User login
Predicting treatment response in leiomyosarcoma, liposarcoma
Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma, based on an analysis of whole-exome sequencing of tumor samples from patients in a completed phase 3 randomized trial comparing trabectedin and dacarbazine.
In that trial, trabectedin benefit was mostly seen in patients with leiomyosarcoma, as well as in patients with myxoid/round cell sarcomas, and less so in those with dedifferentiated and pleomorphic liposarcomas.
Gurpreet Kapoor, PhD, of LabConnect, Seattle, and colleagues examined aberrations in oncogenic pathways (DNA damage response, PI3K, MDM2-p53) and in immune modulation and then correlated the genomic aberrations with prospective data on clinical outcomes in the trial.
For the study, presented at the annual meeting of the American Society of Clinical Oncology in Chicago, archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas.
Peripheral blood samples from a subset of 346 patients were also analyzed as matched normal to filter noise from nonpathogenic variants in the whole-exome sequencing.
Consistent with sarcoma data from The Cancer Genome Atlas, frequent homozygous gene deletions with relatively low mutational load were noted in these leiomyosarcoma and liposarcoma samples. TP53 and RB1 alterations were more frequent in leiomyosarcomas than in liposarcomas and were not associated with clinical outcomes. Analyses of 103 DNA damage-response genes found somatic alterations exceeded 20% across subtypes and correlated with improved progression-free survival in only uterine leiomyosarcomas (hazard ratio, 0.63; P = .03).
Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
A trend towards better overall survival was noted in dedifferentiated liposarcoma patients with MDM2 amplification as compared with normal MDM2 copy number.
Certain subtype-specific genomic aberrations in immune modulation pathways were associated with worse clinical outcomes in patients with uterine leiomyosarcoma or dedifferentiated liposarcoma. Alterations in immune suppressors were associated with improved clinical outcomes in nonuterine leiomyosarcomas and alterations in lipid metabolism were associated with improved clinical outcomes in dedifferentiated liposarcomas.
The invited discussant for the study, Mark Andrew Dickson, MD, of Memorial Sloan Kettering Cancer Center, New York, noted that “the real take-home here is that the TMBs (tumor mutation burdens) are relatively low across all of the L-type sarcomas.
“The pattern and prevalence of genomic aberrations that we’re seeing in this cohort of patients prospectively analyzed on a clinical trial are consistent with prior reports. ... including CDK4 and MDM2 in dedifferentiated liposarcoma, PI3-kinase in some myxoid/round cells, p53 in leiomyosarcoma and liposarcoma, and so on.”
Generally, tumor mutation burden is low in L-type sarcomas, and there are some intriguing associations with benefit to therapies, such as PI3-kinase pathway and potential resistance to trabectedin and high tumor mutation burden and potential sensitivity to trabectedin, that need to be explored and validated in another larger cohort, he said.
“I also am increasingly coming to terms with the fact that the tumors like leiomyosarcoma, which have low tumor mutation burden, and which so far have proven fairly immune to immunotherapy, based on all of the negative PD-1 data that we’ve seen, and that also have recurrent, relatively unactionable mutations, like p53 and Rb, remain very difficult to treat,” Dr. Dickson concluded.
SOURCE: Kapoor G et al. ASCO 2018, Abstract 11513.
Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma, based on an analysis of whole-exome sequencing of tumor samples from patients in a completed phase 3 randomized trial comparing trabectedin and dacarbazine.
In that trial, trabectedin benefit was mostly seen in patients with leiomyosarcoma, as well as in patients with myxoid/round cell sarcomas, and less so in those with dedifferentiated and pleomorphic liposarcomas.
Gurpreet Kapoor, PhD, of LabConnect, Seattle, and colleagues examined aberrations in oncogenic pathways (DNA damage response, PI3K, MDM2-p53) and in immune modulation and then correlated the genomic aberrations with prospective data on clinical outcomes in the trial.
For the study, presented at the annual meeting of the American Society of Clinical Oncology in Chicago, archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas.
Peripheral blood samples from a subset of 346 patients were also analyzed as matched normal to filter noise from nonpathogenic variants in the whole-exome sequencing.
Consistent with sarcoma data from The Cancer Genome Atlas, frequent homozygous gene deletions with relatively low mutational load were noted in these leiomyosarcoma and liposarcoma samples. TP53 and RB1 alterations were more frequent in leiomyosarcomas than in liposarcomas and were not associated with clinical outcomes. Analyses of 103 DNA damage-response genes found somatic alterations exceeded 20% across subtypes and correlated with improved progression-free survival in only uterine leiomyosarcomas (hazard ratio, 0.63; P = .03).
Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
A trend towards better overall survival was noted in dedifferentiated liposarcoma patients with MDM2 amplification as compared with normal MDM2 copy number.
Certain subtype-specific genomic aberrations in immune modulation pathways were associated with worse clinical outcomes in patients with uterine leiomyosarcoma or dedifferentiated liposarcoma. Alterations in immune suppressors were associated with improved clinical outcomes in nonuterine leiomyosarcomas and alterations in lipid metabolism were associated with improved clinical outcomes in dedifferentiated liposarcomas.
The invited discussant for the study, Mark Andrew Dickson, MD, of Memorial Sloan Kettering Cancer Center, New York, noted that “the real take-home here is that the TMBs (tumor mutation burdens) are relatively low across all of the L-type sarcomas.
“The pattern and prevalence of genomic aberrations that we’re seeing in this cohort of patients prospectively analyzed on a clinical trial are consistent with prior reports. ... including CDK4 and MDM2 in dedifferentiated liposarcoma, PI3-kinase in some myxoid/round cells, p53 in leiomyosarcoma and liposarcoma, and so on.”
Generally, tumor mutation burden is low in L-type sarcomas, and there are some intriguing associations with benefit to therapies, such as PI3-kinase pathway and potential resistance to trabectedin and high tumor mutation burden and potential sensitivity to trabectedin, that need to be explored and validated in another larger cohort, he said.
“I also am increasingly coming to terms with the fact that the tumors like leiomyosarcoma, which have low tumor mutation burden, and which so far have proven fairly immune to immunotherapy, based on all of the negative PD-1 data that we’ve seen, and that also have recurrent, relatively unactionable mutations, like p53 and Rb, remain very difficult to treat,” Dr. Dickson concluded.
SOURCE: Kapoor G et al. ASCO 2018, Abstract 11513.
Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma, based on an analysis of whole-exome sequencing of tumor samples from patients in a completed phase 3 randomized trial comparing trabectedin and dacarbazine.
In that trial, trabectedin benefit was mostly seen in patients with leiomyosarcoma, as well as in patients with myxoid/round cell sarcomas, and less so in those with dedifferentiated and pleomorphic liposarcomas.
Gurpreet Kapoor, PhD, of LabConnect, Seattle, and colleagues examined aberrations in oncogenic pathways (DNA damage response, PI3K, MDM2-p53) and in immune modulation and then correlated the genomic aberrations with prospective data on clinical outcomes in the trial.
For the study, presented at the annual meeting of the American Society of Clinical Oncology in Chicago, archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas.
Peripheral blood samples from a subset of 346 patients were also analyzed as matched normal to filter noise from nonpathogenic variants in the whole-exome sequencing.
Consistent with sarcoma data from The Cancer Genome Atlas, frequent homozygous gene deletions with relatively low mutational load were noted in these leiomyosarcoma and liposarcoma samples. TP53 and RB1 alterations were more frequent in leiomyosarcomas than in liposarcomas and were not associated with clinical outcomes. Analyses of 103 DNA damage-response genes found somatic alterations exceeded 20% across subtypes and correlated with improved progression-free survival in only uterine leiomyosarcomas (hazard ratio, 0.63; P = .03).
Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
A trend towards better overall survival was noted in dedifferentiated liposarcoma patients with MDM2 amplification as compared with normal MDM2 copy number.
Certain subtype-specific genomic aberrations in immune modulation pathways were associated with worse clinical outcomes in patients with uterine leiomyosarcoma or dedifferentiated liposarcoma. Alterations in immune suppressors were associated with improved clinical outcomes in nonuterine leiomyosarcomas and alterations in lipid metabolism were associated with improved clinical outcomes in dedifferentiated liposarcomas.
The invited discussant for the study, Mark Andrew Dickson, MD, of Memorial Sloan Kettering Cancer Center, New York, noted that “the real take-home here is that the TMBs (tumor mutation burdens) are relatively low across all of the L-type sarcomas.
“The pattern and prevalence of genomic aberrations that we’re seeing in this cohort of patients prospectively analyzed on a clinical trial are consistent with prior reports. ... including CDK4 and MDM2 in dedifferentiated liposarcoma, PI3-kinase in some myxoid/round cells, p53 in leiomyosarcoma and liposarcoma, and so on.”
Generally, tumor mutation burden is low in L-type sarcomas, and there are some intriguing associations with benefit to therapies, such as PI3-kinase pathway and potential resistance to trabectedin and high tumor mutation burden and potential sensitivity to trabectedin, that need to be explored and validated in another larger cohort, he said.
“I also am increasingly coming to terms with the fact that the tumors like leiomyosarcoma, which have low tumor mutation burden, and which so far have proven fairly immune to immunotherapy, based on all of the negative PD-1 data that we’ve seen, and that also have recurrent, relatively unactionable mutations, like p53 and Rb, remain very difficult to treat,” Dr. Dickson concluded.
SOURCE: Kapoor G et al. ASCO 2018, Abstract 11513.
REPORTING FROM ASCO 2018
Key clinical point: Aberrations in oncogenic pathways and immune modulation influence treatment response in patients with metastatic leiomyosarcoma or liposarcoma.
Major finding: Genomic alterations in PI3K pathway genes were noted in 30% of myxoid liposarcomas and were associated with a worse rate of progression-free survival (HR, 3.0; P = .045).
Study details: Archival tumor samples were collected from 456 of the 518 patients; 180 had uterine leiomyosarcomas, 149 had nonuterine leiomyosarcomas, 66 had dedifferentiated liposarcomas, 46 had myxoid liposarcomas, and 15 had pleomorphic liposarcomas in the completed phase 3 randomized trial comparing trabectedin and dacarbazine.
Disclosures: Dr. Kapoor is employed by LabConnect, Seattle. Research funding was supplied by Janssen Research & Development.
Source: Kapoor G et al. ASCO 2018, Abstract 11513.
Sexual assault, harassment linked to depression
Also today, experts recommend anti-TNFs for severe psoriasis in pregnancy, the FDA approves first-of-its-kind lung disease treatment, and do you know what the five oncological emergencies are?
Amazon Alexa
Apple Podcasts
Spotify
Also today, experts recommend anti-TNFs for severe psoriasis in pregnancy, the FDA approves first-of-its-kind lung disease treatment, and do you know what the five oncological emergencies are?
