User login
Preventing sepsis alert fatigue
If they’re too infrequent, alerts can delay sepsis identification and treatment. If they’re too abundant, the alerts can overwhelm providers. Finding the sweet spot for sepsis alerts, QI leaders say, can require time, technology, patience – and sometimes trial and error.
University Hospital in Salt Lake City wanted to broaden its sepsis recognition system to ensure that decompensating patients were seen and resuscitated quickly, regardless of the cause. Another hospital offered a lesson in what not to do when a staff member cautioned that a sepsis alert system based on SIRS alone had been a “total disaster” and left providers fuming. One report suggested that nearly half of all ward patients meet SIRS criteria at some point during their hospitalization, and that using the criteria for sepsis screening in hospital wards is both “time consuming and impractical.”1
Instead, University Hospital tweaked its MEWS or Modified Early Warning System, based on consultations with hospitalists, ICU physicians, and other providers about the appropriate thresholds for vital signs. “It’s kind of like asking someone, ‘Well, when are you really scared of the heart rate and when are you sort of scared and when are you not scared at all?’ ” said project coleader Devin J. Horton, MD, an academic hospitalist.
The team also analyzed the number of alerts per week per unit and their sensitivity and specificity in detecting sepsis. As junior faculty members, Dr. Horton and his collaborator, academic hospitalist Kencee K. Graves, MD, were mindful to avoid angering other doctors over being alerted too often. For their MEWS scoring system, they sacrificed a bit of sensitivity to ensure that the number of alerts remained manageable.
Before going live with its own new alert system, Middlesex Hospital in Middletown, Conn., had a subgroup spend several weeks testing the system in silent mode and tweaking different parameters such as respiratory rate and heart rate to reduce the potential for too many alerts. “If you look at each and every alert, then you can identify how to make your adjustment so that it’s not overly sensitive,” said Terri Savino, MSN, RN, CPHQ, the hospital’s manager of patient experience and service excellence.
A sepsis task force also shared data showing the hospital’s significant reductions in sepsis mortality, total hospital mortality, and sepsis length of stay. “Medical staff were willing to accept the frequency and high sensitivity of the alert because the data demonstrated that it was making a difference in the lives of our patients,” said David M. Cosentino, MD, the hospital’s chief medical information officer.
Other alert systems’ mixed performances have yielded important lessons. At the University of Pennsylvania, Philadelphia, one prototype detected clinically deteriorating patients and sent an alert to the nurse, physician, and a rapid response team. Alerted providers converged on the patient’s bedside within 30 minutes and decided whether to elevate the level of care. Craig Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine, said the system was associated with a suggestion of reduced mortality.2 But it was noisy and less helpful than it could have been, he said, because it didn’t separate out declining patients already known to the team from those who were still unrecognized.
Tools to predict which patients may develop severe sepsis or septic shock have worked even less well, he said. One triggered an alarm before patients showed signs of clinical deterioration. “The team didn’t know what to do with that prediction,” Dr. Umscheid said. As a result, the alert didn’t improve mortality or discharges to home. “If you’re making this prediction too early and providers don’t know what to do with the information, it’s not going to change care or affect patient outcomes,” he said. “It’s just going to frustrate providers.”
References
1. Churpek MM et al. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015 Oct 15;192(8):958-64.
2. Umscheid CA et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med. 2015 Jan;10: 26-31.
If they’re too infrequent, alerts can delay sepsis identification and treatment. If they’re too abundant, the alerts can overwhelm providers. Finding the sweet spot for sepsis alerts, QI leaders say, can require time, technology, patience – and sometimes trial and error.
University Hospital in Salt Lake City wanted to broaden its sepsis recognition system to ensure that decompensating patients were seen and resuscitated quickly, regardless of the cause. Another hospital offered a lesson in what not to do when a staff member cautioned that a sepsis alert system based on SIRS alone had been a “total disaster” and left providers fuming. One report suggested that nearly half of all ward patients meet SIRS criteria at some point during their hospitalization, and that using the criteria for sepsis screening in hospital wards is both “time consuming and impractical.”1
Instead, University Hospital tweaked its MEWS or Modified Early Warning System, based on consultations with hospitalists, ICU physicians, and other providers about the appropriate thresholds for vital signs. “It’s kind of like asking someone, ‘Well, when are you really scared of the heart rate and when are you sort of scared and when are you not scared at all?’ ” said project coleader Devin J. Horton, MD, an academic hospitalist.
The team also analyzed the number of alerts per week per unit and their sensitivity and specificity in detecting sepsis. As junior faculty members, Dr. Horton and his collaborator, academic hospitalist Kencee K. Graves, MD, were mindful to avoid angering other doctors over being alerted too often. For their MEWS scoring system, they sacrificed a bit of sensitivity to ensure that the number of alerts remained manageable.
Before going live with its own new alert system, Middlesex Hospital in Middletown, Conn., had a subgroup spend several weeks testing the system in silent mode and tweaking different parameters such as respiratory rate and heart rate to reduce the potential for too many alerts. “If you look at each and every alert, then you can identify how to make your adjustment so that it’s not overly sensitive,” said Terri Savino, MSN, RN, CPHQ, the hospital’s manager of patient experience and service excellence.
A sepsis task force also shared data showing the hospital’s significant reductions in sepsis mortality, total hospital mortality, and sepsis length of stay. “Medical staff were willing to accept the frequency and high sensitivity of the alert because the data demonstrated that it was making a difference in the lives of our patients,” said David M. Cosentino, MD, the hospital’s chief medical information officer.
Other alert systems’ mixed performances have yielded important lessons. At the University of Pennsylvania, Philadelphia, one prototype detected clinically deteriorating patients and sent an alert to the nurse, physician, and a rapid response team. Alerted providers converged on the patient’s bedside within 30 minutes and decided whether to elevate the level of care. Craig Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine, said the system was associated with a suggestion of reduced mortality.2 But it was noisy and less helpful than it could have been, he said, because it didn’t separate out declining patients already known to the team from those who were still unrecognized.
Tools to predict which patients may develop severe sepsis or septic shock have worked even less well, he said. One triggered an alarm before patients showed signs of clinical deterioration. “The team didn’t know what to do with that prediction,” Dr. Umscheid said. As a result, the alert didn’t improve mortality or discharges to home. “If you’re making this prediction too early and providers don’t know what to do with the information, it’s not going to change care or affect patient outcomes,” he said. “It’s just going to frustrate providers.”
References
1. Churpek MM et al. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015 Oct 15;192(8):958-64.
2. Umscheid CA et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med. 2015 Jan;10: 26-31.
If they’re too infrequent, alerts can delay sepsis identification and treatment. If they’re too abundant, the alerts can overwhelm providers. Finding the sweet spot for sepsis alerts, QI leaders say, can require time, technology, patience – and sometimes trial and error.
University Hospital in Salt Lake City wanted to broaden its sepsis recognition system to ensure that decompensating patients were seen and resuscitated quickly, regardless of the cause. Another hospital offered a lesson in what not to do when a staff member cautioned that a sepsis alert system based on SIRS alone had been a “total disaster” and left providers fuming. One report suggested that nearly half of all ward patients meet SIRS criteria at some point during their hospitalization, and that using the criteria for sepsis screening in hospital wards is both “time consuming and impractical.”1
Instead, University Hospital tweaked its MEWS or Modified Early Warning System, based on consultations with hospitalists, ICU physicians, and other providers about the appropriate thresholds for vital signs. “It’s kind of like asking someone, ‘Well, when are you really scared of the heart rate and when are you sort of scared and when are you not scared at all?’ ” said project coleader Devin J. Horton, MD, an academic hospitalist.
The team also analyzed the number of alerts per week per unit and their sensitivity and specificity in detecting sepsis. As junior faculty members, Dr. Horton and his collaborator, academic hospitalist Kencee K. Graves, MD, were mindful to avoid angering other doctors over being alerted too often. For their MEWS scoring system, they sacrificed a bit of sensitivity to ensure that the number of alerts remained manageable.
Before going live with its own new alert system, Middlesex Hospital in Middletown, Conn., had a subgroup spend several weeks testing the system in silent mode and tweaking different parameters such as respiratory rate and heart rate to reduce the potential for too many alerts. “If you look at each and every alert, then you can identify how to make your adjustment so that it’s not overly sensitive,” said Terri Savino, MSN, RN, CPHQ, the hospital’s manager of patient experience and service excellence.
A sepsis task force also shared data showing the hospital’s significant reductions in sepsis mortality, total hospital mortality, and sepsis length of stay. “Medical staff were willing to accept the frequency and high sensitivity of the alert because the data demonstrated that it was making a difference in the lives of our patients,” said David M. Cosentino, MD, the hospital’s chief medical information officer.
Other alert systems’ mixed performances have yielded important lessons. At the University of Pennsylvania, Philadelphia, one prototype detected clinically deteriorating patients and sent an alert to the nurse, physician, and a rapid response team. Alerted providers converged on the patient’s bedside within 30 minutes and decided whether to elevate the level of care. Craig Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine, said the system was associated with a suggestion of reduced mortality.2 But it was noisy and less helpful than it could have been, he said, because it didn’t separate out declining patients already known to the team from those who were still unrecognized.
Tools to predict which patients may develop severe sepsis or septic shock have worked even less well, he said. One triggered an alarm before patients showed signs of clinical deterioration. “The team didn’t know what to do with that prediction,” Dr. Umscheid said. As a result, the alert didn’t improve mortality or discharges to home. “If you’re making this prediction too early and providers don’t know what to do with the information, it’s not going to change care or affect patient outcomes,” he said. “It’s just going to frustrate providers.”
References
1. Churpek MM et al. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015 Oct 15;192(8):958-64.
2. Umscheid CA et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med. 2015 Jan;10: 26-31.
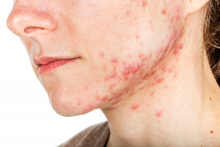
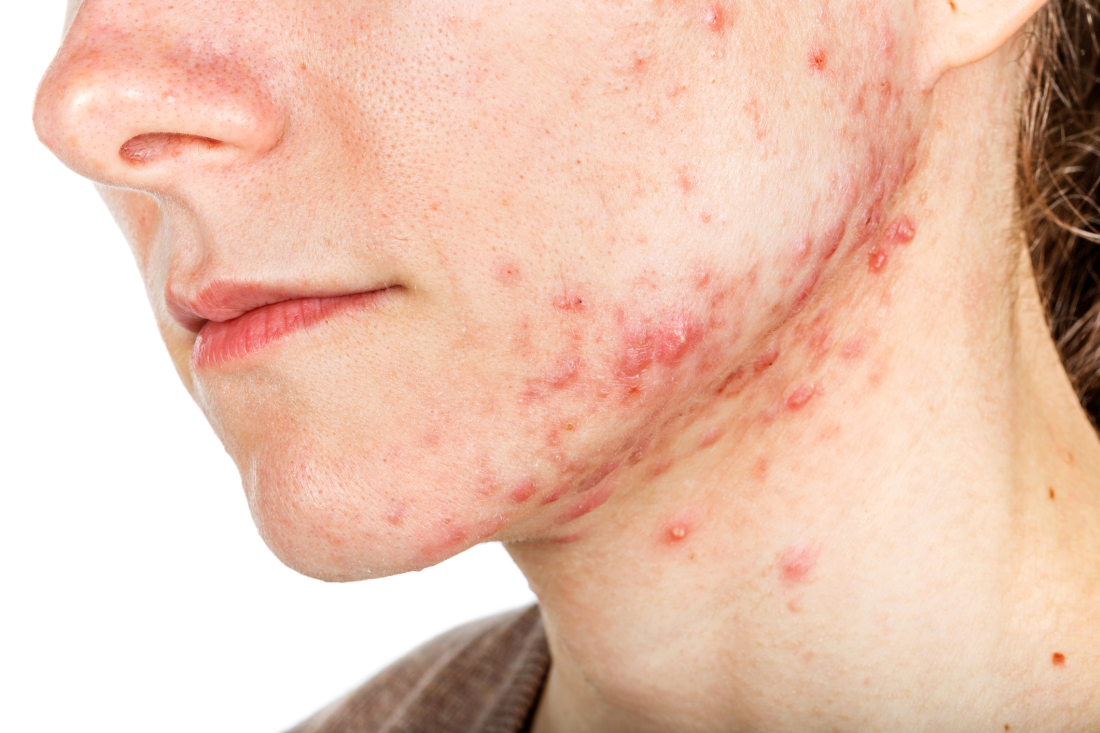
Women with acne more likely to have hyperkeratinization, study finds
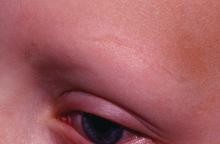
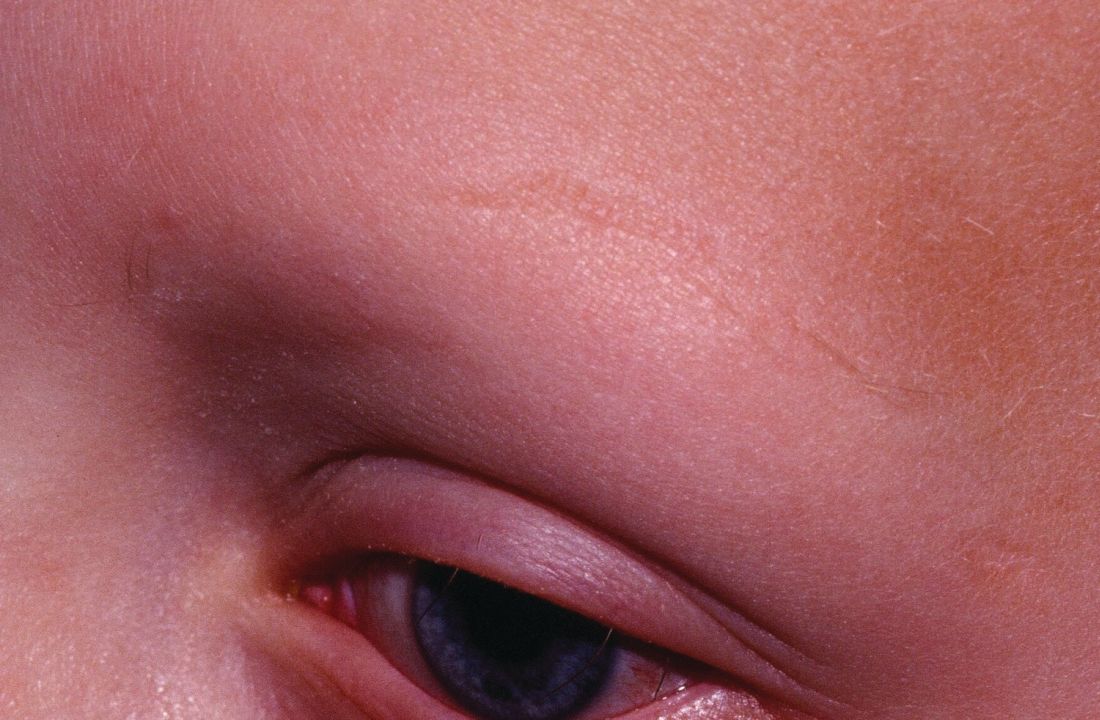
The finding was seen in women with both types of acne – diffuse and mandibular – and in patients with both inflammatory and hyperkeratinization features, wrote Louise M. Guenot, MD, of the dermatology department at CHU Nantes, France, and her coauthors. In the women with mandibular acne, there were significantly more follicle abnormalities on the mandibular area of the face, compared with the forehead area, with no acne lesions.
