User login
Primary Cutaneous Follicle Center Lymphoma Mimicking Folliculitis
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
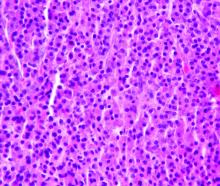
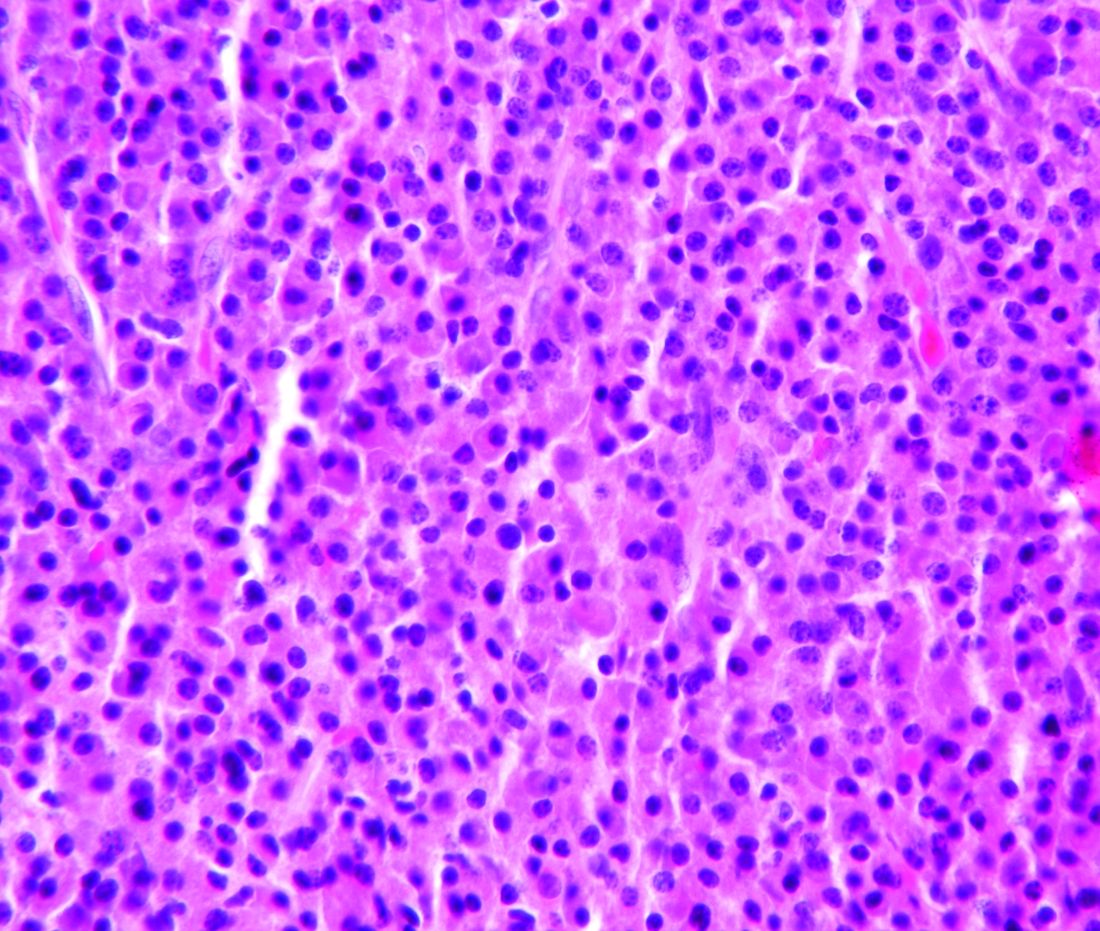
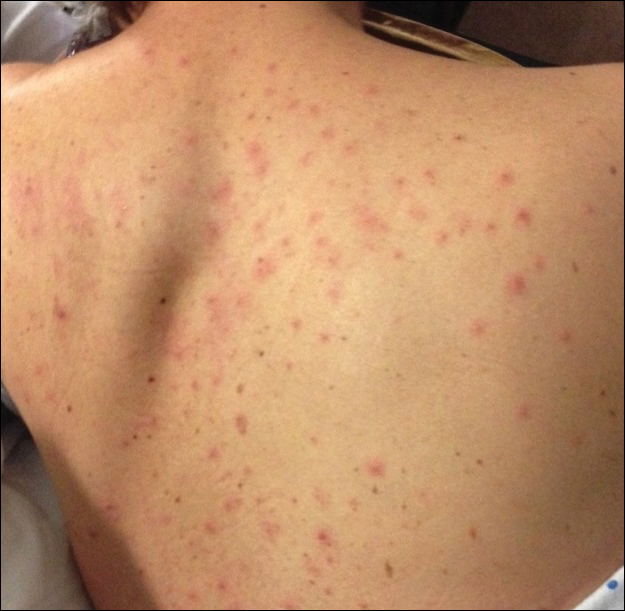
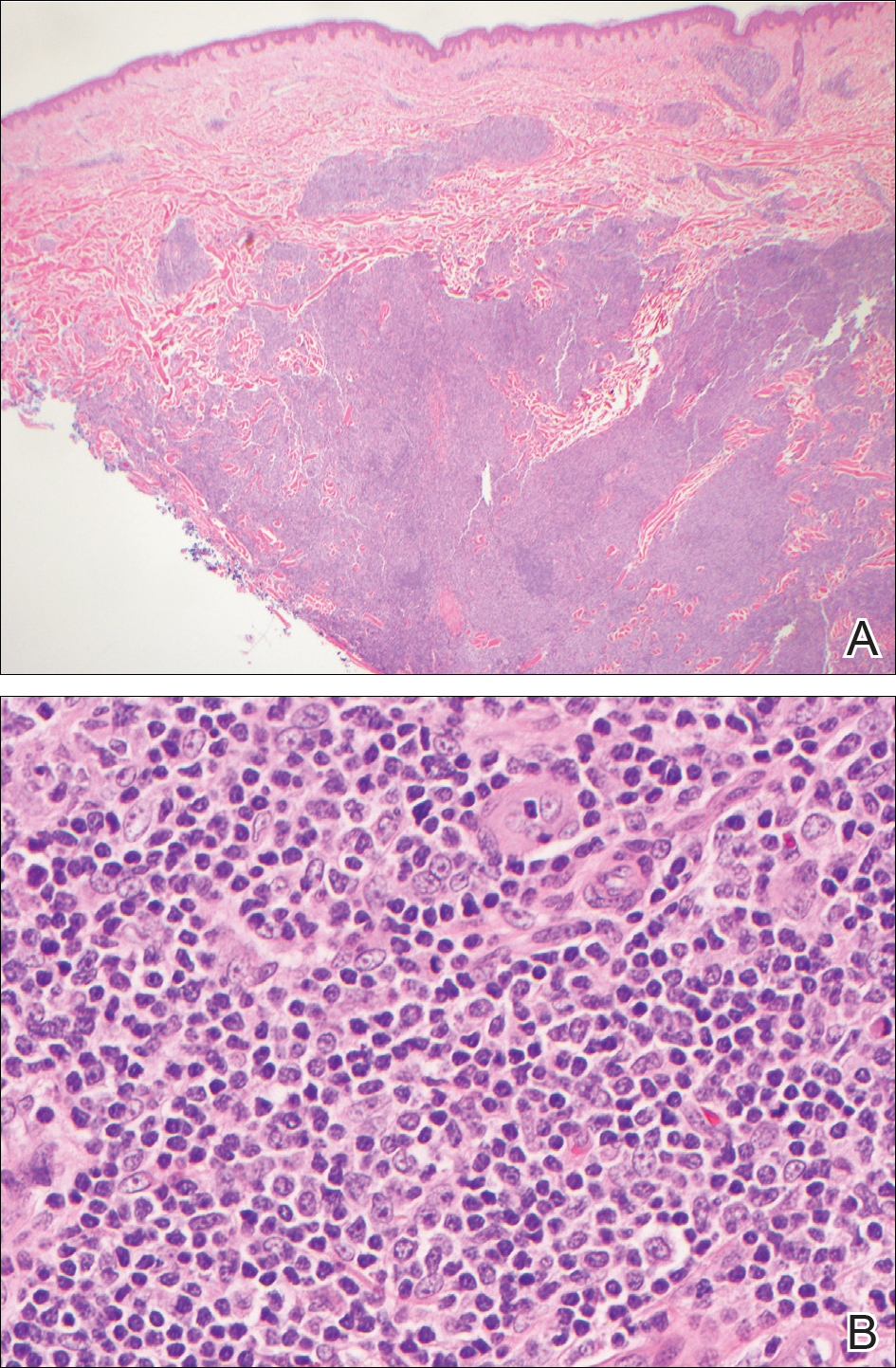
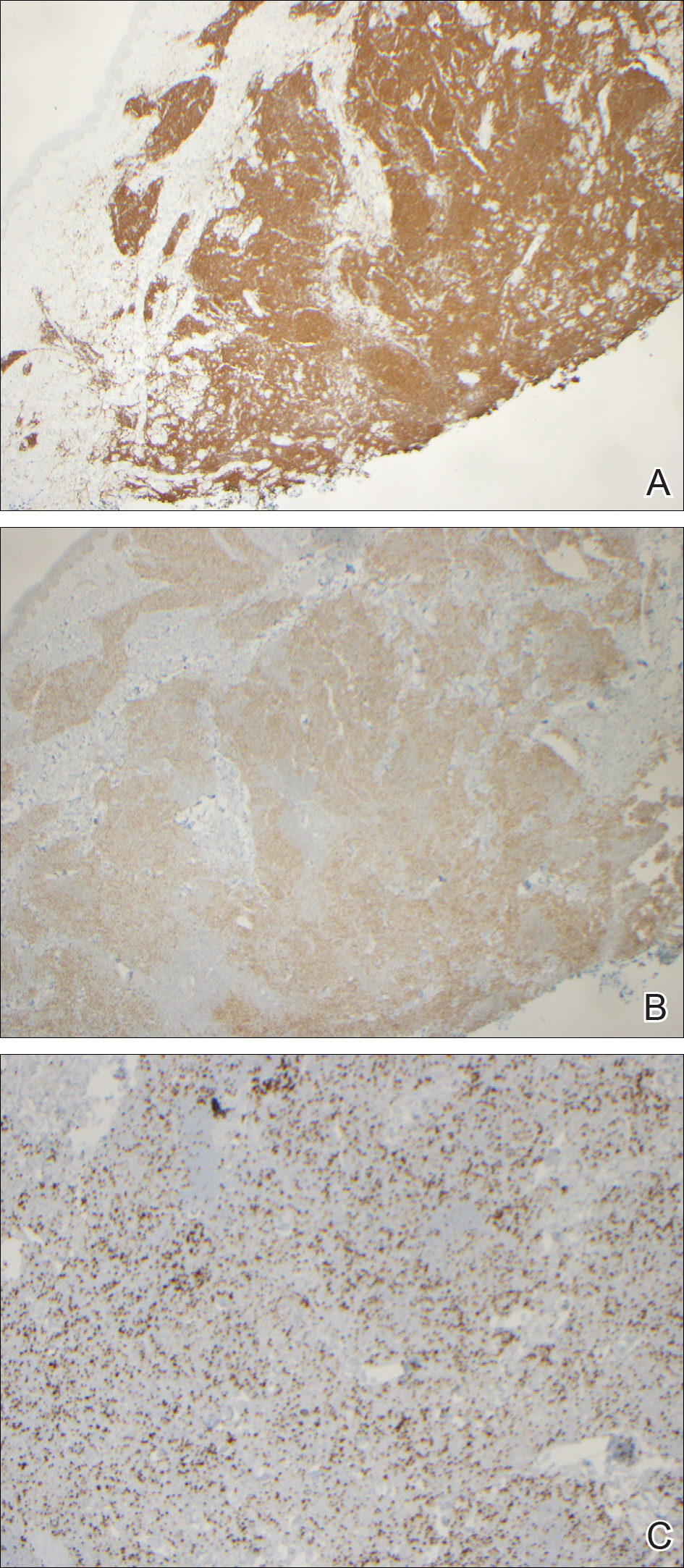
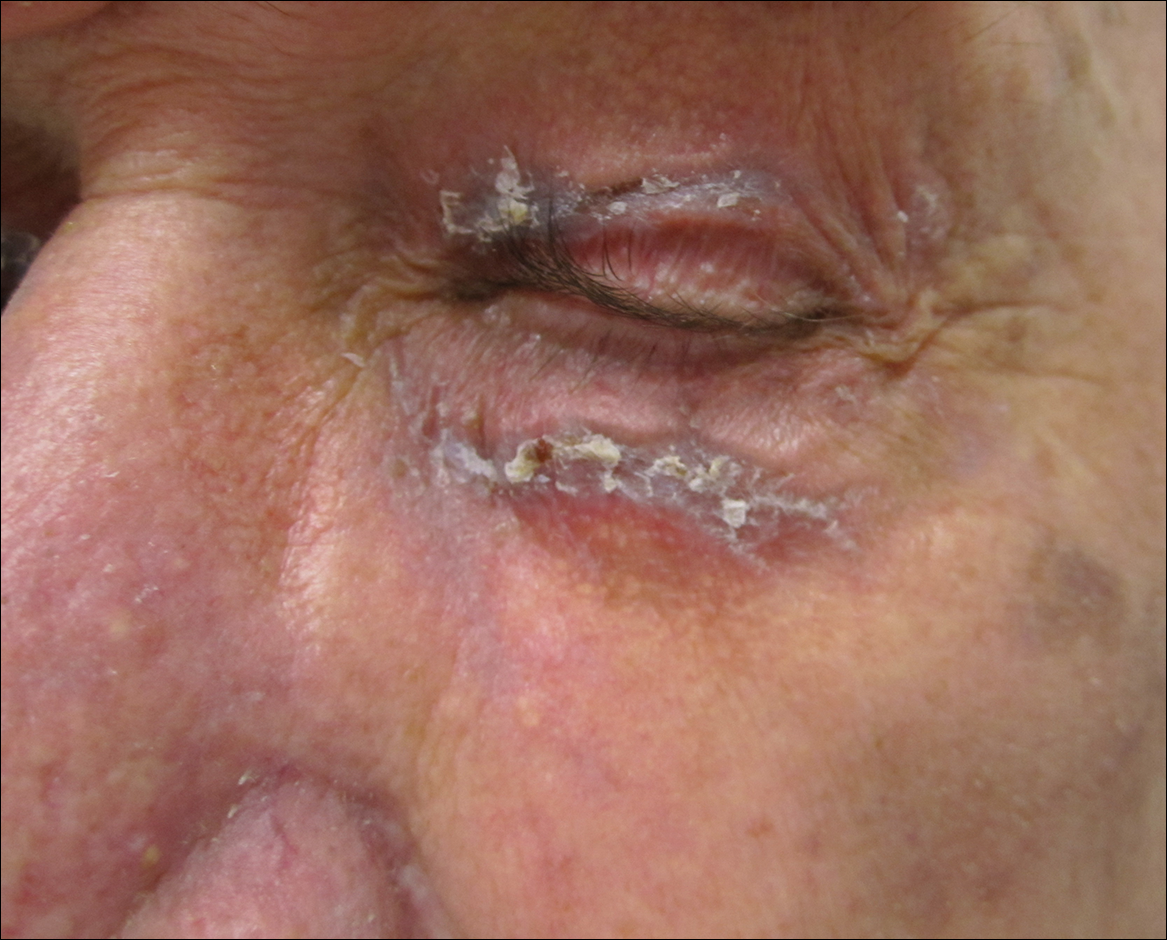
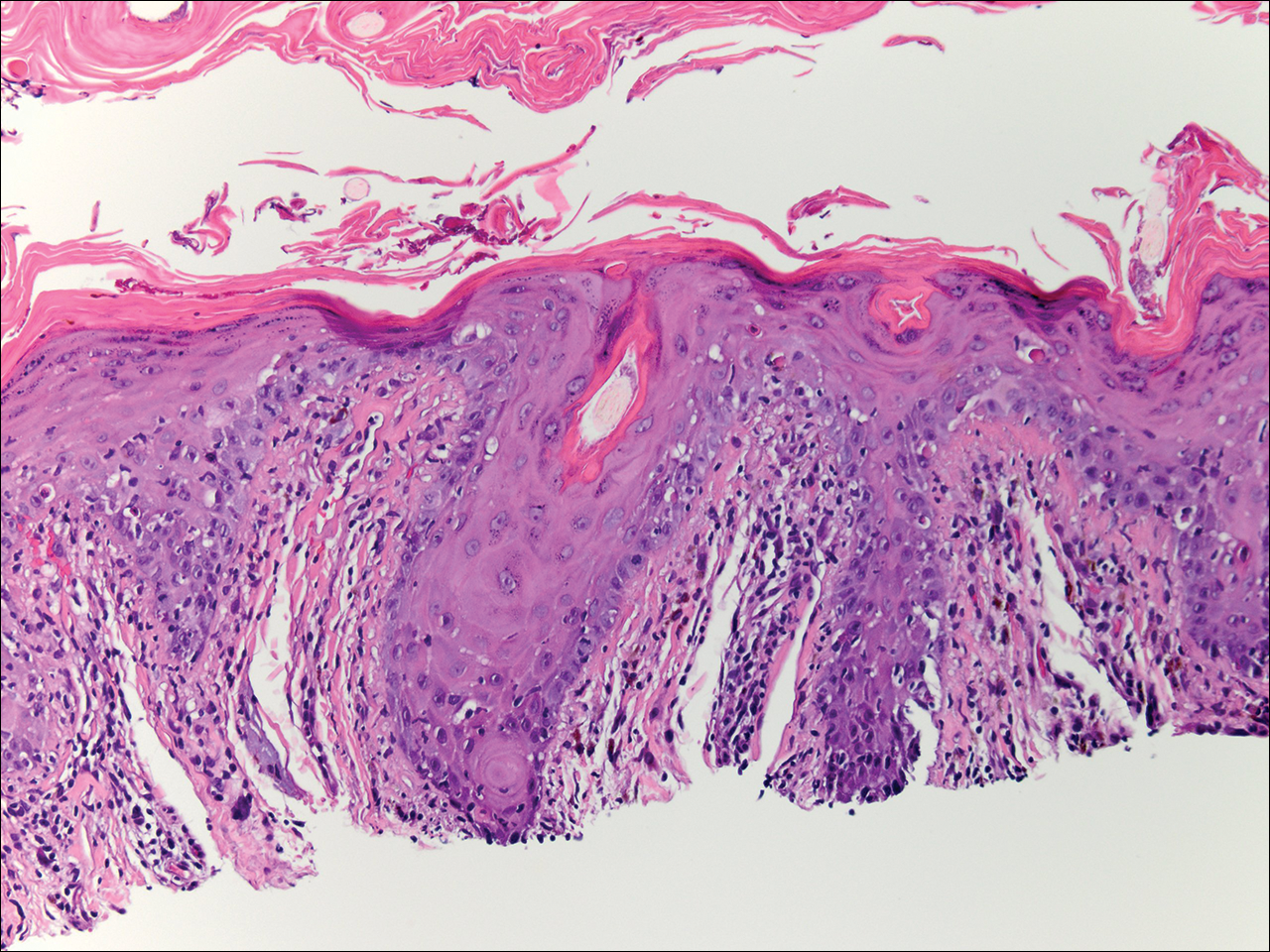
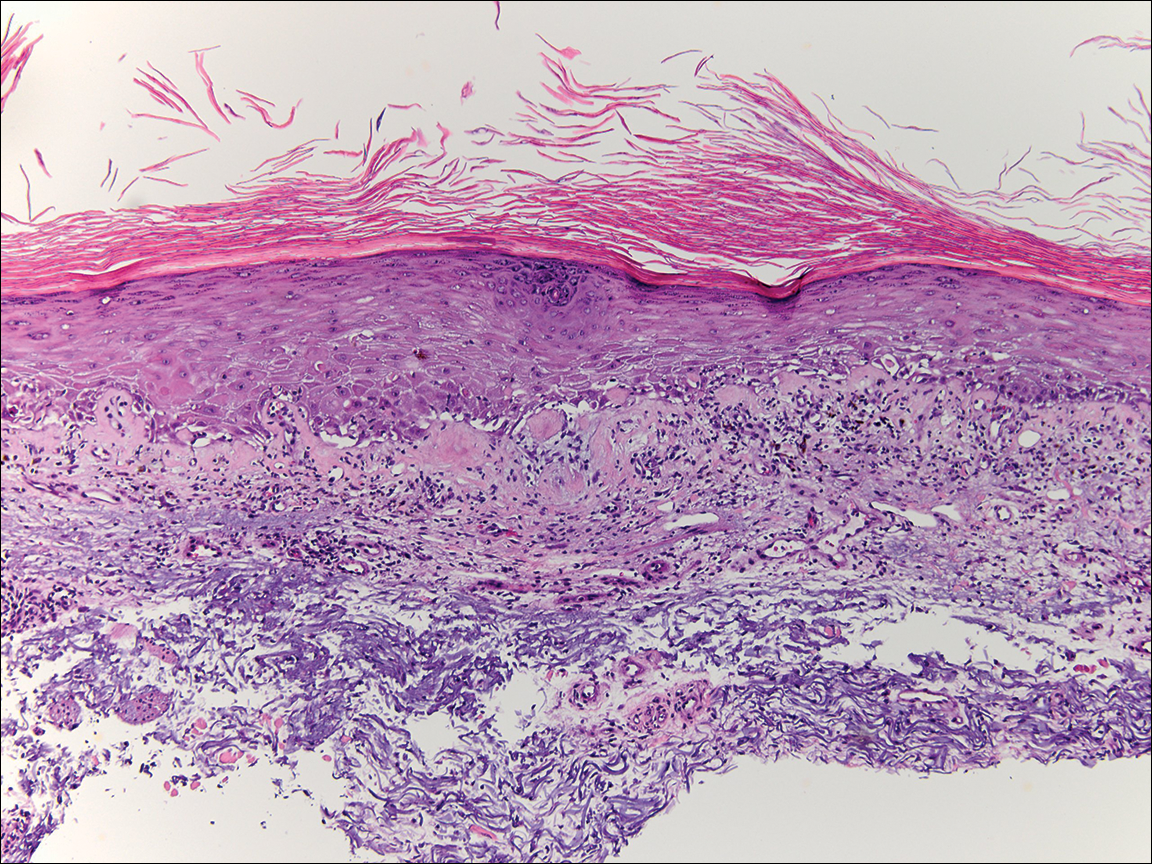
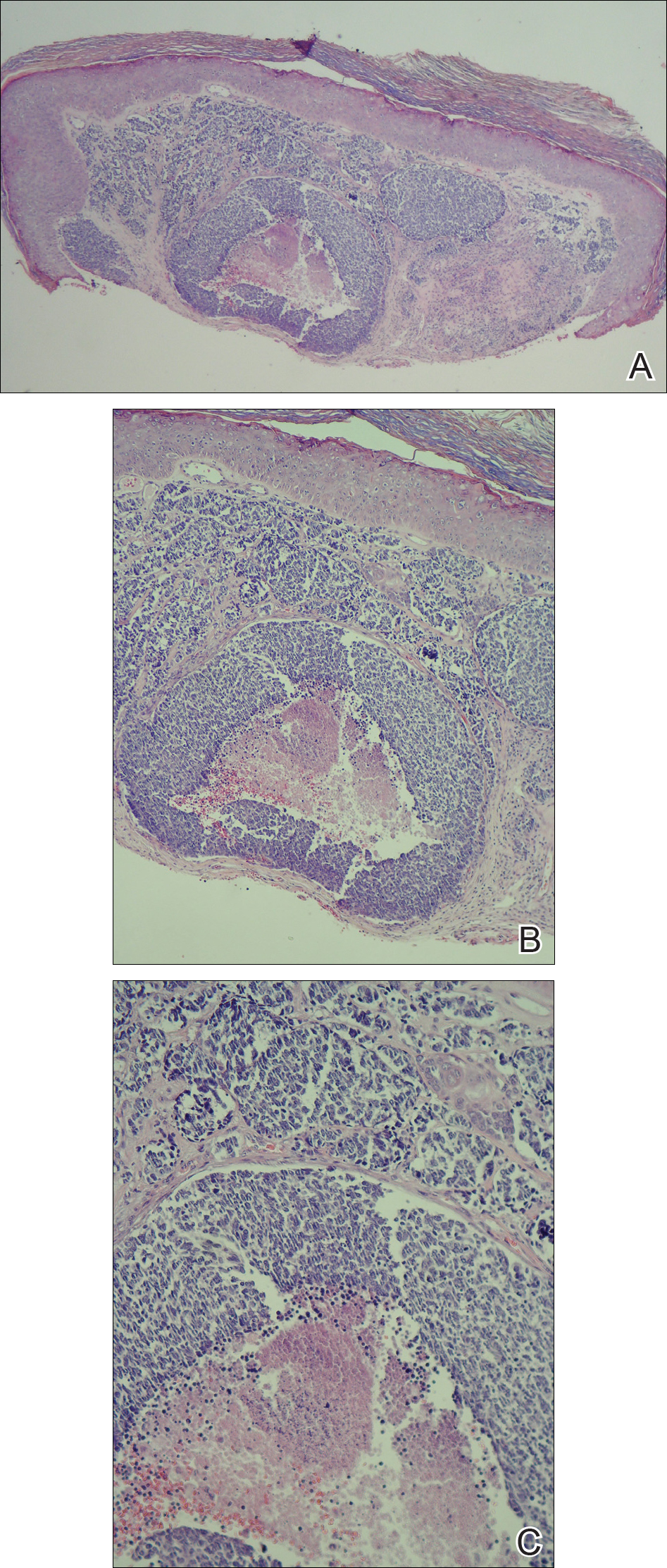
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.
Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
The 2008 World Health Organization and European Organization for Treatment of Cancer joint classification has distinguished 3 categories of primary cutaneous B-cell lymphomas: primary cutaneous follicle center lymphoma (PCFCL), primary cutaneous diffuse large B-cell lymphoma, and primary cutaneous marginal zone lymphoma.1-3 Primary cutaneous follicle center lymphoma is the most common type of cutaneous B-cell lymphoma, accounting for approximately 60% of cases worldwide.4 The median age at diagnosis is 60 years, and most lesions are located on the scalp, forehead, neck, and trunk.5 Histologically, PCFCL is characterized by dermal proliferation of centrocytes and centroblasts derived from germinal center B cells that are arranged in either a follicular, diffuse, or mixed growth pattern.1 The cutaneous manifestations of PCFCL include solitary erythematous or violaceous plaques, nodules, or tumors of varying sizes.4 Grouped lesions also may be observed, but multifocal disease is rare.1 We report a rare presentation of PCFCL mimicking folliculitis with multiple multifocal papules on the back.
Case Report
A 54-year-old woman presented with fever and leukocytosis of 4 days’ duration and was admitted to the hospital for presumed sepsis. She had a history of mastectomy for treatment of ductal carcinoma in situ of the right breast 5 years prior to the current presentation and endocrine therapy with tamoxifen. Her symptoms were thought to be a complication from a surgery for implantation of a tissue expander in the right breast 5 years prior to presentation.
During her hospital admission, she developed a papular and cystic eruption on the back that was clinically suggestive of folliculitis, transient acantholytic dermatosis (Grover disease), or miliaria rubra (Figure 1). This papular and cystic eruption initially was managed conservatively with observation as she recovered from an occult infection. Due to the persistent nature of the eruption on the back, an excisional biopsy of the cystic component was performed 2 months after her discharge from the hospital. Histologic studies showed a dense infiltrate of lymphocytes, which expanded into the deep dermis in a nodular and diffuse growth pattern that was accentuated in the periadnexal areas. The B lymphocytes were small and hyperchromatic with few scattered centroblasts (Figure 2). Further immunohistochemical studies demonstrated that the neoplastic cells were positive for CD20, CD79a, BCL-2, and BCL-6; CD3, CD5, and cyclin D1 were negative. Staining for antigen Ki-67 revealed a proliferation index of 15% to 20% among the neoplastic cells (Figure 3). These findings were consistent with either PCFCL or secondary cutaneous follicle center lymphoma.



Further evaluation for systemic disease was unremarkable. Positron emission tomography–computed tomography revealed no evidence of nodal lymphoma, and a bone marrow biopsy was negative. Other laboratory studies including lactate dehydrogenase were within reference range, which conferred a diagnosis of PCFCL. The patient was treated with localized electron beam radiation therapy to the skin of the mid back for a total dose of 24 Gy in 12 fractions at 2 Gy per fraction once daily over a 12-day period. She tolerated the treatment well and has remained clinically and radiographically without evidence of disease for more than 3 years.
Comment
Because the incidence of cutaneous B-cell lymphomas has been increasing, especially among males, non-Hispanic whites, and adults older than 50 years,1 it is important for clinicians to have a high index of suspicion for this entity. In our patient, the clinical findings of a papular, largely asymptomatic eruption on the back with acute onset were initially thought to be consistent with folliculitis; the differential diagnosis included transient acantholytic dermatosis and miliaria rubra. Lymphoma was not in the initial clinical differential, and we only arrived at this diagnosis based on histopathologic evaluation.
The neoplastic cells typically are positive for CD20, CD79a, and BCL-6, and negative for BCL-2.4 Most cases of PCFCL do not express the t(14;18) translocation involving the BCL-2 locus, in contrast to systemic follicular lymphoma.1 Systemic imaging and evaluation is needed to definitively differentiate PCFCL from systemic lymphoma with cutaneous involvement. Our patient was unusual in that BCL-2 was strongly staining in the setting of a negative systemic workup.
With regard to treatment of PCFCL, electron beam radiation therapy is highly effective and safe in patients with solitary lesions, as the remission rate is close to 100%.1 For patients with multiple lesions confined to one area, electron beam radiation therapy also can be helpful, as in our patient. In patients with more extensive skin involvement, rituximab therapy may be preferable. Relapse following treatment with either radiation or rituximab occurs in approximately one-third of patients, but these relapses generally are limited to the skin.1 The International Extranodal Lymphoma Study Group has noted that elevated lactate dehydrogenase, presence of more than 2 skin lesions, and presence of nodular lesions are negative prognostic factors in patients with PCFCL6; however, PCFCL has an excellent prognosis overall with a 5-year survival rate of 95%.1
Other rare heterogeneous presentations of PCFCL have been reported in the literature. A large multinodular mass on the scalp with multifocal facial lesions has been described in a patient with essential thrombocytopenia.7 Another report identified a variant of PCFCL characterized by multiple erythematous firm papules that were distributed in a miliary pattern, predominantly on the forehead and cheeks.8 Barzilai et al9 described 4 patients with PCFCL who developed lesions that were clinically similar to rosacea or rhinophyma, including papulonodular eruptions on the cheeks; infiltrated erythematous nasal plaques; and small flesh-colored to erythematous papules on the cheeks, nose, helices, and upper back. Hodak et al10 identified 2 cases of PCFCL that manifested as anetoderma, a condition characterized by the focal loss of elastic tissue. In the setting of chronic lymphocytic leukemia, PCFCL has been observed as a red or violaceous nodule with a centrally depressed scar on the legs.11 In one case, PCFCL manifested as recurrent episodes of extraorbital swelling and a multifocal red-blue macular lesion that extended from the inferior orbital rim to the nasojugal fold.12 An interesting presentation of PCFCL was noted as a small, recurring, blood-filled blister on the cheek with perineural spread of the tumor along cranial nerves V2, V3, VII, and VIII.13 In the pediatric literature, PCFCL has been reported to present as an erythematous nodule with a smooth surface and a hard elastic consistency that appeared on the nose and nasolabial fold and spread to the ipsilateral cheek, maxillary sinus, and soft palate.14 In many of these unusual cases, the diagnosis of PCFCL was made after treatment with topical or systemic anti-inflammatory therapies failed.
