User login
Psychiatric pharmacogenomics not ‘ready for prime time’
NEW YORK – Pharmacogenomics testing for guiding drug choices in psychiatric disease is “not completely ready for prime time,” based on a critical review of published guidelines and expert opinions on the use of those tests, according to Erika L. Nurmi, MD, PhD.
It is important to understand the limitations of such tests because many patients or family members are asking clinicians to be guided by the results of tests they have ordered on their own, said Dr. Nurmi, a researcher and clinician at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
Published guidelines and expert opinions based on objective data support these conclusions, she said. Dr. Nurmi suggested that .
“Basically, what it says is if you do not have the testing in hand, don’t order it. If you have the testing in hand when a poor metabolizer of CYP2D6 or CYP2C19 has been identified, switch to a med that is not metabolized by those enzymes. That’s it,” Dr. Nurmi reported at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
The guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC) were only modestly more detailed. Only a moderate level of evidence supported most recommendations, she said, and these were labeled optional. The exception regarded treating ultrafast metabolizers of CYP2D6 who are taking paroxetine: In these, the use of a drug metabolized by a different enzyme was strongly recommended.
Similar recommendations in the CPIC guidelines were made for fluoxetine, fluvoxamine, and sertraline. In patients on citalopram or escitalopram, ultrafast metabolizers of CYP2C19 are considered candidates for a non-CYP2C19 drug. If they are poor metabolizers, the CPIC guidelines recommended a non-CYP2C19 drug or reducing the starting dose by 50%.
However, in all of these cases, pharmacogenomics testing is best reserved for patients who have had an inadequate response to therapy or, in the case of poor metabolizers, have had unacceptable adverse events.
Of the limitations Dr. Nurmi outlined for pharmacogenomics testing, one of the most important is that these tests typically focus on a single genetic variant. According to Dr. Nurmi, the problem with a single variant is that “our bodies are more complex.” She said she believes that genetic information for drug selection will not be useful until testing is able to synthesize information from multiple genetic variants and place this in context with confounders such as age and exposure to other substances, such as hormones, caffeine, or grapefruit juice.
This complexity is likely to be mastered eventually, Dr. Nurmi said, but patients now have unrealistic expectations. For their part, clinicians need to develop an understanding of the limitations of these tests in order to provide informed counsel. As pharmacogenomics testing is being marketed directly to consumers with inflated claims about its value, clinicians often must defend their decision to use or not use this information.
“Commercially available products combine variants of widely discrepant validity using proprietary, undisclosed algorithms into sweeping treatment recommendations,” said Dr. Nurmi, who noted that she has found some of her own data misappropriated to make claims. Often, the companies that develop the tests have conducted the validation studies without any replication by independent investigators. She noted that many studies have been declared positive on the basis of secondary outcomes after the primary outcome was negative.
“There are very few positive prospective, randomized, double-blind trials,” Dr. Nurmi said. Even when such trials have been conducted, they typically are not designed to show a clinically meaningful outcome.
By attempting to look at a single or a limited number of variants in which to guide choice of medication in psychiatric disease, pharmacogenomics testing is being “vastly oversimplified,” Dr. Nurmi said. Although she said she believes this field is enormously promising and that medical records for each patient eventually will contain the genome sequence, she emphasized that, at this time, pharmacogenomics testing has a very limited role to play for the management of psychiatric diseases.
Dr. Nurmi reported she had no financial relationships relevant to this topic.
NEW YORK – Pharmacogenomics testing for guiding drug choices in psychiatric disease is “not completely ready for prime time,” based on a critical review of published guidelines and expert opinions on the use of those tests, according to Erika L. Nurmi, MD, PhD.
It is important to understand the limitations of such tests because many patients or family members are asking clinicians to be guided by the results of tests they have ordered on their own, said Dr. Nurmi, a researcher and clinician at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
Published guidelines and expert opinions based on objective data support these conclusions, she said. Dr. Nurmi suggested that .
“Basically, what it says is if you do not have the testing in hand, don’t order it. If you have the testing in hand when a poor metabolizer of CYP2D6 or CYP2C19 has been identified, switch to a med that is not metabolized by those enzymes. That’s it,” Dr. Nurmi reported at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
The guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC) were only modestly more detailed. Only a moderate level of evidence supported most recommendations, she said, and these were labeled optional. The exception regarded treating ultrafast metabolizers of CYP2D6 who are taking paroxetine: In these, the use of a drug metabolized by a different enzyme was strongly recommended.
Similar recommendations in the CPIC guidelines were made for fluoxetine, fluvoxamine, and sertraline. In patients on citalopram or escitalopram, ultrafast metabolizers of CYP2C19 are considered candidates for a non-CYP2C19 drug. If they are poor metabolizers, the CPIC guidelines recommended a non-CYP2C19 drug or reducing the starting dose by 50%.
However, in all of these cases, pharmacogenomics testing is best reserved for patients who have had an inadequate response to therapy or, in the case of poor metabolizers, have had unacceptable adverse events.
Of the limitations Dr. Nurmi outlined for pharmacogenomics testing, one of the most important is that these tests typically focus on a single genetic variant. According to Dr. Nurmi, the problem with a single variant is that “our bodies are more complex.” She said she believes that genetic information for drug selection will not be useful until testing is able to synthesize information from multiple genetic variants and place this in context with confounders such as age and exposure to other substances, such as hormones, caffeine, or grapefruit juice.
This complexity is likely to be mastered eventually, Dr. Nurmi said, but patients now have unrealistic expectations. For their part, clinicians need to develop an understanding of the limitations of these tests in order to provide informed counsel. As pharmacogenomics testing is being marketed directly to consumers with inflated claims about its value, clinicians often must defend their decision to use or not use this information.
“Commercially available products combine variants of widely discrepant validity using proprietary, undisclosed algorithms into sweeping treatment recommendations,” said Dr. Nurmi, who noted that she has found some of her own data misappropriated to make claims. Often, the companies that develop the tests have conducted the validation studies without any replication by independent investigators. She noted that many studies have been declared positive on the basis of secondary outcomes after the primary outcome was negative.
“There are very few positive prospective, randomized, double-blind trials,” Dr. Nurmi said. Even when such trials have been conducted, they typically are not designed to show a clinically meaningful outcome.
By attempting to look at a single or a limited number of variants in which to guide choice of medication in psychiatric disease, pharmacogenomics testing is being “vastly oversimplified,” Dr. Nurmi said. Although she said she believes this field is enormously promising and that medical records for each patient eventually will contain the genome sequence, she emphasized that, at this time, pharmacogenomics testing has a very limited role to play for the management of psychiatric diseases.
Dr. Nurmi reported she had no financial relationships relevant to this topic.
NEW YORK – Pharmacogenomics testing for guiding drug choices in psychiatric disease is “not completely ready for prime time,” based on a critical review of published guidelines and expert opinions on the use of those tests, according to Erika L. Nurmi, MD, PhD.
It is important to understand the limitations of such tests because many patients or family members are asking clinicians to be guided by the results of tests they have ordered on their own, said Dr. Nurmi, a researcher and clinician at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
Published guidelines and expert opinions based on objective data support these conclusions, she said. Dr. Nurmi suggested that .
“Basically, what it says is if you do not have the testing in hand, don’t order it. If you have the testing in hand when a poor metabolizer of CYP2D6 or CYP2C19 has been identified, switch to a med that is not metabolized by those enzymes. That’s it,” Dr. Nurmi reported at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
The guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC) were only modestly more detailed. Only a moderate level of evidence supported most recommendations, she said, and these were labeled optional. The exception regarded treating ultrafast metabolizers of CYP2D6 who are taking paroxetine: In these, the use of a drug metabolized by a different enzyme was strongly recommended.
Similar recommendations in the CPIC guidelines were made for fluoxetine, fluvoxamine, and sertraline. In patients on citalopram or escitalopram, ultrafast metabolizers of CYP2C19 are considered candidates for a non-CYP2C19 drug. If they are poor metabolizers, the CPIC guidelines recommended a non-CYP2C19 drug or reducing the starting dose by 50%.
However, in all of these cases, pharmacogenomics testing is best reserved for patients who have had an inadequate response to therapy or, in the case of poor metabolizers, have had unacceptable adverse events.
Of the limitations Dr. Nurmi outlined for pharmacogenomics testing, one of the most important is that these tests typically focus on a single genetic variant. According to Dr. Nurmi, the problem with a single variant is that “our bodies are more complex.” She said she believes that genetic information for drug selection will not be useful until testing is able to synthesize information from multiple genetic variants and place this in context with confounders such as age and exposure to other substances, such as hormones, caffeine, or grapefruit juice.
This complexity is likely to be mastered eventually, Dr. Nurmi said, but patients now have unrealistic expectations. For their part, clinicians need to develop an understanding of the limitations of these tests in order to provide informed counsel. As pharmacogenomics testing is being marketed directly to consumers with inflated claims about its value, clinicians often must defend their decision to use or not use this information.
“Commercially available products combine variants of widely discrepant validity using proprietary, undisclosed algorithms into sweeping treatment recommendations,” said Dr. Nurmi, who noted that she has found some of her own data misappropriated to make claims. Often, the companies that develop the tests have conducted the validation studies without any replication by independent investigators. She noted that many studies have been declared positive on the basis of secondary outcomes after the primary outcome was negative.
“There are very few positive prospective, randomized, double-blind trials,” Dr. Nurmi said. Even when such trials have been conducted, they typically are not designed to show a clinically meaningful outcome.
