User login
See VAM 2018 Presentations with VAM on Demand
VAM on Demand, a library of hundreds of presentations from the 2018 Vascular Annual Meeting is now available. Those who attended VAM can review sessions at their own pace and watch others that they missed. Those who did not attend now get the chance to learn from the sessions offered. Cost is $199 for VAM attendees and $499 for non-attendees. Access, including the ability to download materials, is good for one year. Learn more and purchase VAM on Demand here. Contact the SVS Education Department for more information at 312-334-2300, or at [email protected].
VAM on Demand, a library of hundreds of presentations from the 2018 Vascular Annual Meeting is now available. Those who attended VAM can review sessions at their own pace and watch others that they missed. Those who did not attend now get the chance to learn from the sessions offered. Cost is $199 for VAM attendees and $499 for non-attendees. Access, including the ability to download materials, is good for one year. Learn more and purchase VAM on Demand here. Contact the SVS Education Department for more information at 312-334-2300, or at [email protected].
VAM on Demand, a library of hundreds of presentations from the 2018 Vascular Annual Meeting is now available. Those who attended VAM can review sessions at their own pace and watch others that they missed. Those who did not attend now get the chance to learn from the sessions offered. Cost is $199 for VAM attendees and $499 for non-attendees. Access, including the ability to download materials, is good for one year. Learn more and purchase VAM on Demand here. Contact the SVS Education Department for more information at 312-334-2300, or at [email protected].
VRIC Set for May 13, 2019
The Society for Vascular Surgery has set the date for the 2019 Vascular Research Initiatives Conference: May 13, 2019. Mark your calendars and plan to attend this conference, which focuses on emerging vascular science. VRIC will feature abstracts, the Alexander W. Clowes Distinguished Lecture, a Translational Panel, reception and posters. As in the past, VRIC will be held in conjunction with the American Heart Association's Scientific Sessions. Those take place May 14 to 16, 2019.
The Society for Vascular Surgery has set the date for the 2019 Vascular Research Initiatives Conference: May 13, 2019. Mark your calendars and plan to attend this conference, which focuses on emerging vascular science. VRIC will feature abstracts, the Alexander W. Clowes Distinguished Lecture, a Translational Panel, reception and posters. As in the past, VRIC will be held in conjunction with the American Heart Association's Scientific Sessions. Those take place May 14 to 16, 2019.
The Society for Vascular Surgery has set the date for the 2019 Vascular Research Initiatives Conference: May 13, 2019. Mark your calendars and plan to attend this conference, which focuses on emerging vascular science. VRIC will feature abstracts, the Alexander W. Clowes Distinguished Lecture, a Translational Panel, reception and posters. As in the past, VRIC will be held in conjunction with the American Heart Association's Scientific Sessions. Those take place May 14 to 16, 2019.
Attend VAM 2019, Tour Clinics, With Scholarship
Apply for the SVS International Scholars Program by Sept. 1. Up to four qualified vascular surgeons younger than 40, from countries other than the United States or Canada, will receive $5,000 each, to attend the 2019 Vascular Annual Meeting and to visit clinical, teaching and research facilities in the U.S. and Canada. Watch a past recipient discuss the impact on her career here.
Apply for the SVS International Scholars Program by Sept. 1. Up to four qualified vascular surgeons younger than 40, from countries other than the United States or Canada, will receive $5,000 each, to attend the 2019 Vascular Annual Meeting and to visit clinical, teaching and research facilities in the U.S. and Canada. Watch a past recipient discuss the impact on her career here.
Apply for the SVS International Scholars Program by Sept. 1. Up to four qualified vascular surgeons younger than 40, from countries other than the United States or Canada, will receive $5,000 each, to attend the 2019 Vascular Annual Meeting and to visit clinical, teaching and research facilities in the U.S. and Canada. Watch a past recipient discuss the impact on her career here.
CBT for depression: What the evidence says
Major depressive disorder (MDD) has a devastating impact on individuals and society because of its high prevalence, its recurrent nature, its frequent comorbidity with other disorders, and the functional impairment it causes. Compared with other chronic diseases, such as arthritis, asthma, and diabetes, MDD produces the greatest decrement in health worldwide.1 The goals in treating MDD should be not just to reduce symptom severity but also to achieve continuing remission and lower the risk for relapse.2
Antidepressants are the most common treatment for depression.3 Among psychotherapies used to treat MDD, cognitive-behavioral therapy (CBT) has been identified as an effective treatment.4 Collaborative care models have been reported to manage MDD more effectively.5 In this article, we review the evidence supporting the use of CBT as monotherapy and in combination with antidepressants for acute and long-term treatment of MDD.
Acute treatment: Not too soon for CBT
Mild to moderate depression
Research has indicated that for the treatment of mild MDD, antidepressants are unlikely to be more effective than placebo.6,7 Studies also have reported that response to antidepressants begins to outpace response to placebo only when symptoms are no longer mild. Using antidepressants for patients with mild depression could therefore place them at risk of overtreatment.8 In keeping with these findings, the American Psychiatric Association (APA) has recommended the use of evidence-based psychotherapies, such as CBT, as an initial treatment choice for patients with mild to moderate MDD.9
Two recent studies have suggested that the combination of CBT plus antidepressants could boost improvement in psychosocial functioning for patients with mild MDD.10,11 However, neither study included a group of patients who received only CBT to evaluate if CBT alone could have also produced similar effects. Other limitations include the lack of a control group in one study and small sample sizes in both studies. However, both studies had a long follow-up period and specifically studied the impact on psychosocial functioning.
Moderate to severe depression
Earlier depression treatment guidelines suggested that antidepressants should be used to treat more severe depression, while psychotherapy should be used mainly for mild depression.12 This recommendation was influenced by the well-known National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program, a multicenter randomized controlled trial (RCT) that used a placebo control.13 In this study, CBT was compared with antidepressants and found to be no more effective than placebo for more severely depressed patients.13 However, this finding was not consistent across the 3 sites where the study was conducted; at the site where CBT was provided by more experienced CBT therapists, patients with more severe depression who received CBT fared as well as patients treated with antidepressants.14 A later double-blind RCT that used experienced therapists found that CBT was as effective as antidepressants (monoamine oxidase inhibitors), and both treatments were superior to placebo in reducing symptoms of atypical depression.15
Another placebo-controlled RCT conducted at 2 sites found that CBT was as effective as antidepressants in the treatment of moderately to severely depressed patients. As in the NIMH Treatment of Depression Collaborative Research Program trial,13 in this study, there were indications that the results were dependent on therapist experience.16 These findings suggest that the experience of the therapist is an important factor.
A recent meta-analysis of treatments of the acute phase of MDD compared 11 RCTs of CBT and second-generation antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other medications with related mechanisms of action).17 It found that as a first-step treatment, CBT and antidepressants had a similar impact on symptom relief in patients with moderate to severe depression. Patients treated with antidepressants also had a higher risk of experiencing adverse events or discontinuing treatment because of adverse events. However, this meta-analysis included trials that had methodological shortcomings, which reduces the strength of these conclusions.
Continue to: Patients with MDD and comorbid personality disorders have been...
Patients with MDD and comorbid personality disorders have been reported to have poorer outcomes, regardless of the treatment used.18 Fournier et al19 examined the impact of antidepressants and CBT in moderately to severely depressed patients with and without a personality disorder. They found that a combination of antidepressants and CBT was suitable for patients with personality disorders because antidepressants would boost the initial response and CBT would help sustain improvement in the long term.
Presently, the APA suggests that the combination of psychotherapy and antidepressants may be used as an initial treatment for patients with moderate to severe MDD.9 As research brings to light other factors that affect treatment outcomes, these guidelines could change.
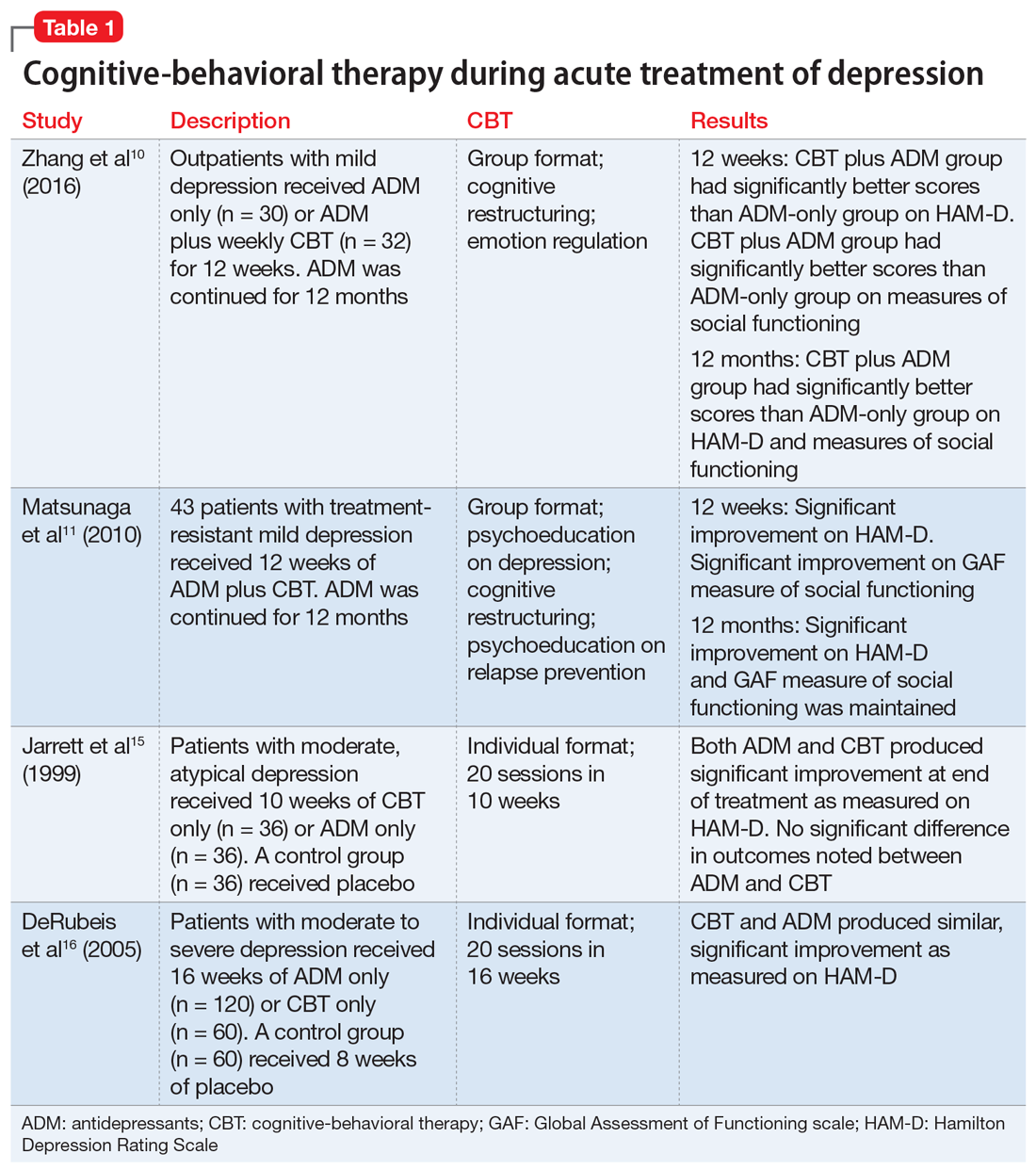
Table 110,11,15,16 summarizes the findings of select studies evaluating the use of CBT for the acute treatment of depression.
CBT’s role in long-term treatment
Recurrence and relapse are major problems associated with MDD. The large majority of individuals who experience an episode of depression go on to experience more episodes of depression,20 and the risk of recurrence increases after each successive episode.21
To reduce the risk of relapse and the return of symptoms, it is recommended that patients treated with antidepressants continue pharmacotherapy for 4 to 9 months after remission.9 Maintenance pharmacotherapy, which involves keeping patients on antidepressants beyond the point of recovery, is intended to reduce the risk of recurrence, and is standard treatment for patients with chronic or recurrent MDD.22 However, this preventive effect exists only while the patient continues to take the medication. Rates of symptom recurrence following medication withdrawal are often high regardless of how long patients have taken medications.23
Continue to: Studies examining CBT as a maintenance treatment...
Studies examining CBT as a maintenance treatment—provided alone or in combination with or sequentially with antidepressants—have found it has an enduring effect that extends beyond the end of treatment and equals the impact of continuing antidepressants.24-27 A recent meta-analysis of 10 trials where CBT had been provided to patients after acute treatment found that the risk of relapse was reduced by 21% in the first year and by 28% in the first 2 years.28
Studies have compared the prophylactic impact of maintenance CBT and antidepressants. In an early study, 40 patients who had been successfully treated with antidepressants but had residual symptoms were randomly assigned to 20 weeks of CBT or to clinical management.29 By the end of 20 weeks, patients were tapered off their antidepressant. All patients were then followed for 2 years, during which time they received no treatment. At the 2-year follow-up, the CBT group had a relapse rate of 25%, compared with 80% in the antidepressant group.29 Weaknesses of this study include a small sample size, and the fact that a single therapist provided the CBT.
This study was extended to a 6-year follow-up; antidepressants were prescribed only to patients who relapsed. The CBT group continued to have a significantly lower relapse rate (40%) compared with the antidepressant group (90%).30
In another RCT, patients with depression who had recovered with CBT or medication continued with the same treatment during a maintenance phase.26 The CBT group received 3 booster sessions during the next year and antidepressant group received medication. At the end of the second year (without CBT or medication) CBT patients were less likely to relapse compared with patients receiving antidepressants. The adjusted relapse rates were 17.3% for CBT and 53.6% for antidepressants.26
An RCT that included 452 patients with severe depression used a long intervention period (up to 42 weeks) and a flexible treatment algorithm to more closely model the strategies used in clinical practice.31 Patients were randomly assigned to antidepressants only or in combination with CBT. At the end of 12 months, outcome assessment by blinded interviewers indicated that patients with more severe depression were more likely to benefit from the combination of antidepressants and CBT (76.9% vs 60.3%) and those with severe, non-chronic depression received the most benefit (79.5% vs 62.8%). The lack of a CBT-only group limits the generalizability of these findings. Neither patients nor clinicians were blinded to the treatment assignment, which is a common limitation in psychotherapy studies but could have contributed to the finding that combined treatment was more effective.
Continue to: Some evidence suggests...
Some evidence suggests that augmenting treatment as usual (TAU) with CBT can have a resilient protective impact that also intensifies with the number of depressive episodes experienced. In an RCT, 172 patients with depression in remission were randomly assigned to TAU or to TAU augmented with CBT.32 The time to recurrence was assessed over the course of 10 years. Augmenting TAU with CBT had a significant protective impact that was greater for patients who had >3 previous episodes.32
Another long-term study assessed the longitudinal course of 158 patients who received CBT, medication, and clinical management, or medication and clinical management alone.33 Patients were followed 6 years after randomization (4.5 years after completion of CBT). Researchers found the effects of CBT in preventing relapse and recurrence persisted for several years.33
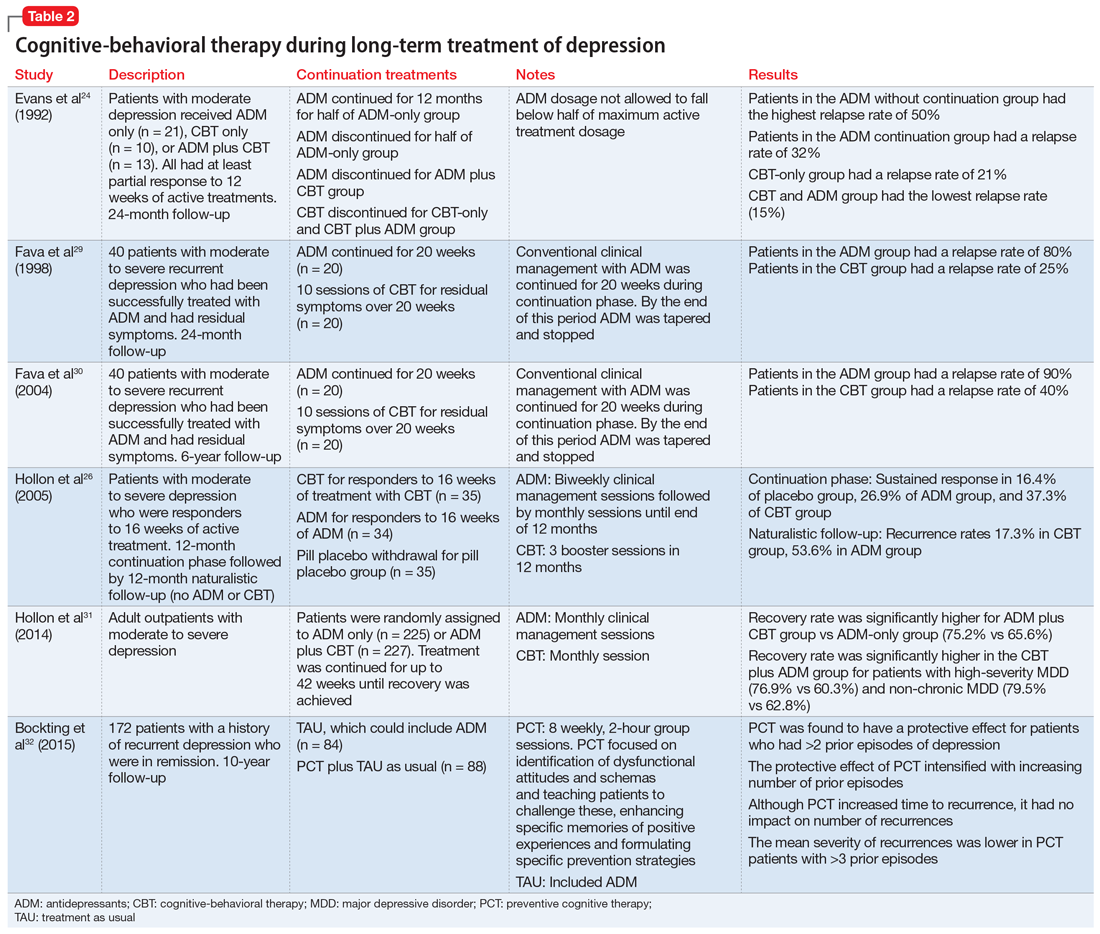
Table 224,26,29-32 summarizes the findings of select studies evaluating the use of CBT for the long-term treatment of depression.
Limitations of long-term studies
Studies that have examined the efficacy of adding CBT to antidepressants in the continuation and maintenance treatment of patients with MDD have had some limitations. The definitions of relapse and recurrence have not always been clearly delineated in all studies. This is important because recurrence rates tend to be lower, and long-term follow-up would be needed to detect multiple recurrences so that their incidence is not underestimated. In addition, the types of CBT interventions utilized has varied across studies. Some studies have employed standard interventions such as cognitive restructuring, while others have added strategies that focus on enhancing memories for positive experiences or interventions to encourage medication adherence. Despite these limitations, research has shown promising results and suggests that adding CBT to the maintenance treatment of patients with depression—with or without antidepressants—is likely to reduce the rate of relapse and recurrence.
Consider CBT for all depressed patients
Research indicates that CBT can be the preferred treatment for patients with mild to moderate MDD. Antidepressants significantly reduce depressive symptoms in patients with moderate to severe MDD. Some research suggests that CBT can be as effective as antidepressants for moderate and severe MDD. However, as the severity and chronicity of depression increase, other moderating factors need to be considered. The expertise of the CBT therapist has an impact on outcomes. Treatment protocols that utilize CBT plus antidepressants are likely to be more effective than CBT or antidepressants alone. Incorporating CBT in the acute phase of depression treatment, with or without antidepressants, can have a long-term impact. For maintenance treatment, CBT alone and CBT plus antidepressants have been found to help sustain remission.
Continue to: Bottom Line
Bottom Line
Cognitive-behavioral therapy (CBT) can be an effective treatment for patients with major depressive disorder, regardless of symptom severity. The expertise of the clinician who provides CBT has a substantial impact on outcomes. Combination treatment with CBT plus antidepressants is more likely to be effective than either treatment alone.
Related Resources
- Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5:CD010558.
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
2. Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152-3160.
3. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
4. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
5. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am. 2005;28(4):1061-1077.
6. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
7. Paykel ES, Hollyman JA, Freeling P, et al. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo-controlled trial. J Affect Disord. 1988;14(1):83-95
8. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
9. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
10. Zhang B, Ding X, Lu W, et al. Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Arch Psychiatry. 2016;28(1):18-27.
11. Matsunaga M, Okamoto Y, Suzuki S et.al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22.
12. American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry. 1993;150(suppl 4):1-26.
13. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
14. Jacobson NS, Hollon SD. Prospects for future comparisons between drugs and psychotherapy: lessons from the CBT-versus-pharmacotherapy exchange. J Consult Clin Psychol. 1996;64(1):104-108.
