User login
Study using U.K. data quantifies infection risk associated with psoriasis
Psoriasis was linked to increased risk of serious infection, with more severe disease associated with increased infection risk, in a study that used electronic medical records of patients in the United Kingdom.
The most common serious infections were lower respiratory tract, skin and soft tissue, and upper respiratory tract infections; and the most common opportunistic infection was tuberculosis, reported Junko Takeshita, MD, PhD, of the departments of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, and her coauthors.
They identified 199,700 psoriasis patients and 954,315 healthy patients from THIN (the Health Improvement Network), a medical records database in the United Kingdom. Of the psoriasis patients, 187,258 had mild disease and 12,442 had moderate to severe disease; almost 70% of patients with moderate to severe disease were treated with methotrexate.
Adjusted hazard ratios for serious infection were 1.21 (95% confidence interval, 1.18-1.23) for psoriasis patients overall, 1.18 (95% confidence interval, 1.16-1.21) for those with mild psoriasis, and 1.63 (95% CI, 1.52-1.75) for those with moderate to severe psoriasis, Dr. Takeshita and her coauthors wrote in the Journal of Investigative Dermatology.
Among all psoriasis patients, the attributable risk of serious infection was 16.2 per 10,000 person-years, compared with 14.4 per 10,000 person-years among those with mild psoriasis, and 49.5 per 10,000 person-years, among those with moderate to severe disease.
The investigators also analyzed data from a nested cohort – the iHOPE (Incident Health Outcomes and Psoriasis Events) study – of 8,569 psoriasis patients, with mild (less than 3% of body surface area involvement) or moderate to severe disease (3% or greater BSA), and 83,540 matched patients without psoriasis.
The adjusted HR for serious infection was 1.21 (95% CI, 1.09-1.35) for all psoriasis patients, 1.16 (95% CI, 0.99-1.35) for those with mild disease, and 1.27 (95% CI, 1.10-1.47) for those with moderate to severe disease. When patients who had received immunosuppressive treatment were excluded from the analysis, hazard ratios were similar among the different psoriasis groups, at 1.18 for all psoriasis patients (95% CI, 1.05-1.32), 1.15 among those with mild disease (95% CI, 0.99-1.34), and 1.21 for those with moderate to severe disease (95% CI, 1.03-1.42).
“Importantly, the risk of serious infection was observed to be similar in both the full THIN and iHOPE cohorts with the exception of the moderate to severe psoriasis subgroup among whom the risk of serious infection was attenuated but still significantly elevated in the iHOPE versus full THIN cohort,” they observed.
In the THIN cohort, the most common opportunistic infection “by far” was tuberculosis, with incidence rates of 1.05, 0.94, and 3.00 per 10,000 person-years among all psoriasis patients, patients with mild disease, and patients with moderate to severe disease, respectively, compared with 1.15 for those without psoriasis.
Patients with moderate to severe disease had an increased risk of opportunistic infection (HR, 1.57; 95% CI, 1.06-2.34), but rates were similar among those with mild disease and those without psoriasis, Dr. Takeshita and her colleagues reported. But the opportunistic infection risk was “substantially attenuated” when patients who had received immunosuppressive treatment were excluded (HR, 1.17; 95% CI, 0.44-3.12).
Patients with moderate to severe disease also had the greatest risk of herpes zoster (HR, 1.17; 95% CI, 1.06-1.30). While the increased risk of herpes zoster was smaller in patients with mild psoriasis, it was still significant (HR, 1.07; 95% CI, 1.05-1.10). Again, when exclusion of patients who had received immunosuppressive therapies, the risk for herpes zoster associated with moderate to severe psoriasis no longer was elevated (HR, 0.97; 95% CI, 0.76-1.23).
“Our findings suggest that psoriasis is associated with an increased risk of serious infection, and more severe psoriasis, whether defined by treatment pattern or by BSA involvement, is a predictor of greater serious infection risk,” the authors wrote. Clinicians should ensure that patients, especially those with severe disease and those who receive immunosuppressive treatment, are vaccinated against influenza and pneumonia, and “should also consider herpes zoster vaccination with the new nonlive vaccine.”
“Future studies will be important to further characterize the risk of various infections among patients with psoriasis, compare the risk of infection associated with psoriasis to that of other chronic diseases, and delineate the pathophysiologic mechanisms that contribute to the increased risk of infections associated with psoriasis and its therapies,” they concluded.
The study was funded by an unrestricted Pfizer grant. Dr. Takeshita has received a research grant (to the Trustees of the University of Pennsylvania) from Pfizer for unrelated work payment for continuing medical education work related to psoriasis supported indirectly by Eli Lilly and Novartis. Other authors’ disclosures included servings as a consultant for Bristol-Myers Squibb, Novartis, Pfizer, Coherus, and other pharmaceutical companies.
[email protected]
SOURCE: Takeshita J et al. J Invest Dermatol. 2018 Mar 2. doi: 10.1016/j.jid.2018.01.039.
Psoriasis was linked to increased risk of serious infection, with more severe disease associated with increased infection risk, in a study that used electronic medical records of patients in the United Kingdom.
The most common serious infections were lower respiratory tract, skin and soft tissue, and upper respiratory tract infections; and the most common opportunistic infection was tuberculosis, reported Junko Takeshita, MD, PhD, of the departments of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, and her coauthors.
They identified 199,700 psoriasis patients and 954,315 healthy patients from THIN (the Health Improvement Network), a medical records database in the United Kingdom. Of the psoriasis patients, 187,258 had mild disease and 12,442 had moderate to severe disease; almost 70% of patients with moderate to severe disease were treated with methotrexate.
Adjusted hazard ratios for serious infection were 1.21 (95% confidence interval, 1.18-1.23) for psoriasis patients overall, 1.18 (95% confidence interval, 1.16-1.21) for those with mild psoriasis, and 1.63 (95% CI, 1.52-1.75) for those with moderate to severe psoriasis, Dr. Takeshita and her coauthors wrote in the Journal of Investigative Dermatology.
Among all psoriasis patients, the attributable risk of serious infection was 16.2 per 10,000 person-years, compared with 14.4 per 10,000 person-years among those with mild psoriasis, and 49.5 per 10,000 person-years, among those with moderate to severe disease.
The investigators also analyzed data from a nested cohort – the iHOPE (Incident Health Outcomes and Psoriasis Events) study – of 8,569 psoriasis patients, with mild (less than 3% of body surface area involvement) or moderate to severe disease (3% or greater BSA), and 83,540 matched patients without psoriasis.
The adjusted HR for serious infection was 1.21 (95% CI, 1.09-1.35) for all psoriasis patients, 1.16 (95% CI, 0.99-1.35) for those with mild disease, and 1.27 (95% CI, 1.10-1.47) for those with moderate to severe disease. When patients who had received immunosuppressive treatment were excluded from the analysis, hazard ratios were similar among the different psoriasis groups, at 1.18 for all psoriasis patients (95% CI, 1.05-1.32), 1.15 among those with mild disease (95% CI, 0.99-1.34), and 1.21 for those with moderate to severe disease (95% CI, 1.03-1.42).
“Importantly, the risk of serious infection was observed to be similar in both the full THIN and iHOPE cohorts with the exception of the moderate to severe psoriasis subgroup among whom the risk of serious infection was attenuated but still significantly elevated in the iHOPE versus full THIN cohort,” they observed.
In the THIN cohort, the most common opportunistic infection “by far” was tuberculosis, with incidence rates of 1.05, 0.94, and 3.00 per 10,000 person-years among all psoriasis patients, patients with mild disease, and patients with moderate to severe disease, respectively, compared with 1.15 for those without psoriasis.
Patients with moderate to severe disease had an increased risk of opportunistic infection (HR, 1.57; 95% CI, 1.06-2.34), but rates were similar among those with mild disease and those without psoriasis, Dr. Takeshita and her colleagues reported. But the opportunistic infection risk was “substantially attenuated” when patients who had received immunosuppressive treatment were excluded (HR, 1.17; 95% CI, 0.44-3.12).
Patients with moderate to severe disease also had the greatest risk of herpes zoster (HR, 1.17; 95% CI, 1.06-1.30). While the increased risk of herpes zoster was smaller in patients with mild psoriasis, it was still significant (HR, 1.07; 95% CI, 1.05-1.10). Again, when exclusion of patients who had received immunosuppressive therapies, the risk for herpes zoster associated with moderate to severe psoriasis no longer was elevated (HR, 0.97; 95% CI, 0.76-1.23).
“Our findings suggest that psoriasis is associated with an increased risk of serious infection, and more severe psoriasis, whether defined by treatment pattern or by BSA involvement, is a predictor of greater serious infection risk,” the authors wrote. Clinicians should ensure that patients, especially those with severe disease and those who receive immunosuppressive treatment, are vaccinated against influenza and pneumonia, and “should also consider herpes zoster vaccination with the new nonlive vaccine.”
“Future studies will be important to further characterize the risk of various infections among patients with psoriasis, compare the risk of infection associated with psoriasis to that of other chronic diseases, and delineate the pathophysiologic mechanisms that contribute to the increased risk of infections associated with psoriasis and its therapies,” they concluded.
The study was funded by an unrestricted Pfizer grant. Dr. Takeshita has received a research grant (to the Trustees of the University of Pennsylvania) from Pfizer for unrelated work payment for continuing medical education work related to psoriasis supported indirectly by Eli Lilly and Novartis. Other authors’ disclosures included servings as a consultant for Bristol-Myers Squibb, Novartis, Pfizer, Coherus, and other pharmaceutical companies.
[email protected]
SOURCE: Takeshita J et al. J Invest Dermatol. 2018 Mar 2. doi: 10.1016/j.jid.2018.01.039.
Psoriasis was linked to increased risk of serious infection, with more severe disease associated with increased infection risk, in a study that used electronic medical records of patients in the United Kingdom.
The most common serious infections were lower respiratory tract, skin and soft tissue, and upper respiratory tract infections; and the most common opportunistic infection was tuberculosis, reported Junko Takeshita, MD, PhD, of the departments of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, and her coauthors.
They identified 199,700 psoriasis patients and 954,315 healthy patients from THIN (the Health Improvement Network), a medical records database in the United Kingdom. Of the psoriasis patients, 187,258 had mild disease and 12,442 had moderate to severe disease; almost 70% of patients with moderate to severe disease were treated with methotrexate.
Adjusted hazard ratios for serious infection were 1.21 (95% confidence interval, 1.18-1.23) for psoriasis patients overall, 1.18 (95% confidence interval, 1.16-1.21) for those with mild psoriasis, and 1.63 (95% CI, 1.52-1.75) for those with moderate to severe psoriasis, Dr. Takeshita and her coauthors wrote in the Journal of Investigative Dermatology.
Among all psoriasis patients, the attributable risk of serious infection was 16.2 per 10,000 person-years, compared with 14.4 per 10,000 person-years among those with mild psoriasis, and 49.5 per 10,000 person-years, among those with moderate to severe disease.
The investigators also analyzed data from a nested cohort – the iHOPE (Incident Health Outcomes and Psoriasis Events) study – of 8,569 psoriasis patients, with mild (less than 3% of body surface area involvement) or moderate to severe disease (3% or greater BSA), and 83,540 matched patients without psoriasis.
The adjusted HR for serious infection was 1.21 (95% CI, 1.09-1.35) for all psoriasis patients, 1.16 (95% CI, 0.99-1.35) for those with mild disease, and 1.27 (95% CI, 1.10-1.47) for those with moderate to severe disease. When patients who had received immunosuppressive treatment were excluded from the analysis, hazard ratios were similar among the different psoriasis groups, at 1.18 for all psoriasis patients (95% CI, 1.05-1.32), 1.15 among those with mild disease (95% CI, 0.99-1.34), and 1.21 for those with moderate to severe disease (95% CI, 1.03-1.42).
“Importantly, the risk of serious infection was observed to be similar in both the full THIN and iHOPE cohorts with the exception of the moderate to severe psoriasis subgroup among whom the risk of serious infection was attenuated but still significantly elevated in the iHOPE versus full THIN cohort,” they observed.
In the THIN cohort, the most common opportunistic infection “by far” was tuberculosis, with incidence rates of 1.05, 0.94, and 3.00 per 10,000 person-years among all psoriasis patients, patients with mild disease, and patients with moderate to severe disease, respectively, compared with 1.15 for those without psoriasis.
Patients with moderate to severe disease had an increased risk of opportunistic infection (HR, 1.57; 95% CI, 1.06-2.34), but rates were similar among those with mild disease and those without psoriasis, Dr. Takeshita and her colleagues reported. But the opportunistic infection risk was “substantially attenuated” when patients who had received immunosuppressive treatment were excluded (HR, 1.17; 95% CI, 0.44-3.12).
Patients with moderate to severe disease also had the greatest risk of herpes zoster (HR, 1.17; 95% CI, 1.06-1.30). While the increased risk of herpes zoster was smaller in patients with mild psoriasis, it was still significant (HR, 1.07; 95% CI, 1.05-1.10). Again, when exclusion of patients who had received immunosuppressive therapies, the risk for herpes zoster associated with moderate to severe psoriasis no longer was elevated (HR, 0.97; 95% CI, 0.76-1.23).
“Our findings suggest that psoriasis is associated with an increased risk of serious infection, and more severe psoriasis, whether defined by treatment pattern or by BSA involvement, is a predictor of greater serious infection risk,” the authors wrote. Clinicians should ensure that patients, especially those with severe disease and those who receive immunosuppressive treatment, are vaccinated against influenza and pneumonia, and “should also consider herpes zoster vaccination with the new nonlive vaccine.”
“Future studies will be important to further characterize the risk of various infections among patients with psoriasis, compare the risk of infection associated with psoriasis to that of other chronic diseases, and delineate the pathophysiologic mechanisms that contribute to the increased risk of infections associated with psoriasis and its therapies,” they concluded.
The study was funded by an unrestricted Pfizer grant. Dr. Takeshita has received a research grant (to the Trustees of the University of Pennsylvania) from Pfizer for unrelated work payment for continuing medical education work related to psoriasis supported indirectly by Eli Lilly and Novartis. Other authors’ disclosures included servings as a consultant for Bristol-Myers Squibb, Novartis, Pfizer, Coherus, and other pharmaceutical companies.
[email protected]
SOURCE: Takeshita J et al. J Invest Dermatol. 2018 Mar 2. doi: 10.1016/j.jid.2018.01.039.
Key clinical point: Psoriasis is linked to risk of serious infection, with increased risk in more severe disease.
Major finding: Hazard ratios for serious infection were 1.21 (95% CI, 1.18-1.23) for psoriasis overall, 1.18 (95% CI, 1.16-1.21) for mild psoriasis, and 1.63 (95% CI, 1.52-1.75) for moderate to severe psoriasis.
Study details: The evaluation included data on 199,700 patients with psoriasis and 954,315 patients without psoriasis in a U.K. electronic medical records database.
Disclosures: The study was funded by an unrestricted Pfizer grant. Dr. Takeshita has received a research grant (to the Trustees of the University of Pennsylvania) from Pfizer for unrelated work payment for continuing medical education work related to psoriasis supported indirectly by Eli Lilly and Novartis. Other authors’ disclosures included servings as a consultant for Bristol-Myers Squibb, Novartis, Pfizer, Coherus, and other pharmaceutical companies.
Source: Takeshita J et al. J Invest Dermatol. 2018 Mar 2. doi: 10.1016/j.jid.2018.01.039.
mainbar
Psoriasis was linked to increased risk of serious infection, with more severe disease associated with increased infection risk, in a study that used electronic medical records of patients in the United Kingdom.
The most common serious infections were lower respiratory tract, skin and soft tissue, and upper respiratory tract infections; and the most common opportunistic infection was tuberculosis, reported Junko Takeshita, MD, PhD, of the departments of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, and her coauthors.
Adjusted hazard ratios for serious infection were 1.21 (95% confidence interval, 1.18-1.23) for psoriasis patients overall, 1.18 (95% confidence interval, 1.16-1.21) for those with mild psoriasis, and 1.63 (95% CI, 1.52-1.75) for those with moderate to severe psoriasis, Dr. Takeshita and her coauthors wrote in the Journal of Investigative Dermatology.
Among all psoriasis patients, the attributable risk of serious infection was 16.2 per 10,000 person-years, compared with 14.4 per 10,000 person-years among those with mild psoriasis, and 49.5 per 10,000 person-years, among those with moderate to severe disease.
The investigators also analyzed data from a nested cohort – the iHOPE (Incident Health Outcomes and Psoriasis Events) study – of 8,569 psoriasis patients, with mild (less than 3% of body surface area involvement) or moderate to severe disease (3% or greater BSA), and 83,540 matched patients without psoriasis. The adjusted HR for serious infection was 1.21 (95% CI, 1.09-1.35) for all psoriasis patients, 1.16 (95% CI, 0.99-1.35) for those with mild disease, and 1.27 (95% CI, 1.10-1.47) for those with moderate to severe disease. When patients who had received immunosuppressive treatment were excluded from the analysis, hazard ratios were similar among the different psoriasis groups, at 1.18 for all psoriasis patients (95% CI, 1.05-1.32), 1.15 among those with mild disease (95% CI, 0.99-1.34), and 1.21 for those with moderate to severe disease (95% CI, 1.03-1.42).
