User login
Stubborn derm condition? Don’t just rely on emails and data
MONTEREY, CALIF. – Looking for a better treatment for a stubborn dermatologic condition? Pick up the telephone, work closely with nondermatologists, and find a dermatology specialty pharmacy. Seek out clinical trials that fit your patient’s needs. And be aware that data may not provide the best dosage protocols.
These tips and more came from Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, who spoke about best practices in drug therapy at the annual Coastal Dermatology Symposium. Dr. Bhatia urged colleagues not to give up on conditions such as vitiligo, alopecia areata, severe atopic dermatitis (AD), granulomatous disorders, recalcitrant urticaria, and itching, even when there is resistance from insurance companies. Instead, he said, rely on persistence and the power of a united front with other specialists.
Rheumatologists, oncologists, and allergists may be helpful allies in certain cases, he said, as can dermatologic specialty pharmacies. And if you’re dealing with a medical director of an insurance company, he said, make sure to call. Don’t write a letter or send a fax.
Because some treatments never get evaluated in clinical trials because of cost or lack of interest, he also recommended that dermatologists keep an eye on anecdotal protocols, which can provide helpful “real-world options,” he said in an interview. “Searching for conclusions from case reports and small independent studies can be just as informative and beneficial to patient care as pivotal data from large late-phase studies. Practical information, pearls on management, and other important tips can be found in anecdotes that were either too small or short in duration to be conducted as a validated trial.”
One option is to check ClinicalTrials.gov for a trial; another is to pursue an investigator-initiated study. “Some companies will offer the option to fund a small study for one or several patients to get them treatment and drugs without a high expense for the study or funding for the site’s costs,” Dr. Bhatia said.
In his presentation, Dr. Bhatia referred to a variety of dermatologic medications are showing promising results in trials, and some relatively new options.
Topical hypochlorous acid (Sebuderm gel) for dermatoses, such as seborrheic dermatitis. This prescription nonsteroidal gel is now available in the United States and was cleared by the Food and Drug Administration for seborrhea and seborrheic dermatitis in 2015. Dr. Bhatia referred to a study presented in a poster at a meeting in 2017 that showed improvement in 20 of 24 patients with mild to moderate seborrheic dermatitis treated with this product after 28 days. None of the patients worsened, and overall disease activity fell by more than half.
Loyon lotion (Cetiol oil and dimethicone), a nonmedicated descaling treatment for scaly patches in infants with cradle cap (seborrheic dermatitis). A 2014 pilot study found that it improved scaling in 80% of 20 infants and children aged 3-36 months over 8 days, and it reached “treatment success” in 50% (Dermatol Ther [Heidelb]. 2014 Dec;4[2]:221-32).
(As for cost, GoodRx.com states that various pharmacies sell one 8-ounce [227-gram] bottle of Sebuderm gel for about $300 with a coupon. Loyon is also expensive, with a GoodRx.com listing its price at about $300, with coupon, for one 50-ml spray bottle.)
Dr. Bhatia also reviewed drugs in the research pipeline. Several drugs for AD are in early phases of research, while some phase 3 trials involving Janus kinase (JAK) inhibitors for AD are starting – or are close to starting.
He pointed to other research that’s underway on treatments for acne and rosacea. Three acne drugs that he said are “almost here” are minocycline gel and cortexolone 17 alpha-propionate 1% cream. He also referred to sarecycline, a tetracycline-derived antibiotic taken orally, which was approved by the Food and Drug Administration on October 2 for moderate to severe acne vulgaris in patients aged 9 years and older.
This symposium was jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education. This publication and the Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Bhatia reported affiliations with multiple drugmakers: Abbvie, Aclaris, Almirall, Bayer, Biofrontera, BioPharmX, Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, IntraDerm, ISDIN, La Roche-Posay, Leo, Mayne, Menlo, Novartis, Ortho, Pfizer, Pierre Fabre, Promius, Regeneron, Sanofi, Skinfix, Soligenix, Sun, and Vidac.
MONTEREY, CALIF. – Looking for a better treatment for a stubborn dermatologic condition? Pick up the telephone, work closely with nondermatologists, and find a dermatology specialty pharmacy. Seek out clinical trials that fit your patient’s needs. And be aware that data may not provide the best dosage protocols.
These tips and more came from Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, who spoke about best practices in drug therapy at the annual Coastal Dermatology Symposium. Dr. Bhatia urged colleagues not to give up on conditions such as vitiligo, alopecia areata, severe atopic dermatitis (AD), granulomatous disorders, recalcitrant urticaria, and itching, even when there is resistance from insurance companies. Instead, he said, rely on persistence and the power of a united front with other specialists.
Rheumatologists, oncologists, and allergists may be helpful allies in certain cases, he said, as can dermatologic specialty pharmacies. And if you’re dealing with a medical director of an insurance company, he said, make sure to call. Don’t write a letter or send a fax.
Because some treatments never get evaluated in clinical trials because of cost or lack of interest, he also recommended that dermatologists keep an eye on anecdotal protocols, which can provide helpful “real-world options,” he said in an interview. “Searching for conclusions from case reports and small independent studies can be just as informative and beneficial to patient care as pivotal data from large late-phase studies. Practical information, pearls on management, and other important tips can be found in anecdotes that were either too small or short in duration to be conducted as a validated trial.”
One option is to check ClinicalTrials.gov for a trial; another is to pursue an investigator-initiated study. “Some companies will offer the option to fund a small study for one or several patients to get them treatment and drugs without a high expense for the study or funding for the site’s costs,” Dr. Bhatia said.
In his presentation, Dr. Bhatia referred to a variety of dermatologic medications are showing promising results in trials, and some relatively new options.
Topical hypochlorous acid (Sebuderm gel) for dermatoses, such as seborrheic dermatitis. This prescription nonsteroidal gel is now available in the United States and was cleared by the Food and Drug Administration for seborrhea and seborrheic dermatitis in 2015. Dr. Bhatia referred to a study presented in a poster at a meeting in 2017 that showed improvement in 20 of 24 patients with mild to moderate seborrheic dermatitis treated with this product after 28 days. None of the patients worsened, and overall disease activity fell by more than half.
Loyon lotion (Cetiol oil and dimethicone), a nonmedicated descaling treatment for scaly patches in infants with cradle cap (seborrheic dermatitis). A 2014 pilot study found that it improved scaling in 80% of 20 infants and children aged 3-36 months over 8 days, and it reached “treatment success” in 50% (Dermatol Ther [Heidelb]. 2014 Dec;4[2]:221-32).
(As for cost, GoodRx.com states that various pharmacies sell one 8-ounce [227-gram] bottle of Sebuderm gel for about $300 with a coupon. Loyon is also expensive, with a GoodRx.com listing its price at about $300, with coupon, for one 50-ml spray bottle.)
Dr. Bhatia also reviewed drugs in the research pipeline. Several drugs for AD are in early phases of research, while some phase 3 trials involving Janus kinase (JAK) inhibitors for AD are starting – or are close to starting.
He pointed to other research that’s underway on treatments for acne and rosacea. Three acne drugs that he said are “almost here” are minocycline gel and cortexolone 17 alpha-propionate 1% cream. He also referred to sarecycline, a tetracycline-derived antibiotic taken orally, which was approved by the Food and Drug Administration on October 2 for moderate to severe acne vulgaris in patients aged 9 years and older.
This symposium was jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education. This publication and the Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Bhatia reported affiliations with multiple drugmakers: Abbvie, Aclaris, Almirall, Bayer, Biofrontera, BioPharmX, Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, IntraDerm, ISDIN, La Roche-Posay, Leo, Mayne, Menlo, Novartis, Ortho, Pfizer, Pierre Fabre, Promius, Regeneron, Sanofi, Skinfix, Soligenix, Sun, and Vidac.
MONTEREY, CALIF. – Looking for a better treatment for a stubborn dermatologic condition? Pick up the telephone, work closely with nondermatologists, and find a dermatology specialty pharmacy. Seek out clinical trials that fit your patient’s needs. And be aware that data may not provide the best dosage protocols.
These tips and more came from Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, who spoke about best practices in drug therapy at the annual Coastal Dermatology Symposium. Dr. Bhatia urged colleagues not to give up on conditions such as vitiligo, alopecia areata, severe atopic dermatitis (AD), granulomatous disorders, recalcitrant urticaria, and itching, even when there is resistance from insurance companies. Instead, he said, rely on persistence and the power of a united front with other specialists.
Rheumatologists, oncologists, and allergists may be helpful allies in certain cases, he said, as can dermatologic specialty pharmacies. And if you’re dealing with a medical director of an insurance company, he said, make sure to call. Don’t write a letter or send a fax.
Because some treatments never get evaluated in clinical trials because of cost or lack of interest, he also recommended that dermatologists keep an eye on anecdotal protocols, which can provide helpful “real-world options,” he said in an interview. “Searching for conclusions from case reports and small independent studies can be just as informative and beneficial to patient care as pivotal data from large late-phase studies. Practical information, pearls on management, and other important tips can be found in anecdotes that were either too small or short in duration to be conducted as a validated trial.”
