User login
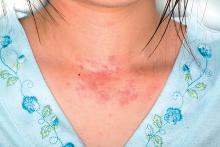
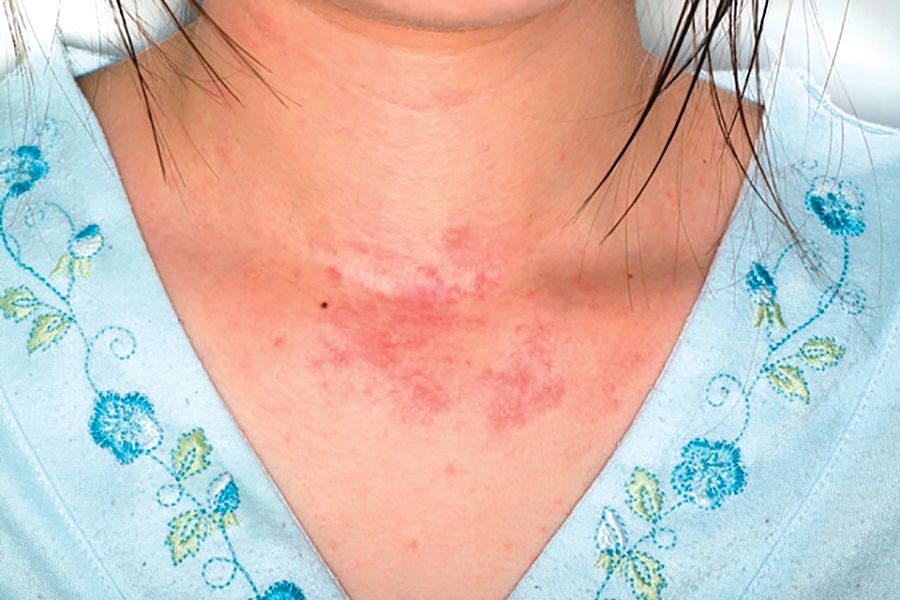
Antihistamines still are prescribed inappropriately for atopic dermatitis
said Alice He, MD, at the Center for Dermatology Research, Wake Forest University, Winston-Salem, N.C., and her associates.
Results of double-blind, randomized trials have found that oral antihistamines do not effectively treat itch associated with atopic dermatitis (AD), and American Academy of Dermatology guidelines state that intermittent short-term use of sedating antihistamines may help insomnia secondary to itch, but should not be substituted for topical treatments in atopic dermatitis management (J Am Acad Dermatol. 2014;71[2]:327-49), the investigators said. Nonetheless, physicians continue to prescribe antihistamines to AD patients.
Sedating antihistamines accounted for the majority of antihistamines prescribed by pediatricians (58%) and dermatologists (70%) for AD. Nonsedating antihistamines accounted for the majority of the antihistamines prescribed by family physicians (84%), internists (100%), and other specialists (55%), the investigators said.
(The sedating antihistamines prescribed included hydroxyzine, diphenhydramine, chlorpheniramine, brompheniramine, and cyproheptadine; nonsedating antihistamines include cetirizine, desloratadine, levocetirizine, loratadine, and fexofenadine.)
Although the AAD guidelines allow intermittent, short-term use of sedating antihistamines to treat AD-associated itch, these medications may be harmful to sleep in terms of reduced sleep quality and decreased REM sleep. Also, studies have shown that their effects can persist into the daytime and have the potential to impair cognitive function, including learning and memory.
“Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis,” Dr. He and her associates warned in the article in the Journal of the American Academy of Dermatology.
The researchers took a look at the top ten comorbidities reported among visits for AD, because nonsedating antihistamines might have been prescribed for a different diagnosis. There were three conditions for which antihistamines may be indicated: allergic rhinitis (13%), dermatitis attributable to food (8.5%), and conjunctivitis (3%). This “validates antihistamine prescriptions for a maximum of only 24.5% of patients who received antihistamines, indicating that a large proportion of antihistamine prescriptions were prescribed specifically for AD,” they said.
The authors said that, “while histamine does not play a role in AD via H1R [histamine-1 receptor], it may still be relevant in AD pathogenesis through actions on its most recently described receptor, histamine-4 receptor (H4R).”
“The advent of new targeted systemic therapies, such as T-helper 2 cytokine antagonists and H4R antagonists,” may one day help fill “the unmet need for effective treatments for atopic dermatitis,” Dr. He and her associates concluded.
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her coauthors disclosed research, speaking, and/or consulting support from a variety of pharmaceutical companies; one author is an employee of Abbvie.
SOURCE: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
said Alice He, MD, at the Center for Dermatology Research, Wake Forest University, Winston-Salem, N.C., and her associates.
Results of double-blind, randomized trials have found that oral antihistamines do not effectively treat itch associated with atopic dermatitis (AD), and American Academy of Dermatology guidelines state that intermittent short-term use of sedating antihistamines may help insomnia secondary to itch, but should not be substituted for topical treatments in atopic dermatitis management (J Am Acad Dermatol. 2014;71[2]:327-49), the investigators said. Nonetheless, physicians continue to prescribe antihistamines to AD patients.
Sedating antihistamines accounted for the majority of antihistamines prescribed by pediatricians (58%) and dermatologists (70%) for AD. Nonsedating antihistamines accounted for the majority of the antihistamines prescribed by family physicians (84%), internists (100%), and other specialists (55%), the investigators said.
(The sedating antihistamines prescribed included hydroxyzine, diphenhydramine, chlorpheniramine, brompheniramine, and cyproheptadine; nonsedating antihistamines include cetirizine, desloratadine, levocetirizine, loratadine, and fexofenadine.)
Although the AAD guidelines allow intermittent, short-term use of sedating antihistamines to treat AD-associated itch, these medications may be harmful to sleep in terms of reduced sleep quality and decreased REM sleep. Also, studies have shown that their effects can persist into the daytime and have the potential to impair cognitive function, including learning and memory.
“Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis,” Dr. He and her associates warned in the article in the Journal of the American Academy of Dermatology.
The researchers took a look at the top ten comorbidities reported among visits for AD, because nonsedating antihistamines might have been prescribed for a different diagnosis. There were three conditions for which antihistamines may be indicated: allergic rhinitis (13%), dermatitis attributable to food (8.5%), and conjunctivitis (3%). This “validates antihistamine prescriptions for a maximum of only 24.5% of patients who received antihistamines, indicating that a large proportion of antihistamine prescriptions were prescribed specifically for AD,” they said.
The authors said that, “while histamine does not play a role in AD via H1R [histamine-1 receptor], it may still be relevant in AD pathogenesis through actions on its most recently described receptor, histamine-4 receptor (H4R).”
“The advent of new targeted systemic therapies, such as T-helper 2 cytokine antagonists and H4R antagonists,” may one day help fill “the unmet need for effective treatments for atopic dermatitis,” Dr. He and her associates concluded.
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her coauthors disclosed research, speaking, and/or consulting support from a variety of pharmaceutical companies; one author is an employee of Abbvie.
SOURCE: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
said Alice He, MD, at the Center for Dermatology Research, Wake Forest University, Winston-Salem, N.C., and her associates.
Results of double-blind, randomized trials have found that oral antihistamines do not effectively treat itch associated with atopic dermatitis (AD), and American Academy of Dermatology guidelines state that intermittent short-term use of sedating antihistamines may help insomnia secondary to itch, but should not be substituted for topical treatments in atopic dermatitis management (J Am Acad Dermatol. 2014;71[2]:327-49), the investigators said. Nonetheless, physicians continue to prescribe antihistamines to AD patients.
Sedating antihistamines accounted for the majority of antihistamines prescribed by pediatricians (58%) and dermatologists (70%) for AD. Nonsedating antihistamines accounted for the majority of the antihistamines prescribed by family physicians (84%), internists (100%), and other specialists (55%), the investigators said.
(The sedating antihistamines prescribed included hydroxyzine, diphenhydramine, chlorpheniramine, brompheniramine, and cyproheptadine; nonsedating antihistamines include cetirizine, desloratadine, levocetirizine, loratadine, and fexofenadine.)
Although the AAD guidelines allow intermittent, short-term use of sedating antihistamines to treat AD-associated itch, these medications may be harmful to sleep in terms of reduced sleep quality and decreased REM sleep. Also, studies have shown that their effects can persist into the daytime and have the potential to impair cognitive function, including learning and memory.
“Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis,” Dr. He and her associates warned in the article in the Journal of the American Academy of Dermatology.
The researchers took a look at the top ten comorbidities reported among visits for AD, because nonsedating antihistamines might have been prescribed for a different diagnosis. There were three conditions for which antihistamines may be indicated: allergic rhinitis (13%), dermatitis attributable to food (8.5%), and conjunctivitis (3%). This “validates antihistamine prescriptions for a maximum of only 24.5% of patients who received antihistamines, indicating that a large proportion of antihistamine prescriptions were prescribed specifically for AD,” they said.
The authors said that, “while histamine does not play a role in AD via H1R [histamine-1 receptor], it may still be relevant in AD pathogenesis through actions on its most recently described receptor, histamine-4 receptor (H4R).”
“The advent of new targeted systemic therapies, such as T-helper 2 cytokine antagonists and H4R antagonists,” may one day help fill “the unmet need for effective treatments for atopic dermatitis,” Dr. He and her associates concluded.
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her coauthors disclosed research, speaking, and/or consulting support from a variety of pharmaceutical companies; one author is an employee of Abbvie.
SOURCE: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis.
Major finding: Physicians from specialties other than dermatology prescribed antihistamines at a greater proportion of AD visits than dermatologists (26%-44% of visits vs. 22%), with the exception of pediatricians (16%).
Study details: Data from the National Ambulatory Medical Care Survey on 9.9 million physician visits for AD from 2003 to 2012.
Disclosures: The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her associates disclosed research, speaking and/or consulting support from a variety of pharmaceutical companies. One author was an employee of Abbvie.
Source: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
Doctors to Congress: Keep Part B drug payments out of MIPS adjustment
Physician specialists are calling on Congress to isolate Medicare Part B drug reimbursements from payment adjustments under the Merit-Based Incentive Payment System (MIPS).
A coalition of medical societies, large group practices, and patient advocacy groups has asked for an “intervention this year with a technical correction that ensures the [MIPS] score adjustment is not applied to Part B drug payments,” according to Jan. 18 letter sent to the leaders of the Senate Finance Committee, House Energy and Commerce Committee, and the House Ways and Means Committee. “Since the 2018 MIPS year has begun, it is imperative that Congress acts quickly to ensure that patient access to critical treatments is not negatively impacted.”
Under MIPS, physicians are scored based on their performance across three categories: quality, improvement activities, and advancing care information. A fourth category, cost, is planned but not yet included in the score (although cost doesn’t impact adjustments until 2020, it is part of the 2018 program year). Medicare payments, which currently include Part B drug reimbursements, are subject to bonuses and penalties based on performance scores.
In their November 2017 update to the Quality Payment Program, which includes MIPS, officials at the Centers for Medicare & Medicaid Services said they would be moving forward with including Part B drug payments in the MIPS adjustment.
“This application of the adjustment ... is a significant departure from current policy and would disproportionately affect certain specialties,” according to the coalition’s letter.
Certain specialties, including rheumatology, oncology, and ophthalmology, have more to lose under the current policy because these specialists administer more Part B drugs than other specialists, according to health care consultancy Avalere Health.
For gastroenterologists, the primary concern with the application of the MIPS payment adjustment to Part B drugs is patient access to the biologics and biosimilars used to treat irritable bowel disease including Crohn’s disease and ulcerative colitis. Under CMS’s policy, gastroenterologists facing a penalty or negative payment adjustment may have Part B drug payments fall below what it costs to buy the Part B drug. Since Medicare payment for Part B drugs is based on average sales price (ASP), affected practices are more likely to be small, have a smaller number of patients on a Part B drug or prescribe a broader range of Part B drugs to patients. As MIPS payment adjustments increase each year the impact of the policy is expected to touch more practices and bring larger deficits further eroding access to the biologics and biosimilars used to treat inflammatory bowel disease.
“Certain specialists administer more Part B drugs than others and, therefore, may be exposed to significant financial risk and payment swings year-over-year under the CMS [Centers for Medicare & Medicaid Services] proposal,” John Feore, director at Avalere, said in a statement.
In 2018, physicians in those specialties could see drug payments increase or decrease by as much as 16%, according to Avalere research.
The policy likely will have an even greater effect on smaller practices and those in rural settings and could lead to access issues, according to the coalition letter.
“Some patients already face access challenges because the budget sequester has eroded reimbursements to physicians, and this policy would exacerbate these problems,” the letter states. “Patients would be left with fewer locations where they could receive care, resulting in less access and higher costs. A growing number of patients would then have to seek care in a hospital, which would result in higher out-of-pocket expenses and, particularly in rural communities, may require traveling longer distances to receive care.”
Physician specialists are calling on Congress to isolate Medicare Part B drug reimbursements from payment adjustments under the Merit-Based Incentive Payment System (MIPS).
A coalition of medical societies, large group practices, and patient advocacy groups has asked for an “intervention this year with a technical correction that ensures the [MIPS] score adjustment is not applied to Part B drug payments,” according to Jan. 18 letter sent to the leaders of the Senate Finance Committee, House Energy and Commerce Committee, and the House Ways and Means Committee. “Since the 2018 MIPS year has begun, it is imperative that Congress acts quickly to ensure that patient access to critical treatments is not negatively impacted.”
Under MIPS, physicians are scored based on their performance across three categories: quality, improvement activities, and advancing care information. A fourth category, cost, is planned but not yet included in the score (although cost doesn’t impact adjustments until 2020, it is part of the 2018 program year). Medicare payments, which currently include Part B drug reimbursements, are subject to bonuses and penalties based on performance scores.