Amazon Alexa
Apple Podcasts
Spotify
Also today, experts recommend anti-TNFs for severe psoriasis in pregnancy, the FDA approves first-of-its-kind lung disease treatment, and do you know what the five oncological emergencies are?
Amazon Alexa
Apple Podcasts
Spotify
Duvelisib bests ofatumumab as monotherapy for treatment of CLL/SLL
Final analysis of the phase 3 DUO trial has shown monotherapy with oral duvelisib results in a statistically significant improvement in progression-free survival (PFS) and overall response rate (ORR) compared to monotherapy with ofatumumab for patients with relapsed or refractory chronic lymphocytic leukemia/small lympchocytic lymphoma (CLL/SLL).
PFS for all patients as assessed by Independent Review Committee (IRC) was a median 13.3 months with duvelisib compared to 9.9 months with ofatumumab (P<0.0001).
ORR was significantly higher with duvelisib, 74% compared to 45%, P<0.0001, regardless of deletion 17p status.
Duvelisib (Copiktra™) was recently approved by the U.S. Food and Drug Administration for CLL/SLL based in part on this head-to-head trial.
The investigators reported the results in Blood.
"The way we treat patients with CLL is changing rapidly as we move from standard chemotherapy-based approaches to more targeted therapies," said principal investigator Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute in Nashville.
"Based on these data, duvelisib may offer a new treatment option for patients who otherwise may have limited options."
Duvelisib is an oral, dual inhibitor of phosphoinositide 3-kinase (PI3K)-δ and -γ, which means it blocks the survival and proliferation of malignant B cells and also disrupts the recruitment and differentiation of T cells and macrophages within the tumor microenvironment.
Ofatumumab is a humanized anti-CD20 antibody with single-agent efficacy in refractory CLL. It is approved by the FDA as a treatment option in CLL.
Study design
Investigators randomized 319 relapsed or refractory CLL/SLL patients, 160 to the duvelisib arm and 159 to the ofatumumab arm.
Patients in the duvelisib arm self-administered 25 mg capsules twice daily continuously in 28-day cycles. They could take duvelisib for up to 18 cycles, until disease progression or unacceptable toxicity.
Ofatumumab-treated patients received infusions as approved in the product labeling for monotherapy in relapsed CLL. Dosing of ofatumumab could not exceed 12 doses in 7 cycles.
Prophylaxis for Pneumocystis jirovecci was required for all patients on both treatment arms.
Patients were allowed to crossover to a separate extension study to receive the opposite therapy if they had progressive disease.
They were followed for a median of 22.4 months.
Patient characteristics
According to the investigators, patient characteristics were well balanced between the arms.
The majority (60%) were male and the median age in both arms was 69. Most had an ECOG performance status of 0 or 1; 7% in the duvelisib arm and 10% in the ofatumumab arm had a performance status of 2.
Other patient characteristics in the duvelisib and ofatumumab arms, respectively, were:
- Time from initial diagnosis: 7.5 years, 6.7 years
- CLL/SLL, %: 97/5, 99/2
- Bulky disease: 46%, 45%
- Baseline lymphocyte counts: 38x109/L, 35x109/L
- Deletion 17p and/or TP53 mutation: 31%, 33%
- Median number of prior therapies: 2 in each arm
- Previous alkylating agent: 93%, 95%
- Previous monoclonal antibody: 78%, 83%
- Prior purine analog: 60%, 71%
Of the total patients enrolled, 158 patients in the duvelisib arm and 155 in the ofatumumab arm received treatment, for a median exposure of 50 weeks and 23 weeks, respectively.
Efficacy
In addition to the significantly improved overall PFS and ORR with duvelisib, further analysis revealed that PFS also improved for all predefined subgroups.
High-risk patients with deletion 17p/TP53 mutations also experienced a significant improvement in PFS with duvelisib of 12.7 months compared to 9.0 months with ofatumumab by IRC (P=0.0002).
The estimated probability of being progression-free for these patients at 6 and 12 moths was 73% and 55% with duvelisib and 63% and 30% with ofatumumab.
The investigators pointed out that duvelisib treatment was particularly effective in eliciting a lymph node response—85.0% compared to 15.7% with ofatumumab as assessed by IRC (P<0.0001).
Median overall survival was not reached in either arm. The 12-month probability of survival was 86% for both treatments.
Safety
Median treatment exposure was almost twice as long in the duvelisib arm because ofatumumab treatment was not allowed to exceed 12 doses as specified in the prescribing information.
The investigators explained this resulted in a longer adverse event (AE) reporting period for duvelisib.
One hundred twenty-four duvelisib-treated patients discontinued treatment, most commonly due to AEs (35%), disease progression (22%), subject withdrawal (8%), and death (8%).
All ofatumumab-treated patients discontinued treatment by the time of data cutoff, and 67% had completed treatment as per protocol. Others discontinued due to disease progression (20%), subject withdrawal (5%), and AEs (4%).
Eight (5%) duvelisib patients crossed over to ofatumumab therapy at the time of disease progression, and 89 (57%) ofatumumab-treated patients crossed over to duvelisib.
Nearly all patients in both arms experienced an AE.
The most common hematologic malignancies with duvelisib and ofatumumab, respectively, occurring in 10% or more patients were neutropenia (33%, 21%), anemia (23%, 10%), and thrombocytopenia (15%, 6%).
The most common nonhematologic AES with duvelisib were diarrhea (51%), pyrexia (29%), nausea (23%), and cough (21%).
With ofatumumab, the most common nonhematologic AES were infusion-related reaction (19%), cough (14%), and diarrhea, rash, and fatigue (12% each).
Grade 3 or greater AEs occurred in 87% of duvelisib-treated patients and 48% in the ofatumumab arm.
The most common grade 3 or greater events with duvelisib were neutropenia (30%), diarrhea (15%), pneumonia (14%), and anemia (13%).
With ofatumumab, only neutropenia (17%) of grade 3 or higher occurred in 10% or more patients.
Severe immune-related toxicities with duvelisib included colitis (12%) and pneumonitis, alanine transaminase (ALT) or aspartate transaminase (AST) increase (3% each). The events were managed with dose interruptions and steroid therapy for pneumonitis or colitis. All reported events resolved, and none was fatal.
Infectious AEs occurred more frequently with duvelisib, 69% compared to 43% in the ofatumumab arm. Pneumonia (18%) and upper respiratory tract infection (16%) were the most common events.
Three patients in the duvelisib arm and 1 in the ofatumumab arm contracted Pneumocystis jirovecii.
The most frequently reported serious AE was pneumonia (duvelisib 15%; ofatumumab 3%).
Nineteen fatal AEs occurred in patients on the duvelisib arm, 4 of which were related to the study drug: staphylococcal pneumonia (n = 2), sepsis (n=1), and general health deterioration (n = 1).
Seven fatal AEs occurred in patients on the ofatumumab arm, although none was attributed to ofatumumab.
The DUO trial was sponsored by Verastem Oncology and Infinity Pharmaceuticals , Inc.
Final analysis of the phase 3 DUO trial has shown monotherapy with oral duvelisib results in a statistically significant improvement in progression-free survival (PFS) and overall response rate (ORR) compared to monotherapy with ofatumumab for patients with relapsed or refractory chronic lymphocytic leukemia/small lympchocytic lymphoma (CLL/SLL).
PFS for all patients as assessed by Independent Review Committee (IRC) was a median 13.3 months with duvelisib compared to 9.9 months with ofatumumab (P<0.0001).
ORR was significantly higher with duvelisib, 74% compared to 45%, P<0.0001, regardless of deletion 17p status.
Duvelisib (Copiktra™) was recently approved by the U.S. Food and Drug Administration for CLL/SLL based in part on this head-to-head trial.
The investigators reported the results in Blood.
"The way we treat patients with CLL is changing rapidly as we move from standard chemotherapy-based approaches to more targeted therapies," said principal investigator Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute in Nashville.
"Based on these data, duvelisib may offer a new treatment option for patients who otherwise may have limited options."
Duvelisib is an oral, dual inhibitor of phosphoinositide 3-kinase (PI3K)-δ and -γ, which means it blocks the survival and proliferation of malignant B cells and also disrupts the recruitment and differentiation of T cells and macrophages within the tumor microenvironment.
Ofatumumab is a humanized anti-CD20 antibody with single-agent efficacy in refractory CLL. It is approved by the FDA as a treatment option in CLL.
Study design
Investigators randomized 319 relapsed or refractory CLL/SLL patients, 160 to the duvelisib arm and 159 to the ofatumumab arm.
Patients in the duvelisib arm self-administered 25 mg capsules twice daily continuously in 28-day cycles. They could take duvelisib for up to 18 cycles, until disease progression or unacceptable toxicity.
Ofatumumab-treated patients received infusions as approved in the product labeling for monotherapy in relapsed CLL. Dosing of ofatumumab could not exceed 12 doses in 7 cycles.
Prophylaxis for Pneumocystis jirovecci was required for all patients on both treatment arms.
Patients were allowed to crossover to a separate extension study to receive the opposite therapy if they had progressive disease.
They were followed for a median of 22.4 months.
Patient characteristics
According to the investigators, patient characteristics were well balanced between the arms.
The majority (60%) were male and the median age in both arms was 69. Most had an ECOG performance status of 0 or 1; 7% in the duvelisib arm and 10% in the ofatumumab arm had a performance status of 2.
Other patient characteristics in the duvelisib and ofatumumab arms, respectively, were:
- Time from initial diagnosis: 7.5 years, 6.7 years
- CLL/SLL, %: 97/5, 99/2
- Bulky disease: 46%, 45%
- Baseline lymphocyte counts: 38x109/L, 35x109/L
- Deletion 17p and/or TP53 mutation: 31%, 33%
- Median number of prior therapies: 2 in each arm
- Previous alkylating agent: 93%, 95%
- Previous monoclonal antibody: 78%, 83%
- Prior purine analog: 60%, 71%
Of the total patients enrolled, 158 patients in the duvelisib arm and 155 in the ofatumumab arm received treatment, for a median exposure of 50 weeks and 23 weeks, respectively.
Efficacy
In addition to the significantly improved overall PFS and ORR with duvelisib, further analysis revealed that PFS also improved for all predefined subgroups.
High-risk patients with deletion 17p/TP53 mutations also experienced a significant improvement in PFS with duvelisib of 12.7 months compared to 9.0 months with ofatumumab by IRC (P=0.0002).
The estimated probability of being progression-free for these patients at 6 and 12 moths was 73% and 55% with duvelisib and 63% and 30% with ofatumumab.