One option is to check ClinicalTrials.gov for a trial; another is to pursue an investigator-initiated study. “Some companies will offer the option to fund a small study for one or several patients to get them treatment and drugs without a high expense for the study or funding for the site’s costs,” Dr. Bhatia said.
In his presentation, Dr. Bhatia referred to a variety of dermatologic medications are showing promising results in trials, and some relatively new options.
Topical hypochlorous acid (Sebuderm gel) for dermatoses, such as seborrheic dermatitis. This prescription nonsteroidal gel is now available in the United States and was cleared by the Food and Drug Administration for seborrhea and seborrheic dermatitis in 2015. Dr. Bhatia referred to a study presented in a poster at a meeting in 2017 that showed improvement in 20 of 24 patients with mild to moderate seborrheic dermatitis treated with this product after 28 days. None of the patients worsened, and overall disease activity fell by more than half.
Loyon lotion (Cetiol oil and dimethicone), a nonmedicated descaling treatment for scaly patches in infants with cradle cap (seborrheic dermatitis). A 2014 pilot study found that it improved scaling in 80% of 20 infants and children aged 3-36 months over 8 days, and it reached “treatment success” in 50% (Dermatol Ther [Heidelb]. 2014 Dec;4[2]:221-32).
(As for cost, GoodRx.com states that various pharmacies sell one 8-ounce [227-gram] bottle of Sebuderm gel for about $300 with a coupon. Loyon is also expensive, with a GoodRx.com listing its price at about $300, with coupon, for one 50-ml spray bottle.)
Dr. Bhatia also reviewed drugs in the research pipeline. Several drugs for AD are in early phases of research, while some phase 3 trials involving Janus kinase (JAK) inhibitors for AD are starting – or are close to starting.
He pointed to other research that’s underway on treatments for acne and rosacea. Three acne drugs that he said are “almost here” are minocycline gel and cortexolone 17 alpha-propionate 1% cream. He also referred to sarecycline, a tetracycline-derived antibiotic taken orally, which was approved by the Food and Drug Administration on October 2 for moderate to severe acne vulgaris in patients aged 9 years and older.
This symposium was jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education. This publication and the Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Bhatia reported affiliations with multiple drugmakers: Abbvie, Aclaris, Almirall, Bayer, Biofrontera, BioPharmX, Dermira, Encore, EPI Health, Ferndale, Foamix, Galderma, IntraDerm, ISDIN, La Roche-Posay, Leo, Mayne, Menlo, Novartis, Ortho, Pfizer, Pierre Fabre, Promius, Regeneron, Sanofi, Skinfix, Soligenix, Sun, and Vidac.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
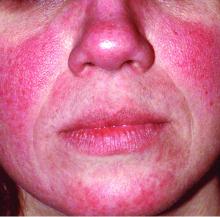
In rosacea, a single treatment may not be enough
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
MONTEREY, CALIF. – , a dermatologist said at the annual Coastal Dermatology Symposium. Don’t assume you can just prescribe one drug like you might with acne, she advised.
“Treat everything that you see,” said dermatologist Julie C. Harper, MD, of Birmingham, Ala. “That may mean a laser or something you’re using off-label. Different lesions and signs of rosacea will require multiple modes of treatment.”
Dr. Harper offered these other pearls to consider when treating rosacea:
- Don’t get hung up on subtypes.
The four subtypes of rosacea should be used to classify lesions, not people, she said. That’s because patients can fall into more than one of the four categories – erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea, she noted.
“Document the redness you see and ask them what’s bothering them the most,” she said. And ask yourself, she added, “Do I have them on everything that I should have them on?”
- Talk to patients about triggers.
For the first visit, “we have to talk to patients about skin care and triggers,” Dr. Harper noted. According to the American Academy of Dermatology, common rosacea triggers include sunlight, hairspray, heat, stress, alcohol, and spicy foods.
- Consider an ivermectin-brimonidine combination.
“Targeting inflammation in papules and pustules doesn’t necessarily translate to less background erythema,” Dr. Harper said. What to do? She pointed to a 2017 study that examined a combination treatment of ivermectin 1% topical cream (Soolantra) and brimonidine 0.33% topical gel (Mirvaso) for patients with rosacea with moderate to severe persistent erythema and inflammatory lesions. Ivermectin is indicated for inflammatory lesions, while brimonidine treats persistent erythema.
At week 12, the proportion of patients who achieved investigator global assessment of clear or almost clear was 55.8% in the combination group, versus 36.8% of those in the vehicle group (P = .007), according to the study (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16). Dr. Harper highlighted the effect of brimonidine when added to ivermectin. “In a period of 3 hours,” she said, “we had twice as many people fall into clear or almost clear.”
- Consider adding botulinum toxin to your toolbox.
This “really does work,” Dr. Harper said. She pointed to a 2015 report of botulinum toxin use in two cases of refractory flushing and erythema and a 2012 report of 13 cases in patients with the same symptoms (Dermatology. 2015;230:299-301; J Drugs Dermatol. 2012 Dec;11[12]:e76-9). Dr. Harper said that she usually uses the full 50-unit dose of Botox.
- Consider a beta-blocker.
According to a 2018 report, the beta-blocker carvedilol (Coreg) showed benefit when added to other treatments in five patients with facial flushing and persistent erythema.
- Keep isotretinoin in mind.
A 2016 report suggested low-dose isotretinoin had value for difficult-to-treat papulopustular rosacea. As Dr. Harper noted, 57% of those who took isotretinoin reached the primary endpoint, versus 10% of those taking the placebo. However, relapses over 4 months were common, which is a sign that it may be wise to prescribe low doses over the long term, but not in females of child-bearing potential, she said.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Harper disclosed speaker/advisor relationships with Allergan, Bayer, BioPharmX, Galderma, LaRoche Posay, and Ortho and has served as investigator for Bayer.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
CAR T therapy being explored in Hodgkin lymphoma
New York—Although the data set is small and not yet mature, chimeric antigen receptor (CAR) T-cell therapy appears to be a promising approach for Hodgkin lymphoma, according to Philippe Armand, MD, PhD, of Dana-Farber/Brigham and Women’s Cancer Center and the Massachusetts General Hospital Cancer Center.
While based on a handful of patients, the data do suggest this approach may play a role either by targeting CD30 or Epstein Barr virus (EBV), Dr. Armand said in a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies.
“Most importantly perhaps, like it's experience outside of Hodgkin lymphoma, it may really have curative potential, based on the long CR rates that have been already exhibited,” he told attendees at the NCCN conference.
Much of the published clinical experience to date is with CD30-directed CAR Ts, Dr. Armand said, noting that in Hodgkin lymphoma, results so far show promise for this particular approach.
In a recent phase 1 dose escalation study, 9 patients with relapsed/refractory Hodgkin lymphoma or anaplastic large-cell lymphoma (ALCL) received infusions of autologous T cells modified to express CD30-specific CAR T cells encoding the CD28 costimulatory domain, with no conditioning regimen.
Out of 7 relapsed Hodgkin patients, one had a complete response (CR) lasting beyond 2.5 years following a second infusion. Another had a CR persisting almost 2 years and 3 had transient stable disease.
One of the 2 ALCL patients had a CR lasting 9 months after a fourth infusion. No toxicities attributable to the therapy were seen, according to investigators.
The CD30 CAR T cells are being evaluated with a conditioning regimen in the phase 1 RELY-30 trial. According to Dr. Armand, preliminary results presented at the EBMT 2018 meeting showed better expansion of CAR T cells and responses in 3 out of 5 patients, including 2 CRs.
A CD30-directed CAR T-cell therapy with a 4-1bb costimulatory domain has also been tested in a small group of Hodgkin patients with a response rate of 35%, including some CRs. Response rates were lower in patients with extranodal involvement, although that needs to be validated with further study, according to Dr. Armand.
A considerable amount of active research is ongoing in China, Dr. Armand said, while a phase 1 study of T cells expressing a fully human anti-CD30 CAR is being evaluated in the United States in CD30-expressing lymphomas, he added.
Among non-CD30-targeted products, a CD19 CAR-T approach has been tried in Hodgkin lymphoma, though preliminary results suggest only transient activity.
An interesting approach has been the targeting of EBV, Dr. Armand noted. Recently reported results showed that two doses of T cells with specificity for EBV-derived tumor antigens induced clinical responses in patients with EBV-positive Hodgkin lymphoma.
The cells were engineered to express dominant-negative TGF-β receptor type 2 (DNRII).
“We know that TGF-β provides a strong immunosuppressant signal in the tumor microenvironment,” Dr. Armand said, noting that some of the responses in the 7 evaluable patients lasted 4 years or more.
New York—Although the data set is small and not yet mature, chimeric antigen receptor (CAR) T-cell therapy appears to be a promising approach for Hodgkin lymphoma, according to Philippe Armand, MD, PhD, of Dana-Farber/Brigham and Women’s Cancer Center and the Massachusetts General Hospital Cancer Center.
