User login
The Effect of Age on the Benefits of Early Decompression for Cervical Spondylotic Myelopathy
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Cervical myelopathy is the most common cause of acquired spinal cord dysfunction in people aged >55 years. Advanced age and duration of symptoms have been implicated in the literature as negative prognostic indicators for postoperative functional improvement, but very few studies have evaluated the interaction of these factors. We retrospectively reviewed 125 patients who underwent surgery for cervical myelopathy. Patients were stratified according to age greater or less than 65 years and duration of symptoms of greater or less than 12 and 24 months. Functional outcomes were assessed using the Nurick score. Simple regression and multiple regression analyses were done, controlling for sex, preoperative Nurick score, surgical approach, smoking status, diabetes status, prior surgery, number of levels fused, ethanol use, and signal change on preoperative magnetic resonance imaging. The average change in Nurick score in all patients was 1.36, with a significant difference between patients with symptoms for <24 months and those with symptoms for >24 months (1.54 vs 0.98, P = .03). Multiple regression analysis revealed that older patients had a significant difference at 24 months (1.69 vs 1.25, P = .01), whereas younger patients showed slightly lower improvement overall and a change in Nurick score at both thresholds that was statistically nonsignificant.
Continue to: Cervical spondylotic myelopathy...
Cervical spondylotic myelopathy (CSM) is the most common acquired cause of spinal cord dysfunction in people aged >55 years.1 It is a slowly progressive disorder usually caused by spinal cord compression and ischemia due to age-related changes in the spine and is characterized by neck pain, radicular arm pain, paresthesia, weakness, lower extremity hyperreflexia, and gait and balance abnormalities and may also present with bowel and bladder dysfunction. The majority of cases progress in a stepwise manner, but about 5% of cases decline rapidly, and the prognosis of nonoperative treatment is poor once the patient is truly myelopathic. The objective of surgery is to decompress the spinal cord before permanent damage has set in.2-4
Several studies have attempted to describe the prognostic significance of duration of symptoms in surgical decompression of CSM. Some studies have found that there is no association with outcomes,5-7 but most of the studies have concluded that there is an association. Several of these studies specify that duration of symptoms is significant beyond particular time points, typically of 12 months8-12 or 24 months.13,14 At least 2 review studies have found low evidence for the influence of symptom duration on postoperative outcomes.15,16
Age has also been cited as an important prognostic factor in surgical decompression of CSM by some of these same studies. Only a few studies have concluded that age itself does not affect outcomes.17-19 However, most of the studies conclude that advanced age is a significant factor. Most of these cite a cutoff of 60 years of age,14,20 65 years of age,21 or 70 years of age,10 but at least 1 study has cited a cutoff as young as 40 years of age,9 and at least 1 other has cited 50 years of age.8
Most of the available literature has evaluated the effects of age and duration of symptoms separately. However, at least 2 studies have discussed the interplay between these variables, and both found that outcomes are associated with duration of symptoms only in the elderly, defined as above either 65 or 70 years of age.5,19 This study is an attempt to clarify this relationship.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
Institutional Review Board approval was obtained for this study. Informed consent was waived due to the retrospective nature of the work. The medical records of 212 patients who underwent surgery for CSM by the senior author were reviewed. All surgeries were performed at the University Hospital or the Veterans Administration (VA) between March 2005 and July 2012. CSM was diagnosed by magnetic resonance imaging (MRI) and based on the presence of upper motor signs, clonus, gait abnormalities, or difficulty with fine motor movements such as buttoning a shirt. Nurick score (Table 1) was assessed at presentation and at follow-up, and was the only outcome measure recorded in this cohort. Inclusion criteria were the diagnosis of CSM with a Nurick score, surgical intervention, and at least 2 years of follow-up. Age at presentation, sex, preoperative Nurick score, postoperative Nurick score, duration of symptoms preoperatively, duration of follow-up, procedure performed, approach (anterior vs posterior vs anterior and posterior), prior surgery, number of levels fused, diabetes status, cocaine use, ethanol use, tobacco use, signal change on preoperative MRI, and whether the patient belonged to the VA were recorded. Posterior cervical surgery was performed in patients who had ossification of the posterior longitudinal ligament, had multiple prior anterior cervical procedures, or had involvement of 3 or more levels with anatomy that would make an extensive exposure difficult. Surgeries were performed anteriorly for cases of 1- or 2-level stenosis in the absence of ossification of the posterior longitudinal ligament.
Anterior surgery was also considered in patients with 3-level disease who did not have anatomy that precluded a more extensive exposure.
Patients were stratified according to duration of symptoms by cutoffs of 12 or 24 months and according to age <65 years or >65 years. The age cutoff was chosen because this was the youngest cohort in which stratification revealed a significant difference in change in the Nurick score according to duration of symptoms, and because this age is consistent with the literature. Data were blinded, and outcomes according to duration of symptoms and age were analyzed. The analysis was conducted using simple linear regression and multiple regression.
SURGICAL TECHNIQUE
Patients were evaluated through a complete neurological examination and Nurick scores preoperatively and postoperatively at 6 weeks, 3 months, 6 months, 1 year, and annually thereafter. Decompression procedures performed included single or multilevel corpectomy, anterior decompression with strut grafting and instrumentation, posterior cervical laminoplasty, and posterior cervical laminectomy and fusion. Patients were placed in a Miami J collar (Össur) postoperatively and sent to physical and occupational therapy when able. All procedures were performed by the senior author with the assistance of residents and fellows.
RESULTS
Of the 125 patients who met all the inclusion criteria, 44 were females and 81 were males. The average follow-up duration was 30.9 months (standard deviation [SD], 13.23). The average age of all patients was 55.2 years (range, 27-89 years), and there was no difference in age according to gender (55.0 years for females vs 55.2 years for males). The average preoperative Nurick score was 2.61 (SD, 1.16), and there was no difference in preoperative Nurick score according to cutoff of duration of symptoms. Males had a higher preoperative Nurick score than females (2.73 vs 2.41, P = .12) and a longer but statistically nonsignificant duration of symptoms (25.7 vs 16.9 months, P = .1). There were 97 patients aged ≤65 years (average, 49.6 years) and 28 patients aged >65 years (average, 73.7 years). The younger cohort had a lower preoperative Nurick score than the older cohort, but this difference was not statistically significant (2.52 vs 3.0, P = .06). The younger cohort also had a longer duration of symptoms, but this difference was not significant (21.8 vs 26.2 months, P > .1). The initial analysis of the change in Nurick score in all patients according to duration of symptoms revealed an average change of 1.36 points (SD, 1.13) and a difference in postoperative change in Nurick score for the duration of symptom cutoffs of 12 and 24 months. This pattern was also present when patients were stratified according to age (Tables 2 and 3). The most common procedures performed were anterior cervical discectomy and fusion (ACDF) (58) and corpectomy (49). Data according to the procedure performed are recorded in Table 4. No significant complications were recorded. Simple regression and multiple regression analyses were undertaken to further evaluate these relationships.
Table 1. Nurick Score
0 | Signs or symptoms of nerve root involvement by no signs or symptoms of spinal cord involvement |
1 | Signs of spinal cord compression but no gait abnormalities |
2 | Gait abnormalities but no interference on employment |
3 | Gait abnormalities that prevent full time employment |
4 | Unable to walk without assistance |
5 | Wheelchair bound or bedbound |
Table 2. Change in Nurick According to Threshold of Duration of Symptoms
| <12 months | >12 months | <24 months | >24 months | Total |
Number | 58 | 67 | 85 | 40 | 125 |
Preoperative (SD) | 2.54 (1.22) | 2.70 (1.11) | 2.56 (1.19) | 2.75 (1.09) | 2.61 (1.16) |
Change (SD) | 1.59 (1.12) | 1.17 (1.11) | 1.54 (1.21) | 0.98 (0.87) | 1.36 (1.13) |
Abbreviation: SD, standard deviation.
Table 3. Change in Nurick According to Threshold of Duration of Symptoms, by Age
Age <65 Years | Age >65 Years | |||||||
Months | <12 | >12 | <24 | >24 | <12 | >12 | <24 | >24 |
Number | 49 | 48 | 69 | 28 | 9 | 19 | 16 | 12 |
Preoperative (SD) | 2.53 (1.17) | 2.5 (1.11) | 2.49 (1.17) | 2.57 (1.07) | 2.56 (1.51) | 3.2 (1.03) | 2.88 (1.31) | 3.16 (1.11) |
Change (SD) | 1.61 (1.15) | 1.04 (1.11) | 1.51 (1.22) | 0.89 (0.88) | 1.44 (1.01) | 1.53 (1.12) | 1.69 (1.2) | 1.25 (0.87) |
Abbreviation: SD, standard deviation.
Abbreviations: ACDF, anterior cervical discectomy and fusion; SD, standard deviation.
Continue to: Simple regression analysis of data...
Simple regression analysis of data of all patients revealed a statistically significant negative relationship between duration of symptoms and postoperative change in Nurick score (P = .044). There was no relationship between duration of symptoms and preoperative Nurick score (P = .482). When stratified according to duration of preoperative symptoms by 12 or 24 months, the relationship between duration of symptoms and change in Nurick score was statistically significant for cutoffs of 12 months (P = .03) and 24 months (P = .007). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration. When these results were stratified according to age, patients aged ≤65 years showed a statistically significant association between duration of preoperative symptoms and change in Nurick score for cutoffs of 12 months (P = .016) and 24 months (P = .019). However, patients aged >65 years did not show a statistically significant association for cutoffs of 12 or 24 months (P = .85 and .29, respectively). There was also no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
Multiple regression analysis of the previously described findings was undertaken to assess the influence of potential confounding variables. These included age, gender, diabetes, cocaine use, alcohol use, tobacco use, signal change on preoperative MRI, severity of myelopathy, total levels fused, prior surgery, surgical approach (anterior vs posterior), and procedure performed (Table 4). Analysis of the relationship between duration of symptoms and change in Nurick score for all patients initially revealed a statistically nonsignificant correlation (P = .22). Significant factors in this model included diabetes status and tobacco use that correlated with decreasing change in Nurick score (P = .02 and .0001, respectively) and severity of myelopathy that correlated with increasing change in Nurick score (P = .0002). Notably, combined procedures also correlated with decreasing change in Nurick score (P = .03), but the performance of individual procedures did not correlate with change in Nurick score. There was no association between duration of symptoms and preoperative Nurick score (P = .76). When stratified according to duration of symptoms of 12 or 24 months, only 24 months was found to be statistically significant (P = .03). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of symptom duration. When further stratified according to age, the younger cohort did not show a statistically significant association between duration of preoperative symptoms and change in Nurick score for either threshold of symptom duration (P = .15 and .43, respectively). Diabetes status, tobacco use, number of levels fused, severity of myelopathy, and combined procedures remained significant predictors of change in Nurick score for both thresholds of symptom duration. In contrast, the older cohort showed a statistically significant association between duration of symptoms and postoperative change in Nurick score only for a threshold of 24 months (P = .01). In contrast to the younger cohort, the only other significant predictors in this group were preoperative severity of myelopathy, anterior approach (all ACDF procedures), and signal change on preoperative MRI (P = .02, .04, and .03, respectively). There was no relationship between duration of symptoms and preoperative Nurick score for any threshold of preoperative symptom duration in either age cohort.
DISCUSSION
Several studies have attempted to describe the prognostic influence of preoperative symptom duration on surgical outcomes for CSM. Few studies suggest that duration of symptoms does not correlate with functional outcomes. For example, Naderi and colleagues6 concluded in a retrospective study of 27 patients that there is no correlation as assessed by the modified Japanese Orthopedic Association scale. Handa and colleagues5 similarly concluded in a retrospective study of 61 patients that duration of symptoms was not significant, but only in patients aged <70 years. Furlan and colleagues7 conducted a prospective study of 81 patients with a mean follow-up of 10 months and concluded that there is no association as assessed using the modified Japanese Orthopedic Association (mJOA) and Nurick score. In contrast, the majority of studies support the notion that duration of symptoms adversely affects outcomes. Several of these studies do not provide a clear cutoff beyond which outcomes are significantly affected.17-19,22
Of the studies that provide a cutoff, a fair number of studies suggest a limit of 12 months and a few suggest 24 months. In a retrospective study of 109 patients with cervical radiculopathy and 55 with cervical myelopathy, Bertalanffy and Eggert8 found that duration of symptoms beyond 12 months significantly correlated with worse outcomes as assessed by the evaluation criteria set forth by Roosen and Grote.23 Using the more common European Myelopathy Score, Heidecke and colleagues9 arrived at the same conclusion from a retrospective review of 106 patients. In a large retrospective review of 248 patients, Pumberger and colleagues11 found that patients who did not improve following surgical decompression for CSM, where improvement was defined as a reduction of at least 1 Nurick grade, had an average of 17.85 months of preoperative symptoms, whereas those who did improve had symptoms for an average of 11.21 months. In a prospective study of 98 patients, Suzuki and colleagues10 found that recovery rate of the JOA scale was significantly decreased in those with >1 year of preoperative symptoms. Both Chagas and colleagues14 and Suri and colleagues13 conducted prospective studies that revealed a significant difference in Nurick score improvement in patients with >2 years of symptoms. In reviews of the literature, both Holly and colleagues15 and Yoon and colleagues16 found a low level of evidence for the significance of symptom duration on outcomes. Similarly, Tetreault and colleagues24 found that duration of symptoms was predictive of outcomes as assessed by both mJOA and Nurick score.
Continue to: Our results in all patients showed...
Our results in all patients showed a clear difference in outcomes at the 12-month cutoff as revealed by the simple regression and a trend that reached significance at the 24-month cutoff as assessed by the multiple regression. These results are consistent with those discussed, especially those that specifically used the Nurick score. We further showed that the influence of duration of symptoms on outcomes is dependent on age. Our simple regression analysis suggested that this dependence was evident for symptom durations of 12 and 24 months only in the younger cohort. However, our multiple regression analysis showed that the effect of symptom duration on outcomes is evident only in patients aged >65 years who have had symptoms for 24 months. The stark difference in results between the simple and multiple regressions is probably due to the several potentially confounding variables that were controlled for in the multiple regression analysis. Of course, it should be noted that a statistically nonsignificant difference does not necessarily translate into a clinically nonsignificant difference.
Our results are consistent with the few studies that describe the influence of the interplay between age and duration of symptoms on postoperative outcomes in CSM. For example, Handa and colleagues5 retrospectively reviewed 61 patients who underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 70 years. Compared with the younger patients, duration of symptoms in the 22 elderly patients correlated with a significant difference in outcomes as assessed by the mJOA, with a cutoff of 1 year.5 Similarly, Yamazaki and colleagues19 evaluated 64 patients who also underwent expansive laminoplasty for CSM and stratified them according to age greater or less than 65 years. Duration of symptoms in 35 elderly patients significantly correlated with outcomes as assessed by the JOA scale, such that those considered to have an excellent outcome had a mean duration of symptoms of 11.1 months compared to the 39 months of symptoms in those considered to have a fair outcome.19 In contrast to those studies, we found that 24 months rather than 12 months was significant. However, we also evaluated outcomes using the Nurick score rather than the JOA. The JOA is a more detailed instrument, and this may be the reason for the discrepancy. Nonetheless, our results are consistent with the extant literature and add to the limited number of studies that have commented on the combined interactions of symptom duration and age in postoperative outcomes for CSM.
There are several strengths and limitations to this study. One strength is the relatively large sample size of patients. However, there was an uneven distribution in the number of patients in each age cohort. Ideally, there would have been an equal number of patients in each age group. The fact that all patients were operated on by the same surgeon minimizes variability in outcomes due to surgeon skill. We also controlled for multiple variables that are known to affect CSM outcomes, but we did not have quantitative data with respect to degree of compression or cross-sectional area of the affected spinal cord, which have been described as significant variables in outcomes of CSM. Furthermore, we did not evaluate the results using several outcome measures such as the JOA in addition to the Nurick score, and this limits the comparability of our work to some of the existing literature. This study also suffers from the inherent biases and shortcomings of retrospective studies, and the fact that this was not a multicenter study may limit generalizability of the results. However, given the dearth of literature on this topic, our work adds to the literature. Further studies will be needed to more clearly elucidate this topic.
CONCLUSION
This study demonstrated that duration of symptoms may be a significant factor in the recovery of patients undergoing surgical decompression for CSM, but only in patients aged >65 years who have had symptoms for 24 months.
This paper will be judged for the Resident Writer’s Award.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
1. Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl.):190S-197S. doi:10.1016/j.spinee.2006.04.024.
2. Emery S. Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376-688.
3. Matz PG, Anderson PA, Holly LT, et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):104-111. doi:10.3171/2009.1.SPINE08716.
4. Tracy JA, Bartleson JD. Cervical spondylotic myelopathy. Neurologist. 2010;16(3):176-187 doi:10.1097/NRL.0b013e3181da3a29.
5. Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2):173-179. doi:10.3171/spi.2002.96.2.0173.
6. Naderi S, Ozgen S, Pamir MN, Ozek MM, Erzen C. Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43-49.
7. Furlan JC, Kalsi-Ryan S, Kailaya-Vasan A, Massicotte EM, Fehlings MG. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases. J Neurosurg Spine. 2011;14(3):348-355. doi:10.3171/2010.10.SPINE091029.
8. Bertalanffy H, Eggert HR. Clinical long-term results of anterior discectomy Without fusion for treatment of cervical radiculopathy and myelopathy. Acta Neurochir. 1988;90(3-4):127-135. doi:10.1007/BF01560567.
9. Heidecke V, Rainov NG, Marx T, Burkert W. Outcome in Cloward anterior fusion for degenerative cervical spinal disease. Acta Neurochir (Wien). 2000;142(3):283-291.
10. Suzuki A, Misawa H, Simogata M, Tsutsumimoto T, Takaoka K, Nakamura H. Recovery process following cervical laminoplasty in patients with cervical compression myelopathy: prospective cohort study. Spine (Phila Pa 1976). 2009;34(26):2874-2879. doi:10.1097/BRS.0b013e3181bb0e33.
