User login
ED visits related to psychiatric complaints are up 20% among elderly
SAN DIEGO – Between 2011 and 2015, the proportion of elderly patients presenting to the emergency department with psychiatric complaints increased by 20%, according to a retrospective analysis of national hospital data.
In addition, 10-year increases in age, male sex, nursing home status, and Medicare insurance were associated with an increased likelihood of hospital admission.
“The growing geriatric patient population is a well-known phenomenon across every developed country,” lead researcher Derrick Huang said in an interview in advance of the annual meeting of the American College of Emergency Physicians. “A potent mix of increasing life expectancy, greater disease severity and comorbidities in the elderly, and large-scale demographic shifts has placed a significant strain on both our financial and health care resources. This study corroborates these trends in the emergency department and is a preliminary exploration of potential, newly evolving clinical challenges that the ED team will increasingly face into the future.”
For the study, Mr. Huang, a fourth-year medical student at Oakland University William Beaumont School of Medicine in Rochester, Mich., and his colleagues examined National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2015. They limited the analysis to emergency department visits with patients in the age groups of 65-74, 75-84, and 85 or older. For the primary outcome of interest, the researchers evaluated demographic variables of age group, sex, residential status, race and ethnicity, and insurance for association with hospital admission. For the secondary outcome of interest, they evaluated presenting ED complaints related to the clinical domains of cardiopulmonary disease, psychiatric disease, and fractures and dislocations for potential trends in the ED geriatric age group between 2011 and 2015.
Mr. Huang and his associates found that, as a percentage of total ED visits, those among patients aged 65 or older rose from 14.9% in 2011 to 15.6% in 2015, an increase of 4.7%. By age group, the proportion of visits during the study period was highest for those aged 65-74 years (43.8%), followed by those aged 75-84 years (34.7%) and those aged 85 and older (21.5%). On multivariate analysis, the 75-84 and age-85-and-older groups were 1.30 and 1.71 times more likely to be admitted to the hospital, compared with the 65-74 group, respectively (P less than .000 for both).
Men were 1.19 times more likely than were women to be admitted (P less than .000). In addition, elderly patients who reside in nursing homes were 1.70 times more likely to be admitted to the hospital, compared with those who lived in private homes (P less than .000), while those with Medicare insurance were 1.57 more likely to be admitted, compared with those who did not have insurance (P = .004).
On trend analysis, ED psychiatric complaints rose incrementally during the study period, from 3.9% in 2011 to 4.7% in 2015, a relative increase of 20.5%. The researchers identified no consistent trend with visit complaints related to cardiopulmonary disease, and fractures and dislocations.
“This was not too surprising, because these difficulties with older patients are not new and many investigators have sought out solutions,” Mr. Huang said. “For example, there have been many interventions both in the ED as well as in the primary care setting designed to identify risk factors and facilitate postdischarge care to prevent falls. These approaches are constantly evolving and will be of increasing importance.”
“For example, we may be seeing a larger of proportion of patients with acute mental health complaints,” Mr. Huang said. “We will need to continue developing our multidisciplinary approach to care by improving coordination with different specialties – and especially outpatient and community health care providers.”
The study’s senior author was Jason Wasserman, PhD of Oakland University William Beaumont School of Medicine. The researchers reported having no financial disclosures.
SOURCE: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
SAN DIEGO – Between 2011 and 2015, the proportion of elderly patients presenting to the emergency department with psychiatric complaints increased by 20%, according to a retrospective analysis of national hospital data.
In addition, 10-year increases in age, male sex, nursing home status, and Medicare insurance were associated with an increased likelihood of hospital admission.
“The growing geriatric patient population is a well-known phenomenon across every developed country,” lead researcher Derrick Huang said in an interview in advance of the annual meeting of the American College of Emergency Physicians. “A potent mix of increasing life expectancy, greater disease severity and comorbidities in the elderly, and large-scale demographic shifts has placed a significant strain on both our financial and health care resources. This study corroborates these trends in the emergency department and is a preliminary exploration of potential, newly evolving clinical challenges that the ED team will increasingly face into the future.”
For the study, Mr. Huang, a fourth-year medical student at Oakland University William Beaumont School of Medicine in Rochester, Mich., and his colleagues examined National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2015. They limited the analysis to emergency department visits with patients in the age groups of 65-74, 75-84, and 85 or older. For the primary outcome of interest, the researchers evaluated demographic variables of age group, sex, residential status, race and ethnicity, and insurance for association with hospital admission. For the secondary outcome of interest, they evaluated presenting ED complaints related to the clinical domains of cardiopulmonary disease, psychiatric disease, and fractures and dislocations for potential trends in the ED geriatric age group between 2011 and 2015.
Mr. Huang and his associates found that, as a percentage of total ED visits, those among patients aged 65 or older rose from 14.9% in 2011 to 15.6% in 2015, an increase of 4.7%. By age group, the proportion of visits during the study period was highest for those aged 65-74 years (43.8%), followed by those aged 75-84 years (34.7%) and those aged 85 and older (21.5%). On multivariate analysis, the 75-84 and age-85-and-older groups were 1.30 and 1.71 times more likely to be admitted to the hospital, compared with the 65-74 group, respectively (P less than .000 for both).
Men were 1.19 times more likely than were women to be admitted (P less than .000). In addition, elderly patients who reside in nursing homes were 1.70 times more likely to be admitted to the hospital, compared with those who lived in private homes (P less than .000), while those with Medicare insurance were 1.57 more likely to be admitted, compared with those who did not have insurance (P = .004).
On trend analysis, ED psychiatric complaints rose incrementally during the study period, from 3.9% in 2011 to 4.7% in 2015, a relative increase of 20.5%. The researchers identified no consistent trend with visit complaints related to cardiopulmonary disease, and fractures and dislocations.
“This was not too surprising, because these difficulties with older patients are not new and many investigators have sought out solutions,” Mr. Huang said. “For example, there have been many interventions both in the ED as well as in the primary care setting designed to identify risk factors and facilitate postdischarge care to prevent falls. These approaches are constantly evolving and will be of increasing importance.”
“For example, we may be seeing a larger of proportion of patients with acute mental health complaints,” Mr. Huang said. “We will need to continue developing our multidisciplinary approach to care by improving coordination with different specialties – and especially outpatient and community health care providers.”
The study’s senior author was Jason Wasserman, PhD of Oakland University William Beaumont School of Medicine. The researchers reported having no financial disclosures.
SOURCE: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
SAN DIEGO – Between 2011 and 2015, the proportion of elderly patients presenting to the emergency department with psychiatric complaints increased by 20%, according to a retrospective analysis of national hospital data.
In addition, 10-year increases in age, male sex, nursing home status, and Medicare insurance were associated with an increased likelihood of hospital admission.
“The growing geriatric patient population is a well-known phenomenon across every developed country,” lead researcher Derrick Huang said in an interview in advance of the annual meeting of the American College of Emergency Physicians. “A potent mix of increasing life expectancy, greater disease severity and comorbidities in the elderly, and large-scale demographic shifts has placed a significant strain on both our financial and health care resources. This study corroborates these trends in the emergency department and is a preliminary exploration of potential, newly evolving clinical challenges that the ED team will increasingly face into the future.”
For the study, Mr. Huang, a fourth-year medical student at Oakland University William Beaumont School of Medicine in Rochester, Mich., and his colleagues examined National Ambulatory Medical Care Survey (NAMCS) data between 2011 and 2015. They limited the analysis to emergency department visits with patients in the age groups of 65-74, 75-84, and 85 or older. For the primary outcome of interest, the researchers evaluated demographic variables of age group, sex, residential status, race and ethnicity, and insurance for association with hospital admission. For the secondary outcome of interest, they evaluated presenting ED complaints related to the clinical domains of cardiopulmonary disease, psychiatric disease, and fractures and dislocations for potential trends in the ED geriatric age group between 2011 and 2015.
Mr. Huang and his associates found that, as a percentage of total ED visits, those among patients aged 65 or older rose from 14.9% in 2011 to 15.6% in 2015, an increase of 4.7%. By age group, the proportion of visits during the study period was highest for those aged 65-74 years (43.8%), followed by those aged 75-84 years (34.7%) and those aged 85 and older (21.5%). On multivariate analysis, the 75-84 and age-85-and-older groups were 1.30 and 1.71 times more likely to be admitted to the hospital, compared with the 65-74 group, respectively (P less than .000 for both).
Men were 1.19 times more likely than were women to be admitted (P less than .000). In addition, elderly patients who reside in nursing homes were 1.70 times more likely to be admitted to the hospital, compared with those who lived in private homes (P less than .000), while those with Medicare insurance were 1.57 more likely to be admitted, compared with those who did not have insurance (P = .004).
On trend analysis, ED psychiatric complaints rose incrementally during the study period, from 3.9% in 2011 to 4.7% in 2015, a relative increase of 20.5%. The researchers identified no consistent trend with visit complaints related to cardiopulmonary disease, and fractures and dislocations.
“This was not too surprising, because these difficulties with older patients are not new and many investigators have sought out solutions,” Mr. Huang said. “For example, there have been many interventions both in the ED as well as in the primary care setting designed to identify risk factors and facilitate postdischarge care to prevent falls. These approaches are constantly evolving and will be of increasing importance.”
“For example, we may be seeing a larger of proportion of patients with acute mental health complaints,” Mr. Huang said. “We will need to continue developing our multidisciplinary approach to care by improving coordination with different specialties – and especially outpatient and community health care providers.”
The study’s senior author was Jason Wasserman, PhD of Oakland University William Beaumont School of Medicine. The researchers reported having no financial disclosures.
SOURCE: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
REPORTING FROM ACEP18
Key clinical point: An increasing proportion of elderly patients are presenting to the emergency department with mental health complaints.
Major finding: Emergency department psychiatric complaints among elderly patients rose from 3.9% in 2011, to 4.7% in 2015, a relative increase of 20.5%.
Study details: A retrospective analysis of National Ambulatory Medical Care Survey data between 2011 and 2015.
Disclosures: The researchers reported having no financial disclosures.
