User login
B-cell maturation antigen targeted in myeloma trials
NEW YORK – Three novel treatment strategies that target B-cell maturation antigen (BCMA) have shown promise in recent multiple myeloma clinical trials, according to Shaji K. Kumar, MD, of the Mayo Clinic Cancer Center in Rochester, Minn.
These strategies include B-cell maturation antigen (BCMA)–specific chimeric antigen receptor (CAR) T-cell therapies, bispecific T-cell engagers (BiTEs), and a BCMA antibody–drug conjugate, Dr. Kumar said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these three platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar said.
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent that has produced an overall response rate in 67% in a group of myeloma patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, including in a phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone, or bortezomib plus dexamethasone, in patients with relapsed or refractory disease.
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma involves bb2121, which showed durable clinical responses in heavily pretreated patients, according to data presented at the 2017 annual meeting of the American Society of Hematology.
“The overall response rate is quite significant,” said Dr. Kumar, who related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the response were lasting, he said, with five patients in ongoing response for more than 1 year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar said.
Additionally, promising results have been presented on a novel CAR T-cell product, LCAR-B38M, which principally targets BCMA and led to a significant number of patients who achieved stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and costimulatory molecules are currently undergoing clinical trials, Dr. Kumar said.
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short time frame, so having an approach that is off the shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies under investigation include AMG 420 and PF-06863135, he added.
Dr. Kumar reported one disclosure related to Dr. Reddy’s Laboratories.
NEW YORK – Three novel treatment strategies that target B-cell maturation antigen (BCMA) have shown promise in recent multiple myeloma clinical trials, according to Shaji K. Kumar, MD, of the Mayo Clinic Cancer Center in Rochester, Minn.
These strategies include B-cell maturation antigen (BCMA)–specific chimeric antigen receptor (CAR) T-cell therapies, bispecific T-cell engagers (BiTEs), and a BCMA antibody–drug conjugate, Dr. Kumar said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these three platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar said.
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent that has produced an overall response rate in 67% in a group of myeloma patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, including in a phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone, or bortezomib plus dexamethasone, in patients with relapsed or refractory disease.
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma involves bb2121, which showed durable clinical responses in heavily pretreated patients, according to data presented at the 2017 annual meeting of the American Society of Hematology.
“The overall response rate is quite significant,” said Dr. Kumar, who related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the response were lasting, he said, with five patients in ongoing response for more than 1 year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar said.
Additionally, promising results have been presented on a novel CAR T-cell product, LCAR-B38M, which principally targets BCMA and led to a significant number of patients who achieved stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and costimulatory molecules are currently undergoing clinical trials, Dr. Kumar said.
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short time frame, so having an approach that is off the shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies under investigation include AMG 420 and PF-06863135, he added.
Dr. Kumar reported one disclosure related to Dr. Reddy’s Laboratories.
NEW YORK – Three novel treatment strategies that target B-cell maturation antigen (BCMA) have shown promise in recent multiple myeloma clinical trials, according to Shaji K. Kumar, MD, of the Mayo Clinic Cancer Center in Rochester, Minn.
These strategies include B-cell maturation antigen (BCMA)–specific chimeric antigen receptor (CAR) T-cell therapies, bispecific T-cell engagers (BiTEs), and a BCMA antibody–drug conjugate, Dr. Kumar said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
“Clearly, there are a lot of exciting drugs that are currently in clinical trials, but these three platforms appear to be much more advanced than the others, and hopefully we will see that in the clinic in the near future,” Dr. Kumar said.
The antibody-drug conjugate, GSK2857916, is a humanized IgG1 anti-BCMA antibody conjugated to a microtubule-disrupting agent that has produced an overall response rate in 67% in a group of myeloma patients who had previously received multiple standard-of-care agents.
“Some of the responses were quite durable, lasting several months,” he said.
Now, GSK2857916 is being evaluated in a variety of different combinations, including in a phase 2 study of the antibody-drug conjugate in combination with lenalidomide plus dexamethasone, or bortezomib plus dexamethasone, in patients with relapsed or refractory disease.
Some of the most “exciting” data with anti-BCMA CAR T-cell therapy in myeloma involves bb2121, which showed durable clinical responses in heavily pretreated patients, according to data presented at the 2017 annual meeting of the American Society of Hematology.
“The overall response rate is quite significant,” said Dr. Kumar, who related a 94% rate of overall response that was even higher in patients treated with doses of 150 x 106 CAR+ T cells or more. Many of the response were lasting, he said, with five patients in ongoing response for more than 1 year.
“The results are exciting enough that this is actually moving forward with registration trials,” Dr. Kumar said.
Additionally, promising results have been presented on a novel CAR T-cell product, LCAR-B38M, which principally targets BCMA and led to a significant number of patients who achieved stringent complete response that lasted beyond 1 year.
Multiple BCMA-targeting CAR T-cell products that use different vectors and costimulatory molecules are currently undergoing clinical trials, Dr. Kumar said.
In contrast to CAR T-cell products that must be customized to each patient in a process that takes weeks, BiTEs are a ready-made approach to allow T cells to engage with tumor cells.
“In patients with advanced disease, a lot can change in that short time frame, so having an approach that is off the shelf, which is not patient specific, is quite attractive,” Dr. Kumar said.
BCMA-directed BiTE therapies under investigation include AMG 420 and PF-06863135, he added.
Dr. Kumar reported one disclosure related to Dr. Reddy’s Laboratories.
EXPERT ANALYSIS FROM THE NCCN HEMATOLOGIC MALIGNANCIES CONGRESS
Pazopanib is active against renal, other neoplasms of von Hippel-Lindau disease
The oral, multitargeted tyrosine kinase inhibitor pazopanib (Votrient) is active and safe in patients with renal cell carcinoma and other neoplasms caused by von Hippel-Lindau disease, a phase 2 trial has found.
Eric Jonasch, MD, and his coinvestigators at the University of Texas MD Anderson Cancer Center, Houston, conducted the single-arm trial among 31 adult patients with clinical manifestations of von Hippel-Lindau disease, an autosomal dominant disorder that currently has no approved treatment.
All patients were treated with open-label pazopanib (800 mg daily with dose reductions permitted) for 24 weeks, with an option to continue thereafter. Pazopanib derives its antiangiogenic and antineoplastic activity from its selective inhibition of vascular endothelial growth factor receptors (VEGFR)–1, –2, and –3; c-KIT; and platelet-derived growth factor receptor (PDGFR). It is currently approved by the FDA for treatment of advanced renal cell carcinoma and advanced soft-tissue sarcoma.
The trial was stopped before attaining planned enrollment because accrual slowed and it met a prespecified toxicity stopping threshold, according to results reported in Lancet Oncology.
At a median follow-up of 12 months, 42% of patients overall had an objective response to pazopanib. By site, response was seen in 52% of 59 renal cell carcinomas, 53% of 17 pancreatic lesions (mainly serous cystadenomas), and 4% of 49 CNS hemangioblastomas; the median reduction in tumor size was 40.5%, 30.5%, and 13%, respectively.
Slightly more than half of patients, 52%, opted to stay on pazopanib after 24 weeks, with the longest duration being 60 months at data cutoff.
Some 13% of patients withdrew from the study because of grade 3 or 4 transaminitis, and 10% stopped treatment because of general intolerance related to multiple grade 1 and 2 toxicities. There were three treatment-related serious adverse events: one case of appendicitis, one case of gastritis, and one case of fatal CNS bleeding after a fall.
“Pazopanib was associated with encouraging preliminary activity in von Hippel-Lindau disease, with a side effect profile consistent with that seen in previous trials,” the investigators concluded. “Pazopanib could be considered as a treatment choice for patients with von Hippel-Lindau disease and growing lesions, or to reduce the size of unresectable lesions in these patients.”
Dr. Jonasch disclosed that he receives research support and honoraria from Novartis. The study was funded by Novartis and by a National Institutes of Health National Cancer Institute core grant.
SOURCE: Jonasch E et al. Lancet Oncol. 2018 Sep 17. doi: 10.1016/S1470-2045(18)30487-X,
The oral, multitargeted tyrosine kinase inhibitor pazopanib (Votrient) is active and safe in patients with renal cell carcinoma and other neoplasms caused by von Hippel-Lindau disease, a phase 2 trial has found.
Eric Jonasch, MD, and his coinvestigators at the University of Texas MD Anderson Cancer Center, Houston, conducted the single-arm trial among 31 adult patients with clinical manifestations of von Hippel-Lindau disease, an autosomal dominant disorder that currently has no approved treatment.
All patients were treated with open-label pazopanib (800 mg daily with dose reductions permitted) for 24 weeks, with an option to continue thereafter. Pazopanib derives its antiangiogenic and antineoplastic activity from its selective inhibition of vascular endothelial growth factor receptors (VEGFR)–1, –2, and –3; c-KIT; and platelet-derived growth factor receptor (PDGFR). It is currently approved by the FDA for treatment of advanced renal cell carcinoma and advanced soft-tissue sarcoma.
The trial was stopped before attaining planned enrollment because accrual slowed and it met a prespecified toxicity stopping threshold, according to results reported in Lancet Oncology.
At a median follow-up of 12 months, 42% of patients overall had an objective response to pazopanib. By site, response was seen in 52% of 59 renal cell carcinomas, 53% of 17 pancreatic lesions (mainly serous cystadenomas), and 4% of 49 CNS hemangioblastomas; the median reduction in tumor size was 40.5%, 30.5%, and 13%, respectively.
Slightly more than half of patients, 52%, opted to stay on pazopanib after 24 weeks, with the longest duration being 60 months at data cutoff.
Some 13% of patients withdrew from the study because of grade 3 or 4 transaminitis, and 10% stopped treatment because of general intolerance related to multiple grade 1 and 2 toxicities. There were three treatment-related serious adverse events: one case of appendicitis, one case of gastritis, and one case of fatal CNS bleeding after a fall.
“Pazopanib was associated with encouraging preliminary activity in von Hippel-Lindau disease, with a side effect profile consistent with that seen in previous trials,” the investigators concluded. “Pazopanib could be considered as a treatment choice for patients with von Hippel-Lindau disease and growing lesions, or to reduce the size of unresectable lesions in these patients.”
Dr. Jonasch disclosed that he receives research support and honoraria from Novartis. The study was funded by Novartis and by a National Institutes of Health National Cancer Institute core grant.
SOURCE: Jonasch E et al. Lancet Oncol. 2018 Sep 17. doi: 10.1016/S1470-2045(18)30487-X,
The oral, multitargeted tyrosine kinase inhibitor pazopanib (Votrient) is active and safe in patients with renal cell carcinoma and other neoplasms caused by von Hippel-Lindau disease, a phase 2 trial has found.
Eric Jonasch, MD, and his coinvestigators at the University of Texas MD Anderson Cancer Center, Houston, conducted the single-arm trial among 31 adult patients with clinical manifestations of von Hippel-Lindau disease, an autosomal dominant disorder that currently has no approved treatment.
