User login
Stress linked to disease markers in CLL
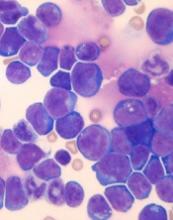
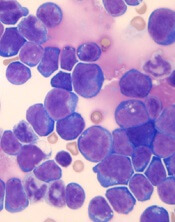
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
NICE rejects DLBCL indication for CAR T-cell therapy
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
OBI-3424 receives orphan designation for ALL
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
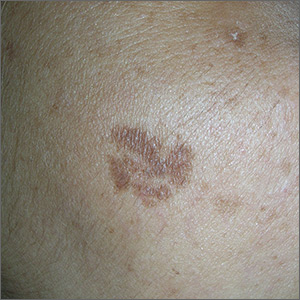
Brown spot on cheek
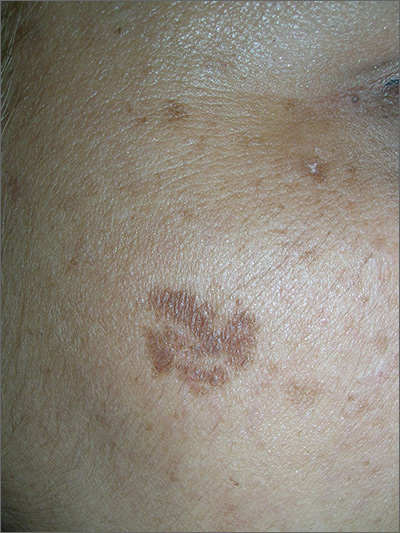
The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP noted that the patient had a dominant brown patch on her right cheek that was larger and darker than the other light brown spots found on her face. Fortunately, he had a dermatoscope and examined the spot closely, finding only features of a benign solar lentigo. There were no suspicious features for melanoma.
The FP gave the patient a choice between a broad shave biopsy that day, or having the lesion monitored (safe with a flat lesion) using photography and dermoscopy.
The patient didn’t want a biopsy on her face and was willing to have the area monitored. The clinical and dermoscopic photographs were taken and stored. The patient was given a follow-up appointment in 4 to 6 months and instructions to avoid the sun as much as possible. She was also told to use sunscreen and hats when out in the sun.
After 5 months, the previous photos were compared with the new photos on a computer screen and there were no changes. Everyone was reassured, and the patient indicated that she was being careful about her sun exposure.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine, R. Lentigo maligna. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:981-984.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Dr. Valerie W. Rusch to receive ACS Distinguished Service Award
The Board of Regents of the American College of Surgeons (ACS) has selected Valerie W. Rusch, MD, FACS, an esteemed thoracic surgeon in New York, NY, as the recipient of the 2018 Distinguished Service Award (DSA)--the College's highest honor. The Board of Regents will present the award to Dr. Rusch, vice-chair, clinical research, department of surgery; Miner Family Chair in Intrathoracic Cancers; attending surgeon, thoracic service, department of surgery, Memorial Sloan-Kettering Cancer Center; and professor of surgery, Weill Cornell Medical College, New York, NY, at the Convocation ceremony at 6:00 pm October 21 at the Clinical Congress 2018 in Boston, MA.
The Board of Regents is presenting the DSA to Dr. Rusch for "her exemplary leadership of many professional organizations and as a mentor, teacher, and trainer of the next generation of surgeons in clinical trial development and her dedication to expand access to surgical care to underserved global populations," according to the award citation.
The award also is being presented to Dr. Rusch "in admiration of her natural leadership, integrity, vision, and steadfast commitment to the College's initiatives and principles, serving as a role model to surgeons everywhere to always do the right thing for patients."
Leadership in the ACS
An ACS Fellow since 1986, Dr. Rusch has led several prominent ACS bodies, including serving as Chair of the Board of Governors (2006−2008) and Board of Regents (2015−2016). A Regent from 2008 to 2017, she chaired the Central Judiciary Committee (2009–2013), the Program Committee (2011–2017), the Board of Regents Nominating Committee (2011–2012), and the Committee on Global Engagement (2016−2017). She served on the Board of Regents Honors Committee (2012−2016), Executive Committee (2013−2016), and Finance Committee (2014−2016).
