User login
Introduction
Dyspnea, the sensation of difficult or labored breathing, is the most common symptom in chronic obstructive pulmonary disease (COPD) and the primary symptom that limits physical activity in more advanced disease.1 According to the American Thoracic Society, dyspnea may be measured according to 3 domains2:
- what breathing feels like for the patient
- how distressed the patient feels when breathing
- how dyspnea affects functional ability, employment, health-related quality of life, or health status.
As disease severity increases, breathlessness becomes more disabling at lower activity levels. These changes further impact the quality of life of patients, and can lead to anxiety and depression.11
Physical inactivity is often considered to be a major contributor to the progression of COPD,6 and is linked to hospitalizations and increased all-cause mortality.12 There is therefore a need to recognize symptoms early and treat them accordingly.
CASE STUDY:
KD, a 64-year-old woman, presented to her primary care physician’s office for a routine visit. Upon assessment, KD revealed that she used to enjoy going on walks with her neighbor, but she cannot walk up the hills in her neighborhood anymore without feeling “incredibly breathless.” She has become increasingly concerned that she is “having trouble getting a full breath.” KD informed her doctor that these symptoms had worsened since her last visit, and so she had stopped going on neighborhood walks. She was diagnosed with COPD 4 years ago, and is currently using a long-acting muscarinic antagonist (LAMA) bronchodilator. KD has a 40 pack-year smoking history, and has previously been advised to stop smoking, but has relapsed several times. She has a medical history of hypertension and depression, and a notable family history of emphysema, breast cancer, and diabetes.
The relationship between lung hyperinflation and dyspnea in COPD
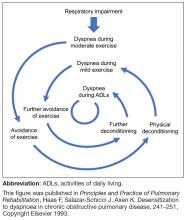
In COPD, pathologic changes give rise to physiologic abnormalities such as mucus hypersecretion and ciliary dysfunction, gas exchange abnormalities, pulmonary hypertension, and airflow limitation and lung hyperinflation.13 Lung hyperinflation, an increase in resting functional residual volume above a normal level, represents a mechanical link between the characteristic expiratory airflow impairment, dyspnea, and physical activity limitation in COPD.1
Although patients can compensate for several of the negative consequences of hyperinflation (eg, altering the chest wall due to overdistended lungs), such compensatory mechanisms are unable to cope with large increases in ventilation, such as those that occur during exercise.1 Air trapping, together with ineffectiveness of respiratory muscle function, leads to increased ventilation requirements and dynamic pulmonary hyperinflation, resulting in dyspnea.1
Patients with COPD describe a sensation of “air hunger,” reporting “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” and a feeling that they “cannot get a deep breath,”18 whereas, in fact, they are limited in their ability to fully exhale. Verbal descriptors (eg, “air hunger” or “chest tightness”) are important tools in understanding a patient’s experience with dyspnea, and a patient’s choice of descriptor may be related to dyspnea severity, and the level of distress that dyspnea causes a given patient.19 Air hunger in turn encourages faster breathing, leading to further shortness of breath and more dynamic hyperinflation.1,20
To deflate the lungs of patients with COPD, physiologic, pharmacologic, and possibly surgical interventions are required:
- Controlled breathing techniques (eg, purse-lipped breathing) that encourage slow and deep breathing can correct abnormal chest wall motion, decrease the work of breathing, increase breathing efficiency, and improve the distribution of ventilation to empty the lungs.21
- Bronchodilators can help to achieve lung deflation by improving ventilatory mechanics, as shown by increases in inspiratory capacity and vital capacity.22
- Lung volume reduction surgery can also be considered to treat severe hyperinflation in emphysematous patients5; bronchoscopic interventions that lower lung volumes are also in development.23
The impact of lung hyperinflation and dyspnea on physical activity in COPD
Dyspnea and hyperinflation are closely interrelated with physical activity limitation,16,29,30 and so can be viewed as significant contributors to patient disability. During an acute exacerbation, patients with COPD will experience worsening airway obstruction, dynamic hyperinflation, and dyspnea.31 Patients with a greater number of comorbid conditions may also have greater shortness of breath.32 In addition, patients with COPD and hyperinflation perform less physical activity than individuals without hyperinflation, regardless of COPD severity, as assessed using the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging (stage I, mild; stage II, moderate; stage III, severe; stage IV, very severe) and BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) index.33 These patients also exhibit increases in dyspnea perception during commonly performed ADLs, which may limit physical activity and worsen lung hyperinflation.33 More limited physical activity also contributes to higher dyspnea scores during ADLs.8
Furthermore, the ability to perform typical ADLs may be significantly altered or eliminated altogether in patients with COPD.11 Leisure activities are often the first to be dropped by patients, as they generally require greater effort than simpler tasks, and are not critical to daily life.11 Eventually, these activities become progressively more difficult, and most patients with moderate or severe COPD can struggle to complete even the most basic daily activities.11
In addition to the morbidity burden and impact on ADLs, lower levels of physical activity in patients with COPD have also been shown to increase the risk of mortality and exacerbations, and elevate the risk of comorbidities such as heart disease and metabolic disease.34 In light of these observations, improving exercise capacity should be a key goal in COPD management.
