User login
GBS in T2DM patients: Study highlights pros and cons, need for better patient selection
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
REPORTING FROM ADA 2018
Key clinical point: Bariatric surgery lowers mortality, CVD, and renal and other risks in obese T2DM patients but also has high complication rates.
Major finding: All-cause mortality, CVD, and renal disease risks were 49%, 34%, and 42% lower, respectively, in cases vs. controls.
Study details: A matched observational cohort study of 5,321 cases and 5,321 controls.
Disclosures: Dr. Liakopoulos reported having no disclosures.
Source: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
Arthritis prevalent in older adults with any degree of depression
Arthritis is highly prevalent in older adults with any degree of depression, results of a recent study suggest.
Doctor-diagnosed arthritis was reported by more than 50% of older adults with mild depression, according to results of the study, which was based on data from the National Health and Nutrition Examination Survey (NHANES).
The prevalence of arthritis exceeded 60% in participants with moderate depression, and approached 70% for those with severe depression, according to the study, published in the International Journal of Geriatric Psychiatry.
Based on those findings, arthritis and depression need to be viewed as frequently co-occurring physical and psychosocial issues, reported Jessica M. Brooks, PhD, of the department of psychiatry at Dartmouth College, Lebanon, N.H., and her coauthors.
“It may be critical for mental health care providers to provide regular arthritis-related pain assessments and evidence‐based treatments for co‐occurring arthritis in older adults with or at risk for depression,” Dr. Brooks and her colleagues said in their report.
Their analysis was based on 2,483 women and 2,309 men aged 50 years and older (mean age, 64.5 years) who had participated in the NHANES survey between 2011 and 2014. Out of that sample, 2,094 participants (43.7%) said they had been told by a doctor that they had arthritis, the researchers said.
The rate of arthritis was 38.2% for participants with no depressive symptoms as indicated by a 0-4 score on the 9-item Patient Health Questionnaire (PHQ-9). By comparison, rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression by PHQ-9.
Individuals with arthritis had a significantly higher mean PHQ-9 score, at 4.6, compared with 2.6 for those without arthritis (P less than .001), the investigators said.
that controlled for age, gender, comorbid conditions, and other factors such as smoking history.
Establishing prevalence rates of arthritis in older adults with depression is an “important step” toward informing mental health professionals on the need to identify and treat arthritis-related pain, Dr. Brooks and her coauthors said.
“Addressing arthritis in mental health treatment and behavioral medicine may also help to reduce the overlapping cognitive, behavioral, and somatic symptoms in older adults with depressive symptoms and arthritis, which may be difficult for providers to disentangle through brief screening procedures and treat through conventional depression care,” they wrote.
The investigators cited several limitations. For example, the cross-sectional nature of the study makes it difficult to draw conclusions about causality. In addition, Dr. Brooks and her colleagues did not distinguish between different types of arthritis.
The researchers declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
SOURCE: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1022/gps.4971.
Arthritis is highly prevalent in older adults with any degree of depression, results of a recent study suggest.
Doctor-diagnosed arthritis was reported by more than 50% of older adults with mild depression, according to results of the study, which was based on data from the National Health and Nutrition Examination Survey (NHANES).
The prevalence of arthritis exceeded 60% in participants with moderate depression, and approached 70% for those with severe depression, according to the study, published in the International Journal of Geriatric Psychiatry.
Based on those findings, arthritis and depression need to be viewed as frequently co-occurring physical and psychosocial issues, reported Jessica M. Brooks, PhD, of the department of psychiatry at Dartmouth College, Lebanon, N.H., and her coauthors.
“It may be critical for mental health care providers to provide regular arthritis-related pain assessments and evidence‐based treatments for co‐occurring arthritis in older adults with or at risk for depression,” Dr. Brooks and her colleagues said in their report.
Their analysis was based on 2,483 women and 2,309 men aged 50 years and older (mean age, 64.5 years) who had participated in the NHANES survey between 2011 and 2014. Out of that sample, 2,094 participants (43.7%) said they had been told by a doctor that they had arthritis, the researchers said.
The rate of arthritis was 38.2% for participants with no depressive symptoms as indicated by a 0-4 score on the 9-item Patient Health Questionnaire (PHQ-9). By comparison, rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression by PHQ-9.
Individuals with arthritis had a significantly higher mean PHQ-9 score, at 4.6, compared with 2.6 for those without arthritis (P less than .001), the investigators said.
that controlled for age, gender, comorbid conditions, and other factors such as smoking history.
Establishing prevalence rates of arthritis in older adults with depression is an “important step” toward informing mental health professionals on the need to identify and treat arthritis-related pain, Dr. Brooks and her coauthors said.
“Addressing arthritis in mental health treatment and behavioral medicine may also help to reduce the overlapping cognitive, behavioral, and somatic symptoms in older adults with depressive symptoms and arthritis, which may be difficult for providers to disentangle through brief screening procedures and treat through conventional depression care,” they wrote.
The investigators cited several limitations. For example, the cross-sectional nature of the study makes it difficult to draw conclusions about causality. In addition, Dr. Brooks and her colleagues did not distinguish between different types of arthritis.
The researchers declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
SOURCE: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1022/gps.4971.
Arthritis is highly prevalent in older adults with any degree of depression, results of a recent study suggest.
Doctor-diagnosed arthritis was reported by more than 50% of older adults with mild depression, according to results of the study, which was based on data from the National Health and Nutrition Examination Survey (NHANES).
The prevalence of arthritis exceeded 60% in participants with moderate depression, and approached 70% for those with severe depression, according to the study, published in the International Journal of Geriatric Psychiatry.
Based on those findings, arthritis and depression need to be viewed as frequently co-occurring physical and psychosocial issues, reported Jessica M. Brooks, PhD, of the department of psychiatry at Dartmouth College, Lebanon, N.H., and her coauthors.
“It may be critical for mental health care providers to provide regular arthritis-related pain assessments and evidence‐based treatments for co‐occurring arthritis in older adults with or at risk for depression,” Dr. Brooks and her colleagues said in their report.
Their analysis was based on 2,483 women and 2,309 men aged 50 years and older (mean age, 64.5 years) who had participated in the NHANES survey between 2011 and 2014. Out of that sample, 2,094 participants (43.7%) said they had been told by a doctor that they had arthritis, the researchers said.
The rate of arthritis was 38.2% for participants with no depressive symptoms as indicated by a 0-4 score on the 9-item Patient Health Questionnaire (PHQ-9). By comparison, rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression by PHQ-9.
Individuals with arthritis had a significantly higher mean PHQ-9 score, at 4.6, compared with 2.6 for those without arthritis (P less than .001), the investigators said.
that controlled for age, gender, comorbid conditions, and other factors such as smoking history.
Establishing prevalence rates of arthritis in older adults with depression is an “important step” toward informing mental health professionals on the need to identify and treat arthritis-related pain, Dr. Brooks and her coauthors said.
“Addressing arthritis in mental health treatment and behavioral medicine may also help to reduce the overlapping cognitive, behavioral, and somatic symptoms in older adults with depressive symptoms and arthritis, which may be difficult for providers to disentangle through brief screening procedures and treat through conventional depression care,” they wrote.
