User login
Transgender health survey provides data on nearly 28,000 individuals
WASHINGTON – Respondents to the 2015 United States Transgender Survey (USTS) reported living with HIV at nearly five times the rate in the U.S. population. Reported HIV rates were even higher among transgender women, especially transgender women of color, according to Sandy James, JD, PhD, the lead author of the USTS and its former research director (2014-2017).
In addition, the survey results detailed high rates of physical and mental health issues, difficulties accessing health care, and negative experiences when receiving medical care.
“There [had been] a dearth of data available about trans people,” said Dr. James, and hard data are required to make any meaningful changes to health care systems, but “now we have numbers.”
The nationwide USTS was the largest survey ever to document the experiences of transgender adults in the United States, comprising 27,715 respondents from all 50 states, the District of Columbia, American Samoa, Guam, Puerto Rico, and U.S. military bases overseas.
The USTS provided a comprehensive examination of a wide range of life outcomes, including those related to health, employment, income, and education. This survey of transgender adults (18 years of age and older) was anonymous, was available in both English and Spanish, and was conducted in the summer of 2015 by the National Center for Transgender Equality.
The document details the stresses and dangers that transgender people face in their daily lives, including attempted suicide rates higher than the norm (40% having attempted suicide in their lifetime, nearly nine times the 4.6% rate in the U.S. population). Nearly 1 in 10 respondents were physically attacked in the past year because of being transgender, and nearly half (47%) of respondents reported having been sexually assaulted during their lifetime.
Respondents reported living with HIV (1.4%) at nearly five times the rate in the U.S. population (0.3%), with HIV rates higher among transgender women (3.4%), especially transgender women of color. Nearly one in five black transgender women were living with HIV, and Native American Indian and Latina women also reported higher rates of infection: 4.6% and 4.4%, respectively.
A total of 25% of respondents experienced a problem in the past year with their insurance related to being transgender, such as being denied coverage for care related to gender transition or being denied coverage for routine care because they were transgender.
In terms of the health care environment, 33% of those who saw a health care provider in the past year reported having at least one negative experience related to being transgender, with higher rates for people of color and people with disabilities. This included being refused treatment, being verbally harassed or physically or sexually assaulted, or having to teach the provider about transgender people to get appropriate care, according to the survey.
In addition, 23% of respondents reported that they did not see a doctor when they needed to in the past year because of fear of being mistreated as a transgender person, and 33% did not see a doctor when needed because they could not afford care.
“I urge you to go and find the survey and look at all of the results, it is really important,” Dr. James stated. He stressed the fact that the breakout reports, including the report on black respondents, the Latino/a response report (in both English and Spanish), and the other minority and individual state reports, can all provide a more detailed view of what is going on in the transgender community than anything previously available.
Dr. James reported having no disclosures.
SOURCE: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S289.
WASHINGTON – Respondents to the 2015 United States Transgender Survey (USTS) reported living with HIV at nearly five times the rate in the U.S. population. Reported HIV rates were even higher among transgender women, especially transgender women of color, according to Sandy James, JD, PhD, the lead author of the USTS and its former research director (2014-2017).
In addition, the survey results detailed high rates of physical and mental health issues, difficulties accessing health care, and negative experiences when receiving medical care.
“There [had been] a dearth of data available about trans people,” said Dr. James, and hard data are required to make any meaningful changes to health care systems, but “now we have numbers.”
The nationwide USTS was the largest survey ever to document the experiences of transgender adults in the United States, comprising 27,715 respondents from all 50 states, the District of Columbia, American Samoa, Guam, Puerto Rico, and U.S. military bases overseas.
The USTS provided a comprehensive examination of a wide range of life outcomes, including those related to health, employment, income, and education. This survey of transgender adults (18 years of age and older) was anonymous, was available in both English and Spanish, and was conducted in the summer of 2015 by the National Center for Transgender Equality.
The document details the stresses and dangers that transgender people face in their daily lives, including attempted suicide rates higher than the norm (40% having attempted suicide in their lifetime, nearly nine times the 4.6% rate in the U.S. population). Nearly 1 in 10 respondents were physically attacked in the past year because of being transgender, and nearly half (47%) of respondents reported having been sexually assaulted during their lifetime.
Respondents reported living with HIV (1.4%) at nearly five times the rate in the U.S. population (0.3%), with HIV rates higher among transgender women (3.4%), especially transgender women of color. Nearly one in five black transgender women were living with HIV, and Native American Indian and Latina women also reported higher rates of infection: 4.6% and 4.4%, respectively.
A total of 25% of respondents experienced a problem in the past year with their insurance related to being transgender, such as being denied coverage for care related to gender transition or being denied coverage for routine care because they were transgender.
In terms of the health care environment, 33% of those who saw a health care provider in the past year reported having at least one negative experience related to being transgender, with higher rates for people of color and people with disabilities. This included being refused treatment, being verbally harassed or physically or sexually assaulted, or having to teach the provider about transgender people to get appropriate care, according to the survey.
In addition, 23% of respondents reported that they did not see a doctor when they needed to in the past year because of fear of being mistreated as a transgender person, and 33% did not see a doctor when needed because they could not afford care.
“I urge you to go and find the survey and look at all of the results, it is really important,” Dr. James stated. He stressed the fact that the breakout reports, including the report on black respondents, the Latino/a response report (in both English and Spanish), and the other minority and individual state reports, can all provide a more detailed view of what is going on in the transgender community than anything previously available.
Dr. James reported having no disclosures.
SOURCE: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S289.
WASHINGTON – Respondents to the 2015 United States Transgender Survey (USTS) reported living with HIV at nearly five times the rate in the U.S. population. Reported HIV rates were even higher among transgender women, especially transgender women of color, according to Sandy James, JD, PhD, the lead author of the USTS and its former research director (2014-2017).
In addition, the survey results detailed high rates of physical and mental health issues, difficulties accessing health care, and negative experiences when receiving medical care.
“There [had been] a dearth of data available about trans people,” said Dr. James, and hard data are required to make any meaningful changes to health care systems, but “now we have numbers.”
The nationwide USTS was the largest survey ever to document the experiences of transgender adults in the United States, comprising 27,715 respondents from all 50 states, the District of Columbia, American Samoa, Guam, Puerto Rico, and U.S. military bases overseas.
The USTS provided a comprehensive examination of a wide range of life outcomes, including those related to health, employment, income, and education. This survey of transgender adults (18 years of age and older) was anonymous, was available in both English and Spanish, and was conducted in the summer of 2015 by the National Center for Transgender Equality.
The document details the stresses and dangers that transgender people face in their daily lives, including attempted suicide rates higher than the norm (40% having attempted suicide in their lifetime, nearly nine times the 4.6% rate in the U.S. population). Nearly 1 in 10 respondents were physically attacked in the past year because of being transgender, and nearly half (47%) of respondents reported having been sexually assaulted during their lifetime.
Respondents reported living with HIV (1.4%) at nearly five times the rate in the U.S. population (0.3%), with HIV rates higher among transgender women (3.4%), especially transgender women of color. Nearly one in five black transgender women were living with HIV, and Native American Indian and Latina women also reported higher rates of infection: 4.6% and 4.4%, respectively.
A total of 25% of respondents experienced a problem in the past year with their insurance related to being transgender, such as being denied coverage for care related to gender transition or being denied coverage for routine care because they were transgender.
In terms of the health care environment, 33% of those who saw a health care provider in the past year reported having at least one negative experience related to being transgender, with higher rates for people of color and people with disabilities. This included being refused treatment, being verbally harassed or physically or sexually assaulted, or having to teach the provider about transgender people to get appropriate care, according to the survey.
In addition, 23% of respondents reported that they did not see a doctor when they needed to in the past year because of fear of being mistreated as a transgender person, and 33% did not see a doctor when needed because they could not afford care.
“I urge you to go and find the survey and look at all of the results, it is really important,” Dr. James stated. He stressed the fact that the breakout reports, including the report on black respondents, the Latino/a response report (in both English and Spanish), and the other minority and individual state reports, can all provide a more detailed view of what is going on in the transgender community than anything previously available.
Dr. James reported having no disclosures.
SOURCE: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S289.
FROM THE STD PREVENTION CONFERENCE 2018
Key clinical point: The 2015 U.S. Transgender Survey provides more data on transgender life and health than ever previously available.
Major finding: Transgender respondents reported living with HIV at nearly five times the rate in the U.S. population.
Study details: Results from an anonymous, online survey of nearly 28,000 transgender individuals in the United States and its territories.