11. Pumberger M, Froemel D, Aichmair A, et al. Clinical predictors of surgical outcome in cervical spondylotic myelopathy: an analysis of 248 patients. Bone Joint J. 2013;95B(7):966-971. doi:10.1302/0301-620X.95B7.31363.
12. Saunders RL, Bernini PM, Shirreffs TG Jr, Reeves AG. Central corpectomy for cervical spondylotic myelopathy: A consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74(2):163-170. doi:10.3171/jns.1991.74.2.0163.
13. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3(1):33-45. doi:10.1016/S1529-9430(02)00448-5.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64 Suppl 1:S1:30-35; discussion:S1:35-36.
15. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11(2):112-118. doi:10.3171/2009.1.SPINE08718.
16. Yoon ST, Raich A, Hashimoto RE, et al. Predictive factors affecting outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S232-S252. doi:10.1097/BRS.0b013e3182a7eb55.
17. Ebersold M, Pare M, Quast LM. Surgical treatment for cervical spondylotic myelopathy. J Neurosurg. 1995;82(5):745-751. doi:10.3171/jns.1995.82.5.0745.
18. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95(18):1659-1666. doi:10.2106/JBJS.L.01323.
19. Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. Neurosurgery. 2003;52(1):122-126.
20. Lee TT, Manzano GR, Green BA. Modified open-door cervical expansive laminoplasty for spondylotic myelopathy: operative technique, outcome, and predictors for gait improvement. J Neurosurg. 1997;86(1):64-68. doi:10.3171/jns.1997.86.1.0064.
21. Karpova A, Arun R, Davis AM, et al. Predictors of surgical outcome in cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(5):392-400. doi:10.1097/BRS.0b013e3182715bc3.
22. Fujiwara K, Ebara YK, S, Ono K. The prognosis of surgery for cervical compression myelopathy. J Bone Joint Surg Br. 1989;71(3):393-398.
23. Roosen K, Grote W. Late results of operative treatment of cervical myelopathy. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, eds. Surgery of Cervical Myelopathy. Advances in Neurosurgery, vol 8. Heidelberg, Berlin: Springer; 1980:69-77.
24. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24 Suppl 2:236-251. doi:10.1007/s00586-013-2658-z.
TAKE-HOME POINTS
- Decompression of cervical myelopathy within 24 months of symptom onset results in greater functional improvement compared to delayed decompression.
- The improvement with respect to time is more significant for patients older than 65 years compared to younger patients.
- Duration of symptoms does not seem to influence the severity of the preoperative Nurick score.
- Preoperative severity of symptoms is related to postoperative outcomes.
- Other significant predictors of worse outcomes include tobacco use, diabetes, and number of levels fused.
Black patients miss out on promising cancer drugs
This story was co-published with Stat.
It’s a promising new drug for multiple myeloma, one of the most savage blood cancers. Called Ninlaro (ixazomib), it can be taken as a pill, sparing patients painful injections or cumbersome IV treatments. In a video sponsored by the manufacturer, Takeda Pharmaceutical, one patient even hailed Ninlaro as “my savior.”
The U.S. Food and Drug Administration approved it in 2015 after patients in a clinical trial gained an average of 6 months without their cancer spreading. That trial, though, had a major shortcoming: its racial composition. One out of five people diagnosed with multiple myeloma in the United States is black, and African Americans are more than twice as likely as white Americans to be diagnosed with the blood cancer.
Yet, of the 722 participants in the trial, only 13 – or 1.8% – were black.
The scarcity of black patients in Ninlaro’s testing left unanswered the vital question of whether the drug would work equally well for them. “Meaningful differences may exist” in how multiple myeloma affects black patients, what symptoms they experience, and how they respond to medications, FDA scientists wrote in a 2017 journal article.
The racial disparity in the Ninlaro study isn’t unusual. Reflecting the reluctance of the FDA to force drugmakers to enroll more minority patients, and the failure of most manufacturers to do so voluntarily, stark underrepresentation of blacks is widespread in clinical trials for cancer drugs, even when the type of cancer disproportionately affects them. A ProPublica analysis of data recently made public by the FDA found that in trials for 24 of the 31 cancer drugs approved since 2015, fewer than 5% of the patients were black. African Americans make up 13.4% of the U.S. population.
As a result, desperately ill black patients who have exhausted other treatment options aren’t getting early access to experimental drugs that could extend their life spans or improve their quality of life. While unapproved treatments also carry a risk of setbacks or side effects, new cancer drugs have dramatically shifted outcomes for some patients.
Recently approved lung cancer treatments are “revolutionary,” said Karen Kelly, MD, associate director for research at UC Davis Comprehensive Cancer Center. Even in the first phase of clinical testing, which is aimed at making sure a drug is safe, 20% of cancer patients now see their tumors shrink or disappear, up from 5% in the early 1990s, Dr. Kelly said.
Kashif Ali, MD, research head at Maryland Oncology Hematology, has spent 7 years recruiting patients for about 30 cancer and blood disease trials a year. He said he’s often seen minorities, including African Americans, miss out on trials because of financial hurdles, logistical challenges, and their lingering distrust of the medical community because of a history of being victimized by medical experimentation.
“They’re potentially losing out on life-extending opportunities because it’s one more option they no longer have,” Dr. Ali said. “Especially when patients are in advanced stages of cancer, treatments are like stepping-stones: When one stops working, you move on to the next.”
Not joining a trial can mean “you’ve lost life expectancy,” he said.
Pat Conley, a 72-year-old retired business analyst whose multiple myeloma relapsed last year, has never participated in a clinical trial. She was interested several times and didn’t meet the criteria. Now she’s eligible for one, but worries about the burden of regular hour-long trips from her home in Fayetteville, Georgia, to the trial site in Atlanta, as well as the copays for medical tests. “They’ll want a new biopsy, and Lordy, biopsies are not cheap,” she said.
Still, two drug regimens haven’t halted her cancer, and she wants something positive to come from her illness. “If they don’t have African Americans to test it on, how will they know it’s going to work?” she asked. “If it doesn’t help me, it’ll help my children; it’ll help somebody else.”
Pharmaceutical companies contacted by ProPublica all said diversity in clinical trials is important to ensure that drugs meet patients’ needs. The issue “is not elevated high enough in the discussion on clinical studies,” said John Maraganore, PhD, chair of the industry group Biotechnology Innovation Organization. But he added that enrolling minorities is challenging, often for reasons beyond the manufacturer’s control, and that it would require a “public-private partnership, working with the FDA and NIH [National Institutes of Health].”
Black participation reached 10% in trials for only 2 of the 31 cancer drugs: the multiple myeloma drug Darzalex (daratumumab), where the figure was exactly 10%, and Yondelis (trabectedin), which treats two types of soft-tissue cancer. A total of 12% of patients in the Yondelis trial were black, the highest proportion in the ProPublica study. Both drugs are made by Johnson & Johnson, which said it has an internal working group on trial diversity. The working group trains site leaders in best practices for diverse recruitment and seeks minority physicians to help run trials, since some patients prefer being treated by doctors of their own race.
Not enrolling in clinical trials is just one of many ways that African Americans trail white Americans in the quality of their health care. From diagnosis to death, they often experience inferior care and worse outcomes. Because some black Americans can’t afford the health insurance mandated under the Affordable Care Act, they remain less likely to have coverage than do non-Hispanic white Americans. African Americans are 30% more likely than white Americans to die from heart disease. Black mothers are three to four times as likely as white mothers to die in pregnancy or childbirth, and black children are diagnosed with autism later than white children are.
While the scarcity of African Americans stands out in ProPublica’s analysis, there appear to be gaps in participation of other minority groups as well. Asians were well represented in trials held in some foreign countries, but they made up only 1.7% of patients for drugs for which at least 70% of trials were conducted within the United States. By comparison, about 6% of the U.S. population identifies as Asian. Almost two-thirds of the trials didn’t report any Native Americans or Alaska Natives, who together make up about 2%of the U.S. population. ProPublica’s analysis excluded Hispanics, because the FDA reports did not have a separate category for them until 2017 and do not distinguish between white and nonwhite Hispanics.
The very relationship of race to drug development is fraught with controversy. Race is primarily seen as a social concept, rather than as a product of measurable biological traits. Yet there’s growing evidence that, whether for environmental or genetic reasons, drugs may have different effects on different populations.
In 2014, the state of Hawaii sued Bristol-Myers Squibb and Sanofi, the manufacturers of the blood thinner Plavix, accusing them of deceptive marketing for failing to disclose that the drug was less effective for some patients of East Asian or Pacific Islander descent. The drugmakers have denied allegations of misconduct and argue that genetic traits have not been proven to affect how well Plavix works. That case is pending. In the meantime, the FDA has added a warning to the label saying that Chinese patients are more likely to have a gene variation that renders the drug ineffective. Researchers at the University of California, San Francisco, have found that a commonly used asthma medication, albuterol, doesn’t work as well for black and Puerto Rican children as it does for European American or Mexican children.
Inadequate minority representation in drug trials means that “we aren’t doing good science,” said Jonathan Jackson, MD, founding director of the Community Access, Recruitment and Engagement Research Center at Massachusetts General Hospital in Boston. “If we aren’t doing good science and releasing these drugs out into the public, then we are at best being inefficient, at worst being irresponsible.”
The National Black Church Initiative, a coalition of 34,000 U.S. churches, urged the FDA in 2017 to mandate diversity in all clinical trials before approving a drug or device. “Simply put, the pharmaceutical community is not going to improve minority participation in clinical trials until the FDA compels them to do so via regulations,” it wrote to Commissioner Scott Gottlieb.
The FDA hasn’t done so. Although it noted in a 2013 report that a lack of diversity betrays a key ethical principle of medical research – equal justice for all – the agency has shied away from setting quotas or numerical guidelines for participation by race.
Instead, it has relied on persuasion. In its 2014 Action Plan, it said it aimed to “share best practices,” to “support” industry efforts at improving diversity in clinical trials, and to “encourage” patients to participate in trials via social media, emails, and blog posts.
“The FDA believes that enrollment should reflect the patients most likely to use a medical product,” spokeswoman Gloria Sanchez-Contreras said. The FDA “does not have the regulatory authority to require specific levels of minority representation in clinical trials,” although it may ask drugmakers for additional data, she said.
Rachel Sherman, MD, the FDA’s principal deputy commissioner, said that she’s “not entirely satisfied” with minority enrollment but that clinical trials have become more diverse in other ways. Two decades ago, women and children were rarely included in drug studies. Now those groups are better represented and the FDA is working on including more minorities, she said.
Dr. Sherman added that the agency has to balance how much information it demands from drugmakers against the need to get a drug onto the market, where it will be broadly available for all patients.
“When it comes to clinical research in this country, there’s a credit card, and there’s a limit on the credit card,” she said. “If we spend on one thing, it won’t get spent on another. We have to be judicious in what we require and what we demand and what we encourage.”
Diversity has its trade-offs. Clinical trials already cost hundreds of millions of dollars, and drugmakers say that requiring participants to be racially representative would likely add more time and expense.
“If you have a significant delay in enrollment, that would delay the medication advancing to the whole patient population, hurting everybody including the black population,” said Dr. Maraganore, who also is CEO of drugmaker Alnylam Pharmaceuticals.
To offset costs caused by these delays, manufacturers might reduce the number of drugs in development, depriving some patients of experimental treatments, or raise prices, which would translate into higher insurance premiums and make new drugs even less affordable for the uninsured. Dr. Maraganore favors improving diversity through patient education – “a carrot-based approach” – rather than government regulation.
Despite these short-term expenses, eliminating racial disparities in clinical trials would ultimately save costs through disease prevention and improved treatment, according to a 2015 analysis by researchers at the University of California, San Francisco. Without FDA pressure, though, manufacturers may be unlikely to increase efforts to recruit blacks, especially if their sights are set on a worldwide market. They can use the same clinical trial data to gain approval in the European Union or Japan.
Takeda, which is based in Tokyo, tested Ninlaro in the United States, Japan, and 24 other countries, including Australia, Austria, Denmark, New Zealand, Sweden, and others with small black populations. “Clinical trials are reflective of the ethnicity distribution in the population where the study takes place,” Phil Rowlands, head of Takeda’s oncology unit, said in an email. “While we cannot control enrollment eligibility based on the strict clinical criteria, our recruitment efforts extend to diverse patient populations, including minorities.”
Asked why Takeda didn’t pick sites with higher black populations, Mr. Rowlands said that Takeda does not “select sites with ethnicity as an eligibility criteria unless specific risk factors require that.”
Income is another reason for sparse African American representation. Clinical trials are largely a middle-class option. A 2015 study found that patients with an annual household income below $50,000 had 32% lower odds of participating in a trial than did patients with income above that threshold. The median household income of black Americans in 2016 was $39,490, compared with $65,041 for non-Hispanic white Americans.
Patients who can’t afford to travel long distances to a trial, take time off work, or find child care are at a disadvantage. They are usually reimbursed for travel and food costs, but drugmakers are careful not to pay too much because they do not want to appear to be enticing patients to join when it isn’t in their medical interests, said Laurie Halloran, founder and CEO of a consulting firm for the drug industry.
While the experimental drug used in a study is free, any approved treatments that are part of the trial typically need to be covered by a patient’s own insurance, according to Dr. Ali.
He recalled one black patient who was eligible for a study that entailed taking an already approved drug in combination with a new treatment. The approved drug cost $10,000 a month and the patient’s insurance charged 20% as his copay, Dr. Ali recalled. The patient decided that joining the trial would be too expensive. He instead underwent chemotherapy, which was cheaper, but he didn’t tolerate it well. The patient is now considering hospice, Dr. Ali said.
Criteria for admission to clinical trials have become more stringent over the years, Mass. General’s Dr. Jackson said, and patients of color increasingly are excluded. They are more likely than white people are to have other conditions such as stroke, hypertension, and diabetes, which could complicate research results. Trials often want to enroll “the healthiest sick people they can find” and preclude patients with other conditions. “The wall basically gets taller and taller,” he said.
Aredia Taylor didn’t qualify for a clinical trial on other grounds. A former U.S. Department of Agriculture supervisor for food-safety inspections, she was diagnosed with multiple myeloma in 2014 and had undergone a gamut of treatment including various chemotherapy drugs and a stem cell transplant.
While the transplant drove her cancer into remission, she still needs to take Revlimid (lenalidomide) – a standard treatment – daily to keep the cancer at bay. The drug has given her side effects that she describes as devastating to her daily life, including diarrhea, muscle spasms, and an inability to concentrate. She said she wants to stop taking Revlimid and would be willing to try an experimental treatment.
Ms. Taylor, 58, said she saw pamphlets at her doctor’s office encouraging patients to ask about clinical trials, so she asked her oncologist, Larry Anderson, MD, at the University of Texas Southwestern Medical Center in Dallas, whether she should join a study. He told her that she “wasn’t a fit,” she said. Dr. Anderson told ProPublica that most trials are seeking patients with either newly diagnosed or relapsed multiple myeloma, and since Ms. Taylor is currently in remission, she wouldn’t be accepted.
Ms. Taylor was disappointed. Knowing that blacks like herself are especially susceptible to multiple myeloma, she’d like to be a part of developing more effective treatments.
“I want to pay it forward and be a blessing to somebody else,” she said. “I want to be one of the people that they try to do a clinical trial for, so they find a way to a cure.”
Spurred by Congress, the FDA began in January 2015 to regularly publish a “Drug Trials Snapshot” for every new drug approved, delineating the demographic breakdown for clinical trial participants by sex, race and age subgroups. ProPublica’s analysis focused on participants in trials for the 31 cancer drugs approved since then, comparing their demographics with data from the National Cancer Institute on the incidence of various cancers by race.
Eighteen of those drugs targeted cancers that are at least as likely to afflict black Americans as white Americans. On average, only 4.1% of patients in those trials were black. Trials for four multiple myeloma drugs, including Ninlaro, averaged 5% black participation.
An FDA study over a longer time period corroborated ProPublica’s findings. In a 2017 article in the journal Blood coauthored by Richard Pazdur, the FDA’s cancer chief, the agency reported that black patients on average made up 4.5% of participants in trials for multiple myeloma drugs since 2003. Higher enrollment of minorities in myeloma trials would have “multiple benefits,” the FDA scientists noted. Not only would “underserved populations” gain access to new therapies, but additional data could help researchers identify subtypes of the blood cancer and develop targeted treatments.
A similar pattern emerges for treatments of non–small cell lung cancer. It occurs in 56 out of 100,000 black Americans, versus 49 out of 100,000 white Americans. Yet ProPublica found that in trials for two recently approved drugs for a type of non–small cell lung cancer driven by a mutation in what is known as the ALK gene, less than 2% of participants were black.
Those drugs, Takeda’s Alunbrig (brigatinib) and Genentech’s Alecensa (alectinib), are approved for patients whose lung cancer has spread to other parts of the body. In trials, both drugs were able to shrink tumors, including lesions in the brain. Patients taking Alecensa lived without the disease progressing for an average of 25.7 months, more than double the 10.4 months for patients on another treatment.
“We believe that we must consider differences across all populations to deliver on the promise of personalized health care,” said Meghan Cox, a Genentech spokeswoman, adding that the drugmaker is “continuing to study patient response to Alecensa across populations in the postmarketing setting.”
Similarly, prostate cancer affects 178 out of every 100,000 African Americans, more than for any other race in the United States, compared with 106 out of every 100,000 white Americans. Black Americans are twice as likely as white Americans to die from prostate cancer.
Yet during 2009-2015, in seven trials conducted for five new prostate cancer therapies, only 3% of participants were black, according to a study conducted by Daniel Spratt, MD, vice chair of research in the department of radiation oncology at the University of Michigan, Ann Arbor. More recently, 11 times as many white as black participants, or 66% – compared with 6% – joined trials for Johnson & Johnson’s new prostate cancer treatment, Erleada (apalutamide).