In addition, she has been a member of the College’s Advisory Council for Cardiothoracic Surgery (2002−2017), International Relations Committee (2007−2013, Executive Committee, 2009−2012), Commission on Cancer Executive Committee (2012−2017), Scholarships Committee (2008−2012), and Research and Optimal Patient Care Committee (2008−2015).
Renowned thoracic surgeon
Dr. Rusch specializes in the diagnosis and treatment of patients with cancers of the lung, airways (trachea, bronchi), esophagus, mediastinum, chest wall, and pleura (malignant pleural mesothelioma). She was among the first women in the U.S. to be board certified in thoracic surgery.
For more than 30 years, she has emphasized a multidisciplinary approach to treating patients with thoracic malignancy. Her research has focused on the molecular behaviors of asbestos cancers and the genetic tendencies of lung cancer as a means to identify certain cancers in the earlier stages.
Dr. Rusch has been a leader in national and international clinical trials for the treatment of thoracic malignancies and played a pivotal role in establishing the ACS Oncology Group--now the ACS Clinical Research Program. Among her many honors, in 2007, Dr. Rusch received the Thoracic Surgery Foundation for Research and Education Socrates Award, and in 2012, the Association of Women Surgeons awarded her the Nina Starr Braunwald Award for lifetime contributions to the advancement of women in surgery.
She has held 25 visiting professorships and lectureships and given more than 300 major lectures on thoracic cancers at medical conferences around the world. Her curriculum vitae boasts more than 400 peer-reviewed publications.
In addition to the ACS, Dr. Rusch has been a leader of other surgical organizations. More specifically, she served as chair of the American Board of Thoracic Surgery, chair of the Lung and Esophagus Task Force of the American Joint Commission on Cancer, and chair of the Mesothelioma Subcommittee of the International Association for the Study of Lung Cancer Staging Committee.
Dr. Rusch is fluent in both French and English, having graduated from the Lycée Français de New York. She graduated from Vassar College, Poughkeepsie, NY, with a degree in biochemistry. She earned her medical degree from the Columbia University College of Physicians and Surgeons, New York, and she completed surgical residency training in general surgery and thoracic surgery at the University of Washington, Seattle, followed by a fellowship at the University of Texas MD Anderson Cancer Center, Houston.
The Board of Regents of the American College of Surgeons (ACS) has selected Valerie W. Rusch, MD, FACS, an esteemed thoracic surgeon in New York, NY, as the recipient of the 2018 Distinguished Service Award (DSA)--the College's highest honor. The Board of Regents will present the award to Dr. Rusch, vice-chair, clinical research, department of surgery; Miner Family Chair in Intrathoracic Cancers; attending surgeon, thoracic service, department of surgery, Memorial Sloan-Kettering Cancer Center; and professor of surgery, Weill Cornell Medical College, New York, NY, at the Convocation ceremony at 6:00 pm October 21 at the Clinical Congress 2018 in Boston, MA.
The Board of Regents is presenting the DSA to Dr. Rusch for "her exemplary leadership of many professional organizations and as a mentor, teacher, and trainer of the next generation of surgeons in clinical trial development and her dedication to expand access to surgical care to underserved global populations," according to the award citation.
The award also is being presented to Dr. Rusch "in admiration of her natural leadership, integrity, vision, and steadfast commitment to the College's initiatives and principles, serving as a role model to surgeons everywhere to always do the right thing for patients."
Leadership in the ACS
An ACS Fellow since 1986, Dr. Rusch has led several prominent ACS bodies, including serving as Chair of the Board of Governors (2006−2008) and Board of Regents (2015−2016). A Regent from 2008 to 2017, she chaired the Central Judiciary Committee (2009–2013), the Program Committee (2011–2017), the Board of Regents Nominating Committee (2011–2012), and the Committee on Global Engagement (2016−2017). She served on the Board of Regents Honors Committee (2012−2016), Executive Committee (2013−2016), and Finance Committee (2014−2016).