The investigators evaluated two groups of women: 15 women with diffuse acne lesions across the forehead, cheeks, and chin (mean age 38 years) and 15 women with mandibular acne only (mean age 31 years). Skin that appeared to be acne free among those in the diffuse acne group was compared with the skin of a control group of six women with no acne history. In the mandibular acne group, patients served as their own controls: Healthy forehead skin was compared with mandibular skin. In the diffuse acne group, 791 forehead follicles were evaluated, and in the mandibular group, 569 mandible follicles and 475 forehead follicles were evaluated.
The investigators studied several features, including diameter of the infundibulum, thickness of the border, onion-like appearance, keratin plugs, signs of inflammation, vascularization, and presence of Demodex mites.
In the diffuse acne group, infundibulum was larger, compared with controls (94.9 mcm vs. 74.9 mcm; P = .047). The follicle border was thicker, was more hyperkeratinized, and had more keratin plugs in the infundibulum compared with controls, all statistically significant differences. Signs of inflammation, vascularization, and the presence of Demodex mites did not differ significantly between those with acne and controls.
In the mandibular acne group, where each patient served as her own control, follicles in the mandibular area were larger than those on the forehead (99 mcm vs. 91 mcm, P = .03), and the follicle border in the mandibular area was significantly thicker, with an “onion-like hyperkeratinized appearance” that was significantly more common in the mandibular area. In addition, images showed a keratin plug in the infundibulum was present more often in the follicles in the mandibular area compared with the forehead (46.7% vs. 36.8%, P = .002). Inflammation and vascularization were also significantly more common in the mandibular area compared with the forehead.
Among controls, though, no differences in infundibulum diameter, follicle thickness, or quantity of keratin plugs were seen on the forehead compared with the mandibular area. There was no more onion-like appearance or keratin plugs on the forehead than in the mandibular area, Dr. Guenot and her coauthors reported. Their findings show that “the follicle morphology on apparently healthy skin of adult women with acne was significantly different from that of follicles on the normal skin of healthy subjects.”
The results also “support the concept that the management of acne with mandibular involvement, even if the main clinical lesions are inflammatory, should take into account the hyperkeratinization process, and the treatment should thus be both comedolytic and anti-inflammatory,” they added.
The authors had no financial disclosures, and there was no funding source.
SOURCE: Guenot et al. Int J Dermatol. 2018 Jan 25. doi: 10.1111/ijd.13910.
The finding was seen in women with both types of acne – diffuse and mandibular – and in patients with both inflammatory and hyperkeratinization features, wrote Louise M. Guenot, MD, of the dermatology department at CHU Nantes, France, and her coauthors. In the women with mandibular acne, there were significantly more follicle abnormalities on the mandibular area of the face, compared with the forehead area, with no acne lesions.
The investigators evaluated two groups of women: 15 women with diffuse acne lesions across the forehead, cheeks, and chin (mean age 38 years) and 15 women with mandibular acne only (mean age 31 years). Skin that appeared to be acne free among those in the diffuse acne group was compared with the skin of a control group of six women with no acne history. In the mandibular acne group, patients served as their own controls: Healthy forehead skin was compared with mandibular skin. In the diffuse acne group, 791 forehead follicles were evaluated, and in the mandibular group, 569 mandible follicles and 475 forehead follicles were evaluated.
The investigators studied several features, including diameter of the infundibulum, thickness of the border, onion-like appearance, keratin plugs, signs of inflammation, vascularization, and presence of Demodex mites.
In the diffuse acne group, infundibulum was larger, compared with controls (94.9 mcm vs. 74.9 mcm; P = .047). The follicle border was thicker, was more hyperkeratinized, and had more keratin plugs in the infundibulum compared with controls, all statistically significant differences. Signs of inflammation, vascularization, and the presence of Demodex mites did not differ significantly between those with acne and controls.
In the mandibular acne group, where each patient served as her own control, follicles in the mandibular area were larger than those on the forehead (99 mcm vs. 91 mcm, P = .03), and the follicle border in the mandibular area was significantly thicker, with an “onion-like hyperkeratinized appearance” that was significantly more common in the mandibular area. In addition, images showed a keratin plug in the infundibulum was present more often in the follicles in the mandibular area compared with the forehead (46.7% vs. 36.8%, P = .002). Inflammation and vascularization were also significantly more common in the mandibular area compared with the forehead.
Among controls, though, no differences in infundibulum diameter, follicle thickness, or quantity of keratin plugs were seen on the forehead compared with the mandibular area. There was no more onion-like appearance or keratin plugs on the forehead than in the mandibular area, Dr. Guenot and her coauthors reported. Their findings show that “the follicle morphology on apparently healthy skin of adult women with acne was significantly different from that of follicles on the normal skin of healthy subjects.”
The results also “support the concept that the management of acne with mandibular involvement, even if the main clinical lesions are inflammatory, should take into account the hyperkeratinization process, and the treatment should thus be both comedolytic and anti-inflammatory,” they added.
The authors had no financial disclosures, and there was no funding source.
SOURCE: Guenot et al. Int J Dermatol. 2018 Jan 25. doi: 10.1111/ijd.13910.
The finding was seen in women with both types of acne – diffuse and mandibular – and in patients with both inflammatory and hyperkeratinization features, wrote Louise M. Guenot, MD, of the dermatology department at CHU Nantes, France, and her coauthors. In the women with mandibular acne, there were significantly more follicle abnormalities on the mandibular area of the face, compared with the forehead area, with no acne lesions.
The investigators evaluated two groups of women: 15 women with diffuse acne lesions across the forehead, cheeks, and chin (mean age 38 years) and 15 women with mandibular acne only (mean age 31 years). Skin that appeared to be acne free among those in the diffuse acne group was compared with the skin of a control group of six women with no acne history. In the mandibular acne group, patients served as their own controls: Healthy forehead skin was compared with mandibular skin. In the diffuse acne group, 791 forehead follicles were evaluated, and in the mandibular group, 569 mandible follicles and 475 forehead follicles were evaluated.
The investigators studied several features, including diameter of the infundibulum, thickness of the border, onion-like appearance, keratin plugs, signs of inflammation, vascularization, and presence of Demodex mites.
In the diffuse acne group, infundibulum was larger, compared with controls (94.9 mcm vs. 74.9 mcm; P = .047). The follicle border was thicker, was more hyperkeratinized, and had more keratin plugs in the infundibulum compared with controls, all statistically significant differences. Signs of inflammation, vascularization, and the presence of Demodex mites did not differ significantly between those with acne and controls.
In the mandibular acne group, where each patient served as her own control, follicles in the mandibular area were larger than those on the forehead (99 mcm vs. 91 mcm, P = .03), and the follicle border in the mandibular area was significantly thicker, with an “onion-like hyperkeratinized appearance” that was significantly more common in the mandibular area. In addition, images showed a keratin plug in the infundibulum was present more often in the follicles in the mandibular area compared with the forehead (46.7% vs. 36.8%, P = .002). Inflammation and vascularization were also significantly more common in the mandibular area compared with the forehead.
Among controls, though, no differences in infundibulum diameter, follicle thickness, or quantity of keratin plugs were seen on the forehead compared with the mandibular area. There was no more onion-like appearance or keratin plugs on the forehead than in the mandibular area, Dr. Guenot and her coauthors reported. Their findings show that “the follicle morphology on apparently healthy skin of adult women with acne was significantly different from that of follicles on the normal skin of healthy subjects.”
The results also “support the concept that the management of acne with mandibular involvement, even if the main clinical lesions are inflammatory, should take into account the hyperkeratinization process, and the treatment should thus be both comedolytic and anti-inflammatory,” they added.
The authors had no financial disclosures, and there was no funding source.
SOURCE: Guenot et al. Int J Dermatol. 2018 Jan 25. doi: 10.1111/ijd.13910.
FROM THE INTERNATIONAL JOURNAL OF DERMATOLOGY
Key clinical point: Imaging performed with confocal microscopy showed hyperkeratinization and other abnormalities in healthy-looking skin of acne patients.
Major finding: Follicle abnormalities were significantly more common in the healthy appearing skin of adult women with acne, compared with the skin of controls with no acne.
Data source: A study evaluated skin structures with confocal microscopy in 15 women with diffuse acne and 15 with mandibular acne.
Disclosures: The authors had no financial disclosures, and there was no funding source.
Source: Guenot LM et al. Int J Dermatol. 2018 Jan 25. doi: 10.1111/ijd.13910.
Children’s behavioral problems tied to mothers’ postpartum depression
Persistent and severe postpartum depression in mothers may be associated with behavioral problems, poor mathematics grades, and a higher risk of depression in their offspring, research published Jan. 31 in JAMA Psychiatry showed.
In the study, Elena Netsi, DPhil, and her associates presented an analysis of data from 9,848 mothers and 8,287 children enrolled in the observational Avon Longitudinal Study of Parents and Children.
This analysis revealed that postpartum depression of any severity or level of persistence was associated with a two- to fourfold increase in the risk of children showing behavioral problems at age 3.5 years. In women with marked but not persistent postpartum depression, the odds ratio for child behavioral disturbance was 1.91, in mothers with severe but not persistent depression it was 2.39, and in mothers with severe persistent depression, the odds ratio was 4.84 – all of which were highly significant (P less than .001).
However, the authors reported.
It was associated with a 2.65-fold increase in the likelihood of a child having mathematics grades of D or below (P = .01), and a more than sevenfold increase in the prevalence of depression in offspring at 18 years (P less than .001). There also was a 2.3-fold increase in depression at 18 years in the children of mothers who experienced marked but not persistent postpartum depression (P = .04).
Dr. Netsi of the department of psychiatry at the University of Oxford (England) and her coauthors noted that, in women with persistent postpartum depression, mean Edinburgh Postpartum Depression Scale scores remained relatively stable, from 21 months to 11 years, suggesting an increased risk for prolonged depression.
“Identification of women with [postpartum depression] may be associated with increased treatment costs, but the overall cost to the public sector of perinatal mental health problems is five times more than the cost of improving services, further highlighting that early intervention and effective treatment of perinatal depression are a public health priority,” they wrote.
However, they acknowledged that there were mixed findings in the literature with respect to the impact on child outcomes of treating maternal depression.
“Treatments for [postpartum depression] have been relatively brief in duration and moderate in intensity; therefore, it is perhaps unsurprising that such interventions have not shown long-term benefits for either the mother or the child,” the investigators wrote.
The Avon Longitudinal Study of Parents and Children is supported by the U.K. Medical Research Council, the Wellcome Trust, and the University of Bristol. This study was supported by the Wellcome Trust, the National Institute for Health Research Biomedical Research Centre, the University of Bristol, and the NIHR Oxford Health Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Netsi E et al. JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4363.
This study raises new and interesting questions about the clinical effects of maternal depression on offspring, and identifies a particularly vulnerable group of mothers and their offspring. If anything, the findings are likely an underestimation of the true effect, because of high rates of study attrition, especially within the most vulnerable group.
However, this does not answer the question of what interventions to use, whom to treat, when to do so, and how to treat. Some would argue that clinicians should treat maternal depression first, as a woman with acute depression needs care before she can be helped with parenting. Others suggest that a better approach is to engage the unique mother-infant experience, as this can be a positive therapeutic interaction in itself.
Whatever the approach, the treatment of maternal depression should be evidence based and available early, particularly in new mothers with persistent depression.
Myrna M. Weissman, PhD, is affiliated with the division of epidemiology at Columbia University, New York. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4265). Dr. Weissman declared funding from a variety of funding bodies and publishing companies outside the submitted work.
This study raises new and interesting questions about the clinical effects of maternal depression on offspring, and identifies a particularly vulnerable group of mothers and their offspring. If anything, the findings are likely an underestimation of the true effect, because of high rates of study attrition, especially within the most vulnerable group.
However, this does not answer the question of what interventions to use, whom to treat, when to do so, and how to treat. Some would argue that clinicians should treat maternal depression first, as a woman with acute depression needs care before she can be helped with parenting. Others suggest that a better approach is to engage the unique mother-infant experience, as this can be a positive therapeutic interaction in itself.
Whatever the approach, the treatment of maternal depression should be evidence based and available early, particularly in new mothers with persistent depression.
Myrna M. Weissman, PhD, is affiliated with the division of epidemiology at Columbia University, New York. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4265). Dr. Weissman declared funding from a variety of funding bodies and publishing companies outside the submitted work.
This study raises new and interesting questions about the clinical effects of maternal depression on offspring, and identifies a particularly vulnerable group of mothers and their offspring. If anything, the findings are likely an underestimation of the true effect, because of high rates of study attrition, especially within the most vulnerable group.
However, this does not answer the question of what interventions to use, whom to treat, when to do so, and how to treat. Some would argue that clinicians should treat maternal depression first, as a woman with acute depression needs care before she can be helped with parenting. Others suggest that a better approach is to engage the unique mother-infant experience, as this can be a positive therapeutic interaction in itself.
Whatever the approach, the treatment of maternal depression should be evidence based and available early, particularly in new mothers with persistent depression.
Myrna M. Weissman, PhD, is affiliated with the division of epidemiology at Columbia University, New York. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4265). Dr. Weissman declared funding from a variety of funding bodies and publishing companies outside the submitted work.
Persistent and severe postpartum depression in mothers may be associated with behavioral problems, poor mathematics grades, and a higher risk of depression in their offspring, research published Jan. 31 in JAMA Psychiatry showed.
In the study, Elena Netsi, DPhil, and her associates presented an analysis of data from 9,848 mothers and 8,287 children enrolled in the observational Avon Longitudinal Study of Parents and Children.
This analysis revealed that postpartum depression of any severity or level of persistence was associated with a two- to fourfold increase in the risk of children showing behavioral problems at age 3.5 years. In women with marked but not persistent postpartum depression, the odds ratio for child behavioral disturbance was 1.91, in mothers with severe but not persistent depression it was 2.39, and in mothers with severe persistent depression, the odds ratio was 4.84 – all of which were highly significant (P less than .001).
However, the authors reported.
It was associated with a 2.65-fold increase in the likelihood of a child having mathematics grades of D or below (P = .01), and a more than sevenfold increase in the prevalence of depression in offspring at 18 years (P less than .001). There also was a 2.3-fold increase in depression at 18 years in the children of mothers who experienced marked but not persistent postpartum depression (P = .04).
Dr. Netsi of the department of psychiatry at the University of Oxford (England) and her coauthors noted that, in women with persistent postpartum depression, mean Edinburgh Postpartum Depression Scale scores remained relatively stable, from 21 months to 11 years, suggesting an increased risk for prolonged depression.
“Identification of women with [postpartum depression] may be associated with increased treatment costs, but the overall cost to the public sector of perinatal mental health problems is five times more than the cost of improving services, further highlighting that early intervention and effective treatment of perinatal depression are a public health priority,” they wrote.
However, they acknowledged that there were mixed findings in the literature with respect to the impact on child outcomes of treating maternal depression.
“Treatments for [postpartum depression] have been relatively brief in duration and moderate in intensity; therefore, it is perhaps unsurprising that such interventions have not shown long-term benefits for either the mother or the child,” the investigators wrote.
The Avon Longitudinal Study of Parents and Children is supported by the U.K. Medical Research Council, the Wellcome Trust, and the University of Bristol. This study was supported by the Wellcome Trust, the National Institute for Health Research Biomedical Research Centre, the University of Bristol, and the NIHR Oxford Health Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Netsi E et al. JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4363.
Persistent and severe postpartum depression in mothers may be associated with behavioral problems, poor mathematics grades, and a higher risk of depression in their offspring, research published Jan. 31 in JAMA Psychiatry showed.
In the study, Elena Netsi, DPhil, and her associates presented an analysis of data from 9,848 mothers and 8,287 children enrolled in the observational Avon Longitudinal Study of Parents and Children.
This analysis revealed that postpartum depression of any severity or level of persistence was associated with a two- to fourfold increase in the risk of children showing behavioral problems at age 3.5 years. In women with marked but not persistent postpartum depression, the odds ratio for child behavioral disturbance was 1.91, in mothers with severe but not persistent depression it was 2.39, and in mothers with severe persistent depression, the odds ratio was 4.84 – all of which were highly significant (P less than .001).
