User login
Rituximab Still Proves Safe Long Term
Rituximab, a B-cell–depleting agent, has been found safe and effective in clinical trials of patients with multiple sclerosis and patients with rheumatoid arthritis, among others. However, progressive multifocal leukoencephalopathy (PML) and malignancies have been reported in patients with lymphoma, rheumatoid arthritis, and lupus who also received multiple immunosuppressive therapies, say researchers from Wayne State University in Michigan and University of Chicago in Illinois. In studies with ocrelizumab, which also depletes B-cells, adverse effects (AEs) have included infections, such as, herpes virus-associated infection, and neoplasms.
Although most research has found rituximab and ocrelizumab safe and effective, there is a “paucity of literature” on the safety of continuous B-cell depletion over a long period, the researchers say. They conducted a retrospective study involving 29 patients with immune-mediated neurologic disorders who received continuous cycles of rituximab infusions every 6 to 9 months for up to 7 years. Although small, the study was longer than the trials with ocrelizumab in multiple sclerosis . The mean duration of treatment was 51 months; with a mean of 9 treatment cycles.
The researchers found a low incidence of adverse events and prolonged rituximab-induced B-cell depletion did not lead to any life-threatening AEs, including malignancy. Overall, 32 AEs were reported. Four were serious; 3 were noted after 9 cycles (48 months), and 1 after 11 cycles (60 months). There were no cases of PML or malignancies. Repeated rituximab infusions were well tolerated The rate of AEs remained low over the 7-year observation period.
Source:
Memon AB, Javed A, Caon C, et al. PLoS ONE. 2018;13(1):e0190425.
doi: 10.1371/journal.pone.0190425.
Rituximab, a B-cell–depleting agent, has been found safe and effective in clinical trials of patients with multiple sclerosis and patients with rheumatoid arthritis, among others. However, progressive multifocal leukoencephalopathy (PML) and malignancies have been reported in patients with lymphoma, rheumatoid arthritis, and lupus who also received multiple immunosuppressive therapies, say researchers from Wayne State University in Michigan and University of Chicago in Illinois. In studies with ocrelizumab, which also depletes B-cells, adverse effects (AEs) have included infections, such as, herpes virus-associated infection, and neoplasms.
Although most research has found rituximab and ocrelizumab safe and effective, there is a “paucity of literature” on the safety of continuous B-cell depletion over a long period, the researchers say. They conducted a retrospective study involving 29 patients with immune-mediated neurologic disorders who received continuous cycles of rituximab infusions every 6 to 9 months for up to 7 years. Although small, the study was longer than the trials with ocrelizumab in multiple sclerosis . The mean duration of treatment was 51 months; with a mean of 9 treatment cycles.
The researchers found a low incidence of adverse events and prolonged rituximab-induced B-cell depletion did not lead to any life-threatening AEs, including malignancy. Overall, 32 AEs were reported. Four were serious; 3 were noted after 9 cycles (48 months), and 1 after 11 cycles (60 months). There were no cases of PML or malignancies. Repeated rituximab infusions were well tolerated The rate of AEs remained low over the 7-year observation period.
Source:
Memon AB, Javed A, Caon C, et al. PLoS ONE. 2018;13(1):e0190425.
doi: 10.1371/journal.pone.0190425.
Rituximab, a B-cell–depleting agent, has been found safe and effective in clinical trials of patients with multiple sclerosis and patients with rheumatoid arthritis, among others. However, progressive multifocal leukoencephalopathy (PML) and malignancies have been reported in patients with lymphoma, rheumatoid arthritis, and lupus who also received multiple immunosuppressive therapies, say researchers from Wayne State University in Michigan and University of Chicago in Illinois. In studies with ocrelizumab, which also depletes B-cells, adverse effects (AEs) have included infections, such as, herpes virus-associated infection, and neoplasms.
Although most research has found rituximab and ocrelizumab safe and effective, there is a “paucity of literature” on the safety of continuous B-cell depletion over a long period, the researchers say. They conducted a retrospective study involving 29 patients with immune-mediated neurologic disorders who received continuous cycles of rituximab infusions every 6 to 9 months for up to 7 years. Although small, the study was longer than the trials with ocrelizumab in multiple sclerosis . The mean duration of treatment was 51 months; with a mean of 9 treatment cycles.
The researchers found a low incidence of adverse events and prolonged rituximab-induced B-cell depletion did not lead to any life-threatening AEs, including malignancy. Overall, 32 AEs were reported. Four were serious; 3 were noted after 9 cycles (48 months), and 1 after 11 cycles (60 months). There were no cases of PML or malignancies. Repeated rituximab infusions were well tolerated The rate of AEs remained low over the 7-year observation period.
Source:
Memon AB, Javed A, Caon C, et al. PLoS ONE. 2018;13(1):e0190425.
doi: 10.1371/journal.pone.0190425.
VIP an unwelcome contributor to eosinophilic esophagitis
Vasoactive intestinal peptide (VIP) appears to play an important role in the pathology of eosinophilic esophagitis (EoE) by recruiting mast cells and eosinophils that contribute to EoE’s hallmark symptoms of dysphagia and esophageal dysmotility, investigators reported in the February issue of Cellular and Molecular Gastroenterology and Hepatology.
Blocking one of three VIP receptors – chemoattractant receptor-homologous molecule expressed on Th2 (CRTH2) – could reduce eosinophil infiltration and mast cell numbers in the esophagus, wrote Alok K. Verma, PhD, a postodoctoral fellow at Tulane University in New Orleans, and his colleagues.
“We suggest that inhibiting the VIP–CRTH2 axis may ameliorate the dysphagia, stricture, and motility dysfunction of chronic EoE,” they wrote in a research letter to Cellular and Molecular Gastroenterology and Hepatology.
Several cytokines and chemokines, notably interleukin-5 and eotaxin-3, have been fingered as suspects in eosinophil infiltration, but whether chemokines other than eotaxin play a role has not been well documented, the investigators noted.
They hypothesized that VIP may be a chemoattractant that draws eosinophils into perineural areas of the muscular mucosa of the esophagus.
To test this idea, they looked at VIP-expression in samples from patients both with and without EoE and found that VIP expression was low among controls (without EoE); they also found that eosinophils were seen to accumulate near VIP-expressing nerve cells in biopsy samples from patients with EoE.
When they performed in vitro studies of VIP binding and immunologic functions, they found that eosinophils primarily express the CRTH2 receptor rather than the vasoactive intestinal peptide receptor 1 (VPAC-1) or VPAC-2.
They also demonstrated that VIP’s effects on eosinophil motility was similar to that of eotaxin and that, when they pretreated eosinophils with a CRTH2 inhibitor, esoinophil motility was hampered.
The investigators next looked at biopsy specimens from patients with EoE and found that eosinophils that express CRTH2 accumulated in the epithelial mucosa.
To see whether (as they and other researchers had suspected) VIP and its interaction with the CRTH2 receptor might play a role in mast cell recruitment, they performed immunofluorescence analyses and confirmed the presence of the CRTH2 receptor on tryptase-positive mast cells in the esophageal mucosa of patients with EoE.