Increased recognition of anomalous presentations of PCFCL among dermatologists can lead to more timely diagnoses and treatment. Based on our experience with this patient, we recommend considering biopsy for histopathologic evaluation when treating patients with presumed folliculitis or transient acantholytic dermatosis that does not improve with routine treatment or is accompanied by systemic symptoms.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
- Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:73-76.
- Kim YH, Willemze R, Pimpinelli N, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:479-484.
- World Health Organization. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: World Health Organization; 2008: 227.
- Dilly M, Ben-Rejeb H, Vergier B, et al. Primary cutaneous follicle center lymphoma with Hodgkin and Reed-Sternberg-like cells: a new histopathologic variant. J Cutan Pathol. 2014;41:797-801.
- Suárez AL, Pulitzer M, Horwitz S, et al. Primary cutaneous B-cell lymphomas: part I. clinical features, diagnosis, and classification. J Am Acad Dermatol. 2013;69:329.e1-13; quiz 341-342.
- Mian M, Marcheselli L, Luminari S, et al. CLIPI: a new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11) [published online September 25, 2010]. Ann Hematol. 2011;90:401-408.
- Tirefort Y, Pham XC, Ibrahim YL, et al. A rare case of primary cutaneous follicle centre lymphoma presenting as a giant tumour of the scalp and combined with JAK2V617F positive essential thrombocythaemia. Biomark Res. 2014;2:7.
- Massone C, Fink-Puches R, Laimer M, et al. Miliary and agminated-type primary cutaneous follicle center lymphoma: report of 18 cases.J Am Acad Dermatol. 2011;65:749-755.
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148:824-831.
- Hodak E, Feuerman H, Barzilai A, et al. Anetodermic primary cutaneous B-cell lymphoma: a unique clinicopathological presentation of lymphoma possibly associated with antiphospholipid antibodies. Arch Dermatol. 2010;146:175-182.
- Konda S, Beckford A, Demierre MF, et al. Primary cutaneous follicle center lymphoma in the setting of chronic lymphocytic leukemia. Indian J Dermatol Venereol Leprol. 2011;77:314-317.
- Pandya VB, Conway RM, Taylor SF. Primary cutaneous B cell lymphoma presenting as recurrent eyelid swelling. Clin Exp Ophthalmol. 2008;36:672-674.
- Buda-Okreglak EM, Walden MJ, Brissette MD. Perineural CNS invasion in primary cutaneous follicular center lymphoma. J Clin Oncol. 2007;25:4684-4686.
- Ghislanzoni M, Gambini D, Perrone T, et al. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 suppl 1):S73-S75.
Practice Points
- Atypical or unresponsive folliculitis should be biopsied.
- Primary cutaneous follicle center lymphoma can mimic folliculitis or Grover disease.
Surgery team scorecard improved patient satisfaction
JACKSONVILLE, FLA. – A scorecard enables spectators at a baseball game to keep track of who the players are, and a scorecard of the surgery team can do the same for inpatients, researchers at Johns Hopkins University in Baltimore found.
They gave patients a “facesheet” that included photographs and biographies of all members of their surgery team, which helped patients to better understand the team members’ roles in their care and led to improvements in overall satisfaction scores, according to a study reported at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
The study involved two intervals: a prefacesheet phase of 153 patients and a postfacesheet phase of 100 patients. The two groups, all gastrointestinal surgery inpatients, were administered preintervention discharge surveys to evaluate their level of patient satisfaction according to a 5-point Likert scale (1 = strongly disagree and 5 = strongly agree).
“We found that using these facesheets helped patients know the roles of their team members, and the patients felt that it was important to [have this information],” Dr. DiBrito said.
The share of patients answering 4 (agreed) or 5 (strongly agreed) for overall satisfaction rose from 83% before the facesheet intervention to 88% afterward (P = .5). The number of patients agreeing that they understood their providers’ roles increased from 72% to 83% (P = .05), and the number who agreed that it was important to know who their surgical team members were increased from 85% to 94% (P = .04). The latter finding somewhat surprised the researchers. Dr. DiBrito said, “That’s not exactly what we were anticipating.”
The study also revealed a trend in patients’ feeling more confident in their team overall after the facesheet intervention, rising from 89% to 95%, Dr. DiBrito said.
She said the Johns Hopkins team is not continuing the initiative currently but would like to roll it out more broadly to other hospital services. Other groups within the hospital, including nursing and clinical customer services, must get on board, she said. “We really need buy-in from higher levels in the hospital, and this was part of the proof that we needed,” Dr. DiBrito said.
The premise of the study was that patients need to identify a member of their care team as a point person, she added. “We’re trying to give the patients, and their family members as well, some people to look out for,” Dr. DiBrito said.
Dr. DiBrito and her coauthors had no financial relationships to disclose.
SOURCE: Dibrito SR et al. Academic Surgical Congress 2018, Abstract 09.04.
JACKSONVILLE, FLA. – A scorecard enables spectators at a baseball game to keep track of who the players are, and a scorecard of the surgery team can do the same for inpatients, researchers at Johns Hopkins University in Baltimore found.
They gave patients a “facesheet” that included photographs and biographies of all members of their surgery team, which helped patients to better understand the team members’ roles in their care and led to improvements in overall satisfaction scores, according to a study reported at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
The study involved two intervals: a prefacesheet phase of 153 patients and a postfacesheet phase of 100 patients. The two groups, all gastrointestinal surgery inpatients, were administered preintervention discharge surveys to evaluate their level of patient satisfaction according to a 5-point Likert scale (1 = strongly disagree and 5 = strongly agree).