In their November 2017 update to the Quality Payment Program, which includes MIPS, officials at the Centers for Medicare & Medicaid Services said they would be moving forward with including Part B drug payments in the MIPS adjustment.
“This application of the adjustment ... is a significant departure from current policy and would disproportionately affect certain specialties,” according to the coalition’s letter.
Certain specialties, including rheumatology, oncology, and ophthalmology, have more to lose under the current policy because these specialists administer more Part B drugs than other specialists, according to health care consultancy Avalere Health.
For gastroenterologists, the primary concern with the application of the MIPS payment adjustment to Part B drugs is patient access to the biologics and biosimilars used to treat irritable bowel disease including Crohn’s disease and ulcerative colitis. Under CMS’s policy, gastroenterologists facing a penalty or negative payment adjustment may have Part B drug payments fall below what it costs to buy the Part B drug. Since Medicare payment for Part B drugs is based on average sales price (ASP), affected practices are more likely to be small, have a smaller number of patients on a Part B drug or prescribe a broader range of Part B drugs to patients. As MIPS payment adjustments increase each year the impact of the policy is expected to touch more practices and bring larger deficits further eroding access to the biologics and biosimilars used to treat inflammatory bowel disease.
“Certain specialists administer more Part B drugs than others and, therefore, may be exposed to significant financial risk and payment swings year-over-year under the CMS [Centers for Medicare & Medicaid Services] proposal,” John Feore, director at Avalere, said in a statement.
In 2018, physicians in those specialties could see drug payments increase or decrease by as much as 16%, according to Avalere research.
The policy likely will have an even greater effect on smaller practices and those in rural settings and could lead to access issues, according to the coalition letter.
“Some patients already face access challenges because the budget sequester has eroded reimbursements to physicians, and this policy would exacerbate these problems,” the letter states. “Patients would be left with fewer locations where they could receive care, resulting in less access and higher costs. A growing number of patients would then have to seek care in a hospital, which would result in higher out-of-pocket expenses and, particularly in rural communities, may require traveling longer distances to receive care.”
Physician specialists are calling on Congress to isolate Medicare Part B drug reimbursements from payment adjustments under the Merit-Based Incentive Payment System (MIPS).
A coalition of medical societies, large group practices, and patient advocacy groups has asked for an “intervention this year with a technical correction that ensures the [MIPS] score adjustment is not applied to Part B drug payments,” according to Jan. 18 letter sent to the leaders of the Senate Finance Committee, House Energy and Commerce Committee, and the House Ways and Means Committee. “Since the 2018 MIPS year has begun, it is imperative that Congress acts quickly to ensure that patient access to critical treatments is not negatively impacted.”
Under MIPS, physicians are scored based on their performance across three categories: quality, improvement activities, and advancing care information. A fourth category, cost, is planned but not yet included in the score (although cost doesn’t impact adjustments until 2020, it is part of the 2018 program year). Medicare payments, which currently include Part B drug reimbursements, are subject to bonuses and penalties based on performance scores.
In their November 2017 update to the Quality Payment Program, which includes MIPS, officials at the Centers for Medicare & Medicaid Services said they would be moving forward with including Part B drug payments in the MIPS adjustment.
“This application of the adjustment ... is a significant departure from current policy and would disproportionately affect certain specialties,” according to the coalition’s letter.
Certain specialties, including rheumatology, oncology, and ophthalmology, have more to lose under the current policy because these specialists administer more Part B drugs than other specialists, according to health care consultancy Avalere Health.
For gastroenterologists, the primary concern with the application of the MIPS payment adjustment to Part B drugs is patient access to the biologics and biosimilars used to treat irritable bowel disease including Crohn’s disease and ulcerative colitis. Under CMS’s policy, gastroenterologists facing a penalty or negative payment adjustment may have Part B drug payments fall below what it costs to buy the Part B drug. Since Medicare payment for Part B drugs is based on average sales price (ASP), affected practices are more likely to be small, have a smaller number of patients on a Part B drug or prescribe a broader range of Part B drugs to patients. As MIPS payment adjustments increase each year the impact of the policy is expected to touch more practices and bring larger deficits further eroding access to the biologics and biosimilars used to treat inflammatory bowel disease.
“Certain specialists administer more Part B drugs than others and, therefore, may be exposed to significant financial risk and payment swings year-over-year under the CMS [Centers for Medicare & Medicaid Services] proposal,” John Feore, director at Avalere, said in a statement.
In 2018, physicians in those specialties could see drug payments increase or decrease by as much as 16%, according to Avalere research.
The policy likely will have an even greater effect on smaller practices and those in rural settings and could lead to access issues, according to the coalition letter.
“Some patients already face access challenges because the budget sequester has eroded reimbursements to physicians, and this policy would exacerbate these problems,” the letter states. “Patients would be left with fewer locations where they could receive care, resulting in less access and higher costs. A growing number of patients would then have to seek care in a hospital, which would result in higher out-of-pocket expenses and, particularly in rural communities, may require traveling longer distances to receive care.”
Genetic Screens Yield Potential Therapies for Neurodegenerative Diseases
SAN DIEGO—Cross-species genetic screens are helping researchers find molecules that modulate the proteins that cause adult neurodegenerative disease, according to a lecture delivered at the 142nd Annual Meeting of the American Neurological Association. Such screening thus reveals potential therapeutic targets and augments scientific understanding of the biology of these proteins.
The research raises the possibility that clinicians will be able to delay or prevent neurodegenerative disease in the future through the early administration of molecules that target these proteins. “We need to identify those at risk and begin the therapy … before the symptoms develop, just like you would treat somebody with statins if they have high cholesterol before they have a heart attack,” said Huda Y. Zoghbi, MD, an investigator with the Howard Hughes Medical Institute; Professor of Pediatrics, Molecular and Human Genetics, Neuroscience, and Neurology at Baylor College of Medicine; and Director of the Jan and Dan Duncan Neurological Research Institute in Houston.
Research Into a Rare Disorder
Studying the rare disorder spinocerebellar ataxia type 1 (SCA1) has yielded information that could be applicable to more common neurodegenerative diseases, said Dr. Zoghbi. SCA1 is characterized by a loss of Purkinje cells and brainstem neurons that impairs balance and coordination and increases the risk of premature death. In 1993, Dr. Zoghbi; Harry Orr, PhD, Professor and Tulloch Chair in Genetics at the University of Minnesota in Minneapolis; and colleagues discovered that a CAG repeat expansion in ATXN1 causes SCA1 by producing an abnormally long version of the ataxin-1 protein. They also found that neurodegeneration results if levels of normal ataxin-1 are increased by 20% or 30%. The brain thus is highly sensitive to ataxin-1 levels, said Dr. Zoghbi.
Borrowing a technique from cancer research, the investigators performed genetic screening using fruit fly and human cells to find targets that would reduce ataxin-1 levels when inhibited. They found approximately 30 relevant genes in each organism, and about 12 were common to both organisms. All of the shared genes operate in the mitogen-activated protein (MAP) kinase pathway, and inhibiting each gene lowered ataxin-1 in cells and flies.
Inhibiting Enzymes
The researchers also observed that the enzymes MSK1 and MSK2 intervene in the pathway and promote ataxin-1 accumulation. Inhibiting MSK1 produced clinical improvement in SCA1 mouse models, and inhibiting MSK1 and MSK2 together produced still more improvement. A small molecule that inhibited MSK1 therefore could help patients with SCA1, said Dr. Zoghbi.
Inhibiting enzymes such as MSK1 and MSK2 for years at a time could raise safety concerns, however. The investigators thus decided to look for other modulators of ataxin-1, on the theory that targeting modulators that function in different pathways could reduce the amount of inhibition required and decrease the risk of adverse events.
Further screening revealed that PKA1 appeared to modulate ataxin-1 by a mechanism similar to that of MSK1. An animal study indicated that inhibiting MSK1 and inhibiting PKA1 produced equivalent reductions on ataxin-1, but that inhibiting both in tandem yielded a greater reduction. The investigators then found that the PAK1 enzyme promoted ataxin-1 accumulation through a pathway different from that of MSK1 and PKA1. Inhibiting PAK1 reduced ataxin-1 levels, and inhibiting PAK1 and MSK1 simultaneously had a still greater effect.
Targeting Tauopathies
Their research into kinases and enzymes prompted Dr. Zoghbi and colleagues to consider whole genome screening as a method of targeting proteins that cause neurodegenerative diseases other than SCA1. Overexpression of tau, for example, causes neurodegeneration, regardless of whether the overexpression results from a mutation in tau-encoding genes. “Tau is a true culprit in dementias, and we thought that if we could find something to lower it, we could help patients with these disorders,” said Dr. Zoghbi.
Another genetic screen suggested that the enzyme Nuak1 stabilizes tau by phosphorylating it at Ser356. The investigators observed that inhibiting Nuak1 reduced tau levels and suppressed neurodegeneration in human cells and in fruit flies. Tau accumulation decreases fruit flies’ climbing ability, and inhibiting Nuak1 improved this ability in flies with tau accumulation.
In a subsequent mouse study, Dr. Zoghbi and colleagues found that inhibiting Nuak1 significantly reduced levels of phosphorylated tau and provided smaller reductions in total tau and endogenous tau. They also observed that mice with tauopathy took longer than wild-type mice to complete a water maze task. Inhibiting Nuak1 in mice with tauopathy improved their performance on this task. Nuak1 inhibition also restored synaptic plasticity in these mice to a level similar to that of wild-type mice. Finally, Nuak1 inhibition reduced tau tangle pathology and increased survival. Dr. Zoghbi and colleagues are now searching for small-molecule Nuak1 inhibitors that could provide protection against tauopathy.
In Search of More Targets
The investigators next looked for genes that influence tau. Successive levels of genetic screening identified 59 genes that “robustly lower tau levels,” said Dr. Zoghbi. She and her colleagues prioritized 12 of the genes for investigation.
They used adenoassociated viral vectors to deliver therapies that can knock out mouse genes for as long as a year. With this technique, the investigators confirmed that all of the initial 12 genes decreased tau levels. In principle, this strategy could enable researchers to scan the whole genome for genes that modulate tau, said Dr. Zoghbi.
—Erik Greb
Suggested Reading
Lasagna-Reeves CA, de Haro M, Hao S, et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron. 2016; 92(2):407-418.
Park J, Al-Ramahi I, Tan Q, et al. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498(7454):325-331.
Rousseaux MW, de Haro M, Lasagna-Reeves CA, et al. TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife. 2016 Oct 25;5. pii: e19809
SAN DIEGO—Cross-species genetic screens are helping researchers find molecules that modulate the proteins that cause adult neurodegenerative disease, according to a lecture delivered at the 142nd Annual Meeting of the American Neurological Association. Such screening thus reveals potential therapeutic targets and augments scientific understanding of the biology of these proteins.
The research raises the possibility that clinicians will be able to delay or prevent neurodegenerative disease in the future through the early administration of molecules that target these proteins. “We need to identify those at risk and begin the therapy … before the symptoms develop, just like you would treat somebody with statins if they have high cholesterol before they have a heart attack,” said Huda Y. Zoghbi, MD, an investigator with the Howard Hughes Medical Institute; Professor of Pediatrics, Molecular and Human Genetics, Neuroscience, and Neurology at Baylor College of Medicine; and Director of the Jan and Dan Duncan Neurological Research Institute in Houston.
Research Into a Rare Disorder
Studying the rare disorder spinocerebellar ataxia type 1 (SCA1) has yielded information that could be applicable to more common neurodegenerative diseases, said Dr. Zoghbi. SCA1 is characterized by a loss of Purkinje cells and brainstem neurons that impairs balance and coordination and increases the risk of premature death. In 1993, Dr. Zoghbi; Harry Orr, PhD, Professor and Tulloch Chair in Genetics at the University of Minnesota in Minneapolis; and colleagues discovered that a CAG repeat expansion in ATXN1 causes SCA1 by producing an abnormally long version of the ataxin-1 protein. They also found that neurodegeneration results if levels of normal ataxin-1 are increased by 20% or 30%. The brain thus is highly sensitive to ataxin-1 levels, said Dr. Zoghbi.
Borrowing a technique from cancer research, the investigators performed genetic screening using fruit fly and human cells to find targets that would reduce ataxin-1 levels when inhibited. They found approximately 30 relevant genes in each organism, and about 12 were common to both organisms. All of the shared genes operate in the mitogen-activated protein (MAP) kinase pathway, and inhibiting each gene lowered ataxin-1 in cells and flies.
Inhibiting Enzymes
The researchers also observed that the enzymes MSK1 and MSK2 intervene in the pathway and promote ataxin-1 accumulation. Inhibiting MSK1 produced clinical improvement in SCA1 mouse models, and inhibiting MSK1 and MSK2 together produced still more improvement. A small molecule that inhibited MSK1 therefore could help patients with SCA1, said Dr. Zoghbi.
Inhibiting enzymes such as MSK1 and MSK2 for years at a time could raise safety concerns, however. The investigators thus decided to look for other modulators of ataxin-1, on the theory that targeting modulators that function in different pathways could reduce the amount of inhibition required and decrease the risk of adverse events.
Further screening revealed that PKA1 appeared to modulate ataxin-1 by a mechanism similar to that of MSK1. An animal study indicated that inhibiting MSK1 and inhibiting PKA1 produced equivalent reductions on ataxin-1, but that inhibiting both in tandem yielded a greater reduction. The investigators then found that the PAK1 enzyme promoted ataxin-1 accumulation through a pathway different from that of MSK1 and PKA1. Inhibiting PAK1 reduced ataxin-1 levels, and inhibiting PAK1 and MSK1 simultaneously had a still greater effect.
