User login
CAR T-cell therapy produces durable CRs in ALL
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
Updated results from the phase 2 ELIANA study have shown that tisagenlecleucel can produce durable complete responses (CRs) in children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
Sixty percent of patients who received the chimeric antigen receptor (CAR) T-cell therapy achieved a CR, and 21% had a CR with incomplete hematologic recovery (CRi).
The median duration of CR/CRi was not reached at a median follow-up of 13.1 months.
The most common treatment-related adverse event (AE) was cytokine release syndrome (CRS), occurring in 77% of patients.
Researchers reported these results in NEJM. The study was sponsored by Novartis.
“This expanded, global study of CAR T-cell therapy gives us further evidence of how remarkable this treatment can be for our young patients in whom all other treatments failed,” said study author Shannon L. Maude, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania.
“Our data show not only can we can achieve longer-term durable remissions and longer-term survival for our patients but that these personalized, cancer-fighting cells can remain in the body for months or even years, effectively doing their job.”
The trial included 75 patients who received tisagenlecleucel. At enrollment, the patients’ median age was 11 (range, 3 to 23).
Patients had received a median of 3 prior therapies (range, 1 to 8), and they had a median marrow blast percentage of 74% (range, 5 to 99).
All patients received a single infusion of tisagenlecleucel. Most (n=72) received lymphodepleting chemotherapy prior to the CAR T cells.
Results
The median duration of follow-up was 13.1 months.
The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CRi within 3 months. The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The researchers said tisagenlecleucel persisted in the blood for as long as 20 months.
The relapse-free survival rate among patients with a CR/CRi was 80% at 6 months and 59% at 12 months.
Seventeen patients who had achieved a CR relapsed before receiving subsequent treatment. Three patients went on to subsequent therapy before relapse but ultimately relapsed.
Relapse was also reported in 2 patients who had been classified as non-responders because they did not maintain a response for at least 28 days.
Eight patients underwent allogeneic hematopoietic stem cell transplant while in remission, and all 8 were alive when the manuscript for this study was submitted. Four patients had not relapsed, and the other 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the overall survival rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. Grade 3/4 AEs occurred in 88% of patients. In 73% of patients, these AEs were thought to be related to treatment.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The median duration of CRS was 8 days (range, 1-36). Forty-seven patients were admitted to the intensive care unit to receive treatment for CRS, with a median stay of 7 days (range, 1-34).
“One of our more challenging questions—‘Can we manage the serious side effects of CAR T-cell therapy?’—was asked and answered in this global study,” said author Stephan A. Grupp, MD, PhD, of Children’s Hospital of Philadelphia.
“Some of our patients get very sick, but we showed that most toxic effects can be short-lived and reversible, with the potential for our patients to achieve durable complete remissions. That’s a pretty amazing turnaround for the high-risk child who, up until now, had little chance of surviving.” ![]()
FDA places T-cell therapy on clinical hold
The US Food and Drug Administration (FDA) has placed BPX-501, a T-cell therapy being evaluated in patients who undergo haploidentical hematopoietic stem cell transplants (HSCTs), on clinical hold.
Three cases of encephalopathy possibly related to BPX-501 prompted the agency to impose the hold.
Bellicum Pharmaceuticals is the developer of BPX-501, and the company was conducting 4 trials in the US in children and adults with hematologic disorders.
The BPX-501 registration trial in Europe is not affected by the clinical hold.
BPX-501 is designed to fight infection, support engraftment, prevent disease relapse, and potentially stop graft-versus-host disease (GVHD) should it occur.
BPX-501 contains a safety switch, CaspaCIDe®, that can be activated with the administration of rimiducid to kill the toxic T cells in the event of GVHD.
The 3 cases of encephalopathy are complex, according to a company press release, and have confounding factors. These include prior failed transplants, prior history of immunodeficiency, concurrent infection, and administration of rimiducid in combination with other medications.
Encephalopathy had not emerged as an adverse event in 240 patients treated with the cell therapy, until now.
BPX-501 had produced encouraging results, according to trial data presented at EHA 2017 and ASH 2017 (abstract 211*).
In this trial, 112 pediatric patients were transfused with BPX-501 cells about 2 weeks after transplant. Patients had acute leukemia (n=53), primary immune deficiencies (n=26), erythroid disorders (n=17), Fanconi anemia (n=7), and other diseases (n=9).
Investigators reported that infused cells expanded and persisted, with peak expansion reached at 9 months after infusion. Investigators continued to detect BPX-501 cells after 2 years.
The European Commission granted BPX-501 orphan drug designation for the agent for treatment in HSCT, and for the activator agent rimiducid for the treatment of GVHD.
And the FDA had granted the agents orphan drug status as a combination replacement T-cell therapy for the treatment of immunodeficiency and GVHD after HSCT.
Bellicum says it is working with the FDA to evaluate the risk of encephalopathy in patients receiving BPX-501. ![]()
* Data in the abstract were updated in the oral presentation and reported on the company’s website.
The US Food and Drug Administration (FDA) has placed BPX-501, a T-cell therapy being evaluated in patients who undergo haploidentical hematopoietic stem cell transplants (HSCTs), on clinical hold.
Three cases of encephalopathy possibly related to BPX-501 prompted the agency to impose the hold.
Bellicum Pharmaceuticals is the developer of BPX-501, and the company was conducting 4 trials in the US in children and adults with hematologic disorders.
The BPX-501 registration trial in Europe is not affected by the clinical hold.
BPX-501 is designed to fight infection, support engraftment, prevent disease relapse, and potentially stop graft-versus-host disease (GVHD) should it occur.
BPX-501 contains a safety switch, CaspaCIDe®, that can be activated with the administration of rimiducid to kill the toxic T cells in the event of GVHD.
The 3 cases of encephalopathy are complex, according to a company press release, and have confounding factors. These include prior failed transplants, prior history of immunodeficiency, concurrent infection, and administration of rimiducid in combination with other medications.
Encephalopathy had not emerged as an adverse event in 240 patients treated with the cell therapy, until now.
BPX-501 had produced encouraging results, according to trial data presented at EHA 2017 and ASH 2017 (abstract 211*).
In this trial, 112 pediatric patients were transfused with BPX-501 cells about 2 weeks after transplant. Patients had acute leukemia (n=53), primary immune deficiencies (n=26), erythroid disorders (n=17), Fanconi anemia (n=7), and other diseases (n=9).
Investigators reported that infused cells expanded and persisted, with peak expansion reached at 9 months after infusion. Investigators continued to detect BPX-501 cells after 2 years.
The European Commission granted BPX-501 orphan drug designation for the agent for treatment in HSCT, and for the activator agent rimiducid for the treatment of GVHD.
And the FDA had granted the agents orphan drug status as a combination replacement T-cell therapy for the treatment of immunodeficiency and GVHD after HSCT.
Bellicum says it is working with the FDA to evaluate the risk of encephalopathy in patients receiving BPX-501. ![]()
* Data in the abstract were updated in the oral presentation and reported on the company’s website.
The US Food and Drug Administration (FDA) has placed BPX-501, a T-cell therapy being evaluated in patients who undergo haploidentical hematopoietic stem cell transplants (HSCTs), on clinical hold.
Three cases of encephalopathy possibly related to BPX-501 prompted the agency to impose the hold.
Bellicum Pharmaceuticals is the developer of BPX-501, and the company was conducting 4 trials in the US in children and adults with hematologic disorders.
The BPX-501 registration trial in Europe is not affected by the clinical hold.
BPX-501 is designed to fight infection, support engraftment, prevent disease relapse, and potentially stop graft-versus-host disease (GVHD) should it occur.
BPX-501 contains a safety switch, CaspaCIDe®, that can be activated with the administration of rimiducid to kill the toxic T cells in the event of GVHD.
The 3 cases of encephalopathy are complex, according to a company press release, and have confounding factors. These include prior failed transplants, prior history of immunodeficiency, concurrent infection, and administration of rimiducid in combination with other medications.
Encephalopathy had not emerged as an adverse event in 240 patients treated with the cell therapy, until now.
BPX-501 had produced encouraging results, according to trial data presented at EHA 2017 and ASH 2017 (abstract 211*).
In this trial, 112 pediatric patients were transfused with BPX-501 cells about 2 weeks after transplant. Patients had acute leukemia (n=53), primary immune deficiencies (n=26), erythroid disorders (n=17), Fanconi anemia (n=7), and other diseases (n=9).
Investigators reported that infused cells expanded and persisted, with peak expansion reached at 9 months after infusion. Investigators continued to detect BPX-501 cells after 2 years.
The European Commission granted BPX-501 orphan drug designation for the agent for treatment in HSCT, and for the activator agent rimiducid for the treatment of GVHD.
And the FDA had granted the agents orphan drug status as a combination replacement T-cell therapy for the treatment of immunodeficiency and GVHD after HSCT.
Bellicum says it is working with the FDA to evaluate the risk of encephalopathy in patients receiving BPX-501. ![]()
* Data in the abstract were updated in the oral presentation and reported on the company’s website.
Persistent rash on feet
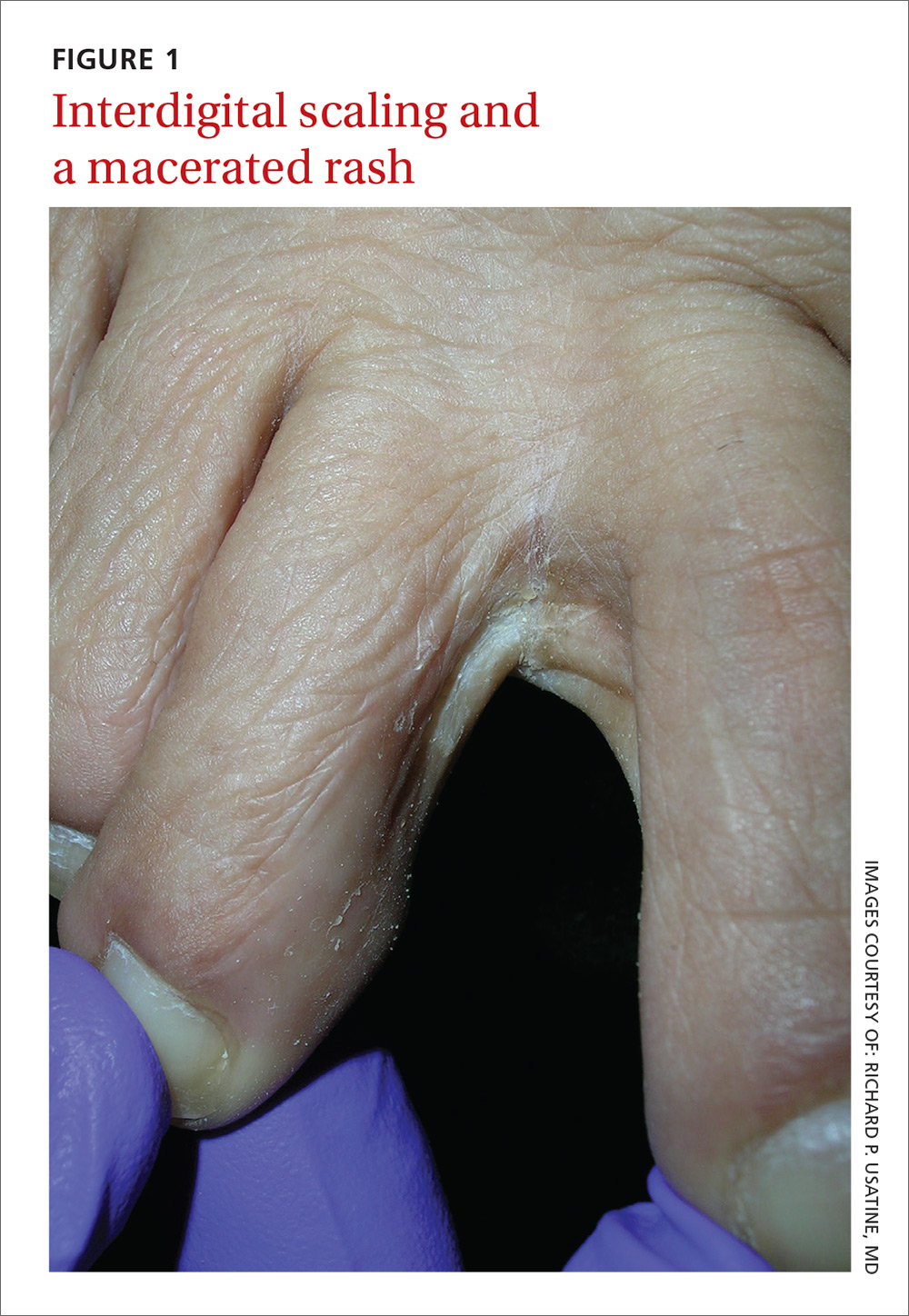
A 49-year-old Hispanic woman presented with a 4-month history of scaling and a macerated rash localized between her toes (FIGURE 1). The rash was malodorous, mildly erythematous, and sometimes associated with pruritus. The patient had no relevant medical history. Potassium hydroxide (KOH) testing was performed and found to be negative. So a Wood’s lamp was used to examine the patient’s toes—and it revealed the diagnosis.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythrasma
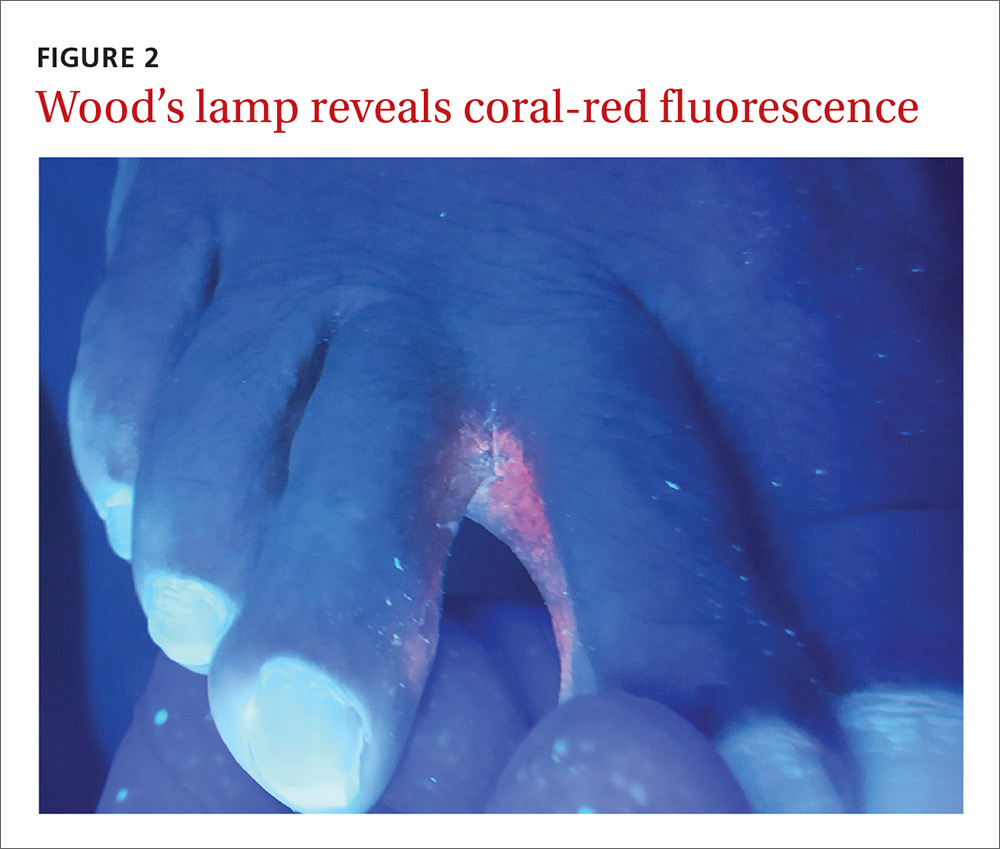
The Wood’s lamp revealed a coral-red fluorescence in the interdigital spaces (FIGURE 2), which led us to a diagnosis of erythrasma.
The coral-red fluorescence seen under the Wood’s lamp is due to porphyrins produced by Corynebacterium minutissimum. The organism invades the stratum corneum where it proliferates and causes erythrasma. Erythrasma typically appears as delineated, dry, red-brown patches in intertriginous areas, such as the axilla, groin, interdigital spaces, intergluteal cleft, perianal skin, and inframammary area.1,2
Interdigital erythrasma is more common than previously thought; in one study of 151 patients with erythrasma, the most common site was the toe webs (64.9%), followed by the inguinal region (17.9%), the axillary region (14.6%), and the inframammary region (2.6%).2 Erythrasma affects 4% of the population; risk factors include poor hygiene, hyperhidrosis, obesity, warm climate, diabetes, and an immunocompromised state.3
Differential includes “athlete’s foot”
The differential diagnosis for a pruritic rash between the toes includes:
Tinea pedis. Erythrasma is often mistaken for tinea pedis, because both conditions cause scaling between the toes. A Wood’s lamp exam can quickly differentiate between the 2,1 as tinea pedis does not fluoresce under ultraviolet light.
Contact dermatitis mimics many conditions, but a negative Wood’s lamp exam and history of worsening with contact to specific substances helps to make this diagnosis.
Prevention and Tx hinge on good hygiene, topical agents
First-line management of erythrasma includes both nonpharmacologic and pharmacologic modalities. Good hygiene and, depending on the area affected, loose-fitting cotton undergarments can help treat and prevent erythrasma.
Topical 2% miconazole bid for 2 weeks has resulted in clearance rates as high as 88%.4 Its affordable price, over-the-counter availability, and lack of adverse effects make miconazole a reasonable choice.4,5 It is also a smart treatment choice when erythrasma is coexisting with tinea, because it can treat both conditions. This is not uncommon in the interdigital spaces between the toes and in the groin.
Topical 1% clindamycin or 2% erythromycin solution or gel bid for 2 weeks can also be used to treat the condition.3,6 However, given that topical antibiotics are more expensive than single-dose oral treatment and are no better than the oral formulations of these antibiotics,6 clarithromycin 1 g taken once orally may be preferred.2,6
Our patient was treated with a single dose of clarithromycin 1 g. At follow-up, her erythrasma was clear.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229; [email protected].
1. Polat M, lhan MN. The prevalence of interdigital erythrasma: a prospective study from an outpatient clinic in Turkey. J Am Podiatr Med Assoc. 2015;105:121-124.
2. Avci O, Tanyildizi T, Kusku E. A comparison between the effectiveness of erythromycin, single-dose clarithromycin and topical fusidic acid in the treatment of erythrasma. J Dermatolog Treat. 2013;24:70-74.
3. Kibbi AG, Sleiman M. Erythrasma. Available at: http://emedicine.medscape.com/article/1052532-overview#a0199. Accessed December 10, 2016.
4. Pitcher DG, Noble WC, Seville RH. Treatment of erythrasma with miconazole. Clin Exp Dermatol. 1979;4:453-456.
5. Clayton YM, Knight AG. A clinical double-blind trial of topical miconazole and clotrimazole against superficial fungal infections and erythrasma. Clin Exp Dermatol. 1976;1:225-232.
6. Holdiness MR. Management of cutaneous erythrasma. Drugs. 2002;62:1131-1141.
A 49-year-old Hispanic woman presented with a 4-month history of scaling and a macerated rash localized between her toes (FIGURE 1). The rash was malodorous, mildly erythematous, and sometimes associated with pruritus. The patient had no relevant medical history. Potassium hydroxide (KOH) testing was performed and found to be negative. So a Wood’s lamp was used to examine the patient’s toes—and it revealed the diagnosis.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythrasma
The Wood’s lamp revealed a coral-red fluorescence in the interdigital spaces (FIGURE 2), which led us to a diagnosis of erythrasma.
The coral-red fluorescence seen under the Wood’s lamp is due to porphyrins produced by Corynebacterium minutissimum. The organism invades the stratum corneum where it proliferates and causes erythrasma. Erythrasma typically appears as delineated, dry, red-brown patches in intertriginous areas, such as the axilla, groin, interdigital spaces, intergluteal cleft, perianal skin, and inframammary area.1,2
Interdigital erythrasma is more common than previously thought; in one study of 151 patients with erythrasma, the most common site was the toe webs (64.9%), followed by the inguinal region (17.9%), the axillary region (14.6%), and the inframammary region (2.6%).2 Erythrasma affects 4% of the population; risk factors include poor hygiene, hyperhidrosis, obesity, warm climate, diabetes, and an immunocompromised state.3
Differential includes “athlete’s foot”
The differential diagnosis for a pruritic rash between the toes includes:
Tinea pedis. Erythrasma is often mistaken for tinea pedis, because both conditions cause scaling between the toes. A Wood’s lamp exam can quickly differentiate between the 2,1 as tinea pedis does not fluoresce under ultraviolet light.
Contact dermatitis mimics many conditions, but a negative Wood’s lamp exam and history of worsening with contact to specific substances helps to make this diagnosis.
Prevention and Tx hinge on good hygiene, topical agents
First-line management of erythrasma includes both nonpharmacologic and pharmacologic modalities. Good hygiene and, depending on the area affected, loose-fitting cotton undergarments can help treat and prevent erythrasma.
Topical 2% miconazole bid for 2 weeks has resulted in clearance rates as high as 88%.4 Its affordable price, over-the-counter availability, and lack of adverse effects make miconazole a reasonable choice.4,5 It is also a smart treatment choice when erythrasma is coexisting with tinea, because it can treat both conditions. This is not uncommon in the interdigital spaces between the toes and in the groin.
Topical 1% clindamycin or 2% erythromycin solution or gel bid for 2 weeks can also be used to treat the condition.3,6 However, given that topical antibiotics are more expensive than single-dose oral treatment and are no better than the oral formulations of these antibiotics,6 clarithromycin 1 g taken once orally may be preferred.2,6
Our patient was treated with a single dose of clarithromycin 1 g. At follow-up, her erythrasma was clear.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229; [email protected].
A 49-year-old Hispanic woman presented with a 4-month history of scaling and a macerated rash localized between her toes (FIGURE 1). The rash was malodorous, mildly erythematous, and sometimes associated with pruritus. The patient had no relevant medical history. Potassium hydroxide (KOH) testing was performed and found to be negative. So a Wood’s lamp was used to examine the patient’s toes—and it revealed the diagnosis.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Erythrasma
The Wood’s lamp revealed a coral-red fluorescence in the interdigital spaces (FIGURE 2), which led us to a diagnosis of erythrasma.