“Importantly, the risk of serious infection was observed to be similar in both the full THIN and iHOPE cohorts with the exception of the moderate to severe psoriasis subgroup among whom the risk of serious infection was attenuated but still significantly elevated in the iHOPE versus full THIN cohort,” they observed.
In the THIN cohort, the most common opportunistic infection “by far” was tuberculosis, with incidence rates of 1.05, 0.94, and 3.00 per 10,000 person-years among all psoriasis patients, patients with mild disease, and patients with moderate to severe disease, respectively, compared with 1.15 for those without psoriasis.
Patients with moderate to severe disease had an increased risk of opportunistic infection (HR, 1.57; 95% CI, 1.06-2.34), but rates were similar among those with mild disease and those without psoriasis, Dr. Takeshita and her colleagues reported. But the opportunistic infection risk was “substantially attenuated” when patients who had received immunosuppressive treatment were excluded (HR, 1.17; 95% CI, 0.44-3.12).
Patients with moderate to severe disease also had the greatest risk of herpes zoster (HR, 1.17; 95% CI, 1.06-1.30). While the increased risk of herpes zoster was smaller in patients with mild psoriasis, it was still significant (HR, 1.07; 95% CI, 1.05-1.10). Again, when exclusion of patients who had received immunosuppressive therapies, the risk for herpes zoster associated with moderate to severe psoriasis no longer was elevated (HR, 0.97; 95% CI, 0.76-1.23).
“Our findings suggest that psoriasis is associated with an increased risk of serious infection, and more severe psoriasis, whether defined by treatment pattern or by BSA involvement, is a predictor of greater serious infection risk,” the authors wrote. Clinicians should ensure that patients, especially those with severe disease and those who receive immunosuppressive treatment, are vaccinated against influenza and pneumonia, and “should also consider herpes zoster vaccination with the new nonlive vaccine.”
“Future studies will be important to further characterize the risk of various infections among patients with psoriasis, compare the risk of infection associated with psoriasis to that of other chronic diseases, and delineate the pathophysiologic mechanisms that contribute to the increased risk of infections associated with psoriasis and its therapies,” they concluded.
The study was funded by an unrestricted Pfizer grant. Dr. Takeshita has received a research grant (to the Trustees of the University of Pennsylvania) from Pfizer for unrelated work payment for continuing medical education work related to psoriasis supported indirectly by Eli Lilly and Novartis. Other authors’ disclosures included servings as a consultant for Bristol-Myers Squibb, Novartis, Pfizer, Coherus, and other pharmaceutical companies.
SOURCE: Takeshita J et al. J Invest Dermatol. 2018 Mar 2. doi: 10.1016/j.jid.2018.01.039.
Drug pricing proposals raise red flags with specialists
White House proposals to help lower drug prices are being met with concerns by a group of specialists.
In a March 14 letter to Department of Health & Human Services Secretary Alex Azar, a group of nine specialty medical organizations highlighted four recent proposals that could have an unintended consequence of limiting access.
The specialty groups that signed onto the letter are the American Academy of Dermatology Association, American Academy of Neurology, American Academy of Ophthalmology, American Academy of Physical Medicine and Rehabilitation, American College of Gastroenterology, American College of Rheumatology, American Gastroenterological Association, American Urological Association, and the Infectious Diseases Society of America.
The groups focused on issues pertaining to four proposals highlighted in the White House’s proposed fiscal year 2019 budget and the February 2018 report from the Council of Economic Advisors titled, “Reforming Biopharmaceutical Pricing at Home and Abroad.”
The first proposal relates to changing the requirement that Medicare Part D prescription drug plans cover at least two drugs per category to covering only one as a way to provide more flexibility and potential negotiation power, while at the same time expanding the ability to use utilization management tools.
“We worry this could create access issues for patients on high cost biologic medications,” the groups said in the letter. “We believe Part D benefits should not limit patients’ access to the medical therapy judged by the treating physician to be the most efficacious choice.”
“We worry that moving Part B drugs into Part D may lead to access issues and force patients into higher cost sites of care,” the groups said.
Third, the White House is proposing to cut Part B drug reimbursement to physicians from the current average sales price plus 6% down to ASP+3% for new drugs.
The groups said that with the budget sequestration currently in place, “the existing Part B payment structure does not adequately cover the costs of obtaining and providing these complex therapies in an outpatient setting. If additional payment cuts or negative changes are implemented or activated through demonstration projects, many patients would be forced into more expensive, less convenient settings to receive their therapies – if an alternative setting is available at all in their areas.”
Finally, the groups mention a proposal that would introduce physician reimbursement that is not tied to drug pricing. But this option is not expanded upon in the Council of Economic Advisors report.
“We request more clarity on any potential policies that would affect physician reimbursement,” the groups said. “Physicians have no control over the cost of drugs or ancillary services, nor over the severity of illness and comorbidities that drive the need for such services.”
The groups did support a few of the recommendations from the two documents, including requiring Medicare Part D plans to apply a substantial portion of the rebate at point of sale, establishing a Part D out-of-pocket maximum in the catastrophic phase to better protect beneficiaries against high drug costs, decreasing the consolidation by pharmacy benefit managers and others in the supply chain, and providing the Centers for Medicare & Medicaid Services with guidance on how drug-related value-based contracts and price reporting would affect other price regulations.
“We appreciate HHS’ continued focus on transparency and patient-centered care,” the groups said. “Knowing that HHS is committed to transforming the health care delivery system and the Medicare program by putting a strong focus on patient-centered care, so providers can direct their time and resources to patients and improving outcomes, is reassuring to our providers.”
White House proposals to help lower drug prices are being met with concerns by a group of specialists.
In a March 14 letter to Department of Health & Human Services Secretary Alex Azar, a group of nine specialty medical organizations highlighted four recent proposals that could have an unintended consequence of limiting access.
The specialty groups that signed onto the letter are the American Academy of Dermatology Association, American Academy of Neurology, American Academy of Ophthalmology, American Academy of Physical Medicine and Rehabilitation, American College of Gastroenterology, American College of Rheumatology, American Gastroenterological Association, American Urological Association, and the Infectious Diseases Society of America.
The groups focused on issues pertaining to four proposals highlighted in the White House’s proposed fiscal year 2019 budget and the February 2018 report from the Council of Economic Advisors titled, “Reforming Biopharmaceutical Pricing at Home and Abroad.”
The first proposal relates to changing the requirement that Medicare Part D prescription drug plans cover at least two drugs per category to covering only one as a way to provide more flexibility and potential negotiation power, while at the same time expanding the ability to use utilization management tools.
“We worry this could create access issues for patients on high cost biologic medications,” the groups said in the letter. “We believe Part D benefits should not limit patients’ access to the medical therapy judged by the treating physician to be the most efficacious choice.”
“We worry that moving Part B drugs into Part D may lead to access issues and force patients into higher cost sites of care,” the groups said.
Third, the White House is proposing to cut Part B drug reimbursement to physicians from the current average sales price plus 6% down to ASP+3% for new drugs.
The groups said that with the budget sequestration currently in place, “the existing Part B payment structure does not adequately cover the costs of obtaining and providing these complex therapies in an outpatient setting. If additional payment cuts or negative changes are implemented or activated through demonstration projects, many patients would be forced into more expensive, less convenient settings to receive their therapies – if an alternative setting is available at all in their areas.”
Finally, the groups mention a proposal that would introduce physician reimbursement that is not tied to drug pricing. But this option is not expanded upon in the Council of Economic Advisors report.
“We request more clarity on any potential policies that would affect physician reimbursement,” the groups said. “Physicians have no control over the cost of drugs or ancillary services, nor over the severity of illness and comorbidities that drive the need for such services.”
The groups did support a few of the recommendations from the two documents, including requiring Medicare Part D plans to apply a substantial portion of the rebate at point of sale, establishing a Part D out-of-pocket maximum in the catastrophic phase to better protect beneficiaries against high drug costs, decreasing the consolidation by pharmacy benefit managers and others in the supply chain, and providing the Centers for Medicare & Medicaid Services with guidance on how drug-related value-based contracts and price reporting would affect other price regulations.
“We appreciate HHS’ continued focus on transparency and patient-centered care,” the groups said. “Knowing that HHS is committed to transforming the health care delivery system and the Medicare program by putting a strong focus on patient-centered care, so providers can direct their time and resources to patients and improving outcomes, is reassuring to our providers.”
White House proposals to help lower drug prices are being met with concerns by a group of specialists.
In a March 14 letter to Department of Health & Human Services Secretary Alex Azar, a group of nine specialty medical organizations highlighted four recent proposals that could have an unintended consequence of limiting access.
The specialty groups that signed onto the letter are the American Academy of Dermatology Association, American Academy of Neurology, American Academy of Ophthalmology, American Academy of Physical Medicine and Rehabilitation, American College of Gastroenterology, American College of Rheumatology, American Gastroenterological Association, American Urological Association, and the Infectious Diseases Society of America.
The groups focused on issues pertaining to four proposals highlighted in the White House’s proposed fiscal year 2019 budget and the February 2018 report from the Council of Economic Advisors titled, “Reforming Biopharmaceutical Pricing at Home and Abroad.”
The first proposal relates to changing the requirement that Medicare Part D prescription drug plans cover at least two drugs per category to covering only one as a way to provide more flexibility and potential negotiation power, while at the same time expanding the ability to use utilization management tools.
“We worry this could create access issues for patients on high cost biologic medications,” the groups said in the letter. “We believe Part D benefits should not limit patients’ access to the medical therapy judged by the treating physician to be the most efficacious choice.”
“We worry that moving Part B drugs into Part D may lead to access issues and force patients into higher cost sites of care,” the groups said.
Third, the White House is proposing to cut Part B drug reimbursement to physicians from the current average sales price plus 6% down to ASP+3% for new drugs.
The groups said that with the budget sequestration currently in place, “the existing Part B payment structure does not adequately cover the costs of obtaining and providing these complex therapies in an outpatient setting. If additional payment cuts or negative changes are implemented or activated through demonstration projects, many patients would be forced into more expensive, less convenient settings to receive their therapies – if an alternative setting is available at all in their areas.”
Finally, the groups mention a proposal that would introduce physician reimbursement that is not tied to drug pricing. But this option is not expanded upon in the Council of Economic Advisors report.
“We request more clarity on any potential policies that would affect physician reimbursement,” the groups said. “Physicians have no control over the cost of drugs or ancillary services, nor over the severity of illness and comorbidities that drive the need for such services.”
The groups did support a few of the recommendations from the two documents, including requiring Medicare Part D plans to apply a substantial portion of the rebate at point of sale, establishing a Part D out-of-pocket maximum in the catastrophic phase to better protect beneficiaries against high drug costs, decreasing the consolidation by pharmacy benefit managers and others in the supply chain, and providing the Centers for Medicare & Medicaid Services with guidance on how drug-related value-based contracts and price reporting would affect other price regulations.
“We appreciate HHS’ continued focus on transparency and patient-centered care,” the groups said. “Knowing that HHS is committed to transforming the health care delivery system and the Medicare program by putting a strong focus on patient-centered care, so providers can direct their time and resources to patients and improving outcomes, is reassuring to our providers.”
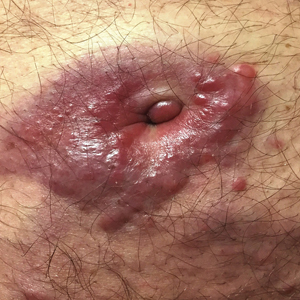
Violaceous Plaques and Papulonodules on the Umbilicus
The Diagnosis: Cutaneous Deposits Of Myeloma
Cutaneous deposits of myeloma are a rare skin manifestation of multiple myeloma that typically occur in less than 5% of patients.1,2 The lesions represent monoclonal proliferations of plasma cells and arise from direct extension of a neoplastic mass or less commonly from hematogenous or lymphatic spread. This secondary cutaneous involvement by plasma cell myeloma has been referred to in the literature as metastatic or extramedullary cutaneous plasmacytoma.1,2 This condition must be distinguished from cutaneous plasma cell infiltrates without underlying bone marrow involvement, classified by the World Health Organization as primary cutaneous marginal zone B-cell lymphoma and previously referred to as primary cutaneous plasmacytoma.3
Clinically, cutaneous deposits of myeloma manifest as erythematous to violaceous papules, plaques, or nodules with a smooth surface and firm consistency.1,2 The lesions typically occur on the trunk and less commonly on the head, neck, arms, and legs. In a review of 83 cases of metastatic cutaneous plasmacytoma and primary cutaneous plasmacytoma in multiple myeloma, Kato et al4 found that 52% (43/83) of cases occurred in IgG myelomas and 23% (19/83) in IgA myelomas.
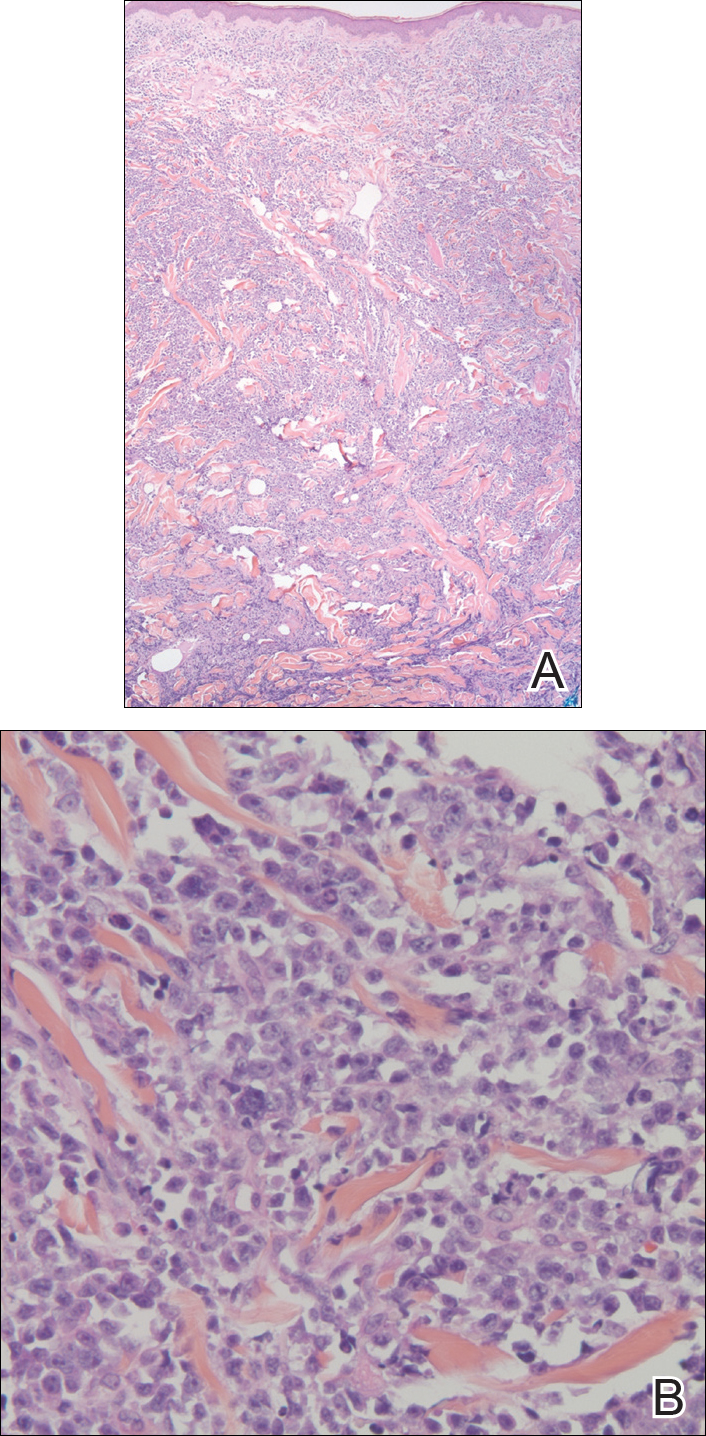
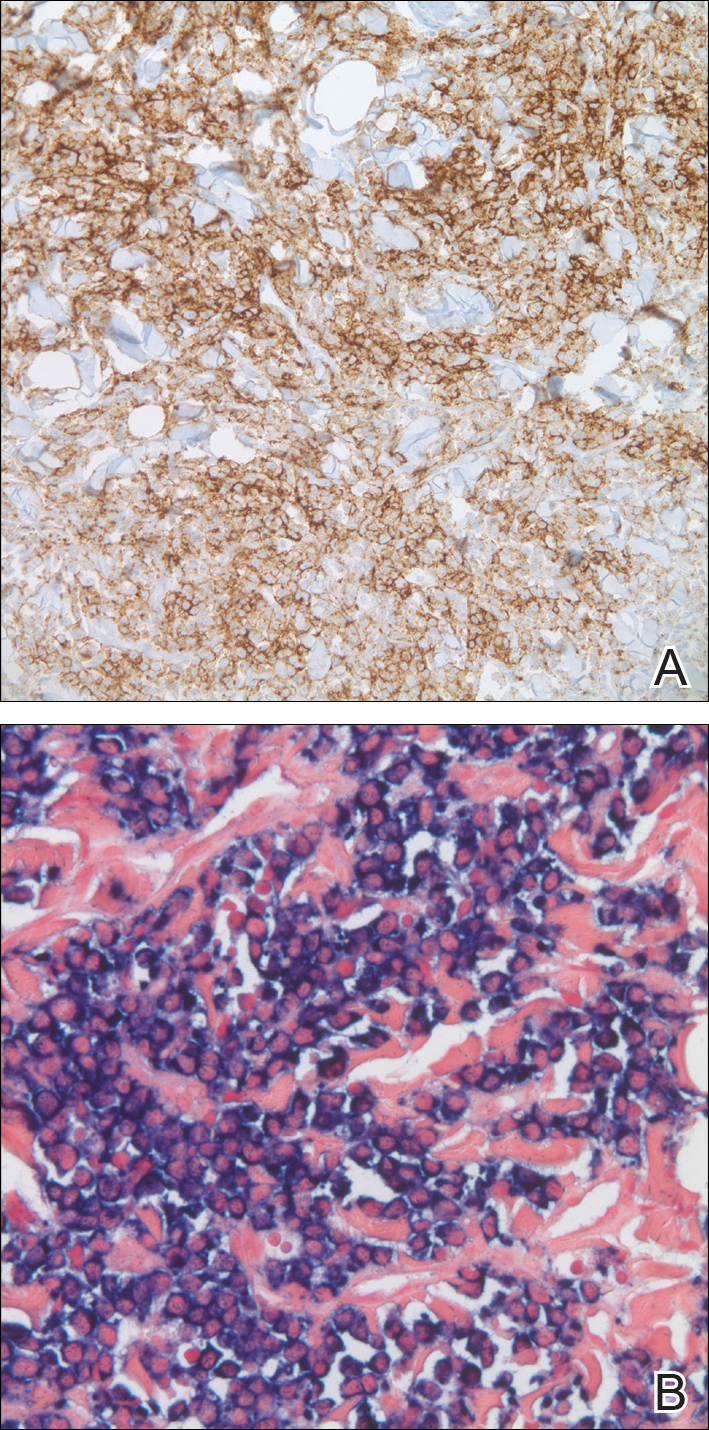
In our patient, a 4-mm punch biopsy of an umbilical plaque demonstrated a dense infiltrate of atypical plasmacytoid cells through the full thickness of the dermis with nuclear pleomorphism, prominent nucleoli, and frequent mitoses (Figure 1). Immunohistochemical staining was positive for IgA λ light chain (Figure 2A) and CD138 (Figure 2B) and was negative for CD20, which was consistent with the patient's known plasma cell myeloma. Positron emission tomography revealed progression of underlying disease compared to prior studies with hypermetabolic mediastinal, retroperitoneal, and pelvic side wall lymphadenopathy, as well as extensive hypermetabolic soft tissue masses with involvement of the periumbilical region.