The FDA approved Erleada in February after trials showed patients on the drug lived an average of 2 years longer without their cancer spreading into other organs than if they were taking a placebo. In a J&J press release, physicians hailed Erleada’s “impressive clinical benefits” for prostate cancer patients.
J&J is “confident in the efficacy and safety data that have supported the approval” of Erleada, J&J spokeswoman Satu Glawe said.
Like Genentech, J&J and Takeda said they track drugs after approval to see if any racial differences emerge. For example, after another J&J drug for prostate cancer, Zytiga (abiraterone), went on the market in 2011, the company ran a new study of 100 patients, 50 black and 50 white.
“We were aware of the low number of African American men” in the preapproval drug studies of Zytiga, Ms. Glawe said. The postmarketing study was intended “to ensure the medication was also providing clinical benefit to these patients.” In fact, it showed that black men responded better than white men did to the drug.
The FDA is able to look for signs that an approved drug isn’t helping a specific population through a surveillance system launched in 2016, called the Sentinel Initiative, which provides access to medical claims and other data on 200 million Americans obtained from insurers and other health providers, said the FDA’s Dr. Sherman.
Still, postmarketing surveillance doesn’t compensate for lack of diversity in clinical trials. Minorities still miss out on experimental treatments – and, if they take a drug once it’s approved, may suffer unanticipated side effects.
Leaving analysis of a drug’s effect on minorities until it’s already on the market is “a hubristic assumption, unnecessarily arrogant,” Dr. Jackson said. Drugs should “work for the individuals who are the most vulnerable,” he said. “That necessarily includes racial and ethnic minorities.”
Like African Americans, Native Americans rarely enroll in clinical trials. Among the drug trials analyzed by ProPublica, 64.5% did not report any Native American participants, even for types of cancer that Native Americans get at similar rates as other races. (It’s possible that some Native Americans were enrolled but lumped into a generic “other” category.)
Native Americans are at higher risk of colorectal cancer than are white or Asian Americans. Yet the drugmaker Taiho Oncology didn’t report a single Native American among the 800 participants in its trials for the colorectal cancer treatment Lonsurf (trifluridine and tipiracil). Taiho didn’t respond to requests for comment.
To understand how a small minority group responds to a drug, researchers might have to enroll more patients than what would be a nationally representative sample. In a trial of 100 people, two Native American or Alaska Native patients would reflect those groups’ proportion of the U.S. population but wouldn’t give doctors enough information about race-related impacts.
Linda Burhansstipanov, DrPH, founder of the Native American Cancer Research Corp., estimated that 85% of Native Americans want to participate in clinical trials when informed of the opportunity. She knows several Native American patients who drove 3 hours each way to participate in a trial, despite losing an entire day of work.
Still, trial protocols are rarely designed with minority communities in mind, Dr. Burhansstipanov said. It has long been rumored among Native Americans, she said, that a clinical trial in the 1990s required patients to take a medication upon rising in the morning. In many Native American tribes, the first thing people do when they wake up is greet the sun with morning prayers. For some tribes, prayers can take more than half an hour. Because of the delay, the tale goes, Native American patients were kicked out of the clinical trial for violating the protocol. “The story spread and became a barrier for people to take part in clinical trials,” she said.
In addition, the history of unscrupulous medical experimentation on minorities feeds wariness of clinical trials. Angela M. Marshall, MD, an internal medicine doctor and board member of Black Women’s Health Imperative, said she sees a “lack of excitement among minority communities for clinical trials, coming from a mistrust of the medical community.”
Her black patients often cite the infamous Tuskegee study, conducted from 1932 to 1972 by the U.S. Public Health Service, in which researchers knowingly withheld treatment from African American sharecroppers with syphilis in order to study the progression of the disease. Some Native Americans are similarly suspicious of the medical community, Dr. Burhansstipanov said, because the Indian Health Service (a unit of the U.S. Department of Health and Human Services) sterilized thousands of Native American women in the 1970s without their consent.
“The medical community has to engage in some serious trust-building initiatives,” Dr. Marshall said.
That trust is what has helped to keep Thomas Goode alive. An African American diagnosed with multiple myeloma in 2005 when he was 34 years old, Mr. Goode has endured three stem cell transplants. Between each transplant, he participated in clinical trials, where he gained access to drug cocktails that helped bridge him to the next procedure. He counted himself lucky that he lived close to Durham, North Carolina, which is a research hub, so he was able to see specialists and take part in trials.
“I never knew anything about clinical trials, but a lot of my trust and faith was in the doctor,” he said. “I said, ‘I trust you, doctor, whatever you say.’ ”
Now, Mr. Goode has gone 6 years without a relapse. “I’m in a good place right now,” he said, “But if I didn’t try a trial, who’s to say I would still be here?”
ProPublica is a Pulitzer Prize–winning investigative newsroom. Stat is a news organization reporting from the frontiers of health and medicine.
This story was co-published with Stat.
It’s a promising new drug for multiple myeloma, one of the most savage blood cancers. Called Ninlaro (ixazomib), it can be taken as a pill, sparing patients painful injections or cumbersome IV treatments. In a video sponsored by the manufacturer, Takeda Pharmaceutical, one patient even hailed Ninlaro as “my savior.”
The U.S. Food and Drug Administration approved it in 2015 after patients in a clinical trial gained an average of 6 months without their cancer spreading. That trial, though, had a major shortcoming: its racial composition. One out of five people diagnosed with multiple myeloma in the United States is black, and African Americans are more than twice as likely as white Americans to be diagnosed with the blood cancer.
Yet, of the 722 participants in the trial, only 13 – or 1.8% – were black.
The scarcity of black patients in Ninlaro’s testing left unanswered the vital question of whether the drug would work equally well for them. “Meaningful differences may exist” in how multiple myeloma affects black patients, what symptoms they experience, and how they respond to medications, FDA scientists wrote in a 2017 journal article.
The racial disparity in the Ninlaro study isn’t unusual. Reflecting the reluctance of the FDA to force drugmakers to enroll more minority patients, and the failure of most manufacturers to do so voluntarily, stark underrepresentation of blacks is widespread in clinical trials for cancer drugs, even when the type of cancer disproportionately affects them. A ProPublica analysis of data recently made public by the FDA found that in trials for 24 of the 31 cancer drugs approved since 2015, fewer than 5% of the patients were black. African Americans make up 13.4% of the U.S. population.
As a result, desperately ill black patients who have exhausted other treatment options aren’t getting early access to experimental drugs that could extend their life spans or improve their quality of life. While unapproved treatments also carry a risk of setbacks or side effects, new cancer drugs have dramatically shifted outcomes for some patients.
Recently approved lung cancer treatments are “revolutionary,” said Karen Kelly, MD, associate director for research at UC Davis Comprehensive Cancer Center. Even in the first phase of clinical testing, which is aimed at making sure a drug is safe, 20% of cancer patients now see their tumors shrink or disappear, up from 5% in the early 1990s, Dr. Kelly said.
Kashif Ali, MD, research head at Maryland Oncology Hematology, has spent 7 years recruiting patients for about 30 cancer and blood disease trials a year. He said he’s often seen minorities, including African Americans, miss out on trials because of financial hurdles, logistical challenges, and their lingering distrust of the medical community because of a history of being victimized by medical experimentation.
“They’re potentially losing out on life-extending opportunities because it’s one more option they no longer have,” Dr. Ali said. “Especially when patients are in advanced stages of cancer, treatments are like stepping-stones: When one stops working, you move on to the next.”
Not joining a trial can mean “you’ve lost life expectancy,” he said.
Pat Conley, a 72-year-old retired business analyst whose multiple myeloma relapsed last year, has never participated in a clinical trial. She was interested several times and didn’t meet the criteria. Now she’s eligible for one, but worries about the burden of regular hour-long trips from her home in Fayetteville, Georgia, to the trial site in Atlanta, as well as the copays for medical tests. “They’ll want a new biopsy, and Lordy, biopsies are not cheap,” she said.
Still, two drug regimens haven’t halted her cancer, and she wants something positive to come from her illness. “If they don’t have African Americans to test it on, how will they know it’s going to work?” she asked. “If it doesn’t help me, it’ll help my children; it’ll help somebody else.”
Pharmaceutical companies contacted by ProPublica all said diversity in clinical trials is important to ensure that drugs meet patients’ needs. The issue “is not elevated high enough in the discussion on clinical studies,” said John Maraganore, PhD, chair of the industry group Biotechnology Innovation Organization. But he added that enrolling minorities is challenging, often for reasons beyond the manufacturer’s control, and that it would require a “public-private partnership, working with the FDA and NIH [National Institutes of Health].”
Black participation reached 10% in trials for only 2 of the 31 cancer drugs: the multiple myeloma drug Darzalex (daratumumab), where the figure was exactly 10%, and Yondelis (trabectedin), which treats two types of soft-tissue cancer. A total of 12% of patients in the Yondelis trial were black, the highest proportion in the ProPublica study. Both drugs are made by Johnson & Johnson, which said it has an internal working group on trial diversity. The working group trains site leaders in best practices for diverse recruitment and seeks minority physicians to help run trials, since some patients prefer being treated by doctors of their own race.
Not enrolling in clinical trials is just one of many ways that African Americans trail white Americans in the quality of their health care. From diagnosis to death, they often experience inferior care and worse outcomes. Because some black Americans can’t afford the health insurance mandated under the Affordable Care Act, they remain less likely to have coverage than do non-Hispanic white Americans. African Americans are 30% more likely than white Americans to die from heart disease. Black mothers are three to four times as likely as white mothers to die in pregnancy or childbirth, and black children are diagnosed with autism later than white children are.
While the scarcity of African Americans stands out in ProPublica’s analysis, there appear to be gaps in participation of other minority groups as well. Asians were well represented in trials held in some foreign countries, but they made up only 1.7% of patients for drugs for which at least 70% of trials were conducted within the United States. By comparison, about 6% of the U.S. population identifies as Asian. Almost two-thirds of the trials didn’t report any Native Americans or Alaska Natives, who together make up about 2%of the U.S. population. ProPublica’s analysis excluded Hispanics, because the FDA reports did not have a separate category for them until 2017 and do not distinguish between white and nonwhite Hispanics.
The very relationship of race to drug development is fraught with controversy. Race is primarily seen as a social concept, rather than as a product of measurable biological traits. Yet there’s growing evidence that, whether for environmental or genetic reasons, drugs may have different effects on different populations.
In 2014, the state of Hawaii sued Bristol-Myers Squibb and Sanofi, the manufacturers of the blood thinner Plavix, accusing them of deceptive marketing for failing to disclose that the drug was less effective for some patients of East Asian or Pacific Islander descent. The drugmakers have denied allegations of misconduct and argue that genetic traits have not been proven to affect how well Plavix works. That case is pending. In the meantime, the FDA has added a warning to the label saying that Chinese patients are more likely to have a gene variation that renders the drug ineffective. Researchers at the University of California, San Francisco, have found that a commonly used asthma medication, albuterol, doesn’t work as well for black and Puerto Rican children as it does for European American or Mexican children.
Inadequate minority representation in drug trials means that “we aren’t doing good science,” said Jonathan Jackson, MD, founding director of the Community Access, Recruitment and Engagement Research Center at Massachusetts General Hospital in Boston. “If we aren’t doing good science and releasing these drugs out into the public, then we are at best being inefficient, at worst being irresponsible.”
The National Black Church Initiative, a coalition of 34,000 U.S. churches, urged the FDA in 2017 to mandate diversity in all clinical trials before approving a drug or device. “Simply put, the pharmaceutical community is not going to improve minority participation in clinical trials until the FDA compels them to do so via regulations,” it wrote to Commissioner Scott Gottlieb.
The FDA hasn’t done so. Although it noted in a 2013 report that a lack of diversity betrays a key ethical principle of medical research – equal justice for all – the agency has shied away from setting quotas or numerical guidelines for participation by race.
Instead, it has relied on persuasion. In its 2014 Action Plan, it said it aimed to “share best practices,” to “support” industry efforts at improving diversity in clinical trials, and to “encourage” patients to participate in trials via social media, emails, and blog posts.
“The FDA believes that enrollment should reflect the patients most likely to use a medical product,” spokeswoman Gloria Sanchez-Contreras said. The FDA “does not have the regulatory authority to require specific levels of minority representation in clinical trials,” although it may ask drugmakers for additional data, she said.
Rachel Sherman, MD, the FDA’s principal deputy commissioner, said that she’s “not entirely satisfied” with minority enrollment but that clinical trials have become more diverse in other ways. Two decades ago, women and children were rarely included in drug studies. Now those groups are better represented and the FDA is working on including more minorities, she said.
Dr. Sherman added that the agency has to balance how much information it demands from drugmakers against the need to get a drug onto the market, where it will be broadly available for all patients.
“When it comes to clinical research in this country, there’s a credit card, and there’s a limit on the credit card,” she said. “If we spend on one thing, it won’t get spent on another. We have to be judicious in what we require and what we demand and what we encourage.”
Diversity has its trade-offs. Clinical trials already cost hundreds of millions of dollars, and drugmakers say that requiring participants to be racially representative would likely add more time and expense.
“If you have a significant delay in enrollment, that would delay the medication advancing to the whole patient population, hurting everybody including the black population,” said Dr. Maraganore, who also is CEO of drugmaker Alnylam Pharmaceuticals.
To offset costs caused by these delays, manufacturers might reduce the number of drugs in development, depriving some patients of experimental treatments, or raise prices, which would translate into higher insurance premiums and make new drugs even less affordable for the uninsured. Dr. Maraganore favors improving diversity through patient education – “a carrot-based approach” – rather than government regulation.
Despite these short-term expenses, eliminating racial disparities in clinical trials would ultimately save costs through disease prevention and improved treatment, according to a 2015 analysis by researchers at the University of California, San Francisco. Without FDA pressure, though, manufacturers may be unlikely to increase efforts to recruit blacks, especially if their sights are set on a worldwide market. They can use the same clinical trial data to gain approval in the European Union or Japan.
Takeda, which is based in Tokyo, tested Ninlaro in the United States, Japan, and 24 other countries, including Australia, Austria, Denmark, New Zealand, Sweden, and others with small black populations. “Clinical trials are reflective of the ethnicity distribution in the population where the study takes place,” Phil Rowlands, head of Takeda’s oncology unit, said in an email. “While we cannot control enrollment eligibility based on the strict clinical criteria, our recruitment efforts extend to diverse patient populations, including minorities.”
Asked why Takeda didn’t pick sites with higher black populations, Mr. Rowlands said that Takeda does not “select sites with ethnicity as an eligibility criteria unless specific risk factors require that.”
Income is another reason for sparse African American representation. Clinical trials are largely a middle-class option. A 2015 study found that patients with an annual household income below $50,000 had 32% lower odds of participating in a trial than did patients with income above that threshold. The median household income of black Americans in 2016 was $39,490, compared with $65,041 for non-Hispanic white Americans.
Patients who can’t afford to travel long distances to a trial, take time off work, or find child care are at a disadvantage. They are usually reimbursed for travel and food costs, but drugmakers are careful not to pay too much because they do not want to appear to be enticing patients to join when it isn’t in their medical interests, said Laurie Halloran, founder and CEO of a consulting firm for the drug industry.
While the experimental drug used in a study is free, any approved treatments that are part of the trial typically need to be covered by a patient’s own insurance, according to Dr. Ali.
He recalled one black patient who was eligible for a study that entailed taking an already approved drug in combination with a new treatment. The approved drug cost $10,000 a month and the patient’s insurance charged 20% as his copay, Dr. Ali recalled. The patient decided that joining the trial would be too expensive. He instead underwent chemotherapy, which was cheaper, but he didn’t tolerate it well. The patient is now considering hospice, Dr. Ali said.
Criteria for admission to clinical trials have become more stringent over the years, Mass. General’s Dr. Jackson said, and patients of color increasingly are excluded. They are more likely than white people are to have other conditions such as stroke, hypertension, and diabetes, which could complicate research results. Trials often want to enroll “the healthiest sick people they can find” and preclude patients with other conditions. “The wall basically gets taller and taller,” he said.
Aredia Taylor didn’t qualify for a clinical trial on other grounds. A former U.S. Department of Agriculture supervisor for food-safety inspections, she was diagnosed with multiple myeloma in 2014 and had undergone a gamut of treatment including various chemotherapy drugs and a stem cell transplant.
While the transplant drove her cancer into remission, she still needs to take Revlimid (lenalidomide) – a standard treatment – daily to keep the cancer at bay. The drug has given her side effects that she describes as devastating to her daily life, including diarrhea, muscle spasms, and an inability to concentrate. She said she wants to stop taking Revlimid and would be willing to try an experimental treatment.
Ms. Taylor, 58, said she saw pamphlets at her doctor’s office encouraging patients to ask about clinical trials, so she asked her oncologist, Larry Anderson, MD, at the University of Texas Southwestern Medical Center in Dallas, whether she should join a study. He told her that she “wasn’t a fit,” she said. Dr. Anderson told ProPublica that most trials are seeking patients with either newly diagnosed or relapsed multiple myeloma, and since Ms. Taylor is currently in remission, she wouldn’t be accepted.
Ms. Taylor was disappointed. Knowing that blacks like herself are especially susceptible to multiple myeloma, she’d like to be a part of developing more effective treatments.
“I want to pay it forward and be a blessing to somebody else,” she said. “I want to be one of the people that they try to do a clinical trial for, so they find a way to a cure.”
Spurred by Congress, the FDA began in January 2015 to regularly publish a “Drug Trials Snapshot” for every new drug approved, delineating the demographic breakdown for clinical trial participants by sex, race and age subgroups. ProPublica’s analysis focused on participants in trials for the 31 cancer drugs approved since then, comparing their demographics with data from the National Cancer Institute on the incidence of various cancers by race.
Eighteen of those drugs targeted cancers that are at least as likely to afflict black Americans as white Americans. On average, only 4.1% of patients in those trials were black. Trials for four multiple myeloma drugs, including Ninlaro, averaged 5% black participation.
