User login
Inherited mutations drive 12% of Nigerian breast cancer
About one in eight Nigerian women with breast cancer has an inherited mutation of the BRCA1, BRCA2, PALB2, or TP53 gene.
A new analysis of the Nigerian Breast Cancer Study confirmed that these inherited mutations drive about 12% of the country’s breast cancer cases. The findings could pave the way for the first large-scale national breast cancer gene screening program, wrote Olufunmilayo I. Olopade, MD, and her colleagues. The report is in the Journal of Clinical Oncology.
“We suggest that genomic sequencing to identify women at extremely high risk of breast cancer could be a highly innovative approach to tailored risk management and life-saving interventions,” wrote Dr. Olopade, director of the Center for Clinical Cancer Genetics at the University of Chicago, and her colleagues. “Nigeria now has data to prioritize the integration of genetic testing into its cancer control plan. Women with an extremely high risk of breast cancer because of mutations in these genes can be identified inexpensively and unambiguously and offered interventions to reduce cancer risk.”
And, since about half of the sisters and daughters of affected women will carry the same mutation, such a screening program could reach far beyond every index patient identified, the investigators noted.
“If these women at very high risk can be identified either through their relatives with breast cancer or in the general population, resources can be focused particularly on their behalf. For as-yet unaffected women at high genetic risk, these resources would be intensive surveillance for early detection of breast cancer and, after childbearing is completed, the possibility of preventive salpingo-oophorectomy. Integrated population screening for cancer for all women is the goal, but focused outreach to women at extremely high risk represents an especially efficient use of resources and an attainable evidence-based global health approach.”
The Nigerian Breast Cancer Study enrolled 1,136 women with invasive breast cancer from 1998 to 2014. These were compared with 997 women without cancer, matched from the same communities. Genetic sequencing searched for mutations in both known and breast cancer genes.
Cases and controls were a mean of 47 years old; only 6% of cases reported a family history of breast cancer. Of 577 patients with information on tumor stage, 86% (497) were diagnosed at stage III (241) or IV (256).
Among the cases, 167 (14.7%) carried a mutation in a breast cancer risk gene, compared with 1.8% of controls. BRCA1 was the most common mutation, occurring in 7% of patients; these women were 23 times more likely to develop breast cancer than were those without the gene (odds ratio, 23.4). BRCA2 was the next most common, occurring in 4% of cases and conferring a nearly 11-fold increased risk (OR, 10.76). PALB2 occurred in 11 cases (1%) and no controls, and TP53 in four cases (0.4%).
Women with the BRCA1 mutation were diagnosed at a significantly younger age than were other patients (42.6 vs. 47.9 years), as were carriers of the TP53 mutation (32.8 vs. 47.6 years).
Ten other genes (ATM, BARD1, BRIP1, CHEK1, CHEK2, GEN1, NBN, RAD51C, RAD51D, and XRCC2) carried a mutation in at least one patient each. “When limited to mutations in the four high-risk genes, 11%-12% of cases in this study carried a loss-of-function variant.”
Dr. Olopade had no financial disclosures.
SOURCE: Olopade et al. J Clin Oncol. 2018 Aug 21. doi: 10.1200/JCO.2018.78.3977.
The findings of the Nigerian Breast Cancer Study make a case for large-scale breast cancer gene screening. But even in a wealthy country with good infrastructure, such a program would be dauntingly complex, Ophira Ginsburg, MD, and Paul Brennan, PhD, wrote in an accompanying editorial.
“Given the estimated 40,983 women in Nigeria younger than age 65 years who will be newly diagnosed with breast cancer in 2030, the estimated mutation carrier frequency for a high-risk gene of 11%-12% translates to approximately 5,000 women with breast cancer each year who might benefit directly from tailored risk-reducing strategies. Moreover, 50% of these women’s sisters and daughters would also stand to benefit,” they wrote.
However, 32 million women would need to be screened to find the 220,000 with one of the mutations – a task that is “clearly beyond the scope of most countries.
“Furthermore, women with pathogenic variants would require intensive follow-up and intervention strategies to reduce their risk of developing breast, ovarian/fallopian tube, and potentially other cancers depending on the gene involved. Importantly, this approach would not address the larger problem of the high breast cancer mortality among the vast majority of women without a pathogenic variant but who make up approximately 85% of the breast cancer burden.”
The World Health Organization recognizes this challenge; the agency doesn’t even recommend mammogram-based population screening unless there is a basic, reliable infrastructure including electricity, quality-assurance measures, referral and recall mechanisms, and monitoring and evaluation frameworks. But WHO does suggest some core elements to guide a country’s comprehensive cancer management strategy, including:
• Considering the whole continuum from prevention to palliation.
• Providing a sustainable strategic plan on the basis of the country’s cancer burden, risk factor prevalence, and the resources available to implement the plan.
• Developing an evidence-based approach generated by population-based cancer registries.
“As many countries improve their cancer systems, investing in human resources, infrastructure, monitoring, and evaluation, it is timely to consider how to evaluate readiness to undertake a population-level cancer genetics intervention and consider the core elements that should be in place to make a substantive effect on cancer mortality.”
Dr. Ginsburg is with the Perlmutter Cancer Center of New York University. Dr. Brennan is with the International Agency for Research on Cancer, Lyon, France.
The findings of the Nigerian Breast Cancer Study make a case for large-scale breast cancer gene screening. But even in a wealthy country with good infrastructure, such a program would be dauntingly complex, Ophira Ginsburg, MD, and Paul Brennan, PhD, wrote in an accompanying editorial.
“Given the estimated 40,983 women in Nigeria younger than age 65 years who will be newly diagnosed with breast cancer in 2030, the estimated mutation carrier frequency for a high-risk gene of 11%-12% translates to approximately 5,000 women with breast cancer each year who might benefit directly from tailored risk-reducing strategies. Moreover, 50% of these women’s sisters and daughters would also stand to benefit,” they wrote.
However, 32 million women would need to be screened to find the 220,000 with one of the mutations – a task that is “clearly beyond the scope of most countries.
“Furthermore, women with pathogenic variants would require intensive follow-up and intervention strategies to reduce their risk of developing breast, ovarian/fallopian tube, and potentially other cancers depending on the gene involved. Importantly, this approach would not address the larger problem of the high breast cancer mortality among the vast majority of women without a pathogenic variant but who make up approximately 85% of the breast cancer burden.”
The World Health Organization recognizes this challenge; the agency doesn’t even recommend mammogram-based population screening unless there is a basic, reliable infrastructure including electricity, quality-assurance measures, referral and recall mechanisms, and monitoring and evaluation frameworks. But WHO does suggest some core elements to guide a country’s comprehensive cancer management strategy, including:
• Considering the whole continuum from prevention to palliation.
• Providing a sustainable strategic plan on the basis of the country’s cancer burden, risk factor prevalence, and the resources available to implement the plan.
• Developing an evidence-based approach generated by population-based cancer registries.
“As many countries improve their cancer systems, investing in human resources, infrastructure, monitoring, and evaluation, it is timely to consider how to evaluate readiness to undertake a population-level cancer genetics intervention and consider the core elements that should be in place to make a substantive effect on cancer mortality.”
Dr. Ginsburg is with the Perlmutter Cancer Center of New York University. Dr. Brennan is with the International Agency for Research on Cancer, Lyon, France.
The findings of the Nigerian Breast Cancer Study make a case for large-scale breast cancer gene screening. But even in a wealthy country with good infrastructure, such a program would be dauntingly complex, Ophira Ginsburg, MD, and Paul Brennan, PhD, wrote in an accompanying editorial.
“Given the estimated 40,983 women in Nigeria younger than age 65 years who will be newly diagnosed with breast cancer in 2030, the estimated mutation carrier frequency for a high-risk gene of 11%-12% translates to approximately 5,000 women with breast cancer each year who might benefit directly from tailored risk-reducing strategies. Moreover, 50% of these women’s sisters and daughters would also stand to benefit,” they wrote.
However, 32 million women would need to be screened to find the 220,000 with one of the mutations – a task that is “clearly beyond the scope of most countries.
“Furthermore, women with pathogenic variants would require intensive follow-up and intervention strategies to reduce their risk of developing breast, ovarian/fallopian tube, and potentially other cancers depending on the gene involved. Importantly, this approach would not address the larger problem of the high breast cancer mortality among the vast majority of women without a pathogenic variant but who make up approximately 85% of the breast cancer burden.”
The World Health Organization recognizes this challenge; the agency doesn’t even recommend mammogram-based population screening unless there is a basic, reliable infrastructure including electricity, quality-assurance measures, referral and recall mechanisms, and monitoring and evaluation frameworks. But WHO does suggest some core elements to guide a country’s comprehensive cancer management strategy, including:
• Considering the whole continuum from prevention to palliation.
• Providing a sustainable strategic plan on the basis of the country’s cancer burden, risk factor prevalence, and the resources available to implement the plan.
• Developing an evidence-based approach generated by population-based cancer registries.
“As many countries improve their cancer systems, investing in human resources, infrastructure, monitoring, and evaluation, it is timely to consider how to evaluate readiness to undertake a population-level cancer genetics intervention and consider the core elements that should be in place to make a substantive effect on cancer mortality.”
Dr. Ginsburg is with the Perlmutter Cancer Center of New York University. Dr. Brennan is with the International Agency for Research on Cancer, Lyon, France.
About one in eight Nigerian women with breast cancer has an inherited mutation of the BRCA1, BRCA2, PALB2, or TP53 gene.
A new analysis of the Nigerian Breast Cancer Study confirmed that these inherited mutations drive about 12% of the country’s breast cancer cases. The findings could pave the way for the first large-scale national breast cancer gene screening program, wrote Olufunmilayo I. Olopade, MD, and her colleagues. The report is in the Journal of Clinical Oncology.
“We suggest that genomic sequencing to identify women at extremely high risk of breast cancer could be a highly innovative approach to tailored risk management and life-saving interventions,” wrote Dr. Olopade, director of the Center for Clinical Cancer Genetics at the University of Chicago, and her colleagues. “Nigeria now has data to prioritize the integration of genetic testing into its cancer control plan. Women with an extremely high risk of breast cancer because of mutations in these genes can be identified inexpensively and unambiguously and offered interventions to reduce cancer risk.”
And, since about half of the sisters and daughters of affected women will carry the same mutation, such a screening program could reach far beyond every index patient identified, the investigators noted.
“If these women at very high risk can be identified either through their relatives with breast cancer or in the general population, resources can be focused particularly on their behalf. For as-yet unaffected women at high genetic risk, these resources would be intensive surveillance for early detection of breast cancer and, after childbearing is completed, the possibility of preventive salpingo-oophorectomy. Integrated population screening for cancer for all women is the goal, but focused outreach to women at extremely high risk represents an especially efficient use of resources and an attainable evidence-based global health approach.”
The Nigerian Breast Cancer Study enrolled 1,136 women with invasive breast cancer from 1998 to 2014. These were compared with 997 women without cancer, matched from the same communities. Genetic sequencing searched for mutations in both known and breast cancer genes.
Cases and controls were a mean of 47 years old; only 6% of cases reported a family history of breast cancer. Of 577 patients with information on tumor stage, 86% (497) were diagnosed at stage III (241) or IV (256).
Among the cases, 167 (14.7%) carried a mutation in a breast cancer risk gene, compared with 1.8% of controls. BRCA1 was the most common mutation, occurring in 7% of patients; these women were 23 times more likely to develop breast cancer than were those without the gene (odds ratio, 23.4). BRCA2 was the next most common, occurring in 4% of cases and conferring a nearly 11-fold increased risk (OR, 10.76). PALB2 occurred in 11 cases (1%) and no controls, and TP53 in four cases (0.4%).
