User login
Extramedullary plasmacytoma of the thyroid, refractory to radiation therapy and treated with bortezomib
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
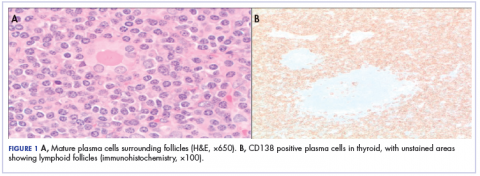
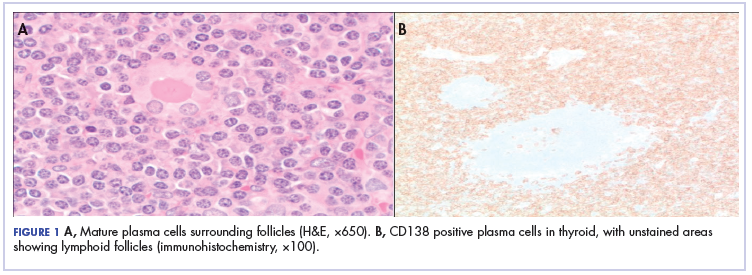
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
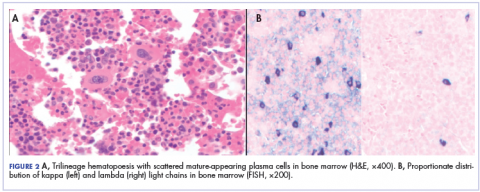
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
Plasma cell neoplasms involving tissues other than the bone marrow are known as extramedullary plasmacytoma (EMP).1 EMPs mostly involve the head and neck region.2 Solitary EMP involving only the thyroid gland is very rare.3,4 Because of the limited knowledge about this condition and its rarity, its management can be challenging and is often extrapolated from plasma cell myeloma.5,6 In general, surgery or radiation are considered as front-line therapy.3,5 EMPs usually respond well to radiotherapy with almost complete remission. No definite guidelines outlining the treatment of radio-resistant EMP of the thyroid have yet been published. Data supporting the use of chemotherapy is particularly limited.4,7,8
Here, we describe the case of a 53-year-old woman with a long history of thyroiditis who presented with rapidly worsening symptomatic thyroid enlargement. She was diagnosed with EMP of the thyroid gland that was not amenable to surgery and was refractory to radiotherapy but responded to adjuvant chemotherapy with bortezomib. This report highlights 2 unique aspects of this condition: it focuses on a rare case of EMP and, as far as we know, it reports for the first time on EMP that was resistant to radiotherapy. It also highlights the need for guidelines for the treatment of EMPs.
Case presentation and summary
A 53-year-old woman presented to the emergency department with complaints of difficulty swallowing, hoarseness, and neck pain during the previous 1 month. She had a known history of Hashimoto’s thyroiditis, and an ultrasound scan of her neck 6 years previously had demonstrated diffuse thyromegaly without discrete nodules. On presentation, the patient’s vitals were stable, and a neck examination revealed a firm and enlarged thyroid without any cervical adenopathy. Laboratory investigations revealed a normal complete blood count and comprehensive metabolic panel. She had an elevated thyroid-stimulating hormone level of 13.40 mIU/L (reference range, 0.47-4.68 mIU/L) and normal thyroxine level of 4.5 pmol/L (reference range, 4.5-12.0 pmol/L). A computerized tomography (CT) scan of the neck revealed an enlarged thyroid gland (right lobe length, 10.3 cm; isthmus, 2 cm; left lobe, 8 cm) with a focal area of increased echogenicity in the midpole of the left lobe measuring 9.5 mm × 5.5 mm. The patient was discharged to home with pain medications, and urgent follow-up with an otolaryngologist was arranged. A flexible laryngoscopy was done in the otolaryngology clinic, which revealed retropharyngeal bulging that correlated with the thyromegaly evident on the CT scan.
Because of the patient’s significant symptoms, we decided to proceed with surgery with a clinical diagnosis of likely thyroiditis. A left subtotal thyroidectomy with extension to the superior mediastinum was performed, but a right thyroidectomy could not be done safely. On gross examination, a well-capsulated left lobe with a tan-white, lobulated, soft cut surface was seen. Microscopic examination revealed replacement of thyroid parenchyma with sheets of mature-appearing plasma cells with eccentric round nuclei, abundant eosinophilic cytoplasm without atypia, and few scattered thyroid follicles with lymphoepithelial lesions (Figure 1A). Immunohistochemistry confirmed plasma cells with expression of CD138 (Figure 1B).
Fluorescence in situ hybridization (FISH) showed that the neoplastic plasma cells contained monotypic kappa immunoglobulin light chain messenger RNA. Clonal immunoglobulin gene rearrangement was detected on polymerase chain reaction. A diagnosis of plasmacytoma of the thyroid gland in a background of thyroiditis was made on the basis of the aforementioned observations.
After that diagnosis, we performed an extensive work-up for plasma cell myeloma. Bone marrow biopsy showed normal maturing trilineage hematopoiesis with scattered mature-appearing plasma cells Figure 2A. Flow cytometry showed a rare (0.2%) population of polytypic plasma cells and was confirmed by CD138 immunohistochemistry. FISH showed proportionate distribution (2-5:1) of kappa and lambda light chains in plasma cells (Figure 2B).
Serum protein electrophoresis showed normal levels of serum proteins with no M spike. Serum total protein was 7.9 g/dL, albumin 5.0 g/dL, α1-globulin 0.3 g/dL, α2-globulin 0.8 g/dL, β-globulin 0.7 g/dL, and γ-globulin 1.6 g/dL, with an albumin–globulin ratio of 1.47. Calcium and β2-microglobulin were also in the normal ranges. Serum-free kappa light chain was found to be elevated (20.9 mg/L; reference range, 3.3-19.4 mg/L). The immunoglobulin G level was also elevated at 3,104 mg/dL (reference range, 700-1,600 mg/dL).
A positron-emission tomographic (PET) scan done 1 month after the surgery showed no other sites of disease except the thyroid. No lytic bone lesions were present. The patient was treated with 50.4 Gy of radiation by external beam radiotherapy to the thyroid in 28 fractions as definitive therapy. Despite treatment with surgery and radiation, she continued to have pain around the neck, and a repeat PET scan 3 months after completion of radiation showed persistent uptake in the thyroid. Because of her refractoriness to radiotherapy, she was started on systemic therapy with a weekly regimen of bortezomib and dexamethasone for 9 cycles. Her symptoms began to resolve, and a repeat PET scan done after completion of chemotherapy showed no evidence of uptake, suggesting adequate response to chemotherapy, and chemotherapy was therefore stopped. The patient was scheduled a regular follow-up in 3 months. Because of continued local symptoms in this follow-up period, the decision was made to perform surgical gland removal, and she underwent completion of
Discussion
Plasma cells are well-differentiated B-lymphocytes that secrete antibodies and provide protective immunity to the human body.9 Plasma cell neoplasms are clonal proliferation of plasma cells, producing monoclonal immunoglobulins. They are of the following different types: plasma cell myeloma, monoclonal gammopathy of unknown significance, immunoglobulin deposition disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes) syndrome, and plasmacytomas, which are divided into 2 types – solitary plasmacytoma of the bone, and extramedullary plasmacytoma (EMP).10 EMP is a rare condition and encompasses 3% to 5% of all plasma cell neoplasms, depending on the study.1,2,5 It is more common in men than in women (2.6:1, respectively), with equal incidence among black and white patients. Median age at diagnosis is 62 years, and it is more common among those aged 40 to 70 years.2,11 The most common sites of occurrence are the respiratory tract, the mouth, and the pharynx, but other sites such as the eyes, brain, skin, and lymph nodes may also be involved.2
EMP involving the thyroid gland is a very rare occurrence, but plasma cell myeloma has been shown to secondarily involve the thyroid.4 Similar to our report, EMP of the thyroid in the setting of thyroiditis has been reported by other authors.3,4 The incidence of EMP occurring in the thyroid varies according to different authors. Wiltshaw found 7 cases involving the thyroid out of 272 cases of EMP.1 Galieni and colleagues reported only 1 case that involved the thyroid out of 46 described cases of EMP.12
El- Siemińska and colleagues showed that levels of interleukin (IL)-6 are elevated in thyroiditis.13 IL-6 promotes monoclonal as well as polyclonal proliferation of plasma cells. Kovalchuk and colleagues showed an increase in EMP in IL-6 transgenic mice, suggesting a pathophysiologic explanation.14
The diagnostic requirements of EMP include the following: histology showing monoclonal plasma cell infiltration in tissue; bone marrow biopsy with normal plasma cell aspirate and biopsy (plasma cells, <5%); no lytic lesions on skeletal survey; no anemia, renal impairment, or hypercalcemia; and absent or low serum M protein.12
Our case fulfilled those criteria.
The treatment options for EMP include surgery, radiotherapy, or a combined approach including both. Usually, EMPs are very sensitive to radiotherapy, and complete remission can be achieved by radiotherapy alone in 80% to 100% of cases.6,11,15 Surgery is considered if the tumor is diffuse or is causing symptoms secondary to pressure on surrounding structures. A combined approach is recommended in cases with incomplete surgical margin or lymph node involvement.5,6
There is limited evidence about and experience with the use of chemotherapy in the treatment of EMP. It has been recommended that chemotherapy be considered in patients with refractory or relapsed disease using the same regimen used in plasma cell myeloma.5 Katodritou and colleagues have reported using bortezomib and dexamethasone without surgery in a solitary gastric plasmacytoma to avoid the toxicity of gastrointestinal irradiation.7 Wei and colleagues treated a patient with EMP in the pancreas with bortezomib and achieved a near-complete remission.8 To our knowledge, there is no documented literature about the treatment of EMP of the thyroid with chemotherapy. In our patient, despite the treatment with surgery and radiation, there was persistent uptake on the PET scan, so we treated her with bortezomib and dexamethasone. Because there is an 11% to 30% risk of progression to multiple myeloma, long-term follow-up is recommended in EMP.11
Conclusions
Solitary EMP of the thyroid gland is a rare condition. Plasma cell myeloma must be ruled out to make a diagnosis. Data on the incidence of EMP and its clinicopathological features are sparse, and literature describing proper guidelines on treatment is limited. It can be treated with radiotherapy, surgery, or a combined approach. There is limited data on the role of chemotherapy; our case adds to the available literature on using myeloma-based therapy in refractory disease and, to our knowledge, is the only case report using this in the literature on cases of EMP of the thyroid. Regular follow-up, even after the disease is in remission, is necessary because of the high risk of progression to plasma cell myeloma.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
1. Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore). 1976;55(3):217-238.
2. Dores GM, Landgren O, McGlynn KA, Curtis RE, Linet MS, Devesa SS. Plasmacytoma of bone, extramedullary plasmacytoma, and multiple myeloma: incidence and survival in the United States, 1992-2004. Br J Haematol. 2009;144(1):86-94.
3. Kovacs CS, Mant MJ, Nguyen GK, Ginsberg J. Plasma cell lesions of the thyroid: report of a case of solitary plasmacytoma and a review of the literature. Thyroid. 1994;4(1):65-71.
4. Avila A, Villalpando A, Montoya G, Luna MA. Clinical features and differential diagnoses of solitary extramedullary plasmacytoma of the thyroid: a case report. Ann Diagn Pathol. 2009;13(2):119-123.
5. Hughes M, Soutar R, Lucraft H, Owen R, Bird J. Guidelines on the diagnosis and management of solitary plasmacytoma of bone, extramedullary plasmacytoma and multiple solitary plasmacytomas: 2009 update. London, United Kingdom: British Committee for Standards in Haematology; 2009.
6. Weber DM. Solitary bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. 2005;373-376.
7. Katodritou E, Kartsios C, Gastari V, et al. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32(2):339-341.
8. Wei JY, Tong HY, Zhu WF, et al. Bortezomib in treatment of extramedullary plasmacytoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2009;8(3):329-331.
9. Roth K, Oehme L, Zehentmeier S, Zhang Y, Niesner R, Hauser AE. Tracking plasma cell differentiation and survival. Cytometry A. 2014;85(1):15-24.
10. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008.
11. Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85(11):2305-2314.
12. Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85(1):47-51.
13. Siemińska L, Wojciechowska C, Kos-Kudła B, et al. Serum concentrations of leptin, adiponectin, and interleukin-6 in postmenopausal women with Hashimoto’s thyroiditis. Endokrynol Pol. 2010;61(1):112-116.
14. Kovalchuk AL, Kim JS, Park SS, et al. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci US
15. Chao MW, Gibbs P, Wirth A, Quong G, Guiney MJ, Liew KH. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35(4):211-215.
