User login
Study eyed natural history of branch-duct intraductal papillary mucinous neoplasms
Branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) grew at a median annual rate of 0.8 mm in a retrospective study of 1,369 patients.
While most of these cysts were “indolent and dormant,” some grew rapidly and developed “other worrisome features,” Youngmin Han, MS, of Seoul (South Korea) National University reported with his associates in the February issue of Gastroenterology. Therefore, clinicians should plan follow-up surveillance based on initial cyst size and growth rate, they concluded.
Based on their findings, the researchers recommended surgery for young, fit, asymptomatic patients who have BD-IPMNs with a diameter of least 30 mm or with thickened cyst walls or those who have a main pancreatic duct measuring 5-9 mm. Surgery also should be considered when patients have lymphadenopathy, high tumor marker levels, or an abrupt change in pancreatic duct caliber with distal pancreatic atrophy or a rapidly growing cyst, they said.
For asymptomatic patients whose cysts are under 10 mm and who do not have worrisome features, they recommended follow-up with CT or MRI at 6 months and then every 2 years after that. Cysts of 10-20 mm should be imaged at 6 months, at 12 months, and then every 1.5-2 years after that, they said. Patients with cyst diameters greater than 20 mm “should undergo MRI or CT or EUS [endoscopic ultrasound] every 6 months for 1 year and then annually thereafter, until the cyst size and features become stable,” they added. Patients whose cysts have a diameter of 30 mm or greater “should be closely monitored with MRI or CT or EUS every 6 months. Surgical resection can be considered in younger patients or those with other combined worrisome features.”
To characterize the natural history of BD-IPMN, the investigators evaluated clinical and imaging data collected between 2001 and 2016 from patients with classical features of BD-IPMN. Each patient included in the study provided 3 or more years of CT, MRI, EUS, and endoscopic retrograde cholangiopancreatography data. The researchers used regression models to estimate changes in sizes of cysts and main pancreatic ducts.
Median follow-up time was 61 months (range, 36-189 months). Cyst diameter averaged 12.8 mm (standard deviation, 6.5 mm) at baseline and 17 mm (SD, 9.2 mm) at final measurement. Larger baseline diameter was associated with faster growth (P = .046): Cysts measuring less than 10 mm at baseline grew at a median annual rate of 0.8 mm (SD, 1.1 mm), while those measuring at least 30 mm grew at a median annual rate of 1.2 mm (SD, 2.1 mm).
Worrisome features were present in 59 patients at baseline and emerged in another 150 patients during follow-up. At baseline, only 2.3% of cysts exceeded 30 mm in diameter, but 8.0% did at final measurement. Cyst wall thickening was found in 0.5% of patients at baseline and 3.7% of patients at final measurement. Main pancreatic ducts measured 5-9 mm in 1.9% of patients at baseline and in 5.6% of patients at final measurement. Additionally, the prevalence of mural nodules rose from 0.4% at baseline to 3.1% at final measurement.
Main pancreatic ducts averaged 1.8 mm (SD, 1.0 mm) at baseline and 2.4 mm (SD, 1.8 mm) at final measurement. Compared with the values seen with smaller cysts, larger baseline cyst diameter correlated significantly with larger main pancreatic ducts, more cases of cyst wall thickening, and more cases with mural nodules (P less than .001 for all comparisons).
The study was funded by a grant from Korean Health Technology R&D Project of Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
SOURCE: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
The appropriate management of branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs), a precursor cystic lesion to pancreatic cancer, has been a controversial issue since their initial description in 1982. Current national and international guidelines are primarily based on surgical series with potential selection bias and on observational studies with short surveillance periods. Consequently, there is limited information on the natural history and, more importantly, the malignant potential of BD-IPMNs.
The study by Youngmin Han and colleagues represents a comprehensive analysis of over 1,000 patients, each with at least 3 years of follow-up for a suspected BD-IPMN. In addition, the authors identified an optimal screening method for patients based on cyst size. Their data largely validates prior reports and will undoubtedly serve as the basis for future pancreatic cyst guidelines.
However, as the authors note, limitations of their study include its retrospective design and validation of their screening protocol. Moreover, several lingering questions remain for patients with BD-IPMNs: What is the best method of measuring a BD-IPMN (for example, CT, MRI, or endoscopic ultrasound)? How long should surveillance continue? And what is the role for cytopathology and ancillary studies, such as carcinoembryonic antigen testing, molecular testing, and testing for other pancreatic cyst biomarkers? At the risk of enouncing a cliché, “further studies are needed” to identify an optimal treatment algorithm and, considering the increasingly frequent detection of pancreatic cysts, a cost-effective approach to the evaluation of patients with BD-IPMNs.
Aatur D. Singhi, MD, PhD, is in the division of anatomic pathology in the department of pathology at the University of Pittsburgh Medical Center. He has no conflicts of interest.
The appropriate management of branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs), a precursor cystic lesion to pancreatic cancer, has been a controversial issue since their initial description in 1982. Current national and international guidelines are primarily based on surgical series with potential selection bias and on observational studies with short surveillance periods. Consequently, there is limited information on the natural history and, more importantly, the malignant potential of BD-IPMNs.
The study by Youngmin Han and colleagues represents a comprehensive analysis of over 1,000 patients, each with at least 3 years of follow-up for a suspected BD-IPMN. In addition, the authors identified an optimal screening method for patients based on cyst size. Their data largely validates prior reports and will undoubtedly serve as the basis for future pancreatic cyst guidelines.
However, as the authors note, limitations of their study include its retrospective design and validation of their screening protocol. Moreover, several lingering questions remain for patients with BD-IPMNs: What is the best method of measuring a BD-IPMN (for example, CT, MRI, or endoscopic ultrasound)? How long should surveillance continue? And what is the role for cytopathology and ancillary studies, such as carcinoembryonic antigen testing, molecular testing, and testing for other pancreatic cyst biomarkers? At the risk of enouncing a cliché, “further studies are needed” to identify an optimal treatment algorithm and, considering the increasingly frequent detection of pancreatic cysts, a cost-effective approach to the evaluation of patients with BD-IPMNs.
Aatur D. Singhi, MD, PhD, is in the division of anatomic pathology in the department of pathology at the University of Pittsburgh Medical Center. He has no conflicts of interest.
The appropriate management of branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs), a precursor cystic lesion to pancreatic cancer, has been a controversial issue since their initial description in 1982. Current national and international guidelines are primarily based on surgical series with potential selection bias and on observational studies with short surveillance periods. Consequently, there is limited information on the natural history and, more importantly, the malignant potential of BD-IPMNs.
The study by Youngmin Han and colleagues represents a comprehensive analysis of over 1,000 patients, each with at least 3 years of follow-up for a suspected BD-IPMN. In addition, the authors identified an optimal screening method for patients based on cyst size. Their data largely validates prior reports and will undoubtedly serve as the basis for future pancreatic cyst guidelines.
However, as the authors note, limitations of their study include its retrospective design and validation of their screening protocol. Moreover, several lingering questions remain for patients with BD-IPMNs: What is the best method of measuring a BD-IPMN (for example, CT, MRI, or endoscopic ultrasound)? How long should surveillance continue? And what is the role for cytopathology and ancillary studies, such as carcinoembryonic antigen testing, molecular testing, and testing for other pancreatic cyst biomarkers? At the risk of enouncing a cliché, “further studies are needed” to identify an optimal treatment algorithm and, considering the increasingly frequent detection of pancreatic cysts, a cost-effective approach to the evaluation of patients with BD-IPMNs.
Aatur D. Singhi, MD, PhD, is in the division of anatomic pathology in the department of pathology at the University of Pittsburgh Medical Center. He has no conflicts of interest.
Branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) grew at a median annual rate of 0.8 mm in a retrospective study of 1,369 patients.
While most of these cysts were “indolent and dormant,” some grew rapidly and developed “other worrisome features,” Youngmin Han, MS, of Seoul (South Korea) National University reported with his associates in the February issue of Gastroenterology. Therefore, clinicians should plan follow-up surveillance based on initial cyst size and growth rate, they concluded.
Based on their findings, the researchers recommended surgery for young, fit, asymptomatic patients who have BD-IPMNs with a diameter of least 30 mm or with thickened cyst walls or those who have a main pancreatic duct measuring 5-9 mm. Surgery also should be considered when patients have lymphadenopathy, high tumor marker levels, or an abrupt change in pancreatic duct caliber with distal pancreatic atrophy or a rapidly growing cyst, they said.
For asymptomatic patients whose cysts are under 10 mm and who do not have worrisome features, they recommended follow-up with CT or MRI at 6 months and then every 2 years after that. Cysts of 10-20 mm should be imaged at 6 months, at 12 months, and then every 1.5-2 years after that, they said. Patients with cyst diameters greater than 20 mm “should undergo MRI or CT or EUS [endoscopic ultrasound] every 6 months for 1 year and then annually thereafter, until the cyst size and features become stable,” they added. Patients whose cysts have a diameter of 30 mm or greater “should be closely monitored with MRI or CT or EUS every 6 months. Surgical resection can be considered in younger patients or those with other combined worrisome features.”
To characterize the natural history of BD-IPMN, the investigators evaluated clinical and imaging data collected between 2001 and 2016 from patients with classical features of BD-IPMN. Each patient included in the study provided 3 or more years of CT, MRI, EUS, and endoscopic retrograde cholangiopancreatography data. The researchers used regression models to estimate changes in sizes of cysts and main pancreatic ducts.
Median follow-up time was 61 months (range, 36-189 months). Cyst diameter averaged 12.8 mm (standard deviation, 6.5 mm) at baseline and 17 mm (SD, 9.2 mm) at final measurement. Larger baseline diameter was associated with faster growth (P = .046): Cysts measuring less than 10 mm at baseline grew at a median annual rate of 0.8 mm (SD, 1.1 mm), while those measuring at least 30 mm grew at a median annual rate of 1.2 mm (SD, 2.1 mm).
Worrisome features were present in 59 patients at baseline and emerged in another 150 patients during follow-up. At baseline, only 2.3% of cysts exceeded 30 mm in diameter, but 8.0% did at final measurement. Cyst wall thickening was found in 0.5% of patients at baseline and 3.7% of patients at final measurement. Main pancreatic ducts measured 5-9 mm in 1.9% of patients at baseline and in 5.6% of patients at final measurement. Additionally, the prevalence of mural nodules rose from 0.4% at baseline to 3.1% at final measurement.
Main pancreatic ducts averaged 1.8 mm (SD, 1.0 mm) at baseline and 2.4 mm (SD, 1.8 mm) at final measurement. Compared with the values seen with smaller cysts, larger baseline cyst diameter correlated significantly with larger main pancreatic ducts, more cases of cyst wall thickening, and more cases with mural nodules (P less than .001 for all comparisons).
The study was funded by a grant from Korean Health Technology R&D Project of Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
SOURCE: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
Branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) grew at a median annual rate of 0.8 mm in a retrospective study of 1,369 patients.
While most of these cysts were “indolent and dormant,” some grew rapidly and developed “other worrisome features,” Youngmin Han, MS, of Seoul (South Korea) National University reported with his associates in the February issue of Gastroenterology. Therefore, clinicians should plan follow-up surveillance based on initial cyst size and growth rate, they concluded.
Based on their findings, the researchers recommended surgery for young, fit, asymptomatic patients who have BD-IPMNs with a diameter of least 30 mm or with thickened cyst walls or those who have a main pancreatic duct measuring 5-9 mm. Surgery also should be considered when patients have lymphadenopathy, high tumor marker levels, or an abrupt change in pancreatic duct caliber with distal pancreatic atrophy or a rapidly growing cyst, they said.
For asymptomatic patients whose cysts are under 10 mm and who do not have worrisome features, they recommended follow-up with CT or MRI at 6 months and then every 2 years after that. Cysts of 10-20 mm should be imaged at 6 months, at 12 months, and then every 1.5-2 years after that, they said. Patients with cyst diameters greater than 20 mm “should undergo MRI or CT or EUS [endoscopic ultrasound] every 6 months for 1 year and then annually thereafter, until the cyst size and features become stable,” they added. Patients whose cysts have a diameter of 30 mm or greater “should be closely monitored with MRI or CT or EUS every 6 months. Surgical resection can be considered in younger patients or those with other combined worrisome features.”
To characterize the natural history of BD-IPMN, the investigators evaluated clinical and imaging data collected between 2001 and 2016 from patients with classical features of BD-IPMN. Each patient included in the study provided 3 or more years of CT, MRI, EUS, and endoscopic retrograde cholangiopancreatography data. The researchers used regression models to estimate changes in sizes of cysts and main pancreatic ducts.
Median follow-up time was 61 months (range, 36-189 months). Cyst diameter averaged 12.8 mm (standard deviation, 6.5 mm) at baseline and 17 mm (SD, 9.2 mm) at final measurement. Larger baseline diameter was associated with faster growth (P = .046): Cysts measuring less than 10 mm at baseline grew at a median annual rate of 0.8 mm (SD, 1.1 mm), while those measuring at least 30 mm grew at a median annual rate of 1.2 mm (SD, 2.1 mm).
Worrisome features were present in 59 patients at baseline and emerged in another 150 patients during follow-up. At baseline, only 2.3% of cysts exceeded 30 mm in diameter, but 8.0% did at final measurement. Cyst wall thickening was found in 0.5% of patients at baseline and 3.7% of patients at final measurement. Main pancreatic ducts measured 5-9 mm in 1.9% of patients at baseline and in 5.6% of patients at final measurement. Additionally, the prevalence of mural nodules rose from 0.4% at baseline to 3.1% at final measurement.
Main pancreatic ducts averaged 1.8 mm (SD, 1.0 mm) at baseline and 2.4 mm (SD, 1.8 mm) at final measurement. Compared with the values seen with smaller cysts, larger baseline cyst diameter correlated significantly with larger main pancreatic ducts, more cases of cyst wall thickening, and more cases with mural nodules (P less than .001 for all comparisons).
The study was funded by a grant from Korean Health Technology R&D Project of Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
SOURCE: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
FROM GASTROENTEROLOGY
Key clinical point: Tailor the surveillance of BD-IPMNs based on initial diameter and the presence or absence of high-risk features.
Major finding: Median annual growth rate was 0.8 mm.
Data source: A retrospective study of 1,369 patients with BD-IPMNs.
Disclosures: The study was funded by a grant from the Korean Health Technology R&D Project of the Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
Source: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
One in five Crohn’s disease patients have major complications after infliximab withdrawal
About , according to research published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.09.061).
About 70% of patients remained free of both infliximab restart failure and major complications, said Catherine Reenaers, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium), and her associates. Significant predictors of major complications included upper gastrointestinal disease at the time of infliximab withdrawal, white blood cell count of at least 5.0 x 109 per L, and hemoglobin level under 12.5 g per dL. “Patients with at least two of these factors had a more than 40% risk of major complication in the 7 years following infliximab withdrawal,” the researchers reported.
Little is known about long-term outcomes after patients with Crohn’s disease withdraw from infliximab. Therefore, Dr. Reenaers and her associates retrospectively studied 102 patients with Crohn’s disease who had received infliximab and an antimetabolite (azathioprine, mercaptopurine, or methotrexate) for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and then withdrew from infliximab. Patients were recruited from 19 centers in Belgium and France and were originally part of a prospective cohort study of infliximab withdrawal in Crohn’s disease (Gastroenterology. 2012;142[1]:63-70.e5).
About half of patients relapsed and restarted infliximab within 12 months, which is in line with other studies, the researchers noted. Over a median follow-up of 83 months (interquartile range, 71-93 months), 21% (95% confidence interval, 13.1%-30.3%) of patients had no complications, did not restart infliximab, and started no other biologics. In all, 70.2% of patients (95% CI, 60.2%-80.1%) had no major complications and did not fail to respond after restarting infliximab.
Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: 14 who required surgery and 4 who developed new complex perianal lesions. In a multivariable model, the strongest independent predictor of major complications was leukocytosis (hazard ratio, 10.5; 95% CI, 1.3-83; P less than .002), followed by upper gastrointestinal disease (HR, 5.8; 95% CI, 1.5-22) and low hemoglobin level (HR, 4.1; 95% CI, 1.5-21.8; P less than .01). The 13 patients who lacked these risk factors had no major complications of infliximab withdrawal. Among 72 patients who had at least one risk factor, 16.3% (95% CI, 7%-25%) developed major complications over 7 years. Strikingly, among 17 with at least two risk factors, 43% (95% CI, 17%-69%) developed major complications over 7 years, the researchers noted.
Complications emerged a median of 50 months (interquartile range, 41-73 months) after patients received their last infliximab infusion, highlighting the need for close long-term monitoring even if patients show no signs of early clinical relapse after infliximab withdrawal, the investigators said. “One strength of this cohort was the homogeneity of the population,” they stressed. “Most studies of anti–tumor necrosis factor withdrawal after clinical remission were limited by heterogeneous populations, variable lengths of infliximab treatment before discontinuation, and variable use of immunomodulators and corticosteroids. In [our] cohort, the population was homogenous, infliximab withdrawal was standardized, and the disease characteristics at the time of stopping were collected prospectively.” Although follow-up times varied, less than 5% of patients were followed for less than 3 years, they noted.
The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
SOURCE: Reenaers C et al. Clin Gastro Hepatol 2018 February (in press).
The option of stopping a biologic agent is an attractive prospect for most Crohn's disease (CD) patients in stable clinical remission. The STORI trial, published in 2012, was among the earliest and select few studies addressing withdrawal of biologic therapy in CD among patients in sustained clinical remission with combination therapy (infliximab and thiopurine/methotrexate) for at least 6 months. Almost 50% of patients experienced disease relapse within a year of stopping infliximab in the trial.
Reenaers et al. recently published long-term follow-up of the original STORI cohort. After a median follow-up time of 7 years; four out five patients previously in clinical remission with combination therapy experienced worsening disease activity following withdrawal of infliximab. While the majority (70%) were able to resume infliximab and recapture disease response without any untoward adverse effects; one in five patients experienced major disease-related complications such as complex perianal disease or need for abdominal surgery. Upper GI tract involvement, high white blood cell count, and low hemoglobin concentration were associated with increased likelihood of a major complication. Notably, median time to a major complication was almost 4 years.
These results are similar to long-term relapse rates reported in other studies of withdrawal of therapy in CD. While biomarkers such as C-reactive protein, fecal calprotectin, along with endoscopic disease activity are reliable predictors of short-term relapse; clinical factors such as family history of CD, disease extent, stricturing or penetrating disease, and cigarette smoking are more relevant predictors of long-term disease activity. It is important to consider both types of predictors when considering withdrawal of therapy in CD.
Lastly, while the majority of patients who relapse following withdrawal of a biologic agent will do so within a year or two, a subset may not experience disease-related complications for several years - underscoring the need for long-term follow-up.
Manreet Kaur, MD, is assistant professor in the division of gastroenterology and hepatology; medical director, Inflammatory Bowel Disease Center, and medical director, faculty group practice, Baylor College of Medicine, Houston.
The option of stopping a biologic agent is an attractive prospect for most Crohn's disease (CD) patients in stable clinical remission. The STORI trial, published in 2012, was among the earliest and select few studies addressing withdrawal of biologic therapy in CD among patients in sustained clinical remission with combination therapy (infliximab and thiopurine/methotrexate) for at least 6 months. Almost 50% of patients experienced disease relapse within a year of stopping infliximab in the trial.
Reenaers et al. recently published long-term follow-up of the original STORI cohort. After a median follow-up time of 7 years; four out five patients previously in clinical remission with combination therapy experienced worsening disease activity following withdrawal of infliximab. While the majority (70%) were able to resume infliximab and recapture disease response without any untoward adverse effects; one in five patients experienced major disease-related complications such as complex perianal disease or need for abdominal surgery. Upper GI tract involvement, high white blood cell count, and low hemoglobin concentration were associated with increased likelihood of a major complication. Notably, median time to a major complication was almost 4 years.