An FDA study over a longer time period corroborated ProPublica’s findings. In a 2017 article in the journal Blood coauthored by Richard Pazdur, the FDA’s cancer chief, the agency reported that black patients on average made up 4.5% of participants in trials for multiple myeloma drugs since 2003. Higher enrollment of minorities in myeloma trials would have “multiple benefits,” the FDA scientists noted. Not only would “underserved populations” gain access to new therapies, but additional data could help researchers identify subtypes of the blood cancer and develop targeted treatments.
A similar pattern emerges for treatments of non–small cell lung cancer. It occurs in 56 out of 100,000 black Americans, versus 49 out of 100,000 white Americans. Yet ProPublica found that in trials for two recently approved drugs for a type of non–small cell lung cancer driven by a mutation in what is known as the ALK gene, less than 2% of participants were black.
Those drugs, Takeda’s Alunbrig (brigatinib) and Genentech’s Alecensa (alectinib), are approved for patients whose lung cancer has spread to other parts of the body. In trials, both drugs were able to shrink tumors, including lesions in the brain. Patients taking Alecensa lived without the disease progressing for an average of 25.7 months, more than double the 10.4 months for patients on another treatment.
“We believe that we must consider differences across all populations to deliver on the promise of personalized health care,” said Meghan Cox, a Genentech spokeswoman, adding that the drugmaker is “continuing to study patient response to Alecensa across populations in the postmarketing setting.”
Similarly, prostate cancer affects 178 out of every 100,000 African Americans, more than for any other race in the United States, compared with 106 out of every 100,000 white Americans. Black Americans are twice as likely as white Americans to die from prostate cancer.
Yet during 2009-2015, in seven trials conducted for five new prostate cancer therapies, only 3% of participants were black, according to a study conducted by Daniel Spratt, MD, vice chair of research in the department of radiation oncology at the University of Michigan, Ann Arbor. More recently, 11 times as many white as black participants, or 66% – compared with 6% – joined trials for Johnson & Johnson’s new prostate cancer treatment, Erleada (apalutamide).
The FDA approved Erleada in February after trials showed patients on the drug lived an average of 2 years longer without their cancer spreading into other organs than if they were taking a placebo. In a J&J press release, physicians hailed Erleada’s “impressive clinical benefits” for prostate cancer patients.
J&J is “confident in the efficacy and safety data that have supported the approval” of Erleada, J&J spokeswoman Satu Glawe said.
Like Genentech, J&J and Takeda said they track drugs after approval to see if any racial differences emerge. For example, after another J&J drug for prostate cancer, Zytiga (abiraterone), went on the market in 2011, the company ran a new study of 100 patients, 50 black and 50 white.
“We were aware of the low number of African American men” in the preapproval drug studies of Zytiga, Ms. Glawe said. The postmarketing study was intended “to ensure the medication was also providing clinical benefit to these patients.” In fact, it showed that black men responded better than white men did to the drug.
The FDA is able to look for signs that an approved drug isn’t helping a specific population through a surveillance system launched in 2016, called the Sentinel Initiative, which provides access to medical claims and other data on 200 million Americans obtained from insurers and other health providers, said the FDA’s Dr. Sherman.
Still, postmarketing surveillance doesn’t compensate for lack of diversity in clinical trials. Minorities still miss out on experimental treatments – and, if they take a drug once it’s approved, may suffer unanticipated side effects.
Leaving analysis of a drug’s effect on minorities until it’s already on the market is “a hubristic assumption, unnecessarily arrogant,” Dr. Jackson said. Drugs should “work for the individuals who are the most vulnerable,” he said. “That necessarily includes racial and ethnic minorities.”
Like African Americans, Native Americans rarely enroll in clinical trials. Among the drug trials analyzed by ProPublica, 64.5% did not report any Native American participants, even for types of cancer that Native Americans get at similar rates as other races. (It’s possible that some Native Americans were enrolled but lumped into a generic “other” category.)
Native Americans are at higher risk of colorectal cancer than are white or Asian Americans. Yet the drugmaker Taiho Oncology didn’t report a single Native American among the 800 participants in its trials for the colorectal cancer treatment Lonsurf (trifluridine and tipiracil). Taiho didn’t respond to requests for comment.
To understand how a small minority group responds to a drug, researchers might have to enroll more patients than what would be a nationally representative sample. In a trial of 100 people, two Native American or Alaska Native patients would reflect those groups’ proportion of the U.S. population but wouldn’t give doctors enough information about race-related impacts.
Linda Burhansstipanov, DrPH, founder of the Native American Cancer Research Corp., estimated that 85% of Native Americans want to participate in clinical trials when informed of the opportunity. She knows several Native American patients who drove 3 hours each way to participate in a trial, despite losing an entire day of work.
Still, trial protocols are rarely designed with minority communities in mind, Dr. Burhansstipanov said. It has long been rumored among Native Americans, she said, that a clinical trial in the 1990s required patients to take a medication upon rising in the morning. In many Native American tribes, the first thing people do when they wake up is greet the sun with morning prayers. For some tribes, prayers can take more than half an hour. Because of the delay, the tale goes, Native American patients were kicked out of the clinical trial for violating the protocol. “The story spread and became a barrier for people to take part in clinical trials,” she said.
In addition, the history of unscrupulous medical experimentation on minorities feeds wariness of clinical trials. Angela M. Marshall, MD, an internal medicine doctor and board member of Black Women’s Health Imperative, said she sees a “lack of excitement among minority communities for clinical trials, coming from a mistrust of the medical community.”
Her black patients often cite the infamous Tuskegee study, conducted from 1932 to 1972 by the U.S. Public Health Service, in which researchers knowingly withheld treatment from African American sharecroppers with syphilis in order to study the progression of the disease. Some Native Americans are similarly suspicious of the medical community, Dr. Burhansstipanov said, because the Indian Health Service (a unit of the U.S. Department of Health and Human Services) sterilized thousands of Native American women in the 1970s without their consent.
“The medical community has to engage in some serious trust-building initiatives,” Dr. Marshall said.
That trust is what has helped to keep Thomas Goode alive. An African American diagnosed with multiple myeloma in 2005 when he was 34 years old, Mr. Goode has endured three stem cell transplants. Between each transplant, he participated in clinical trials, where he gained access to drug cocktails that helped bridge him to the next procedure. He counted himself lucky that he lived close to Durham, North Carolina, which is a research hub, so he was able to see specialists and take part in trials.
“I never knew anything about clinical trials, but a lot of my trust and faith was in the doctor,” he said. “I said, ‘I trust you, doctor, whatever you say.’ ”
Now, Mr. Goode has gone 6 years without a relapse. “I’m in a good place right now,” he said, “But if I didn’t try a trial, who’s to say I would still be here?”
ProPublica is a Pulitzer Prize–winning investigative newsroom. Stat is a news organization reporting from the frontiers of health and medicine.
This story was co-published with Stat.
It’s a promising new drug for multiple myeloma, one of the most savage blood cancers. Called Ninlaro (ixazomib), it can be taken as a pill, sparing patients painful injections or cumbersome IV treatments. In a video sponsored by the manufacturer, Takeda Pharmaceutical, one patient even hailed Ninlaro as “my savior.”
The U.S. Food and Drug Administration approved it in 2015 after patients in a clinical trial gained an average of 6 months without their cancer spreading. That trial, though, had a major shortcoming: its racial composition. One out of five people diagnosed with multiple myeloma in the United States is black, and African Americans are more than twice as likely as white Americans to be diagnosed with the blood cancer.
Yet, of the 722 participants in the trial, only 13 – or 1.8% – were black.
The scarcity of black patients in Ninlaro’s testing left unanswered the vital question of whether the drug would work equally well for them. “Meaningful differences may exist” in how multiple myeloma affects black patients, what symptoms they experience, and how they respond to medications, FDA scientists wrote in a 2017 journal article.
The racial disparity in the Ninlaro study isn’t unusual. Reflecting the reluctance of the FDA to force drugmakers to enroll more minority patients, and the failure of most manufacturers to do so voluntarily, stark underrepresentation of blacks is widespread in clinical trials for cancer drugs, even when the type of cancer disproportionately affects them. A ProPublica analysis of data recently made public by the FDA found that in trials for 24 of the 31 cancer drugs approved since 2015, fewer than 5% of the patients were black. African Americans make up 13.4% of the U.S. population.
As a result, desperately ill black patients who have exhausted other treatment options aren’t getting early access to experimental drugs that could extend their life spans or improve their quality of life. While unapproved treatments also carry a risk of setbacks or side effects, new cancer drugs have dramatically shifted outcomes for some patients.
Recently approved lung cancer treatments are “revolutionary,” said Karen Kelly, MD, associate director for research at UC Davis Comprehensive Cancer Center. Even in the first phase of clinical testing, which is aimed at making sure a drug is safe, 20% of cancer patients now see their tumors shrink or disappear, up from 5% in the early 1990s, Dr. Kelly said.
Kashif Ali, MD, research head at Maryland Oncology Hematology, has spent 7 years recruiting patients for about 30 cancer and blood disease trials a year. He said he’s often seen minorities, including African Americans, miss out on trials because of financial hurdles, logistical challenges, and their lingering distrust of the medical community because of a history of being victimized by medical experimentation.
“They’re potentially losing out on life-extending opportunities because it’s one more option they no longer have,” Dr. Ali said. “Especially when patients are in advanced stages of cancer, treatments are like stepping-stones: When one stops working, you move on to the next.”
Not joining a trial can mean “you’ve lost life expectancy,” he said.
Pat Conley, a 72-year-old retired business analyst whose multiple myeloma relapsed last year, has never participated in a clinical trial. She was interested several times and didn’t meet the criteria. Now she’s eligible for one, but worries about the burden of regular hour-long trips from her home in Fayetteville, Georgia, to the trial site in Atlanta, as well as the copays for medical tests. “They’ll want a new biopsy, and Lordy, biopsies are not cheap,” she said.
Still, two drug regimens haven’t halted her cancer, and she wants something positive to come from her illness. “If they don’t have African Americans to test it on, how will they know it’s going to work?” she asked. “If it doesn’t help me, it’ll help my children; it’ll help somebody else.”
Pharmaceutical companies contacted by ProPublica all said diversity in clinical trials is important to ensure that drugs meet patients’ needs. The issue “is not elevated high enough in the discussion on clinical studies,” said John Maraganore, PhD, chair of the industry group Biotechnology Innovation Organization. But he added that enrolling minorities is challenging, often for reasons beyond the manufacturer’s control, and that it would require a “public-private partnership, working with the FDA and NIH [National Institutes of Health].”
Black participation reached 10% in trials for only 2 of the 31 cancer drugs: the multiple myeloma drug Darzalex (daratumumab), where the figure was exactly 10%, and Yondelis (trabectedin), which treats two types of soft-tissue cancer. A total of 12% of patients in the Yondelis trial were black, the highest proportion in the ProPublica study. Both drugs are made by Johnson & Johnson, which said it has an internal working group on trial diversity. The working group trains site leaders in best practices for diverse recruitment and seeks minority physicians to help run trials, since some patients prefer being treated by doctors of their own race.
Not enrolling in clinical trials is just one of many ways that African Americans trail white Americans in the quality of their health care. From diagnosis to death, they often experience inferior care and worse outcomes. Because some black Americans can’t afford the health insurance mandated under the Affordable Care Act, they remain less likely to have coverage than do non-Hispanic white Americans. African Americans are 30% more likely than white Americans to die from heart disease. Black mothers are three to four times as likely as white mothers to die in pregnancy or childbirth, and black children are diagnosed with autism later than white children are.
While the scarcity of African Americans stands out in ProPublica’s analysis, there appear to be gaps in participation of other minority groups as well. Asians were well represented in trials held in some foreign countries, but they made up only 1.7% of patients for drugs for which at least 70% of trials were conducted within the United States. By comparison, about 6% of the U.S. population identifies as Asian. Almost two-thirds of the trials didn’t report any Native Americans or Alaska Natives, who together make up about 2%of the U.S. population. ProPublica’s analysis excluded Hispanics, because the FDA reports did not have a separate category for them until 2017 and do not distinguish between white and nonwhite Hispanics.
The very relationship of race to drug development is fraught with controversy. Race is primarily seen as a social concept, rather than as a product of measurable biological traits. Yet there’s growing evidence that, whether for environmental or genetic reasons, drugs may have different effects on different populations.
In 2014, the state of Hawaii sued Bristol-Myers Squibb and Sanofi, the manufacturers of the blood thinner Plavix, accusing them of deceptive marketing for failing to disclose that the drug was less effective for some patients of East Asian or Pacific Islander descent. The drugmakers have denied allegations of misconduct and argue that genetic traits have not been proven to affect how well Plavix works. That case is pending. In the meantime, the FDA has added a warning to the label saying that Chinese patients are more likely to have a gene variation that renders the drug ineffective. Researchers at the University of California, San Francisco, have found that a commonly used asthma medication, albuterol, doesn’t work as well for black and Puerto Rican children as it does for European American or Mexican children.
Inadequate minority representation in drug trials means that “we aren’t doing good science,” said Jonathan Jackson, MD, founding director of the Community Access, Recruitment and Engagement Research Center at Massachusetts General Hospital in Boston. “If we aren’t doing good science and releasing these drugs out into the public, then we are at best being inefficient, at worst being irresponsible.”
The National Black Church Initiative, a coalition of 34,000 U.S. churches, urged the FDA in 2017 to mandate diversity in all clinical trials before approving a drug or device. “Simply put, the pharmaceutical community is not going to improve minority participation in clinical trials until the FDA compels them to do so via regulations,” it wrote to Commissioner Scott Gottlieb.
The FDA hasn’t done so. Although it noted in a 2013 report that a lack of diversity betrays a key ethical principle of medical research – equal justice for all – the agency has shied away from setting quotas or numerical guidelines for participation by race.
Instead, it has relied on persuasion. In its 2014 Action Plan, it said it aimed to “share best practices,” to “support” industry efforts at improving diversity in clinical trials, and to “encourage” patients to participate in trials via social media, emails, and blog posts.
“The FDA believes that enrollment should reflect the patients most likely to use a medical product,” spokeswoman Gloria Sanchez-Contreras said. The FDA “does not have the regulatory authority to require specific levels of minority representation in clinical trials,” although it may ask drugmakers for additional data, she said.
Rachel Sherman, MD, the FDA’s principal deputy commissioner, said that she’s “not entirely satisfied” with minority enrollment but that clinical trials have become more diverse in other ways. Two decades ago, women and children were rarely included in drug studies. Now those groups are better represented and the FDA is working on including more minorities, she said.
Dr. Sherman added that the agency has to balance how much information it demands from drugmakers against the need to get a drug onto the market, where it will be broadly available for all patients.
“When it comes to clinical research in this country, there’s a credit card, and there’s a limit on the credit card,” she said. “If we spend on one thing, it won’t get spent on another. We have to be judicious in what we require and what we demand and what we encourage.”
Diversity has its trade-offs. Clinical trials already cost hundreds of millions of dollars, and drugmakers say that requiring participants to be racially representative would likely add more time and expense.
“If you have a significant delay in enrollment, that would delay the medication advancing to the whole patient population, hurting everybody including the black population,” said Dr. Maraganore, who also is CEO of drugmaker Alnylam Pharmaceuticals.
To offset costs caused by these delays, manufacturers might reduce the number of drugs in development, depriving some patients of experimental treatments, or raise prices, which would translate into higher insurance premiums and make new drugs even less affordable for the uninsured. Dr. Maraganore favors improving diversity through patient education – “a carrot-based approach” – rather than government regulation.
Despite these short-term expenses, eliminating racial disparities in clinical trials would ultimately save costs through disease prevention and improved treatment, according to a 2015 analysis by researchers at the University of California, San Francisco. Without FDA pressure, though, manufacturers may be unlikely to increase efforts to recruit blacks, especially if their sights are set on a worldwide market. They can use the same clinical trial data to gain approval in the European Union or Japan.
Takeda, which is based in Tokyo, tested Ninlaro in the United States, Japan, and 24 other countries, including Australia, Austria, Denmark, New Zealand, Sweden, and others with small black populations. “Clinical trials are reflective of the ethnicity distribution in the population where the study takes place,” Phil Rowlands, head of Takeda’s oncology unit, said in an email. “While we cannot control enrollment eligibility based on the strict clinical criteria, our recruitment efforts extend to diverse patient populations, including minorities.”
Asked why Takeda didn’t pick sites with higher black populations, Mr. Rowlands said that Takeda does not “select sites with ethnicity as an eligibility criteria unless specific risk factors require that.”
Income is another reason for sparse African American representation. Clinical trials are largely a middle-class option. A 2015 study found that patients with an annual household income below $50,000 had 32% lower odds of participating in a trial than did patients with income above that threshold. The median household income of black Americans in 2016 was $39,490, compared with $65,041 for non-Hispanic white Americans.
Patients who can’t afford to travel long distances to a trial, take time off work, or find child care are at a disadvantage. They are usually reimbursed for travel and food costs, but drugmakers are careful not to pay too much because they do not want to appear to be enticing patients to join when it isn’t in their medical interests, said Laurie Halloran, founder and CEO of a consulting firm for the drug industry.
While the experimental drug used in a study is free, any approved treatments that are part of the trial typically need to be covered by a patient’s own insurance, according to Dr. Ali.
He recalled one black patient who was eligible for a study that entailed taking an already approved drug in combination with a new treatment. The approved drug cost $10,000 a month and the patient’s insurance charged 20% as his copay, Dr. Ali recalled. The patient decided that joining the trial would be too expensive. He instead underwent chemotherapy, which was cheaper, but he didn’t tolerate it well. The patient is now considering hospice, Dr. Ali said.
