User login
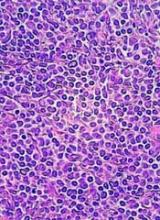
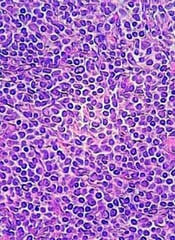
5-year remission rates with combo prove durable in MCL
Long-term results of a phase 2 clinical trial of the lenalidomide and rituximab combination as first-line therapy for mantle cell lymphoma (MCL) show continued durable responses with manageable toxicities after 5 years.
With a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University in New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote.
Their report was published in Blood.
In the multicenter, phase 2 single-arm study (NCT01472562), 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase.
Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Results
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P=0.04).
Safety
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption.
Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting,” the investigators wrote, “lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach.”
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation.
Long-term results of a phase 2 clinical trial of the lenalidomide and rituximab combination as first-line therapy for mantle cell lymphoma (MCL) show continued durable responses with manageable toxicities after 5 years.
With a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University in New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote.
Their report was published in Blood.
In the multicenter, phase 2 single-arm study (NCT01472562), 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase.
Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Results
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P=0.04).
Safety
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption.
Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting,” the investigators wrote, “lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach.”
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation.
Long-term results of a phase 2 clinical trial of the lenalidomide and rituximab combination as first-line therapy for mantle cell lymphoma (MCL) show continued durable responses with manageable toxicities after 5 years.
With a median follow-up of 64 months, 21 of 33 patients with initial responses remained in durable, minimal residual disease (MRD)-negative remission following induction with lenalidomide and rituximab and maintenance with those same two agents for at least 3 years.
The patients with durable responses included five who opted to discontinue maintenance after 3 years, reported Jia Ruan, MD, PhD, of Cornell University in New York, and her colleagues.
“Our long-term data provide proof of concept that an outpatient-based induction and maintenance strategy free of conventional chemotherapy is effective, safe, and feasible as first-line therapy for MCL,” they wrote.
Their report was published in Blood.
In the multicenter, phase 2 single-arm study (NCT01472562), 38 patients with untreated MCL were enrolled and treated with lenalidomide 20 mg daily on days 1-21 of each 28-day cycle for 12 cycles during induction, followed by dose reduction to 15 mg during the maintenance phase.
Patients also received standard dose rituximab 375 mg/m2 weekly for 4 weeks during cycle 1, then once every other cycle.
Patients remained on treatment until disease progression, unacceptable toxicities, or study withdrawal. Patients who remained in remission after 3 years, based on routine surveillance CT scans, had the option to discontinue maintenance.
Results
Of the original 38 patients enrolled, 36 were evaluable for response, including 23 with a complete response (CR) and 10 with a partial response.
At the 64-month median follow-up, neither the median progression-free survival (PFS) nor duration of response had been reached.
Overall, 21 of the 33 patients with responses (64%) had ongoing responses, including six patients with responses beyond 6 years.
Estimated 3-year and 5-year PFS rates were 80.3% and 63.9%, respectively. Respective estimated 3- and 5-year overall survival rates were 89.5% and 77.4%.
Mantle cell lymphoma international prognostic index (MIPI) scores were not associated with either response or PFS rates, but patients with high-risk MIPI scores were significantly more likely to have worse overall survival (P=0.04).
Safety
Grade 3 or greater hematologic toxicities included neutropenia in 42% of patients in both induction and maintenance, anemia in 8% and 3%, thrombocytopenia in 11% and 5%, and febrile neutropenia in 3% and 5%.
Secondary primary malignancies occurred in six patients. These included five noninvasive skin cancers requiring only local therapy without the need for study interruption.
Two patients, including one with a skin cancer, died from the secondary malignancies, including one from Merkel cell carcinoma and one from pancreatic cancer.
“The efficacy and survival outcome observed in our study compared favorably to those reported with lenalidomide either as single agent, or in combination with rituximab in relapsed and refractory setting,” the investigators wrote, “lending support for prioritizing novel agents such as lenalidomide early in the treatment sequence, to compare to conventional chemotherapy-based approach.”
The study was supported in part by Celgene Corporation, a Clinical Translational Science Center grant, and the Lymphoma Foundation.
Communication and consent
We knew that the case would be a difficult one. The patient was a man in his mid-40s who had several serious chronic conditions and was on high-dose steroids. He had been operated on 10 days earlier by one of my partners for a bowel obstruction and had required a resection of a small portion of the terminal ileum. Unfortunately, on the day after surgery, it became obvious that the patient needed a reexploration for bleeding. He had developed clear evidence of a significant anastomotic leak and had to be taken emergently back to the operating room.
His condition had been worsening during the day. We had booked the case in the OR but had been put off by a trauma emergency and a neurosurgical emergency. During the 3 hours of waiting to take him to the OR, the patient’s sister and mother came to the hospital and were now waiting with him in the preop area. I was on my way up to see him when my resident called. Despite the patient having signed an operative consent form a few hours earlier when we booked the case, he was now “declining” an operation. I was surprised. This man had undergone several operations in the last few years and two in the last 2 weeks. I arrived to find the patient stating that he did not want surgery. Lying in bed, he was adamant that he should not have surgery. The surgical resident who had spoken with the patient several times over the last few hours was also surprised. The patient’s family members were yelling that, of course, he wanted surgery and why would he change his mind.
This is a difficult situation since one of the central tenets of the ethical practice of surgery is to allow patients to make decisions about their own care. The right to make autonomous choices even extends to circumstances in which patients make what we might consider “bad” decisions. As long as the patient has the capacity to make an autonomous choice, he or she should have that choice respected.
This patient, who just a few hours ago had agreed to surgery, now seemed to have changed his mind. Although it can be frustrating, we do allow patients to change their minds. On the one hand, this was a straightforward case. The patient was refusing a potentially life-saving operation. Such a situation is never pleasant for a surgeon, but as long as the patient understands the risks, we respect such choices.
However, my resident made an astute observation. She pointed out that, when asked why he now did not want surgery, he replied that “this is all a movie – it’s not really happening.” The patient appeared to be oriented to person and place, but nevertheless, his reasoning seemed to have been altered. It appeared that this patient was no longer making sense because his underlying medical condition had deteriorated. We considered whether he was becoming septic and that this change in medical condition had rendered him unable to make an informed decision. My resident, who had discussed the operation with the patient several times, stated that the patient’s decision making seemed very different than even an hour ago. His family members agreed, stating that, up until a few minutes before, he was in favor of surgery. They pleaded with us to just take him into the operating room.
We considered our options. We could delay surgery and consult psychiatry to ask them to assess his competency. However, on a weekend night, this would likely take several hours. We considered the option of waiting in the preop area for the patient’s medical condition to further worsen. If he became overtly septic and lost consciousness, then we could readily turn to the family members – his surrogate decision makers – and ask them to consent to the procedure. Although this “by the book” approach might take away any worry that we were overriding an autonomous patient’s choice, we knew that it would unnecessarily expose him to greater operative risks. This option was not in his best interest and therefore not much of an option.
Ultimately, the surgical resident, the attending anesthesiologist, the family, and I decided that his decision to not have surgery at this moment was not consistent with his prior decisions, and he could provide no reason for changing his mind. We brought the patient into the operating room and explored him. He did have a large anastomotic leak with a large volume of enteric contents in the peritoneal cavity. He survived the operation and, not unexpectedly, required a long postoperative stay in the hospital. Once he was a few days out, I inquired about whether he was glad that he had surgery. He was quick to state his confidence that it had been the right choice for him. He did not even remember having ever refused the surgery.
Although this case raised many concerns for all of us involved in the patient’s care, one overriding lesson that came through to me. Informed consent should not be viewed as a solitary event, but a conversation. This patient had expressed his desire to have surgery multiple times to my surgical resident and to his family. Even though we should never take the position that patients cannot change their minds, we should carefully question those choices that are inconsistent with the prior discussions that have been undertaken. Good communication skills – including listening to the patient, understanding the patient’s reasoning, and reflecting on the entire conversation – are essential in obtaining informed consent.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
We knew that the case would be a difficult one. The patient was a man in his mid-40s who had several serious chronic conditions and was on high-dose steroids. He had been operated on 10 days earlier by one of my partners for a bowel obstruction and had required a resection of a small portion of the terminal ileum. Unfortunately, on the day after surgery, it became obvious that the patient needed a reexploration for bleeding. He had developed clear evidence of a significant anastomotic leak and had to be taken emergently back to the operating room.
His condition had been worsening during the day. We had booked the case in the OR but had been put off by a trauma emergency and a neurosurgical emergency. During the 3 hours of waiting to take him to the OR, the patient’s sister and mother came to the hospital and were now waiting with him in the preop area. I was on my way up to see him when my resident called. Despite the patient having signed an operative consent form a few hours earlier when we booked the case, he was now “declining” an operation. I was surprised. This man had undergone several operations in the last few years and two in the last 2 weeks. I arrived to find the patient stating that he did not want surgery. Lying in bed, he was adamant that he should not have surgery. The surgical resident who had spoken with the patient several times over the last few hours was also surprised. The patient’s family members were yelling that, of course, he wanted surgery and why would he change his mind.
This is a difficult situation since one of the central tenets of the ethical practice of surgery is to allow patients to make decisions about their own care. The right to make autonomous choices even extends to circumstances in which patients make what we might consider “bad” decisions. As long as the patient has the capacity to make an autonomous choice, he or she should have that choice respected.
This patient, who just a few hours ago had agreed to surgery, now seemed to have changed his mind. Although it can be frustrating, we do allow patients to change their minds. On the one hand, this was a straightforward case. The patient was refusing a potentially life-saving operation. Such a situation is never pleasant for a surgeon, but as long as the patient understands the risks, we respect such choices.
However, my resident made an astute observation. She pointed out that, when asked why he now did not want surgery, he replied that “this is all a movie – it’s not really happening.” The patient appeared to be oriented to person and place, but nevertheless, his reasoning seemed to have been altered. It appeared that this patient was no longer making sense because his underlying medical condition had deteriorated. We considered whether he was becoming septic and that this change in medical condition had rendered him unable to make an informed decision. My resident, who had discussed the operation with the patient several times, stated that the patient’s decision making seemed very different than even an hour ago. His family members agreed, stating that, up until a few minutes before, he was in favor of surgery. They pleaded with us to just take him into the operating room.
We considered our options. We could delay surgery and consult psychiatry to ask them to assess his competency. However, on a weekend night, this would likely take several hours. We considered the option of waiting in the preop area for the patient’s medical condition to further worsen. If he became overtly septic and lost consciousness, then we could readily turn to the family members – his surrogate decision makers – and ask them to consent to the procedure. Although this “by the book” approach might take away any worry that we were overriding an autonomous patient’s choice, we knew that it would unnecessarily expose him to greater operative risks. This option was not in his best interest and therefore not much of an option.