“We found that using these facesheets helped patients know the roles of their team members, and the patients felt that it was important to [have this information],” Dr. DiBrito said.
The share of patients answering 4 (agreed) or 5 (strongly agreed) for overall satisfaction rose from 83% before the facesheet intervention to 88% afterward (P = .5). The number of patients agreeing that they understood their providers’ roles increased from 72% to 83% (P = .05), and the number who agreed that it was important to know who their surgical team members were increased from 85% to 94% (P = .04). The latter finding somewhat surprised the researchers. Dr. DiBrito said, “That’s not exactly what we were anticipating.”
The study also revealed a trend in patients’ feeling more confident in their team overall after the facesheet intervention, rising from 89% to 95%, Dr. DiBrito said.
She said the Johns Hopkins team is not continuing the initiative currently but would like to roll it out more broadly to other hospital services. Other groups within the hospital, including nursing and clinical customer services, must get on board, she said. “We really need buy-in from higher levels in the hospital, and this was part of the proof that we needed,” Dr. DiBrito said.
The premise of the study was that patients need to identify a member of their care team as a point person, she added. “We’re trying to give the patients, and their family members as well, some people to look out for,” Dr. DiBrito said.
Dr. DiBrito and her coauthors had no financial relationships to disclose.
SOURCE: Dibrito SR et al. Academic Surgical Congress 2018, Abstract 09.04.
JACKSONVILLE, FLA. – A scorecard enables spectators at a baseball game to keep track of who the players are, and a scorecard of the surgery team can do the same for inpatients, researchers at Johns Hopkins University in Baltimore found.
They gave patients a “facesheet” that included photographs and biographies of all members of their surgery team, which helped patients to better understand the team members’ roles in their care and led to improvements in overall satisfaction scores, according to a study reported at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
The study involved two intervals: a prefacesheet phase of 153 patients and a postfacesheet phase of 100 patients. The two groups, all gastrointestinal surgery inpatients, were administered preintervention discharge surveys to evaluate their level of patient satisfaction according to a 5-point Likert scale (1 = strongly disagree and 5 = strongly agree).
“We found that using these facesheets helped patients know the roles of their team members, and the patients felt that it was important to [have this information],” Dr. DiBrito said.
The share of patients answering 4 (agreed) or 5 (strongly agreed) for overall satisfaction rose from 83% before the facesheet intervention to 88% afterward (P = .5). The number of patients agreeing that they understood their providers’ roles increased from 72% to 83% (P = .05), and the number who agreed that it was important to know who their surgical team members were increased from 85% to 94% (P = .04). The latter finding somewhat surprised the researchers. Dr. DiBrito said, “That’s not exactly what we were anticipating.”
The study also revealed a trend in patients’ feeling more confident in their team overall after the facesheet intervention, rising from 89% to 95%, Dr. DiBrito said.
She said the Johns Hopkins team is not continuing the initiative currently but would like to roll it out more broadly to other hospital services. Other groups within the hospital, including nursing and clinical customer services, must get on board, she said. “We really need buy-in from higher levels in the hospital, and this was part of the proof that we needed,” Dr. DiBrito said.
The premise of the study was that patients need to identify a member of their care team as a point person, she added. “We’re trying to give the patients, and their family members as well, some people to look out for,” Dr. DiBrito said.
Dr. DiBrito and her coauthors had no financial relationships to disclose.
SOURCE: Dibrito SR et al. Academic Surgical Congress 2018, Abstract 09.04.
REPORTING FROM THE ACADEMIC SURGICAL CONGRESS
Key clinical point: A “facesheet” that includes photographs and biographies of the surgical care team improves patient satisfaction.
Major finding: Overall satisfaction scores increased from 83% preintervention to 88% postintervention.
Data source: Analysis of the survey responses from 253 gastrointestinal surgery patients pre- and postintervention from February 2017 to May 2017.
Disclosures: Dr. DiBrito and her coauthors had no financial relationships to disclose.
Source: Dibrito SR et al. Academic Surgical Congress 2018, Abstract 09.04.
FDA issues safety alert for loperamide
The Food and Drug Administration announced Jan. 30 that is has issued a MedWatch safety alert on the use of the over-the-counter (OTC) antidiarrhea drug, loperamide.
Currently, the FDA is working with manufacturers to use blister packs or other single-dose packaging and to limit the number of doses in a package.
The alert comes after receiving continuous reports of serious heart problems and deaths with the use of much higher than recommended doses of loperamide, mainly among people who are intentionally misusing or abusing the product, regardless of the addition of a warning to the medicine label and a previous communication. The FDA states that loperamide is a safe drug when used as directed.
Loperamide is approved to help control symptoms of diarrhea. The maximum recommended daily dose for adults is 8 mg per day for OTC use and 16 mg per day for prescription use. It acts on opioid receptors in the gut to slow the movement in the intestines and decrease the number of bowel movements.
It is noted that much higher than recommended doses of loperamide, either intentionally or unintentionally, can result in serious cardiac adverse events, including QT interval prolongation, torsade de pointes or other ventricular arrhythmias, syncope, and cardiac arrest. Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
In 2016, the FDA issued a Drug Safety Communication and added warnings about serious heart problems to the drug label of prescription loperamide and to the Drug Facts label of OTC loperamide products. The FDA is working to evaluate this safety issue and will update the public when more information is available.
Read the full safety alert here.
The Food and Drug Administration announced Jan. 30 that is has issued a MedWatch safety alert on the use of the over-the-counter (OTC) antidiarrhea drug, loperamide.
Currently, the FDA is working with manufacturers to use blister packs or other single-dose packaging and to limit the number of doses in a package.
The alert comes after receiving continuous reports of serious heart problems and deaths with the use of much higher than recommended doses of loperamide, mainly among people who are intentionally misusing or abusing the product, regardless of the addition of a warning to the medicine label and a previous communication. The FDA states that loperamide is a safe drug when used as directed.
Loperamide is approved to help control symptoms of diarrhea. The maximum recommended daily dose for adults is 8 mg per day for OTC use and 16 mg per day for prescription use. It acts on opioid receptors in the gut to slow the movement in the intestines and decrease the number of bowel movements.
It is noted that much higher than recommended doses of loperamide, either intentionally or unintentionally, can result in serious cardiac adverse events, including QT interval prolongation, torsade de pointes or other ventricular arrhythmias, syncope, and cardiac arrest. Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
In 2016, the FDA issued a Drug Safety Communication and added warnings about serious heart problems to the drug label of prescription loperamide and to the Drug Facts label of OTC loperamide products. The FDA is working to evaluate this safety issue and will update the public when more information is available.
Read the full safety alert here.
The Food and Drug Administration announced Jan. 30 that is has issued a MedWatch safety alert on the use of the over-the-counter (OTC) antidiarrhea drug, loperamide.
Currently, the FDA is working with manufacturers to use blister packs or other single-dose packaging and to limit the number of doses in a package.
The alert comes after receiving continuous reports of serious heart problems and deaths with the use of much higher than recommended doses of loperamide, mainly among people who are intentionally misusing or abusing the product, regardless of the addition of a warning to the medicine label and a previous communication. The FDA states that loperamide is a safe drug when used as directed.
Loperamide is approved to help control symptoms of diarrhea. The maximum recommended daily dose for adults is 8 mg per day for OTC use and 16 mg per day for prescription use. It acts on opioid receptors in the gut to slow the movement in the intestines and decrease the number of bowel movements.
It is noted that much higher than recommended doses of loperamide, either intentionally or unintentionally, can result in serious cardiac adverse events, including QT interval prolongation, torsade de pointes or other ventricular arrhythmias, syncope, and cardiac arrest. Health care professionals and patients can report adverse events or side effects related to the use of these products to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
In 2016, the FDA issued a Drug Safety Communication and added warnings about serious heart problems to the drug label of prescription loperamide and to the Drug Facts label of OTC loperamide products. The FDA is working to evaluate this safety issue and will update the public when more information is available.
Read the full safety alert here.
Periorbital Lupuslike Presentation of Graft-versus-host Disease
To the Editor:
A 79-year-old man presented with a scaling eruption in the periorbital area, on the bilateral forearms, and on the chest of 4 weeks’ duration. The patient denied systemic symptoms including lethargy, muscle weakness, and fevers. His medical history was notable for blastic plasmacytoid dendritic cell neoplasm, a form of acute myeloid leukemia, diagnosed 3 years prior to presentation. The patient received an allogeneic hematopoietic stem cell transplant 8 months later. His posttransplant course was complicated by gastrointestinal graft-versus-host disease (GVHD); progressive graft loss requiring a donor lymphocyte infusion after 1 month; and leukemia cutis, which spontaneously resolved after 1 month. The patient was taken off all immunosuppressive therapy 5 months after the transplant and had been doing well for 2 years with only mild mucosal GVHD affecting the oral mucosa and the head of the penis.
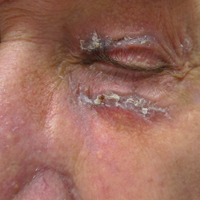
Physical examination at the current presentation revealed linear, atrophic, scaling, purplish plaques with adherent white scale on the upper and lower eyelids (Figure 1). The patient also had scattered purple scaling patches on the bilateral forearms and chest. Laboratory tests including complete blood cell count, comprehensive metabolic panel, and lactate dehydrogenase demonstrated no gross abnormalities. Two shave biopsies of the right lower eyelid (Figure 2) and left arm (Figure 3) were performed for histologic examination and revealed basket weave hyperkeratosis, irregular acanthosis, sawtooth rete ridges, and scattered dyskeratotic cells. Vacuolar changes and smudging of the basement membrane zone along with a bandlike lymphocytic infiltrate in the upper dermis also were noted in both biopsies. A diagnosis of lupuslike grade 1 GVHD was made.
Graft-versus-host disease remains a notable cause of morbidity and mortality in allogenic hematopoietic stem cell transplant patients.1 Skin manifestations represent the most common manifestation of GVHD and have been reclassified as acute or chronic disease based on clinical and histologic findings rather than time of onset. Although acute GVHD classically presents as diffuse morbilliform papules and macules, chronic GVHD has a large range of clinical presentations most commonly mimicking the skin findings of lichen planus, morphea, scleroderma, or lichen sclerosus.1
Lupuslike GVHD is a rarely reported manifestation of chronic GVHD that predominantly affects the lower eyelids and malar regions.2,3 Our case documents extensive involvement of both the upper and lower eyelids. A lupuslike manifestation of GVHD may portend a poor prognosis. In a case series of 5 patients with chronic GVHD presenting as facial lupuslike plaques, 1 patient died from a relapse of leukemia and 3 patients developed sclerodermatous GVHD. The fifth patient was lost to follow-up.2 In another case series, a retrospective analysis discovered that 3 of 7 patients with sclerodermatous GVHD initially presented with hyperpigmented periorbital plaques.4 Resolution of skin findings with topical steroids and oral tacrolimus was reported in a case of GVHD presenting with periorbital lupuslike plaques.3 Although further reports are needed to validate the relationship, a lupuslike presentation of chronic GVHD may be an important harbinger for the development of extensive sclerodermatous GVHD.
A diagnosis of lupuslike GVHD is made based on the correlation of a comprehensive medical history, clinical examination, and histopathologic findings. Although other cases of chronic GVHD resembling dermatomyositis presented with purple periorbital plaques, these patients demonstrated dermatomyositislike systemic symptoms including muscle weakness and fatigue, which were not present in our patient.5,6 Antinuclear antibody (ANA) testing is unlikely to be helpful in the diagnosis of this uncommon presentation, as 65% (41/63) of chronic GVHD patients developed ANA antibodies in one study.7 Also, other patients with lupuslike GVHD who progressed to sclerodermatous GVHD have had both positive and negative ANA serology.2 The histopathology of GVHD and lupus erythematosus can exhibit overlapping features, such as lymphocytic infiltrate with interface changes; however, in lupus erythematosus, mucin usually is present, the infiltrate usually is denser and deeper, and a thickened basement membrane zone may be present. Necrotic keratinocytes also usually are not seen in lupus erythematosus unless the patient’s photosensitivity has led to a sunburn reaction.
After his initial presentation, our patient’s mucosal GVHD flared in the mouth and on the penis, and he was started on prednisone 50 mg once daily and mycophenolate mofetil 1 g twice daily. With this treatment, our patient’s periorbital scaling plaques resolved to residual hyperpigmentation along with remarkable improvement of the mucosal GVHD. He has not manifested any signs of leukemia relapse or sclerodermatous GVHD; however, he remains under close clinical evaluation.
This case highlights an unusual presentation of GVHD with periorbital plaques mimicking hypertrophic lupus erythematous. A greater recognition of this rare entity is essential to further elucidate its prognosis and its relationship with sclerodermatous GVHD.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-5.15e18; quiz 533-534.
- Goiriz R, Peñas PF, Delgado-Jiménez Y, et al. Cutaneous lichenoid graft-versus-host disease mimicking lupus erythematosus. Lupus. 2008;17:591-595.
- Hu SW, Myskowski PL, Papadopoulos EB, et al. Chronic cutaneous graft-versus host disease simulating hypertrophic lupus erythematosus—a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34:E81-E83.
- Chosidow O, Bagot M, Vernant JP, et al. Sclerodermatous chronic graft-versus-host disease. J Am Acad Dermatol. 1992;26:49-55.
- Ollivier I, Wolkenstein P, Gherardi R, et al. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558-559.
- Arin MJ, Scheid C, Hübel K, et al. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31:141-143.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
To the Editor:
A 79-year-old man presented with a scaling eruption in the periorbital area, on the bilateral forearms, and on the chest of 4 weeks’ duration. The patient denied systemic symptoms including lethargy, muscle weakness, and fevers. His medical history was notable for blastic plasmacytoid dendritic cell neoplasm, a form of acute myeloid leukemia, diagnosed 3 years prior to presentation. The patient received an allogeneic hematopoietic stem cell transplant 8 months later. His posttransplant course was complicated by gastrointestinal graft-versus-host disease (GVHD); progressive graft loss requiring a donor lymphocyte infusion after 1 month; and leukemia cutis, which spontaneously resolved after 1 month. The patient was taken off all immunosuppressive therapy 5 months after the transplant and had been doing well for 2 years with only mild mucosal GVHD affecting the oral mucosa and the head of the penis.
Physical examination at the current presentation revealed linear, atrophic, scaling, purplish plaques with adherent white scale on the upper and lower eyelids (Figure 1). The patient also had scattered purple scaling patches on the bilateral forearms and chest. Laboratory tests including complete blood cell count, comprehensive metabolic panel, and lactate dehydrogenase demonstrated no gross abnormalities. Two shave biopsies of the right lower eyelid (Figure 2) and left arm (Figure 3) were performed for histologic examination and revealed basket weave hyperkeratosis, irregular acanthosis, sawtooth rete ridges, and scattered dyskeratotic cells. Vacuolar changes and smudging of the basement membrane zone along with a bandlike lymphocytic infiltrate in the upper dermis also were noted in both biopsies. A diagnosis of lupuslike grade 1 GVHD was made.



Graft-versus-host disease remains a notable cause of morbidity and mortality in allogenic hematopoietic stem cell transplant patients.1 Skin manifestations represent the most common manifestation of GVHD and have been reclassified as acute or chronic disease based on clinical and histologic findings rather than time of onset. Although acute GVHD classically presents as diffuse morbilliform papules and macules, chronic GVHD has a large range of clinical presentations most commonly mimicking the skin findings of lichen planus, morphea, scleroderma, or lichen sclerosus.1
Lupuslike GVHD is a rarely reported manifestation of chronic GVHD that predominantly affects the lower eyelids and malar regions.2,3 Our case documents extensive involvement of both the upper and lower eyelids. A lupuslike manifestation of GVHD may portend a poor prognosis. In a case series of 5 patients with chronic GVHD presenting as facial lupuslike plaques, 1 patient died from a relapse of leukemia and 3 patients developed sclerodermatous GVHD. The fifth patient was lost to follow-up.2 In another case series, a retrospective analysis discovered that 3 of 7 patients with sclerodermatous GVHD initially presented with hyperpigmented periorbital plaques.4 Resolution of skin findings with topical steroids and oral tacrolimus was reported in a case of GVHD presenting with periorbital lupuslike plaques.3 Although further reports are needed to validate the relationship, a lupuslike presentation of chronic GVHD may be an important harbinger for the development of extensive sclerodermatous GVHD.
A diagnosis of lupuslike GVHD is made based on the correlation of a comprehensive medical history, clinical examination, and histopathologic findings. Although other cases of chronic GVHD resembling dermatomyositis presented with purple periorbital plaques, these patients demonstrated dermatomyositislike systemic symptoms including muscle weakness and fatigue, which were not present in our patient.5,6 Antinuclear antibody (ANA) testing is unlikely to be helpful in the diagnosis of this uncommon presentation, as 65% (41/63) of chronic GVHD patients developed ANA antibodies in one study.7 Also, other patients with lupuslike GVHD who progressed to sclerodermatous GVHD have had both positive and negative ANA serology.2 The histopathology of GVHD and lupus erythematosus can exhibit overlapping features, such as lymphocytic infiltrate with interface changes; however, in lupus erythematosus, mucin usually is present, the infiltrate usually is denser and deeper, and a thickened basement membrane zone may be present. Necrotic keratinocytes also usually are not seen in lupus erythematosus unless the patient’s photosensitivity has led to a sunburn reaction.
After his initial presentation, our patient’s mucosal GVHD flared in the mouth and on the penis, and he was started on prednisone 50 mg once daily and mycophenolate mofetil 1 g twice daily. With this treatment, our patient’s periorbital scaling plaques resolved to residual hyperpigmentation along with remarkable improvement of the mucosal GVHD. He has not manifested any signs of leukemia relapse or sclerodermatous GVHD; however, he remains under close clinical evaluation.
This case highlights an unusual presentation of GVHD with periorbital plaques mimicking hypertrophic lupus erythematous. A greater recognition of this rare entity is essential to further elucidate its prognosis and its relationship with sclerodermatous GVHD.
To the Editor:
A 79-year-old man presented with a scaling eruption in the periorbital area, on the bilateral forearms, and on the chest of 4 weeks’ duration. The patient denied systemic symptoms including lethargy, muscle weakness, and fevers. His medical history was notable for blastic plasmacytoid dendritic cell neoplasm, a form of acute myeloid leukemia, diagnosed 3 years prior to presentation. The patient received an allogeneic hematopoietic stem cell transplant 8 months later. His posttransplant course was complicated by gastrointestinal graft-versus-host disease (GVHD); progressive graft loss requiring a donor lymphocyte infusion after 1 month; and leukemia cutis, which spontaneously resolved after 1 month. The patient was taken off all immunosuppressive therapy 5 months after the transplant and had been doing well for 2 years with only mild mucosal GVHD affecting the oral mucosa and the head of the penis.