Criteria for admission to clinical trials have become more stringent over the years, Mass. General’s Dr. Jackson said, and patients of color increasingly are excluded. They are more likely than white people are to have other conditions such as stroke, hypertension, and diabetes, which could complicate research results. Trials often want to enroll “the healthiest sick people they can find” and preclude patients with other conditions. “The wall basically gets taller and taller,” he said.
Aredia Taylor didn’t qualify for a clinical trial on other grounds. A former U.S. Department of Agriculture supervisor for food-safety inspections, she was diagnosed with multiple myeloma in 2014 and had undergone a gamut of treatment including various chemotherapy drugs and a stem cell transplant.
While the transplant drove her cancer into remission, she still needs to take Revlimid (lenalidomide) – a standard treatment – daily to keep the cancer at bay. The drug has given her side effects that she describes as devastating to her daily life, including diarrhea, muscle spasms, and an inability to concentrate. She said she wants to stop taking Revlimid and would be willing to try an experimental treatment.
Ms. Taylor, 58, said she saw pamphlets at her doctor’s office encouraging patients to ask about clinical trials, so she asked her oncologist, Larry Anderson, MD, at the University of Texas Southwestern Medical Center in Dallas, whether she should join a study. He told her that she “wasn’t a fit,” she said. Dr. Anderson told ProPublica that most trials are seeking patients with either newly diagnosed or relapsed multiple myeloma, and since Ms. Taylor is currently in remission, she wouldn’t be accepted.
Ms. Taylor was disappointed. Knowing that blacks like herself are especially susceptible to multiple myeloma, she’d like to be a part of developing more effective treatments.
“I want to pay it forward and be a blessing to somebody else,” she said. “I want to be one of the people that they try to do a clinical trial for, so they find a way to a cure.”
Spurred by Congress, the FDA began in January 2015 to regularly publish a “Drug Trials Snapshot” for every new drug approved, delineating the demographic breakdown for clinical trial participants by sex, race and age subgroups. ProPublica’s analysis focused on participants in trials for the 31 cancer drugs approved since then, comparing their demographics with data from the National Cancer Institute on the incidence of various cancers by race.
Eighteen of those drugs targeted cancers that are at least as likely to afflict black Americans as white Americans. On average, only 4.1% of patients in those trials were black. Trials for four multiple myeloma drugs, including Ninlaro, averaged 5% black participation.
An FDA study over a longer time period corroborated ProPublica’s findings. In a 2017 article in the journal Blood coauthored by Richard Pazdur, the FDA’s cancer chief, the agency reported that black patients on average made up 4.5% of participants in trials for multiple myeloma drugs since 2003. Higher enrollment of minorities in myeloma trials would have “multiple benefits,” the FDA scientists noted. Not only would “underserved populations” gain access to new therapies, but additional data could help researchers identify subtypes of the blood cancer and develop targeted treatments.
A similar pattern emerges for treatments of non–small cell lung cancer. It occurs in 56 out of 100,000 black Americans, versus 49 out of 100,000 white Americans. Yet ProPublica found that in trials for two recently approved drugs for a type of non–small cell lung cancer driven by a mutation in what is known as the ALK gene, less than 2% of participants were black.
Those drugs, Takeda’s Alunbrig (brigatinib) and Genentech’s Alecensa (alectinib), are approved for patients whose lung cancer has spread to other parts of the body. In trials, both drugs were able to shrink tumors, including lesions in the brain. Patients taking Alecensa lived without the disease progressing for an average of 25.7 months, more than double the 10.4 months for patients on another treatment.
“We believe that we must consider differences across all populations to deliver on the promise of personalized health care,” said Meghan Cox, a Genentech spokeswoman, adding that the drugmaker is “continuing to study patient response to Alecensa across populations in the postmarketing setting.”
Similarly, prostate cancer affects 178 out of every 100,000 African Americans, more than for any other race in the United States, compared with 106 out of every 100,000 white Americans. Black Americans are twice as likely as white Americans to die from prostate cancer.
Yet during 2009-2015, in seven trials conducted for five new prostate cancer therapies, only 3% of participants were black, according to a study conducted by Daniel Spratt, MD, vice chair of research in the department of radiation oncology at the University of Michigan, Ann Arbor. More recently, 11 times as many white as black participants, or 66% – compared with 6% – joined trials for Johnson & Johnson’s new prostate cancer treatment, Erleada (apalutamide).
The FDA approved Erleada in February after trials showed patients on the drug lived an average of 2 years longer without their cancer spreading into other organs than if they were taking a placebo. In a J&J press release, physicians hailed Erleada’s “impressive clinical benefits” for prostate cancer patients.
J&J is “confident in the efficacy and safety data that have supported the approval” of Erleada, J&J spokeswoman Satu Glawe said.
Like Genentech, J&J and Takeda said they track drugs after approval to see if any racial differences emerge. For example, after another J&J drug for prostate cancer, Zytiga (abiraterone), went on the market in 2011, the company ran a new study of 100 patients, 50 black and 50 white.
“We were aware of the low number of African American men” in the preapproval drug studies of Zytiga, Ms. Glawe said. The postmarketing study was intended “to ensure the medication was also providing clinical benefit to these patients.” In fact, it showed that black men responded better than white men did to the drug.
The FDA is able to look for signs that an approved drug isn’t helping a specific population through a surveillance system launched in 2016, called the Sentinel Initiative, which provides access to medical claims and other data on 200 million Americans obtained from insurers and other health providers, said the FDA’s Dr. Sherman.
Still, postmarketing surveillance doesn’t compensate for lack of diversity in clinical trials. Minorities still miss out on experimental treatments – and, if they take a drug once it’s approved, may suffer unanticipated side effects.
Leaving analysis of a drug’s effect on minorities until it’s already on the market is “a hubristic assumption, unnecessarily arrogant,” Dr. Jackson said. Drugs should “work for the individuals who are the most vulnerable,” he said. “That necessarily includes racial and ethnic minorities.”
Like African Americans, Native Americans rarely enroll in clinical trials. Among the drug trials analyzed by ProPublica, 64.5% did not report any Native American participants, even for types of cancer that Native Americans get at similar rates as other races. (It’s possible that some Native Americans were enrolled but lumped into a generic “other” category.)
Native Americans are at higher risk of colorectal cancer than are white or Asian Americans. Yet the drugmaker Taiho Oncology didn’t report a single Native American among the 800 participants in its trials for the colorectal cancer treatment Lonsurf (trifluridine and tipiracil). Taiho didn’t respond to requests for comment.
To understand how a small minority group responds to a drug, researchers might have to enroll more patients than what would be a nationally representative sample. In a trial of 100 people, two Native American or Alaska Native patients would reflect those groups’ proportion of the U.S. population but wouldn’t give doctors enough information about race-related impacts.
Linda Burhansstipanov, DrPH, founder of the Native American Cancer Research Corp., estimated that 85% of Native Americans want to participate in clinical trials when informed of the opportunity. She knows several Native American patients who drove 3 hours each way to participate in a trial, despite losing an entire day of work.
Still, trial protocols are rarely designed with minority communities in mind, Dr. Burhansstipanov said. It has long been rumored among Native Americans, she said, that a clinical trial in the 1990s required patients to take a medication upon rising in the morning. In many Native American tribes, the first thing people do when they wake up is greet the sun with morning prayers. For some tribes, prayers can take more than half an hour. Because of the delay, the tale goes, Native American patients were kicked out of the clinical trial for violating the protocol. “The story spread and became a barrier for people to take part in clinical trials,” she said.
In addition, the history of unscrupulous medical experimentation on minorities feeds wariness of clinical trials. Angela M. Marshall, MD, an internal medicine doctor and board member of Black Women’s Health Imperative, said she sees a “lack of excitement among minority communities for clinical trials, coming from a mistrust of the medical community.”
Her black patients often cite the infamous Tuskegee study, conducted from 1932 to 1972 by the U.S. Public Health Service, in which researchers knowingly withheld treatment from African American sharecroppers with syphilis in order to study the progression of the disease. Some Native Americans are similarly suspicious of the medical community, Dr. Burhansstipanov said, because the Indian Health Service (a unit of the U.S. Department of Health and Human Services) sterilized thousands of Native American women in the 1970s without their consent.
“The medical community has to engage in some serious trust-building initiatives,” Dr. Marshall said.
That trust is what has helped to keep Thomas Goode alive. An African American diagnosed with multiple myeloma in 2005 when he was 34 years old, Mr. Goode has endured three stem cell transplants. Between each transplant, he participated in clinical trials, where he gained access to drug cocktails that helped bridge him to the next procedure. He counted himself lucky that he lived close to Durham, North Carolina, which is a research hub, so he was able to see specialists and take part in trials.
“I never knew anything about clinical trials, but a lot of my trust and faith was in the doctor,” he said. “I said, ‘I trust you, doctor, whatever you say.’ ”
Now, Mr. Goode has gone 6 years without a relapse. “I’m in a good place right now,” he said, “But if I didn’t try a trial, who’s to say I would still be here?”
ProPublica is a Pulitzer Prize–winning investigative newsroom. Stat is a news organization reporting from the frontiers of health and medicine.
Maternal infanticide and postpartum psychosis
Postpartum psychosis is extremely rare, but when it does occur, the consequences can prove catastrophic.
An article in The Atlantic chronicles the nightmare suffered by mothers driven to kill their children.
Cara Angelotta, MD, a forensic psychiatrist affiliated with Northwestern University in Chicago, says the presence of postpartum psychosis also puts mothers’ lives at risk. “Suicide is a major contributor to maternal death in the first year postpartum. [And] postpartum psychosis increases the risk of suicide,” she says.
“People don’t get it,” says Susan Benjamin Feingold, PsyD, a psychologist at the Illinois School of Professional Psychology in Chicago and an expert in postpartum disorders. “They see baby killers. These women are really sick and need treatment, not punishment.”
On June 1, Illinois became the first state to implement a law that allows judges to consider postpartum psychosis during sentencing. When left untreated, postpartum psychosis might raise the risk of infanticide by about 4%, the article in The Atlantic noted.
Thank you for being a friend
Some senior adults are benefiting from the friendship, security, and invigoration provided by an initiative of several universities in which they share living spaces with students. The arrangement, according to the Canadian Broadcasting Corporation, saves the student on housing costs and provides the senior with companionship and assistance with household chores.
But these arrangements have deeper and more enriching benefits.
About 25% of older adults lack family assistance with everyday tasks and live without the companionship that is a hallmark of multiperson households. “It actually gives some peace of mind to that older person,” says Samir Sinha, MD, director of geriatrics at Toronto’s Mount Sinai Hospital.
“It gives them a greater sense of security and the ability to feel like they can maintain their independence for that much longer.”
Jobs and the deaf community
Hearing loss can cause a retreat from the world. But in one San Francisco neighborhood, it is not a barrier to work. As reported by National Public Radio (NPR), every staff member at Mozzeria is partly or fully deaf. Using pen-and-paper ordering or the tried and true pointing at the menu that eases the interchange between hearing-enabled patrons not versed in American Sign Language and hearing-challenged staff, Mozzeria owners Melody and Russ Stein provide solid employment for people for whom a job could otherwise be elusive. Only about 48% of the deaf community is employed in the United States (compared with 72% of hearing individuals), according to the National Deaf Center.
“We envision that each Mozzeria location will be looked at as a source of both local and national pride,” Melody Stein wrote in a recent article.
An alternative to opioids?
Jelena M. Janjic, PhD, a pain researcher at Duquesne University in Pittsburgh, is drawing on her expertise in nanotechnology and her personal experience to develop a means of delivering nonopioid drugs directly to cells in the body. If successful in humans, as it has been in rats, the method would free people from using addictive opioids for pain relief.
As reported by NPR, a motivation for the tact is Dr. Janjic’s own decades-long battle with chronic pain. Personal introspection and literature searches led her to the view that chronic pain can be rooted in inflammation. Quelling the inflammation without disabling the good that the immune system does in fighting infections and other harms might help ease the pain.
“As a patient, I want an answer,” Dr. Janjic says in the interview. “I want to figure out this.”
Marshmallows and self-control
The world recently bid goodbye to Walter Mischel, PhD, who died on Sept. 12. Dr. Mischel is chiefly remembered for his ionic marshmallow test.
In the test of delayed gratification, children were presented with a treat (sometimes the famous marshmallow) then asked to hold off gobbling down the treat in exchange for an even more treats later.
As described in The New Yorker, an inspiration for Dr. Mischel was his repeated attempts to quit his prodigious smoking addiction. His research was one of the first glimpses into the role of self-control as a beneficial strategy in life. Learning to mentally temper that got-to-have-it moment is, according to Dr. Mischel, key to self-control.
“If we have the skills to allow us to make discriminations about when we do or don’t do something, when we do or don’t drink something, and when we do and when we don’t wait for something, we are no longer victims of our desires,” he said in the New Yorker interview.
Postpartum psychosis is extremely rare, but when it does occur, the consequences can prove catastrophic.
An article in The Atlantic chronicles the nightmare suffered by mothers driven to kill their children.
Cara Angelotta, MD, a forensic psychiatrist affiliated with Northwestern University in Chicago, says the presence of postpartum psychosis also puts mothers’ lives at risk. “Suicide is a major contributor to maternal death in the first year postpartum. [And] postpartum psychosis increases the risk of suicide,” she says.
“People don’t get it,” says Susan Benjamin Feingold, PsyD, a psychologist at the Illinois School of Professional Psychology in Chicago and an expert in postpartum disorders. “They see baby killers. These women are really sick and need treatment, not punishment.”
On June 1, Illinois became the first state to implement a law that allows judges to consider postpartum psychosis during sentencing. When left untreated, postpartum psychosis might raise the risk of infanticide by about 4%, the article in The Atlantic noted.
Thank you for being a friend
Some senior adults are benefiting from the friendship, security, and invigoration provided by an initiative of several universities in which they share living spaces with students. The arrangement, according to the Canadian Broadcasting Corporation, saves the student on housing costs and provides the senior with companionship and assistance with household chores.
But these arrangements have deeper and more enriching benefits.
About 25% of older adults lack family assistance with everyday tasks and live without the companionship that is a hallmark of multiperson households. “It actually gives some peace of mind to that older person,” says Samir Sinha, MD, director of geriatrics at Toronto’s Mount Sinai Hospital.
“It gives them a greater sense of security and the ability to feel like they can maintain their independence for that much longer.”
Jobs and the deaf community
Hearing loss can cause a retreat from the world. But in one San Francisco neighborhood, it is not a barrier to work. As reported by National Public Radio (NPR), every staff member at Mozzeria is partly or fully deaf. Using pen-and-paper ordering or the tried and true pointing at the menu that eases the interchange between hearing-enabled patrons not versed in American Sign Language and hearing-challenged staff, Mozzeria owners Melody and Russ Stein provide solid employment for people for whom a job could otherwise be elusive. Only about 48% of the deaf community is employed in the United States (compared with 72% of hearing individuals), according to the National Deaf Center.
“We envision that each Mozzeria location will be looked at as a source of both local and national pride,” Melody Stein wrote in a recent article.
An alternative to opioids?
Jelena M. Janjic, PhD, a pain researcher at Duquesne University in Pittsburgh, is drawing on her expertise in nanotechnology and her personal experience to develop a means of delivering nonopioid drugs directly to cells in the body. If successful in humans, as it has been in rats, the method would free people from using addictive opioids for pain relief.
As reported by NPR, a motivation for the tact is Dr. Janjic’s own decades-long battle with chronic pain. Personal introspection and literature searches led her to the view that chronic pain can be rooted in inflammation. Quelling the inflammation without disabling the good that the immune system does in fighting infections and other harms might help ease the pain.
“As a patient, I want an answer,” Dr. Janjic says in the interview. “I want to figure out this.”
Marshmallows and self-control
The world recently bid goodbye to Walter Mischel, PhD, who died on Sept. 12. Dr. Mischel is chiefly remembered for his ionic marshmallow test.
In the test of delayed gratification, children were presented with a treat (sometimes the famous marshmallow) then asked to hold off gobbling down the treat in exchange for an even more treats later.
As described in The New Yorker, an inspiration for Dr. Mischel was his repeated attempts to quit his prodigious smoking addiction. His research was one of the first glimpses into the role of self-control as a beneficial strategy in life. Learning to mentally temper that got-to-have-it moment is, according to Dr. Mischel, key to self-control.
“If we have the skills to allow us to make discriminations about when we do or don’t do something, when we do or don’t drink something, and when we do and when we don’t wait for something, we are no longer victims of our desires,” he said in the New Yorker interview.
Postpartum psychosis is extremely rare, but when it does occur, the consequences can prove catastrophic.
An article in The Atlantic chronicles the nightmare suffered by mothers driven to kill their children.
Cara Angelotta, MD, a forensic psychiatrist affiliated with Northwestern University in Chicago, says the presence of postpartum psychosis also puts mothers’ lives at risk. “Suicide is a major contributor to maternal death in the first year postpartum. [And] postpartum psychosis increases the risk of suicide,” she says.
“People don’t get it,” says Susan Benjamin Feingold, PsyD, a psychologist at the Illinois School of Professional Psychology in Chicago and an expert in postpartum disorders. “They see baby killers. These women are really sick and need treatment, not punishment.”
On June 1, Illinois became the first state to implement a law that allows judges to consider postpartum psychosis during sentencing. When left untreated, postpartum psychosis might raise the risk of infanticide by about 4%, the article in The Atlantic noted.
Thank you for being a friend
Some senior adults are benefiting from the friendship, security, and invigoration provided by an initiative of several universities in which they share living spaces with students. The arrangement, according to the Canadian Broadcasting Corporation, saves the student on housing costs and provides the senior with companionship and assistance with household chores.
But these arrangements have deeper and more enriching benefits.
About 25% of older adults lack family assistance with everyday tasks and live without the companionship that is a hallmark of multiperson households. “It actually gives some peace of mind to that older person,” says Samir Sinha, MD, director of geriatrics at Toronto’s Mount Sinai Hospital.
“It gives them a greater sense of security and the ability to feel like they can maintain their independence for that much longer.”