Source: Huang D et al. Ann Emerg Med. 2018 Oct. doi: 10.1016/j.annemergmed.2018.08.212.
The In Vivo Impact of Leukocyte Injections on Normal Rat Achilles Tendons: Potential Detriment to Tendon Morphology, Cellularity, and Vascularity
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
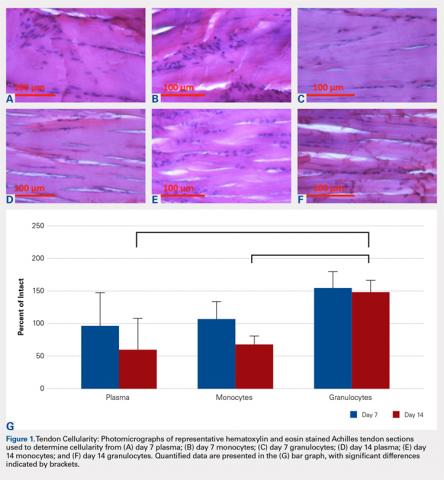
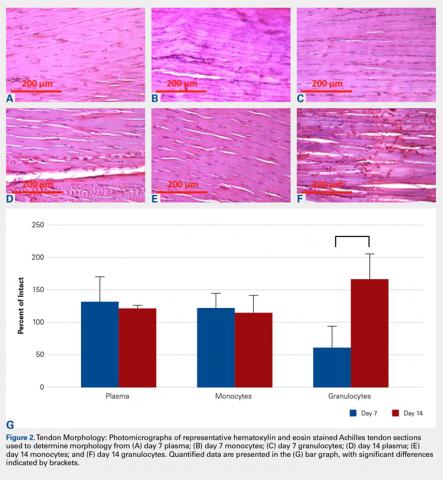
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
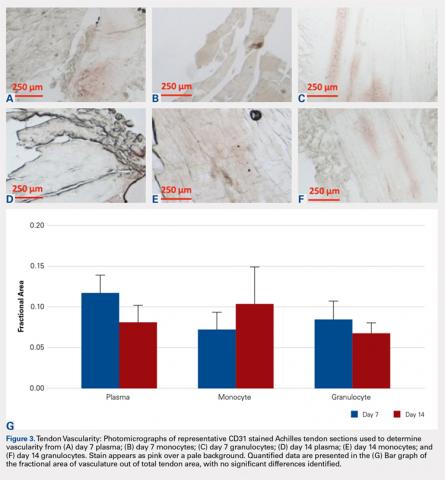
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
TAKE-HOME POINTS
- Injection of leukocytes into healthy rat Achilles tendons increases inflammation.
- Injection of leukocytes into healthy rat Achilles tendons does not affect vascularity.
- Leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis.
- Leukocyte-rich PRP preparations may be useful for chronic tendinosis.
- The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Drug-coated balloons shown noninferior to DES in thin coronaries
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Drug-coated balloon treatment worked as well as drug-eluting stents in thin coronaries.
Major finding: Twelve-month MACE occurred in 7.33% of balloon-treated patients and in 7.45% of stent-treated patients.
Study details: BASKET-SMALL 2, an international, multicenter randomized trial with 758 patients.
Disclosures: The investigator-initiated study received partial funding from B. Braun, the company that markets the drug-coated balloon (SeQuent Please) tested in the study. Dr. Jeger has received research funding from B. Braun. Dr. Mehran has been a consultant to Abbott, Bayer, BSC, and CSL Behring and has received research funding from Abbott, Astra Zeneca, Bayer, BCC, DSI, and Janssen.
The hidden cost of excellence
SAN DIEGO – Readiness and excellence in trauma services come at a high cost.
Level I trauma centers in Georgia lay out an average of $10 million each year to maintain the essential services necessary to operate at full throttle 24 hours a day, 7 days a week. At the Medical Center of Central Georgia, Macon, that amounts to about $4,480 per patient, Dennis W. Ashley, MD, said at the annual meeting of American Association for the Surgery of Trauma. The yearly tab comes to about $5 million for level II centers.
Medical staff pay was the biggest single driver of that cost, accounting for an average $5.5 million annually for level I centers and $3 million for level II centers.
“These readiness costs are incurred well before the first patient is even treated,” said Dr. Ashley, director of trauma and adult critical care at the Medical Center of Central Georgia. “These are costs required by trauma center regulations to maintain essential infrastructure – nonpatient care costs that the hospital would not have to pay if it were not a trauma center.”
Hospitals incur these costs to comply with standards outlined in “Resources for Optimal Care of the Injured Patient,” otherwise known as the “Orange Book.”
To assess these by center, the Georgia Trauma Commission created a cost-reporting survey that was distributed to the state’s 6 level I centers and 10 level II centers in 2017. The surveys examined 2016 costs, and were carried out in conjunction with independent financial reviews to guarantee accurate reporting. Data were gathered in four general areas: administrative/program support staff, clinical medical staff, in-house operating room, and outreach and education.
During 2016, the 16 centers treated a total of 24,488 trauma patients: 15,660 in level I centers and 8,828 in level II centers.
Overall, the six level I centers spent a total of about $60.5 million in 2017 on resource readiness expense. The 10 level II centers spent a total of about $51 million. In all, trauma center status costs these institutions more than $112 million that year.
Salary and benefits for clinical and medical staff were the biggest cost driver for both types of trauma centers. Level I centers laid out a total of $33.2 million – about $5.5 million for each center. Level II centers paid a total of $31.7 million – about $3.1 million each.
Administrative and program support staff comprised the next largest expense. Level I centers paid abut $21.6 million altogether – $3.6 million each. Level II centers paid $13.9 million – about $1.4 million each.
The cost of maintaining constant operating room coverage accounted for $4.9 million in the level I centers ($830,000 each), and $2.5 million in level II centers (about $252,000 each).
Outreach and education comprised the smallest portion of spending. This accounted for a total of about $700,000 for level I centers and $1 million for level II centers ($115,000 and $109,000, respectively).
Dr. Ashley further broke down each general category. In the administrative and program support bucket, trauma program managers and trauma coordinators were the main cost drivers for both types of center. Trauma managers cost a grand total of $1.56 million: $113,000 for each level I center and $88,169 for level II centers. Trauma coordinators cost a grand total of $921,800 and senior administrative support accounted for a total of about $590,000.
Program support staff consisted of physical, occupational, and speech therapists, as well as research coordinators and others. The big-ticket items here were case managers, and staff providing physical, occupational, and speech therapy. Each of those services cost a total of about $6 million for all centers (around $600,000 for each level I center and $240,000 for each level II center).
Staffing trauma surgery made up the bulk of costs for clinical medical staff (a total of almost $19 million), followed by orthopedics ($13.8 million) and neurosurgery ($6.5 million).
Education and outreach were comparatively poorly funded, Dr. Ashley said. “We should do a better job on this,” he noted. All the centers combined spent about $340,000 on injury prevention, for example. Educational programs for emergency department staff made up about $590,000, and for intensive care unit staff, the total tab was about $174,000.
The survey plainly shows the financial commitment necessary to maintain high-quality trauma care, he said. “The significant cost of trauma center readiness highlights the need for additional trauma funding.”
He had no financial disclosures.
[email protected]
SAN DIEGO – Readiness and excellence in trauma services come at a high cost.
Level I trauma centers in Georgia lay out an average of $10 million each year to maintain the essential services necessary to operate at full throttle 24 hours a day, 7 days a week. At the Medical Center of Central Georgia, Macon, that amounts to about $4,480 per patient, Dennis W. Ashley, MD, said at the annual meeting of American Association for the Surgery of Trauma. The yearly tab comes to about $5 million for level II centers.
Medical staff pay was the biggest single driver of that cost, accounting for an average $5.5 million annually for level I centers and $3 million for level II centers.
“These readiness costs are incurred well before the first patient is even treated,” said Dr. Ashley, director of trauma and adult critical care at the Medical Center of Central Georgia. “These are costs required by trauma center regulations to maintain essential infrastructure – nonpatient care costs that the hospital would not have to pay if it were not a trauma center.”
Hospitals incur these costs to comply with standards outlined in “Resources for Optimal Care of the Injured Patient,” otherwise known as the “Orange Book.”
To assess these by center, the Georgia Trauma Commission created a cost-reporting survey that was distributed to the state’s 6 level I centers and 10 level II centers in 2017. The surveys examined 2016 costs, and were carried out in conjunction with independent financial reviews to guarantee accurate reporting. Data were gathered in four general areas: administrative/program support staff, clinical medical staff, in-house operating room, and outreach and education.
During 2016, the 16 centers treated a total of 24,488 trauma patients: 15,660 in level I centers and 8,828 in level II centers.
Overall, the six level I centers spent a total of about $60.5 million in 2017 on resource readiness expense. The 10 level II centers spent a total of about $51 million. In all, trauma center status costs these institutions more than $112 million that year.
Salary and benefits for clinical and medical staff were the biggest cost driver for both types of trauma centers. Level I centers laid out a total of $33.2 million – about $5.5 million for each center. Level II centers paid a total of $31.7 million – about $3.1 million each.
Administrative and program support staff comprised the next largest expense. Level I centers paid abut $21.6 million altogether – $3.6 million each. Level II centers paid $13.9 million – about $1.4 million each.
The cost of maintaining constant operating room coverage accounted for $4.9 million in the level I centers ($830,000 each), and $2.5 million in level II centers (about $252,000 each).
Outreach and education comprised the smallest portion of spending. This accounted for a total of about $700,000 for level I centers and $1 million for level II centers ($115,000 and $109,000, respectively).
Dr. Ashley further broke down each general category. In the administrative and program support bucket, trauma program managers and trauma coordinators were the main cost drivers for both types of center. Trauma managers cost a grand total of $1.56 million: $113,000 for each level I center and $88,169 for level II centers. Trauma coordinators cost a grand total of $921,800 and senior administrative support accounted for a total of about $590,000.
Program support staff consisted of physical, occupational, and speech therapists, as well as research coordinators and others. The big-ticket items here were case managers, and staff providing physical, occupational, and speech therapy. Each of those services cost a total of about $6 million for all centers (around $600,000 for each level I center and $240,000 for each level II center).
