User login
Anti-TNF agents preferred for severe psoriasis in pregnancy
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
CHICAGO – according to Kenneth B. Gordon, MD, professor and chair of dermatology at Medical College of Wisconsin in Milwaukee.
“We always had this concept that psoriasis gets better during pregnancy, that you might have 20% or 30% of patients who might have a little bit of a flare or maintain, but most keep on getting better,” Dr. Gordon told attendees at the American Academy of Dermatology summer meeting.
But the majority doesn’t mean everyone. He shared the case of one pregnant woman who came to him with severe psoriasis, covering the whole of her inner thigh, to underscore that severe cases do happen in pregnancy.
“These are real situations, and when you talk about maternal health, this woman is uncomfortable, she can’t sleep, and she’s having huge stressors that are not only going to impact her and her pregnancy but also that impact her child,” Dr. Gordon said.
Dr. Gordon clarified that he is not referring to patients with limited psoriasis or those who respond to topicals or phototherapy. But because methotrexate or acitretin are “hands-off during pregnancy,” he said, the only systemic therapy available for serious cases besides biologics is cyclosporine, which has its own risks. “We know that [cyclosporine] is associated with preterm labor and preterm birth and significant low birth weight, so even in the best scenario, when we have someone with persistent severe psoriasis in pregnancy, our best agent has a lot of downsides.”
Too few data exist on anti–interleukin (IL)-17 or anti-IL-23 therapies to draw conclusions about their use, he said, and but gastroenterology and rheumatology have a fair amount of evidence on anti–tumor necrosis factor (TNF) therapies during pregnancy because it’s usually too risky to stop treating conditions such as Crohn’s with these drugs. Still, Dr. Gordon cautioned, much of the data on biologics in pregnancy are conflicting.
The question of what medications to use, and in whom, centers on balancing risks to the fetus from the medication versus risks from the condition.
“There are impacts on the fetus of having severe psoriasis, and it varies with severity of disease,” Dr. Gordon said. For example, data suggest an increased likelihood of low birth weight in children born to mothers with severe psoriasis, and that risk may extend to preterm birth as well, although “we don’t know exactly the magnitude of that effect.”
Meanwhile, the consensus from the literature throughout dermatology, rheumatology, and gastroenterology is that anti-TNF agents do not cause birth defects or affect risk of preterm birth or low birth weight.
“The bigger question is what’s the impact on the immune system of the child,” Dr. Gordon said. Data from a small Scandinavian study suggested no increased risk of allergies, infections, or similar immunologic outcomes, but evidence remains limited.
Research has shown that infants’ exposure to anti-TNF medications persists for 3-6 months after delivery, and the American Academy of Pediatrics recommends delaying immunization in children exposed to anti-TNF agents in pregnancy. But actual evidence on immunization outcomes shows no reduced immunogenicity in such children.
“Clearly there is persistence of drug in the child, but in fact you have normal responses to immunization,” Dr. Gordon said. “The pediatricians’ argument is not based on data of what actually happens in immunization; it’s based on the fact that the drug is there.”
So what’s the bottom line?
The National Psoriasis Foundation recommends moisturizers and topical corticosteroids as first-line therapy in pregnant women with psoriasis, followed by phototherapy for second-line treatment.
But some patients will need systemic therapy during pregnancy, although it’s “best not to introduce more medications than needed in pregnancy,” Dr. Gordon said. For women with a significant flare-up or very persistent volatile disease, NPF first recommends cyclosporine, but Dr. Gordon disagrees and would go with anti-TNF agents before cyclosporine.
Data show that certolizumab is not actively transported across the placenta therefore reducing fetal exposure, so Dr. Gordon would specifically use certolizumab first, all other things being equal.
“But if the patient has been on another anti-TNF that’s been working, I don’t really have an issue with staying with it,” he added.
Existing evidence so far shows no impact in terms of genetic abnormalities, birth weight, premature birth, or even infant immunizations from anti-TNF agents. But beyond those, “there is simply not enough information on pregnancy with other forms of biologic therapy to draw conclusions.” Dr. Gordon said.
Dr. Gordon disclosed that he has received grant support and/or honoraria from Abbvie, Amgen, Almirall, and Boehringer Ingelheim.
EXPERT ANALYSIS FROM SUMMER AAD 2018
Now is the time to be heard: October is Advocacy Month!
The American College of Obstetricians and Gynecologists (ACOG) and specifically the Junior Fellow College Advisory Council (JFCAC) are rolling out steps to help you make your voice heard. Starting October 1, head to acog.org/advocacy to check out the ACOG Physician Advocacy video to get inspired. (Or watch it here!) Whether you are a seasoned advocate or just getting started, ACOG and women across the country are counting on you!
Week 1 (October 1–7): Why I advocate
The focus of this week is on delving into topics that interest you, learning why advocacy is critically important, and developing your own message to advocate for women’s health.
- View advocacy videos here to understand what advocacy is and why it is so important.
- See ACOG’s 2018 list of legislative priorities here to find topics that inspire you.
Week 2 (October 8–14): Use your voice
Explore the multitude of platforms available today for amplifying your message. Learn to use social media smartly, get advice for how to write op-eds for local outlets, add your name to support current legislative efforts, and find out who your representatives are to schedule sit-down meetings.
- For tips on communicating with elected officials, click here.
- Connect with ACOG and your district on social media, and remember to use social media responsibly to advocate effectively. See this link for more information!
- Don’t forget to include #JFadvoMonth in your posts while highlighting your advocacy work on social media!
Continued to: Week 3 (October 15–19): Empower your patients
Week 3 (October 15–21): Empower your patients
As a physician, advocating for your patient extends into the clinic itself. Access toolkits, patient websites, handouts, and resources available through ACOG.
- Familiarize yourself with the Patient Page for videos, infographics, and FAQs that are useful resources for your patients.
- Toolkits for providers are available here—use these to enhance your practice and empower your patients!
Week 4 (October 22–28): Take it forward
Advocacy happens year-round. Be sure you are actively involved in ACOG’s efforts. Participate in calls to action and remember on November 6 to GET OUT THE VOTE!
- Participate in the annual Congressional Leadership Conference (March 10–12, 2019) in Washington, DC. Descend on Washington with hundreds of fellow ObGyns to advocate to Congress on important issues. For more information, click here.
- Donate to the Ob-GynPAC, ACOG’s political action committee dedicated to electing officials who support our specialty.
- Run for office! ACOG has resources to support you. Be on the lookout for opportunities to attend candidate workshops sponsored by the Ob-GynPAC!
Get active now!
We are at a critical moment for women’s health and the future of our specialty. Key issues nationally include advocating to Congress to move forward with bills in the Senate (S 1112) and House (HR 1318) to support efforts to reduce maternal mortality. (See this article for background information on these bills.)
To find your elected officials and take action now, click here and tell Congress to help prevent maternal mortality, defend patient protections, and improve access and quality of maternity care.
You can be an advocate for your patients and your profession. Your voice matters. Now is the time to be heard.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The American College of Obstetricians and Gynecologists (ACOG) and specifically the Junior Fellow College Advisory Council (JFCAC) are rolling out steps to help you make your voice heard. Starting October 1, head to acog.org/advocacy to check out the ACOG Physician Advocacy video to get inspired. (Or watch it here!) Whether you are a seasoned advocate or just getting started, ACOG and women across the country are counting on you!
Week 1 (October 1–7): Why I advocate
The focus of this week is on delving into topics that interest you, learning why advocacy is critically important, and developing your own message to advocate for women’s health.
- View advocacy videos here to understand what advocacy is and why it is so important.
- See ACOG’s 2018 list of legislative priorities here to find topics that inspire you.
Week 2 (October 8–14): Use your voice
Explore the multitude of platforms available today for amplifying your message. Learn to use social media smartly, get advice for how to write op-eds for local outlets, add your name to support current legislative efforts, and find out who your representatives are to schedule sit-down meetings.
- For tips on communicating with elected officials, click here.
- Connect with ACOG and your district on social media, and remember to use social media responsibly to advocate effectively. See this link for more information!
- Don’t forget to include #JFadvoMonth in your posts while highlighting your advocacy work on social media!
Continued to: Week 3 (October 15–19): Empower your patients
Week 3 (October 15–21): Empower your patients
As a physician, advocating for your patient extends into the clinic itself. Access toolkits, patient websites, handouts, and resources available through ACOG.
- Familiarize yourself with the Patient Page for videos, infographics, and FAQs that are useful resources for your patients.
- Toolkits for providers are available here—use these to enhance your practice and empower your patients!
Week 4 (October 22–28): Take it forward
Advocacy happens year-round. Be sure you are actively involved in ACOG’s efforts. Participate in calls to action and remember on November 6 to GET OUT THE VOTE!
- Participate in the annual Congressional Leadership Conference (March 10–12, 2019) in Washington, DC. Descend on Washington with hundreds of fellow ObGyns to advocate to Congress on important issues. For more information, click here.
- Donate to the Ob-GynPAC, ACOG’s political action committee dedicated to electing officials who support our specialty.
- Run for office! ACOG has resources to support you. Be on the lookout for opportunities to attend candidate workshops sponsored by the Ob-GynPAC!
Get active now!
We are at a critical moment for women’s health and the future of our specialty. Key issues nationally include advocating to Congress to move forward with bills in the Senate (S 1112) and House (HR 1318) to support efforts to reduce maternal mortality. (See this article for background information on these bills.)
To find your elected officials and take action now, click here and tell Congress to help prevent maternal mortality, defend patient protections, and improve access and quality of maternity care.
You can be an advocate for your patients and your profession. Your voice matters. Now is the time to be heard.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The American College of Obstetricians and Gynecologists (ACOG) and specifically the Junior Fellow College Advisory Council (JFCAC) are rolling out steps to help you make your voice heard. Starting October 1, head to acog.org/advocacy to check out the ACOG Physician Advocacy video to get inspired. (Or watch it here!) Whether you are a seasoned advocate or just getting started, ACOG and women across the country are counting on you!
Week 1 (October 1–7): Why I advocate
The focus of this week is on delving into topics that interest you, learning why advocacy is critically important, and developing your own message to advocate for women’s health.
- View advocacy videos here to understand what advocacy is and why it is so important.
- See ACOG’s 2018 list of legislative priorities here to find topics that inspire you.
Week 2 (October 8–14): Use your voice
Explore the multitude of platforms available today for amplifying your message. Learn to use social media smartly, get advice for how to write op-eds for local outlets, add your name to support current legislative efforts, and find out who your representatives are to schedule sit-down meetings.
- For tips on communicating with elected officials, click here.
- Connect with ACOG and your district on social media, and remember to use social media responsibly to advocate effectively. See this link for more information!
- Don’t forget to include #JFadvoMonth in your posts while highlighting your advocacy work on social media!
Continued to: Week 3 (October 15–19): Empower your patients
Week 3 (October 15–21): Empower your patients
As a physician, advocating for your patient extends into the clinic itself. Access toolkits, patient websites, handouts, and resources available through ACOG.
- Familiarize yourself with the Patient Page for videos, infographics, and FAQs that are useful resources for your patients.
- Toolkits for providers are available here—use these to enhance your practice and empower your patients!
Week 4 (October 22–28): Take it forward
Advocacy happens year-round. Be sure you are actively involved in ACOG’s efforts. Participate in calls to action and remember on November 6 to GET OUT THE VOTE!
- Participate in the annual Congressional Leadership Conference (March 10–12, 2019) in Washington, DC. Descend on Washington with hundreds of fellow ObGyns to advocate to Congress on important issues. For more information, click here.