All patients were treated with open-label pazopanib (800 mg daily with dose reductions permitted) for 24 weeks, with an option to continue thereafter. Pazopanib derives its antiangiogenic and antineoplastic activity from its selective inhibition of vascular endothelial growth factor receptors (VEGFR)–1, –2, and –3; c-KIT; and platelet-derived growth factor receptor (PDGFR). It is currently approved by the FDA for treatment of advanced renal cell carcinoma and advanced soft-tissue sarcoma.
The trial was stopped before attaining planned enrollment because accrual slowed and it met a prespecified toxicity stopping threshold, according to results reported in Lancet Oncology.
At a median follow-up of 12 months, 42% of patients overall had an objective response to pazopanib. By site, response was seen in 52% of 59 renal cell carcinomas, 53% of 17 pancreatic lesions (mainly serous cystadenomas), and 4% of 49 CNS hemangioblastomas; the median reduction in tumor size was 40.5%, 30.5%, and 13%, respectively.
Slightly more than half of patients, 52%, opted to stay on pazopanib after 24 weeks, with the longest duration being 60 months at data cutoff.
Some 13% of patients withdrew from the study because of grade 3 or 4 transaminitis, and 10% stopped treatment because of general intolerance related to multiple grade 1 and 2 toxicities. There were three treatment-related serious adverse events: one case of appendicitis, one case of gastritis, and one case of fatal CNS bleeding after a fall.
“Pazopanib was associated with encouraging preliminary activity in von Hippel-Lindau disease, with a side effect profile consistent with that seen in previous trials,” the investigators concluded. “Pazopanib could be considered as a treatment choice for patients with von Hippel-Lindau disease and growing lesions, or to reduce the size of unresectable lesions in these patients.”
Dr. Jonasch disclosed that he receives research support and honoraria from Novartis. The study was funded by Novartis and by a National Institutes of Health National Cancer Institute core grant.
SOURCE: Jonasch E et al. Lancet Oncol. 2018 Sep 17. doi: 10.1016/S1470-2045(18)30487-X,
FROM LANCET ONCOLOGY
Key clinical point: Pazopanib appears efficacious and safe for treating neoplasms associated with von Hippel-Lindau disease.
Major finding: The objective response rate was 42% overall, with response seen in 52% of renal cell carcinomas.
Study details: Single-center, single-arm, open-label, phase 2 trial among 31 adult patients with clinical manifestations of von Hippel-Lindau disease who were treated with pazopanib for at least 24 weeks.
Disclosures: Dr. Jonasch disclosed that he receives research support and honoraria from Novartis. The study was funded by Novartis and by a National Institutes of Health National Cancer Institute core grant.
Source: Jonasch E et al. Lancet Oncol. 2018 Sep 17. doi: 10.1016/S1470-2045(18)30487-X.
Mobile App Rankings in Dermatology
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
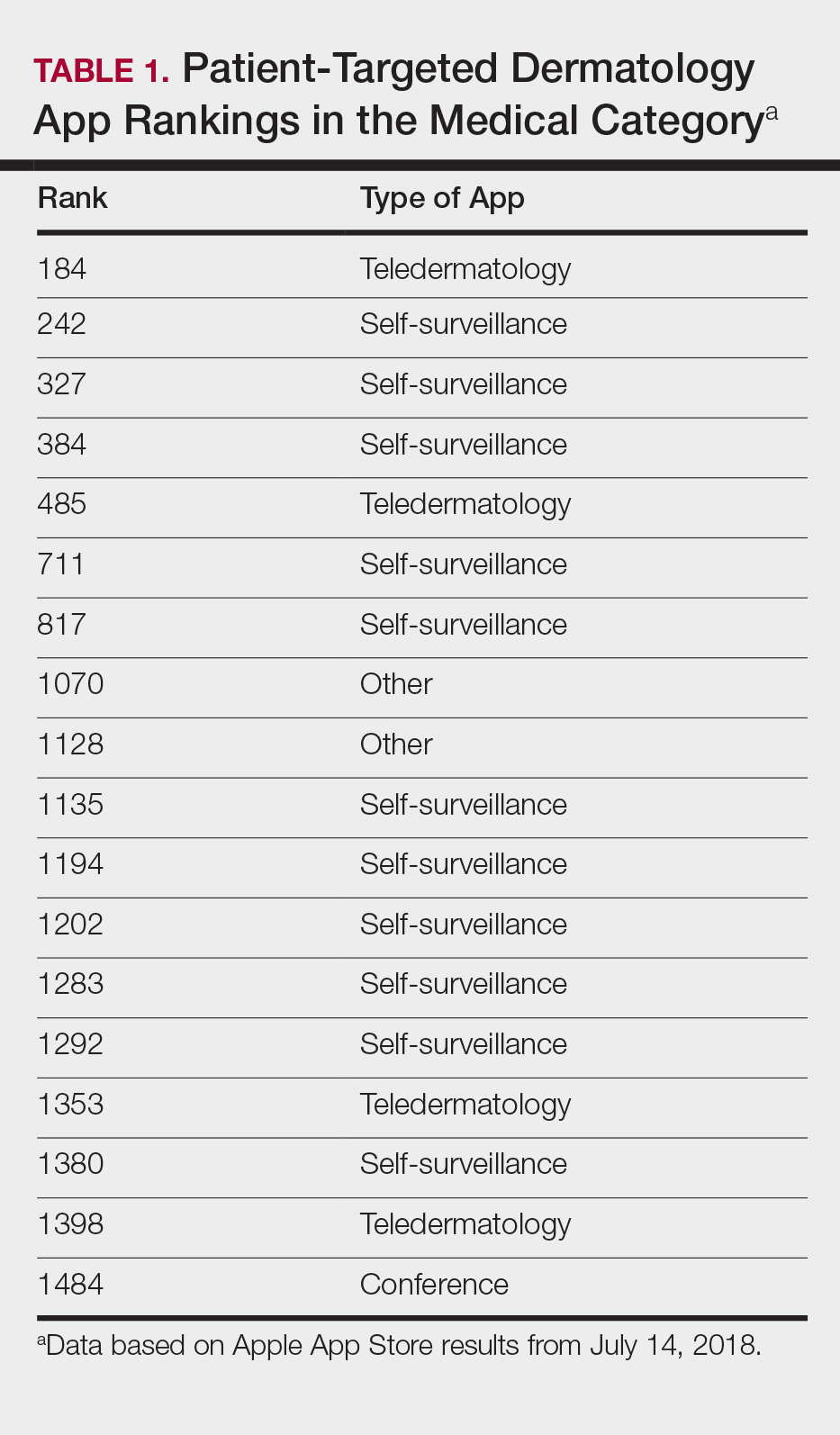
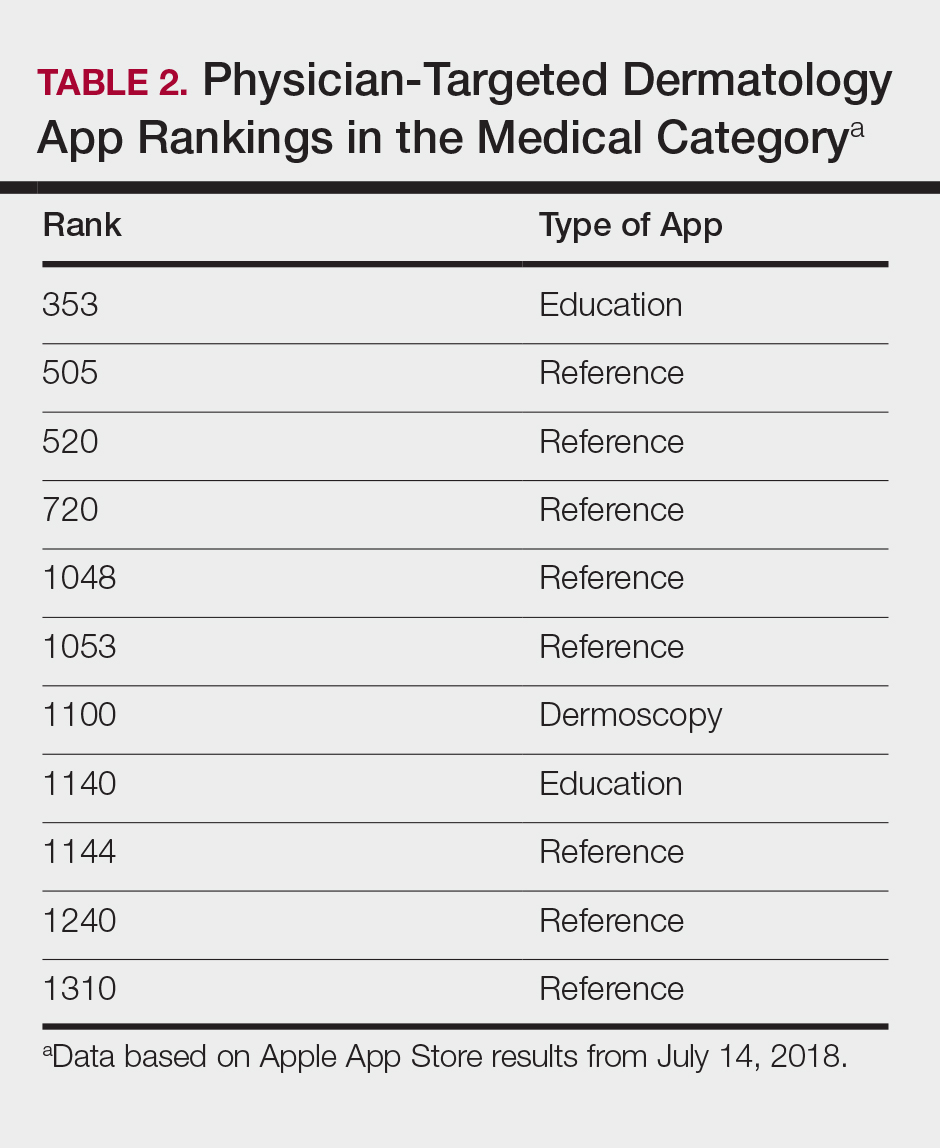
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
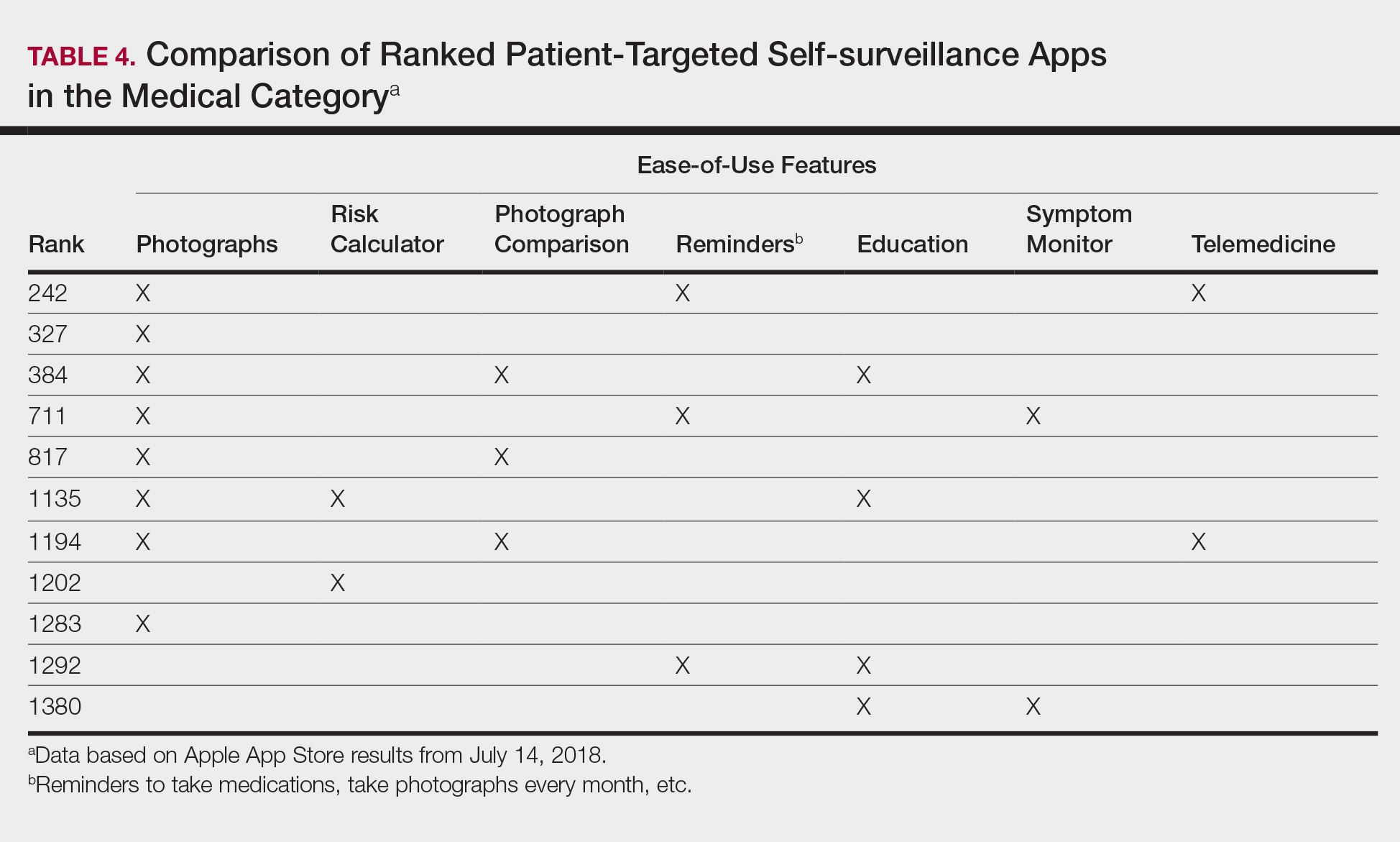
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
Practice Points
- As mobile application (app) usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in dermatology education. As such, it will become more critical to develop formal scientific standards.