Staffing trauma surgery made up the bulk of costs for clinical medical staff (a total of almost $19 million), followed by orthopedics ($13.8 million) and neurosurgery ($6.5 million).
Education and outreach were comparatively poorly funded, Dr. Ashley said. “We should do a better job on this,” he noted. All the centers combined spent about $340,000 on injury prevention, for example. Educational programs for emergency department staff made up about $590,000, and for intensive care unit staff, the total tab was about $174,000.
The survey plainly shows the financial commitment necessary to maintain high-quality trauma care, he said. “The significant cost of trauma center readiness highlights the need for additional trauma funding.”
He had no financial disclosures.
[email protected]
SAN DIEGO – Readiness and excellence in trauma services come at a high cost.
Level I trauma centers in Georgia lay out an average of $10 million each year to maintain the essential services necessary to operate at full throttle 24 hours a day, 7 days a week. At the Medical Center of Central Georgia, Macon, that amounts to about $4,480 per patient, Dennis W. Ashley, MD, said at the annual meeting of American Association for the Surgery of Trauma. The yearly tab comes to about $5 million for level II centers.
Medical staff pay was the biggest single driver of that cost, accounting for an average $5.5 million annually for level I centers and $3 million for level II centers.
“These readiness costs are incurred well before the first patient is even treated,” said Dr. Ashley, director of trauma and adult critical care at the Medical Center of Central Georgia. “These are costs required by trauma center regulations to maintain essential infrastructure – nonpatient care costs that the hospital would not have to pay if it were not a trauma center.”
Hospitals incur these costs to comply with standards outlined in “Resources for Optimal Care of the Injured Patient,” otherwise known as the “Orange Book.”
To assess these by center, the Georgia Trauma Commission created a cost-reporting survey that was distributed to the state’s 6 level I centers and 10 level II centers in 2017. The surveys examined 2016 costs, and were carried out in conjunction with independent financial reviews to guarantee accurate reporting. Data were gathered in four general areas: administrative/program support staff, clinical medical staff, in-house operating room, and outreach and education.
During 2016, the 16 centers treated a total of 24,488 trauma patients: 15,660 in level I centers and 8,828 in level II centers.
Overall, the six level I centers spent a total of about $60.5 million in 2017 on resource readiness expense. The 10 level II centers spent a total of about $51 million. In all, trauma center status costs these institutions more than $112 million that year.
Salary and benefits for clinical and medical staff were the biggest cost driver for both types of trauma centers. Level I centers laid out a total of $33.2 million – about $5.5 million for each center. Level II centers paid a total of $31.7 million – about $3.1 million each.
Administrative and program support staff comprised the next largest expense. Level I centers paid abut $21.6 million altogether – $3.6 million each. Level II centers paid $13.9 million – about $1.4 million each.
The cost of maintaining constant operating room coverage accounted for $4.9 million in the level I centers ($830,000 each), and $2.5 million in level II centers (about $252,000 each).
Outreach and education comprised the smallest portion of spending. This accounted for a total of about $700,000 for level I centers and $1 million for level II centers ($115,000 and $109,000, respectively).
Dr. Ashley further broke down each general category. In the administrative and program support bucket, trauma program managers and trauma coordinators were the main cost drivers for both types of center. Trauma managers cost a grand total of $1.56 million: $113,000 for each level I center and $88,169 for level II centers. Trauma coordinators cost a grand total of $921,800 and senior administrative support accounted for a total of about $590,000.
Program support staff consisted of physical, occupational, and speech therapists, as well as research coordinators and others. The big-ticket items here were case managers, and staff providing physical, occupational, and speech therapy. Each of those services cost a total of about $6 million for all centers (around $600,000 for each level I center and $240,000 for each level II center).
Staffing trauma surgery made up the bulk of costs for clinical medical staff (a total of almost $19 million), followed by orthopedics ($13.8 million) and neurosurgery ($6.5 million).
Education and outreach were comparatively poorly funded, Dr. Ashley said. “We should do a better job on this,” he noted. All the centers combined spent about $340,000 on injury prevention, for example. Educational programs for emergency department staff made up about $590,000, and for intensive care unit staff, the total tab was about $174,000.
The survey plainly shows the financial commitment necessary to maintain high-quality trauma care, he said. “The significant cost of trauma center readiness highlights the need for additional trauma funding.”
He had no financial disclosures.
[email protected]
REPORTING FROM THE AAST ANNUAL MEETING
Key clinical point: Meeting excellence requirements costs trauma centers millions of dollars.
Major finding: A level I center invests about $10 million annually to meet readiness requirements.
Study details: The Georgia survey queried six level I centers and 10 level II centers.
Disclosures: Dr. Ashley had no financial disclosures.
Source: Ashely DW et al. AAST 2018. Abstract 18.
Eat, toke or vape: Teens not too picky when it comes to pot’s potpourri
There is no doubt that some high school students will try to get high. However, the ways they’re doing it might be changing.
A survey of more than 3,000 10th-graders from 10 high schools in Los Angeles showed that, while traditional combustible marijuana is still the most popular method, kids are turning to edible and vaporized weed, according to a study published in JAMA Network Open Sept. 28.
Because of L.A.’s size and diversity, the researchers said, the patterns of cannabis use they tracked provide insights about “a wide cross-section” of American teens. They found that 62% of students who had ever tried marijuana had used multiple kinds and around 8% tried all three forms.
Those findings were consistent with the impressions of Andrew G., a 15-year-old from the Washington metropolitan area. (Kaiser Health News is not fully identifying him because he is a minor.) While he doesn’t use marijuana, he guessed that more than half of his peers have tried it. He also knows people who have used it in all three forms.
“I feel like, because it’s being incorporated in new ways – edibles, vaping – the stigma has been broken,” he said.
This attitude of wider acceptance and greater access worries researchers. “We are concerned about the developing teen brain, the potential effects on cognitive development, mood and exposure to cannabinoids and chemicals in these various products,” said Adam Leventhal, PhD, an author on the study and a professor at the University of Southern California, Los Angeles.
Dr. Leventhal called this “polyproduct use” alarming for a number of reasons.
A major one is that the body processes these forms of cannabis differently. Smoking or vaping likely has a more immediate effect, while eating it takes a longer time for the body to process. Teens might not realize how much they’re ingesting, Dr. Leventhal said.
Also, “these novel products could be drawing in youth who could be otherwise be deterred,” he added, referring to a range of commercially available cannabinoid-infused products such as gummy bears and energy drinks.
If kids are consuming many different products, he said, it increases the potential risk for addiction.
“It’s a parallel issue. We definitely do see teens who use more different forms of nicotine and tobacco products are at more risk of addiction,” Dr. Leventhal said.
The study was a cross-sectional survey of 10th-graders in the Los Angeles area. It was conducted during Jan. 2, 2015–Oct. 6, 2015. The location and timing of the survey was important, noted the researchers, because California legalized medical cannabis in 1996. In 2018, it became one of nine states to allow the sale for recreational use.
The findings, Dr. Leventhal said, could be an early warning for a trend that will only increase as more states legalize marijuana. Specifically, cannabis products appear to be gaining ground among teens, compared with other substances.
Teen alcohol and nicotine use, for example, has been on the decline for years, according to Monitoring the Future, a multidecade survey conducted by the University of Michigan, Ann Arbor.
“It’s really a public health and policy success story,” Richard Miech, PhD, the survey’s principal investigator, wrote in an email. “In contrast, marijuana use hasn’t declined much at all in the past two decades.” He was not involved in the JAMA study.
Dr. Leventhal also highlighted a socioeconomic element in the findings.
Traditionally, kids who were in a higher socioeconomic bracket were at a lower risk of using marijuana, he said. That’s still true for traditional and edible cannabis, but wealthier kids were more likely to use vaporized weed, according to the study.
Leventhal thinks the popularity of vaping is pulling more kids and teenagers into marijuana use. It removes some of the common barriers that keeps kids away from drugs: There’s no telltale smell that would alert parents, no harshness in their lungs, and a perception that it’s safer than traditional smoke.
“The same vaporizer could be used, teens can load in a liquid on one day with nicotine and the next day a liquid that has THC or another cannabinoid,” Dr. Leventhal said.
And then it might be harder for parents or teachers to detect kids’ drug use.
Getting caught with a bag of marijuana plants could immediately get a kid in trouble. But if a parent finds a vape that looks like a normal e-cigarette or a package of gummy bears laced with THC, they might not realize what they’re seeing.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
There is no doubt that some high school students will try to get high. However, the ways they’re doing it might be changing.
A survey of more than 3,000 10th-graders from 10 high schools in Los Angeles showed that, while traditional combustible marijuana is still the most popular method, kids are turning to edible and vaporized weed, according to a study published in JAMA Network Open Sept. 28.
Because of L.A.’s size and diversity, the researchers said, the patterns of cannabis use they tracked provide insights about “a wide cross-section” of American teens. They found that 62% of students who had ever tried marijuana had used multiple kinds and around 8% tried all three forms.
Those findings were consistent with the impressions of Andrew G., a 15-year-old from the Washington metropolitan area. (Kaiser Health News is not fully identifying him because he is a minor.) While he doesn’t use marijuana, he guessed that more than half of his peers have tried it. He also knows people who have used it in all three forms.
“I feel like, because it’s being incorporated in new ways – edibles, vaping – the stigma has been broken,” he said.
This attitude of wider acceptance and greater access worries researchers. “We are concerned about the developing teen brain, the potential effects on cognitive development, mood and exposure to cannabinoids and chemicals in these various products,” said Adam Leventhal, PhD, an author on the study and a professor at the University of Southern California, Los Angeles.
Dr. Leventhal called this “polyproduct use” alarming for a number of reasons.
A major one is that the body processes these forms of cannabis differently. Smoking or vaping likely has a more immediate effect, while eating it takes a longer time for the body to process. Teens might not realize how much they’re ingesting, Dr. Leventhal said.
Also, “these novel products could be drawing in youth who could be otherwise be deterred,” he added, referring to a range of commercially available cannabinoid-infused products such as gummy bears and energy drinks.