The differential diagnosis for violaceous periumbilical plaques includes cutaneous marginal zone B-cell lymphoma (primary or secondary) or T-cell lymphoma (primary or secondary), cutaneous metastases from solid organ or hematologic malignancies (eg, Sister Mary Joseph nodule), AIDS-associated Kaposi sarcoma (plum-colored plaques that may be extensive), and cutaneous endometriosis (umbilical nodules that may develop in women after surgical excision of endometrial tissue).
The mainstay of therapy for secondary cutaneous involvement of plasma cell myeloma includes treatment with chemotherapy and local radiotherapy.1,2,5 After the diagnosis of cutaneous deposits of myeloma was made in our patient, he was treated with bortezomib, cyclophosphamide with dexamethasone, and local radiotherapy to symptomatic bony lesions; however, he was unresponsive to therapy and the disease progressed with numerous extramedullary lesions of the mediastinum, gastrointestinal tract, and retroperitoneum 2 months later. The patient developed hydronephrosis from external renal compression necessitating nephrostomy tube and malignant pleural effusions requiring intubation. He experienced rapid clinical decline and died 3 months after the initial presentation due to multiorgan failure.
Cutaneous deposits of myeloma are a sign of underlying disease progression in plasma cell myeloma and often herald a fulminant course (eg, death within 12 months of presentation), as seen in our patient.5 Clinicians should be aware of this rare manifestation of plasma cell myeloma and pursue aggressive therapy given the poor prognostic nature of these cutaneous findings.
- Jorizzo JL, Gammon WR, Briggaman RA. Cutaneous plasmacytomas: a review and presentation of an unusual case. J Am Acad Dermatol. 1979;1:59-66.
- Bayer-Garner IB, Smoller BR. The spectrum of cutaneous disease in multiple myeloma. J Am Acad Dermatol. 2003;48:497-507.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kato N, Kimura K, Yasukawa K, et al. Metastatic cutaneous plasmacytoma: a case report associated with IgA lambda multiple myeloma and a review of the literature of metastatic cutaneous plasmacytomas associated with multiple myeloma and primary cutaneous plasmacytomas. J Dermatol. 1999;26:587-594.
- Sanal SM, Yaylaci M, Mangold KA, et al. Extensive extramedullary disease in myeloma. an uncommon variant with features of poor prognosis and dedifferentiation. Cancer. 1996;77:1298-1302.
The Diagnosis: Cutaneous Deposits Of Myeloma
Cutaneous deposits of myeloma are a rare skin manifestation of multiple myeloma that typically occur in less than 5% of patients.1,2 The lesions represent monoclonal proliferations of plasma cells and arise from direct extension of a neoplastic mass or less commonly from hematogenous or lymphatic spread. This secondary cutaneous involvement by plasma cell myeloma has been referred to in the literature as metastatic or extramedullary cutaneous plasmacytoma.1,2 This condition must be distinguished from cutaneous plasma cell infiltrates without underlying bone marrow involvement, classified by the World Health Organization as primary cutaneous marginal zone B-cell lymphoma and previously referred to as primary cutaneous plasmacytoma.3
Clinically, cutaneous deposits of myeloma manifest as erythematous to violaceous papules, plaques, or nodules with a smooth surface and firm consistency.1,2 The lesions typically occur on the trunk and less commonly on the head, neck, arms, and legs. In a review of 83 cases of metastatic cutaneous plasmacytoma and primary cutaneous plasmacytoma in multiple myeloma, Kato et al4 found that 52% (43/83) of cases occurred in IgG myelomas and 23% (19/83) in IgA myelomas.
In our patient, a 4-mm punch biopsy of an umbilical plaque demonstrated a dense infiltrate of atypical plasmacytoid cells through the full thickness of the dermis with nuclear pleomorphism, prominent nucleoli, and frequent mitoses (Figure 1). Immunohistochemical staining was positive for IgA λ light chain (Figure 2A) and CD138 (Figure 2B) and was negative for CD20, which was consistent with the patient's known plasma cell myeloma. Positron emission tomography revealed progression of underlying disease compared to prior studies with hypermetabolic mediastinal, retroperitoneal, and pelvic side wall lymphadenopathy, as well as extensive hypermetabolic soft tissue masses with involvement of the periumbilical region.


The differential diagnosis for violaceous periumbilical plaques includes cutaneous marginal zone B-cell lymphoma (primary or secondary) or T-cell lymphoma (primary or secondary), cutaneous metastases from solid organ or hematologic malignancies (eg, Sister Mary Joseph nodule), AIDS-associated Kaposi sarcoma (plum-colored plaques that may be extensive), and cutaneous endometriosis (umbilical nodules that may develop in women after surgical excision of endometrial tissue).
The mainstay of therapy for secondary cutaneous involvement of plasma cell myeloma includes treatment with chemotherapy and local radiotherapy.1,2,5 After the diagnosis of cutaneous deposits of myeloma was made in our patient, he was treated with bortezomib, cyclophosphamide with dexamethasone, and local radiotherapy to symptomatic bony lesions; however, he was unresponsive to therapy and the disease progressed with numerous extramedullary lesions of the mediastinum, gastrointestinal tract, and retroperitoneum 2 months later. The patient developed hydronephrosis from external renal compression necessitating nephrostomy tube and malignant pleural effusions requiring intubation. He experienced rapid clinical decline and died 3 months after the initial presentation due to multiorgan failure.
Cutaneous deposits of myeloma are a sign of underlying disease progression in plasma cell myeloma and often herald a fulminant course (eg, death within 12 months of presentation), as seen in our patient.5 Clinicians should be aware of this rare manifestation of plasma cell myeloma and pursue aggressive therapy given the poor prognostic nature of these cutaneous findings.
The Diagnosis: Cutaneous Deposits Of Myeloma
Cutaneous deposits of myeloma are a rare skin manifestation of multiple myeloma that typically occur in less than 5% of patients.1,2 The lesions represent monoclonal proliferations of plasma cells and arise from direct extension of a neoplastic mass or less commonly from hematogenous or lymphatic spread. This secondary cutaneous involvement by plasma cell myeloma has been referred to in the literature as metastatic or extramedullary cutaneous plasmacytoma.1,2 This condition must be distinguished from cutaneous plasma cell infiltrates without underlying bone marrow involvement, classified by the World Health Organization as primary cutaneous marginal zone B-cell lymphoma and previously referred to as primary cutaneous plasmacytoma.3
Clinically, cutaneous deposits of myeloma manifest as erythematous to violaceous papules, plaques, or nodules with a smooth surface and firm consistency.1,2 The lesions typically occur on the trunk and less commonly on the head, neck, arms, and legs. In a review of 83 cases of metastatic cutaneous plasmacytoma and primary cutaneous plasmacytoma in multiple myeloma, Kato et al4 found that 52% (43/83) of cases occurred in IgG myelomas and 23% (19/83) in IgA myelomas.
In our patient, a 4-mm punch biopsy of an umbilical plaque demonstrated a dense infiltrate of atypical plasmacytoid cells through the full thickness of the dermis with nuclear pleomorphism, prominent nucleoli, and frequent mitoses (Figure 1). Immunohistochemical staining was positive for IgA λ light chain (Figure 2A) and CD138 (Figure 2B) and was negative for CD20, which was consistent with the patient's known plasma cell myeloma. Positron emission tomography revealed progression of underlying disease compared to prior studies with hypermetabolic mediastinal, retroperitoneal, and pelvic side wall lymphadenopathy, as well as extensive hypermetabolic soft tissue masses with involvement of the periumbilical region.


The differential diagnosis for violaceous periumbilical plaques includes cutaneous marginal zone B-cell lymphoma (primary or secondary) or T-cell lymphoma (primary or secondary), cutaneous metastases from solid organ or hematologic malignancies (eg, Sister Mary Joseph nodule), AIDS-associated Kaposi sarcoma (plum-colored plaques that may be extensive), and cutaneous endometriosis (umbilical nodules that may develop in women after surgical excision of endometrial tissue).
The mainstay of therapy for secondary cutaneous involvement of plasma cell myeloma includes treatment with chemotherapy and local radiotherapy.1,2,5 After the diagnosis of cutaneous deposits of myeloma was made in our patient, he was treated with bortezomib, cyclophosphamide with dexamethasone, and local radiotherapy to symptomatic bony lesions; however, he was unresponsive to therapy and the disease progressed with numerous extramedullary lesions of the mediastinum, gastrointestinal tract, and retroperitoneum 2 months later. The patient developed hydronephrosis from external renal compression necessitating nephrostomy tube and malignant pleural effusions requiring intubation. He experienced rapid clinical decline and died 3 months after the initial presentation due to multiorgan failure.
Cutaneous deposits of myeloma are a sign of underlying disease progression in plasma cell myeloma and often herald a fulminant course (eg, death within 12 months of presentation), as seen in our patient.5 Clinicians should be aware of this rare manifestation of plasma cell myeloma and pursue aggressive therapy given the poor prognostic nature of these cutaneous findings.
- Jorizzo JL, Gammon WR, Briggaman RA. Cutaneous plasmacytomas: a review and presentation of an unusual case. J Am Acad Dermatol. 1979;1:59-66.
- Bayer-Garner IB, Smoller BR. The spectrum of cutaneous disease in multiple myeloma. J Am Acad Dermatol. 2003;48:497-507.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kato N, Kimura K, Yasukawa K, et al. Metastatic cutaneous plasmacytoma: a case report associated with IgA lambda multiple myeloma and a review of the literature of metastatic cutaneous plasmacytomas associated with multiple myeloma and primary cutaneous plasmacytomas. J Dermatol. 1999;26:587-594.
- Sanal SM, Yaylaci M, Mangold KA, et al. Extensive extramedullary disease in myeloma. an uncommon variant with features of poor prognosis and dedifferentiation. Cancer. 1996;77:1298-1302.
- Jorizzo JL, Gammon WR, Briggaman RA. Cutaneous plasmacytomas: a review and presentation of an unusual case. J Am Acad Dermatol. 1979;1:59-66.
- Bayer-Garner IB, Smoller BR. The spectrum of cutaneous disease in multiple myeloma. J Am Acad Dermatol. 2003;48:497-507.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kato N, Kimura K, Yasukawa K, et al. Metastatic cutaneous plasmacytoma: a case report associated with IgA lambda multiple myeloma and a review of the literature of metastatic cutaneous plasmacytomas associated with multiple myeloma and primary cutaneous plasmacytomas. J Dermatol. 1999;26:587-594.
- Sanal SM, Yaylaci M, Mangold KA, et al. Extensive extramedullary disease in myeloma. an uncommon variant with features of poor prognosis and dedifferentiation. Cancer. 1996;77:1298-1302.
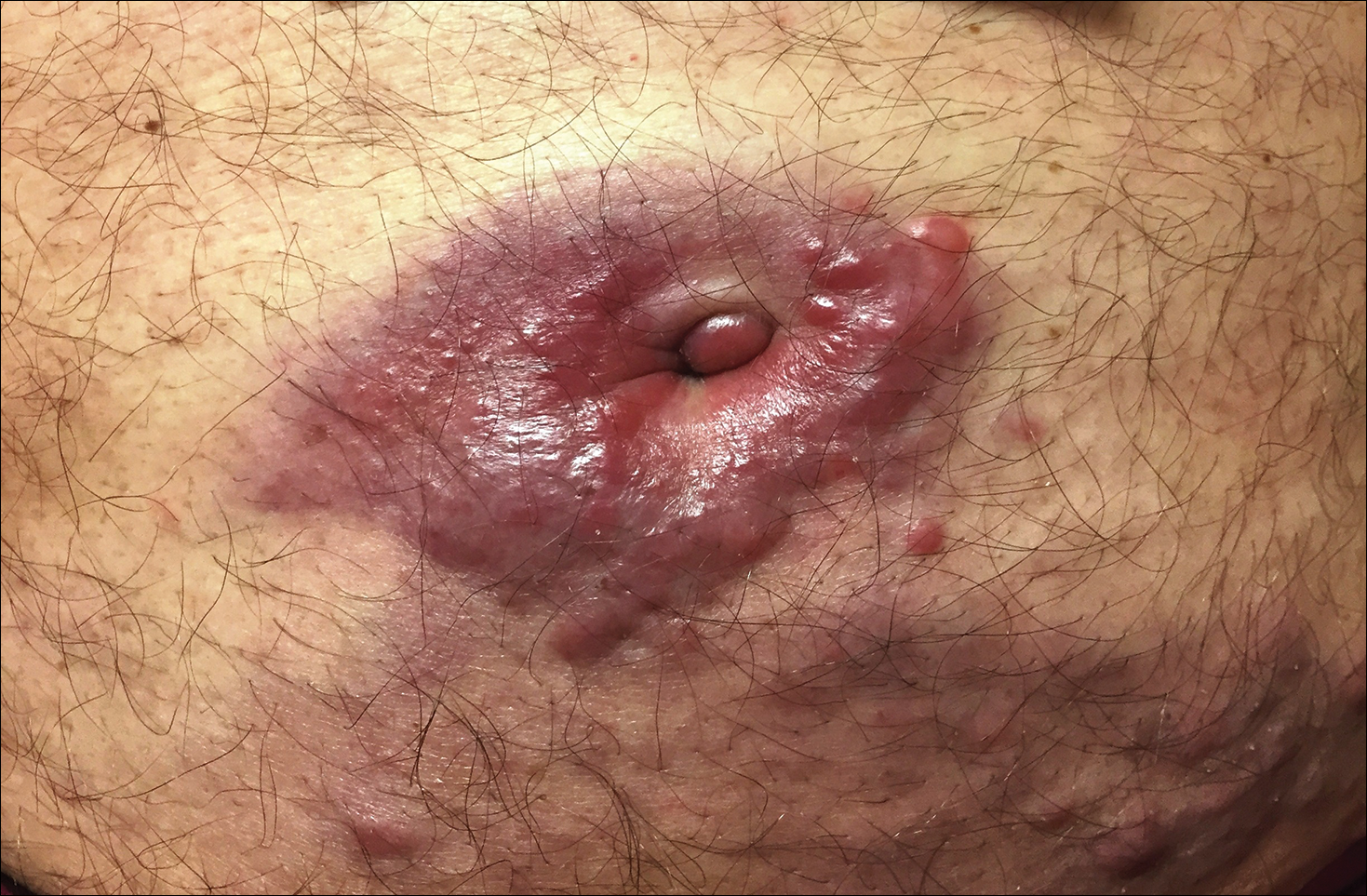
A 75-year-old man presented for evaluation of lesions on the umbilicus and lower abdomen that had developed over the past 4 weeks and were asymptomatic. His medical history was notable for plasma cell myeloma (stage III, IgA λ light chain restricted), deep vein thrombosis, and a 30-year history of smoking (20 packs per year). On physical examination, violaceous plaques and papulonodules were noted on the umbilicus. The lesions had a firm consistency and smooth surface without epidermal change. Violaceous papulonodules and subcutaneous plaques were noted on the lower abdomen. The lesions were nontender to palpation. Bilateral edema of the legs also was noted. The remainder of the skin was normal and there was no cervical, axillary, or inguinal lymphadenopathy.