Women with the BRCA1 mutation were diagnosed at a significantly younger age than were other patients (42.6 vs. 47.9 years), as were carriers of the TP53 mutation (32.8 vs. 47.6 years).
Ten other genes (ATM, BARD1, BRIP1, CHEK1, CHEK2, GEN1, NBN, RAD51C, RAD51D, and XRCC2) carried a mutation in at least one patient each. “When limited to mutations in the four high-risk genes, 11%-12% of cases in this study carried a loss-of-function variant.”
Dr. Olopade had no financial disclosures.
SOURCE: Olopade et al. J Clin Oncol. 2018 Aug 21. doi: 10.1200/JCO.2018.78.3977.
About one in eight Nigerian women with breast cancer has an inherited mutation of the BRCA1, BRCA2, PALB2, or TP53 gene.
A new analysis of the Nigerian Breast Cancer Study confirmed that these inherited mutations drive about 12% of the country’s breast cancer cases. The findings could pave the way for the first large-scale national breast cancer gene screening program, wrote Olufunmilayo I. Olopade, MD, and her colleagues. The report is in the Journal of Clinical Oncology.
“We suggest that genomic sequencing to identify women at extremely high risk of breast cancer could be a highly innovative approach to tailored risk management and life-saving interventions,” wrote Dr. Olopade, director of the Center for Clinical Cancer Genetics at the University of Chicago, and her colleagues. “Nigeria now has data to prioritize the integration of genetic testing into its cancer control plan. Women with an extremely high risk of breast cancer because of mutations in these genes can be identified inexpensively and unambiguously and offered interventions to reduce cancer risk.”
And, since about half of the sisters and daughters of affected women will carry the same mutation, such a screening program could reach far beyond every index patient identified, the investigators noted.
“If these women at very high risk can be identified either through their relatives with breast cancer or in the general population, resources can be focused particularly on their behalf. For as-yet unaffected women at high genetic risk, these resources would be intensive surveillance for early detection of breast cancer and, after childbearing is completed, the possibility of preventive salpingo-oophorectomy. Integrated population screening for cancer for all women is the goal, but focused outreach to women at extremely high risk represents an especially efficient use of resources and an attainable evidence-based global health approach.”
The Nigerian Breast Cancer Study enrolled 1,136 women with invasive breast cancer from 1998 to 2014. These were compared with 997 women without cancer, matched from the same communities. Genetic sequencing searched for mutations in both known and breast cancer genes.
Cases and controls were a mean of 47 years old; only 6% of cases reported a family history of breast cancer. Of 577 patients with information on tumor stage, 86% (497) were diagnosed at stage III (241) or IV (256).
Among the cases, 167 (14.7%) carried a mutation in a breast cancer risk gene, compared with 1.8% of controls. BRCA1 was the most common mutation, occurring in 7% of patients; these women were 23 times more likely to develop breast cancer than were those without the gene (odds ratio, 23.4). BRCA2 was the next most common, occurring in 4% of cases and conferring a nearly 11-fold increased risk (OR, 10.76). PALB2 occurred in 11 cases (1%) and no controls, and TP53 in four cases (0.4%).
Women with the BRCA1 mutation were diagnosed at a significantly younger age than were other patients (42.6 vs. 47.9 years), as were carriers of the TP53 mutation (32.8 vs. 47.6 years).
Ten other genes (ATM, BARD1, BRIP1, CHEK1, CHEK2, GEN1, NBN, RAD51C, RAD51D, and XRCC2) carried a mutation in at least one patient each. “When limited to mutations in the four high-risk genes, 11%-12% of cases in this study carried a loss-of-function variant.”
Dr. Olopade had no financial disclosures.
SOURCE: Olopade et al. J Clin Oncol. 2018 Aug 21. doi: 10.1200/JCO.2018.78.3977.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Loss-of-function mutations in four breast cancer risk genes account for much of the disease among Nigerian women with the disease.
Major finding: Inherited mutations of the BRCA1, BRCA2, PALB2, or TP53 gene account for 12% of breast cancer in Nigerian women.
Study details: The Nigerian Breast Cancer Study comprised 1,136 women with invasive breast cancer and 997 controls.
Disclosures: Dr. Olopade had no financial disclosures. The study was largely funded by the National Institutes of Health and the Susan G Komen Foundation.
Source: Olopade et al. J Clin Oncol. 2018 Aug 21. doi: 10.1200/JCO.2018.78.3977.
RA, JIA may raise risk of preterm delivery
according to a study examining autoimmune disease in pregnancy. Corticosteroid use in any trimester increased that risk from 100%-400%, regardless of how active the arthritis was.
The study found that women with RA had more than double the risk for preterm delivery, compared with a cohort without autoimmune disease (relative risk, 2.09; 95% confidence interval, 1.50-2.91). For women with juvenile idiopathic arthritis (JIA), the relative risk was 1.81 for preterm delivery (95% CI, 1.14-2.89).
The prospective cohort study, part of the Organization of Teratology Information Specialists Autoimmune Disease in Pregnancy Project, enrolled 657 women with RA and 170 women with JIA. The study also included a comparison group of 564 women without autoimmune disease. All of those included in the study were enrolled before 19 weeks’ gestation and delivered live-born infants during 2004-2017.
The study adds to a clinically important area of research that has yielded sometimes conflicting results; clarity has also been impeded by a variety of methodologies. Though several analyses have shown higher risk of preterm delivery in women with RA, not all studies have adjusted for medication use and disease activity, Chelsey F. Smith, MD, and her coauthors wrote in Arthritis Care & Research. Further, how women with JIA fare in pregnancy has not been well studied, they said.
Dr. Smith, a rheumatologist at the University of California, San Diego, and her colleagues included many baseline covariates in their analysis of pregnancy outcomes; these included maternal age and race, socioeconomic status, body mass index, previous adverse pregnancy outcomes, and comorbidities, including autoimmune disease. Adverse pregnancy outcomes during the studied pregnancy were also included as covariates, and deliveries were considered preterm if labor began before 37 weeks’ gestation.
For women with RA, a higher active disease score at any point during pregnancy was associated with a significantly higher risk of preterm delivery, even after adjustment for other potential risk factors, including first-trimester corticosteroid use (adjusted risk ratio, 1.52; 95% CI, 1.06-2.18). The persistence of this association, wrote Dr. Smith and her colleagues, implies “that active disease in RA may contribute to [preterm delivery] independent of medications,” perhaps through the action of proinflammatory cytokines that may stimulate prostaglandins and provoke uterine contractions.
The researchers found, though, that this association between disease activity and risk for preterm birth did not hold true for women with JIA, leaving part of the mystery unsolved.
However, women with both RA and JIA who used corticosteroids in any trimester were more likely to have a preterm delivery, as were women with JIA who used NSAIDs in the first trimester of pregnancy. The use of disease-modifying antirheumatic drugs (DMARDs) and biologics in any trimester did not confer increased risk for preterm delivery in women with either disease state.
There were other differences between the groups: Women with JIA were overall younger, but had more prepregnancy hypertension, which “may have contributed to the elevated incidence of preeclampsia seen in this group,” the investigators wrote. Fever was more common in women with JIA, and had an independent association with preterm delivery, as did first trimester NSAID use in this group alone.
Dr. Smith and her colleagues hypothesized that the relative heterogeneity of the JIA group may mean that disease activity still influenced outcomes.
Among other comorbidities, gestational diabetes (GDM) was more common in the RA group than in the JIA group or the comparison cohort, and was associated with a significantly higher risk for preterm delivery in women with RA, even after accounting for preeclampsia and hypertension in a multivariate analysis.
Dr. Smith and her colleagues pointed out that it was difficult to account for physician behavior in managing pregnancy in these high-risk women. “Additionally, given that women with both RA and GDM are at a particularly high risk for perinatal complications, we can speculate that the obstetricians in this group were perhaps more aggressive about inducing at an earlier gestational age than in other groups, but this information was not available in the dataset.”
Through phone interviews, investigators obtained information about prescription and nonprescription medication use during pregnancy; women were also asked about use of other substances and occupational exposures, infections, and prenatal testing and other medical procedures. Another telephone interview conducted soon after delivery asked about birth outcomes. Abstracted medical record data were used to verify and supplement the interview information.
When looking at treatments used, Dr. Smith and her colleagues grouped autoimmune disease medications into DMARDs, non-DMARD biologic medications, corticosteroids, and NSAIDs.
Disease activity assessment, conducted at intake and at 32 weeks’ gestation, used the Health Assessment Questionnaire, pain scores, and patient global disease activity to calculate a Patient Activity Scale score ranging from 0 to 10. Patients with a score over 3.7 were classified as having high disease activity.
Dr. Smith and her colleagues said that the study’s strengths included its prospective design and robust statistical schema. Also, using data about corticosteroid use and disease activity earlier in pregnancy avoided the inclusion of a reverse causation effect, where systemic inflammatory changes associated with preterm delivery might provoke more disease activity and a consequent boost in corticosteroid use.
However, the researchers said, the overall numbers of participants with preterm delivery was relatively small, and the JIA cohort was small as well.
“Further analyses are necessary to look at other categories of arthritis affecting women of childbearing age, racial disparities in these populations, as well as the influence of disease activity in the later stages of pregnancy on other perinatal factors” that can contribute to preterm delivery, said Dr. Smith and her colleagues.
The collaborative research group that collected data for the study receives research funding from several pharmaceutical companies. None of the authors reported any personal conflicts of interest.
SOURCE: Smith CF et al. Arthritis Care Res. 2018 Aug 21. doi: 10.1002/acr.23730.
according to a study examining autoimmune disease in pregnancy. Corticosteroid use in any trimester increased that risk from 100%-400%, regardless of how active the arthritis was.
The study found that women with RA had more than double the risk for preterm delivery, compared with a cohort without autoimmune disease (relative risk, 2.09; 95% confidence interval, 1.50-2.91). For women with juvenile idiopathic arthritis (JIA), the relative risk was 1.81 for preterm delivery (95% CI, 1.14-2.89).
The prospective cohort study, part of the Organization of Teratology Information Specialists Autoimmune Disease in Pregnancy Project, enrolled 657 women with RA and 170 women with JIA. The study also included a comparison group of 564 women without autoimmune disease. All of those included in the study were enrolled before 19 weeks’ gestation and delivered live-born infants during 2004-2017.
The study adds to a clinically important area of research that has yielded sometimes conflicting results; clarity has also been impeded by a variety of methodologies. Though several analyses have shown higher risk of preterm delivery in women with RA, not all studies have adjusted for medication use and disease activity, Chelsey F. Smith, MD, and her coauthors wrote in Arthritis Care & Research. Further, how women with JIA fare in pregnancy has not been well studied, they said.
Dr. Smith, a rheumatologist at the University of California, San Diego, and her colleagues included many baseline covariates in their analysis of pregnancy outcomes; these included maternal age and race, socioeconomic status, body mass index, previous adverse pregnancy outcomes, and comorbidities, including autoimmune disease. Adverse pregnancy outcomes during the studied pregnancy were also included as covariates, and deliveries were considered preterm if labor began before 37 weeks’ gestation.
For women with RA, a higher active disease score at any point during pregnancy was associated with a significantly higher risk of preterm delivery, even after adjustment for other potential risk factors, including first-trimester corticosteroid use (adjusted risk ratio, 1.52; 95% CI, 1.06-2.18). The persistence of this association, wrote Dr. Smith and her colleagues, implies “that active disease in RA may contribute to [preterm delivery] independent of medications,” perhaps through the action of proinflammatory cytokines that may stimulate prostaglandins and provoke uterine contractions.
The researchers found, though, that this association between disease activity and risk for preterm birth did not hold true for women with JIA, leaving part of the mystery unsolved.