15. Jarrett RB, Schaffer M, McIntire D, et al. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):431-437.
16. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
17. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019.
18. Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry. 2006;188(1):13-20.
19. Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124-129.
20. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006.
21. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233.
22. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855.
23. Thase ME. Relapse and recurrence of depression: an updated practical approach for prevention. In: Palmer KJ, ed. Drug treatment issues in depression. Auckland, New Zealand: Adis International; 2000:35-52.
24. Evans MD, Hollon, SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49(10):802-808.
25. Vittengal JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475-488.
26. Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417-422.
27. Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829-835.
28. Clarke K, Mayo-Wilson E, Kenny J, et al. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2015;39:58-70.
29. Fava GA, Rafanelli C, Grandi, S, et al. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55(9):816-820.
30. Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876.
31. Hollon SD, DeRubeis RJ, Fawcett J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157-1164.
32. Bockting CL, Smid NH, Koeter MW, et al. Enduring effects of preventive cognitive therapy in adults remitted from recurrent depression: a 10 year follow-up of a randomized controlled trial. J Affect Disord. 2015;185:188-194.
33. Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59-68.
Major depressive disorder (MDD) has a devastating impact on individuals and society because of its high prevalence, its recurrent nature, its frequent comorbidity with other disorders, and the functional impairment it causes. Compared with other chronic diseases, such as arthritis, asthma, and diabetes, MDD produces the greatest decrement in health worldwide.1 The goals in treating MDD should be not just to reduce symptom severity but also to achieve continuing remission and lower the risk for relapse.2
Antidepressants are the most common treatment for depression.3 Among psychotherapies used to treat MDD, cognitive-behavioral therapy (CBT) has been identified as an effective treatment.4 Collaborative care models have been reported to manage MDD more effectively.5 In this article, we review the evidence supporting the use of CBT as monotherapy and in combination with antidepressants for acute and long-term treatment of MDD.
Acute treatment: Not too soon for CBT
Mild to moderate depression
Research has indicated that for the treatment of mild MDD, antidepressants are unlikely to be more effective than placebo.6,7 Studies also have reported that response to antidepressants begins to outpace response to placebo only when symptoms are no longer mild. Using antidepressants for patients with mild depression could therefore place them at risk of overtreatment.8 In keeping with these findings, the American Psychiatric Association (APA) has recommended the use of evidence-based psychotherapies, such as CBT, as an initial treatment choice for patients with mild to moderate MDD.9
Two recent studies have suggested that the combination of CBT plus antidepressants could boost improvement in psychosocial functioning for patients with mild MDD.10,11 However, neither study included a group of patients who received only CBT to evaluate if CBT alone could have also produced similar effects. Other limitations include the lack of a control group in one study and small sample sizes in both studies. However, both studies had a long follow-up period and specifically studied the impact on psychosocial functioning.
Moderate to severe depression
Earlier depression treatment guidelines suggested that antidepressants should be used to treat more severe depression, while psychotherapy should be used mainly for mild depression.12 This recommendation was influenced by the well-known National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program, a multicenter randomized controlled trial (RCT) that used a placebo control.13 In this study, CBT was compared with antidepressants and found to be no more effective than placebo for more severely depressed patients.13 However, this finding was not consistent across the 3 sites where the study was conducted; at the site where CBT was provided by more experienced CBT therapists, patients with more severe depression who received CBT fared as well as patients treated with antidepressants.14 A later double-blind RCT that used experienced therapists found that CBT was as effective as antidepressants (monoamine oxidase inhibitors), and both treatments were superior to placebo in reducing symptoms of atypical depression.15
Another placebo-controlled RCT conducted at 2 sites found that CBT was as effective as antidepressants in the treatment of moderately to severely depressed patients. As in the NIMH Treatment of Depression Collaborative Research Program trial,13 in this study, there were indications that the results were dependent on therapist experience.16 These findings suggest that the experience of the therapist is an important factor.
A recent meta-analysis of treatments of the acute phase of MDD compared 11 RCTs of CBT and second-generation antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other medications with related mechanisms of action).17 It found that as a first-step treatment, CBT and antidepressants had a similar impact on symptom relief in patients with moderate to severe depression. Patients treated with antidepressants also had a higher risk of experiencing adverse events or discontinuing treatment because of adverse events. However, this meta-analysis included trials that had methodological shortcomings, which reduces the strength of these conclusions.
Continue to: Patients with MDD and comorbid personality disorders have been...
Patients with MDD and comorbid personality disorders have been reported to have poorer outcomes, regardless of the treatment used.18 Fournier et al19 examined the impact of antidepressants and CBT in moderately to severely depressed patients with and without a personality disorder. They found that a combination of antidepressants and CBT was suitable for patients with personality disorders because antidepressants would boost the initial response and CBT would help sustain improvement in the long term.
Presently, the APA suggests that the combination of psychotherapy and antidepressants may be used as an initial treatment for patients with moderate to severe MDD.9 As research brings to light other factors that affect treatment outcomes, these guidelines could change.
Table 110,11,15,16 summarizes the findings of select studies evaluating the use of CBT for the acute treatment of depression.

CBT’s role in long-term treatment
Recurrence and relapse are major problems associated with MDD. The large majority of individuals who experience an episode of depression go on to experience more episodes of depression,20 and the risk of recurrence increases after each successive episode.21
To reduce the risk of relapse and the return of symptoms, it is recommended that patients treated with antidepressants continue pharmacotherapy for 4 to 9 months after remission.9 Maintenance pharmacotherapy, which involves keeping patients on antidepressants beyond the point of recovery, is intended to reduce the risk of recurrence, and is standard treatment for patients with chronic or recurrent MDD.22 However, this preventive effect exists only while the patient continues to take the medication. Rates of symptom recurrence following medication withdrawal are often high regardless of how long patients have taken medications.23
Continue to: Studies examining CBT as a maintenance treatment...
Studies examining CBT as a maintenance treatment—provided alone or in combination with or sequentially with antidepressants—have found it has an enduring effect that extends beyond the end of treatment and equals the impact of continuing antidepressants.24-27 A recent meta-analysis of 10 trials where CBT had been provided to patients after acute treatment found that the risk of relapse was reduced by 21% in the first year and by 28% in the first 2 years.28
Studies have compared the prophylactic impact of maintenance CBT and antidepressants. In an early study, 40 patients who had been successfully treated with antidepressants but had residual symptoms were randomly assigned to 20 weeks of CBT or to clinical management.29 By the end of 20 weeks, patients were tapered off their antidepressant. All patients were then followed for 2 years, during which time they received no treatment. At the 2-year follow-up, the CBT group had a relapse rate of 25%, compared with 80% in the antidepressant group.29 Weaknesses of this study include a small sample size, and the fact that a single therapist provided the CBT.
This study was extended to a 6-year follow-up; antidepressants were prescribed only to patients who relapsed. The CBT group continued to have a significantly lower relapse rate (40%) compared with the antidepressant group (90%).30
In another RCT, patients with depression who had recovered with CBT or medication continued with the same treatment during a maintenance phase.26 The CBT group received 3 booster sessions during the next year and antidepressant group received medication. At the end of the second year (without CBT or medication) CBT patients were less likely to relapse compared with patients receiving antidepressants. The adjusted relapse rates were 17.3% for CBT and 53.6% for antidepressants.26
An RCT that included 452 patients with severe depression used a long intervention period (up to 42 weeks) and a flexible treatment algorithm to more closely model the strategies used in clinical practice.31 Patients were randomly assigned to antidepressants only or in combination with CBT. At the end of 12 months, outcome assessment by blinded interviewers indicated that patients with more severe depression were more likely to benefit from the combination of antidepressants and CBT (76.9% vs 60.3%) and those with severe, non-chronic depression received the most benefit (79.5% vs 62.8%). The lack of a CBT-only group limits the generalizability of these findings. Neither patients nor clinicians were blinded to the treatment assignment, which is a common limitation in psychotherapy studies but could have contributed to the finding that combined treatment was more effective.
Continue to: Some evidence suggests...
Some evidence suggests that augmenting treatment as usual (TAU) with CBT can have a resilient protective impact that also intensifies with the number of depressive episodes experienced. In an RCT, 172 patients with depression in remission were randomly assigned to TAU or to TAU augmented with CBT.32 The time to recurrence was assessed over the course of 10 years. Augmenting TAU with CBT had a significant protective impact that was greater for patients who had >3 previous episodes.32
Another long-term study assessed the longitudinal course of 158 patients who received CBT, medication, and clinical management, or medication and clinical management alone.33 Patients were followed 6 years after randomization (4.5 years after completion of CBT). Researchers found the effects of CBT in preventing relapse and recurrence persisted for several years.33
Table 224,26,29-32 summarizes the findings of select studies evaluating the use of CBT for the long-term treatment of depression.

Limitations of long-term studies
Studies that have examined the efficacy of adding CBT to antidepressants in the continuation and maintenance treatment of patients with MDD have had some limitations. The definitions of relapse and recurrence have not always been clearly delineated in all studies. This is important because recurrence rates tend to be lower, and long-term follow-up would be needed to detect multiple recurrences so that their incidence is not underestimated. In addition, the types of CBT interventions utilized has varied across studies. Some studies have employed standard interventions such as cognitive restructuring, while others have added strategies that focus on enhancing memories for positive experiences or interventions to encourage medication adherence. Despite these limitations, research has shown promising results and suggests that adding CBT to the maintenance treatment of patients with depression—with or without antidepressants—is likely to reduce the rate of relapse and recurrence.
Consider CBT for all depressed patients
Research indicates that CBT can be the preferred treatment for patients with mild to moderate MDD. Antidepressants significantly reduce depressive symptoms in patients with moderate to severe MDD. Some research suggests that CBT can be as effective as antidepressants for moderate and severe MDD. However, as the severity and chronicity of depression increase, other moderating factors need to be considered. The expertise of the CBT therapist has an impact on outcomes. Treatment protocols that utilize CBT plus antidepressants are likely to be more effective than CBT or antidepressants alone. Incorporating CBT in the acute phase of depression treatment, with or without antidepressants, can have a long-term impact. For maintenance treatment, CBT alone and CBT plus antidepressants have been found to help sustain remission.
Continue to: Bottom Line
Bottom Line
Cognitive-behavioral therapy (CBT) can be an effective treatment for patients with major depressive disorder, regardless of symptom severity. The expertise of the clinician who provides CBT has a substantial impact on outcomes. Combination treatment with CBT plus antidepressants is more likely to be effective than either treatment alone.
Related Resources
- Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5:CD010558.
Major depressive disorder (MDD) has a devastating impact on individuals and society because of its high prevalence, its recurrent nature, its frequent comorbidity with other disorders, and the functional impairment it causes. Compared with other chronic diseases, such as arthritis, asthma, and diabetes, MDD produces the greatest decrement in health worldwide.1 The goals in treating MDD should be not just to reduce symptom severity but also to achieve continuing remission and lower the risk for relapse.2
Antidepressants are the most common treatment for depression.3 Among psychotherapies used to treat MDD, cognitive-behavioral therapy (CBT) has been identified as an effective treatment.4 Collaborative care models have been reported to manage MDD more effectively.5 In this article, we review the evidence supporting the use of CBT as monotherapy and in combination with antidepressants for acute and long-term treatment of MDD.
Acute treatment: Not too soon for CBT
Mild to moderate depression
Research has indicated that for the treatment of mild MDD, antidepressants are unlikely to be more effective than placebo.6,7 Studies also have reported that response to antidepressants begins to outpace response to placebo only when symptoms are no longer mild. Using antidepressants for patients with mild depression could therefore place them at risk of overtreatment.8 In keeping with these findings, the American Psychiatric Association (APA) has recommended the use of evidence-based psychotherapies, such as CBT, as an initial treatment choice for patients with mild to moderate MDD.9
Two recent studies have suggested that the combination of CBT plus antidepressants could boost improvement in psychosocial functioning for patients with mild MDD.10,11 However, neither study included a group of patients who received only CBT to evaluate if CBT alone could have also produced similar effects. Other limitations include the lack of a control group in one study and small sample sizes in both studies. However, both studies had a long follow-up period and specifically studied the impact on psychosocial functioning.
Moderate to severe depression
Earlier depression treatment guidelines suggested that antidepressants should be used to treat more severe depression, while psychotherapy should be used mainly for mild depression.12 This recommendation was influenced by the well-known National Institute of Mental Health (NIMH) Treatment of Depression Collaborative Research Program, a multicenter randomized controlled trial (RCT) that used a placebo control.13 In this study, CBT was compared with antidepressants and found to be no more effective than placebo for more severely depressed patients.13 However, this finding was not consistent across the 3 sites where the study was conducted; at the site where CBT was provided by more experienced CBT therapists, patients with more severe depression who received CBT fared as well as patients treated with antidepressants.14 A later double-blind RCT that used experienced therapists found that CBT was as effective as antidepressants (monoamine oxidase inhibitors), and both treatments were superior to placebo in reducing symptoms of atypical depression.15
Another placebo-controlled RCT conducted at 2 sites found that CBT was as effective as antidepressants in the treatment of moderately to severely depressed patients. As in the NIMH Treatment of Depression Collaborative Research Program trial,13 in this study, there were indications that the results were dependent on therapist experience.16 These findings suggest that the experience of the therapist is an important factor.
A recent meta-analysis of treatments of the acute phase of MDD compared 11 RCTs of CBT and second-generation antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and other medications with related mechanisms of action).17 It found that as a first-step treatment, CBT and antidepressants had a similar impact on symptom relief in patients with moderate to severe depression. Patients treated with antidepressants also had a higher risk of experiencing adverse events or discontinuing treatment because of adverse events. However, this meta-analysis included trials that had methodological shortcomings, which reduces the strength of these conclusions.
Continue to: Patients with MDD and comorbid personality disorders have been...
Patients with MDD and comorbid personality disorders have been reported to have poorer outcomes, regardless of the treatment used.18 Fournier et al19 examined the impact of antidepressants and CBT in moderately to severely depressed patients with and without a personality disorder. They found that a combination of antidepressants and CBT was suitable for patients with personality disorders because antidepressants would boost the initial response and CBT would help sustain improvement in the long term.
Presently, the APA suggests that the combination of psychotherapy and antidepressants may be used as an initial treatment for patients with moderate to severe MDD.9 As research brings to light other factors that affect treatment outcomes, these guidelines could change.
Table 110,11,15,16 summarizes the findings of select studies evaluating the use of CBT for the acute treatment of depression.

CBT’s role in long-term treatment
Recurrence and relapse are major problems associated with MDD. The large majority of individuals who experience an episode of depression go on to experience more episodes of depression,20 and the risk of recurrence increases after each successive episode.21
To reduce the risk of relapse and the return of symptoms, it is recommended that patients treated with antidepressants continue pharmacotherapy for 4 to 9 months after remission.9 Maintenance pharmacotherapy, which involves keeping patients on antidepressants beyond the point of recovery, is intended to reduce the risk of recurrence, and is standard treatment for patients with chronic or recurrent MDD.22 However, this preventive effect exists only while the patient continues to take the medication. Rates of symptom recurrence following medication withdrawal are often high regardless of how long patients have taken medications.23
Continue to: Studies examining CBT as a maintenance treatment...
Studies examining CBT as a maintenance treatment—provided alone or in combination with or sequentially with antidepressants—have found it has an enduring effect that extends beyond the end of treatment and equals the impact of continuing antidepressants.24-27 A recent meta-analysis of 10 trials where CBT had been provided to patients after acute treatment found that the risk of relapse was reduced by 21% in the first year and by 28% in the first 2 years.28
Studies have compared the prophylactic impact of maintenance CBT and antidepressants. In an early study, 40 patients who had been successfully treated with antidepressants but had residual symptoms were randomly assigned to 20 weeks of CBT or to clinical management.29 By the end of 20 weeks, patients were tapered off their antidepressant. All patients were then followed for 2 years, during which time they received no treatment. At the 2-year follow-up, the CBT group had a relapse rate of 25%, compared with 80% in the antidepressant group.29 Weaknesses of this study include a small sample size, and the fact that a single therapist provided the CBT.
This study was extended to a 6-year follow-up; antidepressants were prescribed only to patients who relapsed. The CBT group continued to have a significantly lower relapse rate (40%) compared with the antidepressant group (90%).30
In another RCT, patients with depression who had recovered with CBT or medication continued with the same treatment during a maintenance phase.26 The CBT group received 3 booster sessions during the next year and antidepressant group received medication. At the end of the second year (without CBT or medication) CBT patients were less likely to relapse compared with patients receiving antidepressants. The adjusted relapse rates were 17.3% for CBT and 53.6% for antidepressants.26
An RCT that included 452 patients with severe depression used a long intervention period (up to 42 weeks) and a flexible treatment algorithm to more closely model the strategies used in clinical practice.31 Patients were randomly assigned to antidepressants only or in combination with CBT. At the end of 12 months, outcome assessment by blinded interviewers indicated that patients with more severe depression were more likely to benefit from the combination of antidepressants and CBT (76.9% vs 60.3%) and those with severe, non-chronic depression received the most benefit (79.5% vs 62.8%). The lack of a CBT-only group limits the generalizability of these findings. Neither patients nor clinicians were blinded to the treatment assignment, which is a common limitation in psychotherapy studies but could have contributed to the finding that combined treatment was more effective.
Continue to: Some evidence suggests...
Some evidence suggests that augmenting treatment as usual (TAU) with CBT can have a resilient protective impact that also intensifies with the number of depressive episodes experienced. In an RCT, 172 patients with depression in remission were randomly assigned to TAU or to TAU augmented with CBT.32 The time to recurrence was assessed over the course of 10 years. Augmenting TAU with CBT had a significant protective impact that was greater for patients who had >3 previous episodes.32
Another long-term study assessed the longitudinal course of 158 patients who received CBT, medication, and clinical management, or medication and clinical management alone.33 Patients were followed 6 years after randomization (4.5 years after completion of CBT). Researchers found the effects of CBT in preventing relapse and recurrence persisted for several years.33
Table 224,26,29-32 summarizes the findings of select studies evaluating the use of CBT for the long-term treatment of depression.

Limitations of long-term studies
Studies that have examined the efficacy of adding CBT to antidepressants in the continuation and maintenance treatment of patients with MDD have had some limitations. The definitions of relapse and recurrence have not always been clearly delineated in all studies. This is important because recurrence rates tend to be lower, and long-term follow-up would be needed to detect multiple recurrences so that their incidence is not underestimated. In addition, the types of CBT interventions utilized has varied across studies. Some studies have employed standard interventions such as cognitive restructuring, while others have added strategies that focus on enhancing memories for positive experiences or interventions to encourage medication adherence. Despite these limitations, research has shown promising results and suggests that adding CBT to the maintenance treatment of patients with depression—with or without antidepressants—is likely to reduce the rate of relapse and recurrence.
Consider CBT for all depressed patients
Research indicates that CBT can be the preferred treatment for patients with mild to moderate MDD. Antidepressants significantly reduce depressive symptoms in patients with moderate to severe MDD. Some research suggests that CBT can be as effective as antidepressants for moderate and severe MDD. However, as the severity and chronicity of depression increase, other moderating factors need to be considered. The expertise of the CBT therapist has an impact on outcomes. Treatment protocols that utilize CBT plus antidepressants are likely to be more effective than CBT or antidepressants alone. Incorporating CBT in the acute phase of depression treatment, with or without antidepressants, can have a long-term impact. For maintenance treatment, CBT alone and CBT plus antidepressants have been found to help sustain remission.
Continue to: Bottom Line
Bottom Line
Cognitive-behavioral therapy (CBT) can be an effective treatment for patients with major depressive disorder, regardless of symptom severity. The expertise of the clinician who provides CBT has a substantial impact on outcomes. Combination treatment with CBT plus antidepressants is more likely to be effective than either treatment alone.
Related Resources
- Flynn HA, Warren R. Using CBT effectively for treating depression and anxiety. Current Psychiatry. 2014;13(6):45-53.
- Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5:CD010558.
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
2. Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152-3160.
3. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
4. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
5. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am. 2005;28(4):1061-1077.
6. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
7. Paykel ES, Hollyman JA, Freeling P, et al. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo-controlled trial. J Affect Disord. 1988;14(1):83-95
8. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
9. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
10. Zhang B, Ding X, Lu W, et al. Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Arch Psychiatry. 2016;28(1):18-27.
11. Matsunaga M, Okamoto Y, Suzuki S et.al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22.
12. American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry. 1993;150(suppl 4):1-26.
13. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
14. Jacobson NS, Hollon SD. Prospects for future comparisons between drugs and psychotherapy: lessons from the CBT-versus-pharmacotherapy exchange. J Consult Clin Psychol. 1996;64(1):104-108.
15. Jarrett RB, Schaffer M, McIntire D, et al. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):431-437.
16. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
17. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019.
18. Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry. 2006;188(1):13-20.
19. Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124-129.
20. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006.
21. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233.
22. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855.
23. Thase ME. Relapse and recurrence of depression: an updated practical approach for prevention. In: Palmer KJ, ed. Drug treatment issues in depression. Auckland, New Zealand: Adis International; 2000:35-52.
24. Evans MD, Hollon, SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49(10):802-808.
25. Vittengal JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475-488.
26. Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417-422.
27. Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829-835.
28. Clarke K, Mayo-Wilson E, Kenny J, et al. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2015;39:58-70.
29. Fava GA, Rafanelli C, Grandi, S, et al. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55(9):816-820.
30. Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876.
31. Hollon SD, DeRubeis RJ, Fawcett J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157-1164.
32. Bockting CL, Smid NH, Koeter MW, et al. Enduring effects of preventive cognitive therapy in adults remitted from recurrent depression: a 10 year follow-up of a randomized controlled trial. J Affect Disord. 2015;185:188-194.
33. Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59-68.
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858.
2. Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289(23):3152-3160.
3. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
4. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol. 2001;52:685-716.
5. Oxman TE, Dietrich AJ, Schulberg HC. Evidence-based models of integrated management of depression in primary care. Psychiatr Clin North Am. 2005;28(4):1061-1077.
6. Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
7. Paykel ES, Hollyman JA, Freeling P, et al. Predictors of therapeutic benefit from amitriptyline in mild depression: a general practice placebo-controlled trial. J Affect Disord. 1988;14(1):83-95
8. Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265-1273.
9. Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
10. Zhang B, Ding X, Lu W, et al. Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Arch Psychiatry. 2016;28(1):18-27.
11. Matsunaga M, Okamoto Y, Suzuki S et.al. Psychosocial functioning in patients with treatment-resistant depression after group cognitive behavioral therapy. BMC Psychiatry. 2010;10:22.
12. American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry. 1993;150(suppl 4):1-26.
13. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
14. Jacobson NS, Hollon SD. Prospects for future comparisons between drugs and psychotherapy: lessons from the CBT-versus-pharmacotherapy exchange. J Consult Clin Psychol. 1996;64(1):104-108.
15. Jarrett RB, Schaffer M, McIntire D, et al. Treatment of atypical depression with cognitive therapy or phenelzine: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):431-437.
16. DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):409-416.
17. Amick HR, Gartlehner G, Gaynes BN, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019.
18. Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br J Psychiatry. 2006;188(1):13-20.
19. Fournier JC, DeRubeis RJ, Shelton RC, et al. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry. 2008;192(2):124-129.
20. Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000-1006.
21. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229-233.
22. Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48(9):851-855.
23. Thase ME. Relapse and recurrence of depression: an updated practical approach for prevention. In: Palmer KJ, ed. Drug treatment issues in depression. Auckland, New Zealand: Adis International; 2000:35-52.
24. Evans MD, Hollon, SD, DeRubeis RJ, et al. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49(10):802-808.
25. Vittengal JR, Clark LA, Dunn TW, et al. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75(3):475-488.
26. Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62(4):417-422.
27. Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56(9):829-835.
28. Clarke K, Mayo-Wilson E, Kenny J, et al. Can non-pharmacological interventions prevent relapse in adults who have recovered from depression? A systematic review and meta-analysis of randomised controlled trials. Clin Psychol Rev. 2015;39:58-70.
29. Fava GA, Rafanelli C, Grandi, S, et al. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55(9):816-820.
30. Fava GA, Ruini C, Rafanelli C, et al. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161(10):1872-1876.
31. Hollon SD, DeRubeis RJ, Fawcett J, et al. Effect of cognitive therapy with antidepressant medications vs antidepressants alone on the rate of recovery in major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(10):1157-1164.
32. Bockting CL, Smid NH, Koeter MW, et al. Enduring effects of preventive cognitive therapy in adults remitted from recurrent depression: a 10 year follow-up of a randomized controlled trial. J Affect Disord. 2015;185:188-194.
33. Paykel ES, Scott J, Cornwall PL, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med. 2005;35(1):59-68.
Rivaroxaban no help for heart failure outcomes
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
SOURCE: Zannad F et al. NEJM/ESC.
.
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
SOURCE: Zannad F et al. NEJM/ESC.
.
MUNICH – For patients with heart disease, coronary artery disease, and normal sinus rhythm, giving , investigators in the COMMANDER trial said.
Rivaroxaban did not improve rehospitalization rates either, reported lead author Faiez Zannad, MD, PhD, from the University of Henri Poincaré in Nancy, France, and his co-investigators.
“After an episode of worsening chronic heart failure, rates of readmission to the hospital and of death are high, especially in the first few months,” they said in a presentation at the annual congress of the European Society of Cardiology. The report of the research was published simultaneously in the New England Journal of Medicine.
Findings from previous research have suggested that, for patients with coronary artery disease, a combination of antiplatelet agents and low-dose rivaroxaban (2.5 mg twice daily) reduced incidence of death, myocardial infarction, and stroke. The authors designed the COMMANDER trial to test a similar regimen in patients with chronic heart failure and coronary heart disease without an arrhythmia. Results were published simultaneously in the New England Journal of Medicine.
The COMMANDER trial involved 5,022 patients with coronary artery disease, reduced left ventricular ejection fraction (less than or equal to 40%), worsening chronic heart failure (index event within past 21 days), and normal splasma concentration of brain natriuretic peptide (BNP) of at least 200 ng per liter or N-terminal pro-brain natriuretic peptide (NT-proBNP) of at least 800 ng per liter.
Patients were randomly assigned to receive rivaroxaban 2.5 mg twice daily (n = 2,507) or placebo (n = 2,515). Treatment was given in addition to standard care for coronary disease or heart failure (single or dual antiplatelet therapy was allowed). Patients were assessed at week 4 and week 12, then every 12 weeks.
The primary efficacy outcome was a composite of stroke, myocardial infarction, or death from any cause. Secondary efficacy outcomes included death from cardiovascular disease, rehospitalization for heart failure, a composite of either, or rehospitalization for cardiovascular events. The principal safety outcome was a composite of bleeding into a critical space with potential for permanent disability or fatal bleeding.
Death, myocardial failure, or stroke occurred in 626 patients (25%) in the rivaroxaban group compared with 658 patients (26.2%) in the placebo group (P = .27). Secondary efficacy outcomes were also highly similar between groups, differing at most by 0.9%. The principal safety outcome (fatal bleeding or bleeding into a critical space) occurred in 18 patients (0.7%) in the rivaroxaban group and 23 patients (0.9%) in the placebo group (P = .25). Again, no significant difference was found between groups.
These results suggest that while low-dose rivaroxaban may be safe, it also offers no treatment benefit. “The most likely reason for the failure … is that thrombin-mediated events are not the major driver of heart failure-related events in patients with recent hospitalization for heart failure,” the authors wrote.
“Whether a higher dose of rivaroxaban could have led to a more favorable outcome remains unknown,” they concluded.
The COMMANDER trial was funded by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
SOURCE: Zannad F et al. NEJM/ESC.
.
Key clinical point: For patients with heart failure and coronary artery disease, rivaroxaban does not significantly reduce the risk of death, myocardial infarction, or stroke.
Major finding: Death, myocardial failure, or stroke occurred in 25.0% of patients in the rivaroxaban group compared with 26.2% of patients in the placebo group (P = .27).
Study details: The COMMANDER study was a double-blind, randomized trial involving 5,022 patients. Patients had heart failure, normal sinus rhythm, and coronary artery disease.
Disclosures: Funding was provided by Janssen Research and Development. Authors reported compensation from Bayer, Servier, Novartis, Impulse Dynamics, and others.
Source: Zannad F et al. NEJM/ESC.
Innovative Devices Could Help Keep Suicidal Vets Safer
In 2014, 68% of male veterans and 41% of female veterans who committed suicide used a firearm. Research suggests that most suicidal crises pass within minutes to hours: Building in time and space between a suicidal impulse and access to a gun can be a safety valve.
The VA Challenge Team launched an innovation contest for novel and effective approaches using gun-safety mechanisms to prevent suicide, injury, and accidents. One main criterion: the device or system must allow for 100% voluntary control (implementation, suspension, decommissioning) by the veteran.
The Challenge team was looking for solutions that would be inexpensive, applicable for at-home use, not impede responsible access to the weapon, and provide education about gun safety. The winners were:
- First place—Barret Schlegelmilch, for the DuoBox, a mechanical device that provides inexpensive, secure and reliable weapon storage and encourages responsible weapon access with 2 people present.
- Second place—Timothy Oh, Christine Tate, and Jorel Lalicki, for their “open environment” biometric safe that includes fingerprint authentication and other features to enhance control of access.
- Third place—Kathleen Gilligan and Leslie Bodi, for the Sentinel, a mobile application that helps veterans connect with peers in an innovative “buddy system” so they know they are not alone in a crisis. The app also controls Bluetooth-enabled gun-lock boxes and has unique features such as a time-lock and automated emergency calling.
The winners received cash awards, as well as access to VA resources, such as subject matter experts, for any potential follow-on design and development.
In 2014, 68% of male veterans and 41% of female veterans who committed suicide used a firearm. Research suggests that most suicidal crises pass within minutes to hours: Building in time and space between a suicidal impulse and access to a gun can be a safety valve.
The VA Challenge Team launched an innovation contest for novel and effective approaches using gun-safety mechanisms to prevent suicide, injury, and accidents. One main criterion: the device or system must allow for 100% voluntary control (implementation, suspension, decommissioning) by the veteran.
The Challenge team was looking for solutions that would be inexpensive, applicable for at-home use, not impede responsible access to the weapon, and provide education about gun safety. The winners were:
- First place—Barret Schlegelmilch, for the DuoBox, a mechanical device that provides inexpensive, secure and reliable weapon storage and encourages responsible weapon access with 2 people present.
- Second place—Timothy Oh, Christine Tate, and Jorel Lalicki, for their “open environment” biometric safe that includes fingerprint authentication and other features to enhance control of access.
- Third place—Kathleen Gilligan and Leslie Bodi, for the Sentinel, a mobile application that helps veterans connect with peers in an innovative “buddy system” so they know they are not alone in a crisis. The app also controls Bluetooth-enabled gun-lock boxes and has unique features such as a time-lock and automated emergency calling.
The winners received cash awards, as well as access to VA resources, such as subject matter experts, for any potential follow-on design and development.
In 2014, 68% of male veterans and 41% of female veterans who committed suicide used a firearm. Research suggests that most suicidal crises pass within minutes to hours: Building in time and space between a suicidal impulse and access to a gun can be a safety valve.
The VA Challenge Team launched an innovation contest for novel and effective approaches using gun-safety mechanisms to prevent suicide, injury, and accidents. One main criterion: the device or system must allow for 100% voluntary control (implementation, suspension, decommissioning) by the veteran.
The Challenge team was looking for solutions that would be inexpensive, applicable for at-home use, not impede responsible access to the weapon, and provide education about gun safety. The winners were:
- First place—Barret Schlegelmilch, for the DuoBox, a mechanical device that provides inexpensive, secure and reliable weapon storage and encourages responsible weapon access with 2 people present.
- Second place—Timothy Oh, Christine Tate, and Jorel Lalicki, for their “open environment” biometric safe that includes fingerprint authentication and other features to enhance control of access.
- Third place—Kathleen Gilligan and Leslie Bodi, for the Sentinel, a mobile application that helps veterans connect with peers in an innovative “buddy system” so they know they are not alone in a crisis. The app also controls Bluetooth-enabled gun-lock boxes and has unique features such as a time-lock and automated emergency calling.
The winners received cash awards, as well as access to VA resources, such as subject matter experts, for any potential follow-on design and development.
Rivaroxaban doesn’t reduce risk of fatal VTE
MUNICH—Extended thromboprophylaxis with rivaroxaban does not significantly reduce the risk of fatal venous thromboembolism (VTE) in patients hospitalized for medical illness, according to new research.
In the MARINER trial, the combined rate of symptomatic VTE and VTE-related death was similar in patients who received placebo and those who received rivaroxaban for 45 days after hospital discharge.
Rates of VTE-related death were similar between the treatment groups, but the rate of nonfatal VTE was lower with rivaroxaban.
The researchers therefore concluded that some medically ill patients may benefit from extended thromboprophylaxis with rivaroxaban, although more research is needed.
Alex C. Spyropoulos, MD, of Northwell Health at Lenox Hill Hospital in New York, New York, presented these results at ESC Congress 2018.
The research was also published in NEJM. The study was funded by Janssen Research and Development.
The MARINER trial included 12,019 medically ill patients who had an increased risk of VTE and had been hospitalized for 3 to 10 days.
The patients were randomized to receive rivaroxaban (n=6007) at 10 mg daily (7.5 mg in patients with renal impairment) or daily placebo (n=6012) for 45 days after hospital discharge. In all, 11,962 patients (99.5%) received at least one dose of assigned treatment.
Results
The study’s primary endpoint was a composite of symptomatic VTE and VTE-related death. This endpoint was met in 0.83% (n=50) of patients in the rivaroxaban arm and 1.10% (n=66) of patients in the placebo arm (hazard ratio [HR]=0.76; P=0.136).
The incidence of VTE-related death was 0.72% (n=43) in the rivaroxaban arm and 0.77% (n=46) in the placebo arm (HR=0.93, P=0.751).
The incidence of symptomatic VTE was 0.18% (n=11) in the rivaroxaban arm and 0.42% (n=25) in the placebo arm (HR=0.44, P=0.023).
“We were able to reduce instances of non-fatal blood clots and pulmonary embolism by more than half, which shows that the use of direct oral anticoagulants . . . after the hospitalization of medically ill patients could help prevent clots from forming,” Dr. Spyropoulos said.
He and his colleagues also examined an exploratory secondary composite endpoint of symptomatic VTE and all-cause mortality and found that 1.30% (n=78) of patients in the rivaroxaban arm experienced an event, compared to 1.78% (n=107) of patients in the placebo arm (HR=0.73, P=0.033).
The study’s principal safety outcome was major bleeding. It occurred in 0.28% (n=17) of patients in the rivaroxaban arm and 0.15% (n=9) of those in the placebo arm (HR=1.88, P=0.124).
The difference in risk of major bleeding with rivaroxaban compared to placebo was 0.28 percentage points, and the difference in risk of symptomatic VTE with rivaroxaban vs placebo was -0.24 percentage points.
This suggests the number of patients needed to prevent one symptomatic VTE event is 430, and the number needed to cause one major bleed is 856.
Dr. Spyropoulos therefore concluded that thromboprophylaxis, when used in appropriate medically ill patients, might reduce the population health burden of symptomatic VTE with little serious bleeding.
“Our next course of research is to further identify and refine a post-discharge treatment program which would maximize the net clinical benefit across a defined spectrum of medically ill patients,” he said.
MUNICH—Extended thromboprophylaxis with rivaroxaban does not significantly reduce the risk of fatal venous thromboembolism (VTE) in patients hospitalized for medical illness, according to new research.
In the MARINER trial, the combined rate of symptomatic VTE and VTE-related death was similar in patients who received placebo and those who received rivaroxaban for 45 days after hospital discharge.
Rates of VTE-related death were similar between the treatment groups, but the rate of nonfatal VTE was lower with rivaroxaban.
The researchers therefore concluded that some medically ill patients may benefit from extended thromboprophylaxis with rivaroxaban, although more research is needed.
Alex C. Spyropoulos, MD, of Northwell Health at Lenox Hill Hospital in New York, New York, presented these results at ESC Congress 2018.
The research was also published in NEJM. The study was funded by Janssen Research and Development.
The MARINER trial included 12,019 medically ill patients who had an increased risk of VTE and had been hospitalized for 3 to 10 days.
The patients were randomized to receive rivaroxaban (n=6007) at 10 mg daily (7.5 mg in patients with renal impairment) or daily placebo (n=6012) for 45 days after hospital discharge. In all, 11,962 patients (99.5%) received at least one dose of assigned treatment.
Results
The study’s primary endpoint was a composite of symptomatic VTE and VTE-related death. This endpoint was met in 0.83% (n=50) of patients in the rivaroxaban arm and 1.10% (n=66) of patients in the placebo arm (hazard ratio [HR]=0.76; P=0.136).
The incidence of VTE-related death was 0.72% (n=43) in the rivaroxaban arm and 0.77% (n=46) in the placebo arm (HR=0.93, P=0.751).
The incidence of symptomatic VTE was 0.18% (n=11) in the rivaroxaban arm and 0.42% (n=25) in the placebo arm (HR=0.44, P=0.023).
“We were able to reduce instances of non-fatal blood clots and pulmonary embolism by more than half, which shows that the use of direct oral anticoagulants . . . after the hospitalization of medically ill patients could help prevent clots from forming,” Dr. Spyropoulos said.
He and his colleagues also examined an exploratory secondary composite endpoint of symptomatic VTE and all-cause mortality and found that 1.30% (n=78) of patients in the rivaroxaban arm experienced an event, compared to 1.78% (n=107) of patients in the placebo arm (HR=0.73, P=0.033).
The study’s principal safety outcome was major bleeding. It occurred in 0.28% (n=17) of patients in the rivaroxaban arm and 0.15% (n=9) of those in the placebo arm (HR=1.88, P=0.124).
The difference in risk of major bleeding with rivaroxaban compared to placebo was 0.28 percentage points, and the difference in risk of symptomatic VTE with rivaroxaban vs placebo was -0.24 percentage points.
This suggests the number of patients needed to prevent one symptomatic VTE event is 430, and the number needed to cause one major bleed is 856.
Dr. Spyropoulos therefore concluded that thromboprophylaxis, when used in appropriate medically ill patients, might reduce the population health burden of symptomatic VTE with little serious bleeding.
“Our next course of research is to further identify and refine a post-discharge treatment program which would maximize the net clinical benefit across a defined spectrum of medically ill patients,” he said.
MUNICH—Extended thromboprophylaxis with rivaroxaban does not significantly reduce the risk of fatal venous thromboembolism (VTE) in patients hospitalized for medical illness, according to new research.
In the MARINER trial, the combined rate of symptomatic VTE and VTE-related death was similar in patients who received placebo and those who received rivaroxaban for 45 days after hospital discharge.
Rates of VTE-related death were similar between the treatment groups, but the rate of nonfatal VTE was lower with rivaroxaban.
The researchers therefore concluded that some medically ill patients may benefit from extended thromboprophylaxis with rivaroxaban, although more research is needed.
Alex C. Spyropoulos, MD, of Northwell Health at Lenox Hill Hospital in New York, New York, presented these results at ESC Congress 2018.
The research was also published in NEJM. The study was funded by Janssen Research and Development.
The MARINER trial included 12,019 medically ill patients who had an increased risk of VTE and had been hospitalized for 3 to 10 days.
The patients were randomized to receive rivaroxaban (n=6007) at 10 mg daily (7.5 mg in patients with renal impairment) or daily placebo (n=6012) for 45 days after hospital discharge. In all, 11,962 patients (99.5%) received at least one dose of assigned treatment.
Results
The study’s primary endpoint was a composite of symptomatic VTE and VTE-related death. This endpoint was met in 0.83% (n=50) of patients in the rivaroxaban arm and 1.10% (n=66) of patients in the placebo arm (hazard ratio [HR]=0.76; P=0.136).
The incidence of VTE-related death was 0.72% (n=43) in the rivaroxaban arm and 0.77% (n=46) in the placebo arm (HR=0.93, P=0.751).
The incidence of symptomatic VTE was 0.18% (n=11) in the rivaroxaban arm and 0.42% (n=25) in the placebo arm (HR=0.44, P=0.023).
“We were able to reduce instances of non-fatal blood clots and pulmonary embolism by more than half, which shows that the use of direct oral anticoagulants . . . after the hospitalization of medically ill patients could help prevent clots from forming,” Dr. Spyropoulos said.
He and his colleagues also examined an exploratory secondary composite endpoint of symptomatic VTE and all-cause mortality and found that 1.30% (n=78) of patients in the rivaroxaban arm experienced an event, compared to 1.78% (n=107) of patients in the placebo arm (HR=0.73, P=0.033).
The study’s principal safety outcome was major bleeding. It occurred in 0.28% (n=17) of patients in the rivaroxaban arm and 0.15% (n=9) of those in the placebo arm (HR=1.88, P=0.124).
The difference in risk of major bleeding with rivaroxaban compared to placebo was 0.28 percentage points, and the difference in risk of symptomatic VTE with rivaroxaban vs placebo was -0.24 percentage points.
This suggests the number of patients needed to prevent one symptomatic VTE event is 430, and the number needed to cause one major bleed is 856.
Dr. Spyropoulos therefore concluded that thromboprophylaxis, when used in appropriate medically ill patients, might reduce the population health burden of symptomatic VTE with little serious bleeding.