Ultimately, the surgical resident, the attending anesthesiologist, the family, and I decided that his decision to not have surgery at this moment was not consistent with his prior decisions, and he could provide no reason for changing his mind. We brought the patient into the operating room and explored him. He did have a large anastomotic leak with a large volume of enteric contents in the peritoneal cavity. He survived the operation and, not unexpectedly, required a long postoperative stay in the hospital. Once he was a few days out, I inquired about whether he was glad that he had surgery. He was quick to state his confidence that it had been the right choice for him. He did not even remember having ever refused the surgery.
Although this case raised many concerns for all of us involved in the patient’s care, one overriding lesson that came through to me. Informed consent should not be viewed as a solitary event, but a conversation. This patient had expressed his desire to have surgery multiple times to my surgical resident and to his family. Even though we should never take the position that patients cannot change their minds, we should carefully question those choices that are inconsistent with the prior discussions that have been undertaken. Good communication skills – including listening to the patient, understanding the patient’s reasoning, and reflecting on the entire conversation – are essential in obtaining informed consent.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
We knew that the case would be a difficult one. The patient was a man in his mid-40s who had several serious chronic conditions and was on high-dose steroids. He had been operated on 10 days earlier by one of my partners for a bowel obstruction and had required a resection of a small portion of the terminal ileum. Unfortunately, on the day after surgery, it became obvious that the patient needed a reexploration for bleeding. He had developed clear evidence of a significant anastomotic leak and had to be taken emergently back to the operating room.
His condition had been worsening during the day. We had booked the case in the OR but had been put off by a trauma emergency and a neurosurgical emergency. During the 3 hours of waiting to take him to the OR, the patient’s sister and mother came to the hospital and were now waiting with him in the preop area. I was on my way up to see him when my resident called. Despite the patient having signed an operative consent form a few hours earlier when we booked the case, he was now “declining” an operation. I was surprised. This man had undergone several operations in the last few years and two in the last 2 weeks. I arrived to find the patient stating that he did not want surgery. Lying in bed, he was adamant that he should not have surgery. The surgical resident who had spoken with the patient several times over the last few hours was also surprised. The patient’s family members were yelling that, of course, he wanted surgery and why would he change his mind.
This is a difficult situation since one of the central tenets of the ethical practice of surgery is to allow patients to make decisions about their own care. The right to make autonomous choices even extends to circumstances in which patients make what we might consider “bad” decisions. As long as the patient has the capacity to make an autonomous choice, he or she should have that choice respected.
This patient, who just a few hours ago had agreed to surgery, now seemed to have changed his mind. Although it can be frustrating, we do allow patients to change their minds. On the one hand, this was a straightforward case. The patient was refusing a potentially life-saving operation. Such a situation is never pleasant for a surgeon, but as long as the patient understands the risks, we respect such choices.
However, my resident made an astute observation. She pointed out that, when asked why he now did not want surgery, he replied that “this is all a movie – it’s not really happening.” The patient appeared to be oriented to person and place, but nevertheless, his reasoning seemed to have been altered. It appeared that this patient was no longer making sense because his underlying medical condition had deteriorated. We considered whether he was becoming septic and that this change in medical condition had rendered him unable to make an informed decision. My resident, who had discussed the operation with the patient several times, stated that the patient’s decision making seemed very different than even an hour ago. His family members agreed, stating that, up until a few minutes before, he was in favor of surgery. They pleaded with us to just take him into the operating room.
We considered our options. We could delay surgery and consult psychiatry to ask them to assess his competency. However, on a weekend night, this would likely take several hours. We considered the option of waiting in the preop area for the patient’s medical condition to further worsen. If he became overtly septic and lost consciousness, then we could readily turn to the family members – his surrogate decision makers – and ask them to consent to the procedure. Although this “by the book” approach might take away any worry that we were overriding an autonomous patient’s choice, we knew that it would unnecessarily expose him to greater operative risks. This option was not in his best interest and therefore not much of an option.
Ultimately, the surgical resident, the attending anesthesiologist, the family, and I decided that his decision to not have surgery at this moment was not consistent with his prior decisions, and he could provide no reason for changing his mind. We brought the patient into the operating room and explored him. He did have a large anastomotic leak with a large volume of enteric contents in the peritoneal cavity. He survived the operation and, not unexpectedly, required a long postoperative stay in the hospital. Once he was a few days out, I inquired about whether he was glad that he had surgery. He was quick to state his confidence that it had been the right choice for him. He did not even remember having ever refused the surgery.
Although this case raised many concerns for all of us involved in the patient’s care, one overriding lesson that came through to me. Informed consent should not be viewed as a solitary event, but a conversation. This patient had expressed his desire to have surgery multiple times to my surgical resident and to his family. Even though we should never take the position that patients cannot change their minds, we should carefully question those choices that are inconsistent with the prior discussions that have been undertaken. Good communication skills – including listening to the patient, understanding the patient’s reasoning, and reflecting on the entire conversation – are essential in obtaining informed consent.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
Upcoming CPT® Changes
Pulmonary, critical care, and sleep physicians often provide services to patients, as well as consultative services to other health-care professionals, without a patient being present. This can be done via telephone or electronic (internet or electronic health record) communications. Many are not aware that Current Procedural Terminology (CPT®) codes were published to describe and define the work involved in these services. In 2019, there will be additional CPT codes available for health-care workers to use for these non-face-to-face services.
Telephone services are reported using CPT codes 99441-99443 and may be used for evaluation and management (E/M) services provided by telephone for an established patient that do not result in a patient visit within the next 24 hours or are associated with an E/M visit from the last 7 days.
99441 Telephone evaluation and management service by a physician or other qualified health-care professional who may report evaluation and management services provided to an established patient, parent, or guardian not originating from a related E/M serv ice provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion
99442 11-20 minutes of medical discussion
99443 21-30 minutes of medical discussion
These codes may not be reported by a provider more frequently than every 7 days. The details of the service should be documented in the medical record.
If the E/M service is prompted by an online patient request, then CPT code 99444 can be used.
99444 Online evaluation and management service provided by a physician or other qualified health care professional who may report evaluation and management services provided to an established patient or guardian, not originating from a related E/M service provided within the previous 7 days, using the internet or similar electronic communications network.
This code may be reported only every 7 days and can not be related to a previous E/M evaluation in the last 7 days or to a previous surgical procedure. The service includes all of the communication (eg, related telephone calls, prescription provision, laboratory orders) pertaining to the online patient encounter.
There are also CPT codes for Interprofessional Telephone/Internet/Electronic Health Record Consultations. These codes are used when one health-care provider requests the opinion and/or treatment advice of another provider (consultant) for either a new or established patient without face-to-face contact between the patient and the consultant.
99446 Interprofessional telephone/Internet/electronic health record assessment and management service provided by a consultative physician, including a verbal and written report to the patient’s treating/requesting physician or other qualified health care professional; 5-10 minutes of medical consultative discussion and review.
99447 11-20 minutes of medical consultative discussion and review
99448 21-30 minutes of medical consultative discussion and review
99449 31 minutes or more of medical consultative discussion and review
These codes are not used if the consultant has seen the patient in a face-to-face encounter within the last 14 days or the consultation results in a transfer of care or other face-to-face service with the consultant within the next 14 days. In addition, greater than 50% of the service time reported must be devoted to the medical consultative verbal or internet discussion. The request and reason for telephone/internet/electronic health record consultation by the requesting health-care professional should be documented in the patient’s medical record. After an oral report from the consultant is provided to the treating/requesting physician, a written report should be documented in the medical record. Consultations of less than 5 minutes should not be reported.
As noted, CPT codes 99446-49 require an oral and written report. A new code is added for 2019.
99451 Interprofessional telephone/Internet/electronic health record assessment and management service provided by a consultative physician, including a written report to the patient’s treating/requesting physician or other qualified health care professional, 5 minutes or more of medical consultative time.
CPT code 99451 describes a consultative service lasting more than 5 minutes and requires only a written report to the requesting physician. This was added recognizing that oral communications do not always occur between healthcare professionals and may facilitate consultative services in geographic areas with no specialists available.
99452 Interprofessional telephone/Internet/electronic health record referral service(s) provided by a treating/requesting physician or other qualified health care professional, 30 minutes.
CPT code 99452 is reported for 16-30 minutes preparing for the referral and/or communicating with a consultant. If more than 30 minutes is spent by the treating/requesting healthcare provider, then one would use a prolonged services code (99358-59).
As with all coding and billing issues, review the CPT manual for parentheticals that describe coding rules not included in the code description. In addition, not all CPT codes are paid by all providers. Knowledge of payer policies is, therefore, important for appropriate reimbursement.
Pulmonary, critical care, and sleep physicians often provide services to patients, as well as consultative services to other health-care professionals, without a patient being present. This can be done via telephone or electronic (internet or electronic health record) communications. Many are not aware that Current Procedural Terminology (CPT®) codes were published to describe and define the work involved in these services. In 2019, there will be additional CPT codes available for health-care workers to use for these non-face-to-face services.
Telephone services are reported using CPT codes 99441-99443 and may be used for evaluation and management (E/M) services provided by telephone for an established patient that do not result in a patient visit within the next 24 hours or are associated with an E/M visit from the last 7 days.
99441 Telephone evaluation and management service by a physician or other qualified health-care professional who may report evaluation and management services provided to an established patient, parent, or guardian not originating from a related E/M serv ice provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion
99442 11-20 minutes of medical discussion
99443 21-30 minutes of medical discussion
These codes may not be reported by a provider more frequently than every 7 days. The details of the service should be documented in the medical record.
If the E/M service is prompted by an online patient request, then CPT code 99444 can be used.
99444 Online evaluation and management service provided by a physician or other qualified health care professional who may report evaluation and management services provided to an established patient or guardian, not originating from a related E/M service provided within the previous 7 days, using the internet or similar electronic communications network.
This code may be reported only every 7 days and can not be related to a previous E/M evaluation in the last 7 days or to a previous surgical procedure. The service includes all of the communication (eg, related telephone calls, prescription provision, laboratory orders) pertaining to the online patient encounter.
There are also CPT codes for Interprofessional Telephone/Internet/Electronic Health Record Consultations. These codes are used when one health-care provider requests the opinion and/or treatment advice of another provider (consultant) for either a new or established patient without face-to-face contact between the patient and the consultant.
99446 Interprofessional telephone/Internet/electronic health record assessment and management service provided by a consultative physician, including a verbal and written report to the patient’s treating/requesting physician or other qualified health care professional; 5-10 minutes of medical consultative discussion and review.
99447 11-20 minutes of medical consultative discussion and review
99448 21-30 minutes of medical consultative discussion and review
99449 31 minutes or more of medical consultative discussion and review
These codes are not used if the consultant has seen the patient in a face-to-face encounter within the last 14 days or the consultation results in a transfer of care or other face-to-face service with the consultant within the next 14 days. In addition, greater than 50% of the service time reported must be devoted to the medical consultative verbal or internet discussion. The request and reason for telephone/internet/electronic health record consultation by the requesting health-care professional should be documented in the patient’s medical record. After an oral report from the consultant is provided to the treating/requesting physician, a written report should be documented in the medical record. Consultations of less than 5 minutes should not be reported.
As noted, CPT codes 99446-49 require an oral and written report. A new code is added for 2019.