Focusing on Inattention: The Diagnostic Accuracy of Brief Measures of Inattention for Detecting Delirium
Delirium is an acute neurocognitive disorder1 that affects up to 25% of older emergency department (ED) and hospitalized patients.2-4 The relationship between delirium and adverse outcomes is well documented.5-7 Delirium is a strong predictor of increased length of mechanical ventilation, longer intensive care unit and hospital stays, increased risk of falls, long-term cognitive impairment, and mortality.8-13 Delirium is frequently missed by healthcare professionals2,14-16 and goes undetected in up to 3 out of 4 patients by bedside nurses and medical practitioners in many hospital settings.14,17-22 A significant barrier to recognizing delirium is the absence of brief delirium assessments.
In an effort to improve delirium recognition in the acute care setting, there has been a concerted effort to develop and validate brief delirium assessments. To address this unmet need, 4 ‘A’s Test (4AT), the Brief Confusion Assessment Method (bCAM), and the 3-minute diagnostic assessment for CAM-defined delirium (3D-CAM) are 1- to 3-minute delirium assessments that were validated in acutely ill older patients.23 However, 1 to 3 minutes may still be too long in busy clinical environments, and briefer (<30 seconds) delirium assessments may be needed.
One potential more-rapid method to screen for delirium is to specifically test for the presence of inattention, which is a cardinal feature of delirium.24,25 Inattention can be ascertained by having the patient recite the months backwards, recite the days of the week backwards, or spell a word backwards.26 Recent studies have evaluated the diagnostic accuracy of reciting the months of the year backwards for delirium. O’Regan et al.27 evaluated the diagnostic accuracy of the month of the year backwards from December to July (MOTYB-6) and observed that this task was 84% sensitive and 90% specific for delirium in older patients. However, they performed the reference standard delirium assessments in patients who had a positive MOTYB-6, which can overestimate sensitivity and underestimate specificity (verification bias).28 Fick et al.29 examined the diagnostic accuracy of 20 individual elements of the 3D-CAM and observed that reciting the months of the year backwards from December to January (MOTYB-12) was 83% sensitive and 69% specific for delirium. However, this was an exploratory study that was designed to identify an element of the 3D-CAM that had the best diagnostic accuracy.
To address these limitations, we sought to evaluate the diagnostic performance of the MOTYB-6 and MOTYB-12 for delirium as diagnosed by a reference standard. We also explored other brief tests of inattention such as spelling a word (“LUNCH”) backwards, reciting the days of the week backwards, 10-letter vigilance “A” task, and 5 picture recognition task.
METHODS
Study Design and Setting
This was a preplanned secondary analysis of a prospective observational study that validated 3 delirium assessments.30,31 This study was conducted at a tertiary care, academic ED. The local institutional review board (IRB) reviewed and approved this study. Informed consent from the patient or an authorized surrogate was obtained whenever possible. Because this was an observational study and posed minimal risk to the patient, the IRB granted a waiver of consent for patients who were both unable to provide consent and were without an authorized surrogate available in the ED or by phone.
Selection of Participants
We enrolled a convenience sample of patients between June 2010 and February 2012 Monday through Friday from 8
Research assistants approached patients who met inclusion criteria and determined if any exclusion criteria were present. If none of the exclusion criteria were present, then the research assistant reviewed the informed consent document with the patient or authorized surrogate if the patient was not capable of providing consent. If a patient was not capable of providing consent and no authorized surrogate was available, then the patient was enrolled (under the waiver of consent) as long as the patient assented to be a part of the study. Once the patient was enrolled, the research assistant contacted the physician rater and reference standard psychiatrists to approach the patient.
Measures of Inattention
An emergency physician (JHH) who had no formal training in the mental status assessment of elders administered a cognitive battery to the patient, including tests of inattention. The following inattention tasks were administered:
- Spell the word “LUNCH” backwards.30 Patients were initially allowed to spell the word “LUNCH” forwards. Patients who were unable to perform the task were assigned 5 errors.
- Recite the months of the year backwards from December to July.23,26,27,30,32 Patients who were unable to perform the task were assigned 6 errors.
- Recite the days of the week backwards.23,26,33 Patients who were unable to perform the task were assigned 7 errors.
- Ten-letter vigilance “A” task.34 The patient was given a series of 10 letters (“S-A-V-E-A-H-A-A-R-T”) every 3 seconds and was asked to squeeze the rater’s hand every time the patient heard the letter “A.” Patients who were unable to perform the task were assigned 10 errors.
- Five picture recognition task.34 Patients were shown 5 objects on picture cards. Afterwards, patients were shown 10 pictures with the previously shown objects intermingled. The patient had to identify which objects were seen previously in the first 5 pictures. Patients who were unable to perform the task were assigned 10 errors.
- Recite the months of the year backwards from December to January.29 Patients who were unable to perform the task were assigned 12 errors.
Reference Standard for Delirium
A comprehensive consultation-liaison psychiatrist assessment was the reference standard for delirium; the diagnosis of delirium was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria.35 Three psychiatrists who each had an average of 11 years of clinical experience and regularly diagnosed delirium as part of their daily clinical practice were available to perform these assessments. To arrive at the diagnosis of delirium, they interviewed those who best understood the patient’s mental status (eg, the patient’s family members or caregivers, physician, and nurses). They also reviewed the patient’s medical record and radiology and laboratory test results. They performed bedside cognitive testing that included, but was not limited to, the Mini-Mental State Examination, Clock Drawing Test, Luria hand sequencing task, and tests for verbal fluency. A focused neurological examination was also performed (ie, screening for paraphasic errors, tremors, tone, asterixis, frontal release signs, etc.), and they also evaluated the patient for affective lability, hallucinations, and level of alertness. If the presence of delirium was still questionable, then confrontational naming, proverb interpretation or similarities, and assessments for apraxias were performed at the discretion of the psychiatrist. The psychiatrists were blinded to the physician’s assessments, and the assessments were conducted within 3 hours of each other.
Additional Variables Collected
Using medical record review, comorbidity burden, severity of illness, and premorbid cognition were ascertained. The Charlson Comorbidity Index, a weighted index that takes into account the number and seriousness of 19 preexisting comorbid conditions, was used to quantify comorbidity burden; higher scores indicate higher comorbid burden.36,37 The Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II was used to quantify severity of illness.38 This score is based upon the initial values of 12 routine physiologic measurements such as vital sign and laboratory abnormalities; higher scores represent higher severities of illness.38 The medical record was reviewed to ascertain the presence of premorbid cognitive impairment; any documentation of dementia in the patient’s clinical problem list or physician history and physical examination from the outpatient or inpatient settings was considered positive. The medical record review was performed by a research assistant and was double-checked for accuracy by one of the investigators (JHH).
Data Analyses
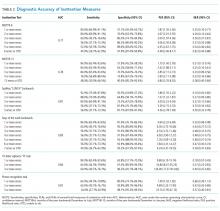
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. Receiver operating characteristic curves were constructed for each inattention task. Area under the receiver operating characteristic curves (AUC) was reported to provide a global measure of diagnostic accuracy. Sensitivities, specificities, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with their 95% CIs were calculated using the psychiatrist’s assessment as the reference standard.39 Cut-points with PLRs greater than 10 (strongly increased the likelihood of delirium) or NLRs less than 0.1 (strongly decreased the likelihood of delirium) were preferentially reported whenever possible.
All statistical analyses were performed with open source R statistical software version 3.0.1 (http://www.r-project.org/), SAS 9.4 (SAS Institute, Cary, NC), and Microsoft Excel 2010 (Microsoft Inc., Redmond, WA).
RESULTS
DISCUSSION
Delirium is frequently missed by healthcare providers because it is not routinely screened for in the acute care setting. To help address this deficiency of care, we evaluated several brief measures of inattention that take less than 30 seconds to complete. We observed that any errors made on the MOTYB-6 and MOTYB-12 tasks had very good sensitivities (80% and 84%) but were limited by their modest specificities (approximately 50%) for delirium. As a result, these assessments have limited clinical utility as standalone delirium screens. We also explored other commonly used brief measures of inattention and at a variety of error cutoffs. Reciting the days of the week backwards appeared to best balance sensitivity and specificity. None of the inattention measures could convincingly rule out delirium (NLR < 0.10), but the vigilance “A” and picture recognition tasks may have clinical utility in ruling in delirium (PLR > 10). Overall, all the inattention tasks, including MOTYB-6 and MOTYB-12, had very good diagnostic performances based upon their AUC. However, achieving a high sensitivity often had to be sacrificed for specificity or, alternatively, achieving a high specificity had to be sacrificed for sensitivity.
Inattention has been shown to be the cardinal feature for delirium,40 and its assessment using cognitive testing has been recommended to help identify the presence of delirium according to an expert consensus panel.26 The diagnostic performance of the MOTYB-12 observed in our study is similar to a study by Fick et al., who reported that MOTYB-12 had very good sensitivity (83%) but had modest specificity (69%) with a cutoff of 1 or more errors. Hendry et al. observed that the MOTYB-12 was 91% sensitive and 50% specific using a cutoff of 4 or more errors. With regard to the MOTYB-6, our reported specificity was different from what was observed by O’Regan et al.27 Using 1 or more errors as a cutoff, they observed a much higher specificity for delirium than we did (90% vs 57%). Discordant observations regarding the diagnostic accuracy for other inattention tasks also exist. We observed that making any error on the days of the week backwards task was 84% sensitive and 82% specific for delirium, whereas Fick et al. observed a sensitivity and specificity of 50% and 94%, respectively. For the vigilance “A” task, we observed that making 2 or more errors over a series of 10 letters was 64.0% sensitive and 91.4% specific for delirium, whereas Pompei et al.41 observed that making 2 or more errors over a series of 60 letters was 51% sensitive and 77% specific for delirium.
The abovementioned discordant findings may be driven by spectrum bias, wherein the sensitivities and specificities for each inattention task may differ in different subgroups. As a result, differences in the age distribution, proportion of college graduates, history of dementia, and susceptibility to delirium can influence overall sensitivity and specificity. Objective measures of delirium, including the inattention screens studied, are particularly prone to spectrum bias.31,34 However, the strength of this approach is that the assessment of inattention becomes less reliant upon clinical judgment and allows it to be used by raters from a wide range of clinical backgrounds. On the other hand, a subjective interpretation of these inattention tasks may allow the rater to capture the subtleties of inattention (ie, decreased speed of performance in a highly intelligent and well-educated patient without dementia). The disadvantage of this approach, however, is that it is more dependent on clinical judgment and may have decreased diagnostic accuracy in those with less clinical experience or with limited training.14,42,43 These factors must be carefully considered when determining which delirium assessment to use.
Additional research is required to determine the clinical utility of these brief inattention assessments. These findings need to be further validated in larger studies, and the optimal cutoff of each task for different subgroup of patients (eg, demented vs nondemented) needs to be further clarified. It is not completely clear whether these inattention tests can serve as standalone assessments. Depending on the cutoff used, some of these assessments may have unacceptable false negative or false positive rates that may lead to increased adverse patient outcomes or increased resource utilization, respectively. Additional components or assessments may be needed to improve the diagnostic accuracy of these assessments. In addition to understanding these inattention assessments’ diagnostic accuracies, their ability to predict adverse outcomes also needs to be investigated. While a previous study observed that making any error on the MOTYB-12 task was associated with increased physical restraint use and prolonged hospital length of stay,44 these assessments’ ability to prognosticate long-term outcomes such as mortality or long-term cognition or function need to be studied. Lastly, studies should also evaluate how easily implementable these assessments are and whether improved delirium recognition leads to improved patient outcomes.
This study has several notable limitations. Though planned a priori, this was a secondary analysis of a larger investigation designed to validate 3 delirium assessments. Our sample size was also relatively small, causing our 95% CIs to overlap in most cases and limiting the statistical power to truly determine whether one measure is better than the other. We also asked the patient to recite the months backwards from December to July as well as recite the months backwards from December to January. It is possible that the patient may have performed better at going from December to January because of learning effect. Our reference standard for delirium was based upon DSM-IV-TR criteria. The new DSM-V criteria may be more restrictive and may slightly change the sensitivities and specificities of the inattention tasks. We enrolled a convenience sample and enrolled patients who were more likely to be male, have cardiovascular chief complaints, and be admitted to the hospital; as a result, selection bias may have been introduced. Lastly, this study was conducted in a single center and enrolled patients who were 65 years and older. Our findings may not be generalizable to other settings and in those who are less than 65 years of age.
CONCLUSIONS
The MOTYB-6 and MOTYB-12 tasks had very good sensitivities but modest specificities (approximately 50%) using any error made as a cutoff; increasing cutoff to 2 errors and 3 errors, respectively, improved their specificities (approximately 70%) with minimal impact to their sensitivities. Reciting the days of the week backwards, spelling the word “LUNCH” backwards, and the 10-letter vigilance “A” task appeared to perform the best in ruling out delirium but only moderately decreased the likelihood of delirium. The 10-letter Vigilance “A” and picture recognition task
Disclosure
This study was funded by the Emergency Medicine Foundation Career Development Award, National Institutes of Health K23AG032355, and National Center for Research Resources, Grant UL1 RR024975-01. The authors report no financial conflicts of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
33. Hamrick I, Hafiz R, Cummings DM. Use of days of the week in a modified mini-mental state exam (M-MMSE) for detecting geriatric cognitive impairment. J Am Board Fam Med. 2013;26(4):429-435.
Delirium is an acute neurocognitive disorder1 that affects up to 25% of older emergency department (ED) and hospitalized patients.2-4 The relationship between delirium and adverse outcomes is well documented.5-7 Delirium is a strong predictor of increased length of mechanical ventilation, longer intensive care unit and hospital stays, increased risk of falls, long-term cognitive impairment, and mortality.8-13 Delirium is frequently missed by healthcare professionals2,14-16 and goes undetected in up to 3 out of 4 patients by bedside nurses and medical practitioners in many hospital settings.14,17-22 A significant barrier to recognizing delirium is the absence of brief delirium assessments.
In an effort to improve delirium recognition in the acute care setting, there has been a concerted effort to develop and validate brief delirium assessments. To address this unmet need, 4 ‘A’s Test (4AT), the Brief Confusion Assessment Method (bCAM), and the 3-minute diagnostic assessment for CAM-defined delirium (3D-CAM) are 1- to 3-minute delirium assessments that were validated in acutely ill older patients.23 However, 1 to 3 minutes may still be too long in busy clinical environments, and briefer (<30 seconds) delirium assessments may be needed.
One potential more-rapid method to screen for delirium is to specifically test for the presence of inattention, which is a cardinal feature of delirium.24,25 Inattention can be ascertained by having the patient recite the months backwards, recite the days of the week backwards, or spell a word backwards.26 Recent studies have evaluated the diagnostic accuracy of reciting the months of the year backwards for delirium. O’Regan et al.27 evaluated the diagnostic accuracy of the month of the year backwards from December to July (MOTYB-6) and observed that this task was 84% sensitive and 90% specific for delirium in older patients. However, they performed the reference standard delirium assessments in patients who had a positive MOTYB-6, which can overestimate sensitivity and underestimate specificity (verification bias).28 Fick et al.29 examined the diagnostic accuracy of 20 individual elements of the 3D-CAM and observed that reciting the months of the year backwards from December to January (MOTYB-12) was 83% sensitive and 69% specific for delirium. However, this was an exploratory study that was designed to identify an element of the 3D-CAM that had the best diagnostic accuracy.
To address these limitations, we sought to evaluate the diagnostic performance of the MOTYB-6 and MOTYB-12 for delirium as diagnosed by a reference standard. We also explored other brief tests of inattention such as spelling a word (“LUNCH”) backwards, reciting the days of the week backwards, 10-letter vigilance “A” task, and 5 picture recognition task.
METHODS
Study Design and Setting
This was a preplanned secondary analysis of a prospective observational study that validated 3 delirium assessments.30,31 This study was conducted at a tertiary care, academic ED. The local institutional review board (IRB) reviewed and approved this study. Informed consent from the patient or an authorized surrogate was obtained whenever possible. Because this was an observational study and posed minimal risk to the patient, the IRB granted a waiver of consent for patients who were both unable to provide consent and were without an authorized surrogate available in the ED or by phone.