Salivary ductal adenocarcinoma with complete response to androgen blockade
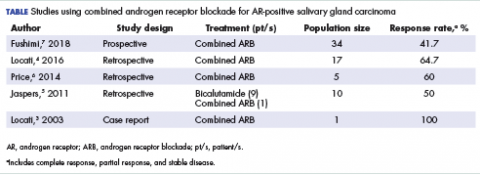
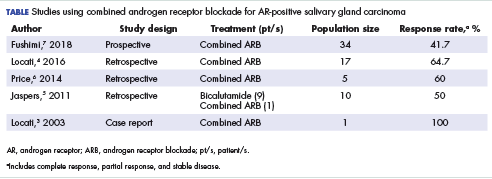
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
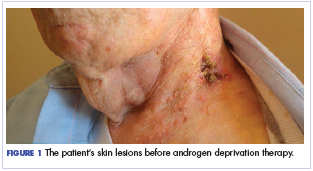
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
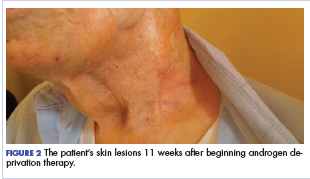
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
Salivary ductal adenocarcinomas make up about 9% of malignant salivary gland tumors and occur mostly in men older than 50 years, with a peak incidence in the sixth and seventh decades. It is the most aggressive of salivary gland tumors and is histologically similar to high-grade, invasive ductal carcinoma of the breast. In all, 65% of patients will die of the disease, and most will experience skin ulceration and nerve palsy.1 With such an aggressive clinical picture, the temptation for many oncologists and patients is to use aggressive cytotoxic chemotherapies. Considering the lack of large trials exploring treatment options in this less-common subtype of salivary gland carcinoma, practice guidelines also recommend the use of aggressive chemotherapies. Unlike other types of malignant cancers of the salivary glands, 70% to 90% of ductal adenocarcinomas express the androgen receptor (AR) by immunohistochemistry.2 There are reported cases of androgen deprivation therapy (ADT) as a successful treatment for salivary ductal adenocarcinomas that express the AR (Table).In 2003, Locati and colleagues reported the case of a man with salivary ductal adenocarcinomas who had a complete response with ADT.3 In 2016, the same group of authors published a retrospective analysis of 17 patients with recurrent or metastatic AR-positive salivary gland cancers who were treated with ADT and reported a 64.7% overall response rate among the patients.4 A 10-patient case series in the Netherlands demonstrated a 50% response rate to ADT plus bicalutamide, including a palliative effect in the form of pain relief.5 A retrospective analysis by Price and colleagues of 5 patients with AR-positive metastatic salivary duct adenocarcinoma showed a 60% response rate to a combination of leuprolide and bicalutamide.6
Case presentation and summary
A 91-year-old man was diagnosed with salivary ductal adenocarcinoma of the left parotid gland in September 2013 and underwent left parotidectomy and lymph node dissection, which revealed AJCC stage IVA (pT2 pN3 M0) disease. The following year, in December 2014, he had an enlarging left neck mass that was pathologically confirmed to be recurrent disease, and he underwent left level V neck dissection in February 2015. Five months after surgery, in July 2015, he presented with left neck fullness and new skin nodules, and the results of a biopsy confirmed recurrent disease. Given his relatively asymptomatic state and advanced age, the oncology care team decided to follow the patient without any pharmacologic therapy.
The patient felt relatively well for 11 months but slowly developed increasing pain in the left neck in June 2016. The skin nodules also began to spread inferiorly from his left neck to his upper chest with the development of open sores that wept serous fluid with scab formation (Figure 1). He and his wife lived independently and managed all their own instrumental activities of daily living (IADL). Eventually, the pain in his neck became so severe that it began to interfere with his ability to drive. He declined radiation therapy because of side effects and transportation issues, but he desired something to alleviate the burden of the disease. During a multidisciplinary cancer conference, the staff pathologist and oncologist discussed AR immunohistochemistry to assist with management. In June 2016, the patient’s tumor was found to have AR immunostaining (nuclear pattern) in 100% of cells, and he was treated with combined androgen blockade, consisting of monthly 3.6 mg goserelin injections and daily bicalutamide 50 mg orally.
Within a week, the patient noticed that the skin lesions stopped weeping fluid. Within 2 weeks, the pain had begun to resolve. At his formal follow-up visit 11 weeks after starting treatment, he was not taking any pain medications and reported no pain. In addition, his visually apparent disease had almost completely resolved (Figure 2). He was fully able to manage his own IADL and reported a marked increase in satisfaction with the quality of his life.
Discussion
The oncology care team clearly defined the goal of care for this patient as palliative and conveyed as such to the patient. The team considered the risks and side effects of cytotoxic chemotherapy agents to be contrary to the patient’s stated primary goal of independence. We selected the combined androgen blockade because it has a low toxicity rate and thus met the primary goals of therapy.
The European Organization for Research and Treatment of Cancer is presently conducting a trial in which cytotoxic chemotherapy is being compared with ADT in AR-positive salivary duct tumors. Findings from a recent prospective, phase-2 trial conducted in Japan suggested that combined AR blockade has similar efficacy and less toxicity than conventional cytotoxic chemotherapy for recurrent and/or metastatic and unresectable locally advanced AR-positive salivary gland carcinoma.7 As more data become available from other studies, it is possible that practice guidelines will be revised to recommend this treatment approach for these cancers.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
1. Eveson JW, Thompson LDR. Malignant neoplasms of the salivary glands. In: Thompson LDR, ed. Head and neck pathology. 2nd ed. Philadelphia, PA: Elsevier Inc; 2013:304-305.
2. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(suppl 1):E1838-E1847.
3. Locati LD, Quattrone P, Bossi P, Marchianò AV, Cantù G, Licitra L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann Oncol. 2003;14(8):1327-1328.
4. Locati LD, Perrone F, Cortelazzi B, et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck. 2016;38(5):724-731.
5. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473-e476.
6. Price KAR, Okuno SH, Molina JR, Garcia JJ. Treatment of metastatic salivary duct carcinoma with combined androgen blockade (CAB) with leuprolide acetate and bicalutamide. Int J Radiat Oncol Biol Phys. 2014;88(2):521-522.
7. Fushimi C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979-984.
Characteristics of urgent palliative cancer care consultations encountered by radiation oncologists
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
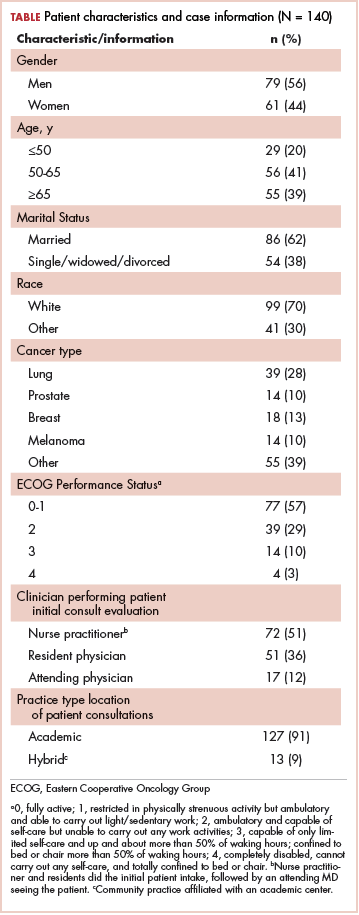
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
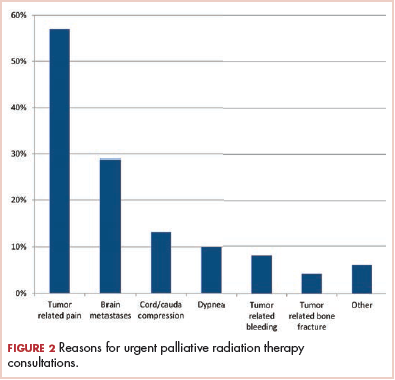
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
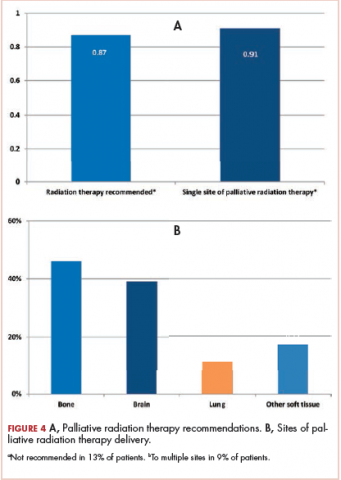
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
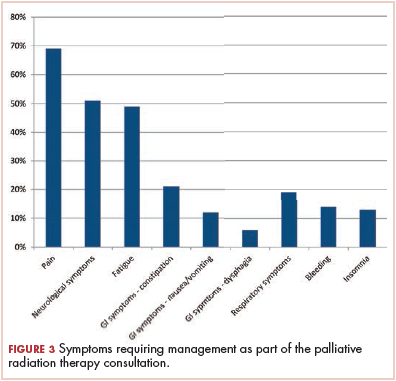
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
Neratinib extends adjuvant treatment of patients with HER2-positive breast cancer
The small-molecule tyrosine kinase inhibitor neratinib is now approved for the extended adjuvant treatment of patients with early-stage HER2 [human epidermal growth factor receptor]-positive breast cancer following postoperative trastuzumab. Trastuzumab is a HER2-targeted monoclonal antibody that has become standard of care in combination with chemotherapy for the treatment of this patient population in which it significantly improves survival. However, disease recurrence will occur in about a quarter of trastuzumab-treated patients owing to the development of resistance.
Neratinib may help overcome trastuzumab resistance thanks to its potent inhibition of the downstream phosphorylation of HER2 and other members of the HER family. Its approval was based on the phase 3 ExteNET trial, in which extended adjuvant treatment with neratinib was compared with placebo among 2,840 patients who remained disease free after 1 year of adjuvant trastuzumab.1
The ExteNET trial was performed at 495 centers in Europe, Asia, Australia, New Zealand, and South America. Patients aged 18 years or older (≥20 years in Japan), with stage 1-3 HER2-positive breast cancer, who completed neoadjuvant and adjuvant trastuzumab therapy up to 1 year before randomization were eligible. Patients also had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (range, 0-5; 0, fully active, and 5, dead), normal organ function, and a left ventricular ejection fraction within normal institutional range. Patients with clinically significant cardiac, gastrointesintal or psychiatric comorbidities and those who were not able to swallow oral medication were excluded from the study.
Patients randomly received oral neratinib 240 mg per day or matching placebo, and randomization was stratified according to HR status (positive or negative), nodal status (0, 1-3, or ≥4) and trastuzumab-adjuvant regimen (sequentially or concurrently with chemotherapy).
The primary outcome was invasive disease-free survival (iDFS). The 2-year iDFS rate was 93.9% for neratinib, compared with 91.6% for placebo (hazard ratio [HR], 0.66; P < .008). Recently, a 5-year analysis of the ExteNET trial showed that after a median follow-up of 5.2 years, the iDFS rates were 90.2% vs 87.7% (HR, 0.73; P = .0083).2
Adverse events
The most common adverse event (AE) was diarrhea, in 95% of patients, 40% of whom had grade 3 diarrhea, leading to dose reduction in 26% of patients and discontinuation in 16.8% of patients. Serious AEs occurred in 7% of patients in the neratinib and 6% of those in the placebo arms. In the 5-year analysis, there was no evidence of increased risk of long-term toxicity or adverse consequences of neratinib-associated diarrhea. Furthermore, the ongoing, open-label phase 2 CONTROL trial suggests that the occurrence and severity of neratinib-associated diarrhea can be effectively controlled with antidiarrheal prophylaxis, with drugs such as loperamide.3
At the January 2017 cut-off, 137 patients treated with neratinib (240 mg/day) for 1 year had also received treatment with loperamide monotherapy, 64 patients had received loperamide and budesonide, and 10 patients had received loperamide and colestipol. The safety data from the loperamide monotherapy arm were compared with the safety data from the ExteNET trial, which was based in a similar population of patients who did not receive antidiarrheal prophylaxis. The incidence of all-grade diarrhea was 77% vs 95%, respectively, for those who received antidiarrheal prophylaxis in the CONTROL trial compared with those in the ExteNET trial who did not, and the repective rates of grade 3 diarrhea were 31% and 40%. The rate of dose reductions and holds owing to diarrhea were also lower among those who received antidiarrheal prophylaxis, but the rate of discontinuation due to diarrhea was higher in the loperamide-treated cohort.
Warnings and precautions
Neratinib is marketed as Nerlynx by Puma Biotechnology Inc. The prescribing information describes warnings and precautions relating to diarrhea, hepatotoxicity, and embryofetal toxicity. Patients should be monitored for diarrhea and treated with antidiarrheals as needed. Severe diarrhea with dehydration should be treated with fluids and electrolytes as needed, treatment should be interrupted and resumed at a reduced dose. For grade 3/4 diarrhea or diarrhea with complicating features (eg, dehydration, fever, neutropenia), stool cultures should be performed to rule out infectious causes.
Total bilirubin, aspartate and alanine aminotransferase, and alkaline phosphatase levels should be measured before starting treatment, every 3 months during treatment, or as clinically indicated. Neratinib can cause fetal harm, so pregnant women should be advised of the risk to the fetus and patients of reproductive potential should be counseled on the need for effective contraception during treatment and for at least 1 month after the last dose.4
1. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17: 367-377.
2. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab- based adjuvant therapy in HER2-positive breast cancer (ExteNET): a 5-year analysis of a randomised, double-blind, placebo- controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700.
3. Ibrahim E, Tripathy D, Wilkinson M, et al. E£ects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients (pts) with HER2+ early-stage breast cancer (EBC): The CONTROL trial. Cancer Res. 2017; 77(13 supplement): Abstract CT128.
4. Nerlynx (neratinib) tablets, for oral use. Prescribing information. Puma Biotechnology Inc. https://nerlynx.com/pdf/full-prescribinginformation. pdf. Revised July 2017. Accessed November 20th, 2017.
The small-molecule tyrosine kinase inhibitor neratinib is now approved for the extended adjuvant treatment of patients with early-stage HER2 [human epidermal growth factor receptor]-positive breast cancer following postoperative trastuzumab. Trastuzumab is a HER2-targeted monoclonal antibody that has become standard of care in combination with chemotherapy for the treatment of this patient population in which it significantly improves survival. However, disease recurrence will occur in about a quarter of trastuzumab-treated patients owing to the development of resistance.
Neratinib may help overcome trastuzumab resistance thanks to its potent inhibition of the downstream phosphorylation of HER2 and other members of the HER family. Its approval was based on the phase 3 ExteNET trial, in which extended adjuvant treatment with neratinib was compared with placebo among 2,840 patients who remained disease free after 1 year of adjuvant trastuzumab.1
The ExteNET trial was performed at 495 centers in Europe, Asia, Australia, New Zealand, and South America. Patients aged 18 years or older (≥20 years in Japan), with stage 1-3 HER2-positive breast cancer, who completed neoadjuvant and adjuvant trastuzumab therapy up to 1 year before randomization were eligible. Patients also had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (range, 0-5; 0, fully active, and 5, dead), normal organ function, and a left ventricular ejection fraction within normal institutional range. Patients with clinically significant cardiac, gastrointesintal or psychiatric comorbidities and those who were not able to swallow oral medication were excluded from the study.
Patients randomly received oral neratinib 240 mg per day or matching placebo, and randomization was stratified according to HR status (positive or negative), nodal status (0, 1-3, or ≥4) and trastuzumab-adjuvant regimen (sequentially or concurrently with chemotherapy).