Disclosures: Dr. James reported having no disclosures.
Source: James S. Sexually Transmitted Diseases 2018. 45 [Supplement 2] Session 5D. S28.
New perspectives keep SHM relevant
Atashi Mandal, MD, finds committee work illuminating and gratifying
Editor’s note: SHM occasionally puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Atashi Mandal, MD , a Med-Peds hospitalist in Huntington Beach, Calif. Dr. Mandal has been a member of SHM since for more than a decade, has served on the Public Policy Committee, and is currently serving on the Patient Experience Committee.
How did you initially hear about SHM, and why did you become a member?
I was a newly minted hospitalist and eagerly searching for a way to use my CME allowance, when I discovered SHM’s annual conference, which happened to be nearby in San Diego that year. I also was intrigued by, and excited to learn more about, an organization that dedicated itself only to hospital medicine. After attending the conference, I was hooked!
As a member of more than a decade, what aspects of your membership have you found to be most valuable?
I’ve always been very impressed by the quality and variety of the educational offerings. As a Med-Peds hospitalist, I can happily attest to greater inclusion of pediatric-specific content and a more robust presence of pediatric hospitalists over the years. Moreover, I am very appreciative of SHM’s progressive attitude as demonstrated by incorporating topics such as gender disparities, LGBTQ health, and the opioid crisis into our curriculum. I also have greatly enjoyed the networking opportunities with fellow hospitalists, some of whom I am happy to say have also become good friends over the years. More recently over the past few years, I’ve participated on committees, which has been an illuminating and gratifying way to help shape SHM’s current and future directives.
Describe your role on the Public Policy Committee. What did the committee accomplish during your term?
I was very honored to serve as a member of this committee for three terms. The staff is truly superhuman and amazing, considering how well they stay abreast of the swiftly changing administrative and legislative currents in health care. Just during my tenure as an SHM member, we’ve witnessed paramount shifts in our practice and culture, from the passage of MACRA, [the Medicare Access and CHIP Reauthorization Act] to the opioid epidemic. The Public Policy Committee identifies issues that affect our practice as hospitalists and advocates on our behalf through various means, from submitting comments and letters as well as personally meeting with our regulatory agencies such as CMS [Centers for Medicare & Medicaid Services], and our federal legislators. Some major victories were the acquisition of our specialty billing code and approval of an advanced care billing code. Additionally, the committee has been tirelessly advocating for reform with observation status. We have submitted comments to legislative committees regarding the opioid crisis and continue to work with MACRA as it affects our membership. While I served, I took a special interest in mental health and pediatric issues, including CHIP [Children’s Health Insurance Program] reauthorization and the 21st Century Cures Act.
What is Hill Day, and what can Hospital Medicine 2019 attendees expect to gain from participating?
Hill Day is a truly educational, exciting – and most important – fun opportunity to hone our advocacy skills and gain some real-world experience interacting with legislators and their staffs. On the last day of the annual conference attendees can travel to D.C., where we will spend about a half-day meeting with our respective state’s legislators or their staff. We typically discuss two or three preselected bills that can directly impact our practice as hospitalists. The legislators and their staffers generally are not aware of how certain legislative items can greatly benefit or adversely affect our patients, and they therefore rely on front-line clinicians like us to provide this narrative, much to their gratitude. I learn a lot and have even more fun each time I go to Capitol Hill, so I strongly encourage everyone to participate in this unique opportunity.
Do you have any advice for early-career hospitalists looking to gain experience and get involved with SHM?
I would encourage you to find your voice and participate! Whether by joining a committee or a Special Interest Group or just chatting on one of the many stimulating forums, we each have something to bring to the table, irrespective of our tenure as hospitalists. The new perspectives mingling with those that are well established is what keeps our organization relevant, so I look forward to new ideas and fresh faces!
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Atashi Mandal, MD, finds committee work illuminating and gratifying
Atashi Mandal, MD, finds committee work illuminating and gratifying
Editor’s note: SHM occasionally puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Atashi Mandal, MD , a Med-Peds hospitalist in Huntington Beach, Calif. Dr. Mandal has been a member of SHM since for more than a decade, has served on the Public Policy Committee, and is currently serving on the Patient Experience Committee.
How did you initially hear about SHM, and why did you become a member?
I was a newly minted hospitalist and eagerly searching for a way to use my CME allowance, when I discovered SHM’s annual conference, which happened to be nearby in San Diego that year. I also was intrigued by, and excited to learn more about, an organization that dedicated itself only to hospital medicine. After attending the conference, I was hooked!
As a member of more than a decade, what aspects of your membership have you found to be most valuable?
I’ve always been very impressed by the quality and variety of the educational offerings. As a Med-Peds hospitalist, I can happily attest to greater inclusion of pediatric-specific content and a more robust presence of pediatric hospitalists over the years. Moreover, I am very appreciative of SHM’s progressive attitude as demonstrated by incorporating topics such as gender disparities, LGBTQ health, and the opioid crisis into our curriculum. I also have greatly enjoyed the networking opportunities with fellow hospitalists, some of whom I am happy to say have also become good friends over the years. More recently over the past few years, I’ve participated on committees, which has been an illuminating and gratifying way to help shape SHM’s current and future directives.
Describe your role on the Public Policy Committee. What did the committee accomplish during your term?
I was very honored to serve as a member of this committee for three terms. The staff is truly superhuman and amazing, considering how well they stay abreast of the swiftly changing administrative and legislative currents in health care. Just during my tenure as an SHM member, we’ve witnessed paramount shifts in our practice and culture, from the passage of MACRA, [the Medicare Access and CHIP Reauthorization Act] to the opioid epidemic. The Public Policy Committee identifies issues that affect our practice as hospitalists and advocates on our behalf through various means, from submitting comments and letters as well as personally meeting with our regulatory agencies such as CMS [Centers for Medicare & Medicaid Services], and our federal legislators. Some major victories were the acquisition of our specialty billing code and approval of an advanced care billing code. Additionally, the committee has been tirelessly advocating for reform with observation status. We have submitted comments to legislative committees regarding the opioid crisis and continue to work with MACRA as it affects our membership. While I served, I took a special interest in mental health and pediatric issues, including CHIP [Children’s Health Insurance Program] reauthorization and the 21st Century Cures Act.
What is Hill Day, and what can Hospital Medicine 2019 attendees expect to gain from participating?
Hill Day is a truly educational, exciting – and most important – fun opportunity to hone our advocacy skills and gain some real-world experience interacting with legislators and their staffs. On the last day of the annual conference attendees can travel to D.C., where we will spend about a half-day meeting with our respective state’s legislators or their staff. We typically discuss two or three preselected bills that can directly impact our practice as hospitalists. The legislators and their staffers generally are not aware of how certain legislative items can greatly benefit or adversely affect our patients, and they therefore rely on front-line clinicians like us to provide this narrative, much to their gratitude. I learn a lot and have even more fun each time I go to Capitol Hill, so I strongly encourage everyone to participate in this unique opportunity.
Do you have any advice for early-career hospitalists looking to gain experience and get involved with SHM?
I would encourage you to find your voice and participate! Whether by joining a committee or a Special Interest Group or just chatting on one of the many stimulating forums, we each have something to bring to the table, irrespective of our tenure as hospitalists. The new perspectives mingling with those that are well established is what keeps our organization relevant, so I look forward to new ideas and fresh faces!
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Editor’s note: SHM occasionally puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Visit www.hospitalmedicine.org for more information on how you can lend your expertise to help improve the care of hospitalized patients.
This month, The Hospitalist spotlights Atashi Mandal, MD , a Med-Peds hospitalist in Huntington Beach, Calif. Dr. Mandal has been a member of SHM since for more than a decade, has served on the Public Policy Committee, and is currently serving on the Patient Experience Committee.
How did you initially hear about SHM, and why did you become a member?
I was a newly minted hospitalist and eagerly searching for a way to use my CME allowance, when I discovered SHM’s annual conference, which happened to be nearby in San Diego that year. I also was intrigued by, and excited to learn more about, an organization that dedicated itself only to hospital medicine. After attending the conference, I was hooked!
As a member of more than a decade, what aspects of your membership have you found to be most valuable?
I’ve always been very impressed by the quality and variety of the educational offerings. As a Med-Peds hospitalist, I can happily attest to greater inclusion of pediatric-specific content and a more robust presence of pediatric hospitalists over the years. Moreover, I am very appreciative of SHM’s progressive attitude as demonstrated by incorporating topics such as gender disparities, LGBTQ health, and the opioid crisis into our curriculum. I also have greatly enjoyed the networking opportunities with fellow hospitalists, some of whom I am happy to say have also become good friends over the years. More recently over the past few years, I’ve participated on committees, which has been an illuminating and gratifying way to help shape SHM’s current and future directives.
