User login
A Recalcitrant Case of Toxic Epidermal Necrolysis
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
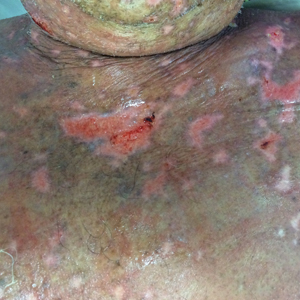
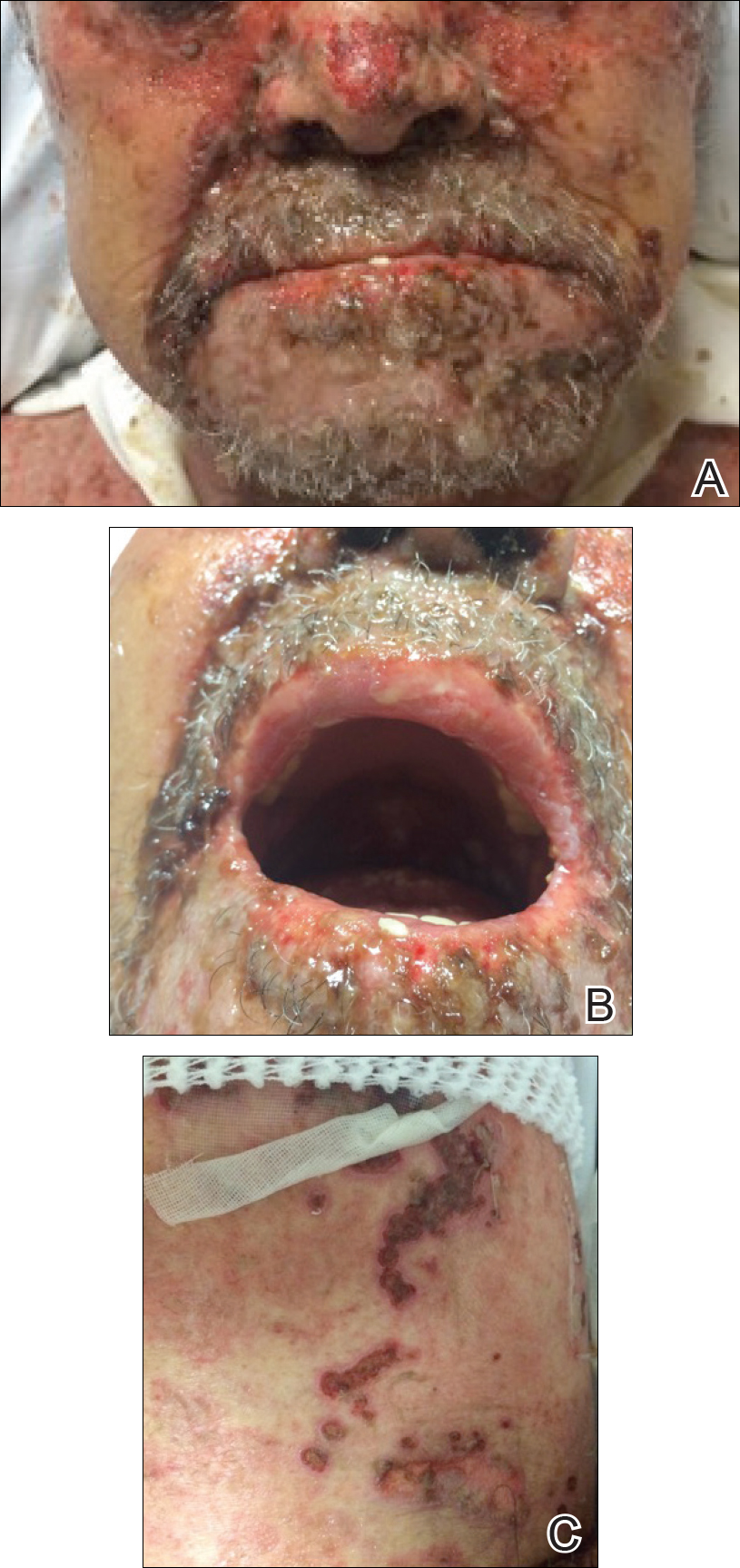
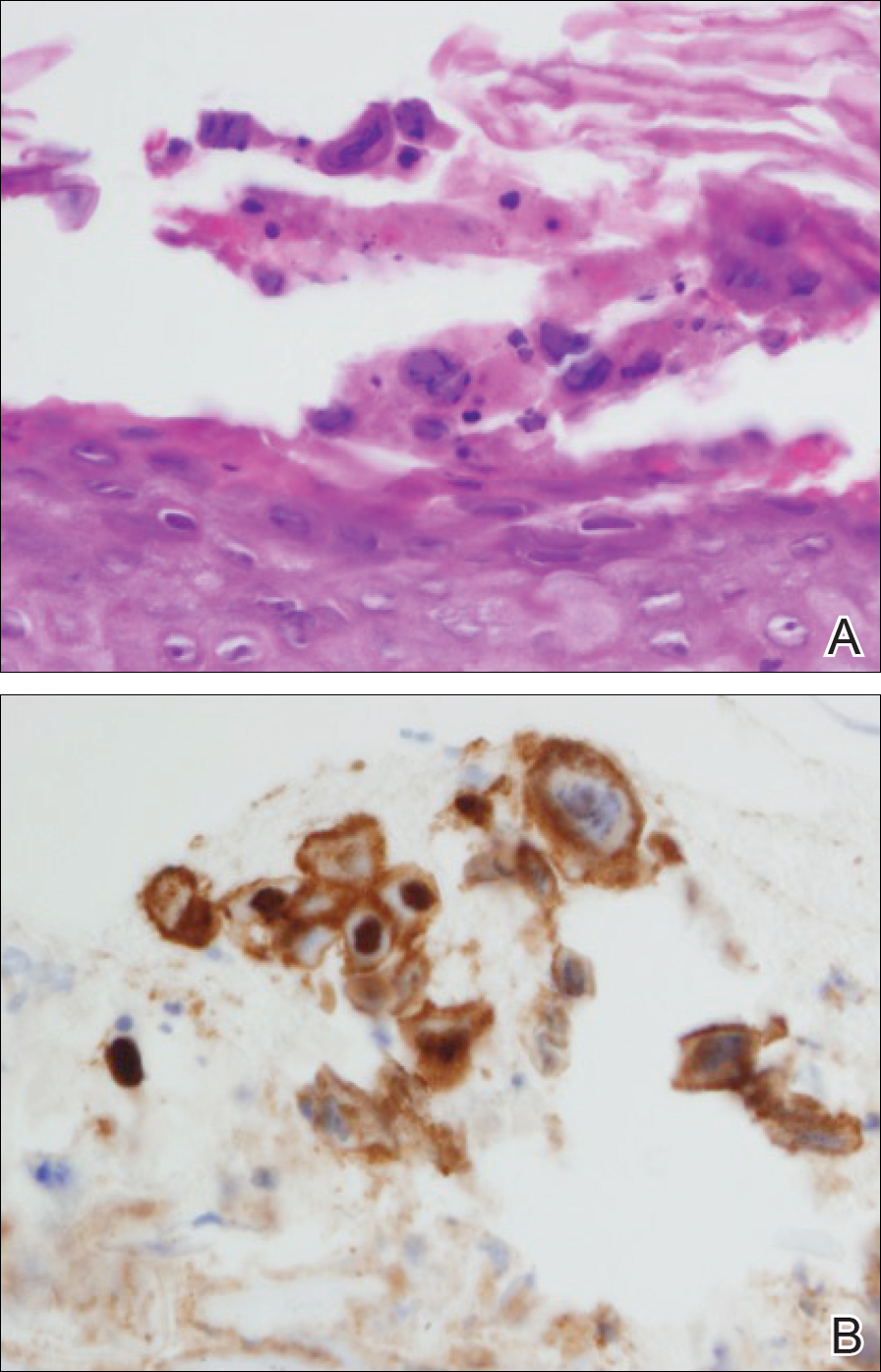
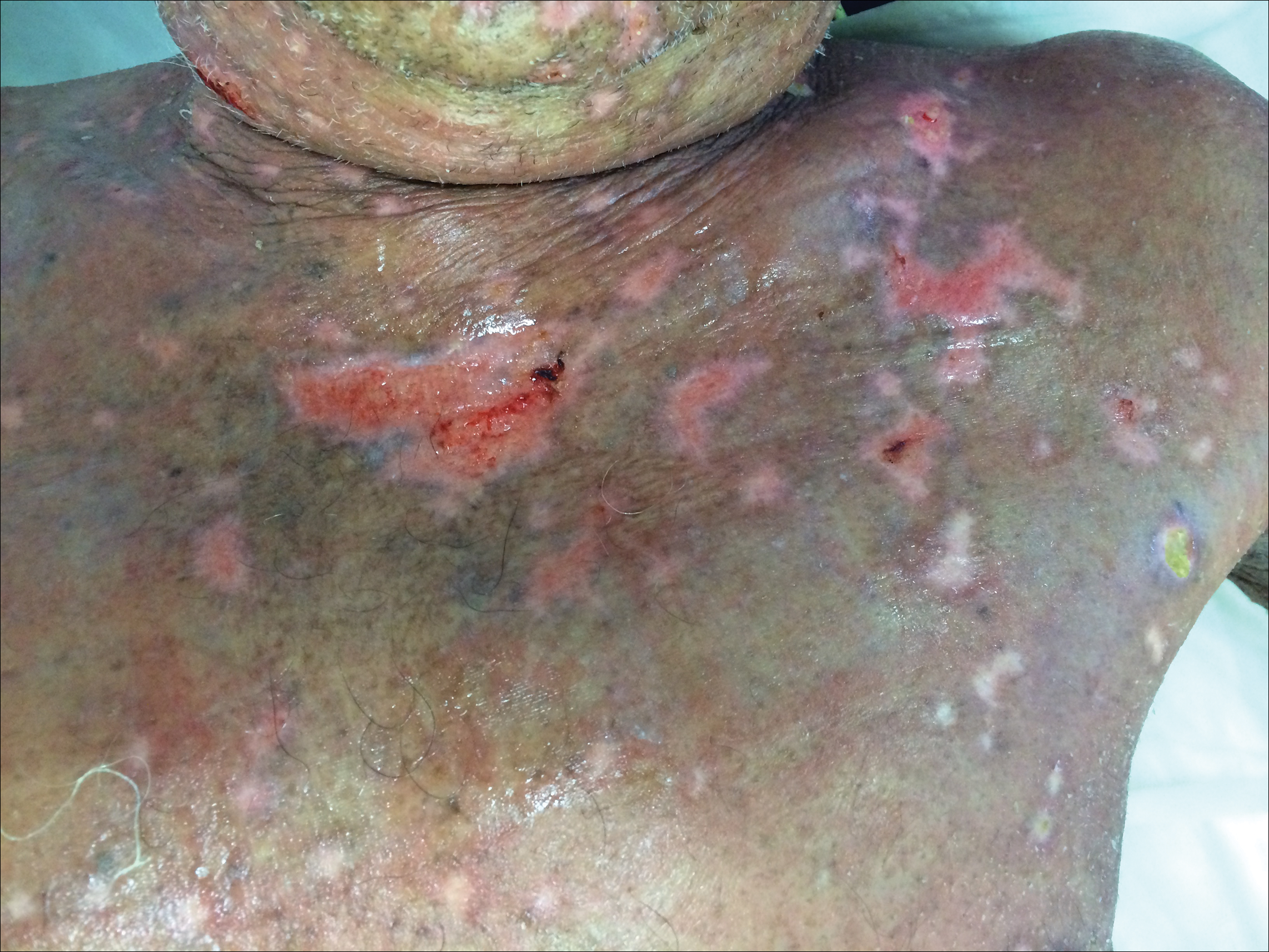
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.
Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
Practice Points
- Toxic epidermal necrolysis can be difficult to diagnose and treat.
- Patients who are refractory to treatment should prompt further management considerations.
The retirement horizon creeps up
My lease is up later this year, after 5 1/2 years. It doesn’t seem that long. Some days it feels like I just moved in.
As a result, I had an email exchange recently with the building’s manager and we hashed out an agreement on a new 10-year contract. In the process, I realized that sort of time frame might (and, again, I say might) take me into retirement.
And now I’m starting to think about retiring and the career endgame.
Granted, it’s still 10 years away, and knowing me I’ll probably want to work another 5 years or so beyond that if I can. I like what I do and would probably go stir crazy without this job. Besides, given the current anti-doctor financial climate, I may not be able to retire in 10 years, even if I want to.
But still, it’s an odd realization to think that, after all those applications, and classes, and tests, and rotations, and all the other things you went through ... that your career is closer to wrapping up than it is to the beginning.