By attempting to look at a single or a limited number of variants in which to guide choice of medication in psychiatric disease, pharmacogenomics testing is being “vastly oversimplified,” Dr. Nurmi said. Although she said she believes this field is enormously promising and that medical records for each patient eventually will contain the genome sequence, she emphasized that, at this time, pharmacogenomics testing has a very limited role to play for the management of psychiatric diseases.
Dr. Nurmi reported she had no financial relationships relevant to this topic.
EXPERT ANALYSIS FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
PCOS may influence the diversity of the gut microbiome
according to a study from the Journal of Clinical Endocrinology and Metabolism.
“This study demonstrated that Caucasian women diagnosed with PCOS using the Rotterdam criteria had a reduction in overall species richness [alpha diversity] of the gut microbiome, compared to healthy women, and changes in the composition of the microbial community [beta diversity]” wrote Pedro J. Torres and his associates. “Interestingly, our study found that the biodiversity of the microbiome strongly correlated with hyperandrogenism.”
Dr. Torres of the University of California, San Diego, and his colleagues recruited 163 women at the University of Poznan (Poland) and conducted analysis on fecal samples to determine the effects of PCOS on the gut microbiome. Each woman underwent a battery of tests to determine whether she had PCOS or polycystic ovarian morphology (PCOM). Ovarian morphology was determined from a transvaginal ultrasound evaluation. The women were assessed for body mass index and hirsutism. Blood samples were taken to test for hormonal abnormalities common with PCOS and metabolic issues, like type 2 diabetes mellitus and glucose tolerance. Fecal samples were taken to analyze the gut microbiota of each study participant; analysis of the fecal samples generated gut microbial diversity profiles for each of the 163 women. Analysis of the samples was conducted at the University of California, San Diego.
Of the subjects, 48 were healthy, 42 had PCOM, and 73 were diagnosed with PCOS. The researches noted that, compared with healthy women and those with PCOM, women with PCOS had higher levels of serum total and free testosterone, as well as higher rates of hirsutism and fewer menses per year. These women also had higher levels of serum luteinizing hormone and increased ratios of luteinizing hormone to follicle stimulating hormone.
The DNA analysis of fecal samples yielded 481 sequence variants from the fecal swabs. Women with PCOS were found to have lower alpha diversity in their gut microbiome, as evidenced by abundance (P = .04) and Faith’s phylogenetic diveristy (P = .02). The luteinizing hormone to follicle stimulating hormone ratio also appeared to affect the alpha diversity of women with PCOS, as seen in observed sequence variants and Faith’s phylogenetic diversity (P = .08).
Beta diversity analysis, or the biodiversity between samples, revealed that hyperandrogenism could be a primary driver of changes in the gut microbiome. Using permutational multivariate analysis of variance, researchers determined that hyperandrogenism significantly affected beta diversity (P = .0009).
Androgens may help affect the gut microbiome in important ways, and changes in the gut microbiome may influence how the pathology of PCOS develops, according to Mr. Torres and his colleagues; however, more studies should be conducted to determine the effects of androgens on the gut microbiome.
“If hyperandrogenism drives the microbial composition of the gut, it would be interesting to determine if treatment of PCOS with androgen antagonists or oral contraceptives results in recovery of the gut microbiome and improvement of the PCOS metabolic phenotype” wrote Mr. Torres and his colleagues. “Moreover, it would be informative to determine whether the gut microbiome of women diagnosed with PCOS using the criteria of oligomenorrhea and polycystic ovaries is distinct from that of women diagnosed with the other subtypes of PCOS that include hyperandrogenism.”
The authors had no relevant financial disclosures to report.
SOURCE: Torres PJ et al. J Clin Endocrinol Metab. 2018 Jan 23. doi: 10.1210/jc.2017-02153.
Polycystic ovarian syndrome (PCOS) can manifest itself in many ways, but this study reveals that it can directly affect the metabolism of those who have the disorder.
“We’re still early days in studying this, but this study suggests that one of the clinical characteristics of these women with this disorder – their elevated testosterone – is correlated with changes in the gut microbiome,” Varykina G. Thackray, PhD, of the department of reproductive medicine and the center for reproductive science and medicine at the University of California, San Diego, said in an interview. “That means that these women are in a different group than other people with metabolic disorders, and it potentially gives us a way to think of new therapies that might be helpful for this specific group of women.”
When asked whether fecal transplants may be a potential therapy to help treat the metabolic issues associated with PCOS, Dr. Thackray stated that she did not believe a lot of women would use that as a therapy because of the “ick” factor. She stated the goal is to identify some beneficial bacteria that could be taken as a probiotic to help restore the gut microbiome.
Unfortunately, researchers still do not understand what causes PCOS. Some studies suggest that there are environmental and genetic factors, but there is nothing definitive. Dr. Thackray stated that getting more funding and conducting more research are the best ways to understand and combat this disorder.
Dr. Thackray is an associate professor of reproductive medicine at the University of California, San Diego.
Polycystic ovarian syndrome (PCOS) can manifest itself in many ways, but this study reveals that it can directly affect the metabolism of those who have the disorder.
“We’re still early days in studying this, but this study suggests that one of the clinical characteristics of these women with this disorder – their elevated testosterone – is correlated with changes in the gut microbiome,” Varykina G. Thackray, PhD, of the department of reproductive medicine and the center for reproductive science and medicine at the University of California, San Diego, said in an interview. “That means that these women are in a different group than other people with metabolic disorders, and it potentially gives us a way to think of new therapies that might be helpful for this specific group of women.”
When asked whether fecal transplants may be a potential therapy to help treat the metabolic issues associated with PCOS, Dr. Thackray stated that she did not believe a lot of women would use that as a therapy because of the “ick” factor. She stated the goal is to identify some beneficial bacteria that could be taken as a probiotic to help restore the gut microbiome.
Unfortunately, researchers still do not understand what causes PCOS. Some studies suggest that there are environmental and genetic factors, but there is nothing definitive. Dr. Thackray stated that getting more funding and conducting more research are the best ways to understand and combat this disorder.
Dr. Thackray is an associate professor of reproductive medicine at the University of California, San Diego.
Polycystic ovarian syndrome (PCOS) can manifest itself in many ways, but this study reveals that it can directly affect the metabolism of those who have the disorder.
“We’re still early days in studying this, but this study suggests that one of the clinical characteristics of these women with this disorder – their elevated testosterone – is correlated with changes in the gut microbiome,” Varykina G. Thackray, PhD, of the department of reproductive medicine and the center for reproductive science and medicine at the University of California, San Diego, said in an interview. “That means that these women are in a different group than other people with metabolic disorders, and it potentially gives us a way to think of new therapies that might be helpful for this specific group of women.”
When asked whether fecal transplants may be a potential therapy to help treat the metabolic issues associated with PCOS, Dr. Thackray stated that she did not believe a lot of women would use that as a therapy because of the “ick” factor. She stated the goal is to identify some beneficial bacteria that could be taken as a probiotic to help restore the gut microbiome.
Unfortunately, researchers still do not understand what causes PCOS. Some studies suggest that there are environmental and genetic factors, but there is nothing definitive. Dr. Thackray stated that getting more funding and conducting more research are the best ways to understand and combat this disorder.
Dr. Thackray is an associate professor of reproductive medicine at the University of California, San Diego.
according to a study from the Journal of Clinical Endocrinology and Metabolism.
“This study demonstrated that Caucasian women diagnosed with PCOS using the Rotterdam criteria had a reduction in overall species richness [alpha diversity] of the gut microbiome, compared to healthy women, and changes in the composition of the microbial community [beta diversity]” wrote Pedro J. Torres and his associates. “Interestingly, our study found that the biodiversity of the microbiome strongly correlated with hyperandrogenism.”
Dr. Torres of the University of California, San Diego, and his colleagues recruited 163 women at the University of Poznan (Poland) and conducted analysis on fecal samples to determine the effects of PCOS on the gut microbiome. Each woman underwent a battery of tests to determine whether she had PCOS or polycystic ovarian morphology (PCOM). Ovarian morphology was determined from a transvaginal ultrasound evaluation. The women were assessed for body mass index and hirsutism. Blood samples were taken to test for hormonal abnormalities common with PCOS and metabolic issues, like type 2 diabetes mellitus and glucose tolerance. Fecal samples were taken to analyze the gut microbiota of each study participant; analysis of the fecal samples generated gut microbial diversity profiles for each of the 163 women. Analysis of the samples was conducted at the University of California, San Diego.
Of the subjects, 48 were healthy, 42 had PCOM, and 73 were diagnosed with PCOS. The researches noted that, compared with healthy women and those with PCOM, women with PCOS had higher levels of serum total and free testosterone, as well as higher rates of hirsutism and fewer menses per year. These women also had higher levels of serum luteinizing hormone and increased ratios of luteinizing hormone to follicle stimulating hormone.
The DNA analysis of fecal samples yielded 481 sequence variants from the fecal swabs. Women with PCOS were found to have lower alpha diversity in their gut microbiome, as evidenced by abundance (P = .04) and Faith’s phylogenetic diveristy (P = .02). The luteinizing hormone to follicle stimulating hormone ratio also appeared to affect the alpha diversity of women with PCOS, as seen in observed sequence variants and Faith’s phylogenetic diversity (P = .08).