In addition, she has been a member of the College’s Advisory Council for Cardiothoracic Surgery (2002−2017), International Relations Committee (2007−2013, Executive Committee, 2009−2012), Commission on Cancer Executive Committee (2012−2017), Scholarships Committee (2008−2012), and Research and Optimal Patient Care Committee (2008−2015).
Renowned thoracic surgeon
Dr. Rusch specializes in the diagnosis and treatment of patients with cancers of the lung, airways (trachea, bronchi), esophagus, mediastinum, chest wall, and pleura (malignant pleural mesothelioma). She was among the first women in the U.S. to be board certified in thoracic surgery.
For more than 30 years, she has emphasized a multidisciplinary approach to treating patients with thoracic malignancy. Her research has focused on the molecular behaviors of asbestos cancers and the genetic tendencies of lung cancer as a means to identify certain cancers in the earlier stages.
Dr. Rusch has been a leader in national and international clinical trials for the treatment of thoracic malignancies and played a pivotal role in establishing the ACS Oncology Group--now the ACS Clinical Research Program. Among her many honors, in 2007, Dr. Rusch received the Thoracic Surgery Foundation for Research and Education Socrates Award, and in 2012, the Association of Women Surgeons awarded her the Nina Starr Braunwald Award for lifetime contributions to the advancement of women in surgery.
She has held 25 visiting professorships and lectureships and given more than 300 major lectures on thoracic cancers at medical conferences around the world. Her curriculum vitae boasts more than 400 peer-reviewed publications.
In addition to the ACS, Dr. Rusch has been a leader of other surgical organizations. More specifically, she served as chair of the American Board of Thoracic Surgery, chair of the Lung and Esophagus Task Force of the American Joint Commission on Cancer, and chair of the Mesothelioma Subcommittee of the International Association for the Study of Lung Cancer Staging Committee.
Dr. Rusch is fluent in both French and English, having graduated from the Lycée Français de New York. She graduated from Vassar College, Poughkeepsie, NY, with a degree in biochemistry. She earned her medical degree from the Columbia University College of Physicians and Surgeons, New York, and she completed surgical residency training in general surgery and thoracic surgery at the University of Washington, Seattle, followed by a fellowship at the University of Texas MD Anderson Cancer Center, Houston.
The Board of Regents of the American College of Surgeons (ACS) has selected Valerie W. Rusch, MD, FACS, an esteemed thoracic surgeon in New York, NY, as the recipient of the 2018 Distinguished Service Award (DSA)--the College's highest honor. The Board of Regents will present the award to Dr. Rusch, vice-chair, clinical research, department of surgery; Miner Family Chair in Intrathoracic Cancers; attending surgeon, thoracic service, department of surgery, Memorial Sloan-Kettering Cancer Center; and professor of surgery, Weill Cornell Medical College, New York, NY, at the Convocation ceremony at 6:00 pm October 21 at the Clinical Congress 2018 in Boston, MA.
The Board of Regents is presenting the DSA to Dr. Rusch for "her exemplary leadership of many professional organizations and as a mentor, teacher, and trainer of the next generation of surgeons in clinical trial development and her dedication to expand access to surgical care to underserved global populations," according to the award citation.
The award also is being presented to Dr. Rusch "in admiration of her natural leadership, integrity, vision, and steadfast commitment to the College's initiatives and principles, serving as a role model to surgeons everywhere to always do the right thing for patients."
Leadership in the ACS
An ACS Fellow since 1986, Dr. Rusch has led several prominent ACS bodies, including serving as Chair of the Board of Governors (2006−2008) and Board of Regents (2015−2016). A Regent from 2008 to 2017, she chaired the Central Judiciary Committee (2009–2013), the Program Committee (2011–2017), the Board of Regents Nominating Committee (2011–2012), and the Committee on Global Engagement (2016−2017). She served on the Board of Regents Honors Committee (2012−2016), Executive Committee (2013−2016), and Finance Committee (2014−2016).