Jobs and the deaf community
Hearing loss can cause a retreat from the world. But in one San Francisco neighborhood, it is not a barrier to work. As reported by National Public Radio (NPR), every staff member at Mozzeria is partly or fully deaf. Using pen-and-paper ordering or the tried and true pointing at the menu that eases the interchange between hearing-enabled patrons not versed in American Sign Language and hearing-challenged staff, Mozzeria owners Melody and Russ Stein provide solid employment for people for whom a job could otherwise be elusive. Only about 48% of the deaf community is employed in the United States (compared with 72% of hearing individuals), according to the National Deaf Center.
“We envision that each Mozzeria location will be looked at as a source of both local and national pride,” Melody Stein wrote in a recent article.
An alternative to opioids?
Jelena M. Janjic, PhD, a pain researcher at Duquesne University in Pittsburgh, is drawing on her expertise in nanotechnology and her personal experience to develop a means of delivering nonopioid drugs directly to cells in the body. If successful in humans, as it has been in rats, the method would free people from using addictive opioids for pain relief.
As reported by NPR, a motivation for the tact is Dr. Janjic’s own decades-long battle with chronic pain. Personal introspection and literature searches led her to the view that chronic pain can be rooted in inflammation. Quelling the inflammation without disabling the good that the immune system does in fighting infections and other harms might help ease the pain.
“As a patient, I want an answer,” Dr. Janjic says in the interview. “I want to figure out this.”
Marshmallows and self-control
The world recently bid goodbye to Walter Mischel, PhD, who died on Sept. 12. Dr. Mischel is chiefly remembered for his ionic marshmallow test.
In the test of delayed gratification, children were presented with a treat (sometimes the famous marshmallow) then asked to hold off gobbling down the treat in exchange for an even more treats later.
As described in The New Yorker, an inspiration for Dr. Mischel was his repeated attempts to quit his prodigious smoking addiction. His research was one of the first glimpses into the role of self-control as a beneficial strategy in life. Learning to mentally temper that got-to-have-it moment is, according to Dr. Mischel, key to self-control.
“If we have the skills to allow us to make discriminations about when we do or don’t do something, when we do or don’t drink something, and when we do and when we don’t wait for something, we are no longer victims of our desires,” he said in the New Yorker interview.
Opioid use predicts longer sleep latency in chronic pain patients
Patients with chronic pain who took opioids reported significantly more difficulty falling asleep, compared with those who didn’t use opioids, a study of 144 adults has found.
“Identification of factors that influence insomnia symptoms among adults with chronic pain may inform prevention and treatment efforts for both disorders,” wrote Mary Beth Miller, PhD, of the University of Missouri School of Medicine, Columbia, and her colleagues.
To identify the potential impact of opioid use on sleep among chronic pain patients, the researchers recruited adults reporting symptoms of both insomnia and fibromyalgia. The average age of the participants was 52 years, and 95% were women. The study findings were published in Sleep Medicine.
The participants completed sleep diaries for 14 days, during which they recorded data including when they went to bed, how long it took them to fall asleep (SOL), how often they woke up during the night and how long they stayed awake (WASO), what time they woke up, and what time they got out of bed. Patients also reported “yes” or “no” on opioid use and their dosage each day and rated their pain on a scale of 0-100 each night before retiring.
The study participants wore wrist actigraphy devices for baseline assessment and underwent 1 night of ambulatory polysomnography.
The researchers used a multiple regression model to examine how pain intensity affected the association between opioid use and insomnia.
Overall, “opioid use was not associated with improvements in insomnia symptoms across any level of pain intensity, and was associated with worse insomnia symptoms among those reporting less intense pain,” the researchers said.
Opioid use was associated with significantly longer time to sleep onset in participants with low levels of pain (P = .02) but not among those with moderate to high levels; average sleep onset latency appeared unaffected by pain level among participants who did not use opioids.
The study findings were limited by several factors including the small number of male participants, the use of paper forms for the sleep diaries, which prevented confirmation of timely reporting, and the cross-sectional nature of the analysis, the researchers noted. However, from a clinical perspective, the “findings suggest that it may be important to advise patients reporting symptoms of insomnia about the risks of extending time in bed when providing them with opioid pain medication and that the use of behavioral or cognitive-behavioral treatment for insomnia may be recommended,” they said. The researchers also recommended that future studies address the longitudinal associations between opioid use and insomnia.
The researchers had no financial conflicts to disclose.
SOURCE: Miller M et al., Sleep Med. 2018; doi: 10.1016/j.sleep.2018.08.015.
Patients with chronic pain who took opioids reported significantly more difficulty falling asleep, compared with those who didn’t use opioids, a study of 144 adults has found.
“Identification of factors that influence insomnia symptoms among adults with chronic pain may inform prevention and treatment efforts for both disorders,” wrote Mary Beth Miller, PhD, of the University of Missouri School of Medicine, Columbia, and her colleagues.
To identify the potential impact of opioid use on sleep among chronic pain patients, the researchers recruited adults reporting symptoms of both insomnia and fibromyalgia. The average age of the participants was 52 years, and 95% were women. The study findings were published in Sleep Medicine.
The participants completed sleep diaries for 14 days, during which they recorded data including when they went to bed, how long it took them to fall asleep (SOL), how often they woke up during the night and how long they stayed awake (WASO), what time they woke up, and what time they got out of bed. Patients also reported “yes” or “no” on opioid use and their dosage each day and rated their pain on a scale of 0-100 each night before retiring.
The study participants wore wrist actigraphy devices for baseline assessment and underwent 1 night of ambulatory polysomnography.
The researchers used a multiple regression model to examine how pain intensity affected the association between opioid use and insomnia.
Overall, “opioid use was not associated with improvements in insomnia symptoms across any level of pain intensity, and was associated with worse insomnia symptoms among those reporting less intense pain,” the researchers said.
Opioid use was associated with significantly longer time to sleep onset in participants with low levels of pain (P = .02) but not among those with moderate to high levels; average sleep onset latency appeared unaffected by pain level among participants who did not use opioids.
The study findings were limited by several factors including the small number of male participants, the use of paper forms for the sleep diaries, which prevented confirmation of timely reporting, and the cross-sectional nature of the analysis, the researchers noted. However, from a clinical perspective, the “findings suggest that it may be important to advise patients reporting symptoms of insomnia about the risks of extending time in bed when providing them with opioid pain medication and that the use of behavioral or cognitive-behavioral treatment for insomnia may be recommended,” they said. The researchers also recommended that future studies address the longitudinal associations between opioid use and insomnia.
The researchers had no financial conflicts to disclose.
SOURCE: Miller M et al., Sleep Med. 2018; doi: 10.1016/j.sleep.2018.08.015.
Patients with chronic pain who took opioids reported significantly more difficulty falling asleep, compared with those who didn’t use opioids, a study of 144 adults has found.
“Identification of factors that influence insomnia symptoms among adults with chronic pain may inform prevention and treatment efforts for both disorders,” wrote Mary Beth Miller, PhD, of the University of Missouri School of Medicine, Columbia, and her colleagues.
To identify the potential impact of opioid use on sleep among chronic pain patients, the researchers recruited adults reporting symptoms of both insomnia and fibromyalgia. The average age of the participants was 52 years, and 95% were women. The study findings were published in Sleep Medicine.
The participants completed sleep diaries for 14 days, during which they recorded data including when they went to bed, how long it took them to fall asleep (SOL), how often they woke up during the night and how long they stayed awake (WASO), what time they woke up, and what time they got out of bed. Patients also reported “yes” or “no” on opioid use and their dosage each day and rated their pain on a scale of 0-100 each night before retiring.
The study participants wore wrist actigraphy devices for baseline assessment and underwent 1 night of ambulatory polysomnography.
The researchers used a multiple regression model to examine how pain intensity affected the association between opioid use and insomnia.
Overall, “opioid use was not associated with improvements in insomnia symptoms across any level of pain intensity, and was associated with worse insomnia symptoms among those reporting less intense pain,” the researchers said.
Opioid use was associated with significantly longer time to sleep onset in participants with low levels of pain (P = .02) but not among those with moderate to high levels; average sleep onset latency appeared unaffected by pain level among participants who did not use opioids.
The study findings were limited by several factors including the small number of male participants, the use of paper forms for the sleep diaries, which prevented confirmation of timely reporting, and the cross-sectional nature of the analysis, the researchers noted. However, from a clinical perspective, the “findings suggest that it may be important to advise patients reporting symptoms of insomnia about the risks of extending time in bed when providing them with opioid pain medication and that the use of behavioral or cognitive-behavioral treatment for insomnia may be recommended,” they said. The researchers also recommended that future studies address the longitudinal associations between opioid use and insomnia.
The researchers had no financial conflicts to disclose.
SOURCE: Miller M et al., Sleep Med. 2018; doi: 10.1016/j.sleep.2018.08.015.
FROM SLEEP MEDICINE
Key clinical point: Adults taking opioids for chronic pain reported more difficulty falling asleep, compared with those who didn’t use opioids.
Major finding: Approximately one-third of adults with chronic pain reported using an opioid.
Study details: The data come from 144 adults who reported both insomnia and fibromyalgia.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Miller M et al. Sleep Medicine. 2018. doi: 10.1016/j.sleep.2018.08.015.
Age, risk factors should guide chlamydia, gonorrhea screening of HIV-infected women
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
WASHINGTON – Among HIV-infected women, those aged 18-29 years had the highest rates of gonorrhea and chlamydia. These results suggest that screening for these sexually transmitted infections (STIs) should be based on age and risk in HIV-infected women, said Jodie Dionne-Odom, MD, of the University of Alabama at Birmingham.
Annual screening for gonorrhea and chlamydia is recommended for all sexually active adults with HIV, but prevalence varies by gender, age, and risk behavior, Dr. Dionne-Odom said at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Dr. Odom and her colleagues calculated annual testing and positivity rates during 2007-2016 for chlamydia and gonorrhea among women engaged in HIV care in eight U.S. cities as part of the Centers for AIDS Research (CFAR) Clinical Networks and Integrated Clinical Services (CNICS) longitudinal cohort.
They assessed demographic data based on the most recent year the patient was in care and used validated surveys (AUDIT-C and ASSIST) to assess risk behaviors in the past 3-6 months. They collected information from 5,084 women and 158,745 HIV primary care and women’s health visits.
The median patient age was 47 years; 62.1% of the patients were black; 70% had CD4 counts greater than 350; and 73.6% had HIV viral loads of less than 500 copies/mL. In terms of reported risk, 60.6% of the women were sexually active, (85.5% of whom reported monogamy); 13.1% had problem alcohol use, and 11.6% had active drug use.
Sampling for gonorrhea and chlamydia were mostly from urogenital sites (86.6%), 6.6% were extragenital, and 6.8% were “other.” Nearly all (98.5%) of 23,492 chlamydia tests and 95.7% of 23,324 gonorrhea tests used nucleic acid amplification, Dr. Dionne-Odom said.
During the most recent year in care, 42.7% of women were tested for gonorrhea and chlamydia, and 3.4% were positive, with the annual positivity rates over the study ranging from 1.5% to 3.2% for chlamydia and 0.9% to 1.5% for gonorrhea. However, Dr. Dionne-Odom and her colleagues found that the prevalence of STIs was inversely related to patient age, with gonorrhea and chlamydia positivity in 2016 being 16% for chlamydia and 3.9% for gonorrhea among women aged 18-24 years, compared with 1.1% and 0.7%, respectively, for women older than 50 years.
“As with national data on women, HIV-infected women aged 18-29 years had the highest rates of gonorrhea and chlamydia. Our results show that targeted screening for chlamydia and gonorrhea in women with HIV based on age and risk is warranted,” Dr. Dionne-Odom concluded.
Dr. Dionne-Odom reported that she had no relevant disclosures.
REPORTING FROM THE 2018 STD PREVENTION CONFERENCE
Key clinical point: Targeted screening for chlamydia and gonorrhea in women with HIV based on age, risk is warranted.
Major finding: Chlamydia infections were seen in 16% and gonorrhea in 3.9% of HIV-infected women aged 18-24 years and in 1.1% and 0.7%, respectively, in women over age 50.
Study details: Data analysis of 5,084 women in 8 U.S. cities during 2007-2016.
Disclosures: Dr. Dionne-Odom reported that she had no relevant disclosures.
Most in-hospital pneumonia deaths may not be preventable
Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy, according to an analysis of deaths at five U.S. hospitals with expertise in pneumonia care.
Adults who are hospitalized with community-acquired pneumonia (CAP) are at high risk for short-term mortality but it is unclear whether an improvement in care could lower this risk, noted the study authors led by Grant W. Waterer, MBBS, PhD, of Northwestern University, Chicago.
“Understanding the circumstances in which CAP patients die could facilitate improvements in the management of CAP by enabling future improvement efforts to focus on common preventable causes of death,” they wrote. Their report was published in CHEST®.
They therefore performed a secondary analysis of the Etiology of Pneumonia in the Community (EPIC) study involving adults hospitalized with CAP between January 2010 and June 2012 across five tertiary-care hospitals in the United States.
The clinical characteristics of patients who died in the hospital were compared with those of patients who survived to hospital discharge. Chronic heart failure, chronic obstructive pulmonary disease, coronary artery disease, chronic liver disease, cerebrovascular disease, cancer (excluding skin cancer), and diabetes were considered as severe chronic comorbidities based on their association with increased mortality and ICU admission in CAP severity scores.
Deaths caused by septic shock, respiratory failure, multisystem organ failure, cardiopulmonary arrest prior to stabilization of CAP, and endocarditis, were considered to be directly related to CAP.
Conversely, causes of death indirectly related to CAP included acute cardiovascular disease, stroke, acute renal failure, and secondary infections developed after hospitalization. Deaths caused by cancer, cirrhosis, and chronic neurologic conditions were considered unrelated to CAP.
Medical notes were assessed to determine whether the patient received management consistent with current recommendations; for example, antibiotics consistent with guidelines from the Infectious Diseases Society of America.
End-of-life limitations in care, such as patient/family decision not to proceed with full medical treatment, also were considered by the research team.
Results showed that among the 2,320 patients with radiographically confirmed CAP, 52 died during initial hospitalization, 33 of whom were aged 65 years or older, and 32 of whom had two or more chronic comorbidities.
Most of the in-hospital deaths occurred early in the hospitalization: 35 within the first 10 days of admission, and 5 after 30 days in hospital.
CAP was judged by an expert physician review panel to be the direct cause of death in 27 of the patients, 10 with CAP having an indirect role with major contribution, 9 with CAP having an indirect role with minor contribution, and 6 with CAP having no role in death.
Do-not-resuscitate orders were present at the time of death for 21 of the patients.
Forty-five of the patients were admitted to an ICU, with 37 dying in the ICU. The eight patients who died on the ward after transfer out of the ICU had end-of-life limitations of care in place.
The researchers noted that the number of patients dying in the ICU was greater in the United States, possibly because in Europe fewer patients are admitted to an ICU.
“This discrepancy likely reflects cultural differences between the U.S. and Europe in the role of intensive care for patients with advanced age and/or advanced comorbid conditions,” they noted.
Overall, the physician review panel identified nine patients who had a lapse in quality of in-hospital CAP care, with four of the deaths potentially linked to this lapse in care.
However, two of the patients had end-of-life limitations of care in place, which according to the authors meant that “only two patients undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care potentially contributing to in-hospital death, including one with a delay in antibiotics for over an hour in the presence of shock and one with initial antibiotics not consistent with IDSA/ATS guidelines.”
The research team concluded that most in-hospital deaths among adult patients admitted with CAP in their study would not have been preventable with higher quality in-hospital pneumonia care.
“Many of the in-hospital deaths among patients admitted with CAP occurred in older patients with severe comorbidities and end-of-life limitations in care,” they noted.
They said the influence of end-of-life limitations on care short of full palliation was an important finding, with all patients who died outside the ICU having end-of-life limitations in care.
“Current diagnostic related group (DRG) and international classification of diseases (ICD) coding systems do not have the necessary nuances to capture these limitations of care, yet they are clearly important factors in determining whether patients experience in-hospital death,” they added.
Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
SOURCE: Waterer G. et al. CHEST 2018;154(3):628-35. doi: 10.1016/j.chest.2018.05.021.
Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy, according to an analysis of deaths at five U.S. hospitals with expertise in pneumonia care.
Adults who are hospitalized with community-acquired pneumonia (CAP) are at high risk for short-term mortality but it is unclear whether an improvement in care could lower this risk, noted the study authors led by Grant W. Waterer, MBBS, PhD, of Northwestern University, Chicago.
“Understanding the circumstances in which CAP patients die could facilitate improvements in the management of CAP by enabling future improvement efforts to focus on common preventable causes of death,” they wrote. Their report was published in CHEST®.
They therefore performed a secondary analysis of the Etiology of Pneumonia in the Community (EPIC) study involving adults hospitalized with CAP between January 2010 and June 2012 across five tertiary-care hospitals in the United States.
The clinical characteristics of patients who died in the hospital were compared with those of patients who survived to hospital discharge. Chronic heart failure, chronic obstructive pulmonary disease, coronary artery disease, chronic liver disease, cerebrovascular disease, cancer (excluding skin cancer), and diabetes were considered as severe chronic comorbidities based on their association with increased mortality and ICU admission in CAP severity scores.
Deaths caused by septic shock, respiratory failure, multisystem organ failure, cardiopulmonary arrest prior to stabilization of CAP, and endocarditis, were considered to be directly related to CAP.
Conversely, causes of death indirectly related to CAP included acute cardiovascular disease, stroke, acute renal failure, and secondary infections developed after hospitalization. Deaths caused by cancer, cirrhosis, and chronic neurologic conditions were considered unrelated to CAP.
Medical notes were assessed to determine whether the patient received management consistent with current recommendations; for example, antibiotics consistent with guidelines from the Infectious Diseases Society of America.
End-of-life limitations in care, such as patient/family decision not to proceed with full medical treatment, also were considered by the research team.