The investigators cited several limitations. For example, the cross-sectional nature of the study makes it difficult to draw conclusions about causality. In addition, Dr. Brooks and her colleagues did not distinguish between different types of arthritis.
The researchers declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
SOURCE: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1022/gps.4971.
FROM THE INTERNATIONAL JOURNAL OF GERIATRIC PSYCHIATRY
Key clinical point: “It may be critical for mental health care providers to provide regular arthritis-related pain assessments” for older adults with or at risk for depression.
Major finding: Rates of arthritis were 55.0%, 62.9%, and 67.8% for those with mild, moderate, or severe depression, respectively, according to the 9-item Patient Health Questionnaire-9 scores.
Study details: Findings on 2,483 women and 2,309 men aged 50 years and older who participated in the National Health and Nutrition Examination Survey.
Disclosures: Dr. Brooks and her coauthors declared no conflicts of interest. The study was supported by several U.S. institutes, including the National Institute of Mental Health, and by numerous entities related to Dartmouth, including the Dartmouth Health Promotion and Disease Prevention Research Center. The Howard and Phyllis Schwartz Philanthropic Fund also provided funding.
Source: Brooks JM et al. Int J Geriatr Psychiatry. 2018 Sep 19. doi: 10.1002/gps.4971.
Cannabinoids may raise pain tolerance, not relieve pain itself
The belief that cannabinoids address chronic pain by relieving it has been challenged by a meta-analysis that finds cannabinoids have effects on pain thresholds that may, instead, make pain more tolerable.
The systematic review and meta-analysis comprised 18 placebo-controlled studies looking at the effect of plant-based or synthetic cannabinoids on experimental pain in 442 healthy participants. The conclusions were published Sept. 19 in JAMA Psychiatry.
“Cannabinoid drugs may prevent the onset of pain by producing small increases in pain thresholds,” wrote Martin J. De Vita and his colleagues, “but may not reduce the intensity of experimental pain already being experienced; instead, cannabinoids may make experimental pain feel less unpleasant and more tolerable, suggesting an influence on affective processes.”
Ten of the studies analyzed measured changes in pain thresholds and showed a small but significant association between cannabinoids and higher pain thresholds (95% confidence interval, 0.054-0.318; P = .006). Five studies examined pain unpleasantness, and showed that cannabinoids were associated with reduced unpleasantness ratings, compared with placebo (95% CI, 0.104-0.472; P = .002).
Among the eight studies that measured pain tolerance, a significant association was found between cannabinoid administration and higher pain tolerance (95% CI, 0.015-0.436; P = .04).
However, the 13 studies looking at pain intensity found no association between cannabinoid use and changes in the intensity of pain (CI, –0.120-0.154), nor were reductions in pain sensitivity to mechanical stimulation found in the three studies that measured mechanical hyperalgesia (95% CI, –0.059-0.244; P = .23).
The analysis did see significant effects according to the type of cannabinoid; plant-based cannabis had significantly stronger associations with reductions in pain unpleasantness compared to dronabinol and other synthetic THC preparations. However, both plant-based cannabis and dronabinol were associated with increases in pain tolerance, whereas other synthetic THC preparations were associated with significant reductions in pain tolerance.
Mr. De Vita, of the department of psychology at Syracuse (N.Y.) University, and his colleagues stressed that the systematic review and meta-analysis focused solely on studies of experimental pain, which “merely approximates features of clinical pain,” and specifically excluded individuals with chronic pain.
The absence of neuropathic pain data is “especially limiting, given that neuropathic pain is the primary condition for which modest empirical evidence exists that supports cannabinoid analgesia,” they wrote.
In particular, they drew attention to the finding that cannabinoids did not appear to have any effect on mechanical hyperalgesia, which they said suggests that cannabinoids might not address central sensitization in people with neuropathic pain.
The authors also raised the question of whether cannabinoids simply relieve pain by making people feel good or “high,” much like other intoxicating substances such as alcohol. They argued that the answer depends on what outcome is desired from treatment.
“If treatment aims to relieve pain without producing intoxication, psychoactive cannabinoids may not suffice,” they wrote. Ultimately, they said, the relief from pain experienced by some patients might be driven largely by an “affective rather than a sensory component.”
Among the limitations cited by the researchers is the focus on studies of experimental pain. “To produce evidence that supports the generalizability of the current findings, pain reactivity research must be conduced in clinical samples,” Mr. De Vita and his colleagues wrote.
The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
SOURCE: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
The belief that cannabinoids address chronic pain by relieving it has been challenged by a meta-analysis that finds cannabinoids have effects on pain thresholds that may, instead, make pain more tolerable.
The systematic review and meta-analysis comprised 18 placebo-controlled studies looking at the effect of plant-based or synthetic cannabinoids on experimental pain in 442 healthy participants. The conclusions were published Sept. 19 in JAMA Psychiatry.
“Cannabinoid drugs may prevent the onset of pain by producing small increases in pain thresholds,” wrote Martin J. De Vita and his colleagues, “but may not reduce the intensity of experimental pain already being experienced; instead, cannabinoids may make experimental pain feel less unpleasant and more tolerable, suggesting an influence on affective processes.”
Ten of the studies analyzed measured changes in pain thresholds and showed a small but significant association between cannabinoids and higher pain thresholds (95% confidence interval, 0.054-0.318; P = .006). Five studies examined pain unpleasantness, and showed that cannabinoids were associated with reduced unpleasantness ratings, compared with placebo (95% CI, 0.104-0.472; P = .002).
Among the eight studies that measured pain tolerance, a significant association was found between cannabinoid administration and higher pain tolerance (95% CI, 0.015-0.436; P = .04).
However, the 13 studies looking at pain intensity found no association between cannabinoid use and changes in the intensity of pain (CI, –0.120-0.154), nor were reductions in pain sensitivity to mechanical stimulation found in the three studies that measured mechanical hyperalgesia (95% CI, –0.059-0.244; P = .23).
The analysis did see significant effects according to the type of cannabinoid; plant-based cannabis had significantly stronger associations with reductions in pain unpleasantness compared to dronabinol and other synthetic THC preparations. However, both plant-based cannabis and dronabinol were associated with increases in pain tolerance, whereas other synthetic THC preparations were associated with significant reductions in pain tolerance.
Mr. De Vita, of the department of psychology at Syracuse (N.Y.) University, and his colleagues stressed that the systematic review and meta-analysis focused solely on studies of experimental pain, which “merely approximates features of clinical pain,” and specifically excluded individuals with chronic pain.
The absence of neuropathic pain data is “especially limiting, given that neuropathic pain is the primary condition for which modest empirical evidence exists that supports cannabinoid analgesia,” they wrote.
In particular, they drew attention to the finding that cannabinoids did not appear to have any effect on mechanical hyperalgesia, which they said suggests that cannabinoids might not address central sensitization in people with neuropathic pain.
The authors also raised the question of whether cannabinoids simply relieve pain by making people feel good or “high,” much like other intoxicating substances such as alcohol. They argued that the answer depends on what outcome is desired from treatment.
“If treatment aims to relieve pain without producing intoxication, psychoactive cannabinoids may not suffice,” they wrote. Ultimately, they said, the relief from pain experienced by some patients might be driven largely by an “affective rather than a sensory component.”