Assessment and measurement of dyspnea and hyperinflation
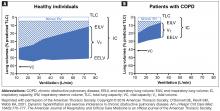
Reducing hyperinflation and dyspnea is essential for improving physical activity endurance and overall physical activity in patients with COPD; therefore, measuring the degree of impairment is important.22 Clinicians should be aware that some patients may have relief of dyspnea due to improvements in hyperinflation, despite relatively mild changes in FEV1.35 Lung volume measures, including total lung capacity, residual volume and functional residual capacity, are valuable tools in the assessment of lung hyperinflation in COPD, and therefore constitute a key component of pulmonary function testing.36 However, expanded pulmonary function testing may be required for patients with severe dyspnea that does not correspond to spirometric findings, or cases in which diagnosis is uncertain.37
Lung volumes are evaluated primarily by body plethysmography, during which a patient sits inside an airtight “body box” equipped to measure pressure and volume changes.14,38 Helium dilution and nitrogen washing can also be used to measure functional residual capacity in patients with COPD,14 but body plethysmography is considered to be a more accurate method of lung volume evaluation in patients with severe airflow obstruction.14,38 Radiographic techniques can also be used, but due to a lack of standardization, they are not typically utilized in clinical practice.14 Measurement of IC may complement other lung volume measures as part of assessment of hyperinflation.16 This can be measured using either spirometry or body plethysmography.39,40
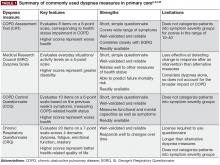
In addition to evaluating hyperinflation, ADLs, physical activity, exercise capacity, and dyspnea should all be assessed in patients with COPD in primary care. It is known that patients may self-limit ADLs to avoid symptoms of COPD; in doing so, worsening symptoms may be underappreciated, and subsequently underreported, by the patient. Thus, it is essential that physicians ask patients with COPD, as well as individuals at risk of COPD, questions about changes in their physical activity or ability to perform common tasks. There are a number of methods to measure functional performance, but for a simple assessment of ADLs, clinicians can ask the patient or caregiver questions related to basic daily tasks.11 In early COPD, patients who experience mild dyspnea during exercise should be able to perform most productive activities. Patients with stable COPD and moderate dyspnea during exercise should be able to carry out most of the higher functioning ADLs, whereas patients with severe COPD may struggle to complete basic ADLs without assistance.11 It should be noted, however, that patients may experience dyspnea with fairly routine activities, and even reduce physical activity at relatively early stages of airflow limitation.41,42
Other tests may be useful in assessing the impact of an intervention, be it pharmacologic or nonpharmacologic, on dyspnea severity. For example, increases in the 6-minute-walk distance (6MWD) have been shown to correlate with improvements in dyspnea.46 The 6MWD has also been shown to be an important predictor of hospitalization and mortality in patients with COPD.47 However, it is important to note that improvements in 6MWD show only a very weak correlation with patient-reported outcomes,48 and may be a less sensitive measure for patients with less disability than those with more profound functional limitation.49 Moreover, 6MWD can be affected by a patient’s psychologic motivation,6,50 as well as other comorbidities observed in patients with COPD, such as osteoporosis, heart failure, and peripheral vascular disease.46,51 Although not used for COPD diagnosis or evaluation of dyspnea or physical activity limitation, a chest X-ray can also be a useful tool for excluding alternative diagnoses, as well as for detecting significant comorbidities in patients with COPD, such as concomitant respiratory, cardiac, and skeletal diseases.5
Management of dyspnea and hyperinflation in primary care
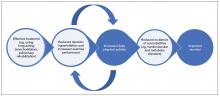
Pulmonary rehabilitation is a tailored intervention that encompasses exercise training, education, and self-management support for people with chronic respiratory disease, based on detailed assessment of their exercise capacity and symptoms.52 Pulmonary rehabilitation is as important as medication in COPD management, providing a cost-effective intervention with minimal adverse effects.53 Moreover, pulmonary rehabilitation has been shown to benefit patients with mild to severe dyspnea (as classified according to the Medical Research Council dyspnea scale), demonstrating the value of successful execution of these programs in patients with COPD, irrespective of disease severity.54 Although the most significant improvements in patient quality of life are observed when a multimodality approach is used, exercise and proper pulmonary rehabilitation programs have been shown to improve quality of life more than medication alone.5,55 Notably, there are few supporting data for the use of supplemental oxygen in patients experiencing dyspnea without hypoxemia. Oxygen supplementation is only of minimal benefit to relieving the sensation of dyspnea.56,57
The relationship between the impact of pulmonary rehabilitation in patients with COPD and frailty scores has also been evaluated. Frailty scores are calculated based on an individual’s level of physical activity, and other key criteria that are indicative of their ability to self-manage their medical condition.58 These scores are particularly relevant in the context of COPD, given the high prevalence of the condition in older people.58 Although frailty is a strong independent predictor of noncompletion of pulmonary rehabilitation, completion of a pulmonary rehabilitation program in patients who are frail has been shown to reverse their frailty in the short term.58 It is therefore important that physicians guide and encourage these patients for the duration of a pulmonary rehabilitation program, from initiation through to completion, to ensure that those who are likely to derive the greatest benefit from pulmonary rehabilitation are supported to do so.
In addition to pulmonary rehabilitation, other nonpharmacologic interventions have emerged in recent years that may help to relieve dyspnea in patients with COPD. Airway clearance devices, such as acapella (Smiths Medical; Minneapolis, MN), Flutter (Allergan; Dublin, Ireland), Lung Flute (Medical Acoustics; Buffalo, NY), Quake (Thayer Medical; Tucson, AZ), and Aerobika (Monaghan Medical; Plattsburgh, NY) promote the clearance of sputum through the application of positive expiratory pressure, possibly allowing medicines to penetrate the lungs more effectively, and improving diffuse airflow obstruction.59-61 Incorporating an airway clearance device into a bronchodilator therapy regimen has been shown to improve dyspnea scores, both before and after exercise, compared with bronchodilator therapy combined with a nonfunctional control device in patients with severe COPD.59 In addition, noninvasive forms of ventilation, such as continuous positive airway pressure and bi-level positive airway pressure (BiPAP), have been shown to effectively reduce dyspnea in patients with COPD.62,63 In a 24-month study in patients with severe COPD, resting dyspnea improved significantly in patients using the BiPAP Auto-Trak (Philips Respironics, Best, The Netherlands) in conjunction with their regular bronchodilator therapy, compared with those receiving long-term oxygen therapy in addition to their typical therapeutic regimen.63 Further studies are required to establish the impact of these devices in the management of dyspnea and other symptoms of COPD.
These nonpharmacologic interventions can be supplemented with pharmacologic treatments to help patients achieve their treatment goals of improved dyspnea and increased exercise performance. Bronchodilators, which form the basis of various COPD treatment options, include5:
- short-acting muscarinic antagonists (SAMAs), such as ipratropium
- short-acting β2-agonists (SABAs), such as albuterol, levalbuterol, and terbutaline
- SAMA/SABA combinations
- LAMAs, such as aclidinium, glycopyrrolate, tiotropium, and umeclidinium
- long-acting β 2-agonists (LABAs), such as arformoterol, indacaterol, formoterol, olodaterol, salmeterol, and vilanterol
- LAMA/LABA combinations (umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, glycopyrrolate/indacaterol)
Inhaled corticosteroids can also be used in a fixed-dose combination with a LABA, which can be combined with a LAMA, in select patients5; however, these combination products may have minimal value in treating dyspnea unless asthma is concomitantly present.5,64 Further discussion of the different treatment options available for patients with COPD can be found in the final article of this supplement.
In addition to improving quality of life, long-acting bronchodilators, such as LAMAs, LABAs, and LAMA/LABA combinations, increase expiratory flow, reduce dynamic hyperinflation, and improve exercise capacity of patients.65-67 As disease severity worsens, physicians may opt for long-acting bronchodilator options that have twice-daily dosing, which may confer a benefit in improving night-time symptom control.68
As well as active pharmacologic and nonpharmacologic interventions, physicians should always encourage smoking cessation in patients with COPD, as this has the greatest capacity to influence the natural course of the disease.5 It is essential that health care providers continually deliver smoking cessation messages to patients with COPD; patients can also be supported to stop smoking by using nicotine replacement therapy, pharmacologic interventions, attending smoking cessation programs, and counseling.5
Lung volume reduction surgery may also be considered as a strategy for the management of dyspnea in severe, refractory COPD.69 Similarly, nonsurgical bronchoscopic interventions are being developed that look to achieve similar results to lung volume reduction surgery, including endobronchial one-way valves, lung volume reduction coils, airway bypasses, adhesives, and vapor therapy.23
CASE STUDY:
The primary care physician assessed KD’s dyspnea using the CAT and ordered a chest X-ray to identify any significant comorbidities, such as concomitant respiratory, skeletal, or cardiac diseases. As KD’s CAT score was 17, and her symptoms were uncontrolled on LAMA monotherapy, her physician prescribed a long-acting LAMA/LABA combination, along with pulmonary rehabilitation. The physician also counseled KD on the importance of smoking cessation, and referred her to a local smoking cessation program.