- Donate to the Ob-GynPAC, ACOG’s political action committee dedicated to electing officials who support our specialty.
- Run for office! ACOG has resources to support you. Be on the lookout for opportunities to attend candidate workshops sponsored by the Ob-GynPAC!
Get active now!
We are at a critical moment for women’s health and the future of our specialty. Key issues nationally include advocating to Congress to move forward with bills in the Senate (S 1112) and House (HR 1318) to support efforts to reduce maternal mortality. (See this article for background information on these bills.)
To find your elected officials and take action now, click here and tell Congress to help prevent maternal mortality, defend patient protections, and improve access and quality of maternity care.
You can be an advocate for your patients and your profession. Your voice matters. Now is the time to be heard.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Vaginal and bilateral thigh removal of a transobturator sling

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This video is brought to you by
Better therapy, not earlier diagnosis, explains improved RCC survival
Credit improvements in therapy rather than diagnosis at an earlier stage for improved survival of renal cell carcinoma in recent years, investigators say.
A review of records on nearly 263,000 patients diagnosed with renal cell carcinoma (RCC ) from 2004 through 2015 showed that better 5-year overall survival (OS) in later years was likely attributable to better treatments rather than an uptick in detection of cancers at an earlier stage, a trend known as “stage migration,” reported Hiten D. Patel, MD, of the Brady Urological Institute at Johns Hopkins Medicine in Baltimore, and his colleagues.
“While survival has improved over time when considering all RCC patients, the primary benefit was observed in advanced RCC (stage III–IV), with 5-year survival increasing from 9.8% in 2004 to 13.2% in 2010 for patients with distant metastatic disease. The results indicate that stage migration no longer contributes to improvements in survival for RCC, and additional gains reflect improvements in advanced treatment options,” they wrote in European Urology Oncology.
Dr. Patel and colleagues noted that the incidence of RCC has been on the rise worldwide for nearly 3 decades because of both environmental risk factors and improvements in medical imaging that have resulted in an increase in incidental cancers.
“Data from the National Cancer Database (NCDB) indicated an increase in the proportion of patients presenting with cT1 RCC from 40% before 1993 to 60% through 2004. However, it is unknown if clinical stage migration has continued into recent years, which has implications for patient outcomes,” they wrote.
To try to answer this question, they sifted through data on 262,597 patients diagnosed with RCC from 2004 through 2015 at more than 1,500 facilities covered by the U.S. National Cancer Database.
They found that, up to 2007, there were statistically significant trends toward more frequent diagnosis of clinical stage I disease (70% of cases) and less frequent diagnoses of stage III (8%) and stage IV (11%; P less than .001 for all comparisons). From 2008 through 2015, however, the respective rates stabilized.
They also noticed a trend throughout the study period for decreased size of localized tumors at diagnosis, with a mean decrease of 0.22 cm for stage I lesions, and 1.24 cm for stage II tumors.
When they looked at 5-year overall survival by Kaplan-Meier analysis, they saw that it improved from 67.9% in 2004 to 72.3% in 2010. As noted before, most of the benefit was attributable to gains in survival among patients with stage III or IV disease.
In multivariable Cox proportional hazard models, diagnosis in recent years was a statistically significant predictor of improved survival, even after adjustment for stage distribution. In addition, receipt of systemic therapy was associated with improved survival, with a hazard ratio of 0.811 (P less than .001).
The authors acknowledged that a limitation of the findings is the reliance on the NCDB, which includes data on most cancer diagnoses in the United States, but is not a population-based sample.
No study funding source was reported. Dr. Patel and coauthors reported having no conflicts of interest to disclose.
SOURCE: Patel HD et al. Eur Urol Oncol. 2018 Sep 25. doi: 10.1016/j.euo.2018.08.023.
Credit improvements in therapy rather than diagnosis at an earlier stage for improved survival of renal cell carcinoma in recent years, investigators say.
A review of records on nearly 263,000 patients diagnosed with renal cell carcinoma (RCC ) from 2004 through 2015 showed that better 5-year overall survival (OS) in later years was likely attributable to better treatments rather than an uptick in detection of cancers at an earlier stage, a trend known as “stage migration,” reported Hiten D. Patel, MD, of the Brady Urological Institute at Johns Hopkins Medicine in Baltimore, and his colleagues.
“While survival has improved over time when considering all RCC patients, the primary benefit was observed in advanced RCC (stage III–IV), with 5-year survival increasing from 9.8% in 2004 to 13.2% in 2010 for patients with distant metastatic disease. The results indicate that stage migration no longer contributes to improvements in survival for RCC, and additional gains reflect improvements in advanced treatment options,” they wrote in European Urology Oncology.
Dr. Patel and colleagues noted that the incidence of RCC has been on the rise worldwide for nearly 3 decades because of both environmental risk factors and improvements in medical imaging that have resulted in an increase in incidental cancers.
“Data from the National Cancer Database (NCDB) indicated an increase in the proportion of patients presenting with cT1 RCC from 40% before 1993 to 60% through 2004. However, it is unknown if clinical stage migration has continued into recent years, which has implications for patient outcomes,” they wrote.
To try to answer this question, they sifted through data on 262,597 patients diagnosed with RCC from 2004 through 2015 at more than 1,500 facilities covered by the U.S. National Cancer Database.
They found that, up to 2007, there were statistically significant trends toward more frequent diagnosis of clinical stage I disease (70% of cases) and less frequent diagnoses of stage III (8%) and stage IV (11%; P less than .001 for all comparisons). From 2008 through 2015, however, the respective rates stabilized.
They also noticed a trend throughout the study period for decreased size of localized tumors at diagnosis, with a mean decrease of 0.22 cm for stage I lesions, and 1.24 cm for stage II tumors.
When they looked at 5-year overall survival by Kaplan-Meier analysis, they saw that it improved from 67.9% in 2004 to 72.3% in 2010. As noted before, most of the benefit was attributable to gains in survival among patients with stage III or IV disease.
In multivariable Cox proportional hazard models, diagnosis in recent years was a statistically significant predictor of improved survival, even after adjustment for stage distribution. In addition, receipt of systemic therapy was associated with improved survival, with a hazard ratio of 0.811 (P less than .001).
The authors acknowledged that a limitation of the findings is the reliance on the NCDB, which includes data on most cancer diagnoses in the United States, but is not a population-based sample.
No study funding source was reported. Dr. Patel and coauthors reported having no conflicts of interest to disclose.
SOURCE: Patel HD et al. Eur Urol Oncol. 2018 Sep 25. doi: 10.1016/j.euo.2018.08.023.
Credit improvements in therapy rather than diagnosis at an earlier stage for improved survival of renal cell carcinoma in recent years, investigators say.
A review of records on nearly 263,000 patients diagnosed with renal cell carcinoma (RCC ) from 2004 through 2015 showed that better 5-year overall survival (OS) in later years was likely attributable to better treatments rather than an uptick in detection of cancers at an earlier stage, a trend known as “stage migration,” reported Hiten D. Patel, MD, of the Brady Urological Institute at Johns Hopkins Medicine in Baltimore, and his colleagues.
“While survival has improved over time when considering all RCC patients, the primary benefit was observed in advanced RCC (stage III–IV), with 5-year survival increasing from 9.8% in 2004 to 13.2% in 2010 for patients with distant metastatic disease. The results indicate that stage migration no longer contributes to improvements in survival for RCC, and additional gains reflect improvements in advanced treatment options,” they wrote in European Urology Oncology.
Dr. Patel and colleagues noted that the incidence of RCC has been on the rise worldwide for nearly 3 decades because of both environmental risk factors and improvements in medical imaging that have resulted in an increase in incidental cancers.
“Data from the National Cancer Database (NCDB) indicated an increase in the proportion of patients presenting with cT1 RCC from 40% before 1993 to 60% through 2004. However, it is unknown if clinical stage migration has continued into recent years, which has implications for patient outcomes,” they wrote.
To try to answer this question, they sifted through data on 262,597 patients diagnosed with RCC from 2004 through 2015 at more than 1,500 facilities covered by the U.S. National Cancer Database.
They found that, up to 2007, there were statistically significant trends toward more frequent diagnosis of clinical stage I disease (70% of cases) and less frequent diagnoses of stage III (8%) and stage IV (11%; P less than .001 for all comparisons). From 2008 through 2015, however, the respective rates stabilized.
They also noticed a trend throughout the study period for decreased size of localized tumors at diagnosis, with a mean decrease of 0.22 cm for stage I lesions, and 1.24 cm for stage II tumors.
When they looked at 5-year overall survival by Kaplan-Meier analysis, they saw that it improved from 67.9% in 2004 to 72.3% in 2010. As noted before, most of the benefit was attributable to gains in survival among patients with stage III or IV disease.
In multivariable Cox proportional hazard models, diagnosis in recent years was a statistically significant predictor of improved survival, even after adjustment for stage distribution. In addition, receipt of systemic therapy was associated with improved survival, with a hazard ratio of 0.811 (P less than .001).
The authors acknowledged that a limitation of the findings is the reliance on the NCDB, which includes data on most cancer diagnoses in the United States, but is not a population-based sample.
No study funding source was reported. Dr. Patel and coauthors reported having no conflicts of interest to disclose.
SOURCE: Patel HD et al. Eur Urol Oncol. 2018 Sep 25. doi: 10.1016/j.euo.2018.08.023.
FROM EUROPEAN UROLOGY ONCOLOGY
Key clinical point: Improved renal cell carcinoma survival appears to be attributable to improvements in therapy rather than increased diagnosis of early-stage disease.
Major finding: 5-year survival increased from 9.8% in 2004 to 13.2% in 2010 for patients with distant metastatic disease.
Study details: Retrospective analysis of data from the National Cancer Database on 262,597 patients diagnosed with RCC from 2004 through 2015.
Disclosures: No study funding source was reported. Dr. Patel and coauthors reported having no conflicts of interest to disclose.
Source: Patel HD et al. Eur Urol Oncol. 2018 Sep 25. doi: 10.1016/j.euo.2018.08.023.
Could group CBT help survivors of Florence?
Rising waters forced hundreds of people, mainly in the Carolinas, to call for emergency rescues, and some people were forced to abandon their cars because of flooding. One man reportedly died by electrocution while trying to hook up a generator. Another man died after going out to check the status of hunting dogs, according to media reports. And in one of the most heart-wrenching tragedies, a mother and her infant were killed when a tree fell on their home.
Watching the TV reports and listening to the news of Hurricane Florence’s devastating impact on so many millions of people has been shocking. The death toll from this catastrophic weather event as of this writing stands at 39. Besides the current and future physical problems and illnesses left in Florence’s wake, the extent of property damage and loss must be overwhelming for the survivors.
I worry about the extent of the emotional toll left behind by Florence, just as Hurricane Maria did last year in Puerto Rico. The storm and its subsequent damage to the individual psyche – including the loss of identity and the fracturing of social structures and networks – almost certainly will lead to posttraumatic stress disorder, depression, and utter despair for many survivors.
While monitoring Florence’s impact, I thought about Hurricane Sandy, which upended me personally when it hit New York in 2012. As I’ve written previously, Sandy’s impact left me without power, running water, or toilet facilities. Almost 3 days of this uncertainty shook me from my comfort zone and truly affected my emotions. Before day 3, I left my home and drove (yes, I could still use my car; the roads were clear and my garage was not flooded) to my older son’s home – where I had a great support system and was able to continue to live a relatively normal life while watching the storm’s developments on TV. To this day, many areas of New York, New Jersey, and Connecticut that were hit by Sandy have not fully recovered.