Physical examination at the current presentation revealed linear, atrophic, scaling, purplish plaques with adherent white scale on the upper and lower eyelids (Figure 1). The patient also had scattered purple scaling patches on the bilateral forearms and chest. Laboratory tests including complete blood cell count, comprehensive metabolic panel, and lactate dehydrogenase demonstrated no gross abnormalities. Two shave biopsies of the right lower eyelid (Figure 2) and left arm (Figure 3) were performed for histologic examination and revealed basket weave hyperkeratosis, irregular acanthosis, sawtooth rete ridges, and scattered dyskeratotic cells. Vacuolar changes and smudging of the basement membrane zone along with a bandlike lymphocytic infiltrate in the upper dermis also were noted in both biopsies. A diagnosis of lupuslike grade 1 GVHD was made.



Graft-versus-host disease remains a notable cause of morbidity and mortality in allogenic hematopoietic stem cell transplant patients.1 Skin manifestations represent the most common manifestation of GVHD and have been reclassified as acute or chronic disease based on clinical and histologic findings rather than time of onset. Although acute GVHD classically presents as diffuse morbilliform papules and macules, chronic GVHD has a large range of clinical presentations most commonly mimicking the skin findings of lichen planus, morphea, scleroderma, or lichen sclerosus.1
Lupuslike GVHD is a rarely reported manifestation of chronic GVHD that predominantly affects the lower eyelids and malar regions.2,3 Our case documents extensive involvement of both the upper and lower eyelids. A lupuslike manifestation of GVHD may portend a poor prognosis. In a case series of 5 patients with chronic GVHD presenting as facial lupuslike plaques, 1 patient died from a relapse of leukemia and 3 patients developed sclerodermatous GVHD. The fifth patient was lost to follow-up.2 In another case series, a retrospective analysis discovered that 3 of 7 patients with sclerodermatous GVHD initially presented with hyperpigmented periorbital plaques.4 Resolution of skin findings with topical steroids and oral tacrolimus was reported in a case of GVHD presenting with periorbital lupuslike plaques.3 Although further reports are needed to validate the relationship, a lupuslike presentation of chronic GVHD may be an important harbinger for the development of extensive sclerodermatous GVHD.
A diagnosis of lupuslike GVHD is made based on the correlation of a comprehensive medical history, clinical examination, and histopathologic findings. Although other cases of chronic GVHD resembling dermatomyositis presented with purple periorbital plaques, these patients demonstrated dermatomyositislike systemic symptoms including muscle weakness and fatigue, which were not present in our patient.5,6 Antinuclear antibody (ANA) testing is unlikely to be helpful in the diagnosis of this uncommon presentation, as 65% (41/63) of chronic GVHD patients developed ANA antibodies in one study.7 Also, other patients with lupuslike GVHD who progressed to sclerodermatous GVHD have had both positive and negative ANA serology.2 The histopathology of GVHD and lupus erythematosus can exhibit overlapping features, such as lymphocytic infiltrate with interface changes; however, in lupus erythematosus, mucin usually is present, the infiltrate usually is denser and deeper, and a thickened basement membrane zone may be present. Necrotic keratinocytes also usually are not seen in lupus erythematosus unless the patient’s photosensitivity has led to a sunburn reaction.
After his initial presentation, our patient’s mucosal GVHD flared in the mouth and on the penis, and he was started on prednisone 50 mg once daily and mycophenolate mofetil 1 g twice daily. With this treatment, our patient’s periorbital scaling plaques resolved to residual hyperpigmentation along with remarkable improvement of the mucosal GVHD. He has not manifested any signs of leukemia relapse or sclerodermatous GVHD; however, he remains under close clinical evaluation.
This case highlights an unusual presentation of GVHD with periorbital plaques mimicking hypertrophic lupus erythematous. A greater recognition of this rare entity is essential to further elucidate its prognosis and its relationship with sclerodermatous GVHD.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-5.15e18; quiz 533-534.
- Goiriz R, Peñas PF, Delgado-Jiménez Y, et al. Cutaneous lichenoid graft-versus-host disease mimicking lupus erythematosus. Lupus. 2008;17:591-595.
- Hu SW, Myskowski PL, Papadopoulos EB, et al. Chronic cutaneous graft-versus host disease simulating hypertrophic lupus erythematosus—a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34:E81-E83.
- Chosidow O, Bagot M, Vernant JP, et al. Sclerodermatous chronic graft-versus-host disease. J Am Acad Dermatol. 1992;26:49-55.
- Ollivier I, Wolkenstein P, Gherardi R, et al. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558-559.
- Arin MJ, Scheid C, Hübel K, et al. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31:141-143.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
- Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I. pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66:515.e1-5.15e18; quiz 533-534.
- Goiriz R, Peñas PF, Delgado-Jiménez Y, et al. Cutaneous lichenoid graft-versus-host disease mimicking lupus erythematosus. Lupus. 2008;17:591-595.
- Hu SW, Myskowski PL, Papadopoulos EB, et al. Chronic cutaneous graft-versus host disease simulating hypertrophic lupus erythematosus—a case report of a new morphologic variant of graft-versus-host disease. Am J Dermatopathol. 2012;34:E81-E83.
- Chosidow O, Bagot M, Vernant JP, et al. Sclerodermatous chronic graft-versus-host disease. J Am Acad Dermatol. 1992;26:49-55.
- Ollivier I, Wolkenstein P, Gherardi R, et al. Dermatomyositis-like graft-versus-host disease. Br J Dermatol. 1998;138:558-559.
- Arin MJ, Scheid C, Hübel K, et al. Chronic graft-versus-host disease with skin signs suggestive of dermatomyositis. Clin Exp Dermatol. 2006;31:141-143.
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396.
Cold snare polypectomy works for large SSPs
Piecemeal cold snare polypectomy was effective and safe for sessile, serrated colon polyps larger than 10 mm in a series from the University of Sydney.
Endoscopic mucosal resection (EMR) is the usual choice for lesions that size, but it comes with the risks of electrocautery, including delayed bleeding in perhaps 10% of patients. The Sydney investigators took a gamble to see if cold snare polypectomy, a technique usually reserved for smaller sessile, serrated polyps (SSPs), worked as well as EMR for larger ones, but without the risks. That seemed to be the case in their pilot study; . The median lesion size was 15 mm, and ranged from 10 to 35 mm. The procedure took a median of 4.5 minutes, much quicker than EMR, and didn’t require submucosal lifting injections. There were no perforations, deep injuries to the colon wall, or intraprocedural bleeding. There were no significant adverse events at 2 weeks, including no delayed bleeding or postpolypectomy syndrome.
Most importantly, there was no evidence of recurrence in the 15 lesions that had surveillance colonoscopy by press time at a median of 6 months. “We suggest, cautiously, that this is related to the wide margin of [normal] tissue [2-3 mm] removed during the initial procedures and the meticulous examination of the defect and margin for residual tissue,” said investigators led by David Tate, MD, of the University of Sydney.
“We have demonstrated the safety and feasibility of pCSP in a tertiary referral cohort of patients referred for the removal of large SSPs. There is potential for pCSP to become the standard of care for nondysplastic SSPs. This could reduce the burden of removing SSPs on patients and health care systems, particularly by avoidance of clinically significant postendoscopic bleeding,” the investigators wrote.
“Because SSPs commonly lack high-grade histology, have a long dwell time prior to developing dysplasia, and recur less frequently than conventional adenomas, they represent comparatively indolent disease and are excellent targets for piecemeal mucosal resection,” the researchers said.
Resection was performed with a stiff thin-wire snare (TeleMed 10-mm hexagonal, TeleMed Systems). “A thin-wire snare is paramount, both to aid tissue capture and to create a crisp resection margin that can be examined for residual serrated tissue. Each progressive resection utilizes this margin to ensure snare purchase and avoid tissue islands,” the researchers said.
It took a median of three cuts to remove an SSP; complete resection was achieved in all cases.
The team used high-definition endoscopic imaging to assess the lesion and margins before the procedure, and again to assess the defect margin to ensure the absence of residual serrated tissue. “We did not use submucosal injection or a chromic dye. While we acknowledge their utility for delineation of serrated tissue, we found that high-definition imaging was sufficient for this purpose and for detecting residual serrated tissue at the resection margin,” they said.
Patients were a mean age of 69 years old; almost 80% were women. About two-thirds of the lesions were proximal to the transverse colon.
The work was supported by the Cancer Institute New South Wales. The investigators had no conflicts of interest.
[email protected]
SOURCE: Tate DJ, et al. Endoscopy. 2017 Nov 23. doi: 10.1055/s-0043-121219.
Piecemeal cold snare polypectomy was effective and safe for sessile, serrated colon polyps larger than 10 mm in a series from the University of Sydney.
Endoscopic mucosal resection (EMR) is the usual choice for lesions that size, but it comes with the risks of electrocautery, including delayed bleeding in perhaps 10% of patients. The Sydney investigators took a gamble to see if cold snare polypectomy, a technique usually reserved for smaller sessile, serrated polyps (SSPs), worked as well as EMR for larger ones, but without the risks. That seemed to be the case in their pilot study; . The median lesion size was 15 mm, and ranged from 10 to 35 mm. The procedure took a median of 4.5 minutes, much quicker than EMR, and didn’t require submucosal lifting injections. There were no perforations, deep injuries to the colon wall, or intraprocedural bleeding. There were no significant adverse events at 2 weeks, including no delayed bleeding or postpolypectomy syndrome.
Most importantly, there was no evidence of recurrence in the 15 lesions that had surveillance colonoscopy by press time at a median of 6 months. “We suggest, cautiously, that this is related to the wide margin of [normal] tissue [2-3 mm] removed during the initial procedures and the meticulous examination of the defect and margin for residual tissue,” said investigators led by David Tate, MD, of the University of Sydney.
“We have demonstrated the safety and feasibility of pCSP in a tertiary referral cohort of patients referred for the removal of large SSPs. There is potential for pCSP to become the standard of care for nondysplastic SSPs. This could reduce the burden of removing SSPs on patients and health care systems, particularly by avoidance of clinically significant postendoscopic bleeding,” the investigators wrote.
“Because SSPs commonly lack high-grade histology, have a long dwell time prior to developing dysplasia, and recur less frequently than conventional adenomas, they represent comparatively indolent disease and are excellent targets for piecemeal mucosal resection,” the researchers said.
Resection was performed with a stiff thin-wire snare (TeleMed 10-mm hexagonal, TeleMed Systems). “A thin-wire snare is paramount, both to aid tissue capture and to create a crisp resection margin that can be examined for residual serrated tissue. Each progressive resection utilizes this margin to ensure snare purchase and avoid tissue islands,” the researchers said.
It took a median of three cuts to remove an SSP; complete resection was achieved in all cases.
The team used high-definition endoscopic imaging to assess the lesion and margins before the procedure, and again to assess the defect margin to ensure the absence of residual serrated tissue. “We did not use submucosal injection or a chromic dye. While we acknowledge their utility for delineation of serrated tissue, we found that high-definition imaging was sufficient for this purpose and for detecting residual serrated tissue at the resection margin,” they said.
Patients were a mean age of 69 years old; almost 80% were women. About two-thirds of the lesions were proximal to the transverse colon.
The work was supported by the Cancer Institute New South Wales. The investigators had no conflicts of interest.
[email protected]
SOURCE: Tate DJ, et al. Endoscopy. 2017 Nov 23. doi: 10.1055/s-0043-121219.
Piecemeal cold snare polypectomy was effective and safe for sessile, serrated colon polyps larger than 10 mm in a series from the University of Sydney.
Endoscopic mucosal resection (EMR) is the usual choice for lesions that size, but it comes with the risks of electrocautery, including delayed bleeding in perhaps 10% of patients. The Sydney investigators took a gamble to see if cold snare polypectomy, a technique usually reserved for smaller sessile, serrated polyps (SSPs), worked as well as EMR for larger ones, but without the risks. That seemed to be the case in their pilot study; . The median lesion size was 15 mm, and ranged from 10 to 35 mm. The procedure took a median of 4.5 minutes, much quicker than EMR, and didn’t require submucosal lifting injections. There were no perforations, deep injuries to the colon wall, or intraprocedural bleeding. There were no significant adverse events at 2 weeks, including no delayed bleeding or postpolypectomy syndrome.
Most importantly, there was no evidence of recurrence in the 15 lesions that had surveillance colonoscopy by press time at a median of 6 months. “We suggest, cautiously, that this is related to the wide margin of [normal] tissue [2-3 mm] removed during the initial procedures and the meticulous examination of the defect and margin for residual tissue,” said investigators led by David Tate, MD, of the University of Sydney.
“We have demonstrated the safety and feasibility of pCSP in a tertiary referral cohort of patients referred for the removal of large SSPs. There is potential for pCSP to become the standard of care for nondysplastic SSPs. This could reduce the burden of removing SSPs on patients and health care systems, particularly by avoidance of clinically significant postendoscopic bleeding,” the investigators wrote.
“Because SSPs commonly lack high-grade histology, have a long dwell time prior to developing dysplasia, and recur less frequently than conventional adenomas, they represent comparatively indolent disease and are excellent targets for piecemeal mucosal resection,” the researchers said.
Resection was performed with a stiff thin-wire snare (TeleMed 10-mm hexagonal, TeleMed Systems). “A thin-wire snare is paramount, both to aid tissue capture and to create a crisp resection margin that can be examined for residual serrated tissue. Each progressive resection utilizes this margin to ensure snare purchase and avoid tissue islands,” the researchers said.
It took a median of three cuts to remove an SSP; complete resection was achieved in all cases.
The team used high-definition endoscopic imaging to assess the lesion and margins before the procedure, and again to assess the defect margin to ensure the absence of residual serrated tissue. “We did not use submucosal injection or a chromic dye. While we acknowledge their utility for delineation of serrated tissue, we found that high-definition imaging was sufficient for this purpose and for detecting residual serrated tissue at the resection margin,” they said.
Patients were a mean age of 69 years old; almost 80% were women. About two-thirds of the lesions were proximal to the transverse colon.
The work was supported by the Cancer Institute New South Wales. The investigators had no conflicts of interest.
[email protected]
SOURCE: Tate DJ, et al. Endoscopy. 2017 Nov 23. doi: 10.1055/s-0043-121219.
FROM ENDOSCOPY
Key clinical point: Piecemeal cold snare polypectomy is effective and safe for sessile, serrated colon polyps larger than 10 mm.
Major finding: There was no delayed bleeding, and no evidence of recurrence, in 15 patients who had surveillance colonoscopies at 6 months.
Study details: A case series of 34 patients.
Disclosures: The work was supported by the Cancer Institute New South Wales, Australia. The investigators had no conflicts of interest.
Source: Tate DJ et al. Endoscopy. 2017 Nov 23. doi: 10.1055/s-0043-121219
Complete Remission of Metastatic Merkel Cell Carcinoma in a Patient With Severe Psoriasis
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
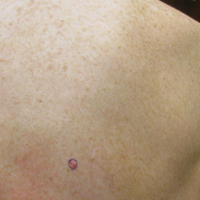
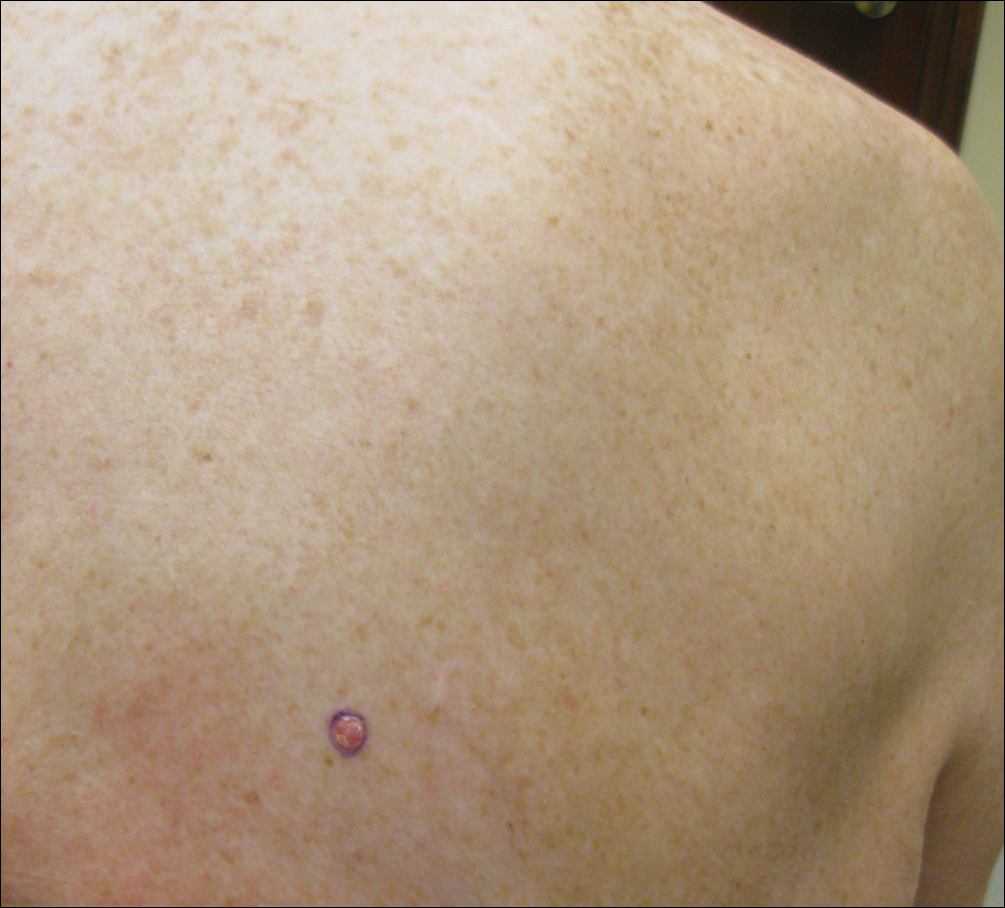
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.
A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.
The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
To the Editor:
A 69-year-old white man presented with a skin lesion on the back of 1 to 2 weeks’ duration. The patient stated he was unaware of it, but his wife had recently noticed the new spot. He denied any bleeding, pain, pruritus, or other associated symptoms with the lesion. He also denied any prior treatment to the area. The patient’s medical history was remarkable for severe psoriasis involving more than 80% body surface area, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, coronary artery disease, squamous cell carcinoma, and actinic keratoses. He had been on multiple treatment regimens over the last 20 years for control of psoriasis including topical corticosteroids, psoralen plus UVA and UVB phototherapy, gold injections, acitretin, prednisone, efalizumab, ustekinumab, and alefacept upon evaluation of this new skin lesion. Utilization of immunosuppressive agents also provided an additional benefit of controlling the patient’s inflammatory arthritic disease.