However, the authors reported.
It was associated with a 2.65-fold increase in the likelihood of a child having mathematics grades of D or below (P = .01), and a more than sevenfold increase in the prevalence of depression in offspring at 18 years (P less than .001). There also was a 2.3-fold increase in depression at 18 years in the children of mothers who experienced marked but not persistent postpartum depression (P = .04).
Dr. Netsi of the department of psychiatry at the University of Oxford (England) and her coauthors noted that, in women with persistent postpartum depression, mean Edinburgh Postpartum Depression Scale scores remained relatively stable, from 21 months to 11 years, suggesting an increased risk for prolonged depression.
“Identification of women with [postpartum depression] may be associated with increased treatment costs, but the overall cost to the public sector of perinatal mental health problems is five times more than the cost of improving services, further highlighting that early intervention and effective treatment of perinatal depression are a public health priority,” they wrote.
However, they acknowledged that there were mixed findings in the literature with respect to the impact on child outcomes of treating maternal depression.
“Treatments for [postpartum depression] have been relatively brief in duration and moderate in intensity; therefore, it is perhaps unsurprising that such interventions have not shown long-term benefits for either the mother or the child,” the investigators wrote.
The Avon Longitudinal Study of Parents and Children is supported by the U.K. Medical Research Council, the Wellcome Trust, and the University of Bristol. This study was supported by the Wellcome Trust, the National Institute for Health Research Biomedical Research Centre, the University of Bristol, and the NIHR Oxford Health Biomedical Research Centre. No conflicts of interest were declared.
SOURCE: Netsi E et al. JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4363.
FROM JAMA PSYCHIATRY
Key clinical point: Persistent and severe postpartum depression in mothers is associated with behavioral problems, poor mathematics grades, and a higher risk of depression in their offspring.
Major finding: Maternal postpartum depression of any severity or level of persistence was associated with a two- to fourfold increase in the risk of children showing behavioral problems at age 3.5 years.
Data source: An analysis of data from 9,848 mothers and 8,287 children enrolled in the observational Avon Longitudinal Study of Parents and Children.
Disclosures: The Avon Longitudinal Study of Parents and Children is supported by the U.K. Medical Research Council, the Wellcome Trust, and the University of Bristol. This study was supported by the Wellcome Trust, the National Institute for Health Research Biomedical Research Centre, the University of Bristol, and the NIHR Oxford Health Biomedical Research Centre. No conflicts of interest were declared.
Source: Netsi E et al. JAMA Psychiatry. 2018 Jan 31. doi: 10.1001/jamapsychiatry.2017.4363.
Turmeric-, frankincense-derived supplement shows OA benefit
A combination of curcumin extracted from the turmeric rhizome and boswellic acid extracted from Indian frankincense root beat placebo for reducing pain-related symptoms from knee osteoarthritis in a 12-week clinical trial from Armenia with 201 patients 40-70 years old.
The combination (Curamin) also beat a standalone curcumin preparation (CuraMed), according to a report in BMC Complementary and Alternative Medicine.
It appears that combining the two “increases the efficacy of treatment of OA presumably due to synergistic effects of curcumin and boswellic acid”; it’s also possible that boswellic acid increases curcumin bioavailability, said investigators led by Armine Haroyan, PhD, head of rheumatology at Erebuni Medical Center in Yerevan, Armenia.
The subjects were randomized evenly to the combination, curcumin alone, or placebo, all in 500-mg capsules taken three times daily. They had been diagnosed with degenerative hypertrophic OA of knee bone joints.
At the end of 12 weeks, patients on the combination outperformed those on placebo in physical performance tests and joint pain scores; curcumin outperformed placebo in only a few of the physical tests.
For instance, patients on the combination were a mean of 2.03 seconds quicker than baseline in a stair-climbing exercise by the end of the study, versus 0.22 seconds in the placebo group and 1.66 seconds in the curcumin group. Combination patients had a mean 7.38-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index, versus 2.26 points in the placebo arm and 6.34 points in the curcumin group. The differences versus placebo were statistically significant for the combination, but not for stand-alone curcumin.
The treatments were well tolerated, with only a few patients in each arm reporting nausea, gastroesophageal reflux, and similar problems.
The work was funded in part by EuroPharma USA, maker of the supplements. The authors said they had no competing interests.
SOURCE: Haroyan A et al. BMC Complement Altern Med. 2018 Jan 9;18:7. doi: 10.1186/s12906-017-2062-z
A combination of curcumin extracted from the turmeric rhizome and boswellic acid extracted from Indian frankincense root beat placebo for reducing pain-related symptoms from knee osteoarthritis in a 12-week clinical trial from Armenia with 201 patients 40-70 years old.
The combination (Curamin) also beat a standalone curcumin preparation (CuraMed), according to a report in BMC Complementary and Alternative Medicine.
It appears that combining the two “increases the efficacy of treatment of OA presumably due to synergistic effects of curcumin and boswellic acid”; it’s also possible that boswellic acid increases curcumin bioavailability, said investigators led by Armine Haroyan, PhD, head of rheumatology at Erebuni Medical Center in Yerevan, Armenia.
The subjects were randomized evenly to the combination, curcumin alone, or placebo, all in 500-mg capsules taken three times daily. They had been diagnosed with degenerative hypertrophic OA of knee bone joints.
At the end of 12 weeks, patients on the combination outperformed those on placebo in physical performance tests and joint pain scores; curcumin outperformed placebo in only a few of the physical tests.
For instance, patients on the combination were a mean of 2.03 seconds quicker than baseline in a stair-climbing exercise by the end of the study, versus 0.22 seconds in the placebo group and 1.66 seconds in the curcumin group. Combination patients had a mean 7.38-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index, versus 2.26 points in the placebo arm and 6.34 points in the curcumin group. The differences versus placebo were statistically significant for the combination, but not for stand-alone curcumin.
The treatments were well tolerated, with only a few patients in each arm reporting nausea, gastroesophageal reflux, and similar problems.
The work was funded in part by EuroPharma USA, maker of the supplements. The authors said they had no competing interests.
SOURCE: Haroyan A et al. BMC Complement Altern Med. 2018 Jan 9;18:7. doi: 10.1186/s12906-017-2062-z
A combination of curcumin extracted from the turmeric rhizome and boswellic acid extracted from Indian frankincense root beat placebo for reducing pain-related symptoms from knee osteoarthritis in a 12-week clinical trial from Armenia with 201 patients 40-70 years old.
The combination (Curamin) also beat a standalone curcumin preparation (CuraMed), according to a report in BMC Complementary and Alternative Medicine.
It appears that combining the two “increases the efficacy of treatment of OA presumably due to synergistic effects of curcumin and boswellic acid”; it’s also possible that boswellic acid increases curcumin bioavailability, said investigators led by Armine Haroyan, PhD, head of rheumatology at Erebuni Medical Center in Yerevan, Armenia.
The subjects were randomized evenly to the combination, curcumin alone, or placebo, all in 500-mg capsules taken three times daily. They had been diagnosed with degenerative hypertrophic OA of knee bone joints.
At the end of 12 weeks, patients on the combination outperformed those on placebo in physical performance tests and joint pain scores; curcumin outperformed placebo in only a few of the physical tests.
For instance, patients on the combination were a mean of 2.03 seconds quicker than baseline in a stair-climbing exercise by the end of the study, versus 0.22 seconds in the placebo group and 1.66 seconds in the curcumin group. Combination patients had a mean 7.38-point improvement on the Western Ontario and McMaster Universities Osteoarthritis Index, versus 2.26 points in the placebo arm and 6.34 points in the curcumin group. The differences versus placebo were statistically significant for the combination, but not for stand-alone curcumin.
The treatments were well tolerated, with only a few patients in each arm reporting nausea, gastroesophageal reflux, and similar problems.
The work was funded in part by EuroPharma USA, maker of the supplements. The authors said they had no competing interests.
SOURCE: Haroyan A et al. BMC Complement Altern Med. 2018 Jan 9;18:7. doi: 10.1186/s12906-017-2062-z
FROM BMC COMPLEMENTARY AND ALTERNATIVE MEDICINE
Cosmetic Corner: Dermatologists Weigh in on Bar Soaps
To improve patient care and outcomes, leading dermatologists offered their recommendations on bar soaps. Consideration must be given to:
- Avène Cold Cream Ultra-Rich Cleansing Bar
Pierre Fabre Dermo-Cosmetique USA
“This gentle cleansing bar is not only hypoallergenic, soap free, and lanolin free, it also has Avène’s soothing Thermal Spring Water, plus white beeswax and a noncomedogenic, pharmaceutical-grade paraffin oil to protect the skin.”—Jeannette Graf, MD, Great Neck, New York
- Hydrating Cleanser Bar
CeraVe
Recommended by Shari Lipner, MD, PhD, New York, New York
- Vanicream Cleansing Bar
Pharmaceutical Specialties, Inc
“This is a great option for patients with eczema or dry, sensitive skin.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Lip plumpers, shaving lotions for men, and night creams will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on bar soaps. Consideration must be given to:
- Avène Cold Cream Ultra-Rich Cleansing Bar
Pierre Fabre Dermo-Cosmetique USA
“This gentle cleansing bar is not only hypoallergenic, soap free, and lanolin free, it also has Avène’s soothing Thermal Spring Water, plus white beeswax and a noncomedogenic, pharmaceutical-grade paraffin oil to protect the skin.”—Jeannette Graf, MD, Great Neck, New York
- Hydrating Cleanser Bar
CeraVe
Recommended by Shari Lipner, MD, PhD, New York, New York
- Vanicream Cleansing Bar
Pharmaceutical Specialties, Inc
“This is a great option for patients with eczema or dry, sensitive skin.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Lip plumpers, shaving lotions for men, and night creams will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on bar soaps. Consideration must be given to:
- Avène Cold Cream Ultra-Rich Cleansing Bar
Pierre Fabre Dermo-Cosmetique USA
“This gentle cleansing bar is not only hypoallergenic, soap free, and lanolin free, it also has Avène’s soothing Thermal Spring Water, plus white beeswax and a noncomedogenic, pharmaceutical-grade paraffin oil to protect the skin.”—Jeannette Graf, MD, Great Neck, New York
- Hydrating Cleanser Bar
CeraVe
Recommended by Shari Lipner, MD, PhD, New York, New York
- Vanicream Cleansing Bar
Pharmaceutical Specialties, Inc
“This is a great option for patients with eczema or dry, sensitive skin.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Lip plumpers, shaving lotions for men, and night creams will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Expert advice for the corporate titans taking on health care
An announcement Jan. 30 by three of the nation’s corporate titans – Amazon, Berkshire Hathaway, and JPMorgan Chase & Co. – that they are joining forces to address the high costs of employee health care has stirred the health policy pot. It immediately sent shock waves through the health sector of the stock market and reinvigorated talk about health care technology, value, and quality.
Though details regarding the undertaking are thin, the companies said in a statement that their partnership’s intent is to improve employee satisfaction and hold down costs by bringing “their scale and complementary expertise to this long-term effort.”
They plan to create an independent company, “free from profit-making incentives and constraints,” to focus on “technology solutions.”
Berkshire Hathaway CEO Warren Buffett described health care costs as “a hungry tapeworm on the American economy,” and Amazon founder and CEO Jeff Bezos said the partnership was “open-eyed about the degree of difficulty” ahead. Jamie Dimon, chairman and CEO of JPMorgan, said the results could benefit the employees of these companies and possibly all Americans.
But what does all of this mean and how can it be successful when so many other initiatives have fallen short? Kaiser Health News asked a variety of health policy experts for their thoughts on this venture, and what advice they would offer these CEOs as they go forward. Some of the advice has been edited for clarity and length.
Tom Miller, resident fellow, American Enterprise Institute:
“It’s great that someone theoretically with resources would try to build a better mousetrap. But it’s been difficult to do, and part of it is regulatory and competitive barriers are well-constructed in the health care sphere, which tend to make it less receptive or subject to competitive pressures.
“I welcome any new capital trying to disrupt health care. … The incumbents are comfortable and could use disruption. If Amazon has an idea, and is willing to put some money behind it, that’s wonderful. What they are willing to do other than fly low-cost providers for home visits in drones – I don’t know. They’d probably have to miniaturize them, wouldn’t they?”
Stan Dorn, senior fellow, Families USA:
“Number one, look at prices. America doesn’t use more health care than European countries, but we pay a lot more and that’s because of prices more than anything else. Look at hospital prices and prescription drug prices. I would also say, look to eliminate middlemen operating in darkness. I’m thinking in particular of pharmacy benefit managers. Often, the supply chain is hidden and complex, and every step along the way the middlemen are taking their share, and it winds up costing a huge amount of money.”
Bob Kocher, MD, partner, Venrock:
“It has been said that health care is complicated. One thing that is not complicated is that the way to save money is to focus on the sickest patients. And that’s the only thing that has proven to work in great primary care. I hope Amazon realizes this early and does not think that [its smart digital assistant] Alexa and apps are going to make us healthier and save any money.
“It would sure be nice if they invest in a ‘post-CPT-ICD-10-and-many-bills-per-visit’ world where we know prices, can easily know what is known about quality and experience, and have same-day service.”
Tracy Watts, senior partner, Mercer:
“Everyone thinks millennials want to do everything on their phones. But that’s not necessarily the case.
“[There was a recent] survey about this – specifically, millennials are the most interested in new health care offerings, but it wasn’t as much high-tech as it is convenience they are interested in – same-day appointments with a family doctor, guaranteed appointments with specialists, home visits, a wider array of services available at retail clinics. That was kind of an ‘aha’ – this kind of convenience and high-touch experience is what they’re looking for. And when you think of ‘health care of the future,’ that’s not what comes to mind.”
John Rother, president and CEO, National Coalition on Health Care:
“Health care is complex and expensive, so the aim should always be simplicity and affordability. Three keys to success: Manage chronic conditions recognizing the life context of the patient, emphasize primary care-based medical homes, and aggressively negotiate prescription drug costs.”
Suzanne Delbanco, executive director, Catalyst for Payment Reform:
“The biggest driver of health care costs is prices. Those are being driven up by health care providers who have consolidated and will continue to consolidate and amass more market power.
“It sounds like they [the companies] are limiting the use of health plans, but if they’re going to get into that business, they’re going to come up with the same challenges health plans face. What would be really innovative would be to build some provider systems from the ground up where they can truly get a handle on the actual costs and eliminate the market power that drives the prices up, and they can have control over their prices.”
Brian Marcotte, president and CEO, National Business Group on Health:
“They recognize this is [a] long-term play to get involved in this. I’d have to say, this industry is ripe for disruption.
“I think we know technology will continue to play an increasing role in how consumers access and receive health care. We’ve also learned most consumers do not touch the health care delivery system with enough frequency to ever be a sophisticated consumer. What’s intriguing about this partnership is Amazon for many consumers has become part of their day-to-day world, part of their routine. It’s intriguing to consider the possibilities of integrating health care into consumer routine.
“And I think that therein lies the opportunity. Employers offer a lot of resources to their employees to help them maximize their experience, and their No. 1 challenge is engagement.”
Joseph Antos, health economist, American Enterprise Institute:
“My first suggestion is to look at what other employers have done (some unsuccessfully) and consider how to adapt those ideas for the three companies and more broadly. Change incentives for providers. Change incentives for consumers. Work on ways to reduce the effects of market consolidation. The bottom line: Don’t keep doing what we are doing now. I don’t see that these three companies have enough presence in health markets to pull this off anytime soon, but perhaps this should be viewed as the private-sector version of the Affordable Care Act’s Innovation Center – except, this time, there may be some new ideas to test.”