How did that happen?
And I’ll continue to try. Even after the halfway point I still get up each morning wanting to help people. The same sentiments I expressed in a personal statement so long ago are still there, and hopefully always will be. When they’re gone, it’s time to leave. Hopefully, they’ll be with me until I’m ready to sign off.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My lease is up later this year, after 5 1/2 years. It doesn’t seem that long. Some days it feels like I just moved in.
As a result, I had an email exchange recently with the building’s manager and we hashed out an agreement on a new 10-year contract. In the process, I realized that sort of time frame might (and, again, I say might) take me into retirement.
And now I’m starting to think about retiring and the career endgame.
Granted, it’s still 10 years away, and knowing me I’ll probably want to work another 5 years or so beyond that if I can. I like what I do and would probably go stir crazy without this job. Besides, given the current anti-doctor financial climate, I may not be able to retire in 10 years, even if I want to.
But still, it’s an odd realization to think that, after all those applications, and classes, and tests, and rotations, and all the other things you went through ... that your career is closer to wrapping up than it is to the beginning.
How did that happen?
And I’ll continue to try. Even after the halfway point I still get up each morning wanting to help people. The same sentiments I expressed in a personal statement so long ago are still there, and hopefully always will be. When they’re gone, it’s time to leave. Hopefully, they’ll be with me until I’m ready to sign off.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My lease is up later this year, after 5 1/2 years. It doesn’t seem that long. Some days it feels like I just moved in.
As a result, I had an email exchange recently with the building’s manager and we hashed out an agreement on a new 10-year contract. In the process, I realized that sort of time frame might (and, again, I say might) take me into retirement.
And now I’m starting to think about retiring and the career endgame.
Granted, it’s still 10 years away, and knowing me I’ll probably want to work another 5 years or so beyond that if I can. I like what I do and would probably go stir crazy without this job. Besides, given the current anti-doctor financial climate, I may not be able to retire in 10 years, even if I want to.
But still, it’s an odd realization to think that, after all those applications, and classes, and tests, and rotations, and all the other things you went through ... that your career is closer to wrapping up than it is to the beginning.
How did that happen?
And I’ll continue to try. Even after the halfway point I still get up each morning wanting to help people. The same sentiments I expressed in a personal statement so long ago are still there, and hopefully always will be. When they’re gone, it’s time to leave. Hopefully, they’ll be with me until I’m ready to sign off.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
FDA updates breast implant–associated lymphoma cases, risk
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
(BIA-ALCL), including nine deaths.
This figure includes all medical device reports received by the agency between 2011 and September 2017. The FDA recently provided an update on ALCL linked to breast implants and an estimate of lifetime risk of developing ALCL.
Based on available medical literature, the lifetime risk of developing BIA-ALCL for patients with textured breast implants ranges from 1 in 3,817 to 1 in 30,000, according to the update.
Of the 272 reports with data on surface type, 242 were textured implants and 30 were smooth implants. In addition, 413 reports include information on the implant fill type: 234 used silicone gel and 179 were saline filled.
“The FDA has been closely tracking the relationship between breast implants and a rare type of non-Hodgkin’s lymphoma since we first identified this possible association. We’ve been working to gather additional information to better characterize and quantify the risk so that patients and providers can have more informed discussions about breast implants,” said Binita Ashar, MD, director of the division of surgical devices in the FDA’s Center for Devices and Radiological Health. “As part of that effort, we are working to update and enhance the information we have on this association, including updating the total number of known cases of BIA-ALCL and the lifetime risk of developing BIA-ALCL as reported in medical literature.”
The possible association between breast implants and the development of anaplastic large cell lymphoma (ALCL) was first identified in 2011. At that time, there were not enough cases of to determine what factors increased a patient’s risk of developing the disease. As more information became available, the World Health Organization designated BIA-ALCL as a T-cell lymphoma that can develop following breast implants.
Serial entrepreneur examines the risk-to-reward ratio balance in GI innovation
BOSTON – Just out of fellowship, Christopher C. Thompson, MD, director of therapeutic endoscopy, Brigham and Women’s Hospital, Boston, adapted an antireflux suturing device for use in a bariatric procedure. It worked so well he began using it routinely and taught others the technique. That was the first step in a journey that has taken him from consulting with industry to a founder of start-ups.
“The device company heard about what we were doing and were interested,” Dr. Thompson recounted during his How-I-Did-It lecture at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. In the end, he served as a consultant in the development of a new suturing device specific for the bariatric procedure. This included helping secure a patent and learning first-hand what steps are needed to get a device to market.
“I do not have any regrets. It was good for my career and fun to be involved, but there was not much financial gain for me or for my department,” Dr. Thompson said.
His subsequent experience with licensing was an incremental step forward. In one example, he worked on developing an endoscopic simulator, an important unmet need both for teaching and evaluating skills in diagnostic colonoscopy, including a kinematic analysis that helped identify techniques that are associated with high levels of skill.
“We developed the technology in-house through a series of grants. The risks were low, but the rewards were better because the money helped fund activities in our department,” he said.
That device, too, has been very successful, but Dr. Thompson said it is important to recognize how far innovation can go when the work stays in the academic setting and the goal is licensing the technology. More recently, he took a nonsurgical anastomosis device through preclinical testing, but he was then unable to attract a device company for the next steps of development.
“With no one interested, we created a start-up,” Dr. Thompson said. The company, GI Windows, has now taken this product, a magnetic endo-luminal anastomosis bypass device for the treatment of diabetes mellitus, into advanced stages of clinical testing. Relative to licensing arrangements, this involved a different level of participation.
“A start-up means creating a board, raising money, and being involved in details that can involve a lot of heavy lifting,” Dr. Thompson said. “It is basically a second job.”
The ongoing clinical studies in patients with diabetes have been very encouraging. Dr. Thompson reported that a large proportion of patients with diabetes fitted with the device have been able to reduce or discontinue their antidiabetic medications, and high rates of excess weight loss have been documented.
GI Windows was created for the sole purpose of developing the anastomosis device, but Dr. Thompson was also involved in creating another company, now sold, that started without a specific device in mind.
“The products we developed were just from brainstorming on unmet needs, and we had several successes. That was a chance to learn new areas of the business, including building a sales force and learning how to get involved in international distribution, which were separate from trying simply to produce a viable clinical tool,” he said.
Creating companies, rather than licensing ideas, trades higher risk for greater reward, but Dr. Thompson emphasized that these rewards are not just financial.
“It is exciting to develop a team you trust, get a successful company off the ground, and watch it grow,” Dr. Thompson said. He indicated that the risk-to-reward calculation should not be undertaken independent of the value of the learning experience.
BOSTON – Just out of fellowship, Christopher C. Thompson, MD, director of therapeutic endoscopy, Brigham and Women’s Hospital, Boston, adapted an antireflux suturing device for use in a bariatric procedure. It worked so well he began using it routinely and taught others the technique. That was the first step in a journey that has taken him from consulting with industry to a founder of start-ups.
“The device company heard about what we were doing and were interested,” Dr. Thompson recounted during his How-I-Did-It lecture at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. In the end, he served as a consultant in the development of a new suturing device specific for the bariatric procedure. This included helping secure a patent and learning first-hand what steps are needed to get a device to market.
“I do not have any regrets. It was good for my career and fun to be involved, but there was not much financial gain for me or for my department,” Dr. Thompson said.
His subsequent experience with licensing was an incremental step forward. In one example, he worked on developing an endoscopic simulator, an important unmet need both for teaching and evaluating skills in diagnostic colonoscopy, including a kinematic analysis that helped identify techniques that are associated with high levels of skill.
“We developed the technology in-house through a series of grants. The risks were low, but the rewards were better because the money helped fund activities in our department,” he said.
That device, too, has been very successful, but Dr. Thompson said it is important to recognize how far innovation can go when the work stays in the academic setting and the goal is licensing the technology. More recently, he took a nonsurgical anastomosis device through preclinical testing, but he was then unable to attract a device company for the next steps of development.
“With no one interested, we created a start-up,” Dr. Thompson said. The company, GI Windows, has now taken this product, a magnetic endo-luminal anastomosis bypass device for the treatment of diabetes mellitus, into advanced stages of clinical testing. Relative to licensing arrangements, this involved a different level of participation.
“A start-up means creating a board, raising money, and being involved in details that can involve a lot of heavy lifting,” Dr. Thompson said. “It is basically a second job.”
The ongoing clinical studies in patients with diabetes have been very encouraging. Dr. Thompson reported that a large proportion of patients with diabetes fitted with the device have been able to reduce or discontinue their antidiabetic medications, and high rates of excess weight loss have been documented.
GI Windows was created for the sole purpose of developing the anastomosis device, but Dr. Thompson was also involved in creating another company, now sold, that started without a specific device in mind.
“The products we developed were just from brainstorming on unmet needs, and we had several successes. That was a chance to learn new areas of the business, including building a sales force and learning how to get involved in international distribution, which were separate from trying simply to produce a viable clinical tool,” he said.
Creating companies, rather than licensing ideas, trades higher risk for greater reward, but Dr. Thompson emphasized that these rewards are not just financial.
“It is exciting to develop a team you trust, get a successful company off the ground, and watch it grow,” Dr. Thompson said. He indicated that the risk-to-reward calculation should not be undertaken independent of the value of the learning experience.
BOSTON – Just out of fellowship, Christopher C. Thompson, MD, director of therapeutic endoscopy, Brigham and Women’s Hospital, Boston, adapted an antireflux suturing device for use in a bariatric procedure. It worked so well he began using it routinely and taught others the technique. That was the first step in a journey that has taken him from consulting with industry to a founder of start-ups.
“The device company heard about what we were doing and were interested,” Dr. Thompson recounted during his How-I-Did-It lecture at the 2018 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology. In the end, he served as a consultant in the development of a new suturing device specific for the bariatric procedure. This included helping secure a patent and learning first-hand what steps are needed to get a device to market.
“I do not have any regrets. It was good for my career and fun to be involved, but there was not much financial gain for me or for my department,” Dr. Thompson said.
His subsequent experience with licensing was an incremental step forward. In one example, he worked on developing an endoscopic simulator, an important unmet need both for teaching and evaluating skills in diagnostic colonoscopy, including a kinematic analysis that helped identify techniques that are associated with high levels of skill.
“We developed the technology in-house through a series of grants. The risks were low, but the rewards were better because the money helped fund activities in our department,” he said.
That device, too, has been very successful, but Dr. Thompson said it is important to recognize how far innovation can go when the work stays in the academic setting and the goal is licensing the technology. More recently, he took a nonsurgical anastomosis device through preclinical testing, but he was then unable to attract a device company for the next steps of development.
“With no one interested, we created a start-up,” Dr. Thompson said. The company, GI Windows, has now taken this product, a magnetic endo-luminal anastomosis bypass device for the treatment of diabetes mellitus, into advanced stages of clinical testing. Relative to licensing arrangements, this involved a different level of participation.
“A start-up means creating a board, raising money, and being involved in details that can involve a lot of heavy lifting,” Dr. Thompson said. “It is basically a second job.”
The ongoing clinical studies in patients with diabetes have been very encouraging. Dr. Thompson reported that a large proportion of patients with diabetes fitted with the device have been able to reduce or discontinue their antidiabetic medications, and high rates of excess weight loss have been documented.
GI Windows was created for the sole purpose of developing the anastomosis device, but Dr. Thompson was also involved in creating another company, now sold, that started without a specific device in mind.
“The products we developed were just from brainstorming on unmet needs, and we had several successes. That was a chance to learn new areas of the business, including building a sales force and learning how to get involved in international distribution, which were separate from trying simply to produce a viable clinical tool,” he said.
Creating companies, rather than licensing ideas, trades higher risk for greater reward, but Dr. Thompson emphasized that these rewards are not just financial.
“It is exciting to develop a team you trust, get a successful company off the ground, and watch it grow,” Dr. Thompson said. He indicated that the risk-to-reward calculation should not be undertaken independent of the value of the learning experience.
REPORTING FROM 2018 AGA TECH SUMMIT
Study links RA flares after joint replacement to disease activity, not medications
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
Patients with the most severe cases of rheumatoid arthritis are more likely to suffer flares after knee or hip replacement surgery, a new study finds, and it doesn’t seem to matter whether they stop taking biologics before their operation.
“We found that the majority of patients had active disease at the time of surgery, contrary to prior statements that RA patients have inactive disease at the time they go for hip or knee replacement. In fact, the majority – 65% of the patients – reported a flare of RA within 6 weeks of surgery,” lead author Susan M. Goodman, MD, of Cornell University and the Hospital for Special Surgery, New York, said in an interview. “Surprisingly, although more of the flaring patients were taking potent biologics that had been withheld preoperatively, the major risk factor for flares was their baseline disease activity.”