“Our next course of research is to further identify and refine a post-discharge treatment program which would maximize the net clinical benefit across a defined spectrum of medically ill patients,” he said.
AAP cautions against marijuana use during pregnancy, breastfeeding
, according to a recent clinical report published in the journal Pediatrics.
“The fact that marijuana is legal in many states may give the impression the drug is harmless during pregnancy, especially with stories swirling on social media about using it for nausea with morning sickness,” Sheryl A. Ryan, MD, FAAP, Chair of the American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention, stated in a press release. “But in fact, this is still a big question. We do not have good safety data on prenatal exposure to marijuana. Based on the limited data that do exist, as pediatricians, we believe there is cause to be concerned about how the drug will impact the long-term development of children.”
The rate of marijuana use is increasing among pregnant women 18 years to 44 years old is increasing, the committee said, with 3.84% of women in 2014 within that age range using marijuana within the past month compared with 2.37% in 2002. Among women who were between 18 years and 25 years old, the rate of marijuana use within the past month was 7.47% in 2014.
The committee also noted research has shown cannabidiol exposure in the short term may impact placental permeability to “pharmacologic agents and recreational substances, potentially placing the fetus at risk from these agents or drugs.” A more well-known substance in marijuana, delta-9-tetrahydrocannabinol (THC) crosses the placental barrier and can appear in fetal blood. Studies have reported any level of marijuana use among pregnant women put the mothers at risk of anemia, while their newborns had an increased risk of low-birth weight and neonatal intensive care unit (NICU) use. Further research has shown impaired mental development, executive function deficits, increased impulsivity and hyperactivity, behavioral problems, depressive symptoms, and greater rates of substance abuse among children exposed to marijuana.
“Many of these effects may not show up right away, but they can impact how well a child can maneuver in the world,” Dr. Ryan stated in the release. “Children’s and teens’ cognitive ability to manage their time and school work might be harmed down the line from marijuana use during their mother’s pregnancy.”
In a related study, Kerri A. Bertrand, MPH, from the department of pediatrics at the University of California in San Diego, Calif., and her colleagues studied cannabinoid concentrations in breastmilk donated to a human milk biorepository. The investigators analyzed 54 samples donated by 50 women who used marijuana while breastfeeding between 2014 and 2017 and determined whether substances such as delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), cannabidiol, and cannabinol were present in breastmilk by performing liquid chromatography mass spectrometry electrospray ionization on the samples.
They found 34 of 54 samples (64%) had detectable delta-9-THC approximately 6 days after marijuana use (median concentration, 9.47 ng/mL; range, 1.01-323 ng/mL), while 5 of 54 samples (9%) had measurable concentrations of 11-OH-THC (range, 1.33-12.80 ng/mL) and 5 of 54 samples (9%) contained measurable cannabidiol (range, 1.32-8.56 ng/mL). Predictors of log delta-9-THC concentrations included number of hours since last use (-0.03; 95% confidence interval, -0.04 to -0.01; P equals .005), the number of times per day marijuana was used (0.51; 95% CI, 0.03-0.99; P equals .039), and the amount of time between sample donation and analysis (0.08; 95% CI, 0.00-0.15; P equals .038), researchers said.
“Because marijuana is the most commonly used recreational drug among breastfeeding women, information regarding risks to breastfeeding infants is urgently needed,” Dr. Bertrand and colleagues wrote in their study.
The authors of the AAP clinical report acknowledge no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
SOURCE: Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
The study by Bertrand and colleagues should be commended for being among the first to analyze cannabinoids in breast milk, but there are still very important questions to be answered about marijuana use among women who breast-feed, Sheryl A. Ryan, MD, FAAP, wrote in a related editorial.
Questions remain about why one-third of participants in the study had no detectable cannabinoids in their breast milk, and a frame of reference is needed for the levels that did appear in the study, Dr. Ryan said. Data are also needed on how the cannabinoids “accumulate in the infant, how the infant metabolizes these substances, how quickly they are excreted, whether they accumulate, and thus how long these metabolites remain in the infant,” she said.
Dr. Ryan also questioned what to tell mothers who use marijuana but want to breastfeed their newborns, and noted guidelines from the AAP and the American College of Obstetricians currently recommend avoiding marijuana use entirely while breastfeeding.
“With their study, Bertrand et al. have provided additional and valuable support for those current recommendations. But the picture is incomplete without our understanding of what is happening at the level of those infants exposed to cannabinoid–containing breast milk,” Dr. Ryan said. “Hopefully, the calls for research to answer these important questions will not go unheeded.”
Dr. Ryan is from the Division of Adolescent Medicine and Department of Pediatrics at Penn State Health Children’s Hospital in Hershey, Penn. These comments summarize her editorial in response to Bertrand and colleagues. She reports no relevant conflicts of interest (Ryan SA. Pediatrics. 2018;142[3]:e20181921).
The study by Bertrand and colleagues should be commended for being among the first to analyze cannabinoids in breast milk, but there are still very important questions to be answered about marijuana use among women who breast-feed, Sheryl A. Ryan, MD, FAAP, wrote in a related editorial.
Questions remain about why one-third of participants in the study had no detectable cannabinoids in their breast milk, and a frame of reference is needed for the levels that did appear in the study, Dr. Ryan said. Data are also needed on how the cannabinoids “accumulate in the infant, how the infant metabolizes these substances, how quickly they are excreted, whether they accumulate, and thus how long these metabolites remain in the infant,” she said.
Dr. Ryan also questioned what to tell mothers who use marijuana but want to breastfeed their newborns, and noted guidelines from the AAP and the American College of Obstetricians currently recommend avoiding marijuana use entirely while breastfeeding.
“With their study, Bertrand et al. have provided additional and valuable support for those current recommendations. But the picture is incomplete without our understanding of what is happening at the level of those infants exposed to cannabinoid–containing breast milk,” Dr. Ryan said. “Hopefully, the calls for research to answer these important questions will not go unheeded.”
Dr. Ryan is from the Division of Adolescent Medicine and Department of Pediatrics at Penn State Health Children’s Hospital in Hershey, Penn. These comments summarize her editorial in response to Bertrand and colleagues. She reports no relevant conflicts of interest (Ryan SA. Pediatrics. 2018;142[3]:e20181921).
The study by Bertrand and colleagues should be commended for being among the first to analyze cannabinoids in breast milk, but there are still very important questions to be answered about marijuana use among women who breast-feed, Sheryl A. Ryan, MD, FAAP, wrote in a related editorial.
Questions remain about why one-third of participants in the study had no detectable cannabinoids in their breast milk, and a frame of reference is needed for the levels that did appear in the study, Dr. Ryan said. Data are also needed on how the cannabinoids “accumulate in the infant, how the infant metabolizes these substances, how quickly they are excreted, whether they accumulate, and thus how long these metabolites remain in the infant,” she said.
Dr. Ryan also questioned what to tell mothers who use marijuana but want to breastfeed their newborns, and noted guidelines from the AAP and the American College of Obstetricians currently recommend avoiding marijuana use entirely while breastfeeding.
“With their study, Bertrand et al. have provided additional and valuable support for those current recommendations. But the picture is incomplete without our understanding of what is happening at the level of those infants exposed to cannabinoid–containing breast milk,” Dr. Ryan said. “Hopefully, the calls for research to answer these important questions will not go unheeded.”
Dr. Ryan is from the Division of Adolescent Medicine and Department of Pediatrics at Penn State Health Children’s Hospital in Hershey, Penn. These comments summarize her editorial in response to Bertrand and colleagues. She reports no relevant conflicts of interest (Ryan SA. Pediatrics. 2018;142[3]:e20181921).
, according to a recent clinical report published in the journal Pediatrics.
“The fact that marijuana is legal in many states may give the impression the drug is harmless during pregnancy, especially with stories swirling on social media about using it for nausea with morning sickness,” Sheryl A. Ryan, MD, FAAP, Chair of the American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention, stated in a press release. “But in fact, this is still a big question. We do not have good safety data on prenatal exposure to marijuana. Based on the limited data that do exist, as pediatricians, we believe there is cause to be concerned about how the drug will impact the long-term development of children.”
The rate of marijuana use is increasing among pregnant women 18 years to 44 years old is increasing, the committee said, with 3.84% of women in 2014 within that age range using marijuana within the past month compared with 2.37% in 2002. Among women who were between 18 years and 25 years old, the rate of marijuana use within the past month was 7.47% in 2014.
The committee also noted research has shown cannabidiol exposure in the short term may impact placental permeability to “pharmacologic agents and recreational substances, potentially placing the fetus at risk from these agents or drugs.” A more well-known substance in marijuana, delta-9-tetrahydrocannabinol (THC) crosses the placental barrier and can appear in fetal blood. Studies have reported any level of marijuana use among pregnant women put the mothers at risk of anemia, while their newborns had an increased risk of low-birth weight and neonatal intensive care unit (NICU) use. Further research has shown impaired mental development, executive function deficits, increased impulsivity and hyperactivity, behavioral problems, depressive symptoms, and greater rates of substance abuse among children exposed to marijuana.
“Many of these effects may not show up right away, but they can impact how well a child can maneuver in the world,” Dr. Ryan stated in the release. “Children’s and teens’ cognitive ability to manage their time and school work might be harmed down the line from marijuana use during their mother’s pregnancy.”
In a related study, Kerri A. Bertrand, MPH, from the department of pediatrics at the University of California in San Diego, Calif., and her colleagues studied cannabinoid concentrations in breastmilk donated to a human milk biorepository. The investigators analyzed 54 samples donated by 50 women who used marijuana while breastfeeding between 2014 and 2017 and determined whether substances such as delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), cannabidiol, and cannabinol were present in breastmilk by performing liquid chromatography mass spectrometry electrospray ionization on the samples.
They found 34 of 54 samples (64%) had detectable delta-9-THC approximately 6 days after marijuana use (median concentration, 9.47 ng/mL; range, 1.01-323 ng/mL), while 5 of 54 samples (9%) had measurable concentrations of 11-OH-THC (range, 1.33-12.80 ng/mL) and 5 of 54 samples (9%) contained measurable cannabidiol (range, 1.32-8.56 ng/mL). Predictors of log delta-9-THC concentrations included number of hours since last use (-0.03; 95% confidence interval, -0.04 to -0.01; P equals .005), the number of times per day marijuana was used (0.51; 95% CI, 0.03-0.99; P equals .039), and the amount of time between sample donation and analysis (0.08; 95% CI, 0.00-0.15; P equals .038), researchers said.
“Because marijuana is the most commonly used recreational drug among breastfeeding women, information regarding risks to breastfeeding infants is urgently needed,” Dr. Bertrand and colleagues wrote in their study.
The authors of the AAP clinical report acknowledge no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
SOURCE: Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
, according to a recent clinical report published in the journal Pediatrics.
“The fact that marijuana is legal in many states may give the impression the drug is harmless during pregnancy, especially with stories swirling on social media about using it for nausea with morning sickness,” Sheryl A. Ryan, MD, FAAP, Chair of the American Academy of Pediatrics (AAP) Committee on Substance Use and Prevention, stated in a press release. “But in fact, this is still a big question. We do not have good safety data on prenatal exposure to marijuana. Based on the limited data that do exist, as pediatricians, we believe there is cause to be concerned about how the drug will impact the long-term development of children.”
The rate of marijuana use is increasing among pregnant women 18 years to 44 years old is increasing, the committee said, with 3.84% of women in 2014 within that age range using marijuana within the past month compared with 2.37% in 2002. Among women who were between 18 years and 25 years old, the rate of marijuana use within the past month was 7.47% in 2014.
The committee also noted research has shown cannabidiol exposure in the short term may impact placental permeability to “pharmacologic agents and recreational substances, potentially placing the fetus at risk from these agents or drugs.” A more well-known substance in marijuana, delta-9-tetrahydrocannabinol (THC) crosses the placental barrier and can appear in fetal blood. Studies have reported any level of marijuana use among pregnant women put the mothers at risk of anemia, while their newborns had an increased risk of low-birth weight and neonatal intensive care unit (NICU) use. Further research has shown impaired mental development, executive function deficits, increased impulsivity and hyperactivity, behavioral problems, depressive symptoms, and greater rates of substance abuse among children exposed to marijuana.
“Many of these effects may not show up right away, but they can impact how well a child can maneuver in the world,” Dr. Ryan stated in the release. “Children’s and teens’ cognitive ability to manage their time and school work might be harmed down the line from marijuana use during their mother’s pregnancy.”
In a related study, Kerri A. Bertrand, MPH, from the department of pediatrics at the University of California in San Diego, Calif., and her colleagues studied cannabinoid concentrations in breastmilk donated to a human milk biorepository. The investigators analyzed 54 samples donated by 50 women who used marijuana while breastfeeding between 2014 and 2017 and determined whether substances such as delta-9-THC, 11-hydroxy-delta-9-tetrahydrocannabinol (11-OH-THC), cannabidiol, and cannabinol were present in breastmilk by performing liquid chromatography mass spectrometry electrospray ionization on the samples.
They found 34 of 54 samples (64%) had detectable delta-9-THC approximately 6 days after marijuana use (median concentration, 9.47 ng/mL; range, 1.01-323 ng/mL), while 5 of 54 samples (9%) had measurable concentrations of 11-OH-THC (range, 1.33-12.80 ng/mL) and 5 of 54 samples (9%) contained measurable cannabidiol (range, 1.32-8.56 ng/mL). Predictors of log delta-9-THC concentrations included number of hours since last use (-0.03; 95% confidence interval, -0.04 to -0.01; P equals .005), the number of times per day marijuana was used (0.51; 95% CI, 0.03-0.99; P equals .039), and the amount of time between sample donation and analysis (0.08; 95% CI, 0.00-0.15; P equals .038), researchers said.
“Because marijuana is the most commonly used recreational drug among breastfeeding women, information regarding risks to breastfeeding infants is urgently needed,” Dr. Bertrand and colleagues wrote in their study.
The authors of the AAP clinical report acknowledge no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
SOURCE: Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
PEDIATRICS
Key clinical point: More studies are needed to analyze the long-term effects marijuana has on mother and child during pregnancy and while breastfeeding.
Major finding: Of women between 18 years and 44 years old, 3.84% used marijuana during pregnancy in 2014 compared with 2.37% in 2002; 64% of samples in Bertrand and colleagues’ study had THC traceable in breastmilk approximately 6 days after marijuana use.
Study details:A clinical report on marijuana use during pregnancy and while breastfeeding, and a study of 50 women who used marijuana while breastfeeding and donated samples to a human milk biorepository.
Disclosures:The authors of the AAP clinical report no relevant conflicts of interest. The study by Bertrand and colleagues was supported by the University of California San Diego Center for Better Beginnings, a grant from the National Institutes of Health, and the Gerber Foundation.
Source: Ryan SA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1889. Bertrand KA et al. Pediatrics. 2018 Aug 27;doi:10.1542/peds.2018-1076.
Immunotherapy-related adverse effects: how to identify and treat them in the emergency department
DR HENRY I am pleased to be talking with Dr Maura Sammon, an emergency department (ED) physician, about identifying and treating immunotherapy-related side effects in the ED. This is a hot topic in oncology, and I was very interested in having an ED physician talk about what happens when treating oncologists send their patients to the ED, because a physician may think it is chemotherapy when it is immunotherapy. Let’s start with the example of an oncology patient going to the ED with some symptoms, and the ED physician asks the patient what they’re being treated with. The patient may or may not say the right thing – that is, inform you whether they are being treated with chemotherapy or immunotherapy. How do you morph over into knowing that they are not getting chemotherapy?
DR SAMMON Yes, that’s a big problem in the ED. Patients come to the ED and say they’re being treated for cancer. They say they’re on chemotherapy, when they’re actually on immunotherapy, and it can really send the treatment team down the wrong path. I have a metaphor to explain this. They say that Great Britain and the United States are two nations separated by a common language. For example, when a British person talks about football, they mean something very different than when an American talks about football. If someone in Great Britain asks you to come play football, you might show up with shoulder pads and a helmet rather than shin guards, and you’re left without having the right tools to participate in the game.
How this sometimes plays out with immunotherapy, unfortunately, is that a patient will present to the ED and say they’re having a cough and that they’re on chemotherapy for melanoma. Usually, this patient would be worked up for being in a potentially immunosuppressed state. You might get a white blood cell count. You might get a chest X-ray. You might see what looks to you like a new infiltrate on this chest X-ray and then start going down the path of treating someone whom you think is immunosuppressed with pneumonia and giving them antibiotics rather than what could be life-saving steroids, as would be the case if the patient were on immunotherapy.
It’s a real problem, because you have one word that patients may use meaning two very different things. It can get you into trouble if you are treating someone for potentially infectious causes rather than immunotherapy-related adverse reactions, which are much more similar to graft-versus-host disease than in the case of traditional chemotherapy.
DR HENRY That’s a very good point. I think, as we in oncology use these immunotherapies/checkpoint inhibitors more often, you will see them more often in the ED. Let’s get right into that. You’ve identified this patient as not getting a traditional chemotherapy – hopefully, all our records on these patients are available. You’ve decided to follow onto what might be a side effect of the immunotherapy, so I’m going to name the side effects that always occur to me: lung, gastrointestinal (GI) – which could be loose bowels or liver function – rash, endocrine problems. Let’s start with lung symptoms. You see the patient is short of breath and you identified immunotherapy. What’s your next step?
DR SAMMON That’s a great example, because the problem is that you see these patients with cough or shortness of breath and pulmonary complications, and pulmonary complications of immunotherapy, while rare, are potentially life threatening if they’re not identified quickly.
You can start with a chest X-ray on these patients knowing, however, that for a good percentage of them you won’t see findings on their chest X-ray (Figure 1, from Sammon M, Tobin T. Identification and management of immune-related adverse events in the emergency setting. Presented at: Advances in Cancer Immunotherapy – Society for Immunotherapy of Cancer (SITC); August 4, 2017; Philadelphia, PA). You need to proceed to computed tomography (CT), because the issue is that you can have protean findings on the CT related to immunotherapy treatment/adverse reactions. You need to have a very high index of suspicion regardless of what abnormal findings you’re seeing on this CT and erring toward withholding the drugs, starting treatment, and being more aggressive with this type of finding.
DR HENRY I’ve heard you talk at a conference about a patient with metastatic lung cancer, or some other tumor that may have existing disease in lung. The patient is aware of that, and the chart reflects that. Then you have this difficulty where the CT scan shows a pneumonitis, and it may not be tumor progression at all – it may be the drug. How do you work through that? Of course, your additional problem is you don’t have a whole lot of time. You must decide to whether you’re going to keep them in the ED, admit them, or send them home.
DR SAMMON Right. One of the first things is to get the oncologist involved at an early stage in treating this. We are all a team. We are all working together, and it’s very important to have that communication occur very early. I’m going to err on admitting those folks who have had symptoms and who have had findings on a chest CT, because they can progress. They can get much worse. I’m going to be getting the oncologist involved very early. We’re going to have the conversation about whether we should be starting steroids on them in the ED, getting them upstairs, and being aggressive in treating this.
DR HENRY So, time and therapy are very important.
DR SAMMON Yes.
DR HENRY You’ll get that steroid started as an antidote right away.
DR SAMMON Absolutely. For grade 2, you’re going to use methylprednisolone, 1 mg /kg daily. For anything higher than that – grade 3 or grade 4 – we’re going to start higher. We’re going to start at 2-4 mg/kg a day and get the patient upstairs and taper them slowly.
DR HENRY That’s worth empathizing. I, sadly, had a patient who ultimately did well, but had severe grade 4 pneumonitis. T
DR SAMMON Absolutely, but it’s very important to work together as a team to make these patients have good outcomes.
DR HENRY Agree. Let’s change over to the GI symptoms. The patient comes in and misidentifies him- or herself as having chemotherapy and diarrhea. We are used to causing nausea, vomiting, and diarrhea with some of our therapies. You realize, in talking to the oncologist, the patient is taking a checkpoint inhibitor. How would you approach the patient with diarrhea?
DR SAMMON First, I would talk to the patient. I would try to establish the baseline number of stools per day, because it’s not defined as a definite number of stools per day, it’s the number of stools above their baseline per day. If they’re having fewer than 4 stools above their baseline per day, I would send off some tests. We can send off a C diff (Clostridium difficile) test, we can send off a stool culture – all the parasites. Make sure the oncologist is going to be able to get these results and get them followed up, because these are results I’m not going to get back myself in the ED.
Then I’m going to talk to the patient about symptomatic treatment. I’m going to talk to them about oral hydration, a bland diet. Avoid using loperamide or any other antidiarrheal medicines, because that could decrease the frequency of stools but mask more severe symptoms that they may be having.
If I have a patient who is having more than 4 stools above their daily baseline and it’s been happening 4 to 6 stools a day for more than a week, I’m going to be sending those studies off, and I’m going to be having a conversation with the treating oncologist to find out if they want me to start the patient on steroids immediately, or if they want to wait for the test results to come back and have the steroids started as an outpatient.
