User login
Parents surveyed about underage drinking
Most parents of children aged 10-17 years have talked to them about alcohol consumption, but many do not consider brain health to be an important reason to avoid underage drinking, according to a recent survey of 1,000 parents.
The 76% of parents who reported having at least one conversation about alcohol was up by 7% from a survey conducted in 2003, the Foundation for Advancing Alcohol Responsibility said.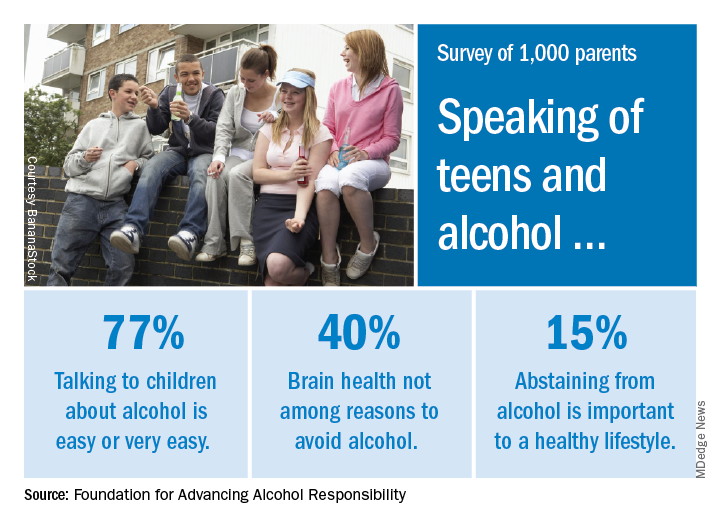
As for the most important reasons to avoid alcohol, 79% said that it interferes with judgment and the ability to make good decisions, and 77% mentioned the unintended consequences of consuming too much. Slightly more than 40% did not include its effects on brain development, the report said.
“Adolescence includes critical phases in brain development. The area of the brain that controls reasoning – helps us think before we act – matures later in the third decade of life. The sooner that parents speak with their children about the dangers of drinking alcohol underage, the better,” said Deborah Gilboa, MD, a Pittsburgh family physician who serves on the foundation’s education advisory board.
The survey was conducted online by GfK between Nov. 10 and 12, 2017, among adults aged 18 years and over with at least one child aged 10-17 years. The margin of error is plus or minus 3 percentage points for the full sample.
Most parents of children aged 10-17 years have talked to them about alcohol consumption, but many do not consider brain health to be an important reason to avoid underage drinking, according to a recent survey of 1,000 parents.
The 76% of parents who reported having at least one conversation about alcohol was up by 7% from a survey conducted in 2003, the Foundation for Advancing Alcohol Responsibility said.
As for the most important reasons to avoid alcohol, 79% said that it interferes with judgment and the ability to make good decisions, and 77% mentioned the unintended consequences of consuming too much. Slightly more than 40% did not include its effects on brain development, the report said.
“Adolescence includes critical phases in brain development. The area of the brain that controls reasoning – helps us think before we act – matures later in the third decade of life. The sooner that parents speak with their children about the dangers of drinking alcohol underage, the better,” said Deborah Gilboa, MD, a Pittsburgh family physician who serves on the foundation’s education advisory board.
The survey was conducted online by GfK between Nov. 10 and 12, 2017, among adults aged 18 years and over with at least one child aged 10-17 years. The margin of error is plus or minus 3 percentage points for the full sample.
Most parents of children aged 10-17 years have talked to them about alcohol consumption, but many do not consider brain health to be an important reason to avoid underage drinking, according to a recent survey of 1,000 parents.
The 76% of parents who reported having at least one conversation about alcohol was up by 7% from a survey conducted in 2003, the Foundation for Advancing Alcohol Responsibility said.
As for the most important reasons to avoid alcohol, 79% said that it interferes with judgment and the ability to make good decisions, and 77% mentioned the unintended consequences of consuming too much. Slightly more than 40% did not include its effects on brain development, the report said.
“Adolescence includes critical phases in brain development. The area of the brain that controls reasoning – helps us think before we act – matures later in the third decade of life. The sooner that parents speak with their children about the dangers of drinking alcohol underage, the better,” said Deborah Gilboa, MD, a Pittsburgh family physician who serves on the foundation’s education advisory board.
The survey was conducted online by GfK between Nov. 10 and 12, 2017, among adults aged 18 years and over with at least one child aged 10-17 years. The margin of error is plus or minus 3 percentage points for the full sample.
Bleeding episodes more common in boys with VWD
SAN DIEGO – Among children with types 1 and 2 von Willebrand disease (VWD), a higher proportion of boys than girls reported ever having a bleeding episode and using more treatment products. But the trend did not continue among children with type 3 disease.
Those are some of the key findings from a never-before-published analysis of surveillance data from the Centers for Disease Control and Prevention presented by Karon Abe, PhD, during a poster session at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The researchers retrieved data from the UDC (Universal Data Collection System), a federally funded surveillance system of people with hemophilia and other bleeding disorders treated at 130 U.S. Hemophilia Treatment Centers (HTCs) during 1998-2011. Although UDC data collection ended in 2011, a current CDC bleeding surveillance project called Community Counts continues and expands on the work of the UDC.
Between 1998 and 2011, data were collected on 2,413 children with VWD aged 2-12 years. Of these, 2,070 had type 1, 224 had type 2, and 119 had type 3 VWD. The researchers used chi-square analysis and Wilcoxon rank sum tests to assess differences in bleeding characteristics by sex and by type of VWD. Next, they used a multivariate regression model to examine the association between demographic and clinical characteristics and a history of ever having had a bleeding episode among type 1 VWD patients.
Nearly two-thirds of children (65%) were non-Hispanic, 17% were Hispanic, 8% were black, and the remainder were from other ethnicities. In addition, 40% of the children had no family history of a bleeding disorder.
The median age of first bleed was lower among children with type 3 VWD, compared with other VWD types, and was lower among boys than girls with type 1 VWD (36 months vs. 48 months, respectively; P less than .001) and type 3 VWD (9 months vs. 12 months; P = .04), Dr. Abe reported.
A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P = .01) and type 2 VWD (90% vs. 75%; P = .01), but not among children with type 3 VWD (97% vs. 96%; P = .77).
A higher prevalence of treatment-product use was reported among children with type 3 VWD, compared with those with the other VWD types (a mean of 95% vs. 79% and 71% among types 2 and 1, respectively). A significantly higher prevalence of the use of treatment product was seen among boys than girls with type 1 VWD (73% vs. 68%, P = .03) and type 2 VWD (87% vs. 72%, P =.01), but not type 3 VWD (94% vs. 96%, P = .87).
The most common sites of the first bleed among all patients regardless of gender or VWD type were epistaxis and oral cavity bleeding.
“To our surprise, the boys were showing more bleeding and were receiving more product than the females,” Dr. Abe said in an interview. “This is a fairly large population.”
Multivariate regression analysis revealed independent associations between the following patient characteristics and ever having a bleed among children with type 1 VWD: male gender (adjusted odds ratio, 1.23); being aged 7-9 years at registration, compared with being aged 2-6 years (aOR, 1.5); being black (aOR, 1.7); being Asian, Native Hawaiian or Pacific Islander (aOR, 2.4), being Hispanic (aOR, 2.8), and being some other race/ethnicity (aOR, 1.8). However, family history of a bleeding disorder was protective (aOR, 0.721).
Dr. Abe said she hopes that the findings will raise awareness and help physicians to educate families about bleeding symptoms and intervene to treat bleeding episodes appropriately. She and her associates are planning to compare the data with Community Counts, “so it’s more up to date,” she said.
Dr. Abe reported having no financial disclosures.
SOURCE: Abe K et al. THSNA 2018, Poster 145.
SAN DIEGO – Among children with types 1 and 2 von Willebrand disease (VWD), a higher proportion of boys than girls reported ever having a bleeding episode and using more treatment products. But the trend did not continue among children with type 3 disease.
Those are some of the key findings from a never-before-published analysis of surveillance data from the Centers for Disease Control and Prevention presented by Karon Abe, PhD, during a poster session at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The researchers retrieved data from the UDC (Universal Data Collection System), a federally funded surveillance system of people with hemophilia and other bleeding disorders treated at 130 U.S. Hemophilia Treatment Centers (HTCs) during 1998-2011. Although UDC data collection ended in 2011, a current CDC bleeding surveillance project called Community Counts continues and expands on the work of the UDC.
Between 1998 and 2011, data were collected on 2,413 children with VWD aged 2-12 years. Of these, 2,070 had type 1, 224 had type 2, and 119 had type 3 VWD. The researchers used chi-square analysis and Wilcoxon rank sum tests to assess differences in bleeding characteristics by sex and by type of VWD. Next, they used a multivariate regression model to examine the association between demographic and clinical characteristics and a history of ever having had a bleeding episode among type 1 VWD patients.
Nearly two-thirds of children (65%) were non-Hispanic, 17% were Hispanic, 8% were black, and the remainder were from other ethnicities. In addition, 40% of the children had no family history of a bleeding disorder.
The median age of first bleed was lower among children with type 3 VWD, compared with other VWD types, and was lower among boys than girls with type 1 VWD (36 months vs. 48 months, respectively; P less than .001) and type 3 VWD (9 months vs. 12 months; P = .04), Dr. Abe reported.
A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P = .01) and type 2 VWD (90% vs. 75%; P = .01), but not among children with type 3 VWD (97% vs. 96%; P = .77).
A higher prevalence of treatment-product use was reported among children with type 3 VWD, compared with those with the other VWD types (a mean of 95% vs. 79% and 71% among types 2 and 1, respectively). A significantly higher prevalence of the use of treatment product was seen among boys than girls with type 1 VWD (73% vs. 68%, P = .03) and type 2 VWD (87% vs. 72%, P =.01), but not type 3 VWD (94% vs. 96%, P = .87).
The most common sites of the first bleed among all patients regardless of gender or VWD type were epistaxis and oral cavity bleeding.
“To our surprise, the boys were showing more bleeding and were receiving more product than the females,” Dr. Abe said in an interview. “This is a fairly large population.”
Multivariate regression analysis revealed independent associations between the following patient characteristics and ever having a bleed among children with type 1 VWD: male gender (adjusted odds ratio, 1.23); being aged 7-9 years at registration, compared with being aged 2-6 years (aOR, 1.5); being black (aOR, 1.7); being Asian, Native Hawaiian or Pacific Islander (aOR, 2.4), being Hispanic (aOR, 2.8), and being some other race/ethnicity (aOR, 1.8). However, family history of a bleeding disorder was protective (aOR, 0.721).
Dr. Abe said she hopes that the findings will raise awareness and help physicians to educate families about bleeding symptoms and intervene to treat bleeding episodes appropriately. She and her associates are planning to compare the data with Community Counts, “so it’s more up to date,” she said.
Dr. Abe reported having no financial disclosures.
SOURCE: Abe K et al. THSNA 2018, Poster 145.
SAN DIEGO – Among children with types 1 and 2 von Willebrand disease (VWD), a higher proportion of boys than girls reported ever having a bleeding episode and using more treatment products. But the trend did not continue among children with type 3 disease.
Those are some of the key findings from a never-before-published analysis of surveillance data from the Centers for Disease Control and Prevention presented by Karon Abe, PhD, during a poster session at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
The researchers retrieved data from the UDC (Universal Data Collection System), a federally funded surveillance system of people with hemophilia and other bleeding disorders treated at 130 U.S. Hemophilia Treatment Centers (HTCs) during 1998-2011. Although UDC data collection ended in 2011, a current CDC bleeding surveillance project called Community Counts continues and expands on the work of the UDC.
Between 1998 and 2011, data were collected on 2,413 children with VWD aged 2-12 years. Of these, 2,070 had type 1, 224 had type 2, and 119 had type 3 VWD. The researchers used chi-square analysis and Wilcoxon rank sum tests to assess differences in bleeding characteristics by sex and by type of VWD. Next, they used a multivariate regression model to examine the association between demographic and clinical characteristics and a history of ever having had a bleeding episode among type 1 VWD patients.
Nearly two-thirds of children (65%) were non-Hispanic, 17% were Hispanic, 8% were black, and the remainder were from other ethnicities. In addition, 40% of the children had no family history of a bleeding disorder.
The median age of first bleed was lower among children with type 3 VWD, compared with other VWD types, and was lower among boys than girls with type 1 VWD (36 months vs. 48 months, respectively; P less than .001) and type 3 VWD (9 months vs. 12 months; P = .04), Dr. Abe reported.
A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P = .01) and type 2 VWD (90% vs. 75%; P = .01), but not among children with type 3 VWD (97% vs. 96%; P = .77).
A higher prevalence of treatment-product use was reported among children with type 3 VWD, compared with those with the other VWD types (a mean of 95% vs. 79% and 71% among types 2 and 1, respectively). A significantly higher prevalence of the use of treatment product was seen among boys than girls with type 1 VWD (73% vs. 68%, P = .03) and type 2 VWD (87% vs. 72%, P =.01), but not type 3 VWD (94% vs. 96%, P = .87).
The most common sites of the first bleed among all patients regardless of gender or VWD type were epistaxis and oral cavity bleeding.
“To our surprise, the boys were showing more bleeding and were receiving more product than the females,” Dr. Abe said in an interview. “This is a fairly large population.”
Multivariate regression analysis revealed independent associations between the following patient characteristics and ever having a bleed among children with type 1 VWD: male gender (adjusted odds ratio, 1.23); being aged 7-9 years at registration, compared with being aged 2-6 years (aOR, 1.5); being black (aOR, 1.7); being Asian, Native Hawaiian or Pacific Islander (aOR, 2.4), being Hispanic (aOR, 2.8), and being some other race/ethnicity (aOR, 1.8). However, family history of a bleeding disorder was protective (aOR, 0.721).