While based on a handful of patients, the data do suggest this approach may play a role either by targeting CD30 or Epstein Barr virus (EBV), Dr. Armand said in a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies.
“Most importantly perhaps, like it's experience outside of Hodgkin lymphoma, it may really have curative potential, based on the long CR rates that have been already exhibited,” he told attendees at the NCCN conference.
Much of the published clinical experience to date is with CD30-directed CAR Ts, Dr. Armand said, noting that in Hodgkin lymphoma, results so far show promise for this particular approach.
In a recent phase 1 dose escalation study, 9 patients with relapsed/refractory Hodgkin lymphoma or anaplastic large-cell lymphoma (ALCL) received infusions of autologous T cells modified to express CD30-specific CAR T cells encoding the CD28 costimulatory domain, with no conditioning regimen.
Out of 7 relapsed Hodgkin patients, one had a complete response (CR) lasting beyond 2.5 years following a second infusion. Another had a CR persisting almost 2 years and 3 had transient stable disease.
One of the 2 ALCL patients had a CR lasting 9 months after a fourth infusion. No toxicities attributable to the therapy were seen, according to investigators.
The CD30 CAR T cells are being evaluated with a conditioning regimen in the phase 1 RELY-30 trial. According to Dr. Armand, preliminary results presented at the EBMT 2018 meeting showed better expansion of CAR T cells and responses in 3 out of 5 patients, including 2 CRs.
A CD30-directed CAR T-cell therapy with a 4-1bb costimulatory domain has also been tested in a small group of Hodgkin patients with a response rate of 35%, including some CRs. Response rates were lower in patients with extranodal involvement, although that needs to be validated with further study, according to Dr. Armand.
A considerable amount of active research is ongoing in China, Dr. Armand said, while a phase 1 study of T cells expressing a fully human anti-CD30 CAR is being evaluated in the United States in CD30-expressing lymphomas, he added.
Among non-CD30-targeted products, a CD19 CAR-T approach has been tried in Hodgkin lymphoma, though preliminary results suggest only transient activity.
An interesting approach has been the targeting of EBV, Dr. Armand noted. Recently reported results showed that two doses of T cells with specificity for EBV-derived tumor antigens induced clinical responses in patients with EBV-positive Hodgkin lymphoma.
The cells were engineered to express dominant-negative TGF-β receptor type 2 (DNRII).
“We know that TGF-β provides a strong immunosuppressant signal in the tumor microenvironment,” Dr. Armand said, noting that some of the responses in the 7 evaluable patients lasted 4 years or more.
New York—Although the data set is small and not yet mature, chimeric antigen receptor (CAR) T-cell therapy appears to be a promising approach for Hodgkin lymphoma, according to Philippe Armand, MD, PhD, of Dana-Farber/Brigham and Women’s Cancer Center and the Massachusetts General Hospital Cancer Center.
While based on a handful of patients, the data do suggest this approach may play a role either by targeting CD30 or Epstein Barr virus (EBV), Dr. Armand said in a presentation at the NCCN 13th Annual Congress: Hematologic Malignancies.
“Most importantly perhaps, like it's experience outside of Hodgkin lymphoma, it may really have curative potential, based on the long CR rates that have been already exhibited,” he told attendees at the NCCN conference.
Much of the published clinical experience to date is with CD30-directed CAR Ts, Dr. Armand said, noting that in Hodgkin lymphoma, results so far show promise for this particular approach.
In a recent phase 1 dose escalation study, 9 patients with relapsed/refractory Hodgkin lymphoma or anaplastic large-cell lymphoma (ALCL) received infusions of autologous T cells modified to express CD30-specific CAR T cells encoding the CD28 costimulatory domain, with no conditioning regimen.
Out of 7 relapsed Hodgkin patients, one had a complete response (CR) lasting beyond 2.5 years following a second infusion. Another had a CR persisting almost 2 years and 3 had transient stable disease.
One of the 2 ALCL patients had a CR lasting 9 months after a fourth infusion. No toxicities attributable to the therapy were seen, according to investigators.
The CD30 CAR T cells are being evaluated with a conditioning regimen in the phase 1 RELY-30 trial. According to Dr. Armand, preliminary results presented at the EBMT 2018 meeting showed better expansion of CAR T cells and responses in 3 out of 5 patients, including 2 CRs.
A CD30-directed CAR T-cell therapy with a 4-1bb costimulatory domain has also been tested in a small group of Hodgkin patients with a response rate of 35%, including some CRs. Response rates were lower in patients with extranodal involvement, although that needs to be validated with further study, according to Dr. Armand.
A considerable amount of active research is ongoing in China, Dr. Armand said, while a phase 1 study of T cells expressing a fully human anti-CD30 CAR is being evaluated in the United States in CD30-expressing lymphomas, he added.
Among non-CD30-targeted products, a CD19 CAR-T approach has been tried in Hodgkin lymphoma, though preliminary results suggest only transient activity.
An interesting approach has been the targeting of EBV, Dr. Armand noted. Recently reported results showed that two doses of T cells with specificity for EBV-derived tumor antigens induced clinical responses in patients with EBV-positive Hodgkin lymphoma.
The cells were engineered to express dominant-negative TGF-β receptor type 2 (DNRII).
“We know that TGF-β provides a strong immunosuppressant signal in the tumor microenvironment,” Dr. Armand said, noting that some of the responses in the 7 evaluable patients lasted 4 years or more.
Pharmacist-led clinic boosted hypertension control after discharge
SAN DIEGO – Pharmacist-physician collaboration on hypertension management was effective for controlling blood pressure in patients discharged from an urban ED, results from a pilot study showed.
“Hypertension is the leading risk factor for cardiovascular events and stroke in this country,” Brittany Stewart, RD, PharmD, said at the annual meeting of the American College of Emergency Physicians. “The good news is that we’re not trying to figure out how to treat this disease, but the bad news is that we have 50% of people with uncontrolled high blood pressure.”
“Pharmacists are highly accessible in the community in outpatient settings,” Dr. Stewart of the department of pharmacy practice at Wayne State University, Detroit, said. “This is where ED providers can really integrate and work on a team to refer patients for their chronic diseases on an outpatient basis.”
In a prospective pilot trial, Dr. Stewart and her colleagues recruited 89 patients with uncontrolled hypertension who presented to the ED at Wayne State during May 24, 2017–May 18, 2018. Their average age was 43 years, 51% were male, 94% were black, 51% were current smokers, the mean body mass index was 34.5 kg/m2, and 18% had no health insurance.
“In Detroit, we have significant health disparities with our patient population,” she said. “They are high utilizers of the emergency department, not only for their acute illness but for chronic illness as well. There are several studies showing that team-based care has improved hypertension and blood pressure control. However, the adoption and sustainability of these models haven’t really taken off in our health care landscape yet.”
Over a period of 1.5 years, Dr. Stewart and two physicians developed a transitional care clinic, based on a collaborative practice agreement with emergency physicians. Per protocol, five follow-up visits were planned in an outpatient pharmacy clinic, where Dr. Stewart initiated and titrated antihypertensive medications and handled refills.
“The physician does not have to physically be at the clinic,” she said. “We work closely over the phone to make the best decisions, but it’s not typical ambulatory care where the physician has to be sitting right next to the pharmacist to make the best decisions for the patients.” The primary outcome was the transitional care clinic’s impact on blood pressure.
Dr. Stewart reported results from 47 medication interventions that were provided over 97 follow-up visits. The researchers found that the median blood pressure dropped from an initial reading of 160/102 mm Hg to 130/93 mm Hg by the fifth transitional care clinic visit. Across all patients, systolic blood pressure decreased by an average of 48 mm Hg. “I don’t think we were surprised by these results; we are getting people very much in need down to their blood pressure goals,” she said. “By the end of the study, patients were on an average of three antihypertensive medications.”
She and her colleagues plan to conduct a larger randomized, clinical trial of this care model. The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
SAN DIEGO – Pharmacist-physician collaboration on hypertension management was effective for controlling blood pressure in patients discharged from an urban ED, results from a pilot study showed.
“Hypertension is the leading risk factor for cardiovascular events and stroke in this country,” Brittany Stewart, RD, PharmD, said at the annual meeting of the American College of Emergency Physicians. “The good news is that we’re not trying to figure out how to treat this disease, but the bad news is that we have 50% of people with uncontrolled high blood pressure.”
“Pharmacists are highly accessible in the community in outpatient settings,” Dr. Stewart of the department of pharmacy practice at Wayne State University, Detroit, said. “This is where ED providers can really integrate and work on a team to refer patients for their chronic diseases on an outpatient basis.”
In a prospective pilot trial, Dr. Stewart and her colleagues recruited 89 patients with uncontrolled hypertension who presented to the ED at Wayne State during May 24, 2017–May 18, 2018. Their average age was 43 years, 51% were male, 94% were black, 51% were current smokers, the mean body mass index was 34.5 kg/m2, and 18% had no health insurance.
