User login
Brown spot on cheek
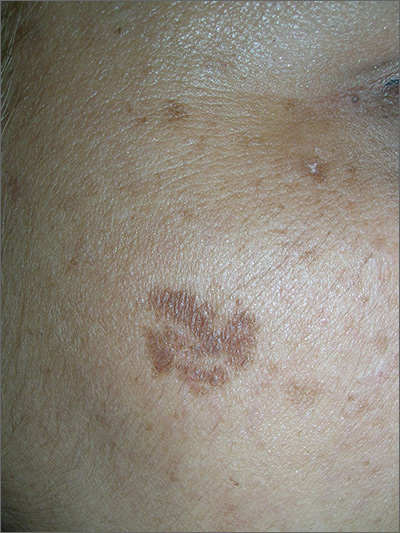
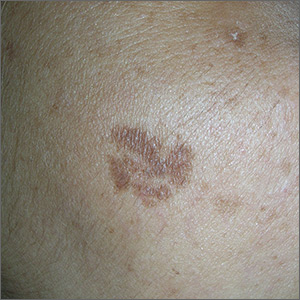
The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Dr. Valerie W. Rusch to receive ACS Distinguished Service Award
The Board of Regents of the American College of Surgeons (ACS) has selected Valerie W. Rusch, MD, FACS, an esteemed thoracic surgeon in New York, NY, as the recipient of the 2018 Distinguished Service Award (DSA)--the College's highest honor. The Board of Regents will present the award to Dr. Rusch, vice-chair, clinical research, department of surgery; Miner Family Chair in Intrathoracic Cancers; attending surgeon, thoracic service, department of surgery, Memorial Sloan-Kettering Cancer Center; and professor of surgery, Weill Cornell Medical College, New York, NY, at the Convocation ceremony at 6:00 pm October 21 at the Clinical Congress 2018 in Boston, MA.
The Board of Regents is presenting the DSA to Dr. Rusch for "her exemplary leadership of many professional organizations and as a mentor, teacher, and trainer of the next generation of surgeons in clinical trial development and her dedication to expand access to surgical care to underserved global populations," according to the award citation.
The award also is being presented to Dr. Rusch "in admiration of her natural leadership, integrity, vision, and steadfast commitment to the College's initiatives and principles, serving as a role model to surgeons everywhere to always do the right thing for patients."
Leadership in the ACS
An ACS Fellow since 1986, Dr. Rusch has led several prominent ACS bodies, including serving as Chair of the Board of Governors (2006−2008) and Board of Regents (2015−2016). A Regent from 2008 to 2017, she chaired the Central Judiciary Committee (2009–2013), the Program Committee (2011–2017), the Board of Regents Nominating Committee (2011–2012), and the Committee on Global Engagement (2016−2017). She served on the Board of Regents Honors Committee (2012−2016), Executive Committee (2013−2016), and Finance Committee (2014−2016).
In addition, she has been a member of the College’s Advisory Council for Cardiothoracic Surgery (2002−2017), International Relations Committee (2007−2013, Executive Committee, 2009−2012), Commission on Cancer Executive Committee (2012−2017), Scholarships Committee (2008−2012), and Research and Optimal Patient Care Committee (2008−2015).
Renowned thoracic surgeon
Dr. Rusch specializes in the diagnosis and treatment of patients with cancers of the lung, airways (trachea, bronchi), esophagus, mediastinum, chest wall, and pleura (malignant pleural mesothelioma). She was among the first women in the U.S. to be board certified in thoracic surgery.
For more than 30 years, she has emphasized a multidisciplinary approach to treating patients with thoracic malignancy. Her research has focused on the molecular behaviors of asbestos cancers and the genetic tendencies of lung cancer as a means to identify certain cancers in the earlier stages.
Dr. Rusch has been a leader in national and international clinical trials for the treatment of thoracic malignancies and played a pivotal role in establishing the ACS Oncology Group--now the ACS Clinical Research Program. Among her many honors, in 2007, Dr. Rusch received the Thoracic Surgery Foundation for Research and Education Socrates Award, and in 2012, the Association of Women Surgeons awarded her the Nina Starr Braunwald Award for lifetime contributions to the advancement of women in surgery.
She has held 25 visiting professorships and lectureships and given more than 300 major lectures on thoracic cancers at medical conferences around the world. Her curriculum vitae boasts more than 400 peer-reviewed publications.
In addition to the ACS, Dr. Rusch has been a leader of other surgical organizations. More specifically, she served as chair of the American Board of Thoracic Surgery, chair of the Lung and Esophagus Task Force of the American Joint Commission on Cancer, and chair of the Mesothelioma Subcommittee of the International Association for the Study of Lung Cancer Staging Committee.
Dr. Rusch is fluent in both French and English, having graduated from the Lycée Français de New York. She graduated from Vassar College, Poughkeepsie, NY, with a degree in biochemistry. She earned her medical degree from the Columbia University College of Physicians and Surgeons, New York, and she completed surgical residency training in general surgery and thoracic surgery at the University of Washington, Seattle, followed by a fellowship at the University of Texas MD Anderson Cancer Center, Houston.
The Board of Regents of the American College of Surgeons (ACS) has selected Valerie W. Rusch, MD, FACS, an esteemed thoracic surgeon in New York, NY, as the recipient of the 2018 Distinguished Service Award (DSA)--the College's highest honor. The Board of Regents will present the award to Dr. Rusch, vice-chair, clinical research, department of surgery; Miner Family Chair in Intrathoracic Cancers; attending surgeon, thoracic service, department of surgery, Memorial Sloan-Kettering Cancer Center; and professor of surgery, Weill Cornell Medical College, New York, NY, at the Convocation ceremony at 6:00 pm October 21 at the Clinical Congress 2018 in Boston, MA.
The Board of Regents is presenting the DSA to Dr. Rusch for "her exemplary leadership of many professional organizations and as a mentor, teacher, and trainer of the next generation of surgeons in clinical trial development and her dedication to expand access to surgical care to underserved global populations," according to the award citation.
The award also is being presented to Dr. Rusch "in admiration of her natural leadership, integrity, vision, and steadfast commitment to the College's initiatives and principles, serving as a role model to surgeons everywhere to always do the right thing for patients."
Leadership in the ACS
An ACS Fellow since 1986, Dr. Rusch has led several prominent ACS bodies, including serving as Chair of the Board of Governors (2006−2008) and Board of Regents (2015−2016). A Regent from 2008 to 2017, she chaired the Central Judiciary Committee (2009–2013), the Program Committee (2011–2017), the Board of Regents Nominating Committee (2011–2012), and the Committee on Global Engagement (2016−2017). She served on the Board of Regents Honors Committee (2012−2016), Executive Committee (2013−2016), and Finance Committee (2014−2016).
In addition, she has been a member of the College’s Advisory Council for Cardiothoracic Surgery (2002−2017), International Relations Committee (2007−2013, Executive Committee, 2009−2012), Commission on Cancer Executive Committee (2012−2017), Scholarships Committee (2008−2012), and Research and Optimal Patient Care Committee (2008−2015).