The coral-red fluorescence seen under the Wood’s lamp is due to porphyrins produced by Corynebacterium minutissimum. The organism invades the stratum corneum where it proliferates and causes erythrasma. Erythrasma typically appears as delineated, dry, red-brown patches in intertriginous areas, such as the axilla, groin, interdigital spaces, intergluteal cleft, perianal skin, and inframammary area.1,2
Interdigital erythrasma is more common than previously thought; in one study of 151 patients with erythrasma, the most common site was the toe webs (64.9%), followed by the inguinal region (17.9%), the axillary region (14.6%), and the inframammary region (2.6%).2 Erythrasma affects 4% of the population; risk factors include poor hygiene, hyperhidrosis, obesity, warm climate, diabetes, and an immunocompromised state.3
Differential includes “athlete’s foot”
The differential diagnosis for a pruritic rash between the toes includes:
Tinea pedis. Erythrasma is often mistaken for tinea pedis, because both conditions cause scaling between the toes. A Wood’s lamp exam can quickly differentiate between the 2,1 as tinea pedis does not fluoresce under ultraviolet light.
Contact dermatitis mimics many conditions, but a negative Wood’s lamp exam and history of worsening with contact to specific substances helps to make this diagnosis.
Prevention and Tx hinge on good hygiene, topical agents
First-line management of erythrasma includes both nonpharmacologic and pharmacologic modalities. Good hygiene and, depending on the area affected, loose-fitting cotton undergarments can help treat and prevent erythrasma.
Topical 2% miconazole bid for 2 weeks has resulted in clearance rates as high as 88%.4 Its affordable price, over-the-counter availability, and lack of adverse effects make miconazole a reasonable choice.4,5 It is also a smart treatment choice when erythrasma is coexisting with tinea, because it can treat both conditions. This is not uncommon in the interdigital spaces between the toes and in the groin.
Topical 1% clindamycin or 2% erythromycin solution or gel bid for 2 weeks can also be used to treat the condition.3,6 However, given that topical antibiotics are more expensive than single-dose oral treatment and are no better than the oral formulations of these antibiotics,6 clarithromycin 1 g taken once orally may be preferred.2,6
Our patient was treated with a single dose of clarithromycin 1 g. At follow-up, her erythrasma was clear.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229; [email protected].
1. Polat M, lhan MN. The prevalence of interdigital erythrasma: a prospective study from an outpatient clinic in Turkey. J Am Podiatr Med Assoc. 2015;105:121-124.
2. Avci O, Tanyildizi T, Kusku E. A comparison between the effectiveness of erythromycin, single-dose clarithromycin and topical fusidic acid in the treatment of erythrasma. J Dermatolog Treat. 2013;24:70-74.
3. Kibbi AG, Sleiman M. Erythrasma. Available at: http://emedicine.medscape.com/article/1052532-overview#a0199. Accessed December 10, 2016.
4. Pitcher DG, Noble WC, Seville RH. Treatment of erythrasma with miconazole. Clin Exp Dermatol. 1979;4:453-456.
5. Clayton YM, Knight AG. A clinical double-blind trial of topical miconazole and clotrimazole against superficial fungal infections and erythrasma. Clin Exp Dermatol. 1976;1:225-232.
6. Holdiness MR. Management of cutaneous erythrasma. Drugs. 2002;62:1131-1141.
1. Polat M, lhan MN. The prevalence of interdigital erythrasma: a prospective study from an outpatient clinic in Turkey. J Am Podiatr Med Assoc. 2015;105:121-124.
2. Avci O, Tanyildizi T, Kusku E. A comparison between the effectiveness of erythromycin, single-dose clarithromycin and topical fusidic acid in the treatment of erythrasma. J Dermatolog Treat. 2013;24:70-74.
3. Kibbi AG, Sleiman M. Erythrasma. Available at: http://emedicine.medscape.com/article/1052532-overview#a0199. Accessed December 10, 2016.
4. Pitcher DG, Noble WC, Seville RH. Treatment of erythrasma with miconazole. Clin Exp Dermatol. 1979;4:453-456.
5. Clayton YM, Knight AG. A clinical double-blind trial of topical miconazole and clotrimazole against superficial fungal infections and erythrasma. Clin Exp Dermatol. 1976;1:225-232.
6. Holdiness MR. Management of cutaneous erythrasma. Drugs. 2002;62:1131-1141.
Mild cough • wheezing • loud heart sounds • Dx?
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.
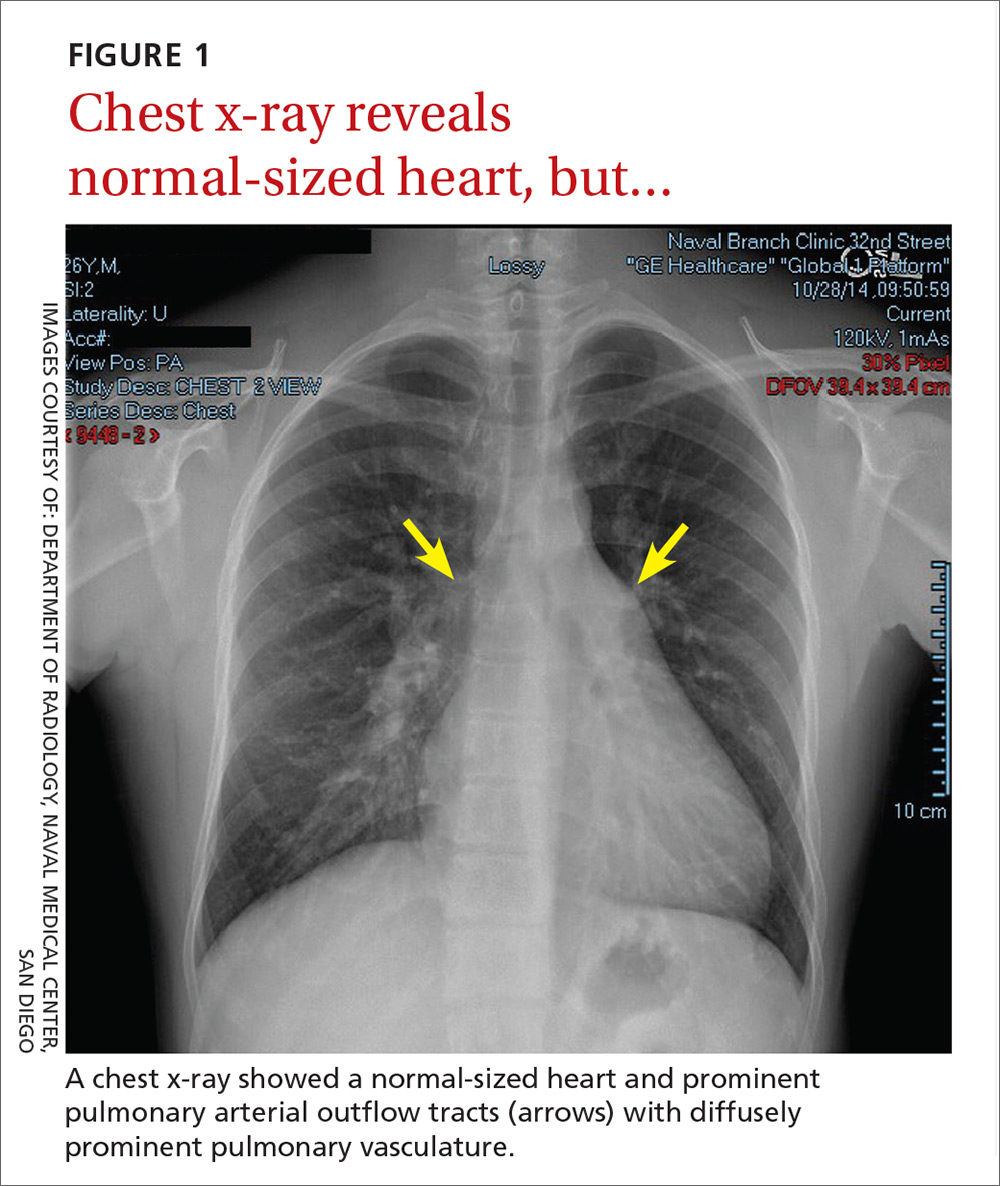
In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.
THE DIAGNOSIS
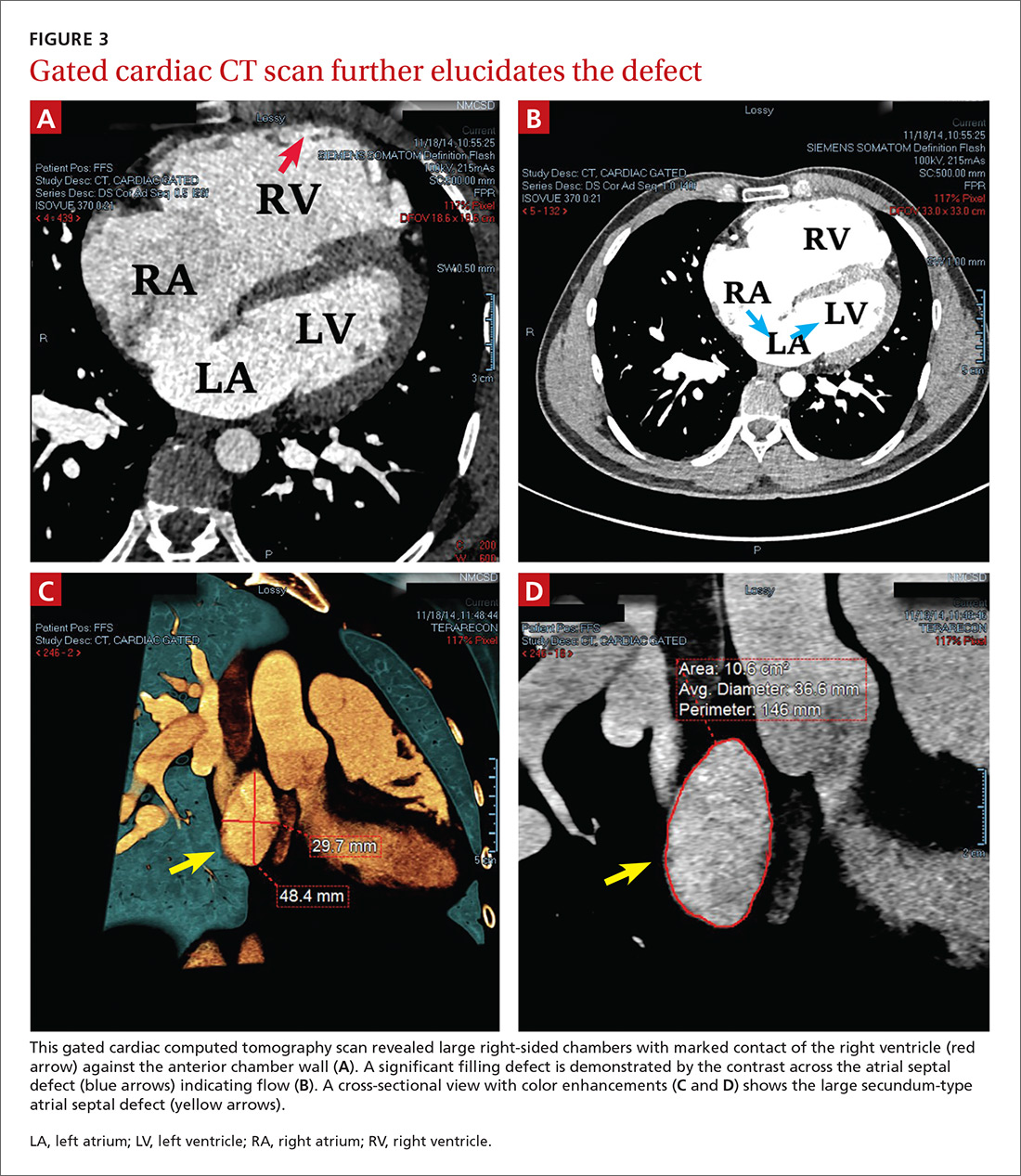
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.
In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.

THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
THE CASE
A 25-year-old man, who was an active duty US Navy sailor, went to his ship’s medical department complaining of a mild cough that he’d had for 2 days. He denied having any fevers, chills, night sweats, angina, or dyspnea. He said he hadn’t experienced any exertional fatigue or difficulty completing the rigorous physical tasks of his occupation as an engineman on the ship. The patient had no medical or surgical history of significance, and he wasn’t taking any medications or supplements.
On exam, he was not in acute distress and his vital signs were within normal limits. Auscultation revealed mild wheezing throughout the upper lung fields and loud heart sounds throughout his chest that were audible even with gentle contact of the stethoscope diaphragm. He had no discernible murmurs, rubs, or gallops.
In light of the unusually loud heart sounds heard on exam, we performed an electrocardiogram. The EKG revealed a normal sinus rhythm, slight right axis deviation indicated by tall R-waves in V1 (also suggestive of right ventricular hypertrophy), an incomplete right bundle branch block, and a crochetage sign (a notch in the R-waves of the inferior leads).1 A chest x-ray (FIGURE 1) revealed a normal-sized heart and dilated pulmonary vasculature suggestive of pulmonary hypertension.

THE DIAGNOSIS
To further evaluate the cardiopulmonary findings, ultrasound studies (transthoracic and transesophageal echocardiography) were performed. These demonstrated a very large secundum-type atrial septal defect (ASD), measuring at its largest point about 30 × 48 mm (FIGURE 2 and FIGURE 3C). Doppler flow analysis and a bubble study (VIDEOS 1 and 2) demonstrated significant shunting across the ASD. Gated cardiac computed tomography (CT) was also used to characterize the ASD (FIGURE 3). It revealed that the superior and posterior rims of the ASD were essentially absent and that the right atrium and ventricle were severely enlarged, while the left chambers were normal in size and function with an ejection fraction >55%. The notching of the R-waves of the inferior leads, seen in our patient’s EKG, is typically seen with large ASDs.1,2
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with color Doppler flow (red) demonstrated significant shunting across a large atrial septal defect (white box). The largest white dot is positioned near the center of the defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Transthoracic echocardiography with a bubble study showed injected air bubbles traversing the atrial septal defect.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
DISCUSSION
ASDs are typically uncovered on exam via auscultation of heart sounds, which might reveal a split of the second heart sound (S2) and diastolic murmurs. ASDs are typically classified by size, and their management depends on this factor, along with the patient’s age and symptoms. In children with small defects (<6 mm), treatment usually consists of conservative observation, as more than half of these ASDs will spontaneously close.3 But, as children age, they are more likely to engage in exertional activity (work, recreational sports) and an unrepaired ASD may yield symptoms (angina, dyspnea, fatigue, other cardiopulmonary strain). With such symptoms and when closure is not spontaneously achieved by adolescence or adulthood, an invasive approach is often necessary to correct the defect.
ASD repair. Traditionally, repair has involved some form of open thoracotomy. More recently, several minimally invasive techniques have been developed. Catheter-based device closure, in which a catheter is percutaneously guided to the defect and a patch is deployed to seal the ASD, is a technique that has been shown to successfully correct large ASDs of up to 40 mm in size.4 Robotic procedures have also been developed to correct ASDs through much smaller incisions.5 Both of these techniques require a significant rim of residual septal tissue around the defect.
Individualized approach. Since our patient had a rather large ASD that did not have sufficient residual septal rim tissue, percutaneous and robotic approaches were not feasible. Instead, he required more invasive cardiothoracic surgery. In cases such as this, the exact technique and type of incision (sternotomy vs access through the lateral chest wall) depend on age, gender, and the presence of other comorbidities.6
Our patient. Because there was concern that any approach other than a median one might not afford enough space to fix an ASD of such considerable size, our patient underwent a median sternotomy by a pediatric cardiothoracic surgeon who specialized in these repairs (in children as well as young adults). During the procedure, the ASD was accessed and confirmed to be as large as predicted by diagnostic imaging. A surgical patch was sutured in place to correct the defect. There were no intra-operative or postop complications.
Four weeks later, the patient had a mild pericardial effusion that was managed medically with daily furosemide and aspirin. At his 8-week postop appointment, the fluid accumulation had resolved, and he was completely asymptomatic. The patient returned to full-time active duty in the US Navy.
Adults with rather large ASDs can present in a relatively asymptomatic manner and report none of the classic complaints (angina, dyspnea, fatigue). They may even engage in heavy exertional activity with no difficulty. The underlying defect may be discovered incidentally on exam by noting a split of the S2 on auscultation. If pulmonary hypertension exists, the clinician may also note a loud S2. An exam that raises suspicion for an ASD can then be followed by tests that solidify the diagnosis. Surgery is usually necessary to correct an ASD in an adult who is symptomatic or exhibits significant cardiopulmonary strain.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
1. Heller J, Hagège AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: a new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol. 1996;27:877-882.
2. Kuijpers JM, Mulder BJM, Bouma BJ. Secundum atrial septal defect in adults: a practical review and recent developments. Neth Heart J. 2015;23:205-211.
3. McMahon CJ, Feltes TF, Fraley JK, et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart. 2002;87:256-259.
4. Lopez K, Dalvi BV, Balzer D, et al. Transcatheter closure of large secundum atrial septal defects using the 40 mm amplatzer septal occluder: results of an international registry. Catheter Cardiovasc Interv. 2005;66:580-584.
5. Argenziano M, Oz MC, Kohmoto T, et al. Totally endoscopic atrial septal defect repair with robotic assistance. Circulation. 2003;108 Suppl 1:II191-II194.
6. Hopkins RA, Bert AA, Buchholz B, et al. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144-2149.
Elevated serum alkaline phosphatase • generalized pruritus • Dx?
THE CASE
A 34-year-old woman was referred to the hepatology clinic for evaluation of an increased serum alkaline phosphatase (ALP) level. She was gravida 5 and in her 38th week of gestation. Her obstetric history was significant for 2 uncomplicated spontaneous term vaginal deliveries resulting in live births and 2 spontaneous abortions. The patient reported generalized pruritus for 2 months prior to the visit. She had no comorbidities and denied any other symptoms. She reported no family history of liver disease or complications during pregnancy in relatives. The patient did not smoke or drink, and had come to our hospital for her prenatal care visits.
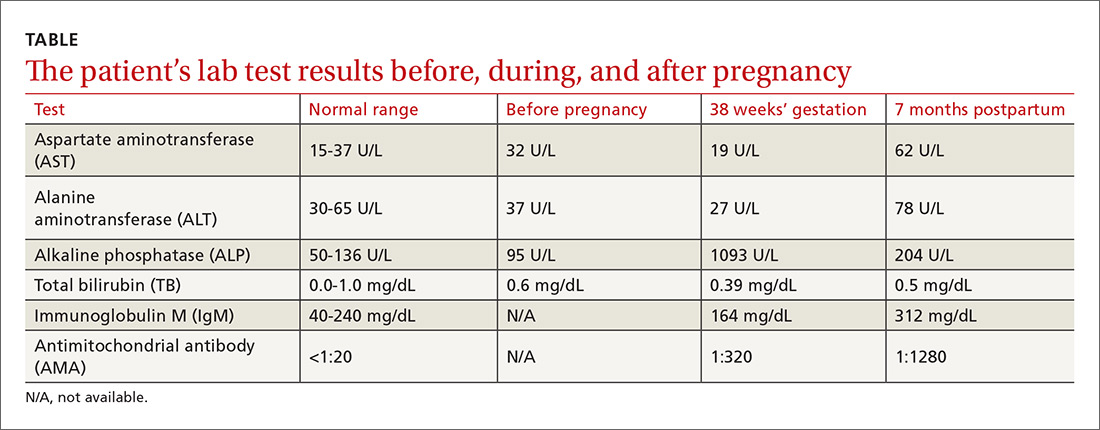
The physical exam revealed normal vital signs, no jaundice, a gravid uterus, and acanthosis nigricans on the neck and axilla with scattered excoriations on the arms, legs, and abdomen. Her serum ALP level was 1093 U/L (normal: 50-136 U/L). Immediately before this pregnancy, her serum ALP had been normal at 95 U/L, but it had since been increasing with a peak value of 1134 U/L by 37 weeks’ gestation. Serum transaminase activities and albumin and bilirubin concentrations were normal, as was her prothrombin time. The rest of her lab tests were also normal, including her fasting serum bile acid concentration, which was 9 mcmol/L (normal: 4.5-19.2 mcmol/L).
THE DIAGNOSIS
Although cholestasis of pregnancy was considered, the patient’s markedly elevated serum ALP level suggested the presence of another cholestatic liver disease. Additional tests revealed an antimitochondrial antibody (AMA) titer of 1:320 (normal: <1:20) and immunoglobulin A, G, and M levels within normal limits. Accordingly, we diagnosed primary biliary cholangitis (PBC).
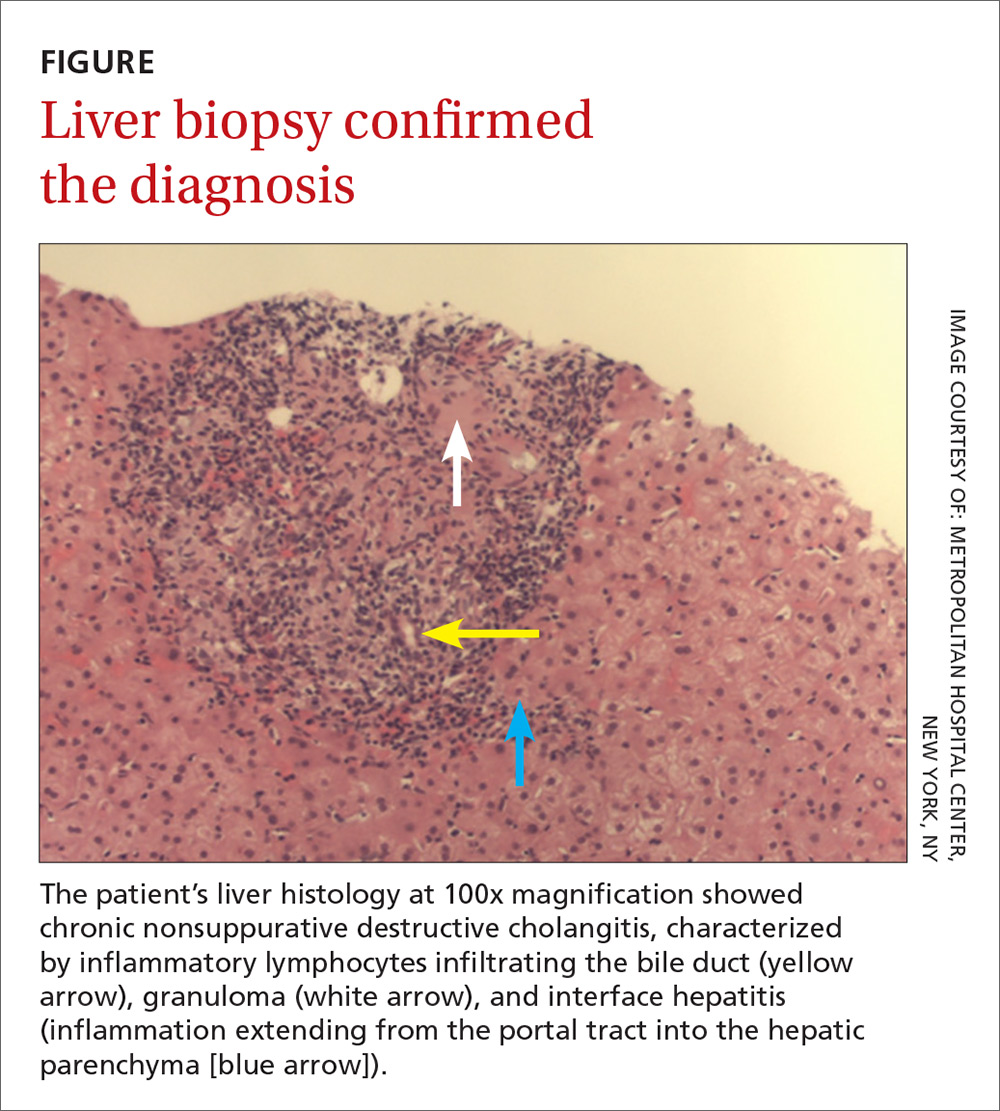
The patient delivered vaginally at another institution uneventfully and returned to the hepatology clinic 7 months postpartum. Repeat laboratory tests (TABLE) revealed increased AMA titer and immunoglobulin M levels from baseline (38 weeks’ gestation). The physical exam was notable for the absence of both jaundice and stigmata of chronic liver disease. A liver ultrasound was normal. The patient still reported pruritus, as well as a new symptom—fatigue. A liver biopsy was performed, and findings were consistent with PBC, stage 1 (FIGURE).