The investigators pointed out that duvelisib treatment was particularly effective in eliciting a lymph node response—85.0% compared to 15.7% with ofatumumab as assessed by IRC (P<0.0001).
Median overall survival was not reached in either arm. The 12-month probability of survival was 86% for both treatments.
Safety
Median treatment exposure was almost twice as long in the duvelisib arm because ofatumumab treatment was not allowed to exceed 12 doses as specified in the prescribing information.
The investigators explained this resulted in a longer adverse event (AE) reporting period for duvelisib.
One hundred twenty-four duvelisib-treated patients discontinued treatment, most commonly due to AEs (35%), disease progression (22%), subject withdrawal (8%), and death (8%).
All ofatumumab-treated patients discontinued treatment by the time of data cutoff, and 67% had completed treatment as per protocol. Others discontinued due to disease progression (20%), subject withdrawal (5%), and AEs (4%).
Eight (5%) duvelisib patients crossed over to ofatumumab therapy at the time of disease progression, and 89 (57%) ofatumumab-treated patients crossed over to duvelisib.
Nearly all patients in both arms experienced an AE.
The most common hematologic malignancies with duvelisib and ofatumumab, respectively, occurring in 10% or more patients were neutropenia (33%, 21%), anemia (23%, 10%), and thrombocytopenia (15%, 6%).
The most common nonhematologic AES with duvelisib were diarrhea (51%), pyrexia (29%), nausea (23%), and cough (21%).
With ofatumumab, the most common nonhematologic AES were infusion-related reaction (19%), cough (14%), and diarrhea, rash, and fatigue (12% each).
Grade 3 or greater AEs occurred in 87% of duvelisib-treated patients and 48% in the ofatumumab arm.
The most common grade 3 or greater events with duvelisib were neutropenia (30%), diarrhea (15%), pneumonia (14%), and anemia (13%).
With ofatumumab, only neutropenia (17%) of grade 3 or higher occurred in 10% or more patients.
Severe immune-related toxicities with duvelisib included colitis (12%) and pneumonitis, alanine transaminase (ALT) or aspartate transaminase (AST) increase (3% each). The events were managed with dose interruptions and steroid therapy for pneumonitis or colitis. All reported events resolved, and none was fatal.
Infectious AEs occurred more frequently with duvelisib, 69% compared to 43% in the ofatumumab arm. Pneumonia (18%) and upper respiratory tract infection (16%) were the most common events.
Three patients in the duvelisib arm and 1 in the ofatumumab arm contracted Pneumocystis jirovecii.
The most frequently reported serious AE was pneumonia (duvelisib 15%; ofatumumab 3%).
Nineteen fatal AEs occurred in patients on the duvelisib arm, 4 of which were related to the study drug: staphylococcal pneumonia (n = 2), sepsis (n=1), and general health deterioration (n = 1).
Seven fatal AEs occurred in patients on the ofatumumab arm, although none was attributed to ofatumumab.
The DUO trial was sponsored by Verastem Oncology and Infinity Pharmaceuticals , Inc.
Final analysis of the phase 3 DUO trial has shown monotherapy with oral duvelisib results in a statistically significant improvement in progression-free survival (PFS) and overall response rate (ORR) compared to monotherapy with ofatumumab for patients with relapsed or refractory chronic lymphocytic leukemia/small lympchocytic lymphoma (CLL/SLL).
PFS for all patients as assessed by Independent Review Committee (IRC) was a median 13.3 months with duvelisib compared to 9.9 months with ofatumumab (P<0.0001).
ORR was significantly higher with duvelisib, 74% compared to 45%, P<0.0001, regardless of deletion 17p status.
Duvelisib (Copiktra™) was recently approved by the U.S. Food and Drug Administration for CLL/SLL based in part on this head-to-head trial.
The investigators reported the results in Blood.
"The way we treat patients with CLL is changing rapidly as we move from standard chemotherapy-based approaches to more targeted therapies," said principal investigator Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute in Nashville.
"Based on these data, duvelisib may offer a new treatment option for patients who otherwise may have limited options."
Duvelisib is an oral, dual inhibitor of phosphoinositide 3-kinase (PI3K)-δ and -γ, which means it blocks the survival and proliferation of malignant B cells and also disrupts the recruitment and differentiation of T cells and macrophages within the tumor microenvironment.
Ofatumumab is a humanized anti-CD20 antibody with single-agent efficacy in refractory CLL. It is approved by the FDA as a treatment option in CLL.
Study design
Investigators randomized 319 relapsed or refractory CLL/SLL patients, 160 to the duvelisib arm and 159 to the ofatumumab arm.
Patients in the duvelisib arm self-administered 25 mg capsules twice daily continuously in 28-day cycles. They could take duvelisib for up to 18 cycles, until disease progression or unacceptable toxicity.
Ofatumumab-treated patients received infusions as approved in the product labeling for monotherapy in relapsed CLL. Dosing of ofatumumab could not exceed 12 doses in 7 cycles.
Prophylaxis for Pneumocystis jirovecci was required for all patients on both treatment arms.
Patients were allowed to crossover to a separate extension study to receive the opposite therapy if they had progressive disease.
They were followed for a median of 22.4 months.
Patient characteristics
According to the investigators, patient characteristics were well balanced between the arms.
The majority (60%) were male and the median age in both arms was 69. Most had an ECOG performance status of 0 or 1; 7% in the duvelisib arm and 10% in the ofatumumab arm had a performance status of 2.
Other patient characteristics in the duvelisib and ofatumumab arms, respectively, were:
- Time from initial diagnosis: 7.5 years, 6.7 years
- CLL/SLL, %: 97/5, 99/2
- Bulky disease: 46%, 45%
- Baseline lymphocyte counts: 38x109/L, 35x109/L
- Deletion 17p and/or TP53 mutation: 31%, 33%
- Median number of prior therapies: 2 in each arm
- Previous alkylating agent: 93%, 95%
- Previous monoclonal antibody: 78%, 83%
- Prior purine analog: 60%, 71%
Of the total patients enrolled, 158 patients in the duvelisib arm and 155 in the ofatumumab arm received treatment, for a median exposure of 50 weeks and 23 weeks, respectively.
Efficacy
In addition to the significantly improved overall PFS and ORR with duvelisib, further analysis revealed that PFS also improved for all predefined subgroups.
High-risk patients with deletion 17p/TP53 mutations also experienced a significant improvement in PFS with duvelisib of 12.7 months compared to 9.0 months with ofatumumab by IRC (P=0.0002).
The estimated probability of being progression-free for these patients at 6 and 12 moths was 73% and 55% with duvelisib and 63% and 30% with ofatumumab.
The investigators pointed out that duvelisib treatment was particularly effective in eliciting a lymph node response—85.0% compared to 15.7% with ofatumumab as assessed by IRC (P<0.0001).
Median overall survival was not reached in either arm. The 12-month probability of survival was 86% for both treatments.
Safety
Median treatment exposure was almost twice as long in the duvelisib arm because ofatumumab treatment was not allowed to exceed 12 doses as specified in the prescribing information.
The investigators explained this resulted in a longer adverse event (AE) reporting period for duvelisib.
One hundred twenty-four duvelisib-treated patients discontinued treatment, most commonly due to AEs (35%), disease progression (22%), subject withdrawal (8%), and death (8%).
All ofatumumab-treated patients discontinued treatment by the time of data cutoff, and 67% had completed treatment as per protocol. Others discontinued due to disease progression (20%), subject withdrawal (5%), and AEs (4%).
Eight (5%) duvelisib patients crossed over to ofatumumab therapy at the time of disease progression, and 89 (57%) ofatumumab-treated patients crossed over to duvelisib.
Nearly all patients in both arms experienced an AE.
The most common hematologic malignancies with duvelisib and ofatumumab, respectively, occurring in 10% or more patients were neutropenia (33%, 21%), anemia (23%, 10%), and thrombocytopenia (15%, 6%).
The most common nonhematologic AES with duvelisib were diarrhea (51%), pyrexia (29%), nausea (23%), and cough (21%).
With ofatumumab, the most common nonhematologic AES were infusion-related reaction (19%), cough (14%), and diarrhea, rash, and fatigue (12% each).
Grade 3 or greater AEs occurred in 87% of duvelisib-treated patients and 48% in the ofatumumab arm.
The most common grade 3 or greater events with duvelisib were neutropenia (30%), diarrhea (15%), pneumonia (14%), and anemia (13%).
With ofatumumab, only neutropenia (17%) of grade 3 or higher occurred in 10% or more patients.
Severe immune-related toxicities with duvelisib included colitis (12%) and pneumonitis, alanine transaminase (ALT) or aspartate transaminase (AST) increase (3% each). The events were managed with dose interruptions and steroid therapy for pneumonitis or colitis. All reported events resolved, and none was fatal.
Infectious AEs occurred more frequently with duvelisib, 69% compared to 43% in the ofatumumab arm. Pneumonia (18%) and upper respiratory tract infection (16%) were the most common events.
Three patients in the duvelisib arm and 1 in the ofatumumab arm contracted Pneumocystis jirovecii.
The most frequently reported serious AE was pneumonia (duvelisib 15%; ofatumumab 3%).
Nineteen fatal AEs occurred in patients on the duvelisib arm, 4 of which were related to the study drug: staphylococcal pneumonia (n = 2), sepsis (n=1), and general health deterioration (n = 1).
Seven fatal AEs occurred in patients on the ofatumumab arm, although none was attributed to ofatumumab.
The DUO trial was sponsored by Verastem Oncology and Infinity Pharmaceuticals , Inc.
Emicizumab now also approved for hemophilia A without inhibitors
The U.S. Food and Drug Administration (FDA) approved emicizumab-kxwh (Hemlibra) for prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients, including newborns, with hemophilia A with or without factor VIII (FVIII) inhibitors.
Emicizumab is a humanized bispecific factor IXa- and factor X-directed antibody for patients with congenital FVIII deficiency.
It was first approved in 2017 for hemophilia A patients with FVIII inhibitors.
The current approval expands the indication to include patients without FVIII inhibitors and provides new dosing regimens.
The FDA based the current approval on the HAVEN 3 and HAVEN 4 trials.
HAVEN 3 (NCT02847637)
This multicenter trial randomized 89 patients with severe hemophilia A without FVIII inhibitors to receive emicizumab prophylaxis at one of 3 dose levels: 1.5 mg/kg once weekly (ARM A), 3 mg/kg once every two weeks (Arm B), or no prophylaxis (Arm C). Patients had previously received on-demand treatment with FVIII.
Before the start of the trial, investigators stratified patients by 24-week bleed rate—fewer than 9 bleeds and 9 or more bleeds.
Patients were treated with emicizumab for a minimum of 24 weeks.