Results showed that among the 2,320 patients with radiographically confirmed CAP, 52 died during initial hospitalization, 33 of whom were aged 65 years or older, and 32 of whom had two or more chronic comorbidities.
Most of the in-hospital deaths occurred early in the hospitalization: 35 within the first 10 days of admission, and 5 after 30 days in hospital.
CAP was judged by an expert physician review panel to be the direct cause of death in 27 of the patients, 10 with CAP having an indirect role with major contribution, 9 with CAP having an indirect role with minor contribution, and 6 with CAP having no role in death.
Do-not-resuscitate orders were present at the time of death for 21 of the patients.
Forty-five of the patients were admitted to an ICU, with 37 dying in the ICU. The eight patients who died on the ward after transfer out of the ICU had end-of-life limitations of care in place.
The researchers noted that the number of patients dying in the ICU was greater in the United States, possibly because in Europe fewer patients are admitted to an ICU.
“This discrepancy likely reflects cultural differences between the U.S. and Europe in the role of intensive care for patients with advanced age and/or advanced comorbid conditions,” they noted.
Overall, the physician review panel identified nine patients who had a lapse in quality of in-hospital CAP care, with four of the deaths potentially linked to this lapse in care.
However, two of the patients had end-of-life limitations of care in place, which according to the authors meant that “only two patients undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care potentially contributing to in-hospital death, including one with a delay in antibiotics for over an hour in the presence of shock and one with initial antibiotics not consistent with IDSA/ATS guidelines.”
The research team concluded that most in-hospital deaths among adult patients admitted with CAP in their study would not have been preventable with higher quality in-hospital pneumonia care.
“Many of the in-hospital deaths among patients admitted with CAP occurred in older patients with severe comorbidities and end-of-life limitations in care,” they noted.
They said the influence of end-of-life limitations on care short of full palliation was an important finding, with all patients who died outside the ICU having end-of-life limitations in care.
“Current diagnostic related group (DRG) and international classification of diseases (ICD) coding systems do not have the necessary nuances to capture these limitations of care, yet they are clearly important factors in determining whether patients experience in-hospital death,” they added.
Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
SOURCE: Waterer G. et al. CHEST 2018;154(3):628-35. doi: 10.1016/j.chest.2018.05.021.
Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy, according to an analysis of deaths at five U.S. hospitals with expertise in pneumonia care.
Adults who are hospitalized with community-acquired pneumonia (CAP) are at high risk for short-term mortality but it is unclear whether an improvement in care could lower this risk, noted the study authors led by Grant W. Waterer, MBBS, PhD, of Northwestern University, Chicago.
“Understanding the circumstances in which CAP patients die could facilitate improvements in the management of CAP by enabling future improvement efforts to focus on common preventable causes of death,” they wrote. Their report was published in CHEST®.
They therefore performed a secondary analysis of the Etiology of Pneumonia in the Community (EPIC) study involving adults hospitalized with CAP between January 2010 and June 2012 across five tertiary-care hospitals in the United States.
The clinical characteristics of patients who died in the hospital were compared with those of patients who survived to hospital discharge. Chronic heart failure, chronic obstructive pulmonary disease, coronary artery disease, chronic liver disease, cerebrovascular disease, cancer (excluding skin cancer), and diabetes were considered as severe chronic comorbidities based on their association with increased mortality and ICU admission in CAP severity scores.
Deaths caused by septic shock, respiratory failure, multisystem organ failure, cardiopulmonary arrest prior to stabilization of CAP, and endocarditis, were considered to be directly related to CAP.
Conversely, causes of death indirectly related to CAP included acute cardiovascular disease, stroke, acute renal failure, and secondary infections developed after hospitalization. Deaths caused by cancer, cirrhosis, and chronic neurologic conditions were considered unrelated to CAP.
Medical notes were assessed to determine whether the patient received management consistent with current recommendations; for example, antibiotics consistent with guidelines from the Infectious Diseases Society of America.
End-of-life limitations in care, such as patient/family decision not to proceed with full medical treatment, also were considered by the research team.
Results showed that among the 2,320 patients with radiographically confirmed CAP, 52 died during initial hospitalization, 33 of whom were aged 65 years or older, and 32 of whom had two or more chronic comorbidities.
Most of the in-hospital deaths occurred early in the hospitalization: 35 within the first 10 days of admission, and 5 after 30 days in hospital.
CAP was judged by an expert physician review panel to be the direct cause of death in 27 of the patients, 10 with CAP having an indirect role with major contribution, 9 with CAP having an indirect role with minor contribution, and 6 with CAP having no role in death.
Do-not-resuscitate orders were present at the time of death for 21 of the patients.
Forty-five of the patients were admitted to an ICU, with 37 dying in the ICU. The eight patients who died on the ward after transfer out of the ICU had end-of-life limitations of care in place.
The researchers noted that the number of patients dying in the ICU was greater in the United States, possibly because in Europe fewer patients are admitted to an ICU.
“This discrepancy likely reflects cultural differences between the U.S. and Europe in the role of intensive care for patients with advanced age and/or advanced comorbid conditions,” they noted.
Overall, the physician review panel identified nine patients who had a lapse in quality of in-hospital CAP care, with four of the deaths potentially linked to this lapse in care.
However, two of the patients had end-of-life limitations of care in place, which according to the authors meant that “only two patients undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care potentially contributing to in-hospital death, including one with a delay in antibiotics for over an hour in the presence of shock and one with initial antibiotics not consistent with IDSA/ATS guidelines.”
The research team concluded that most in-hospital deaths among adult patients admitted with CAP in their study would not have been preventable with higher quality in-hospital pneumonia care.
“Many of the in-hospital deaths among patients admitted with CAP occurred in older patients with severe comorbidities and end-of-life limitations in care,” they noted.
They said the influence of end-of-life limitations on care short of full palliation was an important finding, with all patients who died outside the ICU having end-of-life limitations in care.
“Current diagnostic related group (DRG) and international classification of diseases (ICD) coding systems do not have the necessary nuances to capture these limitations of care, yet they are clearly important factors in determining whether patients experience in-hospital death,” they added.
Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
SOURCE: Waterer G. et al. CHEST 2018;154(3):628-35. doi: 10.1016/j.chest.2018.05.021.
FROM CHEST
Key clinical point: Most in-hospital deaths from community-acquired pneumonia are not preventable with current medical therapy.
Major finding: Two out of 52 patients who died in-hospital from community-acquired pneumonia (CAP) who were undergoing full medical treatment without end-of-life limitations of care had an identified lapse in quality of in-hospital pneumonia care that potentially contributed to their death.
Study details: A secondary analysis of the prospective multicenter Etiology of Pneumonia in the Community (EPIC) study involving 2,320 adults with radiographically confirmed CAP.
Disclosures: Dr. Waterer reported no conflicts. Two coauthors reported potential conflicts of interest in relation to consulting fees from several pharmaceutical companies.
Source: Waterer G. et al. CHEST 2018;154(3):628-35.
Short sleep linked to elevated blood pressure
CHICAGO – Consider 24-hour ambulatory blood pressure monitoring when patients complain about not getting enough sleep. You might catch hypertension early, according to researchers from the University of Pennsylvania, Philadelphia, and elsewhere.
They found a less than 7 hours a night and a mean in the study of 5.5 hours. Every 2-2.5 minutes of lost sleep was associated with an increase of 1 mm Hg in 24-hour mean systolic blood pressure and a increase of 1 beat per minute in heart rate.
The relationship was independent of office BP, nocturnal dipping status, and BP variability. It held in both the obese and nonobese, and in patients with and without obstructive sleep apnea (OSA). However, the relationship was found only among subjects who were not on antihypertensive medications.
“Adults with shorter sleep duration may benefit from screening with 24-hour ambulatory BP monitoring to promote earlier detection of hypertension and potentially mitigate the” the risk of cardiovascular disease. “This may be particularly important in screening for masked hypertension,” meaning normal pressures in the office, but elevated pressures at home, said investigators led by Jordana Cohen, MD, of the department of medicine at the University of Pennsylvania in a presentation at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Dr. Cohen suggested that perhaps the sympathetic and endothelial derangements that drive hypertension in OSA also affect people with insufficient sleep. It may be that the normal morning surge in blood pressure persists longer into the day, she suggested. The investigative team analyzed data from two studies. The first, LIMBS (Lifestyle Modification in BP Lowering Study), was a phase 2 trial assessing yoga for blood pressure lowering. It was conducted in West Philadelphia and excluded people with diabetes, hypertension, OSA, and kidney or cardiovascular disease. The new analysis included 66 LIMBS subjects who had 24-hour blood pressure monitoring and kept sleep diaries to record their sleep duration (J Clin Hypertens (Greenwich). 2016 Aug;18[8]:809-16).
The team also analyzed 153 subjects from the PISA (Penn Icelandic Sleep Apnea) cohort, an ongoing project assessing continuous positive airway pressure for OSA, among other things. PISA includes patients with OSA, diabetes, hypertension, and kidney or cardiovascular disease. Sleep duration in the 153 subjects was again self-reported, but corroborated by actigraphy (J Sleep Res. 2015 Jun;24[3]:328-38).
The new findings were driven mostly by higher daytime systolic BP among short sleepers in LIMBS, and higher systolic pressures during both day and night among short sleepers in PISA, compared with subjects who slept at least 7 hours, and a mean of 8.5 hours, with napping included in overall sleep duration assessment.
In LIMBS, the mean 24-hour systolic BP was 12.7 mm Hg higher and the average heart rate 8 bpm faster among short sleepers; in PISA, the mean 24-hour systolic BP was 4.7 mm Hg higher and the heart rate 2 bpm faster.
Every 2.57 minutes of sleep lost in LIMBS and every 1.99 minute of lost sleep in PISA was associated with a 1–mm Hg gain in mean systolic BP and about a 1-bpm increase in heart rate. The findings were statistically significant and adjusted for age, race, body mass index, nocturnal dipping status, and office systolic blood pressure.
Baseline characteristics were generally well matched between short and long sleepers in both studies. However, while mean office systolic BP in LIMBS was the same in both sleep groups at about 139 mm Hg, the mean office systolic BP among long sleepers in PISA was 130 mm Hg versus 136 mm Hg among short sleepers, a significant difference.
It’s unclear why some people slept less, Dr. Cohen said, and the use of sleeping pills wasn’t considered in the analysis. Patients were an average of about 50 years old, with a body mass index of about 30 mg/m2. The same model of 24-hour blood pressure monitor was used in both studies.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
CHICAGO – Consider 24-hour ambulatory blood pressure monitoring when patients complain about not getting enough sleep. You might catch hypertension early, according to researchers from the University of Pennsylvania, Philadelphia, and elsewhere.
They found a less than 7 hours a night and a mean in the study of 5.5 hours. Every 2-2.5 minutes of lost sleep was associated with an increase of 1 mm Hg in 24-hour mean systolic blood pressure and a increase of 1 beat per minute in heart rate.
The relationship was independent of office BP, nocturnal dipping status, and BP variability. It held in both the obese and nonobese, and in patients with and without obstructive sleep apnea (OSA). However, the relationship was found only among subjects who were not on antihypertensive medications.
“Adults with shorter sleep duration may benefit from screening with 24-hour ambulatory BP monitoring to promote earlier detection of hypertension and potentially mitigate the” the risk of cardiovascular disease. “This may be particularly important in screening for masked hypertension,” meaning normal pressures in the office, but elevated pressures at home, said investigators led by Jordana Cohen, MD, of the department of medicine at the University of Pennsylvania in a presentation at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Dr. Cohen suggested that perhaps the sympathetic and endothelial derangements that drive hypertension in OSA also affect people with insufficient sleep. It may be that the normal morning surge in blood pressure persists longer into the day, she suggested. The investigative team analyzed data from two studies. The first, LIMBS (Lifestyle Modification in BP Lowering Study), was a phase 2 trial assessing yoga for blood pressure lowering. It was conducted in West Philadelphia and excluded people with diabetes, hypertension, OSA, and kidney or cardiovascular disease. The new analysis included 66 LIMBS subjects who had 24-hour blood pressure monitoring and kept sleep diaries to record their sleep duration (J Clin Hypertens (Greenwich). 2016 Aug;18[8]:809-16).
The team also analyzed 153 subjects from the PISA (Penn Icelandic Sleep Apnea) cohort, an ongoing project assessing continuous positive airway pressure for OSA, among other things. PISA includes patients with OSA, diabetes, hypertension, and kidney or cardiovascular disease. Sleep duration in the 153 subjects was again self-reported, but corroborated by actigraphy (J Sleep Res. 2015 Jun;24[3]:328-38).
The new findings were driven mostly by higher daytime systolic BP among short sleepers in LIMBS, and higher systolic pressures during both day and night among short sleepers in PISA, compared with subjects who slept at least 7 hours, and a mean of 8.5 hours, with napping included in overall sleep duration assessment.
In LIMBS, the mean 24-hour systolic BP was 12.7 mm Hg higher and the average heart rate 8 bpm faster among short sleepers; in PISA, the mean 24-hour systolic BP was 4.7 mm Hg higher and the heart rate 2 bpm faster.
Every 2.57 minutes of sleep lost in LIMBS and every 1.99 minute of lost sleep in PISA was associated with a 1–mm Hg gain in mean systolic BP and about a 1-bpm increase in heart rate. The findings were statistically significant and adjusted for age, race, body mass index, nocturnal dipping status, and office systolic blood pressure.
Baseline characteristics were generally well matched between short and long sleepers in both studies. However, while mean office systolic BP in LIMBS was the same in both sleep groups at about 139 mm Hg, the mean office systolic BP among long sleepers in PISA was 130 mm Hg versus 136 mm Hg among short sleepers, a significant difference.
It’s unclear why some people slept less, Dr. Cohen said, and the use of sleeping pills wasn’t considered in the analysis. Patients were an average of about 50 years old, with a body mass index of about 30 mg/m2. The same model of 24-hour blood pressure monitor was used in both studies.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
CHICAGO – Consider 24-hour ambulatory blood pressure monitoring when patients complain about not getting enough sleep. You might catch hypertension early, according to researchers from the University of Pennsylvania, Philadelphia, and elsewhere.
They found a less than 7 hours a night and a mean in the study of 5.5 hours. Every 2-2.5 minutes of lost sleep was associated with an increase of 1 mm Hg in 24-hour mean systolic blood pressure and a increase of 1 beat per minute in heart rate.
The relationship was independent of office BP, nocturnal dipping status, and BP variability. It held in both the obese and nonobese, and in patients with and without obstructive sleep apnea (OSA). However, the relationship was found only among subjects who were not on antihypertensive medications.
“Adults with shorter sleep duration may benefit from screening with 24-hour ambulatory BP monitoring to promote earlier detection of hypertension and potentially mitigate the” the risk of cardiovascular disease. “This may be particularly important in screening for masked hypertension,” meaning normal pressures in the office, but elevated pressures at home, said investigators led by Jordana Cohen, MD, of the department of medicine at the University of Pennsylvania in a presentation at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Dr. Cohen suggested that perhaps the sympathetic and endothelial derangements that drive hypertension in OSA also affect people with insufficient sleep. It may be that the normal morning surge in blood pressure persists longer into the day, she suggested. The investigative team analyzed data from two studies. The first, LIMBS (Lifestyle Modification in BP Lowering Study), was a phase 2 trial assessing yoga for blood pressure lowering. It was conducted in West Philadelphia and excluded people with diabetes, hypertension, OSA, and kidney or cardiovascular disease. The new analysis included 66 LIMBS subjects who had 24-hour blood pressure monitoring and kept sleep diaries to record their sleep duration (J Clin Hypertens (Greenwich). 2016 Aug;18[8]:809-16).
The team also analyzed 153 subjects from the PISA (Penn Icelandic Sleep Apnea) cohort, an ongoing project assessing continuous positive airway pressure for OSA, among other things. PISA includes patients with OSA, diabetes, hypertension, and kidney or cardiovascular disease. Sleep duration in the 153 subjects was again self-reported, but corroborated by actigraphy (J Sleep Res. 2015 Jun;24[3]:328-38).
The new findings were driven mostly by higher daytime systolic BP among short sleepers in LIMBS, and higher systolic pressures during both day and night among short sleepers in PISA, compared with subjects who slept at least 7 hours, and a mean of 8.5 hours, with napping included in overall sleep duration assessment.
In LIMBS, the mean 24-hour systolic BP was 12.7 mm Hg higher and the average heart rate 8 bpm faster among short sleepers; in PISA, the mean 24-hour systolic BP was 4.7 mm Hg higher and the heart rate 2 bpm faster.
Every 2.57 minutes of sleep lost in LIMBS and every 1.99 minute of lost sleep in PISA was associated with a 1–mm Hg gain in mean systolic BP and about a 1-bpm increase in heart rate. The findings were statistically significant and adjusted for age, race, body mass index, nocturnal dipping status, and office systolic blood pressure.
Baseline characteristics were generally well matched between short and long sleepers in both studies. However, while mean office systolic BP in LIMBS was the same in both sleep groups at about 139 mm Hg, the mean office systolic BP among long sleepers in PISA was 130 mm Hg versus 136 mm Hg among short sleepers, a significant difference.
It’s unclear why some people slept less, Dr. Cohen said, and the use of sleeping pills wasn’t considered in the analysis. Patients were an average of about 50 years old, with a body mass index of about 30 mg/m2. The same model of 24-hour blood pressure monitor was used in both studies.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
REPORTING FROM JOINT HYPERTENSION 2018
Key clinical point: Ambulatory blood pressure monitoring in patients complaining about lack of sleep could detect hypertension.
Major finding: Every 2-2.5 minutes of lost sleep was associated with a 1–mm Hg increase in 24-hour mean systolic blood pressure and a 1-bpm increase in heart rate.
Study details: Post-hoc review of 219 patients in two trials
Disclosures: The work was funded by the National Institutes of Health. The investigators didn’t have any disclosures.