Describe your role on the Public Policy Committee. What did the committee accomplish during your term?
I was very honored to serve as a member of this committee for three terms. The staff is truly superhuman and amazing, considering how well they stay abreast of the swiftly changing administrative and legislative currents in health care. Just during my tenure as an SHM member, we’ve witnessed paramount shifts in our practice and culture, from the passage of MACRA, [the Medicare Access and CHIP Reauthorization Act] to the opioid epidemic. The Public Policy Committee identifies issues that affect our practice as hospitalists and advocates on our behalf through various means, from submitting comments and letters as well as personally meeting with our regulatory agencies such as CMS [Centers for Medicare & Medicaid Services], and our federal legislators. Some major victories were the acquisition of our specialty billing code and approval of an advanced care billing code. Additionally, the committee has been tirelessly advocating for reform with observation status. We have submitted comments to legislative committees regarding the opioid crisis and continue to work with MACRA as it affects our membership. While I served, I took a special interest in mental health and pediatric issues, including CHIP [Children’s Health Insurance Program] reauthorization and the 21st Century Cures Act.
What is Hill Day, and what can Hospital Medicine 2019 attendees expect to gain from participating?
Hill Day is a truly educational, exciting – and most important – fun opportunity to hone our advocacy skills and gain some real-world experience interacting with legislators and their staffs. On the last day of the annual conference attendees can travel to D.C., where we will spend about a half-day meeting with our respective state’s legislators or their staff. We typically discuss two or three preselected bills that can directly impact our practice as hospitalists. The legislators and their staffers generally are not aware of how certain legislative items can greatly benefit or adversely affect our patients, and they therefore rely on front-line clinicians like us to provide this narrative, much to their gratitude. I learn a lot and have even more fun each time I go to Capitol Hill, so I strongly encourage everyone to participate in this unique opportunity.
Do you have any advice for early-career hospitalists looking to gain experience and get involved with SHM?
I would encourage you to find your voice and participate! Whether by joining a committee or a Special Interest Group or just chatting on one of the many stimulating forums, we each have something to bring to the table, irrespective of our tenure as hospitalists. The new perspectives mingling with those that are well established is what keeps our organization relevant, so I look forward to new ideas and fresh faces!
Ms. Steele is a marketing communications specialist at the Society of Hospital Medicine.
Burden of dementia will shift more to minorities by 2060
, according to a study in Alzheimer’s and Dementia.
Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
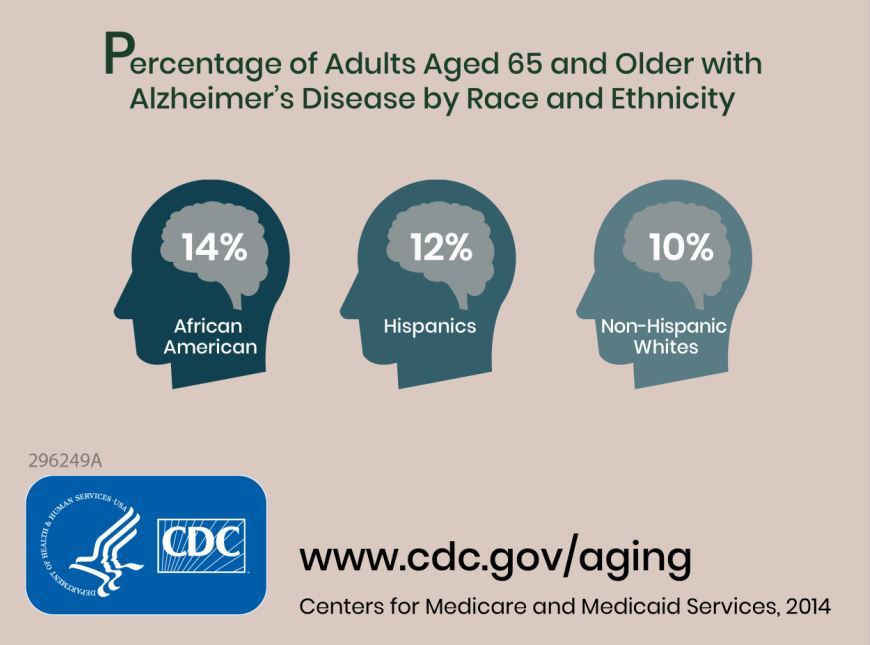
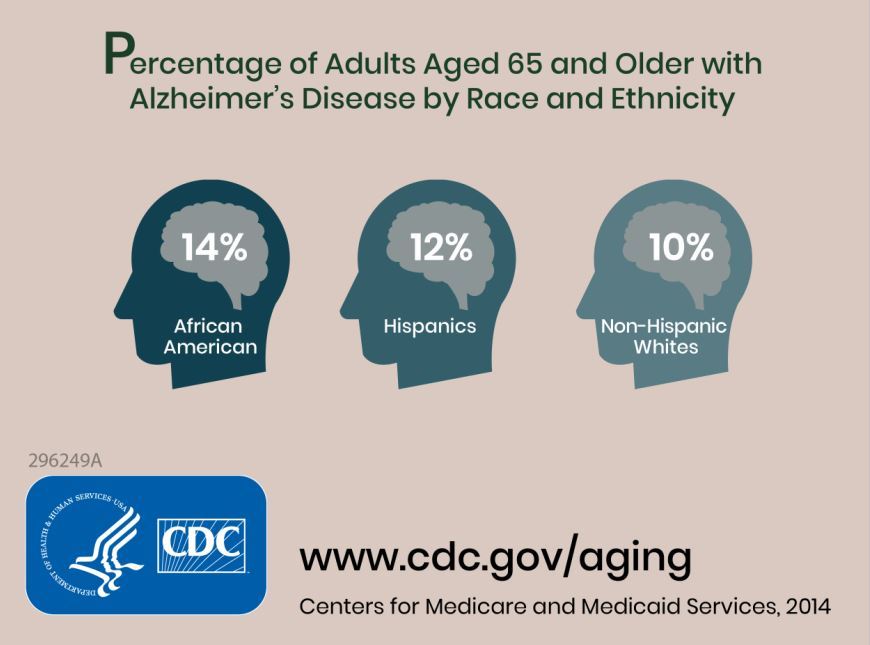
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
, according to a study in Alzheimer’s and Dementia.

Prior to this study, no research had defined future estimates based on projected changes among demographic groups.
Researchers combined information about the prevalence of Alzheimer’s disease and related dementias (ADRD) by demographic group in 2014 Medicare-Fee-for-Service data with population projections data from the U.S. Census Bureau to assess how existing disparities by demographic group will change as those demographic groups become more or less represented in the U.S. population. They estimated the future prevalence of ADRD for 70 subgroups; these groups were defined by sex, seven racial and ethnic groups, and five age groups. The researchers estimated that in 2014 African Americans had the highest prevalence of ADRD at 13.8%, followed by Hispanics at 12.2%, non-Hispanic whites at 10.3%, American Indian and Alaska Natives at 9.1%, and Asian and Pacific Islanders at 8.4%.
The researchers estimated an overall increase from about 5.0 million people (1.9% of the U.S. population) in 2014 to about 13.9 million (3.3% of the population) in 2060. The non-Hispanic whites group will have the largest total number of cases of ADRD in 2060 because of its relative size, compared with other subgroups, going from about 3.7 million in 2014 to 7.1 million. The ADRD prevalence in non-Hispanic whites will begin to plateau around 2030, whereas the Hispanic population is expected to see the greatest increase, going from 430,000 in 2014 to 3.2 million in 2060.
Some of the limitations of the study include the assumption that the Medicare Fee-for-Service population is representative of the U.S. population and that these prevalences will remain constant over time.
“These estimates can be used for public health planning related to providing culturally competent care for the ADRD population and supporting caregivers from diverse backgrounds,” the researchers concluded.
SOURCE: Matthews KA et al. Alzheimers Dement. 2018 Sep 19. doi: 10.1016/j.jalz.2018.06.3063.
This article was updated 10/4/18.
FROM ALZHEIMER’S AND DEMENTIA
Is CMS becoming more open to PTAC recommendations?
Recommendations on potential physician-focused alternative payment models so far have gained little traction with officials at the Centers for Medicare & Medicaid Services, but that could be changing.