Renowned thoracic surgeon
Dr. Rusch specializes in the diagnosis and treatment of patients with cancers of the lung, airways (trachea, bronchi), esophagus, mediastinum, chest wall, and pleura (malignant pleural mesothelioma). She was among the first women in the U.S. to be board certified in thoracic surgery.
For more than 30 years, she has emphasized a multidisciplinary approach to treating patients with thoracic malignancy. Her research has focused on the molecular behaviors of asbestos cancers and the genetic tendencies of lung cancer as a means to identify certain cancers in the earlier stages.
Dr. Rusch has been a leader in national and international clinical trials for the treatment of thoracic malignancies and played a pivotal role in establishing the ACS Oncology Group--now the ACS Clinical Research Program. Among her many honors, in 2007, Dr. Rusch received the Thoracic Surgery Foundation for Research and Education Socrates Award, and in 2012, the Association of Women Surgeons awarded her the Nina Starr Braunwald Award for lifetime contributions to the advancement of women in surgery.
She has held 25 visiting professorships and lectureships and given more than 300 major lectures on thoracic cancers at medical conferences around the world. Her curriculum vitae boasts more than 400 peer-reviewed publications.
In addition to the ACS, Dr. Rusch has been a leader of other surgical organizations. More specifically, she served as chair of the American Board of Thoracic Surgery, chair of the Lung and Esophagus Task Force of the American Joint Commission on Cancer, and chair of the Mesothelioma Subcommittee of the International Association for the Study of Lung Cancer Staging Committee.
Dr. Rusch is fluent in both French and English, having graduated from the Lycée Français de New York. She graduated from Vassar College, Poughkeepsie, NY, with a degree in biochemistry. She earned her medical degree from the Columbia University College of Physicians and Surgeons, New York, and she completed surgical residency training in general surgery and thoracic surgery at the University of Washington, Seattle, followed by a fellowship at the University of Texas MD Anderson Cancer Center, Houston.
The Board of Regents of the American College of Surgeons (ACS) has selected Valerie W. Rusch, MD, FACS, an esteemed thoracic surgeon in New York, NY, as the recipient of the 2018 Distinguished Service Award (DSA)--the College's highest honor. The Board of Regents will present the award to Dr. Rusch, vice-chair, clinical research, department of surgery; Miner Family Chair in Intrathoracic Cancers; attending surgeon, thoracic service, department of surgery, Memorial Sloan-Kettering Cancer Center; and professor of surgery, Weill Cornell Medical College, New York, NY, at the Convocation ceremony at 6:00 pm October 21 at the Clinical Congress 2018 in Boston, MA.
The Board of Regents is presenting the DSA to Dr. Rusch for "her exemplary leadership of many professional organizations and as a mentor, teacher, and trainer of the next generation of surgeons in clinical trial development and her dedication to expand access to surgical care to underserved global populations," according to the award citation.
The award also is being presented to Dr. Rusch "in admiration of her natural leadership, integrity, vision, and steadfast commitment to the College's initiatives and principles, serving as a role model to surgeons everywhere to always do the right thing for patients."
Leadership in the ACS
An ACS Fellow since 1986, Dr. Rusch has led several prominent ACS bodies, including serving as Chair of the Board of Governors (2006−2008) and Board of Regents (2015−2016). A Regent from 2008 to 2017, she chaired the Central Judiciary Committee (2009–2013), the Program Committee (2011–2017), the Board of Regents Nominating Committee (2011–2012), and the Committee on Global Engagement (2016−2017). She served on the Board of Regents Honors Committee (2012−2016), Executive Committee (2013−2016), and Finance Committee (2014−2016).
In addition, she has been a member of the College’s Advisory Council for Cardiothoracic Surgery (2002−2017), International Relations Committee (2007−2013, Executive Committee, 2009−2012), Commission on Cancer Executive Committee (2012−2017), Scholarships Committee (2008−2012), and Research and Optimal Patient Care Committee (2008−2015).
Renowned thoracic surgeon
Dr. Rusch specializes in the diagnosis and treatment of patients with cancers of the lung, airways (trachea, bronchi), esophagus, mediastinum, chest wall, and pleura (malignant pleural mesothelioma). She was among the first women in the U.S. to be board certified in thoracic surgery.
For more than 30 years, she has emphasized a multidisciplinary approach to treating patients with thoracic malignancy. Her research has focused on the molecular behaviors of asbestos cancers and the genetic tendencies of lung cancer as a means to identify certain cancers in the earlier stages.
Dr. Rusch has been a leader in national and international clinical trials for the treatment of thoracic malignancies and played a pivotal role in establishing the ACS Oncology Group--now the ACS Clinical Research Program. Among her many honors, in 2007, Dr. Rusch received the Thoracic Surgery Foundation for Research and Education Socrates Award, and in 2012, the Association of Women Surgeons awarded her the Nina Starr Braunwald Award for lifetime contributions to the advancement of women in surgery.
She has held 25 visiting professorships and lectureships and given more than 300 major lectures on thoracic cancers at medical conferences around the world. Her curriculum vitae boasts more than 400 peer-reviewed publications.
In addition to the ACS, Dr. Rusch has been a leader of other surgical organizations. More specifically, she served as chair of the American Board of Thoracic Surgery, chair of the Lung and Esophagus Task Force of the American Joint Commission on Cancer, and chair of the Mesothelioma Subcommittee of the International Association for the Study of Lung Cancer Staging Committee.
Dr. Rusch is fluent in both French and English, having graduated from the Lycée Français de New York. She graduated from Vassar College, Poughkeepsie, NY, with a degree in biochemistry. She earned her medical degree from the Columbia University College of Physicians and Surgeons, New York, and she completed surgical residency training in general surgery and thoracic surgery at the University of Washington, Seattle, followed by a fellowship at the University of Texas MD Anderson Cancer Center, Houston.
Produce and promises
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
New insight into celiac disease: What you should know
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
IgA vasculitis may be more common in adults than assumed
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
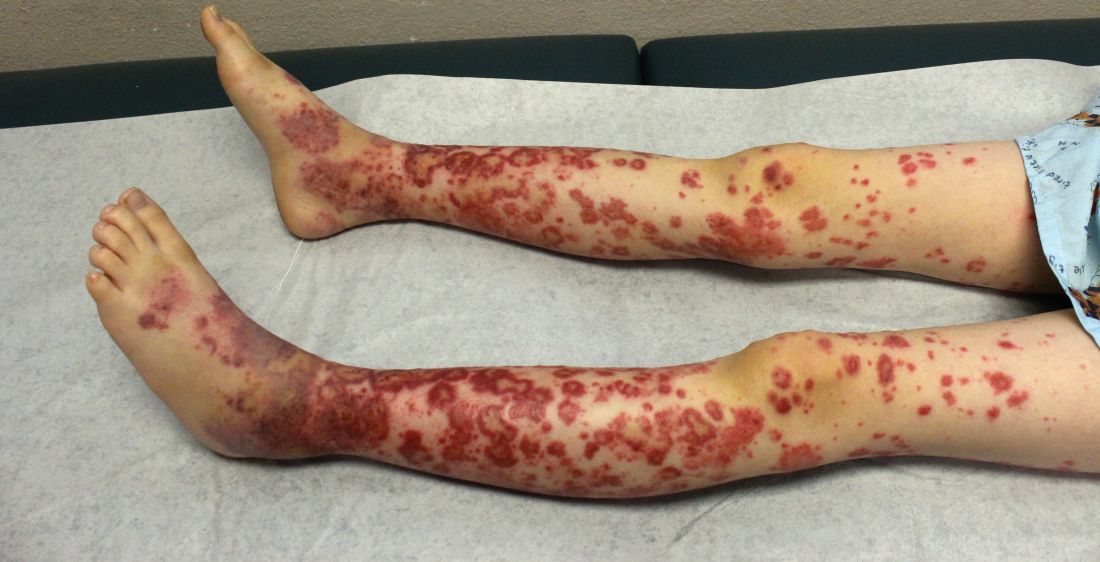
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Global bronchial thermoplasty registry for asthma documents benefits
PARIS – A global registry to track the safety and efficacy of shows benefits comparable to those previously reported in randomized trials, according to 1-year results presented at the annual congress of the European Respiratory Society.