If kids are consuming many different products, he said, it increases the potential risk for addiction.
“It’s a parallel issue. We definitely do see teens who use more different forms of nicotine and tobacco products are at more risk of addiction,” Dr. Leventhal said.
The study was a cross-sectional survey of 10th-graders in the Los Angeles area. It was conducted during Jan. 2, 2015–Oct. 6, 2015. The location and timing of the survey was important, noted the researchers, because California legalized medical cannabis in 1996. In 2018, it became one of nine states to allow the sale for recreational use.
The findings, Dr. Leventhal said, could be an early warning for a trend that will only increase as more states legalize marijuana. Specifically, cannabis products appear to be gaining ground among teens, compared with other substances.
Teen alcohol and nicotine use, for example, has been on the decline for years, according to Monitoring the Future, a multidecade survey conducted by the University of Michigan, Ann Arbor.
“It’s really a public health and policy success story,” Richard Miech, PhD, the survey’s principal investigator, wrote in an email. “In contrast, marijuana use hasn’t declined much at all in the past two decades.” He was not involved in the JAMA study.
Dr. Leventhal also highlighted a socioeconomic element in the findings.
Traditionally, kids who were in a higher socioeconomic bracket were at a lower risk of using marijuana, he said. That’s still true for traditional and edible cannabis, but wealthier kids were more likely to use vaporized weed, according to the study.
Leventhal thinks the popularity of vaping is pulling more kids and teenagers into marijuana use. It removes some of the common barriers that keeps kids away from drugs: There’s no telltale smell that would alert parents, no harshness in their lungs, and a perception that it’s safer than traditional smoke.
“The same vaporizer could be used, teens can load in a liquid on one day with nicotine and the next day a liquid that has THC or another cannabinoid,” Dr. Leventhal said.
And then it might be harder for parents or teachers to detect kids’ drug use.
Getting caught with a bag of marijuana plants could immediately get a kid in trouble. But if a parent finds a vape that looks like a normal e-cigarette or a package of gummy bears laced with THC, they might not realize what they’re seeing.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
There is no doubt that some high school students will try to get high. However, the ways they’re doing it might be changing.
A survey of more than 3,000 10th-graders from 10 high schools in Los Angeles showed that, while traditional combustible marijuana is still the most popular method, kids are turning to edible and vaporized weed, according to a study published in JAMA Network Open Sept. 28.
Because of L.A.’s size and diversity, the researchers said, the patterns of cannabis use they tracked provide insights about “a wide cross-section” of American teens. They found that 62% of students who had ever tried marijuana had used multiple kinds and around 8% tried all three forms.
Those findings were consistent with the impressions of Andrew G., a 15-year-old from the Washington metropolitan area. (Kaiser Health News is not fully identifying him because he is a minor.) While he doesn’t use marijuana, he guessed that more than half of his peers have tried it. He also knows people who have used it in all three forms.
“I feel like, because it’s being incorporated in new ways – edibles, vaping – the stigma has been broken,” he said.
This attitude of wider acceptance and greater access worries researchers. “We are concerned about the developing teen brain, the potential effects on cognitive development, mood and exposure to cannabinoids and chemicals in these various products,” said Adam Leventhal, PhD, an author on the study and a professor at the University of Southern California, Los Angeles.
Dr. Leventhal called this “polyproduct use” alarming for a number of reasons.
A major one is that the body processes these forms of cannabis differently. Smoking or vaping likely has a more immediate effect, while eating it takes a longer time for the body to process. Teens might not realize how much they’re ingesting, Dr. Leventhal said.
Also, “these novel products could be drawing in youth who could be otherwise be deterred,” he added, referring to a range of commercially available cannabinoid-infused products such as gummy bears and energy drinks.
If kids are consuming many different products, he said, it increases the potential risk for addiction.
“It’s a parallel issue. We definitely do see teens who use more different forms of nicotine and tobacco products are at more risk of addiction,” Dr. Leventhal said.
The study was a cross-sectional survey of 10th-graders in the Los Angeles area. It was conducted during Jan. 2, 2015–Oct. 6, 2015. The location and timing of the survey was important, noted the researchers, because California legalized medical cannabis in 1996. In 2018, it became one of nine states to allow the sale for recreational use.
The findings, Dr. Leventhal said, could be an early warning for a trend that will only increase as more states legalize marijuana. Specifically, cannabis products appear to be gaining ground among teens, compared with other substances.
Teen alcohol and nicotine use, for example, has been on the decline for years, according to Monitoring the Future, a multidecade survey conducted by the University of Michigan, Ann Arbor.
“It’s really a public health and policy success story,” Richard Miech, PhD, the survey’s principal investigator, wrote in an email. “In contrast, marijuana use hasn’t declined much at all in the past two decades.” He was not involved in the JAMA study.
Dr. Leventhal also highlighted a socioeconomic element in the findings.
Traditionally, kids who were in a higher socioeconomic bracket were at a lower risk of using marijuana, he said. That’s still true for traditional and edible cannabis, but wealthier kids were more likely to use vaporized weed, according to the study.
Leventhal thinks the popularity of vaping is pulling more kids and teenagers into marijuana use. It removes some of the common barriers that keeps kids away from drugs: There’s no telltale smell that would alert parents, no harshness in their lungs, and a perception that it’s safer than traditional smoke.
“The same vaporizer could be used, teens can load in a liquid on one day with nicotine and the next day a liquid that has THC or another cannabinoid,” Dr. Leventhal said.
And then it might be harder for parents or teachers to detect kids’ drug use.
Getting caught with a bag of marijuana plants could immediately get a kid in trouble. But if a parent finds a vape that looks like a normal e-cigarette or a package of gummy bears laced with THC, they might not realize what they’re seeing.
KHN’s coverage of children’s health care issues is supported in part by the Heising-Simons Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Postpartum hemorrhage: Aortic compression to reduce pelvic bleeding
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11
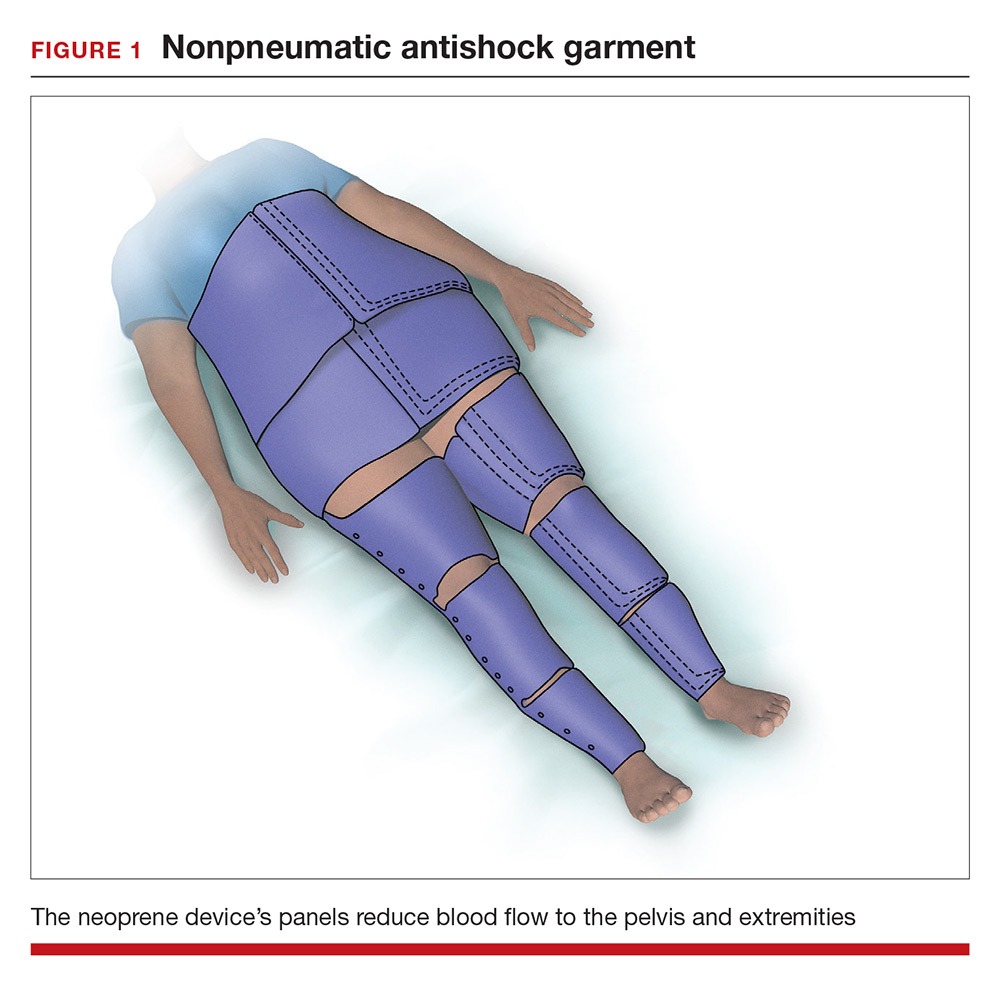
In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.