At a recent meeting of the Physician-Focused Payment Model Technical Advisory Committee (PTAC), Alex Azar, secretary of the Department of Health & Human Services, and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, attended briefly, according to officials at the American College of Emergency Physicians. ACEP was having its proposal evaluated by PTAC.
Physician organizations have been puzzled as to why federal health officials have yet to approve a physician-developed alternative payment model (APM) under the Quality Payment Program, despite several being recommended by PTAC. In fact, no physician-developed APM has even been sent to the Center for Medicare and Medicaid Innovation for further testing and refinement.
ACEP’s model, the Acute Unscheduled Care Model, “provides incentives to safely discharge Medicare beneficiaries from the ED by facilitating and rewarding postdischarge care coordination,” the organizations notes in the model description submitted to PTAC. “The model ensures that emergency physicians who make the decision to provide safe, efficient outpatient care have the necessary tools to support this transformation and are rewarded for their decision making.”
Going into the meeting, the preliminary evaluation report had the PTAC reviewers agreeing unanimously that the model met 7 of the secretary’s 10 criteria for a physician-focused APM, that the majority agreed on the 8th criterion, and a majority agreed that the model did not meet criteria on the remaining two items.
PTAC reviewers “thought that we met all 10 criteria for models that the secretary put forth for evaluating physician-focused payment models,” Jeffrey Davis, ACEP Director of Regulatory Affairs, said in an interview, adding that the attendance of Mr. Azar and Ms. Verma at the meeting was a positive development.
“I think we were especially inspired by the fact that Secretary Azar, Administrator Verma, and Adam Boehler, the new head of [Center for Medicare and Medicaid Innovation], spoke at the beginning of the PTAC meeting,” he said, adding that while no models have been formally implemented or even designated for testing, they said that ideas are being incorporated into model development going on at the CMS.
Ms. Verma went so far as to tweet that “this group of leading experts volunteer their time to improve our healthcare system, and we greatly value their input.”
“We still think [PTAC] is a great avenue to get our model into the public arena,” Mr. Davis said. “We are optimistic.”
Recommendations on potential physician-focused alternative payment models so far have gained little traction with officials at the Centers for Medicare & Medicaid Services, but that could be changing.
At a recent meeting of the Physician-Focused Payment Model Technical Advisory Committee (PTAC), Alex Azar, secretary of the Department of Health & Human Services, and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, attended briefly, according to officials at the American College of Emergency Physicians. ACEP was having its proposal evaluated by PTAC.
Physician organizations have been puzzled as to why federal health officials have yet to approve a physician-developed alternative payment model (APM) under the Quality Payment Program, despite several being recommended by PTAC. In fact, no physician-developed APM has even been sent to the Center for Medicare and Medicaid Innovation for further testing and refinement.
ACEP’s model, the Acute Unscheduled Care Model, “provides incentives to safely discharge Medicare beneficiaries from the ED by facilitating and rewarding postdischarge care coordination,” the organizations notes in the model description submitted to PTAC. “The model ensures that emergency physicians who make the decision to provide safe, efficient outpatient care have the necessary tools to support this transformation and are rewarded for their decision making.”
Going into the meeting, the preliminary evaluation report had the PTAC reviewers agreeing unanimously that the model met 7 of the secretary’s 10 criteria for a physician-focused APM, that the majority agreed on the 8th criterion, and a majority agreed that the model did not meet criteria on the remaining two items.
PTAC reviewers “thought that we met all 10 criteria for models that the secretary put forth for evaluating physician-focused payment models,” Jeffrey Davis, ACEP Director of Regulatory Affairs, said in an interview, adding that the attendance of Mr. Azar and Ms. Verma at the meeting was a positive development.
“I think we were especially inspired by the fact that Secretary Azar, Administrator Verma, and Adam Boehler, the new head of [Center for Medicare and Medicaid Innovation], spoke at the beginning of the PTAC meeting,” he said, adding that while no models have been formally implemented or even designated for testing, they said that ideas are being incorporated into model development going on at the CMS.
Ms. Verma went so far as to tweet that “this group of leading experts volunteer their time to improve our healthcare system, and we greatly value their input.”
“We still think [PTAC] is a great avenue to get our model into the public arena,” Mr. Davis said. “We are optimistic.”
Recommendations on potential physician-focused alternative payment models so far have gained little traction with officials at the Centers for Medicare & Medicaid Services, but that could be changing.
At a recent meeting of the Physician-Focused Payment Model Technical Advisory Committee (PTAC), Alex Azar, secretary of the Department of Health & Human Services, and Seema Verma, administrator of the Centers for Medicare & Medicaid Services, attended briefly, according to officials at the American College of Emergency Physicians. ACEP was having its proposal evaluated by PTAC.
Physician organizations have been puzzled as to why federal health officials have yet to approve a physician-developed alternative payment model (APM) under the Quality Payment Program, despite several being recommended by PTAC. In fact, no physician-developed APM has even been sent to the Center for Medicare and Medicaid Innovation for further testing and refinement.
ACEP’s model, the Acute Unscheduled Care Model, “provides incentives to safely discharge Medicare beneficiaries from the ED by facilitating and rewarding postdischarge care coordination,” the organizations notes in the model description submitted to PTAC. “The model ensures that emergency physicians who make the decision to provide safe, efficient outpatient care have the necessary tools to support this transformation and are rewarded for their decision making.”
Going into the meeting, the preliminary evaluation report had the PTAC reviewers agreeing unanimously that the model met 7 of the secretary’s 10 criteria for a physician-focused APM, that the majority agreed on the 8th criterion, and a majority agreed that the model did not meet criteria on the remaining two items.
PTAC reviewers “thought that we met all 10 criteria for models that the secretary put forth for evaluating physician-focused payment models,” Jeffrey Davis, ACEP Director of Regulatory Affairs, said in an interview, adding that the attendance of Mr. Azar and Ms. Verma at the meeting was a positive development.
“I think we were especially inspired by the fact that Secretary Azar, Administrator Verma, and Adam Boehler, the new head of [Center for Medicare and Medicaid Innovation], spoke at the beginning of the PTAC meeting,” he said, adding that while no models have been formally implemented or even designated for testing, they said that ideas are being incorporated into model development going on at the CMS.
Ms. Verma went so far as to tweet that “this group of leading experts volunteer their time to improve our healthcare system, and we greatly value their input.”
“We still think [PTAC] is a great avenue to get our model into the public arena,” Mr. Davis said. “We are optimistic.”
Medicare gets input on revamping Part B drug program
The Centers for Medicare & Medicaid Services has an uphill climb if it is hoping to sell the idea of a revamped competitive acquisition program (CAP) for prescription drugs administered in the office under Medicare Part B.
The CMS has been seeking comments on bringing the program back. Given the responses to the proposal for simply revising the program without specific operational details as presented in the American Patients First blueprint for lowering drug prices and costs, the final program design will need to be something special to attract physicians to participate.
Under the original CAP, participating physicians would order drugs from an approved vendor, who would then bill Medicare and collect copayments from the beneficiary. The original program was in effect for 18 months and ended on Dec. 31, 2008, after it had little participation and faced other concerns.
More recently, the Medicare Payment Advisory Committee (MedPAC) recommended a revised version of the program, which they dubbed the Part B Drug Value Program (DVP). Under this proposal, private vendors would acquire drugs at lower prices using various negotiation tools, and physicians would be encouraged to make more value-based use decisions based on opportunities for shared savings under Part B.
The CMS is asking for feedback in its proposed 2019 update to the outpatient prospective payment system on a wide range of questions on how the revamped CAP program should be designed, including program design, which suppliers and drugs to include, how to encourage participation, how to structure outcomes-based arrangements, and whether indication-based pricing should be used.
Any demonstration project to test the provisions of a revised CAP program “must be voluntary, as we cannot predict the outcome and the impacts on patients and practice,” officials from the American College of Rheumatology insisted in a July 13 comment letter. “Making any programs and demonstrations voluntary, rather than compulsory, is centrally important to protecting patients.”
The ACR noted that it has “concerns regarding recreating a [CAP] for Part B drugs that was previously unworkable,” adding that it would “oppose a CAP program if it were unchanged or similar to the previous program, or if it would not ensure adequate protections for patient access to medicines.”
The ACR expressly opposed any CAP program that would include utilization management techniques such as prior authorization, step therapy, or other formulary limitation methods.
