and without rotator cuff pathology (c2 = .624, P = .430).
Failures. According to ASES scores, no throwers with RCTs failed, while 9.4% (3/32) with posterior instability alone failed. Regarding stability, 8.3% (2/24) of athletes with RCTs failed, while 6.3% (2/32) with posterior instability alone failed.
SURGICAL FINDINGS AND PROCEDURES
Of the 24 throwing athletes with rotator cuff pathology, 92% (22/24) had labral tears, while 78% (25/32) of those without RCTs had labral tears. The majority of RCTs were in the posterior supraspinatus and anterior infraspinatus regions. This was not significantly different between groups (c2 = 1.86, P = .172). All labral pathology was posterior-inferior, and all RCTs were <50% thickness, and therefore were débrided. Fifty-four percent (13/24) of those with RCTs had a patulous capsule and 63% (20/32) of throwing athletes without rotator cuff pathology had a patulous capsule. There was no significant difference between groups (c2 = .393, P = .530). Of those with RCTs, 92% (22/24) had surgical fixation with anchors, while 78% (25/32) of those without rotator cuff pathology underwent repair with anchor fixation. There was no statistically significant difference in anchor use between groups (c2 = 1.86, P = .172).
Continue to: Discussion
DISCUSSION
Throwing athletes with and without RCTs had similar rates of recovery and return to play after arthroscopic capsular labral repair, with rotator cuff débridement if a tear was present. The mean follow-up was 3.2 years. Further, there was no difference in return to play (92% vs 84%), ASES score, stability, pain, function, ROM, or strength between the 2 groups before or after surgery. In this cohort of 56 patients, 24 throwing athletes (43%) were found to have RCTs.
Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport, and 67% (16/24) returned to the same level. Eight percent of throwing athletes with RCTs were unable to return to sport after surgery. These return-to-play rates are an improvement over most previously reported rates in throwing athletes and in posterior shoulder instability in general.1-4,11 When these athletes are compared with their counterparts with combined SLAP tears and RCTs, return-to-play rates are notably higher. There may be discrepancies in interpreting return-to-play between the two studies, but in the current study, 67% of those with concomitant RCTs achieved return to preinjury level of play. This is 10% higher than the rate reported in athletes with SLAP tears alone (57%) and even higher than in those with concomitant SLAP and RCTs. It is also essential to note that a number of this cohort’s athletes who did not return to play did so for factors (eg, graduation) unrelated to the shoulder. However, the study by Neri and colleagues5 included professional athletes who likely all attempted to return to play and, if unable to perform at the same level, likely were unable to continue their professional career.5
All patients with RCTs had a good or excellent outcome (ASES score), and 70.8% had an excellent outcome. Similarly, 97% of those without rotator cuff pathology had a good or excellent outcome, and 81.3% had an excellent outcome. There was no significant difference between the two groups. These results parallel those of Neri and colleagues’5 study of SLAP tears with RCTs, where 96% (22/23) of throwing athletes had a good or excellent outcome. Despite these high outcome scores in patients with SLAP tears, only 57% were able to return to elite pitching.5 In the current study, pain was slightly higher for those with rotator cuff pathology before surgery—a finding consistent with pain frequently being found in patients with isolated partial-thickness RCTs. Their postoperative pain scores were actually lower on average than those of patients without RCTs, which suggests simple débridement of undersurface tears adequately addressed the pathology. The authors theorize that the main pain generator in this population may be posterior instability, and that the rotator cuff has less of an influence. In the SLAP population, the main pain generator likely is the RCT.
Failures by ASES score or strength were fairly rare in this cohort. Many patients opted to have revision surgery because of continued instability, pain, decreased function, or reinjury. One potential cause of failure in this cohort is inadequate capsular shift. However, capsular plication in throwing athletes is difficult to address, as overtensioning the repair can lead to the inability to adequately perform overhead activites.3,4 This cannot be overemphasized, particularly with pitchers.
Partial-thickness RCTs, particularly those on the articular side, are common in throwing athletes because of high tensile and compressive loads.12 Despite the known risk of RCTs with posterior shoulder instability in throwing athletes, the authors are unaware of reports of the incidence or treatment of this pathology. RCTs in this posterior instability group likely represent a pathology other than internal impingement. The high proportion of throwing athletes with RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes. Ide et al found that 75% of patients with SLAP tears had partial articular-sided RCTs.13 In the current study, all RCTs were small partial tears, and arthroscopic débridement was performed. It is unknown whether repair of these RCTs would impact return to play. However, rotator cuff repair in this population has been shown to have poor outcomes. Tear thickness typically is used to determine treatment, with débridement performed if <50% tendon thickness is affected. More recently, many have advocated having greater tendon involvement in throwers before repair, because of poor outcomes. Although studies are limited, tear size does seem to correlate with outcomes.14
Continue to: Study Limitations
STUDY LIMITATIONS
Limitations of this study include its small number of professional throwing athletes, with the majority being high school athletes. Further, although ASES scores are consistently used in posterior shoulder instability studies, these scores are influenced highly by pain scores, and some argue that other scoring systems may provide more useful information. However, none of the more modern scoring systems have been studied extensively in posterior glenohumeral instability. Further, because the authors used the present scoring systems previously,1-4 they were continued to be used for comparison and consistency. Outcomes such as ROM and strength may carry more weight if measured and documented by clinical examination. Further testing, such as clinical evaluation of the jerk test or the posterior load-and-shift test, and their comparison before and after surgery may provide more objective data.
CONCLUSION
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike a previous study of throwing athletes’ outcomes after surgery for concomitant SLAP tears and RCTs,5 this study of throwing athletes with concomitant posterior shoulder instability and RCTs found no difference in patient-reported outcome measures or return to play. In throwing athletes with posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
1. Bradley JP, Baker CL 3rd, Kline AJ, Armfield DR, Chhabra A. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 100 shoulders. Am J Sports Med. 2006;34(7):1061-1071.
2. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014.
3. McClincy MP, Arner JW, Bradley JP. Posterior shoulder instability in throwing athletes: a case-matched comparison of throwers and non-throwers. Arthroscopy. 2015;31(6):1041-1051.
4. Radkowski CA, Chhabra A, Baker CL 3rd, Tejwani SG, Bradley JP. Arthroscopic capsulolabral repair for posterior shoulder instability in throwing athletes compared with nonthrowing athletes. Am J Sports Med. 2008;36(4):693-699.
5. Neri BR, ElAttrache NS, Owsley KC, Mohr K, Yocum LA. Outcome of type II superior labral anterior posterior repairs in elite overhead athletes: effect of concomitant partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(1):114-120.
6. Kim SH, Park JS, Jeong WK, Shin SK. The Kim test: a novel test for posteroinferior labral lesion of the shoulder—a comparison to the jerk test. Am J Sports Med. 2005;33(8):1188-1192.
7. Antoniou J, Duckworth DT, Harryman DT 2nd. Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg Am. 2000;82(9):1220-1230.
8. Altchek DW, Hobbs WR. Evaluation and management of shoulder instability in the elite overhead thrower. Orthop Clin North Am. 2001;32(3):423-430, viii.
9. Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82(1):16-25.
10. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
11. Arner JW, McClincy MP, Bradley JP. Arthroscopic stabilization of posterior shoulder instability is successful in American football players. Arthroscopy. 2015;31(8):1466-1471.
12. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
13. Ide J, Maeda S, Takagi K. Sports activity after arthroscopic superior labral repair using suture anchors in overhead-throwing athletes. Am J Sports Med. 2005;33(4):507-514.
14. Economopoulos KJ, Brockmeier SF. Rotator cuff tears in overhead athletes. Clin Sports Med. 2012;31(4):675-692.
ABSTRACT
In a previous study, compared with throwing athletes with superior labral anterior posterior (SLAP) tears, those with concomitant SLAP tears and rotator cuff tears (RCTs) had significantly poorer outcome scores and return to play. Posterior shoulder instability also occurs in throwing athletes, but no studies currently exist regarding outcomes of these patients with concomitant RCTs.
The authors hypothesized that throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent rotator cuff pathology would have poorer outcome scores and return to play.
Fifty-six consecutive throwing athletes with unidirectional posterior shoulder instability underwent arthroscopic capsulolabral repair. Preoperative and postoperative patient-centered outcomes of pain, stability, function, range of motion, strength, and American Shoulder and Elbow Surgeons Shoulder (ASES) scores, as well as return to play, were evaluated. Patients with and without rotator cuff pathology were compared.
Forty-three percent (24/56) of throwing athletes had rotator cuff pathology in addition to posterior capsulolabral pathology. All RCTs were débrided. At a mean of 3 years, there were no differences in preoperative and postoperative patient-centered outcomes between those with and without RCTs. Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport (P = .414) and 67% (16/24) returned to the same level (P = .430).
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike the previous study evaluating throwers outcomes after surgical treatment for concomitant SLAP tears and RCTs, the authors found no difference in patient-reported outcome measures or return to play for throwing athletes with concomitant posterior shoulder instability and RCTs. In throwing athletes with concomitant posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
Continue to: Posterior shoulder instability...
Posterior shoulder instability is an important and increasingly recognized pathology among throwers. Like the superior labrum, the posterior capsulolabral complex is also susceptible to injury during the throwing motion; the posterior labrum being most at risk during the late cocking and follow-through phases. Recent studies have found that arthroscopic capsulolabral reconstruction in posterior shoulder instability is successful in allowing athletes to return to their preinjury sports activities, with 2 studies detailing outcomes in throwing athletes.1-4 However, superior labral anterior posterior (SLAP) tears are common in throwing athletes and have been treated with varying and limited success. Further, in a study of outcomes of arthroscopic repair of SLAP lesions, Neri and colleagues5 found that, compared with throwing athletes with SLAP tears, throwing athletes with concomitant SLAP tears and partial-thickness rotator cuff tears (RCTs) had significantly poorer outcomes and return-to-play rates after surgical repair.
The purpose of this study was to determine outcome scores and return to play of throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent RCTs and to compare them with outcome scores as well as return to play of throwing athletes with isolated posterior shoulder instability. It was hypothesized that throwing athletes with a combination of posterior shoulder instability and RCT would have poorer outcomes and poorer return to play after surgery.5
METHODS
PATIENT SELECTION
After Institutional Review Board approval, informed consent was obtained, and consecutive throwing athletes who underwent arthroscopic posterior capsulolabral reconstruction for posterior shoulder instability were followed in the perioperative period. Inclusion criteria were throwing athletes participating in competitive sports at the high school, collegiate, or professional level, minimum 1-year follow-up, presence of unidirectional posterior instability, and absence of symptoms of instability in any direction other than posterior. Patients with inferior instability, SLAP pathology on examination and on magnetic resonance imaging, multidirectional instability, or habitual or psychogenic voluntary shoulder subluxations were excluded. Patients with diagnoses of both posterior shoulder instability and impingement treated with subacromial decompression and distal clavicle resection were also excluded.
After this cohort was identified, patient records were reviewed for pertinent operative data, such as procedure, complications, and evidence of RCT by operative report and arthroscopic photographs. A partial RCT was defined as a tear of 10% to 50%; those with rotator cuff fraying were determined not to be significant.
PATIENT EVALUATION
Surgeries were performed between January 1998 and December 2009 by the senior author (JPB). All patients were followed with clinical examinations, radiographs, and subjective grading scales. Recorded patient demographic data included age, sex, sport, position, competition level, and follow-up duration.
Continue to: All patients had...
All patients had symptomatic posterior shoulder instability, including posterior shoulder pain, clicking, a sensation of subluxation, or instability/apprehension with motion. Each athlete’s shoulder was palpated for tenderness and tested for impingement. Specific posterior glenohumeral instability tests, including the Kim test,6 the circumduction test, the jerk test,7 the posterior load-and-shift test,8 and the posterior stress test,9 were performed on all patients. Patients with multidirectional instability on the sulcus test, as well as provocative tests indicating SLAP pathology, such as the Crank test and the active compression test, were not included. Standard radiography and magnetic resonance arthrography (MRA) were performed to further narrow inclusion and exclusion criteria.