The primary outcome was invasive disease-free survival (iDFS). The 2-year iDFS rate was 93.9% for neratinib, compared with 91.6% for placebo (hazard ratio [HR], 0.66; P < .008). Recently, a 5-year analysis of the ExteNET trial showed that after a median follow-up of 5.2 years, the iDFS rates were 90.2% vs 87.7% (HR, 0.73; P = .0083).2
Adverse events
The most common adverse event (AE) was diarrhea, in 95% of patients, 40% of whom had grade 3 diarrhea, leading to dose reduction in 26% of patients and discontinuation in 16.8% of patients. Serious AEs occurred in 7% of patients in the neratinib and 6% of those in the placebo arms. In the 5-year analysis, there was no evidence of increased risk of long-term toxicity or adverse consequences of neratinib-associated diarrhea. Furthermore, the ongoing, open-label phase 2 CONTROL trial suggests that the occurrence and severity of neratinib-associated diarrhea can be effectively controlled with antidiarrheal prophylaxis, with drugs such as loperamide.3
At the January 2017 cut-off, 137 patients treated with neratinib (240 mg/day) for 1 year had also received treatment with loperamide monotherapy, 64 patients had received loperamide and budesonide, and 10 patients had received loperamide and colestipol. The safety data from the loperamide monotherapy arm were compared with the safety data from the ExteNET trial, which was based in a similar population of patients who did not receive antidiarrheal prophylaxis. The incidence of all-grade diarrhea was 77% vs 95%, respectively, for those who received antidiarrheal prophylaxis in the CONTROL trial compared with those in the ExteNET trial who did not, and the repective rates of grade 3 diarrhea were 31% and 40%. The rate of dose reductions and holds owing to diarrhea were also lower among those who received antidiarrheal prophylaxis, but the rate of discontinuation due to diarrhea was higher in the loperamide-treated cohort.
Warnings and precautions
Neratinib is marketed as Nerlynx by Puma Biotechnology Inc. The prescribing information describes warnings and precautions relating to diarrhea, hepatotoxicity, and embryofetal toxicity. Patients should be monitored for diarrhea and treated with antidiarrheals as needed. Severe diarrhea with dehydration should be treated with fluids and electrolytes as needed, treatment should be interrupted and resumed at a reduced dose. For grade 3/4 diarrhea or diarrhea with complicating features (eg, dehydration, fever, neutropenia), stool cultures should be performed to rule out infectious causes.
Total bilirubin, aspartate and alanine aminotransferase, and alkaline phosphatase levels should be measured before starting treatment, every 3 months during treatment, or as clinically indicated. Neratinib can cause fetal harm, so pregnant women should be advised of the risk to the fetus and patients of reproductive potential should be counseled on the need for effective contraception during treatment and for at least 1 month after the last dose.4
The small-molecule tyrosine kinase inhibitor neratinib is now approved for the extended adjuvant treatment of patients with early-stage HER2 [human epidermal growth factor receptor]-positive breast cancer following postoperative trastuzumab. Trastuzumab is a HER2-targeted monoclonal antibody that has become standard of care in combination with chemotherapy for the treatment of this patient population in which it significantly improves survival. However, disease recurrence will occur in about a quarter of trastuzumab-treated patients owing to the development of resistance.
Neratinib may help overcome trastuzumab resistance thanks to its potent inhibition of the downstream phosphorylation of HER2 and other members of the HER family. Its approval was based on the phase 3 ExteNET trial, in which extended adjuvant treatment with neratinib was compared with placebo among 2,840 patients who remained disease free after 1 year of adjuvant trastuzumab.1
The ExteNET trial was performed at 495 centers in Europe, Asia, Australia, New Zealand, and South America. Patients aged 18 years or older (≥20 years in Japan), with stage 1-3 HER2-positive breast cancer, who completed neoadjuvant and adjuvant trastuzumab therapy up to 1 year before randomization were eligible. Patients also had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (range, 0-5; 0, fully active, and 5, dead), normal organ function, and a left ventricular ejection fraction within normal institutional range. Patients with clinically significant cardiac, gastrointesintal or psychiatric comorbidities and those who were not able to swallow oral medication were excluded from the study.
Patients randomly received oral neratinib 240 mg per day or matching placebo, and randomization was stratified according to HR status (positive or negative), nodal status (0, 1-3, or ≥4) and trastuzumab-adjuvant regimen (sequentially or concurrently with chemotherapy).
The primary outcome was invasive disease-free survival (iDFS). The 2-year iDFS rate was 93.9% for neratinib, compared with 91.6% for placebo (hazard ratio [HR], 0.66; P < .008). Recently, a 5-year analysis of the ExteNET trial showed that after a median follow-up of 5.2 years, the iDFS rates were 90.2% vs 87.7% (HR, 0.73; P = .0083).2
Adverse events
The most common adverse event (AE) was diarrhea, in 95% of patients, 40% of whom had grade 3 diarrhea, leading to dose reduction in 26% of patients and discontinuation in 16.8% of patients. Serious AEs occurred in 7% of patients in the neratinib and 6% of those in the placebo arms. In the 5-year analysis, there was no evidence of increased risk of long-term toxicity or adverse consequences of neratinib-associated diarrhea. Furthermore, the ongoing, open-label phase 2 CONTROL trial suggests that the occurrence and severity of neratinib-associated diarrhea can be effectively controlled with antidiarrheal prophylaxis, with drugs such as loperamide.3
At the January 2017 cut-off, 137 patients treated with neratinib (240 mg/day) for 1 year had also received treatment with loperamide monotherapy, 64 patients had received loperamide and budesonide, and 10 patients had received loperamide and colestipol. The safety data from the loperamide monotherapy arm were compared with the safety data from the ExteNET trial, which was based in a similar population of patients who did not receive antidiarrheal prophylaxis. The incidence of all-grade diarrhea was 77% vs 95%, respectively, for those who received antidiarrheal prophylaxis in the CONTROL trial compared with those in the ExteNET trial who did not, and the repective rates of grade 3 diarrhea were 31% and 40%. The rate of dose reductions and holds owing to diarrhea were also lower among those who received antidiarrheal prophylaxis, but the rate of discontinuation due to diarrhea was higher in the loperamide-treated cohort.
Warnings and precautions
Neratinib is marketed as Nerlynx by Puma Biotechnology Inc. The prescribing information describes warnings and precautions relating to diarrhea, hepatotoxicity, and embryofetal toxicity. Patients should be monitored for diarrhea and treated with antidiarrheals as needed. Severe diarrhea with dehydration should be treated with fluids and electrolytes as needed, treatment should be interrupted and resumed at a reduced dose. For grade 3/4 diarrhea or diarrhea with complicating features (eg, dehydration, fever, neutropenia), stool cultures should be performed to rule out infectious causes.
Total bilirubin, aspartate and alanine aminotransferase, and alkaline phosphatase levels should be measured before starting treatment, every 3 months during treatment, or as clinically indicated. Neratinib can cause fetal harm, so pregnant women should be advised of the risk to the fetus and patients of reproductive potential should be counseled on the need for effective contraception during treatment and for at least 1 month after the last dose.4
1. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17: 367-377.
2. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab- based adjuvant therapy in HER2-positive breast cancer (ExteNET): a 5-year analysis of a randomised, double-blind, placebo- controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700.
3. Ibrahim E, Tripathy D, Wilkinson M, et al. E£ects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients (pts) with HER2+ early-stage breast cancer (EBC): The CONTROL trial. Cancer Res. 2017; 77(13 supplement): Abstract CT128.
4. Nerlynx (neratinib) tablets, for oral use. Prescribing information. Puma Biotechnology Inc. https://nerlynx.com/pdf/full-prescribinginformation. pdf. Revised July 2017. Accessed November 20th, 2017.
1. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17: 367-377.
2. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab- based adjuvant therapy in HER2-positive breast cancer (ExteNET): a 5-year analysis of a randomised, double-blind, placebo- controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700.
3. Ibrahim E, Tripathy D, Wilkinson M, et al. E£ects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients (pts) with HER2+ early-stage breast cancer (EBC): The CONTROL trial. Cancer Res. 2017; 77(13 supplement): Abstract CT128.
4. Nerlynx (neratinib) tablets, for oral use. Prescribing information. Puma Biotechnology Inc. https://nerlynx.com/pdf/full-prescribinginformation. pdf. Revised July 2017. Accessed November 20th, 2017.
More biosimilars reach the market in efforts to improve access and cut costs
Biosimilars are copies of FDA-approved biologic drugs (those generally derived from a living organism) that cannot be identical to the reference drug but demonstrate a high similarity to it. As patents on the reference drugs expire, biosimilars are being developed to increase competition in the marketplace to reduce costs and improve patient access to therapy. Although the US Food and Drug Administration (FDA) has no regulatory power over drug prices, it recently announced efforts to streamline the biosimilar approval process to facilitate access to therapies and curb the associated skyrocketing costs.
Several biosimilars have been approved by the agency in recent years, and earlier this year they were joined by 2 more: the approval in May of epoetin alfa-epbx (Retacrit; Hospira, a Pfizer company) for all indications of the reference product (epoetin alfa; Epogen/Procrit, Amgen), including the treatment of anemia caused by myelosuppressive chemotherapy, when there is a minimum of 2 additional months of planned chemotherapy;1 and the June approval of pegfilgrastim-jmdb (Fulphila, Mylan and Biocon) for the treatment of patients undergoing myelosuppressive chemotherapy to help reduce the chance of infection as suggested by febrile neutropenia (fever, often with other signs of infection, associated with an abnormally low number of infection-fighting white blood cells).2 The reference product for pegfilgrastim-jmdb is pegfilgrastim (Neulasta, Amgen).
The approval of both biosimilars was based on a review of a body of evidence that included structural and functional characterization, animal study data, human pharmacokinetic (PK) and pharmacodynamic (PD) data, clinical immunogenicity data, and other clinical safety and efficacy data. This evidence established that the biosimilars were highly similar to the already FDA-approved reference products, with no clinically relevant differences.
Biocon and Mylan-GmBH, which jointly developed pegfilgrastim-jmdb, originally filed for approval in 2017; and Hospira Inc, a Pfizer company that developed epoetin alfa-epbx, filed for the first time in 2015. They subsequently received complete response letters from the FDA, twice in the case of the epoetin alfa biosimilar, rejecting their approval. For pegfilgrastim-jmdb, the complete response letter was related to a pending update of the Biologic License Application as the result of requalification activities taken because of modifications at their manufacturing plant. For epoetin alfa-epbx, the FDA expressed concerns relating to a manufacturing facility. The companies addressed the concerns in the complete response letters and submitted corrective and preventive action plans.3,4
Pegfilgrastim-jmdb
The results from a phase 3, multicenter, randomized, double-blind parallel-group trial of pegfilgrastim-jmdb compared with European Union-approved pegfilgrastim were published in 2016. Chemotherapy and radiation-naïve patients with newly diagnosed breast cancer (n = 194) received the biosimilar or reference product every 3 weeks for 6 cycles. The primary endpoint was duration of severe neutropenia in cycle 1, defined as days with absolute neutrophil count <0.5 x 109/L. The mean standard deviation was 1.2 [0.93] in the pegfilgrastim-jmdb arm and 1.2 [1.10] in the EU-pegfilgrastim arm, and the 95% confidence interval of least squares means differences was within the -1 day, +1 day range, indicating equivalency.5
A characterization and similarity assessment of pegfilgrastim-jmdb compared with US- and EU-approved pegfilgrastim was presented at the 2018 Annual Meeting of the American Society of Clinical Oncology. G-CSF receptor (G-CSFR) binding was assessed by surface plasmon resonance and potency was measured by in vitro stimulated proliferation in a mouse myelogenous leukemia cell line. In vivo rodent studies were also performed and included a PD study with a single dose of up to 3 mg/kg.6
There was high similarity in the structure, molecular mass, impurities and functional activity of the biosimilar and reference products, as well as similar G-CSFR binding and equivalent relative potency. Neutrophil and leukocyte counts were increased to a similar degree, and toxicology and drug kinetics were also comparable.
The recommended dose of pegfilgrastim-jmdb is a 6 mg/0.6 ml injection in a single-dose prefilled syringe for manual use only, administered subcutaneously once per chemotherapy cycle. The prescribing information also has dosing guidelines for administration in pediatric patients who weigh less than 45 kg. Pegfilgrastim-jmdb should not be administered between 14 days before and 24 hours after administration of chemotherapy.
The prescribing information details warnings and precautions relating to splenic rupture, acute respiratory distress syndrome (ARDS), serious allergic reactions, potential for severe/fatal sickle cell crises in patients with sickle cell disorders, glomerulonephritis, leukocytosis, capillary leak syndrome, and the potential for tumor growth or recurrence.7
Patients should be evaluated for an enlarged spleen or splenic rupture if they report upper left abdominal or shoulder pain. Patients who develop fever and lung infiltrates or respiratory distress should be evaluated for ARDS and treatment discontinued if a diagnosis is confirmed. Pegfilgrastim-jmdb should be permanently discontinued in patients who develop serious allergic reactions and should not be used in patients with a history of serious allergic reactions to pegfilgrastim or filgrastim products.
Dose-reduction or interruption should be considered in patients who develop glomerulonephritis. Complete blood counts should be monitored throughout treatment. Patients should be monitored closely for capillary leak syndrome and treated with standard therapy. Pegfilgrastim-jmdb is marketed as Fulphila.
Epoetin alfa-epbx
Epoetin alfa-epbx was evaluated in 2 clinical trials in healthy individuals. The EPOE-12-02 trial established the PK and PD following a single subcutaneous dose of 100 U/kg in 81 participants. The EPOE-14-1 study was designed to determine the PK and PD of multiple doses of subcutaneous 100 U/kg 3 times weekly for 3 weeks in 129 participants. Both studies met prespecified criteria
Evidence of efficacy and safety were also evaluated using pooled data from EPOE-10-13 and EPOE-10-01, conducted in patients with chronic kidney disease, which was considered the most sensitive population in which to evaluate clinically meaningful differences between the biosimilar and reference product.8,9
There were no clinically meaningful differences in efficacy and a similar adverse event profile. The most common side effects include high blood pressure, joint pain, muscle spasm, fever, dizziness, respiratory infection, and cough, among others.
