User login
Slowing down
This past Labor Day weekend, I did something radical. I slowed down. Way down. My wife slowed down with me, which helped. We spent the weekend close to home walking, talking, reading, contemplating, planning, assessing, doing puzzles and crosswords, and imbibing a craft beer or two, slowly, of course. Why? Because of Adam Grant, PhD, the organizational psychologist at the University of Pennsylvania’s Wharton School of Business, Philadelphia. I had recently reread his 2016 book I’m a big fan; he’s one of those professors who makes you fervently wish you were a student again, someone who will provoke you and challenge your way of thinking.
Dr. Grant’s basic premise, which he has proved through research, is that procrastination boosts productivity. Here’s how: Let’s say you’re facing a challenge or difficult task. He says to start working on it immediately, then take some time away for reflection. This “quick to start and slow to finish” method allows your brain to continually percolate on the problem. An incomplete task stays partially active in your brain. When you come back to it you often see it with fresh eyes. You will experience your highest productivity when you are toggling between these two modes.
This makes sense, and Dr. Grant cites numerous examples from Leonardo da Vinci to the founders of Warby-Parker, as examples of success. But how can it benefit physicians? Many of us are “precrastinators,” people who tend to complete or at least begin tasks as soon as possible, even when it’s unnecessary or not urgent. Unlike some jobs in which it’s easier to take a break from a project and return to it with more creative solutions, we often are racing against a clock to see more patients, read more slides, answer more emails, and make more phone calls. We are perpetually frenetic, which is not conducive to original thinking.
If this sounds like you, then you are likely to benefit from deliberate procrastination. Here are a few ways to slow down:
- Put it on your calendar. Yes, I see the irony, but it works. Start by scheduling one hour a week where you are to accomplish nothing. You can fill this time with whatever your mind wants to do at that moment.
- When faced with a diagnostic dilemma or treatment failure, resist the urge to solve that problem in that moment. Save that note for later, tell the patient you will call him back or bring him back for a visit later. Even if you’re not actively working on it, it will incubate somewhere in your brain, allowing more divergent thought processes to take over. It’s a little like trying to solve a crossword that seems impossible in the moment and then answers suddenly appear without effort.
- Take up a hobby: Play the guitar, learn to make pasta, climb a big rock. When you are fully engaged in such pursuits it requires complete mental focus. When you revisit the difficult problem you’re working on, you will likely see it from different perspectives.
- Meditate: Meditation requires our brains and bodies to slow down. It can help reduce self-doubt and criticism which stifle problem solving.
- Watch Slow TV. Slow TV is a Scandinavian phenomenon where you sit and watch meditative video such as a 7-hour train cam from Bergen, Norway, to Oslo. There’s no dialogue, no plot, no commercials. It’s just 7 hours of track and train and is weirdly comforting.
If you want to learn more, then when you get a chance, Google “slow living” and explore. Of course, some of you precrastinators probably have already started before finishing this column.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This past Labor Day weekend, I did something radical. I slowed down. Way down. My wife slowed down with me, which helped. We spent the weekend close to home walking, talking, reading, contemplating, planning, assessing, doing puzzles and crosswords, and imbibing a craft beer or two, slowly, of course. Why? Because of Adam Grant, PhD, the organizational psychologist at the University of Pennsylvania’s Wharton School of Business, Philadelphia. I had recently reread his 2016 book I’m a big fan; he’s one of those professors who makes you fervently wish you were a student again, someone who will provoke you and challenge your way of thinking.
Dr. Grant’s basic premise, which he has proved through research, is that procrastination boosts productivity. Here’s how: Let’s say you’re facing a challenge or difficult task. He says to start working on it immediately, then take some time away for reflection. This “quick to start and slow to finish” method allows your brain to continually percolate on the problem. An incomplete task stays partially active in your brain. When you come back to it you often see it with fresh eyes. You will experience your highest productivity when you are toggling between these two modes.
This makes sense, and Dr. Grant cites numerous examples from Leonardo da Vinci to the founders of Warby-Parker, as examples of success. But how can it benefit physicians? Many of us are “precrastinators,” people who tend to complete or at least begin tasks as soon as possible, even when it’s unnecessary or not urgent. Unlike some jobs in which it’s easier to take a break from a project and return to it with more creative solutions, we often are racing against a clock to see more patients, read more slides, answer more emails, and make more phone calls. We are perpetually frenetic, which is not conducive to original thinking.
If this sounds like you, then you are likely to benefit from deliberate procrastination. Here are a few ways to slow down:
- Put it on your calendar. Yes, I see the irony, but it works. Start by scheduling one hour a week where you are to accomplish nothing. You can fill this time with whatever your mind wants to do at that moment.
- When faced with a diagnostic dilemma or treatment failure, resist the urge to solve that problem in that moment. Save that note for later, tell the patient you will call him back or bring him back for a visit later. Even if you’re not actively working on it, it will incubate somewhere in your brain, allowing more divergent thought processes to take over. It’s a little like trying to solve a crossword that seems impossible in the moment and then answers suddenly appear without effort.
- Take up a hobby: Play the guitar, learn to make pasta, climb a big rock. When you are fully engaged in such pursuits it requires complete mental focus. When you revisit the difficult problem you’re working on, you will likely see it from different perspectives.
- Meditate: Meditation requires our brains and bodies to slow down. It can help reduce self-doubt and criticism which stifle problem solving.
- Watch Slow TV. Slow TV is a Scandinavian phenomenon where you sit and watch meditative video such as a 7-hour train cam from Bergen, Norway, to Oslo. There’s no dialogue, no plot, no commercials. It’s just 7 hours of track and train and is weirdly comforting.
If you want to learn more, then when you get a chance, Google “slow living” and explore. Of course, some of you precrastinators probably have already started before finishing this column.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This past Labor Day weekend, I did something radical. I slowed down. Way down. My wife slowed down with me, which helped. We spent the weekend close to home walking, talking, reading, contemplating, planning, assessing, doing puzzles and crosswords, and imbibing a craft beer or two, slowly, of course. Why? Because of Adam Grant, PhD, the organizational psychologist at the University of Pennsylvania’s Wharton School of Business, Philadelphia. I had recently reread his 2016 book I’m a big fan; he’s one of those professors who makes you fervently wish you were a student again, someone who will provoke you and challenge your way of thinking.
Dr. Grant’s basic premise, which he has proved through research, is that procrastination boosts productivity. Here’s how: Let’s say you’re facing a challenge or difficult task. He says to start working on it immediately, then take some time away for reflection. This “quick to start and slow to finish” method allows your brain to continually percolate on the problem. An incomplete task stays partially active in your brain. When you come back to it you often see it with fresh eyes. You will experience your highest productivity when you are toggling between these two modes.
This makes sense, and Dr. Grant cites numerous examples from Leonardo da Vinci to the founders of Warby-Parker, as examples of success. But how can it benefit physicians? Many of us are “precrastinators,” people who tend to complete or at least begin tasks as soon as possible, even when it’s unnecessary or not urgent. Unlike some jobs in which it’s easier to take a break from a project and return to it with more creative solutions, we often are racing against a clock to see more patients, read more slides, answer more emails, and make more phone calls. We are perpetually frenetic, which is not conducive to original thinking.
If this sounds like you, then you are likely to benefit from deliberate procrastination. Here are a few ways to slow down:
- Put it on your calendar. Yes, I see the irony, but it works. Start by scheduling one hour a week where you are to accomplish nothing. You can fill this time with whatever your mind wants to do at that moment.
- When faced with a diagnostic dilemma or treatment failure, resist the urge to solve that problem in that moment. Save that note for later, tell the patient you will call him back or bring him back for a visit later. Even if you’re not actively working on it, it will incubate somewhere in your brain, allowing more divergent thought processes to take over. It’s a little like trying to solve a crossword that seems impossible in the moment and then answers suddenly appear without effort.
- Take up a hobby: Play the guitar, learn to make pasta, climb a big rock. When you are fully engaged in such pursuits it requires complete mental focus. When you revisit the difficult problem you’re working on, you will likely see it from different perspectives.
- Meditate: Meditation requires our brains and bodies to slow down. It can help reduce self-doubt and criticism which stifle problem solving.
- Watch Slow TV. Slow TV is a Scandinavian phenomenon where you sit and watch meditative video such as a 7-hour train cam from Bergen, Norway, to Oslo. There’s no dialogue, no plot, no commercials. It’s just 7 hours of track and train and is weirdly comforting.
If you want to learn more, then when you get a chance, Google “slow living” and explore. Of course, some of you precrastinators probably have already started before finishing this column.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
How to screen for, manage FASD in a medical home
providing early intervention and accessing community resources, according to a clinical report from the American Academy of Pediatrics.
After the AAP released its guidelines on fetal alcohol spectrum disorder (FASD) in 2015, some pediatricians asked for further guidance on how to care for patients with FASD within the medical home, as many had a knowledge gap on how to best manage these patients.
“For some pediatricians, it can seem like a daunting task to care for an individual with an FASD, but there are aspects of integrated care and providing a medical home that can be instituted as with all children with complex medical diagnoses,” wrote Renee M. Turchi, MD, MPH, of the department of pediatrics at St. Christopher’s Hospital for Children and Drexel Dornsife School of Public Health in Philadelphia, and her colleagues on the AAP Committee on Substance Abuse and the Council on Children with Disabilities. Their report is in Pediatrics. “In addition, not recognizing an FASD can lead to inadequate treatment and less-than-optimal outcomes for the patient and family.”
Dr. Turchi and her colleagues released the FASD clinical report with “strategies to support families who are interacting with early intervention services, the educational system, the behavioral and/or mental health system, other community resources, and the transition to adult-oriented heath care systems when appropriate.” They noted the prevalence of FASD is increasing, with 1 in 10 pregnant women using alcohol within the past 30 days and 1 in 33 pregnant women reporting binge drinking in the past 30 days. They reaffirmed the AAP’s endorsement from the 2015 clinical report on FASD regarding abstinence of alcohol for pregnant women, emphasizing that there is no amount or kind of alcohol that is risk free during pregnancy, nor is there a time in pregnancy when drinking alcohol is risk free.
Providers in a medical home should communicate any prenatal alcohol exposure (PAE) to obstetric providers so they can review risk factors, optimize screening, and monitor children, Dr. Turchi and her colleagues said. They also should understand the diagnostic criteria and classifications for FASDs, including physical features such as low weight, short palpebral features, smooth philtrum, a thin upper lip, abnormalities in the central nervous system, and any alcohol use during pregnancy. Any child – regardless of age – is a candidate for universal PAE screening at initial visits or when “additional cognitive and behavioral concerns arise.”
The federal Child Abuse Prevention and Treatment Act “does not require clinicians to report to child protective services if a child has been exposed prenatally to alcohol (i.e., for a positive PAE screening result). Referral to child protective services is required if the child has been diagnosed with an FASD in the period between birth and 3 years. The intent of this referral is to develop safe care and possible treatment plans for the infant and caregiver if needed, not to initiate punitive actions,” according to the report. States have their own definitions about child abuse and neglect, so the report encourages providers to know the mandates and reporting laws in the states where they practice.
Monitoring children in a medical home for the signs and symptoms of FASD is important, the authors said, because research has shown an increased chance at reducing adverse life outcomes if a child is diagnosed before age 6 and is in a stable home with access to support services.