Beta diversity analysis, or the biodiversity between samples, revealed that hyperandrogenism could be a primary driver of changes in the gut microbiome. Using permutational multivariate analysis of variance, researchers determined that hyperandrogenism significantly affected beta diversity (P = .0009).
Androgens may help affect the gut microbiome in important ways, and changes in the gut microbiome may influence how the pathology of PCOS develops, according to Mr. Torres and his colleagues; however, more studies should be conducted to determine the effects of androgens on the gut microbiome.
“If hyperandrogenism drives the microbial composition of the gut, it would be interesting to determine if treatment of PCOS with androgen antagonists or oral contraceptives results in recovery of the gut microbiome and improvement of the PCOS metabolic phenotype” wrote Mr. Torres and his colleagues. “Moreover, it would be informative to determine whether the gut microbiome of women diagnosed with PCOS using the criteria of oligomenorrhea and polycystic ovaries is distinct from that of women diagnosed with the other subtypes of PCOS that include hyperandrogenism.”
The authors had no relevant financial disclosures to report.
SOURCE: Torres PJ et al. J Clin Endocrinol Metab. 2018 Jan 23. doi: 10.1210/jc.2017-02153.
according to a study from the Journal of Clinical Endocrinology and Metabolism.
“This study demonstrated that Caucasian women diagnosed with PCOS using the Rotterdam criteria had a reduction in overall species richness [alpha diversity] of the gut microbiome, compared to healthy women, and changes in the composition of the microbial community [beta diversity]” wrote Pedro J. Torres and his associates. “Interestingly, our study found that the biodiversity of the microbiome strongly correlated with hyperandrogenism.”
Dr. Torres of the University of California, San Diego, and his colleagues recruited 163 women at the University of Poznan (Poland) and conducted analysis on fecal samples to determine the effects of PCOS on the gut microbiome. Each woman underwent a battery of tests to determine whether she had PCOS or polycystic ovarian morphology (PCOM). Ovarian morphology was determined from a transvaginal ultrasound evaluation. The women were assessed for body mass index and hirsutism. Blood samples were taken to test for hormonal abnormalities common with PCOS and metabolic issues, like type 2 diabetes mellitus and glucose tolerance. Fecal samples were taken to analyze the gut microbiota of each study participant; analysis of the fecal samples generated gut microbial diversity profiles for each of the 163 women. Analysis of the samples was conducted at the University of California, San Diego.
Of the subjects, 48 were healthy, 42 had PCOM, and 73 were diagnosed with PCOS. The researches noted that, compared with healthy women and those with PCOM, women with PCOS had higher levels of serum total and free testosterone, as well as higher rates of hirsutism and fewer menses per year. These women also had higher levels of serum luteinizing hormone and increased ratios of luteinizing hormone to follicle stimulating hormone.
The DNA analysis of fecal samples yielded 481 sequence variants from the fecal swabs. Women with PCOS were found to have lower alpha diversity in their gut microbiome, as evidenced by abundance (P = .04) and Faith’s phylogenetic diveristy (P = .02). The luteinizing hormone to follicle stimulating hormone ratio also appeared to affect the alpha diversity of women with PCOS, as seen in observed sequence variants and Faith’s phylogenetic diversity (P = .08).
Beta diversity analysis, or the biodiversity between samples, revealed that hyperandrogenism could be a primary driver of changes in the gut microbiome. Using permutational multivariate analysis of variance, researchers determined that hyperandrogenism significantly affected beta diversity (P = .0009).
Androgens may help affect the gut microbiome in important ways, and changes in the gut microbiome may influence how the pathology of PCOS develops, according to Mr. Torres and his colleagues; however, more studies should be conducted to determine the effects of androgens on the gut microbiome.
“If hyperandrogenism drives the microbial composition of the gut, it would be interesting to determine if treatment of PCOS with androgen antagonists or oral contraceptives results in recovery of the gut microbiome and improvement of the PCOS metabolic phenotype” wrote Mr. Torres and his colleagues. “Moreover, it would be informative to determine whether the gut microbiome of women diagnosed with PCOS using the criteria of oligomenorrhea and polycystic ovaries is distinct from that of women diagnosed with the other subtypes of PCOS that include hyperandrogenism.”
The authors had no relevant financial disclosures to report.
SOURCE: Torres PJ et al. J Clin Endocrinol Metab. 2018 Jan 23. doi: 10.1210/jc.2017-02153.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Key clinical point: Hyperandrogenism may have an effect on the gut microbiome of women with PCOS.
Major finding: Lower bacterial diversity was observed in women with PCOS, compared with healthy women
Study details: Researchers recruited 163 women diagnosed with PCOS. Blood and fecal samples were collected, and ovaries were imaged using ultrasound.
Disclosures: The authors had no relevant financial disclosures to report.
Source: Torres PJ et al. J Clin Endocrinol Metab. 2018 Jan 23. doi: 10.1210/jc.2017-02153.
Abrupt behavior changes in autism? ID medical triggers first
NEW YORK – When treating children with autism spectrum disorder who develop an abrupt increase in symptoms, it is best to identify and treat the precipitating event or events – rather than intensify ASD drug therapy, an expert said.
“These acute behavior changes are almost always triggered by something,” Jeremy Veenstra-VanderWeele, MD, reported at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry. Triggers are not always identifiable, but Dr. Veenstra-VanderWeele said solutions may prove simple when they are.
In ASD patients with an acute change in behavior, caregivers typically think first of environmental triggers, including adverse interactions with peers or siblings. But Dr. Veenstra-VanderWeele emphasized that medical problems should be considered first. This makes sense because of the importance of quickly resolving health problems. However, pain and discomfort, particularly in those with difficulty verbalizing these complaints, can be overlooked.
Moreover, even highly verbal ASD patients may not volunteer physical complaints without prompting, Dr. Veenstra-VanderWeele said. Among the health issues in children, constipation and other gastrointestinal issues are “incredibly common” in ASD patients. Dr. Veenstra-VanderWeele looks for clues, such as body posturing suggesting abdominal pain or flatulence, when a history is ambiguous.
“I will order an abdominal flat plate when I hear enough symptoms to make me wonder when the family is not sure,” Dr. Veenstra-VanderWeele reported. “Almost always it comes back with evidence of constipation. We treat it, and they are less irritable like all of us would be.”
All common conditions in a pediatric population, including ear infections, dental caries, and food allergies, should be considered, according to Dr. Veenstra-VanderWeele, who recommended a practice pathway for evaluating triggers in children with ASD (Pediatrics. 2016 Feb;137 Suppl 2:S136-48). A coauthor on this pathway, Dr. Veenstra-VanderWeele emphasized the importance of pursuing a systematic approach to medical issues before considering other triggers, such as psychosocial stressors.
In adolescents, headache caused by migraine and late-onset epilepsy, often in the form of complex partial seizures, should be added to the list of potential triggers for irritation or aggression, Dr. Veenstra-VanderWeele said. Epilepsy often precedes the diagnosis of ASD in young children, and Dr. Veenstra-VanderWeele noted that a second peak incidence sometimes occurs in late adolescence.
After ruling out medical problems, helping patients recognize and verbalize stressors can serve as both diagnosis and treatment. In ASD patients with limited verbal skills who are suffering from stress, “aggression is one form of communication,” Dr. Veenstra-VanderWeele said.
However, Dr. Veenstra-VanderWeele cautioned that, even if a trigger is successfully addressed, inadvertently reinforced aggression might persist.
“Aggression can be rewarded sometimes by removing the patient from the classroom, sometimes by giving in, and then that becomes a maladaptive reinforcement pattern that needs to be broken,” Dr. Veenstra-VanderWeele said. “Even if you are treating their irritability and agitation with, say, risperidone, you still need to break the maladaptive reinforcement pattern or they will keep engaging in what has become instrumental aggression.”
Dr. Veenstra-VanderWeele reported financial relationships with Hoffmann-La Roche, Novartis, Seaside Therapeutics, and SynapDx.
NEW YORK – When treating children with autism spectrum disorder who develop an abrupt increase in symptoms, it is best to identify and treat the precipitating event or events – rather than intensify ASD drug therapy, an expert said.
“These acute behavior changes are almost always triggered by something,” Jeremy Veenstra-VanderWeele, MD, reported at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry. Triggers are not always identifiable, but Dr. Veenstra-VanderWeele said solutions may prove simple when they are.
In ASD patients with an acute change in behavior, caregivers typically think first of environmental triggers, including adverse interactions with peers or siblings. But Dr. Veenstra-VanderWeele emphasized that medical problems should be considered first. This makes sense because of the importance of quickly resolving health problems. However, pain and discomfort, particularly in those with difficulty verbalizing these complaints, can be overlooked.
Moreover, even highly verbal ASD patients may not volunteer physical complaints without prompting, Dr. Veenstra-VanderWeele said. Among the health issues in children, constipation and other gastrointestinal issues are “incredibly common” in ASD patients. Dr. Veenstra-VanderWeele looks for clues, such as body posturing suggesting abdominal pain or flatulence, when a history is ambiguous.
“I will order an abdominal flat plate when I hear enough symptoms to make me wonder when the family is not sure,” Dr. Veenstra-VanderWeele reported. “Almost always it comes back with evidence of constipation. We treat it, and they are less irritable like all of us would be.”