These moderate patients can maybe wait a day until these test results come back. Those who are having more than 7 stools above baseline per day, peritoneal signs, ileus, or fever, are the patients you should worry about. You need to admit them for IV hydration. You need to do the stools, so you might need to keep them in the hospital until you find the results of the stool studies. You need to rule out perforation.
You may be starting steroids on these patients sooner rather than later. They’re going to be getting systemic corticosteroids at about 1-2 mg/kg of prednisone equivalent, assuming there is no perforation and their stool studies are negative. If they are unstable, though, they are really going to need high-dose corticosteroids. They are going to need methylprednisolone, 125 mg IV, to evaluate for their responsiveness. These folks really need to be treated as inpatients, and they need to have their oncologists involved early on with their treatment.
DR HENRY Yes. I couldn’t agree with you more. When I talk to the diarrhea side effect patients that we see, I tend to think it’s a curse. It’s volume. It’s calories. It’s electrolytes. The number of stools you’re mentioning, it is almost certainly going to need admission to rule out other causes. Then, if it’s the checkpoint inhibitor, the steroid antidotes.
Let’s move on to the rash. This is another organ system that can be affected by immunotherapy. What is your approach when you see a generalized body rash in a patient on one of these drugs who is sent to the ED?
DR SAMMON I am obviously going to be ruling out other causes first, but generally you’re going to see a maculopapular rash. It may be itchy. It may be burning. Patients will often describe it as just sort of having a tight sensation. I’m going to be looking at them a little bit like I look at a burn patient. What is their total body surface area that’s involved? If they’ve got less than 30% of their total body surface area involved, that’s considered a mild reaction.
For those folks, I’m not going to use systemic steroids, but I can give them some topical steroids, and I can give them some Benadryl, some diphenhydramine, and really treat them symptomatically as well as ensuring that they have early follow-up to make sure this isn’t progressing. Once we get between 30% and 50% body area, we’re talking about a moderate toxicity. If these patients are not improving rapidly with just withholding the drug, they need systemic corticosteroids.
We usually treat them at about 0.5-1 mg/kg body weight a day of prednisone equivalent. Just as with burn patients, these patients’ symptoms can become very severe. You can see signs of blistering, dermal ulceration, necrotic, bolus, hemorrhagic lesions (Figure 2, A-D).1 These folks can have very difficult-to-manage fluid balances, and they’re at very high risk for skin infections as well. They need to be treated as inpatients. If possible, you might want to consider sending these patients to a burn unit. They need systemic corticosteroids, 1-2 mg/kg per day, and they need careful monitoring for signs of dehydration, electrolytic abnormalities, and/or skin infections. They need excellent wound care.
DR HENRY That’s very well put and always difficult, because there are so many causes of rash. That takes me to an area that has always been difficult for me, which is therapy-related endocrine problems. It’s interesting to note that these drugs can cause endocrine problems. I’ve heard you speak about the pituitary affecting vision, as well as thyroid or adrenal issues. Let’s start with how you’d approach vision difficulty in a patient on these drugs.
DR SAMMON The endocrinopathies that you can get with these checkpoint inhibitors really have a myriad of symptoms. Your patient may present saying that they’re feeling tired, that they’re feeling weak, or they may have a headache. If your patient is having actual pituitary enlargement, they can present with headaches, visual field defects, or cranial nerve defects. The reason for those symptoms is that the pituitary sits in the cavernous sinus, and you have various cranial nerves passing through that area as well as the optic chiasm just above the pituitary gland (Figure 3).1 Your patient may present with a bitemporal hemianopia. Or with diplopia. You are going to want to very quickly get either a CT scan or an MRI to find out if that is what’s going on (Figure 4).1 These folks need to be treated aggressively as well.
DR HENRY You’ll get your CT scan or your MRI and rule out an enlargement or a change to the visual field. I haven’t seen this yet, but certainly exciting when you see it to treat it. Would you get the radialis brevis involved, steroids involved? How would you manage that?
DR SAMMON It’s interesting, because you do want to use corticosteroids. One of the questions here is, which corticosteroid do you want to use? If you’re talking about someone who may have adrenal insufficiency, you may want to be able to do a stimulation test. In these patients, you may want to choose using dexamethasone, because you can still do the corticotropin stimulation test. However, if your patient is in frank shock because of what you think is an adrenal crisis, you’re going to want to use hydrocortisone. If a patient is truly hypotensive and unstable, the testing is at that point less important than the treatment.
DR HENRY Very interesting. We have covered what I would consider the major aspects of these fascinating drugs. We haven’t covered all of what they do when they work well, which hopefully we’re seeing more and more often, but we have covered very well what can happen when things go wrong in side effects. Anything else that you would like to add from the ED perspective or other side effects worth mentioning?
DR SAMMON The thing that I would most like to share with the oncology office is the importance of communicating with your patients that, when they’re on these drugs, they need to tell emergency physicians that they’re on immunotherapy, not chemotherapy. It might be helpful to give these patients a card stating that they’re on immunotherapy, not chemotherapy, and outlining some of the side effects that ED physicians should be looking out for in these patients.
DR HENRY That’s a great point. I’ve seen that some of the manufacturers have little cheat cards that the patient can carry naming the drug and the side effects, because not all ED doctors are aware of the side effects of these drugs.
DR SAMMON Absolutely. We love those cards.
DR HENRY Yes. I’ve also given some to the ED doctors at Pennsylvania Hospital, and they love it. I think we’ve covered everything in quite a bit of detail. Thank you, Dr Sammon, for sharing this information from the frontlines of the ED.
DR HENRY I am pleased to be talking with Dr Maura Sammon, an emergency department (ED) physician, about identifying and treating immunotherapy-related side effects in the ED. This is a hot topic in oncology, and I was very interested in having an ED physician talk about what happens when treating oncologists send their patients to the ED, because a physician may think it is chemotherapy when it is immunotherapy. Let’s start with the example of an oncology patient going to the ED with some symptoms, and the ED physician asks the patient what they’re being treated with. The patient may or may not say the right thing – that is, inform you whether they are being treated with chemotherapy or immunotherapy. How do you morph over into knowing that they are not getting chemotherapy?
DR SAMMON Yes, that’s a big problem in the ED. Patients come to the ED and say they’re being treated for cancer. They say they’re on chemotherapy, when they’re actually on immunotherapy, and it can really send the treatment team down the wrong path. I have a metaphor to explain this. They say that Great Britain and the United States are two nations separated by a common language. For example, when a British person talks about football, they mean something very different than when an American talks about football. If someone in Great Britain asks you to come play football, you might show up with shoulder pads and a helmet rather than shin guards, and you’re left without having the right tools to participate in the game.
How this sometimes plays out with immunotherapy, unfortunately, is that a patient will present to the ED and say they’re having a cough and that they’re on chemotherapy for melanoma. Usually, this patient would be worked up for being in a potentially immunosuppressed state. You might get a white blood cell count. You might get a chest X-ray. You might see what looks to you like a new infiltrate on this chest X-ray and then start going down the path of treating someone whom you think is immunosuppressed with pneumonia and giving them antibiotics rather than what could be life-saving steroids, as would be the case if the patient were on immunotherapy.
It’s a real problem, because you have one word that patients may use meaning two very different things. It can get you into trouble if you are treating someone for potentially infectious causes rather than immunotherapy-related adverse reactions, which are much more similar to graft-versus-host disease than in the case of traditional chemotherapy.
DR HENRY That’s a very good point. I think, as we in oncology use these immunotherapies/checkpoint inhibitors more often, you will see them more often in the ED. Let’s get right into that. You’ve identified this patient as not getting a traditional chemotherapy – hopefully, all our records on these patients are available. You’ve decided to follow onto what might be a side effect of the immunotherapy, so I’m going to name the side effects that always occur to me: lung, gastrointestinal (GI) – which could be loose bowels or liver function – rash, endocrine problems. Let’s start with lung symptoms. You see the patient is short of breath and you identified immunotherapy. What’s your next step?
DR SAMMON That’s a great example, because the problem is that you see these patients with cough or shortness of breath and pulmonary complications, and pulmonary complications of immunotherapy, while rare, are potentially life threatening if they’re not identified quickly.
You can start with a chest X-ray on these patients knowing, however, that for a good percentage of them you won’t see findings on their chest X-ray (Figure 1, from Sammon M, Tobin T. Identification and management of immune-related adverse events in the emergency setting. Presented at: Advances in Cancer Immunotherapy – Society for Immunotherapy of Cancer (SITC); August 4, 2017; Philadelphia, PA). You need to proceed to computed tomography (CT), because the issue is that you can have protean findings on the CT related to immunotherapy treatment/adverse reactions. You need to have a very high index of suspicion regardless of what abnormal findings you’re seeing on this CT and erring toward withholding the drugs, starting treatment, and being more aggressive with this type of finding.
DR HENRY I’ve heard you talk at a conference about a patient with metastatic lung cancer, or some other tumor that may have existing disease in lung. The patient is aware of that, and the chart reflects that. Then you have this difficulty where the CT scan shows a pneumonitis, and it may not be tumor progression at all – it may be the drug. How do you work through that? Of course, your additional problem is you don’t have a whole lot of time. You must decide to whether you’re going to keep them in the ED, admit them, or send them home.
DR SAMMON Right. One of the first things is to get the oncologist involved at an early stage in treating this. We are all a team. We are all working together, and it’s very important to have that communication occur very early. I’m going to err on admitting those folks who have had symptoms and who have had findings on a chest CT, because they can progress. They can get much worse. I’m going to be getting the oncologist involved very early. We’re going to have the conversation about whether we should be starting steroids on them in the ED, getting them upstairs, and being aggressive in treating this.
DR HENRY So, time and therapy are very important.
DR SAMMON Yes.
DR HENRY You’ll get that steroid started as an antidote right away.
DR SAMMON Absolutely. For grade 2, you’re going to use methylprednisolone, 1 mg /kg daily. For anything higher than that – grade 3 or grade 4 – we’re going to start higher. We’re going to start at 2-4 mg/kg a day and get the patient upstairs and taper them slowly.
DR HENRY That’s worth empathizing. I, sadly, had a patient who ultimately did well, but had severe grade 4 pneumonitis. T
DR SAMMON Absolutely, but it’s very important to work together as a team to make these patients have good outcomes.
DR HENRY Agree. Let’s change over to the GI symptoms. The patient comes in and misidentifies him- or herself as having chemotherapy and diarrhea. We are used to causing nausea, vomiting, and diarrhea with some of our therapies. You realize, in talking to the oncologist, the patient is taking a checkpoint inhibitor. How would you approach the patient with diarrhea?
DR SAMMON First, I would talk to the patient. I would try to establish the baseline number of stools per day, because it’s not defined as a definite number of stools per day, it’s the number of stools above their baseline per day. If they’re having fewer than 4 stools above their baseline per day, I would send off some tests. We can send off a C diff (Clostridium difficile) test, we can send off a stool culture – all the parasites. Make sure the oncologist is going to be able to get these results and get them followed up, because these are results I’m not going to get back myself in the ED.
Then I’m going to talk to the patient about symptomatic treatment. I’m going to talk to them about oral hydration, a bland diet. Avoid using loperamide or any other antidiarrheal medicines, because that could decrease the frequency of stools but mask more severe symptoms that they may be having.
If I have a patient who is having more than 4 stools above their daily baseline and it’s been happening 4 to 6 stools a day for more than a week, I’m going to be sending those studies off, and I’m going to be having a conversation with the treating oncologist to find out if they want me to start the patient on steroids immediately, or if they want to wait for the test results to come back and have the steroids started as an outpatient.
These moderate patients can maybe wait a day until these test results come back. Those who are having more than 7 stools above baseline per day, peritoneal signs, ileus, or fever, are the patients you should worry about. You need to admit them for IV hydration. You need to do the stools, so you might need to keep them in the hospital until you find the results of the stool studies. You need to rule out perforation.
You may be starting steroids on these patients sooner rather than later. They’re going to be getting systemic corticosteroids at about 1-2 mg/kg of prednisone equivalent, assuming there is no perforation and their stool studies are negative. If they are unstable, though, they are really going to need high-dose corticosteroids. They are going to need methylprednisolone, 125 mg IV, to evaluate for their responsiveness. These folks really need to be treated as inpatients, and they need to have their oncologists involved early on with their treatment.
DR HENRY Yes. I couldn’t agree with you more. When I talk to the diarrhea side effect patients that we see, I tend to think it’s a curse. It’s volume. It’s calories. It’s electrolytes. The number of stools you’re mentioning, it is almost certainly going to need admission to rule out other causes. Then, if it’s the checkpoint inhibitor, the steroid antidotes.
Let’s move on to the rash. This is another organ system that can be affected by immunotherapy. What is your approach when you see a generalized body rash in a patient on one of these drugs who is sent to the ED?
DR SAMMON I am obviously going to be ruling out other causes first, but generally you’re going to see a maculopapular rash. It may be itchy. It may be burning. Patients will often describe it as just sort of having a tight sensation. I’m going to be looking at them a little bit like I look at a burn patient. What is their total body surface area that’s involved? If they’ve got less than 30% of their total body surface area involved, that’s considered a mild reaction.
For those folks, I’m not going to use systemic steroids, but I can give them some topical steroids, and I can give them some Benadryl, some diphenhydramine, and really treat them symptomatically as well as ensuring that they have early follow-up to make sure this isn’t progressing. Once we get between 30% and 50% body area, we’re talking about a moderate toxicity. If these patients are not improving rapidly with just withholding the drug, they need systemic corticosteroids.
We usually treat them at about 0.5-1 mg/kg body weight a day of prednisone equivalent. Just as with burn patients, these patients’ symptoms can become very severe. You can see signs of blistering, dermal ulceration, necrotic, bolus, hemorrhagic lesions (Figure 2, A-D).1 These folks can have very difficult-to-manage fluid balances, and they’re at very high risk for skin infections as well. They need to be treated as inpatients. If possible, you might want to consider sending these patients to a burn unit. They need systemic corticosteroids, 1-2 mg/kg per day, and they need careful monitoring for signs of dehydration, electrolytic abnormalities, and/or skin infections. They need excellent wound care.
DR HENRY That’s very well put and always difficult, because there are so many causes of rash. That takes me to an area that has always been difficult for me, which is therapy-related endocrine problems. It’s interesting to note that these drugs can cause endocrine problems. I’ve heard you speak about the pituitary affecting vision, as well as thyroid or adrenal issues. Let’s start with how you’d approach vision difficulty in a patient on these drugs.
DR SAMMON The endocrinopathies that you can get with these checkpoint inhibitors really have a myriad of symptoms. Your patient may present saying that they’re feeling tired, that they’re feeling weak, or they may have a headache. If your patient is having actual pituitary enlargement, they can present with headaches, visual field defects, or cranial nerve defects. The reason for those symptoms is that the pituitary sits in the cavernous sinus, and you have various cranial nerves passing through that area as well as the optic chiasm just above the pituitary gland (Figure 3).1 Your patient may present with a bitemporal hemianopia. Or with diplopia. You are going to want to very quickly get either a CT scan or an MRI to find out if that is what’s going on (Figure 4).1 These folks need to be treated aggressively as well.
DR HENRY You’ll get your CT scan or your MRI and rule out an enlargement or a change to the visual field. I haven’t seen this yet, but certainly exciting when you see it to treat it. Would you get the radialis brevis involved, steroids involved? How would you manage that?
DR SAMMON It’s interesting, because you do want to use corticosteroids. One of the questions here is, which corticosteroid do you want to use? If you’re talking about someone who may have adrenal insufficiency, you may want to be able to do a stimulation test. In these patients, you may want to choose using dexamethasone, because you can still do the corticotropin stimulation test. However, if your patient is in frank shock because of what you think is an adrenal crisis, you’re going to want to use hydrocortisone. If a patient is truly hypotensive and unstable, the testing is at that point less important than the treatment.
DR HENRY Very interesting. We have covered what I would consider the major aspects of these fascinating drugs. We haven’t covered all of what they do when they work well, which hopefully we’re seeing more and more often, but we have covered very well what can happen when things go wrong in side effects. Anything else that you would like to add from the ED perspective or other side effects worth mentioning?
DR SAMMON The thing that I would most like to share with the oncology office is the importance of communicating with your patients that, when they’re on these drugs, they need to tell emergency physicians that they’re on immunotherapy, not chemotherapy. It might be helpful to give these patients a card stating that they’re on immunotherapy, not chemotherapy, and outlining some of the side effects that ED physicians should be looking out for in these patients.
DR HENRY That’s a great point. I’ve seen that some of the manufacturers have little cheat cards that the patient can carry naming the drug and the side effects, because not all ED doctors are aware of the side effects of these drugs.
DR SAMMON Absolutely. We love those cards.
DR HENRY Yes. I’ve also given some to the ED doctors at Pennsylvania Hospital, and they love it. I think we’ve covered everything in quite a bit of detail. Thank you, Dr Sammon, for sharing this information from the frontlines of the ED.
DR HENRY I am pleased to be talking with Dr Maura Sammon, an emergency department (ED) physician, about identifying and treating immunotherapy-related side effects in the ED. This is a hot topic in oncology, and I was very interested in having an ED physician talk about what happens when treating oncologists send their patients to the ED, because a physician may think it is chemotherapy when it is immunotherapy. Let’s start with the example of an oncology patient going to the ED with some symptoms, and the ED physician asks the patient what they’re being treated with. The patient may or may not say the right thing – that is, inform you whether they are being treated with chemotherapy or immunotherapy. How do you morph over into knowing that they are not getting chemotherapy?
DR SAMMON Yes, that’s a big problem in the ED. Patients come to the ED and say they’re being treated for cancer. They say they’re on chemotherapy, when they’re actually on immunotherapy, and it can really send the treatment team down the wrong path. I have a metaphor to explain this. They say that Great Britain and the United States are two nations separated by a common language. For example, when a British person talks about football, they mean something very different than when an American talks about football. If someone in Great Britain asks you to come play football, you might show up with shoulder pads and a helmet rather than shin guards, and you’re left without having the right tools to participate in the game.
How this sometimes plays out with immunotherapy, unfortunately, is that a patient will present to the ED and say they’re having a cough and that they’re on chemotherapy for melanoma. Usually, this patient would be worked up for being in a potentially immunosuppressed state. You might get a white blood cell count. You might get a chest X-ray. You might see what looks to you like a new infiltrate on this chest X-ray and then start going down the path of treating someone whom you think is immunosuppressed with pneumonia and giving them antibiotics rather than what could be life-saving steroids, as would be the case if the patient were on immunotherapy.
It’s a real problem, because you have one word that patients may use meaning two very different things. It can get you into trouble if you are treating someone for potentially infectious causes rather than immunotherapy-related adverse reactions, which are much more similar to graft-versus-host disease than in the case of traditional chemotherapy.
DR HENRY That’s a very good point. I think, as we in oncology use these immunotherapies/checkpoint inhibitors more often, you will see them more often in the ED. Let’s get right into that. You’ve identified this patient as not getting a traditional chemotherapy – hopefully, all our records on these patients are available. You’ve decided to follow onto what might be a side effect of the immunotherapy, so I’m going to name the side effects that always occur to me: lung, gastrointestinal (GI) – which could be loose bowels or liver function – rash, endocrine problems. Let’s start with lung symptoms. You see the patient is short of breath and you identified immunotherapy. What’s your next step?
DR SAMMON That’s a great example, because the problem is that you see these patients with cough or shortness of breath and pulmonary complications, and pulmonary complications of immunotherapy, while rare, are potentially life threatening if they’re not identified quickly.
You can start with a chest X-ray on these patients knowing, however, that for a good percentage of them you won’t see findings on their chest X-ray (Figure 1, from Sammon M, Tobin T. Identification and management of immune-related adverse events in the emergency setting. Presented at: Advances in Cancer Immunotherapy – Society for Immunotherapy of Cancer (SITC); August 4, 2017; Philadelphia, PA). You need to proceed to computed tomography (CT), because the issue is that you can have protean findings on the CT related to immunotherapy treatment/adverse reactions. You need to have a very high index of suspicion regardless of what abnormal findings you’re seeing on this CT and erring toward withholding the drugs, starting treatment, and being more aggressive with this type of finding.
DR HENRY I’ve heard you talk at a conference about a patient with metastatic lung cancer, or some other tumor that may have existing disease in lung. The patient is aware of that, and the chart reflects that. Then you have this difficulty where the CT scan shows a pneumonitis, and it may not be tumor progression at all – it may be the drug. How do you work through that? Of course, your additional problem is you don’t have a whole lot of time. You must decide to whether you’re going to keep them in the ED, admit them, or send them home.