DISCUSSION
PBC, historically known as primary biliary cirrhosis, is a chronic, likely immune-mediated, cholestatic liver disease characterized by the progressive inflammatory destruction of intrahepatic bile ducts. The disease has a female to male predominance of 10:1, with age of diagnosis most often between 40 and 50 years, although about a quarter of female patients present during their reproductive years.1,2
PBC in pregnant women
During pregnancy, the profound physiologic changes and adaptations in the endocrine, metabolic, and immune systems that are necessary for normal fetal development can affect the maternal hepatobiliary system. In patients with prior autoimmune liver disease, the liver is known to adapt itself to these physiologic changes by entering a state of immune tolerance. This is induced by relative hypercortisolism, a shift from predominantly cell-mediated immunity to humoral immunity, and inhibition of T-cell activation. These changes can result in remission of autoimmune disease activity during pregnancy and postpartum flaring when these protective mechanisms are lost (although neither remission nor postpartum flaring occurred in this patient’s case).1-3
While a well-compensated state is associated with better fetal and maternal outcomes than a decompensated condition, cirrhosis is not a contraindication to pregnancy. Vaginal delivery is generally safe for patients with PBC, and studies have reported no childbirth complications or adverse maternal outcomes.1,3,4
The approved treatment for PBC, ursodeoxycholic acid (UDCA), was classified as a category B agent according to the Food and Drug Administration’s now defunct classification system for drugs used during pregnancy and lactation. It’s considered to be the treatment of choice for intrahepatic cholestasis of pregnancy, but there are no recommendations for its use in pregnant patients with PBC. Several studies have observed no significant teratogenic effect in babies whose mothers were treated with UDCA for PBC during pregnancy.1-4 Postpartum, 60% to 70% of PBC patients have been reported to exhibit biochemical disease activity,1,3 and in one case, a liver transplant was required due to liver failure.5
Look for AMA, elevated ALP
The diagnosis of the disease in this case was made by the detection of AMA, which has a specificity of 98% for PBC. However, isolated instances of the presence of AMA are not uncommon; they have been documented in up to 64% of healthy individuals.6 In addition, while one would expect to see a 2- to 4-fold rise in ALP levels during pregnancy (due to placental isoenzyme production),2,7 our patient’s serum ALP level was much higher, suggesting probable cholestatic liver disease such as PBC. The diagnosis in this case was confirmed by liver biopsy.
Our patient was started on UDCA 13 to 15 mg/kg/d. She remained clinically stable at subsequent follow-ups.
THE TAKEAWAY
Typically seen in middle-aged women, PBC can be detected by the presence of AMA and elevated ALP levels. Pregnant patients with chronic liver disease, including PBC, should be followed by a hepatologist and a high-risk obstetrician. They should be carefully monitored and frequently reassessed throughout the pregnancy, delivery, and postpartum period, even though studies have documented favorable outcomes for both mother and baby.1,3,4
1. Trivedi PJ, Kumagi T, Al-Harthy N, et al. Good maternal and fetal outcomes for pregnant women with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1179-1185.
2. Marchioni Beery RM, Vaziri H, Forouhar F. Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women’s health perspective. J Clin Transl Hepatol. 2014;2:266-284.
3. Efe C, Kahramanoğlu-Aksoy E, Yilmaz B, et al. Pregnancy in women with primary biliary cirrhosis. Autoimmun Rev. 2014;13:931-935.
4. Floreani A, Infantolino C, Franceschet I, et al. Pregnancy and primary biliary cirrhosis: a case control study. Clin Rev Allergy Immunol. 2015;48:236-242.
5. Rabinovitz M, Appasamy R, Finkelstein S. Primary biliary cirrhosis diagnosed during pregnancy. Does it have a different outcome? Dig Dis Sci. 1995;40:571-574.
6. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575.
7. The Johns Hopkins School of Medicine Department of Gynecology. Hurt KJ, Guile MW, Bienstock JL, et al, eds. The Johns Hopkins Manual of Gynecology and Obstetrics. 4th edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2011.
THE CASE
A 34-year-old woman was referred to the hepatology clinic for evaluation of an increased serum alkaline phosphatase (ALP) level. She was gravida 5 and in her 38th week of gestation. Her obstetric history was significant for 2 uncomplicated spontaneous term vaginal deliveries resulting in live births and 2 spontaneous abortions. The patient reported generalized pruritus for 2 months prior to the visit. She had no comorbidities and denied any other symptoms. She reported no family history of liver disease or complications during pregnancy in relatives. The patient did not smoke or drink, and had come to our hospital for her prenatal care visits.
The physical exam revealed normal vital signs, no jaundice, a gravid uterus, and acanthosis nigricans on the neck and axilla with scattered excoriations on the arms, legs, and abdomen. Her serum ALP level was 1093 U/L (normal: 50-136 U/L). Immediately before this pregnancy, her serum ALP had been normal at 95 U/L, but it had since been increasing with a peak value of 1134 U/L by 37 weeks’ gestation. Serum transaminase activities and albumin and bilirubin concentrations were normal, as was her prothrombin time. The rest of her lab tests were also normal, including her fasting serum bile acid concentration, which was 9 mcmol/L (normal: 4.5-19.2 mcmol/L).
THE DIAGNOSIS
Although cholestasis of pregnancy was considered, the patient’s markedly elevated serum ALP level suggested the presence of another cholestatic liver disease. Additional tests revealed an antimitochondrial antibody (AMA) titer of 1:320 (normal: <1:20) and immunoglobulin A, G, and M levels within normal limits. Accordingly, we diagnosed primary biliary cholangitis (PBC).
The patient delivered vaginally at another institution uneventfully and returned to the hepatology clinic 7 months postpartum. Repeat laboratory tests (TABLE) revealed increased AMA titer and immunoglobulin M levels from baseline (38 weeks’ gestation). The physical exam was notable for the absence of both jaundice and stigmata of chronic liver disease. A liver ultrasound was normal. The patient still reported pruritus, as well as a new symptom—fatigue. A liver biopsy was performed, and findings were consistent with PBC, stage 1 (FIGURE).
DISCUSSION
PBC, historically known as primary biliary cirrhosis, is a chronic, likely immune-mediated, cholestatic liver disease characterized by the progressive inflammatory destruction of intrahepatic bile ducts. The disease has a female to male predominance of 10:1, with age of diagnosis most often between 40 and 50 years, although about a quarter of female patients present during their reproductive years.1,2
PBC in pregnant women
During pregnancy, the profound physiologic changes and adaptations in the endocrine, metabolic, and immune systems that are necessary for normal fetal development can affect the maternal hepatobiliary system. In patients with prior autoimmune liver disease, the liver is known to adapt itself to these physiologic changes by entering a state of immune tolerance. This is induced by relative hypercortisolism, a shift from predominantly cell-mediated immunity to humoral immunity, and inhibition of T-cell activation. These changes can result in remission of autoimmune disease activity during pregnancy and postpartum flaring when these protective mechanisms are lost (although neither remission nor postpartum flaring occurred in this patient’s case).1-3
While a well-compensated state is associated with better fetal and maternal outcomes than a decompensated condition, cirrhosis is not a contraindication to pregnancy. Vaginal delivery is generally safe for patients with PBC, and studies have reported no childbirth complications or adverse maternal outcomes.1,3,4
The approved treatment for PBC, ursodeoxycholic acid (UDCA), was classified as a category B agent according to the Food and Drug Administration’s now defunct classification system for drugs used during pregnancy and lactation. It’s considered to be the treatment of choice for intrahepatic cholestasis of pregnancy, but there are no recommendations for its use in pregnant patients with PBC. Several studies have observed no significant teratogenic effect in babies whose mothers were treated with UDCA for PBC during pregnancy.1-4 Postpartum, 60% to 70% of PBC patients have been reported to exhibit biochemical disease activity,1,3 and in one case, a liver transplant was required due to liver failure.5
Look for AMA, elevated ALP
The diagnosis of the disease in this case was made by the detection of AMA, which has a specificity of 98% for PBC. However, isolated instances of the presence of AMA are not uncommon; they have been documented in up to 64% of healthy individuals.6 In addition, while one would expect to see a 2- to 4-fold rise in ALP levels during pregnancy (due to placental isoenzyme production),2,7 our patient’s serum ALP level was much higher, suggesting probable cholestatic liver disease such as PBC. The diagnosis in this case was confirmed by liver biopsy.
Our patient was started on UDCA 13 to 15 mg/kg/d. She remained clinically stable at subsequent follow-ups.
THE TAKEAWAY
Typically seen in middle-aged women, PBC can be detected by the presence of AMA and elevated ALP levels. Pregnant patients with chronic liver disease, including PBC, should be followed by a hepatologist and a high-risk obstetrician. They should be carefully monitored and frequently reassessed throughout the pregnancy, delivery, and postpartum period, even though studies have documented favorable outcomes for both mother and baby.1,3,4
THE CASE
A 34-year-old woman was referred to the hepatology clinic for evaluation of an increased serum alkaline phosphatase (ALP) level. She was gravida 5 and in her 38th week of gestation. Her obstetric history was significant for 2 uncomplicated spontaneous term vaginal deliveries resulting in live births and 2 spontaneous abortions. The patient reported generalized pruritus for 2 months prior to the visit. She had no comorbidities and denied any other symptoms. She reported no family history of liver disease or complications during pregnancy in relatives. The patient did not smoke or drink, and had come to our hospital for her prenatal care visits.
The physical exam revealed normal vital signs, no jaundice, a gravid uterus, and acanthosis nigricans on the neck and axilla with scattered excoriations on the arms, legs, and abdomen. Her serum ALP level was 1093 U/L (normal: 50-136 U/L). Immediately before this pregnancy, her serum ALP had been normal at 95 U/L, but it had since been increasing with a peak value of 1134 U/L by 37 weeks’ gestation. Serum transaminase activities and albumin and bilirubin concentrations were normal, as was her prothrombin time. The rest of her lab tests were also normal, including her fasting serum bile acid concentration, which was 9 mcmol/L (normal: 4.5-19.2 mcmol/L).
THE DIAGNOSIS
Although cholestasis of pregnancy was considered, the patient’s markedly elevated serum ALP level suggested the presence of another cholestatic liver disease. Additional tests revealed an antimitochondrial antibody (AMA) titer of 1:320 (normal: <1:20) and immunoglobulin A, G, and M levels within normal limits. Accordingly, we diagnosed primary biliary cholangitis (PBC).
The patient delivered vaginally at another institution uneventfully and returned to the hepatology clinic 7 months postpartum. Repeat laboratory tests (TABLE) revealed increased AMA titer and immunoglobulin M levels from baseline (38 weeks’ gestation). The physical exam was notable for the absence of both jaundice and stigmata of chronic liver disease. A liver ultrasound was normal. The patient still reported pruritus, as well as a new symptom—fatigue. A liver biopsy was performed, and findings were consistent with PBC, stage 1 (FIGURE).
DISCUSSION
PBC, historically known as primary biliary cirrhosis, is a chronic, likely immune-mediated, cholestatic liver disease characterized by the progressive inflammatory destruction of intrahepatic bile ducts. The disease has a female to male predominance of 10:1, with age of diagnosis most often between 40 and 50 years, although about a quarter of female patients present during their reproductive years.1,2
PBC in pregnant women
During pregnancy, the profound physiologic changes and adaptations in the endocrine, metabolic, and immune systems that are necessary for normal fetal development can affect the maternal hepatobiliary system. In patients with prior autoimmune liver disease, the liver is known to adapt itself to these physiologic changes by entering a state of immune tolerance. This is induced by relative hypercortisolism, a shift from predominantly cell-mediated immunity to humoral immunity, and inhibition of T-cell activation. These changes can result in remission of autoimmune disease activity during pregnancy and postpartum flaring when these protective mechanisms are lost (although neither remission nor postpartum flaring occurred in this patient’s case).1-3
While a well-compensated state is associated with better fetal and maternal outcomes than a decompensated condition, cirrhosis is not a contraindication to pregnancy. Vaginal delivery is generally safe for patients with PBC, and studies have reported no childbirth complications or adverse maternal outcomes.1,3,4
The approved treatment for PBC, ursodeoxycholic acid (UDCA), was classified as a category B agent according to the Food and Drug Administration’s now defunct classification system for drugs used during pregnancy and lactation. It’s considered to be the treatment of choice for intrahepatic cholestasis of pregnancy, but there are no recommendations for its use in pregnant patients with PBC. Several studies have observed no significant teratogenic effect in babies whose mothers were treated with UDCA for PBC during pregnancy.1-4 Postpartum, 60% to 70% of PBC patients have been reported to exhibit biochemical disease activity,1,3 and in one case, a liver transplant was required due to liver failure.5
Look for AMA, elevated ALP
The diagnosis of the disease in this case was made by the detection of AMA, which has a specificity of 98% for PBC. However, isolated instances of the presence of AMA are not uncommon; they have been documented in up to 64% of healthy individuals.6 In addition, while one would expect to see a 2- to 4-fold rise in ALP levels during pregnancy (due to placental isoenzyme production),2,7 our patient’s serum ALP level was much higher, suggesting probable cholestatic liver disease such as PBC. The diagnosis in this case was confirmed by liver biopsy.
Our patient was started on UDCA 13 to 15 mg/kg/d. She remained clinically stable at subsequent follow-ups.
THE TAKEAWAY
Typically seen in middle-aged women, PBC can be detected by the presence of AMA and elevated ALP levels. Pregnant patients with chronic liver disease, including PBC, should be followed by a hepatologist and a high-risk obstetrician. They should be carefully monitored and frequently reassessed throughout the pregnancy, delivery, and postpartum period, even though studies have documented favorable outcomes for both mother and baby.1,3,4
1. Trivedi PJ, Kumagi T, Al-Harthy N, et al. Good maternal and fetal outcomes for pregnant women with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1179-1185.
2. Marchioni Beery RM, Vaziri H, Forouhar F. Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women’s health perspective. J Clin Transl Hepatol. 2014;2:266-284.
3. Efe C, Kahramanoğlu-Aksoy E, Yilmaz B, et al. Pregnancy in women with primary biliary cirrhosis. Autoimmun Rev. 2014;13:931-935.
4. Floreani A, Infantolino C, Franceschet I, et al. Pregnancy and primary biliary cirrhosis: a case control study. Clin Rev Allergy Immunol. 2015;48:236-242.
5. Rabinovitz M, Appasamy R, Finkelstein S. Primary biliary cirrhosis diagnosed during pregnancy. Does it have a different outcome? Dig Dis Sci. 1995;40:571-574.
6. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575.
7. The Johns Hopkins School of Medicine Department of Gynecology. Hurt KJ, Guile MW, Bienstock JL, et al, eds. The Johns Hopkins Manual of Gynecology and Obstetrics. 4th edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2011.
1. Trivedi PJ, Kumagi T, Al-Harthy N, et al. Good maternal and fetal outcomes for pregnant women with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1179-1185.
2. Marchioni Beery RM, Vaziri H, Forouhar F. Primary biliary cirrhosis and primary sclerosing cholangitis: a review featuring a women’s health perspective. J Clin Transl Hepatol. 2014;2:266-284.
3. Efe C, Kahramanoğlu-Aksoy E, Yilmaz B, et al. Pregnancy in women with primary biliary cirrhosis. Autoimmun Rev. 2014;13:931-935.
4. Floreani A, Infantolino C, Franceschet I, et al. Pregnancy and primary biliary cirrhosis: a case control study. Clin Rev Allergy Immunol. 2015;48:236-242.
5. Rabinovitz M, Appasamy R, Finkelstein S. Primary biliary cirrhosis diagnosed during pregnancy. Does it have a different outcome? Dig Dis Sci. 1995;40:571-574.
6. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386:1565-1575.
7. The Johns Hopkins School of Medicine Department of Gynecology. Hurt KJ, Guile MW, Bienstock JL, et al, eds. The Johns Hopkins Manual of Gynecology and Obstetrics. 4th edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2011.
Inpatient antibiotic resistance: Everyone’s problem
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
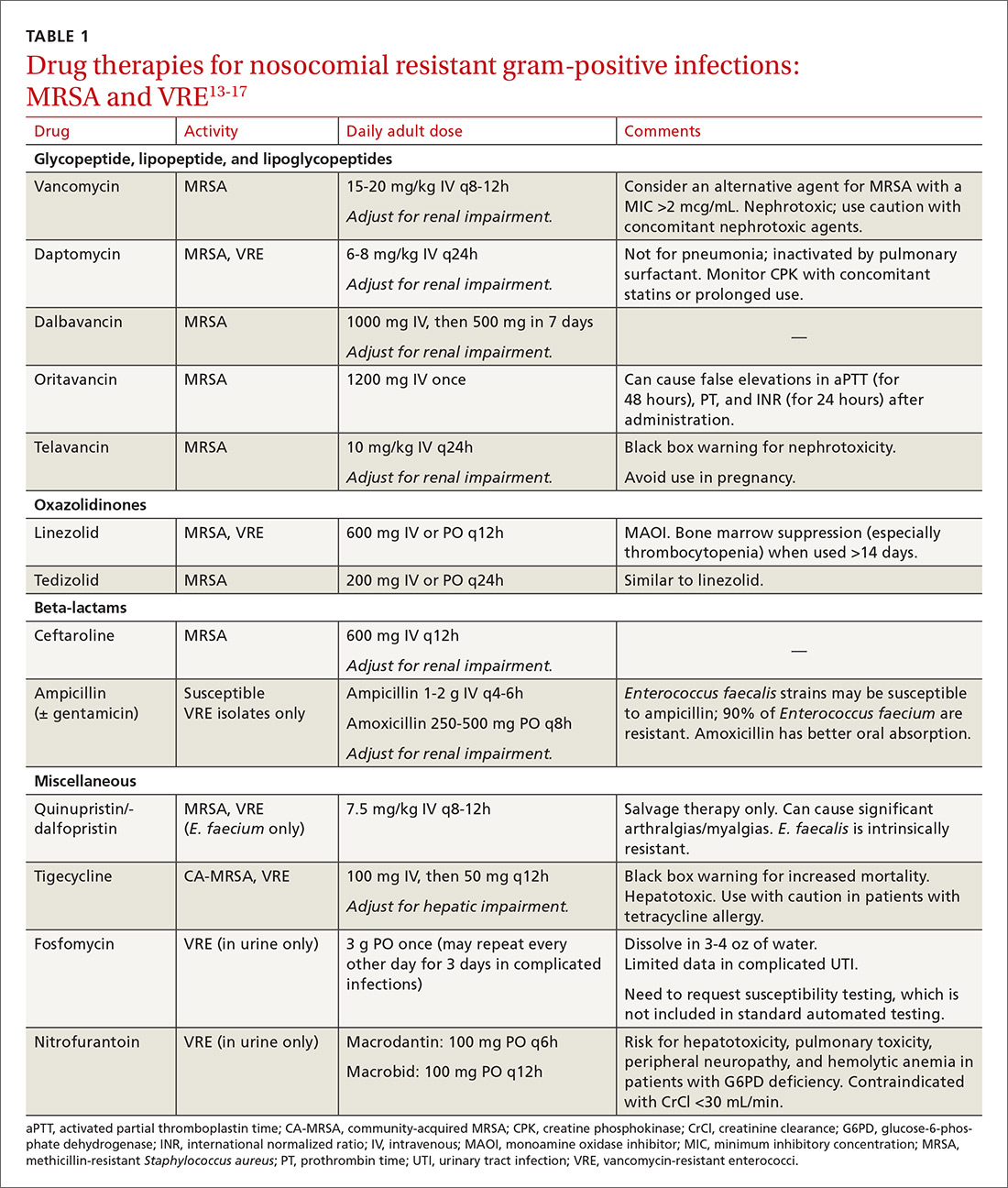
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17).
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting.
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17).
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting.
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17).
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting.
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
From The Journal of Family Practice | 2018;67(2):E1-E11.
PRACTICE RECOMMENDATIONS
› Consider alternatives to vancomycin for health care-associated methicillin-resistant Staphylococcus aureus isolates with a vancomycin minimum inhibitory concentration >2 mcg/mL or in the setting of poor clinical response. A
› Identify colonization vs infection with vancomycin-resistant enterococci (VRE) in the gastrointestinal tract following antibiotic exposure to minimize inappropriate antibiotic prescribing for VRE. C
› Use carbapenems as first-line treatment for severe infections caused by Enterobacteriaceae-producing extended-spectrum beta-lactamases. C
› Treat invasive carbapenem-resistant Enterobacteriaceae infections with combination therapy; site of infection, susceptibility patterns, and patient-specific factors should guide antibiotic selection. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Schizophrenia: Ensuring an accurate Dx, optimizing treatment
THE CASE
Steven R,* a 21-year-old man, visited the clinic accompanied by his mother. He did not speak much, and his mother provided his history. Over the previous 2 months, she had overheard him whispering in an agitated voice, even though no one else was nearby. And, lately, he refused to answer or make calls on his cell phone, claiming that if he did it would activate a deadly chip that had been implanted in his brain by evil aliens. He also stopped attending classes at the community college. He occasionally had a few beers with his friends, but he had never been known to abuse alcohol or use other recreational drugs.