Ceci Connolly, president and CEO, Alliance of Community Health Plans:
“We know that 5% of any population consumes 50% of the health care dollar. I would encourage this group to focus on how to better serve those individuals who need help managing multiple chronic conditions.”
David Lansky, CEO, Pacific Business Group on Health:
“The incumbent providers of services to our members are not doing as much as we need done for affordability and quality. So, we are pleased to see them go down this path. We don’t know what piece of the puzzle they will tackle.
“We know well-intended efforts over the years haven’t added up to material impact on cost and quality. I would suspect they are looking at doing something broader, more disruptive than initiatives we have tried before.
“I think across the board they have the opportunity to set high standards for the health system in whatever platform they use. These companies have a history of raising the bar. Potentially, it could be a help to all of us.”
Staff writers Julie Appleby, Rachel Bluth, Jenny Gold, Jay Hancock, Shefali Luthra, Jordan Rau, Julie Rovner and Chad Terhune contributed to this report. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
An announcement Jan. 30 by three of the nation’s corporate titans – Amazon, Berkshire Hathaway, and JPMorgan Chase & Co. – that they are joining forces to address the high costs of employee health care has stirred the health policy pot. It immediately sent shock waves through the health sector of the stock market and reinvigorated talk about health care technology, value, and quality.
Though details regarding the undertaking are thin, the companies said in a statement that their partnership’s intent is to improve employee satisfaction and hold down costs by bringing “their scale and complementary expertise to this long-term effort.”
They plan to create an independent company, “free from profit-making incentives and constraints,” to focus on “technology solutions.”
Berkshire Hathaway CEO Warren Buffett described health care costs as “a hungry tapeworm on the American economy,” and Amazon founder and CEO Jeff Bezos said the partnership was “open-eyed about the degree of difficulty” ahead. Jamie Dimon, chairman and CEO of JPMorgan, said the results could benefit the employees of these companies and possibly all Americans.
But what does all of this mean and how can it be successful when so many other initiatives have fallen short? Kaiser Health News asked a variety of health policy experts for their thoughts on this venture, and what advice they would offer these CEOs as they go forward. Some of the advice has been edited for clarity and length.
Tom Miller, resident fellow, American Enterprise Institute:
“It’s great that someone theoretically with resources would try to build a better mousetrap. But it’s been difficult to do, and part of it is regulatory and competitive barriers are well-constructed in the health care sphere, which tend to make it less receptive or subject to competitive pressures.
“I welcome any new capital trying to disrupt health care. … The incumbents are comfortable and could use disruption. If Amazon has an idea, and is willing to put some money behind it, that’s wonderful. What they are willing to do other than fly low-cost providers for home visits in drones – I don’t know. They’d probably have to miniaturize them, wouldn’t they?”
Stan Dorn, senior fellow, Families USA:
“Number one, look at prices. America doesn’t use more health care than European countries, but we pay a lot more and that’s because of prices more than anything else. Look at hospital prices and prescription drug prices. I would also say, look to eliminate middlemen operating in darkness. I’m thinking in particular of pharmacy benefit managers. Often, the supply chain is hidden and complex, and every step along the way the middlemen are taking their share, and it winds up costing a huge amount of money.”
Bob Kocher, MD, partner, Venrock:
“It has been said that health care is complicated. One thing that is not complicated is that the way to save money is to focus on the sickest patients. And that’s the only thing that has proven to work in great primary care. I hope Amazon realizes this early and does not think that [its smart digital assistant] Alexa and apps are going to make us healthier and save any money.
“It would sure be nice if they invest in a ‘post-CPT-ICD-10-and-many-bills-per-visit’ world where we know prices, can easily know what is known about quality and experience, and have same-day service.”
Tracy Watts, senior partner, Mercer:
“Everyone thinks millennials want to do everything on their phones. But that’s not necessarily the case.
“[There was a recent] survey about this – specifically, millennials are the most interested in new health care offerings, but it wasn’t as much high-tech as it is convenience they are interested in – same-day appointments with a family doctor, guaranteed appointments with specialists, home visits, a wider array of services available at retail clinics. That was kind of an ‘aha’ – this kind of convenience and high-touch experience is what they’re looking for. And when you think of ‘health care of the future,’ that’s not what comes to mind.”
John Rother, president and CEO, National Coalition on Health Care:
“Health care is complex and expensive, so the aim should always be simplicity and affordability. Three keys to success: Manage chronic conditions recognizing the life context of the patient, emphasize primary care-based medical homes, and aggressively negotiate prescription drug costs.”
Suzanne Delbanco, executive director, Catalyst for Payment Reform:
“The biggest driver of health care costs is prices. Those are being driven up by health care providers who have consolidated and will continue to consolidate and amass more market power.
“It sounds like they [the companies] are limiting the use of health plans, but if they’re going to get into that business, they’re going to come up with the same challenges health plans face. What would be really innovative would be to build some provider systems from the ground up where they can truly get a handle on the actual costs and eliminate the market power that drives the prices up, and they can have control over their prices.”
Brian Marcotte, president and CEO, National Business Group on Health:
“They recognize this is [a] long-term play to get involved in this. I’d have to say, this industry is ripe for disruption.
“I think we know technology will continue to play an increasing role in how consumers access and receive health care. We’ve also learned most consumers do not touch the health care delivery system with enough frequency to ever be a sophisticated consumer. What’s intriguing about this partnership is Amazon for many consumers has become part of their day-to-day world, part of their routine. It’s intriguing to consider the possibilities of integrating health care into consumer routine.
“And I think that therein lies the opportunity. Employers offer a lot of resources to their employees to help them maximize their experience, and their No. 1 challenge is engagement.”
Joseph Antos, health economist, American Enterprise Institute:
“My first suggestion is to look at what other employers have done (some unsuccessfully) and consider how to adapt those ideas for the three companies and more broadly. Change incentives for providers. Change incentives for consumers. Work on ways to reduce the effects of market consolidation. The bottom line: Don’t keep doing what we are doing now. I don’t see that these three companies have enough presence in health markets to pull this off anytime soon, but perhaps this should be viewed as the private-sector version of the Affordable Care Act’s Innovation Center – except, this time, there may be some new ideas to test.”
Ceci Connolly, president and CEO, Alliance of Community Health Plans:
“We know that 5% of any population consumes 50% of the health care dollar. I would encourage this group to focus on how to better serve those individuals who need help managing multiple chronic conditions.”
David Lansky, CEO, Pacific Business Group on Health:
“The incumbent providers of services to our members are not doing as much as we need done for affordability and quality. So, we are pleased to see them go down this path. We don’t know what piece of the puzzle they will tackle.
“We know well-intended efforts over the years haven’t added up to material impact on cost and quality. I would suspect they are looking at doing something broader, more disruptive than initiatives we have tried before.
“I think across the board they have the opportunity to set high standards for the health system in whatever platform they use. These companies have a history of raising the bar. Potentially, it could be a help to all of us.”
Staff writers Julie Appleby, Rachel Bluth, Jenny Gold, Jay Hancock, Shefali Luthra, Jordan Rau, Julie Rovner and Chad Terhune contributed to this report. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
An announcement Jan. 30 by three of the nation’s corporate titans – Amazon, Berkshire Hathaway, and JPMorgan Chase & Co. – that they are joining forces to address the high costs of employee health care has stirred the health policy pot. It immediately sent shock waves through the health sector of the stock market and reinvigorated talk about health care technology, value, and quality.
Though details regarding the undertaking are thin, the companies said in a statement that their partnership’s intent is to improve employee satisfaction and hold down costs by bringing “their scale and complementary expertise to this long-term effort.”
They plan to create an independent company, “free from profit-making incentives and constraints,” to focus on “technology solutions.”
Berkshire Hathaway CEO Warren Buffett described health care costs as “a hungry tapeworm on the American economy,” and Amazon founder and CEO Jeff Bezos said the partnership was “open-eyed about the degree of difficulty” ahead. Jamie Dimon, chairman and CEO of JPMorgan, said the results could benefit the employees of these companies and possibly all Americans.
But what does all of this mean and how can it be successful when so many other initiatives have fallen short? Kaiser Health News asked a variety of health policy experts for their thoughts on this venture, and what advice they would offer these CEOs as they go forward. Some of the advice has been edited for clarity and length.
Tom Miller, resident fellow, American Enterprise Institute:
“It’s great that someone theoretically with resources would try to build a better mousetrap. But it’s been difficult to do, and part of it is regulatory and competitive barriers are well-constructed in the health care sphere, which tend to make it less receptive or subject to competitive pressures.
“I welcome any new capital trying to disrupt health care. … The incumbents are comfortable and could use disruption. If Amazon has an idea, and is willing to put some money behind it, that’s wonderful. What they are willing to do other than fly low-cost providers for home visits in drones – I don’t know. They’d probably have to miniaturize them, wouldn’t they?”
Stan Dorn, senior fellow, Families USA:
“Number one, look at prices. America doesn’t use more health care than European countries, but we pay a lot more and that’s because of prices more than anything else. Look at hospital prices and prescription drug prices. I would also say, look to eliminate middlemen operating in darkness. I’m thinking in particular of pharmacy benefit managers. Often, the supply chain is hidden and complex, and every step along the way the middlemen are taking their share, and it winds up costing a huge amount of money.”
Bob Kocher, MD, partner, Venrock:
“It has been said that health care is complicated. One thing that is not complicated is that the way to save money is to focus on the sickest patients. And that’s the only thing that has proven to work in great primary care. I hope Amazon realizes this early and does not think that [its smart digital assistant] Alexa and apps are going to make us healthier and save any money.
“It would sure be nice if they invest in a ‘post-CPT-ICD-10-and-many-bills-per-visit’ world where we know prices, can easily know what is known about quality and experience, and have same-day service.”
Tracy Watts, senior partner, Mercer:
“Everyone thinks millennials want to do everything on their phones. But that’s not necessarily the case.
“[There was a recent] survey about this – specifically, millennials are the most interested in new health care offerings, but it wasn’t as much high-tech as it is convenience they are interested in – same-day appointments with a family doctor, guaranteed appointments with specialists, home visits, a wider array of services available at retail clinics. That was kind of an ‘aha’ – this kind of convenience and high-touch experience is what they’re looking for. And when you think of ‘health care of the future,’ that’s not what comes to mind.”
John Rother, president and CEO, National Coalition on Health Care:
“Health care is complex and expensive, so the aim should always be simplicity and affordability. Three keys to success: Manage chronic conditions recognizing the life context of the patient, emphasize primary care-based medical homes, and aggressively negotiate prescription drug costs.”
Suzanne Delbanco, executive director, Catalyst for Payment Reform:
“The biggest driver of health care costs is prices. Those are being driven up by health care providers who have consolidated and will continue to consolidate and amass more market power.
“It sounds like they [the companies] are limiting the use of health plans, but if they’re going to get into that business, they’re going to come up with the same challenges health plans face. What would be really innovative would be to build some provider systems from the ground up where they can truly get a handle on the actual costs and eliminate the market power that drives the prices up, and they can have control over their prices.”
Brian Marcotte, president and CEO, National Business Group on Health:
“They recognize this is [a] long-term play to get involved in this. I’d have to say, this industry is ripe for disruption.
“I think we know technology will continue to play an increasing role in how consumers access and receive health care. We’ve also learned most consumers do not touch the health care delivery system with enough frequency to ever be a sophisticated consumer. What’s intriguing about this partnership is Amazon for many consumers has become part of their day-to-day world, part of their routine. It’s intriguing to consider the possibilities of integrating health care into consumer routine.
“And I think that therein lies the opportunity. Employers offer a lot of resources to their employees to help them maximize their experience, and their No. 1 challenge is engagement.”
Joseph Antos, health economist, American Enterprise Institute:
“My first suggestion is to look at what other employers have done (some unsuccessfully) and consider how to adapt those ideas for the three companies and more broadly. Change incentives for providers. Change incentives for consumers. Work on ways to reduce the effects of market consolidation. The bottom line: Don’t keep doing what we are doing now. I don’t see that these three companies have enough presence in health markets to pull this off anytime soon, but perhaps this should be viewed as the private-sector version of the Affordable Care Act’s Innovation Center – except, this time, there may be some new ideas to test.”
Ceci Connolly, president and CEO, Alliance of Community Health Plans:
“We know that 5% of any population consumes 50% of the health care dollar. I would encourage this group to focus on how to better serve those individuals who need help managing multiple chronic conditions.”
David Lansky, CEO, Pacific Business Group on Health:
“The incumbent providers of services to our members are not doing as much as we need done for affordability and quality. So, we are pleased to see them go down this path. We don’t know what piece of the puzzle they will tackle.
“We know well-intended efforts over the years haven’t added up to material impact on cost and quality. I would suspect they are looking at doing something broader, more disruptive than initiatives we have tried before.
“I think across the board they have the opportunity to set high standards for the health system in whatever platform they use. These companies have a history of raising the bar. Potentially, it could be a help to all of us.”
Staff writers Julie Appleby, Rachel Bluth, Jenny Gold, Jay Hancock, Shefali Luthra, Jordan Rau, Julie Rovner and Chad Terhune contributed to this report. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
JAK inhibitors look good for severe alopecia areata treatment
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
FROM JOURNAL OF INVESTIGATIVE DERMATOLOGY SYMPOSIUM PROCEEDINGS
CDC’s Fitzgerald resigns amid tobacco stock brouhaha
Brenda Fitzgerald, MD, director of the Centers for Disease Control and Prevention, resigned Jan. 31 after reports surfaced on the public affairs website Politico that she purchased shares of Japan Tobacco about 1 month after becoming the agency’s director.
Dr. Fitzgerald, an ob.gyn., also bought stock in Merck & Co., Bayer, and Humana after joining the Trump Administration in July 2017, according to the report. Financial disclosure records confirm that she sold the tobacco stock in October and “all of her stock holdings above $1,000 by Nov. 21, more than 4 months after she became CDC director,” according to the Politico report.
According to a spokesperson for the Health and Human Services department, “This morning Secretary Azar accepted Dr. Brenda Fitzgerald’s resignation as Director of the Centers for Disease Control and Prevention.
“Dr. Fitzgerald owns certain complex financial interests that have imposed a broad recusal limiting her ability to complete all of her duties as the CDC Director. Due to the nature of these financial interests, Dr. Fitzgerald could not divest from them in a definitive time period. After advising Secretary Azar of both the status of the financial interests and the scope of her recusal, Dr. Fitzgerald tendered, and the Secretary accepted, her resignation. The Secretary thanks Dr. Brenda Fitzgerald for her service and wishes her the best in all her endeavors,” according to a report on CNBC.
Brenda Fitzgerald, MD, director of the Centers for Disease Control and Prevention, resigned Jan. 31 after reports surfaced on the public affairs website Politico that she purchased shares of Japan Tobacco about 1 month after becoming the agency’s director.
Dr. Fitzgerald, an ob.gyn., also bought stock in Merck & Co., Bayer, and Humana after joining the Trump Administration in July 2017, according to the report. Financial disclosure records confirm that she sold the tobacco stock in October and “all of her stock holdings above $1,000 by Nov. 21, more than 4 months after she became CDC director,” according to the Politico report.
According to a spokesperson for the Health and Human Services department, “This morning Secretary Azar accepted Dr. Brenda Fitzgerald’s resignation as Director of the Centers for Disease Control and Prevention.
“Dr. Fitzgerald owns certain complex financial interests that have imposed a broad recusal limiting her ability to complete all of her duties as the CDC Director. Due to the nature of these financial interests, Dr. Fitzgerald could not divest from them in a definitive time period. After advising Secretary Azar of both the status of the financial interests and the scope of her recusal, Dr. Fitzgerald tendered, and the Secretary accepted, her resignation. The Secretary thanks Dr. Brenda Fitzgerald for her service and wishes her the best in all her endeavors,” according to a report on CNBC.