Bronchothermoplasty has been Food and Drug Administration approved since 2010, but joint 2014 guidelines from the ERS and the American Thoracic Society (ATS) recommended that this procedure be restricted to patients participating in a registry, making these findings an important part of an ongoing assessment, according to Alfons Torrego Fernández, MD, of the pulmonology service at Hospital de la Santa Creu i Sant Pau, Barcelona.
The BT Global Registry (BTGR), created at the end of 2014, involves 18 centers in Europe, South Africa, and Australia. Dr. Fernandez provided data on 123 of the 157 patients enrolled by the end of 2016. All had at least 1 year of follow-up.
Compared with the year prior to bronchial thermoplasty, the proportion of patients with severe exacerbations in the year following this procedure fell from 90.3% to 59.6%, a 34% reduction (P less than .001). The proportion of patients requiring oral corticosteroids fell from 47.8% to 23.5%, a reduction of more than 50%.
Relative to the year prior to bronchial thermoplasty, “there was also a reduction in emergency room visits [21.1% vs. 54.6%] and hospitalizations [20.2% vs. 43%] as well as a reduction in the need for asthma maintenance medications,” Dr. Fernandez reported.
On the Asthma Control Questionnaire (ACQ), quality of life (QOL) was improved on average by 1.2 points from the prior year (4.48 vs. 3.26; P less than .05), according to Dr. Fernandez. The proportion of patients who achieved at least a 0.5-point increase in the ACQ, a level that Dr. Fernandez said is considered clinically relevant, was 67.1%.
However, when lung function measures such as forced expiratory volume in one second and fractional exhaled nitric oxide taken 1 year after bronchothermoplasty were compared with the same measures taken prior to this treatment, there was no significant improvement, according to Dr. Fernandez.
Bronchial thermoplasty involves the use of thermal energy delivered through a bronchoscope to reduce airway smooth muscle mass, thereby eliminating a source of airway restriction. Although nearly 70% of severe asthma patients in this BTGR derived an improvement in quality of life, 30% did not. Asked if the registry has provided any insight about who does or does not respond, Dr. Fernandez acknowledged that this is “the key question,” but added that “no specific profile or biomarker” has yet been identified.
“These are early results, but a 2-year follow-up is planned,” he said.
Although the technical aspects of bronchial thermoplasty “are quite well standardized,” Dr. Fernandez acknowledged that there might be a learning curve that favors experienced operators. He reported that outcomes between high- and low-volume centers in BTGR have not yet been compared. However, he maintained that “these results in real-life patients confirm that severe asthma patients can benefit” from this therapy as shown previously in sham-controlled trials.
Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
PARIS – A global registry to track the safety and efficacy of shows benefits comparable to those previously reported in randomized trials, according to 1-year results presented at the annual congress of the European Respiratory Society.
Bronchothermoplasty has been Food and Drug Administration approved since 2010, but joint 2014 guidelines from the ERS and the American Thoracic Society (ATS) recommended that this procedure be restricted to patients participating in a registry, making these findings an important part of an ongoing assessment, according to Alfons Torrego Fernández, MD, of the pulmonology service at Hospital de la Santa Creu i Sant Pau, Barcelona.
The BT Global Registry (BTGR), created at the end of 2014, involves 18 centers in Europe, South Africa, and Australia. Dr. Fernandez provided data on 123 of the 157 patients enrolled by the end of 2016. All had at least 1 year of follow-up.
Compared with the year prior to bronchial thermoplasty, the proportion of patients with severe exacerbations in the year following this procedure fell from 90.3% to 59.6%, a 34% reduction (P less than .001). The proportion of patients requiring oral corticosteroids fell from 47.8% to 23.5%, a reduction of more than 50%.
Relative to the year prior to bronchial thermoplasty, “there was also a reduction in emergency room visits [21.1% vs. 54.6%] and hospitalizations [20.2% vs. 43%] as well as a reduction in the need for asthma maintenance medications,” Dr. Fernandez reported.
On the Asthma Control Questionnaire (ACQ), quality of life (QOL) was improved on average by 1.2 points from the prior year (4.48 vs. 3.26; P less than .05), according to Dr. Fernandez. The proportion of patients who achieved at least a 0.5-point increase in the ACQ, a level that Dr. Fernandez said is considered clinically relevant, was 67.1%.
However, when lung function measures such as forced expiratory volume in one second and fractional exhaled nitric oxide taken 1 year after bronchothermoplasty were compared with the same measures taken prior to this treatment, there was no significant improvement, according to Dr. Fernandez.
Bronchial thermoplasty involves the use of thermal energy delivered through a bronchoscope to reduce airway smooth muscle mass, thereby eliminating a source of airway restriction. Although nearly 70% of severe asthma patients in this BTGR derived an improvement in quality of life, 30% did not. Asked if the registry has provided any insight about who does or does not respond, Dr. Fernandez acknowledged that this is “the key question,” but added that “no specific profile or biomarker” has yet been identified.
“These are early results, but a 2-year follow-up is planned,” he said.
Although the technical aspects of bronchial thermoplasty “are quite well standardized,” Dr. Fernandez acknowledged that there might be a learning curve that favors experienced operators. He reported that outcomes between high- and low-volume centers in BTGR have not yet been compared. However, he maintained that “these results in real-life patients confirm that severe asthma patients can benefit” from this therapy as shown previously in sham-controlled trials.
Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
PARIS – A global registry to track the safety and efficacy of shows benefits comparable to those previously reported in randomized trials, according to 1-year results presented at the annual congress of the European Respiratory Society.
Bronchothermoplasty has been Food and Drug Administration approved since 2010, but joint 2014 guidelines from the ERS and the American Thoracic Society (ATS) recommended that this procedure be restricted to patients participating in a registry, making these findings an important part of an ongoing assessment, according to Alfons Torrego Fernández, MD, of the pulmonology service at Hospital de la Santa Creu i Sant Pau, Barcelona.
The BT Global Registry (BTGR), created at the end of 2014, involves 18 centers in Europe, South Africa, and Australia. Dr. Fernandez provided data on 123 of the 157 patients enrolled by the end of 2016. All had at least 1 year of follow-up.