Management of children with FASD is individual, as symptoms for each child will uniquely present not just in terms of physical issues such as growth or congenital defects affecting the heart, eyes, kidneys, or bones, but also as developmental, cognitive, and behavioral problems. Children with FASD also may receive a concomitant diagnosis when evaluated, such as ADHD or depression, that will require additional accommodation. The use of evidence-based diagnostic and standard screening approaches and referring when necessary will help reevaluate whether a child has a condition such as ADHD, oppositional defiant disorder, or another diagnosis, or is displaying symptoms of FASD such as a receptive or expressive language disorder.
Pediatricians must work together with the families, educational professionals, the mental health community, and therapists to help manage FASD in children. In cases where a child is in foster care, partnering with the foster care partners and child welfare agencies to gain access to the medical information of the biological parents is important to determine whether there is parental history of substance abuse and to provide appropriate treatment and interventions.
“Given the complex array of systems and services requiring navigation and coordination for children with an FASD and their families, a high-quality primary care medical home with partnerships with families, specialists, therapists, mental and/or behavioral health professionals, and community partners is critical, as it is for all children with special health care needs,” Dr. Turchi and her colleagues said.
The authors reported no relevant conflicts of interest.
SOURCE: Turchi RM et al. Pediatrics. 2018 Sept 10. doi:10.1542/peds.2018-2333.
providing early intervention and accessing community resources, according to a clinical report from the American Academy of Pediatrics.
After the AAP released its guidelines on fetal alcohol spectrum disorder (FASD) in 2015, some pediatricians asked for further guidance on how to care for patients with FASD within the medical home, as many had a knowledge gap on how to best manage these patients.
“For some pediatricians, it can seem like a daunting task to care for an individual with an FASD, but there are aspects of integrated care and providing a medical home that can be instituted as with all children with complex medical diagnoses,” wrote Renee M. Turchi, MD, MPH, of the department of pediatrics at St. Christopher’s Hospital for Children and Drexel Dornsife School of Public Health in Philadelphia, and her colleagues on the AAP Committee on Substance Abuse and the Council on Children with Disabilities. Their report is in Pediatrics. “In addition, not recognizing an FASD can lead to inadequate treatment and less-than-optimal outcomes for the patient and family.”
Dr. Turchi and her colleagues released the FASD clinical report with “strategies to support families who are interacting with early intervention services, the educational system, the behavioral and/or mental health system, other community resources, and the transition to adult-oriented heath care systems when appropriate.” They noted the prevalence of FASD is increasing, with 1 in 10 pregnant women using alcohol within the past 30 days and 1 in 33 pregnant women reporting binge drinking in the past 30 days. They reaffirmed the AAP’s endorsement from the 2015 clinical report on FASD regarding abstinence of alcohol for pregnant women, emphasizing that there is no amount or kind of alcohol that is risk free during pregnancy, nor is there a time in pregnancy when drinking alcohol is risk free.
Providers in a medical home should communicate any prenatal alcohol exposure (PAE) to obstetric providers so they can review risk factors, optimize screening, and monitor children, Dr. Turchi and her colleagues said. They also should understand the diagnostic criteria and classifications for FASDs, including physical features such as low weight, short palpebral features, smooth philtrum, a thin upper lip, abnormalities in the central nervous system, and any alcohol use during pregnancy. Any child – regardless of age – is a candidate for universal PAE screening at initial visits or when “additional cognitive and behavioral concerns arise.”
The federal Child Abuse Prevention and Treatment Act “does not require clinicians to report to child protective services if a child has been exposed prenatally to alcohol (i.e., for a positive PAE screening result). Referral to child protective services is required if the child has been diagnosed with an FASD in the period between birth and 3 years. The intent of this referral is to develop safe care and possible treatment plans for the infant and caregiver if needed, not to initiate punitive actions,” according to the report. States have their own definitions about child abuse and neglect, so the report encourages providers to know the mandates and reporting laws in the states where they practice.
Monitoring children in a medical home for the signs and symptoms of FASD is important, the authors said, because research has shown an increased chance at reducing adverse life outcomes if a child is diagnosed before age 6 and is in a stable home with access to support services.
Management of children with FASD is individual, as symptoms for each child will uniquely present not just in terms of physical issues such as growth or congenital defects affecting the heart, eyes, kidneys, or bones, but also as developmental, cognitive, and behavioral problems. Children with FASD also may receive a concomitant diagnosis when evaluated, such as ADHD or depression, that will require additional accommodation. The use of evidence-based diagnostic and standard screening approaches and referring when necessary will help reevaluate whether a child has a condition such as ADHD, oppositional defiant disorder, or another diagnosis, or is displaying symptoms of FASD such as a receptive or expressive language disorder.
Pediatricians must work together with the families, educational professionals, the mental health community, and therapists to help manage FASD in children. In cases where a child is in foster care, partnering with the foster care partners and child welfare agencies to gain access to the medical information of the biological parents is important to determine whether there is parental history of substance abuse and to provide appropriate treatment and interventions.
“Given the complex array of systems and services requiring navigation and coordination for children with an FASD and their families, a high-quality primary care medical home with partnerships with families, specialists, therapists, mental and/or behavioral health professionals, and community partners is critical, as it is for all children with special health care needs,” Dr. Turchi and her colleagues said.
The authors reported no relevant conflicts of interest.
SOURCE: Turchi RM et al. Pediatrics. 2018 Sept 10. doi:10.1542/peds.2018-2333.
providing early intervention and accessing community resources, according to a clinical report from the American Academy of Pediatrics.
After the AAP released its guidelines on fetal alcohol spectrum disorder (FASD) in 2015, some pediatricians asked for further guidance on how to care for patients with FASD within the medical home, as many had a knowledge gap on how to best manage these patients.
“For some pediatricians, it can seem like a daunting task to care for an individual with an FASD, but there are aspects of integrated care and providing a medical home that can be instituted as with all children with complex medical diagnoses,” wrote Renee M. Turchi, MD, MPH, of the department of pediatrics at St. Christopher’s Hospital for Children and Drexel Dornsife School of Public Health in Philadelphia, and her colleagues on the AAP Committee on Substance Abuse and the Council on Children with Disabilities. Their report is in Pediatrics. “In addition, not recognizing an FASD can lead to inadequate treatment and less-than-optimal outcomes for the patient and family.”
Dr. Turchi and her colleagues released the FASD clinical report with “strategies to support families who are interacting with early intervention services, the educational system, the behavioral and/or mental health system, other community resources, and the transition to adult-oriented heath care systems when appropriate.” They noted the prevalence of FASD is increasing, with 1 in 10 pregnant women using alcohol within the past 30 days and 1 in 33 pregnant women reporting binge drinking in the past 30 days. They reaffirmed the AAP’s endorsement from the 2015 clinical report on FASD regarding abstinence of alcohol for pregnant women, emphasizing that there is no amount or kind of alcohol that is risk free during pregnancy, nor is there a time in pregnancy when drinking alcohol is risk free.
Providers in a medical home should communicate any prenatal alcohol exposure (PAE) to obstetric providers so they can review risk factors, optimize screening, and monitor children, Dr. Turchi and her colleagues said. They also should understand the diagnostic criteria and classifications for FASDs, including physical features such as low weight, short palpebral features, smooth philtrum, a thin upper lip, abnormalities in the central nervous system, and any alcohol use during pregnancy. Any child – regardless of age – is a candidate for universal PAE screening at initial visits or when “additional cognitive and behavioral concerns arise.”
The federal Child Abuse Prevention and Treatment Act “does not require clinicians to report to child protective services if a child has been exposed prenatally to alcohol (i.e., for a positive PAE screening result). Referral to child protective services is required if the child has been diagnosed with an FASD in the period between birth and 3 years. The intent of this referral is to develop safe care and possible treatment plans for the infant and caregiver if needed, not to initiate punitive actions,” according to the report. States have their own definitions about child abuse and neglect, so the report encourages providers to know the mandates and reporting laws in the states where they practice.
Monitoring children in a medical home for the signs and symptoms of FASD is important, the authors said, because research has shown an increased chance at reducing adverse life outcomes if a child is diagnosed before age 6 and is in a stable home with access to support services.
Management of children with FASD is individual, as symptoms for each child will uniquely present not just in terms of physical issues such as growth or congenital defects affecting the heart, eyes, kidneys, or bones, but also as developmental, cognitive, and behavioral problems. Children with FASD also may receive a concomitant diagnosis when evaluated, such as ADHD or depression, that will require additional accommodation. The use of evidence-based diagnostic and standard screening approaches and referring when necessary will help reevaluate whether a child has a condition such as ADHD, oppositional defiant disorder, or another diagnosis, or is displaying symptoms of FASD such as a receptive or expressive language disorder.
Pediatricians must work together with the families, educational professionals, the mental health community, and therapists to help manage FASD in children. In cases where a child is in foster care, partnering with the foster care partners and child welfare agencies to gain access to the medical information of the biological parents is important to determine whether there is parental history of substance abuse and to provide appropriate treatment and interventions.
“Given the complex array of systems and services requiring navigation and coordination for children with an FASD and their families, a high-quality primary care medical home with partnerships with families, specialists, therapists, mental and/or behavioral health professionals, and community partners is critical, as it is for all children with special health care needs,” Dr. Turchi and her colleagues said.
The authors reported no relevant conflicts of interest.
SOURCE: Turchi RM et al. Pediatrics. 2018 Sept 10. doi:10.1542/peds.2018-2333.
FROM PEDIATRICS
Women, older patients at greater risk of more aggressive PBC
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Women, older individuals, and patients with other autoimmune diseases are more likely to have worse primary biliary cholangitis (PBC).
Major finding: More than one-third of patients with PBC have high levels of alkaline phosphatase.
Study details: An analysis of medical records for 15,875 patients with PBC.
Disclosures: Two authors declared research funding or consulting fees from the pharmaceutical industry; one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
Source: Younossi ZM et al. J Clin Gastroenterol. 2018 August 24. doi: 10.1097/MCG.0000000000001120.
CDC: Obesity affects over 35% in 7 states
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.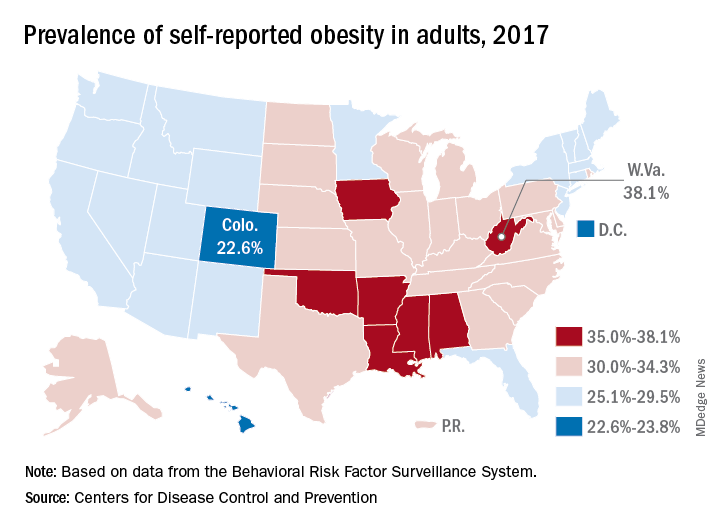
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
Iowa and Oklahoma, the two newest states with prevalences at or exceeding 35%, joined Alabama, Arkansas, Louisiana, Mississippi, and West Virginia, which has the country’s highest rate of adult obesity at 38.1%. Colorado’s 22.6% rate is the lowest prevalence among all states. The District of Columbia and Hawaii also have prevalences under 25%; previously, Massachusetts also was in this group, but its prevalence went up to 25.9% last year, the CDC reported.
Regional disparities in self-reported adult obesity put the South (32.4%) and the Midwest (32.3%) well ahead of the Northeast (27.7%) and the West (26.1%) in 2017. Racial and ethnic disparities also were seen, with large gaps between blacks, who had a prevalence of 39%, and Hispanics (32.4%) and whites (29.3%). Obesity prevalence was 35% or higher among black adults in 31 states and D.C., while this was true among Hispanics in eight states and among whites in one (West Virginia), although the prevalence was at or above 35% for multiple racial groups in some of these states, the CDC reported based on data from the Behavioral Risk Factor Surveillance System.
“Obesity costs the United States health care system over $147 billion a year [and] research has shown that obesity affects work productivity and military readiness,” the CDC said in a written statement. “To protect the health of the next generation, support for healthy behaviors such as healthy eating, better sleep, stress management, and physical activity should start early and expand to reach Americans across the lifespan in the communities where they live, learn, work, and play.”
Recommendations to Improve Asthma Outcomes: Work Group Call to Action
Click here to read the supplement.