The recommended dose of epoetin alfa-epbx, which is marketed as Retacrit, is 40,000 Units weekly or 150 U/kg 3 times weekly in adults and 600 U/kg intravenously weekly in pediatric patients aged 5 years or younger. Epoetin alfa-epbx comes with a boxed warning to alert health care providers to the increased risks of death, heart problems, stroke, and tumor growth, or recurrence. The prescribing information also details warnings and precautions relating to these risks, as well as hypertension, seizures, lack or loss of hemoglobin response, pure red cell aplasia, serious allergic reactions, and severe cutaneous reactions.9
Blood pressure should be appropriately controlled before treatment initiation, treatment should be reduced or withheld if it becomes uncontrollable, and patients should be advised of the importance of compliance with anti-hypertensive medication and dietary restrictions. Patients should be monitored closely for premonitory neurologic symptoms and advised to contact their provider in the event of new-onset seizures, premonitory symptoms, or change in seizure frequency.
The prescribing information has dosing recommendations for lack or loss of hemoglobin response to epoetin alfa-epbx. If severe anemia or low reticulocyte count occur, treatment should be withheld and patients evaluated for neutralizing antibodies to erythropoietin and, in the event that PRCA is confirmed, treatment should be permanently discontinued. Treatment should be immediately and permanently discontinued for serious allergic reactions or severe cutaneous reactions.
1. US Food and Drug Administration website. FDA approves first epoetin alfa biosimilar for the treatment of anemia. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607703.htm. Updated May 15, 2018. Accessed June 22, 2018.
2. US Food and Drug Administration website. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm609805.htm. Updated June 4, 2018. Accessed June 22, 2018.
3. Reuters. BRIEF – Biocon says US FDA issues complete response letter for proposed biosimilar pegfilgrastim. https://www.reuters.com/article/brief-biocon-says-us-fda-issued-complete/brief-biocon-says-us-fda-issued-complete-response-letter-for-proposed-biosimilar-pegfilgrastim-idUSFWN1MK0Q1. Updated October 9, 2017. Accessed June 22, 2018.
4. FiercePharma. Pfizer, on third try, wins nod for biosimilar of blockbuster epogen/procrit. https://www.fiercepharma.com/pharma/pfizer-third-try-wins-fda-nod-for-biosimilar-blockbuster-epogen-procrit. Updated May 15, 2018. Accessed June 22, 2018.
5. Waller CF, Blakeley C, Pennella E. Phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H vs EU-neulasta in the prophylaxis of chemotherapy-induced neutropenia. Ann Oncol. 2016;27(suppl_6):1433O.
6. Sankaran PV, Palanivelu DV, Nair R, et al. Characterization and similarity assessment of a pegfilgrastim biosimilar MYL-1401H. J Clin Oncol. 2018;36(suppl; abstr e19028).
7. Fulphila (pegfilgrastim-jmdb) injection, for subcutaneous use. Prescribing information. Mylan GmBH. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761075s000lbl.pdf. Released June 2018. Accessed June 22, 2018.
8. US Food and Drug Administration website. ‘Epoetin Hospira,’ a proposed biosimilar to US-licensed Epogen/Procrit. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559962.pdf. Updated May 25, 2017. Accessed June 22, 2018.
9. Retacrit (epoetin alfa-epbx) injection, for intravenous or subcutaneous use. Prescribing information. Pfizer. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125545s000lbl.pdf. Released May 2018. Accessed June 22, 2018.
Biosimilars are copies of FDA-approved biologic drugs (those generally derived from a living organism) that cannot be identical to the reference drug but demonstrate a high similarity to it. As patents on the reference drugs expire, biosimilars are being developed to increase competition in the marketplace to reduce costs and improve patient access to therapy. Although the US Food and Drug Administration (FDA) has no regulatory power over drug prices, it recently announced efforts to streamline the biosimilar approval process to facilitate access to therapies and curb the associated skyrocketing costs.
Several biosimilars have been approved by the agency in recent years, and earlier this year they were joined by 2 more: the approval in May of epoetin alfa-epbx (Retacrit; Hospira, a Pfizer company) for all indications of the reference product (epoetin alfa; Epogen/Procrit, Amgen), including the treatment of anemia caused by myelosuppressive chemotherapy, when there is a minimum of 2 additional months of planned chemotherapy;1 and the June approval of pegfilgrastim-jmdb (Fulphila, Mylan and Biocon) for the treatment of patients undergoing myelosuppressive chemotherapy to help reduce the chance of infection as suggested by febrile neutropenia (fever, often with other signs of infection, associated with an abnormally low number of infection-fighting white blood cells).2 The reference product for pegfilgrastim-jmdb is pegfilgrastim (Neulasta, Amgen).
The approval of both biosimilars was based on a review of a body of evidence that included structural and functional characterization, animal study data, human pharmacokinetic (PK) and pharmacodynamic (PD) data, clinical immunogenicity data, and other clinical safety and efficacy data. This evidence established that the biosimilars were highly similar to the already FDA-approved reference products, with no clinically relevant differences.
Biocon and Mylan-GmBH, which jointly developed pegfilgrastim-jmdb, originally filed for approval in 2017; and Hospira Inc, a Pfizer company that developed epoetin alfa-epbx, filed for the first time in 2015. They subsequently received complete response letters from the FDA, twice in the case of the epoetin alfa biosimilar, rejecting their approval. For pegfilgrastim-jmdb, the complete response letter was related to a pending update of the Biologic License Application as the result of requalification activities taken because of modifications at their manufacturing plant. For epoetin alfa-epbx, the FDA expressed concerns relating to a manufacturing facility. The companies addressed the concerns in the complete response letters and submitted corrective and preventive action plans.3,4
Pegfilgrastim-jmdb
The results from a phase 3, multicenter, randomized, double-blind parallel-group trial of pegfilgrastim-jmdb compared with European Union-approved pegfilgrastim were published in 2016. Chemotherapy and radiation-naïve patients with newly diagnosed breast cancer (n = 194) received the biosimilar or reference product every 3 weeks for 6 cycles. The primary endpoint was duration of severe neutropenia in cycle 1, defined as days with absolute neutrophil count <0.5 x 109/L. The mean standard deviation was 1.2 [0.93] in the pegfilgrastim-jmdb arm and 1.2 [1.10] in the EU-pegfilgrastim arm, and the 95% confidence interval of least squares means differences was within the -1 day, +1 day range, indicating equivalency.5
A characterization and similarity assessment of pegfilgrastim-jmdb compared with US- and EU-approved pegfilgrastim was presented at the 2018 Annual Meeting of the American Society of Clinical Oncology. G-CSF receptor (G-CSFR) binding was assessed by surface plasmon resonance and potency was measured by in vitro stimulated proliferation in a mouse myelogenous leukemia cell line. In vivo rodent studies were also performed and included a PD study with a single dose of up to 3 mg/kg.6
There was high similarity in the structure, molecular mass, impurities and functional activity of the biosimilar and reference products, as well as similar G-CSFR binding and equivalent relative potency. Neutrophil and leukocyte counts were increased to a similar degree, and toxicology and drug kinetics were also comparable.
The recommended dose of pegfilgrastim-jmdb is a 6 mg/0.6 ml injection in a single-dose prefilled syringe for manual use only, administered subcutaneously once per chemotherapy cycle. The prescribing information also has dosing guidelines for administration in pediatric patients who weigh less than 45 kg. Pegfilgrastim-jmdb should not be administered between 14 days before and 24 hours after administration of chemotherapy.
The prescribing information details warnings and precautions relating to splenic rupture, acute respiratory distress syndrome (ARDS), serious allergic reactions, potential for severe/fatal sickle cell crises in patients with sickle cell disorders, glomerulonephritis, leukocytosis, capillary leak syndrome, and the potential for tumor growth or recurrence.7
Patients should be evaluated for an enlarged spleen or splenic rupture if they report upper left abdominal or shoulder pain. Patients who develop fever and lung infiltrates or respiratory distress should be evaluated for ARDS and treatment discontinued if a diagnosis is confirmed. Pegfilgrastim-jmdb should be permanently discontinued in patients who develop serious allergic reactions and should not be used in patients with a history of serious allergic reactions to pegfilgrastim or filgrastim products.
Dose-reduction or interruption should be considered in patients who develop glomerulonephritis. Complete blood counts should be monitored throughout treatment. Patients should be monitored closely for capillary leak syndrome and treated with standard therapy. Pegfilgrastim-jmdb is marketed as Fulphila.
Epoetin alfa-epbx
Epoetin alfa-epbx was evaluated in 2 clinical trials in healthy individuals. The EPOE-12-02 trial established the PK and PD following a single subcutaneous dose of 100 U/kg in 81 participants. The EPOE-14-1 study was designed to determine the PK and PD of multiple doses of subcutaneous 100 U/kg 3 times weekly for 3 weeks in 129 participants. Both studies met prespecified criteria
Evidence of efficacy and safety were also evaluated using pooled data from EPOE-10-13 and EPOE-10-01, conducted in patients with chronic kidney disease, which was considered the most sensitive population in which to evaluate clinically meaningful differences between the biosimilar and reference product.8,9
There were no clinically meaningful differences in efficacy and a similar adverse event profile. The most common side effects include high blood pressure, joint pain, muscle spasm, fever, dizziness, respiratory infection, and cough, among others.
The recommended dose of epoetin alfa-epbx, which is marketed as Retacrit, is 40,000 Units weekly or 150 U/kg 3 times weekly in adults and 600 U/kg intravenously weekly in pediatric patients aged 5 years or younger. Epoetin alfa-epbx comes with a boxed warning to alert health care providers to the increased risks of death, heart problems, stroke, and tumor growth, or recurrence. The prescribing information also details warnings and precautions relating to these risks, as well as hypertension, seizures, lack or loss of hemoglobin response, pure red cell aplasia, serious allergic reactions, and severe cutaneous reactions.9
Blood pressure should be appropriately controlled before treatment initiation, treatment should be reduced or withheld if it becomes uncontrollable, and patients should be advised of the importance of compliance with anti-hypertensive medication and dietary restrictions. Patients should be monitored closely for premonitory neurologic symptoms and advised to contact their provider in the event of new-onset seizures, premonitory symptoms, or change in seizure frequency.
The prescribing information has dosing recommendations for lack or loss of hemoglobin response to epoetin alfa-epbx. If severe anemia or low reticulocyte count occur, treatment should be withheld and patients evaluated for neutralizing antibodies to erythropoietin and, in the event that PRCA is confirmed, treatment should be permanently discontinued. Treatment should be immediately and permanently discontinued for serious allergic reactions or severe cutaneous reactions.
Biosimilars are copies of FDA-approved biologic drugs (those generally derived from a living organism) that cannot be identical to the reference drug but demonstrate a high similarity to it. As patents on the reference drugs expire, biosimilars are being developed to increase competition in the marketplace to reduce costs and improve patient access to therapy. Although the US Food and Drug Administration (FDA) has no regulatory power over drug prices, it recently announced efforts to streamline the biosimilar approval process to facilitate access to therapies and curb the associated skyrocketing costs.
Several biosimilars have been approved by the agency in recent years, and earlier this year they were joined by 2 more: the approval in May of epoetin alfa-epbx (Retacrit; Hospira, a Pfizer company) for all indications of the reference product (epoetin alfa; Epogen/Procrit, Amgen), including the treatment of anemia caused by myelosuppressive chemotherapy, when there is a minimum of 2 additional months of planned chemotherapy;1 and the June approval of pegfilgrastim-jmdb (Fulphila, Mylan and Biocon) for the treatment of patients undergoing myelosuppressive chemotherapy to help reduce the chance of infection as suggested by febrile neutropenia (fever, often with other signs of infection, associated with an abnormally low number of infection-fighting white blood cells).2 The reference product for pegfilgrastim-jmdb is pegfilgrastim (Neulasta, Amgen).
The approval of both biosimilars was based on a review of a body of evidence that included structural and functional characterization, animal study data, human pharmacokinetic (PK) and pharmacodynamic (PD) data, clinical immunogenicity data, and other clinical safety and efficacy data. This evidence established that the biosimilars were highly similar to the already FDA-approved reference products, with no clinically relevant differences.
Biocon and Mylan-GmBH, which jointly developed pegfilgrastim-jmdb, originally filed for approval in 2017; and Hospira Inc, a Pfizer company that developed epoetin alfa-epbx, filed for the first time in 2015. They subsequently received complete response letters from the FDA, twice in the case of the epoetin alfa biosimilar, rejecting their approval. For pegfilgrastim-jmdb, the complete response letter was related to a pending update of the Biologic License Application as the result of requalification activities taken because of modifications at their manufacturing plant. For epoetin alfa-epbx, the FDA expressed concerns relating to a manufacturing facility. The companies addressed the concerns in the complete response letters and submitted corrective and preventive action plans.3,4
Pegfilgrastim-jmdb
The results from a phase 3, multicenter, randomized, double-blind parallel-group trial of pegfilgrastim-jmdb compared with European Union-approved pegfilgrastim were published in 2016. Chemotherapy and radiation-naïve patients with newly diagnosed breast cancer (n = 194) received the biosimilar or reference product every 3 weeks for 6 cycles. The primary endpoint was duration of severe neutropenia in cycle 1, defined as days with absolute neutrophil count <0.5 x 109/L. The mean standard deviation was 1.2 [0.93] in the pegfilgrastim-jmdb arm and 1.2 [1.10] in the EU-pegfilgrastim arm, and the 95% confidence interval of least squares means differences was within the -1 day, +1 day range, indicating equivalency.5
A characterization and similarity assessment of pegfilgrastim-jmdb compared with US- and EU-approved pegfilgrastim was presented at the 2018 Annual Meeting of the American Society of Clinical Oncology. G-CSF receptor (G-CSFR) binding was assessed by surface plasmon resonance and potency was measured by in vitro stimulated proliferation in a mouse myelogenous leukemia cell line. In vivo rodent studies were also performed and included a PD study with a single dose of up to 3 mg/kg.6
There was high similarity in the structure, molecular mass, impurities and functional activity of the biosimilar and reference products, as well as similar G-CSFR binding and equivalent relative potency. Neutrophil and leukocyte counts were increased to a similar degree, and toxicology and drug kinetics were also comparable.