99451 Interprofessional telephone/Internet/electronic health record assessment and management service provided by a consultative physician, including a written report to the patient’s treating/requesting physician or other qualified health care professional, 5 minutes or more of medical consultative time.
CPT code 99451 describes a consultative service lasting more than 5 minutes and requires only a written report to the requesting physician. This was added recognizing that oral communications do not always occur between healthcare professionals and may facilitate consultative services in geographic areas with no specialists available.
99452 Interprofessional telephone/Internet/electronic health record referral service(s) provided by a treating/requesting physician or other qualified health care professional, 30 minutes.
CPT code 99452 is reported for 16-30 minutes preparing for the referral and/or communicating with a consultant. If more than 30 minutes is spent by the treating/requesting healthcare provider, then one would use a prolonged services code (99358-59).
As with all coding and billing issues, review the CPT manual for parentheticals that describe coding rules not included in the code description. In addition, not all CPT codes are paid by all providers. Knowledge of payer policies is, therefore, important for appropriate reimbursement.
Pulmonary, critical care, and sleep physicians often provide services to patients, as well as consultative services to other health-care professionals, without a patient being present. This can be done via telephone or electronic (internet or electronic health record) communications. Many are not aware that Current Procedural Terminology (CPT®) codes were published to describe and define the work involved in these services. In 2019, there will be additional CPT codes available for health-care workers to use for these non-face-to-face services.
Telephone services are reported using CPT codes 99441-99443 and may be used for evaluation and management (E/M) services provided by telephone for an established patient that do not result in a patient visit within the next 24 hours or are associated with an E/M visit from the last 7 days.
99441 Telephone evaluation and management service by a physician or other qualified health-care professional who may report evaluation and management services provided to an established patient, parent, or guardian not originating from a related E/M serv ice provided within the previous 7 days nor leading to an E/M service or procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical discussion
99442 11-20 minutes of medical discussion
99443 21-30 minutes of medical discussion
These codes may not be reported by a provider more frequently than every 7 days. The details of the service should be documented in the medical record.
If the E/M service is prompted by an online patient request, then CPT code 99444 can be used.
99444 Online evaluation and management service provided by a physician or other qualified health care professional who may report evaluation and management services provided to an established patient or guardian, not originating from a related E/M service provided within the previous 7 days, using the internet or similar electronic communications network.
This code may be reported only every 7 days and can not be related to a previous E/M evaluation in the last 7 days or to a previous surgical procedure. The service includes all of the communication (eg, related telephone calls, prescription provision, laboratory orders) pertaining to the online patient encounter.
There are also CPT codes for Interprofessional Telephone/Internet/Electronic Health Record Consultations. These codes are used when one health-care provider requests the opinion and/or treatment advice of another provider (consultant) for either a new or established patient without face-to-face contact between the patient and the consultant.
99446 Interprofessional telephone/Internet/electronic health record assessment and management service provided by a consultative physician, including a verbal and written report to the patient’s treating/requesting physician or other qualified health care professional; 5-10 minutes of medical consultative discussion and review.
99447 11-20 minutes of medical consultative discussion and review
99448 21-30 minutes of medical consultative discussion and review
99449 31 minutes or more of medical consultative discussion and review
These codes are not used if the consultant has seen the patient in a face-to-face encounter within the last 14 days or the consultation results in a transfer of care or other face-to-face service with the consultant within the next 14 days. In addition, greater than 50% of the service time reported must be devoted to the medical consultative verbal or internet discussion. The request and reason for telephone/internet/electronic health record consultation by the requesting health-care professional should be documented in the patient’s medical record. After an oral report from the consultant is provided to the treating/requesting physician, a written report should be documented in the medical record. Consultations of less than 5 minutes should not be reported.
As noted, CPT codes 99446-49 require an oral and written report. A new code is added for 2019.
99451 Interprofessional telephone/Internet/electronic health record assessment and management service provided by a consultative physician, including a written report to the patient’s treating/requesting physician or other qualified health care professional, 5 minutes or more of medical consultative time.
CPT code 99451 describes a consultative service lasting more than 5 minutes and requires only a written report to the requesting physician. This was added recognizing that oral communications do not always occur between healthcare professionals and may facilitate consultative services in geographic areas with no specialists available.
99452 Interprofessional telephone/Internet/electronic health record referral service(s) provided by a treating/requesting physician or other qualified health care professional, 30 minutes.
CPT code 99452 is reported for 16-30 minutes preparing for the referral and/or communicating with a consultant. If more than 30 minutes is spent by the treating/requesting healthcare provider, then one would use a prolonged services code (99358-59).
As with all coding and billing issues, review the CPT manual for parentheticals that describe coding rules not included in the code description. In addition, not all CPT codes are paid by all providers. Knowledge of payer policies is, therefore, important for appropriate reimbursement.
Welcome Dr. Cowl!
As we greet our new CHEST President, Clayton T. Cowl, MD, MS, FCCP, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would be one of the many things you would like to accomplish as President of CHEST?
We plan to increase the engagement of our membership, and, in do so, allow for more opportunities to serve in leadership roles, educate as faculty, or to participate in more of the wide array of educational opportunities within CHEST – whether the member is a long-tenured physician, a trainee, an earlier career researcher or educator, or a colleague in the care team, such as a respiratory therapist, advanced practice provider, or a pharmacist. CHEST has been and will continue to be a leader in delivery of education, and will further advance opportunities to present breaking research. Ultimately, the reason we are in medicine is to improve the care that we deliver to our patients, so it is incumbent upon us to keep the mission aimed toward “patient-centric” goals.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
Our greatest strength is our members, who bring a diversity of experience, expertise, and passion for what they do at the forefront. Together, with our incredibly talented and dedicated support staff at CHEST, as well as our industry and publishing partners, our organization is poised to bring medical education in pulmonary, critical care, and sleep medicine globally to the next level. The CHEST Foundation has stimulated important opportunities for research, increased the ability for younger members to attend meetings and actively engage in CHEST activities, and provided valuable information to patients in a language they can understand. Thanks to advances in technology, there are improved platforms for communicating with our membership and for delivering education in novel and more effective ways than ever before. We plan to double down on our strategic focus of utilizing innovation and new technologies to lead trends in education, influence health-care improvements for our patients and their families, and to deliver the latest in medical education to clinicians and investigators worldwide.
What are some challenges facing CHEST, and how will you address these challenges?
Many of our members are facing challenges in their practices – both domestically and internationally. Industry and employer-based sponsorship to attend meetings has declined, travel remains expensive, and time away from the practice has become more and more difficult for a variety of reasons. Our members are being challenged with greater regulatory and administrative burdens and are bombarded with the demands of work overload. In addition to working with other organizations to identify workplace burnout and, more importantly, to offer better solutions, we are focused on leveraging a variety of new technologies to bring our CHEST brand of quality education to all of our members, regardless of location, and to do so in a way that best suits individual needs. The traditional model of attending a large meeting comprised solely of didactic presentations is, frankly, becoming outdated. CHEST will continue to “tip the apple cart” of worn out educational delivery methods and look toward innovating courses that are more accessible, more effective and relevant, more affordable, and more fun.
And finally, what is your charge to the members and new Fellows of CHEST?
We have each been blessed with the opportunity to serve patients and their families in their times of need. Let’s not forget that privilege as we deliver care each and every day. The word “doctor” comes from an agentive noun of the Latin verb docēre (“to teach”). Regardless of where you practice, what your role is in the health-care paradigm, or whether your contribution is directly with patients or indirectly through research, education, or administration, we are all teachers in various ways to various people. That’s why the American College of Chest Physicians (CHEST) needs to listen to your needs, cultivate your collective wisdom, and continue to be the leading organization within our specialties for delivering medical education and, ultimately, for providing outstanding care and compassion to our patients.
As we greet our new CHEST President, Clayton T. Cowl, MD, MS, FCCP, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would be one of the many things you would like to accomplish as President of CHEST?
We plan to increase the engagement of our membership, and, in do so, allow for more opportunities to serve in leadership roles, educate as faculty, or to participate in more of the wide array of educational opportunities within CHEST – whether the member is a long-tenured physician, a trainee, an earlier career researcher or educator, or a colleague in the care team, such as a respiratory therapist, advanced practice provider, or a pharmacist. CHEST has been and will continue to be a leader in delivery of education, and will further advance opportunities to present breaking research. Ultimately, the reason we are in medicine is to improve the care that we deliver to our patients, so it is incumbent upon us to keep the mission aimed toward “patient-centric” goals.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
Our greatest strength is our members, who bring a diversity of experience, expertise, and passion for what they do at the forefront. Together, with our incredibly talented and dedicated support staff at CHEST, as well as our industry and publishing partners, our organization is poised to bring medical education in pulmonary, critical care, and sleep medicine globally to the next level. The CHEST Foundation has stimulated important opportunities for research, increased the ability for younger members to attend meetings and actively engage in CHEST activities, and provided valuable information to patients in a language they can understand. Thanks to advances in technology, there are improved platforms for communicating with our membership and for delivering education in novel and more effective ways than ever before. We plan to double down on our strategic focus of utilizing innovation and new technologies to lead trends in education, influence health-care improvements for our patients and their families, and to deliver the latest in medical education to clinicians and investigators worldwide.
What are some challenges facing CHEST, and how will you address these challenges?
Many of our members are facing challenges in their practices – both domestically and internationally. Industry and employer-based sponsorship to attend meetings has declined, travel remains expensive, and time away from the practice has become more and more difficult for a variety of reasons. Our members are being challenged with greater regulatory and administrative burdens and are bombarded with the demands of work overload. In addition to working with other organizations to identify workplace burnout and, more importantly, to offer better solutions, we are focused on leveraging a variety of new technologies to bring our CHEST brand of quality education to all of our members, regardless of location, and to do so in a way that best suits individual needs. The traditional model of attending a large meeting comprised solely of didactic presentations is, frankly, becoming outdated. CHEST will continue to “tip the apple cart” of worn out educational delivery methods and look toward innovating courses that are more accessible, more effective and relevant, more affordable, and more fun.
And finally, what is your charge to the members and new Fellows of CHEST?
We have each been blessed with the opportunity to serve patients and their families in their times of need. Let’s not forget that privilege as we deliver care each and every day. The word “doctor” comes from an agentive noun of the Latin verb docēre (“to teach”). Regardless of where you practice, what your role is in the health-care paradigm, or whether your contribution is directly with patients or indirectly through research, education, or administration, we are all teachers in various ways to various people. That’s why the American College of Chest Physicians (CHEST) needs to listen to your needs, cultivate your collective wisdom, and continue to be the leading organization within our specialties for delivering medical education and, ultimately, for providing outstanding care and compassion to our patients.
As we greet our new CHEST President, Clayton T. Cowl, MD, MS, FCCP, we asked him for a few thoughts about his upcoming presidential year. He kindly offered these responses:
What would be one of the many things you would like to accomplish as President of CHEST?