Dr. Abe said she hopes that the findings will raise awareness and help physicians to educate families about bleeding symptoms and intervene to treat bleeding episodes appropriately. She and her associates are planning to compare the data with Community Counts, “so it’s more up to date,” she said.
Dr. Abe reported having no financial disclosures.
SOURCE: Abe K et al. THSNA 2018, Poster 145.
REPORTING FROM THSNA 2018
Key clinical point:
Major finding: A higher proportion of boys than girls reported ever having a bleeding episode among children with type 1 VWD (78% vs. 73%; P= .01) and type 2 VWD (90% vs. 75%; P= .01), but not among children with type 3 VWD (97% vs. 96%; P= .77).
Study details: An analysis of 2,413 children with VWD aged 2-12 years.
Disclosures: Dr. Abe reported having no financial disclosures.
Source: Abe K et al. THSNA 2018, Poster 145.
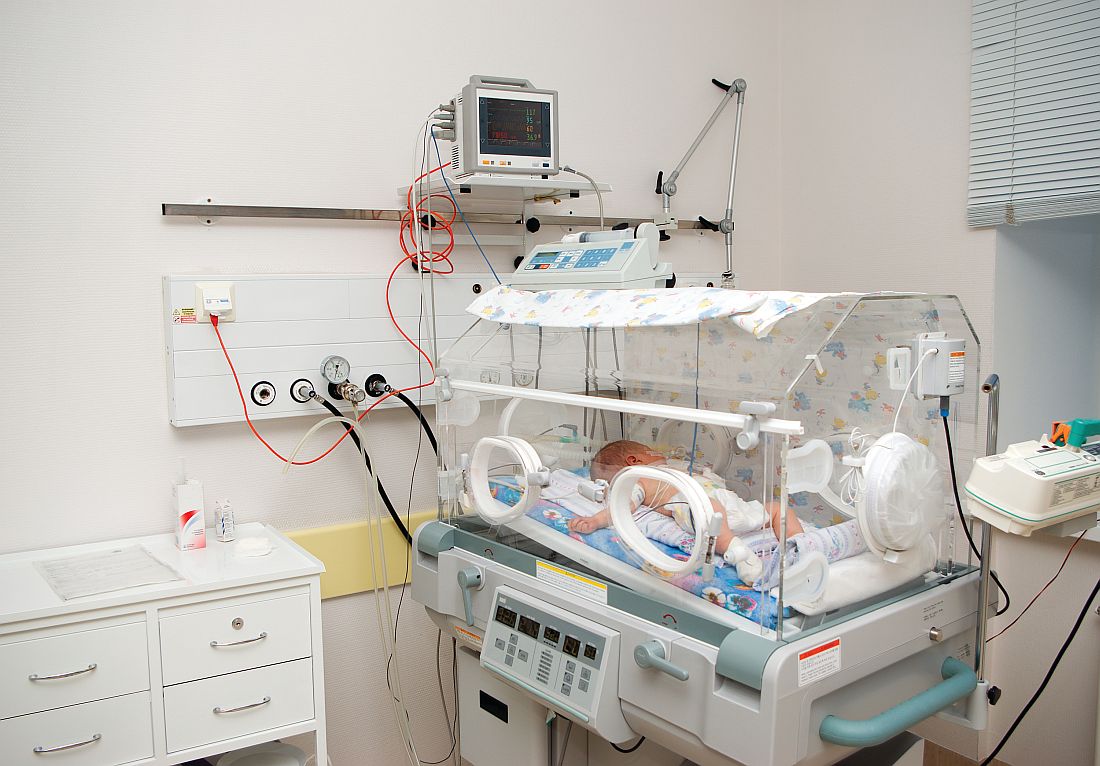
Alternative oxygen therapy reduces treatment failure in bronchiolitis
High-flow oxygen therapy outside the ICU boosts the likelihood that infants with bronchiolitis will avoid treatment failure and an escalation of treatment, a study finds.
“High flow can be safely used in general emergency wards and general pediatric ward settings in regional and metropolitan hospitals that have no immediate direct access to dedicated pediatric intensive care facilities,” study coauthor Andreas Schibler, MD, of University of Queensland in Australia, said in an interview. The findings were published March 22 in the New England Journal of Medicine.
“The typical treatment for bronchiolitis is supportive therapy, providing nutrition, fluids, and if needed respiratory support including provision of oxygen,” Dr. Schibler said.
The prognosis is generally goods thanks to improvements in intensive care, he said, which some infants need because the standard oxygen therapy provided in general pediatric wards is insufficient. The new study examines whether high-flow oxygen therapy through a cannula – which he said has become more common – reduces the risk of treatment failure in non-ICU therapy, compared with standard oxygen treatment.
Dr. Schibler and his colleagues tracked 1,472 patients under 12 months with bronchiolitis and a need for oxygen treatment who were randomly assigned to high-flow or standard oxygen therapy to maintain their oxygen saturation at 92%-98% or 94%-98%, depending on policy at the hospital. The subjects were patients at 17 hospitals in Australia and New Zealand.
A total of 739 infants received high-flow treatment that provided heated and humidified oxygen at a rate of 2 liters per kilogram of body weight per minute. The other 733 infants received standard oxygen therapy up to a maximum 2 liters per minute.
The treatment failed, requiring an escalation of care, in 87 of 739 patients (12%) in the high-flow group and 167 of 733 (23%) in the standard-therapy group. (risk difference = –11% points; 95% confidence interval, –15 to –7; P less than .001).
“The ease to use and simplicity of high flow made us recognize and think that this level of respiratory care can be provided outside intensive care,” Dr. Schibler said. “This was further supported by the observational fact that most of these infants with bronchiolitis showed a dramatically improved respiratory condition once on high flow.”
Dr. Schibler said there haven’t been any signs of adverse effects from high-flow oxygen therapy. As for the cost of the treatment, he said it is “likely offset by a reduced need for intensive care therapy or costs associated with transferring to a children’s hospital.”
What should physicians and hospitals take from the study findings? “If a hospital explores the option to use high flow in bronchiolitis, then start the therapy early in the disease process or once an oxygen requirement is recognized,” Dr. Schibler said. “Implementation of a solid and structured training program with a clear hospital guideline based on the evidence will ensure the staff who care for these patients will be empowered and comfortable to adjust the oxygen levels given by the high-flow equipment. The greater the confidence and comfort level for the nursing and respiratory technician staff the better for these infants, as they will sooner observe those infants who are not responding well and may require a higher level of care such as intensive care or they will recognize the infant who responds well.”
The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment and consumables and travel/accommodation support. Study authors reported various grants and other support.
SOURCE: Franklin D et al. N Engl J Med. 2018;378(12):1112-31.
High-flow oxygen therapy outside the ICU boosts the likelihood that infants with bronchiolitis will avoid treatment failure and an escalation of treatment, a study finds.
“High flow can be safely used in general emergency wards and general pediatric ward settings in regional and metropolitan hospitals that have no immediate direct access to dedicated pediatric intensive care facilities,” study coauthor Andreas Schibler, MD, of University of Queensland in Australia, said in an interview. The findings were published March 22 in the New England Journal of Medicine.
“The typical treatment for bronchiolitis is supportive therapy, providing nutrition, fluids, and if needed respiratory support including provision of oxygen,” Dr. Schibler said.
The prognosis is generally goods thanks to improvements in intensive care, he said, which some infants need because the standard oxygen therapy provided in general pediatric wards is insufficient. The new study examines whether high-flow oxygen therapy through a cannula – which he said has become more common – reduces the risk of treatment failure in non-ICU therapy, compared with standard oxygen treatment.
Dr. Schibler and his colleagues tracked 1,472 patients under 12 months with bronchiolitis and a need for oxygen treatment who were randomly assigned to high-flow or standard oxygen therapy to maintain their oxygen saturation at 92%-98% or 94%-98%, depending on policy at the hospital. The subjects were patients at 17 hospitals in Australia and New Zealand.
A total of 739 infants received high-flow treatment that provided heated and humidified oxygen at a rate of 2 liters per kilogram of body weight per minute. The other 733 infants received standard oxygen therapy up to a maximum 2 liters per minute.
The treatment failed, requiring an escalation of care, in 87 of 739 patients (12%) in the high-flow group and 167 of 733 (23%) in the standard-therapy group. (risk difference = –11% points; 95% confidence interval, –15 to –7; P less than .001).
“The ease to use and simplicity of high flow made us recognize and think that this level of respiratory care can be provided outside intensive care,” Dr. Schibler said. “This was further supported by the observational fact that most of these infants with bronchiolitis showed a dramatically improved respiratory condition once on high flow.”
Dr. Schibler said there haven’t been any signs of adverse effects from high-flow oxygen therapy. As for the cost of the treatment, he said it is “likely offset by a reduced need for intensive care therapy or costs associated with transferring to a children’s hospital.”
What should physicians and hospitals take from the study findings? “If a hospital explores the option to use high flow in bronchiolitis, then start the therapy early in the disease process or once an oxygen requirement is recognized,” Dr. Schibler said. “Implementation of a solid and structured training program with a clear hospital guideline based on the evidence will ensure the staff who care for these patients will be empowered and comfortable to adjust the oxygen levels given by the high-flow equipment. The greater the confidence and comfort level for the nursing and respiratory technician staff the better for these infants, as they will sooner observe those infants who are not responding well and may require a higher level of care such as intensive care or they will recognize the infant who responds well.”
The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment and consumables and travel/accommodation support. Study authors reported various grants and other support.
SOURCE: Franklin D et al. N Engl J Med. 2018;378(12):1112-31.
High-flow oxygen therapy outside the ICU boosts the likelihood that infants with bronchiolitis will avoid treatment failure and an escalation of treatment, a study finds.
“High flow can be safely used in general emergency wards and general pediatric ward settings in regional and metropolitan hospitals that have no immediate direct access to dedicated pediatric intensive care facilities,” study coauthor Andreas Schibler, MD, of University of Queensland in Australia, said in an interview. The findings were published March 22 in the New England Journal of Medicine.
“The typical treatment for bronchiolitis is supportive therapy, providing nutrition, fluids, and if needed respiratory support including provision of oxygen,” Dr. Schibler said.
The prognosis is generally goods thanks to improvements in intensive care, he said, which some infants need because the standard oxygen therapy provided in general pediatric wards is insufficient. The new study examines whether high-flow oxygen therapy through a cannula – which he said has become more common – reduces the risk of treatment failure in non-ICU therapy, compared with standard oxygen treatment.
Dr. Schibler and his colleagues tracked 1,472 patients under 12 months with bronchiolitis and a need for oxygen treatment who were randomly assigned to high-flow or standard oxygen therapy to maintain their oxygen saturation at 92%-98% or 94%-98%, depending on policy at the hospital. The subjects were patients at 17 hospitals in Australia and New Zealand.
A total of 739 infants received high-flow treatment that provided heated and humidified oxygen at a rate of 2 liters per kilogram of body weight per minute. The other 733 infants received standard oxygen therapy up to a maximum 2 liters per minute.
The treatment failed, requiring an escalation of care, in 87 of 739 patients (12%) in the high-flow group and 167 of 733 (23%) in the standard-therapy group. (risk difference = –11% points; 95% confidence interval, –15 to –7; P less than .001).
“The ease to use and simplicity of high flow made us recognize and think that this level of respiratory care can be provided outside intensive care,” Dr. Schibler said. “This was further supported by the observational fact that most of these infants with bronchiolitis showed a dramatically improved respiratory condition once on high flow.”
Dr. Schibler said there haven’t been any signs of adverse effects from high-flow oxygen therapy. As for the cost of the treatment, he said it is “likely offset by a reduced need for intensive care therapy or costs associated with transferring to a children’s hospital.”
What should physicians and hospitals take from the study findings? “If a hospital explores the option to use high flow in bronchiolitis, then start the therapy early in the disease process or once an oxygen requirement is recognized,” Dr. Schibler said. “Implementation of a solid and structured training program with a clear hospital guideline based on the evidence will ensure the staff who care for these patients will be empowered and comfortable to adjust the oxygen levels given by the high-flow equipment. The greater the confidence and comfort level for the nursing and respiratory technician staff the better for these infants, as they will sooner observe those infants who are not responding well and may require a higher level of care such as intensive care or they will recognize the infant who responds well.”
The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment and consumables and travel/accommodation support. Study authors reported various grants and other support.
SOURCE: Franklin D et al. N Engl J Med. 2018;378(12):1112-31.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In non-ICUs, infants under 12 months with bronchiolitis are less likely to fail treatment if they are given high-flow oxygen therapy instead of standard oxygen therapy.
Major finding: Treatment failure occurred in 8 of 739 (12%) patients in the high-flow oxygen therapy group and 167 of 733 (23%) in the standard-therapy group.
Study details: Multicenter, randomized, controlled trial of 1,472 infants.
Disclosures: The National Health and Medical Research Council (Australia) and the Queensland Emergency Medical Research Fund provided funding, and sites received grant funding from various sources. Fisher & Paykel Healthcare, a respiratory care company based in Auckland, New Zealand, donated high-flow equipment/consumables and travel/accommodation support. Study authors reported various grants and other support.
Source: Franklin D et al. N Engl J Med 2018;378(12):1112-31.
H. pylori eradication cuts new gastric cancers by half
Treatment for Helicobacter pylori infection cut the incidence of new gastric cancers in half among patients undergoing endoscopic resection of early gastric cancer, according to results of a recent randomized, placebo-controlled study.
Patients receiving H. pylori treatment also had greater improvement from baseline in grade of gastric corpus atrophy, compared with patients receiving placebo, according to the study. The results were published in the New England Journal of Medicine.
“We speculate that persistent inflammation of gastric mucosa with H. pylori infection promotes carcinogenesis and also increases tumor growth or invasiveness,” said Il Ju Choi, MD, PhD, of the Center for Gastric Cancer, National Cancer Center, Goyang, South Korea, and coauthors.