Targeting Tauopathies
Their research into kinases and enzymes prompted Dr. Zoghbi and colleagues to consider whole genome screening as a method of targeting proteins that cause neurodegenerative diseases other than SCA1. Overexpression of tau, for example, causes neurodegeneration, regardless of whether the overexpression results from a mutation in tau-encoding genes. “Tau is a true culprit in dementias, and we thought that if we could find something to lower it, we could help patients with these disorders,” said Dr. Zoghbi.
Another genetic screen suggested that the enzyme Nuak1 stabilizes tau by phosphorylating it at Ser356. The investigators observed that inhibiting Nuak1 reduced tau levels and suppressed neurodegeneration in human cells and in fruit flies. Tau accumulation decreases fruit flies’ climbing ability, and inhibiting Nuak1 improved this ability in flies with tau accumulation.
In a subsequent mouse study, Dr. Zoghbi and colleagues found that inhibiting Nuak1 significantly reduced levels of phosphorylated tau and provided smaller reductions in total tau and endogenous tau. They also observed that mice with tauopathy took longer than wild-type mice to complete a water maze task. Inhibiting Nuak1 in mice with tauopathy improved their performance on this task. Nuak1 inhibition also restored synaptic plasticity in these mice to a level similar to that of wild-type mice. Finally, Nuak1 inhibition reduced tau tangle pathology and increased survival. Dr. Zoghbi and colleagues are now searching for small-molecule Nuak1 inhibitors that could provide protection against tauopathy.
In Search of More Targets
The investigators next looked for genes that influence tau. Successive levels of genetic screening identified 59 genes that “robustly lower tau levels,” said Dr. Zoghbi. She and her colleagues prioritized 12 of the genes for investigation.
They used adenoassociated viral vectors to deliver therapies that can knock out mouse genes for as long as a year. With this technique, the investigators confirmed that all of the initial 12 genes decreased tau levels. In principle, this strategy could enable researchers to scan the whole genome for genes that modulate tau, said Dr. Zoghbi.
—Erik Greb
Suggested Reading
Lasagna-Reeves CA, de Haro M, Hao S, et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron. 2016; 92(2):407-418.
Park J, Al-Ramahi I, Tan Q, et al. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498(7454):325-331.
Rousseaux MW, de Haro M, Lasagna-Reeves CA, et al. TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife. 2016 Oct 25;5. pii: e19809
SAN DIEGO—Cross-species genetic screens are helping researchers find molecules that modulate the proteins that cause adult neurodegenerative disease, according to a lecture delivered at the 142nd Annual Meeting of the American Neurological Association. Such screening thus reveals potential therapeutic targets and augments scientific understanding of the biology of these proteins.
The research raises the possibility that clinicians will be able to delay or prevent neurodegenerative disease in the future through the early administration of molecules that target these proteins. “We need to identify those at risk and begin the therapy … before the symptoms develop, just like you would treat somebody with statins if they have high cholesterol before they have a heart attack,” said Huda Y. Zoghbi, MD, an investigator with the Howard Hughes Medical Institute; Professor of Pediatrics, Molecular and Human Genetics, Neuroscience, and Neurology at Baylor College of Medicine; and Director of the Jan and Dan Duncan Neurological Research Institute in Houston.
Research Into a Rare Disorder
Studying the rare disorder spinocerebellar ataxia type 1 (SCA1) has yielded information that could be applicable to more common neurodegenerative diseases, said Dr. Zoghbi. SCA1 is characterized by a loss of Purkinje cells and brainstem neurons that impairs balance and coordination and increases the risk of premature death. In 1993, Dr. Zoghbi; Harry Orr, PhD, Professor and Tulloch Chair in Genetics at the University of Minnesota in Minneapolis; and colleagues discovered that a CAG repeat expansion in ATXN1 causes SCA1 by producing an abnormally long version of the ataxin-1 protein. They also found that neurodegeneration results if levels of normal ataxin-1 are increased by 20% or 30%. The brain thus is highly sensitive to ataxin-1 levels, said Dr. Zoghbi.
Borrowing a technique from cancer research, the investigators performed genetic screening using fruit fly and human cells to find targets that would reduce ataxin-1 levels when inhibited. They found approximately 30 relevant genes in each organism, and about 12 were common to both organisms. All of the shared genes operate in the mitogen-activated protein (MAP) kinase pathway, and inhibiting each gene lowered ataxin-1 in cells and flies.
Inhibiting Enzymes
The researchers also observed that the enzymes MSK1 and MSK2 intervene in the pathway and promote ataxin-1 accumulation. Inhibiting MSK1 produced clinical improvement in SCA1 mouse models, and inhibiting MSK1 and MSK2 together produced still more improvement. A small molecule that inhibited MSK1 therefore could help patients with SCA1, said Dr. Zoghbi.
Inhibiting enzymes such as MSK1 and MSK2 for years at a time could raise safety concerns, however. The investigators thus decided to look for other modulators of ataxin-1, on the theory that targeting modulators that function in different pathways could reduce the amount of inhibition required and decrease the risk of adverse events.
Further screening revealed that PKA1 appeared to modulate ataxin-1 by a mechanism similar to that of MSK1. An animal study indicated that inhibiting MSK1 and inhibiting PKA1 produced equivalent reductions on ataxin-1, but that inhibiting both in tandem yielded a greater reduction. The investigators then found that the PAK1 enzyme promoted ataxin-1 accumulation through a pathway different from that of MSK1 and PKA1. Inhibiting PAK1 reduced ataxin-1 levels, and inhibiting PAK1 and MSK1 simultaneously had a still greater effect.
Targeting Tauopathies
Their research into kinases and enzymes prompted Dr. Zoghbi and colleagues to consider whole genome screening as a method of targeting proteins that cause neurodegenerative diseases other than SCA1. Overexpression of tau, for example, causes neurodegeneration, regardless of whether the overexpression results from a mutation in tau-encoding genes. “Tau is a true culprit in dementias, and we thought that if we could find something to lower it, we could help patients with these disorders,” said Dr. Zoghbi.
Another genetic screen suggested that the enzyme Nuak1 stabilizes tau by phosphorylating it at Ser356. The investigators observed that inhibiting Nuak1 reduced tau levels and suppressed neurodegeneration in human cells and in fruit flies. Tau accumulation decreases fruit flies’ climbing ability, and inhibiting Nuak1 improved this ability in flies with tau accumulation.
In a subsequent mouse study, Dr. Zoghbi and colleagues found that inhibiting Nuak1 significantly reduced levels of phosphorylated tau and provided smaller reductions in total tau and endogenous tau. They also observed that mice with tauopathy took longer than wild-type mice to complete a water maze task. Inhibiting Nuak1 in mice with tauopathy improved their performance on this task. Nuak1 inhibition also restored synaptic plasticity in these mice to a level similar to that of wild-type mice. Finally, Nuak1 inhibition reduced tau tangle pathology and increased survival. Dr. Zoghbi and colleagues are now searching for small-molecule Nuak1 inhibitors that could provide protection against tauopathy.
In Search of More Targets
The investigators next looked for genes that influence tau. Successive levels of genetic screening identified 59 genes that “robustly lower tau levels,” said Dr. Zoghbi. She and her colleagues prioritized 12 of the genes for investigation.
They used adenoassociated viral vectors to deliver therapies that can knock out mouse genes for as long as a year. With this technique, the investigators confirmed that all of the initial 12 genes decreased tau levels. In principle, this strategy could enable researchers to scan the whole genome for genes that modulate tau, said Dr. Zoghbi.
—Erik Greb
Suggested Reading
Lasagna-Reeves CA, de Haro M, Hao S, et al. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron. 2016; 92(2):407-418.
Park J, Al-Ramahi I, Tan Q, et al. RAS-MAPK-MSK1 pathway modulates ataxin 1 protein levels and toxicity in SCA1. Nature. 2013;498(7454):325-331.
Rousseaux MW, de Haro M, Lasagna-Reeves CA, et al. TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife. 2016 Oct 25;5. pii: e19809
Ibudilast shows promise in progressive MS
San Diego – In a phase 2 trial, .
“That was a striking result,” one of the study authors, Robert T. Naismith, MD, said in an interview prior to presenting the study at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We’ll need to see how that translates into a possible clinical benefit.”
In a trial known as SPRINT-MS/NN 102, researchers led by principal investigator Robert Fox, MD, a neurologist at the Cleveland Clinic in Ohio, along with colleagues in the NeuroNEXT Network, randomized 255 subjects with primary or secondary progressive MS to receive either ibudilast up to 100 mg/day (50 mg twice daily) or matching placebo and followed them for 96 weeks. They assessed clinical and imaging outcomes every 24 weeks, and the primary outcome was change in brain atrophy as measured by brain parenchymal fraction (BPF) over 96 weeks. Secondary outcomes included magnetization transfer ratio (MTR), diffusion tensor imaging, and optical coherence tomography. Analysis was based on a modified intention-to-treat approach, and linear mixed effects modeling was used to estimate the rate of change within imaging measures for each treatment group.
The 255 subjects were enrolled at 28 U.S. sites and their last follow-up visit was completed on May 11, 2017. The retention rate through week 96 was 86%, or 219 patients. The researchers found that treatment with ibudilast was associated with a 48% slowing in the rate of decline in brain atrophy as measured by BPF. A per-protocol analysis yielded similar results (P = .045). In addition, no increased rate of serious adverse events and no opportunistic infections or cancer signals were observed. As for tolerability, 25% of subjects on placebo and 30% of those on ibudilast discontinued treatment (P = .30). The main treatment-related adverse events were gastrointestinal in nature (20% in the placebo group vs. 67% in the ibudilast group; P = .002), primarily nausea.
In the analysis of key secondary outcomes, the researchers found that the change in MTR in normal-appearing brain tissue was reduced –0.00558 for ibudilast and –0.03064 for placebo, which was a relative reduction of 82% in MTR decline. At the same time, the change in MTR in normal-appearing gray matter was reduced –0.00753 for ibudilast and –0.03210 for placebo, which was a relative reduction of 77% in MTR decline. No significant difference in white matter changes were observed in transverse diffusivity (P = .15) or longitudinal diffusivity (P = .73), compared with placebo.
For the secondary endpoint of disability, a blinded evaluator assessed Expanded Disability Status Scale (EDSS) score at baseline and every 24 weeks. The researchers used a time-to-event Kaplan-Meier analysis and a Cox proportional hazards model to determine time to confirmed EDSS progression not due to relapse. They found that the hazard ratio for progression in the ibudilast group, compared with the placebo group, was 0.74, with a 90% confidence interval of 0.47-1.17 (P = .29).
“Ibudilast is a novel therapy for progressive MS, with a positive phase 2 study,” Dr. Naismith concluded. “Safety and tolerability were favorable. Clinical endpoints from this trial will be forthcoming.”
The NeuroNEXT Network is supported by the National Institute of Neurological Disorders and Stroke. Dr. Naismith reported having no financial disclosures.
SOURCE: Naismith R et al. ACTRIMS Forum 2018 Abstract P029.
San Diego – In a phase 2 trial, .
“That was a striking result,” one of the study authors, Robert T. Naismith, MD, said in an interview prior to presenting the study at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We’ll need to see how that translates into a possible clinical benefit.”
In a trial known as SPRINT-MS/NN 102, researchers led by principal investigator Robert Fox, MD, a neurologist at the Cleveland Clinic in Ohio, along with colleagues in the NeuroNEXT Network, randomized 255 subjects with primary or secondary progressive MS to receive either ibudilast up to 100 mg/day (50 mg twice daily) or matching placebo and followed them for 96 weeks. They assessed clinical and imaging outcomes every 24 weeks, and the primary outcome was change in brain atrophy as measured by brain parenchymal fraction (BPF) over 96 weeks. Secondary outcomes included magnetization transfer ratio (MTR), diffusion tensor imaging, and optical coherence tomography. Analysis was based on a modified intention-to-treat approach, and linear mixed effects modeling was used to estimate the rate of change within imaging measures for each treatment group.
The 255 subjects were enrolled at 28 U.S. sites and their last follow-up visit was completed on May 11, 2017. The retention rate through week 96 was 86%, or 219 patients. The researchers found that treatment with ibudilast was associated with a 48% slowing in the rate of decline in brain atrophy as measured by BPF. A per-protocol analysis yielded similar results (P = .045). In addition, no increased rate of serious adverse events and no opportunistic infections or cancer signals were observed. As for tolerability, 25% of subjects on placebo and 30% of those on ibudilast discontinued treatment (P = .30). The main treatment-related adverse events were gastrointestinal in nature (20% in the placebo group vs. 67% in the ibudilast group; P = .002), primarily nausea.
In the analysis of key secondary outcomes, the researchers found that the change in MTR in normal-appearing brain tissue was reduced –0.00558 for ibudilast and –0.03064 for placebo, which was a relative reduction of 82% in MTR decline. At the same time, the change in MTR in normal-appearing gray matter was reduced –0.00753 for ibudilast and –0.03210 for placebo, which was a relative reduction of 77% in MTR decline. No significant difference in white matter changes were observed in transverse diffusivity (P = .15) or longitudinal diffusivity (P = .73), compared with placebo.
For the secondary endpoint of disability, a blinded evaluator assessed Expanded Disability Status Scale (EDSS) score at baseline and every 24 weeks. The researchers used a time-to-event Kaplan-Meier analysis and a Cox proportional hazards model to determine time to confirmed EDSS progression not due to relapse. They found that the hazard ratio for progression in the ibudilast group, compared with the placebo group, was 0.74, with a 90% confidence interval of 0.47-1.17 (P = .29).
“Ibudilast is a novel therapy for progressive MS, with a positive phase 2 study,” Dr. Naismith concluded. “Safety and tolerability were favorable. Clinical endpoints from this trial will be forthcoming.”
The NeuroNEXT Network is supported by the National Institute of Neurological Disorders and Stroke. Dr. Naismith reported having no financial disclosures.
SOURCE: Naismith R et al. ACTRIMS Forum 2018 Abstract P029.