These results are similar to long-term relapse rates reported in other studies of withdrawal of therapy in CD. While biomarkers such as C-reactive protein, fecal calprotectin, along with endoscopic disease activity are reliable predictors of short-term relapse; clinical factors such as family history of CD, disease extent, stricturing or penetrating disease, and cigarette smoking are more relevant predictors of long-term disease activity. It is important to consider both types of predictors when considering withdrawal of therapy in CD.
Lastly, while the majority of patients who relapse following withdrawal of a biologic agent will do so within a year or two, a subset may not experience disease-related complications for several years - underscoring the need for long-term follow-up.
Manreet Kaur, MD, is assistant professor in the division of gastroenterology and hepatology; medical director, Inflammatory Bowel Disease Center, and medical director, faculty group practice, Baylor College of Medicine, Houston.
The option of stopping a biologic agent is an attractive prospect for most Crohn's disease (CD) patients in stable clinical remission. The STORI trial, published in 2012, was among the earliest and select few studies addressing withdrawal of biologic therapy in CD among patients in sustained clinical remission with combination therapy (infliximab and thiopurine/methotrexate) for at least 6 months. Almost 50% of patients experienced disease relapse within a year of stopping infliximab in the trial.
Reenaers et al. recently published long-term follow-up of the original STORI cohort. After a median follow-up time of 7 years; four out five patients previously in clinical remission with combination therapy experienced worsening disease activity following withdrawal of infliximab. While the majority (70%) were able to resume infliximab and recapture disease response without any untoward adverse effects; one in five patients experienced major disease-related complications such as complex perianal disease or need for abdominal surgery. Upper GI tract involvement, high white blood cell count, and low hemoglobin concentration were associated with increased likelihood of a major complication. Notably, median time to a major complication was almost 4 years.
These results are similar to long-term relapse rates reported in other studies of withdrawal of therapy in CD. While biomarkers such as C-reactive protein, fecal calprotectin, along with endoscopic disease activity are reliable predictors of short-term relapse; clinical factors such as family history of CD, disease extent, stricturing or penetrating disease, and cigarette smoking are more relevant predictors of long-term disease activity. It is important to consider both types of predictors when considering withdrawal of therapy in CD.
Lastly, while the majority of patients who relapse following withdrawal of a biologic agent will do so within a year or two, a subset may not experience disease-related complications for several years - underscoring the need for long-term follow-up.
Manreet Kaur, MD, is assistant professor in the division of gastroenterology and hepatology; medical director, Inflammatory Bowel Disease Center, and medical director, faculty group practice, Baylor College of Medicine, Houston.
About , according to research published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.09.061).
About 70% of patients remained free of both infliximab restart failure and major complications, said Catherine Reenaers, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium), and her associates. Significant predictors of major complications included upper gastrointestinal disease at the time of infliximab withdrawal, white blood cell count of at least 5.0 x 109 per L, and hemoglobin level under 12.5 g per dL. “Patients with at least two of these factors had a more than 40% risk of major complication in the 7 years following infliximab withdrawal,” the researchers reported.
Little is known about long-term outcomes after patients with Crohn’s disease withdraw from infliximab. Therefore, Dr. Reenaers and her associates retrospectively studied 102 patients with Crohn’s disease who had received infliximab and an antimetabolite (azathioprine, mercaptopurine, or methotrexate) for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and then withdrew from infliximab. Patients were recruited from 19 centers in Belgium and France and were originally part of a prospective cohort study of infliximab withdrawal in Crohn’s disease (Gastroenterology. 2012;142[1]:63-70.e5).
About half of patients relapsed and restarted infliximab within 12 months, which is in line with other studies, the researchers noted. Over a median follow-up of 83 months (interquartile range, 71-93 months), 21% (95% confidence interval, 13.1%-30.3%) of patients had no complications, did not restart infliximab, and started no other biologics. In all, 70.2% of patients (95% CI, 60.2%-80.1%) had no major complications and did not fail to respond after restarting infliximab.
Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: 14 who required surgery and 4 who developed new complex perianal lesions. In a multivariable model, the strongest independent predictor of major complications was leukocytosis (hazard ratio, 10.5; 95% CI, 1.3-83; P less than .002), followed by upper gastrointestinal disease (HR, 5.8; 95% CI, 1.5-22) and low hemoglobin level (HR, 4.1; 95% CI, 1.5-21.8; P less than .01). The 13 patients who lacked these risk factors had no major complications of infliximab withdrawal. Among 72 patients who had at least one risk factor, 16.3% (95% CI, 7%-25%) developed major complications over 7 years. Strikingly, among 17 with at least two risk factors, 43% (95% CI, 17%-69%) developed major complications over 7 years, the researchers noted.
Complications emerged a median of 50 months (interquartile range, 41-73 months) after patients received their last infliximab infusion, highlighting the need for close long-term monitoring even if patients show no signs of early clinical relapse after infliximab withdrawal, the investigators said. “One strength of this cohort was the homogeneity of the population,” they stressed. “Most studies of anti–tumor necrosis factor withdrawal after clinical remission were limited by heterogeneous populations, variable lengths of infliximab treatment before discontinuation, and variable use of immunomodulators and corticosteroids. In [our] cohort, the population was homogenous, infliximab withdrawal was standardized, and the disease characteristics at the time of stopping were collected prospectively.” Although follow-up times varied, less than 5% of patients were followed for less than 3 years, they noted.
The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
SOURCE: Reenaers C et al. Clin Gastro Hepatol 2018 February (in press).
About , according to research published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.09.061).
About 70% of patients remained free of both infliximab restart failure and major complications, said Catherine Reenaers, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium), and her associates. Significant predictors of major complications included upper gastrointestinal disease at the time of infliximab withdrawal, white blood cell count of at least 5.0 x 109 per L, and hemoglobin level under 12.5 g per dL. “Patients with at least two of these factors had a more than 40% risk of major complication in the 7 years following infliximab withdrawal,” the researchers reported.
Little is known about long-term outcomes after patients with Crohn’s disease withdraw from infliximab. Therefore, Dr. Reenaers and her associates retrospectively studied 102 patients with Crohn’s disease who had received infliximab and an antimetabolite (azathioprine, mercaptopurine, or methotrexate) for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and then withdrew from infliximab. Patients were recruited from 19 centers in Belgium and France and were originally part of a prospective cohort study of infliximab withdrawal in Crohn’s disease (Gastroenterology. 2012;142[1]:63-70.e5).
About half of patients relapsed and restarted infliximab within 12 months, which is in line with other studies, the researchers noted. Over a median follow-up of 83 months (interquartile range, 71-93 months), 21% (95% confidence interval, 13.1%-30.3%) of patients had no complications, did not restart infliximab, and started no other biologics. In all, 70.2% of patients (95% CI, 60.2%-80.1%) had no major complications and did not fail to respond after restarting infliximab.
Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: 14 who required surgery and 4 who developed new complex perianal lesions. In a multivariable model, the strongest independent predictor of major complications was leukocytosis (hazard ratio, 10.5; 95% CI, 1.3-83; P less than .002), followed by upper gastrointestinal disease (HR, 5.8; 95% CI, 1.5-22) and low hemoglobin level (HR, 4.1; 95% CI, 1.5-21.8; P less than .01). The 13 patients who lacked these risk factors had no major complications of infliximab withdrawal. Among 72 patients who had at least one risk factor, 16.3% (95% CI, 7%-25%) developed major complications over 7 years. Strikingly, among 17 with at least two risk factors, 43% (95% CI, 17%-69%) developed major complications over 7 years, the researchers noted.
Complications emerged a median of 50 months (interquartile range, 41-73 months) after patients received their last infliximab infusion, highlighting the need for close long-term monitoring even if patients show no signs of early clinical relapse after infliximab withdrawal, the investigators said. “One strength of this cohort was the homogeneity of the population,” they stressed. “Most studies of anti–tumor necrosis factor withdrawal after clinical remission were limited by heterogeneous populations, variable lengths of infliximab treatment before discontinuation, and variable use of immunomodulators and corticosteroids. In [our] cohort, the population was homogenous, infliximab withdrawal was standardized, and the disease characteristics at the time of stopping were collected prospectively.” Although follow-up times varied, less than 5% of patients were followed for less than 3 years, they noted.
The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
SOURCE: Reenaers C et al. Clin Gastro Hepatol 2018 February (in press).
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Over 7 years, about one in five patients with remitted Crohn’s disease developed a major complication after withdrawing from infliximab, despite remaining on an antimetabolite.
Major finding: Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: Fourteen needed surgery and four developed new complex perianal lesions.
Data source: A cohort study of 102 patients with Crohn’s disease who had received infliximab and an antimetabolite for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and who then withdrew from infliximab.
Disclosures: The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
Source: Reenaers C et al. Clin Gastroenterol Hepatol. 2018 February (in press).
APOE4: Elders with allele benefit from lifestyle changes
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
FROM JAMA NEUROLOGY
Preparing from the Outside Looking In for Safely Transitioning Pediatric Inpatients to Home
The transition of children from hospital to home introduces a unique set of challenges to patients and families who may not be well-versed in the healthcare system. In addition to juggling the stress and worry of a sick child, which can inhibit the ability to understand complicated discharge instructions prior to leaving the hospital,1 caregivers need to navigate the medical system to ensure continued recovery. The responsibility to fill and administer medications, arrange follow up appointments, and determine when to seek care if the child’s condition changes are burdens we as healthcare providers expect caregivers to manage but may underestimate how frequently they are reliably completed.2-4
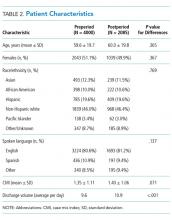
In this issue of the Journal of Hospital Medicine, the article by Rehm et al.5 adds to the growing body of evidence highlighting challenges that caregivers of children face upon discharge from the hospital. The multicenter, retrospective study of postdischarge encounters for over 12,000 patients discharged from 4 children’s hospitals aimed to evaluate the following: (1) various methods for hospital-initiated postdischarge contact of families, (2) the type and frequency of postdischarge issues, and (3) specific characteristics of pediatric patients most commonly affected by postdischarge issues.
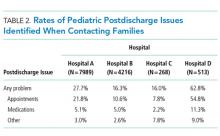
Using standardized questions administered through telephone, text, or e-mail contact, postdischarge issues were identified in 25% of discharges across all hospitals. Notably, there was considerable variation of rates of postdischarge issues among hospitals (from 16% to 62.8%). The hospital with the highest rate of postdischarge issues identified had attending hospitalists calling families after discharge. Thus, postdischarge issues may be most easily identified by providers who are familiar with both the patient and the expected postdischarge care.
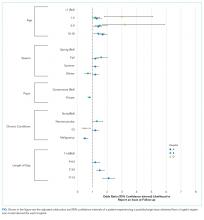
Often, postdischarge issues represented events that could be mitigated with intentional planning to better anticipate and address patient and family needs prior to discharge. The vast majority of postdischarge issues identified across all hospitals were related to appointments, accounting for 76.3% of postdischarge issues, which may be attributed to a variety of causes, from inadequate or unclear provider recommendations to difficulty scheduling the appointments. The most common medication postdischarge issue was difficulty filling prescriptions, accounting for 84.8% of the medication issues. “Other” postdischarge issues (12.7%) as reported by caregivers included challenges with understanding discharge instructions and concerns about changes in their child’s clinical status. Forty percent of included patients had a chronic care condition. Older children, patients with more medication classes, shorter length of stay, and neuromuscular chronic care conditions had higher odds of postdischarge issues. Although a high proportion of postdischarge issues suggests a systemic problem addressing the needs of patients and families after hospital discharge, these data likely underestimate the magnitude of the problem; as such, the need for improvement may be higher.
Postdischarge challenges faced by families are not unique to pediatrics. Pediatric and adult medical patients face similar rates of challenges after
Given the prevalence of postdischarge issues after both pediatric and adult hospitalizations, how should hospitalists proceed? Physicians and health systems should explore approaches to better prepare caregivers, perhaps using models akin to the Seamless Transitions and (Re)admissions Network model of enhanced communication, care coordination, and family engagement.10 Pediatric hospitalists can prepare children for discharge long before departure by delivering medications to patients prior to discharge,11,12 providing discharge instructions that are clear and readable,13,14 as well as utilizing admission-discharge teaching nurses,15 inpatient care managers,16,17 and pediatric nurse practitioners18 to aid transition.
While a variety of interventions show promise in securing a successful transition to home from the hospitalist vantage point, a partnership with primary care physicians (PCPs) in our communities is paramount. Though the evidence linking gaps in primary care after discharge and readmission rates remain elusive, effective partnerships with PCPs are important for ensuring discharge plans are carried out, which may ultimately lead to decreased rates of unanticipated adverse outcomes. Several adult studies note that no single intervention is likely to prevent issues after discharge, but interventions should include high-quality communication with and involvement of community partners.9,19,20 In practice, providing a high-quality, reliable handoff can be difficult given competing priorities of busy outpatient clinic schedules and inpatient bed capacity concerns, necessitating efficient discharge practices. Some of these challenges are amenable to quality improvement efforts to improve discharge communication.21 Innovative ideas include collaborating with PCPs earlier in the admission to design the care plan up front, including PCPs in weekly team meetings for patients with chronic care conditions,16,17 and using telehealth to communicate with PCPs.
Ensuring a safe transition to home is our responsibility as hospitalists, but the solutions to doing so reliably require multi-fold interventions that build teams within hospitals, innovative outreach to those patients recently discharged to ensure their well-being and mitigate postdischarge issues and broad community programs—including greater access to primary care—to meet our urgent imperative.
Disclosure
The authors declare no conflicts of interest. Dr. Auger’s research is funded by the Agency for Healthcare Research and Quality (1K08HS024735).
1. Solan LG, Beck AF, Brunswick SA, et al. The Family Perspective on Hospital to Home Transitions: A Qualitative Study. Pediatrics. 2015;136(6):e1539-1549. PubMed
2. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. PubMed
3. Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124 Suppl 3:S289-298. PubMed
4. Glick AF, Farkas JS, Nicholson J, et al. Parental Management of Discharge Instructions: A Systematic Review. Pediatrics. 2017. [Epub ahead of print]. PubMed
5. Rehm KP, Brittan MS, Stephens JR, et al. Issues Identified by Post-Discharge Contact after Pediatric Hospitalization: A Multi-site Study (published online ahead of print February 2, 2018) J Hosp Med. doi: 10.12788/jhm.2934
6. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
7. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
8. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
9. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
10. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135(1):164-175. PubMed
11. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. PubMed
12. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. PubMed
13. Unaka N, Statile A, Jerardi K, et al. Improving the Readability of Pediatric Hospital Medicine Discharge Instructions. J Hosp Med. 2017;12(7):551-557. PubMed
14. Wu S, Tyler A, Logsdon T, et al. A Quality Improvement Collaborative to Improve the Discharge Process for Hospitalized Children. Pediatrics. 2016;138(2). PubMed
15. Blankenship JS, Winslow SA. Admission-discharge-teaching nurses: bridging the gap in today’s workforce. J Nurs Adm. 2003;33(1):11-13. PubMed
16. White CM, Thomson JE, Statile AM, et al. Development of a New Care Model for Hospitalized Children With Medical Complexity. Hosp Pediatr. 2017;7(7):410-414. PubMed
17. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving Discharge Efficiency in Medically Complex Pediatric Patients. Pediatrics. 2016;138(2). PubMed
18. Dunn K, Rogers J. Discharge Facilitation: An Innovative PNP Role. J Pediatr Health Care. 2016;30(5):499-505. PubMed
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
20. Scott AM, Li J, Oyewole-Eletu S, et al. Understanding Facilitators and Barriers to Care Transitions: Insights from Project ACHIEVE Site Visits. Jt Comm J Qual Patient Saf. 2017;43(9):433-447. PubMed
21. Shen MW, Hershey D, Bergert L, Mallory L, Fisher ES, Cooperberg D. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258-265. PubMed
The transition of children from hospital to home introduces a unique set of challenges to patients and families who may not be well-versed in the healthcare system. In addition to juggling the stress and worry of a sick child, which can inhibit the ability to understand complicated discharge instructions prior to leaving the hospital,1 caregivers need to navigate the medical system to ensure continued recovery. The responsibility to fill and administer medications, arrange follow up appointments, and determine when to seek care if the child’s condition changes are burdens we as healthcare providers expect caregivers to manage but may underestimate how frequently they are reliably completed.2-4
In this issue of the Journal of Hospital Medicine, the article by Rehm et al.5 adds to the growing body of evidence highlighting challenges that caregivers of children face upon discharge from the hospital. The multicenter, retrospective study of postdischarge encounters for over 12,000 patients discharged from 4 children’s hospitals aimed to evaluate the following: (1) various methods for hospital-initiated postdischarge contact of families, (2) the type and frequency of postdischarge issues, and (3) specific characteristics of pediatric patients most commonly affected by postdischarge issues.
Using standardized questions administered through telephone, text, or e-mail contact, postdischarge issues were identified in 25% of discharges across all hospitals. Notably, there was considerable variation of rates of postdischarge issues among hospitals (from 16% to 62.8%). The hospital with the highest rate of postdischarge issues identified had attending hospitalists calling families after discharge. Thus, postdischarge issues may be most easily identified by providers who are familiar with both the patient and the expected postdischarge care.
Often, postdischarge issues represented events that could be mitigated with intentional planning to better anticipate and address patient and family needs prior to discharge. The vast majority of postdischarge issues identified across all hospitals were related to appointments, accounting for 76.3% of postdischarge issues, which may be attributed to a variety of causes, from inadequate or unclear provider recommendations to difficulty scheduling the appointments. The most common medication postdischarge issue was difficulty filling prescriptions, accounting for 84.8% of the medication issues. “Other” postdischarge issues (12.7%) as reported by caregivers included challenges with understanding discharge instructions and concerns about changes in their child’s clinical status. Forty percent of included patients had a chronic care condition. Older children, patients with more medication classes, shorter length of stay, and neuromuscular chronic care conditions had higher odds of postdischarge issues. Although a high proportion of postdischarge issues suggests a systemic problem addressing the needs of patients and families after hospital discharge, these data likely underestimate the magnitude of the problem; as such, the need for improvement may be higher.
Postdischarge challenges faced by families are not unique to pediatrics. Pediatric and adult medical patients face similar rates of challenges after
Given the prevalence of postdischarge issues after both pediatric and adult hospitalizations, how should hospitalists proceed? Physicians and health systems should explore approaches to better prepare caregivers, perhaps using models akin to the Seamless Transitions and (Re)admissions Network model of enhanced communication, care coordination, and family engagement.10 Pediatric hospitalists can prepare children for discharge long before departure by delivering medications to patients prior to discharge,11,12 providing discharge instructions that are clear and readable,13,14 as well as utilizing admission-discharge teaching nurses,15 inpatient care managers,16,17 and pediatric nurse practitioners18 to aid transition.
While a variety of interventions show promise in securing a successful transition to home from the hospitalist vantage point, a partnership with primary care physicians (PCPs) in our communities is paramount. Though the evidence linking gaps in primary care after discharge and readmission rates remain elusive, effective partnerships with PCPs are important for ensuring discharge plans are carried out, which may ultimately lead to decreased rates of unanticipated adverse outcomes. Several adult studies note that no single intervention is likely to prevent issues after discharge, but interventions should include high-quality communication with and involvement of community partners.9,19,20 In practice, providing a high-quality, reliable handoff can be difficult given competing priorities of busy outpatient clinic schedules and inpatient bed capacity concerns, necessitating efficient discharge practices. Some of these challenges are amenable to quality improvement efforts to improve discharge communication.21 Innovative ideas include collaborating with PCPs earlier in the admission to design the care plan up front, including PCPs in weekly team meetings for patients with chronic care conditions,16,17 and using telehealth to communicate with PCPs.