In addition, she has been a member of the College’s Advisory Council for Cardiothoracic Surgery (2002−2017), International Relations Committee (2007−2013, Executive Committee, 2009−2012), Commission on Cancer Executive Committee (2012−2017), Scholarships Committee (2008−2012), and Research and Optimal Patient Care Committee (2008−2015).
Renowned thoracic surgeon
Dr. Rusch specializes in the diagnosis and treatment of patients with cancers of the lung, airways (trachea, bronchi), esophagus, mediastinum, chest wall, and pleura (malignant pleural mesothelioma). She was among the first women in the U.S. to be board certified in thoracic surgery.
For more than 30 years, she has emphasized a multidisciplinary approach to treating patients with thoracic malignancy. Her research has focused on the molecular behaviors of asbestos cancers and the genetic tendencies of lung cancer as a means to identify certain cancers in the earlier stages.
Dr. Rusch has been a leader in national and international clinical trials for the treatment of thoracic malignancies and played a pivotal role in establishing the ACS Oncology Group--now the ACS Clinical Research Program. Among her many honors, in 2007, Dr. Rusch received the Thoracic Surgery Foundation for Research and Education Socrates Award, and in 2012, the Association of Women Surgeons awarded her the Nina Starr Braunwald Award for lifetime contributions to the advancement of women in surgery.
She has held 25 visiting professorships and lectureships and given more than 300 major lectures on thoracic cancers at medical conferences around the world. Her curriculum vitae boasts more than 400 peer-reviewed publications.
In addition to the ACS, Dr. Rusch has been a leader of other surgical organizations. More specifically, she served as chair of the American Board of Thoracic Surgery, chair of the Lung and Esophagus Task Force of the American Joint Commission on Cancer, and chair of the Mesothelioma Subcommittee of the International Association for the Study of Lung Cancer Staging Committee.
Dr. Rusch is fluent in both French and English, having graduated from the Lycée Français de New York. She graduated from Vassar College, Poughkeepsie, NY, with a degree in biochemistry. She earned her medical degree from the Columbia University College of Physicians and Surgeons, New York, and she completed surgical residency training in general surgery and thoracic surgery at the University of Washington, Seattle, followed by a fellowship at the University of Texas MD Anderson Cancer Center, Houston.
Produce and promises
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Most of us are in medicine because we find joy and fulfillment in treating patients. That’s why we signed up for the long educational slog, and why many of us continue to practice medicine long after all the bills have been paid. That is why we all find obstructions between us and our patients so maddening.
I guess Medicare was a great boon for seniors, who found health insurance increasingly more difficult to afford, and for doctors, who now got paid in something other than produce and promises by indigent, elderly patients. The American Medical Association opposed the adoption of Medicare, fearing that it would interfere with the physician-patient relationship. This may sound quaint now, especially at a time when there are calls for Medicare for all. While it is hard to argue against Medicare improving access to health care, the AMA was right about the government’s intrusion into the physician-patient relationship, which has become progressively more intrusive. Medicare has undergone major revisions at least five times; none of these revisions has simplified care. Think about the steadily increasing documentation requirements, audits, inflation-ravaged fee schedules, and MIPS [Merit-Based Incentive Payment System], and MACRA [Medicare Access and CHIP Reauthorization Act of 2015], although the current proposed Medicare rule, with a two-level fee schedule and reduced documentation, claims to eliminate 50 hours of charting per year.
The next big blow was ERISA (the Employee Retirement Income Security Act of 1974), which really did not seem relevant to medical practice at the time. However, embedded in this law was indemnification of insurers from patient lawsuits. Well, OK, insurers don’t practice medicine, right? Fast-forward to today, when critical medical decisions, including which test can be ordered and which drug can be administered, are driven by insurers – who can delay or refuse care and who cannot be legally blamed for the death or harm of the patient. That’s right, step therapy and prior authorizations would not be possible without ERISA.
Of course, absolutely the most onerous intrusion on the physician-patient relationship is the American Recovery and Reinvestment Act of 2014, which mandated electronic health records. I believe this is the major cause of current physician burnout, which has created the worst and most intrusive barrier between physicians and patients to date. Talk about good intentions gone awry!
