User login
Entrepreneurs attempt to convince the Shark Tank experts that they can address unmet needs
BOSTON – At the 2018 AGA Tech Summit, this year’s Shark Tank line up included an automated system for video capture of endoscopy, a feeding tube that prevents aspiration in intubated patients, a tool to accurately measure polyps captured on colonoscopy, a device that targets gastrointestinal cancers with electrical pulses, and a new method for real-time stool testing of infectious pathogens.

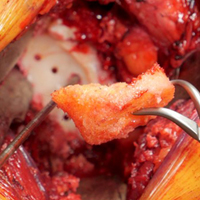
In each case, the presenting entrepreneur vowed to the Shark Tank panel of experts that their innovation is addressing an important clinical need. In announcing the winner, V. Raman Muthusamy, MD, who is the chair of the AGA Center for GI Innovation and Technology (CGIT) and director of interventional endoscopy and general GI endoscopy, David Geffen School of Medicine at UCLA, Los Angeles, said, “We have rarely had such a strong group of candidates.”
New for 2018, both the Sharks and AGA Tech Summit attendees voted on a winner. The unanimous winner was Chang-Hee Kim, PhD, who presented a new rapid test for identifying infectious pathogens in stool. The sharks were looking for 1. Novelty/immediate patient impact, 2. Business plan, and 3. Pitch. While all of the participants were close, the immediate patient impact for Dr. Kim’s innovation gave him a leg up on his competitors.
New device permits real-time stool sample analysis
A new tool for rapid analysis of stool for pathogens may be revolutionary in that it can provide results within 15 minutes rather than the days normally required when stool samples are sent to a laboratory, according to Dr. Chang-Hee Kim, chief executive officer of GoDx, Inc. The patent-pending methodology developed at Dr. Kim’s company permits real-time analysis “at the point of need and without the need for a lab.” In addition to the efficiency, the paper-based test, which Dr. Kim compared to a pregnancy test in that there is a color change with positive results, has the potential to improve outcomes.
Real-time testing “will decrease the loss of patients to follow-up and accelerate the time to treatment,” Dr. Kim asserted. “The test is also likely to reduce the spread of nosocomial pathogens if rapid infection control reduces spread.”
The test, which Dr. Kim expected to be made available at a cost of $100, will also be substantially cheaper than current laboratory analyses. In experimental studies, the accuracy has been at least as accurate as polymerase-chain reaction (PCR) testing, according to Dr. Kim. He sees applications not only in hospitals but at sites where ordering laboratory studies are not normally available, such as on cruise ships, in nursing homes, or in the military.
In the Shark Tank session, the main concerns expressed were about how this test will fit with Clinical Laboratory Improvement Amendments (CLIA) compliance. Herb Lerner, MD, a former officer in the Food and Drug Administration (FDA) and now senior director of medical and regulatory affairs at Hogan Lovells, a Washington, D.C.–based law firm, urged Dr. Kim to approach the FDA as soon as possible for advice on how to address this and other potential obstacles to a commercial product.
GI endoscopy capture, storage, and sharing
In introducing an automated method of capturing videos taken during colonoscopy, Matthew Z. Schwartz, the cofounder of Virgo Surgical Video Solutions, Inc. said, “There is currently no low-cost, user-friendly way to systematically capture GI endoscopy video.” Relative to existing products that “require complicated formatting and time-consuming setup for each procedure recording,” the plug-and-play system developed at Virgo “works with any existing endoscopy system that has a video output.” The videos are designed for cloud storage.
“Our long-term vision is to create the largest and highest quality repository of GI endoscopy videos,” said Mr. Schwartz, who added that support tools, including clinical decision-making aided by artificial intelligence, are being developed to provide even more value for quality control, research, and training.
Several Sharks, including Tom Shehab, MD, managing director, Arboretum Ventures, Ann Arbor, Mich., asked for more information about the value proposition for a proposed cost of $500 per month for the system. The answer was that this value would differ for settings, such as in an academic center that might apply the system for training relative to a community setting where the main purpose might be quality improvement.
New NG tube addresses aspiration risk
Pulmonary aspiration, along with symptomatic gastric reflux, is a common clinical challenge in intubated patients on a feeding tube, but a novel nasogastric (NG) tube equipped with a dual balloon system is designed to solve this problem, according to Talal Sharaiha, MD, the founder of Aspisafe Solutions. He said that reducing the risk of aspiration is important because it is associated with pneumonia, erosive inflammation, and upper GI bleeding. He called it the most common cause of upper GI bleeding in intensive care units.
Of the two balloons, one serves as an anchor and sits in the esophagus. The other serves as an anti-aspiration reservoir and sits in the stomach. The anti-aspiration balloon, inflated after it is inserted in the stomach, blocks reflux of gastric contents. Contending that there is a large market for this device, Dr. Sharaiha said, “having a feeding tube in intubated patients that prevents gastric reflux and aspiration will dramatically reduce complications and likely help reduce length of ventilation time, ICU time, and length of hospitals stay.”
More than one Shark requested more information about safety. Michael L. Kochman, MD, the Wilmott Professor of Medicine and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, Philadelphia, noted that there have been adverse experiences in the past with other types of balloon devices introduced into the GI tract. He indicated that this may be a potential bar to clinician comfort, so a comprehensive approach to safety analyses should be a priority in clinical development.
Virtual tape measure for colonoscopy measures polyp size
There are risks from the current practice of estimating polyp size, according to Avishay Sidlesky, founder of VTM Technologies, Ltd. He called these estimates “inherently inaccurate” and asserted that they “lead to erroneous diagnoses, suboptimal treatment and follow-up.” He believes that a “virtual tape measure” dependent on an endoscopic laser emitter solves this problem and cited a published paper that confirmed the accuracy of polyp measurements conducted with this device in an animal model.
“Employing dedicated software that analyzes the laser curves in the endoscopic image, the system enables reporting of the size of lesions, diameter and profile of polyps, longitudinal cross-section of lumens and more,” Mr. Sidlesky reported, specifying that the tool can be used alone or integrated into third party endoscopes. He believes applications can eventually be developed for a variety of endoscopic procedures in addition to colonoscopy.
Of Shark comments, several regarded concern about how this measuring tool would be integrated with existing endoscopic systems. Even though the measuring tool can be introduced in an unused channel of available scopes, the image is displayed separately. Michael J. Docktor, MD, clinical director of innovation, Boston Children’s Hospital, cautioned that devices that add steps to the workflow in the endoscopy suite may not be as rapidly accepted as one in which the tool is totally integrated with existing systems.
New device treats esophageal cancers with electroporation
There are a variety of tools to treat upper GI cancers endoscopically, including with radiofrequency ablation, cryotechnology, and photodynamic therapy, but morbidity rates are high, according to Declan Soden, founder of Mirai Medical. He described the attributes of a new technology based on electroporation in which electrical pulses painlessly target neoplastic tissue while preserving adjacent healthy tissue. He said that the treatment is performed in a matter of minutes on an outpatient basis.
“Clinical results to date have demonstrated the benefit of the technology in the treatment of late-stage esophageal and colorectal disease,” Mr. Soden said. He believes that the “unique selling point” of this electroporation treatment, which is part of a complete treatment regimen, “is that it renders tumor tissue leaky or porous, allowing absorption and uptake of drugs.” Relative to current standards, the treatment has multiple advantages, not least of which is preservation of quality of life, he believes.
The Sharks posed many technical questions about this strategy that might narrow the applicability of this device. For example, Trey Reed, MD, medical director, Humana Inc., posed questions about the depth of penetration of the electoral pulses and where they can be adjusted for tumors of different sizes. He also questioned whether the device will be too large to penetrate lumens obstructed by tumor. Although Mr. Soden believes that the device will be versatile, he acknowledged that its role might be better understood when phase 2 trials begin later this year.
A moderator at the Shank Tank session and a previous CGIT Chair, Dr. Kochman reported that he was impressed with the crop of entries. As one of the creators of the AGA Tech Summit, Dr. Kochman has the experience to recognize good ideas when he hears them.
“It is gratifying to see the high quality of the Shark Tank presenters. Over the past years, a number of the presenting companies have gone on to obtain additional funding, be acquired, and on to successful launches,” Dr. Kochman observed. “We hope the same successes await this year’s group.”
BOSTON – At the 2018 AGA Tech Summit, this year’s Shark Tank line up included an automated system for video capture of endoscopy, a feeding tube that prevents aspiration in intubated patients, a tool to accurately measure polyps captured on colonoscopy, a device that targets gastrointestinal cancers with electrical pulses, and a new method for real-time stool testing of infectious pathogens.