Selection of Participants
We enrolled a convenience sample of patients between June 2010 and February 2012 Monday through Friday from 8
Research assistants approached patients who met inclusion criteria and determined if any exclusion criteria were present. If none of the exclusion criteria were present, then the research assistant reviewed the informed consent document with the patient or authorized surrogate if the patient was not capable of providing consent. If a patient was not capable of providing consent and no authorized surrogate was available, then the patient was enrolled (under the waiver of consent) as long as the patient assented to be a part of the study. Once the patient was enrolled, the research assistant contacted the physician rater and reference standard psychiatrists to approach the patient.
Measures of Inattention
An emergency physician (JHH) who had no formal training in the mental status assessment of elders administered a cognitive battery to the patient, including tests of inattention. The following inattention tasks were administered:
- Spell the word “LUNCH” backwards.30 Patients were initially allowed to spell the word “LUNCH” forwards. Patients who were unable to perform the task were assigned 5 errors.
- Recite the months of the year backwards from December to July.23,26,27,30,32 Patients who were unable to perform the task were assigned 6 errors.
- Recite the days of the week backwards.23,26,33 Patients who were unable to perform the task were assigned 7 errors.
- Ten-letter vigilance “A” task.34 The patient was given a series of 10 letters (“S-A-V-E-A-H-A-A-R-T”) every 3 seconds and was asked to squeeze the rater’s hand every time the patient heard the letter “A.” Patients who were unable to perform the task were assigned 10 errors.
- Five picture recognition task.34 Patients were shown 5 objects on picture cards. Afterwards, patients were shown 10 pictures with the previously shown objects intermingled. The patient had to identify which objects were seen previously in the first 5 pictures. Patients who were unable to perform the task were assigned 10 errors.
- Recite the months of the year backwards from December to January.29 Patients who were unable to perform the task were assigned 12 errors.
Reference Standard for Delirium
A comprehensive consultation-liaison psychiatrist assessment was the reference standard for delirium; the diagnosis of delirium was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria.35 Three psychiatrists who each had an average of 11 years of clinical experience and regularly diagnosed delirium as part of their daily clinical practice were available to perform these assessments. To arrive at the diagnosis of delirium, they interviewed those who best understood the patient’s mental status (eg, the patient’s family members or caregivers, physician, and nurses). They also reviewed the patient’s medical record and radiology and laboratory test results. They performed bedside cognitive testing that included, but was not limited to, the Mini-Mental State Examination, Clock Drawing Test, Luria hand sequencing task, and tests for verbal fluency. A focused neurological examination was also performed (ie, screening for paraphasic errors, tremors, tone, asterixis, frontal release signs, etc.), and they also evaluated the patient for affective lability, hallucinations, and level of alertness. If the presence of delirium was still questionable, then confrontational naming, proverb interpretation or similarities, and assessments for apraxias were performed at the discretion of the psychiatrist. The psychiatrists were blinded to the physician’s assessments, and the assessments were conducted within 3 hours of each other.
Additional Variables Collected
Using medical record review, comorbidity burden, severity of illness, and premorbid cognition were ascertained. The Charlson Comorbidity Index, a weighted index that takes into account the number and seriousness of 19 preexisting comorbid conditions, was used to quantify comorbidity burden; higher scores indicate higher comorbid burden.36,37 The Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II was used to quantify severity of illness.38 This score is based upon the initial values of 12 routine physiologic measurements such as vital sign and laboratory abnormalities; higher scores represent higher severities of illness.38 The medical record was reviewed to ascertain the presence of premorbid cognitive impairment; any documentation of dementia in the patient’s clinical problem list or physician history and physical examination from the outpatient or inpatient settings was considered positive. The medical record review was performed by a research assistant and was double-checked for accuracy by one of the investigators (JHH).
Data Analyses
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. Receiver operating characteristic curves were constructed for each inattention task. Area under the receiver operating characteristic curves (AUC) was reported to provide a global measure of diagnostic accuracy. Sensitivities, specificities, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with their 95% CIs were calculated using the psychiatrist’s assessment as the reference standard.39 Cut-points with PLRs greater than 10 (strongly increased the likelihood of delirium) or NLRs less than 0.1 (strongly decreased the likelihood of delirium) were preferentially reported whenever possible.
All statistical analyses were performed with open source R statistical software version 3.0.1 (http://www.r-project.org/), SAS 9.4 (SAS Institute, Cary, NC), and Microsoft Excel 2010 (Microsoft Inc., Redmond, WA).
RESULTS
DISCUSSION
Delirium is frequently missed by healthcare providers because it is not routinely screened for in the acute care setting. To help address this deficiency of care, we evaluated several brief measures of inattention that take less than 30 seconds to complete. We observed that any errors made on the MOTYB-6 and MOTYB-12 tasks had very good sensitivities (80% and 84%) but were limited by their modest specificities (approximately 50%) for delirium. As a result, these assessments have limited clinical utility as standalone delirium screens. We also explored other commonly used brief measures of inattention and at a variety of error cutoffs. Reciting the days of the week backwards appeared to best balance sensitivity and specificity. None of the inattention measures could convincingly rule out delirium (NLR < 0.10), but the vigilance “A” and picture recognition tasks may have clinical utility in ruling in delirium (PLR > 10). Overall, all the inattention tasks, including MOTYB-6 and MOTYB-12, had very good diagnostic performances based upon their AUC. However, achieving a high sensitivity often had to be sacrificed for specificity or, alternatively, achieving a high specificity had to be sacrificed for sensitivity.
Inattention has been shown to be the cardinal feature for delirium,40 and its assessment using cognitive testing has been recommended to help identify the presence of delirium according to an expert consensus panel.26 The diagnostic performance of the MOTYB-12 observed in our study is similar to a study by Fick et al., who reported that MOTYB-12 had very good sensitivity (83%) but had modest specificity (69%) with a cutoff of 1 or more errors. Hendry et al. observed that the MOTYB-12 was 91% sensitive and 50% specific using a cutoff of 4 or more errors. With regard to the MOTYB-6, our reported specificity was different from what was observed by O’Regan et al.27 Using 1 or more errors as a cutoff, they observed a much higher specificity for delirium than we did (90% vs 57%). Discordant observations regarding the diagnostic accuracy for other inattention tasks also exist. We observed that making any error on the days of the week backwards task was 84% sensitive and 82% specific for delirium, whereas Fick et al. observed a sensitivity and specificity of 50% and 94%, respectively. For the vigilance “A” task, we observed that making 2 or more errors over a series of 10 letters was 64.0% sensitive and 91.4% specific for delirium, whereas Pompei et al.41 observed that making 2 or more errors over a series of 60 letters was 51% sensitive and 77% specific for delirium.
The abovementioned discordant findings may be driven by spectrum bias, wherein the sensitivities and specificities for each inattention task may differ in different subgroups. As a result, differences in the age distribution, proportion of college graduates, history of dementia, and susceptibility to delirium can influence overall sensitivity and specificity. Objective measures of delirium, including the inattention screens studied, are particularly prone to spectrum bias.31,34 However, the strength of this approach is that the assessment of inattention becomes less reliant upon clinical judgment and allows it to be used by raters from a wide range of clinical backgrounds. On the other hand, a subjective interpretation of these inattention tasks may allow the rater to capture the subtleties of inattention (ie, decreased speed of performance in a highly intelligent and well-educated patient without dementia). The disadvantage of this approach, however, is that it is more dependent on clinical judgment and may have decreased diagnostic accuracy in those with less clinical experience or with limited training.14,42,43 These factors must be carefully considered when determining which delirium assessment to use.
Additional research is required to determine the clinical utility of these brief inattention assessments. These findings need to be further validated in larger studies, and the optimal cutoff of each task for different subgroup of patients (eg, demented vs nondemented) needs to be further clarified. It is not completely clear whether these inattention tests can serve as standalone assessments. Depending on the cutoff used, some of these assessments may have unacceptable false negative or false positive rates that may lead to increased adverse patient outcomes or increased resource utilization, respectively. Additional components or assessments may be needed to improve the diagnostic accuracy of these assessments. In addition to understanding these inattention assessments’ diagnostic accuracies, their ability to predict adverse outcomes also needs to be investigated. While a previous study observed that making any error on the MOTYB-12 task was associated with increased physical restraint use and prolonged hospital length of stay,44 these assessments’ ability to prognosticate long-term outcomes such as mortality or long-term cognition or function need to be studied. Lastly, studies should also evaluate how easily implementable these assessments are and whether improved delirium recognition leads to improved patient outcomes.
This study has several notable limitations. Though planned a priori, this was a secondary analysis of a larger investigation designed to validate 3 delirium assessments. Our sample size was also relatively small, causing our 95% CIs to overlap in most cases and limiting the statistical power to truly determine whether one measure is better than the other. We also asked the patient to recite the months backwards from December to July as well as recite the months backwards from December to January. It is possible that the patient may have performed better at going from December to January because of learning effect. Our reference standard for delirium was based upon DSM-IV-TR criteria. The new DSM-V criteria may be more restrictive and may slightly change the sensitivities and specificities of the inattention tasks. We enrolled a convenience sample and enrolled patients who were more likely to be male, have cardiovascular chief complaints, and be admitted to the hospital; as a result, selection bias may have been introduced. Lastly, this study was conducted in a single center and enrolled patients who were 65 years and older. Our findings may not be generalizable to other settings and in those who are less than 65 years of age.
CONCLUSIONS
The MOTYB-6 and MOTYB-12 tasks had very good sensitivities but modest specificities (approximately 50%) using any error made as a cutoff; increasing cutoff to 2 errors and 3 errors, respectively, improved their specificities (approximately 70%) with minimal impact to their sensitivities. Reciting the days of the week backwards, spelling the word “LUNCH” backwards, and the 10-letter vigilance “A” task appeared to perform the best in ruling out delirium but only moderately decreased the likelihood of delirium. The 10-letter Vigilance “A” and picture recognition task
Disclosure
This study was funded by the Emergency Medicine Foundation Career Development Award, National Institutes of Health K23AG032355, and National Center for Research Resources, Grant UL1 RR024975-01. The authors report no financial conflicts of interest.
Delirium is an acute neurocognitive disorder1 that affects up to 25% of older emergency department (ED) and hospitalized patients.2-4 The relationship between delirium and adverse outcomes is well documented.5-7 Delirium is a strong predictor of increased length of mechanical ventilation, longer intensive care unit and hospital stays, increased risk of falls, long-term cognitive impairment, and mortality.8-13 Delirium is frequently missed by healthcare professionals2,14-16 and goes undetected in up to 3 out of 4 patients by bedside nurses and medical practitioners in many hospital settings.14,17-22 A significant barrier to recognizing delirium is the absence of brief delirium assessments.
In an effort to improve delirium recognition in the acute care setting, there has been a concerted effort to develop and validate brief delirium assessments. To address this unmet need, 4 ‘A’s Test (4AT), the Brief Confusion Assessment Method (bCAM), and the 3-minute diagnostic assessment for CAM-defined delirium (3D-CAM) are 1- to 3-minute delirium assessments that were validated in acutely ill older patients.23 However, 1 to 3 minutes may still be too long in busy clinical environments, and briefer (<30 seconds) delirium assessments may be needed.
One potential more-rapid method to screen for delirium is to specifically test for the presence of inattention, which is a cardinal feature of delirium.24,25 Inattention can be ascertained by having the patient recite the months backwards, recite the days of the week backwards, or spell a word backwards.26 Recent studies have evaluated the diagnostic accuracy of reciting the months of the year backwards for delirium. O’Regan et al.27 evaluated the diagnostic accuracy of the month of the year backwards from December to July (MOTYB-6) and observed that this task was 84% sensitive and 90% specific for delirium in older patients. However, they performed the reference standard delirium assessments in patients who had a positive MOTYB-6, which can overestimate sensitivity and underestimate specificity (verification bias).28 Fick et al.29 examined the diagnostic accuracy of 20 individual elements of the 3D-CAM and observed that reciting the months of the year backwards from December to January (MOTYB-12) was 83% sensitive and 69% specific for delirium. However, this was an exploratory study that was designed to identify an element of the 3D-CAM that had the best diagnostic accuracy.
To address these limitations, we sought to evaluate the diagnostic performance of the MOTYB-6 and MOTYB-12 for delirium as diagnosed by a reference standard. We also explored other brief tests of inattention such as spelling a word (“LUNCH”) backwards, reciting the days of the week backwards, 10-letter vigilance “A” task, and 5 picture recognition task.
METHODS
Study Design and Setting
This was a preplanned secondary analysis of a prospective observational study that validated 3 delirium assessments.30,31 This study was conducted at a tertiary care, academic ED. The local institutional review board (IRB) reviewed and approved this study. Informed consent from the patient or an authorized surrogate was obtained whenever possible. Because this was an observational study and posed minimal risk to the patient, the IRB granted a waiver of consent for patients who were both unable to provide consent and were without an authorized surrogate available in the ED or by phone.
Selection of Participants
We enrolled a convenience sample of patients between June 2010 and February 2012 Monday through Friday from 8
Research assistants approached patients who met inclusion criteria and determined if any exclusion criteria were present. If none of the exclusion criteria were present, then the research assistant reviewed the informed consent document with the patient or authorized surrogate if the patient was not capable of providing consent. If a patient was not capable of providing consent and no authorized surrogate was available, then the patient was enrolled (under the waiver of consent) as long as the patient assented to be a part of the study. Once the patient was enrolled, the research assistant contacted the physician rater and reference standard psychiatrists to approach the patient.
Measures of Inattention
An emergency physician (JHH) who had no formal training in the mental status assessment of elders administered a cognitive battery to the patient, including tests of inattention. The following inattention tasks were administered:
- Spell the word “LUNCH” backwards.30 Patients were initially allowed to spell the word “LUNCH” forwards. Patients who were unable to perform the task were assigned 5 errors.
- Recite the months of the year backwards from December to July.23,26,27,30,32 Patients who were unable to perform the task were assigned 6 errors.
- Recite the days of the week backwards.23,26,33 Patients who were unable to perform the task were assigned 7 errors.
- Ten-letter vigilance “A” task.34 The patient was given a series of 10 letters (“S-A-V-E-A-H-A-A-R-T”) every 3 seconds and was asked to squeeze the rater’s hand every time the patient heard the letter “A.” Patients who were unable to perform the task were assigned 10 errors.
- Five picture recognition task.34 Patients were shown 5 objects on picture cards. Afterwards, patients were shown 10 pictures with the previously shown objects intermingled. The patient had to identify which objects were seen previously in the first 5 pictures. Patients who were unable to perform the task were assigned 10 errors.
- Recite the months of the year backwards from December to January.29 Patients who were unable to perform the task were assigned 12 errors.
Reference Standard for Delirium
A comprehensive consultation-liaison psychiatrist assessment was the reference standard for delirium; the diagnosis of delirium was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria.35 Three psychiatrists who each had an average of 11 years of clinical experience and regularly diagnosed delirium as part of their daily clinical practice were available to perform these assessments. To arrive at the diagnosis of delirium, they interviewed those who best understood the patient’s mental status (eg, the patient’s family members or caregivers, physician, and nurses). They also reviewed the patient’s medical record and radiology and laboratory test results. They performed bedside cognitive testing that included, but was not limited to, the Mini-Mental State Examination, Clock Drawing Test, Luria hand sequencing task, and tests for verbal fluency. A focused neurological examination was also performed (ie, screening for paraphasic errors, tremors, tone, asterixis, frontal release signs, etc.), and they also evaluated the patient for affective lability, hallucinations, and level of alertness. If the presence of delirium was still questionable, then confrontational naming, proverb interpretation or similarities, and assessments for apraxias were performed at the discretion of the psychiatrist. The psychiatrists were blinded to the physician’s assessments, and the assessments were conducted within 3 hours of each other.
Additional Variables Collected
Using medical record review, comorbidity burden, severity of illness, and premorbid cognition were ascertained. The Charlson Comorbidity Index, a weighted index that takes into account the number and seriousness of 19 preexisting comorbid conditions, was used to quantify comorbidity burden; higher scores indicate higher comorbid burden.36,37 The Acute Physiology Score of the Acute Physiology and Chronic Health Evaluation II was used to quantify severity of illness.38 This score is based upon the initial values of 12 routine physiologic measurements such as vital sign and laboratory abnormalities; higher scores represent higher severities of illness.38 The medical record was reviewed to ascertain the presence of premorbid cognitive impairment; any documentation of dementia in the patient’s clinical problem list or physician history and physical examination from the outpatient or inpatient settings was considered positive. The medical record review was performed by a research assistant and was double-checked for accuracy by one of the investigators (JHH).
Data Analyses
Measures of central tendency and dispersion for continuous variables were reported as medians and interquartile ranges. Categorical variables were reported as proportions. Receiver operating characteristic curves were constructed for each inattention task. Area under the receiver operating characteristic curves (AUC) was reported to provide a global measure of diagnostic accuracy. Sensitivities, specificities, positive likelihood ratios (PLRs), and negative likelihood ratios (NLRs) with their 95% CIs were calculated using the psychiatrist’s assessment as the reference standard.39 Cut-points with PLRs greater than 10 (strongly increased the likelihood of delirium) or NLRs less than 0.1 (strongly decreased the likelihood of delirium) were preferentially reported whenever possible.