San Diego – In a phase 2 trial, .
“That was a striking result,” one of the study authors, Robert T. Naismith, MD, said in an interview prior to presenting the study at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “We’ll need to see how that translates into a possible clinical benefit.”
In a trial known as SPRINT-MS/NN 102, researchers led by principal investigator Robert Fox, MD, a neurologist at the Cleveland Clinic in Ohio, along with colleagues in the NeuroNEXT Network, randomized 255 subjects with primary or secondary progressive MS to receive either ibudilast up to 100 mg/day (50 mg twice daily) or matching placebo and followed them for 96 weeks. They assessed clinical and imaging outcomes every 24 weeks, and the primary outcome was change in brain atrophy as measured by brain parenchymal fraction (BPF) over 96 weeks. Secondary outcomes included magnetization transfer ratio (MTR), diffusion tensor imaging, and optical coherence tomography. Analysis was based on a modified intention-to-treat approach, and linear mixed effects modeling was used to estimate the rate of change within imaging measures for each treatment group.
The 255 subjects were enrolled at 28 U.S. sites and their last follow-up visit was completed on May 11, 2017. The retention rate through week 96 was 86%, or 219 patients. The researchers found that treatment with ibudilast was associated with a 48% slowing in the rate of decline in brain atrophy as measured by BPF. A per-protocol analysis yielded similar results (P = .045). In addition, no increased rate of serious adverse events and no opportunistic infections or cancer signals were observed. As for tolerability, 25% of subjects on placebo and 30% of those on ibudilast discontinued treatment (P = .30). The main treatment-related adverse events were gastrointestinal in nature (20% in the placebo group vs. 67% in the ibudilast group; P = .002), primarily nausea.
In the analysis of key secondary outcomes, the researchers found that the change in MTR in normal-appearing brain tissue was reduced –0.00558 for ibudilast and –0.03064 for placebo, which was a relative reduction of 82% in MTR decline. At the same time, the change in MTR in normal-appearing gray matter was reduced –0.00753 for ibudilast and –0.03210 for placebo, which was a relative reduction of 77% in MTR decline. No significant difference in white matter changes were observed in transverse diffusivity (P = .15) or longitudinal diffusivity (P = .73), compared with placebo.
For the secondary endpoint of disability, a blinded evaluator assessed Expanded Disability Status Scale (EDSS) score at baseline and every 24 weeks. The researchers used a time-to-event Kaplan-Meier analysis and a Cox proportional hazards model to determine time to confirmed EDSS progression not due to relapse. They found that the hazard ratio for progression in the ibudilast group, compared with the placebo group, was 0.74, with a 90% confidence interval of 0.47-1.17 (P = .29).
“Ibudilast is a novel therapy for progressive MS, with a positive phase 2 study,” Dr. Naismith concluded. “Safety and tolerability were favorable. Clinical endpoints from this trial will be forthcoming.”
The NeuroNEXT Network is supported by the National Institute of Neurological Disorders and Stroke. Dr. Naismith reported having no financial disclosures.
SOURCE: Naismith R et al. ACTRIMS Forum 2018 Abstract P029.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: Ibudilast’s positive phase 2 trial results in progressive multiple sclerosis patients bode well for future development.
Major finding: Ibudilast treatment was associated with a 48% slowing in the rate of decline in brain atrophy as measured by brain parenchymal fraction.
Study details: A randomized trial of 255 subjects with progressive MS enrolled at 28 U.S. sites.
Disclosures: The NeuroNEXT Network is supported by the National Institute of Neurological Disorders and Stroke. Dr. Naismith reported having no financial disclosures.
Source: Naismith R et al. ACTRIMS Forum 2018 Abstract P029.
Integrating behavioral health into primary care
This is the sixth in a series of articles from the National Center for Excellence in Primary Care Research in the Agency for Healthcare Research and Quality. This series introduces sets of tools and resources designed to help your practice.
Primary care practice is under increasing pressure to evolve. As highlighted in this series, topics such as shared decision making, team-based care, integration of behavioral health into primary care practice, and practice facilitation all offer the potential to enhance your primary care practice. On the other hand, quality of care must remain a top priority during this transformation. While much of the Agency for Healthcare Research and Quality’s (AHRQ) work in quality of care focuses on the inpatient setting, AHRQ offers many tools and resources to evaluate quality of care and to implement quality improvement into your primary care practice.
The NQMC mission is to provide an accessible mechanism for obtaining detailed information on quality measures and to further the dissemination, implementation, and use of these measures to inform health care decisions. NQMC is designed for practitioners, health care providers, health plans, integrated delivery systems, purchasers, and others interested in health care quality measurement. Funding for the NQMC is in question, and the future of this resource is not certain.
Confidential feedback reporting is widely considered to be a precursor to and a foundation for performance improvement. However, to enable change, the clinician responsible for and capable of change must receive, understand, and act on the information. The following publications from the National Center for Excellence in Primary Care Research offer some guidance from the on ways to best do so:
- Confidential Physician Feedback Reports: Designing for Optimal Impact on Performance is a guide that informs developers of feedback reports about evidence-based strategies to consider when they develop or refine a feedback reporting system.
- Will It Work Here? A Decisionmaker’s Guide to Adopting Innovations can help you determine if an innovation would be a good fit – or an appropriate stretch – for your practice or health care organization by asking a series of questions. It links users to actionable Web-based tools and presents case studies that illustrate how other organizations have addressed these questions.
- Improving Your Office Testing Process: A Toolkit for Rapid-Cycle Patient Safety and Quality Improvement provides information and resources to help physicians’ offices, clinics, and other ambulatory care facilities assess and improve the testing process in their offices.
These and other tools can be found at the AHRQ website.
Dr. Ganiats is the director for the National Center for Excellence in Primary Care Research at AHRQ.
This is the sixth in a series of articles from the National Center for Excellence in Primary Care Research in the Agency for Healthcare Research and Quality. This series introduces sets of tools and resources designed to help your practice.
Primary care practice is under increasing pressure to evolve. As highlighted in this series, topics such as shared decision making, team-based care, integration of behavioral health into primary care practice, and practice facilitation all offer the potential to enhance your primary care practice. On the other hand, quality of care must remain a top priority during this transformation. While much of the Agency for Healthcare Research and Quality’s (AHRQ) work in quality of care focuses on the inpatient setting, AHRQ offers many tools and resources to evaluate quality of care and to implement quality improvement into your primary care practice.
The NQMC mission is to provide an accessible mechanism for obtaining detailed information on quality measures and to further the dissemination, implementation, and use of these measures to inform health care decisions. NQMC is designed for practitioners, health care providers, health plans, integrated delivery systems, purchasers, and others interested in health care quality measurement. Funding for the NQMC is in question, and the future of this resource is not certain.
Confidential feedback reporting is widely considered to be a precursor to and a foundation for performance improvement. However, to enable change, the clinician responsible for and capable of change must receive, understand, and act on the information. The following publications from the National Center for Excellence in Primary Care Research offer some guidance from the on ways to best do so:
- Confidential Physician Feedback Reports: Designing for Optimal Impact on Performance is a guide that informs developers of feedback reports about evidence-based strategies to consider when they develop or refine a feedback reporting system.
- Will It Work Here? A Decisionmaker’s Guide to Adopting Innovations can help you determine if an innovation would be a good fit – or an appropriate stretch – for your practice or health care organization by asking a series of questions. It links users to actionable Web-based tools and presents case studies that illustrate how other organizations have addressed these questions.
- Improving Your Office Testing Process: A Toolkit for Rapid-Cycle Patient Safety and Quality Improvement provides information and resources to help physicians’ offices, clinics, and other ambulatory care facilities assess and improve the testing process in their offices.
These and other tools can be found at the AHRQ website.
Dr. Ganiats is the director for the National Center for Excellence in Primary Care Research at AHRQ.
This is the sixth in a series of articles from the National Center for Excellence in Primary Care Research in the Agency for Healthcare Research and Quality. This series introduces sets of tools and resources designed to help your practice.
Primary care practice is under increasing pressure to evolve. As highlighted in this series, topics such as shared decision making, team-based care, integration of behavioral health into primary care practice, and practice facilitation all offer the potential to enhance your primary care practice. On the other hand, quality of care must remain a top priority during this transformation. While much of the Agency for Healthcare Research and Quality’s (AHRQ) work in quality of care focuses on the inpatient setting, AHRQ offers many tools and resources to evaluate quality of care and to implement quality improvement into your primary care practice.
The NQMC mission is to provide an accessible mechanism for obtaining detailed information on quality measures and to further the dissemination, implementation, and use of these measures to inform health care decisions. NQMC is designed for practitioners, health care providers, health plans, integrated delivery systems, purchasers, and others interested in health care quality measurement. Funding for the NQMC is in question, and the future of this resource is not certain.
Confidential feedback reporting is widely considered to be a precursor to and a foundation for performance improvement. However, to enable change, the clinician responsible for and capable of change must receive, understand, and act on the information. The following publications from the National Center for Excellence in Primary Care Research offer some guidance from the on ways to best do so:
- Confidential Physician Feedback Reports: Designing for Optimal Impact on Performance is a guide that informs developers of feedback reports about evidence-based strategies to consider when they develop or refine a feedback reporting system.
- Will It Work Here? A Decisionmaker’s Guide to Adopting Innovations can help you determine if an innovation would be a good fit – or an appropriate stretch – for your practice or health care organization by asking a series of questions. It links users to actionable Web-based tools and presents case studies that illustrate how other organizations have addressed these questions.
- Improving Your Office Testing Process: A Toolkit for Rapid-Cycle Patient Safety and Quality Improvement provides information and resources to help physicians’ offices, clinics, and other ambulatory care facilities assess and improve the testing process in their offices.
These and other tools can be found at the AHRQ website.
Dr. Ganiats is the director for the National Center for Excellence in Primary Care Research at AHRQ.
AHA: Heart health helps optimize breast cancer outcomes
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
FROM CIRCULATION
MS may be a transmissible protein misfolding disorder, study suggests
SAN DIEGO – MS may even be caused by prions, potentially putting it into the same category as Creutzfeldt-Jakob disease.
Researchers don’t think MS is contagious between humans. But their findings in mice do suggest that the disease is transmissible from “a seed of protein misfolding,” Shigeki Tsutsui, DVM, PhD, of the University of Calgary (Alta.), said in an interview in advance of his presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The next question is: ‘What is actually causing primary damage?’ ” he said. “Our hypothesis is that it’s protein misfolding. If protein misfolding targets the central neuron cells, then those damaged cells release a kind of a trigger to start an immune response.”
This isn’t an unusual concept. Protein misfolding is believed to cause several chronic neurodegenerative diseases, including Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis, Dr. Tsutsui said.
The researchers focused on the potential transmissibility of human prion protein “since we thought that the prion protein might be the candidate causing the MS,” he said.
Prion diseases are extremely rare and often deadly. They appear when normal cellular prion proteins are induced to misfold when they come in contact with infectious agents known as prions.
In humans, prion diseases include Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru (a condition spread among cannibals who eat the brains of other humans). Most famously, a variant of Creutzfeldt-Jakob disease has been linked – but not conclusively – to the eating of meat from cows infected with mad cow disease.
Depending on the condition, prion diseases can appear spontaneously, via inheritance or through infection.
In the new study, researchers intracerebrally injected brain homogenate from 10 patients with primary or secondary-progressive MS into 36 transgenic mice. All the mice over-expressed human prion protein. A control group of 23 mice were injected with homogenate from six donor brains.
At 6-12 months, the control mice did not develop pathology. However, the mice injected with brain matter from MS patients developed various levels of significant pathology. The researchers also managed to transmit illness from affected mice into another group of transgenic mice that over-expressed human prion protein via injection of brain homogenate.
While the mice in the study represent “a very extreme case” since their levels of prion protein expression were very high, it’s clear that “MS is actually transmissible,” Dr. Tsutsui said.
The study authors speculate that pathogenic prion protein triggers an autoimmune response by degenerating myelin and causing the release of cellular debris.
Could MS also be contagious? It may not be, even if it’s a prion disease, Dr. Tsutsui said. Some prion disease variants are neither contagious nor infectious.
The researchers plan to study the animal model they’ve created and explore the potential for its use in research, Dr. Tsutsui said.
The authors have no disclosures with the exception of one who disclosed serving as deputy editor of the Multiple Sclerosis Journal. Funding sources include Canada Research Chairs, Alberta Innovates-Health Solutions, and the Alberta Prion Research Institute.
SOURCE: Tsutsui S et al. ACTRIMS Forum 2018 Abstract LB282.
SAN DIEGO – MS may even be caused by prions, potentially putting it into the same category as Creutzfeldt-Jakob disease.
Researchers don’t think MS is contagious between humans. But their findings in mice do suggest that the disease is transmissible from “a seed of protein misfolding,” Shigeki Tsutsui, DVM, PhD, of the University of Calgary (Alta.), said in an interview in advance of his presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The next question is: ‘What is actually causing primary damage?’ ” he said. “Our hypothesis is that it’s protein misfolding. If protein misfolding targets the central neuron cells, then those damaged cells release a kind of a trigger to start an immune response.”
This isn’t an unusual concept. Protein misfolding is believed to cause several chronic neurodegenerative diseases, including Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis, Dr. Tsutsui said.
The researchers focused on the potential transmissibility of human prion protein “since we thought that the prion protein might be the candidate causing the MS,” he said.
Prion diseases are extremely rare and often deadly. They appear when normal cellular prion proteins are induced to misfold when they come in contact with infectious agents known as prions.
In humans, prion diseases include Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru (a condition spread among cannibals who eat the brains of other humans). Most famously, a variant of Creutzfeldt-Jakob disease has been linked – but not conclusively – to the eating of meat from cows infected with mad cow disease.
Depending on the condition, prion diseases can appear spontaneously, via inheritance or through infection.