In each case, the presenting entrepreneur vowed to the Shark Tank panel of experts that their innovation is addressing an important clinical need. In announcing the winner, V. Raman Muthusamy, MD, who is the chair of the AGA Center for GI Innovation and Technology (CGIT) and director of interventional endoscopy and general GI endoscopy, David Geffen School of Medicine at UCLA, Los Angeles, said, “We have rarely had such a strong group of candidates.”
New for 2018, both the Sharks and AGA Tech Summit attendees voted on a winner. The unanimous winner was Chang-Hee Kim, PhD, who presented a new rapid test for identifying infectious pathogens in stool. The sharks were looking for 1. Novelty/immediate patient impact, 2. Business plan, and 3. Pitch. While all of the participants were close, the immediate patient impact for Dr. Kim’s innovation gave him a leg up on his competitors.
New device permits real-time stool sample analysis
A new tool for rapid analysis of stool for pathogens may be revolutionary in that it can provide results within 15 minutes rather than the days normally required when stool samples are sent to a laboratory, according to Dr. Chang-Hee Kim, chief executive officer of GoDx, Inc. The patent-pending methodology developed at Dr. Kim’s company permits real-time analysis “at the point of need and without the need for a lab.” In addition to the efficiency, the paper-based test, which Dr. Kim compared to a pregnancy test in that there is a color change with positive results, has the potential to improve outcomes.
Real-time testing “will decrease the loss of patients to follow-up and accelerate the time to treatment,” Dr. Kim asserted. “The test is also likely to reduce the spread of nosocomial pathogens if rapid infection control reduces spread.”
The test, which Dr. Kim expected to be made available at a cost of $100, will also be substantially cheaper than current laboratory analyses. In experimental studies, the accuracy has been at least as accurate as polymerase-chain reaction (PCR) testing, according to Dr. Kim. He sees applications not only in hospitals but at sites where ordering laboratory studies are not normally available, such as on cruise ships, in nursing homes, or in the military.
In the Shark Tank session, the main concerns expressed were about how this test will fit with Clinical Laboratory Improvement Amendments (CLIA) compliance. Herb Lerner, MD, a former officer in the Food and Drug Administration (FDA) and now senior director of medical and regulatory affairs at Hogan Lovells, a Washington, D.C.–based law firm, urged Dr. Kim to approach the FDA as soon as possible for advice on how to address this and other potential obstacles to a commercial product.
GI endoscopy capture, storage, and sharing
In introducing an automated method of capturing videos taken during colonoscopy, Matthew Z. Schwartz, the cofounder of Virgo Surgical Video Solutions, Inc. said, “There is currently no low-cost, user-friendly way to systematically capture GI endoscopy video.” Relative to existing products that “require complicated formatting and time-consuming setup for each procedure recording,” the plug-and-play system developed at Virgo “works with any existing endoscopy system that has a video output.” The videos are designed for cloud storage.
“Our long-term vision is to create the largest and highest quality repository of GI endoscopy videos,” said Mr. Schwartz, who added that support tools, including clinical decision-making aided by artificial intelligence, are being developed to provide even more value for quality control, research, and training.
Several Sharks, including Tom Shehab, MD, managing director, Arboretum Ventures, Ann Arbor, Mich., asked for more information about the value proposition for a proposed cost of $500 per month for the system. The answer was that this value would differ for settings, such as in an academic center that might apply the system for training relative to a community setting where the main purpose might be quality improvement.
New NG tube addresses aspiration risk
Pulmonary aspiration, along with symptomatic gastric reflux, is a common clinical challenge in intubated patients on a feeding tube, but a novel nasogastric (NG) tube equipped with a dual balloon system is designed to solve this problem, according to Talal Sharaiha, MD, the founder of Aspisafe Solutions. He said that reducing the risk of aspiration is important because it is associated with pneumonia, erosive inflammation, and upper GI bleeding. He called it the most common cause of upper GI bleeding in intensive care units.
Of the two balloons, one serves as an anchor and sits in the esophagus. The other serves as an anti-aspiration reservoir and sits in the stomach. The anti-aspiration balloon, inflated after it is inserted in the stomach, blocks reflux of gastric contents. Contending that there is a large market for this device, Dr. Sharaiha said, “having a feeding tube in intubated patients that prevents gastric reflux and aspiration will dramatically reduce complications and likely help reduce length of ventilation time, ICU time, and length of hospitals stay.”
More than one Shark requested more information about safety. Michael L. Kochman, MD, the Wilmott Professor of Medicine and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, Philadelphia, noted that there have been adverse experiences in the past with other types of balloon devices introduced into the GI tract. He indicated that this may be a potential bar to clinician comfort, so a comprehensive approach to safety analyses should be a priority in clinical development.
Virtual tape measure for colonoscopy measures polyp size
There are risks from the current practice of estimating polyp size, according to Avishay Sidlesky, founder of VTM Technologies, Ltd. He called these estimates “inherently inaccurate” and asserted that they “lead to erroneous diagnoses, suboptimal treatment and follow-up.” He believes that a “virtual tape measure” dependent on an endoscopic laser emitter solves this problem and cited a published paper that confirmed the accuracy of polyp measurements conducted with this device in an animal model.
“Employing dedicated software that analyzes the laser curves in the endoscopic image, the system enables reporting of the size of lesions, diameter and profile of polyps, longitudinal cross-section of lumens and more,” Mr. Sidlesky reported, specifying that the tool can be used alone or integrated into third party endoscopes. He believes applications can eventually be developed for a variety of endoscopic procedures in addition to colonoscopy.
Of Shark comments, several regarded concern about how this measuring tool would be integrated with existing endoscopic systems. Even though the measuring tool can be introduced in an unused channel of available scopes, the image is displayed separately. Michael J. Docktor, MD, clinical director of innovation, Boston Children’s Hospital, cautioned that devices that add steps to the workflow in the endoscopy suite may not be as rapidly accepted as one in which the tool is totally integrated with existing systems.
New device treats esophageal cancers with electroporation
There are a variety of tools to treat upper GI cancers endoscopically, including with radiofrequency ablation, cryotechnology, and photodynamic therapy, but morbidity rates are high, according to Declan Soden, founder of Mirai Medical. He described the attributes of a new technology based on electroporation in which electrical pulses painlessly target neoplastic tissue while preserving adjacent healthy tissue. He said that the treatment is performed in a matter of minutes on an outpatient basis.
“Clinical results to date have demonstrated the benefit of the technology in the treatment of late-stage esophageal and colorectal disease,” Mr. Soden said. He believes that the “unique selling point” of this electroporation treatment, which is part of a complete treatment regimen, “is that it renders tumor tissue leaky or porous, allowing absorption and uptake of drugs.” Relative to current standards, the treatment has multiple advantages, not least of which is preservation of quality of life, he believes.
The Sharks posed many technical questions about this strategy that might narrow the applicability of this device. For example, Trey Reed, MD, medical director, Humana Inc., posed questions about the depth of penetration of the electoral pulses and where they can be adjusted for tumors of different sizes. He also questioned whether the device will be too large to penetrate lumens obstructed by tumor. Although Mr. Soden believes that the device will be versatile, he acknowledged that its role might be better understood when phase 2 trials begin later this year.
A moderator at the Shank Tank session and a previous CGIT Chair, Dr. Kochman reported that he was impressed with the crop of entries. As one of the creators of the AGA Tech Summit, Dr. Kochman has the experience to recognize good ideas when he hears them.
“It is gratifying to see the high quality of the Shark Tank presenters. Over the past years, a number of the presenting companies have gone on to obtain additional funding, be acquired, and on to successful launches,” Dr. Kochman observed. “We hope the same successes await this year’s group.”
BOSTON – At the 2018 AGA Tech Summit, this year’s Shark Tank line up included an automated system for video capture of endoscopy, a feeding tube that prevents aspiration in intubated patients, a tool to accurately measure polyps captured on colonoscopy, a device that targets gastrointestinal cancers with electrical pulses, and a new method for real-time stool testing of infectious pathogens.

