User login
FDA issues new REMS for immediate-release opioids
Opioid prescribers will need to be mindful of a new, expanded Risk Evaluation and Mitigation Strategy issued Sept. 18 by the Food and Drug Administration, covering immediate-release opioid analgesics used in the outpatient setting. The strategy also applies to extended-release and long-acting opioids, which have been subject to REMS since 2012.
The new REMS program requires for the first time that training be made available to health care providers who are involved in pain management. For the purposes of this REMS program, the training is not limited to just the prescriber, but includes nurses and pharmacists.
, including alternatives to opioids for pain management.
The FDA said it is in the process of approving a new label for opioids that will contain information about health care provider education that is now a part of the REMS.
“Our new effort is aimed at arming providers with the most current and comprehensive information on the appropriate management of pain,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Appropriate prescribing practices and education are important steps that we are prioritizing to help address the human and financial toll of this crisis.”
Dr. Gottlieb added that the goal of the new REMS is to help prescribers with the latest evidence on the appropriate amount of doses that should be prescribed for a given condition and that the “aim is to reduce overall dispensing as a way to further reduce exposure to these drugs. Our goal is to help prevent patients from becoming addicted by decreasing unnecessary or inappropriate exposure to opioids and fostering rational prescribing to enable appropriate access to those patients who have legitimate medical need for these medicines.”
The FDA also approved a new guidance document that includes updated educational content.
Opioid prescribers will need to be mindful of a new, expanded Risk Evaluation and Mitigation Strategy issued Sept. 18 by the Food and Drug Administration, covering immediate-release opioid analgesics used in the outpatient setting. The strategy also applies to extended-release and long-acting opioids, which have been subject to REMS since 2012.
The new REMS program requires for the first time that training be made available to health care providers who are involved in pain management. For the purposes of this REMS program, the training is not limited to just the prescriber, but includes nurses and pharmacists.
, including alternatives to opioids for pain management.
The FDA said it is in the process of approving a new label for opioids that will contain information about health care provider education that is now a part of the REMS.
“Our new effort is aimed at arming providers with the most current and comprehensive information on the appropriate management of pain,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Appropriate prescribing practices and education are important steps that we are prioritizing to help address the human and financial toll of this crisis.”
Dr. Gottlieb added that the goal of the new REMS is to help prescribers with the latest evidence on the appropriate amount of doses that should be prescribed for a given condition and that the “aim is to reduce overall dispensing as a way to further reduce exposure to these drugs. Our goal is to help prevent patients from becoming addicted by decreasing unnecessary or inappropriate exposure to opioids and fostering rational prescribing to enable appropriate access to those patients who have legitimate medical need for these medicines.”
The FDA also approved a new guidance document that includes updated educational content.
Opioid prescribers will need to be mindful of a new, expanded Risk Evaluation and Mitigation Strategy issued Sept. 18 by the Food and Drug Administration, covering immediate-release opioid analgesics used in the outpatient setting. The strategy also applies to extended-release and long-acting opioids, which have been subject to REMS since 2012.
The new REMS program requires for the first time that training be made available to health care providers who are involved in pain management. For the purposes of this REMS program, the training is not limited to just the prescriber, but includes nurses and pharmacists.
, including alternatives to opioids for pain management.
The FDA said it is in the process of approving a new label for opioids that will contain information about health care provider education that is now a part of the REMS.
“Our new effort is aimed at arming providers with the most current and comprehensive information on the appropriate management of pain,” FDA Commissioner Scott Gottlieb, MD, said in a statement. “Appropriate prescribing practices and education are important steps that we are prioritizing to help address the human and financial toll of this crisis.”
Dr. Gottlieb added that the goal of the new REMS is to help prescribers with the latest evidence on the appropriate amount of doses that should be prescribed for a given condition and that the “aim is to reduce overall dispensing as a way to further reduce exposure to these drugs. Our goal is to help prevent patients from becoming addicted by decreasing unnecessary or inappropriate exposure to opioids and fostering rational prescribing to enable appropriate access to those patients who have legitimate medical need for these medicines.”
The FDA also approved a new guidance document that includes updated educational content.
Tracking 90-day vascular surgery outcomes: The coming new normal?
The Centers for Medicare and Medicaid Services is test driving a new quality measurement model that pushes hospital readmissions measures out from 30 to 90 days.
Previous research has identified vascular surgery as having twice as high rates of 90-day readmissions, compared with 30-day readmissions (Am J Manag Care. 2014;20[9]:e432-e438), and this could prove problematic in light of the CMS pilot project currently underway, according to Donald E. Fry, MD, of MPA Healthcare Solutions, Chicago, and his colleagues.
They performed a study that found a high level of adverse outcomes for common vascular procedures and that there was a significant variability in risk-adjusted outcomes among best- and poorest-performing hospitals in all major vascular procedures, indicating that a large opportunity exists for improvement in results.
Medicare’s value-based care Readmissions Reduction Model developed financial penalties for hospitals that fail to achieve acceptable performance scores, and in doing so shifted some of the financial risks of care to the providers based on a 30-day readmission model. In contrast, the pilot Bundled Payments for Care Improvement (BPCI) Advanced Program, which the CMS plans to launch in October 2018, will follow a 90-day period of postoperative care as its duration of financial accountability.
“While BPCI Advanced, has, until now, focused upon orthopedics, cardiovascular procedures, and high-volume medical admissions areas, it is anticipated that vascular surgery will be included in the future,” according to the investigators. Therefore, the researchers performed an in-depth analysis to examine the 90-day outcomes of common vascular surgeries across hospitals as a prelude to the vascular surgery field having to potentially confront this new CMS model (Surgery 2018 Jun 22 doi: 10.1016/j.surg.2018.03.025).
Dr. Fry and his colleagues used the Medicare Limited Data Set for 2012-2014 to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postoperative care. A pool of more than 500 aggregated and individual candidate risk factors, including age and sex, was used in model development, based upon data from 359 hospitals with 10,815 patients in the Medicare Limited Data Set.
The researchers examined the risk-adjusted outcomes of four major groupings of vascular surgery procedures: elective open aortic; open peripheral vascular procedures; endovascular aortic; and percutaneous angioplasty procedures.
They found that the total adverse-outcome rate (AO) was 27.8% for open aortic procedures, 31.5% for open peripheral vascular procedures, 19.6% for endovascular aortic procedures, and 36.4% for percutaneous angioplasty procedures. The difference in risk-adjusted adverse-outcome rates between the best- and the poorest- performing deciles was 32.2% for open aortic procedures, 29.5% for open peripheral vascular procedures, 21.5% for endovascular aortic procedures, and 37.1% for percutaneous angioplasty procedures.
The model determined significant risk factors (P less than .001) for inpatient death (including malnutrition, intestinal ischemia, supplemental oxygen, and age greater than or equal to 85 years); prolonged length of stay (including supplemental oxygen, peritoneal adhesions, and chronic lung obstructive disease); 90-day postdischarge death (including heart failure, chronic infection, psychosis, and primary head/neck cancer); and 90-day postdischarge readmission (malnutrition, chronic obstructive lung disease, upper aerodigestive tract cancer, and skin ulceration) for these procedures.
For all cases, the total 90-day postdischarge mortality rate exceeded the inpatient death rate, and readmissions were the major driver of the total AO. They found that 22% of all patients readmitted across the entire 90-day interval had not seen a physician for follow-up after discharge. [This] “begs the question of whether more frequent physician or physician-extender follow-up can reduce this AO,” according to Dr. Fry and his colleagues. “Importantly, first readmissions during days 31-90 following discharge were almost as common as those occurring during the initial 30 days. Over 20% of total readmissions were subsequently repeat events during the 90-day interval,” they added.
They also found that the variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures and indicates a large opportunity for improvement in results.
“Understanding variables associated with higher risk can be used as a decision support tool to identify which patients will need increased vigilance to avoid AOs. Identification of very high risk may become a consideration in the assessment of the appropriateness of the surgical intervention. If providers know their outcomes and those outcomes are benchmarked against the whole population of hospitals, then clinical performance can be improved by specific care redesign initiatives,” the researchers concluded.
Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
SOURCE: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
The Centers for Medicare and Medicaid Services is test driving a new quality measurement model that pushes hospital readmissions measures out from 30 to 90 days.
Previous research has identified vascular surgery as having twice as high rates of 90-day readmissions, compared with 30-day readmissions (Am J Manag Care. 2014;20[9]:e432-e438), and this could prove problematic in light of the CMS pilot project currently underway, according to Donald E. Fry, MD, of MPA Healthcare Solutions, Chicago, and his colleagues.