Compared with the year prior to bronchial thermoplasty, the proportion of patients with severe exacerbations in the year following this procedure fell from 90.3% to 59.6%, a 34% reduction (P less than .001). The proportion of patients requiring oral corticosteroids fell from 47.8% to 23.5%, a reduction of more than 50%.
Relative to the year prior to bronchial thermoplasty, “there was also a reduction in emergency room visits [21.1% vs. 54.6%] and hospitalizations [20.2% vs. 43%] as well as a reduction in the need for asthma maintenance medications,” Dr. Fernandez reported.
On the Asthma Control Questionnaire (ACQ), quality of life (QOL) was improved on average by 1.2 points from the prior year (4.48 vs. 3.26; P less than .05), according to Dr. Fernandez. The proportion of patients who achieved at least a 0.5-point increase in the ACQ, a level that Dr. Fernandez said is considered clinically relevant, was 67.1%.
However, when lung function measures such as forced expiratory volume in one second and fractional exhaled nitric oxide taken 1 year after bronchothermoplasty were compared with the same measures taken prior to this treatment, there was no significant improvement, according to Dr. Fernandez.
Bronchial thermoplasty involves the use of thermal energy delivered through a bronchoscope to reduce airway smooth muscle mass, thereby eliminating a source of airway restriction. Although nearly 70% of severe asthma patients in this BTGR derived an improvement in quality of life, 30% did not. Asked if the registry has provided any insight about who does or does not respond, Dr. Fernandez acknowledged that this is “the key question,” but added that “no specific profile or biomarker” has yet been identified.
“These are early results, but a 2-year follow-up is planned,” he said.
Although the technical aspects of bronchial thermoplasty “are quite well standardized,” Dr. Fernandez acknowledged that there might be a learning curve that favors experienced operators. He reported that outcomes between high- and low-volume centers in BTGR have not yet been compared. However, he maintained that “these results in real-life patients confirm that severe asthma patients can benefit” from this therapy as shown previously in sham-controlled trials.
Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
REPORTING FROM THE ERS CONGRESS 2018
Key clinical point: In a global registry, bronchothermoplasty provided improvement in real-world severe asthma consistent with clinical trials.
Major finding: At 1 year, severe asthma exacerbations were reduced 34% (P less than .001) relative to the year before treatment.
Study details: Open-label observational study.
Disclosures: Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
Guideline offers comprehensive approach for ICU clinicians
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
FROM CRITICAL CARE MEDICINE
Key clinical point: The 2018 PADIS guideline recommends intervention strategies for adult ICU patients with pain, agitation/sedation, delirium, immobility, and sleep disruption.
Major finding: The guideline includes 37 recommendations; 32 ungraded, nonactionable statements; and two good practice statements.
Study details: A clinical practice guideline was created by 32 international experts, four methodologists, and four survivors of critical illness.
Disclosures: The authors declared funding from AstraZeneca, Baxter, Covidien, and others.
Sources: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
Sildenafil added to nintedanib offers no benefit in IPF patients
PARIS – Despite the promise of in IPF patients who have significantly impaired gas exchange did not improve quality of life (QOL), according to a randomized trial presented at the annual congress of the European Respiratory Society and simultaneously published in the New England Journal of Medicine (2018 Sep 15. doi: 10.1056/NEJMoa18117).
“Sildenafil and nintedanib in combination were well tolerated, but there was no significant benefit for these two drugs relative to nintedanib alone in patients with IPF and severe gas exchange impairment,” reported Fernando J. Martinez, MD, of the department of internal medicine, Cornell University, New York.In the multicenter INSTAGE trial, 274 IPF patients with impaired gas exchange, defined as diffusing capacity of the lungs for carbon monoxide of 35% or less, were randomized to 20 mg of sildenafil or placebo three times daily. Both groups received 150 mg twice daily of nintedanib, which is approved for the treatment of IPF.
The primary endpoint of the study was change from baseline in the St. George’s Respiratory Questionnaire (SGRQ), a 50-item quality of life (QOL) survey that captures the impact of symptoms on activities and well-being. With lower scores representing improved QOL, Dr. Martinez reported that a change of 4 or more points is considered to be clinically meaningful.
At week 12, the SGRQ score fell by 1.28 points in the group that received sildenafil and by 0.77 points in the group randomized to placebo, a difference that did not approach significance (P = .72). There was also no significant difference after 12 weeks of treatment in dyspnea, a secondary endpoint measured with the University of California, San Diego Shortness of Breath Questionnaire (UCSD-SOBQ).
When both measures were reassessed after 24 weeks of treatment, the slight persistent numerical advantage in the sildenafil arm remained statistically and clinically insignificant, according to Dr. Martinez. Rates of acute exacerbations (7.3% vs. 7.4%) and all-cause mortality (10.2% vs. 11.0%) were also reported to be similar in the experimental and control arms, respectively.
Nintedanib, which has been shown to slow decline in forced vital capacity in patients with IPF, inhibits tyrosine kinases involved in IPF progression. However, it has not been associated with an improvement in QOL in this population.
In contrast, sildenafil, a phosphodiesterase-5 inhibitor that relaxes smooth muscle, has been associated with an improvement in both SGRQ score and in dyspnea in a large IPF trial even though it failed the primary endpoint of an improvement in the 6-minute walk test (N Engl J Med 2010;363:620-28).
The hypothesis of the INSTAGE trial was that combining sildenafil with nintedanib in patients with severely impaired gas exchange might have an additive or synergistic effect for IPF symptom control by providing two distinct mechanisms of action.
This was not seen, according to Dr. Martinez. Although those receiving the combination had “a lower risk of an absolute 5-percentage-point decline in FVC of the predicted value or death than those who received nintedanib alone,” Dr. Martinez said this was not a study endpoint, and was of unclear clinical significance. Rather, although nintedanib and sildenafil were well tolerated when taken together, Dr. Martinez concluded that this trial was unable to show any definitive clinical benefit from this combination.
Dr. Martinez has financial relationships with AstraZeneca, Boehringer-Ingelheim, Cheisi, ConCert, Genentech, GlaxoSmithKline, Inova, Novartis, Roche, Sunovion, Theravance, and Teva.
.
PARIS – Despite the promise of in IPF patients who have significantly impaired gas exchange did not improve quality of life (QOL), according to a randomized trial presented at the annual congress of the European Respiratory Society and simultaneously published in the New England Journal of Medicine (2018 Sep 15. doi: 10.1056/NEJMoa18117).
“Sildenafil and nintedanib in combination were well tolerated, but there was no significant benefit for these two drugs relative to nintedanib alone in patients with IPF and severe gas exchange impairment,” reported Fernando J. Martinez, MD, of the department of internal medicine, Cornell University, New York.In the multicenter INSTAGE trial, 274 IPF patients with impaired gas exchange, defined as diffusing capacity of the lungs for carbon monoxide of 35% or less, were randomized to 20 mg of sildenafil or placebo three times daily. Both groups received 150 mg twice daily of nintedanib, which is approved for the treatment of IPF.
The primary endpoint of the study was change from baseline in the St. George’s Respiratory Questionnaire (SGRQ), a 50-item quality of life (QOL) survey that captures the impact of symptoms on activities and well-being. With lower scores representing improved QOL, Dr. Martinez reported that a change of 4 or more points is considered to be clinically meaningful.