“In Detroit, we have significant health disparities with our patient population,” she said. “They are high utilizers of the emergency department, not only for their acute illness but for chronic illness as well. There are several studies showing that team-based care has improved hypertension and blood pressure control. However, the adoption and sustainability of these models haven’t really taken off in our health care landscape yet.”
Over a period of 1.5 years, Dr. Stewart and two physicians developed a transitional care clinic, based on a collaborative practice agreement with emergency physicians. Per protocol, five follow-up visits were planned in an outpatient pharmacy clinic, where Dr. Stewart initiated and titrated antihypertensive medications and handled refills.
“The physician does not have to physically be at the clinic,” she said. “We work closely over the phone to make the best decisions, but it’s not typical ambulatory care where the physician has to be sitting right next to the pharmacist to make the best decisions for the patients.” The primary outcome was the transitional care clinic’s impact on blood pressure.
Dr. Stewart reported results from 47 medication interventions that were provided over 97 follow-up visits. The researchers found that the median blood pressure dropped from an initial reading of 160/102 mm Hg to 130/93 mm Hg by the fifth transitional care clinic visit. Across all patients, systolic blood pressure decreased by an average of 48 mm Hg. “I don’t think we were surprised by these results; we are getting people very much in need down to their blood pressure goals,” she said. “By the end of the study, patients were on an average of three antihypertensive medications.”
She and her colleagues plan to conduct a larger randomized, clinical trial of this care model. The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
SAN DIEGO – Pharmacist-physician collaboration on hypertension management was effective for controlling blood pressure in patients discharged from an urban ED, results from a pilot study showed.
“Hypertension is the leading risk factor for cardiovascular events and stroke in this country,” Brittany Stewart, RD, PharmD, said at the annual meeting of the American College of Emergency Physicians. “The good news is that we’re not trying to figure out how to treat this disease, but the bad news is that we have 50% of people with uncontrolled high blood pressure.”
“Pharmacists are highly accessible in the community in outpatient settings,” Dr. Stewart of the department of pharmacy practice at Wayne State University, Detroit, said. “This is where ED providers can really integrate and work on a team to refer patients for their chronic diseases on an outpatient basis.”
In a prospective pilot trial, Dr. Stewart and her colleagues recruited 89 patients with uncontrolled hypertension who presented to the ED at Wayne State during May 24, 2017–May 18, 2018. Their average age was 43 years, 51% were male, 94% were black, 51% were current smokers, the mean body mass index was 34.5 kg/m2, and 18% had no health insurance.
“In Detroit, we have significant health disparities with our patient population,” she said. “They are high utilizers of the emergency department, not only for their acute illness but for chronic illness as well. There are several studies showing that team-based care has improved hypertension and blood pressure control. However, the adoption and sustainability of these models haven’t really taken off in our health care landscape yet.”
Over a period of 1.5 years, Dr. Stewart and two physicians developed a transitional care clinic, based on a collaborative practice agreement with emergency physicians. Per protocol, five follow-up visits were planned in an outpatient pharmacy clinic, where Dr. Stewart initiated and titrated antihypertensive medications and handled refills.
“The physician does not have to physically be at the clinic,” she said. “We work closely over the phone to make the best decisions, but it’s not typical ambulatory care where the physician has to be sitting right next to the pharmacist to make the best decisions for the patients.” The primary outcome was the transitional care clinic’s impact on blood pressure.
Dr. Stewart reported results from 47 medication interventions that were provided over 97 follow-up visits. The researchers found that the median blood pressure dropped from an initial reading of 160/102 mm Hg to 130/93 mm Hg by the fifth transitional care clinic visit. Across all patients, systolic blood pressure decreased by an average of 48 mm Hg. “I don’t think we were surprised by these results; we are getting people very much in need down to their blood pressure goals,” she said. “By the end of the study, patients were on an average of three antihypertensive medications.”
She and her colleagues plan to conduct a larger randomized, clinical trial of this care model. The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
REPORTING FROM ACEP18
Key clinical point: Hypertensive patients discharged from an urban ED benefited from a pharmacist-led clinic.
Major finding: By the last follow-up visit, patients’ mean systolic blood pressure had decreased by 48 mm Hg.
Study details: A pilot study of 89 patients with uncontrolled blood pressure.
Disclosures: The research was supported by a faculty award from the Eugene Applebaum College of Pharmacy and Health Sciences at Wayne State University and by the National Association of Chain Drug Stores Foundation.
Source: Brody A et al. Ann Emerg Med. 2018 Oct;72;4:S36-7. doi. 10.1016/j.annemergmed.2018.08.088.
Half of outpatient antibiotics prescribed with no infectious disease code
based on a review of more than half a million outpatient prescriptions to more than a quarter million patients at 514 clinics around Chicago.
The researchers looked to see if prescriptions had an ICD-10 code that indicated an antibiotic; they were liberal in their approach, considering over 21,000 codes to at least possibly signal the need for an antibiotic.
Almost half the time, there was nothing in the codes related to bacterial infection: 29% of scripts were written in connection with codes for high blood pressure, annual visits, and other noninfectious disorders; 17% of prescriptions were written with no diagnosis code at all.
The study is likely the largest to date to look at outpatient antibiotic prescribing patterns in the United States, and the findings are worrisome. “Nearly half the time, clinicians have either a bad reason for prescribing antibiotics, or don’t provide a reason at all. When you consider about 80% of antibiotics are prescribed on an outpatient basis, that’s a concern,” lead investigator Jeffrey A. Linder, MD, MPH, chief of the division of general internal medicine and geriatrics at Northwestern University, Chicago, said in a written statement.
“At busy clinics, sadly, the most efficient thing to do is just call in an antibiotic prescription. We need to dig into the data more, but we believe there is a lot of antibiotic prescribing for colds, the flu, and non-specific symptoms such as just not feeling well,” he said.
With all the concern in recent years about overuse, it’s hard to imagine that prescribers are still being free and easy with antibiotics, and Dr. Linder’s study will certainly have its skeptics.
Sloppy record keeping could be one explanation for the findings. A patient could really have needed an antibiotic, but it just wasn’t captured in coding. There are also valid reasons for prescribing antibiotics over the phone, such as acne and recurrent UTIs.
Dr. Linder, however, thinks it’s more than that. He explained his study, its implications, and the next steps in an interview at ID Week, an annual scientific meeting on infectious diseases.
The 2,413 prescribers in the study included physicians, surgeons, residents, fellows, nurse practitioners, and physician assistants in general and specialty practices. Patients were a mean of 43 years old: 60% were women and 75% were white. The most common antibiotic classes were penicillins, macrolides, and cephalosporins. Prescriptions were written from November 2015 through October 2017.
The work was funded by the Agency for Healthcare Research and Quality. Dr. Linder did not have any disclosures.
SOURCE: Linder JA et al. ID Week 2018 abstract 1632.
based on a review of more than half a million outpatient prescriptions to more than a quarter million patients at 514 clinics around Chicago.
The researchers looked to see if prescriptions had an ICD-10 code that indicated an antibiotic; they were liberal in their approach, considering over 21,000 codes to at least possibly signal the need for an antibiotic.
Almost half the time, there was nothing in the codes related to bacterial infection: 29% of scripts were written in connection with codes for high blood pressure, annual visits, and other noninfectious disorders; 17% of prescriptions were written with no diagnosis code at all.
The study is likely the largest to date to look at outpatient antibiotic prescribing patterns in the United States, and the findings are worrisome. “Nearly half the time, clinicians have either a bad reason for prescribing antibiotics, or don’t provide a reason at all. When you consider about 80% of antibiotics are prescribed on an outpatient basis, that’s a concern,” lead investigator Jeffrey A. Linder, MD, MPH, chief of the division of general internal medicine and geriatrics at Northwestern University, Chicago, said in a written statement.
“At busy clinics, sadly, the most efficient thing to do is just call in an antibiotic prescription. We need to dig into the data more, but we believe there is a lot of antibiotic prescribing for colds, the flu, and non-specific symptoms such as just not feeling well,” he said.
With all the concern in recent years about overuse, it’s hard to imagine that prescribers are still being free and easy with antibiotics, and Dr. Linder’s study will certainly have its skeptics.
Sloppy record keeping could be one explanation for the findings. A patient could really have needed an antibiotic, but it just wasn’t captured in coding. There are also valid reasons for prescribing antibiotics over the phone, such as acne and recurrent UTIs.
Dr. Linder, however, thinks it’s more than that. He explained his study, its implications, and the next steps in an interview at ID Week, an annual scientific meeting on infectious diseases.
The 2,413 prescribers in the study included physicians, surgeons, residents, fellows, nurse practitioners, and physician assistants in general and specialty practices. Patients were a mean of 43 years old: 60% were women and 75% were white. The most common antibiotic classes were penicillins, macrolides, and cephalosporins. Prescriptions were written from November 2015 through October 2017.