On physical examination a 0.6×0.7-cm, pink to erythematous, pearly papule with superficial telangiectases was noted on the right side of the dorsal thorax (Figure 1). Multiple well-demarcated erythematous plaques with silvery scale and areas of secondary excoriation were noted on the trunk and both legs consistent with the patient’s history of psoriasis.

A shave biopsy was performed on the skin lesion on the right side of the dorsal thorax with a suspected clinical diagnosis of basal cell carcinoma. Two weeks later the patient returned for a discussion of the pathology report, which revealed nodules of basaloid cells with tightly packed vesicular nuclei and scant cytoplasm in sheets within the superficial dermis, as well as areas of nuclear molding, numerous mitotic figures, and areas of focal necrosis (Figure 2). In addition, immunostaining was positive for cytokeratin (CK) 20 antibodies with a characteristic paranuclear dot uptake of the antibody. These findings were consistent with a diagnosis of Merkel cell carcinoma (MCC). At that time, alefacept was discontinued and he was referred to a tertiary referral center for further evaluation and treatment.

The patient subsequently underwent wide excision with 1-cm margins of the MCC, with intraoperative lymphatic mapping/sentinel lymph node biopsy (SLNB) of the right axillary nodal basin 1 month later, which he tolerated well without any associated complications. Further histopathologic examination revealed the deep, medial, and lateral surgical margins to be negative of residual neoplasm. However, one sentinel lymph node indicated positivity for micrometastatic MCC, consistent with stage IIIA disease progression.
He underwent a second procedure the following month for complete right axillary lymph node dissection. Histopathologic examination of the right axillary contents included 28 lymph nodes, which were negative for carcinoma. He continued to do well without any signs of clinical recurrence or distant metastasis at subsequent follow-up visits.
Approximately 2.5 years after the second procedure, the patient began to develop right upper quadrant abdominal pain of an unclear etiology. Computed tomography of the abdomen and pelvis was performed, revealing areas of calcification and findings consistent with malignant lymphadenopathy. Multiple hepatic lesions also were noted including a 9-cm lesion in the posterior right hepatic lobe. Computed tomography–guided biopsy of the liver lesion was performed and the findings were consistent with metastatic MCC, indicating progression to stage IV disease.
The patient was subsequently started on combination chemotherapeutic treatment with carboplatin and VP-16, with a planned treatment course of 4 to 6 cycles. He was able to complete a total of 6 cycles over a 4-month period, tolerating the treatment regimen fairly well. Follow-up positron emission tomography–computed tomography was within normal limits with no evidence of any hypermetabolic activity noted, indicating a complete radiographic remission of MCC. He was seen approximately 1 month after completion of treatment for clinical follow-up and monthly thereafter.
While on chemotherapy, the patient experienced a notable improvement in the psoriasis and psoriatic joint disease. Upon completion of chemotherapy, he was restarted on the same treatment plan that was utilized prior to surgery including topical corticosteroids, calcitriol, intramuscular steroid injections, and UVB phototherapy, which provided substantial control of psoriasis and arthritic joint disease. The patient later died, likely due to his multiple comorbidities.
Merkel cells are slow-responding mechanoreceptors located within the basal layer of the epidermis and are the source of a rare aggressive cutaneous malignancy.1 Merkel cell carcinoma was first noted in 1972 and termed trabecular carcinoma of the skin, and it accounts for less than 1% of all nonmelanoma skin cancer.2,3 This primary neuroendocrine carcinoma has remarkable metastatic potential (34%–75%) and can invade regional lymph nodes, as well as distant metastasis most commonly to the liver, lungs, bones, and brain.2 Approximately 25% of patients present with palpable lymphadenopathy and 5% with distant metastasis at the time of diagnosis. This frequency of metastasis at diagnosis as well as the recurrence after treatment contributes to the poor prognosis of MCC. Local recurrence rates have been reported at 25% with lymph node involvement in 52% and metastasis in 34%, with most recurrences occurring within 2 years of diagnosis. Patient mortality is dependent on the aggressiveness of the tumor, with 5-year survival rates of 83.3% without lymph node involvement, 58.3% with lymph node involvement, and 31.3% in those with metastatic disease.4
The tumor classically presents as a red to violaceous, painless nodule with a smooth shiny surface most often on the head and neck region.4-6 Approximately 50% of MCC cases present in the head and neck region, 32% to 38% on the extremities, and 12% to 14% on the trunk.1 This nonspecific presentation may lead to diagnostic uncertainty and a consequent delay in treatment. Definitive diagnosis of MCC is achieved with a skin biopsy and allows for distinction from other clinically similar–appearing neoplasms. Merkel cell carcinoma presents histologically as small round basophilic cells penetrating through the dermis in 3 histologic patterns: the trabecular, intermediate (80% of cases), and small cell type.5 It may be differentiated immunohistochemically from other neoplasms, as it displays CK20 positivity (showing paranuclear dotlike depositions in the cytoplasm or cell membrane) and is negative for CK7. Chromagranin and synaptophysin positivity also may provide further histologic confirmation. In addition, absence of peripheral palisading, retraction artifact, and a fibromyxoid stroma allow for distinction from cutaneous basal cell carcinoma, which may display these features histologically. Other immunohistochemical markers that may be of value include thyroid transcription factor 1, which is typically positive in cutaneous metastasis of neuroendocrine carcinoma of the lung; S-100 and human melanoma black 45, which are positive in melanoma; and leukocyte common antigen (CD45), which can be positive in lymphoma. These stains are classically negative in MCC.3
Merkel cell carcinoma is commonly associated with the presence of Merkel cell polyomavirus (MCPyV) in tumor specimens, with a prevalence of 70% to 80% in all cases. Merkel cell polyomavirus is a class 2A carcinogen (ie, a probable carcinogen to humans) and is classified among a group of viruses that encode T antigens (ie, an antigen coded by a viral genome associated with transformation of infected cells by tumor viruses), which can lead to initiation of tumorigenesis through interference with cellular tumor suppressing proteins such as p53.5 In addition, several risk factors have been associated with the development of MCC including immunosuppression, older age (>50 years), and UV-exposed fair skin.7 One explanation for this phenomenon is the increase in MCPyV small T antigen transcripts induced by UV irradiation.5 In addition, as with other cancers induced by viruses, host immunity can impede tumor progression and development. Therefore, impairment of normal immune function likely creates a higher risk for MCC development and potential for a worse prognosis.3Although the exact incidence of MCC in immunosuppressed patients appears unclear, chronic immunosuppressive therapy may play a notable role in the pathogenesis of the tumor.3
Although each of these factors was observed in our patient, it also was possible that his associated comorbidities further contributed to disease presentation. In particular, rheumatoid arthritis has been shown to carry an increased risk for the development of MCC.8 In addition, inflammatory monocytes infected with MCPyV, as evidenced in a patient with a history of chronic psoriasis prior to diagnosis of MCC, also may contribute to the pathogenesis of MCC by traveling to inflammatory skin lesions, such as those seen in psoriasis, releasing MCPyV locally and infecting Merkel cells.9 Although MCPyV testing was never performed in our patient, it certainly would be prudent as well as further studies determining the correlation of MCC to these disease processes.
Although regression is rare, multiple cases have documented spontaneous regression of MCC after biopsy of these lesions.4,6,10 The exact mechanism is unclear, but apoptosis induced by T-cell immunity is suspected to play a role. Programmed cell death 1 protein (PD-1)–positive cells play a role. The PD-1 receptor is an inhibitory receptor expressed by T cells and in approximately half of tumor-infiltrating cells in MCC. It was found that in a regressed case of MCC there was a notably lower percentage of PD-1 positivity compared to cases with no apparent regression, suggesting that PD-1–positive cells suppress tumor immunity to MCC and that significant reduction in these cells may induce clinical regression.10 Additional investigation would be beneficial to examine the relationship of this phenomenon to tumor regression.
Initial evaluation of these patients should include a meticulous clinical examination with an emphasis on detection of cutaneous, lymph node, and distant metastasis. Due to the risk of metastatic potential, regional lymph node ultrasonography and computed tomography of the chest, abdomen, and pelvis typically are recommended at baseline. Other imaging modalities may be warranted based on clinical findings.3 Treatment modalities include various approaches, with surgical excision of the primary tumor with more than 1-cm margin to the fascial plane being the primary modality for uncomplicated cases.1,3,7 In addition, SLNB also should be performed at the time of the procedure. In the case of a positive SLNB or suspected regional lymph node involvement upon initial examination, radical regional lymph node dissection also is recommended.3 Although some authorities advocate postsurgical radiation therapy to minimize the risk of local recurrence, there does not appear to be a clear benefit in survival rate.3,5 However, radiation treatment as monotherapy has been advocated in certain instances, particularly in cases of unresectable tumors or patients who are poor surgical candidates.5,7 Cases of distant metastasis (stage IV disease) may include management with surgery, radiation, and/or chemotherapy. Although none of these modalities have consistently shown to improve survival, there appears to be up to a 60% response with chemotherapy in these patients.3
Because MCC tends to affect an older population, often with other notable comorbidities, important considerations involving a treatment plan include the cost, side effects, and convenience for patients. The combination of carboplatin and VP-16 (etoposide) was utilized and tolerated well in our patient, and it has been successful in achieving complete radiologic and clinical remission of his metastatic disease. This combination appears to prolong survival in patients with distant metastasis, as compared to those patients not receiving chemotherapy.1 Our patient has since died, but in these high-risk patients, close clinical monitoring is essential to help optimize their prognosis.
Merkel cell carcinoma is a rare aggressive cutaneous neoplasm that most commonly affects the elderly, immunosuppressed, and those with chronic UV sun damage. An association between the oncogenesis of MCC and infection with MCPyV has been documented, but other underlying diseases also may play a role in this process including rheumatoid arthritis and psoriasis. Although these risk factors were associated with our patient, his history of chronic immunosuppressive therapy for treatment of his psoriasis and inflammatory joint disease likely played a role in the pathogenesis of the tumor and should be an important point of discussion with any patient requiring this type of long-term management for disease control. Our unique clinical case highlights a patient with substantial comorbidities who developed metastatic MCC and achieved complete clinical and radiologic remission after treatment with surgery and chemotherapy.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
- Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment [published online March 11, 2015]. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
- Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis [published online December 1, 2014]. Balkan Med J. 2014;31:356-359.
- Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives [published online Dec 31, 2014]. Semin Oncol. 2015;42:347-358.
- Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging [published online January 30, 2015]. Skeletal Radiol. 2015;44:777-786.
- Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy [published online May 14, 2015]. Dermatol Ther. 2015;28:282-286.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
- Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults [published online June 15, 2010]. Br J Cancer. 2010;103:112-114.
- Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus [published online December 17, 2009]. J Invest Dermatol. 2009;130:1146-1151.
- Fujimoto N, Nakanishi G, Kabuto M, et al. Merkel cell carcinoma showing regression after biopsy: evaluation of programmed cell death 1-positive cells [published online February 24, 2015]. J Dermatol. 2015;42:496-499.
Practice Points
- Merkel cell carcinoma (MCC) has remarkable metastatic potential.
- Initial evaluation of patients with MCC should include clinical examination to detect cutaneous, lymph node, and distant metastasis.
- Risk factors associated with the development of MCC include immunosuppression, older age, and UV-exposed fair skin.
Preventive health: Getting rid of the middleman (uh-oh, that’s us!)
As physicians, we find that preventive health is, frankly, really difficult. It requires thinking about a changing list of recommendations unprompted by the symptoms for which patients present. Compounding that challenge is that, in doing preventive health well, we need to have personalized discussions with our patients and this requires they come into the office, which doesn’t always happen on a regular basis. Furthermore, when patients do come in, they usually are presenting for an acute care visit, so there is little time set aside to discuss preventive health.
For all these reasons and many others, the data suggest that we are not particularly good at performing preventive health maintenance. We are much better at figuring out diagnostic dilemmas and choosing among competing medications or procedures to most effectively address acute and chronic medical problems. Let’s examine the data to see if there is a shred of truth in what we are saying; then let’s look at a potential solution to the dilemma of preventive health that we all believe in and that we carry out less frequently than any of us would like.
First, let’s look at recent data on cancer screening reported by the CDC1:
- Mammography: 72% of women aged 50–74 years reported having had a mammogram within the past 2 years.
- Pap test: 83% of women reported being up to date with cervical cancer screening.
- Colorectal cancer screening: 62% of men and women reported colorectal cancer screening test use consistent with USPSTF recommendations.
Of note, colorectal cancer screening has improved dramatically over he last 15 years, while screening for breast and cervical cancer has largely plateaued.1
Our success with cancer screening – or lack thereof depending upon one’s perspective – looks quite good next to national vaccination rates for adults. The immunization rate for commonly recommended vaccines are as follows2:
- The Tdap vaccination rate is 20%.
- The tetanus-diphtheria vaccination rate is 62%.
- The herpes zoster vaccination rate is 28%.
- The influenza vaccination rate is 43%.
- The pneumococcal vaccination rate among high-risk persons aged 19-64 years is 20% and among adults aged greater than or equal to 65 years is 61%.
Of adults who had health insurance and at least 10 physician contacts within the past year, 23.8%-88.8% reported not having received vaccinations that were recommended.
In the business literature there is a great deal of disagreement about the value of the “middleman.” The term middleman describes someone who brings the product from the producer, or factory, to the consumer. On the one hand, if the factory can sell the product directly to the consumer, the consumer can save money and the factory can make more money. On the other hand, if the middleman can help the consumer make a better choice among the variety of products available, then the middleman provides value and the consumer benefits.3
Traditionally, clinicians have served the role of the middleman for preventive health activities, knowing what to recommend to patients and informing them of the correct preventive health choices that fit their needs. The problem with this concept is that preventive health recommendations are largely demographically based, are tied to population-based risk assessment, and usually require very little individual judgment.
We as physicians are good at – and I believe truly enjoy – exercising judgment. We love thinking things through and helping the person in front of us. We are not as good at remembering unprompted information in the middle of busy visits that are often made for unrelated reasons. Most of the people who have not had a colonoscopy or pneumococcal vaccine have not decided against the procedure after a detailed discussion with their physician. On the contrary, the service was never recommended, or it was recommended, but the patient did not follow up to have the procedure performed.
Let’s now imagine another approach. You’re a patient and once a year you click on an email that shows up in your inbox from your doctor with the words “Preventive Health” in the subject line. The EHR – based on your gender, age, and a query of what has been documented in your chart – has determined the preventive health activities that are recommended for you. You can choose to pursue, opt out, or get more information for each of the recommended preventive services as you read through them.
If you choose to have more information, it is provided in a structured format that allows you to drill down to the level of detail that you desire. In all probability, you will find a greater level of detail and accuracy of information about each preventive service than could possibly be provided during a routine office visit. Specifics about the risks and benefits of the procedure will also be more extensive, as it is unlikely your care providers are able to keep all of the details and risk ratios in their heads. If desired, you as a patient can take your time to read and digest the information, sleep on it, and come back to it to make an informed decision. This is not something you can do during a routine office visit.
If you choose to opt out of the procedure, just click the “declined” box. Otherwise, when you’ve made all of your decisions and indicate that you’re done, the necessary prescriptions for blood work and x-rays, as well as referrals to the appropriate specialists, will print out. An entry will also be made in the electronic record showing you’ve been provided preventive health recommendations that are appropriate for your age and sex and made your preferred choices. At any point, if you feel you’d like further discussion with your physician, you can make an appointment electronically through the interface.
The hurdles for implementing such a system are real, but they are solvable, and the development of such an approach is inevitable, enviable, and will ultimately be good for both patients and their providers. Patients will get more predictable and complete recommendations for preventive care and providers will have more time to do what we enjoy and are most skilled at – talking with patients to clarify diagnoses, decide upon treatment, and clarify questions that come up about preventive health recommendations.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. White A et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201-6.
2. Williams WW et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016 Feb 5;65(1):1-36.
3. Conerly B. Don’t eliminate the middleman – He’s much too valuable. Forbes. Oct 28, 2015.
As physicians, we find that preventive health is, frankly, really difficult. It requires thinking about a changing list of recommendations unprompted by the symptoms for which patients present. Compounding that challenge is that, in doing preventive health well, we need to have personalized discussions with our patients and this requires they come into the office, which doesn’t always happen on a regular basis. Furthermore, when patients do come in, they usually are presenting for an acute care visit, so there is little time set aside to discuss preventive health.
For all these reasons and many others, the data suggest that we are not particularly good at performing preventive health maintenance. We are much better at figuring out diagnostic dilemmas and choosing among competing medications or procedures to most effectively address acute and chronic medical problems. Let’s examine the data to see if there is a shred of truth in what we are saying; then let’s look at a potential solution to the dilemma of preventive health that we all believe in and that we carry out less frequently than any of us would like.
First, let’s look at recent data on cancer screening reported by the CDC1:
- Mammography: 72% of women aged 50–74 years reported having had a mammogram within the past 2 years.
- Pap test: 83% of women reported being up to date with cervical cancer screening.
- Colorectal cancer screening: 62% of men and women reported colorectal cancer screening test use consistent with USPSTF recommendations.
Of note, colorectal cancer screening has improved dramatically over he last 15 years, while screening for breast and cervical cancer has largely plateaued.1
Our success with cancer screening – or lack thereof depending upon one’s perspective – looks quite good next to national vaccination rates for adults. The immunization rate for commonly recommended vaccines are as follows2:
- The Tdap vaccination rate is 20%.
- The tetanus-diphtheria vaccination rate is 62%.
- The herpes zoster vaccination rate is 28%.
- The influenza vaccination rate is 43%.
- The pneumococcal vaccination rate among high-risk persons aged 19-64 years is 20% and among adults aged greater than or equal to 65 years is 61%.
Of adults who had health insurance and at least 10 physician contacts within the past year, 23.8%-88.8% reported not having received vaccinations that were recommended.
In the business literature there is a great deal of disagreement about the value of the “middleman.” The term middleman describes someone who brings the product from the producer, or factory, to the consumer. On the one hand, if the factory can sell the product directly to the consumer, the consumer can save money and the factory can make more money. On the other hand, if the middleman can help the consumer make a better choice among the variety of products available, then the middleman provides value and the consumer benefits.3
Traditionally, clinicians have served the role of the middleman for preventive health activities, knowing what to recommend to patients and informing them of the correct preventive health choices that fit their needs. The problem with this concept is that preventive health recommendations are largely demographically based, are tied to population-based risk assessment, and usually require very little individual judgment.