Among the limitations cited by the researchers is the focus on studies of experimental pain. “To produce evidence that supports the generalizability of the current findings, pain reactivity research must be conduced in clinical samples,” Mr. De Vita and his colleagues wrote.
The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
SOURCE: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
The belief that cannabinoids address chronic pain by relieving it has been challenged by a meta-analysis that finds cannabinoids have effects on pain thresholds that may, instead, make pain more tolerable.
The systematic review and meta-analysis comprised 18 placebo-controlled studies looking at the effect of plant-based or synthetic cannabinoids on experimental pain in 442 healthy participants. The conclusions were published Sept. 19 in JAMA Psychiatry.
“Cannabinoid drugs may prevent the onset of pain by producing small increases in pain thresholds,” wrote Martin J. De Vita and his colleagues, “but may not reduce the intensity of experimental pain already being experienced; instead, cannabinoids may make experimental pain feel less unpleasant and more tolerable, suggesting an influence on affective processes.”
Ten of the studies analyzed measured changes in pain thresholds and showed a small but significant association between cannabinoids and higher pain thresholds (95% confidence interval, 0.054-0.318; P = .006). Five studies examined pain unpleasantness, and showed that cannabinoids were associated with reduced unpleasantness ratings, compared with placebo (95% CI, 0.104-0.472; P = .002).
Among the eight studies that measured pain tolerance, a significant association was found between cannabinoid administration and higher pain tolerance (95% CI, 0.015-0.436; P = .04).
However, the 13 studies looking at pain intensity found no association between cannabinoid use and changes in the intensity of pain (CI, –0.120-0.154), nor were reductions in pain sensitivity to mechanical stimulation found in the three studies that measured mechanical hyperalgesia (95% CI, –0.059-0.244; P = .23).
The analysis did see significant effects according to the type of cannabinoid; plant-based cannabis had significantly stronger associations with reductions in pain unpleasantness compared to dronabinol and other synthetic THC preparations. However, both plant-based cannabis and dronabinol were associated with increases in pain tolerance, whereas other synthetic THC preparations were associated with significant reductions in pain tolerance.
Mr. De Vita, of the department of psychology at Syracuse (N.Y.) University, and his colleagues stressed that the systematic review and meta-analysis focused solely on studies of experimental pain, which “merely approximates features of clinical pain,” and specifically excluded individuals with chronic pain.
The absence of neuropathic pain data is “especially limiting, given that neuropathic pain is the primary condition for which modest empirical evidence exists that supports cannabinoid analgesia,” they wrote.
In particular, they drew attention to the finding that cannabinoids did not appear to have any effect on mechanical hyperalgesia, which they said suggests that cannabinoids might not address central sensitization in people with neuropathic pain.
The authors also raised the question of whether cannabinoids simply relieve pain by making people feel good or “high,” much like other intoxicating substances such as alcohol. They argued that the answer depends on what outcome is desired from treatment.
“If treatment aims to relieve pain without producing intoxication, psychoactive cannabinoids may not suffice,” they wrote. Ultimately, they said, the relief from pain experienced by some patients might be driven largely by an “affective rather than a sensory component.”
Among the limitations cited by the researchers is the focus on studies of experimental pain. “To produce evidence that supports the generalizability of the current findings, pain reactivity research must be conduced in clinical samples,” Mr. De Vita and his colleagues wrote.
The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
SOURCE: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
FROM JAMA PSYCHIATRY
Key clinical point: Cannabinoids might relieve experimental pain by making people feel good or “high,” much like other intoxicating substances such as alcohol.
Major finding: Cannabinoids improve pain thresholds (95% confidence interval, 0.054-0.3,18; P = .006) and pain unpleasantness (95% CI, 0.104-0.472; P = .002).
Study details: Systematic review and meta-analysis of 18 placebo-controlled studies of experimental pain.
Disclosures: The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
Source: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
Connecting at TCT 2018
The 30th anniversary of the Transcatheter Cardiovascular Therapeutics meeting in San Diego, sponsored by the Cardiovascular Research Foundation, will be the temporary home of more than 10,000 attendees. That’s up from about 9,400 last year.
Even so, that leaves a lot of folks who can’t be in sunny California. Keep in touch with friends near and far by using social media. Twitter hashtags for the meeting are #TCT2018 and #TCTConference.
The organizers encourage attendees to share their experiences and the educational content from the meeting, keeping in mind embargoes and some limitations, by posting photos, slides, or short video clips. Also, presentation slides will be online immediately after the sessions.
Attendees also can connect with each other using the the CRF Events app, available for free download here. But we recommend that meet outside if you can, considering the weather report.
The 30th anniversary of the Transcatheter Cardiovascular Therapeutics meeting in San Diego, sponsored by the Cardiovascular Research Foundation, will be the temporary home of more than 10,000 attendees. That’s up from about 9,400 last year.
Even so, that leaves a lot of folks who can’t be in sunny California. Keep in touch with friends near and far by using social media. Twitter hashtags for the meeting are #TCT2018 and #TCTConference.
The organizers encourage attendees to share their experiences and the educational content from the meeting, keeping in mind embargoes and some limitations, by posting photos, slides, or short video clips. Also, presentation slides will be online immediately after the sessions.
Attendees also can connect with each other using the the CRF Events app, available for free download here. But we recommend that meet outside if you can, considering the weather report.
The 30th anniversary of the Transcatheter Cardiovascular Therapeutics meeting in San Diego, sponsored by the Cardiovascular Research Foundation, will be the temporary home of more than 10,000 attendees. That’s up from about 9,400 last year.
Even so, that leaves a lot of folks who can’t be in sunny California. Keep in touch with friends near and far by using social media. Twitter hashtags for the meeting are #TCT2018 and #TCTConference.
The organizers encourage attendees to share their experiences and the educational content from the meeting, keeping in mind embargoes and some limitations, by posting photos, slides, or short video clips. Also, presentation slides will be online immediately after the sessions.
Attendees also can connect with each other using the the CRF Events app, available for free download here. But we recommend that meet outside if you can, considering the weather report.