Patients in Arm A experienced a 96% reduction in annualized bleed rate (ABR) compared to patients with no prophylaxis (ABR ratio=0.04; P<0.0001).
Patients in Arm B had a 97% reduction in ABR compared to patients with no prophylaxis (ABR ratio=0.03; P<0.0001).
The trial met all bleed-related secondary endpoints, such as all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds.
HAVEN 4 (NCT03020160)
This was a single-arm multicenter trial in 48 adult and adolescent males with hemophilia A with or without FVIII inhibitors. The patients had previously received on-demand or prophylactic treatment with FVIII or bypassing agents.
The study was conducted in two parts: a run-in of 7 patients to determine the pharmacokinetics after a single 6 mg/kg dose in four weeks. This was followed by the same dose once every four weeks for at least 24 weeks.
The second part was an expansion cohort of 41 patients who received emicizumab at 3 mg/kg once weekly for the first four weeks followed by 6 mg/kg once every four weeks for at least 24 weeks.
The ABR for treated bleeds was 2.4 (95% CI: 1.38, 4.28) and the median ABR was 0.0 (interquartile range: 0.00, 2.08).
The recommended loading dose is 3 mg/kg once weekly for the first four weeks for all prophylactic regimens.
Safety
The prescribing information for emicizumab includes a warning about thrombotic microangiopathy and thromboembolism.
These events were reported on average when a cumulative amount of >100 U/kg/24 hours of activated prothrombin complex concentrate (aPCC) was administered for 24 hours or more to patients receiving emicizumab prophylaxis.
According to the warning box, patients should be monitored for these events if aPCC is administered. If symptoms occur, aPCC should be discontinued and emicizumab dosing suspended.
The most common adverse reactions reported for emicizumab with an incidence ≥10% were injection site reactions (22%), headache (15%), and arthralgia (15%).
Emicizumab is manufactured by Genentech, Inc., a member of the Roche Group.
Additional data on emicizumab can be found in an earlier Roche media release.
The U.S. Food and Drug Administration (FDA) approved emicizumab-kxwh (Hemlibra) for prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients, including newborns, with hemophilia A with or without factor VIII (FVIII) inhibitors.
Emicizumab is a humanized bispecific factor IXa- and factor X-directed antibody for patients with congenital FVIII deficiency.
It was first approved in 2017 for hemophilia A patients with FVIII inhibitors.
The current approval expands the indication to include patients without FVIII inhibitors and provides new dosing regimens.
The FDA based the current approval on the HAVEN 3 and HAVEN 4 trials.
HAVEN 3 (NCT02847637)
This multicenter trial randomized 89 patients with severe hemophilia A without FVIII inhibitors to receive emicizumab prophylaxis at one of 3 dose levels: 1.5 mg/kg once weekly (ARM A), 3 mg/kg once every two weeks (Arm B), or no prophylaxis (Arm C). Patients had previously received on-demand treatment with FVIII.
Before the start of the trial, investigators stratified patients by 24-week bleed rate—fewer than 9 bleeds and 9 or more bleeds.
Patients were treated with emicizumab for a minimum of 24 weeks.
Patients in Arm A experienced a 96% reduction in annualized bleed rate (ABR) compared to patients with no prophylaxis (ABR ratio=0.04; P<0.0001).
Patients in Arm B had a 97% reduction in ABR compared to patients with no prophylaxis (ABR ratio=0.03; P<0.0001).
The trial met all bleed-related secondary endpoints, such as all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds.
HAVEN 4 (NCT03020160)
This was a single-arm multicenter trial in 48 adult and adolescent males with hemophilia A with or without FVIII inhibitors. The patients had previously received on-demand or prophylactic treatment with FVIII or bypassing agents.
The study was conducted in two parts: a run-in of 7 patients to determine the pharmacokinetics after a single 6 mg/kg dose in four weeks. This was followed by the same dose once every four weeks for at least 24 weeks.
The second part was an expansion cohort of 41 patients who received emicizumab at 3 mg/kg once weekly for the first four weeks followed by 6 mg/kg once every four weeks for at least 24 weeks.
The ABR for treated bleeds was 2.4 (95% CI: 1.38, 4.28) and the median ABR was 0.0 (interquartile range: 0.00, 2.08).
The recommended loading dose is 3 mg/kg once weekly for the first four weeks for all prophylactic regimens.
Safety
The prescribing information for emicizumab includes a warning about thrombotic microangiopathy and thromboembolism.
These events were reported on average when a cumulative amount of >100 U/kg/24 hours of activated prothrombin complex concentrate (aPCC) was administered for 24 hours or more to patients receiving emicizumab prophylaxis.
According to the warning box, patients should be monitored for these events if aPCC is administered. If symptoms occur, aPCC should be discontinued and emicizumab dosing suspended.
The most common adverse reactions reported for emicizumab with an incidence ≥10% were injection site reactions (22%), headache (15%), and arthralgia (15%).
Emicizumab is manufactured by Genentech, Inc., a member of the Roche Group.
Additional data on emicizumab can be found in an earlier Roche media release.
The U.S. Food and Drug Administration (FDA) approved emicizumab-kxwh (Hemlibra) for prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients, including newborns, with hemophilia A with or without factor VIII (FVIII) inhibitors.
Emicizumab is a humanized bispecific factor IXa- and factor X-directed antibody for patients with congenital FVIII deficiency.
It was first approved in 2017 for hemophilia A patients with FVIII inhibitors.
The current approval expands the indication to include patients without FVIII inhibitors and provides new dosing regimens.
The FDA based the current approval on the HAVEN 3 and HAVEN 4 trials.
HAVEN 3 (NCT02847637)
This multicenter trial randomized 89 patients with severe hemophilia A without FVIII inhibitors to receive emicizumab prophylaxis at one of 3 dose levels: 1.5 mg/kg once weekly (ARM A), 3 mg/kg once every two weeks (Arm B), or no prophylaxis (Arm C). Patients had previously received on-demand treatment with FVIII.
Before the start of the trial, investigators stratified patients by 24-week bleed rate—fewer than 9 bleeds and 9 or more bleeds.
Patients were treated with emicizumab for a minimum of 24 weeks.
Patients in Arm A experienced a 96% reduction in annualized bleed rate (ABR) compared to patients with no prophylaxis (ABR ratio=0.04; P<0.0001).
Patients in Arm B had a 97% reduction in ABR compared to patients with no prophylaxis (ABR ratio=0.03; P<0.0001).
The trial met all bleed-related secondary endpoints, such as all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds.
HAVEN 4 (NCT03020160)
This was a single-arm multicenter trial in 48 adult and adolescent males with hemophilia A with or without FVIII inhibitors. The patients had previously received on-demand or prophylactic treatment with FVIII or bypassing agents.
The study was conducted in two parts: a run-in of 7 patients to determine the pharmacokinetics after a single 6 mg/kg dose in four weeks. This was followed by the same dose once every four weeks for at least 24 weeks.
The second part was an expansion cohort of 41 patients who received emicizumab at 3 mg/kg once weekly for the first four weeks followed by 6 mg/kg once every four weeks for at least 24 weeks.
The ABR for treated bleeds was 2.4 (95% CI: 1.38, 4.28) and the median ABR was 0.0 (interquartile range: 0.00, 2.08).
The recommended loading dose is 3 mg/kg once weekly for the first four weeks for all prophylactic regimens.
Safety
The prescribing information for emicizumab includes a warning about thrombotic microangiopathy and thromboembolism.
These events were reported on average when a cumulative amount of >100 U/kg/24 hours of activated prothrombin complex concentrate (aPCC) was administered for 24 hours or more to patients receiving emicizumab prophylaxis.
According to the warning box, patients should be monitored for these events if aPCC is administered. If symptoms occur, aPCC should be discontinued and emicizumab dosing suspended.
The most common adverse reactions reported for emicizumab with an incidence ≥10% were injection site reactions (22%), headache (15%), and arthralgia (15%).
Emicizumab is manufactured by Genentech, Inc., a member of the Roche Group.
Additional data on emicizumab can be found in an earlier Roche media release.
No falls, fractures, or bone density benefits from vitamin D supplements
There is little justification for the use of vitamin D supplementation for the prevention of fractures or falls or for increasing bone density, according to the authors of a meta-analysis that found no benefits from supplementation.
A systematic review and meta-analysis, published in the Oct. 4 edition of Lancet Diabetes & Endocrinology, examined 81 randomized controlled trials – involving 53,537 participants – of the effects of vitamin D supplementation on fractures, falls, or bone mineral density.
In the pooled analyses, researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day. Their results were similar when researchers compared high doses and low doses in their trials.
Similarly, vitamin D supplementation was not associated with any clinically relevant improvements in bone mineral density at any site; lumbar spine, total hip, femoral neck, forearm, or total body.
Even a post hoc analysis of randomized, controlled trials that compared daily high doses with daily low doses, as well as trials that compared intermittent high doses with intermittent low doses found no significant interactions for any outcome.
The paper also explored whether baseline vitamin D levels might influence outcomes. Eighteen trials in the analysis reported the results of subgroup analyses using baseline serum 25-hydroxyvitamin D (25[OH]D); three found no effects of vitamin D supplements in different subgroups of baseline, five studies found no effects of subgroups or interaction with baseline serum 25[OH]D, and one found mixed effects with respect to falls.
The outcomes for bone mineral density, as related to baseline serum 25[OH]D, were slightly more mixed. One trial found a positive effect of vitamin D supplements a bone mineral density for different subgroups of baseline serum, five trials reported mixed effects, and eight trials found no effects.
“The strengths of the current analyses are that they are comprehensive, include all available data from a large number of new trials, and concomitantly assess the major clinical and surrogate endpoints for musculoskeletal health,” wrote Mark J. Bolland, MD, of the department of medicine at the University of Auckland (New Zealand), and his coauthors. “Therefore, there is little justification for the use of vitamin D supplements to maintain or improve musculoskeletal health, and clinical guidelines should reflect these findings.”
They also conducted trial sequential analyses, which is a type of cumulative meta-analysis. For each outcome, they set a relative risk reduction threshold, then progressively reduced that threshold until the optimum sample size for that threshold exceeded the actual sample size.
“The trial sequential analyses are important because they provide estimates about the reliability of current evidence and the likelihood of future trials to change current conclusions,” the authors wrote.
Using this approach, they once again found clear evidence that vitamin D supplementation did not reduce fractures or falls for any measure of relative risk reduction. For hip fracture, the trial sequential analysis even found some uncertainty as to whether vitamin D supplementation might increase the risk of hip fractures.
Given the results of the trial sequential analyses, the authors argued that further similar trials were unlikely to alter their conclusion.