According to Dr. Goodman, the researchers launched the study to better understand how medical decisions prior to joint replacement surgery affect the progress of RA afterward.
In terms of continuing RA drug treatment, she said, “the decision really hinges on the risk of infection versus the risk of flare, and we didn’t know the usual course of events for these patients.”
In addition, she said, “many doctors incorrectly think that the majority of patients with RA have ‘burnt-out’ or inactive disease at the time of hip or knee replacement surgery.”
For the study, the researchers prospectively followed 120 patients who were to undergo joint replacement surgery. (The researchers initially approached 354 patients, of whom 169 declined to participate. Another 65 were dropped from the study for various reasons, including 42 who did not sufficiently fill out questionnaires and were deleted from the final analysis.)
The researchers tracked the patients before surgery and for 6 weeks after surgery. A majority of the patients were female (83%) and white (81%), with a mean age of 62 and a median RA symptom duration of 15 years. A total of 44% underwent hip replacement surgery while the rest underwent knee replacement surgery. Just over half of the patients were taking biologics, which were stopped prior to surgery, while glucocorticoids and methotrexate were usually continued.
Just under two-thirds of the patients flared within the first 6 weeks after surgery. The researchers didn’t find any connection between the flares and stopping biologics or using methotrexate. They did, however, link higher baseline RA activity to postsurgery flaring (odds ratio, 2.11; P = .015).
Dr. Goodman said that she and her colleagues continue to collect data to better understand flares and the link to disease severity. “The long-term implications of this are not yet known. We would like to know the effect on long-term functional outcome and complication rate.”
The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. Dr. Goodman disclosed receiving research funding from Novartis and Roche.
SOURCE: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point:
Major finding: Sixty-five percent of RA patients developed flares after joint replacement surgery, and it was more common in those with higher baseline RA activity (odds ratio, 2.11; P = .015).
Study details: Prospective study of 120 patients with RA who underwent hip replacement (44%) or knee replacement (56%).
Disclosures: The National Institutes of Health, the Weill Cornell Clinical Translational Science Center, and the Block Family Foundation supported the study. The lead author disclosed receiving research funding from Novartis and Roche.
Source: Goodman S et al. J Rheumatol. 2018 Mar 15. doi: 10.3899/jrheum.170366.
Low microbiota diversity linked to poor survival after transplant
SALT LAKE CITY – A multicenter study confirmed that diversity of gut microbiota is associated with better survival after allogeneic hematopoietic cell transplantation (HCT), while low diversity and the predominance of pathogenic bacteria are linked to graft versus host disease (GVHD).
Lower calorie intake and exposure to broad-spectrum antibiotics were both associated with lower diversity, the study found.
“One of the striking findings early on was this association between diversity in the gut and overall survival,” said Jonathan Peled, MD, PhD, noting that his research group also saw that high gut diversity was associated with lower rates of GVHD-related mortality.
“The first question that I want to ask today is ‘Are the patterns of microbiota injury that have been described in single-center studies and their association with clinical outcomes consistent across geography?’” Dr. Peled said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
To answer this, Dr. Peled and his associates at Memorial Sloan Kettering Cancer Center (MSKCC), New York, teamed up with a research group at Duke University, Durham, N.C., and with investigators in Regensburg, Germany. The international group devised a study that would use centralized sequencing and analysis to examine patient fecal samples from all three centers.
In all, 5,310 samples were obtained from 1,034 HCT patients. MSKCC contributed most of the samples (n = 908, 87.8%), with Regensburg contributing 79 (7.6%) and Duke contributing 47 (4.5%).
The most common malignancies treated were acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. The balance of graft sources and conditioning intensity varied between centers, but overall, more than three-quarters of grafts were from peripheral blood stem cells and just over half of patients received myeloablative conditioning.
The centralized microbiota profiling involved extracting bacterial DNA, and then using polymerase chain reaction to amplify 16sRNA for sequencing and subsequent taxonomic identification.
“Samples can be segregated into clusters according to microbiota composition,” said Dr. Peled, a medical oncologist at MSKCC. The investigators used an algorithm called t-distributed stochastic neighbor embedding, or tSNE, to help detect patterns in microbiota composition and diversity before and throughout the HCT process. Visualizations using tSNE allow for two-dimensional representations of complicated associations and interrelatedness in data.
“Color-coded by diversity and time, we see that these early samples tend to be more diverse,” in the tSNE analyses, Dr. Peled said. The later clusters, he said, show evidence of lower diversity and injury.
Individual samples can also be coded in a way that shows clusters by abundance of various bacterial taxa, Dr. Peled said. “The early, diverse cluster tends to be dominated, or filled, by anaerobic commensals such as Firmicutes and Clostridia, which we and others have found are associated with good outcomes after transplant.”
The lower-diversity states seen later, after transplant, tend to be dominated by a variety of pathogenic bacteria, Dr. Peled said. These include Enterococcus and Proteobacteria, a phylum that includes Klebsiella and Escherichia coli species. This predominance has been associated with subsequent bacteremia, he said.
“Patients tend to enter transplant with a relatively diverse flora, and a frequent event in the posttransplant samples is domination by these pathobiomes,” Dr. Peled said. “In some cases, almost the entire composition of the gut is [composed] of a single species.” This loss of diversity and single-species domination was seen across the three geographically diverse research sites, he said.
This decimation of diversity is linked to poor transplant outcomes. In particular, Dr. Peled said, an enterococcus-dominated gut had previously been associated with higher risk for acute GVHD and with gastrointestinal GVHD.
Here, the multisite data showed that at Regensburg, higher enterococcus abundance on days 7-14 post HCT was associated with increased risk of GI GVHD. At MSKCC, enterococcus domination was associated with a hazard ratio of 1.4 for acute GVHD (P = .008). The MSKCC group used data from 503 patients, defining domination as at least 30% relative abundance in any sample from post-HCT days 7-21.
Patients at both MSKCC and Regensburg had a better chance of overall survival if they had high intestinal microbial diversity around the period of neutrophil engraftment, as seen in a sample collected within 7 days of post-HCT day 14. At MSKCC, data for 651 patients showed a statistically significant association (P = .006); this finding was reproduced at Regensburg, which also saw a significant association (P = .015) for the 59 patients studied, Dr. Peled reported.
Increased treatment-related mortality was seen for patients who had low microbial diversity following neutrophil engraftment as well. Of 372 MSKCC patients who had samples available 7-50 days after engraftment, high diversity was associated with better overall survival, and with lower treatment-related mortality (P = .03 for both).
Dr. Peled and his collaborators also divided patients into quartiles by amount of biodiversity. They found that comparing the highest to the lowest biodiversity quartile showed significantly overall survival benefits for the highest-diversity group (P = .007).
The problem starts before transplant, Dr. Peled explained. The researchers found that compared with healthy controls at MSKCC and data from the Human Microbiome Project, HCT patients entered their transplant with significantly less gut biodiversity.
The second question to be addressed is “What are the key environmental determinants of intestinal microbiota composition?” said Dr. Peled.
“Peri-HCT exposure to broad-spectrum antibiotics is associated with lower intestinal microbial diversity,” he said. For 5,936 samples taken from 976 patients receiving allogeneic HCT, the most significant difference in diversity between those with and without broad-spectrum antibiotic exposure was seen at day 15 post transplant (P = .008).
Higher calorie intake was also associated with greater diversity (P less than .001). Higher dietary fiber intake was associated with higher abundance of Blautia, a genus considered to be a healthy commensal microorganism, Dr. Peled said.
“Conditioning intensity is associated with the magnitude of diversity loss, and with distinct microbiome configurations,” said Dr. Peled. Using 4,311 samples from 908 patients, a myeloablative conditioning regimen (n = 508) was associated with significantly less diversity when compared with reduced intensity (n = 316) and nonmyeloablative regimens (n = 84; P =.002 and P less than .001, respectively).
To answer a third question – What is the natural history of recovery from microbiota injury after HCT? – the investigators looked at trends over time for 28 allogeneic HCT recipients. With a total of 294 samples for analysis, Dr. Peled and his group found that “diversity increases, but often to a configuration distinct from the pre-HCT state.” It took some patients nearly a year to return to their pretransplant level of diversity.
Patients in the subset of those who go on to develop lower gastrointestinal GVHD have an intestinal microbiota composition that is distinct from those patients whose GVHD exclusively involved the upper gastrointestinal tract, the skin, or the liver (P = .019), Dr. Peled said.
He and his team are currently enrolling patients for a phase 2 randomized clinical trial (NCT03078010) that will explore strategies to deescalate the use of broad-spectrum antibiotics for febrile neutropenia in patients with allogeneic HCT. The trial will randomize patients to receive either piperacillin-tazobactam, the current standard of care at MSKCC, or cefepime with deescalation to aztreonam with vancomycin, the microbiota-sparing strategy. The trial will examine the abundance of Clostridiales and Blautia species, gut biodiversity, the rate of GVHD, bacteremia, and survival rates.
The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics
SOURCE: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
SALT LAKE CITY – A multicenter study confirmed that diversity of gut microbiota is associated with better survival after allogeneic hematopoietic cell transplantation (HCT), while low diversity and the predominance of pathogenic bacteria are linked to graft versus host disease (GVHD).
Lower calorie intake and exposure to broad-spectrum antibiotics were both associated with lower diversity, the study found.
“One of the striking findings early on was this association between diversity in the gut and overall survival,” said Jonathan Peled, MD, PhD, noting that his research group also saw that high gut diversity was associated with lower rates of GVHD-related mortality.
“The first question that I want to ask today is ‘Are the patterns of microbiota injury that have been described in single-center studies and their association with clinical outcomes consistent across geography?’” Dr. Peled said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
To answer this, Dr. Peled and his associates at Memorial Sloan Kettering Cancer Center (MSKCC), New York, teamed up with a research group at Duke University, Durham, N.C., and with investigators in Regensburg, Germany. The international group devised a study that would use centralized sequencing and analysis to examine patient fecal samples from all three centers.
In all, 5,310 samples were obtained from 1,034 HCT patients. MSKCC contributed most of the samples (n = 908, 87.8%), with Regensburg contributing 79 (7.6%) and Duke contributing 47 (4.5%).
The most common malignancies treated were acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. The balance of graft sources and conditioning intensity varied between centers, but overall, more than three-quarters of grafts were from peripheral blood stem cells and just over half of patients received myeloablative conditioning.
The centralized microbiota profiling involved extracting bacterial DNA, and then using polymerase chain reaction to amplify 16sRNA for sequencing and subsequent taxonomic identification.
“Samples can be segregated into clusters according to microbiota composition,” said Dr. Peled, a medical oncologist at MSKCC. The investigators used an algorithm called t-distributed stochastic neighbor embedding, or tSNE, to help detect patterns in microbiota composition and diversity before and throughout the HCT process. Visualizations using tSNE allow for two-dimensional representations of complicated associations and interrelatedness in data.
“Color-coded by diversity and time, we see that these early samples tend to be more diverse,” in the tSNE analyses, Dr. Peled said. The later clusters, he said, show evidence of lower diversity and injury.
Individual samples can also be coded in a way that shows clusters by abundance of various bacterial taxa, Dr. Peled said. “The early, diverse cluster tends to be dominated, or filled, by anaerobic commensals such as Firmicutes and Clostridia, which we and others have found are associated with good outcomes after transplant.”
The lower-diversity states seen later, after transplant, tend to be dominated by a variety of pathogenic bacteria, Dr. Peled said. These include Enterococcus and Proteobacteria, a phylum that includes Klebsiella and Escherichia coli species. This predominance has been associated with subsequent bacteremia, he said.
“Patients tend to enter transplant with a relatively diverse flora, and a frequent event in the posttransplant samples is domination by these pathobiomes,” Dr. Peled said. “In some cases, almost the entire composition of the gut is [composed] of a single species.” This loss of diversity and single-species domination was seen across the three geographically diverse research sites, he said.
This decimation of diversity is linked to poor transplant outcomes. In particular, Dr. Peled said, an enterococcus-dominated gut had previously been associated with higher risk for acute GVHD and with gastrointestinal GVHD.
Here, the multisite data showed that at Regensburg, higher enterococcus abundance on days 7-14 post HCT was associated with increased risk of GI GVHD. At MSKCC, enterococcus domination was associated with a hazard ratio of 1.4 for acute GVHD (P = .008). The MSKCC group used data from 503 patients, defining domination as at least 30% relative abundance in any sample from post-HCT days 7-21.
Patients at both MSKCC and Regensburg had a better chance of overall survival if they had high intestinal microbial diversity around the period of neutrophil engraftment, as seen in a sample collected within 7 days of post-HCT day 14. At MSKCC, data for 651 patients showed a statistically significant association (P = .006); this finding was reproduced at Regensburg, which also saw a significant association (P = .015) for the 59 patients studied, Dr. Peled reported.