The work was funded by the Agency for Healthcare Research and Quality. Dr. Linder did not have any disclosures.
SOURCE: Linder JA et al. ID Week 2018 abstract 1632.
based on a review of more than half a million outpatient prescriptions to more than a quarter million patients at 514 clinics around Chicago.
The researchers looked to see if prescriptions had an ICD-10 code that indicated an antibiotic; they were liberal in their approach, considering over 21,000 codes to at least possibly signal the need for an antibiotic.
Almost half the time, there was nothing in the codes related to bacterial infection: 29% of scripts were written in connection with codes for high blood pressure, annual visits, and other noninfectious disorders; 17% of prescriptions were written with no diagnosis code at all.
The study is likely the largest to date to look at outpatient antibiotic prescribing patterns in the United States, and the findings are worrisome. “Nearly half the time, clinicians have either a bad reason for prescribing antibiotics, or don’t provide a reason at all. When you consider about 80% of antibiotics are prescribed on an outpatient basis, that’s a concern,” lead investigator Jeffrey A. Linder, MD, MPH, chief of the division of general internal medicine and geriatrics at Northwestern University, Chicago, said in a written statement.
“At busy clinics, sadly, the most efficient thing to do is just call in an antibiotic prescription. We need to dig into the data more, but we believe there is a lot of antibiotic prescribing for colds, the flu, and non-specific symptoms such as just not feeling well,” he said.
With all the concern in recent years about overuse, it’s hard to imagine that prescribers are still being free and easy with antibiotics, and Dr. Linder’s study will certainly have its skeptics.
Sloppy record keeping could be one explanation for the findings. A patient could really have needed an antibiotic, but it just wasn’t captured in coding. There are also valid reasons for prescribing antibiotics over the phone, such as acne and recurrent UTIs.
Dr. Linder, however, thinks it’s more than that. He explained his study, its implications, and the next steps in an interview at ID Week, an annual scientific meeting on infectious diseases.
The 2,413 prescribers in the study included physicians, surgeons, residents, fellows, nurse practitioners, and physician assistants in general and specialty practices. Patients were a mean of 43 years old: 60% were women and 75% were white. The most common antibiotic classes were penicillins, macrolides, and cephalosporins. Prescriptions were written from November 2015 through October 2017.
The work was funded by the Agency for Healthcare Research and Quality. Dr. Linder did not have any disclosures.
SOURCE: Linder JA et al. ID Week 2018 abstract 1632.
REPORTING FROM ID WEEK 2018
Telehealth: States broaden options for locations, providers
More states are expanding their telehealth policies to reach patients, and pulling back on rigid in-person requirements.
Several state Medicaid programs now explicitly allow the home to serve as an originating site for telehealth, with 10 states – Delaware, Colorado, Maryland, Michigan, Minnesota, Montana, New York, Texas, Washington, and Wyoming – adding the home as an approved site since 2016.
In addition, 16 jurisdictions now allow schools to serve as originating sites for telehealth, although some have restrictions about when the sites are acceptable, said Mei Kwong, an attorney and executive director for the Center for Connected Health Policy and the author of the center’s Spring 2018 report on telehealth trends.
At the same time, nearly all states have now dropped Medicaid restrictions that limited reimbursable telehealth services to rural or underserved areas. Colorado, Idaho, Nebraska, New Hampshire, Nevada, and Missouri are the most recent states to remove such geographic restrictions.
“[The expanded locations are] extremely helpful in providing greater access for patients to needed services,” Ms. Kwong said in an interview. “For example, a person who has difficulty leaving his or her home for a physical or other reason, they can get care, [or] a child having a rough time in school, can seek out a mental health counselor while at school.”
More telehealth providers
In addition to expanding teleheath sites, states are increasing acceptance for telehealth providers beyond physicians. Most recently, New Jersey enacted a broad telemedicine law that includes doctors, nurses, psychologists, social workers, physician assistants, counselors, respiratory therapists, speech pathologists, and optometrists, among others. The New Jersey law addresses telemedicine practice standards, prescribing, patient consent, privacy, and other requirements for providers.
In addition, more states are carving out telehealth regulations. Since 2016, 11 states have revised or adopted new scope of practice restrictions for counselors providing telemedicine, according to a state telehealth analysis published in July 2018 by law firm Epstein, Becker, Green.
Arkansas, Idaho, Maine, New Jersey, and Rhode Island recently imposed regulations for the practice of telepsychology. In addition, seven states have new or revised scope of practice restrictions for advanced practice registered nurses (APRN) providing telehealth services, while eight states have new licensing requirements specific to telehealth practice by APRNs, according to the Epstein analysis.
A telehealth compact that would allow APRNs to practice nursing via telemedicine across state lines is also in the works. Similar to the physician Interstate Medical Licensure Compact, the APRN Compact would establish an interstate commission and guidelines for uniform licensing requirements and criminal background checks. The compact will become effective when 10 states enact the compact legislation. So far, three states – Wyoming, North Dakota, and Idaho – have enacted the model legislation.
Another telemedicine compact for psychologists is getting ready to launch. In August, Illinois became the seventh state to join the Psychology Interjurisdictional Compact (PSYPACT). The pact requires seven states to enact compact legislation to become effective, however Illinois law does not go into effect until 2020.
The developments highlight the rise in more mid- and lower-level providers practicing telemedicine, said Anjali B. Dooley, an attorney and chief legal and compliance officer for Forefront Telecare, a telehealth platform for behavioral health services. While the increase allows greater care access, the expansion also poses scope of practice challenges, she notes.
“Increasing scope of practice extensions also increases risk if physician extenders are not trained properly in telehealth technology use and protocols,” Ms. Dooley said in an interview. “Providers and provider extenders need to be educated and learned in human factors such as communication, empathy, and etiquette.”
A greater number of nonphysician telemedicine providers is beneficial as long as the providers are adhering to appropriate standards of care and consulting with supervising physicians when necessary, adds Jean R. Sumner, MD, dean of the School of Medicine at Mercer University in Macon, Georgia, and a telemedicine internist.
“The standard should always be equal to an in-person visit,” she said in an interview. “The patient has a right to know who is seeing them, too, to understand [their credentials]. They need to know the person on the telehealth unit is qualified to provide the care.”
Responding to the opioid crisis
The need for increased access to mental health care is a primary driver behind state efforts to expand the pool of telemedicine providers, adds Amy Lerman, an attorney at Epstein, Becker, Green and lead author of her firm’s report.
“The reason it is important for states to continue expanding the scope of health professionals, other than physicians, who can provide behavioral health telemedicine services, is not only to address an overall nationwide shortage of behavioral health providers, but also to expand access to behavioral health services because a wider range of providers are equipped to provide these services,” she said in an interview.
In the same vein, more states are using telehealth to address the opioid crisis, according to both the Epstein report and the Center for Connected Health Policy analysis.
In September, California enacted a law that would allow Medicaid reimbursement for certified substance use disorder counselors who provide treatment via telehealth. In August, Illinois approved a similar law that mandates reimbursement for behavioral and mental health experts who treat Medicaid patients through telehealth technologies.
The laws come after a June 2018 letter from the Centers for Medicare & Medicaid Services that encouraged states to utilize health technology efforts to address the opioid crisis, including through telemedicine and telepsychiatry, said Daniel Kim, an attorney with Epstein, Becker, Green and a coauthor of his firm’s report.
At the same time, a number of states have expanded their controlled substance laws to allow remote prescribing through telehealth for the treatment of psychiatric or substance use disorders. Connecticut’s law, for instance, allows providers to prescribe Schedule I-III controlled substances through telehealth platforms, while banning opioid prescribing. In Indiana, 2017 legislation expanded the types of controlled medications that providers can prescribe through telehealth platforms, primarily drugs used to treat or manage opioid dependence. The states join an increasing number that have enacted laws allowing the remote prescribing of controlled substances, including Delaware, Florida, Indiana, Michigan, New Hampshire, Ohio, and West Virginia.
The new laws will enhance the availability of behavioral health services, while allowing more treatment flexibility and privacy for patients, said Ms. Dooley.
“Treatment in one’s own environment where the addiction takes place is often more effective,” she said. People with addiction disorders “can also receive treatment without having to drive long distances.”
The disappearing in-person requirement
As states define their telehealth policies, they are fading out a once-prevalent requirement – the in-person visit. There is no longer a single state that requires physicians to meet with patients in-person before providing telemedicine services, according to the Epstein report.
States realized that requiring in-person visits before doctors can provide telemedicine creates a barrier to care, said Mr. Kim. A move to eliminate the requirement in Texas influenced other states in phasing out the common regulation. In the widely publicized Teladoc case, the national telemedicine company sued the Texas Medical Board in 2011 over its rule requiring Texas physicians to conduct a face-to-face evaluation before treating a patient via telemedicine. The legal battle continued for years, until Teladoc voluntarily dropped its lawsuit in 2017 after Texas adopted a new law that allowed doctors to treat first-time patients through telemedicine.