At week 12, the SGRQ score fell by 1.28 points in the group that received sildenafil and by 0.77 points in the group randomized to placebo, a difference that did not approach significance (P = .72). There was also no significant difference after 12 weeks of treatment in dyspnea, a secondary endpoint measured with the University of California, San Diego Shortness of Breath Questionnaire (UCSD-SOBQ).
When both measures were reassessed after 24 weeks of treatment, the slight persistent numerical advantage in the sildenafil arm remained statistically and clinically insignificant, according to Dr. Martinez. Rates of acute exacerbations (7.3% vs. 7.4%) and all-cause mortality (10.2% vs. 11.0%) were also reported to be similar in the experimental and control arms, respectively.
Nintedanib, which has been shown to slow decline in forced vital capacity in patients with IPF, inhibits tyrosine kinases involved in IPF progression. However, it has not been associated with an improvement in QOL in this population.
In contrast, sildenafil, a phosphodiesterase-5 inhibitor that relaxes smooth muscle, has been associated with an improvement in both SGRQ score and in dyspnea in a large IPF trial even though it failed the primary endpoint of an improvement in the 6-minute walk test (N Engl J Med 2010;363:620-28).
The hypothesis of the INSTAGE trial was that combining sildenafil with nintedanib in patients with severely impaired gas exchange might have an additive or synergistic effect for IPF symptom control by providing two distinct mechanisms of action.
This was not seen, according to Dr. Martinez. Although those receiving the combination had “a lower risk of an absolute 5-percentage-point decline in FVC of the predicted value or death than those who received nintedanib alone,” Dr. Martinez said this was not a study endpoint, and was of unclear clinical significance. Rather, although nintedanib and sildenafil were well tolerated when taken together, Dr. Martinez concluded that this trial was unable to show any definitive clinical benefit from this combination.
Dr. Martinez has financial relationships with AstraZeneca, Boehringer-Ingelheim, Cheisi, ConCert, Genentech, GlaxoSmithKline, Inova, Novartis, Roche, Sunovion, Theravance, and Teva.
.
PARIS – Despite the promise of in IPF patients who have significantly impaired gas exchange did not improve quality of life (QOL), according to a randomized trial presented at the annual congress of the European Respiratory Society and simultaneously published in the New England Journal of Medicine (2018 Sep 15. doi: 10.1056/NEJMoa18117).
“Sildenafil and nintedanib in combination were well tolerated, but there was no significant benefit for these two drugs relative to nintedanib alone in patients with IPF and severe gas exchange impairment,” reported Fernando J. Martinez, MD, of the department of internal medicine, Cornell University, New York.In the multicenter INSTAGE trial, 274 IPF patients with impaired gas exchange, defined as diffusing capacity of the lungs for carbon monoxide of 35% or less, were randomized to 20 mg of sildenafil or placebo three times daily. Both groups received 150 mg twice daily of nintedanib, which is approved for the treatment of IPF.
The primary endpoint of the study was change from baseline in the St. George’s Respiratory Questionnaire (SGRQ), a 50-item quality of life (QOL) survey that captures the impact of symptoms on activities and well-being. With lower scores representing improved QOL, Dr. Martinez reported that a change of 4 or more points is considered to be clinically meaningful.
At week 12, the SGRQ score fell by 1.28 points in the group that received sildenafil and by 0.77 points in the group randomized to placebo, a difference that did not approach significance (P = .72). There was also no significant difference after 12 weeks of treatment in dyspnea, a secondary endpoint measured with the University of California, San Diego Shortness of Breath Questionnaire (UCSD-SOBQ).
When both measures were reassessed after 24 weeks of treatment, the slight persistent numerical advantage in the sildenafil arm remained statistically and clinically insignificant, according to Dr. Martinez. Rates of acute exacerbations (7.3% vs. 7.4%) and all-cause mortality (10.2% vs. 11.0%) were also reported to be similar in the experimental and control arms, respectively.
Nintedanib, which has been shown to slow decline in forced vital capacity in patients with IPF, inhibits tyrosine kinases involved in IPF progression. However, it has not been associated with an improvement in QOL in this population.
In contrast, sildenafil, a phosphodiesterase-5 inhibitor that relaxes smooth muscle, has been associated with an improvement in both SGRQ score and in dyspnea in a large IPF trial even though it failed the primary endpoint of an improvement in the 6-minute walk test (N Engl J Med 2010;363:620-28).
The hypothesis of the INSTAGE trial was that combining sildenafil with nintedanib in patients with severely impaired gas exchange might have an additive or synergistic effect for IPF symptom control by providing two distinct mechanisms of action.
This was not seen, according to Dr. Martinez. Although those receiving the combination had “a lower risk of an absolute 5-percentage-point decline in FVC of the predicted value or death than those who received nintedanib alone,” Dr. Martinez said this was not a study endpoint, and was of unclear clinical significance. Rather, although nintedanib and sildenafil were well tolerated when taken together, Dr. Martinez concluded that this trial was unable to show any definitive clinical benefit from this combination.
Dr. Martinez has financial relationships with AstraZeneca, Boehringer-Ingelheim, Cheisi, ConCert, Genentech, GlaxoSmithKline, Inova, Novartis, Roche, Sunovion, Theravance, and Teva.
.
REPORTING FROM THE ERS CONGRESS 2018
Key clinical point: Sildenafil does not improve quality of life in patients with idiopathic pulmonary fibrosis who are taking nintedanib.
Major finding: At week 12, the modest QOL benefit with sildenafil plus nintedanib relative to nintedanib alone was statistically insignificant.
Study details: Randomized double-blind multicenter trial.
Disclosures: Dr. Kolb has financial relationships with Alkermes, Actelion, Boehringer-Ingelheim, GlaxoSmithKline, Gilead, Prometic, RespiVert, Roche and Synairgen.
Fatal toxicities from checkpoint inhibitors vary by agent
Fatal adverse events are uncommon with immune checkpoint inhibitors (ICI), but they are not unknown, and it’s important to recognize rare, potentially lethal side effects with these agents, investigators say.
A systematic review and meta-analysis of records from academic medical centers and from a pharmacovigilance database found that fatal side adverse events varied in nature and severity according to the agent and immune checkpoint targeted, reported Daniel Y. Wang, MD, of Vanderbilt University Medical Center, Nashville, Tenn., and his colleagues.
“This study underscores that the risk of death associated with complications of ICI therapy is real, but within or well below fatality rates for common oncologic interventions,” they wrote in JAMA Oncology.
In their study, rates of fatal toxic effects associated with immune checkpoint inhibitors ranged from 0.3% to 1.3%. In contrast, the investigators noted, platinum doublet chemotherapy is associated with a 0.9% rate of fatal toxicities, targeted therapies with angiogenesis inhibitors or tyrosine kinase inhibitors have had fatal toxicity rates up to 4%, and allogeneic hematopoietic stem cell transplants are associated with a fatal adverse event rate of approximately 15%. In addition, the death rate associated with complex oncologic surgeries such as the Whipple procedure or esophagectomy ranges from 1% to 10%.