In each case, the presenting entrepreneur vowed to the Shark Tank panel of experts that their innovation is addressing an important clinical need. In announcing the winner, V. Raman Muthusamy, MD, who is the chair of the AGA Center for GI Innovation and Technology (CGIT) and director of interventional endoscopy and general GI endoscopy, David Geffen School of Medicine at UCLA, Los Angeles, said, “We have rarely had such a strong group of candidates.”
New for 2018, both the Sharks and AGA Tech Summit attendees voted on a winner. The unanimous winner was Chang-Hee Kim, PhD, who presented a new rapid test for identifying infectious pathogens in stool. The sharks were looking for 1. Novelty/immediate patient impact, 2. Business plan, and 3. Pitch. While all of the participants were close, the immediate patient impact for Dr. Kim’s innovation gave him a leg up on his competitors.
New device permits real-time stool sample analysis
A new tool for rapid analysis of stool for pathogens may be revolutionary in that it can provide results within 15 minutes rather than the days normally required when stool samples are sent to a laboratory, according to Dr. Chang-Hee Kim, chief executive officer of GoDx, Inc. The patent-pending methodology developed at Dr. Kim’s company permits real-time analysis “at the point of need and without the need for a lab.” In addition to the efficiency, the paper-based test, which Dr. Kim compared to a pregnancy test in that there is a color change with positive results, has the potential to improve outcomes.
Real-time testing “will decrease the loss of patients to follow-up and accelerate the time to treatment,” Dr. Kim asserted. “The test is also likely to reduce the spread of nosocomial pathogens if rapid infection control reduces spread.”
The test, which Dr. Kim expected to be made available at a cost of $100, will also be substantially cheaper than current laboratory analyses. In experimental studies, the accuracy has been at least as accurate as polymerase-chain reaction (PCR) testing, according to Dr. Kim. He sees applications not only in hospitals but at sites where ordering laboratory studies are not normally available, such as on cruise ships, in nursing homes, or in the military.
In the Shark Tank session, the main concerns expressed were about how this test will fit with Clinical Laboratory Improvement Amendments (CLIA) compliance. Herb Lerner, MD, a former officer in the Food and Drug Administration (FDA) and now senior director of medical and regulatory affairs at Hogan Lovells, a Washington, D.C.–based law firm, urged Dr. Kim to approach the FDA as soon as possible for advice on how to address this and other potential obstacles to a commercial product.
GI endoscopy capture, storage, and sharing
In introducing an automated method of capturing videos taken during colonoscopy, Matthew Z. Schwartz, the cofounder of Virgo Surgical Video Solutions, Inc. said, “There is currently no low-cost, user-friendly way to systematically capture GI endoscopy video.” Relative to existing products that “require complicated formatting and time-consuming setup for each procedure recording,” the plug-and-play system developed at Virgo “works with any existing endoscopy system that has a video output.” The videos are designed for cloud storage.
“Our long-term vision is to create the largest and highest quality repository of GI endoscopy videos,” said Mr. Schwartz, who added that support tools, including clinical decision-making aided by artificial intelligence, are being developed to provide even more value for quality control, research, and training.
Several Sharks, including Tom Shehab, MD, managing director, Arboretum Ventures, Ann Arbor, Mich., asked for more information about the value proposition for a proposed cost of $500 per month for the system. The answer was that this value would differ for settings, such as in an academic center that might apply the system for training relative to a community setting where the main purpose might be quality improvement.
New NG tube addresses aspiration risk
Pulmonary aspiration, along with symptomatic gastric reflux, is a common clinical challenge in intubated patients on a feeding tube, but a novel nasogastric (NG) tube equipped with a dual balloon system is designed to solve this problem, according to Talal Sharaiha, MD, the founder of Aspisafe Solutions. He said that reducing the risk of aspiration is important because it is associated with pneumonia, erosive inflammation, and upper GI bleeding. He called it the most common cause of upper GI bleeding in intensive care units.
Of the two balloons, one serves as an anchor and sits in the esophagus. The other serves as an anti-aspiration reservoir and sits in the stomach. The anti-aspiration balloon, inflated after it is inserted in the stomach, blocks reflux of gastric contents. Contending that there is a large market for this device, Dr. Sharaiha said, “having a feeding tube in intubated patients that prevents gastric reflux and aspiration will dramatically reduce complications and likely help reduce length of ventilation time, ICU time, and length of hospitals stay.”
More than one Shark requested more information about safety. Michael L. Kochman, MD, the Wilmott Professor of Medicine and director of the Center for Endoscopic Innovation, Research and Training at the University of Pennsylvania, Philadelphia, noted that there have been adverse experiences in the past with other types of balloon devices introduced into the GI tract. He indicated that this may be a potential bar to clinician comfort, so a comprehensive approach to safety analyses should be a priority in clinical development.
Virtual tape measure for colonoscopy measures polyp size
There are risks from the current practice of estimating polyp size, according to Avishay Sidlesky, founder of VTM Technologies, Ltd. He called these estimates “inherently inaccurate” and asserted that they “lead to erroneous diagnoses, suboptimal treatment and follow-up.” He believes that a “virtual tape measure” dependent on an endoscopic laser emitter solves this problem and cited a published paper that confirmed the accuracy of polyp measurements conducted with this device in an animal model.
“Employing dedicated software that analyzes the laser curves in the endoscopic image, the system enables reporting of the size of lesions, diameter and profile of polyps, longitudinal cross-section of lumens and more,” Mr. Sidlesky reported, specifying that the tool can be used alone or integrated into third party endoscopes. He believes applications can eventually be developed for a variety of endoscopic procedures in addition to colonoscopy.
Of Shark comments, several regarded concern about how this measuring tool would be integrated with existing endoscopic systems. Even though the measuring tool can be introduced in an unused channel of available scopes, the image is displayed separately. Michael J. Docktor, MD, clinical director of innovation, Boston Children’s Hospital, cautioned that devices that add steps to the workflow in the endoscopy suite may not be as rapidly accepted as one in which the tool is totally integrated with existing systems.
New device treats esophageal cancers with electroporation
There are a variety of tools to treat upper GI cancers endoscopically, including with radiofrequency ablation, cryotechnology, and photodynamic therapy, but morbidity rates are high, according to Declan Soden, founder of Mirai Medical. He described the attributes of a new technology based on electroporation in which electrical pulses painlessly target neoplastic tissue while preserving adjacent healthy tissue. He said that the treatment is performed in a matter of minutes on an outpatient basis.
“Clinical results to date have demonstrated the benefit of the technology in the treatment of late-stage esophageal and colorectal disease,” Mr. Soden said. He believes that the “unique selling point” of this electroporation treatment, which is part of a complete treatment regimen, “is that it renders tumor tissue leaky or porous, allowing absorption and uptake of drugs.” Relative to current standards, the treatment has multiple advantages, not least of which is preservation of quality of life, he believes.
The Sharks posed many technical questions about this strategy that might narrow the applicability of this device. For example, Trey Reed, MD, medical director, Humana Inc., posed questions about the depth of penetration of the electoral pulses and where they can be adjusted for tumors of different sizes. He also questioned whether the device will be too large to penetrate lumens obstructed by tumor. Although Mr. Soden believes that the device will be versatile, he acknowledged that its role might be better understood when phase 2 trials begin later this year.
A moderator at the Shank Tank session and a previous CGIT Chair, Dr. Kochman reported that he was impressed with the crop of entries. As one of the creators of the AGA Tech Summit, Dr. Kochman has the experience to recognize good ideas when he hears them.
“It is gratifying to see the high quality of the Shark Tank presenters. Over the past years, a number of the presenting companies have gone on to obtain additional funding, be acquired, and on to successful launches,” Dr. Kochman observed. “We hope the same successes await this year’s group.”
REPORTING FROM 2018 AGA TECH SUMMIT
Medical treatment of perianal fistulae often warranted, despite limited evidence
PHILADELPHIA – Perianal fistulae are a common and difficult-to-treat complication of Crohn’s disease that may often require medical therapy, though not all treatment options have robust data supporting their use in this setting, according to Mark T. Osterman, MD.
“Most studies done on fistulae actually didn’t have that as the primary endpoint – it was a secondary endpoint, so they weren’t really designed to look at fistulae, specifically,” said Dr. Osterman, associate professor of medicine in the division of gastroenterology at the University of Pennsylvania in Philadelphia.
Dr. Osterman shared his own approach to medical treatment of perianal fistulae in a presentation he gave at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
For simple perianal fistula with no rectal inflammation, a fistulotomy is reasonable, but medical treatment may be preferable, Dr. Osterman said.
“I always favor medical therapy because it attacks the root of the problem, which is the immune system,” he explained.
Helpful treatments in this scenario include antibiotics, along with anti–tumor necrosis factor therapy with or without immunomodulators, he said.
Antibiotic use in this setting is based on uncontrolled data, and efficacy is modest at best, according to Dr. Osterman, who noted that the treatments may reduce fistula drainage but likely do not heal fistulae.
The most commonly used antibiotics are metronidazole and ciprofloxacin given for up to 2-4 months, he added.
Infliximab is the drug that has by far the most robust fistula data, and one of only two drugs where a fistula was the primary outcome of the studies, according to Dr. Osterman.
In a randomized trial, infliximab induction treatment more than doubled fistula-related response and remission rates, compared with placebo, he said.
Maintenance infliximab treatment likewise showed an approximate doubling of both response and remission of fistula versus placebo, he added.
While not designed to look at fistulae as a primary outcome, the randomized CHARM study of adalimumab versus placebo for maintenance of Crohn’s disease remission did demonstrate remission rates about twice as high with the use of adalimumab, compared with placebo, in 117 patients who had draining fistulae at baseline, Dr. Osterman recounted.
For patients with complex fistulae, as well as patients with rectal inflammation, a seton and aggressive medical therapy are likely needed, Dr. Osterman said in his presentation.
An advancement flap or medical therapies such as vedolizumab or tacrolimus might be warranted for patients who fail other medical approaches, he added.
Relevant vedolizumab data come from GEMINI 2, a large clinical trial for Crohn’s disease that included 57 patients who had draining fistulas at baseline.
“We see an improvement in remission rates with fistula with vedolizumab, compared to placebo, but again, (GEMINI 2) wasn’t designed to look at fistula, but we do use it,” said Dr. Osterman.
Tacrolimus is the only drug besides infliximab that has randomized data for fistula, according to Dr. Osterman.
In the small randomized study, 48 patients with Crohn’s disease and draining perianal or enterocutaneous fistulae were treated for 10 weeks with oral tacrolimus at 0.2 mg/kg per day or placebo.
Fistula improvement was seen in 43% of tacrolimus-treated patients and 8% of placebo-treated patients (P = .004), while remission was seen in 10% and 8% of those groups, respectively (P = .86), according to published data on the trial.
“[Tacrolimus] showed a nice improvement in response rates, but very similar remission rates,” Dr. Osterman said, “but it does represent an option for us, for our patients.”
Dr. Osterman reported grant/research support from UCB and serving as a consultant for AbbVie, Janssen, Lycera, Merck, Pfizer, Takeda, and UCB.
Global Academy and this news organization are owned by the same company.
PHILADELPHIA – Perianal fistulae are a common and difficult-to-treat complication of Crohn’s disease that may often require medical therapy, though not all treatment options have robust data supporting their use in this setting, according to Mark T. Osterman, MD.