Brenda Fitzgerald, MD, director of the Centers for Disease Control and Prevention, resigned Jan. 31 after reports surfaced on the public affairs website Politico that she purchased shares of Japan Tobacco about 1 month after becoming the agency’s director.
Dr. Fitzgerald, an ob.gyn., also bought stock in Merck & Co., Bayer, and Humana after joining the Trump Administration in July 2017, according to the report. Financial disclosure records confirm that she sold the tobacco stock in October and “all of her stock holdings above $1,000 by Nov. 21, more than 4 months after she became CDC director,” according to the Politico report.
According to a spokesperson for the Health and Human Services department, “This morning Secretary Azar accepted Dr. Brenda Fitzgerald’s resignation as Director of the Centers for Disease Control and Prevention.
“Dr. Fitzgerald owns certain complex financial interests that have imposed a broad recusal limiting her ability to complete all of her duties as the CDC Director. Due to the nature of these financial interests, Dr. Fitzgerald could not divest from them in a definitive time period. After advising Secretary Azar of both the status of the financial interests and the scope of her recusal, Dr. Fitzgerald tendered, and the Secretary accepted, her resignation. The Secretary thanks Dr. Brenda Fitzgerald for her service and wishes her the best in all her endeavors,” according to a report on CNBC.
2018 Update on fertility
Clinicians always should consider endometriosis in the diagnostic work-up of an infertility patient. But the diagnosis of endometriosis is often difficult, and management is complex. In this Update, we summarize international consensus documents on endometriosis with the aim of enhancing clinicians’ ability to make evidence-based decisions. In addition, we explore the interesting results of a large hysterosalpingography trial in which 2 different contrast mediums were used. Finally, we urge all clinicians to adapt the new standardized lexicon of infertility and fertility care terms that comprise the recently revised international glossary.
Endometriosis and infertility: The knowns and unknowns
Johnson NP, Hummelshoj L, Adamson GD, et al; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315-324.
Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552-1568.
Rogers PA, Adamson GD, Al-Jefout M, et al; WES/WERF Consortium for Research Priorities in Endometriosis. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202-226.
Endometriosis is defined as "a disease characterized by the presence of endometrium-like epithelium and stroma outside the endometrium and myometrium. Intrapelvic endometriosis can be located superficially on the peritoneum (peritoneal endometriosis), can extend 5 mm or more beneath the peritoneum (deep endometriosis) or can be present as an ovarian endometriotic cyst (endometrioma)."1 Always consider endometriosis in the infertile patient.
Although many professional societies and numerous Cochrane Database Systematic Reviews have provided guidelines on endometriosis, controversy and uncertainty remain. The World Endometriosis Society (WES) and the World Endometriosis Research Foundation (WERF), however, have now published several consensus documents that assess the global literature and professional organization guidelines in a structured, consensus-driven process.2-4 These WES and WERF documents consolidate known information and can be used to inform the clinician in making evidence-linked diagnostic and treatment decisions. Recommendations offered in this discussion are based on those documents.
Establishing the diagnosis can be difficult
Diagnosis of endometriosis is often difficult and is delayed an average of 7 years from onset of symptoms. These include severe dysmenorrhea, deep dyspareunia, chronic pelvic pain, ovulation pain, cyclical or perimenstrual symptoms (bowel or bladder associated) with or without abnormal bleeding, chronic fatigue, and infertility. A major difficulty is that the predictive value of any one symptom or set of symptoms remains uncertain, as each of these symptoms can have other causes, and a significant proportion of affected women are asymptomatic.
For a definitive diagnosis of endometriosis, visual inspection of the pelvis at laparoscopy is the gold standard investigation, unless disease is visible in the vagina or elsewhere. Positive histology confirms the diagnosis of endometriosis; negative histology does not exclude it. Whether histology should be obtained if peritoneal disease alone is present is controversial: visual inspection usually is adequate, but histologic confirmation of at least one lesion is ideal. In cases of ovarian endometrioma (>4 cm in diameter) and in deeply infiltrating disease, histology should be obtained to identify endometriosis and to exclude rare instances of malignancy.
Compared with laparoscopy, transvaginal ultrasonography (TVUS) has no value in diagnosing peritoneal endometriosis, but it is a useful tool for both making and excluding the diagnosis of an ovarian endometrioma. TVUS may have a role in the diagnosis of disease involving the bladder or rectum.
At present, evidence is insufficient to indicate that magnetic resonance imaging (MRI) is useful for diagnosing or excluding endometriosis compared with laparoscopy. MRI should be reserved for when ultrasound results are equivocal in cases of rectovaginal or bladder endometriosis.
Serum cancer antigen 125 (CA 125) levels may be elevated in endometriosis. However, measuring serum CA 125 levels has no value as a diagnostic tool.
No fertility benefit with ovarian suppression
More than 2 dozen randomized controlled trials (RCTs) provide strong evidence that there is no fertility benefit from ovarian suppression. The drug costs and delayed time to pregnancy mean that ovarian suppression with oral contraceptives, other progestational agents, or gonadotropin-releasing hormone (GnRH) agonists before fertility treatment is not indicated, with the possible exception of using it prior to in vitro fertilization (IVF).
Ovarian suppression also has been suggested as beneficial in conjunction with surgery. However, at least 16 RCTs have failed to show fertility improvement when ovarian suppression is given either preoperatively or postoperatively. Again, the delay in attempting pregnancy, drug costs, and adverse effects render ovarian suppression not appropriate.
While ovarian suppression has not been shown to increase pregnancy rates, ovarian stimulation (OS) likely does, especially when combined with intrauterine insemination (IUI).5
Laparoscopy: Appropriate for selected patients
A major decision for clinicians and patients dealing with infertility is whether to perform a laparoscopy, both for diagnostic and for treatment reasons. Currently, data are insufficient to recommend laparoscopic surgery prior to OS/IUI unless there is a history of evidence of anatomic disease and/or the patient has sufficient pain to justify the physical, emotional, financial, and time costs of laparoscopy. Laparoscopy therefore can be considered as possibly appropriate in younger women (<37 years of age) with short duration of infertility (<4 years), normal male factor, normal or treatable uterus, normal or treatable ovulation disorder, and limited prior treatment.
It is important to consider what disease might be found and how much of an increase in fertility can be obtained by treatment, so that the number needed to treat (NNT) can be used as an estimate of the potential value of laparoscopy in a given patient. A patient also should have no contraindications to laparoscopy and accept 9 to 15 months of attempting pregnancy before undergoing IVF treatment.
When laparoscopy is performed for minimal to mild disease, the odds ratio for pregnancy is 1.66 with treatment. It is important to remove all visible disease without injuring healthy tissue. When disease is moderate to severe, there is often severe anatomic distortion and a very low background pregnancy rate. Numerous uncontrolled trials show benefit of operative laparoscopy, especially for invasive, adhesive, and cystic endometriosis. However, repeat surgery is rarely indicated. After surgery, the Endometriosis Fertility Index (EFI) can be used to determine prognosis and plan management (FIGURE 1).6 An easy-to-use electronic EFI calculator is available online at www.endometriosisefi.com.
Management of endometriomas
Endometriomas are often operated on because of pain. Initial pain relief occurs in 60% to 100% of patients, but cysts recur following stripping about 10% of the time, and drainage without stripping, about 20%. With recurrence, pain is present about 75% of the time.
Pregnancy rates following endometrioma treatment depend on patient age and the status of the pelvis following operative intervention. This can be determined from the EFI. Often, the dilemma with endometriomas is how aggressive to be in removing them. The principles involved are to remove all the cyst wall if possible, but absolutely to minimize ovarian tissue damage, because reduced ovarian reserve is a possible major negative consequence of ovarian surgery.
Recommendations
While endometriosis is often a cause of infertility, often infertile patients do not have endometriosis. A careful history, physical examination, and ultrasonography, and possibly other imaging studies, are prerequisites to careful clinical judgment in diagnosing and treating infertile patients who might or do have endometriosis.
When pelvic pain is present, initially nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives (OCs), progestational agents, or an intrauterine device can be helpful. These ovarian suppression medications do not increase fertility, however, and should be stopped in any patient who desires to get pregnant.
When pelvic and male fertility factors appear reasonably normal (even if minimal or mild endometriosis is suspected), treatment with clomiphene 100 mg on cycle days 3 through 7 and IUI for 3 to 6 cycles is an effective first step. However, if the patient has persistent pain and/or infertility without other significant infertility factors, then diagnostic laparoscopy with intraoperative treatment of disease is indicated.
Surgery well performed is effective treatment for all stages of endometriosis and endometriomas, both for infertility and for pain. Repeat surgery, however, is rarely indicated because of limited results, so it is important to obtain the best possible result on the first surgery. Surgery is indicated for large endometriomas (>4 cm). Endometriosis has almost no effect on the IVF live birth rate unless ovarian reserve has been reduced by endometriomas or surgery, so endometriosis surgery should be performed by skilled and experienced surgeons.
Endometriosis is a complex disease that can cause infertility. Its diagnosis and management are frequently difficult, requiring knowledge, experience, and good medical judgment and surgical skills. However, if evidence-linked principles are followed, effective treatment plans and good outcomes can be obtained for most patients.
Read about why oil-based contrast may be better than water-based contrast with HSG.
Oil-based contrast medium use in hysterosalpingography is associated with higher pregnancy rates compared with water-based contrast
Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043-2052.
Hysterosalpingography (HSG) to assess tubal patency has been a mainstay of infertility diagnosis for decades. Some, but not all, studies also have suggested that pregnancy rates are higher after this tubal flushing procedure, especially if performed with oil contrast.7,8 A recent multicenter, randomized, controlled trial by Dreyer and colleagues that compared ongoing pregnancy rates and other outcomes among women who had HSG with oil contrast versus with water contrast provides additional valuable information.9
Trial details
In this study, 1,294 infertile women in 27 academic, teaching and nonteaching hospitals were screened for trial eligibility; 1,119 women provided written informed consent. Of these, 557 women were randomly assigned to HSG with oil contrast and 562 to water contrast. The women had spontaneous menstrual cycles, had been attempting pregnancy for at least 1 year, and had indications for HSG.
Exclusion criteria were known endocrine disorders, fewer than 8 menstrual cycles per year, a high risk of tubal disease, iodine allergy, and a total motile sperm count after sperm wash of less than 3 million/mL in the male partner (or a total motile sperm count of less than 1 million/mL when an analysis after sperm wash was not performed).
Just prior to undergoing HSG, the women were randomly assigned to receive either oil contrast or water contrast medium. (The trial was not blinded to participants or caregivers.) HSG was performed according to local protocols using cervical vacuum cup, metal cannula (hysterophore), or balloon catheter and approximately 5 to 10 mL of contrast medium.
After HSG, couples received expectant management when the predicted likelihood of pregnancy within 12 months, based on the prognostic model of Hunault, was 30% or greater.10 IUI was offered for pregnancy likelihood less than 30%, mild male infertility, or failure after a period of expectant management. IUI with or without mild ovarian stimulation (2-3 follicles) with clomiphene or gonadotropins was initiated after a minimum of 2 months of expectant management after HSG.
The primary outcome measure was ongoing pregnancy, defined as a positive fetal heartbeat on ultrasonographic examination after 12 weeks of gestation, with the first day of the last menstrual cycle for the pregnancy within 6 months after randomization. Secondary outcome measures were clinical pregnancy, live birth, miscarriage, ectopic pregnancy, time to pregnancy, and pain scores after HSG. All data were analyzed according to intention-to-treat.
Pregnancy rates increased with oil-contrast HSG
The baseline characteristics of the 2 groups were similar. HSG showed bilateral tubal patency in 477 of 554 women (86.1%) in the oil contrast group and in 491 of 554 women (88.6%) who received the water contrast (rate ratio, 0.97; 95% confidence interval [CI], 0.93-1.02). Bilateral tubal occlusion occurred in 9 women in the oil group (1.6%) and in 13 in the water group (2.3%) (relative risk, 0.69; 95% CI, 0.30-1.61).
A total of 58.3% of the women assigned to oil contrast and 57.2% of those assigned to water contrast received expectant management. Similar percentages of women in the oil group and in the water group underwent IUI (39.7% and 41.0%, respectively), IVF or intracytoplasmic sperm injection (ICSI) (2.3% and 2.2%), laparoscopy (6.2% in each group), and hysteroscopy (4.4% and 4.2%).
Ongoing pregnancy occurred in 220 of 554 women (39.7%) in the oil contrast group and in 161 of 554 women (29.1%) in the water contrast group (rate ratio, 1.37; 95% CI, 1.16-1.61; P<.001). The median time to the onset of pregnancy in the oil group was 2.7 months (interquartile range, 1.5-4.7) (FIGURE 2), while in the water group it was 3.1 months (interquartile range, 1.6-4.8) (P = .44).
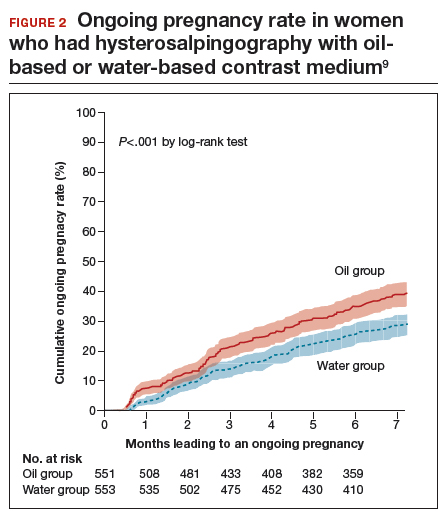
While the proportion of women getting pregnant with or without the different interventions was similar in both groups, the live birth rate was 38.8% in the oil group versus 28.1% in the water group (rate ratio, 1.38; 95% CI, 1.17-1.64; P<.001). Three of 554 women (0.5%) assigned to oil contrast and 4 of 554 women (0.7%) in the water contrast group had an adverse event during the trial period. Three women (1.4%), all in the oil group, delivered a child with a congenital anomaly.
Why this study is important
This is the largest and best methodologic study on this clinical issue. It showed higher pregnancy and live birth rates within 6 months of HSG performed with oil compared with water. Although the study was not blinded, the group similarities and objective outcomes support minimal bias. Importantly, these results can be generalized only to women with similar inclusion characteristics.
It is unclear why oil HSG might enhance fertility. Suggested mechanisms include flushing of debris and/or mucous plugs or an effect on peritoneal macrophages or endometrial receptivity. Since HSG is minimally invasive and inexpensive, and the 10% increase in pregnancy rates corresponds to an NNT of 10, it is reasonable to consider, although formal cost-effectiveness data are lacking.
Concerns include the rare theoretical risk of intravasation with subsequent allergic reaction or fat embolism. Three infants in the oil group and none in the water group had congenital anomalies. This is likely due to chance, since this rate is not higher than that in the general population and no other data suggest an increased risk. Comparison of these results with other new techniques, such as sonohysterography (saline infusion sonogram), awaits further studies.
Recommendation
HSG with oil contrast should be considered a potential therapeutic as well as diagnostic intervention in selected patients.
HSG is an important diagnostic test for most infertility patients. The fact that a therapeutic benefit probably also is associated with oil-based HSG increases the clinical indications for this test.
Read about new definitions of infertility terminology you should know.
Infertility glossary is newly updated
Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393-406.
Terms and definitions used in infertility and fertility care frequently have had different meanings for different stakeholders, especially on a global basis. This can result in misunderstandings and inappropriate interpretation and comparison of published information and research. To help address these issues, international fertility organizations recently developed an updated glossary on infertilityterminology.
The consensus process for updating the glossary
The International Glossary on Infertility and Fertility Care, 2017, was recently published simultaneously in Fertility and Sterility and Human Reproduction. This is the second revision; the first glossary was published in 2006 and revised in 2009. This revision's 25 lead experts began work in 2014. Their teams of professionals interacted by electronic mail, at international and regional society meetings, and at 2 consultations held in Geneva, Switzerland. This glossary represents consensus agreement reached on 283 evidence-driven terms and definitions.