Conclusions
Dyspnea, the most common symptom of COPD and the primary consequence of the condition’s characteristic lung hyperinflation, is a heavy burden on the lives of patients. The impact of dyspnea is perhaps most apparent in the context of physical activity, with activity limitation observed frequently in patients with COPD, regardless of disease stage. This can affect patients’ quality of life significantly, and has long-term consequences on disease progression. Improving dyspnea and increasing exercise endurance should therefore be a key goal for COPD management, which should encompass both nonpharmacologic interventions, such as pulmonary rehabilitation, and pharmacologic interventions, such as use of bronchodilator therapy.
- O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180-184.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272.
- Agusti A, Hedner J, Marin J, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://gold.copd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed November 27, 2017.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577-588.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-1394.
- Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131-137.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977.
- Haas F, Salazar-Schicci J, Axen K. Desensitization to dyspnoea in chronic obstructive pulmonary disease. In: Casaburi R, Petty TL, eds. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: W.B. Saunders; 1993:241-251.
- Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268-278.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187-201.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176-179.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
- Dubé BP, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1.
- Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385.
- Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259-264.
- Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101-111.
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003;40(5 Suppl 2):25-33.
- O’Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Pract. 2015;1:4.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(Suppl 4):S407-S415.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
- O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-549.
- Light RW. Mechanics of respiration. In: George RB, ed. Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:24-38.
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591-597.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753-762.
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181-188.
- Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506-512.
- Di Marco F, Santus P, Sotgiu G, Blasi F, Centanni S. Does improving exercise capacity and daily activity represent the holistic perspective of a new COPD approach? COPD. 2015;12(5):575-581.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042-1050.
- Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745-752.
- Burkhardt R, Pankow W. The diagnosis of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2014;111(49):834-845, quiz 846.
- O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108-1115.
- Criée CP, Sorichter S, Smith HJ, et al; Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959-971.
- Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir Med. 2017;12:3.
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834-840.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005-1011.
- Calverley PMA, Georgopoulos D. Symptoms and signs of COPD. In: Siafakas NM, ed. Management of Chronic Obstructive Pulmonary Disease: European Respiratory Society Journals; 2006.
- Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447-456.
- Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD—revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725-738.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382-386.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225.
- Grant A, Moore L. Pulmonary rehabilitation. In: Blackler L, Jones C, Mooney C, eds. Managing Chronic Obstructive Pulmonary Disease. West Sussex, England: John Wiley & Sons; 2007.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042-1048.
- Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
- Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103(7):1070-1075.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179-187.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429.
- Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988-995.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA Jr. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest. 2002;121(3):702-707.
- Chatburn RL. High-frequency assisted airway clearance. Respir Care. 2007;52(9):1224-1235; discussion 1235-1227.
- Clini E. Positive expiratory pressure techniques in respiratory patients: old evidence and new insights. Breathe. 2009;6(2):153-159.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(2):281-289.
- Clini E, Sturani C, Rossi A, et al; Rehabilitation and Chronic Care Study Group; Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529-538.
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(6):477-482.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288-1296.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
- O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients [published correction appears in Respir Res. 2017;18(1):35]. Respir Res. 2017;18(1):19.
- Shah AA, D’Amico TA. Lung volume reduction surgery for the management of refractory dyspnea in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3(2):107-111.
Introduction
Dyspnea, the sensation of difficult or labored breathing, is the most common symptom in chronic obstructive pulmonary disease (COPD) and the primary symptom that limits physical activity in more advanced disease.1 According to the American Thoracic Society, dyspnea may be measured according to 3 domains2:
- what breathing feels like for the patient
- how distressed the patient feels when breathing
- how dyspnea affects functional ability, employment, health-related quality of life, or health status.
As disease severity increases, breathlessness becomes more disabling at lower activity levels. These changes further impact the quality of life of patients, and can lead to anxiety and depression.11
Physical inactivity is often considered to be a major contributor to the progression of COPD,6 and is linked to hospitalizations and increased all-cause mortality.12 There is therefore a need to recognize symptoms early and treat them accordingly.
CASE STUDY:
KD, a 64-year-old woman, presented to her primary care physician’s office for a routine visit. Upon assessment, KD revealed that she used to enjoy going on walks with her neighbor, but she cannot walk up the hills in her neighborhood anymore without feeling “incredibly breathless.” She has become increasingly concerned that she is “having trouble getting a full breath.” KD informed her doctor that these symptoms had worsened since her last visit, and so she had stopped going on neighborhood walks. She was diagnosed with COPD 4 years ago, and is currently using a long-acting muscarinic antagonist (LAMA) bronchodilator. KD has a 40 pack-year smoking history, and has previously been advised to stop smoking, but has relapsed several times. She has a medical history of hypertension and depression, and a notable family history of emphysema, breast cancer, and diabetes.