The American Medical Association said in July 16 comments on the blueprint that a “hasty resurrection of the original CAP with a few cosmetic changes is bound to fail. A CAP descendant cannot succeed without a significant redesign, consultation with earlier program participants, and adequate public input. Any successful redesign will need to provide physicians with a true choice of whether to participate or not; ensure that patient access to necessary drugs is not harmed; and avoid the temptation to add burdensome new administrative procedures aimed at enhancing CAP vendors’ negotiating power and cost-constraints.”
The AMA raised a number of questions that would need to be addressed to get the program up and running in the areas of patient protections and operational issues.
The American Academy of Dermatology Association offered support for the current Part B payment scheme of average sales price plus 6%, but said simply on CAP that it “should be restructured to provide greater flexibility and appeal to providers and vendors.”
The Alliance of Specialty Physicians offered a suggestions in its comments, including
- Allowing physicians to remain in the current buy-and-bill system.
- Ensuring a minimum of three vendor choices per physician.
- Allowing physicians to easily switch between vendors and/or back to the current buy-and-bill system.
- Prohibiting CAP vendors from engaging in any utilization management techniques or medical review work.
The American Gastroenterological Association, which also supported to the Alliance of Specialty Physicians suggestions, said in separately filed comments that it is “unclear that the marketplace has changed in ways that would support the success of a new CAP program,” though the AGA “does not oppose” the idea of a new, voluntary CAP program.
The AGA noted that consolidation has improved the negotiation and purchasing power of physicians in the Part B space, while practices that remained independent as small and mid-sized groups “mostly shifted their Part B drug and biologic patients to hospital outpatient–based departments and infusion clinics because of the significant upfront costs of administering Part B drugs and biologics.”
The Centers for Medicare & Medicaid Services has an uphill climb if it is hoping to sell the idea of a revamped competitive acquisition program (CAP) for prescription drugs administered in the office under Medicare Part B.
The CMS has been seeking comments on bringing the program back. Given the responses to the proposal for simply revising the program without specific operational details as presented in the American Patients First blueprint for lowering drug prices and costs, the final program design will need to be something special to attract physicians to participate.
Under the original CAP, participating physicians would order drugs from an approved vendor, who would then bill Medicare and collect copayments from the beneficiary. The original program was in effect for 18 months and ended on Dec. 31, 2008, after it had little participation and faced other concerns.
More recently, the Medicare Payment Advisory Committee (MedPAC) recommended a revised version of the program, which they dubbed the Part B Drug Value Program (DVP). Under this proposal, private vendors would acquire drugs at lower prices using various negotiation tools, and physicians would be encouraged to make more value-based use decisions based on opportunities for shared savings under Part B.
The CMS is asking for feedback in its proposed 2019 update to the outpatient prospective payment system on a wide range of questions on how the revamped CAP program should be designed, including program design, which suppliers and drugs to include, how to encourage participation, how to structure outcomes-based arrangements, and whether indication-based pricing should be used.
Any demonstration project to test the provisions of a revised CAP program “must be voluntary, as we cannot predict the outcome and the impacts on patients and practice,” officials from the American College of Rheumatology insisted in a July 13 comment letter. “Making any programs and demonstrations voluntary, rather than compulsory, is centrally important to protecting patients.”
The ACR noted that it has “concerns regarding recreating a [CAP] for Part B drugs that was previously unworkable,” adding that it would “oppose a CAP program if it were unchanged or similar to the previous program, or if it would not ensure adequate protections for patient access to medicines.”
The ACR expressly opposed any CAP program that would include utilization management techniques such as prior authorization, step therapy, or other formulary limitation methods.
The American Medical Association said in July 16 comments on the blueprint that a “hasty resurrection of the original CAP with a few cosmetic changes is bound to fail. A CAP descendant cannot succeed without a significant redesign, consultation with earlier program participants, and adequate public input. Any successful redesign will need to provide physicians with a true choice of whether to participate or not; ensure that patient access to necessary drugs is not harmed; and avoid the temptation to add burdensome new administrative procedures aimed at enhancing CAP vendors’ negotiating power and cost-constraints.”
The AMA raised a number of questions that would need to be addressed to get the program up and running in the areas of patient protections and operational issues.
The American Academy of Dermatology Association offered support for the current Part B payment scheme of average sales price plus 6%, but said simply on CAP that it “should be restructured to provide greater flexibility and appeal to providers and vendors.”
The Alliance of Specialty Physicians offered a suggestions in its comments, including
- Allowing physicians to remain in the current buy-and-bill system.
- Ensuring a minimum of three vendor choices per physician.
- Allowing physicians to easily switch between vendors and/or back to the current buy-and-bill system.
- Prohibiting CAP vendors from engaging in any utilization management techniques or medical review work.
The American Gastroenterological Association, which also supported to the Alliance of Specialty Physicians suggestions, said in separately filed comments that it is “unclear that the marketplace has changed in ways that would support the success of a new CAP program,” though the AGA “does not oppose” the idea of a new, voluntary CAP program.
The AGA noted that consolidation has improved the negotiation and purchasing power of physicians in the Part B space, while practices that remained independent as small and mid-sized groups “mostly shifted their Part B drug and biologic patients to hospital outpatient–based departments and infusion clinics because of the significant upfront costs of administering Part B drugs and biologics.”
The Centers for Medicare & Medicaid Services has an uphill climb if it is hoping to sell the idea of a revamped competitive acquisition program (CAP) for prescription drugs administered in the office under Medicare Part B.
The CMS has been seeking comments on bringing the program back. Given the responses to the proposal for simply revising the program without specific operational details as presented in the American Patients First blueprint for lowering drug prices and costs, the final program design will need to be something special to attract physicians to participate.
Under the original CAP, participating physicians would order drugs from an approved vendor, who would then bill Medicare and collect copayments from the beneficiary. The original program was in effect for 18 months and ended on Dec. 31, 2008, after it had little participation and faced other concerns.
More recently, the Medicare Payment Advisory Committee (MedPAC) recommended a revised version of the program, which they dubbed the Part B Drug Value Program (DVP). Under this proposal, private vendors would acquire drugs at lower prices using various negotiation tools, and physicians would be encouraged to make more value-based use decisions based on opportunities for shared savings under Part B.
The CMS is asking for feedback in its proposed 2019 update to the outpatient prospective payment system on a wide range of questions on how the revamped CAP program should be designed, including program design, which suppliers and drugs to include, how to encourage participation, how to structure outcomes-based arrangements, and whether indication-based pricing should be used.
Any demonstration project to test the provisions of a revised CAP program “must be voluntary, as we cannot predict the outcome and the impacts on patients and practice,” officials from the American College of Rheumatology insisted in a July 13 comment letter. “Making any programs and demonstrations voluntary, rather than compulsory, is centrally important to protecting patients.”
The ACR noted that it has “concerns regarding recreating a [CAP] for Part B drugs that was previously unworkable,” adding that it would “oppose a CAP program if it were unchanged or similar to the previous program, or if it would not ensure adequate protections for patient access to medicines.”
The ACR expressly opposed any CAP program that would include utilization management techniques such as prior authorization, step therapy, or other formulary limitation methods.
The American Medical Association said in July 16 comments on the blueprint that a “hasty resurrection of the original CAP with a few cosmetic changes is bound to fail. A CAP descendant cannot succeed without a significant redesign, consultation with earlier program participants, and adequate public input. Any successful redesign will need to provide physicians with a true choice of whether to participate or not; ensure that patient access to necessary drugs is not harmed; and avoid the temptation to add burdensome new administrative procedures aimed at enhancing CAP vendors’ negotiating power and cost-constraints.”
The AMA raised a number of questions that would need to be addressed to get the program up and running in the areas of patient protections and operational issues.
The American Academy of Dermatology Association offered support for the current Part B payment scheme of average sales price plus 6%, but said simply on CAP that it “should be restructured to provide greater flexibility and appeal to providers and vendors.”
The Alliance of Specialty Physicians offered a suggestions in its comments, including
- Allowing physicians to remain in the current buy-and-bill system.
- Ensuring a minimum of three vendor choices per physician.
- Allowing physicians to easily switch between vendors and/or back to the current buy-and-bill system.
- Prohibiting CAP vendors from engaging in any utilization management techniques or medical review work.
The American Gastroenterological Association, which also supported to the Alliance of Specialty Physicians suggestions, said in separately filed comments that it is “unclear that the marketplace has changed in ways that would support the success of a new CAP program,” though the AGA “does not oppose” the idea of a new, voluntary CAP program.
The AGA noted that consolidation has improved the negotiation and purchasing power of physicians in the Part B space, while practices that remained independent as small and mid-sized groups “mostly shifted their Part B drug and biologic patients to hospital outpatient–based departments and infusion clinics because of the significant upfront costs of administering Part B drugs and biologics.”