Ensuring a safe transition to home is our responsibility as hospitalists, but the solutions to doing so reliably require multi-fold interventions that build teams within hospitals, innovative outreach to those patients recently discharged to ensure their well-being and mitigate postdischarge issues and broad community programs—including greater access to primary care—to meet our urgent imperative.
Disclosure
The authors declare no conflicts of interest. Dr. Auger’s research is funded by the Agency for Healthcare Research and Quality (1K08HS024735).
The transition of children from hospital to home introduces a unique set of challenges to patients and families who may not be well-versed in the healthcare system. In addition to juggling the stress and worry of a sick child, which can inhibit the ability to understand complicated discharge instructions prior to leaving the hospital,1 caregivers need to navigate the medical system to ensure continued recovery. The responsibility to fill and administer medications, arrange follow up appointments, and determine when to seek care if the child’s condition changes are burdens we as healthcare providers expect caregivers to manage but may underestimate how frequently they are reliably completed.2-4
In this issue of the Journal of Hospital Medicine, the article by Rehm et al.5 adds to the growing body of evidence highlighting challenges that caregivers of children face upon discharge from the hospital. The multicenter, retrospective study of postdischarge encounters for over 12,000 patients discharged from 4 children’s hospitals aimed to evaluate the following: (1) various methods for hospital-initiated postdischarge contact of families, (2) the type and frequency of postdischarge issues, and (3) specific characteristics of pediatric patients most commonly affected by postdischarge issues.
Using standardized questions administered through telephone, text, or e-mail contact, postdischarge issues were identified in 25% of discharges across all hospitals. Notably, there was considerable variation of rates of postdischarge issues among hospitals (from 16% to 62.8%). The hospital with the highest rate of postdischarge issues identified had attending hospitalists calling families after discharge. Thus, postdischarge issues may be most easily identified by providers who are familiar with both the patient and the expected postdischarge care.
Often, postdischarge issues represented events that could be mitigated with intentional planning to better anticipate and address patient and family needs prior to discharge. The vast majority of postdischarge issues identified across all hospitals were related to appointments, accounting for 76.3% of postdischarge issues, which may be attributed to a variety of causes, from inadequate or unclear provider recommendations to difficulty scheduling the appointments. The most common medication postdischarge issue was difficulty filling prescriptions, accounting for 84.8% of the medication issues. “Other” postdischarge issues (12.7%) as reported by caregivers included challenges with understanding discharge instructions and concerns about changes in their child’s clinical status. Forty percent of included patients had a chronic care condition. Older children, patients with more medication classes, shorter length of stay, and neuromuscular chronic care conditions had higher odds of postdischarge issues. Although a high proportion of postdischarge issues suggests a systemic problem addressing the needs of patients and families after hospital discharge, these data likely underestimate the magnitude of the problem; as such, the need for improvement may be higher.
Postdischarge challenges faced by families are not unique to pediatrics. Pediatric and adult medical patients face similar rates of challenges after
Given the prevalence of postdischarge issues after both pediatric and adult hospitalizations, how should hospitalists proceed? Physicians and health systems should explore approaches to better prepare caregivers, perhaps using models akin to the Seamless Transitions and (Re)admissions Network model of enhanced communication, care coordination, and family engagement.10 Pediatric hospitalists can prepare children for discharge long before departure by delivering medications to patients prior to discharge,11,12 providing discharge instructions that are clear and readable,13,14 as well as utilizing admission-discharge teaching nurses,15 inpatient care managers,16,17 and pediatric nurse practitioners18 to aid transition.
While a variety of interventions show promise in securing a successful transition to home from the hospitalist vantage point, a partnership with primary care physicians (PCPs) in our communities is paramount. Though the evidence linking gaps in primary care after discharge and readmission rates remain elusive, effective partnerships with PCPs are important for ensuring discharge plans are carried out, which may ultimately lead to decreased rates of unanticipated adverse outcomes. Several adult studies note that no single intervention is likely to prevent issues after discharge, but interventions should include high-quality communication with and involvement of community partners.9,19,20 In practice, providing a high-quality, reliable handoff can be difficult given competing priorities of busy outpatient clinic schedules and inpatient bed capacity concerns, necessitating efficient discharge practices. Some of these challenges are amenable to quality improvement efforts to improve discharge communication.21 Innovative ideas include collaborating with PCPs earlier in the admission to design the care plan up front, including PCPs in weekly team meetings for patients with chronic care conditions,16,17 and using telehealth to communicate with PCPs.
Ensuring a safe transition to home is our responsibility as hospitalists, but the solutions to doing so reliably require multi-fold interventions that build teams within hospitals, innovative outreach to those patients recently discharged to ensure their well-being and mitigate postdischarge issues and broad community programs—including greater access to primary care—to meet our urgent imperative.
Disclosure
The authors declare no conflicts of interest. Dr. Auger’s research is funded by the Agency for Healthcare Research and Quality (1K08HS024735).
1. Solan LG, Beck AF, Brunswick SA, et al. The Family Perspective on Hospital to Home Transitions: A Qualitative Study. Pediatrics. 2015;136(6):e1539-1549. PubMed
2. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. PubMed
3. Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124 Suppl 3:S289-298. PubMed
4. Glick AF, Farkas JS, Nicholson J, et al. Parental Management of Discharge Instructions: A Systematic Review. Pediatrics. 2017. [Epub ahead of print]. PubMed
5. Rehm KP, Brittan MS, Stephens JR, et al. Issues Identified by Post-Discharge Contact after Pediatric Hospitalization: A Multi-site Study (published online ahead of print February 2, 2018) J Hosp Med. doi: 10.12788/jhm.2934
6. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
7. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
8. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
9. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
10. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135(1):164-175. PubMed
11. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. PubMed
12. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. PubMed
13. Unaka N, Statile A, Jerardi K, et al. Improving the Readability of Pediatric Hospital Medicine Discharge Instructions. J Hosp Med. 2017;12(7):551-557. PubMed
14. Wu S, Tyler A, Logsdon T, et al. A Quality Improvement Collaborative to Improve the Discharge Process for Hospitalized Children. Pediatrics. 2016;138(2). PubMed
15. Blankenship JS, Winslow SA. Admission-discharge-teaching nurses: bridging the gap in today’s workforce. J Nurs Adm. 2003;33(1):11-13. PubMed
16. White CM, Thomson JE, Statile AM, et al. Development of a New Care Model for Hospitalized Children With Medical Complexity. Hosp Pediatr. 2017;7(7):410-414. PubMed
17. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving Discharge Efficiency in Medically Complex Pediatric Patients. Pediatrics. 2016;138(2). PubMed
18. Dunn K, Rogers J. Discharge Facilitation: An Innovative PNP Role. J Pediatr Health Care. 2016;30(5):499-505. PubMed
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
20. Scott AM, Li J, Oyewole-Eletu S, et al. Understanding Facilitators and Barriers to Care Transitions: Insights from Project ACHIEVE Site Visits. Jt Comm J Qual Patient Saf. 2017;43(9):433-447. PubMed
21. Shen MW, Hershey D, Bergert L, Mallory L, Fisher ES, Cooperberg D. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258-265. PubMed
1. Solan LG, Beck AF, Brunswick SA, et al. The Family Perspective on Hospital to Home Transitions: A Qualitative Study. Pediatrics. 2015;136(6):e1539-1549. PubMed
2. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. PubMed
3. Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124 Suppl 3:S289-298. PubMed
4. Glick AF, Farkas JS, Nicholson J, et al. Parental Management of Discharge Instructions: A Systematic Review. Pediatrics. 2017. [Epub ahead of print]. PubMed
5. Rehm KP, Brittan MS, Stephens JR, et al. Issues Identified by Post-Discharge Contact after Pediatric Hospitalization: A Multi-site Study (published online ahead of print February 2, 2018) J Hosp Med. doi: 10.12788/jhm.2934
6. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
7. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
8. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
9. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
10. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135(1):164-175. PubMed
11. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. PubMed
12. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. PubMed
13. Unaka N, Statile A, Jerardi K, et al. Improving the Readability of Pediatric Hospital Medicine Discharge Instructions. J Hosp Med. 2017;12(7):551-557. PubMed
14. Wu S, Tyler A, Logsdon T, et al. A Quality Improvement Collaborative to Improve the Discharge Process for Hospitalized Children. Pediatrics. 2016;138(2). PubMed
15. Blankenship JS, Winslow SA. Admission-discharge-teaching nurses: bridging the gap in today’s workforce. J Nurs Adm. 2003;33(1):11-13. PubMed
16. White CM, Thomson JE, Statile AM, et al. Development of a New Care Model for Hospitalized Children With Medical Complexity. Hosp Pediatr. 2017;7(7):410-414. PubMed
17. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving Discharge Efficiency in Medically Complex Pediatric Patients. Pediatrics. 2016;138(2). PubMed
18. Dunn K, Rogers J. Discharge Facilitation: An Innovative PNP Role. J Pediatr Health Care. 2016;30(5):499-505. PubMed
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
20. Scott AM, Li J, Oyewole-Eletu S, et al. Understanding Facilitators and Barriers to Care Transitions: Insights from Project ACHIEVE Site Visits. Jt Comm J Qual Patient Saf. 2017;43(9):433-447. PubMed
21. Shen MW, Hershey D, Bergert L, Mallory L, Fisher ES, Cooperberg D. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258-265. PubMed
© 2018 Society of Hospital Medicine
Accuracy Comparisons between Manual and Automated Respiratory Rate for Detecting Clinical Deterioration in Ward Patients
Respiratory rate is the most accurate vital sign for predicting adverse outcomes in ward patients.1,2 Though other vital signs are typically collected by using machines, respiratory rate is collected manually by caregivers counting the breathing rate. However, studies have shown significant discrepancies between a patient’s respiratory rate documented in the medical record, which is often 18 or 20, and the value measured by counting the rate over a full minute.3 Thus, despite the high accuracy of respiratory rate, it is possible that these values do not represent true patient physiology. It is unknown whether a valid automated measurement of respiratory rate would be more predictive than a manually collected respiratory rate for identifying patients who develop deterioration. The aim of this study was to compare the distribution and predictive accuracy of manually and automatically recorded respiratory rates.
METHODS
In this prospective cohort study, adult patients admitted to one oncology ward at the University of Chicago from April 2015 to May 2016 were approached for consent (Institutional Review Board #14-0682). Enrolled patients were fit with a cableless, FDA-approved respiratory pod device (Philips IntelliVue clResp Pod; Philips Healthcare, Andover, MA) that automatically recorded respiratory rate and heart rate every 15 minutes while they remained on the ward. Pod data were paired with vital sign data documented in the electronic health record (EHR) by taking the automated value closest, but prior to, the manual value up to a maximum of 4 hours. Automated and manual respiratory rate were compared by using the area under the receiver operating characteristic curve (AUC) for whether an intensive care unit (ICU) transfer occurred within 24 hours of each paired observation without accounting for patient-level clustering.
RESULTS
DISCUSSION
In this prospective cohort study, we found that manual respiratory rates were different than those collected from an automated system and, yet, were significantly more accurate for predicting ICU transfer. These results suggest that the predictive accuracy of respiratory rates documented in the EHR is due to more than just physiology. Our findings have important implications for the risk stratification of ward patients.
Though previous literature has suggested that respiratory rate is the most accurate predictor of deterioration, this may not be true.1 Respiratory rates manually recorded by clinical staff may contain information beyond pure physiology, such as a proxy of clinician concern, which may inflate the predictive value. Nursing staff may record standard respiratory rate values for patients that appear to be well (eg, 18) but count actual rates for those patients they suspect have a more severe disease, which is one possible explanation for our findings. In addition, automated assessments are likely to be more sensitive to intermittent fluctuations in respiratory rate associated with patient movement or emotion. This might explain the improved accuracy at higher rates for manually recorded vital signs.
Although limited by its small sample size, our results have important implications for patient monitoring and early warning scores designed to identify high-risk ward patients given that both simple scores and statistically derived models include respiratory rates as a predictor.4 As hospitals move to use newer technologies to automate vital sign monitoring and decrease nursing workload, our findings suggest that accuracy for identifying high-risk patients may be lost. Additional methods for capturing subjective assessments from clinical providers may be necessary and could be incorporated into risk scores.5 For example, the 7-point subjective Patient Acuity Rating has been shown to augment the Modified Early Warning Score for predicting ICU transfer, rapid response activation, or cardiac arrest within 24 hours.6
Manually recorded respiratory rate may include information beyond pure physiology, which inflates its predictive value. This has important implications for the use of automated monitoring technology in hospitals and the integration of these measurements into early warning scores.
Acknowledgments
The authors thank Pamela McCall, BSN, OCN for her assistance with study implementation, Kevin Ig-Izevbekhai and Shivraj Grewal for assistance with data collection, UCM Clinical Engineering for technical support, and Timothy Holper, MS, Julie Johnson, MPH, RN, and Thomas Sutton for assistance with data abstraction.
Disclosure
Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from Chest for invited speaking engagements. Dr. Churpek and Dr. Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and research support from EarlySense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. This study was supported by a grant from Philips Healthcare in Andover, MA. The sponsor had no role in data collection, interpretation of results, or drafting of the manuscript.
1. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170-1176. PubMed
2. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. PubMed
3. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. PubMed
4. Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758-1765. PubMed
5. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. PubMed
6. Patel AR, Zadravecz FJ, Young RS, Williams MV, Churpek MM, Edelson DP. The value of clinical judgment in the detection of clinical deterioration. JAMA Intern Med. 2015;175(3):456-458. PubMed
Respiratory rate is the most accurate vital sign for predicting adverse outcomes in ward patients.1,2 Though other vital signs are typically collected by using machines, respiratory rate is collected manually by caregivers counting the breathing rate. However, studies have shown significant discrepancies between a patient’s respiratory rate documented in the medical record, which is often 18 or 20, and the value measured by counting the rate over a full minute.3 Thus, despite the high accuracy of respiratory rate, it is possible that these values do not represent true patient physiology. It is unknown whether a valid automated measurement of respiratory rate would be more predictive than a manually collected respiratory rate for identifying patients who develop deterioration. The aim of this study was to compare the distribution and predictive accuracy of manually and automatically recorded respiratory rates.
METHODS
In this prospective cohort study, adult patients admitted to one oncology ward at the University of Chicago from April 2015 to May 2016 were approached for consent (Institutional Review Board #14-0682). Enrolled patients were fit with a cableless, FDA-approved respiratory pod device (Philips IntelliVue clResp Pod; Philips Healthcare, Andover, MA) that automatically recorded respiratory rate and heart rate every 15 minutes while they remained on the ward. Pod data were paired with vital sign data documented in the electronic health record (EHR) by taking the automated value closest, but prior to, the manual value up to a maximum of 4 hours. Automated and manual respiratory rate were compared by using the area under the receiver operating characteristic curve (AUC) for whether an intensive care unit (ICU) transfer occurred within 24 hours of each paired observation without accounting for patient-level clustering.
RESULTS
DISCUSSION
In this prospective cohort study, we found that manual respiratory rates were different than those collected from an automated system and, yet, were significantly more accurate for predicting ICU transfer. These results suggest that the predictive accuracy of respiratory rates documented in the EHR is due to more than just physiology. Our findings have important implications for the risk stratification of ward patients.
Though previous literature has suggested that respiratory rate is the most accurate predictor of deterioration, this may not be true.1 Respiratory rates manually recorded by clinical staff may contain information beyond pure physiology, such as a proxy of clinician concern, which may inflate the predictive value. Nursing staff may record standard respiratory rate values for patients that appear to be well (eg, 18) but count actual rates for those patients they suspect have a more severe disease, which is one possible explanation for our findings. In addition, automated assessments are likely to be more sensitive to intermittent fluctuations in respiratory rate associated with patient movement or emotion. This might explain the improved accuracy at higher rates for manually recorded vital signs.
Although limited by its small sample size, our results have important implications for patient monitoring and early warning scores designed to identify high-risk ward patients given that both simple scores and statistically derived models include respiratory rates as a predictor.4 As hospitals move to use newer technologies to automate vital sign monitoring and decrease nursing workload, our findings suggest that accuracy for identifying high-risk patients may be lost. Additional methods for capturing subjective assessments from clinical providers may be necessary and could be incorporated into risk scores.5 For example, the 7-point subjective Patient Acuity Rating has been shown to augment the Modified Early Warning Score for predicting ICU transfer, rapid response activation, or cardiac arrest within 24 hours.6
Manually recorded respiratory rate may include information beyond pure physiology, which inflates its predictive value. This has important implications for the use of automated monitoring technology in hospitals and the integration of these measurements into early warning scores.
Acknowledgments
The authors thank Pamela McCall, BSN, OCN for her assistance with study implementation, Kevin Ig-Izevbekhai and Shivraj Grewal for assistance with data collection, UCM Clinical Engineering for technical support, and Timothy Holper, MS, Julie Johnson, MPH, RN, and Thomas Sutton for assistance with data abstraction.
Disclosure
Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from Chest for invited speaking engagements. Dr. Churpek and Dr. Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and research support from EarlySense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. This study was supported by a grant from Philips Healthcare in Andover, MA. The sponsor had no role in data collection, interpretation of results, or drafting of the manuscript.
Respiratory rate is the most accurate vital sign for predicting adverse outcomes in ward patients.1,2 Though other vital signs are typically collected by using machines, respiratory rate is collected manually by caregivers counting the breathing rate. However, studies have shown significant discrepancies between a patient’s respiratory rate documented in the medical record, which is often 18 or 20, and the value measured by counting the rate over a full minute.3 Thus, despite the high accuracy of respiratory rate, it is possible that these values do not represent true patient physiology. It is unknown whether a valid automated measurement of respiratory rate would be more predictive than a manually collected respiratory rate for identifying patients who develop deterioration. The aim of this study was to compare the distribution and predictive accuracy of manually and automatically recorded respiratory rates.
METHODS
In this prospective cohort study, adult patients admitted to one oncology ward at the University of Chicago from April 2015 to May 2016 were approached for consent (Institutional Review Board #14-0682). Enrolled patients were fit with a cableless, FDA-approved respiratory pod device (Philips IntelliVue clResp Pod; Philips Healthcare, Andover, MA) that automatically recorded respiratory rate and heart rate every 15 minutes while they remained on the ward. Pod data were paired with vital sign data documented in the electronic health record (EHR) by taking the automated value closest, but prior to, the manual value up to a maximum of 4 hours. Automated and manual respiratory rate were compared by using the area under the receiver operating characteristic curve (AUC) for whether an intensive care unit (ICU) transfer occurred within 24 hours of each paired observation without accounting for patient-level clustering.
RESULTS
DISCUSSION
In this prospective cohort study, we found that manual respiratory rates were different than those collected from an automated system and, yet, were significantly more accurate for predicting ICU transfer. These results suggest that the predictive accuracy of respiratory rates documented in the EHR is due to more than just physiology. Our findings have important implications for the risk stratification of ward patients.
Though previous literature has suggested that respiratory rate is the most accurate predictor of deterioration, this may not be true.1 Respiratory rates manually recorded by clinical staff may contain information beyond pure physiology, such as a proxy of clinician concern, which may inflate the predictive value. Nursing staff may record standard respiratory rate values for patients that appear to be well (eg, 18) but count actual rates for those patients they suspect have a more severe disease, which is one possible explanation for our findings. In addition, automated assessments are likely to be more sensitive to intermittent fluctuations in respiratory rate associated with patient movement or emotion. This might explain the improved accuracy at higher rates for manually recorded vital signs.
Although limited by its small sample size, our results have important implications for patient monitoring and early warning scores designed to identify high-risk ward patients given that both simple scores and statistically derived models include respiratory rates as a predictor.4 As hospitals move to use newer technologies to automate vital sign monitoring and decrease nursing workload, our findings suggest that accuracy for identifying high-risk patients may be lost. Additional methods for capturing subjective assessments from clinical providers may be necessary and could be incorporated into risk scores.5 For example, the 7-point subjective Patient Acuity Rating has been shown to augment the Modified Early Warning Score for predicting ICU transfer, rapid response activation, or cardiac arrest within 24 hours.6
Manually recorded respiratory rate may include information beyond pure physiology, which inflates its predictive value. This has important implications for the use of automated monitoring technology in hospitals and the integration of these measurements into early warning scores.