We as physicians are good at – and I believe truly enjoy – exercising judgment. We love thinking things through and helping the person in front of us. We are not as good at remembering unprompted information in the middle of busy visits that are often made for unrelated reasons. Most of the people who have not had a colonoscopy or pneumococcal vaccine have not decided against the procedure after a detailed discussion with their physician. On the contrary, the service was never recommended, or it was recommended, but the patient did not follow up to have the procedure performed.
Let’s now imagine another approach. You’re a patient and once a year you click on an email that shows up in your inbox from your doctor with the words “Preventive Health” in the subject line. The EHR – based on your gender, age, and a query of what has been documented in your chart – has determined the preventive health activities that are recommended for you. You can choose to pursue, opt out, or get more information for each of the recommended preventive services as you read through them.
If you choose to have more information, it is provided in a structured format that allows you to drill down to the level of detail that you desire. In all probability, you will find a greater level of detail and accuracy of information about each preventive service than could possibly be provided during a routine office visit. Specifics about the risks and benefits of the procedure will also be more extensive, as it is unlikely your care providers are able to keep all of the details and risk ratios in their heads. If desired, you as a patient can take your time to read and digest the information, sleep on it, and come back to it to make an informed decision. This is not something you can do during a routine office visit.
If you choose to opt out of the procedure, just click the “declined” box. Otherwise, when you’ve made all of your decisions and indicate that you’re done, the necessary prescriptions for blood work and x-rays, as well as referrals to the appropriate specialists, will print out. An entry will also be made in the electronic record showing you’ve been provided preventive health recommendations that are appropriate for your age and sex and made your preferred choices. At any point, if you feel you’d like further discussion with your physician, you can make an appointment electronically through the interface.
The hurdles for implementing such a system are real, but they are solvable, and the development of such an approach is inevitable, enviable, and will ultimately be good for both patients and their providers. Patients will get more predictable and complete recommendations for preventive care and providers will have more time to do what we enjoy and are most skilled at – talking with patients to clarify diagnoses, decide upon treatment, and clarify questions that come up about preventive health recommendations.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. White A et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201-6.
2. Williams WW et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016 Feb 5;65(1):1-36.
3. Conerly B. Don’t eliminate the middleman – He’s much too valuable. Forbes. Oct 28, 2015.
As physicians, we find that preventive health is, frankly, really difficult. It requires thinking about a changing list of recommendations unprompted by the symptoms for which patients present. Compounding that challenge is that, in doing preventive health well, we need to have personalized discussions with our patients and this requires they come into the office, which doesn’t always happen on a regular basis. Furthermore, when patients do come in, they usually are presenting for an acute care visit, so there is little time set aside to discuss preventive health.
For all these reasons and many others, the data suggest that we are not particularly good at performing preventive health maintenance. We are much better at figuring out diagnostic dilemmas and choosing among competing medications or procedures to most effectively address acute and chronic medical problems. Let’s examine the data to see if there is a shred of truth in what we are saying; then let’s look at a potential solution to the dilemma of preventive health that we all believe in and that we carry out less frequently than any of us would like.
First, let’s look at recent data on cancer screening reported by the CDC1:
- Mammography: 72% of women aged 50–74 years reported having had a mammogram within the past 2 years.
- Pap test: 83% of women reported being up to date with cervical cancer screening.
- Colorectal cancer screening: 62% of men and women reported colorectal cancer screening test use consistent with USPSTF recommendations.
Of note, colorectal cancer screening has improved dramatically over he last 15 years, while screening for breast and cervical cancer has largely plateaued.1
Our success with cancer screening – or lack thereof depending upon one’s perspective – looks quite good next to national vaccination rates for adults. The immunization rate for commonly recommended vaccines are as follows2:
- The Tdap vaccination rate is 20%.
- The tetanus-diphtheria vaccination rate is 62%.
- The herpes zoster vaccination rate is 28%.
- The influenza vaccination rate is 43%.
- The pneumococcal vaccination rate among high-risk persons aged 19-64 years is 20% and among adults aged greater than or equal to 65 years is 61%.
Of adults who had health insurance and at least 10 physician contacts within the past year, 23.8%-88.8% reported not having received vaccinations that were recommended.
In the business literature there is a great deal of disagreement about the value of the “middleman.” The term middleman describes someone who brings the product from the producer, or factory, to the consumer. On the one hand, if the factory can sell the product directly to the consumer, the consumer can save money and the factory can make more money. On the other hand, if the middleman can help the consumer make a better choice among the variety of products available, then the middleman provides value and the consumer benefits.3
Traditionally, clinicians have served the role of the middleman for preventive health activities, knowing what to recommend to patients and informing them of the correct preventive health choices that fit their needs. The problem with this concept is that preventive health recommendations are largely demographically based, are tied to population-based risk assessment, and usually require very little individual judgment.
We as physicians are good at – and I believe truly enjoy – exercising judgment. We love thinking things through and helping the person in front of us. We are not as good at remembering unprompted information in the middle of busy visits that are often made for unrelated reasons. Most of the people who have not had a colonoscopy or pneumococcal vaccine have not decided against the procedure after a detailed discussion with their physician. On the contrary, the service was never recommended, or it was recommended, but the patient did not follow up to have the procedure performed.
Let’s now imagine another approach. You’re a patient and once a year you click on an email that shows up in your inbox from your doctor with the words “Preventive Health” in the subject line. The EHR – based on your gender, age, and a query of what has been documented in your chart – has determined the preventive health activities that are recommended for you. You can choose to pursue, opt out, or get more information for each of the recommended preventive services as you read through them.
If you choose to have more information, it is provided in a structured format that allows you to drill down to the level of detail that you desire. In all probability, you will find a greater level of detail and accuracy of information about each preventive service than could possibly be provided during a routine office visit. Specifics about the risks and benefits of the procedure will also be more extensive, as it is unlikely your care providers are able to keep all of the details and risk ratios in their heads. If desired, you as a patient can take your time to read and digest the information, sleep on it, and come back to it to make an informed decision. This is not something you can do during a routine office visit.
If you choose to opt out of the procedure, just click the “declined” box. Otherwise, when you’ve made all of your decisions and indicate that you’re done, the necessary prescriptions for blood work and x-rays, as well as referrals to the appropriate specialists, will print out. An entry will also be made in the electronic record showing you’ve been provided preventive health recommendations that are appropriate for your age and sex and made your preferred choices. At any point, if you feel you’d like further discussion with your physician, you can make an appointment electronically through the interface.
The hurdles for implementing such a system are real, but they are solvable, and the development of such an approach is inevitable, enviable, and will ultimately be good for both patients and their providers. Patients will get more predictable and complete recommendations for preventive care and providers will have more time to do what we enjoy and are most skilled at – talking with patients to clarify diagnoses, decide upon treatment, and clarify questions that come up about preventive health recommendations.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records.
References
1. White A et al. Cancer screening test use – United States, 2015. MMWR Morb Mortal Wkly Rep. 2017 Mar 3;66(8):201-6.
2. Williams WW et al. Surveillance of vaccination coverage among adult populations – United States, 2014. MMWR Surveill Summ. 2016 Feb 5;65(1):1-36.
3. Conerly B. Don’t eliminate the middleman – He’s much too valuable. Forbes. Oct 28, 2015.
Real-world study makes case for continuous treatment in multiple myeloma
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Each additional month of second-line therapy was associated with a reduced risk of death at 1 year (aOR, 0.78).
Study details: A retrospective study of 628 newly diagnosed multiple myeloma patients between January 2008 and June 2015.
Disclosures: Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
Source: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
FDA approves irritable bowel syndrome treatment
(IBS-C).
Plecanatide had previously been approved to treat adults with chronic idiopathic constipation (CIC).
Plecanatide was approved on the findings of two randomized, double-blind, 12-week, placebo-controlled clinical trials. More than 2,100 adult patients across both trials received either a 3-mg or 6-mg once-daily tablet of plecanatide, or a placebo. The primary endpoints of both studies were greater than 30% reduction in worst abdominal pain and an increase of at least one complete spontaneous bowel movement (CSBM) for at least half of the 12 treatment weeks.
Plecanatide met both of its primary endpoints, with reductions in abdominal pain in both studies, compared with placebo (30.2% vs. 17.8% in study 1, P < .001; 21.5% vs. 14.2% in study 2, P = .009).
Plecanatide is the only prescription, once-daily medication that treats both CIC and IBS-C in adults. The drug should be available in the first quarter of 2018.
AGA offers free patient education materials to help your patients better understand how to live with and manage their IBS. Visit www.gastro.org/ibs.
(IBS-C).
Plecanatide had previously been approved to treat adults with chronic idiopathic constipation (CIC).
Plecanatide was approved on the findings of two randomized, double-blind, 12-week, placebo-controlled clinical trials. More than 2,100 adult patients across both trials received either a 3-mg or 6-mg once-daily tablet of plecanatide, or a placebo. The primary endpoints of both studies were greater than 30% reduction in worst abdominal pain and an increase of at least one complete spontaneous bowel movement (CSBM) for at least half of the 12 treatment weeks.
Plecanatide met both of its primary endpoints, with reductions in abdominal pain in both studies, compared with placebo (30.2% vs. 17.8% in study 1, P < .001; 21.5% vs. 14.2% in study 2, P = .009).
Plecanatide is the only prescription, once-daily medication that treats both CIC and IBS-C in adults. The drug should be available in the first quarter of 2018.
AGA offers free patient education materials to help your patients better understand how to live with and manage their IBS. Visit www.gastro.org/ibs.
(IBS-C).
Plecanatide had previously been approved to treat adults with chronic idiopathic constipation (CIC).
Plecanatide was approved on the findings of two randomized, double-blind, 12-week, placebo-controlled clinical trials. More than 2,100 adult patients across both trials received either a 3-mg or 6-mg once-daily tablet of plecanatide, or a placebo. The primary endpoints of both studies were greater than 30% reduction in worst abdominal pain and an increase of at least one complete spontaneous bowel movement (CSBM) for at least half of the 12 treatment weeks.
Plecanatide met both of its primary endpoints, with reductions in abdominal pain in both studies, compared with placebo (30.2% vs. 17.8% in study 1, P < .001; 21.5% vs. 14.2% in study 2, P = .009).
Plecanatide is the only prescription, once-daily medication that treats both CIC and IBS-C in adults. The drug should be available in the first quarter of 2018.
AGA offers free patient education materials to help your patients better understand how to live with and manage their IBS. Visit www.gastro.org/ibs.
Postsurgical pain: Optimizing relief while minimizing use of opioids
CASE Managing pain associated with prolapse and SUI surgery
A 46-year-old woman (G4P4) described 3 years of worsening symptoms related to recurrent stage-3 palpable uterine prolapse. She had associated symptomatic stress urinary incontinence. She had been treated for uterine prolapse 5 years ago with vaginal hysterectomy, bilateral salpingectomy, and high uterosacral-ligament suspension.
After consultation, the patient elected to undergo laparoscopic sacral colpopexy, a mid-urethral sling, and possible anterior and posterior colporrhaphy. Appropriate discussion about the risks and benefits of mesh was provided preoperatively. The surgical team judged her to be highly motivated; she wanted same-day outpatient surgery so that she could go home and then return to work. She had excellent support at home.
How would you counsel this patient about expected postoperative pain? Which medications would you administer to her preoperatively and perioperatively? Which ones would you prescribe for her to manage pain postoperatively?
Adverse impact of prescription opioids in the United States
Although fewer than 5% of the world’s population live in the United States, nearly 80% of the world’s opioids are written for them.1 In 2012, 259 million prescriptions were written for opioids in the United States—more than enough to give every American adult their own bottle of pills.2 Sadly, drug overdose is now a leading cause of accidental death in the United States, with 52,404 lethal drug overdoses in 2015. A startling statistic is that prescription opioid abuse is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers and 12,990 overdose deaths related to heroin in 2015.3
It is likely that there are multiple reasons prescribing of opioids is epidemic. Surgical pain is a common indication for opioid prescriptions; fewer than half of patients who undergo surgery report adequate postoperative pain relief.4 Recognition of these deficits in pain management has inspired national campaigns to improve patients’ experience with pain and aggressively address pain with drugs such as opioids.5
At the same time, marketing efforts by the pharmaceutical industry sought to reassure the medical community that patients would not become addicted to prescription opioid pain relievers if physical pain was the indication for such prescriptions. In response, health care providers began to prescribe opioids at a greater rate. As providers were encouraged to increase prescriptions, opioid medications began to be misused—and only then did it become clear that these medications are, in fact, highly addictive.6 Opioid abuse and overdose rates began to increase; in 2015, more than 33,000 Americans died because of an opioid overdose, including prescription opioids and heroin7 (FIGURE). In fact, although most people recognize the threat posed by illegal heroin, most of the 2 million who abused opioids in 2015 in the United States suffered from prescription abuse; only about a quarter, or about 600,000, abused heroin.8 In addition, more than 80% of people who abuse heroin initially abused prescription opioids.9
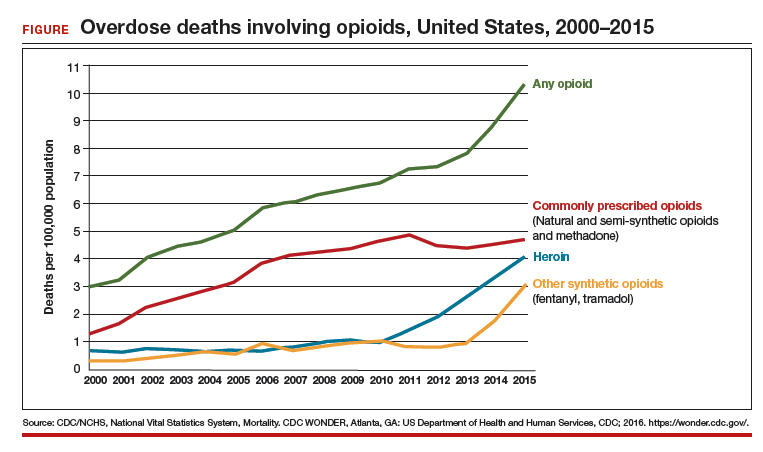
Read about medications and strategies for multimodal pain management.
Multimodal approach to pain management
The goals of postsurgical pain treatment are to relieve suffering, optimize bodily functioning after surgery, limit length of the stay, and optimize patient satisfaction. Pain-control regimens should consider the specific surgical procedure and the patient’s medical, psychological, and physical conditions; age; level of fear or anxiety; personal preference; and response to previous treatments.10
Optimally, postsurgical pain management starts well before the day of surgery. Employing such strategies as Enhanced Recovery after Surgery (ERAS) protocols does not necessarily mean providing the same care for every patient, every time. Rather, ERAS serves as a checklist to ensure that all applicable categories of pain medication and pain-control strategies are considered, selected, and dosed according to individual needs.11 (See “Preoperative management of pain expectations.”)
Ideally, before surgery, provide the patient with an opportunity to learn that:
- Her expectations about postsurgical pain should be realistic, and that freedom from pain is not realistic.
- Pain-reduction options should optimize her bodily function and mobility, reduce the degree to which pain interferes with activities, and relieve associated psychological stressors.
- Inherent in the pain management plan should be a goal of minimizing the risks of opioid misuse, abuse, and addiction—for the patient and for her family members and friends.
Opioids
Opioids have been employed to treat pain for 700 years.12 They are powerful pain relievers because they target central mechanisms involved in the perception of pain. Regrettably, because of their central action, opioids have many adverse effects in addition to being highly addictive.
Nonopioid alternatives
Expert consensus, including recommendations of the World Health Organization,11 favors using nonopioids as first-line medications to address surgical pain. Nonopioid analgesic options are acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications. In addition, nonanalgesic medications such as sedatives, sleep aids, and muscle relaxants can relieve postsurgical pain. Optimal use of these nonopioid medications can significantly reduce or eliminate the need for opioid medications to treat pain. Goals are to 1) reserve opioids for the most severe pain and 2) minimize the number of doses/pills of opioids required to control postsurgical pain.
Acetaminophen. At dosages of 325 to 1,000 mg orally every 4 to 6 hours, to a maximum dosage of 4,000 mg/d, acetaminophen can be used to treat mild pain and, in combination with other medications, moderate-to-severe pain. The drug also can be administered intravenously (IV), although use of the IV route is limited in many hospitals because of its significantly higher expense compared to the oral form.
The mechanism of action of acetaminophen is unique among pain relievers; it can therefore be used in combination with other pain relievers to more effectively treat pain with fewer concerns about medication-induced adverse effects or opioid overdose. However, keep in mind when considering combining analgesics, that acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription formulations, and that a combination of more than one acetaminophen-containing product can create the risk of overdose.
Acetaminophen should be used with caution in patients with liver disease. That being said, multiple trials have documented safe use in normal body weight adults who do not have hepatic disease, at dosages as high as 4,000 mg over a 24-hour period.13
NSAIDs. A combination of an NSAID and acetaminophen has been documented to reduce the amount of opioid medications required to treat postsurgical pain. In most circumstances, especially for minor surgery, acetaminophen and NSAIDS can be administered just before surgery starts. This preoperative treatment, called “preventive analgesia” or “preemptive analgesia,” has been demonstrated in multiple clinical trials to reduce postoperative pain.14
Adjuvant pain medications. Antidepressants, antiepileptic agents, and muscle relaxants—agents that have a primary indication for a condition (or conditions) other than pain and do not directly provide analgesia—have been used as adjuvant pain medications. When employed with traditional analgesics, they have been demonstrated to reduce postsurgical pain scores and the amount of opioids required. These medications need to be used cautiously because some are associated with serious sedation and vertigo (TABLE). Take caution when using adjuvant pain medications in patients older than 65 years; guidance on their use in older patients has been outlined by the American Geriatrics Society and other professional organizations.15
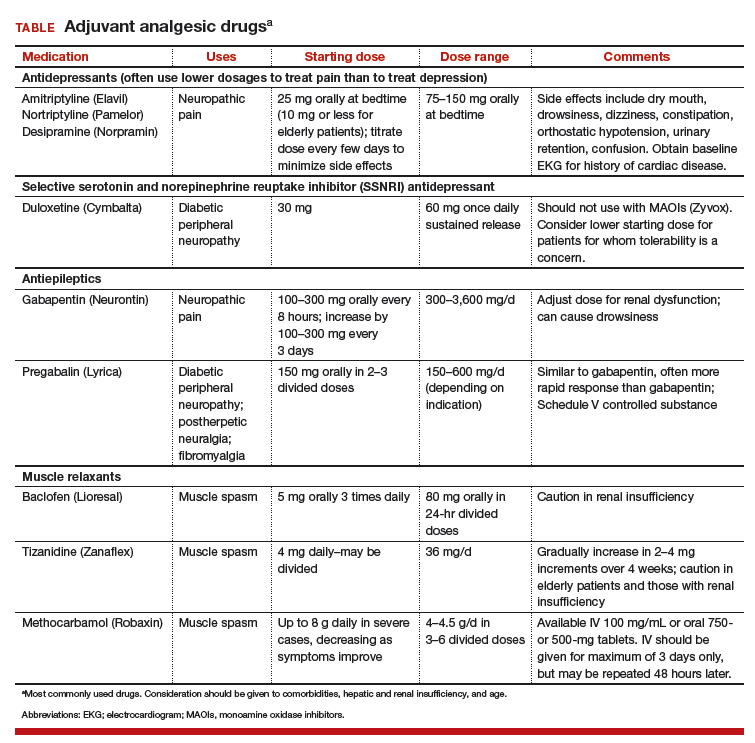
Case Continued
The patient was given the expectation that the 11-mm left lower-quadrant port site would likely be the most bothersome site of pain—a rating of 4 or 5 on a visual analogue scale of 1 to 10, on postoperative day 1, while standing. The other 3 (5-mm) laparoscopic ports, she was told, would, typically, be less bothersome. The patient was educated regarding the role of analgesics and adjuvant medications and cautioned not to exceed 4,000 mg of acetaminophen in any 24-hour period. She was told that gabapentin may make her feel sedated or dizzy, or both; she was encouraged to hold this medication if she found these adverse effects bothersome or limiting.