The work was led by the International Committee for Monitoring Assisted Reproductive Technologies in partnership with the American Society for Reproductive Medicine, European Society of Human Reproduction and Embryology, International Federation of Fertility Societies, March of Dimes, African Fertility Society, Groupe Inter-africain d'Etude de Recherche et d'Application sur la Fertilité, Asian Pacific Initiative on Reproduction, Middle East Fertility Society, Red Latinoamericana de Reproducción Asistida, and the International Federation of Gynecology and Obstetrics.
All together, 108 international professional experts (clinicians, basic scientists, epidemiologists, and social scientists), along with national and regional representatives of infertile persons, participated in the development of this evidence-base driven glossary. As such, these definitions now set the standard for international communication among clinicians, scientists, and policymakers.
Definition of infertility is broadened
The definitions take account of ethics, human rights, cultural sensitivities, ethnic minorities, and gender equality. For example, the first modification included broadening the concept of infertility to be an "impairment of individuals" in their capacity to reproduce, irrespective of whether the individual has a partner. (See “Broadened definition of infertility” below). Reproductive rights are individual human rights and do not depend on a relationship with another individual. The revised definition also reinforces the concept of infertility as a disease that can generate an impairment of function.
Infertility: A disease characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person’s capacity to reproduce either as an individual or with his/her partner. Fertility interventions may be initiated in less than 1 year based on medical, sexual and reproductive history, age, physical findings and diagnostic testing. Infertility is a disease, which generates disability as an impairment of function.
Reference
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406
New--and changed--definitions
Certain terms need to be consistent with those used currently internationally, for example, at which gestational age a miscarriage/abortion becomes a stillbirth.
Some terms are confusing, such as subfertility, which does not define a different or less severe fertility status than infertility, does not exist before infertility is diagnosed, and should not be confused with sterility, which is a permanent state of infertility. The term subfertility therefore is redundant and has been removed and replaced by infertility (See “Some terms with an important new definition” below).
- Clinical pregnancy
- Conception (removed from glossary)
- Diminished ovarian reserve
- Fertility care
- Hypospermia (replaces oligospermia)
- Ovarian reserve
- Pregnancy
- Preimplantation genetic testing
- Spontaneous abortion/miscarriage
- Subfertility (should be used interchangeably with infertility)
Reference
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406.
In a different context, the term conception, and its derivatives such as conceiving or conceived, was removed because it cannot be described biologically during the process of reproduction. Instead, terms such as fertilization, implantation, pregnancy, and live birth should be used.
Important male terms also changed: oligospermia is a term for low semen volume that is now replaced by hypospermia to avoid confusion with oligozoospermia, which is low concentration of spermatozoa in the ejaculate below the lower reference limit. When reporting results, the reference criteria should be specified.
Lastly, owing to the lack of standardization in determining the burden of infertility, and to better ensure comparability of prevalence data published globally, this glossary includes definitions for terms frequently used in epidemiology and public health. Examples include voluntary and involuntary childlessness, primary and secondary infertility, fertility care, fecundity, and fecundability, among others.
Getting the word out
The glossary has been approved by all of the participating organizations who are assisting in its distribution. It is being presented at national and international meetings and is used in The FIGO Fertility Toolbox (www.fertilitytool.com). It is hoped that all professionals and other stakeholders will begin to use its terminology globally to provide quality care and ensure consistency in registering specific fertility care interventions and more accurate reporting of their outcomes.
The language we use determines our individual and collective understanding of the scientific and clinical care of our patients. This glossary provides an essential and comprehensive standardization of terms and definitions essential to quality reproductive health care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406.
- Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–1568.
- Rogers PA, Adamson GD, Al-Jefout M, et al; WES/WERF Consortium for Research Priorities in Endometriosis. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202–226.
- Johnson NP, Hummelshoj L, Adamson GD, et al; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–324.
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598.
- Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94(5):1609–1615.
- Weir WC, Weir DR. Therapeutic value of salpingograms in infertility. Fertil Steril. 1951;2(6);514–522.
- Johnson NP, Farquhar CM, Hadden WE, Suckling J, Yu Y, Sadler L. The FLUSH trial—flushing with lipiodol for unexplained (and endometriosis-related) subfertility by hysterosalpingography: a randomized trial. Hum Reprod. 2004;19(9):2043–2051.
- Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043–2052.
- Van der Steeg JW, Steures P, Eijkemans MJ, et al; Collaborative Effort for Clinical Evaluation in Reproductive Medicine. Pregnancy is predictable: a large-scale prospective external validation of the prediction of spontaneous pregnancy in sub-fertile couples. Hum Reprod. 2007;22(2):536–542.
Clinicians always should consider endometriosis in the diagnostic work-up of an infertility patient. But the diagnosis of endometriosis is often difficult, and management is complex. In this Update, we summarize international consensus documents on endometriosis with the aim of enhancing clinicians’ ability to make evidence-based decisions. In addition, we explore the interesting results of a large hysterosalpingography trial in which 2 different contrast mediums were used. Finally, we urge all clinicians to adapt the new standardized lexicon of infertility and fertility care terms that comprise the recently revised international glossary.
Endometriosis and infertility: The knowns and unknowns
Johnson NP, Hummelshoj L, Adamson GD, et al; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315-324.
Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552-1568.
Rogers PA, Adamson GD, Al-Jefout M, et al; WES/WERF Consortium for Research Priorities in Endometriosis. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202-226.
Endometriosis is defined as "a disease characterized by the presence of endometrium-like epithelium and stroma outside the endometrium and myometrium. Intrapelvic endometriosis can be located superficially on the peritoneum (peritoneal endometriosis), can extend 5 mm or more beneath the peritoneum (deep endometriosis) or can be present as an ovarian endometriotic cyst (endometrioma)."1 Always consider endometriosis in the infertile patient.
Although many professional societies and numerous Cochrane Database Systematic Reviews have provided guidelines on endometriosis, controversy and uncertainty remain. The World Endometriosis Society (WES) and the World Endometriosis Research Foundation (WERF), however, have now published several consensus documents that assess the global literature and professional organization guidelines in a structured, consensus-driven process.2-4 These WES and WERF documents consolidate known information and can be used to inform the clinician in making evidence-linked diagnostic and treatment decisions. Recommendations offered in this discussion are based on those documents.
Establishing the diagnosis can be difficult
Diagnosis of endometriosis is often difficult and is delayed an average of 7 years from onset of symptoms. These include severe dysmenorrhea, deep dyspareunia, chronic pelvic pain, ovulation pain, cyclical or perimenstrual symptoms (bowel or bladder associated) with or without abnormal bleeding, chronic fatigue, and infertility. A major difficulty is that the predictive value of any one symptom or set of symptoms remains uncertain, as each of these symptoms can have other causes, and a significant proportion of affected women are asymptomatic.
For a definitive diagnosis of endometriosis, visual inspection of the pelvis at laparoscopy is the gold standard investigation, unless disease is visible in the vagina or elsewhere. Positive histology confirms the diagnosis of endometriosis; negative histology does not exclude it. Whether histology should be obtained if peritoneal disease alone is present is controversial: visual inspection usually is adequate, but histologic confirmation of at least one lesion is ideal. In cases of ovarian endometrioma (>4 cm in diameter) and in deeply infiltrating disease, histology should be obtained to identify endometriosis and to exclude rare instances of malignancy.
Compared with laparoscopy, transvaginal ultrasonography (TVUS) has no value in diagnosing peritoneal endometriosis, but it is a useful tool for both making and excluding the diagnosis of an ovarian endometrioma. TVUS may have a role in the diagnosis of disease involving the bladder or rectum.
At present, evidence is insufficient to indicate that magnetic resonance imaging (MRI) is useful for diagnosing or excluding endometriosis compared with laparoscopy. MRI should be reserved for when ultrasound results are equivocal in cases of rectovaginal or bladder endometriosis.
Serum cancer antigen 125 (CA 125) levels may be elevated in endometriosis. However, measuring serum CA 125 levels has no value as a diagnostic tool.
No fertility benefit with ovarian suppression
More than 2 dozen randomized controlled trials (RCTs) provide strong evidence that there is no fertility benefit from ovarian suppression. The drug costs and delayed time to pregnancy mean that ovarian suppression with oral contraceptives, other progestational agents, or gonadotropin-releasing hormone (GnRH) agonists before fertility treatment is not indicated, with the possible exception of using it prior to in vitro fertilization (IVF).
Ovarian suppression also has been suggested as beneficial in conjunction with surgery. However, at least 16 RCTs have failed to show fertility improvement when ovarian suppression is given either preoperatively or postoperatively. Again, the delay in attempting pregnancy, drug costs, and adverse effects render ovarian suppression not appropriate.
While ovarian suppression has not been shown to increase pregnancy rates, ovarian stimulation (OS) likely does, especially when combined with intrauterine insemination (IUI).5
Laparoscopy: Appropriate for selected patients
A major decision for clinicians and patients dealing with infertility is whether to perform a laparoscopy, both for diagnostic and for treatment reasons. Currently, data are insufficient to recommend laparoscopic surgery prior to OS/IUI unless there is a history of evidence of anatomic disease and/or the patient has sufficient pain to justify the physical, emotional, financial, and time costs of laparoscopy. Laparoscopy therefore can be considered as possibly appropriate in younger women (<37 years of age) with short duration of infertility (<4 years), normal male factor, normal or treatable uterus, normal or treatable ovulation disorder, and limited prior treatment.
It is important to consider what disease might be found and how much of an increase in fertility can be obtained by treatment, so that the number needed to treat (NNT) can be used as an estimate of the potential value of laparoscopy in a given patient. A patient also should have no contraindications to laparoscopy and accept 9 to 15 months of attempting pregnancy before undergoing IVF treatment.
When laparoscopy is performed for minimal to mild disease, the odds ratio for pregnancy is 1.66 with treatment. It is important to remove all visible disease without injuring healthy tissue. When disease is moderate to severe, there is often severe anatomic distortion and a very low background pregnancy rate. Numerous uncontrolled trials show benefit of operative laparoscopy, especially for invasive, adhesive, and cystic endometriosis. However, repeat surgery is rarely indicated. After surgery, the Endometriosis Fertility Index (EFI) can be used to determine prognosis and plan management (FIGURE 1).6 An easy-to-use electronic EFI calculator is available online at www.endometriosisefi.com.

Management of endometriomas
Endometriomas are often operated on because of pain. Initial pain relief occurs in 60% to 100% of patients, but cysts recur following stripping about 10% of the time, and drainage without stripping, about 20%. With recurrence, pain is present about 75% of the time.
Pregnancy rates following endometrioma treatment depend on patient age and the status of the pelvis following operative intervention. This can be determined from the EFI. Often, the dilemma with endometriomas is how aggressive to be in removing them. The principles involved are to remove all the cyst wall if possible, but absolutely to minimize ovarian tissue damage, because reduced ovarian reserve is a possible major negative consequence of ovarian surgery.
Recommendations
While endometriosis is often a cause of infertility, often infertile patients do not have endometriosis. A careful history, physical examination, and ultrasonography, and possibly other imaging studies, are prerequisites to careful clinical judgment in diagnosing and treating infertile patients who might or do have endometriosis.
When pelvic pain is present, initially nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives (OCs), progestational agents, or an intrauterine device can be helpful. These ovarian suppression medications do not increase fertility, however, and should be stopped in any patient who desires to get pregnant.
When pelvic and male fertility factors appear reasonably normal (even if minimal or mild endometriosis is suspected), treatment with clomiphene 100 mg on cycle days 3 through 7 and IUI for 3 to 6 cycles is an effective first step. However, if the patient has persistent pain and/or infertility without other significant infertility factors, then diagnostic laparoscopy with intraoperative treatment of disease is indicated.
Surgery well performed is effective treatment for all stages of endometriosis and endometriomas, both for infertility and for pain. Repeat surgery, however, is rarely indicated because of limited results, so it is important to obtain the best possible result on the first surgery. Surgery is indicated for large endometriomas (>4 cm). Endometriosis has almost no effect on the IVF live birth rate unless ovarian reserve has been reduced by endometriomas or surgery, so endometriosis surgery should be performed by skilled and experienced surgeons.
Endometriosis is a complex disease that can cause infertility. Its diagnosis and management are frequently difficult, requiring knowledge, experience, and good medical judgment and surgical skills. However, if evidence-linked principles are followed, effective treatment plans and good outcomes can be obtained for most patients.
Read about why oil-based contrast may be better than water-based contrast with HSG.
Oil-based contrast medium use in hysterosalpingography is associated with higher pregnancy rates compared with water-based contrast
Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043-2052.
Hysterosalpingography (HSG) to assess tubal patency has been a mainstay of infertility diagnosis for decades. Some, but not all, studies also have suggested that pregnancy rates are higher after this tubal flushing procedure, especially if performed with oil contrast.7,8 A recent multicenter, randomized, controlled trial by Dreyer and colleagues that compared ongoing pregnancy rates and other outcomes among women who had HSG with oil contrast versus with water contrast provides additional valuable information.9
Trial details
In this study, 1,294 infertile women in 27 academic, teaching and nonteaching hospitals were screened for trial eligibility; 1,119 women provided written informed consent. Of these, 557 women were randomly assigned to HSG with oil contrast and 562 to water contrast. The women had spontaneous menstrual cycles, had been attempting pregnancy for at least 1 year, and had indications for HSG.
Exclusion criteria were known endocrine disorders, fewer than 8 menstrual cycles per year, a high risk of tubal disease, iodine allergy, and a total motile sperm count after sperm wash of less than 3 million/mL in the male partner (or a total motile sperm count of less than 1 million/mL when an analysis after sperm wash was not performed).
Just prior to undergoing HSG, the women were randomly assigned to receive either oil contrast or water contrast medium. (The trial was not blinded to participants or caregivers.) HSG was performed according to local protocols using cervical vacuum cup, metal cannula (hysterophore), or balloon catheter and approximately 5 to 10 mL of contrast medium.
After HSG, couples received expectant management when the predicted likelihood of pregnancy within 12 months, based on the prognostic model of Hunault, was 30% or greater.10 IUI was offered for pregnancy likelihood less than 30%, mild male infertility, or failure after a period of expectant management. IUI with or without mild ovarian stimulation (2-3 follicles) with clomiphene or gonadotropins was initiated after a minimum of 2 months of expectant management after HSG.
The primary outcome measure was ongoing pregnancy, defined as a positive fetal heartbeat on ultrasonographic examination after 12 weeks of gestation, with the first day of the last menstrual cycle for the pregnancy within 6 months after randomization. Secondary outcome measures were clinical pregnancy, live birth, miscarriage, ectopic pregnancy, time to pregnancy, and pain scores after HSG. All data were analyzed according to intention-to-treat.
Pregnancy rates increased with oil-contrast HSG
The baseline characteristics of the 2 groups were similar. HSG showed bilateral tubal patency in 477 of 554 women (86.1%) in the oil contrast group and in 491 of 554 women (88.6%) who received the water contrast (rate ratio, 0.97; 95% confidence interval [CI], 0.93-1.02). Bilateral tubal occlusion occurred in 9 women in the oil group (1.6%) and in 13 in the water group (2.3%) (relative risk, 0.69; 95% CI, 0.30-1.61).
A total of 58.3% of the women assigned to oil contrast and 57.2% of those assigned to water contrast received expectant management. Similar percentages of women in the oil group and in the water group underwent IUI (39.7% and 41.0%, respectively), IVF or intracytoplasmic sperm injection (ICSI) (2.3% and 2.2%), laparoscopy (6.2% in each group), and hysteroscopy (4.4% and 4.2%).