They performed a study that found a high level of adverse outcomes for common vascular procedures and that there was a significant variability in risk-adjusted outcomes among best- and poorest-performing hospitals in all major vascular procedures, indicating that a large opportunity exists for improvement in results.
Medicare’s value-based care Readmissions Reduction Model developed financial penalties for hospitals that fail to achieve acceptable performance scores, and in doing so shifted some of the financial risks of care to the providers based on a 30-day readmission model. In contrast, the pilot Bundled Payments for Care Improvement (BPCI) Advanced Program, which the CMS plans to launch in October 2018, will follow a 90-day period of postoperative care as its duration of financial accountability.
“While BPCI Advanced, has, until now, focused upon orthopedics, cardiovascular procedures, and high-volume medical admissions areas, it is anticipated that vascular surgery will be included in the future,” according to the investigators. Therefore, the researchers performed an in-depth analysis to examine the 90-day outcomes of common vascular surgeries across hospitals as a prelude to the vascular surgery field having to potentially confront this new CMS model (Surgery 2018 Jun 22 doi: 10.1016/j.surg.2018.03.025).
Dr. Fry and his colleagues used the Medicare Limited Data Set for 2012-2014 to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postoperative care. A pool of more than 500 aggregated and individual candidate risk factors, including age and sex, was used in model development, based upon data from 359 hospitals with 10,815 patients in the Medicare Limited Data Set.
The researchers examined the risk-adjusted outcomes of four major groupings of vascular surgery procedures: elective open aortic; open peripheral vascular procedures; endovascular aortic; and percutaneous angioplasty procedures.
They found that the total adverse-outcome rate (AO) was 27.8% for open aortic procedures, 31.5% for open peripheral vascular procedures, 19.6% for endovascular aortic procedures, and 36.4% for percutaneous angioplasty procedures. The difference in risk-adjusted adverse-outcome rates between the best- and the poorest- performing deciles was 32.2% for open aortic procedures, 29.5% for open peripheral vascular procedures, 21.5% for endovascular aortic procedures, and 37.1% for percutaneous angioplasty procedures.
The model determined significant risk factors (P less than .001) for inpatient death (including malnutrition, intestinal ischemia, supplemental oxygen, and age greater than or equal to 85 years); prolonged length of stay (including supplemental oxygen, peritoneal adhesions, and chronic lung obstructive disease); 90-day postdischarge death (including heart failure, chronic infection, psychosis, and primary head/neck cancer); and 90-day postdischarge readmission (malnutrition, chronic obstructive lung disease, upper aerodigestive tract cancer, and skin ulceration) for these procedures.
For all cases, the total 90-day postdischarge mortality rate exceeded the inpatient death rate, and readmissions were the major driver of the total AO. They found that 22% of all patients readmitted across the entire 90-day interval had not seen a physician for follow-up after discharge. [This] “begs the question of whether more frequent physician or physician-extender follow-up can reduce this AO,” according to Dr. Fry and his colleagues. “Importantly, first readmissions during days 31-90 following discharge were almost as common as those occurring during the initial 30 days. Over 20% of total readmissions were subsequently repeat events during the 90-day interval,” they added.
They also found that the variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures and indicates a large opportunity for improvement in results.
“Understanding variables associated with higher risk can be used as a decision support tool to identify which patients will need increased vigilance to avoid AOs. Identification of very high risk may become a consideration in the assessment of the appropriateness of the surgical intervention. If providers know their outcomes and those outcomes are benchmarked against the whole population of hospitals, then clinical performance can be improved by specific care redesign initiatives,” the researchers concluded.
Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
SOURCE: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
The Centers for Medicare and Medicaid Services is test driving a new quality measurement model that pushes hospital readmissions measures out from 30 to 90 days.
Previous research has identified vascular surgery as having twice as high rates of 90-day readmissions, compared with 30-day readmissions (Am J Manag Care. 2014;20[9]:e432-e438), and this could prove problematic in light of the CMS pilot project currently underway, according to Donald E. Fry, MD, of MPA Healthcare Solutions, Chicago, and his colleagues.
They performed a study that found a high level of adverse outcomes for common vascular procedures and that there was a significant variability in risk-adjusted outcomes among best- and poorest-performing hospitals in all major vascular procedures, indicating that a large opportunity exists for improvement in results.
Medicare’s value-based care Readmissions Reduction Model developed financial penalties for hospitals that fail to achieve acceptable performance scores, and in doing so shifted some of the financial risks of care to the providers based on a 30-day readmission model. In contrast, the pilot Bundled Payments for Care Improvement (BPCI) Advanced Program, which the CMS plans to launch in October 2018, will follow a 90-day period of postoperative care as its duration of financial accountability.
“While BPCI Advanced, has, until now, focused upon orthopedics, cardiovascular procedures, and high-volume medical admissions areas, it is anticipated that vascular surgery will be included in the future,” according to the investigators. Therefore, the researchers performed an in-depth analysis to examine the 90-day outcomes of common vascular surgeries across hospitals as a prelude to the vascular surgery field having to potentially confront this new CMS model (Surgery 2018 Jun 22 doi: 10.1016/j.surg.2018.03.025).
Dr. Fry and his colleagues used the Medicare Limited Data Set for 2012-2014 to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postoperative care. A pool of more than 500 aggregated and individual candidate risk factors, including age and sex, was used in model development, based upon data from 359 hospitals with 10,815 patients in the Medicare Limited Data Set.
The researchers examined the risk-adjusted outcomes of four major groupings of vascular surgery procedures: elective open aortic; open peripheral vascular procedures; endovascular aortic; and percutaneous angioplasty procedures.
They found that the total adverse-outcome rate (AO) was 27.8% for open aortic procedures, 31.5% for open peripheral vascular procedures, 19.6% for endovascular aortic procedures, and 36.4% for percutaneous angioplasty procedures. The difference in risk-adjusted adverse-outcome rates between the best- and the poorest- performing deciles was 32.2% for open aortic procedures, 29.5% for open peripheral vascular procedures, 21.5% for endovascular aortic procedures, and 37.1% for percutaneous angioplasty procedures.
The model determined significant risk factors (P less than .001) for inpatient death (including malnutrition, intestinal ischemia, supplemental oxygen, and age greater than or equal to 85 years); prolonged length of stay (including supplemental oxygen, peritoneal adhesions, and chronic lung obstructive disease); 90-day postdischarge death (including heart failure, chronic infection, psychosis, and primary head/neck cancer); and 90-day postdischarge readmission (malnutrition, chronic obstructive lung disease, upper aerodigestive tract cancer, and skin ulceration) for these procedures.
For all cases, the total 90-day postdischarge mortality rate exceeded the inpatient death rate, and readmissions were the major driver of the total AO. They found that 22% of all patients readmitted across the entire 90-day interval had not seen a physician for follow-up after discharge. [This] “begs the question of whether more frequent physician or physician-extender follow-up can reduce this AO,” according to Dr. Fry and his colleagues. “Importantly, first readmissions during days 31-90 following discharge were almost as common as those occurring during the initial 30 days. Over 20% of total readmissions were subsequently repeat events during the 90-day interval,” they added.
They also found that the variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures and indicates a large opportunity for improvement in results.
“Understanding variables associated with higher risk can be used as a decision support tool to identify which patients will need increased vigilance to avoid AOs. Identification of very high risk may become a consideration in the assessment of the appropriateness of the surgical intervention. If providers know their outcomes and those outcomes are benchmarked against the whole population of hospitals, then clinical performance can be improved by specific care redesign initiatives,” the researchers concluded.
Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
SOURCE: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
FROM SURGERY
Key clinical point: The variability in risk-adjusted outcomes among the best and poorest performing hospitals was over 20% in all of the major vascular procedures.
Major finding: The total adverse-outcome rate was 27.8% for open aortic procedures, 31.5% for open peripheral artery, 19.6% for endovascular aortic, and 36.4% for percutaneous angioplasty.
Study details: The Medicare Limited Data Set for 2012-2014 was used to follow the outcomes of major vascular surgery beginning with the inpatient stay and on through 90 days of postop care.
Disclosures: Dr. Fry is executive vice president of MPA Healthcare Solutions, which funded the research.
Source: Fry DE et al. Surgery 2018 Jun 22. doi: 10.1016/j.surg.2018.03.025.
Two red flags spell trouble ahead in spontaneous coronary artery dissection
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
MUNICH –
Women whose spontaneous coronary artery dissection (SCAD) occurred during the peripartum period were at 2.8-fold increased risk of in-hospital major adverse events in a multivariate analysis, while those with a background connective tissue disorder were at 8.7-fold increased risk for major adverse cardiovascular events within 30 days of hospitalization in the Canadian SCAD (CanSCAD) study, Jacqueline Saw, MD, reported at the annual congress of the European Society of Cardiology.