The following multimodal pain management was established.
Preoperatively, the patient was given:
- Acetaminophen 1.5 g orally (as a liquid, 45 mL of a suspension of 500 mg/15 mL liquid), 2 to 3 hours preoperatively; the surgical suite did not stock IV acetaminophen.
- Gabapentin 600 mg orally, with a sip of water, the morning of surgery.
- Celecoxib 100 mg orally, with a sip of water, the morning of surgery.
Prescriptions for home postoperative pain management were provided preoperatively:
- OTC acetaminophen 1,000 mg (as 2 500-mgtablets) taken as a scheduled dose every 8 hours for the first 48 hours postoperatively.
- Meloxicam 15 mg daily as the NSAID, taken as a scheduled dose once per day for the first 48 hours postoperatively, then as needed.
- Gabapentin 300 mg (in addition to the preoperative dose, above), taken as a scheduled dose every 8 hours for the first 48 hours postoperatively, then as needed.
- Oxycodone 5 mg (without acetaminophen) for breakthrough pain.
Intraoperatively:
- Meticulous attention was paid to patient positioning, to reduce the possibility of back and upper- and lower-extremity injury postoperatively.
- A corticosteroid (dexamethasone 8 mg IV) was administered to minimize postoperative nausea and vomiting and as an adjuvant medication for postoperative pain control.
- Careful attention was paid to limit residual CO2 gas and intraoperative intra-abdominal pressures.
- All laparoscopic port sites were injected with 30 mL of 0.25% bupivacaine with epinephrine, extending to subcutaneous, fascial, and peritoneal layers.
Read about why a multimodal approach is best for postsurgical pain.
Why a multimodal plan to treat pain?
Pain following laparoscopy has been associated with many variables, including patient positioning, port size and placement, amount of port manipulation, and gas retention. After a laparoscopic surgical procedure, patients report pain in the abdomen, back, and shoulders.
Postsurgical pain has 3 components:
- Shoulder pain, thought to result from phrenic nerve irritation caused by lingering CO2 in the abdominal cavity.
- Visceral pain, occurring secondary to stretching of the abdominal cavity.
- Somatic pain, caused by the surgical incision; of the 3 components to pain, somatic pain can have the least impact because laparoscopic incisions are small.
For our patient, prior to the incisions being made, she received local anesthesia intraoperatively to the laparoscopic port sites to include the subcutaneous, fascial, and peritoneal layers. Involving these layers allows for more of a block. An ultrasonography-guided transversus abdominis plane (TAP) block, if available, is highly effective at decreasing postoperative pain, but its efficacy is dependent on the anatomy and the skill of the physician (whether anesthesiologist, gynecologist, or surgeon) who is placing it.16
We used dexamethasone 8 mg IV, intraoperatively because this single dose has been shown to decrease the perception of pain postoperatively. Dexamethasone also has been shown to decrease consumption of oxycodone during the 24 hours after laparoscopic gynecologic surgery.17
CO2 used to insufflate the patient’s abdomen can take as long as 2 days to fully resorb, resulting in increased pain. This discomfort has been described as delayed; the patient might not notice it until she goes home. In a study, 70% of patients had shoulder discomfort following laparoscopy 24 hours after their procedure.18 For this reason, we employed several techniques to reduce this effect:
- We reduced the intra-abdominal pressure limit to 10 mm Hg (from 15 mm Hg) once dissection was complete.
- At the end of the procedure, careful attention was paid to removing as much intra-abdominal gas as possible, including placing the patient in the Trendelenburg position and having the anesthesiologist induce a Valsalva maneuver. This action has been shown to significantly improve pain control compared to placebo intervention.19
- We used humidified CO2, which has been demonstrated to reduce pain in laparoscopic surgery.20
Preemptively, we provided this patient with acetaminophen, celecoxib, and gabapentin, which have been demonstrated to be effective in gynecologic patients to decrease the need for postoperative opioids.21 Also, our patient received counseling, with specific expectations for what to expect following the surgical procedure.
CASE Resolved
Our patient did exceptionally well following surgery. She used only one of the oxycodone pills and did not require unplanned interventions. She took gabapentin, acetaminophen, and meloxicam at their scheduled doses for 2 days. She continued to use meloxicam for 4 more days for mild abdominal pain, then discontinued all medications.She flushed her 9 unused oxycodone pills down the toilet. (See “A word about disposal of ‘excess’ opioids”22) The patient returned to her administrative duties at work 2 weeks after the procedure and reported that she was “very satisfied” with her surgical experience.
The US Food and Drug Administration (FDA) recommends disposing of certain drugs through a take-back program or, if such a program is not readily available, by flushing them down a toilet or sink. In a recent study, investigators concluded that opioids on the FDA's so-called flush list include most opioids in clinical use--even if the entire supply prescribed is to be flushed down the drain. Conservative estimates of environmental degradation were employed in the study; the investigators' conclusion was that these drugs pose a "negligible" eco-toxicologic risk.1
Reference
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration "flush list". Sci Total Environ. 2017;609:1023-1040.
In conclusion
Postoperative pain is a complex entity that must be considered to require individualized strategies and, possibly, multiple interventions. Optimally, thorough education, including pain management options, is provided to the patient prior to surgery. Given the current state of opioid abuse in the United States, all gynecologic surgeons should be familiar with multimodal pain therapy and how to employ nonmedical techniques to reduce postsurgical pain without relying solely on opioids. (See “Online resources for pain management”.)
- Drug Disposal Information
(US Department of Justice Drug Enforcement Administration)
https://www.deadiversion.usdoj.gov/drug_disposal/index.html - Surgical Pain Consortium
http://surgicalpainconsortium.org/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- United Nations International Narcotics Control Board. Narcotic drugs: Report 2016: Estimated world requirements for 2017-statistics for 2015. New York, NY. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Published 2017. Accessed January 7, 2018.
- Centers for Disease Control and Prevention. Opioid painkiller prescribing: Where you live makes a difference. Atlanta, GA. https://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf. Published July 2014. Accessed January 5, 2018.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
- Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2015 national survey on drug use and health: Detailed tables. Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Published 2016. Accessed January 5, 2018.
- Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August 2013. Accessed January 5, 2018.
- Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202.
- Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 suppl):6S–11S.
- Brownstein, MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393.
- US Food and Drug Administration. Acetaminophen. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Published November 2017. Accessed January 7, 2018.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications included in the use of high‐risk medications in the elderly and potentially harmful drug–disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18.
- Joshi GP, Janis JE, Haas EM, et al. Surgical site infiltration for abdominal surgery: A novel neuroanatomical-based approach. Plast Reconstr Surg Glob Open. 2016;4(12):e1181. https://insights.ovid.com/crossref?an=01720096-201612000-00021. Accessed January 5, 2018.
- Jokela RM, Ahonen JV, Tallgren MK, et al. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–615.
- Hohlrieder M, Brimacombe J, Eschertzhuber S, et al. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007;62(9):913–918.
- Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95(8):950–956.
- Reagan KM, O’Sullivan DM, Gannon R, Steinberg AC. Decreasing postoperative narcotics in reconstructive pelvic surgery; A randomized controlled trial. Am J Obstet Gynecol. 2017;217(3):325.e1–e10.
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017;609:1023–1040.
CASE Managing pain associated with prolapse and SUI surgery
A 46-year-old woman (G4P4) described 3 years of worsening symptoms related to recurrent stage-3 palpable uterine prolapse. She had associated symptomatic stress urinary incontinence. She had been treated for uterine prolapse 5 years ago with vaginal hysterectomy, bilateral salpingectomy, and high uterosacral-ligament suspension.
After consultation, the patient elected to undergo laparoscopic sacral colpopexy, a mid-urethral sling, and possible anterior and posterior colporrhaphy. Appropriate discussion about the risks and benefits of mesh was provided preoperatively. The surgical team judged her to be highly motivated; she wanted same-day outpatient surgery so that she could go home and then return to work. She had excellent support at home.
How would you counsel this patient about expected postoperative pain? Which medications would you administer to her preoperatively and perioperatively? Which ones would you prescribe for her to manage pain postoperatively?

Adverse impact of prescription opioids in the United States
Although fewer than 5% of the world’s population live in the United States, nearly 80% of the world’s opioids are written for them.1 In 2012, 259 million prescriptions were written for opioids in the United States—more than enough to give every American adult their own bottle of pills.2 Sadly, drug overdose is now a leading cause of accidental death in the United States, with 52,404 lethal drug overdoses in 2015. A startling statistic is that prescription opioid abuse is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers and 12,990 overdose deaths related to heroin in 2015.3
It is likely that there are multiple reasons prescribing of opioids is epidemic. Surgical pain is a common indication for opioid prescriptions; fewer than half of patients who undergo surgery report adequate postoperative pain relief.4 Recognition of these deficits in pain management has inspired national campaigns to improve patients’ experience with pain and aggressively address pain with drugs such as opioids.5
At the same time, marketing efforts by the pharmaceutical industry sought to reassure the medical community that patients would not become addicted to prescription opioid pain relievers if physical pain was the indication for such prescriptions. In response, health care providers began to prescribe opioids at a greater rate. As providers were encouraged to increase prescriptions, opioid medications began to be misused—and only then did it become clear that these medications are, in fact, highly addictive.6 Opioid abuse and overdose rates began to increase; in 2015, more than 33,000 Americans died because of an opioid overdose, including prescription opioids and heroin7 (FIGURE). In fact, although most people recognize the threat posed by illegal heroin, most of the 2 million who abused opioids in 2015 in the United States suffered from prescription abuse; only about a quarter, or about 600,000, abused heroin.8 In addition, more than 80% of people who abuse heroin initially abused prescription opioids.9

Read about medications and strategies for multimodal pain management.
Multimodal approach to pain management
The goals of postsurgical pain treatment are to relieve suffering, optimize bodily functioning after surgery, limit length of the stay, and optimize patient satisfaction. Pain-control regimens should consider the specific surgical procedure and the patient’s medical, psychological, and physical conditions; age; level of fear or anxiety; personal preference; and response to previous treatments.10
Optimally, postsurgical pain management starts well before the day of surgery. Employing such strategies as Enhanced Recovery after Surgery (ERAS) protocols does not necessarily mean providing the same care for every patient, every time. Rather, ERAS serves as a checklist to ensure that all applicable categories of pain medication and pain-control strategies are considered, selected, and dosed according to individual needs.11 (See “Preoperative management of pain expectations.”)
Ideally, before surgery, provide the patient with an opportunity to learn that:
- Her expectations about postsurgical pain should be realistic, and that freedom from pain is not realistic.
- Pain-reduction options should optimize her bodily function and mobility, reduce the degree to which pain interferes with activities, and relieve associated psychological stressors.
- Inherent in the pain management plan should be a goal of minimizing the risks of opioid misuse, abuse, and addiction—for the patient and for her family members and friends.
Opioids
Opioids have been employed to treat pain for 700 years.12 They are powerful pain relievers because they target central mechanisms involved in the perception of pain. Regrettably, because of their central action, opioids have many adverse effects in addition to being highly addictive.
Nonopioid alternatives
Expert consensus, including recommendations of the World Health Organization,11 favors using nonopioids as first-line medications to address surgical pain. Nonopioid analgesic options are acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications. In addition, nonanalgesic medications such as sedatives, sleep aids, and muscle relaxants can relieve postsurgical pain. Optimal use of these nonopioid medications can significantly reduce or eliminate the need for opioid medications to treat pain. Goals are to 1) reserve opioids for the most severe pain and 2) minimize the number of doses/pills of opioids required to control postsurgical pain.
Acetaminophen. At dosages of 325 to 1,000 mg orally every 4 to 6 hours, to a maximum dosage of 4,000 mg/d, acetaminophen can be used to treat mild pain and, in combination with other medications, moderate-to-severe pain. The drug also can be administered intravenously (IV), although use of the IV route is limited in many hospitals because of its significantly higher expense compared to the oral form.
The mechanism of action of acetaminophen is unique among pain relievers; it can therefore be used in combination with other pain relievers to more effectively treat pain with fewer concerns about medication-induced adverse effects or opioid overdose. However, keep in mind when considering combining analgesics, that acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription formulations, and that a combination of more than one acetaminophen-containing product can create the risk of overdose.
Acetaminophen should be used with caution in patients with liver disease. That being said, multiple trials have documented safe use in normal body weight adults who do not have hepatic disease, at dosages as high as 4,000 mg over a 24-hour period.13
NSAIDs. A combination of an NSAID and acetaminophen has been documented to reduce the amount of opioid medications required to treat postsurgical pain. In most circumstances, especially for minor surgery, acetaminophen and NSAIDS can be administered just before surgery starts. This preoperative treatment, called “preventive analgesia” or “preemptive analgesia,” has been demonstrated in multiple clinical trials to reduce postoperative pain.14
Adjuvant pain medications. Antidepressants, antiepileptic agents, and muscle relaxants—agents that have a primary indication for a condition (or conditions) other than pain and do not directly provide analgesia—have been used as adjuvant pain medications. When employed with traditional analgesics, they have been demonstrated to reduce postsurgical pain scores and the amount of opioids required. These medications need to be used cautiously because some are associated with serious sedation and vertigo (TABLE). Take caution when using adjuvant pain medications in patients older than 65 years; guidance on their use in older patients has been outlined by the American Geriatrics Society and other professional organizations.15

Case Continued
The patient was given the expectation that the 11-mm left lower-quadrant port site would likely be the most bothersome site of pain—a rating of 4 or 5 on a visual analogue scale of 1 to 10, on postoperative day 1, while standing. The other 3 (5-mm) laparoscopic ports, she was told, would, typically, be less bothersome. The patient was educated regarding the role of analgesics and adjuvant medications and cautioned not to exceed 4,000 mg of acetaminophen in any 24-hour period. She was told that gabapentin may make her feel sedated or dizzy, or both; she was encouraged to hold this medication if she found these adverse effects bothersome or limiting.
The following multimodal pain management was established.
Preoperatively, the patient was given:
- Acetaminophen 1.5 g orally (as a liquid, 45 mL of a suspension of 500 mg/15 mL liquid), 2 to 3 hours preoperatively; the surgical suite did not stock IV acetaminophen.
- Gabapentin 600 mg orally, with a sip of water, the morning of surgery.
- Celecoxib 100 mg orally, with a sip of water, the morning of surgery.
Prescriptions for home postoperative pain management were provided preoperatively:
- OTC acetaminophen 1,000 mg (as 2 500-mgtablets) taken as a scheduled dose every 8 hours for the first 48 hours postoperatively.
- Meloxicam 15 mg daily as the NSAID, taken as a scheduled dose once per day for the first 48 hours postoperatively, then as needed.
- Gabapentin 300 mg (in addition to the preoperative dose, above), taken as a scheduled dose every 8 hours for the first 48 hours postoperatively, then as needed.
- Oxycodone 5 mg (without acetaminophen) for breakthrough pain.
Intraoperatively:
- Meticulous attention was paid to patient positioning, to reduce the possibility of back and upper- and lower-extremity injury postoperatively.
- A corticosteroid (dexamethasone 8 mg IV) was administered to minimize postoperative nausea and vomiting and as an adjuvant medication for postoperative pain control.
- Careful attention was paid to limit residual CO2 gas and intraoperative intra-abdominal pressures.
- All laparoscopic port sites were injected with 30 mL of 0.25% bupivacaine with epinephrine, extending to subcutaneous, fascial, and peritoneal layers.
Read about why a multimodal approach is best for postsurgical pain.
Why a multimodal plan to treat pain?
Pain following laparoscopy has been associated with many variables, including patient positioning, port size and placement, amount of port manipulation, and gas retention. After a laparoscopic surgical procedure, patients report pain in the abdomen, back, and shoulders.
Postsurgical pain has 3 components:
- Shoulder pain, thought to result from phrenic nerve irritation caused by lingering CO2 in the abdominal cavity.
- Visceral pain, occurring secondary to stretching of the abdominal cavity.
- Somatic pain, caused by the surgical incision; of the 3 components to pain, somatic pain can have the least impact because laparoscopic incisions are small.
For our patient, prior to the incisions being made, she received local anesthesia intraoperatively to the laparoscopic port sites to include the subcutaneous, fascial, and peritoneal layers. Involving these layers allows for more of a block. An ultrasonography-guided transversus abdominis plane (TAP) block, if available, is highly effective at decreasing postoperative pain, but its efficacy is dependent on the anatomy and the skill of the physician (whether anesthesiologist, gynecologist, or surgeon) who is placing it.16
We used dexamethasone 8 mg IV, intraoperatively because this single dose has been shown to decrease the perception of pain postoperatively. Dexamethasone also has been shown to decrease consumption of oxycodone during the 24 hours after laparoscopic gynecologic surgery.17
CO2 used to insufflate the patient’s abdomen can take as long as 2 days to fully resorb, resulting in increased pain. This discomfort has been described as delayed; the patient might not notice it until she goes home. In a study, 70% of patients had shoulder discomfort following laparoscopy 24 hours after their procedure.18 For this reason, we employed several techniques to reduce this effect:
- We reduced the intra-abdominal pressure limit to 10 mm Hg (from 15 mm Hg) once dissection was complete.