Confirmed: Growth in overdose deaths is exponential
Overdose death rates for individual drugs show no particular patterns since the turn of the century, but the exponential growth of overall drug mortality actually started before the opioid epidemic, according to an analysis of almost 600,000 unintentional overdose deaths since 1979.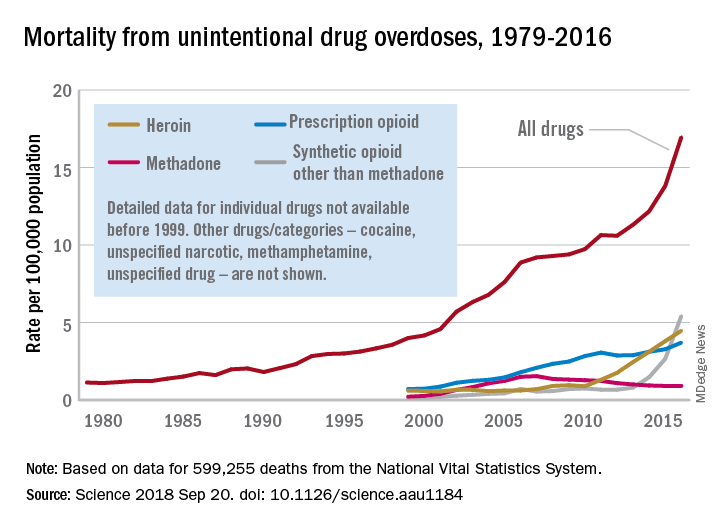
“The current epidemic of overdose deaths due to prescription opioids, heroin, and fentanyl appears to be the most recent manifestation of a more fundamental, longer-term process,” senior author Donald S. Burke, MD, of the University of Pittsburgh, said in a written statement.
Overdose mortality from all types of drugs rose from 1.13 per 100,000 population in 1979 to 16.96 per 100,000 in 2016, based on data for 599,255 deaths from unintentional drug overdoses in the National Vital Statistics System, they reported in Science.
When the investigators plotted annual drug overdose mortality over that 38-year period, they saw a smooth upward exponential curve with a doubling time of about 9 years. “This remarkably smooth, long-term epidemic growth pattern really caught our attention,” Dr. Burke said. “If we can figure it out, we should be able to bend that curve downward.”
The individual drug types that make up the whole, however, are a different story. “There is no regular or predictable pattern to the overdose rates for any of these drugs. Cocaine overdose death rates curved down and up and down and back up over the past 20 years. Methadone deaths have been on a downturn since the mid-2000s. Prescription opioids have been on a fairly steady, steep climb. Heroin deaths shot up in 2010, followed in 2013 by synthetic opioids, such as fentanyl,” lead author Hawre Jalal, MD, PhD, also of the university, said in the statement.
Geographic and demographic analyses produced the same absence of patterns. the researchers wrote.
The study was supported by grants from the Centers for Disease Control and Prevention and the Robert Wood Johnson Foundation. The investigators said they have no competing interests.
SOURCE: Jalal H et al. Science 2018 Sep 20. doi: 10.1126/science.aau1184.
Overdose death rates for individual drugs show no particular patterns since the turn of the century, but the exponential growth of overall drug mortality actually started before the opioid epidemic, according to an analysis of almost 600,000 unintentional overdose deaths since 1979.
“The current epidemic of overdose deaths due to prescription opioids, heroin, and fentanyl appears to be the most recent manifestation of a more fundamental, longer-term process,” senior author Donald S. Burke, MD, of the University of Pittsburgh, said in a written statement.
Overdose mortality from all types of drugs rose from 1.13 per 100,000 population in 1979 to 16.96 per 100,000 in 2016, based on data for 599,255 deaths from unintentional drug overdoses in the National Vital Statistics System, they reported in Science.
When the investigators plotted annual drug overdose mortality over that 38-year period, they saw a smooth upward exponential curve with a doubling time of about 9 years. “This remarkably smooth, long-term epidemic growth pattern really caught our attention,” Dr. Burke said. “If we can figure it out, we should be able to bend that curve downward.”
The individual drug types that make up the whole, however, are a different story. “There is no regular or predictable pattern to the overdose rates for any of these drugs. Cocaine overdose death rates curved down and up and down and back up over the past 20 years. Methadone deaths have been on a downturn since the mid-2000s. Prescription opioids have been on a fairly steady, steep climb. Heroin deaths shot up in 2010, followed in 2013 by synthetic opioids, such as fentanyl,” lead author Hawre Jalal, MD, PhD, also of the university, said in the statement.
Geographic and demographic analyses produced the same absence of patterns. the researchers wrote.
The study was supported by grants from the Centers for Disease Control and Prevention and the Robert Wood Johnson Foundation. The investigators said they have no competing interests.
SOURCE: Jalal H et al. Science 2018 Sep 20. doi: 10.1126/science.aau1184.
Overdose death rates for individual drugs show no particular patterns since the turn of the century, but the exponential growth of overall drug mortality actually started before the opioid epidemic, according to an analysis of almost 600,000 unintentional overdose deaths since 1979.
“The current epidemic of overdose deaths due to prescription opioids, heroin, and fentanyl appears to be the most recent manifestation of a more fundamental, longer-term process,” senior author Donald S. Burke, MD, of the University of Pittsburgh, said in a written statement.
Overdose mortality from all types of drugs rose from 1.13 per 100,000 population in 1979 to 16.96 per 100,000 in 2016, based on data for 599,255 deaths from unintentional drug overdoses in the National Vital Statistics System, they reported in Science.
When the investigators plotted annual drug overdose mortality over that 38-year period, they saw a smooth upward exponential curve with a doubling time of about 9 years. “This remarkably smooth, long-term epidemic growth pattern really caught our attention,” Dr. Burke said. “If we can figure it out, we should be able to bend that curve downward.”
The individual drug types that make up the whole, however, are a different story. “There is no regular or predictable pattern to the overdose rates for any of these drugs. Cocaine overdose death rates curved down and up and down and back up over the past 20 years. Methadone deaths have been on a downturn since the mid-2000s. Prescription opioids have been on a fairly steady, steep climb. Heroin deaths shot up in 2010, followed in 2013 by synthetic opioids, such as fentanyl,” lead author Hawre Jalal, MD, PhD, also of the university, said in the statement.
Geographic and demographic analyses produced the same absence of patterns. the researchers wrote.
The study was supported by grants from the Centers for Disease Control and Prevention and the Robert Wood Johnson Foundation. The investigators said they have no competing interests.
SOURCE: Jalal H et al. Science 2018 Sep 20. doi: 10.1126/science.aau1184.
FROM SCIENCE
HIV patients getting younger ... and older
Men who have sex with men (MSM) were younger at HIV diagnosis in 2016 than in 2008, but those living with the disease were older, according to the Centers for Disease Control and Prevention.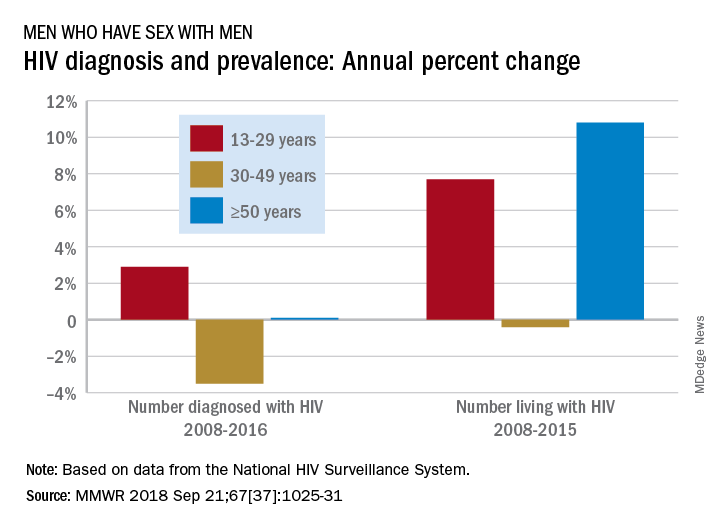
Among MSM aged 13-29 years, the number diagnosed with HIV increased by 2.9% per year from 2008 to 2016 but dropped 3.5% per year for those aged 30-49 and rose just 0.1% annually among those aged 50 and older. Over the period from 2008 to 2015, the number of MSM aged 50 and older who were living with HIV increased by 10.8% per year, compared with an annual percent change of 7.7% for those aged 13-29 and –0.4% for those aged 30-49, Andrew Mitsch, MPH, and his associates at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention reported in Morbidity and Mortality Weekly Report.
The size of the population of MSM living with HIV went from 384,000 in 2008 to 523,000 in 2016, with 13- to 29-year-olds going from 10.7% of the population to 13.3%, 30- to 49-year-olds dropping from 61% to 44%, and the 50-and-older group increasing from 28.3% to 42.7%, they said.
“The increase in annual diagnosis of HIV infections among younger MSM might reflect increased HIV testing, in addition to ongoing transmission,” they suggested, and increased prevalence among older men is probably the “result of increased survival associated with widespread use of antiretroviral therapy, surviving middle age, and advancing to the older group.”
The investigators also noted the persistence of racial/ethnic disparities over the course of the study. Among the three largest groups, whites had the smallest increase in new diagnoses for 13- to 29-year-olds and the largest decrease for 30- to 49-year-olds, and they were second to blacks in the less-than-or-equal-to-50-years-of-age group, according to data from the National HIV Surveillance System.
“Promotion of care and treatment by public health agencies and private sector partners to achieve viral suppression among MSM with diagnosed HIV infection will improve health outcomes and reduce transmission to others, particularly if prevention efforts are tailored to specific age groups,” the researchers wrote.
SOURCE: Mitsch A et al. MMWR 2018 Sep 21;67(37):1025-31.
Men who have sex with men (MSM) were younger at HIV diagnosis in 2016 than in 2008, but those living with the disease were older, according to the Centers for Disease Control and Prevention.
Among MSM aged 13-29 years, the number diagnosed with HIV increased by 2.9% per year from 2008 to 2016 but dropped 3.5% per year for those aged 30-49 and rose just 0.1% annually among those aged 50 and older. Over the period from 2008 to 2015, the number of MSM aged 50 and older who were living with HIV increased by 10.8% per year, compared with an annual percent change of 7.7% for those aged 13-29 and –0.4% for those aged 30-49, Andrew Mitsch, MPH, and his associates at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention reported in Morbidity and Mortality Weekly Report.
The size of the population of MSM living with HIV went from 384,000 in 2008 to 523,000 in 2016, with 13- to 29-year-olds going from 10.7% of the population to 13.3%, 30- to 49-year-olds dropping from 61% to 44%, and the 50-and-older group increasing from 28.3% to 42.7%, they said.
“The increase in annual diagnosis of HIV infections among younger MSM might reflect increased HIV testing, in addition to ongoing transmission,” they suggested, and increased prevalence among older men is probably the “result of increased survival associated with widespread use of antiretroviral therapy, surviving middle age, and advancing to the older group.”
The investigators also noted the persistence of racial/ethnic disparities over the course of the study. Among the three largest groups, whites had the smallest increase in new diagnoses for 13- to 29-year-olds and the largest decrease for 30- to 49-year-olds, and they were second to blacks in the less-than-or-equal-to-50-years-of-age group, according to data from the National HIV Surveillance System.
“Promotion of care and treatment by public health agencies and private sector partners to achieve viral suppression among MSM with diagnosed HIV infection will improve health outcomes and reduce transmission to others, particularly if prevention efforts are tailored to specific age groups,” the researchers wrote.
SOURCE: Mitsch A et al. MMWR 2018 Sep 21;67(37):1025-31.
Men who have sex with men (MSM) were younger at HIV diagnosis in 2016 than in 2008, but those living with the disease were older, according to the Centers for Disease Control and Prevention.
Among MSM aged 13-29 years, the number diagnosed with HIV increased by 2.9% per year from 2008 to 2016 but dropped 3.5% per year for those aged 30-49 and rose just 0.1% annually among those aged 50 and older. Over the period from 2008 to 2015, the number of MSM aged 50 and older who were living with HIV increased by 10.8% per year, compared with an annual percent change of 7.7% for those aged 13-29 and –0.4% for those aged 30-49, Andrew Mitsch, MPH, and his associates at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention reported in Morbidity and Mortality Weekly Report.
The size of the population of MSM living with HIV went from 384,000 in 2008 to 523,000 in 2016, with 13- to 29-year-olds going from 10.7% of the population to 13.3%, 30- to 49-year-olds dropping from 61% to 44%, and the 50-and-older group increasing from 28.3% to 42.7%, they said.
“The increase in annual diagnosis of HIV infections among younger MSM might reflect increased HIV testing, in addition to ongoing transmission,” they suggested, and increased prevalence among older men is probably the “result of increased survival associated with widespread use of antiretroviral therapy, surviving middle age, and advancing to the older group.”
The investigators also noted the persistence of racial/ethnic disparities over the course of the study. Among the three largest groups, whites had the smallest increase in new diagnoses for 13- to 29-year-olds and the largest decrease for 30- to 49-year-olds, and they were second to blacks in the less-than-or-equal-to-50-years-of-age group, according to data from the National HIV Surveillance System.
“Promotion of care and treatment by public health agencies and private sector partners to achieve viral suppression among MSM with diagnosed HIV infection will improve health outcomes and reduce transmission to others, particularly if prevention efforts are tailored to specific age groups,” the researchers wrote.
SOURCE: Mitsch A et al. MMWR 2018 Sep 21;67(37):1025-31.
FROM MMWR
Full disclosure
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].
I have nothing to disclose.
That is the first line on my second slide in just about every talk I give. I have no financial conflicts of interest. I no longer accept meals from pharmaceutical companies, I no longer conduct pharmaceutical company sponsored research, and I no longer give talks that include honoraria from pharmaceutical companies. I turn down payments from pharmaceutical companies when I participate in drug-monitoring safety boards and advisory committees. I do not have a financial conflict of interest.
Or do I?
In preparing to write this essay, I searched the Open Payments website (www.cms.gov/OpenPayments/index.html) for my name. Open Payments is the product of the Physician Payments Sunshine Act passed in 2010 as part of the Affordable Care Act. The website went live in September 2014 with the intention of making public all payments made to physicians from device and drug makers. I was happy to confirm that I have received no “General Payments,” which are payments for meals, travel, honoraria, consulting, and the like. However, I was surprised to learn that I did receive “Associated Research” payments. According to the website, an Associated Research payment is “funding for a research project or study where the physician is named as a principal investigator.”
I still have a few trials open under my name, but none have accrued a patient in more than 7 years. Nonetheless, I am on record, and publicly so, for accepting an Associated payment for research to the tune of $1,308,360.06.
Upon learning this, my thoughts turned to the New York Times. The Times recently published an expose in cooperation with ProPublica. In it, a prominent cancer researcher at Memorial Sloan Kettering was accused of repeatedly failing to disclose his substantial financial conflicts of interest. The payments creating the conflict were listed on the Open Payments website. Since financial disclosure is almost always required for a manuscript listed in PubMed, a simple comparison of two public websites provided the journalists with nearly all the information they needed to conclude malfeasance in disclosure.
In response, the accused admitted the failure to disclose, but attributed it to an unintentional error. In the frenzy that followed, a man of towering stature, a paragon of cancer research, submitted his resignation. The sequence of events was tragic. Had the payments been for research instead of services rendered would the consequences have been the same?
Most of us believe corporate payments for research are less likely to influence our prescribing and consulting habits than are general payments for entertainment and speaking engagements. I remember receiving my first research grant from the now defunct pharma company Immunex. It was for $10,000 – a paltry sum – but enough for me to set up a clinical trial using Immunex’s drugs. I was flattered, indebted, and conflicted from that point forward. Funded research propels our careers forward. Thinking research payments bias our decision making less than direct payments is naive. Money corrupts, and that is why research dollars need to be disclosed whenever we discuss research at the podium or in print.
With appropriate indignation, I explored the Open Payments website to learn more of my hitherto unknown payment. It was attached to a multicenter, randomized clinical trial for which I served as local principal investigator. The payment was made in January 2017 and our research team cannot verify such a payment was ever received. According to the website, the payment was not disputed. I sought to dispute it.
Our friends at the Centers for Medicare and Medicaid Services do not make filing a dispute easy. I first had to register with my home address and create a new password that, of course, needs to be changed every 60 days. I duly registered and logged into the website as instructed. I followed instructions and filled in data fields for about an additional 10 pages before being informed that I needed to logout, then log back in, to access the Open Payments application. When I did that, I was greeted with instructions to register in the Open Payments system. I then realized that all I had done to that point was register with the CMS.gov portal, not Open Payments. In for a dime, in for a dollar, I registered with Open Payments.
I almost gave up when they asked me to provide a Physician Taxonomy Code. It took me a long time to find it. For those interested, the code for Hematology is 207RH0000X. With that code entered in the right box, I was only two pages away from being registered and ready to open the dispute. Failure hit me like a lake effect snow storm. Despite my diligence, I was not “vetted” and could not file a dispute. I must have done something wrong and cannot seem to investigate the payment further, but I’m sure the New York Times could.
Now, I don’t know if I have anything to disclose or not. I do know that I have to investigate my payment the best I can, that I have to disclose it if it is real, and that I have to check Open Payments every so often to make sure I am not surprised by an investigative journalist’s report in the future. Add these to the pantheon of onerous requirements for a successful academic career.
Many wear their entanglements as a badge of honor on slides highlighting a long list of conflicts. One speaker joked that she had so many conflicts that she had no conflicts. Clearly, much like alarm fatigue, the constant display of financial conflict of interest disclosures rarely raises red flags in an audience of peers. To an audience of interested lay persons, though, those conflicts may be very important and relevant.
It is our duty to accurately account for and report them no matter the difficulty in doing so. Failure to do so can carry tragic consequences.
Dr. Kalaycio is editor in chief of Hematology News. He chairs the department of hematologic oncology and blood disorders at Cleveland Clinic Taussig Cancer Institute. Contact him at [email protected].