How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
CHARACTERISTICS AND SCOPE OF SCHIZOPHRENIA
Schizophrenia is a psychotic illness in which the individual loses contact with reality and often experiences hallucinations, delusions, or thought disorders. Criteria for schizophrenia described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) include signs and symptoms of at least 6 months’ duration, as well as at least one month of active-phase positive and negative symptoms.1
Delusions, hallucinations, disorganized speech, and disorganized behavior are examples of positive symptoms. Negative symptoms include a decrease in the range and intensity of expressed emotions (ie, affective flattening) and a diminished initiation of goal-directed activities (ie, avolition).
Approximately 7 in 1000 people will develop the disorder in their lifetime.2 Schizophrenia is considered a “serious mental illness” because of its chronic course and often poor long-term social and vocational outcomes.3,4 Symptom onset is generally between late adolescence and the mid-30s.5
Getting closer to understanding its origin
Both genetic susceptibility and environmental factors influence the incidence of schizophrenia.4 Newer models of the disease have identified genes (ZDHHC8 and DTNBP1) whose mutations may increase the risk of schizophrenia.6 Physiologic insults during fetal life—hypoxia, maternal infection, maternal stress, and maternal malnutrition—account for a small portion of schizophrenia cases.6
Abnormalities in neurotransmission are the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid as part of the neurochemical imbalance of schizophrenia.7
ESTABLISHING A DIAGNOSIS
Although psychotic symptoms may be a prominent part of schizophrenia, not all psychoses indicate a primary psychiatric disorder such as schizophrenia. Broadly, psychoses can be categorized as primary or secondary.
Primary psychoses include schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, and mood disorders (major depressive disorder and borderline personality disorder) with psychotic features.1 Difficulty in distinguishing between these entities can necessitate referral to a psychiatrist.
Secondary psychoses arise from a precursor such as delirium, dementia, medical illness, or adverse effects of medications or illicit substances. Medical illnesses that cause psychotic symptoms include: 5,8
- seizures (especially temporal lobe epilepsy),
- cerebrovascular accidents,
- intracranial space-occupying lesions,
- neuropsychiatric disorders (eg, Wilson’s or Parkinson’s disease),
- endocrine disorders (eg, thyroid or adrenal disease),
- autoimmune disease (eg, systemic lupus erythematosus, Hashimoto encephalopathy),
- deficiencies of vitamins A, B1, B12, or niacin,
- infections (eg, human immunodeficiency virus [HIV], encephalitis, parasites, and prion disease),
- narcolepsy, and
- metabolic disease (eg, acute intermittent porphyria, Tay-Sach’s disease, Niemann-Pick disease).
Several recreational drugs can cause psychotic symptoms: cocaine, amphetamines, cannabis, synthetic cannabinoids, inhalants, opioids, and hallucinogens. Psychotic symptoms can also appear during withdrawal from alcohol (delirium tremens) and from sedative hypnotics such as benzodiazepines. Prescribed medications such as anticholinergics, corticosteroids, dopaminergic agents (L-dopa), stimulants (amphetamines), and interferons can also induce psychotic symptoms.
First rule out causes of secondary psychosis
Rule out causes of secondary psychosis by conducting a detailed history and physical examination and ordering appropriate lab tests and imaging studies. If the patient’s psychosis is of recent onset, make sure the laboratory work-up includes
Consider cranial computed tomography or magnetic resonance imaging if there are focal neurologic deficits or if the patient’s presentation is atypical (eg, new onset psychosis in old age).9 Clinical presentation may also indicate a need for electroencephalography, ceruloplasmin measurement, a dexamethasone suppression test, a corticotropin stimulation test, 24-hour urine porphyrin and copper assays, chest radiography, or cerebrospinal fluid analysis.9
FACTORS TO CONSIDER IN TREATMENT DECISIONS
Although primary care physicians may encounter individuals experiencing their first episode of psychosis, it’s more likely that patients presenting with signs and symptoms of the disorder have been experiencing them for some time and have received no psychiatric care. In both instances, schizophrenia is best managed in conjunction with a psychiatrist until symptoms are stabilized.5 Psychosis does not always require hospitalization. But urgent psychiatry referral is recommended, if possible. Consider admission to a psychiatric inpatient unit for anyone who poses a danger to self or others.8,10
Treatment for schizophrenia is most effective with an interprofessional and collaborative approach that includes medication, psychological treatment, social supports, and primary care clinical management.11,12 The last aspect takes on particular importance given that people with schizophrenia, compared with the general population, have a higher incidence of medical illness, particularly cardiovascular disease.13
Medications (TABLE 15,8) are grouped into first-generation antipsychotics (FGAs) and second-generation, or atypical, antipsychotics (SGAs), with the 2 classes being equally effective.14-16 Quality of life is also similar at one year for patients treated with either drug class.14
Adverse effects can differ. The main difference between these medications is their adverse effect profiles. FGAs cause extrapyramidal symptoms (dystonia, akathisia, and tardive dyskinesia) more often than SGAs. Among the SGAs, olanzapine, asenapine, paliperidone, clozapine, and quetiapine cause significant weight gain, glucose dysregulation, and lipid abnormalities.5,8,12,17 Clozapine is associated with agranulocytosis, as well. Risperidone causes mild to moderate weight gain.5,8,12,17 Aripiprazole, lurasidone, and ziprasidone are considered weight neutral and cause no significant glucose dysregulation or lipid abnormalities.5,8,12,17 All antipsychotics can cause QT prolongation and neuroleptic malignant syndrome.5,8,12,17
Keys to successful treatment. Antipsychotics are most effective in treating positive symptoms of schizophrenia and show limited, if any, effect on negative or cognitive symptoms.18,19 Give patients an adequate trial of therapy (at least 4 weeks at a therapeutic dose) before discontinuing the drug or offering a different medication.20 All patients who report symptom relief while receiving antipsychotics should receive maintenance therapy.12
As with all chronic illnesses, success in managing schizophrenia requires patient adherence to the medication regimen. Discontinuation of antipsychotics is a common problem in schizophrenia, resulting in relapse. Long-acting injectable agents (LAIs) were developed to address this problem (TABLE 2).21 Although LAIs are typically used to ensure adherence during maintenance treatment, recent research has suggested they may also be effective for patients with early-phase or first-episode disease.22
What to watch for. Patients on SGAs may develop metabolic abnormalities, and ongoing monitoring of relevant parameters is key (TABLE 323-27). More frequent monitoring may be necessary in patients with cardiovascular risk factors. Continue antipsychotics for at least 6 months to prevent relapse.12 Also keep in mind the “Choosing Wisely” recommendation from the American Psychiatric Association of not prescribing 2 or more antipsychotics concurrently.28
Adjunctive treatment should also be offered
In addition to receiving medication, patients with schizophrenia should be offered adjunctive therapies such as cognitive behavioral therapy, family intervention, and social skills training.10-12 Among patients with schizophrenia, the incidences of anxiety disorder, panic symptoms, posttraumatic stress disorder, and obsessive compulsive disorder are higher than in the general population.29 To address these conditions, medications such as selective serotonin reuptake inhibitors and anxiolytics can be used simultaneously with antipsychotic agents.
CLINICAL COURSE AND PROGNOSIS CAN VARY
Schizophrenia can have a variable clinical course that includes remissions and exacerbations, or it can follow a more persistently chronic course.
Mortality for patients with schizophrenia is 2 to 3 times higher than that of the general population.30 Most deaths are due to an increased incidence of cardiovascular disease, respiratory illness, cancer, stroke, and other thromboembolic events.30
The lifetime prevalence of suicide attempts among individuals with schizophrenia is 20% to 40%,31 and approximately 5% complete suicide.32 Risk factors include command hallucinations, a history of suicide attempts, intoxication with substances, anxiety, and physical pain.32 Clozapine has been shown to reduce suicide risk and may be considered for patients who are at high risk for suicide.32
Therapeutic response varies among patients with schizophrenia, with one-third remaining symptomatic despite adequate treatment regimens.4
CARE MANAGERS CAN HELP ADDRESS BARRIERS TO CARE
A review of the literature suggests that up to one-third of individuals with serious mental illnesses who have had some contact with the mental health system disengage from care.12 Poor engagement may lead to worse clinical outcomes, with symptom relapse and re-hospitalizations. Disengagement from treatment may indicate a patient’s belief that treatment is not necessary, is not meeting his or her needs, or is not being provided in a collaborative manner.
Although shared decision-making is difficult with patients who have schizophrenia, emerging evidence suggests that this approach coupled with patient-centered care will improve engagement with mental health treatment.12 Models of integrated care are being developed and have shown promise in ensuring access to behavioral health for these patients.34
CASE
The primary care physician talked with Mr. R and his mother about the diagnosis of schizophrenia. He screened for suicide risk, and the patient denied having suicidal thoughts. Both the patient and his mother agreed to his starting medication.
Blood and urine samples were collected for a CBC and ESR, as well as to evaluate renal function, electrolytes, glucose, TSH, vitamin B12, folic acid, ANAs, and HIV antibodies. A serum FTA-ABS test was done, as was a urine culture and sensitivity test and a toxicology screen. Because of the patient’s obesity, the physician decided to prescribe a weight-neutral SGA, aripiprazole 10 mg/d. The physician spoke with the clinic’s care coordinator to schedule an appointment with the psychiatry intake department and to follow up on the phone with the patient and his mother. He also scheduled a follow-up appointment for 2 weeks later.
At the follow-up visit, the patient showed no improvement. His blood and urine test results revealed no metabolic abnormalities or infectious or inflammatory illnesses. His urine toxicology result showed no illicit substances. The physician increased the dosage of aripiprazole to 15 mg/d and asked the patient to return in 2 weeks.
At the next follow-up visit, the patient was more verbal and said he was not hearing voices. His mother also acknowledged an improvement. He had already been scheduled for a psychiatry intake appointment, and he and his mother were reminded about this. Mr. R was also asked to make a follow-up primary care appointment for one month from the current visit.
CORRESPONDENCE
Rajesh (Fnu) Rajesh, MD, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
2. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76.
3. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term and clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716-728.
4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645.
5. Holder SD, Wayhs A. Schizophrenia. Am Fam Phys. 2014;90:775-82.
6. Lakhan SE, Vieira KF. Schizophrenia pathophysiology: are we any closer to a complete model? Ann Gen Psychiatry. 2009;8:12.
7. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014:1019-1046.
8. Viron M, Baggett T, Hill M, et al. Schizophrenia for primary care providers: how to contribute to the care of a vulnerable patient population. Am J Med. 2012;125:223-230.
9. Freudenreich O, Charles Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3:10-18.
10. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: Prevention and management. 2014. Available at: http://www.nice.org.uk/Guidance/CG178. Accessed January 3, 2017.
11. Guo X, Zhai J, Liu Z, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized 1-year study. Arch Gen Psychiatry. 2010;67:895-904.
12. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36:94-103.
13. Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458-465.
14. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
15. Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498-511.
16. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
17. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
18. Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837-855.
19. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892-899.
20. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751-1762.
21. Bera R. Patient outcomes within schizophrenia treatment: a look at the role of long-acting injectable antipsychotics. J Clin Psychiatry. 2014;75(suppl 2):30-33.
22. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
23. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
24. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105.
25. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306-318.
26. Lieberman JA, Merrill D, Parameswaran S. APA guidance on the use of antipsychotic drugs and cardiac sudden death.
27. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
28. American Psychiatric Association. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed February 28, 2017.
29. Buckley PF, Miller BJ, Lehrer DS, et al. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402.
30. Lwin AM, Symeon C, Jan F, et al. Morbidity and mortality in schizophrenia. Br J Hosp Med (Lond). 2011;72:628-630.
31. Pompili M, Amador XF, Girardi P, et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10.
32. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81-90.
33. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151-159.
34. Gerrity M. Evolving models of behavioral health integration: Evidence update 2010-2015. Milbank Memorial Fund. Available at: https://www.milbank.org/wp-content/uploads/2016/05/Evolv ing-Models-of-BHI.pdf. Accessed January 11, 2018.
THE CASE
Steven R,* a 21-year-old man, visited the clinic accompanied by his mother. He did not speak much, and his mother provided his history. Over the previous 2 months, she had overheard him whispering in an agitated voice, even though no one else was nearby. And, lately, he refused to answer or make calls on his cell phone, claiming that if he did it would activate a deadly chip that had been implanted in his brain by evil aliens. He also stopped attending classes at the community college. He occasionally had a few beers with his friends, but he had never been known to abuse alcohol or use other recreational drugs.
How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
CHARACTERISTICS AND SCOPE OF SCHIZOPHRENIA
Schizophrenia is a psychotic illness in which the individual loses contact with reality and often experiences hallucinations, delusions, or thought disorders. Criteria for schizophrenia described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) include signs and symptoms of at least 6 months’ duration, as well as at least one month of active-phase positive and negative symptoms.1
Delusions, hallucinations, disorganized speech, and disorganized behavior are examples of positive symptoms. Negative symptoms include a decrease in the range and intensity of expressed emotions (ie, affective flattening) and a diminished initiation of goal-directed activities (ie, avolition).
Approximately 7 in 1000 people will develop the disorder in their lifetime.2 Schizophrenia is considered a “serious mental illness” because of its chronic course and often poor long-term social and vocational outcomes.3,4 Symptom onset is generally between late adolescence and the mid-30s.5
Getting closer to understanding its origin
Both genetic susceptibility and environmental factors influence the incidence of schizophrenia.4 Newer models of the disease have identified genes (ZDHHC8 and DTNBP1) whose mutations may increase the risk of schizophrenia.6 Physiologic insults during fetal life—hypoxia, maternal infection, maternal stress, and maternal malnutrition—account for a small portion of schizophrenia cases.6
Abnormalities in neurotransmission are the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid as part of the neurochemical imbalance of schizophrenia.7
ESTABLISHING A DIAGNOSIS
Although psychotic symptoms may be a prominent part of schizophrenia, not all psychoses indicate a primary psychiatric disorder such as schizophrenia. Broadly, psychoses can be categorized as primary or secondary.
Primary psychoses include schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, and mood disorders (major depressive disorder and borderline personality disorder) with psychotic features.1 Difficulty in distinguishing between these entities can necessitate referral to a psychiatrist.
Secondary psychoses arise from a precursor such as delirium, dementia, medical illness, or adverse effects of medications or illicit substances. Medical illnesses that cause psychotic symptoms include: 5,8
- seizures (especially temporal lobe epilepsy),
- cerebrovascular accidents,
- intracranial space-occupying lesions,
- neuropsychiatric disorders (eg, Wilson’s or Parkinson’s disease),
- endocrine disorders (eg, thyroid or adrenal disease),
- autoimmune disease (eg, systemic lupus erythematosus, Hashimoto encephalopathy),
- deficiencies of vitamins A, B1, B12, or niacin,
- infections (eg, human immunodeficiency virus [HIV], encephalitis, parasites, and prion disease),
- narcolepsy, and
- metabolic disease (eg, acute intermittent porphyria, Tay-Sach’s disease, Niemann-Pick disease).
Several recreational drugs can cause psychotic symptoms: cocaine, amphetamines, cannabis, synthetic cannabinoids, inhalants, opioids, and hallucinogens. Psychotic symptoms can also appear during withdrawal from alcohol (delirium tremens) and from sedative hypnotics such as benzodiazepines. Prescribed medications such as anticholinergics, corticosteroids, dopaminergic agents (L-dopa), stimulants (amphetamines), and interferons can also induce psychotic symptoms.
First rule out causes of secondary psychosis
Rule out causes of secondary psychosis by conducting a detailed history and physical examination and ordering appropriate lab tests and imaging studies. If the patient’s psychosis is of recent onset, make sure the laboratory work-up includes
Consider cranial computed tomography or magnetic resonance imaging if there are focal neurologic deficits or if the patient’s presentation is atypical (eg, new onset psychosis in old age).9 Clinical presentation may also indicate a need for electroencephalography, ceruloplasmin measurement, a dexamethasone suppression test, a corticotropin stimulation test, 24-hour urine porphyrin and copper assays, chest radiography, or cerebrospinal fluid analysis.9
FACTORS TO CONSIDER IN TREATMENT DECISIONS
Although primary care physicians may encounter individuals experiencing their first episode of psychosis, it’s more likely that patients presenting with signs and symptoms of the disorder have been experiencing them for some time and have received no psychiatric care. In both instances, schizophrenia is best managed in conjunction with a psychiatrist until symptoms are stabilized.5 Psychosis does not always require hospitalization. But urgent psychiatry referral is recommended, if possible. Consider admission to a psychiatric inpatient unit for anyone who poses a danger to self or others.8,10
Treatment for schizophrenia is most effective with an interprofessional and collaborative approach that includes medication, psychological treatment, social supports, and primary care clinical management.11,12 The last aspect takes on particular importance given that people with schizophrenia, compared with the general population, have a higher incidence of medical illness, particularly cardiovascular disease.13
Medications (TABLE 15,8) are grouped into first-generation antipsychotics (FGAs) and second-generation, or atypical, antipsychotics (SGAs), with the 2 classes being equally effective.14-16 Quality of life is also similar at one year for patients treated with either drug class.14
Adverse effects can differ. The main difference between these medications is their adverse effect profiles. FGAs cause extrapyramidal symptoms (dystonia, akathisia, and tardive dyskinesia) more often than SGAs. Among the SGAs, olanzapine, asenapine, paliperidone, clozapine, and quetiapine cause significant weight gain, glucose dysregulation, and lipid abnormalities.5,8,12,17 Clozapine is associated with agranulocytosis, as well. Risperidone causes mild to moderate weight gain.5,8,12,17 Aripiprazole, lurasidone, and ziprasidone are considered weight neutral and cause no significant glucose dysregulation or lipid abnormalities.5,8,12,17 All antipsychotics can cause QT prolongation and neuroleptic malignant syndrome.5,8,12,17
Keys to successful treatment. Antipsychotics are most effective in treating positive symptoms of schizophrenia and show limited, if any, effect on negative or cognitive symptoms.18,19 Give patients an adequate trial of therapy (at least 4 weeks at a therapeutic dose) before discontinuing the drug or offering a different medication.20 All patients who report symptom relief while receiving antipsychotics should receive maintenance therapy.12
As with all chronic illnesses, success in managing schizophrenia requires patient adherence to the medication regimen. Discontinuation of antipsychotics is a common problem in schizophrenia, resulting in relapse. Long-acting injectable agents (LAIs) were developed to address this problem (TABLE 2).21 Although LAIs are typically used to ensure adherence during maintenance treatment, recent research has suggested they may also be effective for patients with early-phase or first-episode disease.22
What to watch for. Patients on SGAs may develop metabolic abnormalities, and ongoing monitoring of relevant parameters is key (TABLE 323-27). More frequent monitoring may be necessary in patients with cardiovascular risk factors. Continue antipsychotics for at least 6 months to prevent relapse.12 Also keep in mind the “Choosing Wisely” recommendation from the American Psychiatric Association of not prescribing 2 or more antipsychotics concurrently.28
Adjunctive treatment should also be offered
In addition to receiving medication, patients with schizophrenia should be offered adjunctive therapies such as cognitive behavioral therapy, family intervention, and social skills training.10-12 Among patients with schizophrenia, the incidences of anxiety disorder, panic symptoms, posttraumatic stress disorder, and obsessive compulsive disorder are higher than in the general population.29 To address these conditions, medications such as selective serotonin reuptake inhibitors and anxiolytics can be used simultaneously with antipsychotic agents.
CLINICAL COURSE AND PROGNOSIS CAN VARY
Schizophrenia can have a variable clinical course that includes remissions and exacerbations, or it can follow a more persistently chronic course.
Mortality for patients with schizophrenia is 2 to 3 times higher than that of the general population.30 Most deaths are due to an increased incidence of cardiovascular disease, respiratory illness, cancer, stroke, and other thromboembolic events.30
The lifetime prevalence of suicide attempts among individuals with schizophrenia is 20% to 40%,31 and approximately 5% complete suicide.32 Risk factors include command hallucinations, a history of suicide attempts, intoxication with substances, anxiety, and physical pain.32 Clozapine has been shown to reduce suicide risk and may be considered for patients who are at high risk for suicide.32
Therapeutic response varies among patients with schizophrenia, with one-third remaining symptomatic despite adequate treatment regimens.4
CARE MANAGERS CAN HELP ADDRESS BARRIERS TO CARE
A review of the literature suggests that up to one-third of individuals with serious mental illnesses who have had some contact with the mental health system disengage from care.12 Poor engagement may lead to worse clinical outcomes, with symptom relapse and re-hospitalizations. Disengagement from treatment may indicate a patient’s belief that treatment is not necessary, is not meeting his or her needs, or is not being provided in a collaborative manner.
Although shared decision-making is difficult with patients who have schizophrenia, emerging evidence suggests that this approach coupled with patient-centered care will improve engagement with mental health treatment.12 Models of integrated care are being developed and have shown promise in ensuring access to behavioral health for these patients.34
CASE
The primary care physician talked with Mr. R and his mother about the diagnosis of schizophrenia. He screened for suicide risk, and the patient denied having suicidal thoughts. Both the patient and his mother agreed to his starting medication.
Blood and urine samples were collected for a CBC and ESR, as well as to evaluate renal function, electrolytes, glucose, TSH, vitamin B12, folic acid, ANAs, and HIV antibodies. A serum FTA-ABS test was done, as was a urine culture and sensitivity test and a toxicology screen. Because of the patient’s obesity, the physician decided to prescribe a weight-neutral SGA, aripiprazole 10 mg/d. The physician spoke with the clinic’s care coordinator to schedule an appointment with the psychiatry intake department and to follow up on the phone with the patient and his mother. He also scheduled a follow-up appointment for 2 weeks later.
At the follow-up visit, the patient showed no improvement. His blood and urine test results revealed no metabolic abnormalities or infectious or inflammatory illnesses. His urine toxicology result showed no illicit substances. The physician increased the dosage of aripiprazole to 15 mg/d and asked the patient to return in 2 weeks.
At the next follow-up visit, the patient was more verbal and said he was not hearing voices. His mother also acknowledged an improvement. He had already been scheduled for a psychiatry intake appointment, and he and his mother were reminded about this. Mr. R was also asked to make a follow-up primary care appointment for one month from the current visit.
CORRESPONDENCE
Rajesh (Fnu) Rajesh, MD, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
Steven R,* a 21-year-old man, visited the clinic accompanied by his mother. He did not speak much, and his mother provided his history. Over the previous 2 months, she had overheard him whispering in an agitated voice, even though no one else was nearby. And, lately, he refused to answer or make calls on his cell phone, claiming that if he did it would activate a deadly chip that had been implanted in his brain by evil aliens. He also stopped attending classes at the community college. He occasionally had a few beers with his friends, but he had never been known to abuse alcohol or use other recreational drugs.