The authors queried the World Health Organization pharmacovigilance database, called Vigilyze, which contains data on more than 16 million adverse drug reactions; records from patients treated with checkpoint inhibitors in seven academic centers in the United States, Germany, and Australia; and published clinical trials.
They combed through the data to identify fatal toxic effects associated with the anti-programmed death 1/ligand-1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo), pembrolizumab (Keytruda), atezolizumab (Tecentriq), avelumab (Bavencio), and durvalumab (Imfinzi), and with the cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitors ipilimumab (Yervoy) and tremelimumab (investigational).
They found in the Vigilyze data that of 193 deaths attributed to CTLA-4 inhibitors, 70% were from colitis, 16% from hepatitis, 8% from pneumonitis, and the remainder from other causes. In contrast, the most common fatal toxicities associated with PD-1/PD-L1 inhibitors were pneumonitis in 35%, hepatitis in 22%, colitis in 17%, and the remainder from other causes.
With the two classes of ICIs used in combination, primarily for treatment of malignant melanoma, colitis accounted for 37% of toxic fatal events, followed by myocarditis in 25%, hepatitis in 22%, pneumonitis in 14%, and others.
The events occurred early in the course of therapy, at a median of 14.5 days from the start of therapy for combination treatment, and 40 days each for anti-PD-1/PD-L1 and anti-CTLA-4 agents.
The complication with highest fatality rate was myocarditis, which killed 39.7% of affected patients. In contrast, endocrine events and colitis were fatal in only 2%-5% of cases. The fatality rates associated with toxic effects in other organ systems ranged from 10% to 17%.
A review of data on 3,545 patients treated with immune checkpoint inhibitors at the seven academic centers found a 0.6% fatality rate, with cardiac and neurologic events accounting for 43% of the deaths.
The investigators also conducted a meta-analysis of data from 112 clinical trials with a total of 19,217 patients treated with ICIs. In these studies, anti-PD-1 agents were associated with a 0.36% rate of fatal toxicities, anti-PD-L1 agents were associated with a 0.38% rate, anti-CTLA-4 agents were linked to a 1.08% rate, and combined PD-1/PD-L1 and CTLA-4 therapy was associated with a 1.23% fatal toxicity rate.
“Each database largely validated the patterns of death from distinct regimens with a few exceptions. Intriguingly, our retrospective analysis at large, experienced academic centers suggested that neurologic and cardiac toxic effects comprised nearly half of deaths. Thus, we surmise that more optimized treatment of these events is urgently needed. In addition, persistent deaths from colitis and pneumonitis, events with standardized treatment algorithms and clear symptoms, suggests that patient and physician education remains a critical objective,” the investigators wrote.
SOURCE: Wang DY et al. JAMA Oncol. 2018 Sept 13. doi: 10.1001/jamaoncol.2018.3923.
Fatal adverse events are uncommon with immune checkpoint inhibitors (ICI), but they are not unknown, and it’s important to recognize rare, potentially lethal side effects with these agents, investigators say.
A systematic review and meta-analysis of records from academic medical centers and from a pharmacovigilance database found that fatal side adverse events varied in nature and severity according to the agent and immune checkpoint targeted, reported Daniel Y. Wang, MD, of Vanderbilt University Medical Center, Nashville, Tenn., and his colleagues.
“This study underscores that the risk of death associated with complications of ICI therapy is real, but within or well below fatality rates for common oncologic interventions,” they wrote in JAMA Oncology.
In their study, rates of fatal toxic effects associated with immune checkpoint inhibitors ranged from 0.3% to 1.3%. In contrast, the investigators noted, platinum doublet chemotherapy is associated with a 0.9% rate of fatal toxicities, targeted therapies with angiogenesis inhibitors or tyrosine kinase inhibitors have had fatal toxicity rates up to 4%, and allogeneic hematopoietic stem cell transplants are associated with a fatal adverse event rate of approximately 15%. In addition, the death rate associated with complex oncologic surgeries such as the Whipple procedure or esophagectomy ranges from 1% to 10%.
The authors queried the World Health Organization pharmacovigilance database, called Vigilyze, which contains data on more than 16 million adverse drug reactions; records from patients treated with checkpoint inhibitors in seven academic centers in the United States, Germany, and Australia; and published clinical trials.
They combed through the data to identify fatal toxic effects associated with the anti-programmed death 1/ligand-1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo), pembrolizumab (Keytruda), atezolizumab (Tecentriq), avelumab (Bavencio), and durvalumab (Imfinzi), and with the cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitors ipilimumab (Yervoy) and tremelimumab (investigational).
They found in the Vigilyze data that of 193 deaths attributed to CTLA-4 inhibitors, 70% were from colitis, 16% from hepatitis, 8% from pneumonitis, and the remainder from other causes. In contrast, the most common fatal toxicities associated with PD-1/PD-L1 inhibitors were pneumonitis in 35%, hepatitis in 22%, colitis in 17%, and the remainder from other causes.
With the two classes of ICIs used in combination, primarily for treatment of malignant melanoma, colitis accounted for 37% of toxic fatal events, followed by myocarditis in 25%, hepatitis in 22%, pneumonitis in 14%, and others.
The events occurred early in the course of therapy, at a median of 14.5 days from the start of therapy for combination treatment, and 40 days each for anti-PD-1/PD-L1 and anti-CTLA-4 agents.
The complication with highest fatality rate was myocarditis, which killed 39.7% of affected patients. In contrast, endocrine events and colitis were fatal in only 2%-5% of cases. The fatality rates associated with toxic effects in other organ systems ranged from 10% to 17%.
A review of data on 3,545 patients treated with immune checkpoint inhibitors at the seven academic centers found a 0.6% fatality rate, with cardiac and neurologic events accounting for 43% of the deaths.
The investigators also conducted a meta-analysis of data from 112 clinical trials with a total of 19,217 patients treated with ICIs. In these studies, anti-PD-1 agents were associated with a 0.36% rate of fatal toxicities, anti-PD-L1 agents were associated with a 0.38% rate, anti-CTLA-4 agents were linked to a 1.08% rate, and combined PD-1/PD-L1 and CTLA-4 therapy was associated with a 1.23% fatal toxicity rate.
“Each database largely validated the patterns of death from distinct regimens with a few exceptions. Intriguingly, our retrospective analysis at large, experienced academic centers suggested that neurologic and cardiac toxic effects comprised nearly half of deaths. Thus, we surmise that more optimized treatment of these events is urgently needed. In addition, persistent deaths from colitis and pneumonitis, events with standardized treatment algorithms and clear symptoms, suggests that patient and physician education remains a critical objective,” the investigators wrote.
SOURCE: Wang DY et al. JAMA Oncol. 2018 Sept 13. doi: 10.1001/jamaoncol.2018.3923.
Fatal adverse events are uncommon with immune checkpoint inhibitors (ICI), but they are not unknown, and it’s important to recognize rare, potentially lethal side effects with these agents, investigators say.
A systematic review and meta-analysis of records from academic medical centers and from a pharmacovigilance database found that fatal side adverse events varied in nature and severity according to the agent and immune checkpoint targeted, reported Daniel Y. Wang, MD, of Vanderbilt University Medical Center, Nashville, Tenn., and his colleagues.