- The most desired dermatology apps for patients were apps that allowed them to be proactive with their health.
- There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps.
On-site coverage of CHEST 2018
CHEST Physician reporting staff will provide on-site coverage of CHEST 2018, the annual meeting of the American College of Chest Physicians, held in San Antonio, Tex., Oct. 6 through Oct. 10. They are planning to report on a wide variety of sessions covering the latest research on treating COPD, sleep medicine, pulmonary hypertension, asthma, and other pulmonary disease. Panels, plenaries, original research presentations, and late-breaking studies will all be covered in depth. Stories will be posted daily during the meeting on the CHEST Physician website. They will also be talking to presenters and discussants about their work, so be sure to watch for video interviews, which also will be published daily.
Among the sessions on the coverage calendar are the following:
The Impact of Obesity on Pulmonary Disorders. Sunday, Oct. 7, 7:30 a.m. to 8:30 a.m., Convention Center 207B
GAMES: Games Augmenting Medical Education. Sunday, Oct. 7, 10:45 a.m. to 11:45 a.m., Convention Center 207B
Current Trends and Controversies in the Practice of Sleep Medicine. Monday, Oct. 8, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Futility? Responding to Nonbeneficial Treatment Requests. Monday, Oct. 8, 11:00 a.m. to 12:00 p.m., Convention Center 212A
Update on Diagnosis and Management of Diffuse Cystic Lung Disease. Tuesday, Oct. 9, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Lung Cancer Screening: News Questions and New Answers. Tuesday, Oct. 9, 8:45 a.m. to 9:45 a.m., Convention Center 207A
Check here on the CHEST Physician website for the latest news from CHEST 2018!
CHEST Physician reporting staff will provide on-site coverage of CHEST 2018, the annual meeting of the American College of Chest Physicians, held in San Antonio, Tex., Oct. 6 through Oct. 10. They are planning to report on a wide variety of sessions covering the latest research on treating COPD, sleep medicine, pulmonary hypertension, asthma, and other pulmonary disease. Panels, plenaries, original research presentations, and late-breaking studies will all be covered in depth. Stories will be posted daily during the meeting on the CHEST Physician website. They will also be talking to presenters and discussants about their work, so be sure to watch for video interviews, which also will be published daily.
Among the sessions on the coverage calendar are the following:
The Impact of Obesity on Pulmonary Disorders. Sunday, Oct. 7, 7:30 a.m. to 8:30 a.m., Convention Center 207B
GAMES: Games Augmenting Medical Education. Sunday, Oct. 7, 10:45 a.m. to 11:45 a.m., Convention Center 207B
Current Trends and Controversies in the Practice of Sleep Medicine. Monday, Oct. 8, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Futility? Responding to Nonbeneficial Treatment Requests. Monday, Oct. 8, 11:00 a.m. to 12:00 p.m., Convention Center 212A
Update on Diagnosis and Management of Diffuse Cystic Lung Disease. Tuesday, Oct. 9, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Lung Cancer Screening: News Questions and New Answers. Tuesday, Oct. 9, 8:45 a.m. to 9:45 a.m., Convention Center 207A
Check here on the CHEST Physician website for the latest news from CHEST 2018!
CHEST Physician reporting staff will provide on-site coverage of CHEST 2018, the annual meeting of the American College of Chest Physicians, held in San Antonio, Tex., Oct. 6 through Oct. 10. They are planning to report on a wide variety of sessions covering the latest research on treating COPD, sleep medicine, pulmonary hypertension, asthma, and other pulmonary disease. Panels, plenaries, original research presentations, and late-breaking studies will all be covered in depth. Stories will be posted daily during the meeting on the CHEST Physician website. They will also be talking to presenters and discussants about their work, so be sure to watch for video interviews, which also will be published daily.
Among the sessions on the coverage calendar are the following:
The Impact of Obesity on Pulmonary Disorders. Sunday, Oct. 7, 7:30 a.m. to 8:30 a.m., Convention Center 207B
GAMES: Games Augmenting Medical Education. Sunday, Oct. 7, 10:45 a.m. to 11:45 a.m., Convention Center 207B
Current Trends and Controversies in the Practice of Sleep Medicine. Monday, Oct. 8, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Futility? Responding to Nonbeneficial Treatment Requests. Monday, Oct. 8, 11:00 a.m. to 12:00 p.m., Convention Center 212A
Update on Diagnosis and Management of Diffuse Cystic Lung Disease. Tuesday, Oct. 9, 7:30 a.m. to 8:30 a.m., Convention Center 214A
Lung Cancer Screening: News Questions and New Answers. Tuesday, Oct. 9, 8:45 a.m. to 9:45 a.m., Convention Center 207A
Check here on the CHEST Physician website for the latest news from CHEST 2018!
IMpower133: Atezolizumab plus standard chemotherapy boosted survival in ES-SCLC
TORONTO – Adding the humanized monoclonal programmed death-ligand 1 (PD-L1) antibody atezolizumab to standard first-line treatment of extensive-stage small cell lung cancer (ES-SCLC) significantly improved overall and progression-free survival in the phase 1/3 IMpower133 trial.
The combination may represent a new standard-of-care regimen for patients with untreated ES-SCLC, which is highly lethal – with 5-year survival of about 1%-3% – and represents about 13% of all lung cancers, Stephen V. Liu, MD, reported at the World Conference on Lung Cancer.
The findings were published simultaneously in the New England Journal of Medicine.
The median overall survival in 201 patients randomized to receive atezolizumab in addition to carboplatin and etoposide was 12.3 months, compared with 10.3 months in 202 patients who received placebo plus carboplatin and etoposide (hazard ratio, 0.7), Dr. Liu of Georgetown University, Washington, and a member of the trial steering committee said at the meeting, sponsored by the International Association for the Study of Lung Cancer.
“That translates to a 30% reduction in the risk of patient death,” he said at a press briefing during the conference. “Patients receiving atezolizumab had a much greater likelihood of being alive at 1 year, with a 1-year survival rate of 51.7% versus 38.2%.”
Median progression-free survival(PFS) also improved with atezolizumab (5.2 months vs. 4.3 months with placebo; HR, 0.77), as did 6-month PFS. At 12 months there was more than a doubling of PFS in the atezolizumab group (5.0% vs. 12.6%), he said.
Participants in the double-blind trial were treatment-naive all-comers with measurable ES-SCLC and good performance status. They received four 21-day cycles of intravenous carboplatin (area under the curve, 5 mg/mL per minute) on day 1 plus intravenous etoposide (100 mg/m2) on days 1-3 with either concurrent 1,200 mg of atezolizumab on day 1 or placebo, followed by maintenance therapy with atezolizumab or placebo until intolerable toxicity or disease progression.
The treatment benefits were seen across many patient subgroups and regardless of tumor mutational burden.
The atezolizumab safety profile was as expected with no new safety signals and did not compromise patients’ ability to complete four treatment cycles, Dr. Liu noted.
The findings are exciting in that they represent the first in decades to show a significant improvement in survival in patients with ES-SCLC, he said. Although most patients have an initial response to standard-of-care chemotherapy, that response isn’t durable. “As much as we expect a response, we also know that it’s transient; we expect a response, we expect relapse. There hasn’t been a change really in the past 20 years, at least, with this regimen that we’ve been using since the 1980s.”
That’s not for lack of trying, he added, noting that more than 40 phase 3 studies have looked at more than 60 different drugs since the 1970s and have “failed to move the needle.”
Immunotherapy, however, has dramatically improved the therapeutic landscape in non–small cell lung cancer, and preclinical data and clinical experience suggest “a possible synergy between checkpoint inhibition and chemotherapy,” which led to this global study, he explained.
“This is the first study in over 20 years to show a significant improvement in survival and progression-free survival in initial treatment of small cell lung cancer. The concurrent administration of atezolizumab with chemotherapy helped people live longer, compared to chemotherapy alone,” Dr. Liu concluded, adding in a press statement that “this is an exciting time in oncology, and we are thrilled to finally see real progress in the SCLC space.”
When questioned about the role of PD-L1 in this population and the possibility of identifying a subgroup in which this treatment may be more cost effective, he noted that tissue samples weren’t required at enrollment in this study, but were collected from some patients, and future analyses will assess those samples to try to determine if there are subsets of patients who derive particular benefit from immunotherapy in this setting.
“But today, in an all-comer population, this combination has improved survival,” he said.