Patients with early gastric cancers not at risk for lymph node metastasis may benefit from endoscopic resection. However, these patients are at high risk of developing new gastric cancer, and usually experience glandular atrophy, or advanced loss of mucosal glandular tissue, the authors said.
One nonrandomized study suggested H. pylori eradication could prevent development of subsequent cancers after endoscopic resection, according to the authors, but subsequent open-label trials were inconsistent on whether the treatment reduced cancer incidence.
Accordingly, Dr. Choi and colleagues conducted a prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Of 396 patients included in an intention-to-treat analysis, 194 were randomized to receive antibiotics for H. pylori eradication, and 202 received placebo.
Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (hazard ratio, 0.50; 95% confidence interval, 0.26-0.94; P = .03).
Histologic analysis, performed in 327 patients, showed that 48.4% of patients in the treatment group had improvement in atrophy grade at the gastric corpus lesser curvature, compared to just 15.0% of the placebo group (P less than .001), the investigators reported.
Mild adverse events were more frequent in the treatment arm (42.0% versus 10.2%; P less than .001), and there were no serious adverse events, they added.
Despite the approximate 50% reduction in incidence of new gastric cancers and histologic improvements, the researchers said that further study would be required to optimize treatment approaches for patients undergoing endoscopic resection for high-grade adenoma or early gastric cancer.
“H. pylori eradication reduces, but cannot completely abolish, the risk of metachronous gastric cancer,” wrote Dr. Choi and colleagues. “Thus, molecular markers, including aberrant methylation at specific genes, might help to identify high-risk patients even after successful eradication.”
The researchers reported that they had nothing to disclose related to the study.
SOURCE: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
The study by Choi and colleagues suggests Helicobacter pylori eradication is effective at stopping the carcinogenic process in patients with severe chronic atrophic gastritis, an advanced precursor lesion to gastric cancer, according to Peter Malfertheiner, MD.
“It is a striking finding that H. pylori eradication may still be effective at this stage, since such therapy decreased the development of gastric cancer by 50% in this trial,” Dr. Malfertheiner wrote in an editorial.
In the randomized, placebo-controlled trial, H. pylori eradication after endoscopic removal of early stage disease effectively prevented metachronous gastric cancers (i.e., those detected on endoscopy at 1-year follow-up or thereafter) with a hazard ratio of 0.50, Dr. Malfertheiner noted.
The results confirm and strengthen previous findings by showing a significant improvement in atrophic gastritis, he added.
“In this endoscopic procedure, removal of early gastric cancer or high-grade adenoma leaves the stomach largely conserved but with the atrophic gastric mucosa remaining in a preneoplastic ‘alarm state,’ ” he noted.
However, the potential link between cancer recurrence and atrophic gastritis was not explored in this particular study report, Dr. Malfertheiner said. Thus, it is unclear whether gastric cancer recurrence was prevented specifically in the subset of patients with atrophic gastritis.
It could be that eradication of H. pylori directly arrests carcinogenic mechanisms directly by ending persistent inflammation, he speculated.
“The beneficial effect may also be mediated by an alteration in the composition of the gastric microbiota because of improvement in the grade of gastric atrophy and a return toward normal gastric acid production,” he added.
Dr. Malfertheiner is with the Clinic of Gastroenterology, Otto von Guericke University, Magdeburg, Germany. These comments are derived from his editorial (N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMe1800147). Dr. Malfertheiner reported personal fees from Allergan, Biohit, and Infai outside the submitted editorial.
The study by Choi and colleagues suggests Helicobacter pylori eradication is effective at stopping the carcinogenic process in patients with severe chronic atrophic gastritis, an advanced precursor lesion to gastric cancer, according to Peter Malfertheiner, MD.
“It is a striking finding that H. pylori eradication may still be effective at this stage, since such therapy decreased the development of gastric cancer by 50% in this trial,” Dr. Malfertheiner wrote in an editorial.
In the randomized, placebo-controlled trial, H. pylori eradication after endoscopic removal of early stage disease effectively prevented metachronous gastric cancers (i.e., those detected on endoscopy at 1-year follow-up or thereafter) with a hazard ratio of 0.50, Dr. Malfertheiner noted.
The results confirm and strengthen previous findings by showing a significant improvement in atrophic gastritis, he added.
“In this endoscopic procedure, removal of early gastric cancer or high-grade adenoma leaves the stomach largely conserved but with the atrophic gastric mucosa remaining in a preneoplastic ‘alarm state,’ ” he noted.
However, the potential link between cancer recurrence and atrophic gastritis was not explored in this particular study report, Dr. Malfertheiner said. Thus, it is unclear whether gastric cancer recurrence was prevented specifically in the subset of patients with atrophic gastritis.
It could be that eradication of H. pylori directly arrests carcinogenic mechanisms directly by ending persistent inflammation, he speculated.
“The beneficial effect may also be mediated by an alteration in the composition of the gastric microbiota because of improvement in the grade of gastric atrophy and a return toward normal gastric acid production,” he added.
Dr. Malfertheiner is with the Clinic of Gastroenterology, Otto von Guericke University, Magdeburg, Germany. These comments are derived from his editorial (N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMe1800147). Dr. Malfertheiner reported personal fees from Allergan, Biohit, and Infai outside the submitted editorial.
The study by Choi and colleagues suggests Helicobacter pylori eradication is effective at stopping the carcinogenic process in patients with severe chronic atrophic gastritis, an advanced precursor lesion to gastric cancer, according to Peter Malfertheiner, MD.
“It is a striking finding that H. pylori eradication may still be effective at this stage, since such therapy decreased the development of gastric cancer by 50% in this trial,” Dr. Malfertheiner wrote in an editorial.
In the randomized, placebo-controlled trial, H. pylori eradication after endoscopic removal of early stage disease effectively prevented metachronous gastric cancers (i.e., those detected on endoscopy at 1-year follow-up or thereafter) with a hazard ratio of 0.50, Dr. Malfertheiner noted.
The results confirm and strengthen previous findings by showing a significant improvement in atrophic gastritis, he added.
“In this endoscopic procedure, removal of early gastric cancer or high-grade adenoma leaves the stomach largely conserved but with the atrophic gastric mucosa remaining in a preneoplastic ‘alarm state,’ ” he noted.
However, the potential link between cancer recurrence and atrophic gastritis was not explored in this particular study report, Dr. Malfertheiner said. Thus, it is unclear whether gastric cancer recurrence was prevented specifically in the subset of patients with atrophic gastritis.
It could be that eradication of H. pylori directly arrests carcinogenic mechanisms directly by ending persistent inflammation, he speculated.
“The beneficial effect may also be mediated by an alteration in the composition of the gastric microbiota because of improvement in the grade of gastric atrophy and a return toward normal gastric acid production,” he added.
Dr. Malfertheiner is with the Clinic of Gastroenterology, Otto von Guericke University, Magdeburg, Germany. These comments are derived from his editorial (N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMe1800147). Dr. Malfertheiner reported personal fees from Allergan, Biohit, and Infai outside the submitted editorial.
Treatment for Helicobacter pylori infection cut the incidence of new gastric cancers in half among patients undergoing endoscopic resection of early gastric cancer, according to results of a recent randomized, placebo-controlled study.
Patients receiving H. pylori treatment also had greater improvement from baseline in grade of gastric corpus atrophy, compared with patients receiving placebo, according to the study. The results were published in the New England Journal of Medicine.
“We speculate that persistent inflammation of gastric mucosa with H. pylori infection promotes carcinogenesis and also increases tumor growth or invasiveness,” said Il Ju Choi, MD, PhD, of the Center for Gastric Cancer, National Cancer Center, Goyang, South Korea, and coauthors.
Patients with early gastric cancers not at risk for lymph node metastasis may benefit from endoscopic resection. However, these patients are at high risk of developing new gastric cancer, and usually experience glandular atrophy, or advanced loss of mucosal glandular tissue, the authors said.
One nonrandomized study suggested H. pylori eradication could prevent development of subsequent cancers after endoscopic resection, according to the authors, but subsequent open-label trials were inconsistent on whether the treatment reduced cancer incidence.
Accordingly, Dr. Choi and colleagues conducted a prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Of 396 patients included in an intention-to-treat analysis, 194 were randomized to receive antibiotics for H. pylori eradication, and 202 received placebo.
Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (hazard ratio, 0.50; 95% confidence interval, 0.26-0.94; P = .03).
Histologic analysis, performed in 327 patients, showed that 48.4% of patients in the treatment group had improvement in atrophy grade at the gastric corpus lesser curvature, compared to just 15.0% of the placebo group (P less than .001), the investigators reported.
Mild adverse events were more frequent in the treatment arm (42.0% versus 10.2%; P less than .001), and there were no serious adverse events, they added.
Despite the approximate 50% reduction in incidence of new gastric cancers and histologic improvements, the researchers said that further study would be required to optimize treatment approaches for patients undergoing endoscopic resection for high-grade adenoma or early gastric cancer.
“H. pylori eradication reduces, but cannot completely abolish, the risk of metachronous gastric cancer,” wrote Dr. Choi and colleagues. “Thus, molecular markers, including aberrant methylation at specific genes, might help to identify high-risk patients even after successful eradication.”
The researchers reported that they had nothing to disclose related to the study.
SOURCE: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
Treatment for Helicobacter pylori infection cut the incidence of new gastric cancers in half among patients undergoing endoscopic resection of early gastric cancer, according to results of a recent randomized, placebo-controlled study.
Patients receiving H. pylori treatment also had greater improvement from baseline in grade of gastric corpus atrophy, compared with patients receiving placebo, according to the study. The results were published in the New England Journal of Medicine.
“We speculate that persistent inflammation of gastric mucosa with H. pylori infection promotes carcinogenesis and also increases tumor growth or invasiveness,” said Il Ju Choi, MD, PhD, of the Center for Gastric Cancer, National Cancer Center, Goyang, South Korea, and coauthors.
Patients with early gastric cancers not at risk for lymph node metastasis may benefit from endoscopic resection. However, these patients are at high risk of developing new gastric cancer, and usually experience glandular atrophy, or advanced loss of mucosal glandular tissue, the authors said.
One nonrandomized study suggested H. pylori eradication could prevent development of subsequent cancers after endoscopic resection, according to the authors, but subsequent open-label trials were inconsistent on whether the treatment reduced cancer incidence.
Accordingly, Dr. Choi and colleagues conducted a prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Of 396 patients included in an intention-to-treat analysis, 194 were randomized to receive antibiotics for H. pylori eradication, and 202 received placebo.
Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (hazard ratio, 0.50; 95% confidence interval, 0.26-0.94; P = .03).
Histologic analysis, performed in 327 patients, showed that 48.4% of patients in the treatment group had improvement in atrophy grade at the gastric corpus lesser curvature, compared to just 15.0% of the placebo group (P less than .001), the investigators reported.
Mild adverse events were more frequent in the treatment arm (42.0% versus 10.2%; P less than .001), and there were no serious adverse events, they added.
Despite the approximate 50% reduction in incidence of new gastric cancers and histologic improvements, the researchers said that further study would be required to optimize treatment approaches for patients undergoing endoscopic resection for high-grade adenoma or early gastric cancer.
“H. pylori eradication reduces, but cannot completely abolish, the risk of metachronous gastric cancer,” wrote Dr. Choi and colleagues. “Thus, molecular markers, including aberrant methylation at specific genes, might help to identify high-risk patients even after successful eradication.”
The researchers reported that they had nothing to disclose related to the study.
SOURCE: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Among patients undergoing endoscopic resection of early gastric cancer, incidence of new gastric cancers was approximately 50% lower for those who received treatment for Helicobacter pylori infection.
Major finding: Over a median follow-up of 5.9 years, new gastric cancers developed in 14 patients (7.2%) who received treatment, and in 27 patients (13.4%) who received placebo (HR, 0.50; 95% CI, 0.26-0.94; P = .03).
Study details: A prospective, double-blind, placebo-controlled, randomized trial of 470 patients who underwent endoscopic resection for high-grade adenoma or early gastric cancer.
Disclosures: The authors reported that they had nothing to disclose related to the study.
Source: Choi et al. N Engl J Med. 2018 Mar 22. doi: 10.1056/NEJMoa1708423.
Report: Abortion in U.S. is safe and effective
, according to a consensus study report by the National Academies of Science, Engineering, and Medicine.
Six private foundations commissioned a comprehensive report from the National Academies, which focused on eight questions related to the safety and quality of U.S. abortion care. The resulting Committee on Reproductive Health Services limited itself to these questions and did not make specific policy or clinical recommendations, though they did note that “state regulations have created barriers to optimizing each dimension of quality care.” The report was released on March 16.
The committee focused on the four legal abortion methods in the United States – medication, aspiration, dilation and evacuation (D&E), and induction – and concluded that all four are safe, but that induction is so rare that there is a lack of quality research on the procedure’s risks in women with prior cesarean deliveries. D&E, though less painful, costly, and time consuming than induction, is banned in Mississippi and West Virginia (with exceptions for emergencies) and limited elsewhere in the country by a lack of physicians trained to perform the procedure.
In attempting to assess the physical and mental health risks of abortion procedures, the committee found that “much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research.” Surveying research that they considered high quality, the committee concluded that there is no increased risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation, preterm birth, breast cancer, or mental health disorders such as depression, anxiety, or posttraumatic stress disorder associated with a woman having an abortion.
The committee was not able to find high-quality research to evaluate the risk of ectopic pregnancy, miscarriage or stillbirth, or long-term mortality associated with abortion. However, it did find an increased risk of preterm birth before 28 weeks’ gestation in a nulliparous women who had had two or more aspiration abortions, compared with women with no abortion history. The risk of preterm birth is greater in women if the interval between their abortion and their next conception is less than 6 months. The same risk exists for short intervals between pregnancy in general, the committee noted.