“The medical board [understood] that the in-person requirement wasn’t really a benefit to patients,” Mr. Kim said in an interview. “Once they changed it, a lot of other states have recognized the same and have moved toward getting rid of the requirement.”
In some states, midlevel providers still must see patients face-to-face before providing telehealth care. Arkansas for instance, requires that psychologists, counselors, and APRNs conduct an in-person exam before rendering telehealth. Professional boards in Colorado and Massachusetts recommend a face-to-face visit by midlevel providers as a best practice.
Reimbursement growing, but restrictions remain
Forty-nine states and the District of Columbia reimburse for some form of telehealth, mainly live video services. At least 20 states now pay providers for remote payment monitoring (RPM), according to the Center for Connected Health Policy report. The reimbursement is often restricted, however, to certain clinical conditions and/or rules that limit the type of monitoring device allowed. Colorado, for instance, only reimburses RPM for patients with congestive heart failure, chronic obstructive pulmonary disease, asthma, or diabetes and requires that the patient was hospitalized at least twice in the last 12 months for reasons associated with one of the conditions. Missouri has similar RPM criteria associated with hospitalizations, but allows for a greater number of conditions including pregnancy, stroke, and cancer.
Most states have yet to pay for store-and-forward services, technologies that enable the electronic transfer of photos, prerecorded videos, or documents. Only about 14 states reimburse for such technology, and many policies include limitations. California, for example, only reimburses for store-and-forward services in teledermatology, teledentistry, and teleophthalmology. Connecticut allows for store-and-forward payment between physicians through email. Missouri allows for store-and-forward services in orthopedics, dermatology, optometry, ophthalmology, and in cases of retinopathy, burn and wound care, dental services, and maternal-fetal ultrasounds.
Reimbursement for telehealth is still a challenge for many physicians, Dr. Sumner said. Part of the problem is the wide discrepancy in how telehealth is defined among states, she said. Some states only consider live or interactive two-way technology as telehealth, excluding services such as store-and-forward and RPM. Other states reimburse for certain technology-based services, but they do not consider them telehealth. Maryland’s Medicaid program for example, does not reimburse for store and forward under its telehealth policy. However, store and forward used in dermatology, radiology, and ophthalmology is reimbursed by Maryland under an alternate billing code, though not considered telehealth.
Establishing best practices in telehealth through careful evaluation and research would improve reimbursement, said S. David McSwain, MD, interim chief medical information officer at the Medical University of South Carolina, Charleston, and medical director for telehealth optimization.
“We can use that evidence to reduce the variation in telehealth payment policies across the states,” Dr. McSwain said in an interview. “By leveraging reimbursement models to promote best practices, we can encourage the spread of telehealth services that have the greatest impacts on patients, their families, and the health care system.”
More states are expanding their telehealth policies to reach patients, and pulling back on rigid in-person requirements.
Several state Medicaid programs now explicitly allow the home to serve as an originating site for telehealth, with 10 states – Delaware, Colorado, Maryland, Michigan, Minnesota, Montana, New York, Texas, Washington, and Wyoming – adding the home as an approved site since 2016.
In addition, 16 jurisdictions now allow schools to serve as originating sites for telehealth, although some have restrictions about when the sites are acceptable, said Mei Kwong, an attorney and executive director for the Center for Connected Health Policy and the author of the center’s Spring 2018 report on telehealth trends.
At the same time, nearly all states have now dropped Medicaid restrictions that limited reimbursable telehealth services to rural or underserved areas. Colorado, Idaho, Nebraska, New Hampshire, Nevada, and Missouri are the most recent states to remove such geographic restrictions.
“[The expanded locations are] extremely helpful in providing greater access for patients to needed services,” Ms. Kwong said in an interview. “For example, a person who has difficulty leaving his or her home for a physical or other reason, they can get care, [or] a child having a rough time in school, can seek out a mental health counselor while at school.”
More telehealth providers
In addition to expanding teleheath sites, states are increasing acceptance for telehealth providers beyond physicians. Most recently, New Jersey enacted a broad telemedicine law that includes doctors, nurses, psychologists, social workers, physician assistants, counselors, respiratory therapists, speech pathologists, and optometrists, among others. The New Jersey law addresses telemedicine practice standards, prescribing, patient consent, privacy, and other requirements for providers.
In addition, more states are carving out telehealth regulations. Since 2016, 11 states have revised or adopted new scope of practice restrictions for counselors providing telemedicine, according to a state telehealth analysis published in July 2018 by law firm Epstein, Becker, Green.
Arkansas, Idaho, Maine, New Jersey, and Rhode Island recently imposed regulations for the practice of telepsychology. In addition, seven states have new or revised scope of practice restrictions for advanced practice registered nurses (APRN) providing telehealth services, while eight states have new licensing requirements specific to telehealth practice by APRNs, according to the Epstein analysis.
A telehealth compact that would allow APRNs to practice nursing via telemedicine across state lines is also in the works. Similar to the physician Interstate Medical Licensure Compact, the APRN Compact would establish an interstate commission and guidelines for uniform licensing requirements and criminal background checks. The compact will become effective when 10 states enact the compact legislation. So far, three states – Wyoming, North Dakota, and Idaho – have enacted the model legislation.
Another telemedicine compact for psychologists is getting ready to launch. In August, Illinois became the seventh state to join the Psychology Interjurisdictional Compact (PSYPACT). The pact requires seven states to enact compact legislation to become effective, however Illinois law does not go into effect until 2020.
The developments highlight the rise in more mid- and lower-level providers practicing telemedicine, said Anjali B. Dooley, an attorney and chief legal and compliance officer for Forefront Telecare, a telehealth platform for behavioral health services. While the increase allows greater care access, the expansion also poses scope of practice challenges, she notes.
“Increasing scope of practice extensions also increases risk if physician extenders are not trained properly in telehealth technology use and protocols,” Ms. Dooley said in an interview. “Providers and provider extenders need to be educated and learned in human factors such as communication, empathy, and etiquette.”
A greater number of nonphysician telemedicine providers is beneficial as long as the providers are adhering to appropriate standards of care and consulting with supervising physicians when necessary, adds Jean R. Sumner, MD, dean of the School of Medicine at Mercer University in Macon, Georgia, and a telemedicine internist.
“The standard should always be equal to an in-person visit,” she said in an interview. “The patient has a right to know who is seeing them, too, to understand [their credentials]. They need to know the person on the telehealth unit is qualified to provide the care.”
Responding to the opioid crisis
The need for increased access to mental health care is a primary driver behind state efforts to expand the pool of telemedicine providers, adds Amy Lerman, an attorney at Epstein, Becker, Green and lead author of her firm’s report.
“The reason it is important for states to continue expanding the scope of health professionals, other than physicians, who can provide behavioral health telemedicine services, is not only to address an overall nationwide shortage of behavioral health providers, but also to expand access to behavioral health services because a wider range of providers are equipped to provide these services,” she said in an interview.
In the same vein, more states are using telehealth to address the opioid crisis, according to both the Epstein report and the Center for Connected Health Policy analysis.
In September, California enacted a law that would allow Medicaid reimbursement for certified substance use disorder counselors who provide treatment via telehealth. In August, Illinois approved a similar law that mandates reimbursement for behavioral and mental health experts who treat Medicaid patients through telehealth technologies.
The laws come after a June 2018 letter from the Centers for Medicare & Medicaid Services that encouraged states to utilize health technology efforts to address the opioid crisis, including through telemedicine and telepsychiatry, said Daniel Kim, an attorney with Epstein, Becker, Green and a coauthor of his firm’s report.
At the same time, a number of states have expanded their controlled substance laws to allow remote prescribing through telehealth for the treatment of psychiatric or substance use disorders. Connecticut’s law, for instance, allows providers to prescribe Schedule I-III controlled substances through telehealth platforms, while banning opioid prescribing. In Indiana, 2017 legislation expanded the types of controlled medications that providers can prescribe through telehealth platforms, primarily drugs used to treat or manage opioid dependence. The states join an increasing number that have enacted laws allowing the remote prescribing of controlled substances, including Delaware, Florida, Indiana, Michigan, New Hampshire, Ohio, and West Virginia.
The new laws will enhance the availability of behavioral health services, while allowing more treatment flexibility and privacy for patients, said Ms. Dooley.
“Treatment in one’s own environment where the addiction takes place is often more effective,” she said. People with addiction disorders “can also receive treatment without having to drive long distances.”
The disappearing in-person requirement
As states define their telehealth policies, they are fading out a once-prevalent requirement – the in-person visit. There is no longer a single state that requires physicians to meet with patients in-person before providing telemedicine services, according to the Epstein report.