IMpower133 was sponsored by F. Hoffman–La Roche. Dr. Liu is a speaker or advisory board member for Genentech, Pfizer, Takeda, Celgene, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb, AstraZeneca, and Ignyta, and has received research or grant support from Genentech, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed Pharmaceuticals, Ignyta, Merck, Lycera, AstraZeneca, and Molecular Partners.
SOURCE: Liu SV et al. WCLC 2018, Abstract PL02.07.
Invited discussant Natasha B. Leighl, MD, said that, while many questions remain, the IMpower133 findings do present a new standard of care for extensive-stage small cell lung cancer given the hazard ratio of 0.70 for survival, the unmet need in this population, and the 4 decades without progress.
“We have a long way to go to catch up with non–small cell lung cancer, and it starts today,” she said, noting, however, that uptake will vary depending on regulatory and economic thresholds in different areas. “I think that really highlights the urgent need for progress in patient selection, biomarker research, and the need to change our culture to one of tissue collection at trial.
“Finally, small cell lung cancer ... is a preventable disease and we need urgent steps in tobacco control to help us eradicate this killer,” she concluded.
Dr. Leighl is a medical oncologist at Princess Margaret Cancer Centre in Toronto. She has received research, grant, or other support from Novartis, AstraZeneca, Bristol-Myers Squibb, Merck, Pfizer, and the Canadian Agency for Drugs and Technologies in Health.
Invited discussant Natasha B. Leighl, MD, said that, while many questions remain, the IMpower133 findings do present a new standard of care for extensive-stage small cell lung cancer given the hazard ratio of 0.70 for survival, the unmet need in this population, and the 4 decades without progress.
“We have a long way to go to catch up with non–small cell lung cancer, and it starts today,” she said, noting, however, that uptake will vary depending on regulatory and economic thresholds in different areas. “I think that really highlights the urgent need for progress in patient selection, biomarker research, and the need to change our culture to one of tissue collection at trial.
“Finally, small cell lung cancer ... is a preventable disease and we need urgent steps in tobacco control to help us eradicate this killer,” she concluded.
Dr. Leighl is a medical oncologist at Princess Margaret Cancer Centre in Toronto. She has received research, grant, or other support from Novartis, AstraZeneca, Bristol-Myers Squibb, Merck, Pfizer, and the Canadian Agency for Drugs and Technologies in Health.
Invited discussant Natasha B. Leighl, MD, said that, while many questions remain, the IMpower133 findings do present a new standard of care for extensive-stage small cell lung cancer given the hazard ratio of 0.70 for survival, the unmet need in this population, and the 4 decades without progress.
“We have a long way to go to catch up with non–small cell lung cancer, and it starts today,” she said, noting, however, that uptake will vary depending on regulatory and economic thresholds in different areas. “I think that really highlights the urgent need for progress in patient selection, biomarker research, and the need to change our culture to one of tissue collection at trial.
“Finally, small cell lung cancer ... is a preventable disease and we need urgent steps in tobacco control to help us eradicate this killer,” she concluded.
Dr. Leighl is a medical oncologist at Princess Margaret Cancer Centre in Toronto. She has received research, grant, or other support from Novartis, AstraZeneca, Bristol-Myers Squibb, Merck, Pfizer, and the Canadian Agency for Drugs and Technologies in Health.
TORONTO – Adding the humanized monoclonal programmed death-ligand 1 (PD-L1) antibody atezolizumab to standard first-line treatment of extensive-stage small cell lung cancer (ES-SCLC) significantly improved overall and progression-free survival in the phase 1/3 IMpower133 trial.
The combination may represent a new standard-of-care regimen for patients with untreated ES-SCLC, which is highly lethal – with 5-year survival of about 1%-3% – and represents about 13% of all lung cancers, Stephen V. Liu, MD, reported at the World Conference on Lung Cancer.
The findings were published simultaneously in the New England Journal of Medicine.
The median overall survival in 201 patients randomized to receive atezolizumab in addition to carboplatin and etoposide was 12.3 months, compared with 10.3 months in 202 patients who received placebo plus carboplatin and etoposide (hazard ratio, 0.7), Dr. Liu of Georgetown University, Washington, and a member of the trial steering committee said at the meeting, sponsored by the International Association for the Study of Lung Cancer.
“That translates to a 30% reduction in the risk of patient death,” he said at a press briefing during the conference. “Patients receiving atezolizumab had a much greater likelihood of being alive at 1 year, with a 1-year survival rate of 51.7% versus 38.2%.”
Median progression-free survival(PFS) also improved with atezolizumab (5.2 months vs. 4.3 months with placebo; HR, 0.77), as did 6-month PFS. At 12 months there was more than a doubling of PFS in the atezolizumab group (5.0% vs. 12.6%), he said.
Participants in the double-blind trial were treatment-naive all-comers with measurable ES-SCLC and good performance status. They received four 21-day cycles of intravenous carboplatin (area under the curve, 5 mg/mL per minute) on day 1 plus intravenous etoposide (100 mg/m2) on days 1-3 with either concurrent 1,200 mg of atezolizumab on day 1 or placebo, followed by maintenance therapy with atezolizumab or placebo until intolerable toxicity or disease progression.
The treatment benefits were seen across many patient subgroups and regardless of tumor mutational burden.
The atezolizumab safety profile was as expected with no new safety signals and did not compromise patients’ ability to complete four treatment cycles, Dr. Liu noted.
The findings are exciting in that they represent the first in decades to show a significant improvement in survival in patients with ES-SCLC, he said. Although most patients have an initial response to standard-of-care chemotherapy, that response isn’t durable. “As much as we expect a response, we also know that it’s transient; we expect a response, we expect relapse. There hasn’t been a change really in the past 20 years, at least, with this regimen that we’ve been using since the 1980s.”
That’s not for lack of trying, he added, noting that more than 40 phase 3 studies have looked at more than 60 different drugs since the 1970s and have “failed to move the needle.”
Immunotherapy, however, has dramatically improved the therapeutic landscape in non–small cell lung cancer, and preclinical data and clinical experience suggest “a possible synergy between checkpoint inhibition and chemotherapy,” which led to this global study, he explained.
“This is the first study in over 20 years to show a significant improvement in survival and progression-free survival in initial treatment of small cell lung cancer. The concurrent administration of atezolizumab with chemotherapy helped people live longer, compared to chemotherapy alone,” Dr. Liu concluded, adding in a press statement that “this is an exciting time in oncology, and we are thrilled to finally see real progress in the SCLC space.”
When questioned about the role of PD-L1 in this population and the possibility of identifying a subgroup in which this treatment may be more cost effective, he noted that tissue samples weren’t required at enrollment in this study, but were collected from some patients, and future analyses will assess those samples to try to determine if there are subsets of patients who derive particular benefit from immunotherapy in this setting.
“But today, in an all-comer population, this combination has improved survival,” he said.
IMpower133 was sponsored by F. Hoffman–La Roche. Dr. Liu is a speaker or advisory board member for Genentech, Pfizer, Takeda, Celgene, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb, AstraZeneca, and Ignyta, and has received research or grant support from Genentech, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed Pharmaceuticals, Ignyta, Merck, Lycera, AstraZeneca, and Molecular Partners.
SOURCE: Liu SV et al. WCLC 2018, Abstract PL02.07.
TORONTO – Adding the humanized monoclonal programmed death-ligand 1 (PD-L1) antibody atezolizumab to standard first-line treatment of extensive-stage small cell lung cancer (ES-SCLC) significantly improved overall and progression-free survival in the phase 1/3 IMpower133 trial.
The combination may represent a new standard-of-care regimen for patients with untreated ES-SCLC, which is highly lethal – with 5-year survival of about 1%-3% – and represents about 13% of all lung cancers, Stephen V. Liu, MD, reported at the World Conference on Lung Cancer.
The findings were published simultaneously in the New England Journal of Medicine.
The median overall survival in 201 patients randomized to receive atezolizumab in addition to carboplatin and etoposide was 12.3 months, compared with 10.3 months in 202 patients who received placebo plus carboplatin and etoposide (hazard ratio, 0.7), Dr. Liu of Georgetown University, Washington, and a member of the trial steering committee said at the meeting, sponsored by the International Association for the Study of Lung Cancer.
“That translates to a 30% reduction in the risk of patient death,” he said at a press briefing during the conference. “Patients receiving atezolizumab had a much greater likelihood of being alive at 1 year, with a 1-year survival rate of 51.7% versus 38.2%.”
Median progression-free survival(PFS) also improved with atezolizumab (5.2 months vs. 4.3 months with placebo; HR, 0.77), as did 6-month PFS. At 12 months there was more than a doubling of PFS in the atezolizumab group (5.0% vs. 12.6%), he said.
Participants in the double-blind trial were treatment-naive all-comers with measurable ES-SCLC and good performance status. They received four 21-day cycles of intravenous carboplatin (area under the curve, 5 mg/mL per minute) on day 1 plus intravenous etoposide (100 mg/m2) on days 1-3 with either concurrent 1,200 mg of atezolizumab on day 1 or placebo, followed by maintenance therapy with atezolizumab or placebo until intolerable toxicity or disease progression.
The treatment benefits were seen across many patient subgroups and regardless of tumor mutational burden.
The atezolizumab safety profile was as expected with no new safety signals and did not compromise patients’ ability to complete four treatment cycles, Dr. Liu noted.
The findings are exciting in that they represent the first in decades to show a significant improvement in survival in patients with ES-SCLC, he said. Although most patients have an initial response to standard-of-care chemotherapy, that response isn’t durable. “As much as we expect a response, we also know that it’s transient; we expect a response, we expect relapse. There hasn’t been a change really in the past 20 years, at least, with this regimen that we’ve been using since the 1980s.”
That’s not for lack of trying, he added, noting that more than 40 phase 3 studies have looked at more than 60 different drugs since the 1970s and have “failed to move the needle.”
Immunotherapy, however, has dramatically improved the therapeutic landscape in non–small cell lung cancer, and preclinical data and clinical experience suggest “a possible synergy between checkpoint inhibition and chemotherapy,” which led to this global study, he explained.
“This is the first study in over 20 years to show a significant improvement in survival and progression-free survival in initial treatment of small cell lung cancer. The concurrent administration of atezolizumab with chemotherapy helped people live longer, compared to chemotherapy alone,” Dr. Liu concluded, adding in a press statement that “this is an exciting time in oncology, and we are thrilled to finally see real progress in the SCLC space.”
When questioned about the role of PD-L1 in this population and the possibility of identifying a subgroup in which this treatment may be more cost effective, he noted that tissue samples weren’t required at enrollment in this study, but were collected from some patients, and future analyses will assess those samples to try to determine if there are subsets of patients who derive particular benefit from immunotherapy in this setting.
“But today, in an all-comer population, this combination has improved survival,” he said.
IMpower133 was sponsored by F. Hoffman–La Roche. Dr. Liu is a speaker or advisory board member for Genentech, Pfizer, Takeda, Celgene, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb, AstraZeneca, and Ignyta, and has received research or grant support from Genentech, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed Pharmaceuticals, Ignyta, Merck, Lycera, AstraZeneca, and Molecular Partners.