However, women with both RA and JIA who used corticosteroids in any trimester were more likely to have a preterm delivery, as were women with JIA who used NSAIDs in the first trimester of pregnancy. The use of disease-modifying antirheumatic drugs (DMARDs) and biologics in any trimester did not confer increased risk for preterm delivery in women with either disease state.
There were other differences between the groups: Women with JIA were overall younger, but had more prepregnancy hypertension, which “may have contributed to the elevated incidence of preeclampsia seen in this group,” the investigators wrote. Fever was more common in women with JIA, and had an independent association with preterm delivery, as did first trimester NSAID use in this group alone.
Dr. Smith and her colleagues hypothesized that the relative heterogeneity of the JIA group may mean that disease activity still influenced outcomes.
Among other comorbidities, gestational diabetes (GDM) was more common in the RA group than in the JIA group or the comparison cohort, and was associated with a significantly higher risk for preterm delivery in women with RA, even after accounting for preeclampsia and hypertension in a multivariate analysis.
Dr. Smith and her colleagues pointed out that it was difficult to account for physician behavior in managing pregnancy in these high-risk women. “Additionally, given that women with both RA and GDM are at a particularly high risk for perinatal complications, we can speculate that the obstetricians in this group were perhaps more aggressive about inducing at an earlier gestational age than in other groups, but this information was not available in the dataset.”
Through phone interviews, investigators obtained information about prescription and nonprescription medication use during pregnancy; women were also asked about use of other substances and occupational exposures, infections, and prenatal testing and other medical procedures. Another telephone interview conducted soon after delivery asked about birth outcomes. Abstracted medical record data were used to verify and supplement the interview information.
When looking at treatments used, Dr. Smith and her colleagues grouped autoimmune disease medications into DMARDs, non-DMARD biologic medications, corticosteroids, and NSAIDs.
Disease activity assessment, conducted at intake and at 32 weeks’ gestation, used the Health Assessment Questionnaire, pain scores, and patient global disease activity to calculate a Patient Activity Scale score ranging from 0 to 10. Patients with a score over 3.7 were classified as having high disease activity.
Dr. Smith and her colleagues said that the study’s strengths included its prospective design and robust statistical schema. Also, using data about corticosteroid use and disease activity earlier in pregnancy avoided the inclusion of a reverse causation effect, where systemic inflammatory changes associated with preterm delivery might provoke more disease activity and a consequent boost in corticosteroid use.
However, the researchers said, the overall numbers of participants with preterm delivery was relatively small, and the JIA cohort was small as well.
“Further analyses are necessary to look at other categories of arthritis affecting women of childbearing age, racial disparities in these populations, as well as the influence of disease activity in the later stages of pregnancy on other perinatal factors” that can contribute to preterm delivery, said Dr. Smith and her colleagues.
The collaborative research group that collected data for the study receives research funding from several pharmaceutical companies. None of the authors reported any personal conflicts of interest.
SOURCE: Smith CF et al. Arthritis Care Res. 2018 Aug 21. doi: 10.1002/acr.23730.
according to a study examining autoimmune disease in pregnancy. Corticosteroid use in any trimester increased that risk from 100%-400%, regardless of how active the arthritis was.
The study found that women with RA had more than double the risk for preterm delivery, compared with a cohort without autoimmune disease (relative risk, 2.09; 95% confidence interval, 1.50-2.91). For women with juvenile idiopathic arthritis (JIA), the relative risk was 1.81 for preterm delivery (95% CI, 1.14-2.89).
The prospective cohort study, part of the Organization of Teratology Information Specialists Autoimmune Disease in Pregnancy Project, enrolled 657 women with RA and 170 women with JIA. The study also included a comparison group of 564 women without autoimmune disease. All of those included in the study were enrolled before 19 weeks’ gestation and delivered live-born infants during 2004-2017.
The study adds to a clinically important area of research that has yielded sometimes conflicting results; clarity has also been impeded by a variety of methodologies. Though several analyses have shown higher risk of preterm delivery in women with RA, not all studies have adjusted for medication use and disease activity, Chelsey F. Smith, MD, and her coauthors wrote in Arthritis Care & Research. Further, how women with JIA fare in pregnancy has not been well studied, they said.
Dr. Smith, a rheumatologist at the University of California, San Diego, and her colleagues included many baseline covariates in their analysis of pregnancy outcomes; these included maternal age and race, socioeconomic status, body mass index, previous adverse pregnancy outcomes, and comorbidities, including autoimmune disease. Adverse pregnancy outcomes during the studied pregnancy were also included as covariates, and deliveries were considered preterm if labor began before 37 weeks’ gestation.
For women with RA, a higher active disease score at any point during pregnancy was associated with a significantly higher risk of preterm delivery, even after adjustment for other potential risk factors, including first-trimester corticosteroid use (adjusted risk ratio, 1.52; 95% CI, 1.06-2.18). The persistence of this association, wrote Dr. Smith and her colleagues, implies “that active disease in RA may contribute to [preterm delivery] independent of medications,” perhaps through the action of proinflammatory cytokines that may stimulate prostaglandins and provoke uterine contractions.
The researchers found, though, that this association between disease activity and risk for preterm birth did not hold true for women with JIA, leaving part of the mystery unsolved.
However, women with both RA and JIA who used corticosteroids in any trimester were more likely to have a preterm delivery, as were women with JIA who used NSAIDs in the first trimester of pregnancy. The use of disease-modifying antirheumatic drugs (DMARDs) and biologics in any trimester did not confer increased risk for preterm delivery in women with either disease state.
There were other differences between the groups: Women with JIA were overall younger, but had more prepregnancy hypertension, which “may have contributed to the elevated incidence of preeclampsia seen in this group,” the investigators wrote. Fever was more common in women with JIA, and had an independent association with preterm delivery, as did first trimester NSAID use in this group alone.
Dr. Smith and her colleagues hypothesized that the relative heterogeneity of the JIA group may mean that disease activity still influenced outcomes.
Among other comorbidities, gestational diabetes (GDM) was more common in the RA group than in the JIA group or the comparison cohort, and was associated with a significantly higher risk for preterm delivery in women with RA, even after accounting for preeclampsia and hypertension in a multivariate analysis.
Dr. Smith and her colleagues pointed out that it was difficult to account for physician behavior in managing pregnancy in these high-risk women. “Additionally, given that women with both RA and GDM are at a particularly high risk for perinatal complications, we can speculate that the obstetricians in this group were perhaps more aggressive about inducing at an earlier gestational age than in other groups, but this information was not available in the dataset.”
Through phone interviews, investigators obtained information about prescription and nonprescription medication use during pregnancy; women were also asked about use of other substances and occupational exposures, infections, and prenatal testing and other medical procedures. Another telephone interview conducted soon after delivery asked about birth outcomes. Abstracted medical record data were used to verify and supplement the interview information.
When looking at treatments used, Dr. Smith and her colleagues grouped autoimmune disease medications into DMARDs, non-DMARD biologic medications, corticosteroids, and NSAIDs.
Disease activity assessment, conducted at intake and at 32 weeks’ gestation, used the Health Assessment Questionnaire, pain scores, and patient global disease activity to calculate a Patient Activity Scale score ranging from 0 to 10. Patients with a score over 3.7 were classified as having high disease activity.
Dr. Smith and her colleagues said that the study’s strengths included its prospective design and robust statistical schema. Also, using data about corticosteroid use and disease activity earlier in pregnancy avoided the inclusion of a reverse causation effect, where systemic inflammatory changes associated with preterm delivery might provoke more disease activity and a consequent boost in corticosteroid use.
However, the researchers said, the overall numbers of participants with preterm delivery was relatively small, and the JIA cohort was small as well.
“Further analyses are necessary to look at other categories of arthritis affecting women of childbearing age, racial disparities in these populations, as well as the influence of disease activity in the later stages of pregnancy on other perinatal factors” that can contribute to preterm delivery, said Dr. Smith and her colleagues.
The collaborative research group that collected data for the study receives research funding from several pharmaceutical companies. None of the authors reported any personal conflicts of interest.
SOURCE: Smith CF et al. Arthritis Care Res. 2018 Aug 21. doi: 10.1002/acr.23730.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The risk of preterm delivery was increased in women with RA and juvenile idiopathic arthritis.
Major finding: The risk ratio for preterm delivery in women with RA was 2.09.
Study details: A prospective cohort study of 657 women with RA, 170 women with juvenile idiopathic arthritis, and 564 women without autoimmune disease.
Disclosures: The study was part of the Organization of Teratology Information Specialists Autoimmune Disease in Pregnancy Project, which receives research funding from several pharmaceutical companies. None of the authors reported any personal conflicts of interest.
Source: Smith CF et al. Arthritis Care Res. 2018 Aug 21. doi: 10.1002/acr.23730.
High-sensitivity cardiac troponin levels linked to cardiovascular outcomes in COPD patients
, according to a post-hoc analysis of a clinical trial.
An increased risk of cardiovascular adverse events and cardiovascular death was seen in COPD patients in the highest quintile of plasma cardiac troponin concentrations at baseline, results of the analysis show.
The findings highlight the potential utility of high-sensitivity cardiac troponin in both clinical trials and clinical practice, according to researcher Nicholas L. Mills, MD, PhD, BHF/University Centre for Cardiovascular Science, The University of Edinburgh, Scotland, and co-investigators.
“Recognizing the risk associated with increased troponin concentrations might encourage clinicians to address cardiovascular risk due to lifestyle choices, and make patients more likely to engage with these recommendations,” Dr. Mills and co-authors wrote in the Journal of the American College of Cardiology.
Improved risk stratification may also help clinicians more appropriately target the use of preventive medications in COPD patients, they added in the report.
The analysis by Dr. Mills and colleagues was based on assessment of cardiac troponin I concentrations for patients in SUMMIT, a randomized trial assessing inhaled corticosteroids and long-acting beta agonists in COPD patients with ele-vated cardiovascular risk.
A total of 1,599 patients in the SUMMIT trial had a baseline cardiac troponin I assessment, and 1,258 had a follow-up assessment at 3 months following randomization.
Compared with those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event, even after adjusting for confounding variables (hazard ratio, 3.67; 95% confidence interval, 1.33-10.13; P = .012)..
Increased risk of cardiovascular death was also seen in the highest quintile as compared with the lowest quintile (HR, 20.06; 95% CI, 2.44-165.15; P = .005), investigators said.
There was no difference in risk of COPD exacerbations between the highest and lowest quintiles, they added.
At 3 months, there were no differences in troponin concentrations related to COPD treatment, consistent with previous observations in the SUMMIT trial that treatment did not impact the cardiovascular composite endpoint, investigators said.
However, patients with a plasma troponin of 5 ng/L or greater recorded at either the baseline or 3-month assessment had an increased rate of the composite cardiovascular endpoint and a “markedly increased” risk of cardiovascular death, they wrote.
The research was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship received by Dr. Mills. Disclosures reported by Dr. Mills included consultancy, research grants, and speaker fees from Abbott Diagnostics, Roche, and Singulex. Study co-authors reported disclosures related to GlaxoSmithKline, Veramed Limited, AstraZeneca, Zambon, Bayer, Novartis, and others.
SOURCE: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
The current study data are “robust” and suggest a strong association between high-sensitivity cardiac troponin values and cardiovascular event risk in these COPD patients, according to authors of an editorial.
The study also showed that a change in high-sensitivity cardiac troponin at 3 months is associated with increased risk, noted editorial authors Allan S. Jaffe, MD, and H. Ari Jaffe, MD.
“Most of these events probably represent acceleration of atherosclerosis, given the effects of smoking on atherosclerotic disease and its progression,” the authors said in the Journal of the American College of Cardiology.