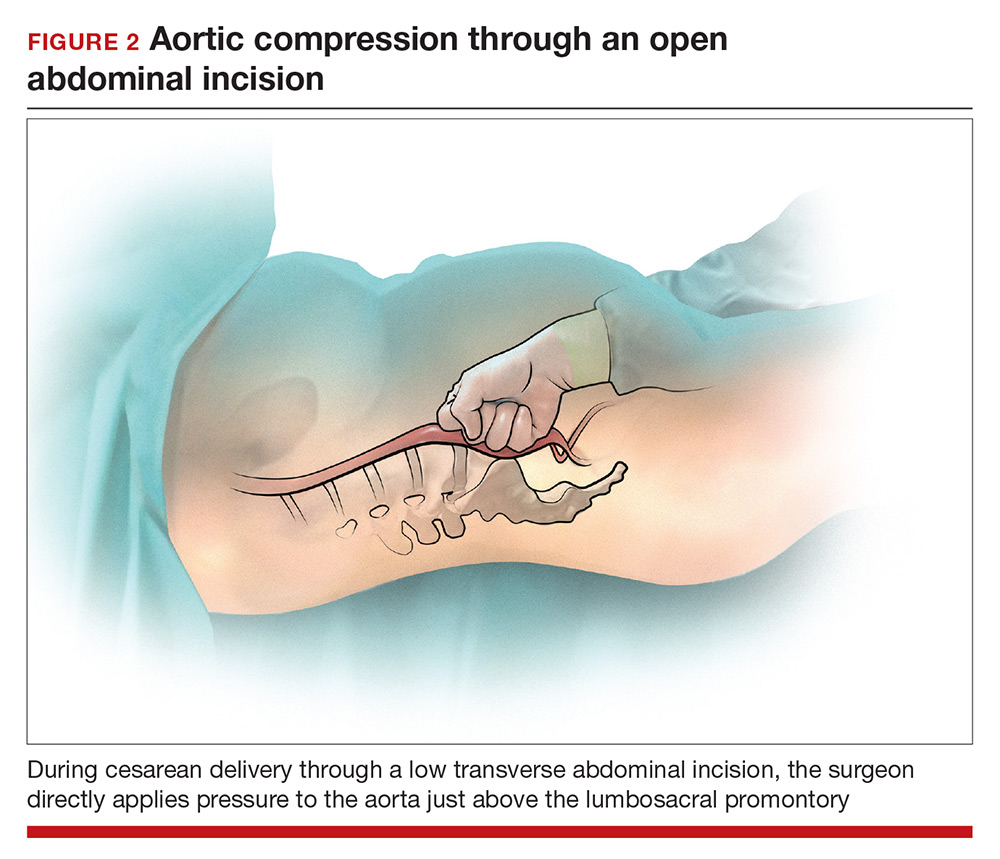
Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
You are performing a repeat cesarean delivery on a 37-year-old G3P2 woman with placenta previa. Immediately after delivery, a postpartum hemorrhage occurs. You order additional uterotonic medications and blood products and prepare for standard surgical interventions including uterine devascularization, uterine compression sutures, and intrauterine balloon tamponade. As the hemorrhage continues, you begin to consider the need to perform a hysterectomy.
Suddenly the anesthesiologist reports that the patient’s blood pressure and heart rate have decreased. She asks you to initiate aortic compression to slow the pelvic bleeding and permit initiation of interventions to restore intravascular volume and optimize cardiovascular status. You have not previously performed this maneuver, and you wonder how to respond to her request.
Preoperative preparation
Anticipating possible adverse outcomes is a key task for every clinician. In the above case, in the setting of a repeat cesarean delivery in a woman with placenta previa, there is an increased risk of postpartum hemorrhage. Therefore, appropriate blood products and equipment should be made available before the operation is initiated. It also may be helpful to review the sequential steps you have found most useful in managing a postpartum hemorrhage prior to starting the procedure.
Rapid response to obstetric hemorrhage
When postpartum hemorrhage occurs during a cesarean delivery, there are many interventions that may successfully control the excessive blood loss, including uterotonics, massive transfusion of blood products, uterine massage, tranexamic acid, uterine devascularization, uterine compression sutures, intrauterine balloon tamponade, uterine artery embolization, uterine tourniquet, internal iliac artery ligation, hysterectomy, and pelvic packing.1 Rapid response to obstetric hemorrhage is important to avoid depletion of coagulation factors and subsequent development of a coagulation disorder. Once a coagulation disorder occurs, it can be very difficult to resolve the problem and complete the surgery.
Abdominal compression
The potentially benefial role of abdominal compression to help reduce blood loss caused by trauma or obstetric hemorrhage has been studied extensively in healthy volunteers. The theory is that abdominal compression will decrease blood flow in the distal aorta, helping to control bleeding in the pelvis and extremities. In one report, 80 to 140 lb of pressure applied to the epigastrium in 9 healthy male participants in a supine position on a rigid surface resulted in decreased blood flow in the common femoral artery as determined by pulsed-wave Doppler ultrasound.2 Abdominal pressure applied above the umbilicus also has been reported to reduce blood pressure in the legs.3 Abdominal compression and tourniquets used on the extremities are not meant to be definitive treatments for traumatic hemorrhages but rather are used to stabilize severely injured patients during transport to emergency surgical care facilities.4
One approach to performing manual abdominal aortic compression involves first gaining a mechanical advantage by positioning yourself above the epigastric area with arms extended. Using one closed fist with the opposite hand providing additional pressure, the equivalent of 80 to 140 lb can be applied to the patient’s upper abdomen.4 To estimate the pressure you can achieve using this method, cover a scale with a towel and use your arms to exert maximum pressure on the scale. What equivalent weight can you reach when applying maximum pressure? What weight can you sustain for a few minutes? Using manual compression, it is difficult for a clinician to exert the equivalent of 140 lb on the epigastrium for the extended period of time needed to transport an injured person to an emergency facility.5 Therefore, mechanical devices such as the abdominal aortic tourniquet (AAT) and the nonpneumatic antishock garment (NASG) have been developed to aid in providing continuous abdominal compression.
Continue to: Abdominal aortic tourniquet
Abdominal aortic tourniquet. The AAT is a corset-like device with an interior pneumatic bladder that is designed to provide sustained compression over the abdomen, therefore compressing the abdominal aorta and reducing blood flow to the pelvis and extremities. In one study with human volunteers, a median pressure of 180 mm Hg (range, 150–230 mm Hg) was associated with cessation of blood flow in the common femoral artery in 7 of 9 volunteers and a decrease in blood flow in all participants as determined by pulsed-wave Doppler ultrasound.6 Participants reported moderate to severe discomfort when the AAT was inflated to a pressure sufficient to stop blood flow in the femoral artery. The AAT device may not be as effective in individuals with an elevated body mass index and excessive abdominal girth.7 In obstetric postpartum hemorrhage, abdominal pressure also has been reported to reduce hemorrhage and femoral artery blood flow. Using a corset-like abdominal binder with an internal spring to provide continuous pressure over the anterior abdomen, Soltan and Sadekreported a beneficial effect of abdominal pressure in the management of severe postpartum hemorrhage in a large observational study in Egypt.8,9
Nonpneumatic antishock garment. The NASG has been studied extensively as a method to help safely transport a woman with severe postpartum hemorrhage to an emergency facility. The NASG is a neoprene and Velcro device with panels for the lower extremities, pelvis, and abdomen (FIGURE 1). The device also has an abdominal segment that includes a compression ball to provide continuous abdominal pressure. When the panels are closed, blood flow to the extremities and pelvis is reduced. In a study of 10 postpartum volunteers, application of the NASG caused decreased blood flow in the internal iliac artery as measured by Doppler ultrasound, but blood flow did not stop completely.10 In an observational study of women with postpartum hemorrhage, use of the NASG device in combination with usual interventions resulted in a decrease in blood loss.11

In a cluster randomized trial, 38 birth centers in Africa were randomly assigned to standard management of obstetric hemorrhage or the same protocol plus use of the NASG prior to transport to a regional emergency surgical center. Compared with the group receiving standard management alone, the women who received standard management plus the NASG device had a nonsignificant reduction in maternal mortality (odds ratio, 0.54; 95% confidence interval [CI], 0.14–2.05; P = .37) and a significantly more rapid recovery from hypovolemic shock (hazard ratio, 1.25; 95% CI, 1.02–1.52; P = .03).12 The International Federation of Gynecology and Obstetrics has issued a guideline supporting the use of the device in the management of obstetric hemorrhage in appropriate settings.13
Aortic compression in the setting of an open abdominal incision
During cesarean delivery, the surgeon has access to the abdominal aorta via the open abdominal incision and can directly apply pressure to the aorta at sites ranging from above the sacral promontory to the subdiaphragmatic aorta. Although aortic compression is occasionally noted as a potential intervention to help with the management of postpartum hemorrhage, there is very little literature on this intervention.1 In one case report of an emergency laparotomy in a Jehovah’s Witness patient with a placenta previa, uterine rupture, massive hemorrhage (hematocrit nadir of 6%), and hypovolemic shock, direct pressure applied to the infradiaphragmatic aorta and pelvic organs permitted the anesthesiologist to stabilize the patient’s cardiovascular status, facilitating the patient’s recovery from shock.14 The authors of the case concluded that compression of the aorta and pelvic organs can be lifesaving and is underutilized in the management of uncontrolled obstetric hemorrhage. Other case reports also recommend considering the use of aortic compression to permit the anesthesia team to resuscitate a bleeding patient.15
There is very little published guidance on how to perform aortic compression at cesarean delivery. Techniques for aortic compression include using a closed fist or the heel of the hand to compress the aorta against the lumbosacral spine. Alternatively, use a moist rolled-up surgical towel or laparotomy sponge to compress the aorta against the lumbosacral spine. With a low transverse abdominal incision, the aorta just above the lumbosacral promontory is closest to the surgeon (aorta zone III) (FIGURE 2). If a vertical abdominal incision has been made, the subdiaphragmatic aorta may be within reach of the surgeon (aorta zone II). If an anesthesiologist asks you to apply aortic compression, it is likely that the patient is hypotensive. In this setting, reducing blood flow through the aorta can be achieved with less pressure than required for successful aortic compression in a healthy volunteer.

Prolonged aortic compression that completely obstructs blood flow may result in downstream ischemia. This is illustrated by leg ischemia and amputation that have occurred following the use of the resuscitative endovascular balloon occlusion of the aorta (REBOA) occlusion device.16 Another strategy that has been used in the management of massive hemorrhage, when immediate replacement of clotting factors is not possible, is damage control surgery, a technique in which capillary and venous bleeding is controlled by placing pelvic packs or a pelvic umbrella pressure pack and sending the patient to the intensive care unit for resuscitation.17 With damage control surgery, a second procedure is planned to remove the packs after the patient has been stabilized.