“Most studies done on fistulae actually didn’t have that as the primary endpoint – it was a secondary endpoint, so they weren’t really designed to look at fistulae, specifically,” said Dr. Osterman, associate professor of medicine in the division of gastroenterology at the University of Pennsylvania in Philadelphia.
Dr. Osterman shared his own approach to medical treatment of perianal fistulae in a presentation he gave at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
For simple perianal fistula with no rectal inflammation, a fistulotomy is reasonable, but medical treatment may be preferable, Dr. Osterman said.
“I always favor medical therapy because it attacks the root of the problem, which is the immune system,” he explained.
Helpful treatments in this scenario include antibiotics, along with anti–tumor necrosis factor therapy with or without immunomodulators, he said.
Antibiotic use in this setting is based on uncontrolled data, and efficacy is modest at best, according to Dr. Osterman, who noted that the treatments may reduce fistula drainage but likely do not heal fistulae.
The most commonly used antibiotics are metronidazole and ciprofloxacin given for up to 2-4 months, he added.
Infliximab is the drug that has by far the most robust fistula data, and one of only two drugs where a fistula was the primary outcome of the studies, according to Dr. Osterman.
In a randomized trial, infliximab induction treatment more than doubled fistula-related response and remission rates, compared with placebo, he said.
Maintenance infliximab treatment likewise showed an approximate doubling of both response and remission of fistula versus placebo, he added.
While not designed to look at fistulae as a primary outcome, the randomized CHARM study of adalimumab versus placebo for maintenance of Crohn’s disease remission did demonstrate remission rates about twice as high with the use of adalimumab, compared with placebo, in 117 patients who had draining fistulae at baseline, Dr. Osterman recounted.
For patients with complex fistulae, as well as patients with rectal inflammation, a seton and aggressive medical therapy are likely needed, Dr. Osterman said in his presentation.
An advancement flap or medical therapies such as vedolizumab or tacrolimus might be warranted for patients who fail other medical approaches, he added.
Relevant vedolizumab data come from GEMINI 2, a large clinical trial for Crohn’s disease that included 57 patients who had draining fistulas at baseline.
“We see an improvement in remission rates with fistula with vedolizumab, compared to placebo, but again, (GEMINI 2) wasn’t designed to look at fistula, but we do use it,” said Dr. Osterman.
Tacrolimus is the only drug besides infliximab that has randomized data for fistula, according to Dr. Osterman.
In the small randomized study, 48 patients with Crohn’s disease and draining perianal or enterocutaneous fistulae were treated for 10 weeks with oral tacrolimus at 0.2 mg/kg per day or placebo.
Fistula improvement was seen in 43% of tacrolimus-treated patients and 8% of placebo-treated patients (P = .004), while remission was seen in 10% and 8% of those groups, respectively (P = .86), according to published data on the trial.
“[Tacrolimus] showed a nice improvement in response rates, but very similar remission rates,” Dr. Osterman said, “but it does represent an option for us, for our patients.”
Dr. Osterman reported grant/research support from UCB and serving as a consultant for AbbVie, Janssen, Lycera, Merck, Pfizer, Takeda, and UCB.
Global Academy and this news organization are owned by the same company.
PHILADELPHIA – Perianal fistulae are a common and difficult-to-treat complication of Crohn’s disease that may often require medical therapy, though not all treatment options have robust data supporting their use in this setting, according to Mark T. Osterman, MD.
“Most studies done on fistulae actually didn’t have that as the primary endpoint – it was a secondary endpoint, so they weren’t really designed to look at fistulae, specifically,” said Dr. Osterman, associate professor of medicine in the division of gastroenterology at the University of Pennsylvania in Philadelphia.
Dr. Osterman shared his own approach to medical treatment of perianal fistulae in a presentation he gave at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
For simple perianal fistula with no rectal inflammation, a fistulotomy is reasonable, but medical treatment may be preferable, Dr. Osterman said.
“I always favor medical therapy because it attacks the root of the problem, which is the immune system,” he explained.
Helpful treatments in this scenario include antibiotics, along with anti–tumor necrosis factor therapy with or without immunomodulators, he said.
Antibiotic use in this setting is based on uncontrolled data, and efficacy is modest at best, according to Dr. Osterman, who noted that the treatments may reduce fistula drainage but likely do not heal fistulae.
The most commonly used antibiotics are metronidazole and ciprofloxacin given for up to 2-4 months, he added.
Infliximab is the drug that has by far the most robust fistula data, and one of only two drugs where a fistula was the primary outcome of the studies, according to Dr. Osterman.
In a randomized trial, infliximab induction treatment more than doubled fistula-related response and remission rates, compared with placebo, he said.
Maintenance infliximab treatment likewise showed an approximate doubling of both response and remission of fistula versus placebo, he added.
While not designed to look at fistulae as a primary outcome, the randomized CHARM study of adalimumab versus placebo for maintenance of Crohn’s disease remission did demonstrate remission rates about twice as high with the use of adalimumab, compared with placebo, in 117 patients who had draining fistulae at baseline, Dr. Osterman recounted.
For patients with complex fistulae, as well as patients with rectal inflammation, a seton and aggressive medical therapy are likely needed, Dr. Osterman said in his presentation.
An advancement flap or medical therapies such as vedolizumab or tacrolimus might be warranted for patients who fail other medical approaches, he added.
Relevant vedolizumab data come from GEMINI 2, a large clinical trial for Crohn’s disease that included 57 patients who had draining fistulas at baseline.
“We see an improvement in remission rates with fistula with vedolizumab, compared to placebo, but again, (GEMINI 2) wasn’t designed to look at fistula, but we do use it,” said Dr. Osterman.
Tacrolimus is the only drug besides infliximab that has randomized data for fistula, according to Dr. Osterman.
In the small randomized study, 48 patients with Crohn’s disease and draining perianal or enterocutaneous fistulae were treated for 10 weeks with oral tacrolimus at 0.2 mg/kg per day or placebo.
Fistula improvement was seen in 43% of tacrolimus-treated patients and 8% of placebo-treated patients (P = .004), while remission was seen in 10% and 8% of those groups, respectively (P = .86), according to published data on the trial.
“[Tacrolimus] showed a nice improvement in response rates, but very similar remission rates,” Dr. Osterman said, “but it does represent an option for us, for our patients.”
Dr. Osterman reported grant/research support from UCB and serving as a consultant for AbbVie, Janssen, Lycera, Merck, Pfizer, Takeda, and UCB.
Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Step-up diet: Less-intensive way to ID eosinophilic esophagitis food triggers?
PHILADELPHIA – While traditional elimination diets for eosinophilic esophagitis are highly restrictive and endoscopically intensive, a new “step-up” approach may offer a superior empiric scheme for identifying food triggers, according to Stuart J. Spechler, MD.
The recently described step-up approach may be preferable to the standard empiric six-food elimination diet, which has a 72% success rate but is challenging to implement, according to Dr. Spechler, chief of the division of gastroenterology, Baylor University Medical Center at Dallas.
“That’s an extremely demanding, time-consuming, inconvenient, and expensive thing to do. It requires at least seven endoscopies, probably more, performed over a period of 42 weeks,” Dr. Spechler said here at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
In contrast, the step-up approach recently described by Molina-Infante et al. in the Journal of Allergy and Clinical Immunology (2018 Mar 6. doi: 10.1016/j.jaci.2018.02.028) consists of a two-food elimination diet escalated to a four- and six-food elimination diet as needed.
This diet, which the researchers described as a “2-4-6” approach, starts with elimination of milk and wheat, the two most common food triggers for eosinophilic esophagitis. If patients do not respond, eggs and soy/legumes are eliminated, and if that fails, nuts and seafood are eliminated.
Once patients do respond, foods are reintroduced one at a time over 6 weeks with repeat endoscopy and biopsy, according to the report.
Molina-Infante and coauthors further described results of the diet in 220 patients with eosinophilic esophagitis. Of that group, investigators said 90 patients refused dietary therapy.
However, for the remaining 130 patients, 74 responded to the diet, the investigators reported.
Of 74 responders, 56 (43%) achieved remission at the first step in which milk and wheat were eliminated, results show, while an additional 10 had remission after the step up to four-food elimination, and another 8 had remission after the final step up to six-food elimination.
Dr. Spechler said his current take on diet therapy for eosinophilic esophagitis would be to start with the two-food elimination diet.
That first step alone identified about three-quarters of the patients who eventually would respond to the step-up approach in the Molina-Infante study, he observed.
For patients who do not respond and are motivated to continue, Dr. Spechler said he would move up to the four-food elimination diet, which identified about 90% of patients who eventually responded.
However, Dr. Spechler said he would consider the final step-up only in “exceptionally highly motivated” patients, since that step seems to identify very few additional responders.
“They got very little benefit here from going all the way up to a six-food elimination diet,” Dr. Spechler said.
Patient acceptance may be one major barrier to any dietary approach to treatment of eosinophilic esophagitis, regardless of how intensive the approach is.
“Ninety patients just flat-out refused to try the diet, so this is not a popular form of therapy,” Dr. Spechler noted.
However, diet is one of three valid treatment options for patients who do have an established diagnosis of eosinophilic esophagitis, the other two being proton pump inhibitors (PPIs) or 6-8 weeks of topical steroids, according to Dr. Spechler.
“If you’re going to use diet, I think you begin with that two-food elimination diet,“ he said. “Fortunately, most of the patients who don’t respond to diet will respond to PPIs or steroids.”
Dr. Spechler reported disclosures related to Ironwood Pharmaceuticals and Takeda Pharmaceuticals.
Global Academy and this news organization are owned by the same company.
PHILADELPHIA – While traditional elimination diets for eosinophilic esophagitis are highly restrictive and endoscopically intensive, a new “step-up” approach may offer a superior empiric scheme for identifying food triggers, according to Stuart J. Spechler, MD.
The recently described step-up approach may be preferable to the standard empiric six-food elimination diet, which has a 72% success rate but is challenging to implement, according to Dr. Spechler, chief of the division of gastroenterology, Baylor University Medical Center at Dallas.
“That’s an extremely demanding, time-consuming, inconvenient, and expensive thing to do. It requires at least seven endoscopies, probably more, performed over a period of 42 weeks,” Dr. Spechler said here at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
In contrast, the step-up approach recently described by Molina-Infante et al. in the Journal of Allergy and Clinical Immunology (2018 Mar 6. doi: 10.1016/j.jaci.2018.02.028) consists of a two-food elimination diet escalated to a four- and six-food elimination diet as needed.
This diet, which the researchers described as a “2-4-6” approach, starts with elimination of milk and wheat, the two most common food triggers for eosinophilic esophagitis. If patients do not respond, eggs and soy/legumes are eliminated, and if that fails, nuts and seafood are eliminated.
Once patients do respond, foods are reintroduced one at a time over 6 weeks with repeat endoscopy and biopsy, according to the report.
Molina-Infante and coauthors further described results of the diet in 220 patients with eosinophilic esophagitis. Of that group, investigators said 90 patients refused dietary therapy.
However, for the remaining 130 patients, 74 responded to the diet, the investigators reported.
Of 74 responders, 56 (43%) achieved remission at the first step in which milk and wheat were eliminated, results show, while an additional 10 had remission after the step up to four-food elimination, and another 8 had remission after the final step up to six-food elimination.
Dr. Spechler said his current take on diet therapy for eosinophilic esophagitis would be to start with the two-food elimination diet.
That first step alone identified about three-quarters of the patients who eventually would respond to the step-up approach in the Molina-Infante study, he observed.
For patients who do not respond and are motivated to continue, Dr. Spechler said he would move up to the four-food elimination diet, which identified about 90% of patients who eventually responded.