Ongoing pregnancy occurred in 220 of 554 women (39.7%) in the oil contrast group and in 161 of 554 women (29.1%) in the water contrast group (rate ratio, 1.37; 95% CI, 1.16-1.61; P<.001). The median time to the onset of pregnancy in the oil group was 2.7 months (interquartile range, 1.5-4.7) (FIGURE 2), while in the water group it was 3.1 months (interquartile range, 1.6-4.8) (P = .44).

While the proportion of women getting pregnant with or without the different interventions was similar in both groups, the live birth rate was 38.8% in the oil group versus 28.1% in the water group (rate ratio, 1.38; 95% CI, 1.17-1.64; P<.001). Three of 554 women (0.5%) assigned to oil contrast and 4 of 554 women (0.7%) in the water contrast group had an adverse event during the trial period. Three women (1.4%), all in the oil group, delivered a child with a congenital anomaly.
Why this study is important
This is the largest and best methodologic study on this clinical issue. It showed higher pregnancy and live birth rates within 6 months of HSG performed with oil compared with water. Although the study was not blinded, the group similarities and objective outcomes support minimal bias. Importantly, these results can be generalized only to women with similar inclusion characteristics.
It is unclear why oil HSG might enhance fertility. Suggested mechanisms include flushing of debris and/or mucous plugs or an effect on peritoneal macrophages or endometrial receptivity. Since HSG is minimally invasive and inexpensive, and the 10% increase in pregnancy rates corresponds to an NNT of 10, it is reasonable to consider, although formal cost-effectiveness data are lacking.
Concerns include the rare theoretical risk of intravasation with subsequent allergic reaction or fat embolism. Three infants in the oil group and none in the water group had congenital anomalies. This is likely due to chance, since this rate is not higher than that in the general population and no other data suggest an increased risk. Comparison of these results with other new techniques, such as sonohysterography (saline infusion sonogram), awaits further studies.
Recommendation
HSG with oil contrast should be considered a potential therapeutic as well as diagnostic intervention in selected patients.
HSG is an important diagnostic test for most infertility patients. The fact that a therapeutic benefit probably also is associated with oil-based HSG increases the clinical indications for this test.
Read about new definitions of infertility terminology you should know.
Infertility glossary is newly updated
Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393-406.
Terms and definitions used in infertility and fertility care frequently have had different meanings for different stakeholders, especially on a global basis. This can result in misunderstandings and inappropriate interpretation and comparison of published information and research. To help address these issues, international fertility organizations recently developed an updated glossary on infertilityterminology.
The consensus process for updating the glossary
The International Glossary on Infertility and Fertility Care, 2017, was recently published simultaneously in Fertility and Sterility and Human Reproduction. This is the second revision; the first glossary was published in 2006 and revised in 2009. This revision's 25 lead experts began work in 2014. Their teams of professionals interacted by electronic mail, at international and regional society meetings, and at 2 consultations held in Geneva, Switzerland. This glossary represents consensus agreement reached on 283 evidence-driven terms and definitions.
The work was led by the International Committee for Monitoring Assisted Reproductive Technologies in partnership with the American Society for Reproductive Medicine, European Society of Human Reproduction and Embryology, International Federation of Fertility Societies, March of Dimes, African Fertility Society, Groupe Inter-africain d'Etude de Recherche et d'Application sur la Fertilité, Asian Pacific Initiative on Reproduction, Middle East Fertility Society, Red Latinoamericana de Reproducción Asistida, and the International Federation of Gynecology and Obstetrics.
All together, 108 international professional experts (clinicians, basic scientists, epidemiologists, and social scientists), along with national and regional representatives of infertile persons, participated in the development of this evidence-base driven glossary. As such, these definitions now set the standard for international communication among clinicians, scientists, and policymakers.
Definition of infertility is broadened
The definitions take account of ethics, human rights, cultural sensitivities, ethnic minorities, and gender equality. For example, the first modification included broadening the concept of infertility to be an "impairment of individuals" in their capacity to reproduce, irrespective of whether the individual has a partner. (See “Broadened definition of infertility” below). Reproductive rights are individual human rights and do not depend on a relationship with another individual. The revised definition also reinforces the concept of infertility as a disease that can generate an impairment of function.
Infertility: A disease characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person’s capacity to reproduce either as an individual or with his/her partner. Fertility interventions may be initiated in less than 1 year based on medical, sexual and reproductive history, age, physical findings and diagnostic testing. Infertility is a disease, which generates disability as an impairment of function.
Reference
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406
New--and changed--definitions
Certain terms need to be consistent with those used currently internationally, for example, at which gestational age a miscarriage/abortion becomes a stillbirth.
Some terms are confusing, such as subfertility, which does not define a different or less severe fertility status than infertility, does not exist before infertility is diagnosed, and should not be confused with sterility, which is a permanent state of infertility. The term subfertility therefore is redundant and has been removed and replaced by infertility (See “Some terms with an important new definition” below).
- Clinical pregnancy
- Conception (removed from glossary)
- Diminished ovarian reserve
- Fertility care
- Hypospermia (replaces oligospermia)
- Ovarian reserve
- Pregnancy
- Preimplantation genetic testing
- Spontaneous abortion/miscarriage
- Subfertility (should be used interchangeably with infertility)
Reference
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406.
In a different context, the term conception, and its derivatives such as conceiving or conceived, was removed because it cannot be described biologically during the process of reproduction. Instead, terms such as fertilization, implantation, pregnancy, and live birth should be used.
Important male terms also changed: oligospermia is a term for low semen volume that is now replaced by hypospermia to avoid confusion with oligozoospermia, which is low concentration of spermatozoa in the ejaculate below the lower reference limit. When reporting results, the reference criteria should be specified.
Lastly, owing to the lack of standardization in determining the burden of infertility, and to better ensure comparability of prevalence data published globally, this glossary includes definitions for terms frequently used in epidemiology and public health. Examples include voluntary and involuntary childlessness, primary and secondary infertility, fertility care, fecundity, and fecundability, among others.
Getting the word out
The glossary has been approved by all of the participating organizations who are assisting in its distribution. It is being presented at national and international meetings and is used in The FIGO Fertility Toolbox (www.fertilitytool.com). It is hoped that all professionals and other stakeholders will begin to use its terminology globally to provide quality care and ensure consistency in registering specific fertility care interventions and more accurate reporting of their outcomes.
The language we use determines our individual and collective understanding of the scientific and clinical care of our patients. This glossary provides an essential and comprehensive standardization of terms and definitions essential to quality reproductive health care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Clinicians always should consider endometriosis in the diagnostic work-up of an infertility patient. But the diagnosis of endometriosis is often difficult, and management is complex. In this Update, we summarize international consensus documents on endometriosis with the aim of enhancing clinicians’ ability to make evidence-based decisions. In addition, we explore the interesting results of a large hysterosalpingography trial in which 2 different contrast mediums were used. Finally, we urge all clinicians to adapt the new standardized lexicon of infertility and fertility care terms that comprise the recently revised international glossary.
Endometriosis and infertility: The knowns and unknowns
Johnson NP, Hummelshoj L, Adamson GD, et al; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315-324.
Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552-1568.
Rogers PA, Adamson GD, Al-Jefout M, et al; WES/WERF Consortium for Research Priorities in Endometriosis. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202-226.
Endometriosis is defined as "a disease characterized by the presence of endometrium-like epithelium and stroma outside the endometrium and myometrium. Intrapelvic endometriosis can be located superficially on the peritoneum (peritoneal endometriosis), can extend 5 mm or more beneath the peritoneum (deep endometriosis) or can be present as an ovarian endometriotic cyst (endometrioma)."1 Always consider endometriosis in the infertile patient.
Although many professional societies and numerous Cochrane Database Systematic Reviews have provided guidelines on endometriosis, controversy and uncertainty remain. The World Endometriosis Society (WES) and the World Endometriosis Research Foundation (WERF), however, have now published several consensus documents that assess the global literature and professional organization guidelines in a structured, consensus-driven process.2-4 These WES and WERF documents consolidate known information and can be used to inform the clinician in making evidence-linked diagnostic and treatment decisions. Recommendations offered in this discussion are based on those documents.
Establishing the diagnosis can be difficult
Diagnosis of endometriosis is often difficult and is delayed an average of 7 years from onset of symptoms. These include severe dysmenorrhea, deep dyspareunia, chronic pelvic pain, ovulation pain, cyclical or perimenstrual symptoms (bowel or bladder associated) with or without abnormal bleeding, chronic fatigue, and infertility. A major difficulty is that the predictive value of any one symptom or set of symptoms remains uncertain, as each of these symptoms can have other causes, and a significant proportion of affected women are asymptomatic.
For a definitive diagnosis of endometriosis, visual inspection of the pelvis at laparoscopy is the gold standard investigation, unless disease is visible in the vagina or elsewhere. Positive histology confirms the diagnosis of endometriosis; negative histology does not exclude it. Whether histology should be obtained if peritoneal disease alone is present is controversial: visual inspection usually is adequate, but histologic confirmation of at least one lesion is ideal. In cases of ovarian endometrioma (>4 cm in diameter) and in deeply infiltrating disease, histology should be obtained to identify endometriosis and to exclude rare instances of malignancy.
Compared with laparoscopy, transvaginal ultrasonography (TVUS) has no value in diagnosing peritoneal endometriosis, but it is a useful tool for both making and excluding the diagnosis of an ovarian endometrioma. TVUS may have a role in the diagnosis of disease involving the bladder or rectum.
At present, evidence is insufficient to indicate that magnetic resonance imaging (MRI) is useful for diagnosing or excluding endometriosis compared with laparoscopy. MRI should be reserved for when ultrasound results are equivocal in cases of rectovaginal or bladder endometriosis.
Serum cancer antigen 125 (CA 125) levels may be elevated in endometriosis. However, measuring serum CA 125 levels has no value as a diagnostic tool.
No fertility benefit with ovarian suppression
More than 2 dozen randomized controlled trials (RCTs) provide strong evidence that there is no fertility benefit from ovarian suppression. The drug costs and delayed time to pregnancy mean that ovarian suppression with oral contraceptives, other progestational agents, or gonadotropin-releasing hormone (GnRH) agonists before fertility treatment is not indicated, with the possible exception of using it prior to in vitro fertilization (IVF).
Ovarian suppression also has been suggested as beneficial in conjunction with surgery. However, at least 16 RCTs have failed to show fertility improvement when ovarian suppression is given either preoperatively or postoperatively. Again, the delay in attempting pregnancy, drug costs, and adverse effects render ovarian suppression not appropriate.
While ovarian suppression has not been shown to increase pregnancy rates, ovarian stimulation (OS) likely does, especially when combined with intrauterine insemination (IUI).5
Laparoscopy: Appropriate for selected patients
A major decision for clinicians and patients dealing with infertility is whether to perform a laparoscopy, both for diagnostic and for treatment reasons. Currently, data are insufficient to recommend laparoscopic surgery prior to OS/IUI unless there is a history of evidence of anatomic disease and/or the patient has sufficient pain to justify the physical, emotional, financial, and time costs of laparoscopy. Laparoscopy therefore can be considered as possibly appropriate in younger women (<37 years of age) with short duration of infertility (<4 years), normal male factor, normal or treatable uterus, normal or treatable ovulation disorder, and limited prior treatment.
It is important to consider what disease might be found and how much of an increase in fertility can be obtained by treatment, so that the number needed to treat (NNT) can be used as an estimate of the potential value of laparoscopy in a given patient. A patient also should have no contraindications to laparoscopy and accept 9 to 15 months of attempting pregnancy before undergoing IVF treatment.
When laparoscopy is performed for minimal to mild disease, the odds ratio for pregnancy is 1.66 with treatment. It is important to remove all visible disease without injuring healthy tissue. When disease is moderate to severe, there is often severe anatomic distortion and a very low background pregnancy rate. Numerous uncontrolled trials show benefit of operative laparoscopy, especially for invasive, adhesive, and cystic endometriosis. However, repeat surgery is rarely indicated. After surgery, the Endometriosis Fertility Index (EFI) can be used to determine prognosis and plan management (FIGURE 1).6 An easy-to-use electronic EFI calculator is available online at www.endometriosisefi.com.

Management of endometriomas
Endometriomas are often operated on because of pain. Initial pain relief occurs in 60% to 100% of patients, but cysts recur following stripping about 10% of the time, and drainage without stripping, about 20%. With recurrence, pain is present about 75% of the time.
Pregnancy rates following endometrioma treatment depend on patient age and the status of the pelvis following operative intervention. This can be determined from the EFI. Often, the dilemma with endometriomas is how aggressive to be in removing them. The principles involved are to remove all the cyst wall if possible, but absolutely to minimize ovarian tissue damage, because reduced ovarian reserve is a possible major negative consequence of ovarian surgery.
Recommendations
While endometriosis is often a cause of infertility, often infertile patients do not have endometriosis. A careful history, physical examination, and ultrasonography, and possibly other imaging studies, are prerequisites to careful clinical judgment in diagnosing and treating infertile patients who might or do have endometriosis.
When pelvic pain is present, initially nonsteroidal anti-inflammatory drugs (NSAIDs), oral contraceptives (OCs), progestational agents, or an intrauterine device can be helpful. These ovarian suppression medications do not increase fertility, however, and should be stopped in any patient who desires to get pregnant.
When pelvic and male fertility factors appear reasonably normal (even if minimal or mild endometriosis is suspected), treatment with clomiphene 100 mg on cycle days 3 through 7 and IUI for 3 to 6 cycles is an effective first step. However, if the patient has persistent pain and/or infertility without other significant infertility factors, then diagnostic laparoscopy with intraoperative treatment of disease is indicated.
Surgery well performed is effective treatment for all stages of endometriosis and endometriomas, both for infertility and for pain. Repeat surgery, however, is rarely indicated because of limited results, so it is important to obtain the best possible result on the first surgery. Surgery is indicated for large endometriomas (>4 cm). Endometriosis has almost no effect on the IVF live birth rate unless ovarian reserve has been reduced by endometriomas or surgery, so endometriosis surgery should be performed by skilled and experienced surgeons.
Endometriosis is a complex disease that can cause infertility. Its diagnosis and management are frequently difficult, requiring knowledge, experience, and good medical judgment and surgical skills. However, if evidence-linked principles are followed, effective treatment plans and good outcomes can be obtained for most patients.
Read about why oil-based contrast may be better than water-based contrast with HSG.
Oil-based contrast medium use in hysterosalpingography is associated with higher pregnancy rates compared with water-based contrast
Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043-2052.
Hysterosalpingography (HSG) to assess tubal patency has been a mainstay of infertility diagnosis for decades. Some, but not all, studies also have suggested that pregnancy rates are higher after this tubal flushing procedure, especially if performed with oil contrast.7,8 A recent multicenter, randomized, controlled trial by Dreyer and colleagues that compared ongoing pregnancy rates and other outcomes among women who had HSG with oil contrast versus with water contrast provides additional valuable information.9
Trial details
In this study, 1,294 infertile women in 27 academic, teaching and nonteaching hospitals were screened for trial eligibility; 1,119 women provided written informed consent. Of these, 557 women were randomly assigned to HSG with oil contrast and 562 to water contrast. The women had spontaneous menstrual cycles, had been attempting pregnancy for at least 1 year, and had indications for HSG.
Exclusion criteria were known endocrine disorders, fewer than 8 menstrual cycles per year, a high risk of tubal disease, iodine allergy, and a total motile sperm count after sperm wash of less than 3 million/mL in the male partner (or a total motile sperm count of less than 1 million/mL when an analysis after sperm wash was not performed).
Just prior to undergoing HSG, the women were randomly assigned to receive either oil contrast or water contrast medium. (The trial was not blinded to participants or caregivers.) HSG was performed according to local protocols using cervical vacuum cup, metal cannula (hysterophore), or balloon catheter and approximately 5 to 10 mL of contrast medium.