What can be done to address the burden of asthma beyond pharmacotherapy? A panel of experts discuss steps for addressing sensitization to allergens that trigger increased asthma burden.
Topics Include:
- Identifying Patients with Allergic Components of Asthma
- Identifying and Addressing Allergen Exposure in Daily Practice
- The Opportunity for Payers and Health Systems for Supporting Trigger Avoidance Education
Click here to read the supplement.
Click here to read the supplement.

What can be done to address the burden of asthma beyond pharmacotherapy? A panel of experts discuss steps for addressing sensitization to allergens that trigger increased asthma burden.
Topics Include:
- Identifying Patients with Allergic Components of Asthma
- Identifying and Addressing Allergen Exposure in Daily Practice
- The Opportunity for Payers and Health Systems for Supporting Trigger Avoidance Education
Click here to read the supplement.
Click here to read the supplement.

What can be done to address the burden of asthma beyond pharmacotherapy? A panel of experts discuss steps for addressing sensitization to allergens that trigger increased asthma burden.
Topics Include:
- Identifying Patients with Allergic Components of Asthma
- Identifying and Addressing Allergen Exposure in Daily Practice
- The Opportunity for Payers and Health Systems for Supporting Trigger Avoidance Education
Click here to read the supplement.
GERD patients who fail PPI often have functional heartburn or hypersensitivity
Abnormal pH results were similar in patients with gastroesophageal reflux disease (GERD) who improved or failed to improve on a once-daily dose of a proton pump inhibitor (PPI), but 75% of patients who failed treatment demonstrated either functional heartburn or reflux hypersensitivity, based on data from 29 adults.
Previous research on PPI failure in GERD patients has focused on twice-daily doses; “the purpose of the study was to compare impedance-pH parameters between patients who failed versus those who responded to PPI once daily,” wrote Jason Abdallah, MD, of Case Western Reserve University in Cleveland and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the investigators reviewed data from adults diagnosed with GERD who were treated with PPI therapy. The 16 who reported heartburn and/or regurgitation at least twice a week for 3 months while on a standard, once-daily PPI dose were classified as the failure group. The 13 patients who reported complete symptom resolution for at least 4 weeks while on the same standard dose were classified as the success group.
Most of the patients in the PPI-failure group (75%) were found to have either functional heartburn or reflux hypersensitivity with GERD. Impedance and pH parameters did not differ significantly between the PPI-failure and -success group, the researchers noted. Abnormal pH test results were similar between the groups, occurring in four of the patients who were successfully treated with PPI (31%) and four of the patients who failed PPI treatment (25%).
All patients completed the Short-Form 36 (SF-36) and GERD Health-Related Quality of Life (GERD-HRQL) questionnaires, and all underwent upper endoscopy and combined 24-hour esophageal impedance and pH monitoring within 2-4 weeks of study enrollment and while following their PPI treatment plans. There were no significant differences in demographic characteristics between the success and failure groups; the mean ages were 55 years and 47 years, respectively.
The patients in the success group averaged higher scores on the SF-36 than the failure group, but the difference was not significant. On the GERD-HRQL, treatment-failure patients reported that overall heartburn and either heartburn or bloating while lying down were the symptoms they found most annoying on a daily basis.
Among the treatment-failure patients, 10 (62%) had normal acid exposure and negative symptom-reflux association, 2 patients (13%) had normal acid exposure and positive symptom-reflux association, and 4 patients (25%) had abnormal esophageal acid exposure. Patients in the treatment failure group reported a total of 315 episodes of either heartburn or regurgitation.
Endoscopy findings were normal in most of the patients in both groups; 81% of the treatment-failure patients and 69% of the treatment-success patients had normal upper endoscopy findings. Abnormal findings in the treatment-success group included one case of erosive esophagitis, two cases of Barrett’s esophagus, three cases of nonobstructive Schatzki rings, and five cases of hiatal hernia. Abnormal findings in the treatment-failure group included two cases of Schatzki rings, one case of esophageal stricture, and three cases of hiatal hernia.
The total number of reflux events was similar between the groups; 1,279 in the treatment-failure group and 1,099 in the treatment-success group, with the number of reflux events per patient averaging 80 and 84, respectively.
“Our results support the hypothesis that PPI failure is primarily driven by esophageal hypersensitivity,” the researchers noted. The similarity in impedance and reflux “implies that the shift to nonacidic reflux is a general PPI phenomenon, as opposed to being unique to PPI-failure patients,” they said.
The study was limited by the small patient population, but the results provide some insight into refractory GERD and suggest that patients who fail to respond to once-daily PPI might benefit from a neuromodulator, as well as psychological interventions including cognitive-behavioral therapy, hypnotherapy, relaxation techniques, mindfulness, and biofeedback, the researchers concluded.
Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
SOURCE: Abdallah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
Abnormal pH results were similar in patients with gastroesophageal reflux disease (GERD) who improved or failed to improve on a once-daily dose of a proton pump inhibitor (PPI), but 75% of patients who failed treatment demonstrated either functional heartburn or reflux hypersensitivity, based on data from 29 adults.
Previous research on PPI failure in GERD patients has focused on twice-daily doses; “the purpose of the study was to compare impedance-pH parameters between patients who failed versus those who responded to PPI once daily,” wrote Jason Abdallah, MD, of Case Western Reserve University in Cleveland and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the investigators reviewed data from adults diagnosed with GERD who were treated with PPI therapy. The 16 who reported heartburn and/or regurgitation at least twice a week for 3 months while on a standard, once-daily PPI dose were classified as the failure group. The 13 patients who reported complete symptom resolution for at least 4 weeks while on the same standard dose were classified as the success group.
Most of the patients in the PPI-failure group (75%) were found to have either functional heartburn or reflux hypersensitivity with GERD. Impedance and pH parameters did not differ significantly between the PPI-failure and -success group, the researchers noted. Abnormal pH test results were similar between the groups, occurring in four of the patients who were successfully treated with PPI (31%) and four of the patients who failed PPI treatment (25%).
All patients completed the Short-Form 36 (SF-36) and GERD Health-Related Quality of Life (GERD-HRQL) questionnaires, and all underwent upper endoscopy and combined 24-hour esophageal impedance and pH monitoring within 2-4 weeks of study enrollment and while following their PPI treatment plans. There were no significant differences in demographic characteristics between the success and failure groups; the mean ages were 55 years and 47 years, respectively.
The patients in the success group averaged higher scores on the SF-36 than the failure group, but the difference was not significant. On the GERD-HRQL, treatment-failure patients reported that overall heartburn and either heartburn or bloating while lying down were the symptoms they found most annoying on a daily basis.
Among the treatment-failure patients, 10 (62%) had normal acid exposure and negative symptom-reflux association, 2 patients (13%) had normal acid exposure and positive symptom-reflux association, and 4 patients (25%) had abnormal esophageal acid exposure. Patients in the treatment failure group reported a total of 315 episodes of either heartburn or regurgitation.
Endoscopy findings were normal in most of the patients in both groups; 81% of the treatment-failure patients and 69% of the treatment-success patients had normal upper endoscopy findings. Abnormal findings in the treatment-success group included one case of erosive esophagitis, two cases of Barrett’s esophagus, three cases of nonobstructive Schatzki rings, and five cases of hiatal hernia. Abnormal findings in the treatment-failure group included two cases of Schatzki rings, one case of esophageal stricture, and three cases of hiatal hernia.
The total number of reflux events was similar between the groups; 1,279 in the treatment-failure group and 1,099 in the treatment-success group, with the number of reflux events per patient averaging 80 and 84, respectively.
“Our results support the hypothesis that PPI failure is primarily driven by esophageal hypersensitivity,” the researchers noted. The similarity in impedance and reflux “implies that the shift to nonacidic reflux is a general PPI phenomenon, as opposed to being unique to PPI-failure patients,” they said.
The study was limited by the small patient population, but the results provide some insight into refractory GERD and suggest that patients who fail to respond to once-daily PPI might benefit from a neuromodulator, as well as psychological interventions including cognitive-behavioral therapy, hypnotherapy, relaxation techniques, mindfulness, and biofeedback, the researchers concluded.
Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
SOURCE: Abdallah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
Abnormal pH results were similar in patients with gastroesophageal reflux disease (GERD) who improved or failed to improve on a once-daily dose of a proton pump inhibitor (PPI), but 75% of patients who failed treatment demonstrated either functional heartburn or reflux hypersensitivity, based on data from 29 adults.
Previous research on PPI failure in GERD patients has focused on twice-daily doses; “the purpose of the study was to compare impedance-pH parameters between patients who failed versus those who responded to PPI once daily,” wrote Jason Abdallah, MD, of Case Western Reserve University in Cleveland and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the investigators reviewed data from adults diagnosed with GERD who were treated with PPI therapy. The 16 who reported heartburn and/or regurgitation at least twice a week for 3 months while on a standard, once-daily PPI dose were classified as the failure group. The 13 patients who reported complete symptom resolution for at least 4 weeks while on the same standard dose were classified as the success group.
Most of the patients in the PPI-failure group (75%) were found to have either functional heartburn or reflux hypersensitivity with GERD. Impedance and pH parameters did not differ significantly between the PPI-failure and -success group, the researchers noted. Abnormal pH test results were similar between the groups, occurring in four of the patients who were successfully treated with PPI (31%) and four of the patients who failed PPI treatment (25%).
All patients completed the Short-Form 36 (SF-36) and GERD Health-Related Quality of Life (GERD-HRQL) questionnaires, and all underwent upper endoscopy and combined 24-hour esophageal impedance and pH monitoring within 2-4 weeks of study enrollment and while following their PPI treatment plans. There were no significant differences in demographic characteristics between the success and failure groups; the mean ages were 55 years and 47 years, respectively.