We plan to increase the engagement of our membership, and, in do so, allow for more opportunities to serve in leadership roles, educate as faculty, or to participate in more of the wide array of educational opportunities within CHEST – whether the member is a long-tenured physician, a trainee, an earlier career researcher or educator, or a colleague in the care team, such as a respiratory therapist, advanced practice provider, or a pharmacist. CHEST has been and will continue to be a leader in delivery of education, and will further advance opportunities to present breaking research. Ultimately, the reason we are in medicine is to improve the care that we deliver to our patients, so it is incumbent upon us to keep the mission aimed toward “patient-centric” goals.
What do you consider to be the greatest strength of CHEST, and how will you build upon this during your Presidency?
Our greatest strength is our members, who bring a diversity of experience, expertise, and passion for what they do at the forefront. Together, with our incredibly talented and dedicated support staff at CHEST, as well as our industry and publishing partners, our organization is poised to bring medical education in pulmonary, critical care, and sleep medicine globally to the next level. The CHEST Foundation has stimulated important opportunities for research, increased the ability for younger members to attend meetings and actively engage in CHEST activities, and provided valuable information to patients in a language they can understand. Thanks to advances in technology, there are improved platforms for communicating with our membership and for delivering education in novel and more effective ways than ever before. We plan to double down on our strategic focus of utilizing innovation and new technologies to lead trends in education, influence health-care improvements for our patients and their families, and to deliver the latest in medical education to clinicians and investigators worldwide.
What are some challenges facing CHEST, and how will you address these challenges?
Many of our members are facing challenges in their practices – both domestically and internationally. Industry and employer-based sponsorship to attend meetings has declined, travel remains expensive, and time away from the practice has become more and more difficult for a variety of reasons. Our members are being challenged with greater regulatory and administrative burdens and are bombarded with the demands of work overload. In addition to working with other organizations to identify workplace burnout and, more importantly, to offer better solutions, we are focused on leveraging a variety of new technologies to bring our CHEST brand of quality education to all of our members, regardless of location, and to do so in a way that best suits individual needs. The traditional model of attending a large meeting comprised solely of didactic presentations is, frankly, becoming outdated. CHEST will continue to “tip the apple cart” of worn out educational delivery methods and look toward innovating courses that are more accessible, more effective and relevant, more affordable, and more fun.
And finally, what is your charge to the members and new Fellows of CHEST?
We have each been blessed with the opportunity to serve patients and their families in their times of need. Let’s not forget that privilege as we deliver care each and every day. The word “doctor” comes from an agentive noun of the Latin verb docēre (“to teach”). Regardless of where you practice, what your role is in the health-care paradigm, or whether your contribution is directly with patients or indirectly through research, education, or administration, we are all teachers in various ways to various people. That’s why the American College of Chest Physicians (CHEST) needs to listen to your needs, cultivate your collective wisdom, and continue to be the leading organization within our specialties for delivering medical education and, ultimately, for providing outstanding care and compassion to our patients.
The importance of diversity and inclusion in medicine
Diversity
There is growing appreciation for diversity and inclusion (DI) as drivers of excellence in medicine. CHEST also promotes excellence in medicine. Therefore, it is intuitive that CHEST promote DI. Diversity encompasses differences in gender, race/ethnicity, vocational training, age, sexual orientation, thought processes, etc.
Academic medicine is rich with examples of how diversity is critical to the health of our nation:
– Diverse student populations have been shown to improve our learners’ satisfaction with their educational experience.
– Diverse teams have been shown to be more capable of solving complex problems than homogenous teams.
– Health care is moving toward a team-based, interprofessional model that values the contributions of a range of providers’ perspectives in improving patient outcomes.
– In biomedical research, investigators ask different research questions based on their own background and experiences. This implies that finding solutions to diseases that affect specific populations will require a diverse pool of biomedical researchers.
– Faculty diversity as a key component of excellence for medical education and research has been documented.
Diversity alone doesn’t drive inclusion. Noted diversity advocate, Verna Myers, stated, “Diversity is being invited to the party. Inclusion is being asked to dance.” In my opinion, diversity is the commencement of work, but inclusion helps complete the task.
Inclusion
An inclusive environment values the unique contributions all members bring. Teams with diversity of thought are more innovative as individual members with different backgrounds and points of view bring an extensive range of ideas and creativity to scientific discovery and decision-making processes. Inclusion leverages the power of our unique differences to accomplish our mutual goals. By valuing everyone’s perspective, we demonstrate excellence.
I recommend an article from the Harvard Business Review (HBR Feb 2017). The authors suggest several ways to promote inclusiveness: (1) ensuring team members speak up and are heard; (2) making it safe to propose novel ideas; (3) empowering team members to make decisions; (4) taking advice and implementing feedback; (5) giving actionable feedback; and ( 6) sharing credit for team success. If the team leader possesses at least three of these traits, 87% of team members say they feel welcome and included in their team; 87% say they feel free to express their views and opinions; and 74% say they feel that their ideas are heard and recognized. If the team leader possessed none of these traits, those percentages dropped to 51%, 46%, and 37%, respectively. I believe this concept is applicable in medicine also.
Sponsors
What can we do to advance diversity and inclusion individually and in our individual institutions? A sponsor is a senior level leader who advocates for key assignments, promotes for and puts his or her reputation on the line for the protégé’s advancement. This invigorates and drives engagement. One key to rising above the playing field for women and people of color is sponsorship. Being a sponsor does not mean one would recommend someone who is not qualified. It means one recommends or supports those who are capable of doing the job but would not otherwise be given the opportunity.
Ask yourself: Have I served as a sponsor? What would prevent me from being a sponsor? Do I believe in this concept?
Cause for Alarm
Numerous publications have recently discussed the crisis of the decline of black men entering medicine. In 1978, there were 1,410 black male applicants to medical school, and in 2014, there were 1,337. Additionally, the number of black male matriculants to medical school over more than 35 years has not surpassed the 1978 numbers. In 1978, there were 542 black male matriculants, and in 2014, there were 515 (J of Racial and Ethnic Health Disparities. 2017, 4:317-321). This report is thorough and insightful and illustrates the work that we must do to help improve this situation.
Dr. Marc Nivet, Association of American Medical Colleges (AAMC) Chief Diversity Officer, stated “No other minority group has experienced such declines. The inability to find, engage, and develop candidates for careers in medicine from all members of our society limits our ability to improve health care for all.” I recommend you read the 2015 AAMC publication entitled: Altering the Course: Black Males in Medicine.
Health-care Disparities
Research suggests that the overall health of Americans has improved; however, disparities continue to persist among many populations within the United States. Racial and ethnic minority populations have poorer access to care and worse outcomes than their white counterparts. Approximately 20% of the nation living in rural areas is less likely than those living in urban areas to receive preventive care and more likely to experience language barriers.
Individuals identifying as lesbian, gay, bisexual, or transgender are likely to experience discrimination in health-care settings. These individuals often face insurance-based barriers and are less likely to have a usual source of care than patients who identify as straight.
A 2002 report by the Institute of Medicine entitled: Unequal Treatment: What Healthcare Providers Need to Know about Racial and Ethnic Disparities in Healthcare is revealing. Salient information reported is: It is generally accepted that a diverse workforce is a key component in the delivery of quality, competent care throughout the nation. Physicians from racial and ethnic backgrounds typically underrepresented in medicine are significantly more likely to practice primary care than white physicians and are more likely to practice in impoverished and medically underserved areas. Diversity in the physician workforce impacts the quality of care received by patients. Race concordance between patient and physician results in longer visits and increased patient satisfaction, and language concordance is positively associated with adherence to treatment among certain racial or ethnic groups.
Improving the patient experience or quality of care received also requires attention to education and training on cultural competence. By weaving together a diverse and culturally responsive pool of physicians working collaboratively with other health-care professionals, access and quality of care can improve throughout the nation.
CHEST cannot attain more racial diversity in our organization if we don’t have this diversity in medical education and training. This is why CHEST must be actively involved in addressing these issues.
Unconscious Bias
Despite many examples of how diversity enriches the quality of health care and health research, there is still much work to be done to address the human biases that impede our ability to benefit from diversity in medicine. While academic medicine has made progress toward addressing overt discrimination, unconscious bias (implicit bias) represents another threat. Unconscious bias describes the prejudices we don’t know we have. While unconscious biases vary from person to person, we all possess them. The existence of unconscious bias in academic medicine, while uncomfortable and unsettling, is a reality. The AAMC developed an unconscious bias learning lab for the health professions and produced an oft-cited video about addressing unconscious bias in the faculty advancement, promotion, and tenure process. We must consider this and other ways in which we can help promote the acknowledgment of unconscious bias. The CHEST staff have undergone unconscious bias training, and I recommend it for all faculty in academic medicine.
Summary
Diversity and inclusion in medicine is of paramount importance. It leads to better patient care and better trainee education and will decrease health-care disparities. Progress has been made, but there is more work to be done.
CHEST is supportive of these efforts and has worked on this previously and with a renewed push in the past 2 years with the DI Task Force initially and, now, the DI Roundtable, which has representatives from each of the standing committees, including the Board of Regents. This roundtable group will help advance the DI initiatives of the organization. I ask that each person reading this article consider what we as individuals can do in helping make DI in medicine a priority.
Dr. Haynes is Professor of Medicine at The University of Mississippi Medical Center in Jackson, MS. He is also the Executive Vice Chair of the Department of Medicine. At CHEST, he is a member of the training and transitions committee, executive scientific program committee, former chair of the diversity and inclusion task force, and is the current chair of the diversity and inclusion roundtable.
Diversity
There is growing appreciation for diversity and inclusion (DI) as drivers of excellence in medicine. CHEST also promotes excellence in medicine. Therefore, it is intuitive that CHEST promote DI. Diversity encompasses differences in gender, race/ethnicity, vocational training, age, sexual orientation, thought processes, etc.
Academic medicine is rich with examples of how diversity is critical to the health of our nation:
– Diverse student populations have been shown to improve our learners’ satisfaction with their educational experience.
– Diverse teams have been shown to be more capable of solving complex problems than homogenous teams.
– Health care is moving toward a team-based, interprofessional model that values the contributions of a range of providers’ perspectives in improving patient outcomes.
– In biomedical research, investigators ask different research questions based on their own background and experiences. This implies that finding solutions to diseases that affect specific populations will require a diverse pool of biomedical researchers.
– Faculty diversity as a key component of excellence for medical education and research has been documented.
Diversity alone doesn’t drive inclusion. Noted diversity advocate, Verna Myers, stated, “Diversity is being invited to the party. Inclusion is being asked to dance.” In my opinion, diversity is the commencement of work, but inclusion helps complete the task.
Inclusion
An inclusive environment values the unique contributions all members bring. Teams with diversity of thought are more innovative as individual members with different backgrounds and points of view bring an extensive range of ideas and creativity to scientific discovery and decision-making processes. Inclusion leverages the power of our unique differences to accomplish our mutual goals. By valuing everyone’s perspective, we demonstrate excellence.