“These findings suggest that, similar to eosinophils, mast cells accumulate via interaction of the CRTH2 receptor with neutrally derived VIP,” they wrote.
Finally, to see whether a reduction in peak eosinophil levels in patients with EoE with a CRTH2 antagonist – as seen in prior studies – could also ameliorate the negative effects of mast cells on esophageal function, they looked at the effects of CRTH2 inhibition in a mouse model of human EoE.
They found that, in the mice treated with a CRTH2 blocker, each segment of the esophagus had significant reductions in both eosinophil infiltration and mast cell numbers (P less than .05 for each).
The work was supported in part by grants from the National Institutes of Health and the Tulane Edward G. Schlieder Educational Foundation. Senior author Anil Mishra, PhD, disclosed serving as a consultant for Axcan Pharma, Aptalis, Elite Biosciences, Calypso Biotech SA, and Enumeral Biomedical. The remaining authors disclosed no conflicts of interest.
SOURCE: Verma AK et al. Cell Mol Gastroenterol Hepatol. 2018;5[1]:99-100.e7.
The rapid increase in the incidence of pediatric and adult eosinophilic esophagitis (EoE) draws immediate attention to the importance of studying the mechanisms underlying this detrimental condition. The lack of preventive or curative therapies for EoE further underscores the importance of research that addresses gaps in our understanding of how eosinophilic inflammation of the esophagus is regulated on the molecular and cellular level. EoE is classified as an allergic immune disorder of the gastrointestinal tract and is characterized by eosinophil-rich, chronic Th2-type inflammation of the esophagus.
In this recent publication, the laboratory of Anil Mishra, PhD, showed that vasoactive intestinal peptide (VIP) serves as a potent chemoattractant for eosinophils and promotes accumulation of these innate immune cells adjacent to nerve cells in the muscular mucosa. Increased VIP expression was documented in EoE patients when compared to controls, and the authors identified the chemoattractant receptor homologous molecule expressed on Th2 lymphocytes (CRTH2) as a main binding receptor for VIP. Interestingly, CRTH2 was not only found to be expressed on eosinophils but also on tissue mast cells – another innate immune cell type that significantly contributes to the inflammatory tissue infiltrate in EoE patients. Based on the human findings, the authors tested whether VIP plays a major role in recruiting eosinophils and mast cells to the inflamed esophagus and whether CRTH2 blockade can modulate experimental EoE. Indeed, EoE pathology improved in animals that were treated with a CRTH2 antagonist.
In conclusion, these observations suggest that inhibiting the VIP-CRTH2 axis may serve as a therapeutic intervention pathway to ameliorate innate tissue inflammation in EoE patients.
Edda Fiebiger, PhD, is in the department of pediatrics in the division of gastroenterology, hepatology and nutrition at Boston Children’s Hospital, as well as in the department of medicine at Harvard Medical School, also in Boston. She had no disclosures.
The rapid increase in the incidence of pediatric and adult eosinophilic esophagitis (EoE) draws immediate attention to the importance of studying the mechanisms underlying this detrimental condition. The lack of preventive or curative therapies for EoE further underscores the importance of research that addresses gaps in our understanding of how eosinophilic inflammation of the esophagus is regulated on the molecular and cellular level. EoE is classified as an allergic immune disorder of the gastrointestinal tract and is characterized by eosinophil-rich, chronic Th2-type inflammation of the esophagus.
In this recent publication, the laboratory of Anil Mishra, PhD, showed that vasoactive intestinal peptide (VIP) serves as a potent chemoattractant for eosinophils and promotes accumulation of these innate immune cells adjacent to nerve cells in the muscular mucosa. Increased VIP expression was documented in EoE patients when compared to controls, and the authors identified the chemoattractant receptor homologous molecule expressed on Th2 lymphocytes (CRTH2) as a main binding receptor for VIP. Interestingly, CRTH2 was not only found to be expressed on eosinophils but also on tissue mast cells – another innate immune cell type that significantly contributes to the inflammatory tissue infiltrate in EoE patients. Based on the human findings, the authors tested whether VIP plays a major role in recruiting eosinophils and mast cells to the inflamed esophagus and whether CRTH2 blockade can modulate experimental EoE. Indeed, EoE pathology improved in animals that were treated with a CRTH2 antagonist.
In conclusion, these observations suggest that inhibiting the VIP-CRTH2 axis may serve as a therapeutic intervention pathway to ameliorate innate tissue inflammation in EoE patients.
Edda Fiebiger, PhD, is in the department of pediatrics in the division of gastroenterology, hepatology and nutrition at Boston Children’s Hospital, as well as in the department of medicine at Harvard Medical School, also in Boston. She had no disclosures.
The rapid increase in the incidence of pediatric and adult eosinophilic esophagitis (EoE) draws immediate attention to the importance of studying the mechanisms underlying this detrimental condition. The lack of preventive or curative therapies for EoE further underscores the importance of research that addresses gaps in our understanding of how eosinophilic inflammation of the esophagus is regulated on the molecular and cellular level. EoE is classified as an allergic immune disorder of the gastrointestinal tract and is characterized by eosinophil-rich, chronic Th2-type inflammation of the esophagus.
In this recent publication, the laboratory of Anil Mishra, PhD, showed that vasoactive intestinal peptide (VIP) serves as a potent chemoattractant for eosinophils and promotes accumulation of these innate immune cells adjacent to nerve cells in the muscular mucosa. Increased VIP expression was documented in EoE patients when compared to controls, and the authors identified the chemoattractant receptor homologous molecule expressed on Th2 lymphocytes (CRTH2) as a main binding receptor for VIP. Interestingly, CRTH2 was not only found to be expressed on eosinophils but also on tissue mast cells – another innate immune cell type that significantly contributes to the inflammatory tissue infiltrate in EoE patients. Based on the human findings, the authors tested whether VIP plays a major role in recruiting eosinophils and mast cells to the inflamed esophagus and whether CRTH2 blockade can modulate experimental EoE. Indeed, EoE pathology improved in animals that were treated with a CRTH2 antagonist.
In conclusion, these observations suggest that inhibiting the VIP-CRTH2 axis may serve as a therapeutic intervention pathway to ameliorate innate tissue inflammation in EoE patients.
Edda Fiebiger, PhD, is in the department of pediatrics in the division of gastroenterology, hepatology and nutrition at Boston Children’s Hospital, as well as in the department of medicine at Harvard Medical School, also in Boston. She had no disclosures.
Vasoactive intestinal peptide (VIP) appears to play an important role in the pathology of eosinophilic esophagitis (EoE) by recruiting mast cells and eosinophils that contribute to EoE’s hallmark symptoms of dysphagia and esophageal dysmotility, investigators reported in the February issue of Cellular and Molecular Gastroenterology and Hepatology.
Blocking one of three VIP receptors – chemoattractant receptor-homologous molecule expressed on Th2 (CRTH2) – could reduce eosinophil infiltration and mast cell numbers in the esophagus, wrote Alok K. Verma, PhD, a postodoctoral fellow at Tulane University in New Orleans, and his colleagues.