CanSCAD is an ongoing, rigorous, prospective, multicenter, observational study of 750 patients with SCAD documented on angiography and confirmed in a core lab. To put the study in perspective, the worldwide medical literature published over the last decade contains fewer than 1,300 other cases of this seriously underdiagnosed, poorly understood disorder, noted Dr. Saw, CanSCAD principal investigator and a cardiologist at the University of British Columbia, Vancouver. Because much remains unclear about SCAD, a condition mistakenly considered to be rare in the past, the Canadian study was undertaken to shed light on predisposing and precipitating factors, optimal management, and clinical outcomes. Although Dr. Saw could present only the in-hospital and 30-day outcomes, follow-up will continue at 6, 12, 24, and 36 months.
SCAD is a nontraumatic, noniatrogenic, nonatherosclerotic separation of the coronary artery wall by intramural hematoma, creating a false lumen which compresses the true arterial lumen. This compromises blood flow with resultant myocardial ischemia or infarction. Intimal tear may or may not be present.
CanSCAD underscored that this is predominantly a disease affecting relatively young women: 89% of SCAD participants were female, 55% of whom were postmenopausal. The mean age at presentation was 52 years, and only 9% of subjects were older than age 65. Seventy percent of subjects presented with non–ST-elevation MI, the other 30% with STEMI. The predominant symptom was chest pain in 92% of patients. The average length of hospital stay was 4 days.
In terms of precipitating factors, half of patients cited high or severe emotional stress, with 41% of subjects scoring 20 or higher on the Perceived Stress Scale. About 30% of patients cited unusually intense physical stress, such as lifting more than 50 pounds, as a precipitating factor.
Of note, one-third of patients had no cardiovascular risk factors.
The in-hospital major adverse event rate – a composite of all-cause mortality, stroke, recurrent MI, cardiogenic shock, heart failure, cardiac arrest, repeat or unplanned revascularization, and heart transplantation – was 8.8%. Mortality through 1 month was reassuringly low, at 0.1%. Nonetheless, 4.9% of patients experienced recurrent symptoms necessitating emergency room visits within 30 days post discharge, and 2.5% required hospitalization because of their chest pain.
Patients who presented with SCAD during the peripartum period were more severely affected. Although they accounted for only 4.5% of subjects, their in-hospital major adverse event rate was 20.6%, compared with 8.2% in the others. They had a 17.6% prevalence of a left ventricular ejection fraction below 35%, as did only 3.1% of patients without peripartum SCAD. They were more than twice as likely to have elevated cardiac troponin levels. Moreover, peripartum SCAD was independently associated with a 2.9-fold increased risk of major adverse cardiovascular events at 30 days, a composite of all-cause mortality, stroke, recurrent MI, heart failure, or revascularization.
The other independent predictor of 30-day major adverse cardiovascular events in a multivariate logistic regression analysis was having a connective tissue disorder, present in 3.6% of participants.
Management was conservative, with no percutaneous coronary intervention used in 84% of patients. Outcomes were worse in the subgroup who underwent PCI, but Dr. Saw cautioned against making much of that.
“Keep in mind that the patients who undergo PCI are typically the higher-risk cohort with ongoing ischemia and chest pain, so there will be some bias there,” according to the cardiologist.
Session chair Patrick W. Serruys, MD, of Erasmus University, Rotterdam, the Netherlands, commented, “It seems like there is a critical period of 30 days, more or less, and beyond that time the situation can be considered as settled and a wait-and-see attitude is fine. It looked like patients with peripartum dissection or connective tissue disease should potentially be kept in hospital for at least 15 days, and maybe 30 days, because I see your cumulative adverse event curve plateauing around 15 days.”
“Are you doing that in your practice?” asked Dr. Serruys.
“It’s true that conservatively managed patients should remain in hospital for typically about 4 days. For patients with a high-risk presentation we do advocate staying in hospital for longer periods,” Dr. Saw replied. “It would be great to keep them for 15 days, although typically if their chest pain has settled by 10 days they can be discharged home.”
Audience members were eager to hear her recommendations regarding dual-antiplatelet therapy. She explained that in her practice patients are generally discharged on aspirin and clopidogrel and typically continue the clopidogrel for at least a month.
“When we follow them in the office at 1 month, if their chest pain has settled, we would discontinue DAPT,” Dr. Saw said.
The ongoing CanSCAD study is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier. Dr. Saw reported serving as a consultant to Abbott Vascular and Boston Scientific.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: SCAD occurring during the peripartum period or in patients with connective tissue disease identifies subgroups at high risk for major adverse cardiovascular events within 30 days.
Major finding: Patients with spontaneous coronary artery dissection and comorbid connective tissue disease were at 8.7-fold increased risk of major adverse cardiovascular events within 30 days.
Study details: CanSCAD is an ongoing, prospective, multicenter, observational study in 750 patients with confirmed spontaneous coronary artery dissection.
Disclosures: CanSCAD is sponsored by the Canadian Institutes of Health Research, the Stroke Foundation of Canada, the National Institutes of Health, Abbott Vascular, Boston Scientific, AstraZeneca, and Servier.
CRP levels may augur RCC prognosis
In patients with renal cell carcinoma, high levels of the inflammation marker C-reactive protein may signal the presence of an immunosuppressive tumor microenvironment, a feature associated with poor prognosis, investigators contend.
Among 111 patients with renal cell carcinoma (RCC) treated with either partial or radical nephrectomy, those with high C-reactive protein (CRP) levels had significantly worse cancer-specific survival (CSS) compared with patients with normal CRP levels, and CRP levels were significantly higher among patients whose tumors showed strong infiltration of immune-suppressor cells, reported Takayuki Nakayama, MD, of Tokyo Medical and Dental Graduate University in Japan, and his colleagues.
“We have found that CRP level is positively associated with the infiltration of Treg and M2 macrophage[s] in patients with RCC, indicating that CRP reflects an immunosuppressive microenvironment. However, further studies are required to confirm these findings and provide a better understanding of the association between host systemic inflammation and the immunosuppressive microenvironment,” they wrote in Clinical Genitourinary Cancer.
The investigators had previously proposed CRP as a biomarker for urologic malignancies, including RCC. The current study was designed to look at the association between CRP and the tumor microenvironment, and to determine whether serum CRP levels correlate with prognosis.
To do this, they performed immunohistochemistry studies of immune cells, including immunosuppressive M2 macrophages (CD4, CD8, and CD163 cells) and Foxp4 regulatory T cells (Tregs) on resected renal tissues from 111 patients with RCC treated in their center.
They found that 33 of the 111 patients (30%) had high CRP levels, defined as 0.5 mg/L or greater. These patients had a 5-year CSS rate of 51%, compared with 91% for patients with CRP levels below the 0.5 mg/L cutoff (P less than .001).
They also found that tumors with strong infiltration of M2 macrophages had significantly worse disease and poorer prognoses. For example, patients with strong infiltration of CD163-positive cells had higher tumor and nodal stages, as well as higher rates of distant metastases and higher Fuhrman nuclear grade (respective P values less than .001, = .003, less than .001, and = .008).
CRP levels were significantly higher among patients with strong infiltration of CD8-positive cells (P = .041), Foxp3-positive cells (P = .001), or CD163-positive (P = .035). These patients also had significantly worse CSS compared with patients with weak tumor infiltration of the respective cells (P = .040, P = .026, and P less than .001, respectively).
Independent prognostic factors for CSS in multivariate analysis included strong CD163-positive cell infiltration (P =.001), pathologic stage T3 (P = .036), lymph-node involvement (P = .007), distant metastasis (P less than .001), and Fuhrman nuclear grade 4 (P = .003).
The authors acknowledged that the study was limited by its retrospective design, small sample size, and limited analysis of components of the tumor microenvironment.
The study was supported by the Japan Society for the Promotion of Science. The authors reported having no conflicts of interest to disclose.
SOURCE: Nakayama T et al. Clin Genitourin Cancer. 2018 Aug 11. doi: 10.1016/j.clgc.2018.07.027.
In patients with renal cell carcinoma, high levels of the inflammation marker C-reactive protein may signal the presence of an immunosuppressive tumor microenvironment, a feature associated with poor prognosis, investigators contend.
Among 111 patients with renal cell carcinoma (RCC) treated with either partial or radical nephrectomy, those with high C-reactive protein (CRP) levels had significantly worse cancer-specific survival (CSS) compared with patients with normal CRP levels, and CRP levels were significantly higher among patients whose tumors showed strong infiltration of immune-suppressor cells, reported Takayuki Nakayama, MD, of Tokyo Medical and Dental Graduate University in Japan, and his colleagues.