All statistical analyses were performed with open source R statistical software version 3.0.1 (http://www.r-project.org/), SAS 9.4 (SAS Institute, Cary, NC), and Microsoft Excel 2010 (Microsoft Inc., Redmond, WA).
RESULTS
DISCUSSION
Delirium is frequently missed by healthcare providers because it is not routinely screened for in the acute care setting. To help address this deficiency of care, we evaluated several brief measures of inattention that take less than 30 seconds to complete. We observed that any errors made on the MOTYB-6 and MOTYB-12 tasks had very good sensitivities (80% and 84%) but were limited by their modest specificities (approximately 50%) for delirium. As a result, these assessments have limited clinical utility as standalone delirium screens. We also explored other commonly used brief measures of inattention and at a variety of error cutoffs. Reciting the days of the week backwards appeared to best balance sensitivity and specificity. None of the inattention measures could convincingly rule out delirium (NLR < 0.10), but the vigilance “A” and picture recognition tasks may have clinical utility in ruling in delirium (PLR > 10). Overall, all the inattention tasks, including MOTYB-6 and MOTYB-12, had very good diagnostic performances based upon their AUC. However, achieving a high sensitivity often had to be sacrificed for specificity or, alternatively, achieving a high specificity had to be sacrificed for sensitivity.
Inattention has been shown to be the cardinal feature for delirium,40 and its assessment using cognitive testing has been recommended to help identify the presence of delirium according to an expert consensus panel.26 The diagnostic performance of the MOTYB-12 observed in our study is similar to a study by Fick et al., who reported that MOTYB-12 had very good sensitivity (83%) but had modest specificity (69%) with a cutoff of 1 or more errors. Hendry et al. observed that the MOTYB-12 was 91% sensitive and 50% specific using a cutoff of 4 or more errors. With regard to the MOTYB-6, our reported specificity was different from what was observed by O’Regan et al.27 Using 1 or more errors as a cutoff, they observed a much higher specificity for delirium than we did (90% vs 57%). Discordant observations regarding the diagnostic accuracy for other inattention tasks also exist. We observed that making any error on the days of the week backwards task was 84% sensitive and 82% specific for delirium, whereas Fick et al. observed a sensitivity and specificity of 50% and 94%, respectively. For the vigilance “A” task, we observed that making 2 or more errors over a series of 10 letters was 64.0% sensitive and 91.4% specific for delirium, whereas Pompei et al.41 observed that making 2 or more errors over a series of 60 letters was 51% sensitive and 77% specific for delirium.
The abovementioned discordant findings may be driven by spectrum bias, wherein the sensitivities and specificities for each inattention task may differ in different subgroups. As a result, differences in the age distribution, proportion of college graduates, history of dementia, and susceptibility to delirium can influence overall sensitivity and specificity. Objective measures of delirium, including the inattention screens studied, are particularly prone to spectrum bias.31,34 However, the strength of this approach is that the assessment of inattention becomes less reliant upon clinical judgment and allows it to be used by raters from a wide range of clinical backgrounds. On the other hand, a subjective interpretation of these inattention tasks may allow the rater to capture the subtleties of inattention (ie, decreased speed of performance in a highly intelligent and well-educated patient without dementia). The disadvantage of this approach, however, is that it is more dependent on clinical judgment and may have decreased diagnostic accuracy in those with less clinical experience or with limited training.14,42,43 These factors must be carefully considered when determining which delirium assessment to use.
Additional research is required to determine the clinical utility of these brief inattention assessments. These findings need to be further validated in larger studies, and the optimal cutoff of each task for different subgroup of patients (eg, demented vs nondemented) needs to be further clarified. It is not completely clear whether these inattention tests can serve as standalone assessments. Depending on the cutoff used, some of these assessments may have unacceptable false negative or false positive rates that may lead to increased adverse patient outcomes or increased resource utilization, respectively. Additional components or assessments may be needed to improve the diagnostic accuracy of these assessments. In addition to understanding these inattention assessments’ diagnostic accuracies, their ability to predict adverse outcomes also needs to be investigated. While a previous study observed that making any error on the MOTYB-12 task was associated with increased physical restraint use and prolonged hospital length of stay,44 these assessments’ ability to prognosticate long-term outcomes such as mortality or long-term cognition or function need to be studied. Lastly, studies should also evaluate how easily implementable these assessments are and whether improved delirium recognition leads to improved patient outcomes.
This study has several notable limitations. Though planned a priori, this was a secondary analysis of a larger investigation designed to validate 3 delirium assessments. Our sample size was also relatively small, causing our 95% CIs to overlap in most cases and limiting the statistical power to truly determine whether one measure is better than the other. We also asked the patient to recite the months backwards from December to July as well as recite the months backwards from December to January. It is possible that the patient may have performed better at going from December to January because of learning effect. Our reference standard for delirium was based upon DSM-IV-TR criteria. The new DSM-V criteria may be more restrictive and may slightly change the sensitivities and specificities of the inattention tasks. We enrolled a convenience sample and enrolled patients who were more likely to be male, have cardiovascular chief complaints, and be admitted to the hospital; as a result, selection bias may have been introduced. Lastly, this study was conducted in a single center and enrolled patients who were 65 years and older. Our findings may not be generalizable to other settings and in those who are less than 65 years of age.
CONCLUSIONS
The MOTYB-6 and MOTYB-12 tasks had very good sensitivities but modest specificities (approximately 50%) using any error made as a cutoff; increasing cutoff to 2 errors and 3 errors, respectively, improved their specificities (approximately 70%) with minimal impact to their sensitivities. Reciting the days of the week backwards, spelling the word “LUNCH” backwards, and the 10-letter vigilance “A” task appeared to perform the best in ruling out delirium but only moderately decreased the likelihood of delirium. The 10-letter Vigilance “A” and picture recognition task
Disclosure
This study was funded by the Emergency Medicine Foundation Career Development Award, National Institutes of Health K23AG032355, and National Center for Research Resources, Grant UL1 RR024975-01. The authors report no financial conflicts of interest.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
33. Hamrick I, Hafiz R, Cummings DM. Use of days of the week in a modified mini-mental state exam (M-MMSE) for detecting geriatric cognitive impairment. J Am Board Fam Med. 2013;26(4):429-435.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013.
33. Hamrick I, Hafiz R, Cummings DM. Use of days of the week in a modified mini-mental state exam (M-MMSE) for detecting geriatric cognitive impairment. J Am Board Fam Med. 2013;26(4):429-435.
© 2018 Society of Hospital Medicine
Statin use is uniformly low in adults with dyslipidemia disorders
Only half of adults with familial hypercholesterolemia are currently on statin therapy, with even fewer receiving a high-intensity statin, according to an analysis of data from the National Health and Nutrition Examination Survey.
“We found that 0.47% of the adult U.S. population has definite/probable FH [familial hypercholesterolemia] but observed a large disconnect between screening and treatment rates in adults with definite/ probable FH and adults with severe dyslipidemia,” wrote Emily Bucholz, MD, of Boston’s Children Hospital, and her colleagues in Circulation.
The analysis found that 14 million adults, or almost 7%, in the population had severe dyslipidemia (LDL cholesterol of at least 190 mg/dL), and that 1 million of these adults, 7.2%, had definite or probable FH. Among the adults with FH, awareness and screening rates were above 80%, but those rates were only moderate among the general population. Adults who were likely to have FH were also more likely to be screened and be aware of hyperlipidemia than were those with only severe dyslipidemia.
Despite high rates of awareness, statin use was low across the board. Just over half (52% ) of patients with definite/probable FH were using a statin. The rate of statin use was even lower in patients with severe dyslipidemia, at only 38%. High-intensity statin use also dwindled in both dyslipidemia and FH patient groups (37% and 30%, respectively).
Rates of cholesterol screening, awareness, and lipid-lowering medication were particularly low in younger patients, uninsured patients, and patients without a usual source of care. Among young patients (20-39 years), 62% reported cholesterol screening in the past 5 years and 64% reported being aware of having hypercholesterolemia. Of this group, 13% were on a statin. Uninsured adults fared only slightly better, with nearly a third (29%) undergoing a recent screening and using a statin.
Limitations to the analysis include the self-reported information on cholesterol screening, awareness, treatment rates, and the lack of information concerning statin dosages.
In light of the results of this study, Dr. Bucholz emphasized that statin use among underserved patient groups without access to care needs to be a priority.
“Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates,” wrote Dr. Bucholz and her colleagues. “Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care.”
Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had an relevant financial disclosures to report.
SOURCE: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
Only half of adults with familial hypercholesterolemia are currently on statin therapy, with even fewer receiving a high-intensity statin, according to an analysis of data from the National Health and Nutrition Examination Survey.
“We found that 0.47% of the adult U.S. population has definite/probable FH [familial hypercholesterolemia] but observed a large disconnect between screening and treatment rates in adults with definite/ probable FH and adults with severe dyslipidemia,” wrote Emily Bucholz, MD, of Boston’s Children Hospital, and her colleagues in Circulation.
The analysis found that 14 million adults, or almost 7%, in the population had severe dyslipidemia (LDL cholesterol of at least 190 mg/dL), and that 1 million of these adults, 7.2%, had definite or probable FH. Among the adults with FH, awareness and screening rates were above 80%, but those rates were only moderate among the general population. Adults who were likely to have FH were also more likely to be screened and be aware of hyperlipidemia than were those with only severe dyslipidemia.
Despite high rates of awareness, statin use was low across the board. Just over half (52% ) of patients with definite/probable FH were using a statin. The rate of statin use was even lower in patients with severe dyslipidemia, at only 38%. High-intensity statin use also dwindled in both dyslipidemia and FH patient groups (37% and 30%, respectively).
Rates of cholesterol screening, awareness, and lipid-lowering medication were particularly low in younger patients, uninsured patients, and patients without a usual source of care. Among young patients (20-39 years), 62% reported cholesterol screening in the past 5 years and 64% reported being aware of having hypercholesterolemia. Of this group, 13% were on a statin. Uninsured adults fared only slightly better, with nearly a third (29%) undergoing a recent screening and using a statin.
Limitations to the analysis include the self-reported information on cholesterol screening, awareness, treatment rates, and the lack of information concerning statin dosages.
In light of the results of this study, Dr. Bucholz emphasized that statin use among underserved patient groups without access to care needs to be a priority.
“Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates,” wrote Dr. Bucholz and her colleagues. “Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care.”
Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had an relevant financial disclosures to report.
SOURCE: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
Only half of adults with familial hypercholesterolemia are currently on statin therapy, with even fewer receiving a high-intensity statin, according to an analysis of data from the National Health and Nutrition Examination Survey.
“We found that 0.47% of the adult U.S. population has definite/probable FH [familial hypercholesterolemia] but observed a large disconnect between screening and treatment rates in adults with definite/ probable FH and adults with severe dyslipidemia,” wrote Emily Bucholz, MD, of Boston’s Children Hospital, and her colleagues in Circulation.
The analysis found that 14 million adults, or almost 7%, in the population had severe dyslipidemia (LDL cholesterol of at least 190 mg/dL), and that 1 million of these adults, 7.2%, had definite or probable FH. Among the adults with FH, awareness and screening rates were above 80%, but those rates were only moderate among the general population. Adults who were likely to have FH were also more likely to be screened and be aware of hyperlipidemia than were those with only severe dyslipidemia.
Despite high rates of awareness, statin use was low across the board. Just over half (52% ) of patients with definite/probable FH were using a statin. The rate of statin use was even lower in patients with severe dyslipidemia, at only 38%. High-intensity statin use also dwindled in both dyslipidemia and FH patient groups (37% and 30%, respectively).
Rates of cholesterol screening, awareness, and lipid-lowering medication were particularly low in younger patients, uninsured patients, and patients without a usual source of care. Among young patients (20-39 years), 62% reported cholesterol screening in the past 5 years and 64% reported being aware of having hypercholesterolemia. Of this group, 13% were on a statin. Uninsured adults fared only slightly better, with nearly a third (29%) undergoing a recent screening and using a statin.
Limitations to the analysis include the self-reported information on cholesterol screening, awareness, treatment rates, and the lack of information concerning statin dosages.
In light of the results of this study, Dr. Bucholz emphasized that statin use among underserved patient groups without access to care needs to be a priority.
“Although rates of statin prescription have been steadily increasing over time, the rate of growth over the past decade has been slow, and there remains a significant gap in treatment rates,” wrote Dr. Bucholz and her colleagues. “Younger and uninsured adults with severe dyslipidemia in addition to those without a usual source of care are significantly less likely to be prescribed statins, highlighting the need for community-based interventions to target these adults with limited access to care.”
Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had an relevant financial disclosures to report.
SOURCE: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
FROM CIRCULATION
Key clinical point: Statin use is uniformly low across multiple patient groups.
Major finding:
Study details: A cross-sectional analysis of 42,471 patients from the National Health and Nutrition Examination Survey.
Disclosures: Apart from Sarah de Ferranti, MD, who receives research funding and royalties related to pediatric cardiology, no other authors had any relevant financial disclosures to report.
Source: Bucholz EM et al. Circulation. 2018 Mar 23. doi: 10.1161/CIRCULATIONAHA.117.032321.
All VA Facilities Now Offer Same-Day Service
Calling it “a major milestone,” the VA announced same-day services for urgent primary and mental health care are now available at 100% of its > 1,000 medical facilities.
Now a veteran can more conveniently have a face-to-face visit with a clinician, call a nurse, have a telehealth or video care visit, get an appointment with a specialist, or have a prescription filled in the same day, “depending upon what best meets the needs of the veteran.”
The VA also has reduced patient wait time, and implemented a new process to ensure timely follow-up appointments for time-sensitive medical needs.
Calling it “a major milestone,” the VA announced same-day services for urgent primary and mental health care are now available at 100% of its > 1,000 medical facilities.
Now a veteran can more conveniently have a face-to-face visit with a clinician, call a nurse, have a telehealth or video care visit, get an appointment with a specialist, or have a prescription filled in the same day, “depending upon what best meets the needs of the veteran.”
The VA also has reduced patient wait time, and implemented a new process to ensure timely follow-up appointments for time-sensitive medical needs.
Calling it “a major milestone,” the VA announced same-day services for urgent primary and mental health care are now available at 100% of its > 1,000 medical facilities.
Now a veteran can more conveniently have a face-to-face visit with a clinician, call a nurse, have a telehealth or video care visit, get an appointment with a specialist, or have a prescription filled in the same day, “depending upon what best meets the needs of the veteran.”
The VA also has reduced patient wait time, and implemented a new process to ensure timely follow-up appointments for time-sensitive medical needs.
CHMP recommends rejection for betrixaban
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a negative opinion recommending refusal of marketing authorization for the factor Xa inhibitor betrixaban (Dexxience).
Portola Pharmaceuticals, Inc. is seeking approval of betrixaban for the prevention of venous thromboembolism (VTE) in adults hospitalized for an acute medical illness with risk factors for VTE, including VTE-related death.
The marketing authorization application for betrixaban was supported by data from the phase 3 APEX study. In this study, researchers compared oral betrixaban to injectable enoxaparin for the prevention of VTE in patients with acute medical illnesses.
The CHMP said APEX did not satisfactorily show that betrixaban’s benefits outweigh its risk in this patient population. The study results were not considered reliable because some test results for VTEs were not available.
In addition, patients treated with betrixaban had more episodes of bleeding than those treated with enoxaparin. This was considered an important concern given that betrixaban was expected to be used in patients with serious underlying conditions.
For these reasons, the CHMP recommended that betrixaban be refused marketing authorization.
Portola Pharmaceuticals said it intends to appeal the CHMP’s opinion and ask the committee for a re-examination.
“We believe we have generated substantial evidence which demonstrates the clinically meaningful benefit of betrixaban in reducing VTE and VTE-related deaths in this vulnerable patient population, and we remain confident in its potential to have a major public health impact,” said Jack Lawrence, MD, chief medical officer of Portola Pharmaceuticals.
“The re-examination process allows us the opportunity to address the CHMP’s questions and provide further clarification as needed, with the goal of making betrixaban available to acute medically ill patients in Europe who are at risk for VTE.”
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a negative opinion recommending refusal of marketing authorization for the factor Xa inhibitor betrixaban (Dexxience).
Portola Pharmaceuticals, Inc. is seeking approval of betrixaban for the prevention of venous thromboembolism (VTE) in adults hospitalized for an acute medical illness with risk factors for VTE, including VTE-related death.
The marketing authorization application for betrixaban was supported by data from the phase 3 APEX study. In this study, researchers compared oral betrixaban to injectable enoxaparin for the prevention of VTE in patients with acute medical illnesses.
The CHMP said APEX did not satisfactorily show that betrixaban’s benefits outweigh its risk in this patient population. The study results were not considered reliable because some test results for VTEs were not available.
In addition, patients treated with betrixaban had more episodes of bleeding than those treated with enoxaparin. This was considered an important concern given that betrixaban was expected to be used in patients with serious underlying conditions.
For these reasons, the CHMP recommended that betrixaban be refused marketing authorization.