“If a large future trial has markedly different results to the current trials, adding its results will substantially increase the heterogeneity of the trial results, which in turn will reduce the weighting the new large trial receives in the pooled analyses,” they wrote. “Thus, adding a positive result from a large randomized, controlled trial will have only a small effect on the pooled result and is unlikely to alter the conclusions of these meta-analyses.”
They also noted that some of the studies had methodological limitations, and smaller studies of shorter duration tended to have “inflated” effect sizes, such that “the results of small, short-duration studies should be interpreted very cautiously, since they might not be replicated in larger, longer studies.”
The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
SOURCE: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
While there have been more than a dozen meta-analyses exploring the effect of vitamin D supplements on fractures, falls, and bone mineral density, this latest one incorporates a large amount of new research information. It also comes at a time when vitamin D often is touted as a cure-all, both in research and on social media.
One of the unanswered questions is that the majority of the daily treatment groups in the studies involved doses less than 1,000 IU per day, so serum 25-hydroxyvitamin D (25[OH]D) concentrations may not have reached the range of interest.
There are still likely to be questions about the extraskeletal benefits of vitamin D supplementation, which may be answered by large randomized, controlled trials currently underway that are expected to report in the next few years.
J. Chris Gallagher, MD, is a professor at the Creighton University Medical Center, Omaha. These comments are taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587[18]30269-9). No conflicts of interest were declared.
While there have been more than a dozen meta-analyses exploring the effect of vitamin D supplements on fractures, falls, and bone mineral density, this latest one incorporates a large amount of new research information. It also comes at a time when vitamin D often is touted as a cure-all, both in research and on social media.
One of the unanswered questions is that the majority of the daily treatment groups in the studies involved doses less than 1,000 IU per day, so serum 25-hydroxyvitamin D (25[OH]D) concentrations may not have reached the range of interest.
There are still likely to be questions about the extraskeletal benefits of vitamin D supplementation, which may be answered by large randomized, controlled trials currently underway that are expected to report in the next few years.
J. Chris Gallagher, MD, is a professor at the Creighton University Medical Center, Omaha. These comments are taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587[18]30269-9). No conflicts of interest were declared.
While there have been more than a dozen meta-analyses exploring the effect of vitamin D supplements on fractures, falls, and bone mineral density, this latest one incorporates a large amount of new research information. It also comes at a time when vitamin D often is touted as a cure-all, both in research and on social media.
One of the unanswered questions is that the majority of the daily treatment groups in the studies involved doses less than 1,000 IU per day, so serum 25-hydroxyvitamin D (25[OH]D) concentrations may not have reached the range of interest.
There are still likely to be questions about the extraskeletal benefits of vitamin D supplementation, which may be answered by large randomized, controlled trials currently underway that are expected to report in the next few years.
J. Chris Gallagher, MD, is a professor at the Creighton University Medical Center, Omaha. These comments are taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587[18]30269-9). No conflicts of interest were declared.
There is little justification for the use of vitamin D supplementation for the prevention of fractures or falls or for increasing bone density, according to the authors of a meta-analysis that found no benefits from supplementation.
A systematic review and meta-analysis, published in the Oct. 4 edition of Lancet Diabetes & Endocrinology, examined 81 randomized controlled trials – involving 53,537 participants – of the effects of vitamin D supplementation on fractures, falls, or bone mineral density.
In the pooled analyses, researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day. Their results were similar when researchers compared high doses and low doses in their trials.
Similarly, vitamin D supplementation was not associated with any clinically relevant improvements in bone mineral density at any site; lumbar spine, total hip, femoral neck, forearm, or total body.
Even a post hoc analysis of randomized, controlled trials that compared daily high doses with daily low doses, as well as trials that compared intermittent high doses with intermittent low doses found no significant interactions for any outcome.
The paper also explored whether baseline vitamin D levels might influence outcomes. Eighteen trials in the analysis reported the results of subgroup analyses using baseline serum 25-hydroxyvitamin D (25[OH]D); three found no effects of vitamin D supplements in different subgroups of baseline, five studies found no effects of subgroups or interaction with baseline serum 25[OH]D, and one found mixed effects with respect to falls.
The outcomes for bone mineral density, as related to baseline serum 25[OH]D, were slightly more mixed. One trial found a positive effect of vitamin D supplements a bone mineral density for different subgroups of baseline serum, five trials reported mixed effects, and eight trials found no effects.
“The strengths of the current analyses are that they are comprehensive, include all available data from a large number of new trials, and concomitantly assess the major clinical and surrogate endpoints for musculoskeletal health,” wrote Mark J. Bolland, MD, of the department of medicine at the University of Auckland (New Zealand), and his coauthors. “Therefore, there is little justification for the use of vitamin D supplements to maintain or improve musculoskeletal health, and clinical guidelines should reflect these findings.”
They also conducted trial sequential analyses, which is a type of cumulative meta-analysis. For each outcome, they set a relative risk reduction threshold, then progressively reduced that threshold until the optimum sample size for that threshold exceeded the actual sample size.
“The trial sequential analyses are important because they provide estimates about the reliability of current evidence and the likelihood of future trials to change current conclusions,” the authors wrote.
Using this approach, they once again found clear evidence that vitamin D supplementation did not reduce fractures or falls for any measure of relative risk reduction. For hip fracture, the trial sequential analysis even found some uncertainty as to whether vitamin D supplementation might increase the risk of hip fractures.
Given the results of the trial sequential analyses, the authors argued that further similar trials were unlikely to alter their conclusion.
“If a large future trial has markedly different results to the current trials, adding its results will substantially increase the heterogeneity of the trial results, which in turn will reduce the weighting the new large trial receives in the pooled analyses,” they wrote. “Thus, adding a positive result from a large randomized, controlled trial will have only a small effect on the pooled result and is unlikely to alter the conclusions of these meta-analyses.”
They also noted that some of the studies had methodological limitations, and smaller studies of shorter duration tended to have “inflated” effect sizes, such that “the results of small, short-duration studies should be interpreted very cautiously, since they might not be replicated in larger, longer studies.”
The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
SOURCE: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
There is little justification for the use of vitamin D supplementation for the prevention of fractures or falls or for increasing bone density, according to the authors of a meta-analysis that found no benefits from supplementation.
A systematic review and meta-analysis, published in the Oct. 4 edition of Lancet Diabetes & Endocrinology, examined 81 randomized controlled trials – involving 53,537 participants – of the effects of vitamin D supplementation on fractures, falls, or bone mineral density.
In the pooled analyses, researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day. Their results were similar when researchers compared high doses and low doses in their trials.
Similarly, vitamin D supplementation was not associated with any clinically relevant improvements in bone mineral density at any site; lumbar spine, total hip, femoral neck, forearm, or total body.
Even a post hoc analysis of randomized, controlled trials that compared daily high doses with daily low doses, as well as trials that compared intermittent high doses with intermittent low doses found no significant interactions for any outcome.
The paper also explored whether baseline vitamin D levels might influence outcomes. Eighteen trials in the analysis reported the results of subgroup analyses using baseline serum 25-hydroxyvitamin D (25[OH]D); three found no effects of vitamin D supplements in different subgroups of baseline, five studies found no effects of subgroups or interaction with baseline serum 25[OH]D, and one found mixed effects with respect to falls.
The outcomes for bone mineral density, as related to baseline serum 25[OH]D, were slightly more mixed. One trial found a positive effect of vitamin D supplements a bone mineral density for different subgroups of baseline serum, five trials reported mixed effects, and eight trials found no effects.
“The strengths of the current analyses are that they are comprehensive, include all available data from a large number of new trials, and concomitantly assess the major clinical and surrogate endpoints for musculoskeletal health,” wrote Mark J. Bolland, MD, of the department of medicine at the University of Auckland (New Zealand), and his coauthors. “Therefore, there is little justification for the use of vitamin D supplements to maintain or improve musculoskeletal health, and clinical guidelines should reflect these findings.”
They also conducted trial sequential analyses, which is a type of cumulative meta-analysis. For each outcome, they set a relative risk reduction threshold, then progressively reduced that threshold until the optimum sample size for that threshold exceeded the actual sample size.
“The trial sequential analyses are important because they provide estimates about the reliability of current evidence and the likelihood of future trials to change current conclusions,” the authors wrote.
Using this approach, they once again found clear evidence that vitamin D supplementation did not reduce fractures or falls for any measure of relative risk reduction. For hip fracture, the trial sequential analysis even found some uncertainty as to whether vitamin D supplementation might increase the risk of hip fractures.
Given the results of the trial sequential analyses, the authors argued that further similar trials were unlikely to alter their conclusion.
“If a large future trial has markedly different results to the current trials, adding its results will substantially increase the heterogeneity of the trial results, which in turn will reduce the weighting the new large trial receives in the pooled analyses,” they wrote. “Thus, adding a positive result from a large randomized, controlled trial will have only a small effect on the pooled result and is unlikely to alter the conclusions of these meta-analyses.”
They also noted that some of the studies had methodological limitations, and smaller studies of shorter duration tended to have “inflated” effect sizes, such that “the results of small, short-duration studies should be interpreted very cautiously, since they might not be replicated in larger, longer studies.”
The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
SOURCE: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
FROM LANCET DIABETES & ENDOCRINOLOGY
Key clinical point: Vitamin D does not reduce the risk of falls or fractures or to improve bone mineral density.
Major finding: Researchers found that vitamin D supplementation did not reduce total fracture, hip fracture, or falls, even in trials in which participants took doses greater than 800 IU/day.
Study details: Systematic review, meta-analysis, and trial sequential analysis of 81 randomized controlled trials of vitamin D supplementation.
Disclosures: The study was funded by the Health Research Council of New Zealand. Two authors declared grants from the Health Research Council during the study, one author is a shareholder in a company that provides bone mineral density measurements, and one reported grants from the Scottish Government Health and Social Care Directorates during the study.
Source: Bolland M et al. Lancet Diabetes Endocrinol. 2018 Oct 4. doi. org/10.1016/S2213-8587(18)30265-1.
Pain doctor explains liberal opioid prescribing; actor uses skills for science
Barry Schultz, MD, once operated a thriving pain clinic in Delray Beach, Fla. Now he is serving a 157-year prison sentence after a conviction of selling opioids on a massive scale.
In an interview with Bill Whitaker of “60 Minutes,” Dr. Schultz explains: “I’m a scapegoat. I mean, I was one of hundreds of doctors that were prescribing medication for chronic pain. I see myself as a healer. … In my mind, what I was doing was legitimate.”
This role included prescribing more than 1,000 opioid pills to a woman during her pregnancy. She and thousands of others sought drugs from Dr. Schultz, who complied. In 2010, he prescribed nearly 17,000 of the highest-potency oxycodone pills to one patient over 7 months. Another patient was prescribed more than 23,000 pills over 8 months – more than 100 pills a day.