The relationship between lung hyperinflation and dyspnea in COPD
In COPD, pathologic changes give rise to physiologic abnormalities such as mucus hypersecretion and ciliary dysfunction, gas exchange abnormalities, pulmonary hypertension, and airflow limitation and lung hyperinflation.13 Lung hyperinflation, an increase in resting functional residual volume above a normal level, represents a mechanical link between the characteristic expiratory airflow impairment, dyspnea, and physical activity limitation in COPD.1
Although patients can compensate for several of the negative consequences of hyperinflation (eg, altering the chest wall due to overdistended lungs), such compensatory mechanisms are unable to cope with large increases in ventilation, such as those that occur during exercise.1 Air trapping, together with ineffectiveness of respiratory muscle function, leads to increased ventilation requirements and dynamic pulmonary hyperinflation, resulting in dyspnea.1
Patients with COPD describe a sensation of “air hunger,” reporting “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” and a feeling that they “cannot get a deep breath,”18 whereas, in fact, they are limited in their ability to fully exhale. Verbal descriptors (eg, “air hunger” or “chest tightness”) are important tools in understanding a patient’s experience with dyspnea, and a patient’s choice of descriptor may be related to dyspnea severity, and the level of distress that dyspnea causes a given patient.19 Air hunger in turn encourages faster breathing, leading to further shortness of breath and more dynamic hyperinflation.1,20
To deflate the lungs of patients with COPD, physiologic, pharmacologic, and possibly surgical interventions are required:
- Controlled breathing techniques (eg, purse-lipped breathing) that encourage slow and deep breathing can correct abnormal chest wall motion, decrease the work of breathing, increase breathing efficiency, and improve the distribution of ventilation to empty the lungs.21
- Bronchodilators can help to achieve lung deflation by improving ventilatory mechanics, as shown by increases in inspiratory capacity and vital capacity.22
- Lung volume reduction surgery can also be considered to treat severe hyperinflation in emphysematous patients5; bronchoscopic interventions that lower lung volumes are also in development.23
The impact of lung hyperinflation and dyspnea on physical activity in COPD
Dyspnea and hyperinflation are closely interrelated with physical activity limitation,16,29,30 and so can be viewed as significant contributors to patient disability. During an acute exacerbation, patients with COPD will experience worsening airway obstruction, dynamic hyperinflation, and dyspnea.31 Patients with a greater number of comorbid conditions may also have greater shortness of breath.32 In addition, patients with COPD and hyperinflation perform less physical activity than individuals without hyperinflation, regardless of COPD severity, as assessed using the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging (stage I, mild; stage II, moderate; stage III, severe; stage IV, very severe) and BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) index.33 These patients also exhibit increases in dyspnea perception during commonly performed ADLs, which may limit physical activity and worsen lung hyperinflation.33 More limited physical activity also contributes to higher dyspnea scores during ADLs.8
Furthermore, the ability to perform typical ADLs may be significantly altered or eliminated altogether in patients with COPD.11 Leisure activities are often the first to be dropped by patients, as they generally require greater effort than simpler tasks, and are not critical to daily life.11 Eventually, these activities become progressively more difficult, and most patients with moderate or severe COPD can struggle to complete even the most basic daily activities.11
In addition to the morbidity burden and impact on ADLs, lower levels of physical activity in patients with COPD have also been shown to increase the risk of mortality and exacerbations, and elevate the risk of comorbidities such as heart disease and metabolic disease.34 In light of these observations, improving exercise capacity should be a key goal in COPD management.
Assessment and measurement of dyspnea and hyperinflation
Reducing hyperinflation and dyspnea is essential for improving physical activity endurance and overall physical activity in patients with COPD; therefore, measuring the degree of impairment is important.22 Clinicians should be aware that some patients may have relief of dyspnea due to improvements in hyperinflation, despite relatively mild changes in FEV1.35 Lung volume measures, including total lung capacity, residual volume and functional residual capacity, are valuable tools in the assessment of lung hyperinflation in COPD, and therefore constitute a key component of pulmonary function testing.36 However, expanded pulmonary function testing may be required for patients with severe dyspnea that does not correspond to spirometric findings, or cases in which diagnosis is uncertain.37
Lung volumes are evaluated primarily by body plethysmography, during which a patient sits inside an airtight “body box” equipped to measure pressure and volume changes.14,38 Helium dilution and nitrogen washing can also be used to measure functional residual capacity in patients with COPD,14 but body plethysmography is considered to be a more accurate method of lung volume evaluation in patients with severe airflow obstruction.14,38 Radiographic techniques can also be used, but due to a lack of standardization, they are not typically utilized in clinical practice.14 Measurement of IC may complement other lung volume measures as part of assessment of hyperinflation.16 This can be measured using either spirometry or body plethysmography.39,40
In addition to evaluating hyperinflation, ADLs, physical activity, exercise capacity, and dyspnea should all be assessed in patients with COPD in primary care. It is known that patients may self-limit ADLs to avoid symptoms of COPD; in doing so, worsening symptoms may be underappreciated, and subsequently underreported, by the patient. Thus, it is essential that physicians ask patients with COPD, as well as individuals at risk of COPD, questions about changes in their physical activity or ability to perform common tasks. There are a number of methods to measure functional performance, but for a simple assessment of ADLs, clinicians can ask the patient or caregiver questions related to basic daily tasks.11 In early COPD, patients who experience mild dyspnea during exercise should be able to perform most productive activities. Patients with stable COPD and moderate dyspnea during exercise should be able to carry out most of the higher functioning ADLs, whereas patients with severe COPD may struggle to complete basic ADLs without assistance.11 It should be noted, however, that patients may experience dyspnea with fairly routine activities, and even reduce physical activity at relatively early stages of airflow limitation.41,42
Other tests may be useful in assessing the impact of an intervention, be it pharmacologic or nonpharmacologic, on dyspnea severity. For example, increases in the 6-minute-walk distance (6MWD) have been shown to correlate with improvements in dyspnea.46 The 6MWD has also been shown to be an important predictor of hospitalization and mortality in patients with COPD.47 However, it is important to note that improvements in 6MWD show only a very weak correlation with patient-reported outcomes,48 and may be a less sensitive measure for patients with less disability than those with more profound functional limitation.49 Moreover, 6MWD can be affected by a patient’s psychologic motivation,6,50 as well as other comorbidities observed in patients with COPD, such as osteoporosis, heart failure, and peripheral vascular disease.46,51 Although not used for COPD diagnosis or evaluation of dyspnea or physical activity limitation, a chest X-ray can also be a useful tool for excluding alternative diagnoses, as well as for detecting significant comorbidities in patients with COPD, such as concomitant respiratory, cardiac, and skeletal diseases.5
Management of dyspnea and hyperinflation in primary care
Pulmonary rehabilitation is a tailored intervention that encompasses exercise training, education, and self-management support for people with chronic respiratory disease, based on detailed assessment of their exercise capacity and symptoms.52 Pulmonary rehabilitation is as important as medication in COPD management, providing a cost-effective intervention with minimal adverse effects.53 Moreover, pulmonary rehabilitation has been shown to benefit patients with mild to severe dyspnea (as classified according to the Medical Research Council dyspnea scale), demonstrating the value of successful execution of these programs in patients with COPD, irrespective of disease severity.54 Although the most significant improvements in patient quality of life are observed when a multimodality approach is used, exercise and proper pulmonary rehabilitation programs have been shown to improve quality of life more than medication alone.5,55 Notably, there are few supporting data for the use of supplemental oxygen in patients experiencing dyspnea without hypoxemia. Oxygen supplementation is only of minimal benefit to relieving the sensation of dyspnea.56,57
The relationship between the impact of pulmonary rehabilitation in patients with COPD and frailty scores has also been evaluated. Frailty scores are calculated based on an individual’s level of physical activity, and other key criteria that are indicative of their ability to self-manage their medical condition.58 These scores are particularly relevant in the context of COPD, given the high prevalence of the condition in older people.58 Although frailty is a strong independent predictor of noncompletion of pulmonary rehabilitation, completion of a pulmonary rehabilitation program in patients who are frail has been shown to reverse their frailty in the short term.58 It is therefore important that physicians guide and encourage these patients for the duration of a pulmonary rehabilitation program, from initiation through to completion, to ensure that those who are likely to derive the greatest benefit from pulmonary rehabilitation are supported to do so.