Overall, they wrote, “serious complications are rare and occur much less frequently than during childbirth.”
The committee identified several kinds of state-level regulations that are obstacles to effective abortion care in the United States. Such regulations “may limit the number of available providers, misinform women of the risks of the procedures they are considering, overrule women’s and clinicians’ medical decision making, or require medically unnecessary services and delays in care.” Some laws “prohibit the abortion method that is most effective for a particular clinical circumstance” (for example, D&E).
Access to care varies widely geographically, and 17% of women must travel more than 50 miles to obtain an abortion. Regulations that required counseling, whether the woman desires counseling or not, are cited as an example of inferior patient-centered care.
The committee delineated safeguards that are necessary to manage emergencies that might arise from an abortion procedure, such as resuscitation and monitoring equipment to be located in a facility for procedures involving moderate and deep sedation. However, they did not find any evidence that it is necessary for clinicians performing abortions to have hospital admitting privileges. In the Whole Woman’s Health v. Hellerstedt decision in 2016, the U.S. Supreme Court ruled that the admitting privileges requirement in Texas was an undue burden on access to care. The committee wrote that it is sufficient for the facility to have an emergency transfer plan in place.
The committee also identified areas for further evaluation. First, whether the Food and Drug Administration should expand the distribution of mifepristone, the only drug currently approved for medication abortions, but which can only be dispensed to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. The committee also called for examining more effective methods of pain management, whether advanced practice clinicians can be trained to perform D&Es, and ways to provide more social and psychological supports for lower-income women or who are at risk of intimate partner or other forms of violence.
On March 16, the National Academies of Science, Engineering, and Medicine released a comprehensive report finding that abortion is safe and effective but inaccessible to many women.
A high-quality foundation of evidence, contributed to by many U.S. family planning researchers, provided the studies on which the conclusions of the National Academies are based. We are fortunate that the Society of Family Planning provides research funding and a forum for family planning researchers to continue to produce the high-quality evidence used by policy makers to improve access to and quality of abortion care.
Eve Espey, MD, MPH, is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. She reported having no relevant financial disclosures.
On March 16, the National Academies of Science, Engineering, and Medicine released a comprehensive report finding that abortion is safe and effective but inaccessible to many women.
A high-quality foundation of evidence, contributed to by many U.S. family planning researchers, provided the studies on which the conclusions of the National Academies are based. We are fortunate that the Society of Family Planning provides research funding and a forum for family planning researchers to continue to produce the high-quality evidence used by policy makers to improve access to and quality of abortion care.
Eve Espey, MD, MPH, is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. She reported having no relevant financial disclosures.
On March 16, the National Academies of Science, Engineering, and Medicine released a comprehensive report finding that abortion is safe and effective but inaccessible to many women.
A high-quality foundation of evidence, contributed to by many U.S. family planning researchers, provided the studies on which the conclusions of the National Academies are based. We are fortunate that the Society of Family Planning provides research funding and a forum for family planning researchers to continue to produce the high-quality evidence used by policy makers to improve access to and quality of abortion care.
Eve Espey, MD, MPH, is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque. She reported having no relevant financial disclosures.
, according to a consensus study report by the National Academies of Science, Engineering, and Medicine.
Six private foundations commissioned a comprehensive report from the National Academies, which focused on eight questions related to the safety and quality of U.S. abortion care. The resulting Committee on Reproductive Health Services limited itself to these questions and did not make specific policy or clinical recommendations, though they did note that “state regulations have created barriers to optimizing each dimension of quality care.” The report was released on March 16.
The committee focused on the four legal abortion methods in the United States – medication, aspiration, dilation and evacuation (D&E), and induction – and concluded that all four are safe, but that induction is so rare that there is a lack of quality research on the procedure’s risks in women with prior cesarean deliveries. D&E, though less painful, costly, and time consuming than induction, is banned in Mississippi and West Virginia (with exceptions for emergencies) and limited elsewhere in the country by a lack of physicians trained to perform the procedure.
In attempting to assess the physical and mental health risks of abortion procedures, the committee found that “much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research.” Surveying research that they considered high quality, the committee concluded that there is no increased risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation, preterm birth, breast cancer, or mental health disorders such as depression, anxiety, or posttraumatic stress disorder associated with a woman having an abortion.
The committee was not able to find high-quality research to evaluate the risk of ectopic pregnancy, miscarriage or stillbirth, or long-term mortality associated with abortion. However, it did find an increased risk of preterm birth before 28 weeks’ gestation in a nulliparous women who had had two or more aspiration abortions, compared with women with no abortion history. The risk of preterm birth is greater in women if the interval between their abortion and their next conception is less than 6 months. The same risk exists for short intervals between pregnancy in general, the committee noted.
Overall, they wrote, “serious complications are rare and occur much less frequently than during childbirth.”
The committee identified several kinds of state-level regulations that are obstacles to effective abortion care in the United States. Such regulations “may limit the number of available providers, misinform women of the risks of the procedures they are considering, overrule women’s and clinicians’ medical decision making, or require medically unnecessary services and delays in care.” Some laws “prohibit the abortion method that is most effective for a particular clinical circumstance” (for example, D&E).
Access to care varies widely geographically, and 17% of women must travel more than 50 miles to obtain an abortion. Regulations that required counseling, whether the woman desires counseling or not, are cited as an example of inferior patient-centered care.
The committee delineated safeguards that are necessary to manage emergencies that might arise from an abortion procedure, such as resuscitation and monitoring equipment to be located in a facility for procedures involving moderate and deep sedation. However, they did not find any evidence that it is necessary for clinicians performing abortions to have hospital admitting privileges. In the Whole Woman’s Health v. Hellerstedt decision in 2016, the U.S. Supreme Court ruled that the admitting privileges requirement in Texas was an undue burden on access to care. The committee wrote that it is sufficient for the facility to have an emergency transfer plan in place.
The committee also identified areas for further evaluation. First, whether the Food and Drug Administration should expand the distribution of mifepristone, the only drug currently approved for medication abortions, but which can only be dispensed to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. The committee also called for examining more effective methods of pain management, whether advanced practice clinicians can be trained to perform D&Es, and ways to provide more social and psychological supports for lower-income women or who are at risk of intimate partner or other forms of violence.
, according to a consensus study report by the National Academies of Science, Engineering, and Medicine.
Six private foundations commissioned a comprehensive report from the National Academies, which focused on eight questions related to the safety and quality of U.S. abortion care. The resulting Committee on Reproductive Health Services limited itself to these questions and did not make specific policy or clinical recommendations, though they did note that “state regulations have created barriers to optimizing each dimension of quality care.” The report was released on March 16.
The committee focused on the four legal abortion methods in the United States – medication, aspiration, dilation and evacuation (D&E), and induction – and concluded that all four are safe, but that induction is so rare that there is a lack of quality research on the procedure’s risks in women with prior cesarean deliveries. D&E, though less painful, costly, and time consuming than induction, is banned in Mississippi and West Virginia (with exceptions for emergencies) and limited elsewhere in the country by a lack of physicians trained to perform the procedure.
In attempting to assess the physical and mental health risks of abortion procedures, the committee found that “much of the published literature on these topics does not meet scientific standards for rigorous, unbiased research.” Surveying research that they considered high quality, the committee concluded that there is no increased risk of secondary infertility, pregnancy-related hypertensive disorders, abnormal placentation, preterm birth, breast cancer, or mental health disorders such as depression, anxiety, or posttraumatic stress disorder associated with a woman having an abortion.
The committee was not able to find high-quality research to evaluate the risk of ectopic pregnancy, miscarriage or stillbirth, or long-term mortality associated with abortion. However, it did find an increased risk of preterm birth before 28 weeks’ gestation in a nulliparous women who had had two or more aspiration abortions, compared with women with no abortion history. The risk of preterm birth is greater in women if the interval between their abortion and their next conception is less than 6 months. The same risk exists for short intervals between pregnancy in general, the committee noted.
Overall, they wrote, “serious complications are rare and occur much less frequently than during childbirth.”
The committee identified several kinds of state-level regulations that are obstacles to effective abortion care in the United States. Such regulations “may limit the number of available providers, misinform women of the risks of the procedures they are considering, overrule women’s and clinicians’ medical decision making, or require medically unnecessary services and delays in care.” Some laws “prohibit the abortion method that is most effective for a particular clinical circumstance” (for example, D&E).
Access to care varies widely geographically, and 17% of women must travel more than 50 miles to obtain an abortion. Regulations that required counseling, whether the woman desires counseling or not, are cited as an example of inferior patient-centered care.
The committee delineated safeguards that are necessary to manage emergencies that might arise from an abortion procedure, such as resuscitation and monitoring equipment to be located in a facility for procedures involving moderate and deep sedation. However, they did not find any evidence that it is necessary for clinicians performing abortions to have hospital admitting privileges. In the Whole Woman’s Health v. Hellerstedt decision in 2016, the U.S. Supreme Court ruled that the admitting privileges requirement in Texas was an undue burden on access to care. The committee wrote that it is sufficient for the facility to have an emergency transfer plan in place.
The committee also identified areas for further evaluation. First, whether the Food and Drug Administration should expand the distribution of mifepristone, the only drug currently approved for medication abortions, but which can only be dispensed to patients in clinics, hospitals, or medical offices under the supervision of a certified prescriber. The committee also called for examining more effective methods of pain management, whether advanced practice clinicians can be trained to perform D&Es, and ways to provide more social and psychological supports for lower-income women or who are at risk of intimate partner or other forms of violence.
Genetic Pap tests catch more cancers
A Pap test combined with assays for gene mutations showed an 81% and 33% sensitivity or identifying endometrial and ovarian cancer, respectively.
In a study published in Science Translational Medicine, Yuxuan Wang, MD, of Johns Hopkins University, Baltimore, and her colleagues used a polymerase chain reaction–based test known as PapSEEK to examine Pap brush samples from 382 women with endometrial cancer, 245 women with ovarian cancer, and 714 women without cancer.
Overall, 81% of the endometrial cancer patients and 29% of the ovarian cancer patients had detectable mutations on Pap smears taken with a Pap brush. Among endometrial cancer patients, the genetic test identified mutations in 78% of patients with early-stage disease and 89% of those with late-stage disease. Among ovarian cancer patients, the genetic test identified 28% of patients with early-stage disease and 30% of those with late-stage disease.
The investigators also used the genetic test on Tao brush samples from the intrauterine cavity from a subset of 123 patients with endometrial cancer, 51 with ovarian cancer, and 125 controls and identified genetic mutations in 93% and 45% of endometrial and ovarian cancers, respectively.
The results demonstrate the potential of mutation-based diagnostic testing, and samples taken with the Tao brush may be especially helpful for ovarian cancers, especially in samples combining Pap and plasma sample data, the researchers said.
The study was limited by several factors including its retrospective design and having a study population confined to women with known cancers, the researchers noted. More research is needed, but results suggest the potential for DNA analysis to catch cancers early in a screening setting, they said. “Our study lays the foundation for evaluating PapSEEK in a large prospective study,” ideally including patients at high risk for gynecologic cancers, they added.
The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
SOURCE: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
A Pap test combined with assays for gene mutations showed an 81% and 33% sensitivity or identifying endometrial and ovarian cancer, respectively.
In a study published in Science Translational Medicine, Yuxuan Wang, MD, of Johns Hopkins University, Baltimore, and her colleagues used a polymerase chain reaction–based test known as PapSEEK to examine Pap brush samples from 382 women with endometrial cancer, 245 women with ovarian cancer, and 714 women without cancer.
Overall, 81% of the endometrial cancer patients and 29% of the ovarian cancer patients had detectable mutations on Pap smears taken with a Pap brush. Among endometrial cancer patients, the genetic test identified mutations in 78% of patients with early-stage disease and 89% of those with late-stage disease. Among ovarian cancer patients, the genetic test identified 28% of patients with early-stage disease and 30% of those with late-stage disease.
The investigators also used the genetic test on Tao brush samples from the intrauterine cavity from a subset of 123 patients with endometrial cancer, 51 with ovarian cancer, and 125 controls and identified genetic mutations in 93% and 45% of endometrial and ovarian cancers, respectively.
The results demonstrate the potential of mutation-based diagnostic testing, and samples taken with the Tao brush may be especially helpful for ovarian cancers, especially in samples combining Pap and plasma sample data, the researchers said.
The study was limited by several factors including its retrospective design and having a study population confined to women with known cancers, the researchers noted. More research is needed, but results suggest the potential for DNA analysis to catch cancers early in a screening setting, they said. “Our study lays the foundation for evaluating PapSEEK in a large prospective study,” ideally including patients at high risk for gynecologic cancers, they added.
The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
SOURCE: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
A Pap test combined with assays for gene mutations showed an 81% and 33% sensitivity or identifying endometrial and ovarian cancer, respectively.
In a study published in Science Translational Medicine, Yuxuan Wang, MD, of Johns Hopkins University, Baltimore, and her colleagues used a polymerase chain reaction–based test known as PapSEEK to examine Pap brush samples from 382 women with endometrial cancer, 245 women with ovarian cancer, and 714 women without cancer.
Overall, 81% of the endometrial cancer patients and 29% of the ovarian cancer patients had detectable mutations on Pap smears taken with a Pap brush. Among endometrial cancer patients, the genetic test identified mutations in 78% of patients with early-stage disease and 89% of those with late-stage disease. Among ovarian cancer patients, the genetic test identified 28% of patients with early-stage disease and 30% of those with late-stage disease.
The investigators also used the genetic test on Tao brush samples from the intrauterine cavity from a subset of 123 patients with endometrial cancer, 51 with ovarian cancer, and 125 controls and identified genetic mutations in 93% and 45% of endometrial and ovarian cancers, respectively.