Minilaparoscopy is the next step in minimally invasive surgery
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
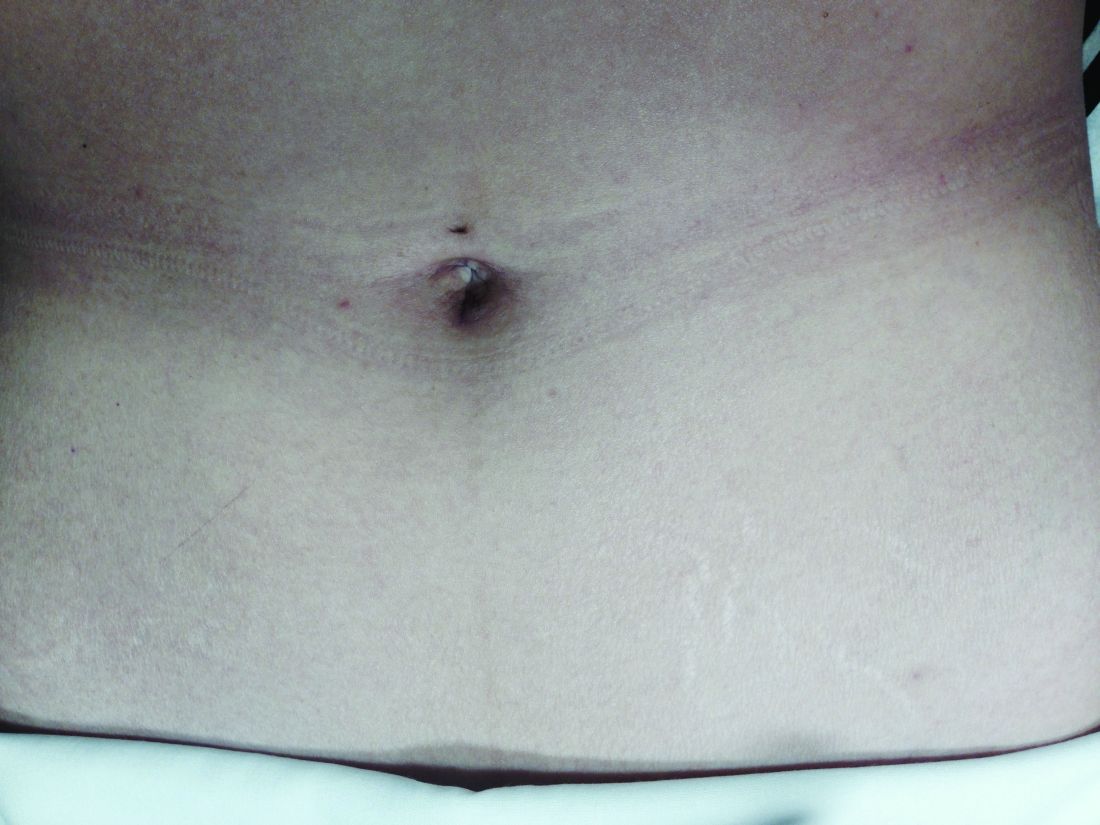
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”

Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”

Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minilaparoscopy is a relevant surgical technique
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
TCT presents new, ‘massive’ integrated, hands-on learning center
pavilions devoted to specific fields.
This major innovation brings TCT back to basics, Juan F. Granada, MD, president and CEO of the Cardiovascular Research Foundation and codirector of Transcatheter Cardiovascular Therapeutics, said in an interview.
Dr. Granada was closely involved with the program and said it’s been in the works for a couple of years. The emphasis of these workshops is to teach physicians an understanding of anatomy. TCT is devoting a massive amount of space to this effort, about 60% of the exhibit hall. The eight pavilions are dedicated to training tracks, and every single tool available to interventional cardiologists will be there, he said.
If you preregistered before all 4,000 spots filled up some days ago, consider yourself lucky. But be assured that the TCT organizers are tracking the registrations, even those submitted after the limit was reached, to help them plan for next year’s meeting in San Francisco.
The workshops will run over 3 days, from Saturday through Monday, Sept. 22-24.
pavilions devoted to specific fields.
This major innovation brings TCT back to basics, Juan F. Granada, MD, president and CEO of the Cardiovascular Research Foundation and codirector of Transcatheter Cardiovascular Therapeutics, said in an interview.
Dr. Granada was closely involved with the program and said it’s been in the works for a couple of years. The emphasis of these workshops is to teach physicians an understanding of anatomy. TCT is devoting a massive amount of space to this effort, about 60% of the exhibit hall. The eight pavilions are dedicated to training tracks, and every single tool available to interventional cardiologists will be there, he said.
If you preregistered before all 4,000 spots filled up some days ago, consider yourself lucky. But be assured that the TCT organizers are tracking the registrations, even those submitted after the limit was reached, to help them plan for next year’s meeting in San Francisco.
The workshops will run over 3 days, from Saturday through Monday, Sept. 22-24.
pavilions devoted to specific fields.
This major innovation brings TCT back to basics, Juan F. Granada, MD, president and CEO of the Cardiovascular Research Foundation and codirector of Transcatheter Cardiovascular Therapeutics, said in an interview.
Dr. Granada was closely involved with the program and said it’s been in the works for a couple of years. The emphasis of these workshops is to teach physicians an understanding of anatomy. TCT is devoting a massive amount of space to this effort, about 60% of the exhibit hall. The eight pavilions are dedicated to training tracks, and every single tool available to interventional cardiologists will be there, he said.
If you preregistered before all 4,000 spots filled up some days ago, consider yourself lucky. But be assured that the TCT organizers are tracking the registrations, even those submitted after the limit was reached, to help them plan for next year’s meeting in San Francisco.
The workshops will run over 3 days, from Saturday through Monday, Sept. 22-24.
Trump, not health care, likely focus of midterm elections
Provider community must be “creative and participative”
Come November 2018, Americans will return to the polls to vote for their representatives in Congress, for governors, and for state legislative seats.
Health care has been a topic of debate since the 2016 elections brought a Republican sweep to the executive and legislative branches, but other issues have since moved to the forefront. Will the midterm elections this year prove health care to be a significant issue at the polls?
Unlikely, said Robert Berenson, MD, FACP, Institute Fellow of the Health Policy Center at the Urban Institute. More likely, the election will be a referendum on President Donald Trump, he said. “Things are so partisan right now and it’s all about Trump. I don’t see serious discussion about health policy.”
Ron Greeno, MD, MHM, FCCP, immediate past president of SHM and former chair of the Public Policy Committee, also doesn’t see health care rising to the top of election year issues. But that doesn’t mean health care doesn’t matter to American voters.
“Whether Democrats control the House or Republicans control the House won’t likely make a big difference in terms of impact on the things we care about,” said Dr. Greeno. “The issues they debate in Washington are not going to save the health care system. They are just debating about who is going to pay for what and for whom. To save our health care system, we have to lower the cost of care and only providers can do that.”
What the government can do, he said, is create the right incentives for providers to move away from fee for service and participate in new models that may lower the cost of care. At the same time, “the economy also has to grow at a robust pace, which will make a huge difference. So, recent increases in economic growth rate are welcomed,” said Dr. Greeno.
In 2015, Republicans and Democrats came together to pass bipartisan legislation aimed at moving the health care system away from fee for service: the Medicare and CHIP Reauthorization Act, or MACRA.
However, the law has not been without frustrations, and these concerns will likely not be part of any candidate campaigns in 2018, Dr. Greeno predicted: “There’s not a lot of appetite to reopen the statute (more than) 2 years after it passed.”
MACRA provides clinicians two pathways to reimbursement. The first track, called MIPS (Merit-Based Incentive Payment System), bases a portion of physician reimbursement on scores measured across several categories, including cost and quality. It still operates largely under a fee-for-service framework but is meant to be budget neutral; for every winner there is a loser.
The second track, called the APMs (Alternative Payment Models), requires physicians to take on substantial risk (with potential for reward), if they can achieve specific patient volumes under approved models. However, few providers qualify, especially among hospitalists, though the structure of the program makes it clear that the Centers for Medicare & Medicaid Services intends to have most providers ultimately transition to APMs.