In addition to pulmonary rehabilitation, other nonpharmacologic interventions have emerged in recent years that may help to relieve dyspnea in patients with COPD. Airway clearance devices, such as acapella (Smiths Medical; Minneapolis, MN), Flutter (Allergan; Dublin, Ireland), Lung Flute (Medical Acoustics; Buffalo, NY), Quake (Thayer Medical; Tucson, AZ), and Aerobika (Monaghan Medical; Plattsburgh, NY) promote the clearance of sputum through the application of positive expiratory pressure, possibly allowing medicines to penetrate the lungs more effectively, and improving diffuse airflow obstruction.59-61 Incorporating an airway clearance device into a bronchodilator therapy regimen has been shown to improve dyspnea scores, both before and after exercise, compared with bronchodilator therapy combined with a nonfunctional control device in patients with severe COPD.59 In addition, noninvasive forms of ventilation, such as continuous positive airway pressure and bi-level positive airway pressure (BiPAP), have been shown to effectively reduce dyspnea in patients with COPD.62,63 In a 24-month study in patients with severe COPD, resting dyspnea improved significantly in patients using the BiPAP Auto-Trak (Philips Respironics, Best, The Netherlands) in conjunction with their regular bronchodilator therapy, compared with those receiving long-term oxygen therapy in addition to their typical therapeutic regimen.63 Further studies are required to establish the impact of these devices in the management of dyspnea and other symptoms of COPD.
These nonpharmacologic interventions can be supplemented with pharmacologic treatments to help patients achieve their treatment goals of improved dyspnea and increased exercise performance. Bronchodilators, which form the basis of various COPD treatment options, include5:
- short-acting muscarinic antagonists (SAMAs), such as ipratropium
- short-acting β2-agonists (SABAs), such as albuterol, levalbuterol, and terbutaline
- SAMA/SABA combinations
- LAMAs, such as aclidinium, glycopyrrolate, tiotropium, and umeclidinium
- long-acting β 2-agonists (LABAs), such as arformoterol, indacaterol, formoterol, olodaterol, salmeterol, and vilanterol
- LAMA/LABA combinations (umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, glycopyrrolate/indacaterol)
Inhaled corticosteroids can also be used in a fixed-dose combination with a LABA, which can be combined with a LAMA, in select patients5; however, these combination products may have minimal value in treating dyspnea unless asthma is concomitantly present.5,64 Further discussion of the different treatment options available for patients with COPD can be found in the final article of this supplement.
In addition to improving quality of life, long-acting bronchodilators, such as LAMAs, LABAs, and LAMA/LABA combinations, increase expiratory flow, reduce dynamic hyperinflation, and improve exercise capacity of patients.65-67 As disease severity worsens, physicians may opt for long-acting bronchodilator options that have twice-daily dosing, which may confer a benefit in improving night-time symptom control.68
As well as active pharmacologic and nonpharmacologic interventions, physicians should always encourage smoking cessation in patients with COPD, as this has the greatest capacity to influence the natural course of the disease.5 It is essential that health care providers continually deliver smoking cessation messages to patients with COPD; patients can also be supported to stop smoking by using nicotine replacement therapy, pharmacologic interventions, attending smoking cessation programs, and counseling.5
Lung volume reduction surgery may also be considered as a strategy for the management of dyspnea in severe, refractory COPD.69 Similarly, nonsurgical bronchoscopic interventions are being developed that look to achieve similar results to lung volume reduction surgery, including endobronchial one-way valves, lung volume reduction coils, airway bypasses, adhesives, and vapor therapy.23
CASE STUDY:
The primary care physician assessed KD’s dyspnea using the CAT and ordered a chest X-ray to identify any significant comorbidities, such as concomitant respiratory, skeletal, or cardiac diseases. As KD’s CAT score was 17, and her symptoms were uncontrolled on LAMA monotherapy, her physician prescribed a long-acting LAMA/LABA combination, along with pulmonary rehabilitation. The physician also counseled KD on the importance of smoking cessation, and referred her to a local smoking cessation program.
Conclusions
Dyspnea, the most common symptom of COPD and the primary consequence of the condition’s characteristic lung hyperinflation, is a heavy burden on the lives of patients. The impact of dyspnea is perhaps most apparent in the context of physical activity, with activity limitation observed frequently in patients with COPD, regardless of disease stage. This can affect patients’ quality of life significantly, and has long-term consequences on disease progression. Improving dyspnea and increasing exercise endurance should therefore be a key goal for COPD management, which should encompass both nonpharmacologic interventions, such as pulmonary rehabilitation, and pharmacologic interventions, such as use of bronchodilator therapy.
Introduction
Dyspnea, the sensation of difficult or labored breathing, is the most common symptom in chronic obstructive pulmonary disease (COPD) and the primary symptom that limits physical activity in more advanced disease.1 According to the American Thoracic Society, dyspnea may be measured according to 3 domains2:
- what breathing feels like for the patient
- how distressed the patient feels when breathing
- how dyspnea affects functional ability, employment, health-related quality of life, or health status.
As disease severity increases, breathlessness becomes more disabling at lower activity levels. These changes further impact the quality of life of patients, and can lead to anxiety and depression.11
Physical inactivity is often considered to be a major contributor to the progression of COPD,6 and is linked to hospitalizations and increased all-cause mortality.12 There is therefore a need to recognize symptoms early and treat them accordingly.
CASE STUDY:
KD, a 64-year-old woman, presented to her primary care physician’s office for a routine visit. Upon assessment, KD revealed that she used to enjoy going on walks with her neighbor, but she cannot walk up the hills in her neighborhood anymore without feeling “incredibly breathless.” She has become increasingly concerned that she is “having trouble getting a full breath.” KD informed her doctor that these symptoms had worsened since her last visit, and so she had stopped going on neighborhood walks. She was diagnosed with COPD 4 years ago, and is currently using a long-acting muscarinic antagonist (LAMA) bronchodilator. KD has a 40 pack-year smoking history, and has previously been advised to stop smoking, but has relapsed several times. She has a medical history of hypertension and depression, and a notable family history of emphysema, breast cancer, and diabetes.