In addition, now private equity has entered into medicine, in part in response to these issues and intrusions. But has this improved the patient-physician relationship, or just made things worse?
A big selling point of these private equity–backed groups is the central handling of administrative issues, such as billing, coding, compliance, human resources, prior authorizations, as well as other back-office functions. Some groups even claim to improve patient care and value, by instituting quality metrics for care (I would love to see these published). These services all must be paid for, and the logical argument is that pooling these services will result in efficiency and cost less overall.
Maybe so, but private equity creates yet another barrier between the patient and the physician while it eliminate others. These businesses are driven by profit; they are private equity after all. They are a more insidious threat to the physician-patient relationship and the future of medicine than are clumsy laws, since private equity commoditizes patients and their care. .
Any barrier between the patient and the physician is bad, and two or three barriers make things logarithmically worse. No wonder physicians have become cynical and disillusioned. It makes you pause and wonder, how much do we currently pay in time and overhead to navigate these barriers? Maybe we should call it all even. Maybe we would come out ahead if we counted in produce, promises, and unobstructed patient care.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
New insight into celiac disease: What you should know
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
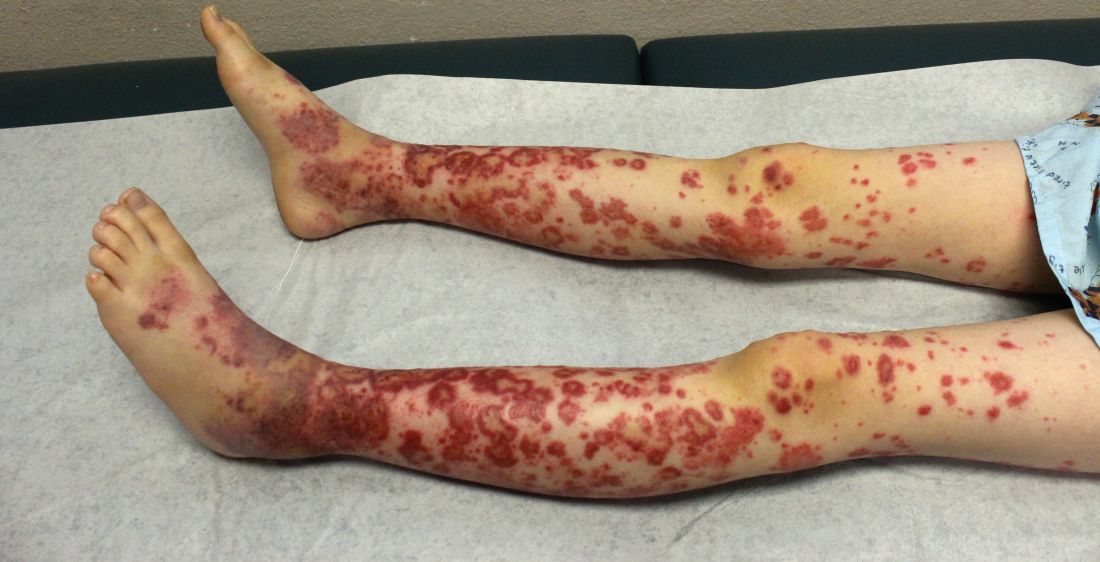
IgA vasculitis may be more common in adults than assumed
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Global bronchial thermoplasty registry for asthma documents benefits
PARIS – A global registry to track the safety and efficacy of shows benefits comparable to those previously reported in randomized trials, according to 1-year results presented at the annual congress of the European Respiratory Society.
Bronchothermoplasty has been Food and Drug Administration approved since 2010, but joint 2014 guidelines from the ERS and the American Thoracic Society (ATS) recommended that this procedure be restricted to patients participating in a registry, making these findings an important part of an ongoing assessment, according to Alfons Torrego Fernández, MD, of the pulmonology service at Hospital de la Santa Creu i Sant Pau, Barcelona.