“We have found that CRP level is positively associated with the infiltration of Treg and M2 macrophage[s] in patients with RCC, indicating that CRP reflects an immunosuppressive microenvironment. However, further studies are required to confirm these findings and provide a better understanding of the association between host systemic inflammation and the immunosuppressive microenvironment,” they wrote in Clinical Genitourinary Cancer.
The investigators had previously proposed CRP as a biomarker for urologic malignancies, including RCC. The current study was designed to look at the association between CRP and the tumor microenvironment, and to determine whether serum CRP levels correlate with prognosis.
To do this, they performed immunohistochemistry studies of immune cells, including immunosuppressive M2 macrophages (CD4, CD8, and CD163 cells) and Foxp4 regulatory T cells (Tregs) on resected renal tissues from 111 patients with RCC treated in their center.
They found that 33 of the 111 patients (30%) had high CRP levels, defined as 0.5 mg/L or greater. These patients had a 5-year CSS rate of 51%, compared with 91% for patients with CRP levels below the 0.5 mg/L cutoff (P less than .001).
They also found that tumors with strong infiltration of M2 macrophages had significantly worse disease and poorer prognoses. For example, patients with strong infiltration of CD163-positive cells had higher tumor and nodal stages, as well as higher rates of distant metastases and higher Fuhrman nuclear grade (respective P values less than .001, = .003, less than .001, and = .008).
CRP levels were significantly higher among patients with strong infiltration of CD8-positive cells (P = .041), Foxp3-positive cells (P = .001), or CD163-positive (P = .035). These patients also had significantly worse CSS compared with patients with weak tumor infiltration of the respective cells (P = .040, P = .026, and P less than .001, respectively).
Independent prognostic factors for CSS in multivariate analysis included strong CD163-positive cell infiltration (P =.001), pathologic stage T3 (P = .036), lymph-node involvement (P = .007), distant metastasis (P less than .001), and Fuhrman nuclear grade 4 (P = .003).
The authors acknowledged that the study was limited by its retrospective design, small sample size, and limited analysis of components of the tumor microenvironment.
The study was supported by the Japan Society for the Promotion of Science. The authors reported having no conflicts of interest to disclose.
SOURCE: Nakayama T et al. Clin Genitourin Cancer. 2018 Aug 11. doi: 10.1016/j.clgc.2018.07.027.
In patients with renal cell carcinoma, high levels of the inflammation marker C-reactive protein may signal the presence of an immunosuppressive tumor microenvironment, a feature associated with poor prognosis, investigators contend.
Among 111 patients with renal cell carcinoma (RCC) treated with either partial or radical nephrectomy, those with high C-reactive protein (CRP) levels had significantly worse cancer-specific survival (CSS) compared with patients with normal CRP levels, and CRP levels were significantly higher among patients whose tumors showed strong infiltration of immune-suppressor cells, reported Takayuki Nakayama, MD, of Tokyo Medical and Dental Graduate University in Japan, and his colleagues.
“We have found that CRP level is positively associated with the infiltration of Treg and M2 macrophage[s] in patients with RCC, indicating that CRP reflects an immunosuppressive microenvironment. However, further studies are required to confirm these findings and provide a better understanding of the association between host systemic inflammation and the immunosuppressive microenvironment,” they wrote in Clinical Genitourinary Cancer.
The investigators had previously proposed CRP as a biomarker for urologic malignancies, including RCC. The current study was designed to look at the association between CRP and the tumor microenvironment, and to determine whether serum CRP levels correlate with prognosis.
To do this, they performed immunohistochemistry studies of immune cells, including immunosuppressive M2 macrophages (CD4, CD8, and CD163 cells) and Foxp4 regulatory T cells (Tregs) on resected renal tissues from 111 patients with RCC treated in their center.
They found that 33 of the 111 patients (30%) had high CRP levels, defined as 0.5 mg/L or greater. These patients had a 5-year CSS rate of 51%, compared with 91% for patients with CRP levels below the 0.5 mg/L cutoff (P less than .001).
They also found that tumors with strong infiltration of M2 macrophages had significantly worse disease and poorer prognoses. For example, patients with strong infiltration of CD163-positive cells had higher tumor and nodal stages, as well as higher rates of distant metastases and higher Fuhrman nuclear grade (respective P values less than .001, = .003, less than .001, and = .008).
CRP levels were significantly higher among patients with strong infiltration of CD8-positive cells (P = .041), Foxp3-positive cells (P = .001), or CD163-positive (P = .035). These patients also had significantly worse CSS compared with patients with weak tumor infiltration of the respective cells (P = .040, P = .026, and P less than .001, respectively).
Independent prognostic factors for CSS in multivariate analysis included strong CD163-positive cell infiltration (P =.001), pathologic stage T3 (P = .036), lymph-node involvement (P = .007), distant metastasis (P less than .001), and Fuhrman nuclear grade 4 (P = .003).
The authors acknowledged that the study was limited by its retrospective design, small sample size, and limited analysis of components of the tumor microenvironment.
The study was supported by the Japan Society for the Promotion of Science. The authors reported having no conflicts of interest to disclose.
SOURCE: Nakayama T et al. Clin Genitourin Cancer. 2018 Aug 11. doi: 10.1016/j.clgc.2018.07.027.
FROM CLINICAL GENITOURINARY CANCER
Key clinical point: The nonspecific inflammatory marker C-reactive protein could be a biomarker for renal cell carcinoma prognosis.
Major finding: 5-year cancer specific survival was 51% for patients with high CRP levels, vs. 91% for patients with normal levels.
Study details: Retrospective analysis of the tumor immune microenvironment in tissue samples from 111 patients with RCC.
Disclosures: The study was supported by the Japan Society for the Promotion of Science. The authors reported having no conflicts of interest to disclose.
Source: Nakayama T et al. Clin Genitourin Cancer. 2018 Aug 11. doi: 10.1016/j.clgc.2018.07.027.
Expert panel updates guidelines on antithrombotic therapy for AF
For patients with , experts said in a comprehensive, updated guideline.
The 113-page guideline, published in the journal CHEST®, provides antithrombotic treatment recommendations for atrial fibrillation based on different levels of risk for stroke and in a variety of clinical presentations.
Altogether, the new guidelines highlight 60 key recommendations from the 12-person expert panel, chaired by Gregory Y.H. Lip, MD, of the Institute of Cardiovascular Sciences, University of Birmingham (England).
To develop the guidelines, the panel conducted a systematic literature review of relevant articles released since the 2012 publication of Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (9th Edition).
Since that time, “there have been substantial developments in atrial fibrillation thromboprophylaxis, whether with regard to risk assessment, antithrombotic drugs, or non-drug approaches,” panelists said in their report.
The panel graded the quality of the new evidence found in the literature review, and then undertook a consensus development process. Each recommendation and statement required at least 80% consensus to pass.
Their treatment recommendations in the report are focused on three topic areas: stroke and bleeding risk assessment, antithrombotic therapy in general, and antithrombotic therapy in special situations, such as acute coronary syndrome and stenting, chronic atrial flutter, pregnancy, and chronic kidney disease.
Stroke prevention is the main priority in a “holistic approach” to management of atrial fibrillation, the panelists said in the report.
“Many of the risk factors leading to incident AF are also risk factors for ischemic stroke, and the promotion of an integrated or holistic approach to AF management is needed, incorporating stroke prevention, addressing symptoms and risk factor management,” they said.
No antithrombotic therapy is needed for patients who have atrial fibrillation without valvular heart disease, the panelists concluded.
For patients with at least one nongender CHA2DS2-VASc stroke risk factor, oral anticoagulation is recommended over aspirin, aspirin and clopidogrel, or no therapy, they said.
In high-risk patients, including males with two or more CHA2DS2-VASc risk factors and females with three or more, novel oral anticoagulants are recommended over adjusted-dose warfarin, they added.
At each patient contact, patients with atrial fibrillation should receive bleeding risk assessment starting with potentially modifiable risk factors such as uncontrolled blood pressure or excessive alcohol intake, according to the expert panel.
High-risk patients, as indicated by a HAS-BLED score of 3 or greater, should have more frequent and regular follow-up, they said.
The expert panel report concludes with a discussion on practical and patient-centered issues.
“Patient education is essential to provide patients with sufficient information to enable them to make an informed decision about whether or not they wish to take oral anticoagulants, and if they do, which oral anticoagulant they would prefer,” Dr. Lip and his colleagues said in their report.
Dr. Lip disclosed a potential conflict of interest with Boehringer Ingelheim. Expert panel members reported disclosures related to Boston Scientific, Medtronic, St. Jude Medical, Biotronik, MSD, Novartis, Pfizer, Bayer, Servier, Gilead, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Lip GYH et al. CHEST. 2018 Aug 21. pii: S0012-3692(18)32244-X.