With knowledge and practice comes preparedness
Hopefully you will never be asked by an anesthesiologist to stop operating and initiate aortic compression. With effective preprocedure preparation and rapid institution of standard postpartum hemorrhage techniques, it is unlikely aortic compression ever will be needed. If an unusually difficult case triggers a request for aortic compression, you have the knowledge and skills to provide that service.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
- Hofmeyr GJ, Qureshi Z. Preventing deaths due to haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2016;36:68–82.
- Blaivas M, Shiver S, Lyon M, et al. Control of hemorrhage in critical femoral or inguinal penetrating wounds—an ultrasound evaluation. Prehosp Disast Med. 2006;21(6):379–382.
- Riley DP, Burgess RW. External abdominal aortic compression: a study of a resuscitation manoeuvre for postpartum hemorrhage. Anaesth Intensive Care. 1994;22(5):571–575.
- Douma M, Smith KE, Brindley PG. Temporization of penetrating abdominal-pelvic trauma with manual external aortic compression: a novel case report. Ann Emerg Med. 2014;64(1):79–81.
- Douma M, Brindley PG. Abdominal aortic and iliac artery compression following penetrating trauma: a study of feasibility. Prehosp Disaster Med. 2014;29:299–302.
- Lyon M, Shiver SA, Greenfield EM, et al. Use of a novel abdominal aortic tourniquet to reduce or eliminate flow in the common femoral artery in human subjects. J Trauma Acute Care Surg. 2012;73(2 suppl 1):S103–S105.
- Taylor DM, Coleman M, Parker PJ. The evaluation of an abdominal aortic tourniquet for the control of pelvic and lower limb hemorrhage. Mil Med. 2013;178(11):1196–1201.
- Soltan MH, Sadek RR. Experience managing postpartum hemorrhage at Minia University Maternity Hospital, Egypt: no mortality using aortic compression. J Obstet Gynaecol Res. 2011;37(11):1557–1563.
- Soltan MH, Faragallah MF, Mosabah MH, et al. External aortic compression device: the first aid for postpartum hemorrhage control. J Obstet Gynaecol Res. 2009;35(3):453–458.
- Lester F, Stenson A, Meyer C, et al. Impact of the non-pneumatic antishock garment on pelvic blood flow in healthy postpartum women. Am J Obstet Gynecol. 2011; 204(5): 409.e1–5.
- Miller S, Hamza S, Bray EH, et al. First aid for obstetric haemorrhage: the pilot study of the non-pneumatic anti-shock garment in Egypt. BJOG. 2006;113(4):424–429.
- Miller S, Bergel EF, El Ayadi AM, et al. Non-pneumatic anti-shock garment (NASG), a first-aid device to decrease maternal mortality from obstetric hemorrhage: a cluster randomized trial. PLoS One. 2013;8(10):e76477.
- FIGO Safe Motherhoood and Newborn Health Committee; International Federation of Gynecology and Obstetrics. Non-pneumatic anti-shock garment to stabilize women with hypovolemic shock secondary to obstetric hemorrhage. Int J Gynaecol Obstet. 2015;128(3):194–195.
- Belfort M, Kofford S, Varner M. Massive obstetric hemorrhage in a Jehovah’s Witness: intraoperative strategies and high-dose erythropoietin use. Am J Perinatol. 2011;28(3):207–210.
- Keogh J, Tsokos N. Aortic compression in massive postpartum hemorrhage—an old but lifesaving technique. Aust N Z J Obstet Gyencol. 1997;37(2):237–238.
- Ribeiro MAF, Feng CYD, Nguyen ATM, et al. The complications associated with resuscitative endovascular balloon occlusion of the aorta (REBOA). World J Emerg Surg. 2018;13:20.
- Pacheco LD, Lozada MJ, Saade GR, et al. Damage-control surgery for obstetric hemorrhage. Obstet Gynecol 2018;132(2):423–427.
Hemorrhage; bladder laceration during hysterectomy
Hemorrhage; bladder laceration during hysterectomy
A 46-year-old woman reported increasingly frequent and painful menstrual periods to her Gyn. Estrogen-progestin contraceptives were relatively contraindicated because of the patient’s hyp
The patient began to hemorrhage immediately after surgery; nurses informed the Gyn of this multiple times over the next several hours. After 7 hours, the Gyn examined the patient, found that she was in hemorrhagic shock, and advised a hysterectomy was necessary. During surgery, the Gyn lacerated the patient’s bladder twice, which required a urologist to repair. Postoperatively, the patient had a stroke, respiratory failure, and kidney failure.
PATIENT'S CLAIM: The Gyn’s morcellation technique was negligent. He did not respond to the nurses for 7 hours. If he had responded earlier, she might not have lost her uterus. He was also negligent for injuring the patient’s bladder during the second surgery.
PHYSICIAN'S DEFENSE: The case was settled during mediation.
VERDICT: A confidential North Carolina settlement was reached.
Bowel injured during BSO
In 2013, a 52-year-old woman underwent bilateral salpingo-oophorectomy (BSO) performed by a Gyn. Postoperatively, she was found to have a 1.5-cm bowel perforation. After surgical repair, she developed a wound infection and wound breakdown. She was treated with a vacuum-assisted wound closure device. She later developed a ventral hernia and an intra-abdominal abscess leading to a colostomy, which eventually was reversed. At trial, she had a low-output bowel-to-skin fistula and extensive abdominal scarring.
PATIENT'S CLAIM: The surgeon should have known to perform open BSO rather than laparoscopic surgery based on her 3 prior abdominal surgeries that would have left severe adhesions. He caused a perforation and/or thermal injury to the sigmoid colon during the BSO. He should have consulted a general surgeon when encountering the adhesions. The surgeon failed to readmit her on a timely basis for treatment of the suspected bowel injury.
PHYSICIAN'S DEFENSE: The severe adhesions encountered during BSO surgery could not have been predicted; no adhesions were noted during a 2004 surgery. The adhesions precluded procedure completion. He attempted to lyse the adhesions to create a visual field for removing the ovaries but they could not be visualized. After using a harmonic scalpel for lysis, he inspected the bowel portions that he could see and found no thermal injury or perforation.
VERDICT: An Illinois defense verdict was returned.
Multiple injuries after LVH
A woman was found to have a 4-cm uterine fibroid in April 2007. She received medical management.
In May 2008, she reported left lower quadrant pain to her Gyn. A pelvic ultrasound showed an increase in the fibroid’s diameter to 5.8 cm. On December 4 she underwent laparoscopic-assisted vaginal hysterectomy (LVH). The Gyn performed intraoperative cystoscopy. The patient was discharged the following day.
Over the next several weeks, the patient experienced urinary tract symptoms that progressed to rust-colored urine and incontinence. On December 31 she was found to have bilateral vesicovaginal fistulas. By early April 2009, urologists had placed ureteral stents on 2 separate occasions and performed 2 bilateral reimplantation procedures. On April 28, 2009, a urologist placed a stent in the right ureter but was unable to place a stent in the left ureter. The right stent was removed prior to another reconstructive surgery on August 18. Two stents were also placed on August 26 and were removed on October 6. She underwent annual ultrasounds that revealed minimal hydronephrosis. Except for urinary frequency, the patient’s symptoms had subsided by trial.
PATIENT'S CLAIM: The Gyn fell below the standard of care during the LVH when he negligently cauterized and/or burned the patient’s ureters.
PHYSICIAN'S DEFENSE: The Gyn denied negligence. She argued that, following the cystoscopy, both of the patient’s ureteral orifices discharged indigo carmine–stained urine, an indication that there was no injury to the ureters.
VERDICT: A Nevada defense verdict was returned.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hemorrhage; bladder laceration during hysterectomy
A 46-year-old woman reported increasingly frequent and painful menstrual periods to her Gyn. Estrogen-progestin contraceptives were relatively contraindicated because of the patient’s hyp
The patient began to hemorrhage immediately after surgery; nurses informed the Gyn of this multiple times over the next several hours. After 7 hours, the Gyn examined the patient, found that she was in hemorrhagic shock, and advised a hysterectomy was necessary. During surgery, the Gyn lacerated the patient’s bladder twice, which required a urologist to repair. Postoperatively, the patient had a stroke, respiratory failure, and kidney failure.
PATIENT'S CLAIM: The Gyn’s morcellation technique was negligent. He did not respond to the nurses for 7 hours. If he had responded earlier, she might not have lost her uterus. He was also negligent for injuring the patient’s bladder during the second surgery.
PHYSICIAN'S DEFENSE: The case was settled during mediation.
VERDICT: A confidential North Carolina settlement was reached.
Bowel injured during BSO
In 2013, a 52-year-old woman underwent bilateral salpingo-oophorectomy (BSO) performed by a Gyn. Postoperatively, she was found to have a 1.5-cm bowel perforation. After surgical repair, she developed a wound infection and wound breakdown. She was treated with a vacuum-assisted wound closure device. She later developed a ventral hernia and an intra-abdominal abscess leading to a colostomy, which eventually was reversed. At trial, she had a low-output bowel-to-skin fistula and extensive abdominal scarring.
PATIENT'S CLAIM: The surgeon should have known to perform open BSO rather than laparoscopic surgery based on her 3 prior abdominal surgeries that would have left severe adhesions. He caused a perforation and/or thermal injury to the sigmoid colon during the BSO. He should have consulted a general surgeon when encountering the adhesions. The surgeon failed to readmit her on a timely basis for treatment of the suspected bowel injury.
PHYSICIAN'S DEFENSE: The severe adhesions encountered during BSO surgery could not have been predicted; no adhesions were noted during a 2004 surgery. The adhesions precluded procedure completion. He attempted to lyse the adhesions to create a visual field for removing the ovaries but they could not be visualized. After using a harmonic scalpel for lysis, he inspected the bowel portions that he could see and found no thermal injury or perforation.
VERDICT: An Illinois defense verdict was returned.
Multiple injuries after LVH
A woman was found to have a 4-cm uterine fibroid in April 2007. She received medical management.
In May 2008, she reported left lower quadrant pain to her Gyn. A pelvic ultrasound showed an increase in the fibroid’s diameter to 5.8 cm. On December 4 she underwent laparoscopic-assisted vaginal hysterectomy (LVH). The Gyn performed intraoperative cystoscopy. The patient was discharged the following day.