However, Dr. Spechler said he would consider the final step-up only in “exceptionally highly motivated” patients, since that step seems to identify very few additional responders.
“They got very little benefit here from going all the way up to a six-food elimination diet,” Dr. Spechler said.
Patient acceptance may be one major barrier to any dietary approach to treatment of eosinophilic esophagitis, regardless of how intensive the approach is.
“Ninety patients just flat-out refused to try the diet, so this is not a popular form of therapy,” Dr. Spechler noted.
However, diet is one of three valid treatment options for patients who do have an established diagnosis of eosinophilic esophagitis, the other two being proton pump inhibitors (PPIs) or 6-8 weeks of topical steroids, according to Dr. Spechler.
“If you’re going to use diet, I think you begin with that two-food elimination diet,“ he said. “Fortunately, most of the patients who don’t respond to diet will respond to PPIs or steroids.”
Dr. Spechler reported disclosures related to Ironwood Pharmaceuticals and Takeda Pharmaceuticals.
Global Academy and this news organization are owned by the same company.
PHILADELPHIA – While traditional elimination diets for eosinophilic esophagitis are highly restrictive and endoscopically intensive, a new “step-up” approach may offer a superior empiric scheme for identifying food triggers, according to Stuart J. Spechler, MD.
The recently described step-up approach may be preferable to the standard empiric six-food elimination diet, which has a 72% success rate but is challenging to implement, according to Dr. Spechler, chief of the division of gastroenterology, Baylor University Medical Center at Dallas.
“That’s an extremely demanding, time-consuming, inconvenient, and expensive thing to do. It requires at least seven endoscopies, probably more, performed over a period of 42 weeks,” Dr. Spechler said here at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
In contrast, the step-up approach recently described by Molina-Infante et al. in the Journal of Allergy and Clinical Immunology (2018 Mar 6. doi: 10.1016/j.jaci.2018.02.028) consists of a two-food elimination diet escalated to a four- and six-food elimination diet as needed.
This diet, which the researchers described as a “2-4-6” approach, starts with elimination of milk and wheat, the two most common food triggers for eosinophilic esophagitis. If patients do not respond, eggs and soy/legumes are eliminated, and if that fails, nuts and seafood are eliminated.
Once patients do respond, foods are reintroduced one at a time over 6 weeks with repeat endoscopy and biopsy, according to the report.
Molina-Infante and coauthors further described results of the diet in 220 patients with eosinophilic esophagitis. Of that group, investigators said 90 patients refused dietary therapy.
However, for the remaining 130 patients, 74 responded to the diet, the investigators reported.
Of 74 responders, 56 (43%) achieved remission at the first step in which milk and wheat were eliminated, results show, while an additional 10 had remission after the step up to four-food elimination, and another 8 had remission after the final step up to six-food elimination.
Dr. Spechler said his current take on diet therapy for eosinophilic esophagitis would be to start with the two-food elimination diet.
That first step alone identified about three-quarters of the patients who eventually would respond to the step-up approach in the Molina-Infante study, he observed.
For patients who do not respond and are motivated to continue, Dr. Spechler said he would move up to the four-food elimination diet, which identified about 90% of patients who eventually responded.
However, Dr. Spechler said he would consider the final step-up only in “exceptionally highly motivated” patients, since that step seems to identify very few additional responders.
“They got very little benefit here from going all the way up to a six-food elimination diet,” Dr. Spechler said.
Patient acceptance may be one major barrier to any dietary approach to treatment of eosinophilic esophagitis, regardless of how intensive the approach is.
“Ninety patients just flat-out refused to try the diet, so this is not a popular form of therapy,” Dr. Spechler noted.
However, diet is one of three valid treatment options for patients who do have an established diagnosis of eosinophilic esophagitis, the other two being proton pump inhibitors (PPIs) or 6-8 weeks of topical steroids, according to Dr. Spechler.
“If you’re going to use diet, I think you begin with that two-food elimination diet,“ he said. “Fortunately, most of the patients who don’t respond to diet will respond to PPIs or steroids.”
Dr. Spechler reported disclosures related to Ironwood Pharmaceuticals and Takeda Pharmaceuticals.
Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM DIGESTIVE DISEASES: NEW ADVANCES
Axial SpA disease activity remains mostly stable throughout pregnancy
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
, according to results of a prospective study.
“In the largest prospective study to date exploring disease activity during pregnancy in women with axSpA, we found that the majority experienced stable, low disease activity,” Kristin Ursin, MD, of the Trondheim (Norway) University Hospital and her associates wrote in Rheumatology. “In accordance with two previous studies, we found a small increase in disease activity in the second trimester.”
All women in the study fulfilled the Assessment of SpondyloArthritis International Society criteria for axSpA and had seven clinic visits throughout their pregnancy: one before conception, one at each trimester, another at 6 weeks, and two visits 6 and 12 months after delivery. No differentiation was made between women with radiographic or nonradiographic axSpA. At each visit, patients’ disease activity was determined using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), which is calculated based on six patient factors scored from 1 to 10, including: fatigue, back pain, peripheral joint pain and swelling, localized tenderness, duration of morning stiffness, and severity of morning stiffness. A disease score of 4 is commonly used to define active disease. Disease activity was also assessed by measuring C-reactive protein (CRP). Women’s function and health was also assessed using the Bath Ankylosing Spondylitis Functional Index (BASFI), scored similarly to the BASDAI, and the RAND-36 questionnaire to assess general health.
The research team found that, across the span of pregnancy, axSpA disease activity was relatively low and consistent throughout the study. But there was a significant relationship between disease activity and time (P = .029) in which disease activity was highest during the second trimester and proved to be significantly higher than 6 weeks after giving birth (mean BASDAI 3.97 vs. 3.46, P = .005). In fact, 45% of women had active disease in their second trimester (BASDAI of 4 or greater). But among women with data from the second trimester and 6 weeks post partum, 42% had a decrease in BASDAI score of 1 or more, and 22% had an equivalent increase. CRP values remained low and stable throughout the study.
Like the BASDAI scores, physical function and well being seemed to correlate with later time points during pregnancy. The lowest level of physical functioning for most women was during the second and third trimesters, and those periods were significantly worse than 6 weeks after giving birth (mean BASFI of 3.2 during the second trimester and 3.6 during the third vs. 2.6 at 6 weeks post partum). Similarly, patients reported overall worse general health during the second and third trimesters, compared with 6 weeks after giving birth, based on RAND-36 scores (mean physical functioning score of 63.1 in the second trimester and 54.5 in the third vs. 71.0 at 6 weeks post partum).
Use of nonbiologic medications remained relatively stable during pregnancy, while biologic disease-modifying antirheumatic drug (DMARD) use (all tumor necrosis factor [TNF] inhibitors) dropped significantly when pregnancy was confirmed. About 20% used NSAIDs during pregnancy, except for during the third trimester when use decreased to 8%. Prednisolone use remained stable at 5%-8%. A total of 11% had used synthetic DMARDs prior to pregnancy, and this rate stayed at 10%-16% during the three trimesters. Overall, 44% had used a biologic DMARD (TNF inhibitor) prior to pregnancy, but this declined sharply to 6% during the first trimester and 1% in the third.
The main limitations of the study included not having data from all of the women for all the time points. In fact, only 22% were included prior to conceiving, which could suggest that women with low disease activity were less likely to visit their rheumatologist, thereby biasing the sample toward an overestimated disease activity at preconception that could have potentially hidden a “more evident deterioration between the preconception visit and the second trimester,” the authors said.
“Future research on pregnancy in women with axSpA should differentiate between subgroups of the disease and aim to include objective assessment of inflammation.”
The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
SOURCE: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
FROM RHEUMATOLOGY
Key clinical point: Higher disease activity and lower physical well being correspond with late stage pregnancy.
Major finding: Disease activity appears to be highest in the second trimester, compared with 6 weeks post partum (mean BASDAI 3.97 vs. 3.46, P = .005).
Study details: A prospective study of 179 pregnancies in 166 Norwegian women with axSpA from a Norwegian health registry between 2006 and 2016.
Disclosures: The study was funded by the Research Fund of the Norwegian Organization for People With Rheumatic Diseases. None of the authors had any conflicts of interest to declare.
Source: Ursin K et al. Rheumatology. 2018 Mar 14. doi: 10.1093/rheumatology/key047.
U.S. adults consumed 17.5 billion total binge drinks in 2015
A new study seeks to elucidate disparities in the binge drinking habits of U.S. adults.
To its authors’ knowledge, the study by Dafna Kanny, PhD, and her coinvestigators is the first to assess total binge drinks consumed, in contrast with other studies that have assessed prevalence alone. This total binge drinks consumed measure is calculated by multiplying the intensity of drinking (greatest number of drinks consumed on one binge-drinking occasion) by the frequency of binge drinking.
Using this measure, Dr. Kanny and her coinvestigators found that 17.1% of U.S. adults reported consuming 17.5 billion total binge drinks, or 470 binge drinks per binge drinker in 2015.
The measure also led to insight into drinking patterns among different U.S. demographic groups. For example, while binge drinking was more common among people aged 18-34 years, half of all binge drinks were consumed by people older than 35 years – which reinforces the notion that binge drinking is a lifelong issue. The researchers also found that, while binge drinking was less common among people with lower incomes and educational attainment, they consumed a higher “total annual number of binge drinks per binge drinker” than did people with higher incomes and educational attainment, Dr. Kanny, of the Centers for Disease Control and Prevention, and her coinvestigators, reported in the American Journal of Preventive Medicine.
In addition to finding disparities among demographic groups, the investigators found disparities between different geographical regions of the country. “The highest annual number of total binge drinks per drinker was reported in Arkansas (841.0), Mississippi (831.8), Kentucky (652.8), and Hawaii (611.7),” they wrote. “Notably, total annual binge drinks per binge drinker and per adult were generally higher in the Mississippi River Valley than in other regions.”
Assessing binge-drinking prevalence alone does not capture some disparities in binge-drinking behavior, the coinvestigators concluded. “Monitoring total binge drinks consumed annually and total binge drinks per binge drinker” could not only overcome those limitations but also help implement approaches to reducing binge drinking and alcohol-related harms.
Dr. Kanny and her coinvestigators reported that their findings “do not necessarily represent the official position” of the CDC. They reported no financial disclosures.
SOURCE: Kanny D et al. Am J Prev Med. 2018;54(4):486-96.
A new study seeks to elucidate disparities in the binge drinking habits of U.S. adults.
To its authors’ knowledge, the study by Dafna Kanny, PhD, and her coinvestigators is the first to assess total binge drinks consumed, in contrast with other studies that have assessed prevalence alone. This total binge drinks consumed measure is calculated by multiplying the intensity of drinking (greatest number of drinks consumed on one binge-drinking occasion) by the frequency of binge drinking.
Using this measure, Dr. Kanny and her coinvestigators found that 17.1% of U.S. adults reported consuming 17.5 billion total binge drinks, or 470 binge drinks per binge drinker in 2015.
The measure also led to insight into drinking patterns among different U.S. demographic groups. For example, while binge drinking was more common among people aged 18-34 years, half of all binge drinks were consumed by people older than 35 years – which reinforces the notion that binge drinking is a lifelong issue. The researchers also found that, while binge drinking was less common among people with lower incomes and educational attainment, they consumed a higher “total annual number of binge drinks per binge drinker” than did people with higher incomes and educational attainment, Dr. Kanny, of the Centers for Disease Control and Prevention, and her coinvestigators, reported in the American Journal of Preventive Medicine.