The results demonstrate the potential of mutation-based diagnostic testing, and samples taken with the Tao brush may be especially helpful for ovarian cancers, especially in samples combining Pap and plasma sample data, the researchers said.
The study was limited by several factors including its retrospective design and having a study population confined to women with known cancers, the researchers noted. More research is needed, but results suggest the potential for DNA analysis to catch cancers early in a screening setting, they said. “Our study lays the foundation for evaluating PapSEEK in a large prospective study,” ideally including patients at high risk for gynecologic cancers, they added.
The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
SOURCE: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Genetics-based Pap test identified endometrial and ovarian cancers.
Major finding: Pap brush samples identified mutations in 81% of women with endometrial cancer and 29% of women with ovarian cancer.
Study details: Analysis of 1,915 Pap samples from 1,658 women; 1,002 were healthy controls, while 656 had gynecologic cancer.
Disclosures: The study was funded by multiple sources including the Virginia and D.K. Ludwig Fund for Cancer Research, the National Institutes of Health, and the Stand Up to Cancer Colorectal Dream Team Translational Research Grant. Dr. Wang and several coauthors disclosed patent, equity, and royalty interest in technologies discussed in the paper. Four coauthors are cofounders of and stockholders in PapGene, which has licensed technologies related to the work described in the paper.
Source: Wang Y et al. Sci Transl Med. 2018 Mar 21;10(433):eaap8793.
Accelerated breast irradiation advocated by ASTRO guideline
Hypofractionation is the preferred means of giving whole breast irradiation to women with invasive breast cancer, according to updated guidance from the American Society for Radiation Oncology.
A dose of 4,000 cGy given in 15 fractions or 4,250 cGy in 16 fractions is recommended, with or without inclusion of the low axilla, and regardless of a variety of factors such as tumor grade, prior chemotherapy, and patient age.
“Previously, accelerated treatment was recommended only for certain patients, including older patients and those with less advanced disease,” Benjamin Smith, MD, one of the cochairs of the guideline task force, said in an ASTRO news release.
Dr. Smith, of the University of Texas MD Anderson Cancer Center, Houston, added that recent long-term data from several large trials “strongly support the safety and efficacy of accelerated treatment for most breast cancer patients.”
Treatment decisions and plans still need to be individualized, but the updated ASTRO guidance notes that whole breast irradiation (WBI) can be offered to most women with invasive breast cancer independent of breast size and whether or not the cancer is in the left or right breast, provided that homogeneous dosing can be achieved. Hormone receptor, HER2 status, and postsurgical margin status also appear not to matter.
Historically, conventional fractionation (CF) with or without a tumor bed boost was used for WBI, Dr. Smith and associates wrote in the guidelines, which were published online in Practical Radiation Oncology. This consisted of daily doses of 180-200 cGy for a total dose of 4,500-5,000 cGy.
“Recognizing the limitations of CF for convenience and cost, randomized trials in the 1990s and 2000s investigated if moderate hypofractionation [HF], defined as daily doses of 265-330 cGy, could yield oncologic and functional/cosmetic outcomes similar to CF-WBI,” they said.
Initial results of these trials “supported the safety and effectiveness of HF-WBI” and were then used to form ASTRO’s 2011 guideline on dose fractionation for WBI. With longer term data from these trials now available, it was time to review the evidence again. A systematic literature review was thus conducted to identify all relevant studies published during 2009-2016, and 100 articles met the task force criteria and were used to create the updated guideline.
Aside from the delivery and dosing of WBI, other key recommendations look at the use of a radiation boost to the tumor bed, and preferred techniques for treatment planning.
With regards to a radiation boost, this needs to be considered on an individual basis but can be independent of any previous WBI. A radiation boost is recommended if patients have any grade invasive cancer and are aged 50 years or younger, have a high-grade tumor and are aged 51-70 years, or if there is a positive margin following surgery. A radiation boost also is recommended in women with ductal carcinoma in situ if they are aged 50 years or younger, have a high-grade tumor, and positive or close postsurgical margins.
As for treatment planning, 3-dimensional conformal treatment planning with a “field-in-field” technique is recommended as the initial approach. This is to minimize the volume of breast tissue that receives more than 105% of the radiation dose. The guideline also covers optimal patient positioning and how to avoid nearby tissues and organs, such as the heart, lungs and contralateral breast.
ASTRO hopes that the updated guideline will increase the use of hypofractionation, which has been reportedly low in recent years, with as few as 35% of eligible patients received hypofractionation in one study (JAMA. 2014;312[23]:2542-50).
“We hope that this guideline encourages providers to counsel their patients on options including hypofractionation,” said Reshma Jagsi, MD, DPhil, professor of radiation oncology at the University of Michigan, Ann Arbor, who cochaired the guideline task force with Dr. Smith.
“Hypofractionated radiation therapy offers patients a more convenient and lower cost option for their treatment without compromising the likelihood that their cancer will return or increasing their risk of side effects,” Dr. Jagsi noted. Furthermore, “a shorter course of radiation equates to more time with family, less time away from work and lower treatment costs.”
SOURCE: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
Hypofractionation is the preferred means of giving whole breast irradiation to women with invasive breast cancer, according to updated guidance from the American Society for Radiation Oncology.
A dose of 4,000 cGy given in 15 fractions or 4,250 cGy in 16 fractions is recommended, with or without inclusion of the low axilla, and regardless of a variety of factors such as tumor grade, prior chemotherapy, and patient age.
“Previously, accelerated treatment was recommended only for certain patients, including older patients and those with less advanced disease,” Benjamin Smith, MD, one of the cochairs of the guideline task force, said in an ASTRO news release.
Dr. Smith, of the University of Texas MD Anderson Cancer Center, Houston, added that recent long-term data from several large trials “strongly support the safety and efficacy of accelerated treatment for most breast cancer patients.”
Treatment decisions and plans still need to be individualized, but the updated ASTRO guidance notes that whole breast irradiation (WBI) can be offered to most women with invasive breast cancer independent of breast size and whether or not the cancer is in the left or right breast, provided that homogeneous dosing can be achieved. Hormone receptor, HER2 status, and postsurgical margin status also appear not to matter.
Historically, conventional fractionation (CF) with or without a tumor bed boost was used for WBI, Dr. Smith and associates wrote in the guidelines, which were published online in Practical Radiation Oncology. This consisted of daily doses of 180-200 cGy for a total dose of 4,500-5,000 cGy.
“Recognizing the limitations of CF for convenience and cost, randomized trials in the 1990s and 2000s investigated if moderate hypofractionation [HF], defined as daily doses of 265-330 cGy, could yield oncologic and functional/cosmetic outcomes similar to CF-WBI,” they said.
Initial results of these trials “supported the safety and effectiveness of HF-WBI” and were then used to form ASTRO’s 2011 guideline on dose fractionation for WBI. With longer term data from these trials now available, it was time to review the evidence again. A systematic literature review was thus conducted to identify all relevant studies published during 2009-2016, and 100 articles met the task force criteria and were used to create the updated guideline.
Aside from the delivery and dosing of WBI, other key recommendations look at the use of a radiation boost to the tumor bed, and preferred techniques for treatment planning.
With regards to a radiation boost, this needs to be considered on an individual basis but can be independent of any previous WBI. A radiation boost is recommended if patients have any grade invasive cancer and are aged 50 years or younger, have a high-grade tumor and are aged 51-70 years, or if there is a positive margin following surgery. A radiation boost also is recommended in women with ductal carcinoma in situ if they are aged 50 years or younger, have a high-grade tumor, and positive or close postsurgical margins.
As for treatment planning, 3-dimensional conformal treatment planning with a “field-in-field” technique is recommended as the initial approach. This is to minimize the volume of breast tissue that receives more than 105% of the radiation dose. The guideline also covers optimal patient positioning and how to avoid nearby tissues and organs, such as the heart, lungs and contralateral breast.
ASTRO hopes that the updated guideline will increase the use of hypofractionation, which has been reportedly low in recent years, with as few as 35% of eligible patients received hypofractionation in one study (JAMA. 2014;312[23]:2542-50).
“We hope that this guideline encourages providers to counsel their patients on options including hypofractionation,” said Reshma Jagsi, MD, DPhil, professor of radiation oncology at the University of Michigan, Ann Arbor, who cochaired the guideline task force with Dr. Smith.
“Hypofractionated radiation therapy offers patients a more convenient and lower cost option for their treatment without compromising the likelihood that their cancer will return or increasing their risk of side effects,” Dr. Jagsi noted. Furthermore, “a shorter course of radiation equates to more time with family, less time away from work and lower treatment costs.”
SOURCE: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
Hypofractionation is the preferred means of giving whole breast irradiation to women with invasive breast cancer, according to updated guidance from the American Society for Radiation Oncology.
A dose of 4,000 cGy given in 15 fractions or 4,250 cGy in 16 fractions is recommended, with or without inclusion of the low axilla, and regardless of a variety of factors such as tumor grade, prior chemotherapy, and patient age.
“Previously, accelerated treatment was recommended only for certain patients, including older patients and those with less advanced disease,” Benjamin Smith, MD, one of the cochairs of the guideline task force, said in an ASTRO news release.
Dr. Smith, of the University of Texas MD Anderson Cancer Center, Houston, added that recent long-term data from several large trials “strongly support the safety and efficacy of accelerated treatment for most breast cancer patients.”
Treatment decisions and plans still need to be individualized, but the updated ASTRO guidance notes that whole breast irradiation (WBI) can be offered to most women with invasive breast cancer independent of breast size and whether or not the cancer is in the left or right breast, provided that homogeneous dosing can be achieved. Hormone receptor, HER2 status, and postsurgical margin status also appear not to matter.
Historically, conventional fractionation (CF) with or without a tumor bed boost was used for WBI, Dr. Smith and associates wrote in the guidelines, which were published online in Practical Radiation Oncology. This consisted of daily doses of 180-200 cGy for a total dose of 4,500-5,000 cGy.
“Recognizing the limitations of CF for convenience and cost, randomized trials in the 1990s and 2000s investigated if moderate hypofractionation [HF], defined as daily doses of 265-330 cGy, could yield oncologic and functional/cosmetic outcomes similar to CF-WBI,” they said.
Initial results of these trials “supported the safety and effectiveness of HF-WBI” and were then used to form ASTRO’s 2011 guideline on dose fractionation for WBI. With longer term data from these trials now available, it was time to review the evidence again. A systematic literature review was thus conducted to identify all relevant studies published during 2009-2016, and 100 articles met the task force criteria and were used to create the updated guideline.
Aside from the delivery and dosing of WBI, other key recommendations look at the use of a radiation boost to the tumor bed, and preferred techniques for treatment planning.
With regards to a radiation boost, this needs to be considered on an individual basis but can be independent of any previous WBI. A radiation boost is recommended if patients have any grade invasive cancer and are aged 50 years or younger, have a high-grade tumor and are aged 51-70 years, or if there is a positive margin following surgery. A radiation boost also is recommended in women with ductal carcinoma in situ if they are aged 50 years or younger, have a high-grade tumor, and positive or close postsurgical margins.
As for treatment planning, 3-dimensional conformal treatment planning with a “field-in-field” technique is recommended as the initial approach. This is to minimize the volume of breast tissue that receives more than 105% of the radiation dose. The guideline also covers optimal patient positioning and how to avoid nearby tissues and organs, such as the heart, lungs and contralateral breast.
ASTRO hopes that the updated guideline will increase the use of hypofractionation, which has been reportedly low in recent years, with as few as 35% of eligible patients received hypofractionation in one study (JAMA. 2014;312[23]:2542-50).
“We hope that this guideline encourages providers to counsel their patients on options including hypofractionation,” said Reshma Jagsi, MD, DPhil, professor of radiation oncology at the University of Michigan, Ann Arbor, who cochaired the guideline task force with Dr. Smith.
“Hypofractionated radiation therapy offers patients a more convenient and lower cost option for their treatment without compromising the likelihood that their cancer will return or increasing their risk of side effects,” Dr. Jagsi noted. Furthermore, “a shorter course of radiation equates to more time with family, less time away from work and lower treatment costs.”
SOURCE: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
FROM PRACTICAL RADIATION ONCOLOGY
Key clinical point: For invasive cancer, the preferred scheme is hypofractionated whole breast irradiation (HF-WBI).
Major finding: HF-WBI should be given to a total dose of 4,000 cGy in 15 fractions or 4,250 cGy in 16 fractions.
Study details: A systematic literature review of all relevant studies published during 2009-2016.
Disclosures: The guidelines were sponsored by the American Society for Radiation Oncology.
Source: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
Hospital boards can promote quality improvement
Hospital boards play an important role in quality improvement (QI), and now researchers in England have developed a framework they can use to help develop their QI capability by comparing 15 health care organizations.
“We already know that certain board practices are associated with higher quality care,” said lead researcher Lorelei Jones, PhD. “For example, hospital boards that regularly review quality performance have better patient outcomes. But we don’t know a lot about what boards actually do, or what ‘good’ looks like in relation to quality governance. There is a lot of guidance for boards on what they should be doing, but very little research evidence.”
In their study, researchers developed an evidence-based measure of QI “maturity” – how developed boards were in how they led and oversaw quality improvement. They applied this measure to various organizations and then looked at the characteristics of organizations that showed a highly developed approach to QI.
“Organizations with higher levels of QI maturity prioritized QI; balanced attention to short-term (external) priorities with a long-term (internal) investment in QI; used data for quality improvement, not just quality assurance; engaged staff and patients in QI; and had a culture of continuous improvement,” Dr. Jones said. These characteristics often seemed to be facilitated by clinical leaders; the study also highlighted the importance of board-level clinical leaders in hospitals, she said.
Researchers found that organizations with a highly developed approach to QI did the following:
- Brought in-depth knowledge and understanding of quality issues and provided the board with meaningful analyses of data.
- Contributed knowledge of relevant developments in national policy and links to external networks.