Both before surgery and at latest follow-up, patient outcomes were evaluated using the American Shoulder and Elbow Surgeons (ASES) score (range, 0-100) which combines a subjective functional scale measuring activities of daily living (0-3 for each of 10 tasks, with a total of 0-30) and a subjective pain scale (0-10, with 10 being worst pain). Values >80 were described as excellent, and failures were defined as scores <60 after surgery.10 A subjective stability scale (0-10, with 0 indicating completely stable and 10 completely unstable), strength scale (0-3, with 0 indicating none, 1 markedly decreased, 2 slightly decreased, and 3 normal), and ROM scale (0-3, with 0 indicating poor, 1 limited, 2 satisfactory, and 3 full) were evaluated both before surgery and at the latest follow-up. A stability score >5 after surgery was defined as a failure.1,2,11 Patients were also asked if, based on their current state, they would undergo surgery again. Intraoperative findings and specific surgical procedures performed were correlated with the aforementioned subjective and objective outcome scores.
OPERATIVE TREATMENT
Throwing athletes who met inclusion criteria and failed nonoperative management underwent surgery by the senior author (JPB). Each patient was examined under anesthesia and, with the patient in the lateral decubitus position, a diagnostic arthroscopy was performed to identify posterior capsulolabral complex pathology, including a patulous capsule, capsular tears, labral fraying, and labral tears. A careful examination for rotator cuff pathology was also performed. Based on preoperative clinical examination, MRA, examination under anesthesia, pathologic findings at diagnostic arthroscopic surgery, and surgeon experience, capsulolabral plication was performed with or without suture anchors.2,5 After capsulolabral repair, the capsule was evaluated for residual laxity, and additional plication sutures were placed, as indicated, with care to avoid overconstraint in these throwing athletes.1 Posterior glenohumeral stability restoration was judged by removing traction and performing posterior load-and-shift and posterior stress tests. Any RCT with <50% thickness was débrided. Postoperative care and rehabilitation were carried out as previously described and were not altered by the presence or absence of a RCT.3
STATISTICAL ANALYSIS
Preoperative and latest follow-up ASES scores, stability scores, functional scores, and pain-level findings were compared using paired-samples Comparisons between groups, including throwing athletes with and without rotator cuff pathology, were done using the Student t test. Outcome comparisons between multiple groups, which included intraoperative findings and surgical fixation methods, were analyzed with c2 modeling for nonparametric data. Statistical significance was set at P < .05. A power analysis found that this study was able to detect a meaningful difference of 10 ASES points.
RESULTS
PATIENT DEMOGRAPHIC CHARACTERISTICS
Of the 56 throwing athletes who met the inclusion criteria, 24 were found to have rotator cuff pathology in addition to posterior capsulolabral pathology, while 32 were found to have capsulolabral pathology alone. Demographic data are listed in Table 1. Mean age was 20.1 years for patients with rotator cuff pathology and 17.8 years for patients without RCTs. All 24 athletes with rotator cuff pathology were treated with arthroscopic débridement. Mean follow-up was 38.6 months (range, 16.5-63.6 months) for patients with RCTs and 39.1 months (range, 12-98.8 months) for patients without RCTs. No significant difference was found in age, sports level, or follow-up between groups.
Table 1. Demographic Data for Athletes With Posterior Instability With and Without Rotator Cuff Tears (N = 56 Shoulders)a
Characteristic | Rotator Cuff Tears | |
Yes | No | |
| Total | 24 | 32 |
| Sex | ||
| Male | 16 | 27 |
| Female | 8 | 5 |
| Mean age, y | 20.1 | 17.8 |
| Mean follow up, mo | 38.6 | 39.1 |
| Participation level | ||
| Professional | 1 | 0 |
| College | 4 | 4 |
| High school | 17 | 26 |
| Recreational | 2 | 2 |
aThe majority of athletes were males in high school and their mean follow-up was 3 years.
Continue to: Outcomes
OUTCOMES
Table 2 lists the preoperative and postoperative scores for shoulder performance in throwing athletes with posterior shoulder instability, with and without RCTs.
Table 2. Preoperative and Postoperative Scores for Shoulder Performance in Throwing Athletes With Posterior Shoulder Instability With and Without Rotator Cuff Tearsa
| With Rotator Cuff Tears (n=24 shoulders) | Without Rotator Cuff Tears (n=32 shoulders) | |||||||||
| Preoperative | Latest Follow-Up | Preoperative | Latest Follow-Up | |||||||
| Outcome Measure | Mean Score | Range | Mean Score | Range | P | Mean Score | Range | Mean Score | Range | P |
ASES 0-100 0 = worst | 41.8 | 20-70 | 85.4 | 67-100 | <.05 | 49.7 | 20-85 | 83.1 | 25-100 | <.05 |
Stability 0-10 0 = most stable | 6.7 | 2-10 | 2.4 | 0-6 | <.05 | 7.8 | 0-10 | 2.4 | 0-8 | <.05 |
Pain 0-10 10 = worst | 7.6 | 5-10 | 1.9 | 0-5 | <.05 | 6.3 | 0-10 | 2.2 | 0-7 | <.05 |
Function 0-30 0 = worst | 18.5 | 6-27 | 27 | 16-30 | <.05 | 19.0 | 8-26 | 26.4 | 6-30 | <.05 |
aThere was no difference in ASES, stability, pain, or functional scores between athletes with posterior instability alone compared with patients with concomitant rotator cuff tears.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
ASES Scores. Mean preoperative ASES scores for patients with RCTs improved significantly (t = –13.8, P < .001), as did those for patients without rotator cuff pathology (t = –8.9, P < .001). No significant differences in ASES score were found between patients with and without rotator cuff pathology before or after surgery (t = 1.9, P = .07; t = .58, P = .06). In addition, 70.8% (17/24) of throwing athletes with rotator cuff pathology had an excellent postoperative outcome (ASES score >80), and 29.2% (7/24) had a satisfactory outcome (ASES score, 60-80). Thus, 100% of those with concomitant posterior shoulder instability and RCTs had a good or excellent outcome after surgical intervention. In those without rotator cuff pathology, 78.1% (25/32) had an excellent outcome, 12.5% (4/32) had a satisfactory outcome, and 9.4% (3/32) had a poor outcome. Thus, 91% of those without rotator cuff pathology had a good or excellent outcome after surgery.
Stability. Preoperative stability scores improved significantly after surgery in both groups (t = 7.2, P < .001; t = 10.5, P < .001). There were no statistical differences between preoperative or postoperative stability scores in those with or without rotator cuff pathology (t = 1.7, P = .095; t = .03, P = .975). Of throwing athletes with RCTs, 54.2% (13/24) had an excellent outcome, 33.3% (8/24) a good outcome, and 12.5% (3/24) a satisfactory outcome. Thus, 87.5% (21/24) of those with RCTs had a good or excellent outcome in terms of stability. In those without rotator cuff pathology, 46.9% (15/32) had excellent stability, 46.9% (15/32) had good stability, and 3.1% (1/32) had satisfactory stability after surgery. Thus, 93.8% (30/32) of throwing athletes without rotator cuff pathology had good or excellent stability after surgery.
Pain. Mean preoperative pain scores for those with and without rotator cuff pathology improved significantly (t = 13.4, P < .001; t = 7.1, P < .001). There was no statistical difference in preoperative or postoperative pain scores between those with and without rotator cuff pathology (t = 1.99, P = .051; t = .49, P = .627).
Function. Mean preoperative function scores for both groups improved significantly (t = 7.7, P < .001; t = 8.0, P < .001). There was no difference in improvement in functional scores between the two groups before or after surgery (t = .36, P = .721; t = .5, P = .622).
Continue to: ROM
ROM. Of those with rotator cuff pathology, 54% (13/24) had normal ROM, 42% (10/24) had satisfactory ROM, and 4% (1/24) had limited ROM. In throwing athletes without rotator cuff pathology, 34% (11/32) had normal ROM, 53.1% (17/32) had satisfactory ROM, and 9% (3/32) had limited ROM after surgery. There was no significant difference in ROM between the groups (c2 = 2.7, P = .260).
Strength. Of those with RCTs, 67% (16/24) reported normal strength, 29% (7/24) slightly decreased strength, and 4% (1/24) markedly decreased strength. Of those throwing athletes without rotator cuff pathology, 50% (16/32) had normal strength, 41% (13/32) had slightly decreased strength, and 9% (3/32) had markedly decreased strength. No statistical difference was noted between the two groups (c2 = 1.7, P = .429).
Return to Sport. Of those with RCTs, 92% (22/24) returned to sport while 84% (27/32) of throwing athletes without RCTs returned to sport. There was no difference between the two groups (c2 = .667, P = .414). Sixty-seven percent (16/24) of those with RCTs and 56% (18/32) of those without RCTs returned to the same level of sport. No statistical difference was found in return to play between throwing athletes with and without rotator cuff pathology (c2 = .624, P = .430).
Failures. According to ASES scores, no throwers with RCTs failed, while 9.4% (3/32) with posterior instability alone failed. Regarding stability, 8.3% (2/24) of athletes with RCTs failed, while 6.3% (2/32) with posterior instability alone failed.
SURGICAL FINDINGS AND PROCEDURES
Of the 24 throwing athletes with rotator cuff pathology, 92% (22/24) had labral tears, while 78% (25/32) of those without RCTs had labral tears. The majority of RCTs were in the posterior supraspinatus and anterior infraspinatus regions. This was not significantly different between groups (c2 = 1.86, P = .172). All labral pathology was posterior-inferior, and all RCTs were <50% thickness, and therefore were débrided. Fifty-four percent (13/24) of those with RCTs had a patulous capsule and 63% (20/32) of throwing athletes without rotator cuff pathology had a patulous capsule. There was no significant difference between groups (c2 = .393, P = .530). Of those with RCTs, 92% (22/24) had surgical fixation with anchors, while 78% (25/32) of those without rotator cuff pathology underwent repair with anchor fixation. There was no statistically significant difference in anchor use between groups (c2 = 1.86, P = .172).
Continue to: Discussion
DISCUSSION
Throwing athletes with and without RCTs had similar rates of recovery and return to play after arthroscopic capsular labral repair, with rotator cuff débridement if a tear was present. The mean follow-up was 3.2 years. Further, there was no difference in return to play (92% vs 84%), ASES score, stability, pain, function, ROM, or strength between the 2 groups before or after surgery. In this cohort of 56 patients, 24 throwing athletes (43%) were found to have RCTs.
Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport, and 67% (16/24) returned to the same level. Eight percent of throwing athletes with RCTs were unable to return to sport after surgery. These return-to-play rates are an improvement over most previously reported rates in throwing athletes and in posterior shoulder instability in general.1-4,11 When these athletes are compared with their counterparts with combined SLAP tears and RCTs, return-to-play rates are notably higher. There may be discrepancies in interpreting return-to-play between the two studies, but in the current study, 67% of those with concomitant RCTs achieved return to preinjury level of play. This is 10% higher than the rate reported in athletes with SLAP tears alone (57%) and even higher than in those with concomitant SLAP and RCTs. It is also essential to note that a number of this cohort’s athletes who did not return to play did so for factors (eg, graduation) unrelated to the shoulder. However, the study by Neri and colleagues5 included professional athletes who likely all attempted to return to play and, if unable to perform at the same level, likely were unable to continue their professional career.5
All patients with RCTs had a good or excellent outcome (ASES score), and 70.8% had an excellent outcome. Similarly, 97% of those without rotator cuff pathology had a good or excellent outcome, and 81.3% had an excellent outcome. There was no significant difference between the two groups. These results parallel those of Neri and colleagues’5 study of SLAP tears with RCTs, where 96% (22/23) of throwing athletes had a good or excellent outcome. Despite these high outcome scores in patients with SLAP tears, only 57% were able to return to elite pitching.5 In the current study, pain was slightly higher for those with rotator cuff pathology before surgery—a finding consistent with pain frequently being found in patients with isolated partial-thickness RCTs. Their postoperative pain scores were actually lower on average than those of patients without RCTs, which suggests simple débridement of undersurface tears adequately addressed the pathology. The authors theorize that the main pain generator in this population may be posterior instability, and that the rotator cuff has less of an influence. In the SLAP population, the main pain generator likely is the RCT.