PD-1/PD-L1 treatment linked to hyperprogressive disease in lung cancer patients
Hyperprogressive disease is a new pattern of progression that may be more prevalent in patients with non–small-cell lung cancer receiving treatment with PD-1/PD-L1 inhibitors than in those receiving chemotherapy, results of a multicenter retrospective study suggest.
Nearly 14% of patients treated with PD/1/PD-L1 inhibitors had hyperprogressive disease in the study, compared with about 5% of patients treated with chemotherapy, the investigators reported.
This acceleration of tumor growth during therapy was associated with a high metastatic burden before treatment, and with a poor prognosis after treatment, wrote Benjamin Besse, MD, PhD, of the cancer medicine department at Gustave Roussy, Villejuif, France, and his colleagues.
Based on these findings, disease evolution should be carefully monitored to identify “hyperprogressors” among non–small-cell lung cancer (NSCLC) patients receiving PD-1/PD-L1 inhibitors, Dr. Besse and his colleagues said.
“Because of the poor overall survival associated with hyperprogressive disease, an early switch to salvage chemotherapy in these patients should be considered,” they wrote in JAMA Oncology.
This is believed to be the first study to look at incidence of hyperprogressive disease after PD-1/PD-L1 therapy specifically in NSCLC patients, and the first in any tumor type to include a chemotherapy treatment control group, according to Dr. Besse and his coauthors.
In one other recent report, hyperprogressive disease was seen in 9% of advanced cancers, and in a second recent investigation, it was seen in 29% of head and neck cancer patients, they said.
Although those proportions appear different, that could be due to differing interpretations of hyperprogressive disease, as there is no consensus on the optimal definition, they noted.
In this study, which included 406 patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors and 59 treated with chemotherapy, hyperprogressive disease was defined as progressive disease observed at the first computed tomography scan and an absolute increase in tumor growth rate of 50% or more per month.
A total of 56 patients (13.8%) in the PD-1/PD-L1 treatment group met that definition for hyperprogressive disease, the investigators found. By comparison, three patients (5.1%) in the chemotherapy treatment group had hyperprogressive disease.
With a median follow-up of 12.1 months in the PD-1/PD-L1 group, the median overall survival was 13.4 months. Median overall survival for those with progressive disease was 6.2 months, and significantly lower at 3.4 months for those who had experienced hyperprogressive disease (P = .002), Dr. Besse and his coinvestigators found.
Moreover, 42.6% patients with progressive disease had two or more metastatic sites, compared with 62.5% of patients with hyperprogressive disease (P = .006), they reported.
The phenomenon of hyperprogressive disease might explain the initial excess in deaths seen in some phase 3 trials, Dr. Besse said in an interview posted on the JAMA Oncology website.
“In most of the second-line trials that compared single-agent immunotherapy to single-agent chemotherapy, overall survival curves crossed, meaning that there were an extra number of deaths in the immunotherapy arm,” he said in the interview. “That led us to evaluate the speed of progression.”
Based on the study results, Dr. Besse recommends early assessment by CT scan at 6 or 8 weeks after the start of immunotherapy. “If a patient has hyperprogressive disease, you don’t have much time to react,” he said. “You have to switch to a new treatment quite early.”
Dr. Besse reported receiving research funding from AstraZeneca, Boehringer, Bristol-Myers Squibb, Clovis Oncology, GlaxoSmithKline, Eli Lilly, Inivata, Merck Sharp & Dohme, Onxeo, OSE Pharma, Pfizer, Roche/Genentech, and Servier.
SOURCE: Ferrara R et al. JAMA Oncol. 2018 Sep 6. doi: 10.1001/jamaoncol.2018.3676.
Hyperprogressive disease is a new pattern of progression that may be more prevalent in patients with non–small-cell lung cancer receiving treatment with PD-1/PD-L1 inhibitors than in those receiving chemotherapy, results of a multicenter retrospective study suggest.
Nearly 14% of patients treated with PD/1/PD-L1 inhibitors had hyperprogressive disease in the study, compared with about 5% of patients treated with chemotherapy, the investigators reported.
This acceleration of tumor growth during therapy was associated with a high metastatic burden before treatment, and with a poor prognosis after treatment, wrote Benjamin Besse, MD, PhD, of the cancer medicine department at Gustave Roussy, Villejuif, France, and his colleagues.
Based on these findings, disease evolution should be carefully monitored to identify “hyperprogressors” among non–small-cell lung cancer (NSCLC) patients receiving PD-1/PD-L1 inhibitors, Dr. Besse and his colleagues said.
“Because of the poor overall survival associated with hyperprogressive disease, an early switch to salvage chemotherapy in these patients should be considered,” they wrote in JAMA Oncology.
This is believed to be the first study to look at incidence of hyperprogressive disease after PD-1/PD-L1 therapy specifically in NSCLC patients, and the first in any tumor type to include a chemotherapy treatment control group, according to Dr. Besse and his coauthors.
In one other recent report, hyperprogressive disease was seen in 9% of advanced cancers, and in a second recent investigation, it was seen in 29% of head and neck cancer patients, they said.
Although those proportions appear different, that could be due to differing interpretations of hyperprogressive disease, as there is no consensus on the optimal definition, they noted.
In this study, which included 406 patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors and 59 treated with chemotherapy, hyperprogressive disease was defined as progressive disease observed at the first computed tomography scan and an absolute increase in tumor growth rate of 50% or more per month.
A total of 56 patients (13.8%) in the PD-1/PD-L1 treatment group met that definition for hyperprogressive disease, the investigators found. By comparison, three patients (5.1%) in the chemotherapy treatment group had hyperprogressive disease.
With a median follow-up of 12.1 months in the PD-1/PD-L1 group, the median overall survival was 13.4 months. Median overall survival for those with progressive disease was 6.2 months, and significantly lower at 3.4 months for those who had experienced hyperprogressive disease (P = .002), Dr. Besse and his coinvestigators found.
Moreover, 42.6% patients with progressive disease had two or more metastatic sites, compared with 62.5% of patients with hyperprogressive disease (P = .006), they reported.
The phenomenon of hyperprogressive disease might explain the initial excess in deaths seen in some phase 3 trials, Dr. Besse said in an interview posted on the JAMA Oncology website.
“In most of the second-line trials that compared single-agent immunotherapy to single-agent chemotherapy, overall survival curves crossed, meaning that there were an extra number of deaths in the immunotherapy arm,” he said in the interview. “That led us to evaluate the speed of progression.”
Based on the study results, Dr. Besse recommends early assessment by CT scan at 6 or 8 weeks after the start of immunotherapy. “If a patient has hyperprogressive disease, you don’t have much time to react,” he said. “You have to switch to a new treatment quite early.”
Dr. Besse reported receiving research funding from AstraZeneca, Boehringer, Bristol-Myers Squibb, Clovis Oncology, GlaxoSmithKline, Eli Lilly, Inivata, Merck Sharp & Dohme, Onxeo, OSE Pharma, Pfizer, Roche/Genentech, and Servier.
SOURCE: Ferrara R et al. JAMA Oncol. 2018 Sep 6. doi: 10.1001/jamaoncol.2018.3676.
Hyperprogressive disease is a new pattern of progression that may be more prevalent in patients with non–small-cell lung cancer receiving treatment with PD-1/PD-L1 inhibitors than in those receiving chemotherapy, results of a multicenter retrospective study suggest.
Nearly 14% of patients treated with PD/1/PD-L1 inhibitors had hyperprogressive disease in the study, compared with about 5% of patients treated with chemotherapy, the investigators reported.
This acceleration of tumor growth during therapy was associated with a high metastatic burden before treatment, and with a poor prognosis after treatment, wrote Benjamin Besse, MD, PhD, of the cancer medicine department at Gustave Roussy, Villejuif, France, and his colleagues.
Based on these findings, disease evolution should be carefully monitored to identify “hyperprogressors” among non–small-cell lung cancer (NSCLC) patients receiving PD-1/PD-L1 inhibitors, Dr. Besse and his colleagues said.
“Because of the poor overall survival associated with hyperprogressive disease, an early switch to salvage chemotherapy in these patients should be considered,” they wrote in JAMA Oncology.
This is believed to be the first study to look at incidence of hyperprogressive disease after PD-1/PD-L1 therapy specifically in NSCLC patients, and the first in any tumor type to include a chemotherapy treatment control group, according to Dr. Besse and his coauthors.