The BT Global Registry (BTGR), created at the end of 2014, involves 18 centers in Europe, South Africa, and Australia. Dr. Fernandez provided data on 123 of the 157 patients enrolled by the end of 2016. All had at least 1 year of follow-up.
Compared with the year prior to bronchial thermoplasty, the proportion of patients with severe exacerbations in the year following this procedure fell from 90.3% to 59.6%, a 34% reduction (P less than .001). The proportion of patients requiring oral corticosteroids fell from 47.8% to 23.5%, a reduction of more than 50%.
Relative to the year prior to bronchial thermoplasty, “there was also a reduction in emergency room visits [21.1% vs. 54.6%] and hospitalizations [20.2% vs. 43%] as well as a reduction in the need for asthma maintenance medications,” Dr. Fernandez reported.
On the Asthma Control Questionnaire (ACQ), quality of life (QOL) was improved on average by 1.2 points from the prior year (4.48 vs. 3.26; P less than .05), according to Dr. Fernandez. The proportion of patients who achieved at least a 0.5-point increase in the ACQ, a level that Dr. Fernandez said is considered clinically relevant, was 67.1%.
However, when lung function measures such as forced expiratory volume in one second and fractional exhaled nitric oxide taken 1 year after bronchothermoplasty were compared with the same measures taken prior to this treatment, there was no significant improvement, according to Dr. Fernandez.
Bronchial thermoplasty involves the use of thermal energy delivered through a bronchoscope to reduce airway smooth muscle mass, thereby eliminating a source of airway restriction. Although nearly 70% of severe asthma patients in this BTGR derived an improvement in quality of life, 30% did not. Asked if the registry has provided any insight about who does or does not respond, Dr. Fernandez acknowledged that this is “the key question,” but added that “no specific profile or biomarker” has yet been identified.
“These are early results, but a 2-year follow-up is planned,” he said.
Although the technical aspects of bronchial thermoplasty “are quite well standardized,” Dr. Fernandez acknowledged that there might be a learning curve that favors experienced operators. He reported that outcomes between high- and low-volume centers in BTGR have not yet been compared. However, he maintained that “these results in real-life patients confirm that severe asthma patients can benefit” from this therapy as shown previously in sham-controlled trials.
Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
PARIS – A global registry to track the safety and efficacy of shows benefits comparable to those previously reported in randomized trials, according to 1-year results presented at the annual congress of the European Respiratory Society.
Bronchothermoplasty has been Food and Drug Administration approved since 2010, but joint 2014 guidelines from the ERS and the American Thoracic Society (ATS) recommended that this procedure be restricted to patients participating in a registry, making these findings an important part of an ongoing assessment, according to Alfons Torrego Fernández, MD, of the pulmonology service at Hospital de la Santa Creu i Sant Pau, Barcelona.
The BT Global Registry (BTGR), created at the end of 2014, involves 18 centers in Europe, South Africa, and Australia. Dr. Fernandez provided data on 123 of the 157 patients enrolled by the end of 2016. All had at least 1 year of follow-up.
Compared with the year prior to bronchial thermoplasty, the proportion of patients with severe exacerbations in the year following this procedure fell from 90.3% to 59.6%, a 34% reduction (P less than .001). The proportion of patients requiring oral corticosteroids fell from 47.8% to 23.5%, a reduction of more than 50%.
Relative to the year prior to bronchial thermoplasty, “there was also a reduction in emergency room visits [21.1% vs. 54.6%] and hospitalizations [20.2% vs. 43%] as well as a reduction in the need for asthma maintenance medications,” Dr. Fernandez reported.
On the Asthma Control Questionnaire (ACQ), quality of life (QOL) was improved on average by 1.2 points from the prior year (4.48 vs. 3.26; P less than .05), according to Dr. Fernandez. The proportion of patients who achieved at least a 0.5-point increase in the ACQ, a level that Dr. Fernandez said is considered clinically relevant, was 67.1%.
However, when lung function measures such as forced expiratory volume in one second and fractional exhaled nitric oxide taken 1 year after bronchothermoplasty were compared with the same measures taken prior to this treatment, there was no significant improvement, according to Dr. Fernandez.