Back to the human tragedy still unfolding for the survivors of Florence: I believe – and the data suggest – that early intervention and treatment of PTSD leads to better outcomes and should be addressed sooner than later. There is no specific medicinal “magic bullet” for PTSD, although some medications may help as well as treat a depressive component of the disorder and other medications may assist in improving sleep and disruptive sleep patterns. It’s been shown, time and again, that cognitive-behavioral therapy, various types of prolonged exposure therapy, and eye movement desensitization therapies work best. The most updated federal guidelines from the Department of Veterans Affairs and the Department of Defense, coauthored by Lori L. Davis, MD, of the University of Alabama at Birmingham, reinforce those treatments.
I also believe that, in situations in which masses of people are affected or potentially affected by PTSD, another first line of care that should be added is supportive, educational, interactive group therapy. In other words, it is possible that a cognitive-behavioral group therapy (CBGT) approach would reach many more people, make psychiatric intervention acceptable, and help the survivors of Florence. A recent study by researchers at the University of Massachusetts Boston that examined the role of “decentering” as part of CBGT for patients with specific anxiety disorders, for example, social anxiety disorder, might provide some hints. Decentering involves learning to observe thoughts and feelings as objective events in the mind rather than identifying with them personally. Aaron T. Beck, MD, and others hypothesized decentering as a mechanism of change in CBT.
In the UMass study, researchers recruited 81 people with a principal diagnosis of social anxiety disorder based on the Anxiety Disorders Interview Scheduled for DSM-IV. Other inclusion criteria for the study included stability on medications for 3 months or 1 month on benzodiazepines (Behav Ther. 2018 Sep;49[5]:809-12). Sixty-three of participants had 12 sessions of CBGT. The researchers found that people who received the CBGT experienced an increase in decentering. An increase in decentering, in turn, predicted improvement on most outcome measures.
Just as primary care physicians and surgeons know how to address serious physical health issues related natural and man-made disasters, psychiatrists must quickly know how to address the mental health aspects of care. Group therapy has the greatest potential to help more people and perhaps treat – and even prevent not only PTSD but many anxiety disorders as well.
Dr. London, a psychiatrist who practices in New York, developed and ran a short-term psychotherapy program for 20 years at NYU Langone Medical Center and has been writing columns for 35 years. His new book about helping people feel better fast is expected to be published in fall 2018. He has no disclosures.
Rising waters forced hundreds of people, mainly in the Carolinas, to call for emergency rescues, and some people were forced to abandon their cars because of flooding. One man reportedly died by electrocution while trying to hook up a generator. Another man died after going out to check the status of hunting dogs, according to media reports. And in one of the most heart-wrenching tragedies, a mother and her infant were killed when a tree fell on their home.
Watching the TV reports and listening to the news of Hurricane Florence’s devastating impact on so many millions of people has been shocking. The death toll from this catastrophic weather event as of this writing stands at 39. Besides the current and future physical problems and illnesses left in Florence’s wake, the extent of property damage and loss must be overwhelming for the survivors.
I worry about the extent of the emotional toll left behind by Florence, just as Hurricane Maria did last year in Puerto Rico. The storm and its subsequent damage to the individual psyche – including the loss of identity and the fracturing of social structures and networks – almost certainly will lead to posttraumatic stress disorder, depression, and utter despair for many survivors.
While monitoring Florence’s impact, I thought about Hurricane Sandy, which upended me personally when it hit New York in 2012. As I’ve written previously, Sandy’s impact left me without power, running water, or toilet facilities. Almost 3 days of this uncertainty shook me from my comfort zone and truly affected my emotions. Before day 3, I left my home and drove (yes, I could still use my car; the roads were clear and my garage was not flooded) to my older son’s home – where I had a great support system and was able to continue to live a relatively normal life while watching the storm’s developments on TV. To this day, many areas of New York, New Jersey, and Connecticut that were hit by Sandy have not fully recovered.
Back to the human tragedy still unfolding for the survivors of Florence: I believe – and the data suggest – that early intervention and treatment of PTSD leads to better outcomes and should be addressed sooner than later. There is no specific medicinal “magic bullet” for PTSD, although some medications may help as well as treat a depressive component of the disorder and other medications may assist in improving sleep and disruptive sleep patterns. It’s been shown, time and again, that cognitive-behavioral therapy, various types of prolonged exposure therapy, and eye movement desensitization therapies work best. The most updated federal guidelines from the Department of Veterans Affairs and the Department of Defense, coauthored by Lori L. Davis, MD, of the University of Alabama at Birmingham, reinforce those treatments.
I also believe that, in situations in which masses of people are affected or potentially affected by PTSD, another first line of care that should be added is supportive, educational, interactive group therapy. In other words, it is possible that a cognitive-behavioral group therapy (CBGT) approach would reach many more people, make psychiatric intervention acceptable, and help the survivors of Florence. A recent study by researchers at the University of Massachusetts Boston that examined the role of “decentering” as part of CBGT for patients with specific anxiety disorders, for example, social anxiety disorder, might provide some hints. Decentering involves learning to observe thoughts and feelings as objective events in the mind rather than identifying with them personally. Aaron T. Beck, MD, and others hypothesized decentering as a mechanism of change in CBT.
In the UMass study, researchers recruited 81 people with a principal diagnosis of social anxiety disorder based on the Anxiety Disorders Interview Scheduled for DSM-IV. Other inclusion criteria for the study included stability on medications for 3 months or 1 month on benzodiazepines (Behav Ther. 2018 Sep;49[5]:809-12). Sixty-three of participants had 12 sessions of CBGT. The researchers found that people who received the CBGT experienced an increase in decentering. An increase in decentering, in turn, predicted improvement on most outcome measures.
Just as primary care physicians and surgeons know how to address serious physical health issues related natural and man-made disasters, psychiatrists must quickly know how to address the mental health aspects of care. Group therapy has the greatest potential to help more people and perhaps treat – and even prevent not only PTSD but many anxiety disorders as well.
Dr. London, a psychiatrist who practices in New York, developed and ran a short-term psychotherapy program for 20 years at NYU Langone Medical Center and has been writing columns for 35 years. His new book about helping people feel better fast is expected to be published in fall 2018. He has no disclosures.
Rising waters forced hundreds of people, mainly in the Carolinas, to call for emergency rescues, and some people were forced to abandon their cars because of flooding. One man reportedly died by electrocution while trying to hook up a generator. Another man died after going out to check the status of hunting dogs, according to media reports. And in one of the most heart-wrenching tragedies, a mother and her infant were killed when a tree fell on their home.
Watching the TV reports and listening to the news of Hurricane Florence’s devastating impact on so many millions of people has been shocking. The death toll from this catastrophic weather event as of this writing stands at 39. Besides the current and future physical problems and illnesses left in Florence’s wake, the extent of property damage and loss must be overwhelming for the survivors.
I worry about the extent of the emotional toll left behind by Florence, just as Hurricane Maria did last year in Puerto Rico. The storm and its subsequent damage to the individual psyche – including the loss of identity and the fracturing of social structures and networks – almost certainly will lead to posttraumatic stress disorder, depression, and utter despair for many survivors.
While monitoring Florence’s impact, I thought about Hurricane Sandy, which upended me personally when it hit New York in 2012. As I’ve written previously, Sandy’s impact left me without power, running water, or toilet facilities. Almost 3 days of this uncertainty shook me from my comfort zone and truly affected my emotions. Before day 3, I left my home and drove (yes, I could still use my car; the roads were clear and my garage was not flooded) to my older son’s home – where I had a great support system and was able to continue to live a relatively normal life while watching the storm’s developments on TV. To this day, many areas of New York, New Jersey, and Connecticut that were hit by Sandy have not fully recovered.
Back to the human tragedy still unfolding for the survivors of Florence: I believe – and the data suggest – that early intervention and treatment of PTSD leads to better outcomes and should be addressed sooner than later. There is no specific medicinal “magic bullet” for PTSD, although some medications may help as well as treat a depressive component of the disorder and other medications may assist in improving sleep and disruptive sleep patterns. It’s been shown, time and again, that cognitive-behavioral therapy, various types of prolonged exposure therapy, and eye movement desensitization therapies work best. The most updated federal guidelines from the Department of Veterans Affairs and the Department of Defense, coauthored by Lori L. Davis, MD, of the University of Alabama at Birmingham, reinforce those treatments.
I also believe that, in situations in which masses of people are affected or potentially affected by PTSD, another first line of care that should be added is supportive, educational, interactive group therapy. In other words, it is possible that a cognitive-behavioral group therapy (CBGT) approach would reach many more people, make psychiatric intervention acceptable, and help the survivors of Florence. A recent study by researchers at the University of Massachusetts Boston that examined the role of “decentering” as part of CBGT for patients with specific anxiety disorders, for example, social anxiety disorder, might provide some hints. Decentering involves learning to observe thoughts and feelings as objective events in the mind rather than identifying with them personally. Aaron T. Beck, MD, and others hypothesized decentering as a mechanism of change in CBT.
In the UMass study, researchers recruited 81 people with a principal diagnosis of social anxiety disorder based on the Anxiety Disorders Interview Scheduled for DSM-IV. Other inclusion criteria for the study included stability on medications for 3 months or 1 month on benzodiazepines (Behav Ther. 2018 Sep;49[5]:809-12). Sixty-three of participants had 12 sessions of CBGT. The researchers found that people who received the CBGT experienced an increase in decentering. An increase in decentering, in turn, predicted improvement on most outcome measures.
Just as primary care physicians and surgeons know how to address serious physical health issues related natural and man-made disasters, psychiatrists must quickly know how to address the mental health aspects of care. Group therapy has the greatest potential to help more people and perhaps treat – and even prevent not only PTSD but many anxiety disorders as well.
Dr. London, a psychiatrist who practices in New York, developed and ran a short-term psychotherapy program for 20 years at NYU Langone Medical Center and has been writing columns for 35 years. His new book about helping people feel better fast is expected to be published in fall 2018. He has no disclosures.
Web-based lifestyle program aids in weight loss for NAFLD patients
A web-based intervention for management of nonalcoholic fatty liver disease – which researchers said may help reach busy or remote patients not able to attend in-person sessions – had a similar number of patients reach a target weight-loss goal of at least 10% of body weight, compared with a group-based intervention.
“The use of web education in the management of noncommunicable diseases has long been suggested, considering the huge number of cases at risk and patients’ needs,” wrote Arianna Mazzotti, MD, of the department of medical and surgical sciences, Alma Mater University, Bologna, Italy, and her colleagues. Their report was published in the Journal of Hepatology.
“The majority of cases are in an age range where job constraints make it difficult to implement a systematic face-to-face or group approach, whereas the eHealth procedures may keep the contact between patients and therapists without disrupting normal daily living.”
Dr. Mazzotti and her colleagues studied 716 patients with nonalcoholic fatty liver disease (NAFLD) at the university between January 2010 and December 2015 who attended either a web-based NAFLD intervention (278 patients) or an in-person, group-based lifestyle modification program (438 patients). Patients in the web-based intervention tended to be younger males with a higher education level, similar mean body mass index (33 kg/m2), and significantly lower blood pressure and rates of type 2 diabetes mellitus. The primary outcome included weight loss of at least 10%; secondary outcomes included changes in weight, changes in lifestyle, surrogate markers of steatosis and fibrosis, and alanine aminotransferase (ALT) within normal limits, researchers said.