Failures by ASES score or strength were fairly rare in this cohort. Many patients opted to have revision surgery because of continued instability, pain, decreased function, or reinjury. One potential cause of failure in this cohort is inadequate capsular shift. However, capsular plication in throwing athletes is difficult to address, as overtensioning the repair can lead to the inability to adequately perform overhead activites.3,4 This cannot be overemphasized, particularly with pitchers.
Partial-thickness RCTs, particularly those on the articular side, are common in throwing athletes because of high tensile and compressive loads.12 Despite the known risk of RCTs with posterior shoulder instability in throwing athletes, the authors are unaware of reports of the incidence or treatment of this pathology. RCTs in this posterior instability group likely represent a pathology other than internal impingement. The high proportion of throwing athletes with RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes. Ide et al found that 75% of patients with SLAP tears had partial articular-sided RCTs.13 In the current study, all RCTs were small partial tears, and arthroscopic débridement was performed. It is unknown whether repair of these RCTs would impact return to play. However, rotator cuff repair in this population has been shown to have poor outcomes. Tear thickness typically is used to determine treatment, with débridement performed if <50% tendon thickness is affected. More recently, many have advocated having greater tendon involvement in throwers before repair, because of poor outcomes. Although studies are limited, tear size does seem to correlate with outcomes.14
Continue to: Study Limitations
STUDY LIMITATIONS
Limitations of this study include its small number of professional throwing athletes, with the majority being high school athletes. Further, although ASES scores are consistently used in posterior shoulder instability studies, these scores are influenced highly by pain scores, and some argue that other scoring systems may provide more useful information. However, none of the more modern scoring systems have been studied extensively in posterior glenohumeral instability. Further, because the authors used the present scoring systems previously,1-4 they were continued to be used for comparison and consistency. Outcomes such as ROM and strength may carry more weight if measured and documented by clinical examination. Further testing, such as clinical evaluation of the jerk test or the posterior load-and-shift test, and their comparison before and after surgery may provide more objective data.
CONCLUSION
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike a previous study of throwing athletes’ outcomes after surgery for concomitant SLAP tears and RCTs,5 this study of throwing athletes with concomitant posterior shoulder instability and RCTs found no difference in patient-reported outcome measures or return to play. In throwing athletes with posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
ABSTRACT
In a previous study, compared with throwing athletes with superior labral anterior posterior (SLAP) tears, those with concomitant SLAP tears and rotator cuff tears (RCTs) had significantly poorer outcome scores and return to play. Posterior shoulder instability also occurs in throwing athletes, but no studies currently exist regarding outcomes of these patients with concomitant RCTs.
The authors hypothesized that throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent rotator cuff pathology would have poorer outcome scores and return to play.
Fifty-six consecutive throwing athletes with unidirectional posterior shoulder instability underwent arthroscopic capsulolabral repair. Preoperative and postoperative patient-centered outcomes of pain, stability, function, range of motion, strength, and American Shoulder and Elbow Surgeons Shoulder (ASES) scores, as well as return to play, were evaluated. Patients with and without rotator cuff pathology were compared.
Forty-three percent (24/56) of throwing athletes had rotator cuff pathology in addition to posterior capsulolabral pathology. All RCTs were débrided. At a mean of 3 years, there were no differences in preoperative and postoperative patient-centered outcomes between those with and without RCTs. Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport (P = .414) and 67% (16/24) returned to the same level (P = .430).
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike the previous study evaluating throwers outcomes after surgical treatment for concomitant SLAP tears and RCTs, the authors found no difference in patient-reported outcome measures or return to play for throwing athletes with concomitant posterior shoulder instability and RCTs. In throwing athletes with concomitant posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
Continue to: Posterior shoulder instability...
Posterior shoulder instability is an important and increasingly recognized pathology among throwers. Like the superior labrum, the posterior capsulolabral complex is also susceptible to injury during the throwing motion; the posterior labrum being most at risk during the late cocking and follow-through phases. Recent studies have found that arthroscopic capsulolabral reconstruction in posterior shoulder instability is successful in allowing athletes to return to their preinjury sports activities, with 2 studies detailing outcomes in throwing athletes.1-4 However, superior labral anterior posterior (SLAP) tears are common in throwing athletes and have been treated with varying and limited success. Further, in a study of outcomes of arthroscopic repair of SLAP lesions, Neri and colleagues5 found that, compared with throwing athletes with SLAP tears, throwing athletes with concomitant SLAP tears and partial-thickness rotator cuff tears (RCTs) had significantly poorer outcomes and return-to-play rates after surgical repair.
The purpose of this study was to determine outcome scores and return to play of throwing athletes treated with arthroscopic capsulolabral repair for posterior shoulder instability with coexistent RCTs and to compare them with outcome scores as well as return to play of throwing athletes with isolated posterior shoulder instability. It was hypothesized that throwing athletes with a combination of posterior shoulder instability and RCT would have poorer outcomes and poorer return to play after surgery.5
METHODS
PATIENT SELECTION
After Institutional Review Board approval, informed consent was obtained, and consecutive throwing athletes who underwent arthroscopic posterior capsulolabral reconstruction for posterior shoulder instability were followed in the perioperative period. Inclusion criteria were throwing athletes participating in competitive sports at the high school, collegiate, or professional level, minimum 1-year follow-up, presence of unidirectional posterior instability, and absence of symptoms of instability in any direction other than posterior. Patients with inferior instability, SLAP pathology on examination and on magnetic resonance imaging, multidirectional instability, or habitual or psychogenic voluntary shoulder subluxations were excluded. Patients with diagnoses of both posterior shoulder instability and impingement treated with subacromial decompression and distal clavicle resection were also excluded.
After this cohort was identified, patient records were reviewed for pertinent operative data, such as procedure, complications, and evidence of RCT by operative report and arthroscopic photographs. A partial RCT was defined as a tear of 10% to 50%; those with rotator cuff fraying were determined not to be significant.
PATIENT EVALUATION
Surgeries were performed between January 1998 and December 2009 by the senior author (JPB). All patients were followed with clinical examinations, radiographs, and subjective grading scales. Recorded patient demographic data included age, sex, sport, position, competition level, and follow-up duration.
Continue to: All patients had...
All patients had symptomatic posterior shoulder instability, including posterior shoulder pain, clicking, a sensation of subluxation, or instability/apprehension with motion. Each athlete’s shoulder was palpated for tenderness and tested for impingement. Specific posterior glenohumeral instability tests, including the Kim test,6 the circumduction test, the jerk test,7 the posterior load-and-shift test,8 and the posterior stress test,9 were performed on all patients. Patients with multidirectional instability on the sulcus test, as well as provocative tests indicating SLAP pathology, such as the Crank test and the active compression test, were not included. Standard radiography and magnetic resonance arthrography (MRA) were performed to further narrow inclusion and exclusion criteria.
Both before surgery and at latest follow-up, patient outcomes were evaluated using the American Shoulder and Elbow Surgeons (ASES) score (range, 0-100) which combines a subjective functional scale measuring activities of daily living (0-3 for each of 10 tasks, with a total of 0-30) and a subjective pain scale (0-10, with 10 being worst pain). Values >80 were described as excellent, and failures were defined as scores <60 after surgery.10 A subjective stability scale (0-10, with 0 indicating completely stable and 10 completely unstable), strength scale (0-3, with 0 indicating none, 1 markedly decreased, 2 slightly decreased, and 3 normal), and ROM scale (0-3, with 0 indicating poor, 1 limited, 2 satisfactory, and 3 full) were evaluated both before surgery and at the latest follow-up. A stability score >5 after surgery was defined as a failure.1,2,11 Patients were also asked if, based on their current state, they would undergo surgery again. Intraoperative findings and specific surgical procedures performed were correlated with the aforementioned subjective and objective outcome scores.
OPERATIVE TREATMENT
Throwing athletes who met inclusion criteria and failed nonoperative management underwent surgery by the senior author (JPB). Each patient was examined under anesthesia and, with the patient in the lateral decubitus position, a diagnostic arthroscopy was performed to identify posterior capsulolabral complex pathology, including a patulous capsule, capsular tears, labral fraying, and labral tears. A careful examination for rotator cuff pathology was also performed. Based on preoperative clinical examination, MRA, examination under anesthesia, pathologic findings at diagnostic arthroscopic surgery, and surgeon experience, capsulolabral plication was performed with or without suture anchors.2,5 After capsulolabral repair, the capsule was evaluated for residual laxity, and additional plication sutures were placed, as indicated, with care to avoid overconstraint in these throwing athletes.1 Posterior glenohumeral stability restoration was judged by removing traction and performing posterior load-and-shift and posterior stress tests. Any RCT with <50% thickness was débrided. Postoperative care and rehabilitation were carried out as previously described and were not altered by the presence or absence of a RCT.3
STATISTICAL ANALYSIS
Preoperative and latest follow-up ASES scores, stability scores, functional scores, and pain-level findings were compared using paired-samples Comparisons between groups, including throwing athletes with and without rotator cuff pathology, were done using the Student t test. Outcome comparisons between multiple groups, which included intraoperative findings and surgical fixation methods, were analyzed with c2 modeling for nonparametric data. Statistical significance was set at P < .05. A power analysis found that this study was able to detect a meaningful difference of 10 ASES points.
RESULTS
PATIENT DEMOGRAPHIC CHARACTERISTICS
Of the 56 throwing athletes who met the inclusion criteria, 24 were found to have rotator cuff pathology in addition to posterior capsulolabral pathology, while 32 were found to have capsulolabral pathology alone. Demographic data are listed in Table 1. Mean age was 20.1 years for patients with rotator cuff pathology and 17.8 years for patients without RCTs. All 24 athletes with rotator cuff pathology were treated with arthroscopic débridement. Mean follow-up was 38.6 months (range, 16.5-63.6 months) for patients with RCTs and 39.1 months (range, 12-98.8 months) for patients without RCTs. No significant difference was found in age, sports level, or follow-up between groups.
Table 1. Demographic Data for Athletes With Posterior Instability With and Without Rotator Cuff Tears (N = 56 Shoulders)a
Characteristic | Rotator Cuff Tears | |
Yes | No | |
| Total | 24 | 32 |
| Sex | ||
| Male | 16 | 27 |
| Female | 8 | 5 |
| Mean age, y | 20.1 | 17.8 |
| Mean follow up, mo | 38.6 | 39.1 |
| Participation level | ||
| Professional | 1 | 0 |
| College | 4 | 4 |
| High school | 17 | 26 |
| Recreational | 2 | 2 |
aThe majority of athletes were males in high school and their mean follow-up was 3 years.
Continue to: Outcomes
OUTCOMES
Table 2 lists the preoperative and postoperative scores for shoulder performance in throwing athletes with posterior shoulder instability, with and without RCTs.
Table 2. Preoperative and Postoperative Scores for Shoulder Performance in Throwing Athletes With Posterior Shoulder Instability With and Without Rotator Cuff Tearsa
| With Rotator Cuff Tears (n=24 shoulders) | Without Rotator Cuff Tears (n=32 shoulders) | |||||||||
| Preoperative | Latest Follow-Up | Preoperative | Latest Follow-Up | |||||||
| Outcome Measure | Mean Score | Range | Mean Score | Range | P | Mean Score | Range | Mean Score | Range | P |
ASES 0-100 0 = worst | 41.8 | 20-70 | 85.4 | 67-100 | <.05 | 49.7 | 20-85 | 83.1 | 25-100 | <.05 |
Stability 0-10 0 = most stable | 6.7 | 2-10 | 2.4 | 0-6 | <.05 | 7.8 | 0-10 | 2.4 | 0-8 | <.05 |
Pain 0-10 10 = worst | 7.6 | 5-10 | 1.9 | 0-5 | <.05 | 6.3 | 0-10 | 2.2 | 0-7 | <.05 |
Function 0-30 0 = worst | 18.5 | 6-27 | 27 | 16-30 | <.05 | 19.0 | 8-26 | 26.4 | 6-30 | <.05 |
aThere was no difference in ASES, stability, pain, or functional scores between athletes with posterior instability alone compared with patients with concomitant rotator cuff tears.