However, study authors could have more extensively addressed how to use that information to improve the care of COPD patients at elevated cardiovascular event risk, they added.
A “pilot algorithm” that could be used to apply this biomarker analysis in clinical practice was proposed in an editorial accompanying the research report.
They suggest repeating high-sensitivity cardiac troponin measurements to reduce variability, as well as repeating samples at 3 months to detect changes that could signal increased risk.
“In addition, one should avoid decisions based on small differences,” they wrote.
Allan S. Jaffe, MD, is with the department of cardiovascular medicine and the department of laboratory medicine and pathology at the Mayo Clinic in Rochester, Minn. He reported serving as a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphingotec, Becton Dickinson, Quindel, and Novartis. H. Ari Jaffe, MD, is with the department of medicine, pulmonary division at University of Illinois at Chicago, Jesse Brown VA Medicine Center, Chicago. He reported he has no relationships to disclose relevant to the contents of the editorial.
The current study data are “robust” and suggest a strong association between high-sensitivity cardiac troponin values and cardiovascular event risk in these COPD patients, according to authors of an editorial.
The study also showed that a change in high-sensitivity cardiac troponin at 3 months is associated with increased risk, noted editorial authors Allan S. Jaffe, MD, and H. Ari Jaffe, MD.
“Most of these events probably represent acceleration of atherosclerosis, given the effects of smoking on atherosclerotic disease and its progression,” the authors said in the Journal of the American College of Cardiology.
However, study authors could have more extensively addressed how to use that information to improve the care of COPD patients at elevated cardiovascular event risk, they added.
A “pilot algorithm” that could be used to apply this biomarker analysis in clinical practice was proposed in an editorial accompanying the research report.
They suggest repeating high-sensitivity cardiac troponin measurements to reduce variability, as well as repeating samples at 3 months to detect changes that could signal increased risk.
“In addition, one should avoid decisions based on small differences,” they wrote.
Allan S. Jaffe, MD, is with the department of cardiovascular medicine and the department of laboratory medicine and pathology at the Mayo Clinic in Rochester, Minn. He reported serving as a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphingotec, Becton Dickinson, Quindel, and Novartis. H. Ari Jaffe, MD, is with the department of medicine, pulmonary division at University of Illinois at Chicago, Jesse Brown VA Medicine Center, Chicago. He reported he has no relationships to disclose relevant to the contents of the editorial.
The current study data are “robust” and suggest a strong association between high-sensitivity cardiac troponin values and cardiovascular event risk in these COPD patients, according to authors of an editorial.
The study also showed that a change in high-sensitivity cardiac troponin at 3 months is associated with increased risk, noted editorial authors Allan S. Jaffe, MD, and H. Ari Jaffe, MD.
“Most of these events probably represent acceleration of atherosclerosis, given the effects of smoking on atherosclerotic disease and its progression,” the authors said in the Journal of the American College of Cardiology.
However, study authors could have more extensively addressed how to use that information to improve the care of COPD patients at elevated cardiovascular event risk, they added.
A “pilot algorithm” that could be used to apply this biomarker analysis in clinical practice was proposed in an editorial accompanying the research report.
They suggest repeating high-sensitivity cardiac troponin measurements to reduce variability, as well as repeating samples at 3 months to detect changes that could signal increased risk.
“In addition, one should avoid decisions based on small differences,” they wrote.
Allan S. Jaffe, MD, is with the department of cardiovascular medicine and the department of laboratory medicine and pathology at the Mayo Clinic in Rochester, Minn. He reported serving as a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphingotec, Becton Dickinson, Quindel, and Novartis. H. Ari Jaffe, MD, is with the department of medicine, pulmonary division at University of Illinois at Chicago, Jesse Brown VA Medicine Center, Chicago. He reported he has no relationships to disclose relevant to the contents of the editorial.
, according to a post-hoc analysis of a clinical trial.
An increased risk of cardiovascular adverse events and cardiovascular death was seen in COPD patients in the highest quintile of plasma cardiac troponin concentrations at baseline, results of the analysis show.
The findings highlight the potential utility of high-sensitivity cardiac troponin in both clinical trials and clinical practice, according to researcher Nicholas L. Mills, MD, PhD, BHF/University Centre for Cardiovascular Science, The University of Edinburgh, Scotland, and co-investigators.
“Recognizing the risk associated with increased troponin concentrations might encourage clinicians to address cardiovascular risk due to lifestyle choices, and make patients more likely to engage with these recommendations,” Dr. Mills and co-authors wrote in the Journal of the American College of Cardiology.
Improved risk stratification may also help clinicians more appropriately target the use of preventive medications in COPD patients, they added in the report.
The analysis by Dr. Mills and colleagues was based on assessment of cardiac troponin I concentrations for patients in SUMMIT, a randomized trial assessing inhaled corticosteroids and long-acting beta agonists in COPD patients with ele-vated cardiovascular risk.
A total of 1,599 patients in the SUMMIT trial had a baseline cardiac troponin I assessment, and 1,258 had a follow-up assessment at 3 months following randomization.
Compared with those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event, even after adjusting for confounding variables (hazard ratio, 3.67; 95% confidence interval, 1.33-10.13; P = .012)..
Increased risk of cardiovascular death was also seen in the highest quintile as compared with the lowest quintile (HR, 20.06; 95% CI, 2.44-165.15; P = .005), investigators said.
There was no difference in risk of COPD exacerbations between the highest and lowest quintiles, they added.
At 3 months, there were no differences in troponin concentrations related to COPD treatment, consistent with previous observations in the SUMMIT trial that treatment did not impact the cardiovascular composite endpoint, investigators said.
However, patients with a plasma troponin of 5 ng/L or greater recorded at either the baseline or 3-month assessment had an increased rate of the composite cardiovascular endpoint and a “markedly increased” risk of cardiovascular death, they wrote.
The research was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship received by Dr. Mills. Disclosures reported by Dr. Mills included consultancy, research grants, and speaker fees from Abbott Diagnostics, Roche, and Singulex. Study co-authors reported disclosures related to GlaxoSmithKline, Veramed Limited, AstraZeneca, Zambon, Bayer, Novartis, and others.
SOURCE: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
, according to a post-hoc analysis of a clinical trial.
An increased risk of cardiovascular adverse events and cardiovascular death was seen in COPD patients in the highest quintile of plasma cardiac troponin concentrations at baseline, results of the analysis show.
The findings highlight the potential utility of high-sensitivity cardiac troponin in both clinical trials and clinical practice, according to researcher Nicholas L. Mills, MD, PhD, BHF/University Centre for Cardiovascular Science, The University of Edinburgh, Scotland, and co-investigators.
“Recognizing the risk associated with increased troponin concentrations might encourage clinicians to address cardiovascular risk due to lifestyle choices, and make patients more likely to engage with these recommendations,” Dr. Mills and co-authors wrote in the Journal of the American College of Cardiology.
Improved risk stratification may also help clinicians more appropriately target the use of preventive medications in COPD patients, they added in the report.
The analysis by Dr. Mills and colleagues was based on assessment of cardiac troponin I concentrations for patients in SUMMIT, a randomized trial assessing inhaled corticosteroids and long-acting beta agonists in COPD patients with ele-vated cardiovascular risk.
A total of 1,599 patients in the SUMMIT trial had a baseline cardiac troponin I assessment, and 1,258 had a follow-up assessment at 3 months following randomization.
Compared with those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event, even after adjusting for confounding variables (hazard ratio, 3.67; 95% confidence interval, 1.33-10.13; P = .012)..
Increased risk of cardiovascular death was also seen in the highest quintile as compared with the lowest quintile (HR, 20.06; 95% CI, 2.44-165.15; P = .005), investigators said.
There was no difference in risk of COPD exacerbations between the highest and lowest quintiles, they added.
At 3 months, there were no differences in troponin concentrations related to COPD treatment, consistent with previous observations in the SUMMIT trial that treatment did not impact the cardiovascular composite endpoint, investigators said.
However, patients with a plasma troponin of 5 ng/L or greater recorded at either the baseline or 3-month assessment had an increased rate of the composite cardiovascular endpoint and a “markedly increased” risk of cardiovascular death, they wrote.
The research was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship received by Dr. Mills. Disclosures reported by Dr. Mills included consultancy, research grants, and speaker fees from Abbott Diagnostics, Roche, and Singulex. Study co-authors reported disclosures related to GlaxoSmithKline, Veramed Limited, AstraZeneca, Zambon, Bayer, Novartis, and others.
SOURCE: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: In patients with COPD and heightened cardiovascular risk, high levels of high-sensitivity cardiac troponin were strongly associated with risk of cardiovascular outcomes.
Major finding: Compared to those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event (hazard ratio, 3.67; 95% CI, 1.33-10.13; P = 0.012).
Study details: Post-hoc analysis of 1,599 patients in the SUMMIT trial who had a baseline cardiac troponin I assessment and 1,258 who had a 3-month follow-up assessment.
Disclosures: The study was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship. Authors reported disclosures related to GlaxoSmithKline, Veramed Limited, Abbott Diagnostics, Roche, Singulex, AstraZeneca, Zambon, Bayer, Novartis, and others.
Source: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
Earnings gap seen among Maryland physicians
Male physicians in Maryland reported higher earnings than did female physicians, even when they all worked 41 or more hours a week, according to a 2018 survey of physicians in the state.
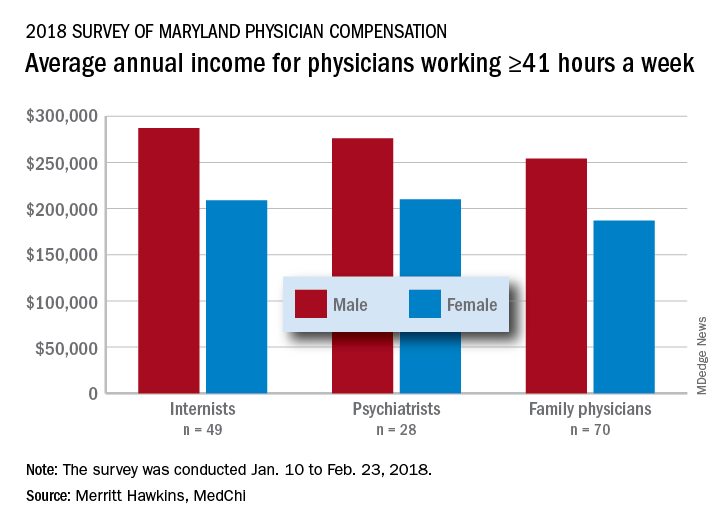
The average pretax income for all 508 respondents was $299,000 in 2016: Male physicians (66.6% of the sample) had an average of $335,000 and women averaged 33% lower at $224,000, MedChi (the Maryland State Medical Society) and Merritt Hawkins reported on July 31. Men did report working a longer week: Their average of 50.5 hours was 11% more than the 45.4-hour average for women.
“The biggest disparities we see in compensation are between male and female physicians in Maryland,” Gene Ransom, MedChi’s chief executive officer, said in a written statement. “Though such disparities have been noted in other research, it is still surprising to see the extent to which they persist.”
Of the respondents who worked an average of 41 or more hours per week – an analysis conducted only for the three largest specialties in the survey – female internists earned 27% less than their male counterparts, female psychiatrists earned 24% less, and female family physicians earned 26% less, the survey results showed.
Earnings were structured somewhat differently for Maryland’s male and female physicians. Women were more likely to be compensated in the form of a straight salary than men (35.0% vs. 30.3%), and men were more likely to paid based on production (22.7% vs. 16.9%) or in the form of an income guarantee (0.9% vs. 0.0%). Proportions receiving a salary with a production bonus were 42.7% for men and 42.5% for women, according to the survey.