“There’s growing recognition that MACRA, at least the MIPS portion, was a big mistake but Congress can’t go back and say we blew it,” Dr. Berenson said. “CMS has now exempted somewhere between 550,000 and 900,000 clinicians from MACRA,” because they cannot meet the requirements of either pathway without significant hardship.1
CMS wasn’t considering hospitalists specifically when implementing the law, though hospitalists admit half of the Medicare patients in the United States, Dr. Greeno said. There are very few hospitalists currently participating in Advanced APMs and those that are, do not see the volume of patients the pathway requires.
“What hospitalists do is very conducive to alternative payment models, and we can help those alternative payment models drive improved quality and lowered costs,” said Dr. Greeno. “Hospitals use hospitalists to help them manage risk, so it’s frustrating that most hospitalists will not meet the thresholds for the APM track and benefit from the incentives created.”
However, the Society of Hospital Medicine continues to work on behalf of hospitalists. Thanks to its efforts, Dr. Greeno explained, CMS is planning in 2019 to allow hospitalists to choose to be scored under MIPS based on their hospital’s performance across reporting categories. Or, they can choose to report on their own and opt out of this new “facility-based” option.
“We are working with (CMS) to figure out how to make this new option work,” said Dr. Greeno.
At the state level, 36 governorships are up for grabs and those outcomes could influence the direction of Medicaid. In Kentucky, the Trump administration approved a waiver allowing the state to enforce work requirements for Medicaid recipients. However, on June 29, 2018, the D.C. federal district court invalidated the Kentucky HEALTH waiver approval (with the exception of Kentucky’s IMD SUD [institutions for mental disease for substance use disorders] payment waiver authority) and sent it back to HHS to reconsider. Ten other states as of August 2018 had applied for similar waivers.2 However, Dr. Berenson believes that most of what could happen to Medicaid will be a topic after the midterm elections and not before.
He also believes drug prices could become an issue in national elections, though there will not be an easy solution from either side. “Democrats will be reluctant to say they’re going to negotiate drug prices; they’re going to want the government to negotiate for Medicare-like pricing.” Republicans, on the other hand, will be reluctant to consider government regulation.
As a general principle leading into the midterms: “Democrats want to avoid an internal war about whether they are for Medicare for all or single payer or not,” Dr. Berenson said. “What I’m hoping doesn’t happen is that it becomes a litmus test for purity where you have to be for single payer. I think would be huge mistake because it’s not realistic that it would ever get there.”
However, he cites an idea from left-leaning Princeton University’s Paul Starr, a professor of sociology and public affairs, that Democrats could consider: so-called Midlife Medicare, an option that could be made available to Americans beginning at age 50 years.3 It would represent a new Medicare option, funded by general revenues and premiums, available to people age 50 years and older and those younger than 65 years who are without employer-sponsored health insurance.
Regardless, as the United States catapults toward another election that could disrupt the political system or maintain the relative status quo, Dr. Greeno said hospitalists continue to play key roles in improving American health care.
“There are programs in place where we can get the job done if we in the provider community are creative and participative,” he said. “Some of the most important work being done is coming out of the CMS Innovation Center. Hospitalists continue to be a big part of that, but we knew it would take decades of really hard work and I don’t see anything happening in the midterms to derail this or bring about a massive increase in the pace of change.”
References
1. Dickson V. CMS gives more small practices a pass on MACRA. Modern Healthcare. Published June 20, 2017.
2. Medicaid Waiver Tracker: Which States Have Approved and Pending Section 1115 Medicaid Waivers? Kaiser Family Foundation. Published Aug. 8, 2018.
3. Starr P. A new strategy for health care. The American Prospect. Published Jan. 4, 2018. Accessed March 5, 2018.
Provider community must be “creative and participative”
Provider community must be “creative and participative”
Come November 2018, Americans will return to the polls to vote for their representatives in Congress, for governors, and for state legislative seats.
Health care has been a topic of debate since the 2016 elections brought a Republican sweep to the executive and legislative branches, but other issues have since moved to the forefront. Will the midterm elections this year prove health care to be a significant issue at the polls?
Unlikely, said Robert Berenson, MD, FACP, Institute Fellow of the Health Policy Center at the Urban Institute. More likely, the election will be a referendum on President Donald Trump, he said. “Things are so partisan right now and it’s all about Trump. I don’t see serious discussion about health policy.”
Ron Greeno, MD, MHM, FCCP, immediate past president of SHM and former chair of the Public Policy Committee, also doesn’t see health care rising to the top of election year issues. But that doesn’t mean health care doesn’t matter to American voters.
“Whether Democrats control the House or Republicans control the House won’t likely make a big difference in terms of impact on the things we care about,” said Dr. Greeno. “The issues they debate in Washington are not going to save the health care system. They are just debating about who is going to pay for what and for whom. To save our health care system, we have to lower the cost of care and only providers can do that.”
What the government can do, he said, is create the right incentives for providers to move away from fee for service and participate in new models that may lower the cost of care. At the same time, “the economy also has to grow at a robust pace, which will make a huge difference. So, recent increases in economic growth rate are welcomed,” said Dr. Greeno.
In 2015, Republicans and Democrats came together to pass bipartisan legislation aimed at moving the health care system away from fee for service: the Medicare and CHIP Reauthorization Act, or MACRA.
However, the law has not been without frustrations, and these concerns will likely not be part of any candidate campaigns in 2018, Dr. Greeno predicted: “There’s not a lot of appetite to reopen the statute (more than) 2 years after it passed.”
MACRA provides clinicians two pathways to reimbursement. The first track, called MIPS (Merit-Based Incentive Payment System), bases a portion of physician reimbursement on scores measured across several categories, including cost and quality. It still operates largely under a fee-for-service framework but is meant to be budget neutral; for every winner there is a loser.
The second track, called the APMs (Alternative Payment Models), requires physicians to take on substantial risk (with potential for reward), if they can achieve specific patient volumes under approved models. However, few providers qualify, especially among hospitalists, though the structure of the program makes it clear that the Centers for Medicare & Medicaid Services intends to have most providers ultimately transition to APMs.
“There’s growing recognition that MACRA, at least the MIPS portion, was a big mistake but Congress can’t go back and say we blew it,” Dr. Berenson said. “CMS has now exempted somewhere between 550,000 and 900,000 clinicians from MACRA,” because they cannot meet the requirements of either pathway without significant hardship.1
CMS wasn’t considering hospitalists specifically when implementing the law, though hospitalists admit half of the Medicare patients in the United States, Dr. Greeno said. There are very few hospitalists currently participating in Advanced APMs and those that are, do not see the volume of patients the pathway requires.
“What hospitalists do is very conducive to alternative payment models, and we can help those alternative payment models drive improved quality and lowered costs,” said Dr. Greeno. “Hospitals use hospitalists to help them manage risk, so it’s frustrating that most hospitalists will not meet the thresholds for the APM track and benefit from the incentives created.”
However, the Society of Hospital Medicine continues to work on behalf of hospitalists. Thanks to its efforts, Dr. Greeno explained, CMS is planning in 2019 to allow hospitalists to choose to be scored under MIPS based on their hospital’s performance across reporting categories. Or, they can choose to report on their own and opt out of this new “facility-based” option.
“We are working with (CMS) to figure out how to make this new option work,” said Dr. Greeno.