The patients in the success group averaged higher scores on the SF-36 than the failure group, but the difference was not significant. On the GERD-HRQL, treatment-failure patients reported that overall heartburn and either heartburn or bloating while lying down were the symptoms they found most annoying on a daily basis.
Among the treatment-failure patients, 10 (62%) had normal acid exposure and negative symptom-reflux association, 2 patients (13%) had normal acid exposure and positive symptom-reflux association, and 4 patients (25%) had abnormal esophageal acid exposure. Patients in the treatment failure group reported a total of 315 episodes of either heartburn or regurgitation.
Endoscopy findings were normal in most of the patients in both groups; 81% of the treatment-failure patients and 69% of the treatment-success patients had normal upper endoscopy findings. Abnormal findings in the treatment-success group included one case of erosive esophagitis, two cases of Barrett’s esophagus, three cases of nonobstructive Schatzki rings, and five cases of hiatal hernia. Abnormal findings in the treatment-failure group included two cases of Schatzki rings, one case of esophageal stricture, and three cases of hiatal hernia.
The total number of reflux events was similar between the groups; 1,279 in the treatment-failure group and 1,099 in the treatment-success group, with the number of reflux events per patient averaging 80 and 84, respectively.
“Our results support the hypothesis that PPI failure is primarily driven by esophageal hypersensitivity,” the researchers noted. The similarity in impedance and reflux “implies that the shift to nonacidic reflux is a general PPI phenomenon, as opposed to being unique to PPI-failure patients,” they said.
The study was limited by the small patient population, but the results provide some insight into refractory GERD and suggest that patients who fail to respond to once-daily PPI might benefit from a neuromodulator, as well as psychological interventions including cognitive-behavioral therapy, hypnotherapy, relaxation techniques, mindfulness, and biofeedback, the researchers concluded.
Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
SOURCE: Abdallah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: PPI failure in GERD patients appears to be driven by esophageal hypersensitivity, not significantly associated with reflux.
Major finding: Most (75%) of the patients who failed PPI treatment had heartburn or reflux hypersensitivity with GERD.
Study details: The data come from a prospective cohort study of 29 adults with GERD.
Disclosures: Dr. Abdullah had no financial conflicts to disclose; a coauthor disclosed relationships with companies including Ironwood Pharmaceuticals, Mederi Therapeutics, and Ethicon Pharmaceuticals.
Source: Abdullah J et al. Clin Gastroenterol Hepatol. 2018; doi: 10.1016/j.cgh.2018.06.018.
Spurring innovation in digital health
Owing to digital advances, we’re experiencing a reimagination of health care delivery. Consumers are now empowered to take more control of their own health information to make better informed decisions about their medical care and healthy living. These advances enable better health outcomes for patients.
This opportunity is supported by a new technological paradigm of digital health tools, like apps, that enable consumers to have more active engagement and access to real-time information about their health and activities. These tools allow consumers and providers to supersede the traditional, physical constraints of health care delivery and make the most of the opportunities offered by mobile technology.
With these advances has come a new swath of companies that are investing in these new opportunities. These firms may be new to health care products and may not be accustomed to navigating the regulatory landscape that has traditionally surrounded these areas. A great example is the announcement of two mobile medical apps designed by Apple to work on the Apple Watch. One app creates an electrocardiogram, similar to traditional electrocardiograms, to detect the presence of atrial fibrillation and regular heart rhythm, while the other app analyzes pulse rate data to identify irregular heart rhythms suggestive of atrial fibrillation and notify the user. The FDA [Food and Drug Administration] worked closely with the company as they developed and tested these software products, which may help millions of users identify health concerns more quickly. Health care products on ubiquitous devices, like smartwatches, may help users seek treatment earlier and will truly empower them with more information about their health.
In the last few years, the FDA has been taking steps to encourage more development and greater innovation in the digital health space. With the launch of our Digital Health Innovation Action Plan last summer, we committed to implementing policies, adding expertise, and exploring a software precertification pilot program to bring clarity and efficiency to how we regulate digital health products.
This commitment is not only reflected in actions like approving or clearing new apps and launching our Digital Health Innovation Action Plan but also in what we hope to do in the future. That’s why in the FDA’s Fiscal Year 2019 Budget, we proposed to create a Center of Excellence for Digital Health that would advance modernizing our regulatory approach to help this industry grow and reach its full potential, while protecting patients.
Dr. Gottlieb is commissioner of the FDA and Dr. Shuren in director of the FDA Center for Devices and Radiological Health. Their comments are excerpted from an FDA statement released Sept. 12, 2018.
Owing to digital advances, we’re experiencing a reimagination of health care delivery. Consumers are now empowered to take more control of their own health information to make better informed decisions about their medical care and healthy living. These advances enable better health outcomes for patients.
This opportunity is supported by a new technological paradigm of digital health tools, like apps, that enable consumers to have more active engagement and access to real-time information about their health and activities. These tools allow consumers and providers to supersede the traditional, physical constraints of health care delivery and make the most of the opportunities offered by mobile technology.
With these advances has come a new swath of companies that are investing in these new opportunities. These firms may be new to health care products and may not be accustomed to navigating the regulatory landscape that has traditionally surrounded these areas. A great example is the announcement of two mobile medical apps designed by Apple to work on the Apple Watch. One app creates an electrocardiogram, similar to traditional electrocardiograms, to detect the presence of atrial fibrillation and regular heart rhythm, while the other app analyzes pulse rate data to identify irregular heart rhythms suggestive of atrial fibrillation and notify the user. The FDA [Food and Drug Administration] worked closely with the company as they developed and tested these software products, which may help millions of users identify health concerns more quickly. Health care products on ubiquitous devices, like smartwatches, may help users seek treatment earlier and will truly empower them with more information about their health.
In the last few years, the FDA has been taking steps to encourage more development and greater innovation in the digital health space. With the launch of our Digital Health Innovation Action Plan last summer, we committed to implementing policies, adding expertise, and exploring a software precertification pilot program to bring clarity and efficiency to how we regulate digital health products.
This commitment is not only reflected in actions like approving or clearing new apps and launching our Digital Health Innovation Action Plan but also in what we hope to do in the future. That’s why in the FDA’s Fiscal Year 2019 Budget, we proposed to create a Center of Excellence for Digital Health that would advance modernizing our regulatory approach to help this industry grow and reach its full potential, while protecting patients.
Dr. Gottlieb is commissioner of the FDA and Dr. Shuren in director of the FDA Center for Devices and Radiological Health. Their comments are excerpted from an FDA statement released Sept. 12, 2018.
Owing to digital advances, we’re experiencing a reimagination of health care delivery. Consumers are now empowered to take more control of their own health information to make better informed decisions about their medical care and healthy living. These advances enable better health outcomes for patients.
This opportunity is supported by a new technological paradigm of digital health tools, like apps, that enable consumers to have more active engagement and access to real-time information about their health and activities. These tools allow consumers and providers to supersede the traditional, physical constraints of health care delivery and make the most of the opportunities offered by mobile technology.
With these advances has come a new swath of companies that are investing in these new opportunities. These firms may be new to health care products and may not be accustomed to navigating the regulatory landscape that has traditionally surrounded these areas. A great example is the announcement of two mobile medical apps designed by Apple to work on the Apple Watch. One app creates an electrocardiogram, similar to traditional electrocardiograms, to detect the presence of atrial fibrillation and regular heart rhythm, while the other app analyzes pulse rate data to identify irregular heart rhythms suggestive of atrial fibrillation and notify the user. The FDA [Food and Drug Administration] worked closely with the company as they developed and tested these software products, which may help millions of users identify health concerns more quickly. Health care products on ubiquitous devices, like smartwatches, may help users seek treatment earlier and will truly empower them with more information about their health.
In the last few years, the FDA has been taking steps to encourage more development and greater innovation in the digital health space. With the launch of our Digital Health Innovation Action Plan last summer, we committed to implementing policies, adding expertise, and exploring a software precertification pilot program to bring clarity and efficiency to how we regulate digital health products.
This commitment is not only reflected in actions like approving or clearing new apps and launching our Digital Health Innovation Action Plan but also in what we hope to do in the future. That’s why in the FDA’s Fiscal Year 2019 Budget, we proposed to create a Center of Excellence for Digital Health that would advance modernizing our regulatory approach to help this industry grow and reach its full potential, while protecting patients.
Dr. Gottlieb is commissioner of the FDA and Dr. Shuren in director of the FDA Center for Devices and Radiological Health. Their comments are excerpted from an FDA statement released Sept. 12, 2018.
E/M comments may fall on deaf ears at CMS
Doctors’ dismay at the proposed flattening of evaluation and management (E/M) payments seems to be falling on deaf ears.
More than 170 medical societies and organizations expressed their concern about the new payment structure for E/M codes proposed as part of the 2019 Medicare Physician Fee Schedule (PFS) in comments on the draft rule.
Yet, in the final days of the comment period, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, took to Twitter to defend her agency’s plan.

The controversial proposal would set the payment rate for a level 1 E/M office visit for a new patient at $44, down from the current $45. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
Offsetting the changes in payment are several new proposed add-on codes, according to CMS.
Despite the lower payment for more complex patient care, Ms. Verma touted the scheme’s budget neutrality.