Data included in the survey may be of interest to policymakers and media members who track physician compensation trends in Maryland and nationwide.
The survey was commissioned by MedChi and conducted by Merritt Hawkins from Jan. 10 to Feb. 23, 2018. The margin of error was plus or minus 4.4%.
Male physicians in Maryland reported higher earnings than did female physicians, even when they all worked 41 or more hours a week, according to a 2018 survey of physicians in the state.

The average pretax income for all 508 respondents was $299,000 in 2016: Male physicians (66.6% of the sample) had an average of $335,000 and women averaged 33% lower at $224,000, MedChi (the Maryland State Medical Society) and Merritt Hawkins reported on July 31. Men did report working a longer week: Their average of 50.5 hours was 11% more than the 45.4-hour average for women.
“The biggest disparities we see in compensation are between male and female physicians in Maryland,” Gene Ransom, MedChi’s chief executive officer, said in a written statement. “Though such disparities have been noted in other research, it is still surprising to see the extent to which they persist.”
Of the respondents who worked an average of 41 or more hours per week – an analysis conducted only for the three largest specialties in the survey – female internists earned 27% less than their male counterparts, female psychiatrists earned 24% less, and female family physicians earned 26% less, the survey results showed.
Earnings were structured somewhat differently for Maryland’s male and female physicians. Women were more likely to be compensated in the form of a straight salary than men (35.0% vs. 30.3%), and men were more likely to paid based on production (22.7% vs. 16.9%) or in the form of an income guarantee (0.9% vs. 0.0%). Proportions receiving a salary with a production bonus were 42.7% for men and 42.5% for women, according to the survey.
Data included in the survey may be of interest to policymakers and media members who track physician compensation trends in Maryland and nationwide.
The survey was commissioned by MedChi and conducted by Merritt Hawkins from Jan. 10 to Feb. 23, 2018. The margin of error was plus or minus 4.4%.
Male physicians in Maryland reported higher earnings than did female physicians, even when they all worked 41 or more hours a week, according to a 2018 survey of physicians in the state.

The average pretax income for all 508 respondents was $299,000 in 2016: Male physicians (66.6% of the sample) had an average of $335,000 and women averaged 33% lower at $224,000, MedChi (the Maryland State Medical Society) and Merritt Hawkins reported on July 31. Men did report working a longer week: Their average of 50.5 hours was 11% more than the 45.4-hour average for women.
“The biggest disparities we see in compensation are between male and female physicians in Maryland,” Gene Ransom, MedChi’s chief executive officer, said in a written statement. “Though such disparities have been noted in other research, it is still surprising to see the extent to which they persist.”
Of the respondents who worked an average of 41 or more hours per week – an analysis conducted only for the three largest specialties in the survey – female internists earned 27% less than their male counterparts, female psychiatrists earned 24% less, and female family physicians earned 26% less, the survey results showed.
Earnings were structured somewhat differently for Maryland’s male and female physicians. Women were more likely to be compensated in the form of a straight salary than men (35.0% vs. 30.3%), and men were more likely to paid based on production (22.7% vs. 16.9%) or in the form of an income guarantee (0.9% vs. 0.0%). Proportions receiving a salary with a production bonus were 42.7% for men and 42.5% for women, according to the survey.
Data included in the survey may be of interest to policymakers and media members who track physician compensation trends in Maryland and nationwide.
The survey was commissioned by MedChi and conducted by Merritt Hawkins from Jan. 10 to Feb. 23, 2018. The margin of error was plus or minus 4.4%.
Insufficient sleep is costing countries billions annually because of low productivity
in other countries worldwide, according to a cross-country comparative analysis.
“Our study shows that the effects from a lack of sleep are massive. Sleep deprivation not only influences an individual’s health and well-being but has a significant impact on a nation’s economy, with lower productivity levels and a higher mortality risk among workers,” Marco Hafner, a research leader at RAND Europe and the report’s main author, stated in a press release.
Mr. Hafner and his colleagues analyzed data from 62,366 employees from the Britain’s Healthiest Workplace competition during 2015 and 2016 to determine factors affecting lack of sleep. Specifically, they found people who were overweight or obese slept an average of 2.5 minutes to 7 minutes less each day, compared with people at a healthy body mass index. Smoking was identified as a factor associated with insufficient sleep, and people who smoke slept 5 fewer minutes per day, compared with nonsmokers. People who had more than two sugary drinks per day slept an average of 3.4 minutes less per day, compared with those who consumed less or no sugary drinks. The authors noted people who performed 120 minutes of physical activity or less per day and people with a medium to high risk of mental health problems slept an average of 2.6 minutes and 17.2 minutes less each day, respectively.
Regarding workplace-associated factors for insufficient sleep, the authors found lack of choice in their work routine was associated with 2.3 minutes less sleep per day, and those who worked irregular hours slept 2.7 minutes less per day on average; people with workplace stress and unrealistic time pressures slept 8 minutes less per day on average. Commuters slept 9.3 minutes less per day if they had a 30- to 60-minute commute to work, while those who had a commute longer than 60 minutes slept 16.5 minutes less per day than people with shorter commutes.
The authors also found the following personal and sociodemographic factors were associated with insufficient sleep:
- People who had financial concerns slept 10 minutes less per day, compared with people who did not have financial concerns.
- Unpaid care was associated with an average of 5 minutes less sleep per day.
- People with dependent children under 18 years old in the same household slept an average of 4.2 minutes less daily.
- Men slept 9 minutes less per day, compared, with women.
- Never being married was associated with sleeping an average of 4.8 minutes less per day, while people who were separated from their partner slept an average of 6.5 minutes less per day.
“At first glance, the estimates of minutes of sleep lost due to the various factors outlined above may seem small,” the authors wrote in their report. “However, it is important to stress that the estimates represent the effect on sleep duration of each single factor, holding all other factors constant.”
That lost sleep can significantly affect a person’s health, the authors noted. Sleeping less than 6 hours per night was associated with a 13% increased risk in all-cause mortality and a person sleeping between 6 hours and 7 hours per night had a 7% increased risk of all-cause mortality, compared with people who slept between 7 hours and 9 hours per night.
Insufficient sleep can also affect workplace productivity, with factors such as absenteeism and presenteeism (working while tired) affecting performance at work because of loss of sleep. There was a 1.5% higher productivity loss among people sleeping between 6 hours and 7 hours of sleep per night, compared with people who slept between 7 hours and 9 hours a night, which the authors estimated would cost an employer 6 working days per year for a person sleeping less than 6 hours per night.
In the United States, the authors reported 1.23 million working days (9.9 million working hours) are lost per year because of lack of sleep. They studied four other member countries in the Organisation for Economic Co-operation and Development and found similar results: Japan loses 0.6 million working days (4.8 million working hours) per year, the United Kingdom loses 207,224 working days (1.6 million working hours), Germany loses 209,024 working days (1.6 million working hours), and Canada loses 78,861 working days (630,886 working hours) annually because of insufficient sleep among workers. In total, the report estimates lack of sleep costs approximately $680 billion for these countries.
The authors encouraged individuals, employers, and countries to adopt policies that would lessen the economic impact of insufficient sleep and improve sleep outcomes. For individuals, recommendations included setting a consistent wake-up time, limiting electronic devices before sleep, limiting intake of substances such as caffeine, alcohol, and nicotine prior to sleep, and increasing physical activity. Employers were encouraged to recognize the benefits that getting a full night’s sleep has for their employees, adopt routines that improve their employees’ sleep outcomes, and limit use of electronic devices outside office hours. Public health authorities were encouraged to create awareness campaigns and activities supporting sleep-related help and implementing more efficient public schedules such as delayed school starting times.
“Improving individual sleep habits and duration has huge implications, with our research showing that simple changes can make a big difference,” Mr. Hafner stated in a press release. “For example, if those who sleep under 6 hours a night increase their sleep to between 6 and 7 hours a night, this could add $226.4 billion to the U.S. economy.”
The authors report no relevant conflicts of interest.
SOURCE: Hafner M et al. RAND Corporation .
in other countries worldwide, according to a cross-country comparative analysis.
“Our study shows that the effects from a lack of sleep are massive. Sleep deprivation not only influences an individual’s health and well-being but has a significant impact on a nation’s economy, with lower productivity levels and a higher mortality risk among workers,” Marco Hafner, a research leader at RAND Europe and the report’s main author, stated in a press release.
Mr. Hafner and his colleagues analyzed data from 62,366 employees from the Britain’s Healthiest Workplace competition during 2015 and 2016 to determine factors affecting lack of sleep. Specifically, they found people who were overweight or obese slept an average of 2.5 minutes to 7 minutes less each day, compared with people at a healthy body mass index. Smoking was identified as a factor associated with insufficient sleep, and people who smoke slept 5 fewer minutes per day, compared with nonsmokers. People who had more than two sugary drinks per day slept an average of 3.4 minutes less per day, compared with those who consumed less or no sugary drinks. The authors noted people who performed 120 minutes of physical activity or less per day and people with a medium to high risk of mental health problems slept an average of 2.6 minutes and 17.2 minutes less each day, respectively.
Regarding workplace-associated factors for insufficient sleep, the authors found lack of choice in their work routine was associated with 2.3 minutes less sleep per day, and those who worked irregular hours slept 2.7 minutes less per day on average; people with workplace stress and unrealistic time pressures slept 8 minutes less per day on average. Commuters slept 9.3 minutes less per day if they had a 30- to 60-minute commute to work, while those who had a commute longer than 60 minutes slept 16.5 minutes less per day than people with shorter commutes.
The authors also found the following personal and sociodemographic factors were associated with insufficient sleep:
- People who had financial concerns slept 10 minutes less per day, compared with people who did not have financial concerns.
- Unpaid care was associated with an average of 5 minutes less sleep per day.
- People with dependent children under 18 years old in the same household slept an average of 4.2 minutes less daily.
- Men slept 9 minutes less per day, compared, with women.
- Never being married was associated with sleeping an average of 4.8 minutes less per day, while people who were separated from their partner slept an average of 6.5 minutes less per day.
“At first glance, the estimates of minutes of sleep lost due to the various factors outlined above may seem small,” the authors wrote in their report. “However, it is important to stress that the estimates represent the effect on sleep duration of each single factor, holding all other factors constant.”
That lost sleep can significantly affect a person’s health, the authors noted. Sleeping less than 6 hours per night was associated with a 13% increased risk in all-cause mortality and a person sleeping between 6 hours and 7 hours per night had a 7% increased risk of all-cause mortality, compared with people who slept between 7 hours and 9 hours per night.
Insufficient sleep can also affect workplace productivity, with factors such as absenteeism and presenteeism (working while tired) affecting performance at work because of loss of sleep. There was a 1.5% higher productivity loss among people sleeping between 6 hours and 7 hours of sleep per night, compared with people who slept between 7 hours and 9 hours a night, which the authors estimated would cost an employer 6 working days per year for a person sleeping less than 6 hours per night.
In the United States, the authors reported 1.23 million working days (9.9 million working hours) are lost per year because of lack of sleep. They studied four other member countries in the Organisation for Economic Co-operation and Development and found similar results: Japan loses 0.6 million working days (4.8 million working hours) per year, the United Kingdom loses 207,224 working days (1.6 million working hours), Germany loses 209,024 working days (1.6 million working hours), and Canada loses 78,861 working days (630,886 working hours) annually because of insufficient sleep among workers. In total, the report estimates lack of sleep costs approximately $680 billion for these countries.