“We suggest that inhibiting the VIP–CRTH2 axis may ameliorate the dysphagia, stricture, and motility dysfunction of chronic EoE,” they wrote in a research letter to Cellular and Molecular Gastroenterology and Hepatology.
Several cytokines and chemokines, notably interleukin-5 and eotaxin-3, have been fingered as suspects in eosinophil infiltration, but whether chemokines other than eotaxin play a role has not been well documented, the investigators noted.
They hypothesized that VIP may be a chemoattractant that draws eosinophils into perineural areas of the muscular mucosa of the esophagus.
To test this idea, they looked at VIP-expression in samples from patients both with and without EoE and found that VIP expression was low among controls (without EoE); they also found that eosinophils were seen to accumulate near VIP-expressing nerve cells in biopsy samples from patients with EoE.
When they performed in vitro studies of VIP binding and immunologic functions, they found that eosinophils primarily express the CRTH2 receptor rather than the vasoactive intestinal peptide receptor 1 (VPAC-1) or VPAC-2.
They also demonstrated that VIP’s effects on eosinophil motility was similar to that of eotaxin and that, when they pretreated eosinophils with a CRTH2 inhibitor, esoinophil motility was hampered.
The investigators next looked at biopsy specimens from patients with EoE and found that eosinophils that express CRTH2 accumulated in the epithelial mucosa.
To see whether (as they and other researchers had suspected) VIP and its interaction with the CRTH2 receptor might play a role in mast cell recruitment, they performed immunofluorescence analyses and confirmed the presence of the CRTH2 receptor on tryptase-positive mast cells in the esophageal mucosa of patients with EoE.
“These findings suggest that, similar to eosinophils, mast cells accumulate via interaction of the CRTH2 receptor with neutrally derived VIP,” they wrote.
Finally, to see whether a reduction in peak eosinophil levels in patients with EoE with a CRTH2 antagonist – as seen in prior studies – could also ameliorate the negative effects of mast cells on esophageal function, they looked at the effects of CRTH2 inhibition in a mouse model of human EoE.
They found that, in the mice treated with a CRTH2 blocker, each segment of the esophagus had significant reductions in both eosinophil infiltration and mast cell numbers (P less than .05 for each).
The work was supported in part by grants from the National Institutes of Health and the Tulane Edward G. Schlieder Educational Foundation. Senior author Anil Mishra, PhD, disclosed serving as a consultant for Axcan Pharma, Aptalis, Elite Biosciences, Calypso Biotech SA, and Enumeral Biomedical. The remaining authors disclosed no conflicts of interest.
SOURCE: Verma AK et al. Cell Mol Gastroenterol Hepatol. 2018;5[1]:99-100.e7.
Vasoactive intestinal peptide (VIP) appears to play an important role in the pathology of eosinophilic esophagitis (EoE) by recruiting mast cells and eosinophils that contribute to EoE’s hallmark symptoms of dysphagia and esophageal dysmotility, investigators reported in the February issue of Cellular and Molecular Gastroenterology and Hepatology.
Blocking one of three VIP receptors – chemoattractant receptor-homologous molecule expressed on Th2 (CRTH2) – could reduce eosinophil infiltration and mast cell numbers in the esophagus, wrote Alok K. Verma, PhD, a postodoctoral fellow at Tulane University in New Orleans, and his colleagues.
“We suggest that inhibiting the VIP–CRTH2 axis may ameliorate the dysphagia, stricture, and motility dysfunction of chronic EoE,” they wrote in a research letter to Cellular and Molecular Gastroenterology and Hepatology.
Several cytokines and chemokines, notably interleukin-5 and eotaxin-3, have been fingered as suspects in eosinophil infiltration, but whether chemokines other than eotaxin play a role has not been well documented, the investigators noted.
They hypothesized that VIP may be a chemoattractant that draws eosinophils into perineural areas of the muscular mucosa of the esophagus.
To test this idea, they looked at VIP-expression in samples from patients both with and without EoE and found that VIP expression was low among controls (without EoE); they also found that eosinophils were seen to accumulate near VIP-expressing nerve cells in biopsy samples from patients with EoE.
When they performed in vitro studies of VIP binding and immunologic functions, they found that eosinophils primarily express the CRTH2 receptor rather than the vasoactive intestinal peptide receptor 1 (VPAC-1) or VPAC-2.
They also demonstrated that VIP’s effects on eosinophil motility was similar to that of eotaxin and that, when they pretreated eosinophils with a CRTH2 inhibitor, esoinophil motility was hampered.
The investigators next looked at biopsy specimens from patients with EoE and found that eosinophils that express CRTH2 accumulated in the epithelial mucosa.
To see whether (as they and other researchers had suspected) VIP and its interaction with the CRTH2 receptor might play a role in mast cell recruitment, they performed immunofluorescence analyses and confirmed the presence of the CRTH2 receptor on tryptase-positive mast cells in the esophageal mucosa of patients with EoE.
“These findings suggest that, similar to eosinophils, mast cells accumulate via interaction of the CRTH2 receptor with neutrally derived VIP,” they wrote.
Finally, to see whether a reduction in peak eosinophil levels in patients with EoE with a CRTH2 antagonist – as seen in prior studies – could also ameliorate the negative effects of mast cells on esophageal function, they looked at the effects of CRTH2 inhibition in a mouse model of human EoE.
They found that, in the mice treated with a CRTH2 blocker, each segment of the esophagus had significant reductions in both eosinophil infiltration and mast cell numbers (P less than .05 for each).
The work was supported in part by grants from the National Institutes of Health and the Tulane Edward G. Schlieder Educational Foundation. Senior author Anil Mishra, PhD, disclosed serving as a consultant for Axcan Pharma, Aptalis, Elite Biosciences, Calypso Biotech SA, and Enumeral Biomedical. The remaining authors disclosed no conflicts of interest.
SOURCE: Verma AK et al. Cell Mol Gastroenterol Hepatol. 2018;5[1]:99-100.e7.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: VIP appears to play an important role in the pathogenesis of eosinophilic esophagitis (EoE).
Major finding: Neurally derived VIP and its interaction with the CRTH2 receptor appear to recruit eosinophils and mast cells into the esophageal mucosa.
Data source: In vitro studies of human EoE biopsy samples and in vivo studies in mouse models of EoE.
Disclosures: The work was supported in part by grants from the National Institutes of Health and the Tulane Edward G. Schlieder Educational Foundation. Senior author Anil Mishra, PhD, disclosed serving as a consultant for Axcan Pharma, Aptalis, Elite Biosciences, Calypso Biotech SA, and Enumeral Biomedical. The remaining authors disclosed no conflicts of interest.
Source: Verma AK et al. Cell Mol Gastroenterol Hepatol. 2018;5[1]:99-100.e7.
Benzodiazepines: Sensible prescribing in light of the risks
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.
Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.
A wide range of risks
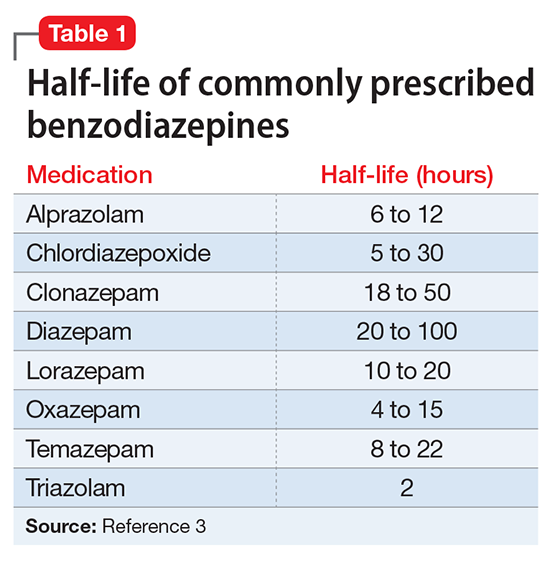
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
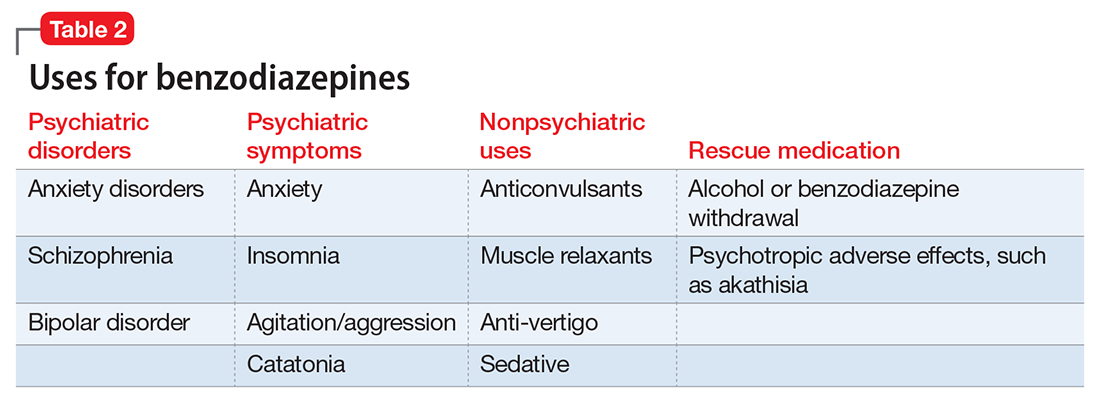
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
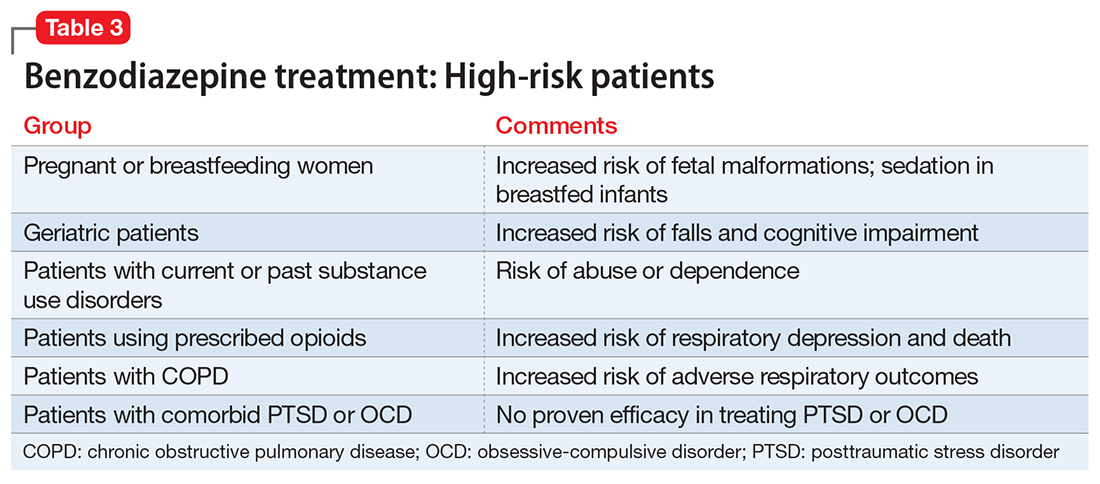
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.
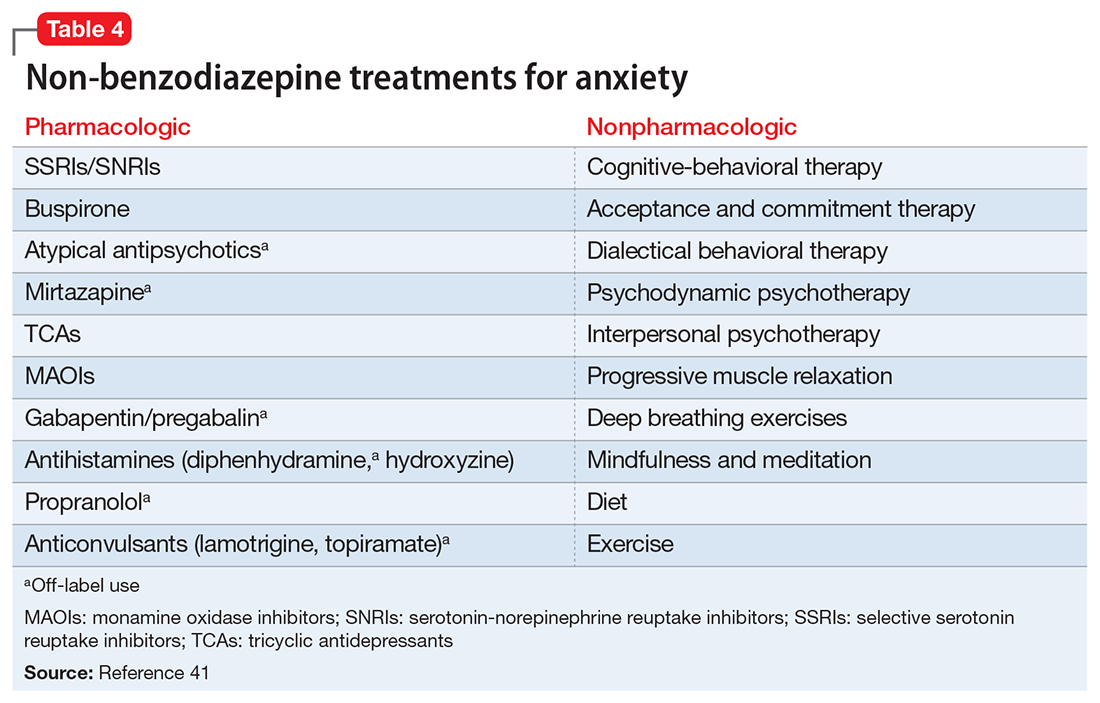
Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).
Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.

Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.

A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.

Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).

Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.

Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.

A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.

Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).

Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
Compulsive sexual behavior: A nonjudgmental approach
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
The toxic zeitgeist of hyper-partisanship: A psychiatric perspective
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
It is always judicious to avoid discussing religious or political issues because inevitably someone will be offended. As a lifetime member of the American Psychiatric Association, I adhere to its "Goldwater Rule," which proscribes the gratuitous diagnosis of any president absent of a formal face-to-face psychiatric evaluation. But it is perfectly permissible to express a psychiatric opinion about the contemporary national political scene.
Frankly, the status of the political arena has become ugly. This should not be surprising, given that at its core, politics is an unquenchable thirst for power, and Machiavelli is its anointed godfather. The current political zeitgeist of the country is becoming downright grotesque and spiteful. Although fierce political rivalry is widely accepted as a tradition to achieve the national goals promulgated by each party, what we are witnessing today is a veritable blood sport fueled by “hyper-partisanship,” where drawing blood, not promoting the public good, has become an undisguised intent.
The intensity of hyper-partisanship has engulfed the collective national psyche and is bordering on the “religification” of politics. What used to be reasonable political views have been transformed into irrefutable articles of faith that do not lend themselves to rational debate or productive compromise. The metastasis of social media into our daily lives over the past decade is catalyzing the venomous crossfire across the political divide that used to be passionate and civil, but recently has degenerated into a raucous cacophony of hateful speech. Thoughtful debate of issues that promote the public good is becoming scarce. Instead of effectively defending the validity of their arguments, extremists focus on spewing accusations and ad hominem insults. It is worrisome that both fringe groups tenaciously uphold fixed and extreme political positions, the tenets of which can never be challenged.
Psychiatrically, those extreme ideological positions appear to be consistent with Jasper’s criteria for a delusion (a belief with an unparalleled degree of subjective feeling of certainty that cannot be influenced by experience or arguments) or McHugh’s definition of an overvalued idea, which resembles an egosyntonic obsession that is relished, amplified, and defended. Given that extremism is not just a “folie à deux” shared by 2 individuals but by many individuals, it may qualify as a “folie en masse.”
Having a political orientation is perfectly normal, a healthy evidence of absence of indolent apathy. However, the unconstrained fervor of political extremism can be as psychologically unhealthy as lethargic passivity. A significant segment of the population may see some merit on both sides of the gaping political chasm, but they are appalled by the intransigence of political extremism, which has become an impediment to the constructive compromise that is vital for progress in politics and in all human interactions.
Beliefs are a transcendent human trait. Homo sapiens represent the only animal species endowed by evolution with a large prefrontal cortex that enables each of its members to harbor a belief system. It prompts me to propose that Descartes’ famous dictum “I think, therefore I am” be revised to “I believe, therefore I am human.” But while many beliefs are reasonable and anchored in reality, irrational beliefs are odd and ambiguous, ranging from superstitions and overvalued ideas to conspiracy theories and cults, which I wrote about a decade ago.1 In fact, epidemiologic research studies have confirmed a high prevalence of subthreshold and pre-psychotic beliefs in the general population.2-5 Thus, radical political partisanship falls on the extreme end of that continuum.
The zeitgeist generated by extreme partisanship is intellectually stunting and emotionally numbing. Psychiatrists may wonder what consequences the intense anger and antipathy and scarcity of compromise between the opposing parties will have for the country’s citizens. Although psychiatrists cannot repair the dysfunctional political fragmentation at the national level, we can help patients who may be negatively affected by the conflicts permeating the national scene when we read or watch the daily news.
Just as it is disturbing for children to watch their parents undermine each other by arguing ferociously and hurling insults, so it is for a populace aghast at how frenzied and intolerant their leaders and their extremist followers have become, failing to work together for the common good and adversely impacting the mental health zeitgeist.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Kelleher I, Wigman JT, Harley M, et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res. 2015;165(1):9-14.
3. Landin-Romero R, McKenna PJ, Romaguera A, et al. Examining the continuum of psychosis: frequency and characteristics of psychotic-like symptoms in relatives and non-relatives of patients with schizophrenia. Schizophr Res. 2016;178(1-3):6-11.
4. Hanssen M, Bak M, Bijl R, et al. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(pt 2):181-191.
5. Nelson B, Fusar-Poli P, Yung AR. Can we detect psychotic-like experiences in the general population? Curr Pharm Des. 2012;18(4):376-385.
Neurodegenerative aspects of psychiatric disorders
Obesity, psychiatric disorders, and pregnancy
Rapid weight loss, irritability, and nausea after restarting ADHD treatment
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.
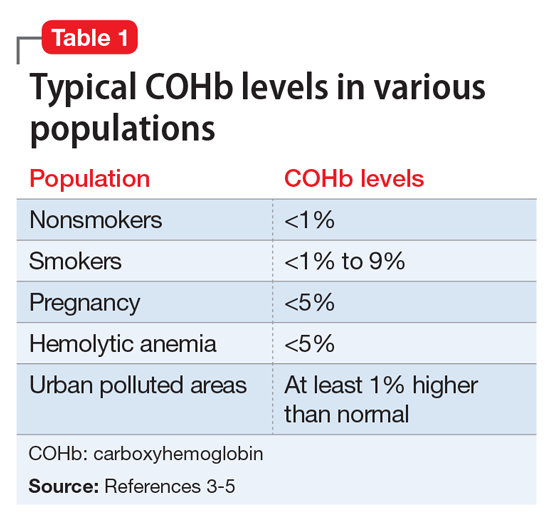
[polldaddy:9928298]
The author’s observations
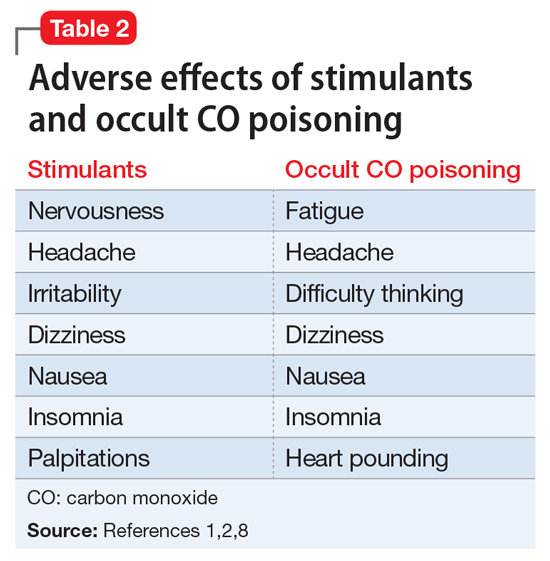
Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.