Increased treatment-related mortality was seen for patients who had low microbial diversity following neutrophil engraftment as well. Of 372 MSKCC patients who had samples available 7-50 days after engraftment, high diversity was associated with better overall survival, and with lower treatment-related mortality (P = .03 for both).
Dr. Peled and his collaborators also divided patients into quartiles by amount of biodiversity. They found that comparing the highest to the lowest biodiversity quartile showed significantly overall survival benefits for the highest-diversity group (P = .007).
The problem starts before transplant, Dr. Peled explained. The researchers found that compared with healthy controls at MSKCC and data from the Human Microbiome Project, HCT patients entered their transplant with significantly less gut biodiversity.
The second question to be addressed is “What are the key environmental determinants of intestinal microbiota composition?” said Dr. Peled.
“Peri-HCT exposure to broad-spectrum antibiotics is associated with lower intestinal microbial diversity,” he said. For 5,936 samples taken from 976 patients receiving allogeneic HCT, the most significant difference in diversity between those with and without broad-spectrum antibiotic exposure was seen at day 15 post transplant (P = .008).
Higher calorie intake was also associated with greater diversity (P less than .001). Higher dietary fiber intake was associated with higher abundance of Blautia, a genus considered to be a healthy commensal microorganism, Dr. Peled said.
“Conditioning intensity is associated with the magnitude of diversity loss, and with distinct microbiome configurations,” said Dr. Peled. Using 4,311 samples from 908 patients, a myeloablative conditioning regimen (n = 508) was associated with significantly less diversity when compared with reduced intensity (n = 316) and nonmyeloablative regimens (n = 84; P =.002 and P less than .001, respectively).
To answer a third question – What is the natural history of recovery from microbiota injury after HCT? – the investigators looked at trends over time for 28 allogeneic HCT recipients. With a total of 294 samples for analysis, Dr. Peled and his group found that “diversity increases, but often to a configuration distinct from the pre-HCT state.” It took some patients nearly a year to return to their pretransplant level of diversity.
Patients in the subset of those who go on to develop lower gastrointestinal GVHD have an intestinal microbiota composition that is distinct from those patients whose GVHD exclusively involved the upper gastrointestinal tract, the skin, or the liver (P = .019), Dr. Peled said.
He and his team are currently enrolling patients for a phase 2 randomized clinical trial (NCT03078010) that will explore strategies to deescalate the use of broad-spectrum antibiotics for febrile neutropenia in patients with allogeneic HCT. The trial will randomize patients to receive either piperacillin-tazobactam, the current standard of care at MSKCC, or cefepime with deescalation to aztreonam with vancomycin, the microbiota-sparing strategy. The trial will examine the abundance of Clostridiales and Blautia species, gut biodiversity, the rate of GVHD, bacteremia, and survival rates.
The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics
SOURCE: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
SALT LAKE CITY – A multicenter study confirmed that diversity of gut microbiota is associated with better survival after allogeneic hematopoietic cell transplantation (HCT), while low diversity and the predominance of pathogenic bacteria are linked to graft versus host disease (GVHD).
Lower calorie intake and exposure to broad-spectrum antibiotics were both associated with lower diversity, the study found.
“One of the striking findings early on was this association between diversity in the gut and overall survival,” said Jonathan Peled, MD, PhD, noting that his research group also saw that high gut diversity was associated with lower rates of GVHD-related mortality.
“The first question that I want to ask today is ‘Are the patterns of microbiota injury that have been described in single-center studies and their association with clinical outcomes consistent across geography?’” Dr. Peled said during a top abstracts session at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
To answer this, Dr. Peled and his associates at Memorial Sloan Kettering Cancer Center (MSKCC), New York, teamed up with a research group at Duke University, Durham, N.C., and with investigators in Regensburg, Germany. The international group devised a study that would use centralized sequencing and analysis to examine patient fecal samples from all three centers.
In all, 5,310 samples were obtained from 1,034 HCT patients. MSKCC contributed most of the samples (n = 908, 87.8%), with Regensburg contributing 79 (7.6%) and Duke contributing 47 (4.5%).
The most common malignancies treated were acute myeloid leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma. The balance of graft sources and conditioning intensity varied between centers, but overall, more than three-quarters of grafts were from peripheral blood stem cells and just over half of patients received myeloablative conditioning.
The centralized microbiota profiling involved extracting bacterial DNA, and then using polymerase chain reaction to amplify 16sRNA for sequencing and subsequent taxonomic identification.
“Samples can be segregated into clusters according to microbiota composition,” said Dr. Peled, a medical oncologist at MSKCC. The investigators used an algorithm called t-distributed stochastic neighbor embedding, or tSNE, to help detect patterns in microbiota composition and diversity before and throughout the HCT process. Visualizations using tSNE allow for two-dimensional representations of complicated associations and interrelatedness in data.
“Color-coded by diversity and time, we see that these early samples tend to be more diverse,” in the tSNE analyses, Dr. Peled said. The later clusters, he said, show evidence of lower diversity and injury.
Individual samples can also be coded in a way that shows clusters by abundance of various bacterial taxa, Dr. Peled said. “The early, diverse cluster tends to be dominated, or filled, by anaerobic commensals such as Firmicutes and Clostridia, which we and others have found are associated with good outcomes after transplant.”
The lower-diversity states seen later, after transplant, tend to be dominated by a variety of pathogenic bacteria, Dr. Peled said. These include Enterococcus and Proteobacteria, a phylum that includes Klebsiella and Escherichia coli species. This predominance has been associated with subsequent bacteremia, he said.
“Patients tend to enter transplant with a relatively diverse flora, and a frequent event in the posttransplant samples is domination by these pathobiomes,” Dr. Peled said. “In some cases, almost the entire composition of the gut is [composed] of a single species.” This loss of diversity and single-species domination was seen across the three geographically diverse research sites, he said.
This decimation of diversity is linked to poor transplant outcomes. In particular, Dr. Peled said, an enterococcus-dominated gut had previously been associated with higher risk for acute GVHD and with gastrointestinal GVHD.
Here, the multisite data showed that at Regensburg, higher enterococcus abundance on days 7-14 post HCT was associated with increased risk of GI GVHD. At MSKCC, enterococcus domination was associated with a hazard ratio of 1.4 for acute GVHD (P = .008). The MSKCC group used data from 503 patients, defining domination as at least 30% relative abundance in any sample from post-HCT days 7-21.
Patients at both MSKCC and Regensburg had a better chance of overall survival if they had high intestinal microbial diversity around the period of neutrophil engraftment, as seen in a sample collected within 7 days of post-HCT day 14. At MSKCC, data for 651 patients showed a statistically significant association (P = .006); this finding was reproduced at Regensburg, which also saw a significant association (P = .015) for the 59 patients studied, Dr. Peled reported.
Increased treatment-related mortality was seen for patients who had low microbial diversity following neutrophil engraftment as well. Of 372 MSKCC patients who had samples available 7-50 days after engraftment, high diversity was associated with better overall survival, and with lower treatment-related mortality (P = .03 for both).
Dr. Peled and his collaborators also divided patients into quartiles by amount of biodiversity. They found that comparing the highest to the lowest biodiversity quartile showed significantly overall survival benefits for the highest-diversity group (P = .007).
The problem starts before transplant, Dr. Peled explained. The researchers found that compared with healthy controls at MSKCC and data from the Human Microbiome Project, HCT patients entered their transplant with significantly less gut biodiversity.
The second question to be addressed is “What are the key environmental determinants of intestinal microbiota composition?” said Dr. Peled.
“Peri-HCT exposure to broad-spectrum antibiotics is associated with lower intestinal microbial diversity,” he said. For 5,936 samples taken from 976 patients receiving allogeneic HCT, the most significant difference in diversity between those with and without broad-spectrum antibiotic exposure was seen at day 15 post transplant (P = .008).
Higher calorie intake was also associated with greater diversity (P less than .001). Higher dietary fiber intake was associated with higher abundance of Blautia, a genus considered to be a healthy commensal microorganism, Dr. Peled said.
“Conditioning intensity is associated with the magnitude of diversity loss, and with distinct microbiome configurations,” said Dr. Peled. Using 4,311 samples from 908 patients, a myeloablative conditioning regimen (n = 508) was associated with significantly less diversity when compared with reduced intensity (n = 316) and nonmyeloablative regimens (n = 84; P =.002 and P less than .001, respectively).
To answer a third question – What is the natural history of recovery from microbiota injury after HCT? – the investigators looked at trends over time for 28 allogeneic HCT recipients. With a total of 294 samples for analysis, Dr. Peled and his group found that “diversity increases, but often to a configuration distinct from the pre-HCT state.” It took some patients nearly a year to return to their pretransplant level of diversity.
Patients in the subset of those who go on to develop lower gastrointestinal GVHD have an intestinal microbiota composition that is distinct from those patients whose GVHD exclusively involved the upper gastrointestinal tract, the skin, or the liver (P = .019), Dr. Peled said.
He and his team are currently enrolling patients for a phase 2 randomized clinical trial (NCT03078010) that will explore strategies to deescalate the use of broad-spectrum antibiotics for febrile neutropenia in patients with allogeneic HCT. The trial will randomize patients to receive either piperacillin-tazobactam, the current standard of care at MSKCC, or cefepime with deescalation to aztreonam with vancomycin, the microbiota-sparing strategy. The trial will examine the abundance of Clostridiales and Blautia species, gut biodiversity, the rate of GVHD, bacteremia, and survival rates.
The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics
SOURCE: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: High microbiota diversity post transplant was associated with better overall survival at two sites (P = .006 and P = .015).
Study details: Multicenter study of 5,310 fecal samples obtained from 1,034 hematopoietic cell transplant recipients.
Disclosures: The research presented was funded by the Parker Institute for Cancer Immunotherapy, the Sawiris Foundation, Empire Clinical Research Investigator Program, and Seres Therapeutics. Dr. Peled reported that he has intellectual property rights and research funding through Seres Therapeutics.
Source: Peled J et al. 2018 BMT Tandem Meetings, Abstract 3.
Chemotherapy, metabolic pathway may affect CAR T-cell potential
Two critical factors – prior exposure to chemotherapy and a glycolytic metabolism – appear to degrade the potential of T cells to become chimeric antigen receptor–T cells.
Chemotherapy, especially with cyclophosphamide and doxorubicin, seems particularly toxic to T cells, damaging the mitochondria and decreasing the cells’ spare respiratory capacity – a measure of mitochondrial health, David Barrett, MD, said during a press briefing held in advance of the annual meeting of the American Association for Cancer Research.
These new findings may help explain why children with acute lymphoblastic leukemia (ALL) tend to respond so vigorously to CAR T treatment, and why T cells from patients with solid tumors simply don’t grow, or die soon after patient infusion, he said in an interview. They also suggest a benefit of harvesting T cells before any chemotherapy, a procedure Dr. Barrett and his colleagues have advocated.
“Based on these data we have altered our practice for T-cell therapy in high-risk leukemia patients. If we have a patient who may have a poor prognosis, we try to collect the cells early and store them before proceeding, because we know chemotherapy will progressively degrade them.”
There still is no successful CAR T-cell protocol for solid tumors, but Dr. Barrett said these findings eventually may help such patients, particularly if more advanced experiments in manipulating the cells’ metabolism prove successful.
He and his colleagues investigated why T cells from some patients result in a poor clinical product that either fails manufacture or does not proliferate in the patient. They examined T cells from 157 pediatric patients with a variety of cancers, including ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, Hodgkin disease, chronic myelogenous leukemia, and Ewing sarcoma. The team obtained cells at diagnosis and after each cycle of chemotherapy.
They examined how well the cells grew in the transformation and expansion process. A “pass” was considered a fivefold expansion in response to CD3/CD28 exposure for 7 days. Normal donor cells typically expand 20- to 30-fold in this time.
Only T cells taken from ALL and Wilms tumor patients before chemotherapy achieved a pass, Dr. Barrett said. Most of the ALL expansions (80%) and half of the Wilms tumor expansions passed. “We noted very poor CAR T-cell potential in all the other tumor types – less than a 30% pass. We noted a decline in potential with cumulative chemotherapy in all cases, though this was particularly significant in children less than 3 years old.”
The team also used RNA profiling to look at the cells’ metabolic pathways. Dr. Barrett noted that T cells are highly metabolically adaptable, capable of using several different fuel types and switching from one to another. Glucose and fatty acids are frequent fuels. Most of the cells from patients with solid tumors exhibited a glycolytic metabolism, while cells from patients with ALL and Wilms tumor appeared to rely more on fatty acids.
“One is not inherently worse than the other,” he said. “But glycolysis appears to be a bad thing when we’re trying to turn them into CAR T cells. Those T cells were too exhausted to do anything.”