In 2009, more than 2,900 people died in Florida of drug overdoses, mostly from prescribed opioid pills. “In one 16-month period, Dr. Schultz dispensed 800,000 opioid pills from his office pharmacy,” the report says. The massive prescribing spree netted Dr. Schultz more than $6,000 a day, “60 Minutes” reported.
“When I started treating people with chronic noncancer pain, I felt it was unethical and discriminatory to limit the dose of medication. And if I had known that the overdose incidents had increased dramatically the way it had, I would have moderated my approach,” he says in the interview.
According to Mr. Whitaker,
Medicine and empathy
For years, actor Alan Alda was TV’s favorite doctor. His 11-season stint as Dr. Hawkeye Pierce on MASH garnered him critical acclaim for his portrayal of the empathetic side of being a physician and human in trying circumstances. In his post-MASH life, Mr. Alda has rechanneled his TV persona and become a spokesperson for the power of empathy for health care professionals and scientists – and anyone who can benefit from better communication.
In a Canadian Broadcasting Corporation interview with Brian Goldman, MD, of “White Coat, Black Art,” Mr. Alda explains that “empathic behavior is medicine.” He cites an example of a physician who had to let a patient know of her cancer diagnosis. “[The doctor] went in and he sat across from her at her level. Took her hand in his hand and talked in very plain language. Didn’t use the word ‘metastasis.’ And, for the first time, she reacted. ... And, for the first time, she asked a question. He came back to us and said: ‘It was just like the mirroring exercise. I was helping her face death, and she was helping me be a better doctor.’ ”
The mirroring exercise he refers to is a part of a workshop Mr. Alda conducts at the Alan Alda Center for Communicating Science at Stony Brook University in New York. The program, which focuses on the role of human connection and communication, has proven popular – and is now taught at 17 medical schools and universities worldwide.
Mr. Alda has proven to be an apt teacher. Now he is a patient, having been diagnosed with Parkinson’s disease about 3 years ago. Only recently did he decide it was time to let everyone in on the news.
“The main reason that I made a statement about it publicly was that … I didn’t want the story to come out in a maudlin way. If somebody saw me, saw my tremor on television then somebody might write an article about isn’t it sad and terrible and awful,” Mr. Alda says.
“I mean [Parkinson’s disease] is not a good thing to have. There’s no doubt about that. But there’s a stigma associated with it which is not helpful to people. And that is as soon as you know you have it, as soon as you get a diagnosis that’s the end of everything, and it’s not.”
Claire Foy’s life with anxiety
Another star of stage and screen has opened up about her troubles. In an interview with freelance writer Tom Lamont for The Guardian, actress Claire Foy explains her struggles with anxiety.
Her condition is not new. Now 34, she has experienced anxiety since childhood. Despite the acclaim and awards, she says she has been plagued by self-doubt and negative thoughts and underestimated her ability. “When you have anxiety, you have anxiety about – I don’t know – crossing the road,” she explains.
But the spotlight that has come with bravura performances, such as her turn as Queen Elizabeth II in the Netflix series “The Crown” and as the antihero Lisbeth Salander in soon-to-be-released “The Girl in the Spider’s Web,” ratcheted up her anxiety.
“The thing about it is, it’s not related to anything that would seem logical. It’s purely about that feeling in the pit of your stomach, and the feeling that you can’t, because you’re ‘this’ or you’re ‘that.’ It’s my mind working at a thousand beats a second and running away with a thought.”
She is currently on hiatus; daily life right now revolves around her daughter. Anxiety remains a part of the day, although time and therapy are easing the burden. “It’s still there, but I guess I don’t believe it so much anymore. I used to think that this was my lot in life, to be anxious,” she said in the interview. “And that I would struggle and struggle and struggle with it, and that it would make me quite miserable, and that I’d always be restricted.”
“But now I’m able to disassociate myself from it more. I know that it’s just something I have – and that I can take care of myself.”
Padma Lakshmi speaks out about rape
Author, cook, TV host, and producer Padma Lakshmi is another celebrity with a seemingly glittering life. But, like Mr. Alda and Ms. Foy, there is darkness. In a recent opinion piece in the New York Times and as reported by Maura Hohman of People magazine, she described being raped at age 16 by her then-boyfriend.
“When we went out, he would park the car and come in and sit on our couch and talk to my mother. He never brought me home late on a school night. We were intimate to a point, but he knew that I was a virgin and that I was unsure of when I would be ready to have sex,” Ms. Lakshmi explains.
“On New Year’s Eve, just a few months after we first started dating, he raped me.”
Ms. Lakshmi says she felt it was her fault at the time. “We had no language in the 1980s for date rape,” she wrote in the opinion piece. Flash ahead to the present and she understands well the current climate around this #WhyIDidn’tReport moment. “I understand why both women would keep this information to themselves for so many years, without involving the police. For years, I did the same thing.”
Her story has inspired victims to come forward with their experiences and, in one case, for an attacker to apologize for his actions.
Are you reading this? Your brain thanks you
For many, reading and thinking deeply can seem a lost pursuit in this ever-changing digital world. But this does not have to be the case, according to cognitive scientist Maryanne Wolf, PhD, incoming director of the Center for Dyslexia, Diverse Learners, and Social Justice at University of Southern California, Los Angeles.
In her book “Reader, Come Home: The Reading Brain in a Digital World,” (Harper, 2018) and in an interview with WBUR’s “On Point,” Dr. Wolf describes her unease over her seeming inability to focus on the printed page other than the tendency to grab snippets of detail.
“At some time impossible to pinpoint, I had begun to read more to be informed than to be immersed, much less to be transported,” she says.
Pondering this led her to realize that she was still up to the mental task of reading but was not devoting as much time to it. She set aside time each day to revisit a novel that she had found daunting reading in her youth, “Magister Ludi” by Hermann Hesse. The novel still proved to be slow going. But optimistically, the experiment made clear to Dr. Wolf that she had “changed in ways I would never have predicted. I now read on the surface and very quickly; in fact, I read too fast to comprehend deeper levels, which forced me constantly to go back and reread the same sentence over and over with increasing frustration.”
The culprit, she concludes, was the instant world of the Internet. Her brain had become used to dabbling in information. In the absence of cognitive impediments, she says, what was lacking in brainpower could be restored. To Dr. Wolf and others who fret over the difficulty in the pleasure of relaxing with a book, there is comfort in knowing that, with some literary exercise, the brain can shift from the digital world to the less frenetic world of the printed page.
Barry Schultz, MD, once operated a thriving pain clinic in Delray Beach, Fla. Now he is serving a 157-year prison sentence after a conviction of selling opioids on a massive scale.
In an interview with Bill Whitaker of “60 Minutes,” Dr. Schultz explains: “I’m a scapegoat. I mean, I was one of hundreds of doctors that were prescribing medication for chronic pain. I see myself as a healer. … In my mind, what I was doing was legitimate.”
This role included prescribing more than 1,000 opioid pills to a woman during her pregnancy. She and thousands of others sought drugs from Dr. Schultz, who complied. In 2010, he prescribed nearly 17,000 of the highest-potency oxycodone pills to one patient over 7 months. Another patient was prescribed more than 23,000 pills over 8 months – more than 100 pills a day.
In 2009, more than 2,900 people died in Florida of drug overdoses, mostly from prescribed opioid pills. “In one 16-month period, Dr. Schultz dispensed 800,000 opioid pills from his office pharmacy,” the report says. The massive prescribing spree netted Dr. Schultz more than $6,000 a day, “60 Minutes” reported.
“When I started treating people with chronic noncancer pain, I felt it was unethical and discriminatory to limit the dose of medication. And if I had known that the overdose incidents had increased dramatically the way it had, I would have moderated my approach,” he says in the interview.
According to Mr. Whitaker,
Medicine and empathy
For years, actor Alan Alda was TV’s favorite doctor. His 11-season stint as Dr. Hawkeye Pierce on MASH garnered him critical acclaim for his portrayal of the empathetic side of being a physician and human in trying circumstances. In his post-MASH life, Mr. Alda has rechanneled his TV persona and become a spokesperson for the power of empathy for health care professionals and scientists – and anyone who can benefit from better communication.
In a Canadian Broadcasting Corporation interview with Brian Goldman, MD, of “White Coat, Black Art,” Mr. Alda explains that “empathic behavior is medicine.” He cites an example of a physician who had to let a patient know of her cancer diagnosis. “[The doctor] went in and he sat across from her at her level. Took her hand in his hand and talked in very plain language. Didn’t use the word ‘metastasis.’ And, for the first time, she reacted. ... And, for the first time, she asked a question. He came back to us and said: ‘It was just like the mirroring exercise. I was helping her face death, and she was helping me be a better doctor.’ ”
The mirroring exercise he refers to is a part of a workshop Mr. Alda conducts at the Alan Alda Center for Communicating Science at Stony Brook University in New York. The program, which focuses on the role of human connection and communication, has proven popular – and is now taught at 17 medical schools and universities worldwide.
Mr. Alda has proven to be an apt teacher. Now he is a patient, having been diagnosed with Parkinson’s disease about 3 years ago. Only recently did he decide it was time to let everyone in on the news.
“The main reason that I made a statement about it publicly was that … I didn’t want the story to come out in a maudlin way. If somebody saw me, saw my tremor on television then somebody might write an article about isn’t it sad and terrible and awful,” Mr. Alda says.
“I mean [Parkinson’s disease] is not a good thing to have. There’s no doubt about that. But there’s a stigma associated with it which is not helpful to people. And that is as soon as you know you have it, as soon as you get a diagnosis that’s the end of everything, and it’s not.”
Claire Foy’s life with anxiety
Another star of stage and screen has opened up about her troubles. In an interview with freelance writer Tom Lamont for The Guardian, actress Claire Foy explains her struggles with anxiety.
Her condition is not new. Now 34, she has experienced anxiety since childhood. Despite the acclaim and awards, she says she has been plagued by self-doubt and negative thoughts and underestimated her ability. “When you have anxiety, you have anxiety about – I don’t know – crossing the road,” she explains.
But the spotlight that has come with bravura performances, such as her turn as Queen Elizabeth II in the Netflix series “The Crown” and as the antihero Lisbeth Salander in soon-to-be-released “The Girl in the Spider’s Web,” ratcheted up her anxiety.
“The thing about it is, it’s not related to anything that would seem logical. It’s purely about that feeling in the pit of your stomach, and the feeling that you can’t, because you’re ‘this’ or you’re ‘that.’ It’s my mind working at a thousand beats a second and running away with a thought.”