How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
CHARACTERISTICS AND SCOPE OF SCHIZOPHRENIA
Schizophrenia is a psychotic illness in which the individual loses contact with reality and often experiences hallucinations, delusions, or thought disorders. Criteria for schizophrenia described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) include signs and symptoms of at least 6 months’ duration, as well as at least one month of active-phase positive and negative symptoms.1
Delusions, hallucinations, disorganized speech, and disorganized behavior are examples of positive symptoms. Negative symptoms include a decrease in the range and intensity of expressed emotions (ie, affective flattening) and a diminished initiation of goal-directed activities (ie, avolition).
Approximately 7 in 1000 people will develop the disorder in their lifetime.2 Schizophrenia is considered a “serious mental illness” because of its chronic course and often poor long-term social and vocational outcomes.3,4 Symptom onset is generally between late adolescence and the mid-30s.5
Getting closer to understanding its origin
Both genetic susceptibility and environmental factors influence the incidence of schizophrenia.4 Newer models of the disease have identified genes (ZDHHC8 and DTNBP1) whose mutations may increase the risk of schizophrenia.6 Physiologic insults during fetal life—hypoxia, maternal infection, maternal stress, and maternal malnutrition—account for a small portion of schizophrenia cases.6
Abnormalities in neurotransmission are the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid as part of the neurochemical imbalance of schizophrenia.7
ESTABLISHING A DIAGNOSIS
Although psychotic symptoms may be a prominent part of schizophrenia, not all psychoses indicate a primary psychiatric disorder such as schizophrenia. Broadly, psychoses can be categorized as primary or secondary.
Primary psychoses include schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, and mood disorders (major depressive disorder and borderline personality disorder) with psychotic features.1 Difficulty in distinguishing between these entities can necessitate referral to a psychiatrist.
Secondary psychoses arise from a precursor such as delirium, dementia, medical illness, or adverse effects of medications or illicit substances. Medical illnesses that cause psychotic symptoms include: 5,8
- seizures (especially temporal lobe epilepsy),
- cerebrovascular accidents,
- intracranial space-occupying lesions,
- neuropsychiatric disorders (eg, Wilson’s or Parkinson’s disease),
- endocrine disorders (eg, thyroid or adrenal disease),
- autoimmune disease (eg, systemic lupus erythematosus, Hashimoto encephalopathy),
- deficiencies of vitamins A, B1, B12, or niacin,
- infections (eg, human immunodeficiency virus [HIV], encephalitis, parasites, and prion disease),
- narcolepsy, and
- metabolic disease (eg, acute intermittent porphyria, Tay-Sach’s disease, Niemann-Pick disease).
Several recreational drugs can cause psychotic symptoms: cocaine, amphetamines, cannabis, synthetic cannabinoids, inhalants, opioids, and hallucinogens. Psychotic symptoms can also appear during withdrawal from alcohol (delirium tremens) and from sedative hypnotics such as benzodiazepines. Prescribed medications such as anticholinergics, corticosteroids, dopaminergic agents (L-dopa), stimulants (amphetamines), and interferons can also induce psychotic symptoms.
First rule out causes of secondary psychosis
Rule out causes of secondary psychosis by conducting a detailed history and physical examination and ordering appropriate lab tests and imaging studies. If the patient’s psychosis is of recent onset, make sure the laboratory work-up includes
Consider cranial computed tomography or magnetic resonance imaging if there are focal neurologic deficits or if the patient’s presentation is atypical (eg, new onset psychosis in old age).9 Clinical presentation may also indicate a need for electroencephalography, ceruloplasmin measurement, a dexamethasone suppression test, a corticotropin stimulation test, 24-hour urine porphyrin and copper assays, chest radiography, or cerebrospinal fluid analysis.9
FACTORS TO CONSIDER IN TREATMENT DECISIONS
Although primary care physicians may encounter individuals experiencing their first episode of psychosis, it’s more likely that patients presenting with signs and symptoms of the disorder have been experiencing them for some time and have received no psychiatric care. In both instances, schizophrenia is best managed in conjunction with a psychiatrist until symptoms are stabilized.5 Psychosis does not always require hospitalization. But urgent psychiatry referral is recommended, if possible. Consider admission to a psychiatric inpatient unit for anyone who poses a danger to self or others.8,10
Treatment for schizophrenia is most effective with an interprofessional and collaborative approach that includes medication, psychological treatment, social supports, and primary care clinical management.11,12 The last aspect takes on particular importance given that people with schizophrenia, compared with the general population, have a higher incidence of medical illness, particularly cardiovascular disease.13
Medications (TABLE 15,8) are grouped into first-generation antipsychotics (FGAs) and second-generation, or atypical, antipsychotics (SGAs), with the 2 classes being equally effective.14-16 Quality of life is also similar at one year for patients treated with either drug class.14
Adverse effects can differ. The main difference between these medications is their adverse effect profiles. FGAs cause extrapyramidal symptoms (dystonia, akathisia, and tardive dyskinesia) more often than SGAs. Among the SGAs, olanzapine, asenapine, paliperidone, clozapine, and quetiapine cause significant weight gain, glucose dysregulation, and lipid abnormalities.5,8,12,17 Clozapine is associated with agranulocytosis, as well. Risperidone causes mild to moderate weight gain.5,8,12,17 Aripiprazole, lurasidone, and ziprasidone are considered weight neutral and cause no significant glucose dysregulation or lipid abnormalities.5,8,12,17 All antipsychotics can cause QT prolongation and neuroleptic malignant syndrome.5,8,12,17
Keys to successful treatment. Antipsychotics are most effective in treating positive symptoms of schizophrenia and show limited, if any, effect on negative or cognitive symptoms.18,19 Give patients an adequate trial of therapy (at least 4 weeks at a therapeutic dose) before discontinuing the drug or offering a different medication.20 All patients who report symptom relief while receiving antipsychotics should receive maintenance therapy.12
As with all chronic illnesses, success in managing schizophrenia requires patient adherence to the medication regimen. Discontinuation of antipsychotics is a common problem in schizophrenia, resulting in relapse. Long-acting injectable agents (LAIs) were developed to address this problem (TABLE 2).21 Although LAIs are typically used to ensure adherence during maintenance treatment, recent research has suggested they may also be effective for patients with early-phase or first-episode disease.22
What to watch for. Patients on SGAs may develop metabolic abnormalities, and ongoing monitoring of relevant parameters is key (TABLE 323-27). More frequent monitoring may be necessary in patients with cardiovascular risk factors. Continue antipsychotics for at least 6 months to prevent relapse.12 Also keep in mind the “Choosing Wisely” recommendation from the American Psychiatric Association of not prescribing 2 or more antipsychotics concurrently.28
Adjunctive treatment should also be offered
In addition to receiving medication, patients with schizophrenia should be offered adjunctive therapies such as cognitive behavioral therapy, family intervention, and social skills training.10-12 Among patients with schizophrenia, the incidences of anxiety disorder, panic symptoms, posttraumatic stress disorder, and obsessive compulsive disorder are higher than in the general population.29 To address these conditions, medications such as selective serotonin reuptake inhibitors and anxiolytics can be used simultaneously with antipsychotic agents.
CLINICAL COURSE AND PROGNOSIS CAN VARY
Schizophrenia can have a variable clinical course that includes remissions and exacerbations, or it can follow a more persistently chronic course.
Mortality for patients with schizophrenia is 2 to 3 times higher than that of the general population.30 Most deaths are due to an increased incidence of cardiovascular disease, respiratory illness, cancer, stroke, and other thromboembolic events.30
The lifetime prevalence of suicide attempts among individuals with schizophrenia is 20% to 40%,31 and approximately 5% complete suicide.32 Risk factors include command hallucinations, a history of suicide attempts, intoxication with substances, anxiety, and physical pain.32 Clozapine has been shown to reduce suicide risk and may be considered for patients who are at high risk for suicide.32
Therapeutic response varies among patients with schizophrenia, with one-third remaining symptomatic despite adequate treatment regimens.4
CARE MANAGERS CAN HELP ADDRESS BARRIERS TO CARE
A review of the literature suggests that up to one-third of individuals with serious mental illnesses who have had some contact with the mental health system disengage from care.12 Poor engagement may lead to worse clinical outcomes, with symptom relapse and re-hospitalizations. Disengagement from treatment may indicate a patient’s belief that treatment is not necessary, is not meeting his or her needs, or is not being provided in a collaborative manner.
Although shared decision-making is difficult with patients who have schizophrenia, emerging evidence suggests that this approach coupled with patient-centered care will improve engagement with mental health treatment.12 Models of integrated care are being developed and have shown promise in ensuring access to behavioral health for these patients.34
CASE
The primary care physician talked with Mr. R and his mother about the diagnosis of schizophrenia. He screened for suicide risk, and the patient denied having suicidal thoughts. Both the patient and his mother agreed to his starting medication.
Blood and urine samples were collected for a CBC and ESR, as well as to evaluate renal function, electrolytes, glucose, TSH, vitamin B12, folic acid, ANAs, and HIV antibodies. A serum FTA-ABS test was done, as was a urine culture and sensitivity test and a toxicology screen. Because of the patient’s obesity, the physician decided to prescribe a weight-neutral SGA, aripiprazole 10 mg/d. The physician spoke with the clinic’s care coordinator to schedule an appointment with the psychiatry intake department and to follow up on the phone with the patient and his mother. He also scheduled a follow-up appointment for 2 weeks later.
At the follow-up visit, the patient showed no improvement. His blood and urine test results revealed no metabolic abnormalities or infectious or inflammatory illnesses. His urine toxicology result showed no illicit substances. The physician increased the dosage of aripiprazole to 15 mg/d and asked the patient to return in 2 weeks.
At the next follow-up visit, the patient was more verbal and said he was not hearing voices. His mother also acknowledged an improvement. He had already been scheduled for a psychiatry intake appointment, and he and his mother were reminded about this. Mr. R was also asked to make a follow-up primary care appointment for one month from the current visit.
CORRESPONDENCE
Rajesh (Fnu) Rajesh, MD, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
2. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76.
3. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term and clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716-728.
4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645.
5. Holder SD, Wayhs A. Schizophrenia. Am Fam Phys. 2014;90:775-82.
6. Lakhan SE, Vieira KF. Schizophrenia pathophysiology: are we any closer to a complete model? Ann Gen Psychiatry. 2009;8:12.
7. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014:1019-1046.
8. Viron M, Baggett T, Hill M, et al. Schizophrenia for primary care providers: how to contribute to the care of a vulnerable patient population. Am J Med. 2012;125:223-230.
9. Freudenreich O, Charles Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3:10-18.
10. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: Prevention and management. 2014. Available at: http://www.nice.org.uk/Guidance/CG178. Accessed January 3, 2017.
11. Guo X, Zhai J, Liu Z, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized 1-year study. Arch Gen Psychiatry. 2010;67:895-904.
12. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36:94-103.
13. Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458-465.
14. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
15. Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498-511.
16. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
17. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
18. Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837-855.
19. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892-899.
20. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751-1762.
21. Bera R. Patient outcomes within schizophrenia treatment: a look at the role of long-acting injectable antipsychotics. J Clin Psychiatry. 2014;75(suppl 2):30-33.
22. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
23. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
24. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105.
25. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306-318.
26. Lieberman JA, Merrill D, Parameswaran S. APA guidance on the use of antipsychotic drugs and cardiac sudden death.
27. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
28. American Psychiatric Association. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed February 28, 2017.
29. Buckley PF, Miller BJ, Lehrer DS, et al. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402.
30. Lwin AM, Symeon C, Jan F, et al. Morbidity and mortality in schizophrenia. Br J Hosp Med (Lond). 2011;72:628-630.
31. Pompili M, Amador XF, Girardi P, et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10.
32. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81-90.
33. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151-159.
34. Gerrity M. Evolving models of behavioral health integration: Evidence update 2010-2015. Milbank Memorial Fund. Available at: https://www.milbank.org/wp-content/uploads/2016/05/Evolv ing-Models-of-BHI.pdf. Accessed January 11, 2018.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
2. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76.
3. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term and clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716-728.
4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645.
5. Holder SD, Wayhs A. Schizophrenia. Am Fam Phys. 2014;90:775-82.
6. Lakhan SE, Vieira KF. Schizophrenia pathophysiology: are we any closer to a complete model? Ann Gen Psychiatry. 2009;8:12.
7. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014:1019-1046.
8. Viron M, Baggett T, Hill M, et al. Schizophrenia for primary care providers: how to contribute to the care of a vulnerable patient population. Am J Med. 2012;125:223-230.
9. Freudenreich O, Charles Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3:10-18.
10. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: Prevention and management. 2014. Available at: http://www.nice.org.uk/Guidance/CG178. Accessed January 3, 2017.
11. Guo X, Zhai J, Liu Z, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized 1-year study. Arch Gen Psychiatry. 2010;67:895-904.
12. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36:94-103.
13. Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458-465.
14. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
15. Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498-511.
16. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
17. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
18. Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837-855.
19. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892-899.
20. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751-1762.
21. Bera R. Patient outcomes within schizophrenia treatment: a look at the role of long-acting injectable antipsychotics. J Clin Psychiatry. 2014;75(suppl 2):30-33.
22. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
23. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
24. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105.
25. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306-318.
26. Lieberman JA, Merrill D, Parameswaran S. APA guidance on the use of antipsychotic drugs and cardiac sudden death.
27. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
28. American Psychiatric Association. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed February 28, 2017.
29. Buckley PF, Miller BJ, Lehrer DS, et al. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402.
30. Lwin AM, Symeon C, Jan F, et al. Morbidity and mortality in schizophrenia. Br J Hosp Med (Lond). 2011;72:628-630.
31. Pompili M, Amador XF, Girardi P, et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10.
32. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81-90.
33. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151-159.
34. Gerrity M. Evolving models of behavioral health integration: Evidence update 2010-2015. Milbank Memorial Fund. Available at: https://www.milbank.org/wp-content/uploads/2016/05/Evolv ing-Models-of-BHI.pdf. Accessed January 11, 2018.
Osteoporosis: A quick update
Researchers estimate that approximately 10.2 million Americans have osteoporosis, and an additional 43 million have low bone density.1 Equally stark are the ramifications of these numbers. About one out of every 2 Caucasian women will experience an osteoporosis-related fracture at some point in their lifetime, as will approximately one in 5 men.2 Although African American women tend to have a higher bone mineral density (BMD) than white women throughout their lives, those who have osteoporosis have the same elevated risk for fractures as Caucasians.
Osteoporotic fractures are associated with increased risk of disability, mortality, and nursing home placement. Given the aging population, researchers expect annual direct costs from osteoporosis to reach $25.3 billion by 2025.3
Family physicians (FPs) can have a meaningful impact on the extent to which this condition affects the population. To that end, we’ve put together a brief summary of the screening recommendations to keep in mind and a comparison of the different agents used to treat and prevent osteoporosis. The reference tables throughout will put these details at your fingertips.
Screening recommendations vary, Dx doesn’t require BMD testing
Guidelines for screening for osteoporosis vary considerably by professional organization. For example, the US Preventive Services Task Force (USPSTF) recommends screening all women ≥65 years, and younger women whose fracture risk is the same, or greater than, that of a 65-year-old white woman who has no additional risk factors (TABLE 14).5 In addition, the USPSTF concludes that the current evidence is insufficient to recommend routine screening for osteoporosis in men.5
The National Osteoporosis Foundation (NOF) recommends that BMD testing be performed in all women ≥65 years and in men ≥70 years.6 In terms of frequency, NOF recommends BMD testing one to 2 years after initiating therapy to reduce fracture risk and every 2 years thereafter. The American College of Obstetricians and Gynecologists recommends BMD screening for women no more than every 2 years starting at age 65 years.7 It also recommends selective screening in women younger than 65 years of age if they are postmenopausal and have other risk factors for osteoporosis.7
The most recent guideline regarding osteoporosis was published in May 2017 by the American College of Physicians (ACP) and endorsed by the American Academy of Family Physicians.8 But the guideline focuses on treatment rather than screening.
Although guidelines vary by society, most experts agree with BMD assessment in all women ≥65 years and postmenopausal women <65 years if one or more of the risk factors identified in TABLE 14 are present.
Diagnosis. Osteoporosis can be diagnosed using dual energy x-ray absorptiometry (DXA) and T-score (TABLE 26),9 but BMD testing is not always necessary to establish the diagnosis. For example, osteoporosis can be diagnosed clinically in both men and women who have sustained a hip fracture (with or without BMD testing). Osteoporosis may also be diagnosed in patients with osteopenia (determined by DXA and T-score) who have had a vertebral, proximal humeral, or pelvic fracture. Generally speaking, a detailed history and physical together with BMD assessment, vertebral imaging to diagnose vertebral fractures, and, when appropriate, the World Health Organization’s 10-year estimated fracture probability, are all utilized to establish patients’ fracture risk.6,10
Treatment: Which agents and for how long?
Once a patient is diagnosed with osteoporosis, answering the following questions will help with selection of the best therapy for the patient:
- Where on the body is BMD the lowest (vertebral, nonvertebral, or hip) and, consequently, at highest risk for a fracture?
- Does the patient have any conditions that would interfere with therapy (difficulty swallowing, esophageal/gastrointestinal irritation)? This is important, as certain agents are associated with severe esophagitis.
- Does the patient have any issues that would prevent adherence? Adherence may improve with therapy that is administered less frequently (weekly, monthly, once every 3 months, or annually).
TABLE 36,11-14 lists the prescription medications used to treat and prevent osteoporosis, their effect on the risk of vertebral, hip, and nonvertebral fractures, and contraindications/major adverse effects. First-line therapies are recommended based on clinical trials comparing the medication to placebo and evaluating their effectiveness in lowering the risk of vertebral, hip, and nonvertebral fractures.15 Given the absence of studies comparing these drugs to one another, TABLE 36,11-14 should not be used to make direct comparisons.
A new monoclonal antibody, romosozumab, has shown statistically significant decreases in the risk of new vertebral and nonvertebral fractures compared to alendronate after 12 months of use.16 However, there was a statistically significant higher number of patients who had a cardiac ischemic event or revascularization while taking romosozumab compared with those taking alendronate in the one-year double-blind period of the study.16 As of press time, the US Food and Drug Administration has not approved romosozumab.
Duration of treatment should be individualized based on specific patient factors, the pharmacologic agent, and, of course, adverse effects. However, no pharmacologic agent should be used indefinitely.6 In its clinical practice guidelines, the ACP recommends that patients be treated for 5 years with an appropriate pharmacologic therapy.8 The American Society for Bone and Mineral Research (ASBMR) Task Force recommends a review of therapy after 3 years with an intravenous bisphosphonate (BP; strength of recommendation [SOR]=C).17
A review of 2 recent long-term trials analyzing the effects of BPs offers some additional guidance regarding duration of therapy in Caucasian postmenopausal women.18 In one study, women who received 10 years of therapy with alendronate reported fewer vertebral fractures than those who were switched to placebo after 5 years of treatment.19
In the second trial, which studied zoledronic acid, there were fewer morphometric vertebral fractures for those participants given annual injections for 6 years vs 3 years.20 This trial found a significant transient increase in serum creatinine >0.5 mg/dL in the zoledronic acid treatment group.
These findings have prompted some experts in the field of osteoporosis to call for physicians to consider longer therapy with a BP (10 years with oral therapy or 6 years with intravenous therapy) in high-risk postmenopausal women (older women, those with a low hip T-score or high fracture risk score, those with a previous major osteoporotic fracture, and those who experienced fracture while on therapy) (SOR=B).18
Two rare adverse effects to keep in mind
The incidence of atypical femoral fracture, although rare (2-100 per 100,000 women), increases with duration of BP use. As a result, a drug holiday of 2 to 3 years should be considered for women with a low risk for fracture after 3 to 5 years of BP therapy (SOR=C).18
Osteonecrosis of the jaw (ONJ), also known as antiresorptive-associated osteonecrosis of the jaw, is a rare adverse effect of BPs that is associated with higher drug potency, higher cumulative dose, and parenteral route of administration, as well as other risk factors.17,21 The American Association of Maxillofacial Surgeons (AAOMS) states that the risk of developing ONJ increases with use of oral BPs for more than 4 years;22 however, the Task Force of the ASBMR states that the evidence to support this is of poor quality.18 No recommendations on duration of therapy based on risk for ONJ have been made; however, AAOMS recommends discontinuation of oral BPs for a period of 2 months prior to, and 3 months following (or until osseous healing has occurred), elective invasive dental surgery for patients who have been taking an oral BP ≥4 years (SOR=C).22
If a long-term drug holiday is selected, patients should be reassessed in 2 years. Shorter duration of follow-up is warranted for patients taking denosumab, teriparatide, or raloxifene, since bone loss will resume once therapy is discontinued.18
Because the benefits of BPs (in terms of reducing the risk of vertebral fracture) are significantly greater than the risks of an atypical fracture or ONJ, therapy should be started in appropriate patients, but duration of therapy should be monitored closely.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lovedhi Aggarwal, MD, 95-390 Kuahelani Avenue, Mililani, HI 96789; [email protected].
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
2. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD); 2004.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164-S169.
4. Kanis JA, McCloskey EV, Johansson H, et al, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57.
5. Screening for Osteoporosis: U.S. Preventive Services Task Force Final Recommendation Statement. Ann Intern Med. 2011;154:356-364.
6. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis (National Osteoporosis Foundation). Osteoporos Int. 2014;25:2359-2381.
7. Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718-734.
8. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839.
9. Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
10. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411.
11. Lexicomp Online. Clinical Drug Information. Available at: https://online.lexi.com/lco/action/home. Accessed June 30, 2016.
12. Crandall CJ, Newberry SJ, Diamant A, et al. Treatment to Prevent Fractures in Men and Women with Low Bone Density and Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/osteoporosis-bone-fracture_research.pdf. Accessed January 10, 2018.
13. O’Connell MB, Borchert JS. Chapter 73. Osteoporosis and other metabolic bone diseases. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy: a pathophysiologic approach. 9th ed. McGraw-Hill Education; 2014.
14. Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161:711-723.
15. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Supp 3):1-37. Available at: https://www.aace.com/files/osteo-guidelines-2010.pdf. Accessed June 17, 2016.
16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
17. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
18. Adler RA. Duration of anti-resorptive therapy for osteoporosis. Endocrine. 2015;51:222-224.
19. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
20. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-254.
21. Denosumab (Xgeva, Prolia); intravenous bisphosphonates: osteonecrosis of the jaw—further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk. Accessed June 30, 2016.
22. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938-1956.