The recommended dose of pegfilgrastim-jmdb is a 6 mg/0.6 ml injection in a single-dose prefilled syringe for manual use only, administered subcutaneously once per chemotherapy cycle. The prescribing information also has dosing guidelines for administration in pediatric patients who weigh less than 45 kg. Pegfilgrastim-jmdb should not be administered between 14 days before and 24 hours after administration of chemotherapy.
The prescribing information details warnings and precautions relating to splenic rupture, acute respiratory distress syndrome (ARDS), serious allergic reactions, potential for severe/fatal sickle cell crises in patients with sickle cell disorders, glomerulonephritis, leukocytosis, capillary leak syndrome, and the potential for tumor growth or recurrence.7
Patients should be evaluated for an enlarged spleen or splenic rupture if they report upper left abdominal or shoulder pain. Patients who develop fever and lung infiltrates or respiratory distress should be evaluated for ARDS and treatment discontinued if a diagnosis is confirmed. Pegfilgrastim-jmdb should be permanently discontinued in patients who develop serious allergic reactions and should not be used in patients with a history of serious allergic reactions to pegfilgrastim or filgrastim products.
Dose-reduction or interruption should be considered in patients who develop glomerulonephritis. Complete blood counts should be monitored throughout treatment. Patients should be monitored closely for capillary leak syndrome and treated with standard therapy. Pegfilgrastim-jmdb is marketed as Fulphila.
Epoetin alfa-epbx
Epoetin alfa-epbx was evaluated in 2 clinical trials in healthy individuals. The EPOE-12-02 trial established the PK and PD following a single subcutaneous dose of 100 U/kg in 81 participants. The EPOE-14-1 study was designed to determine the PK and PD of multiple doses of subcutaneous 100 U/kg 3 times weekly for 3 weeks in 129 participants. Both studies met prespecified criteria
Evidence of efficacy and safety were also evaluated using pooled data from EPOE-10-13 and EPOE-10-01, conducted in patients with chronic kidney disease, which was considered the most sensitive population in which to evaluate clinically meaningful differences between the biosimilar and reference product.8,9
There were no clinically meaningful differences in efficacy and a similar adverse event profile. The most common side effects include high blood pressure, joint pain, muscle spasm, fever, dizziness, respiratory infection, and cough, among others.
The recommended dose of epoetin alfa-epbx, which is marketed as Retacrit, is 40,000 Units weekly or 150 U/kg 3 times weekly in adults and 600 U/kg intravenously weekly in pediatric patients aged 5 years or younger. Epoetin alfa-epbx comes with a boxed warning to alert health care providers to the increased risks of death, heart problems, stroke, and tumor growth, or recurrence. The prescribing information also details warnings and precautions relating to these risks, as well as hypertension, seizures, lack or loss of hemoglobin response, pure red cell aplasia, serious allergic reactions, and severe cutaneous reactions.9
Blood pressure should be appropriately controlled before treatment initiation, treatment should be reduced or withheld if it becomes uncontrollable, and patients should be advised of the importance of compliance with anti-hypertensive medication and dietary restrictions. Patients should be monitored closely for premonitory neurologic symptoms and advised to contact their provider in the event of new-onset seizures, premonitory symptoms, or change in seizure frequency.
The prescribing information has dosing recommendations for lack or loss of hemoglobin response to epoetin alfa-epbx. If severe anemia or low reticulocyte count occur, treatment should be withheld and patients evaluated for neutralizing antibodies to erythropoietin and, in the event that PRCA is confirmed, treatment should be permanently discontinued. Treatment should be immediately and permanently discontinued for serious allergic reactions or severe cutaneous reactions.
1. US Food and Drug Administration website. FDA approves first epoetin alfa biosimilar for the treatment of anemia. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607703.htm. Updated May 15, 2018. Accessed June 22, 2018.
2. US Food and Drug Administration website. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm609805.htm. Updated June 4, 2018. Accessed June 22, 2018.
3. Reuters. BRIEF – Biocon says US FDA issues complete response letter for proposed biosimilar pegfilgrastim. https://www.reuters.com/article/brief-biocon-says-us-fda-issued-complete/brief-biocon-says-us-fda-issued-complete-response-letter-for-proposed-biosimilar-pegfilgrastim-idUSFWN1MK0Q1. Updated October 9, 2017. Accessed June 22, 2018.
4. FiercePharma. Pfizer, on third try, wins nod for biosimilar of blockbuster epogen/procrit. https://www.fiercepharma.com/pharma/pfizer-third-try-wins-fda-nod-for-biosimilar-blockbuster-epogen-procrit. Updated May 15, 2018. Accessed June 22, 2018.
5. Waller CF, Blakeley C, Pennella E. Phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H vs EU-neulasta in the prophylaxis of chemotherapy-induced neutropenia. Ann Oncol. 2016;27(suppl_6):1433O.
6. Sankaran PV, Palanivelu DV, Nair R, et al. Characterization and similarity assessment of a pegfilgrastim biosimilar MYL-1401H. J Clin Oncol. 2018;36(suppl; abstr e19028).
7. Fulphila (pegfilgrastim-jmdb) injection, for subcutaneous use. Prescribing information. Mylan GmBH. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761075s000lbl.pdf. Released June 2018. Accessed June 22, 2018.
8. US Food and Drug Administration website. ‘Epoetin Hospira,’ a proposed biosimilar to US-licensed Epogen/Procrit. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559962.pdf. Updated May 25, 2017. Accessed June 22, 2018.
9. Retacrit (epoetin alfa-epbx) injection, for intravenous or subcutaneous use. Prescribing information. Pfizer. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125545s000lbl.pdf. Released May 2018. Accessed June 22, 2018.
1. US Food and Drug Administration website. FDA approves first epoetin alfa biosimilar for the treatment of anemia. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607703.htm. Updated May 15, 2018. Accessed June 22, 2018.
2. US Food and Drug Administration website. FDA approves first biosimilar to Neulasta to help reduce the risk of infection during cancer treatment. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm609805.htm. Updated June 4, 2018. Accessed June 22, 2018.
3. Reuters. BRIEF – Biocon says US FDA issues complete response letter for proposed biosimilar pegfilgrastim. https://www.reuters.com/article/brief-biocon-says-us-fda-issued-complete/brief-biocon-says-us-fda-issued-complete-response-letter-for-proposed-biosimilar-pegfilgrastim-idUSFWN1MK0Q1. Updated October 9, 2017. Accessed June 22, 2018.
4. FiercePharma. Pfizer, on third try, wins nod for biosimilar of blockbuster epogen/procrit. https://www.fiercepharma.com/pharma/pfizer-third-try-wins-fda-nod-for-biosimilar-blockbuster-epogen-procrit. Updated May 15, 2018. Accessed June 22, 2018.
5. Waller CF, Blakeley C, Pennella E. Phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H vs EU-neulasta in the prophylaxis of chemotherapy-induced neutropenia. Ann Oncol. 2016;27(suppl_6):1433O.
6. Sankaran PV, Palanivelu DV, Nair R, et al. Characterization and similarity assessment of a pegfilgrastim biosimilar MYL-1401H. J Clin Oncol. 2018;36(suppl; abstr e19028).
7. Fulphila (pegfilgrastim-jmdb) injection, for subcutaneous use. Prescribing information. Mylan GmBH. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761075s000lbl.pdf. Released June 2018. Accessed June 22, 2018.
8. US Food and Drug Administration website. ‘Epoetin Hospira,’ a proposed biosimilar to US-licensed Epogen/Procrit. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559962.pdf. Updated May 25, 2017. Accessed June 22, 2018.
9. Retacrit (epoetin alfa-epbx) injection, for intravenous or subcutaneous use. Prescribing information. Pfizer. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125545s000lbl.pdf. Released May 2018. Accessed June 22, 2018.
Finding your practice home base
As summer winds down and we begin to gear up to return to school or work, I was thinking about new and returning hem-onc residents, fellows, and young attendings and a question I routinely get from them: what should I do next in my career? I always answer by holding up 3 fingers and telling them that they can practice 1, at a university hospital; 2, at a university teaching affiliate; or 3, at a community hospital or practice with a little or no university affiliation. These days, trainees in hematology-oncology are often advised to be highly specialty-specific when they plan their long-term careers and to focus on a particular cancer or hematologic disorder. That is fine if you want to remain in an academic or university-based practice, but not if community practice is your preference. So, what are the differences among these 3 options?
Option 1, to remain in a university setting where you can be highly focused and specialized in a single narrowly defined area, could be satisfying, but keep in mind that the institution expects results! You will be carefully monitored for research output and teaching and administration commitments, and your interaction with patients could add up to less than 50% of your time. Publication and grant renewal will also play a role and therefore take up your time.
If you are considering option 2 – to work at a university teaching affiliate hospital – you need to bear in mind that you likely will see a patient population with a much broader range of diagnoses than would be the case with the first option. Patient care for option 2 will take up more than 50% of your time, so it might be a little more challenging to stay current, but perhaps more refreshing if you enjoy contact with patients. Teaching, research, and administration will surely be available, and publication and grant renewal will play as big or small a role as you want.
Option 3 would be to join a community hospital or practice where the primary focus is on patient care and the diagnoses will span the hematology and oncology spectrum. This type of practice can be very demanding of one’s time, but as rewarding as the other options, especially if you value contact with patients. With this option, one is more likely to practice as a generalist, perhaps with an emphasis in one of the hem-onc specialties, but able to treat a cluster of different types of cancer as well.
I always advise trainees to be sure they ask physicians practicing in each of these options to give examples of what their best and worst days are like so that they can get some idea of what the daily humdrum and challenges would encompass. What did I choose? I have always gone with option 2 and have been very happy in that setting.
In this issue…
More biosimilars head our way. Turning to the current issue of the journal, on page e181, Dr Jane de Lartigue discusses 2 new biosimilars recently approved by the United States Food and Drug Administration (FDA) – epoetin alfa-epbx (Retacrit; Hospira, a Pfizer company) for chemotherapy-induced anemia (CIA), and pegfilgrastim-jmdb (Fulphila; Mylan and Biocon) for prevention of febrile neutropenia. As Dr de Lartigue notes, biosimilars are copies of FDA-approved biologic drugs that cannot be identical to the reference drug but demonstrate a high similarity to it. In this case, the reference drug for epoetin alfa-epbx is epoetin alfa (Epogen/Procrit, Amgen) and for pegfilgrastim-jmdb, it is pegfilgrastim (Neulasta, Amgen). As the reference drugs’ patents expire, biosimilars are being developed to increase competition in the marketplace in an effort to reduce costs and improve patient access to these therapies. Indeed, the FDA is working to streamline the biosimilar approval process to facilitate that access.
Reading this article got me thinking about something I often have to consider in the course of my work: transfusion versus erythropoiesis-stimulating agents (ESAs)? Recombinant erythropoietin drugs such as the biosimilar, epoetin alfa-epbx, and its reference drug are grouped together as ESAs, and have been used to treat CIA since the late 1980s. However, there were a few trials that used higher-dose ESA or set high hemoglobin targets, and their findings suggested that ESAs may shorten survival in patients with cancer or increase tumor growth, or both. The use of ESAs took a nosedive after the 2007 decision by the FDA’s Oncologic Drugs Advisory Committee to rein in their use for a hard start of ESA treatment at less than 10 g/dL hemoglobin, and not higher. Subsequent trials addressed the concerns about survival and tumor growth. A meta-analysis of 60 randomized, placebo-controlled trials of ESAs in CIA found that there was no difference in overall survival between the study and control groups.1 Likewise, findings from an FDA-mandated trial with epoetin alfa (Procrit) in patients with metastatic breast cancer have reported that there was no significant difference in overall survival between the study and control groups.2 The results of a second FDA-mandated trial with darbepoetin alfa (Aranesp, Amgen) in patients with metastatic lung cancer are expected to be released soon. The FDA lifted the ESA Risk Evaluation and Mitigation Strategy based on those findings. However, many practitioners, both young and old, continue to shy away from using ESAs because of the FDA black box warning that remains in place despite the latest data.3The use of transfusion ticked up reciprocally with the decline in ESA use, but perhaps we should re-evaluate the use of these agents in our practice, especially now that the less costly, equally safe and effective biosimilars are becoming available and we have the new survival data. Transfusions are time consuming and have side effects, including allergic reaction and infection risk, whereas ESAs are easily administered by injection, which patients might find preferable.
Malignancies in patients with HIV-AIDS. On page e188, Koppaka and colleagues report on a study in India of the patterns of malignancies in patients with HIV-AIDS. I began my career just as the first reports of what became known as HIV-AIDS emerged, and we were all mystified by what was killing these patients and the curious hematologic and oncologic problems they developed. Back then, the patients were profoundly immunosuppressed, and the immunosuppression cancers of non-Hodgkin lymphoma, usually higher grade, and Kaposi sarcoma were most prevalent and today are collectively labeled AIDS-defining malignancies (ADMs).
Fast forward to present day, and we have extremely effective antiretroviral therapies that have resulted in a significant reduction in mortality among HIV-infected individuals who are now living long enough to get what we call non–AIDS-defining malignancies (NADMs) such as anal or cervical cancers, hepatoma (hepatocellular carcinoma), Hodgkin lymphoma, and lung cancer. Of note is that these NADMs are all highly viral associated, with anal and cervical cancers linked to infection with the human papillomavirus; hepatoma linked to the hepatitis B/C viruses; Hodgkin lymphoma to the Epstein-Barr virus; and lung cancer, possibly also HPV. Fortunately, these days we can use standard-dose chemoradiation therapy for all HIV-related cancers because the patients’ immune systems are much better reconstituted and the modern-day antiretroviral therapies have much less drug–drug interaction thanks to the advent of the integrase inhibitors. The researchers give an excellent breakdown of the occurrence of these malignancies, as well as an analysis of the correlation between CD4 counts and the different malignancies.