In addition to finding disparities among demographic groups, the investigators found disparities between different geographical regions of the country. “The highest annual number of total binge drinks per drinker was reported in Arkansas (841.0), Mississippi (831.8), Kentucky (652.8), and Hawaii (611.7),” they wrote. “Notably, total annual binge drinks per binge drinker and per adult were generally higher in the Mississippi River Valley than in other regions.”
Assessing binge-drinking prevalence alone does not capture some disparities in binge-drinking behavior, the coinvestigators concluded. “Monitoring total binge drinks consumed annually and total binge drinks per binge drinker” could not only overcome those limitations but also help implement approaches to reducing binge drinking and alcohol-related harms.
Dr. Kanny and her coinvestigators reported that their findings “do not necessarily represent the official position” of the CDC. They reported no financial disclosures.
SOURCE: Kanny D et al. Am J Prev Med. 2018;54(4):486-96.
A new study seeks to elucidate disparities in the binge drinking habits of U.S. adults.
To its authors’ knowledge, the study by Dafna Kanny, PhD, and her coinvestigators is the first to assess total binge drinks consumed, in contrast with other studies that have assessed prevalence alone. This total binge drinks consumed measure is calculated by multiplying the intensity of drinking (greatest number of drinks consumed on one binge-drinking occasion) by the frequency of binge drinking.
Using this measure, Dr. Kanny and her coinvestigators found that 17.1% of U.S. adults reported consuming 17.5 billion total binge drinks, or 470 binge drinks per binge drinker in 2015.
The measure also led to insight into drinking patterns among different U.S. demographic groups. For example, while binge drinking was more common among people aged 18-34 years, half of all binge drinks were consumed by people older than 35 years – which reinforces the notion that binge drinking is a lifelong issue. The researchers also found that, while binge drinking was less common among people with lower incomes and educational attainment, they consumed a higher “total annual number of binge drinks per binge drinker” than did people with higher incomes and educational attainment, Dr. Kanny, of the Centers for Disease Control and Prevention, and her coinvestigators, reported in the American Journal of Preventive Medicine.
In addition to finding disparities among demographic groups, the investigators found disparities between different geographical regions of the country. “The highest annual number of total binge drinks per drinker was reported in Arkansas (841.0), Mississippi (831.8), Kentucky (652.8), and Hawaii (611.7),” they wrote. “Notably, total annual binge drinks per binge drinker and per adult were generally higher in the Mississippi River Valley than in other regions.”
Assessing binge-drinking prevalence alone does not capture some disparities in binge-drinking behavior, the coinvestigators concluded. “Monitoring total binge drinks consumed annually and total binge drinks per binge drinker” could not only overcome those limitations but also help implement approaches to reducing binge drinking and alcohol-related harms.
Dr. Kanny and her coinvestigators reported that their findings “do not necessarily represent the official position” of the CDC. They reported no financial disclosures.
SOURCE: Kanny D et al. Am J Prev Med. 2018;54(4):486-96.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Different OSA subtypes respond differently to therapy
Patients with obstructive sleep apnea can be grouped into distinct clinical subtypes that differ in response to positive airway pressure treatment, according to two studies published in the March issue of the journal Sleep.
In the first study, investigators evaluated whether patients in different clinical clusters responded differently to positive airway pressure (PAP) treatment. Authors identified 706 patients with moderate to severe obstructive sleep apnea (OSA) from the Icelandic Sleep Apnea Cohort. All patients completed a sleep study prior to starting PAP treatment, and completed questionnaires to assess symptoms. Patients were grouped into one of three clusters based on symptomatology: disturbed sleep, minimally symptomatic, or sleepy, wrote Grace W. Pien, MD, of the division of pulmonary and critical care medicine at Johns Hopkins University, Baltimore, and her coauthors.
Patients in the minimally symptomatic cluster reported symptoms at lower rates than patients in the other clusters at baseline, and they remained relatively asymptomatic at follow-up, the authors noted. By comparison, patients in the sleepy group reported the highest Epworth Sleepiness Scale scores at baseline (16.0 plus or minus 3.4), which fell by five points at follow-up (mean change, −5.3; 95% confidence interval, −5.8 to −4.8). Also, patients in the sleepy group reported higher rates of drowsy driving (37.8%) at baseline, which dropped to 8.1% at follow-up (odds ratio, 0.06; 95% CI, 0.03-0.14).
At baseline, the disturbed-sleep group reported mainly insomnia-related symptoms, including difficulty falling asleep (43.2%), waking often at night (90.8%), restless sleep (74.2%), and waking up early (62.3%). At follow-up, improvements in the frequency of insomnia-related symptoms ranged from 0.28 to 1.25 points, and Epworth Sleepiness Scale scores fell significantly (−2.06; 95% CI, −2.64 to −1.48). Reductions in the proportion of patients with insomnia symptoms ranged from 13.1% (OR, 0.35; 95% CI, 0.20-0.59) for difficulty falling asleep to 39.0% (OR, 0.08; 95% CI, 0.04-0.14) for restless sleep, Dr. Pien and her colleagues reported.
The results “demonstrate that although symptoms improved overall among each of the three clinical phenotypes of moderate to severe OSA, patterns of treatment response … varied based on initial clinical presentation,” the authors wrote. “Our findings underscore the need to consider initial OSA phenotype when designing future trials.”
In the second study, also published in Sleep, investigators confirmed the three clinical OSA subtypes previously identified in the Icelandic Sleep Apnea Cohort. In analysis of an international sample, they also expanded these clusters to include two additional disease subtypes. One of these subtypes consisted of patients with symptoms dominated by indications of upper airway obstruction. The other new subtype, sleepiness dominant OSA, included patients who had excessive sleepiness but no symptoms of upper airway obstruction.
The study authors performed a cluster analysis using data from 972 patients from the Sleep Apnea Global Interdisciplinary Consortium with moderate to severe OSA, with 215 of these patients being from Iceland.
In total, 688 (70.8%) patients were diagnosed using laboratory-based polysomnography and 284 (29.2%) with home-based sleep studies. Patients completed questionnaires related to symptoms including sleepiness, insomnia, sleep disturbance, abnormal behaviors during sleep, upper airway symptoms, and other symptoms such as headaches and excessive sweating, wrote Brendan T. Keenan, of the University of Pennsylvania, Philadelphia, and his coauthors.
In the Icelandic group, results identified 72 (33.5%) patients in the disturbed-sleep cluster, 62 (28.8%) in the minimally symptomatic cluster, and 81 (37.7%) in the excessively sleepy cluster, similar to prior research. The three subtypes were found in the international sample of patients as well, with 150 (19.8%) in the disturbed-sleep cluster, 306 (40.4%) in the minimally symptomatic cluster, and 301 (39.8%) in the excessively sleepy cluster.
“Overall, this study provides a novel approach to better characterize patients with OSA presenting at sleep clinics worldwide,” wrote the authors of the second study. “This information can help inform personalized medicine approaches to OSA treatment by allowing clinicians to focus interventions on the most relevant OSA symptoms and consequences within an individual patient.”
Both studies were funded by the National Institutes of Health.
SOURCES: Pien GW et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx201; Keenan BT et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx214.
The results of these studies “advance the personalization of sleep apnea care by validating distinct symptom-based groups that generalize across nations and assessing how members of these clinical phenotypes respond to therapy,” wrote Vishesh K. Kapur, MD, of the division of pulmonary, critical care and sleep medicine at the University of Washington, Seattle, in an editorial published in the March issue of Sleep (2018 Mar. doi: 10.1093/sleep/zsy042).
“Patients with OSA differ in their presenting symptoms,” he said, and future studies should aim to “elucidate whether the proposed phenotypes will enable a more personalized paradigm of sleep apnea care that results in better tailored and more effective care.”
Dr. Kapur did not report any relevant disclosures.
The results of these studies “advance the personalization of sleep apnea care by validating distinct symptom-based groups that generalize across nations and assessing how members of these clinical phenotypes respond to therapy,” wrote Vishesh K. Kapur, MD, of the division of pulmonary, critical care and sleep medicine at the University of Washington, Seattle, in an editorial published in the March issue of Sleep (2018 Mar. doi: 10.1093/sleep/zsy042).
“Patients with OSA differ in their presenting symptoms,” he said, and future studies should aim to “elucidate whether the proposed phenotypes will enable a more personalized paradigm of sleep apnea care that results in better tailored and more effective care.”
Dr. Kapur did not report any relevant disclosures.
The results of these studies “advance the personalization of sleep apnea care by validating distinct symptom-based groups that generalize across nations and assessing how members of these clinical phenotypes respond to therapy,” wrote Vishesh K. Kapur, MD, of the division of pulmonary, critical care and sleep medicine at the University of Washington, Seattle, in an editorial published in the March issue of Sleep (2018 Mar. doi: 10.1093/sleep/zsy042).
“Patients with OSA differ in their presenting symptoms,” he said, and future studies should aim to “elucidate whether the proposed phenotypes will enable a more personalized paradigm of sleep apnea care that results in better tailored and more effective care.”
Dr. Kapur did not report any relevant disclosures.
Patients with obstructive sleep apnea can be grouped into distinct clinical subtypes that differ in response to positive airway pressure treatment, according to two studies published in the March issue of the journal Sleep.
In the first study, investigators evaluated whether patients in different clinical clusters responded differently to positive airway pressure (PAP) treatment. Authors identified 706 patients with moderate to severe obstructive sleep apnea (OSA) from the Icelandic Sleep Apnea Cohort. All patients completed a sleep study prior to starting PAP treatment, and completed questionnaires to assess symptoms. Patients were grouped into one of three clusters based on symptomatology: disturbed sleep, minimally symptomatic, or sleepy, wrote Grace W. Pien, MD, of the division of pulmonary and critical care medicine at Johns Hopkins University, Baltimore, and her coauthors.
Patients in the minimally symptomatic cluster reported symptoms at lower rates than patients in the other clusters at baseline, and they remained relatively asymptomatic at follow-up, the authors noted. By comparison, patients in the sleepy group reported the highest Epworth Sleepiness Scale scores at baseline (16.0 plus or minus 3.4), which fell by five points at follow-up (mean change, −5.3; 95% confidence interval, −5.8 to −4.8). Also, patients in the sleepy group reported higher rates of drowsy driving (37.8%) at baseline, which dropped to 8.1% at follow-up (odds ratio, 0.06; 95% CI, 0.03-0.14).
At baseline, the disturbed-sleep group reported mainly insomnia-related symptoms, including difficulty falling asleep (43.2%), waking often at night (90.8%), restless sleep (74.2%), and waking up early (62.3%). At follow-up, improvements in the frequency of insomnia-related symptoms ranged from 0.28 to 1.25 points, and Epworth Sleepiness Scale scores fell significantly (−2.06; 95% CI, −2.64 to −1.48). Reductions in the proportion of patients with insomnia symptoms ranged from 13.1% (OR, 0.35; 95% CI, 0.20-0.59) for difficulty falling asleep to 39.0% (OR, 0.08; 95% CI, 0.04-0.14) for restless sleep, Dr. Pien and her colleagues reported.
The results “demonstrate that although symptoms improved overall among each of the three clinical phenotypes of moderate to severe OSA, patterns of treatment response … varied based on initial clinical presentation,” the authors wrote. “Our findings underscore the need to consider initial OSA phenotype when designing future trials.”
In the second study, also published in Sleep, investigators confirmed the three clinical OSA subtypes previously identified in the Icelandic Sleep Apnea Cohort. In analysis of an international sample, they also expanded these clusters to include two additional disease subtypes. One of these subtypes consisted of patients with symptoms dominated by indications of upper airway obstruction. The other new subtype, sleepiness dominant OSA, included patients who had excessive sleepiness but no symptoms of upper airway obstruction.