I recommend an article from the Harvard Business Review (HBR Feb 2017). The authors suggest several ways to promote inclusiveness: (1) ensuring team members speak up and are heard; (2) making it safe to propose novel ideas; (3) empowering team members to make decisions; (4) taking advice and implementing feedback; (5) giving actionable feedback; and ( 6) sharing credit for team success. If the team leader possesses at least three of these traits, 87% of team members say they feel welcome and included in their team; 87% say they feel free to express their views and opinions; and 74% say they feel that their ideas are heard and recognized. If the team leader possessed none of these traits, those percentages dropped to 51%, 46%, and 37%, respectively. I believe this concept is applicable in medicine also.
Sponsors
What can we do to advance diversity and inclusion individually and in our individual institutions? A sponsor is a senior level leader who advocates for key assignments, promotes for and puts his or her reputation on the line for the protégé’s advancement. This invigorates and drives engagement. One key to rising above the playing field for women and people of color is sponsorship. Being a sponsor does not mean one would recommend someone who is not qualified. It means one recommends or supports those who are capable of doing the job but would not otherwise be given the opportunity.
Ask yourself: Have I served as a sponsor? What would prevent me from being a sponsor? Do I believe in this concept?
Cause for Alarm
Numerous publications have recently discussed the crisis of the decline of black men entering medicine. In 1978, there were 1,410 black male applicants to medical school, and in 2014, there were 1,337. Additionally, the number of black male matriculants to medical school over more than 35 years has not surpassed the 1978 numbers. In 1978, there were 542 black male matriculants, and in 2014, there were 515 (J of Racial and Ethnic Health Disparities. 2017, 4:317-321). This report is thorough and insightful and illustrates the work that we must do to help improve this situation.
Dr. Marc Nivet, Association of American Medical Colleges (AAMC) Chief Diversity Officer, stated “No other minority group has experienced such declines. The inability to find, engage, and develop candidates for careers in medicine from all members of our society limits our ability to improve health care for all.” I recommend you read the 2015 AAMC publication entitled: Altering the Course: Black Males in Medicine.
Health-care Disparities
Research suggests that the overall health of Americans has improved; however, disparities continue to persist among many populations within the United States. Racial and ethnic minority populations have poorer access to care and worse outcomes than their white counterparts. Approximately 20% of the nation living in rural areas is less likely than those living in urban areas to receive preventive care and more likely to experience language barriers.
Individuals identifying as lesbian, gay, bisexual, or transgender are likely to experience discrimination in health-care settings. These individuals often face insurance-based barriers and are less likely to have a usual source of care than patients who identify as straight.
A 2002 report by the Institute of Medicine entitled: Unequal Treatment: What Healthcare Providers Need to Know about Racial and Ethnic Disparities in Healthcare is revealing. Salient information reported is: It is generally accepted that a diverse workforce is a key component in the delivery of quality, competent care throughout the nation. Physicians from racial and ethnic backgrounds typically underrepresented in medicine are significantly more likely to practice primary care than white physicians and are more likely to practice in impoverished and medically underserved areas. Diversity in the physician workforce impacts the quality of care received by patients. Race concordance between patient and physician results in longer visits and increased patient satisfaction, and language concordance is positively associated with adherence to treatment among certain racial or ethnic groups.
Improving the patient experience or quality of care received also requires attention to education and training on cultural competence. By weaving together a diverse and culturally responsive pool of physicians working collaboratively with other health-care professionals, access and quality of care can improve throughout the nation.
CHEST cannot attain more racial diversity in our organization if we don’t have this diversity in medical education and training. This is why CHEST must be actively involved in addressing these issues.
Unconscious Bias
Despite many examples of how diversity enriches the quality of health care and health research, there is still much work to be done to address the human biases that impede our ability to benefit from diversity in medicine. While academic medicine has made progress toward addressing overt discrimination, unconscious bias (implicit bias) represents another threat. Unconscious bias describes the prejudices we don’t know we have. While unconscious biases vary from person to person, we all possess them. The existence of unconscious bias in academic medicine, while uncomfortable and unsettling, is a reality. The AAMC developed an unconscious bias learning lab for the health professions and produced an oft-cited video about addressing unconscious bias in the faculty advancement, promotion, and tenure process. We must consider this and other ways in which we can help promote the acknowledgment of unconscious bias. The CHEST staff have undergone unconscious bias training, and I recommend it for all faculty in academic medicine.
Summary
Diversity and inclusion in medicine is of paramount importance. It leads to better patient care and better trainee education and will decrease health-care disparities. Progress has been made, but there is more work to be done.
CHEST is supportive of these efforts and has worked on this previously and with a renewed push in the past 2 years with the DI Task Force initially and, now, the DI Roundtable, which has representatives from each of the standing committees, including the Board of Regents. This roundtable group will help advance the DI initiatives of the organization. I ask that each person reading this article consider what we as individuals can do in helping make DI in medicine a priority.
Dr. Haynes is Professor of Medicine at The University of Mississippi Medical Center in Jackson, MS. He is also the Executive Vice Chair of the Department of Medicine. At CHEST, he is a member of the training and transitions committee, executive scientific program committee, former chair of the diversity and inclusion task force, and is the current chair of the diversity and inclusion roundtable.
Diversity
There is growing appreciation for diversity and inclusion (DI) as drivers of excellence in medicine. CHEST also promotes excellence in medicine. Therefore, it is intuitive that CHEST promote DI. Diversity encompasses differences in gender, race/ethnicity, vocational training, age, sexual orientation, thought processes, etc.
Academic medicine is rich with examples of how diversity is critical to the health of our nation:
– Diverse student populations have been shown to improve our learners’ satisfaction with their educational experience.
– Diverse teams have been shown to be more capable of solving complex problems than homogenous teams.
– Health care is moving toward a team-based, interprofessional model that values the contributions of a range of providers’ perspectives in improving patient outcomes.
– In biomedical research, investigators ask different research questions based on their own background and experiences. This implies that finding solutions to diseases that affect specific populations will require a diverse pool of biomedical researchers.
– Faculty diversity as a key component of excellence for medical education and research has been documented.
Diversity alone doesn’t drive inclusion. Noted diversity advocate, Verna Myers, stated, “Diversity is being invited to the party. Inclusion is being asked to dance.” In my opinion, diversity is the commencement of work, but inclusion helps complete the task.
Inclusion
An inclusive environment values the unique contributions all members bring. Teams with diversity of thought are more innovative as individual members with different backgrounds and points of view bring an extensive range of ideas and creativity to scientific discovery and decision-making processes. Inclusion leverages the power of our unique differences to accomplish our mutual goals. By valuing everyone’s perspective, we demonstrate excellence.
I recommend an article from the Harvard Business Review (HBR Feb 2017). The authors suggest several ways to promote inclusiveness: (1) ensuring team members speak up and are heard; (2) making it safe to propose novel ideas; (3) empowering team members to make decisions; (4) taking advice and implementing feedback; (5) giving actionable feedback; and ( 6) sharing credit for team success. If the team leader possesses at least three of these traits, 87% of team members say they feel welcome and included in their team; 87% say they feel free to express their views and opinions; and 74% say they feel that their ideas are heard and recognized. If the team leader possessed none of these traits, those percentages dropped to 51%, 46%, and 37%, respectively. I believe this concept is applicable in medicine also.
Sponsors
What can we do to advance diversity and inclusion individually and in our individual institutions? A sponsor is a senior level leader who advocates for key assignments, promotes for and puts his or her reputation on the line for the protégé’s advancement. This invigorates and drives engagement. One key to rising above the playing field for women and people of color is sponsorship. Being a sponsor does not mean one would recommend someone who is not qualified. It means one recommends or supports those who are capable of doing the job but would not otherwise be given the opportunity.
Ask yourself: Have I served as a sponsor? What would prevent me from being a sponsor? Do I believe in this concept?
Cause for Alarm
Numerous publications have recently discussed the crisis of the decline of black men entering medicine. In 1978, there were 1,410 black male applicants to medical school, and in 2014, there were 1,337. Additionally, the number of black male matriculants to medical school over more than 35 years has not surpassed the 1978 numbers. In 1978, there were 542 black male matriculants, and in 2014, there were 515 (J of Racial and Ethnic Health Disparities. 2017, 4:317-321). This report is thorough and insightful and illustrates the work that we must do to help improve this situation.
Dr. Marc Nivet, Association of American Medical Colleges (AAMC) Chief Diversity Officer, stated “No other minority group has experienced such declines. The inability to find, engage, and develop candidates for careers in medicine from all members of our society limits our ability to improve health care for all.” I recommend you read the 2015 AAMC publication entitled: Altering the Course: Black Males in Medicine.
Health-care Disparities
Research suggests that the overall health of Americans has improved; however, disparities continue to persist among many populations within the United States. Racial and ethnic minority populations have poorer access to care and worse outcomes than their white counterparts. Approximately 20% of the nation living in rural areas is less likely than those living in urban areas to receive preventive care and more likely to experience language barriers.
Individuals identifying as lesbian, gay, bisexual, or transgender are likely to experience discrimination in health-care settings. These individuals often face insurance-based barriers and are less likely to have a usual source of care than patients who identify as straight.
A 2002 report by the Institute of Medicine entitled: Unequal Treatment: What Healthcare Providers Need to Know about Racial and Ethnic Disparities in Healthcare is revealing. Salient information reported is: It is generally accepted that a diverse workforce is a key component in the delivery of quality, competent care throughout the nation. Physicians from racial and ethnic backgrounds typically underrepresented in medicine are significantly more likely to practice primary care than white physicians and are more likely to practice in impoverished and medically underserved areas. Diversity in the physician workforce impacts the quality of care received by patients. Race concordance between patient and physician results in longer visits and increased patient satisfaction, and language concordance is positively associated with adherence to treatment among certain racial or ethnic groups.
Improving the patient experience or quality of care received also requires attention to education and training on cultural competence. By weaving together a diverse and culturally responsive pool of physicians working collaboratively with other health-care professionals, access and quality of care can improve throughout the nation.
CHEST cannot attain more racial diversity in our organization if we don’t have this diversity in medical education and training. This is why CHEST must be actively involved in addressing these issues.
Unconscious Bias
Despite many examples of how diversity enriches the quality of health care and health research, there is still much work to be done to address the human biases that impede our ability to benefit from diversity in medicine. While academic medicine has made progress toward addressing overt discrimination, unconscious bias (implicit bias) represents another threat. Unconscious bias describes the prejudices we don’t know we have. While unconscious biases vary from person to person, we all possess them. The existence of unconscious bias in academic medicine, while uncomfortable and unsettling, is a reality. The AAMC developed an unconscious bias learning lab for the health professions and produced an oft-cited video about addressing unconscious bias in the faculty advancement, promotion, and tenure process. We must consider this and other ways in which we can help promote the acknowledgment of unconscious bias. The CHEST staff have undergone unconscious bias training, and I recommend it for all faculty in academic medicine.
Summary
Diversity and inclusion in medicine is of paramount importance. It leads to better patient care and better trainee education and will decrease health-care disparities. Progress has been made, but there is more work to be done.
CHEST is supportive of these efforts and has worked on this previously and with a renewed push in the past 2 years with the DI Task Force initially and, now, the DI Roundtable, which has representatives from each of the standing committees, including the Board of Regents. This roundtable group will help advance the DI initiatives of the organization. I ask that each person reading this article consider what we as individuals can do in helping make DI in medicine a priority.