SOURCE: Liu SV et al. WCLC 2018, Abstract PL02.07.
REPORTING FROM WCLC 2018
Key clinical point: Immunotherapy added to standard chemotherapy improves survival outcomes in extensive-stage small cell lung cancer.
Major finding: Median overall survival was 12.3 months with atezolizumab versus 10.3 months with placebo (HR, 0.7).
Study details: A global phase 1/3 study of 403 extensive-stage small cell lung cancer patients.
Disclosures: IMpower 133 was sponsored by F. Hoffman–La Roche. Dr. Liu is a speaker or advisory board member for Genentech, Pfizer, Takeda, Celgene, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb, AstraZeneca, and Ignyta, and has received research or grant support from Genentech, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer, OncoMed Pharmaceuticals, Ignyta, Merck, Lycera, AstraZeneca, and Molecular Partners.
Source: Liu SV et al. WCLC 2018, Abstract PL02.07.
The devil is in the headlines
“Breast Milk From Bottle May Not Be As Beneficial As Feeding Directly From The Breast, Researchers Say.” This was the headline on the AAP Daily Briefing that was sent to American Academy of Pediatrics members on Sept 25, 2018.
I suspect that this finding doesn’t surprise you. I can imagine a dozen factors that could make bottled breast milk less advantageous for a baby than milk received directly from the mother’s breast. Antibodies might adhere to the glass surface. A few degrees above or below body temperature could interfere with gastric emptying. Or a temptation to focus on the level in the bottle and inadvertently overfeed could inflate the baby’s body mass index at 3 months, as was found in the study published online in the September 2018 issue of Pediatrics (“Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food”).
I agree that the title of the actual paper is rather dry; it’s a scientific research paper. But the distillation chosen by the folks at AAP Daily Briefing seems ill advised. They were not alone. CCN-Health chose “Breastfeeding better for babies’ weight gain than pumping, new study says” (Michael Nedelman, Sept. 24, 2018). HealthDay News headlined its story with “Milk straight from breast best for baby’s weight” (Sept. 24, 2018).
The articles themselves were well balanced and accurately described this research based on more than 2,500 Canadian mother-infant dyads. But not everyone – including mothers who are struggling with or considering breastfeeding – reads beyond the headlines. How many realize that “better for babies’ weight gain” means a slower weight gain? For the mother who has found that, for a variety of reasons, pumping is the only way she can provide her baby the benefits of breast milk, what these headlines suggest is another blow to her already fragile sense of self-worth.
This research article is excellent and should be read by all of us who counsel young families. It suggests that one of the contributors to our epidemic of childhood obesity may be that bottle-feeding discourages the infant’s own self-regulation skills. It should prompt us to ask every parent who is bottle-feeding his or her baby – regardless of what is in the bottle – exactly how they decide how much to put in the bottle and how long a feeding takes. Even if we are comfortable with the infant’s weight gain, we should caution parents to be more aware of the baby’s cues that he or she has had enough. Not every baby provides cues that are obvious, and parents may need our coaching in deciding how much to feed. This research paper also suggests that as long as breastfeeding was continued, introduction of solids as early as 5 months was not associated with an unhealthy BMI trajectory.
Unfortunately, the reporting of this research article is another example of the hazards of the explosive growth of the Internet. There really is no reason to keep the results of well-crafted research from the lay public, particularly if they are explained in common sense language. However, this places a burden of responsibility on the editors of websites to consider the damage that can be done by a poorly chosen headline.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“Breast Milk From Bottle May Not Be As Beneficial As Feeding Directly From The Breast, Researchers Say.” This was the headline on the AAP Daily Briefing that was sent to American Academy of Pediatrics members on Sept 25, 2018.
I suspect that this finding doesn’t surprise you. I can imagine a dozen factors that could make bottled breast milk less advantageous for a baby than milk received directly from the mother’s breast. Antibodies might adhere to the glass surface. A few degrees above or below body temperature could interfere with gastric emptying. Or a temptation to focus on the level in the bottle and inadvertently overfeed could inflate the baby’s body mass index at 3 months, as was found in the study published online in the September 2018 issue of Pediatrics (“Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food”).
I agree that the title of the actual paper is rather dry; it’s a scientific research paper. But the distillation chosen by the folks at AAP Daily Briefing seems ill advised. They were not alone. CCN-Health chose “Breastfeeding better for babies’ weight gain than pumping, new study says” (Michael Nedelman, Sept. 24, 2018). HealthDay News headlined its story with “Milk straight from breast best for baby’s weight” (Sept. 24, 2018).
The articles themselves were well balanced and accurately described this research based on more than 2,500 Canadian mother-infant dyads. But not everyone – including mothers who are struggling with or considering breastfeeding – reads beyond the headlines. How many realize that “better for babies’ weight gain” means a slower weight gain? For the mother who has found that, for a variety of reasons, pumping is the only way she can provide her baby the benefits of breast milk, what these headlines suggest is another blow to her already fragile sense of self-worth.
This research article is excellent and should be read by all of us who counsel young families. It suggests that one of the contributors to our epidemic of childhood obesity may be that bottle-feeding discourages the infant’s own self-regulation skills. It should prompt us to ask every parent who is bottle-feeding his or her baby – regardless of what is in the bottle – exactly how they decide how much to put in the bottle and how long a feeding takes. Even if we are comfortable with the infant’s weight gain, we should caution parents to be more aware of the baby’s cues that he or she has had enough. Not every baby provides cues that are obvious, and parents may need our coaching in deciding how much to feed. This research paper also suggests that as long as breastfeeding was continued, introduction of solids as early as 5 months was not associated with an unhealthy BMI trajectory.
Unfortunately, the reporting of this research article is another example of the hazards of the explosive growth of the Internet. There really is no reason to keep the results of well-crafted research from the lay public, particularly if they are explained in common sense language. However, this places a burden of responsibility on the editors of websites to consider the damage that can be done by a poorly chosen headline.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
“Breast Milk From Bottle May Not Be As Beneficial As Feeding Directly From The Breast, Researchers Say.” This was the headline on the AAP Daily Briefing that was sent to American Academy of Pediatrics members on Sept 25, 2018.
I suspect that this finding doesn’t surprise you. I can imagine a dozen factors that could make bottled breast milk less advantageous for a baby than milk received directly from the mother’s breast. Antibodies might adhere to the glass surface. A few degrees above or below body temperature could interfere with gastric emptying. Or a temptation to focus on the level in the bottle and inadvertently overfeed could inflate the baby’s body mass index at 3 months, as was found in the study published online in the September 2018 issue of Pediatrics (“Infant Feeding and Weight Gain: Separating Breast Milk From Breastfeeding and Formula From Food”).
I agree that the title of the actual paper is rather dry; it’s a scientific research paper. But the distillation chosen by the folks at AAP Daily Briefing seems ill advised. They were not alone. CCN-Health chose “Breastfeeding better for babies’ weight gain than pumping, new study says” (Michael Nedelman, Sept. 24, 2018). HealthDay News headlined its story with “Milk straight from breast best for baby’s weight” (Sept. 24, 2018).
The articles themselves were well balanced and accurately described this research based on more than 2,500 Canadian mother-infant dyads. But not everyone – including mothers who are struggling with or considering breastfeeding – reads beyond the headlines. How many realize that “better for babies’ weight gain” means a slower weight gain? For the mother who has found that, for a variety of reasons, pumping is the only way she can provide her baby the benefits of breast milk, what these headlines suggest is another blow to her already fragile sense of self-worth.
This research article is excellent and should be read by all of us who counsel young families. It suggests that one of the contributors to our epidemic of childhood obesity may be that bottle-feeding discourages the infant’s own self-regulation skills. It should prompt us to ask every parent who is bottle-feeding his or her baby – regardless of what is in the bottle – exactly how they decide how much to put in the bottle and how long a feeding takes. Even if we are comfortable with the infant’s weight gain, we should caution parents to be more aware of the baby’s cues that he or she has had enough. Not every baby provides cues that are obvious, and parents may need our coaching in deciding how much to feed. This research paper also suggests that as long as breastfeeding was continued, introduction of solids as early as 5 months was not associated with an unhealthy BMI trajectory.
Unfortunately, the reporting of this research article is another example of the hazards of the explosive growth of the Internet. There really is no reason to keep the results of well-crafted research from the lay public, particularly if they are explained in common sense language. However, this places a burden of responsibility on the editors of websites to consider the damage that can be done by a poorly chosen headline.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
2018 Update on pelvic floor dysfunction
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).
Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).

Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Using cystoscopy to evaluate ureteral efflux and bladder integrity following benign gynecologic surgery increases the detection rate of urinary tract injuries.1 Currently, it is standard of care to perform a cystoscopy following anti-incontinence procedures, but there is no consensus among ObGyns regarding the use of universal cystoscopy following benign gynecologic surgery.2 A number of studies, however, have suggested potential best practices for evaluating urinary tract injury during pelvic surgery for benign gynecologic conditions.
Pelvic surgeries for benign gynecologic conditions, including fibroids, menorrhagia, and pelvic organ prolapse (POP), are common. More than 500,000 hysterectomies are performed annually in the United States, and up to 11% of women will undergo at least one surgery for POP or urinary incontinence in their lifetime.3,4 During gynecologic surgery, the urinary tract is at risk, and the injury rate ranges from 0.02% to 2% for ureteral injury and from 1% to 5% for bladder injury.5,6
In a recent large randomized controlled trial, the rate of intraoperative ureteral obstruction following uterosacral ligament suspension (USLS) was 3.2%.7 Vaginal vault suspensions, as well as other vaginal cuff closure techniques, are common procedures associated with urinary tract injury.8 Additionally, ureteral injury during surgery for POP occurs in as many as 2% of anterior vaginal wall repairs.9
It is well documented that a delay in diagnosis of ureteral and/or bladder injuries is associated with increased morbidity, including the need for additional surgery to repair the injury; in addition, significant delay in identifying an injury may lead to subsequent sequela, such as renal injury and fistula formation.8
A large study in California found that 36.5% of hysterectomies performed for POP were performed by general gynecologists.10 General ObGyns performing these surgeries therefore must understand the risk of urinary tract injury during hysterectomy and reconstructive pelvic procedures so that they can appropriately identify, evaluate, and repair injuries in a timely fashion.
The best way to identify urinary tract injury at the time of gynecologic surgery is by cystoscopy, including a bladder survey and ureteral efflux evaluation. When should a cystoscopy be performed, and what is the best method for visualizing ureteral efflux? Can instituting universal cystoscopy for all gynecologic procedures make a difference in the rate of injury detection? In this Update, we summarize the data from 4 studies that help to answer these questions.
Continue to: About 30% of urinary tract injuries...
About 30% of urinary tract injuries identified prior to cystoscopy at hysterectomy (which detected 5 of 6 injuries)
Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192(5):1599–1604.
Vakili and colleagues conducted a multicenter prospective cohort study of women undergoing hysterectomy for benign indications; cystoscopy was performed in all cases. The 3 hospitals involved were all part of the Louisiana State University Health system. The investigators’ goal was to determine the rate of urinary tract injury in this patient population at the time of intraoperative cystoscopy.