She is currently on hiatus; daily life right now revolves around her daughter. Anxiety remains a part of the day, although time and therapy are easing the burden. “It’s still there, but I guess I don’t believe it so much anymore. I used to think that this was my lot in life, to be anxious,” she said in the interview. “And that I would struggle and struggle and struggle with it, and that it would make me quite miserable, and that I’d always be restricted.”
“But now I’m able to disassociate myself from it more. I know that it’s just something I have – and that I can take care of myself.”
Padma Lakshmi speaks out about rape
Author, cook, TV host, and producer Padma Lakshmi is another celebrity with a seemingly glittering life. But, like Mr. Alda and Ms. Foy, there is darkness. In a recent opinion piece in the New York Times and as reported by Maura Hohman of People magazine, she described being raped at age 16 by her then-boyfriend.
“When we went out, he would park the car and come in and sit on our couch and talk to my mother. He never brought me home late on a school night. We were intimate to a point, but he knew that I was a virgin and that I was unsure of when I would be ready to have sex,” Ms. Lakshmi explains.
“On New Year’s Eve, just a few months after we first started dating, he raped me.”
Ms. Lakshmi says she felt it was her fault at the time. “We had no language in the 1980s for date rape,” she wrote in the opinion piece. Flash ahead to the present and she understands well the current climate around this #WhyIDidn’tReport moment. “I understand why both women would keep this information to themselves for so many years, without involving the police. For years, I did the same thing.”
Her story has inspired victims to come forward with their experiences and, in one case, for an attacker to apologize for his actions.
Are you reading this? Your brain thanks you
For many, reading and thinking deeply can seem a lost pursuit in this ever-changing digital world. But this does not have to be the case, according to cognitive scientist Maryanne Wolf, PhD, incoming director of the Center for Dyslexia, Diverse Learners, and Social Justice at University of Southern California, Los Angeles.
In her book “Reader, Come Home: The Reading Brain in a Digital World,” (Harper, 2018) and in an interview with WBUR’s “On Point,” Dr. Wolf describes her unease over her seeming inability to focus on the printed page other than the tendency to grab snippets of detail.
“At some time impossible to pinpoint, I had begun to read more to be informed than to be immersed, much less to be transported,” she says.
Pondering this led her to realize that she was still up to the mental task of reading but was not devoting as much time to it. She set aside time each day to revisit a novel that she had found daunting reading in her youth, “Magister Ludi” by Hermann Hesse. The novel still proved to be slow going. But optimistically, the experiment made clear to Dr. Wolf that she had “changed in ways I would never have predicted. I now read on the surface and very quickly; in fact, I read too fast to comprehend deeper levels, which forced me constantly to go back and reread the same sentence over and over with increasing frustration.”
The culprit, she concludes, was the instant world of the Internet. Her brain had become used to dabbling in information. In the absence of cognitive impediments, she says, what was lacking in brainpower could be restored. To Dr. Wolf and others who fret over the difficulty in the pleasure of relaxing with a book, there is comfort in knowing that, with some literary exercise, the brain can shift from the digital world to the less frenetic world of the printed page.
Barry Schultz, MD, once operated a thriving pain clinic in Delray Beach, Fla. Now he is serving a 157-year prison sentence after a conviction of selling opioids on a massive scale.
In an interview with Bill Whitaker of “60 Minutes,” Dr. Schultz explains: “I’m a scapegoat. I mean, I was one of hundreds of doctors that were prescribing medication for chronic pain. I see myself as a healer. … In my mind, what I was doing was legitimate.”
This role included prescribing more than 1,000 opioid pills to a woman during her pregnancy. She and thousands of others sought drugs from Dr. Schultz, who complied. In 2010, he prescribed nearly 17,000 of the highest-potency oxycodone pills to one patient over 7 months. Another patient was prescribed more than 23,000 pills over 8 months – more than 100 pills a day.
In 2009, more than 2,900 people died in Florida of drug overdoses, mostly from prescribed opioid pills. “In one 16-month period, Dr. Schultz dispensed 800,000 opioid pills from his office pharmacy,” the report says. The massive prescribing spree netted Dr. Schultz more than $6,000 a day, “60 Minutes” reported.
“When I started treating people with chronic noncancer pain, I felt it was unethical and discriminatory to limit the dose of medication. And if I had known that the overdose incidents had increased dramatically the way it had, I would have moderated my approach,” he says in the interview.
According to Mr. Whitaker,
Medicine and empathy
For years, actor Alan Alda was TV’s favorite doctor. His 11-season stint as Dr. Hawkeye Pierce on MASH garnered him critical acclaim for his portrayal of the empathetic side of being a physician and human in trying circumstances. In his post-MASH life, Mr. Alda has rechanneled his TV persona and become a spokesperson for the power of empathy for health care professionals and scientists – and anyone who can benefit from better communication.
In a Canadian Broadcasting Corporation interview with Brian Goldman, MD, of “White Coat, Black Art,” Mr. Alda explains that “empathic behavior is medicine.” He cites an example of a physician who had to let a patient know of her cancer diagnosis. “[The doctor] went in and he sat across from her at her level. Took her hand in his hand and talked in very plain language. Didn’t use the word ‘metastasis.’ And, for the first time, she reacted. ... And, for the first time, she asked a question. He came back to us and said: ‘It was just like the mirroring exercise. I was helping her face death, and she was helping me be a better doctor.’ ”
The mirroring exercise he refers to is a part of a workshop Mr. Alda conducts at the Alan Alda Center for Communicating Science at Stony Brook University in New York. The program, which focuses on the role of human connection and communication, has proven popular – and is now taught at 17 medical schools and universities worldwide.
Mr. Alda has proven to be an apt teacher. Now he is a patient, having been diagnosed with Parkinson’s disease about 3 years ago. Only recently did he decide it was time to let everyone in on the news.
“The main reason that I made a statement about it publicly was that … I didn’t want the story to come out in a maudlin way. If somebody saw me, saw my tremor on television then somebody might write an article about isn’t it sad and terrible and awful,” Mr. Alda says.
“I mean [Parkinson’s disease] is not a good thing to have. There’s no doubt about that. But there’s a stigma associated with it which is not helpful to people. And that is as soon as you know you have it, as soon as you get a diagnosis that’s the end of everything, and it’s not.”
Claire Foy’s life with anxiety
Another star of stage and screen has opened up about her troubles. In an interview with freelance writer Tom Lamont for The Guardian, actress Claire Foy explains her struggles with anxiety.
Her condition is not new. Now 34, she has experienced anxiety since childhood. Despite the acclaim and awards, she says she has been plagued by self-doubt and negative thoughts and underestimated her ability. “When you have anxiety, you have anxiety about – I don’t know – crossing the road,” she explains.
But the spotlight that has come with bravura performances, such as her turn as Queen Elizabeth II in the Netflix series “The Crown” and as the antihero Lisbeth Salander in soon-to-be-released “The Girl in the Spider’s Web,” ratcheted up her anxiety.
“The thing about it is, it’s not related to anything that would seem logical. It’s purely about that feeling in the pit of your stomach, and the feeling that you can’t, because you’re ‘this’ or you’re ‘that.’ It’s my mind working at a thousand beats a second and running away with a thought.”
She is currently on hiatus; daily life right now revolves around her daughter. Anxiety remains a part of the day, although time and therapy are easing the burden. “It’s still there, but I guess I don’t believe it so much anymore. I used to think that this was my lot in life, to be anxious,” she said in the interview. “And that I would struggle and struggle and struggle with it, and that it would make me quite miserable, and that I’d always be restricted.”
“But now I’m able to disassociate myself from it more. I know that it’s just something I have – and that I can take care of myself.”
Padma Lakshmi speaks out about rape
Author, cook, TV host, and producer Padma Lakshmi is another celebrity with a seemingly glittering life. But, like Mr. Alda and Ms. Foy, there is darkness. In a recent opinion piece in the New York Times and as reported by Maura Hohman of People magazine, she described being raped at age 16 by her then-boyfriend.
“When we went out, he would park the car and come in and sit on our couch and talk to my mother. He never brought me home late on a school night. We were intimate to a point, but he knew that I was a virgin and that I was unsure of when I would be ready to have sex,” Ms. Lakshmi explains.
“On New Year’s Eve, just a few months after we first started dating, he raped me.”
Ms. Lakshmi says she felt it was her fault at the time. “We had no language in the 1980s for date rape,” she wrote in the opinion piece. Flash ahead to the present and she understands well the current climate around this #WhyIDidn’tReport moment. “I understand why both women would keep this information to themselves for so many years, without involving the police. For years, I did the same thing.”
Her story has inspired victims to come forward with their experiences and, in one case, for an attacker to apologize for his actions.
Are you reading this? Your brain thanks you
For many, reading and thinking deeply can seem a lost pursuit in this ever-changing digital world. But this does not have to be the case, according to cognitive scientist Maryanne Wolf, PhD, incoming director of the Center for Dyslexia, Diverse Learners, and Social Justice at University of Southern California, Los Angeles.
In her book “Reader, Come Home: The Reading Brain in a Digital World,” (Harper, 2018) and in an interview with WBUR’s “On Point,” Dr. Wolf describes her unease over her seeming inability to focus on the printed page other than the tendency to grab snippets of detail.
“At some time impossible to pinpoint, I had begun to read more to be informed than to be immersed, much less to be transported,” she says.
Pondering this led her to realize that she was still up to the mental task of reading but was not devoting as much time to it. She set aside time each day to revisit a novel that she had found daunting reading in her youth, “Magister Ludi” by Hermann Hesse. The novel still proved to be slow going. But optimistically, the experiment made clear to Dr. Wolf that she had “changed in ways I would never have predicted. I now read on the surface and very quickly; in fact, I read too fast to comprehend deeper levels, which forced me constantly to go back and reread the same sentence over and over with increasing frustration.”
The culprit, she concludes, was the instant world of the Internet. Her brain had become used to dabbling in information. In the absence of cognitive impediments, she says, what was lacking in brainpower could be restored. To Dr. Wolf and others who fret over the difficulty in the pleasure of relaxing with a book, there is comfort in knowing that, with some literary exercise, the brain can shift from the digital world to the less frenetic world of the printed page.
Knee Injuries in Elite Level Soccer Players
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.
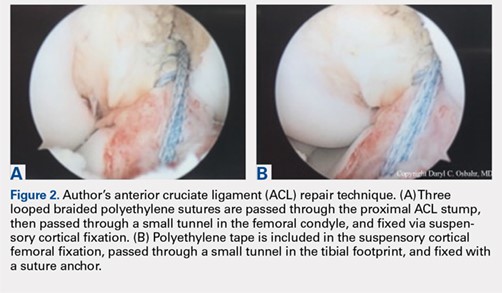
In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.