Over the next several weeks, the patient experienced urinary tract symptoms that progressed to rust-colored urine and incontinence. On December 31 she was found to have bilateral vesicovaginal fistulas. By early April 2009, urologists had placed ureteral stents on 2 separate occasions and performed 2 bilateral reimplantation procedures. On April 28, 2009, a urologist placed a stent in the right ureter but was unable to place a stent in the left ureter. The right stent was removed prior to another reconstructive surgery on August 18. Two stents were also placed on August 26 and were removed on October 6. She underwent annual ultrasounds that revealed minimal hydronephrosis. Except for urinary frequency, the patient’s symptoms had subsided by trial.
PATIENT'S CLAIM: The Gyn fell below the standard of care during the LVH when he negligently cauterized and/or burned the patient’s ureters.
PHYSICIAN'S DEFENSE: The Gyn denied negligence. She argued that, following the cystoscopy, both of the patient’s ureteral orifices discharged indigo carmine–stained urine, an indication that there was no injury to the ureters.
VERDICT: A Nevada defense verdict was returned.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hemorrhage; bladder laceration during hysterectomy
A 46-year-old woman reported increasingly frequent and painful menstrual periods to her Gyn. Estrogen-progestin contraceptives were relatively contraindicated because of the patient’s hyp
The patient began to hemorrhage immediately after surgery; nurses informed the Gyn of this multiple times over the next several hours. After 7 hours, the Gyn examined the patient, found that she was in hemorrhagic shock, and advised a hysterectomy was necessary. During surgery, the Gyn lacerated the patient’s bladder twice, which required a urologist to repair. Postoperatively, the patient had a stroke, respiratory failure, and kidney failure.
PATIENT'S CLAIM: The Gyn’s morcellation technique was negligent. He did not respond to the nurses for 7 hours. If he had responded earlier, she might not have lost her uterus. He was also negligent for injuring the patient’s bladder during the second surgery.
PHYSICIAN'S DEFENSE: The case was settled during mediation.
VERDICT: A confidential North Carolina settlement was reached.
Bowel injured during BSO
In 2013, a 52-year-old woman underwent bilateral salpingo-oophorectomy (BSO) performed by a Gyn. Postoperatively, she was found to have a 1.5-cm bowel perforation. After surgical repair, she developed a wound infection and wound breakdown. She was treated with a vacuum-assisted wound closure device. She later developed a ventral hernia and an intra-abdominal abscess leading to a colostomy, which eventually was reversed. At trial, she had a low-output bowel-to-skin fistula and extensive abdominal scarring.
PATIENT'S CLAIM: The surgeon should have known to perform open BSO rather than laparoscopic surgery based on her 3 prior abdominal surgeries that would have left severe adhesions. He caused a perforation and/or thermal injury to the sigmoid colon during the BSO. He should have consulted a general surgeon when encountering the adhesions. The surgeon failed to readmit her on a timely basis for treatment of the suspected bowel injury.
PHYSICIAN'S DEFENSE: The severe adhesions encountered during BSO surgery could not have been predicted; no adhesions were noted during a 2004 surgery. The adhesions precluded procedure completion. He attempted to lyse the adhesions to create a visual field for removing the ovaries but they could not be visualized. After using a harmonic scalpel for lysis, he inspected the bowel portions that he could see and found no thermal injury or perforation.
VERDICT: An Illinois defense verdict was returned.
Multiple injuries after LVH
A woman was found to have a 4-cm uterine fibroid in April 2007. She received medical management.
In May 2008, she reported left lower quadrant pain to her Gyn. A pelvic ultrasound showed an increase in the fibroid’s diameter to 5.8 cm. On December 4 she underwent laparoscopic-assisted vaginal hysterectomy (LVH). The Gyn performed intraoperative cystoscopy. The patient was discharged the following day.
Over the next several weeks, the patient experienced urinary tract symptoms that progressed to rust-colored urine and incontinence. On December 31 she was found to have bilateral vesicovaginal fistulas. By early April 2009, urologists had placed ureteral stents on 2 separate occasions and performed 2 bilateral reimplantation procedures. On April 28, 2009, a urologist placed a stent in the right ureter but was unable to place a stent in the left ureter. The right stent was removed prior to another reconstructive surgery on August 18. Two stents were also placed on August 26 and were removed on October 6. She underwent annual ultrasounds that revealed minimal hydronephrosis. Except for urinary frequency, the patient’s symptoms had subsided by trial.
PATIENT'S CLAIM: The Gyn fell below the standard of care during the LVH when he negligently cauterized and/or burned the patient’s ureters.
PHYSICIAN'S DEFENSE: The Gyn denied negligence. She argued that, following the cystoscopy, both of the patient’s ureteral orifices discharged indigo carmine–stained urine, an indication that there was no injury to the ureters.
VERDICT: A Nevada defense verdict was returned.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
For solitary renal tumors, RFA looks good at 10 years
Radiofrequency ablation (RFA) of small renal tumors is safe and effective, and is associated with high rates of disease-free survival, according to a study that followed patients for up to 10 years.
In 106 patients who had a total of 112 tumors and who were followed for a median 79 months, 10 recurrences were seen, after an initial procedural success rate of 97%.
Over a 6-year period, Kaplan-Meier disease-free survival (DFS) was 89%, and cancer-specific survival (CSS) was 96%. In the subgroup followed for 10 years, DFS was 82%, CSS was 94%, and overall survival (OS) was 49%.
Tumors were, on average, small (mean 2.5 cm), but for patients whose tumors were larger than 3 cm, the DFS rate fell to 68%. Patients were included in the study if they had a solitary renal mass; those who had metastatic renal cell carcinoma (RCC) or a hereditary kidney cancer syndrome were excluded.
Brett Johnson, MD, and his collaborators from the University of Texas Southwestern, Dallas, noted that an additional 29 patients received RFA but also had partial nephrectomy; these patients were excluded. “Healthier patients with larger tumors may have been advised to undergo partial nephrectomy, thereby selecting for more comorbid patients for RFA,” they noted in discussing their findings. The report was published in The Journal of Urology
Within these parameters, Dr. Johnson and his colleagues conducted a retrospective review of clinic patients whose renal tumors were successfully treated with RFA between the years 2000 and 2007. Patients were followed with yearly imaging, and were considered to have a recurrence if contrast enhancement was seen within the ablation zone at any point after a negative imaging study.
Of the 10 recurrences that were seen, eight were local and two were local and metastatic. An additional patient developed metastatic RCC without evidence of local recurrence; all patients with metastases died of their disease, said Dr. Johnson and his coauthors.
For patients whose tumors recurred, the mean initial tumor size was 3.2 cm, compared with 2.4 cm in those whose tumors didn’t recur (P = .005). Looking at the tumor size data another way, tumor size “was an independent risk factor for cancer recurrence,” with an odds ratio of 3.01 (P = .025), wrote Dr. Johnson and his collaborators.
They noted that it was not routine practice for biopsies to be performed during the initial study period; the seven patients with recurrences who had biopsy data all had clear cell RCC. Median time to local recurrence was 26 months, with no recurrences seen after 5 years.
At the time of the initial procedure, patients were a mean 63.1 years old. The relatively low OS in the subgroup with 10 years of follow-up was likely related to advancing age.
In the subgroup analysis of patients for whom 10-year data were available, the investigators used only data from patients whose initial tumors were biopsied when calculating CSS and metastasis-free survival; these rates were both 94% for those analyzed.
“Age, gender, and time of follow-up had no statistically significant effect on disease-free recurrence” in a univariate analysis, said Dr. Johnson and his colleagues.
“Nephron sparing surgery is the gold standard for treatment of small renal masses,” and the study bolsters the safety and durable efficacy of RFA by using actual survival data rather than actuarial disease survival, said the investigators.
The current study is unique in having such a long duration of follow-up and a subgroup of individuals with 10 years of annual imaging data. In addition, experienced urologists at a single site all used a uniform technique to perform thermal ablation on solitary tumors, noted Dr. Johnson and his coauthors. Successful ablations were followed only by surveillance, in keeping with current American Urological Association recommendations.
However, the study’s retrospective, nonrandomized nature introduces the possibility of selection bias, and variations in follow-up protocols may be a source of ascertainment bias, they said.
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Johnson B et al. J Urol. 2018. doi:10.1016/j.juro.2018.08.045.
Radiofrequency ablation (RFA) of small renal tumors is safe and effective, and is associated with high rates of disease-free survival, according to a study that followed patients for up to 10 years.
In 106 patients who had a total of 112 tumors and who were followed for a median 79 months, 10 recurrences were seen, after an initial procedural success rate of 97%.
Over a 6-year period, Kaplan-Meier disease-free survival (DFS) was 89%, and cancer-specific survival (CSS) was 96%. In the subgroup followed for 10 years, DFS was 82%, CSS was 94%, and overall survival (OS) was 49%.
Tumors were, on average, small (mean 2.5 cm), but for patients whose tumors were larger than 3 cm, the DFS rate fell to 68%. Patients were included in the study if they had a solitary renal mass; those who had metastatic renal cell carcinoma (RCC) or a hereditary kidney cancer syndrome were excluded.
Brett Johnson, MD, and his collaborators from the University of Texas Southwestern, Dallas, noted that an additional 29 patients received RFA but also had partial nephrectomy; these patients were excluded. “Healthier patients with larger tumors may have been advised to undergo partial nephrectomy, thereby selecting for more comorbid patients for RFA,” they noted in discussing their findings. The report was published in The Journal of Urology
Within these parameters, Dr. Johnson and his colleagues conducted a retrospective review of clinic patients whose renal tumors were successfully treated with RFA between the years 2000 and 2007. Patients were followed with yearly imaging, and were considered to have a recurrence if contrast enhancement was seen within the ablation zone at any point after a negative imaging study.
Of the 10 recurrences that were seen, eight were local and two were local and metastatic. An additional patient developed metastatic RCC without evidence of local recurrence; all patients with metastases died of their disease, said Dr. Johnson and his coauthors.