[polldaddy:9928298]
The author’s observations

Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
CASE Medication management
Mr. L, age 58, presents to the outpatient psychiatric clinic seeking treatment for attention-deficit/hyperactivity disorder (ADHD), which was first diagnosed 11 years ago. Since discontinuing his ADHD medication, lisdexamfetamine 60 mg/d, 8 months ago, he has not been completing tasks and has been distracted in his job as a limousine driver. Mr. L says that when he was taking the medication, “I could focus and prioritize.” He reports that he has trouble retaining information and is easily distracted. He says he generally is organized with appointments and keeping track of things but is messy, forgetful, tardy, and impatient. Procrastination is an ongoing problem. He denies misplacing things or being impulsive. Mr. L reports that as a child he was frequently reprimanded for talking in class. He states, “I get in trouble even now for talking too much.”
Mr. L is cooperative and polite, maintains good eye contact, and is alert. No psychomotor abnormalities are noted. His speech is spontaneous and coherent, with normal rate, rhythm, and volume. He reports that his mood is “all right,” and denies suicidal or homicidal ideation. His insight is full, judgment is intact, and thought is linear and logical. Mr. L sleeps 5 hours at night and takes a nap during the day, but his energy varies.
His psychiatric history is negative for suicide attempts or hospitalizations. Mr. L denies a history of major depressive episodes, manic symptoms, hallucinations, or delusions. Anxiety history is negative for excessive worrying, obsessions and compulsions, and panic attacks. Mr. L has no family history of mental illness or substance abuse, and he denies any personal history of drug use. He stopped using tobacco 14 years ago. Mr. L says he drinks 3 caffeinated drinks a day and 2 glasses of wine once a week. Previous medications included
A review of systems is negative. Vital signs are unremarkable. A recent electrocardiogram (EKG) showed normal sinus rhythm. Thyroid-stimulating hormone, comprehensive metabolic panel (CMP), lipids, iron, vitamin B12, folate, complete blood count (CBC), hemoglobin A1c, and urine analysis are normal, except for mildly elevated low-density lipoprotein. Testing for hepatitis C is negative.
The previous diagnosis of ADHD is confirmed, and Mr. L is started on
[polldaddy:9928295]
The author’s observations
Anxiety, irritability, agitation, and palpitations can all be symptoms of stimulant medications.1,2 There are numerous other iatrogenic causes, including steroid-based asthma treatments, thyroid medications, antidepressants in bipolar patients, and caffeine-based migraine treatments. Mr. L’s theory that his 15-lb weight loss was the result of his methylphenidate ER dose being too high was a reasonable one. Often, medication doses need to be adjusted with weight changes. His decrease in energy during the day could be explained by the methylphenidate ER controlling his hyperactive symptoms, which include high energy. At night, when the medication wears off, his hyperactivity symptoms could be returning, which would account for the increase in energy when he gets home from work. Although longer-acting stimulants tend to have a more benign adverse effects profile, they can cause insomnia if they are still in the patient’s system at bedtime. Shorter-acting stimulants wear off quickly but can be advantageous for patients who want to target concentration during certain times of day, such as for school and homework.
TREATMENT A surprising cause
The next month, Mr. L presents to the emergency room complaining of jitteriness, headache, and tingling in his fingers, and is evaluated for suspected carbon monoxide (CO) poisoning. Three months earlier, he had noted the odor of exhaust fumes in the limousine he drives 7 days a week. He took it to the mechanic twice for evaluation, but no cause was found. Despite his concerns, he continued to drive the car until an older client, in frail health, suddenly became short of breath and developed chest pain shortly after entering his vehicle, on a day when the odor was particularly bad. Before that, a family of passengers had complained of headaches upon entering his vehicle. The third time he brought his car to be checked, the mechanic identified an exhaust system leak.