Ms. Verma’s tweets come as medical societies filed their formal complaints on the proposal, mirroring concerns expressed in two letters sent to the agency ahead of the comment deadline. The letters, sent at the end of August and between the two of them signed by more than 170 medical associations, aimed to preempt the comment process. They called for the E/M proposal to be rescinded, claiming that the cuts would reduce access to Medicare services by patients and hurt physicians that treat the sickest patients and those who provide comprehensive primary care because the expected lower reimbursement. One suggested that the changes exacerbate workforce shortages.
In its formal comments, the American Medical Association said that given “the groundswell of opposition from individual physicians and nearly every physician and health professional organization in the country, including the AMA, we ask that CMS set aside its proposal to restructure payment and coding for E/M office and other outpatient visits while an expert physician work group, with input from a broad spectrum of physicians and other health professionals, develops an alternative that could be implemented in 2020.”
The proposed E/M changes “are not an improvement over the current documentation requirements and payment structure. The structure is flawed, and the proposal to reduce payments when E/M services are reported with procedures fails to account for fee schedule reductions that have already been taken on these codes,” according to comments submitted by the American Academy of Dermatology Association.
The American Society of Clinical Oncology said it “supports the Agency’s proposal to reduce documentation burdens for E&M services but pairing it with reductions in payment will negatively impact patient access and should be avoided.”
ASCO also called on the agency to withdraw its proposal to consolidate E/M payments, noting that offsetting payments from add-on codes do “not appear to fully offset the direct and indirect cuts to oncology reimbursement, is ambiguous, and lacks assurances of long-term durability.”
Surgeons “cannot support the collapse of work RVU [relative value unit] values into one single rate under the [physician fee schedule] that would be paid for services using the current CPT codes for level 2 through 5 E/M visits because this single rate is a calculation of several values that were resourced-based, but in and of itself is not a resource-based value. There is no assurance that the underlying math used to derive this single value correctly reflects the resources used to deliver care across a wide spectrum of providers in America,” according to comments submitted by the American College of Surgeons.
ACS also argued that it is “not possible to fully analyze the repercussions and potential distortions to the PFS from these policies individually or taken as a whole during the 60-day comment period.” The comments noted that ACS favors documentation reduction efforts included in the proposal, but urged CMS to delay finalizing any E/M changes until more work can be done in tandem with stakeholders to craft a better solution.
The American College of Cardiology voiced support for the documentation reduction aspects of the E/M proposal but urged CMS to “not finalize any E/M payment changes for 2019. The Agency makes it clear it believes documentation proposals are intrinsically linked to the payment proposals. It is not clear to the ACC exactly why that must be the case.”
ACC also voiced concern over a provision that would halve the least-expensive procedure or the E/M visit code when a physician bills for both simultaneously. “No data are described to indicate that 50% is a correct reduction. Instead, it appears that CMS chose 50% because the reduction is equivalent to the 6.7 million RVUs needed to offset other proposed changes for compressing E/M payment into single levels and allowing use of the new add-on codes.”
A key concern for the American Academy of Family Physicians was collapsing the levels 2 through 5 E/M visits into a single payment level.
Instead, AAFP recommended that CMS work with it and other medical societies to develop new codes and values to ensure proper payment for services. Instead of a primary care add-on code, CMS should increase E/M payments by 15% for services provided “by physicians who list their primary practice designation as family medicine, internal medicine, or geriatrics,” according to the comments.
AAFP is “concerned that the changes included in the proposed rule may harm the quality and cost of care for Medicare beneficiaries,” the comment letter states, adding that it is “possible that beneficiary out-of-pocket costs would increase due to more frequent physician or clinician visits.”
The American College of Rheumatology voiced its support for the focus “on reducing physician burden by simplifying documentation requirements,” but said it had “serious concerns about the changes to evaluation and management (E/M) codes that result in cuts in reimbursement to cognitive specialists for the complex services they provide.” It added that while there is support for the documentation reduction efforts, “we are skeptical that this proposal will simplify the reporting burden on providers in the Quality Payment Program. As proposed, the new plan proposes several ‘add-on’ codes that would likely prevent reduction in audits or documentation.”
The estimated 51 hours per doctor per year of time saved “are insufficient to offset the proposed cuts to reimbursement. For example, if a physician sees around 100 patients a week, this translates to under 40 seconds per patient, which is not a benefit that outweighs the proposed reduction in reimbursement.”
The American College of Physicians voiced its opposition to the E/M proposal, noting that it “strongly believes that cognitive care of more complex patients must be appropriately recognized with higher allowed payment rates than less complex care patients.” ACP said the even with the proposed add-on codes, the proposed changes undervalue cognitive care for the most complex patients.
Doctors’ dismay at the proposed flattening of evaluation and management (E/M) payments seems to be falling on deaf ears.
More than 170 medical societies and organizations expressed their concern about the new payment structure for E/M codes proposed as part of the 2019 Medicare Physician Fee Schedule (PFS) in comments on the draft rule.
Yet, in the final days of the comment period, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, took to Twitter to defend her agency’s plan.

The controversial proposal would set the payment rate for a level 1 E/M office visit for a new patient at $44, down from the current $45. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
Offsetting the changes in payment are several new proposed add-on codes, according to CMS.
Despite the lower payment for more complex patient care, Ms. Verma touted the scheme’s budget neutrality.

Ms. Verma’s tweets come as medical societies filed their formal complaints on the proposal, mirroring concerns expressed in two letters sent to the agency ahead of the comment deadline. The letters, sent at the end of August and between the two of them signed by more than 170 medical associations, aimed to preempt the comment process. They called for the E/M proposal to be rescinded, claiming that the cuts would reduce access to Medicare services by patients and hurt physicians that treat the sickest patients and those who provide comprehensive primary care because the expected lower reimbursement. One suggested that the changes exacerbate workforce shortages.
In its formal comments, the American Medical Association said that given “the groundswell of opposition from individual physicians and nearly every physician and health professional organization in the country, including the AMA, we ask that CMS set aside its proposal to restructure payment and coding for E/M office and other outpatient visits while an expert physician work group, with input from a broad spectrum of physicians and other health professionals, develops an alternative that could be implemented in 2020.”
The proposed E/M changes “are not an improvement over the current documentation requirements and payment structure. The structure is flawed, and the proposal to reduce payments when E/M services are reported with procedures fails to account for fee schedule reductions that have already been taken on these codes,” according to comments submitted by the American Academy of Dermatology Association.
The American Society of Clinical Oncology said it “supports the Agency’s proposal to reduce documentation burdens for E&M services but pairing it with reductions in payment will negatively impact patient access and should be avoided.”
ASCO also called on the agency to withdraw its proposal to consolidate E/M payments, noting that offsetting payments from add-on codes do “not appear to fully offset the direct and indirect cuts to oncology reimbursement, is ambiguous, and lacks assurances of long-term durability.”
Surgeons “cannot support the collapse of work RVU [relative value unit] values into one single rate under the [physician fee schedule] that would be paid for services using the current CPT codes for level 2 through 5 E/M visits because this single rate is a calculation of several values that were resourced-based, but in and of itself is not a resource-based value. There is no assurance that the underlying math used to derive this single value correctly reflects the resources used to deliver care across a wide spectrum of providers in America,” according to comments submitted by the American College of Surgeons.
ACS also argued that it is “not possible to fully analyze the repercussions and potential distortions to the PFS from these policies individually or taken as a whole during the 60-day comment period.” The comments noted that ACS favors documentation reduction efforts included in the proposal, but urged CMS to delay finalizing any E/M changes until more work can be done in tandem with stakeholders to craft a better solution.
The American College of Cardiology voiced support for the documentation reduction aspects of the E/M proposal but urged CMS to “not finalize any E/M payment changes for 2019. The Agency makes it clear it believes documentation proposals are intrinsically linked to the payment proposals. It is not clear to the ACC exactly why that must be the case.”
ACC also voiced concern over a provision that would halve the least-expensive procedure or the E/M visit code when a physician bills for both simultaneously. “No data are described to indicate that 50% is a correct reduction. Instead, it appears that CMS chose 50% because the reduction is equivalent to the 6.7 million RVUs needed to offset other proposed changes for compressing E/M payment into single levels and allowing use of the new add-on codes.”
A key concern for the American Academy of Family Physicians was collapsing the levels 2 through 5 E/M visits into a single payment level.
Instead, AAFP recommended that CMS work with it and other medical societies to develop new codes and values to ensure proper payment for services. Instead of a primary care add-on code, CMS should increase E/M payments by 15% for services provided “by physicians who list their primary practice designation as family medicine, internal medicine, or geriatrics,” according to the comments.
AAFP is “concerned that the changes included in the proposed rule may harm the quality and cost of care for Medicare beneficiaries,” the comment letter states, adding that it is “possible that beneficiary out-of-pocket costs would increase due to more frequent physician or clinician visits.”
The American College of Rheumatology voiced its support for the focus “on reducing physician burden by simplifying documentation requirements,” but said it had “serious concerns about the changes to evaluation and management (E/M) codes that result in cuts in reimbursement to cognitive specialists for the complex services they provide.” It added that while there is support for the documentation reduction efforts, “we are skeptical that this proposal will simplify the reporting burden on providers in the Quality Payment Program. As proposed, the new plan proposes several ‘add-on’ codes that would likely prevent reduction in audits or documentation.”
The estimated 51 hours per doctor per year of time saved “are insufficient to offset the proposed cuts to reimbursement. For example, if a physician sees around 100 patients a week, this translates to under 40 seconds per patient, which is not a benefit that outweighs the proposed reduction in reimbursement.”
The American College of Physicians voiced its opposition to the E/M proposal, noting that it “strongly believes that cognitive care of more complex patients must be appropriately recognized with higher allowed payment rates than less complex care patients.” ACP said the even with the proposed add-on codes, the proposed changes undervalue cognitive care for the most complex patients.
Doctors’ dismay at the proposed flattening of evaluation and management (E/M) payments seems to be falling on deaf ears.
More than 170 medical societies and organizations expressed their concern about the new payment structure for E/M codes proposed as part of the 2019 Medicare Physician Fee Schedule (PFS) in comments on the draft rule.
Yet, in the final days of the comment period, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, took to Twitter to defend her agency’s plan.

The controversial proposal would set the payment rate for a level 1 E/M office visit for a new patient at $44, down from the current $45. Payment for levels 2-5 would be $135. Currently, payments for level 2 new patient visits are set at $76, level 3 at $110, level 4 at $167, and level 5 at $211.
For E/M office visits with established patients, the proposed rate would be $24 for level 1, up from the current $22. Payment for levels 2-5 would be $93. Under the current methodology, payments for established patient level 2 visits are set at $45, level 3 at $74, level 4 at $109, and level 5 at $148.
Offsetting the changes in payment are several new proposed add-on codes, according to CMS.
Despite the lower payment for more complex patient care, Ms. Verma touted the scheme’s budget neutrality.