The group-based program consisted of five 2-hour weekly sessions counseling patients on diet and physical activity, whereas the web-based intervention reproduced these sessions in addition to questionnaires, “highly interactive” slides, examples, and games as well as a mechanism to ask questions. Regardless of intervention, patients attended a 6-month in-person follow-up where they received treatment and reinforcement for comorbidities such as type 2 diabetes mellitus.
In the web-based intervention, 76% of patients attended the 6-month follow-up, 58% attended the 12-month follow-up, and 43% attended the 24-month follow-up, compared with 87%, 80%, and 69% of patients in the group-based intervention, respectively. Patients in the web-based intervention had a significantly decreased intake of calories after 6 months (273 kcal/day vs. 193 kcal/day; P = .006) compared with the group-based intervention. Physical activity significantly increased at 6 months for both groups, but there were no significant differences between groups.
Body weight decreased for the web-based intervention by 3.4% at 6 months, 4.9% at 12 months, and 5.5% at 24 months, compared with 3.1% at 6 months, 4.0% at 12 months, and 4.2% at 24 months in the group-based intervention. There was a nearly two-point reduction in body mass index for both groups, with 20% of web-based intervention patients and 15% of group-based intervention patients achieving the 10% weight-loss target; and, when the researchers performed a logistic regression analysis, the web-based intervention group was not associated with less short- and long-term 10% weight reduction after attrition rates and confounders were adjusted for.
At 24-month follow-up, the researchers found a decrease in ALT levels by an average of 22 ± 32 mU/mL, with the web-based intervention group having normalized ALT levels in 18% of cases at 6 months, 32% at 12 months, and 35% at 24 months, compared with 16% of cases at 6 months, 22% of cases at 12 months, and 29% of cases at 24 months in the group-based intervention. Compared with the group-based intervention, there was a higher reduction in fatty liver index scores at 12-month follow-up (71.3 vs. 78.0; P less than .001) and 24-month follow-up (68.9 vs. 76.3; P = .002) for the web-based intervention group. The researchers noted NAFLD fibrosis score and Fib-4 scores were reduced in both groups.
“The [web-based intervention] program might be extended to other units and/or general practitioners, increasing its impact in the community in prevention and treatment of progressive NAFLD,” Dr. Mazzotti and her colleagues wrote. “It might also be superimposed to drug treatment in the most severe cases, with possible additive effects.”
This study was supported by a grant from the European Community Seventh Framework Program. The authors report no relevant conflicts of interest.
*This story was updated on 10/4/2018.
SOURCE: Mazzotti A et al. J Hepatol. 2018 Oct 2. doi: 10.1016/j.jhep.2018.07.013.
A web-based intervention for management of nonalcoholic fatty liver disease – which researchers said may help reach busy or remote patients not able to attend in-person sessions – had a similar number of patients reach a target weight-loss goal of at least 10% of body weight, compared with a group-based intervention.
“The use of web education in the management of noncommunicable diseases has long been suggested, considering the huge number of cases at risk and patients’ needs,” wrote Arianna Mazzotti, MD, of the department of medical and surgical sciences, Alma Mater University, Bologna, Italy, and her colleagues. Their report was published in the Journal of Hepatology.
“The majority of cases are in an age range where job constraints make it difficult to implement a systematic face-to-face or group approach, whereas the eHealth procedures may keep the contact between patients and therapists without disrupting normal daily living.”
Dr. Mazzotti and her colleagues studied 716 patients with nonalcoholic fatty liver disease (NAFLD) at the university between January 2010 and December 2015 who attended either a web-based NAFLD intervention (278 patients) or an in-person, group-based lifestyle modification program (438 patients). Patients in the web-based intervention tended to be younger males with a higher education level, similar mean body mass index (33 kg/m2), and significantly lower blood pressure and rates of type 2 diabetes mellitus. The primary outcome included weight loss of at least 10%; secondary outcomes included changes in weight, changes in lifestyle, surrogate markers of steatosis and fibrosis, and alanine aminotransferase (ALT) within normal limits, researchers said.
The group-based program consisted of five 2-hour weekly sessions counseling patients on diet and physical activity, whereas the web-based intervention reproduced these sessions in addition to questionnaires, “highly interactive” slides, examples, and games as well as a mechanism to ask questions. Regardless of intervention, patients attended a 6-month in-person follow-up where they received treatment and reinforcement for comorbidities such as type 2 diabetes mellitus.
In the web-based intervention, 76% of patients attended the 6-month follow-up, 58% attended the 12-month follow-up, and 43% attended the 24-month follow-up, compared with 87%, 80%, and 69% of patients in the group-based intervention, respectively. Patients in the web-based intervention had a significantly decreased intake of calories after 6 months (273 kcal/day vs. 193 kcal/day; P = .006) compared with the group-based intervention. Physical activity significantly increased at 6 months for both groups, but there were no significant differences between groups.
Body weight decreased for the web-based intervention by 3.4% at 6 months, 4.9% at 12 months, and 5.5% at 24 months, compared with 3.1% at 6 months, 4.0% at 12 months, and 4.2% at 24 months in the group-based intervention. There was a nearly two-point reduction in body mass index for both groups, with 20% of web-based intervention patients and 15% of group-based intervention patients achieving the 10% weight-loss target; and, when the researchers performed a logistic regression analysis, the web-based intervention group was not associated with less short- and long-term 10% weight reduction after attrition rates and confounders were adjusted for.
At 24-month follow-up, the researchers found a decrease in ALT levels by an average of 22 ± 32 mU/mL, with the web-based intervention group having normalized ALT levels in 18% of cases at 6 months, 32% at 12 months, and 35% at 24 months, compared with 16% of cases at 6 months, 22% of cases at 12 months, and 29% of cases at 24 months in the group-based intervention. Compared with the group-based intervention, there was a higher reduction in fatty liver index scores at 12-month follow-up (71.3 vs. 78.0; P less than .001) and 24-month follow-up (68.9 vs. 76.3; P = .002) for the web-based intervention group. The researchers noted NAFLD fibrosis score and Fib-4 scores were reduced in both groups.
“The [web-based intervention] program might be extended to other units and/or general practitioners, increasing its impact in the community in prevention and treatment of progressive NAFLD,” Dr. Mazzotti and her colleagues wrote. “It might also be superimposed to drug treatment in the most severe cases, with possible additive effects.”
This study was supported by a grant from the European Community Seventh Framework Program. The authors report no relevant conflicts of interest.
*This story was updated on 10/4/2018.
SOURCE: Mazzotti A et al. J Hepatol. 2018 Oct 2. doi: 10.1016/j.jhep.2018.07.013.
A web-based intervention for management of nonalcoholic fatty liver disease – which researchers said may help reach busy or remote patients not able to attend in-person sessions – had a similar number of patients reach a target weight-loss goal of at least 10% of body weight, compared with a group-based intervention.
“The use of web education in the management of noncommunicable diseases has long been suggested, considering the huge number of cases at risk and patients’ needs,” wrote Arianna Mazzotti, MD, of the department of medical and surgical sciences, Alma Mater University, Bologna, Italy, and her colleagues. Their report was published in the Journal of Hepatology.
“The majority of cases are in an age range where job constraints make it difficult to implement a systematic face-to-face or group approach, whereas the eHealth procedures may keep the contact between patients and therapists without disrupting normal daily living.”
Dr. Mazzotti and her colleagues studied 716 patients with nonalcoholic fatty liver disease (NAFLD) at the university between January 2010 and December 2015 who attended either a web-based NAFLD intervention (278 patients) or an in-person, group-based lifestyle modification program (438 patients). Patients in the web-based intervention tended to be younger males with a higher education level, similar mean body mass index (33 kg/m2), and significantly lower blood pressure and rates of type 2 diabetes mellitus. The primary outcome included weight loss of at least 10%; secondary outcomes included changes in weight, changes in lifestyle, surrogate markers of steatosis and fibrosis, and alanine aminotransferase (ALT) within normal limits, researchers said.
The group-based program consisted of five 2-hour weekly sessions counseling patients on diet and physical activity, whereas the web-based intervention reproduced these sessions in addition to questionnaires, “highly interactive” slides, examples, and games as well as a mechanism to ask questions. Regardless of intervention, patients attended a 6-month in-person follow-up where they received treatment and reinforcement for comorbidities such as type 2 diabetes mellitus.
In the web-based intervention, 76% of patients attended the 6-month follow-up, 58% attended the 12-month follow-up, and 43% attended the 24-month follow-up, compared with 87%, 80%, and 69% of patients in the group-based intervention, respectively. Patients in the web-based intervention had a significantly decreased intake of calories after 6 months (273 kcal/day vs. 193 kcal/day; P = .006) compared with the group-based intervention. Physical activity significantly increased at 6 months for both groups, but there were no significant differences between groups.
Body weight decreased for the web-based intervention by 3.4% at 6 months, 4.9% at 12 months, and 5.5% at 24 months, compared with 3.1% at 6 months, 4.0% at 12 months, and 4.2% at 24 months in the group-based intervention. There was a nearly two-point reduction in body mass index for both groups, with 20% of web-based intervention patients and 15% of group-based intervention patients achieving the 10% weight-loss target; and, when the researchers performed a logistic regression analysis, the web-based intervention group was not associated with less short- and long-term 10% weight reduction after attrition rates and confounders were adjusted for.
At 24-month follow-up, the researchers found a decrease in ALT levels by an average of 22 ± 32 mU/mL, with the web-based intervention group having normalized ALT levels in 18% of cases at 6 months, 32% at 12 months, and 35% at 24 months, compared with 16% of cases at 6 months, 22% of cases at 12 months, and 29% of cases at 24 months in the group-based intervention. Compared with the group-based intervention, there was a higher reduction in fatty liver index scores at 12-month follow-up (71.3 vs. 78.0; P less than .001) and 24-month follow-up (68.9 vs. 76.3; P = .002) for the web-based intervention group. The researchers noted NAFLD fibrosis score and Fib-4 scores were reduced in both groups.
“The [web-based intervention] program might be extended to other units and/or general practitioners, increasing its impact in the community in prevention and treatment of progressive NAFLD,” Dr. Mazzotti and her colleagues wrote. “It might also be superimposed to drug treatment in the most severe cases, with possible additive effects.”
This study was supported by a grant from the European Community Seventh Framework Program. The authors report no relevant conflicts of interest.
*This story was updated on 10/4/2018.
SOURCE: Mazzotti A et al. J Hepatol. 2018 Oct 2. doi: 10.1016/j.jhep.2018.07.013.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: A web-based lifestyle modification intervention was similarly effective as a group intervention in reducing weight for patients with nonalcoholic fatty liver disease.
Major finding: In the web-based intervention, body weight decreased by 3.4% at 6 months, 4.9% at 12 months, and 5.5% at 24 months, compared with 3.1% at 6 months, 4.0% at 12 months, and 4.2% at 24 months in the group-based intervention. A similar number of patients in both groups achieved the 10% weight-loss target.
Study details: An observational study of 716 patients participating in web-based or group nonalcoholic fatty liver disease interventions at the University of Bologna between January 2010 and December 2015.
Disclosures: The European Community Seventh Framework Program supported the study. The authors report no conflicts of interest.
Source: Mazzotti A et al. J Hepatol. 2018 Oct 2. doi: 10.1016/j.jhep.2018.07.013.
Antibiotics trigger proteolytic activity that leads to chronic colitis
Antibiotics are associated with increased large intestinal proteolytic activity and gut barrier disruption, thereby raising the risk of chronic colitis in susceptible individuals, a recent study found.