Acknowledgments
The authors thank Pamela McCall, BSN, OCN for her assistance with study implementation, Kevin Ig-Izevbekhai and Shivraj Grewal for assistance with data collection, UCM Clinical Engineering for technical support, and Timothy Holper, MS, Julie Johnson, MPH, RN, and Thomas Sutton for assistance with data abstraction.
Disclosure
Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from Chest for invited speaking engagements. Dr. Churpek and Dr. Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and research support from EarlySense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. This study was supported by a grant from Philips Healthcare in Andover, MA. The sponsor had no role in data collection, interpretation of results, or drafting of the manuscript.
1. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170-1176. PubMed
2. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. PubMed
3. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. PubMed
4. Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758-1765. PubMed
5. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. PubMed
6. Patel AR, Zadravecz FJ, Young RS, Williams MV, Churpek MM, Edelson DP. The value of clinical judgment in the detection of clinical deterioration. JAMA Intern Med. 2015;175(3):456-458. PubMed
1. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170-1176. PubMed
2. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. PubMed
3. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. PubMed
4. Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758-1765. PubMed
5. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. PubMed
6. Patel AR, Zadravecz FJ, Young RS, Williams MV, Churpek MM, Edelson DP. The value of clinical judgment in the detection of clinical deterioration. JAMA Intern Med. 2015;175(3):456-458. PubMed
© 2018 Society of Hospital Medicine
Lean-Based Redesign of Multidisciplinary Rounds on General Medicine Service
Given that multiple disciplines are often involved in caring for patients admitted to the hospital, timely communication, collaboration, and coordination amongst various disciplines is necessary for safe and effective patient care.1 With the focus on improving patient satisfaction and throughput in hospitals, it is also important to make more accurate predictions of the discharge date and allow time for patients and their families to prepare for discharge.2-4
Multidisciplinary rounds (MDR) are defined as structured daily communication amongst key members of the patient’s care team (eg, nurses, physicians, case managers, social workers, pharmacists, and rehabilitation services). MDR have shown to be a useful strategy for ensuring that all members of the care team are updated on the plan of care for the patient.5 During MDR, a brief “check-in” discussing the patient’s plan of care, pending needs, and barriers to discharge allows all team members, patients, and families to effectively coordinate care and plan and prepare for discharge.
Multiple studies have reported increased collaboration and improved communication between disciplines with the use of such multidisciplinary rounding.2,5-7 Additionally, MDR have been shown to improve patient outcomes8 and reduce adverse events,9 length of stay (LOS),6,8 cost of care,8 and readmissions.1
We redesigned MDR on the general medicine wards at our institution in October 2014 by using Lean management techniques. Lean is defined as a set of philosophies and methods that aim to create transformation in thinking, behavior, and culture in each process, with the goal of maximizing the value for the patients and providers, adding efficiency, and reducing waste and waits.10
In this study, we evaluate whether this new model of MDR was associated with a decrease in the LOS. We also evaluate whether this new model of MDR was associated with an increase in discharges before noon, documentation of estimated discharge date (EDD) in our electronic health record (EHR), and patient satisfaction.
METHODS
Setting, Design, and Patients
The study was conducted on the teaching general medicine service at our institution, an urban, 484-bed academic hospital. The general medicine service has patients on 4 inpatient units (total of 95 beds) and is managed by 5 teaching service teams.
We performed a pre-post study. The preperiod (in which the old model of MDR was followed) included 4000 patients discharged between September 1, 2013, and October 22, 2014. The postperiod (in which the new model of MDR was followed) included 2085 patients discharged between October 23, 2014, and April 30, 2015. We excluded 139 patients that died in the hospital prior to discharge and patients on the nonteaching and/or private practice service.
All data were provided by our institution’s Digital Solutions Department. Our institutional review board issued a letter of determination exempting this study from further review because it was deemed to be a quality improvement initiative.
Use of Lean Management to Redesign our MDR
Our institution has incorporated the Lean management system to continually add value to services through the elimination of waste, thus simultaneously optimizing the quality of patient care, cost, and patient satisfaction.11 Lean, derived from the Toyota Production System, has long been used in manufacturing and in recent decades has spread to healthcare.12 We leveraged the following 3 key Lean techniques to redesign our MDR: (1) value stream management (VSM), (2) rapid process improvement workshops (RPIW), and (3) active daily management (ADM), as detailed in supplementary Appendix 1.
Interventions
Outcomes
The primary outcome was mean LOS. The secondary outcomes were (1) discharges before noon, (2) recording of the EDD in our EHR within 24 hours of admission (as time stamped on our EHR), and (3) patient satisfaction.
Data for patient satisfaction were obtained using the Press Ganey survey. We used data on patient satisfaction scores for the following 2 relevant questions on this survey: (1) extent to which the patient felt ready to be discharged and (2) how well staff worked together to care for the patient. Proportions of the “top-box” (“very good”) were used for the analysis. These survey data were available on 467 patients (11.7%) in the preperiod and 188 patients (9.0%) in the postperiod.
Data Analysis
A sensitivity analysis was conducted on a second cohort that included a subset of patients from the preperiod between November 1, 2013, and April 30, 2014, and a subset of patients from the postperiod between November 1, 2014, and April 1, 2015, to control for the calendar period (supplementary Appendix 2).
All analyses were conducted in R version 3.3.0, with the linear mixed-effects model lme4 statistical package.13,14
RESULTS
Table 3 shows the differences in the outcomes between the pre- and postperiods. There was no change in the LOS or LOS adjusted for CMI. There was a 3.9% increase in discharges before noon in the postperiod compared with the preperiod (95% CI, 2.4% to 5.3%; P < .001). There was a 9.9% increase in the percentage of patients for whom the EDD was recorded in our EHR within 24 hours of admission (95% CI, 7.4% to 12.4%; P < .001). There was no change in the “top-box” patient satisfaction scores.
There were only marginal differences in the results between the entire cohort and a second subset cohort used for sensitivity analysis (supplementary Appendix 2).
DISCUSSION
In our study, there was no change in the mean LOS with the new model of MDR. There was an increase in discharges before noon and in recording of the EDD in our EHR within 24 hours of admission in the postperiod when the Lean-based new model of MDR was utilized. There was no change in patient satisfaction. With no change in staffing, we were able to accommodate the increase in the discharge volume in the postperiod.
We believe our results are attributable to several factors, including clearly defined roles and responsibilities for all participants of MDR, the inclusion of more experienced general medicine attending physician (compared with housestaff), Lean management techniques to identify gaps in the patient’s journey from emergency department to discharge using VSM, the development of appropriate workflows and standard work on how the multidisciplinary teams would work together at RPIWs, and ADM to ensure sustainability and engagement among frontline members and institutional leaders. In order to sustain this, we planned to continue monitoring data in daily, weekly, and monthly forums with senior physician and administrative leaders. Planning for additional interventions is underway, including moving MDR to the bedside, instituting an afternoon “check-in” that would enable more detailed action planning, and addressing barriers in a timely manner for patients ready to discharge the following day.
Our study has a few limitations. First, this is an observational study that cannot determine causation. Second, this is a single-center study conducted on patients only on the general medicine teaching service. Third, there were several concurrent interventions implemented at our institution to improve LOS, throughput, and patient satisfaction in addition to MDR, thus making it difficult to isolate the impact of our intervention. Fourth, in the new model of MDR, rounds took place only 5 days per week, thereby possibly limiting the potential impact on our outcomes. Fifth, while we showed improvements in the discharges before noon and recording of EDD in the post period, we were not able to achieve our target of 25% discharges before noon or 100% recording of EDD in this time period. We believe the limited amount of time between the pre- and postperiods to allow for adoption and learning of the processes might have contributed to the underestimation of the impact of the new model of MDR, thereby limiting our ability to achieve our targets. Sixth, the response rate on the Press Ganey survey was low, and we did not directly survey patients or families for their satisfaction with MDR.
Our study has several strengths. To our knowledge, this is the first study to embed Lean management techniques in the design of MDR in the inpatient setting. While several studies have demonstrated improvements in discharges before noon through the implementation of MDR, they have not incorporated Lean management techniques, which we believe are critical to ensure the sustainability of results.1,3,5,6,8,15 Second, while it was not measured, there was a high level of provider engagement in the process in the new model of MDR. Third, because the MDR were conducted at the nurse’s station on each inpatient unit in the new model instead of in a conference room, it was well attended by all members of the multidisciplinary team. Fourth, the presence of a visibility board allowed for all team members to have easy access to visual feedback throughout the day, even if they were not present at the MDR. Fifth, we believe that there was also more accurate estimation of the date and time of discharge in the new model of MDR because the discussion was facilitated by the case manager, who is experienced in identifying barriers to discharge (compared with the housestaff in the old model of MDR), and included the more experienced attending physician. Finally, the consistent presence of a multidisciplinary team at MDR allowed for the incorporation of everyone’s concerns at one time, thereby limiting the need for paging multiple disciplines throughout the day, which led to quicker resolution of issues and assignment of pending tasks.
In conclusion, our study shows no change in the mean LOS when the Lean-based model of MDR was utilized. Our study demonstrates an increase in discharges before noon and in recording of EDD on our EHR within 24 hours of admission in the post period when the Lean-based model of MDR was utilized. There was no change in patient satisfaction. While this study was conducted at an academic medical center on the general medicine wards, we believe our new model of MDR, which leveraged Lean management techniques, may successfully impact patient flow in all inpatient clinical services and nonteaching hospitals.
Disclosure
The authors report no financial conflicts of interest and have nothing to disclose.
1. Townsend-Gervis M, Cornell P, Vardaman JM. Interdisciplinary Rounds and Structured Communication Reduce Re-Admissions and Improve Some Patient Outcomes. West J Nurs Res. 2014;36(7):917-928. PubMed
2. Vazirani S, Hays RD, Shapiro MF, Cowan M. Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71-77. PubMed
3. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. PubMed
4. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. PubMed
5. Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M. Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17(3):133-142. PubMed
6. O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073-1079. PubMed
7. Reimer N, Herbener L. Round and round we go: rounding strategies to impact exemplary professional practice. Clin J Oncol Nurs. 2014;18(6):654-660. PubMed
8. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 Suppl):AS4-AS12. PubMed
9. Baggs JG, Ryan SA, Phelps CE, Richeson JF, Johnson JE. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung. 1992;21(1):18-24. PubMed
10. Lawal AK, Rotter T, Kinsman L, et al. Lean management in health care: definition, concepts, methodology and effects reported (systematic review protocol). Syst Rev. 2014;3:103. PubMed
11. Liker JK. Toyota Way: 14 Management Principles from the World’s Greatest Manufacturer. New York, Chicago, San Francisco, Athens, London, Madrid, Mexico City, Milan, New Delhi, Singapore, Sydney, Toronto: McGraw-Hill Education; 2004.
12. Kane M, Chui K, Rimicci J, et al. Lean Manufacturing Improves Emergency Department Throughput and Patient Satisfaction. J Nurs Adm. 2015;45(9):429-434. PubMed
13. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. http://www.R-project.org/. Accessed November 7, 2017.
14. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1-48.
15. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684. PubMed
Given that multiple disciplines are often involved in caring for patients admitted to the hospital, timely communication, collaboration, and coordination amongst various disciplines is necessary for safe and effective patient care.1 With the focus on improving patient satisfaction and throughput in hospitals, it is also important to make more accurate predictions of the discharge date and allow time for patients and their families to prepare for discharge.2-4
Multidisciplinary rounds (MDR) are defined as structured daily communication amongst key members of the patient’s care team (eg, nurses, physicians, case managers, social workers, pharmacists, and rehabilitation services). MDR have shown to be a useful strategy for ensuring that all members of the care team are updated on the plan of care for the patient.5 During MDR, a brief “check-in” discussing the patient’s plan of care, pending needs, and barriers to discharge allows all team members, patients, and families to effectively coordinate care and plan and prepare for discharge.
Multiple studies have reported increased collaboration and improved communication between disciplines with the use of such multidisciplinary rounding.2,5-7 Additionally, MDR have been shown to improve patient outcomes8 and reduce adverse events,9 length of stay (LOS),6,8 cost of care,8 and readmissions.1
We redesigned MDR on the general medicine wards at our institution in October 2014 by using Lean management techniques. Lean is defined as a set of philosophies and methods that aim to create transformation in thinking, behavior, and culture in each process, with the goal of maximizing the value for the patients and providers, adding efficiency, and reducing waste and waits.10
In this study, we evaluate whether this new model of MDR was associated with a decrease in the LOS. We also evaluate whether this new model of MDR was associated with an increase in discharges before noon, documentation of estimated discharge date (EDD) in our electronic health record (EHR), and patient satisfaction.
METHODS
Setting, Design, and Patients
The study was conducted on the teaching general medicine service at our institution, an urban, 484-bed academic hospital. The general medicine service has patients on 4 inpatient units (total of 95 beds) and is managed by 5 teaching service teams.
We performed a pre-post study. The preperiod (in which the old model of MDR was followed) included 4000 patients discharged between September 1, 2013, and October 22, 2014. The postperiod (in which the new model of MDR was followed) included 2085 patients discharged between October 23, 2014, and April 30, 2015. We excluded 139 patients that died in the hospital prior to discharge and patients on the nonteaching and/or private practice service.
All data were provided by our institution’s Digital Solutions Department. Our institutional review board issued a letter of determination exempting this study from further review because it was deemed to be a quality improvement initiative.
Use of Lean Management to Redesign our MDR
Our institution has incorporated the Lean management system to continually add value to services through the elimination of waste, thus simultaneously optimizing the quality of patient care, cost, and patient satisfaction.11 Lean, derived from the Toyota Production System, has long been used in manufacturing and in recent decades has spread to healthcare.12 We leveraged the following 3 key Lean techniques to redesign our MDR: (1) value stream management (VSM), (2) rapid process improvement workshops (RPIW), and (3) active daily management (ADM), as detailed in supplementary Appendix 1.
Interventions
Outcomes
The primary outcome was mean LOS. The secondary outcomes were (1) discharges before noon, (2) recording of the EDD in our EHR within 24 hours of admission (as time stamped on our EHR), and (3) patient satisfaction.
Data for patient satisfaction were obtained using the Press Ganey survey. We used data on patient satisfaction scores for the following 2 relevant questions on this survey: (1) extent to which the patient felt ready to be discharged and (2) how well staff worked together to care for the patient. Proportions of the “top-box” (“very good”) were used for the analysis. These survey data were available on 467 patients (11.7%) in the preperiod and 188 patients (9.0%) in the postperiod.
Data Analysis
A sensitivity analysis was conducted on a second cohort that included a subset of patients from the preperiod between November 1, 2013, and April 30, 2014, and a subset of patients from the postperiod between November 1, 2014, and April 1, 2015, to control for the calendar period (supplementary Appendix 2).
All analyses were conducted in R version 3.3.0, with the linear mixed-effects model lme4 statistical package.13,14
RESULTS
Table 3 shows the differences in the outcomes between the pre- and postperiods. There was no change in the LOS or LOS adjusted for CMI. There was a 3.9% increase in discharges before noon in the postperiod compared with the preperiod (95% CI, 2.4% to 5.3%; P < .001). There was a 9.9% increase in the percentage of patients for whom the EDD was recorded in our EHR within 24 hours of admission (95% CI, 7.4% to 12.4%; P < .001). There was no change in the “top-box” patient satisfaction scores.
There were only marginal differences in the results between the entire cohort and a second subset cohort used for sensitivity analysis (supplementary Appendix 2).
DISCUSSION
In our study, there was no change in the mean LOS with the new model of MDR. There was an increase in discharges before noon and in recording of the EDD in our EHR within 24 hours of admission in the postperiod when the Lean-based new model of MDR was utilized. There was no change in patient satisfaction. With no change in staffing, we were able to accommodate the increase in the discharge volume in the postperiod.
We believe our results are attributable to several factors, including clearly defined roles and responsibilities for all participants of MDR, the inclusion of more experienced general medicine attending physician (compared with housestaff), Lean management techniques to identify gaps in the patient’s journey from emergency department to discharge using VSM, the development of appropriate workflows and standard work on how the multidisciplinary teams would work together at RPIWs, and ADM to ensure sustainability and engagement among frontline members and institutional leaders. In order to sustain this, we planned to continue monitoring data in daily, weekly, and monthly forums with senior physician and administrative leaders. Planning for additional interventions is underway, including moving MDR to the bedside, instituting an afternoon “check-in” that would enable more detailed action planning, and addressing barriers in a timely manner for patients ready to discharge the following day.
Our study has a few limitations. First, this is an observational study that cannot determine causation. Second, this is a single-center study conducted on patients only on the general medicine teaching service. Third, there were several concurrent interventions implemented at our institution to improve LOS, throughput, and patient satisfaction in addition to MDR, thus making it difficult to isolate the impact of our intervention. Fourth, in the new model of MDR, rounds took place only 5 days per week, thereby possibly limiting the potential impact on our outcomes. Fifth, while we showed improvements in the discharges before noon and recording of EDD in the post period, we were not able to achieve our target of 25% discharges before noon or 100% recording of EDD in this time period. We believe the limited amount of time between the pre- and postperiods to allow for adoption and learning of the processes might have contributed to the underestimation of the impact of the new model of MDR, thereby limiting our ability to achieve our targets. Sixth, the response rate on the Press Ganey survey was low, and we did not directly survey patients or families for their satisfaction with MDR.
Our study has several strengths. To our knowledge, this is the first study to embed Lean management techniques in the design of MDR in the inpatient setting. While several studies have demonstrated improvements in discharges before noon through the implementation of MDR, they have not incorporated Lean management techniques, which we believe are critical to ensure the sustainability of results.1,3,5,6,8,15 Second, while it was not measured, there was a high level of provider engagement in the process in the new model of MDR. Third, because the MDR were conducted at the nurse’s station on each inpatient unit in the new model instead of in a conference room, it was well attended by all members of the multidisciplinary team. Fourth, the presence of a visibility board allowed for all team members to have easy access to visual feedback throughout the day, even if they were not present at the MDR. Fifth, we believe that there was also more accurate estimation of the date and time of discharge in the new model of MDR because the discussion was facilitated by the case manager, who is experienced in identifying barriers to discharge (compared with the housestaff in the old model of MDR), and included the more experienced attending physician. Finally, the consistent presence of a multidisciplinary team at MDR allowed for the incorporation of everyone’s concerns at one time, thereby limiting the need for paging multiple disciplines throughout the day, which led to quicker resolution of issues and assignment of pending tasks.
In conclusion, our study shows no change in the mean LOS when the Lean-based model of MDR was utilized. Our study demonstrates an increase in discharges before noon and in recording of EDD on our EHR within 24 hours of admission in the post period when the Lean-based model of MDR was utilized. There was no change in patient satisfaction. While this study was conducted at an academic medical center on the general medicine wards, we believe our new model of MDR, which leveraged Lean management techniques, may successfully impact patient flow in all inpatient clinical services and nonteaching hospitals.
Disclosure
The authors report no financial conflicts of interest and have nothing to disclose.
Given that multiple disciplines are often involved in caring for patients admitted to the hospital, timely communication, collaboration, and coordination amongst various disciplines is necessary for safe and effective patient care.1 With the focus on improving patient satisfaction and throughput in hospitals, it is also important to make more accurate predictions of the discharge date and allow time for patients and their families to prepare for discharge.2-4
Multidisciplinary rounds (MDR) are defined as structured daily communication amongst key members of the patient’s care team (eg, nurses, physicians, case managers, social workers, pharmacists, and rehabilitation services). MDR have shown to be a useful strategy for ensuring that all members of the care team are updated on the plan of care for the patient.5 During MDR, a brief “check-in” discussing the patient’s plan of care, pending needs, and barriers to discharge allows all team members, patients, and families to effectively coordinate care and plan and prepare for discharge.