The authors encouraged individuals, employers, and countries to adopt policies that would lessen the economic impact of insufficient sleep and improve sleep outcomes. For individuals, recommendations included setting a consistent wake-up time, limiting electronic devices before sleep, limiting intake of substances such as caffeine, alcohol, and nicotine prior to sleep, and increasing physical activity. Employers were encouraged to recognize the benefits that getting a full night’s sleep has for their employees, adopt routines that improve their employees’ sleep outcomes, and limit use of electronic devices outside office hours. Public health authorities were encouraged to create awareness campaigns and activities supporting sleep-related help and implementing more efficient public schedules such as delayed school starting times.
“Improving individual sleep habits and duration has huge implications, with our research showing that simple changes can make a big difference,” Mr. Hafner stated in a press release. “For example, if those who sleep under 6 hours a night increase their sleep to between 6 and 7 hours a night, this could add $226.4 billion to the U.S. economy.”
The authors report no relevant conflicts of interest.
SOURCE: Hafner M et al. RAND Corporation .
in other countries worldwide, according to a cross-country comparative analysis.
“Our study shows that the effects from a lack of sleep are massive. Sleep deprivation not only influences an individual’s health and well-being but has a significant impact on a nation’s economy, with lower productivity levels and a higher mortality risk among workers,” Marco Hafner, a research leader at RAND Europe and the report’s main author, stated in a press release.
Mr. Hafner and his colleagues analyzed data from 62,366 employees from the Britain’s Healthiest Workplace competition during 2015 and 2016 to determine factors affecting lack of sleep. Specifically, they found people who were overweight or obese slept an average of 2.5 minutes to 7 minutes less each day, compared with people at a healthy body mass index. Smoking was identified as a factor associated with insufficient sleep, and people who smoke slept 5 fewer minutes per day, compared with nonsmokers. People who had more than two sugary drinks per day slept an average of 3.4 minutes less per day, compared with those who consumed less or no sugary drinks. The authors noted people who performed 120 minutes of physical activity or less per day and people with a medium to high risk of mental health problems slept an average of 2.6 minutes and 17.2 minutes less each day, respectively.
Regarding workplace-associated factors for insufficient sleep, the authors found lack of choice in their work routine was associated with 2.3 minutes less sleep per day, and those who worked irregular hours slept 2.7 minutes less per day on average; people with workplace stress and unrealistic time pressures slept 8 minutes less per day on average. Commuters slept 9.3 minutes less per day if they had a 30- to 60-minute commute to work, while those who had a commute longer than 60 minutes slept 16.5 minutes less per day than people with shorter commutes.
The authors also found the following personal and sociodemographic factors were associated with insufficient sleep:
- People who had financial concerns slept 10 minutes less per day, compared with people who did not have financial concerns.
- Unpaid care was associated with an average of 5 minutes less sleep per day.
- People with dependent children under 18 years old in the same household slept an average of 4.2 minutes less daily.
- Men slept 9 minutes less per day, compared, with women.
- Never being married was associated with sleeping an average of 4.8 minutes less per day, while people who were separated from their partner slept an average of 6.5 minutes less per day.
“At first glance, the estimates of minutes of sleep lost due to the various factors outlined above may seem small,” the authors wrote in their report. “However, it is important to stress that the estimates represent the effect on sleep duration of each single factor, holding all other factors constant.”
That lost sleep can significantly affect a person’s health, the authors noted. Sleeping less than 6 hours per night was associated with a 13% increased risk in all-cause mortality and a person sleeping between 6 hours and 7 hours per night had a 7% increased risk of all-cause mortality, compared with people who slept between 7 hours and 9 hours per night.
Insufficient sleep can also affect workplace productivity, with factors such as absenteeism and presenteeism (working while tired) affecting performance at work because of loss of sleep. There was a 1.5% higher productivity loss among people sleeping between 6 hours and 7 hours of sleep per night, compared with people who slept between 7 hours and 9 hours a night, which the authors estimated would cost an employer 6 working days per year for a person sleeping less than 6 hours per night.
In the United States, the authors reported 1.23 million working days (9.9 million working hours) are lost per year because of lack of sleep. They studied four other member countries in the Organisation for Economic Co-operation and Development and found similar results: Japan loses 0.6 million working days (4.8 million working hours) per year, the United Kingdom loses 207,224 working days (1.6 million working hours), Germany loses 209,024 working days (1.6 million working hours), and Canada loses 78,861 working days (630,886 working hours) annually because of insufficient sleep among workers. In total, the report estimates lack of sleep costs approximately $680 billion for these countries.
The authors encouraged individuals, employers, and countries to adopt policies that would lessen the economic impact of insufficient sleep and improve sleep outcomes. For individuals, recommendations included setting a consistent wake-up time, limiting electronic devices before sleep, limiting intake of substances such as caffeine, alcohol, and nicotine prior to sleep, and increasing physical activity. Employers were encouraged to recognize the benefits that getting a full night’s sleep has for their employees, adopt routines that improve their employees’ sleep outcomes, and limit use of electronic devices outside office hours. Public health authorities were encouraged to create awareness campaigns and activities supporting sleep-related help and implementing more efficient public schedules such as delayed school starting times.
“Improving individual sleep habits and duration has huge implications, with our research showing that simple changes can make a big difference,” Mr. Hafner stated in a press release. “For example, if those who sleep under 6 hours a night increase their sleep to between 6 and 7 hours a night, this could add $226.4 billion to the U.S. economy.”
The authors report no relevant conflicts of interest.
SOURCE: Hafner M et al. RAND Corporation .
FROM RAND CORPORATION REPORT
Key clinical point: Lack of sleep is economically costly and increases mortality risk for a country’s workforce.
Major finding: The United States loses approximately $411 billion per year and 1.23 million working days from insufficient sleeps among workers.
Study details: A research report of the effects of insufficient sleep in the United States, Canada, United Kingdom, Germany, and Japan.
Disclosures: The authors report no relevant conflicts of interest.
Source: Hafner M et al. RAND Corporation.
EC approves CAR T-cell therapy for ALL, DLBCL
The European Commission (EC) has granted approval for tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy targeting CD19.
Tisagenlecleucel (formerly CTL019) is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
The EC’s approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
JULIET trial
Updated results from JULIET were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached.
At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least one AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Commission (EC) has granted approval for tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy targeting CD19.
Tisagenlecleucel (formerly CTL019) is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
The EC’s approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
JULIET trial
Updated results from JULIET were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached.
At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least one AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Commission (EC) has granted approval for tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy targeting CD19.
Tisagenlecleucel (formerly CTL019) is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
The EC’s approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
JULIET trial
Updated results from JULIET were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached.
At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least one AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
FDA approves new use for ibrutinib in WM
The US Food and Drug Administration (FDA) has approved ibrutinib (Imbruvica®) for use in combination with rituximab to treat adults with Waldenström’s macroglobulinemia (WM).
This is the ninth FDA approval for ibrutinib and the second approval for the drug in WM.
The latest approval was supported by the phase 3 iNNOVATE trial, in which researchers compared ibrutinib plus rituximab to rituximab alone in patients with previously untreated or relapsed/refractory WM.
Results from iNNOVATE were presented at the 2018 ASCO Annual Meeting (abstract 8003) and simultaneously published in NEJM.
The trial enrolled 150 patients. They received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm. Serious AEs occurred in 43% and 33%, respectively.
There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
About ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that is FDA-approved to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic graft-versus-host disease (cGVHD), and WM.
In November 2013, ibrutinib was approved to treat adults with MCL who have received at least one prior therapy.
In February 2014, ibrutinib was approved to treat adults with CLL who have received at least one prior therapy. In July 2014, ibrutinib received approval for adult CLL patients with 17p deletion, and, in March 2016, ibrutinib was approved as a frontline CLL treatment.
Ibrutinib was approved for use as a single agent in adults with WM in January 2015.
In May 2016, ibrutinib was approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
In January 2017, ibrutinib was approved for adults with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
In August 2017, ibrutinib was approved to treat adults with cGVHD that did not respond to one or more lines of systemic therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved ibrutinib (Imbruvica®) for use in combination with rituximab to treat adults with Waldenström’s macroglobulinemia (WM).
This is the ninth FDA approval for ibrutinib and the second approval for the drug in WM.
The latest approval was supported by the phase 3 iNNOVATE trial, in which researchers compared ibrutinib plus rituximab to rituximab alone in patients with previously untreated or relapsed/refractory WM.
Results from iNNOVATE were presented at the 2018 ASCO Annual Meeting (abstract 8003) and simultaneously published in NEJM.
The trial enrolled 150 patients. They received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm. Serious AEs occurred in 43% and 33%, respectively.
There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
About ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that is FDA-approved to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic graft-versus-host disease (cGVHD), and WM.
In November 2013, ibrutinib was approved to treat adults with MCL who have received at least one prior therapy.
In February 2014, ibrutinib was approved to treat adults with CLL who have received at least one prior therapy. In July 2014, ibrutinib received approval for adult CLL patients with 17p deletion, and, in March 2016, ibrutinib was approved as a frontline CLL treatment.
Ibrutinib was approved for use as a single agent in adults with WM in January 2015.
In May 2016, ibrutinib was approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
In January 2017, ibrutinib was approved for adults with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
In August 2017, ibrutinib was approved to treat adults with cGVHD that did not respond to one or more lines of systemic therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved ibrutinib (Imbruvica®) for use in combination with rituximab to treat adults with Waldenström’s macroglobulinemia (WM).
This is the ninth FDA approval for ibrutinib and the second approval for the drug in WM.
The latest approval was supported by the phase 3 iNNOVATE trial, in which researchers compared ibrutinib plus rituximab to rituximab alone in patients with previously untreated or relapsed/refractory WM.
Results from iNNOVATE were presented at the 2018 ASCO Annual Meeting (abstract 8003) and simultaneously published in NEJM.
The trial enrolled 150 patients. They received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm. Serious AEs occurred in 43% and 33%, respectively.
There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
About ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that is FDA-approved to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic graft-versus-host disease (cGVHD), and WM.
In November 2013, ibrutinib was approved to treat adults with MCL who have received at least one prior therapy.
In February 2014, ibrutinib was approved to treat adults with CLL who have received at least one prior therapy. In July 2014, ibrutinib received approval for adult CLL patients with 17p deletion, and, in March 2016, ibrutinib was approved as a frontline CLL treatment.
Ibrutinib was approved for use as a single agent in adults with WM in January 2015.
In May 2016, ibrutinib was approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
In January 2017, ibrutinib was approved for adults with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
In August 2017, ibrutinib was approved to treat adults with cGVHD that did not respond to one or more lines of systemic therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Medicare donut hole: Fewer enrollees, more spending in 2016

Those 5.2 million enrollees in 2016 represented a reversal from the rising number of those reaching the coverage-gap over the last 3 years and the potential start of a trend toward increasing out-of-pocket costs. The number of part D enrollees without low-income subsidies (the coverage gap does not apply to those who receive the subsidies) had risen from 2013 to 2015 after being fairly stable from 2007 to 2012. The average out-of-pocket cost for enrollees with low-income subsidies, on the other hand, dropped by 20% in 2011 – from $1,858 to $1,485 – with a smaller drop in 2013 before two consecutive years of increases in 2015 and 2016, Kaiser reported.
“As of 2019, there will no longer be a coverage gap for brand-name drugs, as a result of changes in the” Bipartisan Budget Act of 2018, which reduced enrollees’ share of costs and increased the manufacturer discount, Kaiser explained, but a proposal from the Trump administration “to exclude the manufacturer discount from the calculation of out-of-pocket spending would substantially increase part D enrollees’ out-of-pocket costs and would lead to fewer enrollees qualifying for catastrophic coverage.”