After HSG, couples received expectant management when the predicted likelihood of pregnancy within 12 months, based on the prognostic model of Hunault, was 30% or greater.10 IUI was offered for pregnancy likelihood less than 30%, mild male infertility, or failure after a period of expectant management. IUI with or without mild ovarian stimulation (2-3 follicles) with clomiphene or gonadotropins was initiated after a minimum of 2 months of expectant management after HSG.
The primary outcome measure was ongoing pregnancy, defined as a positive fetal heartbeat on ultrasonographic examination after 12 weeks of gestation, with the first day of the last menstrual cycle for the pregnancy within 6 months after randomization. Secondary outcome measures were clinical pregnancy, live birth, miscarriage, ectopic pregnancy, time to pregnancy, and pain scores after HSG. All data were analyzed according to intention-to-treat.
Pregnancy rates increased with oil-contrast HSG
The baseline characteristics of the 2 groups were similar. HSG showed bilateral tubal patency in 477 of 554 women (86.1%) in the oil contrast group and in 491 of 554 women (88.6%) who received the water contrast (rate ratio, 0.97; 95% confidence interval [CI], 0.93-1.02). Bilateral tubal occlusion occurred in 9 women in the oil group (1.6%) and in 13 in the water group (2.3%) (relative risk, 0.69; 95% CI, 0.30-1.61).
A total of 58.3% of the women assigned to oil contrast and 57.2% of those assigned to water contrast received expectant management. Similar percentages of women in the oil group and in the water group underwent IUI (39.7% and 41.0%, respectively), IVF or intracytoplasmic sperm injection (ICSI) (2.3% and 2.2%), laparoscopy (6.2% in each group), and hysteroscopy (4.4% and 4.2%).
Ongoing pregnancy occurred in 220 of 554 women (39.7%) in the oil contrast group and in 161 of 554 women (29.1%) in the water contrast group (rate ratio, 1.37; 95% CI, 1.16-1.61; P<.001). The median time to the onset of pregnancy in the oil group was 2.7 months (interquartile range, 1.5-4.7) (FIGURE 2), while in the water group it was 3.1 months (interquartile range, 1.6-4.8) (P = .44).

While the proportion of women getting pregnant with or without the different interventions was similar in both groups, the live birth rate was 38.8% in the oil group versus 28.1% in the water group (rate ratio, 1.38; 95% CI, 1.17-1.64; P<.001). Three of 554 women (0.5%) assigned to oil contrast and 4 of 554 women (0.7%) in the water contrast group had an adverse event during the trial period. Three women (1.4%), all in the oil group, delivered a child with a congenital anomaly.
Why this study is important
This is the largest and best methodologic study on this clinical issue. It showed higher pregnancy and live birth rates within 6 months of HSG performed with oil compared with water. Although the study was not blinded, the group similarities and objective outcomes support minimal bias. Importantly, these results can be generalized only to women with similar inclusion characteristics.
It is unclear why oil HSG might enhance fertility. Suggested mechanisms include flushing of debris and/or mucous plugs or an effect on peritoneal macrophages or endometrial receptivity. Since HSG is minimally invasive and inexpensive, and the 10% increase in pregnancy rates corresponds to an NNT of 10, it is reasonable to consider, although formal cost-effectiveness data are lacking.
Concerns include the rare theoretical risk of intravasation with subsequent allergic reaction or fat embolism. Three infants in the oil group and none in the water group had congenital anomalies. This is likely due to chance, since this rate is not higher than that in the general population and no other data suggest an increased risk. Comparison of these results with other new techniques, such as sonohysterography (saline infusion sonogram), awaits further studies.
Recommendation
HSG with oil contrast should be considered a potential therapeutic as well as diagnostic intervention in selected patients.
HSG is an important diagnostic test for most infertility patients. The fact that a therapeutic benefit probably also is associated with oil-based HSG increases the clinical indications for this test.
Read about new definitions of infertility terminology you should know.
Infertility glossary is newly updated
Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393-406.
Terms and definitions used in infertility and fertility care frequently have had different meanings for different stakeholders, especially on a global basis. This can result in misunderstandings and inappropriate interpretation and comparison of published information and research. To help address these issues, international fertility organizations recently developed an updated glossary on infertilityterminology.
The consensus process for updating the glossary
The International Glossary on Infertility and Fertility Care, 2017, was recently published simultaneously in Fertility and Sterility and Human Reproduction. This is the second revision; the first glossary was published in 2006 and revised in 2009. This revision's 25 lead experts began work in 2014. Their teams of professionals interacted by electronic mail, at international and regional society meetings, and at 2 consultations held in Geneva, Switzerland. This glossary represents consensus agreement reached on 283 evidence-driven terms and definitions.
The work was led by the International Committee for Monitoring Assisted Reproductive Technologies in partnership with the American Society for Reproductive Medicine, European Society of Human Reproduction and Embryology, International Federation of Fertility Societies, March of Dimes, African Fertility Society, Groupe Inter-africain d'Etude de Recherche et d'Application sur la Fertilité, Asian Pacific Initiative on Reproduction, Middle East Fertility Society, Red Latinoamericana de Reproducción Asistida, and the International Federation of Gynecology and Obstetrics.
All together, 108 international professional experts (clinicians, basic scientists, epidemiologists, and social scientists), along with national and regional representatives of infertile persons, participated in the development of this evidence-base driven glossary. As such, these definitions now set the standard for international communication among clinicians, scientists, and policymakers.
Definition of infertility is broadened
The definitions take account of ethics, human rights, cultural sensitivities, ethnic minorities, and gender equality. For example, the first modification included broadening the concept of infertility to be an "impairment of individuals" in their capacity to reproduce, irrespective of whether the individual has a partner. (See “Broadened definition of infertility” below). Reproductive rights are individual human rights and do not depend on a relationship with another individual. The revised definition also reinforces the concept of infertility as a disease that can generate an impairment of function.
Infertility: A disease characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or due to an impairment of a person’s capacity to reproduce either as an individual or with his/her partner. Fertility interventions may be initiated in less than 1 year based on medical, sexual and reproductive history, age, physical findings and diagnostic testing. Infertility is a disease, which generates disability as an impairment of function.
Reference
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406
New--and changed--definitions
Certain terms need to be consistent with those used currently internationally, for example, at which gestational age a miscarriage/abortion becomes a stillbirth.
Some terms are confusing, such as subfertility, which does not define a different or less severe fertility status than infertility, does not exist before infertility is diagnosed, and should not be confused with sterility, which is a permanent state of infertility. The term subfertility therefore is redundant and has been removed and replaced by infertility (See “Some terms with an important new definition” below).
- Clinical pregnancy
- Conception (removed from glossary)
- Diminished ovarian reserve
- Fertility care
- Hypospermia (replaces oligospermia)
- Ovarian reserve
- Pregnancy
- Preimplantation genetic testing
- Spontaneous abortion/miscarriage
- Subfertility (should be used interchangeably with infertility)
Reference
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406.
In a different context, the term conception, and its derivatives such as conceiving or conceived, was removed because it cannot be described biologically during the process of reproduction. Instead, terms such as fertilization, implantation, pregnancy, and live birth should be used.
Important male terms also changed: oligospermia is a term for low semen volume that is now replaced by hypospermia to avoid confusion with oligozoospermia, which is low concentration of spermatozoa in the ejaculate below the lower reference limit. When reporting results, the reference criteria should be specified.
Lastly, owing to the lack of standardization in determining the burden of infertility, and to better ensure comparability of prevalence data published globally, this glossary includes definitions for terms frequently used in epidemiology and public health. Examples include voluntary and involuntary childlessness, primary and secondary infertility, fertility care, fecundity, and fecundability, among others.
Getting the word out
The glossary has been approved by all of the participating organizations who are assisting in its distribution. It is being presented at national and international meetings and is used in The FIGO Fertility Toolbox (www.fertilitytool.com). It is hoped that all professionals and other stakeholders will begin to use its terminology globally to provide quality care and ensure consistency in registering specific fertility care interventions and more accurate reporting of their outcomes.
The language we use determines our individual and collective understanding of the scientific and clinical care of our patients. This glossary provides an essential and comprehensive standardization of terms and definitions essential to quality reproductive health care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406.
- Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–1568.
- Rogers PA, Adamson GD, Al-Jefout M, et al; WES/WERF Consortium for Research Priorities in Endometriosis. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202–226.
- Johnson NP, Hummelshoj L, Adamson GD, et al; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–324.
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598.
- Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94(5):1609–1615.
- Weir WC, Weir DR. Therapeutic value of salpingograms in infertility. Fertil Steril. 1951;2(6);514–522.
- Johnson NP, Farquhar CM, Hadden WE, Suckling J, Yu Y, Sadler L. The FLUSH trial—flushing with lipiodol for unexplained (and endometriosis-related) subfertility by hysterosalpingography: a randomized trial. Hum Reprod. 2004;19(9):2043–2051.
- Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043–2052.
- Van der Steeg JW, Steures P, Eijkemans MJ, et al; Collaborative Effort for Clinical Evaluation in Reproductive Medicine. Pregnancy is predictable: a large-scale prospective external validation of the prediction of spontaneous pregnancy in sub-fertile couples. Hum Reprod. 2007;22(2):536–542.
- Zegers-Hochchild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406.
- Johnson NP, Hummelshoj L; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–1568.
- Rogers PA, Adamson GD, Al-Jefout M, et al; WES/WERF Consortium for Research Priorities in Endometriosis. Research priorities for endometriosis. Reprod Sci. 2017;24(2):202–226.
- Johnson NP, Hummelshoj L, Adamson GD, et al; World Endometriosis Society Sao Paulo Consortium. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–324.
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598.
- Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94(5):1609–1615.
- Weir WC, Weir DR. Therapeutic value of salpingograms in infertility. Fertil Steril. 1951;2(6);514–522.
- Johnson NP, Farquhar CM, Hadden WE, Suckling J, Yu Y, Sadler L. The FLUSH trial—flushing with lipiodol for unexplained (and endometriosis-related) subfertility by hysterosalpingography: a randomized trial. Hum Reprod. 2004;19(9):2043–2051.
- Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043–2052.
- Van der Steeg JW, Steures P, Eijkemans MJ, et al; Collaborative Effort for Clinical Evaluation in Reproductive Medicine. Pregnancy is predictable: a large-scale prospective external validation of the prediction of spontaneous pregnancy in sub-fertile couples. Hum Reprod. 2007;22(2):536–542.
Health care gets little attention in State of the Union address
President Trump reaffirmed his campaign promise to lower prescription drug prices during his first State of the Union address – but gave no details on how he plans to do so.
“One of my greatest priorities is to reduce the price of prescription drugs,” President Trump said in his Jan. 30 address to a joint session of Congress. “In many other countries, these drugs cost far less than what we pay in the United States, and it is very, very unfair. That is why I have directed my administration to make fixing the injustice of high drug prices one of my top priorities for the year.”
His words followed the confirmation of Alex Azar as Health & Human Services secretary. Mr. Azar’s nomination was criticized by some who questioned whether the former president of Eli Lilly’s U.S. operations could be effective at tackling the surging prices of pharmaceuticals.
President Trump also expressed his support for allowing terminally ill patients to access experimental drugs prior to Food and Drug Administration approval, the so-called right to try.
“We also believe that patients with terminal conditions, terminal illness, should have access to experimental treatment immediately that could potentially save their lives,” he said. “People who are terminally ill should not have to go from country to country to seek a cure. I want to give them a chance right here at home. It’s time for the Congress to give these wonderful incredible Americans the right to try.”
The Senate passed a right to try bill (S. 204) in 2017 by unanimous consent, but the House has yet to act upon it.
President Trump reaffirmed his commitment to fighting the opioid epidemic and made a loose connection between it and his overall platform for immigration reform, saying that “these reforms will also support our response to the terrible crisis of opioid and drug addiction.”
As far as addressing the epidemic itself, Mr. Trump said that his administration “is committed to fighting the drug epidemic and helping get treatment for those in need, for those who have been so terribly hurt. The struggle will be long and it will be difficult, but, as Americans always do, in the end we will succeed. We will prevail.”
The president also commended Congress for effectively eliminating the Affordable Care Act’s individual mandate that required people to have health insurance or suffer a financial penalty.
President Trump reaffirmed his campaign promise to lower prescription drug prices during his first State of the Union address – but gave no details on how he plans to do so.
“One of my greatest priorities is to reduce the price of prescription drugs,” President Trump said in his Jan. 30 address to a joint session of Congress. “In many other countries, these drugs cost far less than what we pay in the United States, and it is very, very unfair. That is why I have directed my administration to make fixing the injustice of high drug prices one of my top priorities for the year.”
His words followed the confirmation of Alex Azar as Health & Human Services secretary. Mr. Azar’s nomination was criticized by some who questioned whether the former president of Eli Lilly’s U.S. operations could be effective at tackling the surging prices of pharmaceuticals.
President Trump also expressed his support for allowing terminally ill patients to access experimental drugs prior to Food and Drug Administration approval, the so-called right to try.
“We also believe that patients with terminal conditions, terminal illness, should have access to experimental treatment immediately that could potentially save their lives,” he said. “People who are terminally ill should not have to go from country to country to seek a cure. I want to give them a chance right here at home. It’s time for the Congress to give these wonderful incredible Americans the right to try.”
The Senate passed a right to try bill (S. 204) in 2017 by unanimous consent, but the House has yet to act upon it.
President Trump reaffirmed his commitment to fighting the opioid epidemic and made a loose connection between it and his overall platform for immigration reform, saying that “these reforms will also support our response to the terrible crisis of opioid and drug addiction.”
As far as addressing the epidemic itself, Mr. Trump said that his administration “is committed to fighting the drug epidemic and helping get treatment for those in need, for those who have been so terribly hurt. The struggle will be long and it will be difficult, but, as Americans always do, in the end we will succeed. We will prevail.”
The president also commended Congress for effectively eliminating the Affordable Care Act’s individual mandate that required people to have health insurance or suffer a financial penalty.
President Trump reaffirmed his campaign promise to lower prescription drug prices during his first State of the Union address – but gave no details on how he plans to do so.
“One of my greatest priorities is to reduce the price of prescription drugs,” President Trump said in his Jan. 30 address to a joint session of Congress. “In many other countries, these drugs cost far less than what we pay in the United States, and it is very, very unfair. That is why I have directed my administration to make fixing the injustice of high drug prices one of my top priorities for the year.”
His words followed the confirmation of Alex Azar as Health & Human Services secretary. Mr. Azar’s nomination was criticized by some who questioned whether the former president of Eli Lilly’s U.S. operations could be effective at tackling the surging prices of pharmaceuticals.
President Trump also expressed his support for allowing terminally ill patients to access experimental drugs prior to Food and Drug Administration approval, the so-called right to try.
“We also believe that patients with terminal conditions, terminal illness, should have access to experimental treatment immediately that could potentially save their lives,” he said. “People who are terminally ill should not have to go from country to country to seek a cure. I want to give them a chance right here at home. It’s time for the Congress to give these wonderful incredible Americans the right to try.”
The Senate passed a right to try bill (S. 204) in 2017 by unanimous consent, but the House has yet to act upon it.
President Trump reaffirmed his commitment to fighting the opioid epidemic and made a loose connection between it and his overall platform for immigration reform, saying that “these reforms will also support our response to the terrible crisis of opioid and drug addiction.”
As far as addressing the epidemic itself, Mr. Trump said that his administration “is committed to fighting the drug epidemic and helping get treatment for those in need, for those who have been so terribly hurt. The struggle will be long and it will be difficult, but, as Americans always do, in the end we will succeed. We will prevail.”
The president also commended Congress for effectively eliminating the Affordable Care Act’s individual mandate that required people to have health insurance or suffer a financial penalty.