[polldaddy:9928298]
The author’s observations

Work-up for suspected CO poisoning includes ABG, COHb level, CBC, basic metabolic panel, EKG, cardiac enzymes, and chest radiography, as well as other laboratory tests as deemed appropriate. Treatment includes oxygen by mask for low-level poisoning.
High levels of poisoning may require hyperbaric oxygen, which should be considered for patients who are unconscious or have an abnormal score on the Carbon Monoxide Neuropsychological Screening Battery, COHb of >40%, signs of cardiac ischemia or arrhythmia, history of ischemic heart disease with COHb level >20%, recurrent symptoms for up to 3 weeks, or symptoms that have not resolved with normobaric oxygen after 4 to 6 hours.9 Any pregnant woman with CO poisoning should receive hyperbaric therapy.10
OUTCOME Lasting improvement
Mr. L presents for follow-up in the psychiatric clinic 3 weeks after his emergency room visit. After his limousine was repaired, his symptoms resolved. He no longer experiences fatigue during the day with higher energy at night, palpitations, jitteriness, headache, or tingling. His concentration has improved, so he opts to stick with the 18-mg dose of methylphenidate ER rather than increase it to the initial dose. He places a CO detector in his vehicle, which proves to be a good decision when it gives him a warning that the exhaust leak had not been properly repaired.
[polldaddy:9928299]
The author’s observations
Although the correct cause of Mr. L’s symptoms was found incidentally, this case is an important reminder to always consider medical causes in the differential diagnosis. We are taught in medical school to look first for horses (more likely causes), not zebras (less likely causes), but sometimes zebras do occur. Be mindful that medical causes should be considered not only for symptoms of primary illnesses, but also for symptoms thought to be caused by adverse effects of medications. The differential diagnosis for Mr. L’s symptoms (palpitations, agitation, anxiety, irritability, weight loss, fatigue, nausea, and headache) included metabolic and endocrine abnormalities (thyroid disease, pheochromocytoma, hypoglycemia); psychiatric conditions (panic, bipolar disorder, depression); substance abuse (caffeine, cocaine, amphetamines); immune disorders; cardiac disorders; malignancy; toxic exposure; infectious sources; and nutritional deficiencies. CO poisoning can cause many of these symptoms (Table 2).1,2,8
Intentional CO poisoning should be considered in an obtunded or unconscious patient with depression. Patients may consider CO poisoning a more peaceful way to complete suicide than shooting, cutting, or hanging. As for unintentional poisoning, clinical suspicion can be increased by time of year, occupation, locale, and smoking status. Winter months increase risk because of the high use of heating devices, cars warming up in the garage, closed fireplace flues, and vehicle tailpipes blocked by snow. As in Mr. L’s case, occupation also may increase suspicion; drivers, mechanics, tollbooth operators, parking attendants, miners, and firefighters are all at increased risk for CO poisoning. Regarding locale, polluted urban environments as well as cold climates requiring heating sources cause higher risks for CO exposure. Rarely, excessive smoking can result in CO poisoning. The author once had a patient with schizophrenia who was admitted to the hospital with delirium. It was determined that he had CO poisoning from his 5-pack-a-day smoking habit.
Psychiatric patients often have the frustrating experience of their physical symptoms being attributed to psychiatric causes, which results in major medical issues being overlooked. We psychiatrists can fall into the same trap of overlooking medical illnesses, as indicated in this case, where Mr. L’s CO poisoning initially was attributed to adverse effects of his psychiatric medication.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
1. Drugs.com. Amphetamine side effects. https://www.drugs.com/sfx/amphetamine-side-effects.html. Accessed December 7, 2017.
2. Golmirzaei J, Mahboobi H, Yazdanparast M, et al. Psychopharmacology of attention-deficit hyperactivity disorder: effects and side effects. Curr Pharm Des. 2016;22(5):590-594.
3. Bleecker ML. Carbon monoxide intoxication. Handb Clin Neurol. 2015;131(3):191-203.
4. Carter D. Carbon monoxide: the forgotten killer. http://scot.nhs.uk/sehd/cmo/CMO(1998)19.pdf. Published September 7, 1998. Accessed January 10, 2018.
5. Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187-1195.
6. AA1Car. Troubleshoot odors & smells inside your car. http://www.aa1car.com/library/troubleshoot_odors.htm. Accessed December 7, 2017.
7. Rodkey FL, O’Neal JD, Collison HA, et al. Relative affinity of hemoglobin S and hemoglobin A for carbon monoxide and oxygen. Clin Chem. 1974;20(1):83-84.
8. Kirkpatrick JN. Occult carbon monoxide poisoning. West J Med. 1987;146(1):52-56.
9. Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603-1608.
10. Guzman JA. Carbon monoxide poisoning. Critical Care Clin. 2012;28(4):537-548.
4 Ways to help your patients with schizophrenia quit smoking
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
Decreasing suicide risk with math
Suicide is a common reality, accounting for approximately 800,000 deaths per year worldwide.1 Properly assessing and minimizing suicide risk can be challenging. We are taught that lithium and clozapine can decrease suicidality, and many psychiatrists prescribe these medications with the firm, “evidence-based” belief that doing so reduces suicide risk. Paradoxically, what they in fact might be doing is the exact opposite; they may be giving high-risk patients the opportunity and the means to attempt suicide with a lethal amount of medication.
One patient diagnosed with a mood disorder who attempted suicide had a surprising point of view. After taking a large qu
Operations research is a subfield of mathematics that tries to optimize one or more variables when multiple variables are in play. One example would be to maximize profit while minimizing cost. During World War II, operations research was used to decrease the number of munitions used to shoot down airplanes, and to sink submarines more efficiently.
Focusing on the patient who attempted suicide by overdose, the question was: If she was discharged from the psychiatry unit with a 30-day supply of medication, how lethal would that prescription be if deliberately taken all at once? And what can be done to minimize this suicide risk? Psychiatrists know that some medications are more dangerous than others, but few have performed quantitative analysis to determine the potential lethality of these medications. The math analysis did not involve multivariable calculus or differential equations, only multiplication and division. The results were eye-opening.