Researchers estimate that approximately 10.2 million Americans have osteoporosis, and an additional 43 million have low bone density.1 Equally stark are the ramifications of these numbers. About one out of every 2 Caucasian women will experience an osteoporosis-related fracture at some point in their lifetime, as will approximately one in 5 men.2 Although African American women tend to have a higher bone mineral density (BMD) than white women throughout their lives, those who have osteoporosis have the same elevated risk for fractures as Caucasians.
Osteoporotic fractures are associated with increased risk of disability, mortality, and nursing home placement. Given the aging population, researchers expect annual direct costs from osteoporosis to reach $25.3 billion by 2025.3
Family physicians (FPs) can have a meaningful impact on the extent to which this condition affects the population. To that end, we’ve put together a brief summary of the screening recommendations to keep in mind and a comparison of the different agents used to treat and prevent osteoporosis. The reference tables throughout will put these details at your fingertips.
Screening recommendations vary, Dx doesn’t require BMD testing
Guidelines for screening for osteoporosis vary considerably by professional organization. For example, the US Preventive Services Task Force (USPSTF) recommends screening all women ≥65 years, and younger women whose fracture risk is the same, or greater than, that of a 65-year-old white woman who has no additional risk factors (TABLE 14).5 In addition, the USPSTF concludes that the current evidence is insufficient to recommend routine screening for osteoporosis in men.5
The National Osteoporosis Foundation (NOF) recommends that BMD testing be performed in all women ≥65 years and in men ≥70 years.6 In terms of frequency, NOF recommends BMD testing one to 2 years after initiating therapy to reduce fracture risk and every 2 years thereafter. The American College of Obstetricians and Gynecologists recommends BMD screening for women no more than every 2 years starting at age 65 years.7 It also recommends selective screening in women younger than 65 years of age if they are postmenopausal and have other risk factors for osteoporosis.7
The most recent guideline regarding osteoporosis was published in May 2017 by the American College of Physicians (ACP) and endorsed by the American Academy of Family Physicians.8 But the guideline focuses on treatment rather than screening.
Although guidelines vary by society, most experts agree with BMD assessment in all women ≥65 years and postmenopausal women <65 years if one or more of the risk factors identified in TABLE 14 are present.
Diagnosis. Osteoporosis can be diagnosed using dual energy x-ray absorptiometry (DXA) and T-score (TABLE 26),9 but BMD testing is not always necessary to establish the diagnosis. For example, osteoporosis can be diagnosed clinically in both men and women who have sustained a hip fracture (with or without BMD testing). Osteoporosis may also be diagnosed in patients with osteopenia (determined by DXA and T-score) who have had a vertebral, proximal humeral, or pelvic fracture. Generally speaking, a detailed history and physical together with BMD assessment, vertebral imaging to diagnose vertebral fractures, and, when appropriate, the World Health Organization’s 10-year estimated fracture probability, are all utilized to establish patients’ fracture risk.6,10

Treatment: Which agents and for how long?
Once a patient is diagnosed with osteoporosis, answering the following questions will help with selection of the best therapy for the patient:
- Where on the body is BMD the lowest (vertebral, nonvertebral, or hip) and, consequently, at highest risk for a fracture?
- Does the patient have any conditions that would interfere with therapy (difficulty swallowing, esophageal/gastrointestinal irritation)? This is important, as certain agents are associated with severe esophagitis.
- Does the patient have any issues that would prevent adherence? Adherence may improve with therapy that is administered less frequently (weekly, monthly, once every 3 months, or annually).
TABLE 36,11-14 lists the prescription medications used to treat and prevent osteoporosis, their effect on the risk of vertebral, hip, and nonvertebral fractures, and contraindications/major adverse effects. First-line therapies are recommended based on clinical trials comparing the medication to placebo and evaluating their effectiveness in lowering the risk of vertebral, hip, and nonvertebral fractures.15 Given the absence of studies comparing these drugs to one another, TABLE 36,11-14 should not be used to make direct comparisons.
A new monoclonal antibody, romosozumab, has shown statistically significant decreases in the risk of new vertebral and nonvertebral fractures compared to alendronate after 12 months of use.16 However, there was a statistically significant higher number of patients who had a cardiac ischemic event or revascularization while taking romosozumab compared with those taking alendronate in the one-year double-blind period of the study.16 As of press time, the US Food and Drug Administration has not approved romosozumab.
Duration of treatment should be individualized based on specific patient factors, the pharmacologic agent, and, of course, adverse effects. However, no pharmacologic agent should be used indefinitely.6 In its clinical practice guidelines, the ACP recommends that patients be treated for 5 years with an appropriate pharmacologic therapy.8 The American Society for Bone and Mineral Research (ASBMR) Task Force recommends a review of therapy after 3 years with an intravenous bisphosphonate (BP; strength of recommendation [SOR]=C).17
A review of 2 recent long-term trials analyzing the effects of BPs offers some additional guidance regarding duration of therapy in Caucasian postmenopausal women.18 In one study, women who received 10 years of therapy with alendronate reported fewer vertebral fractures than those who were switched to placebo after 5 years of treatment.19
In the second trial, which studied zoledronic acid, there were fewer morphometric vertebral fractures for those participants given annual injections for 6 years vs 3 years.20 This trial found a significant transient increase in serum creatinine >0.5 mg/dL in the zoledronic acid treatment group.
These findings have prompted some experts in the field of osteoporosis to call for physicians to consider longer therapy with a BP (10 years with oral therapy or 6 years with intravenous therapy) in high-risk postmenopausal women (older women, those with a low hip T-score or high fracture risk score, those with a previous major osteoporotic fracture, and those who experienced fracture while on therapy) (SOR=B).18
Two rare adverse effects to keep in mind
The incidence of atypical femoral fracture, although rare (2-100 per 100,000 women), increases with duration of BP use. As a result, a drug holiday of 2 to 3 years should be considered for women with a low risk for fracture after 3 to 5 years of BP therapy (SOR=C).18
Osteonecrosis of the jaw (ONJ), also known as antiresorptive-associated osteonecrosis of the jaw, is a rare adverse effect of BPs that is associated with higher drug potency, higher cumulative dose, and parenteral route of administration, as well as other risk factors.17,21 The American Association of Maxillofacial Surgeons (AAOMS) states that the risk of developing ONJ increases with use of oral BPs for more than 4 years;22 however, the Task Force of the ASBMR states that the evidence to support this is of poor quality.18 No recommendations on duration of therapy based on risk for ONJ have been made; however, AAOMS recommends discontinuation of oral BPs for a period of 2 months prior to, and 3 months following (or until osseous healing has occurred), elective invasive dental surgery for patients who have been taking an oral BP ≥4 years (SOR=C).22
If a long-term drug holiday is selected, patients should be reassessed in 2 years. Shorter duration of follow-up is warranted for patients taking denosumab, teriparatide, or raloxifene, since bone loss will resume once therapy is discontinued.18
Because the benefits of BPs (in terms of reducing the risk of vertebral fracture) are significantly greater than the risks of an atypical fracture or ONJ, therapy should be started in appropriate patients, but duration of therapy should be monitored closely.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lovedhi Aggarwal, MD, 95-390 Kuahelani Avenue, Mililani, HI 96789; [email protected].
Researchers estimate that approximately 10.2 million Americans have osteoporosis, and an additional 43 million have low bone density.1 Equally stark are the ramifications of these numbers. About one out of every 2 Caucasian women will experience an osteoporosis-related fracture at some point in their lifetime, as will approximately one in 5 men.2 Although African American women tend to have a higher bone mineral density (BMD) than white women throughout their lives, those who have osteoporosis have the same elevated risk for fractures as Caucasians.
Osteoporotic fractures are associated with increased risk of disability, mortality, and nursing home placement. Given the aging population, researchers expect annual direct costs from osteoporosis to reach $25.3 billion by 2025.3
Family physicians (FPs) can have a meaningful impact on the extent to which this condition affects the population. To that end, we’ve put together a brief summary of the screening recommendations to keep in mind and a comparison of the different agents used to treat and prevent osteoporosis. The reference tables throughout will put these details at your fingertips.
Screening recommendations vary, Dx doesn’t require BMD testing
Guidelines for screening for osteoporosis vary considerably by professional organization. For example, the US Preventive Services Task Force (USPSTF) recommends screening all women ≥65 years, and younger women whose fracture risk is the same, or greater than, that of a 65-year-old white woman who has no additional risk factors (TABLE 14).5 In addition, the USPSTF concludes that the current evidence is insufficient to recommend routine screening for osteoporosis in men.5
The National Osteoporosis Foundation (NOF) recommends that BMD testing be performed in all women ≥65 years and in men ≥70 years.6 In terms of frequency, NOF recommends BMD testing one to 2 years after initiating therapy to reduce fracture risk and every 2 years thereafter. The American College of Obstetricians and Gynecologists recommends BMD screening for women no more than every 2 years starting at age 65 years.7 It also recommends selective screening in women younger than 65 years of age if they are postmenopausal and have other risk factors for osteoporosis.7
The most recent guideline regarding osteoporosis was published in May 2017 by the American College of Physicians (ACP) and endorsed by the American Academy of Family Physicians.8 But the guideline focuses on treatment rather than screening.
Although guidelines vary by society, most experts agree with BMD assessment in all women ≥65 years and postmenopausal women <65 years if one or more of the risk factors identified in TABLE 14 are present.
Diagnosis. Osteoporosis can be diagnosed using dual energy x-ray absorptiometry (DXA) and T-score (TABLE 26),9 but BMD testing is not always necessary to establish the diagnosis. For example, osteoporosis can be diagnosed clinically in both men and women who have sustained a hip fracture (with or without BMD testing). Osteoporosis may also be diagnosed in patients with osteopenia (determined by DXA and T-score) who have had a vertebral, proximal humeral, or pelvic fracture. Generally speaking, a detailed history and physical together with BMD assessment, vertebral imaging to diagnose vertebral fractures, and, when appropriate, the World Health Organization’s 10-year estimated fracture probability, are all utilized to establish patients’ fracture risk.6,10

Treatment: Which agents and for how long?
Once a patient is diagnosed with osteoporosis, answering the following questions will help with selection of the best therapy for the patient:
- Where on the body is BMD the lowest (vertebral, nonvertebral, or hip) and, consequently, at highest risk for a fracture?
- Does the patient have any conditions that would interfere with therapy (difficulty swallowing, esophageal/gastrointestinal irritation)? This is important, as certain agents are associated with severe esophagitis.
- Does the patient have any issues that would prevent adherence? Adherence may improve with therapy that is administered less frequently (weekly, monthly, once every 3 months, or annually).
TABLE 36,11-14 lists the prescription medications used to treat and prevent osteoporosis, their effect on the risk of vertebral, hip, and nonvertebral fractures, and contraindications/major adverse effects. First-line therapies are recommended based on clinical trials comparing the medication to placebo and evaluating their effectiveness in lowering the risk of vertebral, hip, and nonvertebral fractures.15 Given the absence of studies comparing these drugs to one another, TABLE 36,11-14 should not be used to make direct comparisons.
A new monoclonal antibody, romosozumab, has shown statistically significant decreases in the risk of new vertebral and nonvertebral fractures compared to alendronate after 12 months of use.16 However, there was a statistically significant higher number of patients who had a cardiac ischemic event or revascularization while taking romosozumab compared with those taking alendronate in the one-year double-blind period of the study.16 As of press time, the US Food and Drug Administration has not approved romosozumab.
Duration of treatment should be individualized based on specific patient factors, the pharmacologic agent, and, of course, adverse effects. However, no pharmacologic agent should be used indefinitely.6 In its clinical practice guidelines, the ACP recommends that patients be treated for 5 years with an appropriate pharmacologic therapy.8 The American Society for Bone and Mineral Research (ASBMR) Task Force recommends a review of therapy after 3 years with an intravenous bisphosphonate (BP; strength of recommendation [SOR]=C).17
A review of 2 recent long-term trials analyzing the effects of BPs offers some additional guidance regarding duration of therapy in Caucasian postmenopausal women.18 In one study, women who received 10 years of therapy with alendronate reported fewer vertebral fractures than those who were switched to placebo after 5 years of treatment.19
In the second trial, which studied zoledronic acid, there were fewer morphometric vertebral fractures for those participants given annual injections for 6 years vs 3 years.20 This trial found a significant transient increase in serum creatinine >0.5 mg/dL in the zoledronic acid treatment group.
These findings have prompted some experts in the field of osteoporosis to call for physicians to consider longer therapy with a BP (10 years with oral therapy or 6 years with intravenous therapy) in high-risk postmenopausal women (older women, those with a low hip T-score or high fracture risk score, those with a previous major osteoporotic fracture, and those who experienced fracture while on therapy) (SOR=B).18
Two rare adverse effects to keep in mind
The incidence of atypical femoral fracture, although rare (2-100 per 100,000 women), increases with duration of BP use. As a result, a drug holiday of 2 to 3 years should be considered for women with a low risk for fracture after 3 to 5 years of BP therapy (SOR=C).18
Osteonecrosis of the jaw (ONJ), also known as antiresorptive-associated osteonecrosis of the jaw, is a rare adverse effect of BPs that is associated with higher drug potency, higher cumulative dose, and parenteral route of administration, as well as other risk factors.17,21 The American Association of Maxillofacial Surgeons (AAOMS) states that the risk of developing ONJ increases with use of oral BPs for more than 4 years;22 however, the Task Force of the ASBMR states that the evidence to support this is of poor quality.18 No recommendations on duration of therapy based on risk for ONJ have been made; however, AAOMS recommends discontinuation of oral BPs for a period of 2 months prior to, and 3 months following (or until osseous healing has occurred), elective invasive dental surgery for patients who have been taking an oral BP ≥4 years (SOR=C).22
If a long-term drug holiday is selected, patients should be reassessed in 2 years. Shorter duration of follow-up is warranted for patients taking denosumab, teriparatide, or raloxifene, since bone loss will resume once therapy is discontinued.18
Because the benefits of BPs (in terms of reducing the risk of vertebral fracture) are significantly greater than the risks of an atypical fracture or ONJ, therapy should be started in appropriate patients, but duration of therapy should be monitored closely.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lovedhi Aggarwal, MD, 95-390 Kuahelani Avenue, Mililani, HI 96789; [email protected].
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
2. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD); 2004.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164-S169.
4. Kanis JA, McCloskey EV, Johansson H, et al, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57.
5. Screening for Osteoporosis: U.S. Preventive Services Task Force Final Recommendation Statement. Ann Intern Med. 2011;154:356-364.
6. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis (National Osteoporosis Foundation). Osteoporos Int. 2014;25:2359-2381.
7. Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718-734.
8. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839.
9. Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
10. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411.
11. Lexicomp Online. Clinical Drug Information. Available at: https://online.lexi.com/lco/action/home. Accessed June 30, 2016.
12. Crandall CJ, Newberry SJ, Diamant A, et al. Treatment to Prevent Fractures in Men and Women with Low Bone Density and Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/osteoporosis-bone-fracture_research.pdf. Accessed January 10, 2018.
13. O’Connell MB, Borchert JS. Chapter 73. Osteoporosis and other metabolic bone diseases. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy: a pathophysiologic approach. 9th ed. McGraw-Hill Education; 2014.
14. Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161:711-723.
15. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Supp 3):1-37. Available at: https://www.aace.com/files/osteo-guidelines-2010.pdf. Accessed June 17, 2016.
16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
17. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
18. Adler RA. Duration of anti-resorptive therapy for osteoporosis. Endocrine. 2015;51:222-224.
19. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
20. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-254.
21. Denosumab (Xgeva, Prolia); intravenous bisphosphonates: osteonecrosis of the jaw—further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk. Accessed June 30, 2016.
22. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938-1956.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
2. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD); 2004.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164-S169.
4. Kanis JA, McCloskey EV, Johansson H, et al, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57.
5. Screening for Osteoporosis: U.S. Preventive Services Task Force Final Recommendation Statement. Ann Intern Med. 2011;154:356-364.
6. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis (National Osteoporosis Foundation). Osteoporos Int. 2014;25:2359-2381.
7. Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718-734.
8. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839.
9. Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
10. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411.
11. Lexicomp Online. Clinical Drug Information. Available at: https://online.lexi.com/lco/action/home. Accessed June 30, 2016.
12. Crandall CJ, Newberry SJ, Diamant A, et al. Treatment to Prevent Fractures in Men and Women with Low Bone Density and Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/osteoporosis-bone-fracture_research.pdf. Accessed January 10, 2018.
13. O’Connell MB, Borchert JS. Chapter 73. Osteoporosis and other metabolic bone diseases. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy: a pathophysiologic approach. 9th ed. McGraw-Hill Education; 2014.
14. Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161:711-723.
15. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Supp 3):1-37. Available at: https://www.aace.com/files/osteo-guidelines-2010.pdf. Accessed June 17, 2016.
16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
17. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
18. Adler RA. Duration of anti-resorptive therapy for osteoporosis. Endocrine. 2015;51:222-224.
19. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
20. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-254.
21. Denosumab (Xgeva, Prolia); intravenous bisphosphonates: osteonecrosis of the jaw—further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk. Accessed June 30, 2016.
22. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938-1956.
From The Journal of Family Practice | 2018;67(2):59-62,64-65.
PRACTICE RECOMMENDATIONS
› Use bisphosphonates (except ibandronate) and denosumab as first-line pharmacologic treatment for osteoporosis. A
› Treat patients for 5 years with oral bisphosphonates and 3 years with intravenous bisphosphonates before reviewing therapy, unless there are complications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Polypharmacy in the Elderly: How to Reduce Adverse Drug Events
CE/CME No: CR-1802
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients who are at the greatest risk for the effects of polypharmacy.
• Recognize which medications are most likely to cause adverse drug events (ADEs) in the elderly population.
• Understand the effects of aging on the pharmacokinetics and pharmacodynamics of medications.
• Learn strategies to reduce the risk for polypharmacy and ADEs, including use of the Beers Criteria and the STOPP/START Criteria.
FACULTY
Kelsey Barclay practices in orthopedic surgery at Stanford Medical Center in Palo Alto, California. Amy Frassetto practices in Ob-Gyn at NewYork-Presbyterian in New York City. Julie Robb practices in emergency medicine at South Nassau Communities Hospital in Oceanside, New York. Ellen D. Mandel is a Clinical Professor in the Department of PA Studies at Pace University-Lenox Hill Hospital in New York City.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through January 31, 2019.
Article begins on next page >>
Managing medications in the elderly can be complicated by the physiologic effects of aging and the prevalence of comorbidities. Consistent use of tools such as the Beers criteria and the STOPP/START criteria, as well as medication reconciliation, can reduce polypharmacy and its adverse drug effects, improving health outcomes in this population.
Older adults (those 65 and older) often have a number of comorbidities requiring pharmacologic intervention, making medication management a complicated but essential part of caring for the elderly. A recent analysis of trends in prescription drug use by community-dwelling adults found that 39% of older adults used five or more prescribed medications.1 Furthermore, about 72% of older adults also take a nonprescription medication (OTC or supplement); while OTC medication use has declined in this population in recent years, dietary supplement use has increased.2
These patients are also more susceptible to adverse drug events (ADEs)—including adverse drug reactions (ADRs)—resulting from the physiologic changes of aging. By one estimate, ADRs are about seven times more common in those older than 70 than in younger persons.3 One out of every 30 urgent hospital admissions in patients ages 65 and older is related to an ADR.4
Providers must therefore be cognizant of drug indications, dosing, and drug interactions when prescribing medications to elderly patients. Fortunately, tools and methods to avoid polypharmacy and the adverse effects of commonly prescribed medications—such as anticholinergics and psychotropic drugs—are available.
POLYPHARMACY AND PRESCRIPTION CASCADING
While there is no specific number of medications required to define polypharmacy, the term is generally used when a nonhospitalized individual is taking five or more medications.5 The more medications a patient is taking, the more at risk he or she will be for ADRs, drug interactions, and prescription cascading.
Prescription cascading begins when an ADR is thought to be a new symptom and a new drug is prescribed to control it. Ultimately, a cascade of prescriptions occurs to control avoidable ADRs, resulting in polypharmacy. As many as 57% of women older than 65 in the United States are currently prescribed five or more medications, with 12% prescribed nine or more drugs.6 Not only do these medications cause independent ADRs, but there is also increased risk for drug interactions—and potentially, additional avoidable ADRs.
The elderly population is at greater risk for ADEs because these patients are more likely to have multiple comorbidities and chronic diseases, requiring multiple therapies.7 Polypharmacy is also more dangerous in the elderly because the physiologic changes that occur during natural aging can affect both the pharmacokinetics and pharmacodynamics of medications. The absorption, distribution, metabolism, and excretion of drugs within the human body changes as a person ages, while certain drug classes can alter the way the body functions. For example, muscle mass naturally declines and the proportion of body fat to muscle increases; this change affects the distribution of drugs such as benzodiazepines or lithium.7 If the medication dosage is not corrected, the toxicity of the drug will be increased.7
Medication excretion is largely controlled by the kidneys. Renal perfusion and function decline with age, leading to a decrease in glomerular filtration rate—which requires closer monitoring of medication selection and dosing. The risk is heightened when the elderly patient becomes acutely ill. An acute decrease in kidney function results in decreased excretion of medications, leading to an increase in ADRs.7
Ultimately, the safety of many medications in the elderly patient is unknown.8 But there is a growing body of knowledge on the adverse effects of some classes of medication in this population.