States realized that requiring in-person visits before doctors can provide telemedicine creates a barrier to care, said Mr. Kim. A move to eliminate the requirement in Texas influenced other states in phasing out the common regulation. In the widely publicized Teladoc case, the national telemedicine company sued the Texas Medical Board in 2011 over its rule requiring Texas physicians to conduct a face-to-face evaluation before treating a patient via telemedicine. The legal battle continued for years, until Teladoc voluntarily dropped its lawsuit in 2017 after Texas adopted a new law that allowed doctors to treat first-time patients through telemedicine.
“The medical board [understood] that the in-person requirement wasn’t really a benefit to patients,” Mr. Kim said in an interview. “Once they changed it, a lot of other states have recognized the same and have moved toward getting rid of the requirement.”
In some states, midlevel providers still must see patients face-to-face before providing telehealth care. Arkansas for instance, requires that psychologists, counselors, and APRNs conduct an in-person exam before rendering telehealth. Professional boards in Colorado and Massachusetts recommend a face-to-face visit by midlevel providers as a best practice.
Reimbursement growing, but restrictions remain
Forty-nine states and the District of Columbia reimburse for some form of telehealth, mainly live video services. At least 20 states now pay providers for remote payment monitoring (RPM), according to the Center for Connected Health Policy report. The reimbursement is often restricted, however, to certain clinical conditions and/or rules that limit the type of monitoring device allowed. Colorado, for instance, only reimburses RPM for patients with congestive heart failure, chronic obstructive pulmonary disease, asthma, or diabetes and requires that the patient was hospitalized at least twice in the last 12 months for reasons associated with one of the conditions. Missouri has similar RPM criteria associated with hospitalizations, but allows for a greater number of conditions including pregnancy, stroke, and cancer.
Most states have yet to pay for store-and-forward services, technologies that enable the electronic transfer of photos, prerecorded videos, or documents. Only about 14 states reimburse for such technology, and many policies include limitations. California, for example, only reimburses for store-and-forward services in teledermatology, teledentistry, and teleophthalmology. Connecticut allows for store-and-forward payment between physicians through email. Missouri allows for store-and-forward services in orthopedics, dermatology, optometry, ophthalmology, and in cases of retinopathy, burn and wound care, dental services, and maternal-fetal ultrasounds.
Reimbursement for telehealth is still a challenge for many physicians, Dr. Sumner said. Part of the problem is the wide discrepancy in how telehealth is defined among states, she said. Some states only consider live or interactive two-way technology as telehealth, excluding services such as store-and-forward and RPM. Other states reimburse for certain technology-based services, but they do not consider them telehealth. Maryland’s Medicaid program for example, does not reimburse for store and forward under its telehealth policy. However, store and forward used in dermatology, radiology, and ophthalmology is reimbursed by Maryland under an alternate billing code, though not considered telehealth.
Establishing best practices in telehealth through careful evaluation and research would improve reimbursement, said S. David McSwain, MD, interim chief medical information officer at the Medical University of South Carolina, Charleston, and medical director for telehealth optimization.
“We can use that evidence to reduce the variation in telehealth payment policies across the states,” Dr. McSwain said in an interview. “By leveraging reimbursement models to promote best practices, we can encourage the spread of telehealth services that have the greatest impacts on patients, their families, and the health care system.”
More states are expanding their telehealth policies to reach patients, and pulling back on rigid in-person requirements.
Several state Medicaid programs now explicitly allow the home to serve as an originating site for telehealth, with 10 states – Delaware, Colorado, Maryland, Michigan, Minnesota, Montana, New York, Texas, Washington, and Wyoming – adding the home as an approved site since 2016.
In addition, 16 jurisdictions now allow schools to serve as originating sites for telehealth, although some have restrictions about when the sites are acceptable, said Mei Kwong, an attorney and executive director for the Center for Connected Health Policy and the author of the center’s Spring 2018 report on telehealth trends.
At the same time, nearly all states have now dropped Medicaid restrictions that limited reimbursable telehealth services to rural or underserved areas. Colorado, Idaho, Nebraska, New Hampshire, Nevada, and Missouri are the most recent states to remove such geographic restrictions.
“[The expanded locations are] extremely helpful in providing greater access for patients to needed services,” Ms. Kwong said in an interview. “For example, a person who has difficulty leaving his or her home for a physical or other reason, they can get care, [or] a child having a rough time in school, can seek out a mental health counselor while at school.”
More telehealth providers
In addition to expanding teleheath sites, states are increasing acceptance for telehealth providers beyond physicians. Most recently, New Jersey enacted a broad telemedicine law that includes doctors, nurses, psychologists, social workers, physician assistants, counselors, respiratory therapists, speech pathologists, and optometrists, among others. The New Jersey law addresses telemedicine practice standards, prescribing, patient consent, privacy, and other requirements for providers.
In addition, more states are carving out telehealth regulations. Since 2016, 11 states have revised or adopted new scope of practice restrictions for counselors providing telemedicine, according to a state telehealth analysis published in July 2018 by law firm Epstein, Becker, Green.
Arkansas, Idaho, Maine, New Jersey, and Rhode Island recently imposed regulations for the practice of telepsychology. In addition, seven states have new or revised scope of practice restrictions for advanced practice registered nurses (APRN) providing telehealth services, while eight states have new licensing requirements specific to telehealth practice by APRNs, according to the Epstein analysis.
A telehealth compact that would allow APRNs to practice nursing via telemedicine across state lines is also in the works. Similar to the physician Interstate Medical Licensure Compact, the APRN Compact would establish an interstate commission and guidelines for uniform licensing requirements and criminal background checks. The compact will become effective when 10 states enact the compact legislation. So far, three states – Wyoming, North Dakota, and Idaho – have enacted the model legislation.
Another telemedicine compact for psychologists is getting ready to launch. In August, Illinois became the seventh state to join the Psychology Interjurisdictional Compact (PSYPACT). The pact requires seven states to enact compact legislation to become effective, however Illinois law does not go into effect until 2020.
The developments highlight the rise in more mid- and lower-level providers practicing telemedicine, said Anjali B. Dooley, an attorney and chief legal and compliance officer for Forefront Telecare, a telehealth platform for behavioral health services. While the increase allows greater care access, the expansion also poses scope of practice challenges, she notes.
“Increasing scope of practice extensions also increases risk if physician extenders are not trained properly in telehealth technology use and protocols,” Ms. Dooley said in an interview. “Providers and provider extenders need to be educated and learned in human factors such as communication, empathy, and etiquette.”
A greater number of nonphysician telemedicine providers is beneficial as long as the providers are adhering to appropriate standards of care and consulting with supervising physicians when necessary, adds Jean R. Sumner, MD, dean of the School of Medicine at Mercer University in Macon, Georgia, and a telemedicine internist.
“The standard should always be equal to an in-person visit,” she said in an interview. “The patient has a right to know who is seeing them, too, to understand [their credentials]. They need to know the person on the telehealth unit is qualified to provide the care.”
Responding to the opioid crisis
The need for increased access to mental health care is a primary driver behind state efforts to expand the pool of telemedicine providers, adds Amy Lerman, an attorney at Epstein, Becker, Green and lead author of her firm’s report.
“The reason it is important for states to continue expanding the scope of health professionals, other than physicians, who can provide behavioral health telemedicine services, is not only to address an overall nationwide shortage of behavioral health providers, but also to expand access to behavioral health services because a wider range of providers are equipped to provide these services,” she said in an interview.
In the same vein, more states are using telehealth to address the opioid crisis, according to both the Epstein report and the Center for Connected Health Policy analysis.
In September, California enacted a law that would allow Medicaid reimbursement for certified substance use disorder counselors who provide treatment via telehealth. In August, Illinois approved a similar law that mandates reimbursement for behavioral and mental health experts who treat Medicaid patients through telehealth technologies.
The laws come after a June 2018 letter from the Centers for Medicare & Medicaid Services that encouraged states to utilize health technology efforts to address the opioid crisis, including through telemedicine and telepsychiatry, said Daniel Kim, an attorney with Epstein, Becker, Green and a coauthor of his firm’s report.
At the same time, a number of states have expanded their controlled substance laws to allow remote prescribing through telehealth for the treatment of psychiatric or substance use disorders. Connecticut’s law, for instance, allows providers to prescribe Schedule I-III controlled substances through telehealth platforms, while banning opioid prescribing. In Indiana, 2017 legislation expanded the types of controlled medications that providers can prescribe through telehealth platforms, primarily drugs used to treat or manage opioid dependence. The states join an increasing number that have enacted laws allowing the remote prescribing of controlled substances, including Delaware, Florida, Indiana, Michigan, New Hampshire, Ohio, and West Virginia.
The new laws will enhance the availability of behavioral health services, while allowing more treatment flexibility and privacy for patients, said Ms. Dooley.
“Treatment in one’s own environment where the addiction takes place is often more effective,” she said. People with addiction disorders “can also receive treatment without having to drive long distances.”
The disappearing in-person requirement
As states define their telehealth policies, they are fading out a once-prevalent requirement – the in-person visit. There is no longer a single state that requires physicians to meet with patients in-person before providing telemedicine services, according to the Epstein report.