In the new study, researchers intracerebrally injected brain homogenate from 10 patients with primary or secondary-progressive MS into 36 transgenic mice. All the mice over-expressed human prion protein. A control group of 23 mice were injected with homogenate from six donor brains.
At 6-12 months, the control mice did not develop pathology. However, the mice injected with brain matter from MS patients developed various levels of significant pathology. The researchers also managed to transmit illness from affected mice into another group of transgenic mice that over-expressed human prion protein via injection of brain homogenate.
While the mice in the study represent “a very extreme case” since their levels of prion protein expression were very high, it’s clear that “MS is actually transmissible,” Dr. Tsutsui said.
The study authors speculate that pathogenic prion protein triggers an autoimmune response by degenerating myelin and causing the release of cellular debris.
Could MS also be contagious? It may not be, even if it’s a prion disease, Dr. Tsutsui said. Some prion disease variants are neither contagious nor infectious.
The researchers plan to study the animal model they’ve created and explore the potential for its use in research, Dr. Tsutsui said.
The authors have no disclosures with the exception of one who disclosed serving as deputy editor of the Multiple Sclerosis Journal. Funding sources include Canada Research Chairs, Alberta Innovates-Health Solutions, and the Alberta Prion Research Institute.
SOURCE: Tsutsui S et al. ACTRIMS Forum 2018 Abstract LB282.
SAN DIEGO – MS may even be caused by prions, potentially putting it into the same category as Creutzfeldt-Jakob disease.
Researchers don’t think MS is contagious between humans. But their findings in mice do suggest that the disease is transmissible from “a seed of protein misfolding,” Shigeki Tsutsui, DVM, PhD, of the University of Calgary (Alta.), said in an interview in advance of his presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“The next question is: ‘What is actually causing primary damage?’ ” he said. “Our hypothesis is that it’s protein misfolding. If protein misfolding targets the central neuron cells, then those damaged cells release a kind of a trigger to start an immune response.”
This isn’t an unusual concept. Protein misfolding is believed to cause several chronic neurodegenerative diseases, including Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis, Dr. Tsutsui said.
The researchers focused on the potential transmissibility of human prion protein “since we thought that the prion protein might be the candidate causing the MS,” he said.
Prion diseases are extremely rare and often deadly. They appear when normal cellular prion proteins are induced to misfold when they come in contact with infectious agents known as prions.
In humans, prion diseases include Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru (a condition spread among cannibals who eat the brains of other humans). Most famously, a variant of Creutzfeldt-Jakob disease has been linked – but not conclusively – to the eating of meat from cows infected with mad cow disease.
Depending on the condition, prion diseases can appear spontaneously, via inheritance or through infection.
In the new study, researchers intracerebrally injected brain homogenate from 10 patients with primary or secondary-progressive MS into 36 transgenic mice. All the mice over-expressed human prion protein. A control group of 23 mice were injected with homogenate from six donor brains.
At 6-12 months, the control mice did not develop pathology. However, the mice injected with brain matter from MS patients developed various levels of significant pathology. The researchers also managed to transmit illness from affected mice into another group of transgenic mice that over-expressed human prion protein via injection of brain homogenate.
While the mice in the study represent “a very extreme case” since their levels of prion protein expression were very high, it’s clear that “MS is actually transmissible,” Dr. Tsutsui said.
The study authors speculate that pathogenic prion protein triggers an autoimmune response by degenerating myelin and causing the release of cellular debris.
Could MS also be contagious? It may not be, even if it’s a prion disease, Dr. Tsutsui said. Some prion disease variants are neither contagious nor infectious.
The researchers plan to study the animal model they’ve created and explore the potential for its use in research, Dr. Tsutsui said.
The authors have no disclosures with the exception of one who disclosed serving as deputy editor of the Multiple Sclerosis Journal. Funding sources include Canada Research Chairs, Alberta Innovates-Health Solutions, and the Alberta Prion Research Institute.
SOURCE: Tsutsui S et al. ACTRIMS Forum 2018 Abstract LB282.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: Translational research suggests the primary damage in MS may be caused by transmissible protein misfolding.
Major finding: Transgenic mice over-expressing human prion protein that were injected with brain matter from MS patients developed various levels of significant pathology, and brain homogenate from these mice could transmit illness to naive transgenic mice.
Study details: Transgenic mice over-expressing human prion protein received intracerebral injection of brain homogenate from patients with primary or secondary-progressive MS
Disclosures: The authors have no disclosures with the exception of one who disclosed serving as deputy editor of the Multiple Sclerosis Journal. Funding sources include Canada Research Chairs, Alberta Innovates-Health Solutions, and the Alberta Prion Research Institute.
Source: Tsutsui S et al. ACTRIMS Forum 2018 Abstract LB282.
Making hospital medicine a lifelong, enjoyable, and engaging career
Editor’s note: Each month, the Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Amith Skandhan, MD, FHM, a hospitalist, a director/physician liaison for clinical documentation improvement and core faculty member in the Internal Medicine Residency Program at Southeast Alabama Medical Center in Dothan, Ala., and clinical faculty member at the Alabama College of Osteopathic Medicine also in Dothan. Dr. Skandhan is the cofounder and current president of the SHM Wiregrass Chapter and is an active member of SHM’s Annual Conference and Performance Measurement Reporting committees.
When did you join SHM, and what prompted you to apply for your current committee roles?
There were many things that were not sufficiently taught during clinical training that were required in my day-to-day practice, like clinical documentation improvement, practice management, billing, coding, and so forth. I also quickly understood how vast and dynamic hospital medicine really was. While looking for an outlet to voice my questions, concerns, and curiosity, I decided to join SHM, which has helped me find and apply the techniques I’d been looking for to further my career as a hospitalist.
I’m now fortunate to be a part of SHM’s national committees, which involve hospitalists of various backgrounds and experiences, who work together to improve the overall quality of inpatient medicine. I currently serve on the Performance Reporting Measurement Committee and the Annual Conference Committee. My interests in reviewing the ever-evolving policies of health care made me apply to be a part of the Performance Reporting Measurement Committee. We work very closely with the Public Policy Committee, analyzing written policies and subsequently offering our recommendations. It’s been fulfilling to be a part of a committee that works towards developing policies that support a good quality of care on such a large scale.
My penchant for organizing events and bringing people together based on common ground led me to apply for the Annual Conference Committee. We meet every week to discuss various topics, choose and invite speakers, and help organize the entire event, which will host close to 5,000 hospitalists later this year. It has made me appreciate being a member of an organization that provides hospitalists with opportunities for education and growth. I’m hopeful that the attendees next year will find the conference to be a worthwhile experience!
As the president of SHM’s Wiregrass Chapter, how has the chapter grown since its establishment in May 2015?
Our chapter is based in Dothan, a small, rural Alabama town where Southeast Alabama Medical Center is located. The chapter covers the counties of lower Alabama and the panhandle of Florida. We named the chapter after a special species of grass that grows in this region.
When we started the chapter, our goal was to bring the best and brightest of hospital medicine to our region to give talks on hot topics in the field and also to use their expertise to guide inpatient care in our hospital system. We aggressively marketed the events to bring in large crowds of medical professionals, and we consistently average around 70-80 attendees in our meetings. Bringing in leaders from the field helped create an atmosphere of learning and inspired us to grow and develop our hospitalist program. We now closely work with hospital medicine groups in surrounding rural areas toward improving inpatient hospital care.
During these past years, we also realized that, for the further growth of our chapter, we would need to nurture an interest in hospital medicine among future generations of doctors, and this realization led to the creation of our medical student and resident wing. So far, the students have been very enthusiastic about participating in SHM-related events, and I hope that continues. We also developed a mentor-mentee program, in which we paired selected medical students with hospitalists to help guide future careers in acute care medicine. This year, we have also been helping the hospital medicine division at Southeast Alabama Medical Center create a clinical research track for medical students. To that end, we have just completed our second annual poster competition where we presented around 50 posters in the areas of clinical vignettes, quality improvement, and original research.
In addition, the chapter is very active with community activities. We took notice of the fact that many of our patients and community members were unaware of what hospitalists did because they could not understand how our work was different from that of primary care physicians. Our members have therefore participated in TV, radio, and newspaper interviews to help elucidate the role of hospitalists in patient care. We have also periodically visited primary care physician offices, nursing homes, senior citizen groups, and cancer support groups to educate these patients on various facets of health care and how hospitalists influence these areas.
In 2014, we organized a “walk with a hospitalist” event, for which we set up a half-mile “admission to discharge” scenario explaining the role of hospitalists and other departments involved in patient care. This year, in hopes of improving patient literacy in our region, we held a “shop with a doc” event, where the Southeast Alabama Medical Center hospitalists teamed up with dietitians and taught patients how food and lifestyle influenced their chronic medical illnesses. This was followed by physicians and dietitians shopping with patients in the grocery store, educating them on healthy choices and label reading.
We’re incredibly grateful for the support that we’ve received from our medical and patient communities; they’ve been critical in helping our chapter grow as much as it has, and they motivate us to work harder and do more with the chapter. We were honored to receive the SHM’s Rising Star Award at the Hospital Medicine 2017 conference in Las Vegas. We never thought that our little chapter in the American countryside would be chosen, but we’re very thankful to have our efforts recognized on the national stage!
Which SHM conferences have you attended? Tell TH about your most memorable highlights or takeaways.
When I started out as a hospitalist in 2014, I decided to attend the annual conference in Las Vegas, and I can honestly say that conference changed the course of my career. I can still remember listening to the opening speech and realizing that I was surrounded by more than 3,000 hospitalists who understood the power we had to influence inpatient care. I’ve attended all the national conferences since then and am grateful that I now get to help organize the Hospital Medicine 2018 annual conference, also known as HM18.
I had been working to find a way to improve documentation within my group, as well as change the culture and perception towards billing and coding practices, which prompted me to attend the Quality and Safety Educators Academy. During one of the problem-solving sessions, I explained the challenges that I faced to my conference group. The exercise required me to explain the problem at hand, and the players of my group then discussed their thoughts while I took notes. It was a fantastic experience, as the participants at my table offered strong solutions to my problems within a matter of minutes. Their advice led to meaningful changes in our group’s hospital documentation practices, and in turn, I’ve been promoted to physician advisor in Southeast Alabama Medical Center.
After such a great experience at Quality and Safety Educators Academy, I went on to attend SHM’s Leadership Academy, where I had the opportunity to meet some of the top leaders and pioneers in the field of hospital medicine. It’s empowering to be mentored by the very people you look up to and aspire to be like. Not only was I able to bring ideas home to my institution, but I was able to reflect and improve my own professional and personal growth. I’m happy to say that I’ve completed all three levels of Leadership Academy.
As I’ve become involved with the medical student and residency programs at my medical center, I recently attended the Academic Hospitalist Academy to help my transition into academic hospital medicine. Meeting and spending time with the faculty at Academic Hospitalist Academy made me further realize the roles that academic hospitalists play in the education of future physicians, emphasizing the idea that we can all be champions in quality and patient safety.
If you’re looking to advance your career as a hospitalist, take advantage of the conferences that SHM offers. I’ve gained so much from each experience, and I’m looking forward to returning to these conferences as a potential facilitator, in hopes of offering what I’ve learned to hospitalists looking to bring about change in their fields and careers.
What can attendees at HM18 expect to see in the area of career development, and how is this different than previous years?
Hospital medicine is only about 2 decades old, making it one of the youngest branches in medicine today. Given this fact, the Annual Conference Committee feels that it is paramount to focus on career development for both new and midcareer hospitalists alike.
One question that we wish to explore and answer this year is: “How do you make hospital medicine a life-long, enjoyable, and engaging career?” In turn, our committee has created several new additions to HM18. This includes a “Seasoning Your Career” track, which will provide ideas on how to advance in leadership, use emotional intelligence to achieve success, change your roles midcareer, and change hospitalist schedules. Another unique addition this year are career development workshops, which will aim to developing various aspects of a hospitalist’s career, such as working on leadership skills, refining presentation and communication skills, providing constructive feedback, promoting women in hospital medicine, preventing burnout, and turning ideas into clinical research. We also plan to incorporate an education track, which will focus on how hospitalists can expand their careers towards educational leadership.
Given your involvement in SHM at both the local and national levels, do you have any advice for young hospital medicine professionals looking to build their professional profiles?
I’ve frequently noticed that young hospitalists don’t realize the potential influence they hold within their own institutions or the power they have to elicit change in health care at the national level.
Though we don’t often admit it, some hospitalists feel like they are glorified residents, which definitely is not the case. As a provider on the front lines, you have the unique opportunity to implement changes pertaining to issues of cost, utilization of resources, process management, quality and patient safety, and bottlenecks in care, to name a few. These are issues that keep the administrators of your organization and leaders of hospital medicine up at night. Don’t sit around and complain about how things could be or should be; look toward creating change. Bring up possible solutions to these problems with your leaders. They will appreciate the effort, and hopefully together you can find ways to tackle these problems.
I will conclude by saying this: Hospital medicine is such a unique specialty in that it’s constantly evolving, and the pioneers of this field are still alive and practicing medicine. You can meet and interact with them during the SHM conferences and look to them as sources of inspiration or guidance. Meeting people you look up to and having them as your mentors can take you places.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, the Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Amith Skandhan, MD, FHM, a hospitalist, a director/physician liaison for clinical documentation improvement and core faculty member in the Internal Medicine Residency Program at Southeast Alabama Medical Center in Dothan, Ala., and clinical faculty member at the Alabama College of Osteopathic Medicine also in Dothan. Dr. Skandhan is the cofounder and current president of the SHM Wiregrass Chapter and is an active member of SHM’s Annual Conference and Performance Measurement Reporting committees.
When did you join SHM, and what prompted you to apply for your current committee roles?