Dr. Haynes is Professor of Medicine at The University of Mississippi Medical Center in Jackson, MS. He is also the Executive Vice Chair of the Department of Medicine. At CHEST, he is a member of the training and transitions committee, executive scientific program committee, former chair of the diversity and inclusion task force, and is the current chair of the diversity and inclusion roundtable.
CHEST Foundation – designated as a Combined Federal Campaign-approved charity
The CHEST Foundation was recently designated as a Combined Federal Campaign-approved charity! The federal campaign started on September 10 and runs through January 11, 2019. If you are a federal employee organizing your workplace giving, you can easily choose the CHEST Foundation as your designated charity! Simply list our CFC number when designating your selected charity! CFC Number: 24565
To set up your CFC account, follow these easy steps outlined below:
1. Visit https://cfcgiving.opm.gov/welcome
2. From the welcome page, select “sign up now,” and fill out the required information if you do not have an account. If you do have an account, simply log in using the email address tied to your CFC account and your password, and skip to step 6.
3. After your account is set up, the CFC will send you an email to the address you provided along with a verification pin number. Select the “CLICK HERE to enter your PIN” option from the verification email, and enter the provided pin on the page provided by the link.
4. On the next page, you will create your security questions to log back into your account, should you lose your password. Select “Save Changes” at the bottom of the page when you are ready to move on.
5. Next, you’ll be asked to fill out some personal information about yourself as a donor, such as your full name, and which department of the federal government you work for. Choose “Save Changes” at the bottom of the page when you are done.
6. Upon completing the profile page (or logging into your account), you will be directed to the welcome page. Select the “Pledge Now” button, located in the center of the page.
7. The next page will ask you questions about the charity you would like to support. Enter the CHEST Foundation’s CFC number: 24565, and click “Search for Charities” to be directed to the next page.
8. Select the “add” button next to the CHEST Foundation’s listing. Then click the “checkout” button that appears in the pop-up window.
9. Fill out the requested information regarding your pledge amount, your pledge frequency, and your annual pledge amount, then select the “Continue with your pledge” option at the bottom of the page.
10. On this final page, you can review your pledge amount and review a brief attestation agreement. After reviewing, check the “I confirm” checkbox, then click “submit pledge.”
That’s it!
Thank you for supporting the CHEST Foundation’s mission-based programming supporting patient education materials, clinical research grants, and community service initiatives.
The CHEST Foundation was recently designated as a Combined Federal Campaign-approved charity! The federal campaign started on September 10 and runs through January 11, 2019. If you are a federal employee organizing your workplace giving, you can easily choose the CHEST Foundation as your designated charity! Simply list our CFC number when designating your selected charity! CFC Number: 24565
To set up your CFC account, follow these easy steps outlined below:
1. Visit https://cfcgiving.opm.gov/welcome
2. From the welcome page, select “sign up now,” and fill out the required information if you do not have an account. If you do have an account, simply log in using the email address tied to your CFC account and your password, and skip to step 6.
3. After your account is set up, the CFC will send you an email to the address you provided along with a verification pin number. Select the “CLICK HERE to enter your PIN” option from the verification email, and enter the provided pin on the page provided by the link.
4. On the next page, you will create your security questions to log back into your account, should you lose your password. Select “Save Changes” at the bottom of the page when you are ready to move on.
5. Next, you’ll be asked to fill out some personal information about yourself as a donor, such as your full name, and which department of the federal government you work for. Choose “Save Changes” at the bottom of the page when you are done.
6. Upon completing the profile page (or logging into your account), you will be directed to the welcome page. Select the “Pledge Now” button, located in the center of the page.
7. The next page will ask you questions about the charity you would like to support. Enter the CHEST Foundation’s CFC number: 24565, and click “Search for Charities” to be directed to the next page.
8. Select the “add” button next to the CHEST Foundation’s listing. Then click the “checkout” button that appears in the pop-up window.
9. Fill out the requested information regarding your pledge amount, your pledge frequency, and your annual pledge amount, then select the “Continue with your pledge” option at the bottom of the page.
10. On this final page, you can review your pledge amount and review a brief attestation agreement. After reviewing, check the “I confirm” checkbox, then click “submit pledge.”
That’s it!
Thank you for supporting the CHEST Foundation’s mission-based programming supporting patient education materials, clinical research grants, and community service initiatives.
The CHEST Foundation was recently designated as a Combined Federal Campaign-approved charity! The federal campaign started on September 10 and runs through January 11, 2019. If you are a federal employee organizing your workplace giving, you can easily choose the CHEST Foundation as your designated charity! Simply list our CFC number when designating your selected charity! CFC Number: 24565
To set up your CFC account, follow these easy steps outlined below:
1. Visit https://cfcgiving.opm.gov/welcome
2. From the welcome page, select “sign up now,” and fill out the required information if you do not have an account. If you do have an account, simply log in using the email address tied to your CFC account and your password, and skip to step 6.
3. After your account is set up, the CFC will send you an email to the address you provided along with a verification pin number. Select the “CLICK HERE to enter your PIN” option from the verification email, and enter the provided pin on the page provided by the link.
4. On the next page, you will create your security questions to log back into your account, should you lose your password. Select “Save Changes” at the bottom of the page when you are ready to move on.
5. Next, you’ll be asked to fill out some personal information about yourself as a donor, such as your full name, and which department of the federal government you work for. Choose “Save Changes” at the bottom of the page when you are done.
6. Upon completing the profile page (or logging into your account), you will be directed to the welcome page. Select the “Pledge Now” button, located in the center of the page.
7. The next page will ask you questions about the charity you would like to support. Enter the CHEST Foundation’s CFC number: 24565, and click “Search for Charities” to be directed to the next page.
8. Select the “add” button next to the CHEST Foundation’s listing. Then click the “checkout” button that appears in the pop-up window.
9. Fill out the requested information regarding your pledge amount, your pledge frequency, and your annual pledge amount, then select the “Continue with your pledge” option at the bottom of the page.
10. On this final page, you can review your pledge amount and review a brief attestation agreement. After reviewing, check the “I confirm” checkbox, then click “submit pledge.”
That’s it!
Thank you for supporting the CHEST Foundation’s mission-based programming supporting patient education materials, clinical research grants, and community service initiatives.
Secondary fractures in older men spike soon after first, but exercise may help
Older men have a higher risk than women of sustaining secondary fractures within a few years of their first fracture, but moderate physical activity may improve bone strength, potentially reducing their risk of fractures, according to two studies presented at the annual meeting of the American Society for Bone and Mineral Research in Montreal.
The first study, a matched historical cohort of 57,783 people aged 50 or older (40,062 women and 17,721 men) in Manitoba, Canada, found that men had a threefold higher risk of sustaining a secondary major osteoporotic fracture (MOF) within 1 year of a first fracture, compared with healthy controls. The risk for women, by comparison, was 1.8 times higher than in age-matched controls who did not experience a fracture. These risks declined over time but remained elevated even as much as 15-25 years after the index fracture, according to primary investigator Suzanne N. Morin, MD, of the department of medicine at McGill University in Montreal.
“Often, men and clinicians don’t think men have skeletal fragility – everybody thinks it’s a women’s disease,” Dr. Morin said. “It’s true that it’s more frequent in women, but men do have osteoporosis, and often when they have it, they tend to have more serious complications following the fractures.” This includes higher risk of subsequent fractures and higher mortality, she said. “If you see an older gentleman with a fracture, it really should be some kind of an alarm signal.”
Using administrative health care databases, Dr. Morin and her colleagues reviewed records of patients who had an index MOF between 1989 and 2006. They compared rates of subsequent MOFs until 2016 with those of age- and sex-matched controls (n = 165,965), allowing for between 10 and 25 years of follow-up.
Researchers identified 29,694 index MOF cases (11,028 to the wrist, 9,313 to the hip, 5,799 to the humerus, and 3,554 to the spine). The annual crude rate of subsequent MOFs per 1,000 person-years was 18.5 in men (95% confidence interval, 17.3-19.8) and 29.6 in women (95% CI, 28.8-30.4). The cumulative incidence of subsequent MOFs up to 25 years later was higher in cases versus controls for both sexes and across all ages except those over 80.
Hazard ratios for subsequent MOFs were higher in men than women, particularly in the first year following the index fracture and remained very high for men during the first 3 years of follow-up. Across all follow-up years, men who had fractures were 2.5 times more likely to experience a secondary MOF (95% CI, 2.3-2.7) and women who had fractures were 1.6 times more likely to experience a secondary MOF (95% CI, 1.6-1.7), compared with controls.
To prevent fractures, clinicians should consider gait or balance training for older men and women, especially those who already have experienced a fracture, Dr. Morin said. Physicians also should note any medications such as sedatives that put patients at higher risk for falls and consider medications like bisphosphonates to reduce fracture risk. Additionally, they should ensure there are no underlying causes for skeletal fragility, such as severe vitamin D deficiency or a hormonal imbalance, she said.
Physical activity could reduce risk
In a second, unrelated study, researchers found that moderate physical activity may have a modest effect on bone strength in older men, accounting for up to a 20% lower fracture risk, according to Lisa Langsetmo, PhD, primary investigator and a senior research associate at the University of Minnesota, Minneapolis. She and her colleagues studied physical activity and bone strength in 994 older men (mean age 83.9) participating in the Osteoporotic Fractures in Men (MrOS) Study, a longitudinal, observational study of musculoskeletal health in older American men that initially enrolled about 6,000 participants.
Participants wore armband activity monitors for 5 days during their year-7 and year-14 assessments; investigators averaged their physical activity over the two time points and used armband data along with factors like height, weight, and smoking status to estimate total energy expenditure (TEE), total steps per day, and level of activity, from sedentary to at least moderate. The men also underwent bone microarchitecture assessments of the distal radius and tibia using high-resolution peripheral quantitative computed tomography (HR-pQCT), a technique that produces detailed pictures of the bones. Investigators used mathematical models to predict failure load, or the force required to break a bone – a predictor of osteoporotic fractures in men. They also computed total, cortical, and trabecular volumetric bone mineral density (BMD).
Overall, researchers found that time spent doing at least moderate activity versus time spent in sedentary activity was related to better bone strength at both sites, whereas time spent in light activity was not. The results suggest that at least moderate physical activity such as vigorous walking averaged over a period of time may have a modest effect on bone strength among older men, Dr. Langsetmo said.
“This is important for older men,” she said. “They may not be able to jog any more but they may be able to do more moderate activity.” Physicians should ask older male patients about their activity levels and any barriers to activity, or consider a referral to a physical therapist to keep them active, she said.
Higher TEE, step count, and peak 30-minute cadence (P30MC), a measure of vigorous activity, were each associated with higher failure load of the distal radius (effect size 0.08-0.13) but not higher volumetric or compartment-specific BMD. These measures also were associated with higher failure load of the distal tibia (effect size 0.19-0.21), higher volumetric BMD (effect size 0.08-0.15), higher trabecular BMD (effect size 0.07-0.11), and higher cortical BMD (0.09-0.13).