Bronchial thermoplasty involves the use of thermal energy delivered through a bronchoscope to reduce airway smooth muscle mass, thereby eliminating a source of airway restriction. Although nearly 70% of severe asthma patients in this BTGR derived an improvement in quality of life, 30% did not. Asked if the registry has provided any insight about who does or does not respond, Dr. Fernandez acknowledged that this is “the key question,” but added that “no specific profile or biomarker” has yet been identified.
“These are early results, but a 2-year follow-up is planned,” he said.
Although the technical aspects of bronchial thermoplasty “are quite well standardized,” Dr. Fernandez acknowledged that there might be a learning curve that favors experienced operators. He reported that outcomes between high- and low-volume centers in BTGR have not yet been compared. However, he maintained that “these results in real-life patients confirm that severe asthma patients can benefit” from this therapy as shown previously in sham-controlled trials.
Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
PARIS – A global registry to track the safety and efficacy of shows benefits comparable to those previously reported in randomized trials, according to 1-year results presented at the annual congress of the European Respiratory Society.
Bronchothermoplasty has been Food and Drug Administration approved since 2010, but joint 2014 guidelines from the ERS and the American Thoracic Society (ATS) recommended that this procedure be restricted to patients participating in a registry, making these findings an important part of an ongoing assessment, according to Alfons Torrego Fernández, MD, of the pulmonology service at Hospital de la Santa Creu i Sant Pau, Barcelona.
The BT Global Registry (BTGR), created at the end of 2014, involves 18 centers in Europe, South Africa, and Australia. Dr. Fernandez provided data on 123 of the 157 patients enrolled by the end of 2016. All had at least 1 year of follow-up.
Compared with the year prior to bronchial thermoplasty, the proportion of patients with severe exacerbations in the year following this procedure fell from 90.3% to 59.6%, a 34% reduction (P less than .001). The proportion of patients requiring oral corticosteroids fell from 47.8% to 23.5%, a reduction of more than 50%.
Relative to the year prior to bronchial thermoplasty, “there was also a reduction in emergency room visits [21.1% vs. 54.6%] and hospitalizations [20.2% vs. 43%] as well as a reduction in the need for asthma maintenance medications,” Dr. Fernandez reported.
On the Asthma Control Questionnaire (ACQ), quality of life (QOL) was improved on average by 1.2 points from the prior year (4.48 vs. 3.26; P less than .05), according to Dr. Fernandez. The proportion of patients who achieved at least a 0.5-point increase in the ACQ, a level that Dr. Fernandez said is considered clinically relevant, was 67.1%.
However, when lung function measures such as forced expiratory volume in one second and fractional exhaled nitric oxide taken 1 year after bronchothermoplasty were compared with the same measures taken prior to this treatment, there was no significant improvement, according to Dr. Fernandez.
Bronchial thermoplasty involves the use of thermal energy delivered through a bronchoscope to reduce airway smooth muscle mass, thereby eliminating a source of airway restriction. Although nearly 70% of severe asthma patients in this BTGR derived an improvement in quality of life, 30% did not. Asked if the registry has provided any insight about who does or does not respond, Dr. Fernandez acknowledged that this is “the key question,” but added that “no specific profile or biomarker” has yet been identified.
“These are early results, but a 2-year follow-up is planned,” he said.
Although the technical aspects of bronchial thermoplasty “are quite well standardized,” Dr. Fernandez acknowledged that there might be a learning curve that favors experienced operators. He reported that outcomes between high- and low-volume centers in BTGR have not yet been compared. However, he maintained that “these results in real-life patients confirm that severe asthma patients can benefit” from this therapy as shown previously in sham-controlled trials.
Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
REPORTING FROM THE ERS CONGRESS 2018
Key clinical point: In a global registry, bronchothermoplasty provided improvement in real-world severe asthma consistent with clinical trials.
Major finding: At 1 year, severe asthma exacerbations were reduced 34% (P less than .001) relative to the year before treatment.