Although the association between antibiotics and chronic colitis has been previously described, this is the first study to demonstrate the causative role of high proteolytic activity, reported lead author Hongsup Yoon, PhD, chair of nutrition and immunology at Technische Universität München in Freising-Weihenstephan, Germany, and colleagues. The team’s experiments support development of antiproteolytic strategies in susceptible humans.
“In the context of IBD, several clinical studies have already revealed that early and frequent antibiotic therapies, especially metronidazole or fluoroquinolone treatments, are associated with increased risk for Crohn’s disease,” the authors wrote in Cellular and Molecular Gastroenterology and Hepatology. “However, the causal role of antibiotic therapies in the disease development and the mechanisms underlying this [potentially] serious long-term adverse effect of antibiotics on the intestinal immune homeostasis remain unknown.”
Previous studies have shown that antibiotic therapy often causes high luminal proteolytic activity in the large intestine, likely because of the elimination of antiproteolytic bacteria that normally control pancreatic protease levels. Other studies have shown that exposing murine colonic mucosa to fecal supernatants with high proteolytic activity increases gut barrier permeability, which triggers chronic inflammation via translocation of luminal antigens.
“In view of these data,” the authors wrote, “we hypothesized that the antibiotic-increased proteolytic activity in the large intestine is a relevant risk factor for the development of colitis in susceptible organisms.”
The first component of the study used transwell experiments to evaluate the impact of high proteolytic activity on gut barrier integrity. High proteolytic activity was induced by several antibiotics, including fluoroquinolones with or without an imidazole (ciprofloxacin and levofloxacin plus or minus metronidazole), a beta-lactam (amoxicillin + clavulanate), cephalosporins with or without a macrolide (azithromycin and ceftriaxone plus or minus azithromycin), and a rifamycin (rifaximin).
“All tested antibiotic classes mediated a major proteolytic activity increase in some patients but not in others,” the authors wrote, “demonstrating individual-specific vulnerability of the intestinal microbiota toward antibiotic therapies, which is likely caused by the high interindividual variability of human microbial ecosystems.”
One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy, and several had an increase greater than 900%. Analysis indicated that proteolytic activity was caused by pancreatic proteases such as chymotrypsin and trypsin.
Subsequent cell line testing showed that stool supernatants with high proteolytic activity damaged the epithelial barrier, but samples with low proteolytic activity did not. Of note, the negative impact of high proteolytic activity on epithelial cells could be mitigated by incubating stool supernatants with a serine protease inhibitor.
In analogous experiments, mice were given a combination of vancomycin and metronidazole (V/M). In contrast with the various proteolytic activity levels observed in humans, all mice had high proteolytic activity levels following treatment, suggesting that V/M eliminated almost all antiproteolytic bacteria.
The loss of antiproteolytic bacteria was clarified by cecal microbiota transplantation tests. Transplants from untreated mice were capable of normalizing proteolytic activity levels in germ-free mice (which have high proteolytic activity levels), but transplants from V/M-treated mice were ineffective, suggesting a near-total loss of antiproteolytic bacteria. The identity of these antiproteolytic bacteria remains a mystery.
“Although our data are in line with published literature suggesting specific strains of the order Bacteroidales to play a role in the physiological inactivation of pancreatic proteases,” the authors wrote, “the identity of relevant antiproteolytic species/strains remains to be elucidated.”
The next part of the study involved wild-type and interleukin (IL)-10–/– mice, the latter of which serves as a model of human colitis. Both types of mice were given V/M with or without an oral serine protease inhibitor, a potential therapy intended to limit proteolytic activity and associated intestinal barrier damage.
Although both wild-type and IL-10–/– mice had increased intestinal permeability after V/M treatment, only IL-10–/– mice showed lasting inflammation. Of note, coadministration of an oral serine protease inhibitor with V/M protected against colitis in IL-10–/– mice.
The protective benefit of an oral serine protease inhibitor in IL-10–/– mice prompts the development of antiproteolytic strategies in humans. These would target “large intestinal proteolytic activity [e.g., oral administration of encapsulated serine protease inhibitors, commensal antiproteolytic bacteria, or genetically modified bacteria expressing protease inhibitors] to protect the large intestinal mucosa from adverse effects of antibiotic-induced or diarrhea-induced high proteolytic activity,” the authors wrote.
The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
SOURCE: Yoon H-S et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
Antibiotics are associated with increased large intestinal proteolytic activity and gut barrier disruption, thereby raising the risk of chronic colitis in susceptible individuals, a recent study found.
Although the association between antibiotics and chronic colitis has been previously described, this is the first study to demonstrate the causative role of high proteolytic activity, reported lead author Hongsup Yoon, PhD, chair of nutrition and immunology at Technische Universität München in Freising-Weihenstephan, Germany, and colleagues. The team’s experiments support development of antiproteolytic strategies in susceptible humans.
“In the context of IBD, several clinical studies have already revealed that early and frequent antibiotic therapies, especially metronidazole or fluoroquinolone treatments, are associated with increased risk for Crohn’s disease,” the authors wrote in Cellular and Molecular Gastroenterology and Hepatology. “However, the causal role of antibiotic therapies in the disease development and the mechanisms underlying this [potentially] serious long-term adverse effect of antibiotics on the intestinal immune homeostasis remain unknown.”
Previous studies have shown that antibiotic therapy often causes high luminal proteolytic activity in the large intestine, likely because of the elimination of antiproteolytic bacteria that normally control pancreatic protease levels. Other studies have shown that exposing murine colonic mucosa to fecal supernatants with high proteolytic activity increases gut barrier permeability, which triggers chronic inflammation via translocation of luminal antigens.
“In view of these data,” the authors wrote, “we hypothesized that the antibiotic-increased proteolytic activity in the large intestine is a relevant risk factor for the development of colitis in susceptible organisms.”
The first component of the study used transwell experiments to evaluate the impact of high proteolytic activity on gut barrier integrity. High proteolytic activity was induced by several antibiotics, including fluoroquinolones with or without an imidazole (ciprofloxacin and levofloxacin plus or minus metronidazole), a beta-lactam (amoxicillin + clavulanate), cephalosporins with or without a macrolide (azithromycin and ceftriaxone plus or minus azithromycin), and a rifamycin (rifaximin).
“All tested antibiotic classes mediated a major proteolytic activity increase in some patients but not in others,” the authors wrote, “demonstrating individual-specific vulnerability of the intestinal microbiota toward antibiotic therapies, which is likely caused by the high interindividual variability of human microbial ecosystems.”
One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy, and several had an increase greater than 900%. Analysis indicated that proteolytic activity was caused by pancreatic proteases such as chymotrypsin and trypsin.
Subsequent cell line testing showed that stool supernatants with high proteolytic activity damaged the epithelial barrier, but samples with low proteolytic activity did not. Of note, the negative impact of high proteolytic activity on epithelial cells could be mitigated by incubating stool supernatants with a serine protease inhibitor.
In analogous experiments, mice were given a combination of vancomycin and metronidazole (V/M). In contrast with the various proteolytic activity levels observed in humans, all mice had high proteolytic activity levels following treatment, suggesting that V/M eliminated almost all antiproteolytic bacteria.
The loss of antiproteolytic bacteria was clarified by cecal microbiota transplantation tests. Transplants from untreated mice were capable of normalizing proteolytic activity levels in germ-free mice (which have high proteolytic activity levels), but transplants from V/M-treated mice were ineffective, suggesting a near-total loss of antiproteolytic bacteria. The identity of these antiproteolytic bacteria remains a mystery.
“Although our data are in line with published literature suggesting specific strains of the order Bacteroidales to play a role in the physiological inactivation of pancreatic proteases,” the authors wrote, “the identity of relevant antiproteolytic species/strains remains to be elucidated.”
The next part of the study involved wild-type and interleukin (IL)-10–/– mice, the latter of which serves as a model of human colitis. Both types of mice were given V/M with or without an oral serine protease inhibitor, a potential therapy intended to limit proteolytic activity and associated intestinal barrier damage.
Although both wild-type and IL-10–/– mice had increased intestinal permeability after V/M treatment, only IL-10–/– mice showed lasting inflammation. Of note, coadministration of an oral serine protease inhibitor with V/M protected against colitis in IL-10–/– mice.
The protective benefit of an oral serine protease inhibitor in IL-10–/– mice prompts the development of antiproteolytic strategies in humans. These would target “large intestinal proteolytic activity [e.g., oral administration of encapsulated serine protease inhibitors, commensal antiproteolytic bacteria, or genetically modified bacteria expressing protease inhibitors] to protect the large intestinal mucosa from adverse effects of antibiotic-induced or diarrhea-induced high proteolytic activity,” the authors wrote.
The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
SOURCE: Yoon H-S et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
Antibiotics are associated with increased large intestinal proteolytic activity and gut barrier disruption, thereby raising the risk of chronic colitis in susceptible individuals, a recent study found.
Although the association between antibiotics and chronic colitis has been previously described, this is the first study to demonstrate the causative role of high proteolytic activity, reported lead author Hongsup Yoon, PhD, chair of nutrition and immunology at Technische Universität München in Freising-Weihenstephan, Germany, and colleagues. The team’s experiments support development of antiproteolytic strategies in susceptible humans.
“In the context of IBD, several clinical studies have already revealed that early and frequent antibiotic therapies, especially metronidazole or fluoroquinolone treatments, are associated with increased risk for Crohn’s disease,” the authors wrote in Cellular and Molecular Gastroenterology and Hepatology. “However, the causal role of antibiotic therapies in the disease development and the mechanisms underlying this [potentially] serious long-term adverse effect of antibiotics on the intestinal immune homeostasis remain unknown.”
Previous studies have shown that antibiotic therapy often causes high luminal proteolytic activity in the large intestine, likely because of the elimination of antiproteolytic bacteria that normally control pancreatic protease levels. Other studies have shown that exposing murine colonic mucosa to fecal supernatants with high proteolytic activity increases gut barrier permeability, which triggers chronic inflammation via translocation of luminal antigens.
“In view of these data,” the authors wrote, “we hypothesized that the antibiotic-increased proteolytic activity in the large intestine is a relevant risk factor for the development of colitis in susceptible organisms.”
The first component of the study used transwell experiments to evaluate the impact of high proteolytic activity on gut barrier integrity. High proteolytic activity was induced by several antibiotics, including fluoroquinolones with or without an imidazole (ciprofloxacin and levofloxacin plus or minus metronidazole), a beta-lactam (amoxicillin + clavulanate), cephalosporins with or without a macrolide (azithromycin and ceftriaxone plus or minus azithromycin), and a rifamycin (rifaximin).
“All tested antibiotic classes mediated a major proteolytic activity increase in some patients but not in others,” the authors wrote, “demonstrating individual-specific vulnerability of the intestinal microbiota toward antibiotic therapies, which is likely caused by the high interindividual variability of human microbial ecosystems.”
One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy, and several had an increase greater than 900%. Analysis indicated that proteolytic activity was caused by pancreatic proteases such as chymotrypsin and trypsin.
Subsequent cell line testing showed that stool supernatants with high proteolytic activity damaged the epithelial barrier, but samples with low proteolytic activity did not. Of note, the negative impact of high proteolytic activity on epithelial cells could be mitigated by incubating stool supernatants with a serine protease inhibitor.
In analogous experiments, mice were given a combination of vancomycin and metronidazole (V/M). In contrast with the various proteolytic activity levels observed in humans, all mice had high proteolytic activity levels following treatment, suggesting that V/M eliminated almost all antiproteolytic bacteria.