For patients with , experts said in a comprehensive, updated guideline.
The 113-page guideline, published in the journal CHEST®, provides antithrombotic treatment recommendations for atrial fibrillation based on different levels of risk for stroke and in a variety of clinical presentations.
Altogether, the new guidelines highlight 60 key recommendations from the 12-person expert panel, chaired by Gregory Y.H. Lip, MD, of the Institute of Cardiovascular Sciences, University of Birmingham (England).
To develop the guidelines, the panel conducted a systematic literature review of relevant articles released since the 2012 publication of Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (9th Edition).
Since that time, “there have been substantial developments in atrial fibrillation thromboprophylaxis, whether with regard to risk assessment, antithrombotic drugs, or non-drug approaches,” panelists said in their report.
The panel graded the quality of the new evidence found in the literature review, and then undertook a consensus development process. Each recommendation and statement required at least 80% consensus to pass.
Their treatment recommendations in the report are focused on three topic areas: stroke and bleeding risk assessment, antithrombotic therapy in general, and antithrombotic therapy in special situations, such as acute coronary syndrome and stenting, chronic atrial flutter, pregnancy, and chronic kidney disease.
Stroke prevention is the main priority in a “holistic approach” to management of atrial fibrillation, the panelists said in the report.
“Many of the risk factors leading to incident AF are also risk factors for ischemic stroke, and the promotion of an integrated or holistic approach to AF management is needed, incorporating stroke prevention, addressing symptoms and risk factor management,” they said.
No antithrombotic therapy is needed for patients who have atrial fibrillation without valvular heart disease, the panelists concluded.
For patients with at least one nongender CHA2DS2-VASc stroke risk factor, oral anticoagulation is recommended over aspirin, aspirin and clopidogrel, or no therapy, they said.
In high-risk patients, including males with two or more CHA2DS2-VASc risk factors and females with three or more, novel oral anticoagulants are recommended over adjusted-dose warfarin, they added.
At each patient contact, patients with atrial fibrillation should receive bleeding risk assessment starting with potentially modifiable risk factors such as uncontrolled blood pressure or excessive alcohol intake, according to the expert panel.
High-risk patients, as indicated by a HAS-BLED score of 3 or greater, should have more frequent and regular follow-up, they said.
The expert panel report concludes with a discussion on practical and patient-centered issues.
“Patient education is essential to provide patients with sufficient information to enable them to make an informed decision about whether or not they wish to take oral anticoagulants, and if they do, which oral anticoagulant they would prefer,” Dr. Lip and his colleagues said in their report.
Dr. Lip disclosed a potential conflict of interest with Boehringer Ingelheim. Expert panel members reported disclosures related to Boston Scientific, Medtronic, St. Jude Medical, Biotronik, MSD, Novartis, Pfizer, Bayer, Servier, Gilead, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Lip GYH et al. CHEST. 2018 Aug 21. pii: S0012-3692(18)32244-X.
For patients with , experts said in a comprehensive, updated guideline.
The 113-page guideline, published in the journal CHEST®, provides antithrombotic treatment recommendations for atrial fibrillation based on different levels of risk for stroke and in a variety of clinical presentations.
Altogether, the new guidelines highlight 60 key recommendations from the 12-person expert panel, chaired by Gregory Y.H. Lip, MD, of the Institute of Cardiovascular Sciences, University of Birmingham (England).
To develop the guidelines, the panel conducted a systematic literature review of relevant articles released since the 2012 publication of Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines (9th Edition).
Since that time, “there have been substantial developments in atrial fibrillation thromboprophylaxis, whether with regard to risk assessment, antithrombotic drugs, or non-drug approaches,” panelists said in their report.
The panel graded the quality of the new evidence found in the literature review, and then undertook a consensus development process. Each recommendation and statement required at least 80% consensus to pass.
Their treatment recommendations in the report are focused on three topic areas: stroke and bleeding risk assessment, antithrombotic therapy in general, and antithrombotic therapy in special situations, such as acute coronary syndrome and stenting, chronic atrial flutter, pregnancy, and chronic kidney disease.
Stroke prevention is the main priority in a “holistic approach” to management of atrial fibrillation, the panelists said in the report.
“Many of the risk factors leading to incident AF are also risk factors for ischemic stroke, and the promotion of an integrated or holistic approach to AF management is needed, incorporating stroke prevention, addressing symptoms and risk factor management,” they said.
No antithrombotic therapy is needed for patients who have atrial fibrillation without valvular heart disease, the panelists concluded.
For patients with at least one nongender CHA2DS2-VASc stroke risk factor, oral anticoagulation is recommended over aspirin, aspirin and clopidogrel, or no therapy, they said.
In high-risk patients, including males with two or more CHA2DS2-VASc risk factors and females with three or more, novel oral anticoagulants are recommended over adjusted-dose warfarin, they added.
At each patient contact, patients with atrial fibrillation should receive bleeding risk assessment starting with potentially modifiable risk factors such as uncontrolled blood pressure or excessive alcohol intake, according to the expert panel.
High-risk patients, as indicated by a HAS-BLED score of 3 or greater, should have more frequent and regular follow-up, they said.
The expert panel report concludes with a discussion on practical and patient-centered issues.
“Patient education is essential to provide patients with sufficient information to enable them to make an informed decision about whether or not they wish to take oral anticoagulants, and if they do, which oral anticoagulant they would prefer,” Dr. Lip and his colleagues said in their report.
Dr. Lip disclosed a potential conflict of interest with Boehringer Ingelheim. Expert panel members reported disclosures related to Boston Scientific, Medtronic, St. Jude Medical, Biotronik, MSD, Novartis, Pfizer, Bayer, Servier, Gilead, Bristol-Myers Squibb, AstraZeneca, and others.
SOURCE: Lip GYH et al. CHEST. 2018 Aug 21. pii: S0012-3692(18)32244-X.
FROM CHEST
Stop treating gout and start curing it, physician urges
LAS VEGAS – Brian F. Mandell, MD, PhD, of Cleveland Clinic, has a message about one of the most devastating conditions that rheumatologists see: Gout isn’t just a treatable disease. It’s a curable one.
Still, research shows time and time again that physicians manage gout “horrendously,” he told colleagues at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “The problem really lies with us,” he said. “We need to do a better job.”
At issue, he believes, is a failure to consider the basic workings of gout when making treatment decisions and advising patients. Lowering serum uric acid (SUA) via medication works, he said, but physicians too frequently don’t go far enough with this approach.
Gout appears to be on the rise in the United States, reflecting increases in related conditions such as obesity and diabetes. A study published this year found that the rate of new-onset gout more than doubled in Olmsted County, Minn., from 1989-1992 to 2009-2010, reaching an adjusted rate of 137/100,000 (J Rheumatol. 2018 Apr;45[4]:574-9).
According to Dr. Mandell, various mysteries regarding gout still need to be cleared up. For one, does resolution of gout also resolve conditions related to hyperuricemia, such as onset of hypertension, progressive chronic kidney disease, and nonalcoholic fatty liver along with higher all-cause mortality?
“We don’t know from interventional studies whether these are as reversible as the gouty arthritis,” he said.
It’s also unknown why so many hyperuricemic patients don’t get flares, with one study estimating that about 50% don’t get them over 15 years (Arthritis Rheumatol. 2017;69[Suppl 10]: Abstract 2843).
One fascinating theory, Dr. Mandell said, suggests “the microbiome is playing a huge [role] in the body’s response to deposits of crystals.”
Fortunately, he said, other mysteries about gout are being solved.
It’s now clear that lowering SUA below 6 mg/dL with medication will reduce flares, Dr. Mandell said. He pointed to a 2017 study of 314 patients with early gout that found 63% of patients who took febuxostat (Uloric) lowered their SUA below 6 mg/dL, compared with just 6% of the placebo group. The overall percentage of patients who had at least one gout flare over 2 years was 29% in the febuxostat group vs. 41% in the placebo group (Arthritis Rheumatol. 2017;69[12]:2386‐95).
It’s also clear that maintenance of lower SUA levels is crucial to prevent recurrence, Dr. Mandell said.
So why is management of hyperuricemia so poor? He ticked off various possible explanations: Maybe it’s the medications. Or perhaps patient compliance is low.
But the drugs are fine, he said, although he cautioned that too-rapid lowering of SUA levels can provoke attacks. He pointed to a 2014 study that suggests allopurinol can help nearly all patients get their SUA below 6 mg/dL, and in the study, the drug was “generally well tolerated” (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
As for compliance, Dr. Mandell said, it can be boosted by patient education. The problem, he said, is that physicians are failing patients by not up-titrating allopurinol despite evidence that this approach works.