Study details: Open-label observational study.
Disclosures: Dr. Fernandez reported having no conflicts of interest. The registry is funded by Boston Scientific.
Guideline offers comprehensive approach for ICU clinicians
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
A new (PADIS).
The guideline builds upon the 2013 Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium (PAD) in Adult Patients in the ICU. Given the comprehensive nature of the PADIS guideline, an accompanying commentary was published simultaneously to help with implementation and interpretation. Both papers are the result of a large-scale, multicenter collaboration and were published in Critical Care Medicine.
A panel of 32 international experts, four methodologists, and four survivors of critical illness used the Grading of Recommendations Assessment, Development and Evaluation approach to develop the PADIS guideline.
“Thousands of hours were invested by these guidelines’ authors, who were in turn were supported by formal and informal collaborators, over the 3.5 years it took to produce this effort,” reported lead author John W. Devlin, PharmD, of the department of pharmacy and health systems sciences, Bouvé College of Health Sciences at Northeastern University, Boston, and his colleagues.
Compared with the 2013 PAD guideline, the PADIS guideline includes new sections regarding rehabilitation/mobility and sleep. “We sought to clarify conceptual definitions within these relatively new critical care research domains,” the panel wrote. “The recommendation rationales, fueled by debate and discussion, circled back to the bedside experience – and the perspective of what was best for patients – held by all panelists and methodology experts.”
The result is extensive and comprehensive, consisting of both broad and specific descriptions of current ICU practices and associated evidence; the guideline includes 37 recommendations, 32 ungraded, nonactionable statements, and two good practice statements. Of note, conditional recommendations far outnumber strong recommendations (34 vs. 3). Reasons for conditional rather than strong recommendations are discussed in rationale sections within the guideline and in the accompanying paper.
“Although our goal was to provide specific recommendations for each question, we suspect some guideline readers may be discouraged by the conditional nature of many recommendations and daunted by the breadth of topics discussed,” wrote Michele C. Balas, PhD, of the Ohio State University College of Nursing in Columbus, and her colleagues. Dr. Balas was on the guideline panel and is the lead author of the accompanying article intended to facilitate implementation and interpretation.
One of the more challenging recommendations surrounds the use of antipsychotics for delirious patients. Although this intervention has become relatively common, the guideline stands against it.
“It should be emphasized that there are few supportive data on ICU antipsychotic use and that the initiation of psychoactive medications during critical illness often results in their inappropriate continuation after ICU discharge,” wrote Dr. Balas and her coauthors.
Along with a hard look at existing practices, the panel actively sought to expand upon the 2013 guideline with new interventions. Discussions ranged from the less conventional, such as aromatherapy, to the more established, such as polypharmacy. Questions, recommendations, and rationale are clearly described for each topic, with clear supporting evidence. Where evidence is missing, the panel recommends future research possibilities.
“One example is the consideration of multiple pharmacologic and nonpharmacologic coanalgesic approaches to the ICU patient,” wrote Dr. Devlin and his coauthors. “When the published evidence was insufficient, limited to a narrow population or specific intervention (e.g., for procedural analgesia), or outright absent to answer the questions we posed, we structured evidence gap descriptors to inform clinicians where the uncertainty lay, and intended to provide sufficient information to apprise and invite researchers to address these gaps.”
The authors disclosed funding from AstraZeneca, Baxter, Covidien, and others.
SOURCE: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.
FROM CRITICAL CARE MEDICINE
Key clinical point: The 2018 PADIS guideline recommends intervention strategies for adult ICU patients with pain, agitation/sedation, delirium, immobility, and sleep disruption.
Major finding: The guideline includes 37 recommendations; 32 ungraded, nonactionable statements; and two good practice statements.
Study details: A clinical practice guideline was created by 32 international experts, four methodologists, and four survivors of critical illness.
Disclosures: The authors declared funding from AstraZeneca, Baxter, Covidien, and others.
Sources: Devlin JW et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003299; Balas MC et al. Crit Care Med. 2018 Sep 1. doi: 10.1097/CCM.0000000000003307.