All common conditions in a pediatric population, including ear infections, dental caries, and food allergies, should be considered, according to Dr. Veenstra-VanderWeele, who recommended a practice pathway for evaluating triggers in children with ASD (Pediatrics. 2016 Feb;137 Suppl 2:S136-48). A coauthor on this pathway, Dr. Veenstra-VanderWeele emphasized the importance of pursuing a systematic approach to medical issues before considering other triggers, such as psychosocial stressors.
In adolescents, headache caused by migraine and late-onset epilepsy, often in the form of complex partial seizures, should be added to the list of potential triggers for irritation or aggression, Dr. Veenstra-VanderWeele said. Epilepsy often precedes the diagnosis of ASD in young children, and Dr. Veenstra-VanderWeele noted that a second peak incidence sometimes occurs in late adolescence.
After ruling out medical problems, helping patients recognize and verbalize stressors can serve as both diagnosis and treatment. In ASD patients with limited verbal skills who are suffering from stress, “aggression is one form of communication,” Dr. Veenstra-VanderWeele said.
However, Dr. Veenstra-VanderWeele cautioned that, even if a trigger is successfully addressed, inadvertently reinforced aggression might persist.
“Aggression can be rewarded sometimes by removing the patient from the classroom, sometimes by giving in, and then that becomes a maladaptive reinforcement pattern that needs to be broken,” Dr. Veenstra-VanderWeele said. “Even if you are treating their irritability and agitation with, say, risperidone, you still need to break the maladaptive reinforcement pattern or they will keep engaging in what has become instrumental aggression.”
Dr. Veenstra-VanderWeele reported financial relationships with Hoffmann-La Roche, Novartis, Seaside Therapeutics, and SynapDx.
NEW YORK – When treating children with autism spectrum disorder who develop an abrupt increase in symptoms, it is best to identify and treat the precipitating event or events – rather than intensify ASD drug therapy, an expert said.
“These acute behavior changes are almost always triggered by something,” Jeremy Veenstra-VanderWeele, MD, reported at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry. Triggers are not always identifiable, but Dr. Veenstra-VanderWeele said solutions may prove simple when they are.
In ASD patients with an acute change in behavior, caregivers typically think first of environmental triggers, including adverse interactions with peers or siblings. But Dr. Veenstra-VanderWeele emphasized that medical problems should be considered first. This makes sense because of the importance of quickly resolving health problems. However, pain and discomfort, particularly in those with difficulty verbalizing these complaints, can be overlooked.
Moreover, even highly verbal ASD patients may not volunteer physical complaints without prompting, Dr. Veenstra-VanderWeele said. Among the health issues in children, constipation and other gastrointestinal issues are “incredibly common” in ASD patients. Dr. Veenstra-VanderWeele looks for clues, such as body posturing suggesting abdominal pain or flatulence, when a history is ambiguous.
“I will order an abdominal flat plate when I hear enough symptoms to make me wonder when the family is not sure,” Dr. Veenstra-VanderWeele reported. “Almost always it comes back with evidence of constipation. We treat it, and they are less irritable like all of us would be.”
All common conditions in a pediatric population, including ear infections, dental caries, and food allergies, should be considered, according to Dr. Veenstra-VanderWeele, who recommended a practice pathway for evaluating triggers in children with ASD (Pediatrics. 2016 Feb;137 Suppl 2:S136-48). A coauthor on this pathway, Dr. Veenstra-VanderWeele emphasized the importance of pursuing a systematic approach to medical issues before considering other triggers, such as psychosocial stressors.
In adolescents, headache caused by migraine and late-onset epilepsy, often in the form of complex partial seizures, should be added to the list of potential triggers for irritation or aggression, Dr. Veenstra-VanderWeele said. Epilepsy often precedes the diagnosis of ASD in young children, and Dr. Veenstra-VanderWeele noted that a second peak incidence sometimes occurs in late adolescence.
After ruling out medical problems, helping patients recognize and verbalize stressors can serve as both diagnosis and treatment. In ASD patients with limited verbal skills who are suffering from stress, “aggression is one form of communication,” Dr. Veenstra-VanderWeele said.
However, Dr. Veenstra-VanderWeele cautioned that, even if a trigger is successfully addressed, inadvertently reinforced aggression might persist.
“Aggression can be rewarded sometimes by removing the patient from the classroom, sometimes by giving in, and then that becomes a maladaptive reinforcement pattern that needs to be broken,” Dr. Veenstra-VanderWeele said. “Even if you are treating their irritability and agitation with, say, risperidone, you still need to break the maladaptive reinforcement pattern or they will keep engaging in what has become instrumental aggression.”
Dr. Veenstra-VanderWeele reported financial relationships with Hoffmann-La Roche, Novartis, Seaside Therapeutics, and SynapDx.
EXPERT ANALYSIS FROM the PSYCHOPHARMACOLOGY UPDATE INSTITUTE
No improvement in sight for Alzheimer’s drug development
Another one bites the dust.
Yet another investigational agent joins intepirdine, verubecestat, solanezumab, bapineuzumab, latrepirdine, and many others on the scrap pile of research: The complete release of trial data on idalopirdine found the drug wasn’t of clinically significant benefit in Alzheimer’s disease (JAMA. 2018;319[2]:130-42).
The numbers are bad enough that a handful of companies, including the giant Pfizer, have decided to leave Alzheimer’s drug development entirely to focus on more promising fields. And I get that. All of us – on any exhausting, fruitless, task – will reach the point where it’s time to cut our losses and move on. I don’t blame these companies for mostly leaving the field. (Pfizer is planning to form a neuroscience venture fund to support further research.)
Optimists will argue that you still learn things from a negative trial, which is true, but nothing to date is on the immediate horizon to help. The five agents we’ve had available for the past 15-20 years are all old enough to have lost their patents, and their benefits are modest, at best.
And all this going on as the overall human population, including myself, gradually ages and dementia becomes a medical-cost time bomb on the horizon. This isn’t an American problem. Every country in the world is facing it.
Politicians love to promise hope for these things: creating fast-track programs to get drugs to market faster, finding ways to bring down costs so more people can afford them, and improving methods to treat those in need. But none of those things matter if the medications don’t work.
Many of these trials test similar molecules because the evidence to date suggests they’re targeting the cause of Alzheimer’s. But so far they aren’t working. What if, as the Firesign Theatre and others have said, everything you know is wrong?
Perhaps our greatest quality as a species is resilience. We go on because we have to. The planet keeps moving around the sun as it has for almost 5 billion years, and we face tomorrow. Caregivers wake up for another day of doing their best for a faltering parent. I wake up for another day of doing my best to help them. And the researchers go back for another day hoping to find the real answer and treatment. Without trying, no treatment for anything will ever be found. We owe our patients, and ourselves, a better future than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Another one bites the dust.
Yet another investigational agent joins intepirdine, verubecestat, solanezumab, bapineuzumab, latrepirdine, and many others on the scrap pile of research: The complete release of trial data on idalopirdine found the drug wasn’t of clinically significant benefit in Alzheimer’s disease (JAMA. 2018;319[2]:130-42).
The numbers are bad enough that a handful of companies, including the giant Pfizer, have decided to leave Alzheimer’s drug development entirely to focus on more promising fields. And I get that. All of us – on any exhausting, fruitless, task – will reach the point where it’s time to cut our losses and move on. I don’t blame these companies for mostly leaving the field. (Pfizer is planning to form a neuroscience venture fund to support further research.)
Optimists will argue that you still learn things from a negative trial, which is true, but nothing to date is on the immediate horizon to help. The five agents we’ve had available for the past 15-20 years are all old enough to have lost their patents, and their benefits are modest, at best.
And all this going on as the overall human population, including myself, gradually ages and dementia becomes a medical-cost time bomb on the horizon. This isn’t an American problem. Every country in the world is facing it.
Politicians love to promise hope for these things: creating fast-track programs to get drugs to market faster, finding ways to bring down costs so more people can afford them, and improving methods to treat those in need. But none of those things matter if the medications don’t work.
Many of these trials test similar molecules because the evidence to date suggests they’re targeting the cause of Alzheimer’s. But so far they aren’t working. What if, as the Firesign Theatre and others have said, everything you know is wrong?
Perhaps our greatest quality as a species is resilience. We go on because we have to. The planet keeps moving around the sun as it has for almost 5 billion years, and we face tomorrow. Caregivers wake up for another day of doing their best for a faltering parent. I wake up for another day of doing my best to help them. And the researchers go back for another day hoping to find the real answer and treatment. Without trying, no treatment for anything will ever be found. We owe our patients, and ourselves, a better future than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Another one bites the dust.
Yet another investigational agent joins intepirdine, verubecestat, solanezumab, bapineuzumab, latrepirdine, and many others on the scrap pile of research: The complete release of trial data on idalopirdine found the drug wasn’t of clinically significant benefit in Alzheimer’s disease (JAMA. 2018;319[2]:130-42).
The numbers are bad enough that a handful of companies, including the giant Pfizer, have decided to leave Alzheimer’s drug development entirely to focus on more promising fields. And I get that. All of us – on any exhausting, fruitless, task – will reach the point where it’s time to cut our losses and move on. I don’t blame these companies for mostly leaving the field. (Pfizer is planning to form a neuroscience venture fund to support further research.)
Optimists will argue that you still learn things from a negative trial, which is true, but nothing to date is on the immediate horizon to help. The five agents we’ve had available for the past 15-20 years are all old enough to have lost their patents, and their benefits are modest, at best.
And all this going on as the overall human population, including myself, gradually ages and dementia becomes a medical-cost time bomb on the horizon. This isn’t an American problem. Every country in the world is facing it.