DR SAMMON Right. One of the first things is to get the oncologist involved at an early stage in treating this. We are all a team. We are all working together, and it’s very important to have that communication occur very early. I’m going to err on admitting those folks who have had symptoms and who have had findings on a chest CT, because they can progress. They can get much worse. I’m going to be getting the oncologist involved very early. We’re going to have the conversation about whether we should be starting steroids on them in the ED, getting them upstairs, and being aggressive in treating this.
DR HENRY So, time and therapy are very important.
DR SAMMON Yes.
DR HENRY You’ll get that steroid started as an antidote right away.
DR SAMMON Absolutely. For grade 2, you’re going to use methylprednisolone, 1 mg /kg daily. For anything higher than that – grade 3 or grade 4 – we’re going to start higher. We’re going to start at 2-4 mg/kg a day and get the patient upstairs and taper them slowly.
DR HENRY That’s worth empathizing. I, sadly, had a patient who ultimately did well, but had severe grade 4 pneumonitis. T
DR SAMMON Absolutely, but it’s very important to work together as a team to make these patients have good outcomes.
DR HENRY Agree. Let’s change over to the GI symptoms. The patient comes in and misidentifies him- or herself as having chemotherapy and diarrhea. We are used to causing nausea, vomiting, and diarrhea with some of our therapies. You realize, in talking to the oncologist, the patient is taking a checkpoint inhibitor. How would you approach the patient with diarrhea?
DR SAMMON First, I would talk to the patient. I would try to establish the baseline number of stools per day, because it’s not defined as a definite number of stools per day, it’s the number of stools above their baseline per day. If they’re having fewer than 4 stools above their baseline per day, I would send off some tests. We can send off a C diff (Clostridium difficile) test, we can send off a stool culture – all the parasites. Make sure the oncologist is going to be able to get these results and get them followed up, because these are results I’m not going to get back myself in the ED.
Then I’m going to talk to the patient about symptomatic treatment. I’m going to talk to them about oral hydration, a bland diet. Avoid using loperamide or any other antidiarrheal medicines, because that could decrease the frequency of stools but mask more severe symptoms that they may be having.
If I have a patient who is having more than 4 stools above their daily baseline and it’s been happening 4 to 6 stools a day for more than a week, I’m going to be sending those studies off, and I’m going to be having a conversation with the treating oncologist to find out if they want me to start the patient on steroids immediately, or if they want to wait for the test results to come back and have the steroids started as an outpatient.
These moderate patients can maybe wait a day until these test results come back. Those who are having more than 7 stools above baseline per day, peritoneal signs, ileus, or fever, are the patients you should worry about. You need to admit them for IV hydration. You need to do the stools, so you might need to keep them in the hospital until you find the results of the stool studies. You need to rule out perforation.
You may be starting steroids on these patients sooner rather than later. They’re going to be getting systemic corticosteroids at about 1-2 mg/kg of prednisone equivalent, assuming there is no perforation and their stool studies are negative. If they are unstable, though, they are really going to need high-dose corticosteroids. They are going to need methylprednisolone, 125 mg IV, to evaluate for their responsiveness. These folks really need to be treated as inpatients, and they need to have their oncologists involved early on with their treatment.
DR HENRY Yes. I couldn’t agree with you more. When I talk to the diarrhea side effect patients that we see, I tend to think it’s a curse. It’s volume. It’s calories. It’s electrolytes. The number of stools you’re mentioning, it is almost certainly going to need admission to rule out other causes. Then, if it’s the checkpoint inhibitor, the steroid antidotes.
Let’s move on to the rash. This is another organ system that can be affected by immunotherapy. What is your approach when you see a generalized body rash in a patient on one of these drugs who is sent to the ED?
DR SAMMON I am obviously going to be ruling out other causes first, but generally you’re going to see a maculopapular rash. It may be itchy. It may be burning. Patients will often describe it as just sort of having a tight sensation. I’m going to be looking at them a little bit like I look at a burn patient. What is their total body surface area that’s involved? If they’ve got less than 30% of their total body surface area involved, that’s considered a mild reaction.
For those folks, I’m not going to use systemic steroids, but I can give them some topical steroids, and I can give them some Benadryl, some diphenhydramine, and really treat them symptomatically as well as ensuring that they have early follow-up to make sure this isn’t progressing. Once we get between 30% and 50% body area, we’re talking about a moderate toxicity. If these patients are not improving rapidly with just withholding the drug, they need systemic corticosteroids.
We usually treat them at about 0.5-1 mg/kg body weight a day of prednisone equivalent. Just as with burn patients, these patients’ symptoms can become very severe. You can see signs of blistering, dermal ulceration, necrotic, bolus, hemorrhagic lesions (Figure 2, A-D).1 These folks can have very difficult-to-manage fluid balances, and they’re at very high risk for skin infections as well. They need to be treated as inpatients. If possible, you might want to consider sending these patients to a burn unit. They need systemic corticosteroids, 1-2 mg/kg per day, and they need careful monitoring for signs of dehydration, electrolytic abnormalities, and/or skin infections. They need excellent wound care.
DR HENRY That’s very well put and always difficult, because there are so many causes of rash. That takes me to an area that has always been difficult for me, which is therapy-related endocrine problems. It’s interesting to note that these drugs can cause endocrine problems. I’ve heard you speak about the pituitary affecting vision, as well as thyroid or adrenal issues. Let’s start with how you’d approach vision difficulty in a patient on these drugs.
DR SAMMON The endocrinopathies that you can get with these checkpoint inhibitors really have a myriad of symptoms. Your patient may present saying that they’re feeling tired, that they’re feeling weak, or they may have a headache. If your patient is having actual pituitary enlargement, they can present with headaches, visual field defects, or cranial nerve defects. The reason for those symptoms is that the pituitary sits in the cavernous sinus, and you have various cranial nerves passing through that area as well as the optic chiasm just above the pituitary gland (Figure 3).1 Your patient may present with a bitemporal hemianopia. Or with diplopia. You are going to want to very quickly get either a CT scan or an MRI to find out if that is what’s going on (Figure 4).1 These folks need to be treated aggressively as well.
DR HENRY You’ll get your CT scan or your MRI and rule out an enlargement or a change to the visual field. I haven’t seen this yet, but certainly exciting when you see it to treat it. Would you get the radialis brevis involved, steroids involved? How would you manage that?
DR SAMMON It’s interesting, because you do want to use corticosteroids. One of the questions here is, which corticosteroid do you want to use? If you’re talking about someone who may have adrenal insufficiency, you may want to be able to do a stimulation test. In these patients, you may want to choose using dexamethasone, because you can still do the corticotropin stimulation test. However, if your patient is in frank shock because of what you think is an adrenal crisis, you’re going to want to use hydrocortisone. If a patient is truly hypotensive and unstable, the testing is at that point less important than the treatment.
DR HENRY Very interesting. We have covered what I would consider the major aspects of these fascinating drugs. We haven’t covered all of what they do when they work well, which hopefully we’re seeing more and more often, but we have covered very well what can happen when things go wrong in side effects. Anything else that you would like to add from the ED perspective or other side effects worth mentioning?
DR SAMMON The thing that I would most like to share with the oncology office is the importance of communicating with your patients that, when they’re on these drugs, they need to tell emergency physicians that they’re on immunotherapy, not chemotherapy. It might be helpful to give these patients a card stating that they’re on immunotherapy, not chemotherapy, and outlining some of the side effects that ED physicians should be looking out for in these patients.
DR HENRY That’s a great point. I’ve seen that some of the manufacturers have little cheat cards that the patient can carry naming the drug and the side effects, because not all ED doctors are aware of the side effects of these drugs.
DR SAMMON Absolutely. We love those cards.
DR HENRY Yes. I’ve also given some to the ED doctors at Pennsylvania Hospital, and they love it. I think we’ve covered everything in quite a bit of detail. Thank you, Dr Sammon, for sharing this information from the frontlines of the ED.
Addressing the rarity and complexities of sarcomas
The rarity and complexities of bone and soft tissue sarcomas pose a major challenge to effective treatment. Historically, there has been a blanket approach to treatment, but more recently that has begun to change thanks to genome profiling studies and novel clinical trial strategies. Here, we discuss the resulting enrichment of the therapeutic armamentarium with molecularly targeted and immune therapies.
A challenging tumor type
Sarcomas are a large group of histologically diverse cancers that arise in the mesenchymal cells. They can be broadly divided into bone and soft tissue sarcomas (STS) but are further subdivided according to the type of cell from which they derive; osteosarcomas in the bone, rhabdomyosarcomas in the skeletal muscle, liposarcomas in the fat tissues, leiomyosarcomas in the smooth muscle, and chondrosarcomas in the cartilaginous tissue, for example.
Each sarcoma subtype itself encompasses a range of different cancers with unique biology. Under the umbrella of liposarcoma, for example, are well/dedifferentiated liposarcomas and myxoid liposarcomas, which have very different pathologies and clinical courses.
As a whole, sarcomas are extremely rare tumors, accounting for less than 1% of all adult cancers, although they disproportionately affect children and young adults, with a prevalence closer to 15%.1,2 Certain sarcoma subtypes are exceptionally rare, with only a few cases diagnosed worldwide each year, whereas liposarcomas are at the other end of the spectrum, comprising the most common form of STS (Figure 1).3
In the early stages, sarcomas are generally highly treatable with a combination of surgical resection, chemotherapy, and radiation therapy. However, many patients develop advanced, metastatic disease, which presents much more of a challenge.4,5
Magic bullet for GIST
Despite their clear heterogeneity and complexity, sarcomas have tended to be treated as a single entity. Chemotherapy has played a central role in the treatment of advanced sarcomas and continues to do so, with 2 newer drugs approved by the United States Food and Drug Administration (FDA) in the past several years.6,7
The development of targeted therapy, on the other hand, for the most part proved unsuccessful. In general, studies examining the somatic mutation landscape in sarcomas found very few that were highly recurrent. The exception was gastrointestinal stromal tumors (GIST), which represent around 8% of STS.8 Frequent mutations in several highly targetable tyrosine kinases, notably KIT, which is mutated in around 85% of cases,9 and platelet-derived growth factor receptor alpha (PDGFRα) were identified in these tumors.10This prompted the development of tyrosine kinase inhibitors (TKIs), targeting these and other kinases, for the treatment of patients with GIST, and culminated in the approval of imatinib for this indication in 2002. This revolutionized the treatment of GIST, which had a poor prognosis and were resistant to chemotherapy, extending median overall survival in patients with metastatic disease almost to 5 years.11-13
Imatinib was also shown to benefit patients with surgically resectable disease and was subsequently approved in the adjuvant setting in 2008. A recent trial demonstrated that 3-year continuation of adjuvant imatinib resulted in a significantly longer progression-free survival (PFS) compared with 1 year of adjuvant imatinib, and even longer time periods are now being evaluated.14,15 The TKIs sunitinib and regorafenib have also been approved for the treatment of patients who become resistant to imatinib.16,17 Avapritinib, a newer, more specific inhibitor of KIT is also being evaluated in patients with GIST (Table).
Long-sought success for STS
Sunitinib and regorafenib include PDGFRα and the vascular endothelial growth factor receptors (VEGFRs) among their targets, receptors that play crucial roles in the formation of new blood vessels (angiogenesis). Many types of non-GIST sarcomas have been shown to be highly vascularized and express high levels of both of those receptors and other angiogenic proteins, which sparked interest in the development of multitargeted TKIs and other anti-angiogenic drugs in patients with STS.18
In 2012, pazopanib became the first FDA-approved molecularly targeted therapy for the treatment of non-GIST sarcomas. Approval in the second-line setting was based on the demonstration of a 3-month improvement in PFS compared with placebo.19 Four years later, the monoclonal antibody olaratumab, a more specific inhibitor of PDGFRα, was approved in combination with doxorubicin, marking the first front-line approval for more than 4 decades.20Numerous other anti-angiogenic drugs continue to be evaluated for the treatment of advanced STS. Among them, anlotinib is being tested in phase 3 clinical trials, and results from the ALTER0203 trial were presented at the 2018 annual meeting of the American Society of Clinical Oncology (ASCO).21 After failure of chemotherapy, 223 patients were randomly assigned to receive either anlotinib or placebo. Anlotinib significantly improved median PFS across all patients, compared with placebo (6.27 vs 1.4 months, respectively; hazard ratio [HR], 0.33; P < .0001), but was especially effective in patients with alveolar soft part sarcoma (ASPS; mPFS: 18.2 vs 3 months) and was well tolerated.21
Sarcoma secrets revealed
Advancements in genome sequencing technologies have made it possible to interrogate the molecular underpinnings of sarcomas in greater detail. However, their rarity presents a significant technical challenge, with a dearth of samples available for genomic testing. Large-scale worldwide collaborative efforts have facilitated the collection of sufficiently large patient populations to provide statistically robust data in many cases. The Cancer Genome Atlas has established a rare tumor characterization project to facilitate the genomic sequencing of rare cancer types like sarcomas.
Genome sequencing studies have revealed 2 types of sarcomas: those with relatively stable genomes and few molecular alterations, exemplified by Ewing sarcoma, which has a mutational load of 0.15 mutations/Megabase (Mb); and those that are much more complex with frequent somatic mutations, the prime example being leiomyosarcoma. The latter are characterized by mutations in the TP53 gene, dubbed the “guardian of the genome” for its essential role in genome stability.
The 2 types are likely to require very different therapeutic strategies. Although genomically complex tumors offer up lots of potential targets for therapy, they also display significant heterogeneity and it can be challenging to find a shared target across different tumor samples. The p53 protein would make a logical target but, to date, tumor suppressor proteins are not readily druggable.
The most common type of molecular alterations in sarcomas are chromosomal translocations, where part of a chromosome breaks off and becomes reattached to another chromosome. This can result in the formation of a gene fusion when parts of 2 different genes are brought together in a way in which the genetic code can still be read, leading to the formation of a fusion protein with altered activity.22-25
In sarcomas, these chromosomal translocations predominantly involve genes encoding transcription factors and the gene fusion results in their aberrant expression and activation of the transcriptional programs that they regulate.
Ewing sarcoma is a prime example of a sarcoma that is defined by chromosomal translocations. Most often, the resulting gene fusions occur between members of theten-eleven translocation (TET) family of RNA-binding proteins and the E26 transformation-specific (ETS) family of transcription factors. The most common fusion is between the EWSR1 and FLI1 genes, observed in between 85% and 90% of cases.
Significant efforts have been made to target EWSR1-FLI1. Since direct targeting of transcription factors is challenging, those efforts focused on targeting the aberrant transcriptional programs that they initiate. A major downstream target is the insulin-like growth factor receptor 1 (IGF1R) and numerous IGF1R inhibitors were developed and tested in patients with Ewing sarcoma, but unfortunately success was limited. Attention turned to the mammalian target of rapamycin (mTOR) as a potential mechanism of resistance to IGF1R inhibitors and explanation for the limited responses. Clinical trials combining mTOR and IGF1R inhibitors also proved unsuccessful.26
Although overall these trials were deemed failures, they were notable for the dramatic responses that were seen in 1 or 2 patients. Researchers are probing these “exceptional responses” using novel N-of-1 clinical trial designs that focus on a single patient (Figure 2).27-30 More recently, the first drug to specifically target the EWSR1-FLI1 fusion protein was developed. TK216 binds to the fusion protein and prevents it from binding to RNA helicase A, thereby blocking its function.31
Another type of gene fusion, involving the neurotrophic tropomyosin receptor kinase (NTRK) genes, has recently come into the spotlight for the treatment of lung cancer. According to a recent study, NTRK fusions may also be common in sarcomas. They were observed in 8% of patients with breast sarcomas, 5% with fibrosarcomas, and 5% with stomach or small intestine sarcomas.32
The NTRK genes encode TRK proteins and several small molecule inhibitors of TRK have been developed to treat patients with NTRK fusion-positive cancers. Another novel clinical trial design – the basket trial – is being used to test these inhibitors. This type of trial uses a tumor-agnostic approach, recruiting patients with all different histological subtypes of cancer that are unified by the shared presence of a specific molecular alteration.33
Repurposing gynecologic cancer drugs
More recently, a third group of sarcomas was categorized, with intermediate genomic complexity. These tumors, including well/dedifferentiated liposarcomas, were characterized by amplifications of chromosome 12, involving genes such as cyclin-dependent kinase 4 (CDK4). In fact, more than 90% of patients with well/dedifferentiated sarcomas display CDK4 amplification, making it a logical therapeutic target.36
CDK4 encodes CDK4 protein, a cell cycle-associated protein that regulates the transition from G1-S phase, known as the restriction point, beyond which the cell commits to undergoing mitosis. Aberrant expression of CDK4 in cancer drives the hallmark process of unchecked cellular proliferation.
Some small molecule CDK4/6 inhibitors have been developed and have shown significant promise in the treatment of breast cancer. They are also being evaluatedin patients with sarcoma whose tumors display CDK4 overexpression. In a recently published phase 2 trial of palbociclib in 60 patients with well/dedifferentiated liposarcomas, there was 1 CR.37
Another group of drugs that has advanced the treatment of gynecologic cancers comprises the poly (ADP-ribose) polymerase (PARP) inhibitors. In this context, PARP inhibitors are used in patients with mutations in the breast cancer susceptibility genes, BRCA1/2. The BRCA and PARP proteins are both involved in DNA repair pathways and the inhibition of PARP in patients who already have a defective BRCA pathway renders a lethal double blow to the cancer cell. According to the Broad Institute Cancer Cell Line Encyclopedia, Ewing sarcomas express high levels of the PARP1 enzyme, which could render them sensitive to PARP inhibition. Preclinical studies seemed to confirm that sensitivity, however, so far this has yet to translate into success in clinical trials, with no objective responses observed as yet.38
Expanding the field
Other treatment strategies being tested in patients with sarcoma are moving the field beyond conventional targeted therapies. There has been substantial focus in recent years on epigenetic alterations and their potential role in the development of cancer. Epigenetics is the secondary layer of regulation that acts on the genome and directs the spatial and temporal expression of genes.
Both DNA and the histone proteins they are packaged up with to form chromatin in nondividing cells can be modified by the attachment of chemical groups, such as acetyl and methyl groups, which can alter access to the DNA for transcription.
EZH2 is an enzyme that participates in histone methylation and thereby regulates transcriptional repression. Some types of sarcoma are characterized by a loss of expression of the INI1 gene, also known as SMARCB1. The INI1 protein is part of a chromatin remodeling complex that relieves transcriptional repression and when INI1 is lost, cells become dependent upon EZH2.39Clinical trials of the EZH2 inhibitor tazemetostat are ongoing in several types of sarcoma. Results from a phase 2 study in adults with INI1-negative tumors were presented at ASCO in 2017. Among 31 patients treated with 800 mg tazemetostat in continuous 28-day cycles, mPFS was 5.7 months, disease control rate was 10%, and confirmed overall response rate was 13%. The FDA has granted tazemetostat orphan drug designation in this indication.40A pediatric basket trial of tazemetostat is also ongoing, but the FDA recently placed it under a clinical hold as a result of a safety update from the trial in which a pediatric patient with advanced poorly differentiated chordoma developed a secondary T-cell lymphoma.41
Targeting the unique metabolism of sarcomas may offer a promising therapeutic strategy, although this is in the preliminary stages of evaluation. A recent study showed that the expression of the argininosuccinate synthase 1 enzyme, which is involved in the generation of arginine through the urea cycle, was lost in up to 90% of STS. A pegylated arginine deaminase (ADI-PEG20), is being evaluated in a phase 2 clinical trial.42
Finally, the concept of using immunotherapy to boost the anti-tumor immune response is also being examined in sarcomas. A significant number of cases of STS, osteosarcoma and GIST have been shown to express programmed cell death protein-ligand 1, therefore the use of immune checkpoint inhibitors that block this ligand or its receptor and help to reactive tumor-infiltrating T cells, could be a beneficial strategy.
Limited activity has been observed in studies conducted to date, however combination therapies, especially with inhibitors of the indoleamine 2,3-dioxygenase (IDO) enzyme, which plays a key role in immunosuppression, could help to harness the power of these drugs. Studies have suggested that sarcomas may be infiltrated by immunosuppressive macrophages that express IDO.43
It is generally believed that immunotherapy is most effective in tumors that are highly mutated because that allows a large number of cancer antigens to provoke an anti-tumor immune response. However, a single highly expressed antigen can also be strongly immunogenic. Synovial sarcomas have a relatively low mutational burden but they do express high levels of the cancer testis antigen NY-ESO-1.
NY-ESO-1 has provided a useful target for the development of adoptive cell therapies and vaccines for the treatment of sarcomas. CMB305 is an NY-ESO-1 vaccine that also incorporates a toll-like receptor 4 agonist. It is being evaluated in the phase 3 Synovate study as maintenance monotherapy in patients with locally advanced, unresectable or metastatic synovial sarcoma. In a phase 1 study, at a median follow-up of just under 18 months, the median OS for all 25 patients was 23.7 months.44
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922-2930.
3. Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2(1):14.
4. Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049-1054.
5. Savina M, Le Cesne A, Blay JY, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15(1):78.
6. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786-793.
7. Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637.
8. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416-421; discussion 421-412.
9. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342-4349.
10. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708-710.
11. Dagher R, Cohen M, Williams G, et al. Approval summary. Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8(10):3034-3038.
12. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626-632.
13. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127-1134.
14. Zhao R, Wang Y, Huang Y, et al. Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Scientific Reports. 2017;7:16834.
15. Raut C, Espat N, Maki R, Araujo D, Williams T, Wolff J. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol. 2017;35(15_suppl):11009.
16. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295-302.
17. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329-1338.
18. Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol. 2014;91(2):172-185.
19. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879-1886.
20. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497.
21. Chi Y, Yao Y, Wang S, et al. Anlotinib for metastatic soft tissue sarcoma: A randomized, double-blind, placebo-controlled and multi-centered clinical trial. J Clin Oncol. 2018;36(suppl):abstr 11503.
22. Brohl AS, Shah HR, Wang Y-C, Kasarskis A, Maki RG. The somatic mutational landscape in soft tissue sarcoma: Early results from TCGA data. J Clin Oncol. 2015;33(15_suppl):10508-10508.
23. Crompton BD, Stewart C, Taylor-Weiner A, et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4(11):1326-1341.
24. Jour G, Scarborough JD, Jones RL, et al. Molecular profiling of soft tissue sarcomas using next-generation sequencing: a pilot study toward precision therapeutics. Hum Pathol. 2014;45(8):1563-1571.
25. Yang J-L. Investigation of osteosarcoma genomics and its impact on targeted therapy: an international collaboration to conquer human osteosarcoma. Chin J Cancer. 2014;33(12):575-580.
26. Cidre-Aranaz F, Alonso J. EWS/FLI1 target genes and therapeutic opportunities in Ewing sarcoma. Front Oncol. 2015;5:162.
27. Savoia C, Volpe M, Grassi G, Borghi C, Agabiti Rosei E, Touyz RM. Personalized medicine-a modern approach for the diagnosis and management of hypertension. Clin Sci (Lond). 2017;131(22):2671-2685.
28. Biswas B, Bakhshi S. Management of Ewing sarcoma family of tumors: Current scenario and unmet need. World J Orthop. 2016;7(9):527-538.
29. van Maldegem AM, Bovée JVMG, Peterse EFP, Hogendoorn PCW, Gelderblom H. Ewing sarcoma: the clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur J Cancer. 2016;53:171-180.
30. Subbiah V, Hess KR, Khawaja MR, et al. Evaluation of novel targeted therapies in aggressive biology sarcoma patients after progression from US FDA approved therapies. Sci Rep. 2016;6:35448.
31. Jessen K, Moseley E, Chung EYL, et al. TK216, a novel, small molecule inhibitor of the ETS-family of transcription factors, displays anti-tumor activity in AML and DLBCL. Blood. 2016;128(22):4035-4035.
32. Sankhala K, Potts S, Christiansen J, et al. Immunohistochemistry screening to increase the efficacy of next-generation sequencing for detection of NTRK, ROS1, and ALK gene rearrangements (fusions) in sarcoma patients. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 9-12, 2016, 2016; Lisbon, Portugal.
33. Renfro LA, An MW, Mandrekar SJ. Precision oncology: a new era of cancer clinical trials. Cancer Lett. 2017;387:121-126.
34. DuBois S, Laetsch T, Federman N, et al. The use of larotrectinib in the management of locally advanced pediatric NTRK-fusion sarcoma. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 8-11, 2017; Maui, Hawaii.
35. Multani P, Manavel E, Hornby Z. Preliminary evidence of clinical response to entrectinib in three sarcome patients. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 8-11, 2017; Maui, Hawaii.
36. Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715-721.
37. Dickson MA, Schwartz GK, Keohan ML, et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: a phase 2 clinical trial. JAMA Oncol. 2016;2(7):937-940.
38. Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603-607.
39. Kenichi K, Yoshinao O. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Science. 2017;108(4):547-552.
40. US Food and Drug Administration. Orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=544416. Designated date September 28, 2017. Accessed July 4, 2018.
41. Press release. Epizyme provides update regarding tazemetostat clinical program. https://globenewswire.com/news-release/2018/04/23/1485765/0/en/Epizyme-Provides-Update-Regarding-Tazemetostat-Clinical-Program.html. Released April 23, 2018. Accessed July 4, 2018.
42. Bean GR, Kremer JC, Prudner BC, et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death &Amp; Disease. 2016;7:e2406.
43. Bourcier K, Italiano A. Newer therapeutic strategies for soft-tissue sarcomas. Pharmacol Ther. 2018;188:118-123.
44. Somaiah N, Chawla SP, Block MS, et al. Immune response, safety, and survival impact from CMB305 in NY-ESO-1+ recurrent soft tissue sarcomas (STS). J Clin Oncol. 2017;35(15_suppl):11006-11006.
The rarity and complexities of bone and soft tissue sarcomas pose a major challenge to effective treatment. Historically, there has been a blanket approach to treatment, but more recently that has begun to change thanks to genome profiling studies and novel clinical trial strategies. Here, we discuss the resulting enrichment of the therapeutic armamentarium with molecularly targeted and immune therapies.
A challenging tumor type
Sarcomas are a large group of histologically diverse cancers that arise in the mesenchymal cells. They can be broadly divided into bone and soft tissue sarcomas (STS) but are further subdivided according to the type of cell from which they derive; osteosarcomas in the bone, rhabdomyosarcomas in the skeletal muscle, liposarcomas in the fat tissues, leiomyosarcomas in the smooth muscle, and chondrosarcomas in the cartilaginous tissue, for example.
Each sarcoma subtype itself encompasses a range of different cancers with unique biology. Under the umbrella of liposarcoma, for example, are well/dedifferentiated liposarcomas and myxoid liposarcomas, which have very different pathologies and clinical courses.
As a whole, sarcomas are extremely rare tumors, accounting for less than 1% of all adult cancers, although they disproportionately affect children and young adults, with a prevalence closer to 15%.1,2 Certain sarcoma subtypes are exceptionally rare, with only a few cases diagnosed worldwide each year, whereas liposarcomas are at the other end of the spectrum, comprising the most common form of STS (Figure 1).3
In the early stages, sarcomas are generally highly treatable with a combination of surgical resection, chemotherapy, and radiation therapy. However, many patients develop advanced, metastatic disease, which presents much more of a challenge.4,5
Magic bullet for GIST
Despite their clear heterogeneity and complexity, sarcomas have tended to be treated as a single entity. Chemotherapy has played a central role in the treatment of advanced sarcomas and continues to do so, with 2 newer drugs approved by the United States Food and Drug Administration (FDA) in the past several years.6,7
The development of targeted therapy, on the other hand, for the most part proved unsuccessful. In general, studies examining the somatic mutation landscape in sarcomas found very few that were highly recurrent. The exception was gastrointestinal stromal tumors (GIST), which represent around 8% of STS.8 Frequent mutations in several highly targetable tyrosine kinases, notably KIT, which is mutated in around 85% of cases,9 and platelet-derived growth factor receptor alpha (PDGFRα) were identified in these tumors.10This prompted the development of tyrosine kinase inhibitors (TKIs), targeting these and other kinases, for the treatment of patients with GIST, and culminated in the approval of imatinib for this indication in 2002. This revolutionized the treatment of GIST, which had a poor prognosis and were resistant to chemotherapy, extending median overall survival in patients with metastatic disease almost to 5 years.11-13
Imatinib was also shown to benefit patients with surgically resectable disease and was subsequently approved in the adjuvant setting in 2008. A recent trial demonstrated that 3-year continuation of adjuvant imatinib resulted in a significantly longer progression-free survival (PFS) compared with 1 year of adjuvant imatinib, and even longer time periods are now being evaluated.14,15 The TKIs sunitinib and regorafenib have also been approved for the treatment of patients who become resistant to imatinib.16,17 Avapritinib, a newer, more specific inhibitor of KIT is also being evaluated in patients with GIST (Table).
Long-sought success for STS
Sunitinib and regorafenib include PDGFRα and the vascular endothelial growth factor receptors (VEGFRs) among their targets, receptors that play crucial roles in the formation of new blood vessels (angiogenesis). Many types of non-GIST sarcomas have been shown to be highly vascularized and express high levels of both of those receptors and other angiogenic proteins, which sparked interest in the development of multitargeted TKIs and other anti-angiogenic drugs in patients with STS.18
In 2012, pazopanib became the first FDA-approved molecularly targeted therapy for the treatment of non-GIST sarcomas. Approval in the second-line setting was based on the demonstration of a 3-month improvement in PFS compared with placebo.19 Four years later, the monoclonal antibody olaratumab, a more specific inhibitor of PDGFRα, was approved in combination with doxorubicin, marking the first front-line approval for more than 4 decades.20Numerous other anti-angiogenic drugs continue to be evaluated for the treatment of advanced STS. Among them, anlotinib is being tested in phase 3 clinical trials, and results from the ALTER0203 trial were presented at the 2018 annual meeting of the American Society of Clinical Oncology (ASCO).21 After failure of chemotherapy, 223 patients were randomly assigned to receive either anlotinib or placebo. Anlotinib significantly improved median PFS across all patients, compared with placebo (6.27 vs 1.4 months, respectively; hazard ratio [HR], 0.33; P < .0001), but was especially effective in patients with alveolar soft part sarcoma (ASPS; mPFS: 18.2 vs 3 months) and was well tolerated.21
Sarcoma secrets revealed
Advancements in genome sequencing technologies have made it possible to interrogate the molecular underpinnings of sarcomas in greater detail. However, their rarity presents a significant technical challenge, with a dearth of samples available for genomic testing. Large-scale worldwide collaborative efforts have facilitated the collection of sufficiently large patient populations to provide statistically robust data in many cases. The Cancer Genome Atlas has established a rare tumor characterization project to facilitate the genomic sequencing of rare cancer types like sarcomas.
Genome sequencing studies have revealed 2 types of sarcomas: those with relatively stable genomes and few molecular alterations, exemplified by Ewing sarcoma, which has a mutational load of 0.15 mutations/Megabase (Mb); and those that are much more complex with frequent somatic mutations, the prime example being leiomyosarcoma. The latter are characterized by mutations in the TP53 gene, dubbed the “guardian of the genome” for its essential role in genome stability.
The 2 types are likely to require very different therapeutic strategies. Although genomically complex tumors offer up lots of potential targets for therapy, they also display significant heterogeneity and it can be challenging to find a shared target across different tumor samples. The p53 protein would make a logical target but, to date, tumor suppressor proteins are not readily druggable.
The most common type of molecular alterations in sarcomas are chromosomal translocations, where part of a chromosome breaks off and becomes reattached to another chromosome. This can result in the formation of a gene fusion when parts of 2 different genes are brought together in a way in which the genetic code can still be read, leading to the formation of a fusion protein with altered activity.22-25
In sarcomas, these chromosomal translocations predominantly involve genes encoding transcription factors and the gene fusion results in their aberrant expression and activation of the transcriptional programs that they regulate.
Ewing sarcoma is a prime example of a sarcoma that is defined by chromosomal translocations. Most often, the resulting gene fusions occur between members of theten-eleven translocation (TET) family of RNA-binding proteins and the E26 transformation-specific (ETS) family of transcription factors. The most common fusion is between the EWSR1 and FLI1 genes, observed in between 85% and 90% of cases.
Significant efforts have been made to target EWSR1-FLI1. Since direct targeting of transcription factors is challenging, those efforts focused on targeting the aberrant transcriptional programs that they initiate. A major downstream target is the insulin-like growth factor receptor 1 (IGF1R) and numerous IGF1R inhibitors were developed and tested in patients with Ewing sarcoma, but unfortunately success was limited. Attention turned to the mammalian target of rapamycin (mTOR) as a potential mechanism of resistance to IGF1R inhibitors and explanation for the limited responses. Clinical trials combining mTOR and IGF1R inhibitors also proved unsuccessful.26
Although overall these trials were deemed failures, they were notable for the dramatic responses that were seen in 1 or 2 patients. Researchers are probing these “exceptional responses” using novel N-of-1 clinical trial designs that focus on a single patient (Figure 2).27-30 More recently, the first drug to specifically target the EWSR1-FLI1 fusion protein was developed. TK216 binds to the fusion protein and prevents it from binding to RNA helicase A, thereby blocking its function.31
Another type of gene fusion, involving the neurotrophic tropomyosin receptor kinase (NTRK) genes, has recently come into the spotlight for the treatment of lung cancer. According to a recent study, NTRK fusions may also be common in sarcomas. They were observed in 8% of patients with breast sarcomas, 5% with fibrosarcomas, and 5% with stomach or small intestine sarcomas.32
The NTRK genes encode TRK proteins and several small molecule inhibitors of TRK have been developed to treat patients with NTRK fusion-positive cancers. Another novel clinical trial design – the basket trial – is being used to test these inhibitors. This type of trial uses a tumor-agnostic approach, recruiting patients with all different histological subtypes of cancer that are unified by the shared presence of a specific molecular alteration.33
Repurposing gynecologic cancer drugs
More recently, a third group of sarcomas was categorized, with intermediate genomic complexity. These tumors, including well/dedifferentiated liposarcomas, were characterized by amplifications of chromosome 12, involving genes such as cyclin-dependent kinase 4 (CDK4). In fact, more than 90% of patients with well/dedifferentiated sarcomas display CDK4 amplification, making it a logical therapeutic target.36
CDK4 encodes CDK4 protein, a cell cycle-associated protein that regulates the transition from G1-S phase, known as the restriction point, beyond which the cell commits to undergoing mitosis. Aberrant expression of CDK4 in cancer drives the hallmark process of unchecked cellular proliferation.
Some small molecule CDK4/6 inhibitors have been developed and have shown significant promise in the treatment of breast cancer. They are also being evaluatedin patients with sarcoma whose tumors display CDK4 overexpression. In a recently published phase 2 trial of palbociclib in 60 patients with well/dedifferentiated liposarcomas, there was 1 CR.37
Another group of drugs that has advanced the treatment of gynecologic cancers comprises the poly (ADP-ribose) polymerase (PARP) inhibitors. In this context, PARP inhibitors are used in patients with mutations in the breast cancer susceptibility genes, BRCA1/2. The BRCA and PARP proteins are both involved in DNA repair pathways and the inhibition of PARP in patients who already have a defective BRCA pathway renders a lethal double blow to the cancer cell. According to the Broad Institute Cancer Cell Line Encyclopedia, Ewing sarcomas express high levels of the PARP1 enzyme, which could render them sensitive to PARP inhibition. Preclinical studies seemed to confirm that sensitivity, however, so far this has yet to translate into success in clinical trials, with no objective responses observed as yet.38
Expanding the field
Other treatment strategies being tested in patients with sarcoma are moving the field beyond conventional targeted therapies. There has been substantial focus in recent years on epigenetic alterations and their potential role in the development of cancer. Epigenetics is the secondary layer of regulation that acts on the genome and directs the spatial and temporal expression of genes.
Both DNA and the histone proteins they are packaged up with to form chromatin in nondividing cells can be modified by the attachment of chemical groups, such as acetyl and methyl groups, which can alter access to the DNA for transcription.
EZH2 is an enzyme that participates in histone methylation and thereby regulates transcriptional repression. Some types of sarcoma are characterized by a loss of expression of the INI1 gene, also known as SMARCB1. The INI1 protein is part of a chromatin remodeling complex that relieves transcriptional repression and when INI1 is lost, cells become dependent upon EZH2.39Clinical trials of the EZH2 inhibitor tazemetostat are ongoing in several types of sarcoma. Results from a phase 2 study in adults with INI1-negative tumors were presented at ASCO in 2017. Among 31 patients treated with 800 mg tazemetostat in continuous 28-day cycles, mPFS was 5.7 months, disease control rate was 10%, and confirmed overall response rate was 13%. The FDA has granted tazemetostat orphan drug designation in this indication.40A pediatric basket trial of tazemetostat is also ongoing, but the FDA recently placed it under a clinical hold as a result of a safety update from the trial in which a pediatric patient with advanced poorly differentiated chordoma developed a secondary T-cell lymphoma.41
Targeting the unique metabolism of sarcomas may offer a promising therapeutic strategy, although this is in the preliminary stages of evaluation. A recent study showed that the expression of the argininosuccinate synthase 1 enzyme, which is involved in the generation of arginine through the urea cycle, was lost in up to 90% of STS. A pegylated arginine deaminase (ADI-PEG20), is being evaluated in a phase 2 clinical trial.42
Finally, the concept of using immunotherapy to boost the anti-tumor immune response is also being examined in sarcomas. A significant number of cases of STS, osteosarcoma and GIST have been shown to express programmed cell death protein-ligand 1, therefore the use of immune checkpoint inhibitors that block this ligand or its receptor and help to reactive tumor-infiltrating T cells, could be a beneficial strategy.
Limited activity has been observed in studies conducted to date, however combination therapies, especially with inhibitors of the indoleamine 2,3-dioxygenase (IDO) enzyme, which plays a key role in immunosuppression, could help to harness the power of these drugs. Studies have suggested that sarcomas may be infiltrated by immunosuppressive macrophages that express IDO.43
It is generally believed that immunotherapy is most effective in tumors that are highly mutated because that allows a large number of cancer antigens to provoke an anti-tumor immune response. However, a single highly expressed antigen can also be strongly immunogenic. Synovial sarcomas have a relatively low mutational burden but they do express high levels of the cancer testis antigen NY-ESO-1.
NY-ESO-1 has provided a useful target for the development of adoptive cell therapies and vaccines for the treatment of sarcomas. CMB305 is an NY-ESO-1 vaccine that also incorporates a toll-like receptor 4 agonist. It is being evaluated in the phase 3 Synovate study as maintenance monotherapy in patients with locally advanced, unresectable or metastatic synovial sarcoma. In a phase 1 study, at a median follow-up of just under 18 months, the median OS for all 25 patients was 23.7 months.44
The rarity and complexities of bone and soft tissue sarcomas pose a major challenge to effective treatment. Historically, there has been a blanket approach to treatment, but more recently that has begun to change thanks to genome profiling studies and novel clinical trial strategies. Here, we discuss the resulting enrichment of the therapeutic armamentarium with molecularly targeted and immune therapies.
A challenging tumor type
Sarcomas are a large group of histologically diverse cancers that arise in the mesenchymal cells. They can be broadly divided into bone and soft tissue sarcomas (STS) but are further subdivided according to the type of cell from which they derive; osteosarcomas in the bone, rhabdomyosarcomas in the skeletal muscle, liposarcomas in the fat tissues, leiomyosarcomas in the smooth muscle, and chondrosarcomas in the cartilaginous tissue, for example.
Each sarcoma subtype itself encompasses a range of different cancers with unique biology. Under the umbrella of liposarcoma, for example, are well/dedifferentiated liposarcomas and myxoid liposarcomas, which have very different pathologies and clinical courses.
As a whole, sarcomas are extremely rare tumors, accounting for less than 1% of all adult cancers, although they disproportionately affect children and young adults, with a prevalence closer to 15%.1,2 Certain sarcoma subtypes are exceptionally rare, with only a few cases diagnosed worldwide each year, whereas liposarcomas are at the other end of the spectrum, comprising the most common form of STS (Figure 1).3
In the early stages, sarcomas are generally highly treatable with a combination of surgical resection, chemotherapy, and radiation therapy. However, many patients develop advanced, metastatic disease, which presents much more of a challenge.4,5
Magic bullet for GIST
Despite their clear heterogeneity and complexity, sarcomas have tended to be treated as a single entity. Chemotherapy has played a central role in the treatment of advanced sarcomas and continues to do so, with 2 newer drugs approved by the United States Food and Drug Administration (FDA) in the past several years.6,7
The development of targeted therapy, on the other hand, for the most part proved unsuccessful. In general, studies examining the somatic mutation landscape in sarcomas found very few that were highly recurrent. The exception was gastrointestinal stromal tumors (GIST), which represent around 8% of STS.8 Frequent mutations in several highly targetable tyrosine kinases, notably KIT, which is mutated in around 85% of cases,9 and platelet-derived growth factor receptor alpha (PDGFRα) were identified in these tumors.10This prompted the development of tyrosine kinase inhibitors (TKIs), targeting these and other kinases, for the treatment of patients with GIST, and culminated in the approval of imatinib for this indication in 2002. This revolutionized the treatment of GIST, which had a poor prognosis and were resistant to chemotherapy, extending median overall survival in patients with metastatic disease almost to 5 years.11-13
Imatinib was also shown to benefit patients with surgically resectable disease and was subsequently approved in the adjuvant setting in 2008. A recent trial demonstrated that 3-year continuation of adjuvant imatinib resulted in a significantly longer progression-free survival (PFS) compared with 1 year of adjuvant imatinib, and even longer time periods are now being evaluated.14,15 The TKIs sunitinib and regorafenib have also been approved for the treatment of patients who become resistant to imatinib.16,17 Avapritinib, a newer, more specific inhibitor of KIT is also being evaluated in patients with GIST (Table).