At the state level, 36 governorships are up for grabs and those outcomes could influence the direction of Medicaid. In Kentucky, the Trump administration approved a waiver allowing the state to enforce work requirements for Medicaid recipients. However, on June 29, 2018, the D.C. federal district court invalidated the Kentucky HEALTH waiver approval (with the exception of Kentucky’s IMD SUD [institutions for mental disease for substance use disorders] payment waiver authority) and sent it back to HHS to reconsider. Ten other states as of August 2018 had applied for similar waivers.2 However, Dr. Berenson believes that most of what could happen to Medicaid will be a topic after the midterm elections and not before.
He also believes drug prices could become an issue in national elections, though there will not be an easy solution from either side. “Democrats will be reluctant to say they’re going to negotiate drug prices; they’re going to want the government to negotiate for Medicare-like pricing.” Republicans, on the other hand, will be reluctant to consider government regulation.
As a general principle leading into the midterms: “Democrats want to avoid an internal war about whether they are for Medicare for all or single payer or not,” Dr. Berenson said. “What I’m hoping doesn’t happen is that it becomes a litmus test for purity where you have to be for single payer. I think would be huge mistake because it’s not realistic that it would ever get there.”
However, he cites an idea from left-leaning Princeton University’s Paul Starr, a professor of sociology and public affairs, that Democrats could consider: so-called Midlife Medicare, an option that could be made available to Americans beginning at age 50 years.3 It would represent a new Medicare option, funded by general revenues and premiums, available to people age 50 years and older and those younger than 65 years who are without employer-sponsored health insurance.
Regardless, as the United States catapults toward another election that could disrupt the political system or maintain the relative status quo, Dr. Greeno said hospitalists continue to play key roles in improving American health care.
“There are programs in place where we can get the job done if we in the provider community are creative and participative,” he said. “Some of the most important work being done is coming out of the CMS Innovation Center. Hospitalists continue to be a big part of that, but we knew it would take decades of really hard work and I don’t see anything happening in the midterms to derail this or bring about a massive increase in the pace of change.”
References
1. Dickson V. CMS gives more small practices a pass on MACRA. Modern Healthcare. Published June 20, 2017.
2. Medicaid Waiver Tracker: Which States Have Approved and Pending Section 1115 Medicaid Waivers? Kaiser Family Foundation. Published Aug. 8, 2018.
3. Starr P. A new strategy for health care. The American Prospect. Published Jan. 4, 2018. Accessed March 5, 2018.
Come November 2018, Americans will return to the polls to vote for their representatives in Congress, for governors, and for state legislative seats.
Health care has been a topic of debate since the 2016 elections brought a Republican sweep to the executive and legislative branches, but other issues have since moved to the forefront. Will the midterm elections this year prove health care to be a significant issue at the polls?
Unlikely, said Robert Berenson, MD, FACP, Institute Fellow of the Health Policy Center at the Urban Institute. More likely, the election will be a referendum on President Donald Trump, he said. “Things are so partisan right now and it’s all about Trump. I don’t see serious discussion about health policy.”
Ron Greeno, MD, MHM, FCCP, immediate past president of SHM and former chair of the Public Policy Committee, also doesn’t see health care rising to the top of election year issues. But that doesn’t mean health care doesn’t matter to American voters.
“Whether Democrats control the House or Republicans control the House won’t likely make a big difference in terms of impact on the things we care about,” said Dr. Greeno. “The issues they debate in Washington are not going to save the health care system. They are just debating about who is going to pay for what and for whom. To save our health care system, we have to lower the cost of care and only providers can do that.”
What the government can do, he said, is create the right incentives for providers to move away from fee for service and participate in new models that may lower the cost of care. At the same time, “the economy also has to grow at a robust pace, which will make a huge difference. So, recent increases in economic growth rate are welcomed,” said Dr. Greeno.
In 2015, Republicans and Democrats came together to pass bipartisan legislation aimed at moving the health care system away from fee for service: the Medicare and CHIP Reauthorization Act, or MACRA.
However, the law has not been without frustrations, and these concerns will likely not be part of any candidate campaigns in 2018, Dr. Greeno predicted: “There’s not a lot of appetite to reopen the statute (more than) 2 years after it passed.”
MACRA provides clinicians two pathways to reimbursement. The first track, called MIPS (Merit-Based Incentive Payment System), bases a portion of physician reimbursement on scores measured across several categories, including cost and quality. It still operates largely under a fee-for-service framework but is meant to be budget neutral; for every winner there is a loser.
The second track, called the APMs (Alternative Payment Models), requires physicians to take on substantial risk (with potential for reward), if they can achieve specific patient volumes under approved models. However, few providers qualify, especially among hospitalists, though the structure of the program makes it clear that the Centers for Medicare & Medicaid Services intends to have most providers ultimately transition to APMs.
“There’s growing recognition that MACRA, at least the MIPS portion, was a big mistake but Congress can’t go back and say we blew it,” Dr. Berenson said. “CMS has now exempted somewhere between 550,000 and 900,000 clinicians from MACRA,” because they cannot meet the requirements of either pathway without significant hardship.1
CMS wasn’t considering hospitalists specifically when implementing the law, though hospitalists admit half of the Medicare patients in the United States, Dr. Greeno said. There are very few hospitalists currently participating in Advanced APMs and those that are, do not see the volume of patients the pathway requires.
“What hospitalists do is very conducive to alternative payment models, and we can help those alternative payment models drive improved quality and lowered costs,” said Dr. Greeno. “Hospitals use hospitalists to help them manage risk, so it’s frustrating that most hospitalists will not meet the thresholds for the APM track and benefit from the incentives created.”
However, the Society of Hospital Medicine continues to work on behalf of hospitalists. Thanks to its efforts, Dr. Greeno explained, CMS is planning in 2019 to allow hospitalists to choose to be scored under MIPS based on their hospital’s performance across reporting categories. Or, they can choose to report on their own and opt out of this new “facility-based” option.
“We are working with (CMS) to figure out how to make this new option work,” said Dr. Greeno.
At the state level, 36 governorships are up for grabs and those outcomes could influence the direction of Medicaid. In Kentucky, the Trump administration approved a waiver allowing the state to enforce work requirements for Medicaid recipients. However, on June 29, 2018, the D.C. federal district court invalidated the Kentucky HEALTH waiver approval (with the exception of Kentucky’s IMD SUD [institutions for mental disease for substance use disorders] payment waiver authority) and sent it back to HHS to reconsider. Ten other states as of August 2018 had applied for similar waivers.2 However, Dr. Berenson believes that most of what could happen to Medicaid will be a topic after the midterm elections and not before.
He also believes drug prices could become an issue in national elections, though there will not be an easy solution from either side. “Democrats will be reluctant to say they’re going to negotiate drug prices; they’re going to want the government to negotiate for Medicare-like pricing.” Republicans, on the other hand, will be reluctant to consider government regulation.
As a general principle leading into the midterms: “Democrats want to avoid an internal war about whether they are for Medicare for all or single payer or not,” Dr. Berenson said. “What I’m hoping doesn’t happen is that it becomes a litmus test for purity where you have to be for single payer. I think would be huge mistake because it’s not realistic that it would ever get there.”
However, he cites an idea from left-leaning Princeton University’s Paul Starr, a professor of sociology and public affairs, that Democrats could consider: so-called Midlife Medicare, an option that could be made available to Americans beginning at age 50 years.3 It would represent a new Medicare option, funded by general revenues and premiums, available to people age 50 years and older and those younger than 65 years who are without employer-sponsored health insurance.