“This study underscores that the risk of death associated with complications of ICI therapy is real, but within or well below fatality rates for common oncologic interventions,” they wrote in JAMA Oncology.
In their study, rates of fatal toxic effects associated with immune checkpoint inhibitors ranged from 0.3% to 1.3%. In contrast, the investigators noted, platinum doublet chemotherapy is associated with a 0.9% rate of fatal toxicities, targeted therapies with angiogenesis inhibitors or tyrosine kinase inhibitors have had fatal toxicity rates up to 4%, and allogeneic hematopoietic stem cell transplants are associated with a fatal adverse event rate of approximately 15%. In addition, the death rate associated with complex oncologic surgeries such as the Whipple procedure or esophagectomy ranges from 1% to 10%.
The authors queried the World Health Organization pharmacovigilance database, called Vigilyze, which contains data on more than 16 million adverse drug reactions; records from patients treated with checkpoint inhibitors in seven academic centers in the United States, Germany, and Australia; and published clinical trials.
They combed through the data to identify fatal toxic effects associated with the anti-programmed death 1/ligand-1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo), pembrolizumab (Keytruda), atezolizumab (Tecentriq), avelumab (Bavencio), and durvalumab (Imfinzi), and with the cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitors ipilimumab (Yervoy) and tremelimumab (investigational).
They found in the Vigilyze data that of 193 deaths attributed to CTLA-4 inhibitors, 70% were from colitis, 16% from hepatitis, 8% from pneumonitis, and the remainder from other causes. In contrast, the most common fatal toxicities associated with PD-1/PD-L1 inhibitors were pneumonitis in 35%, hepatitis in 22%, colitis in 17%, and the remainder from other causes.
With the two classes of ICIs used in combination, primarily for treatment of malignant melanoma, colitis accounted for 37% of toxic fatal events, followed by myocarditis in 25%, hepatitis in 22%, pneumonitis in 14%, and others.
The events occurred early in the course of therapy, at a median of 14.5 days from the start of therapy for combination treatment, and 40 days each for anti-PD-1/PD-L1 and anti-CTLA-4 agents.
The complication with highest fatality rate was myocarditis, which killed 39.7% of affected patients. In contrast, endocrine events and colitis were fatal in only 2%-5% of cases. The fatality rates associated with toxic effects in other organ systems ranged from 10% to 17%.
A review of data on 3,545 patients treated with immune checkpoint inhibitors at the seven academic centers found a 0.6% fatality rate, with cardiac and neurologic events accounting for 43% of the deaths.
The investigators also conducted a meta-analysis of data from 112 clinical trials with a total of 19,217 patients treated with ICIs. In these studies, anti-PD-1 agents were associated with a 0.36% rate of fatal toxicities, anti-PD-L1 agents were associated with a 0.38% rate, anti-CTLA-4 agents were linked to a 1.08% rate, and combined PD-1/PD-L1 and CTLA-4 therapy was associated with a 1.23% fatal toxicity rate.
“Each database largely validated the patterns of death from distinct regimens with a few exceptions. Intriguingly, our retrospective analysis at large, experienced academic centers suggested that neurologic and cardiac toxic effects comprised nearly half of deaths. Thus, we surmise that more optimized treatment of these events is urgently needed. In addition, persistent deaths from colitis and pneumonitis, events with standardized treatment algorithms and clear symptoms, suggests that patient and physician education remains a critical objective,” the investigators wrote.
SOURCE: Wang DY et al. JAMA Oncol. 2018 Sept 13. doi: 10.1001/jamaoncol.2018.3923.
FROM JAMA ONCOLOGY
Key clinical point: Clinicians should be aware of the potential fatal toxic effects associated with immune checkpoint inhibitors.
Major finding: Cardiac and neurologic toxicities accounted for approximately 43% of all toxicity related deaths.
Study details: Systematic review from WHO data on fatal adverse events, data on 3,545 patients treated in academic medical centers, and a meta-analysis from 112 trials involving 19,217 patients treated with immune checkpoint inhibitors.
Disclosures: The study was supported by the French National Alliance for Life and Health Sciences, “Plan Cancer 2014-2019,” the National Institutes of Health, the James C. Bradford Jr. Melanoma Fund, and the Melanoma Research Foundation. Corresponding author Douglas B Johnson, MD, disclosed serving on advisory boards for Array Biopharma, Bristol Myers Squibb, Incyte, Merck, Novartis, and Navigate BP, and research funding from Bristol Myers Squibb and Incyte. Four other coauthors reported similar relationships.
Source: Wang DY et al. JAMA Oncol. 2018 Sept 13. doi: 10.1001/jamaoncol.2018.3923.
Senators unveil legislation to protect patients against surprise medical bills
With frustration growing among Americans who are being charged exorbitant prices for medical treatment, a bipartisan group of senators unveiled a plan on Sept. 18 to protect patients from surprise bills and high charges from hospitals or doctors who are not in their insurance networks.
The draft legislation, which sponsors said is designed to prevent medical bankruptcies, targets three key consumer concerns:
- Treatment for an emergency by a doctor who is not part of the patient’s insurance network at a hospital that is also outside that network. The patients would be required to pay out of pocket the amount required by their insurance plans. The hospital or doctor could not bill the patient for the remainder of the bill, a practice known as “balance billing.” The hospital and doctor could seek additional payments from the patient’s insurer under state regulations or through a formula established in the legislation.
- Treatment by an out-of-network doctor or other provider at a hospital that is in the patient’s insurance network. Patients would pay only what is required by their plans. Again, the doctors could seek more payments from the plans based on formulas set up by state rules or through the federal formula.
- Mandated notification to emergency patients, once they are stabilized, that they could run up excess charges if they are in an out-of-network hospital. The patients would be required to sign a statement acknowledging that they had been told their insurance might not cover their expenses, and they could seek treatment elsewhere.
“Our proposal protects patients in those emergency situations where current law does not, so that they don’t receive a surprise bill that is basically uncapped by anything but a sense of shame,” Sen. Bill Cassidy (R-La.) said in his announcement about the legislation.
Kevin Lucia, a senior research professor at Georgetown University’s Center on Health Insurance Reforms who had not yet read the draft legislation, said the measure was aimed at a big problem.
“Balance billing is ripe for a federal solution,” he said. States regulate only some health plans and that “leaves open a vast number of people that aren’t covered by those laws.”
Federal law regulates health plans offered by many larger companies and unions that are “self-funded.” Sixty-one percent of privately insured employees get their insurance this way. Those plans pay claims out of their own funds, rather than buying an insurance policy. Federal law does not prohibit balance billing in these plans.
Sen. Cassidy’s office said, however, that this legislation would plug that gap.
In addition to Sen. Cassidy, the legislation is being offered by Sens. Michael Bennet (D-Colo.), Chuck Grassley (R-Iowa), Tom Carper (D-Del.), Todd Young (R-Ind.), and Claire McCaskill (D-Mo.).
Sen. Cassidy’s announcement cited two recent articles from Kaiser Health News and NPR’s “Bill of the Month” series, including a $17,850 urine test and a $109,000 bill after a heart attack.