The first study was funded internally; Manitoba Health provided the data. The second study was funded by the National Institutes of Health. Dr. Morin and Dr. Langsetmo reported no relevant financial disclosures.
Older men have a higher risk than women of sustaining secondary fractures within a few years of their first fracture, but moderate physical activity may improve bone strength, potentially reducing their risk of fractures, according to two studies presented at the annual meeting of the American Society for Bone and Mineral Research in Montreal.
The first study, a matched historical cohort of 57,783 people aged 50 or older (40,062 women and 17,721 men) in Manitoba, Canada, found that men had a threefold higher risk of sustaining a secondary major osteoporotic fracture (MOF) within 1 year of a first fracture, compared with healthy controls. The risk for women, by comparison, was 1.8 times higher than in age-matched controls who did not experience a fracture. These risks declined over time but remained elevated even as much as 15-25 years after the index fracture, according to primary investigator Suzanne N. Morin, MD, of the department of medicine at McGill University in Montreal.
“Often, men and clinicians don’t think men have skeletal fragility – everybody thinks it’s a women’s disease,” Dr. Morin said. “It’s true that it’s more frequent in women, but men do have osteoporosis, and often when they have it, they tend to have more serious complications following the fractures.” This includes higher risk of subsequent fractures and higher mortality, she said. “If you see an older gentleman with a fracture, it really should be some kind of an alarm signal.”
Using administrative health care databases, Dr. Morin and her colleagues reviewed records of patients who had an index MOF between 1989 and 2006. They compared rates of subsequent MOFs until 2016 with those of age- and sex-matched controls (n = 165,965), allowing for between 10 and 25 years of follow-up.
Researchers identified 29,694 index MOF cases (11,028 to the wrist, 9,313 to the hip, 5,799 to the humerus, and 3,554 to the spine). The annual crude rate of subsequent MOFs per 1,000 person-years was 18.5 in men (95% confidence interval, 17.3-19.8) and 29.6 in women (95% CI, 28.8-30.4). The cumulative incidence of subsequent MOFs up to 25 years later was higher in cases versus controls for both sexes and across all ages except those over 80.
Hazard ratios for subsequent MOFs were higher in men than women, particularly in the first year following the index fracture and remained very high for men during the first 3 years of follow-up. Across all follow-up years, men who had fractures were 2.5 times more likely to experience a secondary MOF (95% CI, 2.3-2.7) and women who had fractures were 1.6 times more likely to experience a secondary MOF (95% CI, 1.6-1.7), compared with controls.
To prevent fractures, clinicians should consider gait or balance training for older men and women, especially those who already have experienced a fracture, Dr. Morin said. Physicians also should note any medications such as sedatives that put patients at higher risk for falls and consider medications like bisphosphonates to reduce fracture risk. Additionally, they should ensure there are no underlying causes for skeletal fragility, such as severe vitamin D deficiency or a hormonal imbalance, she said.
Physical activity could reduce risk
In a second, unrelated study, researchers found that moderate physical activity may have a modest effect on bone strength in older men, accounting for up to a 20% lower fracture risk, according to Lisa Langsetmo, PhD, primary investigator and a senior research associate at the University of Minnesota, Minneapolis. She and her colleagues studied physical activity and bone strength in 994 older men (mean age 83.9) participating in the Osteoporotic Fractures in Men (MrOS) Study, a longitudinal, observational study of musculoskeletal health in older American men that initially enrolled about 6,000 participants.
Participants wore armband activity monitors for 5 days during their year-7 and year-14 assessments; investigators averaged their physical activity over the two time points and used armband data along with factors like height, weight, and smoking status to estimate total energy expenditure (TEE), total steps per day, and level of activity, from sedentary to at least moderate. The men also underwent bone microarchitecture assessments of the distal radius and tibia using high-resolution peripheral quantitative computed tomography (HR-pQCT), a technique that produces detailed pictures of the bones. Investigators used mathematical models to predict failure load, or the force required to break a bone – a predictor of osteoporotic fractures in men. They also computed total, cortical, and trabecular volumetric bone mineral density (BMD).
Overall, researchers found that time spent doing at least moderate activity versus time spent in sedentary activity was related to better bone strength at both sites, whereas time spent in light activity was not. The results suggest that at least moderate physical activity such as vigorous walking averaged over a period of time may have a modest effect on bone strength among older men, Dr. Langsetmo said.
“This is important for older men,” she said. “They may not be able to jog any more but they may be able to do more moderate activity.” Physicians should ask older male patients about their activity levels and any barriers to activity, or consider a referral to a physical therapist to keep them active, she said.
Higher TEE, step count, and peak 30-minute cadence (P30MC), a measure of vigorous activity, were each associated with higher failure load of the distal radius (effect size 0.08-0.13) but not higher volumetric or compartment-specific BMD. These measures also were associated with higher failure load of the distal tibia (effect size 0.19-0.21), higher volumetric BMD (effect size 0.08-0.15), higher trabecular BMD (effect size 0.07-0.11), and higher cortical BMD (0.09-0.13).
The first study was funded internally; Manitoba Health provided the data. The second study was funded by the National Institutes of Health. Dr. Morin and Dr. Langsetmo reported no relevant financial disclosures.
Older men have a higher risk than women of sustaining secondary fractures within a few years of their first fracture, but moderate physical activity may improve bone strength, potentially reducing their risk of fractures, according to two studies presented at the annual meeting of the American Society for Bone and Mineral Research in Montreal.
The first study, a matched historical cohort of 57,783 people aged 50 or older (40,062 women and 17,721 men) in Manitoba, Canada, found that men had a threefold higher risk of sustaining a secondary major osteoporotic fracture (MOF) within 1 year of a first fracture, compared with healthy controls. The risk for women, by comparison, was 1.8 times higher than in age-matched controls who did not experience a fracture. These risks declined over time but remained elevated even as much as 15-25 years after the index fracture, according to primary investigator Suzanne N. Morin, MD, of the department of medicine at McGill University in Montreal.
“Often, men and clinicians don’t think men have skeletal fragility – everybody thinks it’s a women’s disease,” Dr. Morin said. “It’s true that it’s more frequent in women, but men do have osteoporosis, and often when they have it, they tend to have more serious complications following the fractures.” This includes higher risk of subsequent fractures and higher mortality, she said. “If you see an older gentleman with a fracture, it really should be some kind of an alarm signal.”
Using administrative health care databases, Dr. Morin and her colleagues reviewed records of patients who had an index MOF between 1989 and 2006. They compared rates of subsequent MOFs until 2016 with those of age- and sex-matched controls (n = 165,965), allowing for between 10 and 25 years of follow-up.
Researchers identified 29,694 index MOF cases (11,028 to the wrist, 9,313 to the hip, 5,799 to the humerus, and 3,554 to the spine). The annual crude rate of subsequent MOFs per 1,000 person-years was 18.5 in men (95% confidence interval, 17.3-19.8) and 29.6 in women (95% CI, 28.8-30.4). The cumulative incidence of subsequent MOFs up to 25 years later was higher in cases versus controls for both sexes and across all ages except those over 80.
Hazard ratios for subsequent MOFs were higher in men than women, particularly in the first year following the index fracture and remained very high for men during the first 3 years of follow-up. Across all follow-up years, men who had fractures were 2.5 times more likely to experience a secondary MOF (95% CI, 2.3-2.7) and women who had fractures were 1.6 times more likely to experience a secondary MOF (95% CI, 1.6-1.7), compared with controls.
To prevent fractures, clinicians should consider gait or balance training for older men and women, especially those who already have experienced a fracture, Dr. Morin said. Physicians also should note any medications such as sedatives that put patients at higher risk for falls and consider medications like bisphosphonates to reduce fracture risk. Additionally, they should ensure there are no underlying causes for skeletal fragility, such as severe vitamin D deficiency or a hormonal imbalance, she said.
Physical activity could reduce risk
In a second, unrelated study, researchers found that moderate physical activity may have a modest effect on bone strength in older men, accounting for up to a 20% lower fracture risk, according to Lisa Langsetmo, PhD, primary investigator and a senior research associate at the University of Minnesota, Minneapolis. She and her colleagues studied physical activity and bone strength in 994 older men (mean age 83.9) participating in the Osteoporotic Fractures in Men (MrOS) Study, a longitudinal, observational study of musculoskeletal health in older American men that initially enrolled about 6,000 participants.
Participants wore armband activity monitors for 5 days during their year-7 and year-14 assessments; investigators averaged their physical activity over the two time points and used armband data along with factors like height, weight, and smoking status to estimate total energy expenditure (TEE), total steps per day, and level of activity, from sedentary to at least moderate. The men also underwent bone microarchitecture assessments of the distal radius and tibia using high-resolution peripheral quantitative computed tomography (HR-pQCT), a technique that produces detailed pictures of the bones. Investigators used mathematical models to predict failure load, or the force required to break a bone – a predictor of osteoporotic fractures in men. They also computed total, cortical, and trabecular volumetric bone mineral density (BMD).
Overall, researchers found that time spent doing at least moderate activity versus time spent in sedentary activity was related to better bone strength at both sites, whereas time spent in light activity was not. The results suggest that at least moderate physical activity such as vigorous walking averaged over a period of time may have a modest effect on bone strength among older men, Dr. Langsetmo said.
“This is important for older men,” she said. “They may not be able to jog any more but they may be able to do more moderate activity.” Physicians should ask older male patients about their activity levels and any barriers to activity, or consider a referral to a physical therapist to keep them active, she said.
Higher TEE, step count, and peak 30-minute cadence (P30MC), a measure of vigorous activity, were each associated with higher failure load of the distal radius (effect size 0.08-0.13) but not higher volumetric or compartment-specific BMD. These measures also were associated with higher failure load of the distal tibia (effect size 0.19-0.21), higher volumetric BMD (effect size 0.08-0.15), higher trabecular BMD (effect size 0.07-0.11), and higher cortical BMD (0.09-0.13).
The first study was funded internally; Manitoba Health provided the data. The second study was funded by the National Institutes of Health. Dr. Morin and Dr. Langsetmo reported no relevant financial disclosures.
FROM ASBMR 2018
Obesity in early childhood promotes obese adolescence
Most obese adolescents first became obese between the ages of 2 and 6 years, based on data from approximately 50,000 children in Germany.
Identifying periods of weight gain in childhood can help develop intervention and prevention strategies to reduce the risk of obesity in adolescence, wrote Mandy Geserick, MSc, of the University of Leipzig, Germany, and her colleagues in the New England Journal of Medicine.
To assess the timing of weight gain in early childhood, the researchers reviewed data from a German patient registry designed to monitor growth data. The study population included 51,505 children who had at least one visit to a pediatrician between birth and age 14 years and a second visit between age 15 and 19 years.
Overall, the probability of being overweight or obese in adolescence was 29% among children who gained more weight in the preschool years, between the ages of 2 and 6 years (defined as a change in body mass index [BMI] of 0.2 or more to less than 2.0), compared with 20% among children whose preschool weight remained stable (defined as a change in BMI of more than −0.2 to less than 0.2) – a relative risk of 1.43.