- Played an important role as “boundary spanners,” providing a link between “the board and the ward,” making connections between sources of data and aligning external demands with internal priorities.
“Boards can use our framework to help develop their QI capability,” Dr. Jones said. “For example, boards can use it to do a gap analysis to explore areas that might need strengthening and for ideas on how they could do this.”
Reference
Jones L et al. How do hospital boards govern for quality improvement? A mixed methods study of 15 organisations in England. BMJ Qual Saf. 2017 Dec;26(12):978-86.
Hospital boards play an important role in quality improvement (QI), and now researchers in England have developed a framework they can use to help develop their QI capability by comparing 15 health care organizations.
“We already know that certain board practices are associated with higher quality care,” said lead researcher Lorelei Jones, PhD. “For example, hospital boards that regularly review quality performance have better patient outcomes. But we don’t know a lot about what boards actually do, or what ‘good’ looks like in relation to quality governance. There is a lot of guidance for boards on what they should be doing, but very little research evidence.”
In their study, researchers developed an evidence-based measure of QI “maturity” – how developed boards were in how they led and oversaw quality improvement. They applied this measure to various organizations and then looked at the characteristics of organizations that showed a highly developed approach to QI.
“Organizations with higher levels of QI maturity prioritized QI; balanced attention to short-term (external) priorities with a long-term (internal) investment in QI; used data for quality improvement, not just quality assurance; engaged staff and patients in QI; and had a culture of continuous improvement,” Dr. Jones said. These characteristics often seemed to be facilitated by clinical leaders; the study also highlighted the importance of board-level clinical leaders in hospitals, she said.
Researchers found that organizations with a highly developed approach to QI did the following:
- Brought in-depth knowledge and understanding of quality issues and provided the board with meaningful analyses of data.
- Contributed knowledge of relevant developments in national policy and links to external networks.
- Played an important role as “boundary spanners,” providing a link between “the board and the ward,” making connections between sources of data and aligning external demands with internal priorities.
“Boards can use our framework to help develop their QI capability,” Dr. Jones said. “For example, boards can use it to do a gap analysis to explore areas that might need strengthening and for ideas on how they could do this.”
Reference
Jones L et al. How do hospital boards govern for quality improvement? A mixed methods study of 15 organisations in England. BMJ Qual Saf. 2017 Dec;26(12):978-86.
Hospital boards play an important role in quality improvement (QI), and now researchers in England have developed a framework they can use to help develop their QI capability by comparing 15 health care organizations.
“We already know that certain board practices are associated with higher quality care,” said lead researcher Lorelei Jones, PhD. “For example, hospital boards that regularly review quality performance have better patient outcomes. But we don’t know a lot about what boards actually do, or what ‘good’ looks like in relation to quality governance. There is a lot of guidance for boards on what they should be doing, but very little research evidence.”
In their study, researchers developed an evidence-based measure of QI “maturity” – how developed boards were in how they led and oversaw quality improvement. They applied this measure to various organizations and then looked at the characteristics of organizations that showed a highly developed approach to QI.
“Organizations with higher levels of QI maturity prioritized QI; balanced attention to short-term (external) priorities with a long-term (internal) investment in QI; used data for quality improvement, not just quality assurance; engaged staff and patients in QI; and had a culture of continuous improvement,” Dr. Jones said. These characteristics often seemed to be facilitated by clinical leaders; the study also highlighted the importance of board-level clinical leaders in hospitals, she said.
Researchers found that organizations with a highly developed approach to QI did the following:
- Brought in-depth knowledge and understanding of quality issues and provided the board with meaningful analyses of data.
- Contributed knowledge of relevant developments in national policy and links to external networks.
- Played an important role as “boundary spanners,” providing a link between “the board and the ward,” making connections between sources of data and aligning external demands with internal priorities.
“Boards can use our framework to help develop their QI capability,” Dr. Jones said. “For example, boards can use it to do a gap analysis to explore areas that might need strengthening and for ideas on how they could do this.”
Reference
Jones L et al. How do hospital boards govern for quality improvement? A mixed methods study of 15 organisations in England. BMJ Qual Saf. 2017 Dec;26(12):978-86.
VIDEO: Andexanet alfa effectively reverses factor Xa anticoagulant
ORLANDO – Andexanet alfa, a new agent that reverses the anticoagulant effect of direct factor Xa inhibitors, showed an acceptable level of efficacy and safety in 227 patients who received the drug in the agent’s pivotal trial.
These results, which placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind), approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa), suggest that andexanet is likely on track for its own Food and Drug Administration marketing approval, Stuart Connolly, MD, said at the annual meeting of the American College of Cardiology.
Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa) previously announced that it expected Food and Drug Administration action on its marketing application by May 2018.
Andexanet reversal “has similar efficacy and safety as seen with other reversal agents” for other types of anticoagulants, said Dr. Connolly, a professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont. In the trial results he reported, andexanet treatment of patients who were bleeding while on treatment with a direct factor Xa inhibitor had an 83% rate of hemostatic efficacy and an 11% rate of thrombotic events. By comparison, idarucizumab, the FDA-approved reversal agent for the anticoagulant dabigatran, produced a 68% hemostatic efficacy and a 6% rate of thrombotic events in the idarucizumab pivotal trial, RE-VERSE AD (N Engl J Med. 2015 Aug 6;373[6]:511-20).
The Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding (ANNEXA-4) enrolled 227 patients at any of 60 centers, with efficacy data available from 132 of the patients. About 60% of the patients had an intracranial bleed, and about 30% had a gastrointestinal bleed, and their average age was 77 years. Roughly three-quarters of patients were on an anticoagulant for atrial fibrillation, with the rest treated for venous thromboembolism, with 4% having both conditions. The most commonly used direct factor Xa inhbitors in these patients were apixaban (Eliquis) in 105 and rivaroxaban (Xarelto) in 75. The ANNEXA-4 study has not enrolled patients treated with a direct factor Xa inhibitor anticoagulant and undergoing surgery, a setting that will be the subject of a future study, Dr. Connolly said.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by about 75%-90% within minutes of starting the bolus and remained depressed at that level during the infusion but then began recovering by 2 hours after the stop of infusion. Andexanet is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops.
“There is no doubt that andexanet rapidly decreases anti–factor Xa activity,” he said.
Adjudicated efficacy results were available for 132 patients and showed good or excellent hemostasis achieved on andexanet in 109 patients (83%), Dr. Connolly reported. The effect on hemostasis was consistent regardless of patient age, sex, bleeding site, type of anticoagulant, and dosage tested.
Thrombotic events during the 30 days following treatment occurred in 24 of 227 patients (11%) who received andexanet and were evaluable for safety. Notably, no clustering of thrombotic events occurred early, even among the 129 patients who restarted on an anticoagulant during the 30 days after treatment. Among the 129 patients who restarted on an anticoagulant, 9 (7%) had a thrombotic event during the 30-day follow-up, compared with 15 events among 98 patients (15%) who did not restart on an anticoagulant.
Dr. Connolly acknowledged that a limitation of the ANNEXA-4 study is the absence of a control group, but he added that he and his associates believed randomizing patients with a serious bleed to placebo control would not have been “practical, feasible, or ethical.”
ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola, and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis. Dr. Kirtane has received research support from several device manufacturers.
SOURCE: Connolly S. ACC 2018.
Treatment with andexanet alfa produced good or excellent hemostasis in 83% of patients in the ANNEXA-4 study, which is what matters when patients are bleeding. Clinicians want to know that you can restore coagulation to a level where you can stop bleeding, and that’s what the results show.
The lack of a reversal agent until now for direct-acting factor Xa inhibitor drugs has probably been a modest but real obstacle to widespread adoption of these agents. We can look at the example of another new oral anticoagulant, dabigatran (Pradaxa), which works by a different mechanism, specifically by inhibiting thrombin. After a reversal agent for dabigatran, idarucizumab (Praxbind) received Food and Drug Administration approval and became available in late 2015, an uptick in dabigatran prescriptions occurred. That experience shows that patients and providers want the safety net of a reversal agent. They want to know that, if there is bleeding or need for urgent surgery, there is a way to facilitate restoration of hemostasis.
It’s the same with direct factor Xa inhibitors: Some patients are concerned about the lack of a reversal agent, and having such an agent may help increase access to these agents for such patients. I think that, once andexanet becomes available for routine U.S. practice, we’ll see an uptick in prescribing of direct factor Xa inhibitors. Also, some patients who have opted for treatment with warfarin will switch to a safer class of drugs, the direct factor X a inhibitors. A myth exists that reversal agents can easily negate the anticoagulant effect of warfarin. The reality is that, despite having treatments that reverse warfarin’s effect, this is often not an easy process in actual practice.
On the safety side, there was no indication in the ANNEXA-4 results of rebound thrombosis with andexanet alfa treatment. Patients receiving a direct factor Xa inhibitor are prothrombotic – that’s why they are on an anticoagulant – so their risk for a thrombotic event is always there, especially when they are not fully anticoagulated, such as when a reversal agent is administered. We need to look to restarting treatment with an anticoagulant because these patients have a high thrombotic risk.
Gregory Piazza, MD , is a cardiologist at Brigham and Women’s Hospital in Boston. He has been an advisor to Portola Pharmaceuticals, the company developing andexanet alfa, as well as to Bayer and Pfizer, and he has received research funding from Bristol-Myers Squibb, Janssen, and Daiichi Sankyo. He made these comments in an interview .
Treatment with andexanet alfa produced good or excellent hemostasis in 83% of patients in the ANNEXA-4 study, which is what matters when patients are bleeding. Clinicians want to know that you can restore coagulation to a level where you can stop bleeding, and that’s what the results show.
The lack of a reversal agent until now for direct-acting factor Xa inhibitor drugs has probably been a modest but real obstacle to widespread adoption of these agents. We can look at the example of another new oral anticoagulant, dabigatran (Pradaxa), which works by a different mechanism, specifically by inhibiting thrombin. After a reversal agent for dabigatran, idarucizumab (Praxbind) received Food and Drug Administration approval and became available in late 2015, an uptick in dabigatran prescriptions occurred. That experience shows that patients and providers want the safety net of a reversal agent. They want to know that, if there is bleeding or need for urgent surgery, there is a way to facilitate restoration of hemostasis.
It’s the same with direct factor Xa inhibitors: Some patients are concerned about the lack of a reversal agent, and having such an agent may help increase access to these agents for such patients. I think that, once andexanet becomes available for routine U.S. practice, we’ll see an uptick in prescribing of direct factor Xa inhibitors. Also, some patients who have opted for treatment with warfarin will switch to a safer class of drugs, the direct factor X a inhibitors. A myth exists that reversal agents can easily negate the anticoagulant effect of warfarin. The reality is that, despite having treatments that reverse warfarin’s effect, this is often not an easy process in actual practice.
On the safety side, there was no indication in the ANNEXA-4 results of rebound thrombosis with andexanet alfa treatment. Patients receiving a direct factor Xa inhibitor are prothrombotic – that’s why they are on an anticoagulant – so their risk for a thrombotic event is always there, especially when they are not fully anticoagulated, such as when a reversal agent is administered. We need to look to restarting treatment with an anticoagulant because these patients have a high thrombotic risk.
Gregory Piazza, MD , is a cardiologist at Brigham and Women’s Hospital in Boston. He has been an advisor to Portola Pharmaceuticals, the company developing andexanet alfa, as well as to Bayer and Pfizer, and he has received research funding from Bristol-Myers Squibb, Janssen, and Daiichi Sankyo. He made these comments in an interview .
Treatment with andexanet alfa produced good or excellent hemostasis in 83% of patients in the ANNEXA-4 study, which is what matters when patients are bleeding. Clinicians want to know that you can restore coagulation to a level where you can stop bleeding, and that’s what the results show.
The lack of a reversal agent until now for direct-acting factor Xa inhibitor drugs has probably been a modest but real obstacle to widespread adoption of these agents. We can look at the example of another new oral anticoagulant, dabigatran (Pradaxa), which works by a different mechanism, specifically by inhibiting thrombin. After a reversal agent for dabigatran, idarucizumab (Praxbind) received Food and Drug Administration approval and became available in late 2015, an uptick in dabigatran prescriptions occurred. That experience shows that patients and providers want the safety net of a reversal agent. They want to know that, if there is bleeding or need for urgent surgery, there is a way to facilitate restoration of hemostasis.
It’s the same with direct factor Xa inhibitors: Some patients are concerned about the lack of a reversal agent, and having such an agent may help increase access to these agents for such patients. I think that, once andexanet becomes available for routine U.S. practice, we’ll see an uptick in prescribing of direct factor Xa inhibitors. Also, some patients who have opted for treatment with warfarin will switch to a safer class of drugs, the direct factor X a inhibitors. A myth exists that reversal agents can easily negate the anticoagulant effect of warfarin. The reality is that, despite having treatments that reverse warfarin’s effect, this is often not an easy process in actual practice.
On the safety side, there was no indication in the ANNEXA-4 results of rebound thrombosis with andexanet alfa treatment. Patients receiving a direct factor Xa inhibitor are prothrombotic – that’s why they are on an anticoagulant – so their risk for a thrombotic event is always there, especially when they are not fully anticoagulated, such as when a reversal agent is administered. We need to look to restarting treatment with an anticoagulant because these patients have a high thrombotic risk.
Gregory Piazza, MD , is a cardiologist at Brigham and Women’s Hospital in Boston. He has been an advisor to Portola Pharmaceuticals, the company developing andexanet alfa, as well as to Bayer and Pfizer, and he has received research funding from Bristol-Myers Squibb, Janssen, and Daiichi Sankyo. He made these comments in an interview .
ORLANDO – Andexanet alfa, a new agent that reverses the anticoagulant effect of direct factor Xa inhibitors, showed an acceptable level of efficacy and safety in 227 patients who received the drug in the agent’s pivotal trial.