Multiple studies have reported increased collaboration and improved communication between disciplines with the use of such multidisciplinary rounding.2,5-7 Additionally, MDR have been shown to improve patient outcomes8 and reduce adverse events,9 length of stay (LOS),6,8 cost of care,8 and readmissions.1
We redesigned MDR on the general medicine wards at our institution in October 2014 by using Lean management techniques. Lean is defined as a set of philosophies and methods that aim to create transformation in thinking, behavior, and culture in each process, with the goal of maximizing the value for the patients and providers, adding efficiency, and reducing waste and waits.10
In this study, we evaluate whether this new model of MDR was associated with a decrease in the LOS. We also evaluate whether this new model of MDR was associated with an increase in discharges before noon, documentation of estimated discharge date (EDD) in our electronic health record (EHR), and patient satisfaction.
METHODS
Setting, Design, and Patients
The study was conducted on the teaching general medicine service at our institution, an urban, 484-bed academic hospital. The general medicine service has patients on 4 inpatient units (total of 95 beds) and is managed by 5 teaching service teams.
We performed a pre-post study. The preperiod (in which the old model of MDR was followed) included 4000 patients discharged between September 1, 2013, and October 22, 2014. The postperiod (in which the new model of MDR was followed) included 2085 patients discharged between October 23, 2014, and April 30, 2015. We excluded 139 patients that died in the hospital prior to discharge and patients on the nonteaching and/or private practice service.
All data were provided by our institution’s Digital Solutions Department. Our institutional review board issued a letter of determination exempting this study from further review because it was deemed to be a quality improvement initiative.
Use of Lean Management to Redesign our MDR
Our institution has incorporated the Lean management system to continually add value to services through the elimination of waste, thus simultaneously optimizing the quality of patient care, cost, and patient satisfaction.11 Lean, derived from the Toyota Production System, has long been used in manufacturing and in recent decades has spread to healthcare.12 We leveraged the following 3 key Lean techniques to redesign our MDR: (1) value stream management (VSM), (2) rapid process improvement workshops (RPIW), and (3) active daily management (ADM), as detailed in supplementary Appendix 1.
Interventions
Outcomes
The primary outcome was mean LOS. The secondary outcomes were (1) discharges before noon, (2) recording of the EDD in our EHR within 24 hours of admission (as time stamped on our EHR), and (3) patient satisfaction.
Data for patient satisfaction were obtained using the Press Ganey survey. We used data on patient satisfaction scores for the following 2 relevant questions on this survey: (1) extent to which the patient felt ready to be discharged and (2) how well staff worked together to care for the patient. Proportions of the “top-box” (“very good”) were used for the analysis. These survey data were available on 467 patients (11.7%) in the preperiod and 188 patients (9.0%) in the postperiod.
Data Analysis
A sensitivity analysis was conducted on a second cohort that included a subset of patients from the preperiod between November 1, 2013, and April 30, 2014, and a subset of patients from the postperiod between November 1, 2014, and April 1, 2015, to control for the calendar period (supplementary Appendix 2).
All analyses were conducted in R version 3.3.0, with the linear mixed-effects model lme4 statistical package.13,14
RESULTS
Table 3 shows the differences in the outcomes between the pre- and postperiods. There was no change in the LOS or LOS adjusted for CMI. There was a 3.9% increase in discharges before noon in the postperiod compared with the preperiod (95% CI, 2.4% to 5.3%; P < .001). There was a 9.9% increase in the percentage of patients for whom the EDD was recorded in our EHR within 24 hours of admission (95% CI, 7.4% to 12.4%; P < .001). There was no change in the “top-box” patient satisfaction scores.
There were only marginal differences in the results between the entire cohort and a second subset cohort used for sensitivity analysis (supplementary Appendix 2).
DISCUSSION
In our study, there was no change in the mean LOS with the new model of MDR. There was an increase in discharges before noon and in recording of the EDD in our EHR within 24 hours of admission in the postperiod when the Lean-based new model of MDR was utilized. There was no change in patient satisfaction. With no change in staffing, we were able to accommodate the increase in the discharge volume in the postperiod.
We believe our results are attributable to several factors, including clearly defined roles and responsibilities for all participants of MDR, the inclusion of more experienced general medicine attending physician (compared with housestaff), Lean management techniques to identify gaps in the patient’s journey from emergency department to discharge using VSM, the development of appropriate workflows and standard work on how the multidisciplinary teams would work together at RPIWs, and ADM to ensure sustainability and engagement among frontline members and institutional leaders. In order to sustain this, we planned to continue monitoring data in daily, weekly, and monthly forums with senior physician and administrative leaders. Planning for additional interventions is underway, including moving MDR to the bedside, instituting an afternoon “check-in” that would enable more detailed action planning, and addressing barriers in a timely manner for patients ready to discharge the following day.
Our study has a few limitations. First, this is an observational study that cannot determine causation. Second, this is a single-center study conducted on patients only on the general medicine teaching service. Third, there were several concurrent interventions implemented at our institution to improve LOS, throughput, and patient satisfaction in addition to MDR, thus making it difficult to isolate the impact of our intervention. Fourth, in the new model of MDR, rounds took place only 5 days per week, thereby possibly limiting the potential impact on our outcomes. Fifth, while we showed improvements in the discharges before noon and recording of EDD in the post period, we were not able to achieve our target of 25% discharges before noon or 100% recording of EDD in this time period. We believe the limited amount of time between the pre- and postperiods to allow for adoption and learning of the processes might have contributed to the underestimation of the impact of the new model of MDR, thereby limiting our ability to achieve our targets. Sixth, the response rate on the Press Ganey survey was low, and we did not directly survey patients or families for their satisfaction with MDR.
Our study has several strengths. To our knowledge, this is the first study to embed Lean management techniques in the design of MDR in the inpatient setting. While several studies have demonstrated improvements in discharges before noon through the implementation of MDR, they have not incorporated Lean management techniques, which we believe are critical to ensure the sustainability of results.1,3,5,6,8,15 Second, while it was not measured, there was a high level of provider engagement in the process in the new model of MDR. Third, because the MDR were conducted at the nurse’s station on each inpatient unit in the new model instead of in a conference room, it was well attended by all members of the multidisciplinary team. Fourth, the presence of a visibility board allowed for all team members to have easy access to visual feedback throughout the day, even if they were not present at the MDR. Fifth, we believe that there was also more accurate estimation of the date and time of discharge in the new model of MDR because the discussion was facilitated by the case manager, who is experienced in identifying barriers to discharge (compared with the housestaff in the old model of MDR), and included the more experienced attending physician. Finally, the consistent presence of a multidisciplinary team at MDR allowed for the incorporation of everyone’s concerns at one time, thereby limiting the need for paging multiple disciplines throughout the day, which led to quicker resolution of issues and assignment of pending tasks.
In conclusion, our study shows no change in the mean LOS when the Lean-based model of MDR was utilized. Our study demonstrates an increase in discharges before noon and in recording of EDD on our EHR within 24 hours of admission in the post period when the Lean-based model of MDR was utilized. There was no change in patient satisfaction. While this study was conducted at an academic medical center on the general medicine wards, we believe our new model of MDR, which leveraged Lean management techniques, may successfully impact patient flow in all inpatient clinical services and nonteaching hospitals.
Disclosure
The authors report no financial conflicts of interest and have nothing to disclose.
1. Townsend-Gervis M, Cornell P, Vardaman JM. Interdisciplinary Rounds and Structured Communication Reduce Re-Admissions and Improve Some Patient Outcomes. West J Nurs Res. 2014;36(7):917-928. PubMed
2. Vazirani S, Hays RD, Shapiro MF, Cowan M. Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71-77. PubMed
3. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. PubMed
4. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. PubMed
5. Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M. Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17(3):133-142. PubMed
6. O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073-1079. PubMed
7. Reimer N, Herbener L. Round and round we go: rounding strategies to impact exemplary professional practice. Clin J Oncol Nurs. 2014;18(6):654-660. PubMed
8. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 Suppl):AS4-AS12. PubMed
9. Baggs JG, Ryan SA, Phelps CE, Richeson JF, Johnson JE. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung. 1992;21(1):18-24. PubMed
10. Lawal AK, Rotter T, Kinsman L, et al. Lean management in health care: definition, concepts, methodology and effects reported (systematic review protocol). Syst Rev. 2014;3:103. PubMed
11. Liker JK. Toyota Way: 14 Management Principles from the World’s Greatest Manufacturer. New York, Chicago, San Francisco, Athens, London, Madrid, Mexico City, Milan, New Delhi, Singapore, Sydney, Toronto: McGraw-Hill Education; 2004.
12. Kane M, Chui K, Rimicci J, et al. Lean Manufacturing Improves Emergency Department Throughput and Patient Satisfaction. J Nurs Adm. 2015;45(9):429-434. PubMed
13. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. http://www.R-project.org/. Accessed November 7, 2017.
14. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1-48.
15. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684. PubMed
1. Townsend-Gervis M, Cornell P, Vardaman JM. Interdisciplinary Rounds and Structured Communication Reduce Re-Admissions and Improve Some Patient Outcomes. West J Nurs Res. 2014;36(7):917-928. PubMed
2. Vazirani S, Hays RD, Shapiro MF, Cowan M. Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71-77. PubMed
3. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. PubMed
4. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: Effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. PubMed
5. Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M. Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17(3):133-142. PubMed
6. O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22(8):1073-1079. PubMed
7. Reimer N, Herbener L. Round and round we go: rounding strategies to impact exemplary professional practice. Clin J Oncol Nurs. 2014;18(6):654-660. PubMed
8. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36(8 Suppl):AS4-AS12. PubMed
9. Baggs JG, Ryan SA, Phelps CE, Richeson JF, Johnson JE. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung. 1992;21(1):18-24. PubMed
10. Lawal AK, Rotter T, Kinsman L, et al. Lean management in health care: definition, concepts, methodology and effects reported (systematic review protocol). Syst Rev. 2014;3:103. PubMed
11. Liker JK. Toyota Way: 14 Management Principles from the World’s Greatest Manufacturer. New York, Chicago, San Francisco, Athens, London, Madrid, Mexico City, Milan, New Delhi, Singapore, Sydney, Toronto: McGraw-Hill Education; 2004.
12. Kane M, Chui K, Rimicci J, et al. Lean Manufacturing Improves Emergency Department Throughput and Patient Satisfaction. J Nurs Adm. 2015;45(9):429-434. PubMed
13. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. http://www.R-project.org/. Accessed November 7, 2017.
14. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1-48.
15. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684. PubMed
© 2018 Society of Hospital Medicine
Issues Identified by Postdischarge Contact after Pediatric Hospitalization: A Multisite Study
Many hospitals are considering or currently employing initiatives to contact patients after discharge. Whether conducted via telephone or other means, the purpose of the contact is to help patients adhere to discharge plans, fulfill discharge needs, and alleviate postdischarge issues (PDIs). The effectiveness of hospital-initiated postdischarge phone calls has been studied in adult patients after hospitalization, and though some studies report positive outcomes,1-3 a 2006 Cochrane review found insufficient evidence to recommend for or against the practice.4
Little is known about follow-up contact after hospitalization for children.5-11 Rates of PDI vary substantially across hospitals. For example, one single-center study of postdischarge telephone contact after hospitalization on a general pediatric ward identified PDIs in ~20% of patients.10 Another study identified PDIs in 84% of patients discharged from a pediatric rehabilitation facility.11 Telephone follow-up has been associated with reduced health resource utilization and improved patient satisfaction for children discharged after an elective surgical procedure6 and for children discharged home from the emergency department.7-9
More information is needed on the clinical experiences of postdischarge contact in hospitalized children to improve the understanding of how the contact is made, who makes it, and which patients are most likely to report a PDI. These experiences are crucial to understand given the expense and time commitment involved in postdischarge contact, as many hospitals may not be positioned to contact all discharged patients. Therefore, we conducted a pragmatic, retrospective, naturalistic study of differing approaches to postdischarge contact occurring in multiple hospitals. Our main objective was to describe the prevalence and types of PDIs identified by the different approaches for follow-up contact across 4 children’s hospitals. We also assessed the characteristics of children who have the highest likelihood of having a PDI identified from the contact within each hospital.
METHODS
Study Design, Setting, and Population
Main Outcome Measures
The main outcome measure was identification of a PDI, defined as a medication, appointment, or other discharge-related issue, that was reported and recorded by the child’s caregiver during conversation from the standardized questions that were asked during follow-up contact as part of routine discharge care (Table 1). Medication PDIs included issues filling prescriptions and tolerating medications. Appointment PDIs included not having a follow-up appointment scheduled. Other PDIs included issues with the child’s health condition, discharge instructions, or any other concerns. All PDIs had been recorded prospectively by hospital contact personnel (hospitals A, B, and D) or through an automated texting system into a database (hospital C). Where available, free text comments that were recorded by contact personnel were reviewed by one of the authors (KB) and categorized via an existing framework of PDI designed by Heath et al.10 in order to further understand the problems that were reported.
Patient Characteristics
Patient hospitalization, demographic, and clinical characteristics were obtained from administrative health data at each institution and compared between children with versus without a PDI. Hospitalization characteristics included length of stay, season of admission, and reason for admission. Reason for admission was categorized by using 3M Health’s All Patient Refined Diagnosis Related Groups (APR-DRG) (3M, Maplewood, MN). Demographic characteristics included age at admission in years, insurance type (eg, public, private, and other), and race/ethnicity (Asian/Pacific Islander, Hispanic, non-Hispanic black, non-Hispanic white, and other).
Clinical characteristics included a count of the different classes of medications (eg, antibiotics, antiepileptic medications, digestive motility medications, etc.) administered to the child during admission, the type and number of chronic conditions, and assistance with medical technology (eg, gastrostomy, tracheostomy, etc.). Except for medications, these characteristics were assessed with International Classification of Diseases, Ninth Revision-Clinical Modification (ICD-9-CM) diagnosis codes.
We used the Agency for Healthcare Research and Quality Chronic Condition Indicator classification system, which categorizes over 14,000 ICD-9-CM diagnosis codes into chronic versus nonchronic conditions to identify the presence and number of chronic conditions.12 Children hospitalized with a chronic condition were further classified as having a complex chronic condition (CCC) by using the ICD-9-CM diagnosis classification scheme of Feudtner et al.13 CCCs represent defined diagnosis groupings of conditions expected to last longer than 12 months and involve either multiple organ systems or a single organ system severely enough to require specialty pediatric care and hospitalization.13,14 Children requiring medical technology were identified by using ICD-9-CM codes indicating their use of a medical device to manage and treat a chronic illness (eg, ventricular shunt to treat hydrocephalus) or to maintain basic body functions necessary for sustaining life (eg a tracheostomy tube for breathing).15,16
Statistical Analysis
Given that the primary purpose for this study was to leverage the natural heterogeneity in the approach to follow-up contact across hospitals, we assessed and reported the prevalence and type of PDIs independently for each hospital. Relatedly, we assessed the relationship between patient characteristics and PDI likelihood independently within each hospital as well rather than pool the data and perform a central analysis across hospitals. Of note, APR-DRG and medication class were not assessed for hospital D, as this information was unavailable. We used χ2 tests for univariable analysis and logistic regression with a backwards elimination derivation process (for variables with P ≥ .05) for multivariable analysis; all patient demographic, clinical, and hospitalization characteristics were entered initially into the models. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC), and P < .05 was considered statistically significant. This study was approved by the institutional review board at all hospitals.
RESULTS
Study Population
There were 12,986 (51.4%) of 25,259 patients reached by follow-up contact after discharge across the 4 hospitals. Median age at admission for contacted patients was 4.0 years (interquartile range [IQR] 0-11). Of those contacted, 45.2% were female, 59.9% were non-Hispanic white, 51.0% used Medicaid, and 95.4% were discharged to home. Seventy-one percent had a chronic condition (of any complexity) and 40.8% had a CCC. Eighty percent received a prescribed medication during the hospitalization. Median (IQR) length of stay was 2.0 days (IQR 1-4 days). The top 5 most common reasons for admission were bronchiolitis (6.3%), pneumonia (6.2%), asthma (5.2%), seizure (4.9%), and tonsil and adenoid procedures (4.1%).
PDIs
Characteristics Associated with PDIs
Age
Older age was a consistent characteristic associated with PDIs in 3 hospitals. For example, PDI rates in children 10 to 18 years versus <1 year were 30.8% versus 21.4% (P < .001) in hospital A, 19.4% versus 13.7% (P = .002) in hospital B, and 70.3% versus 62.8% (P < .001) in hospital D. In multivariable analysis, age 10 to 18 years versus <1 year at admission was associated with an increased likelihood of PDI in hospital A (odds ratio [OR] 1.7; 95% CI, 1.4-2.0), hospital B (OR 1.4; 95% CI, 1.1-1.8), and hospital D (OR 1.7; 95% CI, 0.9-3.0) (Table 3 and Figure).
Medications
Length of Stay
Shorter length of stay was associated with PDI in 1 hospital. In hospital A, the PDI rate increased significantly (P < .001) from 19.0% to 33.9% as length of stay decreased from ≥7 days to ≤1 day (Table 3). In multivariable analysis, length of stay to ≤1 day versus ≥7 days was associated with increased likelihood of PDI (OR 2.1; 95% CI, 1.7-2.5) in hospital A (Table 3 and Figure).
CCCs
A neuromuscular CCC was associated with PDI in 2 hospitals. In hospital B, the PDI rate was higher in children with a neuromuscular CCC compared with a malignancy CCC (21.3% vs 11.2%). In hospital D, the PDI rates were higher in children with a neuromuscular CCC compared with a respiratory CCC (68.9% vs 40.6%) (Table 3). In multivariable analysis, children with versus without a neuromuscular CCC had an increased likelihood of PDI (OR 1.3; 95% CI, 1.0-1.7) in hospital B (Table 3 and Figure).
DISCUSSION
In this retrospective, pragmatic, multicentered study of follow-up contact with a standardized set of questions asked after discharge for hospitalized children, we found that PDIs were identified often, regardless of who made the contact or how the contact was made. The PDI rates varied substantially across hospitals and were likely influenced by the different follow-up approaches that were used. Most PDIs were related to appointments; fewer PDIs were related to medications and other problems. Older age, shorter length of stay, and neuromuscular CCCs were among the identified risk factors for PDIs.
Our assessment of PDIs was, by design, associated with variation in methods and approach for detection across sites. Further investigation is needed to understand how different approaches for follow-up contact after discharge may influence the identification of PDIs. For example, in the current study, the hospital with the highest PDI rate (hospital D) used hospitalists who provided inpatient care for the patient to make follow-up contact. Although not determined from the current study, this approach could have led the hospitalists to ask questions beyond the standardized ones when assessing for PDIs. Perhaps some of the hospitalists had a better understanding of how to probe for PDIs specific to each patient; this understanding may not have been forthcoming for staff in the other hospitals who were unfamiliar with the patients’ hospitalization course and medical history.
Similar to previous studies in adults, our study reported that appointment PDIs in children may be more common than other types of PDIs.17 Appointment PDIs could have been due to scheduling difficulties, inadequate discharge instructions, lack of adherence to recommended follow-up, or other reasons. Further investigation is needed to elucidate these reasons and to determine how to reduce PDIs related to postdischarge appointments. Some children’s hospitals schedule follow-up appointments prior to discharge to mitigate appointment PDIs that might arise.18 However, doing that for every hospitalized child is challenging, especially for very short admissions or for weekend discharges when many outpatient and community practices are not open to schedule appointments. Additional exploration is necessary to assess whether this might help explain why some children in the current study with a short versus long length of stay had a higher likelihood of PDI.