He added that hyperuricemia can be managed even in patients on diuretic therapy (Arthritis Res Ther. 2018;20:53).
What about patients who are intolerant to allopurinol or don’t fully respond to it on the SUA front? Dr. Mandell said he likes to try febuxostat, although he noted that it’s tremendously more expensive than allopurinol in the United States with a price that could be 10 times higher.
The nonscored design of febuxostat pills makes dose adjustment difficult in patients, he said, and there are concerns about heart-related and all-cause deaths.
Lesinurad (Zurampic) may be helpful for patients with hyperuricemia that doesn’t response to high doses of xanthine oxidase inhibitors (XOI) or if they’re intolerant to lower inadequate doses, he said. Avoid the drug in patients with chronic kidney disease, he cautioned, and be aware that it’s not approved as a monotherapy. Instead, it’s approved by the Food and Drug Administration for use with an XOI.
As for other gout issues, Dr. Mandell said pegloticase (Krystexxa) via infusion can help patients who don’t respond to an XOI but infusion reactions can occur (mainly in nonresponders), and it’s extremely expensive (about $20,000 per month).
He added that anti–IL-1 therapy is effective in hospitalized patients with gout and doesn’t exacerbate other conditions.
Dr. Mandell disclosed various links to drug makers that produce treatments for gout. He has served as clinical investigator for Horizon, has been a consultant to AstraZeneca, Ironwood, and Horizon, and has received honoraria (unrestricted grants) for continuing medical education activities from Takeda and Horizon. He also reported soliciting advertisements for a journal and educational grants for CME activities.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Brian F. Mandell, MD, PhD, of Cleveland Clinic, has a message about one of the most devastating conditions that rheumatologists see: Gout isn’t just a treatable disease. It’s a curable one.
Still, research shows time and time again that physicians manage gout “horrendously,” he told colleagues at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “The problem really lies with us,” he said. “We need to do a better job.”
At issue, he believes, is a failure to consider the basic workings of gout when making treatment decisions and advising patients. Lowering serum uric acid (SUA) via medication works, he said, but physicians too frequently don’t go far enough with this approach.
Gout appears to be on the rise in the United States, reflecting increases in related conditions such as obesity and diabetes. A study published this year found that the rate of new-onset gout more than doubled in Olmsted County, Minn., from 1989-1992 to 2009-2010, reaching an adjusted rate of 137/100,000 (J Rheumatol. 2018 Apr;45[4]:574-9).
According to Dr. Mandell, various mysteries regarding gout still need to be cleared up. For one, does resolution of gout also resolve conditions related to hyperuricemia, such as onset of hypertension, progressive chronic kidney disease, and nonalcoholic fatty liver along with higher all-cause mortality?
“We don’t know from interventional studies whether these are as reversible as the gouty arthritis,” he said.
It’s also unknown why so many hyperuricemic patients don’t get flares, with one study estimating that about 50% don’t get them over 15 years (Arthritis Rheumatol. 2017;69[Suppl 10]: Abstract 2843).
One fascinating theory, Dr. Mandell said, suggests “the microbiome is playing a huge [role] in the body’s response to deposits of crystals.”
Fortunately, he said, other mysteries about gout are being solved.
It’s now clear that lowering SUA below 6 mg/dL with medication will reduce flares, Dr. Mandell said. He pointed to a 2017 study of 314 patients with early gout that found 63% of patients who took febuxostat (Uloric) lowered their SUA below 6 mg/dL, compared with just 6% of the placebo group. The overall percentage of patients who had at least one gout flare over 2 years was 29% in the febuxostat group vs. 41% in the placebo group (Arthritis Rheumatol. 2017;69[12]:2386‐95).
It’s also clear that maintenance of lower SUA levels is crucial to prevent recurrence, Dr. Mandell said.
So why is management of hyperuricemia so poor? He ticked off various possible explanations: Maybe it’s the medications. Or perhaps patient compliance is low.
But the drugs are fine, he said, although he cautioned that too-rapid lowering of SUA levels can provoke attacks. He pointed to a 2014 study that suggests allopurinol can help nearly all patients get their SUA below 6 mg/dL, and in the study, the drug was “generally well tolerated” (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
As for compliance, Dr. Mandell said, it can be boosted by patient education. The problem, he said, is that physicians are failing patients by not up-titrating allopurinol despite evidence that this approach works.
He added that hyperuricemia can be managed even in patients on diuretic therapy (Arthritis Res Ther. 2018;20:53).
What about patients who are intolerant to allopurinol or don’t fully respond to it on the SUA front? Dr. Mandell said he likes to try febuxostat, although he noted that it’s tremendously more expensive than allopurinol in the United States with a price that could be 10 times higher.
The nonscored design of febuxostat pills makes dose adjustment difficult in patients, he said, and there are concerns about heart-related and all-cause deaths.
Lesinurad (Zurampic) may be helpful for patients with hyperuricemia that doesn’t response to high doses of xanthine oxidase inhibitors (XOI) or if they’re intolerant to lower inadequate doses, he said. Avoid the drug in patients with chronic kidney disease, he cautioned, and be aware that it’s not approved as a monotherapy. Instead, it’s approved by the Food and Drug Administration for use with an XOI.
As for other gout issues, Dr. Mandell said pegloticase (Krystexxa) via infusion can help patients who don’t respond to an XOI but infusion reactions can occur (mainly in nonresponders), and it’s extremely expensive (about $20,000 per month).
He added that anti–IL-1 therapy is effective in hospitalized patients with gout and doesn’t exacerbate other conditions.
Dr. Mandell disclosed various links to drug makers that produce treatments for gout. He has served as clinical investigator for Horizon, has been a consultant to AstraZeneca, Ironwood, and Horizon, and has received honoraria (unrestricted grants) for continuing medical education activities from Takeda and Horizon. He also reported soliciting advertisements for a journal and educational grants for CME activities.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Brian F. Mandell, MD, PhD, of Cleveland Clinic, has a message about one of the most devastating conditions that rheumatologists see: Gout isn’t just a treatable disease. It’s a curable one.
Still, research shows time and time again that physicians manage gout “horrendously,” he told colleagues at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “The problem really lies with us,” he said. “We need to do a better job.”
At issue, he believes, is a failure to consider the basic workings of gout when making treatment decisions and advising patients. Lowering serum uric acid (SUA) via medication works, he said, but physicians too frequently don’t go far enough with this approach.
Gout appears to be on the rise in the United States, reflecting increases in related conditions such as obesity and diabetes. A study published this year found that the rate of new-onset gout more than doubled in Olmsted County, Minn., from 1989-1992 to 2009-2010, reaching an adjusted rate of 137/100,000 (J Rheumatol. 2018 Apr;45[4]:574-9).
According to Dr. Mandell, various mysteries regarding gout still need to be cleared up. For one, does resolution of gout also resolve conditions related to hyperuricemia, such as onset of hypertension, progressive chronic kidney disease, and nonalcoholic fatty liver along with higher all-cause mortality?
“We don’t know from interventional studies whether these are as reversible as the gouty arthritis,” he said.
It’s also unknown why so many hyperuricemic patients don’t get flares, with one study estimating that about 50% don’t get them over 15 years (Arthritis Rheumatol. 2017;69[Suppl 10]: Abstract 2843).
One fascinating theory, Dr. Mandell said, suggests “the microbiome is playing a huge [role] in the body’s response to deposits of crystals.”
Fortunately, he said, other mysteries about gout are being solved.
It’s now clear that lowering SUA below 6 mg/dL with medication will reduce flares, Dr. Mandell said. He pointed to a 2017 study of 314 patients with early gout that found 63% of patients who took febuxostat (Uloric) lowered their SUA below 6 mg/dL, compared with just 6% of the placebo group. The overall percentage of patients who had at least one gout flare over 2 years was 29% in the febuxostat group vs. 41% in the placebo group (Arthritis Rheumatol. 2017;69[12]:2386‐95).
It’s also clear that maintenance of lower SUA levels is crucial to prevent recurrence, Dr. Mandell said.
So why is management of hyperuricemia so poor? He ticked off various possible explanations: Maybe it’s the medications. Or perhaps patient compliance is low.
But the drugs are fine, he said, although he cautioned that too-rapid lowering of SUA levels can provoke attacks. He pointed to a 2014 study that suggests allopurinol can help nearly all patients get their SUA below 6 mg/dL, and in the study, the drug was “generally well tolerated” (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
As for compliance, Dr. Mandell said, it can be boosted by patient education. The problem, he said, is that physicians are failing patients by not up-titrating allopurinol despite evidence that this approach works.