Those 5.2 million enrollees in 2016 represented a reversal from the rising number of those reaching the coverage-gap over the last 3 years and the potential start of a trend toward increasing out-of-pocket costs. The number of part D enrollees without low-income subsidies (the coverage gap does not apply to those who receive the subsidies) had risen from 2013 to 2015 after being fairly stable from 2007 to 2012. The average out-of-pocket cost for enrollees with low-income subsidies, on the other hand, dropped by 20% in 2011 – from $1,858 to $1,485 – with a smaller drop in 2013 before two consecutive years of increases in 2015 and 2016, Kaiser reported.
“As of 2019, there will no longer be a coverage gap for brand-name drugs, as a result of changes in the” Bipartisan Budget Act of 2018, which reduced enrollees’ share of costs and increased the manufacturer discount, Kaiser explained, but a proposal from the Trump administration “to exclude the manufacturer discount from the calculation of out-of-pocket spending would substantially increase part D enrollees’ out-of-pocket costs and would lead to fewer enrollees qualifying for catastrophic coverage.”

Those 5.2 million enrollees in 2016 represented a reversal from the rising number of those reaching the coverage-gap over the last 3 years and the potential start of a trend toward increasing out-of-pocket costs. The number of part D enrollees without low-income subsidies (the coverage gap does not apply to those who receive the subsidies) had risen from 2013 to 2015 after being fairly stable from 2007 to 2012. The average out-of-pocket cost for enrollees with low-income subsidies, on the other hand, dropped by 20% in 2011 – from $1,858 to $1,485 – with a smaller drop in 2013 before two consecutive years of increases in 2015 and 2016, Kaiser reported.
“As of 2019, there will no longer be a coverage gap for brand-name drugs, as a result of changes in the” Bipartisan Budget Act of 2018, which reduced enrollees’ share of costs and increased the manufacturer discount, Kaiser explained, but a proposal from the Trump administration “to exclude the manufacturer discount from the calculation of out-of-pocket spending would substantially increase part D enrollees’ out-of-pocket costs and would lead to fewer enrollees qualifying for catastrophic coverage.”
ASCEND: Aspirin, fish oil flop in diabetes
MUNICH – In a double blow to the current, widespread routine use of low-dose aspirin and fish oil supplements for primary cardiovascular prevention in patients with diabetes, neither treatment provided any net clinical benefit in the massive, long-term ASCEND study, investigators reported at the annual congress of the European Society of Cardiology.
And, in yet another bitter pill to swallow, low-dose aspirin failed to reduce the incidence of GI cancer or any other type of cancer, compared with placebo in the study. Earlier, nondefinitive meta-analyses of much smaller randomized trials had raised the possibility of a roughly 30% reduction in the incidence of colorectal cancer in long-time users, said Jane Armitage, MD, professor of clinical trials and epidemiology at the University of Oxford (England).
“We saw no suggestion of an anticancer effect emerging over time,” Dr. Armitage said. “We did see a significant reduction in the risk of vascular events but also an increase in major bleeding such that the absolute benefits from avoiding a serious vascular event were largely counterbalanced by increased risk of bleeding. And there was no subgroup in which we could clearly define that the benefit outweighs the risk,” she added in a video interview.
ASCEND (A Study of Cardiovascular Events in Diabetes) was a randomized, blinded trial in which 15,480 U.K. patients with diabetes and no known cardiovascular disease were placed on 100 mg/day of enteric-coated aspirin or placebo and 1 g/day of omega-3 fatty acids in capsule form or placebo in a 2 x 2 factorial design and followed prospectively for a mean of 7.4 years.
The study was undertaken because, even though the value of low-dose aspirin for secondary prevention is supported by strong evidence, the medication’s value for primary prevention in patients with diabetes has long been uncertain. American Diabetes Association guidelines give a Grade C recommendation to consideration of low-dose aspirin as a primary prevention strategy in patients with diabetes having a 10-year cardiovascular risk estimated at 10% or greater.
The primary efficacy endpoint in ASCEND was the occurrence of a first serious vascular event, defined as MI, stroke, transient ischemic attack, or death from any vascular cause other than intracranial hemorrhage. The rate was 8.5% in the aspirin group and 9.6% with placebo, for a statistically significant 12% relative risk reduction. However, the rate of the primary composite safety endpoint, comprising intracranial hemorrhage, GI bleeding, sight-threatening bleeding in the eye, or other bleeding serious enough to entail a trip to the hospital, was 4.1% in the aspirin group and 3.2% with placebo, a 29% increase in risk.
Aspirin reduced the absolute risk of a serious vascular event by 1.1%, compared with placebo, while boosting the risk of major bleeding by an absolute 0.9% – essentially a wash. The number needed to treat to avoid a serious vascular event was 91 patients, with 112 being the number needed to cause a major bleeding event. The risk of major bleeding rose with increasing baseline 5-year vascular event risk.
ASCEND provided no support for a recent report suggesting the benefit of low-dose aspirin for cardioprotection is largely confined to individuals weighing less than 70 kg (Lancet. 2018; 392:387-99); in fact, the opposite appeared to be true, according to Dr. Armitage.
The incidence of cancer of the GI tract was 2.0% in both study arms. The overall cancer rate was 11.6% in the aspirin arm and 11.5% with placebo. Additional follow-up focused on cancer is planned for 5 and 10 years after the end of ASCEND in order to examine the possibility of a delayed anticancer effect, she added.
Dr. Armitage said one reason aspirin may have failed to show a significant net benefit was the high rate of background cardioprotective medication usage, especially statins and antihypertensive drugs.
“I think if your diabetes is well managed and you’ve got your other risk factors under control, you have to consider very carefully whether or not, for you, the benefits of aspirin outweigh the risks,” she concluded.
Cardiologists respond
ASCEND’s two designated discussants, both cardiologists, took a more positive view of the results than Dr. Armitage, who isn’t a cardiologist.
Sigrun Halvorsen, MD, professor of cardiology at the University of Oslo, noted that most ASCEND participants were at relatively low cardiovascular risk, with an event rate of about 1.3% per year. She indicated that she’d like to see more data on higher-risk individuals before excluding any role for aspirin in primary cardiovascular prevention in patients with diabetes.
“Serious vascular events and bleeding are not necessarily equally weighted,” she added. “Most major bleeds in ASCEND were GI bleeds, and these can be largely prevented by PPIs [proton pump inhibitors], in contrast to death and stroke, which are irreversible events.”
“I think this study could be interpreted more favorably,” agreed Christopher P. Cannon, MD, professor of medicine at Harvard Medical School, Boston. “Bleeds are reversible, but MIs and whatnot are not.”
Fish oil flounders in ASCEND
Louise Bowman, MD, also at the University of Oxford, presented the ASCEND fish oil findings. Over an average of 7.4 years, omega-3 fatty acid supplementation had no effect on the rate of serious vascular events, no effect on cancer, no impact on all-cause or cause-specific mortality, and raised no safety issues in patients with diabetes. She argued that current guidelines recommending fish oil supplements for cardiovascular prevention should be reconsidered.
“Certainly there doesn’t seem to be any justification for the use of this dose of omega-3 fatty acids – 1 g/day – for the prevention of cardiovascular disease,” Dr. Bowman said.
However, Dr. Cannon saw a sliver of hope within the ASCEND findings. One component of the serious vascular event composite endpoint – vascular death – occurred in 2.4% of the fish oil group and 2.9% of placebo-treated controls, for a statistically significant 19% relative risk reduction. It could be a fluke, given that none of the other vascular endpoints followed suit. But physicians and patients won’t have to wait long to find out. Another randomized trial of low-dose fish oil supplements for primary cardiovascular prevention – the nearly 26,000-patient VITAL trial – is due to report later in 2018.
In addition, two major, randomized trials of higher-dose fish oil supplementation for secondary prevention are ongoing. The roughly 8,000-patient REDUCE-IT trial is expected to report results later this year, and the STRENGTH trial, featuring more than 13,000 patients, should be completed in 2020. Both studies are heavily loaded with diabetes patients, Dr. Cannon noted.
Simultaneous with presentation of the ASCEND results at the ESC congress, the study was published online at the New England Journal of Medicine website (doi: 10.1056/NEJMoa1804988 and doi: 10.1056/NEJMoa1804989).
The ASCEND study was funded by the British Heart Foundation and the U.K. Medical Research Council with support provided by Abbott Laboratories, Bayer, Mylan, and Solvay. Dr. Armitage and Dr. Bowman reported receiving research grants from the Medicines Company, Bayer, and Mylan. Dr. Halvorsen reported no financial conflicts. Dr. Cannon reported serving as a consultant to roughly a dozen pharmaceutical companies, including the Amarin Corporation, which markets a fish oil supplement and sponsored the REDUCE-IT trial.
MUNICH – In a double blow to the current, widespread routine use of low-dose aspirin and fish oil supplements for primary cardiovascular prevention in patients with diabetes, neither treatment provided any net clinical benefit in the massive, long-term ASCEND study, investigators reported at the annual congress of the European Society of Cardiology.
And, in yet another bitter pill to swallow, low-dose aspirin failed to reduce the incidence of GI cancer or any other type of cancer, compared with placebo in the study. Earlier, nondefinitive meta-analyses of much smaller randomized trials had raised the possibility of a roughly 30% reduction in the incidence of colorectal cancer in long-time users, said Jane Armitage, MD, professor of clinical trials and epidemiology at the University of Oxford (England).
“We saw no suggestion of an anticancer effect emerging over time,” Dr. Armitage said. “We did see a significant reduction in the risk of vascular events but also an increase in major bleeding such that the absolute benefits from avoiding a serious vascular event were largely counterbalanced by increased risk of bleeding. And there was no subgroup in which we could clearly define that the benefit outweighs the risk,” she added in a video interview.
ASCEND (A Study of Cardiovascular Events in Diabetes) was a randomized, blinded trial in which 15,480 U.K. patients with diabetes and no known cardiovascular disease were placed on 100 mg/day of enteric-coated aspirin or placebo and 1 g/day of omega-3 fatty acids in capsule form or placebo in a 2 x 2 factorial design and followed prospectively for a mean of 7.4 years.
The study was undertaken because, even though the value of low-dose aspirin for secondary prevention is supported by strong evidence, the medication’s value for primary prevention in patients with diabetes has long been uncertain. American Diabetes Association guidelines give a Grade C recommendation to consideration of low-dose aspirin as a primary prevention strategy in patients with diabetes having a 10-year cardiovascular risk estimated at 10% or greater.
The primary efficacy endpoint in ASCEND was the occurrence of a first serious vascular event, defined as MI, stroke, transient ischemic attack, or death from any vascular cause other than intracranial hemorrhage. The rate was 8.5% in the aspirin group and 9.6% with placebo, for a statistically significant 12% relative risk reduction. However, the rate of the primary composite safety endpoint, comprising intracranial hemorrhage, GI bleeding, sight-threatening bleeding in the eye, or other bleeding serious enough to entail a trip to the hospital, was 4.1% in the aspirin group and 3.2% with placebo, a 29% increase in risk.
Aspirin reduced the absolute risk of a serious vascular event by 1.1%, compared with placebo, while boosting the risk of major bleeding by an absolute 0.9% – essentially a wash. The number needed to treat to avoid a serious vascular event was 91 patients, with 112 being the number needed to cause a major bleeding event. The risk of major bleeding rose with increasing baseline 5-year vascular event risk.
ASCEND provided no support for a recent report suggesting the benefit of low-dose aspirin for cardioprotection is largely confined to individuals weighing less than 70 kg (Lancet. 2018; 392:387-99); in fact, the opposite appeared to be true, according to Dr. Armitage.