In one other recent report, hyperprogressive disease was seen in 9% of advanced cancers, and in a second recent investigation, it was seen in 29% of head and neck cancer patients, they said.
Although those proportions appear different, that could be due to differing interpretations of hyperprogressive disease, as there is no consensus on the optimal definition, they noted.
In this study, which included 406 patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors and 59 treated with chemotherapy, hyperprogressive disease was defined as progressive disease observed at the first computed tomography scan and an absolute increase in tumor growth rate of 50% or more per month.
A total of 56 patients (13.8%) in the PD-1/PD-L1 treatment group met that definition for hyperprogressive disease, the investigators found. By comparison, three patients (5.1%) in the chemotherapy treatment group had hyperprogressive disease.
With a median follow-up of 12.1 months in the PD-1/PD-L1 group, the median overall survival was 13.4 months. Median overall survival for those with progressive disease was 6.2 months, and significantly lower at 3.4 months for those who had experienced hyperprogressive disease (P = .002), Dr. Besse and his coinvestigators found.
Moreover, 42.6% patients with progressive disease had two or more metastatic sites, compared with 62.5% of patients with hyperprogressive disease (P = .006), they reported.
The phenomenon of hyperprogressive disease might explain the initial excess in deaths seen in some phase 3 trials, Dr. Besse said in an interview posted on the JAMA Oncology website.
“In most of the second-line trials that compared single-agent immunotherapy to single-agent chemotherapy, overall survival curves crossed, meaning that there were an extra number of deaths in the immunotherapy arm,” he said in the interview. “That led us to evaluate the speed of progression.”
Based on the study results, Dr. Besse recommends early assessment by CT scan at 6 or 8 weeks after the start of immunotherapy. “If a patient has hyperprogressive disease, you don’t have much time to react,” he said. “You have to switch to a new treatment quite early.”
Dr. Besse reported receiving research funding from AstraZeneca, Boehringer, Bristol-Myers Squibb, Clovis Oncology, GlaxoSmithKline, Eli Lilly, Inivata, Merck Sharp & Dohme, Onxeo, OSE Pharma, Pfizer, Roche/Genentech, and Servier.
SOURCE: Ferrara R et al. JAMA Oncol. 2018 Sep 6. doi: 10.1001/jamaoncol.2018.3676.
FROM JAMA ONCOLOGY
Key clinical point: Hyperprogressive disease is a new pattern of progression that appears to be associated with PD-1/PD-L1 treatment of various cancers.
Major finding: Nearly 14% of non–small-cell lung cancer patients had hyperprogression in this study, compared with about 5% of patients receiving chemotherapy.
Study details: A retrospective, multicenter study comprising 406 patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors and 59 treated with chemotherapy.
Disclosures: The authors reported disclosures related to AstraZeneca, Boehringer, Bristol-Myers Squibb, Clovis Oncology, GlaxoSmithKline, Eli Lilly, Inivata, Merck Sharp & Dohme, Onxeo, OSE Pharma, Pfizer, Roche/Genentech, and Servier, among others.
Source: Ferrara R et al. JAMA Oncol. 2018 Sep 6. doi: 10.1001/jamaoncol.2018.3676.
Patient outcome questionnaires take a beating in talk
LAS VEGAS – The purpose of patient-reported outcome data is to help physicians understand how patients are faring so they can make better clinical decisions. But commonly used assessments in rheumatoid arthritis aren’t doing their job, rheumatologist Clifton O. Bingham III, MD, said at the annual Perspectives in Rheumatic Diseases.
“We’re really missing the boat in terms of all these things that are important to our patients with rheumatoid arthritis,” said Dr. Bingham of Johns Hopkins Arthritis Center, Baltimore. For example, multiple instruments used in RA ignore important topics such as pain assessment, fatigue, and physical function, he explained at the conference, held by Global Academy for Medical Education.
There’s good news, however. “Can we come up with something that can overcome these barriers?” he asked. “Yes we can.”
First, Dr. Bingham described the weaknesses of patient questionnaires. “Just because an instrument has been used before, like the SF-36, doesn’t necessarily mean that it is measuring what it’s supposed to be measuring or that the information it gathers is important,” he said.
Even instruments that ask about issues important to RA patients can fail to produce a full picture. As Dr. Bingham noted, the Multidimensional Health Assessment Questionnaire (MD-HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) instruments do ask about joint stiffness, but they’re only interested in whether patients felt stiff in the morning and how long the stiffness lasted.
In fact, Dr. Bingham said, a 2013 study found that 23% of 288 patients with RA reported having stiffness only in the morning; most had it all day and night or in the morning and evening (Young KO, et al. ACR 2013, Abstract 2273).
Dr. Bingham listed other problems with patient-reported outcome instruments, such as a question on the MD-HAQ about “fatigue or tiredness.” He said patients actually distinguish between the two: “Patients say they’re tired when they don’t get a good night’s sleep, but that’s not the same as fatigue from RA.”
Is there a better way? Dr. Bingham has explored this issue while serving on the executive committee of Outcome Measures in Rheumatology, an international group supported by drugmakers and other entities. (The funding is arms-length, and he is not compensated.)
Dr. Bingham supports the use of the recently developed Patient-Reported Outcome Measurement Information System (PROMIS), which was established by the National Institutes of Health.
“It lets us see how our patients are doing in their real lives and gives us information about their disease in terms of their activity,” Dr. Bingham said.
He pointed to the upcoming results of a survey of patients with RA that found a wide majority of patients believed many questions on the PROMIS-29 and PROMIS short form questionnaires – regarding pain interference, fatigue and physical function – to be sufficient. However, they did want to see more questions about physical function.
A survey of doctors also revealed that PROMIS results often led them to identify new symptoms or comorbidities, and they frequently took action as a result. In some cases, the results spurred adjustments to RA treatment or medication.
“These measures have the right stuff,” Dr. Bingham said.
He suggested exploring the use of PROMIS-29 in conjunction with other assessments as needed.
“You can administer the questionnaires on tablet computers,” said Dr. Bingham, who added that styluses with built-up handles can be useful for patients with RA when they respond to the questions.
“The information comes straight into your medical record,” he said, “and it can be shown to you and your patient at the same time.”
For more about PROMIS, visit healthmeasures.net.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – The purpose of patient-reported outcome data is to help physicians understand how patients are faring so they can make better clinical decisions. But commonly used assessments in rheumatoid arthritis aren’t doing their job, rheumatologist Clifton O. Bingham III, MD, said at the annual Perspectives in Rheumatic Diseases.
“We’re really missing the boat in terms of all these things that are important to our patients with rheumatoid arthritis,” said Dr. Bingham of Johns Hopkins Arthritis Center, Baltimore. For example, multiple instruments used in RA ignore important topics such as pain assessment, fatigue, and physical function, he explained at the conference, held by Global Academy for Medical Education.
There’s good news, however. “Can we come up with something that can overcome these barriers?” he asked. “Yes we can.”
First, Dr. Bingham described the weaknesses of patient questionnaires. “Just because an instrument has been used before, like the SF-36, doesn’t necessarily mean that it is measuring what it’s supposed to be measuring or that the information it gathers is important,” he said.
Even instruments that ask about issues important to RA patients can fail to produce a full picture. As Dr. Bingham noted, the Multidimensional Health Assessment Questionnaire (MD-HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) instruments do ask about joint stiffness, but they’re only interested in whether patients felt stiff in the morning and how long the stiffness lasted.
In fact, Dr. Bingham said, a 2013 study found that 23% of 288 patients with RA reported having stiffness only in the morning; most had it all day and night or in the morning and evening (Young KO, et al. ACR 2013, Abstract 2273).
Dr. Bingham listed other problems with patient-reported outcome instruments, such as a question on the MD-HAQ about “fatigue or tiredness.” He said patients actually distinguish between the two: “Patients say they’re tired when they don’t get a good night’s sleep, but that’s not the same as fatigue from RA.”
Is there a better way? Dr. Bingham has explored this issue while serving on the executive committee of Outcome Measures in Rheumatology, an international group supported by drugmakers and other entities. (The funding is arms-length, and he is not compensated.)
Dr. Bingham supports the use of the recently developed Patient-Reported Outcome Measurement Information System (PROMIS), which was established by the National Institutes of Health.
“It lets us see how our patients are doing in their real lives and gives us information about their disease in terms of their activity,” Dr. Bingham said.
He pointed to the upcoming results of a survey of patients with RA that found a wide majority of patients believed many questions on the PROMIS-29 and PROMIS short form questionnaires – regarding pain interference, fatigue and physical function – to be sufficient. However, they did want to see more questions about physical function.