He added that hyperuricemia can be managed even in patients on diuretic therapy (Arthritis Res Ther. 2018;20:53).
What about patients who are intolerant to allopurinol or don’t fully respond to it on the SUA front? Dr. Mandell said he likes to try febuxostat, although he noted that it’s tremendously more expensive than allopurinol in the United States with a price that could be 10 times higher.
The nonscored design of febuxostat pills makes dose adjustment difficult in patients, he said, and there are concerns about heart-related and all-cause deaths.
Lesinurad (Zurampic) may be helpful for patients with hyperuricemia that doesn’t response to high doses of xanthine oxidase inhibitors (XOI) or if they’re intolerant to lower inadequate doses, he said. Avoid the drug in patients with chronic kidney disease, he cautioned, and be aware that it’s not approved as a monotherapy. Instead, it’s approved by the Food and Drug Administration for use with an XOI.
As for other gout issues, Dr. Mandell said pegloticase (Krystexxa) via infusion can help patients who don’t respond to an XOI but infusion reactions can occur (mainly in nonresponders), and it’s extremely expensive (about $20,000 per month).
He added that anti–IL-1 therapy is effective in hospitalized patients with gout and doesn’t exacerbate other conditions.
Dr. Mandell disclosed various links to drug makers that produce treatments for gout. He has served as clinical investigator for Horizon, has been a consultant to AstraZeneca, Ironwood, and Horizon, and has received honoraria (unrestricted grants) for continuing medical education activities from Takeda and Horizon. He also reported soliciting advertisements for a journal and educational grants for CME activities.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Laser treatment tips for pigmented lesions
SAN DIEGO –
Victor Ross, MD, turns to the Q-switched alexandrite laser as his device of choice for most pigmented lesions. “I also use the Q-switched 1,064 nm Nd:YAG and sometimes use the Q-switched 532 nm Nd:YAG, particularly for lighter-skinned patients with lighter lesions,” he said at the annual Masters of Aesthetics Symposium.
Compared with long-pulsed devices, the Q-switched 532 nm neodymium:YAG laser is better for one-time pigment reduction and better for treating lighter pigmented spots, yet it’s associated with a higher risk of postinflammatory hyperpigmentation and short-term crusting. “The Q-switched 532 nm Nd:YAG laser will even treat very tight lentigines, but vascular effects tend to cause an immediate bright red color and more postinflammatory hyperpigmentation,” said Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego. He cautioned that the Q-switched 532 nm Nd:YAG laser may cause prolonged redness on the legs and arms of some patients. “This laser is best reserved for lighter skinned patients with very light lentigines – the brisk purpura can prove distasteful short term for cosmetic patients,” he said. “For darker lentigines, I prefer the IPL [intense pulse light], KTP [potassium titanyl phosphate] laser, or Q-switched alexandrite lasers.”
Meanwhile, treating pigmented lesions treated with long-pulse IPL, KTP, and pulsed dye lasers show less risk of postinflammatory hyperpigmentation and better coverage rates. However, they are sensitive to background color and are less likely to achieve complete one-time removal. The first treatment works the best because the “low hanging fruit” (darker lesions) will do well, he said.
For clinicians looking to improve their skills in treating pigmented lesions with lasers, Dr. Ross recommended using a skin meter such as Cynosure’s Skintel Melanin Reader, which measures the real-time pigment of skin. “You measure the pigment, and it gives you a reading,” he said. “It gives you a recommended setting based on the hand piece and the pulse duration.”
Melasma remains a difficult condition to treat with laser and light. In fact, Dr. Ross joked that he wouldn’t mind if the words “He cured melasma” graced his tombstone one day. “I have been treating melasma patients for 29 years now, and I’m not closer to a cure than when I started out,” he said. “I’ve tried lots of things. In my defense, I’ve made more people better than worse.”
His approach to treating melasma is to begin with a KTP laser or a gentle IPL if discrete lesions or telangiectasia are present. Next, he applies hydroquinone followed by a series of treatment sessions with the Q-switched Nd:YAG laser or a conservative fractional laser. “This tends to induce remission, but is associated with a high rate of relapse,” he said.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
SAN DIEGO –
Victor Ross, MD, turns to the Q-switched alexandrite laser as his device of choice for most pigmented lesions. “I also use the Q-switched 1,064 nm Nd:YAG and sometimes use the Q-switched 532 nm Nd:YAG, particularly for lighter-skinned patients with lighter lesions,” he said at the annual Masters of Aesthetics Symposium.
Compared with long-pulsed devices, the Q-switched 532 nm neodymium:YAG laser is better for one-time pigment reduction and better for treating lighter pigmented spots, yet it’s associated with a higher risk of postinflammatory hyperpigmentation and short-term crusting. “The Q-switched 532 nm Nd:YAG laser will even treat very tight lentigines, but vascular effects tend to cause an immediate bright red color and more postinflammatory hyperpigmentation,” said Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego. He cautioned that the Q-switched 532 nm Nd:YAG laser may cause prolonged redness on the legs and arms of some patients. “This laser is best reserved for lighter skinned patients with very light lentigines – the brisk purpura can prove distasteful short term for cosmetic patients,” he said. “For darker lentigines, I prefer the IPL [intense pulse light], KTP [potassium titanyl phosphate] laser, or Q-switched alexandrite lasers.”
Meanwhile, treating pigmented lesions treated with long-pulse IPL, KTP, and pulsed dye lasers show less risk of postinflammatory hyperpigmentation and better coverage rates. However, they are sensitive to background color and are less likely to achieve complete one-time removal. The first treatment works the best because the “low hanging fruit” (darker lesions) will do well, he said.
For clinicians looking to improve their skills in treating pigmented lesions with lasers, Dr. Ross recommended using a skin meter such as Cynosure’s Skintel Melanin Reader, which measures the real-time pigment of skin. “You measure the pigment, and it gives you a reading,” he said. “It gives you a recommended setting based on the hand piece and the pulse duration.”
Melasma remains a difficult condition to treat with laser and light. In fact, Dr. Ross joked that he wouldn’t mind if the words “He cured melasma” graced his tombstone one day. “I have been treating melasma patients for 29 years now, and I’m not closer to a cure than when I started out,” he said. “I’ve tried lots of things. In my defense, I’ve made more people better than worse.”
His approach to treating melasma is to begin with a KTP laser or a gentle IPL if discrete lesions or telangiectasia are present. Next, he applies hydroquinone followed by a series of treatment sessions with the Q-switched Nd:YAG laser or a conservative fractional laser. “This tends to induce remission, but is associated with a high rate of relapse,” he said.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
SAN DIEGO –
Victor Ross, MD, turns to the Q-switched alexandrite laser as his device of choice for most pigmented lesions. “I also use the Q-switched 1,064 nm Nd:YAG and sometimes use the Q-switched 532 nm Nd:YAG, particularly for lighter-skinned patients with lighter lesions,” he said at the annual Masters of Aesthetics Symposium.
Compared with long-pulsed devices, the Q-switched 532 nm neodymium:YAG laser is better for one-time pigment reduction and better for treating lighter pigmented spots, yet it’s associated with a higher risk of postinflammatory hyperpigmentation and short-term crusting. “The Q-switched 532 nm Nd:YAG laser will even treat very tight lentigines, but vascular effects tend to cause an immediate bright red color and more postinflammatory hyperpigmentation,” said Dr. Ross, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego. He cautioned that the Q-switched 532 nm Nd:YAG laser may cause prolonged redness on the legs and arms of some patients. “This laser is best reserved for lighter skinned patients with very light lentigines – the brisk purpura can prove distasteful short term for cosmetic patients,” he said. “For darker lentigines, I prefer the IPL [intense pulse light], KTP [potassium titanyl phosphate] laser, or Q-switched alexandrite lasers.”
Meanwhile, treating pigmented lesions treated with long-pulse IPL, KTP, and pulsed dye lasers show less risk of postinflammatory hyperpigmentation and better coverage rates. However, they are sensitive to background color and are less likely to achieve complete one-time removal. The first treatment works the best because the “low hanging fruit” (darker lesions) will do well, he said.
For clinicians looking to improve their skills in treating pigmented lesions with lasers, Dr. Ross recommended using a skin meter such as Cynosure’s Skintel Melanin Reader, which measures the real-time pigment of skin. “You measure the pigment, and it gives you a reading,” he said. “It gives you a recommended setting based on the hand piece and the pulse duration.”
Melasma remains a difficult condition to treat with laser and light. In fact, Dr. Ross joked that he wouldn’t mind if the words “He cured melasma” graced his tombstone one day. “I have been treating melasma patients for 29 years now, and I’m not closer to a cure than when I started out,” he said. “I’ve tried lots of things. In my defense, I’ve made more people better than worse.”