COMMONLY PRESCRIBED MEDICATIONS—AND RISKS
ADEs result from medication errors, ADRs, allergic reactions, and overdoses. The incidence of ADEs—specifically ADRs and medication errors—is elevated in elderly patients who are prescribed certain classes of medications or multiple drugs simultaneously.8 Anticholinergic drugs and psychotropic drugs (specifically antipsychotics and benzodiazepines) are among the medications most commonly prescribed to elderly patients—and among the most likely to contribute to ADEs.9 Diabetes is a chronic condition whose treatment may also put elderly patients at risk for ADEs.10
Anticholinergic medications
Anticholinergic drugs—commonly prescribed for Parkinson disease, depression, urinary incontinence, pulmonary disorders, intestinal motility, and muscle spasms—competitively inhibit the binding of acetylcholine to muscarinic acetylcholine receptors.9 Because this mechanism tends to be nonselective, the adverse effects may be widespread. Central adverse effects include cognitive impairment, confusion, and delirium; peripheral adverse effects include constipation, urinary retention, dry mouth, blurred vision, peristaltic reduction, and tachycardia.9
Anticholinergic drugs are commonly prescribed to elderly patients for cardiovascular (CV) and neurologic disorders. (Medications for the former include ß-blockers, calcium channel blockers, diuretics, and ACE inhibitors; for the latter, amitriptyline, quetiapine, nortriptyline, prochlorperazine, haloperidol, and paroxetine.) An assessment of anticholinergic activity classified most neurologic medications as high activity and most CV medications as low—however, the latter are usually given in conjunction with other anticholinergic medications, increasing their ability to cause ADRs.11
In many cases, patients are prescribed anticholinergic medications to control symptoms of a disease, not to cure it—which means patients may be taking these medications for years. This cumulative exposure is called the anticholinergic burden. Many studies show that the anticholinergic burden is a predictor of cognitive and physical decline; a 2016 study of adults older than 65 who were exposed to 5 mg/d of oxybutynin for more than three years had a 23% increased risk for dementia, compared to low-risk or no exposure groups.9
In a retrospective, population-level study conducted in New Zealand, researchers assessed the anticholinergic effects of delirium, urinary retention, and constipation in 2,248 patients (65 and older) who were admitted to the hospital with at least one prescribed medication. Anticholinergic burden was found to be a significant independent predictor; patients taking five anticholinergic medications were more than three times as likely to develop an anticholinergic effect than those taking just one such medication (adjusted odds ratio, 3.21).11
Psychotropic drugs
Another often-prescribed medication group is psychotropic drugs, specifically antipsychotics and benzodiazepines, for agitation and behavioral disturbances in dementia. A year-long study of 851 patients in two long-term care nursing homes in Boston found that risk for ADRs—specifically, falls—was increased in those who had a change (initiation or dose increase) in psychotropic medication (ie, benzodiazepine, antipsychotic, or antidepressant).12
Second-generation antipsychotics, which are more commonly prescribed than first-generation agents, work on a postsynaptic blockade of brain dopamine D2 receptors and have an increased affinity for serotonin 5-HT2A receptors (see Table 1 for pharmacology of these medications).13,14 Adverse effects of these drugs include hypotension, sedation, and anticholinergic effects. Second-generation antipsychotics also carry a “black box warning” for increased risk for death in elderly patients with dementia-related psychosis.15
Benzodiazepines bind to receptors in the gamma-aminobutyric acid receptor complex, which enhances the binding of this inhibitory neurotransmitter (see Table 2 for pharmacology). Of this class of drugs, lorazepam has the highest potency, whereas midazolam and diazepam have lower potencies. Use of benzodiazepines increases risk for delirium and respiratory depression.16
Diabetes treatment
People with diabetes have an increased risk for ADEs; this risk is elevated in older adults due to comorbidities such as peripheral neuropathy, retinopathy, coronary artery disease, and peripheral vascular disease.10 Hypoglycemic agents, such as insulin and insulin secretagogues, confer a higher risk for falls due to their hypoglycemic effect.10 Furthermore, metformin is known to increase risk for cognitive impairment in patients with diabetes.10
PREVENTING ADEs AND UNNECESSARY POLYPHARMACY
Predicting and preventing ADEs should be a health care provider’s priority when treating an elderly patient taking multiple medications—but it is often overlooked. Electronic medical records (EMRs) are helpful in preventing ADEs, specifically prescription errors, by flagging the patient’s chart when potentially problematic medications are ordered; however, this captures only a portion of ADEs occurring in this population.7
Other options to evaluate a patient for polypharmacy and possible ADRs include the Beers Criteria and the STOPP/START Criteria.17,18 Additionally, performing thorough and frequent medication reviews helps ensure that patients are prescribed essential medications to treat their comorbidities with the most opportunistic risk-benefit ratio. Patients’ medication lists across settings (eg, hospital, primary care, urgent care) can be accessed more easily, efficiently, and accurately with the integration of EMRs.
Beers Criteria
First published by Dr. Mark Beers in 1991 and endorsed by the American Geriatrics Society, the Beers Criteria identifies possible harmful effects of certain commonly prescribed medications to help guide and modify pharmacologic treatments, particularly in adults older than 65. The Beers Criteria classifies medications into three categories:
- Drugs that should be avoided or dose-adjusted
- Drugs that are potentially inappropriate in patients with certain conditions or syndromes
- Drugs that should be prescribed with caution in older adults.17
In the most recent update (2015), possible adverse effects of medications based on a patient’s hepatic or renal function, the effectiveness of the medication, and possible drug interactions were added. For example, nitrofurantoin and antiarrhythmics (eg, amiodarone and digoxin) should be avoided at a lower threshold of hepatic and renal impairment than previously recommended. The criteria suggest avoiding use of zolpidem, a nonbenzodiazepine receptor agonist, because of its elevated risk for adverse effects and minimal effectiveness in treating insomnia. More information about the 2015 criteria is available from the American Geriatrics Society (http://online library.wiley.com/doi/10.1111/jgs. 13702/full).19
The latest update also takes into account recently published evidence of increased ADEs resulting from drugs such as antipsychotics and proton pump inhibitors (PPIs).20 Antipsychotics are associated with an increased risk for morbidity and mortality, and PPIs are now recommended only for treatment duration of up to two months because of the possible increased risk for Clostridium difficile infection, as well as falls and fractures in patients older than 65.20 (PPIs indirectly reduce calcium absorption, which may lead to an increased fracture risk, particularly in postmenopausal women.20)
As with any guideline, the Beers Criteria was designed to supplement, not replace, clinical expertise and judgment. The risks and benefits of a medication should be weighed for the individual patient.
STOPP/START Criteria
Less widely used is the STOPP/START Criteria, an evidence-based set of guidelines consisting of 65 STOPP (Screening Tool of Older Person’s potentially inappropriate Prescriptions) and 22 START (Screening Tool to Alert doctors to the Right Treatment) criteria. Although they may be used individually, STOPP and START are best used together to determine the most appropriate medications for an elderly patient.
The STOPP guidelines help determine when the risks of a medication may outweigh the benefits in a given patient. STOPP includes recommendations for the appropriate length of time to use a medication; for example, PPIs should not be used for more than eight weeks (similar to the Beers recommendation) and benzodiazepines and neuroleptics for more than four weeks.18
START helps clinicians recognize potential prescribing omissions and to identify when a medication regimen should be implemented based on a patient’s history.18 Examples of START criteria include suggestions of when to initiate calcium and vitamin D supplementation for prevention of osteoporosis and when to begin statins in patients with diabetes, coronary artery disease, and cardiovascular disease.18
STOPP/START is organized by physiologic system, which allows for greater usability, and it addresses medications by class rather than specific medications. (The Beers Criteria was criticized for these reasons, as well as its limited transferability outside the United States.) When assessed in systematic reviews, the STOPP/START criteria were found to be fundamentally more sensitive than the Beers Criteria. Overall, it was concluded that the use of the STOPP/START criteria resulted in an absolute risk reduction of 21.2% to 35.7% and greatly improved the appropriateness of prescribing medication to the elderly. Its use also resulted in fewer follow-up appointments with a primary care physician (PCP).18
iPhone and Android applications such as iGeriatrics and Medstopper provide clinicians with easy access to Beers Criteria and STOPP/START Criteria, respectively.
Medication reconciliation
Medication reconciliation—in which health care providers review a patient’s medication list at hospital admission and discharge, and even at routine office visits—is an increasingly common practice, especially with the implementation of EMRs. The patient’s prescribed and OTC medications, as well as dose, route, frequency, and indication, are updated, with the goal of maintaining the most accurate list. Health care providers can utilize both the Beers Criteria and the STOPP/START criteria in their reconciliation process to help reduce polypharmacy in the elderly. It is an essential step in maintaining communication between providers and ultimately decreasing the incidence of ADEs.17
IMPROVE … continuity of care
Polypharmacy can decrease patient likelihood to adhere to the regimen, whether due to confusion or intolerance.8 Patients should be included, along with caregivers and all medical providers, in a holistic assessment of the patient’s best interests in terms of long-term care and pharmacologic treatment, since those who have a sense of control in their treatment goals and expectations often achieve a better understanding of their medical status.10
However, educating patients about their medications is time-consuming, and time is often at a premium during a typical office visit. A pilot study of 28 male veterans (ages 85 and older)—the Integrated Management and Polypharmacy Review of Vulnerable Elders (IMPROVE) project—devised a model to combat this problem.21 As an adjunct to a visit with the PCP, a clinical pharmacist trained in patient education and medication management performed face-to-face clinical consults with patients and their caregivers. The results indicated that medical management by both the PCP and the pharmacist resulted in better medication management. The pharmacist was able to spend time with the patient and caregiver, resulting in individualized instructions, education, and strategies for safe and effective medication use. The PCP remained involved by cosigning the note with the pharmacist and was available for consultation, if needed.
In IMPROVE, 79% of patients had at least one medication discontinued and 75% had one or more dosing or timing adjustments made. Potentially inappropriate medications were reduced by 14%.21 When the researchers compared the six-month period before the trial with the six-month period afterward, they found an average pharmacy cost savings of $64 per veteran per month. There was also a decreasing trend in phone calls and visits to the PCP. Cost savings were comparable to or greater than those reported for similar interventions.21 There has not been sufficient long-term follow-up to assess this method’s effects on ADEs, morbidity, and mortality, however.
CONCLUSION
Managing medications in the elderly population is difficult, and polypharmacy is common due to the prevalence of patients with comorbidities. It is important for providers to be aware of possible drug interactions, prescribing cascades, and ADEs. Medications such as anticholinergics and antipsychotics pose an increased risk for ADEs, but the regular implementation of criteria such as Beers or STOPP/START in clinical practice will minimize overprescribing and improve health outcomes. These criteria should be used to supplement the clinical judgment and expertise of providers as a mainstay of patient care in the elderly.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818-1830.
2. Qato DM, Wilder J, Schumm LP. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
3. Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992;2(4):356-367.
4. Pedros C, Formiga F, Corbella X, Arnau J. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016:72(2):219-226.
5. Maher RL Jr, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
6. Nguyen PV-Q, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016;149(3):122-124.
7. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866.
8. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137.
9. Koronkowski M, Eisenhower C, Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann Longterm Care. 2016; 24(3):37-40.
10. Peron EP, Ogbonna KC, Donohoe KL. Diabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1): 17-vii.
11. Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3): 530-541.
12. Echt MA, Samelson EJ, Hannan MT, et al. Psychotropic drug initiation or increased dosage and the acute risk of falls: a prospective cohort study of nursing home residents. BMC Geriatrics. 2013;13:19.
13. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of antipsychotics: an update. EXCLI J. 2014;13: 1163-1191.
14. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:29-40.
15. FDA. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances (2005). www. fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 28, 2017.
16. Griffin CE III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-223.
17. Flanagan N, Beizer J. Medication reconciliation and education for older adults: using the 2015 AGS Beers Criteria as a guide. Home Healthc Now. 2016;34(10): 542-549.
18. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360-372.
19. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227-2246.
20. Salbu RL, Feuer J. A closer look at the 2015 Beers criteria. J Pharm Pract. 2017;30(4):419-424.
21. Mirk A, Echt KV, Vandenberg AE, et al. Polypharmacy review of vulnerable elders: can we IMPROVE outcomes? Fed Pract. 2016;33(3):39-41.
22. Saphris [package insert]. Irvine, CA: Allergan, USA, Inc; 2017.
23. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc; 2017.
24. Zyprexa [package insert]. Indianapolis, IN: Lilly USA LLC; 2017.
25. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017.
26. Midazolam hydrochloride injection solution [package insert]. Lake Forest, IL: Hospira Inc; 2017.
27. Diazepam oral solution and Diazepam Intensol oral solution concentrate [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corp; 2016.
28. Ativan tablet [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2013.
CE/CME No: CR-1802
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients who are at the greatest risk for the effects of polypharmacy.
• Recognize which medications are most likely to cause adverse drug events (ADEs) in the elderly population.
• Understand the effects of aging on the pharmacokinetics and pharmacodynamics of medications.
• Learn strategies to reduce the risk for polypharmacy and ADEs, including use of the Beers Criteria and the STOPP/START Criteria.
FACULTY
Kelsey Barclay practices in orthopedic surgery at Stanford Medical Center in Palo Alto, California. Amy Frassetto practices in Ob-Gyn at NewYork-Presbyterian in New York City. Julie Robb practices in emergency medicine at South Nassau Communities Hospital in Oceanside, New York. Ellen D. Mandel is a Clinical Professor in the Department of PA Studies at Pace University-Lenox Hill Hospital in New York City.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through January 31, 2019.
Article begins on next page >>
Managing medications in the elderly can be complicated by the physiologic effects of aging and the prevalence of comorbidities. Consistent use of tools such as the Beers criteria and the STOPP/START criteria, as well as medication reconciliation, can reduce polypharmacy and its adverse drug effects, improving health outcomes in this population.
Older adults (those 65 and older) often have a number of comorbidities requiring pharmacologic intervention, making medication management a complicated but essential part of caring for the elderly. A recent analysis of trends in prescription drug use by community-dwelling adults found that 39% of older adults used five or more prescribed medications.1 Furthermore, about 72% of older adults also take a nonprescription medication (OTC or supplement); while OTC medication use has declined in this population in recent years, dietary supplement use has increased.2
These patients are also more susceptible to adverse drug events (ADEs)—including adverse drug reactions (ADRs)—resulting from the physiologic changes of aging. By one estimate, ADRs are about seven times more common in those older than 70 than in younger persons.3 One out of every 30 urgent hospital admissions in patients ages 65 and older is related to an ADR.4
Providers must therefore be cognizant of drug indications, dosing, and drug interactions when prescribing medications to elderly patients. Fortunately, tools and methods to avoid polypharmacy and the adverse effects of commonly prescribed medications—such as anticholinergics and psychotropic drugs—are available.
POLYPHARMACY AND PRESCRIPTION CASCADING
While there is no specific number of medications required to define polypharmacy, the term is generally used when a nonhospitalized individual is taking five or more medications.5 The more medications a patient is taking, the more at risk he or she will be for ADRs, drug interactions, and prescription cascading.
Prescription cascading begins when an ADR is thought to be a new symptom and a new drug is prescribed to control it. Ultimately, a cascade of prescriptions occurs to control avoidable ADRs, resulting in polypharmacy. As many as 57% of women older than 65 in the United States are currently prescribed five or more medications, with 12% prescribed nine or more drugs.6 Not only do these medications cause independent ADRs, but there is also increased risk for drug interactions—and potentially, additional avoidable ADRs.
The elderly population is at greater risk for ADEs because these patients are more likely to have multiple comorbidities and chronic diseases, requiring multiple therapies.7 Polypharmacy is also more dangerous in the elderly because the physiologic changes that occur during natural aging can affect both the pharmacokinetics and pharmacodynamics of medications. The absorption, distribution, metabolism, and excretion of drugs within the human body changes as a person ages, while certain drug classes can alter the way the body functions. For example, muscle mass naturally declines and the proportion of body fat to muscle increases; this change affects the distribution of drugs such as benzodiazepines or lithium.7 If the medication dosage is not corrected, the toxicity of the drug will be increased.7
Medication excretion is largely controlled by the kidneys. Renal perfusion and function decline with age, leading to a decrease in glomerular filtration rate—which requires closer monitoring of medication selection and dosing. The risk is heightened when the elderly patient becomes acutely ill. An acute decrease in kidney function results in decreased excretion of medications, leading to an increase in ADRs.7
Ultimately, the safety of many medications in the elderly patient is unknown.8 But there is a growing body of knowledge on the adverse effects of some classes of medication in this population.
COMMONLY PRESCRIBED MEDICATIONS—AND RISKS
ADEs result from medication errors, ADRs, allergic reactions, and overdoses. The incidence of ADEs—specifically ADRs and medication errors—is elevated in elderly patients who are prescribed certain classes of medications or multiple drugs simultaneously.8 Anticholinergic drugs and psychotropic drugs (specifically antipsychotics and benzodiazepines) are among the medications most commonly prescribed to elderly patients—and among the most likely to contribute to ADEs.9 Diabetes is a chronic condition whose treatment may also put elderly patients at risk for ADEs.10
Anticholinergic medications
Anticholinergic drugs—commonly prescribed for Parkinson disease, depression, urinary incontinence, pulmonary disorders, intestinal motility, and muscle spasms—competitively inhibit the binding of acetylcholine to muscarinic acetylcholine receptors.9 Because this mechanism tends to be nonselective, the adverse effects may be widespread. Central adverse effects include cognitive impairment, confusion, and delirium; peripheral adverse effects include constipation, urinary retention, dry mouth, blurred vision, peristaltic reduction, and tachycardia.9
Anticholinergic drugs are commonly prescribed to elderly patients for cardiovascular (CV) and neurologic disorders. (Medications for the former include ß-blockers, calcium channel blockers, diuretics, and ACE inhibitors; for the latter, amitriptyline, quetiapine, nortriptyline, prochlorperazine, haloperidol, and paroxetine.) An assessment of anticholinergic activity classified most neurologic medications as high activity and most CV medications as low—however, the latter are usually given in conjunction with other anticholinergic medications, increasing their ability to cause ADRs.11
In many cases, patients are prescribed anticholinergic medications to control symptoms of a disease, not to cure it—which means patients may be taking these medications for years. This cumulative exposure is called the anticholinergic burden. Many studies show that the anticholinergic burden is a predictor of cognitive and physical decline; a 2016 study of adults older than 65 who were exposed to 5 mg/d of oxybutynin for more than three years had a 23% increased risk for dementia, compared to low-risk or no exposure groups.9
In a retrospective, population-level study conducted in New Zealand, researchers assessed the anticholinergic effects of delirium, urinary retention, and constipation in 2,248 patients (65 and older) who were admitted to the hospital with at least one prescribed medication. Anticholinergic burden was found to be a significant independent predictor; patients taking five anticholinergic medications were more than three times as likely to develop an anticholinergic effect than those taking just one such medication (adjusted odds ratio, 3.21).11
Psychotropic drugs
Another often-prescribed medication group is psychotropic drugs, specifically antipsychotics and benzodiazepines, for agitation and behavioral disturbances in dementia. A year-long study of 851 patients in two long-term care nursing homes in Boston found that risk for ADRs—specifically, falls—was increased in those who had a change (initiation or dose increase) in psychotropic medication (ie, benzodiazepine, antipsychotic, or antidepressant).12
Second-generation antipsychotics, which are more commonly prescribed than first-generation agents, work on a postsynaptic blockade of brain dopamine D2 receptors and have an increased affinity for serotonin 5-HT2A receptors (see Table 1 for pharmacology of these medications).13,14 Adverse effects of these drugs include hypotension, sedation, and anticholinergic effects. Second-generation antipsychotics also carry a “black box warning” for increased risk for death in elderly patients with dementia-related psychosis.15
Benzodiazepines bind to receptors in the gamma-aminobutyric acid receptor complex, which enhances the binding of this inhibitory neurotransmitter (see Table 2 for pharmacology). Of this class of drugs, lorazepam has the highest potency, whereas midazolam and diazepam have lower potencies. Use of benzodiazepines increases risk for delirium and respiratory depression.16
Diabetes treatment
People with diabetes have an increased risk for ADEs; this risk is elevated in older adults due to comorbidities such as peripheral neuropathy, retinopathy, coronary artery disease, and peripheral vascular disease.10 Hypoglycemic agents, such as insulin and insulin secretagogues, confer a higher risk for falls due to their hypoglycemic effect.10 Furthermore, metformin is known to increase risk for cognitive impairment in patients with diabetes.10
PREVENTING ADEs AND UNNECESSARY POLYPHARMACY
Predicting and preventing ADEs should be a health care provider’s priority when treating an elderly patient taking multiple medications—but it is often overlooked. Electronic medical records (EMRs) are helpful in preventing ADEs, specifically prescription errors, by flagging the patient’s chart when potentially problematic medications are ordered; however, this captures only a portion of ADEs occurring in this population.7
Other options to evaluate a patient for polypharmacy and possible ADRs include the Beers Criteria and the STOPP/START Criteria.17,18 Additionally, performing thorough and frequent medication reviews helps ensure that patients are prescribed essential medications to treat their comorbidities with the most opportunistic risk-benefit ratio. Patients’ medication lists across settings (eg, hospital, primary care, urgent care) can be accessed more easily, efficiently, and accurately with the integration of EMRs.
Beers Criteria
First published by Dr. Mark Beers in 1991 and endorsed by the American Geriatrics Society, the Beers Criteria identifies possible harmful effects of certain commonly prescribed medications to help guide and modify pharmacologic treatments, particularly in adults older than 65. The Beers Criteria classifies medications into three categories:
- Drugs that should be avoided or dose-adjusted
- Drugs that are potentially inappropriate in patients with certain conditions or syndromes
- Drugs that should be prescribed with caution in older adults.17
In the most recent update (2015), possible adverse effects of medications based on a patient’s hepatic or renal function, the effectiveness of the medication, and possible drug interactions were added. For example, nitrofurantoin and antiarrhythmics (eg, amiodarone and digoxin) should be avoided at a lower threshold of hepatic and renal impairment than previously recommended. The criteria suggest avoiding use of zolpidem, a nonbenzodiazepine receptor agonist, because of its elevated risk for adverse effects and minimal effectiveness in treating insomnia. More information about the 2015 criteria is available from the American Geriatrics Society (http://online library.wiley.com/doi/10.1111/jgs. 13702/full).19
The latest update also takes into account recently published evidence of increased ADEs resulting from drugs such as antipsychotics and proton pump inhibitors (PPIs).20 Antipsychotics are associated with an increased risk for morbidity and mortality, and PPIs are now recommended only for treatment duration of up to two months because of the possible increased risk for Clostridium difficile infection, as well as falls and fractures in patients older than 65.20 (PPIs indirectly reduce calcium absorption, which may lead to an increased fracture risk, particularly in postmenopausal women.20)
As with any guideline, the Beers Criteria was designed to supplement, not replace, clinical expertise and judgment. The risks and benefits of a medication should be weighed for the individual patient.
STOPP/START Criteria
Less widely used is the STOPP/START Criteria, an evidence-based set of guidelines consisting of 65 STOPP (Screening Tool of Older Person’s potentially inappropriate Prescriptions) and 22 START (Screening Tool to Alert doctors to the Right Treatment) criteria. Although they may be used individually, STOPP and START are best used together to determine the most appropriate medications for an elderly patient.
The STOPP guidelines help determine when the risks of a medication may outweigh the benefits in a given patient. STOPP includes recommendations for the appropriate length of time to use a medication; for example, PPIs should not be used for more than eight weeks (similar to the Beers recommendation) and benzodiazepines and neuroleptics for more than four weeks.18
START helps clinicians recognize potential prescribing omissions and to identify when a medication regimen should be implemented based on a patient’s history.18 Examples of START criteria include suggestions of when to initiate calcium and vitamin D supplementation for prevention of osteoporosis and when to begin statins in patients with diabetes, coronary artery disease, and cardiovascular disease.18
STOPP/START is organized by physiologic system, which allows for greater usability, and it addresses medications by class rather than specific medications. (The Beers Criteria was criticized for these reasons, as well as its limited transferability outside the United States.) When assessed in systematic reviews, the STOPP/START criteria were found to be fundamentally more sensitive than the Beers Criteria. Overall, it was concluded that the use of the STOPP/START criteria resulted in an absolute risk reduction of 21.2% to 35.7% and greatly improved the appropriateness of prescribing medication to the elderly. Its use also resulted in fewer follow-up appointments with a primary care physician (PCP).18
iPhone and Android applications such as iGeriatrics and Medstopper provide clinicians with easy access to Beers Criteria and STOPP/START Criteria, respectively.