Abbreviation: ASES, American Shoulder and Elbow Surgeons.
ASES Scores. Mean preoperative ASES scores for patients with RCTs improved significantly (t = –13.8, P < .001), as did those for patients without rotator cuff pathology (t = –8.9, P < .001). No significant differences in ASES score were found between patients with and without rotator cuff pathology before or after surgery (t = 1.9, P = .07; t = .58, P = .06). In addition, 70.8% (17/24) of throwing athletes with rotator cuff pathology had an excellent postoperative outcome (ASES score >80), and 29.2% (7/24) had a satisfactory outcome (ASES score, 60-80). Thus, 100% of those with concomitant posterior shoulder instability and RCTs had a good or excellent outcome after surgical intervention. In those without rotator cuff pathology, 78.1% (25/32) had an excellent outcome, 12.5% (4/32) had a satisfactory outcome, and 9.4% (3/32) had a poor outcome. Thus, 91% of those without rotator cuff pathology had a good or excellent outcome after surgery.
Stability. Preoperative stability scores improved significantly after surgery in both groups (t = 7.2, P < .001; t = 10.5, P < .001). There were no statistical differences between preoperative or postoperative stability scores in those with or without rotator cuff pathology (t = 1.7, P = .095; t = .03, P = .975). Of throwing athletes with RCTs, 54.2% (13/24) had an excellent outcome, 33.3% (8/24) a good outcome, and 12.5% (3/24) a satisfactory outcome. Thus, 87.5% (21/24) of those with RCTs had a good or excellent outcome in terms of stability. In those without rotator cuff pathology, 46.9% (15/32) had excellent stability, 46.9% (15/32) had good stability, and 3.1% (1/32) had satisfactory stability after surgery. Thus, 93.8% (30/32) of throwing athletes without rotator cuff pathology had good or excellent stability after surgery.
Pain. Mean preoperative pain scores for those with and without rotator cuff pathology improved significantly (t = 13.4, P < .001; t = 7.1, P < .001). There was no statistical difference in preoperative or postoperative pain scores between those with and without rotator cuff pathology (t = 1.99, P = .051; t = .49, P = .627).
Function. Mean preoperative function scores for both groups improved significantly (t = 7.7, P < .001; t = 8.0, P < .001). There was no difference in improvement in functional scores between the two groups before or after surgery (t = .36, P = .721; t = .5, P = .622).
Continue to: ROM
ROM. Of those with rotator cuff pathology, 54% (13/24) had normal ROM, 42% (10/24) had satisfactory ROM, and 4% (1/24) had limited ROM. In throwing athletes without rotator cuff pathology, 34% (11/32) had normal ROM, 53.1% (17/32) had satisfactory ROM, and 9% (3/32) had limited ROM after surgery. There was no significant difference in ROM between the groups (c2 = 2.7, P = .260).
Strength. Of those with RCTs, 67% (16/24) reported normal strength, 29% (7/24) slightly decreased strength, and 4% (1/24) markedly decreased strength. Of those throwing athletes without rotator cuff pathology, 50% (16/32) had normal strength, 41% (13/32) had slightly decreased strength, and 9% (3/32) had markedly decreased strength. No statistical difference was noted between the two groups (c2 = 1.7, P = .429).
Return to Sport. Of those with RCTs, 92% (22/24) returned to sport while 84% (27/32) of throwing athletes without RCTs returned to sport. There was no difference between the two groups (c2 = .667, P = .414). Sixty-seven percent (16/24) of those with RCTs and 56% (18/32) of those without RCTs returned to the same level of sport. No statistical difference was found in return to play between throwing athletes with and without rotator cuff pathology (c2 = .624, P = .430).
Failures. According to ASES scores, no throwers with RCTs failed, while 9.4% (3/32) with posterior instability alone failed. Regarding stability, 8.3% (2/24) of athletes with RCTs failed, while 6.3% (2/32) with posterior instability alone failed.
SURGICAL FINDINGS AND PROCEDURES
Of the 24 throwing athletes with rotator cuff pathology, 92% (22/24) had labral tears, while 78% (25/32) of those without RCTs had labral tears. The majority of RCTs were in the posterior supraspinatus and anterior infraspinatus regions. This was not significantly different between groups (c2 = 1.86, P = .172). All labral pathology was posterior-inferior, and all RCTs were <50% thickness, and therefore were débrided. Fifty-four percent (13/24) of those with RCTs had a patulous capsule and 63% (20/32) of throwing athletes without rotator cuff pathology had a patulous capsule. There was no significant difference between groups (c2 = .393, P = .530). Of those with RCTs, 92% (22/24) had surgical fixation with anchors, while 78% (25/32) of those without rotator cuff pathology underwent repair with anchor fixation. There was no statistically significant difference in anchor use between groups (c2 = 1.86, P = .172).
Continue to: Discussion
DISCUSSION
Throwing athletes with and without RCTs had similar rates of recovery and return to play after arthroscopic capsular labral repair, with rotator cuff débridement if a tear was present. The mean follow-up was 3.2 years. Further, there was no difference in return to play (92% vs 84%), ASES score, stability, pain, function, ROM, or strength between the 2 groups before or after surgery. In this cohort of 56 patients, 24 throwing athletes (43%) were found to have RCTs.
Return-to-play rates showed no between-group differences; 92% (22/24) of athletes with concomitant RCTs returned to sport, and 67% (16/24) returned to the same level. Eight percent of throwing athletes with RCTs were unable to return to sport after surgery. These return-to-play rates are an improvement over most previously reported rates in throwing athletes and in posterior shoulder instability in general.1-4,11 When these athletes are compared with their counterparts with combined SLAP tears and RCTs, return-to-play rates are notably higher. There may be discrepancies in interpreting return-to-play between the two studies, but in the current study, 67% of those with concomitant RCTs achieved return to preinjury level of play. This is 10% higher than the rate reported in athletes with SLAP tears alone (57%) and even higher than in those with concomitant SLAP and RCTs. It is also essential to note that a number of this cohort’s athletes who did not return to play did so for factors (eg, graduation) unrelated to the shoulder. However, the study by Neri and colleagues5 included professional athletes who likely all attempted to return to play and, if unable to perform at the same level, likely were unable to continue their professional career.5
All patients with RCTs had a good or excellent outcome (ASES score), and 70.8% had an excellent outcome. Similarly, 97% of those without rotator cuff pathology had a good or excellent outcome, and 81.3% had an excellent outcome. There was no significant difference between the two groups. These results parallel those of Neri and colleagues’5 study of SLAP tears with RCTs, where 96% (22/23) of throwing athletes had a good or excellent outcome. Despite these high outcome scores in patients with SLAP tears, only 57% were able to return to elite pitching.5 In the current study, pain was slightly higher for those with rotator cuff pathology before surgery—a finding consistent with pain frequently being found in patients with isolated partial-thickness RCTs. Their postoperative pain scores were actually lower on average than those of patients without RCTs, which suggests simple débridement of undersurface tears adequately addressed the pathology. The authors theorize that the main pain generator in this population may be posterior instability, and that the rotator cuff has less of an influence. In the SLAP population, the main pain generator likely is the RCT.
Failures by ASES score or strength were fairly rare in this cohort. Many patients opted to have revision surgery because of continued instability, pain, decreased function, or reinjury. One potential cause of failure in this cohort is inadequate capsular shift. However, capsular plication in throwing athletes is difficult to address, as overtensioning the repair can lead to the inability to adequately perform overhead activites.3,4 This cannot be overemphasized, particularly with pitchers.
Partial-thickness RCTs, particularly those on the articular side, are common in throwing athletes because of high tensile and compressive loads.12 Despite the known risk of RCTs with posterior shoulder instability in throwing athletes, the authors are unaware of reports of the incidence or treatment of this pathology. RCTs in this posterior instability group likely represent a pathology other than internal impingement. The high proportion of throwing athletes with RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes. Ide et al found that 75% of patients with SLAP tears had partial articular-sided RCTs.13 In the current study, all RCTs were small partial tears, and arthroscopic débridement was performed. It is unknown whether repair of these RCTs would impact return to play. However, rotator cuff repair in this population has been shown to have poor outcomes. Tear thickness typically is used to determine treatment, with débridement performed if <50% tendon thickness is affected. More recently, many have advocated having greater tendon involvement in throwers before repair, because of poor outcomes. Although studies are limited, tear size does seem to correlate with outcomes.14
Continue to: Study Limitations
STUDY LIMITATIONS
Limitations of this study include its small number of professional throwing athletes, with the majority being high school athletes. Further, although ASES scores are consistently used in posterior shoulder instability studies, these scores are influenced highly by pain scores, and some argue that other scoring systems may provide more useful information. However, none of the more modern scoring systems have been studied extensively in posterior glenohumeral instability. Further, because the authors used the present scoring systems previously,1-4 they were continued to be used for comparison and consistency. Outcomes such as ROM and strength may carry more weight if measured and documented by clinical examination. Further testing, such as clinical evaluation of the jerk test or the posterior load-and-shift test, and their comparison before and after surgery may provide more objective data.
CONCLUSION
Arthroscopic capsulolabral reconstruction is successful in throwing athletes with RCTs treated with arthroscopic débridement. Unlike a previous study of throwing athletes’ outcomes after surgery for concomitant SLAP tears and RCTs,5 this study of throwing athletes with concomitant posterior shoulder instability and RCTs found no difference in patient-reported outcome measures or return to play. In throwing athletes with posterior instability and RCTs, arthroscopic posterior capsulolabral repair with rotator cuff débridement is successful.
1. Bradley JP, Baker CL 3rd, Kline AJ, Armfield DR, Chhabra A. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 100 shoulders. Am J Sports Med. 2006;34(7):1061-1071.
2. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014.
3. McClincy MP, Arner JW, Bradley JP. Posterior shoulder instability in throwing athletes: a case-matched comparison of throwers and non-throwers. Arthroscopy. 2015;31(6):1041-1051.
4. Radkowski CA, Chhabra A, Baker CL 3rd, Tejwani SG, Bradley JP. Arthroscopic capsulolabral repair for posterior shoulder instability in throwing athletes compared with nonthrowing athletes. Am J Sports Med. 2008;36(4):693-699.
5. Neri BR, ElAttrache NS, Owsley KC, Mohr K, Yocum LA. Outcome of type II superior labral anterior posterior repairs in elite overhead athletes: effect of concomitant partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(1):114-120.
6. Kim SH, Park JS, Jeong WK, Shin SK. The Kim test: a novel test for posteroinferior labral lesion of the shoulder—a comparison to the jerk test. Am J Sports Med. 2005;33(8):1188-1192.
7. Antoniou J, Duckworth DT, Harryman DT 2nd. Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg Am. 2000;82(9):1220-1230.
8. Altchek DW, Hobbs WR. Evaluation and management of shoulder instability in the elite overhead thrower. Orthop Clin North Am. 2001;32(3):423-430, viii.
9. Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82(1):16-25.
10. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
11. Arner JW, McClincy MP, Bradley JP. Arthroscopic stabilization of posterior shoulder instability is successful in American football players. Arthroscopy. 2015;31(8):1466-1471.
12. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
13. Ide J, Maeda S, Takagi K. Sports activity after arthroscopic superior labral repair using suture anchors in overhead-throwing athletes. Am J Sports Med. 2005;33(4):507-514.
14. Economopoulos KJ, Brockmeier SF. Rotator cuff tears in overhead athletes. Clin Sports Med. 2012;31(4):675-692.
1. Bradley JP, Baker CL 3rd, Kline AJ, Armfield DR, Chhabra A. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 100 shoulders. Am J Sports Med. 2006;34(7):1061-1071.