However, Dr. Barrett encouraged the cells to switch fuels by treating them in vitro with palmitic acid, the most common fatty acid in plants and animals.
“We were growing the cells in a media containing sugar, fatty acids, and amino acids,” he explained. “We just started overloading them with palmitic acid, which has a natural transporter on the T-cell surface, so it already had a good pathway to get into the cell. It helped restore some of the performance of these T cells in some assays, although it wasn’t a complete reversal. But it was encouraging that something as simple as providing an alternate fuel was enough to get some positive effect. Whether or not we would also have to block glucose use to get it to really work is something we continue to study.”
T cells that had been exposed to chemotherapy also did poorly. Cyclophosphamide and doxorubicin seemed particularly toxic. Cells with exposure to these two agents had severely depleted CAR T cell potential with very poor spare respiratory capacity. This is a marker of mitochondrial injury, Dr. Barrett said. “That wasn’t a huge surprise. We already knew that cyclophosphamide is very toxic to T cells.”
But the finding did suggest the simple intervention of harvesting T cells before chemotherapy, which is what Dr. Barrett and his colleagues now do in their high-risk ALL patients. Whether or not this would improve response in patients with solid tumors is still unknown.
He had no financial disclosures. This study was supported by the AACR, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award.
SOURCE: Barrett DM et al. AACR 2018, Abstract 1631.
Two critical factors – prior exposure to chemotherapy and a glycolytic metabolism – appear to degrade the potential of T cells to become chimeric antigen receptor–T cells.
Chemotherapy, especially with cyclophosphamide and doxorubicin, seems particularly toxic to T cells, damaging the mitochondria and decreasing the cells’ spare respiratory capacity – a measure of mitochondrial health, David Barrett, MD, said during a press briefing held in advance of the annual meeting of the American Association for Cancer Research.
These new findings may help explain why children with acute lymphoblastic leukemia (ALL) tend to respond so vigorously to CAR T treatment, and why T cells from patients with solid tumors simply don’t grow, or die soon after patient infusion, he said in an interview. They also suggest a benefit of harvesting T cells before any chemotherapy, a procedure Dr. Barrett and his colleagues have advocated.
“Based on these data we have altered our practice for T-cell therapy in high-risk leukemia patients. If we have a patient who may have a poor prognosis, we try to collect the cells early and store them before proceeding, because we know chemotherapy will progressively degrade them.”
There still is no successful CAR T-cell protocol for solid tumors, but Dr. Barrett said these findings eventually may help such patients, particularly if more advanced experiments in manipulating the cells’ metabolism prove successful.
He and his colleagues investigated why T cells from some patients result in a poor clinical product that either fails manufacture or does not proliferate in the patient. They examined T cells from 157 pediatric patients with a variety of cancers, including ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, Hodgkin disease, chronic myelogenous leukemia, and Ewing sarcoma. The team obtained cells at diagnosis and after each cycle of chemotherapy.
They examined how well the cells grew in the transformation and expansion process. A “pass” was considered a fivefold expansion in response to CD3/CD28 exposure for 7 days. Normal donor cells typically expand 20- to 30-fold in this time.
Only T cells taken from ALL and Wilms tumor patients before chemotherapy achieved a pass, Dr. Barrett said. Most of the ALL expansions (80%) and half of the Wilms tumor expansions passed. “We noted very poor CAR T-cell potential in all the other tumor types – less than a 30% pass. We noted a decline in potential with cumulative chemotherapy in all cases, though this was particularly significant in children less than 3 years old.”
The team also used RNA profiling to look at the cells’ metabolic pathways. Dr. Barrett noted that T cells are highly metabolically adaptable, capable of using several different fuel types and switching from one to another. Glucose and fatty acids are frequent fuels. Most of the cells from patients with solid tumors exhibited a glycolytic metabolism, while cells from patients with ALL and Wilms tumor appeared to rely more on fatty acids.
“One is not inherently worse than the other,” he said. “But glycolysis appears to be a bad thing when we’re trying to turn them into CAR T cells. Those T cells were too exhausted to do anything.”
However, Dr. Barrett encouraged the cells to switch fuels by treating them in vitro with palmitic acid, the most common fatty acid in plants and animals.
“We were growing the cells in a media containing sugar, fatty acids, and amino acids,” he explained. “We just started overloading them with palmitic acid, which has a natural transporter on the T-cell surface, so it already had a good pathway to get into the cell. It helped restore some of the performance of these T cells in some assays, although it wasn’t a complete reversal. But it was encouraging that something as simple as providing an alternate fuel was enough to get some positive effect. Whether or not we would also have to block glucose use to get it to really work is something we continue to study.”
T cells that had been exposed to chemotherapy also did poorly. Cyclophosphamide and doxorubicin seemed particularly toxic. Cells with exposure to these two agents had severely depleted CAR T cell potential with very poor spare respiratory capacity. This is a marker of mitochondrial injury, Dr. Barrett said. “That wasn’t a huge surprise. We already knew that cyclophosphamide is very toxic to T cells.”
But the finding did suggest the simple intervention of harvesting T cells before chemotherapy, which is what Dr. Barrett and his colleagues now do in their high-risk ALL patients. Whether or not this would improve response in patients with solid tumors is still unknown.
He had no financial disclosures. This study was supported by the AACR, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award.
SOURCE: Barrett DM et al. AACR 2018, Abstract 1631.
Two critical factors – prior exposure to chemotherapy and a glycolytic metabolism – appear to degrade the potential of T cells to become chimeric antigen receptor–T cells.
Chemotherapy, especially with cyclophosphamide and doxorubicin, seems particularly toxic to T cells, damaging the mitochondria and decreasing the cells’ spare respiratory capacity – a measure of mitochondrial health, David Barrett, MD, said during a press briefing held in advance of the annual meeting of the American Association for Cancer Research.
These new findings may help explain why children with acute lymphoblastic leukemia (ALL) tend to respond so vigorously to CAR T treatment, and why T cells from patients with solid tumors simply don’t grow, or die soon after patient infusion, he said in an interview. They also suggest a benefit of harvesting T cells before any chemotherapy, a procedure Dr. Barrett and his colleagues have advocated.
“Based on these data we have altered our practice for T-cell therapy in high-risk leukemia patients. If we have a patient who may have a poor prognosis, we try to collect the cells early and store them before proceeding, because we know chemotherapy will progressively degrade them.”
There still is no successful CAR T-cell protocol for solid tumors, but Dr. Barrett said these findings eventually may help such patients, particularly if more advanced experiments in manipulating the cells’ metabolism prove successful.
He and his colleagues investigated why T cells from some patients result in a poor clinical product that either fails manufacture or does not proliferate in the patient. They examined T cells from 157 pediatric patients with a variety of cancers, including ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms tumor, Hodgkin disease, chronic myelogenous leukemia, and Ewing sarcoma. The team obtained cells at diagnosis and after each cycle of chemotherapy.
They examined how well the cells grew in the transformation and expansion process. A “pass” was considered a fivefold expansion in response to CD3/CD28 exposure for 7 days. Normal donor cells typically expand 20- to 30-fold in this time.
Only T cells taken from ALL and Wilms tumor patients before chemotherapy achieved a pass, Dr. Barrett said. Most of the ALL expansions (80%) and half of the Wilms tumor expansions passed. “We noted very poor CAR T-cell potential in all the other tumor types – less than a 30% pass. We noted a decline in potential with cumulative chemotherapy in all cases, though this was particularly significant in children less than 3 years old.”
The team also used RNA profiling to look at the cells’ metabolic pathways. Dr. Barrett noted that T cells are highly metabolically adaptable, capable of using several different fuel types and switching from one to another. Glucose and fatty acids are frequent fuels. Most of the cells from patients with solid tumors exhibited a glycolytic metabolism, while cells from patients with ALL and Wilms tumor appeared to rely more on fatty acids.
“One is not inherently worse than the other,” he said. “But glycolysis appears to be a bad thing when we’re trying to turn them into CAR T cells. Those T cells were too exhausted to do anything.”
However, Dr. Barrett encouraged the cells to switch fuels by treating them in vitro with palmitic acid, the most common fatty acid in plants and animals.
“We were growing the cells in a media containing sugar, fatty acids, and amino acids,” he explained. “We just started overloading them with palmitic acid, which has a natural transporter on the T-cell surface, so it already had a good pathway to get into the cell. It helped restore some of the performance of these T cells in some assays, although it wasn’t a complete reversal. But it was encouraging that something as simple as providing an alternate fuel was enough to get some positive effect. Whether or not we would also have to block glucose use to get it to really work is something we continue to study.”
T cells that had been exposed to chemotherapy also did poorly. Cyclophosphamide and doxorubicin seemed particularly toxic. Cells with exposure to these two agents had severely depleted CAR T cell potential with very poor spare respiratory capacity. This is a marker of mitochondrial injury, Dr. Barrett said. “That wasn’t a huge surprise. We already knew that cyclophosphamide is very toxic to T cells.”
But the finding did suggest the simple intervention of harvesting T cells before chemotherapy, which is what Dr. Barrett and his colleagues now do in their high-risk ALL patients. Whether or not this would improve response in patients with solid tumors is still unknown.
He had no financial disclosures. This study was supported by the AACR, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award.
SOURCE: Barrett DM et al. AACR 2018, Abstract 1631.
FROM AACR 2018
Key clinical point: Prior exposure to chemotherapy may degrade the potential of T cells to become CAR T cells, suggesting a benefit of harvesting T cells before any chemotherapy.
Major finding: Only T cells taken from ALL and Wilm’s tumor patients before chemotherapy achieved a fivefold expansion in response to CD3/CD28 exposure for 7 days.
Study details: An examination of T cells from 157 pediatric patients with a variety of cancers at diagnosis and after each cycle of chemotherapy.
Disclosures: The study was supported by the American Association of Cancer Research, the Doris Duke Charitable Foundation Clinical Science Development Award, the Jeffrey Pride Foundation Research Award, and the St. Baldrick’s Foundation Scholar Award. Dr. Barrett and his coauthors had no financial disclosures.
Source: Barrett DM et al. AACR 2018, Abstract 1631.
Maternal biologic therapy does not affect infant vaccine responses
MAUI, HAWAII – The infants of inflammatory bowel disease patients on biologic therapy during pregnancy and breastfeeding do not have a diminished response rate to the inactivated vaccines routinely given during the first 6 months of life, Uma Mahadevan, MD, said at the 2018 Rheumatology Winter Clinical Symposium.
“Those babies are going to have detectable levels of drug on board, but they respond to vaccines just as well as infants born to mothers with IBD who were not on biologic therapy. The rates are the same, albeit lower than in the general population,” according to Dr. Mahadevan, professor of medicine and medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
Previous reports from the national registry have established that continuation of biologics in IBD patients throughout pregnancy and breastfeeding to maintain disease control poses no increased risks to the fetus in terms of rates of congenital anomalies, spontaneous abortion, intrauterine growth restriction, low birth weight, or longer-term developmental delay, compared with unexposed babies whose mothers have IBD.
Dr. Ananthakrishnan’s analysis focused on response rates to tetanus and Haemophilus influenzae B vaccines in the infants of 179 PIANO patients. Sixty-five percent of the IBD patients were on various biologic agents during pregnancy, 8% were on a thiopurine, 21% were on combination therapy, and 6% weren’t exposed to any IBD medications. Serologic studies showed that there was no difference across the four groups in terms of infant rates of protective titers in response to the vaccines. However, the 69%-84% rates of protective titers in the four groups fell short of the 90%-plus rate expected in the general population.
Live virus vaccines are contraindicated in the first 6 months of life in infants exposed to maternal biologics in utero. The only live virus vaccine given during that time frame in the United States is rotavirus, administered at months 2 and 3. Dr. Mahadevan and others recommend skipping that vaccine in babies exposed in utero to any IBD biologic other than certolizumab pegol (Cimzia), which uniquely doesn’t cross the placenta.
“That being said, infants born to 71 of our PIANO participants on anti-TNF therapy in pregnancy inadvertently got the rotavirus vaccine, and they were all just fine, even with very high drug levels,” the gastroenterologist said.
The live virus varicella and MMR vaccines can safely be given as scheduled at 1 year of age. By that time the biologics are long gone from the child.
Dr. Mahadevan reported receiving research funding from the Crohn’s and Colitis Foundation of America, which sponsors the PIANO registry. She also has financial relationships with several pharmaceutical companies.
MAUI, HAWAII – The infants of inflammatory bowel disease patients on biologic therapy during pregnancy and breastfeeding do not have a diminished response rate to the inactivated vaccines routinely given during the first 6 months of life, Uma Mahadevan, MD, said at the 2018 Rheumatology Winter Clinical Symposium.