Ms. Verma’s tweets come as medical societies filed their formal complaints on the proposal, mirroring concerns expressed in two letters sent to the agency ahead of the comment deadline. The letters, sent at the end of August and between the two of them signed by more than 170 medical associations, aimed to preempt the comment process. They called for the E/M proposal to be rescinded, claiming that the cuts would reduce access to Medicare services by patients and hurt physicians that treat the sickest patients and those who provide comprehensive primary care because the expected lower reimbursement. One suggested that the changes exacerbate workforce shortages.
In its formal comments, the American Medical Association said that given “the groundswell of opposition from individual physicians and nearly every physician and health professional organization in the country, including the AMA, we ask that CMS set aside its proposal to restructure payment and coding for E/M office and other outpatient visits while an expert physician work group, with input from a broad spectrum of physicians and other health professionals, develops an alternative that could be implemented in 2020.”
The proposed E/M changes “are not an improvement over the current documentation requirements and payment structure. The structure is flawed, and the proposal to reduce payments when E/M services are reported with procedures fails to account for fee schedule reductions that have already been taken on these codes,” according to comments submitted by the American Academy of Dermatology Association.
The American Society of Clinical Oncology said it “supports the Agency’s proposal to reduce documentation burdens for E&M services but pairing it with reductions in payment will negatively impact patient access and should be avoided.”
ASCO also called on the agency to withdraw its proposal to consolidate E/M payments, noting that offsetting payments from add-on codes do “not appear to fully offset the direct and indirect cuts to oncology reimbursement, is ambiguous, and lacks assurances of long-term durability.”
Surgeons “cannot support the collapse of work RVU [relative value unit] values into one single rate under the [physician fee schedule] that would be paid for services using the current CPT codes for level 2 through 5 E/M visits because this single rate is a calculation of several values that were resourced-based, but in and of itself is not a resource-based value. There is no assurance that the underlying math used to derive this single value correctly reflects the resources used to deliver care across a wide spectrum of providers in America,” according to comments submitted by the American College of Surgeons.
ACS also argued that it is “not possible to fully analyze the repercussions and potential distortions to the PFS from these policies individually or taken as a whole during the 60-day comment period.” The comments noted that ACS favors documentation reduction efforts included in the proposal, but urged CMS to delay finalizing any E/M changes until more work can be done in tandem with stakeholders to craft a better solution.
The American College of Cardiology voiced support for the documentation reduction aspects of the E/M proposal but urged CMS to “not finalize any E/M payment changes for 2019. The Agency makes it clear it believes documentation proposals are intrinsically linked to the payment proposals. It is not clear to the ACC exactly why that must be the case.”
ACC also voiced concern over a provision that would halve the least-expensive procedure or the E/M visit code when a physician bills for both simultaneously. “No data are described to indicate that 50% is a correct reduction. Instead, it appears that CMS chose 50% because the reduction is equivalent to the 6.7 million RVUs needed to offset other proposed changes for compressing E/M payment into single levels and allowing use of the new add-on codes.”
A key concern for the American Academy of Family Physicians was collapsing the levels 2 through 5 E/M visits into a single payment level.
Instead, AAFP recommended that CMS work with it and other medical societies to develop new codes and values to ensure proper payment for services. Instead of a primary care add-on code, CMS should increase E/M payments by 15% for services provided “by physicians who list their primary practice designation as family medicine, internal medicine, or geriatrics,” according to the comments.
AAFP is “concerned that the changes included in the proposed rule may harm the quality and cost of care for Medicare beneficiaries,” the comment letter states, adding that it is “possible that beneficiary out-of-pocket costs would increase due to more frequent physician or clinician visits.”
The American College of Rheumatology voiced its support for the focus “on reducing physician burden by simplifying documentation requirements,” but said it had “serious concerns about the changes to evaluation and management (E/M) codes that result in cuts in reimbursement to cognitive specialists for the complex services they provide.” It added that while there is support for the documentation reduction efforts, “we are skeptical that this proposal will simplify the reporting burden on providers in the Quality Payment Program. As proposed, the new plan proposes several ‘add-on’ codes that would likely prevent reduction in audits or documentation.”
The estimated 51 hours per doctor per year of time saved “are insufficient to offset the proposed cuts to reimbursement. For example, if a physician sees around 100 patients a week, this translates to under 40 seconds per patient, which is not a benefit that outweighs the proposed reduction in reimbursement.”
The American College of Physicians voiced its opposition to the E/M proposal, noting that it “strongly believes that cognitive care of more complex patients must be appropriately recognized with higher allowed payment rates than less complex care patients.” ACP said the even with the proposed add-on codes, the proposed changes undervalue cognitive care for the most complex patients.
The Flint Lock: A Novel Technique in Total Knee Arthroplasty Closure
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.
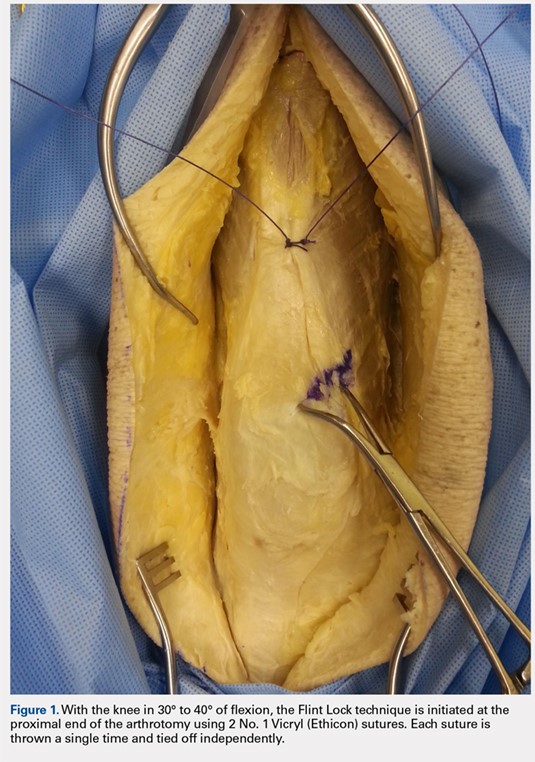
The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.
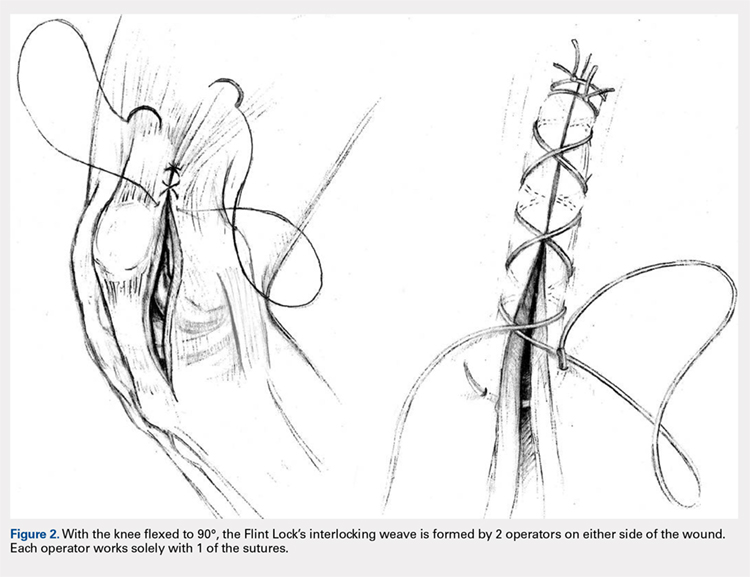
When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).
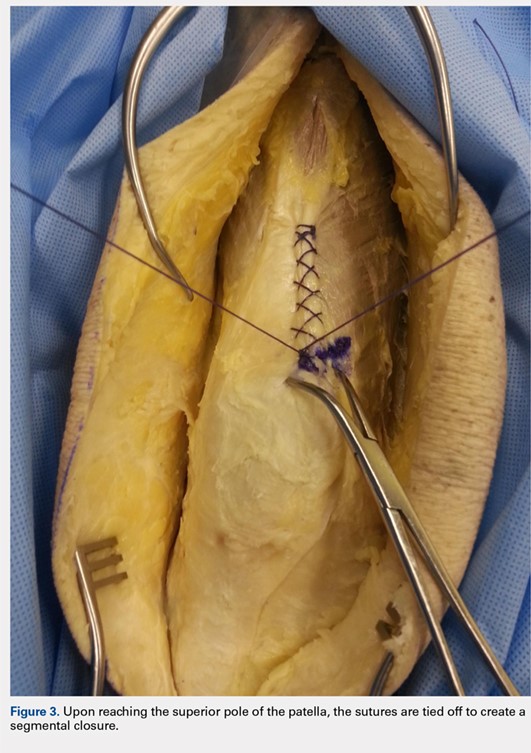
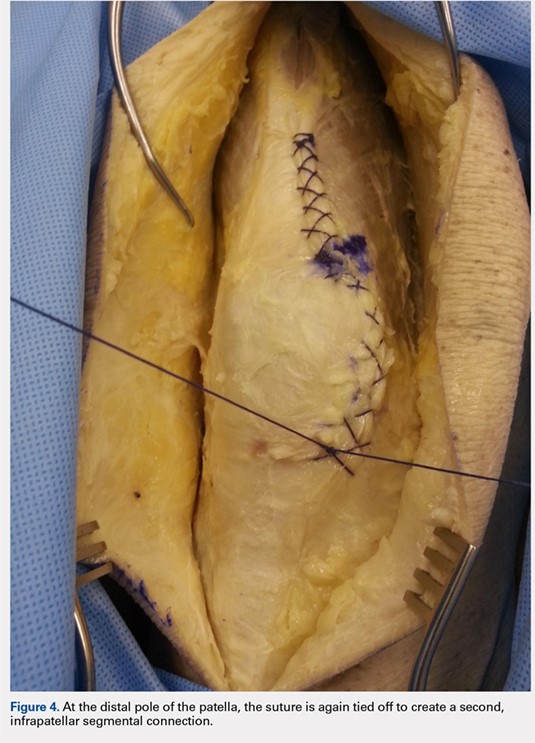
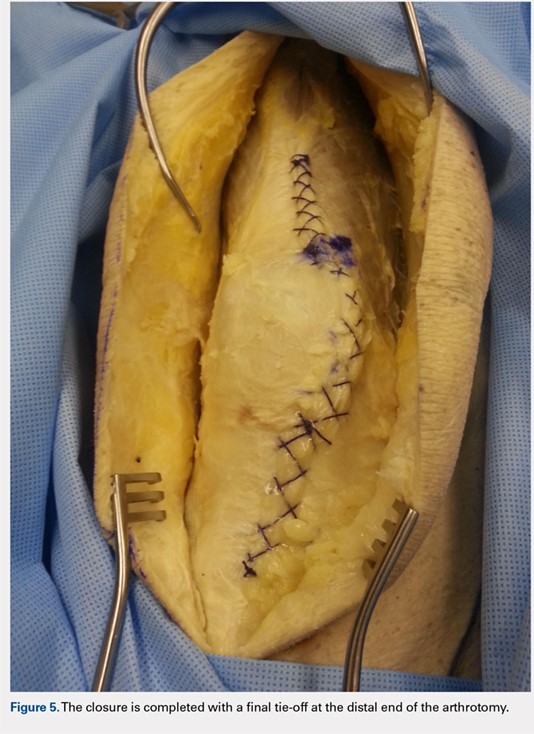
Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.

The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.

When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).



Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.

The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.

When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).



Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
TAKE-HOME POINTS
- The Flint Lock is a novel technique in TKA closure.
- Its continuous nature provides a tight seal with extensor mechanism closure.
- The utilization of a segmental closure with double suture provides a safeguard for suture failure.
- The suture used in the technique is less expensive than barbed suture.
- Future investigation is warranted to further validate the use of the Flint Lock.
The Effect of Insurance Type on Patient Access to Ankle Fracture Care Under the Affordable Care Act
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The purpose of this study is to assess the effect of insurance type (Medicaid, Medicare, private insurance) on the ability for patients with operative ankle fractures to access orthopedic traumatologists. The research team called 245 board-certified orthopedic surgeons specializing in orthopedic trauma within 8 representative states. The caller requested an appointment for their fictitious mother in order to be evaluated for an ankle fracture which was previously evaluated by her primary care physician and believed to require surgery. Each office was called 3 times to assess the response for each insurance type. For each call, information was documented regarding whether the patient was able to receive an appointment and the barriers the patient confronted to receive an appointment. Overall, 35.7% of offices scheduled an appointment for a patient with Medicaid, in comparison to 81.4%and 88.6% for Medicare and BlueCross, respectively (P < .0001). Medicaid patients confronted more barriers for receiving appointments. There was no statistically significant difference in access for Medicaid patients in states that had expanded Medicaid eligibility vs states that had not expanded Medicaid. Medicaid reimbursement for open reduction and internal fixation of an ankle fracture did not significantly correlate with appointment success rates or wait times. Despite the passage of the Affordable Care Act, patients with Medicaid have reduced access to orthopedic surgeons and more complex barriers to receiving appointments. A more robust strategy for increasing care-access for patients with Medicaid would be more equitable.
Continue to: In 2010, the Patient Protection and Affordable Care Act...
In 2010, the Patient Protection and Affordable Care Act (PPACA) expanded the eligibility criteria for Medicaid to all individuals with an income up to 138% of the poverty level.1 A Supreme Court ruling stated that the decision to expand Medicaid was to be decided by individual states.2 Currently, 31 states have chosen to expand Medicaid eligibility to their residents.2 This expansion has allowed an additional 11.7 million people to enroll in Medicaid and the Children’s Health Insurance Program by May 2015.3-5
Even with the passage of the PPACA, Medicaid patients seeking specialty orthopedic care have experienced more barriers to accessing care than Medicare or commercially-insured patients.2,6-10 One major cited reason is Medicaid’s low reimbursement, which may discourage physicians from open panel participation in Medicaid.11,12
A common fundamental teaching for orthopedic traumatologists is the notion that they should be available to treat all injuries regardless of the patient’s ability to pay.13 This has resulted in both trauma centers and trauma surgeons becoming financially challenged due to the higher proportion of Medicaid and uninsured trauma patients and lower Medicaid reimbursement levels.14,15
This study focuses on the effect of different types of insurance (Medicaid, Medicare, or commercial insurance) on the ability of patients to obtain care for operative ankle fractures. The purpose of this study is to evaluate, in the context of the PPACA, patient access to orthopedic surgeons for operative ankle fractures based on insurance-type. We hypothesized that patients with Medicaid would face a greater volume of obstacles when seeking appointments for an ankle fracture, even after the PPACA.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
The study population included board-certified orthopedic surgeons who belonged to the Orthopaedic Trauma Association (OTA) from 8 representative states; 4 states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and 4 states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas). These states were selected due to their ability to represent diverse healthcare marketplaces throughout the country. Using the OTA website’s “Find a Surgeon” search tool,16 we created a list of surgeons for each state and matched each surgeon with a random number. The list of surgeons was ordered according to the value of the surgeon’s associated random number, and surgeons were called in ascending order. We excluded disconnected or inaccurate numbers from the calling list. Surgeons who did not manage ankle fractures were removed from the dataset. Approximately 30 orthopedic trauma surgeons per state were contacted.
Each office was called to make an appointment for the caller’s mother. Every surgeon’s office was specifically asked if the surgeon would accept the patient to be evaluated for an ankle fracture that occurred out-of-state. The caller had a standardized protocol to limit intra- and inter-office variations (Appendix). The scenario involved a request to be evaluated for an unstable ankle fracture, with the patient having Medicaid, Medicare, or BlueCross insurance. The scenario required 3 separate calls to the same surgeon in order to obtain data regarding each insurance-type. The calls were separated by at least 1 week to avoid caller recognition by the surgeon’s office.
Appendix
Scenario
1. Date of Birth: Medicaid–2/07/55; BlueCross PPO–2/09/55; Medicare–7/31/45.
2. Ankle fracture evaluated by primary care physician 1 or 2 days ago
3. Not seen previously by your clinic or hospital, she would be a new patient
4. Asked how early she could be scheduled for an appointment
5. Script:
“I’m calling for my mother who injured her ankle a few days ago. Her family doctor took an X-ray and believes she has a fracture and needs surgery. Is Dr. X accepting new patients for evaluation and treatment of ankle fractures?” If YES →
“I was wondering if you take Medicaid/Medicare/BlueCross plan?” If YES →
“When is your soonest available appointment?”
The date of each phone call and date of appointment, if provided, were recorded. If the office did not give an appointment, we asked for reasons why. If an appointment was denied for a patient with Medicaid, we asked for a referral to another office that accepted Medicaid. We considered barriers to obtaining an initial appointment, such as requiring a referral from a primary care physician (PCP), as an unsuccessful attempt at making an appointment. We determined the waiting period for an appointment by calculating the time between the date of the call and the date of the appointment. Appointments were not scheduled to ensure that actual patients were not disadvantaged. For both appointment success rates and waiting periods, we stratified the data into 2 groups: states with expanded Medicaid eligibility (California, Massachusetts, New York, Ohio) and states without expanded Medicaid eligibility (Florida, North Carolina, Georgia, Texas).
We obtained Medicaid reimbursement rates for open reduction and internal fixation of an ankle fracture by querying each state’s reimbursement rate using Current Procedural Terminology code 27822.
Chi-square test or Fisher’s exact test was used to analyze acceptance rate differences based on the patient’s type of insurance. To compare the waiting periods for an appointment, we used an independent samples t-test after applying natural log-transformation, as the data was not normally distributed. We performed logistic regression analysis to detect whether reimbursement was a significant predictor of successfully making an appointment for patients, and a linear regression analysis was used to evaluate whether reimbursement predicted waiting periods. Unless otherwise stated, all statistical testing was performed two-tailed at an alpha-level of 0.05.
This study was approved by the Institutional Review Board of Yale University School of Medicine (HIC No. 1363).
Continue to: RESULTS...
RESULTS
In total, 350 offices were contacted across 8 states (4 states with and 4 states without expanded Medicaid eligibility) of which we identified 245 orthopedic surgeons who would surgically treat ankle fractures. The 245 surgeons’ offices were called 3 times for each separate insurance-type.
Table 1. Appointment Success Rate
| Medicaid | Medicare | Private |
All states |
|
| |
Yes (%) | 100 (35.7) | 228 (81.4) | 248 (88.6) |
No (%) | 180 (64.3) | 52 (18.60 | 32 (11.4) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 55 (39.6) | 116 (83.5) | 124 (89.2) |
No (%) | 84 (60.4) | 23 (16.5) | 15 (10.8) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 45 (31.9) | 112 (79.4) | 124 (87.9) |
No (%) | 96 (68.1) | 29 (20.6) | 17 (12.1) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross (Table 1). For states with expanded Medicaid eligibility, the success rate for obtaining an appointment was 39.6%, 83.5%, and 89.2% for Medicaid, Medicare, and BlueCross, respectively. For states without expanded Medicaid eligibility, the success rate for obtaining an appointment was 31.9% for Medicaid, 79.4% for Medicare, and 87.9% for BlueCross. In all cases, the success rate for obtaining an appointment was significantly lower for Medicaid, compared to Medicare (P < .0001) or BlueCross (P < .0001). Medicaid appointment success rate was 39.6% in expanded states vs 31.9% in non-expanded states, however, the difference was not statistically significant (Table 2).
Table 2. Medicaid Appointment Success Rate in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 55 (39.6) | 45 (31.9) | .181 |
No (%) | 84 (60.4) | 96 (68.1) |
|
In 43.7% of occasions, patients with Medicaid did not have their insurance accepted, compared to 7.3% for Medicare and 0% for BlueCross. The majority of offices which did not accept Medicaid were not able to refer patients to another surgeon who would accept Medicaid. The requirement to have a primary care referral was the second most common reason for Medicaid patients not obtaining an appointment. No Medicare (10.4% vs 0.0%, P < .0001) or BlueCross (10.4% vs 0.0%, P < .0001) patients experienced this requirement (Table 3). There was no difference found between the percent of Medicaid patients who were required to have referrals in states with and without expanded Medicaid eligibility (Table 4).
Table 3. Referral Rate
| Medicaid | Medicare | Private |
All states |
|
|
|
Yes (%) | 29 (10.4) | 0 (0) | 0 (0) |
No (%) | 251 (89.6) | 280 (100) | 280 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States with expanded Medicaid eligibility |
|
|
|
Yes (%) | 12 (8.6) | 0 (0) | 0 (0) |
No (%) | 127 (91.4) | 139 (100) | 139 (100) |
P-valuea |
| 0.0001 | 0.0001 |
States without expanded Medicaid eligibility |
|
|
|
Yes (%) | 17 (12.1) | 0 (0) | 0 (0) |
No (%) | 124 (87.9) | 141 (100) | 141 (100) |
P-valuea |
| 0.0001 | 0.0001 |
aComparison to Medicaid.
Table 4. Medicaid Referral Rates in Expanded Vs Non-Expanded States
| Expanded states | Non-expanded states | P-value |
Yes (%) | 12 (9.7) | 17 (14.0) | .35 |
No (%) | 127 (91.4) | 124 (87.9) |
|
Reimbursements for ankle fracture varied across states (Table 5). For Medicaid, Georgia paid the highest reimbursement ($1049.95) and Florida paid the lowest ($469.44). Logistic and linear regression analysis did not demonstrate a significant relationship between reimbursement and appointment success rate or waiting periods.
Table 5. Medicaid Reimbursements for Ankle Fracture Repair (CPT and HCPCS 27822) in 2014
State | Medicaid reimbursement |
Californiaa | $785.55 |
Texas | $678.95 |
Florida | $469.44 |
Ohioa | $617.08 |
New Yorka | $500.02 |
North Carolina | $621.63 |
Massachusettsa | $627.94 |
Georgia | $1,049.95 |
Average | $668.82 |
aStates with expanded Medicaid eligibility.
Abbreviations: CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