Regardless, as the United States catapults toward another election that could disrupt the political system or maintain the relative status quo, Dr. Greeno said hospitalists continue to play key roles in improving American health care.
“There are programs in place where we can get the job done if we in the provider community are creative and participative,” he said. “Some of the most important work being done is coming out of the CMS Innovation Center. Hospitalists continue to be a big part of that, but we knew it would take decades of really hard work and I don’t see anything happening in the midterms to derail this or bring about a massive increase in the pace of change.”
References
1. Dickson V. CMS gives more small practices a pass on MACRA. Modern Healthcare. Published June 20, 2017.
2. Medicaid Waiver Tracker: Which States Have Approved and Pending Section 1115 Medicaid Waivers? Kaiser Family Foundation. Published Aug. 8, 2018.
3. Starr P. A new strategy for health care. The American Prospect. Published Jan. 4, 2018. Accessed March 5, 2018.
Top research at TCT 2018
The research area that rose to the top in terms of importance to practice at the upcoming Transcatheter Cardiovascular Therapeutics meeting in San Diego is in mitral valve interventions, but there’s much more potentially practice-changing original reporting as well.
Of the 155 abstracts considered for Late-Breaking Trials, only 10% were selected. That’s in addition to the other 1,650 abstracts submitted for the meeting.
The research chosen for the Late-Breaking Trial and Late-Breaking Research sessions held on Saturday, Sept. 22, through Tuesday, Sept. 25, fall into four categories, Juan F. Granada, MD, president and CEO of the Cardiovascular Research Foundation and codirector of Transcatheter Cardiovascular Therapeutics, said in an interview.
The first and most important category is mitral intervention, he said, and the studies with the most potential to change U.S. practice will be presented on Sunday morning at 10:15-11:15 in the Late-Breaking Trial session. The session kicks off with COAPT, the long-anticipated trial that randomized patients with severe mitral regurgitation secondary to heart failure to the MitraClip or to optimal medical management. The MitraClip, which doesn’t entail a concomitant annuloplasty, currently is approved by the Food and Drug Administration for patients with primary, degenerative mitral regurgitation not amenable to surgical repair only. But, if COAPT yields positive results, the role of the MitraClip may expand greatly.
Also presented in that Sunday session will be results of REDUCE-FMR, a sham-controlled trial in patients with heart failure–associated functional mitral regurgitation who received either treatment with the CARILLON Mitral Contour System, which is an indirect annuloplasty device, or optimal medical treatment.
Secondly, “we’ve got a lot to learn from two trials in the Absorb bioresorbable scaffold,” Dr. Granada said. Although the device failed commercially on the heels of news of target vessel failure and scaffold thrombosis, detailed results of ABSORB IV and COMPARE ABSORB will inform future designs, he said.The third area Dr. Granada emphasized was direct comparisons of stents. Saturday’s Late-Breaking Trials sessions offer results of several head-to-head trials. BIONYX compared the bioresorbable polymer Orsiro stent with the newer durable polymer Resolute Onyx. TALENT, unusual in its all-comers design, compared clinical outcomes at 12 months between the Supraflex ultrathin coronary stent and Xience everolimus eluting system. Whether strut thickness can make a difference in outcomes will be of great interest as the results of these and other trials are presented at the meeting.
Lastly, an endovascular trial comparing two stents in long lesion, IMPERIAL, will be of interest. It randomized patients with chronic, symptomatic superficial femoral or proximal popliteal artery lesions to treatment with either the Eluvia vascular stent or the Zilver PTX, the first drug-eluting stent approved for use in these patients.
Remember, there also are oral abstract and moderated poster sessions happening throughout the meeting, so there’s something for everyone.
The research area that rose to the top in terms of importance to practice at the upcoming Transcatheter Cardiovascular Therapeutics meeting in San Diego is in mitral valve interventions, but there’s much more potentially practice-changing original reporting as well.
Of the 155 abstracts considered for Late-Breaking Trials, only 10% were selected. That’s in addition to the other 1,650 abstracts submitted for the meeting.
The research chosen for the Late-Breaking Trial and Late-Breaking Research sessions held on Saturday, Sept. 22, through Tuesday, Sept. 25, fall into four categories, Juan F. Granada, MD, president and CEO of the Cardiovascular Research Foundation and codirector of Transcatheter Cardiovascular Therapeutics, said in an interview.
The first and most important category is mitral intervention, he said, and the studies with the most potential to change U.S. practice will be presented on Sunday morning at 10:15-11:15 in the Late-Breaking Trial session. The session kicks off with COAPT, the long-anticipated trial that randomized patients with severe mitral regurgitation secondary to heart failure to the MitraClip or to optimal medical management. The MitraClip, which doesn’t entail a concomitant annuloplasty, currently is approved by the Food and Drug Administration for patients with primary, degenerative mitral regurgitation not amenable to surgical repair only. But, if COAPT yields positive results, the role of the MitraClip may expand greatly.
Also presented in that Sunday session will be results of REDUCE-FMR, a sham-controlled trial in patients with heart failure–associated functional mitral regurgitation who received either treatment with the CARILLON Mitral Contour System, which is an indirect annuloplasty device, or optimal medical treatment.
Secondly, “we’ve got a lot to learn from two trials in the Absorb bioresorbable scaffold,” Dr. Granada said. Although the device failed commercially on the heels of news of target vessel failure and scaffold thrombosis, detailed results of ABSORB IV and COMPARE ABSORB will inform future designs, he said.The third area Dr. Granada emphasized was direct comparisons of stents. Saturday’s Late-Breaking Trials sessions offer results of several head-to-head trials. BIONYX compared the bioresorbable polymer Orsiro stent with the newer durable polymer Resolute Onyx. TALENT, unusual in its all-comers design, compared clinical outcomes at 12 months between the Supraflex ultrathin coronary stent and Xience everolimus eluting system. Whether strut thickness can make a difference in outcomes will be of great interest as the results of these and other trials are presented at the meeting.
Lastly, an endovascular trial comparing two stents in long lesion, IMPERIAL, will be of interest. It randomized patients with chronic, symptomatic superficial femoral or proximal popliteal artery lesions to treatment with either the Eluvia vascular stent or the Zilver PTX, the first drug-eluting stent approved for use in these patients.
Remember, there also are oral abstract and moderated poster sessions happening throughout the meeting, so there’s something for everyone.
The research area that rose to the top in terms of importance to practice at the upcoming Transcatheter Cardiovascular Therapeutics meeting in San Diego is in mitral valve interventions, but there’s much more potentially practice-changing original reporting as well.
Of the 155 abstracts considered for Late-Breaking Trials, only 10% were selected. That’s in addition to the other 1,650 abstracts submitted for the meeting.
The research chosen for the Late-Breaking Trial and Late-Breaking Research sessions held on Saturday, Sept. 22, through Tuesday, Sept. 25, fall into four categories, Juan F. Granada, MD, president and CEO of the Cardiovascular Research Foundation and codirector of Transcatheter Cardiovascular Therapeutics, said in an interview.