There were many things that were not sufficiently taught during clinical training that were required in my day-to-day practice, like clinical documentation improvement, practice management, billing, coding, and so forth. I also quickly understood how vast and dynamic hospital medicine really was. While looking for an outlet to voice my questions, concerns, and curiosity, I decided to join SHM, which has helped me find and apply the techniques I’d been looking for to further my career as a hospitalist.
I’m now fortunate to be a part of SHM’s national committees, which involve hospitalists of various backgrounds and experiences, who work together to improve the overall quality of inpatient medicine. I currently serve on the Performance Reporting Measurement Committee and the Annual Conference Committee. My interests in reviewing the ever-evolving policies of health care made me apply to be a part of the Performance Reporting Measurement Committee. We work very closely with the Public Policy Committee, analyzing written policies and subsequently offering our recommendations. It’s been fulfilling to be a part of a committee that works towards developing policies that support a good quality of care on such a large scale.
My penchant for organizing events and bringing people together based on common ground led me to apply for the Annual Conference Committee. We meet every week to discuss various topics, choose and invite speakers, and help organize the entire event, which will host close to 5,000 hospitalists later this year. It has made me appreciate being a member of an organization that provides hospitalists with opportunities for education and growth. I’m hopeful that the attendees next year will find the conference to be a worthwhile experience!
As the president of SHM’s Wiregrass Chapter, how has the chapter grown since its establishment in May 2015?
Our chapter is based in Dothan, a small, rural Alabama town where Southeast Alabama Medical Center is located. The chapter covers the counties of lower Alabama and the panhandle of Florida. We named the chapter after a special species of grass that grows in this region.
When we started the chapter, our goal was to bring the best and brightest of hospital medicine to our region to give talks on hot topics in the field and also to use their expertise to guide inpatient care in our hospital system. We aggressively marketed the events to bring in large crowds of medical professionals, and we consistently average around 70-80 attendees in our meetings. Bringing in leaders from the field helped create an atmosphere of learning and inspired us to grow and develop our hospitalist program. We now closely work with hospital medicine groups in surrounding rural areas toward improving inpatient hospital care.
During these past years, we also realized that, for the further growth of our chapter, we would need to nurture an interest in hospital medicine among future generations of doctors, and this realization led to the creation of our medical student and resident wing. So far, the students have been very enthusiastic about participating in SHM-related events, and I hope that continues. We also developed a mentor-mentee program, in which we paired selected medical students with hospitalists to help guide future careers in acute care medicine. This year, we have also been helping the hospital medicine division at Southeast Alabama Medical Center create a clinical research track for medical students. To that end, we have just completed our second annual poster competition where we presented around 50 posters in the areas of clinical vignettes, quality improvement, and original research.
In addition, the chapter is very active with community activities. We took notice of the fact that many of our patients and community members were unaware of what hospitalists did because they could not understand how our work was different from that of primary care physicians. Our members have therefore participated in TV, radio, and newspaper interviews to help elucidate the role of hospitalists in patient care. We have also periodically visited primary care physician offices, nursing homes, senior citizen groups, and cancer support groups to educate these patients on various facets of health care and how hospitalists influence these areas.
In 2014, we organized a “walk with a hospitalist” event, for which we set up a half-mile “admission to discharge” scenario explaining the role of hospitalists and other departments involved in patient care. This year, in hopes of improving patient literacy in our region, we held a “shop with a doc” event, where the Southeast Alabama Medical Center hospitalists teamed up with dietitians and taught patients how food and lifestyle influenced their chronic medical illnesses. This was followed by physicians and dietitians shopping with patients in the grocery store, educating them on healthy choices and label reading.
We’re incredibly grateful for the support that we’ve received from our medical and patient communities; they’ve been critical in helping our chapter grow as much as it has, and they motivate us to work harder and do more with the chapter. We were honored to receive the SHM’s Rising Star Award at the Hospital Medicine 2017 conference in Las Vegas. We never thought that our little chapter in the American countryside would be chosen, but we’re very thankful to have our efforts recognized on the national stage!
Which SHM conferences have you attended? Tell TH about your most memorable highlights or takeaways.
When I started out as a hospitalist in 2014, I decided to attend the annual conference in Las Vegas, and I can honestly say that conference changed the course of my career. I can still remember listening to the opening speech and realizing that I was surrounded by more than 3,000 hospitalists who understood the power we had to influence inpatient care. I’ve attended all the national conferences since then and am grateful that I now get to help organize the Hospital Medicine 2018 annual conference, also known as HM18.
I had been working to find a way to improve documentation within my group, as well as change the culture and perception towards billing and coding practices, which prompted me to attend the Quality and Safety Educators Academy. During one of the problem-solving sessions, I explained the challenges that I faced to my conference group. The exercise required me to explain the problem at hand, and the players of my group then discussed their thoughts while I took notes. It was a fantastic experience, as the participants at my table offered strong solutions to my problems within a matter of minutes. Their advice led to meaningful changes in our group’s hospital documentation practices, and in turn, I’ve been promoted to physician advisor in Southeast Alabama Medical Center.
After such a great experience at Quality and Safety Educators Academy, I went on to attend SHM’s Leadership Academy, where I had the opportunity to meet some of the top leaders and pioneers in the field of hospital medicine. It’s empowering to be mentored by the very people you look up to and aspire to be like. Not only was I able to bring ideas home to my institution, but I was able to reflect and improve my own professional and personal growth. I’m happy to say that I’ve completed all three levels of Leadership Academy.
As I’ve become involved with the medical student and residency programs at my medical center, I recently attended the Academic Hospitalist Academy to help my transition into academic hospital medicine. Meeting and spending time with the faculty at Academic Hospitalist Academy made me further realize the roles that academic hospitalists play in the education of future physicians, emphasizing the idea that we can all be champions in quality and patient safety.
If you’re looking to advance your career as a hospitalist, take advantage of the conferences that SHM offers. I’ve gained so much from each experience, and I’m looking forward to returning to these conferences as a potential facilitator, in hopes of offering what I’ve learned to hospitalists looking to bring about change in their fields and careers.
What can attendees at HM18 expect to see in the area of career development, and how is this different than previous years?
Hospital medicine is only about 2 decades old, making it one of the youngest branches in medicine today. Given this fact, the Annual Conference Committee feels that it is paramount to focus on career development for both new and midcareer hospitalists alike.
One question that we wish to explore and answer this year is: “How do you make hospital medicine a life-long, enjoyable, and engaging career?” In turn, our committee has created several new additions to HM18. This includes a “Seasoning Your Career” track, which will provide ideas on how to advance in leadership, use emotional intelligence to achieve success, change your roles midcareer, and change hospitalist schedules. Another unique addition this year are career development workshops, which will aim to developing various aspects of a hospitalist’s career, such as working on leadership skills, refining presentation and communication skills, providing constructive feedback, promoting women in hospital medicine, preventing burnout, and turning ideas into clinical research. We also plan to incorporate an education track, which will focus on how hospitalists can expand their careers towards educational leadership.
Given your involvement in SHM at both the local and national levels, do you have any advice for young hospital medicine professionals looking to build their professional profiles?
I’ve frequently noticed that young hospitalists don’t realize the potential influence they hold within their own institutions or the power they have to elicit change in health care at the national level.
Though we don’t often admit it, some hospitalists feel like they are glorified residents, which definitely is not the case. As a provider on the front lines, you have the unique opportunity to implement changes pertaining to issues of cost, utilization of resources, process management, quality and patient safety, and bottlenecks in care, to name a few. These are issues that keep the administrators of your organization and leaders of hospital medicine up at night. Don’t sit around and complain about how things could be or should be; look toward creating change. Bring up possible solutions to these problems with your leaders. They will appreciate the effort, and hopefully together you can find ways to tackle these problems.
I will conclude by saying this: Hospital medicine is such a unique specialty in that it’s constantly evolving, and the pioneers of this field are still alive and practicing medicine. You can meet and interact with them during the SHM conferences and look to them as sources of inspiration or guidance. Meeting people you look up to and having them as your mentors can take you places.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: Each month, the Society of Hospital Medicine puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Amith Skandhan, MD, FHM, a hospitalist, a director/physician liaison for clinical documentation improvement and core faculty member in the Internal Medicine Residency Program at Southeast Alabama Medical Center in Dothan, Ala., and clinical faculty member at the Alabama College of Osteopathic Medicine also in Dothan. Dr. Skandhan is the cofounder and current president of the SHM Wiregrass Chapter and is an active member of SHM’s Annual Conference and Performance Measurement Reporting committees.
When did you join SHM, and what prompted you to apply for your current committee roles?
There were many things that were not sufficiently taught during clinical training that were required in my day-to-day practice, like clinical documentation improvement, practice management, billing, coding, and so forth. I also quickly understood how vast and dynamic hospital medicine really was. While looking for an outlet to voice my questions, concerns, and curiosity, I decided to join SHM, which has helped me find and apply the techniques I’d been looking for to further my career as a hospitalist.
I’m now fortunate to be a part of SHM’s national committees, which involve hospitalists of various backgrounds and experiences, who work together to improve the overall quality of inpatient medicine. I currently serve on the Performance Reporting Measurement Committee and the Annual Conference Committee. My interests in reviewing the ever-evolving policies of health care made me apply to be a part of the Performance Reporting Measurement Committee. We work very closely with the Public Policy Committee, analyzing written policies and subsequently offering our recommendations. It’s been fulfilling to be a part of a committee that works towards developing policies that support a good quality of care on such a large scale.
My penchant for organizing events and bringing people together based on common ground led me to apply for the Annual Conference Committee. We meet every week to discuss various topics, choose and invite speakers, and help organize the entire event, which will host close to 5,000 hospitalists later this year. It has made me appreciate being a member of an organization that provides hospitalists with opportunities for education and growth. I’m hopeful that the attendees next year will find the conference to be a worthwhile experience!
As the president of SHM’s Wiregrass Chapter, how has the chapter grown since its establishment in May 2015?
Our chapter is based in Dothan, a small, rural Alabama town where Southeast Alabama Medical Center is located. The chapter covers the counties of lower Alabama and the panhandle of Florida. We named the chapter after a special species of grass that grows in this region.
When we started the chapter, our goal was to bring the best and brightest of hospital medicine to our region to give talks on hot topics in the field and also to use their expertise to guide inpatient care in our hospital system. We aggressively marketed the events to bring in large crowds of medical professionals, and we consistently average around 70-80 attendees in our meetings. Bringing in leaders from the field helped create an atmosphere of learning and inspired us to grow and develop our hospitalist program. We now closely work with hospital medicine groups in surrounding rural areas toward improving inpatient hospital care.
During these past years, we also realized that, for the further growth of our chapter, we would need to nurture an interest in hospital medicine among future generations of doctors, and this realization led to the creation of our medical student and resident wing. So far, the students have been very enthusiastic about participating in SHM-related events, and I hope that continues. We also developed a mentor-mentee program, in which we paired selected medical students with hospitalists to help guide future careers in acute care medicine. This year, we have also been helping the hospital medicine division at Southeast Alabama Medical Center create a clinical research track for medical students. To that end, we have just completed our second annual poster competition where we presented around 50 posters in the areas of clinical vignettes, quality improvement, and original research.
In addition, the chapter is very active with community activities. We took notice of the fact that many of our patients and community members were unaware of what hospitalists did because they could not understand how our work was different from that of primary care physicians. Our members have therefore participated in TV, radio, and newspaper interviews to help elucidate the role of hospitalists in patient care. We have also periodically visited primary care physician offices, nursing homes, senior citizen groups, and cancer support groups to educate these patients on various facets of health care and how hospitalists influence these areas.
In 2014, we organized a “walk with a hospitalist” event, for which we set up a half-mile “admission to discharge” scenario explaining the role of hospitalists and other departments involved in patient care. This year, in hopes of improving patient literacy in our region, we held a “shop with a doc” event, where the Southeast Alabama Medical Center hospitalists teamed up with dietitians and taught patients how food and lifestyle influenced their chronic medical illnesses. This was followed by physicians and dietitians shopping with patients in the grocery store, educating them on healthy choices and label reading.
We’re incredibly grateful for the support that we’ve received from our medical and patient communities; they’ve been critical in helping our chapter grow as much as it has, and they motivate us to work harder and do more with the chapter. We were honored to receive the SHM’s Rising Star Award at the Hospital Medicine 2017 conference in Las Vegas. We never thought that our little chapter in the American countryside would be chosen, but we’re very thankful to have our efforts recognized on the national stage!
Which SHM conferences have you attended? Tell TH about your most memorable highlights or takeaways.
When I started out as a hospitalist in 2014, I decided to attend the annual conference in Las Vegas, and I can honestly say that conference changed the course of my career. I can still remember listening to the opening speech and realizing that I was surrounded by more than 3,000 hospitalists who understood the power we had to influence inpatient care. I’ve attended all the national conferences since then and am grateful that I now get to help organize the Hospital Medicine 2018 annual conference, also known as HM18.
I had been working to find a way to improve documentation within my group, as well as change the culture and perception towards billing and coding practices, which prompted me to attend the Quality and Safety Educators Academy. During one of the problem-solving sessions, I explained the challenges that I faced to my conference group. The exercise required me to explain the problem at hand, and the players of my group then discussed their thoughts while I took notes. It was a fantastic experience, as the participants at my table offered strong solutions to my problems within a matter of minutes. Their advice led to meaningful changes in our group’s hospital documentation practices, and in turn, I’ve been promoted to physician advisor in Southeast Alabama Medical Center.
After such a great experience at Quality and Safety Educators Academy, I went on to attend SHM’s Leadership Academy, where I had the opportunity to meet some of the top leaders and pioneers in the field of hospital medicine. It’s empowering to be mentored by the very people you look up to and aspire to be like. Not only was I able to bring ideas home to my institution, but I was able to reflect and improve my own professional and personal growth. I’m happy to say that I’ve completed all three levels of Leadership Academy.