Immunotherapy-related side effects in the ED. What happens when our patients who are on immunotherapy end up in the emergency department (ED) with therapy-related symptoms? And what can the treating oncologist do to help the ED physician achieve the best possible outcome for the patient? I spoke to Dr Maura Sammon, an ED physician, about some of the more common of these side effects – lung, gastrointestinal, rash, and endocrine-related problems – and she describes in detail how physicians in the ED would triage and treat the patient. Dr Sammon also emphasizes the importance of communication: first, between the treating oncologist and patient, about the differences between chemotherapy and immunotherapy; and second, between the ED physician and the treating oncologist as soon as possible after the patient has presented to ensure a good outcome. The interview is part of The JCSO Interview series. It is jam-packed with useful, how-to information, and you can read a transcript of it on page e216 of this issue, or you can listen to it online.4
We round off the issue with a selection of Case Reports (pp. e200-e209), an original report on the characteristics of urgent palliative cancer care consultations encountered by radiation oncologists (p. e193), and a New Therapies feature, also by Dr de Lartigue, focusing on the rarity and complexities of sarcomas (p. e210).
Those are my dog-day-of-summer thoughts as we head toward another Labor Day and a new academic year. Since we are all online now, we encourage you to listen to my bimonthly podcast of each issue on our website at www.jcso-online.com, and of course, follow us on Twitter (@jcs_onc) and Instagram (@jcsoncology) and like us on Facebook.
1. Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102(2):301-315.
2. Leyland-Jones B, Bondarenko I, Nemsadze G, et al. A randomized, open-label, multicenter, phase III study of epoetin alfa versus best standard of care in anemic patients with metastatic breast cancer receiving standard chemotherapy. J Clin Oncol. 2016;34:1197-1207.
3. US Food and Drug Administration release. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Last updated April 13, 2017. Accessed August 20, 2018.
4. Henry D, Sammon M. Treating immunotherapy-related AEs in the emergency department [Audio]. https://www.mdedge.com/jcso/article/171966/patient-survivor-care/treating-immunotherapy-related-aes-emergency-department. Published August 6, 2018.
As summer winds down and we begin to gear up to return to school or work, I was thinking about new and returning hem-onc residents, fellows, and young attendings and a question I routinely get from them: what should I do next in my career? I always answer by holding up 3 fingers and telling them that they can practice 1, at a university hospital; 2, at a university teaching affiliate; or 3, at a community hospital or practice with a little or no university affiliation. These days, trainees in hematology-oncology are often advised to be highly specialty-specific when they plan their long-term careers and to focus on a particular cancer or hematologic disorder. That is fine if you want to remain in an academic or university-based practice, but not if community practice is your preference. So, what are the differences among these 3 options?
Option 1, to remain in a university setting where you can be highly focused and specialized in a single narrowly defined area, could be satisfying, but keep in mind that the institution expects results! You will be carefully monitored for research output and teaching and administration commitments, and your interaction with patients could add up to less than 50% of your time. Publication and grant renewal will also play a role and therefore take up your time.
If you are considering option 2 – to work at a university teaching affiliate hospital – you need to bear in mind that you likely will see a patient population with a much broader range of diagnoses than would be the case with the first option. Patient care for option 2 will take up more than 50% of your time, so it might be a little more challenging to stay current, but perhaps more refreshing if you enjoy contact with patients. Teaching, research, and administration will surely be available, and publication and grant renewal will play as big or small a role as you want.
Option 3 would be to join a community hospital or practice where the primary focus is on patient care and the diagnoses will span the hematology and oncology spectrum. This type of practice can be very demanding of one’s time, but as rewarding as the other options, especially if you value contact with patients. With this option, one is more likely to practice as a generalist, perhaps with an emphasis in one of the hem-onc specialties, but able to treat a cluster of different types of cancer as well.
I always advise trainees to be sure they ask physicians practicing in each of these options to give examples of what their best and worst days are like so that they can get some idea of what the daily humdrum and challenges would encompass. What did I choose? I have always gone with option 2 and have been very happy in that setting.
In this issue…
More biosimilars head our way. Turning to the current issue of the journal, on page e181, Dr Jane de Lartigue discusses 2 new biosimilars recently approved by the United States Food and Drug Administration (FDA) – epoetin alfa-epbx (Retacrit; Hospira, a Pfizer company) for chemotherapy-induced anemia (CIA), and pegfilgrastim-jmdb (Fulphila; Mylan and Biocon) for prevention of febrile neutropenia. As Dr de Lartigue notes, biosimilars are copies of FDA-approved biologic drugs that cannot be identical to the reference drug but demonstrate a high similarity to it. In this case, the reference drug for epoetin alfa-epbx is epoetin alfa (Epogen/Procrit, Amgen) and for pegfilgrastim-jmdb, it is pegfilgrastim (Neulasta, Amgen). As the reference drugs’ patents expire, biosimilars are being developed to increase competition in the marketplace in an effort to reduce costs and improve patient access to these therapies. Indeed, the FDA is working to streamline the biosimilar approval process to facilitate that access.
Reading this article got me thinking about something I often have to consider in the course of my work: transfusion versus erythropoiesis-stimulating agents (ESAs)? Recombinant erythropoietin drugs such as the biosimilar, epoetin alfa-epbx, and its reference drug are grouped together as ESAs, and have been used to treat CIA since the late 1980s. However, there were a few trials that used higher-dose ESA or set high hemoglobin targets, and their findings suggested that ESAs may shorten survival in patients with cancer or increase tumor growth, or both. The use of ESAs took a nosedive after the 2007 decision by the FDA’s Oncologic Drugs Advisory Committee to rein in their use for a hard start of ESA treatment at less than 10 g/dL hemoglobin, and not higher. Subsequent trials addressed the concerns about survival and tumor growth. A meta-analysis of 60 randomized, placebo-controlled trials of ESAs in CIA found that there was no difference in overall survival between the study and control groups.1 Likewise, findings from an FDA-mandated trial with epoetin alfa (Procrit) in patients with metastatic breast cancer have reported that there was no significant difference in overall survival between the study and control groups.2 The results of a second FDA-mandated trial with darbepoetin alfa (Aranesp, Amgen) in patients with metastatic lung cancer are expected to be released soon. The FDA lifted the ESA Risk Evaluation and Mitigation Strategy based on those findings. However, many practitioners, both young and old, continue to shy away from using ESAs because of the FDA black box warning that remains in place despite the latest data.3The use of transfusion ticked up reciprocally with the decline in ESA use, but perhaps we should re-evaluate the use of these agents in our practice, especially now that the less costly, equally safe and effective biosimilars are becoming available and we have the new survival data. Transfusions are time consuming and have side effects, including allergic reaction and infection risk, whereas ESAs are easily administered by injection, which patients might find preferable.
Malignancies in patients with HIV-AIDS. On page e188, Koppaka and colleagues report on a study in India of the patterns of malignancies in patients with HIV-AIDS. I began my career just as the first reports of what became known as HIV-AIDS emerged, and we were all mystified by what was killing these patients and the curious hematologic and oncologic problems they developed. Back then, the patients were profoundly immunosuppressed, and the immunosuppression cancers of non-Hodgkin lymphoma, usually higher grade, and Kaposi sarcoma were most prevalent and today are collectively labeled AIDS-defining malignancies (ADMs).
Fast forward to present day, and we have extremely effective antiretroviral therapies that have resulted in a significant reduction in mortality among HIV-infected individuals who are now living long enough to get what we call non–AIDS-defining malignancies (NADMs) such as anal or cervical cancers, hepatoma (hepatocellular carcinoma), Hodgkin lymphoma, and lung cancer. Of note is that these NADMs are all highly viral associated, with anal and cervical cancers linked to infection with the human papillomavirus; hepatoma linked to the hepatitis B/C viruses; Hodgkin lymphoma to the Epstein-Barr virus; and lung cancer, possibly also HPV. Fortunately, these days we can use standard-dose chemoradiation therapy for all HIV-related cancers because the patients’ immune systems are much better reconstituted and the modern-day antiretroviral therapies have much less drug–drug interaction thanks to the advent of the integrase inhibitors. The researchers give an excellent breakdown of the occurrence of these malignancies, as well as an analysis of the correlation between CD4 counts and the different malignancies.
Immunotherapy-related side effects in the ED. What happens when our patients who are on immunotherapy end up in the emergency department (ED) with therapy-related symptoms? And what can the treating oncologist do to help the ED physician achieve the best possible outcome for the patient? I spoke to Dr Maura Sammon, an ED physician, about some of the more common of these side effects – lung, gastrointestinal, rash, and endocrine-related problems – and she describes in detail how physicians in the ED would triage and treat the patient. Dr Sammon also emphasizes the importance of communication: first, between the treating oncologist and patient, about the differences between chemotherapy and immunotherapy; and second, between the ED physician and the treating oncologist as soon as possible after the patient has presented to ensure a good outcome. The interview is part of The JCSO Interview series. It is jam-packed with useful, how-to information, and you can read a transcript of it on page e216 of this issue, or you can listen to it online.4
We round off the issue with a selection of Case Reports (pp. e200-e209), an original report on the characteristics of urgent palliative cancer care consultations encountered by radiation oncologists (p. e193), and a New Therapies feature, also by Dr de Lartigue, focusing on the rarity and complexities of sarcomas (p. e210).
Those are my dog-day-of-summer thoughts as we head toward another Labor Day and a new academic year. Since we are all online now, we encourage you to listen to my bimonthly podcast of each issue on our website at www.jcso-online.com, and of course, follow us on Twitter (@jcs_onc) and Instagram (@jcsoncology) and like us on Facebook.
As summer winds down and we begin to gear up to return to school or work, I was thinking about new and returning hem-onc residents, fellows, and young attendings and a question I routinely get from them: what should I do next in my career? I always answer by holding up 3 fingers and telling them that they can practice 1, at a university hospital; 2, at a university teaching affiliate; or 3, at a community hospital or practice with a little or no university affiliation. These days, trainees in hematology-oncology are often advised to be highly specialty-specific when they plan their long-term careers and to focus on a particular cancer or hematologic disorder. That is fine if you want to remain in an academic or university-based practice, but not if community practice is your preference. So, what are the differences among these 3 options?
Option 1, to remain in a university setting where you can be highly focused and specialized in a single narrowly defined area, could be satisfying, but keep in mind that the institution expects results! You will be carefully monitored for research output and teaching and administration commitments, and your interaction with patients could add up to less than 50% of your time. Publication and grant renewal will also play a role and therefore take up your time.
If you are considering option 2 – to work at a university teaching affiliate hospital – you need to bear in mind that you likely will see a patient population with a much broader range of diagnoses than would be the case with the first option. Patient care for option 2 will take up more than 50% of your time, so it might be a little more challenging to stay current, but perhaps more refreshing if you enjoy contact with patients. Teaching, research, and administration will surely be available, and publication and grant renewal will play as big or small a role as you want.
Option 3 would be to join a community hospital or practice where the primary focus is on patient care and the diagnoses will span the hematology and oncology spectrum. This type of practice can be very demanding of one’s time, but as rewarding as the other options, especially if you value contact with patients. With this option, one is more likely to practice as a generalist, perhaps with an emphasis in one of the hem-onc specialties, but able to treat a cluster of different types of cancer as well.
I always advise trainees to be sure they ask physicians practicing in each of these options to give examples of what their best and worst days are like so that they can get some idea of what the daily humdrum and challenges would encompass. What did I choose? I have always gone with option 2 and have been very happy in that setting.
In this issue…
More biosimilars head our way. Turning to the current issue of the journal, on page e181, Dr Jane de Lartigue discusses 2 new biosimilars recently approved by the United States Food and Drug Administration (FDA) – epoetin alfa-epbx (Retacrit; Hospira, a Pfizer company) for chemotherapy-induced anemia (CIA), and pegfilgrastim-jmdb (Fulphila; Mylan and Biocon) for prevention of febrile neutropenia. As Dr de Lartigue notes, biosimilars are copies of FDA-approved biologic drugs that cannot be identical to the reference drug but demonstrate a high similarity to it. In this case, the reference drug for epoetin alfa-epbx is epoetin alfa (Epogen/Procrit, Amgen) and for pegfilgrastim-jmdb, it is pegfilgrastim (Neulasta, Amgen). As the reference drugs’ patents expire, biosimilars are being developed to increase competition in the marketplace in an effort to reduce costs and improve patient access to these therapies. Indeed, the FDA is working to streamline the biosimilar approval process to facilitate that access.
Reading this article got me thinking about something I often have to consider in the course of my work: transfusion versus erythropoiesis-stimulating agents (ESAs)? Recombinant erythropoietin drugs such as the biosimilar, epoetin alfa-epbx, and its reference drug are grouped together as ESAs, and have been used to treat CIA since the late 1980s. However, there were a few trials that used higher-dose ESA or set high hemoglobin targets, and their findings suggested that ESAs may shorten survival in patients with cancer or increase tumor growth, or both. The use of ESAs took a nosedive after the 2007 decision by the FDA’s Oncologic Drugs Advisory Committee to rein in their use for a hard start of ESA treatment at less than 10 g/dL hemoglobin, and not higher. Subsequent trials addressed the concerns about survival and tumor growth. A meta-analysis of 60 randomized, placebo-controlled trials of ESAs in CIA found that there was no difference in overall survival between the study and control groups.1 Likewise, findings from an FDA-mandated trial with epoetin alfa (Procrit) in patients with metastatic breast cancer have reported that there was no significant difference in overall survival between the study and control groups.2 The results of a second FDA-mandated trial with darbepoetin alfa (Aranesp, Amgen) in patients with metastatic lung cancer are expected to be released soon. The FDA lifted the ESA Risk Evaluation and Mitigation Strategy based on those findings. However, many practitioners, both young and old, continue to shy away from using ESAs because of the FDA black box warning that remains in place despite the latest data.3The use of transfusion ticked up reciprocally with the decline in ESA use, but perhaps we should re-evaluate the use of these agents in our practice, especially now that the less costly, equally safe and effective biosimilars are becoming available and we have the new survival data. Transfusions are time consuming and have side effects, including allergic reaction and infection risk, whereas ESAs are easily administered by injection, which patients might find preferable.