2. Bradley JP, McClincy MP, Arner JW, Tejwani SG. Arthroscopic capsulolabral reconstruction for posterior instability of the shoulder: a prospective study of 200 shoulders. Am J Sports Med. 2013;41(9):2005-2014.
3. McClincy MP, Arner JW, Bradley JP. Posterior shoulder instability in throwing athletes: a case-matched comparison of throwers and non-throwers. Arthroscopy. 2015;31(6):1041-1051.
4. Radkowski CA, Chhabra A, Baker CL 3rd, Tejwani SG, Bradley JP. Arthroscopic capsulolabral repair for posterior shoulder instability in throwing athletes compared with nonthrowing athletes. Am J Sports Med. 2008;36(4):693-699.
5. Neri BR, ElAttrache NS, Owsley KC, Mohr K, Yocum LA. Outcome of type II superior labral anterior posterior repairs in elite overhead athletes: effect of concomitant partial-thickness rotator cuff tears. Am J Sports Med. 2011;39(1):114-120.
6. Kim SH, Park JS, Jeong WK, Shin SK. The Kim test: a novel test for posteroinferior labral lesion of the shoulder—a comparison to the jerk test. Am J Sports Med. 2005;33(8):1188-1192.
7. Antoniou J, Duckworth DT, Harryman DT 2nd. Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg Am. 2000;82(9):1220-1230.
8. Altchek DW, Hobbs WR. Evaluation and management of shoulder instability in the elite overhead thrower. Orthop Clin North Am. 2001;32(3):423-430, viii.
9. Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82(1):16-25.
10. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3(6):347-352.
11. Arner JW, McClincy MP, Bradley JP. Arthroscopic stabilization of posterior shoulder instability is successful in American football players. Arthroscopy. 2015;31(8):1466-1471.
12. Mazoue CG, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34(2):182-189.
13. Ide J, Maeda S, Takagi K. Sports activity after arthroscopic superior labral repair using suture anchors in overhead-throwing athletes. Am J Sports Med. 2005;33(4):507-514.
14. Economopoulos KJ, Brockmeier SF. Rotator cuff tears in overhead athletes. Clin Sports Med. 2012;31(4):675-692.
TAKE-HOME POINTS
- Arthroscopic capsulolabral reconstruction is successful in throwing athletes with concomitant RCTs treated with arthroscopic débridement.
- A previous study of throwing athletes found poor outcomes after surgery for concomitant SLAP tears and RCTs.
- Throwing athletes with concomitant posterior shoulder instability and RCTs were no different in patient-reported outcomes or return to play.
- The high proportion of throwing athletes with partial thickness RCTs in this study (43%) indicates a need for close evaluation of rotator cuff pathology in young throwing athletes.
- The authors theorize the main pain generator in this population may be posterior instability and that the rotator cuff has less of an influence.
Late thrombectomy for stroke has low number needed to treat for benefit
LOS ANGELES – The number of ischemic stroke patients with a clinical core mismatch showing salvageable tissue who need to be treated with thrombectomy to obtain a significant benefit on functional outcomes is just 2 when the time frame from last known well extends out to 24 hours, according a subanalysis of results from the DAWN trial.
The Jan. 4, 2018, publication of the DAWN trial revealed that patients with ischemic strokes can benefit from thrombectomy long after the time window generally thought to afford benefits had closed (N Engl J Med. 2018;378:11-21). The procedure yielded significant benefits in functional outcomes at 90 days in patients with a clinical core mismatch showing salvageable tissue.
The results are important because health care systems must now make decisions about allocating resources for the treatment of these patients, which will include installing imaging techniques and expertise at various centers. “It will be practical in some primary stroke centers and not in others. We’re going to see a lot of interesting research about what frontline hospitals should do. There are lots of options at that screening step, and we’re going to need experience to see what’s best. It won’t be the same answer for everyone,” Jeffrey Saver, MD, said during a press conference announcing the results at the meeting, which was sponsored by the American Heart Association. Dr. Saver is director of the stroke unit at the University of California, Los Angeles, and professor of clinical neurology at the university.
The DAWN trial randomized 206 patients to thrombectomy plus standard care or standard care alone. The study was halted at an enrollment of 206 patients because of overwhelming efficacy. To be eligible, the patients had to have a mismatch between the severity of clinical deficit and the infarct volume as measured via automated analysis (RAPID software, SchemaView) of diffusion-weighted MRI or perfusion CT. They had to have substantial clinical deficits, but limited infarct size, with specific criteria varying with age, National Institutes of Health Stroke Scale score, and infarct size.
The NNT for an mRS score of 0 (asymptomatic) was 19. For freedom from disability (mRS, 0-1), the NNT was 4. For functional independence (mRS, 0-2), it was 3. To achieve ambulatory status (mRS, 0-3), it was 3. To avoid a requirement for constant care (mRS, 0-4), the NNT was 9.
To achieve any reduction in disability at all, the NNT was 2. This value was identical when looking at patients in the 6- to 12-hour window and those in the 12- to 24-hour window. However, the nature of the benefit was different. “In the late window (12-24 hours), outcomes went from really bad to pretty good. In the early window, it was somewhat bad to very good. So it’s still better to be treated early,” Dr. Saver said.
In short, for every 100 patients treated, 50 would gain an improvement in disability-related quality of life, and 36 would gain functional independence. In the 6- to 12-hour group, 45 of every 100 patients would experience lower disability as a result of treatment, as would 56 of every 100 treated patients in the 12- to 24-hour group.
Stryker Neurovascular funded the study. Dr. Saver has consulted for Stryker and received travel reimbursement.
LOS ANGELES – The number of ischemic stroke patients with a clinical core mismatch showing salvageable tissue who need to be treated with thrombectomy to obtain a significant benefit on functional outcomes is just 2 when the time frame from last known well extends out to 24 hours, according a subanalysis of results from the DAWN trial.
The Jan. 4, 2018, publication of the DAWN trial revealed that patients with ischemic strokes can benefit from thrombectomy long after the time window generally thought to afford benefits had closed (N Engl J Med. 2018;378:11-21). The procedure yielded significant benefits in functional outcomes at 90 days in patients with a clinical core mismatch showing salvageable tissue.
The results are important because health care systems must now make decisions about allocating resources for the treatment of these patients, which will include installing imaging techniques and expertise at various centers. “It will be practical in some primary stroke centers and not in others. We’re going to see a lot of interesting research about what frontline hospitals should do. There are lots of options at that screening step, and we’re going to need experience to see what’s best. It won’t be the same answer for everyone,” Jeffrey Saver, MD, said during a press conference announcing the results at the meeting, which was sponsored by the American Heart Association. Dr. Saver is director of the stroke unit at the University of California, Los Angeles, and professor of clinical neurology at the university.
The DAWN trial randomized 206 patients to thrombectomy plus standard care or standard care alone. The study was halted at an enrollment of 206 patients because of overwhelming efficacy. To be eligible, the patients had to have a mismatch between the severity of clinical deficit and the infarct volume as measured via automated analysis (RAPID software, SchemaView) of diffusion-weighted MRI or perfusion CT. They had to have substantial clinical deficits, but limited infarct size, with specific criteria varying with age, National Institutes of Health Stroke Scale score, and infarct size.
The NNT for an mRS score of 0 (asymptomatic) was 19. For freedom from disability (mRS, 0-1), the NNT was 4. For functional independence (mRS, 0-2), it was 3. To achieve ambulatory status (mRS, 0-3), it was 3. To avoid a requirement for constant care (mRS, 0-4), the NNT was 9.
To achieve any reduction in disability at all, the NNT was 2. This value was identical when looking at patients in the 6- to 12-hour window and those in the 12- to 24-hour window. However, the nature of the benefit was different. “In the late window (12-24 hours), outcomes went from really bad to pretty good. In the early window, it was somewhat bad to very good. So it’s still better to be treated early,” Dr. Saver said.
In short, for every 100 patients treated, 50 would gain an improvement in disability-related quality of life, and 36 would gain functional independence. In the 6- to 12-hour group, 45 of every 100 patients would experience lower disability as a result of treatment, as would 56 of every 100 treated patients in the 12- to 24-hour group.
Stryker Neurovascular funded the study. Dr. Saver has consulted for Stryker and received travel reimbursement.
LOS ANGELES – The number of ischemic stroke patients with a clinical core mismatch showing salvageable tissue who need to be treated with thrombectomy to obtain a significant benefit on functional outcomes is just 2 when the time frame from last known well extends out to 24 hours, according a subanalysis of results from the DAWN trial.
The Jan. 4, 2018, publication of the DAWN trial revealed that patients with ischemic strokes can benefit from thrombectomy long after the time window generally thought to afford benefits had closed (N Engl J Med. 2018;378:11-21). The procedure yielded significant benefits in functional outcomes at 90 days in patients with a clinical core mismatch showing salvageable tissue.
The results are important because health care systems must now make decisions about allocating resources for the treatment of these patients, which will include installing imaging techniques and expertise at various centers. “It will be practical in some primary stroke centers and not in others. We’re going to see a lot of interesting research about what frontline hospitals should do. There are lots of options at that screening step, and we’re going to need experience to see what’s best. It won’t be the same answer for everyone,” Jeffrey Saver, MD, said during a press conference announcing the results at the meeting, which was sponsored by the American Heart Association. Dr. Saver is director of the stroke unit at the University of California, Los Angeles, and professor of clinical neurology at the university.
The DAWN trial randomized 206 patients to thrombectomy plus standard care or standard care alone. The study was halted at an enrollment of 206 patients because of overwhelming efficacy. To be eligible, the patients had to have a mismatch between the severity of clinical deficit and the infarct volume as measured via automated analysis (RAPID software, SchemaView) of diffusion-weighted MRI or perfusion CT. They had to have substantial clinical deficits, but limited infarct size, with specific criteria varying with age, National Institutes of Health Stroke Scale score, and infarct size.
The NNT for an mRS score of 0 (asymptomatic) was 19. For freedom from disability (mRS, 0-1), the NNT was 4. For functional independence (mRS, 0-2), it was 3. To achieve ambulatory status (mRS, 0-3), it was 3. To avoid a requirement for constant care (mRS, 0-4), the NNT was 9.
To achieve any reduction in disability at all, the NNT was 2. This value was identical when looking at patients in the 6- to 12-hour window and those in the 12- to 24-hour window. However, the nature of the benefit was different. “In the late window (12-24 hours), outcomes went from really bad to pretty good. In the early window, it was somewhat bad to very good. So it’s still better to be treated early,” Dr. Saver said.
In short, for every 100 patients treated, 50 would gain an improvement in disability-related quality of life, and 36 would gain functional independence. In the 6- to 12-hour group, 45 of every 100 patients would experience lower disability as a result of treatment, as would 56 of every 100 treated patients in the 12- to 24-hour group.
Stryker Neurovascular funded the study. Dr. Saver has consulted for Stryker and received travel reimbursement.
REPORTING FROM ISC 2018
Key clinical point:
Major finding: To achieve a functional improvement at 90 days, the number needed to treat was 2.
Data source: Subanalysis of the randomized, controlled DAWN trial (n = 206).
Disclosures: Stryker Neurovascular funded the study. Dr. Saver has consulted for Stryker and received travel reimbursement.
Source: Saver J et al. ISC 2018 abstract LB3
LAA occlusion boosts anticoagulants’ protection
ORLANDO – When patients with atrial fibrillation have a history of cardioembolic events despite optimal anticoagulant therapy, treatment with left atrial appendage occlusion can substantially improve protection against future events, according to a multicenter review of 22 patients.
During the 2 years prior to undergoing left atrial appendage (LAA) occlusion, the 22 atrial fibrillation (AF) patients studied had a total of 44 cardioembolic events despite receiving “optimal” treatment with an oral anticoagulant, including nine patients with one event, six patients with two events, five patients with three events, and two patients with four events each, Xavier Freixa, MD, said at the annual International AF Symposium. In contrast, during a median follow-up of 1.8 years after their procedure additional events occurred in just two patients – one with a stroke, the other with a transient ischemic attack, while the remaining 20 patients remained free of any additional events.