The loss of antiproteolytic bacteria was clarified by cecal microbiota transplantation tests. Transplants from untreated mice were capable of normalizing proteolytic activity levels in germ-free mice (which have high proteolytic activity levels), but transplants from V/M-treated mice were ineffective, suggesting a near-total loss of antiproteolytic bacteria. The identity of these antiproteolytic bacteria remains a mystery.
“Although our data are in line with published literature suggesting specific strains of the order Bacteroidales to play a role in the physiological inactivation of pancreatic proteases,” the authors wrote, “the identity of relevant antiproteolytic species/strains remains to be elucidated.”
The next part of the study involved wild-type and interleukin (IL)-10–/– mice, the latter of which serves as a model of human colitis. Both types of mice were given V/M with or without an oral serine protease inhibitor, a potential therapy intended to limit proteolytic activity and associated intestinal barrier damage.
Although both wild-type and IL-10–/– mice had increased intestinal permeability after V/M treatment, only IL-10–/– mice showed lasting inflammation. Of note, coadministration of an oral serine protease inhibitor with V/M protected against colitis in IL-10–/– mice.
The protective benefit of an oral serine protease inhibitor in IL-10–/– mice prompts the development of antiproteolytic strategies in humans. These would target “large intestinal proteolytic activity [e.g., oral administration of encapsulated serine protease inhibitors, commensal antiproteolytic bacteria, or genetically modified bacteria expressing protease inhibitors] to protect the large intestinal mucosa from adverse effects of antibiotic-induced or diarrhea-induced high proteolytic activity,” the authors wrote.
The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
SOURCE: Yoon H-S et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: In patients susceptible to inflammatory bowel disease, antibiotics cause increased proteolytic activity in the large intestine that disrupts the gut barrier, thereby increasing risk of chronic colitis.
Major finding: One-quarter of patients had a 400% or greater increase in large intestinal proteolytic activity following antibiotic therapy.
Study details: A prospective study involving mice and humans treated with antibiotics.
Disclosures: The study was funded by the Deutscher Akademischer Austauschdienst. No conflicts of interest were reported.
Source: Yoon H et al. Cell Mol Gastroenterol Hepatol. 2018 May 29. doi: 10.1016/j.jcmgh.2018.05.008.
Primary Cutaneous Apocrine Carcinoma Arising Within a Nevus Sebaceus
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.
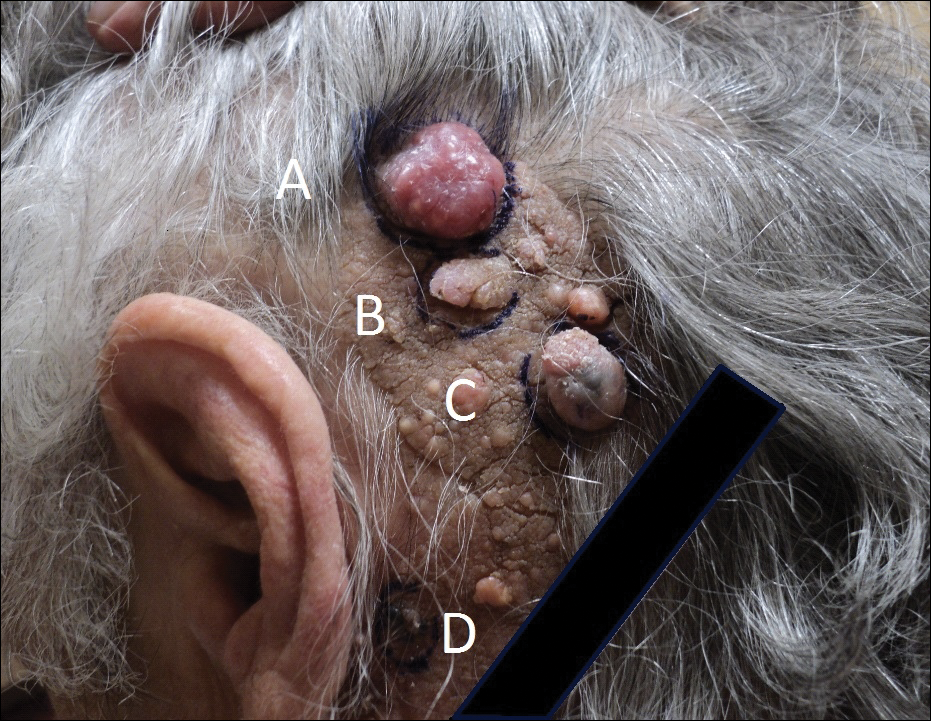

Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.



Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
Nevus sebaceus (NS) is a benign hair follicle neoplasm present in approximately 1.3% of the population, typically involving the scalp, neck, or face.1 These lesions usually are present at birth or identified soon after, during the first year. They present as a yellowish hairless patch or plaque but can develop a more papillomatous appearance, especially after puberty. Historically, the concern with NS was its tendency to transform into basal cell carcinoma (BCC), which prompted surgical excision of the lesion during childhood. This theory has been discounted more recently, as further research has suggested that what was once thought to be BCC may have been confused with the similarly appearing trichoblastoma; however, malignant transformation of NS does still occur, with BCC still being the most common.2 We present the case of a long-standing NS with rare transformation to apocrine carcinoma.
Case Report
A 76-year-old woman presented with several new lesions within a previously diagnosed NS. She reported having the large plaque for as long as she could recall but reported that several new growths developed within the plaque over the last 2 months, slowly increasing in size. She reported a prior biopsy within the growth several years prior, which she described as an irritated seborrheic keratosis.
Physical examination demonstrated 4 distinct lesions within the flesh-colored, verrucous plaque located on the left side of the temporal scalp (Figure 1). The first lesion was a 2.5-cm pearly, pink, exophytic tumor (labeled as A in Figure 1). The next 2 lesions were brown, pedunculated, verrucous papules (labeled as B and C in Figure 1). The last lesion was a purple papule (labeled as D in Figure 1). Four shave biopsies were performed for histologic analysis of the lesions. Lesions B, C, and D were consistent with trichoblastomas, as pathology showed basaloid epithelial tumors that displayed primitive follicular structures, areas of stromal induction, and some pigmentation. Lesion A, originally thought to be suspicious for a BCC, was determined to be a primary cutaneous apocrine adenocarcinoma upon pathologic review. The pathology showed a dermal tumor displaying solid and tubular areas with decapitation secretion. Nuclear pleomorphism and mitoses were present (Figure 2), and staining for carcinoembryonic antigen was positive (Figure 3). Immunoreactivity with epithelial membrane antigen and cytokeratin 7 was noted as well as focal positivity for mammaglobin. Primary apocrine carcinoma was favored over metastatic carcinoma due to the location of the lesion within an NS along with a negative history of internal malignancy. Dermatopathology recommended complete removal of all lesions within the NS.



Upon discussing biopsy results and recommendations with our patient, she agreed to undergo excision with intraoperative pathology by a plastic surgeon within our practice to ensure clear margins. The surgical defect following excision was sizeable and closed utilizing a rhomboid flap, full-thickness skin graft, and a split-thickness skin graft. At surgical follow-up, she was doing well and there have been no signs of local recurrence for 10 months since excision.
Comment
Presentation
Nevus sebaceus is the most common adnexal tumor and is classified as a benign congenital hair follicle tumor that is located most commonly on the scalp but also occurs on the face and neck.1 The lesions usually are present at birth but also can develop during the first year of life.2 Diagnosis may be later, during adolescence, when patients seek medical attention during the lesion’s rapid growth phase.1 Nevus sebaceus also is known as an organoid nevus because it may contain all components of the skin. It was originally identified by Jadassohn in 1895.3 It presents as a yellowish, smooth, hairless patch or plaque in prepubertal patients. During adolescence, the lesion typically becomes more yellowish, as well as papillomatous, scaly, or warty. The reported incidence of NS is 0.05% to 1% in dermatology patients.2
Differential
Nevus sebaceus also is a component of several syndromes that should be kept in mind, including Schimmelpenning-Feuerstein-Mims syndrome, which presents with neurologic, skeletal, genitourinary, cardiovascular, and ophthalmic disorders, in addition to cutaneous features. Others include phacomatosis pigmentokeratotica, didmyosis aplasticosebacea, SCALP syndrome (sebaceus nevus, central nervous system malformations, aplasia cutis congenita, limbal dermoid, and pigmented nevus), and more.4,5
Etiology
The etiology of NS has not been completely determined. One study that evaluated 44 NS tissue samples suggested the presence of human papillomavirus (HPV) in NS formation, finding that 82% of NS lesions studied contained HPV DNA. From these results, Carlson et al6 suggested a possible maternal transmission of HPV and infection of ectodermal cells as a potential cause of NS; however, this hypothesis was soon challenged by a study that showed a complete absence of HPV in 16 samples via histological evaluation and polymerase chain reaction for a broad range of HPV types.7 There were investigations into a patched (PTCH) deletion as the cause of NS and thus explained the historically high rate of secondary BCC.8 Further studies showed no mutations at the PTCH locus in trichoblastomas or other tumors arising from NS.9,10
More recent studies have recognized HRAS and KRAS mutations as a causative factor in NS.11 Nevus sebaceus belongs to a group of syndromes resulting from lethal mutations that survive via mosaicism. Nevus sebaceus is caused by postzygotic HRAS or KRAS mutations and is known as a mosaic RASopathy.12 In fact, there is growing evidence to suggest that other nevoid proliferations including keratinocytic epidermal nevi and melanocytic nevi also fall into the spectrum of mosaic RASopathies.13
Staging
There are 3 clinical stages of NS, originally described by Mehregan and Pinkus.14 In stage I (historically known as the infantile stage), the lesion presents as a yellow to pink, smooth, hairless patch. Histologic features include immature hair follicles and hypoplastic sebaceous glands. In stage II (also known as the puberty stage), the lesion becomes more pronounced. Firmer plaques can develop with hyperkeratosis. Hormonal changes cause sebaceous glands to develop, accompanied by epidermal hyperplasia and maturation of apocrine glands. Stage III (the tumoral stage) is a period that various neoplasms have the highest likelihood of occurring. Nevus sebaceus in an adolescent or adult demonstrates mature adnexal structures and greater epidermal hyperplasia.2,4,15
Malignancy
By virtue of these stages of NS development, malignant transformation is expected most often during stage III. However, cases have been reported of malignant tumor development in NS in children before puberty. Two case reports described a 7-year-old boy and a 10-year-old boy diagnosed with a BCC arising from an NS.16,17 However, secondary BCC formation before 16 years of age is rare. Basal cell carcinoma arising from an NS has been commonly reported and is the most common malignant neoplasm in NS (1.1%).2,3 However, the most common neoplasm overall is trichoblastoma (7.4%). The second most common tumor was syringocystadenoma papilliferum, occurring in approximately 5.2% of NS cases. The neoplasm rate in NS was found to be proportional to the patient age.2,18 Multiple studies have shown the overall rate of secondary neoplasms in NS to be 13% to 21.4%, with malignant tumors composing 0.8% to 2.5%.2,15,19 Other neoplasms that have been reported include keratoacanthoma, trichilemmoma, sebaceoma, nevocellular nevus, squamous cell carcinoma, adnexal carcinoma, apocrine adenocarcinoma, and malignant melanoma.19-21
It is argued that the reported rate of BCC formation is overestimated, as prior studies incorrectly labeled trichoblastomas as BCCs. In fact, the largest studies of NS from the 1990s revealed lower rates of malignant secondary tumors than previously determined.4
The identification of apocrine adenocarcinoma tumors arising from NS is exceedingly rare. A study performed by Cribier et al19 in 2000 retrospect
Histopathology
Histopathologic examination reveals considerable variation in morphology, and an underlying pattern has been difficult to recognize. Unfortunately, some authors have concluded that the diagnosis of apocrine carcinoma is relatively subjective.26 Robson et al26 identified 3 general architectural patterns: tubular, tubulopapillary, and solid. Tubular structures consisted of glands and ducts lined by a single or multilayered epithelium. Tubulopapillary architecture was characterized by epithelium forming papillary folds without a fibrovascular core. The solid morphology showed sheets of cells with limited ductal or tubular formation.26 The most specific criteria of these apocrine carcinomas are identification of decapitation secretion, periodic acid–Schiff–positive diastase-resistant material present in the cells or lumen, and positive immunostaining for gross cystic disease fluid protein-15.27
Robson et al26 reported estrogen receptor positivity and androgen receptor positivity in 62% and 64% of 24 primary apocrine carcinoma cases, respectively. However, whether these markers are as common in NS-related apocrine carcinomas has yet to be noted in the literature. One study reports a case of apocrine carcinoma from NS with positive staining for human epidermal growth factor-2, a cell membrane receptor tyrosine kinase commonly investigated in breast cancers and extramammary Paget disease.22
These apocrine carcinomas do have the potential for lymphatic metastasis, as seen with multiple studies. Domingo and Helwig21 identified regional lymph node metastasis in 2 of its 4 apocrine carcinoma patients. Robson et al26 reported lymphovascular invasion in 4 cases and perineural invasion in 2 of 24 patients studied. However, even in the context of recurrence and regional metastasis, the prognosis was good and seldom fatal.26
Treatment
The most effective treatment of NS is excision of dermal and epidermal components. Excision should be completed with a minimum of 2- to 3-mm margins and full thickness down to the underlying supporting fat.28 Historically, the practice of prophylactic excision of NS was supported by the potential for malignant transformation; however, early excision of NS may be less reasonable in light of these more recent studies showing lower incidence of BCC (0.8%), replaced by benign trichoblastomas.19 In the case of apocrine carcinoma development, excision is undoubtedly recommended, with unclear recommendations regarding further evaluation for metastasis.