Malignancies in patients with HIV-AIDS. On page e188, Koppaka and colleagues report on a study in India of the patterns of malignancies in patients with HIV-AIDS. I began my career just as the first reports of what became known as HIV-AIDS emerged, and we were all mystified by what was killing these patients and the curious hematologic and oncologic problems they developed. Back then, the patients were profoundly immunosuppressed, and the immunosuppression cancers of non-Hodgkin lymphoma, usually higher grade, and Kaposi sarcoma were most prevalent and today are collectively labeled AIDS-defining malignancies (ADMs).
Fast forward to present day, and we have extremely effective antiretroviral therapies that have resulted in a significant reduction in mortality among HIV-infected individuals who are now living long enough to get what we call non–AIDS-defining malignancies (NADMs) such as anal or cervical cancers, hepatoma (hepatocellular carcinoma), Hodgkin lymphoma, and lung cancer. Of note is that these NADMs are all highly viral associated, with anal and cervical cancers linked to infection with the human papillomavirus; hepatoma linked to the hepatitis B/C viruses; Hodgkin lymphoma to the Epstein-Barr virus; and lung cancer, possibly also HPV. Fortunately, these days we can use standard-dose chemoradiation therapy for all HIV-related cancers because the patients’ immune systems are much better reconstituted and the modern-day antiretroviral therapies have much less drug–drug interaction thanks to the advent of the integrase inhibitors. The researchers give an excellent breakdown of the occurrence of these malignancies, as well as an analysis of the correlation between CD4 counts and the different malignancies.
Immunotherapy-related side effects in the ED. What happens when our patients who are on immunotherapy end up in the emergency department (ED) with therapy-related symptoms? And what can the treating oncologist do to help the ED physician achieve the best possible outcome for the patient? I spoke to Dr Maura Sammon, an ED physician, about some of the more common of these side effects – lung, gastrointestinal, rash, and endocrine-related problems – and she describes in detail how physicians in the ED would triage and treat the patient. Dr Sammon also emphasizes the importance of communication: first, between the treating oncologist and patient, about the differences between chemotherapy and immunotherapy; and second, between the ED physician and the treating oncologist as soon as possible after the patient has presented to ensure a good outcome. The interview is part of The JCSO Interview series. It is jam-packed with useful, how-to information, and you can read a transcript of it on page e216 of this issue, or you can listen to it online.4
We round off the issue with a selection of Case Reports (pp. e200-e209), an original report on the characteristics of urgent palliative cancer care consultations encountered by radiation oncologists (p. e193), and a New Therapies feature, also by Dr de Lartigue, focusing on the rarity and complexities of sarcomas (p. e210).
Those are my dog-day-of-summer thoughts as we head toward another Labor Day and a new academic year. Since we are all online now, we encourage you to listen to my bimonthly podcast of each issue on our website at www.jcso-online.com, and of course, follow us on Twitter (@jcs_onc) and Instagram (@jcsoncology) and like us on Facebook.
1. Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102(2):301-315.
2. Leyland-Jones B, Bondarenko I, Nemsadze G, et al. A randomized, open-label, multicenter, phase III study of epoetin alfa versus best standard of care in anemic patients with metastatic breast cancer receiving standard chemotherapy. J Clin Oncol. 2016;34:1197-1207.
3. US Food and Drug Administration release. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Last updated April 13, 2017. Accessed August 20, 2018.
4. Henry D, Sammon M. Treating immunotherapy-related AEs in the emergency department [Audio]. https://www.mdedge.com/jcso/article/171966/patient-survivor-care/treating-immunotherapy-related-aes-emergency-department. Published August 6, 2018.
1. Glaspy J, Crawford J, Vansteenkiste J, et al. Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer. 2010;102(2):301-315.
2. Leyland-Jones B, Bondarenko I, Nemsadze G, et al. A randomized, open-label, multicenter, phase III study of epoetin alfa versus best standard of care in anemic patients with metastatic breast cancer receiving standard chemotherapy. J Clin Oncol. 2016;34:1197-1207.
3. US Food and Drug Administration release. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Last updated April 13, 2017. Accessed August 20, 2018.
4. Henry D, Sammon M. Treating immunotherapy-related AEs in the emergency department [Audio]. https://www.mdedge.com/jcso/article/171966/patient-survivor-care/treating-immunotherapy-related-aes-emergency-department. Published August 6, 2018.
Test Article 8/26
Body text
Body text
Body text
Vitals Text
Lorcaserin shows CV safety in CAMELLIA-TIMI 61
MUNICH – Lorcaserin is the first weight-loss drug proven to have cardiovascular safety, Erin A. Bohula, MD, DPhil, told Mitchel L. Zoler in an interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Bohula reported on the results of the CAMELLIA-TIMI 61 trial, which was designed to evaluate the cardiovascular safety of the weight-loss drug lorcaserin in more than 10,000 patients. She presented the data at the annual congress of the European Society of Cardiology.
In CAMELLIA-TIMI 61, the primary safety endpoint, a composite of cardiovascular death, MI, or stroke, was nearly identical between patients on lorcaserin and those given placebo, 2% and 2.1% per year, at P less than .001 for noninferiority. Similarly, the primary efficacy outcome comprising heart failure, hospitalization for unstable angina, and coronary revascularization, was very close between the treated and placebo patients, occurring in 4.1% and 4.2% per year, respectively.
In addition, “there was a sustained weight loss, more than with lifestyle alone or lifestyle plus placebo, which at its peak was about 3 kg. With that there were small, but significant, reductions in heart rate, blood pressure, triglycerides, and hemoglobin A1c, and there was a significant reduction in new-onset diabetes.”
“Overall, there’s not a lot of use of pharmacologic agents for weight loss in the United States, and a lot of that is based on fear of the historical experience, which is that they were not safe. I suspect that having a drug that is proven safe will now lead people to reach for a pharmacologic agent like lorcaserin,” said Dr. Bohula, a cardiologist at of Brigham and Women’s Hospital and an investigator at the TIMI study group.
MUNICH – Lorcaserin is the first weight-loss drug proven to have cardiovascular safety, Erin A. Bohula, MD, DPhil, told Mitchel L. Zoler in an interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Bohula reported on the results of the CAMELLIA-TIMI 61 trial, which was designed to evaluate the cardiovascular safety of the weight-loss drug lorcaserin in more than 10,000 patients. She presented the data at the annual congress of the European Society of Cardiology.
In CAMELLIA-TIMI 61, the primary safety endpoint, a composite of cardiovascular death, MI, or stroke, was nearly identical between patients on lorcaserin and those given placebo, 2% and 2.1% per year, at P less than .001 for noninferiority. Similarly, the primary efficacy outcome comprising heart failure, hospitalization for unstable angina, and coronary revascularization, was very close between the treated and placebo patients, occurring in 4.1% and 4.2% per year, respectively.
In addition, “there was a sustained weight loss, more than with lifestyle alone or lifestyle plus placebo, which at its peak was about 3 kg. With that there were small, but significant, reductions in heart rate, blood pressure, triglycerides, and hemoglobin A1c, and there was a significant reduction in new-onset diabetes.”
“Overall, there’s not a lot of use of pharmacologic agents for weight loss in the United States, and a lot of that is based on fear of the historical experience, which is that they were not safe. I suspect that having a drug that is proven safe will now lead people to reach for a pharmacologic agent like lorcaserin,” said Dr. Bohula, a cardiologist at of Brigham and Women’s Hospital and an investigator at the TIMI study group.
MUNICH – Lorcaserin is the first weight-loss drug proven to have cardiovascular safety, Erin A. Bohula, MD, DPhil, told Mitchel L. Zoler in an interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Bohula reported on the results of the CAMELLIA-TIMI 61 trial, which was designed to evaluate the cardiovascular safety of the weight-loss drug lorcaserin in more than 10,000 patients. She presented the data at the annual congress of the European Society of Cardiology.
In CAMELLIA-TIMI 61, the primary safety endpoint, a composite of cardiovascular death, MI, or stroke, was nearly identical between patients on lorcaserin and those given placebo, 2% and 2.1% per year, at P less than .001 for noninferiority. Similarly, the primary efficacy outcome comprising heart failure, hospitalization for unstable angina, and coronary revascularization, was very close between the treated and placebo patients, occurring in 4.1% and 4.2% per year, respectively.
In addition, “there was a sustained weight loss, more than with lifestyle alone or lifestyle plus placebo, which at its peak was about 3 kg. With that there were small, but significant, reductions in heart rate, blood pressure, triglycerides, and hemoglobin A1c, and there was a significant reduction in new-onset diabetes.”
“Overall, there’s not a lot of use of pharmacologic agents for weight loss in the United States, and a lot of that is based on fear of the historical experience, which is that they were not safe. I suspect that having a drug that is proven safe will now lead people to reach for a pharmacologic agent like lorcaserin,” said Dr. Bohula, a cardiologist at of Brigham and Women’s Hospital and an investigator at the TIMI study group.
Lorcaserin shows CV safety in CAMELLIA-TIMI 61
Erin A. Bohula, MD, DPhil, told Mitchel L. Zoler in an interview.
Dr. Bohula reported on the results of the CAMELLIA-TIMI 61 trial, which was designed to evaluate the cardiovascular safety of the weight-loss drug lorcaserin in more than 10,000 patients. She presented the data at the annual congress of the European Society of Cardiology.
In CAMELLIA-TIMI 61, the primary safety endpoint, a composite of cardiovascular death, MI, or stroke, was nearly identical between patients on lorcaserin and those given placebo, 2% and 2.1% per year, at P less than .001 for noninferiority. Similarly, the primary efficacy outcome comprising heart failure, hospitalization for unstable angina, and coronary revascularization, was very close between the treated and placebo patients, occurring in 4.1% and 4.2% per year, respectively.
In addition, “there was a sustained weight loss, more than with lifestyle alone or lifestyle plus placebo, which at its peak was about 3 kg. With that there were small, but significant, reductions in heart rate, blood pressure, triglycerides, and hemoglobin A1c, and there was a significant reduction in new-onset diabetes.”
“Overall, there’s not a lot of use of pharmacologic agents for weight loss in the United States, and a lot of that is based on fear of the historical experience, which is that they were not safe. I suspect that having a drug that is proven safe will now lead people to reach for a pharmacologic agent like lorcaserin,” said Dr. Bohula, a cardiologist at of Brigham and Women’s Hospital and an investigator at the TIMI study group.
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
Erin A. Bohula, MD, DPhil, told Mitchel L. Zoler in an interview.
Dr. Bohula reported on the results of the CAMELLIA-TIMI 61 trial, which was designed to evaluate the cardiovascular safety of the weight-loss drug lorcaserin in more than 10,000 patients. She presented the data at the annual congress of the European Society of Cardiology.
In CAMELLIA-TIMI 61, the primary safety endpoint, a composite of cardiovascular death, MI, or stroke, was nearly identical between patients on lorcaserin and those given placebo, 2% and 2.1% per year, at P less than .001 for noninferiority. Similarly, the primary efficacy outcome comprising heart failure, hospitalization for unstable angina, and coronary revascularization, was very close between the treated and placebo patients, occurring in 4.1% and 4.2% per year, respectively.
In addition, “there was a sustained weight loss, more than with lifestyle alone or lifestyle plus placebo, which at its peak was about 3 kg. With that there were small, but significant, reductions in heart rate, blood pressure, triglycerides, and hemoglobin A1c, and there was a significant reduction in new-onset diabetes.”
“Overall, there’s not a lot of use of pharmacologic agents for weight loss in the United States, and a lot of that is based on fear of the historical experience, which is that they were not safe. I suspect that having a drug that is proven safe will now lead people to reach for a pharmacologic agent like lorcaserin,” said Dr. Bohula, a cardiologist at of Brigham and Women’s Hospital and an investigator at the TIMI study group.
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
Erin A. Bohula, MD, DPhil, told Mitchel L. Zoler in an interview.
Dr. Bohula reported on the results of the CAMELLIA-TIMI 61 trial, which was designed to evaluate the cardiovascular safety of the weight-loss drug lorcaserin in more than 10,000 patients. She presented the data at the annual congress of the European Society of Cardiology.
In CAMELLIA-TIMI 61, the primary safety endpoint, a composite of cardiovascular death, MI, or stroke, was nearly identical between patients on lorcaserin and those given placebo, 2% and 2.1% per year, at P less than .001 for noninferiority. Similarly, the primary efficacy outcome comprising heart failure, hospitalization for unstable angina, and coronary revascularization, was very close between the treated and placebo patients, occurring in 4.1% and 4.2% per year, respectively.
In addition, “there was a sustained weight loss, more than with lifestyle alone or lifestyle plus placebo, which at its peak was about 3 kg. With that there were small, but significant, reductions in heart rate, blood pressure, triglycerides, and hemoglobin A1c, and there was a significant reduction in new-onset diabetes.”
“Overall, there’s not a lot of use of pharmacologic agents for weight loss in the United States, and a lot of that is based on fear of the historical experience, which is that they were not safe. I suspect that having a drug that is proven safe will now lead people to reach for a pharmacologic agent like lorcaserin,” said Dr. Bohula, a cardiologist at of Brigham and Women’s Hospital and an investigator at the TIMI study group.
The AGA Obesity Practice Guide provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
REPORTING FROM THE ESC CONGRESS 2018
FOURIER analysis: PCSK9 inhibition helps MetS patients the most
MUNICH – Patients with hypercholesterolemia, atherosclerotic cardiovascular disease, and metabolic syndrome received a bigger benefit from treatment with a PCSK9 inhibitor than did otherwise similar patients without metabolic syndrome in a post hoc analysis of data collected from a placebo-controlled trial of a PCSK9 inhibitor with more than 27,000 total participants.
The analysis showed that patients with metabolic syndrome (MetS) treated with the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitor evolocumab in the FOURIER study had a statistically significant 17% relative risk reduction in the study’s primary efficacy endpoint compared with placebo, while those without MetS had an 11% relative risk reduction that did not reach statistical significance, Prakash Deedwania, MD, said at the annual congress of the European Society of Cardiology. “Given their higher baseline risk [for cardiovascular disease events], patients with metabolic syndrome had a larger absolute reduction in cardiovascular events,” said Dr. Deedwania, a professor of medicine at the University of California, San Francisco.