For patients whose tumors recurred, the mean initial tumor size was 3.2 cm, compared with 2.4 cm in those whose tumors didn’t recur (P = .005). Looking at the tumor size data another way, tumor size “was an independent risk factor for cancer recurrence,” with an odds ratio of 3.01 (P = .025), wrote Dr. Johnson and his collaborators.
They noted that it was not routine practice for biopsies to be performed during the initial study period; the seven patients with recurrences who had biopsy data all had clear cell RCC. Median time to local recurrence was 26 months, with no recurrences seen after 5 years.
At the time of the initial procedure, patients were a mean 63.1 years old. The relatively low OS in the subgroup with 10 years of follow-up was likely related to advancing age.
In the subgroup analysis of patients for whom 10-year data were available, the investigators used only data from patients whose initial tumors were biopsied when calculating CSS and metastasis-free survival; these rates were both 94% for those analyzed.
“Age, gender, and time of follow-up had no statistically significant effect on disease-free recurrence” in a univariate analysis, said Dr. Johnson and his colleagues.
“Nephron sparing surgery is the gold standard for treatment of small renal masses,” and the study bolsters the safety and durable efficacy of RFA by using actual survival data rather than actuarial disease survival, said the investigators.
The current study is unique in having such a long duration of follow-up and a subgroup of individuals with 10 years of annual imaging data. In addition, experienced urologists at a single site all used a uniform technique to perform thermal ablation on solitary tumors, noted Dr. Johnson and his coauthors. Successful ablations were followed only by surveillance, in keeping with current American Urological Association recommendations.
However, the study’s retrospective, nonrandomized nature introduces the possibility of selection bias, and variations in follow-up protocols may be a source of ascertainment bias, they said.
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Johnson B et al. J Urol. 2018. doi:10.1016/j.juro.2018.08.045.
Radiofrequency ablation (RFA) of small renal tumors is safe and effective, and is associated with high rates of disease-free survival, according to a study that followed patients for up to 10 years.
In 106 patients who had a total of 112 tumors and who were followed for a median 79 months, 10 recurrences were seen, after an initial procedural success rate of 97%.
Over a 6-year period, Kaplan-Meier disease-free survival (DFS) was 89%, and cancer-specific survival (CSS) was 96%. In the subgroup followed for 10 years, DFS was 82%, CSS was 94%, and overall survival (OS) was 49%.
Tumors were, on average, small (mean 2.5 cm), but for patients whose tumors were larger than 3 cm, the DFS rate fell to 68%. Patients were included in the study if they had a solitary renal mass; those who had metastatic renal cell carcinoma (RCC) or a hereditary kidney cancer syndrome were excluded.
Brett Johnson, MD, and his collaborators from the University of Texas Southwestern, Dallas, noted that an additional 29 patients received RFA but also had partial nephrectomy; these patients were excluded. “Healthier patients with larger tumors may have been advised to undergo partial nephrectomy, thereby selecting for more comorbid patients for RFA,” they noted in discussing their findings. The report was published in The Journal of Urology
Within these parameters, Dr. Johnson and his colleagues conducted a retrospective review of clinic patients whose renal tumors were successfully treated with RFA between the years 2000 and 2007. Patients were followed with yearly imaging, and were considered to have a recurrence if contrast enhancement was seen within the ablation zone at any point after a negative imaging study.
Of the 10 recurrences that were seen, eight were local and two were local and metastatic. An additional patient developed metastatic RCC without evidence of local recurrence; all patients with metastases died of their disease, said Dr. Johnson and his coauthors.
For patients whose tumors recurred, the mean initial tumor size was 3.2 cm, compared with 2.4 cm in those whose tumors didn’t recur (P = .005). Looking at the tumor size data another way, tumor size “was an independent risk factor for cancer recurrence,” with an odds ratio of 3.01 (P = .025), wrote Dr. Johnson and his collaborators.
They noted that it was not routine practice for biopsies to be performed during the initial study period; the seven patients with recurrences who had biopsy data all had clear cell RCC. Median time to local recurrence was 26 months, with no recurrences seen after 5 years.
At the time of the initial procedure, patients were a mean 63.1 years old. The relatively low OS in the subgroup with 10 years of follow-up was likely related to advancing age.
In the subgroup analysis of patients for whom 10-year data were available, the investigators used only data from patients whose initial tumors were biopsied when calculating CSS and metastasis-free survival; these rates were both 94% for those analyzed.
“Age, gender, and time of follow-up had no statistically significant effect on disease-free recurrence” in a univariate analysis, said Dr. Johnson and his colleagues.
“Nephron sparing surgery is the gold standard for treatment of small renal masses,” and the study bolsters the safety and durable efficacy of RFA by using actual survival data rather than actuarial disease survival, said the investigators.
The current study is unique in having such a long duration of follow-up and a subgroup of individuals with 10 years of annual imaging data. In addition, experienced urologists at a single site all used a uniform technique to perform thermal ablation on solitary tumors, noted Dr. Johnson and his coauthors. Successful ablations were followed only by surveillance, in keeping with current American Urological Association recommendations.
However, the study’s retrospective, nonrandomized nature introduces the possibility of selection bias, and variations in follow-up protocols may be a source of ascertainment bias, they said.
The authors reported no conflicts of interest and no outside sources of funding.
SOURCE: Johnson B et al. J Urol. 2018. doi:10.1016/j.juro.2018.08.045.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Radiofrequency ablation of small renal tumors is safe and effective.
Major finding: In 106 patients with small renal masses, disease-free survival was 89% at 6 years post procedure.
Study details: Retrospective cohort study of 106 patients with 112 tumors, followed up to 10 years.
Disclosures: The authors reported no conflicts of interest and no outside sources of funding.
Source: Johnson B et al. J Urol. 2018. doi: 10.1016/j.juro.2018.08.045.
Zoledronate reduces fracture risk in elderly women with osteopenia
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
REPORTING FROM ASBMR
Key clinical point: Vertebral and nonvertebral fracture risk was significantly lower in osteopenic women who received zoledronate, compared with those who received a placebo.
Major finding: Fragility fractures occurred in 122 women in a zoledronate group and 190 women in a placebo group. The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
Study details: A 6-year randomized, double-blind trial of 2,000 women aged 65 years and older with osteopenia.
Disclosures: The study was supported in part by grants from the Health Research Council of New Zealand; Novartis provided the medication. Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
Source: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
Marking Migraine with Aura from Stroke in Children
Recent findings support the use of multimodal magnetic resonance (MR) imaging to distinguish migraine with aura from stroke and the simultaneous use of these MR imaging sequences to improve understanding of perfusion changes during migraine with aura. Hemiplegic migraine is a common cause of acute brain attack in pediatrics. MR imaging sequences useful in differentiating hemiplegic migraine from other entities include arterial spin-labeling, susceptibility-weighted imaging (SWI), magnetic resonance angiography (MRA), and diffusion-weighted imaging (DWI). Researchers evaluated 12 pediatric patients with acute hemiplegic migraine or migraine with aura who underwent MR imaging within 12 hours of symptom onset. Quantitative and qualitative analyses were performed on arterial spin-labeling, and qualitative analysis, on SWI and MRA sequences. They found:
- All 12 patients had normal DWI and abnormal arterial spin-labeling findings.
- Furthermore, a more rapid transition from hypoperfusion to rebound hyperperfusion was observed in 3 patients compared with prior reports.
Cobb-Pitstick KM, Munjal N, Safier R, Cummings DD, Zuccoli G. Time course of cerebral perfusion changes in children with migraine with aura mimicking stroke. Am J Neuroradiol.
2018;39(9):1751-1755. doi:10.3174/ajnr.A5693.
Recent findings support the use of multimodal magnetic resonance (MR) imaging to distinguish migraine with aura from stroke and the simultaneous use of these MR imaging sequences to improve understanding of perfusion changes during migraine with aura. Hemiplegic migraine is a common cause of acute brain attack in pediatrics. MR imaging sequences useful in differentiating hemiplegic migraine from other entities include arterial spin-labeling, susceptibility-weighted imaging (SWI), magnetic resonance angiography (MRA), and diffusion-weighted imaging (DWI). Researchers evaluated 12 pediatric patients with acute hemiplegic migraine or migraine with aura who underwent MR imaging within 12 hours of symptom onset. Quantitative and qualitative analyses were performed on arterial spin-labeling, and qualitative analysis, on SWI and MRA sequences. They found:
- All 12 patients had normal DWI and abnormal arterial spin-labeling findings.
- Furthermore, a more rapid transition from hypoperfusion to rebound hyperperfusion was observed in 3 patients compared with prior reports.
Cobb-Pitstick KM, Munjal N, Safier R, Cummings DD, Zuccoli G. Time course of cerebral perfusion changes in children with migraine with aura mimicking stroke. Am J Neuroradiol.
2018;39(9):1751-1755. doi:10.3174/ajnr.A5693.
Recent findings support the use of multimodal magnetic resonance (MR) imaging to distinguish migraine with aura from stroke and the simultaneous use of these MR imaging sequences to improve understanding of perfusion changes during migraine with aura. Hemiplegic migraine is a common cause of acute brain attack in pediatrics. MR imaging sequences useful in differentiating hemiplegic migraine from other entities include arterial spin-labeling, susceptibility-weighted imaging (SWI), magnetic resonance angiography (MRA), and diffusion-weighted imaging (DWI). Researchers evaluated 12 pediatric patients with acute hemiplegic migraine or migraine with aura who underwent MR imaging within 12 hours of symptom onset. Quantitative and qualitative analyses were performed on arterial spin-labeling, and qualitative analysis, on SWI and MRA sequences. They found:
- All 12 patients had normal DWI and abnormal arterial spin-labeling findings.
- Furthermore, a more rapid transition from hypoperfusion to rebound hyperperfusion was observed in 3 patients compared with prior reports.
Cobb-Pitstick KM, Munjal N, Safier R, Cummings DD, Zuccoli G. Time course of cerebral perfusion changes in children with migraine with aura mimicking stroke. Am J Neuroradiol.
2018;39(9):1751-1755. doi:10.3174/ajnr.A5693.