As I’ve become involved with the medical student and residency programs at my medical center, I recently attended the Academic Hospitalist Academy to help my transition into academic hospital medicine. Meeting and spending time with the faculty at Academic Hospitalist Academy made me further realize the roles that academic hospitalists play in the education of future physicians, emphasizing the idea that we can all be champions in quality and patient safety.
If you’re looking to advance your career as a hospitalist, take advantage of the conferences that SHM offers. I’ve gained so much from each experience, and I’m looking forward to returning to these conferences as a potential facilitator, in hopes of offering what I’ve learned to hospitalists looking to bring about change in their fields and careers.
What can attendees at HM18 expect to see in the area of career development, and how is this different than previous years?
Hospital medicine is only about 2 decades old, making it one of the youngest branches in medicine today. Given this fact, the Annual Conference Committee feels that it is paramount to focus on career development for both new and midcareer hospitalists alike.
One question that we wish to explore and answer this year is: “How do you make hospital medicine a life-long, enjoyable, and engaging career?” In turn, our committee has created several new additions to HM18. This includes a “Seasoning Your Career” track, which will provide ideas on how to advance in leadership, use emotional intelligence to achieve success, change your roles midcareer, and change hospitalist schedules. Another unique addition this year are career development workshops, which will aim to developing various aspects of a hospitalist’s career, such as working on leadership skills, refining presentation and communication skills, providing constructive feedback, promoting women in hospital medicine, preventing burnout, and turning ideas into clinical research. We also plan to incorporate an education track, which will focus on how hospitalists can expand their careers towards educational leadership.
Given your involvement in SHM at both the local and national levels, do you have any advice for young hospital medicine professionals looking to build their professional profiles?
I’ve frequently noticed that young hospitalists don’t realize the potential influence they hold within their own institutions or the power they have to elicit change in health care at the national level.
Though we don’t often admit it, some hospitalists feel like they are glorified residents, which definitely is not the case. As a provider on the front lines, you have the unique opportunity to implement changes pertaining to issues of cost, utilization of resources, process management, quality and patient safety, and bottlenecks in care, to name a few. These are issues that keep the administrators of your organization and leaders of hospital medicine up at night. Don’t sit around and complain about how things could be or should be; look toward creating change. Bring up possible solutions to these problems with your leaders. They will appreciate the effort, and hopefully together you can find ways to tackle these problems.
I will conclude by saying this: Hospital medicine is such a unique specialty in that it’s constantly evolving, and the pioneers of this field are still alive and practicing medicine. You can meet and interact with them during the SHM conferences and look to them as sources of inspiration or guidance. Meeting people you look up to and having them as your mentors can take you places.
Ms. Steele is the marketing communications specialist at the Society of Hospital Medicine.
Spray-dried plasma inches toward clinical trials
SAN DIEGO – Spray-dried plasma compared well with fresh frozen plasma in two in vitro studies, but clinical studies are needed to confirm the findings, researchers reported at the annual meeting of the American Association of Blood Banks.
The product’s logistical benefits include ease of transport, stability at room temperature, and the ability to be rapidly reconstituted – attributes that make it particularly useful in combat situations and prehospital settings where it is impractical to administer fresh frozen plasma (FFP).
The advantages of reconstituted blood products in combat settings have prompted recent efforts to speed their availability. The Food and Drug Administration and the Department of Defense recently announced a joint program to expedite the FDA’s review of products that could diagnose, treat, or prevent life-threatening conditions facing U.S. military personnel. It would be a fast-track process similar to how the FDA handles the breakthrough designation program.
In the first study, the investigators compared spray-dried plasma (SpDP) and FFP in reconstituted whole blood to test their hypothesis that SpDP is not inferior to FFP in facilitating platelet adhesion and thrombus formation, as evaluated by using a microfusion assay.
“Trauma is frequently associated with the use of plasma,” said Rachel S. Bercovitz, MD, MS, of the BloodCenter of Wisconsin and associate professor of pediatrics (hematology, oncology, and stem cell transplantation) at Northwestern University, Chicago.
Compared with FFP, SpDP can be reconstituted in 5 minutes and has more than 80% of the procoagulation and anticoagulation proteins, she explained. “Factor 8 levels were lower in the spray-dried plasma and were about at the 70% level of FFP. The other factor that was reduced, as compared to the FFP, was the von Willebrand factor (vWF), which was about 60% in SpDP compared to FFP.”
Whole blood was obtained from healthy volunteers and red blood cells (RBCs) were separated from platelet-rich plasma, and following standard procedures, resuspended in either SpDP or FFP and recombined with the packed red blood cells to create reconstituted whole blood with hematocrit of 34%-40% and 150,000-250,000 platelets per mcL.
After fluorescent labeling, the samples were flowed through a type I collagen-coated microchannel and still images of adherent platelets and thrombi were captured in order to calculate surface area coverage along the length of the channel. Next, the investigators used a ratio paired t-test to compare surface area coverage in SpDP versus FFP. The margin of noninferiority was 20% (SpDP/FFP greater than 0.8).
A total of six batches of SpDP and FFP were evaluated with 17 donors, and there was no statistical difference between the SpDP versus FFP pairs (P = .7558).
The mean ratio of SpDP versus FFP was 1.21 with a 95% confidence interval of 0.84-1.57. The surface area coverage in samples that were reconstituted with SpDP were, on average, 20% greater than in samples reconstituted with FFP. The lower limit of the 95% confidence interval was a difference of 16%, and therefore lower than the a priori determined margin of noninferiority of 20%.
“We found that SpDP is not inferior to FFP in supporting platelet adhesion and thrombus formation in our in vitro model,” Dr. Bercovitz said. “We feel that these in vitro assays support further in vivo studies of safety and efficacy of spray dried plasma.”
In a second study, Michael A. Meledeo, PhD, of the U.S. Army Institute of Surgical Research (coagulation and blood research), and his colleagues examined methods of reconstituting SpDP. They noted that a single unit process has been developed that produces a long-lived and readily stored SpDP product, which decreased high-molecular-weight multimers of vWF but increased low-molecular-weight multimers. vWF is critical in the process of platelet adhesion and thrombus formation, Dr. Meledeo said.
The researchers examined different reconstitution solutions: FFP, FFP with glycine, regular SpDP without pretreatment and rehydrated with glycine-hydrochloride:glycine, SpDP pretreated with glycine-HCl, or glycine-HCl:glycine and rehydrated with water.
Several in vitro analyses were performed, including measurement of vWF activity, fibrin polymerization kinetics, thrombin generation, coagulation properties and platelet adhesion to collagen.
Pretreated SpDP had better vWF activity, compared with regular SpDP (P less than .05). As compared with FFP, fibrin polymerization density was slightly lower in regular SpDP (0.879 vs. 0.742 optical density; P less than .01), although generation of thrombin was similar.
The researchers also found that the bicarbonate/base excess were lower in SpDP samples versus FFP (P less than .001). Thromboelastography results (used to measure coagulation properties) remained unchanged in plasma-only samples, but clot strength in reconstructed whole blood was reduced in all SpDP samples, compared with FFP (63.82 vs. 55-59.38; P less than .01).
Finally, platelet adhesion was equivalent in pretreated SpDP samples and FFP, while with regular SpDP, it was improved as compared with all other samples (71.53% surface coverage vs. 30.26%-43.87%; P less than .05).
“Based on these results, spray dried plasma was equivalent or superior to FFP in most of the in vitro hemostasis assays,” Dr. Meledeo said. “Reconstitution with glycine-HCl or glycine-HCl:glycine induced a superior von Willebrand function, but it was inferior in terms of supporting a flowing platelet adhesion to collagen.”
Dr. Bercovitz and Dr. Meledeo reported having no financial disclosures.
SOURCES: Bercovitz R et al. AABB 17 Abstract C20-A02B; Meledeo M et al. AABB 17 Abstract C21-A02B.
SAN DIEGO – Spray-dried plasma compared well with fresh frozen plasma in two in vitro studies, but clinical studies are needed to confirm the findings, researchers reported at the annual meeting of the American Association of Blood Banks.
The product’s logistical benefits include ease of transport, stability at room temperature, and the ability to be rapidly reconstituted – attributes that make it particularly useful in combat situations and prehospital settings where it is impractical to administer fresh frozen plasma (FFP).
The advantages of reconstituted blood products in combat settings have prompted recent efforts to speed their availability. The Food and Drug Administration and the Department of Defense recently announced a joint program to expedite the FDA’s review of products that could diagnose, treat, or prevent life-threatening conditions facing U.S. military personnel. It would be a fast-track process similar to how the FDA handles the breakthrough designation program.
In the first study, the investigators compared spray-dried plasma (SpDP) and FFP in reconstituted whole blood to test their hypothesis that SpDP is not inferior to FFP in facilitating platelet adhesion and thrombus formation, as evaluated by using a microfusion assay.
“Trauma is frequently associated with the use of plasma,” said Rachel S. Bercovitz, MD, MS, of the BloodCenter of Wisconsin and associate professor of pediatrics (hematology, oncology, and stem cell transplantation) at Northwestern University, Chicago.
Compared with FFP, SpDP can be reconstituted in 5 minutes and has more than 80% of the procoagulation and anticoagulation proteins, she explained. “Factor 8 levels were lower in the spray-dried plasma and were about at the 70% level of FFP. The other factor that was reduced, as compared to the FFP, was the von Willebrand factor (vWF), which was about 60% in SpDP compared to FFP.”
Whole blood was obtained from healthy volunteers and red blood cells (RBCs) were separated from platelet-rich plasma, and following standard procedures, resuspended in either SpDP or FFP and recombined with the packed red blood cells to create reconstituted whole blood with hematocrit of 34%-40% and 150,000-250,000 platelets per mcL.
After fluorescent labeling, the samples were flowed through a type I collagen-coated microchannel and still images of adherent platelets and thrombi were captured in order to calculate surface area coverage along the length of the channel. Next, the investigators used a ratio paired t-test to compare surface area coverage in SpDP versus FFP. The margin of noninferiority was 20% (SpDP/FFP greater than 0.8).
A total of six batches of SpDP and FFP were evaluated with 17 donors, and there was no statistical difference between the SpDP versus FFP pairs (P = .7558).
The mean ratio of SpDP versus FFP was 1.21 with a 95% confidence interval of 0.84-1.57. The surface area coverage in samples that were reconstituted with SpDP were, on average, 20% greater than in samples reconstituted with FFP. The lower limit of the 95% confidence interval was a difference of 16%, and therefore lower than the a priori determined margin of noninferiority of 20%.
“We found that SpDP is not inferior to FFP in supporting platelet adhesion and thrombus formation in our in vitro model,” Dr. Bercovitz said. “We feel that these in vitro assays support further in vivo studies of safety and efficacy of spray dried plasma.”
In a second study, Michael A. Meledeo, PhD, of the U.S. Army Institute of Surgical Research (coagulation and blood research), and his colleagues examined methods of reconstituting SpDP. They noted that a single unit process has been developed that produces a long-lived and readily stored SpDP product, which decreased high-molecular-weight multimers of vWF but increased low-molecular-weight multimers. vWF is critical in the process of platelet adhesion and thrombus formation, Dr. Meledeo said.
The researchers examined different reconstitution solutions: FFP, FFP with glycine, regular SpDP without pretreatment and rehydrated with glycine-hydrochloride:glycine, SpDP pretreated with glycine-HCl, or glycine-HCl:glycine and rehydrated with water.
Several in vitro analyses were performed, including measurement of vWF activity, fibrin polymerization kinetics, thrombin generation, coagulation properties and platelet adhesion to collagen.
Pretreated SpDP had better vWF activity, compared with regular SpDP (P less than .05). As compared with FFP, fibrin polymerization density was slightly lower in regular SpDP (0.879 vs. 0.742 optical density; P less than .01), although generation of thrombin was similar.
The researchers also found that the bicarbonate/base excess were lower in SpDP samples versus FFP (P less than .001). Thromboelastography results (used to measure coagulation properties) remained unchanged in plasma-only samples, but clot strength in reconstructed whole blood was reduced in all SpDP samples, compared with FFP (63.82 vs. 55-59.38; P less than .01).
Finally, platelet adhesion was equivalent in pretreated SpDP samples and FFP, while with regular SpDP, it was improved as compared with all other samples (71.53% surface coverage vs. 30.26%-43.87%; P less than .05).
“Based on these results, spray dried plasma was equivalent or superior to FFP in most of the in vitro hemostasis assays,” Dr. Meledeo said. “Reconstitution with glycine-HCl or glycine-HCl:glycine induced a superior von Willebrand function, but it was inferior in terms of supporting a flowing platelet adhesion to collagen.”
Dr. Bercovitz and Dr. Meledeo reported having no financial disclosures.
SOURCES: Bercovitz R et al. AABB 17 Abstract C20-A02B; Meledeo M et al. AABB 17 Abstract C21-A02B.
SAN DIEGO – Spray-dried plasma compared well with fresh frozen plasma in two in vitro studies, but clinical studies are needed to confirm the findings, researchers reported at the annual meeting of the American Association of Blood Banks.
The product’s logistical benefits include ease of transport, stability at room temperature, and the ability to be rapidly reconstituted – attributes that make it particularly useful in combat situations and prehospital settings where it is impractical to administer fresh frozen plasma (FFP).
The advantages of reconstituted blood products in combat settings have prompted recent efforts to speed their availability. The Food and Drug Administration and the Department of Defense recently announced a joint program to expedite the FDA’s review of products that could diagnose, treat, or prevent life-threatening conditions facing U.S. military personnel. It would be a fast-track process similar to how the FDA handles the breakthrough designation program.
In the first study, the investigators compared spray-dried plasma (SpDP) and FFP in reconstituted whole blood to test their hypothesis that SpDP is not inferior to FFP in facilitating platelet adhesion and thrombus formation, as evaluated by using a microfusion assay.