Intraoperative cystoscopy beats visual evaluation
Four hundred and seventy-one women underwent hysterectomy and had intraoperative cystoscopy, including evaluation of ureteral patency with administration of intravenous (IV) indigo carmine. Patients underwent abdominal, vaginal, or laparoscopic hysterectomy, and 54 (11.4%) had concurrent POP or anti-incontinence procedures. The majority underwent an abdominal hysterectomy (59%), 31% had a vaginal hysterectomy, and 10% had a laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy.
Rate of urinary tract injuries. The total urinary tract injury rate detected by cystoscopy was 4.8%. The ureteral injury rate was 1.7%, and the bladder injury rate was 3.6%. A combined ureteral and bladder injury occurred in 2 women.
Surgery for POP significantly increased the risk of ureteral injury (7.3% vs 1.2%; P = .025). All cases of ureteral injury during POP surgery occurred during USLS. There was a trend toward a higher rate of bladder injury in the group with concurrent anti-incontinence surgery (12.5% vs 3.1%; P = .049). Regarding the route of hysterectomy, the vaginal approach had the highest rate of ureteral injury; however, when prolapse cases were removed from the analysis, there were no differences between the abdominal, vaginal, and laparoscopic approaches for ureteral or bladder injuries.
Injury detection with cystoscopy. Importantly, the authors found that only 30% of injuries were identified prior to performing intraoperative cystoscopy. The majority of these were bladder injuries. In addition, despite visual confirmation of ureteral peristalsis during abdominal hysterectomy, when intraoperative cystoscopy was performed with evaluation for ureteral efflux, 5 of 6 ureteral injury cases were identified. The authors reported 1 postoperative vesicovaginal fistula and concluded that it was likely due to an unrecognized bladder injury. No other undetected injuries were identified.
Notably, no complications occurred as a result of cystoscopy.
Multiple surgical indications reflect real-world scenario
The study included physicians from 3 different hospitals and all routes of hysterectomy for multiple benign gynecologic indications as well as concomitant pelvic reconstructive procedures. While this enhances the generalizability of the study results, all study sites were located in Louisiana at hospitals with resident trainee involvement. Additionally, this study confirms previous retrospective studies that reported higher rates of injury with pelvic reconstructive procedures.
The study is limited by the inability to blind surgeons, which may have resulted in the surgeons altering their techniques and/or having a heightened awareness of the urinary tract. However, their rates of ureteral and bladder injuries were slightly higher than previously reported rates, suggesting that the procedures inherently carry risk. The study is further limited by the lack of a retrospective comparison group of hysterectomy without routine cystoscopy and a longer follow-up period that may have revealed additional missed delayed urologic injuries.
The rate of urinary tract injury, including both bladder and ureteral injuries, was more than 4% at the time of hysterectomy for benign conditions. Using intraoperative peristalsis or normal ureteral caliber could result in a false sense of security since these are not reliable signs of ureteral integrity. The majority of urinary tract injuries will not be identified without cystoscopic evaluation.
Continue to: Universal cystoscopy policy...
Universal cystoscopy policy proves protective, surgeon adherence is high
Chi AM, Curran DS, Morgan DM, Fenner DE, Swenson CW. Universal cystoscopy after benign hysterectomy: examining the effects of an institutional policy. Obstet Gynecol. 2016;127(2):369–375.
In a retrospective cohort study, Chi and colleagues evaluated urinary tract injuries at the time of hysterectomy before and after the institution of a universal cystoscopy policy. At the time of policy implementation at the University of Michigan, all faculty who performed hysterectomies attended a cystoscopy workshop. Attending physicians without prior cystoscopy training also were proctored in the operating room for 3 sessions and were required to demonstrate competency with bladder survey, visualizing ureteral efflux, and urethral assessment. Indigo carmine was used to visualize ureteral efflux.
Detection of urologic injury almost doubled with cystoscopy
A total of 2,822 hysterectomies were included in the study, with 973 in the pre–universal cystoscopy group and 1,849 in the post–universal cystoscopy group. The study period was 7 years, and data on complications were abstracted for 1 year after the completion of the study period.
The primary outcome had 3 components:
- the rate of urologic injury before and after the policy
- the cystoscopy detection rate of urologic injury
- the adherence rate to the policy.
The overall rate of bladder and ureteral injury was 2.1%; the rate of injury during pre–universal screening was 2.6%, and during post–universal screening was 1.8%. The intraoperative detection rate of injury nearly doubled, from 24% to 47%, when intraoperative cystoscopy was utilized. In addition, the percentage of delayed urologic complications decreased from 28% to 5.9% (P = .03) following implementation of the universal cystoscopy policy. With regard to surgeon adherence, cystoscopy was documented in 86.1% of the hysterectomy cases after the policy was implemented compared with 35.7% of cases before the policy.
The investigators performed a cost analysis and found that hospital costs were nearly twice as much if a delayed urologic injury was diagnosed.
Study had many strengths
This study evaluated aspects of implementing quality initiatives after proper training and proctoring of a procedure. The authors compared very large cohorts from a busy academic medical center in which surgeon adherence with routine cystoscopy was high. The majority of patient outcomes were tracked for an extended period following surgery, thereby minimizing the risk of missing delayed urologic injuries. Notably, however, there was shorter follow-up time for the post–universal cystoscopy group, which could result in underestimating the rate of delayed urologic injuries in this cohort.
Instituting a universal cystoscopy policy for hysterectomy was associated with a significant decrease in delayed postoperative urinary tract complications and an increase in the intraoperative detection rate of urologic injuries. Intraoperative detection and repair of a urinary tract injury is cost-effective compared with a delayed diagnosis.
Continue to: Cystoscopy reveals ureteral obstruction...
Cystoscopy reveals ureteral obstruction during various vaginal POP repair procedures
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD. The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194(5):1478–1485.
To determine the rate of ureteral obstruction and ureteral injury during vaginal surgery for POP and the accuracy of using intraoperative cystoscopy to prevent upper urinary tract morbidity, Gustilo-Ashby and colleagues performed a retrospective review study of a large patient cohort.
Cystoscopy with indigo carmine is highly sensitive
The study included 700 patients who underwent vaginal surgery for anterior and/or apical POP. Patients had 1 or more of the following anterior and apical prolapse repair procedures: USLS (51%), distal McCall culdeplasty (26%), proximal McCall culdeplasty (29%), anterior colporrhaphy (82%), and colpocleisis (1.4%). Of note, distal McCall culdeplasty was defined as incorporation of the “vaginal epithelium into the uterosacral plication,” while proximal McCall culdeplasty involved plication of “the uterosacral ligaments in the midline proximal to the vaginal cuff.” All patients were given IV indigo carmine to aid in visualizing ureteral efflux.
The majority of patients had a hysterectomy (56%). When accounting for rare false-positive and negative cystoscopy results, the overall ureteral obstruction rate was 5.1% and the ureteral injury rate was 0.9%. The majority of obstructions occurred with USLS (5.9%), proximal McCall culdeplasty (4.4%), and colpocleisis (4.2%). Ureteral injuries occurred only in 6 cases: 3 USLS and 3 proximal McCall culdeplasty procedures.
Based on these findings, the authors calculated that cystoscopy at the time of vaginal surgery for anterior and/or apical prolapse has a sensitivity of 94.4% and a specificity of 99.5% for detecting ureteral obstruction. The positive predictive value of cystoscopy with the use of indigo carmine for detection of ureteral obstruction is 91.9% and the negative predictive value is 99.7%.
Impact of indigo carmine’s unavailability
This study’s strengths include its large sample size and the variety of surgical approaches used for repair of anterior vaginal wall and apical prolapse. Its retrospective design, however, is a limitation; this could result in underreporting of ureteral injuries if patients received care at another institution after surgery. Furthermore, it is unclear if cystoscopy would be as predictive of ureteral injury without the use of indigo carmine, which is no longer available at most institutions.
The utility of cystoscopy with IV indigo carmine as a screening test for ureteral obstruction is highlighted by the fact that most obstructions were relieved by intraoperative suture removal following positive cystoscopy. McCall culdeplasty procedures are commonly performed by general ObGyns at the time of vaginal hysterectomy. It is therefore important to note that rates of ureteral obstruction after proximal McCall culdeplasty were only slightly lower than those after USLS.
Continue to: Sodium fluorescein and 10% dextrose...
Sodium fluorescein and 10% dextrose provide clear visibility of ureteral jets in cystoscopy
Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
In a multicenter randomized controlled trial, Espaillat-Rijo and colleagues compared various methods for visualizing ureteral efflux in participants who underwent gynecologic or urogynecologic procedures in which cystoscopy was performed.
Study compared 4 media
The investigators enrolled 176 participants (174 completed the trial) and randomly assigned them to receive 1 of 4 modalities: 1) normal saline as a bladder distention medium (control), 2) 10% dextrose as a bladder distention medium, 3) 200 mg oral phenazopyridine given 30 minutes prior to cystoscopy, or 4) 50 mg IV sodium fluorescein at the start of cystoscopy. Indigo carmine was not included in this study because it has not been routinely available since 2014.
Surgeons were asked to categorize the ureteral jets as “clearly visible,” “somewhat visible,” or “not visible.”
The primary outcome was subjective visibility of the ureteral jet with each modality during cystoscopy. Secondary outcomes included surgeon satisfaction, adverse reactions to the modality used, postoperative urinary tract infection, postoperative urinary retention, and delayed diagnosis of ureteral injury.
Visibility assessment results. Overall, ureteral jets were “clearly visible” in 125 cases (71%) compared with “somewhat visible” in 45 (25.6%) and “not visible” in 4 (2.3%) cases. There was a statistically significant difference between the 4 groups. Use of sodium fluorescein and 10% dextrose resulted in significantly better visualization of ureteral jets (P < .001 and P = .004, respectively) compared with the control group. Visibility with phenazopyridine was not significantly different from that in the control group or in the 10% dextrose group (FIGURE).

Surgeon satisfaction was highest with 10% dextrose and sodium fluorescein. In 6 cases, the surgeon was not satisfied with visualization of the ureteral jets and relied on fluorescein (5 times) or 10% dextrose (1 time) to ultimately see efflux. No significant adverse events occurred; the rate of urinary tract infection was 24.1% and did not differ between groups.
Results are widely generalizable
This was a well-designed randomized multicenter trial that included both benign gynecologic and urogynecologic procedures, thus strengthening the generalizability of the study. The study was timely since proven methods for visualization of ureteral patency became limited with the withdrawal of commercially available indigo carmine, the previous gold standard.
Intravenous sodium fluorescein and 10% dextrose as bladder distention media can both safely be used to visualize ureteral efflux and result in high surgeon satisfaction. Although 10% dextrose has been associated with higher rates of postoperative urinary tract infection,11 this was not found to be the case in this study. Preoperative administration of oral phenazopyridine was no different from the control modality with regard to visibility and surgeon satisfaction.
Continue to: The cost-effectiveness consideration
The cost-effectiveness consideration
The debate around universal cystoscopy following benign gynecologic surgery is ongoing.
The studies discussed in this Update demonstrate that cystoscopy following hysterectomy for benign indications:
- is superior to visualizing ureteral peristalsis
- increases detection of urinary tract injuries, and
- decreases delayed urologic injuries.