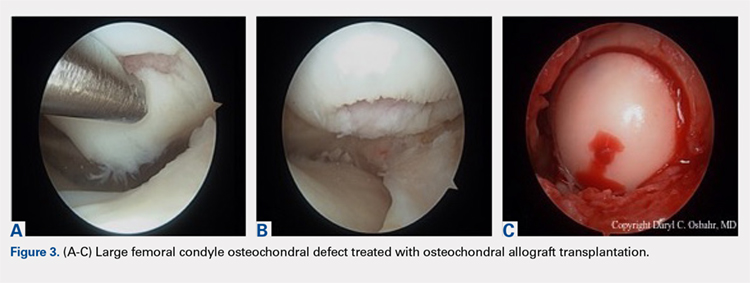
Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.

In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.

Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.

In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.

Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
TAKE-HOME POINTS
- Soccer is one of the most popular sports in the world and has a high incidence of resultant knee injuries.
- Significant, identifiable risk factors put soccer players at risk for serious knee injuries, such as ACL ruptures; age, female sex, and position played influence injury susceptibility.
- ACL injury most commonly occurs via non-contact mechanisms, and female players are at a significantly higher risk of ACL injury than male counterparts.
- The prevalence of osteoarthritis in retired soccer players is high, underscoring the need to be familiar with meniscal and cartilage repair/restoration techniques and associated outcomes.
- The FIFA11+ program reduces injury by 30%, with reported relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of this warm-up program.
October 2018 Highlights
Revised McDonald criteria to be explored at ECTRIMS
The revised McDonald criteria, issued less than a year ago in December 2017, should allow for earlier diagnosis and treatment of multiple sclerosis, but also could be leading to overdiagnosis and misdiagnosis.
A recent study published in JAMA Neurology found that sensitivity for the 2017 criteria was greater (68% vs. 36% for the 2010 criteria) but specificity was not (61% vs. 85%, respectively), based on a study of several hundred patients in the Netherlands with clinically isolated syndrome.
A highlighted session on Saturday, Oct. 10, at 2:30 p.m. (local time) entitled “Burning Debate: The new McDonald diagnostic criteria are controversial making them difficult to use in clinical practice” aims to shed some light. After an introduction from Emmanuelle Waubant, MD, professor of neurology at the University of California, San Francisco, the topic will be debated by Jiwon Oh, MD, of the University of Toronto and Frauke Zipp, MD, of the University of Mainz (Germany). The experts will take questions from the audience as well as via Twitter. Ask your questions using the meeting hashtag #ECTRIMS2018. Find the session in Hall B.
Five new papers on the impact of the revised criteria will be presented in Hall A on Sunday, Oct. 11, at 8:30 a.m. (local time). Among the investigators presenting are Roos M. van der Vuurst de Vries, MD, from the department of neurology at Erasmus Medical Center in Rotterdam, the Netherlands, who authored the recent JAMA Neurology paper, and Wallace Brownlee, MD, of University College London.
The revised McDonald criteria, issued less than a year ago in December 2017, should allow for earlier diagnosis and treatment of multiple sclerosis, but also could be leading to overdiagnosis and misdiagnosis.
A recent study published in JAMA Neurology found that sensitivity for the 2017 criteria was greater (68% vs. 36% for the 2010 criteria) but specificity was not (61% vs. 85%, respectively), based on a study of several hundred patients in the Netherlands with clinically isolated syndrome.
A highlighted session on Saturday, Oct. 10, at 2:30 p.m. (local time) entitled “Burning Debate: The new McDonald diagnostic criteria are controversial making them difficult to use in clinical practice” aims to shed some light. After an introduction from Emmanuelle Waubant, MD, professor of neurology at the University of California, San Francisco, the topic will be debated by Jiwon Oh, MD, of the University of Toronto and Frauke Zipp, MD, of the University of Mainz (Germany). The experts will take questions from the audience as well as via Twitter. Ask your questions using the meeting hashtag #ECTRIMS2018. Find the session in Hall B.
Five new papers on the impact of the revised criteria will be presented in Hall A on Sunday, Oct. 11, at 8:30 a.m. (local time). Among the investigators presenting are Roos M. van der Vuurst de Vries, MD, from the department of neurology at Erasmus Medical Center in Rotterdam, the Netherlands, who authored the recent JAMA Neurology paper, and Wallace Brownlee, MD, of University College London.
The revised McDonald criteria, issued less than a year ago in December 2017, should allow for earlier diagnosis and treatment of multiple sclerosis, but also could be leading to overdiagnosis and misdiagnosis.
A recent study published in JAMA Neurology found that sensitivity for the 2017 criteria was greater (68% vs. 36% for the 2010 criteria) but specificity was not (61% vs. 85%, respectively), based on a study of several hundred patients in the Netherlands with clinically isolated syndrome.
A highlighted session on Saturday, Oct. 10, at 2:30 p.m. (local time) entitled “Burning Debate: The new McDonald diagnostic criteria are controversial making them difficult to use in clinical practice” aims to shed some light. After an introduction from Emmanuelle Waubant, MD, professor of neurology at the University of California, San Francisco, the topic will be debated by Jiwon Oh, MD, of the University of Toronto and Frauke Zipp, MD, of the University of Mainz (Germany). The experts will take questions from the audience as well as via Twitter. Ask your questions using the meeting hashtag #ECTRIMS2018. Find the session in Hall B.
Five new papers on the impact of the revised criteria will be presented in Hall A on Sunday, Oct. 11, at 8:30 a.m. (local time). Among the investigators presenting are Roos M. van der Vuurst de Vries, MD, from the department of neurology at Erasmus Medical Center in Rotterdam, the Netherlands, who authored the recent JAMA Neurology paper, and Wallace Brownlee, MD, of University College London.
REPORTING FROM ECTRIMS 2018
FDA approves emicizumab for hemophilia A without inhibitors
The Food and Drug Administration has approved emicizumab-kxwh (Hemlibra) for subcutaneous prophylactic treatment in hemophilia A without factor VIII inhibitors.
Genentech announced the new approval on Oct. 4 of this year. In 2017, the bispecific antibody, which targets both factors IXa and X, was approved for patients as young as newborns who had factor VIII inhibitors; the latest approval allows it to be used for patients without inhibitors as well.
The approval is based on a pair of trials. HAVEN 3 (NCT02847637) is a phase 3 trial in which investigators looked at emicizumab prophylaxis weekly or every other week, versus on-demand factor VIII treatment in patients without inhibitors. The study included 152 patients aged 12 years and older who were previously treated with factor VIII therapy.
Compared with patients not receiving prophylactic treatments, those receiving weekly doses had a 96% reduction in treated bleeds, and those receiving doses every other week saw a 97% reduction. Investigators also found that 55.6% of patients treated every week, 60% of those treated every other week, and 0% of those treated with no prophylaxis experienced zero bleeds; similarly, 91.7%, 94.3%, and 5.6% experienced three or fewer bleeds.
The single-arm HAVEN 4 (NCT03020160) trial evaluated dosing patients every 4 weeks among 48 patients aged 12 years and older, with or without inhibitors, and results showed that even that dosing regimen could lead to a clinically meaningful control of bleeds: 56.1% had no bleeds, and 90.2% had three or fewer bleeds.
The most common adverse reactions were joint pain, headache, and injection-site reaction. When emicizumab-kxwh is used with activated prothrombin complex concentrate, there’s a risk of thrombotic microangiopathy and thrombotic events. Full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved emicizumab-kxwh (Hemlibra) for subcutaneous prophylactic treatment in hemophilia A without factor VIII inhibitors.
Genentech announced the new approval on Oct. 4 of this year. In 2017, the bispecific antibody, which targets both factors IXa and X, was approved for patients as young as newborns who had factor VIII inhibitors; the latest approval allows it to be used for patients without inhibitors as well.
The approval is based on a pair of trials. HAVEN 3 (NCT02847637) is a phase 3 trial in which investigators looked at emicizumab prophylaxis weekly or every other week, versus on-demand factor VIII treatment in patients without inhibitors. The study included 152 patients aged 12 years and older who were previously treated with factor VIII therapy.
Compared with patients not receiving prophylactic treatments, those receiving weekly doses had a 96% reduction in treated bleeds, and those receiving doses every other week saw a 97% reduction. Investigators also found that 55.6% of patients treated every week, 60% of those treated every other week, and 0% of those treated with no prophylaxis experienced zero bleeds; similarly, 91.7%, 94.3%, and 5.6% experienced three or fewer bleeds.
The single-arm HAVEN 4 (NCT03020160) trial evaluated dosing patients every 4 weeks among 48 patients aged 12 years and older, with or without inhibitors, and results showed that even that dosing regimen could lead to a clinically meaningful control of bleeds: 56.1% had no bleeds, and 90.2% had three or fewer bleeds.
The most common adverse reactions were joint pain, headache, and injection-site reaction. When emicizumab-kxwh is used with activated prothrombin complex concentrate, there’s a risk of thrombotic microangiopathy and thrombotic events. Full prescribing information can be found on the FDA website.
The Food and Drug Administration has approved emicizumab-kxwh (Hemlibra) for subcutaneous prophylactic treatment in hemophilia A without factor VIII inhibitors.
Genentech announced the new approval on Oct. 4 of this year. In 2017, the bispecific antibody, which targets both factors IXa and X, was approved for patients as young as newborns who had factor VIII inhibitors; the latest approval allows it to be used for patients without inhibitors as well.
The approval is based on a pair of trials. HAVEN 3 (NCT02847637) is a phase 3 trial in which investigators looked at emicizumab prophylaxis weekly or every other week, versus on-demand factor VIII treatment in patients without inhibitors. The study included 152 patients aged 12 years and older who were previously treated with factor VIII therapy.
Compared with patients not receiving prophylactic treatments, those receiving weekly doses had a 96% reduction in treated bleeds, and those receiving doses every other week saw a 97% reduction. Investigators also found that 55.6% of patients treated every week, 60% of those treated every other week, and 0% of those treated with no prophylaxis experienced zero bleeds; similarly, 91.7%, 94.3%, and 5.6% experienced three or fewer bleeds.
The single-arm HAVEN 4 (NCT03020160) trial evaluated dosing patients every 4 weeks among 48 patients aged 12 years and older, with or without inhibitors, and results showed that even that dosing regimen could lead to a clinically meaningful control of bleeds: 56.1% had no bleeds, and 90.2% had three or fewer bleeds.
The most common adverse reactions were joint pain, headache, and injection-site reaction. When emicizumab-kxwh is used with activated prothrombin complex concentrate, there’s a risk of thrombotic microangiopathy and thrombotic events. Full prescribing information can be found on the FDA website.