Portola Pharmaceuticals said it intends to appeal the CHMP’s opinion and ask the committee for a re-examination.
“We believe we have generated substantial evidence which demonstrates the clinically meaningful benefit of betrixaban in reducing VTE and VTE-related deaths in this vulnerable patient population, and we remain confident in its potential to have a major public health impact,” said Jack Lawrence, MD, chief medical officer of Portola Pharmaceuticals.
“The re-examination process allows us the opportunity to address the CHMP’s questions and provide further clarification as needed, with the goal of making betrixaban available to acute medically ill patients in Europe who are at risk for VTE.”
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has issued a negative opinion recommending refusal of marketing authorization for the factor Xa inhibitor betrixaban (Dexxience).
Portola Pharmaceuticals, Inc. is seeking approval of betrixaban for the prevention of venous thromboembolism (VTE) in adults hospitalized for an acute medical illness with risk factors for VTE, including VTE-related death.
The marketing authorization application for betrixaban was supported by data from the phase 3 APEX study. In this study, researchers compared oral betrixaban to injectable enoxaparin for the prevention of VTE in patients with acute medical illnesses.
The CHMP said APEX did not satisfactorily show that betrixaban’s benefits outweigh its risk in this patient population. The study results were not considered reliable because some test results for VTEs were not available.
In addition, patients treated with betrixaban had more episodes of bleeding than those treated with enoxaparin. This was considered an important concern given that betrixaban was expected to be used in patients with serious underlying conditions.
For these reasons, the CHMP recommended that betrixaban be refused marketing authorization.
Portola Pharmaceuticals said it intends to appeal the CHMP’s opinion and ask the committee for a re-examination.
“We believe we have generated substantial evidence which demonstrates the clinically meaningful benefit of betrixaban in reducing VTE and VTE-related deaths in this vulnerable patient population, and we remain confident in its potential to have a major public health impact,” said Jack Lawrence, MD, chief medical officer of Portola Pharmaceuticals.
“The re-examination process allows us the opportunity to address the CHMP’s questions and provide further clarification as needed, with the goal of making betrixaban available to acute medically ill patients in Europe who are at risk for VTE.”
CBC data can predict immunotherapy responses in NSCLC
ORLANDO – Information readily available on complete blood count can help predict response to immunotherapy and outcomes in patients with advanced non–small cell lung cancer, according to findings from a review of 157 cases.
Specifically, absolute monocyte count of 0.63 or greater and absolute neutrophil count/absolute lymphocyte count of 5.9 or greater at baseline were significantly associated with poor progression-free survival (hazard ratios, 1.50 and 1.61, respectively) and overall survival (HRs, 1.71 and 1.87, respectively) in patients treated with anti–programmed death-1 (PD-1) antibodies, Aixa E. Soyano, MD, reported in a poster at the annual conference of the National Comprehensive Cancer Network.
Additionally, absolute neutrophil count of at least 7.5 and myeloid to lymphoid ratio of at least 11.3 at baseline were associated with poor overall survival (HRs, 1.86 and 2.31, respectively), according to Dr. Soyano of the Mayo Clinic, Jacksonville, Fla.
“The potential predictive value of these readily available biomarkers might help with risk stratification and treatment strategies,” she and her colleagues wrote.
Cases included in the review involved advanced non–small cell lung cancer (NSCLC) patients with a median age of 66 years who were treated with nivolumab or pembrolizumab at the Mayo Clinic from January 2010 to April 2017. Most (91%) were white, 4.5% were African American, 1.9% were Asian, 0.6% were native Hawaiian/Pacific Islander, and 1.9% were other ethnicities. Slightly more than half (53%) were men, and diagnoses included adenocarcinoma (69%), squamous disease (29%) and other (3%). Half had one prior line of chemotherapy, 22% had two prior lines, and 10% had three or more prior lines. The majority (72%) had Eastern Cooperative Oncology Group performance status of 1 or 2, and 34% had CNS disease.
Pembrolizumab was given intravenously at a dose of 2 mg/kg every 21 days (11 patients), and nivolumab was given intravenously at a dose of 3 mg/kg every 14 days (146 patients). Clinical response was assessed every 8-12 weeks by CT of the chest, abdomen and pelvis, and also – in some cases – by brain MRI.
The findings are notable because, although combination chemotherapy with a platinum-based doublet has, for the last decade, been the backbone of initial systemic therapy for patients whose tumor does not have driver mutations, monoclonal antibodies targeting PD-1 or its ligand PD-L1 have shown improvements in progression-free survival and overall survival in certain patients with metastatic or locally advanced lung cancer, the investigators explained.
Prior studies in melanoma patients treated with immunotherapy targeting the cytotoxic T-lymphocyte antigen 4 pathway and PD-1/PD-L1 pathways have identified predictive or prognostic hematological markers of outcomes. However, data with respect to hematologic markers in lung cancer are sparse, and given the high cost, significant immune-related side effects, and rapidly expanding number of indications for immunotherapy in NSCLC, there is a need for reliable biomarkers to help predict response and outcomes, they said.
In a separate presentation at the NCCN conference, John A. Thompson, MD, of the Fred Hutchinson Cancer Research Center, Seattle, noted that some progress has been made in the area of predicting response to immune checkpoint inhibitors in NSCLC patients. Namely, the value of tumor PD-L1 expression and tumor mutation burden for predicting outcomes was highlighted in a recent study by Rizvi et al., who concluded that “the incorporation of both TMB [tumor mutation burden] and PD-L1 expression into multivariable predictive models should result in greater predictive power.” .
“This is a first step in our evolution and our progress toward a better biomarker. I think when we add in other factors like gene expression, we may be able to develop an even more robust biomarker that will help us select appropriate patients for therapy,” he said.
Dr. Soyano and her colleagues noted, however, that these measures, as well as tumor infiltrating immune cells, which have also been shown to have predictive value, require special testing and/or processing.
Furthermore, the optimal cutoff with PD-L1 expression is debatable, they said.
CBC data is more readily available, and also appears to have predictive and prognostic value, they said, concluding that the findings warrant further investigation in a larger, prospective study.
The authors reported having no disclosures.
SOURCE: Soyano A et al. NCCN Poster 075.
ORLANDO – Information readily available on complete blood count can help predict response to immunotherapy and outcomes in patients with advanced non–small cell lung cancer, according to findings from a review of 157 cases.
Specifically, absolute monocyte count of 0.63 or greater and absolute neutrophil count/absolute lymphocyte count of 5.9 or greater at baseline were significantly associated with poor progression-free survival (hazard ratios, 1.50 and 1.61, respectively) and overall survival (HRs, 1.71 and 1.87, respectively) in patients treated with anti–programmed death-1 (PD-1) antibodies, Aixa E. Soyano, MD, reported in a poster at the annual conference of the National Comprehensive Cancer Network.
Additionally, absolute neutrophil count of at least 7.5 and myeloid to lymphoid ratio of at least 11.3 at baseline were associated with poor overall survival (HRs, 1.86 and 2.31, respectively), according to Dr. Soyano of the Mayo Clinic, Jacksonville, Fla.
“The potential predictive value of these readily available biomarkers might help with risk stratification and treatment strategies,” she and her colleagues wrote.
Cases included in the review involved advanced non–small cell lung cancer (NSCLC) patients with a median age of 66 years who were treated with nivolumab or pembrolizumab at the Mayo Clinic from January 2010 to April 2017. Most (91%) were white, 4.5% were African American, 1.9% were Asian, 0.6% were native Hawaiian/Pacific Islander, and 1.9% were other ethnicities. Slightly more than half (53%) were men, and diagnoses included adenocarcinoma (69%), squamous disease (29%) and other (3%). Half had one prior line of chemotherapy, 22% had two prior lines, and 10% had three or more prior lines. The majority (72%) had Eastern Cooperative Oncology Group performance status of 1 or 2, and 34% had CNS disease.
Pembrolizumab was given intravenously at a dose of 2 mg/kg every 21 days (11 patients), and nivolumab was given intravenously at a dose of 3 mg/kg every 14 days (146 patients). Clinical response was assessed every 8-12 weeks by CT of the chest, abdomen and pelvis, and also – in some cases – by brain MRI.
The findings are notable because, although combination chemotherapy with a platinum-based doublet has, for the last decade, been the backbone of initial systemic therapy for patients whose tumor does not have driver mutations, monoclonal antibodies targeting PD-1 or its ligand PD-L1 have shown improvements in progression-free survival and overall survival in certain patients with metastatic or locally advanced lung cancer, the investigators explained.
Prior studies in melanoma patients treated with immunotherapy targeting the cytotoxic T-lymphocyte antigen 4 pathway and PD-1/PD-L1 pathways have identified predictive or prognostic hematological markers of outcomes. However, data with respect to hematologic markers in lung cancer are sparse, and given the high cost, significant immune-related side effects, and rapidly expanding number of indications for immunotherapy in NSCLC, there is a need for reliable biomarkers to help predict response and outcomes, they said.
In a separate presentation at the NCCN conference, John A. Thompson, MD, of the Fred Hutchinson Cancer Research Center, Seattle, noted that some progress has been made in the area of predicting response to immune checkpoint inhibitors in NSCLC patients. Namely, the value of tumor PD-L1 expression and tumor mutation burden for predicting outcomes was highlighted in a recent study by Rizvi et al., who concluded that “the incorporation of both TMB [tumor mutation burden] and PD-L1 expression into multivariable predictive models should result in greater predictive power.” .
“This is a first step in our evolution and our progress toward a better biomarker. I think when we add in other factors like gene expression, we may be able to develop an even more robust biomarker that will help us select appropriate patients for therapy,” he said.
Dr. Soyano and her colleagues noted, however, that these measures, as well as tumor infiltrating immune cells, which have also been shown to have predictive value, require special testing and/or processing.
Furthermore, the optimal cutoff with PD-L1 expression is debatable, they said.
CBC data is more readily available, and also appears to have predictive and prognostic value, they said, concluding that the findings warrant further investigation in a larger, prospective study.
The authors reported having no disclosures.
SOURCE: Soyano A et al. NCCN Poster 075.
ORLANDO – Information readily available on complete blood count can help predict response to immunotherapy and outcomes in patients with advanced non–small cell lung cancer, according to findings from a review of 157 cases.
Specifically, absolute monocyte count of 0.63 or greater and absolute neutrophil count/absolute lymphocyte count of 5.9 or greater at baseline were significantly associated with poor progression-free survival (hazard ratios, 1.50 and 1.61, respectively) and overall survival (HRs, 1.71 and 1.87, respectively) in patients treated with anti–programmed death-1 (PD-1) antibodies, Aixa E. Soyano, MD, reported in a poster at the annual conference of the National Comprehensive Cancer Network.
Additionally, absolute neutrophil count of at least 7.5 and myeloid to lymphoid ratio of at least 11.3 at baseline were associated with poor overall survival (HRs, 1.86 and 2.31, respectively), according to Dr. Soyano of the Mayo Clinic, Jacksonville, Fla.
“The potential predictive value of these readily available biomarkers might help with risk stratification and treatment strategies,” she and her colleagues wrote.
Cases included in the review involved advanced non–small cell lung cancer (NSCLC) patients with a median age of 66 years who were treated with nivolumab or pembrolizumab at the Mayo Clinic from January 2010 to April 2017. Most (91%) were white, 4.5% were African American, 1.9% were Asian, 0.6% were native Hawaiian/Pacific Islander, and 1.9% were other ethnicities. Slightly more than half (53%) were men, and diagnoses included adenocarcinoma (69%), squamous disease (29%) and other (3%). Half had one prior line of chemotherapy, 22% had two prior lines, and 10% had three or more prior lines. The majority (72%) had Eastern Cooperative Oncology Group performance status of 1 or 2, and 34% had CNS disease.
Pembrolizumab was given intravenously at a dose of 2 mg/kg every 21 days (11 patients), and nivolumab was given intravenously at a dose of 3 mg/kg every 14 days (146 patients). Clinical response was assessed every 8-12 weeks by CT of the chest, abdomen and pelvis, and also – in some cases – by brain MRI.
The findings are notable because, although combination chemotherapy with a platinum-based doublet has, for the last decade, been the backbone of initial systemic therapy for patients whose tumor does not have driver mutations, monoclonal antibodies targeting PD-1 or its ligand PD-L1 have shown improvements in progression-free survival and overall survival in certain patients with metastatic or locally advanced lung cancer, the investigators explained.
Prior studies in melanoma patients treated with immunotherapy targeting the cytotoxic T-lymphocyte antigen 4 pathway and PD-1/PD-L1 pathways have identified predictive or prognostic hematological markers of outcomes. However, data with respect to hematologic markers in lung cancer are sparse, and given the high cost, significant immune-related side effects, and rapidly expanding number of indications for immunotherapy in NSCLC, there is a need for reliable biomarkers to help predict response and outcomes, they said.
In a separate presentation at the NCCN conference, John A. Thompson, MD, of the Fred Hutchinson Cancer Research Center, Seattle, noted that some progress has been made in the area of predicting response to immune checkpoint inhibitors in NSCLC patients. Namely, the value of tumor PD-L1 expression and tumor mutation burden for predicting outcomes was highlighted in a recent study by Rizvi et al., who concluded that “the incorporation of both TMB [tumor mutation burden] and PD-L1 expression into multivariable predictive models should result in greater predictive power.” .
“This is a first step in our evolution and our progress toward a better biomarker. I think when we add in other factors like gene expression, we may be able to develop an even more robust biomarker that will help us select appropriate patients for therapy,” he said.
Dr. Soyano and her colleagues noted, however, that these measures, as well as tumor infiltrating immune cells, which have also been shown to have predictive value, require special testing and/or processing.
Furthermore, the optimal cutoff with PD-L1 expression is debatable, they said.
CBC data is more readily available, and also appears to have predictive and prognostic value, they said, concluding that the findings warrant further investigation in a larger, prospective study.
The authors reported having no disclosures.
SOURCE: Soyano A et al. NCCN Poster 075.
REPORTING FROM THE NCCN ANNUAL CONFERENCE
Key clinical point:
Major finding: Hazard ratios for progression-free survival were 1.50 and 1.61 with absolute monocyte count of 0.63 or greater, absolute neutrophil count/absolute lymphocyte of 5.9 or greater at baseline.
Study details: A review of 157 NSCLC cases.
Disclosures: The authors reported having no disclosures.
Source: Soyano AE et al. NCCN poster 075.
VIDEO: Everolimus/letrozole promising for recurrent endometrial cancer
NEW ORLEANS – Combined treatment for 28 days with the mammalian target of rapamycin inhibitor everolimus plus the aromatase inhibitor letrozole in 37 women with recurrent endometrial cancer produced an overall objective response rate of 24% and an average progression-free survival rate of 6.3 months in a randomized phase 2 study with a total of 74 patients. The treatment was also relatively well tolerated, with more serious adverse vents of anemia and hyperglycemia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the most attention-grabbing finding of this study was that, in the subset of 15 women who had received no prior chemotherapy, the objective response rate on this regimen was 53% and median progression-free survival was 21.6 months, Brian M. Slomovitz, MD, said at the annual meeting of the Society of Gynecologic Oncology.
This level of response in the chemotherapy-naive subgroup was “very high, and not what we expected,” Dr. Slomovitz, , professor of ob.gyn. and human genetics and director of gynecologic oncology at the University of Miami, said in a video interview. “This is something we need to further investigate to see if we can make this part of standard care.”
Although he conceded that the data from this study were too limited to warrant a regulatory indication, he suggested that it might be enough to gain the everolimus plus letrozole combination used in the study citation as a treatment option in clinical guidelines. The next step should be a phase 3 trial that compares the everolimus plus letrozole combination with the traditional chemotherapy regimen of carboplatin plus paclitaxel, Dr. Slomovitz added.
The Everolimus and Letrozole or Hormonal Therapy to Treat Endometrial Cancer phase 2 trial, initiated by the Gynecologic Oncology Group, ran at 26 U.S. centers, and randomized patients with stage III or IV recurrent, advanced, or persistent endometrial cancer who had either no or at most one prior course of chemotherapy. The study design also excluded patients who had previously been treated with an mammalian target of rapamycin inhibitor or hormonal therapy for their endometrial cancer. The control arm placed 37 patients on a standard hormonal therapy regimen of tamoxifen plus medroxyprogesterone, with 36 of patients in this subgroup evaluable.
The overall results in the two treatment arms were roughly similar, except for the striking benefit seen with everolimus(Afinitor) plus letrozole(Femara) in the chemotherapy-naive patents; 15 patients in each arm had not received any chemotherapy before entering the study. In this subgroup, among the patients who received conventional hormonal therapy, the objective response rate was 43% and progression-free survival was 6.6 months.
The two arms also differed by their pattern of grade 3 or 4 adverse events. Among the patients on everolimus plus letrozole, the most common were anemia (24%) and hyperglycemia (14%), both expected consequences of the regimen. The hormonal therapy arm led to an 8% incidence of thromboembolic events, which did not occur in the everolimus plus letrozole arm.
Another attraction of the everolimus and letrozole regimen is that it is oral and avoids the need for drug infusions, Dr. Slomovitz noted.