For the study’s secondary endpoint of the combined rate of cardiovascular disease death, nonfatal MI, and nonfatal stroke, patients with MetS treated with evolocumab had a statistically significant 24% relative risk reduction compared with placebo, while those without MetS had an insignificant 14% reduction. Dr. Deedwania and his associates calculated these relative risk reductions and those for the primary endpoint using adjustments for baseline differences between the MetS and non-MetS subgroups in age, race, sex, history of diabetes, history of MI, heart failure, smoking, renal function, LDL cholesterol levels, and use of a high-intensity dosage of a statin. Among the 27,342 patients enrolled in the trial 16,361 (60%) had MetS at entry into the study, which made this “the largest study ever reported for patients with metabolic syndrome,” he noted.
“Evolocumab was still effective in patients without metabolic syndrome, but it was of lesser magnitude, and that combined with the fewer patients in the non–metabolic syndrome subgroup meant the effect did not reach statistical significance,” Dr. Deedwania explained. “These data help identify the patients who benefit the most from treatment with an expensive drug,” a PCSK9 inhibitor. Based on list prices the annual cost for treatment with a PCSK9 inhibitor such as evolocumab (Repatha) or a second approved drug from this class, alirocumab (Praluent), is roughly $14,000 (JAMA Cardiol. 2017 Oct;2[10]:1066-8).
The analysis run by Dr. Deedwania used data collected in the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial, which had showed an overall relative risk reduction of 15% compared with placebo during a median follow-up of 2.2 years for the primary endpoint of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization for unstable angina, or coronary revascularization (N Engl J Med. 2017 May 4;376[18]:1713-22). The study ran at 1,242 sites in 49 countries. He and his associates identified enrolled patients with MetS using the definition of the 2001 Third Report from the National Cholesterol Education Program: People with at least three of five criteria – fasting blood glucose of at least 100 mg/dL; waist circumference greater than 102 cm in men and 88 cm in women; systolic blood pressure of at least 135 mm Hg, diastolic blood pressure of at least 85 mm Hg, or a history of hypertension diagnosis; a triglyceride level greater than 150 mg/dL; and an HDL cholesterol level of less than 40 mg/dL in men and less than 50 mg/dL in women (JAMA. 2001 May 16;285[19]:2486-97).
The new analysis also showed that treatment with evolocumab led to an incremental, average reduction in LDL cholesterol of 58% in patients with MetS and 61% in patients without MetS at the time of enrollment. The patients with MetS in FOURIER overall had a significantly higher number of primary endpoint events, 15.8%, than enrolled patients without MetS, 12.9%, regardless of whether or not they received evolocumab, a relative risk of 21% more events after adjustment. The analysis also showed that treatment with evolocumab was equally safe and well tolerated by patients regardless of whether or not they had MetS, and that patients with MetS had no increased incidence of diabetes or elevations in fasting blood glucose or hemoglobin A1c while on treatment compared with those without MetS.
Dr. Deedwania has been a consultant to Amgen, the company that markets evolocumab, and to Sanofi, the company that markets a different PCSK9 inhibitor, alirocumab.
[email protected]
On Twitter @mitchelzoler
Given the relatively high cost for the PCSK9 inhibitor drugs, clinicians need to know which patients are likely to get the biggest bang for the buck from these agents. This will be not just patients with the highest risks for cardiovascular disease events, but those with modifiable risk factors.
The finding of greater benefit from evolocumab in patients with metabolic syndrome seen in this new analysis of data from FOURIER is consistent with other reported analyses from this trial, which identified other markers of greater benefit such as peripheral artery disease, recent MIs, and multivessel coronary artery disease. The next step will be to try to put all these findings together and figure out which patients get the most benefit from treatment. If society can’t afford to treat all eligible patients with expensive PCSK9 inhibitors, we need to learn how to use these drugs in the most cost-effective way.
Stephen J. Nicholls, MD , is professor of cardiology at the University of Adelaide, Australia. He has received research funding from and has been a consultant to several drug companies including Amgen and Sanofi/Regeneron, the companies that market PCSK9 inhibitors. He made these comments in an interview.
Given the relatively high cost for the PCSK9 inhibitor drugs, clinicians need to know which patients are likely to get the biggest bang for the buck from these agents. This will be not just patients with the highest risks for cardiovascular disease events, but those with modifiable risk factors.
The finding of greater benefit from evolocumab in patients with metabolic syndrome seen in this new analysis of data from FOURIER is consistent with other reported analyses from this trial, which identified other markers of greater benefit such as peripheral artery disease, recent MIs, and multivessel coronary artery disease. The next step will be to try to put all these findings together and figure out which patients get the most benefit from treatment. If society can’t afford to treat all eligible patients with expensive PCSK9 inhibitors, we need to learn how to use these drugs in the most cost-effective way.
Stephen J. Nicholls, MD , is professor of cardiology at the University of Adelaide, Australia. He has received research funding from and has been a consultant to several drug companies including Amgen and Sanofi/Regeneron, the companies that market PCSK9 inhibitors. He made these comments in an interview.
Given the relatively high cost for the PCSK9 inhibitor drugs, clinicians need to know which patients are likely to get the biggest bang for the buck from these agents. This will be not just patients with the highest risks for cardiovascular disease events, but those with modifiable risk factors.
The finding of greater benefit from evolocumab in patients with metabolic syndrome seen in this new analysis of data from FOURIER is consistent with other reported analyses from this trial, which identified other markers of greater benefit such as peripheral artery disease, recent MIs, and multivessel coronary artery disease. The next step will be to try to put all these findings together and figure out which patients get the most benefit from treatment. If society can’t afford to treat all eligible patients with expensive PCSK9 inhibitors, we need to learn how to use these drugs in the most cost-effective way.
Stephen J. Nicholls, MD , is professor of cardiology at the University of Adelaide, Australia. He has received research funding from and has been a consultant to several drug companies including Amgen and Sanofi/Regeneron, the companies that market PCSK9 inhibitors. He made these comments in an interview.
MUNICH – Patients with hypercholesterolemia, atherosclerotic cardiovascular disease, and metabolic syndrome received a bigger benefit from treatment with a PCSK9 inhibitor than did otherwise similar patients without metabolic syndrome in a post hoc analysis of data collected from a placebo-controlled trial of a PCSK9 inhibitor with more than 27,000 total participants.
The analysis showed that patients with metabolic syndrome (MetS) treated with the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitor evolocumab in the FOURIER study had a statistically significant 17% relative risk reduction in the study’s primary efficacy endpoint compared with placebo, while those without MetS had an 11% relative risk reduction that did not reach statistical significance, Prakash Deedwania, MD, said at the annual congress of the European Society of Cardiology. “Given their higher baseline risk [for cardiovascular disease events], patients with metabolic syndrome had a larger absolute reduction in cardiovascular events,” said Dr. Deedwania, a professor of medicine at the University of California, San Francisco.
For the study’s secondary endpoint of the combined rate of cardiovascular disease death, nonfatal MI, and nonfatal stroke, patients with MetS treated with evolocumab had a statistically significant 24% relative risk reduction compared with placebo, while those without MetS had an insignificant 14% reduction. Dr. Deedwania and his associates calculated these relative risk reductions and those for the primary endpoint using adjustments for baseline differences between the MetS and non-MetS subgroups in age, race, sex, history of diabetes, history of MI, heart failure, smoking, renal function, LDL cholesterol levels, and use of a high-intensity dosage of a statin. Among the 27,342 patients enrolled in the trial 16,361 (60%) had MetS at entry into the study, which made this “the largest study ever reported for patients with metabolic syndrome,” he noted.
“Evolocumab was still effective in patients without metabolic syndrome, but it was of lesser magnitude, and that combined with the fewer patients in the non–metabolic syndrome subgroup meant the effect did not reach statistical significance,” Dr. Deedwania explained. “These data help identify the patients who benefit the most from treatment with an expensive drug,” a PCSK9 inhibitor. Based on list prices the annual cost for treatment with a PCSK9 inhibitor such as evolocumab (Repatha) or a second approved drug from this class, alirocumab (Praluent), is roughly $14,000 (JAMA Cardiol. 2017 Oct;2[10]:1066-8).
The analysis run by Dr. Deedwania used data collected in the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial, which had showed an overall relative risk reduction of 15% compared with placebo during a median follow-up of 2.2 years for the primary endpoint of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization for unstable angina, or coronary revascularization (N Engl J Med. 2017 May 4;376[18]:1713-22). The study ran at 1,242 sites in 49 countries. He and his associates identified enrolled patients with MetS using the definition of the 2001 Third Report from the National Cholesterol Education Program: People with at least three of five criteria – fasting blood glucose of at least 100 mg/dL; waist circumference greater than 102 cm in men and 88 cm in women; systolic blood pressure of at least 135 mm Hg, diastolic blood pressure of at least 85 mm Hg, or a history of hypertension diagnosis; a triglyceride level greater than 150 mg/dL; and an HDL cholesterol level of less than 40 mg/dL in men and less than 50 mg/dL in women (JAMA. 2001 May 16;285[19]:2486-97).
The new analysis also showed that treatment with evolocumab led to an incremental, average reduction in LDL cholesterol of 58% in patients with MetS and 61% in patients without MetS at the time of enrollment. The patients with MetS in FOURIER overall had a significantly higher number of primary endpoint events, 15.8%, than enrolled patients without MetS, 12.9%, regardless of whether or not they received evolocumab, a relative risk of 21% more events after adjustment. The analysis also showed that treatment with evolocumab was equally safe and well tolerated by patients regardless of whether or not they had MetS, and that patients with MetS had no increased incidence of diabetes or elevations in fasting blood glucose or hemoglobin A1c while on treatment compared with those without MetS.
Dr. Deedwania has been a consultant to Amgen, the company that markets evolocumab, and to Sanofi, the company that markets a different PCSK9 inhibitor, alirocumab.
[email protected]
On Twitter @mitchelzoler
MUNICH – Patients with hypercholesterolemia, atherosclerotic cardiovascular disease, and metabolic syndrome received a bigger benefit from treatment with a PCSK9 inhibitor than did otherwise similar patients without metabolic syndrome in a post hoc analysis of data collected from a placebo-controlled trial of a PCSK9 inhibitor with more than 27,000 total participants.
The analysis showed that patients with metabolic syndrome (MetS) treated with the proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitor evolocumab in the FOURIER study had a statistically significant 17% relative risk reduction in the study’s primary efficacy endpoint compared with placebo, while those without MetS had an 11% relative risk reduction that did not reach statistical significance, Prakash Deedwania, MD, said at the annual congress of the European Society of Cardiology. “Given their higher baseline risk [for cardiovascular disease events], patients with metabolic syndrome had a larger absolute reduction in cardiovascular events,” said Dr. Deedwania, a professor of medicine at the University of California, San Francisco.
For the study’s secondary endpoint of the combined rate of cardiovascular disease death, nonfatal MI, and nonfatal stroke, patients with MetS treated with evolocumab had a statistically significant 24% relative risk reduction compared with placebo, while those without MetS had an insignificant 14% reduction. Dr. Deedwania and his associates calculated these relative risk reductions and those for the primary endpoint using adjustments for baseline differences between the MetS and non-MetS subgroups in age, race, sex, history of diabetes, history of MI, heart failure, smoking, renal function, LDL cholesterol levels, and use of a high-intensity dosage of a statin. Among the 27,342 patients enrolled in the trial 16,361 (60%) had MetS at entry into the study, which made this “the largest study ever reported for patients with metabolic syndrome,” he noted.
“Evolocumab was still effective in patients without metabolic syndrome, but it was of lesser magnitude, and that combined with the fewer patients in the non–metabolic syndrome subgroup meant the effect did not reach statistical significance,” Dr. Deedwania explained. “These data help identify the patients who benefit the most from treatment with an expensive drug,” a PCSK9 inhibitor. Based on list prices the annual cost for treatment with a PCSK9 inhibitor such as evolocumab (Repatha) or a second approved drug from this class, alirocumab (Praluent), is roughly $14,000 (JAMA Cardiol. 2017 Oct;2[10]:1066-8).
The analysis run by Dr. Deedwania used data collected in the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial, which had showed an overall relative risk reduction of 15% compared with placebo during a median follow-up of 2.2 years for the primary endpoint of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization for unstable angina, or coronary revascularization (N Engl J Med. 2017 May 4;376[18]:1713-22). The study ran at 1,242 sites in 49 countries. He and his associates identified enrolled patients with MetS using the definition of the 2001 Third Report from the National Cholesterol Education Program: People with at least three of five criteria – fasting blood glucose of at least 100 mg/dL; waist circumference greater than 102 cm in men and 88 cm in women; systolic blood pressure of at least 135 mm Hg, diastolic blood pressure of at least 85 mm Hg, or a history of hypertension diagnosis; a triglyceride level greater than 150 mg/dL; and an HDL cholesterol level of less than 40 mg/dL in men and less than 50 mg/dL in women (JAMA. 2001 May 16;285[19]:2486-97).
The new analysis also showed that treatment with evolocumab led to an incremental, average reduction in LDL cholesterol of 58% in patients with MetS and 61% in patients without MetS at the time of enrollment. The patients with MetS in FOURIER overall had a significantly higher number of primary endpoint events, 15.8%, than enrolled patients without MetS, 12.9%, regardless of whether or not they received evolocumab, a relative risk of 21% more events after adjustment. The analysis also showed that treatment with evolocumab was equally safe and well tolerated by patients regardless of whether or not they had MetS, and that patients with MetS had no increased incidence of diabetes or elevations in fasting blood glucose or hemoglobin A1c while on treatment compared with those without MetS.
Dr. Deedwania has been a consultant to Amgen, the company that markets evolocumab, and to Sanofi, the company that markets a different PCSK9 inhibitor, alirocumab.
[email protected]
On Twitter @mitchelzoler
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: PCSK9 inhibition produced the biggest benefit in patients with metabolic syndrome.
Major finding: In patients with metabolic syndrome, treatment with evolocumab cut the primary endpoint by a relative 17% compared with placebo.
Study details: Post hoc analysis of data from FOURIER, a multicenter, randomized trial with 27,342 patients.
Disclosures: Dr. Deedwania has been a consultant to Amgen, the company that markets evolocumab, and to Sanofi, the company that markets a different PCSK9 inhibitor, alirocumab.