“A total of 83% of the children with obesity at the age of 4 were overweight or obese in adolescence, and only 17% returned to a normal weight,” they wrote. In addition, 44% of children who were born large for gestational age were overweight or obese in adolescence.
“A practical clinical implication of our study results would be surveillance for BMI acceleration, which should be recognized before 6 years of age, even in the absence of obesity,” the researchers wrote.
The study findings were limited by several factors including the variation in the number of visits, the lack of data on many children beyond the age of 14 years, and the lack of data on parental weight and perinatal risk factors associated with obesity, the researchers noted. However, the results were strengthened by the large, population-based design, and support the study hypothesis that obesity develops in early childhood and, once present, persists into adolescence.
“The specific dynamics and patterns of BMI in this early childhood period, rather than the absolute BMI, appear to be important factors in identifying children at risk for obesity later in life,” the researchers wrote. “It is therefore important for health care professionals, educational staff, and parents to become more sensitive to this critical time period.”
The study was supported by the German Research Council for the Clinical Research Center; the Federal Ministry of Education and Research; and the University of Leipzig, which was supported by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative. The CrescNet registry infrastructure was supported by grants from Hexal, Novo Nordisk, Merck Serono, Lilly Deutschland, Pfizer, and Ipsen Pharma. Dr. Geserick had no financial conflicts to report.
SOURCE: Geserick M et al. N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMoa1803527.
Most normal-weight children remained in the normal range throughout childhood, but the association between obesity by the age of 5 years and obese adolescence is a “new and important” finding, Michael S. Freemark, MD, wrote in an accompanying editorial (N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMe1811305).
Although body mass index (BMI) generally decreases by age 5-6 years before increasing through adolescence, data from previous studies have shown that “an early or exaggerated ‘adiposity rebound’ portends an increased risk of obesity in later childhood and adolescence,” he wrote.
In this study, BMI increase between age 2 and 6 years was the strongest predictor of obesity in adolescence. Although the study was not designed to show causality, the results support the idea of a window of opportunity for intervention for children at increased risk for obesity, Dr. Freemark wrote. “The finding that the risk of adolescent obesity manifests by 3 to 5 years of age suggests that nutritional counseling should be considered when exaggerated weight gain persists or emerges after 2 years of age; it would be of value to test the efficacy of early dietary intervention in an appropriate trial.
“Counseling could be applied preemptively for families in which the parents are overweight, particularly if there is a history of maternal diabetes or smoking,” he added.
Dr. Freemark is affiliated with the division of pediatric endocrinology and diabetes at Duke University, Durham, N.C. He disclosed grants from Rhythm Pharmaceuticals, the American Heart Association, and the Humanitarian Innovation Fund and European Commission, as well as personal fees from Springer Publishing outside the submitted work.
Most normal-weight children remained in the normal range throughout childhood, but the association between obesity by the age of 5 years and obese adolescence is a “new and important” finding, Michael S. Freemark, MD, wrote in an accompanying editorial (N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMe1811305).
Although body mass index (BMI) generally decreases by age 5-6 years before increasing through adolescence, data from previous studies have shown that “an early or exaggerated ‘adiposity rebound’ portends an increased risk of obesity in later childhood and adolescence,” he wrote.
In this study, BMI increase between age 2 and 6 years was the strongest predictor of obesity in adolescence. Although the study was not designed to show causality, the results support the idea of a window of opportunity for intervention for children at increased risk for obesity, Dr. Freemark wrote. “The finding that the risk of adolescent obesity manifests by 3 to 5 years of age suggests that nutritional counseling should be considered when exaggerated weight gain persists or emerges after 2 years of age; it would be of value to test the efficacy of early dietary intervention in an appropriate trial.
“Counseling could be applied preemptively for families in which the parents are overweight, particularly if there is a history of maternal diabetes or smoking,” he added.
Dr. Freemark is affiliated with the division of pediatric endocrinology and diabetes at Duke University, Durham, N.C. He disclosed grants from Rhythm Pharmaceuticals, the American Heart Association, and the Humanitarian Innovation Fund and European Commission, as well as personal fees from Springer Publishing outside the submitted work.
Most normal-weight children remained in the normal range throughout childhood, but the association between obesity by the age of 5 years and obese adolescence is a “new and important” finding, Michael S. Freemark, MD, wrote in an accompanying editorial (N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMe1811305).
Although body mass index (BMI) generally decreases by age 5-6 years before increasing through adolescence, data from previous studies have shown that “an early or exaggerated ‘adiposity rebound’ portends an increased risk of obesity in later childhood and adolescence,” he wrote.
In this study, BMI increase between age 2 and 6 years was the strongest predictor of obesity in adolescence. Although the study was not designed to show causality, the results support the idea of a window of opportunity for intervention for children at increased risk for obesity, Dr. Freemark wrote. “The finding that the risk of adolescent obesity manifests by 3 to 5 years of age suggests that nutritional counseling should be considered when exaggerated weight gain persists or emerges after 2 years of age; it would be of value to test the efficacy of early dietary intervention in an appropriate trial.
“Counseling could be applied preemptively for families in which the parents are overweight, particularly if there is a history of maternal diabetes or smoking,” he added.
Dr. Freemark is affiliated with the division of pediatric endocrinology and diabetes at Duke University, Durham, N.C. He disclosed grants from Rhythm Pharmaceuticals, the American Heart Association, and the Humanitarian Innovation Fund and European Commission, as well as personal fees from Springer Publishing outside the submitted work.
Most obese adolescents first became obese between the ages of 2 and 6 years, based on data from approximately 50,000 children in Germany.
Identifying periods of weight gain in childhood can help develop intervention and prevention strategies to reduce the risk of obesity in adolescence, wrote Mandy Geserick, MSc, of the University of Leipzig, Germany, and her colleagues in the New England Journal of Medicine.
To assess the timing of weight gain in early childhood, the researchers reviewed data from a German patient registry designed to monitor growth data. The study population included 51,505 children who had at least one visit to a pediatrician between birth and age 14 years and a second visit between age 15 and 19 years.
Overall, the probability of being overweight or obese in adolescence was 29% among children who gained more weight in the preschool years, between the ages of 2 and 6 years (defined as a change in body mass index [BMI] of 0.2 or more to less than 2.0), compared with 20% among children whose preschool weight remained stable (defined as a change in BMI of more than −0.2 to less than 0.2) – a relative risk of 1.43.
“A total of 83% of the children with obesity at the age of 4 were overweight or obese in adolescence, and only 17% returned to a normal weight,” they wrote. In addition, 44% of children who were born large for gestational age were overweight or obese in adolescence.
“A practical clinical implication of our study results would be surveillance for BMI acceleration, which should be recognized before 6 years of age, even in the absence of obesity,” the researchers wrote.
The study findings were limited by several factors including the variation in the number of visits, the lack of data on many children beyond the age of 14 years, and the lack of data on parental weight and perinatal risk factors associated with obesity, the researchers noted. However, the results were strengthened by the large, population-based design, and support the study hypothesis that obesity develops in early childhood and, once present, persists into adolescence.
“The specific dynamics and patterns of BMI in this early childhood period, rather than the absolute BMI, appear to be important factors in identifying children at risk for obesity later in life,” the researchers wrote. “It is therefore important for health care professionals, educational staff, and parents to become more sensitive to this critical time period.”
The study was supported by the German Research Council for the Clinical Research Center; the Federal Ministry of Education and Research; and the University of Leipzig, which was supported by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative. The CrescNet registry infrastructure was supported by grants from Hexal, Novo Nordisk, Merck Serono, Lilly Deutschland, Pfizer, and Ipsen Pharma. Dr. Geserick had no financial conflicts to report.
SOURCE: Geserick M et al. N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMoa1803527.
Most obese adolescents first became obese between the ages of 2 and 6 years, based on data from approximately 50,000 children in Germany.
Identifying periods of weight gain in childhood can help develop intervention and prevention strategies to reduce the risk of obesity in adolescence, wrote Mandy Geserick, MSc, of the University of Leipzig, Germany, and her colleagues in the New England Journal of Medicine.
To assess the timing of weight gain in early childhood, the researchers reviewed data from a German patient registry designed to monitor growth data. The study population included 51,505 children who had at least one visit to a pediatrician between birth and age 14 years and a second visit between age 15 and 19 years.
Overall, the probability of being overweight or obese in adolescence was 29% among children who gained more weight in the preschool years, between the ages of 2 and 6 years (defined as a change in body mass index [BMI] of 0.2 or more to less than 2.0), compared with 20% among children whose preschool weight remained stable (defined as a change in BMI of more than −0.2 to less than 0.2) – a relative risk of 1.43.
“A total of 83% of the children with obesity at the age of 4 were overweight or obese in adolescence, and only 17% returned to a normal weight,” they wrote. In addition, 44% of children who were born large for gestational age were overweight or obese in adolescence.
“A practical clinical implication of our study results would be surveillance for BMI acceleration, which should be recognized before 6 years of age, even in the absence of obesity,” the researchers wrote.
The study findings were limited by several factors including the variation in the number of visits, the lack of data on many children beyond the age of 14 years, and the lack of data on parental weight and perinatal risk factors associated with obesity, the researchers noted. However, the results were strengthened by the large, population-based design, and support the study hypothesis that obesity develops in early childhood and, once present, persists into adolescence.
“The specific dynamics and patterns of BMI in this early childhood period, rather than the absolute BMI, appear to be important factors in identifying children at risk for obesity later in life,” the researchers wrote. “It is therefore important for health care professionals, educational staff, and parents to become more sensitive to this critical time period.”
The study was supported by the German Research Council for the Clinical Research Center; the Federal Ministry of Education and Research; and the University of Leipzig, which was supported by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative. The CrescNet registry infrastructure was supported by grants from Hexal, Novo Nordisk, Merck Serono, Lilly Deutschland, Pfizer, and Ipsen Pharma. Dr. Geserick had no financial conflicts to report.
SOURCE: Geserick M et al. N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMoa1803527.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Of children who were obese at age 4 years, 83% were overweight or obese in adolescence.
Study details: The data come from a retrospective study of 51,505 children in Germany.
Disclosures: The study was supported by the German Research Council for the Clinical Research Center; the Federal Ministry of Education and Research; and the University of Leipzig, which was supported by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative. The CrescNet registry infrastructure was supported by grants from Hexal, Novo Nordisk, Merck Serono, Lilly Deutschland, Pfizer, and Ipsen Pharma. Dr. Geserick had no financial conflicts to report.
Source: Geserick M et al. N Engl J Med. 2018 Oct 3. doi: 10.1056/NEJMoa1803527.
Addressing your patient's sexual function after cancer
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Platelet-rich plasma injections yield substantial improvement in androgenetic alopecia
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
FROM DERMATOLOGIC SURGERY
Key clinical point: Starting off with monthly PRP injections may yield more hair growth than a protocol that uses less frequently administered injections.
Major finding: Of the patients who completed treatment, 82% were satisfied with the results.
Study details: A prospective, randomized trial comparing two early-phase treatment protocols in 40 patients.
Disclosures: Eclipse Aesthetics provided funding for this study; the authors said they had no significant interest with commercial supporters.
Source: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.