Excision also may be favored for cosmetic purposes, given the visible regions where NS tends to develop. Chepla and Gosain29 argued that surgical intervention should be based on other factors such as location on the scalp, alopecia, and other issues affecting appearance and monitoring rather than incidence of malignant transformation. Close monitoring and biopsy of suspicious areas is a more conservative option.
Other therapies include CO2 laser, as demonstrated by Kiedrowicz et al,30 on linear NS in a patient with Schimmelpenning-Feuerstein-Mims syndrome.31 However, this approach is palliative and not effective in removing the entire lesion. Electrodesiccation and curettage and dermabrasion also are not good options for the same reason.4
Occurrence in Children
Nevus sebaceus in children, accompanied by other findings suggestive of epidermal nevus syndromes, should prompt further investigation. Schimmelpenning-Feuerstein-Mims syndrome includes major neurological abnormalities including hemimegalencephaly and seizures.32
Conclusion
Apocrine carcinomas are malignant neoplasms that may rarely arise within an NS. Their clinical identification is difficult and requires histopathologic evaluation. Upon recognition, prompt excision with tumor-free margins is recommended. As a rare entity, little data is available regarding its metastatic potential or overall survival rates. Further investigation is clearly necessary as new cases arise.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
- Kamyab-Hesari K, Balochi K, Afshar N, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran. 2013;51:879-885.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Ball EA, Hussain M, Moss AL. Squamous cell carcinoma and basal cell carcinoma arising in a naevus sebaceous of Jadassohn: case report and literature review. Clin Exp Dermatol. 2005;30:259-260.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22; quiz 23-24.
- Carlson JA, Cribier B, Nuovo G, et al. Epidermodysplasia verruciformis-associated and genital-mucosal high-risk human papillomavirus DNA are prevalent in nevus sebaceus of Jadassohn. J Am Acad Dermatol. 2008;59:279-294.
- Kim D, Benjamin LT, Sahoo MK, et al. Human papilloma virus is not prevalent in nevus sebaceus [published online November 14, 2013]. Pediatr Dermatol. 2014;31:326-330.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Hafner C, Schmiemann V, Ruetten A, et al. PTCH mutations are not mainly involved in the pathogenesis of sporadic trichoblastomas. Hum Pathol. 2007;38:1496-1500.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus [published online October 25, 2012]. J Invest Dermatol. 2013;133:827-830.
- Happle R. Nevus sebaceus is a mosaic RASopathy. J Invest Dermatol. 2013;133:597-600.
- Luo S, Tsao H. Epidermal, sebaceous, and melanocytic nevoid proliferations are spectrums of mosaic RASopathies. J Invest Dermatol. 2014;134:2493-2496.
- Mehregan AH, Pinkus H. Life history of organoid nevi. special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Altaykan A, Ersoy-Evans S, Erkin G, et al. Basal cell carcinoma arising in nevus sebaceous during childhood. Pediatr Dermatol. 2008;25:616-619.
- Turner CD, Shea CR, Rosoff PM. Basal cell carcinoma originating from a nevus sebaceus on the scalp of a 7-year-old boy. J Pediatr Hematol Oncol. 2001;23:247-249.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2, pt 1):263-268.
- Paudel U, Jha A, Pokhrel DB, et al. Apocrine carcinoma developing in a naevus sebaceous of scalp. Kathmandu Univ Med J (KUMJ). 2012;10:103-105.
- Domingo J, Helwig EB. Malignant neoplasms associated with nevus sebaceus of Jadassohn. J Am Acad Dermatol. 1979;1:545-556.
- Tanese K, Wakabayashi A, Suzuki T, et al. Immunoexpression of human epidermal growth factor receptor-2 in apocrine carcinoma arising in naevus sebaceous, case report [published online August 23, 2009]. J Eur Acad Dermatol Venereol. 2010;24:360-362.
- Dalle S, Skowron F, Balme B, et al. Apocrine carcinoma developed in nevus sebaceus of Jadassohn. Eur J Dermatol. 2003;13:487-489.
- Jacyk WK, Requena L, Sánchez Yus E, et al. Tubular apocrine carcinoma arising in a nevus sebaceus of Jadassohn. Am J Dermatopathol. 1998;20:389-392.
- Ansai S, Koseki S, Hashimoto H, et al. A case of ductal sweat gland carcinoma connected to syringocystadenoma papilliferum arising in nevus sebaceus. J Cutan Pathol. 1994;21:557-563.
- Robson A, Lazar AJ, Ben Nagi J, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008;32:682-690.
- Paties C, Taccagni GL, Papotti M, et al. Apocrine carcinoma of the skin. a clinicopathologic, immunocytochemical, and ultrastructural study. Cancer. 1993;71:375-381.
- Davison SP, Khachemoune A, Yu D, et al. Nevus sebaceus of Jadassohn revisited with reconstruction options. Int J Dermatol. 2005;44:145-150.
- Chepla KJ, Gosain AK. Giant nevus sebaceus: definition, surgical techniques, and rationale for treatment. Plast Reconstr Surg. 2012;130:296E-304E.
- Kiedrowicz M, Kacalak-Rzepka A, Królicki A et al. Therapeutic effects of CO2 laser therapy of linear nevus sebaceous in the course of the Schimmelpenning-Feuerstein-Mims syndrome. Postepy Dermatol Allergol. 2013;30:320-323.
- Ashinoff R. Linear nevus sebaceus of Jadassohn treated with the carbon dioxide laser. Pediatr Dermatol. 1993;10:189-191.
- van de Warrenburg BP, van Gulik S, Renier WO, et al. The linear naevus sebaceus syndrome. Clin Neurol Neurosurg. 1998;100:126-132.
Practice Points
- Nevus sebaceus (NS) in the centrofacial region has been correlated with a higher risk for neurological abnormalities, including intellectual disability and seizures.
- Historically, basal cell carcinomas (BCCs) were considered a common occurrence arising from an NS, prompting prophylactic surgical excision of such lesions.
- More recently, it has been recognized that the most common tumor to arise from NS is trichoblastoma rather than BCC; in fact, BCC and other malignancies have been found to be relatively rare compared to their benign counterparts.
- In light of this discovery, observation of NS may be a more prudent course of treatment versus prophylactic surgical excision.
Adalimumab safety profile similar in children and adults
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The findings of this study underscore the importance of being “aware of the safety profile of this widely used biologic medication,” Philip J. Hashkes, MD, MSc, wrote in an accompanying editorial.
“The major finding was that the safety profile is similar to that seen in adults,” he added. “Although almost all patients developed adverse effects, especially infections, most were usual pediatric infections (including the serious infections) with very few opportunistic infections.” Patients with Crohn’s disease had more serious adverse effects and infections.
Future research should go a step further and focus on “post-marketing surveillance in ‘real life’ settings,” he concluded.
Dr. Hashkes is a pediatric rheumatologist at the Cleveland Clinic. His editorial in response to the article by Horneff et al. appeared in the Journal of Pediatrics (J Pediatr. 2018 Oct;201:2-3).
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
The safety profile for adalimumab in children is similar to that of adults, according to findings published in the Journal of Pediatrics.
In an analysis of data from seven clinical trials from 2002-2015, the most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY). Serious infections were the most frequent adverse events across indications (8% of all patients; 4 events per 100 PY), reported Gerd Horneff, MD, of the department of general pediatrics at Asklepios Klinik Sankt Augustin (Germany), and his coauthors.
All of the clinical trials were funded by AbbVie, and included 577 pediatric patients with juvenile idiopathic arthritis (JIA), psoriasis, or Crohn’s disease. Patients received subcutaneous injection of adalimumab at a dosage of either 40 mg/0.8 mL or 20 mg/0.4 mL.
Adverse events that occurred after the first adalimumab dose and up to 70 days after the last dose were included. Serious adverse events were defined as “events that were fatal or immediately life-threatening; required inpatient or prolonged hospitalization; resulted in persistent or significant disability/incapacity, congenital anomaly, or spontaneous or elective abortion; or required medical or surgical intervention to prevent a serious outcome,” the authors said.
Infections occurred in 82% of JIA patients (151 events per 100 PY), 74% of patients with psoriasis (169 events per 100 PY), and 76% of patients with CD (132 events per 100 PY). The most common events for JIA, psoriasis, and Crohn’s were injection-site pain (22% of patients; 75 events per 100 PY), headache (30% of patients; 47 events per 100 PY), and worsening of Crohn’s disease (55% of patients; 37 events per 100 PY), respectively.
Serious adverse events occurred in 29% of patients. Rates for JIA, psoriasis, and Crohn’s were 14, 7, and 32 events per 100 PY, respectively. Serious infections were the most common serious adverse event, with rates of 3, 1, and 7 events per 100 PY for JIA, psoriasis, and Crohn’s disease, respectively. Pneumonia was the most commonly reported serious infection (1% of patients; 1 event per 100 PY). One death, due to an accidental fall, occurred in an adolescent patient with psoriasis.
The study findings add to “a more complete understanding of the established safety profile of adalimumab,” and suggest that in pediatric patients, “the overall safety profile was comparable and consistent with that in adults,” Dr. Horneff and his associates added.
AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
SOURCE: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point:
Major finding: The most common adverse events across indications were upper respiratory tract infection (24 events per 100 patient-years), nasopharyngitis (17 events per 100 PY), and headache (20 events per 100 PY).
Study details: An analysis of data for 577 pediatric patients from seven clinical trials between September 2002 and December 2015.
Disclosures: AbbVie funded the study. Dr. Horneff has received grants from AbbVie, Chugai, Novartis, Pfizer, and Roche. Seven of the investigators are or were employees of AbbVie and may own AbbVie stock and stock options. Two of the investigators disclosed ties with a number of pharmaceutical companies.
Source: Horneff G et al. J Pediatr. 2018 Oct. doi: 10.1016/j.jpeds.2018.05.042.
Ketamine beats midazolam for agitation control
SAN DIEGO – Following in the wake of a previous prospective comparison of ketamine and haloperidol for control of severe prehospital agitation, a new prospective study by the same group and presented at the annual meeting of the American College of Emergency Physicians found ketamine superior to midazolam for the same indication.
“The difference between the two drugs was larger when agitation was more profound,” reported Jon B. Cole, MD, an emergency medicine physician at Hennepin Healthcare and associate professor of emergency medicine at the University of Minnesota, Minneapolis.
Relative to ketamine (Ketalar), midazolam (Versed) was associated with fewer side effects, “so for less agitated patients, midazolam may still be the preferred therapy,” he added in his late-breaker presentation.
In recent years, ketamine has become a popular drug in emergency medical services for control of agitation, according to Dr. Cole, but he said that this approach has been adopted with relatively limited support from objective evidence. In his literature search, only 2 of 11 original studies that addressed ketamine for agitation involved prospective comparative studies.
One of those prior prospective studies was one Dr. Cole led and published 2 years ago (Clin Toxicol. 2016;54:556-62). In that study, comparing 5 mg/kg of intramuscular (IM) ketamine to 10 mg IM haloperidol, ketamine had a faster onset (median 5 vs. 17 minutes) but produced more side effects, including higher rates of intubation (39% vs. 4%).
In the new prospective study, ketamine and midazolam were compared over separate consecutive 6-month periods in which ketamine and then midazolam were employed as the dominant strategy for agitation control. This was the same open-label, nonrandomized design used for the comparison of ketamine and haloperidol, but with one difference. When the Altered Mental Status Score was +2 or +3, considered severe agitation, patients received 3 mg/kg of IM ketamine or 5 mg of IM midazolam. When the AMS score was +4, called profound agitation, the doses were 5 mg/kg and 15 mg, respectively.
The primary result was that adequate sedation overall was achieved in a median 4.3 minutes on ketamine but 8.8 minutes on midazolam, producing a more than 3-minute advantage for ketamine, which translated into an odds ratio (OR) of 1.8 for adequate agitation control favoring ketamine, according to Dr. Cole. In those with severe agitation, the median advantage was less than 2 minutes, but the OR of 1.6 remained significant. In those with profound agitation, the mean difference climbed above 5 minutes with an OR of 2.5.
but Dr. Cole did report that midazolam was better tolerated. On the lower doses of the two drugs, for example, adverse events that were more common on ketamine than midazolam included vomiting (7% vs. 1%) and hypersalivation (20% vs. 0%).
In addition to the nonrandomized design, one limitation was unequal numbers of patients in the comparative groups. Only 113 were treated with midazolam while 202 patients were treated with ketamine. The reason was that the study of these drugs, which had been in widespread use at Dr. Cole’s institution, was conducted without asking patients to agree to participate. When negative stories in local papers framed this as a study conducted without consent, the institution asked the investigators to halt the study, and they complied.
“Subsequently, we have been assured after multiple evaluations that the rights and safety of our patient population were never violated,” Dr. Cole said, but he acknowledged that closing the study was an appropriate step in order to preserve patient confidence. “What we learned from this experience is that we may need to reconsider how we do waive of consent research at our institution,” he added.
In the meantime, the comparisons of ketamine with haloperidol and midazolam have provided objective evidence of their relative efficacy and safety in the management of prehospital agitation.
Dr. Cole reported no conflicts of interest.
SOURCE: Ann Emerg Med. 2018 Oct. doi. org/10.1016/j.annemergmed.2018.08.007.
SAN DIEGO – Following in the wake of a previous prospective comparison of ketamine and haloperidol for control of severe prehospital agitation, a new prospective study by the same group and presented at the annual meeting of the American College of Emergency Physicians found ketamine superior to midazolam for the same indication.
“The difference between the two drugs was larger when agitation was more profound,” reported Jon B. Cole, MD, an emergency medicine physician at Hennepin Healthcare and associate professor of emergency medicine at the University of Minnesota, Minneapolis.
Relative to ketamine (Ketalar), midazolam (Versed) was associated with fewer side effects, “so for less agitated patients, midazolam may still be the preferred therapy,” he added in his late-breaker presentation.
In recent years, ketamine has become a popular drug in emergency medical services for control of agitation, according to Dr. Cole, but he said that this approach has been adopted with relatively limited support from objective evidence. In his literature search, only 2 of 11 original studies that addressed ketamine for agitation involved prospective comparative studies.
One of those prior prospective studies was one Dr. Cole led and published 2 years ago (Clin Toxicol. 2016;54:556-62). In that study, comparing 5 mg/kg of intramuscular (IM) ketamine to 10 mg IM haloperidol, ketamine had a faster onset (median 5 vs. 17 minutes) but produced more side effects, including higher rates of intubation (39% vs. 4%).
In the new prospective study, ketamine and midazolam were compared over separate consecutive 6-month periods in which ketamine and then midazolam were employed as the dominant strategy for agitation control. This was the same open-label, nonrandomized design used for the comparison of ketamine and haloperidol, but with one difference. When the Altered Mental Status Score was +2 or +3, considered severe agitation, patients received 3 mg/kg of IM ketamine or 5 mg of IM midazolam. When the AMS score was +4, called profound agitation, the doses were 5 mg/kg and 15 mg, respectively.
The primary result was that adequate sedation overall was achieved in a median 4.3 minutes on ketamine but 8.8 minutes on midazolam, producing a more than 3-minute advantage for ketamine, which translated into an odds ratio (OR) of 1.8 for adequate agitation control favoring ketamine, according to Dr. Cole. In those with severe agitation, the median advantage was less than 2 minutes, but the OR of 1.6 remained significant. In those with profound agitation, the mean difference climbed above 5 minutes with an OR of 2.5.
but Dr. Cole did report that midazolam was better tolerated. On the lower doses of the two drugs, for example, adverse events that were more common on ketamine than midazolam included vomiting (7% vs. 1%) and hypersalivation (20% vs. 0%).
In addition to the nonrandomized design, one limitation was unequal numbers of patients in the comparative groups. Only 113 were treated with midazolam while 202 patients were treated with ketamine. The reason was that the study of these drugs, which had been in widespread use at Dr. Cole’s institution, was conducted without asking patients to agree to participate. When negative stories in local papers framed this as a study conducted without consent, the institution asked the investigators to halt the study, and they complied.
“Subsequently, we have been assured after multiple evaluations that the rights and safety of our patient population were never violated,” Dr. Cole said, but he acknowledged that closing the study was an appropriate step in order to preserve patient confidence. “What we learned from this experience is that we may need to reconsider how we do waive of consent research at our institution,” he added.
In the meantime, the comparisons of ketamine with haloperidol and midazolam have provided objective evidence of their relative efficacy and safety in the management of prehospital agitation.
Dr. Cole reported no conflicts of interest.
SOURCE: Ann Emerg Med. 2018 Oct. doi. org/10.1016/j.annemergmed.2018.08.007.
SAN DIEGO – Following in the wake of a previous prospective comparison of ketamine and haloperidol for control of severe prehospital agitation, a new prospective study by the same group and presented at the annual meeting of the American College of Emergency Physicians found ketamine superior to midazolam for the same indication.
“The difference between the two drugs was larger when agitation was more profound,” reported Jon B. Cole, MD, an emergency medicine physician at Hennepin Healthcare and associate professor of emergency medicine at the University of Minnesota, Minneapolis.
Relative to ketamine (Ketalar), midazolam (Versed) was associated with fewer side effects, “so for less agitated patients, midazolam may still be the preferred therapy,” he added in his late-breaker presentation.
In recent years, ketamine has become a popular drug in emergency medical services for control of agitation, according to Dr. Cole, but he said that this approach has been adopted with relatively limited support from objective evidence. In his literature search, only 2 of 11 original studies that addressed ketamine for agitation involved prospective comparative studies.
One of those prior prospective studies was one Dr. Cole led and published 2 years ago (Clin Toxicol. 2016;54:556-62). In that study, comparing 5 mg/kg of intramuscular (IM) ketamine to 10 mg IM haloperidol, ketamine had a faster onset (median 5 vs. 17 minutes) but produced more side effects, including higher rates of intubation (39% vs. 4%).
In the new prospective study, ketamine and midazolam were compared over separate consecutive 6-month periods in which ketamine and then midazolam were employed as the dominant strategy for agitation control. This was the same open-label, nonrandomized design used for the comparison of ketamine and haloperidol, but with one difference. When the Altered Mental Status Score was +2 or +3, considered severe agitation, patients received 3 mg/kg of IM ketamine or 5 mg of IM midazolam. When the AMS score was +4, called profound agitation, the doses were 5 mg/kg and 15 mg, respectively.
The primary result was that adequate sedation overall was achieved in a median 4.3 minutes on ketamine but 8.8 minutes on midazolam, producing a more than 3-minute advantage for ketamine, which translated into an odds ratio (OR) of 1.8 for adequate agitation control favoring ketamine, according to Dr. Cole. In those with severe agitation, the median advantage was less than 2 minutes, but the OR of 1.6 remained significant. In those with profound agitation, the mean difference climbed above 5 minutes with an OR of 2.5.
but Dr. Cole did report that midazolam was better tolerated. On the lower doses of the two drugs, for example, adverse events that were more common on ketamine than midazolam included vomiting (7% vs. 1%) and hypersalivation (20% vs. 0%).
In addition to the nonrandomized design, one limitation was unequal numbers of patients in the comparative groups. Only 113 were treated with midazolam while 202 patients were treated with ketamine. The reason was that the study of these drugs, which had been in widespread use at Dr. Cole’s institution, was conducted without asking patients to agree to participate. When negative stories in local papers framed this as a study conducted without consent, the institution asked the investigators to halt the study, and they complied.
“Subsequently, we have been assured after multiple evaluations that the rights and safety of our patient population were never violated,” Dr. Cole said, but he acknowledged that closing the study was an appropriate step in order to preserve patient confidence. “What we learned from this experience is that we may need to reconsider how we do waive of consent research at our institution,” he added.
In the meantime, the comparisons of ketamine with haloperidol and midazolam have provided objective evidence of their relative efficacy and safety in the management of prehospital agitation.
Dr. Cole reported no conflicts of interest.
SOURCE: Ann Emerg Med. 2018 Oct. doi. org/10.1016/j.annemergmed.2018.08.007.
REPORTING FROM ACEP2018
Key clinical point: In severe agitation involving violent or threatening behavior, ketamine sedates patients more quickly than midazolam.
Major finding: Because of a 3-minute faster onset, ketamine was associated with a 1.8 greater odds ratio than midazolam for adequate sedation control.
Study details: Prospective open-label nonrandomized study.
Disclosures: Dr. Cole reported no conflicts of interest.
Source: Ann Emerg Med. 2018 Oct. doi. org/10.1016/j.annemergmed.2018.08.007.