In a statement to Kaiser Health News, Sen. Bennet said, “In Colorado, we hear from patients facing unexpected bills with astronomical costs even when they’ve received a service from an in-network provider. That’s why Senator Cassidy and I are leading a bipartisan group of senators to address this all-too-common byproduct of limited price transparency.”
Emergency rooms and out-of-network hospitals aren’t the only sources of balance bills, Mr. Lucia said. He mentioned that both ground and air ambulances can leave patients responsible for surprisingly high costs as well.
Mr. Lucia said he was encouraged that both Democrats and Republicans signed on to the draft legislation. “Any effort at the federal level is encouraging because this has been a challenging issue at the state level to make progress on,” he said.
KHN reporter Carmen Heredia Rodriguez contributed to this article. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
With frustration growing among Americans who are being charged exorbitant prices for medical treatment, a bipartisan group of senators unveiled a plan on Sept. 18 to protect patients from surprise bills and high charges from hospitals or doctors who are not in their insurance networks.
The draft legislation, which sponsors said is designed to prevent medical bankruptcies, targets three key consumer concerns:
- Treatment for an emergency by a doctor who is not part of the patient’s insurance network at a hospital that is also outside that network. The patients would be required to pay out of pocket the amount required by their insurance plans. The hospital or doctor could not bill the patient for the remainder of the bill, a practice known as “balance billing.” The hospital and doctor could seek additional payments from the patient’s insurer under state regulations or through a formula established in the legislation.
- Treatment by an out-of-network doctor or other provider at a hospital that is in the patient’s insurance network. Patients would pay only what is required by their plans. Again, the doctors could seek more payments from the plans based on formulas set up by state rules or through the federal formula.
- Mandated notification to emergency patients, once they are stabilized, that they could run up excess charges if they are in an out-of-network hospital. The patients would be required to sign a statement acknowledging that they had been told their insurance might not cover their expenses, and they could seek treatment elsewhere.
“Our proposal protects patients in those emergency situations where current law does not, so that they don’t receive a surprise bill that is basically uncapped by anything but a sense of shame,” Sen. Bill Cassidy (R-La.) said in his announcement about the legislation.
Kevin Lucia, a senior research professor at Georgetown University’s Center on Health Insurance Reforms who had not yet read the draft legislation, said the measure was aimed at a big problem.
“Balance billing is ripe for a federal solution,” he said. States regulate only some health plans and that “leaves open a vast number of people that aren’t covered by those laws.”
Federal law regulates health plans offered by many larger companies and unions that are “self-funded.” Sixty-one percent of privately insured employees get their insurance this way. Those plans pay claims out of their own funds, rather than buying an insurance policy. Federal law does not prohibit balance billing in these plans.
Sen. Cassidy’s office said, however, that this legislation would plug that gap.
In addition to Sen. Cassidy, the legislation is being offered by Sens. Michael Bennet (D-Colo.), Chuck Grassley (R-Iowa), Tom Carper (D-Del.), Todd Young (R-Ind.), and Claire McCaskill (D-Mo.).
Sen. Cassidy’s announcement cited two recent articles from Kaiser Health News and NPR’s “Bill of the Month” series, including a $17,850 urine test and a $109,000 bill after a heart attack.
In a statement to Kaiser Health News, Sen. Bennet said, “In Colorado, we hear from patients facing unexpected bills with astronomical costs even when they’ve received a service from an in-network provider. That’s why Senator Cassidy and I are leading a bipartisan group of senators to address this all-too-common byproduct of limited price transparency.”
Emergency rooms and out-of-network hospitals aren’t the only sources of balance bills, Mr. Lucia said. He mentioned that both ground and air ambulances can leave patients responsible for surprisingly high costs as well.
Mr. Lucia said he was encouraged that both Democrats and Republicans signed on to the draft legislation. “Any effort at the federal level is encouraging because this has been a challenging issue at the state level to make progress on,” he said.
KHN reporter Carmen Heredia Rodriguez contributed to this article. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
With frustration growing among Americans who are being charged exorbitant prices for medical treatment, a bipartisan group of senators unveiled a plan on Sept. 18 to protect patients from surprise bills and high charges from hospitals or doctors who are not in their insurance networks.
The draft legislation, which sponsors said is designed to prevent medical bankruptcies, targets three key consumer concerns:
- Treatment for an emergency by a doctor who is not part of the patient’s insurance network at a hospital that is also outside that network. The patients would be required to pay out of pocket the amount required by their insurance plans. The hospital or doctor could not bill the patient for the remainder of the bill, a practice known as “balance billing.” The hospital and doctor could seek additional payments from the patient’s insurer under state regulations or through a formula established in the legislation.
- Treatment by an out-of-network doctor or other provider at a hospital that is in the patient’s insurance network. Patients would pay only what is required by their plans. Again, the doctors could seek more payments from the plans based on formulas set up by state rules or through the federal formula.
- Mandated notification to emergency patients, once they are stabilized, that they could run up excess charges if they are in an out-of-network hospital. The patients would be required to sign a statement acknowledging that they had been told their insurance might not cover their expenses, and they could seek treatment elsewhere.
“Our proposal protects patients in those emergency situations where current law does not, so that they don’t receive a surprise bill that is basically uncapped by anything but a sense of shame,” Sen. Bill Cassidy (R-La.) said in his announcement about the legislation.
Kevin Lucia, a senior research professor at Georgetown University’s Center on Health Insurance Reforms who had not yet read the draft legislation, said the measure was aimed at a big problem.
“Balance billing is ripe for a federal solution,” he said. States regulate only some health plans and that “leaves open a vast number of people that aren’t covered by those laws.”
Federal law regulates health plans offered by many larger companies and unions that are “self-funded.” Sixty-one percent of privately insured employees get their insurance this way. Those plans pay claims out of their own funds, rather than buying an insurance policy. Federal law does not prohibit balance billing in these plans.
Sen. Cassidy’s office said, however, that this legislation would plug that gap.
In addition to Sen. Cassidy, the legislation is being offered by Sens. Michael Bennet (D-Colo.), Chuck Grassley (R-Iowa), Tom Carper (D-Del.), Todd Young (R-Ind.), and Claire McCaskill (D-Mo.).
Sen. Cassidy’s announcement cited two recent articles from Kaiser Health News and NPR’s “Bill of the Month” series, including a $17,850 urine test and a $109,000 bill after a heart attack.
In a statement to Kaiser Health News, Sen. Bennet said, “In Colorado, we hear from patients facing unexpected bills with astronomical costs even when they’ve received a service from an in-network provider. That’s why Senator Cassidy and I are leading a bipartisan group of senators to address this all-too-common byproduct of limited price transparency.”
Emergency rooms and out-of-network hospitals aren’t the only sources of balance bills, Mr. Lucia said. He mentioned that both ground and air ambulances can leave patients responsible for surprisingly high costs as well.
Mr. Lucia said he was encouraged that both Democrats and Republicans signed on to the draft legislation. “Any effort at the federal level is encouraging because this has been a challenging issue at the state level to make progress on,” he said.
KHN reporter Carmen Heredia Rodriguez contributed to this article. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.