Waiting periods (Table 6) varied significantly by the type of insurance (7.3 days for Medicaid, 6.0 days for Medicare, and 6.0 days for BlueCross; P = .002). For states with expanded Medicaid eligibility, waiting periods varied significantly by insurance (7.7 days for Medicaid, 6.2 days for Medicare, P = .003; and 6.1 days for BlueCross, P = .01). Waiting periods did not vary significantly for states without expanded Medicaid. Additionally, waiting periods did not differ significantly when comparing between states with and without Medicaid expansion.
Table 6. Waiting Period (Days) by Insurance Type.
| Medicaid | Medicare | Private |
Comparison by Insurance Type |
|
|
|
All states |
|
|
|
Waiting period | 7.3 | 6.0 | 6.0 |
P-value |
| 0.002 | 0.002 |
States with expanded Medicaid eligibility |
|
|
|
Waiting period | 7.7 | 6.2 | 6.1 |
P-value |
| 0.003 | 0.01 |
States without expanded Medicaid eligibility |
|
|
|
Waiting period | 6.9 | 5.9 | 5.9 |
P-value |
| 0.15 | 0.15 |
Comparison by Medicaid Expansion |
|
|
|
States with expanded Medicaid eligibility | 7.7 | 6.2 | 6.1 |
States without expanded Medicaid eligibility | 6.9 | 5.9 | 5.9 |
P-value | 0.17 | 0.13 | 0.07 |
Continue to: DISCUSSION...
DISCUSSION
This study assessed how insurance type (Medicaid, Medicare, and BlueCross) affects patient access to orthopedic trauma surgeons in 8 geographically representative states. We selected unstable ankle fractures as they are basic fractures treated by nearly all trauma surgeons and should often be surgically treated to prevent serious long-term consequences. Our hypothesis stated that despite the passage of the PPACA, patients with Medicaid would have reduced access to care. As the PPACA has changed the healthcare marketplace by increasing the number of Medicaid enrollees, it is important to ensure that patient access to care improves.
This nationwide survey of orthopedic trauma surgeons demonstrates that Medicaid patients experience added barriers to care that ultimately results in lower rates of successfully obtaining care. This is consistent with other investigations which have assessed Medicaid patient healthcare access.6,8,10,17-19 This study did not demonstrate a statistically significant difference between Medicaid patients’ ability to obtain appointments in states with expanded Medicaid eligibility vs in states without expanded Medicaid eligibility (39.6% vs 31.9%, P < .18); this has been demonstrated in the literature.6
A barrier that was unique to Medicaid patients was the requirement to have a PCP referral (Table 3). A PCP referral was not a barrier to receiving an appointment for patients with Medicare or BlueCross. One reason to explain why Medicaid patients may be required to have PCP referrals is due to their increased medical complexity, extra documentation requirements, and low reimbursement.4 Patients who have obtained a PCP referral may be characterized as being more medically compliant.
It is important to note that the Medicaid policies for 4 states included in this study (Massachusetts, North Carolina, Texas, and New York) required a PCP referral in order to see a specialist. However, we found that many orthopedic trauma practices in these states scheduled appointments for Medicaid patients without a PCP referral, suggesting that the decision depended on individual policy. In addition, the majority of offices within these states cited that they simply did not accept Medicaid as an insurance policy, and not that they required a referral.
Our regression analysis did not find a significant relationship between being able to successfully obtain an appointment to be evaluated for an ankle fracture and reimbursement rates for Medicaid. Although studies have stressed the importance of Medicaid reimbursements on physician participation, this result is consistent with previous studies regarding carpal tunnel release and total ankle replacements.17,19 Long20 suggested that although reimbursements may help, additional strategies for promoting Medicaid acceptance may be needed, including: lowering the costs of participating in Medicaid by simplifying administrative processes, speeding up reimbursement, and reducing the costs associated with caring for those patients.
Continue to: Previous studies have demonstrated...
Previous studies have demonstrated that more physicians may accept Medicaid if reimbursements increased.4,12 Given the high percentage of trauma patients with Medicaid as their primary insurance or whom are emergently enrolled in Medicaid by hospital systems, it is concerning that the PPACA is reducing payments under the Medicare and Medicaid Disproportionate Share Hospital programs which provide hospitals for uncompensated care given to low-income and uninsured patients.21 Trauma centers generally operate at a deficit due to the higher proportion of Medicaid and uninsured patients.14 This is currently worsened by additional federal funding cuts for supporting trauma service’s humane mission.21
This study has several limitations. While the study evaluated access to care in 8 representative states, a thorough nationwide survey would be more representative. Some results may have become statistically significant if we had performed the study with a larger sample size. In addition, we were unable to control for many factors which could impact appointment wait times, such as physician call schedules and vacations. Socioeconomic factors can influence a patient’s ability to attend an appointment, such as transportation costs, time off from work, and childcare availability. In addition, this study did not assess access for the uninsured, who are predominantly the working poor who cannot afford health insurance, even with federal and state subsidies.
The authors apologize for inconveniencing these offices, however, data collection could not be achieved in a better manner. We hope that the value of this study compensates any inconvenience.
CONCLUSION
Overall, our results demonstrate that despite the ratification of the PPACA, Medicaid patients are confronted with more barriers to accessing care by comparison to patients with Medicare and BlueCross insurance. Medicaid patients have worse baseline health22 and are at an increased risk of complications. These disparities are thought to be due to decreased healthcare access,23,24 as well as socioeconomic challenges. Interventions, such as increasing Medicaid’s reimbursement levels, reducing burdensome administrative responsibilities, and establishing partnerships between trauma centers and trauma surgeons, may enable underinsured patients to be appropriately cared for.
This paper will be judged for the Resident Writer’s Award.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
1. Blumenthal D, Collins SR. Health care coverage under the affordable care act--a progress report. N Engl J Med. 2014;371(3):275-281. doi:10.1056/NEJMhpr1405667.
2. Sommers BD. Health care reform's unfinished work--remaining barriers to coverage and access. N Engl J Med. 2015;373(25):2395-2397. doi:10.1056/NEJMp1509462.
3. US Department of Health and Human Services. Centers for Medicare & Medicaid Services. Medicaid & CHIP: February 2015 monthly applications, eligibility determinations and enrollment report. https://www.medicaid.gov/medicaid/program-information/downloads/medicaid-and-chip-february-2015-application-eligibility-and-enrollment-data.pdf. Published May 1, 2015. Accessed May 2015.
4. Iglehart JK, Sommers BD. Medicaid at 50--from welfare program to nation's largest health insurer. N Engl J Med. 2015;372(22):2152-2159. doi:10.1056/NEJMhpr1500791.
5. Kaiser Family Foundation. Medicaid moving forward. http://kff.org/medicaid/fact-sheet/the-medicaid-program-at-a-glance-update/. Updated 2014. Accessed October 10, 2014.
6. Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the affordable care act. J Arthroplasty. 2015;30(9):1498-1501. doi:10.1016/j.arth.2015.03.015.
7. Draeger RW, Patterson BM, Olsson EC, Schaffer A, Patterson JM. The influence of patient insurance status on access to outpatient orthopedic care for flexor tendon lacerations. J Hand Surg Am. 2014;39(3):527-533. doi:10.1016/j.jhsa.2013.10.031.
8. Patterson BM, Spang JT, Draeger RW, Olsson EC, Creighton RA, Kamath GV. Access to outpatient care for adult rotator cuff patients with private insurance versus Medicaid in North Carolina. J Shoulder Elbow Surg. 2013;22(12):1623-1627. doi:10.1016/j.jse.2013.07.051.
9. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
10. Schwarzkopf R, Phan D, Hoang M, Ross S, Mukamel D. Do patients with income-based insurance have access to total joint arthroplasty? J Arthroplasty. 2014;29(6):1083-1086. doi:10.1016/j.arth.2013.11.022.
11. Decker SL. In 2011 nearly one-third of physicians said they would not accept new Medicaid patients, but rising fees may help. Health Aff (Millwood). 2012;31(8):1673-1679 doi:10.1377/hlthaff.2012.0294.
12. Perloff JD, Kletke P, Fossett JW. Which physicians limit their Medicaid participation, and why. Health Serv Res. 1995;30(1):7-26.
13. Althausen PL. Building a successful trauma practice in a community setting. J Orthop Trauma. 2011;25 Suppl 3:S113-S117. doi:10.1097/BOT.0b013e318237bcce.
14. Greenberg S, Mir HR, Jahangir AA, Mehta S, Sethi MK. Impacting policy change for orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S14-S16. doi:10.1097/BOT.0000000000000216.
15. Wiznia DH, Averbukh L, Kim CY, Goel A, Leslie MP. Motorcycle helmets: The economic burden of an incomplete helmet law to medical care in the state of Connecticut. Conn Med. 2015;79(8):453-459.
16. Orthopaedic Trauma Association. Find a surgeon. https://online.ota.org/otassa/otacenssafindasurgeon.query_page. Updated 2015. Accessed July, 2015.
17. Kim CY, Wiznia DH, Roth AS, Walls RJ, Pelker RR. Survey of patient insurance status on access to specialty foot and ankle care under the affordable care act. Foot Ankle Int. 2016;37(7):776-781. doi:1071100716642015.
18. Patterson BM, Draeger RW, Olsson EC, Spang JT, Lin FC, Kamath GV. A regional assessment of Medicaid access to outpatient orthopaedic care: the influence of population density and proximity to academic medical centers on patient access. J Bone Joint Surg Am. 2014;96(18):e156. doi:10.2106/JBJS.M.01188.
19. Kim CY, Wiznia DH, Wang Y, et al. The effect of insurance type on patient access to carpal tunnel release under the affordable care act. J Hand Surg Am. 2016;41(4):503-509.e1. doi:S0363-5023(16)00104-0.
20. Long SK. Physicians may need more than higher reimbursements to expand Medicaid participation: findings from Washington state. Health Aff (Millwood). 2013;32(9):1560-1567. doi:10.1377/hlthaff.2012.1010.
21. Issar NM, Jahangir AA. The affordable care act and orthopaedic trauma. J Orthop Trauma. 2014;28 Suppl 10:S5-S7. doi:10.1097/BOT.0000000000000211.
22. Hahn B, Flood AB. No insurance, public insurance, and private insurance: do these options contribute to differences in general health? J Health Care Poor Underserved. 1995;6(1):41-59.
23. Hinman A, Bozic KJ. Impact of payer type on resource utilization, outcomes and access to care in total hip arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):9-14. doi:10.1016/j.arth.2008.05.010.
24. Schoenfeld AJ, Tipirneni R, Nelson JH, Carpenter JE, Iwashyna TJ. The influence of race and ethnicity on complications and mortality after orthopedic surgery: A systematic review of the literature. Med Care. 2014;52(9):842-851. doi:10.1097/MLR.0000000000000177.
TAKE-HOME POINTS
- One method in which the PPACA increased the number of individuals with health insurance coverage was by expanding Medicaid eligibility requirements.
- Despite this, Medicaid patients confronted more barriers to accessing care.
- The overall rate of successfully being offered an appointment with Medicaid was 35.7%, 81.4% for Medicare, and 88.6% for BlueCross. Patients with Medicaid also confronted longer appointment wait times.
- The disparity in access for this operative trauma scenario suggests that patients with Medicaid are likely to be excluded from the practice of their choice and may need to make considerably more effort to secure an appointment.
- Ultimately, Medicaid patients may have access to care through federally funded community health centers and public and non-profit safety net hospitals, which generally care for more uninsured and Medicaid patient populations.