Calculating relative lethality
The lethal dose 50 (LD50) is the dose of a medication expressed in mg/kg that results in the death of 50% of the animals (usually rats) used in a controlled experiment. Open-source data for the LD50 of medications is provided by the manufacturers.
I tabulated this data for a wide range of psychiatric medications, including antipsychotics, mood stabilizers, and selective serotonin reuptake inhibitors, in a spreadsheet with columns for maximum daily dose, 30-day supply of the medication, LD50 in mg/kg, LD50 for a 60-kg subject, and percentage of the 30-day supply compared with LD50. I then sorted this data by relative lethality (for my complete data, see Figure 1 and the Table).
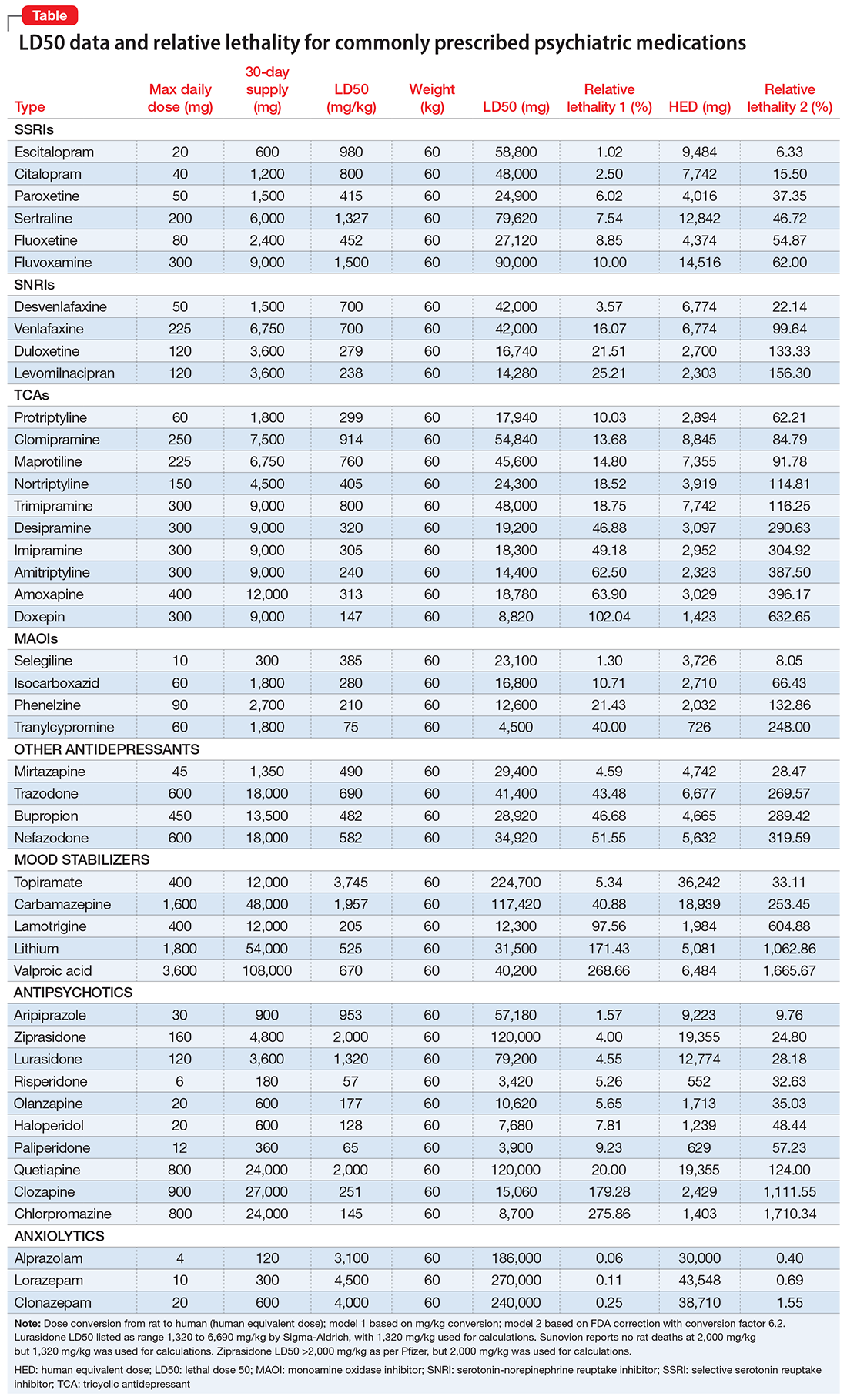
The rat dose in mg/kg was extrapolated to the human equivalent dose (HED) in mg/kg using a conversion factor of 6.2 (for a person who weighs 60 kg, the HED = LD50/6.2) as suggested by the FDA.2 The dose for the first fatality is smaller than the HED, and toxicity occurs at even smaller doses. After simplifying all the terms, the formula for the HED-relative lethality is f(x) = 310x/LD50, where x is the daily dose of a medication prescribed for 30 days. This is the equation of a straight line with a slope inversely proportional to the LD50 of each medication and a y-axis intercept of 0. Each medication line shows that any dose rising above 100% on the y-axis is a quantum higher than the lethal dose.
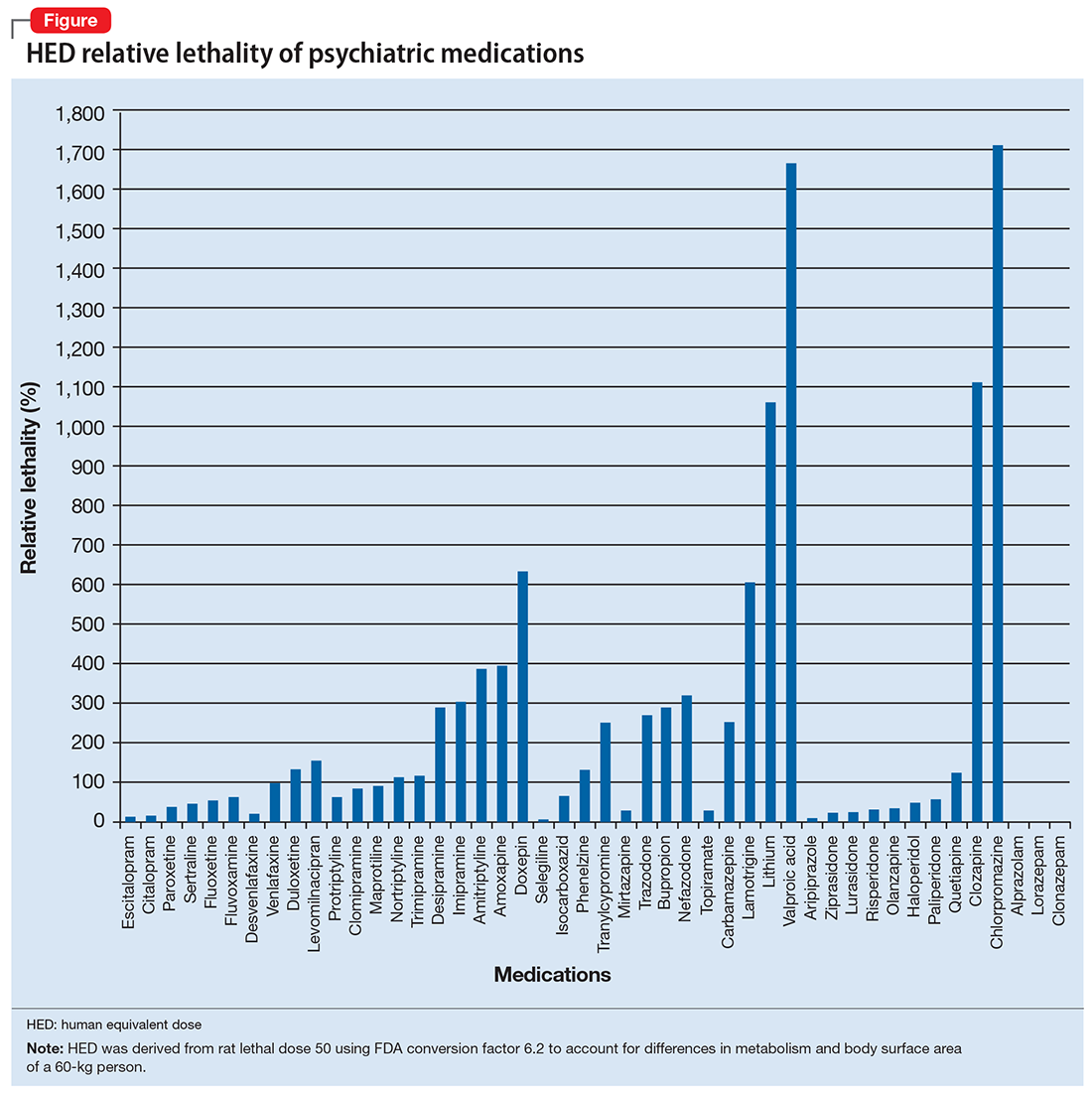
Some commonly prescribed psychotropics are highly lethal
The relative lethality of many commonly prescribed psychiatric medications, including those frequently used to reduce suicidality, varies tremendously. For example, it is widely known that the first-line mood stabilizer lithium has a narrow therapeutic window and can rapidly become toxic. If a patient becomes dehydrated, even a normal lithium dose can be toxic or lethal. Lithium has a relative lethality of 1,063% (Figure 2). Clozapine has a relative lethality of 1,112%. Valproic acid has an even higher relative lethality of 1,666%. By contrast, aripiprazole and olanzapine have a relative lethality of 10% and 35%, respectively. For preventing suicide, prescribing a second-generation antipsychotic with a lower relative lethality may be preferable over prescribing a medication with a higher relative lethality.
According to U.S. poison control centers,3 from 2000 to 2014, there were 15,036 serious outcomes, including 61 deaths, associated with lithium use, and 6,109 serious outcomes, including 37 deaths, associated with valproic acid. In contrast, there were only 1,446 serious outcomes and no deaths associated with aripiprazole use.3 These outcomes may be underreported, but they are consistent with the mathematical model predicting that medications with a higher relative lethality will have higher morbidity and mortality outcomes, regardless of a patient’s intent to overdose.
Many psychiatrists have a preferred antidepressant, mood stabilizer, or antipsychotic, and may prescribe this medication to many of their patients based on familiarity with the agent or other factors. However, simple math can give the decision process of selecting a specific medication for a given patient a more quantitative basis.
Even a small reduction in suicide would save many lives
Ultimately, the math problem comes down to 4 minutes, which is approximately how long the brain can survive without oxygen. By prescribing medications with a lower relative lethality, or by prescribing a less-than-30-day supply of the most lethal medications, it may be possible to decrease overdose morbidity and mortality, and also buy enough time for emergency personnel to save a life. If simple math can put even a 1% dent in the rate of death from suicide, approximately 8,000 lives might be saved every year.
1. World Health Organization. Suicide. Fact sheet. http://www.who.int/mediacentre/factsheets/fs398/en. Updated August 2017. Accessed January 3, 2018.
2. U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Published July 6, 2005. Accessed January 8, 2018.
3. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
Suicide is a common reality, accounting for approximately 800,000 deaths per year worldwide.1 Properly assessing and minimizing suicide risk can be challenging. We are taught that lithium and clozapine can decrease suicidality, and many psychiatrists prescribe these medications with the firm, “evidence-based” belief that doing so reduces suicide risk. Paradoxically, what they in fact might be doing is the exact opposite; they may be giving high-risk patients the opportunity and the means to attempt suicide with a lethal amount of medication.
One patient diagnosed with a mood disorder who attempted suicide had a surprising point of view. After taking a large qu
Operations research is a subfield of mathematics that tries to optimize one or more variables when multiple variables are in play. One example would be to maximize profit while minimizing cost. During World War II, operations research was used to decrease the number of munitions used to shoot down airplanes, and to sink submarines more efficiently.
Focusing on the patient who attempted suicide by overdose, the question was: If she was discharged from the psychiatry unit with a 30-day supply of medication, how lethal would that prescription be if deliberately taken all at once? And what can be done to minimize this suicide risk? Psychiatrists know that some medications are more dangerous than others, but few have performed quantitative analysis to determine the potential lethality of these medications. The math analysis did not involve multivariable calculus or differential equations, only multiplication and division. The results were eye-opening.
Calculating relative lethality
The lethal dose 50 (LD50) is the dose of a medication expressed in mg/kg that results in the death of 50% of the animals (usually rats) used in a controlled experiment. Open-source data for the LD50 of medications is provided by the manufacturers.
I tabulated this data for a wide range of psychiatric medications, including antipsychotics, mood stabilizers, and selective serotonin reuptake inhibitors, in a spreadsheet with columns for maximum daily dose, 30-day supply of the medication, LD50 in mg/kg, LD50 for a 60-kg subject, and percentage of the 30-day supply compared with LD50. I then sorted this data by relative lethality (for my complete data, see Figure 1 and the Table).