Medication reconciliation
Medication reconciliation—in which health care providers review a patient’s medication list at hospital admission and discharge, and even at routine office visits—is an increasingly common practice, especially with the implementation of EMRs. The patient’s prescribed and OTC medications, as well as dose, route, frequency, and indication, are updated, with the goal of maintaining the most accurate list. Health care providers can utilize both the Beers Criteria and the STOPP/START criteria in their reconciliation process to help reduce polypharmacy in the elderly. It is an essential step in maintaining communication between providers and ultimately decreasing the incidence of ADEs.17
IMPROVE … continuity of care
Polypharmacy can decrease patient likelihood to adhere to the regimen, whether due to confusion or intolerance.8 Patients should be included, along with caregivers and all medical providers, in a holistic assessment of the patient’s best interests in terms of long-term care and pharmacologic treatment, since those who have a sense of control in their treatment goals and expectations often achieve a better understanding of their medical status.10
However, educating patients about their medications is time-consuming, and time is often at a premium during a typical office visit. A pilot study of 28 male veterans (ages 85 and older)—the Integrated Management and Polypharmacy Review of Vulnerable Elders (IMPROVE) project—devised a model to combat this problem.21 As an adjunct to a visit with the PCP, a clinical pharmacist trained in patient education and medication management performed face-to-face clinical consults with patients and their caregivers. The results indicated that medical management by both the PCP and the pharmacist resulted in better medication management. The pharmacist was able to spend time with the patient and caregiver, resulting in individualized instructions, education, and strategies for safe and effective medication use. The PCP remained involved by cosigning the note with the pharmacist and was available for consultation, if needed.
In IMPROVE, 79% of patients had at least one medication discontinued and 75% had one or more dosing or timing adjustments made. Potentially inappropriate medications were reduced by 14%.21 When the researchers compared the six-month period before the trial with the six-month period afterward, they found an average pharmacy cost savings of $64 per veteran per month. There was also a decreasing trend in phone calls and visits to the PCP. Cost savings were comparable to or greater than those reported for similar interventions.21 There has not been sufficient long-term follow-up to assess this method’s effects on ADEs, morbidity, and mortality, however.
CONCLUSION
Managing medications in the elderly population is difficult, and polypharmacy is common due to the prevalence of patients with comorbidities. It is important for providers to be aware of possible drug interactions, prescribing cascades, and ADEs. Medications such as anticholinergics and antipsychotics pose an increased risk for ADEs, but the regular implementation of criteria such as Beers or STOPP/START in clinical practice will minimize overprescribing and improve health outcomes. These criteria should be used to supplement the clinical judgment and expertise of providers as a mainstay of patient care in the elderly.
CE/CME No: CR-1802
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients who are at the greatest risk for the effects of polypharmacy.
• Recognize which medications are most likely to cause adverse drug events (ADEs) in the elderly population.
• Understand the effects of aging on the pharmacokinetics and pharmacodynamics of medications.
• Learn strategies to reduce the risk for polypharmacy and ADEs, including use of the Beers Criteria and the STOPP/START Criteria.
FACULTY
Kelsey Barclay practices in orthopedic surgery at Stanford Medical Center in Palo Alto, California. Amy Frassetto practices in Ob-Gyn at NewYork-Presbyterian in New York City. Julie Robb practices in emergency medicine at South Nassau Communities Hospital in Oceanside, New York. Ellen D. Mandel is a Clinical Professor in the Department of PA Studies at Pace University-Lenox Hill Hospital in New York City.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through January 31, 2019.
Article begins on next page >>
Managing medications in the elderly can be complicated by the physiologic effects of aging and the prevalence of comorbidities. Consistent use of tools such as the Beers criteria and the STOPP/START criteria, as well as medication reconciliation, can reduce polypharmacy and its adverse drug effects, improving health outcomes in this population.
Older adults (those 65 and older) often have a number of comorbidities requiring pharmacologic intervention, making medication management a complicated but essential part of caring for the elderly. A recent analysis of trends in prescription drug use by community-dwelling adults found that 39% of older adults used five or more prescribed medications.1 Furthermore, about 72% of older adults also take a nonprescription medication (OTC or supplement); while OTC medication use has declined in this population in recent years, dietary supplement use has increased.2
These patients are also more susceptible to adverse drug events (ADEs)—including adverse drug reactions (ADRs)—resulting from the physiologic changes of aging. By one estimate, ADRs are about seven times more common in those older than 70 than in younger persons.3 One out of every 30 urgent hospital admissions in patients ages 65 and older is related to an ADR.4
Providers must therefore be cognizant of drug indications, dosing, and drug interactions when prescribing medications to elderly patients. Fortunately, tools and methods to avoid polypharmacy and the adverse effects of commonly prescribed medications—such as anticholinergics and psychotropic drugs—are available.
POLYPHARMACY AND PRESCRIPTION CASCADING
While there is no specific number of medications required to define polypharmacy, the term is generally used when a nonhospitalized individual is taking five or more medications.5 The more medications a patient is taking, the more at risk he or she will be for ADRs, drug interactions, and prescription cascading.
Prescription cascading begins when an ADR is thought to be a new symptom and a new drug is prescribed to control it. Ultimately, a cascade of prescriptions occurs to control avoidable ADRs, resulting in polypharmacy. As many as 57% of women older than 65 in the United States are currently prescribed five or more medications, with 12% prescribed nine or more drugs.6 Not only do these medications cause independent ADRs, but there is also increased risk for drug interactions—and potentially, additional avoidable ADRs.
The elderly population is at greater risk for ADEs because these patients are more likely to have multiple comorbidities and chronic diseases, requiring multiple therapies.7 Polypharmacy is also more dangerous in the elderly because the physiologic changes that occur during natural aging can affect both the pharmacokinetics and pharmacodynamics of medications. The absorption, distribution, metabolism, and excretion of drugs within the human body changes as a person ages, while certain drug classes can alter the way the body functions. For example, muscle mass naturally declines and the proportion of body fat to muscle increases; this change affects the distribution of drugs such as benzodiazepines or lithium.7 If the medication dosage is not corrected, the toxicity of the drug will be increased.7
Medication excretion is largely controlled by the kidneys. Renal perfusion and function decline with age, leading to a decrease in glomerular filtration rate—which requires closer monitoring of medication selection and dosing. The risk is heightened when the elderly patient becomes acutely ill. An acute decrease in kidney function results in decreased excretion of medications, leading to an increase in ADRs.7
Ultimately, the safety of many medications in the elderly patient is unknown.8 But there is a growing body of knowledge on the adverse effects of some classes of medication in this population.
COMMONLY PRESCRIBED MEDICATIONS—AND RISKS
ADEs result from medication errors, ADRs, allergic reactions, and overdoses. The incidence of ADEs—specifically ADRs and medication errors—is elevated in elderly patients who are prescribed certain classes of medications or multiple drugs simultaneously.8 Anticholinergic drugs and psychotropic drugs (specifically antipsychotics and benzodiazepines) are among the medications most commonly prescribed to elderly patients—and among the most likely to contribute to ADEs.9 Diabetes is a chronic condition whose treatment may also put elderly patients at risk for ADEs.10
Anticholinergic medications
Anticholinergic drugs—commonly prescribed for Parkinson disease, depression, urinary incontinence, pulmonary disorders, intestinal motility, and muscle spasms—competitively inhibit the binding of acetylcholine to muscarinic acetylcholine receptors.9 Because this mechanism tends to be nonselective, the adverse effects may be widespread. Central adverse effects include cognitive impairment, confusion, and delirium; peripheral adverse effects include constipation, urinary retention, dry mouth, blurred vision, peristaltic reduction, and tachycardia.9
Anticholinergic drugs are commonly prescribed to elderly patients for cardiovascular (CV) and neurologic disorders. (Medications for the former include ß-blockers, calcium channel blockers, diuretics, and ACE inhibitors; for the latter, amitriptyline, quetiapine, nortriptyline, prochlorperazine, haloperidol, and paroxetine.) An assessment of anticholinergic activity classified most neurologic medications as high activity and most CV medications as low—however, the latter are usually given in conjunction with other anticholinergic medications, increasing their ability to cause ADRs.11
In many cases, patients are prescribed anticholinergic medications to control symptoms of a disease, not to cure it—which means patients may be taking these medications for years. This cumulative exposure is called the anticholinergic burden. Many studies show that the anticholinergic burden is a predictor of cognitive and physical decline; a 2016 study of adults older than 65 who were exposed to 5 mg/d of oxybutynin for more than three years had a 23% increased risk for dementia, compared to low-risk or no exposure groups.9
In a retrospective, population-level study conducted in New Zealand, researchers assessed the anticholinergic effects of delirium, urinary retention, and constipation in 2,248 patients (65 and older) who were admitted to the hospital with at least one prescribed medication. Anticholinergic burden was found to be a significant independent predictor; patients taking five anticholinergic medications were more than three times as likely to develop an anticholinergic effect than those taking just one such medication (adjusted odds ratio, 3.21).11
Psychotropic drugs
Another often-prescribed medication group is psychotropic drugs, specifically antipsychotics and benzodiazepines, for agitation and behavioral disturbances in dementia. A year-long study of 851 patients in two long-term care nursing homes in Boston found that risk for ADRs—specifically, falls—was increased in those who had a change (initiation or dose increase) in psychotropic medication (ie, benzodiazepine, antipsychotic, or antidepressant).12
Second-generation antipsychotics, which are more commonly prescribed than first-generation agents, work on a postsynaptic blockade of brain dopamine D2 receptors and have an increased affinity for serotonin 5-HT2A receptors (see Table 1 for pharmacology of these medications).13,14 Adverse effects of these drugs include hypotension, sedation, and anticholinergic effects. Second-generation antipsychotics also carry a “black box warning” for increased risk for death in elderly patients with dementia-related psychosis.15
Benzodiazepines bind to receptors in the gamma-aminobutyric acid receptor complex, which enhances the binding of this inhibitory neurotransmitter (see Table 2 for pharmacology). Of this class of drugs, lorazepam has the highest potency, whereas midazolam and diazepam have lower potencies. Use of benzodiazepines increases risk for delirium and respiratory depression.16
Diabetes treatment
People with diabetes have an increased risk for ADEs; this risk is elevated in older adults due to comorbidities such as peripheral neuropathy, retinopathy, coronary artery disease, and peripheral vascular disease.10 Hypoglycemic agents, such as insulin and insulin secretagogues, confer a higher risk for falls due to their hypoglycemic effect.10 Furthermore, metformin is known to increase risk for cognitive impairment in patients with diabetes.10
PREVENTING ADEs AND UNNECESSARY POLYPHARMACY
Predicting and preventing ADEs should be a health care provider’s priority when treating an elderly patient taking multiple medications—but it is often overlooked. Electronic medical records (EMRs) are helpful in preventing ADEs, specifically prescription errors, by flagging the patient’s chart when potentially problematic medications are ordered; however, this captures only a portion of ADEs occurring in this population.7
Other options to evaluate a patient for polypharmacy and possible ADRs include the Beers Criteria and the STOPP/START Criteria.17,18 Additionally, performing thorough and frequent medication reviews helps ensure that patients are prescribed essential medications to treat their comorbidities with the most opportunistic risk-benefit ratio. Patients’ medication lists across settings (eg, hospital, primary care, urgent care) can be accessed more easily, efficiently, and accurately with the integration of EMRs.
Beers Criteria
First published by Dr. Mark Beers in 1991 and endorsed by the American Geriatrics Society, the Beers Criteria identifies possible harmful effects of certain commonly prescribed medications to help guide and modify pharmacologic treatments, particularly in adults older than 65. The Beers Criteria classifies medications into three categories:
- Drugs that should be avoided or dose-adjusted
- Drugs that are potentially inappropriate in patients with certain conditions or syndromes
- Drugs that should be prescribed with caution in older adults.17
In the most recent update (2015), possible adverse effects of medications based on a patient’s hepatic or renal function, the effectiveness of the medication, and possible drug interactions were added. For example, nitrofurantoin and antiarrhythmics (eg, amiodarone and digoxin) should be avoided at a lower threshold of hepatic and renal impairment than previously recommended. The criteria suggest avoiding use of zolpidem, a nonbenzodiazepine receptor agonist, because of its elevated risk for adverse effects and minimal effectiveness in treating insomnia. More information about the 2015 criteria is available from the American Geriatrics Society (http://online library.wiley.com/doi/10.1111/jgs. 13702/full).19
The latest update also takes into account recently published evidence of increased ADEs resulting from drugs such as antipsychotics and proton pump inhibitors (PPIs).20 Antipsychotics are associated with an increased risk for morbidity and mortality, and PPIs are now recommended only for treatment duration of up to two months because of the possible increased risk for Clostridium difficile infection, as well as falls and fractures in patients older than 65.20 (PPIs indirectly reduce calcium absorption, which may lead to an increased fracture risk, particularly in postmenopausal women.20)
As with any guideline, the Beers Criteria was designed to supplement, not replace, clinical expertise and judgment. The risks and benefits of a medication should be weighed for the individual patient.
STOPP/START Criteria
Less widely used is the STOPP/START Criteria, an evidence-based set of guidelines consisting of 65 STOPP (Screening Tool of Older Person’s potentially inappropriate Prescriptions) and 22 START (Screening Tool to Alert doctors to the Right Treatment) criteria. Although they may be used individually, STOPP and START are best used together to determine the most appropriate medications for an elderly patient.
The STOPP guidelines help determine when the risks of a medication may outweigh the benefits in a given patient. STOPP includes recommendations for the appropriate length of time to use a medication; for example, PPIs should not be used for more than eight weeks (similar to the Beers recommendation) and benzodiazepines and neuroleptics for more than four weeks.18
START helps clinicians recognize potential prescribing omissions and to identify when a medication regimen should be implemented based on a patient’s history.18 Examples of START criteria include suggestions of when to initiate calcium and vitamin D supplementation for prevention of osteoporosis and when to begin statins in patients with diabetes, coronary artery disease, and cardiovascular disease.18
STOPP/START is organized by physiologic system, which allows for greater usability, and it addresses medications by class rather than specific medications. (The Beers Criteria was criticized for these reasons, as well as its limited transferability outside the United States.) When assessed in systematic reviews, the STOPP/START criteria were found to be fundamentally more sensitive than the Beers Criteria. Overall, it was concluded that the use of the STOPP/START criteria resulted in an absolute risk reduction of 21.2% to 35.7% and greatly improved the appropriateness of prescribing medication to the elderly. Its use also resulted in fewer follow-up appointments with a primary care physician (PCP).18
iPhone and Android applications such as iGeriatrics and Medstopper provide clinicians with easy access to Beers Criteria and STOPP/START Criteria, respectively.
Medication reconciliation
Medication reconciliation—in which health care providers review a patient’s medication list at hospital admission and discharge, and even at routine office visits—is an increasingly common practice, especially with the implementation of EMRs. The patient’s prescribed and OTC medications, as well as dose, route, frequency, and indication, are updated, with the goal of maintaining the most accurate list. Health care providers can utilize both the Beers Criteria and the STOPP/START criteria in their reconciliation process to help reduce polypharmacy in the elderly. It is an essential step in maintaining communication between providers and ultimately decreasing the incidence of ADEs.17
IMPROVE … continuity of care
Polypharmacy can decrease patient likelihood to adhere to the regimen, whether due to confusion or intolerance.8 Patients should be included, along with caregivers and all medical providers, in a holistic assessment of the patient’s best interests in terms of long-term care and pharmacologic treatment, since those who have a sense of control in their treatment goals and expectations often achieve a better understanding of their medical status.10
However, educating patients about their medications is time-consuming, and time is often at a premium during a typical office visit. A pilot study of 28 male veterans (ages 85 and older)—the Integrated Management and Polypharmacy Review of Vulnerable Elders (IMPROVE) project—devised a model to combat this problem.21 As an adjunct to a visit with the PCP, a clinical pharmacist trained in patient education and medication management performed face-to-face clinical consults with patients and their caregivers. The results indicated that medical management by both the PCP and the pharmacist resulted in better medication management. The pharmacist was able to spend time with the patient and caregiver, resulting in individualized instructions, education, and strategies for safe and effective medication use. The PCP remained involved by cosigning the note with the pharmacist and was available for consultation, if needed.
In IMPROVE, 79% of patients had at least one medication discontinued and 75% had one or more dosing or timing adjustments made. Potentially inappropriate medications were reduced by 14%.21 When the researchers compared the six-month period before the trial with the six-month period afterward, they found an average pharmacy cost savings of $64 per veteran per month. There was also a decreasing trend in phone calls and visits to the PCP. Cost savings were comparable to or greater than those reported for similar interventions.21 There has not been sufficient long-term follow-up to assess this method’s effects on ADEs, morbidity, and mortality, however.
CONCLUSION
Managing medications in the elderly population is difficult, and polypharmacy is common due to the prevalence of patients with comorbidities. It is important for providers to be aware of possible drug interactions, prescribing cascades, and ADEs. Medications such as anticholinergics and antipsychotics pose an increased risk for ADEs, but the regular implementation of criteria such as Beers or STOPP/START in clinical practice will minimize overprescribing and improve health outcomes. These criteria should be used to supplement the clinical judgment and expertise of providers as a mainstay of patient care in the elderly.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818-1830.
2. Qato DM, Wilder J, Schumm LP. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
3. Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992;2(4):356-367.
4. Pedros C, Formiga F, Corbella X, Arnau J. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016:72(2):219-226.
5. Maher RL Jr, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
6. Nguyen PV-Q, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016;149(3):122-124.
7. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866.
8. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137.
9. Koronkowski M, Eisenhower C, Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann Longterm Care. 2016; 24(3):37-40.
10. Peron EP, Ogbonna KC, Donohoe KL. Diabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1): 17-vii.
11. Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3): 530-541.
12. Echt MA, Samelson EJ, Hannan MT, et al. Psychotropic drug initiation or increased dosage and the acute risk of falls: a prospective cohort study of nursing home residents. BMC Geriatrics. 2013;13:19.
13. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of antipsychotics: an update. EXCLI J. 2014;13: 1163-1191.
14. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:29-40.
15. FDA. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances (2005). www. fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 28, 2017.
16. Griffin CE III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-223.
17. Flanagan N, Beizer J. Medication reconciliation and education for older adults: using the 2015 AGS Beers Criteria as a guide. Home Healthc Now. 2016;34(10): 542-549.
18. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360-372.
19. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227-2246.
20. Salbu RL, Feuer J. A closer look at the 2015 Beers criteria. J Pharm Pract. 2017;30(4):419-424.
21. Mirk A, Echt KV, Vandenberg AE, et al. Polypharmacy review of vulnerable elders: can we IMPROVE outcomes? Fed Pract. 2016;33(3):39-41.
22. Saphris [package insert]. Irvine, CA: Allergan, USA, Inc; 2017.
23. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc; 2017.
24. Zyprexa [package insert]. Indianapolis, IN: Lilly USA LLC; 2017.
25. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017.
26. Midazolam hydrochloride injection solution [package insert]. Lake Forest, IL: Hospira Inc; 2017.
27. Diazepam oral solution and Diazepam Intensol oral solution concentrate [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corp; 2016.
28. Ativan tablet [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2013.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818-1830.
2. Qato DM, Wilder J, Schumm LP. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473-482.
3. Beard K. Adverse reactions as a cause of hospital admission in the aged. Drugs Aging. 1992;2(4):356-367.
4. Pedros C, Formiga F, Corbella X, Arnau J. Adverse drug reactions leading to urgent hospital admission in an elderly population: prevalence and main features. Eur J Clin Pharmacol. 2016:72(2):219-226.
5. Maher RL Jr, Hanlon JT, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65.
6. Nguyen PV-Q, Spinelli C. Prescribing cascade in an elderly woman. Can Pharm J (Ott). 2016;149(3):122-124.
7. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866.
8. Sivagnanam G. Deprescription: the prescription metabolism. J Pharmacol Pharmacother. 2016;7(3):133-137.
9. Koronkowski M, Eisenhower C, Marcum Z. An update on geriatric medication safety and challenges specific to the care of older adults. Ann Longterm Care. 2016; 24(3):37-40.
10. Peron EP, Ogbonna KC, Donohoe KL. Diabetic medications and polypharmacy. Clin Geriatr Med. 2015;31(1): 17-vii.
11. Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3): 530-541.
12. Echt MA, Samelson EJ, Hannan MT, et al. Psychotropic drug initiation or increased dosage and the acute risk of falls: a prospective cohort study of nursing home residents. BMC Geriatrics. 2013;13:19.
13. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of antipsychotics: an update. EXCLI J. 2014;13: 1163-1191.
14. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:29-40.
15. FDA. Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances (2005). www. fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed November 28, 2017.
16. Griffin CE III, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13:214-223.
17. Flanagan N, Beizer J. Medication reconciliation and education for older adults: using the 2015 AGS Beers Criteria as a guide. Home Healthc Now. 2016;34(10): 542-549.
18. Hill-Taylor B, Sketris I, Hayden J, et al. Application of the STOPP/START criteria: a systematic review of the prevalence of potentially inappropriate prescribing in older adults, and evidence of clinical, humanistic and economic impact. J Clin Pharm Ther. 2013;38(5):360-372.
19. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227-2246.
20. Salbu RL, Feuer J. A closer look at the 2015 Beers criteria. J Pharm Pract. 2017;30(4):419-424.
21. Mirk A, Echt KV, Vandenberg AE, et al. Polypharmacy review of vulnerable elders: can we IMPROVE outcomes? Fed Pract. 2016;33(3):39-41.
22. Saphris [package insert]. Irvine, CA: Allergan, USA, Inc; 2017.
23. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc; 2017.
24. Zyprexa [package insert]. Indianapolis, IN: Lilly USA LLC; 2017.
25. Seroquel [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2017.
26. Midazolam hydrochloride injection solution [package insert]. Lake Forest, IL: Hospira Inc; 2017.
27. Diazepam oral solution and Diazepam Intensol oral solution concentrate [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corp; 2016.
28. Ativan tablet [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2013.
Does fish oil during pregnancy help prevent asthma in kids?
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Fish oil supplementation taken by women in the third trimester of pregnancy can reduce the risk of persistent wheeze, asthma, and infections of the lower respiratory tract in their children.1
STRENGTH OF RECOMMENDATION
B: Based on 2 double-blinded randomized controlled trials (RCTs).
Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.1