His approach to treating melasma is to begin with a KTP laser or a gentle IPL if discrete lesions or telangiectasia are present. Next, he applies hydroquinone followed by a series of treatment sessions with the Q-switched Nd:YAG laser or a conservative fractional laser. “This tends to induce remission, but is associated with a high rate of relapse,” he said.
Dr. Ross disclosed having research and financial ties to numerous pharmaceutical and device companies.
EXPERT ANALYSIS FROM MOAS 2018
Task force advises behavioral intervention for obese adults
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
FROM JAMA
UN aims to eradicate TB by 2030
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.
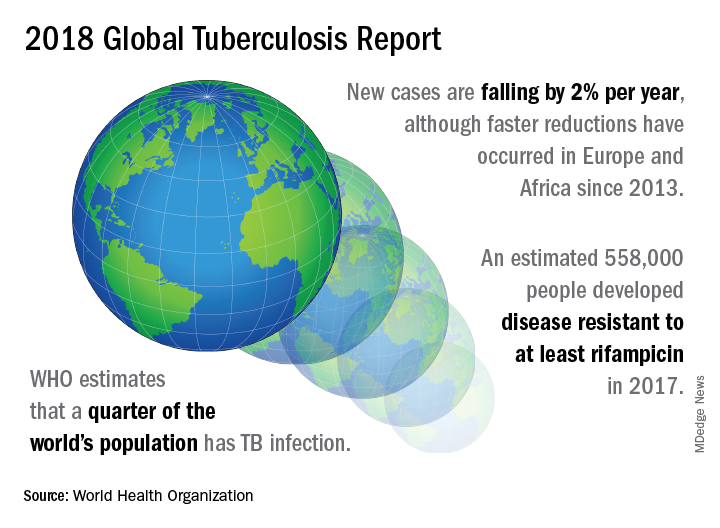
On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
FROM A WORLD HEALTH ORGANIZATION PRESS CONFERENCE
White coats and provider attire: Does it matter to patients?
What is appropriate “ward garb”?
The question of appropriate ward garb is a problem for the ages. Compared with photo stills and films from the 1960s, the doctors of today appear like vagabonds. No ties, no lab coats, and scrub tops have become the norm for a number (a majority?) of hospital-based docs – and even more so on the surgical wards and in the ER.
Past studies have addressed patient preferences for provider dress, but none like the results of a recent survey.
From the University of Michigan, Ann Arbor, comes a physician attire survey of a convenience sample of 4,000 patients at 10 U.S. academic medical centers. It included both inpatients and outpatients, and used the design of many previous studies, showing patients the same doctor dressed seven different ways. After viewing the photographs, the patients received surveys as to their preference of physician based on attire, as well as being asked to rate the physician in the areas of knowledge, trust, care, approachability, and comfort.
You can see the domains: casual, scrubs, and formal, each with and without a lab coat. The seventh category is business attire (future C-suite wannabes – you know who you are).
Over half of the participants indicated that how a physician dresses was important to them, with more than one in three stating that this influenced how happy they were with care received. Overall, respondents indicated that formal attire with white coats was the most preferred form of physician dress.
I found the discussion in the study worthwhile, along with the strengths and weaknesses of the author’s outline. They went to great lengths to design a nonbiased questionnaire and used a consistent approach to shooting their photos. They also discussed lab coats, long sleeves, and hygiene.
But what to draw from the findings? Does patient satisfaction matter or just clinical outcomes? Is patient happiness a means to an end or an end unto itself? Can I even get you exercised about a score of 6 versus 8 (a 25% difference)? For instance, imagine the worst-dressed doc – say shorts and flip-flops. Is that a 5.8 or a 2.3? The anchor matters, and it helps to put the ratings in context.
Read the full post at hospitalleader.org.
Dr. Flansbaum works for Geisinger Health System in Danville, Pa., in both the divisions of hospital medicine and population health. He is a founding member of the Society of Hospital Medicine and served as a board member and officer.
Also in The Hospital Leader
•Hospitalists Can Improve Patient Trust…in Their Colleagues by Chris Moriates, MD, SFHM
•Treatment of Type II MIs by Brad Flansbaum, MD, MPH, MHM
•The $64,000 Question: How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
What is appropriate “ward garb”?
What is appropriate “ward garb”?
The question of appropriate ward garb is a problem for the ages. Compared with photo stills and films from the 1960s, the doctors of today appear like vagabonds. No ties, no lab coats, and scrub tops have become the norm for a number (a majority?) of hospital-based docs – and even more so on the surgical wards and in the ER.
Past studies have addressed patient preferences for provider dress, but none like the results of a recent survey.
From the University of Michigan, Ann Arbor, comes a physician attire survey of a convenience sample of 4,000 patients at 10 U.S. academic medical centers. It included both inpatients and outpatients, and used the design of many previous studies, showing patients the same doctor dressed seven different ways. After viewing the photographs, the patients received surveys as to their preference of physician based on attire, as well as being asked to rate the physician in the areas of knowledge, trust, care, approachability, and comfort.
You can see the domains: casual, scrubs, and formal, each with and without a lab coat. The seventh category is business attire (future C-suite wannabes – you know who you are).
Over half of the participants indicated that how a physician dresses was important to them, with more than one in three stating that this influenced how happy they were with care received. Overall, respondents indicated that formal attire with white coats was the most preferred form of physician dress.
I found the discussion in the study worthwhile, along with the strengths and weaknesses of the author’s outline. They went to great lengths to design a nonbiased questionnaire and used a consistent approach to shooting their photos. They also discussed lab coats, long sleeves, and hygiene.
But what to draw from the findings? Does patient satisfaction matter or just clinical outcomes? Is patient happiness a means to an end or an end unto itself? Can I even get you exercised about a score of 6 versus 8 (a 25% difference)? For instance, imagine the worst-dressed doc – say shorts and flip-flops. Is that a 5.8 or a 2.3? The anchor matters, and it helps to put the ratings in context.
Read the full post at hospitalleader.org.
Dr. Flansbaum works for Geisinger Health System in Danville, Pa., in both the divisions of hospital medicine and population health. He is a founding member of the Society of Hospital Medicine and served as a board member and officer.
Also in The Hospital Leader
•Hospitalists Can Improve Patient Trust…in Their Colleagues by Chris Moriates, MD, SFHM
•Treatment of Type II MIs by Brad Flansbaum, MD, MPH, MHM
•The $64,000 Question: How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM
The question of appropriate ward garb is a problem for the ages. Compared with photo stills and films from the 1960s, the doctors of today appear like vagabonds. No ties, no lab coats, and scrub tops have become the norm for a number (a majority?) of hospital-based docs – and even more so on the surgical wards and in the ER.
Past studies have addressed patient preferences for provider dress, but none like the results of a recent survey.
From the University of Michigan, Ann Arbor, comes a physician attire survey of a convenience sample of 4,000 patients at 10 U.S. academic medical centers. It included both inpatients and outpatients, and used the design of many previous studies, showing patients the same doctor dressed seven different ways. After viewing the photographs, the patients received surveys as to their preference of physician based on attire, as well as being asked to rate the physician in the areas of knowledge, trust, care, approachability, and comfort.
You can see the domains: casual, scrubs, and formal, each with and without a lab coat. The seventh category is business attire (future C-suite wannabes – you know who you are).
Over half of the participants indicated that how a physician dresses was important to them, with more than one in three stating that this influenced how happy they were with care received. Overall, respondents indicated that formal attire with white coats was the most preferred form of physician dress.
I found the discussion in the study worthwhile, along with the strengths and weaknesses of the author’s outline. They went to great lengths to design a nonbiased questionnaire and used a consistent approach to shooting their photos. They also discussed lab coats, long sleeves, and hygiene.
But what to draw from the findings? Does patient satisfaction matter or just clinical outcomes? Is patient happiness a means to an end or an end unto itself? Can I even get you exercised about a score of 6 versus 8 (a 25% difference)? For instance, imagine the worst-dressed doc – say shorts and flip-flops. Is that a 5.8 or a 2.3? The anchor matters, and it helps to put the ratings in context.
Read the full post at hospitalleader.org.
Dr. Flansbaum works for Geisinger Health System in Danville, Pa., in both the divisions of hospital medicine and population health. He is a founding member of the Society of Hospital Medicine and served as a board member and officer.
Also in The Hospital Leader
•Hospitalists Can Improve Patient Trust…in Their Colleagues by Chris Moriates, MD, SFHM
•Treatment of Type II MIs by Brad Flansbaum, MD, MPH, MHM
•The $64,000 Question: How Can Hospitalists Improve Their HCAHPS Scores? by Leslie Flores, MHA, SFHM