The investigator-initiated study received funding from Novartis, the company that markets everolimus and letrozole. Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
SOURCE: Slomovitz BM et al. SGO 2018, abstract 1.
The results from this study are exciting, with fairly compelling response rates. The rate of progression-free survival with everolimus and letrozole treatment is very impressive, compared with traditional chemotherapy using carboplatin and paclitaxel. The median progression-free survival rate of 21.6 months seen among the chemotherapy naive patients who received everolimus and letrozole showed a 7-month edge over the 14-month median progression-free survival previously reported with chemotherapy. This difference is very provocative and could be practice changing, but it of course needs further evaluation.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina in Chapel Hill. She made these comments as designated discussant for the study. She had no disclosures.
The results from this study are exciting, with fairly compelling response rates. The rate of progression-free survival with everolimus and letrozole treatment is very impressive, compared with traditional chemotherapy using carboplatin and paclitaxel. The median progression-free survival rate of 21.6 months seen among the chemotherapy naive patients who received everolimus and letrozole showed a 7-month edge over the 14-month median progression-free survival previously reported with chemotherapy. This difference is very provocative and could be practice changing, but it of course needs further evaluation.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina in Chapel Hill. She made these comments as designated discussant for the study. She had no disclosures.
The results from this study are exciting, with fairly compelling response rates. The rate of progression-free survival with everolimus and letrozole treatment is very impressive, compared with traditional chemotherapy using carboplatin and paclitaxel. The median progression-free survival rate of 21.6 months seen among the chemotherapy naive patients who received everolimus and letrozole showed a 7-month edge over the 14-month median progression-free survival previously reported with chemotherapy. This difference is very provocative and could be practice changing, but it of course needs further evaluation.
Paola A. Gehrig, MD , is professor of ob.gyn. and director of gynecologic oncology at the University of North Carolina in Chapel Hill. She made these comments as designated discussant for the study. She had no disclosures.
NEW ORLEANS – Combined treatment for 28 days with the mammalian target of rapamycin inhibitor everolimus plus the aromatase inhibitor letrozole in 37 women with recurrent endometrial cancer produced an overall objective response rate of 24% and an average progression-free survival rate of 6.3 months in a randomized phase 2 study with a total of 74 patients. The treatment was also relatively well tolerated, with more serious adverse vents of anemia and hyperglycemia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the most attention-grabbing finding of this study was that, in the subset of 15 women who had received no prior chemotherapy, the objective response rate on this regimen was 53% and median progression-free survival was 21.6 months, Brian M. Slomovitz, MD, said at the annual meeting of the Society of Gynecologic Oncology.
This level of response in the chemotherapy-naive subgroup was “very high, and not what we expected,” Dr. Slomovitz, , professor of ob.gyn. and human genetics and director of gynecologic oncology at the University of Miami, said in a video interview. “This is something we need to further investigate to see if we can make this part of standard care.”
Although he conceded that the data from this study were too limited to warrant a regulatory indication, he suggested that it might be enough to gain the everolimus plus letrozole combination used in the study citation as a treatment option in clinical guidelines. The next step should be a phase 3 trial that compares the everolimus plus letrozole combination with the traditional chemotherapy regimen of carboplatin plus paclitaxel, Dr. Slomovitz added.
The Everolimus and Letrozole or Hormonal Therapy to Treat Endometrial Cancer phase 2 trial, initiated by the Gynecologic Oncology Group, ran at 26 U.S. centers, and randomized patients with stage III or IV recurrent, advanced, or persistent endometrial cancer who had either no or at most one prior course of chemotherapy. The study design also excluded patients who had previously been treated with an mammalian target of rapamycin inhibitor or hormonal therapy for their endometrial cancer. The control arm placed 37 patients on a standard hormonal therapy regimen of tamoxifen plus medroxyprogesterone, with 36 of patients in this subgroup evaluable.
The overall results in the two treatment arms were roughly similar, except for the striking benefit seen with everolimus(Afinitor) plus letrozole(Femara) in the chemotherapy-naive patents; 15 patients in each arm had not received any chemotherapy before entering the study. In this subgroup, among the patients who received conventional hormonal therapy, the objective response rate was 43% and progression-free survival was 6.6 months.
The two arms also differed by their pattern of grade 3 or 4 adverse events. Among the patients on everolimus plus letrozole, the most common were anemia (24%) and hyperglycemia (14%), both expected consequences of the regimen. The hormonal therapy arm led to an 8% incidence of thromboembolic events, which did not occur in the everolimus plus letrozole arm.
Another attraction of the everolimus and letrozole regimen is that it is oral and avoids the need for drug infusions, Dr. Slomovitz noted.
The investigator-initiated study received funding from Novartis, the company that markets everolimus and letrozole. Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
SOURCE: Slomovitz BM et al. SGO 2018, abstract 1.
NEW ORLEANS – Combined treatment for 28 days with the mammalian target of rapamycin inhibitor everolimus plus the aromatase inhibitor letrozole in 37 women with recurrent endometrial cancer produced an overall objective response rate of 24% and an average progression-free survival rate of 6.3 months in a randomized phase 2 study with a total of 74 patients. The treatment was also relatively well tolerated, with more serious adverse vents of anemia and hyperglycemia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the most attention-grabbing finding of this study was that, in the subset of 15 women who had received no prior chemotherapy, the objective response rate on this regimen was 53% and median progression-free survival was 21.6 months, Brian M. Slomovitz, MD, said at the annual meeting of the Society of Gynecologic Oncology.
This level of response in the chemotherapy-naive subgroup was “very high, and not what we expected,” Dr. Slomovitz, , professor of ob.gyn. and human genetics and director of gynecologic oncology at the University of Miami, said in a video interview. “This is something we need to further investigate to see if we can make this part of standard care.”
Although he conceded that the data from this study were too limited to warrant a regulatory indication, he suggested that it might be enough to gain the everolimus plus letrozole combination used in the study citation as a treatment option in clinical guidelines. The next step should be a phase 3 trial that compares the everolimus plus letrozole combination with the traditional chemotherapy regimen of carboplatin plus paclitaxel, Dr. Slomovitz added.
The Everolimus and Letrozole or Hormonal Therapy to Treat Endometrial Cancer phase 2 trial, initiated by the Gynecologic Oncology Group, ran at 26 U.S. centers, and randomized patients with stage III or IV recurrent, advanced, or persistent endometrial cancer who had either no or at most one prior course of chemotherapy. The study design also excluded patients who had previously been treated with an mammalian target of rapamycin inhibitor or hormonal therapy for their endometrial cancer. The control arm placed 37 patients on a standard hormonal therapy regimen of tamoxifen plus medroxyprogesterone, with 36 of patients in this subgroup evaluable.
The overall results in the two treatment arms were roughly similar, except for the striking benefit seen with everolimus(Afinitor) plus letrozole(Femara) in the chemotherapy-naive patents; 15 patients in each arm had not received any chemotherapy before entering the study. In this subgroup, among the patients who received conventional hormonal therapy, the objective response rate was 43% and progression-free survival was 6.6 months.
The two arms also differed by their pattern of grade 3 or 4 adverse events. Among the patients on everolimus plus letrozole, the most common were anemia (24%) and hyperglycemia (14%), both expected consequences of the regimen. The hormonal therapy arm led to an 8% incidence of thromboembolic events, which did not occur in the everolimus plus letrozole arm.
Another attraction of the everolimus and letrozole regimen is that it is oral and avoids the need for drug infusions, Dr. Slomovitz noted.
The investigator-initiated study received funding from Novartis, the company that markets everolimus and letrozole. Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
SOURCE: Slomovitz BM et al. SGO 2018, abstract 1.
REPORTING FROM SGO 2018
Key clinical point: Treatment of recurrent endometrial cancer with everolimus and letrozole shows promise.
Major finding: Among 15 chemotherapy-naive patients, objective responses occurred in 53% and median progression-free survival was 21.6 months.
Study details: A multicenter, phase 2 randomized study in 74 patients.
Disclosures: The investigator-initiated study received funding from Novartis, the company that markets everolimus(Afinitor) and letrozole(Femara). Dr. Slomovitz has been an advisor to Advaxis, AstraZeneca, Clovis, Genmab, Jannsen, and Tesaro, and he has received research funding from Novartis.
Source: Slomovitz BM et al. SGO 2018, abstract 1.
Drug coated balloons may match stents for small coronary lesions
WASHINGTON – For treating de novo coronary lesions in vessels smaller than 2.75 mm, drug coated balloon angioplasty is as safe and may be as effective as drug-eluting stents, according to a multicenter randomized trial presented as a late-breaker at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The aim of PCI [percutaneous coronary intervention] without leaving any metal behind seems to be feasible and safe with drug coated balloons,” reported Victor A. Jimenez Diaz, MD, of the department of cardiology at University Hospital, Vigo, Spain.
In this study, 94 patients with de novo coronary lesions in small diameter vessels were randomized to treatment with a paclitaxel drug coated balloon (DCB) (IN.PACT Falcon, Medtronic) or a zotarolimus drug-eluting stent (DES) (Resolute Integrity, Medtronic). Lesions in vessels between 2.0 and 2.75 mm in diameter were eligible; 137 lesions were treated in a study with seven participating centers in Spain.
For entry, target lesions had to have a stenosis of at least 70% by visual estimation or at least 50% by quantitative coronary angiography. Lesion length was limited to less than 25 mm, and severely calcified lesions were excluded.
The primary endpoint was a composite major adverse coronary event (MACE) endpoint of cardiac death, myocardial infarction, or revascularization at 12 months of follow-up. Crossovers by discretion of the interventional team were permitted.
At 12 months, MACE had occurred in 4.4% of those in the DCB group and 11.1% of those in the DES group. All primary endpoint events in both groups involved revascularizations. The two events in the DCB group were clinically driven target vessel revascularizations. Only one of the five events in the DES group was a clinically driven target lesion revascularization; the remaining four were revascularizations performed for nontarget vessels.
Four patients (8%) in the DCB group crossed over, each because of a dissection and managed with a bare-metal stent. There were four other dissections in the DCB group; one type F dissection resulted in a bailout and three dissections were managed conservatively. There were no crossovers in the DES group, and of the two dissections in the group, both were managed with the originally assigned stenting strategy.
Calling DCB a safe strategy for small de novo coronary stenosis, Dr. Jimenez Diaz said, “The procedural success rates were comparable.” However, he acknowledged that because of low enrollment, the study was “underpowered for clinical events.” The original power calculation called for a study of 200 patients.
“We can say results are encouraging,” Dr. Jimenez Diaz said.
Several discussants at the late-breaker abstracts agreed that DCB is an intriguing option for a difficult problem. They also agreed that restoring blood flow without leaving a permanent device is an attractive concept. However, they emphasized that a larger study is needed to declare that DCB and DES are equivalent strategies in regard to risk of MACE.
While agreeing that more data powered for events are needed, Cindy Grines, MD, chief of cardiology, Hofstra University, Hempstead, N.Y., was among those who suggested that this approach might be “worth a try.” She indicated that this study, which involved interventionalists at multiple centers, does provide support for the safety of DCB.
SOURCE: Jimenez Diaz VA. CRT 2018.
WASHINGTON – For treating de novo coronary lesions in vessels smaller than 2.75 mm, drug coated balloon angioplasty is as safe and may be as effective as drug-eluting stents, according to a multicenter randomized trial presented as a late-breaker at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The aim of PCI [percutaneous coronary intervention] without leaving any metal behind seems to be feasible and safe with drug coated balloons,” reported Victor A. Jimenez Diaz, MD, of the department of cardiology at University Hospital, Vigo, Spain.
In this study, 94 patients with de novo coronary lesions in small diameter vessels were randomized to treatment with a paclitaxel drug coated balloon (DCB) (IN.PACT Falcon, Medtronic) or a zotarolimus drug-eluting stent (DES) (Resolute Integrity, Medtronic). Lesions in vessels between 2.0 and 2.75 mm in diameter were eligible; 137 lesions were treated in a study with seven participating centers in Spain.
For entry, target lesions had to have a stenosis of at least 70% by visual estimation or at least 50% by quantitative coronary angiography. Lesion length was limited to less than 25 mm, and severely calcified lesions were excluded.
The primary endpoint was a composite major adverse coronary event (MACE) endpoint of cardiac death, myocardial infarction, or revascularization at 12 months of follow-up. Crossovers by discretion of the interventional team were permitted.
At 12 months, MACE had occurred in 4.4% of those in the DCB group and 11.1% of those in the DES group. All primary endpoint events in both groups involved revascularizations. The two events in the DCB group were clinically driven target vessel revascularizations. Only one of the five events in the DES group was a clinically driven target lesion revascularization; the remaining four were revascularizations performed for nontarget vessels.
Four patients (8%) in the DCB group crossed over, each because of a dissection and managed with a bare-metal stent. There were four other dissections in the DCB group; one type F dissection resulted in a bailout and three dissections were managed conservatively. There were no crossovers in the DES group, and of the two dissections in the group, both were managed with the originally assigned stenting strategy.
Calling DCB a safe strategy for small de novo coronary stenosis, Dr. Jimenez Diaz said, “The procedural success rates were comparable.” However, he acknowledged that because of low enrollment, the study was “underpowered for clinical events.” The original power calculation called for a study of 200 patients.
“We can say results are encouraging,” Dr. Jimenez Diaz said.
Several discussants at the late-breaker abstracts agreed that DCB is an intriguing option for a difficult problem. They also agreed that restoring blood flow without leaving a permanent device is an attractive concept. However, they emphasized that a larger study is needed to declare that DCB and DES are equivalent strategies in regard to risk of MACE.
While agreeing that more data powered for events are needed, Cindy Grines, MD, chief of cardiology, Hofstra University, Hempstead, N.Y., was among those who suggested that this approach might be “worth a try.” She indicated that this study, which involved interventionalists at multiple centers, does provide support for the safety of DCB.
SOURCE: Jimenez Diaz VA. CRT 2018.
WASHINGTON – For treating de novo coronary lesions in vessels smaller than 2.75 mm, drug coated balloon angioplasty is as safe and may be as effective as drug-eluting stents, according to a multicenter randomized trial presented as a late-breaker at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The aim of PCI [percutaneous coronary intervention] without leaving any metal behind seems to be feasible and safe with drug coated balloons,” reported Victor A. Jimenez Diaz, MD, of the department of cardiology at University Hospital, Vigo, Spain.
In this study, 94 patients with de novo coronary lesions in small diameter vessels were randomized to treatment with a paclitaxel drug coated balloon (DCB) (IN.PACT Falcon, Medtronic) or a zotarolimus drug-eluting stent (DES) (Resolute Integrity, Medtronic). Lesions in vessels between 2.0 and 2.75 mm in diameter were eligible; 137 lesions were treated in a study with seven participating centers in Spain.
For entry, target lesions had to have a stenosis of at least 70% by visual estimation or at least 50% by quantitative coronary angiography. Lesion length was limited to less than 25 mm, and severely calcified lesions were excluded.
The primary endpoint was a composite major adverse coronary event (MACE) endpoint of cardiac death, myocardial infarction, or revascularization at 12 months of follow-up. Crossovers by discretion of the interventional team were permitted.
At 12 months, MACE had occurred in 4.4% of those in the DCB group and 11.1% of those in the DES group. All primary endpoint events in both groups involved revascularizations. The two events in the DCB group were clinically driven target vessel revascularizations. Only one of the five events in the DES group was a clinically driven target lesion revascularization; the remaining four were revascularizations performed for nontarget vessels.
Four patients (8%) in the DCB group crossed over, each because of a dissection and managed with a bare-metal stent. There were four other dissections in the DCB group; one type F dissection resulted in a bailout and three dissections were managed conservatively. There were no crossovers in the DES group, and of the two dissections in the group, both were managed with the originally assigned stenting strategy.
Calling DCB a safe strategy for small de novo coronary stenosis, Dr. Jimenez Diaz said, “The procedural success rates were comparable.” However, he acknowledged that because of low enrollment, the study was “underpowered for clinical events.” The original power calculation called for a study of 200 patients.
“We can say results are encouraging,” Dr. Jimenez Diaz said.
Several discussants at the late-breaker abstracts agreed that DCB is an intriguing option for a difficult problem. They also agreed that restoring blood flow without leaving a permanent device is an attractive concept. However, they emphasized that a larger study is needed to declare that DCB and DES are equivalent strategies in regard to risk of MACE.
While agreeing that more data powered for events are needed, Cindy Grines, MD, chief of cardiology, Hofstra University, Hempstead, N.Y., was among those who suggested that this approach might be “worth a try.” She indicated that this study, which involved interventionalists at multiple centers, does provide support for the safety of DCB.
SOURCE: Jimenez Diaz VA. CRT 2018.
REPORTING FROM CRT 2018
Key clinical point: Drug-coated balloon stents were as safe and effective as drug-eluting stents for treatment of small de novo coronary lesions in a randomized trial.
Major finding: (4.4% vs. 11%).
Study details: A multicenter randomized trial.
Disclosures: Dr. Jimenez Diaz reports no potential conflicts of interest.
Source: Jimenez Diaz VA. CRT 2018.