The rate of medication PDIs (5.2%) observed in the current study is lower than the rate that is reported in prior literature. Dudas et al.1 found that medication PDIs occurred in 21% of hospitalized adult patients. One reason for the lower rate of medication PDIs in children may be that they require the use of postdischarge medications less often than adults. Most medication PDIs in the current study involved problems filling a prescription. There was not enough information in the notes taken from the follow-up contact to distinguish the medication PDI etiologies (eg, a prescription was not sent from the hospital team to the pharmacy, prior authorization from an insurance company for a prescription was not obtained, the pharmacy did not stock the medication). To help overcome medication access barriers, some hospitals fill and deliver discharge medications to the patients’ bedside. One study found that children discharged with medication in hand were less likely to have emergency department revisits within 30 days of discharge.19 Further investigation is needed to assess whether initiatives like these help mitigate medication PDIs in children.
Hospitals may benefit from considering how risk factors for PDIs can be used to prioritize which patients receive follow-up contact, especially in hospitals where contact for all hospitalized patients is not feasible. In the current study, there was variation across hospitals in the profile of risk factors that correlated with increased likelihood of PDI. Some of the risk factors are easier to explain than others. For example, as mentioned above, for some hospitalized children, short length of stay might not permit enough time for hospital staff to set up discharge plans that may sufficiently prevent PDIs. Other risk factors, including older age and neuromuscular CCCs, may require additional assessment (eg, through chart review or in-depth patient and provider interviews) to discover the reasons why they were associated with increased likelihood of PDI. There are additional risk factors that might influence the likelihood of PDI that the current study was not positioned to assess, including health literacy, transportation availability, and language spoken.20-23
This study has several other limitations in addition to the ones already mentioned. Some children may have experienced PDIs that were not reported at contact (eg, the respondent was unaware that an issue was present), which may have led to an undercounting of PDIs. Alternatively, some caregivers may have been more likely to respond to the contact if their child was experiencing a PDI, which may have led to overcounting. PDIs of nonrespondents were not measured. PDIs identified by postdischarge outpatient and community providers or by families outside of contact were not measured. The current study was not positioned to assess the severity of the PDIs or what interventions (including additional health services) were needed to address them. Although we assessed medication use during admission, we were unable to assess the number and type of medications that were prescribed for use postdischarge. Information about the number and type of follow-up visits needed for each child was not assessed. Given the variety of approaches for follow-up contact, the findings may generalize best to individual hospitals by using an approach that best matches to one of them. The current study is not positioned to correlate quality of discharge care with the rate of PDI.
Despite these limitations, the findings from the current study reinforce that PDIs identified through follow-up contact in discharged patients appear to be common. Of PDIs identified, appointment problems were more prevalent than medication or other types of problems. Short length of stay, older age, and other patient and/or hospitalization attributes were associated with an increased likelihood of PDI. Hospitals caring for children may find this information useful as they strive to optimize their processes for follow-up contact after discharge. To help further evaluate the value and importance of contacting patients after discharge, additional study of PDI in children is warranted, including (1) actions taken to resolve PDIs, (2) the impact of identifying and addressing PDIs on hospital readmission, and (3) postdischarge experiences and health outcomes of children who responded versus those who did not respond to the follow-up contact. Moreover, future multisite, comparative effectiveness studies of PDI may wish to consider standardization of follow-up contact procedures with controlled manipulation of key processes (eg, contact by administrator vs nurse vs physician) to assess best practices.
Disclosure
Mr. Blaine, Ms. O’Neill, and Drs. Berry, Brittan, Rehm, and Steiner were supported by the Lucile Packard Foundation for Children’s Health. The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest to disclose.
1. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239-248. PubMed
2. Sanchez GM, Douglass MA, Mancuso MA. Revisiting Project Re-Engineered Discharge (RED): The Impact of a Pharmacist Telephone Intervention on Hospital Readmission Rates. Pharmacotherapy. 2015;35(9):805-812. PubMed
3. Jones J, Clark W, Bradford J, Dougherty J. Efficacy of a telephone follow-up system in the emergency department. J Emerg Med. 1988;6(3):249-254. PubMed
4. Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510. PubMed
5. Lushaj EB, Nelson K, Amond K, Kenny E, Badami A, Anagnostopoulos PV. Timely Post-discharge Telephone Follow-Up is a Useful Tool in Identifying Post-discharge Complications Patients After Congenital Heart Surgery. Pediatr Cardiol. 2016;37(6):1106-1110. PubMed
6. McVay MR, Kelley KR, Mathews DL, Jackson RJ, Kokoska ER, Smith SD. Postoperative follow-up: is a phone call enough? J Pediatr Surg. 2008;43(1):83-86. PubMed
7. Chande VT, Exum V. Follow-up phone calls after an emergency department visit. Pediatrics. 1994;93(3):513-514. PubMed
8. Sutton D, Stanley P, Babl FE, Phillips F. Preventing or accelerating emergency care for children with complex healthcare needs. Arch Dis Child. 2008;93(1):17-22. PubMed
9. Patel PB, Vinson DR. Physician e-mail and telephone contact after emergency department visit improves patient satisfaction: a crossover trial. Ann Emerg Med. 2013;61(6):631-637. PubMed
10. Heath J, Dancel R, Stephens JR. Postdischarge phone calls after pediatric hospitalization: an observational study. Hosp Pediatr. 2015;5(5):241-248. PubMed
11. Biffl SE, Biffl WL. Improving transitions of care for complex pediatric trauma patients from inpatient rehabilitation to home: an observational pilot study. Patient Saf Surg. 2015;9:33-37. PubMed
12. AHRQ. Clinical Classifications Software (CCS) for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed on January 31,2012.
13. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205-209. PubMed
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. PubMed
15. Palfrey JS, Walker DK, Haynie M, et al. Technology’s children: report of a statewide census of children dependent on medical supports. Pediatrics. 1991;87(5):611-618. PubMed
16. Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technology-dependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5(1):8-15. PubMed
17. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
18. Brittan M, Tyler A, Martin S, et al. A Discharge Planning Template for the Electronic Medical Record Improves Scheduling of Neurology Follow-up for Comanaged Seizure Patients. Hosp Pediatr. 2014;4(6):366-371. PubMed
19. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. doi:10.1542/peds.2015-0461. PubMed
20. Berry JG, Goldmann DA, Mandl KD, et al. Health information management and perceptions of the quality of care for children with tracheotomy: a qualitative study. BMC Health Serv Res. 2011;11:117-125. PubMed
21. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573-581. PubMed
22. Carusone SC, O’Leary B, McWatt S, Stewart A, Craig S, Brennan DJ. The Lived Experience of the Hospital Discharge “Plan”: A Longitudinal Qualitative Study of Complex Patients. J Hosp Med. 2017;12(1):5-10. PubMed
23. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ Priorities Regarding Hospital-to-Home Transitions for Children With Medical Complexity. Pediatrics. 2017;139(1):e20161581. doi:10.1542/peds.2016-1581. PubMed
Many hospitals are considering or currently employing initiatives to contact patients after discharge. Whether conducted via telephone or other means, the purpose of the contact is to help patients adhere to discharge plans, fulfill discharge needs, and alleviate postdischarge issues (PDIs). The effectiveness of hospital-initiated postdischarge phone calls has been studied in adult patients after hospitalization, and though some studies report positive outcomes,1-3 a 2006 Cochrane review found insufficient evidence to recommend for or against the practice.4
Little is known about follow-up contact after hospitalization for children.5-11 Rates of PDI vary substantially across hospitals. For example, one single-center study of postdischarge telephone contact after hospitalization on a general pediatric ward identified PDIs in ~20% of patients.10 Another study identified PDIs in 84% of patients discharged from a pediatric rehabilitation facility.11 Telephone follow-up has been associated with reduced health resource utilization and improved patient satisfaction for children discharged after an elective surgical procedure6 and for children discharged home from the emergency department.7-9
More information is needed on the clinical experiences of postdischarge contact in hospitalized children to improve the understanding of how the contact is made, who makes it, and which patients are most likely to report a PDI. These experiences are crucial to understand given the expense and time commitment involved in postdischarge contact, as many hospitals may not be positioned to contact all discharged patients. Therefore, we conducted a pragmatic, retrospective, naturalistic study of differing approaches to postdischarge contact occurring in multiple hospitals. Our main objective was to describe the prevalence and types of PDIs identified by the different approaches for follow-up contact across 4 children’s hospitals. We also assessed the characteristics of children who have the highest likelihood of having a PDI identified from the contact within each hospital.
METHODS
Study Design, Setting, and Population
Main Outcome Measures
The main outcome measure was identification of a PDI, defined as a medication, appointment, or other discharge-related issue, that was reported and recorded by the child’s caregiver during conversation from the standardized questions that were asked during follow-up contact as part of routine discharge care (Table 1). Medication PDIs included issues filling prescriptions and tolerating medications. Appointment PDIs included not having a follow-up appointment scheduled. Other PDIs included issues with the child’s health condition, discharge instructions, or any other concerns. All PDIs had been recorded prospectively by hospital contact personnel (hospitals A, B, and D) or through an automated texting system into a database (hospital C). Where available, free text comments that were recorded by contact personnel were reviewed by one of the authors (KB) and categorized via an existing framework of PDI designed by Heath et al.10 in order to further understand the problems that were reported.
Patient Characteristics
Patient hospitalization, demographic, and clinical characteristics were obtained from administrative health data at each institution and compared between children with versus without a PDI. Hospitalization characteristics included length of stay, season of admission, and reason for admission. Reason for admission was categorized by using 3M Health’s All Patient Refined Diagnosis Related Groups (APR-DRG) (3M, Maplewood, MN). Demographic characteristics included age at admission in years, insurance type (eg, public, private, and other), and race/ethnicity (Asian/Pacific Islander, Hispanic, non-Hispanic black, non-Hispanic white, and other).
Clinical characteristics included a count of the different classes of medications (eg, antibiotics, antiepileptic medications, digestive motility medications, etc.) administered to the child during admission, the type and number of chronic conditions, and assistance with medical technology (eg, gastrostomy, tracheostomy, etc.). Except for medications, these characteristics were assessed with International Classification of Diseases, Ninth Revision-Clinical Modification (ICD-9-CM) diagnosis codes.
We used the Agency for Healthcare Research and Quality Chronic Condition Indicator classification system, which categorizes over 14,000 ICD-9-CM diagnosis codes into chronic versus nonchronic conditions to identify the presence and number of chronic conditions.12 Children hospitalized with a chronic condition were further classified as having a complex chronic condition (CCC) by using the ICD-9-CM diagnosis classification scheme of Feudtner et al.13 CCCs represent defined diagnosis groupings of conditions expected to last longer than 12 months and involve either multiple organ systems or a single organ system severely enough to require specialty pediatric care and hospitalization.13,14 Children requiring medical technology were identified by using ICD-9-CM codes indicating their use of a medical device to manage and treat a chronic illness (eg, ventricular shunt to treat hydrocephalus) or to maintain basic body functions necessary for sustaining life (eg a tracheostomy tube for breathing).15,16
Statistical Analysis
Given that the primary purpose for this study was to leverage the natural heterogeneity in the approach to follow-up contact across hospitals, we assessed and reported the prevalence and type of PDIs independently for each hospital. Relatedly, we assessed the relationship between patient characteristics and PDI likelihood independently within each hospital as well rather than pool the data and perform a central analysis across hospitals. Of note, APR-DRG and medication class were not assessed for hospital D, as this information was unavailable. We used χ2 tests for univariable analysis and logistic regression with a backwards elimination derivation process (for variables with P ≥ .05) for multivariable analysis; all patient demographic, clinical, and hospitalization characteristics were entered initially into the models. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC), and P < .05 was considered statistically significant. This study was approved by the institutional review board at all hospitals.
RESULTS
Study Population
There were 12,986 (51.4%) of 25,259 patients reached by follow-up contact after discharge across the 4 hospitals. Median age at admission for contacted patients was 4.0 years (interquartile range [IQR] 0-11). Of those contacted, 45.2% were female, 59.9% were non-Hispanic white, 51.0% used Medicaid, and 95.4% were discharged to home. Seventy-one percent had a chronic condition (of any complexity) and 40.8% had a CCC. Eighty percent received a prescribed medication during the hospitalization. Median (IQR) length of stay was 2.0 days (IQR 1-4 days). The top 5 most common reasons for admission were bronchiolitis (6.3%), pneumonia (6.2%), asthma (5.2%), seizure (4.9%), and tonsil and adenoid procedures (4.1%).
PDIs
Characteristics Associated with PDIs
Age
Older age was a consistent characteristic associated with PDIs in 3 hospitals. For example, PDI rates in children 10 to 18 years versus <1 year were 30.8% versus 21.4% (P < .001) in hospital A, 19.4% versus 13.7% (P = .002) in hospital B, and 70.3% versus 62.8% (P < .001) in hospital D. In multivariable analysis, age 10 to 18 years versus <1 year at admission was associated with an increased likelihood of PDI in hospital A (odds ratio [OR] 1.7; 95% CI, 1.4-2.0), hospital B (OR 1.4; 95% CI, 1.1-1.8), and hospital D (OR 1.7; 95% CI, 0.9-3.0) (Table 3 and Figure).
Medications
Length of Stay
Shorter length of stay was associated with PDI in 1 hospital. In hospital A, the PDI rate increased significantly (P < .001) from 19.0% to 33.9% as length of stay decreased from ≥7 days to ≤1 day (Table 3). In multivariable analysis, length of stay to ≤1 day versus ≥7 days was associated with increased likelihood of PDI (OR 2.1; 95% CI, 1.7-2.5) in hospital A (Table 3 and Figure).
CCCs
A neuromuscular CCC was associated with PDI in 2 hospitals. In hospital B, the PDI rate was higher in children with a neuromuscular CCC compared with a malignancy CCC (21.3% vs 11.2%). In hospital D, the PDI rates were higher in children with a neuromuscular CCC compared with a respiratory CCC (68.9% vs 40.6%) (Table 3). In multivariable analysis, children with versus without a neuromuscular CCC had an increased likelihood of PDI (OR 1.3; 95% CI, 1.0-1.7) in hospital B (Table 3 and Figure).
DISCUSSION
In this retrospective, pragmatic, multicentered study of follow-up contact with a standardized set of questions asked after discharge for hospitalized children, we found that PDIs were identified often, regardless of who made the contact or how the contact was made. The PDI rates varied substantially across hospitals and were likely influenced by the different follow-up approaches that were used. Most PDIs were related to appointments; fewer PDIs were related to medications and other problems. Older age, shorter length of stay, and neuromuscular CCCs were among the identified risk factors for PDIs.
Our assessment of PDIs was, by design, associated with variation in methods and approach for detection across sites. Further investigation is needed to understand how different approaches for follow-up contact after discharge may influence the identification of PDIs. For example, in the current study, the hospital with the highest PDI rate (hospital D) used hospitalists who provided inpatient care for the patient to make follow-up contact. Although not determined from the current study, this approach could have led the hospitalists to ask questions beyond the standardized ones when assessing for PDIs. Perhaps some of the hospitalists had a better understanding of how to probe for PDIs specific to each patient; this understanding may not have been forthcoming for staff in the other hospitals who were unfamiliar with the patients’ hospitalization course and medical history.
Similar to previous studies in adults, our study reported that appointment PDIs in children may be more common than other types of PDIs.17 Appointment PDIs could have been due to scheduling difficulties, inadequate discharge instructions, lack of adherence to recommended follow-up, or other reasons. Further investigation is needed to elucidate these reasons and to determine how to reduce PDIs related to postdischarge appointments. Some children’s hospitals schedule follow-up appointments prior to discharge to mitigate appointment PDIs that might arise.18 However, doing that for every hospitalized child is challenging, especially for very short admissions or for weekend discharges when many outpatient and community practices are not open to schedule appointments. Additional exploration is necessary to assess whether this might help explain why some children in the current study with a short versus long length of stay had a higher likelihood of PDI.
The rate of medication PDIs (5.2%) observed in the current study is lower than the rate that is reported in prior literature. Dudas et al.1 found that medication PDIs occurred in 21% of hospitalized adult patients. One reason for the lower rate of medication PDIs in children may be that they require the use of postdischarge medications less often than adults. Most medication PDIs in the current study involved problems filling a prescription. There was not enough information in the notes taken from the follow-up contact to distinguish the medication PDI etiologies (eg, a prescription was not sent from the hospital team to the pharmacy, prior authorization from an insurance company for a prescription was not obtained, the pharmacy did not stock the medication). To help overcome medication access barriers, some hospitals fill and deliver discharge medications to the patients’ bedside. One study found that children discharged with medication in hand were less likely to have emergency department revisits within 30 days of discharge.19 Further investigation is needed to assess whether initiatives like these help mitigate medication PDIs in children.
Hospitals may benefit from considering how risk factors for PDIs can be used to prioritize which patients receive follow-up contact, especially in hospitals where contact for all hospitalized patients is not feasible. In the current study, there was variation across hospitals in the profile of risk factors that correlated with increased likelihood of PDI. Some of the risk factors are easier to explain than others. For example, as mentioned above, for some hospitalized children, short length of stay might not permit enough time for hospital staff to set up discharge plans that may sufficiently prevent PDIs. Other risk factors, including older age and neuromuscular CCCs, may require additional assessment (eg, through chart review or in-depth patient and provider interviews) to discover the reasons why they were associated with increased likelihood of PDI. There are additional risk factors that might influence the likelihood of PDI that the current study was not positioned to assess, including health literacy, transportation availability, and language spoken.20-23
This study has several other limitations in addition to the ones already mentioned. Some children may have experienced PDIs that were not reported at contact (eg, the respondent was unaware that an issue was present), which may have led to an undercounting of PDIs. Alternatively, some caregivers may have been more likely to respond to the contact if their child was experiencing a PDI, which may have led to overcounting. PDIs of nonrespondents were not measured. PDIs identified by postdischarge outpatient and community providers or by families outside of contact were not measured. The current study was not positioned to assess the severity of the PDIs or what interventions (including additional health services) were needed to address them. Although we assessed medication use during admission, we were unable to assess the number and type of medications that were prescribed for use postdischarge. Information about the number and type of follow-up visits needed for each child was not assessed. Given the variety of approaches for follow-up contact, the findings may generalize best to individual hospitals by using an approach that best matches to one of them. The current study is not positioned to correlate quality of discharge care with the rate of PDI.
Despite these limitations, the findings from the current study reinforce that PDIs identified through follow-up contact in discharged patients appear to be common. Of PDIs identified, appointment problems were more prevalent than medication or other types of problems. Short length of stay, older age, and other patient and/or hospitalization attributes were associated with an increased likelihood of PDI. Hospitals caring for children may find this information useful as they strive to optimize their processes for follow-up contact after discharge. To help further evaluate the value and importance of contacting patients after discharge, additional study of PDI in children is warranted, including (1) actions taken to resolve PDIs, (2) the impact of identifying and addressing PDIs on hospital readmission, and (3) postdischarge experiences and health outcomes of children who responded versus those who did not respond to the follow-up contact. Moreover, future multisite, comparative effectiveness studies of PDI may wish to consider standardization of follow-up contact procedures with controlled manipulation of key processes (eg, contact by administrator vs nurse vs physician) to assess best practices.
Disclosure
Mr. Blaine, Ms. O’Neill, and Drs. Berry, Brittan, Rehm, and Steiner were supported by the Lucile Packard Foundation for Children’s Health. The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest to disclose.