The analysis also revealed that the two patients who had cardioembolic events following their LAA occlusion had been withdrawn from oral anticoagulant treatment by their physicians, who had done this with a “false feeling of comfort,” said Dr. Freixa, an interventional cardiologist at the University Hospital Clinic of Barcelona. These two patients were among three patients maintained on dual-antiplatelet therapy rather than on an oral anticoagulant following LAA occlusion. The remaining 19 patients had remained on either warfarin, a novel oral anticoagulant, or both.
The study included patients from eight Spanish centers who underwent LAA occlusion during June 2009–June 2017, and included 14 with nonvalvular AF and 8 with valvular AF who had all undergone prior valve surgery. None of the 22 patients had a contraindication for treatment with an oral anticoagulant. They averaged about 69 years of age. Prior to their procedure, 18 had at least one stroke or transient ischemic attack, and the remaining 4 patients had at least one systemic embolism. Nineteen patients underwent occlusion with either an Amplatzer Cardiac Plug or Amplatzer Amulet device, two received a Watchman device, and one patient received a LAmbre device. All of the closure procedures were successful, with no complications.
“I think any device will do well for these patients as long as we occlude the LAA,” Dr. Freixa said.
[email protected]
On Twitter @mitchelzoler
SOURCE: Freixa X et al. AF Symposium 2018 Abstract 1821.
ORLANDO – When patients with atrial fibrillation have a history of cardioembolic events despite optimal anticoagulant therapy, treatment with left atrial appendage occlusion can substantially improve protection against future events, according to a multicenter review of 22 patients.
During the 2 years prior to undergoing left atrial appendage (LAA) occlusion, the 22 atrial fibrillation (AF) patients studied had a total of 44 cardioembolic events despite receiving “optimal” treatment with an oral anticoagulant, including nine patients with one event, six patients with two events, five patients with three events, and two patients with four events each, Xavier Freixa, MD, said at the annual International AF Symposium. In contrast, during a median follow-up of 1.8 years after their procedure additional events occurred in just two patients – one with a stroke, the other with a transient ischemic attack, while the remaining 20 patients remained free of any additional events.
The analysis also revealed that the two patients who had cardioembolic events following their LAA occlusion had been withdrawn from oral anticoagulant treatment by their physicians, who had done this with a “false feeling of comfort,” said Dr. Freixa, an interventional cardiologist at the University Hospital Clinic of Barcelona. These two patients were among three patients maintained on dual-antiplatelet therapy rather than on an oral anticoagulant following LAA occlusion. The remaining 19 patients had remained on either warfarin, a novel oral anticoagulant, or both.
The study included patients from eight Spanish centers who underwent LAA occlusion during June 2009–June 2017, and included 14 with nonvalvular AF and 8 with valvular AF who had all undergone prior valve surgery. None of the 22 patients had a contraindication for treatment with an oral anticoagulant. They averaged about 69 years of age. Prior to their procedure, 18 had at least one stroke or transient ischemic attack, and the remaining 4 patients had at least one systemic embolism. Nineteen patients underwent occlusion with either an Amplatzer Cardiac Plug or Amplatzer Amulet device, two received a Watchman device, and one patient received a LAmbre device. All of the closure procedures were successful, with no complications.
“I think any device will do well for these patients as long as we occlude the LAA,” Dr. Freixa said.
[email protected]
On Twitter @mitchelzoler
SOURCE: Freixa X et al. AF Symposium 2018 Abstract 1821.
ORLANDO – When patients with atrial fibrillation have a history of cardioembolic events despite optimal anticoagulant therapy, treatment with left atrial appendage occlusion can substantially improve protection against future events, according to a multicenter review of 22 patients.
During the 2 years prior to undergoing left atrial appendage (LAA) occlusion, the 22 atrial fibrillation (AF) patients studied had a total of 44 cardioembolic events despite receiving “optimal” treatment with an oral anticoagulant, including nine patients with one event, six patients with two events, five patients with three events, and two patients with four events each, Xavier Freixa, MD, said at the annual International AF Symposium. In contrast, during a median follow-up of 1.8 years after their procedure additional events occurred in just two patients – one with a stroke, the other with a transient ischemic attack, while the remaining 20 patients remained free of any additional events.
The analysis also revealed that the two patients who had cardioembolic events following their LAA occlusion had been withdrawn from oral anticoagulant treatment by their physicians, who had done this with a “false feeling of comfort,” said Dr. Freixa, an interventional cardiologist at the University Hospital Clinic of Barcelona. These two patients were among three patients maintained on dual-antiplatelet therapy rather than on an oral anticoagulant following LAA occlusion. The remaining 19 patients had remained on either warfarin, a novel oral anticoagulant, or both.
The study included patients from eight Spanish centers who underwent LAA occlusion during June 2009–June 2017, and included 14 with nonvalvular AF and 8 with valvular AF who had all undergone prior valve surgery. None of the 22 patients had a contraindication for treatment with an oral anticoagulant. They averaged about 69 years of age. Prior to their procedure, 18 had at least one stroke or transient ischemic attack, and the remaining 4 patients had at least one systemic embolism. Nineteen patients underwent occlusion with either an Amplatzer Cardiac Plug or Amplatzer Amulet device, two received a Watchman device, and one patient received a LAmbre device. All of the closure procedures were successful, with no complications.
“I think any device will do well for these patients as long as we occlude the LAA,” Dr. Freixa said.
[email protected]
On Twitter @mitchelzoler
SOURCE: Freixa X et al. AF Symposium 2018 Abstract 1821.
REPORTING FROM THE AF SYMPOSIUM 2018
Key clinical point:
Major finding: Two of 22 patients had a cardioembolic event after left atrial appendage occlusion.
Study details: Review of 22 patients at eight Spanish centers with atrial fibrillation and a history of cardioembolic events despite oral anticoagulation.
Disclosures: Dr. Freixa has been a proctor for Abbott Medical.
Source: Freixa X et al. AF Symposium 2018 Abstract 1821.
Hodgkin lymphoma survivors are at an increased risk of subsequent ER-negative breast cancer
Young women with primary Hodgkin lymphoma had an increased relative risk of estrogen receptor–positive breast cancer if they received radiotherapy and, irrespective of the type of treatment they got, an elevated risk of ER-negative breast cancer, based on results of a study based on patient records from 12 U.S. National Cancer Institute Surveillance, Epidemiology, and End Results registries.
Of 7,355 women diagnosed with primary Hodgkin lymphoma during 1973-2009 and aged 10-39 years, 377 patients subsequently were diagnosed with breast cancer at a mean age of 45 years; 57% of the cancers were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status, Diana R. Withrow, PhD, and her colleagues from the radiation epidemiology branch, division of cancer epidemiology and genetics, National Cancer Institute reported in JAMA Oncology.
Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8; 95% confidence interval, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI, 2.7-3.5; P less than .001 for the difference), the researchers wrote.
For ER-positive disease, the increased SIR was observed only among women who had received radiotherapy for their Hodgkin lymphoma (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than in the no/unknown chemotherapy group (P = .04), said the researchers.
The authors acknowledged that lack of information on patient risk factors such as family history, reproductive factors, and hormone therapy, as well as detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy is a limitation of the current study. Further research, including comprehensive treatment records, will lead to a better understanding of the association between treatment and breast cancer subtype in these patients, the researchers concluded.
None of the study authors reported any conflicts of interest.
SOURCE: Withrow D et al. doi: 10.1001/jamaoncol.2017.4887.
Young women with primary Hodgkin lymphoma had an increased relative risk of estrogen receptor–positive breast cancer if they received radiotherapy and, irrespective of the type of treatment they got, an elevated risk of ER-negative breast cancer, based on results of a study based on patient records from 12 U.S. National Cancer Institute Surveillance, Epidemiology, and End Results registries.
Of 7,355 women diagnosed with primary Hodgkin lymphoma during 1973-2009 and aged 10-39 years, 377 patients subsequently were diagnosed with breast cancer at a mean age of 45 years; 57% of the cancers were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status, Diana R. Withrow, PhD, and her colleagues from the radiation epidemiology branch, division of cancer epidemiology and genetics, National Cancer Institute reported in JAMA Oncology.
Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8; 95% confidence interval, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI, 2.7-3.5; P less than .001 for the difference), the researchers wrote.
For ER-positive disease, the increased SIR was observed only among women who had received radiotherapy for their Hodgkin lymphoma (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than in the no/unknown chemotherapy group (P = .04), said the researchers.
The authors acknowledged that lack of information on patient risk factors such as family history, reproductive factors, and hormone therapy, as well as detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy is a limitation of the current study. Further research, including comprehensive treatment records, will lead to a better understanding of the association between treatment and breast cancer subtype in these patients, the researchers concluded.
None of the study authors reported any conflicts of interest.
SOURCE: Withrow D et al. doi: 10.1001/jamaoncol.2017.4887.
Young women with primary Hodgkin lymphoma had an increased relative risk of estrogen receptor–positive breast cancer if they received radiotherapy and, irrespective of the type of treatment they got, an elevated risk of ER-negative breast cancer, based on results of a study based on patient records from 12 U.S. National Cancer Institute Surveillance, Epidemiology, and End Results registries.
Of 7,355 women diagnosed with primary Hodgkin lymphoma during 1973-2009 and aged 10-39 years, 377 patients subsequently were diagnosed with breast cancer at a mean age of 45 years; 57% of the cancers were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status, Diana R. Withrow, PhD, and her colleagues from the radiation epidemiology branch, division of cancer epidemiology and genetics, National Cancer Institute reported in JAMA Oncology.
Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8; 95% confidence interval, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI, 2.7-3.5; P less than .001 for the difference), the researchers wrote.
For ER-positive disease, the increased SIR was observed only among women who had received radiotherapy for their Hodgkin lymphoma (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than in the no/unknown chemotherapy group (P = .04), said the researchers.
The authors acknowledged that lack of information on patient risk factors such as family history, reproductive factors, and hormone therapy, as well as detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy is a limitation of the current study. Further research, including comprehensive treatment records, will lead to a better understanding of the association between treatment and breast cancer subtype in these patients, the researchers concluded.
None of the study authors reported any conflicts of interest.
SOURCE: Withrow D et al. doi: 10.1001/jamaoncol.2017.4887.
FROM JAMA ONCOLOGY
Key clinical point: .
Major finding: Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8) than ER-positive breast cancer (SIR, 3.1).
Study details: 7,355 women diagnosed with first primary Hodgkin lymphoma during 1973-2009, who were aged 10-39 years, and reported to 12 U.S. National Cancer Institute SEER registries.
Disclosures: None reported.
Source: Withrow D et al. doi: 10.1001/jamaoncolo.2017.4887.
Is it time for health policy M&Ms?
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
Stroke Risk: Estrogen Use in Women with Migraine
The results of a recent study are consistent with an additive increase in stroke risk with combined hormonal contraceptives (CHC) use in women who have migraine with aura. Since the absolute risk of stroke is low even in the presence of these risk factors, use of CHCs in women who have migraine with aura should be based on an individualized assessment of harms and benefits. Researchers conducted a literature search of PubMed, the Cochrane Library, and EMBASE from inception through January 2016 for relevant studies. They included studies that examined exposure to CHCs and reported outcomes of ischemic or hemorrhagic stroke. They found:
- Of 2480 records, 15 studies met inclusion criteria.
- No studies reported odds ratios for stroke risk as a function of estrogen dose in women with migraine, largely due to insufficient sample sizes.
- No interaction effect between migraine and CHCs was seen in the 7 studies that assessed this.
- One study differentiated risk by presence or absence of migraine aura and found an increased risk in the migraine with aura population.
Risk of stroke associated with use of estrogen containing contraceptives in women with migraine: A systematic review. [Published online ahead of print November 15, 2017]. Headache. doi:10.1111/head.13229.