These results, which placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind), approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa), suggest that andexanet is likely on track for its own Food and Drug Administration marketing approval, Stuart Connolly, MD, said at the annual meeting of the American College of Cardiology.
Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa) previously announced that it expected Food and Drug Administration action on its marketing application by May 2018.
Andexanet reversal “has similar efficacy and safety as seen with other reversal agents” for other types of anticoagulants, said Dr. Connolly, a professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont. In the trial results he reported, andexanet treatment of patients who were bleeding while on treatment with a direct factor Xa inhibitor had an 83% rate of hemostatic efficacy and an 11% rate of thrombotic events. By comparison, idarucizumab, the FDA-approved reversal agent for the anticoagulant dabigatran, produced a 68% hemostatic efficacy and a 6% rate of thrombotic events in the idarucizumab pivotal trial, RE-VERSE AD (N Engl J Med. 2015 Aug 6;373[6]:511-20).
The Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding (ANNEXA-4) enrolled 227 patients at any of 60 centers, with efficacy data available from 132 of the patients. About 60% of the patients had an intracranial bleed, and about 30% had a gastrointestinal bleed, and their average age was 77 years. Roughly three-quarters of patients were on an anticoagulant for atrial fibrillation, with the rest treated for venous thromboembolism, with 4% having both conditions. The most commonly used direct factor Xa inhbitors in these patients were apixaban (Eliquis) in 105 and rivaroxaban (Xarelto) in 75. The ANNEXA-4 study has not enrolled patients treated with a direct factor Xa inhibitor anticoagulant and undergoing surgery, a setting that will be the subject of a future study, Dr. Connolly said.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by about 75%-90% within minutes of starting the bolus and remained depressed at that level during the infusion but then began recovering by 2 hours after the stop of infusion. Andexanet is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops.
“There is no doubt that andexanet rapidly decreases anti–factor Xa activity,” he said.
Adjudicated efficacy results were available for 132 patients and showed good or excellent hemostasis achieved on andexanet in 109 patients (83%), Dr. Connolly reported. The effect on hemostasis was consistent regardless of patient age, sex, bleeding site, type of anticoagulant, and dosage tested.
Thrombotic events during the 30 days following treatment occurred in 24 of 227 patients (11%) who received andexanet and were evaluable for safety. Notably, no clustering of thrombotic events occurred early, even among the 129 patients who restarted on an anticoagulant during the 30 days after treatment. Among the 129 patients who restarted on an anticoagulant, 9 (7%) had a thrombotic event during the 30-day follow-up, compared with 15 events among 98 patients (15%) who did not restart on an anticoagulant.
Dr. Connolly acknowledged that a limitation of the ANNEXA-4 study is the absence of a control group, but he added that he and his associates believed randomizing patients with a serious bleed to placebo control would not have been “practical, feasible, or ethical.”
ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola, and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis. Dr. Kirtane has received research support from several device manufacturers.
SOURCE: Connolly S. ACC 2018.
ORLANDO – Andexanet alfa, a new agent that reverses the anticoagulant effect of direct factor Xa inhibitors, showed an acceptable level of efficacy and safety in 227 patients who received the drug in the agent’s pivotal trial.
These results, which placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind), approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa), suggest that andexanet is likely on track for its own Food and Drug Administration marketing approval, Stuart Connolly, MD, said at the annual meeting of the American College of Cardiology.
Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa) previously announced that it expected Food and Drug Administration action on its marketing application by May 2018.
Andexanet reversal “has similar efficacy and safety as seen with other reversal agents” for other types of anticoagulants, said Dr. Connolly, a professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont. In the trial results he reported, andexanet treatment of patients who were bleeding while on treatment with a direct factor Xa inhibitor had an 83% rate of hemostatic efficacy and an 11% rate of thrombotic events. By comparison, idarucizumab, the FDA-approved reversal agent for the anticoagulant dabigatran, produced a 68% hemostatic efficacy and a 6% rate of thrombotic events in the idarucizumab pivotal trial, RE-VERSE AD (N Engl J Med. 2015 Aug 6;373[6]:511-20).
The Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding (ANNEXA-4) enrolled 227 patients at any of 60 centers, with efficacy data available from 132 of the patients. About 60% of the patients had an intracranial bleed, and about 30% had a gastrointestinal bleed, and their average age was 77 years. Roughly three-quarters of patients were on an anticoagulant for atrial fibrillation, with the rest treated for venous thromboembolism, with 4% having both conditions. The most commonly used direct factor Xa inhbitors in these patients were apixaban (Eliquis) in 105 and rivaroxaban (Xarelto) in 75. The ANNEXA-4 study has not enrolled patients treated with a direct factor Xa inhibitor anticoagulant and undergoing surgery, a setting that will be the subject of a future study, Dr. Connolly said.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by about 75%-90% within minutes of starting the bolus and remained depressed at that level during the infusion but then began recovering by 2 hours after the stop of infusion. Andexanet is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops.
“There is no doubt that andexanet rapidly decreases anti–factor Xa activity,” he said.
Adjudicated efficacy results were available for 132 patients and showed good or excellent hemostasis achieved on andexanet in 109 patients (83%), Dr. Connolly reported. The effect on hemostasis was consistent regardless of patient age, sex, bleeding site, type of anticoagulant, and dosage tested.
Thrombotic events during the 30 days following treatment occurred in 24 of 227 patients (11%) who received andexanet and were evaluable for safety. Notably, no clustering of thrombotic events occurred early, even among the 129 patients who restarted on an anticoagulant during the 30 days after treatment. Among the 129 patients who restarted on an anticoagulant, 9 (7%) had a thrombotic event during the 30-day follow-up, compared with 15 events among 98 patients (15%) who did not restart on an anticoagulant.
Dr. Connolly acknowledged that a limitation of the ANNEXA-4 study is the absence of a control group, but he added that he and his associates believed randomizing patients with a serious bleed to placebo control would not have been “practical, feasible, or ethical.”
ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola, and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis. Dr. Kirtane has received research support from several device manufacturers.
SOURCE: Connolly S. ACC 2018.
REPORTING FROM ACC 18
Key clinical point:
Major finding: Hemostatic efficacy of andexanet alfa was 83%, and thrombotic events occurred in 11%.
Study details: ANNEXA-4, a single arm cohort study with 227 patients.
Disclosures: ANNEXA-4 is sponsored by Portola Pharmaceuticals, the company developing andexanet alfa (AndexXa). Dr. Connolly has been a consultant to Portola and also to Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, and Sanofi-Aventis.
Source: Connolly S. ACC 2018.
Women in medicine shout #MeToo about sexual harassment at work
Annette Katz didn’t expect to be part of a major social movement. She didn’t set out to take on a major health organization. But that all began to change when a coworker saw her fighting back tears and joined Katz to report to her union what amounted to a criminal sexual offense at a Cleveland Veterans Affairs Medical Center in 2012 and 2013.
Four years later, Katz, a licensed practical nurse at the hospital, testified in a court deposition that a male nursing assistant had shoved her into a linen closet and groped her and subjected her to an onslaught of lewd comments.
In speaking out and taking legal action, Katz joined a growing group of women who are combating sexual harassment in the medical field at every level, from patients’ bedsides to the executive boardroom.
Much as the #MeToo moment has raised awareness of sexual harassment in business, politics, media, and Hollywood, it is prompting women in medicine to take on a health system where workers have traditionally been discouraged from making waves and where hierarchies are ever-present and all-commanding. While the health care field overall has far more women than men, in many stations of power the top of the pyramid is overwhelmingly male, with women occupying the vast base.
In a recent survey, 30% of women on medical faculties reported experiencing sexual harassment at work within the past 2 years, said Reshma Jagsi, MD, who conducted the poll. That share is comparable to results in other sectors and, as elsewhere, in medicine it had been mostly taboo to discuss before last year.
“We know harassment is more common in fields where there are strong power differentials,” said Dr. Jagsi, director of the Center for Bioethics and Social Sciences in Medicine at the University of Michigan, Ann Arbor. “And we know medicine is very hierarchical.”
Workers in the health care and social assistance field reported 4,738 cases of sexual harassment from fiscal 2005 through 2015, eclipsed only by fields such as hospitality and manufacturing, where men make up a greater proportion of the workforce, according to data gathered by the Equal Employment Opportunity Commission.
A Kaiser Health News review of dozens of legal cases across the U.S. shows similar patterns in the waves of harassment cases that have cropped up in other fields, from entertainment to sports to journalism: The harassers are typically male. The alleged harasser supervises or outranks the alleged victim. There are slaps on the butt, lewd comments, and requests for sex. When superiors are confronted with reports of bad behavior, the victims, mostly women, are disbelieved, demoted, or fired.
But recently, physicians have taken to Twitter using the #MeTooMedicine tag, sharing anecdotes and linking to blogs that chronicle powerful doctors harassing them or disrobing at professional conferences.
Women who work in cardiology recently told the cardiology trade publication TCTMD that they felt the problem was particularly widespread in their specialty, where females account for 14% of the physicians. A Los Angeles anesthesiologist made waves in a blog post urging “prettier” women to adopt a “professional-looking, even severe, hair style” to be taken seriously and to consider self-defense classes.
Among those speaking out is Jennifer Gunter, MD, a San Francisco obstetrician-gynecologist, who recently wrote a blog post about being groped in 2014 by a prominent colleague at a medical conference – even naming him.
“I think nothing will change unless people are able to name people and institutions are held accountable,” she said in an interview. “I don’t think without massive public discourse and exposure that things will change.”
Lawsuits, many settled or still making their way through the courts, describe encounters.
A Florida nurse claimed that in 2014, a surgeon made lewd comments about her breasts, asking her in a room full of people if he should “refer to her as ‘JJ’ or ‘Jugs,’ ” the nurse’s lawsuit says. The nurse said she “responded that she wished to be called by her name.”
In other cases: A phlebotomist in New York alleged in a lawsuit that a doctor in her medical practice gave her a box of Valentine’s Day candy and moved in for an unwanted kiss on the mouth. A Florida medical resident alleged that a supervising doctor told her she looked like a “slutty whore.” A Nebraska nurse claimed that a doctor she traveled with to a professional conference offered to buy her a bikini, if he could see her in it, and an extra night in a hotel, if they could share the room. She declined.
A Pennsylvania nurse described the unsatisfying response she got after reporting that a colleague had pressed his pelvis against her and flipped through her phone for “naked pictures.” A supervisor to whom she reported the conduct expressed exasperation, saying “I can’t deal with this” and “What do you want?”
Kayla Behbahani, DO, chief psychiatry resident at University of Massachusetts Memorial Medical Center, did not file a lawsuit but recently wrote about sexual harassment by a subordinate. In an interview, she said her instincts were to pity the man, and also to follow a dictate that’s drilled into medical students: Don’t make waves. So, she disclosed the harassment only after another woman’s complaint launched an investigation.
“As a professional, I come from a culture where you go with the flow,” Dr. Behbahani said. “You deal with what you’re dealt. In that regard, it was a dilemma for me.”
Annette Katz, the Veterans Affairs nurse, initially didn’t complain about the harassment. A single mother with two children, she needed her job. Her attacker, MD Garrett, was also a nursing assistant but had more seniority, was a veteran, and was friends with her boss.
“I really did feel that I would lose my job,” Ms. Katz said in an interview. “I would be that troublemaker.”
But as the abuse escalated, she went to the VA inspector general and the Cleveland police.
She estimated that five times Mr. Garrett pushed her into a closet where he would ask for sex. She would “tell him ‘no’ and fight my way out of [his] grip,” her statement said. He shoved her into an unconscious patient’s bathroom and would “try to restrain me, but I eventually could break free.”
After one such assault, a colleague noticed tears in Ms. Katz’s eyes. The coworker shared with Ms. Katz that she, too, had been a target of Mr. Garrett’s lewd behavior.
Ms. Katz and the colleague filed complaints in March 2013 with their union, the police, and with their managers. That July, Mr. Garrett was indicted by a grand jury and later pleaded guilty to three counts of sexual imposition and one count of unlawful restraint. He was also dismissed from his job.
Reached by phone, Mr. Garrett said he agreed to the plea because he was facing multiple felonies and didn’t know what a jury would do. He said that even though he pleaded guilty to four misdemeanors, he did not commit the crimes of which he was accused. “There was no harassment; she and I were friends,” he said.
In 2013, Ms. Katz sued the VA, alleging that it failed to protect her from harassment and retaliated against her by refusing to give her a job-site transfer before firing her for not showing up to work.
The VA attorneys argued that the department had no direct knowledge of harassing behavior before Ms. Katz reported it, and that once it was informed, immediate action was taken. Veterans Affairs Deputy Press Secretary Lydia Blaha said in an email that anyone engaged in sexual harassment is swiftly held accountable.
The U.S. Department of Veterans Affairs agreed in February to pay $161,500 to settle Ms. Katz’s lawsuit.
Ms. Katz said it was costly and emotional to press on with her legal case but hopes it helps other women see that seeking justice is worthwhile. “I do think there are a lot of women who just suffer in silence,” she said.
Dr. Gunter, the San Francisco physician-blogger, said that needed change will come only when people who are more established across all professions stand up for those who are more junior. “Speaking quietly, going to HR – if that worked, we wouldn’t be here,” she said.
It’s ironic, she said, that as a gynecologist she’s trained to believe patients’ claims about sexual assault. In the workplace, though, it’s well known that raising such matters can backfire. She added: “Physicians should be setting a standard on this.”
KHN’s coverage of these topics is supported by the John A. Hartford Foundation and The David and Lucile Packard Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Annette Katz didn’t expect to be part of a major social movement. She didn’t set out to take on a major health organization. But that all began to change when a coworker saw her fighting back tears and joined Katz to report to her union what amounted to a criminal sexual offense at a Cleveland Veterans Affairs Medical Center in 2012 and 2013.