Politicians love to promise hope for these things: creating fast-track programs to get drugs to market faster, finding ways to bring down costs so more people can afford them, and improving methods to treat those in need. But none of those things matter if the medications don’t work.
Many of these trials test similar molecules because the evidence to date suggests they’re targeting the cause of Alzheimer’s. But so far they aren’t working. What if, as the Firesign Theatre and others have said, everything you know is wrong?
Perhaps our greatest quality as a species is resilience. We go on because we have to. The planet keeps moving around the sun as it has for almost 5 billion years, and we face tomorrow. Caregivers wake up for another day of doing their best for a faltering parent. I wake up for another day of doing my best to help them. And the researchers go back for another day hoping to find the real answer and treatment. Without trying, no treatment for anything will ever be found. We owe our patients, and ourselves, a better future than that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
VIDEO: Could targeting gut dysbiosis in MS prevent disease?
SAN DIEGO – Compelling findings in a genetically engineered mouse model of multiple sclerosis identify mechanisms of how adolescence and gut dysbiosis contribute to the risk of MS. In addition, disparities in gut microbiome species could explain why some people are at higher risk for developing multiple sclerosis, while others seem to enjoy a protective effect against development of this and other autoimmune diseases.
The hope is that these findings could pave the way for clinicians to potentially prevent development of multiple sclerosis in people at higher risk, perhaps through altering the gut flora and probiotic therapy, Suhayl Dhib-Jalbut, MD, said in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Dhib-Jalbut and his team discovered these findings using humanized transgenic mice – in other words, mice containing risk genes for triggering disease transferred from a patient with multiple sclerosis. The mice were more likely to develop MS-like disease at certain ages and in the presence of an altered gut microbiome or gut dysbiosis (Proc Natl Acad Sci U S A. 2017 Oct 31;114[44]:E9318-27).
Dr. Dhib-Jalbut is past president of ACTRIMS and is professor and chairman of the departments of neurology at Rutgers–Robert Wood Johnson Medical School, New Brunswick, N.J., and New Jersey Medical School, Newark. He has received research grants from Biogen and Teva, and is a consultant for Genzyme, Teva, Celgene, and, Mallinckrodt.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Compelling findings in a genetically engineered mouse model of multiple sclerosis identify mechanisms of how adolescence and gut dysbiosis contribute to the risk of MS. In addition, disparities in gut microbiome species could explain why some people are at higher risk for developing multiple sclerosis, while others seem to enjoy a protective effect against development of this and other autoimmune diseases.
The hope is that these findings could pave the way for clinicians to potentially prevent development of multiple sclerosis in people at higher risk, perhaps through altering the gut flora and probiotic therapy, Suhayl Dhib-Jalbut, MD, said in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Dhib-Jalbut and his team discovered these findings using humanized transgenic mice – in other words, mice containing risk genes for triggering disease transferred from a patient with multiple sclerosis. The mice were more likely to develop MS-like disease at certain ages and in the presence of an altered gut microbiome or gut dysbiosis (Proc Natl Acad Sci U S A. 2017 Oct 31;114[44]:E9318-27).
Dr. Dhib-Jalbut is past president of ACTRIMS and is professor and chairman of the departments of neurology at Rutgers–Robert Wood Johnson Medical School, New Brunswick, N.J., and New Jersey Medical School, Newark. He has received research grants from Biogen and Teva, and is a consultant for Genzyme, Teva, Celgene, and, Mallinckrodt.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Compelling findings in a genetically engineered mouse model of multiple sclerosis identify mechanisms of how adolescence and gut dysbiosis contribute to the risk of MS. In addition, disparities in gut microbiome species could explain why some people are at higher risk for developing multiple sclerosis, while others seem to enjoy a protective effect against development of this and other autoimmune diseases.
The hope is that these findings could pave the way for clinicians to potentially prevent development of multiple sclerosis in people at higher risk, perhaps through altering the gut flora and probiotic therapy, Suhayl Dhib-Jalbut, MD, said in a video interview at ACTRIMS Forum 2018, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Dr. Dhib-Jalbut and his team discovered these findings using humanized transgenic mice – in other words, mice containing risk genes for triggering disease transferred from a patient with multiple sclerosis. The mice were more likely to develop MS-like disease at certain ages and in the presence of an altered gut microbiome or gut dysbiosis (Proc Natl Acad Sci U S A. 2017 Oct 31;114[44]:E9318-27).
Dr. Dhib-Jalbut is past president of ACTRIMS and is professor and chairman of the departments of neurology at Rutgers–Robert Wood Johnson Medical School, New Brunswick, N.J., and New Jersey Medical School, Newark. He has received research grants from Biogen and Teva, and is a consultant for Genzyme, Teva, Celgene, and, Mallinckrodt.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ACTRIMS FORUM 2018
Ticagrelor may be superior to clopidogrel in poor metabolizers
LOS ANGELES – In patients who had experienced a minor stroke or a transient ischemic attack with a moderate to high risk of stroke, the combination of ticagrelor and aspirin reduced the 90-day incidence of high on-treatment platelet reactivity, according to results from the PRINCE trial.
Although the combination outperformed clopidogrel (Plavix) plus aspirin, ticagrelor (Brilinta) was associated with higher bleeding risk.
The researchers also saw a trend toward a reduction in strokes that did not reach statistical significance, but the trial was halted following an interim analysis showing that the high on-treatment platelet reactivity (HOPR) endpoint, defined as P2Y12 reaction unit (PRU) greater than 208, showed a statistically significant difference. “To prove the clinical benefit, we will need a larger sample size,” study first author and presenter Yilong Wang, MD, PhD, of Beijing Tiantan Hospital, Capital Medical University, said in an interview at the International Stroke Conference, sponsored by the American Heart Association.
Previously, the SOCRATES trial found no advantage to treatment with ticagrelor over aspirin, but a prespecified exploratory analysis focusing on Asian patients (Stroke. 2017;48:167-73) found a trend toward reducing vascular events in the ticagrelor group, compared with patients taking aspirin.
In the Platelet Reactivity in Acute Stroke or Transient Ischemic Attack (PRINCE) trial, the researchers sought to examine the safety and efficacy of ticagrelor when compared with clopidogrel in 675 Asian patients (mean age 61 years, one-quarter of whom were female) from 26 centers in China and randomized them to ticagrelor plus aspirin (Tica) or clopidogrel plus aspirin (Clop). Within 24 hours of symptom onset, patients received 180 mg ticagrelor or 300 mg clopidogrel plus 100-300 mg aspirin. During days 1-21, they received 90 mg ticagrelor twice per day or 75 mg clopidogrel once per day. Both groups received 100 mg aspirin once per day. From day 21 to day 90, they received 90 mg ticagrelor twice per day or 75 mg clopidogrel once per day, with no aspirin.
At 90 days, the mean PRU value was 175.44 in the Clop group, compared with 69.24 in the Tica group. Overall, 27.7% of the Clop group experienced HOPR in the first 7 days, compared with 3.9% of the Tica group. At 90 days, 29.7% of the Clop group had experienced HOPR, compared with 12.5% of the Tica group (odds ratio, 0.33; 95% confidence interval, 0.21-0.51; P less than .001).
Ticagrelor was associated with greater benefit among those with impaired ability to metabolize clopidogrel. Among poor metabolizers, HOPR occurred in 10.5% in the Tica group and 42.4% of the Clop group (OR, 0.16; 95% CI, 0.05-0.56; P = .004). A similar favorable effect was seen in intermediate metabolizers in the Tica group (OR, 0.24; 95% CI, 0.12-0.49; P less than .001).
The 90-day stroke rate was no different between the Tica and Clop groups (6.3% vs. 8.8%, respectively; P = .20).
Minimal bleeding was higher in the Tica group (19.0% vs. 10.6%; hazard ratio, 1.86; P = .003), as was any bleeding (22.3% vs. 14.2%; HR, 1.65; P = .007). There were three deaths in the Tica group and two in the Clop group.
Dyspnea was the most common cause of drug discontinuation, and occurred in 4.2% of patients taking ticagrelor but none of the patients taking clopidogrel (P = .0001).
The researchers hope to demonstrate the clinical benefits of the combination in the upcoming PRINCE 2 trial. The results will have an important impact because CYP2C19 loss of function alleles are more common in Asian population. “It’s a very big problem for us,” Dr. Wang said.
The study was funded by the National Natural Science Foundation of China, the Beijing Institute for Brain, and the Beijing Municipal Science & Technology Commission of Cerebral Vascular Disease. AstraZeneca provided study drugs. Dr. Wang reported having no financial disclosures.
SOURCE: Wang Y et al. ISC 2018, abstract LB8
LOS ANGELES – In patients who had experienced a minor stroke or a transient ischemic attack with a moderate to high risk of stroke, the combination of ticagrelor and aspirin reduced the 90-day incidence of high on-treatment platelet reactivity, according to results from the PRINCE trial.
Although the combination outperformed clopidogrel (Plavix) plus aspirin, ticagrelor (Brilinta) was associated with higher bleeding risk.
The researchers also saw a trend toward a reduction in strokes that did not reach statistical significance, but the trial was halted following an interim analysis showing that the high on-treatment platelet reactivity (HOPR) endpoint, defined as P2Y12 reaction unit (PRU) greater than 208, showed a statistically significant difference. “To prove the clinical benefit, we will need a larger sample size,” study first author and presenter Yilong Wang, MD, PhD, of Beijing Tiantan Hospital, Capital Medical University, said in an interview at the International Stroke Conference, sponsored by the American Heart Association.