The results of a recent study are consistent with an additive increase in stroke risk with combined hormonal contraceptives (CHC) use in women who have migraine with aura. Since the absolute risk of stroke is low even in the presence of these risk factors, use of CHCs in women who have migraine with aura should be based on an individualized assessment of harms and benefits. Researchers conducted a literature search of PubMed, the Cochrane Library, and EMBASE from inception through January 2016 for relevant studies. They included studies that examined exposure to CHCs and reported outcomes of ischemic or hemorrhagic stroke. They found:
- Of 2480 records, 15 studies met inclusion criteria.
- No studies reported odds ratios for stroke risk as a function of estrogen dose in women with migraine, largely due to insufficient sample sizes.
- No interaction effect between migraine and CHCs was seen in the 7 studies that assessed this.
- One study differentiated risk by presence or absence of migraine aura and found an increased risk in the migraine with aura population.
Risk of stroke associated with use of estrogen containing contraceptives in women with migraine: A systematic review. [Published online ahead of print November 15, 2017]. Headache. doi:10.1111/head.13229.
The results of a recent study are consistent with an additive increase in stroke risk with combined hormonal contraceptives (CHC) use in women who have migraine with aura. Since the absolute risk of stroke is low even in the presence of these risk factors, use of CHCs in women who have migraine with aura should be based on an individualized assessment of harms and benefits. Researchers conducted a literature search of PubMed, the Cochrane Library, and EMBASE from inception through January 2016 for relevant studies. They included studies that examined exposure to CHCs and reported outcomes of ischemic or hemorrhagic stroke. They found:
- Of 2480 records, 15 studies met inclusion criteria.
- No studies reported odds ratios for stroke risk as a function of estrogen dose in women with migraine, largely due to insufficient sample sizes.
- No interaction effect between migraine and CHCs was seen in the 7 studies that assessed this.
- One study differentiated risk by presence or absence of migraine aura and found an increased risk in the migraine with aura population.
Risk of stroke associated with use of estrogen containing contraceptives in women with migraine: A systematic review. [Published online ahead of print November 15, 2017]. Headache. doi:10.1111/head.13229.
Senate votes on 20-week abortion ban
The U.S. Senate blocked a proposed national ban on abortions after 20 weeks gestation following a closely divided 51-46 vote on Jan. 29.
The Pain-Capable Unborn Children Protection Act, which passed the House last year after a 237-189 vote, did not earn the 60 votes it needed to clear the Senate, marking a defeat for anti-abortion proponents such as Senate Majority Leader Mitch McConnell (R-Ky.).
In a Jan. 29 statement, Sen. McConnell said the Pain-Capable Unborn Child Protection Act reflects a growing consensus that unborn children should not be subjected to elective abortion after 20 weeks.
After the vote, President Trump said in a statement that it was “disappointing that despite support from a bipartisan majority of U.S. Senators, this bill was blocked from further consideration.”
The American College of Obstetricians and Gynecologists (ACOG) denounced the legislation in a Jan. 26 statement, calling it an attack on women’s access to comprehensive health care, including abortion care.
The vote was primarily split along party lines. Only three Democrats voted for the bill – Sens. Robert P. Casey Jr. of Pennsylvania, Joe Donnelly of Indiana, and Joe Manchin III of West Virginia. The three are all up for reelection this year in states in which Trump won in 2016.
The U.S. Senate blocked a proposed national ban on abortions after 20 weeks gestation following a closely divided 51-46 vote on Jan. 29.
The Pain-Capable Unborn Children Protection Act, which passed the House last year after a 237-189 vote, did not earn the 60 votes it needed to clear the Senate, marking a defeat for anti-abortion proponents such as Senate Majority Leader Mitch McConnell (R-Ky.).
In a Jan. 29 statement, Sen. McConnell said the Pain-Capable Unborn Child Protection Act reflects a growing consensus that unborn children should not be subjected to elective abortion after 20 weeks.
After the vote, President Trump said in a statement that it was “disappointing that despite support from a bipartisan majority of U.S. Senators, this bill was blocked from further consideration.”
The American College of Obstetricians and Gynecologists (ACOG) denounced the legislation in a Jan. 26 statement, calling it an attack on women’s access to comprehensive health care, including abortion care.
The vote was primarily split along party lines. Only three Democrats voted for the bill – Sens. Robert P. Casey Jr. of Pennsylvania, Joe Donnelly of Indiana, and Joe Manchin III of West Virginia. The three are all up for reelection this year in states in which Trump won in 2016.
The U.S. Senate blocked a proposed national ban on abortions after 20 weeks gestation following a closely divided 51-46 vote on Jan. 29.
The Pain-Capable Unborn Children Protection Act, which passed the House last year after a 237-189 vote, did not earn the 60 votes it needed to clear the Senate, marking a defeat for anti-abortion proponents such as Senate Majority Leader Mitch McConnell (R-Ky.).
In a Jan. 29 statement, Sen. McConnell said the Pain-Capable Unborn Child Protection Act reflects a growing consensus that unborn children should not be subjected to elective abortion after 20 weeks.
After the vote, President Trump said in a statement that it was “disappointing that despite support from a bipartisan majority of U.S. Senators, this bill was blocked from further consideration.”
The American College of Obstetricians and Gynecologists (ACOG) denounced the legislation in a Jan. 26 statement, calling it an attack on women’s access to comprehensive health care, including abortion care.
The vote was primarily split along party lines. Only three Democrats voted for the bill – Sens. Robert P. Casey Jr. of Pennsylvania, Joe Donnelly of Indiana, and Joe Manchin III of West Virginia. The three are all up for reelection this year in states in which Trump won in 2016.
Does hormonal contraception increase the risk of breast cancer?
Hormonal contraception (HC) has long been utilized safely in this country for a variety of indications, including pregnancy prevention, timing pregnancy appropriately, management of symptoms (dysmenorrhea, irregular menstrual cycles, heavy menstrual bleeding), and to prevent serious diseases (such as ovarian cancer, uterine cancer, osteoporosis in women with premature menopause). Like most prescription medications, there are potential adverse effects. With HC, side effects such as venous thromboembolism, a slight increase in liver cancer, and a possible increase in breast cancer risk have long been recognized.
Danish study compared HC use with breast cancer risk
In the December 7, 2017, issue of New England Journal of Medicine,1 investigators in Denmark published a study of women using HC (oral, transdermal, intravaginal routes, and levonorgestrel intrauterine device [LNG-IUD]) and breast cancer risk compared with women who did not use HC. This retrospective observational country-wide study was very large (1.8 million women followed over an average of 10.9 years), which allowed for the detection of even small changes in breast cancer risk.
Putting results in perspective
It is important to point out that this is an observational study, and small effect sizes (1 in 7,600) should be interpreted with caution. Observational studies can introduce many different types of bias (prescribing bias, confounding bias, etc). Of note, while the LNG-IUD was associated with a small increased risk of breast cancer (relative risk [RR], 1.21; 95% confidence interval [CI], 1.11-1.33]), the higher dose continuous progestin administration (medroxyprogesterone) was not (RR, 0.95; 95% CI, 0.40-2.29).1
Nonetheless, providing patients with a balanced summary of this new study along with other published and reliable information about HC that conveys both benefits and risks is important to assure that each woman makes a decision regarding HC that achieves her health and life goals. See "Counseling talking points" below.
Bottom line
This recent study demonstrated that in Denmark, a woman's risk of developing breast cancer is very slightly elevated on HC1:
- 1 in 7,690 users overall
- 1 in 50,000 women older than age 35 years.
By comparison, the risk of maternal mortality in the United States is 1 in 3,788.2 A substantial reduction in HC use would likely increase unintended and mistimed pregnancies with a potential substantial negative impact on quality of life and personal/societal cost.
The best available data indicate that a woman's risk of developing any cancer is slightly less on HC than not on HC, even with this incremental breast cancer increase.3,4
Breast cancer risk relative to benefits of pregnancy prevention
There was a very slight increase in breast cancer in women using HC in the Danish study.1
Risk of breast cancer
- Overall, the number needed to harm (NNH) was approximately 1 in 7,690, which equates to 13 incremental breast cancers for every 100,000 women using HC (0.013%).
- Breast cancer risk was not evenly distributed across the different age groups. In women younger than 35 years, the risk was 1 extra case for every 50,000 women using HC (0.002%).
Risk of pregnancy prevention failure: Maternal mortality
- By comparison, the rate of maternal mortality is considerably higher than either of these risks in the United States. Specifically, the most recently available rate of maternal mortality (2015) in the United States was 26.4 for every 100,000 women, essentially double that of developing breast cancer on HC.2
-- Most women who develop breast cancer while on HC will survive their cancer long-term.5 And most would agree that while neither is desirable, death is a worse outcome than the development of breast cancer.
Risk of pregnancy prevention failure other than maternal mortality
- Other than the copper IUD and sterilization methods, all other nonhormonal contraceptive methods are by far inferior in terms of the ability to prevent unintended pregnancy.
- Unintended pregnancy has substantial health, social, and economic consequences to women and infants, and contraception use is a well-accepted proximate determinant of unintended pregnancy.6
- Unintended pregnancy is a serious maternal-child health problem with potentially long-term burdens not only for women and families7-10 but also for society.11-13
- Unintended pregnancies generate an estimated $21 billion direct and indirect costs for the US health care system per year,14 and approximately 42% of these pregnancies end in abortion.15
HC cancer risk and HC cancer prevention
- HC use increases risk of breast and liver cancer but reduces risk of ovarian, endometrial, and colorectal cancer; the net effect is a modest reduction in total cancer.3,4
- In addition, there appears to be additional cervical cancer prevention benefit from IUD use.16
- In a recent meta-analysis, IUDs (including LNG-IUD) have been associated with a 33% reduction in cervical cancer.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch, LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775-1812.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193-200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276-2277.
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016. Atlanta, Georgia: American Cancer Society, Inc; 2015.
- Sonfield A. What the Agency for Healthcare Research and Quality forgets to tell Americans about how to protect their sexual and reproductive health. Womens Health Issues. 2015;25(1):1-2.
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the wellbeing of children and families. Washington, DC: National Academy Press; 1995:50-90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281-286.
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. The National Campaign to Prevent Teen Pregnancy and Child Trends. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf. Published May 2007. Accessed January 11, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126-127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Sonfield A, Kost K. Public costs from unintended pregnancy and the role of public insurance program in paying for pregnancy and infant care: Estimates for 2008. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP.pdf. Published October 2013. Accessed January 15, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6-15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010. Guttmacher Institute; 2015. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Accessed January 29, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Cortessis VK, Barrett M, Brown Wade N, et al. Intrauterine device use and cervical cancer risk: A systematic review and meta-analysis. Obstet Gynecol. 2017;130(6):1226-1236.
Hormonal contraception (HC) has long been utilized safely in this country for a variety of indications, including pregnancy prevention, timing pregnancy appropriately, management of symptoms (dysmenorrhea, irregular menstrual cycles, heavy menstrual bleeding), and to prevent serious diseases (such as ovarian cancer, uterine cancer, osteoporosis in women with premature menopause). Like most prescription medications, there are potential adverse effects. With HC, side effects such as venous thromboembolism, a slight increase in liver cancer, and a possible increase in breast cancer risk have long been recognized.
Danish study compared HC use with breast cancer risk
In the December 7, 2017, issue of New England Journal of Medicine,1 investigators in Denmark published a study of women using HC (oral, transdermal, intravaginal routes, and levonorgestrel intrauterine device [LNG-IUD]) and breast cancer risk compared with women who did not use HC. This retrospective observational country-wide study was very large (1.8 million women followed over an average of 10.9 years), which allowed for the detection of even small changes in breast cancer risk.
Putting results in perspective
It is important to point out that this is an observational study, and small effect sizes (1 in 7,600) should be interpreted with caution. Observational studies can introduce many different types of bias (prescribing bias, confounding bias, etc). Of note, while the LNG-IUD was associated with a small increased risk of breast cancer (relative risk [RR], 1.21; 95% confidence interval [CI], 1.11-1.33]), the higher dose continuous progestin administration (medroxyprogesterone) was not (RR, 0.95; 95% CI, 0.40-2.29).1
Nonetheless, providing patients with a balanced summary of this new study along with other published and reliable information about HC that conveys both benefits and risks is important to assure that each woman makes a decision regarding HC that achieves her health and life goals. See "Counseling talking points" below.