The incidence of cancer of the GI tract was 2.0% in both study arms. The overall cancer rate was 11.6% in the aspirin arm and 11.5% with placebo. Additional follow-up focused on cancer is planned for 5 and 10 years after the end of ASCEND in order to examine the possibility of a delayed anticancer effect, she added.
Dr. Armitage said one reason aspirin may have failed to show a significant net benefit was the high rate of background cardioprotective medication usage, especially statins and antihypertensive drugs.
“I think if your diabetes is well managed and you’ve got your other risk factors under control, you have to consider very carefully whether or not, for you, the benefits of aspirin outweigh the risks,” she concluded.
Cardiologists respond
ASCEND’s two designated discussants, both cardiologists, took a more positive view of the results than Dr. Armitage, who isn’t a cardiologist.
Sigrun Halvorsen, MD, professor of cardiology at the University of Oslo, noted that most ASCEND participants were at relatively low cardiovascular risk, with an event rate of about 1.3% per year. She indicated that she’d like to see more data on higher-risk individuals before excluding any role for aspirin in primary cardiovascular prevention in patients with diabetes.
“Serious vascular events and bleeding are not necessarily equally weighted,” she added. “Most major bleeds in ASCEND were GI bleeds, and these can be largely prevented by PPIs [proton pump inhibitors], in contrast to death and stroke, which are irreversible events.”
“I think this study could be interpreted more favorably,” agreed Christopher P. Cannon, MD, professor of medicine at Harvard Medical School, Boston. “Bleeds are reversible, but MIs and whatnot are not.”
Fish oil flounders in ASCEND
Louise Bowman, MD, also at the University of Oxford, presented the ASCEND fish oil findings. Over an average of 7.4 years, omega-3 fatty acid supplementation had no effect on the rate of serious vascular events, no effect on cancer, no impact on all-cause or cause-specific mortality, and raised no safety issues in patients with diabetes. She argued that current guidelines recommending fish oil supplements for cardiovascular prevention should be reconsidered.
“Certainly there doesn’t seem to be any justification for the use of this dose of omega-3 fatty acids – 1 g/day – for the prevention of cardiovascular disease,” Dr. Bowman said.
However, Dr. Cannon saw a sliver of hope within the ASCEND findings. One component of the serious vascular event composite endpoint – vascular death – occurred in 2.4% of the fish oil group and 2.9% of placebo-treated controls, for a statistically significant 19% relative risk reduction. It could be a fluke, given that none of the other vascular endpoints followed suit. But physicians and patients won’t have to wait long to find out. Another randomized trial of low-dose fish oil supplements for primary cardiovascular prevention – the nearly 26,000-patient VITAL trial – is due to report later in 2018.
In addition, two major, randomized trials of higher-dose fish oil supplementation for secondary prevention are ongoing. The roughly 8,000-patient REDUCE-IT trial is expected to report results later this year, and the STRENGTH trial, featuring more than 13,000 patients, should be completed in 2020. Both studies are heavily loaded with diabetes patients, Dr. Cannon noted.
Simultaneous with presentation of the ASCEND results at the ESC congress, the study was published online at the New England Journal of Medicine website (doi: 10.1056/NEJMoa1804988 and doi: 10.1056/NEJMoa1804989).
The ASCEND study was funded by the British Heart Foundation and the U.K. Medical Research Council with support provided by Abbott Laboratories, Bayer, Mylan, and Solvay. Dr. Armitage and Dr. Bowman reported receiving research grants from the Medicines Company, Bayer, and Mylan. Dr. Halvorsen reported no financial conflicts. Dr. Cannon reported serving as a consultant to roughly a dozen pharmaceutical companies, including the Amarin Corporation, which markets a fish oil supplement and sponsored the REDUCE-IT trial.
MUNICH – In a double blow to the current, widespread routine use of low-dose aspirin and fish oil supplements for primary cardiovascular prevention in patients with diabetes, neither treatment provided any net clinical benefit in the massive, long-term ASCEND study, investigators reported at the annual congress of the European Society of Cardiology.
And, in yet another bitter pill to swallow, low-dose aspirin failed to reduce the incidence of GI cancer or any other type of cancer, compared with placebo in the study. Earlier, nondefinitive meta-analyses of much smaller randomized trials had raised the possibility of a roughly 30% reduction in the incidence of colorectal cancer in long-time users, said Jane Armitage, MD, professor of clinical trials and epidemiology at the University of Oxford (England).
“We saw no suggestion of an anticancer effect emerging over time,” Dr. Armitage said. “We did see a significant reduction in the risk of vascular events but also an increase in major bleeding such that the absolute benefits from avoiding a serious vascular event were largely counterbalanced by increased risk of bleeding. And there was no subgroup in which we could clearly define that the benefit outweighs the risk,” she added in a video interview.
ASCEND (A Study of Cardiovascular Events in Diabetes) was a randomized, blinded trial in which 15,480 U.K. patients with diabetes and no known cardiovascular disease were placed on 100 mg/day of enteric-coated aspirin or placebo and 1 g/day of omega-3 fatty acids in capsule form or placebo in a 2 x 2 factorial design and followed prospectively for a mean of 7.4 years.
The study was undertaken because, even though the value of low-dose aspirin for secondary prevention is supported by strong evidence, the medication’s value for primary prevention in patients with diabetes has long been uncertain. American Diabetes Association guidelines give a Grade C recommendation to consideration of low-dose aspirin as a primary prevention strategy in patients with diabetes having a 10-year cardiovascular risk estimated at 10% or greater.
The primary efficacy endpoint in ASCEND was the occurrence of a first serious vascular event, defined as MI, stroke, transient ischemic attack, or death from any vascular cause other than intracranial hemorrhage. The rate was 8.5% in the aspirin group and 9.6% with placebo, for a statistically significant 12% relative risk reduction. However, the rate of the primary composite safety endpoint, comprising intracranial hemorrhage, GI bleeding, sight-threatening bleeding in the eye, or other bleeding serious enough to entail a trip to the hospital, was 4.1% in the aspirin group and 3.2% with placebo, a 29% increase in risk.
Aspirin reduced the absolute risk of a serious vascular event by 1.1%, compared with placebo, while boosting the risk of major bleeding by an absolute 0.9% – essentially a wash. The number needed to treat to avoid a serious vascular event was 91 patients, with 112 being the number needed to cause a major bleeding event. The risk of major bleeding rose with increasing baseline 5-year vascular event risk.
ASCEND provided no support for a recent report suggesting the benefit of low-dose aspirin for cardioprotection is largely confined to individuals weighing less than 70 kg (Lancet. 2018; 392:387-99); in fact, the opposite appeared to be true, according to Dr. Armitage.
The incidence of cancer of the GI tract was 2.0% in both study arms. The overall cancer rate was 11.6% in the aspirin arm and 11.5% with placebo. Additional follow-up focused on cancer is planned for 5 and 10 years after the end of ASCEND in order to examine the possibility of a delayed anticancer effect, she added.
Dr. Armitage said one reason aspirin may have failed to show a significant net benefit was the high rate of background cardioprotective medication usage, especially statins and antihypertensive drugs.
“I think if your diabetes is well managed and you’ve got your other risk factors under control, you have to consider very carefully whether or not, for you, the benefits of aspirin outweigh the risks,” she concluded.
Cardiologists respond
ASCEND’s two designated discussants, both cardiologists, took a more positive view of the results than Dr. Armitage, who isn’t a cardiologist.
Sigrun Halvorsen, MD, professor of cardiology at the University of Oslo, noted that most ASCEND participants were at relatively low cardiovascular risk, with an event rate of about 1.3% per year. She indicated that she’d like to see more data on higher-risk individuals before excluding any role for aspirin in primary cardiovascular prevention in patients with diabetes.
“Serious vascular events and bleeding are not necessarily equally weighted,” she added. “Most major bleeds in ASCEND were GI bleeds, and these can be largely prevented by PPIs [proton pump inhibitors], in contrast to death and stroke, which are irreversible events.”
“I think this study could be interpreted more favorably,” agreed Christopher P. Cannon, MD, professor of medicine at Harvard Medical School, Boston. “Bleeds are reversible, but MIs and whatnot are not.”
Fish oil flounders in ASCEND
Louise Bowman, MD, also at the University of Oxford, presented the ASCEND fish oil findings. Over an average of 7.4 years, omega-3 fatty acid supplementation had no effect on the rate of serious vascular events, no effect on cancer, no impact on all-cause or cause-specific mortality, and raised no safety issues in patients with diabetes. She argued that current guidelines recommending fish oil supplements for cardiovascular prevention should be reconsidered.
“Certainly there doesn’t seem to be any justification for the use of this dose of omega-3 fatty acids – 1 g/day – for the prevention of cardiovascular disease,” Dr. Bowman said.
However, Dr. Cannon saw a sliver of hope within the ASCEND findings. One component of the serious vascular event composite endpoint – vascular death – occurred in 2.4% of the fish oil group and 2.9% of placebo-treated controls, for a statistically significant 19% relative risk reduction. It could be a fluke, given that none of the other vascular endpoints followed suit. But physicians and patients won’t have to wait long to find out. Another randomized trial of low-dose fish oil supplements for primary cardiovascular prevention – the nearly 26,000-patient VITAL trial – is due to report later in 2018.
In addition, two major, randomized trials of higher-dose fish oil supplementation for secondary prevention are ongoing. The roughly 8,000-patient REDUCE-IT trial is expected to report results later this year, and the STRENGTH trial, featuring more than 13,000 patients, should be completed in 2020. Both studies are heavily loaded with diabetes patients, Dr. Cannon noted.
Simultaneous with presentation of the ASCEND results at the ESC congress, the study was published online at the New England Journal of Medicine website (doi: 10.1056/NEJMoa1804988 and doi: 10.1056/NEJMoa1804989).
The ASCEND study was funded by the British Heart Foundation and the U.K. Medical Research Council with support provided by Abbott Laboratories, Bayer, Mylan, and Solvay. Dr. Armitage and Dr. Bowman reported receiving research grants from the Medicines Company, Bayer, and Mylan. Dr. Halvorsen reported no financial conflicts. Dr. Cannon reported serving as a consultant to roughly a dozen pharmaceutical companies, including the Amarin Corporation, which markets a fish oil supplement and sponsored the REDUCE-IT trial.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point:
Major finding: Low-dose aspirin reduced the risk of serious vascular events by 12% versus placebo, but boosted major bleeding events by 29%.
Study details: This prospective, randomized trial included 15,480 patients with diabetes without known cardiovascular disease who were randomized to 100 mg/day of enteric-coated aspirin or placebo and 1 g/day of omega-3 fatty acids or placebo and followed for a mean of 7.4 years.
Disclosures: The study was funded by the British Heart Foundation and the U.K. Medical Research Council with support provided by Abbott Laboratories, Bayer, Mylan, and Solvay. The presenters reported receiving research grants from the Medicines Company, Bayer, and Mylan.
ACS Council Seeks Member; Applications due Aug. 31
The Society for Vascular Surgery is seeking applicants to serve as representative for the American College of Surgeons Advisory Council for Vascular Surgery. Please email letters of interest/nominations for the three-year term by Aug. 31. The nominee must be a Fellow of the ACS and be involved in SVS’ governing boards.
The Society for Vascular Surgery is seeking applicants to serve as representative for the American College of Surgeons Advisory Council for Vascular Surgery. Please email letters of interest/nominations for the three-year term by Aug. 31. The nominee must be a Fellow of the ACS and be involved in SVS’ governing boards.
The Society for Vascular Surgery is seeking applicants to serve as representative for the American College of Surgeons Advisory Council for Vascular Surgery. Please email letters of interest/nominations for the three-year term by Aug. 31. The nominee must be a Fellow of the ACS and be involved in SVS’ governing boards.