The study authors performed a cluster analysis using data from 972 patients from the Sleep Apnea Global Interdisciplinary Consortium with moderate to severe OSA, with 215 of these patients being from Iceland.
In total, 688 (70.8%) patients were diagnosed using laboratory-based polysomnography and 284 (29.2%) with home-based sleep studies. Patients completed questionnaires related to symptoms including sleepiness, insomnia, sleep disturbance, abnormal behaviors during sleep, upper airway symptoms, and other symptoms such as headaches and excessive sweating, wrote Brendan T. Keenan, of the University of Pennsylvania, Philadelphia, and his coauthors.
In the Icelandic group, results identified 72 (33.5%) patients in the disturbed-sleep cluster, 62 (28.8%) in the minimally symptomatic cluster, and 81 (37.7%) in the excessively sleepy cluster, similar to prior research. The three subtypes were found in the international sample of patients as well, with 150 (19.8%) in the disturbed-sleep cluster, 306 (40.4%) in the minimally symptomatic cluster, and 301 (39.8%) in the excessively sleepy cluster.
“Overall, this study provides a novel approach to better characterize patients with OSA presenting at sleep clinics worldwide,” wrote the authors of the second study. “This information can help inform personalized medicine approaches to OSA treatment by allowing clinicians to focus interventions on the most relevant OSA symptoms and consequences within an individual patient.”
Both studies were funded by the National Institutes of Health.
SOURCES: Pien GW et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx201; Keenan BT et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx214.
Patients with obstructive sleep apnea can be grouped into distinct clinical subtypes that differ in response to positive airway pressure treatment, according to two studies published in the March issue of the journal Sleep.
In the first study, investigators evaluated whether patients in different clinical clusters responded differently to positive airway pressure (PAP) treatment. Authors identified 706 patients with moderate to severe obstructive sleep apnea (OSA) from the Icelandic Sleep Apnea Cohort. All patients completed a sleep study prior to starting PAP treatment, and completed questionnaires to assess symptoms. Patients were grouped into one of three clusters based on symptomatology: disturbed sleep, minimally symptomatic, or sleepy, wrote Grace W. Pien, MD, of the division of pulmonary and critical care medicine at Johns Hopkins University, Baltimore, and her coauthors.
Patients in the minimally symptomatic cluster reported symptoms at lower rates than patients in the other clusters at baseline, and they remained relatively asymptomatic at follow-up, the authors noted. By comparison, patients in the sleepy group reported the highest Epworth Sleepiness Scale scores at baseline (16.0 plus or minus 3.4), which fell by five points at follow-up (mean change, −5.3; 95% confidence interval, −5.8 to −4.8). Also, patients in the sleepy group reported higher rates of drowsy driving (37.8%) at baseline, which dropped to 8.1% at follow-up (odds ratio, 0.06; 95% CI, 0.03-0.14).
At baseline, the disturbed-sleep group reported mainly insomnia-related symptoms, including difficulty falling asleep (43.2%), waking often at night (90.8%), restless sleep (74.2%), and waking up early (62.3%). At follow-up, improvements in the frequency of insomnia-related symptoms ranged from 0.28 to 1.25 points, and Epworth Sleepiness Scale scores fell significantly (−2.06; 95% CI, −2.64 to −1.48). Reductions in the proportion of patients with insomnia symptoms ranged from 13.1% (OR, 0.35; 95% CI, 0.20-0.59) for difficulty falling asleep to 39.0% (OR, 0.08; 95% CI, 0.04-0.14) for restless sleep, Dr. Pien and her colleagues reported.
The results “demonstrate that although symptoms improved overall among each of the three clinical phenotypes of moderate to severe OSA, patterns of treatment response … varied based on initial clinical presentation,” the authors wrote. “Our findings underscore the need to consider initial OSA phenotype when designing future trials.”
In the second study, also published in Sleep, investigators confirmed the three clinical OSA subtypes previously identified in the Icelandic Sleep Apnea Cohort. In analysis of an international sample, they also expanded these clusters to include two additional disease subtypes. One of these subtypes consisted of patients with symptoms dominated by indications of upper airway obstruction. The other new subtype, sleepiness dominant OSA, included patients who had excessive sleepiness but no symptoms of upper airway obstruction.
The study authors performed a cluster analysis using data from 972 patients from the Sleep Apnea Global Interdisciplinary Consortium with moderate to severe OSA, with 215 of these patients being from Iceland.
In total, 688 (70.8%) patients were diagnosed using laboratory-based polysomnography and 284 (29.2%) with home-based sleep studies. Patients completed questionnaires related to symptoms including sleepiness, insomnia, sleep disturbance, abnormal behaviors during sleep, upper airway symptoms, and other symptoms such as headaches and excessive sweating, wrote Brendan T. Keenan, of the University of Pennsylvania, Philadelphia, and his coauthors.
In the Icelandic group, results identified 72 (33.5%) patients in the disturbed-sleep cluster, 62 (28.8%) in the minimally symptomatic cluster, and 81 (37.7%) in the excessively sleepy cluster, similar to prior research. The three subtypes were found in the international sample of patients as well, with 150 (19.8%) in the disturbed-sleep cluster, 306 (40.4%) in the minimally symptomatic cluster, and 301 (39.8%) in the excessively sleepy cluster.
“Overall, this study provides a novel approach to better characterize patients with OSA presenting at sleep clinics worldwide,” wrote the authors of the second study. “This information can help inform personalized medicine approaches to OSA treatment by allowing clinicians to focus interventions on the most relevant OSA symptoms and consequences within an individual patient.”
Both studies were funded by the National Institutes of Health.
SOURCES: Pien GW et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx201; Keenan BT et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx214.
FROM SLEEP
Key clinical point: Obstructive sleep apnea patients can be grouped into distinct subtypes depending on disease presentation, and these clusters differ in their responses to positive air pressure treatment.
Major finding: Though improvements were seen in all groups, presentation and changes in symptoms varied widely across clusters; for instance, patients in the sleepy group reported the highest Epworth Sleepiness Scale scores at baseline (16.0 plus or minus 3.4), which fell by five points at follow-up (mean change, −5.3; 95% CI, −5.8 to −4.8); whereas the disturbed-sleep group reported mainly insomnia-related symptoms including difficulty falling asleep (43.2%), waking often at night (90.8%), restless sleep (74.2%), and waking up early (62.3%).
Study details: A study of 706 patients with moderate to severe OSA from the Icelandic Sleep Apnea Cohort, and a study of 972 patients from the Sleep Apnea Global Interdisciplinary Consortium with moderate to severe OSA.
Disclosures: Both studies were funded by the National Institutes of Health.
Sources: Pien GW et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx201; Keenan B et al. Sleep. 2018 Mar. doi: 10.1093/sleep/zsx214.
Lower socioeconomic status linked with poor NSCLC prognosis in those with pretreatment weight loss
, in a retrospective review of medical records.
Investigators identified 1,366 patients with NSCLC who had been consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013, and obtained their insurance status from an institutional tumor registry.
Cancer-associated weight loss was present at the time of diagnosis in 30% of the patients. Among those patients with pretreatment weight loss, uninsured patients had worse survival compared with those who had private insurance (hazard ratio, 1.63; 95% confidence interval, 1.14-2.35). However, no association was found for patients without pretreatment weight loss, wrote Steven Lau, MD, of the University of Texas Southwestern Medical Center, Dallas, and his associates. The report was published in the Journal of Oncology Practice.
After the researchers controlled for other prognostic factors, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42-3.30) and lack of insurance (OR, 2.32; 95% CI, 1.50-3.58) were independently associated with pretreatment weight loss.
“Patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival ... These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival,” Dr. Lau and his associates concluded.
The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
SOURCE: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239
, in a retrospective review of medical records.
Investigators identified 1,366 patients with NSCLC who had been consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013, and obtained their insurance status from an institutional tumor registry.
Cancer-associated weight loss was present at the time of diagnosis in 30% of the patients. Among those patients with pretreatment weight loss, uninsured patients had worse survival compared with those who had private insurance (hazard ratio, 1.63; 95% confidence interval, 1.14-2.35). However, no association was found for patients without pretreatment weight loss, wrote Steven Lau, MD, of the University of Texas Southwestern Medical Center, Dallas, and his associates. The report was published in the Journal of Oncology Practice.
After the researchers controlled for other prognostic factors, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42-3.30) and lack of insurance (OR, 2.32; 95% CI, 1.50-3.58) were independently associated with pretreatment weight loss.
“Patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival ... These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival,” Dr. Lau and his associates concluded.
The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
SOURCE: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239
, in a retrospective review of medical records.
Investigators identified 1,366 patients with NSCLC who had been consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013, and obtained their insurance status from an institutional tumor registry.
Cancer-associated weight loss was present at the time of diagnosis in 30% of the patients. Among those patients with pretreatment weight loss, uninsured patients had worse survival compared with those who had private insurance (hazard ratio, 1.63; 95% confidence interval, 1.14-2.35). However, no association was found for patients without pretreatment weight loss, wrote Steven Lau, MD, of the University of Texas Southwestern Medical Center, Dallas, and his associates. The report was published in the Journal of Oncology Practice.
After the researchers controlled for other prognostic factors, Medicaid insurance (odds ratio, 2.17; 95% CI, 1.42-3.30) and lack of insurance (OR, 2.32; 95% CI, 1.50-3.58) were independently associated with pretreatment weight loss.
“Patients with NSCLC of lower SES as measured by primary payer are disproportionately affected by cancer-associated weight loss, which in turn is prognostic of diminished survival ... These findings suggest early recognition and management of cachexia, even at the time of cancer diagnosis, could result in improved survival,” Dr. Lau and his associates concluded.
The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
SOURCE: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239
FROM JOURNAL OF ONCOLOGY PRACTICE
Key clinical point: Early recognition and management of cancer-associated weight loss in patients with NSCLC and low socioeconomic status may improve outcomes.
Major finding: Lack of insurance was significantly prognostic among patients with NSCLC and pretreatment weight loss (hazard ratio, 1.63 95% CI, 1.14-2.35).
Study details: 1,366 adult patients with NSCLC consecutively treated at a tertiary care health system between Jan. 1, 2006, and Dec. 31, 2013.
Disclosures: The study was supported in part by National Center for Advancing Translational Sciences grants TL1TR001104 and UL1TR001105. Dr. Lau reported employment with LabCorp by an immediate family member. Coauthors reported financial ties to Advenchen Laboratories, Macrogen, Peregrine Pharmaceuticals, and DFINE.
Source: Lau S et al. J Oncol Pract. 2018 Mar 20. doi: 10.1200/JOP.2017.025239.
Glenoid Bone Loss in Reverse Shoulder Arthroplasty Treated with Bone Graft Techniques
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).
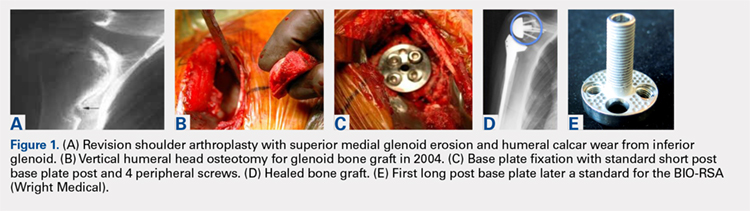
In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8
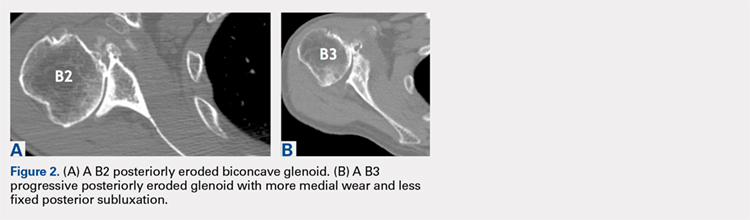
Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9
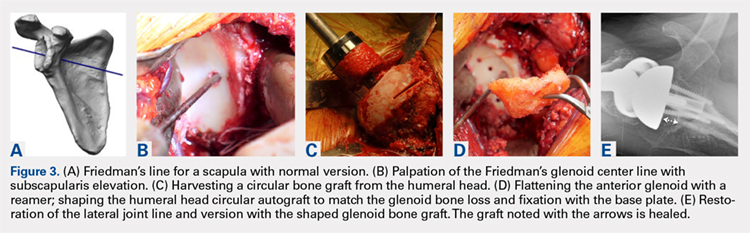
All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.
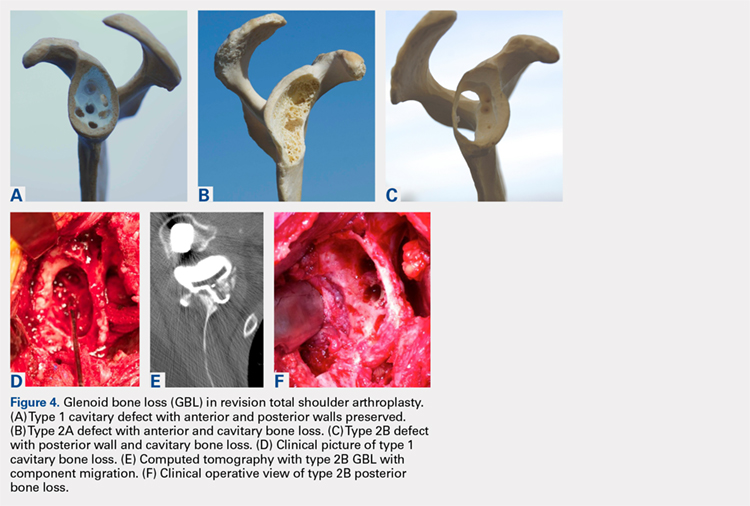
Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).
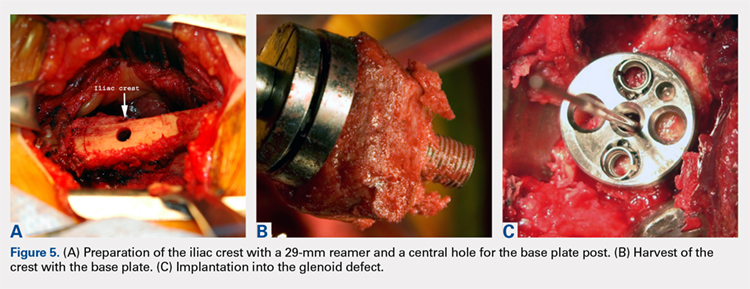
This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).
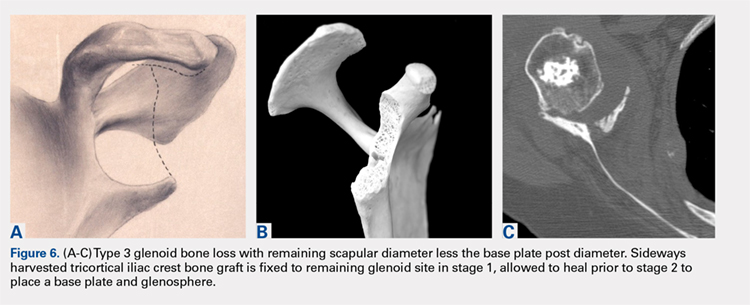
An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26
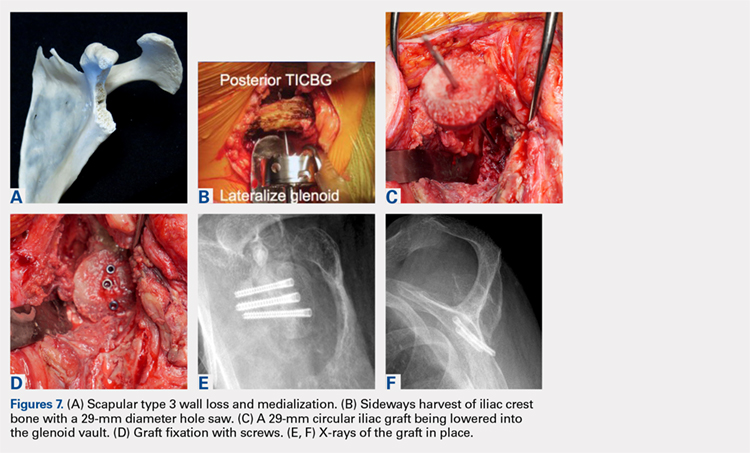
CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.
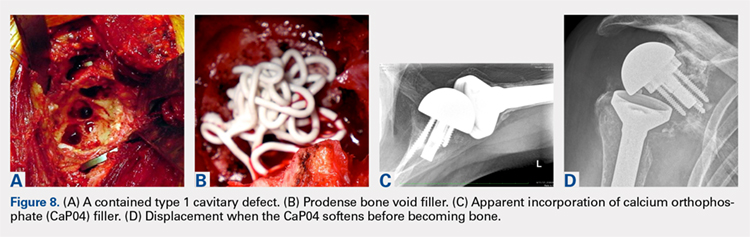
For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).