Many hospitals are considering or currently employing initiatives to contact patients after discharge. Whether conducted via telephone or other means, the purpose of the contact is to help patients adhere to discharge plans, fulfill discharge needs, and alleviate postdischarge issues (PDIs). The effectiveness of hospital-initiated postdischarge phone calls has been studied in adult patients after hospitalization, and though some studies report positive outcomes,1-3 a 2006 Cochrane review found insufficient evidence to recommend for or against the practice.4
Little is known about follow-up contact after hospitalization for children.5-11 Rates of PDI vary substantially across hospitals. For example, one single-center study of postdischarge telephone contact after hospitalization on a general pediatric ward identified PDIs in ~20% of patients.10 Another study identified PDIs in 84% of patients discharged from a pediatric rehabilitation facility.11 Telephone follow-up has been associated with reduced health resource utilization and improved patient satisfaction for children discharged after an elective surgical procedure6 and for children discharged home from the emergency department.7-9
More information is needed on the clinical experiences of postdischarge contact in hospitalized children to improve the understanding of how the contact is made, who makes it, and which patients are most likely to report a PDI. These experiences are crucial to understand given the expense and time commitment involved in postdischarge contact, as many hospitals may not be positioned to contact all discharged patients. Therefore, we conducted a pragmatic, retrospective, naturalistic study of differing approaches to postdischarge contact occurring in multiple hospitals. Our main objective was to describe the prevalence and types of PDIs identified by the different approaches for follow-up contact across 4 children’s hospitals. We also assessed the characteristics of children who have the highest likelihood of having a PDI identified from the contact within each hospital.
METHODS
Study Design, Setting, and Population
Main Outcome Measures
The main outcome measure was identification of a PDI, defined as a medication, appointment, or other discharge-related issue, that was reported and recorded by the child’s caregiver during conversation from the standardized questions that were asked during follow-up contact as part of routine discharge care (Table 1). Medication PDIs included issues filling prescriptions and tolerating medications. Appointment PDIs included not having a follow-up appointment scheduled. Other PDIs included issues with the child’s health condition, discharge instructions, or any other concerns. All PDIs had been recorded prospectively by hospital contact personnel (hospitals A, B, and D) or through an automated texting system into a database (hospital C). Where available, free text comments that were recorded by contact personnel were reviewed by one of the authors (KB) and categorized via an existing framework of PDI designed by Heath et al.10 in order to further understand the problems that were reported.
Patient Characteristics
Patient hospitalization, demographic, and clinical characteristics were obtained from administrative health data at each institution and compared between children with versus without a PDI. Hospitalization characteristics included length of stay, season of admission, and reason for admission. Reason for admission was categorized by using 3M Health’s All Patient Refined Diagnosis Related Groups (APR-DRG) (3M, Maplewood, MN). Demographic characteristics included age at admission in years, insurance type (eg, public, private, and other), and race/ethnicity (Asian/Pacific Islander, Hispanic, non-Hispanic black, non-Hispanic white, and other).
Clinical characteristics included a count of the different classes of medications (eg, antibiotics, antiepileptic medications, digestive motility medications, etc.) administered to the child during admission, the type and number of chronic conditions, and assistance with medical technology (eg, gastrostomy, tracheostomy, etc.). Except for medications, these characteristics were assessed with International Classification of Diseases, Ninth Revision-Clinical Modification (ICD-9-CM) diagnosis codes.
We used the Agency for Healthcare Research and Quality Chronic Condition Indicator classification system, which categorizes over 14,000 ICD-9-CM diagnosis codes into chronic versus nonchronic conditions to identify the presence and number of chronic conditions.12 Children hospitalized with a chronic condition were further classified as having a complex chronic condition (CCC) by using the ICD-9-CM diagnosis classification scheme of Feudtner et al.13 CCCs represent defined diagnosis groupings of conditions expected to last longer than 12 months and involve either multiple organ systems or a single organ system severely enough to require specialty pediatric care and hospitalization.13,14 Children requiring medical technology were identified by using ICD-9-CM codes indicating their use of a medical device to manage and treat a chronic illness (eg, ventricular shunt to treat hydrocephalus) or to maintain basic body functions necessary for sustaining life (eg a tracheostomy tube for breathing).15,16
Statistical Analysis
Given that the primary purpose for this study was to leverage the natural heterogeneity in the approach to follow-up contact across hospitals, we assessed and reported the prevalence and type of PDIs independently for each hospital. Relatedly, we assessed the relationship between patient characteristics and PDI likelihood independently within each hospital as well rather than pool the data and perform a central analysis across hospitals. Of note, APR-DRG and medication class were not assessed for hospital D, as this information was unavailable. We used χ2 tests for univariable analysis and logistic regression with a backwards elimination derivation process (for variables with P ≥ .05) for multivariable analysis; all patient demographic, clinical, and hospitalization characteristics were entered initially into the models. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC), and P < .05 was considered statistically significant. This study was approved by the institutional review board at all hospitals.
RESULTS
Study Population
There were 12,986 (51.4%) of 25,259 patients reached by follow-up contact after discharge across the 4 hospitals. Median age at admission for contacted patients was 4.0 years (interquartile range [IQR] 0-11). Of those contacted, 45.2% were female, 59.9% were non-Hispanic white, 51.0% used Medicaid, and 95.4% were discharged to home. Seventy-one percent had a chronic condition (of any complexity) and 40.8% had a CCC. Eighty percent received a prescribed medication during the hospitalization. Median (IQR) length of stay was 2.0 days (IQR 1-4 days). The top 5 most common reasons for admission were bronchiolitis (6.3%), pneumonia (6.2%), asthma (5.2%), seizure (4.9%), and tonsil and adenoid procedures (4.1%).
PDIs
Characteristics Associated with PDIs
Age
Older age was a consistent characteristic associated with PDIs in 3 hospitals. For example, PDI rates in children 10 to 18 years versus <1 year were 30.8% versus 21.4% (P < .001) in hospital A, 19.4% versus 13.7% (P = .002) in hospital B, and 70.3% versus 62.8% (P < .001) in hospital D. In multivariable analysis, age 10 to 18 years versus <1 year at admission was associated with an increased likelihood of PDI in hospital A (odds ratio [OR] 1.7; 95% CI, 1.4-2.0), hospital B (OR 1.4; 95% CI, 1.1-1.8), and hospital D (OR 1.7; 95% CI, 0.9-3.0) (Table 3 and Figure).
Medications
Length of Stay
Shorter length of stay was associated with PDI in 1 hospital. In hospital A, the PDI rate increased significantly (P < .001) from 19.0% to 33.9% as length of stay decreased from ≥7 days to ≤1 day (Table 3). In multivariable analysis, length of stay to ≤1 day versus ≥7 days was associated with increased likelihood of PDI (OR 2.1; 95% CI, 1.7-2.5) in hospital A (Table 3 and Figure).
CCCs
A neuromuscular CCC was associated with PDI in 2 hospitals. In hospital B, the PDI rate was higher in children with a neuromuscular CCC compared with a malignancy CCC (21.3% vs 11.2%). In hospital D, the PDI rates were higher in children with a neuromuscular CCC compared with a respiratory CCC (68.9% vs 40.6%) (Table 3). In multivariable analysis, children with versus without a neuromuscular CCC had an increased likelihood of PDI (OR 1.3; 95% CI, 1.0-1.7) in hospital B (Table 3 and Figure).
DISCUSSION
In this retrospective, pragmatic, multicentered study of follow-up contact with a standardized set of questions asked after discharge for hospitalized children, we found that PDIs were identified often, regardless of who made the contact or how the contact was made. The PDI rates varied substantially across hospitals and were likely influenced by the different follow-up approaches that were used. Most PDIs were related to appointments; fewer PDIs were related to medications and other problems. Older age, shorter length of stay, and neuromuscular CCCs were among the identified risk factors for PDIs.
Our assessment of PDIs was, by design, associated with variation in methods and approach for detection across sites. Further investigation is needed to understand how different approaches for follow-up contact after discharge may influence the identification of PDIs. For example, in the current study, the hospital with the highest PDI rate (hospital D) used hospitalists who provided inpatient care for the patient to make follow-up contact. Although not determined from the current study, this approach could have led the hospitalists to ask questions beyond the standardized ones when assessing for PDIs. Perhaps some of the hospitalists had a better understanding of how to probe for PDIs specific to each patient; this understanding may not have been forthcoming for staff in the other hospitals who were unfamiliar with the patients’ hospitalization course and medical history.
Similar to previous studies in adults, our study reported that appointment PDIs in children may be more common than other types of PDIs.17 Appointment PDIs could have been due to scheduling difficulties, inadequate discharge instructions, lack of adherence to recommended follow-up, or other reasons. Further investigation is needed to elucidate these reasons and to determine how to reduce PDIs related to postdischarge appointments. Some children’s hospitals schedule follow-up appointments prior to discharge to mitigate appointment PDIs that might arise.18 However, doing that for every hospitalized child is challenging, especially for very short admissions or for weekend discharges when many outpatient and community practices are not open to schedule appointments. Additional exploration is necessary to assess whether this might help explain why some children in the current study with a short versus long length of stay had a higher likelihood of PDI.
The rate of medication PDIs (5.2%) observed in the current study is lower than the rate that is reported in prior literature. Dudas et al.1 found that medication PDIs occurred in 21% of hospitalized adult patients. One reason for the lower rate of medication PDIs in children may be that they require the use of postdischarge medications less often than adults. Most medication PDIs in the current study involved problems filling a prescription. There was not enough information in the notes taken from the follow-up contact to distinguish the medication PDI etiologies (eg, a prescription was not sent from the hospital team to the pharmacy, prior authorization from an insurance company for a prescription was not obtained, the pharmacy did not stock the medication). To help overcome medication access barriers, some hospitals fill and deliver discharge medications to the patients’ bedside. One study found that children discharged with medication in hand were less likely to have emergency department revisits within 30 days of discharge.19 Further investigation is needed to assess whether initiatives like these help mitigate medication PDIs in children.
Hospitals may benefit from considering how risk factors for PDIs can be used to prioritize which patients receive follow-up contact, especially in hospitals where contact for all hospitalized patients is not feasible. In the current study, there was variation across hospitals in the profile of risk factors that correlated with increased likelihood of PDI. Some of the risk factors are easier to explain than others. For example, as mentioned above, for some hospitalized children, short length of stay might not permit enough time for hospital staff to set up discharge plans that may sufficiently prevent PDIs. Other risk factors, including older age and neuromuscular CCCs, may require additional assessment (eg, through chart review or in-depth patient and provider interviews) to discover the reasons why they were associated with increased likelihood of PDI. There are additional risk factors that might influence the likelihood of PDI that the current study was not positioned to assess, including health literacy, transportation availability, and language spoken.20-23
This study has several other limitations in addition to the ones already mentioned. Some children may have experienced PDIs that were not reported at contact (eg, the respondent was unaware that an issue was present), which may have led to an undercounting of PDIs. Alternatively, some caregivers may have been more likely to respond to the contact if their child was experiencing a PDI, which may have led to overcounting. PDIs of nonrespondents were not measured. PDIs identified by postdischarge outpatient and community providers or by families outside of contact were not measured. The current study was not positioned to assess the severity of the PDIs or what interventions (including additional health services) were needed to address them. Although we assessed medication use during admission, we were unable to assess the number and type of medications that were prescribed for use postdischarge. Information about the number and type of follow-up visits needed for each child was not assessed. Given the variety of approaches for follow-up contact, the findings may generalize best to individual hospitals by using an approach that best matches to one of them. The current study is not positioned to correlate quality of discharge care with the rate of PDI.
Despite these limitations, the findings from the current study reinforce that PDIs identified through follow-up contact in discharged patients appear to be common. Of PDIs identified, appointment problems were more prevalent than medication or other types of problems. Short length of stay, older age, and other patient and/or hospitalization attributes were associated with an increased likelihood of PDI. Hospitals caring for children may find this information useful as they strive to optimize their processes for follow-up contact after discharge. To help further evaluate the value and importance of contacting patients after discharge, additional study of PDI in children is warranted, including (1) actions taken to resolve PDIs, (2) the impact of identifying and addressing PDIs on hospital readmission, and (3) postdischarge experiences and health outcomes of children who responded versus those who did not respond to the follow-up contact. Moreover, future multisite, comparative effectiveness studies of PDI may wish to consider standardization of follow-up contact procedures with controlled manipulation of key processes (eg, contact by administrator vs nurse vs physician) to assess best practices.
Disclosure
Mr. Blaine, Ms. O’Neill, and Drs. Berry, Brittan, Rehm, and Steiner were supported by the Lucile Packard Foundation for Children’s Health. The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest to disclose.
1. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239-248. PubMed
2. Sanchez GM, Douglass MA, Mancuso MA. Revisiting Project Re-Engineered Discharge (RED): The Impact of a Pharmacist Telephone Intervention on Hospital Readmission Rates. Pharmacotherapy. 2015;35(9):805-812. PubMed
3. Jones J, Clark W, Bradford J, Dougherty J. Efficacy of a telephone follow-up system in the emergency department. J Emerg Med. 1988;6(3):249-254. PubMed
4. Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510. PubMed
5. Lushaj EB, Nelson K, Amond K, Kenny E, Badami A, Anagnostopoulos PV. Timely Post-discharge Telephone Follow-Up is a Useful Tool in Identifying Post-discharge Complications Patients After Congenital Heart Surgery. Pediatr Cardiol. 2016;37(6):1106-1110. PubMed
6. McVay MR, Kelley KR, Mathews DL, Jackson RJ, Kokoska ER, Smith SD. Postoperative follow-up: is a phone call enough? J Pediatr Surg. 2008;43(1):83-86. PubMed
7. Chande VT, Exum V. Follow-up phone calls after an emergency department visit. Pediatrics. 1994;93(3):513-514. PubMed
8. Sutton D, Stanley P, Babl FE, Phillips F. Preventing or accelerating emergency care for children with complex healthcare needs. Arch Dis Child. 2008;93(1):17-22. PubMed
9. Patel PB, Vinson DR. Physician e-mail and telephone contact after emergency department visit improves patient satisfaction: a crossover trial. Ann Emerg Med. 2013;61(6):631-637. PubMed
10. Heath J, Dancel R, Stephens JR. Postdischarge phone calls after pediatric hospitalization: an observational study. Hosp Pediatr. 2015;5(5):241-248. PubMed
11. Biffl SE, Biffl WL. Improving transitions of care for complex pediatric trauma patients from inpatient rehabilitation to home: an observational pilot study. Patient Saf Surg. 2015;9:33-37. PubMed
12. AHRQ. Clinical Classifications Software (CCS) for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed on January 31,2012.
13. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205-209. PubMed
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. PubMed
15. Palfrey JS, Walker DK, Haynie M, et al. Technology’s children: report of a statewide census of children dependent on medical supports. Pediatrics. 1991;87(5):611-618. PubMed
16. Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technology-dependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5(1):8-15. PubMed
17. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
18. Brittan M, Tyler A, Martin S, et al. A Discharge Planning Template for the Electronic Medical Record Improves Scheduling of Neurology Follow-up for Comanaged Seizure Patients. Hosp Pediatr. 2014;4(6):366-371. PubMed
19. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. doi:10.1542/peds.2015-0461. PubMed
20. Berry JG, Goldmann DA, Mandl KD, et al. Health information management and perceptions of the quality of care for children with tracheotomy: a qualitative study. BMC Health Serv Res. 2011;11:117-125. PubMed
21. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573-581. PubMed
22. Carusone SC, O’Leary B, McWatt S, Stewart A, Craig S, Brennan DJ. The Lived Experience of the Hospital Discharge “Plan”: A Longitudinal Qualitative Study of Complex Patients. J Hosp Med. 2017;12(1):5-10. PubMed
23. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ Priorities Regarding Hospital-to-Home Transitions for Children With Medical Complexity. Pediatrics. 2017;139(1):e20161581. doi:10.1542/peds.2016-1581. PubMed
1. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239-248. PubMed
2. Sanchez GM, Douglass MA, Mancuso MA. Revisiting Project Re-Engineered Discharge (RED): The Impact of a Pharmacist Telephone Intervention on Hospital Readmission Rates. Pharmacotherapy. 2015;35(9):805-812. PubMed
3. Jones J, Clark W, Bradford J, Dougherty J. Efficacy of a telephone follow-up system in the emergency department. J Emerg Med. 1988;6(3):249-254. PubMed
4. Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510. PubMed
5. Lushaj EB, Nelson K, Amond K, Kenny E, Badami A, Anagnostopoulos PV. Timely Post-discharge Telephone Follow-Up is a Useful Tool in Identifying Post-discharge Complications Patients After Congenital Heart Surgery. Pediatr Cardiol. 2016;37(6):1106-1110. PubMed
6. McVay MR, Kelley KR, Mathews DL, Jackson RJ, Kokoska ER, Smith SD. Postoperative follow-up: is a phone call enough? J Pediatr Surg. 2008;43(1):83-86. PubMed
7. Chande VT, Exum V. Follow-up phone calls after an emergency department visit. Pediatrics. 1994;93(3):513-514. PubMed
8. Sutton D, Stanley P, Babl FE, Phillips F. Preventing or accelerating emergency care for children with complex healthcare needs. Arch Dis Child. 2008;93(1):17-22. PubMed
9. Patel PB, Vinson DR. Physician e-mail and telephone contact after emergency department visit improves patient satisfaction: a crossover trial. Ann Emerg Med. 2013;61(6):631-637. PubMed
10. Heath J, Dancel R, Stephens JR. Postdischarge phone calls after pediatric hospitalization: an observational study. Hosp Pediatr. 2015;5(5):241-248. PubMed
11. Biffl SE, Biffl WL. Improving transitions of care for complex pediatric trauma patients from inpatient rehabilitation to home: an observational pilot study. Patient Saf Surg. 2015;9:33-37. PubMed
12. AHRQ. Clinical Classifications Software (CCS) for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed on January 31,2012.
13. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205-209. PubMed
14. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. PubMed
15. Palfrey JS, Walker DK, Haynie M, et al. Technology’s children: report of a statewide census of children dependent on medical supports. Pediatrics. 1991;87(5):611-618. PubMed
16. Feudtner C, Villareale NL, Morray B, Sharp V, Hays RM, Neff JM. Technology-dependency among patients discharged from a children’s hospital: a retrospective cohort study. BMC Pediatr. 2005;5(1):8-15. PubMed
17. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
18. Brittan M, Tyler A, Martin S, et al. A Discharge Planning Template for the Electronic Medical Record Improves Scheduling of Neurology Follow-up for Comanaged Seizure Patients. Hosp Pediatr. 2014;4(6):366-371. PubMed
19. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. doi:10.1542/peds.2015-0461. PubMed
20. Berry JG, Goldmann DA, Mandl KD, et al. Health information management and perceptions of the quality of care for children with tracheotomy: a qualitative study. BMC Health Serv Res. 2011;11:117-125. PubMed
21. Berry JG, Ziniel SI, Freeman L, et al. Hospital readmission and parent perceptions of their child’s hospital discharge. Int J Qual Health Care. 2013;25(5):573-581. PubMed
22. Carusone SC, O’Leary B, McWatt S, Stewart A, Craig S, Brennan DJ. The Lived Experience of the Hospital Discharge “Plan”: A Longitudinal Qualitative Study of Complex Patients. J Hosp Med. 2017;12(1):5-10. PubMed
23. Leyenaar JK, O’Brien ER, Leslie LK, Lindenauer PK, Mangione-Smith RM. Families’ Priorities Regarding Hospital-to-Home Transitions for Children With Medical Complexity. Pediatrics. 2017;139(1):e20161581. doi:10.1542/peds.2016-1581. PubMed
© 2018 Society of Hospital Medicine
Tisagenlecleucel looks effective in phase 2 study of young ALL patients
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Tisagenlecleucel was associated with durable remission and long-term persistence for younger patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL), according to the results of a multicenter, multicontinent, phase 2 trial published in the New England Journal of Medicine.
Shannon L. Maude, MD, PhD, of the Children’s Hospital of Philadelphia and her coauthors reported that the anti-CD19 chimeric antigen receptor (CAR) therapy was highly toxic, but the effects were usually mitigated. Additionally, the investigators showed feasibility of a global supply chain for distribution of the therapy.
The investigators evaluated data from 75 patients with at least 5% lymphoblasts in their bone marrow at the time of screening. Patients were aged 3 years or older at the time of screening but were no older than 21 years of age at the time of diagnosis.
For 50 patients evaluated at the interim analysis, the primary endpoint of overall remission at 3 months was met, and the overall remission rate was 82% (P less than .001).