States realized that requiring in-person visits before doctors can provide telemedicine creates a barrier to care, said Mr. Kim. A move to eliminate the requirement in Texas influenced other states in phasing out the common regulation. In the widely publicized Teladoc case, the national telemedicine company sued the Texas Medical Board in 2011 over its rule requiring Texas physicians to conduct a face-to-face evaluation before treating a patient via telemedicine. The legal battle continued for years, until Teladoc voluntarily dropped its lawsuit in 2017 after Texas adopted a new law that allowed doctors to treat first-time patients through telemedicine.
“The medical board [understood] that the in-person requirement wasn’t really a benefit to patients,” Mr. Kim said in an interview. “Once they changed it, a lot of other states have recognized the same and have moved toward getting rid of the requirement.”
In some states, midlevel providers still must see patients face-to-face before providing telehealth care. Arkansas for instance, requires that psychologists, counselors, and APRNs conduct an in-person exam before rendering telehealth. Professional boards in Colorado and Massachusetts recommend a face-to-face visit by midlevel providers as a best practice.
Reimbursement growing, but restrictions remain
Forty-nine states and the District of Columbia reimburse for some form of telehealth, mainly live video services. At least 20 states now pay providers for remote payment monitoring (RPM), according to the Center for Connected Health Policy report. The reimbursement is often restricted, however, to certain clinical conditions and/or rules that limit the type of monitoring device allowed. Colorado, for instance, only reimburses RPM for patients with congestive heart failure, chronic obstructive pulmonary disease, asthma, or diabetes and requires that the patient was hospitalized at least twice in the last 12 months for reasons associated with one of the conditions. Missouri has similar RPM criteria associated with hospitalizations, but allows for a greater number of conditions including pregnancy, stroke, and cancer.
Most states have yet to pay for store-and-forward services, technologies that enable the electronic transfer of photos, prerecorded videos, or documents. Only about 14 states reimburse for such technology, and many policies include limitations. California, for example, only reimburses for store-and-forward services in teledermatology, teledentistry, and teleophthalmology. Connecticut allows for store-and-forward payment between physicians through email. Missouri allows for store-and-forward services in orthopedics, dermatology, optometry, ophthalmology, and in cases of retinopathy, burn and wound care, dental services, and maternal-fetal ultrasounds.
Reimbursement for telehealth is still a challenge for many physicians, Dr. Sumner said. Part of the problem is the wide discrepancy in how telehealth is defined among states, she said. Some states only consider live or interactive two-way technology as telehealth, excluding services such as store-and-forward and RPM. Other states reimburse for certain technology-based services, but they do not consider them telehealth. Maryland’s Medicaid program for example, does not reimburse for store and forward under its telehealth policy. However, store and forward used in dermatology, radiology, and ophthalmology is reimbursed by Maryland under an alternate billing code, though not considered telehealth.
Establishing best practices in telehealth through careful evaluation and research would improve reimbursement, said S. David McSwain, MD, interim chief medical information officer at the Medical University of South Carolina, Charleston, and medical director for telehealth optimization.
“We can use that evidence to reduce the variation in telehealth payment policies across the states,” Dr. McSwain said in an interview. “By leveraging reimbursement models to promote best practices, we can encourage the spread of telehealth services that have the greatest impacts on patients, their families, and the health care system.”
Beware drug reactions from methotrexate, voriconazole, and BRAF inhibitors
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
MONTEREY, CALIF. – Cutaneous necrosis. Porphyria cutanea tarda, accelerated photoaging, and actinic keratosis (AK). Cutaneous keratinocytic neoplasias. Two drugs – and a class of drugs commonly used in oncologic dermatology – can produce these skin conditions, a dermatologist cautioned his colleagues.
J. Mark Jackson, MD, of the University of Louisville (Ky.), highlighted these drug reactions in a presentation at the annual Coastal Dermatology Symposium. The
Dr. Jackson referred to reports of cutaneous necrosis associated with methotrexate and highlighted a 2017 case series that compared 24 patients who developed the condition with a control population of patients taking methotrexate who did not develop it. The patients with this reaction were more likely to be older, had a higher starting dose, and had signs of kidney problems. They were also less likely to be taking folic acid supplements (J Am Acad Dermatol. 2017 Aug;77[2]:247-55.e2).
“It’s pretty alarming,” he said. “They look like Stevens-Johnson syndrome/TEN [toxic epidermal necrolysis], but the pathology was differentiated,” he pointed out.
He cautioned, though, that this is not “a typical reaction.”
The oral antifungal drug voriconazole is often used in immunosuppressed patients, such as transplant patients, either as prophylaxis or therapy. It is highly photosensitizing and has been linked to porphyria cutanea tarda, accelerated photoaging, development of AKs, and aggressive cutaneous squamous cell carcinoma (Am J Transplant 2008 Apr;8[4]:877-80; AIDS. 2008 Apr 23;22[7]:905-6; J Am Acad Dermatol. 2010 Jan;62[1]:31-7; Dermatol Surg. 2010 Nov;36[11]:1752-5).
The risk of nonmelanoma skin cancer may be quadrupled in patients who take this medication, Dr. Jackson said.
There also are reports of patients on voriconazole developing tense bullae that are suggestive of porphyria cutanea tarda but with normal porphyrin levels, he said. This resolves over time, once therapy has ceased.
The BRAF inhibitor chemotherapy drugs – vemurafenib (Zelboraf), dabrafenib (Tafinlar), and encorafenib (Braftovi) – are used to treat metastatic melanoma. They’ve been linked to rash and cutaneous keratinocytic neoplasias. Patients on these agents should be “closely monitored” for these conditions (Chem Immunol Allergy. 2012;97:191-202). Dr. Jackson emphasized the importance of photoprotection with these patients and noted that it’s crucial to see these patients every month because neoplasias can develop quickly, even within 4 weeks of starting the medication.
The Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
Dr. Jackson reported relationships with AbbVie, Accuitis, Aclaris, Galderma, Janssen, Lilly, Medimetriks, Novartis, Promius, Ralexar, and TopMD.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
In utero efavirenz, dolutegravir exposure linked to childhood neurologic problems
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
SAN FRANCISCO – , according to a review of 3,747 children in the Surveillance Monitoring for ART Toxicities (SMARTT) study, an ongoing effort to monitor children exposed to antiretrovirals in the womb.
Overall, 237 children developed a neurologic complication at a mean age of 2; 16 of them were exposed to efavirenz. The study team estimated that 9.6% of children exposed to efavirenz had a neurological complication, versus 6.2% born to women on ART regimens without efavirenz. There was also a nonsignificant trend toward dolutegravir exposure and later neurological abnormalities, which occurred in four of 94 children exposed to the drug. Results were adjusted for maternal smoking and other risk factors.
No other safety signals were detected with the 19 other antiretrovirals analyzed in the study, lead investigator Claudia S. Crowell, MD, assistant professor of pediatrics at the University of Washington, Seattle, said at the annual scientific meeting on infectious diseases.
Efavirenz isn’t used much in the United States because there are more effective options with fewer side effects, but current guidelines recommend that women who are doing well on the drug stay on it while pregnant. Meanwhile, dolutegravir exposure at the time of conception was recently linked to an increased risk of neural tube defects in infants. The drug is commonly used in the United States, and guidelines have been strengthened to highlight the need for contraception use by women taking dolutegravir.
Dr. Crowell said she was surprised by her study’s findings, in part because efavirenz is not a teratogen. The work highlights how important it is to look beyond birth defects and follow children exposed to antiretrovirals for later problems. “We still haven’t determined what the safest regimen is for use in pregnancy,” she said.
Dr. Crowell explained the problem, and what her work means for practice in an interview at the meeting.
SOURCE: Crowell C et al. ID Week 2018 abstract LB5.
REPORTING FROM ID WEEK 2018
Soccer or Football Medicine? Global Sports Medicine for a Global Game
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
It’s time for universal CMV screening at birth
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
SAN FRANCISCO –
The reason is because most of the time the diagnosis of congenital cytomegalovirus is missed. Only about 10% of infants infected with the virus present with enlarged livers and other classic signs. Too often, the infection isn’t caught until later, when hearing loss and other neurologic sequelae reveal themselves, according to Fatima Kakkar, MD, a pediatric infectious disease specialist and researcher at the University of Montreal.
There are effective treatments – intravenous ganciclovir for 6 weeks or oral valganciclovir (Valcyte) for 6 months – that control the infection and reverse its effects.
People have tried to address the situation by screening children with hearing loss, in utero HIV exposure, or cytomegalovirus symptoms, but in a study Dr. Kakkar presented at IDWeek, an annual scientific meeting on infectious diseases, such targeted efforts still missed a lot of children.
Many think the answer is universal screening, and the Centers for Disease Control and Prevention are considering it. In a video interview at the meeting, Dr. Kakkar explained the issues, her study, and why universal screening is gaining support.
SOURCE: Kakkar F et al. IDWeek 2018, Abstract 115.
REPORTING FROM IDWEEK 2018