Long-sought success for STS
Sunitinib and regorafenib include PDGFRα and the vascular endothelial growth factor receptors (VEGFRs) among their targets, receptors that play crucial roles in the formation of new blood vessels (angiogenesis). Many types of non-GIST sarcomas have been shown to be highly vascularized and express high levels of both of those receptors and other angiogenic proteins, which sparked interest in the development of multitargeted TKIs and other anti-angiogenic drugs in patients with STS.18
In 2012, pazopanib became the first FDA-approved molecularly targeted therapy for the treatment of non-GIST sarcomas. Approval in the second-line setting was based on the demonstration of a 3-month improvement in PFS compared with placebo.19 Four years later, the monoclonal antibody olaratumab, a more specific inhibitor of PDGFRα, was approved in combination with doxorubicin, marking the first front-line approval for more than 4 decades.20Numerous other anti-angiogenic drugs continue to be evaluated for the treatment of advanced STS. Among them, anlotinib is being tested in phase 3 clinical trials, and results from the ALTER0203 trial were presented at the 2018 annual meeting of the American Society of Clinical Oncology (ASCO).21 After failure of chemotherapy, 223 patients were randomly assigned to receive either anlotinib or placebo. Anlotinib significantly improved median PFS across all patients, compared with placebo (6.27 vs 1.4 months, respectively; hazard ratio [HR], 0.33; P < .0001), but was especially effective in patients with alveolar soft part sarcoma (ASPS; mPFS: 18.2 vs 3 months) and was well tolerated.21
Sarcoma secrets revealed
Advancements in genome sequencing technologies have made it possible to interrogate the molecular underpinnings of sarcomas in greater detail. However, their rarity presents a significant technical challenge, with a dearth of samples available for genomic testing. Large-scale worldwide collaborative efforts have facilitated the collection of sufficiently large patient populations to provide statistically robust data in many cases. The Cancer Genome Atlas has established a rare tumor characterization project to facilitate the genomic sequencing of rare cancer types like sarcomas.
Genome sequencing studies have revealed 2 types of sarcomas: those with relatively stable genomes and few molecular alterations, exemplified by Ewing sarcoma, which has a mutational load of 0.15 mutations/Megabase (Mb); and those that are much more complex with frequent somatic mutations, the prime example being leiomyosarcoma. The latter are characterized by mutations in the TP53 gene, dubbed the “guardian of the genome” for its essential role in genome stability.
The 2 types are likely to require very different therapeutic strategies. Although genomically complex tumors offer up lots of potential targets for therapy, they also display significant heterogeneity and it can be challenging to find a shared target across different tumor samples. The p53 protein would make a logical target but, to date, tumor suppressor proteins are not readily druggable.
The most common type of molecular alterations in sarcomas are chromosomal translocations, where part of a chromosome breaks off and becomes reattached to another chromosome. This can result in the formation of a gene fusion when parts of 2 different genes are brought together in a way in which the genetic code can still be read, leading to the formation of a fusion protein with altered activity.22-25
In sarcomas, these chromosomal translocations predominantly involve genes encoding transcription factors and the gene fusion results in their aberrant expression and activation of the transcriptional programs that they regulate.
Ewing sarcoma is a prime example of a sarcoma that is defined by chromosomal translocations. Most often, the resulting gene fusions occur between members of theten-eleven translocation (TET) family of RNA-binding proteins and the E26 transformation-specific (ETS) family of transcription factors. The most common fusion is between the EWSR1 and FLI1 genes, observed in between 85% and 90% of cases.
Significant efforts have been made to target EWSR1-FLI1. Since direct targeting of transcription factors is challenging, those efforts focused on targeting the aberrant transcriptional programs that they initiate. A major downstream target is the insulin-like growth factor receptor 1 (IGF1R) and numerous IGF1R inhibitors were developed and tested in patients with Ewing sarcoma, but unfortunately success was limited. Attention turned to the mammalian target of rapamycin (mTOR) as a potential mechanism of resistance to IGF1R inhibitors and explanation for the limited responses. Clinical trials combining mTOR and IGF1R inhibitors also proved unsuccessful.26
Although overall these trials were deemed failures, they were notable for the dramatic responses that were seen in 1 or 2 patients. Researchers are probing these “exceptional responses” using novel N-of-1 clinical trial designs that focus on a single patient (Figure 2).27-30 More recently, the first drug to specifically target the EWSR1-FLI1 fusion protein was developed. TK216 binds to the fusion protein and prevents it from binding to RNA helicase A, thereby blocking its function.31
Another type of gene fusion, involving the neurotrophic tropomyosin receptor kinase (NTRK) genes, has recently come into the spotlight for the treatment of lung cancer. According to a recent study, NTRK fusions may also be common in sarcomas. They were observed in 8% of patients with breast sarcomas, 5% with fibrosarcomas, and 5% with stomach or small intestine sarcomas.32
The NTRK genes encode TRK proteins and several small molecule inhibitors of TRK have been developed to treat patients with NTRK fusion-positive cancers. Another novel clinical trial design – the basket trial – is being used to test these inhibitors. This type of trial uses a tumor-agnostic approach, recruiting patients with all different histological subtypes of cancer that are unified by the shared presence of a specific molecular alteration.33
Repurposing gynecologic cancer drugs
More recently, a third group of sarcomas was categorized, with intermediate genomic complexity. These tumors, including well/dedifferentiated liposarcomas, were characterized by amplifications of chromosome 12, involving genes such as cyclin-dependent kinase 4 (CDK4). In fact, more than 90% of patients with well/dedifferentiated sarcomas display CDK4 amplification, making it a logical therapeutic target.36
CDK4 encodes CDK4 protein, a cell cycle-associated protein that regulates the transition from G1-S phase, known as the restriction point, beyond which the cell commits to undergoing mitosis. Aberrant expression of CDK4 in cancer drives the hallmark process of unchecked cellular proliferation.
Some small molecule CDK4/6 inhibitors have been developed and have shown significant promise in the treatment of breast cancer. They are also being evaluatedin patients with sarcoma whose tumors display CDK4 overexpression. In a recently published phase 2 trial of palbociclib in 60 patients with well/dedifferentiated liposarcomas, there was 1 CR.37
Another group of drugs that has advanced the treatment of gynecologic cancers comprises the poly (ADP-ribose) polymerase (PARP) inhibitors. In this context, PARP inhibitors are used in patients with mutations in the breast cancer susceptibility genes, BRCA1/2. The BRCA and PARP proteins are both involved in DNA repair pathways and the inhibition of PARP in patients who already have a defective BRCA pathway renders a lethal double blow to the cancer cell. According to the Broad Institute Cancer Cell Line Encyclopedia, Ewing sarcomas express high levels of the PARP1 enzyme, which could render them sensitive to PARP inhibition. Preclinical studies seemed to confirm that sensitivity, however, so far this has yet to translate into success in clinical trials, with no objective responses observed as yet.38
Expanding the field
Other treatment strategies being tested in patients with sarcoma are moving the field beyond conventional targeted therapies. There has been substantial focus in recent years on epigenetic alterations and their potential role in the development of cancer. Epigenetics is the secondary layer of regulation that acts on the genome and directs the spatial and temporal expression of genes.
Both DNA and the histone proteins they are packaged up with to form chromatin in nondividing cells can be modified by the attachment of chemical groups, such as acetyl and methyl groups, which can alter access to the DNA for transcription.
EZH2 is an enzyme that participates in histone methylation and thereby regulates transcriptional repression. Some types of sarcoma are characterized by a loss of expression of the INI1 gene, also known as SMARCB1. The INI1 protein is part of a chromatin remodeling complex that relieves transcriptional repression and when INI1 is lost, cells become dependent upon EZH2.39Clinical trials of the EZH2 inhibitor tazemetostat are ongoing in several types of sarcoma. Results from a phase 2 study in adults with INI1-negative tumors were presented at ASCO in 2017. Among 31 patients treated with 800 mg tazemetostat in continuous 28-day cycles, mPFS was 5.7 months, disease control rate was 10%, and confirmed overall response rate was 13%. The FDA has granted tazemetostat orphan drug designation in this indication.40A pediatric basket trial of tazemetostat is also ongoing, but the FDA recently placed it under a clinical hold as a result of a safety update from the trial in which a pediatric patient with advanced poorly differentiated chordoma developed a secondary T-cell lymphoma.41
Targeting the unique metabolism of sarcomas may offer a promising therapeutic strategy, although this is in the preliminary stages of evaluation. A recent study showed that the expression of the argininosuccinate synthase 1 enzyme, which is involved in the generation of arginine through the urea cycle, was lost in up to 90% of STS. A pegylated arginine deaminase (ADI-PEG20), is being evaluated in a phase 2 clinical trial.42
Finally, the concept of using immunotherapy to boost the anti-tumor immune response is also being examined in sarcomas. A significant number of cases of STS, osteosarcoma and GIST have been shown to express programmed cell death protein-ligand 1, therefore the use of immune checkpoint inhibitors that block this ligand or its receptor and help to reactive tumor-infiltrating T cells, could be a beneficial strategy.
Limited activity has been observed in studies conducted to date, however combination therapies, especially with inhibitors of the indoleamine 2,3-dioxygenase (IDO) enzyme, which plays a key role in immunosuppression, could help to harness the power of these drugs. Studies have suggested that sarcomas may be infiltrated by immunosuppressive macrophages that express IDO.43
It is generally believed that immunotherapy is most effective in tumors that are highly mutated because that allows a large number of cancer antigens to provoke an anti-tumor immune response. However, a single highly expressed antigen can also be strongly immunogenic. Synovial sarcomas have a relatively low mutational burden but they do express high levels of the cancer testis antigen NY-ESO-1.
NY-ESO-1 has provided a useful target for the development of adoptive cell therapies and vaccines for the treatment of sarcomas. CMB305 is an NY-ESO-1 vaccine that also incorporates a toll-like receptor 4 agonist. It is being evaluated in the phase 3 Synovate study as maintenance monotherapy in patients with locally advanced, unresectable or metastatic synovial sarcoma. In a phase 1 study, at a median follow-up of just under 18 months, the median OS for all 25 patients was 23.7 months.44
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922-2930.
3. Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2(1):14.
4. Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049-1054.
5. Savina M, Le Cesne A, Blay JY, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15(1):78.
6. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786-793.
7. Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637.
8. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416-421; discussion 421-412.
9. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342-4349.
10. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708-710.
11. Dagher R, Cohen M, Williams G, et al. Approval summary. Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8(10):3034-3038.
12. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626-632.
13. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127-1134.
14. Zhao R, Wang Y, Huang Y, et al. Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Scientific Reports. 2017;7:16834.
15. Raut C, Espat N, Maki R, Araujo D, Williams T, Wolff J. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol. 2017;35(15_suppl):11009.
16. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295-302.
17. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329-1338.
18. Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol. 2014;91(2):172-185.
19. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879-1886.
20. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497.
21. Chi Y, Yao Y, Wang S, et al. Anlotinib for metastatic soft tissue sarcoma: A randomized, double-blind, placebo-controlled and multi-centered clinical trial. J Clin Oncol. 2018;36(suppl):abstr 11503.
22. Brohl AS, Shah HR, Wang Y-C, Kasarskis A, Maki RG. The somatic mutational landscape in soft tissue sarcoma: Early results from TCGA data. J Clin Oncol. 2015;33(15_suppl):10508-10508.
23. Crompton BD, Stewart C, Taylor-Weiner A, et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4(11):1326-1341.
24. Jour G, Scarborough JD, Jones RL, et al. Molecular profiling of soft tissue sarcomas using next-generation sequencing: a pilot study toward precision therapeutics. Hum Pathol. 2014;45(8):1563-1571.
25. Yang J-L. Investigation of osteosarcoma genomics and its impact on targeted therapy: an international collaboration to conquer human osteosarcoma. Chin J Cancer. 2014;33(12):575-580.
26. Cidre-Aranaz F, Alonso J. EWS/FLI1 target genes and therapeutic opportunities in Ewing sarcoma. Front Oncol. 2015;5:162.
27. Savoia C, Volpe M, Grassi G, Borghi C, Agabiti Rosei E, Touyz RM. Personalized medicine-a modern approach for the diagnosis and management of hypertension. Clin Sci (Lond). 2017;131(22):2671-2685.
28. Biswas B, Bakhshi S. Management of Ewing sarcoma family of tumors: Current scenario and unmet need. World J Orthop. 2016;7(9):527-538.
29. van Maldegem AM, Bovée JVMG, Peterse EFP, Hogendoorn PCW, Gelderblom H. Ewing sarcoma: the clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur J Cancer. 2016;53:171-180.
30. Subbiah V, Hess KR, Khawaja MR, et al. Evaluation of novel targeted therapies in aggressive biology sarcoma patients after progression from US FDA approved therapies. Sci Rep. 2016;6:35448.
31. Jessen K, Moseley E, Chung EYL, et al. TK216, a novel, small molecule inhibitor of the ETS-family of transcription factors, displays anti-tumor activity in AML and DLBCL. Blood. 2016;128(22):4035-4035.
32. Sankhala K, Potts S, Christiansen J, et al. Immunohistochemistry screening to increase the efficacy of next-generation sequencing for detection of NTRK, ROS1, and ALK gene rearrangements (fusions) in sarcoma patients. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 9-12, 2016, 2016; Lisbon, Portugal.
33. Renfro LA, An MW, Mandrekar SJ. Precision oncology: a new era of cancer clinical trials. Cancer Lett. 2017;387:121-126.
34. DuBois S, Laetsch T, Federman N, et al. The use of larotrectinib in the management of locally advanced pediatric NTRK-fusion sarcoma. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 8-11, 2017; Maui, Hawaii.
35. Multani P, Manavel E, Hornby Z. Preliminary evidence of clinical response to entrectinib in three sarcome patients. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 8-11, 2017; Maui, Hawaii.
36. Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715-721.
37. Dickson MA, Schwartz GK, Keohan ML, et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: a phase 2 clinical trial. JAMA Oncol. 2016;2(7):937-940.
38. Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603-607.
39. Kenichi K, Yoshinao O. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Science. 2017;108(4):547-552.
40. US Food and Drug Administration. Orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=544416. Designated date September 28, 2017. Accessed July 4, 2018.
41. Press release. Epizyme provides update regarding tazemetostat clinical program. https://globenewswire.com/news-release/2018/04/23/1485765/0/en/Epizyme-Provides-Update-Regarding-Tazemetostat-Clinical-Program.html. Released April 23, 2018. Accessed July 4, 2018.
42. Bean GR, Kremer JC, Prudner BC, et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death &Amp; Disease. 2016;7:e2406.
43. Bourcier K, Italiano A. Newer therapeutic strategies for soft-tissue sarcomas. Pharmacol Ther. 2018;188:118-123.
44. Somaiah N, Chawla SP, Block MS, et al. Immune response, safety, and survival impact from CMB305 in NY-ESO-1+ recurrent soft tissue sarcomas (STS). J Clin Oncol. 2017;35(15_suppl):11006-11006.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
2. Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer. 2006;119(12):2922-2930.
3. Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2(1):14.
4. Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049-1054.
5. Savina M, Le Cesne A, Blay JY, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15(1):78.
6. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34(8):786-793.
7. Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629-1637.
8. Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416-421; discussion 421-412.
9. Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342-4349.
10. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708-710.
11. Dagher R, Cohen M, Williams G, et al. Approval summary. Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8(10):3034-3038.
12. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626-632.
13. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127-1134.
14. Zhao R, Wang Y, Huang Y, et al. Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Scientific Reports. 2017;7:16834.
15. Raut C, Espat N, Maki R, Araujo D, Williams T, Wolff J. Extended treatment with adjuvant imatinib (IM) for patients (pts) with high-risk primary gastrointestinal stromal tumor (GIST): The PERSIST-5 study. J Clin Oncol. 2017;35(15_suppl):11009.
16. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295-302.
17. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329-1338.
18. Versleijen-Jonkers YM, Vlenterie M, van de Luijtgaarden AC, van der Graaf WT. Anti-angiogenic therapy, a new player in the field of sarcoma treatment. Crit Rev Oncol Hematol. 2014;91(2):172-185.
19. van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879-1886.
20. Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488-497.
21. Chi Y, Yao Y, Wang S, et al. Anlotinib for metastatic soft tissue sarcoma: A randomized, double-blind, placebo-controlled and multi-centered clinical trial. J Clin Oncol. 2018;36(suppl):abstr 11503.
22. Brohl AS, Shah HR, Wang Y-C, Kasarskis A, Maki RG. The somatic mutational landscape in soft tissue sarcoma: Early results from TCGA data. J Clin Oncol. 2015;33(15_suppl):10508-10508.
23. Crompton BD, Stewart C, Taylor-Weiner A, et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov. 2014;4(11):1326-1341.
24. Jour G, Scarborough JD, Jones RL, et al. Molecular profiling of soft tissue sarcomas using next-generation sequencing: a pilot study toward precision therapeutics. Hum Pathol. 2014;45(8):1563-1571.
25. Yang J-L. Investigation of osteosarcoma genomics and its impact on targeted therapy: an international collaboration to conquer human osteosarcoma. Chin J Cancer. 2014;33(12):575-580.
26. Cidre-Aranaz F, Alonso J. EWS/FLI1 target genes and therapeutic opportunities in Ewing sarcoma. Front Oncol. 2015;5:162.
27. Savoia C, Volpe M, Grassi G, Borghi C, Agabiti Rosei E, Touyz RM. Personalized medicine-a modern approach for the diagnosis and management of hypertension. Clin Sci (Lond). 2017;131(22):2671-2685.
28. Biswas B, Bakhshi S. Management of Ewing sarcoma family of tumors: Current scenario and unmet need. World J Orthop. 2016;7(9):527-538.
29. van Maldegem AM, Bovée JVMG, Peterse EFP, Hogendoorn PCW, Gelderblom H. Ewing sarcoma: the clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur J Cancer. 2016;53:171-180.
30. Subbiah V, Hess KR, Khawaja MR, et al. Evaluation of novel targeted therapies in aggressive biology sarcoma patients after progression from US FDA approved therapies. Sci Rep. 2016;6:35448.
31. Jessen K, Moseley E, Chung EYL, et al. TK216, a novel, small molecule inhibitor of the ETS-family of transcription factors, displays anti-tumor activity in AML and DLBCL. Blood. 2016;128(22):4035-4035.
32. Sankhala K, Potts S, Christiansen J, et al. Immunohistochemistry screening to increase the efficacy of next-generation sequencing for detection of NTRK, ROS1, and ALK gene rearrangements (fusions) in sarcoma patients. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 9-12, 2016, 2016; Lisbon, Portugal.
33. Renfro LA, An MW, Mandrekar SJ. Precision oncology: a new era of cancer clinical trials. Cancer Lett. 2017;387:121-126.
34. DuBois S, Laetsch T, Federman N, et al. The use of larotrectinib in the management of locally advanced pediatric NTRK-fusion sarcoma. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 8-11, 2017; Maui, Hawaii.
35. Multani P, Manavel E, Hornby Z. Preliminary evidence of clinical response to entrectinib in three sarcome patients. Paper presented at: Connective Tissue Oncology Society Annual Meeting; November 8-11, 2017; Maui, Hawaii.
36. Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715-721.
37. Dickson MA, Schwartz GK, Keohan ML, et al. Progression-free survival among patients with well-differentiated or dedifferentiated liposarcoma treated with CDK4 inhibitor palbociclib: a phase 2 clinical trial. JAMA Oncol. 2016;2(7):937-940.
38. Barretina J, Caponigro G, Stransky N, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603-607.
39. Kenichi K, Yoshinao O. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Science. 2017;108(4):547-552.
40. US Food and Drug Administration. Orphan drug designations and approvals. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=544416. Designated date September 28, 2017. Accessed July 4, 2018.
41. Press release. Epizyme provides update regarding tazemetostat clinical program. https://globenewswire.com/news-release/2018/04/23/1485765/0/en/Epizyme-Provides-Update-Regarding-Tazemetostat-Clinical-Program.html. Released April 23, 2018. Accessed July 4, 2018.
42. Bean GR, Kremer JC, Prudner BC, et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death &Amp; Disease. 2016;7:e2406.
43. Bourcier K, Italiano A. Newer therapeutic strategies for soft-tissue sarcomas. Pharmacol Ther. 2018;188:118-123.
44. Somaiah N, Chawla SP, Block MS, et al. Immune response, safety, and survival impact from CMB305 in NY-ESO-1+ recurrent soft tissue sarcomas (STS). J Clin Oncol. 2017;35(15_suppl):11006-11006.