“Those babies are going to have detectable levels of drug on board, but they respond to vaccines just as well as infants born to mothers with IBD who were not on biologic therapy. The rates are the same, albeit lower than in the general population,” according to Dr. Mahadevan, professor of medicine and medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
Previous reports from the national registry have established that continuation of biologics in IBD patients throughout pregnancy and breastfeeding to maintain disease control poses no increased risks to the fetus in terms of rates of congenital anomalies, spontaneous abortion, intrauterine growth restriction, low birth weight, or longer-term developmental delay, compared with unexposed babies whose mothers have IBD.
Dr. Ananthakrishnan’s analysis focused on response rates to tetanus and Haemophilus influenzae B vaccines in the infants of 179 PIANO patients. Sixty-five percent of the IBD patients were on various biologic agents during pregnancy, 8% were on a thiopurine, 21% were on combination therapy, and 6% weren’t exposed to any IBD medications. Serologic studies showed that there was no difference across the four groups in terms of infant rates of protective titers in response to the vaccines. However, the 69%-84% rates of protective titers in the four groups fell short of the 90%-plus rate expected in the general population.
Live virus vaccines are contraindicated in the first 6 months of life in infants exposed to maternal biologics in utero. The only live virus vaccine given during that time frame in the United States is rotavirus, administered at months 2 and 3. Dr. Mahadevan and others recommend skipping that vaccine in babies exposed in utero to any IBD biologic other than certolizumab pegol (Cimzia), which uniquely doesn’t cross the placenta.
“That being said, infants born to 71 of our PIANO participants on anti-TNF therapy in pregnancy inadvertently got the rotavirus vaccine, and they were all just fine, even with very high drug levels,” the gastroenterologist said.
The live virus varicella and MMR vaccines can safely be given as scheduled at 1 year of age. By that time the biologics are long gone from the child.
Dr. Mahadevan reported receiving research funding from the Crohn’s and Colitis Foundation of America, which sponsors the PIANO registry. She also has financial relationships with several pharmaceutical companies.
MAUI, HAWAII – The infants of inflammatory bowel disease patients on biologic therapy during pregnancy and breastfeeding do not have a diminished response rate to the inactivated vaccines routinely given during the first 6 months of life, Uma Mahadevan, MD, said at the 2018 Rheumatology Winter Clinical Symposium.
“Those babies are going to have detectable levels of drug on board, but they respond to vaccines just as well as infants born to mothers with IBD who were not on biologic therapy. The rates are the same, albeit lower than in the general population,” according to Dr. Mahadevan, professor of medicine and medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
Previous reports from the national registry have established that continuation of biologics in IBD patients throughout pregnancy and breastfeeding to maintain disease control poses no increased risks to the fetus in terms of rates of congenital anomalies, spontaneous abortion, intrauterine growth restriction, low birth weight, or longer-term developmental delay, compared with unexposed babies whose mothers have IBD.
Dr. Ananthakrishnan’s analysis focused on response rates to tetanus and Haemophilus influenzae B vaccines in the infants of 179 PIANO patients. Sixty-five percent of the IBD patients were on various biologic agents during pregnancy, 8% were on a thiopurine, 21% were on combination therapy, and 6% weren’t exposed to any IBD medications. Serologic studies showed that there was no difference across the four groups in terms of infant rates of protective titers in response to the vaccines. However, the 69%-84% rates of protective titers in the four groups fell short of the 90%-plus rate expected in the general population.
Live virus vaccines are contraindicated in the first 6 months of life in infants exposed to maternal biologics in utero. The only live virus vaccine given during that time frame in the United States is rotavirus, administered at months 2 and 3. Dr. Mahadevan and others recommend skipping that vaccine in babies exposed in utero to any IBD biologic other than certolizumab pegol (Cimzia), which uniquely doesn’t cross the placenta.
“That being said, infants born to 71 of our PIANO participants on anti-TNF therapy in pregnancy inadvertently got the rotavirus vaccine, and they were all just fine, even with very high drug levels,” the gastroenterologist said.
The live virus varicella and MMR vaccines can safely be given as scheduled at 1 year of age. By that time the biologics are long gone from the child.
Dr. Mahadevan reported receiving research funding from the Crohn’s and Colitis Foundation of America, which sponsors the PIANO registry. She also has financial relationships with several pharmaceutical companies.
EXPERT ANALYSIS FROM RWCS 2018
Protons linked to lower urinary AEs, higher costs than with IMRT for prostate cancer
Among men younger than 65 with prostate cancer, proton beam radiotherapy (PBT) was associated with significantly lower rates of urinary toxicities than was intensity-modulated radiotherapy (IMRT), but this safety advantage came at the cost of nearly $60,000 extra per patient, results of an insurance claims study show.
The rate of a composite of urinary toxicities at 2 years among 693 men treated with PBT was 33%, compared with 42% for 3,465 men matched by propensity score who were treated with IMRT. Respective rates of erectile dysfunction were 21% vs. 28%. The mean cost for PBT, however, was nearly double that for IMRT, reported Benjamin D. Smith, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
Although PBT was better at sparing patients from urinary toxicities, it was associated with increased bowel toxicities, and a second comparison of IMRT with stereotactic body radiotherapy (SBRT) showed that SBRT, while slightly cheaper than IMRT, was associated with modest increases in some urinary toxicities, the researchers reported in a study published online in the Journal of Clinical Oncology.
“These key findings, coupled with the real-world private insurance cost reported herein, will be useful for patients selecting the most appropriate treatment and for researchers designing cost-effectiveness models to guide treatment decisions in prostate cancer,” they wrote.
The investigators combed through the MarketScan Commercial Claims and Encounters database to identify men who underwent radiation therapy for prostate cancer between 2008 and 2015. They used propensity-score matching, a technique designed to even out potential confounding factors, to compare those treated with IMRT with others treated with PBT or SBRT.
As noted, PBT was associated with significantly lower urinary toxicities and erectile dysfunction compared with IMRT (P less than .001 for each comparison), but with a higher rate of bowel toxicities at 2 years (20% vs. 15%, P = .02).
The mean cost per patient in 2015 dollars for PBT was $115,501, compared with $59,012 for IMRT.
In a comparison of outcomes and costs for 310 patients who underwent SBRT matched with 3,100 who underwent IMRT, the investigators found no significant differences in composite urinary, erectile dysfunction, or bowel toxicities. However, the risk of urinary fistula, while low, was significantly higher with SBRT compared with IMRT, with rates of 1% vs. 0.1%, respectively (P = .009).
The mean cost of SBRT in this analysis was $49,504, compared with $57,244 for IMRT (P less than .001).
The investigators acknowledged that by using claims data they were unable to plug information about potential confounding factors such as Gleason score, prostate-specific antigen level, clinical stage, or radiation field and dose into their propensity-score models. They also noted that follow-up was relatively short because of the vicissitudes of the U.S. insurance market, which causes many patients to change insurers frequently.
The study was supported by grants from the National Cancer Institute and by Varian Medical Systems. Dr. Smith, lead author Hubert Y. Pan, MD, and others disclosed research support, consulting, and/or travel support from Varian.
SOURCE: Smith BD et al. J Clin Oncol. 2018 Mar 21. doi: 10.1200/JCO.2017.75.5371.
Among men younger than 65 with prostate cancer, proton beam radiotherapy (PBT) was associated with significantly lower rates of urinary toxicities than was intensity-modulated radiotherapy (IMRT), but this safety advantage came at the cost of nearly $60,000 extra per patient, results of an insurance claims study show.
The rate of a composite of urinary toxicities at 2 years among 693 men treated with PBT was 33%, compared with 42% for 3,465 men matched by propensity score who were treated with IMRT. Respective rates of erectile dysfunction were 21% vs. 28%. The mean cost for PBT, however, was nearly double that for IMRT, reported Benjamin D. Smith, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
Although PBT was better at sparing patients from urinary toxicities, it was associated with increased bowel toxicities, and a second comparison of IMRT with stereotactic body radiotherapy (SBRT) showed that SBRT, while slightly cheaper than IMRT, was associated with modest increases in some urinary toxicities, the researchers reported in a study published online in the Journal of Clinical Oncology.
“These key findings, coupled with the real-world private insurance cost reported herein, will be useful for patients selecting the most appropriate treatment and for researchers designing cost-effectiveness models to guide treatment decisions in prostate cancer,” they wrote.
The investigators combed through the MarketScan Commercial Claims and Encounters database to identify men who underwent radiation therapy for prostate cancer between 2008 and 2015. They used propensity-score matching, a technique designed to even out potential confounding factors, to compare those treated with IMRT with others treated with PBT or SBRT.
As noted, PBT was associated with significantly lower urinary toxicities and erectile dysfunction compared with IMRT (P less than .001 for each comparison), but with a higher rate of bowel toxicities at 2 years (20% vs. 15%, P = .02).
The mean cost per patient in 2015 dollars for PBT was $115,501, compared with $59,012 for IMRT.
In a comparison of outcomes and costs for 310 patients who underwent SBRT matched with 3,100 who underwent IMRT, the investigators found no significant differences in composite urinary, erectile dysfunction, or bowel toxicities. However, the risk of urinary fistula, while low, was significantly higher with SBRT compared with IMRT, with rates of 1% vs. 0.1%, respectively (P = .009).
The mean cost of SBRT in this analysis was $49,504, compared with $57,244 for IMRT (P less than .001).
The investigators acknowledged that by using claims data they were unable to plug information about potential confounding factors such as Gleason score, prostate-specific antigen level, clinical stage, or radiation field and dose into their propensity-score models. They also noted that follow-up was relatively short because of the vicissitudes of the U.S. insurance market, which causes many patients to change insurers frequently.
The study was supported by grants from the National Cancer Institute and by Varian Medical Systems. Dr. Smith, lead author Hubert Y. Pan, MD, and others disclosed research support, consulting, and/or travel support from Varian.
SOURCE: Smith BD et al. J Clin Oncol. 2018 Mar 21. doi: 10.1200/JCO.2017.75.5371.
Among men younger than 65 with prostate cancer, proton beam radiotherapy (PBT) was associated with significantly lower rates of urinary toxicities than was intensity-modulated radiotherapy (IMRT), but this safety advantage came at the cost of nearly $60,000 extra per patient, results of an insurance claims study show.
The rate of a composite of urinary toxicities at 2 years among 693 men treated with PBT was 33%, compared with 42% for 3,465 men matched by propensity score who were treated with IMRT. Respective rates of erectile dysfunction were 21% vs. 28%. The mean cost for PBT, however, was nearly double that for IMRT, reported Benjamin D. Smith, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
Although PBT was better at sparing patients from urinary toxicities, it was associated with increased bowel toxicities, and a second comparison of IMRT with stereotactic body radiotherapy (SBRT) showed that SBRT, while slightly cheaper than IMRT, was associated with modest increases in some urinary toxicities, the researchers reported in a study published online in the Journal of Clinical Oncology.
“These key findings, coupled with the real-world private insurance cost reported herein, will be useful for patients selecting the most appropriate treatment and for researchers designing cost-effectiveness models to guide treatment decisions in prostate cancer,” they wrote.
The investigators combed through the MarketScan Commercial Claims and Encounters database to identify men who underwent radiation therapy for prostate cancer between 2008 and 2015. They used propensity-score matching, a technique designed to even out potential confounding factors, to compare those treated with IMRT with others treated with PBT or SBRT.
As noted, PBT was associated with significantly lower urinary toxicities and erectile dysfunction compared with IMRT (P less than .001 for each comparison), but with a higher rate of bowel toxicities at 2 years (20% vs. 15%, P = .02).
The mean cost per patient in 2015 dollars for PBT was $115,501, compared with $59,012 for IMRT.
In a comparison of outcomes and costs for 310 patients who underwent SBRT matched with 3,100 who underwent IMRT, the investigators found no significant differences in composite urinary, erectile dysfunction, or bowel toxicities. However, the risk of urinary fistula, while low, was significantly higher with SBRT compared with IMRT, with rates of 1% vs. 0.1%, respectively (P = .009).
The mean cost of SBRT in this analysis was $49,504, compared with $57,244 for IMRT (P less than .001).
The investigators acknowledged that by using claims data they were unable to plug information about potential confounding factors such as Gleason score, prostate-specific antigen level, clinical stage, or radiation field and dose into their propensity-score models. They also noted that follow-up was relatively short because of the vicissitudes of the U.S. insurance market, which causes many patients to change insurers frequently.
The study was supported by grants from the National Cancer Institute and by Varian Medical Systems. Dr. Smith, lead author Hubert Y. Pan, MD, and others disclosed research support, consulting, and/or travel support from Varian.
SOURCE: Smith BD et al. J Clin Oncol. 2018 Mar 21. doi: 10.1200/JCO.2017.75.5371.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Proton beam therapy is nearly twice as costly as intensity-modulated radiation for prostate cancer.