Bottom line
This recent study demonstrated that in Denmark, a woman's risk of developing breast cancer is very slightly elevated on HC1:
- 1 in 7,690 users overall
- 1 in 50,000 women older than age 35 years.
By comparison, the risk of maternal mortality in the United States is 1 in 3,788.2 A substantial reduction in HC use would likely increase unintended and mistimed pregnancies with a potential substantial negative impact on quality of life and personal/societal cost.
The best available data indicate that a woman's risk of developing any cancer is slightly less on HC than not on HC, even with this incremental breast cancer increase.3,4
Breast cancer risk relative to benefits of pregnancy prevention
There was a very slight increase in breast cancer in women using HC in the Danish study.1
Risk of breast cancer
- Overall, the number needed to harm (NNH) was approximately 1 in 7,690, which equates to 13 incremental breast cancers for every 100,000 women using HC (0.013%).
- Breast cancer risk was not evenly distributed across the different age groups. In women younger than 35 years, the risk was 1 extra case for every 50,000 women using HC (0.002%).
Risk of pregnancy prevention failure: Maternal mortality
- By comparison, the rate of maternal mortality is considerably higher than either of these risks in the United States. Specifically, the most recently available rate of maternal mortality (2015) in the United States was 26.4 for every 100,000 women, essentially double that of developing breast cancer on HC.2
-- Most women who develop breast cancer while on HC will survive their cancer long-term.5 And most would agree that while neither is desirable, death is a worse outcome than the development of breast cancer.
Risk of pregnancy prevention failure other than maternal mortality
- Other than the copper IUD and sterilization methods, all other nonhormonal contraceptive methods are by far inferior in terms of the ability to prevent unintended pregnancy.
- Unintended pregnancy has substantial health, social, and economic consequences to women and infants, and contraception use is a well-accepted proximate determinant of unintended pregnancy.6
- Unintended pregnancy is a serious maternal-child health problem with potentially long-term burdens not only for women and families7-10 but also for society.11-13
- Unintended pregnancies generate an estimated $21 billion direct and indirect costs for the US health care system per year,14 and approximately 42% of these pregnancies end in abortion.15
HC cancer risk and HC cancer prevention
- HC use increases risk of breast and liver cancer but reduces risk of ovarian, endometrial, and colorectal cancer; the net effect is a modest reduction in total cancer.3,4
- In addition, there appears to be additional cervical cancer prevention benefit from IUD use.16
- In a recent meta-analysis, IUDs (including LNG-IUD) have been associated with a 33% reduction in cervical cancer.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hormonal contraception (HC) has long been utilized safely in this country for a variety of indications, including pregnancy prevention, timing pregnancy appropriately, management of symptoms (dysmenorrhea, irregular menstrual cycles, heavy menstrual bleeding), and to prevent serious diseases (such as ovarian cancer, uterine cancer, osteoporosis in women with premature menopause). Like most prescription medications, there are potential adverse effects. With HC, side effects such as venous thromboembolism, a slight increase in liver cancer, and a possible increase in breast cancer risk have long been recognized.
Danish study compared HC use with breast cancer risk
In the December 7, 2017, issue of New England Journal of Medicine,1 investigators in Denmark published a study of women using HC (oral, transdermal, intravaginal routes, and levonorgestrel intrauterine device [LNG-IUD]) and breast cancer risk compared with women who did not use HC. This retrospective observational country-wide study was very large (1.8 million women followed over an average of 10.9 years), which allowed for the detection of even small changes in breast cancer risk.
Putting results in perspective
It is important to point out that this is an observational study, and small effect sizes (1 in 7,600) should be interpreted with caution. Observational studies can introduce many different types of bias (prescribing bias, confounding bias, etc). Of note, while the LNG-IUD was associated with a small increased risk of breast cancer (relative risk [RR], 1.21; 95% confidence interval [CI], 1.11-1.33]), the higher dose continuous progestin administration (medroxyprogesterone) was not (RR, 0.95; 95% CI, 0.40-2.29).1
Nonetheless, providing patients with a balanced summary of this new study along with other published and reliable information about HC that conveys both benefits and risks is important to assure that each woman makes a decision regarding HC that achieves her health and life goals. See "Counseling talking points" below.
Bottom line
This recent study demonstrated that in Denmark, a woman's risk of developing breast cancer is very slightly elevated on HC1:
- 1 in 7,690 users overall
- 1 in 50,000 women older than age 35 years.
By comparison, the risk of maternal mortality in the United States is 1 in 3,788.2 A substantial reduction in HC use would likely increase unintended and mistimed pregnancies with a potential substantial negative impact on quality of life and personal/societal cost.
The best available data indicate that a woman's risk of developing any cancer is slightly less on HC than not on HC, even with this incremental breast cancer increase.3,4
Breast cancer risk relative to benefits of pregnancy prevention
There was a very slight increase in breast cancer in women using HC in the Danish study.1
Risk of breast cancer
- Overall, the number needed to harm (NNH) was approximately 1 in 7,690, which equates to 13 incremental breast cancers for every 100,000 women using HC (0.013%).
- Breast cancer risk was not evenly distributed across the different age groups. In women younger than 35 years, the risk was 1 extra case for every 50,000 women using HC (0.002%).
Risk of pregnancy prevention failure: Maternal mortality
- By comparison, the rate of maternal mortality is considerably higher than either of these risks in the United States. Specifically, the most recently available rate of maternal mortality (2015) in the United States was 26.4 for every 100,000 women, essentially double that of developing breast cancer on HC.2
-- Most women who develop breast cancer while on HC will survive their cancer long-term.5 And most would agree that while neither is desirable, death is a worse outcome than the development of breast cancer.
Risk of pregnancy prevention failure other than maternal mortality
- Other than the copper IUD and sterilization methods, all other nonhormonal contraceptive methods are by far inferior in terms of the ability to prevent unintended pregnancy.
- Unintended pregnancy has substantial health, social, and economic consequences to women and infants, and contraception use is a well-accepted proximate determinant of unintended pregnancy.6
- Unintended pregnancy is a serious maternal-child health problem with potentially long-term burdens not only for women and families7-10 but also for society.11-13
- Unintended pregnancies generate an estimated $21 billion direct and indirect costs for the US health care system per year,14 and approximately 42% of these pregnancies end in abortion.15
HC cancer risk and HC cancer prevention
- HC use increases risk of breast and liver cancer but reduces risk of ovarian, endometrial, and colorectal cancer; the net effect is a modest reduction in total cancer.3,4
- In addition, there appears to be additional cervical cancer prevention benefit from IUD use.16
- In a recent meta-analysis, IUDs (including LNG-IUD) have been associated with a 33% reduction in cervical cancer.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch, LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775-1812.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193-200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276-2277.
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016. Atlanta, Georgia: American Cancer Society, Inc; 2015.
- Sonfield A. What the Agency for Healthcare Research and Quality forgets to tell Americans about how to protect their sexual and reproductive health. Womens Health Issues. 2015;25(1):1-2.
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the wellbeing of children and families. Washington, DC: National Academy Press; 1995:50-90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281-286.
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. The National Campaign to Prevent Teen Pregnancy and Child Trends. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf. Published May 2007. Accessed January 11, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126-127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Sonfield A, Kost K. Public costs from unintended pregnancy and the role of public insurance program in paying for pregnancy and infant care: Estimates for 2008. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP.pdf. Published October 2013. Accessed January 15, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6-15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010. Guttmacher Institute; 2015. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Accessed January 29, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Cortessis VK, Barrett M, Brown Wade N, et al. Intrauterine device use and cervical cancer risk: A systematic review and meta-analysis. Obstet Gynecol. 2017;130(6):1226-1236.
- Mørch, LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775-1812.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193-200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276-2277.
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016. Atlanta, Georgia: American Cancer Society, Inc; 2015.
- Sonfield A. What the Agency for Healthcare Research and Quality forgets to tell Americans about how to protect their sexual and reproductive health. Womens Health Issues. 2015;25(1):1-2.
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the wellbeing of children and families. Washington, DC: National Academy Press; 1995:50-90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281-286.
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. The National Campaign to Prevent Teen Pregnancy and Child Trends. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf. Published May 2007. Accessed January 11, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126-127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Sonfield A, Kost K. Public costs from unintended pregnancy and the role of public insurance program in paying for pregnancy and infant care: Estimates for 2008. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP.pdf. Published October 2013. Accessed January 15, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6-15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010. Guttmacher Institute; 2015. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Accessed January 29, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Cortessis VK, Barrett M, Brown Wade N, et al. Intrauterine device use and cervical cancer risk: A systematic review and meta-analysis. Obstet Gynecol. 2017;130(6):1226-1236.
Examining ED Revisits for Migraine in New York City
More than a quarter of initial emergency department (ED) visits for migraine are followed by headache revisits in less than 6 months, a recent study found. Using the New York City Department of Health and Mental Hygiene Syndromic Surveillance database, researchers conducted a retrospective nested cohort study. They analyzed visits from 18 New York City EDs with discharge diagnoses in the first 6 months of 2015, and conducted descriptive analyses to determine the frequency of headache revisit within 6 months of an index ED visit for migraine and the elapsed time to revisit. They found:
- Of 1052 ED visits with an ED discharge diagnosis of migraine during the first 6 months of 2015, 277 patients (26.3%) had a headache revisit within 6 months of their initial migraine visit and 131 (12.5%) had 2 or more revisits at the same hospital.
- Of the revisits for headache, 9% occurred within 72 hours and 46% occurred within 90 days of the initial migraine visit.
- Sex, age, and poverty level were not associated with an ED revisit.
A retrospective nested cohort study of emergency department revisits for migraine in New York City. [Published online ahead of print November 2, 2017]. Headache. doi:10.1111/head.13216.
More than a quarter of initial emergency department (ED) visits for migraine are followed by headache revisits in less than 6 months, a recent study found. Using the New York City Department of Health and Mental Hygiene Syndromic Surveillance database, researchers conducted a retrospective nested cohort study. They analyzed visits from 18 New York City EDs with discharge diagnoses in the first 6 months of 2015, and conducted descriptive analyses to determine the frequency of headache revisit within 6 months of an index ED visit for migraine and the elapsed time to revisit. They found:
- Of 1052 ED visits with an ED discharge diagnosis of migraine during the first 6 months of 2015, 277 patients (26.3%) had a headache revisit within 6 months of their initial migraine visit and 131 (12.5%) had 2 or more revisits at the same hospital.
- Of the revisits for headache, 9% occurred within 72 hours and 46% occurred within 90 days of the initial migraine visit.
- Sex, age, and poverty level were not associated with an ED revisit.
A retrospective nested cohort study of emergency department revisits for migraine in New York City. [Published online ahead of print November 2, 2017]. Headache. doi:10.1111/head.13216.
More than a quarter of initial emergency department (ED) visits for migraine are followed by headache revisits in less than 6 months, a recent study found. Using the New York City Department of Health and Mental Hygiene Syndromic Surveillance database, researchers conducted a retrospective nested cohort study. They analyzed visits from 18 New York City EDs with discharge diagnoses in the first 6 months of 2015, and conducted descriptive analyses to determine the frequency of headache revisit within 6 months of an index ED visit for migraine and the elapsed time to revisit. They found:
- Of 1052 ED visits with an ED discharge diagnosis of migraine during the first 6 months of 2015, 277 patients (26.3%) had a headache revisit within 6 months of their initial migraine visit and 131 (12.5%) had 2 or more revisits at the same hospital.
- Of the revisits for headache, 9% occurred within 72 hours and 46% occurred within 90 days of the initial migraine visit.
- Sex, age, and poverty level were not associated with an ED revisit.
A retrospective nested cohort study of emergency department revisits for migraine in New York City. [Published online ahead of print November 2, 2017]. Headache. doi:10.1111/head.13216.