A survey of doctors also revealed that PROMIS results often led them to identify new symptoms or comorbidities, and they frequently took action as a result. In some cases, the results spurred adjustments to RA treatment or medication.
“These measures have the right stuff,” Dr. Bingham said.
He suggested exploring the use of PROMIS-29 in conjunction with other assessments as needed.
“You can administer the questionnaires on tablet computers,” said Dr. Bingham, who added that styluses with built-up handles can be useful for patients with RA when they respond to the questions.
“The information comes straight into your medical record,” he said, “and it can be shown to you and your patient at the same time.”
For more about PROMIS, visit healthmeasures.net.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – The purpose of patient-reported outcome data is to help physicians understand how patients are faring so they can make better clinical decisions. But commonly used assessments in rheumatoid arthritis aren’t doing their job, rheumatologist Clifton O. Bingham III, MD, said at the annual Perspectives in Rheumatic Diseases.
“We’re really missing the boat in terms of all these things that are important to our patients with rheumatoid arthritis,” said Dr. Bingham of Johns Hopkins Arthritis Center, Baltimore. For example, multiple instruments used in RA ignore important topics such as pain assessment, fatigue, and physical function, he explained at the conference, held by Global Academy for Medical Education.
There’s good news, however. “Can we come up with something that can overcome these barriers?” he asked. “Yes we can.”
First, Dr. Bingham described the weaknesses of patient questionnaires. “Just because an instrument has been used before, like the SF-36, doesn’t necessarily mean that it is measuring what it’s supposed to be measuring or that the information it gathers is important,” he said.
Even instruments that ask about issues important to RA patients can fail to produce a full picture. As Dr. Bingham noted, the Multidimensional Health Assessment Questionnaire (MD-HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) instruments do ask about joint stiffness, but they’re only interested in whether patients felt stiff in the morning and how long the stiffness lasted.
In fact, Dr. Bingham said, a 2013 study found that 23% of 288 patients with RA reported having stiffness only in the morning; most had it all day and night or in the morning and evening (Young KO, et al. ACR 2013, Abstract 2273).
Dr. Bingham listed other problems with patient-reported outcome instruments, such as a question on the MD-HAQ about “fatigue or tiredness.” He said patients actually distinguish between the two: “Patients say they’re tired when they don’t get a good night’s sleep, but that’s not the same as fatigue from RA.”
Is there a better way? Dr. Bingham has explored this issue while serving on the executive committee of Outcome Measures in Rheumatology, an international group supported by drugmakers and other entities. (The funding is arms-length, and he is not compensated.)
Dr. Bingham supports the use of the recently developed Patient-Reported Outcome Measurement Information System (PROMIS), which was established by the National Institutes of Health.
“It lets us see how our patients are doing in their real lives and gives us information about their disease in terms of their activity,” Dr. Bingham said.
He pointed to the upcoming results of a survey of patients with RA that found a wide majority of patients believed many questions on the PROMIS-29 and PROMIS short form questionnaires – regarding pain interference, fatigue and physical function – to be sufficient. However, they did want to see more questions about physical function.
A survey of doctors also revealed that PROMIS results often led them to identify new symptoms or comorbidities, and they frequently took action as a result. In some cases, the results spurred adjustments to RA treatment or medication.
“These measures have the right stuff,” Dr. Bingham said.
He suggested exploring the use of PROMIS-29 in conjunction with other assessments as needed.
“You can administer the questionnaires on tablet computers,” said Dr. Bingham, who added that styluses with built-up handles can be useful for patients with RA when they respond to the questions.
“The information comes straight into your medical record,” he said, “and it can be shown to you and your patient at the same time.”
For more about PROMIS, visit healthmeasures.net.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES ON RHEUMATIC DISEASES
Stress linked to disease markers in CLL
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
New research has linked stress levels to markers of progressive disease in patients with chronic lymphocytic leukemia (CLL).
Researchers found that CLL patients who reported more stress also had higher absolute lymphocyte counts and elevated levels of three other markers of more advanced disease—tumor necrosis factor-α (TNFα), interleukin 16 (IL-16), and chemokine ligand 3 (CCL3).
“All four variables we measured are related to prognosis in CLL patients, so they have a lot of relevance,” said study author Barbara L. Andersen, PhD, of The Ohio State University in Columbus.
She and her colleagues described this research in Cancer.
The study involved 96 patients with relapsed/refractory CLL who were entering a phase 2 trial of ibrutinib (NCT01589302). Data collection for this study was done before patients received their first dose of ibrutinib.
All patients completed a survey that measured CLL-related stress. They were asked questions like how often they had intrusive thoughts about their disease, how often they tried to avoid thinking about it, and how often they felt jumpy and easily startled.
The researchers used blood samples to determine patients’ absolute lymphocyte counts and to measure levels of eight cytokines known to promote unhealthy levels of inflammation—IL-6, IL-10, IL-16, TNFα, a proliferation‐inducing ligand (APRIL), B‐cell activating factor (BAFF), vascular endothelial growth factor (VEGF), and CCL3.
In an analysis controlling for demographic characteristics, comorbidities, the presence of 17p deletion, and correlates of inflammation, higher stress was significantly associated with higher:
- Absolute lymphocyte counts (P<0.05)
- Levels of TNFα (P<0.05)
- Levels of IL‐16 (P<0.01)
- Levels of CCL3 (P<0.05).
“The fact that stress shows an effect on CLL even after we controlled for other factors suggests it may be relevant to the course of CLL,” Dr. Andersen said.
She added that the researchers are still following these patients and will examine the relationship between stress and disease markers throughout treatment.
This study was supported by the National Cancer Institute, Pharmacylics (the company developing ibrutinib), and a Pelotonia Idea Award from The Ohio State University Comprehensive Cancer Center.
NICE rejects DLBCL indication for CAR T-cell therapy
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance saying it cannot recommend tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission (EC) to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also EC-approved to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Earlier this month, the National Health Service (NHS) of England announced that tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, NICE’s new draft guidance, issued September 19, says tisagenlecleucel cannot be made available for adults with relapsed/refractory DLBCL who have received two or more lines of systemic therapy.
NICE noted that there is no standard treatment for this patient group, and salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial1 suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy.
In addition, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
Furthermore, NICE said all cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is £282,000. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
All of the aforementioned issues aside, NICE said it does recognize that tisagenlecleucel has significant clinical benefits, and the agency welcomes further discussions on the CAR T-cell therapy’s cost-effectiveness.
NICE will consider comments on its draft guidance for tisagenlecleucel, together with any new evidence, at its next meeting on October 23, 2018.
Last month, NICE expressed similar sentiments about another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta).
Axicabtagene ciloleucel is EC-approved to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. Additionally, the cost of axicabtagene ciloleucel is too high for it to be considered a cost-effective use of NHS resources, and the therapy does not meet criteria for inclusion in the Cancer Drugs Fund.
1. Borchmann P et al. AN UPDATED ANALYSIS OF JULIET, A GLOBAL PIVOTAL PHASE 2 TRIAL OF TISAGENLECLEUCEL IN ADULT PATIENTS WITH RELAPSED OR REFRACTORY (R/R) DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL). EHA 2018. Abstract S799.
OBI-3424 receives orphan designation for ALL
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to OBI-3424 for the treatment of acute lymphoblastic leukemia (ALL).
OBI-3424 is a small-molecule prodrug that targets cancers overexpressing aldo-keto reductase 1C3 (AKR1C3) and selectively releases a DNA alkylating agent in the presence of the AKR1C3 enzyme.
AKR1C3 overexpression has been observed in ALL, particularly T-cell ALL.
OBI-3424 demonstrated activity against T-ALL in preclinical research presented as a poster at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics in October 2017.
Researchers reported that OBI-3424 “exerted profound in vivo efficacy” against T-ALL xenografts derived mainly from patients with aggressive and fatal T-ALL.
The researchers said OBI-3424 significantly reduced leukemia bone marrow infiltration in 4 of 6 evaluable T-ALL xenografts, and OBI-3424 was considered well tolerated.
The poster presentation describing this research is available for download from the website of OBI Pharma, the company developing OBI-3424 in cooperation with Ascenta Pharma.
OBI-3424 also has orphan drug designation from the FDA as a treatment for hepatocellular carcinoma.
Enrollment has begun in a phase 1/2 trial (NCT03592264) of OBI-3424 in patients with hepatocellular carcinoma and castrate-resistant prostate cancer.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.