Major finding: Proton therapy was associated with a 9% lower incidence of urinary toxicities, but at a significantly greater cost than IMRT.
Study details: Retrospective claims data study of 693 men treated with protons compared with 3,465 propensity-matched men treated with IMRT, and 310 men treated with SBRT vs. 3,100 matched men treated with IMRT.
Disclosures: The study was supported by grants from the National Cancer Institute and by Varian Medical Systems. Dr. Smith, lead author Hubert Y. Pan, MD, and others disclosed research support, consulting, and/or travel support from Varian.
Source: Smith BD et al. J Clin Oncol. 2018 Mar 21. doi: 10.1200/JCO.2017.75.5371.
Putting IBD medication risks into perspective
MAUI, HAWAII – Prolonged corticosteroid therapy for inflammatory bowel disease (IBD) was associated with a significantly increased mortality risk compared with anti–tumor necrosis factor therapy in a landmark study spotlighted by Edward V. Loftus Jr., MD, at the Gastroenterology Updates, IBD, Liver Disease meeting.
This was one of several key studies on safety issues involving IBD medications published in the past year. Others highlighted by Dr. Loftus and copanelist William J. Sandborn, MD, included a study that provided persuasive evidence that TNF inhibitors modestly increase lymphoma risk in IBD patients to a degree similar to that of thiopurines, and several reports addressing the question of whether preoperative use of vedolizumab in patients undergoing major abdominal operations for IBD boosts postoperative infection risk.
Mortality impact of prolonged steroids vs. anti-TNF therapy
That will come as an unpleasant surprise to many physicians. There is a widespread reluctance to turn to continuous chronic immunosuppression via anti-TNF therapy in patients with challenging IBD, particularly in elderly individuals with multiple comorbid conditions. Many physicians have heard and read so much about the biologics’ risks of serious adverse events that they opt instead for multiple courses of corticosteroids for disease control. This is a serious mistake, emphasized Dr. Loftus, professor of medicine and director of the IBD Interest Group at the Mayo Clinic in Rochester, Minn.
“When you say, ‘Oh, I’ll just give that patient another prednisone taper, he doesn’t want to start taking a TNF inhibitor,’ you’re actually doing the patient harm. You’re actually affecting the patient’s life expectancy when you do that,” he declared. “The message is, yes, steroids are cheap, steroids are easy, nobody’s afraid of steroids, but you should be afraid of steroids.”
The 1,879 Crohn’s disease patients who entered the cohort as new users of anti-TNF therapy had a subsequent mortality incidence rate of 21.4 per 1,000 person-years, compared with a rate of 30.1 per 1,000 person-years in the 7,694 who entered the study period as prolonged steroid users. In a multivariate analysis accounting for 57 potential confounding factors, this translated to a highly significant 22% relative risk reduction in mortality in the patients who went with anti-TNF therapy (Am J Gastroenterol. 2018 Jan 16. doi: 10.1038/ajg.2017.479).
A similar trend was seen in the ulcerative colitis cohort. The 459 ulcerative colitis patients who entered the cohort as new anti-TNF therapy users had a mortality incidence rate of 23.0 per 1,000 person-years, compared with a rate of 30.9 in the 3,224 who received more than 3,000 mg of prednisone in the next 12 months. This represented a 14% relative risk reduction, although this favorable trend did not achieve statistical significance, perhaps because of the smaller size of the ulcerative colitis cohort.
In addition to demonstrably greater life expectancy, anti-TNF therapy offered additional benefits: a 32% reduction in the risk of major adverse cardiovascular events and a 46% lower incidence of hip fracture.
Dr. Sandborn, professor of medicine and chief of the division of gastroenterology at the University of California, San Diego, spun the study data another way: “It shows the number needed to kill is 33. So for every 33 patients you put on prolonged corticosteroids, you’re killing one extra patient by doing that. Of course, you probably blame it on their age and comorbidities, but this is it. This is the data.”
TNF blockers, thiopurines, and lymphoma
The use of thiopurines for treatment of IBD is widely recognized to be associated with a small but real increased risk of lymphoma. Now a large French national study has demonstrated for the first time that anti-TNF therapy for IBD is also associated with an increased risk that needs to be discussed with patients. And in IBD patients on combination therapy with both classes of medication, that risk jumps to 6.1-fold greater than in unexposed IBD patients (JAMA 2017 Nov 7;318[17]:1679-86).
Dr. Loftus and Dr. Sandborn urged their colleagues to keep this increased risk in perspective in counseling patients by focusing on the modest absolute increase in risk rather than the scarier-sounding relative risk. Notably, two-thirds of lymphomas in the French study occurred in patients not on thiopurines or anti-TNF agents.
“The most interesting thing to me is that we worry and worry about lymphoma, and guess what? In this study and in multiple other studies, the majority of lymphomas occurring in IBD patients have nothing to do with their medications. They’re due to the usual risk factors for lymphoma, which include age and male gender,” Dr. Loftus observed.
The French study included more than 189,000 IBD patients followed for a median of 6.7 years, during which 336 cases of lymphoma occurred. The incidence rate was 0.26 cases per 1,000 person-years in unexposed patients. The rate was significantly higher at 0.54 per 1,000 person-years in those on thiopurine monotherapy, increased to a similar extent at 0.41 cases per 1,000 person-years in patients on anti-TNF monotherapy, and 0.95 per 1,000 person-years in those on combination therapy.
In a multivariate analysis, the lymphoma risk was an adjusted 2.6-fold greater in patients exposed to thiopurine monotherapy than in unexposed patients, 2.41-fold greater in patients exposed to anti-TNF monotherapy, and 6.1-fold greater in those exposed to combination therapy.
“The point I want to make is the lymphoma rates in the thiopurine monotherapy and anti-TNF monotherapy groups are not significantly different. So the claim that’s been out there that the increased lymphoma risk in IBD patients can be completely explained by thiopurines is wrong. This study is showing us that with anti-TNF monotherapy there is still a low-level risk of lymphoma,” Dr. Loftus said.
“It is somewhat eyebrow-raising when you see that relative risk of 6.1, and that’s what patients are going to focus on, but when you counsel patients you have to redirect them to the absolute risk. You can say, ‘Even on combination therapy, your risk is 1 in 1,000,’ ” the gastroenterologist said.
Dr. Sandborn said the lymphoma signal hadn’t been spotted previously because the individual registries of IBD patients on anti-TNF agents are too small to allow for identification of a small increase in risk. The French investigators overcame that limitation by tapping into the country’s national health care system.
“This is a huge dataset and I think the message is unequivocal,” Dr. Sandborn said.
He noted that strongly risk-averse patients may find ustekinumab (Stelara) and vedolizumab (Entyvio) to be attractive treatment options. Neither has any link to lymphoma.
Preoperative vedolizumab and postoperative infection risk
“The overall safety profile of vedolizumab is pretty good,” Dr. Loftus observed. “The one unanswered question is its safety when used within 8-12 weeks of a major abdominal operation.”
It’s a clinically relevant question because vedolizumab selectively inhibits leukocyte migration into the intestinal tract, which could provide a mechanism for impaired postoperative wound healing in patients undergoing major abdominal surgery. And sooner or later a high proportion of IBD patients have a major abdominal operation.
Dr. Loftus and his coinvestigators kicked off a controversy by reporting a 37% incidence of surgical site infections in IBD patients who received vedolizumab within 30 days of a major abdominal operation in a retrospective chart review of the Mayo Clinic experience, a postoperative infection rate strikingly higher than in their patients on anti-TNF or nonbiologic therapy (J Crohns Colitis. 2017 Feb;11[2]:185-90).
This prompted investigators at the University of Chicago to look retrospectively at their institutional experience. They reported no increased risk in IBD patients on vedolizumab (Am J Gastroenterol. 2017 Sep;112[9]:1423-9). Neither did Belgian gastroenterologists at the Catholic University of Leuven (J Crohns Colitis. 2017 Oct 27;11[11]:1353-61).
Most recently, the Mayo Clinic group along with gastroenterologists at three other U.S. centers collaborated in a multicenter retrospective review of 146 adult IBD patients who received vedolizumab within 12 weeks before major abdominal surgery and 289 who received anti-TNF therapy. In a multivariate analysis, perioperative use of vedolizumab was independently associated with a 5.8-fold increased risk of developing a surgical site infection (J Inflamm Bowel Dis. 2018 Mar 19. doi: 10.1093/ibd/izx076).
Dr. Sandborn, who like Dr. Loftus was a coauthor of the multicenter study, drew back to look at the big picture.
“Is vedolizumab really causal? I doubt it, although it’s remotely possible. But I bet vedolizumab therapy is a really good marker for sick patients, and sick patients have worse operative outcomes, so we ought to be conservative with their surgery. My read of this is this [postoperative infection risk] isn’t unique to vedolizumab. Just be careful with sick patients when you’re operating and do more conservative surgeries,” he said.
Both gastroenterologists reported serving as consultants to and receiving research grants from numerous pharmaceutical companies.
MAUI, HAWAII – Prolonged corticosteroid therapy for inflammatory bowel disease (IBD) was associated with a significantly increased mortality risk compared with anti–tumor necrosis factor therapy in a landmark study spotlighted by Edward V. Loftus Jr., MD, at the Gastroenterology Updates, IBD, Liver Disease meeting.
This was one of several key studies on safety issues involving IBD medications published in the past year. Others highlighted by Dr. Loftus and copanelist William J. Sandborn, MD, included a study that provided persuasive evidence that TNF inhibitors modestly increase lymphoma risk in IBD patients to a degree similar to that of thiopurines, and several reports addressing the question of whether preoperative use of vedolizumab in patients undergoing major abdominal operations for IBD boosts postoperative infection risk.
Mortality impact of prolonged steroids vs. anti-TNF therapy
That will come as an unpleasant surprise to many physicians. There is a widespread reluctance to turn to continuous chronic immunosuppression via anti-TNF therapy in patients with challenging IBD, particularly in elderly individuals with multiple comorbid conditions. Many physicians have heard and read so much about the biologics’ risks of serious adverse events that they opt instead for multiple courses of corticosteroids for disease control. This is a serious mistake, emphasized Dr. Loftus, professor of medicine and director of the IBD Interest Group at the Mayo Clinic in Rochester, Minn.
“When you say, ‘Oh, I’ll just give that patient another prednisone taper, he doesn’t want to start taking a TNF inhibitor,’ you’re actually doing the patient harm. You’re actually affecting the patient’s life expectancy when you do that,” he declared. “The message is, yes, steroids are cheap, steroids are easy, nobody’s afraid of steroids, but you should be afraid of steroids.”
The 1,879 Crohn’s disease patients who entered the cohort as new users of anti-TNF therapy had a subsequent mortality incidence rate of 21.4 per 1,000 person-years, compared with a rate of 30.1 per 1,000 person-years in the 7,694 who entered the study period as prolonged steroid users. In a multivariate analysis accounting for 57 potential confounding factors, this translated to a highly significant 22% relative risk reduction in mortality in the patients who went with anti-TNF therapy (Am J Gastroenterol. 2018 Jan 16. doi: 10.1038/ajg.2017.479).
A similar trend was seen in the ulcerative colitis cohort. The 459 ulcerative colitis patients who entered the cohort as new anti-TNF therapy users had a mortality incidence rate of 23.0 per 1,000 person-years, compared with a rate of 30.9 in the 3,224 who received more than 3,000 mg of prednisone in the next 12 months. This represented a 14% relative risk reduction, although this favorable trend did not achieve statistical significance, perhaps because of the smaller size of the ulcerative colitis cohort.
In addition to demonstrably greater life expectancy, anti-TNF therapy offered additional benefits: a 32% reduction in the risk of major adverse cardiovascular events and a 46% lower incidence of hip fracture.
Dr. Sandborn, professor of medicine and chief of the division of gastroenterology at the University of California, San Diego, spun the study data another way: “It shows the number needed to kill is 33. So for every 33 patients you put on prolonged corticosteroids, you’re killing one extra patient by doing that. Of course, you probably blame it on their age and comorbidities, but this is it. This is the data.”
TNF blockers, thiopurines, and lymphoma
The use of thiopurines for treatment of IBD is widely recognized to be associated with a small but real increased risk of lymphoma. Now a large French national study has demonstrated for the first time that anti-TNF therapy for IBD is also associated with an increased risk that needs to be discussed with patients. And in IBD patients on combination therapy with both classes of medication, that risk jumps to 6.1-fold greater than in unexposed IBD patients (JAMA 2017 Nov 7;318[17]:1679-86).
Dr. Loftus and Dr. Sandborn urged their colleagues to keep this increased risk in perspective in counseling patients by focusing on the modest absolute increase in risk rather than the scarier-sounding relative risk. Notably, two-thirds of lymphomas in the French study occurred in patients not on thiopurines or anti-TNF agents.