The first and most important category is mitral intervention, he said, and the studies with the most potential to change U.S. practice will be presented on Sunday morning at 10:15-11:15 in the Late-Breaking Trial session. The session kicks off with COAPT, the long-anticipated trial that randomized patients with severe mitral regurgitation secondary to heart failure to the MitraClip or to optimal medical management. The MitraClip, which doesn’t entail a concomitant annuloplasty, currently is approved by the Food and Drug Administration for patients with primary, degenerative mitral regurgitation not amenable to surgical repair only. But, if COAPT yields positive results, the role of the MitraClip may expand greatly.
Also presented in that Sunday session will be results of REDUCE-FMR, a sham-controlled trial in patients with heart failure–associated functional mitral regurgitation who received either treatment with the CARILLON Mitral Contour System, which is an indirect annuloplasty device, or optimal medical treatment.
Secondly, “we’ve got a lot to learn from two trials in the Absorb bioresorbable scaffold,” Dr. Granada said. Although the device failed commercially on the heels of news of target vessel failure and scaffold thrombosis, detailed results of ABSORB IV and COMPARE ABSORB will inform future designs, he said.The third area Dr. Granada emphasized was direct comparisons of stents. Saturday’s Late-Breaking Trials sessions offer results of several head-to-head trials. BIONYX compared the bioresorbable polymer Orsiro stent with the newer durable polymer Resolute Onyx. TALENT, unusual in its all-comers design, compared clinical outcomes at 12 months between the Supraflex ultrathin coronary stent and Xience everolimus eluting system. Whether strut thickness can make a difference in outcomes will be of great interest as the results of these and other trials are presented at the meeting.
Lastly, an endovascular trial comparing two stents in long lesion, IMPERIAL, will be of interest. It randomized patients with chronic, symptomatic superficial femoral or proximal popliteal artery lesions to treatment with either the Eluvia vascular stent or the Zilver PTX, the first drug-eluting stent approved for use in these patients.
Remember, there also are oral abstract and moderated poster sessions happening throughout the meeting, so there’s something for everyone.
FDA review supports Nuplazid’s safety
Pimavanserin (Nuplazid) remains an acceptable treatment for the hallucinations and delusions associated with Parkinson’s disease, according to a statement issued by the Food and Drug Administration after the agency conducted a postmarketing review of deaths and serious adverse events associated with the drug.
“Based on an analysis of all available data, FDA did not identify any new or unexpected safety findings” associated with the drug, according to the Sept. 20 statement.
However, the FDA researchers identified prescribing patterns that might increase the risk of serious adverse events, such as the concomitant use of pimavanserin and other antipsychotic drugs or drugs that can cause QT prolongation. The QT prolongation risk is listed on the drug label, which also includes a Boxed Warning about increased mortality risk in elderly patients.
The FDA statement reminds clinicians to know the risks described in the label and to be aware that no other antipsychotics are currently approved for psychosis in Parkinson’s patients.
The review was prompted by the number of reports of serious adverse events and deaths associated with pimavanserin, based on data obtained from multiple sources including the FDA Adverse Event Reporting System (FAERS), drug utilization data, safety data from the new drug application, the sponsor’s Periodic Adverse Drug Experience Reports, the sponsor’s analysis of fatal adverse event reports, and data from published medical literature.
When conducting the review, the FDA considered several factors, including the fact that Parkinson’s disease patients have higher mortality in general because of older age, advanced disease, and other medical comorbidities. In addition, pimavanserin adverse events and deaths are more likely to be reported because the drug is distributed mainly through a patient-support program and specialty pharmacy. The FDA also found no pattern suggestive of a drug effect on causes of death in patients whose deaths were reported through FAERS.
“Overall, the postmarketing data were consistent with the safety data obtained from the premarketing controlled clinical trials of Nuplazid for Parkinson’s disease psychosis,” according to the FDA statement.
Pimavanserin (Nuplazid) remains an acceptable treatment for the hallucinations and delusions associated with Parkinson’s disease, according to a statement issued by the Food and Drug Administration after the agency conducted a postmarketing review of deaths and serious adverse events associated with the drug.
“Based on an analysis of all available data, FDA did not identify any new or unexpected safety findings” associated with the drug, according to the Sept. 20 statement.
However, the FDA researchers identified prescribing patterns that might increase the risk of serious adverse events, such as the concomitant use of pimavanserin and other antipsychotic drugs or drugs that can cause QT prolongation. The QT prolongation risk is listed on the drug label, which also includes a Boxed Warning about increased mortality risk in elderly patients.
The FDA statement reminds clinicians to know the risks described in the label and to be aware that no other antipsychotics are currently approved for psychosis in Parkinson’s patients.
The review was prompted by the number of reports of serious adverse events and deaths associated with pimavanserin, based on data obtained from multiple sources including the FDA Adverse Event Reporting System (FAERS), drug utilization data, safety data from the new drug application, the sponsor’s Periodic Adverse Drug Experience Reports, the sponsor’s analysis of fatal adverse event reports, and data from published medical literature.
When conducting the review, the FDA considered several factors, including the fact that Parkinson’s disease patients have higher mortality in general because of older age, advanced disease, and other medical comorbidities. In addition, pimavanserin adverse events and deaths are more likely to be reported because the drug is distributed mainly through a patient-support program and specialty pharmacy. The FDA also found no pattern suggestive of a drug effect on causes of death in patients whose deaths were reported through FAERS.
“Overall, the postmarketing data were consistent with the safety data obtained from the premarketing controlled clinical trials of Nuplazid for Parkinson’s disease psychosis,” according to the FDA statement.
Pimavanserin (Nuplazid) remains an acceptable treatment for the hallucinations and delusions associated with Parkinson’s disease, according to a statement issued by the Food and Drug Administration after the agency conducted a postmarketing review of deaths and serious adverse events associated with the drug.
“Based on an analysis of all available data, FDA did not identify any new or unexpected safety findings” associated with the drug, according to the Sept. 20 statement.
However, the FDA researchers identified prescribing patterns that might increase the risk of serious adverse events, such as the concomitant use of pimavanserin and other antipsychotic drugs or drugs that can cause QT prolongation. The QT prolongation risk is listed on the drug label, which also includes a Boxed Warning about increased mortality risk in elderly patients.
The FDA statement reminds clinicians to know the risks described in the label and to be aware that no other antipsychotics are currently approved for psychosis in Parkinson’s patients.
The review was prompted by the number of reports of serious adverse events and deaths associated with pimavanserin, based on data obtained from multiple sources including the FDA Adverse Event Reporting System (FAERS), drug utilization data, safety data from the new drug application, the sponsor’s Periodic Adverse Drug Experience Reports, the sponsor’s analysis of fatal adverse event reports, and data from published medical literature.
When conducting the review, the FDA considered several factors, including the fact that Parkinson’s disease patients have higher mortality in general because of older age, advanced disease, and other medical comorbidities. In addition, pimavanserin adverse events and deaths are more likely to be reported because the drug is distributed mainly through a patient-support program and specialty pharmacy. The FDA also found no pattern suggestive of a drug effect on causes of death in patients whose deaths were reported through FAERS.
“Overall, the postmarketing data were consistent with the safety data obtained from the premarketing controlled clinical trials of Nuplazid for Parkinson’s disease psychosis,” according to the FDA statement.