Although these articles emphasize the importance of detecting urologic injury, the picture would not be complete without mention of cost-effectiveness. Only one study, from 2001, has evaluated the cost-effectiveness of universal cystoscopy.1 Those authors concluded that universal cystoscopy is cost-effective only when the rate of urologic injury is 1.5% to 2%, but this conclusion, admittedly, was limited by the lack of data on medicolegal settlements, outpatient expenses, and nonmedical-related economic loss from decreased productivity. Given the extensive changes that have occurred in medical practice over the last 17 years and the emphasis on quality metrics and safety, an updated analysis would be needed to make definitive conclusions about cost-effectiveness.
While this Update cannot settle the ongoing debate of universal cystoscopy in gynecology, it is important to remember that the American College of Obstetricians and Gynecologists and the American Urogynecologic Society recommend cystoscopy following all surgeries for pelvic organ prolapse and stress urinary incontinence.2
References
- Visco AG, Taber KH, Weidner AD, Barber MD, Myers ER. Cost-effectiveness of universal cystoscopy to identify ureteral injury at hysterectomy. Obstet Gynecol. 2001;97(5 pt 1):685–692.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
- Ibeanu OA, Chesson RR, Echols KT, Nieves M, Busangu F, Nolan TE. Urinary tract injury during hysterectomy based on universal cystoscopy. Obstet Gynecol. 2009;113(1):6–10.
- ACOG Committee on Practice Bulletins–Gynecology and the American Urogynecologic Society. Urinary incontinence in women. Female Pelvic Med Reconstr Surg. 2015;21(6):304–314.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83(4):549–555.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506.
- Mäkinen J, Johansson J, Tomás C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
- Gilmour DT, Dwyer PL, Carey MP. Lower urinary tract injury during gynecologic surgery and its detection by intraoperative cystoscopy. Obstet Gynecol. 1999;94(5 pt 2):883–889.
- Barber MD, Brubaker L, Burgio KL, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial. JAMA. 2014;311(10):1023–1034.
- Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU Int. 2004;94(3):277–289.
- Kwon CH, Goldberg RP, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187(6):1466–1471.
- Adams-Piper ER, Guaderrama NM, Chen Q, Whitcomb EL. Impact of surgical training on the performance of proposed quality measures for hysterectomy for pelvic organ prolapse. Am J Obstet Gynecol. 2017;216(6):588.e1–588.e5.
- Siff LN, Unger CA, Jelovsek JE, Paraiso MF, Ridgeway BM Barber MD. Assessing ureteral patency using 10% dextrose cystoscopy fluid: evaluation of urinary tract infection rates. Am J Obstet Gynecol. 2016;215(1):74.e1–74.e6.
- Espaillat-Rijo L, Siff L, Alas AN, et al. Intraoperative cystoscopic evaluation of ureteral patency: a randomized controlled trial. Obstet Gynecol. 2016;128(6):1378–1383.
The Vampire Study, pathogenic puppies, and carbonated cannabis
What the duck?
Turns out it’s flu shot season for our feathered friends, too. After the bird flu pandemics that began in 2013, Chinese health officials worked hard to vaccinate their chickens and quell the spread of the disease. However, the ducks have outsmarted them: Two new genetic variations of the H7N9 and H7N2 flu subtypes have been found in unvaccinated ducks.
China consumes about 3 billion ducks per year, so officials are working rapidly to eliminate the virus. At press time, there was no word on whether the virus had affected any beloved rubber duckies, but we advise you to use caution when approaching bath time.
Toke-a-Cola
Cannabis is the world’s favorite illicit drug. And Coca-Cola makes the world’s favorite cola. Now there’s news of potential nuptials uniting these two global giants. BNN Bloomberg reports that Coke is talking tie-up with Canada’s Aurora Cannabis in a union that could give birth to cannabis-infused “wellness beverages.”
The fizzy federation would feature products containing cannabidiol, or CBD. Unlike its wilder psychoactive sibling tetrahydrocannabinol, or THC, the more sober-minded CBD is a nonpsychoactive cannabis compound credited with antidepressant, anxiolytic, and anti-inflammatory powers.
And what of Coca-Cola’s mortal cola enemy, Pepsi? Will the Choice of a New Generation let the Real Thing Bogart all the possible market opportunities? Sure, you could soon have a CBD Coke and a smile. But smiles are free. Frito-Lay’s parent, PepsiCo, could offer consumers a doubly profitable, Jeff Spicoli–approved pairing: carbonated cannabinoids and Cheetos.
I vant to suck MY blood
It’s not Halloween quite yet, but get into the spirit with the recently published and aptly named “Vampire Study.” Performed in Zürich, a team of researchers from the division of gastroenterology at Triemli Hospital convinced participants to ingest their own blood, all in the name of science.
Some lucky vamps drank their blood, while others ingested it via nasogastric tube. This isn’t “Saw 17,” though; there was a method to this madness. Researchers were investigating whether the ingestion of blood (as in gastrointestinal bleeding) can result in an increase in fecal calprotectin. Safe to say, though, the ingestion of blood is rarely a good sign – unless your name happens to be Nosferatu.
And they call it Campylobacter love
As any fan of Charles Schulz’s “Peanuts” comic strip can tell you, happiness is a warm puppy. Know what else warm puppies are? Carriers of Campylobacter jejuni.
Every year in the United States, Campylobacter causes an estimated 1.3 million diarrheal illnesses. A recent multistate investigation revealed that 118 people in 18 states across the nation – including 29 employees of an unnamed national pet store chain based in Ohio – got the puppy-borne bug between early 2016 and early 2018.
And the not-so-cuddly infections were resistant to all the antibiotics commonly used to quell Campylobacter. Why? Turns out, of the 149 puppies investigated by health officials, 55% of them got the drugs prophylactically – an approach that may have fueled the resistance. Stung by the canine controversy, the Daisy Hill Puppy Farm has assured Charlie Brown that it never slipped metronidazole into Snoopy’s water bowl.
What the duck?
Turns out it’s flu shot season for our feathered friends, too. After the bird flu pandemics that began in 2013, Chinese health officials worked hard to vaccinate their chickens and quell the spread of the disease. However, the ducks have outsmarted them: Two new genetic variations of the H7N9 and H7N2 flu subtypes have been found in unvaccinated ducks.
China consumes about 3 billion ducks per year, so officials are working rapidly to eliminate the virus. At press time, there was no word on whether the virus had affected any beloved rubber duckies, but we advise you to use caution when approaching bath time.
Toke-a-Cola
Cannabis is the world’s favorite illicit drug. And Coca-Cola makes the world’s favorite cola. Now there’s news of potential nuptials uniting these two global giants. BNN Bloomberg reports that Coke is talking tie-up with Canada’s Aurora Cannabis in a union that could give birth to cannabis-infused “wellness beverages.”
The fizzy federation would feature products containing cannabidiol, or CBD. Unlike its wilder psychoactive sibling tetrahydrocannabinol, or THC, the more sober-minded CBD is a nonpsychoactive cannabis compound credited with antidepressant, anxiolytic, and anti-inflammatory powers.
And what of Coca-Cola’s mortal cola enemy, Pepsi? Will the Choice of a New Generation let the Real Thing Bogart all the possible market opportunities? Sure, you could soon have a CBD Coke and a smile. But smiles are free. Frito-Lay’s parent, PepsiCo, could offer consumers a doubly profitable, Jeff Spicoli–approved pairing: carbonated cannabinoids and Cheetos.
I vant to suck MY blood
It’s not Halloween quite yet, but get into the spirit with the recently published and aptly named “Vampire Study.” Performed in Zürich, a team of researchers from the division of gastroenterology at Triemli Hospital convinced participants to ingest their own blood, all in the name of science.
Some lucky vamps drank their blood, while others ingested it via nasogastric tube. This isn’t “Saw 17,” though; there was a method to this madness. Researchers were investigating whether the ingestion of blood (as in gastrointestinal bleeding) can result in an increase in fecal calprotectin. Safe to say, though, the ingestion of blood is rarely a good sign – unless your name happens to be Nosferatu.
And they call it Campylobacter love
As any fan of Charles Schulz’s “Peanuts” comic strip can tell you, happiness is a warm puppy. Know what else warm puppies are? Carriers of Campylobacter jejuni.
Every year in the United States, Campylobacter causes an estimated 1.3 million diarrheal illnesses. A recent multistate investigation revealed that 118 people in 18 states across the nation – including 29 employees of an unnamed national pet store chain based in Ohio – got the puppy-borne bug between early 2016 and early 2018.
And the not-so-cuddly infections were resistant to all the antibiotics commonly used to quell Campylobacter. Why? Turns out, of the 149 puppies investigated by health officials, 55% of them got the drugs prophylactically – an approach that may have fueled the resistance. Stung by the canine controversy, the Daisy Hill Puppy Farm has assured Charlie Brown that it never slipped metronidazole into Snoopy’s water bowl.
What the duck?
Turns out it’s flu shot season for our feathered friends, too. After the bird flu pandemics that began in 2013, Chinese health officials worked hard to vaccinate their chickens and quell the spread of the disease. However, the ducks have outsmarted them: Two new genetic variations of the H7N9 and H7N2 flu subtypes have been found in unvaccinated ducks.
China consumes about 3 billion ducks per year, so officials are working rapidly to eliminate the virus. At press time, there was no word on whether the virus had affected any beloved rubber duckies, but we advise you to use caution when approaching bath time.
Toke-a-Cola
Cannabis is the world’s favorite illicit drug. And Coca-Cola makes the world’s favorite cola. Now there’s news of potential nuptials uniting these two global giants. BNN Bloomberg reports that Coke is talking tie-up with Canada’s Aurora Cannabis in a union that could give birth to cannabis-infused “wellness beverages.”
The fizzy federation would feature products containing cannabidiol, or CBD. Unlike its wilder psychoactive sibling tetrahydrocannabinol, or THC, the more sober-minded CBD is a nonpsychoactive cannabis compound credited with antidepressant, anxiolytic, and anti-inflammatory powers.
And what of Coca-Cola’s mortal cola enemy, Pepsi? Will the Choice of a New Generation let the Real Thing Bogart all the possible market opportunities? Sure, you could soon have a CBD Coke and a smile. But smiles are free. Frito-Lay’s parent, PepsiCo, could offer consumers a doubly profitable, Jeff Spicoli–approved pairing: carbonated cannabinoids and Cheetos.
I vant to suck MY blood
It’s not Halloween quite yet, but get into the spirit with the recently published and aptly named “Vampire Study.” Performed in Zürich, a team of researchers from the division of gastroenterology at Triemli Hospital convinced participants to ingest their own blood, all in the name of science.
Some lucky vamps drank their blood, while others ingested it via nasogastric tube. This isn’t “Saw 17,” though; there was a method to this madness. Researchers were investigating whether the ingestion of blood (as in gastrointestinal bleeding) can result in an increase in fecal calprotectin. Safe to say, though, the ingestion of blood is rarely a good sign – unless your name happens to be Nosferatu.