“The most interesting thing to me is that we worry and worry about lymphoma, and guess what? In this study and in multiple other studies, the majority of lymphomas occurring in IBD patients have nothing to do with their medications. They’re due to the usual risk factors for lymphoma, which include age and male gender,” Dr. Loftus observed.
The French study included more than 189,000 IBD patients followed for a median of 6.7 years, during which 336 cases of lymphoma occurred. The incidence rate was 0.26 cases per 1,000 person-years in unexposed patients. The rate was significantly higher at 0.54 per 1,000 person-years in those on thiopurine monotherapy, increased to a similar extent at 0.41 cases per 1,000 person-years in patients on anti-TNF monotherapy, and 0.95 per 1,000 person-years in those on combination therapy.
In a multivariate analysis, the lymphoma risk was an adjusted 2.6-fold greater in patients exposed to thiopurine monotherapy than in unexposed patients, 2.41-fold greater in patients exposed to anti-TNF monotherapy, and 6.1-fold greater in those exposed to combination therapy.
“The point I want to make is the lymphoma rates in the thiopurine monotherapy and anti-TNF monotherapy groups are not significantly different. So the claim that’s been out there that the increased lymphoma risk in IBD patients can be completely explained by thiopurines is wrong. This study is showing us that with anti-TNF monotherapy there is still a low-level risk of lymphoma,” Dr. Loftus said.
“It is somewhat eyebrow-raising when you see that relative risk of 6.1, and that’s what patients are going to focus on, but when you counsel patients you have to redirect them to the absolute risk. You can say, ‘Even on combination therapy, your risk is 1 in 1,000,’ ” the gastroenterologist said.
Dr. Sandborn said the lymphoma signal hadn’t been spotted previously because the individual registries of IBD patients on anti-TNF agents are too small to allow for identification of a small increase in risk. The French investigators overcame that limitation by tapping into the country’s national health care system.
“This is a huge dataset and I think the message is unequivocal,” Dr. Sandborn said.
He noted that strongly risk-averse patients may find ustekinumab (Stelara) and vedolizumab (Entyvio) to be attractive treatment options. Neither has any link to lymphoma.
Preoperative vedolizumab and postoperative infection risk
“The overall safety profile of vedolizumab is pretty good,” Dr. Loftus observed. “The one unanswered question is its safety when used within 8-12 weeks of a major abdominal operation.”
It’s a clinically relevant question because vedolizumab selectively inhibits leukocyte migration into the intestinal tract, which could provide a mechanism for impaired postoperative wound healing in patients undergoing major abdominal surgery. And sooner or later a high proportion of IBD patients have a major abdominal operation.
Dr. Loftus and his coinvestigators kicked off a controversy by reporting a 37% incidence of surgical site infections in IBD patients who received vedolizumab within 30 days of a major abdominal operation in a retrospective chart review of the Mayo Clinic experience, a postoperative infection rate strikingly higher than in their patients on anti-TNF or nonbiologic therapy (J Crohns Colitis. 2017 Feb;11[2]:185-90).
This prompted investigators at the University of Chicago to look retrospectively at their institutional experience. They reported no increased risk in IBD patients on vedolizumab (Am J Gastroenterol. 2017 Sep;112[9]:1423-9). Neither did Belgian gastroenterologists at the Catholic University of Leuven (J Crohns Colitis. 2017 Oct 27;11[11]:1353-61).
Most recently, the Mayo Clinic group along with gastroenterologists at three other U.S. centers collaborated in a multicenter retrospective review of 146 adult IBD patients who received vedolizumab within 12 weeks before major abdominal surgery and 289 who received anti-TNF therapy. In a multivariate analysis, perioperative use of vedolizumab was independently associated with a 5.8-fold increased risk of developing a surgical site infection (J Inflamm Bowel Dis. 2018 Mar 19. doi: 10.1093/ibd/izx076).
Dr. Sandborn, who like Dr. Loftus was a coauthor of the multicenter study, drew back to look at the big picture.
“Is vedolizumab really causal? I doubt it, although it’s remotely possible. But I bet vedolizumab therapy is a really good marker for sick patients, and sick patients have worse operative outcomes, so we ought to be conservative with their surgery. My read of this is this [postoperative infection risk] isn’t unique to vedolizumab. Just be careful with sick patients when you’re operating and do more conservative surgeries,” he said.
Both gastroenterologists reported serving as consultants to and receiving research grants from numerous pharmaceutical companies.
MAUI, HAWAII – Prolonged corticosteroid therapy for inflammatory bowel disease (IBD) was associated with a significantly increased mortality risk compared with anti–tumor necrosis factor therapy in a landmark study spotlighted by Edward V. Loftus Jr., MD, at the Gastroenterology Updates, IBD, Liver Disease meeting.
This was one of several key studies on safety issues involving IBD medications published in the past year. Others highlighted by Dr. Loftus and copanelist William J. Sandborn, MD, included a study that provided persuasive evidence that TNF inhibitors modestly increase lymphoma risk in IBD patients to a degree similar to that of thiopurines, and several reports addressing the question of whether preoperative use of vedolizumab in patients undergoing major abdominal operations for IBD boosts postoperative infection risk.
Mortality impact of prolonged steroids vs. anti-TNF therapy
That will come as an unpleasant surprise to many physicians. There is a widespread reluctance to turn to continuous chronic immunosuppression via anti-TNF therapy in patients with challenging IBD, particularly in elderly individuals with multiple comorbid conditions. Many physicians have heard and read so much about the biologics’ risks of serious adverse events that they opt instead for multiple courses of corticosteroids for disease control. This is a serious mistake, emphasized Dr. Loftus, professor of medicine and director of the IBD Interest Group at the Mayo Clinic in Rochester, Minn.
“When you say, ‘Oh, I’ll just give that patient another prednisone taper, he doesn’t want to start taking a TNF inhibitor,’ you’re actually doing the patient harm. You’re actually affecting the patient’s life expectancy when you do that,” he declared. “The message is, yes, steroids are cheap, steroids are easy, nobody’s afraid of steroids, but you should be afraid of steroids.”
The 1,879 Crohn’s disease patients who entered the cohort as new users of anti-TNF therapy had a subsequent mortality incidence rate of 21.4 per 1,000 person-years, compared with a rate of 30.1 per 1,000 person-years in the 7,694 who entered the study period as prolonged steroid users. In a multivariate analysis accounting for 57 potential confounding factors, this translated to a highly significant 22% relative risk reduction in mortality in the patients who went with anti-TNF therapy (Am J Gastroenterol. 2018 Jan 16. doi: 10.1038/ajg.2017.479).
A similar trend was seen in the ulcerative colitis cohort. The 459 ulcerative colitis patients who entered the cohort as new anti-TNF therapy users had a mortality incidence rate of 23.0 per 1,000 person-years, compared with a rate of 30.9 in the 3,224 who received more than 3,000 mg of prednisone in the next 12 months. This represented a 14% relative risk reduction, although this favorable trend did not achieve statistical significance, perhaps because of the smaller size of the ulcerative colitis cohort.
In addition to demonstrably greater life expectancy, anti-TNF therapy offered additional benefits: a 32% reduction in the risk of major adverse cardiovascular events and a 46% lower incidence of hip fracture.
Dr. Sandborn, professor of medicine and chief of the division of gastroenterology at the University of California, San Diego, spun the study data another way: “It shows the number needed to kill is 33. So for every 33 patients you put on prolonged corticosteroids, you’re killing one extra patient by doing that. Of course, you probably blame it on their age and comorbidities, but this is it. This is the data.”
TNF blockers, thiopurines, and lymphoma
The use of thiopurines for treatment of IBD is widely recognized to be associated with a small but real increased risk of lymphoma. Now a large French national study has demonstrated for the first time that anti-TNF therapy for IBD is also associated with an increased risk that needs to be discussed with patients. And in IBD patients on combination therapy with both classes of medication, that risk jumps to 6.1-fold greater than in unexposed IBD patients (JAMA 2017 Nov 7;318[17]:1679-86).
Dr. Loftus and Dr. Sandborn urged their colleagues to keep this increased risk in perspective in counseling patients by focusing on the modest absolute increase in risk rather than the scarier-sounding relative risk. Notably, two-thirds of lymphomas in the French study occurred in patients not on thiopurines or anti-TNF agents.
“The most interesting thing to me is that we worry and worry about lymphoma, and guess what? In this study and in multiple other studies, the majority of lymphomas occurring in IBD patients have nothing to do with their medications. They’re due to the usual risk factors for lymphoma, which include age and male gender,” Dr. Loftus observed.
The French study included more than 189,000 IBD patients followed for a median of 6.7 years, during which 336 cases of lymphoma occurred. The incidence rate was 0.26 cases per 1,000 person-years in unexposed patients. The rate was significantly higher at 0.54 per 1,000 person-years in those on thiopurine monotherapy, increased to a similar extent at 0.41 cases per 1,000 person-years in patients on anti-TNF monotherapy, and 0.95 per 1,000 person-years in those on combination therapy.
In a multivariate analysis, the lymphoma risk was an adjusted 2.6-fold greater in patients exposed to thiopurine monotherapy than in unexposed patients, 2.41-fold greater in patients exposed to anti-TNF monotherapy, and 6.1-fold greater in those exposed to combination therapy.
“The point I want to make is the lymphoma rates in the thiopurine monotherapy and anti-TNF monotherapy groups are not significantly different. So the claim that’s been out there that the increased lymphoma risk in IBD patients can be completely explained by thiopurines is wrong. This study is showing us that with anti-TNF monotherapy there is still a low-level risk of lymphoma,” Dr. Loftus said.
“It is somewhat eyebrow-raising when you see that relative risk of 6.1, and that’s what patients are going to focus on, but when you counsel patients you have to redirect them to the absolute risk. You can say, ‘Even on combination therapy, your risk is 1 in 1,000,’ ” the gastroenterologist said.
Dr. Sandborn said the lymphoma signal hadn’t been spotted previously because the individual registries of IBD patients on anti-TNF agents are too small to allow for identification of a small increase in risk. The French investigators overcame that limitation by tapping into the country’s national health care system.
“This is a huge dataset and I think the message is unequivocal,” Dr. Sandborn said.
He noted that strongly risk-averse patients may find ustekinumab (Stelara) and vedolizumab (Entyvio) to be attractive treatment options. Neither has any link to lymphoma.
Preoperative vedolizumab and postoperative infection risk
“The overall safety profile of vedolizumab is pretty good,” Dr. Loftus observed. “The one unanswered question is its safety when used within 8-12 weeks of a major abdominal operation.”
It’s a clinically relevant question because vedolizumab selectively inhibits leukocyte migration into the intestinal tract, which could provide a mechanism for impaired postoperative wound healing in patients undergoing major abdominal surgery. And sooner or later a high proportion of IBD patients have a major abdominal operation.
Dr. Loftus and his coinvestigators kicked off a controversy by reporting a 37% incidence of surgical site infections in IBD patients who received vedolizumab within 30 days of a major abdominal operation in a retrospective chart review of the Mayo Clinic experience, a postoperative infection rate strikingly higher than in their patients on anti-TNF or nonbiologic therapy (J Crohns Colitis. 2017 Feb;11[2]:185-90).
This prompted investigators at the University of Chicago to look retrospectively at their institutional experience. They reported no increased risk in IBD patients on vedolizumab (Am J Gastroenterol. 2017 Sep;112[9]:1423-9). Neither did Belgian gastroenterologists at the Catholic University of Leuven (J Crohns Colitis. 2017 Oct 27;11[11]:1353-61).
Most recently, the Mayo Clinic group along with gastroenterologists at three other U.S. centers collaborated in a multicenter retrospective review of 146 adult IBD patients who received vedolizumab within 12 weeks before major abdominal surgery and 289 who received anti-TNF therapy. In a multivariate analysis, perioperative use of vedolizumab was independently associated with a 5.8-fold increased risk of developing a surgical site infection (J Inflamm Bowel Dis. 2018 Mar 19. doi: 10.1093/ibd/izx076).
Dr. Sandborn, who like Dr. Loftus was a coauthor of the multicenter study, drew back to look at the big picture.
“Is vedolizumab really causal? I doubt it, although it’s remotely possible. But I bet vedolizumab therapy is a really good marker for sick patients, and sick patients have worse operative outcomes, so we ought to be conservative with their surgery. My read of this is this [postoperative infection risk] isn’t unique to vedolizumab. Just be careful with sick patients when you’re operating and do more conservative surgeries,” he said.
Both gastroenterologists reported serving as consultants to and receiving research grants from numerous pharmaceutical companies.
EXPERT ANALYSIS FROM GUILD 2018