The relationship between lung hyperinflation and dyspnea in COPD
In COPD, pathologic changes give rise to physiologic abnormalities such as mucus hypersecretion and ciliary dysfunction, gas exchange abnormalities, pulmonary hypertension, and airflow limitation and lung hyperinflation.13 Lung hyperinflation, an increase in resting functional residual volume above a normal level, represents a mechanical link between the characteristic expiratory airflow impairment, dyspnea, and physical activity limitation in COPD.1
Although patients can compensate for several of the negative consequences of hyperinflation (eg, altering the chest wall due to overdistended lungs), such compensatory mechanisms are unable to cope with large increases in ventilation, such as those that occur during exercise.1 Air trapping, together with ineffectiveness of respiratory muscle function, leads to increased ventilation requirements and dynamic pulmonary hyperinflation, resulting in dyspnea.1
Patients with COPD describe a sensation of “air hunger,” reporting “unsatisfied” or “unrewarded” inhalation, “shallow breathing,” and a feeling that they “cannot get a deep breath,”18 whereas, in fact, they are limited in their ability to fully exhale. Verbal descriptors (eg, “air hunger” or “chest tightness”) are important tools in understanding a patient’s experience with dyspnea, and a patient’s choice of descriptor may be related to dyspnea severity, and the level of distress that dyspnea causes a given patient.19 Air hunger in turn encourages faster breathing, leading to further shortness of breath and more dynamic hyperinflation.1,20
To deflate the lungs of patients with COPD, physiologic, pharmacologic, and possibly surgical interventions are required:
- Controlled breathing techniques (eg, purse-lipped breathing) that encourage slow and deep breathing can correct abnormal chest wall motion, decrease the work of breathing, increase breathing efficiency, and improve the distribution of ventilation to empty the lungs.21
- Bronchodilators can help to achieve lung deflation by improving ventilatory mechanics, as shown by increases in inspiratory capacity and vital capacity.22
- Lung volume reduction surgery can also be considered to treat severe hyperinflation in emphysematous patients5; bronchoscopic interventions that lower lung volumes are also in development.23
The impact of lung hyperinflation and dyspnea on physical activity in COPD
Dyspnea and hyperinflation are closely interrelated with physical activity limitation,16,29,30 and so can be viewed as significant contributors to patient disability. During an acute exacerbation, patients with COPD will experience worsening airway obstruction, dynamic hyperinflation, and dyspnea.31 Patients with a greater number of comorbid conditions may also have greater shortness of breath.32 In addition, patients with COPD and hyperinflation perform less physical activity than individuals without hyperinflation, regardless of COPD severity, as assessed using the 2007 Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging (stage I, mild; stage II, moderate; stage III, severe; stage IV, very severe) and BODE (Body-mass index, airflow Obstruction, Dyspnea, and Exercise) index.33 These patients also exhibit increases in dyspnea perception during commonly performed ADLs, which may limit physical activity and worsen lung hyperinflation.33 More limited physical activity also contributes to higher dyspnea scores during ADLs.8
Furthermore, the ability to perform typical ADLs may be significantly altered or eliminated altogether in patients with COPD.11 Leisure activities are often the first to be dropped by patients, as they generally require greater effort than simpler tasks, and are not critical to daily life.11 Eventually, these activities become progressively more difficult, and most patients with moderate or severe COPD can struggle to complete even the most basic daily activities.11
In addition to the morbidity burden and impact on ADLs, lower levels of physical activity in patients with COPD have also been shown to increase the risk of mortality and exacerbations, and elevate the risk of comorbidities such as heart disease and metabolic disease.34 In light of these observations, improving exercise capacity should be a key goal in COPD management.
Assessment and measurement of dyspnea and hyperinflation
Reducing hyperinflation and dyspnea is essential for improving physical activity endurance and overall physical activity in patients with COPD; therefore, measuring the degree of impairment is important.22 Clinicians should be aware that some patients may have relief of dyspnea due to improvements in hyperinflation, despite relatively mild changes in FEV1.35 Lung volume measures, including total lung capacity, residual volume and functional residual capacity, are valuable tools in the assessment of lung hyperinflation in COPD, and therefore constitute a key component of pulmonary function testing.36 However, expanded pulmonary function testing may be required for patients with severe dyspnea that does not correspond to spirometric findings, or cases in which diagnosis is uncertain.37
Lung volumes are evaluated primarily by body plethysmography, during which a patient sits inside an airtight “body box” equipped to measure pressure and volume changes.14,38 Helium dilution and nitrogen washing can also be used to measure functional residual capacity in patients with COPD,14 but body plethysmography is considered to be a more accurate method of lung volume evaluation in patients with severe airflow obstruction.14,38 Radiographic techniques can also be used, but due to a lack of standardization, they are not typically utilized in clinical practice.14 Measurement of IC may complement other lung volume measures as part of assessment of hyperinflation.16 This can be measured using either spirometry or body plethysmography.39,40
In addition to evaluating hyperinflation, ADLs, physical activity, exercise capacity, and dyspnea should all be assessed in patients with COPD in primary care. It is known that patients may self-limit ADLs to avoid symptoms of COPD; in doing so, worsening symptoms may be underappreciated, and subsequently underreported, by the patient. Thus, it is essential that physicians ask patients with COPD, as well as individuals at risk of COPD, questions about changes in their physical activity or ability to perform common tasks. There are a number of methods to measure functional performance, but for a simple assessment of ADLs, clinicians can ask the patient or caregiver questions related to basic daily tasks.11 In early COPD, patients who experience mild dyspnea during exercise should be able to perform most productive activities. Patients with stable COPD and moderate dyspnea during exercise should be able to carry out most of the higher functioning ADLs, whereas patients with severe COPD may struggle to complete basic ADLs without assistance.11 It should be noted, however, that patients may experience dyspnea with fairly routine activities, and even reduce physical activity at relatively early stages of airflow limitation.41,42
Other tests may be useful in assessing the impact of an intervention, be it pharmacologic or nonpharmacologic, on dyspnea severity. For example, increases in the 6-minute-walk distance (6MWD) have been shown to correlate with improvements in dyspnea.46 The 6MWD has also been shown to be an important predictor of hospitalization and mortality in patients with COPD.47 However, it is important to note that improvements in 6MWD show only a very weak correlation with patient-reported outcomes,48 and may be a less sensitive measure for patients with less disability than those with more profound functional limitation.49 Moreover, 6MWD can be affected by a patient’s psychologic motivation,6,50 as well as other comorbidities observed in patients with COPD, such as osteoporosis, heart failure, and peripheral vascular disease.46,51 Although not used for COPD diagnosis or evaluation of dyspnea or physical activity limitation, a chest X-ray can also be a useful tool for excluding alternative diagnoses, as well as for detecting significant comorbidities in patients with COPD, such as concomitant respiratory, cardiac, and skeletal diseases.5
Management of dyspnea and hyperinflation in primary care
Pulmonary rehabilitation is a tailored intervention that encompasses exercise training, education, and self-management support for people with chronic respiratory disease, based on detailed assessment of their exercise capacity and symptoms.52 Pulmonary rehabilitation is as important as medication in COPD management, providing a cost-effective intervention with minimal adverse effects.53 Moreover, pulmonary rehabilitation has been shown to benefit patients with mild to severe dyspnea (as classified according to the Medical Research Council dyspnea scale), demonstrating the value of successful execution of these programs in patients with COPD, irrespective of disease severity.54 Although the most significant improvements in patient quality of life are observed when a multimodality approach is used, exercise and proper pulmonary rehabilitation programs have been shown to improve quality of life more than medication alone.5,55 Notably, there are few supporting data for the use of supplemental oxygen in patients experiencing dyspnea without hypoxemia. Oxygen supplementation is only of minimal benefit to relieving the sensation of dyspnea.56,57
The relationship between the impact of pulmonary rehabilitation in patients with COPD and frailty scores has also been evaluated. Frailty scores are calculated based on an individual’s level of physical activity, and other key criteria that are indicative of their ability to self-manage their medical condition.58 These scores are particularly relevant in the context of COPD, given the high prevalence of the condition in older people.58 Although frailty is a strong independent predictor of noncompletion of pulmonary rehabilitation, completion of a pulmonary rehabilitation program in patients who are frail has been shown to reverse their frailty in the short term.58 It is therefore important that physicians guide and encourage these patients for the duration of a pulmonary rehabilitation program, from initiation through to completion, to ensure that those who are likely to derive the greatest benefit from pulmonary rehabilitation are supported to do so.