An updated analysis showed that 81% of 75 patients who had at least 3 months of follow-up experienced overall remission (95% confidence interval, 71-89). A total of 45 of those patients experienced complete remission, and 16 had complete remission with incomplete hematologic recovery.
Event-free survival was experienced by 73% of patients at 6 months and 50% of patients at 12 months. Overall survival was 90% at 6 months and 76% at 12 months, the investigators reported.
Before tisagenlecleucel infusion, 96% of patients received lymphodepleting chemotherapy. The administration of chemotherapy was not done at the discretion of the investigator if a patient had leukopenia.
The median duration of remission was not reached, and the persistence of tisagenlecleucel in the blood was observed for as long as 20 months.
“The remissions were durable, with a 6-month relapse-free survival rate of 80%,” the investigators wrote. “The durability of the clinical response was associated with persistence of tisagenlecleucel in peripheral blood and with persistent B-cell aplasia.”
The phase 1 study of tisagenlecleucel infusion therapy for younger patients with B-cell ALL showed the toxic nature of the therapy, so investigators were not surprised by the safety data they found. Nearly three-quarters of patients who were evaluated in the study experienced a grade 3 or 4 tisagenlecleucel-related adverse event. Cytokine release syndrome occurred in 77% of patients.
Previously reported data regarding anti-CD19 CAR T-cell therapy for ALL came from single-center studies where manufacturing occurred on site, but the current study employed a global, multicenter supply chain, according to the investigators.
“The toxicity and efficacy of tisagenlecleucel [in this study] were consistent with those in the single-center study, and the feasibility of a global supply chain was demonstrated,” they wrote. “Because this study used cryopreserved leukapheresis product, it did not require fresh product and an open manufacture slot for enrollment.”
This research was sponsored and designed by Novartis Pharmaceuticals. Dr. Maude reported having received personal fees from Novartis as well as grant funding from St. Baldrick’s Foundation.
SOURCE: Maude SL, et al. N Engl J Med. 2018;378:439-48.
Key clinical point:
Major finding: The overall remission rate was 81% at 3 months, and 73% of patients experienced grade 3 or 4 adverse events.
Study details: A multicenter, phase 2 study of 75 patients.
Disclosures: Novartis designed and sponsored this research. Dr. Maude reported receiving fees from Novartis and grant funding from St. Baldrick’s Foundation.
Source: Maude SL et al. N Engl J Med. 2018;378:439-48.
CAR T cells produce longest survival in low disease burden ALL patients
Among patients with B-cell acute lymphoblastic leukemia (ALL) who received an infusion of 19-28z CAR T cells, patients with low disease burden had better survival outcomes and fewer toxic effects than did patients with a high disease burden, according to long-term follow-up results of a phase 1 study.
Median overall survival for B-cell ALL patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02), and 12.9 months for the entire cohort, according to results published in the New England Journal of Medicine.
The 12.9-month overall survival for the full study cohort “compares favorably” to results from another recently reported clinical trial showing overall survival of 7.7 months for adult B-cell ALL patients treated with blinatumomab, an anti–CD19/CD3 bispecific T-cell engager, wrote Jae H. Park, MD, of the Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The CAR T-cell and blinatumomab results cannot be directly compared owing to the differences in study design, patient characteristics, and posttreatment consolidation, but “the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies,” Dr. Park and his colleagues wrote in their report.
The phase 1 trial by Dr. Park and his colleagues included 53 adults with relapsed B-cell ALL who received a single infusion of 19-28z CAR T-cell therapy manufactured at Memorial Sloan Kettering Cancer Center.
After the infusion, 41% of patients with high disease burden (at least 5% bone marrow blasts or extramedullary disease) experienced severe cytokine release syndrome, compared with 5% of those with low disease burden, according to the report.
Likewise, neurotoxic effects were seen in 59% of high disease burden B-ALL patients, compared with 14% of those with low disease burden, the investigators reported.
Low disease burden was associated with a higher rate of complete remission, but this finding did not reach statistical significance. However, low disease burden patients not only had improved overall survival, as noted, but also had a significantly longer event-free survival versus high disease burden patients (10.6 and 5.3 months, respectively; P = .01).
Robust expansion of CAR T cells in vivo was a good predictor of short-term response and toxic effects but did not correlate with longer-term efficacy, according to the researchers. Instead, the ratio of peak CAR T-cell expansion to tumor burden correlated significantly with event-free and overall survival.
That finding “raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden,” the investigators wrote.
The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
SOURCE: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
Among patients with B-cell acute lymphoblastic leukemia (ALL) who received an infusion of 19-28z CAR T cells, patients with low disease burden had better survival outcomes and fewer toxic effects than did patients with a high disease burden, according to long-term follow-up results of a phase 1 study.
Median overall survival for B-cell ALL patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02), and 12.9 months for the entire cohort, according to results published in the New England Journal of Medicine.
The 12.9-month overall survival for the full study cohort “compares favorably” to results from another recently reported clinical trial showing overall survival of 7.7 months for adult B-cell ALL patients treated with blinatumomab, an anti–CD19/CD3 bispecific T-cell engager, wrote Jae H. Park, MD, of the Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The CAR T-cell and blinatumomab results cannot be directly compared owing to the differences in study design, patient characteristics, and posttreatment consolidation, but “the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies,” Dr. Park and his colleagues wrote in their report.
The phase 1 trial by Dr. Park and his colleagues included 53 adults with relapsed B-cell ALL who received a single infusion of 19-28z CAR T-cell therapy manufactured at Memorial Sloan Kettering Cancer Center.
After the infusion, 41% of patients with high disease burden (at least 5% bone marrow blasts or extramedullary disease) experienced severe cytokine release syndrome, compared with 5% of those with low disease burden, according to the report.
Likewise, neurotoxic effects were seen in 59% of high disease burden B-ALL patients, compared with 14% of those with low disease burden, the investigators reported.
Low disease burden was associated with a higher rate of complete remission, but this finding did not reach statistical significance. However, low disease burden patients not only had improved overall survival, as noted, but also had a significantly longer event-free survival versus high disease burden patients (10.6 and 5.3 months, respectively; P = .01).
Robust expansion of CAR T cells in vivo was a good predictor of short-term response and toxic effects but did not correlate with longer-term efficacy, according to the researchers. Instead, the ratio of peak CAR T-cell expansion to tumor burden correlated significantly with event-free and overall survival.
That finding “raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden,” the investigators wrote.
The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
SOURCE: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
Among patients with B-cell acute lymphoblastic leukemia (ALL) who received an infusion of 19-28z CAR T cells, patients with low disease burden had better survival outcomes and fewer toxic effects than did patients with a high disease burden, according to long-term follow-up results of a phase 1 study.
Median overall survival for B-cell ALL patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02), and 12.9 months for the entire cohort, according to results published in the New England Journal of Medicine.
The 12.9-month overall survival for the full study cohort “compares favorably” to results from another recently reported clinical trial showing overall survival of 7.7 months for adult B-cell ALL patients treated with blinatumomab, an anti–CD19/CD3 bispecific T-cell engager, wrote Jae H. Park, MD, of the Leukemia Service, Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The CAR T-cell and blinatumomab results cannot be directly compared owing to the differences in study design, patient characteristics, and posttreatment consolidation, but “the observation of patients with durable remissions in these two studies highlights the potential of CD19-targeted immunotherapies,” Dr. Park and his colleagues wrote in their report.
The phase 1 trial by Dr. Park and his colleagues included 53 adults with relapsed B-cell ALL who received a single infusion of 19-28z CAR T-cell therapy manufactured at Memorial Sloan Kettering Cancer Center.
After the infusion, 41% of patients with high disease burden (at least 5% bone marrow blasts or extramedullary disease) experienced severe cytokine release syndrome, compared with 5% of those with low disease burden, according to the report.
Likewise, neurotoxic effects were seen in 59% of high disease burden B-ALL patients, compared with 14% of those with low disease burden, the investigators reported.
Low disease burden was associated with a higher rate of complete remission, but this finding did not reach statistical significance. However, low disease burden patients not only had improved overall survival, as noted, but also had a significantly longer event-free survival versus high disease burden patients (10.6 and 5.3 months, respectively; P = .01).
Robust expansion of CAR T cells in vivo was a good predictor of short-term response and toxic effects but did not correlate with longer-term efficacy, according to the researchers. Instead, the ratio of peak CAR T-cell expansion to tumor burden correlated significantly with event-free and overall survival.
That finding “raises the hypothesis that an effective ratio of CAR T cells to target CD19+ leukemia cells is more likely to occur in patients with a low disease burden than in those with a high disease burden, despite a smaller number of expanded T cells in patients with a low disease burden,” the investigators wrote.
The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
SOURCE: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Median overall survival for patients with low disease burden was 20.1 months, compared with 12.4 months for those with a high disease burden (P = .02).
Study details: A long-term follow-up of a phase 1 trial including 53 adults with relapsed B-cell ALL.
Disclosures: The study was funded by the Commonwealth Foundation for Cancer Research, Juno Therapeutics, and others. Several study authors reported ties to Juno Therapeutics and other pharmaceutical companies.
Source: Park JH et al. N Engl J Med 2018 Feb 1;378:449-59.
Preop physiotherapy training reduces risk of postop pulmonary complications
A single 30-minute coaching session with a physiotherapist within 6 weeks of major upper abdominal surgery significantly reduced postoperative pulmonary complications (PPC), according to the results of a prospective trial.
Ianthe Boden and her colleagues recruited 441 eligible adults scheduled for elective major upper abdominal surgery to participate in the prospective, multicenter, double-blinded, controlled superiority study to assess whether PPC outcomes were affected by preoperative physiotherapy. Consecutive participants were obtained from outpatient preadmission assessment clinics during June 2013 to August 2015; they were assigned randomly in a 1:1 ratio to the control (219) or intervention (222) groups. The median patient age was 68 years for the control and 63 for the intervention group, and each group was composed of 31% women.
Immediately after receiving the booklets, however, participants in the intervention group were also given an added 30-minute education and training session by preoperative physiotherapists. This instruction covered factors contributing to PPC occurrence, strategies to help prevention it, and three coached repetitions of breathing exercises. Emphasis was placed on initiating prescribed breathing exercises upon regaining postoperative consciousness and continuing them every hour until the patients were fully ambulatory.
The primary outcome was evaluated by masked assessors using the Melbourne group score criteria to determine PPC incidence within 14 postoperative days or by the time of hospital discharge, whichever was sooner. Nine participants, 4 from the intervention and 5 from the control group, withdrew from the study. Of the total remaining 432 participants, 85 (20%) had a documented PPC incident, including hospital acquired pneumonia, within the specified postoperative time frame, as reported in the BMJ.
Results showed that the physiotherapy group had significantly fewer PPC occurrences (27/218, 12%) than did the control group (58/214, 27%). The calculated absolute risk reduction was 15% (P less than .001). Adjustment for three of the prespecified covariates (age, respiratory comorbidity, and surgical procedure) showed PPC incidence remained halved (hazard ratio, 0.48; P = .001) for the intervention group with a number needed to treat of 7 (95% confidence interval, 5-14).
Secondary outcomes included incidence of hospital acquired pneumonia, hospital utilization, mobility, patient reported complications at 6 weeks, and mortality rates in hospital, at 6 weeks, and at 12 months. For secondary outcomes in the adjusted analysis, incidences of pneumonia were halved in the physiotherapy intervention group with a number needed to treat of 9 (95% CI, 6-21). No significant differences in secondary outcomes were detected between the control and treatment groups.
Sensitivity analysis that removed participants who had lower abdominal and laparoscopic surgery strengthened both primary and secondary outcome results to favor the preoperative physiotherapy intervention for reducing PPC. The researchers found that, in an adjusted analysis of subgroup effects, there was a gradient in reduction of PPCs according to surgical category.
Shorter lengths hospital stay and lower all-cause 12-month mortality were also associated with more experienced physiotherapists providing the preoperative education and training.
Ms. Boden and her colleagues proposed that the timing for patients to begin breathing exercises after major open upper abdominal surgery could be critical in reducing PPC incidence. Initiating breathing exercises within the first 24 hours after surgery – in contrast to the common practice of waiting 1-2 days to begin postoperative physiotherapy – could prevent general anesthesia-associated mild atelectasis from developing into severe atelectasis and PPCs.
The researchers concluded that “in a general population of patients listed for elective upper abdominal surgery, a 30-minute preoperative physiotherapy session provided within existing hospital multidisciplinary preadmission clinics halves the incidence of PPCs and specifically hospital acquired pneumonia. Further research is required to investigate benefits to mortality and length of stay.”
The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
SOURCE: Boden I et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
A single 30-minute coaching session with a physiotherapist within 6 weeks of major upper abdominal surgery significantly reduced postoperative pulmonary complications (PPC), according to the results of a prospective trial.
Ianthe Boden and her colleagues recruited 441 eligible adults scheduled for elective major upper abdominal surgery to participate in the prospective, multicenter, double-blinded, controlled superiority study to assess whether PPC outcomes were affected by preoperative physiotherapy. Consecutive participants were obtained from outpatient preadmission assessment clinics during June 2013 to August 2015; they were assigned randomly in a 1:1 ratio to the control (219) or intervention (222) groups. The median patient age was 68 years for the control and 63 for the intervention group, and each group was composed of 31% women.
Immediately after receiving the booklets, however, participants in the intervention group were also given an added 30-minute education and training session by preoperative physiotherapists. This instruction covered factors contributing to PPC occurrence, strategies to help prevention it, and three coached repetitions of breathing exercises. Emphasis was placed on initiating prescribed breathing exercises upon regaining postoperative consciousness and continuing them every hour until the patients were fully ambulatory.
The primary outcome was evaluated by masked assessors using the Melbourne group score criteria to determine PPC incidence within 14 postoperative days or by the time of hospital discharge, whichever was sooner. Nine participants, 4 from the intervention and 5 from the control group, withdrew from the study. Of the total remaining 432 participants, 85 (20%) had a documented PPC incident, including hospital acquired pneumonia, within the specified postoperative time frame, as reported in the BMJ.
Results showed that the physiotherapy group had significantly fewer PPC occurrences (27/218, 12%) than did the control group (58/214, 27%). The calculated absolute risk reduction was 15% (P less than .001). Adjustment for three of the prespecified covariates (age, respiratory comorbidity, and surgical procedure) showed PPC incidence remained halved (hazard ratio, 0.48; P = .001) for the intervention group with a number needed to treat of 7 (95% confidence interval, 5-14).
Secondary outcomes included incidence of hospital acquired pneumonia, hospital utilization, mobility, patient reported complications at 6 weeks, and mortality rates in hospital, at 6 weeks, and at 12 months. For secondary outcomes in the adjusted analysis, incidences of pneumonia were halved in the physiotherapy intervention group with a number needed to treat of 9 (95% CI, 6-21). No significant differences in secondary outcomes were detected between the control and treatment groups.
Sensitivity analysis that removed participants who had lower abdominal and laparoscopic surgery strengthened both primary and secondary outcome results to favor the preoperative physiotherapy intervention for reducing PPC. The researchers found that, in an adjusted analysis of subgroup effects, there was a gradient in reduction of PPCs according to surgical category.
Shorter lengths hospital stay and lower all-cause 12-month mortality were also associated with more experienced physiotherapists providing the preoperative education and training.
Ms. Boden and her colleagues proposed that the timing for patients to begin breathing exercises after major open upper abdominal surgery could be critical in reducing PPC incidence. Initiating breathing exercises within the first 24 hours after surgery – in contrast to the common practice of waiting 1-2 days to begin postoperative physiotherapy – could prevent general anesthesia-associated mild atelectasis from developing into severe atelectasis and PPCs.
The researchers concluded that “in a general population of patients listed for elective upper abdominal surgery, a 30-minute preoperative physiotherapy session provided within existing hospital multidisciplinary preadmission clinics halves the incidence of PPCs and specifically hospital acquired pneumonia. Further research is required to investigate benefits to mortality and length of stay.”
The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
SOURCE: Boden I et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
A single 30-minute coaching session with a physiotherapist within 6 weeks of major upper abdominal surgery significantly reduced postoperative pulmonary complications (PPC), according to the results of a prospective trial.
Ianthe Boden and her colleagues recruited 441 eligible adults scheduled for elective major upper abdominal surgery to participate in the prospective, multicenter, double-blinded, controlled superiority study to assess whether PPC outcomes were affected by preoperative physiotherapy. Consecutive participants were obtained from outpatient preadmission assessment clinics during June 2013 to August 2015; they were assigned randomly in a 1:1 ratio to the control (219) or intervention (222) groups. The median patient age was 68 years for the control and 63 for the intervention group, and each group was composed of 31% women.
Immediately after receiving the booklets, however, participants in the intervention group were also given an added 30-minute education and training session by preoperative physiotherapists. This instruction covered factors contributing to PPC occurrence, strategies to help prevention it, and three coached repetitions of breathing exercises. Emphasis was placed on initiating prescribed breathing exercises upon regaining postoperative consciousness and continuing them every hour until the patients were fully ambulatory.
The primary outcome was evaluated by masked assessors using the Melbourne group score criteria to determine PPC incidence within 14 postoperative days or by the time of hospital discharge, whichever was sooner. Nine participants, 4 from the intervention and 5 from the control group, withdrew from the study. Of the total remaining 432 participants, 85 (20%) had a documented PPC incident, including hospital acquired pneumonia, within the specified postoperative time frame, as reported in the BMJ.
Results showed that the physiotherapy group had significantly fewer PPC occurrences (27/218, 12%) than did the control group (58/214, 27%). The calculated absolute risk reduction was 15% (P less than .001). Adjustment for three of the prespecified covariates (age, respiratory comorbidity, and surgical procedure) showed PPC incidence remained halved (hazard ratio, 0.48; P = .001) for the intervention group with a number needed to treat of 7 (95% confidence interval, 5-14).
Secondary outcomes included incidence of hospital acquired pneumonia, hospital utilization, mobility, patient reported complications at 6 weeks, and mortality rates in hospital, at 6 weeks, and at 12 months. For secondary outcomes in the adjusted analysis, incidences of pneumonia were halved in the physiotherapy intervention group with a number needed to treat of 9 (95% CI, 6-21). No significant differences in secondary outcomes were detected between the control and treatment groups.
Sensitivity analysis that removed participants who had lower abdominal and laparoscopic surgery strengthened both primary and secondary outcome results to favor the preoperative physiotherapy intervention for reducing PPC. The researchers found that, in an adjusted analysis of subgroup effects, there was a gradient in reduction of PPCs according to surgical category.
Shorter lengths hospital stay and lower all-cause 12-month mortality were also associated with more experienced physiotherapists providing the preoperative education and training.
Ms. Boden and her colleagues proposed that the timing for patients to begin breathing exercises after major open upper abdominal surgery could be critical in reducing PPC incidence. Initiating breathing exercises within the first 24 hours after surgery – in contrast to the common practice of waiting 1-2 days to begin postoperative physiotherapy – could prevent general anesthesia-associated mild atelectasis from developing into severe atelectasis and PPCs.
The researchers concluded that “in a general population of patients listed for elective upper abdominal surgery, a 30-minute preoperative physiotherapy session provided within existing hospital multidisciplinary preadmission clinics halves the incidence of PPCs and specifically hospital acquired pneumonia. Further research is required to investigate benefits to mortality and length of stay.”
The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
SOURCE: Boden I et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
FROM THE BMJ
Key clinical point: Reduction in PPC incidences corresponded to physiotherapists providing preoperative education and coaching intervention.
Major finding: Compared with the control group, Absolute risk was reduced by 15%, and seven was determined as number needed to treat.
Study details: Prospective, blinded study of 441 adult participants randomly assigned in a 1:1 ratio, comparing PPC outcomes associated with preop practices for upper abdominal surgeries.
Disclosures: The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
Source: Boden I. et al. BMJ. 2018. doi: 10.1136/bmj.j5916.