Previously, the SOCRATES trial found no advantage to treatment with ticagrelor over aspirin, but a prespecified exploratory analysis focusing on Asian patients (Stroke. 2017;48:167-73) found a trend toward reducing vascular events in the ticagrelor group, compared with patients taking aspirin.
In the Platelet Reactivity in Acute Stroke or Transient Ischemic Attack (PRINCE) trial, the researchers sought to examine the safety and efficacy of ticagrelor when compared with clopidogrel in 675 Asian patients (mean age 61 years, one-quarter of whom were female) from 26 centers in China and randomized them to ticagrelor plus aspirin (Tica) or clopidogrel plus aspirin (Clop). Within 24 hours of symptom onset, patients received 180 mg ticagrelor or 300 mg clopidogrel plus 100-300 mg aspirin. During days 1-21, they received 90 mg ticagrelor twice per day or 75 mg clopidogrel once per day. Both groups received 100 mg aspirin once per day. From day 21 to day 90, they received 90 mg ticagrelor twice per day or 75 mg clopidogrel once per day, with no aspirin.
At 90 days, the mean PRU value was 175.44 in the Clop group, compared with 69.24 in the Tica group. Overall, 27.7% of the Clop group experienced HOPR in the first 7 days, compared with 3.9% of the Tica group. At 90 days, 29.7% of the Clop group had experienced HOPR, compared with 12.5% of the Tica group (odds ratio, 0.33; 95% confidence interval, 0.21-0.51; P less than .001).
Ticagrelor was associated with greater benefit among those with impaired ability to metabolize clopidogrel. Among poor metabolizers, HOPR occurred in 10.5% in the Tica group and 42.4% of the Clop group (OR, 0.16; 95% CI, 0.05-0.56; P = .004). A similar favorable effect was seen in intermediate metabolizers in the Tica group (OR, 0.24; 95% CI, 0.12-0.49; P less than .001).
The 90-day stroke rate was no different between the Tica and Clop groups (6.3% vs. 8.8%, respectively; P = .20).
Minimal bleeding was higher in the Tica group (19.0% vs. 10.6%; hazard ratio, 1.86; P = .003), as was any bleeding (22.3% vs. 14.2%; HR, 1.65; P = .007). There were three deaths in the Tica group and two in the Clop group.
Dyspnea was the most common cause of drug discontinuation, and occurred in 4.2% of patients taking ticagrelor but none of the patients taking clopidogrel (P = .0001).
The researchers hope to demonstrate the clinical benefits of the combination in the upcoming PRINCE 2 trial. The results will have an important impact because CYP2C19 loss of function alleles are more common in Asian population. “It’s a very big problem for us,” Dr. Wang said.
The study was funded by the National Natural Science Foundation of China, the Beijing Institute for Brain, and the Beijing Municipal Science & Technology Commission of Cerebral Vascular Disease. AstraZeneca provided study drugs. Dr. Wang reported having no financial disclosures.
SOURCE: Wang Y et al. ISC 2018, abstract LB8
LOS ANGELES – In patients who had experienced a minor stroke or a transient ischemic attack with a moderate to high risk of stroke, the combination of ticagrelor and aspirin reduced the 90-day incidence of high on-treatment platelet reactivity, according to results from the PRINCE trial.
Although the combination outperformed clopidogrel (Plavix) plus aspirin, ticagrelor (Brilinta) was associated with higher bleeding risk.
The researchers also saw a trend toward a reduction in strokes that did not reach statistical significance, but the trial was halted following an interim analysis showing that the high on-treatment platelet reactivity (HOPR) endpoint, defined as P2Y12 reaction unit (PRU) greater than 208, showed a statistically significant difference. “To prove the clinical benefit, we will need a larger sample size,” study first author and presenter Yilong Wang, MD, PhD, of Beijing Tiantan Hospital, Capital Medical University, said in an interview at the International Stroke Conference, sponsored by the American Heart Association.
Previously, the SOCRATES trial found no advantage to treatment with ticagrelor over aspirin, but a prespecified exploratory analysis focusing on Asian patients (Stroke. 2017;48:167-73) found a trend toward reducing vascular events in the ticagrelor group, compared with patients taking aspirin.
In the Platelet Reactivity in Acute Stroke or Transient Ischemic Attack (PRINCE) trial, the researchers sought to examine the safety and efficacy of ticagrelor when compared with clopidogrel in 675 Asian patients (mean age 61 years, one-quarter of whom were female) from 26 centers in China and randomized them to ticagrelor plus aspirin (Tica) or clopidogrel plus aspirin (Clop). Within 24 hours of symptom onset, patients received 180 mg ticagrelor or 300 mg clopidogrel plus 100-300 mg aspirin. During days 1-21, they received 90 mg ticagrelor twice per day or 75 mg clopidogrel once per day. Both groups received 100 mg aspirin once per day. From day 21 to day 90, they received 90 mg ticagrelor twice per day or 75 mg clopidogrel once per day, with no aspirin.
At 90 days, the mean PRU value was 175.44 in the Clop group, compared with 69.24 in the Tica group. Overall, 27.7% of the Clop group experienced HOPR in the first 7 days, compared with 3.9% of the Tica group. At 90 days, 29.7% of the Clop group had experienced HOPR, compared with 12.5% of the Tica group (odds ratio, 0.33; 95% confidence interval, 0.21-0.51; P less than .001).
Ticagrelor was associated with greater benefit among those with impaired ability to metabolize clopidogrel. Among poor metabolizers, HOPR occurred in 10.5% in the Tica group and 42.4% of the Clop group (OR, 0.16; 95% CI, 0.05-0.56; P = .004). A similar favorable effect was seen in intermediate metabolizers in the Tica group (OR, 0.24; 95% CI, 0.12-0.49; P less than .001).
The 90-day stroke rate was no different between the Tica and Clop groups (6.3% vs. 8.8%, respectively; P = .20).
Minimal bleeding was higher in the Tica group (19.0% vs. 10.6%; hazard ratio, 1.86; P = .003), as was any bleeding (22.3% vs. 14.2%; HR, 1.65; P = .007). There were three deaths in the Tica group and two in the Clop group.
Dyspnea was the most common cause of drug discontinuation, and occurred in 4.2% of patients taking ticagrelor but none of the patients taking clopidogrel (P = .0001).
The researchers hope to demonstrate the clinical benefits of the combination in the upcoming PRINCE 2 trial. The results will have an important impact because CYP2C19 loss of function alleles are more common in Asian population. “It’s a very big problem for us,” Dr. Wang said.
The study was funded by the National Natural Science Foundation of China, the Beijing Institute for Brain, and the Beijing Municipal Science & Technology Commission of Cerebral Vascular Disease. AstraZeneca provided study drugs. Dr. Wang reported having no financial disclosures.
SOURCE: Wang Y et al. ISC 2018, abstract LB8
REPORTING FROM ISC 2018
Key clinical point: Ticagrelor may be a better option than clopidogrel in patients with loss-of-function CYP2C19 alleles.
Major finding: Patients on ticagrelor had a lower 90-day risk of high on-treatment platelet reactivity (OR, 0.33).
Data source: Randomized, open-label trial with blinded assessments (n = 675).
Disclosures: The study was funded by the National Natural Science Foundation of China, the Beijing Institute for Brain, and the Beijing Municipal Science & Technology Commission of Cerebral Vascular Disease. AstraZeneca provided study drugs. Dr. Wang reported having no financial disclosures.
Source: Wang Y et al. ISC 2018, abstract LB8
Baby boomers are the hepatitis C generation
Increases in hepatitis C–related inpatient stays for baby boomers from 2005 to 2014 far outpaced those of older adults, while younger adults saw their admissions drop over that period, according to the Agency for Healthcare Research and Quality.
For the baby boomers (adults aged 52-72 years), the rate of inpatient stays involving hepatitis C with or without hepatitis B, HIV, or alcoholic liver disease rose from 300.7 per 100,000 population in 2005 to 503.1 per 100,000 in 2014 – an increase of over 67%. For patients aged 73 years and older, that rate went from 104.4 in 2005 to 117.1 in 2014, which translates to a 12% increase, and for patients aged 18-51 years, it dropped 15%, from 182.5 to 155.4, the AHRQ said in a statistical brief.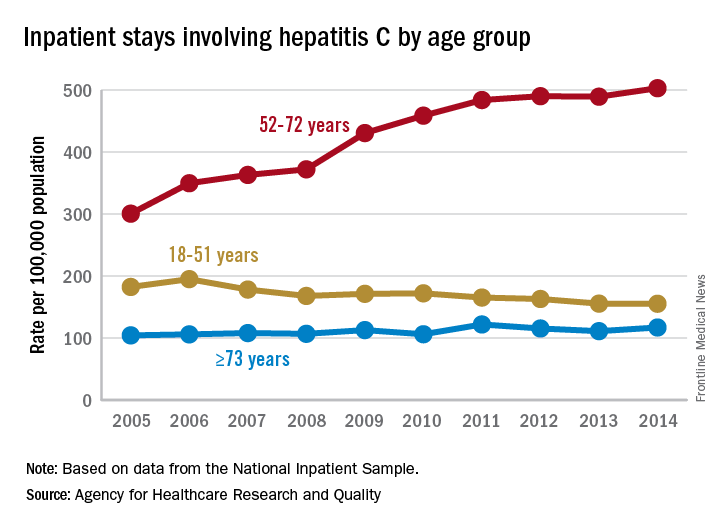
Along with the increased hospitalizations, “acute hepatitis C cases nearly tripled from 2010 through 2015,” the report noted, which was “likely the result of increasing injection drug use due to the growing opioid epidemic.”