In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8

Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9

All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.

Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).

This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).

An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26

CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.

For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).

In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8

Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9

All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.

Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).

This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).

An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26

CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.

For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
TAKE-HOME POINTS
- Glenoid deficiencies that occur from dysplasia, arthritis, or polyethylene osteolysis may be successfully addressed with bone grafting techniques and reverse shoulder arthroplasty.
- The intact humeral head in a primary case is ideal graft to be shaped to fit the glenoid deficits.
- The reverse shoulder with a long post base plate that is fixed securely to the native scapula is the author’s preferred technique.
- As the native humeral head is not available in revision cases, the tricortical iliac crest bone graft may be fixed as a structural graft in 1-stage.
- When the scapular walls are deficient and medial fixation is not secure, 2 stages 4 months to 6 months apart will be necessary before loading the construct.
Takotsubo cardiomyopathy incidence, mortality on the rise
WASHINGTON – Hospitalizations for Takotsubo cardiomyopathy (TCM), a condition that primarily affects postmenopausal women, have been rising steadily, along with rates of in-hospital mortality due to this condition, according to a study conducted with outcomes from the National Inpatient Sample (NIS) database.
The study was presented at CRT 2018, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Importantly, a multivariate analysis of predictors of in-hospital mortality based on this database revealed that there were “several potentially reversible causes,” reported Konstantinos V. Voudris, MD, PhD, who is completing a residency in internal medicine at the University of Illinois at Chicago.
TCM, which is characterized by left ventricular apical akinesis and chest pain that mimics the features of acute coronary syndromes (ACS), was first described in 1990 in Japan. The first U.S. case was reported in 1998, but the condition is now well recognized and an ICD code was created for Takotsubo cardiomyopathy in 2007.
There were 72,559 TCM admissions during the study period. When stratified by year, the annual rate of TCM cases rose significantly, from 11.1 to 43.8 per 100,000 hospitalizations from 2007 to 2013.
Although overall in-hospital mortality was 2.5%, it climbed from 1.4% in 2007 to 3.2% in 2013. When compared to patients who did not die during hospitalizations, those who did die were on average older (69.9 vs. 66.4 years; P less than .0001) and had more comorbidities. When a multivariate adjustment was made for baseline clinical risk, the presence of acute kidney injury (AKI), chronic obstructive pulmonary disease (COPD), peripheral artery disease (PAD), pulmonary hypertension, and arrhythmias remained significant predictors of mortality in patients with TCM.
“We found a number of modifiable and nonmodifiable factors that are associated with a significantly increased mortality, and we think clinicians should be aware of these factors when managing this condition,” Dr. Voudris reported.
Of these risk factors, AKI was associated with the greatest increased odds ratio (OR) with a more than fourfold increased risk of death (OR, 4.18; P less than .001). The presence of PAD (OR, 1.87; P less than .0001) and arrhythmia (OR, 1.88; P less than .0001) almost doubled the risk of in-hospital mortality.
Although females represented 89% of the TCM cases collected during the study period, males with comorbid diseases were at particularly high risk of death, an observation that is consistent with previous reports, according to Dr. Voudris. Relative to women, men were more likely to present with unstable hemodynamics and to develop cardiogenic shock.
Although Dr. Voudris acknowledged that the rising number of hospitalizations for TCM is likely due largely to increasing recognition of this condition, he suggested that there may be other contributing factors to the growing incidence, such as the aging of the U.S. population. He noted that the rise in cases persisted throughout the study period when awareness of TCM might be expected to have improved.
“This is still an uncommon disease, but it is important to recognize,” agreed Sachin Kumar, MD, an interventional cardiologist at the University of Texas Health Science Center at Houston. Moderator of the session in which these data were presented, Dr. Kumar indicated that it is important to increase awareness of the condition to accelerate the time to diagnosis and treatment.
Dr. Voudris reported no financial relationships relevant to this study.
SOURCE: Voudris, KV. CRT 2018.
WASHINGTON – Hospitalizations for Takotsubo cardiomyopathy (TCM), a condition that primarily affects postmenopausal women, have been rising steadily, along with rates of in-hospital mortality due to this condition, according to a study conducted with outcomes from the National Inpatient Sample (NIS) database.
The study was presented at CRT 2018, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Importantly, a multivariate analysis of predictors of in-hospital mortality based on this database revealed that there were “several potentially reversible causes,” reported Konstantinos V. Voudris, MD, PhD, who is completing a residency in internal medicine at the University of Illinois at Chicago.
TCM, which is characterized by left ventricular apical akinesis and chest pain that mimics the features of acute coronary syndromes (ACS), was first described in 1990 in Japan. The first U.S. case was reported in 1998, but the condition is now well recognized and an ICD code was created for Takotsubo cardiomyopathy in 2007.
There were 72,559 TCM admissions during the study period. When stratified by year, the annual rate of TCM cases rose significantly, from 11.1 to 43.8 per 100,000 hospitalizations from 2007 to 2013.
Although overall in-hospital mortality was 2.5%, it climbed from 1.4% in 2007 to 3.2% in 2013. When compared to patients who did not die during hospitalizations, those who did die were on average older (69.9 vs. 66.4 years; P less than .0001) and had more comorbidities. When a multivariate adjustment was made for baseline clinical risk, the presence of acute kidney injury (AKI), chronic obstructive pulmonary disease (COPD), peripheral artery disease (PAD), pulmonary hypertension, and arrhythmias remained significant predictors of mortality in patients with TCM.
“We found a number of modifiable and nonmodifiable factors that are associated with a significantly increased mortality, and we think clinicians should be aware of these factors when managing this condition,” Dr. Voudris reported.
Of these risk factors, AKI was associated with the greatest increased odds ratio (OR) with a more than fourfold increased risk of death (OR, 4.18; P less than .001). The presence of PAD (OR, 1.87; P less than .0001) and arrhythmia (OR, 1.88; P less than .0001) almost doubled the risk of in-hospital mortality.
Although females represented 89% of the TCM cases collected during the study period, males with comorbid diseases were at particularly high risk of death, an observation that is consistent with previous reports, according to Dr. Voudris. Relative to women, men were more likely to present with unstable hemodynamics and to develop cardiogenic shock.
Although Dr. Voudris acknowledged that the rising number of hospitalizations for TCM is likely due largely to increasing recognition of this condition, he suggested that there may be other contributing factors to the growing incidence, such as the aging of the U.S. population. He noted that the rise in cases persisted throughout the study period when awareness of TCM might be expected to have improved.
“This is still an uncommon disease, but it is important to recognize,” agreed Sachin Kumar, MD, an interventional cardiologist at the University of Texas Health Science Center at Houston. Moderator of the session in which these data were presented, Dr. Kumar indicated that it is important to increase awareness of the condition to accelerate the time to diagnosis and treatment.
Dr. Voudris reported no financial relationships relevant to this study.
SOURCE: Voudris, KV. CRT 2018.
WASHINGTON – Hospitalizations for Takotsubo cardiomyopathy (TCM), a condition that primarily affects postmenopausal women, have been rising steadily, along with rates of in-hospital mortality due to this condition, according to a study conducted with outcomes from the National Inpatient Sample (NIS) database.
The study was presented at CRT 2018, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Importantly, a multivariate analysis of predictors of in-hospital mortality based on this database revealed that there were “several potentially reversible causes,” reported Konstantinos V. Voudris, MD, PhD, who is completing a residency in internal medicine at the University of Illinois at Chicago.
TCM, which is characterized by left ventricular apical akinesis and chest pain that mimics the features of acute coronary syndromes (ACS), was first described in 1990 in Japan. The first U.S. case was reported in 1998, but the condition is now well recognized and an ICD code was created for Takotsubo cardiomyopathy in 2007.
There were 72,559 TCM admissions during the study period. When stratified by year, the annual rate of TCM cases rose significantly, from 11.1 to 43.8 per 100,000 hospitalizations from 2007 to 2013.
Although overall in-hospital mortality was 2.5%, it climbed from 1.4% in 2007 to 3.2% in 2013. When compared to patients who did not die during hospitalizations, those who did die were on average older (69.9 vs. 66.4 years; P less than .0001) and had more comorbidities. When a multivariate adjustment was made for baseline clinical risk, the presence of acute kidney injury (AKI), chronic obstructive pulmonary disease (COPD), peripheral artery disease (PAD), pulmonary hypertension, and arrhythmias remained significant predictors of mortality in patients with TCM.
“We found a number of modifiable and nonmodifiable factors that are associated with a significantly increased mortality, and we think clinicians should be aware of these factors when managing this condition,” Dr. Voudris reported.
Of these risk factors, AKI was associated with the greatest increased odds ratio (OR) with a more than fourfold increased risk of death (OR, 4.18; P less than .001). The presence of PAD (OR, 1.87; P less than .0001) and arrhythmia (OR, 1.88; P less than .0001) almost doubled the risk of in-hospital mortality.
Although females represented 89% of the TCM cases collected during the study period, males with comorbid diseases were at particularly high risk of death, an observation that is consistent with previous reports, according to Dr. Voudris. Relative to women, men were more likely to present with unstable hemodynamics and to develop cardiogenic shock.
Although Dr. Voudris acknowledged that the rising number of hospitalizations for TCM is likely due largely to increasing recognition of this condition, he suggested that there may be other contributing factors to the growing incidence, such as the aging of the U.S. population. He noted that the rise in cases persisted throughout the study period when awareness of TCM might be expected to have improved.
“This is still an uncommon disease, but it is important to recognize,” agreed Sachin Kumar, MD, an interventional cardiologist at the University of Texas Health Science Center at Houston. Moderator of the session in which these data were presented, Dr. Kumar indicated that it is important to increase awareness of the condition to accelerate the time to diagnosis and treatment.
Dr. Voudris reported no financial relationships relevant to this study.
SOURCE: Voudris, KV. CRT 2018.
REPORTING FROM CRT 2018
Key clinical point: The Takotsubo cardiomyopathy hospitalization and mortality rates have increased in the United States, according to national data.
Major finding: From 2007 to 2013, Takotsubo cases rose from 11.1 to 43.8 cases per 100,000 hospitalizations and in-hospital mortality more than doubled.
Data source: Retrospective national database analysis.
Disclosures: Dr. Voudris reports no financial relationships relevant to this study.
Source: Voudris, KV. CRT 2018.
The Science Behind Monoclonal Antibodies: What Neurologists Need to Know
Click Here to Read the Content.
Topics in this content include:
- Target-related and non-specific toxicity of therapeutic monoclonal antibodies (mAbs)
- Research in antibody-based therapeutics
- Differences between therapeutic mAbs and small-molecule therapies
Click Here to Read the Content.
Topics in this content include:
- Target-related and non-specific toxicity of therapeutic monoclonal antibodies (mAbs)
- Research in antibody-based therapeutics
- Differences between therapeutic mAbs and small-molecule therapies
Click Here to Read the Content.
Topics in this content include:
- Target-related and non-specific toxicity of therapeutic monoclonal antibodies (mAbs)
- Research in antibody-based therapeutics
- Differences between therapeutic mAbs and small-molecule therapies