And they call it Campylobacter love
As any fan of Charles Schulz’s “Peanuts” comic strip can tell you, happiness is a warm puppy. Know what else warm puppies are? Carriers of Campylobacter jejuni.
Every year in the United States, Campylobacter causes an estimated 1.3 million diarrheal illnesses. A recent multistate investigation revealed that 118 people in 18 states across the nation – including 29 employees of an unnamed national pet store chain based in Ohio – got the puppy-borne bug between early 2016 and early 2018.
And the not-so-cuddly infections were resistant to all the antibiotics commonly used to quell Campylobacter. Why? Turns out, of the 149 puppies investigated by health officials, 55% of them got the drugs prophylactically – an approach that may have fueled the resistance. Stung by the canine controversy, the Daisy Hill Puppy Farm has assured Charlie Brown that it never slipped metronidazole into Snoopy’s water bowl.
FDA approves Seysara for treatment of moderate to severe acne
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
vulgaris for people aged 9 years and older, according to a press release from Paratek, the drug’s manufacturer.
FDA approval is based on results from two phase 3 clinical trials (NCT02320149 and NCT02322866), in which patients received either sarecycline at 1.5 mg/kg per day or placebo for 12 weeks. Patients who received sarecycline were significantly more likely to reach the primary endpoint of improved acne severity based on absolute change in facial lesion counts and percentage of participants with Investigator Global Assessment success.
Common adverse events reported in the sarecycline group were nausea (3.2%), nasopharyngitis (2.8%), and headache (2.8%). The discontinuation rate due to adverse events among sarecycline-treated patients in both studies combined was 1.4%.
Sarecycline is an oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties, and is approved for once-daily use, according to Paratek. Seysara will be marketed in the United States by Almirall SA.
Checkpoint inhibitor linked to antiphospholipid syndrome in melanoma patient
A patient with melanoma experienced antiphospholipid syndrome following multiple infusions of the PD-1 inhibitor pembrolizumab, according to authors of a recent case report.
Presence of Raynaud phenomenon and high levels of antiphospholipid antibodies led to the diagnosis of antiphospholipid syndrome in the patient, who had stage IIIB unresectable melanoma.
This report provides additional evidence that this syndrome is an immune-related adverse event associated with checkpoint inhibitor therapy, said Alexandra Picard, MD, of Hôpital Archet, Nice, France, and coauthors.
“Due to the increased use of anti PD-1 therapies, clinicians should be aware of this new potential immune-mediated toxic effect that manifests as antiphospholipid syndrome,” the researchers wrote. The report is in JAMA Dermatology.
“Great caution” should be exercised when considering use of immune checkpoint inhibitors in patients with a history of antiphospholipid syndrome, the authors added.
The woman in this report was over 60 years of age and had first presented with superficial melanoma on her right calf, followed by recurrent lymph node metastases over the next few years, all of which were surgically treated.
Following a PET-CT scan showing a new metastatic lymph node, the woman started pembrolizumab 2 mg/kg every 3 weeks and had a partial response within 3 months, the investigators reported.
After the tenth infusion, however, the patient developed bilateral secondary Raynaud phenomenon that followed a typical discoloration sequence and resulted in a necrotic lesion at the tip of one finger.
The patient had no personal or family history of Raynaud phenomenon.
While beta2-glycoprotein 1 antibodies were not elevated, laboratory tests did show anticardiolipin antibodies and lupus anticoagulants at elevated levels, the investigators said, noting that repeat testing at 12 weeks confirmed positivity of antiphospholipid antibodies.
The Raynaud phenomenon disappeared and the necrotic lesion healed after pembrolizumab was stopped and prednisolone treatment was started, they added.
No recurrence of either was noted at the last follow-up.
Previous reports have described antiphospholipid syndrome in advanced melanoma patients treated with alfa-2b interferon alone or in combination with anti-interleukin 2, the authors said in their discussion of the case.
In addition, there has been another recent report of antiphospholipid syndrome associated with the CTLA4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab, they said. In that case, testing for antiphospholipid antibodies revealed elevated beta2-glycoprotein 1 antibody levels.
“We hypothesize that [antiphospholipid syndrome] is a kind of autoimmunity induced by anti–PD-1 due to the expansive expression of the immune system against tumor cells,” the researchers wrote.
Although a case of cancer-associated antiphospholipid syndrome could not be ruled out in the present report, the rapid and complete resolution of symptoms after treatment discontinuation suggested that pembrolizumab, a “known immunostimulant,” was the cause, they said.
While antibodies against PD-1 have improved melanoma prognosis, they are associated with a wide range of immune-related adverse effects in the skin, gastrointestinal tract, liver, and endocrine system, Dr. Picard and coauthors noted.
They reported having no conflicts of interest.
SOURCE: Sanchez A, et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
A patient with melanoma experienced antiphospholipid syndrome following multiple infusions of the PD-1 inhibitor pembrolizumab, according to authors of a recent case report.
Presence of Raynaud phenomenon and high levels of antiphospholipid antibodies led to the diagnosis of antiphospholipid syndrome in the patient, who had stage IIIB unresectable melanoma.
This report provides additional evidence that this syndrome is an immune-related adverse event associated with checkpoint inhibitor therapy, said Alexandra Picard, MD, of Hôpital Archet, Nice, France, and coauthors.
“Due to the increased use of anti PD-1 therapies, clinicians should be aware of this new potential immune-mediated toxic effect that manifests as antiphospholipid syndrome,” the researchers wrote. The report is in JAMA Dermatology.
“Great caution” should be exercised when considering use of immune checkpoint inhibitors in patients with a history of antiphospholipid syndrome, the authors added.
The woman in this report was over 60 years of age and had first presented with superficial melanoma on her right calf, followed by recurrent lymph node metastases over the next few years, all of which were surgically treated.
Following a PET-CT scan showing a new metastatic lymph node, the woman started pembrolizumab 2 mg/kg every 3 weeks and had a partial response within 3 months, the investigators reported.
After the tenth infusion, however, the patient developed bilateral secondary Raynaud phenomenon that followed a typical discoloration sequence and resulted in a necrotic lesion at the tip of one finger.
The patient had no personal or family history of Raynaud phenomenon.
While beta2-glycoprotein 1 antibodies were not elevated, laboratory tests did show anticardiolipin antibodies and lupus anticoagulants at elevated levels, the investigators said, noting that repeat testing at 12 weeks confirmed positivity of antiphospholipid antibodies.
The Raynaud phenomenon disappeared and the necrotic lesion healed after pembrolizumab was stopped and prednisolone treatment was started, they added.
No recurrence of either was noted at the last follow-up.
Previous reports have described antiphospholipid syndrome in advanced melanoma patients treated with alfa-2b interferon alone or in combination with anti-interleukin 2, the authors said in their discussion of the case.
In addition, there has been another recent report of antiphospholipid syndrome associated with the CTLA4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab, they said. In that case, testing for antiphospholipid antibodies revealed elevated beta2-glycoprotein 1 antibody levels.
“We hypothesize that [antiphospholipid syndrome] is a kind of autoimmunity induced by anti–PD-1 due to the expansive expression of the immune system against tumor cells,” the researchers wrote.
Although a case of cancer-associated antiphospholipid syndrome could not be ruled out in the present report, the rapid and complete resolution of symptoms after treatment discontinuation suggested that pembrolizumab, a “known immunostimulant,” was the cause, they said.
While antibodies against PD-1 have improved melanoma prognosis, they are associated with a wide range of immune-related adverse effects in the skin, gastrointestinal tract, liver, and endocrine system, Dr. Picard and coauthors noted.
They reported having no conflicts of interest.
SOURCE: Sanchez A, et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
A patient with melanoma experienced antiphospholipid syndrome following multiple infusions of the PD-1 inhibitor pembrolizumab, according to authors of a recent case report.
Presence of Raynaud phenomenon and high levels of antiphospholipid antibodies led to the diagnosis of antiphospholipid syndrome in the patient, who had stage IIIB unresectable melanoma.
This report provides additional evidence that this syndrome is an immune-related adverse event associated with checkpoint inhibitor therapy, said Alexandra Picard, MD, of Hôpital Archet, Nice, France, and coauthors.
“Due to the increased use of anti PD-1 therapies, clinicians should be aware of this new potential immune-mediated toxic effect that manifests as antiphospholipid syndrome,” the researchers wrote. The report is in JAMA Dermatology.
“Great caution” should be exercised when considering use of immune checkpoint inhibitors in patients with a history of antiphospholipid syndrome, the authors added.
The woman in this report was over 60 years of age and had first presented with superficial melanoma on her right calf, followed by recurrent lymph node metastases over the next few years, all of which were surgically treated.
Following a PET-CT scan showing a new metastatic lymph node, the woman started pembrolizumab 2 mg/kg every 3 weeks and had a partial response within 3 months, the investigators reported.
After the tenth infusion, however, the patient developed bilateral secondary Raynaud phenomenon that followed a typical discoloration sequence and resulted in a necrotic lesion at the tip of one finger.
The patient had no personal or family history of Raynaud phenomenon.
While beta2-glycoprotein 1 antibodies were not elevated, laboratory tests did show anticardiolipin antibodies and lupus anticoagulants at elevated levels, the investigators said, noting that repeat testing at 12 weeks confirmed positivity of antiphospholipid antibodies.
The Raynaud phenomenon disappeared and the necrotic lesion healed after pembrolizumab was stopped and prednisolone treatment was started, they added.
No recurrence of either was noted at the last follow-up.
Previous reports have described antiphospholipid syndrome in advanced melanoma patients treated with alfa-2b interferon alone or in combination with anti-interleukin 2, the authors said in their discussion of the case.
In addition, there has been another recent report of antiphospholipid syndrome associated with the CTLA4 inhibitor ipilimumab and the PD-1 inhibitor nivolumab, they said. In that case, testing for antiphospholipid antibodies revealed elevated beta2-glycoprotein 1 antibody levels.
“We hypothesize that [antiphospholipid syndrome] is a kind of autoimmunity induced by anti–PD-1 due to the expansive expression of the immune system against tumor cells,” the researchers wrote.
Although a case of cancer-associated antiphospholipid syndrome could not be ruled out in the present report, the rapid and complete resolution of symptoms after treatment discontinuation suggested that pembrolizumab, a “known immunostimulant,” was the cause, they said.
While antibodies against PD-1 have improved melanoma prognosis, they are associated with a wide range of immune-related adverse effects in the skin, gastrointestinal tract, liver, and endocrine system, Dr. Picard and coauthors noted.
They reported having no conflicts of interest.
SOURCE: Sanchez A, et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.
FROM JAMA DERMATOLOGY
Key clinical point: Antiphospholipid syndrome appears to be an immune-related adverse event associated with anti-PD-1 therapy.
Major finding: A melanoma patient receiving pembrolizumab was diagnosed with antiphospholipid syndrome that resolved following discontinuation of that treatment.
Study details: Case report of a woman in her 60s with stage IIIB unresectable melanoma who was treated with pembrolizumab 2 mg/kg every 3 weeks.
Disclosures: The authors reported no conflicts of interest.
Source: Sanchez A et al. JAMA Derm. 2018 Sep 19. doi: 10.1001/jamadermatol.2018.2770.