AGA offers tools to help you become more efficient, understand quality standards, and improve the process of care for your hepatitis C patients.
Increases in hepatitis C–related inpatient stays for baby boomers from 2005 to 2014 far outpaced those of older adults, while younger adults saw their admissions drop over that period, according to the Agency for Healthcare Research and Quality.
For the baby boomers (adults aged 52-72 years), the rate of inpatient stays involving hepatitis C with or without hepatitis B, HIV, or alcoholic liver disease rose from 300.7 per 100,000 population in 2005 to 503.1 per 100,000 in 2014 – an increase of over 67%. For patients aged 73 years and older, that rate went from 104.4 in 2005 to 117.1 in 2014, which translates to a 12% increase, and for patients aged 18-51 years, it dropped 15%, from 182.5 to 155.4, the AHRQ said in a statistical brief.
Along with the increased hospitalizations, “acute hepatitis C cases nearly tripled from 2010 through 2015,” the report noted, which was “likely the result of increasing injection drug use due to the growing opioid epidemic.”
AGA offers tools to help you become more efficient, understand quality standards, and improve the process of care for your hepatitis C patients.
Increases in hepatitis C–related inpatient stays for baby boomers from 2005 to 2014 far outpaced those of older adults, while younger adults saw their admissions drop over that period, according to the Agency for Healthcare Research and Quality.
For the baby boomers (adults aged 52-72 years), the rate of inpatient stays involving hepatitis C with or without hepatitis B, HIV, or alcoholic liver disease rose from 300.7 per 100,000 population in 2005 to 503.1 per 100,000 in 2014 – an increase of over 67%. For patients aged 73 years and older, that rate went from 104.4 in 2005 to 117.1 in 2014, which translates to a 12% increase, and for patients aged 18-51 years, it dropped 15%, from 182.5 to 155.4, the AHRQ said in a statistical brief.
Along with the increased hospitalizations, “acute hepatitis C cases nearly tripled from 2010 through 2015,” the report noted, which was “likely the result of increasing injection drug use due to the growing opioid epidemic.”
AGA offers tools to help you become more efficient, understand quality standards, and improve the process of care for your hepatitis C patients.
Tenecteplase surpasses alteplase for thrombolysing acute ischemic stroke
LOS ANGELES – Thrombolysis with tenecteplase beat alteplase on an acute imaging endpoint in patients with acute ischemic stroke who were on their way to also get thrombectomy in a randomized, multicenter study with 202 patients in Australia and New Zealand.
The results of the trial, called Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK), showed that when the patients underwent their initial angiogram after receiving thrombolysis and before their thrombectomy procedure, the percentage of patients with robust blood flow – a Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 or no retrievable thrombus – was 22% in the patients treated with tenecteplase 0.25 mg/kg and 10% among those who received alteplase 0.9 mg/kg. After adjustment, the 12% incremental change in robust reperfusion with tenecteplase calculated into a 2.6-fold higher rate of robust reperfusion, statistically significant for both noninferiority and for superiority, Bruce C. Campbell, MBBS, PhD, said at the International Stroke Conference, sponsored by the American Heart Association.
Tenecteplase is a genetically-modified tissue plasminogen activator with enhanced fibrin specificity that increases the drug’s half life and allows for bolus administration, unlike alteplase, which needs continuous infusion (CNS Drugs. 2015 Oct;29[10]:811-8). Tenecteplase also has a U.S. wholesale price that is about $3,000 cheaper per vial than alteplase, said Dr. Campbell, professor of neurology at the University of Melbourne and head of hyperacute stroke at Royal Melbourne Hospital.
But further data are needed before tenecteplase is ready for routine use, conceded Dr. Campbell, an assessment other experts agreed with. Dr. Campbell cited two studies in progress that are comparing tenecteplase with alteplase in acute ischemic stroke patients not headed for endovascular thrombectomy, as well as a study he is leading that compares the tenecteplase dose he just tested, 0.25 mg/kg, with a higher dose, 0.40 mg/kg.
Several reports have appeared in recent years suggesting that treatment with tenecteplase seems to be at least as good as alteplase in ischemic stroke patients. For example, a randomized trial with 75 ischemic stroke patients selected by imaging at three Australian centers showed that treatment with tenecteplase produced a significant 24% improvement in the rate of arterial reperfusion and an average 5-point improvement in NIH Stroke Scale score (N Engl J Med. 2012;366[12]:1099-107). And results from the NOR-TEST study recently showed that among 1,100 patients randomized at 13 Norwegian centers, the primary outcome of a 90-day modified Rankin Scale score of 0-1 was achieved by 64% of the tenecteplase patients and by 63% of those who received alteplase (Lancet Neurol. 2017 Oct;16[10]:781-8).
The EXTEND-IA TNK trial ran during 2015-2017 at 18 hospitals. All enrolled patients received their thrombolytic treatment within 4.5 hours of their stroke onset, and underwent endovascular thrombectomy within 6 hours of onset. The safety outcomes of death, symptomatic intracranial hemorrhage, and parenchymal hematoma occurred at statistically similar rates in both treatment arms.
The study was investigator initiated and funded chiefly by the Australian government. Medtronic provided an unrestricted grant for trial infrastructure but had no role in study design conduct or analysis. Dr. Campbell and Dr. Powers had no disclosures.
SOURCE: Campbell B et al. ISC 2018, abstract LB2.
In the EXTEND-IA TNK study, tenecteplase appeared to act better than alteplase and has the extra advantage of being administered as a bolus injection. Alteplase is delivered as a drip, and it’s often hard to get patients with an intravenous infusion out of the hospital quickly when you have to transport the patient. You need a nurse in the ambulance monitoring the drip. With tenecteplase you administer the bolus and can then send the patient without an intravenous line.
Jeffrey L. Saver, MD, is professor of neurology and director of the stroke unit at the University of California, Los Angeles. He has received research support and personal fees from Medtronic-Abbott and Neuravia. He made these comments in an interview.
In the EXTEND-IA TNK study, tenecteplase appeared to act better than alteplase and has the extra advantage of being administered as a bolus injection. Alteplase is delivered as a drip, and it’s often hard to get patients with an intravenous infusion out of the hospital quickly when you have to transport the patient. You need a nurse in the ambulance monitoring the drip. With tenecteplase you administer the bolus and can then send the patient without an intravenous line.
Jeffrey L. Saver, MD, is professor of neurology and director of the stroke unit at the University of California, Los Angeles. He has received research support and personal fees from Medtronic-Abbott and Neuravia. He made these comments in an interview.
In the EXTEND-IA TNK study, tenecteplase appeared to act better than alteplase and has the extra advantage of being administered as a bolus injection. Alteplase is delivered as a drip, and it’s often hard to get patients with an intravenous infusion out of the hospital quickly when you have to transport the patient. You need a nurse in the ambulance monitoring the drip. With tenecteplase you administer the bolus and can then send the patient without an intravenous line.
Jeffrey L. Saver, MD, is professor of neurology and director of the stroke unit at the University of California, Los Angeles. He has received research support and personal fees from Medtronic-Abbott and Neuravia. He made these comments in an interview.
LOS ANGELES – Thrombolysis with tenecteplase beat alteplase on an acute imaging endpoint in patients with acute ischemic stroke who were on their way to also get thrombectomy in a randomized, multicenter study with 202 patients in Australia and New Zealand.
The results of the trial, called Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK), showed that when the patients underwent their initial angiogram after receiving thrombolysis and before their thrombectomy procedure, the percentage of patients with robust blood flow – a Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 or no retrievable thrombus – was 22% in the patients treated with tenecteplase 0.25 mg/kg and 10% among those who received alteplase 0.9 mg/kg. After adjustment, the 12% incremental change in robust reperfusion with tenecteplase calculated into a 2.6-fold higher rate of robust reperfusion, statistically significant for both noninferiority and for superiority, Bruce C. Campbell, MBBS, PhD, said at the International Stroke Conference, sponsored by the American Heart Association.
Tenecteplase is a genetically-modified tissue plasminogen activator with enhanced fibrin specificity that increases the drug’s half life and allows for bolus administration, unlike alteplase, which needs continuous infusion (CNS Drugs. 2015 Oct;29[10]:811-8). Tenecteplase also has a U.S. wholesale price that is about $3,000 cheaper per vial than alteplase, said Dr. Campbell, professor of neurology at the University of Melbourne and head of hyperacute stroke at Royal Melbourne Hospital.
But further data are needed before tenecteplase is ready for routine use, conceded Dr. Campbell, an assessment other experts agreed with. Dr. Campbell cited two studies in progress that are comparing tenecteplase with alteplase in acute ischemic stroke patients not headed for endovascular thrombectomy, as well as a study he is leading that compares the tenecteplase dose he just tested, 0.25 mg/kg, with a higher dose, 0.40 mg/kg.