“Trauma is frequently associated with the use of plasma,” said Rachel S. Bercovitz, MD, MS, of the BloodCenter of Wisconsin and associate professor of pediatrics (hematology, oncology, and stem cell transplantation) at Northwestern University, Chicago.
Compared with FFP, SpDP can be reconstituted in 5 minutes and has more than 80% of the procoagulation and anticoagulation proteins, she explained. “Factor 8 levels were lower in the spray-dried plasma and were about at the 70% level of FFP. The other factor that was reduced, as compared to the FFP, was the von Willebrand factor (vWF), which was about 60% in SpDP compared to FFP.”
Whole blood was obtained from healthy volunteers and red blood cells (RBCs) were separated from platelet-rich plasma, and following standard procedures, resuspended in either SpDP or FFP and recombined with the packed red blood cells to create reconstituted whole blood with hematocrit of 34%-40% and 150,000-250,000 platelets per mcL.
After fluorescent labeling, the samples were flowed through a type I collagen-coated microchannel and still images of adherent platelets and thrombi were captured in order to calculate surface area coverage along the length of the channel. Next, the investigators used a ratio paired t-test to compare surface area coverage in SpDP versus FFP. The margin of noninferiority was 20% (SpDP/FFP greater than 0.8).
A total of six batches of SpDP and FFP were evaluated with 17 donors, and there was no statistical difference between the SpDP versus FFP pairs (P = .7558).
The mean ratio of SpDP versus FFP was 1.21 with a 95% confidence interval of 0.84-1.57. The surface area coverage in samples that were reconstituted with SpDP were, on average, 20% greater than in samples reconstituted with FFP. The lower limit of the 95% confidence interval was a difference of 16%, and therefore lower than the a priori determined margin of noninferiority of 20%.
“We found that SpDP is not inferior to FFP in supporting platelet adhesion and thrombus formation in our in vitro model,” Dr. Bercovitz said. “We feel that these in vitro assays support further in vivo studies of safety and efficacy of spray dried plasma.”
In a second study, Michael A. Meledeo, PhD, of the U.S. Army Institute of Surgical Research (coagulation and blood research), and his colleagues examined methods of reconstituting SpDP. They noted that a single unit process has been developed that produces a long-lived and readily stored SpDP product, which decreased high-molecular-weight multimers of vWF but increased low-molecular-weight multimers. vWF is critical in the process of platelet adhesion and thrombus formation, Dr. Meledeo said.
The researchers examined different reconstitution solutions: FFP, FFP with glycine, regular SpDP without pretreatment and rehydrated with glycine-hydrochloride:glycine, SpDP pretreated with glycine-HCl, or glycine-HCl:glycine and rehydrated with water.
Several in vitro analyses were performed, including measurement of vWF activity, fibrin polymerization kinetics, thrombin generation, coagulation properties and platelet adhesion to collagen.
Pretreated SpDP had better vWF activity, compared with regular SpDP (P less than .05). As compared with FFP, fibrin polymerization density was slightly lower in regular SpDP (0.879 vs. 0.742 optical density; P less than .01), although generation of thrombin was similar.
The researchers also found that the bicarbonate/base excess were lower in SpDP samples versus FFP (P less than .001). Thromboelastography results (used to measure coagulation properties) remained unchanged in plasma-only samples, but clot strength in reconstructed whole blood was reduced in all SpDP samples, compared with FFP (63.82 vs. 55-59.38; P less than .01).
Finally, platelet adhesion was equivalent in pretreated SpDP samples and FFP, while with regular SpDP, it was improved as compared with all other samples (71.53% surface coverage vs. 30.26%-43.87%; P less than .05).
“Based on these results, spray dried plasma was equivalent or superior to FFP in most of the in vitro hemostasis assays,” Dr. Meledeo said. “Reconstitution with glycine-HCl or glycine-HCl:glycine induced a superior von Willebrand function, but it was inferior in terms of supporting a flowing platelet adhesion to collagen.”
Dr. Bercovitz and Dr. Meledeo reported having no financial disclosures.
SOURCES: Bercovitz R et al. AABB 17 Abstract C20-A02B; Meledeo M et al. AABB 17 Abstract C21-A02B.
REPORTING FROM AABB 17
Key clinical point:
Major finding: Spray-dried plasma was equal to, or superior to, fresh frozen plasma in many of the in vitro assays utilized, especially when pretreated in glycine solutions.
Study details: Two in vitro assays that compared spray-dried plasma with fresh frozen plasma.
Disclosures: Dr. Bercovitz and Dr. Meledeo reported having no financial disclosures.
Sources: Bercovitz R et al. AABB 17 Abstract C20-A02B; Meledeo M et al. AABB 17 Abstract C21-A02B.
Clinical Challenges - February 2018 What's your diagnosis?
The diagnosis: Metastatic insulinoma surrounded by steatotic hepatocytes
The loss of L-FABP expression in steatotic hepatocytes is the hallmark of HNF1alpha-inactivated liver adenoma,1 and clearly suggested this diagnosis. However, the emergence of multiple steatotic lesions over a short period of time was uncommon for liver adenomas. Despite the absence of radiologically detectable metastasis, this diagnosis could not be ruled out, and the patient underwent a surgical liver biopsy (tip of the right lobe). The specimen showed a 0.2 cm greyish nodule surrounded by a steatotic map-like area of 3.5 cm in the largest dimension (Figure E). Histopathologic examination showed neuroendocrine cells (Figures F [hematoxylin and eosin staining] and G [insulin immunostaining]), confirming the diagnosis of metastatic insulinoma surrounded by steatotic hepatocytes.
The key interest of the case is the reduction of L-FABP expression in the steatotic hepatocytes (Figure H [L-FABP immunostaining]), which was an unexpected finding and could have led to an incorrect diagnosis of HNF1α-inactivated liver adenoma.
In contrast with other functional neuroendocrine tumors, insulinomas are frequently benign tumors, and only about 10% of patients develop metastasis. In the liver, they are often surrounded by microscopic or radiologically detectable steatotic areas thanks to the paracrine effect of insulin. Such a feature has been previously described both with liver insulinoma metastases2 and after pancreatic islet transplantation.3 The reduction of L-FABP expression within the steatotic hepatocytes seems to be less frequent because it was not observed in an additional patient with G3 insulinoma (neuroendocrine carcinoma) metastases and in 3 pancreatic islet recipients (data not shown).
The present patient with multiple liver G2 insulinoma metastases illustrates 1) the potential of foci of steatosis to represent early signs of insulinoma liver metastasis, and 2) the presence of a reduction or even a loss of L-FABP expression in other liver lesions than HNF1alpha-inactivated liver adenoma.
Acknowledgment
Claudio De Vito’s current affiliation is Institute of Liver Studies, King’s College Hospital, London, UK.
The authors thank A.M.J. Shapiro from the University of Alberta, Edmonton, Canada and A. Quaglia from the King’s College Hospital, London, UK for sharing the liver samples of transplanted pancreatic islets and G3 insulinoma metastasis. They are also grateful to the members of the Geneva Hepato-Biliary and Pancreatic Center for the discussion of the case.
References
1. Bioulac-Sage P., Cubel G., Taouji S., et al. Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am J Surg Pathol. 2012;36:1691-9.
2. Sohn J., Siegelman E., Osiason, A. Unusual patterns of hepatic steatosis caused by the local effect of insulin revealed on chemical shift MR imaging. AJR Am J Roentgenol. 2001;176:471-4.
3. Toso C., Isse K., Demetris A.J., et al. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88:1286-93.
The diagnosis: Metastatic insulinoma surrounded by steatotic hepatocytes
The loss of L-FABP expression in steatotic hepatocytes is the hallmark of HNF1alpha-inactivated liver adenoma,1 and clearly suggested this diagnosis. However, the emergence of multiple steatotic lesions over a short period of time was uncommon for liver adenomas. Despite the absence of radiologically detectable metastasis, this diagnosis could not be ruled out, and the patient underwent a surgical liver biopsy (tip of the right lobe). The specimen showed a 0.2 cm greyish nodule surrounded by a steatotic map-like area of 3.5 cm in the largest dimension (Figure E). Histopathologic examination showed neuroendocrine cells (Figures F [hematoxylin and eosin staining] and G [insulin immunostaining]), confirming the diagnosis of metastatic insulinoma surrounded by steatotic hepatocytes.
The key interest of the case is the reduction of L-FABP expression in the steatotic hepatocytes (Figure H [L-FABP immunostaining]), which was an unexpected finding and could have led to an incorrect diagnosis of HNF1α-inactivated liver adenoma.
In contrast with other functional neuroendocrine tumors, insulinomas are frequently benign tumors, and only about 10% of patients develop metastasis. In the liver, they are often surrounded by microscopic or radiologically detectable steatotic areas thanks to the paracrine effect of insulin. Such a feature has been previously described both with liver insulinoma metastases2 and after pancreatic islet transplantation.3 The reduction of L-FABP expression within the steatotic hepatocytes seems to be less frequent because it was not observed in an additional patient with G3 insulinoma (neuroendocrine carcinoma) metastases and in 3 pancreatic islet recipients (data not shown).
The present patient with multiple liver G2 insulinoma metastases illustrates 1) the potential of foci of steatosis to represent early signs of insulinoma liver metastasis, and 2) the presence of a reduction or even a loss of L-FABP expression in other liver lesions than HNF1alpha-inactivated liver adenoma.
Acknowledgment
Claudio De Vito’s current affiliation is Institute of Liver Studies, King’s College Hospital, London, UK.
The authors thank A.M.J. Shapiro from the University of Alberta, Edmonton, Canada and A. Quaglia from the King’s College Hospital, London, UK for sharing the liver samples of transplanted pancreatic islets and G3 insulinoma metastasis. They are also grateful to the members of the Geneva Hepato-Biliary and Pancreatic Center for the discussion of the case.
References
1. Bioulac-Sage P., Cubel G., Taouji S., et al. Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am J Surg Pathol. 2012;36:1691-9.
2. Sohn J., Siegelman E., Osiason, A. Unusual patterns of hepatic steatosis caused by the local effect of insulin revealed on chemical shift MR imaging. AJR Am J Roentgenol. 2001;176:471-4.
3. Toso C., Isse K., Demetris A.J., et al. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88:1286-93.
The diagnosis: Metastatic insulinoma surrounded by steatotic hepatocytes
The loss of L-FABP expression in steatotic hepatocytes is the hallmark of HNF1alpha-inactivated liver adenoma,1 and clearly suggested this diagnosis. However, the emergence of multiple steatotic lesions over a short period of time was uncommon for liver adenomas. Despite the absence of radiologically detectable metastasis, this diagnosis could not be ruled out, and the patient underwent a surgical liver biopsy (tip of the right lobe). The specimen showed a 0.2 cm greyish nodule surrounded by a steatotic map-like area of 3.5 cm in the largest dimension (Figure E). Histopathologic examination showed neuroendocrine cells (Figures F [hematoxylin and eosin staining] and G [insulin immunostaining]), confirming the diagnosis of metastatic insulinoma surrounded by steatotic hepatocytes.
The key interest of the case is the reduction of L-FABP expression in the steatotic hepatocytes (Figure H [L-FABP immunostaining]), which was an unexpected finding and could have led to an incorrect diagnosis of HNF1α-inactivated liver adenoma.
In contrast with other functional neuroendocrine tumors, insulinomas are frequently benign tumors, and only about 10% of patients develop metastasis. In the liver, they are often surrounded by microscopic or radiologically detectable steatotic areas thanks to the paracrine effect of insulin. Such a feature has been previously described both with liver insulinoma metastases2 and after pancreatic islet transplantation.3 The reduction of L-FABP expression within the steatotic hepatocytes seems to be less frequent because it was not observed in an additional patient with G3 insulinoma (neuroendocrine carcinoma) metastases and in 3 pancreatic islet recipients (data not shown).
The present patient with multiple liver G2 insulinoma metastases illustrates 1) the potential of foci of steatosis to represent early signs of insulinoma liver metastasis, and 2) the presence of a reduction or even a loss of L-FABP expression in other liver lesions than HNF1alpha-inactivated liver adenoma.
Acknowledgment
Claudio De Vito’s current affiliation is Institute of Liver Studies, King’s College Hospital, London, UK.
The authors thank A.M.J. Shapiro from the University of Alberta, Edmonton, Canada and A. Quaglia from the King’s College Hospital, London, UK for sharing the liver samples of transplanted pancreatic islets and G3 insulinoma metastasis. They are also grateful to the members of the Geneva Hepato-Biliary and Pancreatic Center for the discussion of the case.
References
1. Bioulac-Sage P., Cubel G., Taouji S., et al. Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am J Surg Pathol. 2012;36:1691-9.
2. Sohn J., Siegelman E., Osiason, A. Unusual patterns of hepatic steatosis caused by the local effect of insulin revealed on chemical shift MR imaging. AJR Am J Roentgenol. 2001;176:471-4.
3. Toso C., Isse K., Demetris A.J., et al. Histologic graft assessment after clinical islet transplantation. Transplantation. 2009;88:1286-93.
By Claudio De Vito, MD, PhD, Laura Rubbia-Brandt, MD, PhD, and Christian Toso, MD, PhD. Published previously in Gastroenterology (2016;151[1]32, 330).
A 22-year-old woman with no past medical history was investigated for hypoglycemia episodes. A nodule located in the head of the pancreas was identified, with radiologic features of a neuroendocrine neoplasm. The overall clinical presentation was consistent with an insulinoma. No distant lesion was detected. She underwent a Whipple procedure, and the histopathologic examination reported a 2.2-cm, well-differentiated neuroendocrine tumor (insulinoma) G2 (4% Ki-67 index), with no lymphovascular invasion or lymph node metastasis (0 of 30 lymph nodes).