In addition to pulmonary rehabilitation, other nonpharmacologic interventions have emerged in recent years that may help to relieve dyspnea in patients with COPD. Airway clearance devices, such as acapella (Smiths Medical; Minneapolis, MN), Flutter (Allergan; Dublin, Ireland), Lung Flute (Medical Acoustics; Buffalo, NY), Quake (Thayer Medical; Tucson, AZ), and Aerobika (Monaghan Medical; Plattsburgh, NY) promote the clearance of sputum through the application of positive expiratory pressure, possibly allowing medicines to penetrate the lungs more effectively, and improving diffuse airflow obstruction.59-61 Incorporating an airway clearance device into a bronchodilator therapy regimen has been shown to improve dyspnea scores, both before and after exercise, compared with bronchodilator therapy combined with a nonfunctional control device in patients with severe COPD.59 In addition, noninvasive forms of ventilation, such as continuous positive airway pressure and bi-level positive airway pressure (BiPAP), have been shown to effectively reduce dyspnea in patients with COPD.62,63 In a 24-month study in patients with severe COPD, resting dyspnea improved significantly in patients using the BiPAP Auto-Trak (Philips Respironics, Best, The Netherlands) in conjunction with their regular bronchodilator therapy, compared with those receiving long-term oxygen therapy in addition to their typical therapeutic regimen.63 Further studies are required to establish the impact of these devices in the management of dyspnea and other symptoms of COPD.
These nonpharmacologic interventions can be supplemented with pharmacologic treatments to help patients achieve their treatment goals of improved dyspnea and increased exercise performance. Bronchodilators, which form the basis of various COPD treatment options, include5:
- short-acting muscarinic antagonists (SAMAs), such as ipratropium
- short-acting β2-agonists (SABAs), such as albuterol, levalbuterol, and terbutaline
- SAMA/SABA combinations
- LAMAs, such as aclidinium, glycopyrrolate, tiotropium, and umeclidinium
- long-acting β 2-agonists (LABAs), such as arformoterol, indacaterol, formoterol, olodaterol, salmeterol, and vilanterol
- LAMA/LABA combinations (umeclidinium/vilanterol, tiotropium/olodaterol, glycopyrrolate/formoterol, glycopyrrolate/indacaterol)
Inhaled corticosteroids can also be used in a fixed-dose combination with a LABA, which can be combined with a LAMA, in select patients5; however, these combination products may have minimal value in treating dyspnea unless asthma is concomitantly present.5,64 Further discussion of the different treatment options available for patients with COPD can be found in the final article of this supplement.
In addition to improving quality of life, long-acting bronchodilators, such as LAMAs, LABAs, and LAMA/LABA combinations, increase expiratory flow, reduce dynamic hyperinflation, and improve exercise capacity of patients.65-67 As disease severity worsens, physicians may opt for long-acting bronchodilator options that have twice-daily dosing, which may confer a benefit in improving night-time symptom control.68
As well as active pharmacologic and nonpharmacologic interventions, physicians should always encourage smoking cessation in patients with COPD, as this has the greatest capacity to influence the natural course of the disease.5 It is essential that health care providers continually deliver smoking cessation messages to patients with COPD; patients can also be supported to stop smoking by using nicotine replacement therapy, pharmacologic interventions, attending smoking cessation programs, and counseling.5
Lung volume reduction surgery may also be considered as a strategy for the management of dyspnea in severe, refractory COPD.69 Similarly, nonsurgical bronchoscopic interventions are being developed that look to achieve similar results to lung volume reduction surgery, including endobronchial one-way valves, lung volume reduction coils, airway bypasses, adhesives, and vapor therapy.23
CASE STUDY:
The primary care physician assessed KD’s dyspnea using the CAT and ordered a chest X-ray to identify any significant comorbidities, such as concomitant respiratory, skeletal, or cardiac diseases. As KD’s CAT score was 17, and her symptoms were uncontrolled on LAMA monotherapy, her physician prescribed a long-acting LAMA/LABA combination, along with pulmonary rehabilitation. The physician also counseled KD on the importance of smoking cessation, and referred her to a local smoking cessation program.
Conclusions
Dyspnea, the most common symptom of COPD and the primary consequence of the condition’s characteristic lung hyperinflation, is a heavy burden on the lives of patients. The impact of dyspnea is perhaps most apparent in the context of physical activity, with activity limitation observed frequently in patients with COPD, regardless of disease stage. This can affect patients’ quality of life significantly, and has long-term consequences on disease progression. Improving dyspnea and increasing exercise endurance should therefore be a key goal for COPD management, which should encompass both nonpharmacologic interventions, such as pulmonary rehabilitation, and pharmacologic interventions, such as use of bronchodilator therapy.
- O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180-184.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272.
- Agusti A, Hedner J, Marin J, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://gold.copd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed November 27, 2017.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577-588.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-1394.
- Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131-137.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977.
- Haas F, Salazar-Schicci J, Axen K. Desensitization to dyspnoea in chronic obstructive pulmonary disease. In: Casaburi R, Petty TL, eds. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: W.B. Saunders; 1993:241-251.
- Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268-278.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187-201.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176-179.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
- Dubé BP, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1.
- Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385.
- Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259-264.
- Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101-111.
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003;40(5 Suppl 2):25-33.
- O’Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Pract. 2015;1:4.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(Suppl 4):S407-S415.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
- O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-549.
- Light RW. Mechanics of respiration. In: George RB, ed. Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:24-38.
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591-597.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753-762.
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181-188.
- Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506-512.
- Di Marco F, Santus P, Sotgiu G, Blasi F, Centanni S. Does improving exercise capacity and daily activity represent the holistic perspective of a new COPD approach? COPD. 2015;12(5):575-581.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042-1050.
- Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745-752.
- Burkhardt R, Pankow W. The diagnosis of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2014;111(49):834-845, quiz 846.
- O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108-1115.
- Criée CP, Sorichter S, Smith HJ, et al; Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959-971.
- Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir Med. 2017;12:3.
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834-840.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005-1011.
- Calverley PMA, Georgopoulos D. Symptoms and signs of COPD. In: Siafakas NM, ed. Management of Chronic Obstructive Pulmonary Disease: European Respiratory Society Journals; 2006.
- Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447-456.
- Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD—revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725-738.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382-386.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225.
- Grant A, Moore L. Pulmonary rehabilitation. In: Blackler L, Jones C, Mooney C, eds. Managing Chronic Obstructive Pulmonary Disease. West Sussex, England: John Wiley & Sons; 2007.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042-1048.
- Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
- Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103(7):1070-1075.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179-187.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429.
- Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988-995.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA Jr. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest. 2002;121(3):702-707.
- Chatburn RL. High-frequency assisted airway clearance. Respir Care. 2007;52(9):1224-1235; discussion 1235-1227.
- Clini E. Positive expiratory pressure techniques in respiratory patients: old evidence and new insights. Breathe. 2009;6(2):153-159.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(2):281-289.
- Clini E, Sturani C, Rossi A, et al; Rehabilitation and Chronic Care Study Group; Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529-538.
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(6):477-482.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288-1296.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
- O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients [published correction appears in Respir Res. 2017;18(1):35]. Respir Res. 2017;18(1):19.
- Shah AA, D’Amico TA. Lung volume reduction surgery for the management of refractory dyspnea in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3(2):107-111.
- O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180-184.
- Parshall MB, Schwartzstein RM, Adams L, et al; American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
- Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264-272.
- Agusti A, Hedner J, Marin J, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017. http://gold.copd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed November 27, 2017.
- O’Donnell DE, Gebke KB. Activity restriction in mild COPD: a challenging clinical problem. Int J Chron Obstruct Pulmon Dis. 2014;9:577-588.
- Elbehairy AF, Ciavaglia CE, Webb KA, et al; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-1394.
- Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131-137.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-977.
- Haas F, Salazar-Schicci J, Axen K. Desensitization to dyspnoea in chronic obstructive pulmonary disease. In: Casaburi R, Petty TL, eds. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: W.B. Saunders; 1993:241-251.
- Belfer MH, Reardon JZ. Improving exercise tolerance and quality of life in patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2009;109(5):268-278.
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115.
- Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-946.
- Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187-201.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176-179.
- O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
- Dubé BP, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1.
- Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385.
- Chowienczyk S, Javadzadeh S, Booth S, Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259-264.
- Thomas M, Decramer M, O’Donnell DE. No room to breathe: the importance of lung hyperinflation in COPD. Prim Care Respir J. 2013;22(1):101-111.
- Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD). J Rehabil Res Dev. 2003;40(5 Suppl 2):25-33.
- O’Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Pract. 2015;1:4.
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis. 2014;6(Suppl 4):S407-S415.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434-1440.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
- O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-549.
- Light RW. Mechanics of respiration. In: George RB, ed. Chest Medicine: Essentials of Pulmonary and Critical Care Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:24-38.
- Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591-597.
- O’Donnell DE, Guenette JA, Maltais F, Webb KA. Decline of resting inspiratory capacity in COPD: the impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest. 2012;141(3):753-762.
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4(3):225-236.
- Holland AE. Physiotherapy management of acute exacerbations of chronic obstructive pulmonary disease. J Physiother. 2014;60(4):181-188.
- Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217-227.
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180(6):506-512.
- Di Marco F, Santus P, Sotgiu G, Blasi F, Centanni S. Does improving exercise capacity and daily activity represent the holistic perspective of a new COPD approach? COPD. 2015;12(5):575-581.
- Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042-1050.
- Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am. 2012;96(4):745-752.
- Burkhardt R, Pankow W. The diagnosis of chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2014;111(49):834-845, quiz 846.
- O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137(5):1108-1115.
- Criée CP, Sorichter S, Smith HJ, et al; Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography—its principles and clinical use. Respir Med. 2011;105(7):959-971.
- Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir Med. 2017;12:3.
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Vercoulen JH, Heijdra YF. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med. 2013;107(6):834-840.
- Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005-1011.
- Calverley PMA, Georgopoulos D. Symptoms and signs of COPD. In: Siafakas NM, ed. Management of Chronic Obstructive Pulmonary Disease: European Respiratory Society Journals; 2006.
- Cave AJ, Atkinson L, Tsiligianni IG, Kaplan AG. Assessment of COPD wellness tools for use in primary care: an IPCRG initiative. Int J Chron Obstruct Pulmon Dis. 2012;7:447-456.
- Cazzola M, Hanania NA, MacNee W, Rüdell K, Hackford C, Tamimi N. A review of the most common patient-reported outcomes in COPD—revisiting current knowledge and estimating future challenges. Int J Chron Obstruct Pulmon Dis. 2015;10:725-738.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117.
- Polkey MI, Spruit MA, Edwards LD, et al; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Study Investigators. Six-minute-walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382-386.
- Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32(3):637-643.
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221-225.
- Grant A, Moore L. Pulmonary rehabilitation. In: Blackler L, Jones C, Mooney C, eds. Managing Chronic Obstructive Pulmonary Disease. West Sussex, England: John Wiley & Sons; 2007.
- Crisafulli E, Gorgone P, Vagaggini B, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36(5):1042-1048.
- Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5 Suppl):4S-42S.
- Evans RA, Singh SJ, Collier R, Williams JE, Morgan MD. Pulmonary rehabilitation is successful for COPD irrespective of MRC dyspnoea grade. Respir Med. 2009;103(7):1070-1075.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B; Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179-187.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;11:CD006429.
- Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988-995.
- Wolkove N, Kamel H, Rotaple M, Baltzan MA Jr. Use of a mucus clearance device enhances the bronchodilator response in patients with stable COPD. Chest. 2002;121(3):702-707.
- Chatburn RL. High-frequency assisted airway clearance. Respir Care. 2007;52(9):1224-1235; discussion 1235-1227.
- Clini E. Positive expiratory pressure techniques in respiratory patients: old evidence and new insights. Breathe. 2009;6(2):153-159.
- Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(2):281-289.
- Clini E, Sturani C, Rossi A, et al; Rehabilitation and Chronic Care Study Group; Italian Association of Hospital Pulmonologists (AIPO). The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529-538.
- Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53(6):477-482.
- Berton DC, Reis M, Siqueira AC, et al. Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir Med. 2010;104(9):1288-1296.
- O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
- O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-656.
- Blasi F, Canonica GW, Miravitlles M. Is aclidinium alone or combined with a LABA a rational choice for symptomatic COPD patients [published correction appears in Respir Res. 2017;18(1):35]. Respir Res. 2017;18(1):19.
- Shah AA, D’Amico TA. Lung volume reduction surgery for the management of refractory dyspnea in chronic obstructive pulmonary disease. Curr Opin Support Palliat Care. 2009;3(2):107-111.