Several reports have appeared in recent years suggesting that treatment with tenecteplase seems to be at least as good as alteplase in ischemic stroke patients. For example, a randomized trial with 75 ischemic stroke patients selected by imaging at three Australian centers showed that treatment with tenecteplase produced a significant 24% improvement in the rate of arterial reperfusion and an average 5-point improvement in NIH Stroke Scale score (N Engl J Med. 2012;366[12]:1099-107). And results from the NOR-TEST study recently showed that among 1,100 patients randomized at 13 Norwegian centers, the primary outcome of a 90-day modified Rankin Scale score of 0-1 was achieved by 64% of the tenecteplase patients and by 63% of those who received alteplase (Lancet Neurol. 2017 Oct;16[10]:781-8).
The EXTEND-IA TNK trial ran during 2015-2017 at 18 hospitals. All enrolled patients received their thrombolytic treatment within 4.5 hours of their stroke onset, and underwent endovascular thrombectomy within 6 hours of onset. The safety outcomes of death, symptomatic intracranial hemorrhage, and parenchymal hematoma occurred at statistically similar rates in both treatment arms.
The study was investigator initiated and funded chiefly by the Australian government. Medtronic provided an unrestricted grant for trial infrastructure but had no role in study design conduct or analysis. Dr. Campbell and Dr. Powers had no disclosures.
SOURCE: Campbell B et al. ISC 2018, abstract LB2.
LOS ANGELES – Thrombolysis with tenecteplase beat alteplase on an acute imaging endpoint in patients with acute ischemic stroke who were on their way to also get thrombectomy in a randomized, multicenter study with 202 patients in Australia and New Zealand.
The results of the trial, called Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK), showed that when the patients underwent their initial angiogram after receiving thrombolysis and before their thrombectomy procedure, the percentage of patients with robust blood flow – a Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 or no retrievable thrombus – was 22% in the patients treated with tenecteplase 0.25 mg/kg and 10% among those who received alteplase 0.9 mg/kg. After adjustment, the 12% incremental change in robust reperfusion with tenecteplase calculated into a 2.6-fold higher rate of robust reperfusion, statistically significant for both noninferiority and for superiority, Bruce C. Campbell, MBBS, PhD, said at the International Stroke Conference, sponsored by the American Heart Association.
Tenecteplase is a genetically-modified tissue plasminogen activator with enhanced fibrin specificity that increases the drug’s half life and allows for bolus administration, unlike alteplase, which needs continuous infusion (CNS Drugs. 2015 Oct;29[10]:811-8). Tenecteplase also has a U.S. wholesale price that is about $3,000 cheaper per vial than alteplase, said Dr. Campbell, professor of neurology at the University of Melbourne and head of hyperacute stroke at Royal Melbourne Hospital.
But further data are needed before tenecteplase is ready for routine use, conceded Dr. Campbell, an assessment other experts agreed with. Dr. Campbell cited two studies in progress that are comparing tenecteplase with alteplase in acute ischemic stroke patients not headed for endovascular thrombectomy, as well as a study he is leading that compares the tenecteplase dose he just tested, 0.25 mg/kg, with a higher dose, 0.40 mg/kg.
Several reports have appeared in recent years suggesting that treatment with tenecteplase seems to be at least as good as alteplase in ischemic stroke patients. For example, a randomized trial with 75 ischemic stroke patients selected by imaging at three Australian centers showed that treatment with tenecteplase produced a significant 24% improvement in the rate of arterial reperfusion and an average 5-point improvement in NIH Stroke Scale score (N Engl J Med. 2012;366[12]:1099-107). And results from the NOR-TEST study recently showed that among 1,100 patients randomized at 13 Norwegian centers, the primary outcome of a 90-day modified Rankin Scale score of 0-1 was achieved by 64% of the tenecteplase patients and by 63% of those who received alteplase (Lancet Neurol. 2017 Oct;16[10]:781-8).
The EXTEND-IA TNK trial ran during 2015-2017 at 18 hospitals. All enrolled patients received their thrombolytic treatment within 4.5 hours of their stroke onset, and underwent endovascular thrombectomy within 6 hours of onset. The safety outcomes of death, symptomatic intracranial hemorrhage, and parenchymal hematoma occurred at statistically similar rates in both treatment arms.
The study was investigator initiated and funded chiefly by the Australian government. Medtronic provided an unrestricted grant for trial infrastructure but had no role in study design conduct or analysis. Dr. Campbell and Dr. Powers had no disclosures.
SOURCE: Campbell B et al. ISC 2018, abstract LB2.
REPORTING FROM ISC 2018
Key clinical point: Tenecteplase thrombolysis produced better reperfusion than did alteplase.
Major finding: Robust reperfusion occurred in 22% of tenecteplase patients and in 10% of patients who received alteplase.
Study details: EXTEND-IA TNK, a multicenter, randomized trial with 202 patients.
Disclosures: The study was investigator initiated and funded chiefly by the Australian government. Medtronic provided an unrestricted grant for trial infrastructure but had no role in study design conduct or analysis. Dr. Campbell and Dr. Powers had no disclosures.
Source: Campbell B et al. ISC 2018, abstract LB2
Joint Outpatient Experience Gets an A
The results are in: 93% of soldiers, retirees, and family members report very high overall satisfaction with their experience at Army medical treatment facilities.
Survey responses were for the DoD’s 2017 Joint Outpatient Experience Survey (JOES), which also asked about ease of access to Army providers (83% positive response) and overall experience with Army pharmacies (78% positive).
The results showed an increase in satisfaction of about 2% for those 3 questions compared with the results of 2016, the first time the Army participated in the survey, according to Melissa Gliner, senior health policy analyst with the Office of the Army Surgeon General, in an article for Defense.gov. The survey goes to about 10% of patients who have visited a military health facility.
Besides sharing the survey results with the facilities, Gliner advises them on how to improve the patient experience. For instance, she looks at civilian treatment facilities to see what works. One insight she culled was that it helps to have staff members circulate in the waiting area to chat with patients so they do not feel they are being ignored. Another was that facilities should retrain scheduling clerks to set up appointments without making the patient call back.
Gliner says the U.S. Army Medical Command also is working on a website that will help military health facilities share their ideas and “further elevate patient experience and survey scores.”
The results are in: 93% of soldiers, retirees, and family members report very high overall satisfaction with their experience at Army medical treatment facilities.
Survey responses were for the DoD’s 2017 Joint Outpatient Experience Survey (JOES), which also asked about ease of access to Army providers (83% positive response) and overall experience with Army pharmacies (78% positive).
The results showed an increase in satisfaction of about 2% for those 3 questions compared with the results of 2016, the first time the Army participated in the survey, according to Melissa Gliner, senior health policy analyst with the Office of the Army Surgeon General, in an article for Defense.gov. The survey goes to about 10% of patients who have visited a military health facility.
Besides sharing the survey results with the facilities, Gliner advises them on how to improve the patient experience. For instance, she looks at civilian treatment facilities to see what works. One insight she culled was that it helps to have staff members circulate in the waiting area to chat with patients so they do not feel they are being ignored. Another was that facilities should retrain scheduling clerks to set up appointments without making the patient call back.
Gliner says the U.S. Army Medical Command also is working on a website that will help military health facilities share their ideas and “further elevate patient experience and survey scores.”
The results are in: 93% of soldiers, retirees, and family members report very high overall satisfaction with their experience at Army medical treatment facilities.
Survey responses were for the DoD’s 2017 Joint Outpatient Experience Survey (JOES), which also asked about ease of access to Army providers (83% positive response) and overall experience with Army pharmacies (78% positive).
The results showed an increase in satisfaction of about 2% for those 3 questions compared with the results of 2016, the first time the Army participated in the survey, according to Melissa Gliner, senior health policy analyst with the Office of the Army Surgeon General, in an article for Defense.gov. The survey goes to about 10% of patients who have visited a military health facility.
Besides sharing the survey results with the facilities, Gliner advises them on how to improve the patient experience. For instance, she looks at civilian treatment facilities to see what works. One insight she culled was that it helps to have staff members circulate in the waiting area to chat with patients so they do not feel they are being ignored. Another was that facilities should retrain scheduling clerks to set up appointments without making the patient call back.
Gliner says the U.S. Army Medical Command also is working on a website that will help military health facilities share their ideas and “further elevate patient experience and survey scores.”
Best Practices in Hematology and Oncology (February 2018)
Click here to access the Best Practices in Hematology and Oncology 2018 Digital Edition.
Table of Contents
- Mohs Micrographic Surgery in the VHA
- A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology
- Barriers and Facilitators to the Use of Genomic-Based Targeted Therapy in the VA: Qualitative Findings
- Why Am I Being Treated Like a Female Breast Cancer Patient?
- Breast Cancer Tumor Board
- Advances in CAR T-Cell Therapies

Click here to access the Best Practices in Hematology and Oncology 2018 Digital Edition.
Table of Contents
- Mohs Micrographic Surgery in the VHA
- A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology
- Barriers and Facilitators to the Use of Genomic-Based Targeted Therapy in the VA: Qualitative Findings
- Why Am I Being Treated Like a Female Breast Cancer Patient?
- Breast Cancer Tumor Board
- Advances in CAR T-Cell Therapies

Click here to access the Best Practices in Hematology and Oncology 2018 Digital Edition.
Table of Contents
- Mohs Micrographic Surgery in the VHA
- A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology
- Barriers and Facilitators to the Use of Genomic-Based Targeted Therapy in the VA: Qualitative Findings
- Why Am I Being Treated Like a Female Breast Cancer Patient?
- Breast Cancer Tumor Board
- Advances in CAR T-Cell Therapies