Four years later, Katz, a licensed practical nurse at the hospital, testified in a court deposition that a male nursing assistant had shoved her into a linen closet and groped her and subjected her to an onslaught of lewd comments.
In speaking out and taking legal action, Katz joined a growing group of women who are combating sexual harassment in the medical field at every level, from patients’ bedsides to the executive boardroom.
Much as the #MeToo moment has raised awareness of sexual harassment in business, politics, media, and Hollywood, it is prompting women in medicine to take on a health system where workers have traditionally been discouraged from making waves and where hierarchies are ever-present and all-commanding. While the health care field overall has far more women than men, in many stations of power the top of the pyramid is overwhelmingly male, with women occupying the vast base.
In a recent survey, 30% of women on medical faculties reported experiencing sexual harassment at work within the past 2 years, said Reshma Jagsi, MD, who conducted the poll. That share is comparable to results in other sectors and, as elsewhere, in medicine it had been mostly taboo to discuss before last year.
“We know harassment is more common in fields where there are strong power differentials,” said Dr. Jagsi, director of the Center for Bioethics and Social Sciences in Medicine at the University of Michigan, Ann Arbor. “And we know medicine is very hierarchical.”
Workers in the health care and social assistance field reported 4,738 cases of sexual harassment from fiscal 2005 through 2015, eclipsed only by fields such as hospitality and manufacturing, where men make up a greater proportion of the workforce, according to data gathered by the Equal Employment Opportunity Commission.
A Kaiser Health News review of dozens of legal cases across the U.S. shows similar patterns in the waves of harassment cases that have cropped up in other fields, from entertainment to sports to journalism: The harassers are typically male. The alleged harasser supervises or outranks the alleged victim. There are slaps on the butt, lewd comments, and requests for sex. When superiors are confronted with reports of bad behavior, the victims, mostly women, are disbelieved, demoted, or fired.
But recently, physicians have taken to Twitter using the #MeTooMedicine tag, sharing anecdotes and linking to blogs that chronicle powerful doctors harassing them or disrobing at professional conferences.
Women who work in cardiology recently told the cardiology trade publication TCTMD that they felt the problem was particularly widespread in their specialty, where females account for 14% of the physicians. A Los Angeles anesthesiologist made waves in a blog post urging “prettier” women to adopt a “professional-looking, even severe, hair style” to be taken seriously and to consider self-defense classes.
Among those speaking out is Jennifer Gunter, MD, a San Francisco obstetrician-gynecologist, who recently wrote a blog post about being groped in 2014 by a prominent colleague at a medical conference – even naming him.
“I think nothing will change unless people are able to name people and institutions are held accountable,” she said in an interview. “I don’t think without massive public discourse and exposure that things will change.”
Lawsuits, many settled or still making their way through the courts, describe encounters.
A Florida nurse claimed that in 2014, a surgeon made lewd comments about her breasts, asking her in a room full of people if he should “refer to her as ‘JJ’ or ‘Jugs,’ ” the nurse’s lawsuit says. The nurse said she “responded that she wished to be called by her name.”
In other cases: A phlebotomist in New York alleged in a lawsuit that a doctor in her medical practice gave her a box of Valentine’s Day candy and moved in for an unwanted kiss on the mouth. A Florida medical resident alleged that a supervising doctor told her she looked like a “slutty whore.” A Nebraska nurse claimed that a doctor she traveled with to a professional conference offered to buy her a bikini, if he could see her in it, and an extra night in a hotel, if they could share the room. She declined.
A Pennsylvania nurse described the unsatisfying response she got after reporting that a colleague had pressed his pelvis against her and flipped through her phone for “naked pictures.” A supervisor to whom she reported the conduct expressed exasperation, saying “I can’t deal with this” and “What do you want?”
Kayla Behbahani, DO, chief psychiatry resident at University of Massachusetts Memorial Medical Center, did not file a lawsuit but recently wrote about sexual harassment by a subordinate. In an interview, she said her instincts were to pity the man, and also to follow a dictate that’s drilled into medical students: Don’t make waves. So, she disclosed the harassment only after another woman’s complaint launched an investigation.
“As a professional, I come from a culture where you go with the flow,” Dr. Behbahani said. “You deal with what you’re dealt. In that regard, it was a dilemma for me.”
Annette Katz, the Veterans Affairs nurse, initially didn’t complain about the harassment. A single mother with two children, she needed her job. Her attacker, MD Garrett, was also a nursing assistant but had more seniority, was a veteran, and was friends with her boss.
“I really did feel that I would lose my job,” Ms. Katz said in an interview. “I would be that troublemaker.”
But as the abuse escalated, she went to the VA inspector general and the Cleveland police.
She estimated that five times Mr. Garrett pushed her into a closet where he would ask for sex. She would “tell him ‘no’ and fight my way out of [his] grip,” her statement said. He shoved her into an unconscious patient’s bathroom and would “try to restrain me, but I eventually could break free.”
After one such assault, a colleague noticed tears in Ms. Katz’s eyes. The coworker shared with Ms. Katz that she, too, had been a target of Mr. Garrett’s lewd behavior.
Ms. Katz and the colleague filed complaints in March 2013 with their union, the police, and with their managers. That July, Mr. Garrett was indicted by a grand jury and later pleaded guilty to three counts of sexual imposition and one count of unlawful restraint. He was also dismissed from his job.
Reached by phone, Mr. Garrett said he agreed to the plea because he was facing multiple felonies and didn’t know what a jury would do. He said that even though he pleaded guilty to four misdemeanors, he did not commit the crimes of which he was accused. “There was no harassment; she and I were friends,” he said.
In 2013, Ms. Katz sued the VA, alleging that it failed to protect her from harassment and retaliated against her by refusing to give her a job-site transfer before firing her for not showing up to work.
The VA attorneys argued that the department had no direct knowledge of harassing behavior before Ms. Katz reported it, and that once it was informed, immediate action was taken. Veterans Affairs Deputy Press Secretary Lydia Blaha said in an email that anyone engaged in sexual harassment is swiftly held accountable.
The U.S. Department of Veterans Affairs agreed in February to pay $161,500 to settle Ms. Katz’s lawsuit.
Ms. Katz said it was costly and emotional to press on with her legal case but hopes it helps other women see that seeking justice is worthwhile. “I do think there are a lot of women who just suffer in silence,” she said.
Dr. Gunter, the San Francisco physician-blogger, said that needed change will come only when people who are more established across all professions stand up for those who are more junior. “Speaking quietly, going to HR – if that worked, we wouldn’t be here,” she said.
It’s ironic, she said, that as a gynecologist she’s trained to believe patients’ claims about sexual assault. In the workplace, though, it’s well known that raising such matters can backfire. She added: “Physicians should be setting a standard on this.”
KHN’s coverage of these topics is supported by the John A. Hartford Foundation and The David and Lucile Packard Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Annette Katz didn’t expect to be part of a major social movement. She didn’t set out to take on a major health organization. But that all began to change when a coworker saw her fighting back tears and joined Katz to report to her union what amounted to a criminal sexual offense at a Cleveland Veterans Affairs Medical Center in 2012 and 2013.
Four years later, Katz, a licensed practical nurse at the hospital, testified in a court deposition that a male nursing assistant had shoved her into a linen closet and groped her and subjected her to an onslaught of lewd comments.
In speaking out and taking legal action, Katz joined a growing group of women who are combating sexual harassment in the medical field at every level, from patients’ bedsides to the executive boardroom.
Much as the #MeToo moment has raised awareness of sexual harassment in business, politics, media, and Hollywood, it is prompting women in medicine to take on a health system where workers have traditionally been discouraged from making waves and where hierarchies are ever-present and all-commanding. While the health care field overall has far more women than men, in many stations of power the top of the pyramid is overwhelmingly male, with women occupying the vast base.
In a recent survey, 30% of women on medical faculties reported experiencing sexual harassment at work within the past 2 years, said Reshma Jagsi, MD, who conducted the poll. That share is comparable to results in other sectors and, as elsewhere, in medicine it had been mostly taboo to discuss before last year.
“We know harassment is more common in fields where there are strong power differentials,” said Dr. Jagsi, director of the Center for Bioethics and Social Sciences in Medicine at the University of Michigan, Ann Arbor. “And we know medicine is very hierarchical.”
Workers in the health care and social assistance field reported 4,738 cases of sexual harassment from fiscal 2005 through 2015, eclipsed only by fields such as hospitality and manufacturing, where men make up a greater proportion of the workforce, according to data gathered by the Equal Employment Opportunity Commission.
A Kaiser Health News review of dozens of legal cases across the U.S. shows similar patterns in the waves of harassment cases that have cropped up in other fields, from entertainment to sports to journalism: The harassers are typically male. The alleged harasser supervises or outranks the alleged victim. There are slaps on the butt, lewd comments, and requests for sex. When superiors are confronted with reports of bad behavior, the victims, mostly women, are disbelieved, demoted, or fired.
But recently, physicians have taken to Twitter using the #MeTooMedicine tag, sharing anecdotes and linking to blogs that chronicle powerful doctors harassing them or disrobing at professional conferences.
Women who work in cardiology recently told the cardiology trade publication TCTMD that they felt the problem was particularly widespread in their specialty, where females account for 14% of the physicians. A Los Angeles anesthesiologist made waves in a blog post urging “prettier” women to adopt a “professional-looking, even severe, hair style” to be taken seriously and to consider self-defense classes.
Among those speaking out is Jennifer Gunter, MD, a San Francisco obstetrician-gynecologist, who recently wrote a blog post about being groped in 2014 by a prominent colleague at a medical conference – even naming him.
“I think nothing will change unless people are able to name people and institutions are held accountable,” she said in an interview. “I don’t think without massive public discourse and exposure that things will change.”
Lawsuits, many settled or still making their way through the courts, describe encounters.
A Florida nurse claimed that in 2014, a surgeon made lewd comments about her breasts, asking her in a room full of people if he should “refer to her as ‘JJ’ or ‘Jugs,’ ” the nurse’s lawsuit says. The nurse said she “responded that she wished to be called by her name.”
In other cases: A phlebotomist in New York alleged in a lawsuit that a doctor in her medical practice gave her a box of Valentine’s Day candy and moved in for an unwanted kiss on the mouth. A Florida medical resident alleged that a supervising doctor told her she looked like a “slutty whore.” A Nebraska nurse claimed that a doctor she traveled with to a professional conference offered to buy her a bikini, if he could see her in it, and an extra night in a hotel, if they could share the room. She declined.
A Pennsylvania nurse described the unsatisfying response she got after reporting that a colleague had pressed his pelvis against her and flipped through her phone for “naked pictures.” A supervisor to whom she reported the conduct expressed exasperation, saying “I can’t deal with this” and “What do you want?”
Kayla Behbahani, DO, chief psychiatry resident at University of Massachusetts Memorial Medical Center, did not file a lawsuit but recently wrote about sexual harassment by a subordinate. In an interview, she said her instincts were to pity the man, and also to follow a dictate that’s drilled into medical students: Don’t make waves. So, she disclosed the harassment only after another woman’s complaint launched an investigation.
“As a professional, I come from a culture where you go with the flow,” Dr. Behbahani said. “You deal with what you’re dealt. In that regard, it was a dilemma for me.”
Annette Katz, the Veterans Affairs nurse, initially didn’t complain about the harassment. A single mother with two children, she needed her job. Her attacker, MD Garrett, was also a nursing assistant but had more seniority, was a veteran, and was friends with her boss.
“I really did feel that I would lose my job,” Ms. Katz said in an interview. “I would be that troublemaker.”
But as the abuse escalated, she went to the VA inspector general and the Cleveland police.
She estimated that five times Mr. Garrett pushed her into a closet where he would ask for sex. She would “tell him ‘no’ and fight my way out of [his] grip,” her statement said. He shoved her into an unconscious patient’s bathroom and would “try to restrain me, but I eventually could break free.”
After one such assault, a colleague noticed tears in Ms. Katz’s eyes. The coworker shared with Ms. Katz that she, too, had been a target of Mr. Garrett’s lewd behavior.
Ms. Katz and the colleague filed complaints in March 2013 with their union, the police, and with their managers. That July, Mr. Garrett was indicted by a grand jury and later pleaded guilty to three counts of sexual imposition and one count of unlawful restraint. He was also dismissed from his job.
Reached by phone, Mr. Garrett said he agreed to the plea because he was facing multiple felonies and didn’t know what a jury would do. He said that even though he pleaded guilty to four misdemeanors, he did not commit the crimes of which he was accused. “There was no harassment; she and I were friends,” he said.
In 2013, Ms. Katz sued the VA, alleging that it failed to protect her from harassment and retaliated against her by refusing to give her a job-site transfer before firing her for not showing up to work.
The VA attorneys argued that the department had no direct knowledge of harassing behavior before Ms. Katz reported it, and that once it was informed, immediate action was taken. Veterans Affairs Deputy Press Secretary Lydia Blaha said in an email that anyone engaged in sexual harassment is swiftly held accountable.
The U.S. Department of Veterans Affairs agreed in February to pay $161,500 to settle Ms. Katz’s lawsuit.
Ms. Katz said it was costly and emotional to press on with her legal case but hopes it helps other women see that seeking justice is worthwhile. “I do think there are a lot of women who just suffer in silence,” she said.
Dr. Gunter, the San Francisco physician-blogger, said that needed change will come only when people who are more established across all professions stand up for those who are more junior. “Speaking quietly, going to HR – if that worked, we wouldn’t be here,” she said.
It’s ironic, she said, that as a gynecologist she’s trained to believe patients’ claims about sexual assault. In the workplace, though, it’s well known that raising such matters can backfire. She added: “Physicians should be setting a standard on this.”
KHN’s coverage of these topics is supported by the John A. Hartford Foundation and The David and Lucile Packard Foundation. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.