- At the end of the procedure, careful attention was paid to removing as much intra-abdominal gas as possible, including placing the patient in the Trendelenburg position and having the anesthesiologist induce a Valsalva maneuver. This action has been shown to significantly improve pain control compared to placebo intervention.19
- We used humidified CO2, which has been demonstrated to reduce pain in laparoscopic surgery.20
Preemptively, we provided this patient with acetaminophen, celecoxib, and gabapentin, which have been demonstrated to be effective in gynecologic patients to decrease the need for postoperative opioids.21 Also, our patient received counseling, with specific expectations for what to expect following the surgical procedure.
CASE Resolved
Our patient did exceptionally well following surgery. She used only one of the oxycodone pills and did not require unplanned interventions. She took gabapentin, acetaminophen, and meloxicam at their scheduled doses for 2 days. She continued to use meloxicam for 4 more days for mild abdominal pain, then discontinued all medications.She flushed her 9 unused oxycodone pills down the toilet. (See “A word about disposal of ‘excess’ opioids”22) The patient returned to her administrative duties at work 2 weeks after the procedure and reported that she was “very satisfied” with her surgical experience.
The US Food and Drug Administration (FDA) recommends disposing of certain drugs through a take-back program or, if such a program is not readily available, by flushing them down a toilet or sink. In a recent study, investigators concluded that opioids on the FDA's so-called flush list include most opioids in clinical use--even if the entire supply prescribed is to be flushed down the drain. Conservative estimates of environmental degradation were employed in the study; the investigators' conclusion was that these drugs pose a "negligible" eco-toxicologic risk.1
Reference
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration "flush list". Sci Total Environ. 2017;609:1023-1040.
In conclusion
Postoperative pain is a complex entity that must be considered to require individualized strategies and, possibly, multiple interventions. Optimally, thorough education, including pain management options, is provided to the patient prior to surgery. Given the current state of opioid abuse in the United States, all gynecologic surgeons should be familiar with multimodal pain therapy and how to employ nonmedical techniques to reduce postsurgical pain without relying solely on opioids. (See “Online resources for pain management”.)
- Drug Disposal Information
(US Department of Justice Drug Enforcement Administration)
https://www.deadiversion.usdoj.gov/drug_disposal/index.html - Surgical Pain Consortium
http://surgicalpainconsortium.org/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Managing pain associated with prolapse and SUI surgery
A 46-year-old woman (G4P4) described 3 years of worsening symptoms related to recurrent stage-3 palpable uterine prolapse. She had associated symptomatic stress urinary incontinence. She had been treated for uterine prolapse 5 years ago with vaginal hysterectomy, bilateral salpingectomy, and high uterosacral-ligament suspension.
After consultation, the patient elected to undergo laparoscopic sacral colpopexy, a mid-urethral sling, and possible anterior and posterior colporrhaphy. Appropriate discussion about the risks and benefits of mesh was provided preoperatively. The surgical team judged her to be highly motivated; she wanted same-day outpatient surgery so that she could go home and then return to work. She had excellent support at home.
How would you counsel this patient about expected postoperative pain? Which medications would you administer to her preoperatively and perioperatively? Which ones would you prescribe for her to manage pain postoperatively?

Adverse impact of prescription opioids in the United States
Although fewer than 5% of the world’s population live in the United States, nearly 80% of the world’s opioids are written for them.1 In 2012, 259 million prescriptions were written for opioids in the United States—more than enough to give every American adult their own bottle of pills.2 Sadly, drug overdose is now a leading cause of accidental death in the United States, with 52,404 lethal drug overdoses in 2015. A startling statistic is that prescription opioid abuse is driving this epidemic, with 20,101 overdose deaths related to prescription pain relievers and 12,990 overdose deaths related to heroin in 2015.3
It is likely that there are multiple reasons prescribing of opioids is epidemic. Surgical pain is a common indication for opioid prescriptions; fewer than half of patients who undergo surgery report adequate postoperative pain relief.4 Recognition of these deficits in pain management has inspired national campaigns to improve patients’ experience with pain and aggressively address pain with drugs such as opioids.5
At the same time, marketing efforts by the pharmaceutical industry sought to reassure the medical community that patients would not become addicted to prescription opioid pain relievers if physical pain was the indication for such prescriptions. In response, health care providers began to prescribe opioids at a greater rate. As providers were encouraged to increase prescriptions, opioid medications began to be misused—and only then did it become clear that these medications are, in fact, highly addictive.6 Opioid abuse and overdose rates began to increase; in 2015, more than 33,000 Americans died because of an opioid overdose, including prescription opioids and heroin7 (FIGURE). In fact, although most people recognize the threat posed by illegal heroin, most of the 2 million who abused opioids in 2015 in the United States suffered from prescription abuse; only about a quarter, or about 600,000, abused heroin.8 In addition, more than 80% of people who abuse heroin initially abused prescription opioids.9

Read about medications and strategies for multimodal pain management.
Multimodal approach to pain management
The goals of postsurgical pain treatment are to relieve suffering, optimize bodily functioning after surgery, limit length of the stay, and optimize patient satisfaction. Pain-control regimens should consider the specific surgical procedure and the patient’s medical, psychological, and physical conditions; age; level of fear or anxiety; personal preference; and response to previous treatments.10
Optimally, postsurgical pain management starts well before the day of surgery. Employing such strategies as Enhanced Recovery after Surgery (ERAS) protocols does not necessarily mean providing the same care for every patient, every time. Rather, ERAS serves as a checklist to ensure that all applicable categories of pain medication and pain-control strategies are considered, selected, and dosed according to individual needs.11 (See “Preoperative management of pain expectations.”)
Ideally, before surgery, provide the patient with an opportunity to learn that:
- Her expectations about postsurgical pain should be realistic, and that freedom from pain is not realistic.
- Pain-reduction options should optimize her bodily function and mobility, reduce the degree to which pain interferes with activities, and relieve associated psychological stressors.
- Inherent in the pain management plan should be a goal of minimizing the risks of opioid misuse, abuse, and addiction—for the patient and for her family members and friends.
Opioids
Opioids have been employed to treat pain for 700 years.12 They are powerful pain relievers because they target central mechanisms involved in the perception of pain. Regrettably, because of their central action, opioids have many adverse effects in addition to being highly addictive.
Nonopioid alternatives
Expert consensus, including recommendations of the World Health Organization,11 favors using nonopioids as first-line medications to address surgical pain. Nonopioid analgesic options are acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant medications. In addition, nonanalgesic medications such as sedatives, sleep aids, and muscle relaxants can relieve postsurgical pain. Optimal use of these nonopioid medications can significantly reduce or eliminate the need for opioid medications to treat pain. Goals are to 1) reserve opioids for the most severe pain and 2) minimize the number of doses/pills of opioids required to control postsurgical pain.
Acetaminophen. At dosages of 325 to 1,000 mg orally every 4 to 6 hours, to a maximum dosage of 4,000 mg/d, acetaminophen can be used to treat mild pain and, in combination with other medications, moderate-to-severe pain. The drug also can be administered intravenously (IV), although use of the IV route is limited in many hospitals because of its significantly higher expense compared to the oral form.
The mechanism of action of acetaminophen is unique among pain relievers; it can therefore be used in combination with other pain relievers to more effectively treat pain with fewer concerns about medication-induced adverse effects or opioid overdose. However, keep in mind when considering combining analgesics, that acetaminophen is an active ingredient in hundreds of over-the-counter (OTC) and prescription formulations, and that a combination of more than one acetaminophen-containing product can create the risk of overdose.
Acetaminophen should be used with caution in patients with liver disease. That being said, multiple trials have documented safe use in normal body weight adults who do not have hepatic disease, at dosages as high as 4,000 mg over a 24-hour period.13
NSAIDs. A combination of an NSAID and acetaminophen has been documented to reduce the amount of opioid medications required to treat postsurgical pain. In most circumstances, especially for minor surgery, acetaminophen and NSAIDS can be administered just before surgery starts. This preoperative treatment, called “preventive analgesia” or “preemptive analgesia,” has been demonstrated in multiple clinical trials to reduce postoperative pain.14
Adjuvant pain medications. Antidepressants, antiepileptic agents, and muscle relaxants—agents that have a primary indication for a condition (or conditions) other than pain and do not directly provide analgesia—have been used as adjuvant pain medications. When employed with traditional analgesics, they have been demonstrated to reduce postsurgical pain scores and the amount of opioids required. These medications need to be used cautiously because some are associated with serious sedation and vertigo (TABLE). Take caution when using adjuvant pain medications in patients older than 65 years; guidance on their use in older patients has been outlined by the American Geriatrics Society and other professional organizations.15

Case Continued
The patient was given the expectation that the 11-mm left lower-quadrant port site would likely be the most bothersome site of pain—a rating of 4 or 5 on a visual analogue scale of 1 to 10, on postoperative day 1, while standing. The other 3 (5-mm) laparoscopic ports, she was told, would, typically, be less bothersome. The patient was educated regarding the role of analgesics and adjuvant medications and cautioned not to exceed 4,000 mg of acetaminophen in any 24-hour period. She was told that gabapentin may make her feel sedated or dizzy, or both; she was encouraged to hold this medication if she found these adverse effects bothersome or limiting.
The following multimodal pain management was established.
Preoperatively, the patient was given:
- Acetaminophen 1.5 g orally (as a liquid, 45 mL of a suspension of 500 mg/15 mL liquid), 2 to 3 hours preoperatively; the surgical suite did not stock IV acetaminophen.
- Gabapentin 600 mg orally, with a sip of water, the morning of surgery.
- Celecoxib 100 mg orally, with a sip of water, the morning of surgery.
Prescriptions for home postoperative pain management were provided preoperatively:
- OTC acetaminophen 1,000 mg (as 2 500-mgtablets) taken as a scheduled dose every 8 hours for the first 48 hours postoperatively.
- Meloxicam 15 mg daily as the NSAID, taken as a scheduled dose once per day for the first 48 hours postoperatively, then as needed.
- Gabapentin 300 mg (in addition to the preoperative dose, above), taken as a scheduled dose every 8 hours for the first 48 hours postoperatively, then as needed.
- Oxycodone 5 mg (without acetaminophen) for breakthrough pain.
Intraoperatively:
- Meticulous attention was paid to patient positioning, to reduce the possibility of back and upper- and lower-extremity injury postoperatively.
- A corticosteroid (dexamethasone 8 mg IV) was administered to minimize postoperative nausea and vomiting and as an adjuvant medication for postoperative pain control.
- Careful attention was paid to limit residual CO2 gas and intraoperative intra-abdominal pressures.
- All laparoscopic port sites were injected with 30 mL of 0.25% bupivacaine with epinephrine, extending to subcutaneous, fascial, and peritoneal layers.
Read about why a multimodal approach is best for postsurgical pain.
Why a multimodal plan to treat pain?
Pain following laparoscopy has been associated with many variables, including patient positioning, port size and placement, amount of port manipulation, and gas retention. After a laparoscopic surgical procedure, patients report pain in the abdomen, back, and shoulders.
Postsurgical pain has 3 components:
- Shoulder pain, thought to result from phrenic nerve irritation caused by lingering CO2 in the abdominal cavity.
- Visceral pain, occurring secondary to stretching of the abdominal cavity.
- Somatic pain, caused by the surgical incision; of the 3 components to pain, somatic pain can have the least impact because laparoscopic incisions are small.
For our patient, prior to the incisions being made, she received local anesthesia intraoperatively to the laparoscopic port sites to include the subcutaneous, fascial, and peritoneal layers. Involving these layers allows for more of a block. An ultrasonography-guided transversus abdominis plane (TAP) block, if available, is highly effective at decreasing postoperative pain, but its efficacy is dependent on the anatomy and the skill of the physician (whether anesthesiologist, gynecologist, or surgeon) who is placing it.16
We used dexamethasone 8 mg IV, intraoperatively because this single dose has been shown to decrease the perception of pain postoperatively. Dexamethasone also has been shown to decrease consumption of oxycodone during the 24 hours after laparoscopic gynecologic surgery.17
CO2 used to insufflate the patient’s abdomen can take as long as 2 days to fully resorb, resulting in increased pain. This discomfort has been described as delayed; the patient might not notice it until she goes home. In a study, 70% of patients had shoulder discomfort following laparoscopy 24 hours after their procedure.18 For this reason, we employed several techniques to reduce this effect:
- We reduced the intra-abdominal pressure limit to 10 mm Hg (from 15 mm Hg) once dissection was complete.
- At the end of the procedure, careful attention was paid to removing as much intra-abdominal gas as possible, including placing the patient in the Trendelenburg position and having the anesthesiologist induce a Valsalva maneuver. This action has been shown to significantly improve pain control compared to placebo intervention.19
- We used humidified CO2, which has been demonstrated to reduce pain in laparoscopic surgery.20
Preemptively, we provided this patient with acetaminophen, celecoxib, and gabapentin, which have been demonstrated to be effective in gynecologic patients to decrease the need for postoperative opioids.21 Also, our patient received counseling, with specific expectations for what to expect following the surgical procedure.
CASE Resolved
Our patient did exceptionally well following surgery. She used only one of the oxycodone pills and did not require unplanned interventions. She took gabapentin, acetaminophen, and meloxicam at their scheduled doses for 2 days. She continued to use meloxicam for 4 more days for mild abdominal pain, then discontinued all medications.She flushed her 9 unused oxycodone pills down the toilet. (See “A word about disposal of ‘excess’ opioids”22) The patient returned to her administrative duties at work 2 weeks after the procedure and reported that she was “very satisfied” with her surgical experience.
The US Food and Drug Administration (FDA) recommends disposing of certain drugs through a take-back program or, if such a program is not readily available, by flushing them down a toilet or sink. In a recent study, investigators concluded that opioids on the FDA's so-called flush list include most opioids in clinical use--even if the entire supply prescribed is to be flushed down the drain. Conservative estimates of environmental degradation were employed in the study; the investigators' conclusion was that these drugs pose a "negligible" eco-toxicologic risk.1
Reference
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration "flush list". Sci Total Environ. 2017;609:1023-1040.
In conclusion
Postoperative pain is a complex entity that must be considered to require individualized strategies and, possibly, multiple interventions. Optimally, thorough education, including pain management options, is provided to the patient prior to surgery. Given the current state of opioid abuse in the United States, all gynecologic surgeons should be familiar with multimodal pain therapy and how to employ nonmedical techniques to reduce postsurgical pain without relying solely on opioids. (See “Online resources for pain management”.)
- Drug Disposal Information
(US Department of Justice Drug Enforcement Administration)
https://www.deadiversion.usdoj.gov/drug_disposal/index.html - Surgical Pain Consortium
http://surgicalpainconsortium.org/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- United Nations International Narcotics Control Board. Narcotic drugs: Report 2016: Estimated world requirements for 2017-statistics for 2015. New York, NY. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Published 2017. Accessed January 7, 2018.
- Centers for Disease Control and Prevention. Opioid painkiller prescribing: Where you live makes a difference. Atlanta, GA. https://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf. Published July 2014. Accessed January 5, 2018.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
- Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2015 national survey on drug use and health: Detailed tables. Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Published 2016. Accessed January 5, 2018.
- Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August 2013. Accessed January 5, 2018.
- Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202.
- Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 suppl):6S–11S.
- Brownstein, MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393.
- US Food and Drug Administration. Acetaminophen. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Published November 2017. Accessed January 7, 2018.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications included in the use of high‐risk medications in the elderly and potentially harmful drug–disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18.
- Joshi GP, Janis JE, Haas EM, et al. Surgical site infiltration for abdominal surgery: A novel neuroanatomical-based approach. Plast Reconstr Surg Glob Open. 2016;4(12):e1181. https://insights.ovid.com/crossref?an=01720096-201612000-00021. Accessed January 5, 2018.
- Jokela RM, Ahonen JV, Tallgren MK, et al. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–615.
- Hohlrieder M, Brimacombe J, Eschertzhuber S, et al. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007;62(9):913–918.
- Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95(8):950–956.
- Reagan KM, O’Sullivan DM, Gannon R, Steinberg AC. Decreasing postoperative narcotics in reconstructive pelvic surgery; A randomized controlled trial. Am J Obstet Gynecol. 2017;217(3):325.e1–e10.
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017;609:1023–1040.
- United Nations International Narcotics Control Board. Narcotic drugs: Report 2016: Estimated world requirements for 2017-statistics for 2015. New York, NY. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/Narcotic_Drugs_Publication_2016.pdf. Published 2017. Accessed January 7, 2018.
- Centers for Disease Control and Prevention. Opioid painkiller prescribing: Where you live makes a difference. Atlanta, GA. https://www.cdc.gov/vitalsigns/pdf/2014-07-vitalsigns.pdf. Published July 2014. Accessed January 5, 2018.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625.
- Van Zee A. The promotion and marketing of OxyContin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221–227.
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445–1452.
- US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2015 national survey on drug use and health: Detailed tables. Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm. Published 2016. Accessed January 5, 2018.
- Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. http://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm. Published August 2013. Accessed January 5, 2018.
- Joshi GP. Multimodal analgesia techniques and postoperative rehabilitation. Anesthesiol Clin North America. 2005;23(1):185–202.
- Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 suppl):6S–11S.
- Brownstein, MJ. A brief history of opiates, opioid peptides, and opioid receptors. Proc Natl Acad Sci USA. 1993;90(12):5391–5393.
- US Food and Drug Administration. Acetaminophen. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm165107.htm. Published November 2017. Accessed January 7, 2018.
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal anti-inflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110(4):1170–1179.
- Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications included in the use of high‐risk medications in the elderly and potentially harmful drug–disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18.
- Joshi GP, Janis JE, Haas EM, et al. Surgical site infiltration for abdominal surgery: A novel neuroanatomical-based approach. Plast Reconstr Surg Glob Open. 2016;4(12):e1181. https://insights.ovid.com/crossref?an=01720096-201612000-00021. Accessed January 5, 2018.
- Jokela RM, Ahonen JV, Tallgren MK, et al. The effective analgesic dose of dexamethasone after laparoscopic hysterectomy. Anesth Analg. 2009;109(2):607–615.
- Hohlrieder M, Brimacombe J, Eschertzhuber S, et al. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007;62(9):913–918.
- Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy-induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155–1160.
- Sammour T, Kahokehr A, Hill AG. Meta‐analysis of the effect of warm humidified insufflation on pain after laparoscopy. Br J Surg. 2008;95(8):950–956.
- Reagan KM, O’Sullivan DM, Gannon R, Steinberg AC. Decreasing postoperative narcotics in reconstructive pelvic surgery; A randomized controlled trial. Am J Obstet Gynecol. 2017;217(3):325.e1–e10.
- Khan U, Bloom RA, Nicell JA, Laurenson JP. Risks associated with the environmental release of pharmaceuticals on the U.S. Food and Drug Administration “flush list”. Sci Total Environ. 2017;609:1023–1040.