The rat dose in mg/kg was extrapolated to the human equivalent dose (HED) in mg/kg using a conversion factor of 6.2 (for a person who weighs 60 kg, the HED = LD50/6.2) as suggested by the FDA.2 The dose for the first fatality is smaller than the HED, and toxicity occurs at even smaller doses. After simplifying all the terms, the formula for the HED-relative lethality is f(x) = 310x/LD50, where x is the daily dose of a medication prescribed for 30 days. This is the equation of a straight line with a slope inversely proportional to the LD50 of each medication and a y-axis intercept of 0. Each medication line shows that any dose rising above 100% on the y-axis is a quantum higher than the lethal dose.

Some commonly prescribed psychotropics are highly lethal
The relative lethality of many commonly prescribed psychiatric medications, including those frequently used to reduce suicidality, varies tremendously. For example, it is widely known that the first-line mood stabilizer lithium has a narrow therapeutic window and can rapidly become toxic. If a patient becomes dehydrated, even a normal lithium dose can be toxic or lethal. Lithium has a relative lethality of 1,063% (Figure 2). Clozapine has a relative lethality of 1,112%. Valproic acid has an even higher relative lethality of 1,666%. By contrast, aripiprazole and olanzapine have a relative lethality of 10% and 35%, respectively. For preventing suicide, prescribing a second-generation antipsychotic with a lower relative lethality may be preferable over prescribing a medication with a higher relative lethality.

According to U.S. poison control centers,3 from 2000 to 2014, there were 15,036 serious outcomes, including 61 deaths, associated with lithium use, and 6,109 serious outcomes, including 37 deaths, associated with valproic acid. In contrast, there were only 1,446 serious outcomes and no deaths associated with aripiprazole use.3 These outcomes may be underreported, but they are consistent with the mathematical model predicting that medications with a higher relative lethality will have higher morbidity and mortality outcomes, regardless of a patient’s intent to overdose.
Many psychiatrists have a preferred antidepressant, mood stabilizer, or antipsychotic, and may prescribe this medication to many of their patients based on familiarity with the agent or other factors. However, simple math can give the decision process of selecting a specific medication for a given patient a more quantitative basis.
Even a small reduction in suicide would save many lives
Ultimately, the math problem comes down to 4 minutes, which is approximately how long the brain can survive without oxygen. By prescribing medications with a lower relative lethality, or by prescribing a less-than-30-day supply of the most lethal medications, it may be possible to decrease overdose morbidity and mortality, and also buy enough time for emergency personnel to save a life. If simple math can put even a 1% dent in the rate of death from suicide, approximately 8,000 lives might be saved every year.
Suicide is a common reality, accounting for approximately 800,000 deaths per year worldwide.1 Properly assessing and minimizing suicide risk can be challenging. We are taught that lithium and clozapine can decrease suicidality, and many psychiatrists prescribe these medications with the firm, “evidence-based” belief that doing so reduces suicide risk. Paradoxically, what they in fact might be doing is the exact opposite; they may be giving high-risk patients the opportunity and the means to attempt suicide with a lethal amount of medication.
One patient diagnosed with a mood disorder who attempted suicide had a surprising point of view. After taking a large qu
Operations research is a subfield of mathematics that tries to optimize one or more variables when multiple variables are in play. One example would be to maximize profit while minimizing cost. During World War II, operations research was used to decrease the number of munitions used to shoot down airplanes, and to sink submarines more efficiently.
Focusing on the patient who attempted suicide by overdose, the question was: If she was discharged from the psychiatry unit with a 30-day supply of medication, how lethal would that prescription be if deliberately taken all at once? And what can be done to minimize this suicide risk? Psychiatrists know that some medications are more dangerous than others, but few have performed quantitative analysis to determine the potential lethality of these medications. The math analysis did not involve multivariable calculus or differential equations, only multiplication and division. The results were eye-opening.
Calculating relative lethality
The lethal dose 50 (LD50) is the dose of a medication expressed in mg/kg that results in the death of 50% of the animals (usually rats) used in a controlled experiment. Open-source data for the LD50 of medications is provided by the manufacturers.
I tabulated this data for a wide range of psychiatric medications, including antipsychotics, mood stabilizers, and selective serotonin reuptake inhibitors, in a spreadsheet with columns for maximum daily dose, 30-day supply of the medication, LD50 in mg/kg, LD50 for a 60-kg subject, and percentage of the 30-day supply compared with LD50. I then sorted this data by relative lethality (for my complete data, see Figure 1 and the Table).

The rat dose in mg/kg was extrapolated to the human equivalent dose (HED) in mg/kg using a conversion factor of 6.2 (for a person who weighs 60 kg, the HED = LD50/6.2) as suggested by the FDA.2 The dose for the first fatality is smaller than the HED, and toxicity occurs at even smaller doses. After simplifying all the terms, the formula for the HED-relative lethality is f(x) = 310x/LD50, where x is the daily dose of a medication prescribed for 30 days. This is the equation of a straight line with a slope inversely proportional to the LD50 of each medication and a y-axis intercept of 0. Each medication line shows that any dose rising above 100% on the y-axis is a quantum higher than the lethal dose.

Some commonly prescribed psychotropics are highly lethal
The relative lethality of many commonly prescribed psychiatric medications, including those frequently used to reduce suicidality, varies tremendously. For example, it is widely known that the first-line mood stabilizer lithium has a narrow therapeutic window and can rapidly become toxic. If a patient becomes dehydrated, even a normal lithium dose can be toxic or lethal. Lithium has a relative lethality of 1,063% (Figure 2). Clozapine has a relative lethality of 1,112%. Valproic acid has an even higher relative lethality of 1,666%. By contrast, aripiprazole and olanzapine have a relative lethality of 10% and 35%, respectively. For preventing suicide, prescribing a second-generation antipsychotic with a lower relative lethality may be preferable over prescribing a medication with a higher relative lethality.

According to U.S. poison control centers,3 from 2000 to 2014, there were 15,036 serious outcomes, including 61 deaths, associated with lithium use, and 6,109 serious outcomes, including 37 deaths, associated with valproic acid. In contrast, there were only 1,446 serious outcomes and no deaths associated with aripiprazole use.3 These outcomes may be underreported, but they are consistent with the mathematical model predicting that medications with a higher relative lethality will have higher morbidity and mortality outcomes, regardless of a patient’s intent to overdose.
Many psychiatrists have a preferred antidepressant, mood stabilizer, or antipsychotic, and may prescribe this medication to many of their patients based on familiarity with the agent or other factors. However, simple math can give the decision process of selecting a specific medication for a given patient a more quantitative basis.
Even a small reduction in suicide would save many lives
Ultimately, the math problem comes down to 4 minutes, which is approximately how long the brain can survive without oxygen. By prescribing medications with a lower relative lethality, or by prescribing a less-than-30-day supply of the most lethal medications, it may be possible to decrease overdose morbidity and mortality, and also buy enough time for emergency personnel to save a life. If simple math can put even a 1% dent in the rate of death from suicide, approximately 8,000 lives might be saved every year.
1. World Health Organization. Suicide. Fact sheet. http://www.who.int/mediacentre/factsheets/fs398/en. Updated August 2017. Accessed January 3, 2018.
2. U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Published July 6, 2005. Accessed January 8, 2018.
3. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.
1. World Health Organization. Suicide. Fact sheet. http://www.who.int/mediacentre/factsheets/fs398/en. Updated August 2017. Accessed January 3, 2018.
2. U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. https://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf. Published July 6, 2005. Accessed January 8, 2018.
3. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450.




